Exhibit 99.1

A proprietary in vivo testing and information platform for personalizing cancer care January 2013

Notice of Forward Looking Statements This presentation contains certain “forward - looking statements,” which include information relating to future events, future financial performance, strategies, expectations, competitive environment, regulation, and availability of resources . These forward - looking statements include, without limitation, statements regarding projections , predictions, expectations, estimates, or forecasts as to our business, financial and operational results, and future economic performance ; and statements of management’s goals and objectives and other similar expressions concerning matters that are not historical facts . Forward - looking statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved . Forward - looking statements are based on information available at the time those statements are made or management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward - looking statements . Forward - looking statements speak only as of the date the statements are made . Factors that could cause actual results to differ from those discussed in the forward - looking statements include, but are not limited to, those described the “Risk Factors” section of our Annual Report on Form 10 - K for the fiscal year ended April 30 , 2012 , as updated in our subsequent reports filed with the SEC, including reports on Form 10 - Q . You should not put undue reliance on any forward - looking statements . We assume no obligation to update forward - looking statements to reflect actual results, changes in assumptions, or changes in other factors affecting forward - looking information, except to the extent required by applicable securities laws . If we do update one or more forward - looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward - looking statements . �

Diversified business model C ombines advantages of technology and service business Company Overview Experienced leadership team Diverse experience with shareholder focus Proprietary approach to personalized oncology Predictive technology serving patients and drug development companies �

Build living bank of tumors with patient specific clinical, genomic and response data. Leverage technology, tumors and information to generate technology revenue and IP. Build patient diagnostic business utilizing TumorGraft technology 2 X + 7 Z + 3 A + 16 D + 2 E + 4 F + 1 G + 0 Y + 3 Z + 56 A + 2 B + 11 C + 7 I + 12 J + 13 X + 0 Y + 3 Z + 56 A + 2 B + 7 I + 12 J + 12 X + 4 Y + 2 Z + 8 A + 0 B + 3 C + 4 D+ 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 24 J + 4 X + 1 Y + 12 Z + 1 A+ 12 I + 4 J + 15 X + 8 Y + 15 Z + 3 A + 11 B + 5 C + 11 D + 9 E + 4 F + 0 G + 9 H + 2 F + 1 G + 1 H + 1 I + 2 J + 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 1 D + 2 E + 4 F + 2 G+ 5 F + 1 G + 11 H + 7 I + 5 J + 2 X + 14 Y + 7 Z + 3 A + 5 B + 0 C + 16 D + 2 E + 4 F + 1 G + 9 H + 0 I + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J + 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C 1 D + 6 E + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J + 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J + 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 24 J + 4 X + 1 Y + 12 Z + 1 A + 18 B + 8 C + 7 D + 8 E + 2 F + 2 G + 3 H + 12 I + 4 J + 15 X + 8 Y + 15 Z + 3 A + 11 B + 5 C + 11 D + 9 E + 4 F + 0 G + 9 H + 9 I + 1 J + 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J + 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 1 D + 2 E + 4 F + 2 G + 9 H + 46 I + 22 J + 3 X + 4 Y + 17 Z + 5 A + 11 B + 3 C + 4 D + 6 E + 5 F + 1 G + 11 H + 7 I + 5 J + 2 X + 14 Y + 7 Z + 3 A + 5 B + 0 C + 16 D + 2 E + 4 F + 1 G + 9 H + 0 I + 23 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J + 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 12 X + 4 Y + 2 Z + 8 A + 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J + 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D Champions Strategy � Drug Development Solutions Biomarker Discovery Personalized TumorGrafts Living TumorBank Utilizing Personalized TumorGrafts , we are developing a Living TumorBank that drives multiple revenue streams.

