UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. )
| Filed by the Registrantý |
Filed by a Party other than the Registranto |
Check the appropriate box: |
o |
|
Preliminary Proxy Statement |
o |
|
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
ý |
|
Definitive Proxy Statement |
o |
|
Definitive Additional Materials |
o |
|
Soliciting Material Pursuant to §240.14a-12
|
IMCOR Pharmaceutical Co. |
(Name of Registrant as Specified In Its Charter) |
|
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
| | | | | |
| Payment of Filing Fee (Check the appropriate box): |
ý |
|
No fee required. |
o |
|
Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | | (1) | | Title of each class of securities to which transaction applies:
|
| | | (2) | | Aggregate number of securities to which transaction applies:
|
| | | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
|
| | | (4) | | Proposed maximum aggregate value of transaction:
|
| | | (5) | | Total fee paid:
|
o |
|
Fee paid previously with preliminary materials. |
o |
|
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
|
|
(1) |
|
Amount Previously Paid:
|
| | | (2) | | Form, Schedule or Registration Statement No.:
|
| | | (3) | | Filing Party:
|
| | | (4) | | Date Filed:
|
|
|
|
|
Persons who are to respond to the collection of information contained in this form are not required to respond unless the form displays a currently valid OMB control number. |
IMCOR Pharmaceutical Co.
6175 Lusk Boulevard
San Diego, CA 92121
Notice of 2004 Annual Meeting
of Stockholders and Proxy Statement | | December 27, 2004 |
The 2004 annual meeting of stockholders of IMCOR Pharmaceutical Co. (formerly Photogen Technologies, Inc.) (the "Company") will be held on January 20, 2005, at 10:00 a.m., Chicago time, at the Union League Club of Chicago, 65 West Jackson Boulevard, Chicago, Illinois. The purposes of the meeting are to:
- 1.
- Ratify the issuance of more than 20% of the Company's voting stock in connection with a financing transaction with certain institutional and accredited investors and the conversion of the aggregate principal amount of $8,559,500, plus accrued interest, under the Company's secured promissory notes into the Company's common stock;
- 2.
- Amend our 2000 Long Term Incentive Compensation Plan to (a) increase the number of shares of common stock reserved for issuance from 4,568,750 to 30,000,000 shares, (b) provide for the cashless exercise of options, and (c) provide that acceleration of vesting of options on a change of control is discretionary not mandatory;
- 3.
- Amend our Restated Articles of Incorporation to increase the number of shares of common stock authorized for issuance from 150,000,000 to 200,000,000 shares;
- 4.
- Amend our Restated Articles of Incorporation to effect, as determined by our Board of Directors in its sole discretion, one or more reverse stock splits in a range of a total of one-for-two up to one-for-twenty;
- 5.
- Elect seven directors; and
- 6.
- Transact such other business as may properly come before the meeting or any adjournment.
Stockholders are cordially invited to attend the meeting.If you plan to attend, please notify the Company by January 13, 2005. Space is limited. Only stockholders holding common stock of record at the close of business on November 26, 2004 will be entitled to notice of the annual meeting and to vote at the meeting or any adjournment. A list of stockholders of the Company entitled to vote at the meeting will be available for inspection by stockholders at the Company's office, for ten days before the annual meeting during normal business hours.
Please complete, date, sign and return the enclosed proxy card in the envelope provided, which requires no postage if mailed in the United States. If you decide to attend the meeting, you may, if desired, revoke the proxy and vote the shares in person. Attendance at this meeting does not itself serve to revoke your proxy.
| | | By Order of the Board of Directors, |
|
|
Taffy J. Williams, President and Chief Executive Officer |
TABLE OF CONTENTS
| INTRODUCTION | | 1 |
| | General Information | | 1 |
| | Shareholders Entitled to Vote | | 1 |
| | How to Vote | | 1 |
| | Changing Your Vote | | 1 |
| | Votes Required to Approve Proposals | | 2 |
| | Board Recommendation; Interest of Certain Persons in Matters to Be Acted Upon | | 2 |
| | Agreements to Vote in Favor of Proposals | | 2 |
| | Proxy Solicitation | | 3 |
| | Dissenters' Rights of Appraisal | | 3 |
| | Stockholder Proposals | | 3 |
| | Licensing Transaction | | 3 |
| PRO FORMA INFORMATION | | 3 |
| FORWARD LOOKING STATEMENTS | | 4 |
| PROPOSAL 1 Ratify Issuance of More Than 20% of Our Outstanding Stock in a Financing Transaction and Conversion of Notes | | 4 |
| | Background of Financing Transaction | | 6 |
| | Summary of Financing Transaction | | 6 |
| | Use of Proceeds | | 7 |
| | Dilution | | 7 |
| | Exhibits Relating to Proposal 1 | | 7 |
| PROPOSAL 2 Amend the 2000 Long Term Incentive Compensation Plan | | 8 |
| | Introduction | | 8 |
| | Summary of the 2000 Long Term Incentive Compensation Plan | | 9 |
| | | Introduction | | 9 |
| | | Nature Of Awards To Be Granted Pursuant to the 2000 Long Term Incentive Compensation Plan | | 9 |
| | | Common Stock Subject to the 2000 Long Term Incentive Compensation Plan | | 9 |
| | | Administration of the 2000 Long Term Incentive Compensation Plan | | 10 |
| | | Purchase of Common Stock Under the 2000 Long Term Incentive Compensation Plan | | 10 |
| | | Termination of Employment, Death or Disability of Option holder | | 11 |
| | | Expiration, Termination and Amendment of the 2000 Long Term Incentive Compensation Plan | | 11 |
| | | Federal Tax Consequences | | 11 |
| | | New Plan Benefits | | 12 |
| | | Equity Compensation Plan Information | | 12 |
| PROPOSAL 3 Amend Articles of Incorporation to Increase Authorized Shares of Common Stock | | 13 |
| PROPOSAL 4 Amend Articles of Incorporation to Effect a Reverse Stock Split | | 14 |
| | | Introduction | | 14 |
| | | Reasons for the Reverse Split | | 15 |
| | | Effecting the Reverse Split | | 15 |
| | | No Fractional Shares | | 16 |
| | | Exchange of Stock Certificates | | 16 |
| | | Effect on Outstanding Shares | | 16 |
| | | Accounting Consequences | | 17 |
| | | Federal Income Tax Consequences | | 17 |
| PROPOSAL 5 Election of Seven Directors to the Company's Board of Directors | | 17 |
| | Directors, Executive Officers, Promoters and Control Persons | | 18 |
| | | |
i
| | | Director Nominees | | 18 |
| | Executive Officers and Significant Employees | | 19 |
| | | Directors' Meetings and Committees | | 20 |
| | | Corporate Governance | | 22 |
| | | Security Ownership of Certain Beneficial Owners and Management | | 22 |
| | | Compensation Committee Interlocks and Insider Participation | | 25 |
| | | Certain Relationships and Related Transactions | | 25 |
| | | Executive Compensation | | 26 |
| | | Stock Options | | 27 |
| | Compliance with Section 16(a) of the Exchange Act | | 29 |
| | | Change in Control Agreements | | 30 |
| | Securities Authorized for Issuance Under Equity Compensation Plans. | | 30 |
| | Director Compensation. | | 31 |
| | Compensation Committee Report | | 31 |
| | Stock Performance Chart | | 32 |
| Financial and Other Information | | 33 |
| | | Financial Statements and Supplementary Financial Information | | 33 |
| | | Management's Discussion and Analysis of Financial Condition and Result of Operations | | 33 |
| | | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | | 33 |
| | | Quantitative and Qualitative Disclosures About Market Risk | | 33 |
| | | Other Information | | 33 |
| | Audit Committee Report | | 33 |
| | Independent Auditors' Fees | | 35 |
| | | Audit Fees | | 35 |
| | | Audit-Related Fees. | | 35 |
| | | Tax Fees. | | 35 |
| | | All Other Fees. | | 35 |
| | | Audit Committee Pre-Approval Policy. | | 36 |
| Other Matters | | 36 |
| Information Incorporated By Reference | | 36 |
ii
EXHIBIT LIST
| A | | Form of Securities Purchase Agreement |
| B | | Form of Warrant Agreement |
| C | | Form of Registration Rights Agreement |
| D | | April Voting Agreement |
| E | | Amended 2000 Long-Term Incentive Compensation Plan |
| F | | Form 10-KSB/A for fiscal year ended December 31, 2003, as amended |
| G | | Audit Committee Charter |
| H | | Form 10-QSB/A for the quarterly period ended September 30, 2004 |
iii
IMCOR PHARMACEUTICAL CO.
6175 LUSK BOULEVARD
SAN DIEGO, CA 92121
PROXY STATEMENT
FOR
2004 ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON JANUARY 20, 2005
INTRODUCTION
General Information. IMCOR Pharmaceutical Co. (formerly known as Photogen Technologies, Inc.) ("we," "us," "our," or the "Company") is furnishing this Proxy Statement to holders of our common stock, par value $.001 per share ("Common Stock"), in connection with the solicitation of Proxies on behalf of the Board of Directors for the Annual Meeting of Stockholders to be held at 10:00 a.m., Chicago time, on January 20, 2005 (the "Annual Meeting"). This Annual Meeting is for 2004. The Annual Meeting will be held at the Union League Club of Chicago, 65 West Jackson Boulevard, Chicago, Illinois. We mailed this Proxy Statement to stockholders on approximately December 27, 2004.
Shareholders Entitled to Vote. Only stockholders of record at the close of business on November 26, 2004 are entitled to notice of and to vote at the Annual Meeting. Stockholders will be solicited by mail on or about December 27, 2004. On all matters that may come before the Annual Meeting, each holder of Common Stock will be entitled to one vote for each share of Common Stock he or she holds at the close of business on November 26, 2004.
Holders of a majority of the outstanding Common Stock entitled to vote and present in person or represented by proxy will constitute a quorum at the Annual Meeting. We will count abstentions and broker non-votes for purposes of determining the presence of a quorum, but we will not count them to determine whether the stockholders have approved any given proposal. A broker "non-vote" occurs when a nominee holding shares for a beneficial owner does not vote on a particular proposal because the nominee does not have discretionary voting power with respect to that item and has not received instructions from the beneficial owner.
At November 26, 2004 there were approximately 80,065,300 shares of Common Stock issued and outstanding and entitled to one vote per share. The holders of Common Stock vote as a group on all matters to be considered at the Annual Meeting, except on Proposal 1 for which certain shares are excluded as described under "Vote Required to Approve Proposals" below. We also have 4,500 shares of newly issued Series A Convertible Preferred Stock outstanding but those shares are not entitled to vote on the matters being considered at the Annual Meeting.
We are the record holder of 5,033 shares of Common Stock (less than 0.007% of the outstanding Common Stock) as exchange agent for stockholders who have not turned in their shares of our predecessor (Bemax Corporation) in exchange for Company shares in connection with previous reverse splits of the Common Stock. Proxy materials will be delivered to the last known addresses of those stockholders. If any of those stockholders vote at the Annual Meeting (by Proxy or attending the Annual Meeting), we will record their vote in accordance with their direction. We will treat shares held by such stockholders who do not vote as broker non-votes.
How to Vote. Please sign, date and return the enclosed Proxy promptly. If your shares are held in the name of a bank, broker or other holder of record (that is, in "street name") you will receive instructions from the holder of record that you must follow for your shares to be voted.
Changing Your Vote. Returning your completed Proxy will not prevent you from voting in person at the Annual Meeting if you are present and wish to vote. You may attend the Annual Meeting, revoke your Proxy and vote in person if you desire to do so, but attending the Annual Meeting will not
1
by itself revoke your Proxy. If your shares are held in the name of a bank, broker or other holder of record, you must obtain a Proxy, executed in your favor, from the holder of record to be able to vote at the Annual Meeting. You may revoke your Proxy at any time before it is exercised by either giving written notice of revocation to the Company's Secretary or by submitting a new Proxy dated after the revoked Proxy to us before the Annual Meeting.
Votes Required to Approve Proposals. Shares represented by executed Proxies that are not revoked will be voted in accordance with the instructions in the Proxy or, in the absence of instructions, in accordance with the recommendations of the Board of Directors. Assuming a quorum is present at the Annual Meeting, the following table sets forth the votes required to approve each Proposal:
Proposal
| | Vote Required to Approve
|
|---|
| Proposal 1 (Ratify issuance of more than 20% of stock in connection with a financing transaction and conversion of Secured Promissory Notes) | | Holders of a majority of Common Stock present in person or represented by proxy (excluding shares issued in the initial and second closings of the financing and upon the conversion of the Secured Notes) |
Proposal 2 (Amend the 2000 Long Term Incentive Compensation Plan) |
|
Holders of a majority of Common Stock present in person or represented by proxy |
Proposal 3 (Amend Articles of Incorporation to Increase Authorized Shares) |
|
Holders of a majority of Common Stock present in person or represented by proxy |
Proposal 4 (Reverse Stock Split) |
|
Holders of a majority of Common Stock present in person or represented by proxy |
Proposal 5 (Elect 7 directors) |
|
A plurality of the votes cast by the holders of Common Stock (a "plurality" means that the individuals who receive the largest number of affirmative votes cast are elected as directors up to the maximum number of directors to be chosen) |
Other Business |
|
Holders of a majority of Common Stock present in person or represented by proxy |
Board Recommendation; Interest of Certain Persons in Matters to Be Acted Upon. The Board of Directors unanimously approved each of the Proposals to be considered at the Annual Meeting and recommends that stockholders also vote FOR approval of each Proposal. Because they may be deemed to have an indirect financial interest in certain aspects of the financing transaction, Messrs. Fleming and McPhee abstained from the Board's vote on the financing transaction. Mr. Fleming is associated with Oxford Bioscience Partners IV L.P. ("Oxford") and MRNA Fund II L.P. ("MRNA") and Mr. McPhee is associated with Mi3, L.P. ("Mi3"). Those entities are participating in the financing transaction.
Agreements to Vote in Favor of Proposals. Pursuant to a Voting, Drag-Along and Right of First Refusal Agreement dated as of November 12, 2002 (the "Voting Agreement") and a separate Voting Agreement dated as of April 7, 2004, as amended by Amendment No. 1 to this separate Voting Agreement dated as of April 19, 2004 (the "April Voting Agreement"), Dr. Robert Weinstein, Stuart
2
Levine, Tannebaum, LLC, Mi3, Oxford and MRNA (collectively, the "Stockholders"), who collectively own approximately 51,384,623 shares of Common Stock (approximately 66.2%, as of November 26, 2004, of the Common Stock eligible to vote on Proposal 1 and approximately 64.2%, as of November 26, 2004, of the shares of Common Stock eligible to vote for Proposal 2 and for directors), agreed to vote in favor of Proposal 1 (ratify issuance of more than 20% of stock and conversion of notes in connection with a financing transaction), Proposal 2 (amend 2000 Long Term Incentive Compensation Plan) and Proposal 5 (election of seven director nominees). A copy of the April Voting Agreement is attached as Exhibit D. Because the record date of November 26, 2004 follows the initial and second closings of the financing transaction, the shares issued in the initial and second closings, upon the conversion of the Secured Notes and to reflect the adjusted conversion price of the conversion of the Convertible Notes will not vote at the Annual Meeting on Proposal 1.
As a result of the Voting Agreement and the April Voting Agreement, there are sufficient votes to approve Proposals 1 and 2 and approve the director nominees.
Proxy Solicitation. We will pay all expenses of the solicitation, including the cost of preparing, assembling and mailing the proxy solicitation materials. We expect to reimburse brokerage houses, custodians, nominees and fiduciaries on request for reasonable out-of-pocket expenses they incur in connection with forwarding solicitation materials to the beneficial owners of our Common Stock.
Dissenters' Rights of Appraisal. There are no dissenters' rights of appraisal in connection with any of the matters to be voted on at the Annual Meeting.
Stockholder Proposals. Stockholders interested in presenting a proposal for consideration at our annual meeting of stockholders in 2005 may do so by following the procedures prescribed in Rule 14a-8 under the Securities Exchange Act of 1934 and our Bylaws. To be eligible for inclusion, the Company's Corporate Secretary must receive stockholder proposals no later than March 31, 2005.
Licensing Transaction. On October 29, 2004, we entered into a non-exclusive, cross-licensing agreement with Bristol-Myers Squibb Medical Imaging, Inc. (“BMSMI”) covering ultrasound contrast agent patents. Under the terms of the agreement, BMSMI and the Company will have the right to further develop and commercialize their respective ultrasound contrast imaging agents without risk of infringing upon the other company’s patent rights. We received a payment of $4,000,000 under the terms of the license agreement, and will have the right of first negotiation to license select compounds from Bristol-Myers Squibb Medical Imaging, Inc.
In connection with the license agreement, we entered into a securities purchase agreement with BMSMI and Bristol-Myers Squibb Company pursuant to which we sold an aggregate of 4,500 shares of its recently authorized Series A Convertible Preferred Stock for a total purchase price of $4,500,000. Each such share is entitled to a liquidation preference if not otherwise converted to common stock of $1,000, and may also be redeemed at the same price beginning no earlier than the year 2034. These Series A shares may be convertible under certain conditions into 16,666,667 shares of the our common stock at a conversion rate of $0.27 per share.
An investment banking institution was paid $680,000 in cash and has been issued a 5-year warrant to purchase up to 1,333,333 shares of our common stock at a purchase price of $0.27 per underlying share as compensation for assistance it provided to us in this transaction with BMSMI.
Utilizing a portion of the proceeds received under the BMSMI agreements, on November 2, 2004, we repaid the remaining balance outstanding under the Secured Notes held by Xmark Fund Ltd. and Xmark Fund L.P., amounting to $1,183,835. These notes were secured by a lien on substantially all of our assets. The security interest has now been terminated and at this time there are no blanket liens on any of our owned assets.
We estimate the amount of additional financing required to fund operations and meet continuing obligations through December 31, 2005 could be an additional $12,000,000, in addition to the $8,500,000 resulting from transactions with BMSMI. At our current rate of expenditures (which can be reduced), we may not be able to continue our current level of operations beyond the first quarter of 2005. Therefore, substantial additional capital resources will be required to fund our planned ongoing operations related to clinical development, marketing and manufacturing of Imagent, research and business development activities, debt repayment and other working capital needs.
PRO FORMA INFORMATION
The table below shows the beneficial ownership of certain stockholders: (i) before the transactions in Proposals 1 and 2; (ii) after the consummation of the issuances of Common Stock, warrants and Agent Warrants (defined below) in connection with the financing transaction (Proposal 1); and (iii) after the increase in the shares reserved under the 2000 Long Term Incentive Compensation Plan (the "2000 Plan") (Proposal 2).
Pro Forma Ownership
As of November 26, 2004
| | Pre-Financing—
April 18, 2004(1)(5)
| | Actual as of November 26, 2004 reflecting $10.15 MM Financing Debt Conversion(5)
| | Pro-Forma Increase in Options 2000 Plan
| |
|---|
| | Shares
| | Percent
Ownership
| | Shares
| | Percent
Ownership
| | Shares
| | Percent
Ownership
| |
|---|
| Parties to voting agreements(2) | | 11,918,951 | | 49.2 | % | 54,325,874 | | 54.6 | % | 54,325,874 | | 42.3 | % |
| All Other Officers, Directors, 5% Holders(2) | | 3,268,581 | | 13.5 | % | 1,865,292 | | 1.9 | % | 1,865,292 | | 1.5 | % |
| Other Options, Warrants(2) | | 1,027,355 | | 4.2 | % | 14,586,884 | (3) | 14.7 | % | 14,586,884 | | 11.4 | % |
| $10.15 Million Financing, Debt Conversion | | — | | 0.0 | % | 19,612,499 | | 19.7 | % | 19,612,499 | | 15.3 | % |
| Other Investors | | 8,002,085 | | 33.1 | % | 9,038,928 | | 9.1 | % | 9,038,928 | | 7.0 | % |
| Reserve for issued warrants and options not exercisable in 60 days and reserve for ungranted stock options | | 0 | | 0 | % | 0 | | 0 | % | 28,877,501 | (4) | 22.5 | % |
| | |
| |
| |
| |
| |
| |
| |
| Total | | 24,216,972 | | 100.0 | % | 99,429,477 | | 100.0 | % | 128,306,978 | | 100.0 | % |
- (1)
- Does not include the originally issued Series A Preferred Stock (which was converted into common stock as of June 10, 2004).
3
- (2)
- Includes options and warrants exercisable within 60 days.
- (3)
- Includes warrants representing 11,836,251 shares issued as part of the $10.15 million financing, not otherwise included under the voting agreement.
- (4)
- Includes stock options not exercisable within 60 days representing 7,618,790 shares (including 6,311,852 attributable to Taffy Williams, 502,500 attributable to other officers and directors, and 804,438 attributable to other parties); warrants not exercisable in 60 days representing 152,000 shares and reserve for ungranted options representing 21,106,711 shares.
- (5)
- Excludes reserve for issued warrants and options not exercisable in 60 days and reserve for ungranted stock options because this column reflects beneficially held voting shares.
FORWARD LOOKING STATEMENTS
This Proxy Statement contains (and press releases and other public statements we may issue from time to time may contain) forward-looking statements regarding our business and operations. Statements that are not historical facts are forward-looking statements. Forward-looking statements include those relating to:
- •
- our current business and product development plans,
- •
- our future business, product development and acquisition plans,
- •
- the timing and results of regulatory approval for proposed products, and
- •
- projected capital needs, working capital, liquidity, revenues, interest costs and income.
Examples of forward-looking statements include predictive statements, statements that depend on or refer to future events or conditions, and statements that may include words such as "expects," "anticipates," "intends," "plans," "believes," "estimates," "should," "may," or similar expressions, or statements that imply uncertainty or involve hypothetical events.
Forward-looking statements involve known and unknown risks and uncertainties that may cause our actual results in future periods to differ materially from what is currently anticipated. We made cautionary statements in certain sections of our Form 10-KSB/A for the year ended December 31, 2003, as filed with the Securities and Exchange Commission ("SEC"), including under "Risk Factors." A copy of the Form 10-KSB/A will accompany this Proxy Statement and is included in this Proxy Statement. You should read these cautionary statements as being applicable to all related forward-looking statements wherever they appear in this Proxy Statement. In light of these risks, uncertainties and assumptions, the forward-looking events discussed in this Proxy Statement or other documents incorporated by reference might not occur. No forward-looking statement is a guarantee of future performance and you should not place undue reliance on any forward-looking statement. We do not undertake any obligation to release publicly any revisions to these forward looking statements, to reflect events or circumstances after the date of this Proxy Statement, or to reflect the occurrence of unanticipated events, except as may be required under applicable securities laws.
PROPOSAL 1
Ratify Issuance of More Than 20% of Our Outstanding Stock in a Financing Transaction and Conversion of Notes
At this Annual Meeting we are asking stockholders to consider and vote upon a proposal to ratify the $10.15 million financing we recently completed. We had planned to obtain stockholder approval of this financing prior to issuing more than 20% of our shares, but Nasdaq's decision to delist our shares from the SmallCap Market gave us an opportunity to close prior to obtaining stockholder approval. We nonetheless want to give stockholders an opportunity to consider the financing at this Annual Meeting.
Until June 28, 2004, our Common Stock was listed on the Nasdaq SmallCap Market ("Nasdaq"). Because we did not hold an annual meeting in 2003 we were operating under a temporary exemption from the annual meeting listing requirement granted by Nasdaq that was to expire in June, 2004. In order to finalize our proxy statement and the accompanying Form 10-KSB/A we had to delay the
4
Annual Meeting until mid-July. We requested that Nasdaq extend the exemption until July 19, 2004 in order to complete our response to the SEC's comments. We received notice on June 24, 2004 that Nasdaq would not agree to an extension of the time in which to hold the Annual Meeting.
Accordingly, Nasdaq delisted our shares effective June 28, 2004. Nasdaq denied our appeal of their decision to delist our shares. We cannot provide assurances as to whether our shares can be listed on another exchange or Nasdaq at a later date.
NASD Marketplace Rule 4350(i)(1)(D)(ii) requires companies to seek stockholder approval prior to the sale, issuance or potential issuance by the company of common stock (or securities convertible or exercisable into common stock) equal to 20% or more of the common stock or voting power outstanding before the issuance for less than the greater of book or market value of the stock. For that reason, we divided the financing into two tranches, the first involving the issuance of 19.5% of our Common Stock and the balance of the securities to be issued in a second tranche which would close after we obtained stockholder approval.
Because the necessity of having a shareholder vote prior to issuing more than 20% of our common stock is a Nasdaq rule, the delisting enabled us to close the second tranche of our financing before obtaining a shareholder vote. We consulted with the investors in this financing prior to closing the second tranche and, based on the feedback we received and to reflect changes since the original pricing of the transaction, we decided to reduce the purchase price for the Common Stock to $0.40 per share (from $0.75) and to lower the exercise price of the warrants to $0.50 per share (from $1.00). The warrants continue to be exercisable for 50% of the shares each investor purchased, but covers a greater number of shares given the reduction in the purchase price and corresponding increase in the number of shares issued to each investor.
We closed the second tranche of the financing on June 30, 2004. Holders of over 87% of the Common Stock to be purchased in the financing waived any condition to closing that would not have been met due to Nasdaq delisting our shares and the Company not holding a shareholder vote to approve issuing more than 20% of its stock in the second closing (and any representations, warranties and covenants that may have been breached by these events).
In essence, the financing transaction involved the following:
- •
- We issued 25,374,999 shares of Common Stock for a total purchase price of $10,150,000 and warrants to acquire a total of 12,687,502 shares of Common Stock at an exercise price of $0.50 per share of our Common Stock in a private placement to accredited investors.
- •
- We converted an aggregate principal amount of $8,559,500 plus accrued interest we owed to three existing investors pursuant to our Secured Promissory Notes (the "Secured Notes"), each bearing interest at a rate of 7.25% per annum, into 22,670,952 shares of Common Stock. No warrants were issued in connection with this conversion.
- •
- We converted $4,160,000 principal amount, plus accrued interest, of the Revolving Convertible Senior Secured Promissory Notes (the "Convertible Notes") into 11,069,720 shares of Common Stock pursuant to stockholder approval we received on February 5, 2004 (5,903,851 shares issued at the initial closing and 5,165,869 shares issued to reflect the adjusted conversion price at the second closing). No warrants were issued in connection with this conversion.
5
| • | | We issued to our co-placement agents for the financing, Roth Capital Partners, LLC and Rodman & Renshaw, LLC, as part of their fees for services rendered in the financing transaction, warrants to purchase 2,030,000 shares of our Common Stock (8% of the shares sold in the financing). The warrants issued to our placement agents in connection with the financing are referred to as the "Agent Warrants." |
Background of Financing Transaction. We have been seeking additional financing since 2003. We were not successful in raising additional capital on acceptable terms during that time. During this period, our three principal institutional investors, Oxford, Mi3 and MRNA, provided us with capital through secured loans. As we believed that the capital markets were becoming more receptive to companies such as ours, we reinitiated our efforts to seek additional equity financing. We retained Roth Capital Partners, LLC and Rodman & Renshaw, LLC, as co-placement agents, to assist us in this financing.
Summary of Financing Transaction. We sold in a private transaction $10.15 million of our Common Stock to accredited investors in accordance with the Securities Purchase Agreement entered into between the Company and each of the purchasers signatory thereto (including Oxford and MRNA, affiliates of Mr. Fleming, a Company director, and Mi3, an affiliate of Mr. McPhee, a Company director) (collectively, the "Purchasers"). A copy of the Securities Purchase Agreement is attached as Exhibit A. The transaction is intended to qualify as a private placement under Section 4(2) of the Securities Act of 1933 and/or Rule 506 of Regulation D under the Securities Act. The purchase price was $0.40 per share (the "Offering Price").
We also issued warrants with an exercise price of $0.50 per share to the investors for an aggregate number of shares of our Common Stock equal to the product of 50% multiplied by the issue amount divided by the Offering Price (resulting in warrants carrying one half of the Common Stock purchased at the closing for cash). The warrants may be exercised for a period of five years and are immediately exercisable. We have the right to demand that the holders of the warrants exercise the warrants at any time if the price of our Common Stock exceeds $3.50 per share for at least twenty consecutive trading days. The exercise price and the number of shares issuable upon exercise of the warrant are subject to anti-dilution adjustments. A copy of the Warrant Agreement is attached as Exhibit B.
We filed a registration statement on August 3, 2004 covering the resale of the shares sold to the investors. A copy of the Registration Rights Agreement is attached as Exhibit C. We paid all expenses associated with the registration statement. If the registration statement is not declared effective by the SEC within the first to occur of (i) the 60th day following the second closing (or 90th day after the second closing if the filed registration statement is reviewed and commented upon by the SEC), (ii) the 60th day following termination of the obligations to complete the second closing in accordance with the Securities Purchase Agreement (or the 90th day after such event if the filed registration statement is reviewed and commented upon by the SEC), or (iii) the fifth business day following the date on which we are is notified by the SEC that the registration statement will not be reviewed or is no longer subject to further review and comments, we must make pro rata payments to each investor of 2% of the aggregate amount that investor invested as partial compensation for the delay. We may pay these amounts by delivering additional shares of Common Stock valued at the monthly closing price.
Three institutional investors (Oxford, Mi3 and MRNA) loaned us (i) an aggregate principal amount of $4,160,000 as evidenced by Convertible Notes, bearing interest at 7.25% per annum and (ii) an aggregate principal amount of $8,559,500 as evidenced by Secured Notes, bearing interest at 7.25% per annum.
The Convertible Notes (including accrued interest) were convertible by their terms into shares of our Common Stock on the same terms that shares were issued in a qualified financing. The conversion of the Convertible Notes into our Common Stock was previously approved by the stockholders at the Special Meeting of our Stockholders held on February 5, 2004. The Convertible Notes plus accrued interest were converted into 11,069,720 shares of our Common Stock at the conversion price of $0.40 per share (5,903,851 shares issued at the initial closing and 5,165,869 shares issued to reflect the adjusted conversion price at the second closing).
6
The three institutional investors agreed to also convert the Secured Notes (including accrued interest) into 22,670,952 shares of our Common Stock at the conversion price of $0.40 per share (with no warrants). The Stockholders have or will receive registration rights with respect to all of the shares issued in connection with the conversion of the Convertible Notes and the Secured Notes.
The Common Stock issued in the financing transaction will be entitled to one vote per share on all matters the holders of Common Stock may vote on; provided, however that because the record date of November 26, 2004 follows the initial and second closings of the financing transaction, the shares issued in the initial and second closings, upon the conversion of the Secured Notes and to reflect the adjusted conversion price of the conversion of the Convertible Notes will not vote at the Annual Meeting on Proposal 1. This is a requirement under Nasdaq rules which we are following in connection with this ratification.
Use of Proceeds. Proceeds from the sale of our Common Stock to the Purchasers must be used for the following purposes unless otherwise authorized by a majority of our Board:
- •
- manufacturing, marketing and continued development of Imagent®;
- •
- research and development of PH-50;
- •
- research and development of N1177;
- •
- payment of commissions and other fees associated with the financing transaction;
- •
- payment of certain obligations to vendors of goods or services and other creditors; and
- •
- working capital and general corporate purposes.
Pending use of the proceeds for these purposes, we will invest the funds in securities issued or guaranteed by the United States, national bank repurchase agreements secured by those securities or other investments approved by a majority of the Board, but not in any way that would result in the Company being subject to the Investment Company Act of 1940.
Dilution. As a consequence of the shares to be issued in the financing transaction, current holders of Common Stock will incur dilution of their shares. The table under the heading "Pro Forma Information" on page 3 above illustrates the dilution.
Exhibits Relating to Proposal 1. The following exhibits to this Proxy Statement are pertinent to Proposal 1:
Exhibit
| | Description
|
|---|
| Exhibit A | | Form of Securities Purchase Agreement |
| Exhibit B | | Form of Warrant Agreement |
| Exhibit C | | Form of Registration Rights Agreement |
| Exhibit D | | April Voting Agreement |
7
PROPOSAL 2
Amend the 2000 Long Term Incentive Compensation Plan
Introduction
We adopted the 2000 Plan at the Annual Meeting held in May, 2000. As of November 26, 2004, there were 4,568,750 shares reserved for issuance under the 2000 Plan and options covering 3,899,137 shares of Common Stock have been granted and remain outstanding.
We have two other stock option plans, the 1998 Long Term Incentive Compensation Plan (the "1998 Plan") and the Senior Executive Long Term Incentive Compensation Plan (the "Senior Executive Plan"). As of November 26, 2004, there were 500,000 shares reserved for issuance under the 1998 Plan and options covering 414,625 shares of Common Stock have been granted and remain outstanding. As of November 26, 2004, there were 1,000,000 shares reserved for issuance under the Senior Executive Plan and options covering 1,000,000 shares of Common Stock have been granted and remain outstanding. We have no current intentions to increase the number of shares available for grant under either of these two plans.
Our Board of Directors believes the availability of additional options to purchase Common Stock is necessary to enable us to continue to provide our key employees with equity ownership as an incentive to contribute to our success. Thus, our Compensation Committee voted to amend the 2000 Plan, subject to stockholder approval, to increase the shares covered by the 2000 Plan by 25,431,250 shares, from 4,568,750 to 30,000,000 shares. This increase includes the award of a non-qualified option to purchase 1,050,000 shares of Common Stock granted to Dr. Williams, our President and Chief Executive Officer, pursuant to his current employment agreement. The option granted to Dr. Williams has an exercise price of $1.08 per share and a term of 10 years. It vests upon the earlier of a Change of Control, as defined therein, and Dr. Williams' ceasing to be a salaried employee of the Company. Stockholders holding approximately 64.2% of our Common Stock outstanding as of November 26, 2004 have agreed in writing to approve the issuance of the option to Dr. Williams. Pursuant to the terms of Dr. Williams' employment agreement because the Company closed a financing transaction by July 23, 2004 he was also awarded a non-qualified option to purchase 4,028,402 shares (i.e., the number of shares, which taken together with all other options Dr. Williams has been awarded (other than his options to purchase 750,000 shares with an exercise price or $60 per share and 300,000 shares at $11 per share), equaled 6% of our outstanding Common Stock on a fully diluted basis). The additional option has a 10-year term, exercisable at $.40 per share (i.e., the same price as the shares issued in the financing).
We are now asking the stockholders to approve the amendment of the 2000 Plan to increase the aggregate number of shares of Common Stock issuable under the 2000 Plan from 4,568,750 to 30,000,000. We are also planning to amend the vesting and payment provisions of the Plan. Currently, vesting of option awards accelerates if there is a Change of Control of the Company. Because we will be seeking new capital there is a possibility that such a transaction would result in a Change of Control as defined in the 2000 Plan but under circumstances where it would not be appropriate to accelerate vesting. Therefore, we would like to amend the 2000 Plan so that, upon a Change of Control, vesting of awards would be accelerated in the Compensation Committee's discretion rather than automatically. This change would only affect awards granted after the effective date of the amendment. Each option receipient's award agreement shall specify whether or not such options vest upon a Change of Control.
The exercise price for options granted under the 2000 Plan currently may be paid in cash. We would like to amend the 2000 Plan to provide for the cashless exercise of options, allowing the option recipient to exercise options by transferring to the Company shares he or she has held for a period of at least six months. Each option recipient’s award agreement will specify whether or not such option holder has a cashless exercise right, and will apply only to option awards granted after the effective date of the 2000 Plan amendment.
8
The Board of Directors believes that it is in the Company's best interests to continue the practice of making stock options available to those key employees responsible for significant contributions to our business. The Board believes that the equity stake in our development and success afforded by stock options provides key employees with an incentive to continue to energetically apply their talents to our business.
Summary of the 2000 Long Term Incentive Compensation Plan
The following is a brief and non-comprehensive summary of the 2000 Plan. The complete text of the 2000 Plan, as amended, is attached as Exhibit E to this Proxy Statement and you should refer to that Exhibit for a complete statement of the Plan's provisions.
Introduction. The 2000 Plan is intended to advance our interests by providing key employees with additional incentives to promote the success of our business, to increase their proprietary interest in our success and to encourage them to remain in our employ. The Board and its Compensation Committee believe that the 2000 Plan is a necessary tool to help us compete effectively with other enterprises for the services of new employees and to retain key employees and directors, all as may be required for the future development of our business. Currently, options covering 3,899,137 shares which remain outstanding have been granted to employees under the 2000 Plan. We have granted options covering 414,625 shares which remain outstanding under the 1998 Plan, and options covering 1,000,000 shares which remain outstanding under the Senior Executive Plan to employees, outside directors and others. We cannot determine at this time how many other persons will be eligible for receipt of awards under the 2000 Plan.
Each senior executive officer of the Company, by reason of being eligible to receive awards under the 2000 Plan, has an interest in seeing that the stockholders increase the shares covered by the 2000 Plan.
Nature Of Awards To Be Granted Pursuant to the 2000 Long Term Incentive Compensation Plan. The 2000 Plan provides for the grant of options intended to qualify as "incentive stock options" ("ISOs") under Section 422(b) of the Internal Revenue Code of 1986 (the "Code") and non-qualified stock options ("NQSOs"). The 2000 Plan also permits awards of restricted stock. Any award may be subject to various performance goals established by the Compensation Committee.
Common Stock Subject to the 2000 Long Term Incentive Compensation Plan. Currently, the aggregate number of shares of Common Stock covered by the 2000 Plan is 4,568,750 shares. If Proposal 2 is approved, 30,000,000 shares will be covered by the 2000 Plan.
Shares issued upon exercise of options or awards of Restricted Stock under the 2000 Plan may be either authorized but unissued shares or shares re-acquired by the Company. If, on or prior to the termination of the 2000 Plan, an award expires or is terminated for any reason without having been exercised or vested in full, the unpurchased or unvested shares will again become available for the grant of awards under the 2000 Plan.
The Compensation Committee will determine the purchase price of the shares of Common Stock covered by each NQSO and will be at least the par value of the shares. ISOs will have an exercise price of at least 100% of the fair market value of the Common Stock at the time the option is granted, determined by reference to the closing sale price at which the Common Stock trades on the principal securities exchange on which the shares are traded.
No option granted to any person who, at the time of the grant, owns (taking into account the attribution rules of Section 424(d) of the Code) stock possessing more than 10% of the total combined voting power of all classes of our stock or of the stock of any of our corporate subsidiaries, may be designated as an ISO unless at the time of such grant the purchase price of the shares of Common Stock covered by such option is at least 110% of the fair market value, but in no event less than the
9
par value, of such shares. The aggregate fair market value (determined at the time each ISO is granted) of the shares of Common Stock with respect to which ISOs issued to any one person are exercisable for the first time during any calendar year may not exceed $100,000.
Awards of restricted stock under the 2000 Plan will be made at the discretion of the Compensation Committee and will consist of shares of Common Stock granted to a participant and covered by a restricted stock award agreement. At the time of an award, a participant will have the benefits of ownership in respect of such shares, including the right to vote such shares and receive dividends thereon and other distributions, subject to the restrictions in the 2000 Plan and in the restricted stock award agreement. The shares will be legended and, at our option, may be held in escrow and may not be sold, transferred or disposed of until such restrictions have lapsed. Each award may be subject to a lapsing formula pursuant to which the restrictions on transferability lapse over a particular time period. The Compensation Committee has broad discretion as to the specific terms and conditions of each award, including applicable rights upon certain terminations of employment.
Administration of the 2000 Long Term Incentive Compensation Plan. The Compensation Committee will administer the 2000 Plan. The Compensation Committee has full and exclusive authority to, among other things, determine the grant of awards under the 2000 Plan. Drs. Dean and Watson served as the members of the Compensation Committee during the 2003 fiscal year. Prior to their resignation from the Board, Lester McKeever and Robert J. Weinstein, M.D. were on the Compensation Committee and, until he resigned from the Compensation Committee, William McPhee was also a member. Mr. McPhee rejoined the Compensation Committee in 2004.
Purchase of Common Stock Under the 2000 Long Term Incentive Compensation Plan. Each award granted under the 2000 Plan will be granted pursuant to and subject to the terms and conditions of an award agreement (an "Award Agreement") to be entered into between us and the award holder at the time of the grant. Any Award Agreement will incorporate by reference all of the terms and provisions of the 2000 Plan as in effect at the time of grant and may contain other terms and provisions approved by the Compensation Committee.
The Compensation Committee will determine the expiration date of an award granted under the 2000 Plan at the time of grant. Each award will become exercisable or vested in whole, in part or in installments, at the time or times as the Compensation Committee may prescribe and specify in the Award Agreement.
In the event of a "Change in Control" (defined in the 2000 Plan) of the Company, awards granted under the 2000 Plan, which, by their terms, vest or are exercisable in installments, currently will become immediately exercisable or vested in full. These provisions of the 2000 Plan may have some deterrent effect on certain mergers, tender offers or other takeover attempts, thereby having some potential adverse effect on the market price of our Common Stock. We propose to amend this so that accelerated vesting is left to the Compensation Committee's discretion.
The exercise price for options granted under the 2000 Plan currently may be paid in cash. At the discretion of the Compensation Committee, subject to applicable requirements of the Securities Exchange Act of 1934, the option holder may currently deliver with his or her exercise notice irrevocable instructions to a broker to promptly deliver to us an amount of sale or loan proceeds sufficient to pay the exercise price. We would like to amend the 2000 Plan to provide that the exercise price may be paid in cash or by using the shares underlying the options as consideration for the exercise price by reducing the number of shares underlying the options under a formula. Option holders would instruct the Company to withhold that number of shares of common stock otherwise issuable pursuant to an exercise of the option determined by dividing (A) the product determined by multiplying (1) the aggregate number of shares of common stock specified in the exercise notice, times (2) the exercise price, by (B) the fair market value of one share of common stock as of the date of the exercise notice.
10
Awards granted under the 2000 Plan are assignable or transferable by will or pursuant to the laws of descent and distribution or as otherwise specifically permitted by the Award Agreement or by the Compensation Committee, and options will be exercisable during the award holder's lifetime by the award holder himself or herself or any permitted transferee. No holder of any option will have any rights to dividends or other rights of a stockholder with respect to shares subject to an option before exercising the option.
Termination of Employment, Death or Disability of Option holder. Each option, to the extent it has not been previously exercised, will terminate upon the earliest to occur of: (i) the expiration of the option period set forth in the Award Agreement; (ii) for ISOs, the expiration of three months following the Participant's retirement (following the Participant's retirement, NQSOs will terminate upon the expiration of the option period set forth in the Award Agreement); (iii) the expiration of 12 months following the Participant's death or disability; (iv) immediately upon termination for cause; (v) the expiration of 90 days following the Participant's termination of employment for any reason other than cause, Change in Control, death, disability, or retirement; or (vi) at such other time and on such conditions provided for in the Award Agreement. Upon a termination of employment related to a Change in Control, options will be treated in the manner set forth in the Change of Control provisions.
Upon a Participant's death, disability, or retirement, all restricted stock will vest immediately. Each Award Agreement will set forth the extent to which the Participant will have the right to retain unvested restricted shares following termination of the Participant's employment with the Company in all other circumstances. Such provisions will be determined in the sole discretion of the Compensation Committee, will be included in the Award Agreement entered into with each Participant, need not be uniform among all shares of restricted stock issued pursuant to the 2000 Plan, and may reflect distinctions based on the reasons for termination of employment.
Expiration, Termination and Amendment of the 2000 Long Term Incentive Compensation Plan. The 2000 Plan may at any time be terminated, modified, altered, suspended or amended by the Board of Directors or the Compensation Committee. The Board of Directors or the Compensation Committee may, insofar as permitted by law, from time to time with respect to any shares of Common Stock at the time not subject to options or other awards, suspend or discontinue the 2000 Plan or revise or amend it in any respect whatsoever, except that, without approval of the stockholders, no such revision or amendment may increase the number of shares available for grants of ISOs under the 2000 Plan or alter the class of participants in the 2000 Plan. Subject to the provisions described above, the Board of Directors or the Compensation Committee will have the power to amend the 2000 Plan and any outstanding awards granted thereunder in such respects as the Board of Directors or the Compensation Committee may, in its sole discretion, deem advisable in order to incorporate in the 2000 Plan or any such award any new provision or change designed to comply with or take advantage of requirements or provisions of the Code or other statute, or rules or regulations of the Internal Revenue Service or other federal or state governmental agency enacted or promulgated after the adoption of the 2000 Plan.
Federal Tax Consequences. We believe that the following generally summarizes the federal income tax consequences of awards under the Plan for participants and us. A participant will not be deemed to have received any income subject to tax at the time a stock option (including an ISO) or Restricted Stock award that is subject to conditions, restrictions or contingencies is awarded, nor will we be entitled to a tax deduction at that time. When a stock option (other than an ISO) is exercised, the participant will be deemed to have received an amount of ordinary income equal to the excess of the fair market value of the shares purchased over the exercise price. The income tax consequences of Restricted Stock Awards will depend on how the awards are structured, but generally, a participant will be deemed to have ordinary income at the time a Restricted Stock Award is distributed to him free of all conditions, restrictions and contingencies. In each case, we will be allowed a tax deduction in the
11
year and in an amount equal to the amount of ordinary income that the participant is deemed to have received. The amount of the deduction for certain highly compensated employees may be limited to $1 million annually for performance-based awards under Section 162(m) of the Internal Revenue Code.
If an ISO is exercised by a participant who satisfies certain employment requirements at the time of exercise, the participant will not be deemed to have received any income subject to tax at such time, although the excess of the fair market value of the Common Stock so acquired on the date of exercise over the exercise price will be an item of tax preference for purposes of the alternative minimum tax. Section 422 of the Code provides that if the Common Stock is held at least one year after the exercise date and two years after the date of grant, the participant will realize a long-term capital gain or loss upon a subsequent sale, measured as the difference between the exercise price and the sales price. If that portion of Common Stock acquired upon the exercise of an ISO which is not held for one year, a "disqualifying disposition" results, at which time, the participant is deemed to have received an amount of ordinary income equal to the lesser of (a) the excess of the fair market value of the Common Stock on the date of exercise over the exercise price or (b) the excess of the amount realized on the disposition of the shares over the exercise price. If the amount realized on the "disqualifying disposition" of the Common Stock exceeds the fair market value on the date of exercise, the gain on the excess of the ordinary income portion will be treated as a capital gain. Any loss on the disposition of Common Stock acquired through the exercise of an ISO is a capital loss. No income tax deduction will be allowed to us with respect to shares of Common Stock purchased by a participant through the exercise of an ISO, provided there is no "disqualifying disposition" as described above. In the event of a "disqualifying disposition", we are entitled to a tax deduction equal to the amount of ordinary income recognized by the participant.
New Plan Benefits. To date, options to acquire 3,899,137 shares of Common Stock have been granted and remain outstanding under the 2000 Plan. During the 2004 and future fiscal years, we may consider granting Awards to existing employees or hiring additional personnel and others who may become eligible for receipt of Awards under the 2000 Plan. At this point, however, we cannot provide information as to the amount of the Awards, if any, that would be granted under the 2000 Plan.
Equity Compensation Plan Information. The following table gives information about our Common Stock that may be issued upon the exercise of options under all of our existing equity compensation plans as of December 31, 2003.
Plan Category
| | (a) Number of securities to be issued upon exercise of outstanding options, warrants and rights
| | (b) Weighted-average exercise price of outstanding options, warrants and rights
| | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
|
|---|
| Equity compensation plans approved by security holders | | 5,889,470 | | $ | 10.90 | | 178,155 |
| Equity compensation plans not approved by security holders | | 1,252,431 | (1) | $ | 1.75 | | 0 |
| Total | | 7,141,901 | | $ | 9.30 | | 178,155 |
- (1)
- In addition to shares we may issue under the plans approved by the stockholders, we have granted options and warrants to consultants, the placement agent for our Series B Preferred Stock and to Dr. Williams (which is subject to stockholder approval in Proposal 2) pursuant to arrangements that were not submitted to stockholders for approval. Each of these grants is on different terms under specific agreements negotiated with each recipient. Exercise prices range from $1.08 to $47.78 per share, with terms expiring between 2004 and 2013. Each agreement contains anti-dilution features.
12
We have options outstanding under three plans, the 1998 Plan, the Senior Executive Plan and the 2000 Plan. Each of these Plans was approved by the stockholders. If Proposal 2 is approved, we will increase the number of shares authorized for issuance under the 2000 Plan to 30,000,000 shares.
PROPOSAL 3
Amend Articles of Incorporation to Increase Authorized Shares of Common Stock
We believe that it may be in the best interests of the Company to amend our Restated Articles of Incorporation to increase the number of authorized shares of Common Stock to 200,000,000 from 150,000,000. The Board of Directors does not believe that the current number of shares available is adequate to serve the Company's need for potential future equity financing.
Our current Restated Articles of Incorporation provide for the authorization of 150,000,000 shares of Common Stock. The proposed amendment provides for the authorization of 200,000,000 shares of Common Stock. As of November 26, 2004, based on the number of shares of Common Stock currently outstanding and the number of shares eligible for issuance under all of our stock options plans, warrant agreements and upon the conversion of our newly issued Series A Convertible Preferred Stock, there were 119,542,395 shares of Common Stock either outstanding or reserved, leaving 30,457,605 authorized, but unissued, and unreserved shares of Common Stock available for other corporate purposes or opportunities. The Board of Directors believes that such number may not be adequate to provide for our financing needs.
This amendment increases by 50,000,000 the number of authorized and unissued shares of Common Stock which may be used for any proper corporate purpose by the Company, including issuance of shares in public or private offerings, issuance of securities convertible into shares of Common Stock for cash as a means of obtaining additional capital for use in our business and operations, or issuance of shares of Common Stock as part or all of the consideration required to be paid by the Company in the acquisition of other businesses or properties.
With this increase, we believe that we will have sufficient shares of Common Stock available for corporate purposes or opportunities as are likely to arise in the reasonably foreseeable future particularly if stockholders grant authority to effect up to a 1 for 20 reverse split as contemplated by Proposal 4. Although we have no current plan or proposal to issue any of the currently authorized and unissued shares of Common Stock not already reserved or the additional shares of the Common Stock proposed to be authorized, additional shares could be issued in connection with one or more equity financings.
If the amendment is approved, the newly authorized shares of Common Stock would have all of the rights and privileges as the shares of Common Stock presently authorized. Once shares of Common Stock are authorized, the Board of Directors can issue shares of Common Stock without stockholder approval, except as may be required by law or regulations or by the rules of any stock exchange on which our securities may then be listed.
The issuance of the additional shares of Common Stock, may, under certain circumstances, make attempts to acquire control of the Company more difficult. For example, an issuance of additional shares of Common Stock may make it more difficult to obtain stockholder approval of actions such as removal of directors or amendments to the Bylaws. In addition, shares of Common Stock could be issued in other transactions that could make a change of control of the Company more difficult. We are not aware of any effort to obtain control of the Company by a tender offer, proxy contest, or otherwise, and we have no present intention to use the additional shares of authorized Common Stock for anti-takeover purposes.
Although the Board of Directors would issue additional shares based on its judgment as to the best interests of the Company and its stockholders, the issuance of additional shares of Common Stock
13
would have the effect of diluting the voting power per share and could have the effect of diluting the book value per share of the outstanding shares of Common Stock.
The text of the first sentence of the FOURTH Article of the Restated Articles of Incorporation, as it is proposed to be amended pursuant to this proposal, is as follows:
"The total number of shares of all classes of stock which the Corporation shall have the authority to issue is two hundred million (200,000,000) shares of Common Stock, par value $.001 per share."
If approved by the stockholders, the proposed amendment will become effective upon its filing with the Secretary of State of Nevada, which is expected to take place as soon as practicable after the Annual Meeting.
PROPOSAL 4
Amend Articles of Incorporation to Effect a Reverse Stock Split
Introduction. We believe that it may be in the best interests of the Company to amend our Restated Articles of Incorporation to effect one or more reverse stock splits of our outstanding Common Stock (the "Reverse Split") which could range from an aggregate of a one-for-two up to one-for-twenty reverse stock split, if and as determined by our Board of Directors in its sole discretion within the 12 months after the Annual Meeting, on the terms described in this Proxy Statement. The principal effect of the Reverse Split would be to decrease the number of issued and outstanding shares of our Common Stock (but not the total shares authorized for issuance). Except for adjustments that may result from the treatment of fractional shares as described below, each stockholder would hold the same percentage of Common Stock outstanding immediately following the Reverse Split as such stockholder held immediately prior to the Reverse Split. The relative voting and other rights that accompany the shares of Common Stock would not be affected by the Reverse Split. In the event that our Board of Directors determines that it is in the best interests of the Company to effect a Reverse Split, we will file a Form 8-K with the SEC detailing the specific terms of such split.
This Reverse Split includes the one-for-five reverse split previously approved by stockholders at the February 5, 2004 special meeting. The Board has not implemented the one-for-five reverse split and we seek authority to increase the size of the split to up to one-for-twenty.
The Reverse Split would be accomplished by amending our Restated Articles of Incorporation on one or more occasions so long as, during the 12 months after the Annual Meeting, the total Reverse Split does not exceed one-for-twenty shares. Each amendment would be set forth in a paragraph in the following form (assuming Proposal 3 is approved):
"Effective as of the close of business on the date of filing this Amendment to the Restated Articles of Incorporation with the Nevada Secretary of State (the "Effective Time"), the filing of this Amendment shall effect a reverse split (the "Reverse Split") pursuant to which [ ] shares of Common Stock, par value $0.001 per share, issued and outstanding and held by a single holder, shall be combined into one validly issued, fully paid and nonassessable share of Common Stock, par value $0.001 per share. Each stock certificate that prior to the Effective Time represented shares of Common Stock shall, following the Effective Time represent the number of shares into which the shares of Common Stock represented by such certificate shall be combined as a result of the Reverse Split. The Corporation shall not issue fractional shares or scrip as a result of the Reverse Split, but shall round up to the nearest whole share any fractional share that would otherwise result from the Reverse Split; the number of authorized shares of Common Stock shall continue to be 200,000,000; and the par value of the Common Stock shall be $0.001 per share."
14
The Reverse Split will become effective on a date or dates determined by our Board of Directors (the "Effective Date").
Stockholders do not have the statutory right to dissent and obtain an appraisal of their shares under Nevada law in connection with the amendment to our Restated Articles of Incorporation to complete the Reverse Split.
We may use the authorized and unissued shares of Common Stock to raise capital in a public or private offering, to enter into a strategic transaction or to grant options or warrants to employees or others. We have no current agreements to enter into any stock offerings or strategic transactions although we plan to explore various strategic options concerning our businesses, including securing additional financing, joint ventures, licensing or selling all or a portion of our technology and other possible strategic transactions. In addition, 39,477,095 shares of the Company's authorized and unissued shares are reserved for future grants and the issuance upon exercise of outstanding warrants and options previously granted under our various stock option plans and agreements and the conversion of our newly issued Series A Convertible Preferred Stock.
Reasons for the Reverse Split. If effected, the Reverse Split should enable us to reduce the number of shares outstanding to a more appropriate number for a company at our stage of development as well as attempt to increase the market price of our Common Stock in an effort to ensure meeting the listing requirements of Nasdaq (if we are relisted) or another exchange. A Reverse Split may also increase the attractiveness of our Common Stock to prospective investors and the financial community.
If effected, the Reverse Split and resulting anticipated increase in the price of our Common Stock should also enhance the acceptability and marketability of our Common Stock to the financial community and investing public. Many institutional investors have policies prohibiting them from holding lower-priced stocks in their portfolios, which reduces the number of potential buyers of our Common Stock. Additionally, analysts at many brokerage firms are reluctant to recommend lower-priced stocks to their clients or monitor the activity of lower-priced stocks. Brokerage houses also frequently have internal policies that discourage individual brokers from dealing in lower-priced stocks. Further, because brokers' commissions on lower-priced stock generally represent a higher percentage of the stock price than commissions on higher priced stock, investors in lower-priced stocks pay transaction costs which are a higher percentage of their total share value, which may limit the willingness of individual investors and institutions to purchase our Common Stock.
If implemented, the Reverse Split may result in some stockholders owning "odd-lots" of less than 100 shares. Brokerage commissions and other costs of transactions in odd-lots may be higher, particularly on a per-share basis, than the cost of transactions in even multiples of 100 shares. In addition, if effected, the Reverse Split may make it more difficult for us to meet other requirements for listing on Nasdaq or another exchange relating to the minimum number of shares that must be in the public float and the minimum number of round lot holders.
We cannot assure stockholders that the Reverse Split will have any of the desired consequences described above. Specifically, we cannot assure stockholders that, if effected, the post- Reverse Split market price of our Common Stock will increase proportionately to the ratio for the Reverse Split, that the market price of our Common Stock will not decrease to its pre-split level, that our market capitalization will be equal to the market capitalization before the Reverse Split or that the Reverse Split will enable us to become relisted with Nasdaq or listed with another exchange.
Effecting the Reverse Split. If approved by the stockholders at the Annual Meeting and our Board of Directors determines that it is in the best interests of the Company to effect the Reverse Split on one or more occasions in the twelve months following this Annual Meeting, we will file Amendments to our Restated Articles of Incorporation with the Nevada Secretary of State to become effective at a time determined by our Board of Directors. Upon effectiveness of the Amendment to our
15
Restated Articles of Incorporation, without further action by us or our stockholders, the outstanding shares of Common Stock held by stockholders of record as of the Effective Date would be converted into the right to receive a number of shares of Common Stock (the "New Common Stock") calculated based on a reverse split ratio. For example, if a stockholder presently holds 10,000 shares of Common Stock, that stockholder would hold 5,000 shares of Common Stock following a one-for two Reverse Split or 500 shares following a one-for-twenty Reverse Split.
No Fractional Shares. We will not issue any fractional shares in connection with the Reverse Split, if effected. Instead, any fractional share resulting from the Reverse Split will be rounded up to the nearest whole share.
Exchange of Stock Certificates. If approved by the Board of Directors, the conversion of the shares of our Common Stock under the Reverse Split would occur automatically on the Effective Date. This would occur regardless of when stockholders physically surrender their stock certificates for new stock certificates.
If effected, our transfer agent, Computershare Investor Services LLC, will act as exchange agent ("Exchange Agent") to implement the exchange of stock certificates. As soon as practicable after the Effective Date, the Company or the Exchange Agent would send a letter to each stockholder of record at the Effective Date for use in transmitting certificates representing shares of our Common Stock ("Old Certificates") to the Exchange Agent. The letter of transmittal would contain instructions for the surrender of Old Certificates to the Exchange Agent in exchange for certificates representing the appropriate number of whole shares of New Common Stock. No new stock certificates would be issued to a stockholder until such stockholder has surrendered all Old Certificates, together with a properly completed and executed letter of transmittal, to the Exchange Agent.
Stockholders would then receive a new certificate in exchange for certificates representing the number of whole shares of New Common Stock into which their shares of Common Stock have been converted as a result of the Reverse Split. Until surrendered, we would deem outstanding Old Certificates held by stockholders to be canceled and only to represent the number of whole shares of New Common Stock to which these stockholders are entitled. All expenses of the exchange of certificates would be borne by us.
YOU SHOULD NOT SEND YOUR OLD CERTIFICATES TO THE EXCHANGE AGENT UNTIL YOU HAVE RECEIVED THE LETTER OF TRANSMITTAL.
Effect on Outstanding Shares. If the Reverse Split is completed, the number of shares of our Common Stock owned by each stockholder will be reduced in the same proportion as the reduction in the total number of shares outstanding, such that the percentage of our Common Stock owned by each stockholder will remain unchanged. The number of shares of Common Stock that may be purchased upon exercise of outstanding options, warrants, and other securities convertible into, or exercisable or exchangeable for, shares of our Common Stock, and the exercise or conversion prices for these securities, will be adjusted in accordance with their terms as of the Effective Date.
If Proposal 3 is approved and a Reserve Split is consummated the number of authorized shares of Common Stock will continue to be 200,000,000 shares. The following chart indicates the number of authorized, but unissued shares of Common Stock that would be outstanding if a one-for-five, one-for-ten, one-for-fifteen or one-for-twenty Reserve Split were effected (based upon 80,065,300 issued and outstanding shares of Common Stock as of November 26, 2004). We may effect Reverse Splits in
16
any amount (not necessarily in multiples of five) so long as the total Reverse Split during the 12 months following the Annual Meeting does not exceed one-for-twenty.
| | Pre-Split
| | 1-for-5
| | 1-for-10
| | 1-for-15
| | 1-for-20
|
|---|
| Authorized but unissued Shares of Common Stock | | 69,934,700 | | 13,986,940 | | 6,993,470 | | 4,662,314 | | 3,496,735 |
Accounting Consequences. The par value of our Common Stock will remain unchanged at $.001 per share after the Reverse Split. However, the Common Stock as designated on our Balance Sheet would be adjusted downward in respect of the shares of the New Common Stock to be issued in the Reverse Split such that the Common Stock would become an amount equal to the aggregate par value of the shares of New Common Stock being issued in the Reverse Split, and that the Additional Paid-in Capital as designated on our Balance Sheet would be increased by an amount equal to the amount by which the Common Stock was decreased. Additionally, net loss per share would increase proportionately as a result of the Reverse Split. We do not anticipate that any other accounting consequence would arise as a result of the Reverse Split, if effected.
Federal Income Tax Consequences. The following is a summary of the material anticipated United States federal income tax consequences of the Reverse Split to our stockholders. This summary is based on the United States federal income tax laws now in effect and as currently interpreted, and does not take into account possible changes in such laws or interpretations. This summary is provided for general information only and does not address all aspects of the possible federal income tax consequences of the Reverse Split and is not intended as tax advice to any person. In particular, this summary does not consider the federal income tax consequences to our stockholders in light of their individual investment circumstances or to holders subject to special treatment under the federal income tax laws, and does not address any consequences of the Reverse Split under any state, local, or foreign tax laws.
We believe that our stockholders who exchange their Common Stock solely for New Common Stock should generally recognize no gain or loss for federal income tax purposes. A stockholder's aggregate tax basis in its shares of New Common Stock received should be the same as the stockholder's aggregate tax basis in the Common Stock exchanged therefor. The holding period of the New Common Stock received by that stockholder should include the period during which the surrendered Common Stock was held, provided all such Common Stock was held as a capital asset at the Effective Date.
We will not recognize any gain or loss as a result of the Reverse Split, if effected.
PROPOSAL 5
Election of Seven Directors to the Company's Board of Directors
Our Board of Directors consists of seven directors. Currently, seven directors are serving on the Board. Nominees for election at the Annual Meeting to the Board of Directors are listed below. If elected, each nominee will serve as a director until our Annual Meeting of Stockholders in 2005, and until his or her successor is elected and qualified or until his or her earlier death, retirement, resignation or removal. In the event of a vacancy between Annual Meetings, the vacancy may be filled by the remaining directors. If a nominee declines to serve or becomes unavailable for any reason, or if a vacancy occurs before the election at the Annual Meeting, the Proxies may be voted for a substitute nominee as the Board may designate. Proxies cannot be voted for a greater number of persons than the number of nominees listed below.
17
Directors, Executive Officers, Promoters and Control Persons
Director Nominees. The table below sets forth the seven director nominees to be elected at this meeting, and information concerning their age as of November 26, 2004 and position with the Company. Six of the following director nominees are incumbent directors and none of these individuals is a director of any other company subject to the reporting requirements under the federal securities laws.
Name
| | Age
| | Position
|
|---|
| Brian M. Gallagher, Ph.D.(1) | | 56 | | Director, Chairman of the Board |
| Taffy J. Williams, Ph.D.(1) | | 55 | | Director, President and Chief Executive Officer |
| Robert A. Ashley(3) | | 46 | | Director |
| Richard T. Dean, Ph.D.(2)(3) | | 57 | | Director |
| Darlene M. Deptula-Hicks, M.B.A.(2) | | 46 | | Director |
| Jonathan J. Fleming(1) | | 47 | | Director |
| Alan D. Watson, Ph.D., M.B.A.(2)(3) | | 52 | | Director |
| | | | | |
- (1)
- Member of the Executive Committee
- (2)
- Member of the Audit Committee
- (3)
- Member or proposed member of the Compensation Committee
Ms. Deptula-Hicks is the Chairperson of our Audit Committee. Our Board has determined that she is an "audit committee financial expert" and is "independent" as contemplated by SEC rules. A brief discussion of the business experience of each director is set forth below.
Brian M. Gallagher, Ph.D. is Chairman of the Board and has served as a director since July 22, 2004. Dr. Gallagher received his B.S. is biology from St. Louis University, his M.S. in marine sciences from Long Island University, and his Ph.D. in biology from St. John's University. Dr. Gallagher currently serves as a Director of CollaGenex Pharmaceuticals, Inc. and ImaRx Therapeutics, Inc., a privately held, drug delivery/specialty pharmaceutical company with a focus on cardiovascular diseases. From 1994 to 2003, he was the Chairman, President and Chief Executive Officer of CollaGenex Pharmaceuticals, Inc.
Robert A. Ashley is nominated to serve as a director beginning at the Annual Meeting. Mr. Ashley received his MA in Biochemistry from St. Peter's College, Oxford University. Since 2004, he has served as President, Chief Executive Officer and Chairman of the Board of Amplimed Corporation. From 1994 to 2003, he served in various capacities with CollaGenex Pharmaceuticals Inc., including as Senior Vice President, Vice President of Commercial Development and Managing Director of CollaGenex International Ltd.
Taffy J. Williams, Ph.D. has served as our President, Chief Executive Officer and a director since May 17, 2000. Prior to joining the company, Dr. Williams served as CEO and President of PANAX Pharmaceuticals for two and half years and President of InKine Pharmaceuticals for two years. He received his Bachelor of Science in Chemistry from the University of Notre Dame and his Ph.D. in Chemistry from the University of South Carolina.
Richard T. Dean, Ph.D. has served as a director since October 27, 2003. Dr. Dean received his B.S. from Cornell University, his M.S. from the University of Michigan and his Ph.D. from the University of California at Berkeley. Since 2004, Dr. Dean has served as the Chief Executive Officer of Xanthus Life Sciences, Inc., a company founded to develop novel oncology drugs. From 1999 to 2003, Dr. Dean served in various capacities with Schering AG, including as Head of Strategic Business Development,
18
Diagnostics and Radiopharmaceuticals. From 1990 to 1999, Dr. Dean was the Chief Executive Officer of Diatide Inc. Dr. Dean has over 25 years experience in the pharmaceutical and biotechnology field.
Darlene M. Deptula-Hicks, M.B.A. has served as a director since July 22, 2004. Ms. Deptula-Hicks received her B.S. in accounting from New Hampshire College and her M.B.A. from Rivier College. Since 2002, she has served as Executive Vice President and Chief Financial Officer of ONI Medical Systems, Inc. Prior to that, she was the Executive Vice President, Chief Financial Officer and Treasurer of Implant Sciences Corporation from 1998 to 2001. Ms. Deptula-Hicks has over 20 years of experience in financial management positions, primarily in the life sciences sector.
Jonathan J. Fleming has served as a director since August 15, 2003. Mr. Fleming has been a Partner of Oxford Bioscience Partners since 1996 and its Managing Partner since 2001. Oxford Bioscience Partners is an international venture capital firm with committed capital of more than $800 million specializing in life science technology based investments. Mr. Fleming holds a Master's degree in Public Administration from Princeton University and a Bachelor of Arts degree from the University of California, Berkeley. Mr. Fleming is a co-founder and is Chairman of the Board of Memory Pharmaceuticals. He is also Chairman of the Board of BioProcessors Corporation and Dynogen Corporation, and is a director of several private companies, including Leerink Swann. Mr. Fleming is a Trustee of the Museum of Science in Boston and is a Senior Lecturer at MIT's Sloan School of Management.
William D. McPhee has served as a director since November 12, 2002. Mr. McPhee, age 51, received his B.S. degree in pre-med and an L.L.B. degree in law, both from McGill University. Since 1998, he has been Managing General Partner of Mi3, L.P. where he has been responsible for managing the venture fund. Prior to that, he was Chief Executive Officer of Praxis Advisors, Inc., a strategy consulting firm. Mr. McPhee is not standing for reelection at the Annual Meeting.
Alan D. Watson, Ph.D., M.B.A. has served as a director since November 12, 2002. Dr. Watson received his B.S. from the University of N.S.W., his Ph.D. in Chemistry from Australian National University, and his M.B.A. from Northeastern University. Since 2002, Dr. Watson has served as Executive Vice President and Chief Business Officer of Elixir Pharmaceuticals, Inc. Prior to that, he was the Senior Vice President, Corporate Development of Cubist Pharmaceuticals, Inc. from 1999 to 2002. From 1997 to 1999, he was Senior Vice President, Licensing and Intellectual Property of Nycomed Amersham plc.
The holders of over 64.2% of the currently issued and outstanding Common Stock (as of November 26, 2004) are parties to the Voting Agreement. The Voting Agreement requires the parties to vote for certain director nominees. The Voting Agreement is summarized in Proposal 1 under the heading "Agreements to Vote in Favor of Proposals" on page 2. Specifically, as part of the institutional financing transaction that closed on November 12, 2002, the current holders of 51,384,623 shares of our Common Stock (approximately 64.2% of the outstanding Common Stock eligible to vote for Proposal 3 as of November 26, 2004) entered into the Voting Agreement. The Voting Agreement requires the parties to vote for certain director nominees.
Executive Officers and Significant Employees. The recent business experience of our other executive officers and significant employees follows.
Jack DeFranco, age 59, has served as Senior Vice President of our IMCOR Division since June 18, 2003. For the five years prior to joining the Company, Mr. DeFranco served as Vice President of Marketing and Business Development for Alliance Pharmaceutical Corp. Mr. DeFranco received his B.S. from Stephen F. Austin University and his M.B.A. and M.A. from Fairleigh Dickenson University.
We also have a consultant, Larry D. Grant, who is a certified public accountant and who has 30 years of experience in providing accounting and financial services.
19
Directors' Meetings and Committees. During the fiscal year ended December 31, 2003, the Board of Directors held four meetings. Each director serving on the Board of Directors in fiscal year 2003 attended at least 75% of all meetings of the Board of Directors and the Committees on which he serves. The following table reflects our standing Committees, the members of each Committee, each Committee's functions and the number of meetings each Committee held during the fiscal year ended December 31, 2003.
Name of Committee and Members
| | Functions of Committee
| | Meetings
|
|---|
| Executive Committee 2003: | | • | | Responsible for management of | | |
| Taffy J. Williams, Ph.D. | | | | operations delegated to it from time to | | 2003: 0 meetings |
Robert J. Weinstein, M.D.
(resigned on August 13, 2002) | | | | time by the Board of Directors | | 2 actions by written consent |
William D. McPhee
Jonathan J. Fleming
(appointed on September , 2003 |
| • |
| Provides general oversight of certain business functions |
|
|
| | | | | | |
|
|
|
|
|
|
|
|
| Audit Committee 2003: | | • | | Responsible for meeting with our independent public accountants and reviewing the scope and results of | | |
| Alan D. Watson, Ph.D., M.B.A., Chairman | | | | auditing procedures and our accounting procedures and controls | | 2003: 5 meetings |
Richard T. Dean, Ph.D.
(appointed on December 1, 2003) | | • | | Provides general oversight for the accounting principles employed in our financial reporting | | |
William D. McPhee
(resigned on September 25, 2003 | | • | | Responsible for our compliance with laws and regulations and functions as | | 1 action by written consent |
Eugene Golub
(resigned on August 13, 2003) | | | | our Qualified Legal Compliance Committee. | | |
Lester H. McKeever, Jr.
(resigned on August 12, 2003) |
| |
| |
|
|
| | | | | | | |
| | | | | | |
|
| |
| |
|
|
| | | | | | | |
| | | | | | |
| | | | | | | |
| | | | | | |
20
Compensation Committee 2003: |
|
• |
|
Responsible for reviewing the performance and total compensation package for executive officers, |
|
|
Robert J. Weinstein, M.D.
(resigned on August 13, 2003) | | | | including our President and Chief Executive Officer | | 2003: 1 meeting |
William D. McPhee
(resigned on
December 1, 2003) | | • | | Considers modification of existing compensation and employee benefit programs and the adoption of new plans | | |
Lester H. McKeever, Jr.
(resigned August 13, 2003) | | • | | Administers our 1998 and 2000 Long Senior Executive Long Term Incentive Compensation Plan, 401(k), profit sharing plan and similar employee benefit plans | | 4 actions by written consent |
Alan D. Watson, Ph.D., M.B.A.
(appointed on December 1, 2003) | | • | | Reviews compensation and benefits, of
consultants and non-employee directors | | |
Richard T. Dean, Ph.D.
(appointed on December 1, 2003) |
|
|
| |
|
|
| | | | | | | |
| | | | | | | |
|
|
|
| |
|
|
| | | | | | | |
| | | | | | |
| | | | | | | |
| | | | | | | |
|
| |
| |
|
|
| | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21
Corporate Governance. We operate within a plan of corporate governance for the purpose of defining responsibilities, setting high standards of professional and personal conduct and assuring compliance with such responsibilities and standards. We regularly monitor developments in the area of corporate governance. In July, 2002, Congress passed the Sarbanes-Oxley Act of 2002 which, among other things, establishes, or provides the basis for, a number of new corporate governance standards and disclosure requirements. In addition, Nasdaq has recently changed its corporate governance and listing requirements. In some cases the transitional provisions had not yet expired for the requirements of the Sarbanes-Oxley Act and the Nasdaq rules as of the date of this proxy statement. Nevertheless, the Board of Directors has initiated actions consistent with certain of these rules.
Independent Directors
- •
- Assuming our stockholders elect all of the directors nominated for election at the Annual Meeting, a majority of the members of our Board of Directors will not be Company employees.
Audit Committee
- •
- All Audit Committee members possess the required level of financial literacy and at least two members of the Audit Committee, Dr. Watson and Ms. Deptula-Hicks, meet the current standard of requisite financial management expertise as required by Nasdaq and applicable SEC rules and regulations.
- •
- The Audit Committee operates under a formal charter that governs its duties and conduct. The charter has been amended to incorporate additional concepts proposed in the Nasdaq proposed rules and applicable SEC rules and regulations. A copy of the amended charter is attached to this Proxy Statement as Exhibit G.
- •
- Moss Adams, LLP, our independent auditors, reports directly to the Audit Committee.
- •
- The Audit Committee, consistent with the Sarbanes-Oxley Act of 2002 and the rules adopted thereunder, meets with management and the auditors prior to the filing of officers' certifications with the SEC to receive information concerning, among other things, significant deficiencies in the design or operation of internal controls.
- •
- The Audit Committee has adopted a policy for reporting improper activity to enable confidential and anonymous reporting of improper activities to the Audit Committee.
Compensation Committee
- •
- Assuming our stockholders elect all of the directors nominated for election at the Annual Meeting, all members of the Compensation Committee will meet the appropriate tests for independence.
Security Ownership of Certain Beneficial Owners and Management. The following table sets forth the approximate beneficial ownership of our common stock as of November 26, 2004 by directors and executive officers of the Company and any person or group known to us to be the owner of more than five percent of our common stock. Shares beneficially owned by the individuals below through
22
family partnerships or other entities they control are included in the number of shares listed in the table below for that individual.
Name and Address
of Beneficial Owner(1)
| | Shares of
Common Stock
Beneficially Owned(2)
| | Percent of Class
Outstanding(3)
|
|---|
| EXECUTIVE OFFICERS AND DIRECTORS: | | | | |
| |
Robert A. Ashley
(director nominee) |
|
0 |
|
* |
| |
Brooks Boveroux
(former chief financial officer) |
|
558,542 |
(4) |
* |
| |
Richard T. Dean
230 Cushing Road
Newmarket, NH 03857 |
|
37,500 |
(5) |
* |
| |
Jack DeFranco |
|
52,500 |
(6) |
* |
| |
Darlene M. Deptula-Hicks, M.B.A. |
|
22,500 |
(18) |
* |
| |
Jonathan Fleming/Oxford Bioscience Partners IV L.P.
225 Berkeley St., Suite 1650
Boston, MA 02216 |
|
46,484,770 |
(7) |
46.8 |
| |
Brian M. Gallagher, Ph.D. |
|
25,000 |
(19) |
* |
| |
Gene Golub
625 North Michigan Avenue, Ste. 2000
Chicago, IL 60611 |
|
108,015 |
(8)(17) |
* |
| |
Reinhard Koenig, Ph.D., M.D. |
|
417,360 |
(9) |
* |
| |
Lester H. McKeever, Jr.
819 South Wabash Avenue
Chicago, IL 60605 |
|
16,250 |
(10)(17) |
* |
| |
William D. McPhee/Mi3 L.P.
One Hollis Street, Suite 232
Wellesley, MA 02482 |
|
2,904,098 |
(11) |
2.9 |
| |
Alan D. Watson, Ph.D.
One Kendall Square
Building 1000, Fifth Floor
Cambridge, MA 02139 |
|
115,000 |
(12) |
* |
| |
Robert J. Weinstein, M.D. and Lois Weinstein
500 Lake Cook Road, Suite 130
Deerfield, IL 60015 |
|
1,314,663 |
(14)(16) |
1.3 |
| |
Taffy J. Williams, Ph.D. |
|
1,054,250 |
(13) |
1.1 |
| |
All directors and executive officers as a group—2003 fiscal year (7 persons) |
|
51,254,160 |
|
51.5 |
| | | | | |
23
OTHER SHAREHOLDERS: |
|
|
|
|
| |
Stuart P. Levine
500 Lake Cook Road, Suite 130
Deerfield, IL 60015 |
|
1,277,386 |
(14)(16) |
1.3 |
| |
Oxford Bioscience Partners IV L.P.
225 Berkeley Street, Suite 1650
Boston, MA 02216 |
|
See above. |
|
|
| |
Tannebaum, LLC
875 N. Michigan Avenue
Suite 2930
Chicago, IL 60611-1901 |
|
2,344,957 |
(15) |
2.4 |
| |
Parties to Voting Agreement
(6 persons) |
|
54,325,874 |
(16) |
54.6 |
- *
- Constitutes one percent or less of the percent of class outstanding.
- (1)
- Unless otherwise indicated, the address of each person named in the table is c/o: IMCOR Pharmaceutical Co., 6175 Lusk Boulevard, San Diego, California 92121.
- (2)
- With respect to directors and executive officers, based on information furnished by the director or executive officer listed.
- (3)
- The percent of class outstanding includes Common Stock outstanding as of November 26, 2004 (including shares issued in the initial and second closings and shares issued upon conversion of debt), and the shares of Common Stock subject to warrants and options exercisable within 60 days of November 26, 2004 (including the warrants issued in the initial and second closings and pursuant to the October 29, 2004 transaction with Bristol-Myers Squibb Medical Imaging, Inc.).
- (4)
- Includes 558,542 options exercisable within 60 days of November 26, 2004. Employment with the Company terminated as of August 29, 2004.
- (5)
- Includes 37,500 options exercisable within 60 days of November 26, 2004.
- (6)
- Includes 52,500 options exercisable within 60 days of November 26, 2004.
- (7)
- Includes 401,387 shares (including 5,706 shares issued in the initial closing, 48,929 shares issued in the second closing, 96,866 shares issued in connection with the conversion of debt in the initial closing (includes additional issuance at second closing to reflect conversion at $0.40 per share) and 185,501 shares issued in connection with the conversion of debt in the second closing) and 27,318 warrants (issued in the initial and second closings) held by MRNA Fund II L.P. and 43,333,382 shares (including 568,678 shares in the initial closing, 4,876,687 shares issued in the second closing, 9,654,004 shares issued in connection with the conversion of debt in the initial closing (includes additional issuance at second closing to reflect conversion at $0.40 per share) and 21,816,916 shares issued in connection with the conversion of debt in the second closing) and 2,722,683 warrants (issued in the initial and second closings) held by Oxford Bioscience Partners IV L.P., which Mr. Fleming may be deemed to control. Mr. Fleming disclaims beneficial ownership of these shares for all other purposes. Oxford and MRNA are parties to the Voting Agreement described in footnote 16 below. The shares held by the other parties to the Voting Agreement are not included here.
- (8)
- Includes 108,015 shares of Common Stock.
24
- (9)
- Includes 21,500 shares of Common Stock and 395,860 options exercisable within 60 days of November 26, 2004. Dr. Koenig's employment was terminated, without cause, effective May 1, 2003.
- (10)
- Includes 16,250 shares of Common Stock.
- (11)
- Includes 2,712,848 shares (including 27,414 shares issued in the initial closing, 235,086 shares issued in the second closing, 1,318,850 shares issued in connection with the conversion of debt in the initial closing (includes additional issuance at second closing to reflect conversion at $0.40 per share) and 668,535 shares issued in connection with the conversion of debt in the second closing) and 131,250 warrants (issued in the initial and second closings) held by Mi3, L.P., which Mr. McPhee may be deemed to control. Mr. McPhee disclaims beneficial ownership of Mi3's shares for all other purposes. Mi3, L.P. is a party to the Voting Agreement described in footnote 16 below. The shares held by the other parties to the Voting Agreement are not included here. Includes 60,000 options exercisable within 60 days of November 26, 2004.
- (12)
- Includes 25,000 shares of Common Stock and options to acquire 90,000 shares of Common Stock exercisable within 60 days of November 26, 2004.
- (13)
- Includes 4,250 shares of Common Stock and 1,050,000 options exercisable within 60 days of November 26, 2004.
- (14)
- Dr. Weinstein and Mr. Levine are parties to the Voting Agreement described in footnote 16 below. The shares held by the other parties to the Voting Agreement are not included here.
- (15)
- Includes 2,344,957 shares of Common Stock. Tannebaum, LLC is a party to the Voting Agreement described in footnote 16 below. The shares held by the other parties to the Voting Agreement are not included here.
- (16)
- Dr. Weinstein, Mr. Levine, Mi3, L.P. (of which Mr. McPhee is Managing General Partner), MRNA Fund II, L.P. (an affiliate of Oxford Bioscience Partners IV L.P.), Oxford Bioscience Partners IV L.P. (of which Mr. Fleming is the Managing Partner), and Tannebaum, LLC are parties to the Voting Agreement described above at page 2. The parties to the Voting Agreement may be deemed to be a "group" within the meaning of Section 13(d)(3) of the Securities Exchange Act of 1934, as amended. The provisions of the Voting Agreement include an agreement by the parties to vote their shares for the election of certain directors nominated by the parties to the Voting Agreement.
- (17)
- Resigned from the Company's Board of Directors in August, 2003.
- (18)
- Includes 22,500 options exercisable within 60 days of November 26, 2004.
- (19)
- Includes 25,000 options exercisable within 60 days of November 26, 2004.
Compensation Committee Interlocks and Insider Participation. During the 2003 fiscal year, executive compensation was administered by the Compensation Committee comprised of two outside members of the Board of Directors, Drs. Dean and Watson.
Certain Relationships and Related Transactions. We were involved in a joint venture transaction with affiliates of Elan Corporation, plc ("Elan"). That joint venture, called Sentigen Ltd., was involved in the development of lymphography diagnostic products and is described under the heading "Joint Venture/Investment in Affiliate" in Note 6 to our Financial Statements included in our 10-QSB/A for the quarterly period ended June 30, 2004.
25
Mi3, Oxford and MRNA purchased shares in the institutional financing which closed on November 12, 2002. Jonathan Fleming (a director) is General Partner of OBP Management IV L.P. which is the general partner of Oxford and MRNA. William McPhee (a director) is president of Mi3 Services L.L.C., which is the general partner of Mi3. Tannebaum, LLC also acquired shares of our Common Stock in the financing which closed on November, 12, 2002 through the conversion of outstanding debt into shares of our Common Stock.
Three of our existing investors, Oxford, MRNA and Mi3 are purchasing shares of our Common Stock in the financing transaction described in Proposal 1 above. These investors have also provided capital to us evidenced by the Secured Notes, which will be converted into shares of our Common Stock at $0.40 in connection with the financing.
Executive Compensation. The following table sets forth compensation we paid to our executive officers (the "Named Officers") for services rendered us in all capacities during the year ended December 31, 2003.
SUMMARY COMPENSATION TABLE
| |
| |
| |
| |
| | Long Term Compensation
Awards
| |
| |
| |
|---|
| |
| |
| |
| |
| | Payouts
| |
|---|
| | Annual Compensation
| |
| |
|---|
Name and Principal Position
| | Other
Annual
Compensation ($)
| | Restricted
Stock
Award(s) ($)
| | Securities
Underlying
Options (#)(5)
| | LTIP
Payouts ($)
| | All Other
Compensation ($)
| |
|---|
| | Year
| | Salary($)
| | Bonus($)
| |
|---|
Taffy J. Williams, Ph.D(1)
Director, President and Chief Executive Officer | | 2003
2002
2001 | | $
$
$ | 275,000
265,000
260,000 | | $
$
$ | 0
60,000
30,000 | | $
| 0
—
— | | 0
—
— | | 1,050,000
1,233,450
— | | 0
—
— | | $
| 0
—
— | |
Brooks Boveroux(1)(3)
Senior Vice President—Finance, Chief Financial Officer, Treasurer and Secretary |
|
2003
2002
2001 |
|
$
$
$ |
225,000
216,250
215,000 |
|
$
$
$ |
25,000
32,250
32,250 |
|
$
|
0
—
— |
|
0
—
— |
|
—
320,860
— |
|
0
—
— |
|
$
|
0
—
— |
|
Reinhard Koenig, M.D., Ph.D.(2)
Senior Vice President—Medical and Regulatory Affairs |
|
2003
2002
2001 |
|
$
$
|
75,000
280,625
200,020 |
|
$
|
0
—
— |
|
$
|
0
—
— |
|
0
—
— |
|
0
513,375
100,000 |
|
0
—
— |
|
$
$
|
0
50,000 |
(6) |
Jack DeFranco(1)(4)
Senior Vice President—Business Development, Marketing and Sales |
|
2003
2002
2001 |
|
$
$
$ |
107,145
0
0 |
|
$
$
$ |
60,000
0
0 |
|
$
|
0
—
— |
|
0
—
— |
|
170,000
—
— |
|
0
—
— |
|
$
|
0
—
— |
|
- (1)
- Dr. Williams and Messrs. Boveroux and DeFranco are entitled to severance if their employment is terminated without cause, as discussed below.
- (2)
- Dr. Koenig resigned from the Company as of May 1, 2003
- (3)
- Mr. Boveroux's employment with the Company terminated as of August 29, 2004.
26
- (4)
- Mr. DeFranco joined the Company on June 18, 2003 as part of the acquisition of the medical imaging business of Alliance Pharmaceutical Corp.
- (5)
- Reflects a one-for-four reverse split which occurred in November, 2002.
- (6)
- Reimbursement of moving expenses.
Stock Options. The following table contains information concerning the grant of stock options during the 2003 fiscal year under our 1998 and 2000 Plans to the Named Officers. To date, none of these options have been exercised.
2003 Stock Option Grants
Name
| | Individual Grants Number of Securities Underlying Options Granted(#)(1)
| | % of Total Options Granted to Employees in 2003
| | Exercise Price ($/Sh)
| | Expiration Date
| | Grant Date
Present Value
($)(3)
|
|---|
| Taffy J. Williams, Ph.D.(1) | | 1,050,000 | | 35.8 | % | $ | 1.08 | | 7/23/2013 | | $ | 1,995,525 |
| Brooks Boveroux | | 0 | | 0 | % | $ | | | | | | |
| Jack DeFranco(2) | | 170,000 | | 5.8 | % | | 1.25 | | 6/19/2013 | | $ | 303,178 |
| Reinhard Koenig, M.D., Ph.D. | | 0 | | 0 | % | | | | | | | |
- (1)
- The options were granted at an exercise price equal to the purchase price paid by the institutional investors in the institutional financing which closed on November 12, 2002. The market price of our Common Stock on the date of the option grant was $2.52. The options become exercisable on the earlier of a change in control of the Company or the date that Dr. Williams no longer is an employee of the Company in accordance with the terms of the respective Award Agreements covering the options. These options are subject to stockholder approval of Proposal 2.
- (2)
- The options were granted at an exercise price of $1.25 per share. The market price of our Common Stock on the date of the option grant was $2.20. A portion of the options becomes exercisable upon various dates in accordance with the terms of the respective Award Agreements covering the options.
- (3)
- In accordance with SEC rules, grant date present value is determined using the Black-Scholes Model. The Black-Scholes Model is a complicated mathematical formula widely used to value exchange-traded options. However, stock options granted by the company are long-term, non-transferable and subject to vesting restrictions, while exchange-traded options are short-term and can be exercised or sold immediately in a liquid market. The Black-Scholes Model relies on several key assumptions to estimate the present value of options, including the market price, volatility of, and dividend yield on, the security underlying the option, the risk-free rate of return on the date of grant and the estimated time period until exercise of the option. For the purposes of the calculation of the fair value of the options at grant date, the following assumptions were used: risk-free interest rate at grant date: 2.56%; expected stock price volatility: 98%; dividend yield: 0% and expected time until exercise of the option: 5 years. The shares subject to the options granted are identical to the publicly-traded securities of the company except for restrictions on trading, vesting periods prior to which the options may not be exercised and the presence of an expiration date of the grant. Due to these restrictions the shares subject to the options granted are worth less than their publicly-traded counterparts. The actual value, if any, that an executive officer may realize will depend on his continued employment through the options vesting period, and the excess of the market price over the exercise price on the date the option is exercised so that there is no assurance that the value realized by an executive officer will be at or near the value estimated by the Black-Scholes Model.
27
The following table sets forth information about the Named Officers concerning the exercise of options during the last fiscal year and unexercised options held as of the end of the 2003 fiscal year.
2003 Option Exercises and Year-End Values
| |
| |
| | Number of Securities Underlying Unexercised Options at Year
End 2003 (#)
| | Fair Value of Unexercised In-the-Money Options
at Year
End 2003 ($)(1)
|
|---|
Name
| | Shares Acquired On
Exercise (#)
| | Value
Realized ($)
|
|---|
| | Exercisable
| | Unexercisable
| | Exercisable
| | Unexercisable
|
|---|
| Taffy J. Williams, Ph.D.(2) | | 0 | | 0 | | 975,000 | | 1,308,450 | | $ | 0 | | $ | 703,066 |
| Brooks Boveroux | | 0 | | 0 | | 558,542 | | 304,833 | | $ | 149,150 | | $ | 164,849 |
| Jack DeFranco | | 0 | | 0 | | 0 | | 170,000 | | $ | 0 | | $ | 68,000 |
| Reinhard Koenig, M.D., Ph.D. | | 0 | | 0 | | 274,602 | | 146,258 | | $ | 128,023 | | $ | 54,867 |
- (1)
- For purposes of this table, the "value" of stock options was determined by the difference between the exercise price and $1.65, the closing price for common stock on the last business day of the fiscal year. Shares acquired on exercise of these options are not registered under the Securities Act of 1933 and are not freely saleable except in compliance with exemptions from registration, including the holding period and other requirements of Rule 144 thereunder. Consequently, the values set forth in the table are only theoretical values and may not accurately represent actual market value.
