We incurred the following costs in connection with our cost-reduction initiatives:
| | | | | | | | | | | |
|
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
|
|
|
|
Implementation costs(a) | | $ | 1,389 | | | $ | 788 | | $ | 325 | |
Restructuring charges(b) | | | 2,523 | | | | 1,296 | | | 438 | |
|
|
|
|
|
|
|
|
|
|
|
|
Total costs related to our cost-reduction initiatives | | $ | 3,912 | | | $ | 2,084 | | $ | 763 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
(a) | For 2007, included inCost of sales($700 million),Selling, informational and administrative expenses($334 million),Research and development expenses($416 million) and inOther (income)/deductions—net ($61 million income). For 2006, included inCost of sales($392 million),Selling, informational and administrative expenses($243 million),Research and development expenses($176 million) and inOther (income)/deductions—net($23 million income). For 2005, included inCost of sales($124 million),Selling, informational and administrative expenses($151 million), andResearch and development expenses($50 million). |
| |
(b) | Included inRestructuring charges and acquisition-related costs. |
Through December 31, 2007, the restructuring charges primarily relate to our plant network optimization efforts and the restructuring of our worldwide marketing and research and development operations, and the implementation costs primarily relate to accelerated depreciation of certain assets, as well as system and process standardization and the expansion of shared services.
The components of restructuring charges associated with our cost-reduction initiatives follow:
| | | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | COSTS INCURRED | | ACTIVITY
THROUGH
DEC. 31,
2007(a) | | ACCRUAL
AS OF
DEC. 31,
2007 | |
| |
|
|
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | | TOTAL | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee termination costs | | $ | 2,034 | | | $ | 809 | | $ | 303 | | $ | 3,146 | | | $1,957 | | | $1,189 | |
Asset | | | | | | | | | | | | | | | | | | | | |
impairments | | | 260 | | | | 368 | | | 122 | | | 750 | | | 750 | | | — | |
Other | | | 229 | | | | 119 | | | 13 | | | 361 | | | 261 | | | 100 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | $ | 2,523 | | | $ | 1,296 | | $ | 438 | | $ | 4,257 | | | $2,968 | | | $1,289 | (b) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | Includes adjustments for foreign currency translation. |
| | |
| (b) | Included inOther current liabilities($1.1 billion) andOther noncurrent liabilities($186 million). |
From the beginning of the cost-reduction initiatives in 2005 through December 31, 2007,Employee termination costsrepresent the expected reduction of the workforce by 20,800 employees, mainly in research, manufacturing and sales. As of December 31, 2007, approximately 13,000 of these employees have been formally terminated.Employee termination costsare recorded when the actions are probable and estimable and include accrued severance benefits, pension and postretirement benefits.Asset impairmentsprimarily include charges to write down property, plant and equipment.Otherprimarily includes costs to exit certain activities.
Acquisition-Related Costs
We recorded inRestructuring charges and acquisition-related costs$11 million in 2007, $27 million in 2006 and $918 million in 2005, for acquisition-related costs. Amounts in 2005 were primarily related to our acquisition of Pharmacia on April 16, 2003 and
included integration costs of $543 million and restructuring charges of $375 million. As of December 31, 2007, virtually all restructuring charges incurred have been utilized.
Integration costs represent external, incremental costs directly related to an acquisition, including expenditures for consulting and systems integration. Restructuring charges can include severance, costs of vacating duplicative facilities, contract termination and other exit costs.
Other (Income)/Deductions—Net
In 2007, we recorded higher net interest income compared to 2006, due primarily to higher net financial assets during 2007 compared to 2006, reflecting proceeds of $16.6 billion from the sale of our Consumer Healthcare business in late December 2006, and higher interest rates. Also in 2007, we recorded a gain of $211 million related to the sale of a building in Korea. In 2006, we recorded a charge of $320 million related to the impairment of our Depo-Provera intangible asset. In 2005, we recorded charges of $1.2 billion primarily related to the impairment of our Bextra intangible asset. See also Notes to Consolidated Financial Statements—Note 7. Other (Income)/Deductions—Net.
Provision for Taxes on Income
Our overall effective tax rate for continuing operations was 11.0% in 2007, 15.3% in 2006 and 29.4% in 2005. The lower tax rate in 2007 is primarily due to the impact of charges associated with our decision to exit Exubera (see the “Our 2007 Performance: Decision to Exit Exubera” section of this Financial Review), higher charges related to our cost-reduction initiatives in 2007, lower non-deductible charges for acquisition-related IPR&D, and the volume and geographic mix of product sales and restructuring charges in 2007 compared to 2006, partially offset by certain one-time tax benefits in 2006, all discussed below.
The lower tax rate in 2006 compared to 2005 is primarily due to certain one-time tax benefits associated with favorable tax legislation and the resolution of certain tax positions, and a decrease in the 2005 estimated U.S. tax provision related to the repatriation of foreign earnings, all as discussed below, and the impact of the sale of our Consumer Healthcare business.
In the third quarter of 2006, we recorded a decrease to the 2005 estimated U.S. tax provision related to the repatriation of foreign earnings, due primarily to the receipt of information that raised our assessment of the likelihood of prevailing on the technical merits of a certain position, and we recognized a tax benefit of $124 million.
In the first quarter of 2006, we were notified by the Internal Revenue Service (IRS) Appeals Division that a resolution had been reached on the matter that we were in the process of appealing related to the tax deductibility of an acquisition-related breakup fee paid by the Warner-Lambert Company in 2000. As a result, in the first quarter of 2006, we recorded a tax benefit of approximately $441 million related to the resolution of this issue.
On January 23, 2006, the IRS issued final regulations on Statutory Mergers and Consolidations, which impacted certain prior-period transactions. In the first quarter of 2006, we recorded a tax benefit of $217 million, reflecting the total impact of these regulations.
|
Financial Review |
Pfizer Inc and Subsidiary Companies |
|
|
In 2005, we recorded an income tax charge of $1.7 billion, included inProvision for taxes on income,in connection with our decision to repatriate approximately $37 billion of foreign earnings in accordance with the American Jobs Creation Act of 2004 (the Jobs Act). The Jobs Act created a temporary incentive for U.S. corporations to repatriate accumulated income earned abroad by providing an 85% dividend-received deduction for certain dividends from controlled foreign corporations in 2005. In addition, during 2005, we recorded a tax benefit of $586 million, primarily related to the resolution of certain tax positions.
Discontinued Operations—Net of Tax
For further discussion about our dispositions, see the “Our Strategic Initiatives—Strategy and Recent Transactions: Dispositions” section of this Financial Review. The following amounts, primarily related to our former Consumer Healthcare business, have been segregated from continuing operations and included inDiscontinued operations—net of taxin the consolidated statements of income:
| | | | | | | | | | | |
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Revenues | | $ | — | | | $ | 4,044 | | $ | 3,948 | |
|
|
|
|
|
|
|
|
|
|
| |
Pre-tax income/loss | | | (5 | ) | | | 643 | | | 695 | |
(Benefit)/provision for taxes on income(a) | | | 2 | | | | (210 | ) | | (244 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Income/loss from operations of discontinued businesses—net of tax | | | (3 | ) | | | 433 | | | 451 | |
|
|
|
|
|
|
|
|
|
|
| |
Pre-tax gains/(losses) on sales of discontinued businesses | | | (168 | ) | | | 10,243 | | | 77 | |
(Benefit)/provision for taxes on
gains(b) | | | 102 | | | | (2,363 | ) | | (30 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Gains/(losses) on sales of discontinued businesses—net of tax | | | (66 | ) | | | 7,880 | | | 47 | |
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations—net of tax | | $ | (69 | ) | | $ | 8,313 | | $ | 498 | |
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Includes a deferred tax expense of nil in 2007, $24 million in 2006 and $25 million in 2005. |
| | |
| (b) | Includes a deferred tax benefit of nil in 2007, $444 million in 2006, and nil in 2005. |
Adjusted Income
General Description of Adjusted Income Measure
Adjusted income is an alternative view of performance used by management and we believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. We report Adjusted income in order to portray the results of our major operations—the discovery, development, manufacture, marketing and sale of prescription medicines for humans and animals—prior to considering certain income statement elements. We have defined Adjusted income as Net income before the impact of purchase accounting for acquisitions, acquisition-related costs, discontinued operations, the cumulative effect of a change in accounting principles and certain significant items. The Adjusted income measure is not, and should not be viewed as, a substitute for U.S. GAAP Net income.
The Adjusted income measure is an important internal measurement for Pfizer. We measure the performance of the overall Company on this basis. The following are examples of how the Adjusted income measure is utilized.
| |
• | Senior management receives a monthly analysis of our operating results that is prepared on an Adjusted income basis; |
| |
• | Our annual budgets are prepared on an Adjusted income basis; and |
| |
• | Annual and long-term compensation, including annual cash bonuses, merit-based salary adjustments and share-based payments for various levels of management, is based on financial measures that include Adjusted income. The Adjusted income measure currently represents a significant portion of target objectives that are utilized to determine the annual compensation for various levels of management, although the actual weighting of the objective may vary by level of management and job responsibility and may be considered in the determination of certain long-term compensation plans. The portion of senior management’s bonus, merit-based salary increase and share-based awards based on the Adjusted income measure ranges from 10% to 30%. |
Despite the importance of this measure to management in goal setting and performance measurement, we stress that Adjusted income is a non-U.S. GAAP financial measure that has no standardized meaning prescribed by U.S. GAAP and, therefore, has limits in its usefulness to investors. Because of its non-standardized definition, Adjusted income (unlike U.S. GAAP Net income) may not be comparable with the calculation of similar measures for other companies. Adjusted income is presented solely to permit investors to more fully understand how management assesses our performance.
We also recognize that, as an internal measure of performance, the Adjusted income measure has limitations and we do not restrict our performance-management process solely to this metric. A limitation of the Adjusted income measure is that it provides a view of our operations without including all events during a period, such as the effects of an acquisition or amortization of purchased intangibles and does not provide a comparable view of our performance to other companies in the pharmaceutical industry. We also use other specifically tailored tools designed to ensure the highest levels of our performance. For example, our R&D organization has productivity targets, upon which its effectiveness is measured. In addition, Performance Share Awards grants made in 2006, 2007 and future years will be paid based on a non-discretionary formula that measures our performance using relative total shareholder return.
Purchase Accounting Adjustments
Adjusted income is calculated prior to considering certain significant purchase-accounting impacts, such as those related to our acquisitions of BioRexis, Embrex, Rinat, sanofi-aventis’ rights to Exubera, PowderMed, Idun and Vicuron, as well as net asset acquisitions. These impacts can include charges for purchased in-process R&D, the incremental charge to cost of sales from the sale of acquired inventory that was written up to fair value and the incremental charges related to the amortization of finite-lived
2007 Financial Report | 25
|
Financial Review |
Pfizer Inc and Subsidiary Companies |
|
|
intangible assets for the increase to fair value. Therefore, the Adjusted income measure includes the revenues earned upon the sale of the acquired products without considering the aforementioned significant charges.
Certain of the purchase-accounting adjustments associated with a business combination, such as the amortization of intangibles acquired in connection with our acquisition of Pharmacia in 2003, can occur for up to 40 years (these assets have a weighted-average useful life of approximately nine years), but this presentation provides an alternative view of our performance that is used by management to internally assess business performance. We believe the elimination of amortization attributable to acquired intangible assets provides management and investors an alternative view of our business results by trying to provide a degree of parity to internally developed intangible assets for which research and development costs have been previously expensed.
However, a completely accurate comparison of internally developed intangible assets and acquired intangible assets cannot be achieved through Adjusted income. This component of Adjusted income is derived solely with the impacts of the items listed in the first paragraph of this section. We have not factored in the impacts of any other differences in experience that might have occurred if we had discovered and developed those intangible assets on our own, and this approach is not intended to be representative of the results that would have occurred in those circumstances. For example, our research and development costs in total, and in the periods presented, may have been different; our speed to commercialization and resulting sales, if any, may have been different; or our costs to manufacture may have been different. In addition, our marketing efforts may have been received differently by our customers. As such, in total, there can be no assurance that our Adjusted income amounts would have been the same as presented had we discovered and developed the acquired intangible assets.
Acquisition-Related Costs
Adjusted income is calculated prior to considering integration and restructuring costs associated with business combinations because these costs are unique to each transaction and represent costs that were incurred to restructure and integrate two businesses as a result of the acquisition decision. For additional clarity, only restructuring and integration activities that are associated with a purchase business combination or a net-asset acquisition are included in acquisition-related costs. We have made no adjustments for the resulting synergies.
We believe that viewing income prior to considering these charges provides investors with a useful additional perspective because the significant costs incurred in a business combination result primarily from the need to eliminate duplicate assets, activities or employees—a natural result of acquiring a fully integrated set of activities. For this reason, we believe that the costs incurred to convert disparate systems, to close duplicative facilities or to eliminate duplicate positions (for example, in the context of a business combination) can be viewed differently from those costs incurred in other, more normal business contexts.
The integration and restructuring costs associated with a business combination may occur over several years, with the more significant impacts ending within three years of the transaction. Because of the need for certain external approvals for some actions, the span of time needed to achieve certain restructuring and integration activities can be lengthy. For example, due to the highly regulated nature of the pharmaceutical business, the closure of excess facilities can take several years, as all manufacturing changes are subject to extensive validation and testing and must be approved by the FDA.
Discontinued Operations
Adjusted income is calculated prior to considering the results of operations included in discontinued operations, such as our Consumer Healthcare business, which we sold in December 2006, as well as any related gains or losses on the sale of such operations. We believe that this presentation is meaningful to investors because, while we review our businesses and product lines periodically for strategic fit with our operations, we do not build or run our businesses with an intent to sell them.
Cumulative Effect of a Change in Accounting Principles
Adjusted income is calculated prior to considering the cumulative effect of a change in accounting principles. The cumulative effect of a change in accounting principles is generally one time in nature and not expected to occur as part of our normal business on a regular basis.
Certain Significant Items
Adjusted income is calculated prior to considering certain significant items. Certain significant items represent substantive, unusual items that are evaluated on an individual basis. Such evaluation considers both the quantitative and the qualitative aspect of their unusual nature. Unusual, in this context, may represent items that are not part of our ongoing business; items that, either as a result of their nature or size, we would not expect to occur as part of our normal business on a regular basis; items that would be non-recurring; or items that relate to products we no longer sell. While not all-inclusive, examples of items that could be included as certain significant items would be a major non-acquisition-related restructuring charge and associated implementation costs for a program which is specific in nature with a defined term, such as those related to our cost-reduction initiatives; charges related to sales or disposals of products or facilities that do not qualify as discontinued operations as defined by U.S. GAAP; amounts associated with transition service agreements in support of discontinued operations after sale; certain intangible asset impairments; adjustments related to the resolution of certain tax positions; the impact of adopting certain significant, event-driven tax legislation, such as adjustments associated with charges attributable to the repatriation of foreign earnings in accordance with the American Jobs Creation Act of 2004; or possible charges related to legal matters, such as certain of those discussed inLegal Proceedingsin our Form 10-K and inPart II: Other Information; Item 1, Legal Proceedingsin our Form 10-Q filings. Normal, ongoing defense costs of the Company or settlements and accruals on legal matters made in the normal course of our business would not be considered certain significant items.
26 | 2007 Financial Report
|
Financial Review |
Pfizer Inc and Subsidiary Companies |
|
|
Reconciliation
A reconciliation betweenNet income, as reported under U.S. GAAP, and Adjusted income follows:
| | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | | % CHANGE | |
| |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | | 07/06 | | | 06/05 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Reported net income | | $ | 8,144 | | | $ | 19,337 | | $ | 8,085 | | | (58 | ) | | | 139 | |
Purchase accounting adjustments—net of tax | | | 2,511 | | | | 3,131 | | | 3,967 | | | (20 | ) | | | (21 | ) |
Acquisition-related costs—net of tax | | | 10 | | | | 14 | | | 599 | | | (30 | ) | | | (98 | ) |
Discontinued operations—net of tax | | | 69 | | | | (8,313 | ) | | (498 | ) | | * | | | | M+ | |
Cumulative effect of a change in accounting principles—net of tax | | | — | | | | — | | | 23 | | | — | | | | * | |
Certain significant items—net of tax | | | 4,379 | | | | 813 | | | 2,293 | | | 438 | | | | (65 | ) |
|
|
|
|
|
|
|
|
|
|
| | | | | | | | |
Adjusted income | | $ | 15,113 | | | $ | 14,982 | | $ | 14,469 | | | 1 | | | | 4 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | |
| * | Calculation not meaningful. |
| | |
| M+ Change greater than 1,000%. |
| | |
| Certain amounts and percentages may reflect rounding adjustments. |
2007 Financial Report | 27
|
Financial Review |
Pfizer Inc and Subsidiary Companies |
|
|
|
Adjusted income as shown above excludes the following items: |
|
|
| | | | | | | | | | | |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Purchase accounting adjustments: | | | | | | | | | | | |
Intangible amortization and other(a) | | $ | 3,101 | | | $ | 3,220 | | $ | 3,289 | |
In-process research and development charges(b) | | | 283 | | | | 835 | | | 1,652 | |
|
|
|
|
|
|
|
|
|
|
| |
Total purchase accounting adjustments, pre-tax | | | 3,384 | | | | 4,055 | | | 4,941 | |
Income taxes | | | (873 | ) | | | (924 | ) | | (974 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Total purchase accounting adjustments—net of tax | | | 2,511 | | | | 3,131 | | | 3,967 | |
|
|
|
|
|
|
|
|
|
|
| |
Acquisition-related costs: | | | | | | | | | | | |
Integration costs(c) | | | 17 | | | | 21 | | | 543 | |
Restructuring charges(c) | | | (6 | ) | | | 6 | | | 375 | |
|
|
|
|
|
|
|
|
|
|
| |
Total acquisition-related costs, pre-tax | | | 11 | | | | 27 | | | 918 | |
Income taxes | | | (1 | ) | | | (13 | ) | | (319 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Total acquisition-related costs—net of tax | | | 10 | | | | 14 | | | 599 | |
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations: | | | | | | | | | | | |
(Income)/loss from discontinued operations(d) | | | 5 | | | | (643 | ) | | (695 | ) |
(Gains)/losses on sales of discontinued operations(d) | | | 168 | | | | (10,243 | ) | | (77 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Total discontinued operations, pre-tax | | | 173 | | | | (10,886 | ) | | (772 | ) |
Income taxes | | | (104 | ) | | | 2,573 | | | 274 | |
|
|
|
|
|
|
|
|
|
|
| |
Total discontinued operations—net of tax | | | 69 | | | | (8,313 | ) | | (498 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Cumulative effect of a change in accounting principles—net of tax | | | — | | | | — | | | 23 | |
|
|
|
|
|
|
|
|
|
|
| |
Certain significant items: | | | | | | | | | | | |
Restructuring charges—cost-reduction initiatives(c) | | | 2,523 | | | | 1,296 | | | 438 | |
Implementation costs—cost-reduction initiatives(e) | | | 1,389 | | | | 788 | | | 325 | |
Asset impairment charges and other associated costs(f) | | | 2,798 | | | | 320 | | | 1,240 | |
Consumer Healthcare business transition activity(g) | | | (26 | ) | | | — | | | — | |
sanofi-aventis research and development milestone(h) | | | — | | | | (118 | ) | | — | |
Other(i) | | | (174 | ) | | | (173 | ) | | (134 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Total certain significant items, pre-tax | | | 6,510 | | | | 2,113 | | | 1,869 | |
Income taxes | | | (2,131 | ) | | | (735 | ) | | (654 | ) |
Resolution of certain tax positions(j) | | | — | | | | (441 | ) | | (586 | ) |
Tax impact of the repatriation of foreign earnings(j) | | | — | | | | (124 | ) | | 1,664 | |
|
|
|
|
|
|
|
|
|
|
| |
Total certain significant items—net of tax | | | 4,379 | | | | 813 | | | 2,293 | |
|
|
|
|
|
|
|
|
|
|
| |
Total purchase accounting adjustments, acquisition-related costs, discontinued operations, cumulative effect of a change in accounting principles and certain significant items—net of tax | | $ | 6,969 | | | $ | (4,355 | ) | $ | 6,384 | |
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Included primarily inAmortization of intangible assets.(See Notes to Consolidated Financial Statements—Note 13. Goodwill and Other Intangible Assets.) |
| | |
| (b) | Included inAcquisition-related in-process research and development charges.(See Notes to Consolidated Financial Statements—Note 2. Acquisitions.) |
| | |
| (c) | Included inRestructuring charges and acquisition-related costs. (See Notes to Consolidated Financial Statements—Note 5. Cost-Reduction InitiativesandNote 6. Acquisition-Related Costs.) |
| | |
| (d) | Discontinued operations—net of taxis primarily related to our Consumer Healthcare business. (See Notes to Consolidated Financial Statements—Note 3. Discontinued Operations.) |
| | |
| (e) | Included inCost of sales($700 million),Selling, informational and administrative expenses($334 million),Research and development expenses($416 million) and inOther (income)/deductions—net($61 million income) for 2007. Included inCost of sales($392 million),Selling, informational and administrative expenses($243 million),Research and development expenses($176 million) and inOther (income)/deductions—net ($23 million income) for 2006. Included inCost of sales($124 million),Selling, informational and administrative expenses($151 million),Research and development expenses($50 million) for 2005. (See Notes to Consolidated Financial Statements—Note 5. Cost-Reduction Initiatives.) |
| | |
| (f) | In 2007, these charges primarily related to the decision to exit Exubera and comprise approximately $1.1 billion of intangible asset impairments, $661 million of inventory write-offs, $454 million of fixed asset impairments and $578 million of other exit costs and are included inCost of sales($2.6 billion),Selling, informational and administrative expenses($85 million),Research and development expenses($100 million) andRevenues($10 million for an estimate of customer returns) for 2007. See the “Our 2007 Performance: Decision to Exit Exubera” section of this Financial Review. In 2006, $320 million related to the impairment of the Depo-Provera intangible asset is included inOther (income)/deductions—net. In 2005, included primarily inOther (income)/deductions—netand includes $1.2 billion related to the impairment of the Bextra intangible asset. (See Notes to Consolidated Financial Statements—Note 13B. Goodwill and Other Intangible Assets: Other Intangible Assets.) |
| | |
| (g) | Included inRevenues($219 million),Cost of sales($194 million),Selling, informational and administrative expenses($15 million) andOther (income)/deductions—net($16 million income) for 2007. |
| | |
| (h) | Included inResearch and development expenses. |
| | |
| (i) | Primarily included inOther (income)/deductions—net. (See Notes to Consolidated Financial Statements—Note 7. Other (Income)/Deductions—Net.) |
| | |
| (j) | Included inProvision for taxes on income. (See Notes to Consolidated Financial Statements—Note 8. Taxes on Income.) |
28 | 2007 Financial Report
|
Financial Review |
Pfizer Inc and Subsidiary Companies |
|
|
Financial Condition, Liquidity and
Capital Resources
Net Financial Assets
Our net financial asset position as of December 31 follows:
| | | | | | | | |
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | |
|
|
|
|
|
| |
Financial assets: | | | | | | | | |
Cash and cash equivalents | | $ | 3,406 | | | $ | 1,827 | |
Short-term investments | | | 22,069 | | | | 25,886 | |
Short-term loans | | | 617 | | | | 514 | |
Long-term investments and loans | | | 4,856 | | | | 3,892 | |
|
|
|
|
|
|
|
| |
Total financial assets | | | 30,948 | | | | 32,119 | |
|
|
|
|
|
|
|
| |
Debt: | | | | | | | | |
Short-term borrowings, including current portion of long-term debt | | | 5,825 | | | | 2,434 | |
Long-term debt | | | 7,314 | | | | 5,546 | |
|
|
|
|
|
|
|
| |
Total debt | | | 13,139 | | | | 7,980 | |
|
|
|
|
|
|
|
| |
Net financial assets | | $ | 17,809 | | | $ | 24,139 | |
|
|
|
|
|
|
|
| |
Short-term investments as of December 31, 2006, reflect the receipt of proceeds of $16.6 billion from the sale of our Consumer Healthcare business on December 20, 2006.
We rely largely on operating cash flow, short-term investments, long-term debt and short-term commercial paper borrowings to provide for the working capital needs of our operations, including our R&D activities. We believe that we have the ability to obtain both short-term and long-term debt to meet our financing needs for the foreseeable future.
Investments
Our short-term and long-term investments consist primarily of high-quality, investment-grade available-for-sale debt securities. Our long-term investments include debt securities that totaled $2.6 billion as of December 31, 2007, which have maturities ranging substantially from one to five years. Wherever possible, cash management is centralized and intercompany financing is used to provide working capital to our operations. Where local restrictions prevent intercompany financing, working capital needs are met through operating cash flows and/or external borrowings. Our portfolio of short-term investments as of December 31, 2006, reflects the receipt of proceeds from the sale of our Consumer Healthcare business of $16.6 billion. Our portfolio of short-term investments was reduced in 2007 and the proceeds were used to fund items such as the taxes due on the gain from the sale of our Consumer Healthcare business, completed in December 2006, share repurchases, dividends and capital expenditures in 2007.
Long-Term Debt Issuance
On December 10, 2007, we issued the following notes to be used for general corporate purposes, including the payment of maturing debt:
| |
• | $1.3 billion equivalent, senior, unsecured, euro-denominated notes, due December 15, 2014, which pay interest annually, beginning December 15, 2008, at a fixed rate of 4.75%. |
| |
On May 11, 2007, we issued the following notes to be used for general corporate purposes: |
| |
• | $1.2 billion equivalent, senior, unsecured, euro-denominated notes, due May 15, 2017, which pay interest annually, beginning May 15, 2008, at a fixed rate of 4.55%. |
The notes were issued under a securities registration statement filed with the Securities and Exchange Commission (SEC) in March 2007.
Credit Ratings
Two major corporate debt-rating organizations, Moody’s Investors Service (Moody’s) and Standard & Poor’s (S&P), assign ratings to our short-term and long-term debt. The following chart reflects the current ratings assigned to our senior, unsecured non-credit enhanced long-term debt and commercial paper issued directly by us by each of these agencies:
| | | | | | | | |
|
|
|
|
|
|
|
|
|
NAME OF
RATING AGENCY | | COMMERCIAL
PAPER | | LONG-TERM DEBT | | DATE OF LAST
ACTION |
| |
|
|
| |
| | RATING | | OUTLOOK | |
|
|
|
|
|
|
|
|
|
Moody’s | | P-1 | | Aa1 | | Negative | | October 2007 |
S&P | | A1+ | | AAA | | Negative | | December 2006 |
|
|
|
|
|
|
|
|
|
On October 19, 2007, Moody’s affirmed our Aa1 rating, its second-highest investment grade rating, but revised our ratings outlook to negative from stable. Moody’s cited: (i) our announcement on October 18, 2007, related to recorded charges totaling $2.8 billion ($2.1 billion, net of tax), associated with the impairment of Exubera assets and other exit costs associated with Exubera (see the “Our 2007 Performance: Decision to Exit Exubera” section of this Financial Review); (ii) continuing pressure on U.S. Lipitor sales and market share; and (iii) the loss of U.S. exclusivity for Lipitor in either 2010 or 2011. The negative outlook reflects Moody’s assessment of challenges we face as we head into the 2010-2012 period when the U.S. patents on certain key products expire.
Our access to financing at favorable rates would be affected by a substantial downgrade in our credit ratings.
Debt Capacity
We have available lines of credit and revolving-credit agreements with a group of banks and other financial intermediaries. We maintain cash and cash equivalent balances and short-term investments in excess of our commercial paper and other short-term borrowings. As of December 31, 2007, we had access to $3.7 billion of lines of credit, of which $1.5 billion expire within one year. Of these lines of credit, $3.6 billion are unused, of which our lenders have committed to loan us $2.1 billion at our request. $2.0 billion of the unused lines of credit, which expire in 2012, may be used to support our commercial paper borrowings.
In March 2007, we filed a securities registration statement with the SEC. This registration statement was filed under the automatic shelf registration process available to well-known seasoned issuers and is effective for three years. We can issue securities of various types under that registration statement at any time, subject to approval by our Board of Directors in certain circumstances.
2007 Financial Report | 29
Financial Review
Pfizer Inc and Subsidiary Companies
|
Goodwill and Other Intangible Assets |
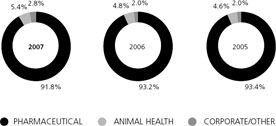
As of December 31, 2007,Goodwilltotaled $21.4 billion (19% of our total assets) and other identifiable intangible assets, net of accumulated amortization, totaled $20.5 billion (18% of our total assets).
The components of goodwill and other identifiable intangible assets, by segment, as of December 31, 2007, follow:
| | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
| |
(MILLIONS OF
DOLLARS) | | PHARMACEUTICAL | | ANIMAL
HEALTH | | OTHER | | TOTAL | |
|
|
|
|
|
|
|
|
| |
Goodwill | | | $ 21,256 | | $ | 108 | | $ | 18 | | $ | 21,382 | |
Finite-lived intangible assets, net(a) | | | 17,188 | | | 322 | | | 52 | | | 17,562 | |
Indefinite-lived intangible assets(b) | | | 2,826 | | | 109 | | | 1 | | | 2,936 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Includes $16.6 billion related to developed technology rights and $565 million related to brands. |
| | |
| (b) | Includes $2.9 billion related to brands. |
Developed Technology Rights — Developed technology rights represent the amortized value associated with developed technology, which has been acquired from third parties, and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. We possess a well-diversified portfolio of hundreds of developed technology rights across therapeutic categories, primarily representing the amortized value of the commercialized products included in our Pharmaceutical segment that we acquired in connection with our Pharmacia acquisition in 2003. While the Arthritis and Pain therapeutic category represents about 30% of the total amortized value of developed technology rights as of December 31, 2007, the balance of the amortized value is evenly distributed across the following Pharmaceutical therapeutic product categories: Ophthalmology; Oncology; Urology; Infectious and Respiratory Diseases; Endocrine Disorders categories; and, as a group, Cardiovascular and Metabolic Diseases; Central Nervous System Disorders and All Other categories. The significant components include values determined for Celebrex, Detrol/Detrol LA, Xalatan, Genotropin, Zyvox, and Campto/Camptosar. Also included in this category are the post-approval milestone payments made under our alliance agreements for certain Pharmaceutical products, such as Rebif and Spiriva. These rights are all subject to our impairment review process explained in the “Accounting Policies: Long-Lived Assets” section of this Financial Review.
In 2007, we recorded a charge of $1.1 billion for the impairment of intangible assets (primarily developed technology rights) associated with Exubera. See the “Our 2007 Performance: Decision to Exit Exubera” section of this Financial Review.
Brands— Significant components of brands include values determined for Depo-Provera contraceptive, Xanax and Medrol.
In 2006, we recorded impairment charges of approximately $320 million related to the Depo-Provera brand (see Notes to Consolidated Financial Statements—Note 7. Other (Income)/ Deductions—Net).
|
Selected Measures of Liquidity and Capital Resources |
|
The following table sets forth certain relevant measures of our liquidity and capital resources as of December 31: |
| | | | | | | |
|
|
| |
| | AS OF DECEMBER 31, | |
| |
| |
(MILLIONS OF DOLLARS, EXCEPT RATIOS AND PER COMMON SHARE DATA) | | 2007 | | | 2006 | |
|
|
|
|
|
| |
Cash and cash equivalents and short-term investments and loans | | $ | 26,092 | | | $ | 28,227 | |
Working capital(a) | | $ | 25,014 | | | $ | 25,559 | |
Ratio of current assets to current liabilities | | | 2.15:1 | | | | 2.16:1 | |
Shareholders’ equity per common share(b) | | $ | 9.65 | | | $ | 10.05 | |
|
|
|
|
|
|
|
| |
| | |
| (a) | Working capital includes assets held for sale of $114 million as of December 31, 2007, and $62 million as of December 31, 2006. Working capital also includes liabilities held for sale of nil as of December 31, 2007, and $2 million as of December 31, 2006. |
| | |
| (b) | Represents total shareholders’ equity divided by the actual number of common shares outstanding (which excludes treasury shares and those held by our employee benefit trust). |
| | |
Working capital and the ratio of current assets to current liabilities in 2007 were comparable to 2006, primarily due to: |
| | |
• | inventory write-offs ($661 million) related to Exubera (See the “Our 2007 Performance: Decision to Exit Exubera” section of this Financial Review), as well as liabilities of $375 million accrued in connection with this decision; |
| | |
• | an increase inOther current liabilitiesrelated to our cost-reduction initiatives of $702 million; and |
| | |
• | the funding of share purchases, dividends and capital expenditures in part through the use of the proceeds from the redemption of short-term investments and the use of short-term borrowings, |
| | |
offset by: |
| | |
• | the reclassification to noncurrent of certain amounts associated with uncertain tax positions of about $3.6 billion ($4.0 billion upon adoption on January 1, 2007, of a new accounting standard, partially offset by $0.4 billion of activity in 2007). |
Summary of Cash Flows
| | | | | | | | | | |
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
|
|
| |
Cash provided by/(used in): | | | | | | | | | | | |
Operating activities | | $ | 13,353 | | | $ | 17,594 | | $ | 14,733 | |
Investing activities | | | 795 | | | | 5,101 | | | (5,072 | ) |
Financing activities | | | (12,610 | ) | | | (23,100 | ) | | (9,222 | ) |
Effect of exchange-rate changes on cash and cash equivalents | | | 41 | | | | (15 | ) | | — | |
|
|
|
|
|
|
|
|
|
|
| |
Net increase/(decrease) in cash and cash equivalents | | $ | 1,579 | | | $ | (420 | ) | $ | 439 | |
|
|
|
|
|
|
|
|
|
|
| |
30 | 2007 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
Operating Activities
Our net cash provided by continuing operating activities was $13.4 billion in 2007, compared to $17.6 billion in 2006. The decrease in net cash provided by operating activities was primarily attributable to:
| |
• | higher tax payments ($2.2 billion) in 2007, related primarily to the gain on the sale of our Consumer Healthcare business in December 2006; and |
| |
• | the timing of other receipts and payments in the ordinary course of business. |
| |
Our net cash provided by continuing operating activities was $17.6 billion in 2006, compared to $14.7 billion in 2005. The increase in net cash provided by operating activities was primarily attributable to: |
| |
• | the payment of $1.7 billion in taxes in 2005 associated with the repatriation of approximately $37 billion of foreign earnings under the Jobs Act in 2005; and |
| |
• | the timing of other receipts and payments in the ordinary course of business. |
In 2007 and 2006, the cash flow line item calledIncome taxes payableprimarily reflects the taxes provided in 2006 on the gain on the sale of our Consumer Healthcare business that were paid in 2007.
Investing Activities
Our net cash provided by investing activities was $795 million in 2007, compared to $5.1 billion in 2006. The decrease in net cash provided by investing activities was primarily attributable to:
| |
• | lower net sales and redemptions of investments in 2007 (a negative change in cash and cash equivalents of $6.1 billion), |
| |
partially offset by: |
| |
• | the acquisitions of BioRexis and Embrex in 2007, compared to the acquisitions of PowderMed, Rinat and sanofi-aventis’ rights associated with Exubera in 2006 (a decreased use of cash of $1.9 billion). |
| |
| Our net cash provided by investing activities was $5.1 billion in 2006, compared to net cash used by investing activities of $5.1 billion in 2005. The increase in net cash provided by investing activities was primarily attributable to: |
| |
• | higher net sales and redemptions of short-term investments in 2006 (an increased source of cash of $12.4 billion), primarily used to pay down short-term borrowings, |
partially offset by:
| |
• | an increase in net purchases of long-term investments (an increased use of cash of $2.3 billion); and |
| |
• | the acquisitions of PowderMed, Rinat and sanofi-aventis’ rights to Exubera in 2006, compared to the acquisitions of Vicuron and Idun in 2005 (an increased use of cash of $216 million). |
Financing Activities
Our net cash used in financing activities was $12.6 billion in 2007, compared to $23.1 billion in 2006. The decrease in net cash used in financing activities was primarily attributable to:
| |
• | net borrowings of $4.9 billion in 2007, compared to net repayments of $9.9 billion on total borrowings in 2006, |
| |
partially offset by: |
| |
• | higher purchases of common stock in 2007 of $10.0 billion, compared to $7.0 billion in 2006; and |
| |
• | an increase in cash dividends paid of $1.1 billion, reflecting an increase in the dividend rate, partially offset by lower shares outstanding. |
| |
Our net cash used in financing activities was $23.1 billion in 2006, compared to $9.2 billion in 2005. The increase in net cash used in financing activities was primarily attributable to: |
| |
• | net repayments of $9.9 billion on total borrowings in 2006, compared to $321 million in 2005; |
| |
• | an increase in cash dividends paid of $1.4 billion in 2006, compared to 2005, reflecting an increase in the dividend rate; and |
| |
• | higher purchases of common stock in 2006 of $7.0 billion, compared to $3.8 billion in 2005, |
| |
partially offset by: |
| |
• | higher proceeds of $243 million from the exercise of employee stock options. |
In June 2005, we announced a $5 billion share-purchase program, which is primarily being funded by operating cash flows and a portion of the proceeds from the sale of our Consumer Healthcare business. In June 2006, the Board of Directors increased our share-purchase authorization from $5 billion to $18 billion. In total, under the June 2005 program, through December 31, 2007, we purchased approximately 683 million shares for approximately $17.5 billion.
