UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________________________________________
Form 10-K
____________________________________________________________________________
(Mark One)
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
Or
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____________ to______________
Commission File Number: 001-36046
AXOGEN, INC.
(Exact name of registrant as specified in its charter)
Minnesota
(State or other jurisdiction of
incorporation or organization)
13631 Progress Blvd., Suite 400 Alachua, FL
(Address of principal executive offices)
41-1301878
(I.R.S. Employer
Identification No.)
32615
(Zip Code)
Registrant’s telephone number, including area code: (386) 462-6800
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of exchange on which registered |
| Common Stock, $0.01 par value | | AXGN | | The Nasdaq Stock Market |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐
Non-accelerated filer ☐
Accelerated filer x
Smaller reporting company ☐
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. □
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). Yes ☐ No ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
As of June 30, 2024, the last day of the registrant's most recently completed second quarter, the aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $186,789,502 based upon the last reported sale price of the common stock on the Nasdaq Capital Market.
The number of shares outstanding of the Registrant’s common stock as of February 19, 2025, was 44,343,785 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy statement to be filed pursuant to Regulation 14A within 120 days after the end of the Registrant’s fiscal year are incorporated by reference into Part III of this Form 10-K.
TABLE OF CONTENTS
FORWARD-LOOKING STATEMENTS
From time to time, in reports filed with the U.S. Securities and Exchange Commission (the “SEC”) (including this Annual Report on Form 10-K), in press releases, and in other communications to shareholders or the investment community, Axogen, Inc. (including Axogen, Inc.’s wholly owned subsidiaries, Axogen Corporation, Axogen Processing Corporation, Axogen Europe GmbH, and Axogen Germany GmbH, (the “Company,” “Axogen,” “we,” “our,” or “us”) may provide forward-looking statements, as defined in the Private Securities Litigation Reform Act of 1995, concerning possible or anticipated future results of operations or business developments. These statements are based on management's current expectations or predictions of future conditions, events, or results based on various assumptions and management's estimates of trends and economic factors in the markets in which we are active, as well as its business plans. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “projects,” “forecasts,” “continue,” “may,” “should,” “will,” “goals,” and variations of such words and similar expressions are intended to identify such forward-looking statements.
The forward-looking statements in this Form 10-K include, but are not limited to the following:
•Our expectations regarding our ability to market Avive+ Soft Tissue Matrix and our expectations that Avive+ Soft Tissue Matrix will, and will continue to be, regulated solely under Section 361 of the Public Health Service Act ("PHS Act");
•Statements regarding estimates of the total addressable market for our current portfolio, our belief that our total addressable market is comprised of four categories: (i) trauma ("Trauma"), (ii) oral maxillofacial ("OMF") and head and neck ("Head and Neck"), collectively ("OMF/H&N"), (iii) breast reconstruction neurotization ("Breast"), and (iv) Upper Extremity Compression, (together the "Total Addressable Market") and statements regarding estimates of the market for our current portfolio in each of the four categories;
•Statements regarding our belief that there is an additional 10-15% of the breast reconstructions done with implants that can also be neurotized and thus offer us expanded revenue opportunities;
•Statements regarding our beliefs that expanding our products into lower extremity surgery, urology, and the surgical treatment of pain could offer us expanded revenue opportunities;
•Our expectation that the Biologics License Application ("BLA") will be approved in September 2025;
•Our belief that we met the clinical end point required for our pivotal study to support the BLA and that only one study is sufficient to support the BLA;
•Our belief that further innovation and product development is needed to maintain our leadership position and provide expansion opportunities;
•Our belief that additional opportunities exist to develop or acquire complementary products in peripheral nerve repair as well as opportunities to expand our existing portfolio of products in new applications of peripheral nerve repair;
•Our belief that there is a global need for surgical repair of damaged or transected nerves and that a global market exists for our product; and
•Statements regarding our ability to monitor product utilization within accounts and generate improved estimates of our revenue by application.
The forward-looking statements are and will be subject to risks and uncertainties, which may cause actual results to differ materially from those expressed or implied in such forward-looking statements. Forward-looking statements contained in this Form 10-K should be evaluated together with the many uncertainties that affect our business and its market, particularly those discussed in the risk factors and cautionary statements set forth in our filings with the SEC, including as described in “Risk Factors” included in Item 1A of this Form 10-K and “Risk Factor Summary” included in this Form 10-K. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements contained in this report are based on information that is currently available to us and expectations and assumptions that we deem reasonable at the time the statements were made. The forward-looking statements are representative only as of the date they are made and, except as required by applicable law, we assume no responsibility to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or otherwise.
PART I
ITEM 1. BUSINESS
General
We are the leading company focused specifically on the science, development, and commercialization of technologies for peripheral nerve regeneration and repair. We are passionate about providing the opportunity to restore nerve function and quality of life for patients with peripheral nerve injuries. We provide innovative, clinically proven, and economically effective repair solutions for surgeons and healthcare providers. Peripheral nerves provide the pathways for both motor and sensory signals throughout the body. Every day, people suffer traumatic injuries or undergo surgical procedures that impact the function of their peripheral nerves. Physical damage to a peripheral nerve or the inability to properly reconnect peripheral nerves can result in the loss of muscle or organ function, the loss of sensory feeling, or the initiation of pain.
Our platform for peripheral nerve repair features a comprehensive portfolio of products, including:
•Avance® Nerve Graft, a biologically active off-the-shelf processed human nerve allograft for bridging severed peripheral nerves without the comorbidities associated with a second surgical site.
•Axoguard Nerve Connector®, a porcine (pig) submucosa extracellular matrix ("ECM") coaptation aid for tensionless repair of severed peripheral nerves.
•Axoguard Nerve Protector®, a porcine submucosa ECM product used to wrap and protect damaged peripheral nerves and reinforce the nerve reconstruction while minimizing soft tissue attachments.
•Axoguard HA+ Nerve Protector™, a porcine submucosa ECM base layer coated with a proprietary hyaluronate-alginate gel, a next-generation technology designed to enhance nerve gliding and provide short- and long-term protection for peripheral nerve injuries.
•Axoguard Nerve Cap®, a porcine submucosa ECM product used to protect a peripheral nerve end and separate the nerve from the surrounding environment to reduce the development of symptomatic or painful neuroma.
•Avive+ Soft Tissue Matrix™, a multi-layer amniotic membrane allograft used to protect and separate tissues in the surgical bed during the critical phase of tissue healing.
On June 24, 2024, we announced the launch of Avive+ Soft Tissue Matrix. Avive+ Soft Tissue Matrix is processed and distributed in accordance with United States Food and Drug Administration ("FDA") requirements for Human Cellular and Tissue-based Products ("HCT/P") under the Code of Federal Regulations ("CFR") Title 21 ("21 CFR") Part 1271 regulations and United States PHS regulations as a Section 361 human tissue product. Products regulated solely under Section 361 of the PHS Act are a product category under close scrutiny by the FDA for compliance with the regulatory requirements and are potentially subject to regulatory change in the future.
Our portfolio of products is currently available in the United States ("U.S."), and 19 other countries including Canada, Germany, United Kingdom ("UK"), Spain, and several other European, Asian, and Latin American countries.
Revenue from the distribution of our nerve repair products, Avance Nerve Graft, Axoguard Nerve Connector, Axoguard Nerve Protector, Axoguard HA+ Nerve Protector, Axoguard Nerve Cap and Avive+ Soft Tissue Matrix in the U.S. is the main contributor to our total reported sales and have been the key component of our growth to date.
Nerves can be damaged in several ways. When a nerve is cut due to a traumatic injury or inadvertently during a surgical procedure, functionality of the nerve may be compromised, causing the nerve to no longer carry the signals to and from the brain to the muscles and skin, thereby reducing or eliminating functionality. The loss of function can impact a person’s ability to work and perform daily tasks, to properly be aware and respond to their environment (e.g., heat, cold or other dangers), and could negatively impact their ability to experience and enjoy life.
Nerve damage or transection of the type described above generally requires a surgical repair. Traditionally, the standard has been to either suture the nerve ends together directly without tension or to bridge the gap between the nerve ends with a less important nerve surgically removed from elsewhere in the patient’s own body, referred to as a nerve autograft. More recently, synthetic or collagen conduits have been used for the repair of short gaps. Nerves that are not repaired or heal abnormally may result in a permanent loss of motor and/or sensory function. Additionally, abnormal healing can form a neuroma that may send
altered signals to the brain resulting in the sensation of pain. This abnormal section of the nerve can, under certain circumstances, be surgically removed and the nerve can be managed by capping, burying, or surgically repairing the nerve.