- (2)
- Does not include the grant of 1,050,000 options shares subject to stockholder approval in Proposal 2 and the $598,000 fair value of these unexercisable options.
We have employment agreements with Dr. Williams and with Messrs. Boveroux and DeFranco. Each of these employment agreements is terminable at-will and can be terminated by either party at any time with or without cause. Mr. Boveroux's employment with the Company terminated effective August 29, 2004. In addition, Dr. Williams and Messrs. Boveroux and DeFranco are bound by their respective Employee Confidentiality, Inventions and Noncompetition Agreements, as amended (each of these agreements is referred to as a "Confidentiality Agreement"). Each Confidentiality Agreement provides that while we employ the respective individual and for a period of two years after termination he will not engage in activities that compete with our business. Each Confidentiality Agreement also requires the respective employee to disclose to our Board of Directors all inventions or other intellectual property discovered or made by the employee during his employment and twelve months thereafter, if those inventions are related to or useful in our business, or result from duties assigned to that individual or from the use of any of our assets or facilities.
In addition, pursuant to the terms of Dr. Williams' current employment agreement, he was awarded a non-qualified option to acquire 1,050,000 shares of our Common Stock at an exercise price of $1.08 per share. The option has a 10-year term and vests upon the earlier of a Change of Control, as defined therein, or when Dr. Williams ceases to be a salaried employee of the Company. The award was made subject to the approval of our stockholders, and if the increase in the number of shares available for issuance under the Company's 2000 Plan as set forth in Proposal 2 is approved by the stockholders, the option will be awarded under the 2000 Plan. Stockholders holding approximately 64.2% of our outstanding Common Stock (as of November 26, 2004) have agreed in writing to vote in favor of the issuance of Dr. Williams' option. Pursuant to the terms of Dr. Williams' employment agreement because the Company closed a financing transaction by July 23, 2004 he was also entitled to a cash bonus of $206,250 (which he elected to defer to a later date) and awarded a non-qualified option to purchase 4,028,402 shares (i.e., the number of shares, which taken together with all other options Dr. Williams has been awarded (other than his options to purchase 750,000 shares with an
28
exercise price of $60 per share and 300,000 shares at $11 per share), equals 6% of our outstanding Common Stock on a fully diluted basis). The additional option has a 10-year term, exercisable at $.40 per share (i.e., the same price as shares are issued in the financing).
Dr. Williams and Mr. DeFranco each are eligible to receive typical health, life and disability insurance benefits that are available to our other salaried employees. These individuals are also eligible to defer a portion of their salary through our 401(k) plan, but the Company did not match or make any contributions to the 401(k) plan during the 2003 fiscal year. The Company does not maintain a pension plan other than its 401(k) plan.
We have a consulting agreement with Dr. Watson pursuant to which he provides business and product development services to us. The consulting agreement commenced in November, 2002. The term is on a month-to-month basis (unless terminated earlier by notice or cause). The consulting agreement provides that Dr. Watson will be paid $175 per hour of service, be reimbursed for all reasonable out-of-pocket expenses, and receive options to purchase up to 10,000 shares of the Company's Common Stock on February 3, 2003 and each January 1 thereafter so long as the agreement is in effect. The options have an exercise price of the fair market value of the Company's Common Stock on the date of grant. However, in no event will Dr. Watson receive more than $60,000 annually while he is on our Audit Committee.
We entered into a consulting agreement with Dr. Koenig which commenced as of May 1, 2003 and was for a period of three months. Under the terms of the consulting agreement Dr. Koenig was available to provide advice and consultation relating to the transition of his former duties regarding the medical and regulatory affairs of the Company to others after his termination. Dr. Koenig received a total of $56,250 under the terms of the consulting agreement.
Officers generally serve at the discretion of the Board of Directors. We do not have any compensatory plans or arrangements resulting from resignation, retirement or any other termination of an executive officer's employment with us. However, the employment agreements for Dr. Williams and Messrs. Boveroux and DeFranco each provide for severance in the event we terminate their employment without cause. Upon termination without cause, Dr. Williams would be entitled to a severance payment equal to one year's salary. If terminated without cause, Mr. DeFranco would be entitled to receive severance equal to one month's salary for each year or partial year he was employed at Alliance and/or the Company at his annual base salary in effect at the time of termination. Mr. Boveroux's employment was terminated without cause effective August 29, 2004 and is entitled to a severance payment equal to six month's salary.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our executive officers, directors and persons who beneficially own more than 10% of our Common Stock to file initial reports of ownership and changes in ownership with the SEC. Those persons are required by SEC regulations to furnish us with copies of all Section 16(a) forms that they file. To our knowledge, based solely on a review of the copies of those reports furnished to us during the fiscal year ended December 31, 2003, we believe that our executive officers, directors and beneficial owners of more than 10% of our common stock complied with all Section 16(a) filing requirements, except as follows:
- •
- Oxford failed to timely file a Form 4 reporting the purchase of shares; it filed a Form 4 for this transaction on January 3, 2003.
- •
- Alan D. Watson, Ph.D., M.B.A. and Lester McKeever failed to timely file Form 4s reporting the purchase of shares and the receipt on an option to purchase shares; they filed Form 4s for this transaction on January 7, 2003.
- •
- Gene Golub failed to timely file a Form 4 reflecting an option grant; he filed a Form 4 for this transaction on January 8, 2003.
29
- •
- Reinhard Koenig failed to timely file a Form 4 reflecting the purchase of shares; he filed a Form 4 for this transaction on January 31, 2003.
- •
- Alan D. Watson failed to timely file a Form 4 reflecting the receipt of an option to purchase shares; he filed a Form 4 for this transaction on February 19, 2003.
- •
- Stuart Levine failed to timely file a Form 4 reflecting the gift of shares; he filed a Form 4 for this transaction on February 19, 2003.
- •
- Brooks Boveroux and Taffy J. Williams, Ph.D. failed to timely file Form 4s reflecting the receipt of options to purchase shares; Mr. Boveroux and Dr. Williams filed Form 4s for these transactions on September 18, 2003 and September 26, 2003, respectively.
The obligation to file these reports rests with the person obligated to make the filing and not with the Company. These late filings were due in part to a lack of resources by the Company to assist the reporting persons with these filings, in part due to oversights by the relevant individuals, and in part due to oversights by Company counsel. The Company has modified its reporting compliance procedures to avoid these delinquencies in the future.
Change in Control Agreements. We know of no arrangements which could result in a change of control of the Company.
Securities Authorized for Issuance Under Equity Compensation Plans.
The information provided in the following table is as of December 31, 2003.
Plan Category
| | Number of securities to be
issued upon exercise of
outstanding options, warrants
and rights (a)
| | Weighted-average exercise
price of outstanding options,
warrants and rights (b)
| | Number of securities remaining
available for future issuance
under equity compensation
plans (excluding securities
reflected in column (a)) (c)
|
|---|
| Equity compensation plans approved by security holders | | 5,889,470 | | $ | 10.90 | | 178,155 |
| Equity compensation plans not approved by security holders | | 1,252,431 | (1) | $ | 1.75 | | 0 |
- (1)
- Reflects an option grant to Dr. Williams that is subject to stockholder approval in Proposal 2.
We have issued the following options and warrants to purchase shares of our Common Stock without the prior approval of our stockholders:
- •
- a warrant to purchase 190,000 shares at $2.52 per share to Clinical Regulatory Strategies, LLC in connection with our August 12, 2002 engagement of Clinical Regulatory Strategies, LLC to provide us with certain clinical, regulatory and new product development services.
- •
- a warrant to purchase 3,750 shares at $44.23 per share to a consultant as of January 24, 2000 in connection with certain consulting services provided by the consultant.
30
- •
- warrants to purchase an aggregate of 8,181 shares at $47.78 per share to Gilford Securities Incorporated and certain of its employees in connection with certain placement agent services provided to us by Gilford Securities Incorporated in February, 2000.
- •
- a warrant to purchase 500 shares at $47.04 per share to Aqua Partners, Inc. in connection with certain investor services provided by Aqua Partners, Inc. in November, 1999.
- •
- An option to purchase 1,050,000 shares at $1.08 per share to Taffy Williams pursuant to an agreement dated July 23, 2003; provided, however, that we are now seeking stockholder approval of this option grant as part of the amendment to the 2000 Plan.
Director Compensation.
All directors receive reimbursement of their out of pocket expenses incurred in the course of their duties as directors. Drs. Dean and Watson and Mr. McPhee receive compensation for serving on the Board or on the Audit Committee or the Compensation Committee of the Board. Each of these individuals receives $20,000 annually for serving as a member of the Board. In addition, each of these persons received a stock option to purchase 60,000 shares of the Company's Common Stock. These options vest quarterly over two years. As members of the Audit Committee, Drs. Dean and Watson receive $5,000 annual compensation, and as members of the Compensation Committee receive $3,000 annual compensation in addition to any fee that they receive as Board members.
Compensation Committee Report
To the Board of Directors of IMCOR Pharmaceutical Co.:
The Compensation Committee is comprised of two non-employee directors: Drs. Dean and Watson. This Committee reviews and oversees the Company's various incentive plans, evaluates performance and reviews compensation levels and other related matters for the Company's senior executives. The Committee met one time in fiscal year 2003.
The Committee's policy regarding executive compensation reflects a commitment to offer competitive compensation opportunities for the Company's executive officers, so as to attract and retain quality executives, to provide incentives for executives to achieve performance objectives that enhance shareholder value, and to reward excellent performance. Accordingly, the Committee reviews and discusses with the Company (i) competitive base salaries, (ii) bonus arrangements to encourage that performance objectives are met, and (iii) stock option grants on terms designed to facilitate long-term strategic management and enhancement of shareholder value.
The Committee believes that the current base salary of $275,000 for the Company's Chief Executive Officer for fiscal year 2003 is appropriate in light of his contributions to the Company's management restructuring, establishing product development objectives, strengthening the research and development team, and providing the framework for obtaining equity financing. In considering the Chief Executive Officer's contribution to the Company's success, the Committee considers, among other things, his role in enhancing the Company's growth, controlling costs, making efficient use of the Company's assets and employees, and monitoring the Company's operations. Additionally, to retain the Company's highly skilled work force, the Company strives to remain competitive with the pay of other highly respected employers who compete with the Company for talent.
The Committee may from time to time grant options to executive officers to further align their long-term interests with those of other shareholders. The Committee believes that stock options encourage the executives to employ strategies designed to enhance the long-term value of the Company's Common Stock. During fiscal year 2003, the Committee approved awards of options under the 2000 Plan to Gene Golub (options to acquire 10,000 shares of Common Stock), Lester McKeever (options to acquire 10,000 shares of Common Stock), Jack DeFranco (options to acquire 170,000 shares of Common Stock), and Alan Watson (options to acquire 20,000 shares of Common Stock). The
31
Committee based the grants on a variety of factors, including expected future contribution to the Company's performance. The Committee may also grant stock options in the future based on these and other factors.
Disclaimer. This report is being provided to the Company's stockholders solely for informational purposes. Stockholders should not consider this report or the stock price performance graph that follows to be "soliciting materials" or to be "filed" with the SEC. They are not subject to the SEC's proxy rules or to the liabilities of Section 18 of the U.S. Securities Exchange Act of 1934. In addition, this report and the performance graph shall not be deemed to be incorporated by reference into any prior or subsequent filing by the Company under the Federal securities laws.
| | | Compensation Committee |
|
|
Alan D. Watson, Ph.D., MBA
Richard T. Dean, Ph.D. |
|
|
Dated: April 27, 2004 |
Stock Performance Chart
The following graph and chart compares our cumulative total shareholder rate of return on our Common Stock for the period from December 31, 1999 to December 31, 2003 (the date our 2003 fiscal year ended), with the cumulative total return of (i) the Nasdaq Stock Market (U.S.) Index and (ii) the Nasdaq Pharmaceutical Index. The comparison assumes $100 was invested on December 31, 1999 in our Common Stock and in each of the foregoing indices and the re-investment of dividends (we issued no dividends on our Common Stock during this time).
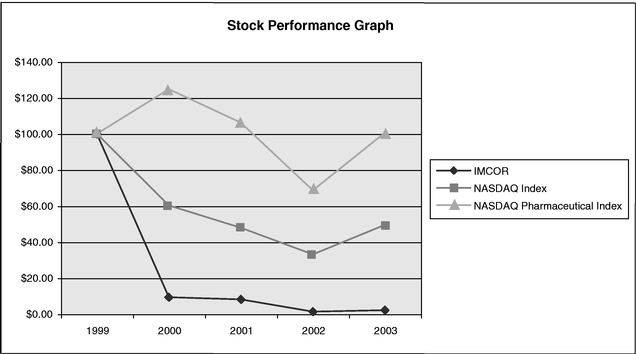
| | 1999
| | 2000
| | 2001
| | 2002
| | 2003
|
|---|
| IMCOR Index | | $ | 100 | | $ | 9.46 | | $ | 8.22 | | $ | 1.34 | | $ | 2.23 |
| NASDAQ Index | | $ | 100 | | $ | 60.31 | | $ | 47.84 | | $ | 33.08 | | $ | 49.45 |
| NASDAQ Pharmaceutical Index | | $ | 100 | | $ | 124.74 | | $ | 106.31 | | $ | 68.69 | | $ | 100.69 |
32
Financial and Other Information
Financial Statements and Supplementary Financial Information. Information is provided in Item 7 of our Form 10-KSB/A for the fiscal year ended December 31, 2003, which is attached as Exhibit F.
Management's Discussion and Analysis of Financial Condition and Result of Operations. Information is provided in Item 6 of our Form 10-KSB/A for the fiscal year ended December 31, 2003, which is attached as Exhibit F.
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. On November 14, 2003 BDO Seidman, LLP informed IMCOR that they were declining to stand for re-election as IMCOR's auditors. IMCOR's Board of Directors did not believe that it was necessary to take any action in response to BDO Seidman, LLP's decision not to stand for re-election and accordingly did not recommend or approve this decision.
On November 18, 2003, IMCOR's Board of Directors, upon recommendation of the Audit Committee, engaged Moss Adams LLP as independent auditor, replacing BDO Seidman, LLP. At the Special Meeting of our stockholders held on February 5, 2004, the stockholders ratified the appointment of Moss Adams LLP as our independent certified public accountants for 2004.
BDO Seidman, LLP's reports on IMCOR's financial statements for the past two years did not contain an adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles other than a qualification related to the entity's ability to continue as going concern.
During IMCOR's two most recent fiscal years and through the date of BDO Seidman, LLP's declination to stand for re-election, there were no disagreements with BDO Seidman, LLP on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure which, if not resolved to BDO Seidman, LLP's satisfaction, would have caused BDO Seidman, LLP to make reference to the subject matter in connection with its report of the financial statements for such years; and there were no reportable events as defined in Item 304(a)(1)(v) of Regulation S-B.
BDO Seidman has stated its agreement with the above statements in its letter to IMCOR dated November 18, 2003.
Quantitative and Qualitative Disclosures About Market Risk. Information is provided in Item 7A of our Form 10-KSB/A for the fiscal year ended December 31, 2003, which is attached as Exhibit F.
Other Information. Representatives of Moss Adams, LLP, our principal accountants for the current year and for the most recently completed fiscal year, (i) are expected to be present or available by conference call at the security holders' meeting; (ii) will have the opportunity to make a statement if they desire to do so; and (iii) are expected to be available to respond to appropriate questions.
Audit Committee Report
To the Board of Directors of IMCOR Pharmaceutical Co.:
The Board of Directors has appointed an Audit Committee consisting of two directors. The Board, in its business judgment, has determined that each current member of this Committee is "independent," as required by applicable listing standards of the Nasdaq National Market and the Sarbanes-Oxley Act of 2002 and applicable SEC rules.
The Audit Committee oversees the Company's financial reporting process on behalf of the Board of Directors. Management has the primary responsibility for the financial statements and their
33
reporting process, including the systems of internal controls. In fulfilling its oversight responsibilities, the Committee reviewed the audited financial statements in the Annual Report with management, including a discussion of the quality (not just the acceptability) of the accounting principles, the reasonableness of significant judgment and the clarity of disclosures in the financial statements.
Moss Adams, LLP is the Company's independent auditor. The independent auditors are responsible for expressing an opinion on the conformity of the audited financial statements with generally accepted accounting principles. The Committee reviewed and discussed with the Company's management, internal accounting personnel and with Moss Adams, LLP the following:
- •
- Moss Adams's judgments as to the quality (not just the acceptability) of the Company's auditing principles.
- •
- The auditors' independence from the Company's management, including the matters in the written disclosures required by the Independent Standards Board in Standard No. 1, as may be modified or supplemented.
- •
- The overall scope and plans for Moss Adams's audits.
- •
- Moss Adams's evaluation of the Company's internal controls.
- •
- The overall quality of the Company's financial reporting.
- •
- Other matters that are required to be discussed with the Committee under generally accepted auditing standards, including the Statement on Auditing Standards No. 61 ("SAS 61").
The Committee met with Moss Adams, with and without management present, to discuss these matters and the results of Moss Adams's examinations. The Committee held two meetings during fiscal 2003.
The directors who serve on the Audit Committee are all "independent" for purposes of the NASDAQ listing standards. That is, the Board has determined that none of the directors who serve on the Audit Committee has a relationship to the Company that may interfere with his independence from the Company or its management.
The Committee has discussed with the Company's independent auditors the matters required to be discussed by SAS 61. SAS 61 requires the Company's independent auditors to discuss with the Company's Audit Committee, among other things, the following:
- •
- Method to account for significant unusual transactions
- •
- The effect of significant accounting policies
- •
- The process used by management in formulating particularly sensitive accounting estimates and the basis for the auditor's conclusions regarding the reasonableness of those estimates
- •
- Disagreements with management over the application of accounting principles, the basis for management's accounting estimates and the disclosures in the financial statements
The Committee has received, reviewed and discussed by written disclosures and the letter from the independent auditors required by Independence Standard No. 1, Independence Discussions with Audit Committees, as amended, by the Independence Standards Board ("Independence Standards Board Standard No. 1"), and have discussed with the auditors the auditors' independence. Independence Standards Board Standard No. 1 requires auditors annually to disclose in writing all relationships that, in the auditor's professional opinion, may be reasonably thought to bear on independence, confirm their perceived independence and engage in a discussion of independence.
34
The Committee has considered whether the non-audit services provided by the independent auditors, as set forth in the section of this Proxy Statement entitled, "Independent Auditor's Fees and Other Matters," are compatible with maintaining the public accountants' independence.
Based on the reviews and discussions referred to above, the Committee recommends to the Board of Directors that the financial statements referred to above be included in the Company's Annual Report on Form 10-KSB for the year ended December 31, 2003.
Disclaimer. This report is being provided to the Company's stockholders solely for informational purposes. Stockholders should not consider this report to be "soliciting material" or to be "filed" with the SEC. It also is not subject to the SEC's proxy rules or to the liabilities of Section 18 of the U.S. Securities Exchange Act of 1934. In addition, this report shall not be deemed to be incorporated by reference into any prior or subsequent filing by the Company under the Federal securities laws.
| | | Audit Committee |
|
|
Alan D. Watson, Ph.D., M.B.A. (Chairman)
Richard T. Dean, Ph.D. |
|
|
Dated: April 27, 2004 |
Independent Auditors' Fees
Audit Fees. In the fiscal year ended December 31, 2003 BDO Seidman, LLP billed us an aggregate of $80,970 in fees for professional services rendered in connection with the audit of our financial statements and the reviews of the financial statements included in our Quarterly Reports on Form 10-Q/10-QSB. Moss Adams LLP billed us an aggregate of $66,551 (as of April 10, 2004) in fees for professional services rendered in connection with our fiscal year 2003 audit of our financial statements and the review of the financial statements included in our Quarterly Report on Form 10-QSB for the quarter ended September 30, 2003, respectively.
Audit-Related Fees. For the fiscal year ended December 31, 2003 Moss Adams LLP billed us $3,248 (as of April 10, 2004) for audit-related fees.
Tax Fees. In the fiscal year ended December 31, 2003 BDO Seidman, LLP billed us an aggregate of $10,881 for professional services rendered in connection with tax compliance, tax advice and tax planning. Moss Adams, LLP billed us an aggregate of $7,682 (as of April 10, 2004) in connection with tax compliance, tax advice or tax planning.
All Other Fees. In the fiscal year ended December 31, 2003 neither BDO Seidman, LLP nor Moss Adams, LLP billed us for any products or services, other than those disclosed above under the headings "Audit Fees" and "Tax Fees."
Audit Committee Pre-Approval Policy. Pursuant to the terms of our Audit Committee's charter, our Audit Committee pre-approves all auditing services (which may entail providing comfort letters in connection with securities underwritings) and non-audit services proposed to be provided by the Company's independent certified public accountant, except for non-audit services within the de minimus exception under Section 10A(i)(B) of the Exchange Act. In this connection, the Audit Committee has the authority to appoint one or more of its members to approve services, provided that the decisions made by such designees between meetings of the Audit Committee shall be presented to the full Audit Committee at the next meeting thereof. All of the non-audit services provided to the Company by BDO Seidman LLP during the fiscal year ended December 31, 2003 were pre-approved by the Audit Committee.
Other Matters
If any other matters are properly presented at the Annual Meeting, including a motion to adjourn, the persons named as Proxies will have discretion to vote on those matters according to their best judgment to the same extent as a person delivering a Proxy would be entitled to vote. At the date this Proxy Statement went to press, we did not anticipate that any other matters would be raised at the Annual Meeting.
Information Incorporated By Reference
The Company's Form 10-QSB/A (including the financial statements) for the quarterly period ended September 30, 2004 (attached as Exhibit H) and Form 10-KSB/A (including the financial statements) for the fiscal year ended December 31, 2003 accompany this Proxy Statement as mailed to stockholders.An additional copy of the Company's Form 10-QSB/A and Form 10-KSB/A and the amendments thereto as filed with the SEC, without exhibits, will be provided without charge following the receipt of a written request to Taffy J. Williams, Ph.D., Chief Executive Officer, IMCOR Pharmaceutical Co., 6175 Lusk Boulevard, San Diego, CA 92121.
35
Exhibit A
SECURITIES PURCHASE AGREEMENT
THIS SECURITIES PURCHASE AGREEMENT (the or this "Agreement") dated as of April 14 2004 is entered into by and among IMCOR Pharmaceutical Co., a Nevada corporation (the "Company"), and the purchasers identified on the signature pages hereto (each a "Purchaser" and collectively, the "Purchasers").
WHEREAS, the Company and the Purchasers are executing and delivering this Agreement in reliance upon the exemption from securities registration afforded by the provisions of Regulation D ("Regulation D"), as promulgated by the U.S. Securities and Exchange Commission (the "SEC") under the Securities Act of 1933, as amended (the "1933 Act"); and
WHEREAS, the Purchasers wish to purchase from the Company, and the Company wishes to sell and issue to the Purchasers, shares of Common Stock of the Company, $0.001 par value per share (the "Common Stock"), together with Warrants (the "Warrants") to purchase Common Stock, upon the terms and conditions set forth in this Agreement;
NOW, THEREFORE, in consideration of the mutual promises and covenants contained in this Agreement, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, intending to be legally bound by the terms and conditions of this Agreement, the parties hereto hereby agree as follows.
1. Purchase and Sale of Securities.
1.1. Offering. Subject to the terms and conditions of this Agreement, the Company shall sell and issue to each of the Purchasers, and each of the Purchasers shall severally, and not jointly, purchase from the Company, the aggregate number of shares of Common Stock (the "Shares") equal to the investment amount set forth opposite the Purchaser's name onSchedule I divided by $0.75 per share (the "Per Share Purchase Price") plus the Warrants set forth opposite such Purchaser's name (50% Warrant coverage). The Shares, the Warrants and the Common Stock issuable upon exercise of the Warrants are collectively referred to as the "Securities." Additional investors may be added by the Company toSchedule I at the discretion of the Company following the First Closing and prior to the filing of a Registration Statement (as defined in the Registration Rights Agreement, defined below), and upon execution by such additional investors of a counterpart signature page to this Agreement, such additional investors shall be deemed a Purchaser hereunder and shall be subject to and may rely upon the representations, warranties, terms and conditions contained herein;provided, that such investors may only acquire Securities at the Second Closing.
1.2. Proceeds. Net proceeds from the sale of the Securities will be used for the following purposes and appropriately related administrative costs, subject to changes authorized by a majority of the Company's Board of Directors (the"Board"):
- •
- manufacturing, marketing and continued development of Imagent;
- •
- research and development of PH-50;
- •
- research and development of N1177;
- •
- payment of commissions and other fees associated with the transactions in this Agreement;
- •
- payment of obligations to vendors of goods or services and other creditors (except as described in the last sentence of this Section 1.2) not to exceed the amounts set forth in the Disclosure Schedule; and
A-1
- •
- working capital and general corporate purposes.
Pending use of the proceeds in accordance with the preceding sentence, the proceeds will be invested in securities issued or guaranteed by the United States, national bank repurchase agreements secured by such securities or such other types of investments as may be approved by a majority of the Company's Board of Directors, but in no event in such a manner that would cause the Company to be deemed an Investment Company as defined in the Investment Company Act of 1940, as amended. The Company shall not use any portion of the net proceeds to redeem any capital stock of the Company or to repay indebtedness described in Section 5.3.14 below.
2. Delivery of the Shares at the Closings.
2.1. Initial Closing. The initial closing (the "Initial Closing") shall take place at the offices of Grippo & Elden, LLC, 227 W. Monroe Street, Chicago, Illinois 60606 on the second business day following the date on which the applicable conditions set forth in Section 5.3 (excluding Section 5.3.9) and Section 6 shall have been satisfied or waived by the appropriate parties, or at such other time, date and place as are mutually agreeable to the Company and the Purchasers subscribing for a majority of the Shares. At the Initial Closing:
(a) in accordance with the terms of the Escrow Agreement (as defined in Section 3.2), the Escrow Agent shall disburse to the Company by wire transfer of immediately available funds payment of the purchase price of the Securities delivered to the Purchasers at the Initial Closing (which shall not exceed 19.9% of the Company's outstanding Common Stock), as set forth onSchedule I hereto; and
(b) the Company shall deliver to the Purchasers certificates for the number of Securities to be delivered at the Initial Closing, as set forth opposite their respective names onSchedule I hereto, registered in the name of such Purchasers.
2.2. Second Closing. The second closing (the "Second Closing") shall take place at the offices of Grippo & Elden LLC, 227 W. Monroe Street, Chicago, Illinois 60606 on the second business day following the date on which the applicable conditions set forth in Section 5.3 (including Section 5.3.9) and Section 6. At the Second Closing:
(a) in accordance with the terms of the Escrow Agreement, the Escrow Agent, shall disburse to the Company the purchase price of the Securities, together with any interest accrued thereon, to be delivered to the Purchasers at the Second Closing, as set forth onSchedule I hereto; and
(b) the Company shall deliver to the Purchasers certificates for the number of Securities to be delivered at the Second Closing, as set forth opposite their respective names onSchedule I hereto, registered in the name of such Purchasers.
The Initial Closing and the Second Closing are collectively referred to herein as the"Closings" and individually as a"Closing." The date on which the Closings occur are referred to herein as a"Closing Date."
2.3. Termination. If by the applicable dates set forth in Section 5.3.13 the conditions set forth in Section 5.3 that apply to the applicable Closing shall not have been fulfilled by the Company, then each Purchaser to receive Securities at such Closing shall, at its election, be relieved of all of its obligations under this Agreement as to such Closing without thereby waiving any other right it may have by reason of such failure or such non-fulfillment. In connection therewith, such Purchaser shall have the right to require the Escrow Agent to disburse to such Purchasers the funds delivered by such Purchaser to the Escrow Account in respect of the applicable Closing (plus any interest thereon). If by the applicable date set forth in Section 6.5, any of the conditions set forth in Section 6 of this Agreement that apply to the applicable Closing shall not have been fulfilled by any Purchaser, the Company shall, at its
A-2
election, be relieved of all of its obligations to such Purchaser under this Agreement as to such Closing without thereby waiving any other right it may have by reason of such failure or such non-fulfillment.
3. Representations and Warranties of the Company. Except as disclosed in the Disclosure Schedule attached hereto (which Disclosure Schedule makes explicit reference to, and shall only modify, the particular Section containing the representation or warranty as to which exception is taken), the Company hereby represents and warrants to the Purchasers as follows:
3.1. Organization; Amended Articles. The Company is a corporation duly organized, validly existing and in corporate good standing under the laws of the State of Nevada and is qualified to do business as a foreign corporation in each jurisdiction in which such qualification is required, except where the failure to be so qualified would not have, either individually or in the aggregate, a material adverse effect on the business, operations, financial condition, assets, prospects, liabilities or contractual rights of the Company, whether individually or taken as a whole (a "Material Adverse Effect"). The Company has, or prior to each Closing will have, duly authorized the sale and issuance, pursuant to the terms of this Agreement an adequate number shares of its Common Stock authorized and available for purchase hereunder and upon exercise of the Warrants issuable at such Closing, having the rights, restrictions, privileges and preferences set forth in the Amended and Restated Certificate of Incorporation (the "Amended Articles").
3.2. Corporate Power. The Company has all required corporate power and authority to own its property, to carry on its business as presently conducted or contemplated, to enter into and perform this Agreement, the Escrow Agreement, in the form attached asExhibit A (the"Escrow Agreement"), the Warrant Agreements by and between the Company and each of the Purchasers, each in the form attached asExhibit B (the "Warrant Agreement"), the Registration Rights Agreement by and among the Company and the Purchasers in the form attached asExhibit C (the "Registration Rights Agreement"), the Voting Agreement (as defined in Section 5.15), and the other agreements, documents and instruments contemplated hereby (collectively with this Agreement, the "Financing Documents"), and to carry out the transactions contemplated hereby. The copies of the Amended Articles and Bylaws of the Company, as amended to date, which have been furnished by the Company to counsel for the Purchasers, are correct and complete at the date hereof. The Company is not in violation of any term of its Amended Articles or Bylaws, each as amended, or in violation of any term of any agreement, instrument, judgment, decree, order, statute, rule or government regulation applicable to the Company or to which the Company is a party, where any violation, noncompliance or default would result in a Material Adverse Effect.
3.3. Authorization. The Financing Documents are valid and binding obligations of the Company, enforceable in accordance with their terms. The execution, delivery and performance of the Financing Documents have been duly authorized by all necessary corporate or other action of the Company. Subject to obtaining stockholder approval for the issuance of more than 20% of the Company's Common Stock pursuant to Nasdaq Marketplace Rule 4350 with respect to Securities to be issued at the Second Closing, the issuance, sale and delivery of the Securities in accordance with this Agreement or the Warrant Agreement have been, or will be prior to each Closing, duly authorized by all necessary corporate action on the part of the Company. The Securities when so issued, sold and delivered against payment therefor in accordance with the provisions of this Agreement or the Warrant Agreement will be duly and validly issued, fully paid and non-assessable. No consent, approval or authorization of, or designation, declaration or filing with, any governmental authority or any other person or entity is required of the Company in connection with the execution and delivery of the Financing Documents, or the issuance, sale and delivery of the Securities in accordance with the terms of this Agreement or the Warrants, or the consummation of any other transaction contemplated hereby or by the other Financing Documents, except (i) the filing with the SEC of one or more Registration Statements in accordance with the requirements Registration Rights Agreement and the filing of a Form D with the SEC, (ii) any filings required by state securities laws, (iii) the filings required in accordance with
A-3
Section 8.12, (iv) the filing of the Proxy Statement in accordance with Section 5.1.4, and (v) those that have been made or obtained prior to or as of the date of this Agreement.
3.4. Capitalization. The authorized capital stock of the Company consists of Series A Preferred Stock, $0.001 par value per share, of which 12,856 shares are authorized, issued and outstanding as of December 31, 2003, and 150,000,000 shares of Common Stock, $0.001 par value per share, of which 21,541,476 shares are issued and outstanding as of March 31, 2004. All of the issued and outstanding shares of the Company's capital stock have been duly authorized and validly issued and are fully paid and non-assessable. The Company will have reserved, immediately prior to the First Closing, a total of 6,068,740 shares of its Common Stock for issuance pursuant to the Option Plans (defined below). Except as contemplated by this Agreement, the other Financing Documents, the Amended Articles, (i) no subscription, warrant, option, convertible security or other right (contingent or otherwise) to purchase or acquire any shares of capital stock of the Company is authorized or outstanding, (ii) there is not any commitment or offer of the Company to issue any subscription, warrant, option, convertible security or other such right or to issue or distribute to holders of any shares of its capital stock any evidences of indebtedness or assets of the Company, (iii) the Company has no obligation (contingent or otherwise) to purchase, redeem or otherwise acquire any shares of its capital stock or any interest therein or to pay any dividend or make any other distribution in respect thereof, and (iv) there are no restrictions on the transfer of the Company's capital stock other than those arising from securities laws or contemplated by this Agreement and the other Financing Documents. There are no voting trusts or agreements, stockholders' agreements, pledge agreements, buy-sell agreements, rights of first refusal, preemptive rights or proxies relating to any securities of the Company to which the Company is a party. All of the outstanding securities of the Company were issued in compliance with all applicable federal and state securities laws. Set forth in the Disclosure Schedule is a capitalization table for the Company showing (i) the capitalization of the Company on a fully diluted basis immediately prior to the issuance of the Shares; and (ii) the capitalization of the Company on a fully diluted basis giving effect to the issuance of the Shares. For the purposes of this Agreement, the term "on a fully diluted basis" means that for purposes of calculating the percentage interest of the Company's capital stock represented by a specified number of shares, there shall be deemed outstanding (i) all shares of Common Stock, $0.001 per value per share, of the Company currently outstanding; (ii) all shares of Common Stock issuable pursuant to the exercise of options granted and capable of being granted under the Company's 1998 Long Term Incentive Compensation Plan, 2000 Long Term Incentive Compensation Plan, and Senior Executive Long Term Incentive Compensation Plan (the "Option Plans"); and (iii) all shares of Common Stock issuable pursuant to other existing options, warrants, convertible debt securities and other instruments and agreements whatsoever. The issue and sale of the Securities will not, immediately or with the passage of time, obligate the Company to issue shares of Common Stock or other securities to any Person and will not result in a right of any holder of Company securities to adjust the exercise, conversion, exchange or reset price under such securities.
3.5. Subsidiaries. Except for Sentigen Ltd., the Company does not control, directly or indirectly, any other entity (a "Subsidiary") and does not own of record or beneficially, directly or indirectly, any shares of capital stock or securities convertible into capital stock of any other corporation, or any participating interest in any partnership, joint venture or other non-corporate business enterprise.
3.6. SEC Filings; Business. The Company has filed all reports required to be filed by it under the 1933 Act and the Securities Exchange Act of 1934, as amended (the "1934 Act"), including pursuant to Section 13(a) or 15(d) thereof, for the twelve months preceding the date hereof (or such shorter period as the Company was required by law to file such reports) (collectively, the "SEC Filings") on a timely basis or has timely filed a valid extension of such time of filing and has filed any such SEC Filings prior to the expiration of any such extension.. The SEC Filings are the only filings required of the Company pursuant to the 1934 Act for such period. At the time of filing thereof, the SEC Filings complied as to form in all material respects with the requirements of the 1934 Act and did
A-4
not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. During the preceding two years, each registration statement and any amendment thereto filed by the Company pursuant to the 1933 Act and the rules and regulations thereunder, as of the date such statement or amendment became effective, complied as to form in all material respects with the 1933 Act and did not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading; and each prospectus filed pursuant to Rule 424(b) under the 1933 Act, as of its issue date and as of the closing of any sale of securities pursuant thereto did not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. The Company and its Subsidiaries are engaged only in the business described in the SEC Filings and the SEC Filings contain a complete and accurate description in all material respects of the business of the Company and its Subsidiaries, taken as a whole, as required to be disclosed.
3.7. No Material Adverse Change. Since December 31, 2002, except as identified and described in the SEC Filings, there has not been:
(a) any change in the consolidated assets, liabilities, financial condition or operating results of the Company from that reflected in the financial statements included in the 2002 10-K, except for changes in the ordinary course of business which have not and could not reasonably be expected to have a Material Adverse Effect, individually or in the aggregate;
(b) any declaration or payment of any dividend, or any authorization or payment of any distribution, on any of the capital stock of the Company, or any redemption or repurchase of any securities of the Company;
(c) any material damage, destruction or loss, whether or not covered by insurance to any assets or properties of the Company;
(d) any waiver, not in the ordinary course of business, by the Company or any Subsidiary of a material right or of a material debt owed to it;
(e) any satisfaction or discharge of any lien, claim or encumbrance or payment of any obligation by the Company or a Subsidiary, except in the ordinary course of business and which is not material to the assets, properties, financial condition, operating results or business of the Company and its Subsidiaries taken as a whole (as such business is presently conducted and as it is proposed to be conducted);
(f) any change or amendment to the Company's Amended Articles or Bylaws, or material change to any material contract or arrangement by which the Company or any Subsidiary is bound or to which any of their respective assets or properties is subject;
(g) any material labor difficulties or labor union organizing activities with respect to employees of the Company or any Subsidiary;
(h) any transaction entered into by the Company or a Subsidiary other than in the ordinary course of business;
(i) any loss of the services of any key employee, or material change in the composition or duties of the senior management of the Company or any Subsidiary;
(j) any loss or threatened loss of any customer which has had or could reasonably be expected to have a Material Adverse Effect; or
A-5
(k) any other event or condition of any character that has had or could reasonably be expected to have a Material Adverse Effect.
3.8. Financial Statements. The financial statements included in each SEC Filing present fairly, in all material respects, the consolidated financial position of the Company as of the dates shown and its consolidated results of operations and cash flows for the periods shown, and such financial statements have been prepared in conformity with United States generally accepted accounting principles applied on a consistent basis (except as may be disclosed therein or in the notes thereto, and, in the case of quarterly financial statements, as permitted by Form 10-Q or Form 10-QSB, as applicable, under the 1934 Act). Except as set forth in the financial statements of the Company included in the SEC Filings filed prior to the date hereof, neither the Company nor any of its Subsidiaries has incurred any liabilities, contingent or otherwise, except those incurred in the ordinary course of business, consistent (as to amount and nature) with past practices since the date of such financial statements, none of which, individually or in the aggregate, have had or could reasonably be expected to have a Material Adverse Effect.
3.9. Contracts and Commitments. Except as identified and described in the SEC Filings, the Company (i) is not a party to any oral or written contract, obligation, instrument, corporate restriction or commitment which involves a potential obligation or liability in excess of $25,000 and which is otherwise material and not entered into in the ordinary course of business, (ii) does not have any oral or written employment contracts, financing agreements, licenses, distributor or sales representative agreements, agreements with officers, directors, employees or shareholders of the Company or persons or organizations related to or affiliated with any such persons, leases, agreements relating to product development; and (iii) does not have any pension, profit sharing, retirement or stock option plans other than the Option Plans and the Company's 401(k) Plan.
3.10. Proprietary Rights, Employee Restrictions.
(a) The Company owns or has the right to use all copyrights, copyright registrations and copyright applications, trademark registrations and applications for registration, patents and patent applications, trademarks, service marks, trade names, or other proprietary rights (collectively, "Intellectual Property Rights") as described in the SEC Filings used or useful in the Company's business as presently conducted or currently contemplated to be conducted and all licenses, assignments and releases of Intellectual Property Rights of others in material works embodied in the Company's products. All Intellectual Property Rights held by any employee, officer or consultant have been assigned to the Company. The Company has exclusive ownership of, or exclusive license to use, all Intellectual Property Rights. The Company has obtained any licenses, releases or assignments necessary to use all third parties' Intellectual Property Rights in works embodied in its products. All such licenses, releases and assignments are specifically identified in the Disclosure Schedule. To the Company's knowledge, neither the present nor currently contemplated business activities or products of the Company infringe any Intellectual Property Rights of others. The Company has not received any notice or other written claim from any person asserting that any of the Company's present or currently contemplated activities infringe or may infringe any Intellectual Property Rights of such person. The Company has the right to use, free and clear of known or asserted claims or rights of others, all trade secrets, customer lists, hardware designs, programming processes, software and other information required for or incident to its products or its business as presently conducted or contemplated to be conducted, except for claims which would not have a Material Adverse Effect. The Company has taken all reasonable measures to protect and preserve the security, confidentiality and value of its Intellectual Property Rights, including its trade secrets and other confidential information. The Company is not aware of any infringement by others of its copyrights or other Intellectual Proprietary Rights in any of its products, technology or services, or any violation of the confidentiality of any of its proprietary information. The activities of the Company's employees on behalf of the Company do not violate
A-6
any agreements or arrangements known to the Company which any such employees have with former employers or any other entity to whom such employees may have rendered consulting services. For the purposes of this Section 3.10, Intellectual Property Rights also includes any and all material licenses, databases, computer programs and other computer software user interfaces (other than those generally available to the public), know-how, trade secrets, customer lists, proprietary technology, processes and formulae, source code, object code, algorithms, architecture, structure, display screens, layouts, development tools, instructions, templates, marketing materials, inventions, trade dress, logos and designs and all documentation and media constituting, describing or relating to the foregoing.
(b) All key employees, including all consultants (contract or otherwise), of or to the Company have executed and delivered to and in favor of the Company an agreement regarding the protection of confidential and proprietary information and the assignment to the Company of all Intellectual Property Rights arising from the services performed for the Company by such Persons.
3.11. Litigation; Compliance with Law. There is no (i) action, suit, claim, proceeding or investigation pending or, to the best of the Company's knowledge, threatened against or affecting the Company, at law or in equity, or before or by any federal, state, municipal or other governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, (ii) arbitration or mediation proceeding relating to the Company, pending or, to the best of the Company's knowledge, threatened, or (iii) governmental inquiry pending or, to the best of the Company's knowledge, threatened against or affecting the Company (including without limitation any inquiry as to the qualification of the Company to hold or receive any license or permit). The Company has not received any opinion or memorandum or legal advice from legal counsel to the effect that it is exposed, from a legal standpoint, to any liability which may have a Material Adverse Effect. The Company is not in default with respect to any order, writ, injunction or decree known to or served upon the Company of any court or of any federal, state, municipal or other governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign. There is no action or suit by the Company, and to the best of the Company's knowledge, pending, threatened or contemplated against others. To the best of the Company's knowledge, the Company has complied and will continue to comply, in all material respects, with all laws, rules, regulations and orders applicable to its present and contemplated business, operations, properties, assets, products and services. The Company has all necessary permits, licenses and other authorizations required to conduct its business as conducted and as proposed to be conducted, except where the failure to obtain such permits, licenses or authorization would not have a Material Adverse Effect, and the Company has been and will continue to be operating its business pursuant to and in compliance with the terms of all such permits, licenses and other authorizations, except where the failure to so operate the business would not have a Material Adverse Effect. The Company has all franchises, permits, licenses and other rights and privileges necessary to permit it to own its property and to conduct its business as it is presently conducted or proposed to be conducted. The Company is not subject to any pending or threatened investigation, inquiry or proceeding by the SEC, any state securities commission or any other federal, state, municipal or other governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, or any securities exchange or securities market, with respect to any matter. The Company is in compliance with all effective requirements of the Sarbanes-Oxley Act of 2002, as amended, and the rules and regulations thereunder, that are applicable to it, except where such noncompliance could not have or reasonably be expected to result in a Material Adverse Effect.
3.12. Leasehold Interests. Each lease or agreement to which the Company is a party under which it is a lessee of any property, real or personal, is a valid and subsisting agreement, duly authorized and entered into, without any default of the Company thereunder and, to the best of the Company's knowledge, without any default thereunder of any other party thereto. No event has occurred and is continuing which, with due notice or lapse of time or both, would constitute a default
A-7
or event of default by the Company under any such lease or agreement or, to the best of the Company's knowledge, by any other party thereto. The Company's possession of such property has not been disturbed and, to the best of the Company's knowledge, no claim has been asserted against the Company adverse to its rights in such leasehold interests.
3.13. Insurance. The Company and each Subsidiary maintain in full force and effect insurance coverage that is customary for comparably situated companies for the business being conducted and properties owned or leased by the Company and each Subsidiary, and the Company reasonably believes such insurance coverage to be adequate against all liabilities, claims and risks against which it is customary for comparably situated companies to insure.
3.14. Loans and Advances. The Company does not have any outstanding loans or advances to any person and is not obligated to make any such loans or advances.
3.15. Assumptions, Guaranties, Etc. of Indebtedness of Other Persons. The Company has not assumed, guaranteed, endorsed or otherwise become directly or contingently liable on any indebtedness of any other person (including, without limitation, liability by way of agreement, contingent or otherwise, to purchase, to provide funds for payment, to supply funds to or otherwise invest in the debtor, or otherwise to assure the creditor against loss), except for guaranties by endorsement of negotiable instruments for deposit or collection in the ordinary course of business.
3.16. Governmental Approvals. Subject to the accuracy of the representations and warranties of the Purchasers set forth in Section 4, no registration or filing with, or consent or approval of or other action by, any federal, state or other governmental agency or instrumentality is or will be necessary for the valid execution, delivery and performance by the Company of any of the Financing Documents, the issuance, sale and delivery of the Shares other than filings pursuant to federal and state securities laws (all of which filings have been made by the Company, other than those which are required to be made after the Closing and which will be duly made on a timely basis) in connection with the sale of the Shares.
3.17. Tax Matters. Each of the Company and each Subsidiary has timely prepared and filed all tax returns required to have been filed by the Company or such Subsidiary with all appropriate governmental agencies and timely paid all taxes shown thereon or otherwise owed by it. The charges, accruals and reserves on the books of the Company in respect of taxes for all fiscal periods are adequate in all material respects, and there are no material unpaid assessments against the Company or any Subsidiary nor any basis for the assessment of any additional taxes, penalties or interest for any fiscal period or audits by any federal, state or local taxing authority except for any assessment which is not material to the Company and its Subsidiaries, taken as a whole. All taxes and other assessments and levies that the Company or any Subsidiary is required to withhold or to collect for payment have been duly withheld and collected and paid to the proper governmental entity or third party when due. There are no tax liens or claims pending or threatened against the Company or any Subsidiary or any of their respective assets or property. There are no outstanding tax sharing agreements or other such arrangements between the Company and any Subsidiary or other corporation or entity.
3.18. Title to Properties. The Company and each Subsidiary has good and marketable title to all real properties and all other properties and assets owned by it, in each case free and clear from any and all liens, security interests, encumbrances and defects, and the Company and each Subsidiary holds any leased real or personal property under valid and enforceable leases with no exceptions that would materially interfere with the use made or currently planned to be made thereof by them.
3.19. No Labor Disputes. No material labor dispute with the employees of the Company or any Subsidiary exists or is imminent.
3.20. Environmental Matters. Neither the Company nor any Subsidiary is in violation of any statute, rule, regulation, decision or order of any governmental agency or body or any court, domestic
A-8
or foreign, relating to the use, disposal or release of hazardous or toxic substances or relating to the protection or restoration of the environment or human exposure to hazardous or toxic substances (collectively, "Environmental Laws"), owns or operates any real property contaminated with any substance that is subject to any Environmental Laws, is liable for any off-site disposal or contamination pursuant to any Environmental Laws, and is subject to any claim relating to any Environmental Laws, which violation, contamination, liability or claim has had or could reasonably be expected to have a Material Adverse Effect, individually or in the aggregate; and there is no pending or threatened investigation that might lead to such a claim.
3.21. Compliance with Nasdaq Continued Listing Requirements. The Company is in compliance with applicable Nasdaq SmallCap Market continued listing requirements. There are no proceedings pending or threatened against the Company relating to the continued listing of the Company's Common Stock on the Nasdaq SmallCap Market and the Company has not received any notice of, nor is there any basis for, the delisting of the Common Stock from the Nasdaq SmallCap Market.
3.22. Brokers. The Company has no contract, arrangement or understanding with any broker, finder or similar agent with respect to the transactions contemplated by this Agreement. No person or entity will have, as a result of the transactions contemplated by this Agreement, any right, interest or claim against or upon the Company, any Subsidiary or a Purchaser for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of the Company.
3.23. Certain Registration Matters. Assuming the accuracy of the Purchaser' representations and warranties set forth in Section 4.1-4.5, no registration under the Securities Act is required for the offer and sale of the Securities by the Company to the Purchaser under the Financing Documents. The Company has not granted or agreed to grant to any Person any rights (including "piggy-back" registration rights) to have any securities of the Company registered with the SEC or any other governmental authority that have not been satisfied or deferred during the period between the date hereof and the date the Company's registration statement pursuant to the Registration Rights Agreement is declared effective. Other than the Registration Statement (as defined in the Registration Rights Agreement), prior to the date that a Registration Statement is first declared effective by the SEC, the Company may not file any registration statement (other than on Form S-8) with the SEC with respect to any securities of the Company.
3.24. Transactions With Affiliates. Except as identified and described in the SEC Filings, no director, officer, employee or stockholder of the Company, or member of the family of any such person, or any corporation, partnership, trust or other entity in which any such person, or any member of the family of any such person, has a substantial interest or is an officer, director, trustee, partner or holder of more than 5% of the outstanding capital stock thereof, is a party to any transaction with the Company, including any contract, agreement or other arrangement providing for the employment of, furnishing of services by, rental of real or personal property from or otherwise requiring payments to any such person or firm, other than employment-at-will arrangements in the ordinary course of business.
3.25. Questionable Payments. Neither the Company nor any of its Subsidiaries nor any of their respective current or former shareholders, directors, officers, employees, agents or other persons or entities acting on behalf of the Company or any Subsidiary, has on behalf of the Company or any Subsidiary or in connection with their respective businesses: (a) used any corporate funds for unlawful contributions, gifts, entertainment or other unlawful expenses relating to political activity; (b) made any direct or indirect unlawful payments to any governmental officials or employees from corporate funds; (c) established or maintained any unlawful or unrecorded fund of corporate monies or other assets; (d) made any false or fictitious entries on the books and records of the Company or any Subsidiary; or
A-9
(e) made any unlawful bribe, rebate, payoff, influence payment, kickback or other unlawful payment of any nature.
3.26. Information Supplied to Purchasers. Neither this Agreement, the Disclosure Schedule and Exhibits attached hereto, the Private Placement Memorandum of the Company, dated as of April 1, 2004 (the"Private Placement Memorandum"), nor any other Financing Documents furnished to the Purchasers by or on behalf of the Company contains any untrue statement of a material fact, and none of this Agreement, the Disclosure Schedule and Exhibits attached hereto, or such other Financing Documents omits to state a material fact necessary in order to make the statements contained herein or therein not misleading. None of the matters described on the Disclosure Schedules have had, or could reasonably be expected to have, a Material Adverse Effect, individually or in the aggregate.
3.27. Internal Accounting Controls. The Company and the Subsidiaries maintain a system of internal accounting controls sufficient to provide reasonable assurance that (i) transactions are executed in accordance with management's general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with generally accepted accounting principles and to maintain asset accountability, (iii) access to assets is permitted only in accordance with management's general or specific authorization, and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences. The Company has established disclosure controls and procedures (as defined in 1934 Act Rules 13a-14 and 15d-14) for the Company and designed such disclosure controls and procedures to ensure that material information relating to the Company, including its Subsidiaries, is made known to the certifying officers by others within those entities, particularly during the period in which the Company's Form 10-KSB or 10-QSB, as the case may be, is being prepared.
3.28. No Additional Agreements. The Company does not have any agreement or understanding with any Purchaser with respect to the transactions contemplated by the Financing Documents other than as specified in the Financing Documents.
3.29. Disclosure. The Company confirms that neither it nor any person acting on its behalf has provided any Purchaser or its respective agents or counsel with any information that the Company believes constitutes material, non-public information except insofar as the existence and terms of the proposed transactions hereunder may constitute such information. The Company understands and confirms that the Purchasers will rely on the foregoing representations and covenants in effecting transactions in securities of the Company.
4. Representations of the Purchasers. Each of the Purchasers represents and warrants to the Company as to itself and no other Purchaser as follows:
4.1. Validity. Assuming the due execution and delivery by the Company of this Agreement and the Financing Documents, this Agreement and the Financing Documents to which such Purchaser is a party constitute legal, valid and binding obligations of the Purchaser, enforceable against such Purchaser in accordance with their respective terms.
4.2. Investor Status. Such Purchaser is an "accredited investor" as such term is defined in Regulation D under the 1933 Act. . Such Investor is not a registered broker-dealer under Section 15 of the 1934 Act.
4.3. Investment. The Securities to be received by such Purchaser hereunder will be acquired for the Purchaser's own account, not as nominee or agent, and not with a view to the resale or distribution of any part thereof in violation of the 1933 Act, and the Purchaser has no intention of selling, granting any participation in, or otherwise distributing the same in violation of the 1933 Act. Such Purchaser is acquiring the Securities hereunder in the ordinary course of its business. Such Purchaser does not have any agreement or understanding, directly or indirectly, with any Person to distribute any of the Securities.
A-10
4.4. Investment Experience. Such Purchaser acknowledges that it can bear the economic risk and complete loss of its investment in the Securities and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment contemplated hereby.
4.5. Restricted Securities. Such Purchaser understands that the Securities are characterized as "restricted securities" under the U.S. federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that under such laws and applicable regulations such securities may be resold without registration under the 1933 Act only in certain limited circumstances.
4.6. Access to Management. Such Purchaser has had an opportunity to receive additional information related to the Company requested by it and to ask questions of and receive answers from the Company regarding the Company, its business and the terms and conditions of the offering of the Securities. Neither such inquiries nor any other investigation conducted by or on behalf of such Purchaser or its representatives or counsel shall modify, amend or affect such Purchaser's right to rely on the truth, accuracy and completeness of the Company's representations and warranties contained in the Financing Documents and upon the Private Placement Memorandum. Such Purchaser has reviewed the SEC Filings and acknowledges that, as of the date of execution of this Agreement, the Company has not filed its Annual Report on Form 10-KSB for the fiscal-year ended December 31, 2003.
4.7. No General Solicitation. Such Purchaser did not learn of the investment in the Securities as a result of any public advertising or general solicitation.
4.8. Limited Ownership. The purchase by such Purchaser of the Securities issuable to it at each Closing will not immediately upon such Closing result in such Purchaser (individually or together with other Persons with whom such Purchaser has identified, or will have identified, itself as part of a "group" in a public filing made with the Commission involving the Company's securities), acquiring, or obtaining the right to acquire, in excess of 19.999% of the Common Stock or the voting power of the Company on a post transaction basis that assumes that such Closing shall have occurred. Such Purchaser does not currently intend to, alone or together with others, make a public filing with the Commission to disclose that it has (or that it together with such other Persons have) acquired, or obtained the right to acquire, as a result of a Closing (when added to any other securities of the Company that it or they then own or have the right to acquire), in excess of 19.999% of the Common Stock or the voting power of the Company on a post transaction basis that assumes that such Closing shall have occurred.
4.9. Independent Investment Decision. Such Purchaser has independently evaluated the merits of its decision to purchase Securities pursuant to this Agreement, such decision has been independently made by such Purchaser and such Purchaser confirms that it has only relied on the advice of its own business and/or legal counsel (which such Purchaser acknowledges is not Bryan Cave LLP) and not on the advice of any other Purchaser's business or legal counsel nor upon any investment banker, placement agent, or their respective counsel in making such decision.
5. Pre-Closing Covenants; Conditions to the Obligations of the Purchasers.
5.1. Pre-Closing Covenants. The parties covenant and agree that they will perform and observe the following covenants and provisions until the Second Closing Date, as applicable.
5.1.1. Dealings with Affiliates. Except as contemplated herein, the Company will not enter into any transaction, including, without limitation, any loans, leases, extension of credit or royalty agreements with any employee, officer or director of the Company or holder of ten percent (10%) or more of any class of capital stock of the Company, or any member of their respective immediate families or any corporation or other entity directly or indirectly controlled by one or more of such employees, officers, directors or stockholders or members of their immediate families.
A-11
5.1.2. Distributions. Except as contemplated herein, the Company will not declare or pay any dividends, purchase, redeem, retire, or otherwise acquire for value any of its capital stock (or rights, options or warrants to purchase such shares) now or hereafter outstanding, return any capital to its stockholders as such, or make any distribution of assets to its stockholders as such, or pay into or set aside a sinking fund for such purpose (such transactions being hereinafter referred to as "Distributions"); provided, however, that nothing herein contained shall prevent the Company from:
(i) complying with any specific provision of the terms of this Agreement;
(ii) redeeming any stock of a deceased stockholder out of insurance held by the Company on that stockholder's life; or
(iii) repurchasing any stock of an officer, employee or consultant subject to a stock repurchase agreement under which the Company has the right or obligation to repurchase such shares in the event of termination of employment or of a consulting arrangement.
5.1.3. Extraordinary Corporate Transactions. Except as contemplated herein, the Company will not take any corporate action, enter into any agreement to take such action, or obligate itself to take any such action, if such action would:
(i) cause the Company to create, authorize or issue any class or series of capital stock with equity features or convertible into equity ranking senior to the Common Stock with respect to liquidation preferences, dilution protection, redemption rights, or payment of dividends, or otherwise having terms and conditions superior to the terms of the Common Stock, or that would impair or limit the Company's obligations under this Agreement;
(ii) enter into any transaction that expressly prohibits or limits the Company's right to perform its obligations under this Agreement;
(iii) cause or authorize any transaction, whether by consolidation or merger of the Company, which results in the holders of the Company's capital stock holding less than 50% of the voting securities of the resulting or surviving entity, or a sale of all or substantially all of the capital stock or assets of the Company, or any other form of business combination or acquisition in which the Company is the object of the acquisition and in which control of the voting securities or assets of the Corporation are transferred in any way;
(iv) create or incur any additional indebtedness (other than replacement of existing indebtedness for borrowed money) for money borrowed which is secured by assets of the Company or any subsidiary or otherwise mortgage or pledge, or create a security interest in all or substantially all of the property of the Company;
(v) change the principal business of the Company, enter new lines of business, or exit its current and proposed line of business;
(vi) make investments in, or loans to, any third parties other than for employee travel or relocation; or
(vii) make any single capital expenditure in excess of $50,000.
5.1.4. Proxy Statement. The Company shall (a) prepare and file with the SEC, promptly after the date hereof and in any event by the 10th business day following the Initial Closing, preliminary proxy materials with respect to a meeting of the Company's stockholders for the purpose of approving the issuance of the Securities to be issued at the Second Closing, as contemplated by this Agreement. Promptly after comments are received from the SEC with respect to the preliminary proxy materials and after the furnishing by the Company of all information required to be contained therein, the Company shall file with the SEC the definitive proxy statement and (b) acting through the Company's Board of Directors, (i) call a meeting of the holders of the Common Stock for the purpose of
A-12
approving the issuance of the Securities to be issued at the Second Closing as contemplated by this Agreement and (ii) include in the proxy statement the recommendation of its Board of Directors that holders of the Common Stock approve such issuance as contemplated by this Agreement.
5.1.5. Stockholders Agreement. The Company shall obtain on or before the date of the Initial Closing, a duly executed and delivered Voting Agreement (the "Voting Agreement") among the holders of at least 50% of the Common Stock to approve the issuance of the Securities at the Second Closing as contemplated by this Agreement. The parties agree and understand that Purchasers may not vote their respective Shares or the shares of Common Stock issuable upon exercise of their respective Warrants in any such vote.
5.2. Funding of Escrow Account for Second Closing. Concurrently with their respective execution and delivery of this Agreement, each Purchaser will deliver to the Escrow Agent for deposit into the escrow account (the "Escrow Account") established pursuant to the terms and conditions of the Escrow Agreement, the full amount of such Purchaser's investment amount as listed onSchedule I hereto for each of the Initial Closing and the Second Closing, by wire transfer of immediately available funds.
5.3. Conditions to the Obligations of the Purchasers. The obligations of the Purchasers under this Agreement are subject to the fulfillment, or the waiver by the Purchasers, of the conditions set forth in this Section 5.3 on or before each Closing Date. All such documents and actions shall be satisfactory in form and substance to such Purchaser and its counsel.
5.3.1. Accuracy of Representations and Warranties; Performance. Each representation and warranty of the Company contained in this Agreement shall be true and correct in all material respects on and as of each Closing Date, with the same effect as though such representation and warranty had been made on and as of that date. The Company shall have performed in all material respects all agreements and covenants required to be performed by it under the Financing Documents prior to such Closing Date.
5.3.2. Consents. The Company shall have obtained any and all consents, permits, approvals, registrations and waivers necessary or appropriate for consummation of the purchase and sale of the Securities to be offered and sold at such Closing. No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the transactions contemplated hereby or in the other Financing Documents.
5.3.3. Certificates and Documents. The Company shall have delivered to counsel to the Purchasers:
(a) the Amended Articles, certified by the Secretary of State of the State of Nevada;
(b) a certificate, as of the most recent practicable date, of the Secretary of State of the State of Nevada as to the Company's legal existence and corporate good standing;
(c) the Bylaws of the Company in effect on and as of the First Closing Date; and
5.3.4. Opinion. The Company shall have delivered to counsel to the Purchasers an original opinion from Grippo & Elden LLC, counsel to the Company, dated as of the Closing Date, addressed to the Purchasers, and in the form attached hereto asExhibit D.
5.3.5. SEC Approvals; Effective Registration; Blue Sky. Any SEC approval required to consummate the transactions contemplated by the Financing Documents (other than the effectiveness of registration statements) shall have been obtained.
A-13
5.3.6. Registration Rights Agreement. The Company shall have executed and delivered to the Purchasers the Registration Rights Agreement.
5.3.7. Warrant Agreements. The Company shall have executed and delivered to the Purchasers the Warrant Agreements.
5.3.8. Voting Agreement. The Company shall have delivered to the Purchasers a copy of the duly executed and delivered Voting Agreement.
5.3.9. Stockholder Approval. With respect to the Second Closing, on or before July 19, 2004 the Company's stockholders shall have approved the issuance of the Securities at the Second Closing, as contemplated by this Agreement and the conversion of the demand notes issued to three Company stockholders (Oxford Bioscience Partners IV L.P., Mi3 L.P. and MRNA Fund II L.P.) (the "Demand Notes") into Common Stock at $0.75 per share.
5.3.10. Nasdaq Listing. The Nasdaq Stock Market shall have waived application of the 15 day prior notice contained in NASD Marketplace Rule 4310(c)(17)(D) or such timeframe shall have expired without objection. Trading in the Common Stock shall not have been suspended by the SEC or the Nasdaq Stock Market (except for any suspensions of trading of not more than one trading day solely to permit dissemination of material information regarding the Company) at any time since the date of execution of this Agreement, and the Common Stock shall have been at all times since such date listed for trading on the Nasdaq Small Cap Market or Nasdaq National Market.
5.3.11. No Adverse Changes. Between the execution of this Agreement and such Closing, no event or series of events (other than stock price fluctuations) shall have occurred which reasonably would be expected to have or result in a material adverse effect on the results of operations, assets, prospects, business or condition (financial or otherwise) of the Company and the Subsidiaries, taken as a whole.
5.3.12. Minimum Subscriptions. The aggregate of all Purchaser investment amounts delivered to the Escrow Account in accordance with Section 5.2 shall not be less than $10,000,000 (provided, that such Purchaser shall have complied with Section 5.2).
5.3.13. Timing. The Initial Closing shall have occurred no later than April 19th, 2004. The Second Closing shall have occurred no later than July 19, 2004.
5.3.14. Conversion of Outstanding Notes. Between the date of execution of this Agreement and the Initial Closing Date, the holders of the Company's Revolving Convertible Senior Secured Promissory Note in the aggregate principal amount of $4,160,000 have converted all principal and interest due thereunder into Common Stock at $0.75 per share and shall have agreed in writing to convert all Demand Notes into Common Stock at $0.75 per share at or before the Second Closing and in any event, the Demand Notes shall automatically convert upon approval of the stockholders to issue 20% or more of the Company's stock.
6. Conditions to the Obligations of the Company. The obligations of the Company under this Agreement are subject to the fulfillment, or the waiver in writing by the Company, of the conditions set forth in this Section 6 on or before each Closing Date.
6.1. Accuracy of Representations and Warranties. The representations and warranties of the Purchasers contained in Section 4 shall be true and correct in all material respects on and as of each Closing Date, with the same effect as though such representations and warranties had been made on and as of that date.
6.2. Performance. The Purchasers shall have performed and complied with all agreements contained in this Agreement required to be performed and complied with by it prior to or at the Closing.
A-14
6.3. Stockholder Approval. With respect to the Second Closing, the Company's stockholders shall have approved the issuance of the Securities at the Second Closing, as contemplated by this Agreement.
6.4. Nasdaq Listing. The Nasdaq Stock Market shall have waived application of the 15 day prior notice contained in NASD Marketplace Rule 4310(c)(17)(D) or such timeframe shall have expired without objection.
6.5. Timing. The Initial Closing shall have occurred no later than April 19th, 2004. The Second Closing shall have occurred no later than July 19, 2004.
6.6. Minimum Subscriptions. The aggregate of all Purchaser investment amounts delivered to the Escrow Account in accordance with Section 5.2 shall not be less than $10,000,000.
7. Post Closing Covenants.
7.1. No Integration. Neither the Company nor any of its affiliates, nor any person or entity acting on its or their behalf will make any offers or sales of any Company security or solicit any offers to buy any security, under circumstances that would adversely affect reliance by the Company on Section 4(2) of the 1933 Act for the exemption from registration for the transactions contemplated hereby or would require registration of the Shares under the 1933 Act.
7.2. Non-Contravention. The Company will not take any action, enter into any agreement or make any commitment that would conflict or interfere with the obligations to the Purchasers under the Financing Documents.
7.3. Listing of Shares. The Company shall take such action as may be required to cause the Shares to be listed on the Nasdaq SmallCap Market no later than the date required under the rules and regulations thereof. Further, if the Company applies to have its Common Stock or other securities traded on any other principal stock exchange or market, it shall include the Shares in such application and will take such other action as is necessary to cause such Common Stock to be so listed. The Company will use reasonable best efforts to continue the listing and trading of its Common Stock on The Nasdaq SmallCap Market or on the Nasdaq National Market and, in accordance, therewith, will use its best efforts to comply in all respects with the Company's reporting, filing and other obligations under the bylaws or rules of such exchange, as applicable.
7.4. Non-Public Information. The Company covenants and agrees that neither it nor any other Person acting on its behalf will provide any Purchaser or its agents or counsel with any information that the Company believes constitutes material non-public information, unless prior thereto such Purchaser shall have executed a written agreement regarding the confidentiality and use of such information. The Company understands and confirms that each Purchaser shall be relying on the foregoing representations in effecting transactions in securities of the Company.
8. General.
8.1. Successors and Assigns. The provisions of this Agreement shall bind and inure to the benefit of the respective successors, assigns, heirs, executors, and administrators of the parties hereto, including but not limited to assigns of the Purchasers pursuant to Section 1.1 of this Agreement.
8.2. Survival of Representations and Warranties. All representations and warranties shall survive and remain in full force and effect after the Closing with respect to the Company.
8.3. Indemnification. The Company agrees to indemnify and hold harmless each Purchaser and its affiliates and their respective directors, officers, employees and agents from and against any and all losses, claims, damages, liabilities and expenses (including without limitation reasonable attorney fees and disbursements and other expenses incurred in connection with investigating, preparing or defending any action, claim or proceeding, pending or threatened and the costs of enforcement hereof) to which
A-15
such person or entity may become subject as a result of and will reimburse any such person or entity for all such amounts as they are incurred by such person or entity as a result of any breach of representation, warranty, covenant or agreement made by or to be performed on the part of the Company under the Financing Documents.
8.4. Conduct of Indemnification Proceedings. Promptly after receipt by any person or entity (the "Indemnified Person") of notice of any demand, claim or circumstances which would or might give rise to a claim or the commencement of any action, proceeding or investigation by a third party in respect of which indemnity may be sought pursuant to Section 8.3 hereof, such Indemnified Person shall promptly notify the Company in writing and the Company shall assume the defense thereof, including the employment of counsel reasonably satisfactory to such Indemnified Person, and shall assume the payment of all fees and expenses; provided, however, that the failure of any Indemnified Person so to notify the Company shall not relieve the Company of its obligations hereunder except to the extent that the Company is materially prejudiced by such failure to notify. In any such proceeding, any Indemnified Person shall have the right to retain its own counsel, but the fees and expenses of such counsel shall be at the expense of such Indemnified Person unless: (i) the Company and the Indemnified Person shall have mutually agreed to the retention of such counsel; or (ii) in the reasonable judgment of counsel to such Indemnified Person representation of both parties by the same counsel would be inappropriate due to actual or potential differing interests between them. The Company shall not be liable for any settlement of any proceeding effected without its written consent, which consent shall not be unreasonably withheld, but if settled with such consent, or if there be a final judgment for the plaintiff, the Company shall indemnify and hold harmless such Indemnified Person from and against any loss or liability (to the extent stated above) by reason of such settlement or judgment. Without the prior written consent of the Indemnified Person, which consent shall not be unreasonably withheld, the Company shall not effect any settlement of any pending or threatened proceeding in respect of which any Indemnified Person is or could have been a party and indemnity could have been sought hereunder by such Indemnified Party, unless such settlement includes an unconditional release of such Indemnified Person from all liability arising out of such proceeding.
8.5. Notices. All notices, requests, consents and other communications under this Agreement shall be in writing and shall be delivered by hand, by telecopier, by express overnight courier service or mailed by first class mail, postage prepaid, to the respective addresses set forth on the signature pages of this Agreement, as such addresses may be modified by notice given pursuant to this Section 8.5, with copies provided simultaneously to counsel as set forth on the signature pages of this Agreement. Notices provided in accordance with this Section 8.5 shall be deemed delivered upon personal delivery, receipt by telecopy or overnight mail, or 48 hours after deposit in the mail in accordance with the above.
8.6. Entire Agreement. This Agreement, together with the instruments and other documents hereby contemplated to be executed and delivered in connection herewith, contains the entire agreement and understanding of the parties hereto, and supersedes any prior agreements or understandings between or among them, with respect to the subject matter hereof. Except as expressly set forth in this Agreement, the Disclosure Schedule and the Exhibits attached hereto, the Private Placement Memorandum and the Financing Documents, the Company makes no representation or warranty, express or implied, with respect to the transactions contemplated by this Agreement or the other Financing Documents, the business of the Company, the Company, the Company's assets or its future prospects. No party is relying on any understandings, agreements or representations other than those expressly contained this Agreement, the Disclosure Schedule and the Exhibits attached hereto, the Private Placement Memorandum, the SEC Filings and the Financing Documents.
8.7. Amendments and Waivers. No provision of this Agreement may be waived or amended except in a written instrument signed by the Company and the Purchasers holding a majority of the Shares. No waiver of any default with respect to any provision, condition or requirement of this
A-16
Agreement shall be deemed to be a continuing waiver in the future or a waiver of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of either party to exercise any right hereunder in any manner impair the exercise of any such right. No consideration shall be offered or paid to any Investor to amend or consent to a waiver or modification of any provision of any Financing Document unless the same consideration is also offered to all Investors who then hold Shares.
8.8. Counterparts. This Agreement may be executed in several counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
8.9. Captions. The captions of the sections, subsections and paragraphs of this Agreement have been added for convenience only and shall not be deemed to be a part of this Agreement.
8.10. Severability. Each provision of this Agreement shall be interpreted in such manner as to validate and give effect thereto to the fullest lawful extent, but if any provision of this Agreement is determined by a court of competent jurisdiction to be invalid or unenforceable under applicable law, such provision shall be ineffective only to the extent so determined and such invalidity or unenforceability shall not affect the remainder of such provision or the remaining provisions of this Agreement.
8.11. Governing Law. This Agreement shall be governed by and interpreted and construed in accordance with the laws of the State of New York;provided, however, that matters relating to the authorization, issuance and enforceability of the terms of the Securities shall be governed and interpreted and construed in accordance with the Nevada General Corporation Law. The Company irrevocably submits to the exclusive jurisdiction of the state and federal courts located in the State of New York for the purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in connection with any such suit, action or proceeding may be served on each party hereto anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. The Company irrevocably consents to the jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. The Company irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such courts and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum. If either party shall commence an action to enforce any provisions of a Financing Document, then the prevailing party in such action shall be reimbursed by the other party for its reasonable attorneys' fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action.
8.12. Independent Nature of Purchasers. The obligations of each Purchaser under any Financing Document are several and not joint with the obligations of any other Purchaser, and no Purchaser shall be responsible in any way for the performance of the obligations of any other Purchaser under any Financing Document. The decision of each Purchaser to purchase Securities pursuant to the Financing Documents has been made by such Purchaser independently of any other Purchaser. Nothing contained herein or in any Financing Document, and no action taken by any Purchaser pursuant thereto, shall be deemed to constitute the Purchasers as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Purchasers are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by the Financing Documents. Each Purchaser acknowledges that no other Purchaser has acted as agent for such Purchaser in connection with making its investment hereunder and that no Purchaser will be acting as agent of such Purchaser in connection with monitoring its investment in the Securities or enforcing its rights under the Financing Documents. Each Purchaser shall be entitled to independently protect and enforce its rights, including without limitation the rights arising out of this Agreement or out of the other Financing Documents, and it shall not be necessary for any other Purchaser to be joined as an additional party in any proceeding for such purpose. The Company acknowledges that
A-17
each of the Purchasers has been provided with the same Financing Documents for the purpose of closing a transaction with multiple Purchasers and not because it was required or requested to do so by any Purchaser.
8.13. Securities Laws Disclosure; Publicity. By 9:00 a.m. (New York time) on the business day following the execution of this Agreement, and by 9:00 a.m. (New York time) on each Closing Date, the Company shall issue press releases disclosing the transactions contemplated hereby and each Closing. On the business day following the execution of this Agreement the Company will file a Current Report on Form 8-K disclosing the material terms of the Financing Documents (and attach as exhibits thereto the Financing Documents), and on each Closing Date the Company will file an additional Current Report on Form 8-K to disclose such Closing. In addition, the Company will make such other filings and notices in the manner and time required by the SEC and the Nasdaq Stock Market. Notwithstanding the foregoing, the Company shall not publicly disclose the name of any Purchaser, or include the name of any Purchaser in any filing with the SEC (other than the Registration Statements and any exhibits to filings made in respect of this transaction in accordance with periodic filing requirements under the 1934 Act) or any regulatory agency or the Nasdaq Stock Market, without the prior written consent of such Purchaser, except to the extent such disclosure is required by law or Nasdaq Stock Market regulations.
8.14. Remedies. In addition to being entitled to exercise all rights provided herein or granted by law, including recovery of damages, each of the Investors and the Company will be entitled to specific performance under the Financing Documents. The parties agree that monetary damages may not be adequate compensation for any loss incurred by reason of any breach of obligations described in the foregoing sentence and hereby agrees to waive in any action for specific performance of any such obligation the defense that a remedy at law would be adequate.
8.15. Payment Set Aside. To the extent that the Company makes a payment or payments to any Purchaser pursuant to any Financing Document or a Purchaser enforces or exercises its rights thereunder, and such payment or payments or the proceeds of such enforcement or exercise or any part thereof are subsequently invalidated, declared to be fraudulent or preferential, set aside, recovered from, disgorged by or are required to be refunded, repaid or otherwise restored to the Company, a trustee, receiver or any other person under any law (including, without limitation, any bankruptcy law, state or federal law, common law or equitable cause of action), then to the extent of any such restoration the obligation or part thereof originally intended to be satisfied shall be revived and continued in full force and effect as if such payment had not been made or such enforcement or setoff had not occurred.
8.16. Limitation of Liability. Notwithstanding anything herein to the contrary, the Company acknowledges and agrees that the liability of a Purchaser arising directly or indirectly, under any Financing Document of any and every nature whatsoever shall be satisfied solely out of the assets of such Purchaser, and that no trustee, officer, other investment vehicle or any other Affiliate of such Purchaser or any investor, shareholder or holder of shares of beneficial interest of such a Purchaser shall be personally liable for any liabilities of such Purchaser.
[Signature Pages Follow]
A-18
IN WITNESS WHEREOF, the parties have executed this Securities Purchase Agreement as of the day and year first above written.
| | | IMCOR Pharmaceutical Co.
|
|
|
By: |
|
|
| | | Taffy J. Williams, Ph.D.,President |
|
|
Notice Address: 6175 Lusk Boulevard
San Diego, CA 92121 |
A-19
IN WITNESS WHEREOF, the parties have executed this Securities Purchase Agreement as of the day and year first above written.
| | | Purchaser: |
|
|
By: |
|
|
| | | | | Name:
Title: |
|
|
Investment Amount: $ |
|
|
Address for Notice: |
A-20
Schedule I
Purchaser
Name
| | Investment
Amount
| | Shares to be Issued
at Initial Closing
| | Warrants to be
Issued at Initial
Closing
| | Shares to be Issued
at Second Closing
| | Warrants to be
Issued at Second
Closing
|
|---|
1
Exhibit B
WARRANT AGREEMENT
WARRANT AGREEMENT dated as of April 14, 2004 (this "Warrant Agreement"), between IMCOR Pharmaceutical Co., a Nevada corporation (the "Company"), and[Purchaser] (together with its successors and assigns, the "Holder").
WHEREAS, the Company and the Holder have entered into that certain Securities Purchase Agreement dated as of April 14, 2004 (the "Purchase Agreement") pursuant to which the Company agreed to sell and the Holder agreed to purchase warrants, as hereinafter described (the "Warrants"), to purchase shares of the Company's common stock, par value $0.001 per share (the "Common Stock");
WHEREAS, the Common Stock issuable upon the exercise of the Warrants is hereinafter referred to as the "Warrant Shares"; and
WHEREAS, each Warrant entitles the Holder to purchase one Warrant Share at the exercise price set forth herein.
NOW, THEREFORE, in consideration of the premises and the mutual agreements herein set forth and as set forth in the Purchase Agreement, the parties hereto agree as follows:
SECTION 1. Warrant Certificate. The certificate evidencing the Warrants (the "Warrant Certificate") to be delivered pursuant to this Agreement shall be in registered form only and shall be substantially in the form set forth in Annex A attached hereto.
SECTION 2. Execution of Warrant Certificates. Warrant Certificates shall be signed on behalf of the Company by its Chairman of the Board or its President or a Vice President and by its Secretary or an Assistant Secretary. Each such signature upon the Warrant Certificates may be in the form of a facsimile signature of the present or any future Chairman of the Board, President, Vice President, Secretary or Assistant Secretary and may be imprinted or otherwise reproduced on the Warrant Certificates and for that purpose the Company may adopt and use the facsimile signature of any person who shall have been Chairman of the Board, President, Vice President, Secretary or Assistant Secretary, notwithstanding the fact that at the time the Warrant Certificates shall be countersigned and delivered or disposed of he shall have ceased to hold such office.
Any Warrant Certificate may be signed on behalf of the Company by any person who, at the actual date of the execution of such Warrant Certificate, shall be a proper officer of the Company to sign such Warrant Certificate, although at the date of the execution of this Warrant Agreement any such person was not such officer.
SECTION 3. Registration. The Company shall number and register the Warrant Certificates in the name of the record holder in a register to be maintained by the Company as they are issued by the Company. The Company may deem and treat the registered holder(s) of the Warrant Certificates as the absolute owner(s) thereof (notwithstanding any notation of ownership or other writing thereon made by anyone), for all purposes, absent actual notice to the contrary.
SECTION 4. Registration of Transfers and Exchanges. The Company shall register the transfer of any outstanding Warrant Certificates upon the records to be maintained by it for that purpose, upon surrender thereof accompanied (if so required by it) by a written instrument or instruments of transfer duly executed by the registered holder or holders thereof or by the duly appointed legal representative thereof or by a duly authorized attorney. Upon any such registration of transfer, a new Warrant Certificate shall be issued to the transferee(s) and the surrendered Warrant Certificate shall be canceled by the Company. Canceled Warrant Certificates shall thereafter be disposed of in a manner satisfactory to the Company. The acceptance of the new Warrant Certificate by the transferee thereof
B-1
shall be deemed the acceptance by such transferee of all of the rights and obligations of a holder of a Warrant Certificate.
The Holder agrees that each certificate representing Warrant Shares will bear the following legend until the Warrant Shares are registered or freely tradeable under the Securities Act of 1933, as amended and the regulations promulgated thereunder:
NEITHER THESE SECURITIES NOR THE SECURITIES ISSUABLE UPON EXERCISE OF THESE SECURITIES HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE "SECURITIES ACT"), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY. THESE SECURITIES AND THE SECURITIES ISSUABLE UPON EXERCISE OF THESE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT SECURED BY SUCH SECURITIES.
Warrant Certificates may be exchanged at the option of the holder(s) thereof, when surrendered to the Company at its office for another Warrant Certificate or other Warrant Certificates of like tenor and representing in the aggregate a like number of Warrants. Warrant Certificates surrendered for exchange shall be canceled by the Company.
SECTION 5. Terms of Warrants, Exercise of Warrants. Subject to the terms of this Agreement and the Warrant Certificate set forth as Annex A, the Warrant holder shall have the right, commencing on the date hereof and until 5:00 p.m., Pacific time on April 19, 2009 (the "Exercise Period"), to receive from the Company the number of fully paid and nonassessable Warrant Shares which the Holder may at the time be entitled to receive on exercise of such Warrants and payment of the Exercise Price (defined herein) then in effect for such Warrant Shares in cash or by certified or official bank check payable to the order of the Company, or through exercise of the cashless exercise provisions hereof if available with respect to such exercise. Each Warrant not exercised prior to 5:00 p.m., Pacific time, on April 19, 2009 (the "Expiration Date") shall become void and all rights thereunder and all rights in respect thereof under this agreement shall cease as of such time.
A Warrant may be exercised upon surrender to the Company at its principal office of the certificate or certificates evidencing the Warrants to be exercised with the form of election to purchase duly filled in and signed, and upon payment to the Company of the exercise price (the "Exercise Price") which is set forth in the form of Warrant Certificate attached hereto as Annex A as adjusted as herein provided, for the number of Warrant Shares in respect of which such Warrants are then exercised. For purposes of this Agreement, an "Exercise Date" means the business day on which a Holder tenders by facsimile to the Company an Exercise Notice, together with the payment of the Exercise Price in respect of such exercise (whether or not actual Warrant Certificates have yet been received by the Company.
The Holder may pay the Exercise Price in one of the following manners:
(1) Cash Exercise. The Holder may deliver immediately available funds; or
(2) Cashless Exercise. If an Exercise Notice is delivered after the first to occur of (1) the date that a registration statement covering the resale of the Warrant Shares is required to be
B-2
declared effective in accordance with terms of the Registration Rights Agreement (as defined in the Purchase Agreement) or (2) the date that such registration statement is required to have been declared effective in accordance with the Registration Rights Agreement, and a registration statement permitting the Holder to resell the Warrant Shares subject to such exercise is not then effective or the prospectus forming a part thereof is not then available to the Holder for the resale of such Warrant Shares, then the Holder may notify the Company in a form of election to purchase of its election to utilize cashless exercise, in which event the Company shall issue to the Holder the number of Warrant Shares determined as follows:
| | | X = Y [(A-B)/A] |
where: |
|
|
|
|
X = the number of Warrant Shares to be issued to the Holder. |
|
|
Y = the number of Warrant Shares with respect to which this Warrant is being exercised. |
|
|
A = the average of the closing prices for the five trading days immediately prior to (but not including) the Exercise Date. |
|
|
B = the Exercise Price. |
For purposes of Rule 144 promulgated under the Securities Act, it is intended, understood and acknowledged that the Warrant Shares issued in a cashless exercise transaction shall be deemed to have been acquired by the Holder, and the holding period for the Warrant Shares shall be deemed to have commenced, on the date this Warrant was originally issued.
Upon such surrender of Warrants and payment of the Exercise Price the Company shall promptly issue and cause to be delivered with all reasonable dispatch to or upon the written order of the Holder in the name of the Holder, a certificate or certificates for the number of full Warrant Shares issuable upon the exercise of such Warrants together with cash as provided in SECTION 14;provided,however, that if any consolidation, merger or lease or sale of assets is proposed to be effected by the Company as described in subsection (m) of SECTION 13 hereof, or a tender offer or an exchange offer for shares of Common Stock of the Company shall be made, upon such surrender of Warrants and payment of the Exercise Price as aforesaid, the Company shall, as soon as possible, but in any event not later than two business days thereafter, issue and cause to be delivered the full number of Warrant Shares issuable upon the exercise of such Warrants in the manner described in this sentence together with cash as provided in SECTION 14. Such certificate or certificates shall be deemed to have been issued to the holder of record of such Warrant Shares as of the applicable Exercise Date.
If by the third business day after an Exercise Date the Company fails to deliver the required number of Warrant Shares in the manner set forth above, then the Holder will have the right to rescind such exercise.
If by the third business day after an Exercise Date the Company fails to deliver the required number of Warrant Shares in the manner required above, and if after such third business day and prior to the receipt of such Warrant Shares, the Holder purchases (in an open market transaction or otherwise) shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a "Buy-In"), then the Company shall (1) pay in cash to the Holder the amount by which (x) the Holder's total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (A) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue by (B) the closing bid price of the Common Stock at the time of the obligation giving rise to such purchase obligation and (2) at the option of the
B-3
Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honored or deliver to the Holder the number of shares of Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In.
The Company's obligations to issue and deliver Warrant Shares in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any Person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by the Holder or any other Person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other Person, and irrespective of any other circumstance which might otherwise limit such obligation of the Company to the Holder in connection with the issuance of Warrant Shares. Nothing herein shall limit a Holder's right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company's failure to timely deliver certificates representing shares of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
The Warrants shall be exercisable, at the election of the Holders, either in full or from time to time in part and, in the event that a certificate evidencing Warrants is exercised in respect of fewer than all of the Warrant Shares issuable on such exercise at any time prior to the date of expiration of the Warrants, a new certificate evidencing the remaining Warrant or Warrants will be issued pursuant to the provisions of this Section. All Warrant Certificates surrendered upon exercise of Warrants shall be canceled by the Company. Such canceled Warrant Certificates shall then be disposed of by the Company.
SECTION 6. Mandatory Exercise. Subject to the provisions of this Section 6, if at any time following the issuance of the Warrant Certificates: (i) the VWAP (as defined below) of the Common Stock for each of 20 consecutive trading days is greater than $3.50 (subject to adjustments due to stock splits, recapitalizations or similar events), (ii) the Warrant Shares are either registered for resale pursuant to an effective registration statement naming the Holders as selling stockholders thereunder (and the prospectus thereunder is available for use by the Holders as to all Warrant Shares) or freely transferable without volume restrictions pursuant to Rule 144(k) promulgated under the Securities Act, as determined by counsel to the Company pursuant to a written opinion letter addressed and in form and substance reasonably acceptable to the Holders and the transfer agent for the Common Stock, during the entire 20 trading day period referenced in (i) above through the expiration of the Call Date as set forth in the Company's notice pursuant to this Section (the "Call Condition Period"), and (iii) the Company shall have complied in all material respects with its obligations under this Warrant Agreement and the Financing Documents and the Common Stock shall at all times be listed or quoted on a the Nasdaq National Market or Nasdaq SmallCap Market, then, subject to the conditions set forth in this Section, the Company may, in its sole discretion, elect to require the exercise of all (but not less than all) of the then unexercised portion of the Warrants, on the date that is the 10th day after written notice thereof (a "Call Notice") is received by the Holders (the "Call Date") at the address last shown on the records of the Company for the Holders or given by Holders to the Company for the purpose of notice;provided, that the conditions to giving such notice must be in effect at all times during the Call Condition Period or any such Call Notice shall be null and void. The Company agrees that, if and to the extent Section 15 hereof would restrict the ability of a Holder to exercise its Warrants in the event of a delivery of a Call Notice, then notwithstanding anything to the contrary set forth in the Call Notice, the Call Notice shall be deemed automatically amended as to such Holder to apply only to such portion of its Warrants as may be exercised by such Holder by the Call Date in accordance with such Section. A Holder will promptly (and, in any event, prior to the Call Date) notify the Company in
B-4
writing following receipt of a Call Notice if Section 15 would restrict its exercise of Warrants, specifying therein the number of Warrant Shares so restricted. The Company covenants and agrees that it will honor all Exercise Notices tendered through 5:00 p.m. (Pacific time) on the Call Date. "VWAP" means on any particular trading day or for any particular period, the volume weighted average trading price per share of Common Stock on such date or for such period as reported by the Bloomberg L.P., by any successor performing similar functions.
Upon such surrender of Warrants and payment of the Exercise Price the Company shall promptly issue and cause to be delivered with all reasonable dispatch to or upon the written order of the Holder in the name of the Holder, a certificate or certificates for the number of full Warrant Shares issuable upon the exercise of such Warrants together with cash as provided in SECTION 14.
SECTION 7. Registration Rights. The Company shall register the Warrant Shares for resale under the Securities Act of 1933, as amended, pursuant to the terms and conditions of that certain Registration Rights Agreement dated even date herewith by and among the Company, the Holder and the other "Purchasers" named therein.
SECTION 8. Payment of Taxes. Issuance and delivery of certificates for shares of Common Stock upon exercise of a Warrant Certificate shall be made without charge to the Holder for any issue or transfer tax, withholding tax, transfer agent fee or other incidental tax or expense in respect of the issuance of such certificates, all of which such taxes and expenses shall be paid by the Company; provided, however, that the Company shall not be required to pay any tax or taxes which may be payable in respect of any transfer involved in the issue of any Warrant Certificates or any certificates for Warrant Shares in a name other than that of the registered holder of a Warrant Certificate surrendered upon the exercise of a Warrant, and the Company shall not be required to issue or deliver such Warrant Certificates unless or until the person or persons requesting the issuance thereof shall have paid to the Company the amount of such tax or shall have established to the satisfaction of the Company that such tax has been paid.
SECTION 9. Mutilated or Missing Warrant Certificates. In case any of the Warrant Certificates shall be mutilated, lost, stolen or destroyed, the Company may, in its discretion, issue in exchange and substitution for and upon cancellation of the mutilated Warrant Certificate, or in lieu of and substitution for the Warrant Certificate lost, stolen or destroyed, a new Warrant Certificate of like tenor and representing an equivalent number of Warrants, but only upon receipt of evidence satisfactory to the Company of such loss, theft or destruction of such Warrant Certificate and indemnity, if requested, also satisfactory to them. Applicants for such substitute Warrant Certificates shall also comply with such other reasonable regulations and pay such other reasonable charges as the Company may prescribe.
SECTION 10. Reservation of Warrant Shares. The Company covenants that it will at all times reserve and keep available, free from preemptive rights, out of the aggregate of its authorized but unissued Common Stock or its authorized and issued Common Stock held in its treasury, for the purpose of enabling it to satisfy any obligation to issue Warrant Shares upon exercise of Warrants, the maximum number of shares of Common Stock which may then be deliverable upon the exercise of all outstanding Warrants. The Company covenants that all Warrant Shares so issuable and deliverable shall, upon issuance and the payment of the applicable Exercise Price in accordance with the terms hereof, be duly and validly authorized, issued and fully paid and nonassessable.