Fundamental Problem of Oncology: Most Drugs Don’t Work For Most Patients Source: Spear et al TRENDS in molecular Medicine Vol. 7 No. 5 May 2001 ; PMC Nov 2006 , Life Technologies 0 10 20 30 40 50 60 Response Rates (%) Analgesics Statins Depression (SSRI) Schizophrenia Cardiac Arrythmias Asthma Diabetes Migraine (acute) Rheumatoid Arthritis Migraine (Prophylaxis) Oseteoporosis HCV Incontinence Ace Inhibitors Alzheimer’s Beta Blockers Oncology Select therapeutics Most cancer patients receive limited or no clinical benefit from most of the drugs they take despite the high cost and toxic exposure they endure. �

� The Complexity of Cancer The genetic, germline and epigenetic heterogeneity of cancer, within each patient and across the a population of patients, has limited the efficacy of modeling cancer in vivo . 1 New Engl J Med 2012 ; 366 : 883 - 892 March 8 , 2012 2 LabMed June 2010 vol. 41 no. 6 364 - 372 Intra - tumor Heterogeneity 1 Cross Tumor Heterogeneity 2

Champions TumorGrafts are Superior to Cell Line Xenografts � Champions TumorGrafts maintain the heterogeneity and preserve the microenvironment that supports the tumor growth. Limited number of passes used to preserve human characteristics of tumors .
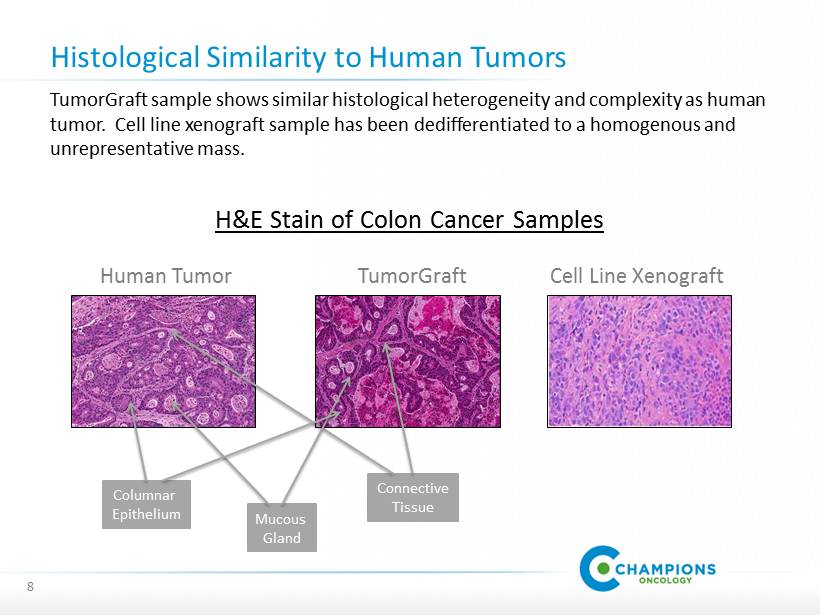
Histological Similarity to Human Tumors 8 TumorGraft sample shows similar histological heterogeneity and complexity as human tumor. Cell line xenograft sample has been dedifferentiated to a homogenous and unrepresentative mass. H&E Stain of Colon Cancer Samples Human Tumor TumorGraft Cell Line Xenograft Connective Tissue Columnar Epithelium Mucous Gland

� Published Results Demonstrate Predictive Accuracy 1 A Pilot Clinical Study of Treatment Guided by Personalized Tumorgrafts in Patients with Advanced Cancer Hidalgo, et al . Molecular Cancer Therapeutics 2011 ; 10 : 1311 - 1316 “The objective response rate was 88 % for treatments deemed effective by the model and tested in the patients. Overall , 11 of 14 patients achieved a PR. The expected response rate with phase I agents, the only available option for some of these subjects, is less than 10 %.” 1

10 Comparable Predictive Results have been Observed in our Patient Studies 63 Patient Drug Studies 1 7 Predictions of Resistance 6 56 Predictions of Sensitivity 52 4 10 False Negatives True Negatives True Positives False Positives Champions TumorGraft platform has accurately predicted sensitivity and resistance in completed studies with corresponding patient response data . 1 1 Source: company analysis.