In addition, compression on a nerve, blunt force trauma or other physical irritations to a nerve can cause nerve damage that may alter the signal conduction of the nerve, resulting in pain, and may, in some instances, require surgical intervention to address the resulting nerve compression. Finally, when a patient undergoes a mastectomy due to breast cancer or prophylactically due to a genetic predisposition for breast cancer, the nerves are cut to allow the removal of the breast tissue. This can result in a loss of sensation, the potential risk of a symptomatic neuroma, and could negatively impact the patient’s quality of life. When a patient chooses a breast reconstruction after a mastectomy, sensation and quality of life can, in certain cases, be returned through surgical nerve repair.
To improve the options available for the surgical repair and regeneration of peripheral nerves, we have developed and licensed regenerative medicine technologies. Our innovative approach to regenerative medicine has resulted in first-in-class products that we believe are redefining the peripheral nerve repair market. Our products are used by surgeons during surgical interventions to repair a wide variety of physical nerve damage throughout the body, which can range from a simple laceration of a finger to a complex brachial plexus injury (an injury to the network of nerves that control the movement and sensation of the shoulder, arm, and hand) as well as nerve injuries caused by dental, orthopedic, and other surgical procedures.
Peripheral Nerve Regeneration Market Overview
Peripheral nerve injury (“PNI”) through damage or transection is a major source of physical disability impairing the ability to move muscles or to feel normal sensations. Patients suffer traumatic bodily injuries every day that may result in damage or transection to peripheral nerves severe enough to require surgical treatment. We break our total addressable market into four categories: (i) Trauma, (ii) OMF and Head and Neck, collectively OMF/H&N, (iii) Breast, and (iv) Upper Extremity Compression, together the Total Addressable Market.
We previously estimated that U.S. PNI has a potential Total Addressable Market for our current product portfolio and believe it is presently at least $2.7 billion. Estimating the Total Addressable Market for nerve repair is challenging as there is not a simple data source for the incidence of peripheral nerve issues. This is further complicated by the fact that nerves can be injured through a variety of traumatic and surgical injuries and can impact a patient from head to toe. In addition, we believe nerves are often one of many structures injured in a trauma (e.g., amputation) or in surgery and the incidence of these nerve injuries are often not coded or tracked. Quantifying the procedures involving nerve repair may also be challenging. While selected trauma and surgical procedures are dedicated to the repair of nerves, most of the incidence of nerve repair is a step in a larger trauma or surgical procedure. Current Procedural Terminology ("CPT") codes exist for surgeons to code for nerve repair; however, we believe the data substantially underestimates the total number of nerves repaired. Physicians are encouraged to document all steps of procedures, but open trauma often involves many surgical steps, and CPT codes may be inclusive of each other or may not be documented or reported in billing records. As a result, we believe CPT coding underrepresents the total number of nerve repairs performed in trauma. Because we believe CPT claims are not fully representative of the true volumes of nerve repair surgery, we follow an “empirical” methodology to estimate the Total Addressable Market – using published clinical literature and procedure databases to make what we believe are the most objective assumptions.
Trauma
The Trauma portion of the Total Addressable Market encompasses traumatic PNI throughout the body, with approximately 95% of injuries affecting upper and lower extremity nerves. We previously estimated that the Trauma portion of the Total Addressable Market and presently believe it is at least $1.9 billion based upon epidemiological studies regarding the general number of trauma patients, clinical literature review reporting PNI incidence, and physician interviews. We have estimated the portion of these nerve repair procedures due to trauma that would require gap repair, primary repair and/or nerve protection and applied, as we believed was appropriate in each procedure segment, the number of units and average sales price of Avance Nerve Graft and the average market price for nerve connectors and nerve protectors to determine the probable Total Addressable Market.
OMF and Head and Neck
We previously estimated the OMF portion of the Total Addressable Market and presently believe it is at least $300 million annually, based upon research indicating that approximately 56,000 PNI occur in the U.S. each year related to third molar surgeries, anesthetic injections, dental implants, orthognathic surgery, and mandibular resection procedures. We have applied the average sales price of the Avance Nerve Graft, Axoguard Nerve Connector, and Axoguard Nerve Protector that address such PNI to derive the OMF portion of the estimated Total Addressable Market.
In Head and Neck, we are focused on addressing nerve injuries in parotidectomy, thyroidectomy and radical neck dissections, which we believe presently represent a significant opportunity with more than 200,000 procedures performed annually, highly concentrated in large academic hospital centers.
Breast
We previously estimated the Breast portion of the Total Addressable Market based on autologous flap reconstructions (i.e. DIEP flaps) and presently believe it is at least $350 million annually. In 2023, we launched Resensation® to implant-based procedures with neurotization of the nipple area complex. We estimate that there is an additional 10-15% of the breast reconstructions done with implants that can also be neurotized which adds at least $250 million to increase the estimated Total Addressable Market to at least $600 million. Currently, when a patient undergoes autologous or implant-based breast reconstruction after a mastectomy, the patient receives the shape of a natural breast, but often times experiences little to no return of sensory feeling. In certain cases, sensation can be returned to the breast area with the use of our products through an innovative surgical technique called Resensation. We believe that the ideal breast reconstruction should restore breast size, shape, symmetry, and softness, as well as sensation, without the potential risks and comorbidity associated with autograft. We believe the Resensation technique incorporates a patient's desire for the opportunity to return sensation to their breasts with a reproducible and efficient surgical approach for reconstructive plastic surgery.
Upper Extremity Compression
PNI caused by recurrent carpal tunnel syndrome and cubital tunnel syndrome constitutes the "Upper Extremity Compression" portion of the Total Addressable Market. We believe the Upper Extremity Compression portion of the Total Addressable Market is approximately $350 million annually, or 145,000 procedures. We estimate there are approximately 488,000 primary carpal tunnel and 150,000 primary cubital tunnel relief surgeries performed annually in the U.S. We estimate that approximately 96,500 carpal tunnel revision surgeries and 51,600 total cubital tunnel procedures are addressable each year in the U.S. to mitigate the recurrence of symptoms. These revision and primary surgeries are required due to compression of the peripheral nerve associated with soft tissue attachments from the surrounding tissue or tissue infiltration entrapping the nerve. To prevent additional recurrences, surgeons will opt for a nerve protection which includes products such as the Axoguard Nerve Protector, Axoguard HA+ Nerve Protector ("HA+") and Avive+ Soft Tissue Matrix. To derive the carpal and cubital tunnel revision portion of the Total Addressable Market, we multiplied the average market sales price of Axoguard Nerve Protectors by the number of estimated procedures.
Although distribution and sales of products in the Trauma, OMF/H&N, Breast and Upper Extremity Compression portions of the Total Addressable Market constitute our primary revenue sources today, market expansion opportunities in lower extremity surgery, urology, and the surgical treatment of pain could offer us expanded revenue opportunities. We have begun an expansion into the surgical treatment of pain with an initial focus in the treatment of neuroma in our existing call points. The size of the pain market opportunity is challenging to identify as the cause of the chronic pain is often not diagnosed and there has not historically been a surgical treatment to resolve the cause of the pain. We believe the market opportunity is sufficient to apply selected resources to the opportunity and there is a significant patient and societal need to reduce the use of pharmacologic solutions, including opioids.
Our Product Portfolio
Avance Nerve Graft
Avance Nerve Graft is a biologically active nerve implant with more than sixteen years of comprehensive clinical evidence and more than 100,000 implants since launch. Avance Nerve Graft is processed nerve allograft intended for the surgical repair of peripheral nerve discontinuities to support regeneration across the defect. It is intended to act as a structural bridge in order to guide and support axonal regeneration across a peripheral nerve gap caused by traumatic injury or surgical intervention. Avance Nerve Graft is decellularized and sterile processed human peripheral nerve tissue. We developed Avance Nerve Graft by following the guiding principle that the human body created the optimal peripheral nerve structure. We, through our licensing efforts and research, developed our Avance Method®, a proprietary method for processing recovered human peripheral nerve tissue in a manner that preserves the essential structure of the ECM while cleansing away cellular and noncellular debris. Avance Nerve Graft provides the natural peripheral nerve structure of a nerve, including the native laminin, to guide the regenerating nerve fibers. The nerve ECM is additionally processed to remove a natural inhibitor to regeneration called chondroitin sulphate proteoglycan.
We believe that Avance Nerve Graft is the first off-the-shelf nerve allograft for bridging nerve transections. Avance Nerve Graft is comprised of bundles of small diameter endoneurial tubes that are held together by an outer sheath called the epineurium. Avance Nerve Graft has been processed to remove cellular and noncellular factors such as cells, fat, blood, and
axonal debris, while preserving the three-dimensional laminin lined tubular bioscaffold (i.e., microarchitecture), epineurium, and microvasculature of the peripheral nerve. After processing, Avance Nerve Graft is flexible and pliable, and its epineurium can be sutured in place allowing for a tension-free approximation of the proximal and distal peripheral nerve stumps. During the healing process, the body revascularizes and gradually remodels the graft into the patient’s own tissue while allowing the processed peripheral nerve allograft to physically support axonal regeneration across the peripheral nerve discontinuities. Avance Nerve Graft does not require immunosuppression for use.
