UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 0-22332
INSITE VISION INCORPORATED
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 94-3015807 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
| 965 Atlantic Avenue, Alameda CA | | 94501 |
| (Address of principal executive offices) | | (Zip Code) |
(510)-865-8800
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
| | |
Title of each class | | Name of each exchange on which registered |
| Common Stock, $0.01 par value per share | | OTC Bulletin Board |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| | |
| Large accelerated filer ¨ | | Accelerated filer ¨ |
| Non-accelerated filer ¨ | | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of registrant’s Common Stock, $0.01 par value, held by non-affiliates of the Registrant as of June 30, 2011 was approximately $46,454,671 (based upon the closing sale price of the Common Stock on the last business day of the registrant’s most recently completed second fiscal quarter). Shares of Common Stock held by each officer and director and by each person who owns 5% or more of the Common Stock have been excluded from such calculation as such persons may be deemed affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
Number of shares of Common Stock, $0.01 par value, outstanding as of February 27, 2012: 131,951,033.
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2011
TABLE OF CONTENTS
Except for the historical information contained herein, the discussion in this Annual Report on Form 10-K contains certain forward-looking statements that involve risks and uncertainties, such as statements of our plans, beliefs, objectives, expectations and intentions. Our actual results could differ materially from those discussed herein. Factors that could cause or contribute to such differences include those discussed below in “Risk Factors,” as well as those discussed elsewhere herein. The cautionary statements made in this document should be read as applicable to all related forward-looking statements wherever they appear in this document. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. References in this Annual Report on Form 10-K to the “Company,” “InSite,” “we,” “our” and “us” refer to InSite Vision, Incorporated and its consolidated subsidiaries, unless we state, or the context indicates, otherwise.
PART I
THE COMPANY
We are an ophthalmic product development company advancing ophthalmic pharmaceutical products to address unmet eye care needs. Our current portfolio of products is based on our proprietary DuraSite® sustained drug delivery technology.
Our DuraSite sustained drug delivery technology is a proven synthetic polymer-based formulation designed to extend the residence time of a drug relative to conventional topical therapies. It enables topical delivery of a drug as a solution, gel or suspension and can be customized for delivering a wide variety of drug candidates. We have focused our research and development and commercial support efforts on the following topical products formulated with our DuraSite drug delivery technology. We may also utilize our DuraSite technology platform for the formulation of new ocular product candidates using either non-proprietary drugs or compounds originally developed by others for non-ophthalmic indications.
| | • | | AzaSite® (azithromycin ophthalmic solution) 1% is a DuraSite formulation of azithromycin, a broad spectrum ocular antibiotic approved by the U.S. Food and Drug Administration (FDA) in April 2007 to treat bacterial conjunctivitis (pink eye). It was commercialized in the United States by Inspire Pharmaceuticals, Inc. (Inspire) beginning in August 2007. The key advantages of AzaSite are a significantly reduced dosing regimen leading to better compliance and outcome, with a broad spectrum antibiotic, and a lowered probability of bacterial resistance based on high tissue concentration. On May 16, 2011, Merck & Co. (Merck) acquired Inspire and Inspire became a wholly-owned subsidiary of Merck. Merck is now responsible for commercializing AzaSite in North America. We receive a 25% royalty on net sales of AzaSite in North America. |
| | • | | Besivance® (besifloxacin ophthalmic suspension) 0.6% is a DuraSite formulation of besifloxacin, a broad spectrum ocular antibiotic approved by the FDA in May 2009 to treat bacterial conjunctivitis (pink eye). An advantage of Besivance is a faster rate of resolution of the infection that may reduce the duration of the illness and reduce the chances of infecting others. Besivance was developed by Bausch & Lomb Incorporated (Bausch & Lomb) and launched in the United States in the second half of 2009. In 2011, Besivance was launched internationally in select countries. We receive a middle single-digit royalty on net sales of Besivance globally. |
| | • | | AzaSite PlusTM (ISV-502) is a fixed combination of azithromycin and dexamethasone in DuraSite for the treatment of ocular inflammation and infection (blepharitis and/or blepharoconjunctivitis) for which there is no FDA approved indicated treatment. We completed a Phase 3 trial in November 2008 and AzaSite Plus was very well tolerated. Although efficacious, the trial did not achieve its primary clinical endpoint as defined by the previous protocol. We discussed the results of this trial with the FDA and |
1
| | determined a new development plan for this product candidate. In May 2011, we reached an agreement with the FDA on a Special Protocol Assessment (SPA) for the design of a Phase 3 clinical trial of AzaSite Plus in patients with blepharitis. A SPA is a written agreement with the FDA that the study design and planned analysis of the sponsor’s Phase 3 clinical trial adequately addresses the objectives necessary to support a regulatory submission. We initiated a new Phase 3 clinical trial for this product candidate in the fourth quarter of 2011. |
| | • | | DexaSiteTM (ISV-305) is a DuraSite formulation of dexamethasone in development for the treatment of ocular inflammation. We have met with the FDA to discuss the development pathway for this product candidate. DexaSite is included in the Phase 3 clinical trial SPA for AzaSite Plus. We initiated a Phase 3 clinical trial for this product candidate in the fourth quarter of 2011. |
| | • | | BromSiteTM (ISV-303) is a DuraSite formulation of bromfenac in development for the treatment of post-operative inflammation and eye pain. We initiated a Phase 1/2 clinical trial for this product candidate in August 2010 and completed patient enrollment in December 2010. In the first quarter of 2011, we received positive top-line results from this study, which demonstrated the efficacy and safety of BromSite. In the third quarter of 2011, we completed an additional Phase 2 clinical trial to investigate the pharmacokinetics (PK) of BromSite in humans. We received positive top-line results that showed that the mean concentration of bromfenac in the aqueous humor of patients using BromSite was more than double compared to the currently available bromfenac eye product. We have discussed the design of the Phase 3 clinical trial with the FDA. We plan to start a Phase 3 clinical trial for this product candidate in mid-2012. |
| | • | | ISV-101 is a DuraSite formulation with a low concentration of bromfenac for the treatment of dry eye disease. We filed an Investigational New Drug Application (IND) with the FDA for this product candidate in the first quarter of 2011. We anticipate a Phase 1/2 clinical trial for this product candidate. |
Business Strategy.
Our business strategy consists of the following:
1. Develop our pipeline of ocular product candidates. We seek to identify new product candidates from proven drugs that can be improved by formulation in DuraSite, which substantially reduces the clinical risk in these product candidates. We plan to conduct preclinical and clinical testing of our portfolio product candidates.
2. Monetize our product candidates. At the appropriate time, we seek to partner with larger pharmaceutical companies to possibly complete the clinical development of, and to manufacture and market, these products. Partnering agreements generally include upfront and milestone payments, as well as on-going royalty payments upon commercialization, payable to us.
Corporate Information.Our principal executive offices are located at 965 Atlantic Avenue, Alameda, California 94501. Our telephone number is (510) 865-8800. We were incorporated in 1986 as a California corporation and reincorporated in Delaware in 1987. We make our periodic and current reports available, free of charge, through our website (http:///www.insitevision.com) under “Investor Relations—SEC Filings” as soon as reasonably practicable after such material is electronically filed with the Securities and Exchange Commission (SEC). Additionally, copies of materials filed by us with the SEC may be accessed at the SEC’s Public Reference Room at 100 F Street NE, Washington D.C. or at the SEC’s website athttp://www.sec.gov. For information about the SEC’s Public Reference Room, the public may contact 1-800-SEC-0330.
2
Ophthalmic Anti-Infective Market
Today, eye infections are routinely treated with topical antibiotics as well as antibiotic/corticosteroid fixed combination products. The ocular anti-infective market represented global sales of approximately $2.0 billion in 2011 and comprises two separate product segments:
| | • | | Ocular antibiotic products; and |
| | • | | Ocular antibiotic/corticosteroid fixed combination products |
We are developing topical products to treat eye and eye-lid infections. Some of these infections are either under-treated or do not have an FDA-approved product indication. These infections can be both acute and chronic. Our goal is to provide highly effective, safe and differentiated therapeutics for the treatment of acute and chronic ocular infection and inflammation. There are two general areas where our topical ocular anti-infective products have been utilized by eye-care physicians, or where we believe our product candidates may be well suited to improving patient care:
| | • | | Acute bacterial conjunctivitis (pink eye) is a common condition experienced by most people at some point in their lives, but is especially prevalent among children. The conjunctiva is the transparent lining on the inside of the eyelids. In bacterial conjunctivitis, bacteria infects this lining and the white part of the eye may look pink from the inflammation. As it is an extremely contagious condition, immediate treatment is recommended. Our developed ocular antibiotics, AzaSite and Besivance, are targeted at treating this disease with significantly lower dosing than competing products. |
| | • | | Blepharitis(also known as lid margin disease) is an inflammation of the eyelids, particularly the eyelid margins where the eyelashes grow. It is a common disorder, particularly among the elderly, that may be caused by bacterial growth, viral infection, allergies, environmental conditions or systemic disease. An eyelid with blepharitis may become itchy and appear red and swollen with scaly, greasy debris along the lid margin. Blepharitis can be a chronic condition with periodic acute episodes that is difficult to treat and it is believed to be both under-diagnosed and under-treated. There are no approved pharmaceutical products for the treatment of blepharitis. Patients are typically advised to use lid scrubs, hot compresses, lid massage, antibiotics, corticosteroids and fixed-combination products. We are advancing two novel ophthalmic products to alleviate the signs and symptoms of blepharitis; AzaSite Plus, a topical anti-bacterial and anti-inflammatory product; and DexaSite, a corticosteroid anti-inflammatory agent. |
| | • | | Blepharoconjunctivitis occurs when conjunctivitis accompanies blepharitis, as it frequently does. A unilateral or bilateral conjunctivitis that persists for four or more weeks is considered chronic. There is a considerable overlap of symptoms of all types of blepharitis and it frequently leads to associated ocular surface inflammation, including conjunctivitis, function tear deficiency and keratitis (an inflammation of the cornea which can develop into corneal ulcers). Blepharoconjunctivitis is a disease with no approved drug therapy indicated for the relief of its chronic symptoms. Typical treatment includes eye hygiene using lid scrubs, topical and/or systemic antibiotics and topical corticosteroids. |
3
Ocular Inflammation and Pain Market
We are developing novel ophthalmic therapeutics for the treatment of conditions associated with ocular inflammation. These efforts are initially focused on two indications, post-operative inflammation and pain, and dry eye disease.
| | • | | Post-Operative Ocular Inflammation and Painis typically treated using non-steroidal anti-inflammatories (NSAIDs). An estimated 60 percent of ophthalmic surgeons deploy topical NSAIDs before and after cataract and other procedures to address patient pain and prevent complications, such as cystoid macular edema. Cystoid macular edema (CME) is a serious post-surgical complication that occurs when inflammation and swelling develop in the center of the retina. Although rare, it is the most common cause of decreased vision following cataract surgery. Cataract surgery is one of the most frequent surgical procedures conducted in the United States, with approximately 3.1 million cataract surgeries performed annually. The ophthalmic NSAID market is estimated to be $320 million, with more than 2.6 million prescriptions written annually. As the population in the U.S. ages, the market for ocular NSAIDs that are efficacious and easy for patients to use is expected to grow 7.1 percent between 2007 and 2014. Our BromSite product candidate is intended to relieve post-operative ocular inflammation and pain while providing superior tissue penetration of drug to the eye to reduce the incidences of CME with convenient twice-daily dosing. |
| | • | | Dry Eye Disease occurs when the ocular surface and/or tear film is compromised. While causes of dry eye may vary, it is frequently associated with inflammation of the surface of the eye, the lacrimal gland, or the conjunctiva. A potentially chronic condition that can occur at any age, dry eye disease is most prevalent among the elderly. Symptoms of dry eye disease include a scratchy feeling as if something is in the eye, stinging or burning of the eye; episodes of excess tearing that follow periods of very dry sensation; stringy discharge from the eye and pain or redness. According to the National Eye Institute, dry eye disease is estimated to affect five million people age 50 and older in the U.S. alone, with tens of millions more experiencing less severe symptoms. We have developed a low-dose topical NSAID, known as ISV-101, intended for the treatment of dry eye disease. |
4
Products and Product Candidates
The following table summarizes the current status of our principal products and product candidates in our development pipeline. A more detailed description of each product and product candidate follows the table.
Principal DuraSite Products and Product Candidates
Active Programs
| | | | | | |
Product | | Indications | | Anticipated Benefits | | Status |
AzaSite | | Bacterial conjunctivitis (pink eye) | | Broad-spectrum macrolide antibiotic with reduced dosing frequency | | *Approved and launched in U.S. *Approved in Canada |
| | | | |
Besivance | | Bacterial conjunctivitis (pink eye) | | Broad-spectrum fluoroquinolone antibiotic with reduced dosing frequency | | *Approved and launched in the U.S. and select countries internationally |
| | | | |
AzaSite Plus | | Blepharitis | | Broad-spectrum antibiotic combined with a potent corticosteroid with reduced dosing frequency to treat both inflammation and infection | | Phase 3 trial completed in blepharoconjunctivitis SPA-approved Phase 3 trial underway in blepharitis |
| | | | |
DexaSite | | Ocular inflammation | | A potent corticosteroid with reduced dosing frequency to treat inflammation | | Phase 3 trial completed in blepharoconjunctivitis SPA-approved Phase 3 trial underway in blepharitis |
| | | | |
BromSite | | Post-operative inflammation and eye pain | | A non-steriodal anti-inflammatory to treat pain and inflammation | | Phase 2 clinical trials completed |
| | | | |
ISV-101 | | Dry eye disease | | A non-steriodal anti-inflammatory to treat dry eye disease | | Filed an IND with the FDA |
The DuraSite Product Family of Topical Anti-infectives and Product Candidates
AzaSite: Launched commercially in the United States by Inspire (acquired by Merck) in August 2007 for Bacterial Conjunctivitis (pink eye)
We developed a topical formulation of the antibiotic azithromycin to treat bacterial conjunctivitis and other infections of the eye. Bacterial conjunctivitis is a common ocular surface disease characterized by inflammation of the delicate skin and mucosa on the inside of the eyelids. These bacterial infections are contagious and are generally accompanied by irritation, itching, foreign body sensation, watering, mucus discharge and redness. The bacterial form of the disease is generally more common in children than adults.
Azithromycin has a broad spectrum of antibiotic activity and is widely used to treat respiratory and other infections in its oral and parenteral forms. AzaSite is an eye drop of 1% azithromycin formulated to deliver sufficient tissue concentrations over a seven-day dosing period using our proprietary DuraSite technology. The
5
eye drop is designed to enable superior bactericidal activity against common ocular pathogens and even difficult bacteria such as pseudomonas. We believe the key advantages of AzaSite include its once-a-day dosing after the first two days of twice-a-day dosing and the high and persistent levels of azithromycin achieved in the tissues of the eye. Clinical studies have shown that AzaSite is well tolerated and efficacious. AzaSite was approved by the FDA in April 2007. In August 2007, Inspire Pharmaceuticals commercially launched AzaSite in the United States pursuant to its license from InSite. On May 16, 2011, Merck acquired Inspire and Inspire became a wholly-owned subsidiary of Merck. AzaSite is currently commercialized by Merck in the United States.
Besivance: Launched commercially in the United States by Bausch & Lomb in second half of 2009 for Bacterial Conjunctivitis (pink eye) and now available in select countries internationally
Besivance (besifloxacin ophthalmic suspension 0.6%) is indicated for the treatment of bacterial conjunctivitis in patients one year or older and is marketed by Bausch & Lomb.
Besivance is the first fluoroquinolone specifically developed for ophthalmic use and is the first and only ophthalmic fluoroquinolone with no previous systemic use. It offers broad-spectrum antibacterial activity, including activity against the strains that are the most common causes of bacterial conjunctivitis.
The FDA approval of Besivance was based on a series of eight clinical trials. These studies were designed to test the efficacy, safety, tolerability, pharmacokinetics and pharmacodynamics with the topical antibacterial. Its efficacy was evaluated in three multi-center, randomized, double-masked trials involving nearly 2,400 patients with a clinical diagnosis of bacterial conjunctivitis. In clinical trials, investigators found that Besivance treatment resulted in a greater proportion of patients experiencing clinical resolution and microbial eradication when compared to its vehicle.
Besivance was approved by the FDA in May 2009 following a unanimous recommendation by an FDA Advisory Committee in December 2008. The product was launched commercially in the second half of 2009 in the United States. In 2011, Besivance was launched internationally in select countries.
AzaSite Plus: Phase 3 Clinical Trials for Blepharitis/Blepharoconjunctivitis
Expansion of our AzaSite product into a larger franchise includes a fixed combination of azithromycin with dexamethasone for the treatment of blepharitis/blepharoconjunctivitis, an infection of the eyelid and the conjunctiva, as well as other ophthalmic infections. In 2006, we completed our preclinical development of this combination product candidate, filed an IND with the FDA and conducted a Phase 1 clinical trial.
In February 2007, we announced that the preliminary safety data from our Phase 1 clinical trial indicated that AzaSite Plus was well tolerated and no serious adverse events were reported. Treatment-related ocular adverse events were minimal in frequency and equivalent between the treatment and placebo groups. There were no significant differences in intraocular pressure between the AzaSite Plus group and placebo group after 14 days of treatment.
In the fall of 2007, we conducted a pilot study to evaluate endpoints and time points for use in the Phase 3 trial for AzaSite Plus. There were 32 patients with blepharoconjunctivitis who completed the double-masked and randomized trial and received eye drops two times a day for 14 days. The results led to the selection of endpoints for the first Phase 3 trial, which included lid margin redness, lid swelling, conjunctival redness, ocular discharge and lid irritation in at least one eye.
The Phase 3 trial tested a total of 417 patients with blepharoconjunctivitis. The dosing regimen consisted of one drop in the eye and one on the eyelid, two times a day for 14 days. The trial design included three treatment arms with the objective of demonstrating the superiority of AzaSite Plus in treating blepharoconjunctivitis over AzaSite alone or dexamethasone alone.
Results from the Phase 3 trial indicated that AzaSite Plus improved clinical outcomes as compared to treatment with AzaSite alone in the reduction of inflammatory signs and symptoms and dexamethasone alone in
6
bacterial eradication. AzaSite Plus was very well tolerated. However, an evaluation of the data indicated that the trial did not achieve its primary clinical endpoint as the reduction of inflammatory signs and symptoms between AzaSite Plus and dexamethasone was statistically equivalent.
In April 2009, we discussed the results of this trial with the FDA and, based on this meeting, we developed a protocol for the treatment of blepharitis that would seek to demonstrate AzaSite Plus’s ability to delay exacerbation and/or recurrence of acute episodes of blepharitis. This study would serve as a basis for revisions to the pivotal Phase 3 clinical trial protocols.
In 2010, we discussed a development pathway for this product candidate with the FDA. In May 2011, we reached an agreement with the FDA on a SPA for the design of a Phase 3 clinical trial of AzaSite Plus in patients with blepharitis, and we initiated a Phase 3 clinical trial for this product candidate in the fourth quarter of 2011. Under the SPA-approved trial protocol, AzaSite Plus will be evaluated for safety and efficacy against AzaSite for the primary clinical endpoint of resolution of the clinical signs and symptoms of blepharitis and against DexaSite to compare the length of time to recurrence or exacerbation of symptoms following the treatment period.
DexaSite: A second candidate for blepharitis in Phase 3 development
We developed a topical formulation of the corticosteroid dexamethasone to treat eye inflammation caused by infections, injury, surgery or other conditions. In 2007, we completed our preclinical development of this product candidate. This is a second product candidate originating from the IND filed for AzaSite Plus.
In 2008, the data from our Phase 3 clinical trial indicated that DexaSite was efficacious and well tolerated with no serious adverse events. Treatment-related ocular adverse events were minimal in frequency and equivalent between all groups. There were no significant differences in intraocular pressure between the DexaSite group and the other group containing dexamethasone after 14 days of treatment.
In 2010, we discussed a development pathway for this product candidate with the FDA. DexaSite is included in the Phase 3 clinical trial SPA for AzaSite Plus, which allows us to simultaneously evaluate both agents in a single Phase 3 clinical trial for the treatment of blepharitis. We initiated a Phase 3 clinical trial for this product candidate in the fourth quarter of 2011. Per the SPA-approved protocol, the efficacy and safety of DexaSite will be measured against the DuraSite vehicle for the primary clinical endpoint of resolution of clinical signs and symptoms of blepharitis at the end of the dosing period. DexaSite will also be evaluated for the secondary clinical endpoint of clinical improvement of signs and symptoms of blepharitis.
BromSite: Phase 1/2 clinical trial for post-operative inflammation and eye pain completed
We developed a topical formulation of the non-steriodal anti-inflammatory bromfenac to treat post-operative inflammation and eye pain in patients who have undergone cataract surgery. In the first half of 2010, we completed our preclinical development of this product candidate and filed an IND with the FDA. In the second half of 2010, we initiated and completed enrollment for the Phase 1/2 clinical trial for this product candidate. In the first quarter of 2011, we received positive top-line results from this study, which demonstrated the efficacy and safety of BromSite. In the third quarter of 2011, we completed an additional Phase 2 clinical trial to investigate the PK of BromSite in humans. We received positive top-line results that showed that the mean concentration of bromfenac in the aqueous humor of patients using BromSite was more than double compared to the currently available bromfenac eye product. We have discussed the design of the Phase 3 clinical trial with the FDA and we plan to start a Phase 3 clinical trial for this product candidate in mid-2012.
ISV-101: Filed an IND for this product candidate
We developed a topical formulation of the non-steriodal anti-inflammatory bromfenac to treat dry eye disease. In January 2011, we filed an IND with the FDA. We anticipate a Phase 1/2 clinical trial for this product candidate.
7
DuraSite Sustained Delivery Technology
At the core of our AzaSite franchise is our proprietary DuraSite drug delivery technology. Our DuraSite sustained drug delivery technology is a synthetic polymer-based formulation designed to extend the residence time of a drug relative to conventional topical therapies. It enables topical delivery of a drug as a solution, gel, or suspension and can be customized for delivering a wide variety of potential drug candidates.
The combination of DuraSite and proven drug products results in differentiated products that have increased efficacy and improved compliance through a reduced dosing frequency that yields better outcomes, lowers the development risk by using the proven DuraSite technology with a proven drug product and lowers development costs. In addition to its formulation with azithromycin in our AzaSite family of products, our DuraSite technology may be used in the formulation of new ocular product candidates using either non-proprietary drugs or compounds developed by others for non-ophthalmic indications.
Physical Properties.DuraSite is composed of a cross-linked polyacrylic acid polymer, water and salts. We have developed considerable knowledge of how to formulate DuraSite for topical applications that have a range of viscosities and physical forms including gels, suspensions and solutions. The size of the dry polymer particle averages 5 microns. The molecular weight of the polymer exceeds 3x107 Daltons. Upon the addition of water, DuraSite swells to ~100x its original weight.
The polymer entraps water and the active drug product in a bioadhesive matrix. The viscosity of the matrix is controlled by pH. The bioavailability and release characteristics of the drug can be adjusted by altering the chemical environment. The resulting drug delivery system is bioadhesive, demonstrates sustained release and is compatible with both water soluble and water insoluble molecules.
Regulatory Status.The ingredients in the DuraSite sustained release technology are classified by the FDA as Category 1 GRAS (generally regarded as safe). It has been approved by many pharmacopeias, which helps to facilitate worldwide approvals of drugs that contain DuraSite. DuraSite has been used commercially in AquaSite, an ophthalmic product for dry eye syndrome; AzaSite, a topical anti-infective product for the treatment of bacterial conjunctivitis; and Besivance, besifloxacin ophthalmic suspension, 0.6%. Besivance was approved by the FDA in 2009 and commercialized by Bausch & Lomb in 2009 in the United States. In 2011, Besivance was launched internationally in select countries.
Additional Research and Development Opportunities
In addition to products leveraging our DuraSite technology, we will seek to in-license or acquire promising product candidates and technologies from other companies and universities and research institutions and to apply our expertise to create novel differentiated ophthalmic products.
Collaborative, Licensing and Service Agreements
As part of our business strategy, we have entered into, and will continue to pursue additional licensing agreements, corporate collaborations and service contracts. There can be no assurance that we will be able to negotiate acceptable collaborative, licensing or service agreements, or that our existing arrangements will be successful or renewed or that it will not be terminated.
Pfizer and Pfizer Products, Inc. In February 2007, we entered into a worldwide, exclusive, royalty-bearing licensing agreement with Pfizer (Pfizer License) under Pfizer’s patent family titled “Method of Treating Eye Infections with Azithromycin” for ocular anti-infective product candidates known as AzaSite and AzaSite Plus. Under the Pfizer License, we are required to pay Pfizer a low single-digit royalty based on net sales of the licensed products and to use reasonable commercial efforts to seek regulatory approval for and market licensed products. We have the right to grant sublicenses, subject to Pfizer’s prior approval which shall not be unreasonably withheld.
8
Merck. In February 2007, we entered into a license agreement with Inspire. On May 16, 2011, Merck acquired Inspire and Inspire is currently a wholly-owned subsidiary of Merck. Under the license agreement (Merck License), we licensed exclusive development and commercialization rights under our AzaSite patent rights and certain know-how for topical anti-infective products containing azithromycin as the sole active ingredient for human ocular or ophthalmic indications in the United States and Canada and their respective territories. The Merck License also provides for nonexclusive licenses under our DuraSite patent rights, container patent rights, Columbia Laboratories, Inc. polymer technology patent rights and certain know-how in the same field of use as described above. We also granted Merck an exclusive sublicense under the Pfizer patent rights that we have licensed under the Pfizer License discussed above. Merck has the right to grant sublicenses under the terms of the Merck License.
Under the Merck License, Inspire paid us license fees and a milestone payment totaling $32 million through FDA approval in April 2007. Merck also pays us a royalty on net sales. The royalty rate was 20% on net sales in the first two years of commercialization and is now 25%. Merck is obligated to pay us royalties under the Merck License for the longer of (i) eleven years from the launch of the first product (August 13, 2007) or (ii) the period during which a valid claim under a patent licensed from us covers a licensed product. For five years after the first year of commercial sale, Merck is required to pay us the greater of the royalty discussed above or certain tiered minimum royalties. The royalties under the Merck License are subject to certain reductions in the event of patent invalidity, third party licenses, generic competition and uncured material breach.
Under the Merck License, we were responsible for obtaining regulatory approval of AzaSite in the United States, which occurred in April 2007. We subsequently transferred regulatory documentation regarding AzaSite, including the NDA, to Merck. We were also responsible for obtaining regulatory approval of AzaSite in Canada. In March 2009, the Therapeutic Products Directorate of Health Canada approved the new drug submission (NDS) for AzaSite in Canada. After obtaining regulatory approval in Canada, we transferred regulatory documentation regarding AzaSite to Merck. Thereafter, Merck has been responsible for all regulatory obligations and strategies relating to the further development and commercialization of products in each country. Merck is also responsible for commercialization in both the U.S. and Canada.
We were obligated to provide to Merck with certain future developments, including know-how and patent rights, developed up to the effective transfer date of the regulatory documentation in the United States and Canada that were necessary or useful to develop or commercialize any product for bacterial conjunctivitis in those countries. Such developments were provided without additional fees but any product that includes such developments will be subject to the same royalty rates described above. For certain further developments after such regulatory transfer date, Merck has a time-limited exclusive option to license such further developments upon terms and conditions to be separately negotiated.
We also entered into a trademark license agreement with Merck in February 2007 under which we granted Merck an exclusive license to the AzaSite trademark and domain name and a nonexclusive license to the DuraSite trademark in connection with the commercialization of products in the United States and Canada under the terms of the Merck License.
We also entered into a supply agreement (Supply Agreement) with Merck in February 2007 for azithromycin. We had previously entered into a third-party supply agreement for the production of azithromycin. Under the Supply Agreement, we agreed to supply Merck’s requirements of azithromycin pursuant to certain forecasting and ordering procedures. The initial term of the Supply Agreement expires in 2012, subject to customary termination provisions, such as termination for material breach. Either party may terminate the Supply Agreement upon 180 days notice to the other party. In addition, Merck may terminate the Supply Agreement if our third party supplier moves the location at which the active ingredient is manufactured. After 2012, the Supply Agreement automatically renews for successive three-year periods unless terminated pursuant to the foregoing termination provisions. If we are in breach of our supply obligations under the Supply Agreement, Merck is permitted to qualify a second source supplier, at our expense, and obtain the active ingredient from such second
9
source. We are obligated under the Supply Agreement to maintain a minimum quantity of the active ingredient in inventory for Merck’s use in manufacturing the licensed products and to maintain the quality agreement negotiated with the supplier. The Supply Agreement also contains certain provisions regarding the rights and responsibilities of the parties with respect to manufacturing specifications, delivery arrangements, quality assurance, regulatory compliance, product recall and indemnification, as well as certain other customary matters.
During the years ended December 31, 2011, 2010 and 2009, Merck royalties and supply revenues represented approximately 90%, 92% and 85%, respectively, of our total revenues.
Catalent Pharma Solutions, formerly Cardinal Health PTS, L.L.C. In September 2005, we entered into a commercial manufacturing supply agreement with Catalent Pharma Solutions (Catalent) for the manufacture of AzaSite commercial units. The agreement had a term of four years subsequent to the approval by the FDA of Catalent as a manufacturer of AzaSite. Payments under the contract are dependent upon rolling production forecasts we provide to Catalent and are subject to certain minimum purchase commitments which escalate over the term of the contract. The AzaSite NDA was transferred to Merck and manufacturing responsibilities for AzaSite were transferred to Merck for manufacturing AzaSite for the United States and Canada. We continue to have a relationship with Catalent for the manufacture of AzaSite, as well as for other products in our pipeline.
Bausch and Lomb Incorporated. In December 2003, we completed the sale of a drug candidate for the treatment of ocular infections to Bausch & Lomb pursuant to a purchase agreement and a license agreement (License Agreement) (collectively, the Asset Sale). The drug candidate, Besivance, was developed by Bausch & Lomb. In May 2009, the FDA approved Besivance to treat bacterial conjunctivitis (pink eye). Besivance was launched in the United States by Bausch & Lomb in 2009. In 2011, Besivance was launched internationally in select countries.
We are entitled to a middle single-digit royalty on Besivance net product sales, globally, ending upon the later of the expiration of the patent rights underlying Besivance or ten years from the date of the first Besivance product sale by Bausch & Lomb. Bausch & Lomb has assumed all future Besivance development and commercialization expenses and is responsible for all development activities.
The License Agreement provides Bausch & Lomb a license to certain of our patents related to our DuraSite delivery system for use with Besivance and to other non-patented intellectual property used in Besivance. The License Agreement provides for Bausch & Lomb to complete development of the SS734 fluoroquinolone products that combine certain compounds we licensed from SSP Co., Ltd. (SSP) with the DuraSite delivery system and to commercialize any such products. The patent license is exclusive in the particular field of developing, testing, manufacturing, obtaining regulatory approval of, marketing, selling and otherwise disposing of such products. The license of non-patented intellectual property granted to Bausch & Lomb is nonexclusive.
In connection with the Asset Sale, we also assigned to Bausch & Lomb an agreement between SSP and us under which we were licensed to commercialize SSP’s SS734 fluoroquinolone.
Other.We continually pursue agreements with other companies, universities and research institutions concerning additional therapeutic agents and drug delivery technologies to complement and expand our family of proprietary ophthalmic products as well as collaborative agreements for the further development and marketing of our current products and product candidates. We intend to continue exploring licensing and collaborative opportunities, although there is no certainty that we can successfully enter into, or maintain, any such agreements.
Patents and Proprietary Rights
Patents and other proprietary rights are important to our business. Our policy is to file patent applications seeking to protect technology, inventions and improvements to our inventions that we consider valuable. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop
10
and maintain our competitive position. Our DuraSite drug delivery products are made under patents and applications, and we have filed a number of patent applications in the United States relating to our DuraSite technology with delivery tips and drug compounds. Of these applications, six U.S. patents have been issued. We have four U.S. patents on our retinal drug delivery device that have been issued. Six U.S. patents have been issued related to our antibiotic programs with two applications pending. At least seven other patent applications by us relating to the foregoing and other aspects of our business and potential business are also pending. Foreign counterparts of our patents have also been filed or issued in many countries.
The patent positions of pharmaceutical companies, including ours, are uncertain and involve complex legal and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before a patent is issued. Consequently, we do not know whether any of our pending patent applications will result in the issuance of patents or if any of our patents will provide significant proprietary protection. Since patent applications are maintained in secrecy until they are published, we cannot be certain that we or any licensor was the first to file patent applications for such inventions or that patents issued to our competitors will not block or limit our ability to exploit our technology. Moreover, we might have to participate in interference proceedings declared by the U.S. Patent and Trademark Office (USPTO) to determine priority of invention, which could result in substantial cost to us, even if the eventual outcome is favorable. There can be no assurance that our patents will be held valid or enforceable by a court or that a competitor’s technology or product would be found to infringe such patents. See Item 3. “Legal Proceedings.”
A number of pharmaceutical companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to our business. Some of these technologies, applications or patents may conflict with our technologies or patent applications. These conflicts, whether actual or perceived, could limit the scope of the patents, if any, that we may be able to obtain or result in the denial of our patent applications. In addition, if patents that cover our activities have been or are issued to other companies, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, or at all, or be able to develop or obtain alternative technology. If we do not obtain such licenses, we could encounter delays in or be precluded altogether from introducing products to the market.
