SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
FILED BY THE REGISTRANTo
FILED BY A PARTY OTHER THAN THE REGISTRANTþ
Check the appropriate box:
o Preliminary Proxy Statement
o Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
o Definitive Proxy Statement
þ Definitive Additional Materials
o Soliciting Material under Rule 14a-12
INSITE VISION INCORPORATED
(Name of the Registrant as Specified In Its Charter)
PINTO TECHNOLOGY VENTURES, L.P.
PINTO TECHNOLOGY VENTURES GP, L.P.
PINTO TV GP COMPANY LLC
EVAN S. MELROSE, M.D.
MATTHEW S. CRAWFORD
RICK D. ANDERSON
TIMOTHY P. LYNCH
TIMOTHY McINERNEY
ROBERT O’HOLLA
ANTHONY J. YOST
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
PAYMENT OF FILING FEE (CHECK THE APPROPRIATE BOX):
| þ | | No fee required. |
| |
| o | | Fee computed on table below per Exchange Act Rules 14a-6(i)(4) and 0-11. |
| | 1. | | Title of each class of securities to which transaction applies: |
| |
| | | | |
| |
| | 2. | | Aggregate number of securities to which transaction applies: |
| |
| | | | |
| |
| | 3. | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): |
| |
| | | | |
| |
| | 4. | | Proposed maximum aggregate value of transaction: |
| |
| | | | |
| |
| | 5. | | Total fee paid: |
| |
| | | | |
| o | | Fee paid previously with preliminary materials. |
| |
| o | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | 1. | | Amount Previously Paid: |
| |
| | | | |
| |
| | 2. | | Form, Schedule or Registration Statement No.: |
| |
| | | | |
| |
| | 3. | | Filing Party: |
| |
| | | | |
| |
| | 4. | | Date Filed: |
| |
| | | | |
PLEASE VOTE FOR OUR NOMINEES BY SIGNING, DATING AND RETURNING THE ENCLOSED
GOLD PROXY CARD, OR VOTING BY TELEPHONE OR OVER THE INTERNET AS DESCRIBED
ON THE ENCLOSED GOLD PROXY CARD, TODAY!
September 8, 2008
Dear Fellow Stockholder:
| | | |
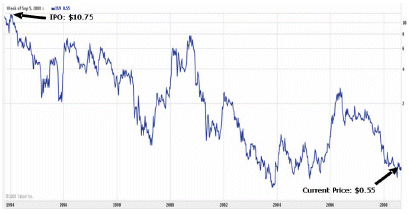
| Ask yourself: Are you better off now ($0.55) than you were... | | one year ago ($1.09)? |
| | | 2 years ago ($1.81)? |
| | | 15 years ago ($10.75)? |
Have you madeANYreturn on your investment? Unfortunately, the answer for most of us is...NO!
* * * * * * * *
What have InSite Vision stockholders received with the current Board and CEO?
| • | | A SERIES OF UNFULFILLED PROMISES AND MISSED MILESTONES: |
During the last 15 years, in spite of its valuable technology and intellectual property, InSite Vision has repeatedly failed to achieve publicly disclosed milestones. Here is a very brief representative sampling of these failures:
| | • | | On March 31, 2003, ISV stated in an SEC filing that Phase III clinical trials for AzaSite would begin within the following three months.The trials eventually began in the third quarter of 2004. |
| |
| | • | | In May 2004, ISV stated that it would submit a New Drug Application to the FDA in the second half of 2005.The company finally submitted the application in June 2006. |
| |
| | • | | In numerous public communications, ISV predicted that it would commercialization Azasite first in 20051, then in 20062, and then in early 2007.3The product was finally commercialized in August 2007. |
For a much longer list of examples of ISV’s inability to accurately set and meet public expectations, please see “ISV’S FAILURE TO ACCURATELY PREDICT AND DELIVER” attached asAppendix A to this letter.
| • | | FISCAL IRRESPONSIBILITY: |
The repeated delays and missed milestones described above have cost ISV and its stockholders millions of dollars. Since inception, ISV has raised approximately $150 million in equity, and we, as stockholders, have little to show for it.