With lengths up to 70 mm and diameters up to 5 mm, Avance Nerve Graft allows surgeons to choose and trim the implant to the correct length for reconstructing the relevant peripheral nerve gap, as well as to match the diameter to the proximal and distal end of the severed peripheral nerve. Avance Nerve Graft is stored frozen and utilizes packaging that maintains the implant in a sterile condition. The packaging is typical for medical products, so the surgical staff is familiar with opening the package for transfer of Avance Nerve Graft into the sterile surgical field. The packaging also provides protection during shipment and storage and a reservoir for the addition of sterile fluid to aid in thawing the product. Avance Nerve Graft thaws in less than 10 minutes, and once thawed, it is ready for implantation.
Avance Nerve Graft provides the following key advantages:
•A three-dimensional bioscaffold for bridging a peripheral nerve gap;
•A biologically active nerve therapy with more than 10 years of comprehensive clinical evidence;
•No patient donor-nerve surgery, therefore no comorbidities associated with a secondary surgical site;
•Available in a variety of diameters up to 5mm to meet a range of anatomical needs;
•Available in a variety of lengths up to 70mm to meet a range of gap lengths;
•Decellularized and cleansed ECM;
•Implanted without the need for immunosuppression, remodels into patient’s own tissue;
•Structurally supports the body’s own regeneration process;
•Handles similar to an autograft, and is flexible and pliable;
•Alleviates tension at the repair site;
•Three-year shelf life; and
•Supplied sterile.
Axoguard Nerve Connector
Axoguard Nerve Connector is a coaptation aid used to align and connect severed peripheral nerve ends in a tensionless repair. The product is in a tubular shape with an open lumen on each end where the severed peripheral nerve ends are inserted. It is typically used when the gap between the peripheral nerve ends is 5mm or less in length. Axoguard Nerve Connector is made from a processed porcine ECM that allows the body’s natural healing process to repair the peripheral nerve while its tube shape isolates and protects the transected nerves during the healing process. During healing, the patient’s own cells incorporate into the ECM product to remodel and form a tissue similar to the outermost layer of the peripheral nerve (nerve epineurium). Axoguard Nerve Connector is provided sterile, for single use only, and in a variety of sizes to meet the surgeon’s needs.
Axoguard Nerve Connector can be used:
•As an alternative to direct suture repair;
•As a peripheral nerve coaptation; Connector-Assisted Repair®;
•To aid coaptation in direct repair, grafting, or cable grafting repairs; and
•To reinforce the coaptation site.
Axoguard Nerve Connector has the following advantages:
•Processed intact porcine ECM with an open, porous structure that allows for cell infiltration and remodeling;
•Designed as a coaptation aid for tensionless repair of transected or severed peripheral nerves;
•Alleviates tension at the repair site;
•Remodels into the patient’s own tissue;
•Reduces the number of required sutures (versus direct repair with suture);
•Allows surgeon to move sutures away from the repair site which may minimize inflammation and aid nerve regeneration;
•Reduces potential for fascicular mismatch;
•Allows visualization of underlying peripheral nerve ends;
•Available in seven different diameters and two different lengths to address a variety of nerve repair situations;
•Strong and flexible, easy to suture; and
•Stored at room temperature with a minimum of 18-month shelf life for the sizes with 6-layers and 24-month shelf life for those with 5-layers.
Axoguard Nerve Protector
Axoguard Nerve Protector is a product used to protect and wrap damaged peripheral nerves and reinforce reconstructed nerve gaps while minimizing soft tissue attachments. It is designed to protect and isolate the peripheral nerve during the healing process after surgery by creating a barrier between the nerve tissue and the surrounding tissue bed. The product is delivered in a slit tube format allowing it to be wrapped around peripheral nerve structures. Axoguard Nerve Protector is made from a processed porcine ECM. During healing, the ECM remodels allowing the protector to separate the peripheral nerve from the surrounding tissue. Axoguard Nerve Protector competes against off-the-shelf biomaterials such as reconstituted bovine collagen as well as the use of the patient’s own tissue such as vein and hypothenar fat pad wrapping. Axoguard Nerve Protector is provided sterile, for single use only, and in a variety of sizes to meet the surgeon’s needs.
Axoguard Nerve Protector can be used to:
•Separate and protect the nerve from the surrounding tissue during the healing process;
•Minimize risk of soft tissue attachments and entrapment in compressed peripheral nerves;
•Protect peripheral nerves in a traumatized wound bed; and
•Reinforce a coaptation site.
Axoguard Nerve Protector has the following advantages:
•Processed porcine submucosa ECM used to reinforce a coaptation site, wrap a partially severed peripheral nerve or protect peripheral nerve tissue;
•Creates a protective layer that isolates and protects the peripheral nerve in a traumatized wound bed;
•Remodels to form a new soft tissue layer;
•Easily conforms and provides 360-degree wrapping of damaged peripheral nerve tissue;
•Allows the body's natural healing process to repair the nerve;
•Minimizes the potential for soft tissue attachments and peripheral nerve entrapment by physically isolating the nerve during the healing process;
•Allows peripheral nerve gliding;
•Strong and flexible, plus easy to suture;
•Is available in five different widths and two different lengths to address a variety of peripheral nerve repair situations; and
•Stored at room temperature with a minimum of 24-month shelf life.
HA+
HA+ is a surgical implant that provides non-constricting protection for peripheral nerves. HA+ is designed to be an interface between the nerve and the surrounding tissue. HA+ is comprised of an ECM and is fully remodeled during the healing process. The lubricant coating on HA+ is composed of sodium hyaluronate and sodium alginate. When hydrated, the lubricant coating reduces friction between the nerve and the surrounding tissue. HA+ is flexible to accommodate movement of the joint and associated tendons and has sufficient mechanical strength to hold sutures. HA+ is provided sterile, for single use only, and in a variety of sizes to meet surgeons’ needs.
HA+ can be used to:
•Separate and protect the nerve from the surrounding tissue during the healing process;
•Minimize risk of soft tissue attachments and entrapment in compressed peripheral nerves;
•Protect peripheral nerves in a traumatized wound bed; and
•Provide tension relief when used in aiding a coaptation.
HA+ has the following advantages:
•Processed porcine submucosa ECM layer is vascularized and remodeled by the patient into new site-specific tissue;
•Double-sided HA+ gel coating to reduce friction and enhance nerve gliding through traumatic tissue beds;
•Formulated for optimized handling and flexibility of surgical application—quick hydration, flat sheet configuration and is easy to suture if needed;
•Allows the body's natural healing process to repair the nerve;
•Minimizes the potential for soft tissue attachments and peripheral nerve entrapment by physically isolating the nerve during the healing process;
•Allows peripheral nerve gliding;
•Is available in five different sizes up to 4cm x 8cm to address a variety of peripheral nerve repair situations; and
•Stored at room temperature with a minimum of 24-month shelf life.
Axoguard Nerve Cap
Axoguard Nerve Cap is a proprietary porcine submucosa ECM product used to protect a peripheral nerve end and separate the nerve from the surrounding environment to reduce the development of symptomatic or painful neuroma.
Nerves are often cut in a variety of surgeries and a nerve that is cut and not reconstructed may form an entangled mass of disorganized nerve and fibrous tissue that could cause debilitating pain called a symptomatic neuroma. Neuromas are a potential cause of pain for those patients who complain of chronic post-surgical pain, including in amputees, which may lead to an inability to use their prosthesis. Despite more than 30 different treatment methods, it is our belief that neuromas continue to be an unresolved problem in microsurgery. We believe the Axoguard Nerve Cap can address these painful neuromas and
address nerve pain without the complications of traditional methods, including pharmacotherapy and chemical injections, among others. Axoguard Nerve Cap can be used to reduce the development of symptomatic or painful neuroma formation.
Axoguard Nerve Cap has the following advantages:
•Separates the nerve end from surrounding tissue, neurotrophic factors and mechanical stimulation;
•Reduces painful neuroma formation;
•Allows for anchoring of a nerve end or stump to nearby tissue structure;
•Material gradually remodels into the patient’s own tissue to protect the nerve end;
•Semi-translucence allows for visualization of nerve ends or stumps and easy visualization for suture placement;
•Is available in six different sizes to address a variety of situations; and
•Stored at room temperature with a minimum of 18-month shelf life.
