Exhibit 99.2
| AMAG PHARMACEUTICALS JP Morgan Healthcare Conference January 2013 |
| FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to: (i) our statement that AMAG is well positioned for growth in 2013 and beyond; (ii) our plans to grow Feraheme revenues in 2013 and thereafter through a combination of volume and price growth; (iii) expectations regarding the status, expiry and extension of our Orange Book listed patents for Feraheme; (iv) the potential for approval and potential launch of the supplemental new drug application in the U.S. and the timing and potential regulatory submission in the EU for Feraheme/Rienso for the broad iron deficiency anemia indication; (v) statements regarding our plans to acquire and/or in-license additional commercial products or assets; (vi) statements regarding the potential size and expansion of the U.S. IV iron market opportunity; (vii) our expected financial results for 2013; (viii) our expected cash flows in 2013 and beyond; (ix) our plans to expand the reach of Feraheme to new indications and geographic territories; (x) statements regarding any potential milestone payments or royalties we may receive; (xi) statements regarding future annual sales for Feraheme/Rienso; (xii) our expected fourth quarter 2012 and full year 2012 financial results; (xiii) our expectation to increase our market share of the IV iron market in 2013; and (xiv) statements regarding our expectation to become the market leader in the current IV iron market, are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include: (1) uncertainties regarding our and Takeda's ability to successfully compete in the intravenous iron replacement market both in the US and outside the US, including the EU, (2) uncertainties regarding our ability to successfully and timely complete our clinical development programs and obtain regulatory approval for Feraheme/Rienso in the broader IDA indication both in the US and outside of the US, including the EU, (3) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso, (4) uncertainties regarding the manufacture of