In addition to patent protection, we also rely upon trade secret protection for our confidential and proprietary information. There can be no assurance that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets, that such trade secrets will not be disclosed or that we can effectively protect our rights to unpatented trade secrets.
We believe our drug delivery technology may expand the ophthalmic pharmaceutical market by permitting the novel use of drugs for ophthalmic indications that are currently used or being developed for non-ophthalmic indications. However, we may be required to obtain licenses from third parties that have rights to these compounds in order to conduct research, to develop or to market products that contain such compounds. There can be no assurance that such licenses will be available on commercially reasonable terms, if at all.
11
Research and Development
On December 31, 2011, our research and development staff numbered 17 people, of whom five have Ph.D.s. Our research and development expenses for the years ended December 31, 2011, 2010 and 2009 were, as follows:
Research and Development Cost by Program
(in millions)
| | | | | | | | | | | | |
Program | | 2011 | | | 2010 | | | 2009 | |
AzaSite Plus/DexaSite | | $ | 1.6 | | | $ | 0.1 | | | $ | 0.2 | |
BromSite | | | 1.0 | | | | 1.3 | | | | 0.2 | |
New products and other | | | 0.7 | | | | 0.7 | | | | 0.2 | |
Programs—non-specific | | | 4.0 | | | | 2.9 | | | | 4.8 | |
| | | | | | | | | | | | |
Total | | $ | 7.3 | | | $ | 5.0 | | | $ | 5.4 | |
| | | | | | | | | | | | |
In 2011, program expenses primarily consisted of non-specific program costs which comprised facility, internal personnel and stock-based compensation costs that are not allocated to a specific development program. Our AzaSite Plus/DexaSite program expenses primarily related to costs for a new Phase 3 clinical trial that we initiated in the fourth quarter of 2011. Our BromSite program expenses primarily related to the Phase 1/2 clinical trial that concluded in the first quarter of 2011 and the Phase 2 PK Study performed in the third quarter of 2011. In addition, we continue to develop new product candidates.
In 2010, program expenses primarily consisted of non-specific program costs which comprised facility, internal personnel and stock-based compensation costs that are not allocated to a specific development program. The decrease in non-specific costs in 2010 as compared to 2009 was primarily due to savings resulting from the Company’s corporate restructuring in March 2009. Our BromSite program expenses primarily related to preclinical experiments and the Phase 1/2 clinical trial that was initiated in August 2010. Other program activities consisted primarily of new product development.
In 2009, program expenses primarily consisted of non-specific program costs which comprised facility, internal personnel, and stock-based compensation costs that are not allocated to a specific development program.
Manufacturing
We have no experience or facilities for the manufacture of products for commercial purposes and we currently have no intention of developing such experience or building such facilities. We have a pilot facility, licensed by the State of California, to produce potential products for Phase 1 and some of our Phase 2 clinical trials. However, we rely on third parties for supplies and materials necessary for our Phase 3 clinical trials and commercial needs. If we should encounter delays or difficulties in establishing and maintaining our relationship with qualified manufacturers to produce, package and distribute our finished products, then clinical trials, regulatory filings, market introduction and subsequent sales of such products would be harmed.
Under the Merck License, Merck is responsible for the manufacture of AzaSite for the United States and Canada. The AzaSite NDA was transferred to Merck and manufacturing responsibilities for AzaSite were transferred to Merck for the United States and Canada. We have a relationship with Catalent for the manufacture of AzaSite, as well as for other products in our pipeline.
Marketing and Sales
The cost to develop and maintain a marketing organization and sales force is significant and would result in the reallocation of our limited resources needed for the development of our product candidates. We do not currently plan on establishing a dedicated sales force or a marketing organization for our AzaSite, AzaSite Plus or other product candidates.
12
We have entered into corporate collaborations, and we may continue to pursue additional collaborations with one or more additional pharmaceutical companies in the United States and abroad, to market our products.
Merck & Co. In February 2007, we entered into the Merck License under which Merck has the right to exclusively market AzaSite in the United States and Canada. We received a licensing fee, a milestone payment when AzaSite was approved by the FDA and received the first royalty payment based on net sales of the product in the third quarter 2007.
Bausch & Lomb. In December 2003, we sold our product candidate for the treatment of ocular infections to Bausch & Lomb. Bausch & Lomb has the exclusive marketing rights for the world except for Japan, which were retained by SSP, and shared rights in the rest of Asia with SSP.Bausch & Lomb has also assumed the development and manufacturing responsibilities for the product formulation for their sales and distribution and we are entitled to royalties based on net sales of the product, if any. The drug candidate, Besivance, was developed by Bausch & Lomb and approved by the FDA in May 2009. Besivance was launched in the United States by Bausch & Lomb in 2009. In 2011, Besivance was launched internationally in select countries.
Competition
The pharmaceutical industry is highly competitive and requires an ongoing commitment to the pursuit of technological innovation. Such commitment requires significant investment in the resources necessary to discover, develop, test and obtain regulatory approvals for products. It also involves the need to effectively commercialize, market and promote approved products, including communicating the effectiveness, safety and value of products to customers and medical professionals.
The global ophthalmic market is anticipated to grow and will become even more competitive going forward as the prevalence of eye disease increases, leading to increased demand for new and novel ophthalmic products. The market segments that treat diseases and conditions of the eye are subject to ongoing technological change and evolution.
Many companies are engaged in activities similar to our own. Some of these companies have substantially greater financial, technical, marketing and human resources available to them, which may allow them to succeed in developing technologies and products at a faster rate, thereby gaining greater market acceptance than the therapies that we are developing or have developed with our more limited resources. By being first to the market, these competitors may also succeed in obtaining cost advantages or intellectual property rights that would limit our ability to develop and commercialize our own product opportunities. Consequently, they might obtain a more timely and effective regulatory approval for the commercialization of their products in comparison to our timeline.
The global ophthalmic pharmaceutical market is currently dominated by a number of large and well-established companies, including Alcon Laboratories, Inc., Allergan, Inc., Bausch & Lomb, Novartis Ophthalmics, Johnson & Johnson, Merck, and Pfizer. While there are many other large- and medium-sized companies participating in the ophthalmic market, smaller companies such as our own find it challenging to successfully develop and market products without entering into collaborations.
Certain segments of the greater ophthalmic market, such as those for glaucoma, anti-infective and anti-inflammatory agents, already have well-established competing products currently available as well as many in development by prominent competitors. Therefore, in order to penetrate these competitive and mature markets, new products must exhibit improved efficacy and safety profiles, be supported by strong marketing and sales initiatives, and have the support of industry thought leaders.
In summary, our competitive position will depend on our ability to develop enhanced and innovative products, maintain a proprietary position in our technology, obtain required government approvals for our products on a timely basis, attract and retain key personnel and enter into effective collaborations for the manufacture, commercial marketing and distribution of our products in key worldwide markets.
13
Government Regulation
The manufacturing and marketing of our products and our research and development activities are subject to regulation by numerous governmental authorities in the United States and other countries. In the United States, drugs are subject to rigorous FDA regulation. The Federal Food, Drug and Cosmetic Act and regulations promulgated thereunder govern the testing, manufacture, labeling, storage, record keeping, approval, advertising and promotion in the United States of our products. In addition to FDA regulations, we are also subject to other federal and state regulations such as the Occupational Safety and Health Act and the Environmental Protection Act. Product development and approval within this regulatory framework takes a number of years and involves the expenditure of substantial resources.
The steps required before a pharmaceutical agent may be marketed in the United States include:
| | • | | preclinical laboratory testing; |
| | • | | submission to the FDA of an IND; |
| | • | | adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug; |
| | • | | the submission of an NDA or Biological License Application (BLA) to the FDA; and |
| | • | | the FDA approval of the NDA or BLA, prior to any commercial sale or shipment of the drug. |
In addition to obtaining FDA approval for each product, each domestic drug manufacturer and facility must be registered with, and approved by, the FDA. Drug product manufacturing establishments located in California also must be licensed by the State of California in compliance with separate regulatory requirements.
Preclinical tests include laboratory evaluation of product chemistry and animal studies to assess the potential safety and efficacy of the product and its formulation. The results of the preclinical tests are submitted to the FDA as part of an IND and, unless the FDA objects, the IND will become effective 30 days following its receipt by the FDA.
Clinical trials involve the administration of the drug to healthy volunteers or to patients under the supervision of a qualified principal investigator. Clinical trials are conducted in accordance with protocols that detail the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Before any clinical trial can commence, each protocol is submitted to the FDA as part of the IND. Each clinical study is conducted under the auspices of an independent Institutional Review Board that considers, among other things, ethical factors and the rights, welfare and safety of human subjects.
Clinical trials are typically conducted in three sequential phases, but the phases may involve multiple studies and may overlap. In Phase 1, the initial introduction of the drug into human subjects, the drug is tested for safety (adverse effects), dosage tolerance, metabolism, distribution, excretion and clinical pharmacology. Phase 2 involves studies in a limited patient population to (i) determine the efficacy of the drug for specific targeted indications, (ii) determine dosage tolerance and optimal dosage and (iii) identify possible adverse effects and safety risks. When a compound is found to be effective and to have an acceptable safety profile in Phase 2 evaluations, Phase 3 clinical trials are undertaken to further evaluate clinical efficacy and to further test for safety within an expanded patient population at multiple clinical study sites. The FDA reviews both the clinical plans and the results of the trials and may discontinue the trials at any time if there are significant safety issues.
The results of the preclinical studies and clinical studies are submitted to the FDA in the form of an NDA or BLA for marketing approval. The testing and approval process requires substantial time and effort and there can be no assurance that any approval will be granted on a timely basis, if at all. Additional animal studies or clinical trials may be requested during the FDA review period and may delay marketing approval. After FDA approval for the initial indications, further clinical trials are necessary to gain approval for the use of the product for additional indications. The FDA may also require post-marketing testing to monitor for adverse effects, which can involve significant expense.
14
Among the conditions for manufacture of clinical drug supplies and for NDA or BLA approval is the requirement that the prospective manufacturer’s quality control and manufacturing procedures conform to current Good Manufacturing Practice, or cGMP. Prior to approval, manufacturing facilities are subject to FDA and/or other regulatory agency inspection to ensure compliance with cGMP. Manufacturing facilities are subject to periodic regulatory inspection to ensure ongoing compliance.
For marketing outside the United States, we or our licensees are also subject to foreign regulatory requirements governing human clinical trials and marketing approval for drugs. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary widely from country to country and in some cases are even more rigorous than in the United States.
Scientific and Business Advisors
We have access to a number of academic and industry advisors with expertise in clinical ophthalmology and pharmaceutical development, marketing and sales. Our advisors meet with our management and key scientific employees on an ad hoc basis to provide advice in their respective areas of expertise and further assist us by periodically reviewing with management our preclinical, clinical and marketing activities. In 2011, we established the Scientific Advisory Board (SAB) to help guide and shape our research programs in the development of novel ophthalmic medicines. Members of the SAB represent leaders in ophthalmic research, treatment and clinical drug development, including Richard Lindstrom, M.D., Gary Foulks, M.D., Michael Lemp, M.D., and Kelly Nichols, O.D., M.P.H., Ph.D. The SAB is led by our Chief Medical Officer, Kamran Hosseini, M.D., Ph.D., and Brian Levy, O.D. M.Sc., a member of our Board of Directors, will also participate in all SAB meetings. Our SAB members possess deep insight into the etiology of important ocular diseases, which will be instrumental in advancing our therapeutic programs. Our SAB members have already made significant contributions to our current clinical development programs, providing input on trial protocols and endpoint design.
We plan to add additional advisors as appropriate. Although we expect to receive guidance from our advisors, all of our advisors are employed on a full-time basis by other entities, or are primarily engaged in outside business activities, and may have other commitments to, or consulting or advisory contracts with, other entities that may conflict or compete with their obligations to us.
Employees
As of December 31, 2011, we had 25 employees, 21 of whom were full time. None of our employees are covered by a collective bargaining agreement. We believe we have good employee relations. We also utilize independent consultants to provide services in certain areas of our scientific and business operations.
15
Risks Relating to Our Business
It is difficult to evaluate our business because we are in an early stage of commercializing our products, our technology is untested and successful development of pharmaceutical products is highly uncertain and requires significant expenditures and time
We are in the early stages of commercializing our products. AzaSite received regulatory approval in the U.S. in April 2007 and commercial sales of AzaSite began in the third quarter of 2007. Besivance received regulatory approval in May 2009 and commercial sales of Besivance began in the second half of 2009. We must receive approval in other countries prior to marketing AzaSite in such countries. Before regulatory authorities grant us marketing approval for additional products, we need to conduct significant additional research and development and preclinical and clinical testing and submit a New Drug Application (NDA). Successful development of pharmaceutical products is highly uncertain. Products that appear promising in research or development, may be delayed or fail to reach later stages of development or the market for several reasons, including:
| | • | | preclinical tests may show the product to be toxic or lack efficacy in animal models; |
| | • | | failure to receive the necessary U.S. and international regulatory approvals or a delay in receiving such approvals due to, among other things, slow enrollment in clinical studies, failure to achieve study endpoints within the time period prescribed by the study, or at all, additional time requirements for data analysis or BLA or NDA preparation, discussions with the FDA, FDA requests for additional preclinical or clinical data, analyses or changes to study design; or unexpected safety, efficacy or manufacturing issues; |
| | • | | clinical trial results may show the product to be less effective than desired or to have harmful or problematic side effects; |
| | • | | difficulties in formulating the product, scaling the manufacturing process, or getting approval for manufacturing; |
| | • | | even if safe and effective, manufacturing costs, pricing, reimbursement issues or other factors may make the product uneconomical; |
| | • | | proprietary rights of others and their competing products and technologies may prevent the product from being developed or commercialized; or |
| | • | | inability to compete with superior, equivalent, more cost-effective or more effectively promoted products offered by competitors. |
Therefore, our research and development activities may not result in any commercially viable products.
We have a history of operating losses and we expect to continue to have losses in the future
We have incurred significant operating losses since our inception in 1986 and have pursued numerous drug development candidates that failed to achieve clinical end points or did not prove to have commercial potential. We expect to incur net losses for the foreseeable future or until we are able to achieve significant royalties or other revenues from sales of our products. Attaining significant revenue or profitability depends upon our ability, alone or with third parties, to develop our potential products successfully, conduct clinical trials, obtain required regulatory approvals and manufacture and market our products successfully. We may not ever achieve or be able to maintain significant revenue or profitability, including with respect to AzaSite, our lead product which has not yet been commercially launched outside the United States.
16
Clinical trials, such as the recently completed BromSite clinical trial, are expensive, time-consuming and difficult to design, enroll and implement and there can never be any assurance that the results of such clinical trials will be favorable
Human clinical trials for our product candidates are very expensive and difficult to design, enroll and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is also time-consuming. We may experience difficulties or delays in enrolling our clinical trials, which can delay the trials and our ability to obtain ultimate approval of our product candidates. In addition, we require various clinical materials to conduct our clinical trials and any unavailability or delay in our ability to obtain these materials may delay our trials, cause them to be more expensive or preclude us from completing these trials, which would harm our ability to obtain approval for our product candidates and therefore harm our business. We estimate that any particular clinical trial may take over a year to complete. Furthermore, we could encounter problems that might cause us to abandon or repeat clinical trials resulting in additional expense, further delays and potentially preventing the completion of such trials. The commencement and completion of clinical trials may be delayed or terminated due to several factors, including, among others:
| | • | | unforeseen safety issues; |
| | • | | lack of effectiveness during clinical trials; |
| | • | | difficulty in determining dosing and trial protocols; |
| | • | | slower than expected rates of patient recruitment; |
| | • | | difficulties in obtaining clinical materials or participants necessary for the conduct of our clinical trials; |
| | • | | inability to monitor patients adequately during or after treatment; and |
| | • | | inability or unwillingness of clinical investigators to follow our clinical protocols. |
In addition, we or the FDA may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the FDA finds deficiencies in our submissions or the conduct of these trials. In any such case, we may not be able to obtain regulatory approval for our product candidates, in which case we would not obtain any benefit from our substantial investment in developing the product and conducting clinical trials for such products.
The results of our clinical trials may not support our product candidate claims
Even if our clinical trials are completed as planned, we cannot be certain that the results will support our product candidate claims. Even if pre-clinical testing and early clinical trials for a product candidate are successful, this does not ensure that later clinical trials will be successful and we cannot be sure that the results of later clinical trials will replicate the results of prior clinical trials and pre-clinical testing, meet our expectations or satisfy the FDA or other regulatory bodies. The clinical trial process may fail to demonstrate that our product candidates are safe for humans or effective for indicated uses. In addition, our clinical trials involve relatively small patient populations. Because of the small sample size, the results of these clinical trials may not be indicative of future results. Any such failure would likely cause us to abandon the product candidate and may delay development of other product candidates. Any delay in, or termination of, our clinical trials will delay or preclude the filing of our NDAs with the FDA and, ultimately, our ability to commercialize our product candidates and generate product revenues.
For example, results from our Phase 3 clinical trial of AzaSite Plus for the treatment of blepharoconjunctivitis showed improved clinical outcomes as compared to treatment with a corticosteroid alone or antibiotic alone in the reduction of inflammatory signs and symptoms and bacterial eradication, respectively. In addition, AzaSite Plus was very well tolerated. However, the trial did not achieve its primary clinical endpoint as defined by the protocol. We discussed the results of this trial with the FDA and determined a development plan for AzaSite Plus. We cannot assure you that any additional clinical trials for AzaSite Plus will meet the prescribed clinical endpoint as defined in the protocol for that study or that we will ultimately achieve FDA approval for the commercialization of AzaSite Plus.
17
Our strategy for commercialization of our products requires us to enter into successful arrangements with corporate collaborators
We generally intend to enter into partnering and collaborative arrangements with respect to the commercialization of our product candidates, such as AzaSite Plus, DexaSite and BromSite. However, we cannot assure you that we will be able to enter into such arrangements or that they will be beneficial to us. The success of our partnering and collaboration arrangements will depend upon many factors, including, among others:
| | • | | the progress and results of our preclinical and clinical testing and research and development programs; |
| | • | | the time and cost involved in obtaining regulatory approvals; |
| | • | | our ability to negotiate favorable terms with potential collaborators; |
| | • | | the efforts and success of our collaborators in further developing or marketing the product; |
| | • | | our ability to prosecute, defend and enforce patent claims and other intellectual property rights; |
| | • | | the outcome of possible future legal actions; and |
| | • | | competing technological and market developments. |
We may not be able to conclude arrangements with third parties to support the commercialization of our products on acceptable terms, or at all, and may not be able to maintain any arrangement that we do enter into. If we pursue a partnership for AzaSite Plus, DexaSite or our other product candidates prior to completing the Phase 3 trials, we will likely receive less favorable economic terms than if we completed successful Phase 3 trials.
The commercial success of our products is dependent on the diligent efforts of our corporate collaborators
Because we generally rely on third parties for the marketing and sale of our products, revenues that we receive will be highly dependent on the efforts and success of these third parties, particularly our partner Merck. Upon Merck’s acquisition of Inspire, we are now reliant on Merck’s efforts to commercialize AzaSite. Since Merck’s acquisition of Inspire, net sales of AzaSite have decreased. There can be no assurance that our royalty revenues from net sales of AzaSite will return to prior levels or will grow as originally anticipated. Our partners, including Merck, may terminate their relationships with us and/or may not diligently or successfully market or sell our products. These partners may also pursue alternative or competing technologies or develop alternative products either on their own or in collaboration with others, including our competitors. In addition, marketing consultants and contract sales organizations that we may use in the future for our products may market products that compete with our products and we must rely on their efforts and ability to market and sell our products effectively.
If we fail to enter into future collaborations or our current collaborations are terminated, we will need to enter into new collaborations or establish our own sales and marketing organization
We may not be able to enter into or maintain collaborative arrangements with third parties, including Merck. If we are not successful in entering into future collaborations or maintaining our existing collaborations, particularly with Merck, we may be required to find new corporate collaborators or establish our own sales and marketing organization. We do not have a long-standing relationship with Merck and have limited information with respect to AzaSite and its marketing. There can be no assurance that we will receive higher, or equivalent, royalty revenues from Merck than we did from Inspire as an independent company. The number of monthly prescriptions for AzaSite has recently significantly declined as Merck has taken over marketing and sales of AzaSite after its acquisition of Inspire. While the minimum royalty payments from Merck have made up for this decline, there can be no assurance that Merck will devote significant resources to, or successfully market and sell, AzaSite. In addition, under the terms of the Merck License, Merck’s obligation to make minimum royalty payments may be suspended upon the occurrence of certain events, such as a requirement by the FDA or other governmental agency to suspend the marketing of AzaSite or withdraw it from the market in the United States or
18
if we are unable to obtain commercial quantities of AzaSite for Merck to market and sell. Furthermore, Merck may terminate the Merck License at any time. If Merck were to terminate the Merck License, we would have to find a new marketing partner or market AzaSite ourselves. There can be no assurance that any new partnership would be on similar terms as the Merck License. We have no experience in sales, marketing or distribution and establishing such an organization would be costly. Moreover, there is no guarantee that a sales and marketing organization established by us would be successful. If we are unable to maintain existing collaborations, enter into additional collaborations or successfully market our products ourselves, our revenues and financial results would be significantly harmed.
Our future success depends on our ability to engage third parties to assist us with the development of new products, new indications for existing products and the conduct of our clinical trials to achieve regulatory approval for commercialization and any failure or delay by those parties to fulfill their obligations could adversely affect our development and commercialization plans
For our business model to succeed, we must continually develop new products or discover new indications for our existing products. As part of that process, we rely on third parties such as clinical research organizations, clinical investigators and outside testing labs for development activities, such as Phase 2 and/or Phase 3 clinical testing, and to assist us in obtaining regulatory approvals for our product candidates. We rely heavily on these parties for successful execution of their responsibilities but have no control over how these parties manage their businesses and cannot assure you that such parties will diligently or effectively perform their activities. For example, the clinical investigators that are conducting our clinical trials, including our current Phase 3 clinical trial for AzaSite Plus and DexaSite are not our employees and we anticipate that any future clinical trials of AzaSite Plus, DexaSite or BromSite will also be conducted by a third party. However, we are responsible for ensuring that each of our clinical trials is conducted in accordance with applicable protocols, rules and regulations or in accordance with the general investigational plan and protocols for the trial as well as the various rules and regulations governing clinical trials in the United States and abroad. Any failure by those parties to perform their duties effectively and on a timely basis could harm our ability to develop and commercialize new products, harm our business and subject us to potential liabilities.
Physicians and patients may not accept or use our products
Even if the FDA approves our product candidates, physicians and patients may not accept or use them. Acceptance and use of our products will depend upon a number of factors including:
| | • | | perceptions by members of the health care community, including physicians, about the safety and effectiveness of our products, among others; |
| | • | | effectiveness of marketing and distribution efforts by us and our licensees and distributors; |
| | • | | cost-effectiveness of our products relative to competing products or treatments; |
| | • | | actual or perceived benefits of competing products or treatments; |
| | • | | physicians’ comfort level and prior experience with and use of competing products or treatments; and |
| | • | | availability of reimbursement for our products from government or other healthcare payers. |
We may require additional licenses or be subject to expensive and uncertain patent litigation in order to sell our products
A number of pharmaceutical and biotechnology companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to our business. Some of these technologies, applications or patents may conflict with our technologies or patent applications. As is common in the pharmaceutical and biotech industry, from time to time we receive notices from third parties alleging various challenges to our patent rights. Such conflicts, if proven, could invalidate our issued patents, limit the scope of the patents, if any, that we may be able to obtain, result in the denial of our
19
patent applications or block our rights to exploit our technology. If the USPTO or foreign patent agencies have issued or in the future issue patents to other companies that cover our activities, we may not be able to obtain licenses to these patents at a reasonable cost, or at all, or be able to develop or obtain alternative technology. If we do not obtain such licenses, we could encounter delays in or be precluded altogether from introducing products to the market. If we are required to obtain additional licenses from third parties for the sale by Merck of AzaSite in the United States and Canada, we will be required to pay for such additional licenses from our existing cash under the terms of the $60 million in aggregate principal amount of non-convertible, non-recourse promissory notes due in 2019 (AzaSite Notes).
In addition, we may need to litigate in order to defend against claims of infringement by others, to enforce patents issued to us or to protect trade secrets or know-how owned or licensed by us. Litigation could result in substantial cost to and diversion of effort by us, which may harm our business, prospects, financial condition and results of operations. Such costs can be particularly harmful to companies such as ours, without significant existing revenue streams or cash resources. We have also agreed to indemnify our licensees against infringement claims by third parties related to our technology, which could result in additional litigation costs and liability for us. In addition, our efforts to protect or defend our proprietary rights may not be successful or, even if successful, may result in substantial cost to us, thereby utilizing our limited resources for purposes other than product development and commercialization.
If our products, methods, processes and other technologies infringe the proprietary rights of other parties, we could incur substantial costs and we may have to:
| | • | | obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| | • | | redesign our products or processes to avoid infringement; |
| | • | | stop using the subject matter claimed in the patents held by others, which could preclude us from commercializing our products; |
| | • | | defend litigation or administrative proceedings, which may be costly whether we win or lose, and which could result in a substantial diversion of our valuable management resources. |
Our business depends upon our proprietary rights and we may not be able to protect, enforce, or secure our intellectual property rights adequately
Our future success will depend in large part on our ability to obtain patents, protect trade secrets, obtain and maintain rights to technology developed by others, and operate without infringing upon the proprietary rights of others. A substantial number of patents in the field of ophthalmology and genetics have been issued to pharmaceutical, biotechnology and biopharmaceutical companies. Moreover, competitors may have filed patent applications, may have been issued patents or may obtain additional patents and proprietary rights relating to products or processes competitive with ours. Our patent applications may not be approved. We may not be able to develop additional proprietary products that are patentable. Even if we receive patent issuances, those issued patents may not be able to provide us with adequate protection for our inventions or may be challenged by others.
A patent interference was declared before the Board of Patent Appeals and Interferences on certain U.S. patents covering AzaSite. Regents (Regents) of the University of California (University) assert that the inventions contained in these patents were made by a former employee of the University alone, and without collaboration with us. They are asserting that they own those inventions, and that they are entitled to an award of priority of invention and a judgment that the inventions are not patentable to us. On November 25, 2011, the USPTO entered judgment against the University. On December 23, 2011, the University filed a Notice of Appeal to the Court of Appeals for the Federal Circuit in Washington, D.C., and on January 4, 2012, we filed a Notice of Cross-Appeal. We continue to believe the University’s assertions are without merit and intend to vigorously contest those assertions. An adverse outcome of this interference could impact our royalty stream from Merck for AzaSite.
20
We received a letter (Notice Letter) dated April 15, 2011 from Sandoz, Inc. (Sandoz) providing notice that Sandoz has filed an Abbreviated New Drug Application (ANDA) with the FDA seeking marketing approval for a 1% azithromycin ophthalmic solution (Sandoz Product) prior to the expiration of the five U.S. patents listed in the Orange Books for AzaSite which include four of our patents and one patent licensed to us by Pfizer. In the paragraph IV Certification accompanying the Sandoz ANDA filing, Sandoz alleges that the claims of the Orange Book listed patents are invalid, unenforceable and/or will not be infringed by the Sandoz Product. On May 26, 2011, we, Merck and Pfizer filed a patent infringement lawsuit against Sandoz and related entities. The plaintiff companies have agreed that Merck will take the lead in prosecuting this lawsuit. Merck, with the assistance of Pfizer and us, will vigorously defend the five U.S. patents related to AzaSite. We own four U.S. patents covering AzaSite and its use, and have an exclusive license to a Pfizer-owned azithromycin patent. The filing of this lawsuit triggered an automatic stay, or bar, of the FDA’s approval of the ANDA for up to 30 months or until a final district court decision of the infringement lawsuit, whichever comes first. We believe that our four patents and the Pfizer patent were properly prosecuted with the USPTO and are valid, and that three of these patents will provide AzaSite with exclusivity until March 2019. We and the other plaintiffs intend to vigorously enforce our patent rights relating to AzaSite and vigorously contest any Sandoz assertions that these patents are invalid or unenforceable. An adverse decision on the issues of validity or enforceability would significantly harm our royalty revenue stream from AzaSite.
Furthermore, the patents of others may impair our ability to commercialize our products. The patent positions of firms in the pharmaceutical and genetic industries generally are highly uncertain, involve complex legal and factual questions, and have recently been the subject of significant litigation. The USPTO and the courts have not developed, formulated, or presented a consistent policy regarding the breadth of claims allowed or the degree of protection afforded under pharmaceutical and genetic patents. Despite our efforts to protect our proprietary rights, others may independently develop similar products, duplicate any of our products or design around any of our patents. In addition, third parties from whom we have licensed or otherwise obtained technology may attempt to terminate or scale back our rights.
We also depend upon unpatented trade secrets to maintain our competitive position. Others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets. Our trade secrets may also be disclosed, and we may not be able to protect our rights to unpatented trade secrets effectively. To the extent that we, our consultants or our research collaborators use intellectual property owned by others, disputes also may arise as to the rights in related or resulting know-how and inventions.
In certain circumstances, we may lose the potential to receive future royalty payments after the AzaSite Notes are repaid in full or we may be required to pay damages for breaches of representations, warranties or covenants under certain of the AzaSite Note financing agreements
In February 2008, through a wholly-owned subsidiary, we issued $60 million in aggregate principal amount of AzaSite Notes, which are secured principally by royalty payments from future sales of AzaSite in North America, but not the right to receive such payments and by a pledge by us of all the outstanding equity interest in our subsidiary. If the AzaSite royalty payments are insufficient to repay the AzaSite Notes or if an event of default occurs under the indenture governing the AzaSite Notes, in certain circumstances, we would have to make payments on the AzaSite Notes out of our own cash resources, the royalty payments and our equity interest in our subsidiary may be foreclosed upon and we would lose the potential to receive future royalty payments after the AzaSite Notes are repaid in full and our intellectual property and other rights related to AzaSite. In addition, in connection with the issuance of the AzaSite Notes, we have made certain representations, warranties and covenants to our subsidiary and the holders of the AzaSite Notes (Noteholders). If we breach these representations, warranties or covenants, such breach could trigger an event of default under the indenture and we could also be liable to our subsidiary or the Noteholders for substantial damages in respect of any such breach, which could harm our financial condition and ability to conduct our business as currently planned. See Note 7 of the Consolidated Financial Statements in our Annual Report on Form 10-K for a more complete description of the terms of the AzaSite Notes.
21
Merck’s failure to successfully market and commercialize AzaSite would harm sales of AzaSite and, therefore, would delay or prevent repayment of the AzaSite Notes, which would delay or prevent us from receiving future revenue from sales of AzaSite
The AzaSite Notes issued by our subsidiary will be repaid solely from royalties on net sales of AzaSite in the United States and Canada by Merck under the Merck License. Merck has full control of all promotional, sales and marketing activities for AzaSite in these territories, and has sole control over the pricing of AzaSite. The number of monthly prescriptions for AzaSite has recently declined as Merck has taken over marketing and sales of AzaSite after its acquisition of Inspire. While the minimum royalty payments from Merck have made up for this decline, there can be no assurance that Merck will devote significant resources to, or successfully market and sell, AzaSite. Accordingly, royalty payments in respect of net sales of AzaSite in the United States and Canada, if Merck markets AzaSite in Canada, will be entirely dependent on the actions, efforts and success of Merck, over whom neither we nor our subsidiary have control. The success of Merck’s commercialization of AzaSite and the timely repayment of the AzaSite Notes will depend on a number of factors, including, among others:
| | • | | the scope of Merck’s launch of AzaSite in the United States and Canada; |
| | • | | the effectiveness and extent of Merck’s promotional, sales and marketing efforts; |
| | • | | Merck’s ability to successfully market AzaSite to physicians and patients; |
| | • | | Merck’s deployment of resources to market and sell AzaSite; |
| | • | | Merck’s ability to build, train and retain an effective sales force; |
| | • | | Merck’s marketing efforts outside of ophthalmologists; |
| | • | | Merck’s pricing decisions regarding AzaSite; |
| | • | | Merck’s marketing and selling of any current or future competing products; |
| | • | | Merck’s ability to compete against competitors; |
| | • | | the discovery of any side effects or negative efficacy findings for AzaSite; |
| | • | | product recalls or product liability claims relating to AzaSite; |
| | • | | the introduction of generic competition; |
| | • | | the extent to which competing products for the treatment of bacterial conjunctivitis obtain more favorable formulary status than AzaSite; and |
| | • | | the relevant parties’ ability to adequately maintain or enforce the intellectual property rights relevant to AzaSite. |
Merck may determine to focus its resources on its other products, internal development and other activities, which may harm its successful marketing of AzaSite and therefore impact our royalty payments.
Inspire has historically promoted AzaSite to eye care professionals. Pediatricians and primary care physicians write more than 67% of prescriptions for ophthalmic antibiotics. However, Inspire had no experience calling on pediatricians and primary care physicians. To date, Merck has continued to market AzaSite exclusively to ophthalmologists. There can be no assurance that Merck will more broadly market AzaSite. Inspire’s focus on eye care professionals rather than pediatricians and primary care providers may have resulted, and if continued by Merck, may in the future result in lower AzaSite sales and therefore lower royalties paid to us. A large number of pharmaceutical companies, including those with competing products and those with products for indications that are completely unrelated to AzaSite, compete for the time and attention of eye care professionals, pediatricians and primary care physicians. Neither we nor our subsidiary have any control over how Merck manages and operates its sales force, how effective Merck’s sales efforts will be or Merck’s pricing decisions regarding AzaSite.