In October 2004, we announced a $5 billion share-purchase program, which we completed in the second quarter of 2005 and was funded from operating cash flows. In total, under the October 2004 program, we purchased approximately 185 million shares.
In January 2008, we announced a new $5 billion share-purchase program, which will be funded by operating cash flows as circumstances and prices warrant.
A summary of common stock purchases follows:
| | | | | | | | | | |
|
|
|
|
|
|
| |
(MILLIONS OF SHARES AND DOLLARS,
EXCEPT PER-SHARE DATA) | | SHARES OF
COMMON
STOCK
PURCHASED | | AVERAGE
PER-SHARE
PRICE PAID | | TOTAL COST OF
COMMON
STOCK
PURCHASED | |
|
|
|
|
|
|
|
|
|
| |
2007: | | | | | | | | | | |
June 2005 program | | | 395 | | $ | 25.27 | | $ | 9,994 | |
|
|
|
|
|
|
|
|
|
| |
Total | | | 395 | | | | | $ | 9,994 | |
|
|
|
|
|
|
|
|
|
| |
2006: | | | | | | | | | | |
June 2005 program | | | 266 | | $ | 26.19 | | $ | 6,979 | |
|
|
|
|
|
|
|
|
|
| |
Total | | | 266 | | | | | $ | 6,979 | |
|
|
|
|
|
|
|
|
|
| |
2007 Financial Report | 31
Financial Review
Pfizer Inc and Subsidiary Companies
Contractual Obligations
Payments due under contractual obligations as of December 31, 2007, mature as follows:
| | | | | | | | | | | | | | | |
| | YEARS |
| |
|
(MILLIONS OF DOLLARS) | | TOTAL | | WITHIN 1 | | OVER 1
TO 3 | | OVER 3
TO 5 | | AFTER 5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt(a) | | $ | 11,203 | | $ | 1,358 | | $ | 1,498 | | $ | 1,061 | | $ | 7,286 |
Other long-term liabilities reflected on our balance sheet under U.S. GAAP(b) | | | 3,407 | | | 480 | | | 615 | | | 635 | | | 1,677 |
Lease commitments(c) | | | 1,518 | | | 212 | | | 343 | | | 175 | | | 788 |
Purchase obligations(d) | | | 826 | | | 403 | | | 248 | | | 142 | | | 33 |
Uncertain tax positions(e) | | | 408 | | | 408 | | | — | | | — | | | — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
(a) | Our long-term debt obligations include both our expected principal and interest obligations. Our calculations of expected interest payments incorporates only current period assumptions for interest rates, foreign currency translations rates and hedging strategies. (SeeNote 10. Financial Instruments.) Long-term debt consists of senior, unsecured notes, floating rate, unsecured notes, foreign currency denominated notes, and other borrowings and mortgages. |
| |
(b) | Includes expected payments relating to our unfunded U.S. supplemental (non-qualified) pension plans, postretirement plans and deferred compensation plans. |
| |
(c) | Includes operating and capital lease obligations. |
| |
(d) | Purchase obligations represent agreements to purchase goods and services that are enforceable and legally binding and include amounts relating to advertising, information technology services and employee benefit administration services. |
| |
(e) | Reflects the adoption as of January 1, 2007, of Financial Accounting Standards Board (FASB) Interpretation No. 48 (FIN 48),Accounting for Uncertainty in Income Taxes, an interpretation of SFAS 109, Accounting for Income Taxes, and supplemented by FASB Financial Staff Position FIN 48-1,Definition of Settlement of FASB Interpretation No. 48, issued May 2, 2007, (see Notes to Consolidated Financial Statements—Note 1D. Significant Accounting Policies: New Accounting Standards). Except for amounts reflected inIncome taxes payable, we are unable to predict the timing of tax settlements, as tax audits can involve complex issues and the resolution of those issues may span multiple years, particularly if subject to negotiation or litigation. |
In 2008, we expect to spend approximately $2.0 billion on property, plant and equipment.
Off-Balance Sheet Arrangements
In the ordinary course of business and in connection with the sale of assets and businesses, we often indemnify our counterparties against certain liabilities that may arise in connection with a transaction or that are related to activities prior to a transaction. These indemnifications typically pertain to environmental, tax, employee and/or product-related matters, and patent infringement claims. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications are generally subject to threshold amounts, specified claim periods and other restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2007, recorded amounts for the estimated fair value of these indemnifications are not significant.
Certain of our co-promotion or license agreements give our licensors or partners the rights to negotiate for, or in some cases to obtain, under certain financial conditions, co-promotion or
other rights in specified countries with respect to certain of our products.
Dividends on Common Stock
We declared dividends of $8.2 billion in 2007 and $7.3 billion in 2006 on our common stock. In 2007, we increased our annual dividend to $1.16 per share from $0.96 per share in 2006. In December 2007, our Board of Directors declared a first-quarter 2008 dividend of $0.32 per share. The 2008 cash dividend marks the 41st consecutive year of dividend increases.
Our current dividend provides a return to shareholders while maintaining sufficient capital to invest in growing our businesses. Our dividends are funded from operating cash flows, our financial asset portfolio and short-term commercial paper borrowings and are not restricted by debt covenants. To the extent we have additional capital in excess of investment opportunities, we typically offer a return to our shareholders through a stock-purchase program. We believe that our profitability and access to financial markets provide sufficient capability for us to pay current and future dividends.
New Accounting Standards
Recently Adopted Accounting Standards
As of January 1, 2007, we adopted FIN 48, which provides guidance on the recognition, derecognition and measurement of tax positions for financial statement purposes. Prior to 2007, our policy had been to account for income tax contingencies based on whether we determined our tax position to be ‘probable’ under current tax law of being sustained, as well as an analysis of potential outcomes under a given set of facts and circumstances. FIN 48 requires that tax positions be sustainable based on a ‘more likely than not’ standard of benefit recognition under current tax law, and adjusted to reflect the largest amount of benefit that is greater than 50% likely of being realized upon settlement, presuming that the tax position is examined by the appropriate taxing authority that has full knowledge of all relevant information. As a result of the implementation of FIN 48, we reduced our existing liabilities for uncertain tax positions by approximately $11 million, which has been recorded as a direct adjustment to the opening balance ofRetained earnings, and changed the classification of virtually all amounts associated with uncertain tax positions, including the associated accrued interest, from current to noncurrent, as of the date of adoption.
Recently Issued Accounting Standards, Not Adopted as of December 31, 2007
In September 2006, the FASB issued Statement of Financial Accounting Standards No. 157 (SFAS 157),Fair Value Measurements. SFAS 157 provides guidance for, among other things, the definition of fair value and the methods used to measure fair value. In February 2008, the FASB issued FASB Staff Position (FSP) 157-2Effective Date of FASB Statement No. 157.Under the terms of FSP 157-2, the provisions of SFAS 157 will be adopted for financial instruments in 2008 and, when required, for nonfinancial assets and nonfinancial liabilities in 2009 (except for those that are recognized or disclosed at fair value in the financial statements on a recurring basis). We do not expect that the provisions to be adopted in 2008 will have a significant impact on our financial statements and we are in the process of evaluating the impact of provisions to be adopted in 2009.
32 | 2007 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
In December 2007, the FASB issued SFAS No. 141(R),Business Combinations. (SFAS 141(R) replaced SFAS No. 141,Business Combinations, originally issued in June 2001.) SFAS 141(R) retains the purchase method of accounting for acquisitions, but requires a number of changes, including changes in the way assets and liabilities are recognized in purchase accounting. It also changes the recognition of assets acquired and liabilities assumed arising from contingencies, requires the capitalization of in-process research and development at fair value, and requires the expensing of acquisition-related costs as incurred. Generally, SFAS 141(R) is effective on a prospective basis for all business combinations completed on or after January 1, 2009. We are currently in the process of evaluating the extent of those potential impacts.
In December 2007, the FASB issued SFAS 160,Noncontrolling Interests in Consolidated Financial Statements, an amendment of ARB 51,Consolidated Financial Statements. SFAS 160 provides guidance for the accounting, reporting and disclosure of noncontrolling interests, also called minority interest. A minority interest represents the portion of equity (net assets) in a subsidiary not attributable, directly or indirectly, to a parent. The provisions of SFAS 160 will be adopted in 2009. The provisions of SFAS 160 will impact our current accounting for minority interests, which are not significant, and will impact our accounting for future acquisitions, if any, where we do not acquire 100% of the entity. We are currently in the process of evaluating the extent of those potential impacts.
In December 2007, the Emerging Issues Task Force (EITF) issued EITF Issue No. 07-1,Accounting for Collaborative Arrangements. EITF 07-1 provides guidance concerning: determining whether an arrangement constitutes a collaborative arrangement within the scope of the Issue; how costs incurred and revenue generated on sales to third parties should be reported in the income statement; how an entity should characterize payments on the income statement; and what participants should disclose in the notes to the financial statements about a collaborative arrangement. The provisions of EITF 07-1 will be adopted in 2009. We are in the process of evaluating the impact of adopting EITF 07-1 on our financial statements.
In June 2007, the EITF issued EITF Issue No. 07-3,Accounting for Nonrefundable Advance Payments for Goods or Services to be Used in Future Research and Development Activities.EITF Issue No. 07-3 provides guidance concerning the accounting for non-refundable advance payments for goods and services that will be used in future R&D activities and requires that they be expensed when the research and development activity has been performed and not at the time of payment. The provisions of EITF Issue No. 07-3 will be adopted in 2008. We do not expect that the adoption of EITF Issue No. 07-3 will have a significant impact on our financial statements.
Forward-Looking Information and Factors
That May Affect Future Results
The Securities and Exchange Commission encourages companies to disclose forward-looking information so that investors can better understand a company’s future prospects and make informed investment decisions. This report and other written or oral statements that we make from time to time contain such forward-looking statements that set forth anticipated results based
on management’s plans and assumptions. Such forward-looking statements involve substantial risks and uncertainties. We have tried, wherever possible, to identify such statements by using words such as “will,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” “target,” “forecast” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or business plans and prospects. In particular, these include statements relating to future actions, business plans and prospects, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, interest rates, foreign exchange rates, the outcome of contingencies, such as legal proceedings, and financial results. Among the factors that could cause actual results to differ materially are the following:
| |
• | Success of research and development activities; |
| |
• | Decisions by regulatory authorities regarding whether and when to approve our drug applications as well as their decisions regarding labeling and other matters that could affect the availability or commercial potential of our products; |
| |
• | Speed with which regulatory authorizations, pricing approvals and product launches may be achieved; |
| |
• | Success of external business development activities; |
| |
• | Competitive developments, including with respect to competitor drugs and drug candidates that treat diseases and conditions similar to those treated by our in-line drugs and drug candidates; |
| |
• | Ability to successfully market both new and existing products domestically and internationally; |
| |
• | Difficulties or delays in manufacturing; |
| |
• | Trade buying patterns; |
| |
• | Ability to meet generic and branded competition after the loss of patent protection for our products and competitor products; |
| |
• | Impact of existing and future legislation and regulatory provisions on product exclusivity; |
| |
• | Trends toward managed care and healthcare cost containment; |
| |
• | U.S. legislation or regulatory action affecting, among other things, pharmaceutical product pricing, reimbursement or access, including under Medicaid and Medicare, the importation of prescription drugs from outside the U.S. at prices that are regulated by governments of various foreign countries, and the involuntary approval of prescription medicines for over-the-counter use; |
| |
• | Impact of the Medicare Prescription Drug, Improvement and Modernization Act of 2003; |
| |
• | Legislation or regulatory action in markets outside the U.S. affecting pharmaceutical product pricing, reimbursement or access; |
| |
• | Contingencies related to actual or alleged environmental contamination; |
2007 Financial Report | 33
Financial Review
Pfizer Inc and Subsidiary Companies
| |
• | Claims and concerns that may arise regarding the safety or efficacy of in-line products and product candidates; |
| |
• | Significant breakdown, infiltration or interruption of our information technology systems and infrastructure; |
| |
• | Legal defense costs, insurance expenses, settlement costs and the risk of an adverse decision or settlement related to product liability, patent protection, governmental investigations, ongoing efforts to explore various means for resolving asbestos litigation, and other legal proceedings; |
| |
• | Ability to protect our patents and other intellectual property both domestically and internationally; |
| |
• | Interest rate and foreign currency exchange rate fluctuations; |
| |
• | Governmental laws and regulations affecting domestic and foreign operations, including tax obligations; |
| |
• | Changes in generally accepted accounting principles; |
| |
• | Any changes in business, political and economic conditions due to the threat of terrorist activity in the U.S. and other parts of the world, and related U.S. military action overseas; |
| |
• | Growth in costs and expenses; |
| |
• | Changes in our product, segment and geographic mix; and |
| |
• | Impact of acquisitions, divestitures, restructurings, product withdrawals and other unusual items, including our ability to realize the projected benefits of our cost-reduction initiatives. |
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Achievement of anticipated results is subject to substantial risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from past results and those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements.
We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise. You are advised, however, to consult any further disclosures we make on related subjects in our Forms 10-Q, 8-K and 10-K reports to the Securities and Exchange Commission.
Certain risks, uncertainties and assumptions are discussed here and under the heading entitled “Risk Factors and Cautionary Factors That May Affect Future Results” in Item 1A of our Annual Report on Form 10-K for the year ended December 31, 2007, which will be filed in February 2008. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider any such list to be a complete set of all potential risks or uncertainties.
This report includes discussion of certain clinical studies relating to various in-line products and/or product candidates. These studies typically are part of a larger body of clinical data relating to such products or product candidates, and the discussion herein should be considered in the context of the larger body of data.
Financial Risk Management
The overall objective of our financial risk management program is to seek a reduction in the potential negative earnings effects from changes in foreign exchange and interest rates arising in our business activities. We manage these financial exposures through operational means and by using various financial instruments. These practices may change as economic conditions change.
Foreign Exchange Risk—A significant portion of our revenues and earnings is exposed to changes in foreign exchange rates. We seek to manage our foreign exchange risk in part through operational means, including managing same currency revenues in relation to same currency costs, and same currency assets in relation to same currency liabilities.
Foreign exchange risk is also managed through the use of foreign currency forward-exchange contracts. These contracts are used to offset the potential earnings effects from mostly intercompany short-term foreign currency assets and liabilities that arise from operations. Foreign currency swaps are used to offset the potential earnings effects from foreign currency debt. We also use foreign currency forward-exchange contracts and foreign currency swaps to hedge the potential earnings effects from short and long-term foreign currency investments, third-party loans and intercompany loans.
In addition, under certain market conditions, we protect against possible declines in the reported net assets of our Japanese yen, Swedish krona and certain euro functional-currency subsidiaries. In these cases, we use currency swaps or foreign currency debt.
Our financial instrument holdings at year-end were analyzed to determine their sensitivity to foreign exchange rate changes. The fair values of these instruments were determined as follows:
| |
• | foreign currency forward-exchange contracts and currency swaps—net present values |
| |
• | foreign receivables, payables, debt and loans—changes in exchange rates |
In this sensitivity analysis, we assumed that the change in one currency’s rate relative to the U.S. dollar would not have an effect on other currencies’ rates relative to the U.S. dollar. All other factors were held constant.
If there were an adverse change in foreign exchange rates of 10%, the expected effect on net income related to our financial instruments would be immaterial. For additional details, see Notes to Consolidated Financial Statements—Note 10D. Financial Instruments: Derivative Financial Instruments and Hedging Activities.
Interest Rate Risk—Our U.S. dollar interest-bearing investments, loans and borrowings are subject to interest rate risk. We are also subject to interest rate risk on euro debt, investments and currency swaps, Swedish krona currency swaps, and on Japanese yen short and long-term borrowings and currency swaps. We invest, loan and borrow primarily on a short-term or variable-rate basis. From time to time, depending on market conditions, we will fix interest rates either through entering into fixed-rate investments and borrowings or through the use of derivative financial instruments such as interest rate swaps.
34 | 2007 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
Our financial instrument holdings at year-end were analyzed to determine their sensitivity to interest rate changes. The fair values of these instruments were determined by net present values.
In this sensitivity analysis, we used a one hundred basis point change (decreased 1% from the rate of the yield of the financial instrument) in interest rates for all maturities. All other factors were held constant. This represents a change in the key model characteristic from last year. The change was made to better reflect the potential impact of a significant change in interest rates. Applying this new model characteristic to our financial instruments last year had no material effect.
In 2007 and 2006, if there were an adverse change of one hundred basis points in interest rates, the expected effect on net income related to our financial instruments would be immaterial.
Legal Proceedings and Contingencies
We and certain of our subsidiaries are involved in various patent, product liability, consumer, commercial, securities, environmental and tax litigations and claims; government investigations; and other legal proceedings that arise from time to time in the ordinary course of our business. We do not believe any of them will have a material adverse effect on our financial position.
Beginning in 2007 upon the adoption of a new accounting standard, we record accruals for income tax contingencies to the extent that we conclude that a tax position is not sustainable under a ‘more likely than not’ standard and we record our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction when we conclude that the potential recovery is more likely than not. (See Notes to Consolidated Financial Statements—Note 1D. Significant Accounting Policies: New Accounting StandardsandNote 8E. Taxes on Income: Tax Contingencies.) We record accruals for all other contingencies to the extent that we conclude their occurrence is probable and the related damages are estimable, and we record anticipated recoveries under existing insurance contracts when assured of recovery. If a range of liability is probable and estimable and some amount within the range appears to be a better estimate than any other amount within the range, we accrue that amount. If a range of liability is probable and estimable and no amount within the range appears to be a better estimate than any other amount within the range, we accrue the minimum of such probable range. Many claims involve highly complex issues relating to causation, label warnings, scientific evidence, actual damages and other matters. Often these issues are subject to substantial uncertainties and, therefore, the probability of loss and an estimation of damages are difficult to ascertain. Consequently, we cannot reasonably estimate the maximum potential exposure or the range of possible loss in excess of amounts accrued for these contingencies. These assessments can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions (see Notes to Consolidated Financial Statements—Note 1B. Significant Accounting Policies: Estimates and Assumptions). Our assessments are based on estimates and assumptions that have been deemed reasonable by management. Litigation is inherently unpredictable, and excessive verdicts do occur. Although we believe we have substantial defenses in these matters, we could in the future incur judgments or enter into
settlements of claims that could have a material adverse effect on our results of operations in any particular period.
Patent claims include challenges to the coverage and/or validity of our patents on various products or processes. Although we believe we have substantial defenses to these challenges with respect to all our material patents, there can be no assurance as to the outcome of these matters, and a loss in any of these cases could result in a loss of patent protection for the drug at issue, which could lead to a significant loss of sales of that drug and could materially affect future results of operations.
2007 Financial Report | 35
| | |
Management’s Report on Internal Control | | Audit Committee’s Report |
Over Financial Reporting | | |
Management’s Report
We prepared and are responsible for the financial statements that appear in our 2007 Financial Report. These financial statements are in conformity with accounting principles generally accepted in the United States of America and, therefore, include amounts based on informed judgments and estimates. We also accept responsibility for the preparation of other financial information that is included in this document.
Report on Internal Control Over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. The Company’s internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2007. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework. Based on our assessment and those criteria, management believes that the Company maintained effective internal control over financial reporting as of December 31, 2007.
The Company’s independent auditors have issued their auditors’ report on the Company’s internal control over financial reporting. That report appears in our 2007 Financial Report under the heading,Report of Independent Registered Public Accounting Firm on Internal Control Over Financial Reporting.

Jeffrey B. Kindler
Chairman and Chief Executive Officer
| | |
| | 
|
| | |
Frank A. D’Amelio | | Loretta V. Cangialosi |
Principal Financial Officer | | Principal Accounting Officer |
| | |
February 29, 2008 | | |
The Audit Committee reviews the Company’s financial reporting process on behalf of the Board of Directors. Management has the primary responsibility for the financial statements and the reporting process, including the system of internal controls.
In this context, the Committee has met and held discussions with management and the independent registered public accounting firm regarding the fair and complete presentation of the Company’s results and the assessment of the Company’s internal control over financial reporting. The Committee has discussed significant accounting policies applied by the Company in its financial statements, as well as alternative treatments. Management represented to the Committee that the Company’s consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States of America, and the Committee has reviewed and discussed the consolidated financial statements with management and the independent registered public accounting firm. The Committee discussed with the independent registered public accounting firm matters required to be discussed by Statement of Auditing Standards No. 61,Communication with Audit Committees.
In addition, the Committee has reviewed and discussed with the independent registered public accounting firm the auditors’ independence from the Company and its management. As part of that review, the Committee received the written disclosures and letter required by the Independence Standards Board Standard No. 1,Independence Discussions with Audit Committeesand by all relevant professional and regulatory standards relating to KPMG’s independence from the Company. The Committee also has considered whether the independent registered public accounting firm’s provision of non-audit services to the Company is compatible with the auditors’ independence. The Committee has concluded that the independent registered public accounting firm is independent from the Company and its management.
The Committee reviewed and discussed Company policies with respect to risk assessment and risk management.
The Committee discussed with the Company’s internal auditors and the independent registered public accounting firm the overall scope and plans for their respective audits. The Committee met with the internal auditors and the independent registered public accounting firm, with and without management present, to discuss the results of their examinations, the evaluations of the Company’s internal controls, and the overall quality of the Company’s financial reporting.
In reliance on the reviews and discussions referred to above, the Committee recommended to the Board of Directors, and the Board has approved, that the audited financial statements be included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2007, for filing with the Securities and Exchange Commission. The Committee has selected and the Board of Directors has ratified, subject to shareholder ratification, the selection of the Company’s independent registered public accounting firm.

W. Don Cornwell
Chair, Audit Committee
February 29, 2008
The Audit Committee’s Report shall not be deemed to be filed or incorporated by reference into any Company filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Company specifically incorporates the Audit Committee’s Report by reference therein.
36 | 2007 Financial Report
Report of Independent Registered Public Accounting Firm on the
Consolidated Financial Statements
The Board of Directors and Shareholders of Pfizer Inc:
We have audited the accompanying consolidated balance sheets of Pfizer Inc and Subsidiary Companies as of December 31, 2007 and 2006, and the related consolidated statements of income, shareholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2007. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Pfizer Inc and Subsidiary Companies as of December 31, 2007 and 2006, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2007, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Pfizer Inc and Subsidiary Companies’ internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated February 29, 2008 expressed an unqualified opinion on the effective operation of the Company’s internal control over financial reporting.
As discussed in the Notes to the Consolidated Financial Statements—Note 1D. Significant Accounting Policies: New Accounting Standards,effective January 1, 2007, Pfizer Inc adopted the provisions of Financial Accounting Standards Board Interpretation (FASB) No. 48,Accounting for Uncertainty in Income Taxes, an interpretation of SFAS 109, Accounting for Income Taxes,and supplemented by FASB Financial Staff Position FIN 48-1,Definition of Settlement in FASB Interpretation No. 48,issued May 2, 2007.
As discussed in the Notes to the Consolidated Financial Statements—Note 1D. Significant Accounting Policies: New Accounting Standards,effective January 1, 2006, Pfizer Inc adopted the provisions of Statement of Financial Accounting Standards No. 123R,Share-Based Payment.
As discussed in the Notes to the Consolidated Financial Statements—Note 1D. Significant Accounting Policies: New Accounting Standards,effective December 31, 2006, Pfizer Inc adopted the provisions of Statement of Financial Accounting Standards No. 158,Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans (an amendment of Financial Accounting Standards Board Statements No. 87, 88, 106 and 132R).
As discussed in the Notes to the Consolidated Financial Statements—Note 1D. Significant Accounting Policies: New Accounting Standards,effective December 31, 2005, Pfizer Inc adopted the provisions of Financial Accounting Standards Board (FASB) Interpretation No. 47 (FIN 47),Accounting for Conditional Asset Retirement Obligations (an interpretation of FASB Statement No. 143).
KPMG LLP
New York, New York
February 29, 2008
2007 Financial Report | 37
Report of Independent Registered Public Accounting Firm on
Internal Control Over Financial Reporting
The Board of Directors and Shareholders of Pfizer Inc:
We have audited the internal control over financial reporting of Pfizer Inc and Subsidiary Companies as of December 31, 2007, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Pfizer Inc and Subsidiary Companies’ management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control, based on risk assessment. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial
statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Pfizer Inc and Subsidiary Companies maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Pfizer Inc and Subsidiary Companies as of December 31, 2007 and 2006, and the related consolidated statements of income, shareholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2007, and our report dated February 29, 2008 expressed an unqualified opinion on those consolidated financial statements.
KPMG LLP
New York, New York
February 29, 2008
38 | 2007 Financial Report
Consolidated Statements of Income
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DECEMBER 31, | |
| |
| |
(MILLIONS, EXCEPT PER COMMON SHARE DATA) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Revenues | | $ | 48,418 | | | $ | 48,371 | | $ | 47,405 | |
Costs and expenses: | | | | | | | | | | | |
Cost of sales(a) | | | 11,239 | | | | 7,640 | | | 7,232 | |
Selling, informational and administrative expenses(a) | | | 15,626 | | | | 15,589 | | | 15,313 | |
Research and development expenses(a) | | | 8,089 | | | | 7,599 | | | 7,256 | |
Amortization of intangible assets | | | 3,128 | | | | 3,261 | | | 3,399 | |
Acquisition-related in-process research and development charges | | | 283 | | | | 835 | | | 1,652 | |
Restructuring charges and acquisition-related costs | | | 2,534 | | | | 1,323 | | | 1,356 | |
Other (income)/deductions—net | | | (1,759 | ) | | | (904 | ) | | 397 | |
|
|
|
|
|
|
|
|
|
|
| |
Income from continuing operations before provision for taxes on income, minority interests and cumulative effect of a change in accounting principles | | | 9,278 | | | | 13,028 | | | 10,800 | |
Provision for taxes on income | | | 1,023 | | | | 1,992 | | | 3,178 | |
Minority interests | | | 42 | | | | 12 | | | 12 | |
|
|
|
|
|
|
|
|
|
|
| |
Income from continuing operations before cumulative effect of a change in accounting principles | | | 8,213 | | | | 11,024 | | | 7,610 | |
Discontinued operations: | | | | | | | | | | | |
Income/(loss) from discontinued operations—net of tax | | | (3 | ) | | | 433 | | | 451 | |
Gains/(losses) on sales of discontinued operations—net of tax | | | (66 | ) | | | 7,880 | | | 47 | |
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations—net of tax | | | (69 | ) | | | 8,313 | | | 498 | |
|
|
|
|
|
|
|
|
|
|
| |
Income before cumulative effect of a change in accounting principles | | | 8,144 | | | | 19,337 | | | 8,108 | |
Cumulative effect of a change in accounting principles—net of tax | | | — | | | | — | | | (23 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Net income | | $ | 8,144 | | | $ | 19,337 | | $ | 8,085 | |
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | |
Earnings per common share—basic | | | | | | | | | | | |
Income from continuing operations before cumulative effect of a change in accounting principles | | $ | 1.19 | | | $ | 1.52 | | $ | 1.03 | |
Discontinued operations | | | (0.01 | ) | | | 1.15 | | | 0.07 | |
|
|
|
|
|
|
|
|
|
|
| |
Income before cumulative effect of a change in accounting principles | | | 1.18 | | | | 2.67 | | | 1.10 | |
Cumulative effect of a change in accounting principles | | | — | | | | — | | | — | |
|
|
|
|
|
|
|
|
|
|
| |
Net income | | $ | 1.18 | | | $ | 2.67 | | $ | 1.10 | |
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | |
Earnings per common share—diluted | | | | | | | | | | | |
Income from continuing operations before cumulative effect of a change in accounting principles | | $ | 1.18 | | | $ | 1.52 | | $ | 1.02 | |
Discontinued operations | | | (0.01 | ) | | | 1.14 | | | 0.07 | |
|
|
|
|
|
|
|
|
|
|
| |
Income before cumulative effect of a change in accounting principles | | | 1.17 | | | | 2.66 | | | 1.09 | |
Cumulative effect of a change in accounting principles | | | — | | | | — | | | — | |
|
|
|
|
|
|
|
|
|
|
| |
Net income | | $ | 1.17 | | | $ | 2.66 | | $ | 1.09 | |
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | |
Weighted-average shares—basic | | | 6,917 | | | | 7,242 | | | 7,361 | |
Weighted-average shares—diluted | | | 6,939 | | | | 7,274 | | | 7,411 | |
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Exclusive of amortization of intangible assets, except as disclosed inNote 1K. Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets. |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
2007 Financial Report | 39
Consolidated Balance Sheets
Pfizer Inc and Subsidiary Companies
| | | | | | | | |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
| | AS OF DECEMBER 31, | |
| |
| |
(MILLIONS, EXCEPT PREFERRED STOCK ISSUED AND PER COMMON SHARE DATA) | | 2007 | | | 2006 | |
|
|
|
|
|
| |
Assets | | | | | | | | |
Cash and cash equivalents | | $ | 3,406 | | | $ | 1,827 | |
Short-term investments | | | 22,069 | | | | 25,886 | |
Accounts receivable, less allowance for doubtful accounts: 2007—$223; 2006—$204 | | | 9,843 | | | | 9,392 | |
Short-term loans | | | 617 | | | | 514 | |
Inventories | | | 5,302 | | | | 6,111 | |
Prepaid expenses and taxes | | | 5,498 | | | | 3,866 | |
Assets held for sale | | | 114 | | | | 62 | |
|
|
|
|
|
|
|
| |
Total current assets | | | 46,849 | | | | 47,658 | |
Long-term investments and loans | | | 4,856 | | | | 3,892 | |
Property, plant and equipment, less accumulated depreciation | | | 15,734 | | | | 16,632 | |
Goodwill | | | 21,382 | | | | 20,876 | |
Identifiable intangible assets, less accumulated amortization | | | 20,498 | | | | 24,350 | |
Other assets, deferred taxes and deferred charges | | | 5,949 | | | | 2,138 | |
|
|
|
|
|
|
|
| |
Total assets | | $ | 115,268 | | | $ | 115,546 | |
|
|
|
|
|
|
|
| |
Liabilities and Shareholders’ Equity | | | | | | | | |
Short-term borrowings, including current portion of long-term debt: 2007—$1,024; 2006—$712 | | $ | 5,825 | | | $ | 2,434 | |
Accounts payable | | | 2,270 | | | | 2,019 | |
Dividends payable | | | 2,163 | | | | 2,055 | |
Income taxes payable | | | 1,380 | | | | 7,176 | |
Accrued compensation and related items | | | 1,974 | | | | 1,903 | |
Other current liabilities | | | 8,223 | | | | 6,510 | |
Liabilities held for sale | | | — | | | | 2 | |
|
|
|
|
|
|
|
| |
Total current liabilities | | | 21,835 | | | | 22,099 | |
Long-term debt | | | 7,314 | | | | 5,546 | |
Pension benefit obligations | | | 2,599 | | | | 3,632 | |
Postretirement benefit obligations | | | 1,708 | | | | 1,970 | |
Deferred taxes | | | 7,696 | | | | 8,015 | |
Other taxes payable | | | 6,246 | | | | — | |
Other noncurrent liabilities | | | 2,746 | | | | 2,852 | |
|
|
|
|
|
|
|
| |
Total liabilities | | | 50,144 | | | | 44,114 | |
|
|
|
|
|
|
|
| |
Minority interests | | | 114 | | | | 74 | |
|
|
|
|
|
|
|
| |
Preferred stock, without par value, at stated value; 27 shares authorized; issued: 2007—2,302; 2006—3,497 | | | 93 | | | | 141 | |
Common stock, $0.05 par value; 12,000 shares authorized; issued: 2007—8,850; 2006—8,819 | | | 442 | | | | 441 | |
Additional paid-in capital | | | 69,913 | | | | 69,104 | |
Employee benefit trust | | | (550 | ) | | | (788 | ) |
Treasury stock, shares at cost; 2007—2,089; 2006—1,695 | | | (56,847 | ) | | | (46,740 | ) |
Retained earnings | | | 49,660 | | | | 49,669 | |
Accumulated other comprehensive income/(expense) | | | 2,299 | | | | (469 | ) |
|
|
|
|
|
|
|
| |
Total shareholders’ equity | | | 65,010 | | | | 71,358 | |
|
|
|
|
|
|
|
| |
Total liabilities and shareholders’ equity | | $ | 115,268 | | | $ | 115,546 | |
|
|
|
|
|
|
|
| |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
40 | 2007 Financial Report
Consolidated Statements of Shareholders’ Equity
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | PREFERRED STOCK | | COMMON STOCK | | ADDITIONAL
PAID-IN
CAPITAL | | EMPLOYEE
BENEFIT TRUST | | TREASURY STOCK | | | | | | |
| |
| |
| | |
| |
| | RETAINED
EARNINGS | | ACCUM. OTHER
COMPRE-
HENSIVE
INC./(EXP.) | | | |
(MILLIONS, EXCEPT PREFERRED SHARES) | | SHARES | | STATED
VALUE | | SHARES | | PAR
VALUE | | | SHARES | | FAIR
VALUE | | SHARES | | COST | | | | TOTAL | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, January 1, 2005 | | 4,779 | | $ | 193 | | 8,754 | | $ | 438 | | $ | 67,253 | | (46 | ) | $ | (1,229 | ) | (1,281 | ) | $ | (35,992 | ) | $ | 35,492 | | $ | 2,278 | | $ | 68,433 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | | | | | | | | | | | | | | | | | | | | | | | 8,085 | | | | | | 8,085 | |
Total other comprehensive expense—net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,799 | ) | | (1,799 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 6,286 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| |
Cash dividends declared— | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
common stock | | | | | | | | | | | | | | | | | | | | | | | | | | (5,960 | ) | | | | | (5,960 | ) |
preferred stock | | | | | | | | | | | | | | | | | | | | | | | | | | (9 | ) | | | | | (9 | ) |
Stock option transactions | | | | | | | 24 | | | 1 | | | 342 | | 7 | | | 193 | | — | | | (6 | ) | | | | | | | | 530 | |
Purchases of common stock | | | | | | | | | | | | | | | | | | | | (143 | ) | | (3,797 | ) | | | | | | | | (3,797 | ) |
Employee benefit trust transactions—net | | | | | | | | | | | | | (113 | ) | (1 | ) | | 113 | | 1 | | | — | | | | | | | | | — | |
Preferred stock conversions and redemptions | | (586 | ) | | (24 | ) | | | | | | | 37 | | | | | | | — | | | 6 | | | | | | | | | 19 | |
Other | | | | | | | 6 | | | — | | | 240 | | | | | | | — | | | 22 | | | | | | | | | 262 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2005 | | 4,193 | | | 169 | | 8,784 | | | 439 | | | 67,759 | | (40 | ) | | (923 | ) | (1,423 | ) | | (39,767 | ) | | 37,608 | | | 479 | | | 65,764 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | | | | | | | | | | | | | | | | | | | | | | | 19,337 | | | | | | 19,337 | |
Total other comprehensive income—net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,192 | | | 1,192 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 20,529 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| |
Adoption of new accounting standard—net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (2,140 | ) | | (2,140 | ) |
Cash dividends declared— | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
common stock | | | | | | | | | | | | | | | | | | | | | | | | | | (7,268 | ) | | | | | (7,268 | ) |
preferred stock | | | | | | | | | | | | | | | | | | | | | | | | | | (8 | ) | | | | | (8 | ) |
Stock option transactions | | | | | | | 28 | | | 1 | | | 896 | | 11 | | | 286 | | (6 | ) | | (8 | ) | | | | | | | | 1,175 | |
Purchases of common stock | | | | | | | | | | | | | | | | | | | | (266 | ) | | (6,979 | ) | | | | | | | | (6,979 | ) |
Employee benefit trust transactions—net | | | | | | | | | | | | | 152 | | (1 | ) | | (151 | ) | | | | | | | | | | | | | 1 | |
Preferred stock conversions and redemptions | | (696 | ) | | (28 | ) | | | | | | | 12 | | | | | | | — | | | 6 | | | | | | | | | (10 | ) |
Other | | | | | | | 7 | | | 1 | | | 285 | | | | | | | — | | | 8 | | | | | | | | | 294 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2006 | | 3,497 | | | 141 | | 8,819 | | | 441 | | | 69,104 | | (30 | ) | | (788 | ) | (1,695 | ) | | (46,740 | ) | | 49,669 | | | (469 | ) | | 71,358 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | | | | | | | | | | | | | | | | | | | | | | | | 8,144 | | | | | | 8,144 | |
Total other comprehensive income—net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 2,768 | | | 2,768 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 10,912 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| |
Adoption of new accounting standard | | | | | | | | | | | | | | | | | | | | | | | | | | 11 | | | | | | 11 | |
Cash dividends declared— | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
common stock | | | | | | | | | | | | | | | | | | | | | | | | | | (8,156 | ) | | | | | (8,156 | ) |
preferred stock | | | | | | | | | | | | | | | | | | | | | | | | | | (8 | ) | | | | | (8 | ) |
Stock option transactions | | | | | | | 23 | | | 1 | | | 738 | | 5 | | | 121 | | | | | (7 | ) | | | | | | | | 853 | |
Purchases of common stock | | | | | | | | | | | | | | | | | | | | (395 | ) | | (9,994 | ) | | | | | | | | (9,994 | ) |
Employee benefit trust transactions—net | | | | | | | | | | | | | (49 | ) | 1 | | | 117 | | | | | | | | | | | | | | 68 | |
Preferred stock conversions and redemptions | | (1,195 | ) | | (48 | ) | | | | | | | (25 | ) | | | | | | 1 | | | 5 | | | | | | | | | (68 | ) |
Other | | | | | | | 8 | | | — | | | 145 | | | | | | | — | | | (111 | ) | | | | | | | | 34 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2007 | | 2,302 | | $ | 93 | | 8,850 | | $ | 442 | | $ | 69,913 | | (24 | ) | $ | (550 | ) | (2,089 | ) | $ | (56,847 | ) | $ | 49,660 | | $ | 2,299 | | $ | 65,010 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
2007 Financial Report | 41
Consolidated Statements of Cash Flows
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DECEMBER 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | 2006 | | 2005 | |
|
|
|
|
|
|
| |
Operating Activities | | | | | | | | | | |
Net income | | $ | 8,144 | | $ | 19,337 | | $ | 8,085 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | |
Depreciation and amortization | | | 5,200 | | | 5,293 | | | 5,576 | |
Share-based compensation expense | | | 437 | | | 655 | | | 157 | |
Acquisition-related in-process research and development charges | | | 283 | | | 835 | | | 1,652 | |
Intangible asset impairments and other associated non-cash charges | | | 2,220 | | | 320 | | | 1,240 | |
Gains on disposals | | | (326 | ) | | (280 | ) | | (172 | ) |
(Gains)/losses on sales of discontinued operations | | | 168 | | | (10,243 | ) | | (77 | ) |
Cumulative effect of a change in accounting principles | | | — | | | — | | | 40 | |
Deferred taxes from continuing operations | | | (2,788 | ) | | (1,525 | ) | | (1,465 | ) |
Other deferred taxes | | | — | | | (420 | ) | | 8 | |
Other non-cash adjustments | | | 815 | | | 606 | | | 486 | |
Changes in assets and liabilities, net of effect of businesses acquired and divested: | | | | | | | | | | |
Accounts receivable | | | (320 | ) | | (172 | ) | | (803 | ) |
Inventories | | | 720 | | | 118 | | | 72 | |
Prepaid and other assets | | | (647 | ) | | 314 | | | 615 | |
Accounts payable and accrued liabilities | | | 1,509 | | | (450 | ) | | (1,054 | ) |
Income taxes payable | | | (2,002 | ) | | 2,909 | | | 254 | |
Other liabilities | | | (60 | ) | | 297 | | | 119 | |
|
|
|
|
|
|
|
|
|
| |
Net cash provided by operating activities | | | 13,353 | | | 17,594 | | | 14,733 | |
|
|
|
|
|
|
|
|
|
| |
Investing Activities | | | | | | | | | | |
Purchases of property, plant and equipment | | | (1,880 | ) | | (2,050 | ) | | (2,106 | ) |
Purchases of short-term investments | | | (25,426 | ) | | (9,597 | ) | | (28,040 | ) |
Proceeds from sales and redemptions of short-term investments | | | 30,288 | | | 20,771 | | | 26,779 | |
Purchases of long-term investments | | | (1,635 | ) | | (1,925 | ) | | (687 | ) |
Proceeds from sales and redemptions of long-term investments | | | 172 | | | 233 | | | 1,309 | |
Purchases of other assets | | | (111 | ) | | (153 | ) | | (431 | ) |
Proceeds from sales of other assets | | | 30 | | | 3 | | | 12 | |
Proceeds from sales of businesses, products and product lines | | | 24 | | | 200 | | | 127 | |
Acquisitions, net of cash acquired | | | (464 | ) | | (2,320 | ) | | (2,104 | ) |
Other investing activities | | | (203 | ) | | (61 | ) | | 69 | |
|
|
|
|
|
|
|
|
|
| |
Net cash provided by/(used in) investing activities | | | 795 | | | 5,101 | | | (5,072 | ) |
|
|
|
|
|
|
|
|
|
| |
Financing Activities | | | | | | | | | | |
Increase in short-term borrowings, net | | | 3,155 | | | 1,040 | | | 1,124 | |
Principal payments on short-term borrowings | | | (764 | ) | | (11,969 | ) | | (1,427 | ) |
Proceeds from issuances of long-term debt | | | 2,573 | | | 1,050 | | | 1,021 | |
Principal payments on long-term debt | | | (64 | ) | | (55 | ) | | (1,039 | ) |
Purchases of common stock | | | (9,994 | ) | | (6,979 | ) | | (3,797 | ) |
Cash dividends paid | | | (7,975 | ) | | (6,919 | ) | | (5,555 | ) |
Stock option transactions and other | | | 459 | | | 732 | | | 451 | |
|
|
|
|
|
|
|
|
|
| |
Net cash used in financing activities | | | (12,610 | ) | | (23,100 | ) | | (9,222 | ) |
|
|
|
|
|
|
|
|
|
| |
Effect of exchange-rate changes on cash and cash equivalents | | | 41 | | | (15 | ) | | — | |
|
|
|
|
|
|
|
|
|
| |
Net increase/(decrease) in cash and cash equivalents | | | 1,579 | | | (420 | ) | | 439 | |
Cash and cash equivalents at beginning of year | | | 1,827 | | | 2,247 | | | 1,808 | |
|
|
|
|
|
|
|
|
|
| |
Cash and cash equivalents at end of year | | $ | 3,406 | | $ | 1,827 | | $ | 2,247 | |
|
|
|
|
|
|
|
|
|
| |
Supplemental Cash Flow Information | | | | | | | | | | |
Non-cash transactions: | | | | | | | | | | |
Sale of the Consumer Healthcare business(a) | | $ | — | | $ | 16,429 | | $ | — | |
|
|
|
|
|
|
|
|
|
| |
Cash paid during the period for: | | | | | | | | | | |
Income taxes | | $ | 5,617 | | $ | 3,443 | | $ | 4,713 | |
Interest | | | 643 | | | 715 | | | 649 | |
|
|
|
|
|
|
|
|
|
| |
| |
(a) | Reflects portion of proceeds received in the form of short-term investments. |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
42 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
1.Significant Accounting Policies
A. Consolidation and Basis of Presentation
The consolidated financial statements include our parent company and all subsidiaries, including those operating outside the U.S., and are prepared in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP). For subsidiaries operating outside the U.S., the financial information is included as of and for the year ended November 30 for each year presented. Substantially all unremitted earnings of international subsidiaries are free of legal and contractual restrictions. All significant transactions among our businesses have been eliminated.