Personalized TumorGraft : Product Overview Process Tumor fragments implanted directly into group of 5 - 10 mice 2 Implant Groups of mice are dosed with compounds chosen for testing 4 Testing Tumor growth inhibition results delivered to oncologist 5 Results Colony of mice is expanded based on patient’s needs 3 Expansion Tumor is removed from patient surgically or with a biopsy 1 Resection ��

Villarroel et al. Mol Cancer Ther , 2011 : 10 : 3 - 8 . Personalized TumorGraft - Pancreatic Case Study TumorGraft sensitive to Mitomycin C and Cisplatin . TumorGraft ™ Insights : Mitomycin C response maintained for 22 months. Patient remains asymptomatic as of last follow - up ( 5 years post - resection). Mutation identified in the PALB 2 gene explains the observed sensitivity to DNA - damaging agents TumorGraft ™ Results: TumorGraft response to drug testing A 61 year old male with pancreatic cancer, poorly differentiated adenocarcinoma with metastases to lymph nodes 2 months after surgery, the patient was found to have lymph node metastasis, and CA 19 - 9 rose to 10 , 132 U/mL Case: ��

�� Personalized TumorGraft Commercialization: Current Status Product Detail: • Available for most solid tumors • Used for second line therapy or later Sales and Marketing Efforts • Active sales and marketing efforts in US and UK • Expanding into SE Asia this quarter • Increasing sampling effort to introduce new physicians and drive implant volumes Patient Volumes: • Approximately 200 patients implanted • Current run rate: 45 new implants per quarter • Approximately 600 studies completed • 70 patients; average of 9 studies per patient 1 Hidalgo et al . Mol Cancer Ther 2011 ; 10 : 1311 - 1316

�� Three Pronged Approach to Clinical Validation Champions is leveraging the existing patient base and academic interest to enhance clinical validation in a cost effective manner. Publications • Growing base of study results from individual patients • Aggregating by tumor type for publication Pilot Studies • Externally funded, investigator initiated studies • 10 – 30 patients per study • Sarcoma, Ovarian and Unknown Primary Multi - Center Clinical Trial • Platinum resistant ovarian patients • 300 patient implants for 50 enrolled patients • Powered to show 40 % ORR vs 15 % ORR in literature contol • Study design and lead institution identified • IRB, CRF and legal in process • Total cost to Champions: $ 1.5 million • Time Frame Expectations: • First implant: Q 2 2013 • Final implant: Q 3 2014 • Initial results: Q 4 2014 • Final results: Q 1 2016