The Company or, if appointed, the transfer agent for the Common Stock (the "Transfer Agent") and every subsequent transfer agent for any shares of the Company's capital stock issuable upon the exercise of any of the rights of purchase aforesaid will be irrevocably authorized and directed at all times to reserve such number of authorized shares as shall be required for such purpose.
Before taking any action which would cause an adjustment pursuant to SECTION 12 hereof to reduce the Exercise Price below the then par value (if any) of the Warrant Shares, the Company will
B-5
take any corporate action which may, in the opinion of its counsel (which may be counsel employed by the Company), be necessary in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares at the Exercise Price as so adjusted.
SECTION 11. Obtaining Stock Exchange Listings. The Company will from time to time take all action which may be necessary so that the Warrant Shares, immediately upon their issuance upon the exercise of Warrants, will be listed on the principal securities exchanges and markets within the United States of America, if any, on which other shares of Common Stock are then listed.
SECTION 12. Adjustment of Exercise Price and Number of Warrant Shares Issuable. The Exercise Price and the number of Warrant Shares issuable upon the exercise of each Warrant are subject to adjustment from time to time upon the occurrence of the events enumerated in this SECTION 12 but subject in all events to the Company receiving approval from its stockholders in accordance with Nasdaq rules to issue more than 20% of the its stock in connection with the transactions in the Purchase Agreement (including this Warrant Agreement). For purposes of this SECTION 12, "Common Stock" means shares now or hereafter authorized of any class of common stock of the Company and any other stock of the Company, however designated, that has the right (subject to any prior rights of any class or series of preferred stock) to participate in any distribution of the assets or earnings of the Company without limit as to per share amount.
(a) Adjustment for Change in Capital Stock.
If the Company:
(1) pays a dividend or makes a distribution on its Common Stock in shares of its Common Stock;
(2) subdivides its outstanding shares of Common Stock into a greater number of shares;
(3) combines its outstanding shares of Common Stock into a smaller number of shares;
(4) makes a distribution on its Common Stock in shares of its capital stock other than Common Stock; or
(5) issues by reclassification of its Common Stock any shares of its capital stock;
then the Exercise Price in effect immediately prior to such action shall be proportionately adjusted so that the holder of any Warrant thereafter exercised may receive the aggregate number and kind of shares of capital stock of the Company which he would have owned immediately following such action if such Warrant had been exercised immediately prior to such action.
The adjustment shall become effective immediately after the record date in the case of a dividend or distribution and immediately after the effective date in the case of a subdivision, combination or reclassification.
If after an adjustment a Holder upon exercise may receive shares of two or more classes of capital stock of the Company, the Company shall determine the allocation of the adjusted Exercise Price between the classes of capital stock. After such allocation, the exercise privilege and the Exercise Price of each class of capital stock shall thereafter be subject to adjustment on terms comparable to those applicable to Common Stock in this Section.
Such adjustment shall be made successively whenever any event listed above shall occur.
(b) Adjustment for Rights Issue.
If the Company distributes any rights, options or warrants to all holders of its Common Stock entitling them to purchase shares of Common Stock at a price per share less than the current market
B-6
price per share on that record date, the Exercise Price shall be adjusted in accordance with the formula:
| | | | O + N × P |
| | | E' = E × | M |
| | | | O + N |
where:
E' = the adjusted Exercise Price.
E = the current Exercise Price.
O = the number of shares of Common Stock outstanding on the record date.
N = the number of additional shares of Common Stock offered.
P = the offering price per share of the additional shares.
M = the current market price per share of Common Stock on the record date.
The adjustment shall be made successively whenever any such rights, options or warrants are issued and shall become effective immediately after the record date for the determination of stockholders entitled to receive the rights, options or warrants. If, at the end of the period during which such rights, options or warrants are exercisable, not all rights, options or warrants shall have been exercised, the Exercise Price shall be immediately readjusted to what it would have been if "N" in the above formula had been the number of shares actually issued.
This subsection (b) does not apply to any of the transactions described in subsection (a) of this Section.
(c) Adjustment for Other Distributions.
If the Company distributes to all holders of its Common Stock any of its assets or debt securities or any rights or warrants to purchase debt securities, assets or other securities of the Company, the Exercise Price shall be adjusted in accordance with the formula:
where:
E' = the adjusted Exercise Price.
E = the current Exercise Price.
M = the current market price per share of Common Stock on the record date mentioned below.
F = the fair market value on the record date of the assets, securities, rights or warrants applicable to one share of Common Stock. The Board of Directors shall determine the fair market value.
The adjustment shall be made successively whenever any such distribution is made and shall become effective immediately after the record date for the determination of stockholders entitled to receive the distribution.
This subsection (c) does not apply to cash dividends or cash distributions paid out of consolidated current or retained earnings as shown on the books of the Company prepared in accordance with generally accepted accounting principles. Also, this subsection does not apply to any transaction in subsection (a) or rights, options or warrants referred to in subsection (b) of this SECTION 12.
B-7
(d) Adjustment for Common Stock Issue.
If the Company issues shares of Common Stock for a consideration per share less than the Exercise Price per share on the date the Company fixes the offering price of such additional shares, the Exercise Price shall be adjusted in accordance with the formula:
where:
E' = the adjusted Exercise Price.
E = the then current Exercise Price.
O = the number of shares outstanding immediately prior to the issuance of such additional shares.
P = the aggregate consideration received for the issuance of such additional shares.
M = the current market price per share on the date of issuance of such additional shares.
A = the number of shares outstanding immediately after the issuance of such additional shares.
The adjustment shall be made successively whenever any such issuance is made, and shall become effective immediately after such issuance.
This subsection (d) does not apply to:
(1) any of the transactions described in subsections (a), (b) or (c) of this SECTION 12,
(2) the exercise of Warrants, or the conversion or exchange of other securities convertible or exchangeable for Common Stock,
(3) Common Stock issued to the Company's employees under bona fide employee benefit plans adopted by the Board of Directors and approved by the holders of Common Stock when required by law, if such Common Stock would otherwise be covered by this subsection (d) (but only to the extent that the aggregate number of shares excluded hereby are issued pursuant to new plans (or amendments to existing plans) authorized after the date of this Warrant Agreement),
(4) Common Stock upon the exercise of rights or warrants issued to the holders of Common Stock,
(5) Common Stock issued to shareholders of any person which merges into the Company in proportion to their stock holdings of such person immediately prior to such merger, upon such merger,
(6) Common Stock issued in a bona fide public offering pursuant to a firm commitment underwriting or
(7) Common Stock issued in a bona fide private placement (except to the extent that any discount from the current market price attributable to restrictions on transferability of the Common Stock, as determined in good faith by the Board of Directors and described in a Board resolution which shall be filed with the Trustee, shall exceed 25%).
(e) Adjustment for Convertible Securities Issue.
If the Company issues any securities convertible into or exchangeable for Common Stock (other than securities issued in transactions described in subsections (b) and (c) of this SECTION 12) for a consideration per share of Common Stock initially deliverable upon conversion or exchange of such
B-8
securities less than the current market price per share on the date of issuance of such securities, the Exercise Price shall be adjusted in accordance with this formula:
where:
E' = the adjusted Exercise Price.
E = the then current Exercise Price.
O = the number of shares outstanding immediately prior to the issuance of such securities.
P = the aggregate consideration received for the issuance of such securities.
M = the current market price per share on the date of issuance of such securities.
D = the maximum number of shares deliverable upon conversion or in exchange for such securities at the initial conversion or exchange rate.
The adjustment shall be made successively whenever any such issuance is made, and shall become effective immediately after such issuance.
If all of the Common Stock deliverable upon conversion or exchange of such securities have not been issued when such securities are no longer outstanding, then the Exercise Price shall promptly be readjusted to the Exercise Price which would then be in effect had the adjustment upon the issuance of such securities been made on the basis of the actual number of shares of Common Stock issued upon conversion or exchange of such securities.
This subsection (e) does not apply to:
(1) any of the transaction referred to in subsection (a), (b), (c) or (d) of this SECTION 12.
(2) convertible securities issued to shareholders of any person which merges into the Company, or with a subsidiary of the Company, in proportion to their stock holdings of such person immediately prior to such merger, upon such merger,
(3) convertible securities issued in a bona fide public offering pursuant to a firm commitment underwriting or
(4) convertible securities issued in a bona fide private placement (except to the extent that any discount from the current market price attributable to restrictions on transferability of Common Stock issuable upon conversion, as determined in good faith by the Board of Directors and described in a Board resolution, shall exceed 25% of the then current market price).
(f) Current Market Price.
In subsections (b), (c), (d) and (e) of this SECTION 12 the current market price per share of Common Stock on any date shall be the fair market value per Warrant Share which shall mean (i) if the Common Stock is in the over-the-counter market and not in The Nasdaq National Market nor on any national securities exchange, the average of the per share closing price on the 10 consecutive trading days immediately preceding the date in question, as reported by The Nasdaq Small Cap Market (or an equivalent generally accepted reporting service if quotations are not reported on The Nasdaq Small Cap Market), or (ii) if the Common Stock is traded in The Nasdaq National Market or on a national securities exchange, the average for the 10 consecutive trading days immediately preceding the date in question of the daily per share closing prices in The Nasdaq National Market or on the
B-9
principal stock exchange on which it is listed, as the case may be. For purposes of clause (i) above, if trading in the Common Stock is not reported by The Nasdaq Small Cap Market, the applicable closing price referred to in said clause shall be the last sale price as reported on the OTC Electronic Bulletin Board of the National Association of Securities Dealers, Inc. or, if not reported thereon, as reported in the "pink sheets" published by National Quotation Bureau, Incorporated, and, if such securities are not so reported, shall be the price of a share of Common Stock determined by the Company's Board of Directors in good faith. The closing price referred to in clauses (i) and (ii) above shall be the last reported sale price or, in case no such reported sale takes place on such day, the average of the reported closing bid and asked prices, in any case in The Nasdaq Small Cap Market (or the equivalent generally accepted reporting service if quotations are not reported on The Nasdaq Small Cap Market), The Nasdaq National Market or on the national securities exchange on which the Common Stock is then listed in accordance with SECTION 11 of this Agreement.
(g) Consideration Received.
For purposes of any computation respecting consideration received pursuant to subsections (d) and (e) of this SECTION 10, the following shall apply:
(1) in the case of the issuance of shares of Common Stock for cash, the consideration shall be the amount of such cash, provided that in no case shall any deduction be made for any commissions, discounts or other expenses incurred by the Company for any underwriting of the issue or otherwise in connection therewith;
(2) in the case of the issuance of shares of Common Stock for a consideration in whole or in part other than cash, the consideration other than cash shall be deemed to be the fair market value thereof as determined in good faith by the Board of Directors (irrespective of the accounting treatment thereof), whose determination shall be conclusive, and described in a Board resolution, a copy of which shall be mailed to each holder; and
(3) in the case of the issuance of securities convertible into or exchangeable for shares, the aggregate consideration received therefor shall be deemed to be the consideration received by the Company for the issuance of such securities plus the additional minimum consideration, if any, to be received by the Company upon the conversion or exchange thereof (the consideration in each case to be determined in the same manner as provided in clauses (1) and (2) of this subsection).
(h) When De Minimis Adjustment May Be Deferred.
No adjustment in the Exercise Price need be made unless the adjustment would require an increase or decrease of at least 1% in the Exercise Price. Any adjustments that are not made shall be carried forward and taken into account in any subsequent adjustment.
(i) All calculations under this Section shall be made to the nearest cent or to the nearest 1/100th of a share, as the case may be.
(j) When No Adjustment Required.
No adjustment need be made for a transaction referred to in subsections (b) or (c) of this SECTION 12 if Warrant holders are to participate in the transaction on a basis and with notice that the Board of Directors determines to be fair and appropriate in light of the basis and notice on which holders of Common Stock participate in the transaction.
No adjustment need be made for rights to purchase Common Stock pursuant to a Company plan for reinvestment of dividends or interest.
B-10
No adjustment need be made for a change in the par value or no par value of the Common Stock.
To the extent the Warrants become convertible into cash, no adjustment need be made thereafter as to the cash. Interest will not accrue on the cash.
(k) Notice of Adjustment.
Whenever the Exercise Price is adjusted, the Company shall provide the notices required by SECTION 14 hereof.
(l) Voluntary Reduction.
The Company from time to time may reduce the Exercise Price by any amount for any period of time if the period is at least 20 days and if the reduction is irrevocable during the period;provided,however, that in no event may the Exercise Price be less than the par value of a share of Common Stock and in no event may any such reduction apply to less than all of the Warrants then outstanding.
Whenever the Exercise Price is reduced, the Company shall mail to Holders a notice of the reduction. The Company shall mail the notice at least 15 days before the date the reduced Exercise Price takes effect. The notice shall state the reduced Exercise Price and the period it will be in effect.
A reduction of the Exercise Price does not change or adjust the Exercise Price otherwise in effect for purposes of this Section 12.
(m) Notice of Certain Transactions.
If the Company:
(1) takes any action that would require an adjustment in the Exercise Price pursuant to this SECTION 12 and if the Company does not arrange for Warrant holders to participate pursuant to subsection (i) of this SECTION 12;
(2) takes any action that would require a supplemental Warrant Agreement pursuant to subsection (m) of this SECTION 12; or
(3) liquidates or dissolves,
then the Company shall mail to Warrant holders a notice stating the proposed record date for a dividend or distribution or the proposed effective date of a subdivision, combination, reclassification, consolidation, merger, transfer, lease, liquidation or dissolution. The Company shall mail the notice at least 20 days before such date. Failure to mail the notice or any defect in it shall not affect the validity of the transaction.
(n) Reorganization of Company.
If the Company consolidates or merges with or into, or transfers or leases all or substantially all its assets to, any person, upon consummation of such transaction the Warrants shall automatically become exercisable for the kind and amount of securities, cash or other assets which the holder of a Warrant would have owned immediately after such transaction if the holder had exercised the Warrant immediately before the effective date of such transaction. Concurrently with the consummation of such transaction, the corporation formed by or surviving any such consolidation or merger if other than the Company, or the person to which such sale or conveyance shall have been made, shall enter into a supplemental Warrant Agreement so providing and further providing for adjustments which shall be as nearly equivalent as may be practical to the adjustments provided for in this Section. The successor Company shall mail to Warrant holders a notice describing the supplemental Warrant Agreement. In the event the surviving company to any such transaction does not have a class of securities registered for and trading on the New York Stock Exchange, American Stock Exchange, Nasdaq Stock Market or OTC Bulletin Board, then, at the option of each Holder, such Holder may require that the Company,
B-11
as a condition precedent to consummating any such transaction, redeem the remaining portion of such Holder's Warrant for an amount in cash equal to the Black Scholes value of such Warrant.
If the issuer of securities deliverable upon exercise of Warrants under the supplemental Warrant Agreement is an Affiliate of the formed, surviving, transferee or lessee corporation, that issuer shall join in the supplemental Warrant Agreement.
If this subsection (l) applies, subsections (a), (b), (c), (d) and (e) of this SECTION 12 do not apply.
(o) When Issuance or Payment May Be Deferred.
In any case in which this SECTION 12 shall require that an adjustment in the Exercise Price be made effective as of a record date for a specified event, the Company may elect to defer until the occurrence of such event (i) issuing to the holder of any Warrant exercised after such record date the Warrant Shares and other capital stock of the Company, if any, issuable upon such exercise over and above the Warrant Shares and other capital stock of the Company, if any, issuable upon such exercise on the basis of the Exercise Price and (ii) paying to such holder any amount in cash in lieu of a fractional share pursuant to SECTION 13;provided,however, that the Company shall deliver to such holder a due bill or other appropriate instrument evidencing such holder's right to receive such additional Warrant Shares, other capital stock and cash upon the occurrence of the event requiring such adjustment.
(p) Adjustment in Number of Shares.
Upon each adjustment of the Exercise Price pursuant to this SECTION 12, each Warrant outstanding prior to the making of the adjustment in the Exercise Price shall thereafter evidence the right to receive upon payment of the adjusted Exercise Price that number of shares of Common Stock (calculated to the nearest hundredth) obtained from the following formula:
where:
N' = the adjusted number of Warrant Shares issuable upon exercise of a Warrant by payment of the adjusted Exercise Price.
N = the number or Warrant Shares previously issuable upon exercise of a Warrant by payment of the Exercise Price prior to adjustment.
E' = the adjusted Exercise Price.
E = the Exercise Price prior to adjustment.
(q) Form of Warrants.
Irrespective of any adjustments in the Exercise Price or the number or kind of shares purchasable upon the exercise of the Warrants, Warrants theretofore or thereafter issued may continue to express the same price and number and kind of shares as are stated in the Warrants initially issuable pursuant to this Agreement.
(r) Any determination that the Company or its Board of Directors must make pursuant to this SECTION 12 is conclusive (absent manifest error) if made reasonably and in good faith.
SECTION 13. Fractional Interests. The Company shall not be required to issue fractional Warrant Shares on the exercise of Warrants. If more than one Warrant shall be presented for exercise in full at the same time by the same holder, the number of full Warrant Shares which shall be issuable
B-12
upon the exercise thereof shall be computed on the basis of the aggregate number of Warrant Shares purchasable on exercise of the Warrants so presented. If any fraction of a Warrant Share would, except for the provisions of this SECTION 13, be issuable on the exercise of any Warrants (or specified portion thereof), the Company shall pay an amount in cash equal to the Exercise Price on the day immediately preceding the date the Warrant is presented for exercise, multiplied by such fraction.
SECTION 14. Notices to Warrant Holders. Upon any adjustment of the Exercise Price pursuant to SECTION 12, the Company shall promptly thereafter (i) cause to be given to each of the registered holders of the Warrant Certificates at his address appearing on the Warrant register a certificate of a firm of independent public accountants of recognized standing selected by the Board of Directors of the Company (who may be the regular auditors of the Company) setting forth the Exercise Price after such adjustment and setting forth in reasonable detail the method of calculation and the facts upon which such calculations are based and setting forth the number of Warrant Shares (or portion thereof) issuable after such adjustment in the Exercise Price, upon exercise of a Warrant and payment of the adjusted Exercise Price, which certificate shall be conclusive evidence of the correctness of the matters set forth therein, by first-class mail, postage prepaid. Where appropriate, such notice may be given in advance and included as a part of the notice required to be mailed under the other provisions of this SECTION 14.
In case:
(a) the Company shall authorize the issuance to all holders of shares of Common Stock of rights, options or warrants to subscribe for or purchase shares of Common Stock or of any other subscription rights or warrants; or
(b) the Company shall authorize the distribution to all holders of shares of Common Stock of evidences of its indebtedness or assets (other than cash dividends or cash distributions payable out of consolidated earnings or earned surplus or dividends payable in shares of Common Stock or distributions referred to in subsection (a) of SECTION 12 hereof); or
(c) of any consolidation or merger to which the Company is a party and for which approval of any shareholders of the Company is required, or of the conveyance or transfer of the properties and assets of the Company substantially as an entirety, or of any reclassification or change of Common Stock issuable upon exercise of the Warrants (other than a change in par value, or from par value to no par value, or from no par value to par value, or as a result of a subdivision or combination), or a tender offer or exchange offer for shares of Common Stock; or
(d) of the voluntary or involuntary dissolution, liquidation or winding up of the Company; or
(e) the Company proposes to take any action (other than actions of the character described in Section 10(a)) which would require an adjustment of the Exercise Price pursuant to SECTION 12; then the Company shall cause to be given to each of the registered holders of the Warrant Certificates at his address appearing on the Warrant register, at least 20 days (or 10 days in any case specified in clauses (a) or (b) above) prior to the applicable record date hereinafter specified, or promptly in the case of events for which there is no record date, by first-class mail, postage prepaid, a written notice stating (i) the date as of which the holders of record of shares of Common Stock to be entitled to receive any such rights, options, warrants or distribution are to be determined, or (ii) the initial expiration date set forth in any tender offer or exchange offer for shares of Common Stock, or (iii) the date on which any such consolidation, merger, conveyance, transfer, dissolution, liquidation or winding up is expected to become effective or consummated, and the date as of which it is expected that holders of record of shares of Common Stock shall be entitled to exchange such shares for securities or other property, if any, deliverable upon such reclassification, consolidation, merger, conveyance, transfer, dissolution, liquidation or winding up. The failure to give the notice required by this SECTION 14 or any defect therein shall not affect
B-13
the legality or validity of any distribution, right, option, warrant, consolidation, merger, conveyance, transfer, dissolution, liquidation or winding up, or the vote upon any action.
Nothing contained in this Agreement or in any of the Warrant Certificates shall be construed as conferring upon the holders thereof the right to vote or to consent or to receive notice as shareholders in respect of the meetings of shareholders or the election of Directors of the Company or any other matter, or any rights whatsoever as shareholders of the Company.
SECTION 15. Limitations on Exercise. Notwithstanding anything to the contrary contained herein, the number of shares of Common Stock that may be acquired by the Holder upon any exercise of a Warrant Certificate (or otherwise in respect hereof) shall be limited to the extent necessary to insure that, following such exercise (or other issuance), the total number of shares of Common Stock then beneficially owned by such Holder and its Affiliates and any other Persons whose beneficial ownership of Common Stock would be aggregated with the Holder's for purposes of Section 13(d) of the Exchange Act, does not exceed 9.999% of the total number of issued and outstanding shares of Common Stock (including for such purpose the shares of Common Stock issuable upon such exercise). For such purposes, beneficial ownership shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. This provision shall not restrict the number of shares of Common Stock which a Holder may receive or beneficially own in order to determine the amount of securities or other consideration that such Holder may receive in the event of a merger or other business combination or reclassification involving the Company as contemplated in Section 12 of this Warrant. This restriction may not be waived, but will expire automatically on the 65th day prior to the Expiration Date.
SECTION 16. Notices to Company. Any notice or demand authorized by this Agreement to be given or made by the Company or by a Holder to or on the Company shall be sufficiently given or made when and if deposited in the mail, first class or registered, postage prepaid, addressed (until another address is filed in writing by the Company), or by facsimile, as follows:
IMCOR Pharmaceutical Co.
6175 Lusk Boulevard
San Diego, CA 92121
858-410-5602
Attention: Taffy J. Williams, Ph.D., President
With copy to (other than for Exercise Notices)
Theodore W. Grippo, Esq.
Grippo & Elden, LLC
227 West Monroe Street, Suite 3600
Chicago, IL 60606
312-558-1195
In case the Company shall fail to maintain such office or agency or shall fail to give such notice of the location or of any change in the location thereof, presentations may be made and notices and demands may be served at the principal office of the Transfer Agent.
SECTION 17. Supplements and Amendments. The Company and the Warrant holders may from time to time supplement or amend this Agreement with the approval of not less than the Holders of at least a majority of all then remaining Warrants.
SECTION 18. Successors. All the covenants and provisions of this Agreement by or for the benefit of the Company or the Holder shall bind and inure to the benefit of their respective successors and assigns hereunder.
B-14
SECTION 19. Termination. This Agreement shall terminate at 5:00 p.m., Pacific time on the Expiration Date, or such earlier date on which all Warrants have been exercised and the Company shall have complied with its obligations as to such exercises.
SECTION 20. Governing Law; Jurisdiction and Venue. This Agreement and each Warrant Certificate issued hereunder shall be deemed to be a contract made under the laws of the State of New York and for all purposes shall be construed in accordance with the internal laws of said State,provided, however, that if, as a result of the Company's incorporation in the State of Nevada the corporate laws of that State should govern a particular issue, the internal laws of the State of Nevada shall govern that issue.
SECTION 21. Transferability and Nonnegotiability of Warrant. The Warrants may not be transferred or assigned in whole or in part without compliance with all applicable federal and state securities laws by the transferor and the transferee. Subject to compliance with such laws, title to the Warrants may be transferred by endorsement (by the Holder executing an Assignment Form reasonably acceptable to the Company) and delivery in the same manner as a negotiable instrument transferable by endorsement and delivery.
SECTION 22. Exchange of Warrant Upon a Transfer. On surrender of the Warrant Certificate for exchange, properly endorsed on the Assignment Form and subject to the provisions of this Agreement with respect to compliance with applicable securities laws and with the limitations on assignments and transfers and contained in SECTION 21, the Company at its expense shall issue to or on the order of the Holder a new Warrant Certificate of like tenor, in the name of the Holder or as the Holder may direct, for the number of shares issuable upon exercise hereof.
SECTION 23. Compliance with Securities Laws. The Holder agrees that the Holder will not offer, sell or otherwise dispose of this Warrant or any shares of Common Stock to be issued upon exercise hereof except under circumstances that will not result in a violation of the federal or any state securities laws. Prior to any proposed transfer of this Warrant, the holder thereof shall give written notice to the Company of its intention to effect such transfer. Each such notice shall describe the manner of the proposed transfer and, if requested by the Company, shall be accompanied by an opinion of counsel reasonably satisfactory to the Company to the effect that the proposed transfer may be effected without registration under the Securities Act, whereupon the Holder shall be entitled to transfer this Warrant in accordance with the terms of its notice; provided, however, that no such opinion of counsel shall be required for a transfer to one or more partners of the transferor (in the case of a transferor that is a partnership) or to an affiliated corporation (in the case of a transferor that is a corporation). Each Warrant transferred as above provided shall bear the legend set forth at the beginning of the form Warrant Certificates annexed hereto as Annex A and B, as the case may be.
SECTION 24. Benefits of This Agreement. Nothing in this Agreement shall be construed to give to any person or corporation other than the Company, the Holder and the registered holders of the Warrant Certificates any legal or equitable right, remedy or claim under this Agreement; but this Agreement shall be for the sole and exclusive benefit of the Company, the Holder and the registered holders of the Warrant Certificates.
SECTION 25. Counterparts. This Agreement may be executed in any number of counterparts and each of such counterparts shall for all purposes be deemed to be an original, and all such counterparts shall together constitute but one and the same instrument.
Signature page follows.
B-15
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed, as of the day and year first above written.
| | | IMCOR Pharmaceutical Co. |
|
|
By: |
|
|
| | | | |
|
| | | | | Taffy J. Williams, Ph.D.,President |
|
|
By: |
|
|
| | | | |
|
| | | | | Brooks Boveroux,Secretary |
|
|
[PURCHASER] |
|
|
By: |
|
|
| | | | |
|
| | | Name: | | |
| | | Title: | | |
B-16
Annex A
[Form of Warrant Certificate]
NEITHER THESE SECURITIES NOR THE SECURITIES ISSUABLE UPON EXERCISE OF THESE SECURITIES HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE "SECURITIES ACT"), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY. THESE SECURITIES AND THE SECURITIES ISSUABLE UPON EXERCISE OF THESE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT SECURED BY SUCH SECURITIES.
EXERCISABLE ON OR BEFORE , 2009
Warrant Certificate
IMCOR PHARMACEUTICAL CO.
This Warrant Certificate certifies that[PURCHASER] or registered assigns ("Holder"), is the registered holder of Warrants expiring , 2009 (the "Warrants") to purchase Common Stock, par value $0.001 per share (the "Common Stock"), of IMCOR PHARMACEUTICAL CO., a Nevada corporation (the "Company"). Each Warrant entitles the Holder upon exercise to receive from the Company at any time from the date hereof and on or before 5:00 p.m. Pacific Time on , 2009 (the "Expiration Date"), one fully paid and nonassessable share of Common Stock (a "Warrant Share") at the initial exercise price (the "Exercise Price") of $1.00 per share payable in accordance with that certain Warrant Agreement dated as of , 2004 (the "Warrant Agreement") between the Company and the original Holder of this Warrant Certificate upon surrender of this Warrant Certificate and payment of the Exercise Price, in accordance with the terms and conditions set forth herein and in the Warrant Agreement. The Exercise Price and number of Warrant Shares issuable upon exercise of the Warrants are subject to adjustment upon the occurrence of certain events set forth in the Warrant Agreement.
No Warrant may be exercised after 5:00 p.m., Pacific Time on the Expiration Date, and to the extent not exercised by such time such Warrants shall become void.
This Warrant Certificate shall be governed and construed in accordance with the internal laws of the State of New York,provided, however, that if, as a result of the Company's incorporation in the State of Nevada the corporate laws of that State should govern a particular issue, the internal laws of the State of Nevada shall govern that issue.
The Warrants evidenced by this Warrant Certificate are part of a duly authorized issue of Warrants expiring on the Expiration Date entitling the Holder on exercise to receive shares of Common Stock, par value $0.001 per share, of the Company (the "Common Stock"), and are issued or to be issued pursuant to the Warrant Agreement, which Warrant Agreement is hereby incorporated by reference in and made a part of this instrument and is hereby referred to for a description of the rights, limitation
Annex A-1
of rights, obligations, duties and immunities thereunder of the Company, the Holder and the Holders. A copy of the Warrant Agreement may be obtained by the Holder upon written request to the Company.
Warrants may be exercised at any time on or before the Expiration Date. The Holder of Warrants evidenced by this Warrant Certificate may exercise them by surrendering this Warrant Certificate, with the form of election to purchase set forth hereon completed and executed, together with payment of the Exercise Price in accordance with the Warrant Agreement. In the event that upon any exercise of Warrants evidenced hereby the number of Warrants exercised shall be less than the total number of Warrants evidenced hereby, there shall be issued to the Holder or its assignee a new Warrant Certificate evidencing the number of Warrants not exercised.
The Warrant Agreement provides that upon the occurrence of certain events the Exercise Price set forth on the face hereof may, subject to certain conditions, be adjusted. If the Exercise Price is adjusted, the Warrant Agreement provides that the number of shares of Common Stock issuable upon the exercise of each Warrant shall be adjusted. No fractions of a share of Common Stock will be issued upon the exercise of any Warrant, but the Company will pay the cash value thereof determined as provided in the Warrant Agreement.
Warrant Certificates, when surrendered at the office of the Company by the registered holder thereof may be exchanged, in the manner and subject to the limitations provided in the Warrant Agreement, but without payment of any service charge, for another Warrant Certificate or Warrant Certificates of like tenor evidencing in the aggregate a like number of Warrants.
Upon due presentation for registration of transfer of this Warrant Certificate at the office of the Company a new Warrant Certificate or Warrant Certificates of like tenor and evidencing in the aggregate a like number of Warrants shall be issued to the transferee(s) in exchange for this Warrant Certificate, subject to the limitations provided in the Warrant Agreement, without charge except for any tax or other governmental charge imposed in connection therewith.
The Company may deem and treat the registered Holder as the absolute owner of this Warrant Certificate (notwithstanding any notation of ownership or other writing hereon made by anyone), for the purpose of any exercise hereof, of any distribution to the Holder hereunder, and for all other purposes, and the Company shall not be affected by any notice to the contrary other than as delivered by or on behalf of the Holder. Neither the Warrants nor this Warrant Certificate entitles the Holder hereof to any rights of a stockholder of the Company.
IN WITNESS WHEREOF, IMCOR Pharmaceutical Co. has caused this Warrant Certificate to be signed by its President and by its Secretary.
| Dated: , 2004 | | | | |
| | | IMCOR PHARMACEUTICAL CO. |
| | | By: | |
|
| | | Name: Taffy J. Williams, Ph.D. |
| | | Title: President |
|
|
By: |
|
|
| | | Name: Brooks Boveroux |
| | | Title: Secretary |
Annex A-2
[EXERCISE NOTICE]
The undersigned hereby elects to exercise the right, represented by this Warrant Certificate, to receive shares of Common Stock and herewith and tenders payment for such shares in cash, by certified or official bank check payable or through the use of the cashless exercise provisions of the Warrant Agreement relating to this Exercise Notice, in accordance with the Warrant Agreement.
The undersigned requests that a certificate for such shares be registered in the name of , whose address is and that such shares be delivered to whose address is . If said number of shares is less than all of the shares of Common Stock purchasable hereunder, the undersigned requests that a new Warrant Certificate representing the remaining balance of such shares be registered in the name of , whose address is and that such Warrant Certificate be delivered to , whose address is .
| Dated: | | | | |
| | | Signature: | | |
| | | | |
|
| | | (Signature must confirm in all respects to name of holder as specified on the face of the Warrant Certificate.)
|
Annex A-3
Exhibit C
REGISTRATION RIGHTS AGREEMENT
This Registration Rights Agreement (the "Agreement") is made and entered into as of this 14th day of April, 2004 by and among IMCOR Pharmaceutical Co., a Nevada corporation (the "Company"), and the "Purchasers" named in that certain Securities Purchase Agreement, of even date herewith, by and among the Company and the Purchasers (the "Purchase Agreement").
The parties hereby agree as follows:
1. Certain Definitions. Capitalized terms used and not otherwise defined herein that are defined in the Purchase Agreement will have the meanings given such terms in the Purchase Agreement. As used in this Agreement, the following terms shall have the following meanings:
"Affiliate" means, with respect to any person, any other person which directly or indirectly controls, is controlled by, or is under common control with, such person.
"Business Day" means a day, other than a Saturday or Sunday, on which banks in New York City are open for the general transaction of business.
"Closing Price" as of any date means (a) the closing bid price of one share of Common Stock as reported on The Nasdaq SmallCap Market ("Nasdaq") or other national securites exchange or OTC bulletin board on which the Company's shares may be quoted or listed on such date, (b) if no closing bid price is available, the average of the high bid and the low asked price quoted on Nasdaq on such date, or (c) if the shares of Common Stock are not then quoted on Nasdaq, the value of one share of Common Stock on such date as shall be determined in good faith by the Board of Directors of the Company and the Purchasers,provided, that if the Board of Directors of the Company and the Purchasers are unable to agree upon the value of a share of Common Stock pursuant to this subpart (c), the Company and the Purchasers shall jointly select an appraiser who is experienced in such matters to determine the Closing Price. The decision of such appraiser shall be final and conclusive, and the cost of such appraiser shall be borne one-half by the Company and one-half by the Purchasers.
"Common Stock" shall mean the Company's common stock, par value $0.001 per share.
"Holder" or "Holders" shall mean the holder or holders, as the case may be, from time to time of Registrable Securities.
"Prospectus" shall mean the prospectus included in any Registration Statement, as amended or supplemented by any prospectus supplement, with respect to the terms of the offering of any portion of the Registrable Securities covered by such Registration Statement and by all other amendments and supplements to the prospectus, including post-effective amendments and all material incorporated by reference in such prospectus.
"Purchasers" shall mean the Purchasers identified in the Purchase Agreement and any Affiliate or permitted transferee of any Purchaser who is a subsequent Holder.
"Register," "registered" and "registration" refer to a registration made by preparing and filing a Registration Statement or similar document in compliance with the 1933 Act, and the declaration or ordering of effectiveness of such Registration Statement or document.
"Registrable Securities" shall mean the shares of Common Stock issued or issuable (i) to the Purchasers pursuant to the Purchase Agreement, (ii) upon exercise of the Warrants issued or issuable to the Purchasers pursuant to the Purchase Agreement, and (iii) upon exercise of compensation warrants issued or issuable to the placement agents identified in the Disclosure
C-1
Schedule to the Purchase Agreement, and any other securities issued or issuable with respect to or in exchange for Registrable Securities;provided,that, a security shall cease to be a Registrable Security upon (A) sale pursuant to a Registration Statement or Rule 144 under the 1933 Act, or (B) such security becoming eligible for sale by the Holder pursuant to Rule 144(k).
"Registration Statement" shall mean any registration statement of the Company filed under the 1933 Act that covers the resale of the Registrable Securities pursuant to the provisions of this Agreement, amendments and supplements to such Registration Statement, including post-effective amendments, all exhibits and all material incorporated by reference in such Registration Statement.
"SEC" means the U.S. Securities and Exchange Commission.
"Warrants" means those warrants issued or issuable pursuant to the terms and conditions of those certain Warrant Agreements dated even date with the Purchase Agreement entered into pursuant to the Purchase Agreement.
"1933 Act" means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
"1934 Act" means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
2. Registration.
(a) Registration Statements. Not later than the first to occur of (i) the 30th day following the Second Closing and (ii) the 15th day following termination of the obligations to complete the Second Closing in accordance with the Purchase Agreement, the Company shall prepare and file with the SEC a Registration Statement on Form S-1 covering the resale of all Registrable Securities. The Registration Statement shall contain (except if otherwise required pursuant to written comments received from the SEC upon a review of such Registration Statement) the "Plan of Distribution" attached hereto asAnnex A. Such Registration Statement also shall cover, to the extent allowable under the 1933 Act and the rules promulgated thereunder (including Rule 416), such indeterminate number of additional shares of Common Stock resulting from stock splits, stock dividends or similar transactions with respect to the Registrable Securities. The "Selling Stockholders" and "Plan of Distribution" sections of the Registration Statement (and each amendment or supplement thereto, and each request for acceleration of effectiveness thereof) and any risk factor contained in such document that addresses specifically this transaction or the Selling Stockholders, shall be provided in accordance withSection 3(c) to the Holders prior to its filing or other submission. Promptly following any date on which the Company becomes eligible to use a registration statement on Form S-3 to register Registrable Securities for resale, but in no event more than 20 days after such date, the Company shall file a Registration Statement on Form S-3 covering the Registrable Securities (or a post-effective amendment on Form S-3 to the then effective Registration Statement) and shall cause such Registration Statement to be declared effective as soon as possible thereafter, but in any event by the 90th day following the date on which the Company becomes eligible to utilize Form S-3 for the registration of the resale of its securities by selling stockholders.
(b) Expenses. The Company will pay all expenses associated with each registration hereunder, including filing and printing fees, counsel and accounting fees and expenses, costs associated with clearing the Registrable Securities for sale under applicable state securities laws, listing fees and the Purchasers' reasonable expenses in connection with the registration, but excluding discounts, commissions, fees of Holder' counsel, and fees of underwriters, selling brokers, dealer managers or similar securities industry professionals with respect to the Registrable Securities being sold.
C-2
(c) Effectiveness.
(i) The Company shall use its reasonable best efforts to have the Registration Statement declared effective as soon as possible, but in any event by the "Effectiveness Date" defined below for such Registration Statement. "Effectiveness Date" for the initial Registration Statement required hereunder means the first to occur of (a) the 60th day following the Second Closing (or 90th day after the Second Closing if the filed Registration Statement is reviewed and commented upon by the SEC), (b) the 60th day following termination of the obligations to complete the Second Closing in accordance with the Purchase Agreement (or 90th day after such event if the filed Registration Statement is reviewed and commented upon by the SEC), and (c) the fifth Business Day following the date on which the Company is notified by the SEC that such Registration Statement will not be reviewed or is no longer subject to further review and comments. If a Registration Statement covering all Registrable Securities is not declared effective by the SEC by the Effectiveness Date, then the Company will, in addition to all other rights of the Holders hereunder and under applicable law, as partial liquidated damages and not as a penalty, make payments to each Purchaser in accordance with Section 2(e) hereof.
(ii) For not more than twenty-five (25) consecutive days or for a total of not more than forty (40) days in any twelve (12) month period, the Company may delay the disclosure of material non-public information concerning the Company, by suspending the use of any Prospectus included in any registration contemplated by this Section containing such information, the disclosure of which at the time would be, in the good faith opinion of the Company, materially detrimental to the Company (an "Allowed Delay");provided, that the Company shall promptly (a) notify the Holders in writing of the existence of (but in no event, without the prior written consent of a Purchaser, shall the Company disclose to such Purchaser any of the facts or circumstances regarding) material non-public information giving rise to an Allowed Delay, and (b) advise the Holders in writing to cease all sales under the Registration Statement until the end of the Allowed Delay. Liquidated damages set forth in Section 2(e) will be due and payable notwithstanding any Allowed Delays hereunder.
(d) Underwritten Offering. If any offering pursuant to a Registration Statement pursuant toSection 2(a) hereof involves an underwritten offering, the Company shall have the right to select an investment banker and manager to administer the offering, which investment banker or manager shall be reasonably satisfactory to the Holders holding a majority of the Registrable Securities.
(e) Liquidated Damages. If: (i) a Registration Statement is not timely filed in accordance with Section 2(a) hereof (if the Company files a Registration Statement without affording the Holders the opportunity to review and comment on the same as required by Section 3(c) hereof, the Company shall not be deemed to have satisfied this clause (i)), or (ii) a Registration Statement is not timely declared effective by the SEC in accordance with Section 2(c) hereof, or (iii) after the date upon which the Registration Statement is declared effective by the SEC, without regard for the reason thereunder or efforts therefore and without regard to any Allowed Delays, such Registration Statement ceases for any reason to be effective and available to the Holders as to all Registrable Securities to which it is required to cover at any time prior to the time when such Registrable Securities have ceased to be "Registrable Securities" in accordance with the definition of Registrable Securities for more than an aggregate of 20 Business Days (which need not be consecutive) (any such failure or breach being referred to as an"Event," and for purposes of clauses (i) or (ii) the date on which such Event occurs, or for purposes of clause (iii) the date which such 20 Business Day-period is exceeded, being referred to as"Event Date"), then, in addition to any other rights available to the Holders under this Agreement or under applicable law, on each such Event Date, and each monthly anniversary thereof, the Company shall issue to each Holder an amount in freely tradeable shares of validly issued, fully paid and non-assessable shares of Common Stock (which shall become Registrable Shares), as liquidated damages and not as a penalty, equal to 2% of the aggregate investment amount paid by such
C-3
Holder pursuant to the Purchase Agreement (valued at the Closing Price). The partial liquidated damages pursuant to this Section shall apply on a pro rata basis for any portion of a month prior to the cure of an Event, other with respect to the first Event Date (for which the entire partial liquidated damages for that month will be due in full on the initial Event Date).
3. Company Obligations. The Company will effect the registration of the Registrable Securities in accordance with the terms hereof, and pursuant thereto the Company will, as expeditiously as possible:
(a) use its reasonable best efforts to cause such Registration Statement to become effective and to remain continuously effective until such time as the Registrable Securities covered thereby are no longer "Registrable Securities" under the definition thereof;
(b) prepare and file with the SEC such amendments and post-effective amendments to the Registration Statement and the Prospectus as may be necessary to keep the Registration Statement effective for the period specified inSection 3(a) hereof and to comply with the provisions of the 1933 Act and the 1934 Act with respect to the distribution of all of the Registrable Securities covered thereby;
(c) provide copies to and permit each Holder to review the "Selling Stockholders" and "Plan of Distribution" sections of each Registration Statement and any risk factor that addresses specifically this transaction or the Selling Stockholders under the Registration Statement and all amendments and supplements thereto, no fewer than five (5) Business Days prior to their filing with the SEC and not, without the consent of such Holder, file any Registration Statement that contains disclosure regarding a Holder that differs in any material respect with the disclosure set forth in the Selling Holder Questionnaire (as hereinafter defined) provided by such Holder for use in such Registration Statement;
(d) furnish to the Holders (i) promptly after the same is prepared and publicly distributed, filed with the SEC, or received by the Company (but not later than two (2) Business Days after the filing date, receipt date or sending date, as the case may be) one (1) copy of any Registration Statement and any amendment thereto, each preliminary prospectus and Prospectus and each amendment or supplement thereto, and each letter written by or on behalf of the Company to the SEC or the staff of the SEC, and each item of correspondence from the SEC or the staff of the SEC, in each case relating to such Registration Statement (other than any portion of any thereof which contains information for which the Company has sought confidential treatment or believes might constitute material and non-public information concerning the Company), and (ii) such number of copies of a Prospectus, including a preliminary prospectus, and all amendments and supplements thereto and such other documents as each Holder may reasonably request in order to facilitate the disposition of the Registrable Securities owned by such Holder that are covered by the related Registration Statement;
(e) in the event of an underwritten offering hereunder wherein the Company selects an underwriter, the Company shall enter into and perform its reasonable obligations under an underwriting agreement, in usual and customary form, including, without limitation, customary indemnification and contribution obligations, with the underwriter of such offering;
(f) if required by the underwriter, or if any Holder is described in the Registration Statement as an underwriter, the Company shall furnish, on the effective date of the Registration Statement (except with respect to clause (i) below) and on the date that Registrable Securities are delivered to an underwriter, if any, for sale in connection with the Registration Statement (including any Holder deemed to be an underwriter), (i) (A) in the case of an underwritten offering, an opinion, dated as of the closing date of the sale of Registrable Securities to the underwriters, from legal counsel representing the Company for purposes of such Registration Statement, in form, scope and substance as is customarily given in an underwritten public offering, addressed to the underwriters
C-4
and the Holders participating in such underwritten offering or (B) in the case of an "at the market" offering, an opinion, dated as of or promptly after the effective date of the Registration Statement to the Holders, from legal counsel representing the Company for purposes of such Registration Statement, in form, scope and substance as is customarily given in a public offering, addressed to the Holders, and (ii) a letter, dated as of the effective date of such Registration Statement and confirmed as of the applicable dates described above, from the Company's independent certified public accountants in form and substance as is customarily given by independent certified public accountants to underwriters in an underwritten public offering, addressed to the underwriters (including any Holder deemed to be an underwriter);
(g) use its reasonable best efforts to (i) prevent the issuance of any stop order or other suspension of effectiveness and, (ii) if such order is issued, obtain the withdrawal of any such order at the earliest possible moment;
(h) prior to any public offering of Registrable Securities, use its reasonable best efforts to register or qualify or cooperate with the Holders and their counsel in connection with the registration or qualification of such Registrable Securities for offer and sale under the securities or blue sky laws of such jurisdictions requested by the Holders and do any and all other commercially reasonable acts or things necessary or advisable to enable the distribution in such jurisdictions of the Registrable Securities covered by the Registration Statement;
(i) use its reasonable best efforts to cause all Registrable Securities covered by a Registration Statement to be listed on each securities exchange, interdealer quotation system or other market on which similar securities issued by the Company are then listed;
(j) immediately notify the Holders, at any time when a Prospectus relating to Registrable Securities is required to be delivered under the 1933 Act, upon discovery that, or upon the happening of any event as a result of which, the Prospectus included in a Registration Statement, as then in effect, includes an untrue statement of a material fact or omits to state any material fact required to be stated therein or necessary to make the statements therein not misleading in light of the circumstances then existing, and at the request of any such Holder, promptly prepare and furnish to such Holder a reasonable number of copies of a supplement to or an amendment of such Prospectus as may be necessary so that, as thereafter delivered to the Holders of such Registrable Securities, such Prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein not misleading in light of the circumstances then existing; and
(k) otherwise comply with all applicable rules and regulations of the SEC under the 1933 Act and the 1934 Act, use its reasonable best efforts to take such other actions as may be reasonably necessary to facilitate the registration of the Registrable Securities hereunder; and make available to its security holders, as soon as reasonably practicable, but not later than the Availability Date (as defined below), an earnings statement covering a period of at least twelve (12) months, beginning after the effective date of each Registration Statement, which earnings statement shall satisfy the provisions of Section 11(a) of the 1933 Act (for the purpose of thissection 3(k), "Availability Date" means the 45th day following the end of the fourth fiscal quarter that includes the effective date of such Registration Statement, except that, if such fourth fiscal quarter is the last quarter of the Company's fiscal year, "Availability Date" means the 90th day after the end of such fourth fiscal quarter).
4. Obligations of the Holders.
(a) Each Holder agrees to furnish to the Company a completed Questionnaire in the form attached to this Agreement asAnnex B (a "Selling Holder Questionnaire"). The Company shall not be required to include the Registrable Securities of a Holder in a Registration Statement and shall
C-5
not be required to pay any partial liquidated or other damages under Section 2(e) hereof to a Holder who fails to furnish to the Company a completed Selling Holder Questionnaire at least two (2) Business Days prior to the date a Registration Statement is required to be filed in accordance with the requirements set forth in Section 2(a).
(b) Each Holder, by its acceptance of the Registrable Securities agrees to cooperate with the Company as reasonably requested by the Company in connection with the preparation and filing of a Registration Statement hereunder, unless such Holder has notified the Company in writing of its election to exclude all of its Registrable Securities from such Registration Statement.
(c) Each Holder covenants and agrees that it will comply with the prospectus delivery requirements of the 1933 Act as applicable to it in connection with sales of Registrable Securities pursuant to the Registration Statement.
(d) Each Holder agrees that, upon receipt of any notice from the Company of either (A) the commencement of an Allowed Delay pursuant toSection 2(c)(ii) or (B) the happening of an event pursuant toSection 3(j) hereof, such Holder will immediately discontinue disposition of Registrable Securities pursuant to the Registration Statement covering such Registrable Securities, until the Holder's receipt of the copies of the supplemented or amended prospectus filed with the SEC and declared effective and, if so directed by the Company, the Holder shall deliver to the Company (at the expense of the Company) or destroy (and deliver to the Company a certificate of destruction) all copies in the Holder's possession of the Prospectus covering the Registrable Securities current at the time of receipt of such notice.
(e) No Holder may participate in any third party underwritten registration hereunder unless it (i) agrees to sell the Registrable Securities on the basis provided in any underwriting arrangements in usual and customary form entered into by the Company, (ii) completes and executes all questionnaires, powers of attorney, indemnities, underwriting agreements and other documents reasonably required under the terms of such underwriting arrangements, and (iii) agrees to pay its pro rata share of all underwriting discounts and commissions. Notwithstanding the foregoing, no Holder shall be required to make any representations to such underwriter, other than those with respect to itself and the Registrable Securities owned by it, including its right to sell the Registrable Securities, and any indemnification in favor of the underwriter by the Holders shall be several and not joint and limited in the case of any Holder, to the proceeds received by such Holder from the sale of its Registrable Securities. The scope of any such indemnification in favor of an underwriter shall be limited to the same extent as the indemnity provided in Section 5(a) hereof.
5. Indemnification.
(a) Indemnification by the Company. The Company will indemnify and hold harmless each Holder and its officers, directors, members, employees and agents, successors and assigns, and each other person, if any, who controls such Holder within the meaning of the 1933 Act, against any losses, claims, damages or liabilities, joint or several, to which such Holder, officer, director, member, or controlling person may become subject under the 1933 Act or otherwise, insofar as such losses, claims, damages or liabilities (or actions in respect thereof) arise out of or are based upon: (i) any untrue statement or alleged untrue statement of any material fact contained in any Registration Statement, any preliminary prospectus or final prospectus contained therein, or any amendment or supplement thereof; (ii) any blue sky application or other document executed by the Company specifically for that purpose or based upon written information furnished by the Company filed in any state or other jurisdiction in order to qualify any or all of the Registrable Securities under the securities laws thereof (any such application, document or information herein called a"Blue Sky Application"); (iii) the omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein not misleading; (iv) any violation by the Company or its agents of any rule or
C-6
regulation promulgated under the 1933 Act applicable to the Company or its agents and relating to action or inaction required of the Company in connection with such registration; or (v) any failure to register or qualify the Registrable Securities included in any such Registration in any state where the Company or its agents has affirmatively undertaken or agreed in writing that the Company will undertake such registration or qualification on a Holder's behalf (the undertaking of any underwriter chosen by the Company being attributed to the Company) and will reimburse such Holder, and each such officer, director or member and each such controlling person for any legal or other expenses reasonably incurred by them in connection with investigating or defending any such loss, claim, damage, liability or action;provided,however, that the Company will not be liable in any such case if and to the extent that any such loss, claim, damage or liability arises out of or is based upon an untrue statement or alleged untrue statement or omission or alleged omission so made in conformity with information furnished by or on behalf of such Holder or any such controlling person in writing specifically for use in such Registration Statement or Prospectus.
(b) Indemnification by the Holders. Each Holder agrees, severally but not jointly, to indemnify and hold harmless, to the fullest extent permitted by law, the Company, its directors, officers, employees, stockholders, and successors and each person who controls the Company (within the meaning of the 1933 Act) against any losses, claims, damages, liabilities and expense (including reasonable attorney fees) resulting from any untrue statement of a material fact or any omission of a material fact required to be stated in the Registration Statement or Prospectus or preliminary prospectus or amendment or supplement thereto or necessary to make the statements therein not misleading, to the extent, but only to the extent that such untrue statement or omission is contained in any information furnished in writing by or on behalf of such Holder to the Company specifically for inclusion in such Registration Statement or Prospectus or amendment or supplement thereto. In no event shall the liability of a Holder be greater in amount than the dollar amount of the proceeds (net of all expense paid by such Holder and the amount of any damages such holder has otherwise been required to pay by reason of such untrue statement or omission) received by such Holder upon the sale of the Registrable Securities giving rise to such indemnification obligation.
(c) Conduct of Indemnification Proceedings. Any person entitled to indemnification hereunder shall (i) give prompt notice to the indemnifying party of any claim with respect to which it seeks indemnification and (ii) permit such indemnifying party to assume the defense of such claim with counsel reasonably satisfactory to the indemnified party;provided that any person entitled to indemnification hereunder shall have the right to employ separate counsel and to participate in the defense of such claim, but the fees and expenses of such separate counsel shall be at the expense of such person unless (a) the indemnifying party has agreed to pay such fees or expenses, or (b) the indemnifying party shall have failed to assume the defense of such claim and employ counsel reasonably satisfactory to such person or (c) in the reasonable judgment of any such person, based upon written advice of its counsel, a conflict of interest exists between such person and the indemnifying party with respect to such claims (in which case, if the person notifies the indemnifying party in writing that such person elects to employ separate counsel at the expense of the indemnifying party, the indemnifying party shall not have the right to assume the defense of such claim on behalf of such person); andprovided,further, that the failure of any indemnified party to give notice as provided herein shall not relieve the indemnifying party of its obligations hereunder, except to the extent that such failure to give notice shall materially adversely affect the indemnifying party in the defense of any such claim or litigation. It is understood that the indemnifying party shall not, in connection with any proceeding in the same jurisdiction, be liable for fees or expenses of more than one separate firm of attorneys at any time for all such indemnified parties. No indemnifying party will, except with the consent of the indemnified party, consent to entry of any judgment or enter into any settlement that does not include as an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a release from all liability in respect of such claim or litigation.
C-7
(d) Contribution. If for any reason the indemnification provided for in the preceding paragraphs (a) and (b) is unavailable to an indemnified party or insufficient to hold it harmless, other than as expressly specified therein, then the indemnifying party shall contribute to the amount paid or payable by the indemnified party as a result of such loss, claim, damage or liability in such proportion as is appropriate to reflect the relative fault of the indemnified party and the indemnifying party, as well as any other relevant equitable considerations. No person guilty of fraudulent misrepresentation within the meaning of Section 11(f) of the 1933 Act shall be entitled to contribution from any person not guilty of such fraudulent misrepresentation. In no event shall the contribution obligation of a holder of Registrable Securities be greater in amount than the dollar amount of the proceeds (net of all expenses paid by such holder and the amount of any damages such holder has otherwise been required to pay by reason of such untrue or alleged untrue statement or omission or alleged omission) received by it upon the sale of the Registrable Securities giving rise to such contribution obligation.
6. Miscellaneous.
(a) Remedies. In the event of a breach by the Company or by a Holder of any of their obligations under this Agreement, each Holder or the Company, as the case may be, in addition to being entitled to exercise all rights granted by law and under this Agreement, including recovery of damages, will be entitled to specific performance of its rights under this Agreement. The Company and each Holder agree that monetary damages may not provide adequate compensation for any losses incurred by reason of a breach by it of any of the provisions of this Agreement and hereby further agrees that, in the event of any action for specific performance in respect of such breach, it shall waive the defense that a remedy at law would be adequate.
(b) No Piggyback on Registrations. Except as and to the extent specified in the Disclosure Schedule to the Purchase Agreement, neither the Company nor any of its security holders (other than the Holders in such capacity pursuant hereto) may include securities of the Company in a Registration Statement hereunder other than the Registrable Securities, and the Company shall not after the date hereof enter into any agreement providing any such right to any of its security holders.
(c) Piggy-Back Registrations. If at any time while a Registration Statement is required to be effective hereunder there is not an effective Registration Statement covering all of the Registrable Securities and the Company shall determine to prepare and file with the SEC a registration statement relating to an offering for its own account or the account of others under the 1933 Act of any of its equity securities, other than with respect to the Company's registration to Dr. Gerald Wolf as described in the Disclosure Schedule or any registration statement on Form S-4 or Form S-8 (each as promulgated under the 1933 Act) or their then equivalents relating to equity securities to be issued solely in connection with any acquisition of any entity or business or equity securities issuable in connection with stock option or other employee benefit plans, then the Company shall send to each Holder written notice of such determination and, if within fifteen days after receipt of such notice, any such Holder shall so request in writing, the Company shall include in such registration statement all or any part of such Registrable Securities such holder requests to be registered, subject to customary underwriter cutbacks applicable to all holders of registration rights.
(e) Amendments and Waivers. The provisions of this Agreement, including the provisions of this section, may not be amended, modified or supplemented, and waivers or consents to departures from the provisions hereof may not be given, unless the same shall be in writing and signed by the Company and the Holder or Holders (as applicable) of no less than a majority in interest of the then outstanding Registrable Securities. Notwithstanding the foregoing, a waiver or consent to depart from the provisions hereof with respect to a matter that relates exclusively to the rights of certain Holders and that does not directly or indirectly affect the rights of other Holders may be given by Holders of at least a majority of the Registrable Securities to which such waiver or consent relates.
C-8
(f) Notices. All notices and other communications provided for or permitted hereunder shall be made as set forth in the Purchase Agreement or, with respect to a Holder, to such other address as provided in writing by a Holder to the Company.
(g) Assignments and Transfers by Holders. The provisions of this Agreement shall be binding upon and inure to the benefit of the Holders and their respective successors and assigns. A Holder may transfer or assign, in whole or from time to time in part, to one or more persons its rights hereunder in connection with the transfer of Registrable Securities by such Holder to such person, provided that such Holder complies with all laws applicable thereto and provides written notice of assignment to the Company promptly after such assignment is effected.
(h) Assignments and Transfers by the Company. This Agreement may not be assigned by the Company (whether by operation of law or otherwise),provided,however, that the Company must assign its rights and delegate its duties hereunder to any surviving or successor corporation in connection with a merger or consolidation of the Company with another corporation, or a sale, transfer or other disposition of all or substantially all of the Company's assets to another corporation.
(i) Benefits of the Agreement. The terms and conditions of this Agreement shall inure to the benefit of and be binding upon the respective permitted successors and assigns of the parties. Other than with respect to indemnified parties hereunder who are not parties to this Agreement, nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective successors and assigns any rights, remedies, obligations, or liabilities under or by reason of this Agreement, except as expressly provided in this Agreement.
(j) Counterparts; Faxes. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. This Agreement may also be executed via facsimile, which shall be deemed an original.
(k) Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing or interpreting this Agreement.
(l) Severability. Any provision of this Agreement that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof but shall be interpreted as if it were written so as to be enforceable to the maximum extent permitted by applicable law, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. To the extent permitted by applicable law, the parties hereby waive any provision of law which renders any provisions hereof prohibited or unenforceable in any respect.
(m) Further Assurances. The parties hereto shall execute and deliver all such further instruments and documents and take all such other actions as may reasonably be required to carry out the transactions contemplated hereby and to evidence the fulfillment of the agreements herein contained.
(n) Entire Agreement. This Agreement and the Purchase Agreement (and the Exhibits and Schedules annexed thereto) are intended by the parties hereto as a final expression of their agreement and intended to be a complete and exclusive statement of the agreement and understanding of the parties hereto in respect of the subject matter contained herein and therein. This Agreement and the Purchase Agreement (and the Exhibits and Schedules annexed thereto) supersede all prior agreements and understandings between the parties with respect to such subject matter.
(o) Governing Law; Consent to Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement (whether brought against a party hereto or
C-9
its respective affiliates, employees or agents) may be commenced non-exclusively in the state and federal courts sitting in the City of New York, Borough of Manhattan, (the "New York Courts"). Each party hereto hereby irrevocably submits to the non-exclusive jurisdiction of the New York Courts for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any proceeding, any claim that it is not personally subject to the jurisdiction of any New York Court, or that such proceeding has been commenced in an improper or inconvenient forum. Each party hereto hereby irrevocably waives personal service of process and consents to process being served in any such proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. Each party hereto hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any proceeding arising out of or relating to this Agreement or the transactions contemplated hereby. If either party shall commence a proceeding to enforce any provisions of this Agreement, then the prevailing party in such proceeding shall be reimbursed by the other party for its attorney's reasonable fees and other costs and expenses incurred with the investigation, preparation and prosecution of such proceeding.
(p) Cumulative Remedies. The remedies provided herein are cumulative and not exclusive of any remedies provided by law.
(q) Independent Nature of Holders Obligations and Rights. The obligations of each Holder hereunder is several and not joint with the obligations of any other Holder hereunder, and no Holder shall be responsible in any way for the performance of the obligations of any other Holder hereunder. Nothing contained herein or in any other agreement or document delivered at any closing, and no action taken by any Holder pursuant hereto or thereto, shall be deemed to constitute the Holder as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Holder are in any way acting in concert with respect to such obligations or the transactions contemplated by this Agreement. Each Holder shall be entitled to protect and enforce its rights, including without limitation the rights arising out of this Agreement, and it shall not be necessary for any other Holder to be joined as an additional party in any proceeding for such purpose. The Company acknowledges that each of the Purchasers has been provided with the same Agreement for the purpose of closing a transaction with multiple Purchasers and not because it was required or requested to do so by any Purchaser.
Signature pages follow.
C-10
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized officers to execute this Agreement as of the date first above written.
| | | Company: |
|
|
IMCOR Pharmaceutical Co. |
|
|
By: |
|
| | | Name: | Taffy J. Williams, Ph.D. |
| | | Title: | President and Chief Executive Officer |
C-11
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized officers to execute this Agreement as of the date first above written.
| | | PURCHASER: |
|
|
By: |
|
| | | Name: | |
| | | Title: | |
C-12
Annex A
Plan of Distribution
The Selling Stockholders and any of their pledgees, donees, transferees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of Common Stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholders may use any one or more of the following methods when selling shares:
- •
- ordinary brokerage transactions and transactions in which the broker-dealer solicits Investors;
- •
- block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
- •
- purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
- •
- an exchange distribution in accordance with the rules of the applicable exchange;
- •
- privately negotiated transactions;
- •
- to cover short sales made after the date that this Registration Statement is declared effective by the Commission;
- •
- broker-dealers may agree with the Selling Stockholders to sell a specified number of such shares at a stipulated price per share;
- •
- a combination of any such methods of sale; and
- •
- any other method permitted pursuant to applicable law.
The Selling Stockholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The Selling Stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
The Selling Stockholders may from time to time pledge or grant a security interest in some or all of the Shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell shares of Common Stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act of 1933 amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
Upon the Company being notified in writing by a Selling Stockholder that any material arrangement has been entered into with a broker-dealer for the sale of Common Stock through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to Rule 424(b) under the Securities Act, disclosing (i) the name of each such Selling Stockholder and of the participating broker-dealer(s), (ii) the number of shares involved, (iii) the price at which such the shares of Common Stock were sold, (iv) the commissions paid or discounts or concessions allowed to such broker-dealer(s), where applicable, (v) that such broker-dealer(s) did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus, and (vi) other facts material to the transaction. In addition, upon the Company being notified in writing by a Selling Stockholder that a
Annex A-1
donee or pledge intends to sell more than 500 shares of Common Stock, a supplement to this prospectus will be filed if then required in accordance with applicable securities law.
The Selling Stockholders also may transfer the shares of Common Stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The Selling Stockholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be "underwriters" within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Discounts, concessions, commissions and similar selling expenses, if any, that can be attributed to the sale of Securities will be paid by the Selling Stockholder and/or the purchasers. Each Selling Stockholder has represented and warranted to the Company that it acquired the securities subject to this registration statement in the ordinary course of such Selling Stockholder's business and, at the time of its purchase of such securities such Selling Stockholder had no agreements or understandings, directly or indirectly, with any person to distribute any such securities.
The Company has advised each Selling Stockholder that it may not use shares registered on this Registration Statement to cover short sales of Common Stock made prior to the date on which this Registration Statement shall have been declared effective by the Commission. If a Selling Stockholder uses this prospectus for any sale of the Common Stock, it will be subject to the prospectus delivery requirements of the Securities Act. The Selling Stockholders will be responsible to comply with the applicable provisions of the Securities Act and Exchange Act, and the rules and regulations thereunder promulgated, including, without limitation, Regulation M, as applicable to such Selling Stockholders in connection with resales of their respective shares under this Registration Statement.
The Company is required to pay all fees and expenses incident to the registration of the shares, but the Company will not receive any proceeds from the sale of the Common Stock. The Company has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act. If the Selling Stockholders use this prospectus for any sale of the Common Stock, they will be subject to the prospectus delivery requirements of the Securities Act.
Annex A-2
Annex B
Selling Securityholder Notice and Questionnaire
The undersigned beneficial owner of common stock (the"Common Stock"), of IMCOR Pharmaceutical Co. (the"Company") understands that the Company has filed or intends to file with the Securities and Exchange Commission (the"SEC") a Registration Statement for the registration and resale of the Registrable Securities, in accordance with the terms of the Registration Rights Agreement, dated as of April , 2004 (the"Registration Rights Agreement"), among the Company and the Purchasers named therein. A copy of the Registration Rights Agreement is available from the Company upon request at the address set forth below. All capitalized terms used and not otherwise defined herein shall have the meanings ascribed thereto in the Registration Rights Agreement.
The undersigned hereby provides the following information to the Company and represents and warrants that such information is accurate:
QUESTIONNAIRE
| 1. | | Name. | | |
|
|
(a) |
|
Full Legal Name of Selling Securityholder |
|
|
|
|
|
|
|
(b) |
|
Full Legal Name of Registered Holder (if not the same as (a) above) through which Registrable Securities Listed in Item 3 below are held: |
|
|
|
|
|
|
|
(c) |
|
Full Legal Name of Natural Control Person (which means a natural person who directly you indirectly alone or with others has power to vote or dispose of the securities covered by the questionnaire): |
|
|
|
|
|
2. Address for Notices to Selling Securityholder:
|
| | |
|
| | |
|
Telephone: |
|
Fax: |
|
Contact Person: |
|
Annex B-1
3. Beneficial Ownership of Registrable Securities:
| | | (a) | | Type and Principal Amount of Registrable Securities beneficially owned: |
|
|
|
|
|
| | | |
|
|
|
|
|
| | | |
|
|
|
|
|
4. Broker-Dealer Status:
| | | (a) | | Are you a broker-dealer? |
|
|
|
|
Yes / / No / / |
|
|
Note: |
|
If yes, the SEC's staff has indicated that you should be identified as an underwriter in the Registration Statement. |
|
|
(b) |
|
Are you an affiliate of a broker-dealer? |
|
|
|
|
Yes / / No / / |
|
|
(c) |
|
If you are an affiliate of a broker-dealer, do you certify that you bought the Registrable Securities in the ordinary course of business, and at the time of the purchase of the Registrable Securities to be resold, you had no agreements or understandings, directly or indirectly, with any person to distribute the Registrable Securities? |
|
|
|
|
Yes / / No / / |
|
|
Note: |
|
If no, the SEC's staff has indicated that you should be identified as an underwriter in the Registration Statement. |
5. Beneficial Ownership of Other Securities of the Company Owned by the Selling Securityholder.
| | | Except as set forth below in this Item 5, the undersigned is not the beneficial or registered owner of any securities of the Company other than the Registrable Securities listed above in Item 3. |
|
|
(a) |
|
Type and Amount of Other Securities beneficially owned by the Selling Securityholder: |
|
|
|
|
|
|
|
|
|
|
6. Relationships with the Company:
| | | | | Except as set forth below, neither the undersigned nor any of its affiliates, officers, directors or principal equity holders (owners of 5% of more of the equity securities of the undersigned) has held any position or office or has had any other material relationship with the Company (or its predecessors or affiliates) during the past three years. |
|
|
|
|
State any exceptions here: |
|
|
|
|
|
|
|
|
|
|
The undersigned agrees to promptly notify the Company of any inaccuracies or changes in the information provided herein that may occur subsequent to the date hereof and prior to the Effective Date for the Registration Statement.
Annex B-2
By signing below, the undersigned consents to the disclosure of the information contained herein in its answers to Items 1 through 6 and the inclusion of such information in the Registration Statement and the related prospectus. The undersigned understands that such information will be relied upon by the Company in connection with the preparation or amendment of the Registration Statement and the related prospectus.
IN WITNESS WHEREOF the undersigned, by authority duly given, has caused this Notice and Questionnaire to be executed and delivered either in person or by its duly authorized agent.
Annex B-3
Exhibit D
VOTING AGREEMENT
THIS VOTING AGREEMENT ("Agreement") is entered into as of April 7, 2004 by and among
- •
- IMCOR Pharmaceutical Co., a Nevada corporation (the "Company"),
- •
- Robert J. Weinstein, M.D. (individually and as General Partner of the W.F. Investment Enterprises Limited Partnership, as Director of the Robert and Lois Weinstein Family Foundation, Inc. and as Trustee of the Robert and Lois Weinstein Joint Revocable Trust) ("Weinstein"),
- •
- Stuart P. Levine (individually and as General Partner of SL Investment Enterprises, L.P. and as President of the Stuart and Sherri Levine Family Foundation, Inc.) ("Levine"),
- •
- Tannebaum, LLC, a Delaware limited liability company ("TLLC"),
- •
- Mi3 L.P., a Delaware limited partnership ("Mi3"),
- •
- Oxford BioScience Partners IV L.P., a Delaware limited partnership ("Oxford"), and
- •
- MRNA Fund II L.P. ("MRNA").
Weinstein, Levine, TLLC, Mi3, Oxford and MRNA are sometimes collectively referred to herein as the "Stockholders."
WHEREAS, the Company intends to issue more than 20% of its outstanding shares of common stock, $.001 par value per share (the "Common Stock"), in connection with the Company's proposed sale of up to $15,000,000 of its Common Stock and warrants to purchase its Common Stock to certain accredited investors (the "Financing Transaction").
WHEREAS, the shares of Common Stock, together with all other capital stock or securities of the Company, whether authorized or outstanding as of the date hereof or at any time hereafter, are collectively referred to as the "Shares."
WHEREAS, the Company intends to hold one or more annual or special meetings of its stockholders to adopt, approve and ratify the transactions in connection with the Financing Transaction, and the Stockholders desire to indicate their agreement to vote all Shares they now own in favor of the Financing Transaction, as hereinafter set forth.
NOW, THEREFORE, in consideration of the mutual promises herein and other good and valuable consideration, the receipt and adequacy of which is acknowledged, the parties hereby agree as follows.
1. Voting Agreement.
(a) The agreement in this Section 1 shall be deemed to constitute a voting agreement among the Stockholders pursuant to Section 78.365(3) of the Nevada General Corporation Law. As used in this Agreement, the definition and determination of a "Beneficial Owner" or "Beneficial Ownership" shall be governed by Regulation 13d-3 under the Securities Exchange Act of 1934, as amended.
(b) At any annual meeting or special meeting of the stockholders of the Company, and at any other time at which stockholders of the Company will have the right to or will vote for or render consent in writing, then and in each event, each Stockholder hereby agrees to take all necessary and
D-1
appropriate actions to cause all Shares he or it Beneficially Owns at the time of such vote or consent (whether now owned or hereafter acquired) to:
i. Be represented in person or by proxy at any such meeting or in any such written consent and to cause a quorum to be present in any such event; and
ii. Be voted to adopt, approve and ratify (A) the issuance of more than 20% of the Company's outstanding Shares in the Financing Transaction, and (B) the execution, delivery and performance of documents relating to the Financing Transaction by the Company and the performance and consummation of the transactions contemplated therein.
2. No Transfers. Until after the stockholders have voted upon the matters described in Section 1(b)(ii), none of the Stockholders shall sell, assign, transfer, grant an option to or for, pledge, hypothecate, mortgage, encumber or dispose of the Beneficial Ownership of all or any of his or its Shares except in compliance with the Voting, Drag-Along and Right of First Refusal Agreement among the parties hereto dated as of November 12, 2002, in which case the transferee of such Shares shall be obligated as set forth in Section 1(b) with regard to the Shares received from the Stockholder.
3. Legend on Certificate. Each certificate representing Shares covered by this Agreement is subject to and shall bear the restrictive legend set forth below:
The stock evidenced by this certificate is subject to a Voting Agreement dated as of April , 2004, as amended from time to time. Copies of the Voting Agreement may be obtained from the Secretary of the Company at no cost by written request of the holder of record of this certificate.
4. Stockholder Holdings. As of the date of this Agreement, each party hereto represents and warrants as to himself or itself only that he or it is the record and beneficial owners of the following Shares: (i) Weinstein owns 1,314,663 Shares; (ii) Levine owns 1,277,388 Shares; (iii) TLLC owns 2,344,957 Shares; (iv) Mi3 owns 462,963 Shares; (v) Oxford owns 6,417,097 Shares; (vi) MRNA owns 64,385 Shares; and (vii) the other stockholders own approximately 9,660,023 Shares. Therefore, the Stockholders hold approximately 55.2% of the Shares prior to the closing of the Financing Transaction.
5. General Provisions.
(a) Each of the parties hereto agrees that he or it will do (or cause to be done) any act or thing and will execute (or cause to be executed) any and all instruments necessary and/or proper to make effective the provisions of this Agreement. Each Stockholder represents and warrants to, and agrees with, each other party hereto that (i) any transferee holding Shares over which such Stockholder remains the Beneficial Owner shall execute and deliver a counterpart of this Agreement and shall be bound by the provisions hereof as if such transferee was an original party hereto; and (ii) such Stockholder shall provide each other party hereto true and complete information concerning the Beneficial Ownership of Shares in the hands of transferees. The parties are executing this Agreement in all applicable individual and representative capacities (including as attorney-in-fact or joint tenant, and as stockholder, officer, director, trustee, member, general partner or limited partner of any entity which Beneficially Owns Common Stock of the Company attributable to that party). This Agreement shall be binding upon and inure to the benefit of the parties hereto and their legal representatives, heirs and legatees.
(b) Each of the parties hereto acknowledge that the execution, delivery and performance of this Agreement is a material inducement to the Purchasers willingness to enter into the Financing Transaction and that each of the parties to this Agreement has been provided with a copy of the Securities Purchase Agreement, the Warrant Agreements, the Registration Rights Agreement and the other agreements, documents and instruments contemplated thereby.
(c) The section headings in this Agreement are inserted for convenience of reference only, and shall not affect the construction or interpretation of this Agreement.
D-2
(d) The failure at any time to enforce any of the provisions of this Agreement shall not be construed as a waiver of such provisions and shall not affect the right of any party thereafter to enforce each and every provision of this Agreement in accordance with its terms.
(e) This Agreement shall be governed by and construed in accordance with the laws of the Commonwealth of Massachusetts without giving effect to conflict of laws principles thereof, except to the extent the Nevada General Corporation Law govern portions hereof.
(f) It is acknowledged that it will be impossible to measure the damages that would be suffered by any party if another party fails to comply with the provisions of this Agreement and that in the event of any such failure, the non-defaulting party will not have an adequate remedy at law. The non-defaulting party (including the Company) shall, therefore, be entitled to obtain specific performance of any defaulting party's obligations hereunder and to obtain immediate injunctive relief. The defaulting party shall not argue, as a defense to any proceeding for such specific performance or injunctive relief, that the non-defaulting party has an adequate remedy at law.
(g) This Agreement may be executed in one or more counterparts, each of which shall be deemed to be an original and shall be enforceable against the party executing the same, and all of which together shall constitute a single Agreement. In making proof of this Agreement, it shall not be necessary to produce or account for more than one such counterpart.
(h) Whenever possible, each provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement shall be held to be invalid by a court of competent jurisdiction, the remaining provisions shall remain in full force and effect and the provision held invalid shall be modified to the extent necessary to be valid and shall be enforced as modified.
(i) Any notice to be served under this Agreement shall be in writing and shall be deemed to be delivered or given upon receipt if delivered personally, by overnight courier or by telecopier, or two days after mailing by registered mail, return receipt requested, addressed to such Stockholder's address on file in the stock records of the Company, or to such other place as a party may specify in writing, delivered in accordance with the provisions of this subsection.
(j) This Agreement constitutes the full and entire understanding and agreement of the parties with regard to the subject hereof, and supersedes any prior agreement or understanding, written or oral, with respect to such subject matter. This Agreement may not be amended until after the Shares have been voted in accordance with Section 1 above. No party shall be liable or bound by any representations, warranties or agreements, or any other information or materials previously delivered, whether written or oral, regarding such subject matter.
[Next Page is Signature Page.]
D-3
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above.
| | | IMCOR Pharmaceutical Co. |
|
|
By: |
|
/s/ TAFFY J. WILLIAMS
Taffy J. Williams, Ph.D.,President |
|
|
/s/ ROBERT J. WEINSTEIN
Robert J. Weinstein, M.D.,
individually and as General Partner of the W.F. Investment Enterprises Limited Partnership, as Director of the Robert and Lois Weinstein Family Foundation, Inc. and as Trustee of the Robert and Lois Weinstein Joint Revocable Trust |
|
|
/s/ STUART LEVINE
Stuart Levine,
individually and as General Partner of SL Investment Enterprises, L.P. and as President of the Stuart and Sherri Levine Family Foundation, Inc. |
|
|
Tannebaum, LLC |
|
|
|
|
By: |
|
Tannebaum Ventures, LLC, its Manager |
|
|
|
|
By: |
|
/s/ LOUIS D. WILLIAMS
|
| | | | | Its: | | Sole Manager |
|
|
Mi3 L.P. |
|
|
|
|
By: |
|
MI3 Services L.L.C., its General Partner |
|
|
|
|
By: |
|
/s/ WILLIAM D. MCPHEE
William D. McPhee,President |
|
|
Oxford Bioscience Partners IV L.P. |
|
|
|
|
By: |
|
OBP Management IV L.P. |
|
|
|
|
By: |
|
/s/ JONATHAN FLEMING
Jonathan J. Fleming—General Partner |
|
|
MRNA Fund II L.P. |
|
|
|
|
By: |
|
OBP Management IV L.P. |
|
|
|
|
By: |
|
/s/ JONATHAN FLEMING
Jonathan J. Fleming—General Partner |
D-4
AMENDMENT NO. 1 TO VOTING AGREEMENT
THIS AMENDMENT NO. 1 (this "Amendment") to the Voting Agreement dated as of April 7, 2004 (the "Agreement") is entered into as of April 19, 2004 by and among
- •
- IMCOR Pharmaceutical Co., a Nevada corporation (the "Company"),
- •
- Robert J. Weinstein, M.D. (individually and as General Partner of the W.F. Investment Enterprises Limited Partnership, as Director of the Robert and Lois Weinstein Family Foundation, Inc. and as Trustee of the Robert and Lois Weinstein Joint Revocable Trust) ("Weinstein"),
- •
- Stuart P. Levine (individually and as General Partner of SL Investment Enterprises, L.P. and as President of the Stuart and Sherri Levine Family Foundation, Inc.) ("Levine"),
- •
- Tannebaum, LLC, a Delaware limited liability company ("TLLC"),
- •
- Mi3 L.P., a Delaware limited partnership ("Mi3"),
- •
- Oxford BioScience Partners IV L.P., a Delaware limited partnership ("Oxford"), and
- •
- MRNA Fund II L.P. ("MRNA").
Weinstein, Levine, TLLC, Mi3, Oxford and MRNA are sometimes collectively referred to herein as the "Stockholders."
WHEREAS, the Stockholders desire to clarify that the definition of "Financing Transaction" as set forth in the Agreement is intended to include: the Company's issuance of more than 20% of its outstanding shares of common stock, $.001 par value per share (the "Common Stock"), in connection with the Company's proposed sale of up to $15,000,000 of its Common Stock and warrants to purchase its Common Stock to certain accredited investors, the issuance of an additional 3,000,000 shares of Common Stock at $0.75 per share (without warrants), the issuance of shares of Common Stock upon the conversion of the outstanding principal and interest under the Company's secured promissory notes held by Oxford, MRNA and Mi3 at the conversion price of $0.75 per share, and the issuance to the Company's co-placement agents of warrants to purchase up to an aggregate of 1,600,000 shares of Common Stock.
NOW, THEREFORE, in consideration of the mutual promises herein and other good and valuable consideration, the receipt and adequacy of which is acknowledged, the parties hereby agree as follows.
6. Amendment to Definition. The definition of "Financing Transaction" set forth in the Agreement is hereby amended and restated in its entirety to mean as follows:
"Financing Transaction" shall mean the Company's issuance of more than 20% of its outstanding shares of common stock, $.001 par value per share (the "Common Stock"), in connection with the Company's proposed sale of up to $15,000,000 of its Common Stock and warrants to purchase its Common Stock to certain accredited investors, the issuance of an additional 3,000,000 shares of Common Stock at $0.75 per share (without warrants), the issuance of shares of Common Stock upon the conversion of the outstanding principal and interest under the Company's secured promissory notes held by Oxford, MRNA and Mi3 at the conversion price of $0.75 per share, and the issuance to the Company's co-placement agents of warrants to purchase up to an aggregate of 1,600,000 shares of Common Stock.
7. Agreement. In all other respects the Agreement shall remain unchanged.
[Next Page is Signature Page.]
D-5
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above.
| | | IMCOR Pharmaceutical Co. |
|
|
By: |
|
/s/ TAFFY J. WILLIAMS
Taffy J. Williams, Ph.D.,President |
|
|
/s/ ROBERT J. WEINSTEIN
Robert J. Weinstein, M.D.,
individually and as General Partner of the W.F. Investment Enterprises Limited Partnership, as Director of the Robert and Lois Weinstein Family Foundation, Inc. and as Trustee of the Robert and Lois Weinstein Joint Revocable Trust |
|
|
/s/ STUART LEVINE
Stuart Levine,
individually and as General Partner of SL Investment Enterprises, L.P. and as President of the Stuart and Sherri Levine Family Foundation, Inc. |
|
|
Tannebaum, LLC |
|
|
|
|
By: |
|
Tannebaum Ventures, LLC, its Manager |
|
|
|
|
By: |
|
/s/ LOUIS D. WILLIAMS
|
| | | | | Its: | | Sole Manager |
|
|
Mi3 L.P. |
|
|
|
|
By: |
|
MI3 Services L.L.C., its General Partner |
|
|
|
|
By: |
|
/s/ WILLIAM D. MCPHEE
William D. McPhee,President |
|
|
Oxford Bioscience Partners IV L.P. |
|
|
|
|
By: |
|
OBP Management IV L.P. |
|
|
|
|
By: |
|
/s/ MICHAEL LYTTON
Michael Lytton—General Partner |
|
|
MRNA Fund II L.P. |
|
|
|
|
By: |
|
OBP Management IV L.P. |
|
|
|
|
By: |
|
/s/ MICHAEL LYTTON
Michael Lytton—General Partner |
D-6
Exhibit E
IMCOR Pharmaceutical Co.
2000 LONG TERM INCENTIVE COMPENSATION PLAN
1. ESTABLISHMENT, OBJECTIVES AND DURATION.
- a.
- ESTABLISHMENT OF THE PLAN. IMCOR Pharmaceutical Co. hereby amends and restates its incentive compensation plan to be known as the "IMCOR Pharmaceutical Co. 2000 Long Term Incentive Compensation Plan" (the "Plan"), as set forth in this document. The Plan permits the grant of Incentive Stock Options, Nonqualified Stock Options and Restricted Stock.
- b.
- OBJECTIVES OF THE PLAN. The objectives of the Plan are to optimize the profitability and growth of the Company through the use of incentives which are consistent with the Company's objectives and which link the interests of Participants to those of the Company's stockholders; to provide Participants with an incentive for excellence in individual performance; and to promote teamwork among Participants. The Plan is further intended to provide flexibility to the Company in its ability to motivate, attract, and retain the services of Participants who make significant contributions to the Company's success and to allow Participants to share in the success of the Company.
- c.
- DURATION OF THE PLAN. The Plan shall become effective as of the date it is approved by the stockholders of IMCOR Pharmaceutical Co. (the "Effective Date"). The Plan shall remain in effect, subject to the right of the Board of Directors or the Committee to amend or terminate the Plan at any time pursuant to Section 11 hereof, until all Shares subject to it shall have been purchased or acquired according to the Plan's provisions. However, in no event may an Incentive Stock Option be granted under the Plan on or after May 17, 2010.
2. DEFINITIONS.
Whenever used in the Plan, the following terms shall have the meanings set forth below:
- a.
- "AFFILIATE" means a "parent corporation" or "subsidiary corporation" as defined in Section 424 of the Code.
- b.
- "AWARD" means, individually or collectively, a grant under this Plan of Incentive Stock Options, Nonqualified Stock Options or Restricted Stock.
- c.
- "AWARD AGREEMENT" means an agreement entered into by the Company and each Participant setting forth the terms and provisions applicable to Awards granted under this Plan.
- d.
- "BENEFICIAL OWNER" or "BENEFICIAL OWNERSHIP" shall have the meaning ascribed to such term in Rule 13d-3 of the General Rules and Regulations under the Exchange Act.
- e.
- "BOARD" or "BOARD OF DIRECTORS" means the Board of Directors of the Company.
- f.
- "CAUSE" shall be determined by the Committee, exercising good faith and reasonable judgment, and shall mean the occurrence of any one or more of the following:
- i.
- The willful and continued failure by the Participant to substantially perform his duties (other than any such failure resulting from the Participant's Disability) after a written demand for substantial performance is delivered by the Committee to the Participant that identifies in reasonable detail the manner in which the Committee believes that the
E-1
- g.
- "CHANGE IN CONTROL" of the Company shall be deemed to have occurred as of the first day that any one or more of the following conditions shall have been satisfied:
- i.
- The acquisition by any Person of Beneficial Ownership of 50% or more of either (1) the then outstanding shares of Common Stock of the Company, or (2) the combined voting power of the outstanding voting securities of the Company entitled to vote generally in the election of Directors; provided, however, that for purposes of this subsection, the following transactions shall not constitute a Change of Control: (A) any acquisition directly from the Company through a public offering of shares of Common Stock of the Company, (B) any acquisition by the Company, (C) any acquisition by any employee benefit plan (or related trust) sponsored or maintained by the Company or any corporation controlled by the Company, or (D) any acquisition by any corporation pursuant to a transaction which complies with clauses (1), (2) and (3) of subsection (iii) below;
- ii.
- The cessation, for any reason, of the individuals who constitute the Company's Board of Directors as of the date hereof ("Incumbent Board") to constitute at least a majority of the Company's Board of Directors; provided, however, that any individual becoming a Director following the date hereof whose election, or nomination for election by the Company's stockholders, was approved by a vote of at least a majority of the Directors then comprising the Incumbent Board shall be considered as though such individual was a member of the Incumbent Board, but excluding, for this purpose, any such individual whose initial assumption of office occurs because of an actual or threatened election contest with respect to the election or removal of Directors or other actual or threatened solicitation of proxies or consents by or on behalf of a Person other than the Company's Board of Directors;
- iii.
- The consummation of a reorganization, merger or consolidation or sale or other disposition of all or substantially all of the assets of the Company ("Business Combination") unless, following such Business Combination, (1) all or substantially all of the individuals and entities who were the Beneficial Owners, respectively, of the outstanding shares of Common Stock of the Company and the outstanding voting securities of the Company immediately before such Business Combination beneficially own, directly or indirectly, more than 50% of, respectively, the then outstanding shares of Common Stock and the combined voting power of the then outstanding voting securities entitled to vote generally in the election of Directors, as the case may be, of the Company resulting from such Business Combination (including, without limitation, a
E-2
corporation which as a result of such transaction owns the Company or all or substantially all of the Company's assets either directly or through one or more subsidiaries) in substantially the same proportions as their ownership immediately before such Business Combination of the outstanding shares of Common Stock and the outstanding voting securities of the Company, as the case may be; (2) no party (excluding any corporation resulting from such Business Combination or any employee benefit plan (or related trust) of the Company or such corporation resulting from such Business Combination) beneficially owns, directly or indirectly, 50%; or more of, respectively, the then outstanding shares of common stock of the corporation resulting from such Business Combination or the combined voting power of the then outstanding voting securities of such corporation except to the extent that such ownership existed before the Business Combination; and (3) at least a majority of the members of the board of directors of the corporation resulting from such Business Combination were members of the Company's Board of Directors at the time of the execution of the initial agreement, or of the action of the Company's Board of Directors, providing for such Business Combination; or
- iv.
- The approval by the stockholders of the Company of a complete liquidation or dissolution of the Company.
- h.
- "CODE" means the Internal Revenue Code of 1986, as amended from time to time.
- i.
- "COMMITTEE" means the Compensation Committee of the Board, as specified in Section 3 herein, or such other Committee appointed by the Board to administer the Plan with respect to grants of Awards.
- j.
- "COMPANY" means IMCOR Pharmaceutical Co., a Nevada corporation, and also means any corporation of which a majority of the voting capital stock is owned directly or indirectly by Photogen Technologies Inc. or by any of its Subsidiaries, and any other corporation designated by the Committee as being a Company hereunder (but only during the period of such ownership or designation).
- k.
- "DIRECTOR" means any individual who is a member of the Board of Directors of the Company.
- l.
- "DISABILITY", as applied to a Participant, means that the Participant (a) has established to the satisfaction of the Committee that the Participant is unable to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment which can be expected to last for a continuous period of not less than 12 months (all within the meaning of Section 22(e)(3) of the Code), and (b) has satisfied any requirement imposed by the Committee in regard to evidence of such disability.
- m.
- "ELIGIBLE PERSON" shall mean all Employees, Directors or consultants of the Company or any Affiliate; provided, however, that no Award may be granted to anyone who is not an "employee" as that term is defined in General Instruction A.(1)(a) of Form S-8, as such definition may be amended from time to time, without first receiving advice and guidance from the Company's outside counsel as to the effect of such grant.
- n.
- "EMPLOYEE" means any officer or employee of the Company.
- o.
- "EXCHANGE ACT" means the Securities Exchange Act of 1934, as amended from time to time, or any successor act thereto.
- p.
- "FAIR MARKET VALUE" Except as otherwise determined by the Committee, the "Fair Market Value" of a share of Common Stock as of any date shall be equal to the closing sale price of a share of Common Stock as reported on The National Association of Securities Dealers' New York Stock Exchange Composite Reporting Tape (or if the Common Stock is
E-3
not traded on The New York Stock Exchange, the closing sale price on the exchange on which it is traded or as reported by an applicable automated quotation system, including the over-the-counter bulletin board) (the "Composite Tape"), on the applicable date or, if no sales of Common Stock are reported on such date, the closing sale price of a share of Common Stock on the date the Common Stock was last reported on the Composite Tape (or such other exchange or automated quotation system, if applicable) as of the date specified by the Committee (and if no date is specified, then on the date of the meeting of the Committee at which the award was granted).
- q.
- "IMMEDIATE FAMILY MEMBERS" means the spouse, children and grandchildren of a Participant.
- r.
- "INCENTIVE STOCK OPTION" or "ISO" means an option to purchase Shares granted under Section 6 herein and which is designated as an Incentive Stock Option and which is intended to meet the requirements of Code Section 422.
