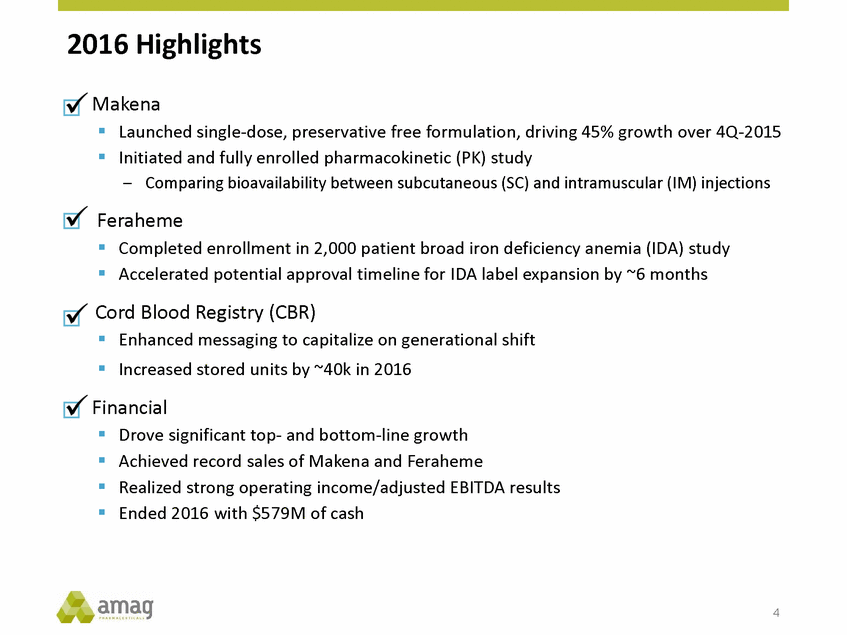
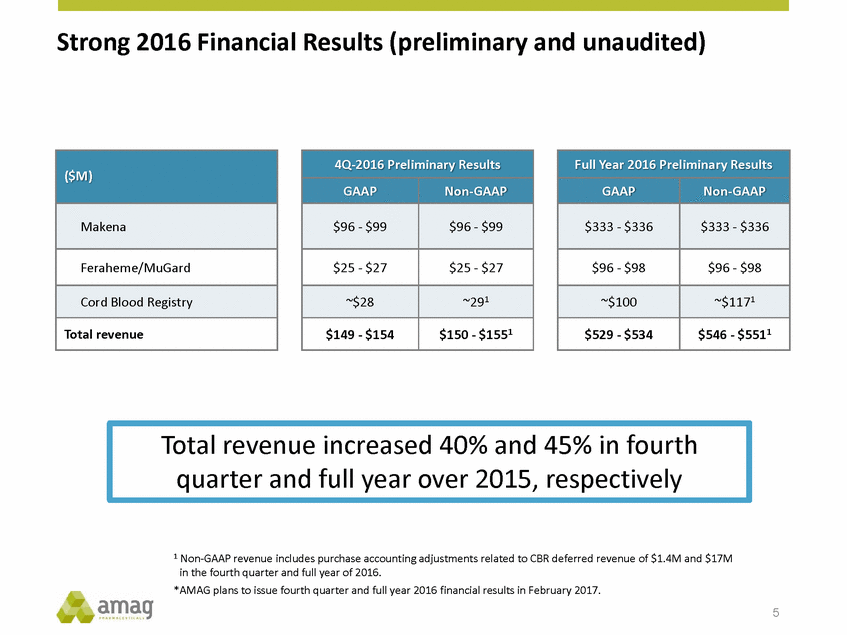
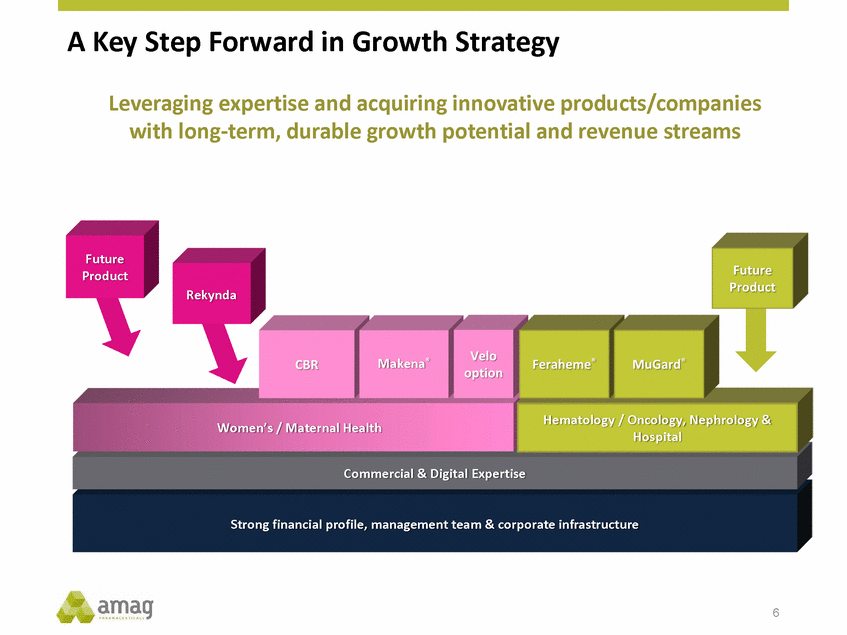
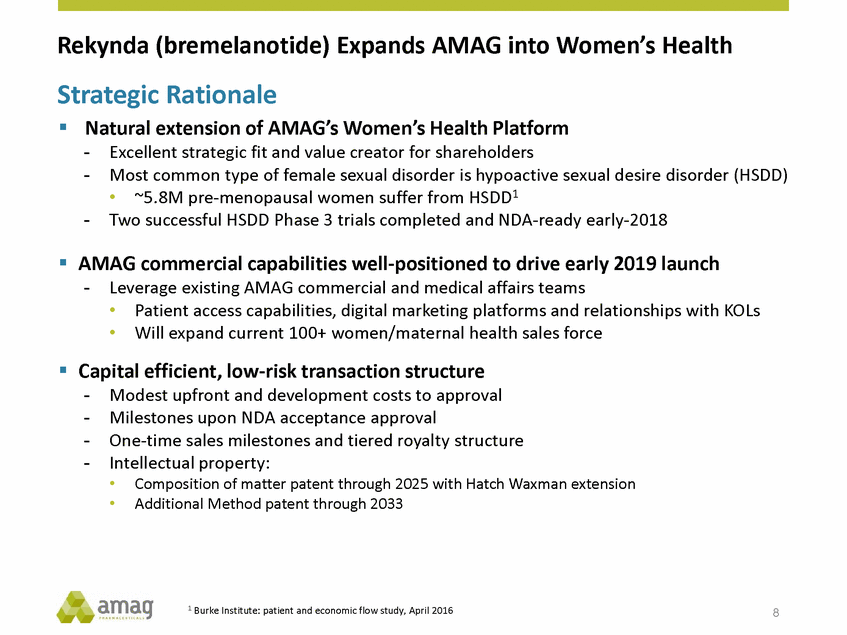
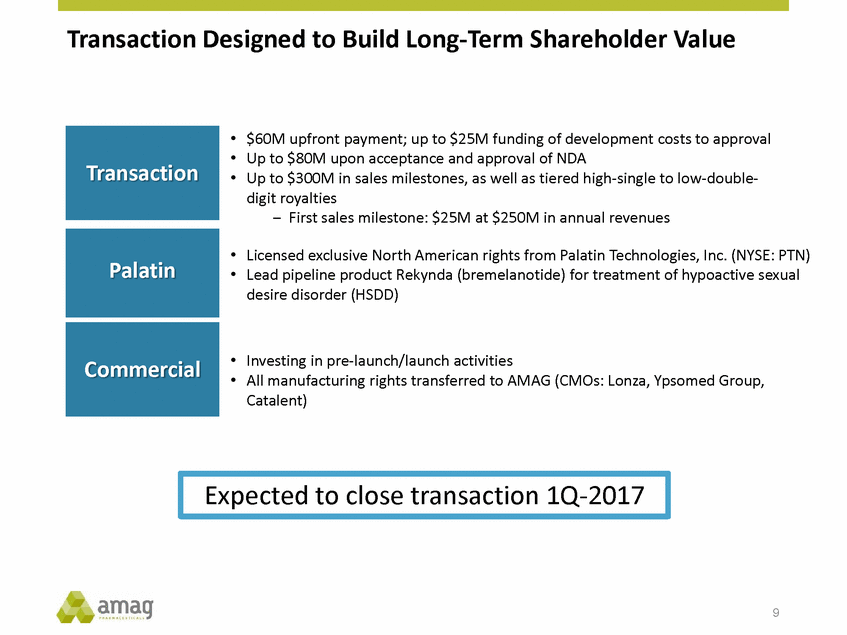
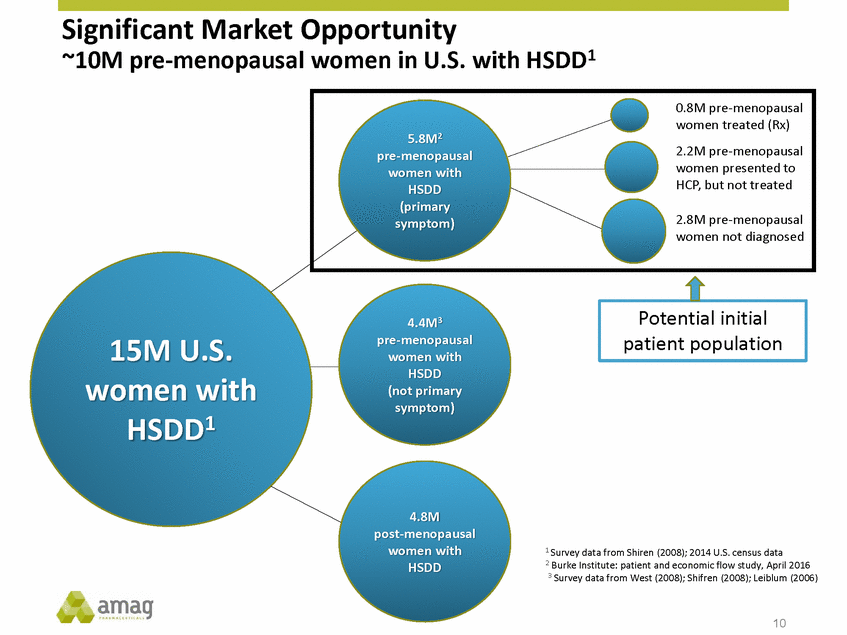
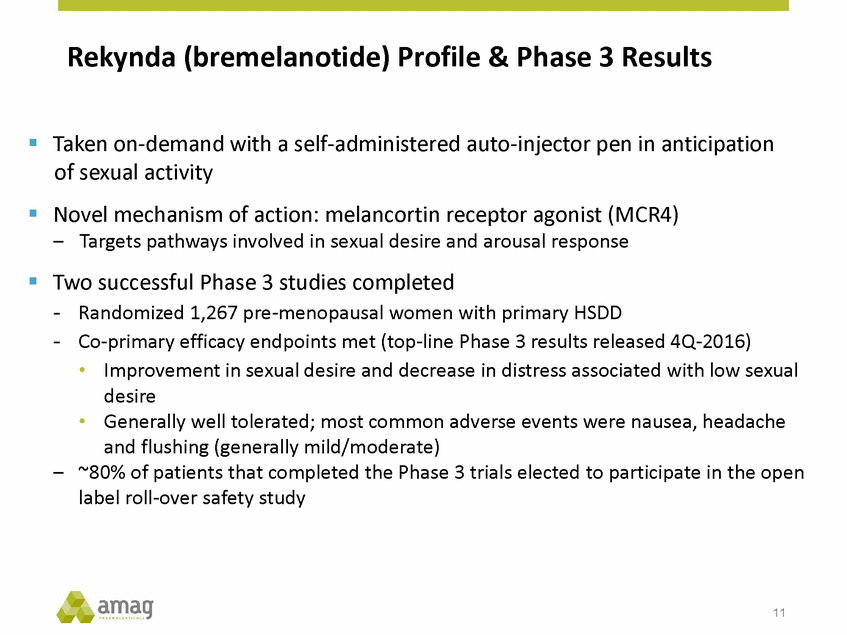
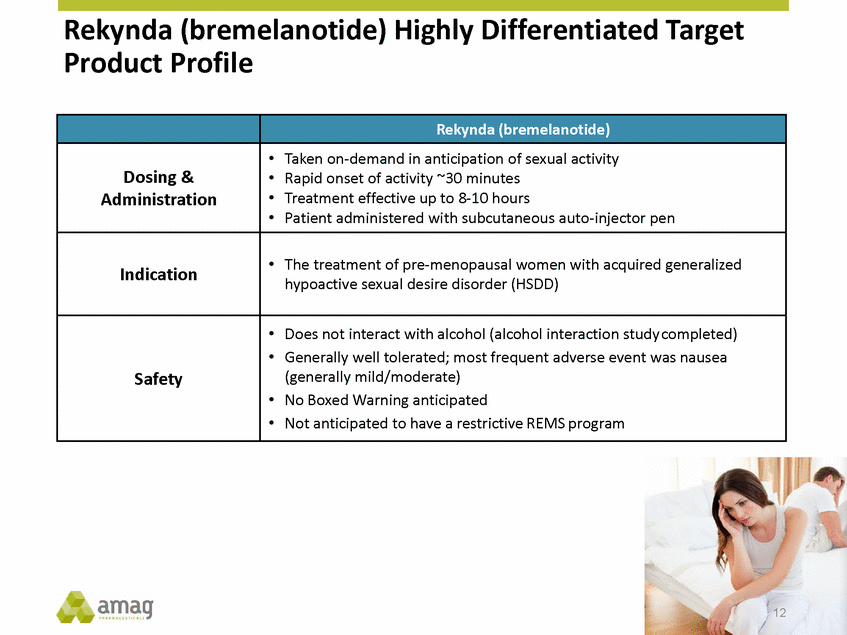
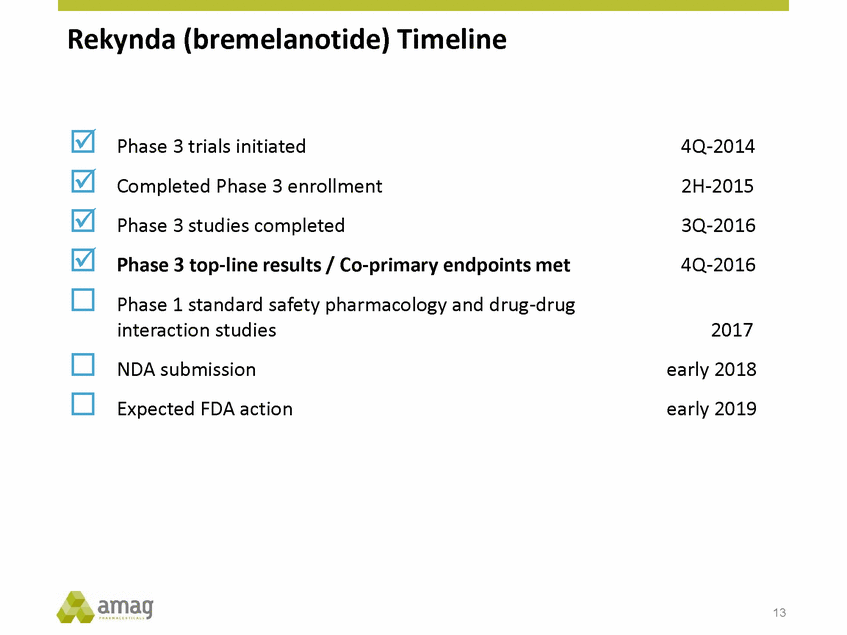
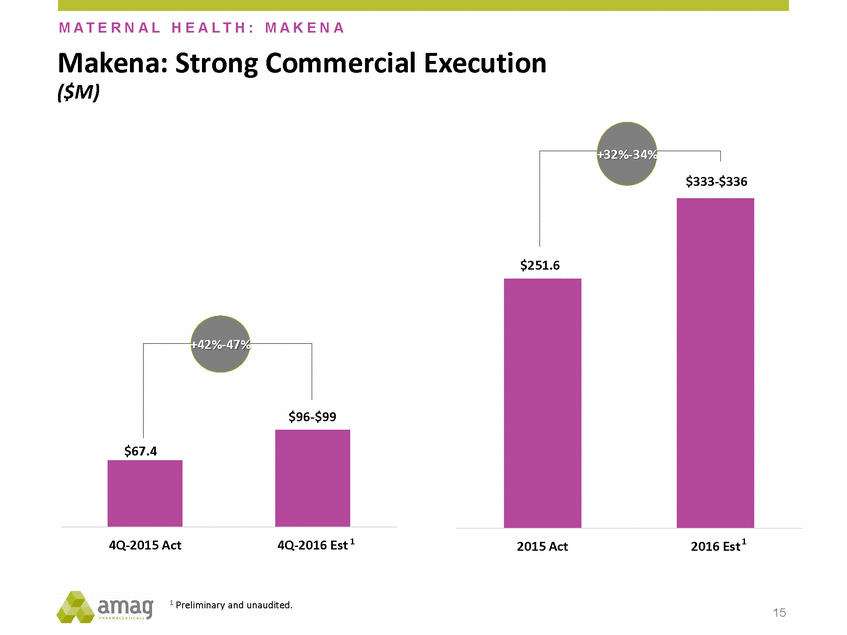
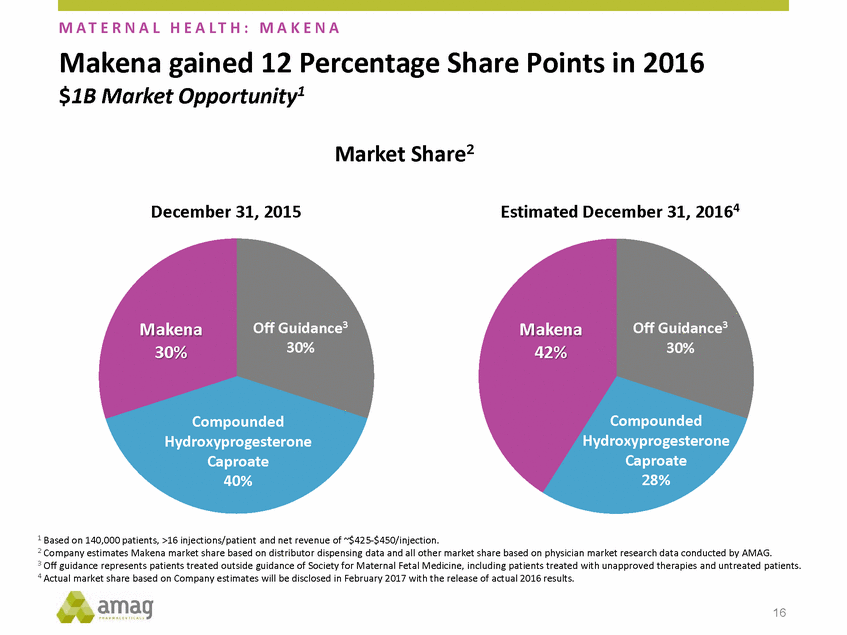
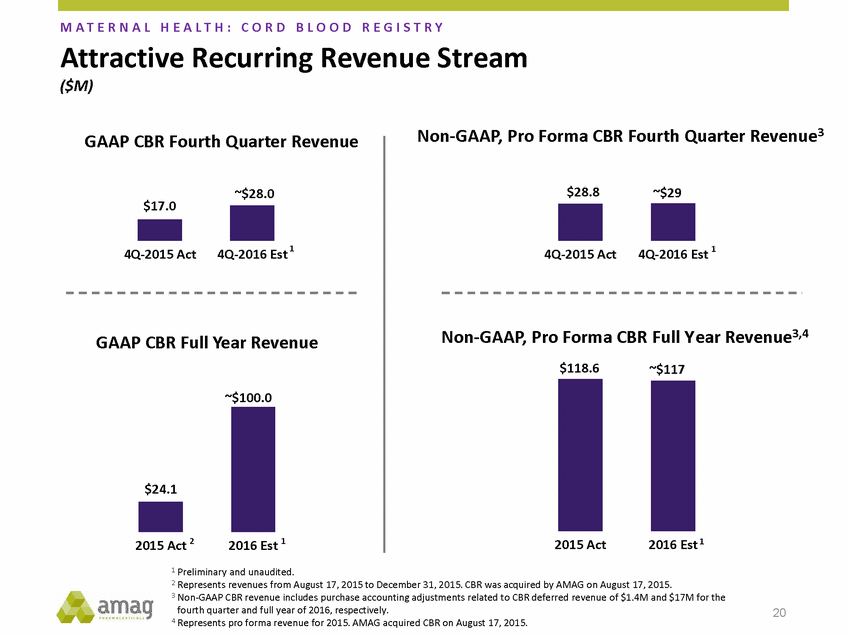
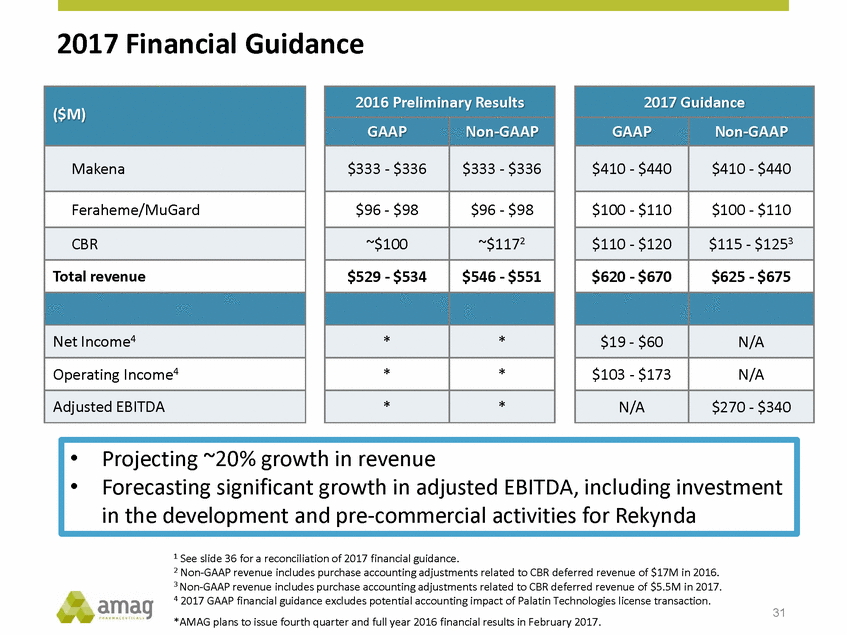
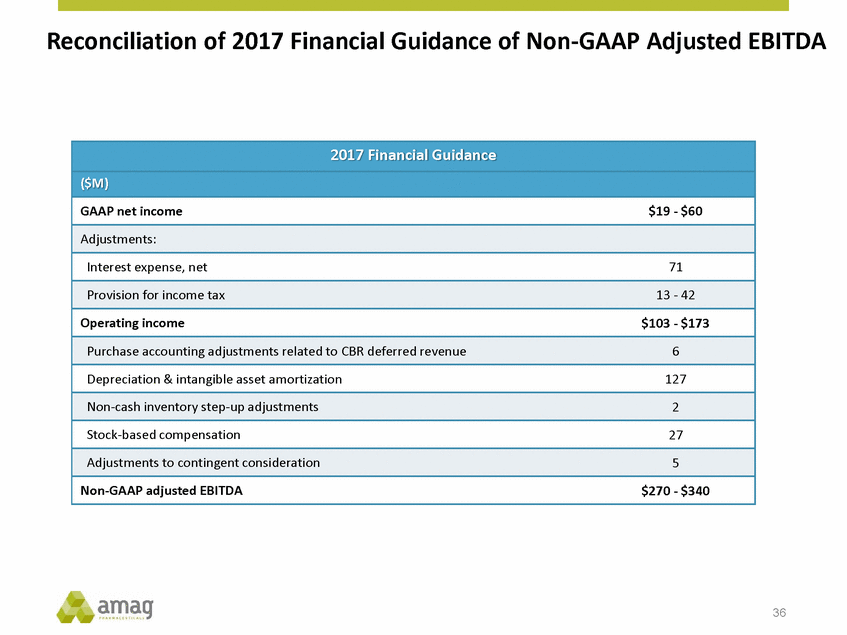
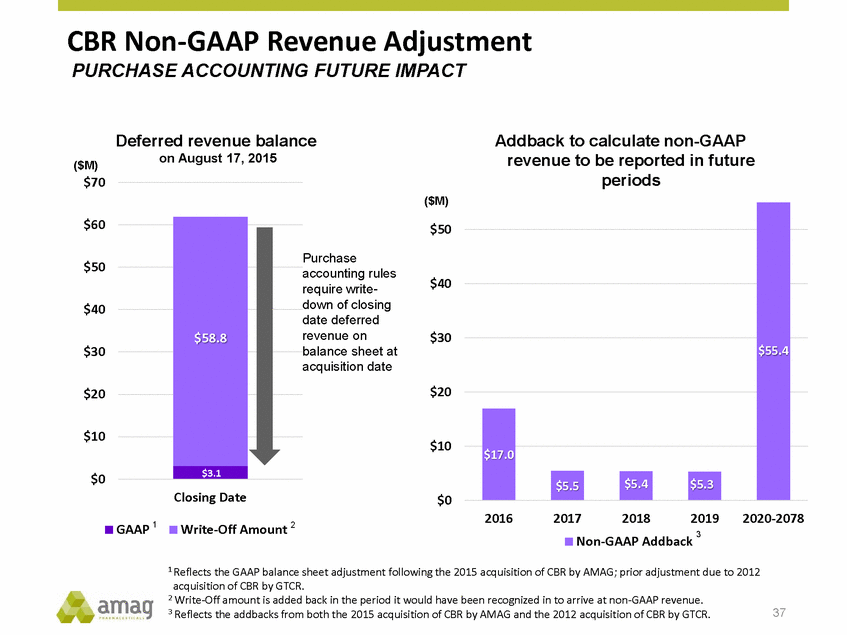
Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including, among others, expectations for AMAG’s growth strategy; AMAG’s anticipated expansion in women’s health through the licensing transaction with Palatin Technologies, Inc. and plans regarding the development activity of Rekynda; the structure of planned and pending clinical trials for Rekynda; the expected timeline, indications and expenditures for clinical development and commercialization of Rekynda, including the timing for the new drug application (NDA), subsequent FDA action and commercial launch; Rekynda’s strategic fit for AMAG’s women’s health business; the competitive landscape and breadth of the female sexual dysfunction (FSD) and hypoactive sexual desire disorder (HSDD) markets and Rekynda’s market potential; expectations for future next-generation formulations of Rekynda; AMAG’s and Palatin’s anticipated intellectual property rights associated with Rekynda; the expected timing for the closing of the Rekynda transaction; the timing and value of payments by AMAG under the Rekynda licensing transaction; the impact of the Rekynda product on AMAG’s financial results; expectations regarding the safety, efficacy and benefits of Rekynda, including that no boxed warning or restrictive REMS programs are anticipated; Makena’s position in the market and future growth drivers for Makena, including the potential market opportunity, progress for the next-generation development programs and customer engagement and outreach; beliefs that the current subcutaneous autoinjector (SC) formulation for Makena offers potential for more convenient and alternative administration; plans to explore alternative injection sites and formulations for Makena; expected timing for AMAG’s supplemental new drug application (sNDA) for Makena (including expected timing for an FDA decision on the sNDA) and collection and analysis of data, and results, from the definitive pharmacokinetic (PK) study; growth drivers for Cord Blood Registry (CBR), including plans to differentiate CBR offerings and increase engagement and communications in the industry; expectations for Feraheme, including growth for the intravenous (IV) iron market and the position of Feraheme within the IV iron market; anticipated