| | • | | The current market capitalization of ISV is approximately $50 million. |
| |
| | • | | Because of the low stock price, ISV has not been able to raise further equity capital at a reasonable stock price. As a result, ISV was recently forced to resort to borrowing $60 million at an annual interest rate of 16%. |
| |
| | • | | Incredibly, the current Board has determined that, because of “the Company’s current needs and financial position,” the Board does not need an audit committee financial expert.4 |
| • | | DEPARTURE OF INSTITUTIONAL STOCKHOLDERS AND LACK OF ANALYST COVERAGE: |
We believe that the chronic underperformance by the CEO and Board over an extended period of time have led to the sale of InSite common stock by many of ISV’s largest institutional stockholders. In fact, in the past year, public records indicate that major institutional stockholders are selling substantial amounts of InSite Vision common stock.5 For example:
| | | | | |
• | | Wellington Asset Management | | Soldall 5,705,900 shares |
• | | Visium Capital Management | | Sold 2,163,442 shares |
• | | Mariner Investment Group | | Soldall 1,781,644 |
• | | First New York | | Soldall 450,800 shares |
• | | Balyasny Asset Management | | Sold 197,100 shares |
We believe that this departure of a meaningful institutional investor base reflects a vote of “no confidence” for the current CEO and Board of Directors. Additionally, the current management team has been unable to attract and maintain any meaningful analyst coverage.
| • | | POOR STOCK PERFORMANCE: |
All of the above have contributed to the dismal performance of the stock price. Since July 2006, the trading price of InSite Vision’s common stock has declined nearly 80%, while broader market indices have been relatively flat and the AMEX Biotechnology Index is up over 20%:
Not only has the stock performed poorly since mid-2006 as reflected in the table above, but since 1993, the stock price has PLUMMETED 95% from the IPO price of $10.75!
| • | | IRRESPONSIBLE COMPENSATION PRACTICES: |
Apparently with blatant disregard for the poor performance of ISV’s common stock, the current “independent” directors, who collectively own only 0.17% of ISV’s outstanding common stock, has generously rewarded the CEO with cash and equity compensation. Despite explicit authorization to engage an outside consultant for purposes of assisting it in determining executive compensation, ISV’s compensation committee refused to do so and instead the Board chose to:6
| | • | | increase the CEO’s total compensation by 91% in 2007; and |
| |
| | • | | provide the CEO with a “golden parachute” worth approximately $2.0 million in 2007. |
At PTV’s request, Compensia, a management consulting firm that provides executive compensation advisory services, reviewed market data for CEO compensation to evaluate the CEO’s 2007 compensation relative to the market. Compensia’s research indicates that, for 2007, the CEO’s total annual cash compensation was over 2 times the median total annual cash compensation of CEOs of life science companies with fewer than 150 employees, and his annual equity compensation approximated 2 times the median annual equity compensation of CEOs of life science companies with fewer than 150 employees. We believe that this compensation is particularly egregious in light of ISV’s poor stock performance, with the trading price of ISV’s stock plummeting nearly 80% since May 2006!
| • | | LACK OF STRATEGIC INITIATIVE AND LEADERSHIP: |
In recognition of the multitude of challenges facing ISV, representatives of PTV have engaged in multiple discussions with the current CEO and Board over the past year. In these discussions, we have encouraged the Board to take steps to:
| | • | | develop and communicate a long-term operation and development plan for ISV; and |
| |
| | • | | transition Kumar into a chief scientist role and recruit an experienced, ophthalmic-focused CEO more appropriate for a public company, in light of ISV’s emergence as a commercial stage enterprise. |
However, the Board failed to take any action. The repeated failures of the current Board and CEO have led us to conclude that they are either unwilling or incapable of making strategic decisions in the long-term interests of ISV’s stockholders. This recognition led PTV to ultimately conclude that it must embark on this proxy contest as a last resort.
| • | | ENTRENCHMENT OF MANAGEMENT AND STATUS QUO: |
In response to PTV’s proxy contest, the InSite Vision Board has offered to move the current CEO into an Executive Chairman position, while allowing him to maintain all major responsibilities. While, it is interesting that the Board refused to act until they were forced to do so, we believe that this “succession” plan:
| | • | | will leave Kumar, with his history of underperformance and lack of capabilities, in control of the major aspects of InSite Vision’s franchise; and |
| |
| | • | | as a result, will not attract the best caliber CEO that ISV’s stockholders deserve, since qualified CEOs will demand to have operational control. |
Instead, the Board’s “plan” virtually insures that whomever Kumar hand-picks to be his successor will be a “yes” man and follow Kumar’s lead. Has the current Board and CEO’s track record over the past 15 years given you confidence that you can trust them to make the necessary changes to lead this commercial-stage company into the future? Or does it mean more of the same? The current Board and management team have had enough chances. As stockholders, we deserve better!