Feraheme/Rienso, (5) uncertainties relating to our patents and proprietary rights both in the US and outside the US, (6) the risk of an Abbreviated New Drug Application (ANDA) filing following the FDA’s recently published draft bioequivalence recommendation for ferumoxytol, and (7) other risks identified in our Securities and Exchange Commission (SEC) filings, including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2012 and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements 2 |
| AMAG: 2012 HIGHLIGHTS Continued growth of Feraheme U.S. CKD business Drove 17% Feraheme volume growth in the US compared to 2011 Exited year with 14% market share in grams Improved net revenue per gram for Feraheme Significant progress in Feraheme expansion efforts Completed phase III program for broad IDA indication; submitted sNDA Launched in Canada and Europe; triggered $33 million in milestones Stabilized organization and turned focus to execution and growth Ready to purchase/in-license additional comercial products Financial Streamlined cost structure with 32% reduction in operating expenses compared to 2011 Exceeded original guidance; delivered on updated financial guidance 3 |
| Marketed Product Feraheme® (ferumoxytol) Injection for intravenous (IV) use, an IV iron replacement therapy Approved in the U.S., Europe, Switzerland and Canada Indicated for adult patients with chronic kidney disease (CKD) with iron deficiency anemia (IDA) 3 Orange Book listed patents; 2020 expiry; anticipated extension in the U.S. to 2023 $200 million market opportunity in the U.S. in non-dialysis CKD setting sNDA submitted in December 2012; if approved, U.S. launch expected in Q4 2013 Commercial Organization Specialty field force of ~50 professionals Targeting hematology-oncology practices, hospitals, and nephrology clinics Full complement of MSLs, managed markets and reimbursement professionals Deep relationships with KOL’s, key physician groups and GPOs International Expansion Feraheme strategic alliance with Takeda in select ex-U.S. territories Canadian and European approvals