Avive+ Soft Tissue Matrix
Avive+ Soft Tissue Matrix, a multi-layer amniotic membrane allograft used to protect and separate tissues in the surgical bed during the critical phase of tissue repair.
Acroval Neurosensory and Motor Testing System
Effective November 2019, we discontinued all sales and effective October 15, 2024, we no longer perform maintenance or calibration of the Acroval Neurosensory and Motor Testing System.
Tissue Recovery and Processing
Avance Nerve Graft Processing Overview
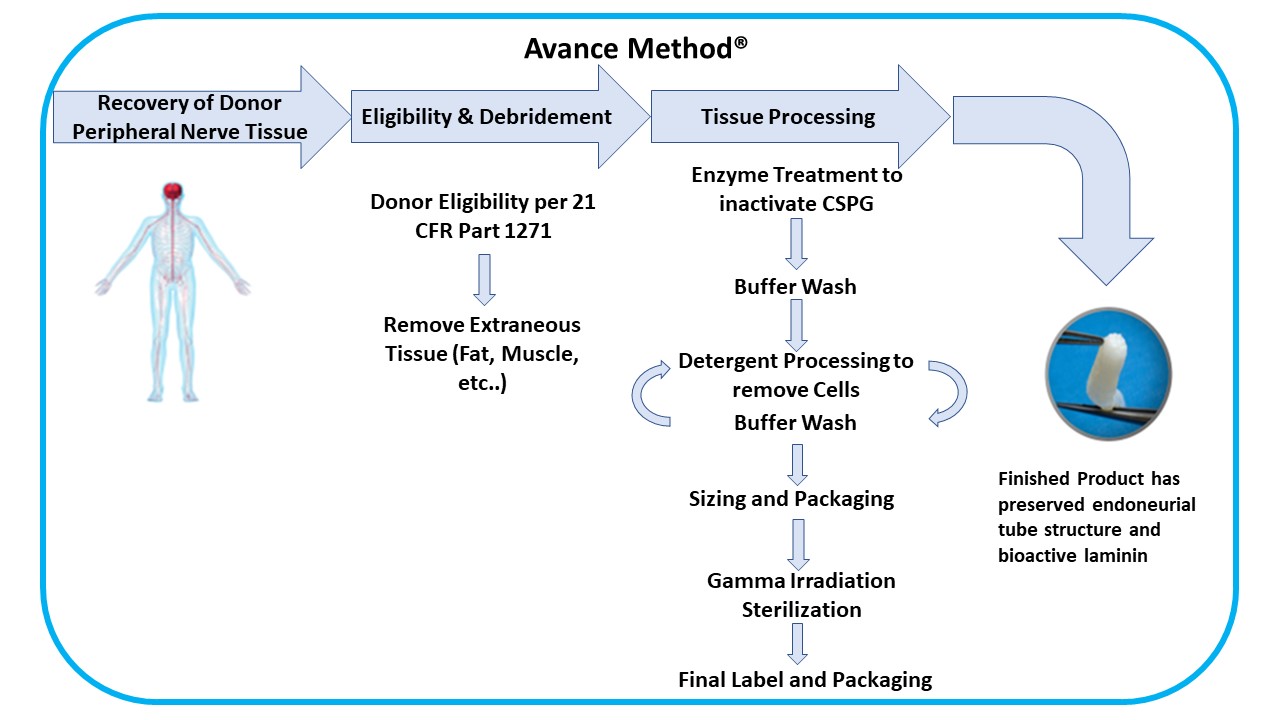
We developed our Avance Method®, an advanced and proprietary technique to process Avance Nerve Graft from donated human peripheral nerve tissue. Our Avance Method requires special training over several months for each manufacturing associate who processes Avance Nerve Grafts. The processing and manufacturing system for Avance Nerve Graft has required significant capital investment, and we seek to continually improve our manufacturing and quality assurance processes and systems. Our Avance Method is depicted as follows:
Tissue Processing
Our Avance Method comprises peripheral nerve tissue recovery/acquisition and testing, donor medical review and release, debridement and other processing steps, packaging, and sterilization to meet or exceed all applicable FDA, state, and international regulations and the American Association of Tissue Banks (“AATB”) standards. Our supply agreements with recovery and acquisition agencies for peripheral nerve tissue and our ability to enter into additional supply contracts, as necessary will provide us with the tissue volumes we require to meet the demand for our Avance implants. As an FDA registered tissue establishment, we use both our own personnel and subcontractors for recovery/acquisition, storage, testing, processing, and sterilization of the donated peripheral nerve tissue and placental tissues. Additionally, we and our subcontractors, have contracted with independent Good Manufacturing Practice ("GMP") and Good Laboratory Practice compliant laboratories to perform testing for product release. The safety of Avance Nerve Graft is supported by donor screening, process validation, process controls, and validated terminal sterilization methods. The Axogen Quality System has built in redundancies that are meant to ensure product release only after such product meets our quality control and product requirements.
Tissue Recovery and Processing Facilities
We partner with other FDA registered tissue establishments and AATB accredited recovery agencies or recovery agencies in compliance with FDA, state and international regulations and AATB standards for human tissue recovery. After consent for donation is obtained, donations are screened and tested in detail for safety in compliance with FDA, state and international regulations and AATB standards on communicable disease transmission.
In 2023, we successfully transferred the Avance Nerve Graft tissue processing and packaging from the Solvita facility to our Axogen Processing Center facility (the "APC Facility"), which is comprised of a 107,000 square foot building on approximately 8.6 acres of land located in Vandalia, Ohio. It is expected that the APC Facility, along with the ability for expansion, will provide processing capabilities that will meet our intended sales growth. We submitted the BLA for Avance Nerve Graft in September of 2024, and, on November 1, 2024, the FDA accepted the BLA for filing and assigned a Prescription Drug User Fee Act goal date of September 5, 2025. The APC Facility is expected to allow us to meet the current Good Manufacturing Practices ("cGMPs") required for processing a biologic product. Biologics manufacturing is highly regulated and subject to scrutiny and inspection by the FDA. Any quality or compliance issues, or manufacturing disruptions for any other reasons, may delay approval of the BLA.
We obtained certain economic development grants from state and local authorities totaling up to $2.7 million including $1.3 million of cash grants to offset costs to acquire and develop the APC Facility. Certain economic development grants were
subject to fixed asset investments and job creation milestones by December 31, 2024, and have clawback clauses if we do not meet the minimum requirements for these milestones. We have not met the job creation milestones and have requested a reduction or waiver of clawbacks or extensions from the grant authorities to extend the job creation milestones evaluation date from December 31, 2024 and the expiration date to December 31, 2026. Certain grant authorities have not approved our request. We are continuing discussions with these grant authorities regarding the evaluation, expiration and clawbacks of the job creation milestones and expect to receive a response during 2025. We could be obligated to pay back up to approximately $950 thousand as of December 31, 2024 related to these grants. See "Part II, Item 8. Financial Statements and Supplementary Data – Notes to Consolidated Financial Statements - Note 15 - Commitments and Contingencies - Service Agreements."
We process and package Avive+ Soft Tissue Matrix using our employees and equipment pursuant to a License and Services Agreement, as amended (the “Solvita Agreement”) with Community Blood Center (doing business as Solvita) ("Solvita"), in Dayton, Ohio at their facility ("Solvita facility"). We expect to continue to rely on the Solvita facility for the processing of Avive+ Soft Tissue Matrix. Solvita is an FDA registered tissue establishment and an AATB accredited organization.
The current Solvita Agreement extends through December 31, 2026. Under the Solvita Agreement, we pay Solvita a facility fee for clean-room, manufacturing, storage, and office space. Solvita also provides services in support of our manufacturing such as routine sterilization of daily supplies, providing disposable supplies and microbial services, and office support. The service fee is based on a per donor batch rate. The Solvita facility provides a cost effective, quality controlled and licensed facility. Our processing methods and process controls have been developed and validated to ensure product uniformity and quality. Pursuant to the Solvita Agreement, we pay license fees on a monthly basis to Solvita. See "Item 8. Financial Statements and Supplementary Data – Notes to Consolidated Financial Statements- Note 15 - Commitments and Contingencies - Service Agreements."
Tissue Packaging
After processing, the packaging operation for Avance Nerve Graft is performed in a controlled environment at the APC Facility and the packaging operation for Avive+ Soft Tissue Matrix is performed in a controlled environment at the Solvita facility. Each Avance Nerve Graft and Avive+ Soft Tissue Matrix are visually inspected and organized by size into finished product codes. The tissue implants are then packaged in primary packaging. The outer pouch acts as the primary sterility and moisture barrier.