We made certain reclassifications to the 2006 and 2005 consolidated financial statements to conform to the 2007 presentation, primarily related to presenting certain tax receivables in current assets.
B. Estimates and Assumptions
In preparing the consolidated financial statements, we use certain estimates and assumptions that affect reported amounts and disclosures. For example, estimates are used when accounting for deductions from revenues (such as rebates, chargebacks, sales returns and sales allowances), depreciation, amortization, employee benefits, contingencies and asset and liability valuations. Our estimates are often based on complex judgments, probabilities and assumptions that we believe to be reasonable but that are inherently uncertain and unpredictable. Assumptions may later prove to be incomplete or inaccurate, or unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions. It is also possible that other professionals, applying reasonable judgment to the same facts and circumstances, could develop and support a range of alternative estimated amounts. We are also subject to other risks and uncertainties that may cause actual results to differ from estimated amounts, such as changes in the healthcare environment, competition, foreign exchange, litigation, legislation and regulations. These and other risks and uncertainties are discussed in the accompanying Financial Review, which is unaudited, under the headings “Our Operating Environment and Response to Key Opportunities and Challenges” and “Forward-Looking Information and Factors That May Affect Future Results.”
C. Contingencies
We and certain of our subsidiaries are involved in various patent, product liability, consumer, commercial, securities, environmental and tax litigations and claims; government investigations; and other legal proceedings that arise from time to time in the ordinary course of our business. Except for income tax contingencies, we record accruals for contingencies to the extent that we conclude that their occurrence is probable and that the related liabilities are estimable and we record anticipated recoveries under existing insurance contracts when assured of recovery. For tax matters, beginning in 2007 upon the adoption of a new accounting standard, we record accruals for income tax contingencies to the extent that we conclude that a tax position is not sustainable under a ‘more likely than not’ standard and we record our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction when we conclude that the potential recovery is more likely than not. (SeeNote 1D. Significant
Accounting Policies: New Accounting Standards and Note 8E. Taxes on Income: Tax Contingencies.) We consider many factors in making these assessments. Because litigation and other contingencies are inherently unpredictable and excessive verdicts do occur, these assessments can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions (seeNote 1B. Significant Accounting Policies: Estimates and Assumptions).
D. New Accounting Standards
As of January 1, 2007, we adopted the provisions of Financial Accounting Standards Board (FASB) Interpretation No. 48 (FIN 48),Accounting for Uncertainty in Income Taxes, an interpretation of SFAS 109, Accounting for Income Taxes,and supplemented by FASB Financial Staff Position FIN 48-1,Definition of Settlement in FASB Interpretation No. 48, issued May 2, 2007, and changed our policy related to the accounting for income tax contingencies. To understand the cumulative effect of these accounting changes, seeNote 8A. Taxes on Income: Adoption of New Accounting Standard.We continue to account for income tax contingencies using a benefit recognition model. Beginning January 1, 2007, if we consider that a tax position is ‘more likely than not’ of being sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that is greater than 50% likely of being realized upon settlement, presuming that the tax position is examined by the appropriate taxing authority that has full knowledge of all relevant information. Under the benefit recognition model, if our initial assessment fails to result in the recognition of a tax benefit, we regularly monitor our position and subsequently recognize the tax benefit: (i) if there are changes in tax law or analogous case law that sufficiently raise the likelihood of prevailing on the technical merits of the position to more likely than not; (ii) if the statute of limitations expires; or (iii) if there is a completion of an audit resulting in a favorable settlement of that tax year with the appropriate agency. We regularly reevaluate our tax positions based on the results of audits of federal, state and foreign income tax filings, statute of limitations expirations, and changes in tax law that would either increase or decrease the technical merits of a position relative to the more likely than not standard. Liabilities associated with uncertain tax positions are now classified as current only when we expect to pay cash within the next 12 months. Interest and penalties, if any, continue to be recorded inProvision for taxes on incomeand are classified on the balance sheet with the related tax liability. Prior to 2007, our policy had been to account for income tax contingencies based on whether we determined our tax position to be ‘probable’ under current tax law of being sustained, as well as an analysis of potential outcomes under a given set of facts and circumstances. In addition, we previously considered all tax liabilities as current once the associated tax year was under audit.
On December 31, 2006, we adopted the provisions of Statement of Financial Accounting Standards (SFAS) No. 158,Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans (an amendment of Financial Accounting Standards Board (FASB) Statements No. 87, 88, 106 and 132R).SFAS 158 requires us to recognize on our balance sheet the difference between our benefit obligations and any plan assets of our benefit plans. In
2007 Financial Report | 43
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
addition, we are required to recognize as part of other comprehensive income/(expense), net of taxes, gains and losses due to differences between our actuarial assumptions and actual experience (actuarial gains and losses) and any effects on prior service due to plan amendments (prior service costs or credits) that arise during the period and which are not yet recognized as net periodic benefit costs. At adoption date, we recognized the previously unrecognized actuarial gains and losses, prior service costs or credits and net transition amounts withinAccumulated other comprehensive income/(expense),net of tax (seeNote 14. Pension and Postretirement Benefit Plans and Defined Contribution Plans).
On January 1, 2006, we adopted the provisions of SFAS No. 123R,Share-Based Payment, as supplemented by the interpretation provided by SEC Staff Accounting Bulletin (SAB) No. 107, issued in March 2005. (SFAS 123R replaced SFAS 123,Stock-Based Compensation, issued in 1995.) We elected the modified prospective application transition method of adoption and, as such, prior-period financial statements were not restated for this change. Under this method, the fair value of all stock options granted or modified after adoption must be recognized in the consolidated statement of income. Total compensation cost related to nonvested awards not yet recognized, determined under the original provisions of SFAS 123, must also be recognized in the consolidated statement of income. The adoption of SFAS 123R primarily impacted our accounting for stock options (seeNote 16. Share-Based Payments). Prior to January 1, 2006, we accounted for stock options under Accounting Principles Board Opinion (APB) No. 25,Accounting for Stock Issued to Employees, an elective accounting policy permitted by SFAS 123. Under this standard, since the exercise price of our stock options granted is set equal to the market price of Pfizer common stock on the date of the grant, we did not record any expense to the consolidated statement of income related to stock options, unless certain original grant date terms were subsequently modified. However, as required, we disclosed, in the Notes to Consolidated Financial Statements, the pro forma expense impact of the stock option grants as if we had applied the fair-value-based recognition provisions of SFAS 123.
As of December 31, 2005, we adopted the provisions of FASB Interpretation No. 47 (FIN 47),Accounting for Conditional Asset Retirement Obligations (an interpretation of FASB Statement No. 143).FIN 47 clarifies that conditional obligations meet the definition of an asset retirement obligation in SFAS No. 143,Accounting for Asset Retirement Obligations, and therefore should be recognized if their fair value is reasonably estimable. As a result of adopting FIN 47, we recorded a non-cash pre-tax charge of $40 million ($23 million, net of tax). This charge was reported inCumulative effect of a change in accounting principles—net of taxin the fourth quarter of 2005. In accordance with these standards, we record accruals for legal obligations associated with the retirement of tangible long-lived assets, including obligations under the doctrine of promissory estoppel and those that are conditional upon the occurrence of future events. We recognize these obligations using management’s best estimate of fair value.
E. Acquisitions
Our consolidated financial statements and results of operations reflect an acquired business after the completion of the acquisition and are not restated. We account for acquired businesses using the purchase method of accounting, which requires that the assets acquired and the liabilities assumed be recorded at the date of acquisition at their respective fair values. Any excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. Amounts allocated to acquired in-process research and development (IPR&D) are expensed at the date of acquisition. When we acquire net assets that do not constitute a business under U.S. GAAP, no goodwill is recognized.
F. Foreign Currency Translation
For most international operations, local currencies have been determined to be the functional currencies. The effects of converting non-functional currency assets and liabilities into the functional currency are recorded inOther (income)/deductions—net. We translate functional currency assets and liabilities to their U.S. dollar equivalents at rates in effect at the balance sheet date and record these translation adjustments inShareholders’ equity—Accumulated other comprehensive income/(expense). We translate functional currency statement of income amounts at average rates for the period.
For operations in highly inflationary economies, we translate monetary items at rates in effect at the balance sheet date, with translation adjustments recorded inOther (income)/deductions—net, and nonmonetary items at historical rates.
G. Revenues
Revenue Recognition—We record revenues from product sales when the goods are shipped and title passes to the customer. At the time of sale, we also record estimates for a variety of sales deductions, such as sales rebates, discounts and incentives, and product returns. When we cannot reasonably estimate the amount of future product returns, we record revenues when the risk of product return has been substantially eliminated.
Deductions from Revenues—Gross product sales are subject to a variety of deductions that are generally estimated and recorded in the same period that the revenues are recognized.
In the U.S., we record provisions for Medicaid, Medicare and contract rebates based upon our actual experience ratio of rebates paid and actual prescriptions during prior quarters. We apply the experience ratio to the respective period’s sales to determine the rebate accrual and related expense. This experience ratio is evaluated regularly to ensure that the historical trends are as current as practicable. As appropriate, we will adjust the ratio to better match our current experience or our expected future experience. In assessing this ratio, we consider current contract terms, such as changes in formulary status and discount rates.
Outside the U.S., the majority of our rebates are contractual or legislatively mandated and our estimates are based on actual invoiced sales within each period; both of these elements help to reduce the risk of variations in the estimation process. Some European countries base their rebates on the government’s unbudgeted pharmaceutical spending and we use an estimated allocation factor based on historical payments against our actual
44 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
invoiced sales to project the expected level of reimbursement. We obtain third-party information that helps us to monitor the adequacy of these accruals.
Our provisions for chargebacks (primarily reimbursements to wholesalers for honoring contracted prices to third parties) closely approximate actual, as we settle these deductions generally within two to three weeks of incurring the liability.
We record sales allowances as a reduction of revenues at the time the related revenues are recorded or when the allowance is offered, whichever is later. We estimate the cost of our sales incentives based on our historical experience with similar incentive programs.
Our accruals for Medicaid rebates, Medicare rebates, performance-based contract rebates and chargebacks were $1.2 billion as of December 31, 2007, and $1.5 billion as of December 31, 2006.
Taxes collected from customers and remitted to governmental authorities are presented on a net basis; that is, they are excluded from revenues.
Alliances—We have agreements to co-promote pharmaceutical products discovered by other companies. Revenues are earned when our co-promotion partners ship the related product and title passes to their customer. Alliance revenues are primarily based upon a percentage of our co-promotion partners’ net sales. Expenses for selling and marketing these products are included inSelling, informational and administrative expenses.
H. Cost of Sales and Inventories
We value inventories at cost or fair value, if lower. Cost is determined as follows:
| |
• | finished goods and work in process at average actual cost; and |
| |
• | raw materials and supplies at average or latest actual cost. |
| |
I. Selling, Informational and Administrative Expenses
Selling, informational and administrative costs are expensed as incurred. Among other things, these expenses include the costs of marketing, advertising, shipping and handling, information technology and non-plant employee compensation.
Advertising expenses relating to production costs are expensed as incurred and the costs of radio time, television time and space in publications are expensed when the related advertising occurs. Advertising expenses totaled approximately $2.7 billion in 2007, $2.6 billion in 2006 and $2.7 billion in 2005.
J. Research and Development Expenses
Research and development (R&D) costs are expensed as incurred. These expenses include the costs of our proprietary R&D efforts, as well as costs incurred in connection with our third-party collaboration efforts. Before a compound receives regulatory approval, we record milestone payments made by us to third parties under contracted R&D arrangements as expense when the specific milestone has been achieved. Once a compound receives regulatory approval, we record any subsequent milestone payments inIdentifiable intangible assets, less accumulated amortizationand, unless the assets are determined to have an indefinite life, we amortize them evenly over the remaining
agreement term or the expected product life cycle, whichever is shorter.
K. Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
Long-lived assets include:
| |
• | Goodwill—Goodwill represents the excess of the purchase price of an acquired business over the fair value of its net assets. Goodwill is not amortized. |
| |
• | Identifiable intangible assets, less accumulated amortization—These acquired assets are recorded at our cost. Intangible assets with finite lives are amortized evenly over their estimated useful lives. Intangible assets with indefinite lives are not amortized. |
| |
• | Property, plant and equipment, less accumulated depreciation—These assets are recorded at original cost and increased by the cost of any significant improvements after purchase. We depreciate the cost evenly over the assets’ estimated useful lives. For tax purposes, accelerated depreciation methods are used as allowed by tax laws. |
Amortization expense related to acquired intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property are included inAmortization of intangible assetsas they benefit multiple business functions. Amortization expense related to intangible assets that are associated with a single function and depreciation of property, plant and equipment are included inCost of sales, Selling, informational and administrative expenses and Researchanddevelopment expenses,as appropriate.
We review all of our long-lived assets, including goodwill and other intangible assets, for impairment indicators at least annually and we perform detailed impairment testing for goodwill and indefinite-lived assets annually and for all other long-lived assets whenever impairment indicators are present. When necessary, we record charges for impairments of long-lived assets for the amount by which the present value of future cash flows, or some other fair value measure, is less than the carrying value of these assets.
L. Acquisition-Related In-Process Research and Development Charges and Restructuring Charges and Acquisition-Related Costs
When recording acquisitions (seeNote 1E. Significant Accounting Policies: Acquisitions), we immediately expense amounts related to acquired IPR&D inAcquisition-related in-process research and development charges.
We may incur restructuring charges in connection with our cost-reduction initiatives, as well as in connection with acquisitions, when we implement plans to restructure and integrate the acquired operations. For restructuring charges associated with a business acquisition that are identified in the first year after the acquisition date, the related costs are recorded as additional goodwill because they are considered to be liabilities assumed in the acquisition. All other restructuring charges, all integration costs and any charges related to our pre-existing businesses impacted by an acquisition are included inRestructuring charges and acquisition-related costs.
2007 Financial Report | 45
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
M. Cash Equivalents
Cash equivalents include items almost as liquid as cash, such as certificates of deposit and time deposits with maturity periods of three months or less when purchased. If items meeting this definition are part of a larger investment pool, we classify them asShort-term investments.
N. Investments
Realized gains or losses on sales of investments are determined by using the specific identification cost method.
O. Income Tax Contingencies
We are subject to income tax in many jurisdictions and a certain degree of estimation is required in recording the assets and liabilities related to income taxes. For a description of our accounting policy associated with accounting for income tax contingencies, seeNote 1D. Significant Accounting Policies: New Accounting Standards. All of our tax positions are subject to audit by the local taxing authorities in each tax jurisdiction. Tax audits can involve complex issues and the resolution of issues may span multiple years, particularly if subject to negotiation or litigations.
P. Share-Based Payments
Our compensation programs can include share-based payments.
Beginning in 2006, all grants under share-based payment programs are accounted for at fair value and these fair values are generally amortized on an even basis over the vesting terms into Cost of sales, Selling, informational and administrative expensesandResearch and development expenses, as appropriate. In 2005 and earlier years, grants under stock option and performance-contingent share award programs were accounted for using the intrinsic value method.
2. Acquisitions
We are committed to capitalizing on new growth opportunities, a strategy that can include acquisitions of companies, products or technologies. During the three years ended December 31, 2007, 2006 and 2005, we acquired the following:
| |
• | In the first quarter of 2007, we acquired BioRexis Pharmaceutical Corp., (BioRexis) a privately held biopharmaceutical company with a number of diabetes candidates and a novel technology platform for developing new protein drug candidates, and Embrex, Inc., (Embrex) an animal health company that possesses a unique vaccine delivery system known as Inovoject that improves consistency and reliability by inoculating chicks while they are still in the egg. In connection with these and other smaller acquisitions, we recorded $283 million inAcquisition-related in-process research and development charges. |
| |
• | In February 2006, we completed the acquisition of the sanofi-aventis worldwide rights, including patent rights and production technology, to manufacture and sell Exubera, an inhaled form of insulin, and the insulin-production business and facilities located in Frankfurt, Germany, previously jointly owned by Pfizer and sanofi-aventis, for approximately $1.4 billion (including transaction costs). Substantially all assets recorded in connection with this acquisition have now been written off. SeeNote 4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera. Prior to the acquisition, |
| |
| in connection with our collaboration agreement with sanofi-aventis, we recorded a research and development milestone due to us from sanofi-aventis of $118 million ($71 million, after tax) in 2006 inResearch and development expensesupon the approval of Exubera in January 2006 by the U.S. Food and Drug Administration (FDA). |
| |
• | In December 2006, we completed the acquisition of PowderMed Ltd. (PowderMed), a U.K. company which specializes in the emerging science of DNA-based vaccines for the treatment of influenza and chronic viral diseases, and in May 2006, we completed the acquisition of Rinat Neurosciences Corp. (Rinat), a biologics company with several new central-nervous-system product candidates. In 2006, the aggregate cost of these and other smaller acquisitions was approximately $880 million (including transaction costs). In connection with those transactions, we recorded $835 million inAcquisition-related in-process research and development charges. |
| |
• | In September 2005, we completed the acquisition of all of the outstanding shares of Vicuron Pharmaceuticals Inc. (Vicuron), a biopharmaceutical company focused on the development of novel anti-infectives, for approximately $1.9 billion in cash (including transaction costs). In connection with the acquisition, as part of our final purchase price allocation, we recorded $1.4 billion inAcquisition-related in-process research and development charges, and $243 million ofGoodwill, which has been allocated to our Pharmaceutical segment. |
| |
• | In April 2005, we completed the acquisition of Idun Pharmaceuticals Inc. (Idun), a biopharmaceutical company focused on the discovery and development of therapies to control apoptosis, and in August 2005, we completed the acquisition of Bioren Inc. (Bioren), which focuses on technology for optimizing antibodies. In 2005, the aggregate cost of these and other smaller acquisitions was approximately $340 million in cash (including transaction costs). In connection with these transactions, we recorded $262 million inAcquisition-related in-process research and development charges. |
3. Discontinued Operations
We evaluate our businesses and product lines periodically for strategic fit within our operations. Recent activity includes:
| | |
• | In the fourth quarter of 2006, we sold our Consumer Healthcare business for $16.6 billion, and recorded a gain of approximately $10.2 billion ($7.9 billion, net of tax) inGains on sales of discontinued operations—net of taxin the consolidated statement of income for 2006. In 2007, we recorded a loss of approximately $70 million, after-tax, primarily related to the resolution of contingencies, such as purchase price adjustments and product warranty obligations, as well as pension settlements. This business was composed of: |
| |
| o | substantially all of our former Consumer Healthcare segment; |
| | |
| o | other associated amounts, such as purchase-accounting impacts, acquisition-related costs and restructuring and implementation costs related to our cost-reduction initiatives that were previously reported in the Corporate/Other segment; and |
46 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
| | |
| o | certain manufacturing facility assets and liabilities, which were previously part of our Pharmaceutical or Corporate/ Other segment but were included in the sale of our Consumer Healthcare business. The net impact to the Pharmaceutical segment was not significant. |
| | |
| The results of this business are included inIncome from discontinued operations—net of taxfor 2006 and 2005. |
| | |
| Legal title to certain assets and legal control of the business in certain non-U.S. jurisdictions did not transfer to the buyer on the closing date of December 20, 2006, because the satisfaction of specific local requirements was pending. These operations represented a small portion of our former Consumer Healthcare business and all of these transactions have now closed. In order to ensure that the buyer was placed in the same economic position as if the assets, operations and activities of those businesses had been transferred on the same date as the rest of the business, we entered into an agreement that passed the risks and rewards of ownership to the buyer from December 20, 2006. We treated these delayed-close businesses as sold for accounting purposes on December 20, 2006. |
| | |
| We continued during 2007, and we will continue for a period of time, to generate cash flows and to report gross revenues, income and expense activity that are associated with our former Consumer Healthcare business, in continuing operations, although at a substantially reduced level. After the transfer of these activities, these cash flows and the income statement activity reported in continuing operations will be eliminated. The activities that give rise to these impacts are transitional in nature and generally result from agreements that ensure and facilitate the orderly transfer of business operations to the new owner. For example, we entered into a number of transition services agreements that allow the buyer sufficient time to prepare for the transfer of activities and to limit the risk of business disruption. The nature, magnitude and duration of the agreements vary depending on the specific circumstances of the service, location and/or business need. The agreements can include the following: manufacturing and product supply, logistics, customer service, support of financial processes, procurement, human resources, facilities management, data collection and information services. Most of these agreements extend for periods generally less than 24 months, but because of the inherent complexity of manufacturing processes and the risk of product flow disruption, the product supply agreements generally extend up to 36 months. Included in continuing operations for 2007 were the following amounts associated with these transition service agreements that will no longer occur after the full transfer of activities to the new owner:Revenuesof $219 million;Cost of Salesof $194 million;Selling, informational and administrative expensesof $15 million; andOther (income)/deductions—netof $16 million in income. |
| | |
| None of these agreements confers upon us the ability to influence the operating and/or financial policies of the Consumer Healthcare business under its new ownership. |
| |
• | In the third quarter of 2005, we sold the last of three European generic pharmaceutical businesses, which we had included in |
| |
| our Pharmaceutical segment, for 4.7 million euro (approximately $5.6 million). This business became a part of Pfizer in April 2003 in connection with our acquisition of Pharmacia. We recorded a loss of $3 million ($2 million, net of tax) inGains on sales of discontinued operations—net of taxin the consolidated statement of income for 2005. |
| |
• | In the first quarter of 2005, we sold the second of three European generic pharmaceutical businesses, which we had included in our Pharmaceutical segment, for 70 million euro (approximately $93 million). This business became a part of Pfizer in April 2003 in connection with our acquisition of Pharmacia. We recorded a gain of $57 million ($36 million, net of tax) inGains on sales of discontinued operations—net of taxin the consolidated statement of income for 2005. In addition, we recorded an impairment charge of $9 million ($6 million, net of tax) related to the third European generic business inIncome from discontinued operations—net of taxin the consolidated statement of income for 2005. |
The following amounts, primarily related to our former Consumer Healthcare business, which was sold in December 2006 for $16.6 billion, have been segregated from continuing operations and included inDiscontinued operations—net of taxin the consolidated statements of income:
| | | | | | | | | | |
|
|
|
|
|
|
|
|
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
Revenues | | $ | — | | $ | 4,044 | | $ | 3,948 | |
|
|
|
|
|
|
|
|
|
|
|
Pre-tax income/(loss) | | $ | (5 | ) | $ | 643 | | $ | 695 | |
Benefit/(provision) for
taxes(a) | | | 2 | | | (210 | ) | | (244 | ) |
|
|
|
|
|
|
|
|
|
|
|
Income/(loss) from operations
of discontinued businesses—
net of tax | | | (3 | ) | | 433 | | | 451 | |
|
|
|
|
|
|
|
|
|
|
|
Pre-tax gains/(losses) on
sales of discontinued
businesses | | $ | (168 | ) | | 10,243 | | | 77 | |
Benefit/(provision) for taxes(b) | | | 102 | | | (2,363 | ) | | (30 | ) |
|
|
|
|
|
|
|
|
|
|
|
Gains/(losses) on sales of
discontinued businesses—
net of tax | | | (66 | ) | | 7,880 | | | 47 | |
|
|
|
|
|
|
|
|
|
|
|
Discontinued operations—net
of tax | | $ | (69 | ) | $ | 8,313 | | $ | 498 | |
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | Includes a deferred tax expense of nil in 2007, $24 million in 2006 and $25 million in 2005. |
| | |
| (b) | Includes a deferred tax benefit of nil in 2007, $444 million in 2006, and nil in 2005. |
Net cash flows of our discontinued operations from each of the categories of operating, investing and financing activities were not significant.
4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera
In the third quarter of 2007, after an assessment of the financial performance of Exubera, an inhalable form of insulin for the treatment of diabetes, as well as its lack of acceptance by patients, physicians and payers, we decided to exit the product.
2007 Financial Report | 47
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
Our Exubera-related exit plans included working with physicians over a three-month period to transition patients to other treatment options, evaluating redeployment options for colleagues, working with our partners and vendors with respect to transition and exit activities, working with regulators on concluding outstanding clinical trials, implementing an extended transition program for those patients unable to transition to other medications within the three-month period, and exploring asset disposal or redeployment opportunities, as appropriate, among other activities.
As part of this exit plan, in 2007, we paid $135 million to one of our partners in satisfaction of all remaining obligations under existing agreements relating to Exubera and a next generation
insulin (NGI) under development. In addition, in the event that a new partner is selected, we have agreed to transfer our remaining rights and all economic benefits for Exubera and NGI This transfer of our interests would include the transfer of the Exubera New Drug Application and Investigational New Drug Applications and all non-U.S. regulatory filings and applications, continuation of ongoing Exubera clinical trials and certain supply chain transition activities.
Total pre-tax charges for 2007 were $2.8 billion, virtually all of which were recorded in the third quarter. The financial statement line items in which the various charges are recorded and related activity are as follows:
| | | | | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(MILLIONS OF DOLLARS) | | CUSTOMER
RETURNS -
REVENUES | | COST OF
SALES | | SELLING,
INFORMATIONAL
& ADMINISTRATIVE
EXPENSES | | RESEARCH &
DEVELOPMENT
EXPENSES | | TOTAL | | ACTIVITY
THROUGH
DEC. 31, 2007(a) | | ACCRUAL
AS OF
DEC. 31, 2007 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible asset impairment charges(b) | | $ | — | | $ | 1,064 | | $ | 41 | | $ | — | | $ | 1,105 | | $ | 1,105 | | $ | — | |
Inventory write-offs | | | — | | | 661 | | | — | | | — | | | 661 | | | 661 | | | — | |
Fixed assets impairment charges and other | | | — | | | 451 | | | — | | | 3 | | | 454 | | | 454 | | | — | |
Other exit costs | | | 10 | | | 427 | | | 44 | | | 97 | | | 578 | | | 164 | | | 414 | (c) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | $ | 10 | | $ | 2,603 | | $ | 85 | | $ | 100 | | $ | 2,798 | | $ | 2,384 | | $ | 414 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | Includes adjustments for foreign currency translation. |
| | |
| (b) | Amortization of these assets had previously been recorded inCost of salesandSelling, informational and administrative expenses. |
| | |
| (c) | Included inOther current liabilities($375 million) andOther noncurrent liabilities($39 million). |
The asset write-offs (intangibles, inventory and fixed assets) represent non-cash charges. The other exit costs, primarily severance, contract and other termination costs, as well as other liabilities, are associated with marketing and research programs, and manufacturing operations related to Exubera. These exit costs resulted in cash expenditures in 2007 (such as the $135 million settlement referred to above) and will result in additional cash expenditures in 2008. We expect that substantially all of the cash spending will be completed within the next year. As a result of exiting this product, certain additional cash costs will be incurred and reported in future periods, such as maintenance-level operating costs. However, those future costs are not expected to be significant. We expect that substantially all exit activities will be completed within the next year.
5. Cost-Reduction Initiatives
In the first quarter of 2005, we launched cost-reduction initiatives to increase efficiency and streamline decision-making across the company. These initiatives, announced in April 2005 and broadened in October 2006 and January 2007, follow the integration of Warner-Lambert and Pharmacia.
We incurred the following costs in connection with our cost-reduction initiatives:
| | | | | | | | | | |
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | 2006 | | 2005 | |
|
|
|
|
|
|
| |
Implementation costs(a) | | $ | 1,389 | | $ | 788 | | $ | 325 | |
Restructuring charges(b) | | | 2,523 | | | 1,296 | | | 438 | |
|
|
|
|
|
|
|
|
|
|
|
Total costs related to our
cost-reduction initiatives | | $ | 3,912 | | $ | 2,084 | | $ | 763 | |
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | For 2007, included inCost of sales($700 million),Selling, informational and administrative expenses($334 million),Research and development expenses($416 million) and inOther (income)/deductions—net($61 million income). For 2006, included inCost of sales($392 million),Selling, informational and administrative expenses($243 million),Research and development expenses($176 million), and inOther (income)/deductions—net($23 million income). For 2005, included inCost of sales($124 million), Selling, informational and administrative expenses($151 million), andResearch and development expenses($50 million). |
| | |
| (b) | Included inRestructuring charges and acquisition-related costs. |
From the beginning of the cost-reduction initiatives in 2005, through December 31, 2007, the restructuring charges primarily relate to our plant network optimization efforts and the restructuring of our worldwide sales, marketing and research and development operations, while the implementation costs primarily relate to accelerated depreciation of certain assets, as well as system and process standardization and the expansion of shared services.