and launches triggered $33 million in milestones $187 million in remaining milestones; double-digit, tiered royalties based on net sales Strategic alliance with 3SBio for development and commercialization of Feraheme in China Business Development Actively pursuing acquisition or in-licensing of commercial assets Leverage existing commercial infrastructure and potential synergies with Feraheme Financial Profile Double-digit revenue growth and aggressive management of operating expenses Cash and investments of $227 million at December 31, 2012; no debt AMAG OVERVIEW 4 |
| AMAG: LEVERAGING OUR ASSETS AND CORE COMPETENCIES TO BUILD A PROFITABLE, MULTI-PRODUCT SPECIALTY PHARMACEUTICAL COMPANY Commercial Infrastructure Focus on Hematology/Oncology, Hospital and Nephrology Strong Balance Sheet Product #2 Product #3 Experienced Management Team Feraheme U.S. CKD Business Feraheme IDA label Expansion IV Iron Market Expansion Feraheme Geographic Expansion 5 |
| U.S. IV IRON MARKET |
| CURRENT US NON-DIALYSIS IV IRON MARKET ~ $400 MILLION (FERAHEME DOLLARS) 50% of market is for treatment of patients with CKD Primary sites of treatment are hematology / oncology clinics and hospitals ~70% of market reimbursed at ASP+ model 7 |
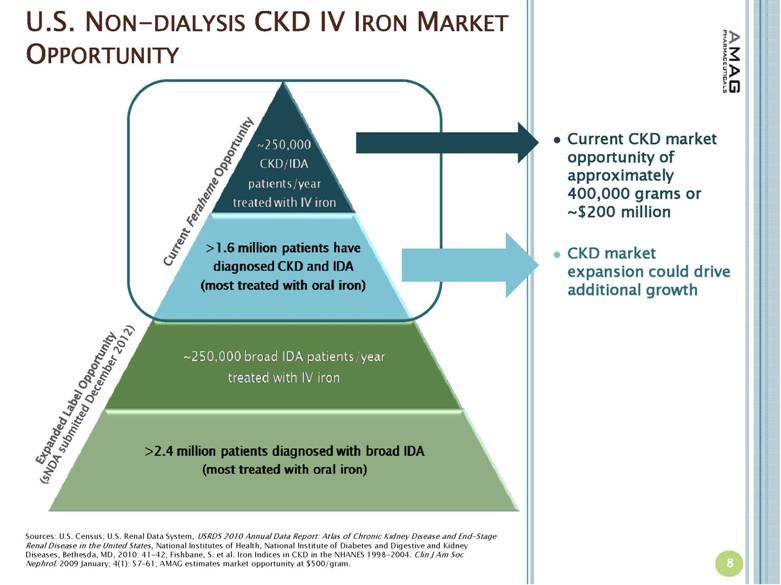
| U.S. NON-DIALYSIS CKD IV IRON MARKET OPPORTUNITY Current Feraheme Opportunity Expanded Label Opportunity (sNDA submitted December 2012) Sources: U.S. Census; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42; Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61; AMAG estimates market opportunity at $500/gram. Current CKD market opportunity of approximately 400,000 grams or ~$200 million CKD market expansion could drive additional growth 8 |
| FERAHEME PERFORMANCE IN THE U.S. |
| ABOUT FERAHEME® (FERUMOXYTOL)/RIENSO In the United States, Feraheme (ferumoxytol) Injection for Intravenous (IV) use is indicated for the treatment of iron deficiency anemia in adult chronic kidney disease (CKD) patients. Feraheme received marketing approval from the U.S. Food and Drug Administration on June 30, 2009 and