Tissue Sterilization and Labeling
After being processed and packaged, Avance Nerve Graft and Avive+ Soft Tissue Matrix are then terminally sterilized at a contract sterilization facility. After sterilization, Avance Nerve Graft and Avive+ Soft Tissue Matrix are shipped back to Axogen where the product lots will undergo quality review to ensure the lots meets specifications and then final packaging and labeling. Orders for Avance Nerve Graft and Avive+ Soft Tissue Matrix are placed with our customer care team, and the products are packaged and shipped from our distribution facilities.
Tissue Product Release
We have established quality procedures for review of tissue recovery, relevant donor medical record review, and release to processing that meet or exceed FDA requirements as defined in 21 CFR Part 1271, state regulations, international regulations, and AATB standards. The Axogen Quality System meets the requirements set forth under 21 CFR Part 1271 for HCT/Ps, including Good Tissue Practices (“GTP”) and is compliant with the 21 CFR Part 820 Quality System Regulation (“QSR”). Furthermore, we utilize validated processes for the handling of raw material components, environmental control, processing, packaging, and terminal sterilization. In addition to ongoing monitoring activities for product conformity to specifications and sterility, shipping methods have been validated in accordance with applicable industry standards.
Manufacturing of Our Medical Device Classified Products
Manufacturing for the Axoguard Product Line
Axoguard Nerve Connector, Axoguard Nerve Protector, HA+, and Axoguard Nerve Cap (the "Axoguard Product Line") is manufactured by Cook Biotech Incorporated (acquired on January 31, 2024 by RTI Surgical, Inc. and rebranded on December 17, 2024 as Evergen), in West Lafayette, Indiana (“Evergen”), which was established in 1995 to develop and manufacture implants utilizing porcine ECM. We do not expect this acquisition to have a material impact on our relationship with Evergen or on our operations. We decided to expand our portfolio of products and felt that the unique ECM material offered by Evergen provided the combination of properties needed in nerve reconstruction. Evergen’s ECM material is pliable, capable of being
sutured, and translucent and allows the patient’s own cells to incorporate into the ECM to remodel and form a tissue similar to the nerve’s epineurium. Evergen has its own source of the raw material for the ECM material and manufactures Axoguard products from such sources.
Axoguard Nerve Connector and Axoguard Nerve Protector
In August 2008, we entered into an agreement with Evergen, amended in February 2012, October 10, 2014, February 26, 2018, and August 4, 2023 (the “Distribution Agreement”), to distribute its ECM technology in the form of the Surgisis® Nerve Cuff, the form of a nerve wrap or patch, or any other mutually agreed to configuration. The Surgisis products were rebranded under our Axoguard name and consist of the Axoguard Nerve Connector and Axoguard Nerve Protector. Our distribution rights are worldwide in the field of the peripheral and central nervous system but excluding use of the products in the oral cavity for endodontic and periodontal applications and OMF surgery solely as they relate to dental, soft or hard tissue repair, or reconstruction. We believe the exclusion does not limit our identified OMF market, but expansion into certain additional OMF market areas could be limited to our other products not subject to the Distribution Agreement.
The Distribution Agreement terminates on December 31, 2030. Although the agreement requires certain minimum purchases, through mutual agreement, the parties have not established such minimums and to date have not enforced such provision. The Distribution Agreement also establishes a formula for the transfer cost of the Axoguard Nerve Connector and Axoguard Nerve Protector.
Axoguard Nerve Cap and HA+
We developed, filed several patent applications, and, on August 8, 2017, obtained FDA 510(k) regulatory clearance for the Axoguard Nerve Cap. We developed, filed several patent applications, and, on April 7, 2023, obtained FDA 510(k) regulatory clearance for HA+ and a second 510(k) regulatory clearance expanding the indication for use of HA+ on October 12, 2023. These devices are made with Evergen’s ECM material. Pursuant to the Nerve End Cap Supply Agreement dated June 27, 2017, as amended on April 6, 2020 and August 4, 2023, (the "Amended Supply Agreement"), Evergen is the exclusive contract manufacturer of the Axoguard Nerve Cap and both parties have provided the other party the necessary licenses to their technologies for operation of the Amended Supply Agreement. The Amended Supply Agreement has a term through December 31, 2030. Pursuant to the HA+ Supply Agreement dated May 2, 2023, Evergen is the exclusive contract manufacturer of HA+ and both parties have provided the other party with the necessary licenses to their technologies for operation under the agreement. The HA+ Supply Agreement has a term through June 30, 2030. Consistent with the Axoguard Nerve Connector and Axoguard Nerve Protector products, we are able to sell the Axoguard Nerve Cap and HA+ worldwide in the field of the peripheral and central nervous system, but subject to the same exclusions as Axoguard Nerve Connector and Axoguard Nerve Protector.
Sales and Marketing
Overview
We are focused on developing the peripheral nerve repair and regeneration market, committed to improving awareness of new surgical peripheral nerve repair options and building additional scientific and clinical data to assist surgeons and patients in making informed choices with respect to the repair of peripheral nerve injuries. We believe that there is an opportunity to improve current approaches to peripheral nerve repair and that our approach will solidify our position as a leader in the field of peripheral nerve repair products. The following provides the key elements of our sales and marketing strategy.
Increase Awareness of Our Products
Prior to the introduction of our portfolio of peripheral nerve repair products, surgeons had a limited number of options available to surgically repair damaged or transected peripheral nerves. We entered the market to improve the standard of care for nerve injury patients. We intend to increase market penetration and share by increasing awareness of the impact of nerve damage on quality of life and improving the adoption of nerve repair techniques and our products through the continued use of educational conferences and presentations, training for surgical residents, fellows and attending physicians, scientific publications, digital communication, and a knowledgeable and professional sales team. We work to increase awareness and the use of our products within high potential accounts that include hospitals that are level 1 trauma centers, academic affiliated and have a large number of microsurgical trained surgeons and large procedural volumes across our focus clinical specialties. Our customer call points are focused on plastic reconstructive surgeons, orthopedic and plastic hand surgeons who perform surgeries on patients suffering traumatic nerve damage or transection, on oral maxillofacial and head and neck surgeons who repair damaged oral and facial nerves, and on plastic reconstructive surgeons who perform breast reconstruction and neurotization.
Expand Clinical and Scientific Data Regarding the Performance of Our Products
Generating robust clinical and scientific data is a cornerstone of our marketing and product development strategy. As of December 31, 2024, there have been over two hundred and fifty peer reviewed clinical publications related to our products, with some publications including data on multiple product offerings. This body of evidence underscores the growing adoption and validation of our technologies in peripheral nerve repair.
Our RANGER® clinical study (defined below in “Government Regulations - Clinical Trials”), a comprehensive utilization registry of the Avance Nerve Graft, enrolled more than 2,800 Avance Nerve Graft repairs to date. Enrollment and follow-up in the primary RANGER arms and the MATCH arm - evaluating autograft and conduit repair options - were completed in December 2023. The analyses from these studies were integral to supporting our BLA submission. Another arm of the RANGER study, Sensation-NOW®, is currently tracking neurotization outcomes in breast reconstruction and continues to enroll patients. To date, the RANGER registry has generated eleven peer-reviewed publications and over 70 scientific conference presentations, reflecting the significant impact of these studies in advancing the field of nerve repair.
In December 2023, we initiated enrollment for COVERED, a multi-center series of protection with HA+ in protection applications. Additionally, several investigator-initiated studies, case reports, and publications have been completed, including breast neurotization, mandible reconstruction, compressive neuropathies, and the surgical treatment of pain. Ongoing case series are being developed for brachial plexus, breast reconstruction neurotization, compression injuries, and the surgical treatment of pain. We remain committed to supporting external research efforts and actively collaborate with investigators on grants with translational and clinical impact.
The RECON study (defined below in "Government Regulations - Clinical Trials"), a pivotal phase 3, multicenter, prospective, randomized, comparative study of hollow tube conduits and Avance Nerve Graft has successfully completed enrollment, follow-up, and analysis. The first peer reviewed publication from this study has been published, marking a significant milestone in transitioning the Avance Nerve Graft to a biologic product.
The REPOSE study pilot phase (defined below in "Government Regulations - Clinical Trials"), a multicenter, prospective, randomized, and subject blinded study of Axoguard Nerve Cap as compared to neurectomy alone for the treatment of symptomatic neuroma, has also been published. The comparative phase has completed enrollment and follow-up, providing critical data to guide treatment strategies. Additionally, enrollment in REPOSE XL was completed in January 2025. This study is evaluating the tolerability and feasibility of a large diameter Axoguard Nerve Cap for protecting and preserving terminated nerve ends.
By continuously generating and disseminating clinical and scientific data, we aim to solidify our leadership in the field of peripheral nerve repair, advance clinical practice, and support the adoption of innovative treatment solutions.