48 | 2007 Financial Report
|
Notes to Consolidated Financial Statements |
Pfizer Inc and Subsidiary Companies |
|
|
The components of restructuring charges associated with our cost-reduction initiatives follow:
| | | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | ACTIVITY
THROUGH
DEC. 31, | | ACCRUAL
AS OF
DEC. 31, | |
| | | | | | | | | | | | | | | |
| | COSTS INCURRED | | | |
| |
| |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | | TOTAL | | 2007(a) | | 2007(b) | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee termination costs | | $ | 2,034 | | | $ | 809 | | $ | 303 | | $ | 3,146 | | $ | 1,957 | | $ | 1,189 | |
Asset impairments | | | 260 | | | | 368 | | | 122 | | | 750 | | | 750 | | | — | |
Other | | | 229 | | | | 119 | | | 13 | | | 361 | | | 261 | | | 100 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total | | $ | 2,523 | | | $ | 1,296 | | $ | 438 | | $ | 4,257 | | $ | 2,968 | | $ | 1,289 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Includes adjustments for foreign currency translation. |
|
| (b) | Included inOther current liabilities($1.1 billion) andOther noncurrent liabilities($186 million). |
From the beginning of the cost-reduction initiatives in 2005, through December 31, 2007,Employee termination costsrepresent the expected reduction of the workforce by approximately 20,800 employees, mainly in research, manufacturing and sales. As of December 31, 2007, approximately 13,000 of these employees have been formally terminated.Employee termination costsare recorded when the actions are probable and estimable and include accrued severance benefits, pension and postretirement benefits.Asset impairmentsprimarily include charges to write down property, plant and equipment.Otherprimarily includes costs to exit certain activities.
6. Acquisition-Related Costs
We recorded inRestructuring charges and acquisition-related costs$11 million in 2007, $27 million in 2006 and $918 million in 2005, for acquisition-related costs. Amounts in 2005 were primarily related to our acquisition of Pharmacia on April 16, 2003, and included integration costs of $543 million and restructuring charges of $375 million. As of December 31, 2007, virtually all restructuring charges incurred have been utilized.
Integration costs represent external, incremental costs directly related to an acquisition, including expenditures for consulting and systems integration. Restructuring charges can include severance, costs of vacating duplicative facilities, contract termination and other exit costs.
7. Other (Income)/Deductions—Net
The components ofOther (income)/deductions—netfollow:
| | | | | | | | | | | |
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Interest income | | $ | (1,496 | ) | | $ | (925 | ) | $ | (740 | ) |
Interest expense | | | 440 | | | | 517 | | | 488 | |
Interest expense capitalized | | | (43 | ) | | | (29 | ) | | (17 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Net interest income(a) | | | (1,099 | ) | | | (437 | ) | | (269 | ) |
Asset impairment charges(b) | | | — | | | | 320 | | | 1,159 | |
Royalty income | | | (224 | ) | | | (395 | ) | | (320 | ) |
Net gains on asset disposals(c) | | | (326 | ) | | | (280 | ) | | (172 | ) |
Other, net | | | (110 | ) | | | (112 | ) | | (1 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Other (income)/deductions—net | | $ | (1,759 | ) | | $ | (904 | ) | $ | 397 | |
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | The increase in net interest income in 2007 compared to 2006 is due primarily to higher net financial assets during 2007 compared to 2006, reflecting proceeds of $16.6 billion from the sale of our Consumer Healthcare business in late December 2006, and higher interest rates. |
| | |
| (b) | In 2006, we recorded a charge of $320 million related to the impairment of our Depo-Provera intangible asset, for which amortization expense is included inAmortization of intangible assets. In 2005, we recorded charges totaling $1.2 billion, primarily related to the impairment of our Bextra intangible asset, for which amortization expense had previously been recorded inAmortization of intangible assets. SeeNote 13B. Goodwill and Other Intangible Assets: Other Intangible Assets. |
| | |
| (c) | In 2007, includes a gain of $211 million related to the sale of a building in Korea. In 2007, gross realized gains were $8 million and gross realized losses were nil on sales of available-for-sale securities. In 2006, gross realized gains were $65 million and gross realized losses were $1 million on sales of available-for-sale securities.In 2005, gross realized gains were $171 million and gross realized losses were $14 million on sales of available-for-sale securities. Proceeds from the sale of available-for-sale securities were $663 million in 2007, $79 million in 2006 and $2.8 billion in 2005. |
8. Taxes on Income
A. Adoption of New Accounting Standard
As of January 1, 2007, we adopted the provisions of FIN 48,Accounting for Uncertainty in Income Taxes, an interpretation of SFAS 109, Accounting for Income Taxes, as supplemented by FASB Financial Staff Position FIN 48-1,Definition of Settlement in FASB Interpretation No. 48, issued May 2, 2007. SeeNote 1D. Significant Accounting Policies: New Accounting Standardsfor a full description of our accounting policy related to the accounting for income tax contingencies. As a result of the implementation of FIN 48, at the date of adoption, we reduced our existing liabilities for uncertain tax positions by approximately $11 million. This has been recorded as a direct adjustment to the opening balance ofRetained earningsand it changed the classification of virtually all amounts associated with uncertain tax positions of approximately $4.0 billion, including the associated accrued interest of approximately $780 million, from current to noncurrent. (SeeNote 8E. Taxes on Income: Tax Contingencies.)
2007 Financial Report | 49
|
Notes to Consolidated Financial Statements |
Pfizer Inc and Subsidiary Companies |
|
|
B. Taxes on Income
Income from continuing operations before provision for taxes on income, minority interests and the cumulative effect of a change in accounting principlesconsists of the following:
| | | | | | | | | | | |
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
United States | | $ | 242 | | | $ | 3,266 | | $ | 985 | |
International | | | 9,036 | | | | 9,762 | | | 9,815 | |
|
|
|
|
|
|
|
|
|
|
| |
Total income from continuing operations before provision for taxes on income, minority interests and cumulative effect of a change in accounting principles | | $ | 9,278 | | | $ | 13,028 | | $ | 10,800 | |
|
|
|
|
|
|
|
|
|
|
| |
The decrease in domestic income from continuing operations before taxes in 2007 compared to 2006 is due primarily to the volume and geographic mix of product sales and restructuring charges in 2007 compared to 2006, as well as the impact of charges associated with Exubera (seeNote 4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera), partially offset by lower IPR&D charges in 2007 of $283 million, primarily related to our acquisitions of Biorexis and Embrex, compared to IPR&D charges in 2006 of $835 million, primarily related to our acquisitions of Rinat and PowderMed.
The increase in domestic income from continuing operations before taxes in 2006 compared to 2005 is due primarily to IPR&D charges in 2005 of $1.7 billion, primarily related to our acquisitions of Vicuron and Idun, the Bextra impairment and changes in product mix, among other factors, partially offset by IPR&D charges recorded in 2006 of $835 million, primarily related to our acquisitions of Rinat and PowderMed, and a 2006 charge of $320 million related to the impairment of the Depo-Provera intangible asset.
The provision for taxes on income from continuing operations before minority interests and the cumulative effect of a change in accounting principles consists of the following:
| | | | | | | | | | | |
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
United States: | | | | | | | | | | | |
Taxes currently payable: | | | | | | | | | | | |
Federal | | $ | 1,393 | | | $ | 1,399 | | $ | 2,572 | |
State and local | | | 243 | | | | 205 | | | 108 | |
Deferred income taxes | | | (1,986 | ) | | | (1,371 | ) | | (1,295 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Total U.S. tax (benefit)/provision | | | (350 | ) | | | 233 | | | 1,385 | |
|
|
|
|
|
|
|
|
|
|
| |
International: | | | | | | | | | | | |
Taxes currently payable | | | 2,175 | | | | 1,913 | | | 1,963 | |
Deferred income taxes | | | (802 | ) | | | (154 | ) | | (170 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Total international tax provision | | | 1,373 | | | | 1,759 | | | 1,793 | |
|
|
|
|
|
|
|
|
|
|
| |
Total provision for taxes on income(a) | | $ | 1,023 | | | $ | 1,992 | | $ | 3,178 | |
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Excludes federal, state and international expense of approximately $1 million in 2007, a benefit of $119 million in 2006 and a benefit of $127 million in 2005, primarily related to the resolution of certain tax positions related to Pharmacia, which were debited or credited toGoodwill, as appropriate. |
In 2006, we were notified by the Internal Revenue Service (IRS) Appeals Division that a resolution had been reached on the matter that we were in the process of appealing related to the tax deductibility of an acquisition-related breakup fee paid by the Warner-Lambert Company in 2000. As a result, we recorded a tax benefit of approximately $441 million related to the resolution of this issue (seeNote 8E. Taxes on Income: Tax Contingencies). Also in 2006, we recorded a decrease to the 2005 estimated U.S. tax provision related to the repatriation of foreign earnings, due primarily to the receipt of information that raised our assessment of the likelihood of prevailing on the technical merits of a certain position, and we recognized a tax benefit of $124 million. Additionally, in 2006, the IRS issued final regulations on Statutory Mergers and Consolidations, which impacted certain prior-period transactions, and we recorded a tax benefit of $217 million, reflecting the total impact of these regulations.
In 2005, we recorded an income tax charge of $1.7 billion, included inProvision for taxes on income, in connection with our decision to repatriate approximately $37 billion of foreign earnings in accordance with theAmerican Jobs Creation Act of 2004(the Jobs Act). The Jobs Act created a temporary incentive for U.S. corporations to repatriate accumulated income earned abroad by providing an 85% dividend-received deduction for certain dividends from controlled foreign corporations, subject to various limitations and restrictions including qualified U.S. reinvestment of such earnings. In addition, in 2005, we recorded a tax benefit of $586 million related to the resolution of certain tax positions (seeNote 8E. Taxes on Income: Tax Contingencies).
Amounts reflected in the preceding tables are based on the location of the taxing authorities. As of December 31, 2007, we have not made a U.S. tax provision on approximately $60 billion of unremitted earnings of our international subsidiaries. As of December 31, 2007, these earnings are intended to be permanently reinvested overseas. Because of the complexity, it is not practical to compute the estimated deferred tax liability on these permanently reinvested earnings.
C. Tax Rate Reconciliation
Reconciliation of the U.S. statutory income tax rate to our effective tax rate for continuing operations before the cumulative effect of a change in accounting principles follows:
| | | | | | | | | | | |
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
| | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
U.S. statutory income tax rate | | | 35.0 | % | | | 35.0 | % | | 35.0 | % |
Earnings taxed at other than U.S. statutory rate | | | (21.6 | ) | | | (15.7 | ) | | (20.6 | ) |
Resolution of certain tax positions | | | — | | | | (3.4 | ) | | (5.4 | ) |
Tax legislation impact | | | — | | | | (1.7 | ) | | — | |
U.S. research tax credit and manufacturing deduction | | | (1.5 | ) | | | (0.5 | ) | | (0.8 | ) |
Repatriation of foreign earnings | | | — | | | | (1.0 | ) | | 15.4 | |
Acquired IPR&D | | | 1.1 | | | | 2.2 | | | 5.4 | |
All other—net | | | (2.0 | ) | | | 0.4 | | | 0.4 | |
|
|
|
|
|
|
|
|
|
|
| |
Effective tax rate for income from continuing operations before cumulative effect of a change in accounting principles | | | 11.0 | % | | | 15.3 | % | | 29.4 | % |
|
|
|
|
|
|
|
|
|
|
| |
50 | 2007 Financial Report
|
Notes to Consolidated Financial Statements |
Pfizer Inc and Subsidiary Companies |
|
|
We operate manufacturing subsidiaries in Puerto Rico, Ireland and Singapore. We benefit from Puerto Rican incentive grants that expire between 2017 and 2027. Under the grants, we are partially exempt from income, property and municipal taxes. Under Section 936 of the U.S. Internal Revenue Code, Pfizer was a “grandfathered” entity and was entitled to the benefits under such statute until September 30, 2006. In Ireland, we benefit from an incentive tax rate effective through 2010 on income from manufacturing operations. In Singapore, we benefit from incentive tax rates effective through 2031 on income from manufacturing operations.
The U.S. research tax credit was effective through December 31, 2007. For a discussion about the repatriation of foreign earnings and the tax legislation impact, seeNote 8B. Taxes on Income: Taxes on Income. For a discussion about the resolution of certain tax positions, seeNote 8E. Taxes on Income: Tax Contingencies. The charges for acquired IPR&D in 2007, 2006 and 2005 are not deductible.
D. Deferred Taxes
Deferred taxes arise because of different timing treatment between financial statement accounting and tax accounting, known as “temporary differences.” We record the tax effect of these temporary differences as “deferred tax assets” (generally items that can be used as a tax deduction or credit in future periods) or “deferred tax liabilities” (generally items for which we received a tax deduction, but that have not yet been recorded in the consolidated statement of income).
The tax effect of the major items recorded as deferred tax assets and liabilities, shown before jurisdictional netting, as of December 31, is as follows:
| | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
| |
| | 2007
DEFERRED TAX | | | 2006
DEFERRED TAX | |
| |
| | |
| |
(MILLIONS OF DOLLARS) | | ASSETS | | (LIABILITIES) | | | ASSETS | | (LIABILITIES) | |
|
|
|
|
|
|
|
|
|
| |
Prepaid/deferred items | | $ | 1,315 | | $ | (431 | ) | | $ | 1,164 | | $ | (312 | ) |
Intangibles | | | 897 | | | (6,737 | ) | | | 841 | | | (7,704 | ) |
Property, plant and equipment | | | 300 | | | (957 | ) | | | 104 | | | (1,105 | ) |
Employee benefits | | | 2,552 | | | (740 | ) | | | 3,141 | | | (804 | ) |
Restructurings and other charges | | | 717 | | | (11 | ) | | | 573 | | | (19 | ) |
Net operating loss/credit carryforwards | | | 1,842 | | | — | | | | 1,061 | | | — | |
Unremitted earnings | | | — | | | (3,550 | ) | | | — | | | (3,567 | ) |
State and local tax
adjustments(a) | | | 529 | | | — | | | | — | | | — | |
All other | | | 848 | | | (37 | ) | | | 912 | | | (392 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Subtotal | | | 9,000 | | | (12,463 | ) | | | 7,796 | | | (13,903 | ) |
Valuation allowance | | | (158 | ) | | — | | | | (194 | ) | | — | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total deferred taxes | | $ | 8,842 | | $ | (12,463 | ) | | $ | 7,602 | | $ | (13,903 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net deferred tax liability | | | | | $ | (3,621 | ) | | | | | $ | (6,301 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Reclassified as a result of the adoption of a new accounting standard. |
The reduction in the net deferred tax liability position in 2007 compared to 2006 is primarily due to amortization of deferred tax liabilities related to identifiable intangibles in connection with our acquisition of Pharmacia in 2003, partially offset by an increase in noncurrent deferred tax assets related to the impairment of Exubera. (SeeNote 4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera.)
We have carryforwards primarily related to foreign tax credit carryovers and net operating losses, which are available to reduce future U.S. federal and state, as well as international, income with either an indefinite life or expiring at various times between 2008 and 2026. Certain of our U.S. net operating losses are subject to limitations under Internal Revenue Code Section 382.
Valuation allowances are provided when we believe that our deferred tax assets are not recoverable, based on an assessment of estimated future taxable income that incorporates ongoing, prudent, feasible tax planning strategies.
Deferred tax assets and liabilities in the preceding table, netted by taxing jurisdiction, are in the following captions in our consolidated balance sheets:
| | | | | | | | |
|
|
|
|
|
| |
| | AS OF DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | |
|
|
|
|
|
| |
Current deferred tax asset(a) | | $ | 1,664 | | | $ | 1,384 | |
Noncurrent deferred tax assets(b) | | | 2,441 | | | | 354 | |
Current deferred tax liability(c) | | | (30 | ) | | | (24 | ) |
Noncurrent deferred tax liability(d) | | | (7,696 | ) | | | (8,015 | ) |
|
|
|
|
|
|
|
| |
Net deferred tax liability | | $ | (3,621 | ) | | $ | (6,301 | ) |
|
|
|
|
|
|
|
| |
| | |
| (a) | Included inPrepaid expenses and taxes. |
| | |
| (b) | Included inOther assets, deferred taxes and deferred charges. |
| | |
| (c) | Included inOther current liabilities. |
| | |
| (d) | Included inDeferred taxes. |
E. Tax Contingencies
We are subject to income tax in many jurisdictions and a certain degree of estimation is required in recording the assets and liabilities related to income taxes. For a description of our accounting policy associated with accounting for income tax contingencies, seeNote 1D. Significant Accounting Policies: New Accounting Standards. All of our tax positions are subject to audit by the local taxing authorities in each tax jurisdiction. Tax audits can involve complex issues and the resolution of issues may span multiple years, particularly if subject to negotiation or litigation.
The United States is one of our major tax jurisdictions and the IRS is currently conducting audits of the Pfizer Inc. tax returns for the years 2002, 2003 and 2004. The 2005, 2006 and 2007 tax years are also currently under audit as part of the IRS Compliance Assurance Process (CAP), a real-time audit process. All other tax years in the U.S. for Pfizer Inc. are closed under the statute of limitations. With respect to Pharmacia Corporation, the IRS is currently conducting an audit for the year 2003 through the date of merger with Pfizer (April 16, 2003). In addition to the open audit years in the U.S., we have open audit years in other major tax jurisdictions, such as Canada (1998-2006), Japan (2006), Europe (1996-2006, primarily reflecting Ireland, the U.K., France, Italy, Spain and Germany), and Puerto Rico (2003-2006).
2007 Financial Report | 51
|
Notes to Consolidated Financial Statements |
Pfizer Inc and Subsidiary Companies |
|
|
We regularly reevaluate our tax positions based on the results of audits of federal, state and foreign income tax filings, statute of limitations expirations, and changes in tax law that would either increase or decrease the technical merits of a position relative to the more likely than not standard. We believe that our accruals for tax liabilities are adequate for all open years. Many factors are considered in making these evaluations, including past history, recent interpretations of tax law, and the specifics of each matter. Because tax regulations are subject to interpretation and tax litigation is inherently uncertain, these evaluations can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions (seeNote 1B. Significant Accounting Policies: Estimates and Assumptions). Our evaluations are based on estimates and assumptions that have been deemed reasonable by management. However, if our estimates and assumptions are not representative of actual outcomes, our results could be materially impacted.
Because tax law is complex and often subject to varied interpretations, it is uncertain whether some of our tax positions will be sustained upon audit. The amounts associated with uncertain tax positions in 2007 are as follows:
| | | | | | | | |
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | DEC. 31,
2007 | | | JAN. 1,
2007 | |
|
|
|
|
|
| |
Noncurrent deferred tax assets(a) | | $ | 529 | | | $ | 395 | |
Other tax assets(a) | | | 890 | | | | 647 | |
Income taxes payable(b)(c) | | | (408 | ) | | | (47 | ) |
Other taxes payable(b) | | | (6,246 | ) | | | (4,962 | ) |
|
|
|
|
|
|
|
| |
Total amounts associated with uncertain tax positions | | $ | (5,235 | ) | | $ | (3,967 | ) |
|
|
|
|
|
|
|
| |
| | |
| (a) | Included inOther assets, deferred taxes and deferred charges. |
| | |
| (b) | Includes gross accrued interest. Accrued penalties are not significant. |
| | |
| (c) | As of December 31, 2007, included inIncome taxes payable($358 million) andPrepaid expenses and taxes($50 million) As of December 31, 2006, included inIncome taxes payable($47 million). |
Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the amounts taken or expected to be taken in a tax return because of the uncertainties described above. These unrecognized tax benefits relate primarily to issues common among multinational corporations. Substantially all of these unrecognized tax benefits, if recognized, would impact our effective income tax rate.
Tax assets associated with uncertain tax positions represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction.
A reconciliation of the beginning and ending amounts of gross unrecognized tax benefits and accrued interest is as follows:
| | | | |
|
|
|
| |
(MILLIONS OF DOLLARS) | | | | |
|
|
|
| |
Balance as of January 1, 2007 | | $ | (5,009 | ) |
Increases based on tax positions taken during a prior period | | | (80 | ) |
Increases based on tax positions taken during the current period | | | (1,089 | ) |
Increases primarily related to currency translation adjustments | | | (191 | ) |
Decreases related to settlements with taxing authorities | | | 32 | |
Decreases as a result of a lapse of the applicable statute of limitations | | | 14 | |
Increases in accrued interest due to the passage of time | | | (331 | ) |
|
|
|
| |
Balance as of December 31, 2007(a) | | $ | (6,654 | ) |
|
|
|
| |
| | |
| (a) | Included inIncome taxes payable($358 million),Prepaid expenses and taxes($50 million) andOther taxes payable($6.2 billion). |
If our estimates of unrecognized tax benefits and potential tax benefits are not representative of actual outcomes, our financial statements could be materially affected in the period of settlement or when the statutes of limitations expire, as we treat these events as discrete items in the period of resolution. Finalizing audits with the relevant taxing authorities can include formal administrative and legal proceedings and, as a result, it is difficult to estimate the timing and range of possible change related to our uncertain tax positions. However, any settlements or statute expirations would likely result in a significant decrease in our uncertain tax positions. We estimate that within the next 12 months, our gross uncertain tax positions could decrease by as much as $800 million, as a result of the settlement of issues common to multinational corporations or the expiration of the statute of limitations in multiple jurisdictions.
52 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
9. Other Comprehensive Income/(Expense) | | | | | | | | | | |
|
Changes, net of tax, in accumulated other comprehensive income/(expense) follow: | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | | | | NET UNREALIZED GAINS/(LOSSES) | | BENEFIT PLANS | | | | |
| | | | |
| |
| | ACCUMULATED | |
| | CURRENCY | | | | | | | | | | | | | | | | | OTHER | |
| | TRANSLATION | | DERIVATIVE | | AVAILABLE- | | | | PRIOR SERVICE | | MINIMUM | | COMPREHENSIVE | |
| | ADJUSTMENT | | FINANCIAL | | FOR-SALE | | ACTUARIAL | | (COSTS)/CREDITS | | PENSION | | INCOME/ | |
(MILLIONS OF DOLLARS) | | AND OTHER | | INSTRUMENTS | | SECURITIES | | GAINS/(LOSSES) | | AND OTHER | | LIABILITY | | (EXPENSE) | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, January 1, 2005 | | $ | 2,594 | | $ | (1 | ) | $ | 266 | | $ | — | | $ | — | | $ | (581 | ) | $ | 2,278 | |
Other comprehensive expense: | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustments | | | (1,476 | ) | | — | | | — | | | — | | | — | | | — | | | (1,476 | ) |
Unrealized holding losses | | | — | | | (148 | ) | | (68 | ) | | — | | | — | | | — | | | (216 | ) |
Reclassification adjustments to income | | | — | | | (11 | ) | | (157 | ) | | — | | | — | | | — | | | (168 | ) |
Other | | | (5 | ) | | — | | | — | | | — | | | — | | | (33 | ) | | (38 | ) |
Income taxes | | | — | | | 53 | | | 42 | | | — | | | — | | | 4 | | | 99 | |
| | | | | | | | | | | | | | | | | | | |
|
| |
| | | | | | | | | | | | | | | | | | | | | (1,799 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2005 | | | 1,113 | | | (107 | ) | | 83 | | | — | | | — | | | (610 | ) | | 479 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustments | | | 1,157 | | | — | | | — | | | — | | | — | | | — | | | 1,157 | |
Unrealized holding gains | | | — | | | 126 | | | 63 | | | — | | | — | | | — | | | 189 | |
Reclassification adjustments to income(a) | | | (40 | ) | | 5 | | | (64 | ) | | — | | | — | | | — | | | (99 | ) |
Other | | | (3 | ) | | — | | | — | | | — | | | — | | | (16 | ) | | (19 | ) |
Income taxes | | | — | | | (50 | ) | | 14 | | | — | | | — | | | — | | | (36 | ) |
| | | | | | | | | | | | | | | | | | | |
|
| |
| | | | | | | | | | | | | | | | | | | | | 1,192 | |
| | | | | | | | | | | | | | | | | | | |
|
| |
Adoption of new accounting standard, net of tax(b) | | | — | | | — | | | — | | | (2,739 | ) | | (27 | ) | | 626 | | | (2,140 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2006 | | | 2,227 | | | (26 | ) | | 96 | | | (2,739 | ) | | (27 | ) | | — | | | (469 | ) |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustments | | | 1,735 | | | — | | | — | | | — | | | — | | | — | | | 1,735 | |
Unrealized holding losses | | | — | | | 3 | | | (43 | ) | | — | | | — | | | — | | | (40 | ) |
Reclassification adjustments to income(a) | | | (96 | ) | | 3 | | | (8 | ) | | — | | | — | | | — | | | (101 | ) |
Actuarial gains and other benefit plan items | | | — | | | — | | | — | | | 1,374 | | | 11 | | | — | | | 1,385 | |
Amortization of actuarial losses and other benefit plan items | | | — | | | — | | | — | | | 248 | | | 7 | | | — | | | 255 | |
Curtailments and settlements—net | | | — | | | — | | | — | | | 268 | | | (5 | ) | | — | | | 263 | |
Other | | | 6 | | | — | | | — | | | (62 | ) | | (6 | ) | | — | | | (62 | ) |
Income taxes | | | — | | | (12 | ) | | 9 | | | (656 | ) | | (8 | ) | | — | | | (667 | ) |
| | | | | | | | | | | | | | | | | | | |
|
| |
| | | | | | | | | | | | | | | | | | | | | 2,768 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2007 | | $ | 3,872 | | $ | (32 | ) | $ | 54 | | $ | (1,567 | ) | $ | (28 | ) | $ | — | | $ | 2,299 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| |
(a) | The currency translation adjustments reclassified to income result from the sale of businesses. |
| |
(b) | Includes pre-tax amounts forActuarial lossesof $4.3 billion andPrior service costs (credits) and otherof $27 million. See alsoNote 14. Pension and Postretirement Benefit Plans and Defined Contribution Plans. |
Income taxes are not provided for foreign currency translation relating to permanent investments in international subsidiaries.
As of December 31, 2007, we estimate that we will reclassify into 2008 income the following pre-tax amounts currently held inAccumulated other comprehensive income/(expense): virtually all of the unrealized holding losses on derivative financial instruments; $138 million ofActuarial gains/(losses)related to benefit plan obligations and plan assets; and $3 million ofPrior service (costs)/credits and otherrelated primarily to benefit plan amendments.
2007 Financial Report | 53
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
| | | | | | | | |
10. Financial Instruments |
A. Investments in Debt and Equity Securities |
|
Information about our investments as of December 31 follows: |
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | |
|
|
|
|
|
| |
Trading investments(a) | | $ | 256 | | | $ | 273 | |
|
|
|
|
|
| |
Amortized cost and fair value of available-for-sale debt securities:(b) | | | | | | | | |
Western European and other government debt | | | 10,848 | | | | 1,606 | |
Corporate debt | | | 6,579 | | | | 8,582 | |
Western European and other government agency debt | | | 4,277 | | | | 4 | |
Supranational debt | | | 1,892 | | | | 460 | |
Corporate asset-backed securities | | | 490 | | | | 700 | |
Certificates of deposit | | | 117 | | | | 45 | |
|
|
|
|
|
|
|
| |
Total available-for-sale debt securities | | | 24,203 | | | | 11,397 | |
|
|
|
|
|
|
|
| |
Amortized cost and fair value of held-to-maturity debt securities:(b) | | | | | | | | |
Certificates of deposit and other | | | 2,609 | | | | 1,189 | |
|
|
|
|
|
|
|
| |
Total held-to-maturity debt securities | | | 2,609 | | | | 1,189 | |
|
|
|
|
|
|
|
| |
Available-for-sale money market fund: | | | | | | | | |
Investing in U.S. government and its agencies’ or instrumentalities’ securities and reverse repurchase agreements involving all of the same investments held | | | 172 | | | | 2,885 | |
Available-for-sale money market fund: | | | | | | | | |
Investing in U.S. government and its agencies’ securities, U.S. and foreign corporate commercial paper, bank deposits, asset-backed securities and reverse repurchase agreements involving virtually all of the same investments held | | | — | | | | 12,300 | |
Available-for-sale money market fund: | | | | | | | | |
Investing in U.S. government securities and reverse repurchase agreements involving U.S. government securities | | | — | | | | 1,246 | |
Available-for-sale money market fund: | | | | | | | | |
Other | | | 125 | | | | 115 | |
|
|
|
|
|
|
|
| |
Total available-for-sale money market funds | | | 297 | | | | 16,546 | |
|
|
|
|
|
|
|
| |
Cost of available-for-sale equity securities, excluding money market funds | | | 202 | | | | 202 | |
Gross unrealized gains | | | 127 | | | | 170 | |
Gross unrealized losses | | | (13 | ) | | | (1 | ) |
|
|
|
|
|
|
|
| |
Fair value of available-for-sale equity securities, excluding money market funds | | | 316 | | | | 371 | |
|
|
|
|
|
|
|
| |
Total fair value of available-for-sale equity securities | | | 613 | | | | 16,917 | |
|
|
|
|
|
|
|
| |
Total investments | | $ | 27,681 | | | $ | 29,776 | |
|
|
|
|
|
|
|
| |
| |
(a) | Trading investments are held in trust for legacy Pharmacia severance benefits. |
| |
(b) | Gross unrealized gains and losses are not significant. |
| | | | | | | | |
These investments are in the following captions in the consolidated balance sheets as of December 31: |
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | |
|
|
|
|
|
|
|
| |
Cash and cash equivalents | | $ | 2,467 | | | $ | 1,118 | |
Short-term investments | | | 22,069 | | | | 25,886 | |
Long-term investments and loans | | | 3,145 | | | | 2,772 | |
|
|
|
|
|
|
|
| |
Total investments | | $ | 27,681 | | | $ | 29,776 | |
|
|
|
|
|
|
|
| |
| | | | | | | | | | | | | | | | |
The contractual maturities of the available-for-sale and held-to-maturity debt securities as of December 31, 2007, follow: | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | YEARS | | | | |
| |
| | | | |
(MILLIONS OF DOLLARS) | | WITHIN 1 | | OVER 1
TO 5 | | OVER 5
TO 10 | | OVER
10 | | | TOTAL | |
|
|
|
|
|
|
|
|
|
|
|
| |
Available-for-sale debt securities: | | | | | | | | | | | | | | | | |
Western European and other government debt | | $ | 10,753 | | $ | 95 | | $ | — | | $ | — | | $ | 10,848 | |
Corporate debt | | | 5,287 | | | 1,292 | | | — | | | — | | | 6,579 | |
Western European and other government agency debt | | | 3,497 | | | 780 | | | — | | | — | | | 4,277 | |
Supranational debt | | | 1,849 | | | 43 | | | — | | | — | | | 1,892 | |
Corporate asset-backed securities | | | 133 | | | 357 | | | — | | | — | | | 490 | |
Certificates of deposit | | | 116 | | | 1 | | | — | | | — | | | 117 | |
Held-to-maturity debt securities: | | | | | | | | | | | | | | | | |
Certificates of deposit and other | | | 2,604 | | | — | | | — | | | 5 | | | 2,609 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total debt securities | | $ | 24,239 | | $ | 2,568 | | $ | — | | $ | 5 | | $ | 26,812 | |
Trading investments | | | | | | | | | | | | | | | 256 | |
Available-for-sale money market funds | | | | | | | | | | | | | | | 297 | |
Available-for-sale equity securities | | | | | | | | | | | | | | | 316 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total investments | | | | | | | | | | | | | | $ | 27,681 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
On an ongoing basis, we evaluate our investments in debt and equity securities to determine if a decline in fair value is other-than-temporary. When a decline in fair value is determined to be other-than-temporary, an impairment charge is recorded and a new cost basis in the investment is established. The aggregate cost and related unrealized losses related to non-traded equity investments are not significant.
B. Short-Term Borrowings
Short-term borrowings include amounts for commercial paper of $4.4 billion as of December 31, 2007, and $1.6 billion as of December 31, 2006. The weighted-average effective interest rate on short-term borrowings outstanding was 3.4% as of December 31, 2007, and 3.0% as of December 31, 2006.
As of December 31, 2007, we had access to $3.7 billion of lines of credit, of which $1.5 billion expire within one year. Of these lines of credit, $3.6 billion are unused, of which our lenders have committed to loan us $2.1 billion at our request. $2.0 billion of the unused lines of credit, which expire in 2012, may be used to support our commercial paper borrowings.
54 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | |
C. Long-Term Debt | | | | | | | | | | | |
Information about our long-term debt as of December 31 follows: |
|
|
|
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | MATURITY DATE | | 2007 | | | 2006 | |
|
|
|
|
|
|
|
|
|
|
| |
Senior unsecured notes: | | | | | | | | | | | |
4.75% euro | | | December 2014 | | $ | 1,296 | | | $ | — | |
4.55% euro | | | May 2017 | | | 1,291 | | | | — | |
6.60% | | | December 2028 | | | 764 | | | | 735 | |
4.50% | | | February 2014 | | | 753 | | | | 720 | |
5.63% | | | April 2009 | | | 612 | | | | 609 | |
1.21% Japanese yen | | | February 2011 | | | 530 | | | | 504 | |
6.50% | | | December 2018 | | | 527 | | | | 506 | |
1.85% Japanese yen | | | February 2016 | | | 484 | | | | 461 | |
4.65% | | | March 2018 | | | 300 | | | | 288 | |
3.30% | | | March 2009 | | | 297 | | | | 290 | |
0.80% Japanese yen | | | March 2008 | | | — | | | | 506 | |
6.00% | | | January 2008 | | | — | | | | 252 | |
Other: | | | | | | | | | | | |
Debentures, notes, borrowings and mortgages | | | | | | 460 | | | | 675 | |
|
|
|
|
|
|
|
|
|
|
| |
Total long-term debt | | | | | $ | 7,314 | | | $ | 5,546 | |
|
|
|
|
|
|
|
|
|
|
| |
Current portion not included above | | | | | $ | 1,024 | | | $ | 712 | |
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | | | | | | |
Long-term debt outstanding as of December 31, 2007, matures in the following years: | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2009 | | 2010 | | 2011 | | 2012 | | AFTER
2012 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Maturities | | $ | 945 | | $ | 6 | | $ | 536 | | $ | 6 | | $ | 5,821 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
In March 2007, we filed a securities registration statement with the SEC. The registration statement was filed under the automatic shelf registration process available to well-known seasoned issuers and is effective for three years. We can issue securities of various types under that registration statement at any time, subject to approval by our Board of Directors in certain circumstances.
D. Derivative Financial Instruments and Hedging Activities
Foreign Exchange Risk—A significant portion of revenues, earnings and net investments in foreign affiliates is exposed to changes in foreign exchange rates. We seek to manage our foreign exchange risk in part through operational means, including managing expected same currency revenues in relation to same currency costs and same currency assets in relation to same currency liabilities. Depending on market conditions, foreign exchange risk is also managed through the use of derivative financial instruments and foreign currency debt. These financial instruments serve to protect net income and net investments against the impact of the translation into U.S. dollars of certain foreign exchange denominated transactions.