22
In addition, Merck depends on three pharmaceutical wholesalers for the vast majority of its AzaSite sales in the United States. These companies are Cardinal Health, McKesson Corporation and AmerisourceBergen. The loss of any of these wholesalers could harm sales of AzaSite. It is also possible that these wholesalers, or others, could decide to change their policies or fees, or both, in the future. This could cause Merck to incur higher product distribution costs, which would result in lower net sales of AzaSite.
Merck could deemphasize or sell or sublicense its rights to AzaSite. Neither we nor our subsidiary can prevent Merck from developing or licensing a product that competes with AzaSite or limiting or withdrawing its support of AzaSite. Our subsidiary’s ability to pay amounts due on the AzaSite Notes may be materially harmed to the extent Merck is unable or unwilling to successfully market and sell AzaSite. To the extent that our subsidiary fails to meet its payment obligations, we will have to make such payments out of our own cash resources in order to avoid a default under the AzaSite Notes, which we have done in the past. Our subsidiary again received insufficient royalties to make the interest payment in full that was due on February 15, 2012. We have the ability to make-up this shortfall with our own cash resources. This shortfall in interest payments constitutes a default under the indenture but not an event of default. To the extent that we pay in full the recent shortfall (plus interest thereon) by May 15, 2012, we will avoid triggering an event of default under the indenture. To the extent that an event of default occurs, the bondholders could seek available remedies, including foreclosure on our subsidiary. Our ability to receive future revenue from sales of AzaSite is dependent on our subsidiary repaying the AzaSite Notes in a timely fashion. If our subsidiary takes longer than anticipated to repay the AzaSite Notes, or if it defaults on the AzaSite Notes, we may not receive future revenue from AzaSite as currently planned, or at all.
Royalties under the Merck License may not be sufficient for our subsidiary to meet its payment obligations under the AzaSite Notes
Merck’s obligation to pay royalties on net sales of AzaSite under the Merck License expires on a country-by-country basis upon the later of 11 years from the first commercial sale of AzaSite or when the last valid claim under one of our licensed patents covering a subject product under the Merck License in the United States and Canada expires. In the United States, first commercial sales occurred in August 2007, therefore, the obligation to pay royalties expires in August 2018. While our subsidiary will be entitled to minimum royalties under the Merck License for five years after the first year of a commercial sale, such minimum royalties will not be sufficient for our subsidiary to meet its payment obligations under the AzaSite Notes and, therefore, it will be dependent on Merck’s successful sales and marketing efforts for AzaSite in order for it to receive royalties in excess of these minimum amounts. In addition, under the terms of the Merck License, Merck’s obligation to make minimum royalty payments may be suspended upon the occurrence of certain events, such as a requirement by the FDA or other governmental agency to suspend the marketing of AzaSite or withdraw it from the market in the United States or if we are unable to obtain commercial quantities of AzaSite for Merck to market and sell. Merck also has the right to terminate the Merck License at any time. To the extent that royalties, including minimum royalties, are insufficient for our subsidiary to meet its payment obligations, we will have to make such payments out of our own cash resources in order to avoid a default under the notes, which we have done in the past. In addition, Merck’s obligation to pay minimum royalties is suspended during any period in which (i) the FDA or any other applicable regulatory authority has required any Merck licensed product to be withdrawn from the market or the marketing thereof otherwise to be suspended in the United States or (ii) Merck is unable, despite use of commercially reasonable efforts, to obtain supply of any Merck licensed product in finished form in commercially reasonable amounts necessary to launch or market such Merck licensed product in the United States.
Royalties under the Merck License are subject to a cumulative reduction or offset in the event of patent invalidity, generic competition, uncured material breaches by us or in the event that Merck is required to pay royalties, milestone payments or license fees to third parties for the continued use of AzaSite. The applicable royalty rate is also subject to reduction by up to 50% in any country during any period in which AzaSite does not have patent protection. These cumulative reductions or offsets could result in our subsidiary receiving
23
significantly reduced or no royalties under the Merck License, which would delay repayment of the AzaSite Notes, or result in a default under the AzaSite Notes. In such circumstances we may not receive future revenue from AzaSite as currently planned, or at all.
If the Merck License is terminated in whole or in part while the AzaSite Notes remain outstanding, we will be forced to find a new third party collaborator for AzaSite, pursue commercialization efforts ourselves or else we will lose our right to certain intellectual property rights related to AzaSite to our subsidiary
In February 2008, in connection with our subsidiary’s issuance of the AzaSite Notes, we entered into the residual license agreement with our subsidiary (Residual License Agreement). Under the terms of the Residual License Agreement, if the Merck License is terminated in the United States or Canada while the AzaSite Notes are outstanding, all of our rights to AzaSite in such country or countries will be licensed to our subsidiary and we have three months under the terms of an interim sublicense (Interim Sublicense), which is a part of the Residual License Agreement, to find a new third party collaborator to undertake commercialization efforts with respect to AzaSite or pursue commercialization efforts ourselves in such country or countries. Merck can terminate the Merck License unilaterally in a variety of circumstances, including at any time in its discretion. If the Merck License is terminated, our efforts to find a new third-party collaborator or pursue direct commercialization efforts ourselves will divert the attention of senior management from our current business operations, which could delay the development or licensing of our other product candidates. If we elect to commercialize AzaSite ourselves, we would have to expend significant resources as we currently have no sales, marketing or distribution capabilities or experience, and have no current plans to establish any such resources, which may not be successful and could harm our financial condition and results of operation. We may not be able to find a new third-party collaborator within the time period allowed and there can be no assurance that any such collaboration will be on acceptable terms or will be successful.
If we are unsuccessful in finding a new third party collaborator for AzaSite or elect not to pursue commercialization efforts ourselves, the Interim Sublicense will terminate and our subsidiary will retain all rights to the intellectual property with respect to AzaSite in the related country or countries in which the Merck License was terminated. If the Interim Sublicense terminates in accordance with the Residual License Agreement, our subsidiary may grant a sublicense under the license granted under the Residual License Agreement or pursue commercialization efforts itself. In any such circumstances, our subsidiary will remit for payment on the AzaSite Notes any royalties and other payments arising from the exercise of the license under the Residual License Agreement. As all economic value arising from the intellectual property subject to the Merck License shall remain with our subsidiary (whether or not the Merck License remains in effect and whether or not our subsidiary continues to be owned by us or our equity in the subsidiary is foreclosed upon by the Noteholders), while the AzaSite Notes are outstanding and following repayment thereof, we may never receive any future royalties or economic benefit from AzaSite and may lose rights to the intellectual property relating thereto.
We rely on a sole source for the supply of the active pharmaceutical ingredient for AzaSite
We currently have a single supplier for azithromycin, the active drug incorporated into AzaSite. Under the Merck License and the Supply Agreement, we have agreed to provide a supply of azithromycin to Merck for the manufacture of AzaSite in the Territory, which we currently arrange through one supplier. The supplier of azithromycin has a drug master file on the compound with the FDA and is subject to the FDA’s review and oversight. The supplier’s manufacturing facility is subject to potential natural disasters, including earthquakes, hurricanes, tornadoes, floods, fires or explosions, and other interruptions in operation due to factors including labor unrest or strikes, failures of utility services or microbial or other contamination. If the supplier failed or refused to continue to supply us, if the FDA were to identify issues in the production of azithromycin that the supplier was unable to resolve quickly and cost-effectively, or if other issues were to arise that impact production, Merck’s ability to manufacture and commercialize AzaSite could be interrupted, and our subsidiary’s ability to pay amounts due on the AzaSite Notes may be materially harmed, which could force us to make such payments out of our own cash resources in order to avoid a default under the AzaSite Notes and prevent or delay
24
our ability to receive future revenue from AzaSite. Additional suppliers for azithromycin exist, but qualification of an alternative source could be time consuming and expensive and, during such qualification process, could negatively impact the sales of AzaSite. There is also no guarantee that these additional suppliers can supply sufficient quantities or quality product at a reasonable price, or at all. While we are required to maintain a certain level of inventory of azithromycin to support Merck’s manufacturing needs, that inventory may not be sufficient to prevent an interruption in the availability of AzaSite.
In addition, certain of the raw materials that we use in formulating DuraSite, the drug delivery system used in AzaSite and our other products, are available only from Lubrizol Advanced Materials, Inc. (Lubrizol). Although we do not have a current supply agreement with Lubrizol, we have not encountered any difficulties obtaining necessary materials from Lubrizol. Any significant interruption in the supply of these raw materials could delay sales of AzaSite, which could then harm our subsidiary’s ability to pay amounts due on the AzaSite Notes and affect our ability to receive future revenue from AzaSite.
We compete in highly competitive markets and our competitors’ financial, technical, marketing, manufacturing and human resources may surpass ours and limit our ability to develop and/or market our products and technologies
Our success depends upon developing and maintaining a competitive advantage in the development of products and technologies in our areas of focus. We have many competitors in the United States and abroad, including pharmaceutical, biotechnology and other companies with varying resources and degrees of concentration in the ophthalmic market. Our competitors may have existing products or products under development which may be technically superior to ours or which may be less costly or more acceptable to the market. Our competitors may obtain cost advantages, patent protection or other intellectual property rights that would block or limit our ability to develop our potential products. Competition from these companies is intense and is expected to increase as new products enter the market and new technologies become available. Many of our competitors have substantially greater financial, technical, marketing, manufacturing and human resources than we do, particularly in light of our current financial condition. In addition, they may succeed in developing technologies and products that are more effective, safer, less expensive or otherwise more commercially acceptable than any that we have or will develop. Our competitors may also obtain regulatory approval for commercialization of their products more effectively or rapidly than we will. If we decide to manufacture and market our products by ourselves, we will be competing in areas in which we have limited or no experience such as manufacturing efficiency and marketing capabilities.
If we cannot compete successfully for market share against other drug companies, we may not achieve sufficient product revenues and our business will suffer
The market for our product candidates is characterized by intense competition and rapid technological advances. If our product candidates receive FDA approval, they will compete with a number of existing and future drugs and therapies developed, manufactured and marketed by others. Existing or future competing products may provide greater therapeutic convenience or clinical or other benefits for a specific indication than our products or may offer comparable performance at a lower cost. If our products fail to capture and maintain market share, we may not achieve sufficient product revenues and our business will be harmed.
We will compete against fully integrated pharmaceutical companies and smaller companies that are collaborating with larger pharmaceutical companies, academic institutions, government agencies and other public and private research organizations. Many of these competitors have products competitive with AzaSite already approved or in development, including Zymar and Ocuflox by Allergan, Vigamox and Ciloxan by Alcon, and Quixin by Johnson & Johnson. In addition, many of these competitors, either alone or together with their collaborative partners, operate larger research and development programs and have substantially greater financial resources than we do, as well as significantly greater experience in:
25
| | • | | undertaking pre-clinical testing and human clinical trials; |
| | • | | obtaining FDA and other regulatory approvals of drugs; |
| | • | | formulating and manufacturing drugs; |
| | • | | launching, marketing and selling drugs; and |
| | • | | attracting qualified personnel, parties for acquisitions, joint ventures or other collaborations. |
We have to attract and retain key employees to be successful
A critical factor to our success will be retaining our personnel or recruiting replacement personnel. Competition for skilled individuals in the biotechnology business, particularly in the San Francisco Bay Area, is highly competitive, and we may not be able to continue to attract and retain personnel necessary for the development of our business. Our ability to attract and retain such individuals may be reduced by our current financial situation and the challenges we face. The loss of key personnel, the failure to recruit replacement personnel or to develop needed expertise would harm our business.
We may need to raise additional funds in the future, which could be difficult to obtain or could dilute the value and rights of our common stock, and if not obtained in satisfactory amounts, may prevent us from developing our products, conducting clinical trials or otherwise taking advantage of future opportunities or growing our business, any of which could harm our business
We may need to raise additional funds through equity or public or private debt, and we cannot be certain that we will be able to obtain additional financing on favorable terms, if at all. The current worldwide financing environment is challenging, particularly for smaller capitalized businesses such as ours, which could make it more difficult for us to raise funds on reasonable terms, or at all. If we issue additional equity securities, our stockholders will experience dilution and the new equity securities may have rights, preferences or privileges senior to those of existing holders of common stock. If we raise funds through debt, we will have to pay interest and may be subject to restrictive covenants, which would restrict operating flexibility and could harm our business. If we cannot raise sufficient funds on acceptable terms, if and when needed, we may not be able to develop our products, conduct clinical trials, have the financial strength and leverage to negotiate favorable terms with potential marketing partners, market our products, take advantage of future opportunities, grow our business or respond to competitive pressures or unanticipated industry changes, any of which could harm our business.
If we engage in acquisitions, we will incur a variety of costs and the anticipated benefits of the acquisitions may never be realized
In the future, we may pursue acquisitions of companies, product lines, technologies or businesses that our management believes may be complementary or otherwise beneficial to us. Any of these acquisitions, if completed, could harm our business. Future acquisitions may result in substantial dilution to our stockholders, the expenditure of our current cash resources, the incurrence of additional debt and amortization expenses related to goodwill, research and development and other intangible assets. In addition, acquisitions would involve many risks for us, including, among others:
| | • | | assimilating employees, operations, technologies and products from the acquired companies with our existing employees, operations, technologies and products; |
| | • | | the potential need for additional funding to support our combined business; |
| | • | | diverting our management’s attention from day-to-day operation of our business; |
| | • | | entering markets in which we have no or limited direct experience; and |
| | • | | potentially losing key employees from the acquired companies. |
If we fail to adequately manage these risks we may not achieve the intended benefits from our acquisitions.
26
Our products are subject to government regulations and approvals which may delay or prevent the marketing of potential products and impose costly procedures upon our activities
The FDA and comparable agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon preclinical and clinical testing, manufacturing and marketing of pharmaceutical products. Lengthy and detailed preclinical and clinical testing, validation of manufacturing and quality control processes, and other costly and time-consuming procedures are required. Satisfaction of these requirements typically takes several years and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product. The effect of government regulation may be to delay or to prevent marketing of potential products for a considerable period of time and to impose costly procedures upon our activities. The FDA or any other regulatory agency may not grant approval on a timely basis, or at all, for any products we develop. Success in preclinical or early stage clinical trials does not assure success in later stage clinical trials. Data obtained from preclinical and clinical activities are susceptible to varying interpretations that could delay, limit or prevent regulatory approval. If regulatory approval of a product is granted, such approval may impose limitations on the indicated uses for which a product may be marketed. Further, even after we have obtained regulatory approval, later discovery of previously unknown problems with a product may result in restrictions on the product, including withdrawal of the product from the market. Moreover, the FDA has recently reduced previous restrictions on the marketing, sale and prescription of products for indications other than those specifically approved by the FDA. Accordingly, even if we receive FDA approval of a product for certain indicated uses, our competitors, including our collaborators, could market products for such indications even if such products have not been specifically approved for such indications. If the FDA determines regulatory approval is required, any delay in obtaining or failure to obtain regulatory approvals would make it difficult or impossible to market our products and would harm our business, prospects, financial condition, and results of operations.
The FDA’s policies may change and additional government regulations may be promulgated which could prevent or delay regulatory approval of our potential products. Moreover, increased attention to the containment of health care costs in the United States could result in new government regulations that could harm our business. Adverse governmental regulation might arise from future legislative or administrative action, either in the United States or abroad. See “Uncertainties regarding healthcare reform and third-party reimbursement may impair our ability to raise capital, form collaborations and sell our products.”
We have no experience in commercial manufacturing and if contract manufacturing is not available to us or does not satisfy regulatory requirements, we will have to establish our own regulatory compliant manufacturing capability and may not have the financial resources to do so
We have no experience manufacturing products for Phase 3 clinical trial and commercial purposes at our own facility. We have a pilot facility licensed by the State of California to manufacture a number of our products for Phase 1 and Phase 2 clinical trials but not for late stage clinical trials or commercial purposes. Therefore, we rely on a single contract manufacturer for a substantial portion of our manufacturing requirements. Any delays or difficulties that we may encounter in establishing and maintaining a relationship with qualified manufacturers to produce, package and distribute our products may harm our clinical trials, regulatory filings, market introduction and subsequent sales of our products.
Contract manufacturers must adhere to cGMP regulations that are strictly enforced by the FDA on an ongoing basis through the FDA’s facilities inspection program, as well as by foreign governmental associations outside the United States. Contract manufacturing facilities must pass a pre-approval plant inspection before the FDA will approve an NDA. Some of the material manufacturing changes that occur after approval are also subject to FDA review and clearance or approval. While the FDA has approved the AzaSite manufacturing process and facility, the FDA or other regulatory agencies may not approve the process or the facilities by which any of our other products may be manufactured or could rescind their approval of the AzaSite manufacturing process or facility. Our dependence on third parties to manufacture our products may harm our ability to develop
27
and deliver products on a timely and competitive basis. To the extent that we change manufacturers or engage additional manufacturers in the United States or abroad, we may experience delays, increased costs, quality-control issues and other issues that could harm our ability to conduct clinical trials and market and sell our products. Should we be required to manufacture products ourselves, we will:
| | • | | be required to expend significant amounts of capital to install a manufacturing capability; |
| | • | | be subject to the regulatory requirements described above; |
| | • | | be subject to similar risks regarding delays or difficulties encountered in manufacturing any such products; and |
| | • | | require substantially more additional capital than we otherwise may require. |
Therefore, we may not be able to manufacture any products successfully or in a cost-effective manner.
Uncertainties regarding healthcare reform and third-party reimbursement may impair our ability to raise capital, form collaborations and sell our products
The continuing efforts of governmental and third-party payers to contain or reduce the costs of healthcare through various means may harm our business. For example, in some foreign markets, the pricing or profitability of healthcare products is subject to government control. In the United States, there have been, and we expect there will continue to be, a number of federal and state proposals to implement similar government control. The implementation or even the announcement of any of these legislative or regulatory proposals or reforms could harm our business by reducing the prices we or our partners are able to charge for our products, impeding our ability to achieve profitability, raise capital or form collaborations. In addition, the availability of reimbursement from third-party payers determines, in large part, the demand for healthcare products in the United States and elsewhere. Examples of such third-party payers are government and private insurance plans. Significant uncertainty exists as to the reimbursement status of newly approved healthcare products and third-party payers are increasingly challenging the prices charged for medical products and services. If we or our partners succeed in bringing one or more products to the market, reimbursement from third-party payers may not be available or may not be sufficient to allow the sale of these products on a competitive or profitable basis.
Our insurance coverage may not adequately cover our potential product liability exposure
We are exposed to potential product liability risks inherent in the development, testing, manufacturing, marketing and sale of human therapeutic products. Product liability insurance for the pharmaceutical industry is expensive. Although we believe our current insurance coverage is adequate to cover likely claims we may encounter given our current stage of development and activities, our present product liability insurance coverage will not be adequate to cover all potential claims we may encounter, particularly as AzaSite is commercialized outside the United States and Canada. Once AzaSite is commercialized in other countries, we may have to increase our coverage, which will be expensive, and we may not be able to obtain or afford adequate insurance coverage against potential claims in sufficient amounts or at a reasonable cost.
Our use of hazardous materials may pose environmental risks and liabilities which may cause us to incur significant costs
Our research, development and manufacturing processes involve the controlled use of small amounts of hazardous solvents used in pharmaceutical development and manufacturing, including acetic acid, acetone, acrylic acid, calcium chloride, chloroform, dimethyl sulfoxide, ethyl alcohol, hydrogen chloride, nitric acid, phosphoric acid and other similar solvents. We retain a licensed outside contractor that specializes in the disposal of hazardous materials used in the biotechnology industry to properly dispose of these materials, but we cannot completely eliminate the risk of accidental contamination or injury from these materials. Our cost for the disposal
28
services rendered by our outside contractor was not material for the years ended 2011, 2010, or 2009, respectively. In the event of an accident involving these materials, we could be held liable for any damages that result and any such liability could exceed our resources. Moreover, as our business develops we may be required to incur significant costs to comply with federal, state and local environmental laws, regulations and policies, especially to the extent that we manufacture our own products.
Management and principal stockholders may be able to exert significant control on matters requiring approval by our stockholders
As of December 31, 2011, our management and principal stockholders (those owning more than 5% of our outstanding shares) together beneficially owned approximately 44% of our shares of common stock. As a result, our management and principal stockholders, acting together or individually, may be able to exert significant control on matters requiring approval by our stockholders, including the election of all or at least a majority of our Board of Directors, the approval of amendments to our charter, and the approval of business combinations and certain financing transactions. In September 2008, a group of our stockholders prevailed in a proxy contest that resulted in the replacement of all members of our Board of Directors.
The market prices for securities of biopharmaceutical and biotechnology companies such as ours have been and are likely to continue to be highly volatile due to reasons that are related and unrelated to our operating performance and progress; we have not paid dividends in the past and do not anticipate doing so in the future
The market prices for securities of biopharmaceutical and biotechnology companies, including ours, have been highly volatile. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. In addition, future announcements and circumstances, the status of our relationships or proposed relationships with third-party collaborators, the results of testing and clinical trials, future sales of equity or debt securities by us, the exercise of outstanding options and warrants that could result in dilution to our current holders of common stock, developments in our patents or other proprietary rights or those of our competitors, our own or Merck’s failure to meet analyst expectations, any litigation regarding the same, technological innovations or new therapeutic products, governmental regulation, or public concern as to the safety of products developed by us or others and general market conditions concerning us, our competitors or other biopharmaceutical companies may have a significant effect on the market price of our common stock. For example, in the twelve months ended December 31, 2011, our closing stock price fluctuated from a high of $0.93 to a low of $0.30. Such fluctuations can lead to securities class action litigation and make it difficult to obtain financing. Securities litigation against us could result in substantial costs and a diversion of our management’s attention and resources, which could have an adverse effect on our business.
We have not paid any cash dividends on our common stock and we do not anticipate paying any dividends on our common stock in the foreseeable future.
Our common stock was delisted from the New York Stock Exchange Alternext US
On April 21, 2009, our common stock was delisted from the NYSE Alternext US LLC (Exchange) for our failure to comply with the Exchange’s stockholders equity requirements. Our common stock currently trades on the over-the-counter bulletin board (OTCBB) market, although there are no assurances that it will continue to trade on this market. Over-the-counter (OTC) transactions involve risks in addition to those associated with transactions on a stock exchange. The delisting and OTC status could harm the trading volume and liquidity of our common stock and, as a result, the market price for our common stock might become more volatile. The delisting and OTC status could also cause a reduction in the number of investors willing or able to hold or acquire our common stock, transactions in our common stock could be delayed and securities analysts’ and news media coverage of us may be reduced. These factors could result in lower prices and larger spreads in the bid and ask prices for shares of common stock. Delisting and OTC status could also make our common stock
29
substantially less attractive as collateral for loans, for investment by potential financing sources under their internal policies or state and federal securities laws or as consideration in future capital raising transactions. Furthermore, the delisting and OTC status may have other negative implications, including the potential loss of confidence by suppliers, partners and employees. Our OTC status may also make it more difficult and expensive for us to comply with state and federal securities laws in connection with future financings, acquisitions or equity issuances to employees and other service providers, thereby making it more difficult and expensive for us to raise capital, acquire other businesses using our stock and compensate our employees using equity.
We have adopted and are subject to anti-takeover provisions that could delay or prevent an acquisition of our Company and could prevent or make it more difficult to replace or remove current management
Provisions of our certificate of incorporation and bylaws may constrain or discourage a third party from acquiring or attempting to acquire control of us. Such provisions could limit the price that investors might be willing to pay in the future for shares of our common stock. In addition, such provisions could also prevent or make it more difficult for our stockholders to replace or remove current management and could adversely affect the price of our common stock if they are viewed as discouraging takeover attempts, business combinations or management changes that stockholders consider in their best interest. Our Board of Directors has the authority to issue up to 5,000,000 shares of our preferred stock (Preferred Stock). Our Board of Directors has the authority to determine the price, rights, preferences, privileges and restrictions, including voting rights, of the unissued shares of Preferred Stock without any further vote or action by the stockholders. The rights of the holders of common stock will be subject to, and may be harmed by, the rights of the holders of any Preferred Stock that may be issued in the future. The issuance of Preferred Stock, while providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock, even if the transaction might be desired by our stockholders. Provisions of Delaware law applicable to us could also delay or make more difficult a merger, tender offer or proxy contest involving us, including Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years unless conditions set forth in the Delaware General Corporation Law are met. The issuance of Preferred Stock or Section 203 of the Delaware General Corporation Law could also be deemed to benefit incumbent management to the extent that these provisions deter offers by persons who would wish to make changes in management or exercise control over management. Other provisions of our certificate of incorporation and bylaws may also have the effect of delaying, deterring or preventing a takeover attempt or management changes that our stockholders might consider in their best interest. For example, our bylaws limit the ability of stockholders to remove directors and fill vacancies on our Board of Directors. Our bylaws also impose advance notice requirements for stockholder proposals and nominations of directors and prohibit stockholders from calling special meetings or acting by written consent.
If earthquakes and other catastrophic events strike, our business may be negatively affected
Our corporate headquarters, including our research and development and pilot plant operations, are located in the San Francisco Bay Area, a region known for seismic activity. A significant natural disaster such as an earthquake would have a material adverse impact on our business, results of operations, and financial condition. If we were able to use the equipment at our contract manufacturing site we could conduct our pilot plant operations although we would incur significant additional costs and delays in our product development timelines.
We face the risk of a decrease in our cash balances and losses in our investment portfolio
Our investment policy is structured to limit credit risk and manage interest rate risk. By policy, we only invest in what we view as very high quality debt securities, such as U.S. government securities. However, the recent uncertainties in the credit markets, including the recent downgrade by Standard & Poor’s of the U.S. debt rating, could negatively affect our ability to liquidate our positions in securities that we currently believe constitute high quality investments and could also result in the loss of some or all of our principal if the issuer of
30
such securities defaults on its credit obligations. Following completion of our $60.0 million financing on February 21, 2008 and our $22.2 million financing in July 2011, investment income has become a more substantial component of our income. The ability to achieve our investment objectives is affected by many factors, some of which are beyond our control. Our interest income will be affected by changes in interest rates, which are highly sensitive to many factors, including governmental monetary policies and domestic and international economic and political conditions. The outlook for our investment income is dependent on the future direction of interest rates and the amount of cash flows from operations, if any, that are available for investment. Any significant decline in our investment income or the value of our investments as a result of falling interest rates, deterioration in the credit of the securities in which we have invested or general market conditions, could harm our ability to liquidate our investments, our cash position and our income.
Sales of shares of common stock issued in our July 2011 financing may cause the market price of our common stock to decline
We issued 36,978,440 shares of common stock and warrants to purchase up to 14,791,376 shares of common stock in our July 2011 financing. We subsequently registered the resale of the shares of common stock issued in the transaction and issuable upon exercise of the warrants with the SEC and, therefore, an aggregate of 51,769,816 shares of common stock issued in the transaction and issuable upon exercise of the warrants may be freely sold in the open market. The sale of a significant amount of these shares of common stock in the open market, or the perception that these sales may occur, could cause the market price of our common stock to decline or become highly volatile.
We may have to pay liquidated damages to the Investors, which would increase our expenses and reduce our cash resources
In connection with the July 2011 financing, we entered into a registration rights agreement with the investors (Registration Rights Agreement). Under the terms of the Registration Rights Agreement, subject to certain limited exceptions, if we fail to keep the registration statement filed with the SEC effective within the time periods specified in the Registration Rights Agreement or we otherwise fail to comply with certain provisions set forth in the Registration Rights Agreement, we will be required to pay the investors, as liquidated damages, 1.0% of the amount invested for each 30-day period (or a pro rata portion thereof) during which such failure continues until the shares are sold or can be sold without restriction under Rule 144 promulgated under the Securities Act of 1933, as amended (Securities Act). There can be no assurance that the registration statement will remain effective for the time periods necessary to avoid payment of liquidated damages. Any payment of liquidated damages would increase our expenses, reduce our cash resources and may limit or preclude us from advancing our product candidates through clinical trials or otherwise growing our business.
31
| Item 1B. | Unresolved Staff Comments |
None.
We currently lease approximately 39,123 square feet of research laboratory and office space located in Alameda, California. The facility includes laboratories for formulation, analytical, microbiology, pharmacology, quality control and development as well as a pilot manufacturing plant. The lease expires on December 31, 2013, and may be renewed by us for an additional 5-year term. In October 2010, we subleased approximately 11,640 square feet of office space at our facility. The sublease expires on December 31, 2013. We believe our existing facilities will be suitable and adequate to meet our needs for the immediate future.
We are subject to various claims and legal actions during the ordinary course of our business. On November 30, 2009, a patent interference was declared before the Board of Patent Appeals and Interferences on certain U.S. patents covering AzaSite. Regents of the University assert that the inventions contained in these patents were made by a former employee of the University alone, and without collaboration with us. They are asserting that they own those inventions, and that they are entitled to an award of priority of invention and a judgment that the inventions are not patentable to us. On November 25, 2011, the USPTO entered judgment against the University. On December 23, 2011, the University filed a Notice of Appeal to the Court of Appeals for the Federal Circuit in Washington, D.C., and on January 4, 2012, we filed a Notice of Cross-Appeal. We continue to believe the University’s assertions are without merit and we will continue to vigorously defend our position.
We received the Notice Letter providing notice that Sandoz has filed an ANDA with the FDA seeking marketing approval for the Sandoz Product prior to the expiration of the five US patents listed in the Orange Books for AzaSite, which include four of our patents and one patent licensed to us by Pfizer. In the paragraph IV Certification accompanying the Sandoz ANDA filing, Sandoz alleges that the claims of the Orange Book listed patents are invalid, unenforceable and/or will not be infringed by the Sandoz Product. On May 26, 2011, we, Merck and Pfizer filed a patent infringement lawsuit against Sandoz and related entities. The plaintiff companies have agreed that Merck will take the lead in prosecuting this lawsuit. The filing of this lawsuit triggered an automatic stay, or bar, of the FDA’s approval of the ANDA for up to 30 months or until a final district court decision of the infringement lawsuit, whichever comes first. We and the other plaintiffs intend to vigorously enforce our patent rights relating to AzaSite and vigorously contest any Sandoz assertions that these patents are invalid or unenforceable.
We believe that there are currently no other claims or legal actions that would have a material adverse impact on our financial position, operations or potential performance.
| Item 4. | Mine Safety Procedures. |
Not applicable.
32
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Since April 21, 2009, our common stock has traded on the OTCBB market under the symbol “INSV.” OTC market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions. From September 30, 2008 to April 20, 2009, our common stock traded on The New York Stock Exchange Alternext US under the symbol “ISV.” From June 10, 1998 to September 29, 2008, our common stock was traded on The American Stock Exchange under the symbol “ISV”. The New York Stock Exchange Euronext acquired the American Stock Exchange on September 30, 2008. From our initial public offering on October 18, 1993 until June 9, 1998, our common stock traded on The Nasdaq National Market under the symbol “INSV.” Prior to our initial public offering, there was no public market for our common stock. The following table sets forth the high and low closing sales prices for our common stock as reported by the OTCBB or The New York Stock Exchange Alternext US for the periods indicated. These prices do not include retail mark-ups, mark-downs or commissions.
| | | | | | | | |
2011 | | High | | | Low | |
First Quarter | | | 0.60 | | | | 0.30 | |
Second Quarter | | | 0.93 | | | | 0.63 | |
Third Quarter | | | 0.70 | | | | 0.48 | |
Fourth Quarter | | | 0.54 | | | | 0.38 | |
| | |
2010 | | High | | | Low | |
First Quarter | | | 0.57 | | | | 0.34 | |
Second Quarter | | | 0.42 | | | | 0.30 | |
Third Quarter | | | 0.36 | | | | 0.25 | |
Fourth Quarter | | | 0.37 | | | | 0.28 | |
Dividends
We have never declared or paid dividends on our common stock and do not anticipate paying any cash dividends in the foreseeable future. It is the present policy of our Board of Directors to retain our earnings, if any, for the development of our business.
Other Information
Information regarding employee stock-based compensation is provided in Note 11 in the Notes to the Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K. The remaining information required by this Item will be included in our Proxy Statement and such required information is incorporated herein by reference.
As of February 27, 2012, we had approximately 171 stockholders of record of our Common Stock. On February 27, 2012, the last sale price reported on the OTCBB for our common stock was $0.44 per share.
33
Stock Performance Graph
The following graph compares the percentage change in (i) the cumulative total stockholder return on our common stock from December 31, 2006 through December 31, 2011 with (ii) the cumulative total return on (a) the American Stock Exchange (U.S. Index) and (b) the American Stock Exchange (biotech) index. The comparison assumes (i) an investment of $100 on December 31, 2006 in each of the foregoing indices and (ii) reinvestment of dividends, if any.
The stock price performance shown on the graph below represents historical price performance and is not necessarily indicative of any future stock price performance.
