
FY2020 Q2 Results May 8, 2020 Exhibit 99.2

The Private Securities Litigation Reform Act of 1995 provides a safe harbor from civil litigation for forward-looking statements accompanied by meaningful cautionary statements. Except for historical information, this presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, which may be identified by words such as “continues”, “estimates”, “anticipates”, “projects”, “plans”, “seeks”, “may”, “will”, “expects”, “intends”, “believes”, “signals”, “should”, “can” and similar expressions or the negative versions thereof and which also may be identified by their context. All statements that address operating performance or events or developments that Meridian expects or anticipates will occur in the future, including, but not limited to, statements relating to per share diluted earnings, sales, product demand, revenue, and the impact of COVID-19 on our business and prospects, are forward-looking statements. Such statements, whether expressed or implied, are based upon current expectations of the Company and speak only as of the date made. Specifically, Meridian’s forward-looking statements are, and will be, based on management’s then-current views and assumptions regarding future events and operating performance. Meridian assumes no obligation to publicly update or revise any forward-looking statements even if experience or future changes make it clear that any projected results expressed or implied therein will not be realized. These statements are subject to various risks, uncertainties and other factors that could cause actual results to differ materially, including, without limitation, the following: Meridian’s operating results, financial condition and continued growth depends, in part, on its ability to introduce into the marketplace enhancements of existing products or new products that incorporate technological advances, meet customer requirements and respond to products developed by Meridian’s competition, its ability to effectively sell such products and its ability to successfully expand and effectively manage increased sales and marketing operations. While Meridian has introduced a number of internally developed products and acquired