2 X+ 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 14 Y + 7 Z + 3 A + 5 B + 0 C + 16 D + 2 E + 4 F + 1 G + 9 H + 0 I + 23 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 12 X + 4 Y + 2 Z + 8 A + 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 24 J 4 X + 1 Y + 12 Z + 1 A + 18 B + 8 C + 7 D + 8 E + 2 F + 2 G + 3 H + 12 I + 4 J 15 X + 8 Y + 15 Z + 3 A + 11 B + 5 C + 11 D + 9 E + 4 F + 0 G + 9 H + 9 I + 1 J 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J + 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 1 D + 2 E + 4 F + 2 G + 9 H + 46 I + 22 J 3 X + 4 Y + 17 Z + 5 A + 11 B + 3 C + 4 D + 6 E + 5 F + 1 G + 11 H + 7 I + 5 J 2 X + 14 Y + 7 Z + 3 A + 5 B + 0 C + 16 D + 2 E + 4 F + 1 G + 9 H + 0 I + 23 J + 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 12 X + 4 Y + 2 Z + 8 A + 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 24 J 4 X + 1 Y + 12 Z + 1 A + 18 B + 8 C + 7 D + 8 E + 2 F + 2 G + 3 H + 12 I + 4 J 15 X + 8 Y + 15 Z + 3 A + 11 B + 5 C + 11 D + 9 E + 4 F + 0 G + 9 H + 9 I + 1 J 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 1 D + 2 E + 4 F + 2 G + 9 H + 46 I + 22 J 3 X + 4 Y + 17 Z + 5 A + 11 B + 3 C + 4 D + 6 E + 5 F + 1 G + 11 H + 7 I + 5 J 2 X + 14 Y + 7 Z + 3 A + 5 B + 0 C + 16 D + 2 E + 4 F + 1 G + 9 H + 0 I + 23 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 12 X + 4 Y + 2 Z + 8 A + 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 24 J 4 X + 1 Y + 12 Z + 1 A + 18 B + 8 C + 7 D + 8 E + 2 F + 2 G + 3 H + 12 I + 4 J 15 X + 8 Y + 15 Z + 3 A + 11 B + 5 C + 11 D + 9 E + 4 F + 0 G + 9 H + 9 I + 1 J 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 1 D + 2 E + 4 F + 2 G + 9 H + 46 I + 22 J 3 X + 4 Y + 17 Z + 5 A + 11 B + 3 C + 4 D + 6 E + 5 F + 1 G + 11 H + 7 I + 5 J 2 X + 14 Y + 7 Z + 3 A + 5 B + 0 C + 16 D + 2 E + 4 F + 1 G + 9 H + 0 I + 23 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J + 12 X + 4 Y + 2 Z + 8 A + 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 24 J 4 X + 1 Y + 12 Z + 1 A + 18 B + 8 C + 7 D + 8 E + 2 F + 2 G + 3 H + 12 I + 4 J 15 X + 8 Y + 15 Z + 3 A + 11 B + 5 C + 11 D + 9 E + 4 F + 0 G + 9 H + 9 I + 1 J 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 1 D + 2 E + 4 F + 2 G + 9 H + 46 I + 22 J 3 X + 4 Y + 17 Z + 5 A + 11 B + 3 C + 4 D + 6 E + 5 F + 1 G + 11 H + 7 I + 5 J + 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J 8 X + 7 Y + 3 Z + 3 A + 9 B + 11 C + 1 D + 2 E + 4 F + 2 G + 9 H + 46 I + 22 J 3 X + 4 Y + 17 Z + 5 A + 11 B + 3 C + 4 D + 6 E + 5 F + 1 G + 11 H + 7 I + 5 J 2 X + 14 Y + 7 Z + 3 A + 5 B + 0 C + 16 D + 2 E + 4 F + 1 G + 9 H + 0 I + 23 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J 13 X + 0 Y + 3 Z + 56 A + 2 B + 11 C + 1 D + 6 E + 8 F + 0 G + 0 H + 7 I + 12 J + 12 X + 4 Y + 2 Z + 8 A + 0 B + 3 C + 4 D + 1 E + 2 F + 1 G + 11 H + 41 I + 3 J 0 X + 2 Y + 4 Z + 1 A + 25 B + 5 C + 21 D + 6 E + 2 F + 2 G + 3 H + 7 I + 11 J 13 X + 1 Y + 8 Z + 2 A + 7 B + 2 C + 3 D + 5 E + 1 F + 1 G + 6 H + 26 I + 24 J 4 X + 1 Y + 12 Z + 1 A + 18 B + 8 C + 7 D + 8 E + 2 F + 2 G + 3 H + 12 I + 4 J + 15 X + 8 Y + 15 Z + 3 A + 11 B + 5 C + 11 D + 9 E + 4 F + 0 G + 9 H + 9 I + 1 J 17 X + 14 Y + 22 Z + 2 A + 0 B + 2 C + 16 D + 0 E + 2 F + 1 G + 1 H + 1 I + 2 J 7 Y + 3 Z + 3 A + 9 B Living TumorBank A Unique Resource for Personalizing Oncology �� Clinical Annotation • Age , sex, race • Site and stage of tumor • Treatment information Response to Drug Treatments • Standard of Care • Off label uses • Combinations Genetic Information • Mutation profile • Gene expression profile The tumors and information that we collect from our patient business is combined and supplemented to create a Living TumorBank that is uniquely positioned to personalize the development and use of oncology drugs.