We entered into financial instruments to hedge or offset by the same currency an appropriate portion of the currency risk and the timing of the hedged or offset item. As of December 31, 2007 and 2006, the more significant financial instruments employed to manage foreign exchange risk follow:
| | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | NOTIONAL AMOUNT | | | |
| | PRIMARY | | | | | | (MILLIONS OF DOLLARS) | | MATURITY
DATE
07/06 | |
| | BALANCE SHEET | | HEDGE | | | |
| | |
INSTRUMENT(a) | | CAPTION(b) | | TYPE(c) | | HEDGED OR OFFSET ITEM | | 2007 | | | 2006 | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Forwards | | OCL | | — | | Short-term foreign currency assets and liabilities(d) | | $ | 10,672 | | | $ | 7,939 | | | 2008/2007 | |
Swaps | | OCL | | NI | | Swedish krona net investments(e) | | | 8,288 | | | | 7,759 | | | 2008 | |
Forwards | | OCL | | CF | | Euro available-for-sale investments | | | 5,297 | | | | — | | | 2008 | |
Swaps | | Prepaid | | CF | | Swedish krona intercompany loan | | | 5,156 | | | | 4,759 | | | 2008 | |
Forwards | | OCL | | CF | | Yen available-for-sale investments | | | 2,666 | | | | — | | | 2008 | |
ST yen borrowings | | STB | | NI | | Yen net investments | | | 1,679 | | | | 1,598 | | | 2008/2007 | |
Forwards | | OCL | | CF | | U.K. pound available-for-sale investments | | | 1,419 | | | | — | | | 2008 | |
Swap | | Other assets | | — | | Euro fixed rate debt | | | 1,321 | | | | — | | | 2014 | |
Swap | | Other assets | | — | | Euro fixed rate debt | | | 1,321 | | | | — | | | 2017 | |
Swaps | | Prepaid | | NI | | Euro net investments | | | 916 | | | | — | | | 2008 | |
Swaps | | OCL | | NI | | Euro net investments | | | — | | | | 1,369 | | | 2007 | |
Swaps | | OCL | | NI | | Yen net investments | | | 686 | | | | 653 | | | 2008/2007 | |
LT yen debt | | LTD | | NI | | Yen net investments | | | 574 | | | | 547 | | | After 2012 | |
ST yen debt | | STB | | NI | | Yen net investments | | | 530 | | | | 506 | | | 2008 | |
LT yen debt | | LTD | | NI | | Yen net investments | | | 530 | | | | 504 | | | 2011 | |
Forwards | | OCL | | — | | Short-term intercompany foreign currency loans(f) | | | — | | | | 3,484 | | | 2007 | |
Forwards | | Prepaid | | CF | | Yen available-for-sale investments | | | — | | | | 1,135 | | | 2007 | |
Swaps | | OCL | | CF | | U.K. pound intercompany loan | | | — | | | | 811 | | | 2007 | |
Forwards | | OCL | | CF | | Euro intercompany loan | | | — | | | | 542 | | | 2007 | |
Forwards | | OCL | | CF | | Euro available-for-sale investments | | | — | | | | 444 | | | 2007 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| |
(a) | Forwards = Forward-exchange contracts; ST yen borrowings = Short-term yen borrowings; ST yen debt = Short-term yen debt; LT yen debt = Long-term yen debt. |
| |
(b) | The primary balance sheet caption indicates the financial statement classification of the amount associated with the financial instrument used to hedge or offset foreign exchange risk. The abbreviations used are defined as follows: Prepaid =Prepaid expenses and taxes; Other assets =Other assets, deferred taxes and deferred charges; STB =Short-term borrowings, including current portion of long-term debt; OCL =Other current liabilities; and LTD =Long-term debt. |
| |
(c) | CF = Cash flow hedge; NI = Net investment hedge. |
| |
(d) | Forward-exchange contracts used to offset short-term foreign currency assets and liabilities were primarily for intercompany transactions in euros, Japanese yen, Swedish krona, U.K. pounds and Canadian dollars for the year ended December 31, 2007, and euros, U.K. pounds, Australian dollars, Canadian dollars, Japanese yen and Swedish krona for the year ended December 31, 2006. |
| |
(e) | Reflects an increase in Swedish krona net investments in 2006 due to the receipt of proceeds related to the sale of our Consumer Healthcare business in Sweden. |
| |
(f) | Forward-exchange contracts used to offset foreign currency loans in 2006 for intercompany contracts arising from the sale of our Consumer Healthcare business, primarily in Canadian dollars, U.K. pounds and euros. |
2007 Financial Report | 55
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
All derivative contracts used to manage foreign currency risk are measured at fair value and reported as assets or liabilities on the balance sheet. Changes in fair value are reported in earnings or deferred, depending on the nature and effectiveness of the offset or hedging relationship, as follows:
| |
• | We recognize the earnings impact of foreign currency swaps and foreign currency forward-exchange contracts designated as cash flow hedges inOther (income)/deductions—netupon the recognition of the foreign exchange gain or loss on the translation to U.S. dollars of the hedged items. |
| |
• | We recognize the earnings impact of foreign currency forward-exchange contracts that are used to offset foreign currency assets or liabilities inOther (income)/deductions—netduring the terms of the contracts, along with the earnings impact of the items they generally offset. |
| |
• | We recognize the earnings impact of foreign currency swaps designated as a hedge of our net investments inOther (income)/deductions—netin three ways: over time-for the periodic net swap payments; immediately-to the extent of any |
| |
| change in the difference between the foreign exchange spot rate and forward rate; and upon sale or substantial liquidation of our net investments-to the extent of change in the foreign exchange spot rates. |
Any ineffectiveness in a hedging relationship is recognized immediately into earnings. There was no significant ineffectiveness in 2007, 2006 or 2005.
Interest Rate Risk—Our interest-bearing investments, loans and borrowings are subject to interest rate risk. We invest, loan and borrow primarily on a short-term or variable-rate basis. From time to time, depending on market conditions, we will fix interest rates either through entering into fixed-rate investments and borrowings or through the use of derivative financial instruments.
We entered into derivative financial instruments to hedge or offset the fixed or variable interest rates on the hedged item, matching the amount and timing of the hedged item. As of December 31, 2007 and 2006, the more significant derivative financial instruments employed to manage interest rate risk follow:
| | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | NOTIONAL AMOUNT | | | | |
| | PRIMARY | | | | | | (MILLIONS OF DOLLARS) | | | |
| | BALANCE SHEET | | HEDGE | | | |
| | MATURITY
DATE | |
INSTRUMENT | | CAPTION(a) | | TYPE(b) | | HEDGED OR OFFSET ITEM | | | 2007 | | | | 2006 | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Swap | | ONCL | | FV | | Euro fixed rate debt(c) | | $ | 1,321 | | | $ | — | | | 2014 | |
Swap | | ONCL | | FV | | Euro fixed rate debt(c) | | | 1,321 | | | | — | | | 2017 | |
Swaps | | Other assets | | — | | U.S. dollar fixed rate debt | | | 1,278 | | | | 1,285 | | | 2018-2028 | |
Swaps | | Other assets | | FV | | U.S. dollar fixed rate debt(c) | | | 1,050 | | | | 1,050 | | | 2014-2018 | |
Swaps | | ONCL | | FV | | U.S. dollar fixed rate debt(c) | | | 900 | | | | 900 | | | 2009 | |
Swaps | | OCL | | FV | | U.S. dollar fixed rate debt(c) | | | 450 | | | | 450 | | | 2008 | |
Swaps | | OCL/ONCL | | FV | | U.S. dollar fixed rate debt(c) | | | — | | | | 700 | | | 2007 | |
Swaps | | ONCL | | — | | Yen LIBOR interest rate related to forecasted issuances of short-term debt | | | — | | | | 1,196 | | | 2009-2013 | |
| | | | | | | | | | | | | | | | | |
| |
(a) | The primary balance sheet caption indicates the financial statement classification of the fair value amount associated with the financial instrument used to hedge or offset interest rate risk. The abbreviations used are defined as follows: OCL =Other current liabilities; ONCL =Other noncurrent liabilities; and Other assets =Other assets, deferred taxes and deferred charges. |
|
(b) | FV = Fair value hedge. |
(c) | Serve to reduce exposure to long-term U.S. dollar and euro interest rates by effectively converting fixed rates associated with long-term debt obligations to floating rates (see alsoNote 10C. Financial Instruments: Long-Term Debt). |
All derivative contracts used to manage interest rate risk are measured at fair value and reported as assets or liabilities on the balance sheet. Changes in fair value are reported in earnings or deferred, depending on the nature and effectiveness of the offset or hedging relationship, as follows:
| |
• | We recognize the earnings impact of interest rate swaps designated as fair value hedges or offsets inOther (income)/deductions—netupon the recognition of the change in fair value for interest rate risk related to the hedged or offset items. |
| |
• | We recognize the earnings impact of interest rate swaps inOther (income)/deductions—net. |
Any ineffectiveness in a hedging relationship is recognized immediately in earnings. There was no significant ineffectiveness in 2007, 2006 or 2005.
E. Fair Value
The following methods and assumptions were used to estimate the fair value of derivative and other financial instruments as of the balance sheet date:
| |
• | short-term financial instruments (cash equivalents, accounts receivable and payable, held-to-maturity debt securities and |
| |
| debt)—we use cost or contract value because of the short maturity period. |
| |
• | available-for-sale debt securities—we use a valuation model that uses observable market quotes and credit ratings of the securities. |
| |
• | available-for-sale equity securities—we use observable market quotes. |
| |
• | derivative contracts—we use valuation models that use observable market quotes and our view of the creditworthiness of the derivative counterparty. |
| |
• | loans—we use cost because of the short interest-reset period. |
| |
• | held-to-maturity long-term investments and long-term debt—we use valuation models that use observable market quotes. |
56 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
The differences between the estimated fair values and carrying values of our financial instruments were not significant as of December 31, 2007 and 2006.
F. Credit Risk
On an ongoing basis, we review the creditworthiness of counterparties to foreign exchange and interest rate agreements and do not expect to incur a loss from failure of any counterparties to perform under the agreements.
There are no significant concentrations of credit risk related to our financial instruments with any individual counterparty. As of December 31, 2007, we had $4.3 billion due from a broad group of banks around the world.
In general, there is no requirement for collateral from customers. However, derivative financial instruments are executed under master netting agreements with financial institutions. These agreements contain provisions that provide for the ability for collateral payments, depending on levels of exposure, our credit rating and the credit rating of the counterparty. As of December 31, 2007, we advanced cash collateral of $460 million and received cash collateral of $364 million against various counterparties. The collateral primarily supports the approximate fair value of our Swedish krona swap contracts. The collateral advanced receivables is reported inPrepaid expenses and taxes,and the collateral received obligation is reported inOther current liabilities.
11.Inventories
The components of inventories as of December 31 follow:
| | | | | | | | | | |
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | | | 2007 | | | 2006 | |
|
|
|
|
|
|
|
| |
Finished goods | | | | $ | 2,064 | | | $ | 1,651 | |
Work-in-process | | | | | 2,353 | | | | 3,198 | |
Raw materials and supplies | | | | | 885 | | | | 1,262 | |
|
|
|
|
|
|
|
|
|
| |
Total inventories(a) | | | | $ | 5,302 | | | $ | 6,111 | |
|
|
|
|
|
|
|
|
|
| |
| |
(a) | Decrease was primarily due to write-off of inventories related to Exubera (seeNote 4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera) and the impact of our inventory-reduction initiatives. |
12.Property, Plant and Equipment
The major categories of property, plant and equipment as of December 31 follow:
| | | | | | | | | | |
|
|
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | USEFUL
LIVES
(YEARS) | | 2007 | | | 2006 | |
|
|
|
|
|
|
|
| |
Land | | — | | $ | 718 | | | $ | 641 | |
Buildings | | 331/3-50 | | | 10,319 | | | | 9,947 | |
Machinery and equipment | | 8-20 | | | 10,441 | | | | 9,969 | |
Furniture, fixtures and other | | 3-121/2 | | | 4,867 | | | | 4,644 | |
Construction in progress | | — | | | 1,758 | | | | 1,862 | |
|
|
|
|
|
|
|
|
|
| |
| | | | | 28,103 | | | | 27,063 | |
Less: accumulated depreciation | | | | | 12,369 | | | | 10,431 | |
|
|
|
|
|
|
|
|
|
| |
Total property, plant and equipment | | | | $ | 15,734 | | | $ | 16,632 | |
|
|
|
|
|
|
|
|
|
| |
13.Goodwill and Other Intangible Assets
A. Goodwill
The changes in the carrying amount of goodwill by segment for the years ended December 31, 2007 and 2006, follow:
| | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | PHARMACEUTICAL | | ANIMAL
HEALTH | | OTHER | | TOTAL | |
|
|
|
|
|
|
|
|
| |
Balance, January 1, 2006 | | $ | 20,919 | | $ | 56 | | $ | 10 | | $ | 20,985 | |
Additions(a) | | | 166 | | | — | | | — | | | 166 | |
Other(b) | | | (287 | ) | | 5 | | | 7 | | | (275 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2006 | | | 20,798 | | | 61 | | | 17 | | | 20,876 | |
Additions(a) | | | — | | | 40 | | | — | | | 40 | |
Other(b) | | | 458 | | | 7 | | | 1 | | | 466 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2007 | | $ | 21,256 | | $ | 108 | | $ | 18 | | $ | 21,382 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Primarily related to Embrex in 2007 and Exubera in 2006. |
| | |
| (b) | In 2007, primarily relates to the impact of foreign exchange. In 2006, includes reductions to goodwill related to the resolution of certain tax positions, adjustments for certain purchase accounting liabilities and the impact of foreign exchange. |
B. Other Intangible Assets
The components of identifiable intangible assets, primarily included in our Pharmaceutical segment, as of December 31 follow:
| | | | | | | | | | | | | | |
|
|
|
|
|
| |
| | 2007 | | | 2006 | |
| |
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | GROSS
CARRYING
AMOUNT | | ACCUMULATED
AMORTIZATION | | | GROSS
CARRYING
AMOUNT | | ACCUMULATED
AMORTIZATION | |
|
|
|
|
|
|
|
|
|
| |
Finite-lived | | | | | | | | | | | | | | |
intangible assets: | | | | | | | | | | | | | | |
Developed | | | | | | | | | | | | | | |
technology rights | | $ | 32,433 | | $ | (15,830 | ) | | $ | 32,769 | | $ | (12,423 | ) |
Brands | | | 1,017 | | | (452 | ) | | | 888 | | | (417 | ) |
License agreements | | | 212 | | | (59 | ) | | | 189 | | | (41 | ) |
Trademarks | | | 128 | | | (82 | ) | | | 113 | | | (73 | ) |
Other(a) | | | 459 | | | (264 | ) | | | 508 | | | (266 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total amortized | | | | | | | | | | | | | | |
finite-lived | | | | | | | | | | | | | | |
intangible assets | | | 34,249 | | | (16,687 | ) | | | 34,467 | | | (13,220 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Indefinite-lived | | | | | | | | | | | | | | |
intangible assets: | | | | | | | | | | | | | | |
Brands | | | 2,864 | | | — | | | | 2,991 | | | — | |
Trademarks | | | 71 | | | — | | | | 77 | | | — | |
Other | | | 1 | | | — | | | | 35 | | | — | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total indefinite-lived | | | | | | | | | | | | | | |
intangible assets | | | 2,936 | | | — | | | | 3,103 | | | — | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total identifiable | | | | | | | | | | | | | | |
intangible assets | | $ | 37,185 | | $ | (16,687 | ) | | $ | 37,570 | | $ | (13,220 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total identifiable | | | | | | | | | | | | | | |
intangible assets, less | | | | | | | | | | | | | | |
accumulated | | | | | | | | | | | | | | |
amortization(b) | | $ 20,498 | | | $ 24,350 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Includes patents, non-compete agreements, customer contracts and other intangible assets. |
| | |
| (b) | Decrease primarily due to amortization, as well as the impairment of intangible assets associated with Exubera (seeNote 4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera), partially offset by the impact of foreign exchange. |
2007 Financial Report | 57
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
Developed technology rights represent the amortized value associated with developed technology, which has been acquired from third parties and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. We possess a well-diversified portfolio of hundreds of developed technology rights across therapeutic categories primarily representing the commercialized products included in our Pharmaceutical segment that we acquired in connection with our Pharmacia acquisition. While the Arthritis and Pain therapeutic category represents about 30% of the total amortized value of developed technology rights as of December 31, 2007, the balance of the amortized value is evenly distributed across the following Pharmaceutical therapeutic product categories: Ophthalmology; Oncology; Urology; Infectious and Respiratory Diseases; Endocrine Disorders categories; and, as a group, the Cardiovascular and Metabolic Diseases; Central Nervous System Disorders and All Other categories. The significant components include values determined for Celebrex, Detrol/Detrol LA, Xalatan, Genotropin, Zyvox and Campto/Camptosar. Also included in this category are the post-approval milestone payments made under our alliance agreements for certain Pharmaceutical products, such as Rebif and Spiriva. These rights are all subject to our review for impairment explained inNote 1K. Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
The weighted-average life of our total finite-lived intangible assets is approximately seven years, which includes developed technology rights at eight years. Total amortization expense for finite-lived intangible assets was $3.2 billion in 2007, $3.4 billion in 2006 and $3.5 billion in 2005.
Brands represent the amortized value associated with tradenames, as the products themselves no longer receive patent protection. Most of these assets are associated with our Pharmaceutical segment and the significant components include values determined for Depo-Provera, Xanax and Medrol.
In 2007, we recorded charges of $1.1 billion inCost of salesandSelling, informational and administrative expensesrelated to the impairment of Exubera (seeNote 4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera). In 2006, we recorded charges of $320 million inOther (income)/deductions—netrelated to the impairment of our Depo-Provera brand, a contraceptive injection, (included in our Pharmaceutical segment). In 2005, we recorded an impairment charge of $1.1 billion inOther (income)/deductions—netrelated to the developed technology rights for Bextra, a selective COX-2 inhibitor (included in our Pharmaceutical segment), in connection with the decision to suspend sales of Bextra. In addition, in connection with the suspension, we recorded $5 million related to the write-off of machinery and equipment included inOther (income)/ deductions—net;$73 million in write-offs of inventory and exit costs, included inCost of sales; $8 million related to the costs of administering the
suspension of sales, included inSelling, informational and administrative expenses;and $212 million for an estimate of customer returns, primarily included againstRevenues.
The annual amortization expense expected for the years 2008 through 2012 is as follows:
| | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2008 | | 2009 | | 2010 | | 2011 | | 2012 | |
|
|
|
|
|
|
|
|
|
|
| |
Amortization expense | | $ | 2,835 | | $ | 2,620 | | $ | 2,611 | | $ | 2,596 | | $ | 2,360 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
14.Pension and Postretirement Benefit Plans and Defined Contribution Plans
We provide defined benefit pension plans and defined contribution plans for the majority of our employees worldwide. In the U.S., we have both qualified and supplemental (non-qualified) defined benefit plans. A qualified plan meets the requirements of certain sections of the Internal Revenue Code and, generally, contributions to qualified plans are tax deductible. A qualified plan typically provides benefits to a broad group of employees and may not discriminate in favor of highly compensated employees in its coverage, benefits or contributions. We also provide benefits through supplemental (non-qualified) retirement plans to certain employees. In addition, we provide medical and life insurance benefits to certain retirees and their eligible dependents through our postretirement plans.
We use a measurement date that coincides with our fiscal yearends; December 31 for our U.S. pension and postretirement plans and November 30 for our international plans. During 2006, pursuant to the divestiture of our Consumer Healthcare business, certain defined benefit obligations and related plan assets, if applicable, were transferred to the purchaser of that business.
A. Adoption of New Accounting Standard
As of December 31, 2006, we adopted the provisions of SFAS No. 158,Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans (an amendment of FASB Statements No. 87, 88, 106 and 132R),which requires us to recognize on our balance sheet the difference between our benefit obligations and any plan assets of our defined benefit plans. In addition, we are required to recognize as part of other comprehensive income/(expense), net of taxes, gains and losses due to differences between our actuarial assumptions and actual experience (actuarial gains and losses) and any effects on prior service due to plan amendments (prior service costs or credits) that arise during the period and which are not being recognized as net periodic benefit costs. Upon adoption, SFAS 158 requires the recognition of previously unrecognized actuarial gains and losses, prior service costs or credits and net transition amounts withinAccumulated other comprehensive income (expense),net of tax. The incremental impact of applying SFAS 158 to our balance sheet as of December 31, 2006, was to reduce our total shareholders’ equity by $2.1 billion, primarily due to the recognition of previously unrecognized actuarial losses.
58 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
|
|
|
B. Components of Net Periodic Benefit Costs and Other Amounts Recognized in Other Comprehensive (Income)/Expense |
|
The annual cost and other amounts recognized in other comprehensive (income)/expense of the U.S. qualified, U.S. supplemental (non-qualified) and international pension plans and postretirement plans for the years ended December 31, 2007, 2006 and 2005, follows: |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
| | PENSION PLANS | | | | | | | |
| |
| | | | | | | |
| | U.S. QUALIFIED | | U.S. SUPPLEMENTAL
(NON-QUALIFIED) | | INTERNATIONAL | | POSTRETIREMENT PLANS | |
| |
| |
| |
| |
|
|
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | | 2007 | | | 2006 | | 2005 | | 2007 | | | 2006 | | 2005 | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service cost | | $ | 282 | | | $ | 368 | | $ | 318 | | $ | 27 | | | $ | 43 | | $ | 37 | | $ | 292 | | | $ | 303 | | $ | 293 | | $ | 42 | | | $ | 47 | | $ | 38 | |
Interest cost | | | 447 | | | | 444 | | | 410 | | | 55 | | | | 60 | | | 59 | | | 349 | | | | 307 | | | 309 | | | 137 | | | | 127 | | | 113 | |
Expected return on plan assets | | | (693 | ) | | | (628 | ) | | (594 | ) | | — | | | | — | | | — | | | (381 | ) | | | (311 | ) | | (297 | ) | | (36 | ) | | | (28 | ) | | (23 | ) |
Amortization of: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Actuarial losses | | | 65 | | | | 119 | | | 101 | | | 45 | | | | 45 | | | 39 | | | 96 | | | | 106 | | | 95 | | | 42 | | | | 36 | | | 21 | |
Prior service costs/(credits) | | | 8 | | | | 9 | | | 10 | | | (2 | ) | | | (3 | ) | | 1 | | | — | | | | 2 | | | (1 | ) | | 1 | | | | 1 | | | 1 | |
Curtailments and | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
settlements—net | | | 58 | | | | 117 | | | 12 | | | 5 | | | | (8 | ) | | 4 | | | (155 | ) | | | (17 | ) | | 19 | | | 5 | | | | 6 | | | — | |
Special termination benefits | | | 16 | | | | 17 | | | 5 | | | — | | | | — | | | — | | | 29 | | | | 14 | | | 29 | | | 17 | | | | 12 | | | 2 | |
Less: amounts included in | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
discontinued operations | | | (27 | ) | | | (81 | ) | | (15 | ) | | — | | | | 4 | | | (2 | ) | | — | | | | 15 | | | (2 | ) | | — | | | | 9 | | | (4 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net periodic benefit costs | | | 156 | | | | 365 | | | 247 | | | 130 | | | | 141 | | | 138 | | | 230 | | | | 419 | | | 445 | | | 208 | | | | 210 | | | 148 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other changes recognized in | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
other comprehensive | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
(income)/expense(a) | | | (582 | ) | | | — | | | — | | | (134 | ) | | | 12 | | | 2 | | | (808 | ) | | | 4 | | | 31 | | | (311 | ) | | | — | | | — | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total recognized in net | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
periodic benefit costs | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
and other comprehensive | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
(income)expense | | $ | (426 | ) | | $ | 365 | | $ | 247 | | $ | (4 | ) | | $ | 153 | | $ | 140 | | $ | (578 | ) | | $ | 423 | | $ | 476 | | $ | (103 | ) | | $ | 210 | | $ | 148 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
(a) | For details, seeNote 9. Other Comprehensive Income/(Expense). |
The decrease in the 2007 U.S. qualified pension plans’ net periodic benefit cost compared to 2006 was largely driven by a higher 2006 actual investment return, the increase in the discount rate and the impact of our cost-reduction initiatives.
The decrease in the 2007 international plans’ net periodic benefit cost compared to 2006 was largely driven by a settlement gain at our Japanese affiliate. Japanese pension regulations permit employers with certain pension obligations to separate the social security benefits portion of those obligations and transfer it,
along with related plan assets, to the Japanese government. During 2007, our Japanese affiliate completed this transfer and effectively received a subsidy from the Japanese government of approximately $168 million. This subsidy was the result of the transfer of pension obligations of approximately $309 million (excluding the effect of any future salary increases of approximately $9 million) along with related plan assets of approximately $141 million. This transfer resulted in a settlement gain of $106 million.
The following table presents the amount inAccumulated other comprehensive income/(expense)expected to be amortized into 2008 net periodic benefit costs:
| | | | | | | | | | | | | |
|
|
|
|
|
|
|
| | PENSION PLANS | | | | |
| |
| | | | |
(MILLIONS OF DOLLARS) | | U.S. QUALIFIED | | U.S. SUPPLEMENTAL
(NON-QUALIFIED) | | INTERNATIONAL | | POSTRETIREMENT
PLANS | |
|
|
|
|
|
|
|
|
|
|
Actuarial losses | | $ | 34 | | $ | 35 | | $ | 44 | | $ | 25 | |
Prior service costs/(credits) and other | | | 3 | | | (3 | ) | | 1 | | | 2 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | $ | 37 | | $ | 32 | | $ | 45 | | $ | 27 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2007 Financial Report | 59
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
C. Actuarial Assumptions
The following table provides the weighted-average actuarial assumptions:
| | | | | | | | | | |
|
|
|
|
|
|
| |
(PERCENTAGES) | | 2007 | | 2006 | | 2005 | |
|
|
|
|
|
|
| |
Weighted-average assumptions used | | | | | | | | | | |
to determine benefit obligations: | | | | | | | | | | |
Discount rate: | | | | | | | | | | |
U.S. qualified pension plans/ | | | | | | | | | | |
non-qualified pension plans | | 6.5 | % | | 5.9 | % | | 5.8 | % | |
International pension plans | | 5.3 | | | 4.4 | | | 4.3 | | |
Postretirement plans | | 6.5 | | | 5.9 | | | 5.8 | | |
Rate of compensation increase: | | | | | | | | | | |
U.S. qualified pension plans/ | | | | | | | | | | |
non-qualified pension plans | | 4.5 | | | 4.5 | | | 4.5 | | |
International pension plans | | 3.3 | | | 3.6 | | | 3.6 | | |
|
|
|
|
|
|
|
|
|
| |
Weighted-average assumptions used | | | | | | | | | | |
to determine net periodic benefit cost: | | | | | | | | | | |
Discount rate: | | | | | | | | | | |
U.S. qualified pension plans/ | | | | | | | | | | |
non-qualified pension plans | | 5.9 | | | 5.8 | | | 6.0 | | |
International pension plans | | 4.4 | | | 4.3 | | | 4.7 | | |
Postretirement plans | | 5.9 | | | 5.8 | | | 6.0 | | |
Expected return on plan assets: | | | | | | | | | | |
U.S. qualified pension plans | | 9.0 | | | 9.0 | | | 9.0 | | |
International pension plans | | 6.6 | | | 6.9 | | | 6.9 | | |
Postretirement plans | | 9.0 | | | 9.0 | | | 9.0 | | |
Rate of compensation increase: | | | | | | | | | | |
U.S. qualified pension plans/ | | | | | | | | | | |
non-qualified pension plans | | 4.5 | | | 4.5 | | | 4.5 | | |
International pension plans | | 3.6 | | | 3.6 | | | 3.6 | | |
|
|
|
|
|
|
|
|
|
|
|
The assumptions above are used to develop the benefit obligations at fiscal year-end and to develop the net periodic benefit cost for the subsequent fiscal year. Therefore, the assumptions used to determine net periodic benefit cost for each year are established at the end of each previous year, while the assumptions used to determine benefit obligations were established at each year-end.
The net periodic benefit cost and the benefit obligations are based on actuarial assumptions that are reviewed on an annual basis. We revise these assumptions based on an annual evaluation of long-term trends, as well as market conditions, that may have an impact on the cost of providing retirement benefits.
The expected rates of return on plan assets for our U.S. qualified, international and postretirement plans represent our long-term assessment of return expectations, which we will change based on significant shifts in economic and financial market conditions. The 2007 expected rates of return for these plans reflect our long-term outlook for a globally diversified portfolio, which is influenced by a combination of return expectations for individual asset classes, actual historical experience and our diversified investment strategy. The historical returns are one of the inputs used to provide context for the development of our expectations for future returns. Using this information, we develop ranges of returns for each asset class and a weighted-average expected return for our targeted portfolio, which includes the impact of portfolio diversification and active portfolio management.
The healthcare cost trend rate assumptions for our U.S. postretirement benefit plans are as follows:
| | | | | | | |
|
|
|
|
|
|
(PERCENTAGES) | | 2007 | | 2006 | |
|
|
|
|
|
|
Healthcare cost trend rate assumed | | | | | | | |
for next year | | 9.9 | % | | | 9.9 | % |
Rate to which the cost trend rate is | | | | | | | |
assumed to decline | | 5.0 | | | | 5.0 | |
|
|
|
|
|
|
|
|
Year that the rate reaches the | | | | | | | |
ultimate trend rate | | 2015 | | | | 2014 | |
|
|
|
|
|
|
|
|
A one-percentage-point increase or decrease in the healthcare cost trend rate assumed for postretirement benefits would have the following effects as of December 31, 2007:
| | | | | | | |
|
|
|
|
|
|
|
|
(MILLIONS OF DOLLARS) | | INCREASE | | DECREASE | |
|
|
|
|
|
|
|
|
Effect on total service and interest | | | | | | | |
cost components | | $ | 17 | | $ | (13 | ) |
Effect on postretirement benefit | | | | | | | |
obligation | | | 181 | | | (151 | ) |
|
|
|
|
|
|
|
|
60 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
|
|
D. Obligations and Funded Status |
|
The following table presents an analysis of the changes in 2007 and 2006 in the benefit obligations, the plan assets and the funded status of our U.S. qualified, U.S. supplemental (non-qualified) and international pension plans, and our postretirement plans: |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| | PENSION PLANS | | | | | | | |
| |
| | | | | | | |
| | U.S. QUALIFIED | | U.S. SUPPLEMENTAL
(NON-QUALIFIED) | | INTERNATIONAL | | POSTRETIREMENT
PLANS | |
| |
|
|
|
|
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2007 | | | 2006 | | 2007 | | | 2006 | | 2007 | | | 2006 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Change in benefit obligation: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Benefit obligation at beginning of year(a) | | $ | 7,792 | | | $ | 7,983 | | $ | 1,045 | | | $ | 1,133 | | $ | 8,144 | | | $ | 6,968 | | $ | 2,416 | | | $ | 2,252 | |
Service cost | | | 282 | | | | 368 | | | 27 | | | | 43 | | | 292 | | | | 303 | | | 42 | | | | 47 | |
Interest cost | | | 447 | | | | 444 | | | 55 | | | | 60 | | | 349 | | | | 307 | | | 137 | | | | 127 | |
Employee contributions | | | — | | | | — | | | — | | | | — | | | 21 | | | | 22 | | | 34 | | | | 34 | |
Plan amendments | | | (47 | ) | | | — | | | (5 | ) | | | — | | | 40 | | | | 10 | | | 1 | | | | 1 | |
Increases/(decreases) arising primarily from changes in actuarial assumptions | | | (412 | ) | | | (137 | ) | | (64 | ) | | | (77 | ) | | (829 | ) | | | 150 | | | (289 | ) | | | 152 | |
Foreign exchange impact | | | — | | | | — | | | — | | | | — | | | 564 | | | | 769 | | | 6 | | | | (1 | ) |
Acquisitions | | | 5 | | | | — | | | (5 | ) | | | — | | | 17 | | | | 11 | | | — | | | | — | |
Curtailments(b) | | | (107 | ) | | | (180 | ) | | (15 | ) | | | (25 | ) | | (80 | ) | | | (42 | ) | | 5 | | | | 9 | |
Settlements(b) | | | (253 | ) | | | (418 | ) | | (11 | ) | | | (13 | ) | | (409 | ) | | | (85 | ) | | — | | | | (23 | ) |
Special termination benefits | | | 16 | | | | 17 | | | — | | | | — | | | 29 | | | | 14 | | | 17 | | | | 12 | |
Benefits paid | | | (267 | ) | | | (285 | ) | | (54 | ) | | | (76 | ) | | (299 | ) | | | (283 | ) | | (191 | ) | | | (194 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Benefit obligation at end of year(a) | | | 7,456 | | | | 7,792 | | | 973 | | | | 1,045 | | | 7,839 | | | | 8,144 | | | 2,178 | | | | 2,416 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in plan assets: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Fair value of plan assets at beginning of year | | | 7,816 | | | | 7,050 | | | — | | | | — | | | 5,880 | | | | 4,595 | | | 396 | | | | 275 | |
Actual gain on plan assets | | | 613 | | | | 1,034 | | | — | | | | — | | | 261 | | | | 552 | | | 16 | | | | 31 | |
Company contributions | | | 106 | | | | 453 | | | 65 | | | | 80 | | | 499 | | | | 533 | | | 158 | | | | 250 | |
Employee contributions | | | — | | | | — | | | — | | | | — | | | 21 | | | | 22 | | | 34 | | | | 34 | |
Foreign exchange impact | | | — | | | | — | | | — | | | | — | | | 435 | | | | 525 | | | — | | | | — | |
Acquisitions | | | — | | | | — | | | — | | | | — | | | 14 | | | | 1 | | | — | | | | — | |
Settlements(b) | | | (279 | ) | | | (436 | ) | | (11 | ) | | | (4 | ) | | (232 | ) | | | (65 | ) | | — | | | | — | |
Benefits paid | | | (267 | ) | | | (285 | ) | | (54 | ) | | | (76 | ) | | (299 | ) | | | (283 | ) | | (191 | ) | | | (194 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of plan assets at end of year | | | 7,989 | | | | 7,816 | | | — | | | | — | | | 6,579 | | | | 5,880 | | | 413 | | | | 396 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Funded status (plan assets greater than (less than) benefit obligation) at end of year | | $ | 533 | | | $ | 24 | | $ | (973 | ) | | $ | (1,045 | ) | $ | (1,260 | ) | | $ | (2,264 | ) | $ | (1,765 | ) | | $ | (2,020 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | For the U.S. and international pension plans, the benefit obligation is the projected benefit obligation. For the postretirement plans, the benefit obligation is the accumulated postretirement benefit obligation. |
| | |
| (b) | For 2006, includes curtailments and settlements associated with the transfer of benefit obligations as part of the sale of our Consumer Healthcare business |
The favorable change in our U.S. qualified plans projected benefit obligations funded status from $24 million overfunded in the aggregate as of December 31, 2006, to $533 million overfunded in the aggregate as of December 31, 2007, was largely driven by the 0.6 percentage-point increase in the discount rate and our voluntary contributions. In 2007, we made required U.S. qualified plan contributions of $6 million and voluntary tax-deductible contributions in excess of minimum requirements of $100 million to certain of our U.S. qualified pension plans. In 2006, we made required U.S. qualified plan contributions of $3 million and voluntary tax-deductible contributions in excess of minimum requirements of $450 million to certain of our U.S. qualified pension plans. In the aggregate, the U.S. qualified pension plans are overfunded on a projected benefit measurement basis and on an accumulated benefit obligation measurement basis as of December 31, 2007 and 2006. In 2006, we made voluntary tax-deductible contributions of $90 million to certain of our U.S. postretirement plans through the establishment of sections 401(h) accounts.
The U.S. supplemental (non-qualified) pension plans are not generally funded, as there are no tax or other incentives that exist, and these obligations, which are substantially greater than the annual cash outlay for these liabilities, are paid from cash generated from operations.
The favorable change in our international plans projected benefit obligations funded status from $2.3 billion underfunded in the aggregate as of December 31, 2006, to $1.3 billion underfunded in the aggregate as of December 31, 2007, was largely driven by the increase in the discount rate in the U.K. and other European plans. Outside the U.S., in general, we fund our defined benefit plans to the extent that tax or other incentives exist and we have accrued liabilities on our consolidated balance sheets to reflect those plans that are not fully funded.
The favorable change in our postretirement plans projected benefit obligations funded status from $2.0 billion underfunded in the aggregate as of December 31, 2006, to $1.8 billion underfunded in the aggregate as of December 31, 2007, was largely driven by the 0.6 percentage-point increase in the discount rate and the impact of our cost-reduction initiatives.
The accumulated benefit obligations (ABO) for our U.S. qualified pension plans were $6.6 billion in 2007 and $6.8 billion in 2006. The ABO for our U.S. supplemental (non-qualified) pension plans was $849 million in 2007 and $883 million in 2006. The ABO for our international pension plans was $6.8 billion in 2007 and $7.1 billion in 2006.