* * * * * * * *
IT IS TIME FOR A NEW CEO AND A NEW BOARD TO IMPLEMENT CHANGE
We believe InSite has the potential, with the right Board and management team, to generate significant value for all InSite stockholders. We need new directors, independent of management, to represent stockholder interests and lead this multi-product, publicly traded, commercial stage company.
| • | | OUR HIGHLY QUALIFIED NOMINEES: |
We have nominated Rick D. Anderson, Timothy P. Lynch, Timothy McInerney, Evan S. Melrose, M.D., Robert O’Holla and Anthony J. Yost as our six highly qualified candidates for election to the InSite Board of Directors. Collectively, PTV and our nominees own 8.8% of InSite Vision’s outstanding common stock. We selected each of our nominees—only two of whom are affiliated with PTV—based on an essential skill set we believe will benefit InSite Vision.
Our highly qualified nominees:
| | • | | collectively have decades of successful experience in ophthalmology, biotechnology and pharmaceuticals. |
| |
| | • | | include individuals with experience ranging from small start up companies to experience as the Company Group Chairman at Johnson & Johnson, a Fortune 50 corporation. |
| |
| | • | | have worldwide regulatory, sales and distribution experience. We believe that the nominees provide a complementary skill set to help transition InSite Vision into a successful commercial stage, specialty pharmaceutical company. |
Our six nominees are committed to creating value forall stockholders. They plan to establish new executive leadership, upgrade corporate governance practices, improve operational performance, develop a fully-integrated strategic plan and improve communication and transparency with stockholders. Additionally, if our nominees are elected, we intend to recruit two additional qualified directors independent of PTV Sciences.
If elected, our nominees intend to create a new vision and strategy that will result in a repositioning of InSite Vision and that we believe will increase stockholder value. The key elements of our plan are set forth below:
| | • | | Develop and communicate a strategic plan to internal and external stakeholders within the first 100 days of new management that will provide a clear path forward for growth and a road map for the future based on InSite Vision’s capabilities and technology. |
| |
| | • | | Recruit a new CEO with extensive commercial and business development experience in ophthalmology. |
| |
| | • | | Conduct an external review of in-licensing opportunities to create additional value. |
| |
| | • | | Review InSite’s product portfolio to both reprioritize and reposition the pipeline to maximize value. |
| |
| | • | | Review management responsibilities and budgetary planning. |
| |
| | • | | Meet with current and perspective partners to discuss accelerated growth strategies for global sales expansion. |
| |
| | • | | Revise selection process for international distribution strategies and partnerships for AzaSite and AzaSite Plus. |
| |
| | • | | Improve communication and transparency by and among the CEO, Board of Directors and stockholders. |
| |
| | • | | Hold an open conference call or town hall meeting to allow an opportunity for stockholders to ask questions of the executive management team and Board of Directors. |
IT IS TIME TO DEMAND WHAT YOU DESERVE!
| | • | | A BOARD THAT IS INDEPENDENT OF MANAGEMENT AND REPRESENTS STOCKHOLDERS |
| |
| | • | | A PROVEN CEO CAPABLE OF LEADING A MULTI-PRODUCT, PUBLICLY TRADED, COMMERCIAL STAGE COMPANY |
| |
| | • | | HONEST COMMUNICATIONS — A REAL PLAN FOR GROWTH FROM PEOPLE YOU CAN BELIEVE |
* * * * * * * *
PLEASE VOTE FOR OUR NOMINEES BY SIGNING, DATING AND RETURNING THE ENCLOSED GOLD PROXY CARD, OR VOTING BY TELEPHONE OR OVER THE INTERNET AS DESCRIBED ON THE ENCLOSED GOLD PROXY CARD, TODAY!
Every vote is important. Regardless of the number of shares you own, we urge you to support our nominees. Every vote counts! If you have any questions or require assistance voting your proxy, please contact MacKenzie Partners, Inc. at (800) 322-2885 or by email at insiteproxy@mackenziepartners.com.
We thank you for your continued support.
Very truly yours,
On Behalf of Pinto Technology Ventures, L.P.
| | | |
| | |  |
| | | |
| By: | | Evan S. Melrose, M.D. |
| Title: | | Managing Director, PTV Sciences |
IMPORTANT
Your vote is important, no matter how many or how few shares you own. To vote your shares, please sign, date and return the GOLD proxy card. You may also vote by phone or on the Internet by following the instructions on the GOLD proxy card. Do not return any WHITE proxy card that you receive from management. If you have any questions or need assistance voting your shares, please contact MacKenzie Partners, Inc., which is assisting us in this matter, toll-free at (800) 322-2885 or insiteproxy@mackenziepartners.com.