Our Models: Cheaper Humans or Better Mice Moderate Response Champions TumorGraft Testing TumorGraft technology is used to match individual patients with specific drugs to increase the response rates to oncology drugs. �� Respond Only in Combination Robust Response Not Likely to Respond

�� An Emerging Paradigm of Oncology Drug Development TumorGraft technology combined with a Living TumorBank enables drug developers to answer the key questions of drug development without the expense and constraints of human clinical trials. Study Type Questions Answered Efficacy: Does this compound inhibit tumor growth ? In which tumor types? Mechanism of Action How does this compound work to shrink tumors? Relative Response: Is this drug more effective than current standards of care? Combination Study: How effective is this compound in combination with other therapies? Patient Enrichment Are there sub - populations with certain molecular characteristics that respond better to this compound?

Translational Oncology Solutions: Case Study 18 Situation Overview Oncology compound in phase 1 clinical trial. Broad mechanism of action. Interest in multiple tumor types. Study Objective Determine response rates across multiple tumor types to pick one for phase II trial. Study Design Measure tumor growth inhibition in 6 colon, 6 lung and 6 pancreatic models. Test drug as stand alone agent AND in combination with standard of care agents. Study Cost $ 500 , 000
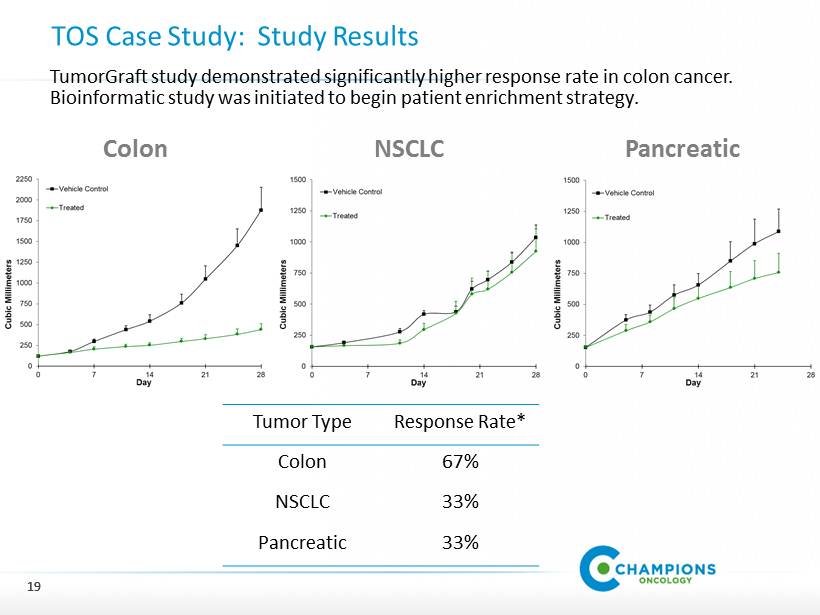
19 19 TOS Case Study: Study Results Colon NSCLC Pancreatic Tumor Type Response Rate* Colon 67 % NSCLC 33 % Pancreatic 33 % TumorGraft study demonstrated significantly higher response rate in colon cancer. Bioinformatic study was initiated to begin patient enrichment strategy.