was commercially launched by AMAG in the U.S. shortly thereafter. Ferumoxytol received marketing approval in Canada in December 2011, where it is marketed by Takeda as Feraheme, and in the European Union in June 2012 and Switzerland in August 2012, where it is marketed by Takeda as Rienso®. For additional product information, please visit www.feraheme.com. Feraheme ® (ferumoxytol) Injection for Intravenous (IV) is indicated for the treatment of iron deficiency anemia in adult patients with chronic kidney disease. Feraheme is contraindicated in patients with known hypersensitivity to Feraheme or any of its components. Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Feraheme. Serious adverse reactions of clinically significant hypotension have been reported. In the post-marketing setting, life-threatening anaphylactic type reactions, cardiac/cardiorespiratory arrest, clinically significant hypotension, syncope, unresponsiveness and other safety events have been reported in patients being treated with Feraheme. In clinical trials, the most commonly occurring adverse reactions for Feraheme-treated patients were nausea, dizziness, hypotension, peripheral edema, headache, edema and vomiting. A full list of adverse events can be found in the full prescribing information for Feraheme. 10 |
| NON-DIALYSIS MARKET SHARE SHIFT SINCE FERAHEME LAUNCH (ASP DOLLARS) 11 ® ® ® ® Demand information is based on data from IMS Health; market share is calculated on published ASP data. |
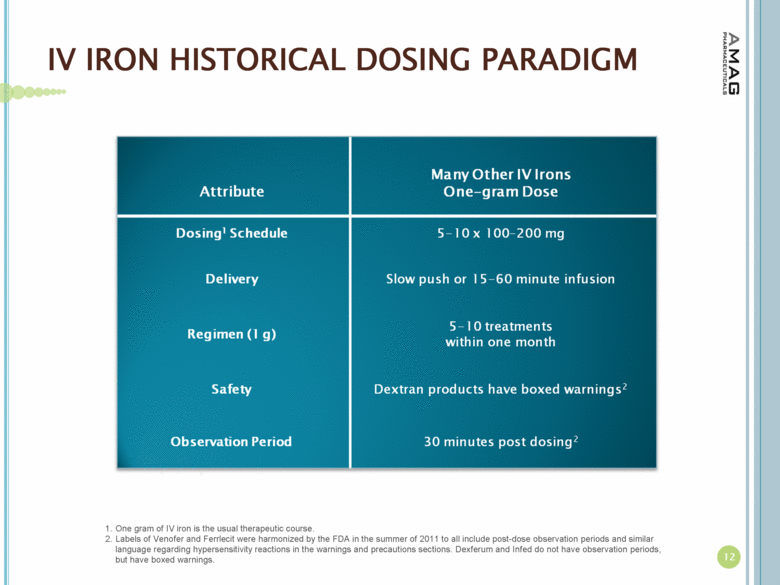
| IV IRON HISTORICAL DOSING PARADIGM One gram of IV iron is the usual therapeutic course. Labels of Venofer and Ferrlecit were harmonized by the FDA in the summer of 2011 to all include post-dose observation periods and similar language regarding hypersensitivity reactions in the warnings and precautions sections. Dexferum and Infed do not have observation periods, but have boxed warnings. 12 |