Commitment to the Education of Best Practices in Peripheral Nerve Repair
We have established educational conferences and presentations and surgical resident and fellow training that we believe have positioned us as a leader in providing peripheral nerve repair best practices. We have historically provided education on peripheral nerve repair through in-person national programs, including our “Advances and Best Practices in Nerve Repair” as well as local and regional educational events. In 2024, we offered multiple educational programs including virtual and in-person surgeon education programs.
Focused on developing deeper penetration with our existing accounts through development of long-term users of our algorithm in our largest market opportunity of extremity trauma
We provide full sales and distribution services. As of December 31, 2024, we had 114 direct sales professionals in the U.S. Our direct sales force continues to be supplemented by independent sales agencies that represent approximately 10% of our total revenue. We believe that near-term growth can be supported first through expanded productivity of our existing sales force as they go into more depth with existing accounts and then by adding additional accounts. We expect the number of direct sales professionals to increase over time. Additionally, we have successfully utilized a hybrid commercial approach that includes the use of independent agencies in more remote geographies to provide appropriate local support for surgeons, without the travel time required of a direct sales representative.
Our products are available and sold in 19 countries outside the U.S. through a number of independent in-country distributors. We provide support and resources for independent agencies and distributors both within and outside the U.S. We provide our products to hospitals, surgery centers and military hospitals, calling on surgeons, including plastic reconstructive surgeons, orthopedic and plastic hand surgeons, and certain oral and maxillofacial surgeons to review the benefits of our
products. While surgeons make the decision to implant our products in appropriate patients, hospitals make the decision to purchase the products from us. In today’s budget constrained environment, hospital committees review new technologies for cost effectiveness as well as quality. We believe that we have been successful in meeting the needs of these hospital committees by demonstrating the cost/benefit of our products and providing a fair value to the hospital.
Expand the Product Pipeline and Applications in Peripheral Nerve Repair
We have developed and continue to develop new and next generation products to support surgeons in their needs for repairing damaged or transected peripheral nerves. We believe additional opportunities exist to develop or acquire complementary products in peripheral nerve repair. In addition, there are opportunities to expand the existing portfolio of products in new applications of peripheral nerve repair in applications such as lower extremity surgery, head and neck surgery, urology, and the surgical treatment of pain.
Avance Nerve Graft Performance
We have worked with leading institutions, researchers, and surgeons to support innovation in the field of surgical peripheral nerve repair. We believe our RANGER study is the largest multi-center clinical study conducted in peripheral nerve gap repair with over 2,800 enrolled repairs. We have completed the RECON study. This study was a phase 3 trial to support our BLA for Avance Nerve Graft. See “Government Regulations - Clinical Trials - Axogen Clinical Trials.”
International Opportunity for Revenue
We currently focus primarily on the U.S. market, with additional foreign distribution and sales in Canada, Germany, UK, Spain, and several other European, Asian and Latin American countries. The need for the surgical repair of damaged or transected nerves is a global opportunity. Through our revenue outside the U.S., we have demonstrated the capability to take our current peripheral nerve repair surgical portfolio into new geographical markets. We currently have European Union (“E.U.”) wide registration only for Axoguard Nerve Connector and Axoguard Nerve Protector as approval/registration for Avance Nerve Graft as human tissue is required in each individual country. Avance Nerve Graft was granted marketing authorization in Germany and direct commercial operations began in 2022. Currently, Axoguard Nerve Cap is available in the U.S. and New Zealand. Introduction of our products into foreign markets is subject to meeting the appropriate regulatory standards of particular countries and any appropriate regional regulation or directive. In addition to regulatory approval, reimbursement approval is necessary to achieve material product adoption in most countries. Avance Nerve Graft has achieved NICE approval in the UK for digital nerve repair and reimbursement approval in South Korea for repairs up to 50mm in length for sensory nerves when an autograft is not possible. To date, revenue from international distribution and sales have not been material, there are no material risks associated with foreign operations, and we do not have dependencies as to international revenue. See " Item 1A. Risk Factors – Our operations must comply with FDA and other governmental requirements."
Research and Development
We are committed to advancing the field of peripheral nerve repair and providing the most comprehensive portfolio of solutions for peripheral nerve injuries. Our development efforts are focused on expanding the clinical evidence base for nerve repair surgical applications, introducing product line extensions of the Avance and Axoguard products, and innovating new technologies and products to address unmet needs in peripheral nerve repair.
In collaboration with leading academic institutions, we actively support the development of novel treatment approaches for peripheral nerve injuries. Our research initiatives are supported by several government-funded grants that explore the application of our technologies in nerve repair and recovery.
For the fiscal year ended December 31, 2024, we invested approximately $27.8 million in research and development activities. This investment includes costs associated with product development, clinical research, and the transition of Avance Nerve Graft to a biological product, reflecting our dedication to both scientific advancement and regulatory excellence.
Competition
The medical device and biotechnology industries are characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. As such, we cannot predict what products may be offered in the future that may compete with our products. In the peripheral nerve repair market, we compete primarily against all transected and non-transected peripheral nerve repair approaches, including direct suture repair, autograft, and hollow-tube nerve conduits and materials used to wrap and protect damaged peripheral nerve tissue.
Because the requirements of the biomaterials used in peripheral nerve repair can vary based on the severity and location of the damaged nerve, the size and function of the nerve, surgical technique, and patient preference, our peripheral nerve repair products compete against both autograft materials (nerve in the case of a bridging repair and vein or fat in the case of a nerve protection repair), and a limited number of off-the-shelf alternatives for repairing and protecting. Competitive aspects of our products focus on their overall value proposition and suitability for specific applications and can include composition and structure of the material, ease of use, clinical evidence, handling, and price. Our major competitors' products include off-the-shelf repair options in hollow-tube conduits, coaptation aids and bio-absorbable wraps.
We believe any current or future competitors face the following important barriers to market entry as it relates to its peripheral nerve repair products. Our intellectual property (“IP”), and that of our partners, including patents, patents-pending, trade secrets, and unique, internal subject matter expertise, is believed to be an important barrier for our Avance Nerve Graft and Axoguard products. We have developed knowledge and experience in understanding and meeting FDA regulatory requirements for Avance Nerve Graft, including having made a substantial investment in conducting the pre-clinical and clinical testing necessary to support a submission for an FDA BLA. Additionally, we believe our ability to offer a portfolio of products focused on peripheral nerve repair and the breadth of clinical data associated with the products provides a unique competitive position versus other entities that do not have this breadth of product offering. However, due to our limited resources, our smaller size, and our relatively early stage, we believe we may face competitive challenges from larger entities and market factors that could negatively impact our growth, including competitors’ introduction of new products and competitors’ bundling of products to achieve pricing benefits. (See “Item 1A. Risk Factors – Technological change and competition for newly developed products could reduce demand for our products”; “Risk Factors –– Our operating results could be adversely impacted if we are unable to effectively manage and sustain our future growth or scale our operations”).
Intellectual Property
Overview
We protect our IP through a combination of patents, trademarks, trade secrets, and copyrights. In addition, we safeguard our trade secrets and other confidential know-how, and carefully protect these and other IP rights when engaging with third parties. For example, we require vendors, contract organizations, consultants, advisors, and employees to execute confidentiality and nondisclosure agreements, and to appropriately protect any information disclosed to them by us so as to preserve confidential and/or trade secret status. We also require consultants, advisors, and employees to assign their rights to any relevant IP arising out of their relationship with us to us.
License Agreements
We have previously entered into license agreements with the University of Florida Research Foundation (the "UFRF") and the University of Texas at Austin ("UTA"). Under the terms of these license agreements, we hold exclusive worldwide licenses to underlying technologies used by us in our Avance Nerve Graft. The license agreements include the right to certain patents and patents pending in the U.S. and international markets. The effective term of the license agreements extends through the term of the related patents. The patents for which royalty obligations exist under the UFRF license agreement expired in December 2023, and the UTA license agreement expired when the last patents licensed thereunder expired in September 2023.
Patents
As of the date of this Annual Report on Form 10-K, we own thirty-two issued U.S. patents, more than fifty pending U.S. patent applications (including those for which we have received a notice of allowance) and three hundred twenty international patents and patent applications with regard to our peripheral nerve products and other related technologies.
In connection with our Avance Nerve Graft, per Section 351(k)(7) and 351(i)(4) of the PHS Act, from the date of BLA approval, we believe we will have a period of 12 years of exclusivity in the U.S. from commercial competition from biosimilars using Avance Nerve Graft as the reference product. Finally, we have Enforcement Discretion from the FDA regarding continued distribution under controls applicable to HCT/Ps with an agreed transition plan to a BLA. We believe a competitive processed peripheral nerve allograft (non-biosimilar) would need to successfully complete BLA Phase I, II and III clinical studies prior to clinical release, the completion of which we believe would take at least eight years.