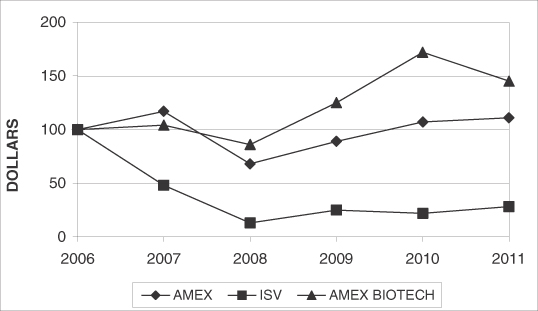
| | | | | | | | | | | | |
| | | AMEX | | | ISV | | | AMEX
BIOTECH | |
12/31/06 | | | 100 | | | | 100 | | | | 100 | |
12/31/07 | | | 117 | | | | 48 | | | | 104 | |
12/31/08 | | | 68 | | | | 13 | | | | 86 | |
12/31/09 | | | 89 | | | | 25 | | | | 125 | |
12/31/10 | | | 107 | | | | 22 | | | | 172 | |
12/31/11 | | | 111 | | | | 28 | | | | 145 | |
Notwithstanding anything to the contrary set forth in any of our previous filings under the Securities Act or the Securities Exchange Act of 1934, as amended (Exchange Act) which might incorporate any of our future filings made under those statutes, the preceding Stock Performance Graph will not be incorporated by reference into any of those prior filings, nor will such graph be incorporated by reference into any of our future filings made under those statues.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Securities
None.
34
| Item 6. | Selected Financial Data |
The comparability of the following selected financial data is affected by a variety of factors, and this data is qualified by reference to and should be read in conjunction with the audited financial statements and notes thereto and the Management’s Discussion and Analysis of Financial Condition and Results of Operations contained elsewhere in this Annual Report on Form 10-K. The following table sets forth selected financial data for us for the five years ended December 31, 2011 (in thousands except per share amounts):
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
(in thousands, except per share data) | | 2011 | | | 2010 | | | 2009 | | | 2008 | | | 2007 | |
Statement of Operations Data | | | | | | | | | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | | | | | | | | | |
Royalties | | $ | 15,138 | | | $ | 11,120 | | | $ | 8,000 | | | $ | 3,596 | | | $ | 701 | |
Licensing fee, milestone amortization and other | | | 785 | | | | 747 | | | | 1,798 | | | | 10,110 | | | | 23,060 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenues | | | 15,923 | | | | 11,867 | | | | 9,798 | | | | 13,706 | | | | 23,761 | |
| | | | | | | | | | | | | | | | | | | | |
Expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 7,337 | | | | 4,974 | | | | 5,436 | | | | 16,242 | | | | 10,384 | |
General and administrative | | | 5,645 | | | | 4,511 | | | | 5,792 | | | | 8,251 | | | | 6,760 | |
Cost of revenues, principally royalties to third parties | | | 1,917 | | | | 1,727 | | | | 1,549 | | | | 630 | | | | 982 | |
Severance | | | — | | | | — | | | | 527 | | | | 1,909 | | | | — | |
Impairment of property and equipment | | | — | | | | — | | | | 615 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total expenses | | | 14,899 | | | | 11,212 | | | | 13,919 | | | | 27,032 | | | | 18,126 | |
Interest expense and other, net | | | (10,167 | ) | | | (10,248 | ) | | | (10,034 | ) | | | (7,984 | ) | | | (100 | ) |
Change in fair value of warrant liability | | | 2,201 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (6,942 | ) | | $ | (9,593 | ) | | $ | (14,155 | ) | | $ | (21,310 | ) | | $ | 5,535 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | |
Income (loss) per share—basic | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | (0.15 | ) | | $ | (0.23 | ) | | $ | 0.06 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) per share—diluted | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | (0.15 | ) | | $ | (0.23 | ) | | $ | 0.06 | |
| | | | | | | | | | | | | | | | | | | | |
Weighted average shares used in per share calculation: | | | | | | | | | | | | | | | | | | | | |
—Basic | | | 111,769 | | | | 94,774 | | | | 94,710 | | | | 94,607 | | | | 94,168 | |
| | | | | | | | | | | | | | | | | | | | |
—Diluted | | | 111,769 | | | | 94,774 | | | | 94,710 | | | | 94,607 | | | | 100,110 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
(in thousands) | | 2011 | | | 2010 | | | 2009 | | | 2008 | | | 2007 | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 26,395 | | | $ | 16,468 | | | $ | 24,721 | | | $ | 37,456 | | | $ | 11,532 | |
Working capital, exclusive of deferred revenues | | | 14,303 | | | | 14,104 | | | | 22,816 | | | | 35,068 | | | | 9,589 | |
Total assets | | | 32,401 | | | | 23,586 | | | | 32,246 | | | | 44,943 | | | | 15,012 | |
Non-recourse secured notes payable | | | 58,558 | | | | 60,000 | | | | 60,000 | | | | 60,000 | | | | — | |
Accumulated deficit | | | (199,527 | ) | | | (192,585 | ) | | | (182,992 | ) | | | (168,837 | ) | | | (147,527 | ) |
Total stockholders’ equity (deficit) | | $ | (34,539 | ) | | $ | (42,220 | ) | | $ | (33,033 | ) | | $ | (19,506 | ) | | $ | 746 | |
No cash dividends have been declared or paid by us since our inception.
35
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion should be read in conjunction with the financial statements and notes thereto included in Item 8 of this Annual Report on Form 10-K.
Overview
We are an ophthalmic product development company advancing ophthalmic pharmaceutical products to address unmet eye care needs. Our current portfolio of products is based on our proprietary DuraSite® sustained drug delivery technology.
Our DuraSite® sustained drug delivery technology is a proven synthetic polymer-based formulation designed to extend the residence time of a drug relative to conventional topical therapies. It enables topical delivery of a drug as a solution, gel or suspension and can be customized for delivering a wide variety of drug candidates. We have focused our research and development and commercial support efforts on the following topical products formulated with our DuraSite® drug delivery technology. We may also utilize our DuraSite technology platform for the formulation of new ocular product candidates using either non-proprietary drugs or compounds originally developed by others for non-ophthalmic indications.
| | • | | AzaSite® (azithromycin ophthalmic solution) 1% is a DuraSite formulation of azithromycin, a broad spectrum ocular antibiotic approved by the FDA in April 2007 to treat bacterial conjunctivitis (pink eye). It was commercialized in the United States by Inspire beginning in August 2007. The key advantages of AzaSite are a significantly reduced dosing regimen leading to better compliance and outcome, with a broad spectrum antibiotic and a lowered probability of bacterial resistance based on high tissue concentration. On May 16, 2011, Merck acquired Inspire and Inspire became a wholly-owned subsidiary of Merck. Merck is now responsible for commercializing AzaSite in North America. We receive a 25% royalty on net sales of AzaSite in North America. |
| | • | | Besivance® (besifloxacin ophthalmic suspension) 0.6% is a DuraSite formulation of besifloxacin, a broad spectrum ocular antibiotic approved by the FDA in May 2009 to treat bacterial conjunctivitis (pink eye). An advantage of Besivance is a faster rate of resolution of the infection that may reduce the duration of the illness and reduce the chances of infecting others. Besivance was developed by Bausch & Lomb and launched in the United States in the second half of 2009. In 2011, Besivance was launched internationally in select countries. We receive a middle single-digit royalty on net sales of Besivance globally. |
| | • | | AzaSite PlusTM (ISV-502) is a fixed combination of azithromycin and dexamethasone in DuraSite for the treatment of ocular inflammation and infection (blepharitis and/or blepharoconjunctivitis) for which there is no FDA approved indicated treatment. We completed a Phase 3 trial in November 2008 and AzaSite Plus was very well tolerated. Although efficacious, the trial did not achieve its primary clinical endpoint as defined by the previous protocol. We discussed the results of this trial with the FDA and determined a new development plan for this product candidate. In May 2011, we reached an agreement with the FDA on a SPA for the design of a Phase 3 clinical trial of AzaSite Plus in patients with blepharitis. A SPA is a written agreement with the FDA that the study design and planned analysis of the sponsor’s Phase 3 clinical trial adequately addresses the objectives necessary to support a regulatory submission. We initiated a new Phase 3 clinical trial for this product candidate in the fourth quarter of 2011. |
| | • | | DexaSiteTM (ISV-305) is a DuraSite formulation of dexamethasone in development for the treatment of ocular inflammation. We have met with the FDA to discuss the development pathway for this product candidate. DexaSite is included in the Phase 3 clinical trial SPA for AzaSite Plus. We initiated a Phase 3 clinical trial for this product candidate in the fourth quarter of 2011. |
| | • | | BromSiteTM (ISV-303) is a DuraSite formulation of bromfenac in development for the treatment of post-operative inflammation and eye pain. We initiated a Phase 1/2 clinical trial for this product candidate in August 2010 and completed patient enrollment in December 2010. In the first quarter of |
36
| | 2011, we received positive top-line results from this study which demonstrated the efficacy and safety of BromSite. In the third quarter of 2011, we completed an additional Phase 2 clinical trial to investigate the PK of BromSite in humans. We received positive top- line results that showed that the mean concentration of bromfenac in the aqueous humor of patients using BromSite was more than double compared to the currently available bromfenac eye product. We have discussed the design of the Phase 3 clinical trial with the FDA. We plan to start a Phase 3 clinical trial for this product candidate in mid-2012. |
| | • | | ISV-101 is a DuraSite formulation with a low concentration of bromfenac for the treatment of dry eye disease. We filed an IND for this product candidate in the first quarter of 2011. We anticipate a Phase 1/2 clinical trial for this product candidate. |
Major Developments and Events in 2011
Our major developments and events in 2011 included:
| | • | | In November 2011, we initiated a Phase 3 clinical trial for AzaSite Plus and DexaSite. |
| | • | | In July 2011, we received $22.2 million in gross proceeds ($20.4 million, net) from a private placement financing transaction; |
| | • | | In May 2011, we received an SPA from the FDA for the Phase 3 clinical trial of AzaSite Plus and DexaSite; |
| | • | | In May 2011, Merck acquired Inspire and became our commercial partner under the Merck License for AzaSite; |
| | • | | In January 2011 and October 2011, we received positive top-line results from the Phase 1/2 clinical trial and Phase 2 PK study for BromSite, respectively; and |
| | • | | In January 2011, we filed an IND for ISV-101. |
Business Strategy
Our business strategy consists of the following:
| | 1. | Develop our pipeline of ocular product candidates.We seek to identify new product candidates from proven drugs that can be improved by formulation in DuraSite, which substantially reduces the clinical risk in these product candidates. We plan to conduct preclinical and clinical testing of our portfolio product candidates. |
| | 2. | Monetize our product candidates.At the appropriate time, we seek to partner with larger pharmaceutical companies to possibly complete the clinical development of, and to manufacture and market, these products. Partnering agreements generally include upfront and milestone payments, as well as on-going royalty payments upon commercialization, payable to us. |
Critical Accounting Policies and Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States requires us to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
We believe the following policies to be the most critical to an understanding of our financial condition and results of operations because they require us to make significant estimates, assumptions and judgments about matters that are uncertain:
Revenue Recognition. We recognize revenue when four basic criteria are met: persuasive evidence of an arrangement exists; delivery has occurred or services have been rendered; the fee is fixed or determinable; and collectability is reasonably assured. We have arrangements with multiple revenue-generating elements. We
37
analyze our multiple element arrangements to determine whether the elements can be separated and accounted for individually as separate units of accounting. An item can generally be considered a separate unit of accounting if both of the following criteria are met: the delivered item(s) has value to the customer on a stand-alone basis and if the arrangement includes a general right of return and delivery or performance of the undelivered item(s) is considered probable and substantially in our control. Items that cannot be divided into separate units are combined with other units of accounting, as appropriate. Consideration received is allocated among the separate units based on the unit’s estimated selling price and is recognized in full when the criteria are met. We deem service to be rendered if no continuing obligation exists on our part.
Our revenues are primarily related to royalties on product sales and licensing agreements, and such agreements may provide for various types of payments, including upfront payments, research funding and related fees during the terms of the agreements, milestone payments based on the achievement of established development objectives and licensing fees.
We receive royalties from licensees based on third-party sales. The royalties are recorded as earned in accordance with the contract terms when third-party results are reliably measured and collectability is reasonably assured.
Revenue associated with non-refundable up-front license fees under arrangements where the license fees cannot be accounted for as separate units of accounting is deferred and recognized as revenue on a straight-line basis over the expected term of our continued involvement. Revenues from the achievement of milestones are recognized as revenue when the milestones are achieved and the milestone payments are due and collectible.
For the year ended December 31, 2011, we adopted amendments to the accounting standard related to multiple-deliverable revenue arrangements. The amendment requires entities to allocate revenue in multiple-deliverable revenue arrangements using estimated selling prices of the delivered goods and services based on a selling price hierarchy. The amendment eliminated the residual method of revenue allocation and requires revenue to be allocated using the relative selling price method. The adoption of this amendment did not impact our consolidated financial position or results of operations since we did not enter into or materially modify revenue arrangements during the year ended December 31, 2011. In addition, there have been no significant changes to the units of accounting, the allocation of consideration received to the various units of accounting, and the timing of revenue recognition based on this amendment. We do not expect the adoption of this amendment to have a material impact on future periods.
Income Taxes. We account for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Realization of deferred tax assets is dependent upon future earnings, the timing and amount of which are uncertain.
We utilize a two-step approach to recognize and measure uncertain tax positions, if any. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained upon tax authority examination, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement.
Stock-Based Compensation. We granted stock-based awards to eligible employees and directors to purchase shares of our common stock under our stock compensation plan approved in 1994 (1994 Plan) and its successor the 2007 Performance Incentive Plan (2007 Plan). In addition, we have a qualified employee stock purchase plan (Purchase Plan) in which eligible employees may elect to withhold up to 15% of their compensation to purchase shares of our common stock on a quarterly basis at a discounted price equal to 85% of the lower of the employee’s offering price or the closing price of the stock on the date of purchase. In August 2009, the Purchase
38
Plan was suspended. The benefits provided by these plans qualify as stock-based compensation which requires us to recognize compensation expense based on their estimated fair values determined on the date of grant for all stock-based awards granted, modified or cancelled.
We estimate the fair value of share-based awards on the date of grant using the Black-Scholes option-pricing method (Black-Scholes method). The determination of fair value of share-based awards using an option-pricing model requires the use of certain estimates and assumptions that affect the reported amount of share-based compensation cost recognized in our Consolidated Statements of Income. These include estimates of the expected term of share-based awards, expected volatility of our stock price, expected dividends and the risk-free interest rate. These estimates and assumptions are highly subjective and may result in materially different amounts should circumstances change and we employ different assumptions in future periods.
For stock-based awards issued, we estimated the expected term by considering various factors including the vesting period of options granted and employees’ historical exercise and post-employment termination behavior. Our estimated volatility was derived using our historical stock price volatility. We have never declared or paid any cash dividends on our common stock and currently do not anticipate paying such cash dividends. We currently anticipate that we will retain all of our future earnings for use in the development and expansion of our business and for general corporate purposes. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and will depend upon our results of operations, financial condition, financial covenants, tax laws and other factors as the Board of Directors, in its discretion, deems relevant. The risk-free interest rate is based upon U.S. Treasury securities with remaining terms similar to the expected term of the stock-based awards.
Results of Operations
Revenues
We had total revenues of $15.9 million, $11.9 million, and $9.8 million for the years ended December 31, 2011, 2010 and 2009, respectively.
In 2011, our revenues primarily consisted of $13.9 million of royalties from net sales of AzaSite by Merck (formerly Inspire prior to its acquisition on May 16, 2011) compared to $10.7 million in 2010. This increase was primarily driven by a $3.9 million minimum royalty true-up payment for AzaSite by Merck for the fiscal year ended September 30, 2011, the measurement period pursuant to the terms of the Merck License. The increase was offset by a 6% decrease in AzaSite net sales. Revenues in 2011 also included $1.2 million of royalties from net sales of Besivance by Bausch & Lomb compared to $0.5 million in 2010. Revenues in 2011 also included $0.5 million from the sale of azithromycin to Merck and certain international partners under supply agreements and $0.3 million from the amortization and recognition of international license fee payments for AzaSite.
In 2010, our revenues primarily consisted of $10.7 million of royalties from net sales of AzaSite by Merck compared to $8.0 million in 2009. The $2.7 million increase in royalty revenues from the prior year was primarily due to a 22% increase of AzaSite net sales in the United States. In addition, the royalty rate for AzaSite increased from 20% to 25% in July 2009. 2010 revenues also included $0.5 million of royalties from net sales of Besivance by Bausch & Lomb, $0.5 million of grant income under the Therapeutic Discovery Program and $0.2 million from the sale of materials to Merck under the Supply Agreement.
In 2009, our revenues primarily consisted of $8.0 million of royalties from net sales of AzaSite by Merck compared to $3.6 million in 2008. The $4.4 million increase in royalty revenues from the prior year was primarily due to a 91% increase of AzaSite net sales and the royalty rate increase for AzaSite in July 2009. 2009 revenues also included $1.4 million of international AzaSite license fees. The remainder of our 2009 revenues represented sales of materials to Merck under the Supply Agreement and contract services provided to Merck related to their AzaSite activities.
39
Research and development.
Our research and development activities can be separated into two major segments, research and clinical development. Research includes activities involved in evaluating a potential product, related preclinical testing and manufacturing. Clinical development includes activities related to filings with the FDA and the related human clinical testing required to obtain marketing approval for a potential product. We estimate that the following represents the approximate cost of these activities for 2011, 2010 and 2009 (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
Research | | $ | 4,468 | | | $ | 3,137 | | | $ | 4,407 | |
Clinical development | | | 2,869 | | | | 1,837 | | | | 1,029 | |
| | | | | | | | | | | | |
Total research and development | | $ | 7,337 | | | $ | 4,974 | | | $ | 5,436 | |
| | | | | | | | | | | | |
Our research and development expenses by program for 2011, 2010 and 2009 (in thousands) were:
| | | | | | | | | | | | |
Program | | 2011 | | | 2010 | | | 2009 | |
AzaSite Plus/DexaSite | | $ | 1.6 | | | $ | 0.1 | | | $ | 0.2 | |
BromSite | | | 1.0 | | | | 1.3 | | | | 0.2 | |
New products and other | | | 0.7 | | | | 0.7 | | | | 0.2 | |
Programs-non-specific | | | 4.0 | | | | 2.9 | | | | 4.8 | |
| | | | | | | | | | | | |
Total | | $ | 7.3 | | | $ | 5.0 | | | $ | 5.4 | |
| | | | | | | | | | | | |
Research and development expenses were $7.3 million in 2011. In 2011, program expenses primarily consisted of non-specific program costs which comprised facility, internal personnel and stock-based compensation costs that are not allocated to a specific development program. Our non-specific program costs increased in 2011 compared to 2010, primarily due to an increase in headcount. Our AzaSite Plus/DexaSite program expenses primarily related to costs for a new Phase 3 clinical trial that we initiated in the fourth quarter of 2011. Our BromSite program expenses primarily related to the Phase 1/2 clinical trial that concluded in the first quarter of 2011 and the Phase 2 PK Study performed in the third quarter of 2011. In addition, we continue to develop new product candidates.
Research and development expenses were $5.0 million in 2010. In 2010, our program expenses primarily consisted of non-specific program costs which comprised facility, internal personnel and stock-based compensation costs that are not allocated to a specific development program. The non-specific costs decreased in 2010 compared to 2009, primarily due to savings resulting from our corporate restructuring in March 2009. Our BromSite program expenses primarily related to preclinical experiments and the Phase 1/2 clinical trial that was initiated in August 2010.
Research and development expenses were $5.4 million in 2009. In 2009, our activities primarily consisted of non-specific program costs which comprised facility, internal personnel, and stock-based compensation costs that are not allocated to a specific development program. Our AzaSite Plus/DexaSite program expenses in 2009 consisted primarily of ongoing consulting and data analysis for our Phase 3 clinical trial.
Our future research and development expenses will depend on the results and magnitude or scope of our clinical, preclinical and research activities and requirements imposed by regulatory agencies. Accordingly, our research and development expense may fluctuate significantly from period to period. In addition, if we in-license or out-license rights to product candidates, our research and development expenses may fluctuate significantly from prior periods.
40
General and administrative.
General and administrative expenses increased to $5.6 million in 2011 from $4.5 million in 2010. In 2011, we incurred higher personnel-related costs due to an increase in headcount and higher legal expenses pertaining to patent litigation with the Regents of the University and Sandoz.
General and administrative expenses decreased to $4.5 million in 2010 from $5.8 million in 2009. This decrease was primarily due to lower personnel-related expenses as a result of our March 2009 corporate restructuring.
Cost of revenues.
Our cost of revenues were $1.9 million, $1.7 million and $1.5 million for 2011, 2010 and 2009, respectively. Cost of revenues were primarily comprised of royalties accrued for third parties, including Pfizer, based on AzaSite net sales from Merck. Cost of revenues also includes the cost of the azithromycin supplied to Merck and international partners under supply agreements.
Severance.
Severance expenses were $0.5 million in 2009 and were associated with our corporate restructurings announced in December 2008 and March 2009. The restructuring plan reduced our personnel by 39 employees. No severance expenses were incurred in 2011 or 2010.
Impairment of property and equipment.
Impairment expenses were $0.6 million in 2009 and were associated with our corporate restructuring announced in March 2009. We impaired laboratory and other equipment, leasehold improvements, and furniture and fixtures located at our corporate headquarters. No impairment expenses were incurred in 2011 or 2010.
Interest expense and other, net.
Interest expense and other, net, was an expense of $10.2 million, $10.2 million and $10.0 million for 2011, 2010 and 2009, respectively. Interest expense was primarily due to the interest expense on the AzaSite Notes issued in February 2008 and related amortization of the debt issuance costs incurred from our issuance of the AzaSite Notes.
Change in fair value of warrant liability.
Change in fair value of warrant liability was income of $2.2 million for 2011. The income resulted from a decrease in the fair value of our warrant liability that was initially valued as of July 18, 2011 and revalued as of December 31, 2011. The decrease in fair value was primarily driven by a decrease in our stock price.
Liquidity and Capital Resources
In recent years, we have financed our operations primarily through private placements of equity securities, debt financings and payments from corporate collaborations. At December 31, 2011 and 2010, our cash, cash equivalents and short-term investments were $26.4 million and $16.5 million, respectively. It is our policy to invest our cash and cash equivalents in highly liquid securities, such as interest-bearing money market funds, treasury and federal agency notes. The current uncertain credit markets may affect the liquidity of such money market funds or other cash investments.
Cash used in operating activities was $8.9 million, $8.1 million and $12.7 million for 2011, 2010 and 2009, respectively. The increase in 2011 compared to 2010 was primarily due to higher personnel costs related to an increase in headcount and costs incurred to prepare for clinical trials related to AzaSite Plus, DexaSite, BromSite
41
and ISV-101. This increase was offset by a $3.9 million AzaSite minimum royalty true-up payment received from Merck. The decrease in 2010 compared to 2009 primarily resulted from lower costs as a result of our corporate restructuring in 2009 and an increase in royalty revenue.
Cash used in investing activities was $19.7 million and $17.5 million for 2011 and 2009, respectively. Cash provided by investing activities was $12.4 million for 2010. In 2011 and 2009, we invested $19.5 million and $17.5 million, respectively, in short-term investments and converted $12.5 million back to cash and cash equivalents in 2010.
Cash provided by financing activities was $19.0 million and $16,000 for 2011 and 2010, respectively. Cash used in financing activities was $7,000 for 2009. In July 2011, we completed a private placement financing transaction in which we sold shares of common stock and warrants to purchase shares of common stock, which resulted in approximately $20.4 million in net proceeds to us after deducting placement agent fees, legal, accounting and other costs associated with the transaction. We intend to use the net proceeds of the transaction to fund clinical trials and for general corporate purposes, including working capital.
The tables below set forth the amount of cash that we raised for fiscal years 2011 through 2009 from equity financings and option exercises.
Cash Received from Private Placements of Equity Securities
| | | | |
Date | | Net Proceeds | | Shares of Common Stock Issued |
July 2011 | | $20.4 million | | 37.0 million plus warrants to purchase 14.8 million shares |
Cash received from Option Exercises
| | | | |
Year | | Net Proceeds | |
2011 | | $ | 58,000 | |
2010 | | $ | 21,000 | |
2009 | | $ | 9,000 | |
Our future capital expenditures and requirements will depend on numerous factors, including the progress of our clinical testing, research and development programs and preclinical testing, the time and costs involved in obtaining regulatory approvals, our ability to successfully commercialize any products that we may launch in the future, our ability to establish collaborative arrangements, the cost of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights, competing technological and market developments, changes in our existing collaborative and licensing relationships, acquisition of new businesses, products and technologies, the completion of commercialization activities and arrangements, and the purchase of additional property and equipment.
We anticipate no material capital expenditures to be incurred for environmental compliance in fiscal year 2012. Based on our environmental compliance record to date, and our belief that we are current in compliance with applicable environmental laws and regulations, environmental compliance is not expected to materially harm our operations.
42
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future material effect.
Contractual Obligations
The following table summarizes our significant contractual obligations as of December 31, 2011 and the effect such obligations are expected to have on our liquidity and cash flows in future periods. Some of these amounts are based on management’s estimates and assumptions about these obligations including their duration, the possibility of renewal and other factors. Because these estimates are necessarily subjective, our actual payments in the future may vary from those listed in this table.
| | | | | | | | | | | | | | | | | | | | |
| | | Payments due by period
(in thousands) | |
| | | Total | | | Less than
1 year | | | 1-3
years | | | 3-5
Years | | | More than
5 years | |
Purchase obligations (1) | | $ | 5,763 | | | $ | 5,763 | | | $ | — | | | $ | — | | | $ | — | |
Facilities lease obligations (2) | | | 1,670 | | | | 822 | | | | 848 | | | | — | | | | — | |
Licensing agreement obligations (3) | | | 8,186 | | | | 1,260 | | | | 2,380 | | | | 2,240 | | | | 2,306 | |
Secured notes payable (4) | | | 58,558 | | | | 6,286 | | | | 9,344 | | | | — | | | | 42,928 | |
Interest payments on secured notes payable (5) | | | 53,909 | | | | 9,453 | | | | 15,265 | | | | 13,737 | | | | 15,454 | |
| | | | | | | | | | | | | | | | | | | | |
Total commitments | | $ | 128,086 | | | $ | 23,584 | | | $ | 27,837 | | | $ | 15,977 | | | $ | 60,688 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Purchase obligations include commitments related to clinical development. |
| (2) | We lease our facilities under a non-cancelable operating lease that expires in 2013. |
| (3) | We have entered into certain license agreements that require us to make royalty payments for the life of the licensed patents. The estimated royalties due under certain of these agreements are as noted for 2012 through 2019. |
| (4) | Principal repayments are limited to royalties received from Merck from net sales of AzaSite in the United States and Canada. When the AzaSite royalties received for any quarter exceed the interest payments and certain expenses due that quarter, the excess will be applied to the repayment of principal of the notes until the notes have been paid in full. Future payments represent an estimate of expected principal repayments based on minimum royalty income covered by the agreement and future projected sales. The note is due in 2019. |
| (5) | Interest repayments represent an estimate based on expected principal repayments. |
Recent Accounting Pronouncements
In May 2011, the Financial Accounting Standards Board issued Accounting Standards Update No. 2011-04 (ASU 2011-04), Fair Value Measurement—Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. Generally Accepted Accounting Principles (U.S. GAAP) and International Financial Reporting Standards (IFRS). The amendments in this update will ensure that fair value has the same meaning in U.S. GAAP and in IFRS and that their respective fair value measurement and disclosure requirements are the same. This update is effective prospectively for interim and annual periods beginning after December 15, 2011. Early adoption by public entities is not permitted and, therefore, we are required to adopt ASU 2011-04 on January 1, 2012. We are currently evaluating the impact to our financial position and results of operations. We expect that the adoption of this amendment will not materially impact our consolidated financial position or results of operations.
43
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
The following discusses our exposure to market risk related to changes in interest rates.
We have debt with fixed interest rates. As a result, our exposure to market risk caused by fluctuations in interest rates is minimal. Our borrowings outstanding as of December 31, 2011 were $58.6 million and the interest rate was 16%. If the market interest rates increased by 10% from the December 31, 2011 levels, it would not result in an increase in interest expense. As of December 31, 2011, the face value of our debt was estimated to be approximately the fair value based on current market rates.
The securities in our investment portfolio are not leveraged and are subject to minimal interest rate risk. Due to their original maturities of twelve months or less, the securities are classified as cash and cash equivalents or short-term investments. They are classified as trading securities principally bought and held for the purpose of selling them in the near term, with unrealized gains and losses included in earnings. Because of the short-term maturities of our investments, we do not believe that a change in market rates would have a significant negative impact on the value of our investment portfolio. While a hypothetical decrease in market interest rates by 10 percent from the December 31, 2011 levels would cause a decrease in interest income, it would not result in loss of principal.
The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve this objective in the current economic environment, we maintain our portfolio in cash equivalents or short-term investments, including obligations of U.S. government-sponsored enterprises and money market funds. These securities are classified as cash and cash equivalents or short-term investments and consequently are recorded on the balance sheet at fair value. We do not utilize derivative financial instruments to manage our interest rate risks.
44
| Item 8. | Financial Statements and Supplementary Data |
The following Consolidated Financial Statements and Report of Independent Registered Public Accounting Firm are included on the pages that follow:
45
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
InSite Vision Incorporated
We have audited the accompanying consolidated balance sheets of InSite Vision Incorporated and subsidiaries (the “Company”) as of December 31, 2011 and 2010, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor have we been engaged to perform, an audit of the Company’s internal control over financial reporting. An audit includes consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of InSite Vision Incorporated as of December 31, 2011 and 2010, and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2011, in conformity with accounting principles generally accepted in the United States of America.
/s/ Burr Pilger Mayer, Inc.
San Francisco, California
March 5, 2012
46
INSITE VISION INCORPORATED
CONSOLIDATED BALANCE SHEETS
| | | | | | | | |
| | | December 31, | |
(in thousands, except share data) | | 2011 | | | 2010 | |
| ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 1,900 | | | $ | 11,469 | |
Short-term investments | | | 24,495 | | | | 4,999 | |
Accounts receivable, net | | | 2,564 | | | | 3,352 | |
Prepaid expenses and other current assets | | | 12 | | | | 15 | |
| | | | | | | | |
Total current assets | | | 28,971 | | | | 19,835 | |
| | |
Property and equipment, net | | | 345 | | | | 247 | |
| | |
Debt issuance costs, net | | | 3,085 | | | | 3,504 | |
| | | | | | | | |
Total assets | | $ | 32,401 | | | $ | 23,586 | |
| | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 703 | | | $ | 332 | |
Accrued liabilities | | | 411 | | | | 511 | |
Accrued compensation and related expense | | | 978 | | | | 620 | |
Accrued royalties | | | 964 | | | | 892 | |
Accrued interest | | | 1,171 | | | | 3,376 | |
Non-recourse secured notes payable, current | | | 6,286 | | | | — | |
Deferred revenues | | | — | | | | 75 | |
Warrant liability | | | 4,155 | | | | — | |
| | | | | | | | |
Total current liabilities | | | 14,668 | | | | 5,806 | |
Non-recourse secured notes payable | | | 52,272 | | | | 60,000 | |
| | | | | | | | |
Total liabilities | | | 66,940 | | | | 65,806 | |
| | | | | | | | |
Commitments and contingencies | | | | | | | | |
Stockholders’ deficit: | | | | | | | | |
Preferred stock, $0.01 par value, 5,000,000 shares authorized, none issued and outstanding | | | — | | | | — | |
Common stock, $0.01 par value, 240,000,000 shares authorized; 131,951,033 and 94,822,593 shares issued and outstanding at December 31, 2011 and 2010, respectively | | | 1,320 | | | | 948 | |
Additional paid-in capital | | | 163,668 | | | | 149,417 | |
Accumulated deficit | | | (199,527 | ) | | | (192,585 | ) |
| | | | | | | | |
Total stockholders’ deficit | | | (34,539 | ) | | | (42,220 | ) |
| | | | | | | | |
Total liabilities and stockholders’ deficit | | $ | 32,401 | | | $ | 23,586 | |
| | | | | | | | |
See accompanying notes to consolidated financial statements.