| FERAHEME IS AS EASY AS 1 – 2 – 3 1 GRAM, 2 DOSES, 3 DAYS APART One gram of IV iron is the usual therapeutic course and that which was studied in the Feraheme clinical trials. Feraheme Indication and contraindications Feraheme ® (ferumoxytol) Injection for Intravenous (IV) is indicated for the treatment of iron deficiency anemia in adult patients with chronic kidney disease. Feraheme is contraindicated in patients with known hypersensitivity to Feraheme or any of its components. Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Feraheme. Anaphylactic type reactions, presenting with cardiac/cardiorespiratory arrest, clinically significant hypotension, syncope, and unresponsiveness have been reported in the post-marketing experience. In clinical trials, the most commonly occurring adverse reactions for Feraheme-treated patients were diarrhea, nausea, dizziness, hypotension, constipation and peripheral edema. A full list of adverse events can be found in the full prescribing information for Feraheme. For full prescribing information, please visit www.feraheme.com. 13 |
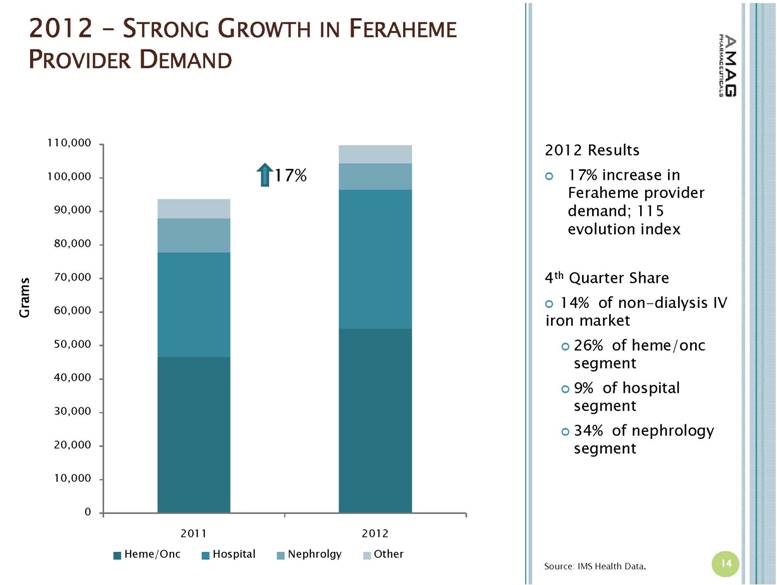
| 2012 – STRONG GROWTH IN FERAHEME PROVIDER DEMAND 14 Source: IMS Health Data. 17% Grams 2012 Results 17% increase in Feraheme provider demand; 115 evolution index 4th Quarter Share 14% of non-dialysis IV iron market 26% of heme/onc segment 9% of hospital segment 34% of nephrology segment |
| EXPANSION OPPORTUNITIES |
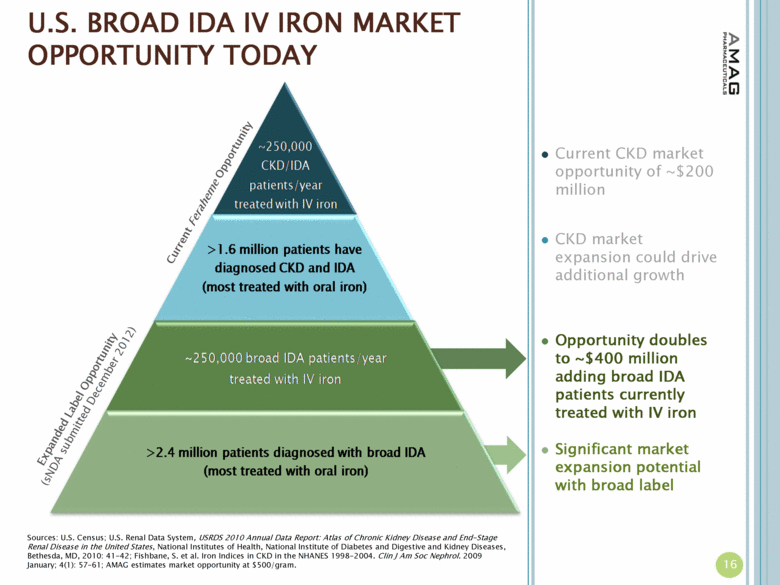
| U.S. BROAD IDA IV IRON MARKET OPPORTUNITY TODAY Current Feraheme Opportunity Sources: U.S. Census; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42; Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61; AMAG estimates market opportunity at $500/gram. Current CKD market opportunity of ~$200 million CKD market expansion could drive additional growth Opportunity doubles to ~$400 million adding broad IDA patients currently treated with IV iron Significant market expansion potential with broad label 16 Expanded Label Opportunity (sNDA submitted December 2012) |
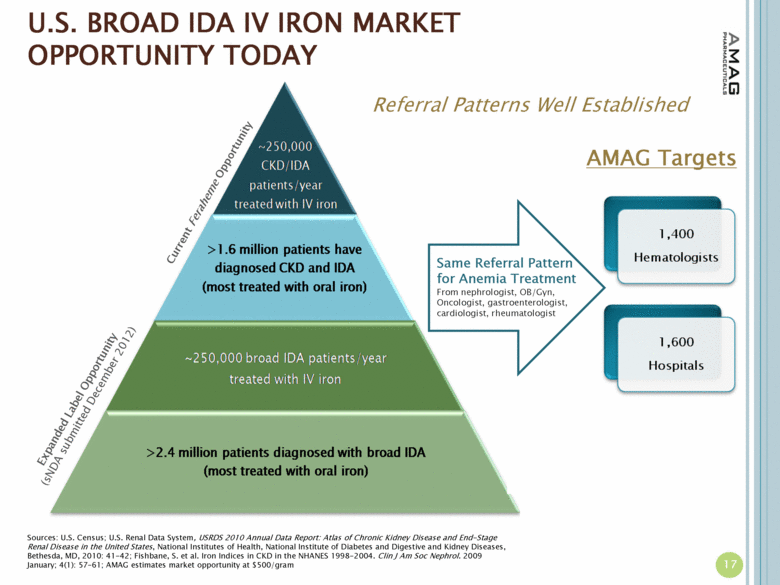
