
Exhibit 99.1


Safe Harbor Statement
Statements contained in this presentation may contain information
that includes or is based upon certain “forward-looking statements”
relating to our business, future anticipated projected plans,
performance and developments, as well as other statements relating
to future operations and results. Any statements in this presentation
that are not statements of historical fact may be considered to be
forward-looking statements. These statements by their nature are
estimates of future results only and involve substantial risks and
uncertainties. Actual results may differ materially from those indicated
by these forward-looking statements. For a discussion of the factors
that could cause actual results to vary from these forward-looking
statements, please see our filings from time to time with the Securities
and Exchange Commission, which are available free of charge on the
SEC’s website at http://www.sec.gov, including our Quarterly Report
on Form 10-QSB for the three months ended March 31, 2006. Except
as required by law, we expressly disclaim any intent or obligation to
update any forward-looking statements.

Scientific Disclaimer
Statements contained in this presentation are based on the
opinion of management. Although management believes
these statements are supported by data and observations
derived from scientific studies performed by the Company, all
of the Company’s research and development efforts are early,
pre-clinical stage and Homspera, Radilex and Viprovex have
only undergone exploratory studies to evaluate its biological
activity in small animals.
There is no guarantee that regulatory authorities will ever
permit large animal or human testing of Radilex, Viprovex or
any other potential products derived from Homspera. Even if
such testing is permitted, none of Radilex, Viprovex or any
other potential drug candidates, if any, derived from Homspera
may be successfully developed or shown to be safe or
effective.
IR BioSciences Holdings, Inc. (OTCBB: IRBO)
A biopharmaceutical company engaged in research
and development of applications for Sar 9, Met (O2 )11-
Substance P with potential applications in the following
markets:
Radiation-Induced Neutropenia and
Thrombocytopenia
Influenza and other Viral and Bacterial Infections
Toxic Industrial Chemical (TIC)-Associated
Respiratory Damage

Russell Bedford Stefanou Mirchandani
Auditor
12.8%
Insider Ownership
NA
Institutional Ownership Shares
4
Full-time Employees
93,400,000
Fully Diluted Shares
78,319,254
Shares Outstanding (5/23/06)1
December 31
$570,000
171,163
$27,846,251
$0.40 || $0.19 to 0.52
Scottsdale, AZ
IRBO
Fiscal Year
Debt (5/23/06)
Volume (daily 90-day average)
Market Capitalization
Stock Price (5/23/06) || 52-Week Range
Corporate Headquarters
Symbol
Key Facts
1 Includes shares approved for issuance, adjustments and penalty shares that are unissued

Management Team
Hal Siegel, Ph.D., Senior Director, Product Development & Regulatory Affairs
Dr. Siegel has over a decade of experience providing scientific, clinical and regulatory
assistance to life science client companies across the medical product spectrum, helping
them meet business needs and FDA requirements from pre-clinical studies through the
regulatory submission process and into the post-approval marketplace.
John N. Fermanis, Chief Financial Officer
Mr. Fermanis is a co-founder of AMPS Wireless Data, Inc., where he served as Chief
Financial Officer from 2001 to 2004. Mr. Fermanis has over 18 years of financial
management experience with both the American Express Corporation and Citigroup in New
York City.
Michael K. Wilhelm, Chief Executive Officer and Director
Mr. Wilhelm has over 14 years of experience in the financial industry, working closely with
early stage companies. He serves as a liaison to government officials at the U.S. Homeland
Security Office as well as the country’s major health agencies. At the behest of the FDA’s
Division of Counter-Terrorism, he recently developed a Bioterrorism Preparedness Advisory
Board comprised of the country’s top bio-defense experts from the military, academia and
private industry.