products, there can be no assurance that it will be successful in the future in introducing such products on a timely basis or in protecting its intellectual property, and unexpected or costly manufacturing costs associated with its introduction of new products or acquired products could cause actual results to differ from expectations. Meridian relies on proprietary, patented and licensed technologies. As such, the Company’s ability to protect its intellectual property rights, as well as the potential for intellectual property litigation, would impact its results. Ongoing consolidations of reference laboratories and formation of multi-hospital alliances may cause adverse changes to pricing and distribution. Recessionary pressures on the economy and the markets in which our customers operate, as well as adverse trends in buying patterns from customers, can change expected results. Costs and difficulties in complying with laws and regulations, including those administered by the United States Food and Drug Administration, can result in unanticipated expenses and delays and interruptions to the sale of new and existing products, as can the uncertainty of regulatory approvals and the regulatory process (including the currently ongoing study and other FDA actions regarding the Company’s LeadCare products). The international scope of Meridian’s operations, including changes in the relative strength or weakness of the U.S. dollar and general economic conditions in foreign countries, can impact results and make them difficult to predict. One of Meridian’s growth strategies is the acquisition of companies and product lines. There can be no assurance that additional acquisitions will be consummated or that, if consummated, will be successful and the acquired businesses will be successfully integrated into Meridian’s operations. There may be risks that acquisitions may disrupt operations and may pose potential difficulties in employee retention, and there may be additional risks with respect to Meridian’s ability to recognize the benefits of acquisitions, including potential synergies and cost savings or the failure of acquisitions to achieve their plans and objectives. Meridian cannot predict the