growth drivers for Feraheme, including plans to optimize net revenue per gram and grow in key segments; expected timing for reporting clinical trial results and submitting the sNDA for the expanded Feraheme label and expectations that the size of the addressable market, if the broader indication is approved, would double; preliminary and unaudited financial results and cash and debt position for 2016 and AMAG’s 2017 finan cial guidance, including forecasted GAAP and non-GAAP revenues, GAAP and non-GAAP net income, GAAP operating income and adjusted EBITDA; expectations for AMAG’s key priorities in 2017, including plans to drive net product sales growth, increase non-GAAP adjusted EBITDA and undertake portfolio expansion activities, including by licensing and acquisition of products or companies; plans to continue to diversify the AMAG portfolio and achieve a mix of commercial assets and development pipeline for long-term growth; and plans to continue to enhance and scale AMAG’s internal capabilities are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others, (1) the possibility that the closing conditions set forth in the license agreement for Rekynda will not be met and that the parties will be unable to consummate the proposed transactions, (2) uncertainties regarding AMAG’s and Palatin’s ability to successfully and timely complete clinical development programs and obtain regulatory approval for Rekynda in North America, (3) the possibility that significant safety or drug interaction problems could arise with respect to Rekynda, (4) the ability of AMAG to raise awareness and understanding of HSDD and the potential benefits of Rekynda, (5) uncertainties regarding the manufacture of Rekynda, (6) uncertainties relating to our and Palatin’s patents and proprietary rights associated with Rekynda in North America, (7) that the cost of the Rekynda transaction to AMAG will be more than planned and/or will not provide the intended positive financial results, (8) that AMAG or Palatin will fail to fully perform under the Rekynda license agreement, (9) uncertainty regarding AMAG’s ability to compete in the FSD market in North America, and (10) as well as those risks identified in AMAG’s filings with the U.S. Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended December 31, 2015, its Quarterly Reports on Form 10-Q for the quarters ended March 31, 2016, June 30, 2016 and September 30, 2016 and subsequent filings with the SEC. Any such risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals® and Feraheme® are registered trademark of AMAG Pharmaceuticals, Inc. MuGard® is a registered trademark of Abeona Therapeutics, Inc. Makena® is a registered trademark of AMAG Pharmaceuticals IP, Ltd. Cord Blood Registry® and CBR® are registered trademarks of CBR Systems, Inc. Rekynda is a trademark of Palatin Technologies, Inc. 2