- s.
- "INSIDER" shall mean an individual who is, on the relevant date, a Director, a 10% Beneficial Owner of any class of the Company's equity securities that is registered pursuant to Section 12 of the Exchange Act or an officer of the Company, as defined under Section 16 of the Exchange Act and as determined by the Board of Directors from time to time.
- t.
- "NONEMPLOYEE DIRECTOR" means an individual who is a member of the Board of Directors of the Company but who is not an Employee of the Company.
- u.
- "NONQUALIFIED STOCK OPTION" or "NQSO" means an option to purchase Shares granted under Section 6 herein and which is not intended to meet the requirements of Code Section 422.
- v.
- "OPTION" means an Incentive Stock Option or a Nonqualified Stock Option, as described in Section 6 herein.
- w.
- "OPTION PRICE" means the price at which a Share may be purchased by a Participant pursuant to an Option.
- x.
- "PARTICIPANT" means an Eligible Person who has outstanding an Award granted under the Plan.
- y.
- "PERIOD OF RESTRICTION" means the period during which the transfer of Shares of Restricted Stock is limited in some way (based on the passage of time, the achievement of performance objectives, or upon the occurrence of other events as determined by the Committee, at its discretion), and the Shares of Restricted Stock are subject to a substantial risk of forfeiture, as provided in Section 7 herein.
- z.
- "PERSON" shall have the meaning ascribed to such term in Section 3(a)(9) of the Exchange Act and used in Sections 13(d) and 14(d) thereof, including a "group" as defined in Section 13(d) thereof.
- aa.
- "RESTRICTED STOCK" means an Award granted to a Participant pursuant to Section 7 herein.
- bb.
- "RETIREMENT" as applied to a Participant, means the Participant's termination of employment in a manner which qualifies the Participant to receive immediately payable retirement benefits under any applicable retirement plan maintained by the Company (the "Retirement Plan"), under the successor or replacement of such Retirement Plan if it is then no longer in effect, or under any other retirement plan maintained or adopted by the Company which is determined by the Committee to be the functional equivalent of such Retirement Plan; or, with respect to a Participant who may not or has not participated in a
E-4
3. ADMINISTRATION.
- a.
- THE COMMITTEE. The Plan shall be administered by the Committee, or by any other committee appointed by the Board, which Committee shall consist solely of two or more "Nonemployee Directors" within the meaning of Rule 16b-3 under the Exchange Act, or any successor provision. The members of the Committee shall be appointed from time to time by, and shall serve at the discretion of, the Board of Directors.
- b.
- AUTHORITY OF THE COMMITTEE. Except as limited by law and subject to the provisions herein, the Committee shall have full power in its discretion to select Eligible Persons who shall participate in the Plan; determine the sizes and types of Awards; determine the terms and conditions of Awards (including vesting periods and restrictions); prescribe the form of, construe and interpret any agreement or instrument entered into under the Plan as they apply to Participants; construe and interpret the terms and conditions of this Plan; establish, amend, or waive rules and regulations for the Plan's administration as they apply to Participants; alter, amend, suspend or terminate the Plan in whole or in part; and (subject to the provisions of Section 11 herein) amend the terms and conditions of any outstanding Award to the extent such terms and conditions are within the discretion of the Committee as provided in the Plan. Further, the Committee shall make all other determinations which may be necessary or advisable for the administration of the Plan. As permitted by law, the Committee may delegate its authority as identified herein.
- c.
- DECISIONS BINDING. All determinations and decisions made by the Committee pursuant to the provisions of the Plan and all related orders and resolutions of the Board shall be final, conclusive and binding on all persons, including the Company, its stockholders, Employees, Participants and their estates and beneficiaries.
- d.
- COSTS OF PLAN. The costs and expenses incurred in the operation and administration of the Plan shall be borne by the Company.
- e.
- INDEMNIFICATION. Each person who is or shall have been a member of the Committee shall be indemnified and held harmless by the Company against and from any loss, cost, liability, or expense that may be imposed upon or reasonably incurred by him in connection with or resulting from any claim, action, suit, or proceeding to which he may be a party or in which he may be involved by reason of any action taken or failure to act under the Plan and against and from any and all amounts paid by him in settlement thereof, to the fullest extent permitted by the Nevada General Corporation Law. The foregoing right of indemnification shall not be exclusive of any other rights of indemnification to which such persons may be entitled under the Company's Articles of Incorporation or Bylaws, as a matter of law, or otherwise, or any power that the Company may have to indemnify them or hold them harmless. In addition, a member of the Committee, in the performance of any act (or in refraining from taking any action) in connection with the Plan, shall not be liable to the Company, its stockholders, Employees, Participants or any person when relying in good faith upon the records of the Company or upon such information or statements presented to the Company by any of its officers, employees or other persons as to matters the member
E-5
4. SHARES SUBJECT TO THE PLAN AND MAXIMUM AWARDS.
- a.
- NUMBER OF SHARES AVAILABLE FOR GRANTS. Subject to adjustment as provided in Section 4(b) herein, the number of Shares hereby reserved for issuance to Participants under the Plan shall be 30,000,000. Shares issued upon exercise of Options or Awards of Restricted Stock under the Plan may be either authorized but unissued Shares or Shares re-acquired by the Company. If, on or prior to the termination of the Plan, an Award granted thereunder expires or is terminated for any reason without having been exercised or vested in full, the unpurchased or unvested Shares covered thereby will again become available for the grant of Awards under the Plan. Shares of Common Stock covered by Options surrendered in connection with the exercise of other Options shall not be deemed to have been exercised and shall again become available for the grant of awards under the Plan.
- b.
- ADJUSTMENTS IN AUTHORIZED SHARES. In the event of any change in corporate capitalization, such as a stock split, or a corporate transaction, such as any merger, consolidation, separation, including a spin-off, or other distribution of stock or property of the Company, any reorganization (whether or not such reorganization comes within the definition of such term in Code Section 368) or any partial or complete liquidation of the Company, such adjustment shall be made in the number and class of Shares which may be delivered under Section 4(a), in the number and class of and/or price of Shares subject to outstanding Awards granted under the Plan, and in the Award limits set forth in Section 4(a), as may be determined to be appropriate and equitable by the Committee, in its sole discretion, to prevent dilution or enlargement of rights; provided, however, that the number of Shares subject to any Award shall always be a whole number.
5. ELIGIBILITY AND PARTICIPATION.
- a.
- ELIGIBILITY. All Eligible Persons are eligible to participate in this Plan.
- b.
- ACTUAL PARTICIPATION. Subject to the provisions of the Plan, the Committee may from time to time in its discretion select Eligible Persons to whom Awards shall be granted and shall determine the nature and amount of each Award.
- c.
- PERFORMANCE-BASED AWARDS. The Committee may, in its discretion, grant Awards that are wholly contingent on the attainment of performance goals established by the Committee from time to time. The performance goals may relate to one or more of the following performance measures, as determined by the Committee for each applicable performance period: (i) return to stockholders, (ii) cash flow, (iii) return on equity, (iv) Company created income (for example, income due to Company initiated cost reductions or productivity improvements), (v) sales growth, (vi) earnings and earnings growth, (vii) return on assets, (viii) stock price, (ix) earnings per share, (x) market share, (xi) customer satisfaction, and (xii) attaining regulatory approvals or other regulatory benchmarks. Any such performance goals and the applicable performance measures will be determined by the Committee at the time of grant and reflected in an Award Agreement. The number or value of performance-based stock Awards that will be paid out to any Participant at the end of the applicable performance period, which may be one year or longer as determined by the Committee, will depend on the extent to which the Company attains the established performance goals. Awards intended to be performance-based stock Awards shall be subject to such restrictions and conditions as may be required under Code Section 162(m) to be performance-based compensation thereunder.
E-6
6. STOCK OPTIONS.
- a.
- GRANT OF OPTIONS. Subject to the terms and provisions of the Plan, Options may be granted to Participants in such number, and upon such terms, and at any time and from time to time as shall be determined by the Committee.
- b.
- AWARD AGREEMENT. Each Option grant shall be evidenced by an Award Agreement that shall specify the Option Price, the duration of the Option, the number of Shares to which the Option pertains, and such other provisions as the Committee shall determine, which need not be uniform for all Participants. The Award Agreement also shall specify whether the Option is intended to be an ISO within the meaning of Code Section 422, or an NQSO whose grant is intended not to fall under the provisions of Code Section 422.
- c.
- PROVISIONS FOR NQSOs. Each Stock Option intended to be a Non-Qualified Stock Option shall be subject to the terms and conditions which the Committee determines to be appropriate, subject to the following minimum standards for any such Non-Qualified Stock Option:
- i.
- The option price (per share) of the Shares covered by each Non-Qualified Stock Option shall be determined by the Committee but shall not be less than the par value per share of Common Stock.
- ii.
- Each Award Agreement shall state the date or dates on which it first is exercisable and the date after which it may no longer be exercised, and may provide that the Stock Option rights accrue or become exercisable in installments over a period of months or years, or upon the occurrence of certain conditions or the attainment of stated goals or events; and
- iii.
- Exercise of any Stock Option may be conditioned upon the Participant's execution of a Share purchase agreement in form satisfactory to the Committee providing, among other things, that:
- (1)
- The Participant's or the Participant's estate, heirs and representatives right to sell the Shares may be restricted; and
- (2)
- The Participant or the Participant's estate, heirs and representatives may be required to execute letters of investment intent and must also acknowledge that the Shares will bear legends noting any applicable restrictions.
- d.
- PROVISIONS FOR ISOs. Each Stock Option intended to be an Incentive Stock Option shall be issued only to an Employee and be subject to at least the following terms and conditions, with such additional restrictions or changes as the Committee determines are appropriate but not in conflict with Code Section 422 and relevant regulations and rulings of the Internal Revenue Service:
- i.
- The Incentive Stock Option shall meet the minimum standards required of Non-Qualified Stock Options, as described in Section 6(c) except clause (i) thereunder.
- ii.
- Immediately before the Incentive Stock Option is granted, if the Participant owns, directly or by reason of the applicable attribution rules in Code Section 424(d):
- (1)
- Ten percent (10%) or less of the total combined voting power of all classes of share capital of the Company or an Affiliate, the option price per share of the Shares covered by each Incentive Stock Option shall not be less than one hundred percent (100%) of the Fair Market Value per share of the Shares on the date of the grant of the Incentive Stock Option.
E-7
- (2)
- More than ten percent (10%) of the total combined voting power of all classes of share capital of the Company or an Affiliate, the option price per share of the Shares covered by each Incentive Stock Option shall not be less than one hundred ten percent (110%) of the said Fair Market Value on the date of grant.
- iii.
- For Participants who own:
- (1)
- Ten percent (10%) or less of the total combined voting power of all classes of share capital of the Company or an Affiliate, each Incentive Stock Option shall terminate not more than ten (10) years from the date of the grant or at such earlier time as the Award Agreement may provide;
- (2)
- More than ten percent (10%) of the total combined voting power of all classes of share capital of the Company or an Affiliate, each Incentive Stock Option shall terminate not more than five (5) years from the date of the grant or at such earlier time as the Award Agreement may provide.
- iv.
- The Award Agreements shall restrict the amount of Options which may be exercisable in any calendar year (under this or any other Incentive Stock Option plan of the Company or an Affiliate) so that the aggregate Fair Market Value (determined at the time each Incentive Stock Option is granted) of the stock with respect to which Incentive Stock Options are exercisable for the first time by the Participant in any calendar year does not exceed one hundred thousand dollars ($100,000), provided that this subparagraph shall have no force or effect if its inclusion in the Plan is not necessary for Options issued as Incentive Stock Options to qualify as Incentive Stock Options pursuant to Section 422(d) of the Code.
- v.
- No Incentive Stock Options shall be granted after the date which is the earlier of ten (10) years from the date of the adoption of the Plan by the Company and the date of the approval of this Plan by the shareholders of the Company.
- vi.
- Each Participant who receives an Incentive Stock Option must agree to notify the Company in writing immediately after the Participant makes a Disqualifying Disposition of any shares acquired pursuant to the exercise of an Incentive Stock Option. A Disqualifying Disposition is any disposition (including any sale) of such shares before the later of (i) two years after the date the Participant was granted the Incentive Stock Option, or (ii) one year after the date the Participant acquired shares by exercising the Incentive Stock Option. If the Participant has died before such stock is sold, these holding period requirements do not apply and no Disqualifying Disposition can occur thereafter.
- e.
- PAYMENT. Options granted under this Section 6 shall be exercised in accordance with the applicable Award Agreement by the delivery of a proper notice of exercise to the Company, setting forth the number of Shares with respect to which the Option is to be exercised and the method of payment of the Option Price for the subject Shares. No shares of Common Stock shall be issued on the exercise of an Option unless the Option Price is paid for in full at the time of exercise. Payment shall be made at the Participant's election by: (i) delivery to the Company of cash, which may be paid by check or other instrument acceptable to the Committee, (ii) subject to compliance with applicable laws and regulations and such conditions as the Committee may impose, the Option holder may deliver with his exercise notice irrevocable instructions to a broker to promptly deliver to the Company an amount of sale or loan proceeds sufficient to pay the exercise price, (iii) in the Committee's discretion and subject to compliance with applicable laws and regulations and such conditions as the Committee may impose with his exercise notice the option holder may deliver shares of Common Stock held for at least six months. As soon as practicable after receipt of proper notification of exercise and full payment, the Company shall deliver to the Participant, in the Participant's name, Share certificates in an appropriate amount based upon the number of Shares purchased under the Option(s).
E-8
- f.
- RESTRICTIONS ON SHARE TRANSFERABILITY. The Committee may impose such restrictions on any Shares acquired pursuant to the exercise of an Option granted under this Section 6 as it may deem advisable, including, without limitation, restrictions under applicable federal securities laws, under the requirements of any stock exchange or market upon which such Shares are then listed and/or traded, and under any state securities laws applicable to such Shares.
- g.
- TERMINATION OF EMPLOYMENT. Each Option, to the extent it has not been previously exercised, shall terminate upon the earliest to occur of: (1) the expiration of the Option period set forth in the Award Agreement; (2) for ISOs, the expiration of three months following the Participant's Retirement (following the Participant's Retirement, NQSOs shall terminate upon the expiration of the Option period set forth in the Option Award Agreement); (3) the expiration of 12 months following the Participant's death or Disability; (4) immediately upon termination for Cause; (5) the expiration of 90 days following the Participant's termination of employment for any reason other than Cause, Change in Control, death, Disability, or Retirement; or (6) at such other time or times and on such conditions provided for in the Award Agreement. Upon a termination of employment related to a Change in Control, Options shall be treated in the manner set forth in Section 10.
- h.
- NONTRANSFERABILITY OF OPTIONS.
- i.
- INCENTIVE STOCK OPTIONS. No ISO granted under the Plan may be sold, transferred, pledged, assigned or otherwise alienated or hypothecated, other than by will or by the laws of descent and distribution. Further, all ISOs granted to a Participant under the Plan shall be exercisable during his or her lifetime only by such Participant.
- ii.
- NONQUALIFIED STOCK OPTIONS. The Committee may, in its discretion, authorize all or a portion of NQSOs granted to a Participant to be on terms which permit transfer by such Participant to (1) Immediate Family Members, (2) a trust or trusts for the exclusive benefit of such Immediate Family Members, or (3) a partnership in which such Immediate Family Members are the only partners, provided that (A) there may be no consideration for any such transfer, (B) the Award Agreement pursuant to which such Options are granted must be approved by the Committee, and must expressly provide for transferability in a manner consistent with this Section, and (C) subsequent transfers of transferred Options shall be prohibited except those by will or the laws of descent and distribution. Following transfer, any such Options shall continue to be subject to the same terms and conditions as were applicable immediately prior to transfer, provided that for purposes of this Plan, the term "Participant" shall be deemed to refer to the transferee. The events of termination of employment shall continue to be applied with respect to the original Participant, following which the Options shall be exercisable by the transferee only to the extent, and for the periods specified in this Section 6(h). Notwithstanding the foregoing, should the Committee provide that Options granted be transferable, the Company by such action incurs no obligation to notify or otherwise provide notice to a transferee of early termination of the Option. In the event of a transfer, as set forth above, the original Participant is and will remain subject to and responsible for any applicable withholding taxes upon the exercise of such Options.
E-9
7. RESTRICTED STOCK.
- a.
- GRANT OF RESTRICTED STOCK. Subject to the terms and provisions of the Plan, the Committee, at any time and from time to time, may grant Shares of Restricted Stock to Participants in such amounts as the Committee shall determine. Without limiting the generality of the foregoing, Restricted Shares may be granted in connection with payouts under other compensation programs of the Company.
- b.
- RESTRICTED STOCK AGREEMENT. Each Restricted Stock grant shall be evidenced by a Restricted Stock Award Agreement that shall specify the Period(s) of Restriction, the number of Shares of Restricted Stock granted, and such other provisions as the Committee shall determine.
- c.
- TRANSFERABILITY. Except as provided in this Section 7, the Shares of Restricted Stock granted herein may not be sold, transferred, pledged, assigned or otherwise alienated or hypothecated until the end of the applicable Period of Restriction established by the Committee and specified in the Restricted Stock Award Agreement, or upon earlier satisfaction of any other conditions, as specified by the Committee in its sole discretion and set forth in the Restricted Stock Award Agreement. All rights with respect to the Restricted Stock granted to a Participant under the Plan shall be available during his or her lifetime only to such Participant.
- d.
- OTHER RESTRICTIONS. Subject to the Plan, the Committee shall impose such other conditions and/or restrictions on any Shares of Restricted Stock granted pursuant to the Plan as it may deem advisable including, without limitation, a requirement that Participants pay a stipulated purchase price for each Share of Restricted Stock, restrictions based upon the achievement of specific performance objectives, time-based restrictions on vesting following the attainment of the performance objectives, and/or restrictions under applicable federal or state securities laws. At the discretion of the Committee, the Company may retain the certificates representing Shares of Restricted Stock in the Company's possession until such time as all conditions and/or restrictions applicable to such Shares have been satisfied. Except as otherwise provided in this Section 7, Shares of Restricted Stock covered by each Restricted Stock grant made under the Plan shall become freely transferable by the Participant after the last day of the applicable Period of Restriction, subject to applicable securities laws.
- e.
- VOTING RIGHTS. During the Period of Restriction, Participants holding Shares of Restricted Stock granted hereunder may exercise full voting rights with respect to those Shares.
- f.
- DIVIDENDS AND OTHER DISTRIBUTIONS. During the Period of Restriction, Participants holding Shares of Restricted Stock granted hereunder may be credited with regular cash dividends paid with respect to the underlying Shares while they are so held. Such dividends may be paid currently, accrued as contingent cash obligations, or converted into additional shares of Restricted Stock, upon such terms as the Committee establishes. The Committee may apply any restrictions to the dividends that the Committee deems appropriate. In the event that any dividend constitutes a "derivative security" or an "equity security" pursuant to Rule 16(a) under the Exchange Act, such dividend shall be subject to a vesting period equal to the remaining vesting period of the Shares of Restricted Stock with respect to which the dividend is paid.
- g.
- TERMINATION OF EMPLOYMENT. Upon a Participant's death, Disability, or Retirement, all Restricted Shares shall vest immediately. Each Restricted Stock Award Agreement shall set forth the extent to which the Participant shall have the right to retain unvested Restricted Shares following termination of the Participant's employment with the Company in all other
E-10
circumstances. Such provisions shall be determined in the sole discretion of the Committee, shall be included in the Award Agreement entered into with each Participant, need not be uniform among all Shares of Restricted Stock issued pursuant to the Plan, and may reflect distinctions based on the reasons for termination of employment.
8. BENEFICIARY DESIGNATION.
A Participant under the Plan may make written designation of a beneficiary on forms prescribed by and filed with the Secretary of the Company. Such beneficiary, or if no such designation of any beneficiary has been made, the legal representative of such Participant or such other person entitled thereto as determined by a court of competent jurisdiction, may exercise, in accordance with and subject to the provisions of Section 6, any unterminated and unexpired Option granted to such Participant to the same extent that the Participant himself could have exercised such Option were he alive or able; provided, however, that no Option granted under the Plan shall be exercisable for more Shares than the Participant could have purchased thereunder on the date his employment by, or other relationship with, the Company and its Subsidiaries was terminated.
9. RIGHTS OF ELIGIBLE PERSONS AND PARTICIPANTS.
Nothing in this Plan or any Award Agreement shall be deemed to: prevent the Company or an Affiliate from terminating the employment, consultancy or director status of a Participant; prevent a Participant from terminating his or her own employment, consultancy or director status; give any Participant a right to be retained in employment or other service by the Company or any Affiliate for any period of time; confer on any person any right to be selected as a Participant.
10. CHANGE IN CONTROL.
- a.
- TREATMENT OF OUTSTANDING AWARDS. Upon the occurrence of a Change in Control, unless otherwise specifically prohibited under applicable laws, or by the rules and regulations of any governing governmental agencies or national securities exchanges:
- i.
- Any and all Options granted hereunder may, in the Committee's discretion, become immediately exercisable, and remain exercisable throughout their entire term; and
- ii.
- Any restriction periods and restrictions imposed on Shares of Restricted Stock shall lapse; provided, however, that the degree of vesting associated with Restricted Stock which has been conditioned upon the achievement of performance conditions pursuant to Section 5(c) herein shall be determined in the manner set forth in Section 7(d) herein.
- b.
- TERMINATION, AMENDMENT, AND MODIFICATIONS OF CHANGE-IN-CONTROL PROVISIONS. Notwithstanding any other provision of this Plan or any Award Agreement provision, the provisions of this Section 10 may not be terminated, amended, or modified on or after the date of a Change in Control to affect adversely any Award theretofore granted under the Plan without the prior written consent of the Participant with respect to said Participant's outstanding Awards.
11. AMENDMENT, MODIFICATION, AND TERMINATION.
- a.
- AMENDMENT, MODIFICATION, AND TERMINATION. Subject to Section 10(b) herein, the Board or the Committee may at any time and from time to time, alter, amend, suspend or terminate the Plan in whole or in part, except that, without approval of the stockholders of the Company, no such revision or amendment shall increase the number of shares available for grants of ISOs under the Plan or alter the class of participants in the Plan. Notwithstanding the foregoing, neither the Company nor the Board or Committee on its
E-11
12. WITHHOLDING.
- a.
- TAX WITHHOLDING. The Company shall have the power and the right to deduct or withhold, or require a Participant to remit to the Company, an amount sufficient to satisfy federal, state, and local taxes, domestic or foreign, required by law or regulation to be withheld with respect to any taxable event arising as a result of this Plan.
- b.
- SHARE WITHHOLDING. To the extent provided by the Committee, a Participant may elect to have any distribution to be made under this Plan to be withheld or to surrender to the Company shares of Common Stock already owned by the Participant to fulfill any tax withholding obligation.
13. SUCCESSORS.
All obligations of the Company under the Plan with respect to Awards granted hereunder shall be binding on any successor to the Company, whether the existence of such successor is the result of a direct or indirect purchase of all or substantially all of the business and/or assets of the Company, or a merger, consolidation or otherwise.
14. LEGAL CONSTRUCTION.
- a.
- GENDER AND NUMBER. Except where otherwise indicated by the context, any masculine term used herein also shall include the feminine; the plural shall include the singular; and, the singular shall include the plural.
- b.
- SEVERABILITY. In the event any provision of the Plan shall be held illegal or invalid for any reason, the illegality or invalidity shall not affect the remaining parts of the Plan, and the illegal or invalid provision shall be modified to the extent necessary to be legal and valid and shall be enforced as modified.
- c.
- REQUIREMENTS OF LAW. The granting of Awards and the issuance of Shares under the Plan shall be subject to all applicable laws, rules, and regulations, and to such approvals by any governmental agencies or national securities exchanges as may be required.
- d.
- SECURITIES LAW COMPLIANCE. With respect to Insiders, transactions under this Plan are intended to comply with all applicable conditions of Rule 16b-3 or its successors under the Exchange Act. To the extent any provision of the Plan or action by the Committee fails to so comply, it shall be deemed null and void, to the extent permitted by law and deemed advisable by the Committee.
E-12
- e.
- GOVERNING LAW. To the extent not preempted by federal law, the Plan, and all agreements hereunder, shall be construed in accordance with and governed by the laws of the state of Nevada.
15. RIGHTS AS A SHAREHOLDER.
No Participant to whom an Award has been granted shall have rights as a shareholder with respect to any Shares covered by such Award, except after due exercise of the Award and tender of the full purchase price for any Shares being purchased pursuant to such exercise and registration of the Shares in the Company's share register in the name of the Participant. Nothing in this Plan shall affect a Participant's employment or other engagement by the Company, including the Company's right to terminate such employment or engagement.
16. PURCHASE FOR INVESTMENT.
- a.
- Unless the offering and sale of the Shares to be issued upon the particular exercise of an Option shall have been effectively registered under the Securities Act of 1933 (the "1933 Act"), the Company shall be under no obligation to issue the Shares covered by such exercise unless and until the person(s) who exercise such Option shall represent and warrant to the Company, prior to the receipt of such Shares, that such person(s) are acquiring such Shares for their own respective accounts, for investment, and not with a view to, or for sale in connection with, the distribution of any such Shares. The person(s) acquiring such Shares shall be bound by the provisions of the following legend which shall be endorsed upon the certificate(s) evidencing their Shares issued pursuant to such exercise or such grant:
- The shares evidenced by this certificate have not been registered under the Securities Act of 1933, as amended, or under state securities laws to the extent applicable. The shares may not be sold, offered for sale, or otherwise transferred in the absence of an effective registration statement under said Act (and any registration or qualification as may be required under such state laws) or an opinion of counsel satisfactory to the company and its counsel that such registration or qualification is not required.
- b.
- The Company may delay issuance of the Shares until completion of any action or obtaining of any consent which the Company deems necessary under any applicable law (including, without limitation, state securities or "blue sky" laws).
E-13
Exhibit F
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-KSB/A
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2003 |
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period From to |
Commission File No. 0-23553
IMCOR PHARMACEUTICAL CO.
(Exact name of Registrant as specified in its Charter)
NEVADA
(State or other jurisdiction of incorporation or organization)
62-1742885
(I.R.S. Employer Identification No.)
6175 LUSK BOULEVARD
SAN DIEGO, CA 92121
(Address of principal executive offices) (Zip Code)
(858) 410-5601
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:None
Securities registered pursuant to Section 12(g) of the Act:common stock, par value $.001 per share
Check whether the issuer (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Check if there is no disclosure of delinquent filers in response to Item 405 of Regulation S-B is not contained in this form, and no disclosure will be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-KSB/A or any amendment to this Form 10-KSB/A. o
The issuer's revenues for its most recent fiscal year: $0
The approximate aggregate market value of voting and non-voting common stock held by non-affiliates computed as of March 30, 2004, based upon the last sale price of the common stock reported on the Nasdaq SmallCap Market was approximately $8,483,000. For purposes of this calculation only, the registrant has assumed that its directors and executive officers, and any person known to the issuer to hold 10% or more of the outstanding common stock, are affiliates.
The number of shares outstanding of each of the registrant's classes of common stock, as of March 30, 2004 was 21,541,476.
Documents Incorporated by Reference
Portions of the registrant's proxy statement to be filed with the Securities and Exchange Commission in connection with its 2004 Annual Meeting are incorporated into Part III of this Report.
PART I
IMCOR Pharmaceutical Co. and its 80.1% owned affiliate, Sentigen, Ltd., are collectively referred to as "IMCOR," the "company," "we," or "us." Portions of the discussion in this Form 10-KSB/A contain forward-looking statements and are subject to the "Risk Factors" described below in Item 1. On February 5, 2004, shareholders of the company approved a change in the company's name from Photogen Technologies, Inc. to IMCOR Pharmaceutical Co.
In November, 2002, we effected a one-for-four reverse split of our common stock and split off our therapeutic line of business. Unless otherwise indicated, all data (including historical numbers) in this Form 10-KSB/A reflect the impact of the reverse split and the split off of our therapeutic line of business.
ITEM 1. DESCRIPTION OF BUSINESS.
INTRODUCTION
We are an emerging, development-stage specialty pharmaceutical company focused on developing and marketing medical imaging pharmaceutical products. We have one FDA approved product,Imagent® (perflexane lipid microspheres), an ultrasound imaging contrast agent that we acquired in June 2003, and two additional contrast agents in development for use in computed tomography (CT) and x-ray imaging with potential applications (1) as a blood pool agent, to diagnose diseased tissue in the cardiovascular system and other organs, which we call PH-50, and (2) to diagnose cancer metastasizing into the lymphatic system, which we call N1177. The product designations PH-50 and N1177 refer to the same chemical entity. We established the two names to distinguish commercialization rights between cardiovascular imaging (and all other indications) (PH-50) to which we retain rights, and the field of lymphography (N1177), which we licensed to our joint venture (Sentigen, Ltd.) with affiliates of Elan. On February 5, 2004, we changed our name to IMCOR Pharmaceutical Co., reflecting the changed nature of our business to imaging pharmaceuticals.
In December 2001, we announced positive preclinical results of PH-50. Based on these studies, the extensive preclinical toxicology and other study work, including the product's use in approximately 65 patients for lymphography, we shifted the strategic research direction of IMCOR to focus on the cardiovascular and lymphography applications. We accomplished this shift in focus also by splitting off our therapeutic line of business, and, in June 2003 acquiring the medical imaging business (the "Imagentbusiness") of Alliance Pharmaceutical Corp. ("Alliance") led byImagent® (perflexane lipid microspheres), an FDA approved diagnostic imaging agent.
For the two years up to the time of our acquisition of theImagent business, substantially all of our research and development efforts were focused on the preclinical testing of PH-50. During that period, we operated with a very small staff and conducted substantially all of our research activities through contracts with academic institutions and contract research laboratories. In addition, we had conducted research and development activities on the development of certain therapeutic photodynamic therapy applications (namely a product we called PH-10 for the treatment of certain dermatological conditions) and the development of a medical laser. These operations were split off to our founding scientists as of November 12, 2002 and have been recorded as discontinued operations in our financial statements.
PH-50 and N1177 are still in early stages of development. Of these products, based on the availability of capital, we intend to focus our research efforts first on the continued development of PH-50 and secondly, N1177.
Pharmaceutical imaging agents are used with echocardiography, computed tomography ("CT"), nuclear medicine and magnetic resonance imaging ("MRI") equipment in order to increase the diagnostic capabilities of these techniques. By enhancing the contrast between different types of tissues, these intravenously administered contrast agents provide critical anatomical and disease state
1
information that is not obtainable using the equipment alone. There are numerous potential applications for a contrast agent used in imaging parts or all of the cardiovascular system as well as other organs. Our products have the potential to enhance a physician's ability to diagnose certain cardiovascular abnormalities and the spread of cancer in the lymphatic system.
IMCOR is based in San Diego, California. We have approximately 30 full time employees and a 53,000 square foot leased building, a portion of which is an FDA approved cGMP (current Good Manufacturing Practices) manufacturing facility, at our San Diego location. Our personnel have expertise in sales, marketing and manufacturing, clinical development, clinical trial design and regulatory approval processes. We also work closely with consultants experienced in drug development and regulatory requirements.
Our History
In May, 1997, IMCOR Pharmaceutical Co. (formerly known as Photogen Technologies, Inc., which was then known as M T Financial Group, Inc. ("M T Financial")), acquired Photogen, Inc. through a subsidiary merger. As a result, Photogen, Inc. became a wholly-owned subsidiary of M T Financial and then M T Financial changed its name to Photogen Technologies, Inc. (then subsequently on February 5, 2004 to IMCOR Pharmaceutical Co.). In November, 2002, we effected the split off of Photogen, Inc. and substantially all of the assets and all of the liabilities related to our therapeutic line of business, including a compound known as PH-10, to our five founding stockholders (Drs. Wachter, Dees, Fisher and Scott and Mr. Smolik, who are collectively referred to as the "Tennessee Stockholders") in exchange for and in cancellation of all of their stock in IMCOR (then known as Photogen Technologies, Inc. (5,137,109 shares)). At that time, we also sold $9.0 million of our common stock to a group of institutional investors at a price of $1.08 per share and effected a one-for-four reverse split of our common stock. In this offering, we received $6,500,000 in cash and converted Tannebaum, LLC's $2,500,000 credit facility into 2,314,815 shares of common stock at $1.08 per share. In December, 2002 and the beginning of January, 2003, we sold an additional approximately $3.0 million of our common stock at $1.08 per share to certain institutional investors and accredited individual investors. See "Corporate Restructuring" below.
Available Information
Our website (www.imcorpharma.com) is under development and at this time you cannot obtain copies of our SEC filings on our website; however, you can obtain, free of charge, a copy of our filed annual reports on Form 10-K or 10-KSB/A, our quarterly reports on Form 10-Q or 10-QSB, our current reports on Form 8-K, and any amendments to those reports, our proxy statements and information statements, by sending a request for those documents to our corporate secretary, Brooks Boveroux, at 6175 Lusk Boulevard, San Diego, CA 92121 or by calling (858) 410-5601. Our filings are also available to the public from the SEC's website at (www.sec.gov) or at the SEC's Public Reference Rooms in Washington D.C., New York, NY and Chicago, IL. The Public Reference Room in Washington, D.C. is located at 450 Fifth Street N.W. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room.
TECHNOLOGY
On June 18, 2003, we acquired assets, including Purchased Technology, related to the diagnostic imaging business, includingImagent, an FDA-approved product, of Alliance.Imagent is an intravenous contrast agent for use with ultrasound imaging equipment to improve visualization of blood vessels for better diagnosis of disease and assessment of organ function.Imagent consists of perfluorochemical-based "microbubbles" that are highly echogenic (reflect ultrasound signals strongly) in the bloodstream, thereby significantly enhancing ultrasound images. We also entered into a number of agreements related to that acquisition. Included in the purchase were an FDA-approved manufacturing facility, the
2
marketing, manufacturing and supporting infrastructure for the product and an intellectual property portfolio of approximately thirty patents in the area of ultrasound imaging.
Imagent has been approved for marketing by the FDA for use in patients with sub optimal echocardiograms to opacify the left ventricle and thereby improve visualization of the main pumping chamber of the heart, and to improve delineation of the endocardial borders of the heart. As a result, ultrasound withImagent may better distinguish normal and abnormal heart structure and function—two critical indicators of cardiac health. Echocardiography is the most widely used imaging modality for the diagnosis of heart disease, the leading cause of death in the U.S. The attractiveness of the ultrasound imaging market potential together with the acquisition of an approved product and an extensive intellectual property portfolio were fundamental reasons for the acquisition and primary determinants of the purchase price of approximately $7,240,000 for plant, property and equipment, $15,638,000 for the purchased technology, plus future earn out payments as described in the purchase agreement based uponImagent revenue. We will manufactureImagent at our facility in San Diego and plan to marketImagent through a sales relationship with units of Cardinal Health. We are currently seeking additional capital to operate theImagent business and to continue our development programs.
In 1999, we entered into an exclusive license for a group of proprietary materials, the lead compound of which was known as WN67722. At that time, the product was being developed only for the field of lymphography. We now call this product N1177 (for use in the field of lymphography) or PH-50 (when referring to cardiovascular and all other indications). The product designations PH-50 and N1177 refer to the same chemical compound. These materials are very small particles designed to travel through the circulatory or lymphatic system (depending on how they are administered) and are called "nanoparticulates." The compound is covered by patents and patent applications filed in the U.S. and elsewhere. As part of the initial acquisition of this product we also acquired patented methods for performing minimally invasive lymphography using x-ray, CT or MRI.
Cardiac and vascular imaging
According to 1998 statistics of the National Heart, Lung, and Blood Institute, ischemic heart disease is the leading cause of death in the U.S., accounting for one of every five deaths. The American Heart Association (www.americanheart.org) estimates that approximately 1.1 million Americans suffer a myocardial infarction ("MI") annually, and over 40 percent will not survive the event. Atherosclerotic coronary artery disease ("CAD") is the underlying cause of most ischemic cardiac events and can result in MI, congestive heart failure, cardiac arrhythmias and sudden cardiac death. Clinically significant CAD is uncommon in men under 40 and pre-menopausal women, but risk increases with advancing age and in the presence of risk factors such as smoking, hypertension, diabetes, high cholesterol and family history of heart disease. Angina is the most common presenting symptom of myocardial ischemia and underlying CAD, but in many persons the first evidence of CAD may be MI or sudden death. The American Heart Association estimates that 1-2 million middle-aged men have asymptomatic but physiologically significant coronary disease, also referred to as silent myocardial ischemia. Over two million angiography procedures are conducted annually in the U.S. Although mortality from heart disease has declined steadily over the past three decades in the U.S., the total burden of coronary disease is predicted to increase substantially over the next 30 years due to the increasing size of the elderly population.
Lymphography
Lymphography is a procedure used to determine if a patient's cancer has spread to the lymph nodes. The presence or absence of cancer in the lymph nodes is an important indication of whether the disease has spread from the original tumor and the seriousness of the disease. It also provides oncologists with critical information to help determine the stage of the disease and the appropriate course of treatment.
3
It is generally accepted that breast cancer and melanoma spread by way of the lymph system as do cancers of the lung, prostate and many other cancers. Clinical studies have demonstrated that patients with cervical cancer have an increased survival rate if lymph nodes with cancerous tissue are removed. Effective treatment of these cancers can be aided by an early assessment of the presence or absence of disease (micro-metastases) in the lymph nodes.
Patients believed to have cancer currently are subjected to injections of blue dye or radioactive particles that track the materials to the general area of the lymph node. In some of these diseases, the location of the lymph node is difficult to access. In the case of breast cancer, oncologists may remove 15-30 underarm lymph nodes for biopsy. Additional treatment will be required if cancer is discovered. Approximately 80% of the patients diagnosed with breast cancer or melanoma who undergo these painful procedures are found to have no sign of disease in their nodes.
The particular properties of N1177 enable it to travel through the lymph system to the lymph nodes most likely to be affected if the cancer has spread. It is recognized by the body's immune system as being a foreign body and thus is normally ingested by macrophage (cells that engulph foreign material in the body for eventual elimination). It is a particular characteristic of tumors that they contain no or a minimal number of macrophage. N1177 is not penetrated by x-rays ("radiopaque"), and thus is seen on a CT scan as white areas. Tumors within a node, as they would not have retained N1177, are seen as dark areas. The difference in contrast created by the presence or absence of the imaging agent serves as the basis to determine the presence or absence of disease. We expect that N1177 would remain in the node to allow for an effective imaging procedure with a good safety profile and acceptable clearance times. A further benefit of these minimally invasive procedures is that they could be used multiple times and therefore provide a means to monitor the results of cancer treatment and detect recurrence.
Products and Product Candidates
Imagent—FDA Approved Product
TheImagent business of Alliance we acquired included:
- •
- Imagent, an FDA-approved product;
- •
- an FDA-approved manufacturing facility;
- •
- the right to hire a 45-person operating organization; and
- •
- a portfolio of intellectual property in imaging technology and ultrasound imaging contrast agents, including over 30 patents.
As part of the acquisition, we also assumed a worldwide development and commercialization agreement forImagent with Schering that was amended in February, 2002 to give Alliance exclusive rights to market the product in the U.S. until February, 2007, with Schering to be paid a nominal royalty on such sales. As part of the assumed agreement we may also obtain exclusive rights to market the product internationally in exchange for the payment of additional royalties to Schering. At the expiration of the period, we have the right to pay all of any remaining royalty obligation to Schering and thus retain all rights to the product on a worldwide basis, or alternatively, to allow Schering the opportunity to obtain co-promotion rights along with us.
The FDA approvedImagent in May 2002 for use in imaging the heart via ultrasound (echocardiography) in patients who are difficult to image using standard non-contrast procedures due to obesity, lung disease, body habitus, or other circumstances. Follow-on indications include use for perfusion (measuring defects in the heart muscle tissues), body imaging for detection of cancer in solid organs (liver, kidney, prostate, etc.), as well as for vascular diseases (carotid arteries, peripheral veins, etc.). Additional clinical studies are planned to support routine use in both cardiology and body
4
imaging applications. Currently,Imagent qualifies for reimbursement by the Centers for Medicare & Medicaid Services in all medical settings for cardiology.
We have assumed the rights and obligations of Alliance under the terms of a long-term supply agreement that Alliance had entered for the principal raw material forImagent. Although some raw materials forImagent are currently qualified from only one source and we have some inventory, we will attempt to acquire additional inventory of these materials and to negotiate long-term supply arrangements. Raw materials used inImagent cannot be changed without equivalency testing of any new material by us and approval of the FDA. Our business could be materially and adversely affected if we are unable to obtain qualified raw materials on a timely basis and at a cost-effective price.
In December 2003 we entered into a license agreement with Kyosei Pharmaceutical Co., Ltd., a unit of Sakai Group, for the development and marketing ofImagent in Japan for all radiology and cardiology indications. Terms of the agreement call for the payment to IMCOR of up to $10 million in fees and development milestone payments plus royalties on commercial sales. Kyosei will also pay IMCOR to manufactureImagent for Kyosei's clinical and commercial requirements.
Development of PH-50
We are developing PH-50 to extend the clinical utility of x-ray based imaging, chiefly CT. The indications we envision for PH-50 include: CT angiography (of the heart and peripheral blood vessels), assessment of whole-body disease burden of atherosclerosis, organ perfusion and cancer staging. PH-50 is a radiopaque contrast agent. When injected systemically into the circulatory system (i.e., the blood stream), it remains within the system mixing with the blood and flowing throughout the circulatory system. As the compound is radiopaque, a physician using a device such as a CT machine along with the contrast agent can create an image of the desired area. In our preclinical studies we have obtained encouraging results that have been demonstrated by imaging heart, liver and other organs with results that are comparable or superior to those obtained by currently available products. We believe that PH-50 could allow the imaging of the cardiovascular system (angiography) and other organs as a non-invasive procedure, at lower cost and with reduced risk and morbidity.
We are completing pre-clinical studies of PH-50 for potentially significant new applications in cardiovascular imaging and have filed for additional patent protection of the use of the compound for vascular and organ imaging applications. To date, we have incurred a total of approximately $2,531,000 of expenses in connection with the development of PH-50 (approximately 438,000, 1,228,000 and 865,000 for the years ended December 31, 2001, 2002 and 2003, respectively). We plan to develop PH-50 as a blood pool contrast agent for several indications. Based on considerable existing preclinical studies and human clinical experience, we believe that we can commence further clinical studies in the near future. Our initial development strategy will be to evaluate PH-50 as a contrast agent in Phase 1 studies for cardiovascular and blood pool imaging using advanced CT. We plan to conduct Phase 1 trials to assess safety and patient tolerance of PH-50 as a cardiovascular imaging agent through intravenous administration following completion of remaining preclinical studies and approval of the FDA to conduct Phase 1 clinical studies. As part of the original acquisition and our further research, we have a substantial body of safety information in both animals and humans. Pre-clinical toxicology and pharmacology data for PH-50/N1177 includes testing in multiple animal models including studies in several models utilizing intravenous administration, which is the route of administration to be utilized in the cardiovascular indications. In animal experiments, our investigators from Stanford University have shown that PH-50 can be used as an effective imaging agent to visualize cardiac structures after a single, low-dose intravenous injection/infusion. We have an open investigational new drug application ("IND") with the FDA for the product in the lymphography indication and have filed an amendment to this application for the intravenous administration of PH-50 for use as a blood pool agent.
5
Following Phase 1, and upon approval of the FDA, we would conduct Phase 2 and, if successful, Phase 3 studies; and then, assuming successful completion of the studies, prepare a New Drug Application submission to the FDA. The timing and costs of this work is dependent upon several factors including the structure of the studies as approved by the FDA, the number of patients that would be required to conduct and achieve statistical significance in these studies, the time required to recruit and study qualified patients, the program priority relative to other activities of the company, and thus the amount of capital to be allocated to the program, and other factors. Due to the significant variability of the nature and timing of these studies, the overall timing and cost is not presently estimable, but we believe that it would take several years with overall costs in the range of $10 to $12 million. We expect that completion of these studies and approval would require at least six years prior to obtaining significant net cash inflows.
The conduct of these studies involves significant risk, and we cannot assure the product's ultimate success. Due to the nature of obtaining FDA approval to continue into any stage of clinical testing and the time required to recruit and test patients, significant delays could result. Should the product fail to achieve clinical success in any stage of development, we would have to carefully review the data to determine whether re-testing should be attempted, re-formulation or other alternatives be considered or whether the program should be terminated. While termination of a program would substantially reduce future capital needs, other than for shut-down expenses, the absence of the product could materially, negatively impact the then future operating plans for the company.
Scaled-up manufacturing of PH-50/N1177 is performed for us by Elan through its Nanosystems division. Nanosystems has undertaken substantial work to manufacture PH-50 in compliance with the FDA's current Good Manufacturing Procedures ("GMP") regulations and in a scalable process sufficient to support commercial quantities. Currently, Nanosystems is our sole supplier of the raw materials necessary to produce PH-50 and N1177. There can be no assurance that Nanosystems will continue to supply us with the raw materials we need on terms that are acceptable to us.
Development of N1177
We are developing N1177 through a joint venture with affiliates of Elan. Our goal is to demonstrate that the use of N1177 will enable physicians to precisely locate tumorous nodes by first injecting the material in the vicinity of the tumor and then taking an image using a CT device. Phase 1 clinical studies to evaluate the safety of N1177 in a total of 45 patients have been completed.
Considerable preclinical testing of N1177 has been conducted in multiple animal models. The results of these studies suggest a favorable safety and efficacy profile for N1177. The results of the Phase I study involving 45 subjects indicate the safety and pharmacokinetic data are consistent with safety, pharmacokinetic and metabolic data obtained in previous nonclinical safety and disposition studies. In addition, the number of nodes visualized showed a statistically significant dose effect (an increase in the number of nodes visualized as the dose increased).
Research and development work has been charged to the joint venture. To date, we have incurred a total of approximately $3,130,000 of expenses in connection with the development of N1177 (approximately $1,160,000, $658,000 and $0 incurred by the joint venture for the years ended December 31, 2001, 2002 and 2003, respectively). Due to capital constraints, no research was conducted on N1177 during 2003.
We plan to commence Phase 2 clinical studies for detection of cancer metastasis in lymph nodes in patients with cervical cancer, subject to the availability of sufficient capital. In this patient population, studies document an increase in survival when lymph nodes containing cancer are removed. Tumor identification by CT imaging will be correlated to the results of biopsy results from patients undergoing surgery to demonstrate effectiveness in locating nodes and identifying those with cancer in them. Following Phase 2, and upon approval of the FDA, we would conduct Phase 3 studies; and then,
6
assuming successful completion of the studies, prepare a New Drug Application submission to the FDA. The timing and costs of this work is dependent upon several factors including the nature of the cancer to be studied, the structure of the studies as approved by the FDA, the number of patients that would be required to conduct and achieve statistical significance in these studies, the time required to recruit and study qualified patients, the program priority relative to other activities of the company, and thus the amount of capital to be allocated to the program, and other factors. Due to the significant variability of the nature and timing of these studies, the overall timing and cost is not presently estimable, but we estimate that it would take several years with overall costs in the range of $10 to $12 million. We expect that completion of these studies and approval would require at least six years prior to obtaining significant net cash inflows, presuming that we obtain adequate capital to conduct the research in a timely manner.
The conduct of these studies involves significant risk, and no assurance can be made of the product's ultimate success. Due to the nature of obtaining FDA approval to continue into any stage of clinical testing and the time required to recruit and test patients, significant delays could result. Should the product fail to achieve clinical success in any stage of development, we would have to carefully review the data to determine whether re-testing should be attempted, re-formulation or other alternatives be considered or whether the program should be terminated. While termination of a program would substantially reduce future capital needs, other than for shut-down expenses, the absence of the product could materially, negatively impact the then future operating plans for the company.
Sentigen Ltd.—Joint Venture with Elan Corporation
In October, 1999, we entered into a joint venture with affiliates of Elan. The joint venture company, Sentigen Ltd., is developing N1177, a nanoparticulate agent used to image lymph nodes to detect cancer. We own 80.1 percent of Sentigen with the balance being owned by affiliates of Elan. We licensed to Sentigen certain methods and other proprietary technology for use in lymphography, and we sub-licensed to Sentigen proprietary compounds we had obtained by license from Massachusetts General Hospital and Nycomed Imaging AS. Elan, through its drug delivery division, Elan Pharmaceutical Technologies, licensed to Sentigen its NanoCrystal™ stabilized nanoparticulate formulation technology to develop the diagnostic imaging agents. Continued development of N1177 is subject to the receipt of adequate capital to finance its operations.
To finance the initial capital requirements of Sentigen, including the licensing by Sentigen of certain technology, we sold to a unit of Elan $12,015,000 of our Series A Convertible Preferred Stock (the "Series A Preferred Stock"). For the first six years following issuance (i.e., through October 2005) Elan may exchange the initial 12,015 shares of Series A Preferred Stock for a number of shares of Sentigen stock that will enable Elan to increase its ownership of Sentigen up to 50 percent. Alternatively, during that period Elan may convert the Series A Preferred Stock into IMCOR common stock at a conversion price of $84.68 per share.
As part of the joint venture agreement, Elan provided us with a $4.8 million credit facility to fund our share of the then anticipated development costs of the joint venture. On November 12, 2002, all of the then outstanding principal and interest ($3,082,487) was converted at a price of $24.00 per share into 128,437 shares of our common stock and the line of credit was cancelled.
Our agreement with Elan provides that Elan has the right to nominate a member to our Board of Directors until October, 2004 or as long as Elan owns 10% of our common stock (Elan currently owns less than 10% of our common stock). Elan has not exercised this right with respect to our current board of directors.
7
CORPORATE RESTRUCTURING—November 2002
Split Off Transaction
From our inception, we were involved in two lines of business: diagnosis of diseased tissue and the treatment of diseased tissue. In the past, our research and development efforts followed this dual focus. We worked to develop therapies to treat cancer, pre-cancerous conditions, psoriasis and other dermatological conditions, using our proprietary multi-photon laser technologies, or using an agent called PH-10 with a variety of energy sources in a regimen called photodynamic therapy, or the activation of a substance by an energy source to achieve a therapeutic effect. We had also been developing products, N1177 and PH-50, to diagnose the spread of cancer to a patient's lymph nodes and to diagnose cardiovascular disease, respectively.
Our pursuit of both diagnostic and therapeutic lines of businesses led to a number of challenges. We found it increasingly difficult to raise capital with such a diverse product pipeline and to concentrate our efforts on a particular application from inception through development. In order to attract capital investment and to take advantage of what we perceived to be the best market opportunities for our proposed products, we concluded that it was necessary to devote our efforts and resources to the development of our medical imaging diagnostic technologies rather than the therapeutic technologies.
We entered into a Separation Agreement with the Tennessee Stockholders in order to enhance our ability to secure additional financing and pursue market opportunities, to enable the Tennessee Stockholders to focus on the development of therapeutic technologies arising from their inventions, and to resolve any differences of opinion with them as to how to allocate financial and management resources. We separated the therapeutic and diagnostic lines of business by first transferring substantially all assets and liabilities associated with the diagnostic business to IMCOR and retaining substantially all assets and liabilities associated with the therapeutic business in Photogen, Inc. Then, IMCOR, Photogen, Inc. and the Tennessee Stockholders engaged in a "split off" transaction in which the Tennessee Stockholders transferred all of their common stock of IMCOR to the company for redemption in exchange for all of the common stock of Photogen, Inc. The Tennessee Stockholders beneficially owned 5,137,109 shares of IMCOR (formerly known as Photogen Technologies, Inc.) common stock (approximately 52.9% of the outstanding shares), and those shares were subsequently cancelled by the company.
Dr. Wachter, one of the Tennessee Stockholders, resigned as a director and officer of IMCOR upon the effectiveness of the split off transaction. Dr. Scott, also a Tennessee stockholder, resigned as an officer of IMCOR upon the effectiveness of the split off transaction.
Financing Transaction
In November, 2002, we sold $9,000,000 of our common stock at a price of $1.08 per share to a group of institutional investors which included Mi3, L.P. ("Mi3"), Oxford BioScience Partners IV L.P. and MRNA Fund II L.P. (collectively, the "Institutional Investors"). In this offering, we received $6,500,000 in cash and converted Tannebaum, LLC's $2,500,000 credit facility into 2,314,815 shares of common stock at $1.08 per share. Tannebaum, LLC was at that time controlled in part by Dr. Weinstein, then one of our directors. In December, 2002, we consummated a second closing of the financing transaction and sold an additional 2,804,539 shares of our common stock at $1.08 per share for an aggregate of $3,028,902 to a group of institutional investors and individual accredited investors. The final closing of the financing took place in January, 2003 at which time we sold an additional 27,778 shares of our common stock at $1.08 per share for an aggregate of $30,000 to two individual accredited investors. As part of the financing transaction, we entered into a Registration Rights Agreement requiring us to file a registration statement with the SEC within 45 days of the closing of the financing transaction to cover the Institutional Investors' sales of the shares. The Institutional
8
Investors in the financing transaction have deferred the implementation of this requirement. As part of the financing transaction, the Institutional Investors entered into a Voting, Drag-Along and Right of First Refusal Agreement ("Voting Agreement") with Dr. Weinstein, Stuart Levine (individually and as co-trustees of the Theodore Tannebaum Trust) and Tannebaum, LLC (collectively, the "Chicago Stockholders"). The Voting Agreement provides, among other things, that each of its parties will vote all shares of which it is the beneficial owner as follows:
- •
- To maintain the total number of directors at seven (we currently have five directors).
- •
- To amend our Articles of Incorporation or Bylaws in accordance with the recommendation of five of the seven directors.
- •
- For the election of the following directors:
- •
- One person nominated by Mi3;
- •
- One person nominated by the holders of 80% of the shares owned by the Institutional Investors;
- •
- One person nominated by the holders of 80% of the shares owned by the Chicago Stockholders;
- •
- One person appointed by our Board of Directors as the Chief Executive Officer;
- •
- One person nominated by Elan International Services, Ltd. ("EIS") in accordance with and subject to our October 20, 1999 Securities Purchase Agreement with EIS (EIS has not exercised this right with respect to our current board of directors);
- •
- Two persons (or three if EIS's right to nominate a director expires) with industry experience in the healthcare, biotech or pharmaceutical industries, as nominated by our Board of Directors and approved by the holders of 80% of the shares beneficially owned by the Institutional Investors; and
- •
- To maintain an Audit Committee and Compensation Committee of the Board, each with three members, and to appoint one director to serve on each Committee nominated by the holders of 80% of the shares owned by the Institutional Investors.
The Voting Agreement also provides that none of its parties will sell, transfer, pledge, grant any option for, or otherwise dispose of any of their shares of company stock except in compliance with the Voting Agreement.
As conditions to the closing of the financing transaction:
- •
- All of our Series B Convertible Preferred Stock (the "Series B Preferred Stock") was converted into common stock at the initial closing on November 12, 2002 of the financing transaction. In exchange for this conversion we agreed that the conversion ratio of the Series B Preferred Stock would change from 1.44703 (the conversion ratio after taking into consideration all issuances of common stock before the institutional financing) to 4.25 (pre-reverse split). The result was that all of the Series B Preferred Stock was converted into a total of approximately 422,316 shares of common stock; the Series B Preferred Stock is no longer outstanding, nor is there any liquidation preference associated with the Series B Preferred Stock, and
- •
- All of the outstanding principal and accrued interest under the Elan line of credit was converted into a total of 128,437 shares of our common stock.
Elan Agreement with the Institutional Investors
We are parties to a joint venture with affiliates of Elan to develop lymphography technologies carried out through Sentigen, Ltd. As part of the joint venture arrangements, among other things, we
9
issued EIS (i) 115,385 shares of our common stock and a warrant to acquire additional shares of common stock, (ii) 12,015 shares of our Series A Preferred Stock, and (iii) a convertible note in the original principal amount of $4,806,000. After issuance, the Series A Preferred Stock was transferred to Elan Pharmaceutical Investments II, Ltd. ("EPI"). The principal and interest outstanding under the promissory note ($3,082,487.30) were converted into 128,437 shares of our common stock in connection with the November, 2002 financing.
The Certificate of Designations, Rights and Preferences (the "Certificate of Designations") of the Series A Preferred Stock provides that upon liquidation, dissolution or winding up of the company (whether voluntary or involuntary), the holder of the Series A Preferred Stock is entitled to a liquidation preference (the "Series A Liquidation Preference") of cash or other property out of funds legally available for distribution to our stockholders, before any distribution is made to the holders of common stock or other securities ranking junior to the Series A Preferred Stock. The amount of the Series A Liquidation Preference is $1,000 per share.
One of the conditions to the closing of the November 2002 financing transaction was that the holder of our Series A Preferred Stock amend the Certificate of Designations to eliminate the Series A Liquidation Preference. As of November 12, 2002, the total Series A Liquidation Preference was $15,892,220. In consideration of eliminating the Series A Liquidation Preference, the Series A conversion price would have been lowered to $12.00 from $84.68 per share.
Immediately before our 2002 annual meeting of stockholders, we were informed that the holder of the Series A Preferred Stock could not proceed with these amendments to the terms of the Series A Preferred Stock. To induce the Institutional Investors to proceed with the closing of the financing, on November 12, 2002, Elan entered into an agreement (the "Elan Agreement") with the Institutional Investors. The Elan Agreement provides that Elan will pay the Institutional Investors an amount in cash (or the fair market value of other property) distributed to the holder of the Series A Preferred Stock on account of the Series A Liquidation Preference that, in the absence of the Series A Liquidation Preference, would have been paid to those Institutional Investors in accordance with applicable law, up to a maximum of $16,000,000. Only the Institutional Investors are beneficiaries of the Elan Agreement. The Elan Agreement was ratified, adopted and approved by the IMCOR common stockholders on January 24, 2003. The Series A Liquidation Preference remains in effect and the conversion price for the Series A Preferred Stock is still $84.68 per share.
Amendment of the 2000 Long Term Incentive Compensation Plan
We adopted the 2000 Long Term Incentive Compensation Plan (the "2000 Plan") at our annual meeting of stockholders held in May, 2000. As of July 15, 2002, 500,000 shares were reserved for issuance under the 2000 Plan and options covering 133,750 of these shares of common stock had been granted. Our Board of Directors believed that the availability of additional options to purchase common stock was necessary to enable us to continue to provide our key employees with equity ownership as an incentive to contribute to our success. Also, increasing the number of shares subject to the 2000 Plan was a condition to the closing of the institutional financing required by the Institutional Investors. Thus, our Compensation Committee voted to amend the 2000 Plan to increase the shares covered by the 2000 Plan by 4,068,750 shares from 500,000 to 4,568,750; and to modify the definition of "cause" in the 2000 Plan to coordinate with employment agreements. The stockholders approved the amendment of the 2000 Plan to increase the aggregate number of shares of common stock issuable under the 2000 Plan from 500,000 to 4,568,750 and approved the amendment of the definition of the term "cause" at our annual meeting of the stockholders held on October 31, 2002. In addition, the Compensation Committed approved a reduction in the shares reserved under our Senior Executive Long Term Incentive Compensation Plan by 250,000 shares (the total number reserved is now capped at 1,000,000 shares which was effective concurrently with the increase under the 2000 Plan).
10
DEVELOPMENT AND COMMERCIALIZATION STRATEGY
Our overall strategy is to leverage our proprietary technologies and to utilize the company as a platform for the acquisition and/or licensing of technologies in related fields using our internal organization supplemented by contractual collaborations with third parties. In particular (based upon the receipt of adequate capital resources), we plan to:
- •
- Manufacture and, through our relationship with units of Cardinal Health, marketImagent in the United States. We expect to negotiate and, if successful, enter into relationships with third parties for the development and marketing ofImagent outside the U.S.
- •
- Conduct preclinical research studies of our products using third party laboratories and/or clinicians to conduct preclinical trials. We intend to use the expertise of these third party clinicians to collect data necessary to obtain IND's (an Investigational New Drug application with the FDA) required to begin human trials.
- •
- Utilize our clinical development team and contractual collaborative arrangements with third parties to access the skills and resources required to design, test, obtain regulatory approval and manufacture our products in development.
- •
- Market potential products through the use of an internal sales force or strategic or distribution collaborations as determined by the specific characteristics of each product application.
We do not have internal marketing and sales capabilities. Our strategy is to establish contract relationships for the marketing, sales, and distribution services or use our collaborative partners to market and sell any products that we develop successfully. Currently,Imagent will be marketed and distributed in the United States through agreements with Cardinal. Various Cardinal companies will provide packaging, sales, and distribution services. To the extent that we enter into co-promotion or other licensing arrangements, any revenues received by us will be dependent on the efforts of third parties, and there can be no assurance that any such efforts will be successful. Further, there can be no assurance that we will be able to enter into future marketing relationships on acceptable terms. The termination of any marketing relationships may limit our ability to market our products, thereby materially adversely affecting our business.
Should we have to market and sell our products directly, we would need to develop a marketing and sales force with technical expertise and distribution capability. The creation of an infrastructure to commercialize pharmaceutical products is an expensive and time-consuming process. There can be no assurance that we would be able to establish the necessary marketing and sales capabilities or be successful in gaining market acceptance for our products.
Imagent is manufactured in San Diego at our commercial-scale facility. It is manufactured using a proprietary process to form dry, PFC vapor-containing microspheres that are reconstituted with an aqueous solution to form microbubbles just prior to use. We have a long-term supply agreement for the principal raw material forImagent. Although some raw materials for our products are currently qualified from only one source, we attempt to acquire a substantial inventory of such materials and to negotiate long-term supply arrangements. Raw materials used in our products cannot be changed without equivalency testing of any new material by us and approval of the FDA. No assurances can be given that we can maintain long-term supplies of these materials on terms acceptable to us. Our business could be materially and adversely affected if we are unable to obtain qualified raw materials on a timely basis and at a cost-effective price.
Our ability to develop and manufacture any product successfully is subject to numerous contingencies and uncertainties, including certain "Risk Factors" described below. Our objectives, business strategy and product development efforts are subject to change based on numerous factors, including the results of preclinical and clinical testing, the availability of suitable collaborative
11
relationships, the nature of competition, regulatory requirements and the availability of capital. The description of our business and business strategy contains forward-looking statements that should be read in conjunction with the "Risk Factors" described below.
PATENTS
We are continuing to pursue patent protection for our proprietary technologies with the U.S. Patent and Trademark Office and in various foreign jurisdictions. We plan to prosecute, assert and defend our patent rights whenever appropriate. However, securing patent protection does not necessarily assure us of competitive success. See "Risk Factors—If we do not obtain and maintain patent or other protection of our core technologies (namelyImagent and PH-50 for cardiovascular imaging and N1177 for our lymphography materials and methods), we may have difficulty commercializing products using these technologies," below.
We have several issued U.S. patents and patent applications related toImagent. The issued patents and pending applications contain claims directed to the composition, manufacture, and use of novel stabilized microbubble compositions based on the discovery that PFC (perfluorocarbon) gases, in combination with appropriate surfactants or other non-PFC gases, can stabilize microbubbles for use in ultrasonic imaging. Foreign applications directed to the same subject matter are also granted or pending. We also have five issued U.S. patents covering the use of various contrast agents, includingImagent, in harmonic imaging.
On June 18, 2003, we filed a complaint against Amersham Health, Inc. and two other Amersham affiliates alleging that certain Amersham products infringe on claims in our patents coveringImagent. The case is captioned asPhotogen Technologies, Inc. and Alliance Pharmaceutical Corp. v. Amersham Health Inc. et. al., Civil Action No. 03CV2853(SRC), in the United States District Court for the District of New Jersey. Our complaint alleges that principally through their Optison® product, Amersham Health Inc. and related Amersham entities infringe on seven patents owned by us. We are also seeking a declaration that the claims of fourteen Amersham patents are invalid and are not infringed by ourImagent product. Alliance, the party from whom we recently acquired theImagent product, is also a plaintiff in the suit. We are seeking damages, an injunction against Amersham, a declaratory judgment and other relief.
Amersham filed an answer dated July 18, 2003 denying the material allegations of our claims and asserting certain affirmative defenses and counterclaims. Amersham's counterclaims against us include claims of patent infringement, breach of contract, breach of good faith and fair dealing, and tortuous interference with contract. We are studying Amersham's answer, defenses and counterclaims in consultation with our counsel.
Intellectual property disputes are often settled through licensing arrangements that could be costly to us. In any intellectual property litigation, it is possible that licenses necessary to settle the dispute would not be available on commercially reasonable terms or that we would not be able to redesign our technologies to avoid any claimed infringement thus resulting in our inability to sell the product.
On July 29, 2003, the European Patent Office revoked Amersham's European Patent (EP) No. 620744 with claims directed to contrast agents. We had brought the opposition proceeding against the EP '744 patent in the European Patent Office. The EP '744 is a counterpart to the patents that are the subject of our suit against Amersham in the United States described above. See "Risk Factors—We filed a suit against Amersham for patent infringement, which, if resolved unfavorably, could impede our ability to utilize our patents forImagent," below.
We also own two U.S. patents related to N1177 and we currently have two patent applications pending in the U.S. and one application filed under the Patent Cooperation Treaty ("PCT") which are
12
directed to the use of nanoparticulates including PH-50 for cardiovascular imaging and for delivery of pharmacologically active substances.
We have an exclusive license (subject to certain limitations) from Massachusetts General Hospital and Nycomed Imaging AS for the world-wide right to make, market, distribute and sell products using (i) the subject matter covered in pending U.S. and foreign patent applications relating to the research, development, manufacture and commercialization of diagnostic imaging agents for medical location, treatment and diagnosis of tumors and other diseased tissues or other material and (ii) the patent rights related to N1177 in the research, development, manufacture and commercialization of human ethical pharmaceutical products containing the diagnostic imaging compound N1177.
In addition, through our joint venture with affiliates of Elan, we have granted Sentigen a license of certain methods we had previously acquired from Alliance and a sublicense of our MGH/Nycomed license for use in the research, development, manufacture and commercialization of nanoparticulate x-ray, CT and/or MRI diagnostic imaging agents using radio-opaque molecules containing iodine that passively target lymph nodes involved in a diseased state following parenteral administration to a mammal to locate, diagnose and/or treat cancer and/or other diseases.
We are continuing to pursue patent protection for our other proprietary technologies with the U.S. Patent and Trademark Office, under the Patent Cooperation Treaty and in various foreign jurisdictions.
Aside from our issued patents, no assurance can be given that any of these applications will result in issued U.S. or foreign patents. Although patents are issued with a presumption of validity and require a challenge with a high degree of proof to establish invalidity, no assurance can be given that any issued patents would survive such a challenge and would be valid and enforceable.
Some of our patented or licensed technology arose from research that was partially funded by the U.S. Government ("Government"). As with other entities whose research is sponsored in any manner by the Government, certain patents that we own or license are subject to Confirmatory Licenses in favor of the Government, as required by applicable federal regulations. These regulations require us or the patent owner to agree to convey to the Government, upon the Government's request, rights in the technology if we decide not to continue prosecution of the patent applications or if the patent owner does not wish to retain title to the inventions covered in the patents. The Government also retains certain "march in rights" that permit the Government to use the technology under certain circumstances, including if that action is necessary because we or the patent owner have not taken or are not expected to take effective steps to achieve practical application of the inventions; it is necessary to alleviate health or safety needs not being met by us or the patent owner; or to meet the requirements for public use specified in certain federal regulations and those requirements are not being met by us or the patent owner. We intend to prosecute patent applications and in other respects develop patents we own so that the Government will not exercise its rights under these Confirmatory Licenses.
We also attempt to protect our proprietary products, processes and other information by relying on trade secret laws and non-disclosure and confidentiality agreements with our employees, consultants, and certain other persons who have access to such products, processes and information. The agreements affirm that all inventions conceived by employees are the exclusive property of the Company. Nevertheless, there can be no assurance that these agreements will afford significant protection against or adequate compensation for misappropriation or unauthorized disclosure of our trade secrets.
"IMCOR" is a trademark andImagent® is a registered trademark of IMCOR. We have filed an application for registration of the mark IMCOR in the U.S. This application is pending. All other trademarks or trade names used in this Form 10-KSB/A are trademarks or trade names of their respective owners.
13
GOVERNMENT REGULATIONS
All of the products we currently contemplate developing require approval by the FDA prior to sales being made within the U.S. and by comparable foreign agencies prior to sales being made outside the U.S. The FDA and comparable regulatory agencies impose substantial requirements on the manufacturing and marketing of pharmaceutical products and medical devices. These agencies and other entities extensively regulate, among other things, research and development activities and the testing, manufacturing, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion of our proposed products. See "Risk Factors—Our proposed products are subject to extensive testing and government approval, and we may not obtain or maintain the approvals necessary to sell our proposed products," below.
The regulatory process required by the FDA through which our products must successfully pass before they may be marketed in the U.S. generally involves the following:
- •
- Preclinical laboratory and animal testing;
- •
- Submission of an application that must become effective before clinical trials may begin;
- •
- Adequate and well-controlled human clinical trials to establish the safety and efficacy of the product for its intended indication; and
- •
- FDA approval of the application to market a given product for a given indication.
For imaging and pharmaceutical products, preclinical tests include laboratory evaluation of the product, its chemistry, formulation and stability, as well as animal studies to assess the potential safety and efficacy of the product. Where appropriate (for example, for human disease indications for which there exist inadequate animal models), we will attempt to obtain preliminary data concerning safety and efficacy of proposed products using carefully designed human pilot studies. We will require sponsored work to be conducted in compliance with pertinent local and international regulatory requirements, including those providing for Institutional Review Board approval, national governing agency approval and patient informed consent, using protocols consistent with ethical principles stated in the Declaration of Helsinki and other internationally recognized standards. We expect any pilot studies to be conducted outside the U.S.; but if any are conducted in the U.S., they will comply with applicable FDA regulations. Data obtained through pilot studies will allow us to make more informed decisions concerning possible expansion into traditional FDA-regulated clinical trials.
If the FDA is satisfied with the results and data from preclinical tests, it will authorize human clinical trials. Human clinical trials are typically conducted in three sequential phases, which may overlap. Each of the three phases involves testing and studying specific aspects of the effects of the pharmaceutical on human subjects, including testing for safety, dosage tolerance, side effects, absorption, metabolism, distribution, excretion and clinical efficacy.
Data from preclinical and clinical trials are submitted to the FDA in an NDA for marketing approval and to foreign regulatory authorities under applicable requirements. Preparing an NDA or foreign application involves considerable data collection, verification, analyses and expense, and there can be no assurance that the applicable regulatory authority will accept the application or grant an approval on a timely basis, if at all. The marketing of pharmaceuticals in the U.S. may not begin without FDA approval. The approval process is affected by a number of factors, including primarily the safety and efficacy demonstrated in clinical trials and the severity of the disease. Regulatory authorities may deny an application in their sole discretion, if they determine that applicable regulatory criteria have not been satisfied or if additional testing or information is required. One of the conditions for initial marketing approval, as well as continued post-approval marketing, is that a prospective manufacturer's quality control and manufacturing procedures conform to the GMP regulations of the regulatory authority. In complying with these regulations, a manufacturer must continue to expend
14
time, money and effort in the area of production, quality control and quality assurance to ensure full compliance. Manufacturing establishments, both foreign and domestic, also are subject to inspections by or under the authority of the FDA and by other federal, state, local or foreign agencies. Discovery of previously unknown problems with a product or manufacturer may result in restrictions on such product or manufacturer, including withdrawal of the product from the market.
We have established a core clinical development team and have been working with outside regulatory consultants to assist us in developing product-specific development and approval strategies, preparing the required submittals, guiding us through the regulatory process, and providing input to the design and site selection of human clinical studies. The testing and approval process requires substantial time, effort and financial resources, and we may not obtain FDA approval on a timely basis, if at all. Success in preclinical or early-stage clinical trials does not assure success in later stage clinical trials. The FDA or the research institution sponsoring the trials may suspend clinical trials or may not permit trials to advance from one phase to another at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Once issued, the FDA may withdraw a product approval if we do not comply with pertinent regulatory requirements and standards or if problems occur after the product reaches the market. If the FDA grants approval of a product, the approval may impose limitations, including limits on the indicated uses for which we may market a product. In addition, the FDA may require additional testing and surveillance programs to monitor the safety and/or effectiveness of approved products that have been commercialized, and the agency has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs. Further, later discovery of previously unknown problems with a product may result in restrictions on the product, including its withdrawal from the market. Marketing our products abroad will require similar regulatory approvals by equivalent national authorities and is subject to similar risks.
In the ordinary course of business, we must also comply with a variety of other federal and state governmental regulations. These regulations impose, among other things, standards of conduct, record keeping, labeling and reporting.
Specific regulations affecting our current and proposed operations include: environmental-type discharge requirements, good laboratory practices governing animal testing, good manufacturing practices regarding the manufacture of drugs and other FDA-regulated products, animal care and use regulations, laws and regulations relating to labor, and required
general business practices. We do not currently anticipate that the cost of compliance in these areas, other than obtaining FDA approval, will present a major obstacle to achieving our goals.
Another area of regulation that will impact our business is the recent developments in health care reimbursement and delivery practices as a means to better control health care costs. See "Risk Factors—Changes in health care reimbursement policies or legislation may make it difficult for patients to use or receive reimbursement for using our products, which could reduce our revenues," below.
COMPETITION
The industry in which we operate is intensely competitive, and subject to significant change with respect to technology for the diagnosis and treatment of disease. Existing or future pharmaceutical and device companies, government entities and universities may create developments that accomplish similar functions to our technologies in ways that are less expensive, receive faster regulatory approval or receive greater market acceptance than our potential products. We expect that competition in the ultrasound contrast imaging agent field (and for our other potential products) will be based primarily on each product's safety profile, efficacy, stability, ease of administration, breadth of approved indications, and physician, healthcare payor and patient acceptance. See "Risk Factors—The markets for imaging products are extremely competitive, and many of our competitors have greater resources
15
and have products that are in more advanced stages of development than we do, which may give them a competitive advantage over us," below.
Our competitors have, in general, been in existence for considerably longer than we have, and may have greater capital resources and access to capital; greater internal resources for activities in research and development, clinical testing and trials, production and distribution; and existing collaborative relationships with third parties. See "Risk Factors—We have to rely on third parties and collaborative relationships for the marketing, manufacturing and clinical testing ofImagent and our proposed products, and it may be difficult to implement our business development plans without these collaborations," below.
The existing market for radiopaque contrast agents is estimated by Frost & Sullivan to be approximately $3.4 billion worldwide. The dominant uses of media, totaling 86 percent, are those employed in conjunction with CT or x-ray scans. Approximately half of the usage is in the U.S. Omnipaque®, marketed by a unit of Amersham, is believed to be the leading agent utilized in coronary angiography. Other companies marketing contrast agents include Mallinckrodt, Inc. (a unit of Tyco International Ltd.), Berlex Laboratories, Inc. (a subsidiary of Schering) and Bracco.
In addition to CT or x-ray scans, other modalities, such as MRI and ultrasound, are also used by physicians to image internal vasculature and organs. These modalities, including CT imaging, each have particular attributes that may make their use applicable to any particular situation.
Competition forImagent
There are two competitive ultrasound contrast agents approved in the U.S. for use in cardiology:Optison is sold by Amersham, andDefinity is sold by Bristol-Myers Squibb Medical Imaging.Optison consists of microspheres comprised of a perfluorocarbon gas surrounded by a shell of human serum albumin (HSA).Definity is a perfluorocarbon gas in a liquid solution that also must be kept refrigerated.
We filed a complaint against Amersham Health, Inc. and two other Amersham affiliates alleging that certain Amersham products infringe on claims in our patents coveringImagent. We also seek a declaration that our patents do not infringe on Amersham's in this area, and other relief. Alliance joined us as a plaintiff in this suit. Amersham filed an answer denying the material allegations of our claims and asserting certain counterclaims. If the suit is resolved unfavorably to us, we may have difficulty utilizing the protections of our patents forImagent and there may be other complications that may make it difficult to implement our commercialization strategy forImagent.
Intellectual property disputes are often settled through licensing arrangements that could be costly to us. In any intellectual property litigation, it is possible that licenses necessary to settle the dispute would not be available on commercially reasonable terms or that we would not be able to redesign our technologies to avoid any claimed infringement thus resulting in our inability to sell the product.
Bracco International has filed an NDA in the U.S. late in 2000 forSonoVue®, and the application is under review. The product is a dry powder of negatively charged liposomal microbubbles containing sulfur hexafluoride gas, to be reconstituted with saline prior to administration.
POINT Biomedical was scheduled to complete a 600-patient Phase 3 myocardial perfusion study forCardioSphere at the end of 2003.CardioSphere consists of a two-layer microspheres with one layer that contains human albumin.
Acusphere initiated a Phase 3 myocardial perfusion study in December 2003 with AI-700. The product is composed of a synthetic biodegradable polymer filled with a perfluorocarbon gas, and requires refrigeration.
16
Competition for PH-50
The existing market for radiopaque contrast agents is estimated to be approximately $3.4 billion worldwide. The dominant uses of media, totaling 86 percent, are those employed in conjunction with CT or x-ray scans. Approximately half of the usage is in the U.S. We believe that Omnipaque®, marketed by a unit of Amersham, is the leading agent utilized in coronary angiography. Other companies marketing contrast agents include Mallinckrodt, Inc. (a unit of Tyco International Ltd.), Berlex Laboratories, Inc. (a subsidiary of Schering AG ("Schering")) and Bracco. Physicians also use other modalities, such as MRI and ultrasound, to image internal vasculature and organs. These modalities, including CT imaging, each have particular attributes that may make their use applicable to any particular situation.
Competition for N1177
As with PH-50, N1177 will be subject to competition with other companies marketing contrast agents such as Amersham, Mallinckrodt, Inc. (a unit of Tyco International Ltd.), Berlex Laboratories, Inc. (a subsidiary of Schering), and Bracco, and with other methods of imaging, such as MRI and ultrasound.
RISK FACTORS
This Form 10-KSB/A contains (and press releases and other public statements we may issue from time to time may contain) a number of forward-looking statements regarding our business and operations. Statements in this document that are not historical facts are forward-looking statements. Such forward-looking statements include those relating to:
- •
- our current business and product development plans,
- •
- our future business and product development plans,
- •
- the timing and results of regulatory approval for proposed products, and
- •
- projected capital needs, working capital, liquidity, revenues, interest costs and income.
Examples of forward-looking statements include predictive statements, statements that depend on or refer to future events or conditions, and statements that may include words such as "expects," "anticipates," "intends," "plans," "believes," "estimates," "should," "may," or similar expressions, or statements that imply uncertainty or involve hypothetical events.
Forward-looking statements involve known and unknown risks and uncertainties that may cause our actual results in future periods to differ materially from what is currently anticipated. We make cautionary statements in certain sections of this Form 10-KSB/A, including under "Risk Factors." You should read these cautionary statements as being applicable to all related forward-looking statements wherever they appear in this Form 10-KSB/A, in the materials referred to in this Form 10-KSB/A, in the materials incorporated by reference into this Form 10-KSB/A, or in our press releases or other public statements. In light of these risks, uncertainties and assumptions, the forward-looking events discussed in this Form 10-KSB/A or other documents incorporated by reference might not occur. No forward-looking statement is a guarantee of future performance and you should not place undue reliance on any forward-looking statement. We do not undertake any obligation to release publicly any revisions to these forward looking statements, to reflect events or circumstances after the date of this Form 10-KSB/A, or to reflect the occurrence of unanticipated events, except as may be required under applicable securities laws.
17
The following are the key risk factors that may affect our future results:
We must raise additional financing in the future and our inability to do so will prevent us from continuing as a going concern and implementing our business development plan.
Our cash and securities on hand at December 31, 2003, following expenditures incurred by our operations and the acquisition of theImagent business, including the financing of that business pending its acquisition, was approximately $1,658,000. We have expended all this cash and are currently funding our operations through the issuance of secured promissory notes to one or more of our principal institutional investors. We will need substantial additional financing to continue operations including our manufacturing, marketing and product development programs.
We cannot accurately estimate the amount of additional financing required to develop our products. In particular, we are currently seeking capital in one or more transactions to fund our immediate and longer-term capital needs through a private placement to accredited investors. Additional funds may not be available on acceptable terms, if at all, and existing stockholders may be diluted as a result of those offerings. The pricing of our common stock, or other securities convertible into common stock, in any such transaction may also result in an increase in the number of shares of common stock issuable upon exercise of warrants in accordance with the anti-dilution provisions in the instruments governing those securities.
We are currently in default under obligations to pay $775,000 to creditors of Alliance Pharmaceutical Co. in connection with our acquisition ofImagent and we are in default under an equipment lease and the lessor has demanded approximately $787,000 in remaining lease payments and other fees. We also have not registered the resale of shares we issued in connection with that acquisition, which in some cases is a condition to the effectiveness of agreements to release us from any claims by the Alliance creditor.
Prior to our acquisition of theImagentassets, we have conducted only limited studies on our products in development and we do not have any revenues from sales; accordingly, we are a development stage company.
Our company and our technologies, other than our recently acquiredImagent product, are in early stages of development. We began our business as a biopharmaceutical company in 1997. To date, we have not generated material revenues from sales or operations. We expect to generate revenue from sales ofImagent, but there can be no assurance that we will be able to generate enough revenue from such sales to be profitable for at least several years, if at all.
The products we currently contemplate developing will require costly and time-consuming research and development, preclinical and clinical testing and regulatory approval before they can be commercially sold. We may not be able to develop our technology into marketable products or develop our technology so it is effective for diagnosis or treatment of human diseases. As a result of changing economic considerations, market, clinical or regulatory conditions, or clinical trial results, we may shift our focus or determine not to continue one or more of the projects we are currently pursuing.
We have a history of losses, we are in a net deficit position (our liabilities, puttable share obligations and mezzanine equity, combined, exceed our assets) and we may not achieve or maintain profitability in the future or pay cash dividends.
We have incurred losses since the beginning of our operations. As of December 31, 2003, we have incurred cumulative net losses (before dividends on preferred stock) of approximately $51,519,000. We expect our losses to increase in the future as our financial resources are used for manufacturing, marketing, research and development, preclinical and clinical testing, regulatory activities, and other related expenses. We may not be able to achieve or maintain profitability in the future. We have never
18
declared or paid any cash dividends to stockholders and do not expect to do so in the foreseeable future.
At December 31, 2003, we had a net deficit position (our liabilities, puttable share obligations and mezzanine equity, combined, exceed our assets) totaling $8,194,743. Among other factors, we have reclassified $12,105,000 of our Series A Preferred Stock from permanent to mezzanine equity to acknowledge the holder's right to exchange that security for that number of additional shares of Sentigen to increase its ownership of Sentigen to 50%. As discussed below, we believe that the probability of the holder exercising the exchange right is remote. Please see "Management's Discussion and Analysis or Plan of Operation—Liquidity; Capital Resources", below.
The markets for imaging products are extremely competitive, and many of our competitors have greater resources and have products that are in more advanced stages of development than we do, which may give them a competitive advantage over us.
In addition toImagent, there are currently two FDA approved ultrasound contrast agents being marketed in the U.S. for certain cardiology applications by Amersham Health, Inc. and Bristol-Myers Squibb. In addition, Bracco International has filed a New Drug Application with the FDA seeking approval to market its product. Two other companies (POINT Biomedical and Acusphere) are in advanced clinical trials for the use of ultrasound contrast agents for assessing certain organs and vascular structures. We expect that competition in the ultrasound contrast imaging field will be based primarily on each product's safety profile, efficacy, stability, ease of administration, breadth of approved indications, and physician, healthcare payer and patient acceptance. We are at the very early stages of launching theImagent product, and we cannot predict whether it will compete successfully with these other products.
We filed a suit against Amersham for patent infringement, which, if resolved unfavorably, could impede our ability to utilize our patents forImagent.
We filed a complaint against Amersham Health, Inc. and two other Amersham affiliates alleging that certain Amersham products infringe on claims in our patents coveringImagent. We also seek a declaration that our patents do not infringe on Amersham's in this area, and other relief. Alliance joined us as a plaintiff in this suit. Amersham filed an answer denying the material allegations of our claims and asserting certain counterclaims. If the suit is resolved unfavorably to us, we may have difficulty utilizing the protections of our patents forImagent and there may be other complications that may make it difficult to implement our commercialization strategy forImagent.
Intellectual property disputes are often settled through licensing arrangements that could be costly to us. In any intellectual property litigation, it is possible that licenses necessary to settle the dispute would not be available on commercially reasonable terms or that we would not be able to redesign our technologies to avoid any claimed infringement thus resulting in our inability to sell the product.
Our common stock could be delisted from the NASDAQ SmallCap Market, which would make trading in our common stock more difficult.
Since November 1999, our common stock has been quoted in the Nasdaq SmallCap Market. Our shares could be delisted if we fail to meet the listing requirements of the Nasdaq SmallCap Market, which would force us to list our shares on the OTC Bulletin Board or some other quotation medium, such as pink sheets, depending upon our ability to meet the specific listing requirements of those quotation systems. As a result, an investor might find it more difficult to dispose of, or to obtain accurate price quotations for, our shares. Delisting might also reduce the visibility, liquidity and price of our common stock.
On August 26, 2003, September 8, 2003, and January 7, 2004 we received notices from Nasdaq that we did not meet specified listing criteria. We have corrected the identified deficiencies to Nasdaq's
19
satisfaction and Nasdaq has notified us that it has withdrawn its delisting notice subject to our meeting the shareholder meeting requirement and all other listing standards by mid-May 2004.
If our common stock were delisted from the Nasdaq SmallCap Market and were not traded on another national securities exchange, we could become subject to penny stock regulations that impose additional sales practice disclosure and market making requirements on broker/dealers who sell or make a market in our stock. The rules of the SEC generally define "penny stock" to be common stock that has a market price of less than $5.00 per share and is not traded on a national exchange. If our stock became subject to penny stock regulations, it could adversely affect the ability and willingness of broker/dealers who sell or make a market in our common stock and of investors to purchase or sell our stock in the secondary market.
Our proposed products are subject to extensive testing and government approval, and we may not obtain or maintain the approvals necessary to sell our proposed products.
Other thanImagent, none of our proposed imaging products has received the FDA's approval for commercial sales. An extensive series of clinical trials and other associated requirements must be completed before our proposed products can be approved and sold in the U.S. or other countries. Requirements for FDA approval of a product include preclinical and clinical testing for effective use and safety in animals and humans, which can be extremely costly. The time frame necessary to perform these tasks for any individual product is long and uncertain, and we may encounter problems or delays that we cannot predict at this time. Even if testing is successful, our proposed products may not demonstrate sufficient effectiveness or safety to warrant approval by the FDA or other regulatory authorities. Any regulatory approval may not cover the clinical symptoms or indications that we may seek. We must also obtain regulatory approvals comparable to those required in the U.S. to market our products in other countries.
Other thanImagent, our proposed technologies and products generally must complete preclinical tests in animals and three phases of tests (also called clinical trials) in humans before we can market them for use. Use of our technology has been limited primarily to laboratory experiments and animal testing; only N1177 has completed Phase 1 human clinical trials. We have not yet conducted substantive studies of our compounds in development on their effectiveness on human subjects.
Our results from early clinical trials may not predict results that we will obtain in large-scale clinical trials, as a number of companies have suffered significant setbacks in advanced clinical trials, even after promising results in early trials.
We may not conduct additional Phase 2 or Phase 3 clinical trials for our products and such trials, if begun, may not demonstrate any efficacy or may not be completed successfully in a timely manner, if at all.
The rate of completion of our clinical trials depends upon, among other things, the rate of patient enrollment. Patient enrollment is a function of many factors, including the size of the patient population for a particular indication, the nature of the clinical protocol under which our products will be studied, the proximity of the patient to a clinical site and the eligibility criteria for the study. Delays in planned patient enrollment may result in increased costs, regulatory filing delays, or both.
Furthermore, we, the FDA or other regulatory authorities may alter, suspend or terminate clinical trials at any time. If we do not successfully complete clinical trials and obtain regulatory approval for a product, we will not be able to market that product.
Any products approved by the FDA are subject to postmarket requirements of the FDA.
Our FDA approved product,Imagent, is subject to numerous postmarket requirements by regulatory authorities. We will be subject to inspection and marketing surveillance by the FDA to
20
determine our compliance with regulatory requirements with respect to any approved products. If the FDA finds that we have failed to comply, it can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
- •
- fines, injunctions and civil penalties;
- •
- recall or seizure of our products;
- •
- operating restrictions, partial suspension or total shutdown of production; and
- •
- criminal prosecution.
Any enforcement action by the FDA may also affect our ability to commercially distribute our products in the U.S.
Product liability claims could increase our costs and adversely affect our results of operations.
The marketing and clinical testing ofImagent and our product candidates may expose us to product liability claims, and we may experience material product liability losses in the future. We maintain certain levels of product liability insurance for the use of our products, but our coverage may not continue to be available on terms acceptable to us or adequate for liabilities we actually incur. A successful claim brought against us in excess of available insurance coverage, or any claim or product recall that results in significant adverse publicity against us, may have a material adverse effect on our business and results of operations.
If we do not obtain and maintain patent or other protection of our core technologies (namely Imagent and PH-50 for cardiovascular imaging and N1177 for lymphography), we may have difficulty commercializing products using these technologies.
Our success depends in part on our ability to obtain, assert and defend our patents, protect trade secrets and operate without infringing the intellectual property of others. Among the important risks in this area are that:
- •
- our patent applications may not result in issued patents. Moreover, any issued patents may not provide us with adequate protection of our intellectual property or competitive advantages, and the law on the scope of patent coverage is continually changing.
- •
- various countries limit the subject matter that can be patented and limit the ability of a patent owner to enforce patents in the medical field. This may limit our ability to obtain or utilize those patents internationally.
- •
- existing or future patents or patent applications (and the products or methods they cover) of our competitors (or others, such as research institutions or universities) may interfere, invalidate, conflict with or infringe our patents or patent applications. Similarly, the use of the methods or technologies contained in our patents, patent applications and other intellectual property may conflict with or infringe the rights of others.
- •
- if an advance is made that qualifies as a joint invention, the joint inventor or his or her employer may have rights in the invention.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that these rights are covered by valid and enforceable patents or effectively maintained as trade secrets. We acquired an extensive patent portfolio related toImagent as part of the acquisition of theImagent business. We also own two patents in the U.S., and certain other patents in foreign countries including Australia, Canada, Japan and Germany that relate to methods for performing lymphography.
21
Other than the patents and patent applications we acquired through the purchase of theImagent Business, we have filed patent applications in the U.S. and under the Patent Cooperation Treaty covering a number of foreign countries. These patent applications relate to the use of nanoparticulates, including PH-50, as cardiovascular imaging agents and to deliver pharmacologically active substances for treatment of various diseases. We are also the exclusive licensee of a group of patented proprietary compounds known as "nanoparticulates," including N1177 and PH-50 from Nycomed Imaging AS. We are the exclusive licensee of U.S. and foreign patent applications from Massachusetts General Hospital which relate to diagnostic imaging agents and methods for using the diagnostic imaging agents for medical location, treatment and diagnosis of tumors and other diseased tissues.