Stockholders can vote by mail, telephone or internet by following the instructions
on the enclosed GOLD proxy card.
If you have questions or need assistance voting your shares please contact:
105 Madison Avenue New York, New York 10016 insiteproxy@mackenziepartners.com Call Collect: (212) 929-5500 orToll-Free (800) 322-2885
Notice
In connection with InSite Vision Incorporated’s upcoming 2008 annual meeting of stockholders (the “Annual Meeting”), Pinto Technology Ventures, L.P. (“PTV”), has filed with the Securities and Exchange Commission (the “SEC”) a proxy statement (the “PTV Proxy Statement”) and related materials for the solicitation of proxies from InSite stockholders for use at the Annual Meeting. PTV, its director nominees and certain of PTV’s affiliates are or may be deemed to be participants in the solicitation of proxies with respect to the Annual Meeting. Information regarding PTV, its nominees and such participants is contained in the Schedule 14A and related materials filed by PTV with the SEC. InSite stockholders should read the PTV Proxy Statement and related materials filed with the SEC with respect to the Annual Meeting because they contain important information. These materials are available without charge at the SEC’s website at www.sec.gov.
| | |
| 1 | | CCBN Healthcare Conference December 10, 2002. |
| |
| 2 | | ISV press release, dated May 9, 2005. |
| |
| 3 | | ISV press release, dated November 8, 2006. |
| |
| 4 | | ISV’s Proxy Statement for the 2008 Annual Meeting. |
| |
| 5 | | Capital IQ database. |
| |
| 6 | | ISV’s Proxy Statement for the 2008 Annual Meeting. |
Appendix A
ISV’S FAILURE TO ACCURATELY PREDICT AND DELIVER
During the last decade, the Company has failed to deliver on its public commitments to investors regarding drug development and commercialization timelines. Below is a sampling of these missed commitments.
ISV-401 (AzaSite)
| | | | | | | |
| Event | | Public Statement | | Source | | Result |
| |
Clinical Trials | | “...phase 3 clinical trials are anticipated to begin in the second quarter of 2003.” | | ISV 10-K filed March 31, 2003. | | |
| | | | | | | |
| | | “...phase 3 clinical trials are anticipated to begin in the second quarter of 2004.” | | ISV 10-K filed March 30, 2004. | | Phase 3 trials eventually began in third quarter 2004. |
| | | | | | | |
| | | “Our intent is to complete both ISV-401 phase 3 trials by the middle of 2005...” | | ISV press release, dated May 14, 2004. | | Phase 3 trials completed in January 2006. |
| | | | | | | |
NDA | | “Our intent is to ... file an NDA in the second half of [2005].” | | ISV press release, dated May 14, 2004. | | |
| | | | | | | |
| | | “This puts us on track to file a New Drug Application (NDA) for AzaSite with the U. S. Food and Drug Administration (FDA) in early 2006.” —Kumar Chandrasekaran, Ph.D., chief executive officer of InSite Vision. | | ISV press release, dated August 15, 2005. | | NDA finally filed in June 2006. |
| | | | | | | |
Commercialization | | “ISV-401, which is a broad spectrum antibiotic we anticipate entering the market in2005.” | | ISV-CCBN Healthcare Conference December 10, 2002. | | |
| | | | | | | |
| | | “The company currently expects to commercially launch AzaSite in the U.S. in2006.” | | ISV press release, dated November 4, 2004. | | |
| | | | | | | |
| | | “The company currently expects to commercially launch AzaSite in the U.S. in2006.” | | ISV press release, dated March 31, 2005. | | |
| | | | | | | |
| Event | | Public Statement | | Source | | Result |
| |
| | | “The company currently expects to commercially launch AzaSite in the U.S. in2006.” | | ISV press release, dated May 9, 2005. | | |
| | | | | | | |
| | | “Pending approval of its NDA with the FDA, InSite Vision currently expects AzaSite to be commercially launched in the United States in early2007.” | | ISV press release, dated November 8, 2006. | | AzaSite finally commercially launched in August 2007. |
ISV-205
| | | | | | | |
| Event | | Public Statement | | Source | | Result |
| |
Clinical Trials | | “The Company expects to complete a Phase II trial of ISV-205 in1998.” | | ISV 10-Q, filed May 14, 1998. | | Phase II trials completed in the second quarter of 1999. |
| | | | | | | |