2007 Financial Report | 61
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Amounts recognized in the consolidated balance sheet as of December 31 follow: |
|
|
| | PENSION PLANS | | | | | | | |
| |
| | | | | | | |
| | U.S. QUALIFIED | | U.S. SUPPLEMENTAL
(NON-QUALIFIED) | | INTERNATIONAL | | POSTRETIREMENT
PLANS | |
| |
|
|
|
|
| |
| |
(MILLIONS OF DOLLARS) | | | 2007 | | | | 2006 | | | 2007 | | | | 2006 | | | 2007 | | | | 2006 | | | 2007 | | | | 2006 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Noncurrent assets(a) | | $ | 862 | | | $ | 441 | | $ | — | | | $ | — | | $ | 327 | | | $ | 40 | | $ | — | | | $ | — | |
Current liabilities(b) | | | — | | | | — | | | (253 | ) | | | (100 | ) | | (37 | ) | | | (34 | ) | | (57 | ) | | | (50 | ) |
Noncurrent liabilities(c) | | | (329 | ) | | | (417 | ) | | (720 | ) | | | (945 | ) | | (1,550 | ) | | | (2,270 | ) | | (1,708 | ) | | | (1,970 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Funded status | | $ | 533 | | | $ | 24 | | $ | (973 | ) | | $ | (1,045 | ) | $ | (1,260 | ) | | $ | (2,264 | ) | $ | (1,765 | ) | | $ | (2,020 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | Included primarily inOther assets, deferred taxes and deferred charges. |
| | |
| (b) | Included inOther current liabilities. |
| | |
| (c) | Included inPension benefit obligationsandPostretirement benefit obligations,as appropriate. |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amounts recognized in Accumulated other comprehensive income/(expense)as of December 31 follow: | |
|
|
| | PENSION PLANS | | | | | | | |
| |
| | | | | | | |
| | U.S. QUALIFIED | | U.S. SUPPLEMENTAL
(NON-QUALIFIED) | | INTERNATIONAL | | POSTRETIREMENT
PLANS | |
| |
|
|
|
|
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2007 | | | 2006 | | 2007 | | | 2006 | | 2007 | | | 2006 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actuarial losses | | $ | 890 | | | $ | 1,418 | | $ | 487 | | | $ | 622 | | $ | 794 | | | $ | 1,649 | | $ | 311 | | | $ | 621 | |
Prior service costs/(credits) and other | | | (4 | ) | | | 50 | | | (26 | ) | | | (27 | ) | | 45 | | | | (2 | ) | | 5 | | | | 6 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | $ | 886 | | | $ | 1,468 | | $ | 461 | | | $ | 595 | | $ | 839 | | | $ | 1,647 | | $ | 316 | | | $ | 627 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The actuarial losses primarily represent the cumulative difference between the actuarial assumptions and actual return on plan assets, changes in discount rates and plan experience. These actuarial losses are recognized inAccumulated other comprehensive income/(expense)and are amortized into income over an average period of 11 years for our U.S. plans and an average period of 14 years for our international plans.
Information related to the U.S. qualified, U.S. supplemental (non-qualified) and international pension plans as of December 31 follows:
| | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | PENSION PLANS | |
| |
|
|
|
|
|
|
| |
| | U.S. QUALIFIED | | U.S. SUPPLEMENTAL
(NON-QUALIFIED) | | INTERNATIONAL | |
| |
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2007 | | | 2006 | | 2007 | | | 2006 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension plans with an accumulated benefit obligation in excess of plan assets: | | | | | | | | | | | | | | | | | | | | | | |
Fair value of plan assets | | $ | 39 | | | $ | 403 | | $ | — | | | $ | — | | $ | 1,052 | | | $ | 2,273 | |
Accumulated benefit obligation | | | 40 | | | | 468 | | | 849 | | | | 883 | | | 2,413 | | | | 4,002 | |
Pension plans with a projected benefit obligation in excess of plan assets: | | | | | | | | | | | | | | | | | | | | | | |
Fair value of plan assets | | | 2,927 | | | | 4,897 | | | — | | | | — | | | 1,445 | | | | 5,265 | |
Projected benefit obligation | | | 3,256 | | | | 5,314 | | | 973 | | | | 1,045 | | | 3,033 | | | | 7,569 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In the aggregate, our U.S. qualified pension plans had assets greater than their ABO
and their projected benefit obligation as of December 31, 2007.
62 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
E. Plan Assets
The following table presents the weighted-average long-term target asset allocations and the percentages of the fair value of plan assets for our U.S. qualified and international pension plans and postretirement plans by investment category as of December 31:
| | | | | | | | | | | | |
|
|
|
|
|
|
| | TARGET
ALLOCATION | | PERCENTAGE OF
PLAN ASSETS | |
| |
|
|
| |
(PERCENTAGES) | | 2007 | | | 2007 | | | | 2006 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. qualified pension plans: | | | | | | | | | | | | |
Global equity securities | | | 55.0 | | | | 61.4 | | | | 68.6 | |
Debt securities | | | 35.0 | | | | 23.6 | | | | 22.8 | |
Alternative investments(a) | | | 10.0 | | | | 10.9 | | | | 8.4 | |
Cash | | | — | | | | 4.1 | | | | 0.2 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | | 100.0 | | | | 100.0 | | | | 100.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
International pension plans: | | | | | | | | | | | | |
Global equity securities | | | 61.1 | | | | 63.2 | | | | 62.2 | |
Debt securities | | | 28.8 | | | | 23.3 | | | | 23.7 | |
Alternative investments(b) | | | 9.4 | | | | 7.9 | | | | 10.3 | |
Cash | | | 0.7 | | | | 5.6 | | | | 3.8 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | | 100.0 | | | | 100.0 | | | | 100.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. postretirement plans(c): | | | | | | | | | | | | |
Global equity securities | | | 75.0 | | | | 72.3 | | | | 74.8 | |
Debt securities | | | 25.0 | | | | 23.8 | | | | 23.1 | |
Alternative investments(a) | | | — | | | | 2.8 | | | | 2.1 | |
Cash | | | — | | | | 1.1 | | | | — | |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total | | | 100.0 | | | | 100.0 | | | | 100.0 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | Private equity, venture capital, private debt and real estate. |
| | |
| (b) | Real estate, insurance contracts and other investments. |
| | |
| (c) | Reflects postretirement plan assets, which support a portion of our U.S. retiree medical plans. |
All long-term asset allocation targets reflect our asset class return expectations and tolerance for investment risk within the context of the respective plans’ long-term benefit obligations. The long-term asset allocation is supported by an analysis that incorporates historical and expected returns by asset class, as well as volatilities and correlations across asset classes and our liability profile. This analysis, referred to as an asset-liability analysis, also provides an estimate of expected returns on plan assets, as well as a forecast of potential future asset and liability balances. Due to market conditions and other factors, actual asset allocations may vary from the target allocation outlined above. For the U.S. qualified pension plans, in late 2007, we modified our strategic asset target allocation to reduce the volatility of our plan funded status and the probability of future contribution requirements. Our target allocations have been revised to increase the debt securities allocation by 10% and to reduce the global equity securities allocation by a corresponding amount. The year-end 2007 cash allocation of 4.1% for U.S. qualified pensions plans and 5.6% for international pension plans was above the target allocation, primarily due to cash raised from the termination of certain investment strategies, which will be redeployed during 2008. The assets are periodically rebalanced back to the target allocation.
The U.S. qualified pension plans held no shares of our common stock as of December 31, 2007, and approximately 10.2 million shares (fair value of approximately $263 million, representing 3.3% of U.S. plan assets) as of December 31, 2006. The plans received approximately $12 million in dividends on shares of our common stock in 2007 and approximately $10 million in dividends on these shares in 2006.
F. Cash Flows
It is our practice to fund amounts for our qualified pension plans that are at least sufficient to meet the minimum requirements set forth in applicable employee benefit laws and local tax laws.
|
The following table presents expected cash flow information: |
|
| | | | | | | | | | | | | |
FOR THE YEAR
ENDED
DECEMBER 31,
(MILLIONS OF DOLLARS) | | PENSION PLANS | | | | |
|
| | | | |
| | | | U.S.
SUPPLEMENTAL
(NON-QUALIFIED) | | INTERNATIONAL | | POST-
RETIREMENT
PLANS | |
| U.S
QUALIFIED | | | | |
| | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employer contributions:
2008 (estimated) | | $ | — | | $ | 253 | | $ | 367 | | $ | 164 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected benefit payments: | | | | | | | | | | | | | |
2008 | | $ | 527 | | $ | 253 | | $ | 328 | | $ | 164 | |
2009 | | | 425 | | | 77 | | | 331 | | | 168 | |
2010 | | | 441 | | | 76 | | | 342 | | | 170 | |
2011 | | | 456 | | | 76 | | | 361 | | | 173 | |
2012 | | | 477 | | | 75 | | | 374 | | | 173 | |
2013–2017 | | | 2,823 | | | 379 | | | 2,102 | | | 812 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The table reflects the total U.S. and international plan benefits projected to be paid from the plans or from our general assets under the current actuarial assumptions used for the calculation of the benefit obligation and, therefore, actual benefit payments may differ from projected benefit payments.
G. Defined Contribution Plans
We have savings and investment plans in several countries, including the U.S., Japan, Spain and the Netherlands. For the U.S. plans, employees may contribute a portion of their salaries and bonuses to the plans, and we match, largely in company stock, a portion of the employee contributions. In the U.S., employees are permitted to diversify all or any portion of their company stock match contribution. The contribution match for certain legacy Pfizer U.S. participants is held in an employee stock ownership plan. We recorded charges related to our plans of $203 million in 2007, $222 million in 2006 and $234 million in 2005.
2007 Financial Report | 63
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
15.Equity
A. Common Stock
We purchase our common stock via privately negotiated transactions or in open market purchases as circumstances and prices warrant. Purchased shares under each of the share-purchase programs, which are authorized by our Board of Directors, are available for general corporate purposes.
A summary of common stock purchases follows:
| | | | | | | | | | |
|
|
|
|
|
|
|
|
|
| |
FOR THE YEAR ENDED DECEMBER 31,
(MILLIONS OF SHARES AND
DOLLARS, EXCEPT PER-SHARE DATA) | | SHARES OF
COMMON STOCK
PURCHASED | | AVERAGE
PER-SHARE
PRICE PAID | | TOTAL COST OF
COMMON STOCK
PURCHASED | |
|
|
|
|
|
|
| |
2007: | | | | | | | | | | |
June 2005 program(a) | | | 395 | | $ | 25.27 | | $ | 9,994 | |
|
|
|
|
|
|
|
|
|
| |
2006: | | | | | | | | | | |
June 2005 program(a) | | | 266 | | $ | 26.19 | | $ | 6,979 | |
|
|
|
|
|
|
|
|
|
| |
2005: | | | | | | | | | | |
June 2005 program(a) | | | 22 | | $ | 22.38 | | $ | 493 | |
October 2004 program(b) | | | 122 | | $ | 27.20 | | | 3,304 | |
|
|
|
|
|
|
|
|
|
| |
Total | | | 144 | | | | | $ | 3,797 | |
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | In June 2005, we announced a $5 billion share-purchase program, which we increased in June 2006 to $18 billion. |
| | |
| (b) | In October 2004, we announced a $5 billion share-purchase program, which we completed in June 2005. |
B. Preferred Stock
The Series A convertible perpetual preferred stock is held by an Employee Stock Ownership Plan (“Preferred ESOP”) Trust and provides dividends at the rate of 6.25%, which are accumulated and paid quarterly. The per-share stated value is $40,300 and the preferred stock ranks senior to our common stock as to dividends and liquidation rights. Each share is convertible, at the holder’s option, into 2,574.87 shares of our common stock with equal voting rights. The conversion option is indexed to our common stock and requires share settlement, and therefore, is reported at the fair value at the date of issuance. We may redeem the preferred stock at any time or upon termination of the Preferred ESOP, at our option, in cash, in shares of common stock or a combination of both at a price of $40,300 per share.
C. Employee Stock Ownership Plans
We have two employee stock ownership plans (collectively the “ESOPs”), a Preferred ESOP and another that holds common stock of the company (“Common ESOP”). A portion of the matching contributions for legacy Pharmacia U.S. savings plan participants is funded through the ESOPs.
In January 2007, we paid the remaining balance of financing, which was outstanding prior to our acquisition of Pharmacia in 2003, relating to the Preferred ESOP. Compensation expense related to the ESOPs totaled approximately $35 million in 2007, $37 million in 2006 and $44 million in 2005.
Allocated shares held by the Common ESOP are considered outstanding for the earnings per share (EPS) calculations and the eventual conversion of allocated preferred shares held by the Preferred ESOP is assumed in the diluted EPS calculation. As of December 31, 2007, the Preferred ESOP held preferred shares with a stated value of approximately $93 million, convertible into approximately six million shares of our common stock. As of
December 31, 2007, the Common ESOP held approximately 6 million shares of our common stock. As of December 31, 2007, all preferred and common shares held by the ESOPs have been allocated to the Pharmacia U.S. and certain Puerto Rico savings plan participants.
D. Employee Benefit Trust
The Pfizer Inc Employee Benefit Trust (EBT) was established in 1999 to fund our employee benefit plans through the use of its holdings of Pfizer Inc stock. The consolidated balance sheets reflect the fair value of the shares owned by the EBT as a reduction ofShareholders’ equity.
16.Share-Based Payments
Our compensation programs can include share-based payments. In 2007, 2006 and 2005, the primary share-based awards and their general terms and conditions are as follows:
| |
• | Stock options, which entitle the holder to purchase, after the end of a vesting term, a specified number of shares of Pfizer common stock at a price per share set equal to the market price of Pfizer common stock on the date of grant. |
| |
• | Restricted stock units (RSUs), which entitle the holder to receive, at the end of a vesting term, a specified number of shares of Pfizer common stock, including shares resulting from dividend equivalents paid on such RSUs. |
| |
• | Performance share awards (PSAs) and performance-contingent share awards (PCSAs), which entitle the holder to receive, at the end of a vesting term, a number of shares of Pfizer common stock, within a range of shares from zero to a specified maximum, calculated using a non-discretionary formula that measures Pfizer’s performance relative to an industry peer group. Dividend equivalents are paid on PSAs. |
| |
• | Restricted stock grants, which entitle the holder to receive, at the end of a vesting term, a specified number of shares of Pfizer common stock, and which also entitle the holder to receive dividends paid on such grants. |
The Company’s shareholders approved the Pfizer Inc. 2004 Stock Plan (the 2004 Plan) at the Annual Meeting of Shareholders held on April 22, 2004 and, effective upon that approval, new stock option and other share-based awards may be granted only under the 2004 Plan. The 2004 Plan allows a maximum of 3 million shares to be awarded to any employee per year and 475 million shares in total. RSUs, PSAs, PCSAs and restricted stock grants count as three shares, while stock options count as one share under the 2004 Plan toward the maximums.
In the past, we had various employee stock and incentive plans under which stock options and other share-based awards were granted. Stock options and other share-based awards that were granted under prior plans and were outstanding on April 22, 2004, continue in accordance with the terms of the respective plans.
As of December 31, 2007, 269 million shares were available for award, which include 40 million shares available for award under the legacy Pharmacia Long-Term Incentive Plan, which reflects award cancellations returned to the pool of available shares for legacy Pharmacia commitments.
64 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
Although not required to do so, historically, we have used authorized and unissued shares and, to a lesser extent, shares held in our Employee Benefit Trust and treasury stock to satisfy our obligations under these programs.
A. Impact on Net Income
The components of share-based compensation expense and the associated tax benefit follow:
| | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Stock option expense(a) | | $ | 286 | | | $ | 410 | | $ | — | |
Restricted stock unit expense | | | 160 | | | | 184 | | | 120 | |
PSA and PCSA (expense reduction)/expense | | | (9 | ) | | | 61 | | | 37 | |
|
|
|
|
|
|
|
|
|
|
| |
Share-based payment expense | | | 437 | | | | 655 | | | 157 | |
Tax benefit for share-based compensation expense | | | (141 | ) | | | (204 | ) | | (50 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Share-based payment expense, net of tax | | $ | 296 | | | $ | 451 | | $ | 107 | |
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | In 2006, we adopted the fair value method of accounting for stock options. |
Amounts capitalized as part of inventory cost were not significant. In 2007 and 2006, the impact of modifications under our cost-reduction initiatives to share-based awards was not significant and, in 2005, the impact of modifications under the Pharmacia restructuring program was not significant. Generally, these modifications resulted in an acceleration of vesting, either in accordance with plan terms or at management’s discretion.
B. Stock Options
Stock options, which entitle the holder to purchase, at the end of a vesting term, a specified number of shares of Pfizer common stock at a price per share set equal to the market price of Pfizer common stock on the date of grant, are accounted for at fair value at the date of grant in the income statement beginning in 2006. These fair values are generally amortized on an even basis over the vesting term intoCost of sales, Selling, informational and administrative expensesandResearch and development expenses,as appropriate.
In 2005 and earlier years, stock options were accounted for under APB No. 25, using the intrinsic value method in the income statement and fair value information was disclosed. In these disclosures of fair value, we allocated stock option compensation expense based on the nominal vesting period, rather than the expected time to achieve retirement eligibility. In 2006, we changed our method of allocating stock option compensation expense to a method based on the substantive vesting period for all new awards, while continuing to allocate outstanding nonvested awards not yet recognized as of December 31, 2005, under the nominal vesting period method. Specifically, under this prospective change in accounting policy, compensation expense related to stock options granted prior to 2006, that are subject to accelerated vesting upon retirement eligibility, is being recognized over the vesting term of the grant, even though the service period after retirement eligibility is not considered to be a substantive vesting requirement. The impact of this change was not significant.
All employees may receive stock option grants. In virtually all instances, stock options vest after three years of continuous
service from the grant date and have a contractual term of ten years; for certain grants to certain members of management, vesting typically occurs in equal annual installments after three, four and five years from the grant date. In all cases, even for stock options that are subject to accelerated vesting upon voluntary retirement, stock options must be held for at least one year from grant date before any vesting may occur. In the event of a divestiture or restructuring, options held by employees are immediately vested and are exercisable from three months to their remaining term, depending on various conditions.
The fair value of each stock option grant is estimated on the grant date using, for virtually all grants, the Black-Scholes-Merton option-pricing model, which incorporates a number of valuation assumptions noted in the following table, shown at their weighted-average values:
| | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
| | YEAR ENDED DEC. 31, | |
| |
|
|
| | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
|
Expected dividend yield(a) | | | 4.49 | % | | | 3.65 | % | | 2.90 | % |
Risk-free interest rate(b) | | | 4.69 | % | | | 4.59 | % | | 3.96 | % |
Expected stock price volatility(c) | | | 21.28 | % | | | 24.47 | % | | 21.93 | % |
Expected term(d)(years) | | | 5.75 | | | | 6.0 | | | 5.75 | |
|
|
|
|
|
|
|
|
|
|
|
|
| | |
| (a) | Determined in 2007 and 2006, using a constant dividend yield during the expected term of the option. In 2005, determined using a historical pattern of dividend payments. |
| | |
| (b) | Determined using the extrapolated yield on U.S. Treasury zero-coupon issues. |
| | |
| (c) | Determined using implied volatility, after consideration of historical volatility. |
| | |
| (d) | Determined using historical exercise and post-vesting termination patterns. |
Starting in the first quarter of 2006, we changed our method of estimating expected stock price volatility to reflect market-based inputs under emerging stock option valuation considerations. We use the implied volatility in a long-term traded option, after consideration of historical volatility. In 2005, we used an average term structure of volatility after consideration of historical volatility.
2007 Financial Report | 65
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
The following table summarizes all stock option activity during 2007, 2006 and 2005:
| | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
| |
| | SHARES
(THOUSANDS) | | | WEIGHTED-
AVERAGE
EXERCISE
PRICE
PER
SHARE | | WEIGHTED-
AVERAGE
REMAINING
CONTRACTUAL
TERM
(YEARS) | | AGGREGATE
INTRINSIC
VALUE
(MILLIONS) | (a)
|
|
|
|
|
|
|
|
|
|
|
|
| |
Outstanding, | | | | | | | | | | | | |
January 1, 2005 | | 635,139 | | | $ | 33.10 | | | | | | |
Granted | | 52,082 | | | | 26.22 | | | | | | |
Exercised | | (31,373 | ) | | | 12.17 | | | | | | |
Forfeited | | (10,072 | ) | | | 32.76 | | | | | | |
Cancelled | | (18,372 | ) | | | 35.40 | | | | | | |
|
|
|
| | | | | | | | | |
Outstanding, | | | | | | | | | | | | |
December 31, 2005 | | 627,404 | | | | 33.51 | | | | | | |
Granted | | 69,300 | | | | 26.20 | | | | | | |
Exercised | | (38,953 | ) | | | 16.09 | | | | | | |
Forfeited | | (9,370 | ) | | | 39.01 | | | | | | |
Cancelled | | (63,591 | ) | | | 32.51 | | | | | | |
|
|
|
| | | | | | | | | |
Outstanding, | | | | | | | | | | | | |
December 31, 2006 | | 584,790 | | | | 33.96 | | | | | | |
Granted | | 51,215 | | | | 25.84 | | | | | | |
Exercised | | (27,391 | ) | | | 19.68 | | | | | | |
Forfeited | | (8,152 | ) | | | 28.00 | | | | | | |
Cancelled | | (77,257 | ) | | | 34.47 | | | | | | |
|
|
|
| | | | | | | | | |
Outstanding, | | | | | | | | | | | | |
December 31, 2007 | | 523,205 | | | | 33.93 | | 4.8 | | $ | 10 | |
Vested and expected to vest(b), | | | | | | | | | | | | |
December 31, 2007 | | 517,032 | | | | 34.02 | | 4.7 | | | 10 | |
Exercisable, | | | | | | | | | | | | |
December 31, 2007 | | 380,823 | | | | 36.74 | | 3.5 | | | 10 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| | |
| (a) | Market price of underlying Pfizer common stock less exercise price. |
| | |
| (b) | The number of options expected to vest takes into account an estimate of expected forfeitures. |
The following table provides data related to all stock option activity:
| | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS, EXCEPT PER
STOCK OPTION AMOUNTS AND YEARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Weighted-average grant date fair value per stock option | | $ | 4.11 | | | $ | 5.42 | | $ | 5.15 | |
Aggregate intrinsic value on exercise | | $ | 173 | | | $ | 380 | | $ | 442 | |
Cash received upon exercise | | $ | 532 | | | $ | 622 | | $ | 378 | |
Tax benefits realized related to exercise | | $ | 54 | | | $ | 114 | | $ | 137 | |
Total compensation cost related to nonvested stock options not yet recognized, pre-tax | | $ | 216 | | | $ | 330 | | | N/A | |
Weighted-average period in years over which stock option compensation cost is expected to be recognized | | | 1.2 | | | | 1.1 | | | N/A | |
|
|
|
|
|
|
|
|
|
|
| |
C. Restricted Stock Units
RSUs, which entitle the holder to receive, at the end of a vesting term, a specified number of shares of Pfizer common stock,
including shares resulting from dividend equivalents paid on such RSUs, are accounted for at fair value at the date of grant. For RSUs granted in 2007, in virtually all instances, the units vest after three years of continuous service from the grant date and the fair values are amortized on an even basis over the vesting term intoCost of sales, Selling, informational and administrative expensesandResearch and development expenses,as appropriate. For RSUs granted in 2006 and 2005, the units vest in substantially equal portions each year over five years of continuous service and the fair value related to each year’s portion is then amortized evenly intoCost of sales, Selling, informational and administrative expensesandResearch and development expenses,as appropriate. For certain members of senior and key management, vesting may occur after three years of continuous service.
The fair value of each RSU grant is estimated on the grant date. For RSUs granted in 2007, the fair value is set using the closing price of Pfizer common stock on the date of grant. For RSUs granted in 2006 and 2005, the fair value is set using the average price of Pfizer common stock on the date of grant.
The following table summarizes all RSU activity during 2007, 2006 and 2005:
| | | | | | | | |
|
|
|
|
|
|
|
|
| |
| | SHARES
(THOUSANDS) | | | WEIGHTED-
AVERAGE
GRANT DATE
FAIR VALUE
PER SHARE | |
|
|
|
|
|
|
|
| |
Nonvested, January 1, 2005 | | | 1,920 | | | $ | 31.27 | |
Granted | | | 11,263 | | | | 26.20 | |
Vested | | | (82 | ) | | | 29.56 | |
Reinvested dividend equivalents | | | 297 | | | | 25.15 | |
Forfeited | | | (595 | ) | | | 26.34 | |
|
|
|
|
| | | | |
Nonvested, December 31, 2005 | | | 12,803 | | | | 26.89 | |
Granted | | | 12,734 | | | | 26.15 | |
Vested | | | (3,573 | ) | | | 27.29 | |
Reinvested dividend equivalents | | | 700 | | | | 25.42 | |
Forfeited | | | (2,334 | ) | | | 26.17 | |
|
|
|
|
| | | | |
Nonvested, December 31, 2006 | | | 20,330 | | | | 26.56 | |
Granted | | | 10,459 | | | | 25.77 | |
Vested | | | (5,337 | ) | | | 27.29 | |
Reinvested dividend equivalents | | | 1,018 | | | | 24.87 | |
Forfeited | | | (3,534 | ) | | | 26.09 | |
|
|
|
|
| | | | |
Nonvested, December 31, 2007 | | | 22,936 | | | | 26.37 | |
|
|
|
|
|
|
|
|
|
The following table provides data related to all RSU activity:
| | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS, EXCEPT PER
RSU AMOUNTS AND YEARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Weighted-average grant date fair value per RSU | | $ | 26.18 | | | $ | 26.34 | | $ | 26.21 | |
Total fair value of shares vested | | $ | 146 | | | $ | 98 | | $ | 2 | |
Total compensation cost related to nonvested RSU awards not yet recognized, pre-tax | | $ | 254 | | | $ | 270 | | $ | 180 | |
Weighted-average period in years over which RSU cost is expected to be recognized | | | 2.1 | | | | 3.8 | | | 4.0 | |
|
|
|
|
|
|
|
|
|
|
| |
66 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
D. Performance Share Awards (PSAs) and Performance-Contingent Share Awards (PCSAs)
PSAs in 2007 and 2006, and PCSAs in 2005 and earlier, entitle the holder to receive, at the end of a vesting term, a number of shares of our common stock, within a specified range of shares, calculated using a non-discretionary formula that measures our performance relative to an industry peer group. PSAs are accounted for at fair value at the date of grant in the income statement beginning with grants in 2006. Further, PSAs are generally amortized on an even basis over the vesting term intoCost of sales, Selling, informational and administrative expensesandResearch and development expenses,as appropriate. For grants in 2005 and earlier years, PCSA grants are accounted for using the intrinsic value method in the income statement. Senior and other key members of management may receive PSA and PCSA grants. In most instances, PSA grants vest after three years and PCSA grants vest after five years of continuous service from the grant date. In certain instances, PCSA grants vest over two to four years of continuous service from the grant date. The vesting terms are equal to the contractual terms.
The 2004 Plan limitations on the maximum amount of share-based awards apply to all awards, including PCSA and PSA grants. In 2001, our shareholders approved the 2001 Performance-Contingent Share Award Plan (the 2001 Plan), allowing a maximum of 12.5 million shares to be awarded to all participants. This maximum was applied to awards for performance periods beginning after January 1, 2002 through 2004. The 2004 Plan is the only plan under which share-based awards may be granted in the future.
PSA grants made in 2007 and 2006 will vest and be paid based on a non-discretionary formula that measures our performance using relative total shareholder return over a performance period relative to an industry peer group. If our minimum performance in the measure is below the threshold level relative to the peer group, then no shares will be paid. PCSA grants made prior to 2006 will vest and be paid based on a non-discretionary formula, which measures our performance using relative total shareholder return and relative change in diluted EPS over a performance period relative to an industry peer group. If our minimum performance in the measures is below the threshold level relative to the peer group, then no shares will be paid.
As of January 1, 2006, we measure PSA grants at fair value, using a Monte Carlo simulation model, times the target number of shares. The target number of shares is determined by reference to the fair value of share-based awards to similar employees in the industry peer group. We measure PCSA grants at intrinsic value whereby the probable award was allocated over the term of the award, then the resultant shares are adjusted to the fair value of our common stock at each accounting period until the date of payment.
The following table summarizes all PSA and PCSA activity during 2007, 2006 and 2005, with the shares granted representing the maximum award that could be achieved:
| | | | | | | | |
|
|
|
|
|
|
|
|
|
| | SHARES
(THOUSANDS) | | | WEIGHTED-
AVERAGE
GRANT DATE
VALUE PER
SHARE | |
|
|
|
|
|
|
|
Nonvested, January 1, 2005 | | | 16,466 | | | $ | 26.89 | |
Granted | | | 2,549 | | | | 26.15 | |
Vested | | | (1,652 | ) | | | 26.20 | |
Forfeited(a) | | | (1,384 | ) | | | 26.28 | |
|
|
|
|
| | | | |
Nonvested, December 31, 2005 | | | 15,979 | | | | 23.32 | |
Granted | | | 1,728 | | | | 34.84 | |
Vested | | | (1,583 | ) | | | 26.20 | |
Reinvested dividend equivalent | | | 44 | | | | 25.36 | |
Forfeited(a) | | | (2,388 | ) | | | 26.11 | |
|
|
|
|
| | | | |
Nonvested, December 31, 2006 | | | 13,780 | | | | 26.78 | |
Granted | | | 1,183 | | | | 28.80 | |
Vested | | | (1,788 | ) | | | 25.87 | |
Reinvested dividend equivalents | | | 22 | | | | 24.82 | |
Forfeited(a) | | | (5,166 | ) | | | 26.44 | |
Modifications(b) | | | 2,192 | | | | 25.66 | |
|
|
|
|
| | | | |
Nonvested, December 31, 2007 | | | 10,223 | | | | 24.81 | |
|
|
|
|
|
|
|
|
|
| | |
| (a) | Forfeited includes nil in 2007, 345 thousand shares in 2006 and 454 thousand shares in 2005 that were forfeited by retirees. At the discretion of the Compensation Committee of our Board of Directors, $9.0 million in 2006 and $11.9 million in 2005 were paid in cash to such retirees, which amounts were equivalent to the fair value of the forfeited shares pro rated for the portion of the performance period that was completed prior to retirement. |
| | |
| (b) | Includes modifications to PCSA and PSA awards to pro rate the awards for services to the date of termination for 34 employees. The modifications were made at the discretion of the Board of Directors, the Executive Leadership Team or the current Chairman. There was no incremental cost related to the modifications. |
The following table provides data related to all PSA and PCSA activity:
| | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS, EXCEPT PER
PCSA AMOUNTS AND YEARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
|
|
| |
Weighted-average grant date fair value per PCSA | | $ | 22.73 | | | $ | 25.90 | | $ | 23.32 | |
Total intrinsic value of vested PCSA shares | | $ | 46 | | | $ | 51 | | $ | 56 | |
Total compensation cost related to nonvested PSA grants not yet recognized, pre-tax | | $ | 15 | | | $ | 10 | | | N/A | |
Weighted-average period in years over which PSA cost is expected to be recognized | | | 2 | | | | 2 | | | N/A | |
|
|
|
|
|
|
|
|
|
|
| |
We entered into forward-purchase contracts that partially offset the potential impact on net income of our obligation under the pre-2006 PCSAs. At settlement date, we would, at the option of the counterparty to each of the contracts, either receive our own stock or settle the contracts for cash. We had contracts for approximately 3 million shares of our stock at a per share price
2007 Financial Report | 67
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
of $33.85 outstanding as of December 31, 2006. The contracts matured early in 2007.
The financial statements include the following items related to these contracts:
Prepaid expenses and taxesin 2006 includes:
| |
• | fair value of these contracts. |
| |
Other (income)/deductions—netincludes: |
|
• | changes in the fair value of these contracts. |
E. Restricted Stock
Restricted stock grants, which entitle the holder to receive, at the end of a vesting term, a specified number of shares of our common stock, and which also entitle the holder to receive dividends paid on such grants, are accounted for at fair value at the date of grant.
Senior and key members of management received restricted stock awards prior to 2005. In most instances, restricted stock grants vest after three years of continuous service from the grant date. The vesting terms are equal to the contractual terms. These awards have not been significant.
F. Transition Information
The following table shows the effect on results for 2005 as if we had applied the fair-value-based recognition provisions to measure stock-based compensation expense for the option grants:
| | | | |
|
|
|
|
|
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | | YEAR
ENDED
DEC. 31,
2005 | |
|
|
|
| |
Net income available to common shareholders used in the calculation of basic earnings per common share: | | | | |
As reported under U.S. GAAP(a) | | $ | 8,079 | |
Compensation expense—net of tax(b) | | | (457 | ) |
|
|
|
| |
Pro forma | | $ | 7,622 | |
|
|
|
| |
Basic earnings per common share: | | | | |
As reported under U.S. GAAP(a) | | $ | 1.10 | |
Compensation expense—net of tax(b) | | | (0.06 | ) |
|
|
|
| |
Pro forma | | $ | 1.04 | |
|
|
|
| |
Net income available to common shareholders used in the calculation of diluted earnings per common share: | | | | |
As reported under U.S. GAAP(a) | | $ | 8,080 | |
Compensation expense—net of tax(b) | | | (457 | ) |
|
|
|
| |
Pro forma | | $ | 7,623 | |
|
|
|
| |
Diluted earnings per common share: | | | | |
As reported under U.S. GAAP(a) | | $ | 1.09 | |
Compensation expense—net of tax(b) | | | (0.06 | ) |
|
|
|
| |
Pro forma | | $ | 1.03 | |
|
|
|
| |
| | |
| (a) | Includes stock-based compensation expense, net of related tax effects, of $107 million (of which $70 million related to RSUs and a nominal amount was a result of acceleration of vesting due to our cost-reduction initiatives). |
| | |
| (b) | Pro forma compensation expense related to stock options that are subject to accelerated vesting upon retirement is recognized over the period of employment up to the vesting date of the grant. |
17. Earnings per Common Share
Basic and diluted EPS were computed using the following common share data:
| | | | | | | | | | | |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
|
|
|
|
EPS Numerator—Basic: | | | | | | | | | | | |
Income from continuing operations before cumulative effect of a change in accounting principles | | $ | 8,213 | | | $ | 11,024 | | $ | 7,610 | |
Less: Preferred stock dividends—net of tax | | | 4 | | | | 5 | | | 6 | |
|
|
|
|
|
|
|
|
|
|
| |
Income available to common shareholders from continuing operations before cumulative effect of a change in accounting principles | | | 8,209 | | | | 11,019 | | | 7,604 | |
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations: | | | | | | | | | | | |
Income/(loss) from discontinued operations—net of tax | | | (3 | ) | | | 433 | | | 451 | |
Gains/(losses) on sales of discontinued operations—net of tax | | | (66 | ) | | | 7,880 | | | 47 | |
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations—net of tax | | | (69 | ) | | | 8,313 | | | 498 | |
|
|
|
|
|
|
|
|
|
|
| |
Income available to common shareholders before cumulative effect of a change in accounting principles | | | 8,140 | | | | 19,332 | | | 8,102 | |
Cumulative effect of a change in accounting principles—net of tax | | | — | | | | — | | | (23 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Net income available to common shareholders | | $ | 8,140 | | | $ | 19,332 | | $ | 8,079 | |
|
|
|
|
|
|
|
|
|
|
| |
EPS Denominator—Basic: | | | | | | | | | | | |
Weighted-average number of common shares outstanding | | | 6,917 | | | | 7,242 | | | 7,361 | |
|
|
|
|
|
|
|
|
|
|
| |
EPS Numerator—Diluted: | | | | | | | | | | | |
Income from continuing operations before cumulative effect of a change in accounting principles | | $ | 8,213 | | | $ | 11,024 | | $ | 7,610 | |
Less: ESOP contribution—net of tax | | | 2 | | | | 3 | | | 5 | |
|
|
|
|
|
|
|
|
|
|
| |
Income available to common shareholders from continuing operations before cumulative effect of a change in accounting principles | | | 8,211 | | | | 11,021 | | | 7,605 | |
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations: | | | | | | | | | | | |
Income/(loss) from discontinued operations—net of tax | | | (3 | ) | | | 433 | | | 451 | |
Gains/(losses) on sales of discontinued operations—net of tax | | | (66 | ) | | | 7,880 | | | 47 | |
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations—net of tax | | | (69 | ) | | | 8,313 | | | 498 | |
|
|
|
|
|
|
|
|
|
|
| |
Income available to common shareholders before cumulative effect of a change in accounting principles | | | 8,142 | | | | 19,334 | | | 8,103 | |
Cumulative effect of a change in accounting principles—net of tax | | | — | | | | — | | | (23 | ) |
|
|
|
|
|
|
|
|
|
|
| |
Net income available to common shareholders | | $ | 8,142 | | | $ | 19,334 | | $ | 8,080 | |
|
|
|
|
|
|
|
|
|
|
| |
EPS Denominator—Diluted: | | | | | | | | | | | |
Weighted-average number of common shares outstanding | | | 6,917 | | | | 7,242 | | | 7,361 | |
Common-share equivalents—stock options, stock issuable under employee compensation plans and convertible preferred stock | | | 22 | | | | 32 | | | 50 | |
|
|
|
|
|
|
|
|
|
|
| |
Weighted-average number of common shares outstanding and common-share equivalents | | | 6,939 | | | | 7,274 | | | 7,411 | |
|
|
|
|
|
|
|
|
|
|
| |
Stock options that had exercise prices greater than the average market price of our common stock issuable under employee compensation plans(a) | | | 514 | | | | 552 | | | 557 | |
|
|
|
|
|
|
|
|
|
|
| |
| |
(a) | These common stock equivalents were outstanding during 2007, 2006 and 2005, but were not included in the computation of diluted EPS for those years because their inclusion would have had an anti-dilutive effect. |
68 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
18. Lease Commitments
We lease properties and equipment for use in our operations. In addition to rent, the leases may require us to pay directly for taxes, insurance, maintenance and other operating expenses, or to pay higher rent when operating expenses increase. Rental expense, net of sublease income, was $398 million in 2007, $420 million in 2006 and $410 million in 2005. This table shows future minimum rental commitments under noncancellable operating leases as of December 31 for the following years:
| | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
(MILLIONS OF DOLLARS) | | 2008 | | 2009 | | 2010 | | 2011 | | 2012 | | AFTER
2012 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Lease commitments | | $ | 212 | | $ | 192 | | $ | 151 | | $ | 99 | | $ | 76 | | $ | 788 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
19. Insurance
Our insurance coverage reflects market conditions (including cost and availability) existing at the time it is written, and our decision to obtain insurance coverage or to self-insure varies accordingly. Depending upon the cost and availability of insurance and the nature of the risk involved, the amount of self-insurance may be significant. The cost and availability of coverage have resulted in our decision to self-insure certain exposures, including product liability. If we incur substantial liabilities that are not covered by insurance or substantially exceed insurance coverage and that are in excess of existing accruals, there could be a material adverse effect on our results of operations in any particular period (see Note 20.Legal Proceedings and Contingencies).