Additional patents may never be issued on any of the patent applications we own or license from third parties. Furthermore, even if such patents are issued, the validity of the patents might successfully be challenged by a third party. The patents might not provide protection against competitive products or otherwise be commercially valuable, or the applications filed by others might result in patents that would be infringed by the manufacture, use or sale of our products.
The patent position of biopharmaceutical companies involves complex legal and factual questions and therefore we cannot assure the enforceability of these patents. Litigation over patents and other intellectual property rights occurs frequently in our industry and there is a risk that we may not prevail in disputes over the ownership of intellectual property. Further, an interference proceeding with the Patent and Trademark Office may be instituted over the rights to certain inventions and there is a risk that we may not prevail in such an interference proceeding. Those disputes can be expensive and time consuming, even if we prevail. Intellectual property disputes are often settled through licensing arrangements that could be costly to us. In any intellectual property litigation, it is possible that licenses necessary to settle the dispute would not be available on commercially reasonable terms or that we would not be able to redesign our technologies to avoid any claimed infringement thus resulting in our inability to sell the product.
Confidentiality agreements covering our intellectual property may be violated and we may not have adequate remedies for any violation. Third parties may challenge our existing patents and seek to hold them invalid or unenforceable. Also, our trade secret intellectual property may in other ways become known or be independently discovered by competitors.
To the extent we use intellectual property through licenses or sub-licenses (as is the case for some of our lymphography technology), our rights are subject to us performing the terms of the license or sub-license agreement with third parties. Our rights are also subject to the actions of third parties we may not be able to control, such as our sub-licensor complying with the terms of its license with the patent owner and the patent owner maintaining the patent.
Where intellectual property results from a research project supported by government funding, the government has limited rights to use the intellectual property without paying us a royalty.
We are highly dependent upon a small number of employees and consultants who provide management expertise, and it may be difficult to implement our business operations and development plans without this expertise.
These individuals have entered into employment or consulting agreements, confidentiality and/or non-competition agreements with us. We could suffer competitive disadvantage, loss of intellectual property or other material adverse effects on our business and results of operations if any employee or consultant violates or terminates these agreements or terminates his or her association with us. Our growth and future success also depends upon the continued involvement and contribution from these individuals, as well as our ability to attract and retain highly qualified personnel now and in the future.
22
We currently employ three senior executive officers: Dr. Williams (our Chief Executive Officer), Mr. Boveroux (our Chief Financial Officer) and Mr. DeFranco (our Sr. Vice President Marketing and Business Development). We also have retained consultants to advise us in regulatory affairs and product development matters. If we lost the services of our executive officers or outside consultants, we could experience a delay in the implementation of our business plan until we arranged for another individual or firm to fulfill the role.
The raw materials forImagent are subject to a long-term supply contract with one supplier. The raw materials necessary for the manufacture of PH-50 and N1177 are supplied by one supplier. An interruption in availability of those materials may impair our ability to market or test those products.
We have assumed the rights and obligations of Alliance under the terms of the long-term supply agreement that Alliance has entered for the principal raw material forImagent. Although some raw materials forImagent are currently qualified from only one source and we have some inventory, we will attempt to acquire additional inventory of these materials and to negotiate long-term supply arrangements. Raw materials used inImagent cannot be changed without equivalency testing of any new material by us and approval of the FDA.
We currently rely on Nanosystems, a division of Élan, as the sole supplier of the raw nanoparticulates used to manufacture PH-50 and N1177. There can be no assurance that Nanosystems will continue to supply us with the raw materials that we need in a timely manner on conditions that are acceptable to us.
We may encounter difficulties starting or expanding our manufacturing operations.
We are leasing a single manufacturing facility located in San Diego, California. This facility is subject to a variety of quality systems regulations, international quality standards and other regulatory requirements, including requirements for good manufacturing practices, or current good manufacturing procedures. We may encounter difficulties expanding our manufacturing operations in compliance with these regulations and standards, which also could result in a delay or termination of manufacturing or an inability to meet product demand.
We will face risks inherent in operating a single facility for the manufacture ofImagent. At this time, we do not have alternative production plans in place or alternative facilities available should the San Diego manufacturing facility cease to function. These risks include unforeseen plant shutdowns due to personnel, equipment or other factors, and the resulting inability to meet customer orders on a timely basis.
We have to rely on third parties and collaborative relationships for the marketing, manufacturing and clinical testing ofImagent and our proposed products, and it may be difficult to implement our business development plans without these collaborations.
We currently have an agreement for sales and marketing ofImagent by Cardinal Health, Inc. We have had and expect to continue in the future to have a variety of research agreements with universities and other research institutions to investigate specific protocols. We also contract and expect to continue to contract with research organizations and other third parties to manage clinical trials of our products. We must obtain and maintain collaborative relationships with third parties for research and development, preclinical and clinical testing, marketing and distribution of our proposed products.
We are currently involved in a joint venture with affiliates of Élan, called Sentigen, Ltd., to develop and commercialize materials in the field of lymphography. Collaborative relationships may limit or restrict our operations or may not result in an adequate supply of necessary resources. Our collaborative partner could also pursue alternative technologies as a means of developing or marketing products for the diseases targeted by our collaborative program. If a third party we are collaborating
23
with fails to perform under its agreement or fails to meet regulatory standards, this could delay or prematurely terminate clinical testing of our proposed products.
Our products may not be fully accepted by physicians, laboratories and health insurance providers, which would reduce our revenue and limit our ability to raise capital.
Our growth and success will depend upon market acceptance by physicians, laboratories and health insurance providers of our products. This requires acceptance of our products as clinically useful and cost-effective alternatives to other medical imaging methods. In addition, physicians may utilize imaging techniques other than those for which our products are being developed (such as magnetic resonance imaging (MRI)) to image internal vasculature and organs and therefore, our products may not be utilized.
Changes in health care reimbursement policies or legislation may make it difficult for patients to use or receive reimbursement for using our products, which could reduce our revenues.
Our success will depend, in part, on the extent to which health insurers, managed care entities and similar organizations provide coverage or reimbursement for using the medical procedures we have and plan to develop. These third-party payers are increasingly challenging the price of medical procedures and services and establishing guidelines that may limit physicians' selections of innovative products and procedures. We also cannot predict the effect of any current or future legislation or regulations relating to third-party coverage or reimbursement on our business. We may not be able to achieve market acceptance of our proposed products or maintain price levels sufficient to achieve or maintain any profits on our products if adequate reimbursement coverage is not available. Failure by physicians, hospitals and other users of our products to obtain sufficient reimbursement from third-party payers for the procedures in which our products would be used or adverse changes in governmental and private third-party payers' policies toward reimbursement for such procedures would have a material adverse effect on our business.
A small group of stockholders control IMCOR, which may make it difficult for stockholders who are not in that group to influence management.
As of December 31, 2003, a small group of stockholders control approximately 55.4% of our outstanding common stock (including shares issuable, following shareholder approval, related to the acquisition of theImagent Business). These stockholders are also parties to a Voting, Drag-Along and Right of First Refusal Agreement (the "voting agreement") concerning the election of certain designees to the Board of Directors and certain other corporate actions. The Voting Agreement requires these stockholders to vote their shares for the election of certain individuals nominated by parties to the Voting Agreement. This concentration of ownership and control may delay or prevent a change in control of IMCOR, and may also result in a small supply of shares available for purchase in the public securities markets. These factors may affect the market and the market price for our common stock in ways that do not reflect the intrinsic value of our common stock.
The price and trading volume of our common stock fluctuates significantly, like that of many biopharmaceutical companies, which may make it difficult for us or a stockholder to sell our common stock at a suitable price and may cause dilution for existing stockholders when we issue additional shares.
During the period from January 1, 2003 through December 31, 2003 the closing trade price of our common stock ranged from $2.73 to $0.90 per share. Daily trading volume ranged from zero shares to approximately 42,900 shares during that period.
24
The market prices for securities of biopharmaceutical companies in general have been highly volatile and may continue to be highly volatile in the future. The following factors may have an impact on the price of our common stock:
- •
- Sales performance ofImagent
- •
- Announcements by us or others regarding scientific discoveries, technological innovations, commercial products, patents or proprietary rights;
- •
- Announcements by us concerning the licensing or other transactions of our products or technologies;
- •
- Private sale of a significant block of our common stock at a price below the then current market price of our common stock and the subsequent registration and sale of those shares;
- •
- The progress of preclinical or clinical testing;
- •
- Developments or outcome of litigation concerning proprietary rights, including patents;
- •
- Changes in government regulation;
- •
- Public concern about the safety of devices or drugs;
- •
- Limited coverage by securities analysts;
- •
- The occurrence of any of the risk factors described in this section;
- •
- Sales of large blocks of stock by an individual or institution;
- •
- Changes in our financial performance from period to period, securities analysts' reports, and general market conditions; and
- •
- Economic and other external factors or a disaster or crisis.
If stockholders holding substantial amounts of our common stock should sell their stock in the public market, the price of our stock could fall and it may be more costly for us to raise capital.
We have reserved shares of common stock for future issuance upon grants of options, or exercise or conversion of outstanding options and warrants and convertible securities. In addition, some of our shares are eligible for sale in the public market free of restriction under Rule 144 of the Securities Act. Others shares of our common stock are subject to agreements requiring us to permit the holders of the shares, under certain circumstances, to join in a public offering of our stock or to demand that we register their shares for resale. The sale of these shares could place downward pressure on the overall market price of our common stock.
If our options and warrants are all issued and exercised, investors may experience significant dilution in the voting power of their common stock. The sale of these shares could also place downward pressure on the overall market price of our common stock.
ITEM 2. DESCRIPTION OF PROPERTY.
We are located in San Diego, California, in a leased facility of approximately 53,000 square feet. At this facility we maintain our executive offices, perform research and development, and manufactureImagent. We have obtained regulatory approval to manufactureImagent at this production facility. To our knowledge, the property and equipment is in good condition.
25
ITEM 3. LEGAL PROCEEDINGS.
On June 18, 2003, we filed a complaint against Amersham Health, Inc. and two other Amersham affiliates alleging that certain Amersham products infringe on claims in our patents coveringImagent. The case is captioned asPhotogen Technologies, Inc. and Alliance Pharmaceutical Corp. v. Amersham Health Inc. et. al., Civil Action No. 03CV2853(SRC), in the United States District Court for the District of New Jersey. Our complaint alleges that principally through their Optison® product, Amersham Health Inc. and related Amersham entities infringe on seven patents owned by us. We are also seeking a declaration that the claims of fourteen Amersham patents are invalid and are not infringed by ourImagent product. Alliance, the party from whom we recently acquired theImagent product, is also a plaintiff in the suit. We are seeking damages, an injunction against Amersham, a declaratory judgment and other relief.
Amersham filed an answer dated July 18, 2003 denying the material allegations of our claims and asserting certain affirmative defenses and counterclaims. Amersham's counterclaims against us include claims of patent infringement, breach of contract, breach of good faith and fair dealing, and tortuous interference with contract. We are studying Amersham's answer, defenses and counterclaims in consultation with our counsel.
On July 29, 2003, the European Patent Office revoked Amersham's European Patent (EP) No. 620744 with claims directed to contrast agents. We had brought the opposition proceeding against the EP '744 patent in the European Patent Office. The EP '744 is a counterpart to the patents that are the subject of our suit against Amersham in the United States described above. See "Risk Factors—We filed a suit against Amersham for patent infringement, which, if resolved unfavorably, could impede our ability to utilize our patents forImagent," above.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
None.
26
PART II.
ITEM 5. MARKET FOR COMMON EQUITY, RELATED STOCKHOLDER MATTERS, AND SMALL BUSINESS ISSUER PURCHASES OF EQUITY SECURITIES.
Our common stock is traded from time to time on the Nasdaq SmallCap Market under the symbol "ICPHC." Upon the timely holding of our 2004 annual shareholder meeting, we expect that our symbol will become "ICPH".
Common Stock
As of March 30, 2004, there were 21,541,476 shares of our common stock outstanding and held by approximately 405 stockholders of record. Holders of our common stock are entitled to receive such dividends as may be declared by the Board of Directors. We have not declared or paid cash dividends on our common stock and we do not anticipate paying any cash dividends in the foreseeable future.
The high and low trading prices (adjusted to reflect our one-for four reverse split) for our common stock during each quarter of the last two fiscal years are set forth below.
| | Year Ended
December 31, 2002
(Amounts in $)
| | Year Ended
December 31, 2003
(Amounts in $)
|
|---|
| | High
| | Low
| | High
| | Low
|
|---|
| 1st Quarter | | 6.36 | | 2.52 | | 2.73 | | 0.90 |
| 2nd Quarter | | 5.20 | | 3.12 | | 2.60 | | 1.50 |
| 3rd Quarter | | 4.20 | | 1.32 | | 2.44 | | 0.97 |
| 4th Quarter | | 2.64 | | 1.00 | | 1.93 | | 1.07 |
For the period January 1, 2004 through March 30, 2004, the high and low trading prices for our common stock were $1.95 and $1.10, respectively. Aggregate trading volume for the period January 1, 2004 through March 30, 2004, was approximately 1,079,660 shares. The foregoing information was obtained from the National Association of Securities Dealers as reported on the Nasdaq SmallCap Market. The quotations generally reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions. The foregoing information reflects trade prices, and not bid or ask prices and has been adjusted to reflect our one-for-four reverse split that was effective November 22, 2002. See "Risk Factors—A small group of stockholders control IMCOR, which may make it difficult for stockholders who are not in that group to influence management" and "The price and trading volume of our common stock fluctuates significantly, like that of many biopharmaceutical companies, which may make it difficult for us or a stockholder to sell our common stock at a suitable price and may cause dilution for existing stockholders when we issue additional shares," above, regarding the possible effects of the concentrated ownership of our stock on the market and price of the stock.
In 2003, we granted qualified and non-qualified stock options pursuant to our 2000 Plan to purchase an aggregate of 1,840,250 shares of common stock each at an exercise price of $1.25 per share to employees joining the Company as part of theImagent business acquisition and two options for 10,000 shares each granted at exercise prices of $0.93 and $2.05, respectively. The options vest at various times up to four years from the date of the grant.
In July 2003, we granted our Chief Executive Officer options to acquire 1,050,000 shares of our common stock at an exercise price of $1.08 per share, subject to shareholder approval. Shareholders holding over 50% of our common stock have committed to vote in favor of this option.
During 2003, we made the following issuances of our common stock. These securities were offered and sold by us in reliance upon exemptions from the registration requirements provided by Section 4(2)
27
of the Securities Act and Regulation D under the Securities Act relating to sales not involving any public offering:
- •
- Pursuant to the terms of the Standstill and Make Whole Agreement dated as of December 30, 2002, in each of January, February and March 2003, we issued 250,000 shares (an aggregate of 750,000 shares) to Xmark in exchange for Xmark's agreement to forbear from exercising its remedies as a creditor against Alliance.
- •
- Pursuant to the terms of a Consulting Agreement dated as of January 30, 2003, we issued up to 75,000 shares of our common stock to a consultant, subject to certain forfeiture provisions, in exchange for consulting services. Forfeiture provisions for 56,250 of these shares have expired through December 31, 2003.
- •
- In January 2003 we sold 27,778 shares of our common stock for cash at $1.08 per share to certain accredited investors.
- •
- On May 27, 2003, we issued an aggregate of 11,250 shares to Xmark as a penalty for failing to register for resale the shares of common stock issued to Xmark.
- •
- In June 2003, we issued an aggregate of 10,000 shares of our common stock pursuant to cashless exercise of options.
- •
- In connection with the acquisition of theImagent business, we issued an aggregate of 2,198,137 shares of our common stock to various creditors of Alliance (who qualify as accredited investors) on June 18, 2003. These shares were issued without registration under the Securities Act at prices ranging from $1.00 to $2.30 per share. An additional 1,987,500 shares (pursuant to this transaction) are issuable at $2.00 to $2.30 per share to certain accredited investors following approval by our shareholders. This approval was obtained at our shareholder meeting of February 5, 2004, and the shares have subsequently been issued.
- •
- In July 2003 we issued 27,216 shares of our common stock as a cashless exercise of certain warrants.
- •
- In July 2003 we issued 82,977 shares of our common stock to Xmark for interest and penalty for failing to timely register shares of common stock issued to Xmark.
- •
- In September 2003 we issued 26,248 shares of our common stock to Xmark as a penalty for failing to timely register shares of common stock issued to Xmark.
In June 2003 we recorded the issuance of 124,627 shares of our common stock that had previously been subject to rescission.
In addition, in May and June, 2003, we issued revolving convertible senior secured promissory notes to three of our investors in an aggregate principal amount of $4,160,000 bearing interest at 7.25% annually, compounded monthly. In the second, third and fourth quarters of 2003 we issued senior secured promissory notes, payable upon demand, to these investors in an aggregate principal amount of $7,257,000 bearing interest at 7.25% annually, compounded monthly.
We are subject to six sets of registration rights agreements. In the aggregate, these agreements cover approximately 15,028,000 shares of common stock (including 2,050,000 shares of common stock issuable pursuant to stock options) and require us to register those shares under various terms and subject to specific conditions in the agreements.
Pursuant to the terms of the Securities Purchase Agreement dated as of October 20, 1999 between IMCOR and Elan International Services, Ltd. ("EIS"), EIS had the right to participate in any equity financing consummated by us in order to maintain its pro rata interest in the company. This right expired on October 20, 2003.
28
As of March 30, 2004, approximately 19,056,000 shares of our common stock are "restricted stock" or are beneficially owned by persons who as of March 30, 2004 were affiliates of the company as defined in Rule 144 under the Securities Act. This excludes approximately 128,437 shares of common stock held by EIS, 115,360 shares of common stock held by Elan Pharma International Limited ("EPIL") and 16,314 shares of Series A Preferred Stock issued or accrued to EPIL. Although Elan has the right to elect one director, they have chosen not to place anyone on the current board of directors at this time. For purposes of this calculation only (and the comparable disclosure on the cover page of this Form 10-KSB/A) we have assumed that officers, directors, 10% stockholders and stockholders subject to the voting agreement dated as of November 12, 2002 are affiliates of the company that stock held by Oxford Bioscience Partners IV L.P. and MRNA Fund II L.P. would be deemed to be beneficially owned by Jonathan Fleming, and stock held by Mi3, L.P. would be deemed beneficially owned by William D. McPhee, each being one of our directors (although both Mr. Fleming and Mr. McPhee disclaim beneficial ownership of those respective shares for all other purposes), and that a person beneficially owns stock subject to an option, warrant or convertible instrument that is exercisable or convertible within 60 days from March 15, 2004. A portion of those shares would be eligible for resale by company affiliates and others who have satisfied the requisite holding periods, subject to the volume limitations and other provisions of Rule 144 and applicable law. As of March 30, 2004, we had approximately 2,486,000 shares eligible for sale free of restriction under Rule 144.
Equity Compensation Plan Information
See Part III Item 11 for information regarding our equity compensation plans.
Series A Convertible Preferred Stock
On October 20, 1999, we issued 12,015 shares of Series A Preferred Stock to EIS, an affiliate of Elan, in conjunction with the formation of Sentigen Ltd., our joint venture with Elan. After issuance, the Series A Preferred Stock was transferred to EPI.
Under the Certificate of Designations, the holder of Series A Preferred Stock has a Series A Liquidation Preference entitling it as of December 31, 2003 to the first $16,036,810 of funds available for distribution to stockholders upon liquidation of the company and has the right to convert shares of Series A Preferred Stock into common stock at any time after October 20, 2001 at a conversion price of $84.68 per share. The conversion price is subject to adjustment for certain dilutive events. Alternatively, the holder of Series A Preferred Stock may exchange the initial 12,015 shares of Series A Preferred Stock for common shares of Sentigen so that the holder of Series A Preferred Stock owns 50% of the equity of Sentigen. The exchange right terminates six years after issuance, or October 19, 2005. As the initial value of the Series A Preferred Stock is exchangeable at the holder's option into additional shares of Sentigen, we have reclassified $12,015,000 as mezzanine equity until such time that the exchange right terminates either by the passage of time or the agreement of the holder to terminate or irrevocably not exercise the right.
Elan entered into an Agreement dated November 12, 2002 with the Institutional Investors in the financing which closed on November 12, 2002 which provides that Elan will pay the Institutional Investors an amount in cash (or the fair market value of other property) distributed to the holder of the Series A Preferred Stock on account of the Series A Liquidation Preference that, in the absence of the Series A Liquidation Preference would have been paid to those Institutional Investors in accordance with applicable law, up to a maximum of $16,000,000.
Each share of Series A Preferred Stock will be paid a mandatory payment-in-kind dividend equal to 7% (i.e., 0.07 additional shares of Series A Preferred Stock). The payment-in-kind dividend is cumulative, compounds on a semi-annual basis and is payable twice a year, beginning April 2000. Pursuant to this dividend requirement, approximately 1,159 shares of Series A Preferred Stock accrued
29
in 2003 and as of March 30, 2004, there were approximately 12,856 shares of Series A Preferred Stock issued and outstanding and 3,458 shares accrued as payment-in-kind dividends. The holder of Series A Preferred Stock is only entitled to vote on matters that adversely affect or change the rights of holders of Series A Preferred Stock. The holder of Series A Preferred Stock does not have the right to vote on the election of directors of the company.
Series B Convertible Preferred Stock
In February, 2000, we issued 327,240 shares of Series B Preferred Stock to 32 accredited investors in a private placement, for a purchase price of $16.88 per share and 9,816 shares to the placement agent in connection with services rendered in our private placement of Series B Preferred Stock. In November, 2002, a majority of the holders of the Series B Preferred Stock consented to the conversion of all of the issued and outstanding shares of Series B Preferred Stock into shares of common stock at the conversion ratio of 4.25 (pre-reverse split) shares of common per share of Series B Preferred Stock. In November, 2002, an aggregate of 422,316 shares of common stock were issued to the former holders of the Series B Preferred Stock and (available to common shareholders) the Series B Preferred Stock is no longer outstanding.
ITEM 6. MANAGEMENT'S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION.
Portions of the discussion in this item contain forward-looking statements and are subject to the "Risk Factors" described above. References to the "Company," "IMCOR," "we" or "our" are to IMCOR Pharmaceutical Co. and, where appropriate, our 80.1% owned joint venture company, Sentigen, Ltd. References to the "Imagent Business", "Technology Purchase" or "Purchased Technology" are to the medical imaging business of Alliance Pharmaceutical Corp. ("Alliance") acquired by us on June 18, 2003.Imagent® is a registered trademark owned by IMCOR.
The accompanying financial statements have been prepared assuming we are a going concern. The company currently receives financing through its use of demand notes payable to its major institutional investor. Without the continuation of the use of these notes or obtaining additional debt or equity financing we will not be able to continue to fund operations. The 2003 financial statements do not include any adjustment to reflect the possible future effects on the recoverability and classification of assets or the amount and classification of liabilities that may result from this uncertainty.
We are an emerging, development-stage specialty pharmaceutical company focused on developing and marketing medical imaging pharmaceutical products. We have one FDA approved product,Imagent® (perflexane lipid microspheres), an ultrasound imaging contrast agent that we acquired in June 2003, and two additional contrast agents in development for use in computed tomography (CT) and x-ray imaging with potential applications (1) as a blood pool agent, to diagnose diseased tissue in the cardiovascular system and other organs, which we call PH-50, and (2) to diagnose cancer metastasizing into the lymphatic system, which we call N1177. The product designations PH-50 and N1177 refer to the same chemical entity. We established the two names to distinguish commercialization rights between cardiovascular imaging (and all other indications) (PH-50) to which we retain rights, and the field of lymphography (N1177), which we licensed to our joint venture (Sentigen, Ltd.) with affiliates of Elan. On February 5, 2004, we changed our name to IMCOR Pharmaceutical Co., reflecting the changed nature of our business to imaging pharmaceuticals.
In December 2001, we announced positive preclinical results of PH-50. Based on these studies, the extensive preclinical toxicology and other study work, including the product's use in approximately 65 patients for lymphography, we shifted the strategic research direction of IMCOR to focus on the cardiovascular and lymphography applications. We accomplished this shift in focus also by splitting off our therapeutic line of business, and, in June 2003 acquiring the medical imaging business (the
30
"Imagentbusiness") of Alliance Pharmaceutical Corp. ("Alliance") led byImagent® (perflexane lipid microspheres), an FDA approved diagnostic imaging agent.
For the two years up to the time of our acquisition of theImagent business, substantially all of our research and development efforts were focused on the preclinical testing of PH-50. During that period, we operated with a very small staff and conducted substantially all of our research activities through contracts with academic institutions and contract research laboratories. In addition, we had conducted research and development activities on the development of certain therapeutic photodynamic therapy applications (namely a product we called PH-10 for the treatment of certain dermatological conditions) and the development of a medical laser. These operations were split off to our founding scientists as of November 12, 2002 and have been recorded as discontinued operations in our financial statements.
PH-50 and N1177 are still in early stages of development. Of these products, based on the availability of capital, we intend to focus our research efforts first on the continued development of PH-50 and secondly, N1177.
Pharmaceutical imaging agents are used with echocardiography, computed tomography ("CT"), nuclear medicine and magnetic resonance imaging ("MRI") equipment in order to increase the diagnostic capabilities of these techniques. By enhancing the contrast between different types of tissues, these intravenously administered contrast agents provide critical anatomical and disease state information that is not obtainable using the equipment alone. There are numerous potential applications for a contrast agent used in imaging parts or all of the cardiovascular system as well as other organs. Our products have the potential to enhance a physician's ability to diagnose certain cardiovascular abnormalities and the spread of cancer in the lymphatic system.
IMCOR is based in San Diego, California. We have approximately 30 full time employees and a 53,000 square foot building, a portion of which is an FDA approved cGMP (current Good Manufacturing Practices) manufacturing facility, at our San Diego location. Our personnel have expertise in sales, marketing and manufacturing, clinical development, clinical trial design and regulatory approval processes. We also work closely with consultants experienced in drug development and regulatory requirements.
As of April 8, 2002, we entered into a credit facility that provided us with a $2,500,000 revolving line of credit, bearing interest at 4.65%, for a period of five years. The loan was secured by a lien on all of our assets. The amount outstanding under the credit facility ($2,500,000) was converted into our common stock at $1.08 per share on November 12, 2002. In aggregate, from November 12, 2002 through January 2, 2003, we consummated the closings of a financing in which we sold 11,165,651 shares of our common stock to a group of institutional investors and individual accredited investors at $1.08 per share, for an aggregate of $12,058,902, including the above conversion of $2.5 million of debt outstanding under the revolving credit line.
On November 12, 2002, we split off our therapeutic business to certain founding shareholders in exchange for all of their 52.9% ownership interest in IMCOR and recognized a gain on the disposition of approximately $11,780,000. This gain was attributable to the value of the common stock held by the founding shareholders that was exchanged for the therapeutic technology, which had been expensed. Our therapeutic business had been focused on the development of certain photodynamic therapy products. Our remaining diagnostic business has been focused on the development of products for medical imaging, with particular emphasis on cardiovascular imaging.
On June 18, 2003, we acquired assets, including Purchased Technology, related to the diagnostic imaging business, includingImagent, an FDA-approved product, of Alliance. We also entered into a number of agreements related to that acquisition. Included in the purchase were an FDA-approved manufacturing facility, the marketing, manufacturing and supporting infrastructure for the product and an intellectual property portfolio of approximately thirty patents in the area of ultrasound imaging.
31
Imagent has been approved for marketing by the FDA for use in patients with sub optimal echocardiograms to opacify the left ventricle and thereby improve visualization of the main pumping chamber of the heart, and to improve delineation of the endocardial borders of the heart. As a result, ultrasound withImagent may better distinguish normal and abnormal heart structure and function—two critical indicators of cardiac health. Echocardiography is the most widely used imaging modality for the diagnosis of heart disease, the leading cause of death in the U.S. The attractiveness of the ultrasound imaging market potential together with the acquisition of an approved product and an extensive intellectual property portfolio were fundamental reasons for the acquisition and primary determinants of the purchase price of approximately $7,240,000 for plant, property and equipment, $15,638,000 for the purchased technology, plus future earn out payments as described in the purchase agreement based uponImagent revenue. The financial statements include the operating impact of this acquisition from June 18, 2003 through the end of the reporting period. We are currently seeking additional capital to operate theImagent business and to continue our development programs.
At the closing of theImagent business acquisition, we paid approximately $669,000 in cash and delivered 2,198,137 shares of our common stock. At the direction of Alliance, we delivered the cash and stock to creditors, employees and vendors of Alliance. In addition to bridge financing we provided to Alliance before the closing, our purchase of a $1,250,000 interest in Alliance's obligations to two of its creditors, Xmark Fund, Ltd. and Xmark Fund, L.P. (collectively "Xmark"), and our assumption of specified Alliance operating liabilities related toImagent, we are obligated to pay additional amounts to Alliance secured and unsecured creditors after the closing (including Xmark, discussed below, and others). At various times between 90 and 365 days after the closing, we must pay, subject to reaching satisfactory agreements with certain Alliance secured and unsecured creditors, an aggregate amount of up to approximately $3,400,000 to creditors other than Xmark and (subject to stockholder approval, which we received on February 5, 2004) deliver an aggregate of approximately 2,054,000 shares of our common stock. The amount of consideration was determined through arms-length negotiations.
In addition, after the closing and through 2010, we must pay Alliance further consideration in the form of an earn out based onImagent revenue we invoice (subject to certain reductions). This earn out equals, for each year of the earn out: 7.5% ofImagent revenue up to $20,000,000; 10% ofImagent revenue between $20,000,000 and $30,000,000; 15% ofImagent revenue between $30,000,000 and $40,000,000; and 20% ofImagent revenue above $40,000,000. The earn out will be reduced by amounts we pay pursuant to a license agreement with Schering Aktiengesellschaft ("Schering"), net of payments we receive from Schering under the license, and amounts of any indemnification claims we have against Alliance. The earn out is subject to three additional offsets (which are to be applied in the manner described in the Asset Purchase Agreement dated as of June 10, 2003 with Alliance) that entitle us to retain portions of the earn out otherwise payable to Alliance:
- •
- Up to approximately $1,600,000 for a fixed price offset, depending on the satisfaction of certain conditions;
- •
- The amount of any payments not committed to at closing that we make after the closing to Alliance's creditors plus up to $1,000,000 of litigation expenses for certain patent and other litigation; and
- •
- Between $4,000,000 and $5,000,000, which is the principal and accrued interest under our bridge loans to Alliance, depending on the satisfaction of certain conditions.
As the amount of the earn out cannot be estimated, it has not been reflected in the purchase price. Amounts paid will be expensed as incurred.
As part of the acquisition of theImagent business, we issued shares of our common stock to two companies, Cardinal Health and inChord, that had been providing services to theImagent business when it had been owned by Alliance and that were creditors of Alliance. As settlement of the Alliance
32
obligations, we issued Cardinal Health 1,100,000 shares and inChord Communications 429,000 shares of our common stock, respectively. As part of the development plan forImagent, we expect to continue to use the services of Cardinal and inChord. Currently,Imagent will be marketed and distributed in the United States through agreements with Cardinal. Various Cardinal companies will also provide packaging, sales support, and distribution services. InChord will provide advertising, medical education and similar services. We also entered into a series of agreements with Xmark. Pursuant to a Standstill and Make Whole Agreement dated as of December 30, 2002, we issued 750,000 shares of our common stock in the first quarter 2003 to Xmark, who agreed to not exercise any rights against Alliance as a creditor for the period of the standstill. On May 2, 2003, we entered into a Going Forward Agreement pursuant to which Xmark agreed to extend the period during which Xmark had refrained from exercising its rights as a creditor against Alliance to permit us to complete the acquisition. On that date, we also purchased a $1,250,000 interest in Alliance's obligations to Xmark.
At the closing of our acquisition of theImagent Business, pursuant to the Going Forward Agreement, we delivered to Xmark our promissory notes in the total principal amount of $2,500,000. These notes were payable in two equal installments on August 5, 2003 and November 3, 2003 or (if sooner) at the time we complete a financing resulting in at least $18,000,000 of gross proceeds to us and bear interest at 3% over the prime rate (payable in shares of our common stock). At the closing of theImagent acquisition, we also issued Xmark an additional 1,056,144 shares that represented payment of interest that was due to Xmark by Alliance through the closing date of the acquisition. The value of these additional 1,056,144 shares has been treated as part of the purchase price of theImagent business.
Our obligations to Xmark are secured by a first priority security interest on theImagent related tangible and intangible assets. Xmark would be entitled to foreclose on its lien covering these assets if we default on our obligations to Xmark, if we lose FDA approval forImagent, if we fail to pay other debts, or if we file for bankruptcy protection or similar matters.
We defaulted on the principal payment due on August 5, 2003 and Xmark delivered a notice to us stating that it was accelerating all amounts due under the notes. We negotiated a new agreement with Xmark dated August 18, 2003 (the "Letter Agreement") pursuant to which we agreed to make an immediate payment of $1,250,000 and Xmark agreed to rescind the default notice. The other key terms of the Letter Agreement are as follows:
- •
- We remain obligated to make a second principal payment of $1,250,000 on the earlier to occur of (i) November 3, 2003 or (ii) the consummation of one or more institutional financings resulting in aggregate gross proceeds to us of at least $18,000,000. We will have the right to extend the November 3rd due date monthly until April 3, 2004 for a fee (and not as a reduction of any amounts due and owing under the promissory notes) ranging from $50,000 to $100,000 per month. We have been exercising our right to extend the November 3, 2003 due date.
- •
- Within twenty days of the execution of the Letter Agreement we will pay to Xmark, in cash or in shares of our common stock (valued at $1.00 per share, subject to certain adjustments), $26,248 for failure to timely register the shares of our common stock issued to Xmark for resale, which payment has been made with shares of our common stock.
- •
- Our obligation to register the shares of common stock issued to Xmark for resale under the terms of the Going Forward Agreement was terminated and no further penalty payments or shares will be due to Xmark as a result of our failure to register the shares of our common stock held by Xmark. In lieu of the demand registration rights Xmark previously had, the Letter Agreement provides that Xmark will have piggyback registration rights.
- •
- The definition of "Event of Default" in the security agreements securing our obligations to Xmark was amended. The provision that an Event of Default included any default by us in the
33
Except as specifically amended by the Letter Agreement, the terms of the Going Forward Agreement and the other agreements governing our obligations to Xmark remain in effect, including Xmark's put right with respect to our common stock that Xmark owns.
Xmark's put right, as set forth in the Going Forward Agreement, requires us to repurchase at $1.00 per share up to 750,000 shares of common stock we issued under the Standstill and Make Whole Agreement and all additional shares we issue to Xmark under the Going Forward Agreement. The put right is fully exercisable beginning on the earlier of 13 months after the closing of the acquisition of theImagent assets or the date on which we close a financing for $20,000,000 or more. Before that time, Xmark can exercise the put right in quarterly installments beginning 90 days after the closing of our acquisition of theImagent assets. In connection with these quarterly installments, we can elect to defer repurchasing the shares if we lack adequate cash to satisfy our operating and working capital requirements for the subsequent 18-month period based on an operating plan approved by our Board. We agreed to use our reasonable efforts to have sufficient cash to meet our operating plan. If we do not repurchase these shares, Xmark is entitled to a payment of 1.5% of the value of the shares not repurchased for any 30-day period or pro rata portion thereof during which such failure is continuing.
We issued 82,977 shares of our common stock to Xmark on July 31, 2003 for interest and various penalties, including the failure to register the shares of our common stock held by Xmark by that date. We issued 26,248 shares of our common stock to Xmark on September 2, 2003, pursuant to the Letter Agreement, for failure to register shares of our common stock held by Xmark as of August 18, 2003. An additional 19,888 shares of our common stock are issuable to Xmark in payment of interest.
In connection with the closing of the acquisition of theImagent assets, we issued approximately 18% of our outstanding common stock. Nasdaq rules applicable under these circumstances require us to obtain prior stockholder approval before issuing more than 20% of our stock. We held a meeting of stockholders on February 5, 2004 and at that meeting obtained approval to issue an additional 1,985,522 shares in connection with the acquisition. In our financial statements, the value of these shares has been included in our equity as "Common Stock to be Issued".
We are currently funding our operations through the use of demand notes we issued to our major institutional investor. Although we are aggressively seeking additional equity or debt financing, we are currently dependant upon the sale of these notes to finance our current operations. Without additional financing we will not have sufficient cash resources to meet our current commitments. We will need substantial additional financing for our research, clinical testing, product development and marketing programs. We cannot accurately estimate the amount of additional financing required; however, the amount could be an additional $30 million or more over the next several years. In particular, we are currently seeking to raise additional capital through the sale of stock to accredited investors. See "Risk Factors—We must raise additional financing in the future and our inability to do so will prevent us from continuing as a going concern and implementing our business development plan," above.
We operate with a staff of approximately 30 people in manufacturing, research and development, regulatory affairs and administration. We contract with third party consultants having expertise in clinical development and regulatory matters to supplement our internal capabilities. We also contract with third party research laboratories, contract research organizations and manufacturers for clinical material supplies and the management of clinical trials. We also contract with academic and other institutions to conduct specified research projects.
34
Currently, the raw materials necessary to produce N1177 and PH-50 are supplied by a sole supplier. We presently have limited supplies of these products on hand. While our raw materials are supplied by a sole supplier, there are additional sources for these materials. However, to make any significant change in the manufacturing process or change in suppliers, we must conduct certain equivalency studies and receive approval by the FDA. Considerable delays in our research and development programs for these products could result from our having to change to a different supplier. While we have no reason to believe that our supplier will be unable or unwilling to provide the necessary supplies for our research, there can be no assurance that this will continue. As we continue to develop these products, we expect to identify and qualify additional suppliers of raw materials.
We expect that our quarterly and annual results of operations will fluctuate based on several factors including, our ability to raise additional capital to implement our sales, marketing and manufacturing programs, the results of our litigation against Amersham, the timing and amount of expenses involved in marketing and manufacturingImagent, conducting our research programs, particularly the conduct of clinical trials, the cost of clinical material used in those trials and required for compliance with FDA regulations and the amount, if any, of fees, milestone payments and research support payments received from potential strategic partners.
We consider the valuation of our investment in the purchased technology ofImagent to be a significant estimate. The carrying value of this purchased technology at December 31, 2003 is approximately $14,980,000 plus approximately $7,240,000 for plant, property and equipment, and a future earn out payments as described in the purchase agreement based uponImagent revenue. The assumptions underlying the financial forecast have been based on currently available information including estimates of market size and penetration, pricing, competitive threats and general operating expenses. We have also instituted litigation against Amersham, and Amersham has responded with counter-claims. Should we lose this litigation, our business and the value of the Purchased Technology would be materially and adversely affected. These estimates may change and such changes may impact future estimates of carrying value.
We also consider our investment in the joint venture with affiliates of Élan Corporation, plc ("Élan") to be subject to a significant estimate. The carrying value of our 80.1% equity interest in Sentigen, Ltd., the joint venture entity, plus advances to Sentigen, Ltd., at December 31, 2003 totaled $2,803,000. Members of Sentigen's management reviewed its financial projections and have concluded that given delays in the development of N1177, resulting from capital constraints, the carrying value of the intangible assets in the joint venture should be reduced by $2,500,000 in 2002. In addition, in the second quarter of 2003, based on the re-prioritization of the development of N1177 and as the development plans are being further delayed pending the acquisition of sufficient capital to allocate to the development of N1177, Sentigen recorded an additional impairment provision of $5,750,000. The assumptions underlying the financial forecasts were based on currently available information, including estimates of development costs, pricing, operating expenses and market sizes and penetration. These estimates may change and any such changes may impact future estimates of appropriate carrying values.
Results of Operations
Prior to our acquisition of theImagent business in June, 2003, our efforts were focused on the development and clinical testing of diagnostic products, specifically PH-50, being developed as a cardiovascular imaging agent, and N1177, being developed for lymphography, the diagnosis of cancer metastasizing to lymph nodes. To date, we have not generated revenues from the sale of any proposed diagnostic products or other operations. Net loss applicable to common shareholders (after preferred dividends) was $12,666,771, $1,649,604 and $23,839,784 for the years ended December 31, 2001, 2002 and 2003, respectively, and our cumulative losses since inception, including "in-kind" preferred stock and beneficial conversion dividends totaling $8,712,509, are approximately $60,231,207.
35
Since inception, IMCOR had been developing products for both therapeutic and medical imaging applications. As one of a series of transactions on November 12, 2002, we split off our therapeutic business to certain founding shareholders. Accordingly, the expenses associated with that business have been reclassified as discontinued operations.
With the acquisition of theImagent business, we hired approximately 35 employees related to this business. These employees were formerly associated with Alliance and bring capabilities in manufacturing, marketing and additional capabilities in research and related administrative infrastructure. We also continue to contract with third party consultants having expertise in clinical development and regulatory matters to supplement our internal capabilities. In addition, we contract with third party research laboratories, contract research organizations and manufacturers for clinical material supplies and the management of clinical trials, and with academic and other institutions to conduct specified research projects.
During 2003 we have conducted limited test marketing ofImagent to certain hospitals and physicians. In December 2003 we entered into a license agreement with Kyosei Pharmaceutical Co., Ltd. ("Kyosei"), a unit of Sakai Group, for the development and marketing ofImagent in Japan for all radiology and cardiology indications. Terms of the agreement call for the payment to IMCOR of up to $10 million in fees and development milestone payments plus royalties on commercial sales. Kyosei will also pay IMCOR to manufactureImagent for Kyosei's clinical and commercial requirements. At the closing, we received a gross payment of $2 million from Kyosei. Recognition of this payment will be made ratably over the anticipated life of the agreement.
In 2004, we received an additional gross payment of $2 million from Kyosei. Additional payments are expected in 2005 and beyond. The future payments from Kyosei are based on certain milestones of clinical development, including the commencement of and completion of clinical studies and approval for sale by Japanese regulatory authorities. Commencement of clinical studies is not expected to be earlier than 2005, and the development program, including regulatory approval, is expected to take four or more years. Accordingly, commercial sales, upon which we will earn royalties, are not expected to commence earlier than 2008. While we believe thatImagent clinical studies will be successful, there can be no assurance that such studies conducted in Japan will be successful or that the product will be approved for sale by Japanese authorities.
Research and development expenses for the years ended December 31, 2001, 2002 and 2003 were $437,958, $1,227,897, and $2,529,679 respectively. These expenses include personnel costs, fees and other compensation paid to outside consultants, and costs associated with conducting preclinical and clinical studies and manufacturing product to be used in these studies. We are completing pre-clinical studies of PH-50 for potentially significant new applications in cardiovascular imaging and have filed for additional patent protection of the use of the compound for vascular and organ imaging applications. To date, we have incurred a total of approximately $2,531,000 of expenses in connection with the development of PH-50 (approximately 438,000, 1,228,000 and 865,000 for the years ended December 31, 2001, 2002 and 2003, respectively). In 2003, we had approximately a $363,000 reduction in PH-50 expenditures due to capital constraints on the Company. Please see "Technology—Development of PH-50" and the discussion below of research and development expenses in 2003.
We have been developing N1177 through a joint venture with affiliates of Elan. Research and development expenses for N1177 have been charged to the joint venture. To date, we have incurred a total of approximately $3,130,000 of expenses in connection with the development of N1177 (approximately $1,160,000, $658,000 and $0 incurred by the joint venture for the years ended December 31, 2001, 2002 and 2003, respectively). Due to capital constraints, no research was conducted on N1177 during 2003. Please see "Technology—Development of N1177" and Note 4 to our financial statements.
36
Research expenses (from continuing operations) in 2002 increased by $789,939 to $1,227,897 primarily due to the shift in research spending from N1177 to the development ofImagent and PH-50, a product having greater market potential than N1177. During 2002, the Company operated with a limited internal staff and utilized third party contract laboratories and consultants to conduct its research programs under the guidance of its internal staff. The increased expenditures were due to the additional contract work conducted by these third party laboratories and consultants.
Substantially all of the $2,529,679 in research expenses in 2003 were oriented to the preclinical development of PH-50, and, since the acquisition of the Imagent Business, expenses associated with regulatory compliance and continued development of Imagent for new indications. The research expenditures were composed of expenses incurred for the development of PH-50 (approximately $865,000) and, since the acquisition of the Imagent Business personnel costs (approximately $1,400,000) and outside service expenses (approximately $279,000) at our San Diego operation. The expenditures incurred in our San Diego operations were directed to product and regulatory support and compliance and not to a particular significant product research program. We expect our development priorities, based on the availability of sufficient capital, to focus first on the development of new indications for Imagent and the continued development of PH-50 and then to the further development of N1177. Expenses associated with the development of N1177 are charged to our joint venture, and expenses associated with the development of our other products are borne completely by us.
Selling, general and administrative expenses were $3,220,584, $4,030,083 and $14,241,767 for the years ended December 31, 2001, 2002, and 2003, respectively. These expenses include (in 2002 and 2003) costs associated with our acquisition of theImagent business prior to firmly committing to the acquisition (costs incurred following our firm commitment to acquire theImagent business have been capitalized as part of the acquisition cost), and for all years costs associated with obtaining patent protection for our intellectual property, costs associated with sales and marketing programs, administrative personnel and corporate management, the amortization of options granted to our employees, legal and related expenses and facilities expenses.
In 2002, selling, general and administrative expenses increased by $809,499 to $4,030,083. As part of the November, 2002 restructuring and financing, stock options were granted to the remaining employees of IMCOR with an exercise price equal to the price at which common stock was sold to the new institutional investors. As the exercise price was below the closing price of the company's common stock at the date of grant, we recorded an expense of approximately $988,000 reflecting the differential between those two prices.
In 2003, selling, general and administrative expenses increased by $10,211,684 to $14,241,767. The primary reasons for the increase were related to our acquisition of theImagent business from Alliance including, (i) provisions we have taken against advances we had made to Alliance and shares of our common stock we had issued to Xmark pursuant to a standstill agreement with Xmark (approximately $2,615,000), (ii) costs of conducting due diligence and other activities related to the acquisition of theImagent business (approximately $775,000), (iii) costs incurred by us to operate theImagent business following its acquisition (approximately ($4,445,000), (iv) depreciation (approximately ($945,000), and (v) amortization of the purchased technology (approximately $658,000). Of the costs to operate the Imagent Business after acquisition, approximately $1,962,000 was for third party contract services, contract sales and marketing and legal expenses, $850,000 for facilities expenses and $775,000 for payroll and related expenses.
As part of the November, 2002 restructuring and financing, stock options were granted to our remaining employees with an exercise price equal to the price at which common stock was sold to the new institutional investors. As part of the acquisition of theImagent business and the related hiring of employees from Alliance in June 2003, we granted these new employees an aggregate of 1,865,750 stock options with an exercise price of $1.25 per share. Pursuant to an agreement in July 2003 with
37
Dr. Taffy Williams, our Chief Executive Officer, an aggregate of 1,050,000 stock options were granted to Dr. Williams, subject to shareholder approval (shareholders representing an excess of 50% of our outstanding common stock have indicated their commitment to vote in favor of approving these options). As the exercise prices were below the closing price of our common stock at the dates of grant, we recorded an expense of approximately $1,236,566 in 2003 reflecting the differentials between those respective two prices.
In September, 2000, we initiated a restructuring of our clinical development operations. We closed our Westborough, Massachusetts office and delivered notices of material breaches and/or dissatisfaction pursuant to the employment agreements with three employees who worked there at that time. Clinical development activities formerly carried on in Westborough were assumed by outside consultants and other company employees. We initially took a charge against earnings of $597,025 for termination of our office lease and associated expenses and termination costs associated with the employees at that location. During 2002, we recorded an additional charge of $807,483 all of which was related to the valuation of certain stock options granted to one of the Westborough employees as part of a settlement of certain litigation. At December 31, 2002, none of this charge was remaining.
In the fourth quarter of 1999, we entered into a joint venture with affiliates of Elan for the development of N1177, a potential product to identify and diagnose lymph nodes for the presence of cancer. We own 80.1% of the joint venture entity, Sentigen, Ltd., with the balance owned by Elan. During 2001, 2002 and 2003 we recorded losses from the joint venture of $1,952,758, $3,452,837, and $5,389,338, respectively. In 2001 and 2002 these losses resulted from our share of development expenses incurred in the joint venture by the two participants, including personnel expenses and costs associated with developing a manufacturing process, including the purchase of drug substance and other materials. As there have been delays, principally due to capital constraints, on the development of N1177, Sentigen recorded in 2002 a $2.5 million charge, of which our 80.1% share is approximately $2.0 million, as an impairment to the carrying value of the Sentigen license. In 2003, these losses were substantially the result of the amortization of the license purchased from Élan and a $4,605,000 charge for further impairment of the license value as, due to the lower priority of the development of N1177 and capital constraints, limited development work was being conducted.
As part of our annual audits, we, on behalf of the Sentigen joint venture, prepared cash flow projections of the product, N1177 including assumptions of research and clinical development costs, market size, market share and resulting potential revenues and the costs (manufacturing, marketing and sales along with administrative costs) to generate the projected revenue stream. These cash flows, assuming that the research is successful, were then discounted to a present value. During the year, we incorporated these projections as part of the analysis of any impairment in the value of the joint venture.
As part of the our review of our financial statements at year end 2002, we evaluated the carrying value of the Sentigen license in light of the projection of cash flows and availability of capital to conduct the research in a timely manner. Accordingly, we advised Sentigen to reduce the carrying value of the Elan license by $2,500,000.
Further, as part of our review of our financial statements for the quarter ended June 30, 2003, we again evaluated the carrying value of the Sentigen license in light of the projection of cash flows, the availability of capital to conduct the research in a timely manner, the knowledge that this area of research was no longer an area stated by Elan to be an area of focus for that company, and the priority of committing capital to the further development of N1177 in light of our other priorities, namely the continued development of PH-50 and the manufacturing and marketing of its recently acquired Imagent Business. Accordingly, we advised Sentigen to reduce the carrying value of the Elan license by $5,750,000 to a value of approximately $4,060,000, it being also determined in the judgment of certain
38
of our directors with experience in these matters, that a value of approximately $4,000,000 could be fair should Sentigen either sell or license N1177 to a third party.
Investment income for the years ended December 31, 2001, 2002 and 2003 was $110,741, $1,563, and $7,394, respectively. Investment income is generated by the amount of capital in our investment portfolio prior to it being used to fund our operations and the rate of interest earned on that capital. We invest funds in the investment portfolio in U.S. Government obligations. In the first half of 2001, we received a total of $1,068,723 in net proceeds of common stock sold under our shelf registration statement that was declared effective in February, 2001. Investment income declined in 2001 due primarily to the decline in the average balances of our investment portfolio as funds were used to support operations and, to a lesser extent, the general decline in short term interest rates. In 2002, investment income declined to $1,563 as the entire portfolio was used to support operating expenses. In 2003 investment income increased to $7,394 reflecting earnings on the investment portfolio following our capital raising efforts in late 2002. We expect investment income to continue to fluctuate both as the size of the investment portfolio decreases or increases and as the rate of interest earned by the portfolio varies due to shifts in short term interest rates.
Since inception and through November 12, 2002, we had been developing products for both therapeutic and medical imaging applications. As one of a series of transactions on November 12, 2002, we split off our therapeutic business to certain founding shareholders. Accordingly, the expenses associated with that business have been reclassified as discontinued operations. Our therapeutic business had been focused on the development of certain photodynamic therapy products, in particular a compound we called PH-10. In the years ended December 31, 2001 and 2002, we incurred losses related to these operations of $2,909,820 and $1,496,430, respectively. On November 12, 2002 this business was split off to certain founding shareholders in exchange for all of their 52.9% ownership interest in IMCOR resulting in a gain from the split off of $11,779,752 based upon the market value of our common stock on that date, net of the net assets that were split off.
Purchases of equipment and leasehold improvements, other than the June 18, 2003 acquisition of theImagent Business including the assumption of the lease of a manufacturing facility located in San Diego, for the years ended December 31, 2001, 2002 and 2003 were $45,938, $0 and $0, respectively. These purchases were primarily for the acquisition of laboratory and other equipment necessary to conduct pre-clinical and clinical studies and leasehold improvements in the Knoxville and Westborough offices that we maintained at that time. During the next twelve months, assuming that we obtain sufficient capital, we expect capital expenditures for equipment to be less than $500,000.
During 2001, as a result of the closing of the Westborough facility and the shift in the research priorities, we determined that certain equipment we were leasing would no longer be useful in our research. Accordingly, we recorded provisions for future lease payments totaling $1,264,208 representing the remaining lease obligations. While we will pursue opportunities to sublease or otherwise mitigate these obligations, there can be no assurance of any success in this regard. We are in default under the lease and have received a demand letter from the lessor for payment of $787,000 for remaining unpaid lease obligations and other fees. We are attempting to negotiate a resolution with the lessor but there can be no assurances that we will be successful in this regard.
We have issued two series of convertible preferred stock: Series A, issued in October 1999 and which remains outstanding and Series B Preferred Stock, issued in February 2000 and which was retired in November 2002. The holder of Series A Preferred Stock is entitled to a mandatory, cumulative, semi-annual payment-in-kind dividend of 7% (i.e., 0.07 additional shares of Series A Preferred Stock). On November 12, 2002, the holders of Series B Preferred Stock converted all of their issued and outstanding shares (including accrued and unpaid dividends) for shares of our common stock at an exchange rate of 4.25 (pre-reverse split) shares of common for each share of Series B Preferred Stock. Additionally, the terms of the Series A Preferred Stock and the Series B Preferred Stock create
39
additional preferred returns for the holders. The Series A Preferred Stock was issued with detachable warrants. The value allocated to those warrants caused the preferred shareholders to receive an additional return. That additional return has been amortized over the period from issuance through October 2001, when the stock was first convertible. The additional return related to the Series A Preferred Stock recorded was $391,154 in 2001. The Series B Preferred contained beneficial conversion features. Emerging Issues Task Force Issue 98-5, "Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios," requires the issuer to assume that the holder will not convert the instrument until the time of the most beneficial conversion which would occur after January 1, 2002. Using the conversion ratio at January 1, 2002 of 1.4 shares of common stock and the stock price at the date of issuance, the maximum beneficial conversion amount was $2,322,316. This amount was recorded as additional preferred stock dividends over the period from issuance until January 1, 2002. As a result of the combination of the mandatory dividends, the preferential value amortization and, in 2002, deemed dividends for our Series B Preferred Stock associated with the beneficial conversion rates offered to holders of these instruments as part of our November 12, 2002 financing, we recorded preferred stock dividends of $2,943,755, $1,997,723 and $1,066,280 in 2001, 2002 and 2003 for Series A Preferred Stock and (for the years 2001 and 2002) Series B Preferred Stock.
As a result of the above factors, the net loss applicable to common stockholders (after preferred dividends) was $12,666,771, $1,649,604 and $23,839,784 for the years ended December 31, 2001, 2002 and 2003 respectively. Basic and diluted loss per common share was $1.33, $0.16 and $1.24, respectively, for these periods.
Liquidity; Capital Resources
At December 31, 2003, we had cash and cash equivalents totaling approximately $1,658,000. Since that date, we have expended all those funds and, as described above, are currently funding our operations through borrowings from two of our principal institutional investors, as cash is required for operations. These investors have continued to provide funding to us, but there can be no assurance that they will continue to provide funding to us.
At December 31, 2003, we had a net deficit position (our liabilities, puttable share obligations and mezzanine equity, combined, exceeded our assets) totaling $8,194,743. Among other factors, we have reclassified $12,015,000 of our Series A Preferred Stock as mezzanine equity to acknowledge the holder's right to exchange that security for that number of additional shares of Sentigen to increase its ownership of Sentigen to 50%. As discussed below, we believe that the probability of the holder exercising the exchange right is remote.
Our ability to conduct operations is entirely dependent on our ability to obtain additional capital. Without additional financing we will not have sufficient cash resources for our entire fiscal year ending December 31, 2004. We have been financing our operations with the proceeds of the financing we closed in November and December 2002 and January 2003, the initial payment that we received from Kyosei (net of taxes and expenses) and currently through borrowings, evidenced by secured promissory notes, payable upon demand, from our principal institutional investors. We will need substantial additional financing for our manufacturing and marketing operations as well as research, clinical testing and product development programs. We cannot accurately estimate the amount of additional financing required; however, the amount could be an additional $30 million or more over the next several years. If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders may result. However, there can be no assurance that such capital will be available under acceptable terms, if at all. See "Risk Factors—We must raise additional financing in the future and our inability to do so will prevent us from continuing as a going concern and implementing our business development plan," above.
40
Under the Certificate of Designations of the Series A Preferred Stock the holder has a Series A Liquidation Preference entitling it as of December 31, 2003 to the first $16,036,810 of funds available for distribution to stockholders upon liquidation of the Company and has the right to convert shares of Series A Preferred Stock into common stock at any time after October 20, 2001 at a conversion price of $84.68 per share. The conversion price is subject to adjustment for certain dilutive events. Alternatively, the holder of Series A Preferred Stock may exchange the initial 12,015 shares of Series A Preferred Stock for common shares of Sentigen so that the holder of Series A Preferred Stock owns 50% of the equity of Sentigen. The exchange right terminates six years after issuance, or October 19, 2005. As the initial value of the Series A Preferred Stock is exchangeable at the holder's option into additional shares of Sentigen, we have reclassified $12,015,000 as mezzanine equity until such time that the exchange right terminates either by the passage of time or the agreement of the holder to terminate or irrevocably to not exercise the right. We believe that as the development program for N1177 will require several years to complete and as no research had been conducted on N1177 during 2003 due to our capital constraints that the probability of the holder exercising its exchange right prior to the expiration of that right is remote.
On April 8, 2002, we entered into a credit facility that provided us with a $2,500,000 revolving line of credit, bearing interest at 4.65%, for a period of five years. The loan was secured by a lien on all of our assets. In connection with our agreement to sell shares of our common stock to a group of institutional investors, both the amount outstanding, including accrued and unpaid interest, under the Elan credit line ($3,082,487 converted at a rate of $24.00 per share) and the $2,500,000 credit facility (at a conversion rate of $1.08 per share) were converted into shares of our common stock on November 12, 2002 and the respective lines of credit were cancelled.
On June 18, 2003, we closed the acquisition of theImagent Business from Alliance. At the closing, we paid approximately $669,000 in cash and delivered 2,198,137 shares of our common stock. At the direction of Alliance, we delivered the cash and stock to creditors, employees and vendors of Alliance. In addition to bridge financing we provided to Alliance before the closing, our purchase of a $1,250,000 interest in Alliance's obligations to Xmark and our assumption of specified Alliance operating liabilities related toImagent, we are obligated to pay additional amounts to Alliance secured and unsecured creditors at various times between 90 and 365 days after the closing, (i) an aggregate of $2,500,000 to Xmark, (ii) subject to reaching satisfactory agreements with certain Alliance secured and unsecured creditors, an aggregate amount of up to approximately $3,000,000 to creditors other than Xmark, (iii) pay certain royalties based upon sales ofImagent through June 2010, subject to certain offsets, and (iv) subject to stockholder approval (which approval was obtained on February 5, 2004) deliver an aggregate of approximately 2,054,000 shares of our common stock.
We are currently in default under obligations to pay $775,000 to creditors of Alliance Pharmaceutical Co. in connection with our acquisition ofImagent. While there can be no assurance of success, we believe that we can resolve these defaults and that they do not give rise to a reduction in the carrying value of Purchased Technology of theImagent Business. We also have not registered the resale of shares we issued in connection with that acquisition, which in some cases is a condition to the effectiveness of agreements to release us from any claims by the Alliance creditor.
We also entered into a series of agreements with Xmark. Prior to our acquisition of theImagent Business, pursuant to a Standstill and Make Whole Agreement with Xmark, we issued a total of 761,250 shares (including 11,250 shares as a penalty for the failure to register those shares by April, 2003) of our common stock to Xmark and Xmark agreed to not exercise any rights against Alliance as a creditor. On May 2, 2003, we entered into a Going Forward Agreement pursuant to which Xmark agreed to extend the period during which Xmark had refrained from exercising its rights as a creditor against Alliance to permit us to complete the acquisition.
41
At the closing of theImagent acquisition, we issued Xmark an additional 1,056,144 shares that represented payment of interest that was due to Xmark by Alliance through the closing date of the acquisition. The value of these additional 1,056,144 shares has been treated as part of the purchase price of theImagent business.
Also at the closing of our acquisition of theImagent assets, pursuant to the Going Forward Agreement, we delivered to Xmark our promissory notes in the total principal amount of $2,500,000. These notes are payable in two equal installments on August 5, 2003 and November 3, 2003 or (if sooner) at the time we complete a financing resulting in at least $18,000,000 of gross proceeds to us and bear interest at 3% over the prime rate. The interest on the notes is payable in shares of our common stock. Our obligations to Xmark are secured by a first priority security interest on theImagent related tangible and intangible assets. Xmark would be entitled to foreclose on its lien covering these assets if we default on our obligations to Xmark, if we lose FDA approval forImagent, if we fail to pay other debts, or if we file for bankruptcy protection or similar matters.
We defaulted on the principal payment due on August 5, 2003 and Xmark delivered a notice to us stating that it was accelerating all amounts due under the notes. We negotiated a new agreement with Xmark dated August 18, 2003 (the "Letter Agreement") pursuant to which we agreed to make an immediate payment of $1,250,000 and Xmark agreed to rescind the default notice. The other key terms of the Letter Agreement are as follows:
- •
- We remain obligated to make a second principal payment of $1,250,000 on the earlier to occur of (i) November 3, 2003 or (ii) the consummation of one or more institutional financings resulting in aggregate gross proceeds to us of at least $18,000,000. We will have the right to extend the November 3rd due date monthly until April 3, 2004 for a fee (and not as a reduction of any amounts due and owing under the promissory notes) ranging from $50,000 to $100,000 per month. We have exercised our right to extend the November 3, 2003 due date by one month.
- •
- Within twenty days of the execution of the Letter Agreement we will pay to Xmark, in cash or in shares of our common stock (valued at $1.00 per share, subject to certain adjustments), $26,248 for failure to timely register the shares of our common stock issued to Xmark for resale, which payment has been made with shares of our common stock.
- •
- Our obligation to register the shares of common stock issued to Xmark for resale under the terms of the Going Forward Agreement was terminated and no further penalty payments or shares will be due to Xmark as a result of our failure to register the shares of our common stock held by Xmark. In lieu of the demand registration rights Xmark previously had, the Letter Agreement provides that Xmark will have piggyback registration rights.
- •
- The definition of "Event of Default" in the security agreements securing our obligations to Xmark was amended. The provision that an Event of Default included any default by us in the payment of borrowed money indebtedness was eliminated. Instead, we agreed that it would be an Event of Default if we made repayments of borrowed money indebtedness.
- •
- With regard to payments to certain specified institutions, we agreed that, when paid, we would also make a pro rata payment to Xmark.
Except as specifically amended by the Letter Agreement, the terms of the Going Forward Agreement and the other agreements governing our obligations to Xmark remain in effect, including Xmark's put right with respect to our common stock that Xmark owns.
Xmark's put right, as set forth in the Going Forward Agreement, requires us to repurchase at $1.00 per share up to 750,000 shares of common stock we issued under the Standstill and Make Whole Agreement and all additional shares we issue to Xmark under the Going Forward Agreement. The put
42
right is fully exercisable beginning on the earlier of 13 months after the closing of the acquisition of theImagent assets or the date on which we close a financing for $20,000,000 or more. Before that time, Xmark can exercise the put right in quarterly installments beginning 90 days after the closing of our acquisition of theImagent assets. In connection with these quarterly installments, we can elect to defer repurchasing the shares if we lack adequate cash to satisfy our operating and working capital requirements for the subsequent 18-month period based on an operating plan approved by our Board. We agreed to use our reasonable efforts to have sufficient cash to meet our operating plan. If we do not repurchase these shares, Xmark is entitled to a payment of 1.5% of the value of the shares not repurchased for any 30-day period or pro rata portion thereof during which such failure is continuing.
In 2001, we determined that certain leased equipment would no longer be useful in our research programs. Accordingly, we recorded a provision of $1,123,740 representing the remaining lease payments for the equipment. We are in default under the equipment lease and in January 2004 the lessor demanded approximately $787,000. We are in discussions with the lessor to settle the demand. There can be no assurance that we will be able to successfully resolve the demand.
In 2002, our accounts payable increased by $531,229 reflecting primarily increases in payables to certain vendors as we managed our cash flow in light of its capital constraints. In 2002, our accrued expense balance decreased by $534,042 primarily due to reductions of accrued payroll and bonuses and accrued interest and restructuring charges. The change in the year end balance of accrued expenses was offset by a non-cash transaction of the conversion of $235,577 of accrued interest into common stock as part of the November 2002 financing resulting in a net cash flow reduction of $298,465.
In 2003, accounts payable increased by $1,049,787 reflecting primarily increases in balances due to certain vendors assumed by us as part of theImagent Business acquisition and to other vendors as we continued to manage our cash flow. In 2003, accrued expense balance increased by $3,247,660 of which $2,530,434 related to liabilities assumed as part of theImagent Business acquisition and the remaining increase was due to the New Hope office abandonment provision and general accruals and payroll liabilities. The 2003 year end accrued liability balance was offset by the non-cash impact of theImagent Business transaction of $3,422,036 resulting in a net cash flow change of $174,375.
Three of our existing institutional investors have provided capital to us in exchange for our Promissory Notes in an aggregate principal amount of up to $11,417,000 (at December 31, 2003). The notes bear interest at a rate of 7.25% per annum, compounded monthly. A total of $4,160,000 of these notes are convertible into our common stock. Unless converted into common stock, amounts due under the notes were payable on the earlier of August 5, 2003 or the date that we commit various defaults or become subject to bankruptcy or similar proceedings. We defaulted on the payment of $4,160,000 of these notes on August 5, 2003; however while under no obligation to do so, these investors continue to loan us capital under demand notes which bear interest at the rate of 7.25% per annum, compounded monthly.
The entire principal and interest outstanding under our convertible notes to these investors automatically converts into shares of our common stock on the same terms and at the same price as the shares issued in a financing for additional long-term capital. To secure the obligations under our notes to these investors, we granted them a security interest in all of our tangible and intangible assets, including intellectual property. This lien covers ourImagent-related assets, but as to these assets the investors' lien is subordinate to the security interest held by Xmark.
In 2003, excluding the 1,926,619 shares of our common stock issued to Xmark and the 1,141,993 shares issued to other creditors of Alliance pursuant to theImagent Business acquisition, we issued an aggregate of 139,994 shares of our common stock pursuant to several factors including cash, compensation for services and the cashless exercise of stock options.
43
In the second quarter of 2003, we recognized the issuance of 124,627 shares of our common stock that previously had been subject to rescission.
We have used, and expect to use capital available from sales of common stock, for general corporate purposes, including activities related to manufacturing and marketingImagent, preparing for and conducting clinical trials, purchase and preparation of clinical material, conduct of preclinical studies, administrative expenses to support our operating activities, capital expenditures and to meet working capital needs.
We are currently seeking to raise capital in one or more transactions to fund our immediate and longer-term capital needs through the private placement of securities to accredited investors. In addition, we expect to evaluate from time to time the acquisition or license of businesses, technologies or products. The purchase of any such licenses or technologies would be funded by a portion of any net proceeds of future offerings or the issuance of debt or equity securities specifically for that purpose. We expect our use of capital to increase as we build the commercial presence ofImagent and conduct further clinical trials of our products in development.
At the time of the acquisition of theImagent business, we entered into a lease agreement with a unit of Baxter International for certain equipment used for the manufacture ofImagent. Our annual cost for this lease is approximately $126,000. Our other long-term commitments that are not recorded on our financial statements are for our office space in San Diego, California. Annual rent for the California lease, which expires in 2008, is approximately $792,000. In addition, we have provided for the remaining lease payments of certain laboratory equipment that is no longer useful to our operations. The lease expires in 2004. We are in default under the equipment lease and have received a demand letter from the lessor for payment of $787,000 for remaining unpaid lease obligations and other fees. We are attempting to negotiate a resolution with the lessor but there can be no assurances that we will be successful in this regard.
Plan of Operation
Management believes it lacks sufficient working capital to fund operations for the entire fiscal year ending December 31, 2004. Therefore, substantial additional capital resources will be required to fund the ongoing operations related to our marketing and manufacturing ofImagent and our research and business development activities. Our financial condition raises substantial doubt about our ability to continue as a going concern. We believe there are a number of potential alternatives available to meet the continuing capital requirements such as public or private financings or collaborative agreements. We are taking continuing actions to reduce our ongoing expenses. If adequate funds are not available, we will be required to significantly curtail our operating plans and may have to sell or license out significant portions of our technology or potential products. The 2002 and 2003 financial statements, including those contained in this Form 10-KSB/A, do not include any adjustment to reflect the possible future effects on the recoverability and classification of assets or the amount and classification of liabilities that may result from the outcome of this uncertainty.
During the next twelve months we will focus our efforts primarily on exploring various options concerning our businesses, including securing additional financing, joint ventures, licensing or selling all or a portion of our technology and other possible strategic transactions. Our ability to commercializeImagent and develop PH-50 and N1177 depends on the successful implementation of one or more of these transactions. In July 2003, given our change into an operating company with the acquisition ofImagent, we entered into an agreement with Taffy Williams, our Chief Executive Officer. Our agreement with Dr. Williams provided, among other things, that we would begin a search for a chief executive officer with greater experience in managing operating companies. Since that time, we have also been working with consultants who have advised us on operational and management issues. In light of the various options for the future of our business currently under consideration—including that
44
if we sellImagent we would return to being focused on development rather than operations—the Company is reevaluating its agreement with Dr. Williams and may seek to extend Dr. Williams' tenure with the Company and amend other provisions of that agreement. We have not selected a new Chief Executive Officer at this time and Dr. Williams will continue in that role until we do.
Depending on the availability of capital and whether or not we pursue a sale or licensing strategy forImagent, we will focus our operating efforts on the sales and marketing ofImagent. If additional capital becomes available, we also expect to conduct preclinical and human clinical studies of our products in development. Subject to the availability of sufficient capital, we expect to continue to incur increasing losses for at least the next three years as we intensify research and development, preclinical and clinical testing and associated regulatory approval activities and engage in or provide for the manufacture and/or sale of any products that we may develop.
Greater capital resources would enable us to quicken and expand our marketing and research and development activities, and our failure to raise additional capital will (absent a suitable collaborative agreement providing for a third party to take over these functions) significantly impair or curtail our ability to conduct further activities. In any event, complete development and commercialization of our technology will require substantial additional funds. See "Risk Factors—We must raise additional financing in the future and our inability to do so will prevent us from continuing as a going concern and implementing our business development plan," above. We are seeking to raise capital through the sale of our common stock or other securities in a private placement to fund our immediate and longer-term capital needs.
As of December 31, 2003, we expect to pay the following contractual obligations and commitments:
| | Payments due by Year
|
|---|
| | 2004
| | 2005-2006
| | 2007-2008
| | Beyond 2008
| | Total
|
|---|
| Recorded Liabilities | | | | | | | | | | | | | | | |
| Long term debt | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — |
| Imagent purchase obligations | | | 3,763,991 | | | — | | | — | | | — | | | 3,763,991 |
| Commitments | | | | | | | | | | | | | | | |
| Operating Lease Commitments | | | 1,455,180 | | | 1,900,569 | | | 1,024,595 | | | — | | | 4,380,344 |
| | |
| |
| |
| |
| |
|
Total Contractual Obligations |
|
$ |
5,219,171 |
|
$ |
1,900,569 |
|
$ |
1,024,595 |
|
$ |
— |
|
$ |
8,144,335 |
| | |
| |
| |
| |
| |
|
Recent Accounting Pronouncements
In May 2003, the Financial Accounting Standards Board ("FASB") issued SFAS No. 150, "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity," effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective as of July 1, 2003. The adoption of SFAS No. 150 did not have a material impact on the results of operations or the financial position of the Company.
In May 2003, the FASB issued SFAS No. 149, "Amendment of Statement 133 on Derivative Instruments and Hedging Activities," effective for contracts entered into or modified after June 30, 2003 and for hedging relationships designated after June 30, 2003. The adoption of SFAS No. 149 did not have an impact on the results of operations or the financial position of the Company.
In December 2002, the FASB issued SFAS No. 148, "Accounting for Stock-Based Compensation—Transition and Disclosure", effective for fiscal years ending after December 15, 2002. This rule amends SFAS No. 123 to provide several alternatives for adopting the stock option expense provisions of SFAS No. 123, as well as additional required interim financial statement disclosures. SFAS No. 148 does not
45
require companies to expense stock options in current earnings. The Company has not adopted the provisions of SFAS No. 123 for expensing stock based compensation (see "—Employee stock option and stock purchase plans"); however, the Company has adopted the additional interim disclosure provisions of the statement. The impact of the adoption of SFAS No. 148 did not have a material impact on the results of operations or the financial position of the Company.
In July 2002, the FASB issued SFAS No. 146, "Accounting for Costs Associated with Exit or Disposal Activities." SFAS No. 146 eliminates Emerging Issues Task Force, or EITF, Issue No. 94-3 "Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (Including Certain Costs Incurred in a Restructuring)." Under SFAS No. 146, liabilities for costs associated with an exit or disposal activity are recognized when the liabilities are incurred, as opposed to being recognized at the date of entity's commitment to an exit plan under EITF No. 94-3. Examples of costs covered by the standard include lease termination cost and certain employee severance costs that are associated with a restructuring; discontinued operation, plant closing or other exit or disposal activity. Furthermore, SFAS No. 146 establishes that fair value is the objective for initial measurement of the liabilities. This Statement is effective for exit or disposal activities that are initiated after December 31, 2002. During 2003 the Company recognized $136,947 of restructuring costs for lease termination and restructuring associated with the closure of its Pennsylvania facility and move to the Alliance facility in San Diego, CA.
In January 2003, the FASB issued FASB Interpretation No. ("FIN") 46, "Consolidation of Variable Interest Entities," which was originally effective on July 1, 2003. In December 2003, the FASB deferred the effective date for applying the provisions of FIN 46 to March 31, 2004 for interests held by public companies in variable interest entities or potential variable interest entities created before February 1, 2003. The Company has completed its evaluation of the provisions of FIN 46 and does not have any significant interests in variable interest entities. Accordingly, the adoption of FIN 46 did not have a material impact on the results of operations or the financial position of the Company.
In November 2002, the FASB issued Interpretation No. (FIN) 45,"Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others." Interpretation No. 45 requires a guarantor to include disclosure of certain obligations, and if applicable, at the inception of the guarantee, recognize a liability for the fair value of other certain obligations undertaken in issuing a guarantee. The recognition requirement is effective for guarantees issued or modified after December 31, 2002 and did not have a material impact on the Company.
46
Report of Independent Registered Public Accounting Firm
Board of Directors
IMCOR Pharmaceutical Co. (formerly Photogen Technologies, Inc.)
We have audited the accompanying consolidated balance sheet of IMCOR Pharmaceutical Co. (formerly Photogen Technologies, Inc.), a development stage company, as of December 31, 2003, and the related consolidated statements of operations, shareholders’ equity and cash flows for the period from November 3, 1996 (inception) to December 31, 2003 and for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of IMCOR Pharmaceutical Co. (formerly Photogen Technologies, Inc.) at December 31, 2003, and the results of its operations and its cash flows for the period from November 3, 1996 (inception) to December 31, 2003 and for the year then ended, in conformity with accounting principles generally accepted in the United States.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has reported accumulated losses since inception of $51,518,698, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As further discussed in Note 1A, The Company restated its financial statements to reclassify certain preferred stock to mezzanine equity.
/s/ Moss Adams LLP
Los Angeles, California
March 31, 2004, except for Note 1A and 1B, which is as of June 30, 2004
Report of Independent Registered Public Accounting Firm
Board of Directors
Photogen Technologies, Inc.
New Hope, Pennsylvania
We have audited the accompanying consolidated balance sheet of Photogen Technologies, Inc., a development stage company, as of December 31, 2002, and the related consolidated statements of operations, shareholders’ equity (deficit) and cash flows for the years ended December 31, 2001 and 2002. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Photogen Technologies, Inc. at December 31, 2002, and the results of its operations and its cash flows for the years ended December 31, 2001 and 2002, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has reported accumulated losses $28,592,845 through December 31, 2002 and without additional financing, lacks sufficient working capital to fund operations for the entire year ending December 31, 2003, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As further discussed in Note 1A, the Company restated its financial statements to reclassify certain preferred stock to mezzanine equity.
/s/ BDO Seidman, LLP
Chicago, Illinois
March 7, 2003, except for Note 1A, which is as of June 30, 2004
F-2
IMCOR Pharmaceutical Co.
(A Development Stage Company)
Consolidated Balance Sheets
December 31,
| | 2002
(restated)
| | 2003
(restated)
| |
|---|
| Assets | | | | | | | |
Current Assets |
|
|
|
|
|
|
|
| Cash and cash equivalents | | $ | 6,090,904 | | $ | 1,657,594 | |
| Deposits | | | 98,721 | | | 108,721 | |
| Prepaid expenses | | | 693,313 | | | 492,718 | |
| | |
| |
| |
| Total Current Assets | | $ | 6,882,938 | | $ | 2,259,033 | |
Property, Plant and Equipment, less accumulated depreciation of $14,651 and $996,091, respectively |
|
|
36,000 |
|
|
6,294,560 |
|
Patent Costs, net of amortization of $142,359 and $183,854, respectively |
|
|
357,641 |
|
|
316,146 |
|
Deposits |
|
|
14,383 |
|
|
338,383 |
|
Investment in Alliance |
|
|
1,255,000 |
|
|
— |
|
Purchased Technology, net of amortization of $0 and $658,217, respectively |
|
|
— |
|
|
14,979,929 |
|
Investment in and Advances to Affiliate |
|
|
8,192,452 |
|
|
2,803,114 |
|
| | |
| |
| |
| Total Assets | | $ | 16,738,414 | | $ | 26,991,165 | |
| | |
| |
| |
| Liabilities and Shareholders' Equity/(Deficit) | | | | | | | |
Current Liabilities |
|
|
|
|
|
|
|
| Accounts payable | | $ | 835,003 | | $ | 1,884,791 | |
| Accrued expenses | | | 34,132 | | | 3,281,792 | |
| Accrued equipment lease | | | 401,664 | | | 586,052 | |
| Lines of Credit | | | — | | | 12,064,716 | |
| Notes Payable | | | — | | | 1,250,000 | |
| | |
| |
| |
| Total Current Liabilities | | | 1,270,799 | | | 19,067,351 | |
Deferred contract revenue |
|
|
— |
|
|
2,000,000 |
|
Accrued Equipment Lease |
|
|
401,664 |
|
|
133,888 |
|
Shares Subject to Rescission |
|
|
650,000 |
|
|
— |
|
Puttable Shares |
|
|
— |
|
|
1,969,668 |
|
Mezzanine Equity: Preferred stock; par value $.01 per share; 5,000,000 shares authorized including: Series A Preferred Stock; 12,015 shares authorized, issued and outstanding, liquidation preference $1,000 per share (in aggregate $12,015,000) |
|
|
12,015,000 |
|
|
12,015,000 |
|
Shareholders' Equity/(Deficit) |
|
|
|
|
|
|
|
| Preferred stock; par value $.01 per share; 5,000,000 shares authorized including: | | | | | | | |
| Series A Preferred Stock; 841 shares authorized, issued and outstanding, liquidation preference $1,000 per share (in aggregate $841,000) | | | 8 | | | 8 | |
| Common stock; par value $.001 per share; 150,000,000 shares authorized; 16,137,465 and 19,464,548 shares issued and outstanding, respectively | | | 16,137 | | | 19,465 | |
| Additional paid-in capital | | | 31,130,000 | | | 38,184,323 | |
| Common Stock to be issued | | | — | | | 5,120,159 | |
| Deficit accumulated during the development stage | | | (28,745,194 | ) | | (51,518,697 | ) |
| | |
| |
| |
| Total Shareholders' Equity/(Deficit) | | | 2,400,951 | | | (8,194,743 | ) |
| | |
| |
| |
| Total Liabilities and Shareholders' Equity/(Deficit) | | $ | 16,738,414 | | $ | 26,991,165 | |
| | |
| |
| |
See accompanying notes to consolidated financial statements.
F-3
IMCOR Pharmaceutical Co.
Consolidated Statements of Operations
| | Year Ended
Dec 31, 2001
| | Year Ended
Dec 31, 2002
| | Year Ended
Dec 31, 2003
| | Cumulative
Amounts From
Nov 3, 1996
(Inception) to
Dec 31, 2003
| |
|---|
| Operating Expenses | | | | | | | | | | | | | |
| Research and development | | $ | 437,958 | | $ | 1,227,897 | | $ | 2,529,679 | | $ | 6,886,316 | |
| Sales, general and administrative | | | 3,220,584 | | | 4,030,083 | | | 14,241,767 | | | 30,889,289 | |
| Restructuring charges | | | — | | | 807,483 | | | 136,947 | | | 1,541,455 | |
| Provision for future lease payments | | | 1,264,208 | | | — | | | — | | | 1,264,208 | |
| | |
| |
| |
| |
| |
| Total operating expenses | | | 4,922,750 | | | 6,065,463 | | | 16,908,393 | | | 40,581,268 | |
Loss From Joint Venture |
|
|
(1,952,758 |
) |
|
(3,452,837 |
) |
|
(5,389,338 |
) |
|
(12,304,354 |
) |
Investment Income |
|
|
110,741 |
|
|
1,563 |
|
|
7,394 |
|
|
1,216,335 |
|
Interest Expense |
|
|
(48,429 |
) |
|
(418,466 |
) |
|
(483,167 |
) |
|
(950,062 |
) |
| | |
| |
| |
| |
| |
Loss from continuing operations |
|
|
(6,813,196 |
) |
|
(9,935,203 |
) |
|
(22,773,504 |
) |
|
(52,619,349 |
) |
Discontinued Operations |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations of discontinued therapeutic business | | | (2,909,820 | ) | | (1,496,430 | ) | | — | | | (10,679,101 | ) |
| Gain from split-off of therapeutic business | | | — | | | 11,779,752 | | | — | | | 11,779,752 | |
| | |
| |
| |
| |
| |
| Income (loss) from discontinued operations | | | (2,909,820 | ) | | 10,283,322 | | | — | | | 1,100,651 | |
| | |
| |
| |
| |
| |
| Net Income (Loss) | | | (9,723,016 | ) | | 348,119 | | | (22,773,504 | ) | $ | (51,518,698 | ) |
| | | | | | | | | | | |
| |
| Dividends on Preferred Stock | | | (2,943,755 | ) | | (1,997,723 | ) | | (1,066,280 | ) | | | |
| | |
| |
| |
| | | | |
| Net Loss Applicable to Common Shareholders | | $ | (12,666,771 | ) | $ | (1,649,604 | ) | $ | (23,839,784 | ) | | | |
| | |
| |
| |
| | | | |
| Basic and Diluted Loss Per Common Share from Continuing Operations | | $ | (1.03 | ) | $ | (1.17 | ) | $ | (1.24 | ) | | | |
| | |
| |
| |
| | | | |
| Basic and Diluted Income (Loss) Per Common Share from Discontinued Operations | | $ | (0.30 | ) | $ | 1.01 | | $ | — | | | | |
| | |
| |
| |
| | | | |
| Basic and Diluted Loss Per Common Share | | $ | (1.33 | ) | $ | (0.16 | ) | $ | (1.24 | ) | | | |
| | |
| |
| |
| | | | |
| Weighted Average Number of Common Shares Outstanding—Basic and Diluted | | | 9,507,693 | | | 10,187,619 | | | 19,265,599 | | | | |
| | |
| |
| |
| | | | |
See accompanying notes to consolidated financial statements.
F-4
IMCOR Pharmaceutical Co.
Consolidated Statement of Shareholders' Equity/(Deficit)
| | Preferred Stock
| |
| |
| |
| |
| |
| |
| |
| |
|---|
| | Series A
| | Series B
| | Common Stock
| |
| |
| |
| | Deficit
Accumulated
Development
Stage
| |
| |
|---|
| | Members'
Capital
| | Common Stock
To be
Issued
| | Additional
Paid-in
Capital
| |
| |
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| | Shares
| | Amount
| | Total
| |
|---|
| Contribution of capital | | — | | $ | — | | — | | $ | — | | — | | $ | — | | $ | 7,268 | | | — | | $ | — | | $ | — | | $ | 7,268 | |
| Net loss for the period ended December 31, 1996 | | — | | | — | | — | | | — | | — | | | — | | | (1,779 | ) | | — | | | — | | | — | | | (1,779 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at May 15, 1997 | | — | | $ | — | | — | | $ | — | | — | | $ | — | | $ | 9,000 | | | — | | $ | — | | $ | (3,511 | ) | $ | 5,489 | |
| |
Issuance of common stock |
|
— |
|
|
— |
|
— |
|
|
— |
|
1,578,208 |
|
|
1,578 |
|
|
— |
|
|
— |
|
|
1,801,872 |
|
|
— |
|
|
1,803,450 |
|
| | Effect of recapitalization and merger | | — | | | — | | — | | | — | | 7,421,792 | | | 7,422 | | | (9,000 | ) | | — | | | 1,203,765 | | | 1,732 | | | 1,203,919 | |
| | Cost associated with recapitalization and merger | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (371,111 | ) | | — | | | (371,111 | ) |
| | Net loss for the period May 16, 1997 to December 31, 1997 | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (554,702 | ) | | (554,702 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at December 31, 1997 | | — | | | — | | — | | | — | | 9,000,000 | | | 9,000 | | | — | | | — | | | 2,634,526 | | | (556,481 | ) | | 2,087,045 | |
| |
Issuance of common stock |
|
— |
|
|
— |
|
— |
|
|
— |
|
218,755 |
|
|
219 |
|
|
— |
|
|
— |
|
|
6,999,781 |
|
|
— |
|
|
7,000,000 |
|
| | Costs associated with common stock issuance | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (50,000 | ) | | — | | | (50,000 | ) |
| | Options issued to consultants | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 45,446 | | | — | | | 45,446 | |
| | Net loss for the year ended December 31, 1998 | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (1,973,913 | ) | | (1,973,913 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at December 31, 1998 | | — | | | — | | — | | | — | | 9,218,755 | | | 9,219 | | | — | | | — | | | 9,629,753 | | | (2,530,394 | ) | | 7,108,578 | |
| |
Exercise of stock options |
|
— |
|
|
— |
|
— |
|
|
— |
|
1,125 |
|
|
1 |
|
|
— |
|
|
— |
|
|
50,062 |
|
|
— |
|
|
50,063 |
|
| | Issuance of warrants and options | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 3,664,749 | | | — | | | 3,664,749 | |
| | Issuance of common stock | | — | | | — | | — | | | — | | 125,967 | | | 126 | | | — | | | — | | | 6,082,528 | | | — | | | 6,082,654 | |
| | Issuance of preferred stock | | 12,015 | | | 120 | | — | | | — | | — | | | — | | | — | | | — | | | 11,578,839 | | | — | | | 11,578,959 | |
| | Reclassification of Series A shares as mezzanine equity in accordance with EITF D-98 | | (12,015 | ) | | (120 | ) | — | | | — | | — | | | — | | | — | | | — | | | (11,578,839 | ) | | — | | | (11,578,959 | ) |
| | Net loss for the year ended December 31, 1999 | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (6,052,841 | ) | | (6,052,841 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at December 31, 1999 (restated) | | — | | | — | | — | | | — | | 9,345,847 | | | 9,346 | | | — | | | — | | | 19,427,092 | | | (8,583,235 | ) | | 10,853,203 | |
| |
Stock option compensation |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
125,020 |
|
|
— |
|
|
125,020 |
|
| | Issuance of warrants and options | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 1,366,050 | | | — | | | 1,366,050 | |
| | Issuance of preferred stock dividend | | 841 | | | 8 | | — | | | — | | — | | | — | | | — | | | — | | | (8 | ) | | — | | | — | |
| | Issuance of preferred stock | | — | | | — | | 337,056 | | | 3,370 | | — | | | — | | | — | | | — | | | 5,272,970 | | | — | | | 5,276,340 | |
| | Beneficial accretion of Series A shares reclassified as mezzanine equity | | | | | | | | | | | | | | | | | | | | | | | | (240,464 | ) | | | | | (240,464 | ) |
| | Net loss for the year ended December 31, 2000 | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (10,787,062 | ) | | (10,787,062 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at December 31, 2000 (restated) | | 841 | | | 8 | | 337,056 | | | 3,370 | | 9,345,847 | | | 9,346 | | | — | | | — | | | 25,950,660 | | | (19,370,297 | ) | | 6,593,087 | |
| |
Stock option compensation |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
64,729 |
|
|
— |
|
|
64,729 |
|
| | Issuance of common stock for cash | | — | | | — | | — | | | — | | 49,245 | | | 49 | | | — | | | — | | | 418,674 | | | — | | | 418,723 | |
| | Issuance of common stock in satisfaction Of anti-dilution provision | | — | | | — | | — | | | — | | 190,856 | | | 191 | | | — | | | — | | | (191 | ) | | — | | | — | |
| | Issuance of preferred stock dividend | | — | | | — | | 20,224 | | | 202 | | — | | | — | | | — | | | — | | | (202 | ) | | — | | | — | |
| | Beneficial accretion of Series A shares reclassified as mezzanine equity | | | | | | | | | | | | | | | | | | | | | | | | (195,577 | ) | | | | | (195,577 | ) |
| | Net loss for the year ended December 31, 2001 | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (9,723,016 | ) | | (9,723,016 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at December 31, 2001 (restated) | | 841 | | | 8 | | 357,280 | | | 3,572 | | 9,585,948 | | | 9,586 | | | — | | | — | | | 26,238,093 | | | (29,093,313 | ) | | (2,842,054 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-5
| Balance, at December 31, 2001 (restated) | | 841 | | $ | 8 | | 357,280 | | $ | 3,572 | | 9,585,948 | | $ | 9,586 | | $ | — | | $ | — | | $ | 26,238,093 | | $ | (29,093,313 | ) | $ | (2,842,054 | ) |
| |
Stock option compensation |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
73,870 |
|
|
— |
|
|
73,870 |
|
| | Issuance of warrants for service | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 322,000 | | | — | | | 322,000 | |
| | Issuance of options in settlement of lawsuit | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 806,415 | | | — | | | 806,415 | |
| | Employee compensation from stock options | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 988,184 | | | — | | | 988,184 | |
| | Issuance of preferred stock dividends | | — | | | — | | 40,194 | | | 402 | | — | | | — | | | — | | | — | | | (402 | ) | | — | | | — | |
| | Conversion of Series B to common stock | | — | | | — | | (397,474 | ) | | (3,974 | ) | 422,316 | | | 422 | | | — | | | — | | | 3,552 | | | — | | | — | |
| | Beneficial inducement costs for convertible debt converted | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 206,348 | | | — | | | 206,348 | |
| | Conversion of line of credit with Élan to common stock | | — | | | — | | — | | | — | | 128,437 | | | 128 | | | — | | | — | | | 3,082,359 | | | — | | | 3,082,487 | |
| | Conversion of line of credit with entity controlled by director of company to common stock | | — | | | — | | — | | | — | | 2,314,815 | | | 2,315 | | | — | | | — | | | 2,497,685 | | | — | | | 2,500,000 | |
| | Retirement of common stock returned in shareholder transaction (Note 6(f)) | | — | | | — | | — | | | — | | (5,137,109 | ) | | (5,137 | ) | | — | | | — | | | (12,221,182 | ) | | — | | | (12,226,319 | ) |
| | Issuance of common stock for cash | | — | | | — | | — | | | — | | 8,823,058 | | | 8,823 | | | — | | | — | | | 9,133,078 | | | — | | | 9,141,901 | |
| | Net loss for the year ended December 31, 2002 | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | 348,119 | | | 348,119 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at December 31, 2002 (restated) | | 841 | | $ | 8 | | — | | $ | — | | 16,137,465 | | $ | 16,137 | | $ | — | | $ | — | | $ | 31,130,000 | | $ | (28,745,194 | ) | $ | 2,400,951 | |
| |
Issuance of common stock for cash |
|
— |
|
|
— |
|
— |
|
|
— |
|
27,778 |
|
|
28 |
|
|
— |
|
|
— |
|
|
29,972 |
|
|
— |
|
|
30,000 |
|
| | Issuance of common stock for standstill agreement | | — | | | — | | — | | | — | | 750,000 | | | 750 | | | — | | | — | | | 1,173,000 | | | — | | | 1,173,750 | |
| | Conversion of Series B to common stock | | — | | | — | | — | | | — | | 100 | | | — | | | — | | | — | | | — | | | — | | | — | |
| | Options issued to consultants for services | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 9,200 | | | — | | | 9,200 | |
| | Shares issued to consultant for services | | — | | | — | | — | | | — | | 68,750 | | | 69 | | | — | | | — | | | 132,193 | | | — | | | 132,262 | |
| | Employee compensation from stock options | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 1,236,566 | | | — | | | 1,236,566 | |
| | Shares issued in Technology Purchase | | — | | | — | | — | | | — | | 2,198,137 | | | 2,198 | | | — | | | — | | | 5,581,070 | | | — | | | 5,583,268 | |
| | Shares to be issued in Technology Purchase | | — | | | — | | — | | | — | | — | | | — | | | — | | | 5,043,226 | | | — | | | — | | | 5,043,226 | |
| | Shares previously subject to rescission | | — | | | — | | — | | | — | | 124,627 | | | 125 | | | — | | | — | | | 649,875 | | | — | | | 650,000 | |
| | Shares issued to Xmark for penalties | | — | | | — | | — | | | — | | 98,826 | | | 99 | | | — | | | — | | | 163,577 | | | — | | | 163,676 | |
| | Shares issued to Xmark for interest | | — | | | — | | — | | | — | | 21,649 | | | 22 | | | — | | | — | | | 48,575 | | | — | | | 48,597 | |
| | Shares to be issued to Xmark and other former Alliance creditors for interest and penalties | | — | | | — | | — | | | — | | — | | | — | | | — | | | 76,933 | | | — | | | — | | | 76,933 | |
| | Options exercised through cashless exercise | | — | | | — | | — | | | — | | 37,216 | | | 37 | | | — | | | — | | | (37 | ) | | — | | | — | |
| | Xmark puttable shares classified as mezzanine equity | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (1,969,668 | ) | | — | | | (1,969,668 | ) |
| | Net loss for the year ended December 31, 2003 | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (22,773,504 | ) | | (22,773,504 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, at December 31, 2003 (restated) | | 841 | | $ | 8 | | — | | $ | — | | 19,464,548 | | $ | 19,465 | | $ | — | | $ | 5,120,159 | | $ | 38,184,323 | | $ | (51,518,698 | ) | $ | (8,194,743 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes to consolidated financial statements.
