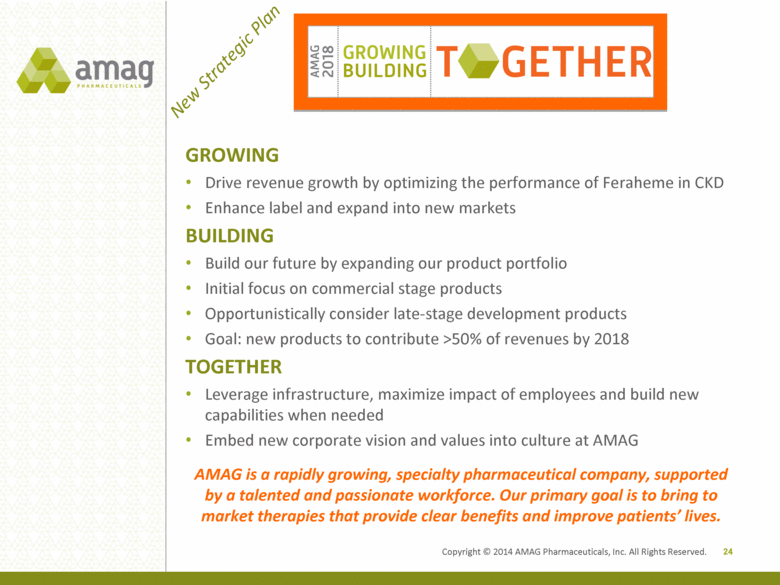
| Forward Looking Statements 1 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to: statements regarding (i) our new strategic plan; (ii) market growth opportunity and demand for Feraheme; (iii) commercial opportunities for Feraheme, including our account base and use by hospitals; (iv) growth in Feraheme’s current indication; (v) plans for the broader iron deficiency anemia indication for Feraheme in the U.S. and Rienso in the EU, if approved, and the expected timing of regulatory action; (vi) plans and strategies for MuGard, including its growth opportunity; (vii) our business development goals and opportunities; (viii) the impact of business development transactions on EBITDA; (ix) our ability to optimize after-tax cash flows with business development transactions; (x) expectations that more than 50% of our revenues will be attributable to new products by 2018; (xi) statements regarding the potential size and expansion of the U.S. IV iron market opportunity and patient population, and shifting practice patterns in the IV iron market; (xii) plans to optimize net revenue per gram; (xiii) plans to drive MuGard growth across the oral mucositis patient population; (xiv) our ability to operate the business with financial discipline, identify unique in-license/acquisition candidates and leverage our balance sheet strength; (xv) our expected financial results for 2013, including revenues, operating expenses and cost of goods sold; (xvi) our expected cash and investments balance for 2013; (xvii) our 2014 goals; and (xviii) our statement that AMAG is well positioned for success in 2014 and beyond are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include: (1) uncertainties regarding our sNDA and our ability to obtain regulatory approval for Feraheme in the U.S. and Canada, and Rienso outside of the U.S. and Canada, in the broader IDA indication , (2) our ability to successfully and timely complete clinical development programs, (3) uncertainties regarding our and Takeda Pharmaceutical’s ability to successfully compete in the intravenous iron replacement market both in the U.S. and outside the U.S., including the EU, (4) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso, (5) uncertainties regarding the manufacture of Feraheme/Rienso or MuGard, (6) uncertainties relating to our patents and proprietary rights both in the U.S. and outside the U.S., (7) the risk of an Abbreviated New Drug Application (ANDA) filing following the FDA’s recently published draft bioequivalence recommendation for ferumoxytol, (8) uncertainties regarding our ability to compete in the oral mucositis market in the U.S. and (9) other risks identified in our filings with the U.S. Securities and Exchange Commission (SEC), including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. |