Utilization of Tumorgraft Technology is Accelerating Rapidly 20 1 Publications using the search term patient derived xenografts 0 200 400 600 800 1000 1200 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 Pub licati ons per Year 1 Recognition of the value of TumorGrafts is growing. Scale and diversity of available models has been a bottleneck for the industy .
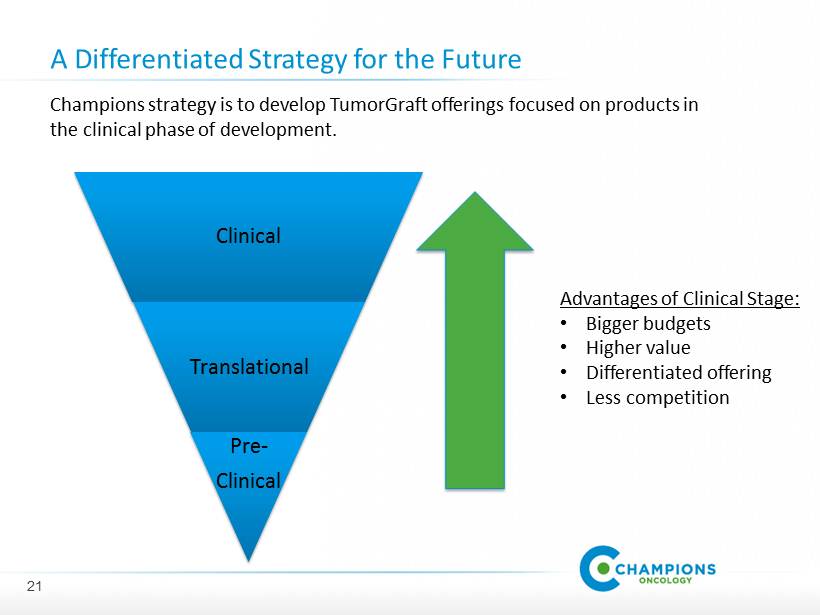
�� A Differentiated Strategy for the Future Clinical Translational Pre - Clinical Champions strategy is to develop TumorGraft offerings focused on products in the clinical phase of development. Advantages of Clinical Stage: • Bigger budgets • Higher value • Differentiated offering • Less competition
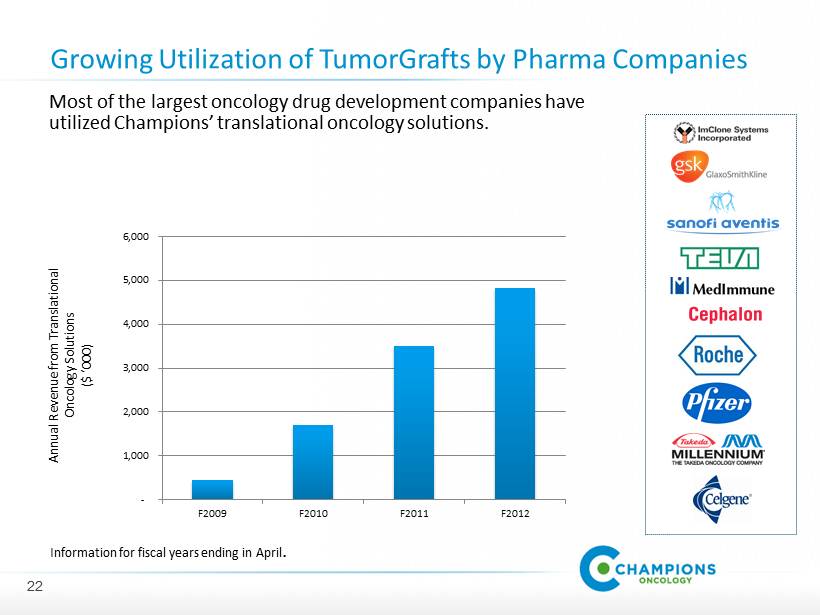
Growing Utilization of TumorGrafts by Pharma Companies Most of the largest oncology drug development companies have utilized Champions’ translational oncology solutions. Annual Revenue from Translational Oncology Solutions ($ ‘ 000 ) �� - 1,000 2,000 3,000 4,000 5,000 6,000 F2009 F2010 F2011 F2012 Information for fiscal years ending in April .

�� An Emerging Consensus on Biomarkers in Oncology “Biomarkers are central to accelerating the identification and adoption of new therapies” AACR - FDA - NCI Cancer Biomarkers Collaborative Consensus Report

�� Champions Offers a Unique Platform for Biomarker Development Challenges of Biomarker Discovery • Small patient populations available for study • Studies limited to drugs or combinations used by patients • Limited availability of tumor tissue for analysis • Must repeat molecular analysis every study Result: Under powered, poorly characterized studies leading to high false negative rate. Champions’ Platform for Biomarker Discovery • Population size limited only by size of bank • Can test multiple drugs alone and in combination • Unlimited amount of tissue for molecular analysis • Invest in characterization once Result: Well powered studies for discovery and clinical validation of biomarkers of response. Biomarker discovery is predicated on correlating genotypic and response data in large samples of tumors. Champions’ platform solves some of the most challenging problems that have prevented the wide scale development of predictive biomarkers in oncology.