F-6
IMCOR Pharmaceutical Co.
Consolidated Statements of Cash Flows
| | Year Ended
Dec 31, 2001
| | Year Ended
Dec 31, 2002
| | Year Ended
Dec 31, 2003
(restated)
| | Cumulative
Amounts From
Nov 3, 1996
(Inception)
to Dec 31, 2003
(restated)
| |
|---|
| Cash Flows From Operating Activities | | | | | | | | | | | | | |
| Net income (loss) | | $ | (9,723,016 | ) | $ | 348,119 | | $ | (22,773,504 | ) | $ | (51,518,698 | ) |
| Loss from discontinued operations | | | 2,909,820 | | | 1,496,430 | | | — | | | 10,679,101 | |
| Depreciation and amortization | | | 442,191 | | | 371,319 | | | 1,023,080 | | | 2,700,984 | |
| Loss (gain) on disposal of equipment and leasehold improvements | | | (4,617 | ) | | — | | | — | | | 38,424 | |
| Gain on sale of marketable securities | | | — | | | — | | | — | | | (18,503 | ) |
| United States Treasury Notes amortization | | | (1,029 | ) | | — | | | — | | | 12,586 | |
| Gain on sale of therapeutic business | | | — | | | (11,779,752 | ) | | — | | | (11,779,752 | ) |
| Allowance for notes receivable | | | — | | | — | | | 658,217 | | | 658,217 | |
| Stock option compensation | | | 64,729 | | | 1,062,054 | | | 1,245,768 | | | 2,924,338 | |
| Accrued interest on line of credit | | | — | | | — | | | 318,035 | | | 318,035 | |
| Note payable for services rendered | | | — | | | — | | | 329,681 | | | 329,681 | |
| Beneficial inducement costs for convertible notes | | | — | | | 206,348 | | | — | | | 206,348 | |
| Issuance of warrants in exchange for services rendered | | | 760,363 | | | 322,000 | | | — | | | 4,317,091 | |
| Issuance of stock options in settlement of lawsuit | | | — | | | 806,415 | | | — | | | 806,415 | |
| Issuance of stock for standstill agreement | | | — | | | — | | | 1,337,426 | | | 1,337,426 | |
| Issuance of stock for services rendered | | | — | | | — | | | 132,262 | | | 132,262 | |
| Issuance of stock for interest payments and penalties | | | — | | | — | | | 125,530 | | | 125,530 | |
| Loss from investment in affiliate | | | 1,952,758 | | | 3,452,837 | | | 5,389,338 | | | 12,304,354 | |
| Changes in operating assets and liabilities | | | | | | | | | | | | | |
| Prepaid expenses | | | 152,340 | | | (663,538 | ) | | 200,595 | | | (492,718 | ) |
| Interest receivable | | | 93,219 | | | — | | | — | | | — | |
| Receipt of deferred contract revenue | | | — | | | — | | | 2,000,000 | | | 2,000,000 | |
| Accounts payable | | | (267,945 | ) | | 531,229 | | | 1,049,787 | | | 1,954,790 | |
| Accrued expenses | | | (11,278 | ) | | (298,465 | ) | | (174,375 | ) | | 95,334 | |
| Accrued equipment lease | | | 1,123,740 | | | 10,237 | | | (83,388 | ) | | 1,050,589 | |
| | |
| |
| |
| |
| |
| Net cash used in continuing operating activities | | | (2,508,725 | ) | | (4,134,767 | ) | | (9,221,549 | ) | | (21,818,167 | ) |
| | |
| |
| |
| |
| |
| Net cash used in discontinued operations | | | (2,909,820 | ) | | (1,496,430 | ) | | — | | | (10,679,101 | ) |
| | |
| |
| |
| |
| |
| Cash Flows From Investing Activities | | | | | | | | | | | | | |
| Sale of marketable securities | | $ | — | | $ | — | | $ | — | | $ | 2,164,464 | |
| Purchases of marketable securities | | | — | | | — | | | — | | | (2,182,967 | ) |
| Purchases of United States Treasury Notes | | | (4,870,000 | ) | | — | | | — | | | (38,656,973 | ) |
| Sales of United States Treasury Notes | | | 9,815,000 | | | — | | | — | | | 39,778,548 | |
| Purchase of equipment and leasehold Improvements | | | (45,938 | ) | | — | | | — | | | (636,877 | ) |
| Proceeds from sale of equipment | | | 145,551 | | | — | | | — | | | 145,551 | |
| Costs to acquire patent | | | — | | | — | | | — | | | (237,335 | ) |
| Investment in and advances to affiliate | | | (2,245,948 | ) | | (650,609 | ) | | — | | | (15,107,468 | ) |
| Increase in note receivable | | | — | | | (1,255,000 | ) | | — | | | (1,255,000 | ) |
| Decrease (increase) in deposit | | | (14,383 | ) | | 100,000 | | | (334,000 | ) | | (777,753 | ) |
| Purchase ofImagent business | | | — | | | — | | | (5,074,761 | ) | | (5,074,761 | ) |
| | |
| |
| |
| |
| |
| Net cash provided by (used in) investing activities | | | 2,784,282 | | | (1,805,609 | ) | | (5,408,761 | ) | | (21,840,571 | |
| | |
| |
| |
| |
| |
| Net cash used in investing activities of discontinued operations | | | — | | | — | | | — | | | (1,306,676 | ) |
| | |
| |
| |
| |
| |
| Cash Flows From Financing Activities | | | | | | | | | | | | | |
| Principal payments on capital leases | | $ | (18,356 | ) | $ | — | | $ | — | | $ | (291,704 | ) |
| Net proceeds from issuance of equity and mezzanine equity | | | 1,068,723 | | | 9,141,901 | | | 30,000 | | | 40,539,340 | |
| Proceeds from capital contributions by shareholders | | | — | | | — | | | — | | | 1,911,674 | |
| Principal payment on acquisition of debt | | | — | | | — | | | (1,250,000 | ) | | (1,250,000 | ) |
| Proceeds from issuance of debt | | | 2,314,005 | | | 3,032,905 | | | 11,417,000 | | | 16,763,910 | |
| Cost of recapitalization | | | — | | | — | | | — | | | (371,111 | ) |
| | |
| |
| |
| |
| |
| Net cash provided by financing activities | | | 3,364,372 | | | 12,174,806 | | | 10,197,000 | | | 57,302,109 | |
| | |
| |
| |
| |
| |
| Net Increase (Decrease) in Cash and Cash Equivalents | | | 730,109 | | | 4,738,000 | | | (4,433,310 | ) | | 1,657,594 | |
| Cash and Cash Equivalents, at beginning of year | | | 622,795 | | | 1,352,904 | | | 6,090,904 | | | — | |
| | |
| |
| |
| |
| |
| Cash and Cash Equivalents, at end of year | | $ | 1,352,904 | | $ | 6,090,904 | | $ | 1,657,594 | | $ | 1,657,594 | |
| | |
| |
| |
| |
| |
See accompanying notes to consolidated financial statements.
F-7
Noncash Investing and Financing Activities
2003
In 2003, the Company assumed $3,422,036 in accrued expenses and $2,500,000 of notes payable from Alliance Pharmaceutical Corp. issued stock valued at $5,583,268 to certain former creditors of Alliance and agreed to issue stock valued at $5,043,225 to such creditors as part of the acquisition of theImagent business.
2002
The split-off of the therapeutic business in 2002 included equipment and leasehold improvements of $516,567 and accounts payable of $70,000 in exchange for the Company's common stock valued at $12,226,319.
In 2002, debt of $5,346,910 and accrued interest of $235,577 were converted to common stock.
Payments due on the accrued equipment lease were made through a reduction in deposits due to the Company in the amount of $330,649.
F-8
IMCOR Pharmaceutical Co.
Notes to Consolidated Financial Statements
1. Organization, Significant Accounting Policies and Restatement of Financial Statements
Nature of Operations
IMCOR Pharmaceutical Co. ((formerly Photogen Technologies, Inc.), the "Company") is an emerging, development-stage specialty pharmaceutical company focused on developing and marketing medical imaging pharmaceutical products. The company has one FDA approved product,Imagent® (perflexane lipid microspheres), an ultrasound imaging contrast agent that it acquired in June 2003, and two additional contrast agents in development for use in computed tomography (CT) and x-ray imaging with potential applications (1) as a blood pool agent, to diagnose diseased tissue in the cardiovascular system and other organs, which is called PH-50, and (2) to diagnose cancer metastasizing into the lymphatic system, which is called N1177. The product designations PH-50 and N1177 refer to the same chemical entity. The two names distinguish commercialization rights between cardiovascular imaging (and all other indications) (PH-50) to which we retain rights, and the field of lymphography (N1177), which is licensed to our joint venture (Sentigen, Ltd.) with affiliates of Elan. On February 5, 2004, we changed our name to IMCOR Pharmaceutical Co., reflecting the changed nature of our business to imaging pharmaceuticals.
In December 2001, we announced positive preclinical results of PH-50. Based on these studies, the extensive preclinical toxicology and other study work, including the product's use in approximately 65 patients for lymphography, we shifted the strategic research direction of IMCOR to focus on the cardiovascular and lymphography applications. We accomplished this shift in focus also by splitting off our therapeutic line of business, and, in June 2003 acquiring the medical imaging business (the "Imagent business") of Alliance Pharmaceutical Corp. ("Alliance") led byImagent® (perflexane lipid microspheres), an FDA approved diagnostic imaging agent. Please see Note 3 "Purchased Technology" below.
For the two years up to the time of our acquisition of theImagent business, substantially all of our research and development efforts were focused on the preclinical testing of PH-50. During that period, we operated with a very small staff and conducted substantially all of our research activities through contracts with academic institutions and contract research laboratories. In addition, we had conducted research and development activities on the development of certain therapeutic photodynamic therapy applications (namely a product we called PH-10 for the treatment of certain dermatological conditions) and the development of a medical laser. These operations were split off to our founding scientists as of November 12, 2002 and have been recorded as discontinued operations in our financial statements.
PH-50 and N1177 are still in early stages of development. Of these products, based on the availability of capital, we intend to focus our research efforts first on the continued development of PH-50 and secondly, N1177.
Pharmaceutical imaging agents are used with echocardiography, computed tomography ("CT"), nuclear medicine and magnetic resonance imaging ("MRI") equipment in order to increase the diagnostic capabilities of these techniques. By enhancing the contrast between different types of tissues, these intravenously administered contrast agents provide critical anatomical and disease state information that is not obtainable using the equipment alone. There are numerous potential applications for a contrast agent used in imaging parts or all of the cardiovascular system as well as other organs. Our products have the potential to enhance a physician's ability to diagnose certain cardiovascular abnormalities and the spread of cancer in the lymphatic system.
F-9
IMCOR Pharmaceutical Co.
Notes to Consolidated Financial Statements (Continued)
1. Organization, Significant Accounting Policies and Restatement of Financial Statements (Continued)
IMCOR is based in San Diego, California. We have approximately 30 full time employees and a 53,000 square foot building, a portion of which is an FDA approved cGMP (current Good Manufacturing Practices) manufacturing facility, at our San Diego location. Our personnel have expertise in sales, marketing and manufacturing, clinical development, clinical trial design and regulatory approval processes. We also work closely with consultants experienced in drug development and regulatory requirements.
Restatement of Financial Statements and Other Information
A. Restatement of Financial Statements
In 1999, the Company entered into a joint venture with units of Elan. As part of that transaction, the Company issued to Elan International Services 12,015 shares of its Series A Preferred Stock, with an initial value and liquidation preference of $1,000 per such share, or $12,015,000. In a concurrent transaction, Elan and the Company formed a joint venture Sentigen, with Elan and the Company holding 19.9% and 80.1% of the shares of common stock of Sentigen, respectively.
The holders of Series A Preferred Stock were entitled to an in-kind 7% dividend payable semi-annually solely in additional shares of Series A Preferred Stock. In addition, if the Company’s Board of Directors declared a dividend or distribution on the common stock, holders of Series A Preferred Stock would have been entitled to receive the amount of dividend or distribution in the same form as received by the common stockholders as if the Series A Preferred Stock had been converted to common stock. No dividends have been declared or paid on the common stock and the Board of Directors had stated its intention not to do so throughout the period during which the Series A Preferred Stock was outstanding. In addition, the holder of the Series A Preferred Stock had the right, exercisable three years after the date of issuance, to exchange it for the number of additional shares of Sentigen Ltd. that would result in the holder of the Series A Preferred Stock owning 50% of Sentigen Ltd. If the holder of the Series A Preferred Stock exercised this exchange right it would have been obligated to pay the Company 30.1% of the total amount of Sentigen Ltd.‘s development funding through the date of the exchange (which could be have been satisfied through reduction of amounts owed by the Company under a convertible promissory note, by payment of cash, or a combination of the two).
These shares of Series A Preferred Stock, plus accreted dividends, were convertible into shares of the Company’s common stock at any time after October 20, 2001 for a conversion price of $84.68 per share of Series A Preferred, which such value at December 31, 2003 having accumulated to $16,036,810 (or 16,036.81 shares of Series A Preferred stock).
As indicated, Elan had the right to exchange only its original 12,015 shares of the Company’s Series A Preferred Stock for additional shares of Sentigen’s common stock of such an amount to increase Elan’s holdings from the aforementioned 19.9% interest in Sentigen to 50%. Any dividends otherwise accreted as attributable to shares of the Series A Preferred Stock and available for potential conversion into the Company’s common stock, however, were not available in the event that in the alternative Elan had elected to exchange the shares of Series A Preferred Stock in order to increase its Sentigen holdings to 50%.
The Company initially accounted for the issuance of the 12,015 shares of Series A Preferred Stock as permanent equity in the Company’s Consolidated Balance Sheets and Consolidated Statements of Shareholders’ Equity/(Deficit) in the year ended December 31, 1999 and all year ends thereafter. Upon the issuance of EITF D-98 in July 2001, the Company evaluated the above-described transaction and made a determination that the elements were not present to warrant a reclassification of any of the value attributed to the shares of Series A Preferred Stock from permanent equity to mezzanine equity.
On June 30, 2004, the Company reevaluated its position with respect to EITF D-98 and determined that the value attributable to the 12,015 shares held by Elan of the Company’s Series A Preferred Stock, or $12,015,000 should be reclassified as mezzanine equity. The Company further determined that, inasmuch as the otherwise accreted dividends could only be considered as (a) preferential over common shareholders in the event of a liquidation of the Company’s assets, or (b) additional value to be realized in the event that the underlying shares of Series A Preferred Stock were converted into common stock of the Company, that such value should not be reclassified as mezzanine equity, but should remain in permanent equity as such.
Therefore, and in accordance with the provision of EITF D-98, the Company has restated its Consolidated Balance Sheets for the years ended December 31, 2002 and December 31, 2003, together with the Consolidated Statements of Shareholders’ Equity/(Deficit) for all years ended December 31, 1999 to and through December 31, 2003, to which these “Notes to Consolidated Financial Statements” relate, by reclassifying $12,015,000 from permanent equity to mezzanine equity. There is no effect on the Company’s Consolidated Statements of Operations as a result of this reclassification.
The effect of the reclassification is as follows: (a) the mezzanine equity was increased by $12,015,000 (from $0 to $12,015,000) for each of the fiscal years ended December 31, 2002 and 2003; (b) the par value of the Series A Preferred Stock was reduced by $120 (from $128 to $8) for each of the fiscal years ended December 31, 2002 and 2003; and (c) the additional paid-in capital was reduced by $12,014,880 for each of the fiscal years ended December 31, 2002 and 2003 (from $43,144,880 to $31,130,000 for the fiscal year ended December 31, 2002 and from $50,199,203 to $38,184,323 for the fiscal year ended December 31, 2003).
Because the Company did not have any accumulated profits for any of the subject periods, the Company did not record any accreted dividends, which accumulated to $4,021,810 as of December 31, 2003, through the apportionment of accumulated losses within the Consolidated Statements of Shareholders’ Equity/(Deficit) for all years ended December 31, 1999 to and through December 31, 2003.
B. Kyosei Agreement.
In April 2004, the Company received a payment of $2,000,000, less Japanese withholding taxes, from a Japanese pharmaceutical company (see “Liquidity and Basis of Presentation – Going Concern” which follows).
Liquidity and Basis of Presentation—Going Concern
The accompanying financial statements are prepared assuming the Company is a going concern. The Company has reported accumulated losses since inception of $51,518,697. The Company believes it lacks sufficient working capital to fund operations for the entire fiscal year ending December 31, 2004. Substantial additional capital resources will be required to fund its ongoing operations. Management believes there are a number of potential alternatives available to meet the continuing capital requirements such as public or private financings or collaborative agreements. The Company is taking continuing actions to reduce its ongoing expenses. If adequate funds are not available, the Company will be required to significantly curtail its operating plans and my have to sell or license out significant portions of the Company's technology or potential products.
At December 31, 2003, we had a net deficit position (our liabilities, puttable share obligations and mezzanine equity, combined, exceeded our assets). Among other factors, we have reclassified $12,015,000 of our Series A Preferred Stock as mezzanine equity to acknowledge the holder's right to exchange that security for that number of additional shares of Sentigen to increase its ownership of Sentigen to 50%. As discussed below, we believe that the probability of the holder exercising the exchange right is remote.
Under the Certificate of Designations of the Series A Preferred Stock the holder has a Series A Liquidation Preference entitling it as of December 31, 2003 to the first $16,036,810 of funds available for distribution to stockholders upon liquidation of the Company and has the right to convert shares of Series A Preferred Stock into common stock at any time after October 20, 2001 at a conversion price of $84.68 per share. The conversion price is subject to adjustment for certain dilutive events. Alternatively, the holder of Series A Preferred Stock may exchange the initial 12,015 shares of Series A Preferred Stock for common shares of Sentigen so that the holder of Series A Preferred Stock owns 50% of the equity of Sentigen. Shares attributable to dividends are not exchangeable and thus not reclassified as mezzanine equity. The exchange right terminates six years after issuance, or October 19, 2005. As the initial value of the Series A Preferred Stock is exchangeable at the holder's option into additional shares of Sentigen, we have reclassified $12,015,000 as mezzanine equity until such time that the exchange right terminates either by the passage of time or the agreement of the holder to terminate or irrevocably to not exercise the right. We believe that as the development program for N1177 will require several years to complete and as no research had been conducted on N1177 during 2003 due to our capital constraints that the probability of the holder exercising his exchange right prior to the expiration of that right is remote.
On December 16, 2003, we entered into a License Agreement (the "Kyosei Agreement") with Kyosei Pharmaceutical Co. Ltd. ("Kyosei"), a subsidiary of the Sakai Group. The Kyosei Agreement gives Kyosei an exclusive license to develop and marketImagent for all indications in Japan. The terms of the Kyosei Agreement provide for the payment to the company of up to $10 million in fees and development milestone payments plus royalties on commercial sales. Kyosei will also pay the company
F-10
to manufactureImagent for Kyosei's clinical and commercial requirements and we will receive royalties based on commercial sales. In each of December 2003 and April 2004, we received a payment of $2,000,000, less withholding taxes. Additional payments are expected in 2005 and beyond. Future payments from Kyosei are based on certain milestones of clinical development, including the commencement of and completion of clinical studies and approval for sale by Japanese regulatory authorities. Commencement of clinical studies is not expected to be earlier than 2005, and the development program, including regulatory approval, is expected to take four or more years. Accordingly, commercial sales, upon which we will earn royalties, are not expected to commence earlier than 2008. While we believe thatImagent clinical studies will be successful, there can be no assurance that such studies conducted in Japan will be successful or that the product will be approved for sale by Japanese authorities. Please see Revenue Recognition and Deferred Revenue, below.
During the next twelve months we will focus our efforts primarily on exploring various options concerning our business, including securing additional financing, joint ventures, licensing or selling all or a portion of our technology and other possible strategic transactions. Our ability to commercializeImagent and develop PH-50 and N1177 depends on the successful implementation of one or more of these transactions.
Depending on the availability of capital and whether or not we pursue a sale or licensing strategy forImagent, we will focus our operating efforts on the sales and marketing ofImagent. If additional capital becomes available, we also expect to conduct preclinical and human clinical studies of our products in development. Subject to the availability of sufficient capital, we expect to continue to incur losses for at least the next three years as we intensify research and development, preclinical and clinical testing and associated regulatory approval activities and engage in or provide for the manufacture and/or sale of any products that we have or may develop.
Greater capital resources would also enable us to quicken and expand our marketing and research and development activities, and our failure to raise additional capital will (absent a suitable collaborative agreement providing for a third party to take over these functions) significantly impair or curtail our ability to conduct further activities. In any event, complete development and commercialization of our technology will require substantial additional funds. We are seeking to raise capital through the sale of our common stock or other securities in a private placement to fund our immediate and longer-term capital needs.
The financial statements do not include any adjustment to reflect the possible future effects on the recoverability and classification of assets or the amount and classification of liabilities that may result from the outcome of this uncertainty.
Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenue and expenses during the reporting period. Actual results could differ materially from the estimates and assumptions that the Company uses in the preparation of its financial statements.
F-11
Cash Equivalents
Highly liquid investments with a maturity of three months or less when purchased are classified as cash equivalents.
Property, Plant and Equipment
Property, plant and equipment and leasehold improvements are stated at cost. Depreciation and amortization of equipment are provided for using the straight-line method over the estimated useful lives of the assets. Computers and laboratory equipment are being depreciated over three years, and furniture and fixtures are being depreciated over seven years. Leasehold improvements are being amortized on a straight-line basis over the lives of the respective leases or the service lives of the improvements, whichever is shorter.
Long-Lived Assets
The Company reviews the carrying values of its long-lived assets for possible impairment whenever an event or change in circumstances indicates that the carrying amount of the assets may not be recoverable. Any long-lived assets held for disposal are reported at the lower of their carrying amounts or fair value less cost to sell.
Patent Costs
Internal patent costs are expensed in the period incurred. Patents purchased are capitalized and amortized over the remaining life of the patent. Patent amortization expense was $41,666 for each of 2001 2002, and 2003. Amortization of patent costs is expected to be $41,666 annually over the remaining life of the patent, approximately 7.5 years at December 31, 2003.
Purchased Technology
On June 18, 2003, we closed on the acquisition of assets, including Purchased Technology, related to the diagnostic imaging business, includingImagent, an FDA-approved product, of Alliance.Imagent is an intravenous contrast agent for use with ultrasound imaging equipment to improve visualization of blood vessels for better diagnosis of disease and assessment of organ function.Imagent consists of perfluorochemical-based "microbubbles" that are highly echogenic (reflect ultrasound signals strongly) in the bloodstream, thereby significantly enhancing ultrasound images. We also entered into a number of agreements related to that acquisition. Included in the purchase were an FDA-approved manufacturing facility, the marketing, manufacturing and supporting infrastructure for the product and an intellectual property portfolio of approximately thirty patents in the area of ultrasound imaging.
Intangible assets resulting from the acquisition of theImagent business are estimated by management based on the fair value of the assets received. These intangible assets consist entirely of purchased technology. Purchased Technology related to theImagent business is amortized on a straight-line basis over the estimated remaining life equal to twelve years. Purchased Technology is stated at cost, less accumulated amortization.
Research and Development
Research and development costs are charged to expense when incurred.
F-12
Revenue Recognition and Deferred Revenue
The company recognizes revenue from license agreements ratably over the period of the agreement. Unamortized revenue is recorded as deferred revenue. Revenue from the Kyosei Agreement is being recognized over ten years.
Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the tax basis and financial reporting basis of certain assets and liabilities based upon currently enacted tax rates expected to be in effect when such amounts are realized or settled.
Basic and Diluted Loss Per Common Share
Basic and diluted loss per common share is computed based on the weighted average number of common shares outstanding. Loss per share excludes the impact of outstanding options, warrants and convertible preferred stock as they are antidilutive. Potential common shares excluded from the calculation at December 31, 2001 are 6,519,000 options, 1,149,724 warrants, 1,124,268 shares issuable upon the conversion of Series A and B Preferred Stock and 130,162 shares issuable upon conversion of long-term debt. Potential common shares excluded from the calculation at December 31, 2002 are 4,535,481 options, 237,431 warrants and 151,819 shares issuable upon the conversion of Series A Preferred Stock. Potential common shares excluded from the calculation at December 31, 2003 are 7,210,280 options, 377,431 warrants and 193,378 shares issuable upon the conversion of Series A Preferred Stock. Basic and diluted loss per common share in 2003 includes 1,985,522 shares to be issued upon shareholder approval (see Note 3).
Financial Instruments
The carrying amounts reported in the consolidated balance sheets for cash, accounts payable and accrued expenses approximate fair value because of the short-term nature of these amounts. The Company's note payable and lines of credit approximate fair value based on instruments with similar terms.
Stock Options
In December 2002, SFAS No. 148,Accounting for Stock-Based Compensation—Transition and Disclosure—an amendment to FASB Statement No. 123 was issued. SFAS No. 148 provides alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation from the intrinsic value-based method of accounting prescribed by Accounting Principles Board (APB) Opinion No. 25,Accounting for Stock Issued to Employees (APB No. 25). In addition, SFAS No. 148 amends the disclosure requirements of SFAS No. 123,Accounting for Stock Based Compensation. The company adopted the disclosure requirements of SFAS No. 123 effective December 31, 2002. As allowed by SFAS 123, the Company has elected to continue to apply the intrinsic value-based method of accounting proscribed in APB No. 25 and, accordingly, does not recognize compensation expense for stock option grants made to employees at an exercise price equal to or in excess of the fair value of the stock a the date of grant.
F-13
If the Company had elected to recognize compensation expense based on the fair value at the grant dates, consistent with the method prescribed by SFAS No. 123, net loss per basic and diluted share would have been changed to the pro forma amount indicated below:
| | 2001
| | 2002
| | 2003
| |
|---|
| Net loss, as reported | | $ | (12,666,771 | ) | $ | (1,649,604 | ) | $ | (22,773,504 | ) |
| Add stock based employee compensation expense included in reported net loss | | | — | | | 988,184 | | | 1,245,768 | |
| Less total stock-based employee compensation expense determined under the fair value based method for all awards | | | (11,314,458 | ) | | (6,084,842 | ) | | (5,771,226 | ) |
| | |
| |
| |
| |
| Pro forma net loss | | $ | (23,981,229 | ) | $ | (6,746,262 | ) | $ | (27,298,962 | ) |
| | |
| |
| |
| |
| Basic and diluted loss per common share, as reported | | $ | (1.33 | ) | $ | (0.16 | ) | $ | (1.24 | ) |
| Basis and diluted loss per common share, pro forma | | $ | (2.52 | ) | $ | (0.66 | ) | $ | (1.42 | ) |
The Company expenses the fair value of stock options granted to nonemployees. During 2002 and 2003, the Company issued stock options to employees with an exercise price less than the market price on the date of grant. Accordingly, compensation expense of $1,245,768 has been recorded in 2003 and $988,184 was recorded in 2002.
The pro forma information regarding net loss is required by SFAS No. 123, and has been determined as if the Company has accounted for its stock-based compensation under the fair value method prescribed in SFAS No. 123. The fair value of the options was estimated at the date of grant using the Black-Scholes pricing model with the following assumptions:
| | 2001
| | 2002
| | 2003
| |
|---|
| Weighted average fair value per options granted | | $ | 3.64 | | $ | 1.31 | | $ | 1.82 | |
| Significant assumptions (weighted average) | | | | | | | | | | |
| Risk-free interest rate at grant date | | | 4.8 | % | | 2.8 | % | | 2.56 | % |
| Expected stock price volatility | | | 95.5 | % | | 98.0 | % | | 98 | % |
| Expected option life (years) | | | 5 | | | 5 | | | 5 | |
Recent Accounting Pronouncements
In May 2003, the Financial Accounting Standards Board ("FASB") issued SFAS No. 150, "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity," effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective as of July 1, 2003. The adoption of SFAS No. 150 did not have a material impact on the results of operations or the financial position of the Company.
In May 2003, the FASB issued SFAS No. 149, "Amendment of Statement 133 on Derivative Instruments and Hedging Activities," effective for contracts entered into or modified after June 30, 2003 and for hedging relationships designated after June 30, 2003. The adoption of SFAS No. 149 did not have an impact on the results of operations or the financial position of the Company.
F-14
In December 2002, the FASB issued SFAS No. 148, "Accounting for Stock-Based Compensation—Transition and Disclosure", effective for fiscal years ending after December 15, 2002. This rule amends SFAS No. 123 to provide several alternatives for adopting the stock option expense provisions of SFAS No. 123, as well as additional required interim financial statement disclosures. SFAS No. 148 does not require companies to expense stock options in current earnings. The Company has not adopted the provisions of SFAS No. 123 for expensing stock based compensation (see "—Employee stock option and stock purchase plans"); however, the Company has adopted the additional interim disclosure provisions of the statement. The impact of the adoption of SFAS No. 148 did not have a material impact on the results of operations or the financial position of the Company.
In July 2002, the FASB issued SFAS No. 146, "Accounting for Costs Associated with Exit or Disposal Activities." SFAS No. 146 eliminates Emerging Issues Task Force, or EITF, Issue No. 94-3 "Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (Including Certain Costs Incurred in a Restructuring)." Under SFAS No. 146, liabilities for costs associated with an exit or disposal activity are recognized when the liabilities are incurred, as opposed to being recognized at the date of entity's commitment to an exit plan under EITF No. 94-3. Examples of costs covered by the standard include lease termination cost and certain employee severance costs that are associated with a restructuring; discontinued operation, plant closing or other exit or disposal activity. Furthermore, SFAS No. 146 establishes that fair value is the objective for initial measurement of the liabilities. This Statement is effective for exit or disposal activities that are initiated after December 31, 2002. During 2003 the Company recognized $136,947 of restructuring costs for lease termination and restructuring associated with the closure of its Pennsylvania facility and move to the Alliance facility in San Diego, CA.
In January 2003, the FASB issued FASB Interpretation No. ("FIN") 46, "Consolidation of Variable Interest Entities," which was originally effective on July 1, 2003. In December 2003, the FASB deferred the effective date for applying the provisions of FIN 46 to March 31, 2004 for interests held by public companies in variable interest entities or potential variable interest entities created before February 1, 2003. The Company has completed its evaluation of the provisions of FIN 46 and does not have any significant interests in variable interest entities. Accordingly, the adoption of FIN 46 did not have a material impact on the results of operations or the financial position of the Company.
In November 2002, the FASB issued Interpretation No. (FIN) 45, "Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others." Interpretation No. 45 requires a guarantor to include disclosure of certain obligations, and if applicable, at the inception of the guarantee, recognize a liability for the fair value of other certain obligations undertaken in issuing a guarantee. The recognition requirement is effective for guarantees issued or modified after December 31, 2002 and did not have a material impact on the Company.
2. Recapitalization and Merger
On May 16, 1997, MT Financial Group, Inc. (an inactive public company), through its wholly owned subsidiary, effected a reverse merger with Photogen, Inc. ("Photogen"), successor to Photogen, L.L.C. Photogen, L.L.C. was formed on September 10, 1996 and commenced operations November 3, 1996. Photogen, L.L.C. was formed to develop proprietary laser-based technologies related to photodynamic therapy. Legally, Photogen was a wholly owned subsidiary of Photogen Technologies, Inc.
F-15
(formerly known as MT Financial Group, Inc.), which is, as of February 5, 2004, now known as IMCOR Pharmaceutical Co.
For financial reporting purposes, Photogen was deemed to be the acquiring entity. The transaction has been reflected in the accompanying financial statements as (1) a recapitalization of Photogen (consisting of a 48,000-for-one stock split and change in par value) and (2) an issuance of shares by Photogen in exchange for all of the outstanding shares of MT Financial Group, Inc.
As part of the recapitalization, the Company sold 1,578,208 shares of common stock for a total purchase price of approximately $1,803,450. Legal and brokerage fees of approximately $371,000 were charged to additional paid-in capital as costs of the recapitalization and merger. Included in the paid-in capital is the net cash of approximately $109,000 from MT Financial Group, Inc.
3. Purchased Technology
On June 18, 2003, the Company acquired the medical imaging business includingImagent® (perflexane lipid microspheres), an FDA-approved product, of Alliance Pharmaceutical Corp. The Company's financial statements include the results of operations for the Purchased Technology from the closing date of the transaction, June 18, 2003, through the end of the reporting period
Included in the purchase were an FDA-approved manufacturing facility, the marketing, manufacturing and supporting infrastructure for the product, and an intellectual property portfolio of approximately thirty patents in the area of ultrasound imaging.Imagent has been approved for marketing by the FDA for use in patients with sub optimal echocardiograms to opacify the left ventricle and thereby improve visualization of the main pumping chamber of the heart, and to improve delineation of the endocardial borders of the heart. As a result, ultrasound withImagent may better distinguish normal and abnormal heart structure and function—two critical indicators of cardiac health. Echocardiography is the most widely used imaging modality for the diagnosis of heart disease, the leading cause of death in the U.S. The attractiveness of the ultrasound imaging market potential together with the acquisition of an approved product and an extensive intellectual property portfolio were fundamental reasons for the acquisition and primary determinants of the purchase price.
F-16
The Purchased Technology acquisition costs and allocation to the assets acquired and liabilities assumed including adjustments in the third and fourth quarters 2003 is as follows:
| Summary of Purchase Transaction: | | | |
| Fair value of common stock issued | | $ | 5,583,268 |
| Fair value of common stock to be issued | | | 5,043,226 |
| Cash advances in prior year applied | | | 1,255,000 |
| Net current year cash advances applied | | | 2,763,052 |
| Cash paid at closing | | | 669,117 |
| Direct current year payments to vendors on behalf of Alliance | | | 388,387 |
| Assumption of debt to Xmark | | | 2,500,000 |
| Assumption of certain Alliance liabilities | | | 3,422,036 |
| Acquisition costs | | | 1,254,205 |
| | |
|
| Purchase Price | | $ | 22,878,291 |
| Fair Value of Assets Acquired and Liabilities Assumed: | | | |
| Property, plant & equipment | | $ | 7,240,145 |
| Purchased technology | | | 15,638,146 |
| | |
|
| Fair Value of Assets Acquired | | $ | 22,878,291 |
At the closing, the Company paid approximately $669,000 in cash and delivered 2,198,137 shares of its common stock. In addition to bridge financing the Company provided to Alliance before the closing, its purchase of a $1,250,000 interest in Alliance's obligations to two of its creditors, Xmark Fund, Ltd. and Xmark Fund, L.P. (collectively "Xmark"), and its assumption of specified Alliance operating liabilities related toImagent, the Company is obligated to pay, at various times between 90 and 365 days after the closing, (i) an aggregate of $2,500,000 to Xmark, (ii) subject to reaching satisfactory agreements with certain Alliance secured and unsecured creditors, an aggregate amount of up to $3,304,793 to creditors other than Xmark and (iii) subject to stockholder approval, deliver an aggregate of approximately 1,985,522 shares of its common stock. On February 5, 2004, the shareholders approved the issuance of the additional shares of common stock.
We are currently in default under obligations to pay $775,000 to creditors of Alliance Pharmaceutical Co. in connection with our acquisition ofImagent and we are in default under an equipment lease and the lessor has demanded approximately $787,000. We also have not registered the resale of shares we issued in connection with that acquisition, which in some cases is a condition to the effectiveness of agreements to release us from any claims by the Alliance creditor.
The Company must also pay Alliance further consideration in the form of an earn out based onImagent revenue, subject to certain reductions, after the closing and through June 2010. This earn out equals, for each year of the earn out: 7.5% ofImagent revenue up to $20,000,000; 10% ofImagent revenue between $20,000,000 and $30,000,000; 15% ofImagent revenue between $30,000,000 and $40,000,000; and 20% ofImagent revenue above $40,000,000. The earn out will be reduced by amounts the Company pays pursuant to a license agreement with Schering Aktiengesellschaft ("Schering"), net of payments received from Schering under the license, and amounts of any indemnification claims the Company has against Alliance. The earn out is subject to three additional offsets (which are to be applied in the manner described in the Asset Purchase Agreement dated as of June 10, 2003 with Alliance) that entitle the Company to retain portions of the earn out otherwise payable to Alliance.
F-17
Due to the absence of current or expected revenue from Imagent, management is not able to, and has not, included the present value of future estimated earn out payments in determination of the consideration paid in the acquisition. Future royalty obligations will be expensed in the period reasonably estimatable.
Intangible assets resulting from the acquisition of theImagent business are estimated by management based on the fair value of the assets received. These intangible assets consist entirely of purchased technology. Purchased Technology related to theImagent business is amortized on a straight-line basis over the estimated remaining life equal to twelve years. Purchased Technology is stated at cost, less accumulated amortization.
Following is summarized statements of revenues and direct expenses for the Purchased Technology for the year ended December 31, 2002 and the approximate six-month period ended June 18, 2003 (date of purchase):
| | Year ended
12/31/02
| | Six-Month
period ended
6/18/03
| | Total for
Periods
|
|---|
| Expenses: | | | | | | | | | |
| | Research & development | | $ | 12,775,041 | | $ | 4,501,299 | | $ | 17,276,341 |
| | Sales, general and administrative | | | 5,080,021 | | | 1,363,973 | | | 6,443,995 |
| | |
| |
| |
|
| Total Expenses | | $ | 17,855,062 | | $ | 5,865,272 | | $ | 23,720,336 |
| | |
| |
| |
|
Below is supplemental unaudited pro forma information that discloses the results of operations for the Purchased Technology for the current three and twelve month periods ending December 31, 2003, as well as the corresponding periods for the preceding year ending December 31, 2002. The pro forma information is as if the acquisition had been completed as of the beginning of the periods presented. The unaudited pro forma information give effect to actual operating results prior to the acquisition,
F-18
adjusted to include the unaudited pro forma effect of amortization of intangibles and weighted shares outstanding:
| | Pro Forma
12 Months
ended
Dec 31,
2003
| | 12 Months
ended
Dec 31,
2002
| |
|---|
| Expenses: | | | | | | | |
| | Research & development | | $ | 7,030,978 | | $ | 14,002,984 | |
| | Sales, general and administrative | | | 16,387,260 | | | 10,399,159 | |
| | Restructuring charge | | | — | | | 807,483 | |
| | |
| |
| |
| Total Expenses | | | 23,418,238 | | | 25,209,626 | |
| Loss from joint venture | | | (5,389,338 | ) | | (3,452,837 | ) |
| Investment income | | | 7,394 | | | 1,563 | |
| Interest expense | | | (483,167 | ) | | (418,466 | ) |
| | |
| |
| |
| Loss from continuing operations | | | (29,283,349 | ) | | (29,079,366 | ) |
| Discontinued operations—net | | | — | | | 10,283,322 | |
| | |
| |
| |
| Net income (Loss) | | $ | (29,283,349 | ) | $ | (18,796,044 | ) |
| | |
| |
| |
| Dividends on Preferred Stock | | | (1,066,280 | ) | | (1,997,723 | ) |
| | |
| |
| |
| Net income (loss) applicable to common shareholder | | | (30,349,629 | ) | | (20,793,767 | ) |
| | |
| |
| |
| Pro forma net income (loss) per share | | $ | (1.58 | ) | $ | (1.45 | ) |
| | |
| |
| |
| Weighted average number of common shares outstanding—basic and diluted | | | 19,265,599 | | | 14,371,265 | |
| | |
| |
| |
The statement of revenues and expenses ofImagent are based upon an audit of that business as filed on Form 8-K/A on October 27, 2003.
As an integrated operation of Alliance, theImagent business did not, in the normal course of operations, prepare separate financial statements in accordance with accounting principles generally accepted in the United States. The accompanying financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission ("SEC") for presentation of less than full financial statements and for inclusion in our Current Report on Form 8-K. These financial statements are not intended to be a complete presentation of theImagent business' financial position and operating results. The Statement of Assets Acquired and Liabilities Assumed include assets and liabilities that are purchased or assumed by the Company. The Statement of Revenues and Direct Expenses include direct charges for expenses and indirect charges for other common expenses and corporate expenses. Common expenses include, but are not limited to, shared services, such as human resources, accounting, information technology, and legal services. Common and corporate expenses are charged to the business unit based on direct labor hours, which is deemed to be a practical and reasonable method. There was no direct interest expense incurred by or allocated to theImagent business, therefore, no interest expense has been reflected in these financial statements. These financial statements are not necessarily indicative of the results of operations that would have occurred if theImagent business had been an independent company.
F-19
The pro forma data give effect to actual operating results prior to the acquisition, adjusted to include the pro forma effect of:
- •
- Amortization of intangibles (totaling $15,136,282) of the Purchased Technology of $1,261,357 and $630,678 for the twelve- and six-month periods ended December 31, 2002 and June 30, 2003, respectively. The estimated life of the purchased technology is twelve years.
- •
- The issuance of 4,183,659 shares of common stock related to the acquisition of the Purchased Technology incorporated into the calculation of weighted shares outstanding.
This unaudited pro forma information is not necessarily indicative of the actual results that would have been achieved had the Purchased Technology been acquired the first day of the Company's twelve- or six-month periods, nor is it necessarily indicative of future results.
4. Joint Venture/Investment in Affiliate
On October 7, 1999, the Company formed a joint venture with Elan International Services, Ltd. ("Elan") to develop and commercialize nanoparticulate diagnostic imaging agents for the detection and treatment of cancer through lymphography. Our goal is to demonstrate that the use of N1177 will enable physicians to precisely locate tumorous nodes by first injecting the material in the vicinity of the tumor and then taking an image (picture) using a CT device.
We plan to commence Phase 2 clinical studies for detection of cancer metastasis in lymph nodes in patients with cervical cancer, subject to the availability of sufficient capital. In this patient population, studies document an increase in survival when lymph nodes containing cancer are removed. Tumor identification by CT imaging will be correlated to the results of biopsy results from patients undergoing surgery to demonstrate effectiveness in locating nodes and identifying those with cancer in them.
Sentigen Ltd. ("Sentigen") was formed to hold the operations, assets and liabilities of the joint venture. Elan purchased 2,980 shares of Sentigen nonvoting convertible preferred stock for $2,985,000 representing a 19.9% ownership interest. Elan also purchased 12,015 shares of the Company's Series A convertible exchangeable preferred stock for $12,015,000. In connection with the issuance of the preferred stock to Elan, the Company granted warrants to Elan to purchase 100,000 shares of the Company's common stock at $84.68 per share which was valued at $678,000. As a result, a preferred stock dividend of $436,041 was recorded for the accretion of the preferred stock to its face value of $12,015,000 over the period until it is first convertible. Beginning in 2000, in accordance with EITF 00-27, the Company recorded an additional preferred stock dividend to represent the beneficial conversion feature associated with the intrinsic value of the Series A Preferred conversion option. As a result, an additional preferred stock dividend of $436,041 was recorded over the period until it is first convertible. As of December 31, 2001, the beneficial conversion feature was fully recorded.
The Company purchased 12,000 shares of Sentigen's common stock for $12,015,000 representing an 80.1% ownership interest.
Sentigen used the $15,000,000 received from the Company and Elan to purchase from Elan a worldwide license to certain patented Elan technology related to the joint venture. The acquisition of the license by Sentigen was for certain issued patents with multiple applications and not for the use of pending or in process research and development, and therefore capitalized. Expenses incurred by the
F-20
Company and Elan, which relate to the development of the diagnostic imaging agents, are charged to Sentigen.
As part of our annual audits, we, on behalf of Sentigen, prepared cash flow projections of the product, N1177 including assumptions of research and clinical development costs, market size, market share and resulting potential revenues and the costs (manufacturing, marketing and sales along with administrative costs) to generate the projected revenue stream. These cash flows, assuming that the research is successful, were then discounted to a present value. During the year, we incorporated these projections as part of the analysis of any impairment in the value of the joint venture.
As part of our review of our financial statements at year end 2002, we evaluated the carrying value of the Sentigen license in light of the projection of cash flows and availability of capital to conduct the research in a timely manner. Accordingly, we advised Sentigen to reduce the carrying value of the Elan license by $2,500,000.
Further, as part of our review of our financial statements for the quarter ended June 30, 2003, we again evaluated the carrying value of the Sentigen license in light of the projection of cash flows, the availability of capital to conduct the research in a timely manner, the knowledge that this area of research was no longer an area stated by Elan to be an area of focus for that company, and the priority of committing capital to the further development of N1177 in light of our other priorities, namely the continued development of PH-50 and the manufacturing and marketing of its recently acquired Imagent Business. Accordingly, we advised Sentigen to reduce the carrying value of the Elan license by $5,750,000 to a value of approximately $4,060,000, it being also determined, in the judgment of certain of our directors with experience in these matters, that a value of approximately $4,000,000 could be fair should Sentigen either sell or license N1177 to a third party.
Elan granted the Company a line of credit of $4,806,000 to be used by the Company to fund its portion of Sentigen's research and development. Principal and interest under the line of credit, if any, become due and payable in 2005 or, at the option of Elan, can be converted into the Company's common stock at $72.60 per share. Any borrowings under the line of credit would bear interest at 8%. At December 31, 2001, borrowings on this line of credit were $2,314,005 and accrued interest was $48,429. During 2002, the Company borrowed an additional $532,905 and incurred additional accrued interest of $187,148. On November 12, 2002, the total borrowings of $2,846,910 and accrued interest of $235,577 were converted to common stock at a conversion price of $24 per share and the line of credit was cancelled. A beneficial conversion amount of $206,348 was recorded as a result of the decrease in the original conversion price.
In connection with the joint venture agreement, Elan also purchased 115,385 shares of the Company's common stock for $6,000,000. The total cash proceeds from the equity issuance to Elan of $18,015,000 were allocated to the common stock, preferred stock and the warrants. If in the Company's next third-party offering after the issuance of the Series A Preferred, it sells common stock at a price less than $52 per share, the Company must issue additional shares to Elan so that Elan's overall price for its common stock equals the effective price in that third-party offering. During 2001, the Company issued 190,857 shares of common stock to satisfy this requirement. Additionally, proceeds received under the line of credit amounting to $4,806,000 was allocated to common stock upon conversion.
Elan has substantive participating rights in Sentigen, including the requirement for approval of the business plan and budgets and equal representation on the management committee which decides all day-to-day functions. As a result, the Company's investment in Sentigen is recorded under the equity method.
F-21
Following is summarized unaudited financial information for Sentigen at December 31, 2002 and 2003 and for the years then ended:
| | 2002
| | 2003
| |
|---|
| Cash | | $ | 410 | | $ | 410 | |
| License purchased from Elan, net of amortization and impairment of $4,701,693 and $11,429,357 respectively | | | 10,298,907 | | | 3,570,643 | |
| | |
| |
| |
| Total assets | | $ | 10,299,317 | | $ | 3,571,053 | |
| | |
| |
| |
| Due to affiliates | | $ | 378,099 | | $ | 378,099 | |
| Total shareholders' equity | | | 9,921,218 | | | 3,192,954 | |
| | |
| |
| |
| Total liabilities and equity | | $ | 10,299,317 | | $ | 3,571,053 | |
| | |
| |
| |
| Research and development expense | | $ | 823,409 | | $ | — | |
| General and administrative expense | | | 18,329 | | | — | |
| Amortization of license | | | 978,264 | | | 978,264 | |
| Impairment of license | | | 2,500,000 | | | 5,750,000 | |
| | |
| |
| |
| Net loss | | $ | (4,320,002 | ) | $ | (6,728,264 | ) |
| | |
| |
| |
5. Property, Plant and Equipment
Property, plant and equipment consist of the following at December 31, 2002 and 2003:
December 31,
| | 2002
| | 2003
| |
|---|
| Leasehold improvements | | $ | 33,131 | | $ | 5,527,738 | |
| Furniture, fixtures and equipment | | | 17,520 | | | 1,762,913 | |
| | |
| |
| |
| | Total | | | 50,651 | | | 7,290,651 | |
| Accumulated depreciation and amortization | | | (14,651 | ) | | (996,091 | ) |
| | |
| |
| |
| | Property, plant and equipment, net | | $ | 36,000 | | $ | 6,294,560 | |
| | |
| |
| |
Maintenance and repairs are charged to operations as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is included in operations.
Depreciation and amortization expense on property, plant and equipment amounted to $400,525, $329,653, and $981,440 during 2001, 2002 and 2003, respectively and $2,517,130 for the period from inception to December 31, 2003.
F-22
6. Accruals
Accrued expenses consist of the following:
December 31,
| | 2002
| | 2003
|
|---|
| Alliance liabilities assumed from acquisition | | $ | — | | $ | 2,530,434 |
| New Hope office abandonment provision | | | — | | | 136,947 |
| Accrued general trade payables | | | — | | | 291,908 |
| Payroll liabilities | | | 34,132 | | | 322,503 |
| | |
| |
|
| | | $ | 34,132 | | $ | 3,281,792 |
| | |
| |
|
7. Commitments and Contingencies
Leases: The Company has leased its facilities and certain equipment under non-cancelable operating leases, which expire at various times through 2008. The leases require the Company to pay for all maintenance, insurance and property taxes.
On August 31, 2003, the Company closed its Pennsylvania corporate headquarters and transferred its headquarters to itsImagent facility in San Diego, California. At December 31, 2003, the remaining obligation under the lease amounts to $136,947.
The Company also has an operating lease for laboratory equipment which expires during 2004. During 2001, the Company determined that equipment leased by the Company under this operating lease would no longer be used by the Company in its research. As a result, in 2001, the Company recorded a provision for all future lease payments of $1,264,208. At December 31, 2003, $786,884 remains to be paid. Minimum future rental payments under these leases are as follows:
Year ending December 31,
| | Operating Leases
|
|---|
| 2004 | | $ | 1,455,180 |
| 2005 | | | 979,944 |
| 2006 | | | 920,625 |
| 2007 | | | 877,514 |
| 2008 | | | 147,081 |
| | |
|
| Total minimum lease payments | | $ | 4,380,344 |
| | |
|
Total rental expense charged to operations for the years ended December 31, 2001, 2002, and 2003 aggregated approximately $358,000, $227,000, and $984,945, respectively.
License Agreements: In September 1997, Alliance entered into the Schering License Agreement, which provided Schering with worldwide exclusive marketing and manufacturing rights, drug compositions, and medical devices and systems related to perfluorocarbon ultrasound imaging products, includingImagent. In February 2002, the agreement with Schering was modified to give Alliance exclusive rights to market the product in the U.S. for cardiology indications for a five-year period, with Schering to be paid a nominal royalty on such sales. At the expiration of the five-year period, Alliance had the option to acquire all of Schering's rights to the product on a worldwide basis or, alternatively, to allow Schering the opportunity to obtain co-promotion rights along with Alliance. The rights Alliance had under the Schering License Agreement were transferred to IMCOR at the consummation of its acquisition of theImagent Business.
F-23
Concurrent with the restructuring of the Schering agreement in 2002, Alliance entered into a five-year exclusive agreement with Cardinal Health, Inc. ("Cardinal") to assist the marketing ofImagent. Under the agreement, Cardinal will perform certain packaging, distribution, and sales services for Alliance under a fee-for-service arrangement. IMCOR assumed the Cardinal agreement from Alliance at the consummation of theImagent acquisition.
Litigation: On June 18, 2003, we filed a complaint against Amersham Health, Inc. and two other Amersham affiliates alleging that certain Amersham products infringe on claims in our patents coveringImagent. The case is captioned asPhotogen Technologies, Inc. and Alliance Pharmaceutical Corp. v. Amersham Health Inc. et. al., Civil Action No. 03CV2853(SRC), in the United States District Court for the District of New Jersey. Our complaint alleges that principally through their Optison® product, Amersham Health Inc. and related Amersham entities infringe on seven patents owned by us. We are also seeking a declaration that the claims of fourteen Amersham patents are invalid and are not infringed by ourImagent product. Alliance, the party from whom we recently acquired theImagent product, is also a plaintiff in the suit. We are seeking damages, an injunction against Amersham, a declaratory judgment and other relief.
Amersham filed an answer dated July 18, 2003 denying the material allegations of our claims and asserting certain affirmative defenses and counterclaims. Amersham's counterclaims against us include claims of patent infringement, breach of contract, breach of good faith and fair dealing, and tortuous interference with contract. We are studying Amersham's answer, defenses and counterclaims in consultation with our counsel.
It is not presently feasible to determine whether there is a reasonable possibility that a loss may have occurred. Such a determination will only be possible after the facts and circumstances of the litigation have been established and, potentially, litigated before a court of competent jurisdiction. Intellectual property disputes are often settled through licensing arrangements, which if resolved unfavorably, could be costly to us or ultimately make it unfeasible to market the product. In any intellectual property litigation, it is possible that licenses necessary to settle the dispute would not be available on commercially reasonable terms or that we would not be able to redesign our technologies to avoid any claimed infringement thus resulting in our inability to sell the product.
On July 29, 2003, the European Patent Office revoked Amersham's European Patent (EP) No. 620744 with claims directed to contrast agents. We had brought the opposition proceeding against the EP "744 patent in the European Patent Office. The EP "744 is a counterpart to the patents that are the subject of our suit against Amersham in the United States described above. See "Risk Factors—We filed a suit against Amersham for patent infringement, which, if resolved unfavorably, could impede our ability to utilize our patents forImagent," above.
8. Secured Debt
Notes payable—At the closing of the Technology Purchase, the Company issued to Xmark promissory notes in the total principal amount of $2,500,000. The notes were payable in two equal installments on August 5 and November 3, 2003 or (if sooner) at the time the Company completed a financing of at least $18,000,000 of gross proceeds and bear interest at 3% over the prime rate. Interest is payable in shares of common stock.
F-24
Obligations to Xmark (including obligations under the put right) are secured by a first priority security interest on theImagent related tangible and intangible assets.
The Company defaulted on the principal payment due to Xmark on August 5, 2003 and Xmark delivered a notice stating that it was accelerating all amounts due under the notes. On August 18, 2003, the Company and Xmark executed a Letter Agreement pursuant to which the Company made an immediate payment of $1,250,000 and Xmark rescinded the default notice. In addition, as part of the Letter Agreement:
- •
- The Company has the right to extend the November 3, 2003 payment due date monthly until April 3, 2004 for a fee (and not as a reduction of any amounts due and owing under the promissory notes) ranging from $50,000 to $100,000 per month. The Company has been exercising its right to extend the November 3, 2003 payment.
- •
- The demand registration rights held by Xmark became piggyback registration rights.
- •
- The definition of "Event of Default" was amended. The provision that an Event of Default included any default by the Company in the payment of borrowed money indebtedness was eliminated. Instead, the Company agreed that it would be an Event of Default if it made any repayments of borrowed money indebtedness.
- •
- If the Company makes payments to certain institutions, it will make a pro-rata payment to Xmark.
Lines of Credit—To obtain funds for operations and certain cash payments related to the Technology Purchase, the Company issued $11,417,000 in notes, of which $4,160,000 are Revolving Convertible Senior Secured Promissory Notes ("Revolving Notes") and the balances are non-convertible ("Demand Notes") (collectively, the "Promissory Notes") to three institutional investors ("Investors"). The Promissory Notes are secured by a first priority security interest on all the Company's assets (but as to the lien onImagent related assets, the lien is subordinate to the security interest held by Xmark see Notes 3, 8 and 9), bear interest at 7.25% per annum, compounded monthly, and were due on the earlier of August 5, 2003 (in the case of the Revolving Notes) or are due upon demand (in the case of the Demand Notes) or are due upon the occurrence of an "acceleration event" (in the case of all Promissory Notes). All principal and interest under the Revolving Notes was to convert into the Company's common stock upon completion of a qualified financing that closes before August 5, 2003 at the same price and on the same terms as are in effect for the other investors in the qualified financing; and the Investors will have the same rights, benefits and obligations as other investors in that financing. No such financing had occurred as of August 5, 2003. The acceleration events include a breach of the Company's obligations or representations under a security agreement in favor of the Investors; if the Company files for bankruptcy protection or similar events occur indicating insolvency; if the Company liquidates, or if the Company incurs any debt senior orpari passu to the debt of the Investors.
The Company defaulted on $4,160,000 of its Revolving Notes due to the Investors on August 5, 2003. The Investors have not asserted any of their rights under the default provisions of the Revolving Notes and (although under no obligation to do so) have continued to advance capital, pursuant to the Demand Notes, to the Company. All other terms and conditions of these Revolving Notes remain unchanged.
F-25
9. Stockholders' Equity Transactions, Series A Preferred Stock Classification and Puttable Shares
(a) The Company applies the recognition provisions of SFAS No. 123, "Accounting for Stock-Based Compensation," and applies the fair value-based method of accounting for stock options issued to nonemployees,. The Company has issued options and warrants in exchange for consulting services rendered. The Company issued 10,000 options in 1998, 2,500 options and 125,500 warrants in 1999, 5,250 options and 128,750 warrants in 2000, 7,500 options in 2001, 200,000 warrants in 2002 and 10,000 options in 2003 to nonemployees. During 2002 the Company issued 190,000 warrants to vest upon achievement of certain milestones. As of December 31, 2003 the warrants are unvested and no expense has been recognized. As the fair market value of these services was not readily determinable, these services were valued based on the fair market value of the options and warrants issued, determined using the Black-Scholes option pricing model (see Note 1). Fair market value for options and warrants issued for consulting services were determined to be $3.08 in 2001, $1.01 to $1.81 in 2002 and $1.60 in 2003. Consulting costs charged to operations were $825,092 in 2001, $395,870 in 2002 and $16,521 in 2003. In 2002, the Company also issued 390,556 options in settlement of a lawsuit associated with restructuring costs. The value of these options under the Black-Scholes option pricing model was $806,415 which was recorded as restructuring charges in 2002.
(b) Series A Preferred Stock—In October 1999 the Company issued 12,015 shares of preferred stock as Series A Preferred Stock ("Series A Preferred"). For the first six years from issuance, the preferred stock has a mandatory dividend of 7%. Such dividends are cumulative and compound on a semiannual basis and are payable semiannually solely by the issuance of additional preferred Series A stock. The Company has a deficit and, as a result, the dividends have been recorded against paid-in capital. In accordance with the provisions of the agreement, the Company issued additional preferred shares of 841 in 2000 in settlement of dividend requirements. Therefore, at December 31, 2003, there were 12,856 shares of Series A Preferred outstanding. The Company has accrued and will issue 3,181 preferred shares in fulfillment of the 2001, 2002 and 2003 dividend requirements. The Series A Preferred shall rank senior to any future series of preferred stock unless the majority of the Series A shareholders agree to rankpari passu with any future issuances. The liquidation preference is $1,000 per share. The Series A Preferred is convertible, after two years but before six years from issuance, into common stock of the Company with an initial conversion price of $84.68.
Under the Certificate of Designations of the Series A Preferred Stock the holder has a Series A Liquidation Preference entitling it as of December 31, 2003 to the first $16,036,810 of funds available for distribution to stockholders upon liquidation of the Company and has the right to convert shares of Series A Preferred Stock into common stock at any time after October 20, 2001 at a conversion price of $84.68 per share. The conversion price is subject to adjustment for certain dilutive events. Alternatively, the holder of the Series A Preferred Stock may exchange the initial 12,015 shares of Series A Preferred Stock for common shares of Sentigen so that the holder of Series A Preferred Stock owns 50% of the equity of Sentigen. The exchange right terminates six years after issuance, or October 19, 2005. As the initial value of the Series A Preferred Stock is exchangeable at the holder's option into additional shares of Sentigen, we have reclassified $12,015,000 as mezzanine equity until such time that the exchange right terminates either by the passage of time or the agreement of the holder to terminate or irrevocably to not exercise the right. We believe that as the development program for N1177 will require several years to complete and as no research had been conducted on N1177 during 2003 due to our capital constraints that the probability of the holder exercising his exchange right prior to the expiration of that right is remote.
F-26
The holder of Series A Preferred is only entitled to vote on matters that adversely affect or change the rights of holders of Series A Preferred and does not have the right to vote on the election of directors of the Company. The holder of Series A Preferred also had a preemptive right until October 2003 to participate in any offering of the Company.
(c) Series B Preferred Stock—In February 2000, the Company completed a private placement of its Series B Convertible Preferred Stock ("Series B Preferred"). The Company received cash proceeds, net of related expenses, of $5,276,340 in exchange for 337,056 shares of the Series B Preferred. The Series B Preferred had an annual dividend rate of 6%. In accordance with the provisions of the agreement, the Company issued additional preferred shares of 20,224 in 2001 and 40,194 in 2002 in settlement of dividend requirements.
In conjunction with the financing on November 12, 2002 all of the Series B Preferred was converted into common stock. In exchange for this conversion, the then conversion ratio of 0.36175 was changed to 1.0625. As a result, the Series B Preferred was converted into 422,316 shares of common stock and a beneficial conversion amount of $668,464 has been recorded. The Series B Preferred is no longer outstanding, nor is there any liquidation preference associated with the Series B Preferred.
(d) Common Stock—The Company is subject to six sets of registration rights agreements covering an aggregate of approximately 15,028,000 shares of the Company's common stock, options and warrants that have vested covering the Company's common stock, its common stock issued/issuable pursuant to the acquisition of the Imagent Business (including shares issued/issuable to Xmark) and common stock issuable upon conversion of the Company's Series A Preferred. Excluded from this total are shares of common stock issuable pursuant to the Company's Revolving Convertible Senior Secured Promissory Notes as this conversion rate is dependant upon the pricing of the Company's next Qualified Equity Financing, as defined in the terms of the notes. All of the agreements contain piggyback registration rights and two contain demand registration rights upon the occurrence of certain events and subject to various terms and conditions. Of the 15,028,000 shares, 4,502,000 shares are entitled to piggyback registration only, 50,000 shares are entitled to both demand and piggyback registration and 10,244,000 shares will be registered by the Company within the time frames set forth in the respective registration agreements. Warrants that have vested covering 187,431 shares of the Company's common stock have weighted average anti-dilution rights.
On March 13, 1998, the Company completed a private placement of 218,755 shares of common stock for $32 per share to a number of accredited investors. The Company received $6,950,000, net of related expenses of approximately $50,000 from this offering.
On February 9, 2001, the Company's shelf registration on Form S-3 was declared effective. Under the shelf registration, the Company could have issued up to $40,000,000 of common stock. As of December 31, 2002, 173,872 shares had been issued under the shelf registration for cash proceeds of $1,068,723, net of related expenses of $56,252. The Company's registration statement is no longer effective.
As of April 2, 2001, the Company no longer met the eligibility requirements to continue selling shares under the S-3. Prior to realizing it was ineligible to continue using the S-3, the Company sold 124,627 shares of common stock for $650,000. Through June 2003, this amount had been reclassified from permanent equity to mezzanine equity as the Company could be required to repay a portion of
F-27
this amount to the purchaser. In the second quarter of 2003 the Company recognized in permanent equity the issuance of these shares.
On November 12, 2002, the Company completed a private placement of 8,333,334 shares of common stock for $1.08 per share to a number of accredited investors. The Company received proceeds of $8,677,000, net of related expenses of $387,000. Proceeds included cash of $6,113,000 and the conversion of a credit facility with an entity controlled by a then director of the Company with borrowings of $2,500,000 which converted into 2,314,815 shares of common stock.
In December 2002, the Company completed a second closing of the November 12, 2002 private placement in which 2,804,539 shares of common stock were sold for $1.08 per share for gross proceeds of $3,028,902.
On November 12, 2002, the Company also effected the split-off of Photogen, Inc. and the assets and liabilities related to the Company's therapeutic line of business to the five founding stockholders of the Company (the "Tennessee Stockholders") in exchange for all of their common stock in the Company. The Tennessee Stockholders owned 5,137,109 shares of the Company and those shares were cancelled prior to December 31, 2002. The market value of the 5,137,109 shares on the date of the split-off was $12,226,319.
In conjunction with the November 12, 2002 transactions, the Company implemented a one-for-four reverse split of its common stock. All amounts have been retroactively stated to reflect this reverse stock split.
Pursuant to a Standstill and Make Whole Agreement dated as of December 30, 2002 with Xmark, secured creditors of Alliance, the Company issued a total of 750,000 shares to Xmark in January through March 2003. Xmark agreed to not exercise any rights against Alliance as a creditor. The value of these shares was treated as an expense at fair market value amounting to $1,173,750.
On May 2, 2003, the Company executed a Going Forward Agreement with Xmark that provided, among other things, for the issuance of shares of the Company's common stock upon the acquisition of the Purchased Technology as payment for interest owed by Alliance to Xmark. On June 18, 2003, the Company issued 2,198,137 shares of its common stock to certain creditors of Alliance, including 1,056,144 shares to Xmark pursuant to the Going Forward Agreement, as partial consideration for the Technology Purchase. Following approval by shareholders, the Company will issue an additional approximately 1,985,522 shares to certain other creditors as part of the Purchased Technology. The value of these shares was treated as acquisition consideration at fair market value (see Note 3). Such approval was obtained at the Company's shareholder meeting held on February 5, 2004.
On May 27, 2003 the Company issued 11,250 shares of its Common Stock to Xmark as a penalty for failure to timely register certain shares held by Xmark. The value of these shares was treated as an expense at fair market value amounting to $25,200.
On July 31, 2003 the Company issued 82,977 shares of its Common Stock to Xmark for interest and penalties, including the failure to timely register certain shares of its Common Stock held by Xmark as of that date. The value of these shares was treated as an expense at fair market value amounting to $158,987.
F-28
On September 2, 2003, pursuant to a Letter Agreement dated as of August 18, 2003 with Xmark, the Company issued 26,248 shares of its Common Stock to Xmark for the failure to timely register certain shares of its Common Stock held by Xmark as of that date. The value of these shares was treated as an expense at fair market value amounting to $28,085.
Total shares issued to Xmark as a result of the agreements noted above are 1,926,619. In addition, an aggregate of 43,048 shares of the Company's Common Stock are issuable to Xmark for payment of accrued interest. Xmark has a put right for all these shares that would, under certain circumstances, require the Company to purchase the shares from Xmark at a price of $1.00 per share. As a result, $1,969,668 has been classified as mezzanine equity at December 31, 2003.
During 2003, in addition to the above issuances, the Company issued an aggregate of 140,094 shares pursuant to several factors including cash, compensation for service and cashless exercise of stock options.
10. Stock Incentive Plans and Warrants
The Company maintains three long-term incentive compensation plans, which are described as follows:
- •
- The 1998 Long-Term Incentive Compensation Plan, which provides for the granting of up to 500,000 stock options to key employees, directors and consultants;
- •
- The 2000 Long-Term Incentive Compensation Plan, which provides for the granting of up to 4,568,750 stock options to key employees, directors and consultants;
- •
- The 2000 Senior Executive Long-Term Incentive Compensation Plan, which provides for the granting of up to 1,000,000 stock options to senior executives. Options exercised under this plan contain certain registration rights.
Options granted under these plans may be either "incentive stock options" within the meaning of Section 422A of the Internal Revenue Code, or nonqualified options. In 2003, 1,050,000 options were issued outside of the option plans in a grant to an executive officer subject to the approval of shareholders. Shareholders representing more than fifty percent of the Company's outstanding stock have agreed to approve this option grant. In 2002, the Company issued 358,000 options outside of the option plans as settlement of certain obligations; these were fully expensed in 2002.
The stock options are exercisable over a period determined by the Board of Directors (through its Compensation Committee), but generally no longer than 10 years after the date they are granted.
F-29
Information with respect to the plans follows:
Year ended December 31,
| | 2001
| | 2002
| | 2003
| |
|---|
| Options outstanding at beginning of year | | | 1,411,000 | | | 1,629,750 | | | 4,535,481 | |
| Options granted | | | 218,750 | | | 2,914,356 | | | 2,930,250 | |
| Options forfeited | | | — | | | (8,625 | ) | | (255,451 | ) |
| | |
| |
| |
| |
| Options outstanding at end of year | | | 1,629,750 | | | 4,535,481 | | | 7,210,280 | |
| | |
| |
| |
| |
| Option prices per share granted | | $ | 4.56 - 21.24 | | $ | 1.08 - 4.20 | | $ | 0.93 - $2.05 | |
| | |
| |
| |
| |
| Weighted average exercise price | | | | | | | | | | |
| Options granted | | $ | 8.12 | | $ | 1.14 | | $ | 1.19 | |
| Options granted at less than market price | | | — | | | 1.08 | | | 1.19 | |
| Options granted at the market price | | | 8.12 | | | 1.55 | | | 1.21 | |
| Options forfeited | | | — | | | 18.41 | | | 0.93 | |
| Options outstanding at end of year | | | 37.16 | | | 14.05 | | | 9.72 | |
| Options exercisable at end of year | | | 43.04 | | | 19.85 | | | 19.74 | |
| Number of shares exercisable | | | 458,333 | | | 2,057,208 | | | 3,077,577 | |
In 2003 all stock options granted, except for 40,000 options granted to directors and a consultant, were granted to employees at an exercise price less than the market value at grant date. The weighted average fair value of the options issued below market is $1.83.
| | Options Outstanding
| | Options Exercisable
|
|---|
Range of
Exercise Price
| | Number
Outstanding at
Dec 31, 2003
| | Weighted
Average
Exercise Price
| | Weighted
Average
Remaining
Contractual
Life (Years)
| | Number
Outstanding at
Dec 31, 2003
| | Weighted
Average
Exercise Price
|
|---|
| $ 0.93 - $ 1.08 | | 3,354,597 | | $ | 1.08 | | 8.32 | | 1,212,425 | | $ | 1.08 |
| $ 1.09 - $ 4.56 | | 2,281,431 | | $ | 1.36 | | 9.24 | | 433,057 | | $ | 1.79 |
| $ 4.57 - $11.00 | | 539,128 | | $ | 10.17 | | 5.56 | | 397,471 | | $ | 10.18 |
| $11.01 - $27.50 | | 13,125 | | $ | 23.04 | | 2.87 | | 13,125 | | $ | 23.04 |
| $27.51 - $53.00 | | 269,499 | | $ | 34.13 | | 6.45 | | 268,999 | | $ | 34.10 |
| $53.01 - $60.25 | | 752,500 | | $ | 60.00 | | 6.38 | | 752,500 | | $ | 60.00 |
| | |
| |
| |
| |
| |
|
| Total | | 7,210,280 | | $ | 9.27 | | 8.12 | | 3,077,577 | | $ | 19.74 |
F-30
Information with respect to the warrants follows:
Year ended December 31,
| | 2001
| | 2002
| | 2003
| |
|---|
| Warrants outstanding at beginning of year | | | 287,431 | | | 287,431 | | | 427,431 | |
| Warrants granted | | | — | | | 390,000 | | | — | |
| Warrants exercised | | | — | | | — | | | (50,000 | ) |
| Warrants forfeited | | | — | | | (250,000 | ) | | — | |
| | |
| |
| |
| |
| Warrants outstanding at end of year | | | 287,431 | | | 427,431 | | | 377,431 | |
| | |
| |
| |
| |
| Warrant prices per share outstanding | | $ | 37.80 - 84.68 | | $ | 1.08 - 84.68 | | $ | 1.08 - 84.68 | |
| | |
| |
| |
| |
| Weighted average exercise price | | | | | | | | | | |
| Warrants granted | | $ | — | | $ | 1.78 | | $ | — | |
| Warrants forfeited | | $ | — | | $ | 37.80 | | $ | — | |
| Warrants outstanding at end of year | | $ | 43.08 | | $ | 7.94 | | $ | 8.84 | |
| Warrants exercisable at end of year | | $ | 43.08 | | $ | 12.27 | | $ | 15.25 | |
| Number of warrants exercisable | | | 287,431 | | | 237,431 | | | 187,431 | |
| | Warrants Outstanding
| | Warrants Exercisable
|
|---|
Range of
Exercise Price
| | Number
Outstanding
at Dec 31,
2003
| | Weighted
Average
Exercise Price
| | Weighted
Average
Remaining
Contractual
Life (Years)
| | Number
Outstanding at
Dec 31, 2003
| | Weighted
Average
Exercise Price
|
|---|
| $ 1.08 - 2.52 | | 340,000 | | $ | 1.78 | | 4.5 | | 150,000 | | $ | 1.08 |
| $44.20 - 47.78 | | 12,431 | | $ | 46.67 | | 3.8 | | 12,431 | | $ | 46.67 |
| $84.68 | | 25,000 | | $ | 84.68 | | 1.7 | | 25,000 | | $ | 84.68 |
As of December 31, 2003, the 1998 Long-Term Incentive Compensation Plan had options outstanding for 490,500 shares with 9,500 shares remaining available, under the 2000 Long-Term Incentive Compensation Plan, options were outstanding for 4,299,220 shares with 269,530 shares remaining available, and under the 2000 Senior Executive Long-Term Incentive Compensation Plan options were outstanding for 1,000,000 shares with zero shares remaining available.
F-31
11. Dividends
Dividends are comprised of the following:
Year ended December 31,
| | 2001
| | 2002
| | 2003
|
|---|
| Accrual of dividend on Series A Convertible Preferred | | $ | 930,090 | | $ | 996,335 | | $ | 1,066,280 |
| Deemed dividend associated with beneficial conversion feature of Series A Convertible Preferred | | | 391,154 | | | — | | | — |
| Accrual of dividend on Series B Convertible Preferred | | | 359,292 | | | 332,924 | | | — |
| Deemed dividend associated with beneficial conversion feature of Series B Convertible Preferred | | | 1,263,219 | | | — | | | — |
| Deemed dividend associated with beneficial conversion of Series B Convertible Preferred to Common Stock | | | — | | | 668,464 | | | — |
| | |
| |
| |
|
| Total | | $ | 2,943,755 | | $ | 1,997,723 | | $ | 1,066,280 |
| | |
| |
| |
|
12. Restructuring
In 2003, the Company closed its Pennsylvania corporate headquarters and transferred its headquarters to itsImagent facility in San Diego, California. The Company recorded a charge of $244,459 in the third quarter ($199,529 for remaining lease payments for the office, $12,103 for certain remaining equipment leases and $32,827 to write off certain leasehold improvements) related to the closing of the office. At December 31, 2003, the remaining obligation under the lease amounts to $136,947.
In 2000, the Company recorded a restructuring charge of $597,025, included in operating expenses, relating to the closure of its operations in Westborough, Massachusetts. The restructuring charge includes accruals related to estimated lease termination costs and accruals related to employee severance and related expenses in connection with their termination. All employees at the Westborough facility were terminated. The activities previously performed at the Westborough facility were continued at other company locations or with outside consultants. In 2002, the Company recorded an additional restructuring charge related to an additional termination costs for one of the Westborough employees. As of December 31, 2002, all restructuring costs have been settled.
13. Income Taxes
In 2002, the Company had taxable income due to the gain from the split-off of the therapeutic business. This resulted in current income tax expense of approximately $2,300,000 which was completely off-set by net operating losses.
The Company has not recorded an income tax benefit for net operating losses remaining of approximately $29,500,000 primarily expiring in 2018 through 2022, or for equity losses in affiliate of approximately $12,000,000. The Company is in the development stage and realization of the losses is not considered more likely than not. An income tax valuation allowance has been provided for the deferred tax assets related to the net operating loss carryforwards and the losses in affiliate.
A portion of the net operating loss carryforwards will not remain with the Company as a result of the split-off of the therapeutic business. However, the amount of these net operating losses is not known.
F-32
Pursuant to Sections 382 and 383 of the Internal Revenue Code, annual use of the Company's net operating losses could be limited in the event of cumulative changes in ownership of more than 50%. However, the Company does not believe such limitation will have a material effect upon the Company's ability to utilize the carryforwards.
14. Selected Quarterly Financial Data (Unaudited)
Summarized quarterly financial data for 2003 and 2002 is as follows:
| | Quarter
| |
|---|
2003
| |
|---|
| | First
| | Second
| | Third(a)
| | Fourth(a)
| |
|---|
| Operating expenses(b) | | $ | 4,749,521 | | $ | 2,536,510 | | $ | 4,868,947 | | $ | 4,753,415 | |
| Loss from continuing operations(c) | | | (4,940,896 | ) | | (7,389,855 | ) | | (5,261,431 | ) | | (5,181,322 | ) |
| Net income (loss) | | | (4,940,896 | ) | | (7,389,855 | ) | | (5,261,431 | ) | | (5,181,322 | ) |
| Basic and diluted loss per share from continuing operations(d) | | | (0.30 | ) | | (0.42 | ) | | (0.25 | ) | | (0.24 | ) |
| Basic and diluted income (loss) per share(d) | | | (0.31 | ) | | (0.44 | ) | | (0.26 | ) | | (0.25 | ) |
| | |
| |
| |
| |
| |
| |
Quarter
| |
|---|
2002
| |
|---|
| | First
| | Second
| | Third
| | Fourth
| |
|---|
| Operating expenses(e) | | $ | 1,208,285 | | $ | 906,957 | | $ | 1,218,083 | | $ | 2,732,138 | |
| Loss from continuing operations(f) | | | (1,813,246 | ) | | (1,341,652 | ) | | (1,594,897 | ) | | (5,185,408 | ) |
| Income (loss) from discontinued operations(g) | | | (502,449 | ) | | (544,657 | ) | | (244,719 | ) | | 11,575,147 | |
| Net income (loss) | | | (2,315,695 | ) | | (1,886,309 | ) | | (1,839,616 | ) | | 6,389,739 | |
| Basic and diluted loss per share from continuing operations(d) | | | (0.23 | ) | | (0.17 | ) | | (0.20 | ) | | (0.51 | ) |
| Basic and diluted income (loss) per share from discontinued operations(d) | | | (0.05 | ) | | (0.06 | ) | | (0.03 | ) | | 0.96 | |
| Basic and diluted income (loss) per share(d) | | | (0.28 | ) | | (0.23 | ) | | (0.23 | ) | | 0.45 | |
- (a)
- Includes the results of operations following the acquisition on June 18, 2003 of theImagent Business from Alliance Pharmaceutical Corp.
- (b)
- Operating expenses for the first quarter 2003 includes a reserve of $1,491,500 for the value of notes receivable from Alliance.
- (c)
- Loss from continuing operations in the second quarter 2003 includes an increase in loss from joint venture of $4,605,000 resulting from the impairment of the value of the joint venture's licensed technology
- (d)
- Earnings (loss) per share calculations for each of the quarters are based on the weighted average shares outstanding for each period. The sum of the quarters may not necessarily be equal to the full year earnings per share amounts.
- (e)
- Operating expenses for the fourth quarter 2002 includes a charge of $988,184 from the issuance of stock options to employees.
F-33
- (f)
- Loss from continuing operations for the fourth quarter 2002 includes an increase in loss from joint venture of $2,000,000 resulting from the impairment of the joint venture's licensed technology.
- (g)
- Income (loss) from discontinued operations for the fourth quarter 2002 includes a gain of $11,779,752 from the sale of the therapeutic business.
F-34
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS.
The primary market risk that could impact us is the fluctuation in interest rates related to our investments in Government bonds, if any. As our investments all have short-term maturities, the investment return reflects the current market rates. To date, we have not engaged in any derivative or hedging activities.
ITEM 8. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
On November 14, 2003 BDO Seidman, LLP informed IMCOR that they were declining to stand for re-election as IMCOR's auditors. IMCOR's Board of Directors did not believe that it was necessary to take any action in response to BDO Seidman, LLP's decision not to stand for re-election and accordingly did not recommend or approve this decision.
On November 18, 2003, IMCOR's Board of Directors, upon recommendation of the Audit Committee, engaged Moss Adams LLP as independent auditor, replacing BDO Seidman, LLP.
BDO Seidman, LLP's reports on IMCOR's financial statements for the past two years did not contain an adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles other than a qualification related to the entity's ability to continue as going concern.
During IMCOR's two most recent fiscal years and through the date of BDO Seidman, LLP's declination to stand for re-election, there were no disagreements with BDO Seidman, LLP on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure which, if not resolved to BDO Seidman, LLP's satisfaction, would have caused BDO Seidman, LLP to make reference to the subject matter in connection with its report of the financial statements for such years; and there were no reportable events as defined in Item 304(a)(1)(v) of Regulation S-B.
BDO Seidman has stated its agreement with the above statements in its letter to IMCOR dated November 18, 2003.
ITEM 8A. CONTROLS AND PROCEDURES.
In September 2004, the Company’s management concluded that there were certain reportable conditions and material weaknesses in its internal controls and procedures related to the period covered by this report. The Company formed this view in response to communications with its independent auditors, Moss Adams LLP. Moss Adams had orally discussed certain matters related to the Company’s internal controls and financial reporting with the Company’s former Chief Financial Officer and the Company’s Audit Committee. After the Company filed its Form 10-QSB for the quarter ended June 30, 2004, Moss Adams concluded that these matters cumulatively amounted to reportable conditions and material weaknesses and formally advised the Company of their overall determination by letter in September 2004.
The reportable conditions noted were that the Company’s financial statement close process was not performed in a timely manner, leading to delays and adjustments in reconciling recurring and non-recurring balances and transactions, and could result in material contracts and transactions being improperly omitted or incorrectly disclosed. In addition, Moss Adams stated that the Company’s use of Quickbooks accounting software, while itself not a reportable condition, when coupled with the lack of adequate internal controls could lead to situations in which errors or irregularities may not be discovered in a timely manner or a risk that users of the software could modify historical data.
The material weaknesses noted were that the Company’s accounting and reporting processes were not completed on a timely basis (in part due to the absence of a full-time on-site chief financial officer); the small size of the Company’s accounting staff results in inadequate segregation of duties; the Company’s limited number of financial and accounting personnel make it difficult to create a backup knowledge base enabling personnel to fill in if there is an absence of one individual; and the Company’s policies and procedures relating to cash disbursements, cash handling, cash receipts and reconciliation should have greater segregation of duties to mitigate the risk of fraud or financial statement misstatement.
Moss Adams recommended taking steps to alleviate understaffing in the Company’s accounting department to improve the timeliness of financial reporting and enable appropriate segregation of duties. Additionally, Moss Adams recommended that the Company require management to assess and report annually on the effectiveness of internal controls.
The Company and its Audit Committee are committed to remediating the reportable conditions and material weakness. The Company does not expect to be able to fully implement all remediation until the Company has sufficient additional financing. Although certain matters may not be fully remediated, the Company believes appropriate mitigating factors are in place to reduce the likelihood of material misstatements or improper disclosure.
During the period beginning in May 2004, the Company began to implement steps to address its internal controls and procedures. The following remediation actions have been completed:
| • | | The Company engaged Larry D. Grant as a financial consultant. Mr. Grant has 30 years of experience in accounting and financial advisory work, both in private practice with major accounting firms and as a consultant and interim executive to various enterprises. He has been working full time for us at the Company’s San Diego headquarters since May 11, 2004. |
| • | | The Company added Darlene Deptula-Hicks to its Audit Committee. Ms. Deptula-Hicks joined the Company’s Board on July 22, 2004, and is Chair of the Company’s Audit Committee. Ms. Deptula-Hicks has over 20 years of experience in financial management positions, primarily in the life sciences sector. She is currently Executive Vice President and Chief Financial Officer of ONI Medical Systems, Inc. |
| • | | The Company has required, since July 2004, the Executive Committee of the Board to ratify routine and/or recurring expenditures and approve significant commitments and non-routine expenditures and payments. |
| • | | The Company required both Larry Grant and its Senior Vice President Business Development and Marketing to review and approve the Company’s incurring all significant obligations and remitting payments. |
| • | | The Company improved controls over its various material agreements and transactions. |
The Company is in the process of developing and implementing the following remediation steps:
| • | | The Company has committed to design an internal control plan, including disclosure controls. The Company presently intends to have this control plan in place by the end of the first quarter of the 2005 fiscal year. |
| • | | The Company is, on an ongoing basis, reviewing significant processes involved in accounting and financial reporting to ensure that they function as designed. |
| • | | The Company is developing a Code of Ethics to address other internal control procedures, such as conflicts of interest. The Company expects to approve and implement the Code of Ethics by the end of the 2004 fiscal year. Once completed, all employees will be made aware of and required to comply with this Code of Ethics. |
| • | | The Company continues to work to improve disclosure controls as they relate to non-routine material agreements and transactions. |
The following remediation actions are in the planning stages. The Company does not expect to be able to fully implement these actions until sufficient additional financing is available:
| • | | The Company intends to hire a full-time on-site Chief Financial Officer. |
| • | | The Company is in a search for additional full time accounting staff, including a Controller, and other staff as necessary. |
| • | | The Company is evaluating the effectiveness of its current accounting software and potential replacement systems. |
As noted, the Company is committed to remediating the reportable conditions and material weaknesses. To mitigate the delay in implementing a formal internal control plan, the Company also intends to specifically monitor internal controls and related procedures until such a plan is put into place.
The Company’s Chief Executive Officer (who is the Company’s acting Principal Financial Officer while the Company searches for a permanent replacement to its former Chief Financial Officer) has consulted with Larry Grant and has participated in the evaluation of the Company’s disclosure controls (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended) required by paragraph (b) of Rule 13a-15 or Rule 15d-15, as of September 30, 2004. Based on that evaluation, as informed by correspondence from Moss Adams received in September 2004, the Company’s Chief Executive Officer concluded that the Company’s disclosure controls and procedures in specific areas were not fully effective to ensure timely collection, evaluation and disclosure of information relating to the Company that would potentially be subject to disclosure under the Securities Exchange Act of 1934, and the rules and regulations thereunder. To the extent corrections and improvements in internal controls and disclosure controls and procedures cannot be immediately implemented, management believes appropriate oversight and review is currently in place to prevent material misstatements in the Company’s financial reporting.
PART III.
ITEM 9. DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS; AND COMPLIANCE WITH SECTION 16(A) OF THE EXCHANGE ACT.
The information required by Item 9 is incorporated by reference to the information under the captions "Directors, Executive Officers, Promoters and Control Persons; and Compliance with
47
Section 16(a) of the Exchange Act" in our definitive proxy statement for the 2004 annual meeting of stockholders.
As of December 31, 2003, we have not adopted a Code of Ethics for financial executives, which include our Company's principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, as required by sections 406 and 407 of the Sarbanes-Oxley Act of 2002.
We are in the infancy stages of our business operations and have not had resources available or all principal executives in place to properly design and adopt a code of ethics. In addition, during fiscal year 2003, our resources and operations were substantially constrained due to the lack of operating capital. We currently have approximately 30 employees, of which only three are either a principal executive or financial officer, and have reduced our overhead expenses in order to operate within the constraints of our limited revenues and capital. Because of our small staff, the involvement of management and the Board of Directors in the business and operations of the Company, and the internal policies of the Company, we have not adopted a separate code of ethics for principal executive and financial officers.
We will revisit this issue in the future to determine if adoption of a code of ethics is appropriate. In the meantime, our management intends to promote honest and ethical conduct, full and fair disclosure in our reports to the SEC, and compliance with applicable governmental laws and regulations.
ITEM 10. EXECUTIVE COMPENSATION.
The information required by Item 10 is incorporated by reference to the information under the caption "Executive Compensation" in our definitive proxy statement for the 2004 annual meeting of stockholders.