Each of Axogen’s other products in the U.S. and abroad, is also protected by multiple patents and local laws providing protection for IP, which provides further barriers to entry for potentially competitive products. Axogen’s Axoguard Nerve Cap is protected by numerous issued Axogen patents in the U.S. and globally. Additional allowed Axogen patent applications, as well as other pending Axogen patent applications that are expected to be issued in the U.S. and abroad, will provide further protection of Axoguard Nerve Cap and thus act as additional obstacles to the commercial introduction of competitive products.
Our HA+ is also the subject of multiple pending Axogen patent applications in the U.S. and abroad. The potential for products competitive with Axogen’s Axoguard line of products, including our Axoguard Nerve Connector and Axoguard Nerve Protector, is further encumbered by the additional IP protections related to their methods of manufacture, as discussed further below in the Trade Secrets section.
Our policy is to seek patent protection for, or where strategically preferable, maintain as trade secret, the inventions that we consider important to our products and the development of our business. We have sought, and will continue to seek, patent protection for select proprietary technologies and other inventions emanating from our research and development ("R&D"), including with respect to uses, methods, and compositions, in an effort to further fortify our IP in areas of importance to us and our growing product portfolio. In instances that patent protection is not possible, product value to our portfolio can still be derived.
Trademarks, Trade Secrets and Copyrights
We hold a significant portfolio of hundreds of registered trademarks and applied-for trademarks in the U.S. and worldwide. Protection of our trademarks allows us to prevent competitors from, for example, using the same or a confusingly similar company name, or the same or confusingly similar product names within identified classes of goods that could otherwise wrongfully allow such competitors to capitalize on our brand, reputation, and goodwill, and thereby improperly bolster their sales or reputations through, for example, consumer confusion, a false indication of our endorsement, or of a false indication of corporate or contractual relationship with us. We police and enforce our marks.
We possess trade secrets and material know-how in the following general subject matters: nerve and tissue processing, nerve repair, product testing methods, and pre-clinical and clinical expertise. We have registered copyrights for training tools and artistic renderings. Additionally, we entered into the Distribution Agreement and Supply Agreement with Evergen for the Axoguard products. Evergen believes it has know-how and trade secrets with respect to its ECM technology that provides certain additional competitive obstacles to third parties, in addition to those obstacles existing in view of Axogen-owned IP.
Government Regulations
U.S. Government Regulation Overview
Our products are subject to regulation throughout their lifecycle by the FDA, as well as other federal and state regulatory bodies in the U.S. and comparable authorities in other countries. In addition, our Avance Nerve Graft must comply with the standards of the tissue bank industry’s accrediting organization, the AATB.
We distribute Axoguard Nerve Connector and Axoguard Nerve Protector products for Evergen, and Evergen is responsible for the regulatory compliance of these products. These Axoguard products are regulated as medical devices and subject to pre-market notification requirements under section 510(k) of the Federal Food, Drug, and Cosmetic Act (the “FD&C Act”), 21 CFR Part 820 QSR, and related laws and regulations. Evergen has obtained a 510(k) pre-market clearance for Axoguard Nerve Connector from the FDA for the use of porcine small intestine submucosa for the repair of peripheral nerve transections where gap closure can be achieved by flexion of the extremity. Evergen has also obtained a 510(k) pre-market clearance for Axoguard Nerve Protector for the repair of peripheral nerve damage in which there is no gap or where a gap closure is achieved by flexion of the extremity. We sell the 510(k) cleared devices under the trade names Axoguard Nerve Connector and Axoguard Nerve Protector.
We are the specification developer and authorization holder of the Axoguard Nerve Cap product, which is classified by the FDA as a Class II device. The Axoguard Nerve Cap was cleared for market under 510(k) K163446. It is classified by FDA under 21 CFR § 882.5275 (Nerve Cuff, product code: JXI). Evergen is the contract manufacturer for our Axoguard Nerve Cap product, and we are responsible for the regulatory compliance, distribution, and sale of this product.
We are the specification developer and authorization holder of the HA+ product, which is classified by the FDA as a Class II device. HA+ was cleared for market under 510(k) K223640 on April 7, 2023, and a second 510(k) K231708 regulatory clearance was obtained on October 12, 2023, expanding the indication for use of HA+. The products are classified by the FDA under 21 CFR § 882.5275 (Nerve Cuff, product code: JXI). Evergen is the contract manufacturer, and we are responsible for the regulatory compliance, distribution, and sale of this product.
Avive+ Soft Tissue Matrix Regulation
We launched Avive+ Soft Tissue Matrix in the second quarter of 2024 after engagement with the FDA through the Tissue Reference Group Rapid Inquiry Program, which resulted in feedback that the product appears to be regulated solely under section 361 of the PHS Act and the regulations in 21 CFR Part 1271. Products regulated solely under Section 361 of the PHS Act are a product category under close scrutiny by the FDA for compliance with the regulatory requirements and potentially subject to regulatory change in the future. Failure to comply with applicable regulatory requirements could expose us to potential compliance actions by the FDA or state regulators and could risk the commercial availability of the product.
FDA — General
FDA regulations govern nearly all the activities that we perform, or that are performed on our behalf, to ensure that medical products distributed domestically or exported internationally are safe and effective for their intended uses. The activities the FDA regulates include the following:
•Product design, development, and manufacture;
•Product safety, testing, labeling, and storage;
•Pre-clinical testing in animals and in the laboratory;
•Clinical investigations in humans;
•Pre-marketing clearance, approval, or licensing;
•Record-keeping and document-retention procedures;
•Advertising and promotion;
•The import and export of products;
•Product marketing, sales, and distribution;
•Post-marketing vigilance, surveillance and medical device reporting, including reporting of deaths, serious injuries, communicable diseases, device malfunctions, or other adverse events; and
•Corrective actions, removals and recalls.
Failure to comply with applicable FDA regulatory requirements may subject us to a variety of administrative or judicially imposed penalties or sanctions and/or prevent us from obtaining or maintaining required approvals, clearances, or licenses to manufacture and market our products. It could also subject us to enforcement actions or sanctions, such as agency refusal to approve pending applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution of products, injunctions, consent decrees or civil monetary penalties or criminal prosecution.
FDA’s Pre-market Clearance and Approval Requirements - Medical Devices
Unless an exemption applies, each medical device distributed commercially in the U.S. requires either a 510(k) pre-market notification submission or a Pre-Market Approval (“PMA”) Application to the FDA, or other FDA regulatory authorization. Medical devices are classified into one of three classes—Class I, Class II, or Class III—depending on the degree of risk, the level of control necessary to assure the safety and effectiveness of each medical device and how much is known about the type of device. For devices first intended for marketing after May 28, 1976, pre-market review and clearance by the FDA for Class I and II medical devices is accomplished through the 510(k) pre-market notification procedure by finding a device substantially equivalent to a legally marketed Class I or II device, unless the device is exempt. The majority of Class I medical devices are exempt from the 510(k) pre-market notification requirement. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting, or implantable devices for which Class II controls are inadequate to assure safety or effectiveness, and novel devices, including devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in Class III. Class III devices generally require an approved PMA prior to marketing, unless classified into Class I or Class II through a de novo request.
A PMA must be supported by extensive data, including, but not limited to, technical, pre-clinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction, and the safety and effectiveness of the device.
Investigational New Drug Application for Drugs and Biologics
Federal law requires that a new drug be the subject of an approved marketing application and that a biological product be properly licensed before each is introduced or delivered for introduction into interstate commerce. Because a sponsor often needs to ship an investigational drug or biological product to clinical investigators in many states, it must seek an exemption from that legal requirement. The Investigational New Drug ("IND") application is the means through which the sponsor obtains this exemption from the FDA. It is additionally the request from a clinical study sponsor to obtain authorization from the FDA to administer an investigational drug or biological product to humans.
There are two IND categories: Commercial and Research (non-commercial). The IND application must contain information in three broad areas:
•Animal Pharmacology and Toxicology Studies - Preclinical data to permit an assessment as to whether the product is reasonably safe for initial testing in humans. Also included are any previous experience with the drug in humans (often foreign use).
•Manufacturing Information - Information pertaining to the composition, manufacturer, stability, and controls used for manufacturing the drug substance and the drug product. This information is assessed to ensure that the company can adequately produce and supply consistent batches of the drug.
•Clinical Protocols and Investigator Information - Detailed protocols for proposed clinical studies to assess whether the initial-phase trials will expose subjects to unnecessary risks. Also, information on the qualifications of clinical investigators--professionals (generally physicians) who oversee the administration of the experimental compound--to assess whether they are qualified to fulfill their clinical trial duties. Finally, commitments to obtain informed consent from the research subjects, to obtain review of the study by an independent institutional review board ("IRB"), and to adhere to the investigational new drug regulations.