Commercialization | | “The ISV-205 glaucoma therapeutic partnership with Pharmacia remains strong.” “Plans are underway for the next phase of the program...” | | ISV 8-K filed February 6, 2001. | | In May 10, 2001 press release, ISV disclosed that Pharmacia had returned global development and commercialization rights to ISV-205 to ISV. |
| | | | | | | |
| | | “We strongly believe that the ISV-205 product is safe and efficacious.” “We are committed to move aggressively on securing a new corporate partner to fund later-stage development and commercialization of this important therapeutic.” | | ISV press release, dated May 10, 2001. | | Clinical trials were abandoned and product development has remained on hold since 2003. |
| | | | | | | |
| | | “We plan to move forward with a pivotal Phase 3 clinical trial program...” | | CEO interview in 4th quarter of 2004 (posted on ISV’s web site). | | Subsequent partnerships were never established, and this product candidate was never commercialized. |
ISV-016 (AzaSite Otic)
| | | | | | | |
| Event | | Public Statement | | Source | | Result |
| |
Clinical Trials | | “This product candidate is currently in preclinical development and we anticipate pursuing it more actively if and when personnel and financial resources become available.” | | ISV 10-K, filed March 15, 2007. | | |
| | | | | | | |
| | | “In 2008, we plan to file an IND and pursue our AzaSite Otic program.”
“Our current plan is to fi[le] an IND in 2008, and start clinical trials soon thereafter.” | | ISV Q1 2008 earnings call, transcript published May 8, 2008. | | In August 2008, ISV disclosed that it had discontinued development of AzaSite Otic. |
ISV-900 (OcuGene)
| | | | | | | |
| Event | | Public Statement | | Source | | Result |
| |
Commercialization | | “[A]s of November 11, 1999, [ISV] signed an agreement to license the ISV-900 technology for diagnostic, prognostic and therapeutic applications to Pharmacia & Upjohn, Inc (“P&U”).” | | ISV press release,
dated November 11, 1999. | | |
| | | | | | | |
| | | The Pharmacia licensing agreement has been terminated. “InSite Vision will accelerate and manage all future development and marketing plans for the product.” “InSite Vision plans to bring ISV-900 to market in 2001 and currently has a comprehensive marketing plan under development.” | | ISV press release,
dated December 14, 2000. | | |
| | | | | | | |
| | | “During the first quarter of 2003, InSite Vision continued its activities to introduce the OcuGene glaucoma genetic test to the U.S. ophthalmic and optometric communities.” | | ISV press release, dated May 14, 2003. | | |
| | | | | | | |
| | | “In December 2002, we entered into an agreement with Società Industria Farmaceutica Italiana (SIFI) that grants them the exclusive right to manufacture/perform, distribute and market OcuGene in Italy for eight years. SIFI introduced the OcuGene test at two Italian ophthalmic meetings in late 2003,and is currently evaluating the market opportunity and feasibility to support a product launch in the second half of 2004.” | | ISV 10-K/A, filed May 7, 2004. | | |
| | | | | | | |
| Event | | Public Statement | | Source | | Result |
| |
| | | “In December 2002, we entered into an agreement with Società Industria Farmaceutica Italiana (SIFI) that grants them the exclusive right to manufacture/perform, distribute and market OcuGene in Italy for eight years. SIFI introduced the OcuGene test at two Italian ophthalmic meetings in late 2003,and is currently evaluating the market opportunity and feasability to support a product launch.” | | ISV 10-K, filed March 31, 2005. | | |
| | | | | | | |
| | | “In December 2002, we entered into an agreement with Società Industria Farmaceutica Italiana, or SIFI, that grants SIFI the exclusive right to manufacture/perform, distribute and market OcuGene in Italy for eight years. SIFI introduced the OcuGene test at two Italian ophthalmic meetings in late 2003,and is currently conducting additional clinical studies to evaluate the technology in the Italian population.” | | ISV 10-Ks, filed March 31, 2006 and March 15, 2007. | | No mention of Italian commercialization in the Form 10-K filed March 17, 2008. |
| | | | | | | |
| | | “Our marketing and sales efforts related to OcuGene glaucoma genetic test have been significantly curtailed.” | | ISV S-3, filed April 11, 2007. | | Based on SEC filings, the product has made no meaningful contribution to revenues. |