ITEM 11. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information required by Item 11 is incorporated by reference to the information under the caption "Security Ownership of Certain Beneficial Owners and Management" in our definitive proxy statement for the 2004 annual meeting of stockholders.
ITEM 12. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS.
The information required by Item 12 is incorporated by reference to the information under the caption "Certain Relationships and Related Transactions" in our definitive proxy statement for the 2004 annual meeting of stockholders.
ITEM 13. EXHIBITS, LIST AND REPORTS ON FORM 8-K.
(a) Exhibits
The following is a list of exhibits filed as part of this Form 10-KSB. Exhibits that were previously filed are incorporated by reference. For exhibits incorporated by reference, the location of the exhibit in the previous filings is indicated in parentheses.
EXHIBIT NO.
| | DESCRIPTION
|
|---|
| +2.1 | | Asset Purchase Agreement dated as of June 10, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Alliance Pharmaceutical Corp. (Filed as Exhibit 2.1 to the company's Form 8-K dated June 20, 2003 and incorporated herein by reference.) |
| | | |
48
+3.1 |
|
Articles of Incorporation of IMCOR Pharmaceutical Co., as amended (Filed as Exhibit E to the company's Proxy Statement on Form DEFM 14A dated September 12, 2002 and incorporated herein by reference.) |
+3.2 |
|
Amended and Restated Certificate of Designations, Preferences and Rights of Series A Preferred Stock. (Filed as Exhibit 3.5 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) |
+3.3 |
|
Amended and Restated Bylaws of IMCOR Pharmaceutical Co. (Filed as Exhibit C to the company's Information Statement on Form DEF 14C dated December 23, 2002 and incorporated herein by reference.) |
+4.1 |
|
Form of Secured Promissory Note by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P., and Xmark Fund, Ltd. dated June 18, 2003. (Filed as Exhibit 10.4 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+4.2 |
|
Form of Revolving Convertible Senior Secured Promissory Note by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each of Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP dated June 18, 2003. (Filed as Exhibit 10.7 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
*4.3 |
|
Form of Secured Promissory Note by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each of Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP. |
+4.4 |
|
Registration Rights Agreement entered into as of November 12, 2002 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Mi3 L.P., Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Tannebaum, LLC. (Filed as Exhibit D to the company's Proxy Statement on Form DEFM 14A dated September 12, 2002 and incorporated herein by reference.) |
+4.5 |
|
Form of Registration Rights Agreement dated as of December 30, 2002 entered into by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each of Oxford Bioscience Partners IV L.P., MRNA Fund II L.P., DMG Legacy Institutional Fund LLC, DMG Legacy Fund LLC, and DMG Legacy International Ltd. (Filed as Exhibit 10.7 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
+4.6 |
|
Incentive Stock Option Award Agreement entered into by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Taffy J. Williams, Ph.D. dated May 17, 2000. (Filed as Exhibit 10.4 to the company's Current Report on Form 8-K dated May 17, 2001 and incorporated herein by reference.) |
+4.7 |
|
Incentive Stock Option Award Agreement entered into by IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Brooks Boveroux dated August 1, 2000. (Filed as Exhibit 10.13 to the company's Annual Report on Form 10-K dated December 31, 2000 and incorporated herein by reference.) |
+4.8 |
|
Amended Warrant Certificates between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Farcap Group LLC dated as of January 31, 2003. (Filed as Exhibit 10.15 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
| | | |
49
+4.9 |
|
Warrant to Purchase Shares of Common Stock dated October 20, 1999 between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Elan International Services, Ltd. (Filed as Exhibit 10.9 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) |
+4.10 |
|
Warrant Agreement dated as of November 12, 2002 between Broadmark Capital LLC and IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.). (Filed as Exhibit 10.27 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
*4.11 |
|
Non-Qualified Stock Option Award Agreement dated as of January 14, 2004 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Taffy J. Williams, Ph.D. |
+9.1 |
|
Voting, Drag-Along and Right of First Refusal Agreement entered into as of November 12, 2002 by and among Robert J. Weinstein, M.D., Stuart P. Levine, Tannebaum, LLC, Mi3 L.P., Oxford Bioscience Partners IV L.P. and MRNA Fund II L.P. (Filed as Exhibit G to the company's Proxy Statement on Form DEFM 14A dated September 12, 2002 and incorporated herein by reference.) |
+10.1 |
|
Letter Agreement entered into as of August 29, 2002 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Clinical Regulatory Strategies, LLC (Filed as Exhibit 10.2 to the company's quarterly report on Form 10-Q for the quarter ended September 30, 2002 and incorporated herein by reference.) |
+10.2 |
|
Employment Agreement entered into by and between the company and Brooks Boveroux, dated October 28, 2002.(Filed as Exhibit 10.9 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
+10.3 |
|
Form of Indemnification Agreement entered into by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each Director and Senior Vice President of the company. (Filed as Exhibit 10.13 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
+10.4 |
|
Tufts University Sponsored Research Agreement dated August 1, 1999 by and between Photogen, Inc. and Tufts University, a/k/a Trustees of Tufts College. (Filed as Exhibit 10.3 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.5 |
|
License Agreement effective as of September 30, 1999 between The General Hospital Corporation and Photogen, Inc. (Filed as Exhibit 10.7 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.6 |
|
Securities Purchase Agreement dated as of October 20, 1999, among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Elan International Services, Ltd. (Filed as Exhibit 10.8 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
| | | |
50
+10.7 |
|
License Agreement dated October 20, 1999 between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Photogen Newco Ltd. and Elan Pharma International Limited. (Filed as Exhibit 10.13 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.8 |
|
License Agreement dated October 20, 1999 between Elan Pharma International Limited, Photogen Newco Ltd. and IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.). (Filed as Exhibit 10.14 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.9 |
|
Subscription, Joint Development and Operating Agreement dated October 20, 1999 between Elan Pharma International Limited, Elan International Services, Ltd., IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Photogen Newco Ltd. (Filed as Exhibit 10.15 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.10 |
|
Equipment Lease between Picker Financial Group, L.L.C. and Photogen, Inc. dated October 25, 1999. (Filed as Exhibit 10.37 to the company's Annual Report on Form 10-KSB for the year ended December 31, 1999 and incorporated herein by reference.) |
+10.11 |
|
Going Forward Agreement dated as of May 2, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P., and Xmark Fund, Ltd. (Filed as Exhibit 10.1 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.12 |
|
Security Agreement dated as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P. and Xmark Fund, Ltd. (Filed as Exhibit 10.2 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.13 |
|
Patent and Trademark Security Agreement dated as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P., and Xmark Fund, Ltd. (Filed as Exhibit 10.3 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.14 |
|
Security Agreement dated as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP. (Filed as Exhibit 10.5 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.15 |
|
Patent and Trademark Security Agreement dated as of as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP. (Filed as Exhibit 10.6 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.16 |
|
Equipment Lease dated as of June 18, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Baxter Healthcare Corporation. (Filed as Exhibit 10.8 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
| | | |
51
+10.17 |
|
Letter Agreement dated as of August 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, Ltd. and Xmark Fund, L.P. (Filed as Exhibit 10.1 to the Company's Form 8-K filed on August 20, 2003 and incorporated herein by reference.) |
*10.18 |
|
Letter Agreement dated June 6, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Jack DeFranco. |
+10.19 |
|
Letter Agreement dated July 23, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Taffy J. Williams, Ph.D. (Filed as Exhibit 10.5 to the company's Form 10-QSB for the quarter ended September 30, 2003 and incorporated herein by reference). |
*10.20 |
|
Form of Letter Agreement by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each outside director. |
+10.21 |
|
License Agreement dated December 16, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Kyosei Pharmaceutical Co. Ltd. (Filed as Exhibit 10.1 to the Company's report on Form 8-K filed on December 18, 2003 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+21 |
|
List of subsidiaries of the company (Filed as Exhibit 21 to the Company's Form 10-K for the year ended December 31, 2002 and incorporated herein by reference.) |
*31.1 |
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
*31.2 |
|
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
*32.1 |
|
Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
*32.2 |
|
Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
- +
- Incorporated by Reference.
- *
- Filed herewith.
- (b)
- Reports On Form 8-K.
During the quarter ended December 31, 2003 we filed the following reports on Form 8-K:
1. On October 27, 2003 we filed a Current Report on Form 8-K with respect to disclosure of pro-forma financial information of the company's acquisition of the medical imaging business of Alliance Pharmaceutical Corp.
2. On November 18, 2003 we filed a Current Report on Form 8-K with respect to the resignation of BDO Seidman as the company's independent auditor and the appointment of Moss Adams LLP as the company's independent auditor.
3. On November 26, 2003 we filed a Current Report on Form 8-K amending the report of November 18, 2003 to include that the Board of Directors did not believe that it was necessary to take any action in response to BDO Seidman, LLP's decision not to stand for re-election and accordingly did not recommend or approve this decision.
52
4. On December 18, 2003 we filed a Current Report on Form 8-K announcing a License Agreement with Kyosei Pharmaceutical Co. Ltd. ("Kyosei"), a subsidiary of the Sakai Group. The Agreement gives Kyosei an exclusive license to develop and marketImagent® (perflexane lipid microspheres) for all indications in Japan
ITEM 14. Principal Accountant Fees and Services.
The information required by Item 14 is incorporated by reference to the information under the caption "Independent Public Accountants" in our definitive proxy statement for the 2004 annual meeting of shareholders.
53
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: January 4, 2005 | | IMCOR Pharmaceutical Co. |
|
|
By: |
/s/ Taffy J. Williams
Taffy J. Williams, Ph.D.
President and Chief Executive Officer |
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature
| | Title
| | Date
|
|---|
|
|
|
|
|
|
|
| By: | | /s/ Taffy J. Williams
Taffy J. Williams, Ph.D. | | President, Chief Executive Officer and Director, Chairman of the Board (principal executive officer and acting principal financial officer) | | January 4, 2005 |
By: |
|
/s/ Darlene M. Deptula-Hicks
Darlene M. Deptula-Hicks |
|
Director |
|
January 4, 2005 |
By: |
|
/s/ Alan D. Watson
Alan D. Watson, Ph.D. |
|
Director |
|
January 4, 2005 |
By: |
|
/s/ Jonathan Fleming
Jonathan Fleming |
|
Director |
|
January 4, 2005 |
By: |
|
/s/ Richard T. Dean
Richard Dean, Ph.D. |
|
Director |
|
January 4, 2005 |
By: |
|
/s/ William D. McPhee
William D. McPhee |
|
Director |
|
January 4, 2005 |
By: |
|
/s/ Brian M. Gallagher
Brian M. Gallagher, Ph.D. |
|
Director |
|
January 4, 2005 |
54
EXHIBIT INDEX
EXHIBIT NO.
| | DESCRIPTION
|
|---|
| +2.1 | | Asset Purchase Agreement dated as of June 10, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Alliance Pharmaceutical Corp. (Filed as Exhibit 2.1 to the company's Form 8-K dated June 20, 2003 and incorporated herein by reference.) |
+3.1 |
|
Articles of Incorporation of IMCOR Pharmaceutical Co., as amended (Filed as Exhibit E to the company's Proxy Statement on Form DEFM 14A dated September 12, 2002 and incorporated herein by reference.) |
+3.2 |
|
Amended and Restated Certificate of Designations, Preferences and Rights of Series A Preferred Stock. (Filed as Exhibit 3.5 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) |
+3.3 |
|
Amended and Restated Bylaws of IMCOR Pharmaceutical Co. (Filed as Exhibit C to the company's Information Statement on Form DEF 14C dated December 23, 2002 and incorporated herein by reference.) |
+4.1 |
|
Form of Secured Promissory Note by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P., and Xmark Fund, Ltd. dated June 18, 2003. (Filed as Exhibit 10.4 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+4.2 |
|
Form of Revolving Convertible Senior Secured Promissory Note by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each of Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP dated June 18, 2003. (Filed as Exhibit 10.7 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
*4.3 |
|
Form of Secured Promissory Note by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each of Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP. |
+4.4 |
|
Registration Rights Agreement entered into as of November 12, 2002 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Mi3 L.P., Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Tannebaum, LLC. (Filed as Exhibit D to the company's Proxy Statement on Form DEFM 14A dated September 12, 2002 and incorporated herein by reference.) |
+4.5 |
|
Form of Registration Rights Agreement dated as of December 30, 2002 entered into by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each of Oxford Bioscience Partners IV L.P., MRNA Fund II L.P., DMG Legacy Institutional Fund LLC, DMG Legacy Fund LLC, and DMG Legacy International Ltd. (Filed as Exhibit 10.7 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
+4.6 |
|
Incentive Stock Option Award Agreement entered into by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Taffy J. Williams, Ph.D. dated May 17, 2000. (Filed as Exhibit 10.4 to the company's Current Report on Form 8-K dated May 17, 2001 and incorporated herein by reference.) |
| | | |
55
+4.7 |
|
Incentive Stock Option Award Agreement entered into by IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Brooks Boveroux dated August 1, 2000. (Filed as Exhibit 10.13 to the company's Annual Report on Form 10-K dated December 31, 2000 and incorporated herein by reference.) |
+4.8 |
|
Amended Warrant Certificates between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Farcap Group LLC dated as of January 31, 2003. (Filed as Exhibit 10.15 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
+4.9 |
|
Warrant to Purchase Shares of Common Stock dated October 20, 1999 between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Elan International Services, Ltd. (Filed as Exhibit 10.9 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) |
+4.10 |
|
Warrant Agreement dated as of November 12, 2002 between Broadmark Capital LLC and IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.). (Filed as Exhibit 10.27 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
*4.11 |
|
Non-Qualified Stock Option Award Agreement dated as of January 14, 2004 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Taffy J. Williams, Ph.D. |
+9.1 |
|
Voting, Drag-Along and Right of First Refusal Agreement entered into as of November 12, 2002 by and among Robert J. Weinstein, M.D., Stuart P. Levine, Tannebaum, LLC, Mi3 L.P., Oxford Bioscience Partners IV L.P. and MRNA Fund II L.P. (Filed as Exhibit G to the company's Proxy Statement on Form DEFM 14A dated September 12, 2002 and incorporated herein by reference.) |
+10.1 |
|
Letter Agreement entered into as of August 29, 2002 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Clinical Regulatory Strategies, LLC (Filed as Exhibit 10.2 to the company's quarterly report on Form 10-Q for the quarter ended September 30, 2002 and incorporated herein by reference.) |
+10.2 |
|
Employment Agreement entered into by and between the company and Brooks Boveroux, dated October 28, 2002. (Filed as Exhibit 10.9 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
+10.3 |
|
Form of Indemnification Agreement entered into by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each Director and Senior Vice President of the company. (Filed as Exhibit 10.13 to the company's Annual Report on Form 10-K for dated December 31, 2002 and incorporated herein by reference.) |
+10.4 |
|
Tufts University Sponsored Research Agreement dated August 1, 1999 by and between Photogen, Inc. and Tufts University, a/k/a Trustees of Tufts College. (Filed as Exhibit 10.3 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.5 |
|
License Agreement effective as of September 30, 1999 between The General Hospital Corporation and Photogen, Inc. (Filed as Exhibit 10.7 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
| | | |
56
+10.6 |
|
Securities Purchase Agreement dated as of October 20, 1999, among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Elan International Services, Ltd. (Filed as Exhibit 10.8 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.7 |
|
License Agreement dated October 20, 1999 between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Photogen Newco Ltd. and Elan Pharma International Limited. (Filed as Exhibit 10.13 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.8 |
|
License Agreement dated October 20, 1999 between Elan Pharma International Limited, Photogen Newco Ltd. and IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.). (Filed as Exhibit 10.14 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.9 |
|
Subscription, Joint Development and Operating Agreement dated October 20, 1999 between Elan Pharma International Limited, Elan International Services, Ltd., IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Photogen Newco Ltd. (Filed as Exhibit 10.15 to the company's Quarterly Report on Form 10-QSB for the quarter ended September 30, 1999 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+10.10 |
|
Equipment Lease between Picker Financial Group, L.L.C. and Photogen, Inc. dated October 25, 1999. (Filed as Exhibit 10.37 to the company's Annual Report on Form 10-KSB for the year ended December 31, 1999 and incorporated herein by reference.) |
+10.11 |
|
Going Forward Agreement dated as of May 2, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P., and Xmark Fund, Ltd. (Filed as Exhibit 10.1 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.12 |
|
Security Agreement dated as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P. and Xmark Fund, Ltd. (Filed as Exhibit 10.2 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.13 |
|
Patent and Trademark Security Agreement dated as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, L.P., and Xmark Fund, Ltd. (Filed as Exhibit 10.3 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.14 |
|
Security Agreement dated as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP. (Filed as Exhibit 10.5 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.15 |
|
Patent and Trademark Security Agreement dated as of as of June 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3 LP. (Filed as Exhibit 10.6 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
| | | |
57
+10.16 |
|
Equipment Lease dated as of June 18, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Baxter Healthcare Corporation. (Filed as Exhibit 10.8 to the Company's 8-K filed on June 20, 2003 and incorporated herein by reference.) |
+10.17 |
|
Letter Agreement dated as of August 18, 2003 by and among IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.), Xmark Fund, Ltd. and Xmark Fund, L.P. (Filed as Exhibit 10.1 to the Company's Form 8-K filed on August 20, 2003 and incorporated herein by reference.) |
*10.18 |
|
Letter Agreement dated June 6, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Jack DeFranco. |
+10.19 |
|
Letter Agreement dated July 23, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Taffy J. Williams, Ph.D. (Filed as Exhibit 10.5 to the company's Form 10-QSB for the quarter ended September 30, 2003 and incorporated herein by reference). |
*10.20 |
|
Form of Letter Agreement by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and each outside director. |
+10.21 |
|
License Agreement dated December 16, 2003 by and between IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.) and Kyosei Pharmaceutical Co. Ltd. (Filed as Exhibit 10.1 to the Company's report on Form 8-K filed on December 18, 2003 and incorporated herein by reference.) Confidential portions of this exhibit were redacted. |
+21 |
|
List of subsidiaries of the company (Filed as Exhibit 21 to the Company's Form 10-K for the year ended December 31, 2002 and incorporated herein by reference.) |
*31.1 |
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
*31.2 |
|
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
*32.1 |
|
Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
*32.2 |
|
Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
- *
- Filed herewith
- +
- Incorporated herein by reference
58
EXHIBIT 31.1
CERTIFICATION
| I, Taffy J. Williams, Ph.D., President and Chief Executive Officer, certify that: |
|
| |
| 1. | I have reviewed this annual report on Form 10-KSB of IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.); |
| | |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| | |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the small business issuer as of, and for, the periods presented in this report; |
| | |
| 4. | The small business issuer’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the small business issuer and have: |
| | (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the small business issuer, including its consolidated subsidiaries, is made known to the Company by others within those entities, particularly during the period in which this report is being prepared; |
| | | |
| | (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| | | |
| | (c) | Evaluated the effectiveness of the small business issuer’s disclosure controls and procedures and presented in this report its conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| | | |
| | (d) | Disclosed in this report any change in the small business issuer’s internal control over financial reporting that occurred during the small business issuer’s most recent fiscal quarter (the small business issuer’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the small business issuer’s internal control over financial reporting; and |
| 5. | The small business issuer’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the small business issuer’s auditors and the audit committee of the small business issuer’s board of directors (or persons performing the equivalent functions): |
| | (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the small business issuer’s ability to record, process, summarize and report financial information; and |
| | | |
| | (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the small business issuer’s internal control over financial reporting. |
| | | |
| | |
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D.Chief Executive Officer |
| | |
| | Date: January 4, 2005 |
|
EXHIBIT 31.2
CERTIFICATION
| I, Taffy J. Williams, Ph.D., acting Principal Financial Officer, certify that: |
| | |
| 1. | I have reviewed this annual report on Form 10-KSB of IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.); |
| | |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| | |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the small business issuer as of, and for, the periods presented in this report; |
| | |
| 4. | The small business issuer’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the small business issuer and have: |
| | (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the small business issuer, including its consolidated subsidiaries, is made known to the Company by others within those entities, particularly during the period in which this report is being prepared; |
| | | |
| | (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| | | |
| | (c) | Evaluated the effectiveness of the small business issuer’s disclosure controls and procedures and presented in this report its conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| | | |
| | (d) | Disclosed in this report any change in the small business issuer’s internal control over financial reporting that occurred during the small business issuer’s most recent fiscal quarter (the small business issuer’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the small business issuer’s internal control over financial reporting; and |
| 5. | The small business issuer’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the small business issuer’s auditors and the audit committee of the small business issuer’s board of directors (or persons performing the equivalent functions): |
| | (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the small business issuer’s ability to record, process, summarize and report financial information; and |
| | | |
| | (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the small business issuer’s internal control over financial reporting. |
| | | |
| | |
|
|
|
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D.Acting Principal Financial Officer |
| | |
| | Date: January 4, 2005 |
EXHIBIT 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of IMCOR Pharmaceutical Co. (the “Company”) on Form 10-KSB for the period ending December 31, 2003 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Taffy J. Williams, Ph.D., Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
| | (1) | The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| | | |
| | (2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| | | |
| | | |
| | |
|
|
|
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D. Chief Executive Officer |
| | |
| | Date: January 4, 2005 |
EXHIBIT 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of IMCOR Pharmaceutical Co. (the “Company”) on Form 10-KSB for the period ending December 31, 2003 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Taffy J. Williams, Ph.D., acting principal financial officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
| | (1) | The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| | | |
| | (2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company |
| | | |
| | |
|
|
|
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D. Acting Principal Financial Officer |
| | |
| | Date: January 4, 2005 |
|
Exhibit G
CHARTER OF
IMCOR PHARMACEUTICAL CO.
AUDIT COMMITTEE
Mission Statement
The Board of Directors has established a committee of its members known as the Audit Committee. The Audit Committee ("Committee") will assist the Board of Directors of IMCOR Pharmaceutical Co. (the "Company") in fulfilling its oversight responsibilities regarding the Company's financial reporting process. The Committee will review the financial reporting process, the system of internal control, the audit process and the Company's process for monitoring compliance with laws and regulations and with the Company's standard operating procedures and policies ("Compliance Policies"). In performing its duties, the Committee will maintain effective working relationships with the Company's Board of Directors, management, external independent public accountants ("External Auditors"), counsel, and internal auditors, if any. To effectively perform his role, each Committee member will obtain an understanding of the detailed responsibilities of Committee membership as well as the Company's business, operations and risks.
I. Organization
- A.
- The Committee shall consist of three (3) or more members to be selected by the Company's Board of Directors.
- B.
- All of the members shall be "independent directors" or qualify under the exceptions thereto, as such are described by National Association of Securities Dealers ("NASD") rule making, as amended from time to time, and the Sarbanes-Oxley Act of 2002 (the "Act"). In order to be considered independent under the Act, each member of the Committee (i) may not, other than in his or her capacity as a member of the Committee, the Company's Board of Directors or another committee of the Board, accept any consulting, advisory or other compensatory fee from the Company or be an affiliated person of the Company or any of its subsidiaries, and (ii) shall be free from any relationship that, in the opinion of the Board, would interfere with the exercise of independent judgment.
- C.
- The Board of Directors shall certify and appoint one (1) member who possesses accounting or related financial management experience and is a "financial expert" having the following attributes, as required by Item 401(h) of Regulation S-K promulgated by the Securities and Exchange Commission ("SEC"): (i) an understanding of generally accepted accounting principles ("GAAP") and financial statements, (ii) the ability to assess the general application of GAAP in connection with the accounting for estimates, accruals and reserves, (iii) some experience preparing, auditing, analyzing or evaluating financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of issues that can reasonably be expected to be raised by the Company's financial statements, or experience actively supervising one or more persons engaged in such activities, (iv) an understanding of internal controls and procedures for financial reporting, and (v) an understanding of audit committee functions. The "financial expert" may also be chairman of the Committee. All members of the Committee shall be able to read and understand fundamental financial statements, including the Company's balance sheets, income statements and cash flow statements.
- D.
- The Committee will disclose in the Company's SEC reports whether the Committee includes at least one member who is a "financial expert" and if not, why not.
G-1
- E.
- The Committee shall have at least the following meetings: a meeting prior to the initiation of the audit of the Company's fiscal year statements; a meeting following the Company's independent auditors' preparation of the Company's fiscal year statements; and meetings at such other times as deemed necessary by the Company's Board of Directors or the Chairman of the Committee.
II. Roles and Responsibilities
The Committee's functions and responsibilities shall encompass those matters required to be performed by audit committees by applicable law, SEC regulations or NASD rules and any other matters customarily performed by audit committees. Unless a separate committee of the Board is established for such purpose, the Committee shall also be and function as the Company's qualified legal compliance committee, and shall perform all acts required by applicable law, SEC regulations or NASD rules of such a committee. Without limiting the generality of the foregoing, the Committee shall do the following:
- A.
- Internal Control
- 1.
- The Committee shall evaluate whether management is setting the appropriate tone by communicating the importance of internal controls and ensuring that all individuals possess an understanding of their roles and responsibilities;
- 2.
- Consider and review with the External Auditor the adequacy of the Company's internal controls, including computerized information systems and security;
- 3.
- Gain an understanding of whether internal control recommendations made by either any internal auditors or External Auditors have been implemented by the management; and
- 4.
- Ensure that External Auditors keep the Committee informed about fraud, illegal acts, deficiencies in internal control and certain other matters.
- B.
- Financial Reporting
- 1.
- General Responsibilities of the Committee:
- (a)
- Review significant accounting and reporting issues, including recent professional and regulatory pronouncements and understand their impact on the financial statements; and
- (b)
- Inquire of management, External Auditors and internal auditors, if any, about significant risks and exposures and the plans to minimize such risks.
- 2.
- With respect to annual financial statements the Committee shall:
- (a)
- Review the annual financial statements and determine whether they are complete and consistent with the information known to Committee members;
- (b)
- Discuss with the External Auditors their qualitative judgments about the appropriateness, not just the acceptability, of accounting principles and financial disclosure practices adopted by the Company;
- (c)
- Meet with management and the External Auditors to review the financial statements and the results of the audit;
- (d)
- Discuss with the External Auditors any significant estimates made by management;
- (e)
- Review management's handling of proposed audit adjustments identified by the External Auditors and the resolution thereof;
G-2
- (f)
- Review the MD&A and other sections of the Company's annual report before its release to determine if the information is adequate and consistent with members' knowledge about the Company and its operations; and
- (g)
- Ensure that the External Auditors communicate certain required matters to the Committee.
- 3.
- With respect to interim financial statements the Committee shall:
- (a)
- Determine how management develops and summarizes quarterly financial information, the extent of internal audit involvement, if any, in the preparation of the financial statements and confirm that External Auditors review quarterly financial information prior to filing the Company's 10-Q;
- (b)
- As a whole, or the Chairman alone, shall meet with management and with the External Auditors, either telephonically or in person, to review the interim financial statements and the results of the review;
- (c)
- Seek to determine the fairness of the interim statements and disclosures, obtaining explanations from management, the External Auditors and the internal auditors, if any, with respect to:
- i.
- Circumstances where the actual financial results for the quarter or interim period varied significantly from budgeted or projected results;
- ii.
- Ensuring that the Company's compliance with generally accepted accounting principles has been consistently applied;
- iii.
- Any actual or proposed changes in accounting or financial reporting practices;
- iv.
- Any significant or unusual events or transactions;
- v.
- Confirming that the Company's financial and operating controls are functioning effectively; and
- vi.
- Confirming that the interim financial statements contain adequate and appropriate disclosures.
- (d)
- Ensure that the External Auditors communicate certain required matters to the Committee.
- C.
- With respect to compliance with laws and regulations and the Committee's function as the Company's qualified legal compliance committee, the Committee shall:
- 1.
- Consist of at least two or more members of the Company's Board of Directors who are not employed, directly or indirectly, by the Company.
- 2.
- Adopt written procedures for the confidential receipt, retention, and consideration of any report of evidence of a material violation under §205.3 of Part 205 of the Code of Federal Regulations (which is incorporated herein by reference).
- 3.
- Have been duly established by the Company's Board of Directors with the authority and responsibility:
- (a)
- To inform the Company's chief legal officer and chief executive officer (or the equivalents thereof) of any report of evidence of a material violation (except in the circumstances described in §205.3(b)(4) of Part 205 of the Code of Federal Regulations).
G-3
- (b)
- To determine whether an investigation is necessary regarding any report of evidence of a material violation by the Company, its officers, directors, employees or agents and, if it determines an investigation is necessary or appropriate, to:
- (i)
- Notify the full Board of Directors.
- (ii)
- Initiate an investigation, which may be conducted either by the chief legal officer (or the equivalent thereof) or by outside attorneys.
- (iii)
- Retain such additional expert personnel as the Committee deems necessary.
- (c)
- At the conclusion of any such investigation, to:
- (i)
- Recommend, by majority vote, that the Company implement an appropriate response to evidence of a material violation.
- (ii)
- Inform the chief legal officer and the chief executive officer (or the equivalents thereof) and the Board of Directors of the results of any such investigation under this paragraph C and the appropriate remedial measures to be adopted.
- 4.
- Have the authority and responsibility, acting by majority vote, to take all other appropriate action, including the authority to notify the SEC in the event that the Company fails in any material respect to implement an appropriate response that the Committee has recommended the Company to take.
- 5.
- Review the effectiveness of the Company's system for monitoring compliance with laws and regulations and the results of management's investigation and action (including disciplinary action) on any fraudulent acts or accounting irregularities;
- 6.
- Periodically obtain updates from management, the Company's counsel and the chief financial officer regarding compliance;
- 7.
- Make reasonable inquiry to determine that all regulatory compliance matters have been considered in the preparation of the financial statements;
- 8.
- Review the findings of any examinations by legal or regulatory agencies, including Nasdaq and the SEC, that have a material effect on the Company's financial statements or related Company compliance policies;
- 9.
- Investigate any matter brought to the Committee's attention within the scope of its duties, with the power to retain outside counsel or experts for this purpose if, in its judgment, that is appropriate.
- D.
- With respect to compliance with the Company's Compliance Policies the Committee shall:
- 1.
- Ensure that the Company's Compliance Policies are disseminated to all employees, including at the initiation of employment of all new employees;
- 2.
- Evaluate whether management is properly communicating the importance of the Compliance Policies and the guidelines for acceptable business practices;
- 3.
- Review the Company's monitoring for compliance with the Compliance Policies; and
- 4.
- Periodically obtain updates from management and counsel regarding compliance.
- E.
- With respect to internal audits the Committee shall monitor and implement an internal audit when appropriate that shall:
- 1.
- Review the activities and organizational structure of the Company's internal audit function;
G-4
- 2.
- Review the qualifications of the internal audit function and concur in the appointment, replacement, reassignment or dismissal of the senior person responsible for any internal audit; and
- 3.
- Review whether an internal audit function is necessary in light of the Company's business and operations, and review the effectiveness of any internal audit function implemented.
- F.
- External Audit and non-audit services, with respect thereto, the Committee shall:
- 1.
- Prior to the commencement of the fiscal year audit, review the External Auditors' proposed audit scope and approach;
- 2.
- Provide sufficient opportunity, including direct separate contact, for the External Auditors to meet or communicate with members of the Committee without members of management present. Among the items to be discussed in these meetings are the External Auditors' evaluation of the Company's financial and accounting personnel, and the cooperation that the External Auditors received during the course of their audit;
- 3.
- Be solely and directly responsible for the appointment, compensation and oversight of the work of any registered public accounting firm employed by the Company (including resolution of any disagreements between management and the External Auditor regarding financial reporting) for purposes of preparing or issuing an audit report. The Company's External Auditor shall report directly to the Committee;
- 4.
- Review and confirm the independence and effectiveness of the External Auditor and determine and provide for the payment of the External Auditor's fees and other compensation. The Committee shall oversee the independence of the External Auditor consistent with the Act, the Securities Act of 1933, the Securities Exchange Act of 1934 (the "Exchange Act") and the rules and regulations of the Public Company Accounting Oversight Board (the "Oversight Board"), the Financial Accounting Standards Board, the NASD, as the same may be amended from time to time, and shall actively engage in a dialogue with the External Auditor with respect to any disclosed relationships or services that may affect the External Auditor's objectivity and independence;
- 5.
- Preapprove all auditing services (which may entail providing comfort letters in connection with securities underwritings) and non-audit services proposed to be provided by the Company's External Auditor, except for non-audit services within the de minimus exception under Section 10A(i)(B) of the Exchange Act. In this connection, the Committee shall have the authority to appoint one or more members to approve services, provided that the decisions made by such designees between meetings of the Committee shall be presented to the full Committee at the next meeting thereof; and
- 6.
- Monitor, and shall have authority to designate employees of the Company or engage agents to act on its behalf to monitor, the non-audit services being performed by the Company's External Auditor to ensure that the External Auditor is not performing services that the Committee does not preapprove or are otherwise prohibited under Section 10A(g) of the Exchange Act, including (i) bookkeeping and related services, (ii) financial systems design and implementation, (iii) appraisal or valuation services and fairness opinions, (iv) actuarial services, (v) internal audit outsourcing services, (vi) management and human resource functions, (vii) broker or dealer, investment advisor or investment banking services, (viii) legal or expert services unrelated to the audit, or (ix) such services as the Oversight Board may determine to be impermissible.
G-5
- G.
- The Committee shall include a Committee report in the Company's proxy statement, including whether the Committee has:
- 1.
- Reviewed and discussed the Company's audited financial statements with management;
- 2.
- Discussed with the External Auditor the matters required to be discussed by Statement of Financial Accounting Standards No. 61;
- 3.
- Received the written disclosures and letter from the Company's External Auditor relating to their independence as required by Independent Standards Board Standard No. 1, and has discussed with the External Auditor its independence; and
- 4.
- Recommended to the Board of Directors, based upon the reviews and discussions referenced above, that the Company's audited financial statements be included in the Company's annual report on Form 10-K.
- H.
- The Committee shall perform the following additional responsibilities:
- 1.
- Meet with the External Auditors, chief financial officer, and management in separate executive sessions to discuss any matters that the Committee or these parties believe should be discussed privately;
- 2.
- Ensure that significant findings and recommendations made by the External Auditors and then by the internal auditors, if any, are received and discussed on a timely basis;
- 3.
- Review, with Company's outside counsel, any legal matters that could have a significant impact on the Company's financial statements;
- 4.
- Review the policies and procedures in effect for considering officers' expenses and management perquisites;
- 5.
- Maintain the authority to conduct or authorize special investigations and to engage independent counsel and other advisors, as the Committee determines necessary to carry out its duties;
- 6.
- Perform other oversight functions as requested by the Board of Directors;
- 7.
- Establish procedures for (i) receiving, retaining and treating complaints received by the Company regarding accounting, internal accounting controls or auditing matters, and (ii) enabling employees to submit on a confidential, anonymous basis their concerns regarding questionable accounting or auditing matters;
- 8.
- Review the Committee's report, containing the information required to be stated therein by the rules of the SEC, to be set forth in the proxy statement for the Company's annual meeting of shareholders, and review other Company disclosures relating to the Committee required to be set forth in such proxy statements;
- 9.
- Prior to any event involving a related party transaction, review and approve such transaction, or notify and request action on the related party transaction by the full Board of Directors of the Company; and
- 10.
- Periodically review and update this Charter, which amendments shall require the approval of the Board of Directors.
III. Reporting Responsibilities
- A.
- The Committee shall report regularly to the Board of Directors about Committee activities, including but not limited to, quarterly and annual financial reporting, and make appropriate recommendations to the Board of Directors, management, the External Auditors and the internal auditors, if any.
G-6
EXHIBIT H
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-QSB
ý | | QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| |
FOR THE QUARTERLY PERIOD ENDED SEPTEMBER 30, 2004 |
| |
OR |
| |
o | | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| |
FOR THE TRANSITION PERIOD FROM TO |
| |
Commission File Number 0-23553 |
| |
IMCOR PHARMACEUTICAL CO. |
| (Exact name of small business issuer as specified in its charter) |
NEVADA | | 62-1742885 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
6175 Lusk Boulevard San Diego, CA 92121 |
| (Address of principal executive offices) (Zip Code) |
| | | |
858/410-5601 |
| (Issuer’s telephone number, including area code) |
Check whether the issuer (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
State the number of shares outstanding of each of the issuer’s classes of common equity, as of the latest practicable date: 80,065,300 shares of common stock, $0.001 par value per share, issued and outstanding at October 20, 2004.
Transitional Small Business Disclosure Format (check one):
Yes o No ý
INDEX
| PART I. | FINANCIAL INFORMATION | |
| ITEM 1. | FINANCIAL STATEMENTS | |
| ITEM 2. | MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION | |
| ITEM 3. | CONTROLS AND PROCEDURES | |
| PART II. | OTHER INFORMATION | |
| ITEM 1. | LEGAL PROCEEDINGS | |
| ITEM 2. | CHANGES IN SECURITIES | |
| ITEM 3. | DEFAULTS UPON SENIOR SECURITIES | |
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS | |
| ITEM 5. | OTHER INFORMATION | |
| ITEM 6. | EXHIBITS AND REPORTS ON FORM 8-K | |
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
IMCOR Pharmaceutical Co.
(Formerly Photogen Technologies, Inc.)
(A Development Stage Company)
Consolidated Condensed Balance Sheets
All amounts in $
| | | | | | | Dec 31, 2003 | |
| | | Sept 30, 2004 (Unaudited) | | | (Audited & Restated) | |
Assets | | | | | | | |
Current Assets | | | | | | | |
| Cash and cash equivalents | | $ | 1,843,493 | | $ | 1,657,594 | |
| Accounts receivable | | | 27,843 | | | — | |
| Deposits | | | — | | | 108,721 | |
| Prepaid expenses | | | 448,989 | | | 492,718 | |
Total Current Assets | | | 2,320,325 | | | 2,259,033 | |
| | | | | | | | |
Property, Plant and Equipment, less accumulated depreciation of $2,300,519 and $996,091 at Sept 30, 2004 and Dec 31, 2003, respectively | | | 5,020,569 | | | 6,294,560 | |
| | | | | | | | |
Prepaid Royalty | | | 378,929 | | | — | |
| | | | | | | | |
Patent Costs, net of amortization of $222,049 and $183,854 at Sept 30, 2004 and Dec 31, 2003, respectively | | | 277,951 | | | 316,146 | |
| | | | | | | | |
Deposits | | | 348,383 | | | 338,383 | |
| | | | | | | | |
Purchased Technology, net of amortization of $1,635,601 and $658,217 at Sept 30, 2004 and Dec 31, 2003, respectively | | | 14,002,545 | | | 14,979,929 | |
| | | | | | | | |
Technology License, net of amortization of $21,818 at Sept 30, 2004 | | | 714,098 | | | — | |
| | | | | | | | |
Investment in and Advances to Affiliate | | | — | | | 2,803,114 | |
| Total Assets | | $ | 23,062,800 | | $ | 26,991,165 | |
| | | | | | | | |
Liabilities and Shareholders’ Equity/(Deficit) | | | | | | | |
| | | | | | | | |
Current Liabilities | | | | | | | |
| Accounts payable | | $ | 2,024,643 | | $ | 1,884,791 | |
| Accrued expenses | | | 3,073,242 | | | 3,281,792 | |
| Obligations pursuant to equipment lease settlement | | | 492,984 | | | 586,052 | |
| Secured bridge loan | | | — | | | — | |
| Secured promissory notes | | | 1,183,835 | | | — | |
| Royalty liability | | | 400,000 | | | — | |
| Promissory notes | | | — | | | 329,679 | |
| Secured lines of credit | | | — | | | 11,735,036 | |
| Secured notes payable | | | — | | | 1,250,000 | |
Total Current Liabilities | | | 7,174,704 | | | 19,067,351 | |
| | | | | | | | |
| | | | | | | | |
Deferred Contract Revenue | | | 3,789,286 | | | 2,000,000 | |
| | | | | | | | |
Accrued Equipment Lease | | | — | | | 133,888 | |
| | | | | | | | |
Puttable Shares | | | — | | | 1,969,668 | |
| | | | | | | | |
Mezzanine Equity: Preferred stock; par value $0.01 per share; 5,000,000 shares authorized includingSeries A Preferred Stock; 0 and 12,015 shares authorized, issued and outstanding at Sept 30, 2004 and Dec 31, 2003, respectively; liquidation preference $1,000 per share (in aggregate $12,015,000) | | | — | | | 12,015,000 | |
| | | | | | | | |
Shareholders’ Equity/(Deficit) | | | | | | | |
| Preferred stock; par value $0.01 per share; 5,000,000 shares authorized including: | | | | | | | |
Series A Preferred Stock; 0 and 841 shares authorized, issued and outstanding at Sept 30, 2004 and Dec 31, 2003, respectively; liquidation preference $1,000 per share (in aggregate $841,000) | | | — | | | 8 | |
| | | | | | | | |
| Common stock; par value $0.001 per share; 150,000,000 shares authorized, 80,065,300 and 19,464,548 shares issued and outstanding atSept 30, 2004 and Dec 31, 2003, respectively | | | 80,065 | | | 19,465 | |
| | | | | | | | |
| Additional paid-in capital | | | 79,524,068 | | | 38,184,323 | |
| Common stock to be issued | | | 375,042 | | | 5,120,159 | |
| Deficit accumulated during the development stage | | | (67,880,365 | ) | | (51,518,697 | |
Total Shareholders’ Equity/(Deficit) | | | 12,098,810 | | | (8,194,743 | |
| Total Liabilities and Shareholders’ Equity | | $ | 23,062,800 | | $ | 26,991,165 | |
IMCOR Pharmaceutical Co.
(Formerly Photogen Technologies, Inc.)
(A Development Stage Company)
Consolidated Condensed Statements of Operations
(Unaudited)
All amounts in $
| | | Three Months Ended Sept 30, 2004 | | | Three Months Ended Sept 30, 2003 | | | Nine Months Ended Sept 30, 2004 | | | Nine Months Ended Sept 30, 2003 | | | Cumulative Amounts From November 3, 1996 (inception) to Sept 30, 2004 | |
| | | | | | | | | | | | | | | | | |
License Revenue | | $ | 84,524 | | $ | — | | $ | 210,714 | | $ | | | $ | 210,714 | |
| | | | | | | | | | | | | | | | | |
Operating Expenses | | | | | | | | | | | | | | | | |
| Research and development | | | 962,604 | | | 562,703 | | | 2,849,511 | | | 1,383,145 | | | 9,735,827 | |
| Sales, general & administrative | | | 3,334,111 | | | 4,306,244 | | | 11,165,872 | | | 10,771,833 | | | 42,055,161 | |
| Restructuring charges | | | -- | | | -- | | | -- | | | -- | | | 1,541,455 | |
| Provision for future lease payments | | | -- | | | -- | | | -- | | | -- | | | 1,264,208 | |
Total Operating Expenses | | | 4,296,715 | | | 4,868,947 | | | 14,015,383 | | | 12,154,978 | | | 54,596,651 | |
| | | | | | | | | | | | | | | | | |
Loss from Joint Venture | | | (0 | ) | | (195,896 | ) | | (2,213,646 | ) | | (5,193,441 | ) | | (14,518,000 | ) |
| | | | | | | | | | | | | | | | | |
Investment and Other Income | | | 28,874 | | | 581 | | | 272,346 | | | 7,394 | | | 1,488,681 | |
Gain on sale of fixed assets | | | 2,200 | | | | | | 2,200 | | | | | | 2,200 | |
Interest Expense | | | (21,145 | ) | | (197,169 | ) | | (617,898 | ) | | (251,157 | ) | | (1,567,960 | ) |
| Loss from continuing operations | | $ | (4,202,262 | ) | $ | (5,261,431 | ) | $ | (16,361,667 | ) | $ | (17,592,182 | ) | $ | (68,981,016 | ) |
| | | | | | | | | | | | | | | | | |
| Discontinued Operations | | | | | | | | | | | | | | | | |
| Loss from operations of discontinued therapeutic business | | | -- | | | -- | | | -- | | | -- | | | (10,679,101 | ) |
| Gain from split-off of therapeutic business | | | -- | | | -- | | | -- | | | -- | | | 11,779,752 | |
| Income (loss) from discontinued operations | | | -- | | | -- | | | -- | | | -- | | | 1,100,651 | |
Net Loss | | $ | (4,202,262 | ) | $ | (5,261,431 | ) | $ | (16,361,667 | ) | $ | (17,592,182 | ) | $ | (67,880,365 | ) |
| | | | | | | | | | | | | | | | | |
Dividends on Preferred Stock | | | -- | | | (267,510 | ) | | (498,030 | ) | | (791,480 | ) | | | |
| | | | | | | | | | | | | | | | | |
Net Loss Applicable to Common Shareholders | | $ | (4,202,262 | ) | $ | (5,528,941 | ) | $ | (16,859,697 | ) | $ | (18,383,662 | ) | | | |
| | | | | | | | | | | | | | | | | |
Basic and Diluted Loss per Common Share | | $ | (0.05 | ) | $ | (0.26 | ) | $ | (0.39 | ) | $ | (0.99 | ) | | | |
| | | | | | | | | | | | | | | | | |
Weighted Average Number of Common Shares
Outstanding - Basic and Diluted | | | 79,979,139 | | | 21,361,817 | | | 43,632,027 | | | 18,562,221 | | | | |
IMCOR Pharmaceutical Co.
(Formerly Photogen Technologies, Inc.)
(A Development Stage Company)
Consolidated Condensed Statements of Shareholders’ Equity/(Deficit)
(Unaudited)
| | | | Preferred Stock | | | | | | | | | | | | | | | | | | | | | | |
| | | Series A | | | Series B | | | Common Stock | | | | | | Common Stock | | | Additional | | | Deficit Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Members’ Capital | | | To be Issued | | | Paid-in Capital | | | Development Stage | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Contribution of capital | | | — | | $ | — | | | — | | $ | — | | | — | | $ | — | | $ | 7,268 | | | — | | $ | — | | $ | — | | $ | 7,268 | |
| Net loss for the period ended December 31, 1996 | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,779 | ) | | — | | | — | | | — | | | (1,779 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at May 15, 1997 | | | — | | $ | — | | | — | | $ | — | | | — | | $ | — | | $ | 9,000 | | | — | | $ | — | | $ | (3,511 | ) | $ | 5,489 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock | | | — | | | — | | | — | | | — | | | 1,578,208 | | | 1,578 | | | — | | | — | | | 1,801,872 | | | — | | | 1,803,450 | |
| Effect of recapitalization and merger | | | — | | | — | | | — | | | — | | | 7,421,792 | | | 7,422 | | | (9,000 | ) | | — | | | 1,203,765 | | | 1,732 | | | 1,203,919 | |
| Cost associated with recapitalization and merger | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (371,111 | ) | | — | | | (371,111 | |
Net loss for the period
May 16, 1997 to December 31, 1997 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (554,702 | ) | | (554,702 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 1997 | | | — | | | — | | | — | | | — | | | 9,000,000 | | | 9,000 | | | — | | | — | | | 2,634,526 | | | (556,481 | ) | | 2,087,045 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock | | | — | | | — | | | — | | | — | | | 218,755 | | | 219 | | | — | | | — | | | 6,999,781 | | | — | | | 7,000,000 | |
| Costs associated with common stock issuance | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (50,000 | ) | | — | | | | |
| Options issued to consultants | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 45,446 | | | — | | | 45,446 | |
| Net loss for the year ended December 31, 1998 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,973,913 | ) | | (1,973,913 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 1998 | | | — | | | — | | | — | | | — | | | 9,218,755 | | | 9,219 | | | — | | | — | | | 9,629,753 | | | (2,530,394 | ) | | 7,108,578 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | — | | | — | | | — | | | — | | | 1,125 | | | 1 | | | — | | | — | | | 50,062 | | | — | | | 50,063 | |
| Issuance of warrants and options | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 3,664,749 | | | — | | | 3,664,749 | |
| Issuance of common stock | | | — | | | — | | | — | | | — | | | 125,967 | | | 126 | | | — | | | — | | | 6,082,528 | | | — | | | 6,082,654 | |
| Issuance of preferred stock | | | 12,015 | | | 120 | | | — | | | — | | | — | | | — | | | — | | | — | | | 11,578,839 | | | — | | | 11,578,959 | |
| Reclassification of Series A shares as mezzanine equity in accordance with EITF D-98 | | | (12,015 | ) | | (120 | ) | | | | | | | | | | | | | | | | | | | | (11,578,839 | ) | | | | | (11,578,959 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss for the year ended December 31, 1999 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (6,052,841 | ) | | (6,052,841 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 1999 (restated) | | | - | | | - | | | — | | | — | | | 9,345,847 | | | 9,346 | | | — | | | — | | | 19,427,092 | | | (8,583,235 | ) | | 10,853,203 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock option compensation | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 125,020 | | | — | | | 125,020 | |
| Issuance of warrants and options | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,366,050 | | | — | | | 1,366,050 | |
| Issuance of preferred stock dividend | | | 841 | | | 8 | | | — | | | — | | | — | | | — | | | — | | | — | | | (8 | ) | | — | | | — | |
| Issuance of preferred stock | | | — | | | — | | | 337,056 | | | 3,370 | | | — | | | — | | | — | | | — | | | 5,272,970 | | | — | | | 5,276,340 | |
| Beneficial accretion of Series A shares reclassified as mezzanine equity | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (240,464 | ) | | — | | | (240,464 | ) |
| Net loss for the year ended December 31, 2000 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (10,787,062 | ) | | (10,787,062 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 2000 (restated) | | | 841 | | | 8 | | | 337,056 | | | 3,370 | | | 9,345,847 | | | 9,346 | | | — | | | — | | | 25,950,660 | | | (19,370,297 | ) | | 6,593,087 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock option compensation | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 64,729 | | | — | | | 64,729 | |
| Issuance of common stock for cash | | | — | | | — | | | — | | | — | | | 49,245 | | | 49 | | | — | | | — | | | 418,674 | | | — | | | 418,723 | |
| Issuance of common stock in satisfaction Of anti-dilution provision | | | — | | | — | | | — | | | — | | | 190,856 | | | 191 | | | — | | | — | | | (191 | ) | | — | | | — | |
| Issuance of preferred stock dividend | | | — | | | — | | | 20,224 | | | 202 | | | — | | | — | | | — | | | — | | | (202 | ) | | — | | | — | |
| Beneficial accretion of Series A shares reclassified as mezzanine equity | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (195,577 | ) | | — | | | (195,577 | ) |
| Net loss for the year ended December 31, 2001 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (9,723,016 | ) | | (9,723,016 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 2001 (restated) | | | 841 | | | 8 | | | 357,280 | | | 3,572 | | | 9,585,948 | | | 9,586 | | | — | | | — | | | 26,238,093 | | | (29,093,313 | ) | | (2,842,054 | ) |
IMCOR Pharmaceutical Co.
(Formerly Photogen Technologies, Inc.)
(A Development Stage Company)
Consolidated Condensed Statements of Shareholders’ Equity/(Deficit)
(Unaudited)
| | | | Preferred Stock | | | | | | | | | | | | | | | | | | | | | | |
| | | | Series A | | | Series B | | | Common Stock | | | | | | Common
Stock | | | Additional | | | Deficit Accumulated | | | | |
| | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Members’ Capital | | | To be Issued | | | Paid-in Capital | | | Development Stage | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 2001 (restated) | | | 841 | | $ | 8 | | | 357,280 | | $ | 3,572 | | | 9,585,948 | | $ | 9,586 | | $ | — | | $ | — | | $ | 26,238,093 | | $ | (29,093,313 | ) | $ | (2,842,054 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock option compensation | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 73,870 | | | — | | | 73,870 | |
| Issuance of warrants for service | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 322,000 | | | — | | | 322,000 | |
| Issuance of options in settlement of lawsuit | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 806,415 | | | — | | | 806,415 | |
| Employee compensation from stock options | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 988,184 | | | — | | | 988,184 | |
| Issuance of preferred stock dividends | | | — | | | — | | | 40,194 | | | 402 | | | — | | | — | | | — | | | — | | | (402 | ) | | — | | | — | |
| Conversion of Series B to common stock | | | — | | | — | | | (397,474 | ) | | (3,974 | ) | | 422,316 | | | 422 | | | — | | | — | | | 3,552 | | | — | | | — | |
Beneficial inducement costs
for convertible debt converted | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 206,348 | | | — | | | 206,348 | |
Conversion of line of credit
with Élan to common stock | | | — | | | — | | | — | | | — | | | 128,437 | | | 128 | | | — | | | — | | | 3,082,359 | | | — | | | 3,082,487 | |
| Conversion of line of credit with entity controlled by director of company to common stock | | | — | | | — | | | — | | | — | | | 2,314,815 | | | 2,315 | | | — | | | — | | | — 2,497,685 | | | — | | | 2,500,000 | |
| Retirement of common stock returned in shareholder transaction (Note 6(f)) | | | — | | | — | | | — | | | — | | | (5,137,109 | ) | | (5,137 | ) | | — | | | — | | | (12,221,182 | ) | | — | | | (12,226,319 | ) |
| Issuance of common stock for cash | | | — | | | — | | | — | | | — | | | 8,823,058 | | | 8,823 | | | — | | | — | | | 9,133,078 | | | — | | | 9,141,901 | |
| Net loss for the year ended December 31, 2002 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 348,119 | | | 348,119 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 2002 (restated) | | | 841 | | $ | 8 | | | — | | $ | — | | | 16,137,465 | | $ | 16,137 | | $ | — | | $ | — | | $ | 31,130,000 | | $ | (28,745,194 | ) | $ | 2,400,951 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for cash | | | — | | | — | | | — | | | — | | | 27,778 | | | 28 | | | — | | | — | | | 29,972 | | | — | | | 30,000 | |
Issuance of common
stock for standstill agreement | | | — | | | — | | | — | | | — | | | 750,000 | | | 750 | | | — | | | — | | | 1,173,000 | | | — | | | 1,173,750 | |
| Conversion of Series B to common stock | | | — | | | — | | | — | | | — | | | 100 | | | — | | | — | | | — | | | — | | | — | | | — | |
| Options issued to consultants for services | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 9,200 | | | — | | | 9,200 | |
| Shares issued to consultant for services | | | — | | | — | | | — | | | — | | | 68,750 | | | 69 | | | — | | | — | | | 132,193 | | | — | | | 132,262 | |
| Employee compensation from stock options | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,236,566 | | | — | | | 1,236,566 | |
Shares issued inImagent Business purchase | | | — | | | — | | | — | | | — | | | 2,198,137 | | | 2,198 | | | — | | | — | | | 5,581,070 | | | — | | | 5,583,268 | |
Shares to be issued i
nImagent Business purchase | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 5,043,226 | | | — | | | - | | | 5,043,226 | |
| Shares previously subject to rescission | | | — | | | — | | | — | | | — | | | 124,627 | | | 125 | | | — | | | — | | | 649,875 | | | — | | | 650,000 | |
| Shares issued to Xmark for penalties | | | — | | | — | | | — | | | — | | | 98,826 | | | 99 | | | — | | | — | | | 163,577 | | | — | | | 163,676 | |
| Shares issued to Xmark for interest | | | — | | | — | | | — | | | — | | | 21,649 | | | 22 | | | — | | | — | | | 48,575 | | | — | | | 48,597 | |
| Shares to be issued to Xmark and other former Alliance creditors for interest and penalties | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 76,933 | | | — | | | — | | | 76,933 | |
| Options exercised through cashless exercise | | | — | | | — | | | — | | | — | | | 37,216 | | | 37 | | | — | | | — | | | (37 | ) | | — | | | — | |
Xmark puttable shares
classified as mezzanine equity | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,969,668 | ) | | — | | | (1,969,668 | ) |
| Net loss for the year ended December 31, 2003 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (22,773,504 | ) | | (22,773,504 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at December 31, 2003 (restated) | | | 841 | | $ | 8 | | | — | | $ | — | | | 19,464,548 | | $ | 19,465 | | $ | — | | $ | 5,120,159 | | $ | 38,184,323 | | $ | (51,518,698 | ) | $ | (8,194,743 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares issued to consultant for services | | | — | | | — | | | — | | | — | | | 231,250 | | | 231 | | | — | | | — | | | 28,456 | | | — | | | 28,687 | |
| Shares to be issued for legal fees | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 116,573 | | | — | | | — | | | 116,573 | |
| Employee compensation from stock options | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 813,724 | | | — | | | 813,724 | |
Shares issued inImagent Business purchase | | | — | | | — | | | — | | | — | | | 1,985,522 | | | 1,985 | | | — | | | (5,043,226 | ) | | 5,041,241 | | | — | | | — | |
| Shares issued for penalties | | | — | | | — | | | — | | | — | | | 68,478 | | | 68 | | | — | | | — | | | 136,888 | | | — | | | 136,956 | |
Shares to be issued for late
stock registration penalties | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 48,775 | | | — | | | — | | | 48,775 | |
| Shares to be issued to Xmark and secured creditors for interest and penalties | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 132,951 | | | — | | | — | | | 132,951 | |
| Shares issued as payment for promissory notes | | | — | | | — | | | — | | | — | | | 33,740,672 | | | 33,741 | | | — | | | — | | | 13,462,528 | | | — | | | 13,496,269 | |
| Shares issued for cash, net | | | — | | | — | | | — | | | — | | | 25,374,999 | | | 25,375 | | | — | | | — | | | 9,299,625 | | | — | | | 9,325,000 | |
Shares to be issued to investors
for late registration | | | | | | | | | | | | | | | | | | | | | | | | 203,000 | | | | | | | | | 203,000 | |
| Issuance of stock in settlement of lease | | | — | | | — | | | — | | | — | | | 616,580 | | | 617 | | | — | | | — | | | — | | | — | | | 617 | |
Xmark puttable shares
issued from mezzanine equity | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,969,668 | | | — | | | 1,969,668 | |
| Conversion of Series A to common stock | | | (841 | ) | | (8 | ) | | — | | | — | | | 195,263 | | | 195 | | | — | | | — | | | 12,161,260 | | | — | | | 12,161,447 | |
| Retirement of puttable shares | | | — | | | — | | | — | | | — | | | (1,611,989 | ) | | (1,612 | ) | | — | | | (203,190 | ) | | (1,573,645 | ) | | — | | | (1,778,447 | ) |
| Adjust shares for prior rounding | | | — | | | — | | | — | | | — | | | (23 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
| Net loss for the nine months ended Sept 30, 2004 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (16,361,667 | ) | | (16,361,667 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, at Sept 30, 2004 | | | — | | $ | — | | | — | | $ | — | | | 80,065,300 | | $ | 80,065 | | $ | — | | $ | 375,042 | | $ | 79,524,068 | | $ | (67,880,365 | ) | $ | 12,098,810 | |
IMCOR Pharmaceutical Co.
(Formerly Photogen Technologies, Inc.)
(A Development Stage Company)
Consolidated Condensed Statements of Cash Flows
(Unaudited)
All amounts in $
| | | | Nine Months Ended Sept 30, 2004 | | | Nine Months Ended Sept 30, 2003 | | | Cumulative Amounts From Nov 3, 1996 (Inception)(restated) | |
| | | | | | | | | | | |
Cash Flows From Operating Activities | | | | | | | | | | |
| Net loss | | $ | (16,361,667 | ) | $ | (17,592,182 | ) | $ | (67,880,365 | ) |
| Loss from discontinued operations | | | — | | | — | | | 10,679,101 | |
| Depreciation and amortization | | | 2,392,476 | | | 569,489 | | | 5,093,460 | |
| Prepaid royalty expense | | | 21,071 | | | — | | | 21,071 | |
| (Gain) Loss on disposal of fixed assets | | | 2,200 | | | — | | | 40,624 | |
| Gain on sale of marketable securities | | | — | | | — | | | (18,503 | ) |
| United States Treasury Notes amortization | | | — | | | — | | | 12,586 | |
| Gain on sale of therapeutic business | | | — | | | — | | | (11,779,752 | ) |
| Gain on equipment lease settlement | | | 126,257 | | | — | | | 126,257 | |
| Allowance for notes receivable | | | — | | | 1,491,500 | | | 658,217 | |
| Stock option compensation | | | 813,724 | | | 905,191 | | | 3,738,062 | |
| Accrued interest on line of credit | | | 495,248 | | | — | | | 813,283 | |
| Note payable for services rendered | | | 53,299 | | | — | | | 382,980 | |
| Recognition of deferred contract revenue | | | (210,714 | ) | | — | | | (210,714 | ) |
| Issuance of stock for standstill agreement | | | — | | | 1,337,426 | | | 1,337,426 | |
| Beneficial inducement costs for convertible notes | | | — | | | — | | | 206,348 | |
| Issuance of warrants in exchange for services rendered | | | — | | | — | | | 4,317,091 | |
| Stock to be issued for services rendered | | | 116,573 | | | — | | | 116,573 | |
| Issuance of stock options in settlement of lawsuit | | | — | | | — | | | 806,415 | |
| Issuance of stock for services rendered | | | 10,688 | | | 99,825 | | | 142,949 | |
| Additional fee per Xmark exchange agreement | | | 350,000 | | | — | | | 350,000 | |
| Loss from investment in affiliate | | | 2,213,646 | | | 5,193,441 | | | 14,518,000 | |
| Issuance of stock for interest payments and penalties | | | 318,681 | | | 85,455 | | | 440,210 | |
| Stock to be issued for late registration | | | 203,000 | | | — | | | 203,000 | |
| Issuance of common stock in equipment lease settlement | | | 617 | | | — | | | 617 | |
| Changes in operating assets and liabilities: | | | | | | | | | | |
| Prepaid expenses | | | 43,729 | | | 110,723 | | | (448,989 | ) |
| Change in accounts receivable | | | (27,843 | ) | | — | | | (27,843 | ) |
| Interest receivable | | | — | | | — | | | — | |
| Accounts payable | | | (21,639 | ) | | 1,675,350 | | | 1,933,151 | |
| Accrued expenses | | | (208,553 | ) | | 80,260 | | | (113,217 | ) |
| Accrued equipment lease | | | — | | | (234,304 | ) | | 1,050,589 | |
| Deferred contract revenue | | | 2,000,000 | | | — | | | 4,000,000 | |
| Net cash used in operating activities(continuing operations) | | | (7,669,207 | ) | | (6,227,828 | ) | | (29,487,373 | ) |
| Net cash used in discontinued operations | | | — | | | — | | | (10,679,101 | ) |
Cash Flows From Investing Activities | | | | | | | | | | |
| Sale of marketable securities | | | — | | | — | | | 2,164,464 | |
| Purchases of marketable securities | | | — | | | — | | | (2,182,967 | ) |
| Purchases of United States Treasury Notes | | | — | | | — | | | (38,656,973 | ) |
| Sales of United States Treasury Notes | | | — | | | — | | | 39,778,548 | |
| Purchase of capital assets | | | (83,287 | ) | | — | | | (720,164 | ) |
| Proceeds from sale of equipment | | | — | | | — | | | 145,551 | |
| Patent costs | | | — | | | — | | | (237,335 | ) |
| Investment in and advances to affiliate | | | — | | | — | | | (15,107,468 | ) |
| Increase in deposit | | | — | | | (10,000 | ) | | (777,753 | ) |
| Investment in Alliance Pharmaceutical Corp. | | | — | | | — | | | (1,255,000 | ) |
Purchase of Imagent business | | | — | | | (6,515,108 | ) | | (5,074,761 | ) |
| Net cash used in investing activities | | | (83,287 | ) | | (6,525,108 | ) | | (21,923,858 | ) |
| Net cash used in investing activities of discontinued operations | | | — | | | — | | | (1,306,676 | ) |
Cash Flows From Financing Activities | | | | | | | | | | |
| Principal payments on capital leases | | | — | | | — | | | (291,704 | ) |
| Net proceeds from issuance of equity and mezzanine equity | | | 9,325,000 | | | 30,000 | | | 49,864,340 | |
| Proceeds from capital contributions by shareholders | | | — | | | — | | | 1,911,674 | |
| Proceeds from issuance of debt | | | 2,102,676 | | | 8,160,000 | | | 18,866,586 | |
| Cost of recapitalization | | | — | | | — | | | (371,111 | ) |
| Payment on lease settlement obligations | | | (75,000 | ) | | — | | | (75,000 | ) |
| Principal payment on acquisition debt | | | (1,350,000 | ) | | (1,250,000 | ) | | (2,600,000 | ) |
| Principal payment on promissory notes | | | (2,064,283 | ) | | — | | | (2,064,283 | ) |
| Net cash provided by financing activities | | | 7,938,393 | | | 6,940,000 | | | 65,240,501 | |
| Increase (decrease) in Cash and Cash Equivalents | | | 185,899 | | | (5,862,934 | ) | | 1,843,493 | |
Cash and Cash Equivalents, at beginning of period | | | 1,657,594 | | | 6,090,904 | | | — | |
Cash and Cash Equivalents, at end of period | | $ | 1,843,493 | | $ | 227,970 | | $ | 1,843,493 | |
Noncash Operating, Investing and FinancingActivities
2004
In the quarter ended September 30, 2004, the Company recorded an increase to shareholders’ equity totaling $18,000 and a decrease to accounts payable as payment to a consultant for prior services.
In the quarter ended June 30, 2004, the Company recorded accrued prepaid royalties of $400,000 which relate to deferred revenues received of $4,000,000 during 2003 and 2004.
In the quarter ended June 30, 2004, the mezzanine equity value of $12,015,000 was reclassified into shareholders’ equity upon the exchange of the Series A Preferred Stock for 195,263 shares of common stock.
In the quarter ended June 30, 2004, an additional increase in shareholders’ equity totaling $146,447, attributed to the above-referenced 195,263 shares of common stock recorded at fair market value (and which represented the 19.9% minority interest in the Sentigen joint venture), together with the Company’s 80.1% majority interest in the Sentigen joint venture, amounting to $589,469 (after reflecting an additional charge off in the joint venture investment of $2,213,646, recorded as “Loss from Joint Venture”), were recorded and reclassified, respectively, as “Technology License” asset totaling $735,916, before amortization.
In the quarter ended June 30, 2004, the Company issued promissory notes in part for $1,778,447 in exchange for Xmark’s 1,611,989 puttable shares valued at $1,611,989 and the release from additional obligations to issue additional shares, valued at $166,458, representing accrued interest ($74,263) and late registration penalties ($92,195).
In the quarter ended June 30, 2004, the Company issued 33,740,672 common shares in settlement of $13,496,269 in promissory notes and accrued interest previously due to Oxford Bioscience Partners IV L.P., MRNA Fund II L.P. and Mi3, L.P.
2003
In 2003, the Company assumed $3,422,036 in accrued expenses and $2,500,000 of notes payable from Alliance Pharmaceutical Corp. issued stock valued at $5,583,268 to certain former creditors of Alliance and agreed to issue stock valued at $5,043,225 to such creditorsas part of the acquisition of theImagent business.
The split-off of the therapeutic business in 2002 included equipment and leasehold improvements of $516,567 and accounts payable of $70,000 in exchange for the Company's common stock valued at $12,226,319.
In 2002, debt of $5,346,910 and accrued interest of $235,577 were converted into common stock.
Payments due on the accrued equipment lease were made through a reduction in deposits due to the Company in the amount of $330,649.
1. Basis of Presentation
The accompanying unaudited financial statements have been prepared in accordance with generally accepted accounting principles for interim financial information pursuant to Regulation S-B. Accordingly, they do not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring accruals as well as adjustments related to the acquisition of the medical imaging business (“Imagent Business”) of Alliance Pharmaceutical Corp. (“Alliance”)) considered necessary for a fair presentation have been included. Operating results for the nine months ended September 30, 2004 are not necessarily indicative of the results that may be expected for the year ending December 31, 2004.
Nature of Operations
IMCOR Pharmaceutical Co. (formerly Photogen Technologies, Inc.) (the “Company”) is an emerging, development-stage pharmaceutical company focused on developing and marketing medical imaging pharmaceutical products. The Company has one FDA approved product,Imagent® (perflexane lipid microspheres), an ultrasound imaging contrast agent that it acquired in June 2003, and two additional contrast agents in development for use in computed tomography (CT) and x-ray imaging with two potential applications (1) as a blood pool agent, to diagnose diseased tissue in the cardiovascular system and other organs, which is called PH-50, and (2) to diagnose cancer metastasizing into the lymphatic system, which is called N1177. On February 5, 2004, the Company changed its name to IMCOR Pharmaceutical Co., reflecting the changed nature of its business to imaging pharmaceuticals.
For the two years up to the time of the Company’s acquisition of theImagent Business, substantially all of its research and development efforts were focused on the preclinical testing of PH-50. PH-50 and N1177 are still in early stages of development. Of these products, based on the availability of capital, the Company intends to focus its research efforts first on the continued development ofImagent for additional indications beyond the current.
As a result of the acquisition of theImagent Business, IMCOR is now based in San Diego, California. The Company has approximately 26 full time employees and leases a 53,000 square foot cGMP (current Good Manufacturing Practice) manufacturing facility at this location.
The Company has realized minimal revenues resulting from the licensing and sale ofImagent to date. Such sales are intermittent and represent selective market efforts and are not necessarily indicative of future revenues or market penetration for current or prospective indications. Currently, the Company’s core business focus remains on raising additional capital, securing extended payment terms with its current creditors, and the continued clinical development of itsImagent products (which will result in continued expenditures for product development). These costs, as well as increased costs associated with relevant sales and marketing activities, will be incurred only as sufficient capital remains available.
The Company’s current manufacturing facility provides significant excess capacity for all clinical development and scaled manufacturing activities, now and for the foreseeable future. Since the excess manufacturing facility capacity provides no significant direct benefit to the Company at the present time, the current costs associated with this excess capacity continue to be charged off against current operations, including a portion attributed to research and development.
Certain financial statement balances have been restated to conform to the September 30, 2004 presentation. These reclassifications have no impact on reported the net losses or the related per share amount. Most notably, we have reclassified our presentation of our Series A preferred stock from permanent equity to mezzanine equity to conform to the requirements of EITF D-98 (see "Restatement of Financial Statements and Other Information" which follows).
Restatement of Financial Statements and Other Information
A. Restatement of Financial Statements.
In 1999, the Company entered into a joint venture with units of Elan. As part of that transaction, the Company issued to Elan International Services 12,015 shares of its Series A Preferred Stock, with an initial value and liquidation preference of $1,000 per such share, or $12,015,000. In a concurrent transaction, Elan and the Company formed a joint venture Sentigen, with Elan and the Company holding 19.9% and 80.1% of the shares of common stock of Sentigen, respectively.
The holders of Series A Preferred Stock were entitled to an in-kind 7% dividend payable semi-annually solely in additional shares of Series A Preferred Stock. In addition, if the Company's Board of Directors declared a dividend or distribution on the common stock, holders of Series A Preferred Stock would have been entitled to receive the amount of dividend or distribution in the same form as received by the common stockholders as if the Series A Preferred Stock had been converted to common stock. No dividends have been declared or paid on the common stock and the Board of Directors had stated its intention not to do so throughout the period during which the Series A Preferred Stock was outstanding. In addition, the holder of the Series A Preferred Stock had the right, exercisable three years after the date of issuance, to exchange it for the number of additional shares of Sentigen Ltd. that would result in the holder of the Series A Preferred Stock owning 50% of Sentigen Ltd. If the holder of the Series A Preferred Stock exercised this exchange right it would have been obligated to pay the Company 30.1% of the total amount of Sentigen Ltd.'s development funding through the date of the exchange (which could be have been satisfied through reduction of amounts owed by the Company under a convertible promissory note, by payment of cash, or a combination of the two).
These shares of Series A Preferred Stock, plus accreted dividends, were convertible into shares of the Company’s common stock at any time after October 20, 2001 for a conversion price of $84.68 per share of Series A Preferred, which such value at December 31, 2003 having accumulated to $16,036,810 (or 16,036.81 shares of Series A Preferred stock).
As indicated, Elan had the right to exchange only its original 12,015 shares of the Company’s Series A Preferred Stock for additional shares of Sentigen’s common stock of such an amount to increase Elan’s holdings from the aforementioned 19.9% interest in Sentigen to 50%. Any dividends otherwise accreted as attributable to shares of the Series A Preferred Stock and available for potential conversion into the Company’s common stock, however, were not available in the event that in the alternative Elan had elected to exchange the shares of Series A Preferred Stock in order to increase its Sentigen holdings to 50%.
The Company initially accounted for the issuance of the 12,015 shares of Series A Preferred Stock as permanent equity in the Company’s Consolidated Balance Sheets and Consolidated Statements of Shareholders’ Equity/(Deficit) in the year ended December 31, 1999 and all year ends thereafter. Upon the issuance of EITF D-98 in July 2001, the Company evaluated the above-described transaction and made a determination that the elements were not present to warrant a reclassification of any of the value attributed to the shares of Series A Preferred Stock from permanent equity to mezzanine equity.
On June 30 2004, the Company reevaluated its position with respect to EITF D-98 and determined that the value attributable to the 12,015 shares held by Elan of the Company’s Series A Preferred Stock, or $12,015,000 should be reclassified as mezzanine equity. The Company further determined that, inasmuch as the otherwise accreted dividends could only be considered as (a) preferential over common shareholders in the event of a liquidation of the Company’s assets, or (b) additional value to be realized in the event that the underlying shares of Series A Preferred Stock were converted into common stock of the Company, that such value should not be reclassified as mezzanine equity, but should remain in permanent equity as such.
Therefore, and in accordance with the provision of EITF D-98, the Company has restated its Consolidated Balance Sheets for the year ended December 31, 2003, together with the Consolidated Statements of Shareholders’ Equity/(Deficit) for all years ended December 31, 1999 to and through December 31, 2003, by reclassifying $12,015,000 from permanent equity to mezzanine equity. There is no effect on the Company’s Consolidated Statements of Operations as a result of this reclassification.
The effect of the reclassification is as follows: (a) the mezzanine equity was increased by $12,015,000 (from $0 to $12,015,000) for each of the fiscal years ended December 31, 2002 and 2003; (b) the par value of the Series A Preferred Stock was reduced by $120 (from $128 to $8) for each of the fiscal years ended December 31, 2002 and 2003; and (c) the additional paid-in capital was reduced by $12,014,880 for each of the fiscal years ended December 31, 2002 and 2003 (from $43,144,880 to $31,130,000 for the fiscal year ended December 31, 2002 and from $50,199,203 to $38,184,323 for the fiscal year ended December 31, 2003).
Because the Company did not have any accumulated profits for any of the subject periods, the Company did not record any accreted dividends, which accumulated to $4,021,810 as of December 31, 2003, through the apportionment of accumulated losses within the Consolidated Statements of Shareholders’ Equity/(Deficit) for all years ended December 31, 1999 to and through December 31, 2003.
B. Kyosei Agreement.
In April 2004, the Company received a payment of $2,000,000, less Japanese withholding taxes, from a Japanese pharmaceutical company (see “Liquidity and Basis of Presentation – Going Concern” which follows).
2. Liquidity and Basis of Presentation - Going Concern
The accompanying financial statements are prepared assuming the Company is a going concern. The Company has reported accumulated losses since inception of $67,880,365. At September 30, 2004, the Company lacked sufficient working capital to fund planned operations through the balance of the fiscal year ending December 31, 2004. However, on October 29, 2004, the Company entered into a non-exclusive cross-licensing agreement and securities purchase agreement with Bristol-Myers Squibb Medical Imaging, Inc. (“BMSMI”) covering ultrasound contrast agent patents and other matters, for which it received gross proceeds of $8,500,000. See Note 12.
Substantial additional capital resources will be required to fund the Company’s ongoing operations and meet its continuing obligations. Management believes there are a number of potential alternatives available to meet the continuing capital requirements such as public or private financings or collaborative agreements. The Company is taking continuing actions to reduce its ongoing expenses. If adequate funds are not available, the Company will be required to significantly curtail its operating plans and may have to sell or license significant portions of the Company’s technology or products. The financial statements do not include any adjustment to reflect the possible future effects on the recoverability and classification of assets or the amount and classification of liabilities that may result from the outcome of this uncertainty.
On April 19 and June 30, 2004, the Company sold 25,374,999 shares of common stock and 14,717,502 warrants, including 2,030,000 warrants issued to its two placement agents, for approximately $10,150,000 at a price of $0.40 per share plus, for each share purchased, a warrant to purchase 0.5 of an additional share exercisable at $0.50 per share. The net proceeds to the Company were approximately $9,325,000. On August 3, 2004, the Company filed a registration statement, which is not yet effective, related to the aforementioned issued common stock and common stock underlying the warrants. The Company also has an obligation to issue additional shares of its common stock pursuant to these resale registration rights as liquidated damages related to delays in the filing or effectiveness of this registration statement, for which it has recorded $203,000 at September 30, 2004.
In addition, holders of an aggregate of approximately $12,719,500 principal amount of the Company’s Secured Promissory Notes and Revolving Convertible Senior Secured Promissory Notes (“Notes”), including accrued interest, converted these Notes into 33,740,672 shares of the Company’s common stock at $0.40 per share.
In June 2004, the Company entered into a bridge loan agreement for up to $750,000 with a bank. The bank held a security interest in substantially all the Company’s assets, other than intellectual property, junior to the security interest of Xmark Fund, Ltd. and Xmark Fund, L.P. (collectively, “Xmark”) and senior to the security interest of the convertible and demand notes held by certain institutional investors. On July 1, 2004, the Company repaid the entire outstanding bridge loan plus accrued interest, then amounting to $702,863.
On December 16, 2003, the Company entered into a License Agreement (the “Kyosei Agreement”) with Kyosei Pharmaceutical Co. Ltd. (“Kyosei”), a member of the Sakai Group in Japan. The Kyosei Agreement gives Kyosei an exclusive license to develop and market Imagent for all indications in Japan. The terms of the Kyosei Agreement provide for the Company to receive up to $10,000,000 in fees and development milestone payments plus royalties on commercial sales. Kyosei will also pay the Company to manufacture Imagent for Kyosei’s clinical and commercial requirements and the Company will receive royalties based on commercial sales. In each of December 2003 and April 2004, the Company received a payment of $2,000,000, less $400,000 in withholding taxes. Additional payments are expected in 2005 and beyond. Future payments from Kyosei are based on certain milestones of clinical development, including the commencement of and completion of clinical studies and approval for sale by Japanese regulatory authorities. Commencement of clinical studies is not expected to be earlier than 2005, and the development program, including regulatory approval, is expected to take four or more years. Accordingly, commercial sales, upon which the Company will earn royalties, are not expected to commence earlier than 2008. While the Company believes thatImagent clinical studies will be successful, there can be no assurance that such studies conducted in Japan will be successful or that the product will be approved for sale by Japanese authorities. See “Note 5 - Licensing and Deferred Revenue and Related Royalty Obligation Claim,” below.
The Company estimates the amount of additional financing required to fund operations and meet continuing obligations could be an additional $12,000,000 or more in order to fund operations through December 31, 2005, in addition to the $8,500,000 resulting from BMSMI subsequent to September 30, 2004 (See Note 12). Therefore, substantial additional capital resources will be required to fund the Company’s planned ongoing operations related to clinical development, marketing and manufacturing of Imagent, research and business development activities, debt repayment and other working capital needs. The Company’s financial condition raises substantial doubt about its ability to continue as a going concern without adjustments to its current planned levels of expenditures. If the Company raises additional funds by issuing equity securities, substantial dilution to existing stockholders may result. There can be no assurance that such capital will be available under acceptable terms, if at all. See “Risk Factors - The Company must raise additional financing in the future and its inability to do so will prevent the Company from continuing as a going concern and implementing its business plan,” below.
During the next twelve months the Company will focus its efforts primarily on exploring various options concerning its business, including securing additional financing, joint ventures, licensing or selling all or a portion of its technology and other possible strategic transactions. The Company’s ability to commercialize Imagent and develop the subsequent indication forImagent (as well as PH-50 and N1177) depends on the successful implementation of one or more of these transactions.
Depending on the availability of capital and whether or not the Company will pursue a sale or licensing strategy for Imagent, the Company will focus its operating efforts first on the additional clinical development for the myocardial perfusion indication, next the development of PH-50 and lastly the development of N1177. Subject to the availability of sufficient capital, the Company expects to continue to incur losses for at least the next three years as the Company intensifies research and development, preclinical and clinical testing and associated regulatory approval activities and engage in or provide for the manufacture and/or sale of any products that the Company has or may develop.
Greater capital resources could also enable the Company to re-consider its marketing strategy, or expansion of research and development activities, and its failure to raise additional capital will (absent a suitable collaborative agreement providing for a third party to take over these functions) significantly impair or curtail its ability to conduct further activities. In any event, complete development and commercialization of the Company’s technology will require substantial additional funds. The Company is seeking to raise capital through the sale of its common stock or other securities to fund its immediate and longer-term capital needs.
3. Basic and Diluted Loss Per Common Share
Basic and diluted loss per common share is computed based on the weighted average number of common shares outstanding. Basic and diluted loss per share excludes the impact of outstanding options, warrants and convertible preferred stock, as they are antidilutive. Potential common shares excluded from the calculation at September 30, 2004 are 10,460,164 shares reserved pursuant to granted options or contractual obligations to grant options and 15,409,470 shares reserved pursuant to warrants. Also excluded from the calculation at this same date are obligations to issue up to 18,000,000 common shares underlying certain rights granted pursuant to the BMSMI transaction subsequent to September 30, 2004. See “Note 12 - Subsequent Event,” below.
4. Purchased Technology
On June 18, 2003, the Company acquired the assets, including purchased technology (“Purchased Technology”), related to the medical imaging business, including Imagent, an FDA-approved product, of Alliance. Imagent is an intravenous contrast agent for use with ultrasound imaging equipment to improve visualization of blood vessels for better diagnosis of disease and assessment of organ function. Imagent consists of perfluorochemical-based “microbubbles” that are highly echogenic (reflect ultrasound signals strongly) in the bloodstream, thereby significantly enhancing ultrasound images. The Company also entered into a number of agreements related to that acquisition. Included in the purchase was the lease of an FDA-approved manufacturing facility, the marketing, manufacturing and supporting infrastructure for the product and an intellectual property portfolio of approximately thirty patents in the area of ultrasound imaging.
Intangible assets resulting from the acquisition of the Imagent Business are estimated by management based on the fair value of the assets received. These intangible assets consist entirely of Purchased Technology. Purchased Technology related to the Imagent Business is amortized on a straight-line basis over the estimated remaining life equal to twelve years. Purchased Technology is stated at cost, less accumulated amortization.
Company management annually evaluates the potential for impairment on the valuation of the Purchased Technology. Based on, among other factors, the recent completion of the cross-licensing agreement with BMSMI subsequent to September 30, 2004 (See “Note 12 - Subsequent Events,” below), equity investment raised since the acquisition of the Purchased Technology, and a recent study conducted by an independent investigator at a leading university utilizingImagent in a novel ultrasound application, management does not believe at this time that any impairment to the carrying values of the Purchased Technology exist at the interim date of September 30, 2004.
Most importantly, the Company plans to expand the label indication forImagentfor myocardial perfusion imaging, (imaging to study and evaluate the characteristics of blood flow to the heart) for the detection of coronary artery disease (“CAD”), using a novel technique of Phasic Changes. The recent study conducted by an independent investigator referenced in the foregoing paragraph has provided preliminary data which suggests thatImagent, using Phasic Changes, demonstrated a strong correlation to the results achieved using coronary angiography, the gold standard of measuring severity in CAD. Based on these encouraging results, the Company plans to conduct myocardial perfusion Phase II clinical trials (assuming it has adequate resources) beginning no later than the first quarter of 2005. In the opinion of Company management, if the proposed new indication forImagent demonstrates to be viable, it will open the use of the product into significant market opportunities well beyond that for the current indication.
5. Licensing and Deferred Revenue and Related Royalty Obligation Claim
In December 2003, the Company entered into a license agreement with Kyosei Pharmaceutical Co., Ltd. (“Kyosei”), a unit of the Sakai Group in Japan, for the development and marketing of Imagent in Japan for all radiology and cardiology indications. Terms of the agreement call for the payment to the Company of up to $10,000,000 in fees and development milestone payments plus royalties on commercial sales. Kyosei will also pay the Company to manufacture Imagentfor Kyosei’s clinical and commercial requirements. At the closing, the Company received a gross payment of $2,000,000 and in April 2004, a second payment of $2,000,000 from Kyosei.
The Company recognizes revenue and related expenses from license agreements based on an evaluation of the period of the agreement over which it is earned. Unamortized revenue is recorded as deferred revenue. Revenue from the first and second payments under the Kyosei Agreement are being recognized over the remaining life ofImagent patents, or approximately twelve years. During the nine month period ended September 30, 2004, the Company recognized $210,714 of license revenue under the Kyosei license agreement.
The Company has received a notice from Schering AG (“Schering”) asserting a royalty claim against a portion of the proceeds received from Kyosei. The Company contests the assertion. Nevertheless, the Company has established a provision of $400,000, recorded as “Royalty liability,” should its position not be sustained. In conjunction with the Company’s revenue recognition policy, it also recognizes a pro rata portion of the “Prepaid Royalty” asset attributed to the Schering royalty claim into expense, which totals $21,071 for the nine months ended September 30, 2004.
Subsequent to September 30, 2004, the company entered into a cross-licensing agreement and securities purchase agreement with BMSMI, for which it received $4,000,000 related to the cross licensing agreement. See “Note 12 - Subsequent Events,” below. The Company will recognize revenue on the $4,000,000 attributed to this agreement based on an evaluation of the period of the agreement over which it is earned, expected to be approximately 12 years, commencing in the fourth quarter ending December 31, 2004. Unamortized revenue will be recorded as deferred revenue.
6. Joint Venture/Investment in Affiliate
On October 7, 1999, the Company formed a joint venture with Elan International Services, Ltd. (“Elan”) to develop and commercialize nanoparticulate diagnostic imaging agents for the detection and treatment of cancer through lymphography. Sentigen Ltd. (“Sentigen”) was formed to hold the operations, assets and liabilities of the joint venture.
Elan purchased 2,980 shares of Sentigen nonvoting convertible preferred stock (“Series A Preferred Stock”) for $2,985,000 representing a 19.9% ownership interest. Elan also purchased 12,015 shares of the Company’s Series A convertible exchangeable preferred stock for $12,015,000. The Series A Preferred Stock was exchangeable for additional shares of Sentigen or convertible into common stock of the Company. The Company purchased 12,000 shares of Sentigen’s common stock for $12,015,000 representing an 80.1% ownership interest. Because Elan had substantive day-to day participating and governance rights in Sentigen the Company’s investment in Sentigen through June 10, 2004 was recorded under the equity method.
On June 10, 2004, the Company and Elan terminated the joint venture. As a result, Sentigen has become a wholly owned subsidiary of the Company, the “investment and advances to affiliate” was eliminated and the assets and liabilities of Sentigen consolidated within the financial statements of the Company.
Separately, in June 2004 the holder of the Company’s Series A Preferred Stock sold that instrument and the purchaser converted all Series A Preferred Stock, including accrued and unpaid dividends, into 195,263 shares of the Company’s common stock at a conversion price of $84.68 per share. See “Note 7 - Equity Transactions,” below; and “Management’s Discussion and Analysis and Plan of Operation - Joint Venture,” below. On the conversion date, the value of the Company’s common stock was $0.75 per share, which was deemed to be the implicit factor to value the 19.9% of Sentigen that Elan sold to the Company at $146,447. Accordingly, the Company wrote down the carrying value of its 80.1% share of the related technology license then held by Sentigen to $589,469. As part of the consolidation of Sentigen, the Company by June 30, 2004 recorded the entire value of the technology license, net of $4,128 of amortization, at $731,788.
7. Equity Transactions
In January 2004, the Company recognized the issuance of 6,250 shares of its common stock as compensation for services provided by a consultant.
On March 5, 2004, the Company issued 2,053,977 shares of the Company’s common stock to certain creditors of Alliance upon approval by the Company’s shareholders. Of the total shares issued, 1,985,522 shares (valued at $5,043,226) were part of the acquisition consideration for theImagent Business and, at December 31, 2003, had been recorded as “Common Stock to be issued.” The same value was correspondingly reclassified out of the “Common Stock to be issued” category upon the issuance of the stock on March 5, 2004. The remaining 68,455 shares, valued at $136,956, was a penalty incurred due to delays in securing shareholder approval of the issuance of the shares to be issued in conjunction with the acquisition of theImagent Business during 2003.
During the six months ended June 30, 2004, Xmark sold 314,630 shares of common stock for which it held a put right. Under certain circumstances, the Company would have been required to purchase these puttable shares (“Puttable Shares”) from Xmark at a price of $1.00 per share. In June 2004, the Company entered into an agreement with Xmark whereby the Company exchanged all remaining 1,611,989 puttable shares held by Xmark plus related obligations and fees for an aggregate of $2,128,447 of Secured Notes, that are payable on or before December 1, 2004. See “Note 12 - Subsequent Events,” below.
The Company accrued 49,681 shares of the Company’s common stock issuable to certain entities for interest and late stock registration penalties.
In June 2004, the holder of the Company’s Series A Preferred Stock converted all shares, including accrued but unpaid dividends, for 195,263 shares of the Company’s common stock. Accordingly, the $12,015,000 of Series A Preferred Stock the Company had classified as mezzanine equity has now been reclassified as permanent equity, and the liquidation preference totaling $16,534,840 attributable to the Series A Preferred Stock has been eliminated.
On April 19 and June 30, 2004, the Company sold 25,374,999 shares of its common stock for $0.40 per share and 14,717,502 warrants exercisable for $0.50 per share (including 2,030,000 warrants issued to its two placement agents), for approximately $10,150,000. The net proceeds to the Company were approximately $9,325,000. In addition, holders of an aggregate of approximately $12,719,500 principal amount of the Company’s Secured Promissory Notes and Revolving Convertible Senior Secured Promissory Notes (“Notes”), including accrued interest, converted these Notes into 33,740,672 shares of the Company’s common stock at $0.40 per share.
In June 2004, the Company issued 616,580 shares of its common stock as a partial settlement of obligations under an equipment lease. As part of the terms of the settlement, the Company remains obligated to make an additional payment equal to the difference (if any) between the net proceeds the lessor receives from sales of these shares of stock during the 12 months after the Company registers the shares and $486,601 (which represents the number of issued shares valued at $0.76 per share). Accordingly, the Company has recorded into equity only that portion of the issued shares’ par value, or $617. The remainder, or $467,984, together with two additional cash obligations totaling $100,000, were recorded as a current liability - “Obligations pursuant to equipment lease settlement,” $75,000 of which was repaid in the quarter ended September 30, 2004.
During the quarter ending September 30, 2004, the Company issued 225,000 shares of its common stock, partially as payment for past services (valued at $18,000) and partially for payment of services to be rendered in the future ($18,000). Of the shares issued representing the future services, $15,000 has been reduced from additional paid-in capital at September 30, 2004, which represents the unearned portion of those services as of that date.