20. Legal Proceedings and Contingencies
We and certain of our subsidiaries are involved in various patent, product liability, consumer, commercial, securities, environmental and tax litigations and claims; government investigations; and other legal proceedings that arise from time to time in the ordinary course of our business. We do not believe any of them will have a material adverse effect on our financial position.
Beginning in 2007 upon the adoption of a new accounting standard, we record accruals for income tax contingencies to the extent that we conclude that a tax position is not sustainable under a ‘more likely than not’ standard and we record our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction when we conclude that the potential recovery is more likely than not. (SeeNote 1D. Significant Accounting Policies: New Accounting StandardsandNote 8E. Taxes on Income: Tax Contingencies.) We record accruals for all other contingencies to the extent that we conclude their occurrence is probable and the related damages are estimable, and we record anticipated recoveries under existing insurance contracts when assured of recovery. If a range of liability is probable and estimable and some amount within the range appears to be a better estimate than any other amount within the range, we accrue that amount. If a range of liability is probable and estimable and no amount within the range appears to be a better estimate than any other amount within the range, we accrue the minimum of such probable range. Many claims involve highly complex issues relating to causation, label warnings, scientific evidence, actual damages and other matters. Often these issues are subject to substantial
uncertainties and, therefore, the probability of loss and an estimation of damages are difficult to ascertain. Consequently, we cannot reasonably estimate the maximum potential exposure or the range of possible loss in excess of amounts accrued for these contingencies. These assessments can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions (seeNote 1B. Significant Accounting Policies: Estimates and Assumptions). Our assessments are based on estimates and assumptions that have been deemed reasonable by management. Litigation is inherently unpredictable, and excessive verdicts do occur. Although we believe we have substantial defenses in these matters, we could in the future incur judgments or enter into settlements of claims that could have a material adverse effect on our results of operations in any particular period.
Patent claims include challenges to the coverage and/or validity of our patents on various products or processes. Although we believe we have substantial defenses to these challenges with respect to all our material patents, there can be no assurance as to the outcome of these matters, and a loss in any of these cases could result in a loss of patent protection for the drug at issue, which could lead to a significant loss of sales of that drug and could materially affect future results of operations.
Among the principal matters pending to which we are a party are the following:
A. Patent Matters
We are involved in a number of suits relating to our U.S. patents, the majority of which involve claims by generic drug manufacturers that patents covering our products, processes or dosage forms are invalid and/or do not cover the product of the generic manufacturer. Pending suits include generic challenges to patents covering, among other products, atorvastatin (Lipitor), atorvastatin/amlodipine combination (Caduet), celecoxib (Celebrex), tolterodine (Detrol and Detrol LA) and donepezil hydrochloride (Aricept). Also, counterclaims as well as various independent actions have been filed claiming that our assertions of, or attempts to enforce, our patent rights with respect to certain products constitute unfair competition and/or violations of the antitrust laws. In addition to the challenges to the U.S. patents on a number of our products that are discussed below, we note that the patent rights to certain of our products, including without limitation Lipitor and Celebrex, are being challenged in various other countries.
Lipitor (atorvastatin)
U.S. – basic patent: In July 2007, a law firm that has represented Ranbaxy Pharmaceuticals Inc. (Ranbaxy) in Lipitor patent litigation filed a request for a reexamination of our basic Lipitor patent with the U.S. Patent and Trademark Office (the Patent Office). The basic patent, including the six-month pediatric exclusivity period, expires in March 2010. In August 2007, the Patent Office granted the request to reexamine the basic patent on the merits. In January 2008, the Patent Office issued its initial official action, rejecting the patent’s claims. We will address the issues raised by the examiner in our response to the Patent Office. An initial rejection of a patent is not unusual in reexamination proceedings, and we continue to believe that the basic patent was properly
2007 Financial Report | 69
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
granted and will be upheld on reexamination. This process could take a few years to complete.
U.S. – enantiomer patent: In January 2007, we filed a reissue application with the Patent Office seeking to correct a technical defect in our patent covering the enantiomer form of atorvastatin. The enantiomer patent, including the six-month pediatric exclusivity period, expires in June 2011. In August 2007, the Patent Office issued its initial official action, which determined that the technical defect had been corrected but rejected the enantiomer patent on other grounds. In October 2007, we submitted our response to the Patent Office. We continue to believe that we have strong arguments for securing the reissued patent. This process also could take a few years to complete.
Separately, in April 2007, Teva Pharmaceuticals USA, Inc. (Teva) notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a generic version of Lipitor. Teva asserts the invalidity of our enantiomer patent and the non-infringement of certain later-expiring patents, but does not challenge our basic patent. In June 2007, we filed suit against Teva in the U.S. District Court for the District of Delaware asserting the validity and infringement of the enantiomer patent.
In addition, in October 2007, Cobalt Pharmaceuticals, Inc. (Cobalt) notified us that it had filed an application with the FDA seeking approval to market a product containing atorvastatin sodium, a salt that is different from atorvastatin calcium, which is used in Lipitor. The notice states that Cobalt is challenging our enantiomer patent and certain later-expiring patents, but not our basic patent. In December 2007, we filed suit against Cobalt in the U.S. District Court for the District of Delaware asserting the validity and infringement of the enantiomer patent.
Canada – enantiomer patent: In January 2007, the Canadian Federal Court in Toronto denied our application to prevent approval of Ranbaxy’s generic atorvastatin product based on our enantiomer patent, which expires in July 2010. In February 2007, we appealed that decision to the Federal Court of Appeal of Canada. The appeal was heard in May 2007, and we are awaiting the decision. We also are seeking to prevent approval of Apotex Inc.’s (Apotex’s) generic atorvastatin product based on our enantiomer patent. A trial was held on this matter in October 2007 in the Canadian Federal Court in Toronto and, on January 2, 2008, the court denied our application. On January 3, 2008, we appealed the decision to the Federal Court of Appeal of Canada.
Canada – certain other patents: In September 2007, in a case against Ranbaxy, the Canadian Federal Court in Toronto issued a decision concerning two other patents. First, the court ruled that our patent covering a crystalline form of atorvastatin would be infringed by Ranbaxy’s process for making its proposed generic atorvastatin product. The court granted our application for an order preventing Ranbaxy from launching its product until the expiration of the patent in July 2016. In October 2007, Ranbaxy appealed this decision to the Federal Court of Appeal of Canada. This decision does not apply to any other generic manufacturer, including Apotex, which is challenging the same patent and other crystalline patents in another proceeding. Second, the Canadian Federal Court in Toronto denied our application for a prohibition order against Ranbaxy in connection with another
patent covering a process for making amorphous atorvastatin, which also expires in July 2016.
Caduet (atorvastatin/amlodipine combination)
In January 2007, Ranbaxy notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a generic version of Caduet and asserting the invalidity of our patents relating to atorvastatin and of our patent covering the atorvastatin/amlodipine combination, which expires in 2018. In March 2007, we filed suit against Ranbaxy in the U.S. District Court for the District of Delaware asserting the validity and/or infringement of the subject patents. In November 2007, the court granted our motion to dismiss Ranbaxy’s challenge to the validity of the atorvastatin (Lipitor) basic patent. The case continues with respect to our assertion of infringement of the patent covering the atorvastatin/amlodipine combination and, at such time as the atorvastatin enantiomer patent is reissued in corrected form, Ranbaxy’s challenge regarding the validity of one claim of that patent.
Norvasc (amlodipine)
In 2006, the Federal Court of Appeal of Canada upheld the validity of our Norvasc patent in Canada in an action involving the generic manufacturer Ratiopharm. The Supreme Court of Canada denied Ratiopharm’s petition to appeal this decision. We also have filed legal challenges against certain other generic manufacturers who are seeking to market their own amlodipine products in Canada. In February 2008, a trial was held in the Federal Court of Canada in Toronto in our challenge against Cobalt, and we are awaiting the decision. Our Norvasc patent in Canada expires in August 2010.
Celebrex (celecoxib)
In January 2004, Teva notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a product containing celecoxib and asserting the non-infringement and invalidity of our patents relating to celecoxib. In February 2004, we filed suit against Teva in the U.S. District Court for the District of New Jersey asserting infringement of our patents relating to celecoxib. In March 2007, the court held that all three of the patents in dispute are valid and infringed and, in April 2007, it issued an injunction prohibiting Teva from marketing its generic celecoxib product before 2015. In April 2007, Teva appealed the decision to the U.S. Court of Appeals for the Federal Circuit. The appeal was heard in January 2008, and we are awaiting the decision.
Neurontin (gabapentin)
In August 2005, the U.S. District Court for the District of New Jersey held that the generic gabapentin (Neurontin) products of a number of generic manufacturers did not infringe our gabapentin low-lactam patent, which expires in 2017, and it granted summary judgment in their favor. Several generic manufacturers launched their gabapentin products in 2004 and 2005. In September 2007, the U.S. Court of Appeals for the Federal Circuit reversed the District Court’s summary judgment decision and remanded the case to the District Court for trial on the patent-infringement issue. If successful at trial, we intend to seek compensation from the generic manufacturers for damages resulting from their at-risk launches of generic gabapentin.
Detrol (tolterodine)
In March 2004, we brought a patent infringement suit in the U.S. District Court for the District of New Jersey against Teva, which had filed an abbreviated new drug application with the FDA seeking approval to market a generic version of Detrol. In January 2007, Teva withdrew its challenge to our patent, and the patent infringement suit was dismissed. At about the same time in January 2007, Ivax Pharmaceuticals, Inc. (Ivax), a wholly owned subsidiary of Teva, amended its previously filed abbreviated new drug application for tolterodine to challenge our tolterodine patent, and we brought a patent infringement action against Ivax in the U.S. District Court for the District of New Jersey.
70 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
Detrol LA (tolterodine)
In October 2007, Teva notified us that it had filed an abbreviated new drug application with the FDA challenging on various grounds four patents relating to Detrol LA, an extended-release formulation of Detrol (tolterodine), and seeking approval to market a generic version of Detrol LA. In December 2007, we filed suit against Teva in the U.S. District Court for the Southern District of New York asserting the infringement of three of the patents relating to Detrol LA. In January 2008, Impax Laboratories Inc. notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a generic version of Detrol LA and challenging the same four patents as Teva.
Aricept (donepezil hydrochloride)
In October 2005, Teva notified Eisai Co., Ltd. (Eisai) that Teva had filed an abbreviated new drug application with the FDA challenging on various grounds Eisai’s U.S. basic product patent for Aricept and seeking approval to market a generic version of Aricept. In December 2005, Eisai filed suit against Teva in the U.S. District Court for the District of New Jersey asserting infringement of that patent. We co-promote Aricept with Eisai in the U.S.
Exubera
In August 2006, Novo Nordisk filed an action against us in the U.S. District Court for the Southern District of New York alleging that our sales of Exubera infringed Novo Nordisk’s patents relating to inhaled insulin and methods of administration of inhaled insulin and seeking damages and injunctive relief. The parties settled this action in December 2007.
B. Product Litigation
Like other pharmaceutical companies, we are defendants in numerous product liability cases, including but not limited to those discussed below, in which the plaintiffs seek relief for personal injuries and other purported damages allegedly caused by our drugs and other products.
Rezulin
Rezulin was a medication that treated insulin resistance and was effective for many patients whose diabetes had not been controlled with other medications. Rezulin was voluntarily withdrawn by Warner-Lambert in March 2000 following approval of two newer medications, which the FDA considered to have similar efficacy and fewer side effects.
In 2003, we took a charge to earnings of $975 million before-tax ($955 million after-tax) in connection with all known personal injury cases and claims relating to Rezulin, and we settled many of those cases and claims. Warner-Lambert continues to defend vigorously the remaining personal injury cases and claims.
Warner-Lambert is also a defendant in a number of suits, including purported class actions, relating to Rezulin that seek relief other than damages for alleged personal injury. These suits are not covered by the charge to earnings that we took in 2003. Motions to certify statewide classes of Rezulin users or purchasers who allegedly incurred economic loss have been denied by state courts in California and Texas and granted by state courts in Illinois and West Virginia. The Illinois action was settled in 2004, as previously reported. The West Virginia action was settled in December 2007 on terms favorable to the Company.
In 2005, the following actions were consolidated for pre-trial proceedings in a Multi-District Litigation(In Re Rezulin Product Liability Litigation MDL-1348)in the U.S. District Court for the Southern District of New York:
| |
• | In April 2001, Louisiana Health Service Indemnity Company and Eastern States Health and Welfare Fund filed a consolidated complaint against Warner-Lambert in the U.S. District Court for the Southern District of New York purportedly on behalf of a class consisting of all health benefit providers that paid for or reimbursed patients for the purchase of Rezulin between February 1997 and April 2001. The action sought to recover amounts paid for Rezulin by the health benefit providers on behalf of their plan participants during the specified period. In September 2005, the court granted Warner-Lambert’s motion for summary judgment and dismissed the complaint. In November 2005, the plaintiffs appealed the decision to the U.S. Court of Appeals for the Second Circuit, and a hearing on the appeal was held in December 2006. In September 2007, the parties voluntarily withdrew the appeal and settled the action on terms favorable to Warner-Lambert. |
| |
• | In May 2005, an action was filed in the U.S. District Court for the Eastern District of Louisiana purportedly on behalf of a nationwide class of third-party payors that asserts claims and seeks damages that are substantially similar to those that had been sought in the Louisiana Health Service Indemnity suit discussed immediately above. This action has been transferred to the Multi-District Litigation. |
| |
• | An action was filed in July 2005 by the Attorney General of the State of Louisiana in the Civil District Court for Orleans Parish, Louisiana, against Warner-Lambert and Pfizer seeking to recover amounts paid by the Louisiana Medicaid program for Rezulin and for medical services to treat persons allegedly injured by Rezulin. This action was removed to the U.S. District Court for the Eastern District of Louisiana and thereafter transferred to the Multi-District Litigation. The court granted our motion for summary judgment and dismissed the complaint in November 2007, and the Louisiana Attorney General appealed the decision to the U.S. Court of Appeals for the Second Circuit in December 2007. |
A number of insurance carriers provided coverage for Rezulin claims against Warner-Lambert. We now have entered into settlements with all of the carriers, resulting in recoveries to us of $397 million.
Asbestos
Quigley Company, Inc. (Quigley), a wholly owned subsidiary, was acquired by Pfizer in 1968 and sold small amounts of products containing asbestos until the early 1970s. In September 2004, Pfizer and Quigley took steps that were intended to resolve all pending and future claims against Pfizer and Quigley in which the claimants allege personal injury from exposure to Quigley products containing asbestos, silica or mixed dust. We took a charge of $369 million before-tax ($229 million after-tax) to third quarter 2004 earnings in connection with these matters.
2007 Financial Report | 71
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
In September 2004, Quigley filed a petition in the U.S. Bankruptcy Court for the Southern District of New York seeking reorganization under Chapter 11 of the U.S. Bankruptcy Code. In March 2005, Quigley filed a reorganization plan in the Bankruptcy Court that needed the approval of both the Bankruptcy Court and the U.S. District Court for the Southern District of New York after receipt of the vote of 75% of the claimants. In connection with that filing, Pfizer entered into settlement agreements with lawyers representing more than 80% of the individuals with claims related to Quigley products against Quigley and Pfizer. The agreements provide for a total of $430 million in payments, of which $215 million became due in December 2005 and is being paid to claimants upon receipt by the Company of certain required documentation from each of the claimants. The reorganization plan provided for the establishment of a Trust (the Trust) for the payment of all remaining pending claims as well as any future claims alleging injury from exposure to Quigley products.
As certified by the balloting agent in May 2006, more than 75% of Quigley’s claimants holding claims that represented more than two-thirds in value of claims against Quigley voted to accept Quigley’s plan of reorganization. In August 2006, in reviewing the voting tabulation methodology, the Bankruptcy Court ruled that certain votes that accepted the plan were not predicated upon the actual value of the claim. As a result, the reorganization plan was not accepted.
In June 2007, Quigley filed an amended plan of reorganization to address the Bankruptcy Court’s concerns regarding the voting tabulation methodology. In July 2007, the Bankruptcy Court held a hearing to consider the adequacy of Quigley’s disclosure statement. In October 2007, the Bankruptcy Court granted Quigley’s application to approve its disclosure statement. On February 26, 2008, the Bankruptcy Court authorized Quigley to solicit its amended reorganization plan for acceptance by claimants. If approved by the claimants and the courts, the amended reorganization plan will result in a permanent injunction directing all pending and future claims alleging personal injury from exposure to Quigley products to the Trust.
Under the amended reorganization plan (as under the original reorganization plan), Pfizer will contribute $405 million to the Trust through a note, which has a present value of $172 million, as well as approximately $100 million in insurance, and will forgive a $30 million secured loan to Quigley. In addition, Pfizer entered into an agreement with the representative of future claimants that provides for the contribution to the Trust of an additional amount with a present value of $88.4 million.
In December 2007, the Bankruptcy Court modified its 2004 preliminary injunction order as it relates to Pfizer. As a result, while asbestos claims against Pfizer that are based on alleged exposure to a Quigley product remain stayed, asbestos claims that are not based on alleged exposure to a Quigley product are no longer stayed.
In a separately negotiated transaction with an insurance company in August 2004, we agreed to a settlement related to certain insurance coverage which provides for payments to us over a ten-year period of amounts totaling $405 million.
Between 1967 and 1982, Warner-Lambert owned American Optical Corporation, which manufactured and sold respiratory protective devices and asbestos safety clothing. In connection with the sale of American Optical in 1982, Warner-Lambert agreed to indemnify the purchaser for certain liabilities, including certain asbestos-related and other claims. As of December 31, 2007, approximately 106,000 claims naming American Optical and numerous other defendants were pending in various federal and state courts seeking damages for alleged personal injury from exposure to asbestos and other allegedly hazardous materials. We are actively engaged in the defense of, and will continue to explore various means to resolve, these claims. Several of the insurance carriers that provided coverage for the American Optical asbestos and other allegedly hazardous materials claims have denied coverage. We believe that these carriers’ position is without merit and are pursuing legal proceedings against such carriers. Separately, there is a small number of lawsuits pending against Pfizer in various federal and state courts seeking damages for alleged personal injury from exposure to products containing asbestos and other allegedly hazardous materials sold by Gibsonburg Lime Products Company, which was acquired by Pfizer in the 1960s and which sold small amounts of products containing asbestos until the early 1970s. There also is a small number of lawsuits pending in various federal and state courts seeking damages for alleged exposure to asbestos in facilities owned or formerly owned by Pfizer or its subsidiaries.
Celebrex and Bextra
In 2003, several purported class action complaints were filed in the U.S. District Court for the District of New Jersey against Pharmacia, Pfizer and certain former officers of Pharmacia. The complaints allege that the defendants violated federal securities laws by misrepresenting the data from a study concerning the gastrointestinal effects of Celebrex. These cases were consolidated for pre-trial proceedings in the District of New Jersey (Alaska Electrical Pension Fund et al. v. Pharmacia Corporation et al.). In January 2007, the court certified a class consisting of all persons who purchased Pharmacia securities from April 17, 2000 through February 6, 2001 and were damaged as a result of the decline in the price of Pharmacia’s securities allegedly attributable to the misrepresentations. Plaintiffs seek damages in an unspecified amount. In October 2007, the court granted defendants’ motion for summary judgment and dismissed the plaintiffs’ claims in their entirety. In November 2007, the plaintiffs appealed the decision to the U.S. Court of Appeals for the Third Circuit.
Pfizer is a defendant in product liability suits, including purported class actions, in various U.S. federal and state courts and in certain other countries alleging personal injury as a result of the use of Celebrex and/or Bextra. These suits include a purported class action filed in 2001 in the U.S. District Court for the Eastern District of New York as well as actions that have been filed since late 2004. In addition, beginning in late 2004, purported class actions have been filed against Pfizer in various U.S. federal and state courts and
72 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
in certain other countries alleging consumer fraud as the result of alleged false advertising of Celebrex and Bextra and the withholding of information from the public regarding the alleged safety risks associated with Celebrex and Bextra. The plaintiffs in these consumer fraud actions seek damages in unspecified amounts for economic loss. In September 2005, the U.S. federal product liability and consumer fraud actions were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Celebrex and Bextra Marketing, Sales Practices and Product Liability Litigation MDL-1699) in the U.S. District Court for the Northern District of California. The majority of the cases involving Celebrex are pending in the Multi-District Litigation and in coordinated proceedings in the Supreme Court of the State of New York. In late 2007 and early 2008, the courts in both of those actions ruled that plaintiffs failed to present reliable scientific evidence necessary to prove that Celebrex can cause heart attacks and strokes at the 200 mg daily dose, which is the most commonly prescribed dose. These rulings render inadmissible certain opinions of plaintiffs’ experts, which we believe could result in the dismissal of many of the Celebrex cases.
In July 2005, an action was filed by the Attorney General of the State of Louisiana in the Civil District Court for Orleans Parish, Louisiana, against Pfizer seeking to recover amounts paid by the Louisiana Medicaid program for Celebrex and Bextra and for medical services to treat persons allegedly injured by Celebrex or Bextra. The action also seeks injunctive relief to prevent the sale of Celebrex and any resumption of the sale of Bextra in Louisiana. This action was removed to the U.S. District Court for the Eastern District of Louisiana and thereafter transferred for consolidated pre-trial proceedings to the same Multi-District Litigation referred to in the preceding paragraph.
Beginning in late 2004, actions, including purported class and shareholder derivative actions, have been filed in various federal and state courts against Pfizer, Pharmacia and certain current and former officers, directors and employees of Pfizer and Pharmacia. These actions include: (i) purported class actions alleging that Pfizer and certain current and former officers of Pfizer violated federal securities laws by misrepresenting the safety of Celebrex and Bextra; (ii) purported shareholder derivative actions alleging that certain of Pfizer’s current and former officers and directors breached fiduciary duties by causing Pfizer to misrepresent the safety of Celebrex and, in certain of the cases, Bextra; and (iii) purported class actions filed by persons who claim to be participants in the Pfizer or Pharmacia Savings Plan alleging that Pfizer and certain current and former officers, directors and employees of Pfizer or, where applicable, Pharmacia and certain former officers, directors and employees of Pharmacia, violated certain provisions of the Employee Retirement Income Security Act of 1974 (ERISA) by selecting and maintaining Pfizer stock as an investment alternative when it allegedly no longer was a suitable or prudent investment option. In June 2005, the federal securities, fiduciary duty and ERISA actions were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Pfizer Inc. Securities, Derivative and “ERISA” Litigation MDL-1688) in the U.S. District Court for the Southern District of New York.
In July 2007, the purported federal shareholder derivative action alleging breach of fiduciary duty was dismissed by the court in the
Multi-District Litigation. In August 2007, the plaintiffs appealed the decision to the U.S. Court of Appeals for the Second Circuit.
Trovan
In May 2007, the Attorney General of the Federation of Nigeria filed civil and criminal actions in the Federal High Court in Abuja against Pfizer, one of our Nigerian subsidiaries, and several current and former U.S. and Nigerian employees, including a current Pfizer director. Also in May 2007, the Attorney General of the State of Kano, Nigeria, filed substantially similar civil and criminal actions in the High Court of Kano State against substantially the same group of defendants. The federal civil action was voluntarily withdrawn by the federal authorities in July 2007, and a new federal civil complaint seeking substantially similar damages against substantially the same group of defendants was filed shortly thereafter.
All of these actions arise out of a 1996 pediatric clinical study of Trovan, an antibiotic then in late-stage development, that was conducted during a severe meningitis epidemic in Kano. The actions allege, among other things, that the study was conducted without proper government authorization and without the informed consent of the parents or guardians of the study participants and resulted in injury or death to a number of study participants. In the civil actions, the federal government is seeking more than $6 billion in damages and the Kano state government is seeking $2.075 billion in damages for, among other things, the costs incurred to provide treatment, compensation and support for the alleged victims and their families; the costs of unrelated health initiatives that failed, allegedly due to societal misgivings attributable to the Trovan study; and general damages. We believe that we have strong defenses in these actions.
The 1996 Trovan clinical study has also been the subject of two civil lawsuits filed against Pfizer in the U.S. District Court for the Southern District of New York on behalf of the study participants. The District Court dismissed both cases in 2005, and those decisions are on appeal to the U.S. Court of Appeals for the Second Circuit.
Hormone-Replacement Therapy
Pfizer and certain wholly owned subsidiaries and limited liability companies, along with several other pharmaceutical manufacturers, have been named as defendants in a number of lawsuits in various federal and state courts alleging personal injury resulting from the use of certain estrogen and progestin medications prescribed for women to treat the symptoms of menopause. Plaintiffs in these suits allege a variety of personal injuries, including breast cancer, stroke and heart disease. Certain co-defendants in some of these actions have asserted indemnification rights against Pfizer and its affiliated companies. The cases against Pfizer and its affiliated companies involve the products femhrt (which Pfizer divested in 2003), Activella and Vagifem (which are Novo Nordisk products that were marketed by a Pfizer affiliate from 2000 to 2004), and Provera, Ogen, Depo-Estradiol, Estring and generic MPA, all of which remain approved by the FDA for use in the treatment of menopause. The federal cases have been transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Prempro Products Liability Litigation MDL-1507) in the U.S. District Court for the Eastern District of Arkansas.
2007 Financial Report | 73
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
This litigation originally included both individual actions as well as various purported nationwide and statewide class actions. However, as a result of the voluntary dismissal of certain purported class actions and the withdrawal of the class action allegations by the plaintiffs in certain other actions, this litigation now consists of individual actions and a few purported statewide class actions.
Viagra
A number of lawsuits, including purported class actions, have been filed against us in various federal and state courts alleging that Viagra causes certain types of visual injuries. The plaintiffs in the purported class actions seek to represent nationwide and certain statewide classes of Viagra users. All of the actions seek damages for personal injury, and the purported class actions also seek medical monitoring. In January 2006, the federal cases were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Viagra Products Liability Litigation MDL-1724) in the U.S. District Court for the District of Minnesota.
Zoloft
A number of individual lawsuits have been filed against us in various federal and state courts alleging personal injury as a result of the purported ingesting of Zoloft.
Mirapex
A number of individual lawsuits seeking damages have been filed against Pfizer and Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) in various U.S. federal and state courts and one purported class action has been filed in Canada alleging that Mirapex, a treatment for Parkinson’s disease, causes certain impulse-control disorders. We co-promoted Mirapex with BIPI until May 2005 but, as a result of the sale of our interests in this product to BIPI, we no longer manufacture or sell Mirapex. In June 2007, all of the U.S. federal cases were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Mirapex Products Liability Litigation MDL-1836) in the U.S. District Court for the District of Minnesota.
Neurontin
A number of lawsuits, including purported class actions, have been filed against us in various federal and state courts alleging claims arising from the promotion and sale of Neurontin. The plaintiffs in the purported class actions seek to represent nationwide and certain statewide classes consisting of persons, including individuals, health insurers, employee benefit plans and other third-party payors, who purchased or reimbursed patients for the purchase of Neurontin that allegedly was used for indications other than those included in the product labeling approved by the FDA. In October 2004, many of the suits pending in federal courts, including individual actions as well as purported class actions, were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Neurontin Marketing, Sales Practices and Product Liability Litigation MDL-1629) in the U.S. District Court for the District of Massachusetts. Purported class actions also have been filed against us in various Canadian provincial courts alleging claims arising from the promotion and sale of Neurontin.
In the Multi-District Litigation, in August 2007, the court denied without prejudice plaintiffs’ motion to certify a nationwide class of all consumers and third-party payors who allegedly purchased or reimbursed patients for the purchase of Neurontin for “off-label” uses from 1994 through 2004. The court indicated that it
would allow plaintiffs to file a renewed motion for class certification under certain circumstances. In December 2007, plaintiffs filed such a motion, which we intend to oppose.
In June 2007, a Pennsylvania state court certified a class of all individuals in Pennsylvania who allegedly purchased Neurontin for “off-label” uses from 1995 to the present. The plaintiffs seek a refund of amounts paid by class members for Neurontin. Plaintiffs also are seeking certification of a statewide class of Neurontin purchasers in an action pending in Kansas state court. State courts in New York and New Mexico have declined to certify statewide classes of Neurontin purchasers.
A number of individual lawsuits have been filed against us in various U.S. federal and state courts and in certain other countries alleging personal injury, suicide and attempted suicide as a result of the purported ingesting of Neurontin. Certain of the U.S. federal actions have been transferred for consolidated pre-trial proceedings to the same Multi-District Litigation referred to in the first paragraph of this section.
Lipitor
Beginning in September 2005, three purported nationwide class actions were filed against us in various federal courts alleging claims relating to the promotion of Lipitor. In January 2006, two of the actions were voluntarily dismissed without prejudice. In the remaining action, which was filed in the U.S. District Court for the Southern District of Florida, the plaintiffs alleged that the Company engaged in false and misleading advertising in violation of state consumer protection laws by allegedly promoting Lipitor for the prevention of heart disease in women (regardless of age) and men over age 55 who in each case had no history of heart disease or diabetes. The action sought monetary and injunctive relief, including treble damages. Effective January 9, 2008, this action was voluntarily dismissed with prejudice without any payment by the Company. In addition, in 2005, a purported class action on behalf of residents of the Province of Quebec was filed against us in Canada that asserts claims under Canadian law and seeks relief substantially similar to the claims that had been asserted and the relief that had been sought in the U.S. action.
In March and April 2006, six purported class actions were filed against us in various federal courts alleging claims relating to the promotion of Lipitor. In May 2006, five of the actions were voluntarily dismissed without prejudice, and the plaintiffs in those actions were added as plaintiffs in the remaining action. The complaint in the remaining action, which was filed in the U.S. District Court for the Northern District of Illinois, alleges that, through patient and medical education programs and other actions, the Company promoted Lipitor for use by certain patients contrary to national cholesterol guidelines that plaintiffs claim are a part of the labeled indications for the product. The plaintiffs seek to represent nationwide and certain statewide classes consisting of health and welfare funds and other third-party payors that purchased Lipitor for such patients or reimbursed such patients for the purchase of Lipitor since January 1, 2002. The plaintiffs allege, among other things, fraud, unjust enrichment and a violation of the federal Racketeer Influenced and Corrupt Organizations Act (“RICO”) and certain state consumer fraud statutes and seek monetary and injunctive relief, including treble damages. In September 2007, plaintiffs filed an amended
74 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
complaint adding allegations that, primarily as the result of the Company’s purported failure to fully disclose the risks of alleged side-effects of Lipitor, the prices that plaintiffs paid for Lipitor were higher than they otherwise would have been.
In 2004, a former employee filed a “whistleblower” action against us in the U.S. District Court for the Eastern District of New York. The complaint remained under seal until September 2007, at which time the U.S. Attorney for the Eastern District of New York declined to intervene in the case. We were served with the complaint on December 19, 2007. Plaintiff alleges that, through patient and medical education programs, written materials and other actions aimed at doctors, consumers, payors and investors, the Company promoted Lipitor for use by certain patients contrary to national cholesterol guidelines that plaintiff claims are a part of the labeled indications for the product. Plaintiff alleges violations of the Federal Civil False Claims Act and the false claims acts of certain states and seeks treble damages and civil penalties on behalf of the U.S. Government and the specified states as the result their purchase, or reimbursement of patients for the purchase, of Lipitor allegedly for such “off-label” uses. Plaintiff also seeks compensation as a whistleblower under those federal and state statutes. In addition, plaintiff alleges that he was wrongfully terminated, in violation of the anti-retaliation provisions of the Federal Civil False Claims Act, the Civil Rights Act of 1964 and applicable New York law, for raising concerns about the alleged “off-label” promotion of Lipitor and about alleged instances of sexual harassment in the workplace, and he seeks damages and the reinstatement of his employment.
C. Commercial and Other Matters
Average Wholesale Price Litigation
A number of states as well as most counties in New York have sued Pharmacia, Pfizer and other pharmaceutical manufacturers alleging that they provided average wholesale price (AWP) information for certain of their products that was higher than the actual prices at which those products were sold. The AWP is used to determine reimbursement levels under Medicare Part B and Medicaid and in many private-sector insurance policies and medical plans. The plaintiffs claim that the alleged spread between the AWPs at which purchasers were reimbursed and the actual prices was promoted by the defendants as an incentive to purchase certain of their products. In addition to suing on their own behalf, many of the plaintiff states seek to recover on behalf of individual Medicare Part B co-payors and private-sector insurance companies and medical plans in their states. These various actions generally assert fraud claims as well as claims under state deceptive trade practice laws, and seek monetary and other relief, including civil penalties and treble damages. Several of the suits also allege that Pharmacia and/or Pfizer did not report to the states their best price for certain products under the Medicaid program.
In addition, Pharmacia, Pfizer and other pharmaceutical manufacturers are defendants in a number of purported class action suits in various federal and state courts brought by employee benefit plans and other third-party payors that assert claims similar to those in the state and county actions. These suits allege, among other things, fraud, unfair competition and unfair trade practices and seek monetary and other relief, including civil penalties and treble damages.
All of these state, county and purported class action suits were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Pharmaceutical Industry Average Wholesale Price Litigation MDL-1456) in the U.S. District Court for the District of Massachusetts. Certain of the state and private suits have been remanded to their respective state courts. In November 2006, the claims against Pfizer in the Multi-District Litigation were dismissed with prejudice; the claims against Pharmacia are still pending.
Monsanto-Related Matters
In 1997, Monsanto Company (Former Monsanto) contributed certain chemical manufacturing operations and facilities to a newly formed corporation, Solutia Inc. (Solutia), and spun off the shares of Solutia. In 2000, Former Monsanto merged with Pharmacia & Upjohn to form Pharmacia Corporation (Pharmacia). Pharmacia then transferred its agricultural operations to a newly created subsidiary, named Monsanto Company (New Monsanto), which it spun off in a two-stage process that was completed in 2002. Pharmacia was acquired by Pfizer in 2003 and is now a wholly owned subsidiary of Pfizer.
In connection with its spin-off that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities related to Pharmacia’s former agricultural business. New Monsanto is defending and indemnifying Pharmacia for various claims and litigation arising out of or related to the agricultural business.