outcome of goodwill impairment testing and the impact of possible goodwill impairments on Meridian’s earnings and financial results. Meridian cannot predict the possible impact of U.S. health care legislation enacted in 2010 – the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act – and any modification or repeal of any of the provisions thereof initiated by Congress or the presidential administration, and any similar initiatives in other countries on its results of operations. Efforts to reduce the U.S. federal deficit, breaches of Meridian’s information technology systems, trade wars, increased tariffs, and natural disasters and other events could have a materially adverse effect on Meridian’s results of operations and revenues. In the past, the Company has identified a material weakness in our internal control over financial reporting, which has been remediated, but the Company can make no assurances that a material weakness will not be identified in the future, which if identified and not properly corrected, could materially adversely affect our operations and result in material misstatements in our financial statements. Meridian also is subject to risks and uncertainties related to disruptions to or reductions in business operations or prospects due to pandemics, epidemics, widespread health emergencies, or outbreaks of infectious diseases such as the coronavirus disease COVID-19. In addition to the factors described in this paragraph, please also refer to additional factors identified from time to time in our filings with the Securities and Exchange Commission, including in Part I, Item 1A Risk Factors of our most recent Annual Report on Form 10-K, which contains a list and description of uncertainties, risks and other matters that may affect the Company. Readers should carefully review these forward-looking statements and risk factors, and not place undue reliance on our forward-looking statements. Forward Looking Statements

