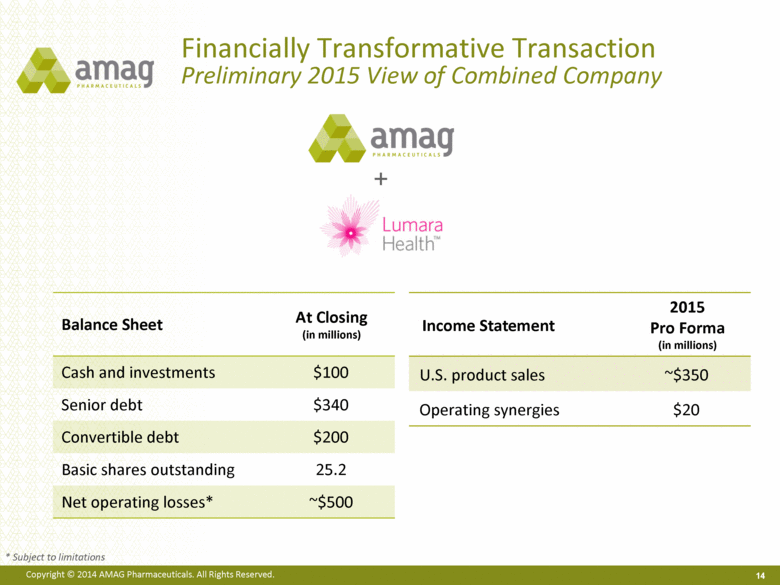
| Forward-Looking Statements (cont.) 2 (13) in connection with the Lumara Health acquisition, we will incur a substantial amount of indebtedness and will have to comply with restrictive and affirmative debt covenants, including a requirement that we reduce our leverage over time, (14) the possibility that we will need to raise additional capital from the sale of our common stock, which will cause significant dilution to our stockholders, in order to satisfy our contractual obligations, including our debt service, milestone payments that may become payable to Lumara Health’s stockholders /or in order to pursue business development activities, (15) upon consummation of the Lumara Health transaction, we will be highly leveraged and have limited cash and cash equivalent resources which may limit our ability to take advantage of attractive business development opportunities and execute on our strategic plan, (16) the possibility that our stockholders will not approve the amendment to our shareholder rights plan and that our tax benefits, including those acquired upon consummation of the Lumara Health acquisition, will not be available in the future, (17) the likelihood and timing of potential approval of Feraheme in the U.S. in the broader IDA indication in light of the complete response letter we received from the FDA informing us that our supplemental new drug application (sNDA) for the broader indication could not be approved in its present form and stating that we had not provided sufficient information to permit labeling of Feraheme for safe and effective use for the proposed broader indication, (18) the possibility that following FDA review of post-marketing safety data, including reports of serious anaphylaxis, cardiovascular events, and death, and/or in light of the label changes requested by the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) and confirmed by the Committee for Medicinal Products for Human Use (CHMP), the FDA (or other regulators) will request additional technical or scientific information, new studies or reanalysis of existing data, on-label warnings, post-marketing requirements/commitments or risk evaluation and mitigation strategies (REMS) in the current indication for Feraheme for IDA in adult patients with chronic kidney disease (CKD) and the additional costs and expenses that will or may be incurred in connection with such activities, (19) whether our proposed label changes will be acceptable to the FDA or other regulatory authorities and what impact such changes, or such additional changes as the FDA, CHMP or other regulators may require, will have on sales of Feraheme/ Rienso™ (Rienso is the trade name for ferumoxytol outside of the U.S. and Canada), (20) our and Takeda Pharmaceutical Limited’s ability to successfully compete in the IV iron replacement market both in the U.S. and outside the U.S., including the EU, as a result of limitations, restrictions or warnings in Feraheme’s/Rienso’s current or future label, including the changes recommended by PRAC and confirmed by CHMP that Rienso be administered to patients by infusion over at least 15-minutes (replacing injection) and that it be contraindicated for patients with any known history of drug allergy, (21) our ability to execute on our long-term strategic plan or to realize the expected results from our long-term strategic plan, (22) Takeda’s ability to obtain regulatory approval for Feraheme in Canada, and Rienso in the EU, in the broader IDA patient population, (23) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso and in turn affect sales, or our ability to market the product both in the U.S. and outside of the U.S., including the EU, (24) the relationship between Takeda and AMAG and the impact on commercialization efforts for Feraheme/Rienso in the EU and Canada, (25) the likelihood and timing of milestone payments, if any, in connection with AMAG’s licensing arrangement with Takeda, (26) the manufacture of Feraheme/Rienso or MuGard (or Makena if the acquisition is consummated), including any significant interruption in the supply of raw materials or finished product, (27) our patents and proprietary rights both in the U.S. and outside the U.S. (including those acquired in the acquisition), (28) the risk of an Abbreviated New Drug Application (ANDA) filing following the FDA’s draft bioequivalence recommendation for ferumoxytol published in December 2012, (29) the possibility that AMAG will disseminate future Dear Healthcare Provider letters in the U.S. (or, working Takeda, in Europe or other markets), (30) uncertainties regarding our ability to compete in the oral mucositis market in the U.S. and in the women’s maternal health market and (31) other risks identified in our filings with the U.S. Securities and Exchange Commission (SEC), including our Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and subsequent filings with the SEC. Any of the above risks and uncertainties could materially and adversely affect our results of operations, our profitability and our cash flows, which would, in turn, have a significant and adverse impact on our stock price. Use of the term “including” in the two paragraphs above shall mean in each case “including, but not limited to.” We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals and Feraheme are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard is a registered trademark of Access Pharmaceuticals, Inc. Rienso is a trademark of Takeda Pharmaceutical Company Limited. Makena is a registered trademark of Lumara Health Inc. Lumara Health is a trademark of Lumara Health Inc. |