�� A New Tool; Not a New Methodology Utilization of in vivo models for biomarker discovery has been used in the past with limited success. The accuracy and scale of our platform will enhance the power of the methodology. Systematic identification of genomic markers of drug sensitivity in cancer cells Garnett et al 483 ( 7391 ): 570 – 575 Identification of genotype - correlated sensitivity to selective kinase inhibitors by using high - throughput tumor cell line profiling McDermott et al . 104 ( 50 ): 19936 – 19941 Genetically Modified Mouse Models for Biomarker Discovery and Preclinical Drug Testing Raju Kucherlapati 18 : 625
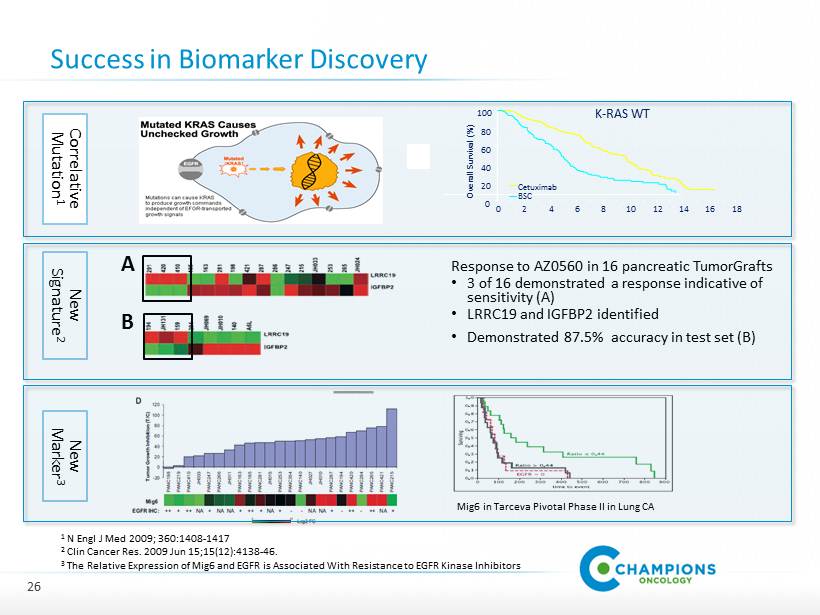
Success in Biomarker Discovery Response to AZ 0560 in 16 pancreatic TumorGrafts • 3 of 16 demonstrated a response indicative of sensitivity (A) • LRRC 19 and IGFBP 2 identified • Demonstrated 87.5 % accuracy in test set (B) 26 A B Mig 6 in Tarceva Pivotal Phase II in Lung CA 0 20 40 60 80 100 0 2 4 6 8 10 12 14 16 18 Overall Survival (%) Cetuximab BSC K - RAS WT Correlative Mutation 1 New Signature 2 New Marker 3 1 N Engl J Med 2009 ; 360 : 1408 - 1417 2 Clin Cancer Res. 2009 Jun 15 ; 15 ( 12 ): 4138 - 46 . 3 The R elative E xpression of Mig 6 and EGFR is Associated With Resistance to EGFR Kinase I nhibitors

�� Strategic Approach to Biomarker Development • Focus on discovery and clinical validation phases Focus • Partnerships that provide added value at all stages of process Partner • Diversification to provide multiple “shots on goal” Diversify Our biomarker development strategy will mitigate the scientific and capital risks inherent in the business while preserving the upside potential.