| U.S. BROAD IDA IV IRON MARKET OPPORTUNITY TODAY Current Feraheme Opportunity Sources: U.S. Census; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42; Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61; AMAG estimates market opportunity at $500/gram Current CKD market opportunity of ~$200 million CKD market expansion could drive additional growth Opportunity doubles to ~$400 million adding broad IDA patients currently treated with IV iron Significant market expansion potential with broad label 17 Same Referral Pattern for Anemia Treatment From nephrologist, OB/Gyn, Oncologist, gastroenterologist, cardiologist, rheumatologist AMAG Targets Referral Patterns Well Established Expanded Label Opportunity (sNDA submitted December 2012) |
| BROAD IDA LABEL EXPANSION PLANS 18 2012 2013 2014 sNDA submitted to FDA Global 1,400 patient phase III program completed Potential U.S. sNDA approval IDA submission in EU Potential IDA approval in EU (milestone to AMAG) |
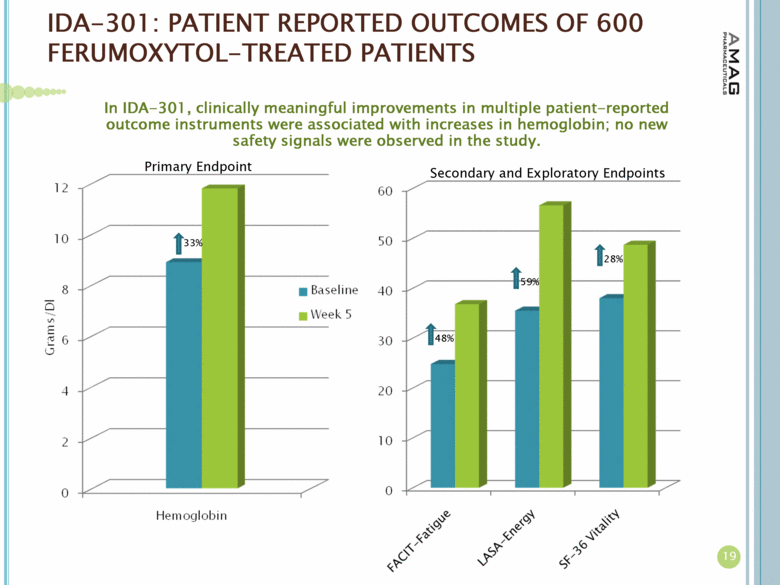
| IDA-301: PATIENT REPORTED OUTCOMES OF 600 FERUMOXYTOL-TREATED PATIENTS 19 In IDA-301, clinically meaningful improvements in multiple patient-reported outcome instruments were associated with increases in hemoglobin; no new safety signals were observed in the study. 48% 59% 28% 33% Primary Endpoint Secondary and Exploratory Endpoints |
| IV IRON MARKET EXPANSION OPPORTUNITY 20 Expansion Opportunity Current IV Iron Market |
| GASTROENTEROLOGY – A PRIME OPPORTUNITY UPON APPROVAL 21 Causes of IDA Chronic GI disorders Inflammatory bowel disease >150,000 with IDA Bleeds of unknown origin Malabsorption disorders Celiac disease Bariatric surgery patients >150,000 with IDA Cannot tolerate or absorb oral iron Chronically iron deficient Relatively small specialty; easily targeted 13,000 GI specialists 150 Hospital-based IBD Centers 570 CMS-approved bariatric surgery centers Recognize anemia as an issue in their patients Have a high level of concern about the impact of anemia on their patients lives Treat IDA today Minority with IV iron Majority unaware of Feraheme Patient Characteristic Physician Characteristics |