Certain financial measures presented in this presentation, such as operating expenses, operating income, net earnings and diluted earnings per share, excluding as applicable the effects of acquisition-related costs, a change in fair value of contingent consideration obligation, restructuring costs and selected legal costs, are not recognized under generally accepted accounting principles in the United States of America, or U.S. GAAP. Management believes this non-GAAP financial information is useful to an investor in evaluating our performance, as these measures: (i) help investors to more meaningfully evaluate and compare the results of operations from period to period by removing the impacts of these non-routine items; and (ii) are used by management for various purposes, including evaluating performance from period to period in presentations to our board of directors, and as a basis for strategic planning and forecasting. While we believe these financial measures are commonly used by investors to evaluate our performance and that of our competitors, the non-GAAP measures in this presentation may be different from non-GAAP measures used by other companies and should not be considered as an alternative to performance measures derived in accordance with U.S. GAAP. In addition, the non-GAAP measures presented herein are not based on any comprehensive set of accounting rules or principles. These non-GAAP measures have limitations, in that they do not reflect all amounts associated with our results as determined in accordance with U.S. GAAP, and they should not be considered as alternatives to information attributable to Meridian Bioscience, Inc. determined in accordance with U.S. GAAP. See the consolidated financial statements included in our reports filed with the U.S. Securities and Exchange Commission for our U.S. GAAP results. Additionally, for reconciliations of the non-GAAP measures included herein to our closest reported U.S. GAAP measures, refer to the reconciliations included in the press release of Meridian Bioscience, Inc. dated May 8, 2020. Non-GAAP Financial Measures

Life Science rapidly responds to COVID-19 & record revenue YoY quarterly revenue growth for Diagnostics segment Curian® analyzer and HpSA ® assay receive FDA clearance Acquisition of Exalenz Bioscience Ltd. and BreathID ® platform Q2 FY2020 Business Highlights Diagnostics Life Science
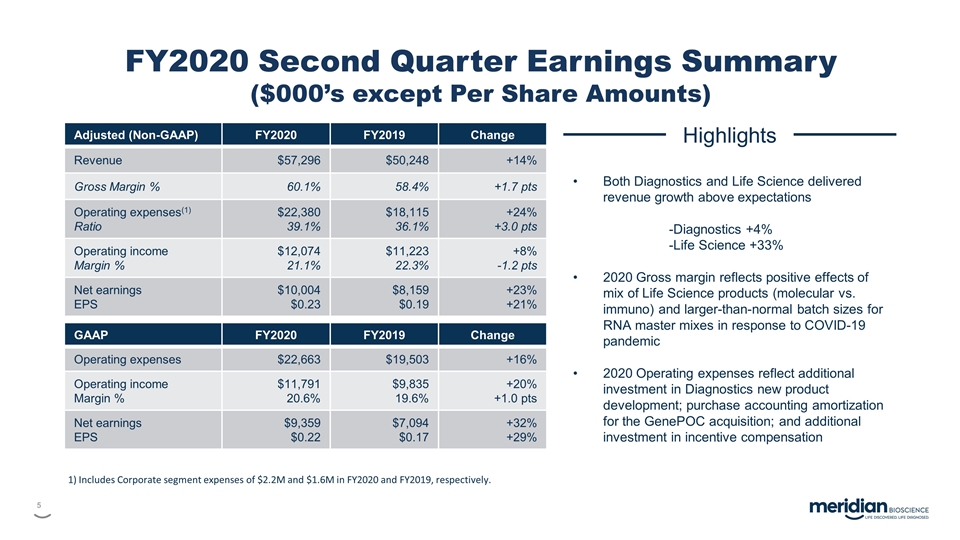