In connection with its spin-off in 1997, Solutia assumed, and agreed to indemnify Pharmacia for, liabilities related to Former Monsanto’s chemical businesses. In addition, in connection with its spinoff that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities primarily related to Former Monsanto’s chemical businesses, including any such liabilities that Solutia assumed. Solutia’s and New Monsanto’s assumption of and agreement to indemnify Pharmacia for these liabilities apply to pending actions and any future actions related to Former Monsanto’s chemical businesses in which Pharmacia is named as a defendant, including, without limitation, actions asserting environmental claims, including alleged exposure to polychlorinated biphenyls.
In December 2003, Solutia filed a petition in the U.S. Bankruptcy Court for the Southern District of New York seeking reorganization under Chapter 11 of the U.S. Bankruptcy Code. Solutia asked the Bankruptcy Court to relieve it from liabilities related to Former Monsanto’s chemical businesses that were assumed by Solutia in 1997. In addition, motions were filed by Solutia in the Chapter 11 proceeding and other actions were filed in the Bankruptcy Court by Solutia and by a committee representing the interests of Solutia’s shareholders that sought to avoid all or a portion of Solutia’s obligations to Pharmacia.
In December 2003, Solutia filed an action, also in the U.S. Bankruptcy Court for the Southern District of New York, seeking a determination that Pharmacia rather than Solutia was responsible for an estimated $475 million in healthcare benefits for certain Solutia retirees. A similar action was filed in May 2004 in the same Bankruptcy Court against Pharmacia and New Monsanto by a committee appointed to represent Solutia retirees in the Bankruptcy Court proceedings.
2007 Financial Report | 75
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
In February 2006, Solutia filed its plan of reorganization in the Bankruptcy Court. In November 2007, the Bankruptcy Court approved the plan of reorganization, and Solutia emerged from bankruptcy in February 2008. Under the reorganization plan, all lawsuits filed against Pharmacia in the Bankruptcy Court by Solutia, the committee representing Solutia retirees and the committee representing Solutia's shareholders have been dismissed or withdrawn with prejudice and without any payment by Pharmacia to Solutia or any other party.
Under the reorganization plan, Solutia's indemnity obligations to Pharmacia that arose in connection with Solutia's 1997 spin-off are shared between Solutia and New Monsanto. New Monsanto is financially responsible for all environmental remediation costs at certain sites that Solutia never owned or operated. Solutia will continue to be financially responsible for all environmental remediation costs at sites that Solutia has owned or operated. The plan also provides that Solutia will indemnify Pharmacia for any environmental remediation costs that Solutia continues to be liable for under the plan. In addition, the plan provides that New Monsanto is financially responsible for all current and future personal injury tort claims related to Former Monsanto's chemical businesses that Solutia assumed in connection with the 1997 spin-off. Finally, under the plan, Pharmacia has been released from all healthcare and other benefit claims of Solutia retirees.
Solutia's reorganization plan does not in any way affect the obligations undertaken by New Monsanto to indemnify Pharmacia for all liabilities that Solutia originally assumed in connection with the 1997 spin-off.
Securities Litigation
In December 2006, a purported class action was filed in the U.S. District Court for the Southern District of New York alleging that Pfizer and certain current officers and one former officer of Pfizer violated federal securities laws by misrepresenting the safety and efficacy of Torcetrapib and the progress of the development program for Torcetrapib, a product candidate whose development program was terminated on December 2, 2006. In April 2007, the plaintiffs filed an amended complaint that, among other things, expanded the purported class period. Pursuant to the amended complaint, the plaintiffs seek to represent a class consisting of all persons who purchased Pfizer securities between January 19, 2005 and December 2, 2006 and were damaged as a result of the decline in the price of Pfizer’s stock, allegedly attributable to the misrepresentations, that followed the announcement of the termination of the Torcetrapib development program. The action seeks compensatory damages in an unspecified amount. On February 28, 2008, the court dismissed the amended complaint and granted the plaintiffs the opportunity to move to replead.
Pharmacia Cash Balance Pension Plan
In 2006, several current and former employees of Pharmacia Corporation filed a purported class action in the U.S. District Court for the Southern District of Illinois against the Pharmacia Cash Balance Pension Plan (the Plan), Pharmacia Corporation, Pharmacia & Upjohn Company and Pfizer Inc. Plaintiffs seek monetary and injunctive relief on behalf of a class consisting of certain current and former participants in the Plan who accrued a benefit in the Monsanto Company Pension Plan prior to its conversion to a cash balance plan in 1997. In January 2002, after various corporate reorganizations, certain of the assets and liabilities of the Monsanto Company Pension Plan were transferred to the Plan. Plaintiffs claim that the Plan violates the age discrimination provisions of the Employee Retirement Income Security Act of 1974 by providing certain credits to such participants only to age 55. This action has been consolidated in the U.S. District Court for the Southern District of Illinois (Walker, et al., v. The Monsanto Company Pension Plan et al.) with purported class actions pending in the same court that make largely similar claims against substantially similar cash balance plans sponsored by Monsanto Company and Solutia Inc., two former affiliates of Pharmacia.
In September 2007, the parties to the action against the Plan submitted to the court an agreed-upon proposed order that would permit the case to proceed as a class action. The court has not yet acted on the proposed order.
Environmental Matters
In August 2007, the U.S. Department of Justice (DOJ) proposed a civil penalty, in an amount that is not material to the Company, to settle certain alleged violations of the Federal Clean Air Act at our Groton, Connecticut manufacturing facility that were identified by the U.S. Environmental Protection Agency (EPA) in 2006. We are in discussions with the DOJ and EPA to resolve this matter, and we have implemented corrective actions to address all of the EPA’s concerns.
We will be required to submit a corrective measures study report to the EPA with regard to Pharmacia’s discontinued industrial chemical facility in North Haven, Connecticut.
We are a party to a number of other proceedings brought under the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended, (CERCLA or Superfund) and other state, local or foreign laws in which the primary relief sought is the cost of past and/or future remediation.
D. Government Investigations and Requests for Information
Like other pharmaceutical companies, we are subject to extensive regulation by national, state and local government agencies in the U.S. and in the other countries in which we operate. As a result, we have interactions with government agencies on an ongoing basis. Among the investigations and requests for information by government agencies are those discussed below. It is possible that criminal charges and fines and/or civil penalties could result from pending government investigations, including but not limited to those discussed below.
76 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
The Department of Justice continues to actively investigate the marketing and safety of our COX-2 medicines, particularly Bextra. The investigation has included requests for information and documents. We also have received requests for information and documents in connection with threatened claims concerning the marketing and safety of Bextra and Celebrex from a group of state attorneys general. We have been considering various ways to resolve these matters.
Separately, the Department of Justice continues to actively investigate certain physician payments budgeted to our prescription pharmaceutical products. The investigation has included requests for information and documents.
The Company has voluntarily provided the Department of Justice and the Securities and Exchange Commission with information concerning potentially improper payments made in connection with certain sales activities outside the U.S. Certain potentially improper payments and other matters are the subject of investigations by government authorities in certain foreign countries, including the following: A wholly owned subsidiary of Pfizer is under criminal investigation by various government authorities in Italy with respect to gifts and payments allegedly provided to certain doctors operating within Italy’s national healthcare system. In Germany, a wholly owned subsidiary of Pfizer is the subject of a civil and criminal investigation with respect to certain tax matters. The Pfizer subsidiaries are fully cooperating in these investigations.
E. Guarantees and Indemnifications
In the ordinary course of business and in connection with the sale of assets and businesses, we often indemnify our counterparties against certain liabilities that may arise in connection with the transaction or related to activities prior to the transaction. These indemnifications typically pertain to environmental, tax, employee and/or product-related matters and patent infringement claims. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications are generally subject to threshold amounts, specified claim periods and other restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2007, recorded amounts for the estimated fair value of these indemnifications were not significant.
| |
21. | Segment, Geographic and Revenue Information |
Business Segments
We operate in the following business segments:
| | |
• | Pharmaceutical |
| | |
| – | The Pharmaceutical segment includes products that prevent and treat cardiovascular and metabolic diseases, central nervous system disorders, arthritis and pain, infectious and respiratory diseases, urogenital conditions, cancer, eye disease, endocrine disorders and allergies. |
| | |
| | |
• | Animal Health |
| | |
| – | The Animal Health segment includes products that prevent and treat diseases in livestock and companion animals. |
For our reportable operating segments (i.e., Pharmaceutical and Animal Health), segment profit/(loss) is measured based on income from continuing operations before provision for taxes on income, minority interests and the cumulative effect of a change in accounting principles. Certain costs, such as significant impacts of purchase accounting for acquisitions, acquisition-related costs and costs related to our cost-reduction initiatives and transition activity associated with our former Consumer Healthcare business, are included inCorporate/Otheronly. This methodology is utilized by management to evaluate our businesses.
Certain income/(expense) items that are excluded from the operating segments’ profit/(loss) are considered corporate items and are included inCorporate/Other. These items include interest income/(expense), corporate expenses (e.g., corporate administration costs), other income/(expense) (e.g., realized gains and losses attributable to our investments in debt and equity securities), certain performance-based and all share-based compensation expenses not allocated to the business segments, significant impacts of purchase accounting for acquisitions, certain milestone payments, acquisition-related costs, intangible asset impairments and costs related to our cost-reduction initiatives.
Each segment is managed separately and offers different products requiring different marketing and distribution strategies.
We sell our products primarily to customers in the wholesale sector. In 2007, sales to our three largest U.S. wholesaler customers represented approximately 18%, 12% and 10% of total revenues and, collectively, represented approximately 20% of accounts receivable as of December 31, 2007. In 2006, sales to our three largest U.S. wholesaler customers represented approximately 20%, 13% and 11% of total revenues and, collectively, represented approximately 26% of accounts receivable as of December 31, 2006. These sales and related accounts receivable were concentrated in the Pharmaceutical segment.
Revenues exceeded $500 million in each of 12 countries outside the U.S. in 2007 and in each of 10 countries outside the U.S. in 2006. The U.S. was the only country to contribute more than 10% of total revenues in each year.
2007 Financial Report | 77
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
|
|
The following tables present segment, geographic and revenue information: |
| | | | | | | | | | | |
Segment | | | | | | | | | | |
|
|
| | FOR/AS OF THE YEAR ENDED DEC. 31, | |
| |
|
(MILLIONS OF DOLLARS) | | 2007 | | 2006 | | 2005 | |
|
|
|
|
|
|
|
|
|
|
|
|
Revenues | | | | | | | | | | | |
Pharmaceutical | | $ | 44,424 | | | $ | 45,083 | | $ | 44,269 | |
Animal Health | | | 2,639 | | | | 2,311 | | | 2,206 | |
Corporate/Other(a) | | | 1,355 | | | | 977 | | | 930 | |
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues | | $ | 48,418 | | | $ | 48,371 | | $ | 47,405 | |
|
|
|
|
|
|
|
|
|
|
|
|
Segment profit/(loss)(b) | | | | | | | | | | | |
Pharmaceutical | | $ | 20,740 | | | $ | 21,615 | | $ | 19,599 | |
Animal Health | | | 620 | | | | 455 | | | 405 | |
Corporate/Other(a)(c) | | | (12,082 | ) | | | (9,042 | ) | | (9,204 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
Total profit/(loss) | | $ | 9,278 | | | $ | 13,028 | | $ | 10,800 | |
|
|
|
|
|
|
|
|
|
|
|
|
Identifiable assets | | | | | | | | | | | |
Pharmaceutical | | $ | 67,431 | | | $ | 72,497 | | $ | 74,056 | |
Animal Health | | | 2,043 | | | | 1,951 | | | 2,098 | |
Discontinued operations/Held for sale | | | 114 | | | | 62 | | | 6,659 | |
Corporate/Other(a)(d) | | | 45,680 | | | | 41,036 | | | 34,157 | |
|
|
|
|
|
|
|
|
|
|
|
|
Total identifiable assets | | $ | 115,268 | | | $ | 115,546 | | $ | 116,970 | |
|
|
|
|
|
|
|
|
|
|
|
|
Property, plant and equipment additions(e) | | | | | | | | | | | |
Pharmaceutical | | $ | 1,608 | | | $ | 1,681 | | $ | 1,703 | |
Animal Health | | | 70 | | | | 51 | | | 61 | |
Discontinued operations/Held for sale | | | — | | | | 162 | | | 189 | |
Corporate/Other(a) | | | 202 | | | | 156 | | | 153 | |
|
|
|
|
|
|
|
|
|
|
|
|
Total property, plant and equipment additions | | $ | 1,880 | | | $ | 2,050 | | $ | 2,106 | |
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization(e) | | | | | | | | | | | |
Pharmaceutical | | $ | 1,886 | | | $ | 1,765 | | $ | 1,880 | |
Animal Health | | | 52 | | | | 49 | | | 59 | |
Discontinued operations/Held for sale | | | — | | | | 71 | | | 78 | |
Corporate/Other(a)(f) | | | 3,262 | | | | 3,408 | | | 3,559 | |
|
|
|
|
|
|
|
|
|
|
|
|
Total depreciation and amortization | | $ | 5,200 | | | $ | 5,293 | | $ | 5,576 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
(a) | Corporate/Otherincludes our gelatin capsules business, our contract manufacturing business and a bulk pharmaceutical chemicals business, and transition activity associated with our former Consumer Healthcare business (sold in December 2006). Corporate/OtherunderSegment profit/(loss)also includes interest income/(expense), corporate expenses (e.g., corporate administration costs), other income/(expense) (e.g., realized gains and losses attributable to our investments in debt and equity securities), certain performance-based and all share-based compensation expenses, significant impacts of purchase accounting for acquisitions, acquisition-related costs, intangible asset impairments and costs related to our cost-reduction initiatives. |
| |
(b) | Segment profit/(loss) equalsIncome from continuing operations before provision for taxes on income, minority interests and the cumulative effect of a change in accounting principles.Certain costs, such as significant impacts of purchase accounting for acquisitions, acquisition-related costs and costs related to our cost-reduction initiatives and transition activity associated with our former Consumer Healthcare business, are included inCorporate/Otheronly. This methodology is utilized by management to evaluate our businesses. |
| |
(c) | In 2007,Corporate/Otherincludes: i) restructuring charges and implementation costs associated with our cost-reduction initiatives of $3.9 billion; (ii) significant impacts of purchase accounting for acquisitions of $3.4 billion, including acquired in-process research and development, intangible asset amortization and other charges; (iii) $2.8 billion of charges associated with Exubera. SeeNote 4. Asset Impairment Charges and Other Costs Associated with Exiting Exubera; (iv) net interest income of $1.1 billion; (v) all share-based compensation expense; (vii) gain on disposal of assets and other of $174 million; (vii) transition activity associated with our |
| |
| former Consumer Healthcare business of $26 million in income; and (viii) acquisition-related costs of $11 million. |
| |
| In 2006,Corporate/Otherincludes: (i) significant impacts of purchase accounting for acquisitions of $4.1 billion, including acquired in-process research and development, intangible asset amortization and other charges; (ii) restructuring charges and implementation costs associated with our cost-reduction initiatives of $2.1 billion; (iii) all share-based compensation expense; (iv) net interest income of $437 million; (v) impairment of the Depo-Provera intangible asset of $320 million; (vi) gain on disposals of investments and other of $173 million; and (vii) a research and development milestone due to us from sanofi-aventis of approximately $118 million; and (viii) acquisition-related costs of $27 million. |
| |
| In 2005,Corporate/Otherincludes: (i) significant impacts of purchase accounting for acquisitions of $4.9 billion, including acquired in-process research and development, intangible asset amortization and other charges; (ii) costs associated with the suspension of Bextra’s sales and marketing of $1.2 billion; (iii) acquisition-related costs of $918 million; (iv) restructuring charges and implementation costs associated with our cost-reduction initiatives of $763 million; (v) net interest income of $269 million; (vi) all share-based compensation expense; and (vii) gain on disposals of investments and other of $134 million. |
| |
(d) | Corporate assets are primarily cash, short-term investments and long-term investments and loans. |
| |
(e) | Certain production facilities are shared by various segments. Property, plant and equipment, as well as capital additions and depreciation, are allocated based on estimates of physical production. |
| |
(f) | Corporate/Otherincludes non-cash charges associated with purchase accounting related to intangible asset amortization of $3.0 billion in 2007, $3.2 billion in 2006 and $3.3 billion in 2005. |
78 | 2007 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | |
Geographic | | | | | | | | | | | |
|
| |
| | FOR/AS OF THE YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Revenues | | | | | | | | | | | |
United States(a) | | $ | 23,153 | | | $ | 25,822 | | $ | 24,751 | |
Europe/Canada(b) | | | 15,918 | | | | 14,194 | | | 14,355 | |
Japan/Asia(c) | | | 6,511 | | | | 5,939 | | | 5,987 | |
Latin America/AFME(d) | | | 2,836 | | | | 2,416 | | | 2,312 | |
|
|
|
|
|
|
|
|
|
|
| |
Consolidated | | $ | 48,418 | | | $ | 48,371 | | $ | 47,405 | |
|
|
|
|
|
|
|
|
|
|
| |
Long-lived assets(e) | | | | | | | | | | | |
United States(a) | | $ | 19,145 | | | $ | 21,795 | | $ | 24,390 | |
Europe/Canada(b) | | | 15,457 | | | | 17,538 | | | 16,492 | |
Japan/Asia(c) | | | 1,177 | | | | 1,205 | | | 1,154 | |
Latin America/AFME(d) | | | 453 | | | | 444 | | | 441 | |
|
|
|
|
|
|
|
|
|
|
| |
Consolidated | | $ | 36,232 | | | $ | 40,982 | | $ | 42,477 | |
|
|
|
|
|
|
|
|
|
|
| |
| |
(a) | Includes operations in Puerto Rico. |
|
(b) | Includes Canada, France, Italy, Spain, Germany, U.K., Ireland, Northern Europe and Central-South Europe. |
|
(c) | Includes Japan, Australia, Korea, China, Taiwan, Thailand and India. |
|
(d) | Includes South America, Central America, Mexico, Africa and the Middle East. |
|
(e) | Long-lived assets include identifiable intangible assets (excluding goodwill) and property, plant and equipment. |
Revenues by Therapeutic Area
| | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
| |
| | YEAR ENDED DEC. 31, | |
| |
| |
(MILLIONS OF DOLLARS) | | 2007 | | | 2006 | | 2005 | |
|
|
|
|
|
|
|
| |
Pharmaceutical | | | | | | | | | | | |
Cardiovascular and metabolic diseases | | $ | 18,853 | | | $ | 19,871 | | $ | 18,732 | |
Central nervous system disorders | | | 5,152 | | | | 6,038 | | | 6,391 | |
Arthritis and pain | | | 2,914 | | | | 2,711 | | | 2,386 | |
Infectious and respiratory diseases | | | 3,552 | | | | 3,474 | | | 4,770 | |
Urology | | | 3,010 | | | | 2,809 | | | 2,684 | |
Oncology | | | 2,640 | | | | 2,191 | | | 1,996 | |
Ophthalmology | | | 1,643 | | | | 1,461 | | | 1,373 | |
Endocrine disorders | | | 1,052 | | | | 985 | | | 1,049 | |
All other | | | 3,819 | | | | 4,169 | | | 3,823 | |
Alliance revenues | | | 1,789 | | | | 1,374 | | | 1,065 | |
|
|
|
|
|
|
|
|
|
|
| |
Total Pharmaceutical | | | 44,424 | | | | 45,083 | | | 44,269 | |
|
|
|
|
|
|
|
|
|
|
| |
Animal Health | | | 2,639 | | | | 2,311 | | | 2,206 | |
Other | | | 1,355 | | | | 977 | | | 930 | |
|
|
|
|
|
|
|
|
|
|
| |
Total revenues | | $ | 48,418 | | | $ | 48,371 | | $ | 47,405 | |
|
|
|
|
|
|
|
|
|
|
| |
2007 Financial Report | 79
Quarterly Consolidated Financial Data (Unaudited)
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | | | |
| | QUARTER | |
| |
| |
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | | FIRST | | SECOND | | THIRD | | FOURTH | |
|
|
|
|
|
|
|
|
|
|
2007 | | | | | | | | | | | | | |
Revenues | | $ | 12,474 | | $ | 11,084 | | $ | 11,990 | | $ | 12,870 | |
Costs and expenses | | | 7,326 | | | 8,414 | | | 10,899 | | | 9,684 | |
Acquisition-related in-process research and development charges | | | 283 | | | — | | | — | | | — | |
Restructuring charges and acquisition-related costs | | | 812 | | | 1,051 | | | 455 | | | 216 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from continuing operations before (benefit)/provision for taxes on income, and minority interests | | | 4,053 | | | 1,619 | | | 636 | | | 2,970 | |
(Benefit)/provision for taxes on income | | | 689 | | | 272 | | | (161 | ) | | 223 | |
Minority interests | | | 3 | | | 2 | | | 1 | | | 36 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from continuing operations | | | 3,361 | | | 1,345 | | | 796 | | | 2,711 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Discontinued operations: | | | | | | | | | | | | | |
Income/(loss) from discontinued operations—net of tax | | | — | | | — | | | — | | | (3 | ) |
Gains/(losses) on sales of discontinued operations—net of tax | | | 31 | | | (78 | ) | | (35 | ) | | 16 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Discontinued operations—net of tax | | | 31 | | | (78 | ) | | (35 | ) | | 13 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income | | $ | 3,392 | | $ | 1,267 | | $ | 761 | | $ | 2,724 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings per common share—basic: | | | | | | | | | | | | | |
Income from continuing operations | | $ | 0.48 | | $ | 0.19 | | $ | 0.12 | | $ | 0.40 | |
Discontinued operations—net of tax | | | — | | | (0.01 | ) | | (0.01 | ) | | — | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income | | $ | 0.48 | | $ | 0.18 | | $ | 0.11 | | $ | 0.40 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings per common share—diluted
Income from continuing operations | | $ | 0.48 | | $ | 0.19 | | $ | 0.12 | | $ | 0.40 | |
Discontinued operations—net of tax | | | — | | | (0.01 | ) | | (0.01 | ) | | — | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income | | $ | 0.48 | | $ | 0.18 | | $ | 0.11 | | $ | 0.40 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash dividends paid per common share | | $ | 0.29 | | $ | 0.29 | | $ | 0.29 | | $ | 0.29 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock prices | | | | | | | | | | | | | |
High | | $ | 27.41 | | $ | 27.73 | | $ | 26.15 | | $ | 25.71 | |
Low | | $ | 24.55 | | $ | 25.23 | | $ | 23.13 | | $ | 22.24 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | |
| Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
Acquisition-related in-process research and development chargesprimarily includes amounts incurred in connection with our acquisitions of BioRexis and Embrex (seeNote 2. Acquisitions). | | Restructuring charges and acquisition-related costsincludes restructuring charges primarily related to our cost-reduction initiatives (seeNote 5. Cost-Reduction Initiatives).
As of January 31, 2008, there were 231,737 holders of record of our common stock (symbol PFE). |
80 | 2007 Financial Report
Quarterly Consolidated Financial Data (Unaudited)
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | | | |
|
|
| |
| | QUARTER | |
| |
| |
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | | FIRST | | SECOND | | THIRD | | FOURTH | |
|
|
| |
2006 | | | | | | | | | | | | | |
Revenues | | $ | 11,747 | | $ | 11,741 | | $ | 12,280 | | $ | 12,603 | |
Costs and expenses | | | 7,178 | | | 7,877 | | | 8,070 | | | 10,060 | |
Acquisition-related in-process research and development charges | | | — | | | 513 | | | — | | | 322 | |
Restructuring charges and acquisition-related costs | | | 299 | | | 268 | | | 249 | | | 507 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Income from continuing operations before provision for taxes on income, and minority interests | | | 4,270 | | | 3,083 | | | 3,961 | | | 1,714 | |
Provision for taxes on income | | | 262 | | | 790 | | | 717 | | | 223 | |
Minority interests | | | 2 | | | 3 | | | 5 | | | 2 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Income from continuing operations | | | 4,006 | | | 2,290 | | | 3,239 | | | 1,489 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations: | | | | | | | | | | | | | |
Income from discontinued operations—net of tax | | | 102 | | | 108 | | | 120 | | | 103 | |
Gains on sales of discontinued operations—net of tax | | | 3 | | | 17 | | | 3 | | | 7,857 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Discontinued operations—net of tax | | | 105 | | | 125 | | | 123 | | | 7,960 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net income | | $ | 4,111 | | $ | 2,415 | | $ | 3,362 | | $ | 9,449 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Earnings per common share—basic: | | | | | | | | | | | | | |
Income from continuing operations | | $ | 0.55 | | $ | 0.31 | | $ | 0.45 | | $ | 0.21 | |
Discontinued operations—net of tax | | | 0.01 | | | 0.02 | | | 0.02 | | | 1.11 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net income | | $ | 0.56 | | $ | 0.33 | | $ | 0.47 | | $ | 1.32 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Earnings per common share—diluted: | | | | | | | | | | | | | |
Income from continuing operations | | $ | 0.55 | | $ | 0.31 | | $ | 0.44 | | $ | 0.21 | |
Discontinued operations—net of tax | | | 0.01 | | | 0.02 | | | 0.02 | | | 1.11 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net income | | $ | 0.56 | | $ | 0.33 | | $ | 0.46 | | $ | 1.32 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Cash dividends paid per common share | | $ | 0.24 | | $ | 0.24 | | $ | 0.24 | | $ | 0.24 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Stock prices | | | | | | | | | | | | | |
High | | $ | 26.84 | | $ | 25.72 | | $ | 28.58 | | $ | 28.60 | |
Low | | $ | 23.60 | | $ | 22.51 | | $ | 22.16 | | $ | 23.75 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | | |
| Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year. | | Acquisition-related in-process research and development charges primarily includes amounts incurred in connection with our acquisitions of PowderMed and Rinat (see Note 2. Acquisitions.) |
|
| All financial information reflects our Consumer Healthcare business as discontinued operations (seeNote 3. Discontinued Operations). | | Restructuring charges and acquisition-related costsincludes restructuring charges primarily related to our cost-reduction initiatives (see Note 5. Cost-Reduction Initiatives.) |
2007 Financial Report | 81
Financial Summary
Pfizer Inc and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| | AS OF/FOR THE YEAR ENDED DECEMBER 31 | |
| |
| |
(MILLIONS, EXCEPT PER COMMON SHARE DATA) | | 2007 | | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Revenues | | $ | 48,418 | | | $ | 48,371 | | $ | 47,405 | | $ | 48,988 | | $ | 41,787 | | $ | 29,758 | |
Research and development expenses(a) | | | 8,089 | | | | 7,599 | | | 7,256 | | | 7,513 | | | 7,279 | | | 5,153 | |
Other costs and expenses | | | 28,234 | | | | 25,586 | | | 26,341 | | | 25,850 | | | 25,652 | | | 12,742 | |
Acquisition-related in-process research and development charges(b) | | | 283 | | | | 835 | | | 1,652 | | | 1,071 | | | 5,052 | | | — | |
Restructuring charges and acquisition-related costs(c) | | | 2,534 | | | | 1,323 | | | 1,356 | | | 1,151 | | | 1,023 | | | 594 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Income from continuing operations before provision for taxes on income, minority interests and cumulative effect of a change in accounting principles | | | 9,278 | | | | 13,028 | | | 10,800 | | | 13,403 | | | 2,781 | | | 11,269 | |
Provision for taxes on income | | | (1,023 | ) | | | (1,992 | ) | | (3,178 | ) | | (2,460 | ) | | (1,614 | ) | | (2,598 | ) |
Income from continuing operations before cumulative effect of a change in accounting principles | | | 8,213 | | | | 11,024 | | | 7,610 | | | 10,936 | | | 1,164 | | | 8,665 | |
Discontinued operations—net of tax | | | (69 | ) | | | 8,313 | | | 498 | | | 425 | | | 2,776 | | | 871 | |
Cumulative effect of a change in accounting principles—net of tax(d) | | | — | | | | — | | | (23 | ) | | — | | | (30 | ) | | (410 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net income | | | 8,144 | | | | 19,337 | | | 8,085 | | | 11,361 | | | 3,910 | | | 9,126 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Effective tax rate—continuing operations | | | 11.0 | % | | | 15.3 | % | | 29.4 | % | | 18.4 | % | | 58.0 | % | | 23.1 | % |
Depreciation and amortization(e) | | | 5,200 | | | | 5,293 | | | 5,576 | | | 5,093 | | | 4,025 | | | 1,030 | |
Property, plant and equipment additions(e) | | | 1,880 | | | | 2,050 | | | 2,106 | | | 2,601 | | | 2,629 | | | 1,758 | |
Cash dividends paid | | | 7,975 | | | | 6,919 | | | 5,555 | | | 5,082 | | | 4,353 | | | 3,168 | |
Working capital(f) | | | 25,014 | | | | 25,559 | | | 18,433 | | | 17,582 | | | 6,059 | | | 5,868 | |
Property, plant and equipment, less accumulated depreciation | | | 15,734 | | | | 16,632 | | | 16,233 | | | 17,593 | | | 17,573 | | | 10,264 | |
Total assets(f) | | | 115,268 | | | | 115,546 | | | 116,970 | | | 125,848 | | | 111,131 | | | 44,251 | |
Long-term debt | | | 7,314 | | | | 5,546 | | | 6,347 | | | 7,279 | | | 5,755 | | | 3,140 | |
Long-term capital(g) | | | 80,134 | | | | 84,993 | | | 81,895 | | | 88,959 | | | 78,866 | | | 21,647 | |
Shareholders’ equity | | | 65,010 | | | | 71,358 | | | 65,764 | | | 68,433 | | | 60,049 | | | 18,099 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Earnings per common share—basic: | | | | | | | | | | | | | | | | | | | | |
Income from continuing operations before cumulative effect of a change in accounting principles | | | 1.19 | | | | 1.52 | | | 1.03 | | | 1.45 | | | 0.16 | | | 1.41 | |
Discontinued operations—net of tax | | | (0.01 | ) | | | 1.15 | | | 0.07 | | | 0.06 | | | 0.38 | | | 0.14 | |
Cumulative effect of a change in accounting principles—net of tax(d) | | | — | | | | — | | | — | | | — | | | — | | | (0.07 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net income | | | 1.18 | | | | 2.67 | | | 1.10 | | | 1.51 | | | 0.54 | | | 1.48 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Earnings per common share—diluted: | | | | | | | | | | | | | | | | | | | | |
Income from continuing operations before cumulative effect of a change in accounting principles | | | 1.18 | | | | 1.52 | | | 1.02 | | | 1.43 | | | 0.16 | | | 1.39 | |
Discontinued operations—net of tax | | | (0.01 | ) | | | 1.14 | | | 0.07 | | | 0.06 | | | 0.38 | | | 0.14 | |
Cumulative effect of a change in accounting principles—net of tax(d) | | | — | | | | — | | | — | | | — | | | — | | | (0.07 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net income | | | 1.17 | | | | 2.66 | | | 1.09 | | | 1.49 | | | 0.54 | | | 1.46 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Market value per share (December 31) | | | 22.73 | | | | 25.90 | | | 23.32 | | | 26.89 | | | 35.33 | | | 30.57 | |
Return on shareholders’ equity | | | 11.94 | % | | | 28.20 | % | | 12.0 | % | | 17.7 | % | | 10.0 | % | | 55.2 | % |
Cash dividends paid per common share | | | 1.16 | | | | 0.96 | | | 0.76 | | | 0.68 | | | 0.60 | | | 0.52 | |
Shareholders’ equity per common share | | | 9.65 | | | | 10.05 | | | 8.98 | | | 9.21 | | | 7.93 | | | 2.97 | |
Current ratio | | | 2.15:1 | | | | 2.16:1 | | | 1.65:1 | | | 1.63:1 | | | 1.26:1 | | | 1.32:1 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Weighted-average shares used to calculate: | | | | | | | | | | | | | | | | | | | | |
Basic earnings per common share amounts | | | 6,917 | | | | 7,242 | | | 7,361 | | | 7,531 | | | 7,213 | | | 6,156 | |
Diluted earnings per common share amounts | | | 6,939 | | | | 7,274 | | | 7,411 | | | 7,614 | | | 7,286 | | | 6,241 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
82 | 2007 Financial Report
Financial Summary
Pfizer Inc and Subsidiary Companies
On April 16, 2003, Pfizer acquired Pharmacia Corporation in a transaction accounted for as a purchase. All financial information reflects the following as discontinued operations: our Consumer Healthcare, in-vitro allergy and autoimmune diagnostic testing, certain European generics, surgical ophthalmic, confectionery, shaving and fish-care products businesses and the femhrt, Loestrin and Estrostep women’s health product lines, as applicable.
| | |
| (a) | Research and development expensesincludes co-promotion charges and milestone payments for intellectual property rights of $603 million in 2007, $292 million in 2006: $156 million in 2005; $160 million in 2004; $380 million in 2003; and $32 million in 2002. |
| | |
| (b) | In 2007, 2006, 2005, 2004 and 2003, we recorded charges for the estimated portion of the purchase price of acquisitions allocated to in-process research and development. |
| | |
| (c) | Restructuring charges and acquisition-related costsprimarily includes the following: |
| | |
| | 2007 — Restructuring charges of $2.5 billion related to our cost-reduction initiatives. |
| | |
| | 2006 — Restructuring charges of $1.3 billion related to our cost-reduction initiatives. |
| | |
| | 2005 — Integration costs of $532 million and restructuring charges of $372 million related to our acquisition of Pharmacia in 2003 and restructuring charges of $438 million related to our cost-reduction initiatives. |
| | |
| | 2004 — Integration costs of $454 million and restructuring charges of $680 million related to our acquisition of Pharmacia in 2003. |
| | |
| | |
| | 2003 — Integration costs of $808 million and restructuring charges of $166 million related to our acquisition of Pharmacia in 2003. |
| | |
| | 2002 — Integration costs of $333 million and restructuring charges of $167 million related to our merger with Warner-Lambert in 2000 and pre-integration costs of $94 million related to our pending acquisition of Pharmacia. |
| | |
| (d) | In 2005, as a result of adopting FIN 47,Accounting for Conditional Asset Retirement Obligations,we recorded a non-cash pre-tax charge of $40 million ($23 million, net of tax). In 2003, as a result of adopting SFAS No. 143,Accounting for Asset Retirement Obligations,we recorded a non-cash pre-tax charge of $47 million ($30 million, net of tax). In 2002, as a result of adopting SFAS No.142,Goodwill and Other Intangible Assets,we recorded pre-tax charges of $565 million ($410 million, net of tax). |
| | |
| (e) | Includes discontinued operations, (see Notes to Consolidated Financial Statements—Note 21. Segment, Geographic and Revenue Information.) |
| | |
| (f) | For 2005 through 2002, includes assets held for sale of our Consumer Healthcare business, and for 2004 through 2002, also includes in-vitro allergy and autoimmune diagnostic testing, surgical ophthalmic, certain European generics, confectionery and shaving businesses and the femhrt, Loestrin and Estrostep women’s health product lines. |
| | |
| (g) | Defined as long-term debt, deferred taxes, minority interests and shareholders’ equity. |
Peer Group Performance Graph
Five Year Performance

| | | | | | | | | | | | | |
| | | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | | 2007 |
|
| |
| |
| |
| |
| |
| |
|
| Pfizer | | 100.0 | | 117.8 | | 91.5 | | 81.8 | | 94.2 | | 86.5 |
| Old Peer Group | | 100.0 | | 103.3 | | 105.1 | | 104.5 | | 122.9 | | 137.1 |
| New Peer Group | | 100.0 | | 113.8 | | 114.5 | | 121.7 | | 135.0 | | 137.2 |
| S&P 500 | | 100.0 | | 128.7 | | 142.7 | | 149.7 | | 173.3 | | 182.8 |
Since 2005, Pfizer’s pharmaceutical peer group has consisted of the following companies: Abbott Laboratories, Amgen, AstraZeneca, Bristol-Myers Squibb Company, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Merck and Co., Schering-Plough Corporation and Wyeth (New Peer Group). Prior to that, Pfizer’s pharmaceutical peer group was comprised of Abbot Laboratories, Baxter International, Bristol-Myers Squibb Company, Colgate-Palmolive Company, Eli Lilly and Company, Johnson & Johnson, Merck and Co., Schering-Plough Corporation and Wyeth (Old Peer Group).
We believe that the companies included in the New Peer Group are more reflective of the Company’s core business, and therefore will provide a more meaningful comparison of stock performance. We have included the New Peer Group in the graph to show what the comparison to those companies would have been if the New Peer Group had been in place during the periods shown on the graph.
2007 Financial Report | 83