�� Future Milestones Building out senior management team First biomarker out licensing deal Insurance reimbursement First biomarker partnership agreement First “mouse phase II clinical trial” Initiation of clinical validation studies Increased implantations and studies Peer reviewed publications Continued sales growth in TOS business The company has both short term and long term milestones that will drive value creation. Time

�� A Deep and Experienced Senior Management Team Dr. David Sidransky – Chairman of the Board • Former Vice Chairman, ImClone Systems • Professor of Oncology, Otolaryngology, Cellular and Molecular Medicine, Urology, Genetics, and Pathology at Johns Hopkins Joel Ackerman – Chief Executive Officer • 15 years at Warburg Pincus investing in healthcare • Board of Directors: Coventry Healthcare, Kindred Healthcare Dr. Ronnie Morris - President • Founder and CMO of MDVIP – leading provider of personalized medicine in US Gary Gemignani – Chief Financial Officer • COO & CFO Coronado Biosciences, Inc . • COO & CFO Gentium SpA
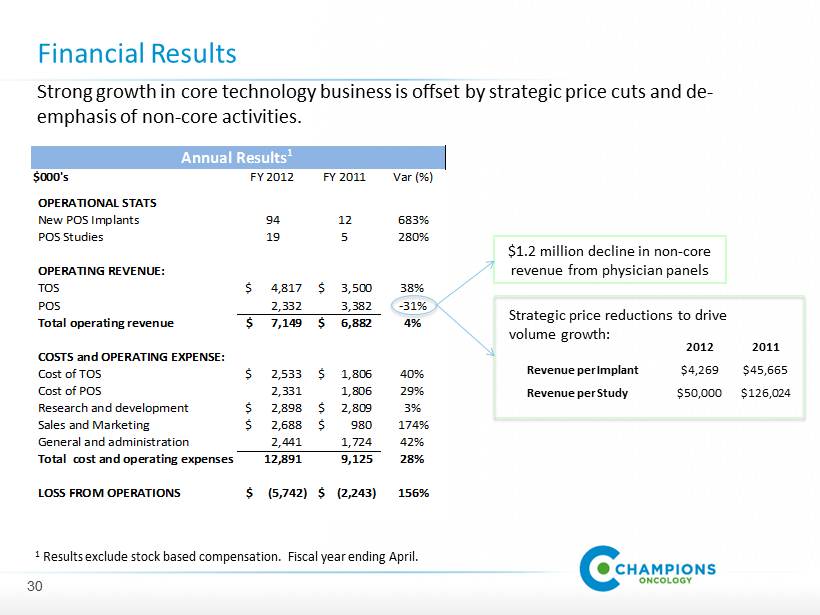
$000's FY 2012 FY 2011 OPERATIONAL STATS New POS Implants 94 12 POS Studies 19 5 OPERATING REVENUE: TOS 4,817$ 3,500$ POS 2,332 3,382 Total operating revenue 7,149$ 6,882$ COSTS and OPERATING EXPENSE: Cost of TOS 2,533$ 1,806$ Cost of POS 2,331 1,806 Research and development 2,898$ 2,809$ Sales and Marketing 2,688$ 980$ General and administration 2,441 1,724 Total cost and operating expenses 12,891 9,125 LOSS FROM OPERATIONS (5,742)$ (2,243)$ Annual Results 1 Var (%) 683% 280% 38% -31% 4% 40% 29% 3% 174% 42% 28% 156% Annual Results 1 Financial Results 1 Results exclude stock based compensation. Fiscal year ending April. �� Strong growth in core technology business is offset by strategic price cuts and de - emphasis of non - core activities. $ 1.2 million decline in non - core revenue from physician panels Strategic price reductions to drive volume growth: 2012 2011 Revenue per Implant $ 4 , 269 $ 45 , 665 Revenue per Study $ 50 , 000 $ 126 , 024

�� Financial Information 1 Ticker Symbol (OTC): CSBR Cash balance: $ 2.5 million Shares outstanding: 47 million shares S tock price: $ 0.30 52 week range: $ 0.21 - $ 0.75 Market Capitalization: $ 14.1 million 1 Financial information as of January 4 , 2013 .

�� Summary of Key Investment Highlights Differentiated technology with multiple applications serving large markets with unmet needs Business model that combines benefits of technology and service businesses Revenue stream demonstrates commercial viability of products Near term milestones to measure progress