Bioterrorism Preparedness
Advisory Board
James R. Campbell, Ph.D., M.P.H, Senior Advisor: Commanding Officer of the Office of Naval Research
Global, United States Navy, Medical Corps, (Ret.), Manager for Biosecurity and Biodefense, in the National
Security Directorate at the Pacific Northwest National Laboratory
The Honorable Asa Hutchinson, J.D. Senior Advisor: former Under Secretary for Border and Transportation
Security at the Department of Homeland Security, Partner and chair of Venable LLP’s Homeland Security Group
Dennis E. Amundson, D.O., Senior Advisor: Captain, United States Navy, Medical Corps, Naval Medical
Center, San Diego, Pulmonary Medicine
Kelly McQueen, M.D., MPH, Senior Advisor: Public Health Consultant specializing in International Health,
Disaster Relief and Humanitarian Aid and Civilian-Military Collaboration
Moshe Arditi, M.D., Senior Advisor: Director, Division of Pediatric Infectious Diseases, Cedars-Sinai Medical
Center, Los Angeles, CA
Mr. Michael Caridi, Senior Advisor: Chairman, MAJIC Development Group, SRC Industries Inc. and Protection
Plus Security Consultants, Inc.
Paul Carlton, M.D., Senior Advisor: Lt. General, USAF, Medical Corps, (Ret.), Director, Homeland Security for
The Health Science Center The Texas A&M University System, Former USAF Surgeon General
William Hoehn, Ph.D., Associate Advisor: Visiting Professor, Georgia Tech, Sam Nunn School of International
Affairs, Center for International Strategy, Technology, and Policy
Col. Kerrie Lindberg (Ret.), Associate Advisor: Colonel, USAF, Nurse Corps, (Ret.), Former Director, Defense
Institute for Medical Operations (DIMO), Brooks City-Base, Texas
John Kalns Ph.D.: Vice President and Chief Scientific Officer Hyperion Biotechnology, Inc. at Brooks City-Base

Contracted R&D Efforts
TGen (Translational Genomics Research Institute) (Phoenix, Arizona)
TGen Drug Development Services (TD2 LLC) is contracted to perform anti-cancer
research designed to assess preclinical efficacy (with the ability to expand to
Phase 1 and Phase 2a clinical studies at the associated Mayo Clinic Scottsdale,
MD Anderson Cancer Center and Arizona Cancer Center Tucson).
Hyperion Laboratory at Brooks City Air Force Base (San Antonio, Texas)
Contracted a series of anthrax studies These studies are being conducted at its
research facility located on the U.S. Air Force School of Aerospace Medicine
(USAFSAM) campus in Brooks City-Base in San Antonio, Texas
Oak Ridge National Laboratory (Oak Ridge, Tennessee)
Contracted to conduct Proof of Concept mouse radiation studies
Assist in the facilitation of additional pre-clinical and future clinical trials with regard to
determining the potential efficacy of Radilex as a treatment for acute radiation syndrome
Battelle (Columbus, Ohio)
Battelle Memorial Institute's Medical Research and Evaluation Facility
(MREF) has issued a letter of intent to support testing of Viprovex as an
Avian Influenza therapeutic in mice
Pacific Northwest National Laboratory (Redmond, Washington)
Developing integrated research plan with ORNL spanning small to large animals
studies utilizing the NIH-supported Proteomics Research Resource for
Integrative Biology for gaining mechanistic insights on Homspera activity
against radiological, biological and chemical threats.
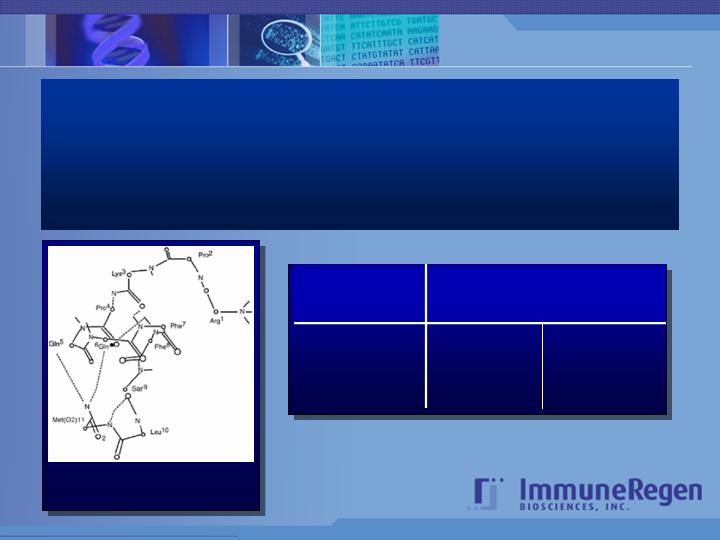
Homspera
The generic name Homspera was selected to refer to the
Sar9, Met (O 2)11-Substance P peptide.
Research and development efforts are centered on two
potential formulations and therapeutic applications for
Homspera – Radilex and Viprovex.
Preferential conformation of Sar9 ,
Met (O2)11-Substance P (Homspera)
in methanol
Viprovex
Radilex
Homspera
Trade names for different
formulations / indications
Generic Name