47
INSITE VISION INCORPORATED
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
(in thousands, except per share data) | | 2011 | | | 2010 | | | 2009 | |
Revenues: | | | | | | | | | | | | |
Royalties | | $ | 15,138 | | | $ | 11,120 | | | $ | 8,000 | |
Licensing fee and milestone amortization | | | 275 | | | | — | | | | 1,423 | |
Other product and service revenues | | | 510 | | | | 747 | | | | 375 | |
| | | | | | | | | | | | |
Total revenues | | | 15,923 | | | | 11,867 | | | | 9,798 | |
| | | | | | | | | | | | |
Expenses: | | | | | | | | | | | | |
Research and development | | | 7,337 | | | | 4,974 | | | | 5,436 | |
General and administrative | | | 5,645 | | | | 4,511 | | | | 5,792 | |
Cost of revenues, principally royalties to third parties | | | 1,917 | | | | 1,727 | | | | 1,549 | |
Severance | | | — | | | | — | | | | 527 | |
Impairment of property and equipment | | | — | | | | — | | | | 615 | |
| | | | | | | | | | | | |
Total expenses | | | 14,899 | | | | 11,212 | | | | 13,919 | |
| | | | | | | | | | | | |
Income (loss) from operations | | | 1,024 | | | | 655 | | | | (4,121 | ) |
Interest expense and other, net | | | (10,167 | ) | | | (10,248 | ) | | | (10,034 | ) |
Change in fair value of warrant liability | | | 2,201 | | | | — | | | | — | |
| | | | | | | | | | | | |
Net loss | | $ | (6,942 | ) | | $ | (9,593 | ) | | $ | (14,155 | ) |
| | | | | | | | | | | | |
Net loss per share: | | | | | | | | | | | | |
Loss per share—basic | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | (0.15 | ) |
| | | | | | | | | | | | |
Loss per share—diluted | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | (0.15 | ) |
| | | | | | | | | | | | |
Weighted average shares used in per share calculation: | | | | | | | | | | | | |
—Basic | | | 111,769 | | | | 94,774 | | | | 94,710 | |
| | | | | | | | | | | | |
—Diluted | | | 111,769 | | | | 94,774 | | | | 94,710 | |
| | | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
48
INSITE VISION INCORPORATED
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
| | | | | | | | | | | | | | | | | | | | |
(in thousands, except share data) | | Common Stock | | | Additional
Paid-in-Capital | | | Accumulated
Deficit | | | Total
Stockholders’
Deficit | |
| | Shares | | | Amount | | | | |
Balances, December 31, 2008 | | | 94,681,618 | | | $ | 947 | | | $ | 148,384 | | | $ | (168,837 | ) | | $ | (19,506 | ) |
Issuance of common stock from employee stock purchase plan | | | 56,782 | | | | — | | | | 9 | | | | — | | | | 9 | |
Stock-based compensation | | | — | | | | — | | | | 619 | | | | — | | | | 619 | |
Net loss | | | — | | | | — | | | | — | | | | (14,155 | ) | | | (14,155 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2009 | | | 94,738,400 | | | | 947 | | | | 149,012 | | | | (182,992 | ) | | | (33,033 | ) |
Issuance of common stock from exercise of stock options | | | 84,193 | | | | 1 | | | | 20 | | | | — | | | | 21 | |
Stock-based compensation | | | — | | | | — | | | | 385 | | | | — | | | | 385 | |
Net loss | | | — | | | | — | | | | — | | | | (9,593 | ) | | | (9,593 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2010 | | | 94,822,593 | | | | 948 | | | | 149,417 | | | | (192,585 | ) | | | (42,220 | ) |
Issuance of common stock from private placement, net of issuance costs | | | 36,978,440 | | | | 370 | | | | 20,029 | | | | — | | | | 20,399 | |
Issuance of common stock from exercise of stock options | | | 150,000 | | | | 2 | | | | 56 | | | | — | | | | 58 | |
Initial value of warrant liability | | | — | | | | — | | | | (6,356 | ) | | | — | | | | (6,356 | ) |
Stock-based compensation | | | — | | | | — | | | | 522 | | | | — | | | | 522 | |
Net loss | | | — | | | | — | | | | — | | | | (6,942 | ) | | | (6,942 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2011 | | | 131,951,033 | | | $ | 1,320 | | | $ | 163,668 | | | $ | (199,527 | ) | | $ | (34,539 | ) |
| | | | | | | | | | | | | | | | | | | | |
See accompanying notes consolidated financial statements.
49
INSITE VISION INCORPORATED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
OPERATING ACTIVITIES: | | | | | | | | | | | | |
Net loss | | $ | (6,942 | ) | | $ | (9,593 | ) | | $ | (14,155 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 86 | | | | 212 | | | | 584 | |
Impairment of assets | | | — | | | | — | | | | 615 | |
Loss (gain) on sale of assets | | | — | | | | — | | | | 1 | |
Amortization of debt issuance costs | | | 419 | | | | 418 | | | | 419 | |
Stock-based compensation | | | 522 | | | | 385 | | | | 619 | |
Change in fair value of warrant liability | | | (2,201 | ) | | | — | | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Accounts receivable, net | | | 788 | | | | (215 | ) | | | (1,682 | ) |
Prepaid expenses and other current assets | | | 3 | | | | 142 | | | | 55 | |
Accounts payable | | | 371 | | | | 46 | | | | (876 | ) |
Accrued liabilities | | | (100 | ) | | | 151 | | | | (369 | ) |
Accrued compensation and related expense | | | 358 | | | | (348 | ) | | | 330 | |
Accrued royalties | | | 72 | | | | 245 | | | | 321 | |
Accrued interest | | | (2,205 | ) | | | 438 | | | | 1,738 | |
Deferred revenues | | | (75 | ) | | | — | | | | (298 | ) |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (8,904 | ) | | | (8,119 | ) | | | (12,698 | ) |
| | | | | | | | | | | | |
INVESTING ACTIVITIES: | | | | | | | | | | | | |
Purchase of property and equipment | | | (184 | ) | | | (150 | ) | | | (34 | ) |
Proceeds from sale of asset | | | — | | | | — | | | | 4 | |
Decrease (increase) in short-term investments | | | (19,496 | ) | | | 12,500 | | | | (17,499 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | (19,680 | ) | | | 12,350 | | | | (17,529 | ) |
| | | | | | | | | | | | |
FINANCING ACTIVITIES: | | | | | | | | | | | | |
Issuance of common stock from private placement, net of issuance costs | | | 20,399 | | | | — | | | | — | |
Issuance of common stock from exercise of options and employee purchase plan, net of issuance costs | | | 58 | | | | 21 | | | | 9 | |
Payment of secured notes payable | | | (1,442 | ) | | | — | | | | — | |
Payment of capital lease obligation | | | — | | | | (5 | ) | | | (16 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 19,015 | | | | 16 | | | | (7 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (9,569 | ) | | | 4,247 | | | | (30,234 | ) |
Cash and cash equivalents at beginning of year | | | 11,469 | | | | 7,222 | | | | 37,456 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of year | | $ | 1,900 | | | $ | 11,469 | | | $ | 7,222 | |
| | | | | | | | | | | | |
| | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
Interest received | | $ | 28 | | | $ | 21 | | | $ | 65 | |
| | | | | | | | | | | | |
Interest paid | | $ | 11,981 | | | $ | 9,411 | | | $ | 7,937 | |
| | | | | | | | | | | | |
Income taxes | | $ | 1 | | | $ | 1 | | | $ | 2 | |
| | | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
50
InSite Vision Incorporated
Notes to Consolidated Financial Statements
For the years ended December 31, 2011, 2010 and 2009
1. Business and Summary of Significant Accounting Policies
InSite Vision Incorporated (“InSite,” the “Company,” “we,” or “our”) is an ophthalmic product development company advancing ophthalmic pharmaceutical products to address unmet eye care needs. The Company’s current portfolio of products is based on its proprietary DuraSite® drug delivery technology.
The Company’s DuraSite sustained drug delivery technology is a proven synthetic polymer-based formulation designed to extend the residence time of a drug relative to conventional topical therapies. It enables topical delivery of a drug as a solution, gel or suspension and can be customized for delivering a wide variety of drug candidates. The Company has focused its research and development and commercial support efforts on topical products formulated with the DuraSite drug delivery technology. The Company may also utilize the DuraSite technology platform for the formulation of new ocular product candidates using either non-proprietary drugs or compounds originally developed by others for non-ophthalmic indications.
Principles of Consolidation. The consolidated financial statements include the accounts of InSite Vision Incorporated as well as its wholly-owned subsidiaries. All intercompany transactions have been eliminated in consolidation.
Industry Segment and Geographic Information. The Company operates in one segment and is focused on developing drugs and drug delivery systems principally for ophthalmic indications. The Company has limited foreign-based operations for the years ended December 31, 2011, 2010 and 2009. All long-lived assets are located in the United States.
Use of Estimates.The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires the Company to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Reclassifications. Certain amounts in prior years’ financial statements have been reclassified to conform to the current presentation. These reclassifications had no impact on previously reported results of operations or stockholders’ deficit.
Cash and cash equivalents.The Company considers all highly liquid investments with maturities of 90 days or less from the date of purchase to be cash equivalents.
Short-term investments.The Company considers all investments with original maturities of 12 months or less from the date of purchase to be short-term investments. They are classified as trading securities principally bought and held for the purpose of selling them in the near term, with unrealized gains and losses included in earnings.
Property and Equipment. Property and equipment is stated at cost, less accumulated depreciation and amortization. Depreciation of property and equipment is provided over the estimated useful lives of the respective assets, which range from three to five years, using the straight-line method. Leasehold improvements and property acquired under capital lease are amortized over the lives of the related leases or their estimated useful lives, whichever is shorter, using the straight-line method. Depreciation and amortization expense for the years ended December 31, 2011, 2010 and 2009 were $86,000, $212,000 and $584,000, respectively. The costs of repairs and maintenance are expensed as incurred.
51
Impairment of Long-Lived Assets. The Company periodically assesses the recoverability of its long-lived assets for which an indicator of impairment exists by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If the Company concludes that the carrying value will not be recovered, the Company measures the amount of such impairment by comparing the fair value to the carrying value. The Company recorded an impairment charge of $615,000 in 2009 associated with a corporate restructuring announced in March 2009. The Company impaired laboratory and other equipment, leasehold improvements, and furniture and fixtures located at the Company’s corporate headquarters.
Patents. As a result of the Company’s research and development efforts, the Company has obtained, or is applying for, a number of patents to protect proprietary technology and inventions. All costs associated with patents for product candidates under development are expensed as incurred. To date, the Company has no capitalized patent costs.
Debt Issuance Costs. Debt issuance costs paid to third parties are capitalized and amortized over the life of the underlying debt, using the straight-line method. Amortization of debt issuance costs for the years ended December 31, 2011, 2010 and 2009 were $419,000, $418,000 and $419,000, respectively, and are included in interest expense and other, net in the Consolidated Statements of Operations. See Note 7, “Non-Recourse Secured Notes Payable” for further discussion of the underlying debt.
Warrant Liability. The Company issued warrants to purchase shares of the Company’s common stock in connection with a private placement financing transaction in July 2011. The Company accounted for these warrants as a liability measured at fair value due to a provision included in the warrant agreements that provides the warrant holders with an option to require the Company (or its successor) to purchase their warrants for cash in the event of a “Fundamental Transaction” (as defined in the warrant agreements). The actual amount of cash required if the option is exercised would be determined using the Black-Scholes Option Pricing Model (the “Black-Scholes Model”) as determined in accordance with the terms of the warrant agreements. The fair value of the warrant liability is estimated using the Black-Scholes Model, which requires inputs such as the remaining term of the warrants, share price volatility and risk-free interest rate. These assumptions are reviewed on a monthly basis and changes in the estimated fair value of the outstanding warrants are recognized each reporting period in the Consolidated Statements of Operations under “Change in fair value of warrant liability.”
Fair Value Measurements. The Company measures assets and liabilities at fair value. The levels of fair value measurements are:
| Level 1 | Inputs are unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. |
| Level 2 | Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. The Company had no Level 2 assets at December 31, 2011. |
| Level 3 | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. The Company had no Level 3 assets at December 31, 2011. |
As of December 31, 2011 and 2010, $26.3 million and $16.3 million, respectively, of the Company’s cash, cash equivalents and short-term investments consisted of Level 1 Treasury-backed money market funds that are measured at fair value on a recurring basis.
The Company’s financial instruments consist mainly of cash and cash equivalents, short-term investments, accounts receivable, accounts payable, accrued liabilities and debt obligations. Accounts receivable and accounts payable are reflected in the accompanying consolidated financial statements at cost, which approximates fair value due to the short-term nature of these instruments. While the Company believes its valuation methodologies
52
are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date.
The Company has debt with fixed interest rates. The Company had $58.6 million in Level 2 borrowings outstanding as of December 31, 2011, measured at fair value on a nonrecurring basis, with an interest rate of 16%. As of December 31, 2011, the face value of the Company’s debt was estimated to be approximately the fair value based on current market rates.
As discussed above, the fair value of the warrant liability (Level 3), which is measured at fair value on a recurring basis, was initially recorded on the issue date and remeasured at December 31, 2011 using the Black-Scholes Model, which requires inputs such as the remaining term of the warrants, share price volatility and risk-free interest rate. These inputs are subjective and generally require significant analysis and judgment to develop.
The fair value of the warrant liability was estimated using the following assumptions, as determined in accordance with the terms of the warrant agreements, at December 31, 2011 and July 18, 2011 (the date of issuance):
| | | | | | | | |
| | | December 31,
2011 | | | July 18,
2011 | |
Risk-free interest rate | | | 0.8 | % | | | 1.5 | % |
Remaining term (years) | | | 4.5 | | | | 5.0 | |
Expected dividends | | | 0.0 | % | | | 0.0 | % |
Volatility | | | 100.0 | % | | | 100.0 | % |
The expected dividend yield is set at zero because the Company has never paid cash dividends and has no present intention to pay cash dividends. Expected volatility is based on the historical volatility of the Company’s common stock and is equal to the greater of 100% or the 30-day volatility rate. The risk-free interest rates are taken from the Daily Federal Yield Curve Rates as published by the Federal Reserve and represent the yields on actively-traded U.S. Treasury securities for a term equal to the remaining term of the warrants.
The following table provides a summary of changes in the fair value of the Company’s Level 3 financial liabilities for the year ended December 31, 2011 (in thousands):
| | | | |
Balance at December 31, 2010 | | $ | — | |
Initial fair value of warrants as of issuance in July 2011 | | | 6,356 | |
Net decrease in fair value of warrant liability on remeasurment | | | (2,201 | ) |
| | | | |
Balance at December 31, 2011 | | $ | 4,155 | |
| | | | |
The net decrease in the estimated fair value of the warrant liability was recognized as income under “Change in fair value of warrant liability” in the Consolidated Statements of Operations.
Revenue Recognition. The Company recognizes revenue when four basic criteria are met: persuasive evidence of an arrangement exists; delivery has occurred or services have been rendered; the fee is fixed or determinable; and collectability is reasonably assured. The Company has arrangements with multiple revenue-generating elements. The Company analyzes its multiple element arrangements to determine whether the elements can be separated and accounted for individually as separate units of accounting. An item can generally be considered a separate unit of accounting if both of the following criteria are met: the delivered item(s) has value to the customer on a stand-alone and if the arrangement includes a general right of return and delivery or performance of the undelivered item(s) is considered probable and substantially in the control of the Company. Items that cannot be divided into separate units are combined with other units of accounting, as appropriate. Consideration received is allocated among the separate units based on the unit’s selling price and is recognized in full when the criteria are met. The Company deems service to be rendered if no continuing obligation exists on the part of the Company.
53
The Company’s revenues are primarily related to royalties on product sales and licensing agreements, and such agreements may provide for various types of payments, including upfront payments, research funding and related fees during the terms of the agreements, milestone payments based on the achievement of established development objectives and licensing fees.
The Company receives royalties from licensees based on third-party sales. The royalties are recorded as earned in accordance with the contract terms when third-party results are reliably measured and collectability is reasonably assured.
Revenue associated with non-refundable up-front license fees under arrangements where the license fees cannot be accounted for as separate units of accounting is deferred and recognized as revenue on a straight-line basis over the expected term of the Company’s continued involvement. Revenues from the achievement of milestones are recognized as revenue when the milestones are achieved and the milestone payments are due and collectible.
For the year ended December 31, 2011, the Company adopted amendments to the accounting standard related to multiple-deliverable revenue arrangements. The amendment requires entities to allocate revenue in multiple-deliverable revenue arrangements using estimated selling prices of the delivered goods and services based on a selling price hierarchy. The amendment eliminated the residual method of revenue allocation and requires revenue to be allocated using the relative selling price method. The adoption of this amendment did not impact the Company’s consolidated financial position or results of operations since the Company did not enter into or materially modify revenue arrangements. In addition, there have been no significant changes to the units of accounting, the allocation of consideration received to the various units of accounting, and the timing of revenue recognition based on this amendment. The Company does not expect the adoption of this amendment to have a material impact on future periods.
Research and Development Expenses. Research and development expenses include salaries, benefits, facility costs, services provided by outside consultants and contractors, administrative costs and materials for the Company’s research and development activities. The Company recognizes such costs as they are incurred.
The Company accrues and expenses the costs for clinical trial activities performed by third parties based upon estimates of the percentage of work completed over the life of the individual study in accordance with agreements established with contract research organizations and clinical trial sites. The Company determines the estimates through discussions with internal clinical personnel and external service providers as to progress or stage of completion of trials or services and the agreed upon fee to be paid for such services. Costs of setting up clinical trial sites for participation in the trials are expensed immediately as research and development expenses. Clinical trial site costs related to patient enrollment are accrued as patients are entered into the trial.
General and Administrative Expenses. General and administrative expenses include salaries, benefits, facility costs, services provided by outside consultants and contractors, advertising and marketing, investor relations, financial reporting, materials and other expenses related to general corporate and sales and marketing activities. The Company recognizes such costs as they are incurred.
Cost of Revenues. The Company recognizes royalties to third parties and the cost of inventory shipped related to the sale of the Company’s products when they are incurred.
Stock-Based Compensation. The Company’s stock-based compensation programs consist of stock options granted to employees as well as our employee stock purchase plan, based on the grant date fair value of those awards.
The grant date fair value of the award is recognized as expense over the requisite service period. The Company uses the Black-Sholes option pricing model to compute the estimated fair value of stock option awards. Using this model, fair value is calculated based on assumptions with respect to: expected volatility of our
54
common stock price: the periods of time over which employees and members of our board are expected to hold their options prior to exercise; expected dividend yield on our common stock; and risk-free interest rates. Stock-based compensation expense also includes an estimate, which is made at the time of grant, of the number of awards that are expected to be forfeited. The estimate is revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
See Note 11, “Employee Stock-Based Compensation” for further discussion of employee stock-based compensation.
Accounting for Stock Options and Warrants Exchanged for Services. The Company occasionally issues stock options and warrants to consultants of the Company in exchange for services. The Company has valued these options and warrants using the Black-Scholes option pricing model, at each reporting period and has recorded charges to operations over the vesting periods of the individual stock options or warrants. Such charges amounted to approximately $20,000 and $3,000 during the years ended 2011 and 2009, respectively. No such charges were incurred in 2010.
Net Loss per Share. Basic net loss per share has been computed using the weighted-average number of common shares outstanding during the period. Dilutive net loss per share is computed using the sum of the weighted-average number of common shares outstanding and the potential number of dilutive common shares outstanding during the period. Potential common shares consist of the shares issuable upon exercise of stock options and warrants. Potentially dilutive securities have been excluded from the computation of diluted net loss per share in 2011, 2010 and 2009 as their inclusion would be anti-dilutive.
The following table sets forth the computation of basic and diluted net loss per share:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
(in thousands, except per share data) | | 2011 | | | 2010 | | | 2009 | |
Numerator: | | | | | | | | | | | | |
Net loss | | $ | (6,942 | ) | | $ | (9,593 | ) | | $ | (14,155 | ) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Weighted-average shares outstanding | | | 111,769 | | | | 94,774 | | | | 94,710 | |
Effect of dilutive securities: | | | | | | | | | | | | |
Stock options and warrants | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Weighted-average shares outstanding for diluted loss | | | 111,769 | | | | 94,774 | | | | 94,710 | |
| | | | | | | | | | | | |
Net loss per share: | | | | | | | | | | | | |
Basic | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | (0.15 | ) |
| | | | | | | | | | | | |
Diluted | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | (0.15 | ) |
| | | | | | | | | | | | |
For the years ended December 31, 2011, 2010 and 2009, due to the loss applicable to common stockholders, loss per share is based on the weighted average number of common shares only, as the effect of including equivalent shares from stock options and warrants would be anti-dilutive. At December 31, 2011, 2010 and 2009, 25,795,339, 10,293,478 and 13,908,611 options and warrants, respectively, were excluded from the calculation of diluted earnings per share because the effect was anti-dilutive.
Accounting for Materials Purchased for Research and Development. The Company expenses materials for research and development activities when the obligation for the items is incurred.
Key Suppliers. The Company is dependent on single or limited source suppliers for certain materials used in its research and development and commercial activities. The Company has generally been able to obtain
55
adequate supplies of these components. However, an extended interruption in the supply of these components currently obtained from single or limited source suppliers could adversely affect the Company’s research and development and commercial efforts.
Income Taxes. The Company accounts for income taxes under the liability method; under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Realization of deferred tax assets is dependent upon future earnings, the timing and amount of which are uncertain.
The Company utilizes a two-step approach to recognize and measure uncertain tax positions, if any. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained upon tax authority examination, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement.
Significant Customers and Risk.All revenues recognized and/or deferred were primarily from AzaSite licensees. The Company is entitled to receive royalty revenue from net sales of AzaSite under the terms of its agreements with Merck and other licenses and, accordingly, all trade receivables are concentrated with these parties. Due to the nature of these agreements, these parties have significant influence over the commercial success of AzaSite. Revenues from Merck represented approximately 90%, 92% and 85% of total revenues for the years ended December 31, 2011, 2010 and 2009, respectively.
Credit Risk.Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash, cash equivalents and short-term investments. The Company’s cash, cash equivalents and short-term investments are primarily deposited in demand accounts with two financial institutions.
Risks from Third Party Manufacturing Concentration.The Company relies on a single source manufacturer for each of its product candidates and on a single source manufacturer for the active pharmaceutical ingredient in its product candidates. Merck is responsible for the manufacturing of AzaSite in North America and relies on a single source manufacturer for the product and on a single source manufacturer for the active pharmaceutical ingredient in the product. Accordingly, delays in the manufacture of AzaSite or the Company’s product candidates could adversely impact the marketing of AzaSite or the development of the Company’s product candidates. Furthermore, the Company has no control over the manufacture and the overall product supply chain of products for which it is entitled to receive revenue.
Recent Accounting Pronouncements.
In May 2011, the Financial Accounting Standards Board issued Accounting Standards Update No. 2011-04 (“ASU 2011-04”), Fair Value Measurement—Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. Generally Accepted Accounting Principles (“U.S. GAAP”) and International Financial Reporting Standards (“IFRS”). The amendments in this update will ensure that fair value has the same meaning in U.S. GAAP and in IFRS and that their respective fair value measurement and disclosure requirements are the same. This update is effective prospectively for interim and annual periods beginning after December 15, 2011. Early adoption by public entities is not permitted, and therefore, the Company is required to adopt ASU 2011-04 on January 1, 2012. The Company is currently evaluating the impact to the Company’s financial position and results of operations. The Company expects that the adoption of this amendment will not materially impact the Company’s consolidated financial position or results of operations.
2.License Agreements
In December 2003, the Company completed the sale of its drug candidate for the treatment of ocular infections to Bausch & Lomb Incorporated (“Bausch & Lomb”), pursuant to a Purchase Agreement and a License Agreement. The drug candidate, Besivance, was developed by Bausch & Lomb. In May 2009, the FDA
56
approved Besivance to treat bacterial conjunctivitis (pink eye). Besivance was launched in the United States by Bausch & Lomb in the last half of 2009. In 2011, Besivance was launched internationally in select countries. During the years ended December 31, 2011and 2010, the Company recognized $1,208,000 and $467,000, respectively, of royalties related to net sales of Besivance by Bausch & Lomb.
On February 15, 2007, the Company entered into a license agreement for AzaSiteTM (the “Merck License”) with Inspire Pharmaceuticals, Inc (“Inspire”). On May 16, 2011, Merck & Co. (“Merck”) acquired Inspire and Inspire became a wholly-owned subsidiary of Merck. Under the Merck License, the Company licensed to Merck, exclusive development and commercialization rights in the United States and Canada, for topical anti-infective products containing azithromycin as the sole active ingredient for human ocular or ophthalmic indications. The Company also granted Merck an exclusive sublicense under the Pfizer patent rights the Company has licensed under the Pfizer License discussed below. Merck has the right to grant sublicenses under the terms of the Merck License.
Merck paid the Company an upfront license fee of $13 million on February 15, 2007 and on May 11, 2007 paid an additional $19 million upon regulatory approval by the U.S. Food and Drug Administration (“FDA”). Merck also pays the Company a royalty on net sales of AzaSite in the United States and Canada. The royalty rate was 20% of net sales in the first two years of commercialization and 25% thereafter. Merck is obligated to pay the Company royalties under the Merck License for the longer of (i) eleven years from the launch of the first product or (ii) the period during which a valid claim under a patent exists. For five years after the first year of commercial sale, Merck will pay the Company certain tiered minimum royalties. The royalties discussed above are subject to certain reductions in the event of patent invalidity, generic competition, uncured material breach under the Merck License or in the event that Merck is required to pay license fees to third parties for the continued use of AzaSite.
The Company also entered into a supply agreement (the “Supply Agreement”) with Merck on February 15, 2007 for the active pharmaceutical ingredient azithromycin. The Company had previously entered into a third-party supply agreement for the production of this active ingredient.
The Company recognized the upfront license fee and milestone payment totaling $32 million ratably over the period that the Company was required to continue to provide services under the Merck License, which ended in April 2008, under the contingency-adjusted performance model of revenue recognition. During the years ended December 31, 2008 and December 31, 2007, the Company recognized $9.9 million and $22.1 million, respectively, of the license fee and milestone payment as revenue.
In August 2007, Merck commercially launched AzaSite in the United States. Correspondingly, during the years ended December 31, 2011, 2010 and 2009, the Company recognized $13,930,000, $10,652,000 and $8,000,000, respectively, of royalties related to net sales of AzaSite by Merck. Additionally, during the years ended December 31, 2011, 2010 and 2009, the Company recognized $449,000, $258,000 and $325,000, respectively, of revenue from Merck for the sales of the active ingredient, azithromycin, under the Supply Agreement, sales of AzaSite inventory and for contract services provided by the Company.
On February 15, 2007, the Company entered into a worldwide, exclusive, royalty-bearing license agreement with Pfizer Inc. (“Pfizer”) under Pfizer’s patent family titled “Method of Treating Eye Infections with Azithromycin” for ocular anti-infective product candidates known as AzaSite and AzaSite Plus (the “Pfizer License”). Under the Pfizer License, the Company is required to pay Pfizer a low single digit royalty based on net sales of the licensed products and to use reasonable commercial efforts to seek regulatory approval for and market licensed products. The Pfizer License provides the Company the right to grant sublicenses thereunder, subject to Pfizer’s prior approval, which approval shall not be unreasonably withheld. Pfizer approved the sublicense granted to Merck. Based on the royalty report provided by Merck, for the years ended December 31, 2011, 2010 and 2009, the Company recorded third-party royalties of $1,407,000, $1,491,000 and $1,224,000, respectively, due primarily under the Pfizer License.
57
The Company has entered into, and will continue to pursue additional licensing agreements, corporate collaborations and service contracts. There can be no assurance that the Company will be able to negotiate acceptable collaborative, licensing or service agreements, or that the existing arrangements will be successful or renewed or will not be terminated.
3. Short-term Investments
As of December 31, 2011 and 2010, the Company had $24.5 million and $5.0 million in short-term investments, respectively. The Company’s investment policy is to limit the risk of principal loss and to ensure safety of invested funds by generally attempting to limit market risk. Accordingly, the Company’s short-term investments were invested in U.S. Treasury securities with original maturities of 12 months or less. They are classified as trading securities principally bought and held for the purpose of selling them in the near term, with unrealized gains and losses included in earnings. At December 31, 2011 and 2010, the Company had less than $4,000 and $1,000, respectively, of unrealized gains included in earnings.
4. Accounts Receivable, net
Accounts receivable, net represent amounts due to the Company from its licensees, including Merck and other third parties. At December 31, 2011, the Company did not record a bad debt allowance related to any accounts receivable as all amounts were reasonably expected to be collected. As of December 31, 2010, the Company recorded a $50,000 bad debt allowance related to these accounts receivable. The need for a bad debt allowance is evaluated each reporting period based on the Company’s assessment of the collectability of the amounts.
5. Property and Equipment, net
Property and equipment, net consisted of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2011 | | | 2010 | |
Laboratory and other equipment | | $ | 1,272 | | | $ | 1,094 | |
Leasehold improvements | | | 45 | | | | 39 | |
Furniture and fixtures | | | 14 | | | | 14 | |
| | | | | | | | |
| | | 1,331 | | | | 1,147 | |
Accumulated depreciation and amortization | | | (986 | ) | | | (900 | ) |
| | | | | | | | |
Property and equipment, net | | $ | 345 | | | $ | 247 | |
| | | | | | | | |
6. Warrant Liability
On July 18, 2011, the Company completed a private placement financing transaction in which it sold shares of its common stock and warrants to purchase shares of its common stock. The Company sold a total of 36,978,440 shares of common stock, at a price of $0.60 per share, and issued warrants to purchase up to 14,791,376 shares of common stock. The warrants are exercisable at $0.75 per share and expire five years from the date of issuance. Piper Jaffray & Co. served as the exclusive placement agent for this transaction which resulted in $22.2 million in gross proceeds and approximately $20.4 million in net proceeds to the Company, after deducting placement agent fees, legal, accounting and other costs associated with the transaction. The Company intends to use the net proceeds of the transaction to fund clinical trials and for general corporate purposes, including working capital.
As discussed in Note 1, the warrants issued in July 2011 include a provision that provides the warrant holders with an option to require the Company (or its successor) to purchase the warrants for cash in an amount equal to the Black-Scholes value in the event of a “Fundamental Transaction” (as defined in the warrant
58
agreements). Accordingly, the fair value of the warrants at the issuance date was estimated using the Black-Scholes Model, as determined in accordance with the terms of the warrant agreements, and the Company recorded a warrant liability of $6.4 million. The Company remeasured the warrant liability at December 31, 2011 and recorded a decrease to the warrant liability of approximately $2.2 million, which was recognized as income in the Company’s Consolidated Statement of Operations for the year ended December 31, 2011. Additional disclosures regarding assumptions used in calculating the fair value of the warrant liability are included in Note 1.
7. Non-Recourse Secured Notes Payable
In February 2008, the Company’s wholly-owned subsidiary, Azithromycin Royalty Sub, LLC completed a private placement of $60.0 million in aggregate principal amount of non-convertible, non-recourse promissory notes due in 2019. Net proceeds from the financing were approximately $55.3 million after transaction costs of approximately $4.7 million. In addition, $5.0 million of the proceeds was set aside for interest reserves. The annual interest rate on the notes is 16% with interest payable quarterly in arrears beginning May 15, 2008. The notes are secured by, and will be repaid from, royalties to be paid to the Company by Merck from net sales of AzaSite in the United States and Canada. The secured notes payable are non-recourse to InSite Vision Incorporated. When the AzaSite royalties received for any quarter exceed the interest payments and certain expenses due that quarter, the excess will be applied to the repayment of principal of the notes until the notes have been paid in full. As of December 31, 2011, no balance remained in the interest reserve. Further shortfalls, if any, can be paid by the Company at its option to avoid default under the agreement. The notes may be redeemed at the Company’s option, subject to the payment of a redemption premium through May 2012. As of December 31, 2011, $52.3 million of secured notes payable was classified as long-term and $6.3 million was classified as current.
At December 31, 2011, the estimated future principal payments on the notes, based on minimum royalty income covered by the agreement and future projected sales, were as follows (in thousands):
| | | | |
Year Ending December 31, | | | |
2012 | | $ | 6,286 | |
2013 | | | 9,344 | |
2014 | | | — | |
2015 | | | — | |
2016 | | | — | |
Thereafter | | | 42,928 | |
| | | | |
Total secured notes payable | | $ | 58,558 | |
| | | | |
8. Commitments and Contingencies
The Company has entered into certain license agreements that require us to make royalty payments for the life of the licensed patents. The estimated royalties due under these agreements is approximately $8.2 million for the periods 2012 through 2019. This contractual obligation is reflected in the Company’s financial statements once the related obligation becomes due.
Capital lease obligations represent the present value of future rental payments under capital lease agreements for telephones and telephone equipment. At December 31, 2010, the Company had $13,000 of capital leased equipment with accumulated depreciation of $13,000. At December 31, 2011, the Company had no remaining capital leased equipment since the contract expired in 2011. There are no future payments.
The Company conducts its operations from leased facilities in Alameda, California under non-cancelable operating lease agreements that expire in 2013. Lease payments include rent and the Company’s pro-rata share of operation expenses. The Company subleases a portion of the facility under a lease agreement that expires in
59
2013. Lease income includes rent and a pro-rata share of operation expenses. For accounting purposes, the Company is amortizing all rent payments and receipts ratably over the life of the lease. Rent expense for the years ended December 31, 2011, 2010 and 2009, was $697,000, $757,000, and $776,000, respectively. Future minimum lease payments under the operating lease and future cash receipts from the sublease, are as follows (in thousands):
| | | | | | | | | | | | |
Year Ending December 31, | | Operating Lease
Cash Payments | | | Operating Sublease
Cash Receipts | | | Operating Lease
Cash Payments, net | |
2012 | | $ | 822 | | | $ | 98 | | | $ | 724 | |
2013 | | | 848 | | | | 106 | | | | 742 | |
| | | | | | | | | | | | |
Total | | $ | 1,670 | | | $ | 204 | | | $ | 1,466 | |
| | | | | | | | | | | | |
9. Income Taxes
Provision for Income Taxes
There was no provision for income taxes for the years ended December 31, 2011, 2010 and 2009 due to the Company’s net operating losses.