| AMAG’S GI PRE-LAUNCH ACTIVITIES Further understand view of anemia management Market research Advisory boards Meet with key opinion leaders in the treatment of IBD and bariatric surgeons who are thought leaders in IDA Meet with advocacy groups to understand the community Educate Present GI-specific data at key GI medical meetings (DDW, ACG) Publish GI-specific data in peer-reviewed journals 22 |
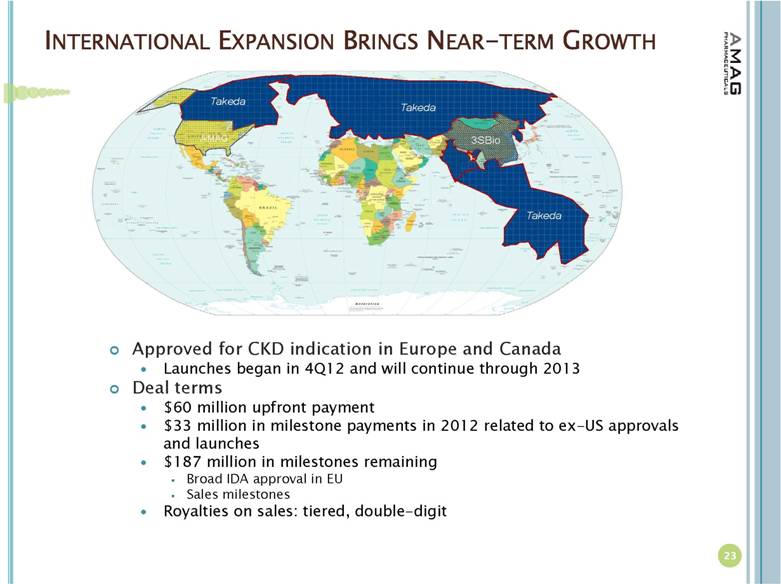
| INTERNATIONAL EXPANSION BRINGS NEAR-TERM GROWTH Approved for CKD indication in Europe and Canada Launches began in 4Q12 and will continue through 2013 Deal terms $60 million upfront payment $33 million in milestone payments in 2012 related to ex-US approvals and launches $187 million in milestones remaining Broad IDA approval in EU Sales milestones Royalties on sales: tiered, double-digit 23 |
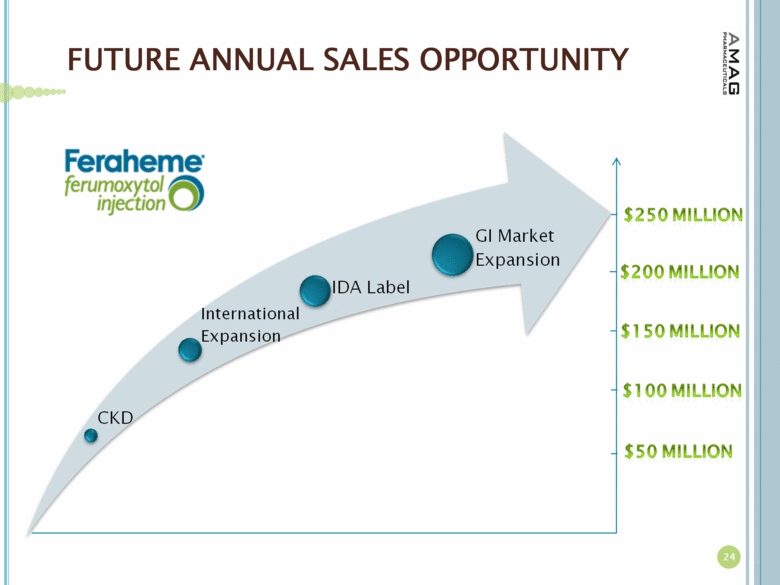
| FUTURE ANNUAL SALES OPPORTUNITY 24 |
| PORTFOLIO EXPANSION Current focus Commercial product acquisitions Assets that can be purchased with AMAG’s existing cash Products with annual revenues of $10 million to $60 million Products with growth potential that leverage our commercial footprint Positive impact on Feraheme Strong intellectual property Accretive to earnings in first year Highly transactable Several opportunities currently in-process (evaluation, due diligence or active negotiations) hematology/oncology assets hospital assets renal assets Future Continue to add commercial products that leverage commercial team Expand to late-stage development or more strategic transactions 25 |
| FINANCIAL OVERVIEW |
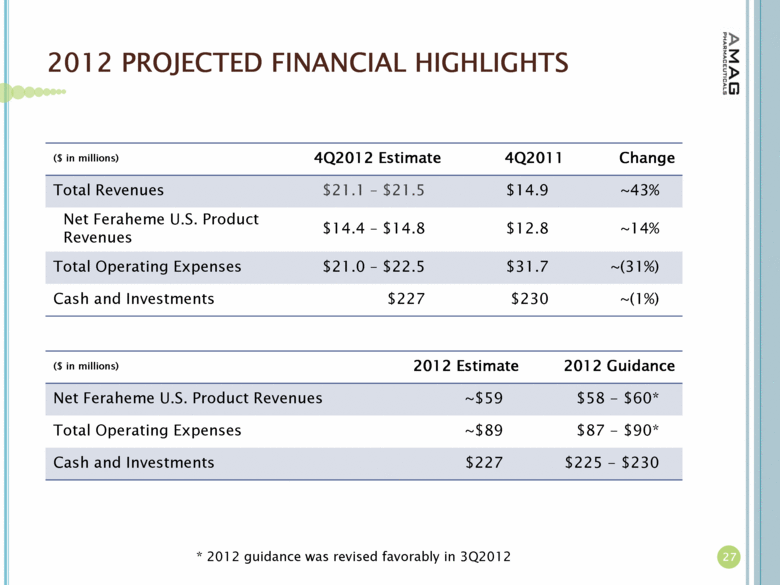
| 2012 PROJECTED FINANCIAL HIGHLIGHTS ($ in millions) 4Q2012 Estimate 4Q2011 Change Total Revenues $21.1 – $21.5 $14.9 ~43% Net Feraheme U.S. Product Revenues $14.4 – $14.8 $12.8 ~14% Total Operating Expenses $21.0 – $22.5 $31.7 ~(31%) Cash and Investments $227 $230 ~(1%) 27 ($ in millions) 2012 Estimate 2012 Guidance Net Feraheme U.S. Product Revenues ~$59 $58 - $60* Total Operating Expenses ~$89 $87 - $90* Cash and Investments $227 $225 - $230 * 2012 guidance was revised favorably in 3Q2012 |