FY2020 Second Quarter Earnings Summary ($000’s except Per Share Amounts) Adjusted (Non-GAAP) FY2020 FY2019 Change Revenue $57,296 $50,248 +14% Gross Margin % 60.1% 58.4% +1.7 pts Operating expenses(1) Ratio $22,380 39.1% $18,115 36.1% +24% +3.0 pts Operating income Margin % $12,074 21.1% $11,223 22.3% +8% -1.2 pts Net earnings EPS $10,004 $0.23 $8,159 $0.19 +23% +21% GAAP FY2020 FY2019 Change Operating expenses $22,663 $19,503 +16% Operating income Margin % $11,791 20.6% $9,835 19.6% +20% +1.0 pts Net earnings EPS $9,359 $0.22 $7,094 $0.17 +32% +29% Highlights Both Diagnostics and Life Science delivered revenue growth above expectations -Diagnostics +4% -Life Science +33% 2020 Gross margin reflects positive effects of mix of Life Science products (molecular vs. immuno) and larger-than-normal batch sizes for RNA master mixes in response to COVID-19 pandemic 2020 Operating expenses reflect additional investment in Diagnostics new product development; purchase accounting amortization for the GenePOC acquisition; and additional investment in incentive compensation 1) Includes Corporate segment expenses of $2.2M and $1.6M in FY2020 and FY2019, respectively.

FY2020 Second Quarter Operating Segment Highlights ($000’s) Diagnostics (Adjusted Non-GAAP) FY2020 FY2019 Change Revenue $34,942 $33,500 +4% Operating income Margin % $3,337 9.6% $7,436 22.2% -55% -12.6 pts Diagnostics revenue by: Technology: Molecular assays $7,238 $7,084 +2% Immunoassays & blood chemistry 27,704 26,416 +5% Disease State: GI (Gastrointestinal) $14,014 $16,177 -13% RI (Respiratory Illnesses) 10,863 7,553 +44% Blood Chemistry (Lead) 4,329 4,330 - % Other 5,736 5,440 +5% Life Science (Adjusted Non-GAAP) FY2020 FY2019 Change Revenue $22,354 $16,748 +33% Operating income Margin % $10,921 48.9% $5,386 32.2% +103% +16.7 pts Life Science revenue by: Technology: Molecular reagents $11,534 $5,390 +114% Immunological reagents 10,820 11,358 -5% Region: Americas $4,612 $5,454 -15% EMEA 9,946 7,852 +27% ROW 7,796 3,442 +126% China (included in ROW) 5,312 1,328 +300% Product / Customer Highlights: RI volume growth strong in Flu, GAS and Mycoplasma GI affected by volume declines in most major assays, as well as Hp contract price changes Blood Chemistry experienced lower order patterns in March (non-critical care assay) Product / Customer Highlights: Record-level molecular reagent shipments ($5.6M related to COVID-19) Shipments to industrial customers increased ~40%