Income tax provision related to continuing operations differ from the amounts computed by applying the statutory income tax rate of 34% to pretax loss as follows (in thousands):
| | | | | | | | | | | | |
| | | 2011 | | | 2010 | | | 2009 | |
Tax provision at federal statutory rate | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % |
State taxes, net of federal benefit | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % |
Warrant Liability | | | 10.8 | % | | | 0.0 | % | | | 0.0 | % |
Other permanent differences | | | -0.1 | % | | | -1.4 | % | | | -1.5 | % |
Credits | | | 2.7 | % | | | 0.9 | % | | | 1.6 | % |
Expiring Net Operating Losses | | | -11.4 | % | | | -46.0 | % | | | -33.1 | % |
True up of Deferred Tax Assets | | | -4.3 | % | | | 0.0 | % | | | 0.0 | % |
Valuation allowance | | | -31.7 | % | | | 12.5 | % | | | -1.0 | % |
| | | | | | | | | | | | |
Effective tax rate | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % |
| | | | | | | | | | | | |
Deferred Tax Assets and Liabilities
Significant components of the Company’s deferred tax assets for federal and state income taxes as of December 31, 2011, 2010 and 2009 were as follows (in thousands):
| | | | | | | | |
| | | 2011 | | | 2010 | |
Deferred tax assets: | | | | | | | | |
Net operating loss carryforwards | | $ | 40,138 | | | $ | 38,761 | |
Tax credit carryforwards | | | 7,523 | | | | 7,115 | |
Capitalized research and development | | | 14,163 | | | | 13,743 | |
Depreciation | | | 263 | | | | 689 | |
Other | | | 291 | | | | 149 | |
| | | | | | | | |
Total deferred tax assets | | | 62,378 | | | | 60,457 | |
Valuation allowance | | | (62,378 | ) | | | (60,457 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | |
During the year ended December 31, 2011, the valuation allowance increased by $1.9 million primarily due to an increase in net operating losses. During the year ended December 31, 2010, the valuation allowance decreased by $0.6 million primarily due to the expiration of net operating losses.
60
At December 31, 2011, the Company had net operating loss carryforwards for federal income tax purposes of approximately $101 million, which expire in the years 2012 through 2031 and federal tax credits of approximately $3.3 million, which expire in the years 2012 through 2031. At December 31, 2011, the Company also has net operating loss carryforwards for state income tax purposes of approximately $96.9 million which expire in the years 2012 through 2031 and state research and development tax credits of approximately $4.3 million which carryforward indefinitely.
The future utilization of the Company’s net operating loss carryforwards to offset future taxable income is subject to an annual limitation as a result of ownership changes that have occurred previously and may be further impacted by future ownership changes. As necessary, the deferred tax assets have been reduced by any carryforwards that expire prior to utilization as a result of such limitations, with a corresponding reduction of the valuation allowance. These carryforwards may be further reduced if the Company has any additional ownership changes in the future.
The valuation allowance includes amounts of benefit at both December 31, 2011 and December 31, 2010 related to stock-based compensation and exercises, prior to the implementation of Accounting Standards Codification 515 and 718 that will be credited to additional paid-in capital when realized.
Unrecognized Tax Benefits
The Company has incurred net operating losses since inception and does not have any unrecognized tax benefits. The Company’s policy is to include interest and penalties related to unrecognized tax benefits, if any, within the provision for taxes in the consolidated statements of operations. If the Company is eventually able to recognize its uncertain positions, its effective tax rate would be reduced. The Company currently has a full valuation allowance against its net deferred tax assets which would impact the timing of the effective tax rate benefit should any uncertain tax positions be favorably settled in the future. Any adjustments to the Company’s uncertain tax positions would result in an adjustment of its net operating loss or tax credit carry forwards.
The Company files income tax returns in the U.S. federal and California jurisdictions. The Company is no longer subject to tax examinations for years before 2008 for federal returns and 2007 for California returns, except to the extent that it utilizes net operating losses or tax credit carryforwards that originated before those years. The Company is not currently under audit by any major tax jurisdiction nor has it been in the past.
Although it is reasonably possible that certain unrecognized tax benefits may increase or decrease within the next twelve months due to tax examination changes, settlement activities, expirations of statute of limitations, or the impact on recognition and measurement considerations related to the results of published tax cases or other similar activities, the Company does not anticipated any significant changes to unrecognized tax benefits over the next 12 months. During the years ended December 31, 2011 and 2010, no interest or penalties were required to be recognized relating to unrecognized tax benefits.
10. Common Stock Warrants
The following table shows outstanding warrants as of December 31, 2011, all of which were issued in the July 2011 private placement financing transaction. All of the outstanding warrants have cashless exercise provisions in the event the registration statement registering the resale of the shares of common stock issuable upon exercise of the warrants is not effective or the prospectus forming a part of the registration statement is not current. All warrants are exercisable for common stock.
| | | | | | | | | | | | | | | | |
Date Issued | | Warrant
Shares | | | Exercise
Price | | | Expiration Date | | | Cash if
Exercised | |
July 18, 2011 | | | 14,791,376 | | | $ | 0.75 | | | | July 18, 2016 | | | $ | 11,093,532 | |
61
11. Stock-based Compensation
Equity Incentive Program
Prior to October 15, 2007, the Company granted options under a stock option plan adopted in 1994 and amended thereafter (the “1994 Plan”), that allowed for the granting of non-qualified stock options, incentive stock options and stock purchase rights to the Company’s employees, directors, and consultants. On October 15, 2007, the Company’s stockholders approved a new equity incentive plan, the 2007 Performance Incentive Plan (the “2007 Plan”), that provides for grants of options and other equity-based awards to the Company’s employees, directors and consultants. The Company’s authority to grant new awards under the 1994 Plan terminated upon stockholder approval of the 2007 Plan. Options granted under these plans expire 10 years after the date of grant and become exercisable at such times and under such conditions as determined by the Company’s Board of Directors or a committee appointed by the Board (generally with 25% vesting after one year and the balance vesting on a daily basis over the next three years of service). Upon termination of the optionee’s service, unvested options terminate, and vested options generally expire at the end of three months. Only nonqualified stock options have been granted under these plans to date. On January 1 of each calendar year during the term of the 2007 Plan, the shares of Common Stock available for issuance will be increased by the lesser of 2% of the total outstanding shares of Common Stock on December 31 of the preceding calendar year, or 3,000,000 shares.
Employee Stock Purchase Plans
The Company maintained an employee stock purchase plan, adopted in 1994 and amended thereafter (the “Purchase Plan”), until August 2009. In August 2009, the Purchase Plan was suspended. No new offering period will commence and no additional shares will be added to the Purchase Plan under its evergreen provision unless and until approved by the Company’s Board of Directors. The Purchase Plan operated in 24-month “offering periods” that are each divided into four six-month “purchase periods.” The Purchase Plan allowed eligible employees to purchase Common Stock at 85% of the lower of the fair market value of the Common Stock on the first day of the applicable offering period or the fair market value of the Common Stock on the last day of the applicable purchase period. Purchases were limited to 10% of each employee’s eligible compensation, subject to certain Internal Revenue Service restrictions. All of the Company’s employees were eligible to participate in the Purchase Plan after certain service periods were met. The number of shares available for issuance under the Purchase Plan was automatically increased on the first trading day in January each calendar year, by an amount equal to 0.5% of the total number of shares of Common Stock outstanding on the last trading day in December in the immediately preceding calendar year, but in no event will any such annual increase exceed 125,000 shares. During the year ended December 31, 2011 and 2010, no shares were issued under the Purchase Plan. As of December 31, 2011, there was no remaining unrecorded deferred stock-based compensation expense related to the Purchase Plan. As of December 31, 2011, 515,183 shares were reserved for issuance under the Purchase Plan.
Stock-based Compensation
Stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized as expense over the requisite service period. All of the Company’s stock compensation is accounted for as an equity instrument.
62
The effect of recording stock-based compensation for the years ended December 31, 2011, 2010 and 2009 was as follows (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
Stock-based compensation expense by type of award: | | | | | | | | | | | | |
Employee stock options | | $ | 502 | | | $ | 385 | | | $ | 588 | |
Employee stock purchase plan | | | — | | | | — | | | | 28 | |
Non-employee stock options | | | 20 | | | | — | | | | 3 | |
| | | | | | | | | | | | |
Total stock-based compensation | | $ | 522 | | | $ | 385 | | | $ | 619 | |
| | | | | | | | | | | | |
Stock-based compensation included in expense line items in the Consolidated Statements of Operations for the year ended December 31, 2011, 2010 and 2009 was as follows (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
Research and development | | $ | 141 | | | $ | 74 | | | $ | 319 | |
General and administrative | | | 381 | | | | 311 | | | | 300 | |
| | | | | | | | | | | | |
| | $ | 522 | | | $ | 385 | | | $ | 619 | |
| | | | | | | | | | | | |
During the years ended December 31, 2011 and 2010, respectively, the Company granted options to purchase 2,955,000 and 5,424,374 shares of common stock with an estimated total grant date fair value of $0.9 million and $1.4 million. Based on the Company’s historical experience of option pre-vesting cancellations and estimates of future forfeiture rates, the Company has assumed an annualized forfeiture rate of 10% for its options for all periods disclosed. Accordingly, for the years ended December 31, 2011 and 2010, the Company estimated that the stock-based compensation for the awards not expected to vest was $0.3 million.
As of December 31, 2011 and 2010, the unrecorded deferred stock-based compensation balances related to stock options were $1.4 million and $1.2 million, respectively, and will be recognized over an estimated weighted-average amortization period of 2.5 years and 3.1 years, respectively.
Fair Value Assumptions
The fair value of each option grant is estimated using the Black-Scholes valuation model on the date of grant and the graded-vesting method with the following weighted-average assumptions:
| | | | | | |
| | | Year ended December 31, |
Stock Options | | 2011 | | 2010 | | 2009 |
Risk-free interest rate | | 0.9% - 2.2% | | 1.3% - 2.6% | | 1.7% - 2.4% |
Expected term (years) | | 5 | | 5 | | 5 |
Expected dividends | | 0.0% | | 0.0% | | 0.0% |
Volatility | | 89.7% | | 87.8% | | 82.1% |
| | | | |
| | | Year ended December 31, | |
Employee Stock Purchase Plan | | 2009 | |
Risk-free interest rate | | | 1.7 | % |
Expected term (years) | | | 1.5 | |
Expected dividends | | | 0.0 | % |
Volatility | | | 80.5 | % |
The dividend yield of zero is based on the fact that the Company has never paid cash dividends and has no present intention to pay cash dividends. Expected volatility is based on the combination of historical volatility of
63
the Company’s common stock and the common stock of the Company’s competitors, the expected moderation in future volatility over the period commensurate with the expected life of the options and other factors. The risk-free interest rates are taken from the Daily Federal Yield Curve Rates as of the grant dates as published by the Federal Reserve and represent the yields on actively traded Treasury securities for terms equal to the expected term of the options. The expected term calculation is based on the terms utilized by the Company’s competitors, observed historical option exercise behavior and post-vesting forfeitures of options by the Company’s employees.
The following is a summary of activity under the Company’s stock option plans for the indicated periods:
| | | | | | | | | | | | | | | | |
| | | Number of
shares | | | Weighted-
Average
Exercise
Price | | | Weighted-Average
Remaining Contractual
Term (Years) | | | Aggregate
Intrinsic
Value (in
thousands) | |
Outstanding at December 31, 2008 | | | 6,214,026 | | | $ | 1.00 | | | | 3.67 | | | $ | — | |
| | | | | | | | | | | | | | | | |
Granted | | | 3,185,000 | | | | 0.26 | | | | | | | | | |
Exercised | | | — | | | | 0.00 | | | | | | | | | |
Forfeited | | | (591,460 | ) | | | 0.25 | | | | | | | | | |
Expired | | | (1,401,281 | ) | | | 1.03 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2009 | | | 7,406,285 | | | | 0.74 | | | | 4.93 | | | | 296 | |
| | | | | | | | | | | | | | | | |
Granted | | | 5,424,374 | | | | 0.37 | | | | | | | | | |
Exercised | | | (84,193 | ) | | | 0.24 | | | | | | | | | |
Forfeited | | | (325,804 | ) | | | 0.34 | | | | | | | | | |
Expired | | | (3,485,199 | ) | | | 1.08 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2010 | | | 8,935,463 | | | | 0.40 | | | | 8.79 | | | | 211 | |
| | | | | | | | | | | | | | | | |
Granted | | | 2,955,000 | | | | 0.44 | | | | | | | | | |
Exercised | | | (150,000 | ) | | | 0.38 | | | | | | | | | |
Forfeited | | | (455,000 | ) | | | 0.33 | | | | | | | | | |
Expired | | | (281,500 | ) | | | 0.70 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2011 | | | 11,003,963 | | | $ | 0.41 | | | | 8.11 | | | $ | 861 | |
| | | | | | | | | | | | | | | | |
Options vested and expected to vest at December 31, 2011 | | | 10,405,710 | | | $ | 0.41 | | | | 8.06 | | | $ | 827 | |
Options exercisable at December 31, 2011 | | | 4,718,014 | | | $ | 0.43 | | | | 7.00 | | | $ | 443 | |
At December 31, 2011, the Company had 1,548,934 shares of common stock available for grant under its 2007 Plan. The weighted average grant date fair value of options granted during the years ended December 31, 2011, 2010 and 2009 were $0.31, $0.25 and $0.17, respectively. The total intrinsic value of options exercised during the year ended December 31, 2011 and 2010 were $24,000 and $13,000, respectively. No options were exercised during the year ended December 31, 2009.
64
The following table summarizes information concerning outstanding and exercisable options as of December 31, 2011:
| | | | | | | | | | | | | | | | | | | | |
| | | Option Outstanding | | | Options Vested and Exercisable | |
| | | Number
Outstanding | | | Weighted-Average | | | Number
Exercisable | | | Weighted-
Average
Exercise Price | |
Range of Exercise Prices | | | Contractual
Life | | | Exercise Price | | | |
$0.20 - $0.22 | | | 1,480,000 | | | | 7.13 | | | $ | 0.21 | | | | 1,061,885 | | | $ | 0.21 | |
$0.28 - $0.33 | | | 500,000 | | | | 8.95 | | | | 0.33 | | | | 465,646 | | | | 0.33 | |
$0.35 - $0.35 | | | 2,844,374 | | | | 8.92 | | | | 0.35 | | | | 769,486 | | | | 0.35 | |
$0.36 - $0.36 | | | 1,700,000 | | | | 8.67 | | | | 0.36 | | | | 300,000 | | | | 0.36 | |
$0.38 - $0.41 | | | 625,000 | | | | 5.83 | | | | 0.38 | | | | 625,000 | | | | 0.38 | |
$0.42 - $0.42 | | | 1,430,000 | | | | 8.25 | | | | 0.42 | | | | 625,625 | | | | 0.42 | |
$0.44 - $0.52 | | | 1,107,500 | | | | 9.38 | | | | 0.46 | | | | 120,000 | | | | 0.52 | |
$0.55 - $1.35 | | | 1,102,088 | | | | 6.65 | | | | 0.69 | | | | 535,371 | | | | 0.76 | |
$1.46 - $1.50 | | | 135,001 | | | | 4.25 | | | | 1.49 | | | | 135,001 | | | | 1.49 | |
$1.59 - $1.59 | | | 80,000 | | | | 5.33 | | | | 1.59 | | | | 80,000 | | | | 1.59 | |
| | | | | | | | | | | | | | | | | | | | |
| | | 11,003,963 | | | | 8.11 | | | $ | 0.41 | | | | 4,718,014 | | | $ | 0.43 | |
| | | | | | | | | | | | | | | | | | | | |
At December 31, 2010 and 2009 options to purchase 2,889,719 and 4,610,202 shares of common stock were exercisable at weighted-average exercise prices of $0.52 and $1.02, per share, respectively.
The following table details the Company’s nonvested stock options activity for the year ended December 31, 2011:
| | | | | | | | |
| | | Number of
shares | | | Weighted-Average
Grant-Date Fair Value | |
Outstanding at December 31, 2010 | | | 6,045,744 | | | $ | 0.23 | |
Granted | | | 2,955,000 | | | | 0.31 | |
Vested | | | (2,259,795 | ) | | | 0.24 | |
Forfeited | | | (455,000 | ) | | | 0.23 | |
| | | | | | | | |
Outstanding at December 31, 2011 | | | 6,285,949 | | | $ | 0.27 | |
| | | | | | | | |
The weighted-average grant date fair value of nonvested stock options is calculated using the Black-Scholes valuation model on the date of grant.
12. Restructuring Charge
In December 2008, the Company announced a corporate restructuring plan to focus on the Company’s growth opportunities and conserve resources. As of December 31, 2009, the restructuring plan reduced the Company’s personnel by 39 employees. When affected employees were notified of their termination, the Company recorded the severance related costs. Severance totaled approximately $2.4 million of which $0.2 million was a non-cash transaction. In 2008, $1.9 million was recorded as severance expense in the Consolidated Statement of Operations. The remaining $0.5 million was recorded as severance expense in the Consolidated Statement of Operations in 2009. Since 2009, the Company has not incurred and does not expect to incur additional severance related costs pertaining to the corporate restructuring. In first quarter of 2010, all remaining severance payments were paid and there is no remaining liability.
In February 2009, the Company announced a plan to develop and implement a new strategy to build shareholder value and further reduce costs. Based on a thorough analysis of the Company’s business and the marketplace, the Company believed the best path forward was a strategy that monetized its current assets and
65
either acquired promising new product opportunities or entered into a sales transaction for the Company. Based on that business strategy, during the first quarter of 2009, the Company determined that the carrying value of its laboratory and other equipment, leasehold improvements, and furniture and fixtures would not be recovered through undiscounted future operating cash flows. To determine the fair value of assets, the Company obtained quoted market prices for similar assets. The Company adjusted the carrying amount of the assets to the lower of the carrying value or fair value and recorded $0.6 million as impairment expense in the Consolidated Statement of Operations in 2009.
13. Legal Proceedings
The Company is subject to various claims and legal actions during the ordinary course of our business. On November 30, 2009, a patent interference was declared before the Board of Patent Appeals and Interferences on certain U.S. patents covering AzaSite. Regents of the University of California (the “University”) assert that the inventions contained in these patents were made by a former employee of the University alone, and without collaboration with us. They are asserting that they own those inventions, and that they are entitled to an award of priority of invention and a judgment that the inventions are not patentable to us. On November 25, 2011, the U.S. Patent and Trademark Office (the “USPTO”) entered judgment against the University. On December 23, 2011, the University filed a Notice of Appeal to the Court of Appeals for the Federal Circuit in Washington, D.C., and on January 4, 2012, the Company filed a Notice of Cross-Appeal. The Company continues to believe the University’s assertions are without merit and we will continue to vigorously defend its position.
The Company received a letter (the “Notice Letter”) dated April 15, 2011 from Sandoz, Inc. (“Sandoz”) providing notice that Sandoz has filed an Abbreviated New Drug Application (“ANDA”) with the FDA seeking marketing approval for a 1% azithromycin ophthalmic solution (the “Sandoz Product”) prior to the expiration of the five US patents listed in the Orange Books for AzaSite, which include four of our patents and one patent licensed to us by Pfizer. In the paragraph IV Certification accompanying the Sandoz ANDA filing, Sandoz alleges that the claims of the Orange Book listed patents are invalid, unenforceable and/or will not be infringed by the Sandoz Product. On May 26, 2011, Merck, Pfizer, and the Company filed a patent infringement lawsuit against Sandoz and related entities. The plaintiff companies have agreed that Merck will take the lead in prosecuting this lawsuit. The filing of this lawsuit triggered an automatic stay, or bar, of the FDA’s approval of the ANDA for up to 30 months or until a final district court decision of the infringement lawsuit, whichever comes first. The Company and the other plaintiffs intend to vigorously enforce our patent rights relating to AzaSite and vigorously contest any Sandoz assertions that these patents are invalid or unenforceable.
The Company believes that there are currently no other claims or legal actions that would have a material adverse impact on our financial position, operations or potential performance.
66
14. Quarterly Results (Unaudited)
The following table is a summary of the quarterly results of operations for the years ended December 31, 2011 and 2010. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
| | | | | | | | | | | | | | | | |
| | | 2011 | |
(In thousands, except per share amounts) | | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter | |
Revenues | | $ | 3,110 | | | $ | 3,069 | | | $ | 6,613 | | | $ | 3,131 | |
Cost of revenues | | | 577 | | | | 368 | | | | 343 | | | | 629 | |
Gross profit | | | 2,533 | | | | 2,701 | | | | 6,270 | | | | 2,502 | |
Income (loss) from operations | | | 124 | | | | (212 | ) | | | 2,695 | | | | (1,583 | ) |
Net income (loss) | | | (2,440 | ) | | | (2,773 | ) | | | 1,511 | | | | (3,240 | ) |
—basic | | $ | (0.03 | ) | | $ | (0.03 | ) | | $ | 0.01 | | | $ | (0.02 | ) |
—diluted | | $ | (0.03 | ) | | $ | (0.03 | ) | | $ | 0.01 | | | $ | (0.02 | ) |
| | | | | | | | | | | | | | | | |
| | | 2010 | |
(In thousands, except per share amounts) | | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter | |
Revenues | | $ | 2,282 | | | $ | 2,470 | | | $ | 3,235 | | | $ | 3,880 | |
Cost of revenues | | | 303 | | | | 336 | | | | 625 | | | | 463 | |
Gross profit | | | 1,979 | | | | 2,134 | | | | 2,610 | | | | 3,417 | |
Income (loss) from operations | | | (384 | ) | | | 111 | | | | 220 | | | | 708 | |
Net loss | | | (2,939 | ) | | | (2,450 | ) | | | (2,345 | ) | | | (1,859 | ) |
—basic | | $ | (0.03 | ) | | $ | (0.03 | ) | | $ | (0.02 | ) | | $ | (0.02 | ) |
—diluted | | $ | (0.03 | ) | | $ | (0.03 | ) | | $ | (0.02 | ) | | $ | (0.02 | ) |
15. Subsequent Events
The Company evaluated subsequent events through the date on which the financial statements were issued, and has determined that there are no subsequent events that require adjustments or disclosure to the financial statements for the year ended December 31, 2011.
67
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures.Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of the end of the period covered by this report (Evaluation Date). Based upon the evaluation, our principal executive officer and principal financial officer concluded as of the Evaluation Date that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms, and (ii) is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Disclosure controls are controls and procedures designed to reasonably ensure that information required to be disclosed in our reports filed under the Exchange Act, such as this report, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls include controls and procedures designed to reasonably ensure that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure. Our quarterly evaluation of disclosure controls includes an evaluation of some components of our internal control over financial reporting, and internal control over financial reporting is also separately evaluated on an annual basis for purposes of providing the management report which is set forth below.
Report of Management on Internal Control Over Financial Reporting.Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
There are inherent limitations in the effectiveness of any system of internal control, including the possibility of human error and the circumvention or overriding of controls. Accordingly, even effective internal controls can provide only reasonable assurances with respect to financial statement preparation. Further, because of changes in conditions, the effectiveness of internal control may vary over time.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2011. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations (COSO) of the Treadway Commission in Internal Control — Integrated Framework. Based on its assessment using those criteria, our management concluded that, as of December 31, 2011, our internal control over financial reporting was effective.
Changes in Internal Control Over Financial Reporting. There were no changes in our internal control over financial reporting (as defined in Exchange act Rule 13a-15(f)) during the quarter ended December 31, 2011 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information |
Not applicable.
68
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
Board of Directors of the Company
Set forth below is information regarding the Board of Directors (Board) as of March 5, 2012, including a brief description of each director’s qualifications:
| | | | | | | | | | |
Name | | Position(s) with the Company | | Age | | | Director Since | |
Timothy McInerney | | Chairman of the Board and Director | | | 51 | | | | 2008 | |
Brian Levy, O.D. M.Sc.. | | Director | | | 60 | | | | 2011 | |
Robert O’Holla. | | Director | | | 60 | | | | 2008 | |
Timothy Ruane | | Director and Chief Executive Officer | | | 47 | | | | 2010 | |
Craig Tooman | | Director | | | 46 | | | | 2011 | |
Anthony J. Yost | | Director | | | 53 | | | | 2008 | |
Business Experience of Board and Qualifications
Timothy McInerney is currently a Partner with Riverbank Capital Securities, an investment banking firm that specializes in providing financing for biotechnology and specialty pharmaceutical companies, a position he has held since June 2007. From 1992 until March 2007, Mr. McInerney was a Managing Director of Paramount Biocapital, Inc. (Paramount) where he oversaw the distribution of Paramount’s private equity product. Prior to 1992, Mr. McInerney was a research analyst focusing on the biotechnology industry at Ladenburg, Thalman & Co. Previously, Mr. McInerney held equity sales positions at Bear, Stearns & Co. and Shearson, Lehman Bros. Mr. McInerney has also worked in sales and marketing for Bristol-Myers Squibb. He received his B.S. in Pharmacy from St. John’s University in New York. He also completed a post-graduate residency in drug information systems at the New York University Medical Center. Mr. McInerney currently serves on the boards of Manhattan Pharmaceuticals, Inc., Ziopharm Oncology, Inc and Edgemont Pharmaceuticals, LLC. Mr. McInerney has valuable experience as a partner with an investment banking firm that specializes in financing for biotechnology companies. Mr. McInerney’s background and skills are important for the Board’s understanding of financial business development, financing and accounting matters. Mr. McInerney also brings interesting and valuable input to the Board due to his extensive knowledge of the Company’s industry obtained while serving as a research analyst for the biotechnology industry.
Brian Levy, O.D. M.Sc. is currently the Chief Medical Officer of Aerie Pharmaceuticals, a venture backed company developing novel technologies for the treatment of glaucoma, a position he has held since January 2012. From June 2010 until October 2011, Dr. Levy was Chief Scientific Officer of Nexis Vision a small company developing technologies for refractive correction of the eye. From June 2008 to November 2009, Dr. Levy was the Chief Operating Officer of Danube Pharmaceuticals, a venture backed company developing therapeutics for the treatment of glaucoma. From September 1994 to June 2008, Dr. Levy held a series of senior leadership positions with Bausch & Lomb, which included Corporate Vice President and Chief Medical Officer responsible for clinical development, medical affairs and product safety for all Bausch & Lomb businesses worldwide. While at Bausch & Lomb, he oversaw the development of Besivance. Dr. Levy is also a Clinical Professor of Ophthalmology at the University of Rochester in New York. He earned a Doctor of Optometry degree from the University of California, Berkeley in 1976 and a Master of Science Degree in comparative anatomy and physiology of the eye from the University of Waterloo, Canada. Dr. Levy’s medical and management background and experience aid the Board in its understanding of our business, planning clinical trials and otherwise developing our product candidates, as well as formulating our business strategy.
Robert O’Holla is currently the President of R.O.H. Consulting, LLC, a regulatory consulting firm specializing in medical devices, a position he has held since May 2008. Mr. O’Holla was the Worldwide Vice President of Regulatory Affairs at Johnson and Johnson from June 1990 until May 2008. Mr. O’Holla is a health
69
products executive who has over thirty years experience, including research and development, quality and compliance, regulatory affairs, policy development and product sterilization. Mr. O’Holla has also written for several publications regarding health products. He received his B.A. from Upsala College, his A.A. from Union College and his M.B.A. from Fairleigh Dickinson University. Mr. O’Holla currently is the Vice President of Regulatory Affairs for Nfocus Neuromedical and CeQur, SA. Mr. O’Holla also serves on the Board of CeQur, SA. Mr. O’Holla brings in-depth knowledge of the important regulatory issues faced by the Company and the regulatory framework in which the Company operates. Mr. O’Holla’s ability as a consultant to skillfully analyze our operations aids the Board in its oversight role.
Timothy Ruane joined the Company on December 1, 2010 as Chief Executive Officer and a member of the Board. Previously, Mr. Ruane served as President and Chief Executive Officer of Tekmira Pharmaceuticals and INEX Pharmaceuticals (which spun-out to Tekmira in 2007), a biopharmaceutical company, from 2005 to 2008. From 2004 to 2005, he served as the Senior Vice President of Corporate Development of INEX. From 2002 to 2004, Mr. Ruane was the Senior Vice President of Business Management of ILEX Oncology. Mr. Ruane has more than 24 years of experience with pharmaceutical and biotechnology companies in various management positions. Mr. Ruane has a Bachelor of Science degree in business finance from Wake Forest University and a Masters in Business Administration from the University of Washington. Over the past five years, Mr. Ruane has also served on the boards of INEX Pharmaceuticals and Tekmira. Mr. Ruane’s experience in leadership positions at biopharmaceutical companies aids the Board in its understanding of our business. Further, Mr. Ruane has valuable experience in business development, financing, strategic alternatives and industry trends. Mr. Ruane, as our Chief Executive Officer, has in depth knowledge of our day-to-day operations, which is essential to the Board’s ability to understand our company.
Craig Tooman is the founder and principal of Stockbourne LLC, a firm that provides strategic business and financial advisory services, a position he has held since January 2011. From July 2010 to January 2011, Mr. Tooman was the Senior Vice President of Finance and Chief Financial Officer of Ikaria Inc., a biotherapeutics company. From January 2005 to July 2010, Mr. Tooman was the Executive Vice President of Finance and Chief Financial Officer at Enzon Pharmaceuticals, a biopharmaceutical company. Prior to that, Mr. Tooman was the Senior Vice President of Strategic Planning and Corporate Communications at ILEX Oncology, Inc and the Vice President of Investor Relations at Pharmacia Corporation, the culmination of a 12-year career that started with the Upjohn Company. He has a Bachelor of Arts degree in Economics from Kalamazoo College and Masters in Business Administration in Finance from the University of Chicago. Mr. Tooman’s financial background and experience serving in various management roles at biotechnology companies is valuable for the Board’s understanding of our financial condition, accounting and operations and aids the Board in developing and implementing our financial strategy.
Anthony J. Yost is currently the Chief Commercial Officer at Prometheus, Inc., a pharmaceutical/diagnostic company, a position he has held since January 2012. From August 2011 to January 2012, Mr. Yost was the Chief Commercial Officer at EKR Therapeutics, an acute-care pharmaceutical company and prior to that, he was the head of Sales and Marketing at PacificCord, a stem cell storage biotechnology company. From October 2008 to June 2010, Mr. Yost was the Executive General Manager at Novartis, a pharmaceutical company engaged in the research, development, manufacture and marketing of healthcare products. From November 2003 to September 2008, he served as the President of Innovex North America, the commercial services unit of Quintiles Transnational Corporation. From February 1998 to November 2003, Mr. Yost had various responsibilities at Schering-Plough Corporation (Schering-Plough), including Vice President of the Acute Coronary Syndromes Business Unit, General Manager of Commercial and Manufacturing Operations in Portugal, Vice President of Managed Care and Vice President of the Cardiovascular Business Unit. Prior to working for Schering-Plough, Mr. Yost worked for Boehringer Mannheim and Eli Lilly and Company. He received his B.S. in Pharmacy from Purdue University. Mr. Yost’s experience in leadership positions at pharmaceutical companies aids the Board in its understanding of our business, including our customers and competitors. Further, Mr. Yost’s international experience is valuable for the Board’s understanding of foreign markets.
70
Executive Officers and Other Officers of the Company
As of March 5, 2012, our executive officers and other officers were as follows:
| | | | | | |
Name | | Title | | Age | |
Timothy Ruane | | Chief Executive Officer and member of the Board | | | 47 | |
Louis Drapeau | | Vice President and Chief Financial Officer | | | 68 | |
Lyle M. Bowman, Ph.D. | | Vice President, Development | | | 63 | |
Kamran Hosseini, M.D., Ph.D. | | Vice President, Clinical & Regulatory Affairs and Chief Medical Officer | | | 47 | |
Surendra Patel | | Vice President, Operations & Quality | | | 57 | |
Timothy Ruane joined us on December 1, 2010 as Chief Executive Officer and was elected as a member of the Board. Previously, Mr. Ruane served as President and Chief Executive Officer of Tekmira Pharmaceuticals and INEX Pharmaceuticals (which spun-out to Tekmira in 2007), a biopharmaceutical company, from 2005 to 2008. From 2004 to 2005, he served as the Senior Vice President of Corporate Development of INEX. From 2002 to 2004, Mr. Ruane was the Senior Vice President of Business Management of ILEX Oncology. Mr. Ruane has more than 24 years of experience with pharmaceutical and biotechnology companies in various management positions. Mr. Ruane has a Bachelor of Science degree in business finance from Wake Forest University and a Masters in Business Administration from the University of Washington.
Louis Drapeau joined us on October 1, 2007 as Vice President and Chief Financial Officer and served as the interim Chief Executive Officer from October 2008 through November 2010. Mr. Drapeau served as Senior Vice President, Finance and Chief Financial Officer of Nektar Therapeutics, a biopharmaceutical company, from January 2006 until September 2007. From August 2002 to August 2005, Mr. Drapeau was Senior Vice President and Chief Financial Officer of BioMarin Pharmaceutical, a fully integrated biopharmaceutical company. From August 2004 to May 2005, Mr. Drapeau also held the position of Acting Chief Executive Officer of BioMarin. Prior to that, Mr. Drapeau spent over 30 years with Arthur Andersen including 19 years as an Audit Partner in Arthur Andersen’s Northern California Audit and Business Consulting practice including 12 years as Managing Partner. He holds an undergraduate degree in mechanical engineering and Masters in Business Administration from Stanford University.
Lyle M. Bowman joined us in October 1988 as Director of Drug Delivery Systems. Previously, Dr. Bowman had worked at Syntex Ophthalmics as Manager/Director of Analytical Polymer Characterization working on contact lenses and solutions from 1979 through September 1988. From 1989 to 1991, Dr. Bowman served as Vice President, Science and Technology. From 1991 to 1995, he served as Vice President, Development and from 1995 to 2008, he served as Vice President Development and Operations. Dr. Bowman currently is Vice President Development, holds a Ph.D. in Physical Chemistry from the University of Utah and has considerable experience in material science as applied to ophthalmic products.