Anthrax : cytokine and apoptosis regulators
Influenza : cytokines and macrophages
TICs : cytokines and immunomodulators
Viprovex
Anti-Apoptosis (proteomics: peroxiredoxin
protection enables ROS scavengering)
Designed to protect / restore Immune
System (neutropenia, thrombocytopenia,
anemia)
Design to protect GI and CNS cells
Radilex
Putative Mechanisms of Action
Mechanisms

Indications
Treatment of pulmonary damage or distress
caused by exposure to various biological and
chemical agents:
Anthrax
Influenza (avian, equine, etc.)
Chemically induced respiratory damage
Viprovex
Treatment of acute radiation exposure:
Acute Radiation Syndrome (ARS)
Neutropenia and Thrombocytopenia
Designed to act as a potential radioprotectant
Radilex
Based on what is believed to be the Mechanisms of
Action, studies are currently being conducted or planned
in the following areas:

Radilex: ARS
No toxicity witnessed in studies to date
Potential of efficacy demonstrated in small animal models
Radilex
Particulate: Potassium iodide, DTPA, Prussian Blue
Irradiation: Filgrastim (Neupogen®); 5-androstenediol
(Neumune®; in development)
Treatment Gold Standards: No approved standard
Particulate: Deposition of radioactive particulates on skin and
ingestion of radioactive materials internally
Irradiation: Exposure to radioactive material

Radilex: Pre-clinical Studies
Mouse Radiation Study No. 1, September 2002, attempted to identify an LD50 value for subsequent standardization of exposure.
Mouse Radiation Study No. 2, January 2003, administered Radilex via aerosol administration to determine efficacy, if any, by
increasing survival time from a lethal dose of radiation. Observed that Radilex treated mice lived an average of two days
longer than untreated, radiation exposed mice.
Mouse Radiation Study No. 3, April 2003, demonstrated 25% survival in mice at 37 days post irradiation with a 50 M dose of
Radilex .
Mouse Radiation Study No. 4, June 2003, analyzed various dose concentrations of Radilex treatment.
Pilot Mouse Radiation Study No. 5, July 2003, further studied radiation lethality and Radilex potential in preventing lethality in
mice. Observed 50% of Radilex-treated mice survived at 90 days post exposure and showed no significant differences in
their immune system.
Pilot Mouse Radiation Study No. 6, June 2004, demonstrated direct muscle injection to be less effective at similar dosing
parameters than inhalation.
Pilot Mouse Radiation Study No. 7, November 2004, studied dosing in attempt to determine a maximum efficacious dose of
Radilex.
Blood Profile Mouse Radiation Study, August 2005, an exploratory pilot study to demonstrate protection of C57BL/6 mice from
neutropenia and thrombocytopenia. Observed treated mice trended toward restored blood and platelet cell numbers with
normalized pathology of bone marrow.
Radiation Sensitivity Animal Model Study, January 2006, further examined the proof of concept for the formulation of Sar9, Met
(O2)11-Substance P.
Radiation Study, February 2006 at Oak Ridge National Laboratory, following the AFRRI model for radiation sickness this study
provided additional support of the potential of Radilex as a treatment to a lethal dose of radiation exposure.

Viprovex: Anthrax
No toxicity witnessed in studies to date
Potential of efficacy demonstrated in protecting
mice from inhaled anthrax (Bacillus Anthracis)
Viprovex
Antibiotics (ciprofloxacin, doxycyclin, penicillin) and
supportive care
Vaccines (BioPort) and anti-toxins (in development)
Treatment Gold Standards:

Viprovex: Anthrax Studies
Sponsoring a series of studies with Hyperion
Biotechnology Inc. at their laboratory facilities located at
Brooks City-Base in San Antonio, Texas with the
cooperation of the U.S. Air Force School of Aerospace
Medicine (USAFSAM).
Studies are being conducted in hope of determining the
efficacy of Viprovex in treating exposure to anthrax bacilli.
The purpose of these studies is to determine if Viprovex
will reduce the mortality rate of an active infection of
pulmonary anthrax.
Potential efficacy demonstrated in small animals
in a pre-treatment model of pulmonary anthrax sickness in
studies with end points of survival.
Viprovex Potentially Activates Innate
Immunity and Neuroprotection Genes
Real time PCR was used to examine cellular
RNA for innate immunity and neuroprotection
genes following Sar 9, Met (O2 )11-Substance P
treatment over a narrow micromolar range of
concentrations:
Defensins increase 50-5000 fold
TLRs increase 7-200 fold
ILs increase 30-1000 fold