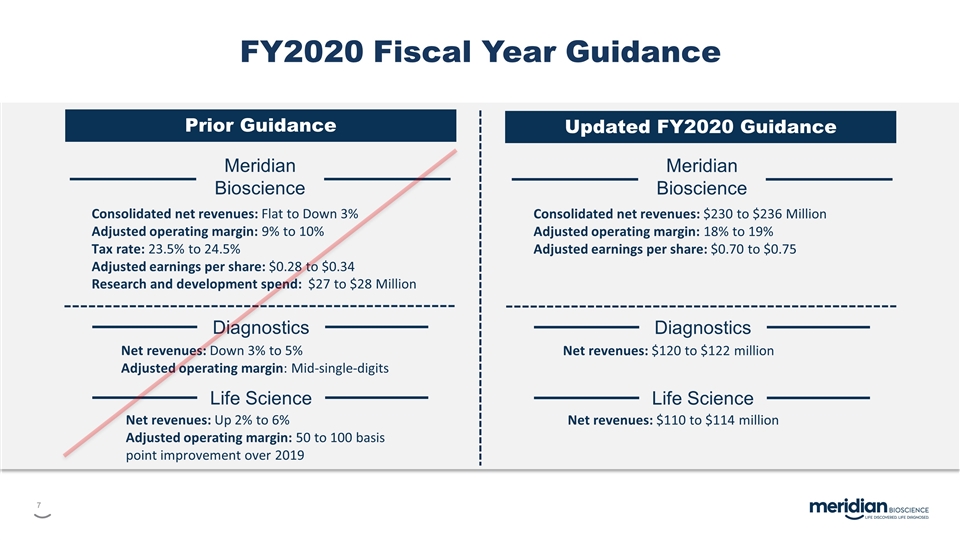
FY2020 Fiscal Year Guidance Meridian Bioscience Consolidated net revenues: Flat to Down 3% Adjusted operating margin: 9% to 10% Tax rate: 23.5% to 24.5% Adjusted earnings per share: $0.28 to $0.34 Research and development spend: $27 to $28 Million Diagnostics Net revenues: Down 3% to 5% Adjusted operating margin: Mid-single-digits Life Science Net revenues: Up 2% to 6% Adjusted operating margin: 50 to 100 basis point improvement over 2019 Prior Guidance Updated FY2020 Guidance Meridian Bioscience Consolidated net revenues: $230 to $236 Million Adjusted operating margin: 18% to 19% Adjusted earnings per share: $0.70 to $0.75 Diagnostics Net revenues: $120 to $122 million Life Science Net revenues: $110 to $114 million

COVID-19 Response
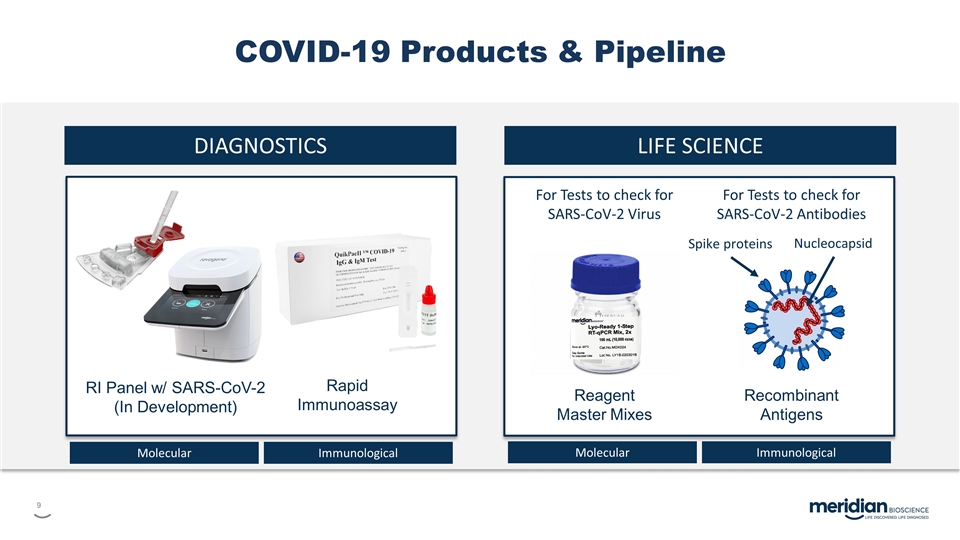
COVID-19 Products & Pipeline DIAGNOSTICS LIFE SCIENCE Reagent Master Mixes Recombinant Antigens RI Panel w/ SARS-CoV-2 (In Development) Rapid Immunoassay For Tests to check for SARS-CoV-2 Antibodies For Tests to check for SARS-CoV-2 Virus Spike proteins Nucleocapsid Molecular Immunological Molecular Immunological

Exalenz Bioscience Ltd. Acquisition
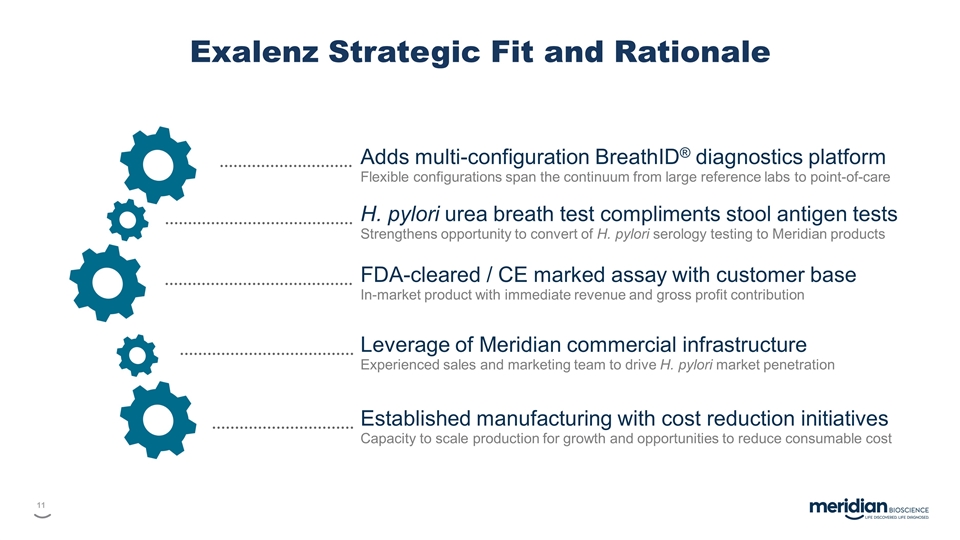
Exalenz Strategic Fit and Rationale Adds multi-configuration BreathID® diagnostics platform Flexible configurations span the continuum from large reference labs to point-of-care H. pylori urea breath test compliments stool antigen tests Strengthens opportunity to convert of H. pylori serology testing to Meridian products FDA-cleared / CE marked assay with customer base In-market product with immediate revenue and gross profit contribution Leverage of Meridian commercial infrastructure Experienced sales and marketing team to drive H. pylori market penetration Established manufacturing with cost reduction initiatives Capacity to scale production for growth and opportunities to reduce consumable cost
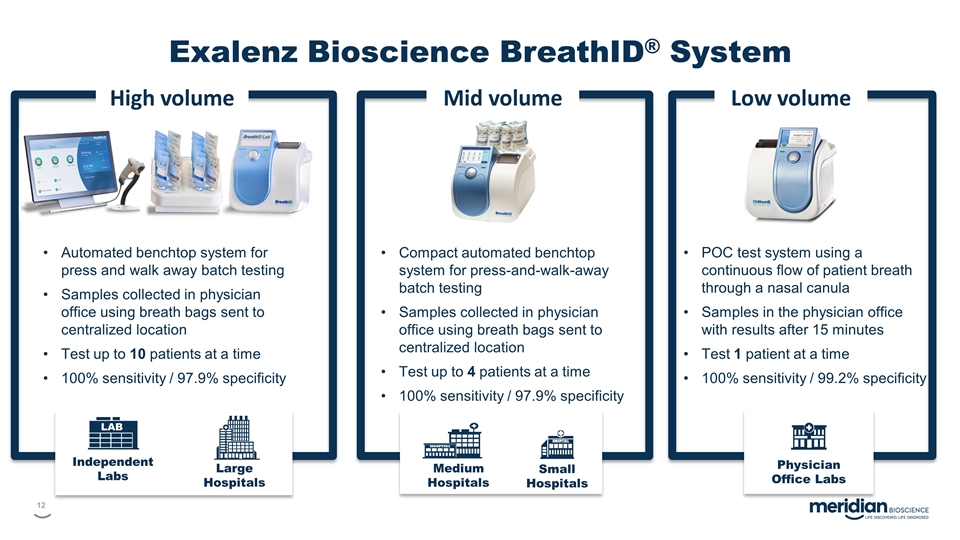