Kamran Hosseini joined us in February 2008 as Vice President, Clinical & Regulatory Affairs and Chief Medical Officer. From November 2007 to February 2008, Dr. Hosseini served as the ophthalmic expert at JGB BioPharma consulting for R&D, preclinical, clinical, and business development projects. From May 2005 to October 2007, he was the director of ophthalmology drug delivery programs at Alza Corporation, a member of the Johnson and Johnson Family of Companies, where he provided ophthalmology and visual science expertise for new technology assessment activities aimed at enhancing the drug/device unit pipeline. From November 2003 to May 2005, he was a post doctoral fellow in retinal degenerative diseases at the University of California, San Francisco. Dr. Hosseini received his M.D. from the University of Groningen Faculty of Medicine, The Netherlands, and his Ph.D. as part of a joint program at the University of Texas, Medical Branch in Galveston and the University of Maastricht, The Netherlands.
Surendra Patel joined us in April 2008 as Vice President, Operations & Quality. From 2002 to 2008, Mr. Patel served as Senior Director, Manufacturing Operations at Nektar Therapeutics where he managed clinical and commercial manufacturing operations and played a strategic role in the selection of domestic and
71
international contract manufacturing sites. Mr. Patel has more than 30 years of development and operational experience in various management positions at pharmaceutical and biotechnology companies, including Syntex, Roche Bioscience, Oread Inc., and DrugAbuse Sciences. Mr. Patel has a Bachelor of Science degree in pharmaceutical formulation from De Montford University, Leicester, United Kingdom.
Officers are appointed to serve at the discretion of the Board until their successors are appointed. There are no family relationships between any members of the Board and our executive officers. Also, no member of the Board currently is, or in the last ten years has been, involved in any legal proceedings that are required to be disclosed by the rules and regulations of the SEC. The Board has determined that each of the members of the Board, other than Mr. Ruane, is “independent” as that term is defined in the rules and regulations of the NYSE Amex (NYSE Amex Rules).
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee currently consists of two directors, Timothy McInerney (Chair) and Brian Levy. The Nominating and Corporate Governance Committee held four meetings during the 2011 fiscal year to evaluate potential candidates to fill vacancies arising on the Board during the fiscal year 2011, recommend such candidates to the Board and recommend to the Board the nomination of directors standing for election at the 2011 Annual Meeting.
The Board has determined that each of the members of the Nominating and Corporate Governance Committee is “independent” as that term is defined in the NYSE Amex Rules. A copy of the Nominating and Corporate Governance Committee charter is available on our website located at www.insitevision.com. A copy of the Nominating and Corporate Governance Committee’s charter is also available to investors free of charge by writing to InSite Vision Incorporated, Investor Relations, 965 Atlantic Avenue, Alameda, CA 94501.
The Nominating and Corporate Governance Committee identifies and recommends director nominees to be selected by the Board for submission to a vote at our annual stockholder meetings or to fill vacancies between such meetings, implements the Board’s criteria for selecting new directors, develops or reviews and recommends corporate governance policies for the Board and oversees any annual evaluation process conducted by the Board.
Consideration of Director Nominees
Stockholder Nominees
The policy of the Nominating and Corporate Governance Committee is to consider properly submitted stockholder nominations for candidates for membership on the Board as described below under “Identifying and Evaluating Nominees for Directors.” In evaluating such nominations, the Nominating and Corporate Governance Committee seeks to achieve a balance of knowledge, experience and capability on the Board and to address the membership criteria set forth under “Director Qualifications.”
The Nominating and Corporate Governance Committee will consider timely suggestions of nominees from stockholders using the same criteria as any other nominee. A stockholder’s notice of nominations must comply with the timing and delivery requirements and include the required information set forth in our Bylaws.
Director Qualifications
The Nominating and Corporate Governance Committee has established the following minimum criteria for evaluating prospective Board candidates:
| | • | | Reputation for integrity, strong moral character and adherence to high ethical standards. |
| | • | | Attainment of a generally recognized position of leadership in the community and/or chosen field of endeavor, and demonstrated high levels of accomplishment. |
72
| | • | | Demonstrated business acumen and experience, and ability to exercise sound business judgment and common sense in matters that relate to our current and long-term objectives. |
| | • | | Ability to read and understand basic financial statements and other financial information pertaining to us. |
| | • | | Commitment to understanding us and our business, industry and strategic objectives. |
| | • | | Commitment and ability to regularly attend and participate in meetings of the Board, committees of the Board and stockholders; and ability to generally fulfill all responsibilities as a director. |
| | • | | Willingness to represent and act in the interests of all of our stockholders rather than the interests of a particular group. |
| | • | | Good health and ability to serve. |
| | • | | For prospective non-employee directors, independence under applicable SEC and stock exchange rules, and an absence of any conflict of interest (whether due to a business or personal relationship) or legal impediment to, or restriction on, the nominee serving as a director. |
| | • | | Willingness to accept the nomination to serve as a director. |
Other Factors for Potential Consideration
The Nominating and Corporate Governance Committee will also consider the following factors in connection with its evaluation of each prospective nominee:
| | • | | Whether the prospective nominee will foster a diversity of skills and experiences and add to or complement the Board’s existing strengths. |
| | • | | For potential Audit Committee members, whether the nominee possesses the requisite education, training and experience to qualify as “financially sophisticated” or as an audit committee “financial expert” under applicable SEC and stock exchange rules. |
| | • | | For incumbent directors standing for re-election, the Nominating and Corporate Governance Committee will assess the incumbent director’s performance during his or her term, including the number of meetings attended, level of participation and overall contribution to the Company. |
| | • | | The number of other boards on which the prospective nominee serves. |
Identifying and Evaluating Nominees for Directors
The Nominating and Corporate Governance Committee initiates the process of identifying and evaluating nominees for director by preparing a slate of potential candidates who, based on their biographical information and other information available to the Nominating and Corporate Governance Committee, appear to meet the criteria specified above and/or have the specific qualities, skills or experience being sought (based on input from the full Board).
| | • | | Outside Advisors. The Nominating and Corporate Governance Committee may engage a third-party search firm or other advisors to assist in identifying prospective nominees. |
| | • | | Nomination of Incumbent Directors. The re-nomination of existing directors is not automatic, but is based on continuing qualification under the criteria set forth above. |
| | • | | For incumbent directors standing for re-election, the Nominating and Corporate Governance Committee will assess the incumbent director’s performance during his or her term, including the number of meetings attended, level of participation and overall contribution to the Company; the number of other company boards on which the individual serves; composition of the Board at that time and any changed circumstances affecting the individual director which may bear on his or her ability to continue to serve on the Board. |
73
| | • | | Management Directors. The number of our officers or employees serving at any time on the Board should be limited such that, at all times, a majority of the directors are “independent” under applicable SEC and NYSE Amex Rules. |
After reviewing appropriate biographical information and qualifications, first-time candidates will be interviewed by at least one member of the Nominating and Corporate Governance Committee and by our Chief Executive Officer. Upon completion of the above procedures, the Nominating and Corporate Governance Committee shall determine the list of potential candidates to be recommended to the full Board for nomination at our Annual Meeting of Stockholders. The Board will select the slate of nominees only from candidates identified, screened and approved by the Nominating and Corporate Governance Committee.
Audit Committee
We have a separately-designated standing audit committee. The Audit Committee currently consists of three directors: Craig Tooman (Chair), Timothy McInerney and Anthony J. Yost. The Audit Committee met six times in 2011.
The Board has determined that each of the members of the Audit Committee is “independent” as that term is defined in the NYSE Amex Rules and also meets the additional criteria for independence of audit committee members set forth in Rule 10A-3(b)(1) under the Exchange Act. In additional, the Board has determined that Mr. Tooman qualifies as an “audit committee financial expert,” within the meaning of SEC regulations. A copy of the Audit Committee’s charter is available on our website located at www.insitevision.com. A copy of the Audit Committee’s charter is also available to investors free of charge by writing to InSite Vision Incorporated, Investor Relations, 965 Atlantic Avenue, Alameda, CA 94501.
The Audit Committee appoints our independent registered public accounting firm; pre-approves all audit and non-audit services to be provided to the Company by the independent registered public accounting firm; oversees the independence of the independent registered public accounting firm; evaluates the independent registered public accounting firm’s performance; receives and considers the independent registered public accounting firm’s comments as to accounting and financial controls; monitors the effectiveness of internal controls; oversees our financial and accounting organization and financial reporting; discusses with management and the independent registered public accounting firm the results of the annual audit and our annual financial statements; and discusses with management and the independent registered public accounting firm, as applicable, the results of the independent registered public accounting firm’s interim review of our quarterly financial statements, as well as our earnings press releases.
Among other things, under the charter of the Audit Committee, the Audit Committee is responsible for reviewing and approving all related party transactions, approving and monitoring our code of ethics for senior finance personnel and code of conduct for all employees and directors (including approving any waivers of such codes for directors, executive officers and senior financial personnel), and establishing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal accounting controls, or auditing matters, and the confidential, anonymous submission by our employees of concerns regarding questionable accounting, auditing or legal matters.
Code of Ethics for the Chief Executive Officer and Senior Financial Officers
Our Code of Ethics for the Chief Executive Officer and Senior Financial Officers can be found on our website located at www.insitevision.com. A copy of our Code of Ethics for the Chief Executive Officer and Senior Financial Officers is also available to investors free of charge by writing to InSite Vision Incorporated, Investor Relations, 965 Atlantic Avenue, Alameda, CA 94501. We will disclose any waivers under the Code of Ethics for the Chief Executive Officer and Senior Financial Officers which are granted to our directors or executive officers in a current report on Form 8-K filed with the SEC within four business days of any such waiver. No such waivers were granted during 2011.
74
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors, executive officers and persons who beneficially own more than 10% of a registered class of our equity securities to file initial reports of beneficial ownership and reports of changes in beneficial ownership of our common stock and our other equity securities with the SEC. Officers, directors and greater than 10% stockholders are required by the Exchange Act to furnish us with copies of all Section 16(a) reports they file.
Based solely upon review of the copies of such reports furnished to us and written representations that no other reports were required, we believe that during the fiscal year ended December 31, 2011, our officers, directors and holders of more than 10% of the Common Stock complied with all Section 16(a) filing requirements with the following exceptions: Mr. McInerney, Mr. O’Holla, and Mr. Yost each received a stock option grant on December 14, 2011 to purchase 150,000 shares of common stock pursuant to the 2007 Plan and the Forms 4 for these grants were filed late with the SEC on December 20, 2011; and Dr. Levy and Mr. Tooman each received a stock option grant on December 14, 2011 to purchase 150,000 shares of common stock pursuant to the 2007 Plan and the Forms 4 for these grants were filed late with the SEC on December 21, 2011.
75
| Item 11. | Executive Compensation |
Director Compensation
The following table presents information regarding the compensation paid for 2011 to members of the Board who are not, or were not during the term, also employees of the Company (Non-Employee Directors).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name (a) | | Fees Earned or
Paid in Cash
($)(1)
(b) | | | Stock Awards
($)(2)
(c) | | | Option Awards
($)(2)(3)
(d) | | | Non-Equity
Incentive Plan
Compensation
($)
(e) | | | Change in Pension
Value and
Nonqualified
Deferred
Compensation
Earnings
($)
(f) | | | All Other
Compensation
($)
(g) | | | Total
($)
(h) | |
Timothy McInerney | | | 46,354 | | | | — | | | | 45,900 | | | | — | | | | — | | | | — | | | | 92,254 | |
Brian Levy, O.D. M. Sc. (4) | | | 18,333 | | | | — | | | | 67,753 | | | | — | | | | — | | | | — | | | | 86,086 | |
Robert O’Holla | | | 45,000 | | | | — | | | | 45,900 | | | | — | | | | — | | | | — | | | | 90,900 | |
Craig A. Tooman (5) | | | 15,313 | | | | — | | | | 62,897 | | | | — | | | | — | | | | — | | | | 78,210 | |
Anthony J. Yost | | | 47,500 | | | | — | | | | 45,900 | | | | — | | | | — | | | | — | | | | 93,400 | |
Evan S. Melrose, M.D. (6) | | | 45,833 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 45,833 | |
Rick D. Anderson (7) | | | 29,688 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 29,688 | |
Timothy P. Lynch (8) | | | 37,188 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 37,188 | |
| (1) | Director compensation is pro-rated based on the service term of each director. |
| (2) | The amount reported in Column (d) of the table above reflects the aggregate grant date fair value of stock option awards (disregarding any estimate of forfeitures related to service-based vesting conditions). Each of these options vests on the first anniversary of the grant date provided the director continues to serve through such date. We have not granted any equity-based awards to Non-Employee Directors other than stock options. For a discussion of the assumptions and methodologies used to value the awards reported in Column (d), please see the discussion of option awards contained in Note 11, Employee Stock-based Compensation, in the Notes to the Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K. |
| (3) | The following table presents, as of December 31, 2011, the number of outstanding and unexercised option awards held by each person who served as a Non-Employee Directors during fiscal year 2011. |
| | | | | | | | | | | | | | | | | | | | |
| | | Option
Grant Date | | | Number of
Securities
Underlying
Unexercised
Options
(#)
Exercisable | | | Number of
Securities
Underlying
Unexercised
Options
(#)
Unexercisable | | | Option
Exercise
Price
($) | | | Option
Expiration
Date | |
Timothy McInerney | | | 12/14/2011 | | | | — | | | | 150,000 | | | | 0.44 | | | | 12/14/2021 | |
| | | 12/20/2010 | | | | 150,000 | | | | — | | | | 0.33 | | | | 12/20/2020 | |
| | | 12/16/2009 | | | | 150,000 | | | | — | | | | 0.38 | | | | 12/16/2019 | |
| | | 9/23/2008 | | | | 30,000 | | | | — | | | | 0.52 | | | | 9/23/2018 | |
Brian Levy, O.D. M. Sc. (4) | | | 12/14/2011 | | | | — | | | | 150,000 | | | | 0.44 | | | | 12/14/2021 | |
| | | 9/16/2011 | | | | — | | | | 56,250 | | | | 0.56 | | | | 9/16/2021 | |
Robert O’Holla | | | 12/14/2011 | | | | — | | | | 150,000 | | | | 0.44 | | | | 12/14/2021 | |
| | | 12/20/2010 | | | | 150,000 | | | | — | | | | 0.33 | | | | 12/20/2020 | |
| | | 12/16/2009 | | | | 150,000 | | | | — | | | | 0.38 | | | | 12/16/2019 | |
| | | 9/23/2008 | | | | 30,000 | | | | — | | | | 0.52 | | | | 9/23/2018 | |
Craig A. Tooman (5) | | | 12/14/2011 | | | | — | | | | 150,000 | | | | 0.44 | | | | 12/14/2021 | |
| | | 9/16/2011 | | | | — | | | | 43,750 | | | | 0.56 | | | | 9/16/2021 | |
Anthony J. Yost | | | 12/14/2011 | | | | — | | | | 150,000 | | | | 0.44 | | | | 12/14/2021 | |
| | | 12/20/2010 | | | | 150,000 | | | | — | | | | 0.33 | | | | 12/20/2020 | |
| | | 12/16/2009 | | | | 150,000 | | | | — | | | | 0.38 | | | | 12/16/2019 | |
| | | 9/23/2008 | | | | 30,000 | | | | — | | | | 0.52 | | | | 9/23/2018 | |
Evan S. Melrose, M.D. (6) | | | 12/16/2009 | | | | 150,000 | | | | — | | | | 0.38 | | | | 2/1/2012 | |
| | | 9/23/2008 | | | | 30,000 | | | | — | | | | 0.52 | | | | 2/1/2012 | |
76
| (4) | Dr. Levy was appointed to the Board on August 15, 2011. |
| (5) | Mr. Tooman was appointed to the Board on September 16, 2011. |
| (6) | Dr. Melrose resigned from the Board on November 2, 2011. |
| (7) | Mr. Anderson resigned from the Board on August 15, 2011. |
| (8) | Mr. Lynch resigned from the Board on September 16, 2011. |
Summary of Director Compensation
Cash and Expenses. The compensation for Non-Employee Directors in fiscal year 2011 consisted of cash fees and retainers and grants of stock options as follows:
| | • | | an annual cash retainer of $45,000 for serving on the Board; |
| | • | | an annual cash retainer of $10,000 for serving as the Chairman of the Board; and |
| | • | | an annual cash retainer for serving as the Chairman of a committee of the Board ($7,500 for the Audit Committee, $5,000 for the SAB, $2,500 for the Stock Plan and Compensation Committee (Compensation Committee), $2,500 for the Nominating and Corporate Governance Committee) |
We also reimburse Non-Employee Directors for reasonable expenses for attending any Board or committee meetings.
Option Grants. Under our current director compensation program, each Non-Employee Director is granted, at the time of his or her initial election or appointment to the Board, an option to purchase a pro rata portion of 150,000 shares of our common stock based on the month in which the Non-Employee Director is initially elected or appointed to the Board. Thereafter, each Non-Employee Director in office on the date of the first Board meeting in December of each year is granted on such date an option to purchase 150,000 shares of our common stock. In the event that there is no Board meeting in December of any year, the annual option grant will be made on December 15 of that year (or, if December 15 is not a trading day, the next succeeding trading day). In addition, Non-Employee Directors are eligible to receive discretionary award grants at any time under the 2007 Plan.
Each option granted to a Non-Employee Director has a per-share exercise price equal to the closing price of our common stock on the date of grant and a maximum term of ten years. These options vest on the first anniversary of the grant date, subject to the director’s continued service as a director, but may vest on an accelerated basis in connection with a change in control. Vested options will generally remain exercisable for three months following the termination of the director’s service or six months following a termination due to death or disability. The options do not include any dividend rights.
On December 14, 2011, each of our Non-Employee Directors received a grant of an option to purchase 150,000 shares of our common stock. Each of these options has a per-share exercise price of $0.44 (the closing price of our common stock on the grant date) and will vest on the first anniversary of the grant date, provided that the director remains in service through such date. Each of these options was granted under, and is subject to, the terms of, the 2007 Plan.
On September 16, 2011, Dr. Levy and Mr. Tooman each were granted an option to purchase 56,250 shares and 43,750 shares, respectively, of our common stock. These grants were a proration of the 150,000 option shares based on their service term for 2011. Each of these options has a per-share exercise price of $0.56 (the closing price of our common stock on the grant date) and will vest on the first anniversary of the grant date. Each of these options was granted under, and is subject to, the terms of the 2007 Plan.
Compensation Discussion and Analysis
This section contains a discussion of the material elements of compensation earned by our principal executive officer, our principal financial officer and our other executive officer for 2011. These individuals are listed in the “Summary Compensation Table” below and referred to as the “Named Executive Officers” in this Annual Report on Form 10-K.
77
Our executive officer compensation program is determined and approved by the Compensation Committee. None of the Named Executive Officers are members of the Compensation Committee or otherwise had any role in determining the compensation of the other Named Executive Officers, although the Compensation Committee does consider the recommendations of our Chief Executive Officer in setting compensation levels for the Named Executive Officers other than the Chief Executive Officer.
Executive Officer Compensation Program Objectives and Overview
The Compensation Committee’s principal goals in making its executive officer compensation decisions are:
| | • | | to help ensure that there exists an appropriate relationship between executive pay and both our performance and the creation of stockholder value through our achievement of long-term strategic goals and initiatives; |
| | • | | to attract, motivate and retain key executives in the face of competition within the biotechnology industry for qualified personnel; and |
| | • | | to align the interests of our executive officers with those of our stockholders. |
Our current executive officer compensation program is based on three components, which are designed to be consistent with our compensation philosophy: (1) base salary, (2) annual incentive bonuses and (3) long-term equity incentive awards that are generally subject to time-based vesting requirements. We also provide Named Executive Officers with severance benefits if the executive’s employment terminates under certain circumstances. In structuring named executive officer compensation packages, the Compensation Committee considers how each component attracts, retains and motivates the best possible executive talent. We do not provide any material perquisites or personal benefits to our Named Executive Officers.
Base salaries and severance benefits are primarily intended to attract and retain highly qualified executives. Annual incentive bonuses are primarily intended to motivate the Named Executive Officers to achieve specific strategic and operating objectives for that year. Long-term equity incentive awards are primarily intended to align the Named Executive Officers’ long-term interests with our stockholders’ long-term interests. We also believe that competitive base salaries, annual bonuses and equity incentives help us attract and retain top executives. These elements of our named executive officer compensation program are designed to reward performance and thus the creation of stockholder value.
As part of our annual review of our named executive officer compensation programs, the Compensation Committee generally reviews Radford surveys of compensation paid to similarly situated executive officers in the technology and biotechnology industries in the San Francisco Bay Area. In setting compensation levels for the Named Executive Officers, the Compensation Committee does not “benchmark” compensation against this survey data. Rather, as discussed below, the Compensation Committee considers a number of factors in making its decisions and uses this market data as a general reference point. The Compensation Committee did not engage any independent consultants in 2011.
Current Named Executive Officer Compensation Program Elements
Base Salaries
The base salaries of the Named Executive Officers are reviewed by the Compensation Committee on an annual basis, as well as at the time of a promotion or other material change in responsibility. Any increases in base salary are based on an evaluation of the particular individual’s performance and base salaries of similarly situated executives at comparable companies based on data from the Radford surveys, as well as the individual’s importance to our future plans.
78
In reviewing the base salaries for each of the Named Executive Officers for 2011 the Compensation Committee took into account:
| | • | | the individual’s performance against our corporate performance goals; |
| | • | | the individual’s particular experience in the biotechnology or pharmaceutical industries; |
| | • | | the scope of the executive’s responsibilities and the executive’s importance in achieving our business goals; and |
| | • | | the base salary data for similarly situated executives (i.e. position, experience and responsibilities) based on data from the Radford surveys referenced above. |
Based on its review, the Compensation Committee approved an increase in Mr. Bowman’s base salary from $263,500 to $271,750 for 2011, and no change was made to the base salaries for Mr. Ruane and Mr. Drapeau.
Annual Bonuses
The InSite Vision Incorporated 2011 Bonus Plan (Bonus Plan) provides bonus opportunities each year for our employees, including each of the Named Executive Officers. Each participant in the Bonus Plan has a target bonus percentage that is expressed as a percentage of the participant’s annual base salary. The Bonus Plan provides that the target bonus percentages for all participants are aggregated to determine our on-target bonus pool. The actual bonus pool for participants in the Bonus Plan is determined based on our achievement of specific performance goals established by the Compensation Committee and approved by the Board for the bonus year. The participant’s actual bonus amount is then subject to increase or decrease based on the participant’s individual performance during the year in meeting such performance goals. In the case of the Named Executive Officers, the Compensation Committee evaluates the participant’s individual performance and determines his or her final bonus amount after the end of the year.
For 2011, the Compensation Committee established a target bonus percentage for Mr. Ruane of 75% of his base salary, with the target bonus percentage for each of the other Named Executive Officers being 30% of his or her base salary. The Compensation Committee established 23 specified corporate goals to measure our performance for 2011 with each goal weighted between 1% and 25% of the total bonus opportunity. The significant goals (i.e. weighted over 50%) were completing a financing transaction, preparing for and initiating a Phase 3 clinical trial for AzaSite Plus/DexaSite and receiving positive top-line results from the Phase 1/2 clinical trial and Phase 2 PK study for BromSite. The amount of the actual bonus pool with respect to each performance goal could range from 50% to 140% of the on-target bonus pool for that goal based on the date by which that particular goal was attained or, in the case of financial performance metrics, our actual performance as compared with the pre-established goals. For 2011, the Compensation Committee set the following financial performance targets: maintaining operating expenses (excluding cost of sales) at less than $5.4 million for the first half of 2011 and maintaining a minimum of one year of cash on hand.
The Compensation Committee evaluated our performance with respect to each of the 23 corporate goals established for the Bonus Plan and determined that the actual bonus pool for 2011 would be 99% of the on-target bonus pool.
Specifically, we achieved the following significant goals established for the Bonus Plan in 2011:
| | • | | Closed a $20.4 million (net proceeds) private placement financing transaction; |
| | • | | Prepared for and initiated a Phase 3 clinical trial for AzaSite Plus & DexaSite; and |
| | • | | Received positive top-line results from the Phase 1/2 clinical trial and Phase 2 PK study for BromSite; |
Based on its assessment of each Named Executive Officer’s individual performance for 2011, the Compensation Committee decided to award the following bonuses to each of the Named Executive Officers (expressed as a percentage of the executive’s base salary), which, in the case of Messrs. Ruane and Drapeau,
79
slightly exceeded their respective target bonus percentages: Mr. Ruane, 76%, Mr. Drapeau, 31%; and Dr. Bowman, 29%. The specific bonus amounts awarded to each of the Named Executive Officers for 2011 under the bonus plan are reported below under the heading “Non-Equity Incentive Plan Compensation” in the Summary Compensation Table.
Long-Term Incentive Equity Awards
Our policy is that the long-term compensation of our Named Executive Officers and other executive officers should be directly linked to the value provided to stockholders. Therefore, we have historically made annual grants of stock options to provide further incentives to our executives to increase stockholder value. The Compensation Committee bases its award grants to our executives under the 2007 Plan on a number of factors, including:
| | • | | the executive’s position with us and his total compensation package; |
| | • | | the executive’s performance of his or her individual responsibilities; |
| | • | | the equity participation levels of comparable executives at comparable companies; and |
| | • | | our achievement of our business objectives and the executive’s contribution to those achievements. |
In addition, the size, frequency and type of long-term incentive grants may be determined on the basis of tax consequences of the grants to the individual and the Company, accounting impact and potential dilution effects.
Our stock option grants to the Named Executive Officers have an exercise price that is equal to the closing price of our common stock on the grant date. Thus, the executives will only realize value on their stock options if our stockholders realize value on their shares. The stock options also function as a retention incentive for executives as they vest ratably over the four-year period after the date of grant.
The Compensation Committee approved the grant of an option to each of Mr. Drapeau and Dr. Bowman in January 2011. The number of options granted was based on the Named Executive Officer’s level within the Company and, in the case of Mr. Drapeau, based on his outstanding service to the Company even though he is employed on less than a full-time basis. The options granted for each level was based upon our performance and grant levels for executives at comparable companies from the Radford survey referenced above.
Appointment of Chief Executive Officer and Director
On November 23, 2010, the Board appointed Mr. Ruane as our Chief Executive Officer, and a member of the Board, effective December 1, 2010. In connection therewith, Mr. Drapeau stepped down from his position as interim Chief Executive Officer while continuing to serve as Vice President and Chief Financial Officer.
Pursuant to the terms of his offer letter effective December 1, 2010, Mr. Ruane’s base salary was set at $350,000 and he is eligible for a discretionary annual bonus to be determined by the Compensation Committee of up to 75% of his current base salary upon achievement of to be agreed upon annual bonus goals. Additionally, pursuant to the terms of Mr. Ruane’s offer letter, he was granted an option to purchase 2,844,374 shares of our common stock under the 2007 Plan. This option grant vests, subject to Mr. Ruane’s continued employment with us, as to 25% of the shares covered by the option on the first anniversary of Mr. Ruane’s hire date and as to the remaining 75% of the shares in daily installments over the three-year period thereafter. Mr. Ruane also received an initial relocation reimbursement of $67,500 pursuant to the terms of his offer letter and was eligible for up to an additional $150,000 relocation reimbursement.
80
Severance Benefits
We believe that severance protections can play a valuable role in attracting and retaining key executive officers. Accordingly, we provide such protections for certain eligible employees, including each of the Named Executive Officers under our Severance Plan (Severance Plan). The Compensation Committee evaluates the level of severance benefits, if any, to provide to a Named Executive Officer on a case-by-case basis, and in general, we consider these severance protections an important part of an executive’s compensation.
As described in more detail under “Potential Payments Upon Termination or Change in Control” below, Named Executive Officers are generally entitled to severance benefits under the Severance Plan in the event of a termination of employment by us without cause. We have determined that it is appropriate to provide these executives with severance benefits in the event of an involuntary termination of the executive’s employment in light of their positions with us and as part of their overall compensation package.
In addition, we believe that the occurrence, or potential occurrence, of a change in control transaction may create uncertainty regarding the continued employment of our executive officers. This uncertainty results from the fact that many change in control transactions result in significant organizational changes, particularly at the senior executive level. In order to encourage the Named Executive Officers to remain employed with us during an important time when their prospects for continued employment following the transaction are often uncertain, we provide the Named Executive Officers with enhanced severance benefits under the Severance Plan if the executive’s employment is terminated by us without cause or by the executive for good reason in connection with a change in control. Because we believe that a termination by an executive for good reason is conceptually the same as a termination by us without cause, and because we believe that in the context of a change in control, potential acquirers would otherwise have an incentive to constructively terminate the executive’s employment to avoid paying severance, we believe it is appropriate to provide severance benefits in these circumstances.
We generally do not believe that the Named Executive Officers should be entitled to severance benefits merely because a change in control transaction occurs. The payment of cash severance benefits is generally only triggered by an actual or constructive termination of employment.
Policy with Respect to Section 162(m)
Section 162(m) of the Internal Revenue Code generally disallows a tax deduction to publicly-held companies for compensation exceeding $1 million paid to their chief executive officers and certain other executive officers unless certain performance and other requirements are met. Our intent generally is to design and administer executive compensation programs in a manner that will preserve the deductibility of compensation paid to our executive officers, and we believe that a substantial portion of our current executive compensation program (including the stock options granted to the Named Executive Officers as described above) satisfies the requirements for exemption from the $1 million deduction limitation. However, we reserve the right to design programs that recognize a full range of performance criteria important to our success, even where the compensation paid under such programs may not be deductible. The Compensation Committee believes that no part of our tax deduction for compensation paid to the Named Executive Officers for 2011 will be disallowed under Section 162(m). The Compensation Committee will continue to monitor the tax and other consequences of our executive compensation program as part of its primary objective of ensuring that compensation paid to executive officers is reasonable, performance-based and consistent with our goals and those of our stockholders.
81
COMPENSATION COMMITTEE REPORT ON EXECUTIVE COMPENSATION(1)
The Compensation Committee has certain duties and powers as described in its charter. The Compensation Committee is currently composed of the three non-employee directors named at the end of this report, each of whom is “independent” as defined by NYSE Amex Rules.