| IMPROVING NET REVENUE PER GRAM OF FERAHAME SOLD 28 Realizing benefit of price increases taken in 2012 Reversed historically deteriorating net revenue per gram in 2012 ASP has increased in each of last two quarters * Net price per gram is calculated by deducting estimated fees, discounts, rebates, and returns from the wholesale acquisition cost (list price) per gram of Feraheme. See AMAG’s quarterly filings with the SEC for more information related to the Company’s gross to net sales adjustment. |
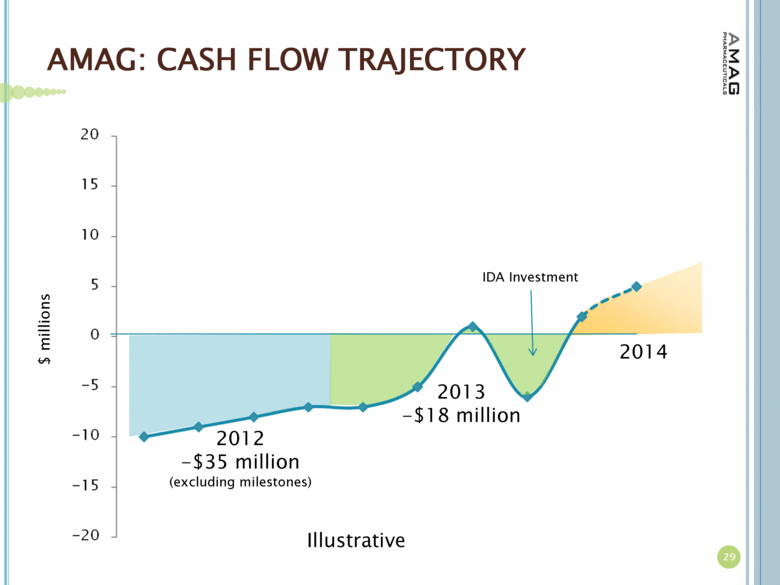
| AMAG: CASH FLOW TRAJECTORY 29 $ millions 2012 -$35 million (excluding milestones) 2013 -$18 million Illustrative IDA Investment 2014 |
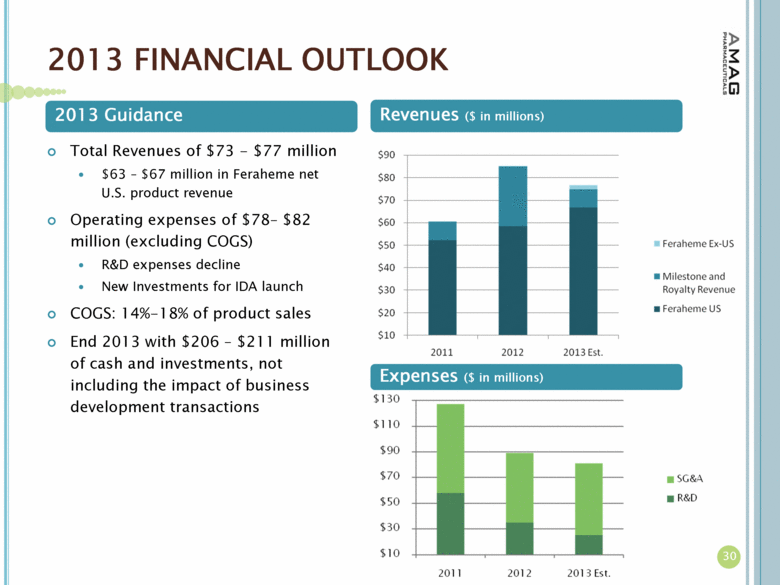
| 2013 FINANCIAL OUTLOOK 30 Expenses ($ in millions) Total Revenues of $73 - $77 million $63 – $67 million in Feraheme net U.S. product revenue Operating expenses of $78– $82 million (excluding COGS) R&D expenses decline New Investments for IDA launch COGS: 14%-18% of product sales End 2013 with $206 – $211 million of cash and investments, not including the impact of business development transactions 2013 Guidance Revenues ($ in millions) |
| BUILDING OFF A BASE OF SUCCESS IN 2013 31 Stabilized organization and turned focus to execution and growth Continued growth of Feraheme US CKD business Drove 17% Feraheme volume growth in the US Improved net revenue per gram for Feraheme Significant progress in Feraheme expansion efforts Completed phase III program for broad IDA indication; submitted sNDA Approved and launched in Canada and Europe; triggered $33 million in milestones Delivered on financial guidance Streamlined cost structure with 32% reduction in operating expenses 2013 Objectives Achieve double-digit growth of Feraheme in CKD patient population Increase net revenue per gram of Feraheme sNDA Formal acceptance by FDA – 1Q13 Approval – 4Q13 Launch – upon approval Execute a business development transaction Prepare for launch in broader IDA patient population Deliver on financial guidance 2012 Accomplishments |
| AMAG PHARMACEUTICALS Well positioned for growth in 2013... and beyond |