High volume Independent Labs LAB Large Hospitals Automated benchtop system for press and walk away batch testing Samples collected in physician office using breath bags sent to centralized location Test up to 10 patients at a time 100% sensitivity / 97.9% specificity Low volume Physician Office Labs Mid volume Medium Hospitals Small Hospitals Compact automated benchtop system for press-and-walk-away batch testing Samples collected in physician office using breath bags sent to centralized location Test up to 4 patients at a time 100% sensitivity / 97.9% specificity POC test system using a continuous flow of patient breath through a nasal canula Samples in the physician office with results after 15 minutes Test 1 patient at a time 100% sensitivity / 99.2% specificity Exalenz Bioscience BreathID® System
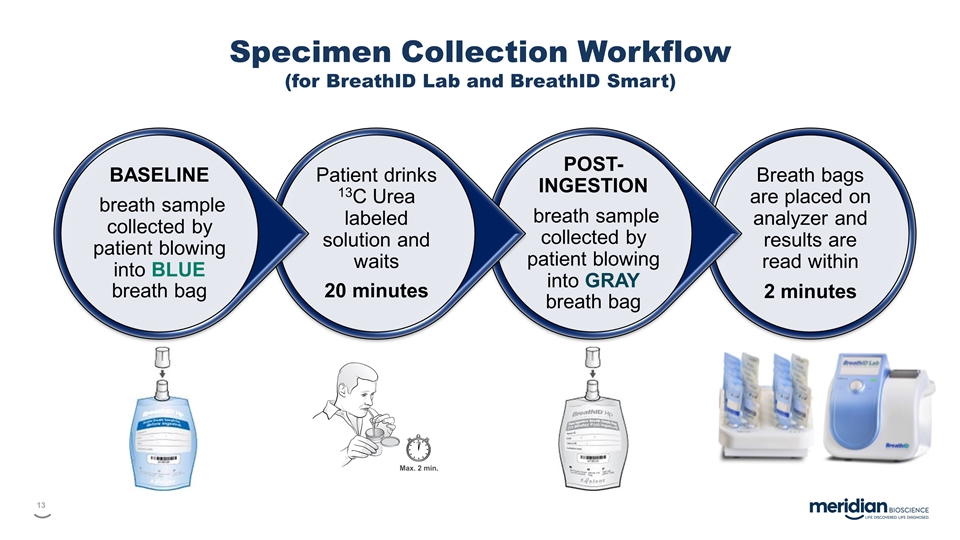
Breath bags are placed on analyzer and results are read within 2 minutes POST-INGESTION breath sample collected by patient blowing into GRAY breath bag Patient drinks 13C Urea labeled solution and waits 20 minutes BASELINE breath sample collected by patient blowing into BLUE breath bag Specimen Collection Workflow (for BreathID Lab and BreathID Smart)
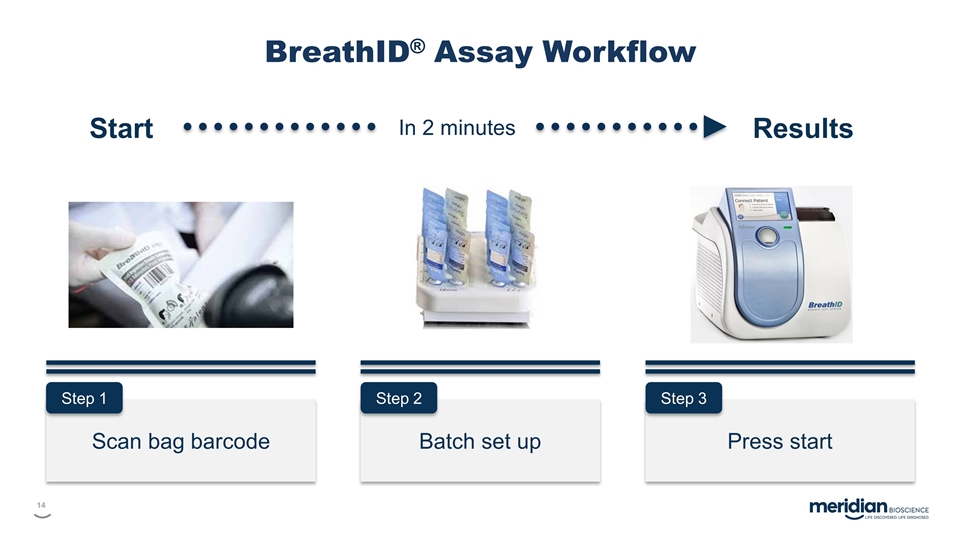
Scan bag barcode Batch set up Press start Step 1 Step 2 Step 3 Start Results In 2 minutes BreathID® Assay Workflow
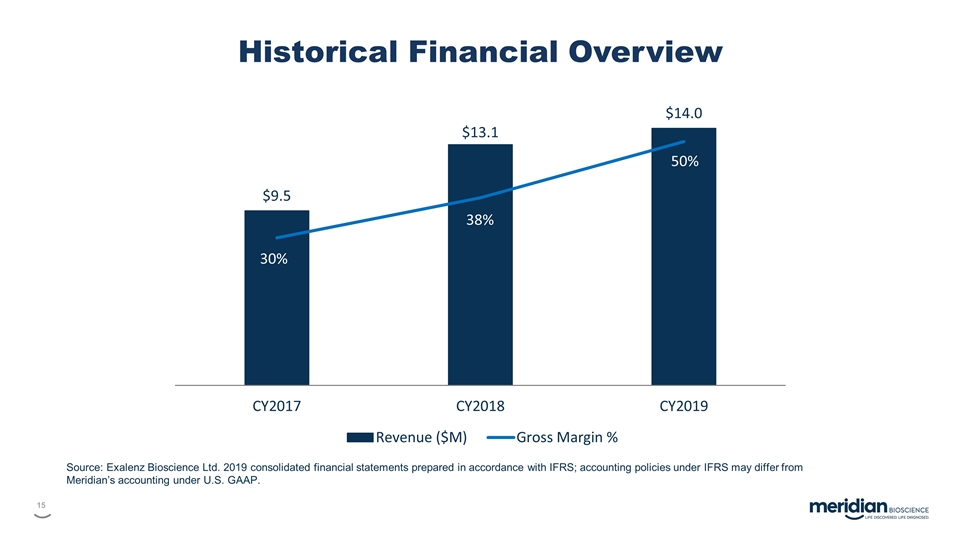
Historical Financial Overview Source: Exalenz Bioscience Ltd. 2019 consolidated financial statements prepared in accordance with IFRS; accounting policies under IFRS may differ from Meridian’s accounting under U.S. GAAP.

Curian® Analyzer
Fluorescent analyzer Improved performance with no subjectivity Intuitive user interface Intuitive, fast, and easy-to-use improves productivity Simple sample prep Clean, comfortable handling of samples Easy, standardized workflow Implement & train only once The Curian® Advantage

C. difficile GDH/Toxin Campy EHEC Future Assay Roadmap Current Menu HpSA® Launched May 1st, 2020 Curian® Present and Future

Discharge sample & vortex Add sample to device card Place card into analyzer and start Step 1 Step 2 Step 3 Curian® Assay Workflow Standardized Simplified Workflow Curian Video

Contact: mbi@meridianbioscience.com



