Viprovex: Avian
Influenza Potential
Potential of efficacy demonstrated in treating Hong Kong
influenza virus in small animal models
No toxicity witnessed in studies to date
Viprovex
Tamiflu (oseltamivir, oral neuraminidase inhibitor) and
Relenza (zanamivir, inhaled neuraminidase inhibitor) and
Symmetrel (amantadine, inhibits viral entry/uncoating)
Vaccination (seasonal; potential pandemic in development)
Treatment Gold Standards:
No FDA-approved vaccines for avian influenza

Viprovex: Viral Studies
In October, 2003 studies were conducted with the Hong Kong
influenza virus (A/Hong Kong/8/68, of the H3N2 avian type)
using mice inoculated with the virus and treated with
Viprovex.
Results demonstrated that Viprovex may have potentially
reduced elevated levels of inflammatory cells in the mice
inoculated with Hong Kong influenza virus and treated with
Viprovex.
Electron micrographs showed no virus particles in the
Viprovex-treated animals in contrast to the controls,
suggesting, that Viprovex may have potential in the treatment
of viral replication and pathology.

Hong Kong Influenza
(Avian) and Viprovex

Development of
Radilex and Viprovex
If the Company is capable of attaining treatment under the
Animal Efficacy Rule the product development process would
be accelerated; however, there are no assurances of such
occurring.
Due to the lethal nature of the ailments that the Company is
developing countermeasures against, management believes
that the development paths of Radilex and Viprovex would fall
under the Animal Efficacy Rule.
All of the Company’s research efforts are at an early, pre-
clinical stage of development.
Animal Efficacy Rule
Adopted by the FDA in 2002
Applies in situations where it would be unethical to conduct
traditional Phase II and Phase III efficacy studies in humans
FDA will review new drugs for approval on the basis of
safety in humans and efficacy in relevant animal models.
Potentially eliminates Phase II and III testing
Potentially accelerates product development timeline
Often reduces capital expenditures associated with the
development process
“Approval of New Drugs When Human Efficacy Studies
Are Not Ethical or Feasible”
(Code of Federal Regulations, Title 21, Part 314, Subpart I)

Summary Financial Data
(3,376,889)
Total stockholder's deficit
3,421,070
Total current liabilities
44,181
Total assets
$ 23,161
Total current assets
44,181
Total liabilities and stockholders' deficit
Selected Balance Sheet Data at March 31, 2006
(Unaudited)
317,242
Options
14,765,569
Warrants
—
Preferred Stock
78,319,254
Common Stock
93,402,065
Total
Capitalization at May 23, 2006

IR BioSciences Holdings, Inc.
4021 N. 75th Street, Suite 201
Scottsdale, AZ 85251
480.922.3926 – Phone
480.922.4781 – Fax
866.922.3926 – Toll Free
investors@immuneregen.com
General Counsel
Jeffrey R. Perry
Law Office of Jeffrey R. Perry
11000 N. 77th Place, Suite 1053
Scottsdale, AZ 85260
480.368.5441
Jeffrey.Perry@azbar.org
Public Relations
Jason Grimley
Spelling Communications
2211 Corinth Avenue, Suite 210
Los Angeles, CA 90064
310.477.9500
jgrimley@spellcom.com
Outside Legal Counsel
Intellectual Property Law
Sarah Kagan, Esq.
Banner & Witcoff, LTD
1001 G. Street NW, Suite 1100
Washington, DC 20001
202.824.3151
skagan@bannerwitcoff.com
www.bannerwitcoff.com
Kent Walker, Esq.
Cooley Godward L.L.P.
4401 Eastgate Mall
San Diego, CA 92121
858.550.6065
walkerkm@cooley.com
www.cooley.com
Corporate Counsel
Thomas J. Poletti, Esq.
Kirkpatrick & Lockhart Nicholson Graham LLP
10100 Santa Monica Blvd., 7th Floor
Los Angeles, CA 90067
310.552.5000
tpoletti@klng.com
www.klng.com