During the quarter ending September 30, 2004, the Company reached an accommodation with a firm providing legal services to compensate that firm in January of 2005 via the issuance of common stock in exchange for the deferral of approximately $116,573 incurred during the quarter. The number of common shares to be issued will be dependent on the market price of the Company’s common stock at this future date, and as such is undeterminable at September 30, 2004. The value of $116,573 has been credited in the Company’s balance sheet at September 30, 2004 as “Common stock to be issued.”
During the quarter ended September 30, 2004, the Company recorded $203,000 as “Common stock to be issued” as liquidated damages owed pursuant to certain registration rights provided to investors in the April 19 and June 30, 2004 sale of the Company’s common stock.
Subsequent to the quarter ending September 30, 2004, the Company issued all of its 4,500 shares of its recently authorized Series A convertible preferred stock (which differs from the previous Series A convertible preferred stock which was cancelled in June 2004) pursuant to the BMSMI non-exclusive cross-licensing agreement executed on October 29, 2004 and in exchange for $4,500,000. The Company is reserving 16,666,667 shares of its common stock pursuant to the conversion rights contained therein. In addition, a five-year warrant to purchase up to 1,333,333 of the Company’s common stock was also issued pursuant to a fee arrangement with an investment banking institution which assisted in the transaction. See “Note 12 - Subsequent Event,” below.
8. Stock Options
The Company expenses the fair value of stock options granted to non-employees based on the “intrinsic value” method. In 2003, the Company issued stock options in connection with the Imagent Business purchase with an exercise price less than the market price on the date of grant, which vest over four years. In 2004, the Company issued stock options to employees with an exercise price less than the market price on the date of grant, which vest over four years. Accordingly, compensation expense of $813,724 has been recorded in the first nine months of 2004.
As of September 30, 2004, the Company has reserved 5,321,762 shares of its common stock pursuant to current outstanding stock options, of which 2,519,241 such shares are exercisable as of that date. An additional 745,863 shares of common stock are reserved upon the exercise of options not yet granted as of September 30, 2004.
In addition, the Company’s Board of Directors recently approved increasing the total shares available for issuance under the 2000 Long Term Incentive Compensation Plan from 4,568,750 to 30,000,000, subject to stockholder approval which is expected to be received at the next meeting of stockholders. The Company is contractually obligated to reserve an additional 5,078,402 shares pursuant to options to be granted to its Chief Executive Officer upon the increase in the shares subject to the 2000 Long Term Incentive Compensation Plan.
For stock options granted to employees and directors during the first nine months of 2004 (all of which were granted prior to the quarter ending September 30, 2004), the Company has estimated the fair value of each option granted using the Black-Scholes option-pricing model with the following assumptions:
| | | | 2004 | |
| | | | | |
| Weighted average fair value per options granted | | $ | 0.27 | |
| Significant assumptions (weighted average) | | | | |
Risk-free interest rate at grant date | | | 3.79 | % |
Expected stock price volatility | | | 98 | % |
Expected option life (years) | | | 5 | |
If the Company had elected to recognize compensation expense based on the fair value at the grant dates, consistent with the method prescribed by SFAS No. 123, net loss per share would have been changed to the pro forma amounts indicated below:
| | | Three Months Ended Sept 30, 2004 | | | Three Months Ended Sept 30, 2003 | | | Nine Months Ended Sept 30, 2004 | | | Nine Months Ended Sept 30, 2003 | |
| | | | | | | | | | | | | | |
| Net Loss Applicable to Common Shareholders, as reported | | $ | (4,202,262 | ) | $ | (5,528,941 | ) | $ | (16,859,697 | ) | $ | (18,383,662 | ) |
| | | | | | | | | | | | | | |
Add stock based employee compensation expense included
in reported net income, based on “intrinsic value” method | | | 151,595 | | | 366,723 | | | 813,724 | | | 895,993 | |
| | | | | | | | | | | | | | |
| Less total stock-based employee compensation expense determined under the fair value based method for all awards | | | (509,668 | ) | | (1,139,987 | ) | | (1,493,300 | ) | | (3,419,962 | ) |
| | | | | | | | | | | | | | |
| Pro forma net loss | | | (4,560,335 | ) | $ | (6,302,205 | ) | $ | (17,539,273 | ) | $ | (20,907,631 | ) |
| | | | | | | | | | | | | | |
| Basic and diluted loss per common share, as reported | | $ | (0.05 | ) | $ | (0.26 | ) | $ | (0.39 | ) | $ | (0.99 | ) |
| | | | | | | | | | | | | | |
| Basic and diluted loss per common share, pro forma | | $ | (0.06 | ) | $ | (0.30 | ) | $ | (0.40 | ) | $ | (1.13 | ) |
9. Secured Debt
Notes payable - The Company has entered into a series of agreements with Xmark. In May 2004 the Company made the final principal payment of $1,250,000 under an obligation assumed in the acquisition of the Imagent Business.
In June 2004, the Company entered into an agreement with Xmark whereby the Company exchanged all remaining Puttable Shares held by Xmark plus related obligations and additional fees for an aggregate of $2,128,447 of Secured Notes, payable in installments on or before December 1, 2004. As of September 30, 2004, the Company had repaid an aggregate of $944,612 of these notes. The balances of these notes, totaling $1,183,835, were repaid on November 2, 2004, and the first priority security interest on all of the Company’s tangible and intangible assets (which Xmark was entitled to foreclose on in the event the Company lost its FDA approval for Imagent, if the Company failed to pay other debts, or if the Company had filed for bankruptcy protection or similar matters) was then terminated. See “Note 12 - Subsequent Event.”
Bridge loan - In June 2004, the Company entered into a 60-day bridge loan agreement with a bank to provide up to $750,000. The advances under the bridge loan agreement bore interest at a rate of 9.0% and were secured by a lien on substantially all the Company’s assets except for intellectual property. The security interest was senior to the liens held by the holders of Promissory Notes and subordinate to the liens held by Xmark. The bridge loan, amounting to $702,863, was repaid on July 1, 2004.
10. Accrued Expenses
Accrued expenses consist of the following at September 30, 2004 and December 31, 2003:
| | | Sept 30,2004 | | | December 31,2003 | |
| | | | | | | | |
| Alliance liabilities assumed from acquisition | | $ | 2,095,441 | | $ | 2,530,434 | |
| Payroll liabilities | | | 690,400 | | | 322,503 | |
| Accrued general trade payables | | | 284,526 | | | 291,908 | |
| New Hope office abandonment provision | | | — | | | 136,947 | |
| | | | | | | | |
| | | $ | 3,070,367 | | $ | 3,281,792 | |
11. Commitments
Leases: The Company has leased its facilities and certain equipment under non-cancelable operating leases, which expire at various times through 2008. The leases require the Company to pay for all maintenance, insurance and property taxes.
Minimum future rental payments under these leases are as follows:
| | | | Operating Leases | |
| Three months ending December 31, 2004 | | $ | 239,182 | |
| Twelve months ending December 31, 2005 | | | 979,944 | |
| Twelve months ending December 31, 2006 | | | 920,625 | |
| Twelve months ending December 31, 2007 | | | 877,514 | |
| Twelve months ending December 31, 2008 | | | 147,081 | |
Total minimum lease payments | | $ | 3,164,346 | |
License Agreements: In September 1997, Alliance entered into an agreement with Schering (the “Schering License Agreement”), that provided Schering with worldwide exclusive marketing and manufacturing rights, drug compositions, and medical devices and systems related to perfluorocarbon ultrasound imaging products, including Imagent. In February 2002, the agreement with Schering was modified to give Alliance exclusive rights to market the product in the U.S. for cardiology indications for a five-year period, with Schering to be paid a royalty on such sales. At the expiration of the five-year period, Alliance had the option to acquire all of Schering’s rights to the product on a worldwide basis for total consideration including cumulative royalties of $20,000,000 or, alternatively, to allow Schering the opportunity to obtain co-promotion rights along with Alliance. The rights Alliance had under the Schering License Agreement were transferred to the Company at the consummation of the Company’s acquisition of the Imagent Business. The Company has received a notice from Schering asserting a royalty claim against a portion of the proceeds received from Kyosei. The Company contests the assertion. Nevertheless, the Company has established a provision of $400,000, recorded as “Royalty liability,” should its position not be sustained. As of September 30, 2004, $21,071 has been recognized as royalty expense, which represents a proportionate writeoff of the corresponding “Prepaid Royalty” asset.
Related Party Agreements: Concurrent with the restructuring of the Schering License Agreement in 2002, Alliance entered into a five-year exclusive agreement with Cardinal Health, Inc. (“Cardinal”) to assist with the marketing of Imagent. Under this agreement, Cardinal performed certain packaging, distribution, and sales services for Alliance under a fee-for-service arrangement. The Company assumed the Cardinal agreement from Alliance at the consummation of theImagent acquisition. At September 30, 2004, the Company owed Cardinal approximately $271,783 for current services, which is included in accounts payable.
Three of the Company’s institutional investors, Oxford Bioscience Partners IV L.P. and MRNA Fund II, L.P. (collectively, “Oxford,”) and Mi3, L.P., have provided capital to the Company in exchange for Investor Notes in an aggregate principal amount of up to $12,719,500, which was included in “Lines of Credit.” As part of the Company’s $10,150,000 financing, holders of the Investor Notes converted these notes, including accrued interest, into 33,740,672 shares of the Company’s common stock at $0.40 per share. In addition, as part of the recent equity financing, these institutional investors purchased an aggregate of 5,762,500 shares of the Company’s common stock plus 2,881,251 warrants. Two directors of the Company may be deemed to have a beneficial interest in these Investor Notes and the purchase of the Company’s common stock and warrants due to their positions as Managing Partner of Oxford and Managing General Partner of Mi3, L.P., respectively.
Litigation: On June 18, 2003, the Company filed a complaint against Amersham Health, Inc. and two other Amersham affiliates alleging that certain Amersham products infringe on claims in the Company’s patents covering Imagent. The case is captioned as Photogen Technologies, Inc. and Alliance Pharmaceutical Corp. v. Amersham Health Inc. et. al., Civil Action No. 03CV2853(SRC), in the United States District Court for the District of New Jersey. The Company’s complaint alleges that principally through their Optison® product, Amersham Health Inc. and related Amersham entities infringe on eight patents owned by the Company. The Company is also seeking a declaration that the claims of fifteen Amersham patents are invalid and are not infringed byImagent. Alliance, the party from whom the Company acquiredImagent, is also a plaintiff in the suit. The Company is seeking damages, an injunction against Amersham, a declaratory judgment and other relief.
Amersham filed an answer dated July 18, 2003 denying the material allegations of the Company’s claims and asserting certain affirmative defenses and counterclaims. Amersham’s counterclaims against the Company include claims of patent infringement, breach of contract, breach of good faith and fair dealing, and tortious interference with contract. The parties are exchanging written discovery at this time.
It is not presently feasible to determine whether there is a reasonable possibility that the assets have been impaired for purposes of Statement of Financial Accounting Standards No. 5, or the extent of damages or gain, if any, that might result depending on whether or not the Company prevails. Such a determination will only be possible after the facts and circumstances of the litigation have been established and, potentially, litigated before a court of competent jurisdiction. In any event, the Company will continue to incur litigation costs as it prosecutes its claims in the litigation. Intellectual property disputes are often settled through licensing arrangements, which if resolved unfavorably, could be costly to the Company or ultimately make it unfeasible to market the product. In any intellectual property litigation, it is possible that licenses necessary to settle the dispute would not be available on commercially reasonable terms or that the Company would not be able to redesign its technologies to avoid any claimed infringement thus resulting in the Company’s inability to sell the product.
On July 29, 2003, the European Patent Office revoked Amersham’s European Patent (EP) No. 620744 with claims directed to contrast agents. The Company had brought the opposition proceeding against the EP ‘744 patent in the European Patent Office. The EP ‘744 is a counterpart to the patents that are the subject of the suit against Amersham in the United States described above. See “Risk Factors - The Company filed a suit against Amersham for patent infringement, which, if resolved unfavorably, could impede its ability to utilize its patents forImagent,” below.
12. Subsequent Events
On October 29, 2004, the Company entered into a non-exclusive, cross-licensing agreement with Bristol-Myers Squibb Medical Imaging, Inc. (“BMSMI”) covering ultrasound contrast agent patents. Under the terms of the agreement, BMSMI and the Company will have the right to further develop and commercialize their respective ultrasound contrast imaging agents without risk of infringing upon the other company’s patent rights. The Company received a payment of $4,000,000 under the terms of the license agreement, and will have the right of first negotiation to license select compounds from Bristol-Myers Squibb Medical Imaging, Inc. In accordance with the Company’s accounting policy related to license agreements, the Company will recognize revenue on the $4,000,000 attributed to this agreement based on an evaluation of the period of the agreement over which it is earned, commencing in the fourth quarter ending December 31, 2004. Unamortized revenue will be recorded as deferred revenue.
In connection with the license agreement, the Company entered into a securities purchase agreement with BMSMI and Bristol-Myers Squibb Company pursuant to which the Company sold an aggregate of 4,500 shares of its recently authorized Series A Convertible Preferred Stock for a total purchase price of $4,500,000. Each such share is entitled to a liquidation preference if not otherwise converted to common stock of $1,000, and may also be redeemed at the same price beginning no earlier than the year 2034. These Series A shares may be convertible under certain conditions into 16,666,667 shares of the Company’s common stock at a conversion rate of $0.27 per share.
An investment banking institution was paid $680,000 in cash and will be issued a 5-year warrant to purchase up to 1,333,333 shares of the Company’s common stock at a purchase price of $0.27 per underlying share as compensation for assistance it provided to the Company in this transaction with BMSMI. The Company has estimated the expense associated with the issuance of the warrant to be $60,400 utilizing the Black-Scholes pricing model. The relevant costs will be allocated pro rata between the license and equity portions of the gross funds received from BMSMI, and will be charged against operating expense and additional paid in capital, respectively, in the quarter ending December 31, 2004.
Utilizing a portion of the proceeds received under the BMSMI agreements, on November 2, 2004, the Company repaid the remaining balance outstanding under the Secured Notes held by Xmark, amounting to $1,183,835. These notes were secured by a lien on substantially all of the Company’s assets. The security interest has now been terminated and at this time there are no blanket liens on any of the Company’s owned assets.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS AND PLAN OF OPERATION
Portions of the discussion in this Form 10-QSB contain forward-looking statements and are subject to the “Risk Factors” described below. All forward-looking statements included in this Form 10-QSB are based on information available to the Company on the date hereof, and the Company assumes no obligation to update any such forward-looking statements. The Company’s actual results could differ materially from those anticipated in these forward-looking statements as a result of a number of factors, including those set forth in the section captioned “RISK FACTORS” and elsewhere in this Form 10-QSB. The following should be read in conjunction with the Company’s unaudited financial statements included above.
References to the “Company,” “IMCOR,” “we” or “our” are to IMCOR Pharmaceutical Co. (formerly known as Photogen Technologies, Inc.) and, where appropriate, our subsidiary, Sentigen, Ltd. (“Sentigen”). References to the “Imagent Business” are to the medical imaging business of Alliance Pharmaceutical Corp. (“Alliance”) the Company acquired on June 18, 2003. Imagent® (perflexane lipid microspheres) is a registered trademark owned by IMCOR.
The financial statements included herein have been prepared assuming the Company is a going concern. The Company had approximately $1,843,493 in cash and securities and current liabilities of $7,174,704 at September 30, 2004. The financial statements for the three- and nine-month periods ending September 30, 2004 do not include any adjustment to reflect the possible future effects on the recoverability and classification of assets or the amount and classification of liabilities that may result from this uncertainty. The Company obtained $8,500,000 through a cross-license with Bristol-Myers Squibb Medical Imaging, Inc. dated October 29, 2004 and as a result has sufficient funds to operate through the end of 2004.
Company Background
General
IMCOR is based in San Diego, California. We have approximately 26 full time employees and lease a 53,000 square foot facility in San Diego, which is an FDA approved cGMP (current Good Manufacturing Practices) manufacturing plant sufficient for our current and projected manufacturing needs of Imagent. Our personnel have expertise in sales, marketing, manufacturing, research and development, clinical development and regulatory approval processes. To supplement our internal capabilities, we work closely with consultants experienced in drug development and regulations, third party research laboratories, contract research organizations and with academic and other institutions to conduct specific research projects.
We are an emerging, development-stage pharmaceutical company focused on developing and marketing medical imaging pharmaceutical products. These imaging agents are used with echocardiography and computed tomography (“CT”) equipment in order to increase the diagnostic capabilities of these techniques. By enhancing the contrast between different types of tissues, these agents provide critical anatomical and disease state information that is not obtainable using the equipment alone. Our products have numerous potential applications including the ability to allow a physician to diagnose certain cardiovascular abnormalities and the spread of cancer in the lymphatic system.
We have one FDA approved product,Imagent, an ultrasound imaging contrast agent we acquired as part of theImagent Business.Imagent has been approved for marketing by the FDA for use in patients with sub-optimal echocardiograms to opacify the left ventricle of the heart and improve delineation of the endocardial borders of the heart. As a result, ultrasound withImagent may better distinguish normal and abnormal heart structure and function - two critical indicators of cardiac health. Echocardiography is the most widely used imaging modality for the diagnosis of heart disease, the leading cause of death in the U.S. The attractiveness of the ultrasound imaging market potential together with the acquisition of an approved product and an extensive intellectual property portfolio were fundamental reasons for the acquisition and primary determinants of the purchase price of approximately $7,240,000 for plant, property and equipment and $15,638,000 for the purchased technology.
We are seeking to expand the label indication forImagent for myocardial perfusion imaging for the detection of coronary artery disease (CAD) using a novel technique called Phasic Changes. Phasic Changes is one of three ultrasound techniques being investigated in the field for myocardial perfusion. The advantage of the Phasic Changes technique, compared to the other two techniques, is that it allows the patient to remain at rest during imaging (as opposed to stressing the patient in order to raise their heart rate). Initial studies of Phasic Changes conducted by an independent investigator has progressed from early feasibility testing in animals to a feasibility study in humans. The initial study provided data on 22 patients, with the results showing that the degree and location of narrowing of blood vessels in and around the heart (known as coronary stenosis) as detected with Phasic Changes were highly correlated with results produced from coronary angiography, the gold standard of measuring the severity of CAD. To protect our patent rights underlying our key product,Imagent, we have filed a complaint against Amersham Health, Inc. alleging certain Amersham products infringe on claims covering certainImagent patents and theft of trade secrets. We intend to continue to vigorously assert our rights on these issues.
Currently,Imagent is marketed in the United States through a contract sales organization, Cardinal Health Sales and Marketing Services with a very limited field force and with very limited agents. Accordingly, our limited recepits are included in “Investment and Other Income.” Various companies provide packaging, technical and reimbursement product support and distribution services on a contract basis.
We have two additional contrast agents in development for use in computed tomography (CT) and x-ray imaging with potential applications, (1) as a blood pool agent to diagnose diseased tissue in the cardiovascular system and other organs, (PH-50), and (2) to diagnose cancer metastasizing into the lymphatic system, N1177. PH-50 and N1177 are iodinated nanoparticulate formulations and are still in early stages of development. Based on the availability of capital and our assessment of market potential, we intend to focus our efforts onImagent and plan to defer the development of PH-50 and N1177 to a later date.
We expect that our quarterly and annual results of operations will fluctuate based on several factors including, our ability to raise additional capital to implement our clinical, sales, marketing and manufacturing programs, the results of our litigation against Amersham Health, Inc., the timing and amount of expenses involved in marketing and manufacturing Imagent, conducting clinical trials, the activities required for compliance with FDA regulations and the amount, if any, of fees, milestone payments and research support payments received from current and potential strategic partners.
Certain Recent Developments
We have completed the following recent transactions:
| · | On October 29, 2004, we entered into a non-exclusive, cross-licensing agreement with Bristol-Myers Squibb Medical Imaging, Inc. covering ultrasound contrast agent patents. Under the terms of the agreement, Bristol-Myers Squibb Medical Imaging, Inc. and the Company will have the right to further develop and commercialize their respective ultrasound contrast imaging agents without risk of infringing upon the other company’s patent rights. We received a payment of $4,000,000 under the terms of the license agreement, and will have the right of first negotiation to license select compounds from Bristol-Myers Squibb Medical Imaging, Inc. In connection with the license agreement, we entered into a securities purchase agreement with Bristol-Myers Squibb Medical Imaging, Inc. and Bristol-Myers Squibb Company pursuant to which we sold an aggregate of 4,500 shares of our recently authorized Series A Convertible Preferred Stock for a total purchase price of $4,500,000. |
| · | On November 2, 2004, we repaid in full the Secured Notes held by Xmark. These notes were secured by a lien on substantially all of our assets. The security interest has now terminated and at this time there are no blanket liens on any of our owned assets. |
On June 18, 2003, we closed the acquisition of theImagent Business. We consider the valuation of our investment in the purchased technology related toImagent (“Purchased Technology”) to be a significant estimate. The carrying value of this Purchased Technology at September 30, 2004 was approximately $14,002,545, net of accumulated amortization. Our current valuation is based upon the purchase price we paid for the acquisition from Alliance. We intend to periodically evaluate the valuation of Purchased Technology and make any necessary adjustments. Our valuation assumptions have been based on currently available information including estimates of market size and penetration, pricing, competitive threats and general operating expenses. These estimates may change and such changes may impact future estimates of carrying value. We instituted litigation against Amersham regarding patents acquired with the Purchased Technology and Amersham has responded with counter-claims. If we lose this litigation, our business and the value of the Purchased Technology would be materially and adversely affected. See “Liquidity; Capital Resources,” below.
Prior to termination, we considered our investment in our technology license with units of Elan to be a significant estimate. The license related to the joint venture formed to develop one of our CT contrast agents, a subcutaneous nanoparticulate formulation for lymphography. In June 2004, the joint venture was terminated and the holder of our then outstanding Series A Preferred Stock converted it, including accrued and unpaid dividends, into 195,263 shares of common stock of IMCOR at a conversion price of $84.68 per share. (This is different from the newly authorized Series A Convertible Preferred Stock recently issued to Bristol-Myers Squibb Company in connection with a cross-license agreement, which remains outstanding and is unrelated to the former Elan joint venture.) Among other factors, the canceled Series A Preferred Stock issued in connection with the Elan joint venture was exchangeable for additional shares of Sentigen. See “Management’s Discussion and Analysis and Plan of Operation, Joint Venture,” above. On the conversion date, the value of our common stock was $0.75 per share, which was deemed to be the implicit factor to value the 19.9% of Sentigen that Elan sold to IMCOR at $146,447. Accordingly, we wrote down the carrying value of our 80.1% share of the related technology license then held by Sentigen to $589,469. As part of the consolidation of Sentigen, by June 30, 2004 we recorded the entire value of the technology license as an asset, valued at $735,916 before amortization. As of September 30, 2004, we are carrying this asset net of $21,818 amortization, as $714,098.
Results of Operations
The financial statements included in this Form 10-QSB present the results of operations of theImagent Business following its acquisition on June 18, 2003. As one of a series of transactions on November 12, 2002, we split off the therapeutic business to certain founding shareholders. Accordingly, the expenses associated with that business have been reclassified as discontinued operations.
Quarters ended September 30, 2004 and 2003
Revenue. To date, we have not generated significant product revenues due to capital constraints limiting our sales and marketing capability. In December 2003 we entered into a license agreement with Kyosei for the development and marketing of Imagent in Japan for all radiology and cardiology indications. At the closing, we received a non-refundable gross payment of $2,000,000 from Kyosei.In April 2004, we received an additionalnon-refundable gross payment of $2,000,000. These are gross amounts; and an aggregate of $400,000 for these payments was withheld for Japanese withholding taxes at the source and charged off to expense in 2003 and 2004 as appropriate. We are recognizing income from these gross payments ratably over the remaining expected life of the Kyosei agreement. Accordingly, we recognized $84,524 as license revenue in the third quarter of 2004. We had no comparable revenue for the third quarter of 2003.
Additional payments from Kyosei are expected in early 2005 and beyond upon successful completion of contractual milestones with total set payments not to exceed $10,000,000, together with royalties based upon future sales. The future payments are based on certain milestones of clinical development, including the commencement of and completion of clinical studies and approval for sale by Japanese regulatory authorities. Commencement of clinical studies is not expected to be earlier than 2005, and the development program, including regulatory approval, is expected to take four or more years. Accordingly, we do not expect commercial sales, upon which we will earn royalties, to commence earlier than 2008. While we believe thatImagent clinical studies will be successful, we cannot assure that such studies conducted in Japan will be successful or that the product will be approved for sale by Japanese authorities.
Research and Development. Research and development expenses for the quarters ended September 30, 2004 and September 30, 2003 were $962,904 and $562,703, respectively.
Prior to the third quarter of 2003, research and development expenses were directed primarily to the development of PH-50, one of our CT contrast agents. Prior to this period, we maintained a small internal management group and conducted research activities through contracts with third party laboratories.
Following theImagent Business acquisition, we gained internal research capabilities and infrastructure. Substantially all of the research expenses in the third quarter 2004 were for infrastructure and expenses associated with regulatory compliance and continued development of Imagent for new indications and the maintenance of our manufacturing capability.
Significant 2004 costs categorized as research and development expenditures (all numbers approximate) include personnel costs (research, development and manufacturing support) ($516,000), contract consulting services ($292,000), patent costs ($52,000), production facilities maintenance ($50,000) and supplies associated with research and development and production activities ($42,000).
Our current manufacturing facility provides significant excess capacity for all clinical development and commercial manufacturing activities, now and for the foreseeable future. Since the excess manufacturing facility capacity provides no significant direct benefit to us at the present time, the current costs associated with this excess capacity continue to be charged off against current operations, including a portion attributed to research and development.
To date we have incurred a total of approximately $2,597,000 of expenses in connection with the development of PH-50 (one of our CT contrast agents), but nothing for the quarter ended September 30, 2004. We had been developing our second CT contrast agent (N1177), a subcutaneous nanoparticulate formulation for imaging of lymph nodes, through a joint venture with affiliates of Elan which was terminated as of June 10, 2004. The associated research and development expenses have previously been charged to the joint venture. We have incurred a total of approximately $3,130,000 of expenses in connection with the development of this formulation through 2002. There have been no such expenditures since that date, including the quarter ended September, 30, 2004.
We expect our development priorities, based on the availability of sufficient capital, to focus on the compliance with regulatory obligations for Imagent and development of new indications for Imagent. Expenses associated with the development of subcutaneous formulation were charged to our joint venture through June 10, 2004, and we bear expenses associated with the development of our other products.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the quarters ended September 30, 2004 and September 30, 2003 were $3,334,111 and $4,306,244, respectively.
Significant third quarter 2004 costs categorized as selling, general and administrative expenses (all numbers approximate) include personnel costs ($324,000), contract consultant costs ($429,000), recognition of the expense of stock options granted at a price below the fair market value for our common stock on the date of grant ($152,000), legal (including general corporate) and accounting ($560,000), facilities costs ($413,000), fees and services primarily related to late registration share charges ($248,000), and depreciation and amortization ($812,000).
In the third quarter of 2003, these expenses include costs associated with obtaining and asserting patent protection for our intellectual property, costs associated with administrative personnel and corporate management, the amortization of certain options granted to our employees, legal and related expenses, facilities expenses at our Pennsylvania offices, which have since been closed, and, following theImagent Business acquisition, costs to operate that business, including amortization of Purchased Technology.
Other Income and Expense. Investment and other income for the quarters ended September 30, 2004 and September 30, 2003 was $28,874 and $581, respectively. Investment income is generated by the amount of capital in our investment portfolio prior to it being used to fund its operations and the rate of interest earned on that capital. In the third quarter of 2004, investment and other income consisted primarily of sales ofImagent.
We expect investment and other income to fluctuate primarily as a result of recording other income items and, to a lesser extent, as the size of the investment portfolio decreases or increases and as the rate of interest earned by the portfolio varies due to shifts in short term interest rates.
Interest expense was $21,145 and $197,169 for the quarters ended September 30, 2004 and 2003. Commencing in May 2003, we financed the operations primarily through the use of Secured Promissory Notes and Revolving Convertible Senior Secured Promissory Notes bearing interest at 7.5%, compounded monthly. In addition, as part of theImagent Business acquisition, we delivered to Xmark promissory notes in the total principal amount of $2,500,000. These notes bore interest at 3% over the prime rate (payable in shares of the Company’s common stock). See “Liquidity; Capital Resources,” below. In the third quarter 2004, the Company also accrued interest expense related to certain other note obligations associated with the acquisition.
Net loss applicable to common shareholders (after preferred dividends) was $4,202,262, or $0.05 per share, and $5,528,941, or $0.26 per share, for the three-month periods ended September 30, 2004 and 2003, respectively. Weighted average shares utilized in these calculations were 79,979,139 and 21,361,817, respectively. The increase in weighted shares outstanding was due primarily to the timing of the Company’s financing, debt conversion and conversion of the Series A Preferred Stock issued in connection with the Elan joint venture. See “Liquidity; Capital Resources,” below.
During the third quarter of 2004 we incurred costs totaling approximately $78,317 related to purchases of laboratory equipment and to upgrade our information technology infrastructure in our San Diego offices. During the third quarter of 2003, we had insignificant purchases of laboratory equipment and leasehold improvements other than those associated with the Purchased Technology. During the next twelve months, assuming that we obtain sufficient capital, we expect capital expenditures for equipment to be less than $250,000.
Nine months ended September 30, 2004 and 2003
Revenue. We recognized $210,714 as license revenue in the first nine months of 2004 pursuant to the amortization of deferred revenue related to the Kyosei agreement. We had no comparable revenue in the first nine months of 2003.
Research and Development. Research and development expenses for the nine months ended September 30, 2004 and 2003 were $2,849,511, and $1,383,145 respectively. The increase in expenses for the first half of 2004 was due primarily to the additional costs incurred related to the Imagent Business. Significant 2004 costs categorized as research and development expenditures (all numbers approximate) include personnel costs (research, development and manufacturing support) ($1,667,000), contract consulting services ($581,000), patent costs ($259,000), production facility maintenance ($236,000) and supplies associated with research and development and production activities ($180,000).
We are planning to initiate additional pre-clinical studies of PH-50 at a later date. We have incurred a total of approximately $2,597,000 of expenses in connection with the development of PH-50, including $66,000 for the nine months ended September 30, 2004.
Selling, General and Administrative Expense. Selling, general and administrative expenses for the nine months ended September 30, 2004 and 2003 were $11,165,872 and $10,771,833, respectively.
Significant 2004 costs categorized as selling, general and administrative expenses (all numbers approximate) include personnel costs ($1,214,000), contract consultant costs ($1,525,000), recognition of the expense of stock options granted at a price below the fair market value for our common stock on the date of grant ($814,000), legal (including general corporate and litigation support) and accounting ($1,468,000), special fees (primarily related to carrying costs associated with various Xmark obligations) ($1,000,000), facilities costs ($1,209,000) and depreciation and amortization ($2,392,000).
These expenses in the nine months of 2003 were comprised primarily of costs associated with the acquisition of the Imagent Business incurred prior to making a firm commitment to the acquisition (costs incurred following our firm commitment to acquire the Imagent Business have been capitalized as part of the acquisition cost) including (i) provisions we took against advances we made to Alliance and shares of the common stock we issued to Xmark pursuant to a standstill agreement with Xmark, (ii) costs of conducting due diligence and other activities related to the acquisition of the Imagent Business, and (iii) costs associated with obtaining patent protection for its intellectual property, costs associated with administrative personnel and corporate management, the amortization of options granted to its employees, legal and related expenses and facilities expenses.
In 2004, the overall increase in expenses for the first nine months of 2004 was due primarily to the additional costs incurred related to the Imagent Business, including costs of litigation, selling and marketingImagent and amortization of Purchased Technology related to the acquisition of the ImagentBusiness. More specifically, significant 2004 costs categorized as selling, general and administrative expenses (all numbers approximate) include personnel costs ($1,214,000), contract consultant costs ($1,525,000), recognition of the expense of stock options granted at a price below the fair market value for our common stock on the date of grant ($814,000), legal (including general corporate) and accounting ($1,468,000), special fees (primarily related to carrying costs associated with various Xmark obligations and late registration share charges) ($1,203,000), facilities costs ($1,209,000) and depreciation and amortization ($2,392,000).
Other Income and Expense. Investment and other income for the nine months ended September 30, 2004 and 2003 was $272,306 and $7,394, respectively.
In 2003, investment and other income primarily reflected earnings on the investment portfolio following our capital raising efforts in late 2002.
In 2004, investment and other income reflected:
| · | reversal of the remaining provision for the closing of our former offices in New Hope, PA upon the landlord’s successful efforts in replacing us on the lease; |
| · | disgorgement of profits by a shareholder deemed to be our affiliate of the Company to the Company resulting from certain sales of our common stock; |
| · | proceeds resulting from modest marketing ofImagent; and |
| · | earnings on the investment portfolio. |
Interest expense was $617,898 and $251,157 for the nine months September 30, 2004 and 2003.
We recorded preferred stock dividends (payable in additional shares of Series A Preferred Stock) of $498,030 and $791,480 in the nine month periods ended September 30, 2004 and 2003 for Series A Preferred Stock issued in connection with the Elan joint venture.
Net loss applicable to common shareholders (after preferred dividends) was $16,859,697, or $0.39 per share, and $18,383,662, or $0.99 per share, for the nine month periods ended September 30, 2004 and 2003, respectively. Weighted average shares utilized in these calculations were 43,632,027 and 18,562,221, respectively. The increase in weighted shares outstanding was due primarily to the timing of our financing, debt conversion and Series A Preferred Stock conversion.
Liquidity; Capital Resources
Capital Resources and Requirements. At September 30, 2004, we had cash and cash equivalents totaling approximately $1,843,493 and current liabilities of approximately $7,174,704 including $1,183,835 (of an original $2,128,447) secured debt that is payable on or before December 1, 2004. The security interest covered substantially all of our assets, including intellectual property. On November 2, 2004, we repaid the final balances owed on these notes, utilizing a portion of the $8,500,000 in gross proceeds we received from BMSMI (see “Certain Recent Developments,” above) and the security interest terminated. We have sufficient funds to continue operations through the end of December, 2004 at current operating levels and assuming we are successful in reaching and maintaining payment term accommodations with our unsecured creditors. Without additional financing, however, we may not have sufficient resources for our entire fiscal year ending December 31, 2005.
At December 31, 2003, we had a net deficit position (our liabilities, puttable share obligations and mezzanine equity exceeded our assets) of $8,194,743.
However, during the quarter ended June 30, 2004, the following three transactions (more fully described in our Form 10-QSB/A for the quarterly period ended June 30, 2004) contributed to increased shareholders’ equity, as follows:
| · | $10,150,000 common stock financing, resulting in approximately $9,325,000 cash proceeds net of costs; |
| · | conversion of various debt instruments with principal values aggregating $12,719,500 plus accrued interest, into common stock for a total value of approximately $13,496,000; and |
| · | reclassification of the $12,015,000 mezzanine equity into shareholders’ equity upon the conversion of the Series A Preferred Stock issued in connection with the Elan joint venture into 195,263 shares of common stock. |
The above three transactions, taken together, resulted in gross increases to shareholders’ equity totaling approximately $34,836,000 during the nine months ended September 30, 2004. The most significant reduction to shareholders’ equity during this same period resulted from continuing net losses, totaling approximately $16,158,667. Accordingly, the net shareholders’ deficit of $8,194,743 at December 31, 2003 was improved to a positive shareholders’ equity of $12,098,810 at September 30, 2004, primarily as a result of the above described transactions and activities.
Shares outstanding have increased from 19,464,548 at December 31, 2003 to 80,065,300 at September 30, 2004 (excluding warrants and options).
We lack sufficient working capital to fund planned operations for the entire fiscal year ending December 31, 2005. We cannot accurately estimate the amount of additional financing required; however, the amount could be an additional $12,000,000 or more in order to fund operations through that date. Therefore, substantial additional capital resources will be required to fund our planned ongoing operations related to clinical development, marketing and manufacturing of Imagent, research and business development activities and debt repayment. Our financial condition raises substantial doubt about its ability to continue as a going concern without adjustments to its current planned levels of expenditures.
We are currently in default under obligations to pay approximately $923,000 to creditors of Alliance in connection with its acquisition of Imagent. While there can be no assurance of success, we believe that we can resolve these defaults and that they do not give rise to a reduction in the carrying value of Purchased Technology of theImagent Business. We also havenot registered the resale of shares we issued in connection with that acquisition, which in some cases is a condition to the effectiveness of agreements to release us from any claims by the Alliance creditor or requires us to issue additional penalty shares.
We believe there are a number of potential alternatives available to meet the continuing capital requirements such as public or private financings or collaborative agreements. We are also taking continuing actions to reduce ongoing expenses. If adequate funds are not available, we will be required to significantly curtail our operating plans and may have to sell or license significant portions of our technology or potential products. Our financial statements, including those contained in this Form 10-QSB, do not include any adjustment to reflect the possible future effects on the recoverability and classification of assets or the amount and classification of liabilities that may result from the outcome of this uncertainty.
If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders may result. However, there can be no assurance that such capital will be available under acceptable terms, if at all. See “Risk Factors - We must raise additional financing in the future and our inability to do so will prevent us from continuing as a going concern and implementing our business plan,” below.
In addition, we expect to evaluate from time to time the acquisition or license of businesses, technologies or products. The purchase of any such licenses or technologies would be funded by a portion of any net proceeds of future offerings or the issuance of debt or equity securities specifically for that purpose. We expect our use of capital to increase as we continue the clinical development ofImagent.
We entered into a series of agreements with Xmark related to its acquisition of theImagent Business. We issued 750,000 shares of our common stock in the first quarter 2003 to Xmark, who agreed to not exercise any rights against Alliance as a creditor for the period of the standstill. On May 2, 2003, we entered into an agreement pursuant to which Xmark agreed to extend the period during which Xmark had refrained from exercising its rights as a creditor against Alliance to permit us to complete the acquisition. On that date, we also purchased a $1,250,000 interest in Alliance's obligations to Xmark.
At the closing of our acquisition of theImagent Business we delivered to Xmark promissory notes in the total principal amount of $2,500,000. These notes were payable in two equal installments on August 5, 2003 and November 3, 2003 and bore interest at 3% over the prime rate (payable in shares of our common stock). At the closing of theImagent Business acquisition, we also issued Xmark an additional 1,056,144 shares that represented payment of interest that was due to Xmark by Alliance through the closing date of the acquisition. We negotiated a new agreement with Xmark dated August 18, 2003 pursuant to which we agreed to make an immediate payment of $1,250,000 and the remaining payment in November 2003.
On May 3, 2004, we repaid to Xmark the last Senior Secured Note in the principal amount of $1,250,000. Following that repayment, Xmark had remaining the right to require us to repurchase 1,611,989 shares of our common stock Xmark held at $1.00 per share. This put right was secured by all the assets of ourImagent Business and was exercisable in full on July 19, 2004. In June 2004, we entered into an agreement with Xmark whereby we exchanged all puttable shares held by Xmark and other related obligations totaling $1,778,447, together with an additional fee of $350,000, for an aggregate of $2,128,447 of Secured Notes payable no later than December 1, 2004. The Secured Notes were secured by all of our assets. As of September 30, 2004 we had repaid $944,612 of these secured notes. On November 2, 2004 we repaid the remaining balances of these notes, amounting to $1,183,835, in full. See “Certain Recent Developments.”
In June 2004 we settled the demand by a lessor of approximately $787,000 for two payments totaling $100,000 and the issuance of 616,580 shares of our common stock. We will make an additional payment equal to the difference (if any) between the net proceeds the lessor receives from sales of the stock during the 12 months after we register the shares and $468,601. Due to the remaining contingency of a payment, we have continued to reflect the amount paid in stock, less the par value of the shares issued, as a liability.
In December 2003, we entered into a license agreement with Kyosei for the development and marketing of Imagent in Japan for all radiology and cardiology indications. We have received a notice from Schering asserting a right against a portion of the proceeds received from Kyosei. We contest the assertion. We have established a provision of $400,000 should our position not be sustained. See “Results of Operations,” above.
We have two long-term commitments that are not recorded on its financial statements. At the time of the acquisition of the Imagent business, we entered into a non-cancelable operating lease agreement with a unit of Baxter International for certain equipment used for the manufacture of Imagent. Our annual cost for this lease is approximately $126,000. We lease the office and manufacturing space in San Diego, California. Annual rent for the California lease, which expires in 2008, is approximately $792,000 (which includes estimated operating costs such as insurance, taxes and maintenance).
On October 29, 2004, we entered into a non-exclusive, cross-licensing agreement with Bristol-Myers Squibb Medical Imaging, Inc. covering ultrasound contrast agent patents. We received a payment of $4,000,000 under the terms of the license agreement. In connection with the license agreement, we entered into a Securities Purchase Agreement with Bristol-Myers Squibb Medical Imaging, Inc. and Bristol-Myers Squibb Company pursuant to which we sold an aggregate of 4,500 shares of our newly issued Series A Convertible Preferred Stock for a total purchase price of $4,500,000.
On November 2, 2004 we repaid the remaining balances of the notes owed to Xmark, amounting to $1,183,835, in full.
After receiving $8,500,000 from Bristol-Myers Squibb Medical Imaging, Inc. and repaying Xmark $1,183,835, we had approximately $7,240,000 in cash as of November 12, 2004. We anticipate a need to raise at least approximately $12,000,000 in order to continue our operations through 2005.
Plan of Operation
During the next twelve months we will focus our efforts primarily on exploring various options concerning our business, including securing additional financing, joint ventures, licensing or selling all or a portion of our technology and other possible strategic transactions. Our ability to continue clinical development ofImagent depends on the successful implementation of one or more of these transactions because our currently available capital is not sufficient to carry out our business plan or fully develop and commercializeImagent.
Depending on the availability of capital and whether we pursue a sale or licensing strategy for Imagent, we intend to focus our operating efforts on the clinical development ofImagent, for an indication to detect CAD.In particular, we plan to focus on Phase II studies for CAD, which we expect to require funding of at least $4,500,000 (including out-of-pocket study costs, staffing and costs to maintain our San Diego production facility at its current level). Longer term, we plan to conduct Phase III clinical trials for the CAD indication forImagent and prepare for a broader commercialization effort, to include investigating the appropriate strategic relationships which might provide additional funding. We also intend to continue prosecuting our claims in the litigation against Amersham as a high priority for our use of funds and other resources. We have asserted that Amersham’s products infringe on our patent claims coveringImagent and theft of trade secrets. Amersham has asserted counterclaims against us.
We plan to focus on thedevelopment of our CT contrast agent (PH-50 and N1177) at a later date, unless other strategic opportunities are presented. Subject to the availability of sufficient capital, we expect to continue to incur losses for at least the next three years as we intensify the clinical development, associated regulatory approval activities and engage in or provide for the manufacture and/or sale of these and any other products that we have or may develop.
Greater capital resources would enable us to quicken and expand our marketing and research and development activities, and our failure to raise additional capital will (absent a suitable collaborative agreement providing for a third party to take over these functions) significantly impair or curtail our ability to conduct further activities. In any event, complete development and commercialization of our technology will require substantial additional funds. We are seeking to raise capital through the sale of our common stock or other securities in a private placement to fund our immediate and longer-term capital needs. See “Risk Factors - We must raise additional financing in the future and our inability to do so will prevent us from continuing as a going concern and implementing our business plan,” below. In the absence of additional funds, we need to reduce our rate of spending by limiting operations, reducing our personnel, seeking accommodations from creditors and other similar resources.
As of September 30, 2004, we expect to pay the following contractual obligations and commitments:
| | | Payments due by Year |
| | | | | | | | | | | | | | | | |
| | | 2004 | | | 2005-2006 | | | 2007-2008 | | | Beyond 2008 | | | Total | |
Recorded Liabilities | | | | | | | | | | | | | | | | |
| Xmark secured promissory notes(Repaid November 2, 2004) | | $ | 1,183,835 | | $ | - | | $ | - | | $ | - | | $ | 1,183,835 | |
Imagent purchase obligations (remaining) | | | 2,095,441 | | | - | | | - | | | - | | | 2,095,441 | |
| Equipment lease settlement obligations | | | 492,984 | | | - | | | - | | | - | | | 492,984 | |
| Accrued royalty liability | | | 400,000 | | | | | | | | | | | | 400,000 | |
| | | | | | | | | | | | | | | | | |
Commitments | | | | | | | | | | | | | | | | |
| Operating Lease Commitments | | | 239,182 | | | 1,900,569 | | | 1,024,595 | | | - | | | 3,164,346 | |
| | | | | | | | | | | | | | | | | |
| Total Contractual Obligations | | $ | 4,411,442 | | $ | 1,900,569 | | $ | 1,024,595 | | $ | - | | $ | 7,336,606 | |
Recent Accounting Pronouncements
During the quarter ended September 30, 2004, there were no new Accounting Pronouncements impacting our accounting, financial position or operating results.
ITEM 3 CONTROLS AND PROCEDURES
In September 2004, our management concluded that there were certain reportable conditions and material weaknesses in our internal controls and procedures. We formed this view in response to communications with our independent auditors, Moss Adams LLP. Moss Adams had orally discussed certain matters related to the Company's internal controls and financial reporting with our former Chief Financial Officer and our Audit Committee. After we filed our Form 10-QSB for the quarter ended June 30, 2004, Moss Adams concluded that these matters cumulatively amounted to reportable conditions and material weaknesses and formally advised us of their overall determination by letter in September 2004.
The reportable conditions noted were that our financial statement close process was not performed in a timely manner, leading to delays and adjustments in reconciling recurring and non-recurring balances and transactions, and could result in material contracts and transactions being improperly omitted or incorrectly disclosed. In addition, Moss Adams stated that our use of Quickbooks accounting software, while itself not a reportable condition, when coupled with the lack of adequate internal controls could lead to situations in which errors or irregularities may not be discovered in a timely manner or a risk that users of the software could modify historical data.
The material weaknesses noted were that our accounting and reporting processes were not completed on a timely basis (in part due to the absence of a full-time on-site chief financial officer); the small size of our accounting staff results in inadequate segregation of duties; our limited number of financial and accounting personnel make it difficult to create a backup knowledge base enabling personnel to fill in if there is an absence of one individual; and our policies and procedures relating to cash disbursements, cash handling, cash receipts and reconciliation should have greater segregation of duties to mitigate the risk of fraud or financial statement misstatement.
Moss Adams recommended taking steps to alleviate understaffing in our accounting department to improve the timeliness of financial reporting and enable appropriate segregation of duties. Additionally, Moss Adams recommended that we require management to assess and report annually on the effectiveness of internal controls.
The Company and our Audit Committee are committed to remediating the reportable conditions and material weakness. We do not expect to be able to fully implement all remediation until the Company has sufficient additional financing. Although certain matters may not be fully remediated, we believe appropriate mitigating factors are in place to reduce the likelihood of material misstatements or improper disclosure.
During the period beginning in May 2004, we began to implement steps to address our internal controls and procedures. The following remediation actions have been completed:
- We engaged Larry D. Grant as a financial consultant. Mr. Grant has 30 years of experience in accounting and financial advisory work, both in private practice with major accounting firms and as a consultant and interim executive to various enterprises. He has been working full time for us at our San Diego headquarters since May 11, 2004.
- We added Darlene Deptula-Hicks to our Audit Committee. Ms. Deptula-Hicks joined our Board on July 22, 2004, and is Chair of our Audit Committee. Ms. Deptula-Hicks has over 20 years of experience in financial management positions, primarily in the life sciences sector. She is currently Executive Vice President and Chief Financial Officer of ONI Medical Systems, Inc.
- We have required, since July 2004, the Executive Committee of the Board to ratify routine and/or recurring expenditures and approve significant commitments and non-routine expenditures and payments.
- We require both Larry Grant and our Senior Vice President Business Development and Marketing to review and approve our incurring all significant obligations and remitting payments.
- We improved controls over our various material agreements and transactions.
We are in the process of developing and implementing the following remediation steps:
- We have committed to design an internal control plan, including disclosure controls. We presently intend to have this control plan in place by the end of the first quarter of the 2005 fiscal year.
- We are, on an ongoing basis, reviewing significant processes involved in accounting and financial reporting to ensure that they function as designed.
- We are developing a Code of Ethics to address other internal control procedures, such as conflicts of interest. We expect to approve and implement the Code of Ethics by the end of the 2004 fiscal year. Once completed, all employees will be made aware of and required to comply with this Code of Ethics.
- We continue to work to improve disclosure controls as they relate to non-routine material agreements and transactions.
The following remediation actions are in the planning stages. We do not expect to be able to fully implement these actions until sufficient additional financing is available:
- We intend to hire a full-time on-site Chief Financial Officer.
- We are in a search for additional full time accounting staff, including a Controller, and other staff as necessary.
- We are evaluating the effectiveness of our current accounting software and potential replacement systems.
As noted, the Company is committed to remediating the reportable conditions and material weaknesses. To mitigate the effects of our current staffing levels in the accounting and finance area, our current personnel have made extra efforts to accurately close the financial statements for the third quarter of 2004 and to complete the necessary steps for this Form 10-QSB. To mitigate the delay in implementing a formal internal control plan, we also intend to specifically monitor internal controls and related procedures until such a plan is put into place.
Our Chief Executive Officer (who is our acting Principal Financial Officer while we search for a permanent replacement to our former Chief Financial Officer) has consulted with Larry Grant and has participated in the evaluation of our disclosure controls (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended) required by paragraph (b) of Rule 13a-15 or Rule 15d-15, as of September 30, 2004 and, based on that evaluation, concluded that notwithstanding the material weaknesses and conditions described above, our disclosure controls and procedures overall were effective to ensure timely collection, evaluation and disclosure of information relating to the Company that would potentially be subject to disclosure under the Securities Exchange Act of 1934, and the rules and regulations thereunder. To the extent corrections and improvements in internal controls and disclosure controls and procedures cannot be immediately implemented, management believes appropriate oversight and review is currently in place to prevent material misstatements in our interim financial reporting.
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
In 2003, we filed a complaint against Amersham and two other Amersham affiliates alleging that certain Amersham products infringe on claims in its patents coveringImagent. The case is captioned asPhotogen Technologies, Inc. and Alliance Pharmaceutical Corp. v. Amersham Health Inc. et. al., Civil Action No. 03CV2853(SRC), in the United States District Court for the District of New Jersey. The Company’s complaint alleges that principally through their Optison® product, Amersham and related Amersham entities infringe on eight patents owned by us. We are also seeking a declaration that the claims of fifteen Amersham patents are invalid and are not infringed by ourImagent product. Amersham denied the material allegations of our claims and asserted certain affirmative defenses and counterclaims. Amersham's counterclaims against us include claims of patent infringement of the fifteen Amersham patents, breach of contract, breach of good faith and fair dealing, and tortious interference with contract.
It is not presently feasible to determine whether there is a reasonable possibility that the assets have been impaired for purposes of Statement of Financial Accounting Standards No. 5, or the extent of damages or gain, if any, that might result depending on whether or not we prevail. In any event, we will continue to incur litigation costs as we prosecute claims in the litigation.
See “Risk Factors—We filed a suit against Amersham for patent infringement, which, if resolved unfavorably, could impede our ability to utilize our patents forImagent,” below.
ITEM 2. CHANGES IN SECURITIES
During the quarter ending September 30, 2004, we issued 225,000 shares of our common stock as compensation for past services provided and future services to be provided by a consultant, without registration under the Securities Act. The portion of the underlying securities are held in escrow and are subject to certain cancellation provisions. These securities were offered and sold by us in reliance upon exemptions from the registration requirements provided by Section 4(2) of the Securities Act and Regulation D under the Securities Act relating to sales not involving any public offering, and/or Rule 701 under the Securities Act relating to transactions occurring under compensatory benefit plans. We relied on representations and warranties from the purchaser of the securities that they were acquiring the securities for their own account and not with a view to, or for sale in connection with, any distribution of the securities in violation of the Securities Act.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
During the quarter ended September 30, 2004 we were in default under obligations to pay an aggregate of $923,000 to creditors of Alliance in connection with our acquisition ofImagent. We did not register the resale of shares delivered to certain of those creditors, which, in some cases, is a condition to the effectiveness of agreements to release us from any claims by that creditor. Although we cannot give assurances of success, we believe the Company can resolve these defaults and that they do not give rise to a reduction in the carrying value of the Purchased Technology of theImagent Business.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted to our shareholders during the quarter ended September 30, 2004.
ITEM 5. OTHER INFORMATION
RISK FACTORS
This Form 10-QSB contains (and press releases and other public statements we may issue from time to time may contain) a number of forward-looking statements regarding its business and operations. Statements in this document that are not historical facts are forward-looking statements. Such forward-looking statements include those relating to:
| · | our current business and product development plans, |
| · | our future business and product development plans, |
| · | the timing and results of regulatory approval for proposed products, and |
| · | projected capital needs, working capital, liquidity, revenues, interest costs and income. |
Examples of forward-looking statements include predictive statements, statements that depend on or refer to future events or conditions, and statements that may include words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” “should,” “may,” or similar expressions, or statements that imply uncertainty or involve hypothetical events.
Forward-looking statements involve known and unknown risks and uncertainties that may cause our actual results in future periods to differ materially from what is currently anticipated. We make cautionary statements in certain sections of this Form 10-QSB, including under “Risk Factors.” You should read these cautionary statements as being applicable to all related forward-looking statements wherever they appear in this Form 10-QSB, in the materials referred to in this Form 10-QSB, in the materials incorporated by reference into this Form 10-QSB, or in its press releases or other public statements. In light of these risks, uncertainties and assumptions, the forward-looking events discussed in this Form 10-QSB or other documents incorporated by reference might not occur. No forward-looking statement is a guarantee of future performance and you should not place undue reliance on any forward-looking statement. We do not undertake any obligation to release publicly any revisions to these forward looking statements, to reflect events or circumstances after the date of this Form 10-QSB, or to reflect the occurrence of unanticipated events, except as may be required under applicable securities laws.
The following are key risk factors that may affect our future results and/or the price of our common stock:
Business and Financial Risks
We must raise additional financing in the future and our inability to do so will prevent us from continuing as a going concern and implementing our business plan.
Our cash and securities on hand at September 30, 2004 was approximately $1,843,493. At September 30, 2004, we had liabilities, including current liabilities of approximately $7,174,704 and deferred revenue of approximately $3,789,286, together totaling $10,963,990. We are in default under obligations to pay $923,000 to former creditors of Alliance in connection with our acquisition of theImagentBusiness. We also have not registered the resale of shares we issued in connection with that acquisition, which in some cases is a condition to the effectiveness of agreements to release us from any claims by the Alliance creditor
We will need substantial additional financing to continue planned operations including its manufacturing, marketing and product development programs, and the repayment of debt. At our current rate of spending, including estimated requirements to service a portion of our current liabilities and without additional funds, we may not be able to continue our current level of operations beyond the first quarter of 2005. We estimate that we will need at least $12,000,000 through December 31, 2005 to fund planned operations, discharge debt and allocate continued resources to the Amersham litigation matter. We are currently seeking capital in one or more transactions to fund our immediate and longer-term capital needs. Additional funds may not be available on acceptable terms, if at all, and existing stockholders may be diluted as a result of those offerings. The pricing of our common stock, or other securities convertible into common stock, in any such transaction may also result in an increase in the number of shares of common stock issuable upon exercise of warrants in accordance with the anti-dilution provisions in the instruments governing those securities.
We have a history of losses, and we may not achieve or maintain profitability in the future or pay cash dividends.
We have incurred losses since the beginning of its operations. As of September 30, 2004, we have incurred cumulative net losses (before dividends on preferred stock) of approximately $67,880,365. We expect our losses to increase in the future as its financial resources are used for litigation, manufacturing, marketing, research and development, preclinical and clinical development, regulatory activities, and other related expenses. We may not be able to achieve or maintain profitability in the future. We have never declared or paid any cash dividends to stockholders and do not expect to do so in the foreseeable future.
We do not have any material revenues from sales; accordingly, we are a development stage company.
We and our technologies, other than our recently acquiredImagent product, are in early stages of development. We began our business as a biopharmaceutical company in 1997. To date, we have not generated material revenues from sales or operations. We expect to generate revenue from sales ofImagent, but there can be no assurance that we will be able to generate enough revenue from such sales to be profitable for at least several years, if at all.
Our inability to expand the label indication ofImagent for the detection of CAD and to continue development of PH-50 and N1177 may impede our ability to raise capital and achieve profitability.
We are seeking to expand the label indication ofImagent for myocardial perfusion imaging for the detection of coronary artery disease (CAD). Our primary focus for clinical development of Imagent is using a novel technique called Phasic Changes. We believe that development ofImagent for the detection of CAD and in particular using the Phasic Changes technique will be important to our ability to attract capital and achieve future profitability. We plan to work toward further clinical development of PH-50 and N1177 at a later date.
There are a number of potential obstacles to these goals:
| · | We may not have sufficient capital to complete the clinical trials and other tasks necessary to obtain regulatory approval. |
| · | The results of studies onImagent for the Phasic Changes technique are preliminary. These studies and others for PH-50 and N1177 may not provide sufficient data to predict the products’ potential for use. |
| · | The FDA may not approve the use ofImagent for the detection of CAD (or PH-50 or N1177 for their planned indications). We have not completed an examination of whetherImagent has sufficient intellectual property protection to use it for the Phasic Changes technique. Intellectual property disputes relating toImagent, PH-50 or N1177 could be costly to resolve and could affect our ability to commercialize products with those technologies. |
| · | We may not attract capital or achieve profitability even if we successfully introduceImagent for the additional indication, PH-50 or N1177. |
| · | As a result of changing economic considerations, market, clinical or regulatory conditions, or clinical trial results, we may shift our focus or determine not to continue one or more of the product development projects we are currently pursuing. |
The markets for imaging products are extremely competitive, and many of our competitors have greater resources and have products that are in more advanced stages of development than we do, which may give them a competitive advantage over us.
In addition toImagent, there are currently two FDA approved ultrasound contrast agents being marketed in the U.S. by Amersham Health, Inc. and Bristol-Myers Squibb Medical Imaging, Inc. for the same indication for sub-optimal ultrasound images as Imagent. In addition, Bracco International has filed a New Drug Application with the FDA seeking approval to market its product in the U.S. Two other companies (POINT Biomedical Corp. and Acusphere, Inc.) are in advanced clinical trials for the use of ultrasound contrast agents for myocardial perfusion. We expect that competition in the ultrasound contrast imaging field will be based primarily on each product's safety profile, efficacy, stability, ease of administration, breadth of approved indications, and physician, healthcare payer and patient acceptance. We are at the very early stages of launchingImagent, and we cannot predict whether it will compete successfully with these other products.
We filed a suit against Amersham for patent infringement, which, if resolved unfavorably could impede our ability to utilize our patents for Imagent.
We filed a complaint against Amersham Health, Inc. and two other Amersham affiliates alleging that certain Amersham products infringe on claims in our patents coveringImagent. We also seek a declaration that our products do not infringe on Amersham's patents covering their products, and other relief. Alliance joined us as a plaintiff in this suit. Amersham filed an answer denying the material allegations of our claims and asserting certain counterclaims. If the suit is resolved unfavorably to us, we may have difficulty utilizing the protections of its patents forImagent and there may be other complications that may make it difficult or impossible to implement its commercialization strategy forImagent.
Intellectual property disputes are often settled through licensing arrangements that could be costly to us. In any intellectual property litigation, it is possible that those licenses necessary to settle the dispute would not be available on commercially reasonable terms or that we would not be able to redesign its technologies to avoid any claimed infringement thus resulting in our inability to sell the product.
Our proposed products are subject to extensive testing and government approval, and we may not obtain or maintain the approvals necessary to sell our proposed products.
Other thanImagent for its current indication, none of our proposed imaging products has received the FDA's approval for commercial sales. An extensive series of clinical trials and other regulatory requirements must be completed before our proposed products can be approved and sold in the U.S. or other countries. Requirements for FDA approval of a product include preclinical safety testing in animals and clinical testing for effective use and safety in humans, which can be extremely costly. The time frame necessary to perform these tasks for any individual product is long and uncertain, and we may encounter problems or delays that we cannot predict at this time. The lack of adequate capital will contribute to delays in this process. We must also obtain regulatory approvals comparable to those required in the U.S. to market its products in other countries. Even if testing is successful, its proposed products may not demonstrate sufficient effectiveness or safety to warrant approval by the FDA or other regulatory authorities.
Development of the nanoparticulate formulations has been limited primarily to laboratory experiments and animal testing. Only the subcutaneous nanoparticulate formulation for lymphography has completed Phase I human clinical trials. The Company has not yet conducted substantive studies of its compounds in development to evaluate their effectiveness on human subjects. Our results from early clinical trials may not predict results that we will obtain in large-scale clinical trials, as even after promising results in early trials, a number of companies have suffered significant setbacks in advanced clinical trials.
We may not conduct additional Phase II or Phase III clinical trials for our products and such trials, if begun, may not demonstrate any efficacy or may not be completed successfully in a timely manner, if at all. The rate of completion of our clinical trials depends upon, among other things, the rate of patient enrollment. Patient enrollment is a function of many factors, including the size of the patient population for a particular indication, the nature of the clinical protocol under which our products will be studied, the proximity of the patient to a clinical site and the eligibility criteria for the study. Delays in planned patient enrollment may result in increased costs, regulatory filing delays, or both. Furthermore, we, the FDA or other regulatory authorities may alter, suspend or terminate clinical trials at any time. If we do not successfully complete clinical trials and obtain regulatory approval for a product, we will not be able to market that product.
Any products approved by the FDA are subject to post-market approval requirements of the FDA.
Our FDA approved product,Imagent, is subject to post-market approval requirements by regulatory authorities. We are subject to inspection and marketing surveillance by the FDA to determine its compliance with regulatory requirements with respect to any approved products. If the FDA finds that we have failed to comply, it can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
| · | recall or seizure of our products; |
| · | fines, injunctions and civil penalties; |
| · | operating restrictions, partial suspension or total shutdown of production; and |
Any enforcement action by the FDA may also affect our ability to commercially distribute its products in the U.S.
Product liability claims could increase our costs and adversely affect its results of operations.
The marketing, sale and clinical testing ofImagent and our product candidates may expose us to product liability claims, and we may experience material product liability losses in the future. We maintain certain levels of product liability insurance for the use of our products, but our coverage may not continue to be available on terms acceptable to us or adequate for liabilities we actually incur. A successful claim brought against us in excess of available insurance coverage, or any claim or product recall that results in significant adverse publicity against us, may have a material adverse effect on its business and results of operations.
If we do not obtain and maintain patent or other protection of its core technologies (namelyImagent, PH-50 and N1177) we may have difficulty commercializing products using these technologies.
Our success depends in part on our ability to obtain, assert and defend its patents, protect trade secrets and operate without infringing the intellectual property of others. Among the important risks in this area are
| · | our patent applications may not result in issued patents. Moreover, any issued patents may not provide us with adequate protection of its intellectual property or competitive advantages, and the law on the scope of patent coverage is continually changing. |
| · | various countries limit the subject matter that can be patented and limit the ability of a patent owner to enforce patents in the medical field. This may limit our ability to obtain or utilize those patents internationally. |
| · | existing or future patents or patent applications (and the products or methods they cover) of our competitors (or others, such as research institutions or universities) may interfere, invalidate, conflict with or infringe our patents or patent applications. Similarly, the use of the methods or technologies contained in our patents, patent applications and other intellectual property may conflict with or infringe the rights of others. |
| · | if an advance is made that qualifies as a joint invention, the joint inventor or his or her employer may have rights in the invention. |
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that these rights are covered by valid and enforceable patents or effectively maintained as trade secrets. We acquired a patent portfolio related toImagent as part of the acquisition of theImagent Business. We have not completed its evaluation of intellectual property rights associated with the use ofImagent for the Phasic Changes technique. We also own two patents in the U.S., and certain other patents in foreign countries including Australia, Canada, Japan and Germany that relate to methods for performing lymphography.
In addition to the patents and patent applications we acquired through the purchase of theImagent Business, we have filed patent applications in the U.S. and under the Patent Cooperation Treaty covering a number of foreign countries. These patent applications relate to the use of nanoparticulates, including PH-50, as cardiovascular imaging agents and to deliver pharmacologically active substances for treatment of various diseases. We are the exclusive licensee from Nycomed Imaging AS of a group of patented proprietary nanoparticulate compounds including N1177 and PH-50. We are the exclusive licensee of U.S. and foreign patent applications from Massachusetts General Hospital that relate to diagnostic imaging agents and methods for using the diagnostic imaging agents for medical location, treatment and diagnosis of tumors and other diseased tissues. Additional patents may never be issued on any of the patent applications we own or license from third parties.
The patent position of biopharmaceutical companies involves complex legal and factual questions and therefore we cannot assure the enforceability of these patents. A third party might successfully challenge the validity or enforceability of our patents or if issued, patents that would result from its patent applications. The patents might not provide protection against competitive products or otherwise be commercially valuable, and applications filed by others might result in patents that would be infringed by the manufacture, use or sale of our products. Litigation over patents and other intellectual property rights occurs frequently in our industry and there is a risk that we may not prevail in disputes over the ownership of intellectual property. Further, an interference proceeding with the Patent and Trademark Office may be instituted over the rights to certain inventions, and there is a risk that we may not prevail in such an interference proceeding. Those disputes can be expensive and time consuming, even if we prevail. Intellectual property disputes are often settled through licensing arrangements that could be costly to us. In any intellectual property litigation, it is possible that licenses necessary to settle the dispute would not be available on commercially reasonable terms or that the Company would not be able to redesign its technologies to avoid any claimed infringement thus resulting in its inability to sell the product.
Confidentiality agreements covering our intellectual property may be violated, and we may not have adequate remedies for any violation. Our trade secret intellectual property may in other ways become known or be independently discovered by competitors. In addition, where intellectual property results from a research project supported by government funding, the government has limited rights to use the intellectual property without paying us a royalty.
To the extent we use intellectual property through licenses or sub-licenses, as is the case for some of its lymphography technology, its rights are subject to us performing the terms of the license or sub-license agreement with third parties. Our rights are also subject to the actions of third parties we may not be able to control, such as its sub-licensor complying with the terms of its license with the patent owner and the patent owner maintaining the patent.
We depend on a small number of employees and consultants to provide management expertise, and the loss of this expertise may interfere with our operations or delay our ability to implement our business plan.
We currently have two senior executive officers: our Chief Executive Officer (Dr. Williams) and our Senior Vice President Business Development and Marketing (Mr. DeFranco). We also have retained consultants to advise us in regulatory affairs, product reimbursement, quality assurance and control matters and other business matters. Larry D. Grant, a consultant, provides advisory and management services on a full-time basis on financial, operational and strategic matters. These individuals have entered into employment or consulting agreements, confidentiality and/or non-competition agreements with us. If an individual performing one of these executive or consulting functions for us terminates his or her association with us or breaches their agreement with us:
| · | We could suffer competitive disadvantage or loss of intellectual property protection. |
| · | We could experience a delay in the implementation of our business plan until we arrange for another individual or firm to fulfill the role. |
The raw materials forImagent are subject to a long-term supply contract with one supplier. An interruption in availability of those materials may impair our ability to market or test those products.
We assumed the rights and obligations of Alliance under the terms of the long-term supply agreement that Alliance had for the principal raw material forImagent. Raw materials used inImagent cannot be changed without equivalency testing of any new material by us and approval of the FDA. Some raw materials forImagentare currently qualified from only one source and, although we have some inventory, we will attempt to acquire additional inventory of these materials and to negotiate long-term supply arrangements. There can be no assurance that we would be successful in negotiating any such long term or other supply agreements.
To make any significant change in the manufacturing process or change in suppliers, we must conduct certain validation studies, which may require review and/or approval by the FDA. Considerable delays in our research and development programs for our products could result from its having to change to a different supplier.There can be no assurance that Nanosystems will continue to supply us with the raw materials that we need in a timely manner on conditions that are acceptable to us.
We may encounter difficulties expanding and/or changing our manufacturing operations.
We lease a single 53,000 square foot facility located in San Diego, California, a portion of which houses our manufacturing operations. This lease expires in three years and we may be required to commitment a significant amount of time and expense in connection with moving to a new facility if the lease is not renewed.
The current facility is subject to a variety of regulatory requirements to maintain compliance with current good manufacturing practices. We may encounter difficulties in maintaining in compliance with these regulations and standards, which also could result in a delay or termination of manufacturing or an inability to meet product demand.
We will face risks inherent in operating a single facility for the manufacture ofImagent. At this time, we do not have alternative production plans in place or alternative facilities available should the San Diego manufacturing facility cease to function. These risks include unforeseen plant shutdowns due to personnel, equipment or other factors, and the resulting inability to meet customer orders on a timely basis.
Our lease for the San Diego, California facility is scheduled to terminate in 2008 with two, three year renewal terms. There will be costs associated with locating and moving to a new facility that meets FDA requirements, assuming we continue to maintain internal manufacturing capabilities.
We have to rely on third parties and collaborative relationships for marketing, distribution pre-clinical and clinical testing ofImagent and our proposed products, and it may be difficult to implement our business plans without these collaborations.
We currently have contract agreement with Cardinal Sales and Marketing Services for marketing, Cardinal Specialty Pharmacy Distribution for distribution and fulfillment, Cardinal PCI for labeling and packaging and RxCrossroads for reimbursement and product technical support. We have had and expect to continue in the future to have a variety of research agreements with universities and other research institutions to investigate specific protocols for the preclinical development and testing of its products or research activities related to other new potential products. We also contract and expect to continue to contract with research organizations and other third parties to manage clinical trials of its products. We must obtain and maintain collaborative relationships with third parties for research and development, preclinical and clinical testing, marketing and distribution of our proposed products. Collaborative relationships may limit or restrict our operations or may not result in an adequate supply of necessary resources. A collaborative partner could also pursue alternative technologies as a means of developing or marketing products for the diseases targeted by our collaborative program. If a third party we are collaborating with fails to perform under its agreement or fails to meet regulatory standards, this could delay or prematurely terminate preclinical or clinical testing of our proposed products.
Our products may not be fully accepted by physicians, laboratories and health insurance providers, which would reduce our revenue and limit our ability to raise capital.
Our growth and success will depend upon market acceptance by physicians, laboratories and health insurance providers of our products. This requires acceptance of our products as clinically useful and cost-effective alternatives to other medical imaging products and methods. In addition, physicians may utilize imaging techniques other than those for which our products are being developed (such as magnetic resonance imaging (MRI) and radionuclear imaging.
Changes in health care reimbursement policies or legislation may make it difficult for physicians and hospitals to use or receive reimbursement for using our products, which could reduce our revenues.
Our success will depend, in part, on the extent to which health insurers, managed care entities and similar organizations provide coverage or reimbursement for using the medical procedures we have and plan to develop. These third-party payers are increasingly challenging the price of medical procedures and services and establishing guidelines that may limit physicians' selections of innovative products and procedures. We also cannot predict the effect of any current or future legislation or regulations relating to third-party coverage or reimbursement on our business. We may not be able to achieve market acceptance of our proposed products or maintain price levels sufficient to achieve or maintain any profits on our products if adequate reimbursement coverage is not available. Failure by physicians, hospitals and other users of our products to obtain sufficient reimbursement from third-party payers for the procedures in which our products would be used or adverse changes in governmental and private third-party payers' policies toward reimbursement for such procedures would have a material adverse effect on our business.
Risks Related To Our Common Stock
A small group of stockholders have significant voting power and may delay or prevent a change of control of IMCOR and may have other adverse effect on the price of our common stock.
As of September 30, 2004, a small group of stockholders control approximately 51,384,623 shares, or 64.2% of our issued and outstanding common stock (our Series A Preferred Stock has no voting rights). These stockholders are also parties to a Voting, Drag-Along and Right of First Refusal Agreement (the “Voting Agreement”) concerning the election of certain designees to the Board of Directors and certain other corporate actions. The Voting Agreement requires these stockholders to vote their shares for the election of certain individuals nominated by parties to the Voting Agreement. This concentration of ownership and control may delay or prevent a change in control of IMCOR, and may also result in a small supply of shares available for purchase in the public securities markets. These factors may affect the market and the market price for its common stock in ways that do not reflect the intrinsic value of our common stock.
The price and trading volume of our common stock fluctuates significantly, like that of many biopharmaceutical companies, which may make it difficult for us or a stockholder to sell common stock at a suitable price and may cause dilution for existing stockholders when we issue additional shares.
During the period from January 1, 2004 through September 30, 2004 the closing trade price of our common stock ranged from $1.95 to $0.11 per share. Daily trading volume ranged from zero shares to approximately 476,141 shares during that period.
The market prices for securities of biopharmaceutical companies in general have been highly volatile and may continue to be highly volatile in the future. The following factors may have an impact on the price of our common stock:
| · | Sales performance ofImagent; |
| · | Announcements by us or others regarding scientific discoveries, technological innovations, commercial products, patents or proprietary rights; |
| · | Announcements by us concerning the licensing or other transactions of its products or technologies; |
| · | Private sales of a significant block of our common stock at a price below the then current market price of our common stock and the subsequent registration and sale of those shares; |
| · | Public Sales of our common stock pursuant to Rule 144 of the Securities Act of 1933; |
| · | The progress of preclinical or clinical testing; |
| · | Developments or outcome of litigation concerning proprietary rights, including patents (and the uncertainty related to the Amersham litigation); |
| · | Changes in government regulation; |
| · | Public concern about the safety of devices or drugs; |
| · | Limited, if any, coverage by securities analysts; |
| · | The occurrence of any of the risk factors described in this section; |
| · | Sales of large blocks of stock by an individual or institution; |
| · | Changes in our financial performance from period to period, securities analysts' reports, and general market conditions; and |
| · | Economic and other external factors or a disaster or crisis. |
If stockholders holding substantial amounts of its common stock should sell their stock in the public market, the price of its stock could fall and it may be more costly for us to raise capital.
We filed a registration statement on Form SB-2 on August 4, 2004 (which is not yet effective) with respect to the sale of 25,374,999 shares of our common stock (plus an additional 14,717,502 shares of our common stock issuable on conversion of warrants) by selling shareholders. We have a commitment to register an additional approximately 62,384,917 shares of our common stock (including shares owned by funds controlled by two of our directors and certain former creditors of Alliance) following the effectiveness of the August 4 registration statement. We may also register the resale of shares issuable pursuant to the exercise of options granted under our various option plans. In addition, some of our shares are eligible for sale in the public market free of restriction under Rule 144 of the Securities Act. Others shares of our common stock are also subject to agreements requiring us to permit the holders of the shares, under certain circumstances, to join in a public offering of our stock or to demand that we register their shares for resale. The sale of these shares could place downward pressure on the overall market price of our common stock.
We have issued a substantial number of securities convertible into shares of our common stock that will result in substantial dilution to the ownership interests of our existing shareholders.
As of September 30, 2004, we had reserved 21,477,095 shares of our common stock for issuance upon exercise or conversion of the following securities:
| · | 15,409,470 shares upon exercise of outstanding stock warrants, including 14,717,502 shares of common stock underlying warrants from our April/June 2004 financing; |
| · | 5,321,762 shares upon exercise of outstanding stock options; and |
| · | 745,863 shares upon exercise of options available for future grants under our existing option plans. |
In addition, our Board of Directors recently approved increasing the total shares available for issuance under the 2000 Long Term Incentive Compensation Plan from 4,568,750 to 30,000,000, subject to stockholder approval which we expect to receive at our next meeting of stockholders. We are contractually obligated to reserve an additional 5,078,402 shares pursuant to options to grant to our Chief Executive Officer upon increase in the shares subject to the 2000 Long Term Incentive Compensation Plan. If our options and warrants are all issued and exercised, investors may experience significant dilution in the voting power of their common stock. The sale of these shares could also place downward pressure on the overall market price of our common stock.
Furthermore, on October 29, 2004, we issued and sold 4,500 shares of our Series A Convertible Preferred Stock which is convertible into 16,667,667 shares of our common stock. We also issued a warrant to purchase 1,333,333 shares of our common stock to our investment banker in connection with the Cross License Agreement with Bristol-Myers Squibb Medical Imaging, Inc. and the related sale of Series A Convertible Preferred Stock.
Applicable Securities and Exchange Commission rules governing the trading of “penny stocks” limits the trading and liquidity of our common stock, which may affect the trading price of our common stock.
Our common stock currently trades on the Pink Sheets. Our appeal to NASDAQ to reconsider its decision to delist our shares was denied. We are considering options to seek re-listing of our shares on an appropriate exchange, but it will be a minimum of at least several months before we can submit an application for re-listing. Because our common stock continues to trade below $5.00 per share, our common stock is considered a “penny stock” and is subject to Securities and Exchange Commission rules and regulations, which impose limitations upon the manner in which our shares can be publicly traded. These regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the associated risks. Under these regulations, certain brokers who recommend such securities to persons other than established customers or certain accredited investors must make a special written suitability determination regarding such a purchaser and receive such purchaser's written agreement to a transaction prior to sale. These regulations have the effect of limiting the trading activity of our common stock and reducing the liquidity of an investment in our common stock.
We do not intend to pay dividends in the foreseeable future.
We have never declared or paid a dividend on its common stock. We intend to retain earnings, if any, for use in the operation and expansion of our business and, therefore, do not anticipate paying any dividends on our common stock in the foreseeable future.
ITEM 6. EXHIBITS AND REPORTS ON FORM 8-K
(a) Exhibits.
The following is a list of exhibits filed as part of this Form 10-QSB. Exhibits that were previously filed are incorporated by reference. For exhibits incorporated by reference, the location of the exhibit in the previous filing is indicated in parenthesis.
EXHIBITNO. | | DESCRIPTION |
| | | |
| +3.1 | | Restated Articles of Incorporation of IMCOR Pharmaceutical Co., as amended, including Certificate of Designation, Preferences and Rights of Series A Convertible Preferred Stock. (Filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on November 8, 2004 and incorporated herein by reference.) |
| | | |
| +3.2 | | Amended and Restated Bylaws of IMCOR Pharmaceutical Co. (Filed as Exhibit C to the Company’s Information Statement on Form DEF 14C dated December 23, 2002 and incorporated herein by reference.) |
| | | |
| *10.1 | | License Agreement originally dated as of September 23, 1997 amended and restated in its entirety as of February 22, 2002 by and between Alliance Pharmaceutical Corp. and Schering Aktiengesellschaft. (Confidential portions redacted.) |
| | | |
| *10.2 | | Settlement and Worldwide License Agreement dated as of January 31, 2001 by and among Bracco International B.V., Schering Aktiengesellschaft, and Alliance Pharmaceutical Corp. (Confidential portions redacted.) |
| | | |
| *31.1 | | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| *31.2 | | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| *32.1 | | Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| *32.2 | | Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
+ Incorporated by Reference.
* Filed herewith.
(b) Reports on Form 8-K.
We filed the following reports on Form 8-K during the three-month period ending September 30, 2004:
On September 16, 2004 we filed a Form 8-K reporting that we expected to exhaust our capital by October 31, 2004.
On September 27, 2004 we filed a Form 8-K reporting that our Chief Financial Officer was leaving IMCOR.
Signatures
In accordance with the requirements of the Exchange Act, the registrant has caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: November 15, 2004 | IMCOR Pharmaceutical Co. |
| | |
| | |
| | /s/ Taffy J. Williams | |
| | Taffy J. Williams, Ph.D., President, Chief Executive Officer and Acting Principal Financial Officer |
EXHIBIT INDEX
EXHIBITNO. | | DESCRIPTION |
| | | |
| +3.1 | | Restated Articles of Incorporation of IMCOR Pharmaceutical Co., as amended, including Certificate of Designation, Preferences and Rights of Series A Convertible Preferred Stock. (Filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on November 8, 2004 and incorporated herein by reference.) |
| | | |
| +3.2 | | Amended and Restated Bylaws of IMCOR Pharmaceutical Co. (Filed as Exhibit C to the Company’s Information Statement on Form DEF 14C dated December 23, 2002 and incorporated herein by reference.) |
| | | |
| *10.1 | | License Agreement originally dated as of September 23, 1997 amended and restated in its entirety as of February 22, 2002 by and between Alliance Pharmaceutical Corp. and Schering Aktiengesellschaft. (Confidential portions redacted.) |
| | | |
| *10.2 | | Settlement and Worldwide License Agreement dated as of January 31, 2001 by and among Bracco International B.V., Schering Aktiengesellschaft, and Alliance Pharmaceutical Corp. (Confidential portions redacted.) |
| | | |
| *31.1 | | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| *31.2 | | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| *32.1 | | Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| *32.2 | | Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
+ Incorporated by Reference.
* Filed herewith.
EXHIBIT 31.1
CERTIFICATION
| I, Taffy J. Williams, Ph.D., President and Chief Executive Officer, certify that: |
|
| |
| 1. | I have reviewed this quarterly report on Form 10-QSB of IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.); |
| | |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| | |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the small business issuer as of, and for, the periods presented in this report; |
| | |
| 4. | The small business issuer’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the small business issuer and have: |
| | (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the small business issuer, including its consolidated subsidiaries, is made known to the Company by others within those entities, particularly during the period in which this report is being prepared; |
| | | |
| | (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| | | |
| | (c) | Evaluated the effectiveness of the small business issuer’s disclosure controls and procedures and presented in this report its conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| | | |
| | (d) | Disclosed in this report any change in the small business issuer’s internal control over financial reporting that occurred during the small business issuer’s most recent fiscal quarter (the small business issuer’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the small business issuer’s internal control over financial reporting; and |
| 5. | The small business issuer’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the small business issuer’s auditors and the audit committee of the small business issuer’s board of directors (or persons performing the equivalent functions): |
| | (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the small business issuer’s ability to record, process, summarize and report financial information; and |
| | | |
| | (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the small business issuer’s internal control over financial reporting. |
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D.Chief Executive Officer |
| | |
| | Date: December 21, 2004 |
|
EXHIBIT 31.2
CERTIFICATION
| I, Taffy J. Williams, Ph.D., acting Principal Financial Officer, certify that: |
| | |
| 1. | I have reviewed this quarterly report on Form 10-QSB of IMCOR Pharmaceutical Co. (f/k/a Photogen Technologies, Inc.); |
| | |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| | |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the small business issuer as of, and for, the periods presented in this report; |
| | |
| 4. | The small business issuer’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the small business issuer and have: |
| | (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the small business issuer, including its consolidated subsidiaries, is made known to the Company by others within those entities, particularly during the period in which this report is being prepared; |
| | | |
| | (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| | | |
| | (c) | Evaluated the effectiveness of the small business issuer’s disclosure controls and procedures and presented in this report its conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| | | |
| | (d) | Disclosed in this report any change in the small business issuer’s internal control over financial reporting that occurred during the small business issuer’s most recent fiscal quarter (the small business issuer’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the small business issuer’s internal control over financial reporting; and |
| 5. | The small business issuer’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the small business issuer’s auditors and the audit committee of the small business issuer’s board of directors (or persons performing the equivalent functions): |
| | (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the small business issuer’s ability to record, process, summarize and report financial information; and |
| | | |
| | (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the small business issuer’s internal control over financial reporting. |
| | | |
| | |
|
|
|
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D.Acting Principal Financial Officer |
| | |
| | Date: December 21, 2004 |
EXHIBIT 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of IMCOR Pharmaceutical Co. (the “Company”) on Form 10-QSB for the period ending September 30, 2004 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Taffy J. Williams, Ph.D., Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
| | (1) | The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| | | |
| | (2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| | | |
| | | |
| | |
|
|
|
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D. Chief Executive Officer |
| | |
| | Date: December 21, 2004 |
EXHIBIT 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of IMCOR Pharmaceutical Co. (the “Company”) on Form 10-QSB for the period ending September 30, 2004 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Taffy J. Williams, Ph.D., acting principal financial officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
| | (1) | The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| | | |
| | (2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company |
| | | |
| | |
|
|
|
| | | /s/ Taffy J. Williams |
| |
Taffy J. Williams, Ph.D. Acting Principal Financial Officer |
| | |
| | Date: December 21, 2004 |
IMCOR PHARMACEUTICAL CO.
ANNUAL MEETING OF STOCKHOLDERS
JANUARY 20, 2005
PROXY
SOLICITED ON BEHALF OF THE BOARD OF DIRECTORS
The undersigned hereby appoints Taffy J. Williams, Ph.D. and Brian Gallagher, Ph.D. or either one of them acting singly in the absence of the other, with full power of substitution, the proxy and proxies of the undersigned to vote the shares of Common Stock, par value $.001 per share, of IMCOR Pharmaceutical Co. (the "Company"), which the undersigned could vote, and with all power the undersigned would possess, if personally present at the Annual Meeting of Stockholders of the Company to be held at the Union League Club of Chicago, 65 West Jackson Boulevard, Chicago, Illinois, on January 20, 2005, at 10:00 a.m., Chicago Time, and any adjournment thereof, all as set forth in the accompanying Proxy Statement:
^ FOLD AND DETACH HERE ^
IMCOR PHARMACEUTICAL CO.
PLEASE MARK VOTE IN OVAL IN THE FOLLOWING MANNER USING DARK INK ONLYo
| 1. | | Ratify the Company issuing more than 20% of its outstanding common stock in connection with a financing transaction and conversion of notes | | For
o | | Against
o | | Abstain
o |
2. |
|
Amend the 2000 Long Term Incentive Compensation Plan to increase the number of shares of Common Stock reserved for issuance to 30,000,000 shares, provide for cashless exercise of options and eliminate automatic vesting on change of control |
|
For
o |
|
Against
o |
|
Abstain
o |
3. |
|
Amend Articles of Incorporation to increase the number of shares of Common Stock authorized for issuance to 200,000,000 |
|
For
o |
|
Against
o |
|
Abstain
o |
4. |
|
Amend Articles of Incorporation to effect up to a 1 for 20 reverse split |
|
For
o |
|
Against
o |
|
Abstain
o |
5. |
|
Election of Directors |
|
For
All
o |
|
Withhold
All
o |
|
Except as noted
o |
INSTRUCTION: TO WITHHOLD AUTHORITY TO VOTE FOR ANY INDIVIDUAL NOMINEE, MARK A LINE THROUGH THE NOMINEE'S NAME IN THE LIST BELOW.
| 01 Robert A. Ashley | | 05 Brian M. Gallagher, Ph.D. |
| 02 Richard T. Dean, Ph.D. | | 06 Alan D. Watson, Ph.D., M.B.A. |
| 03 Darlene Deptula-Hicks | | 07 Taffy J. Williams, Ph.D. |
| 04 Jonathan J. Fleming | | |
| 6. | | In their discretion, the proxies are authorized to act upon any matters incidental to the foregoing and such other business as may properly come before the Annual Meeting, or any adjournment thereof. | | For
o | | Against
o | | Abstain
o |
This Proxy when properly executed, will be voted in the manner directed herein by the undersigned stockholder. If no direction is made, this proxy will be voted FOR Items 1, 2, 3, 4 and 5 above and in the discretion of the appointed proxies upon such other business as may properly come before the meeting.
| | | The signer hereby revokes all proxies heretofore given by the signer to vote at said meeting or any adjournments thereof. |
|
|
Dated |
|
|
|
, 2004 |
|
|
| | | | |
| | | | |
|
|
|
|
|
| | | Signature(s) | | |
| | | (Please date this proxy and sign it exactly and as fully as your name appears on your stock certificate. If shares are held jointly, each stockholder must sign. When signing as an attorney, executor, administrator, or guardian, please give full title as such.)
|
^ FOLD AND DETACH HERE ^
PLEASE VOTE, SIGN, DATE AND RETURN
THIS PROXY CARD PROMPTLY
USING THE ENCLOSED ENVELOPE.
NO POSTAGE IS REQUIRED.
QuickLinks
TABLE OF CONTENTSEXHIBIT LISTINTRODUCTIONPRO FORMA INFORMATIONFORWARD LOOKING STATEMENTSPROPOSAL 1 Ratify Issuance of More Than 20% of Our Outstanding Stock in a Financing Transaction and Conversion of NotesPROPOSAL 2 Amend the 2000 Long Term Incentive Compensation PlanPROPOSAL 3 Amend Articles of Incorporation to Increase Authorized Shares of Common StockPROPOSAL 4 Amend Articles of Incorporation to Effect a Reverse Stock SplitPROPOSAL 5 Election of Seven Directors to the Company's Board of DirectorsFinancial and Other InformationOther MattersInformation Incorporated By ReferenceSECURITIES PURCHASE AGREEMENTSchedule IWARRANT AGREEMENT[Form of Warrant Certificate]REGISTRATION RIGHTS AGREEMENTPlan of DistributionSelling Securityholder Notice and QuestionnaireVOTING AGREEMENTAMENDMENT NO. 1 TO VOTING AGREEMENTIMCOR Pharmaceutical Co. 2000 LONG TERM INCENTIVE COMPENSATION PLANIMCOR Pharmaceutical Co. (A Development Stage Company) Consolidated Balance SheetsIMCOR Pharmaceutical Co. Consolidated Statements of OperationsIMCOR Pharmaceutical Co. Consolidated Statement of Shareholders' Equity/(Deficit)IMCOR Pharmaceutical Co. Consolidated Statements of Cash FlowsIMCOR Pharmaceutical Co. Notes to Consolidated Financial StatementsSIGNATURESEXHIBIT INDEXPART IPhotogen Technologies, Inc. Consolidated Balance SheetsPhotogen Technologies, Inc. Consolidated Statements of OperationsPhotogen Technologies, Inc. Consolidated Statement of Shareholders' EquityPhotogen Technologies, Inc. Consolidated Statements of Cash FlowsPhotogen Technologies, Inc. Notes to Consolidated Financial StatementsSentigen Ltd. Balance SheetsSentigen Ltd. Statements of OperationsSentigen Ltd. Statements of Shareholders' EquitySentigen Ltd. Statements of Cash FlowsSentigen Ltd. Notes to Financial StatementsSIGNATURESCERTIFICATIONEXHIBIT INDEXPhotogen Technologies, Inc. Consolidated Statement of Cash FlowsSIGNATURESCERTIFICATIONEXHIBIT INDEXCHARTER OF IMCOR PHARMACEUTICAL CO. AUDIT COMMITTEEINDEXPART I. FINANCIAL INFORMATIONIMCOR Pharmaceutical Co. (Formerly Photogen Technologies, Inc.) (A Development Stage Company) Consolidated Condensed Balance Sheets All amounts in $IMCOR Pharmaceutical Co. (Formerly Photogen Technologies, Inc.) (A Development Stage Company) Consolidated Condensed Statements of Operations (Unaudited) All amounts in $IMCOR Pharmaceutical Co. (Formerly Photogen Technologies, Inc.) (A Development Stage Company) Consolidated Condensed Statements of Shareholders' Equity/(Deficit) (Unaudited)IMCOR Pharmaceutical Co. (Formerly Photogen Technologies, Inc.) (A Development Stage Company) Consolidated Condensed Statements of Cash Flows (Unaudited) All amounts in $PART II. OTHER INFORMATIONSignaturesEXHIBIT INDEXCERTIFICATIONCERTIFICATIONCERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002