The Compensation Committee has reviewed and discussed with management the disclosures contained in the Compensation Discussion and Analysis section of this Annual Report on Form 10-K. Based upon this review and discussion, the Compensation Committee recommended to the Board that the Compensation Discussion and Analysis section be included in this Annual Report on Form 10-K filed with the SEC.
|
Compensation Committee of the Board of Directors |
| Anthony J. Yost (Chairman) |
| Robert O’Holla |
| Craig Tooman |
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
The Compensation Committee members whose names appear on the Compensation Committee Report above were committee members throughout the 2011 fiscal year, other than Mr. Tooman who was appointed to the Compensation Committee upon his appointment to the Board on September 16, 2011. No member of the Compensation Committee is or at the relevant time was a former or current executive officer of the Company or had any relationships requiring disclosure by us under the SEC’s rules requiring disclosure of certain relationships and related-party transactions. None of our executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, the executive officers of which served as a director or member of the Compensation Committee during the fiscal year ended December 31, 2011.
| (1) | Unless we specifically state otherwise, this report shall not be deemed to be incorporated by reference and shall not constitute soliciting material or otherwise be considered filed under the Securities Act or the Exchange Act. |
82
SUMMARY COMPENSATION TABLE—FISCAL 2011, 2010 and 2009
The following table presents information regarding compensation of the Named Executive Officers for services rendered during 2011, 2010 and 2009.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name and Principal Position (a) | | Year
(b) | | | Salary
($)
(c) | | | Bonus
($)(1)
(d) | | | Stock
Awards
($)
(e) | | | Option
Awards
($)(2)
(f) | | | Non-Equity
Incentive Plan
Compensation
($)(3)
(g) | | | Change in
Pension Value
and
Nonqualified
Deferred
Compensation
Earnings
($)
(h) | | | All Other
Compensation
($)(4)
(i) | | | Total
($)
(j) | |
Timothy Ruane (5) | | | 2011 | | | | 350,000 | | | | — | | | | — | | | | — | | | | 267,671 | | | | — | | | | 96,978 | | | | 714,649 | |
| Chief Executive Officer and | | | 2010 | | | | 29,167 | | | | — | | | | — | | | | 688,907 | | | | — | | | | — | | | | 67,545 | | | | 785,619 | |
Member of the Board | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Louis Drapeau (6) | | | 2011 | | | | 255,000 | | | | — | | | | — | | | | 75,150 | | | | 78,007 | | | | — | | | | — | | | | 408,157 | |
| Vice President, Chief Financial | | | 2010 | | | | 255,000 | | | | — | | | | — | | | | 114,720 | | | | 102,000 | | | | — | | | | 2,713 | | | | 474,433 | |
Officer | | | 2009 | | | | 255,000 | | | | 50,000 | | | | — | | | | 51,760 | | | | 71,400 | | | | — | | | | 7,010 | | | | 435,170 | |
| | | | | | | | | |
Lyle M. Bowman, Ph.D. | | | 2011 | | | | 271,750 | | | | — | | | | — | | | | 50,100 | | | | 79,096 | | | | — | | | | 970 | | | | 401,916 | |
| Vice President, Development | | | 2010 | | | | 263,750 | | | | — | | | | — | | | | 57,360 | | | | 92,300 | | | | — | | | | 2,376 | | | | 415,786 | |
| | | 2009 | | | | 263,750 | | | | — | | | | — | | | | 25,880 | | | | 55,388 | | | | — | | | | 4,374 | | | | 349,392 | |
| (1) | For Mr. Drapeau, the amount reported for 2009 represents payment of a $50,000 interim CEO retention bonus pursuant to his October 2008 offer letter. |
| (2) | In accordance with SEC rules, the amounts reported in Column (f) of the table above reflect the aggregate grant date fair value of option awards granted during each of 2011, 2010 and 2009 in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 718. We have not granted any equity-based awards other than stock options to Named Executive Officers during 2011, 2010 or 2009. For a discussion of the assumptions and methodologies used to value the awards reported in Column (f), please see the discussion of option awards contained in Note 11, Employee Stock-based Compensation, in the Notes to the Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K (or, for years prior to 2011, the corresponding note in the Annual Report on Form 10-K for the applicable fiscal year). For information about the option awards granted to the Named Executive Officers for 2011, please see the discussion under “Grants of Plan-Based Awards” below. |
| (3) | The figures reported in this column reflect the annual performance bonuses that the Named Executive Officers earned for 2011, 2010 and 2009. As described in the “Compensation Discussion and Analysis” above, the Named Executive Officers were paid performance bonuses in 2012, 2011 and 2010 in connection with their contributions to the Company in the previous year. |
| (4) | The amounts reported in Column (i) represent our payments of group term life insurance premiums on behalf of each Named Executive Officer. We are not the beneficiary of the life insurance policies, and the premiums we pay are taxable as income to the applicable officer. For Mr. Ruane, this amount includes a relocation reimbursement of $96,438 and $67,500 for 2011 and 2010, respectively, pursuant to the terms of his December 1, 2010 offer letter. |
| (5) | Mr. Ruane was hired as Chief Executive Officer and elected as a member of the Board effective December 1, 2010. |
| (6) | Mr. Drapeau also served as Interim Chief Executive Officer from October 2008 to November 2010, retaining his position of Vice President and Chief Financial Officer. For all periods, Mr. Drapeau was employed and compensated for a 30 hour work week. In February 2012, Mr. Drapeau became a full time employee of the Company. |
83
GRANTS OF PLAN-BASED AWARDS—FISCAL 2011
The following table presents information regarding the equity incentive awards granted to the Named Executive Officers in 2011.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name (a) | | Grant
Date
(b) | | | Estimated Potential Payouts
Under Non-Equity Incentive
Plan Awards | | | Estimated Future Payouts
Under Equity Incentive
Plan Awards | | | All Other
Stock
Awards:
Number of
Shares of
Stock Units
(#)
(i) | | | All Other
Option
Awards:
Number of
Securities
Underlying
Options
(#)
(j) | | | Exercise
or Base
Price of
Option
Awards
($/Sh)
(k) | | | Grant Date
Fair Value
of Stock
and Option
Awards
($)(1)
(l) | |
| | | Threshold | | | Target | | | Maximum | | | Threshold | | | Target | | | Maximum | | | | | |
| | | ($) | | | ($) | | | ($) | | | ($) | | | ($) | | | ($) | | | | | |
| | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | | | | | |
Timothy Ruane (2) | | | | | | | — | | | | 262,500 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Louis Drapeau | | | | | | | — | | | | 76,500 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | 1/21/2011 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 300,000 | | | | 0.36 | | | | 75,150 | |
Lyle M. Bowman, Ph.D | | | | | | | — | | | | 81,525 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | 1/21/2011 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 200,000 | | | | 0.36 | | | | 50,100 | |
| (1) | The amounts reported in Column (l) reflect the fair value of these awards on the grant date as determined under the principles used to calculate the value of equity awards for purposes of our financial statements. For the assumptions and methodologies used to value the awards reported in Column (l), please see footnote (2) to the Summary Compensation Table. |
| (2) | On December 1, 2010, Mr. Ruane was hired as Chief Executive Officer and granted an option to purchase 2,844,374 shares of our common stock under the 2007 Plan. Mr. Ruane was not eligible for stock option awards in fiscal year 2011. |
Description of Plan-Based Awards
Each of the options reported in the Grants of Plan-Based Awards Table was granted under, and is subject to the terms of the 2007 Plan. The 2007 Plan is administered by the Compensation Committee. The Compensation Committee has the authority to interpret the 2007 Plan’s provisions and make all required determinations under the plans. This authority includes making required proportionate adjustments to outstanding awards upon the occurrence of certain corporate events such as reorganizations, mergers and stock splits, and making provision to ensure that any tax withholding obligations incurred in respect of awards are satisfied. Awards granted under the 2007 Plan are generally only transferable to a beneficiary of a Named Executive Officer upon his or her death. However, the Compensation Committee may establish procedures for the transfer of awards to other persons or entities, provided that such transfers comply with applicable securities laws and, with limited exceptions, are not made for value.
Under the terms of the 2007 Plan, if we dissolve or complete a merger, a sale of substantially all of our assets or any other transaction that we do not survive (or do not survive as a publicly-traded company), all then-outstanding awards granted under the 2007 Plan (including awards held by the Named Executive Officers) will generally become fully vested and, in the case of options, exercisable, unless the Compensation Committee provides for the substitution, assumption, exchange or other continuation of the outstanding awards. Any options that become vested in connection with such a transaction generally must be exercised prior to the transaction, or they will be canceled in exchange for the right to receive a cash payment in connection with the transaction.
Each option reported in the table above was granted with a per-share exercise price equal to the fair market value of a share of our common stock on the grant date. For these purposes, and in accordance with our option grant practices, the fair market value is equal to the closing price of a share of our common stock on the applicable grant date. Each of these options is scheduled to vest as to 25% of the shares covered by the option on the first anniversary of the grant date and as to the remaining 75% of the shares in daily installments over the three-year period thereafter.
Once vested, each option will generally remain exercisable until its normal expiration date. Each of the options granted to the Named Executive Officers in 2011 has a term of ten years. However, vested options may expire earlier in connection with a change in control transaction or a termination of the Named Executive Officer’s employment. Subject to any accelerated vesting that may apply in the circumstances, the unvested portion of the option will immediately terminate upon a termination of the Named Executive Officer’s employment. The Named Executive Officer will generally
84
have three months to exercise the vested portion of the option following a termination of his or her employment for a longer period if such termination is in connection with a change in control of the Company. This period is extended to 12 months if the termination was a result of the Named Executive Officer’s death or disability. The options granted to Named Executive Officers during 2011 do not include any dividend rights.
The terms of the “non-equity incentive plan” awards reflected in the Grants of Plan-Based Awards Table are described in the “Compensation Discussion and Analysis” above.
85
OUTSTANDING EQUITY AWARDS AT 2011 FISCAL YEAR-END
The following table presents information regarding the outstanding stock options held by each of the Named Executive Officers as of December 31, 2011, including the vesting dates for the portions of these options that had not vested as of that date. None of the Named Executive Officers held any outstanding stock awards as of that date.
| | | | | | | | | | | | | | | | | | | | |
Name (a) | | Option
Grant Date
(b) | | | Number of
Securities
Underlying
Unexercised
Options
(#)
Exercisable
(c) | | | Number of
Securities
Underlying
Unexercised
Options
(#)
Unexercisable
(d) | | | Option
Exercise
Price
($)
(e) | | | Option
Expiration
Date
(f) | |
Timothy Ruane | | | 12/1/2010 | | | | 769,486 | | | | 2,844,374 | (1) | | | 0.35 | | | | 12/1/2020 | |
Louis Drapeau | | | 1/21/2011 | | | | — | | | | 300,000 | (2) | | | 0.36 | | | | 1/21/2021 | |
| | | 4/1/2010 | | | | 175,000 | | | | 225,000 | (3) | | | 0.42 | | | | 4/1/2020 | |
| | | 2/17/2009 | | | | 286,678 | | | | 113,322 | (4) | | | 0.20 | | | | 2/17/2019 | |
| | | 10/28/2008 | | | | 300,000 | | | | — | | | | 0.36 | | | | 10/28/2018 | |
| | | 10/15/2007 | | | | 50,000 | | | | — | | | | 1.20 | | | | 10/15/2017 | |
Lyle M. Bowman, Ph.D. | | | 1/21/2011 | | | | — | | | | 200,000 | (2) | | | 0.36 | | | | 1/21/2021 | |
| | | 4/1/2010 | | | | 87,500 | | | | 112,500 | (3) | | | 0.42 | | | | 4/1/2020 | |
| | | 2/17/2009 | | | | 143,339 | | | | 56,661 | (4) | | | 0.20 | | | | 2/17/2019 | |
| | | 5/2/2007 | | | | 80,000 | | | | — | | | | 1.59 | | | | 5/2/2017 | |
| | | 2/1/2006 | | | | 80,000 | | | | — | | | | 1.50 | | | | 2/1/2016 | |
| | | 6/1/2005 | | | | 175,000 | | | | — | | | | 0.63 | | | | 6/1/2015 | |
| | | 6/1/2004 | | | | 40,000 | | | | — | | | | 0.75 | | | | 6/1/2014 | |
| | | 3/30/2004 | | | | 20,000 | | | | — | | | | 0.88 | | | | 3/30/2014 | |
| | | 12/12/2003 | | | | 25,000 | | | | — | | | | 0.41 | | | | 12/12/2013 | |
| | | 9/23/2003 | | | | 15,000 | | | | — | | | | 0.63 | | | | 9/23/2013 | |
| | | 2/14/2003 | | | | 25,000 | | | | — | | | | 0.85 | | | | 2/14/2013 | |
| | | 9/20/2002 | | | | 15,000 | | | | — | | | | 0.93 | | | | 9/20/2012 | |
| (1) | This option is scheduled to vest as to 25% of the shares covered by the option on December 1, 2011 and as to the remaining 75% of the shares in daily installments over the three-year period thereafter. |
| (2) | Each of these options is scheduled to vest as to 25% of the shares covered by the option on January 21, 2012 and as to the remaining 75% of the shares in daily installments over the three-year period thereafter. |
| (3) | Each of these options is scheduled to vest as to 25% of the shares covered by the option on April 1, 2011 and as to the remaining 75% of the shares in daily installments over the three-year period thereafter. |
| (4) | Each of these options is scheduled to vest as to 25% of the shares covered by the option on February 17, 2010 and as to the remaining 75% of the shares in daily installments over the three-year period thereafter. |
86
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
As described in the “Compensation Discussion and Analysis” above, the Compensation Committee approved the Severance Plan in April 2009 to provide severance protections for certain of our eligible employees, including each of the Named Executive Officers. If, during the term of the Severance Plan, a participant’s employment with us is terminated without “cause” (as such term is defined in the Severance Plan), the participant will generally be entitled to receive (1) a lump sum severance payment equal to the participant’s annual base salary rate multiplied by the participant’s “severance multiplier,” and (2) payment by us of the participant’s premiums for continued medical and other welfare benefits pursuant to the Consolidated Omnibus Budget Reconciliation Act (COBRA) for a number of months determined by multiplying the participant’s severance multiplier by twelve. The severance multiplier for each of Messrs. Ruane and Drapeau and Dr. Bowman is one (1).
If, during the term of the Severance Plan, a participant’s employment with us is terminated without cause or by the participant for “good reason” within 90 days before, or within two years after, the occurrence of a “change in control”, then, in lieu of the benefits described above, the participant will generally be entitled to receive (1) a lump sum severance payment equal to the sum of (a) the participant’s annual base salary rate multiplied by the participant’s “change in control severance multiplier” plus (b) the participant’s target bonus for the year in which the termination occurs (or, if the participant does not have a target bonus opportunity for such year, the average annual cash bonus paid to the participant for the three preceding fiscal years), (2) payment by us of the participant’s premiums for continued medical and other welfare benefits pursuant to COBRA for a number of months determined by multiplying the participant’s change in control severance multiplier by twelve and (3) full accelerated vesting of the participant’s stock options and other equity-based awards, with a six-month extension of the period to exercise such stock options. (For these purposes, the term “change in control” is defined in the Severance Plan and the term “good reason” is defined in each participant’s Severance Plan participation agreement). The change in control severance multiplier for Mr. Ruane is one (1). The change in control severance multiplier for each of Mr. Drapeau and Dr. Bowman is one and one-half (1.5).
A participant’s right to receive benefits under the Severance Plan is subject to the participant’s execution of a release of claims in our favor upon the termination of the participant’s employment. Participants are generally not obligated to seek new employment to mitigate our severance obligations under the Severance Plan.
87
Estimated Severance and Change in Control Benefits
Severance Benefits. The following chart presents our estimate of the amount of the benefits to which each of the Named Executive Officers would have been entitled under the Severance Plan had his employment terminated under the circumstances described above (other than in connection with a change in control of the Company) on December 31, 2011.
| | | | | | | | | | | | |
Name and Principal Position | | Cash Severance | | | Continuation of
Health Benefits | | | Total | |
Timothy Ruane | | $ | 350,000 | | | $ | 20,536 | | | $ | 370,536 | |
Chief Executive Officer and Member of the Board | | | | | | | | | | | | |
Louis Drapeau | | $ | 255,000 | | | $ | 45,424 | | | $ | 300,424 | |
Vice President, Chief Financial Officer | | | | | | | | | | | | |
Lyle M. Bowman, Ph.D. | | $ | 271,750 | | | $ | 31,724 | | | $ | 303,474 | |
Vice President, Development | | | | | | | | | | | | |
Change in Control Severance Benefits.The following chart presents our estimate of the amount of the severance benefits to which each of the Named Executive Officers would have been entitled under the Severance Plan had his employment terminated under the circumstances described above following a change in control of the Company, and assuming for purposes of this disclosure that the termination of employment occurred on December 31, 2011.
| | | | | | | | | | | | | | | | |
Name and Principal Position | | Cash Severance | | | Continuation of
Health Benefits | | | Equity Acceleration (1) | | | Total | |
Timothy Ruane | | $ | 612,500 | | | $ | 20,536 | | | $ | 186,740 | | | $ | 819,776 | |
Chief Executive Officer and Member of the Board | | | | | | | | | | | | | | | | |
Louis Drapeau | | $ | 459,000 | | | $ | 68,135 | | | $ | 55,697 | | | $ | 582,833 | |
Vice President, Chief Financial Officer | | | | | | | | | | | | | | | | |
Lyle M. Bowman, Ph.D. | | $ | 489,150 | | | $ | 47,586 | | | $ | 31,849 | | | $ | 568,585 | |
Vice President, Development | | | | | | | | | | | | | | | | |
| (1) | This column reports the intrinsic value of the unvested portions of the Named Executive Officer’s options that would accelerate in the circumstances. For options, this value is calculated by multiplying the amount (if any) by which $0.44 (the closing price of our common stock on December 31, 2011) exceeds the exercise price of the option by the number of shares subject to the accelerated portion of the option. |
88
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information known to us regarding beneficial ownership of our common stock, as of February 22, 2012 unless otherwise noted by (i) each person who is known by us to beneficially own more than five percent of our common stock, (ii) our Chief Executive Officer and each of our other Named Executive Officers, (iii) each director, and (iv) all current executive officers and directors as a group. Unless otherwise indicated, the principal address of each of the stockholders below is: c/o InSite Vision Incorporated, 965 Atlantic Avenue, Alameda, California 94501. Except as otherwise indicated, we believe that each of the beneficial owners of the common stock listed below has sole voting and investment power with respect to such shares, subject to community property laws, where applicable.
The percentage of beneficial ownership is calculated based on the 131,951,033 shares of common stock that were outstanding on February 22, 2012. This percentage also includes common stock of which such individual or entity had the right to acquire beneficial ownership of as of February 22, 2012 or within 60 days thereafter, including, but not limited to, upon the exercise of options and warrants; however, such common stock shall not be deemed outstanding for the purpose of computing the percentage owned by any other individual or entity.
| | | | | | | | |
| | | Beneficially Owned | |
Name of Beneficial Owner | | Number of Shares | | | Percent of Class | |
Coliseum Capital Management, LLC. Coliseum Capital, LLC Coliseum Capital Partners, L.P. Adam Gray Christopher Shackelton Metro Center 1 Station Place, 7th Floor South Stamford, CT 06902 Blackwell Partners, LLC c/o DUMAC, LLC 406 Blackwell Street, Suite 300 Durham, NC 27701 | | | 16,403,338 | (1) | | | 12.43 | % |
Broadfin Capital, LLC 237 Park Avenue, Suite 900 New York, New York 10017 Broadfin Healthcare Master Fund, Ltd. 20 Genesis Close Ansbacher House, 2nd Floor P.O. Box 1344 Grand Cayman KY1-1108 Cayman Islands Kevin Kotler 237 Park Avenue, Suite 900 New York, New York 10017 | | | 13,178,785 | (2) | | | 9.99 | % |
Eli Jacobson 125 Broad Street, 32nd Floor New York, NY 10004 | | | 9,550,674 | (3) | | | 7.24 | % |
89
| | | | | | | | |
| | | Beneficially Owned | |
Name of Beneficial Owner | | Number of Shares | | | Percent of Class | |
Pinto Technology Ventures, L.P. | | | 7,744,621 | (4) | | | 5.87 | % |
Pinto Technology Ventures GP, L.P. | | | | | | | | |
Pinto TV GP Company LLC | | | | | | | | |
Matthew Crawford | | | | | | | | |
Evan S. Melrose | | | | | | | | |
c/o PTV Sciences | | | | | | | | |
221 West 6th Street, Suite 700 | | | | | | | | |
Austin, Texas 78701 | | | | | | | | |
Ayer Capital Management, LP ACM Capital Partners, LLC Jay Venkatesan 230 California, Suite 600 San Francisco, CA 94111 | | | 7,000,000 | (5) | | | 5.30 | % |
Timothy McInerney | | | 997,452 | (6) | | | * | |
Brian Levy, O.D. M.Sc. | | | — | | | | * | |
Robert O’Holla | | | 330,000 | (7) | | | * | |
Craig A. Tooman | | | — | | | | * | |
Anthony Yost | | | 330,000 | (7) | | | * | |
Timothy Ruane | | | 1,269,432 | (8) | | | * | |
Louis Drapeau. | | | 1,307,425 | (9) | | | * | |
Lyle M. Bowman, Ph.D | | | 876,368 | (10) | | | * | |
All current executive officers and directors as a group (9 persons) | | | 5,110,677 | (11) | | | 3.87 | % |
| * | Less than one percent of the outstanding common stock. |
| (1) | Information is based on the Schedule 13G/A filed with the SEC on February 10, 2012 by Coliseum Capital Management, LLC, Coliseum Capital, LLC, Coliseum Capital Partners, L.P., Blackwell Partners, LLC, Adam Gray and Christopher Shackelton. Amount is comprised of 14,403,338 shares over which Coliseum Capital Management, LLC and Messrs. Gray and Shackleton have shared voting and dispositive power, 8,972,456 shares over which Coliseum Capital, LLC has shared voting and dispositive power, 5,222,456 shares over which Coliseum Capital Partners, L.P. has shared voting and dispositive power and 5,430,882 shares over which Blackwell Partners, LLC has shared voting and dispositive power. Amount also includes 2,000,000 warrants issued to Coliseum Capital Management, LLC, |
| (2) | Information is based on the Schedule 13G/A filed with the SEC on February 13, 2012 by Broadfin Capital, LLC, Broadfin Healthcare Master Fund, Ltd., and Kevin Kotler. |
| (3) | Information is based on the Schedule 13G/A filed with the SEC on February 14, 2012 by Eli Jacobson. |
| (4) | Information is based on the Schedule 13D/A filed with the SEC on August 31, 2010 by Pinto Technology Ventures, L.P., Pinto Technology Ventures GP, L.P. and Pinto TV GP Company LLC (Pinto Entities) and Matthew Crawford and Evan Melrose and includes the expiration of 585,015 thereafter. The amount above includes 7,744,621 shares held by Pinto Technology Ventures, L.P. The Pinto Entities and Mr. Crawford and Dr. Melrose share voting and dispositive power with respect to such shares. Mr. Crawford and Dr. Melrose disclaim beneficial ownership in the shares held by the Pinto Entities, except to the extent of their pecuniary interest therein. In addition, the amount above includes 128,000 shares and 179,504 shares for which Mr. Crawford and Dr. Melrose, respectively, have sole voting and dispositive power. |
| (5) | Information is based on the Schedule 13G filed with the SEC on July 12, 2011 by Ayer Capital Management, LP, ACM Capital Partners, LLC and Jay Venkatesan. |
| (6) | Includes 66,666 shares issuable upon the exercise of warrants and 330,000 shares issuable upon the exercise of stock options as of February 22, 2012 or within 60 days thereafter. |
| (7) | Includes 330,000 shares issuable upon the exercise of stock options as of February 22, 2012 or within 60 days thereafter. |
| (8) | Includes 80,000 shares issuable upon the exercise of warrants and 989,432 shares issuable upon the exercise of stock options as of February 22, 2012 or within 60 days thereafter. |
| (9) | Includes 80,000 shares issuable upon the exercise of warrants and 967,425 shares issuable upon the exercise of stock options as of February 22, 2012 or within 60 days thereafter. |
| (10) | Includes 1,320 shares issuable upon the exercise of warrants and 799,360 shares issuable upon the exercise of stock options as of February 22, 2012 or within 60 days thereafter. |
| (11) | Includes 227,986 shares issuable upon the exercise of warrants and 3,746,217 shares issuable upon the exercise of stock options as of February 22, 2012 or within 60 days thereafter. |
90
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
The Audit Committee is responsible for review and approval of “related-party transactions” between us and related parties. Under SEC rules, a related party is a director, officer, nominee for director, or 5% stockholder of a company since the beginning of the last fiscal year and their immediate family members.
Related Transactions
The Company’s Restated Certificate of Incorporation (Certificate) provides for indemnification of directors and officers of the Company to the fullest extent permitted by the General Corporation Law of the State of Delaware, (Delaware Law). Each of the current directors and executive officers of the Company has entered into separate indemnification agreements with the Company. In addition, the Certificate limits the liability of directors to the Company or its stockholders to the fullest extent permitted by Delaware Law.
Director Independence
The Board has determined that each of the members of the Board, other than Mr. Ruane, is “independent” as that term is defined in applicable SEC and NYSE Amex Rules.
| Item 14. | Principal Accounting Fees and Services |
Independent Auditor Fees
The following table shows the fees paid or accrued by the Company for the audit and other services provided by its independent registered public accounting firm, Burr Pilger Mayer, Inc. (BPM), for fiscal years 2011 and 2010 (in thousands):
| | | | | | | | |
| | | 2011 | | | 2010 | |
Audit Fees (1) | | $ | 171 | | | $ | 158 | |
Audit-related Fees | | | — | | | | — | |
Tax Fees | | | — | | | | — | |
All Other Fees | | | — | | | | — | |
| | | | | | | | |
Total | | $ | 171 | | | $ | 158 | |
| | | | | | | | |
| (1) | Audit fees represent fees for professional services provided in connection the audit of our annual consolidated financial statements, review of our quarterly condensed consolidated financial statements and services that are normally provided by BPM in connection with statutory and regulatory filings. |
Independence of Independent Registered Public Accounting Firm and Pre-Approval Policy
The Audit Committee has determined that the provision by BPM of non-audit services is compatible with maintaining the independence of BPM. The Audit Committee has adopted a policy for the pre-approval of audit and non-audit services rendered by our independent registered public accounting firm. The policy generally provides for pre-approval of specified services in the defined categories of audit services, audit-related services and tax services for up to $25,000. Pre-approval may also be given as part of the Audit Committee’s approval of the scope of the engagement of the independent registered public accounting firm or on an individual explicit case-by-case basis before the independent registered public accounting firm is engaged to provide each service. The pre-approval of services may be delegated to one or more of the Audit Committee’s members, but the decision must be reported to the full Audit Committee at its next scheduled meeting. During fiscal 2011, all services provided by BPM were pre-approved by the Audit Committee.
91
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
(a)(1)Financial Statements
The Financial Statements and Report of Independent Auditors are included in Item 8 of Part II of this Annual Report on Form 10-K. See index to consolidated financial statements at Item 8 of Part II of this Annual Report on Form 10-K.
(2)Financial Statement Schedules
The information required under this Item appears in the Financial Statements or notes thereto included in Item 8 of Part II of this Annual Report on Form 10-K. See index to consolidated financial statements at Item 8 of this Annual Report on Form 10-K.
(3)Exhibits
The information required under this Item appears under the heading “Exhibit Index” of this Annual Report on Form 10-K.
92
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: March 5, 2012
| | |
| INSITE VISION INCORPORATED |
| |
| By: | | /S/ LOUIS DRAPEAU |
| | Louis Drapeau Chief Financial Officer (Principal Financial and Accounting Officer) |
POWER OF ATTORNEY
KNOW ALL PEOPLE BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Louis C. Drapeau, his attorney in fact and agent, with the power of substitution and resubstition, for him and in his name, place and stead, in any and all capacities, to sign any amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney in fact, or his substitutes or agents, each acting alone, may do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | |
Name | | Capacity | | Date |
| | |
/S/ TIMOTHY RUANE Timothy Ruane | | Chief Executive Officer and Director
(Principal Executive Officer) | | March 5, 2012 |
| | |
/S/ LOUIS DRAPEAU Louis Drapeau | | Chief Financial Officer
(Principal Financial and Accounting Officer) | | March 5, 2012 |
| | |
/S/ BRIAN LEVY Brian Levy, O.D. M.Sc. | | Director | | March 5, 2012 |
| | |
/S/ TIMOTHY MCINERNEY Timothy McInerney | | Chairman of Board, Director | | March 5, 2012 |
| | |
/S/ ROBERT O’HOLLA Robert O’Holla | | Director | | March 5, 2012 |
| | |
/S/ CRAIG A. TOOMAN Craig A. Tooman | | Director | | March 5, 2012 |
| | |
/S/ ANTHONY J. YOST Anthony J. Yost | | Director | | March 5, 2012 |
93
EXHIBIT INDEX
| | |
Number | | Exhibit Table |
| |
| 3.11 | | Restated Certificate of Incorporation as filed with the Delaware Secretary of State on October 25, 1993. |
| |
| 3.22 | | Certificate of Designations, Preferences and Rights of Series A Convertible Preferred Stock as filed with the Delaware Secretary of State on September 11, 1997. |
| |
| 3.32 | | Certificate of Correction of the Certificate of Designations, Preferences and Rights of Series A Convertible Preferred Stock as filed with the Delaware Secretary of State on September 26, 1997. |
| |
| 3.43 | | Certificate of Designations, Preferences and Rights of Series A-1 Preferred Stock as filed with the Delaware Secretary of State on July 3, 2002. |
| |
| 3.54 | | Certificate of Amendment to Restated Certificate of Incorporation as filed with the Delaware Secretary of State on June 3, 1994. |
| |
| 3.65 | | Certificate of Amendment to Restated Certificate of Incorporation as filed with the Delaware Secretary of State on July 20, 2000. |
| |
| 3.75 | | Certificate of Amendment to Restated Certificate of Incorporation as filed with the Delaware Secretary of State on June 1, 2004. |
| |
| 3.86 | | Certificate of Amendment to Restated Certificate of Incorporation as filed with the Delaware Secretary of State on October 23, 2006 |
| |
| 3.97 | | Amended Bylaws, as amended on December 14, 2011. |
| |
| 4.1 | | Reference is made to Exhibits 3.1 through 3.9. |
| |
| 10.18 | | InSite Vision Incorporated Amended and Restated Employee Stock Purchase Plan adopted October 15, 2007. |
| |
| 10.29HH | | InSite Vision Incorporated 1994 Stock Option Plan (Amended and Restated as of June 8, 1998). |
| |
| 10.38HH | | InSite Vision Incorporated 2007 Performance Incentive Plan. |
| |
| 10.48 | | Form of Nonqualified Stock Option Agreement (2007). |
| |
| 10.58 | | Form of Incentive Stock Option Agreement (2007). |
| |
| 10.610 | | Form of Indemnification Agreement between the Company and its directors and officers. |
| |
| 10.711 | | Form of Employee’s Proprietary Information and Inventions Agreement. |
| |
| 10.812 | | Facilities Lease, dated September 1, 1996, between the Registrant and Alameda Real Estate Investments. |
| |
| 10.913 | | Amendment No. 1 to Marina Village Office Tech Lease, dated July 20, 2001 and effective January 1, 2002. |
| |
| 10.105 | | Amendment No. 3 to Marina Village Office Tech Lease, dated November 28, 2006. |
| |
| 10.1114H | | Exclusive License Agreement, dated as of February 15, 2007, by and between the Company and Pfizer, Inc. and Pfizer Products, Inc. |
| |
| 10.1214H | | License Agreement, dated as of February 15, 2007, by and between the Company and Inspire Pharmaceuticals, Inc. |
| |
| 10.1314H | | Trademark License Agreement, dated as of February 15, 2007, by and between the Company and Inspire Pharmaceuticals, Inc. |
| |
| 10.1414H | | Supply Agreement, dated as of February 15, 2007, by and between the Company and Inspire Pharmaceuticals, Inc. |
94
| | |
Number | | Exhibit Table |
| |
| 10.1514HH | | Change in Control Agreement for S. Kumar Chandrasekaran adopted by InSite Vision Incorporated on May 2, 2007. |
| |
| 10.1615 | | Purchase and Sale Agreement, dated as of February 21, 2008, by and between Azithromycin Royalty Sub LLC and the Company. |
| |
| 10.1715 | | Note Purchase Agreement, dated as of February 21, 2008, by and among Azithromycin Royalty Sub LLC, the Company and the purchasers named therein. |
| |
| 10.1815 | | Indenture, dated as of February 21, 2008, by and between Azithromycin Royalty Sub LLC and U.S. Bank National Association. |
| |
| 10.1915 | | Pledge and Security Agreement made by the Company to U.S. Bank National Association, as Trustee, dated February 21, 2008. |
| |
| 10.2015 | | Residual License Agreement by and between Azithromycin Royalty Sub LLC and the Company dated February 21, 2008. |
| |
| 10.2116 | | InSite Vision Incorporated Annual Bonus Plan. |
| |
| 10.2217 | | InSite Vision Incorporated Severance Plan. |
| |
| 10.2318HH | | Offer letter, by and between the Company and Louis Drapeau, dated October 31, 2008. |
| |
| 10.2419HH | | Offer letter, by and between the Company and Timothy Ruane, dated December 1, 2010. |
| |
| 10.2520 | | Form of Securities Purchase Agreement, dated July 12, 2011. |
| |
| 10.2620 | | Form of Common Stock Warrant issued pursuant to Securities Purchase Agreement, dated July 12, 2011. |
| |
| 10.2720 | | Form of Registration Rights Agreement. |
| |
| 21.1 | | List of Subsidiaries. |
| |
| 23.1 | | Consent of Burr Pilger Mayer, Inc., Independent Registered Public Accounting Firm. |
| |
| 24.1 | | Reference is hereby made to the Power of Attorney included on the signature page to this Annual Report on Form 10-K. |
| |
| 31.1 | | Certification of Principal Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended. |
| |
| 31.2 | | Certification of Principal Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended. |
| |
| 32.1 | | Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| 101 | | The following materials from InSite Vision, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2011, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statements of Income, (ii) the Consolidated Balance Sheets, (iii) the Consolidated Statements of Stockholders’ Deficit, (iv) the Consolidated Statements of Cash Flows, and (v) Notes to Consolidated Financial Statements. |
| 1. | Incorporated by reference to exhibits in the Company’s Annual Report on Form 10-K for the year ended December 31, 1993. |
| 2. | Incorporated by reference to exhibits in the Company’s Registration Statement on Form S-3 (Registration No. 333-36673) as filed with the Securities and Exchange Commission on September 29, 1997. |
| 3. | Incorporated by reference to an exhibit in Amendment No. 1 the Company’s Registration Statement on Form S-1 (Registration No. 33-68024) as filed with the Securities and Exchange Commission on September 16, 1993. |
95
| 4. | Incorporated by reference to an exhibit to the Company’s Registration Statement on Form S-3 (file Number 333-126084) as filed with the Securities and Exchange Commission on June 23, 2005. |
| 5. | Incorporated by reference to an exhibit in the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. |
| 6. | Incorporated by reference to an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2006. |
| 7. | Incorporated by reference to an exhibit in the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 16, 2011. |
| 8. | Incorporated by reference to an exhibit in the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 19, 2007. |
| 9. | Incorporated by reference to exhibits to the Company’s Registration Statement on Form S-8 (Registration No. 333-60057) as filed with the Securities and Exchange Commission on July 28, 1998. |
| 10. | Incorporated by reference to an exhibit in the Company’s Registration Statement on Form S-1 (Registration No. 33-68024) as filed with the Securities and Exchange Commission on August 27, 1993. |
| 11. | Incorporated by reference to an exhibit in the Company’s Annual Report on Form 10-K for the year ended December 31, 1993. |
| 12. | Incorporated by reference to an exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 1996. |
| 13. | Incorporated by reference to an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2001. |
| 14. | Incorporated by reference to an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2007. |
| 15. | Incorporated by reference to an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2008. |
| 16. | Incorporated by reference to an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008. |
| 17. | Incorporated by reference to an exhibit in the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on April 29, 2009. |
| 18. | Incorporated by reference to an exhibit in the Company’s Amendment No. 1 to Annual Report on Form 10-K filed with the Securities and Exchange Commission on April 30, 2009. |
| 19. | Incorporated by reference to an exhibit in the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 30, 2010. |
| 20. | Incorporated by reference to an exhibit in the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on July 18, 2011. |
| H | Confidential treatment has been granted with respect to certain portions of this agreement. |
| HH | Management contract or compensatory plan. |
96