Once the IND is submitted, the sponsor must wait 30 calendar days before initiating any clinical trials. During this time, the FDA has an opportunity to review the IND for safety to assure that research subjects will not be subjected to unreasonable risk. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about issues such as the conduct of the trials and or supporting pre-clinical data as outlined in the IND. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. Therefore, submission of an IND may not result in the FDA allowing clinical trials to commence.
The following regulations apply to the IND application process:
•21 CFR Part 201 Drug Labeling
•21 CFR Part 312 Investigational New Drug Application
•21 CFR Part 314 IND and NDA Applications for FDA Approval to Market a New Drug (New Drug Approval)
•21 CFR Part 316 Orphan Drugs
•21 CFR Part 50 Protection of Human Subjects
•21 CFR Part 54 Financial Disclosure by Clinical Investigators
•21 CFR Part 56 Institutional Review Boards
•21 CFR Part 58 Good Lab Practice for Nonclinical Laboratory Studies
Biological Product License Application Pathway
The BLA is a request for permission to introduce, or deliver for introduction, a biological product into interstate commerce (21 CFR Part 601.2). Form 356h specifies the requirements for a BLA. Biological products require FDA approval of a BLA to be marketed. The application must demonstrate the safety, purity, and potency of the product candidate based on results of pre-clinical studies and clinical trials. A BLA must also contain extensive Chemistry, Manufacturing and Controls ("CMC") and other manufacturing information, as well as labeling information. The applicant must pass an FDA pre-approval inspection of the manufacturing facility or facilities at which the biological product is produced to assess compliance with the FDA’s current cGMP requirements. Satisfaction of FDA approval requirements for biologics typically takes several years and the actual time
required may vary substantially based on the type, complexity, and novelty of the product. We cannot be certain that any BLA approvals for our products will be granted on a timely basis, or at all.
The steps for obtaining FDA approval of a BLA to market a biological product in the U.S. include:
•Completion of pre-clinical laboratory tests, animal studies, and formulation studies under the FDA’s good laboratory practices regulations;
•Submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin and which must include independent IRB approval at each clinical site before the trials may be initiated;
•Performance of an adequate and well-controlled clinical trial in accordance with Good Clinical Practices to establish the safety and efficacy of the product for each indication;
•Submission to the FDA of a BLA, which contains detailed information about the CMC for the product, reports of the outcomes and full data sets from the clinical trials, and proposed labeling and packaging for the product. With agreement from the FDA, sponsors can qualify to submit portions of an application as the information becomes available (“rolling submission”) as an alternative to providing all information in a single submission when it is available;
•Satisfactory review of the contents of the BLA by the FDA, including the satisfactory resolution of any questions raised during the review;
•Satisfactory completion of an FDA Advisory Committee review, if applicable;
•Satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP regulations, to assure that the facilities, methods, and controls are adequate to ensure the product’s identity, strength, quality, and purity; and
•FDA approval of the BLA, including agreement on post-marketing commitments, if applicable.
Avance Nerve Graft Regulatory Classification and Regulatory Pathway
Avance Nerve Graft has been marketed domestically and internationally since 2007. In 2010, the FDA provided us with an enforcement discretion letter, regarding the marketing of Avance so long as we complied with certain terms that focused us on taking the necessary steps to support a BLA submission for the product. The FDA enforcement discretion letter states the FDA will end the period of enforcement discretion upon a final determination of our future BLA submission or if prior to the BLA submission, the FDA finds that we do not meet the conditions for the enforcement discretion terms or are not exercising due diligence in executing the transition plan. If final action on the BLA is negative or we are found to not meet the conditions for the transition plan or its execution, or if FDA were to revoke the enforcement discretion for any other reason, we may not be able to continue to distribute Avance Nerve Graft. We continue to work diligently to execute the transition plan, including maintaining regular communication with the FDA, and, in this context, continue to distribute Avance Nerve Graft.
We met with the FDA Center for Biologics Evaluation and Research ("CBER") in July 2010 and, between July 2010 and November 2010, provided information to CBER that resulted in the FDA issuing a letter stating the agency’s intent to exercise enforcement discretion with respect to the continued introduction or delivery for introduction into interstate commerce of Avance Nerve Graft assuming that certain conditions are met relating to the transition of Avance Nerve Graft from regulation as an HCT/P under Section 361 to a biological product under Section 351 of the PHS Act. Specifically, the FDA transition plan outlined that:
•We transition to compliance with Section 501(a)(2)(B) of the FD&C Act, the current cGMP regulations in 21 CFR Parts 210 and 211 and the applicable regulations and standards in 21 CFR Parts 600-610 prior to initiation of a phase 3 clinical trial designed to demonstrate the safety, purity, and potency of Avance Nerve Graft.
◦We have performed several gap analyses of our quality system for compliance with 21 CFR Parts 210 and 211 and 600-610 regulations. The gap analyses have identified areas in which our quality system could improve with respect to compliance with the regulations. We have created and implemented appropriate changes, including new quality procedures. Through our internal auditing process, we have assessed our compliance to the regulations. We have also retained an external former FDA consultant with experience in
auditing to 21 CFR Parts 210 and 211 and 600-610 regulations to verify quality system compliance with the regulations.
•We conduct a phase 3 clinical trial to demonstrate safety, purity and potency of Avance Nerve Graft under a Special Protocol Assessment (“SPA”). We and the FDA agreed to the SPA in August 2011.
◦In accordance with FDA regulations in 21 CFR Part 312, we submitted IND #15419 to the FDA, and it became effective in March 2015.
◦The phase 3 clinical trial was initiated in the second quarter of 2015. The study completed initial enrollment in January 2019. As required by the SPA and agreed to by FDA and us, an independent statistical analysis was conducted to determine if greater study enrollment was appropriate to maintain the planned statistical power of the trial. As part of that review, the targeted enrollment was increased to 220 subjects, and the number of participating centers was increased to up to 25. The study completed subject enrollment in July 2020. Subject follow-up was completed in August 2021 with topline study data read-out completed during the second quarter of 2022. Topline results showed that this pivotal study met its primary endpoint for the return of nerve function as measured by static two-point discrimination. It also demonstrated that the safety profile was consistent with previously published data. RECON results demonstrated statistical superiority for return of sensory function, as measured by static two-point discrimination, as compared to conduits in gaps greater than 12 mm (p-value <0.05). Avance demonstrated statistical superiority for time to recover of static two-point discrimination over conduits in nerve gaps greater than 10mm (p-value <0.05).
•We continue to comply with the regulations and standards under 21 CFR Part 1271.
◦We were audited by the FDA at the Solvita facility in March 2013, March 2015, October 2016 and September 2022 and at the Avance distribution facility in October 2015 with respect to its Human Tissue Quality System under 21 CFR Part 1271 and in July 2022 with respect to Level 1 Quality System Inspection Technique Medical Device Inspection under 21 CFR Part 820. At each inspection, the quality systems were found to be in compliance with 21 CFR Part 1271, and no FDA Form 483 observations were issued.
◦In February 2018, we were audited by the FDA with respect to its Medical Device Quality System under 21 CFR Part 820 and its Human Tissue Quality System under 21 CFR Part 1271. Such audit resulted in two Form 483 observations on general procedures on our Medical Device Quality System and no Form 483 observations on our Human Tissue Quality System. We took corrective action to correct these observations, and the FDA accepted the corrective action plan.
◦In November 2018, we were inspected by the FDA at our Texas distribution facility with respect to our Human Tissue Quality System under 21 CFR Part 1271. Such inspection resulted in one Form 483 observation on tissue tracking. We took corrective action to correct this observation, and the FDA accepted the corrective action plan.
◦In July 2022, we were inspected by the FDA at our Texas distribution facility with respect to our Medical Device Quality System under 21 CFR Part 820. No FDA Form 483 observations were received.
◦In September 2022, we were inspected by the FDA at the Solvita facility with respect to our Human Tissue Quality System under 21 CFR Part 1271. No FDA Form 483 observations were received.
◦In August 2023, we were inspected by the FDA at our Alachua, Florida Human Tissue Establishment with respect to our Human Tissue Quality System under 21 CFR Part 1271. No FDA Form 483 observations were received.
◦In February 2024, we were inspected by the FDA at our Tampa facility with respect to our medical device specification development under 21 CFR Part 820. No FDA Form 483 observations were received.
◦In September 2024, we were inspected by the FDA at our Vandalia, Ohio APC Facility with respect to our Human Tissue Quality System under 21 CFR Part 1271. No FDA Form 483 observations were received.
In September 2018, the FDA granted a Regenerative Medicine Advanced Therapy ("RMAT") designation for Avance Nerve Graft. A regenerative medicine therapy is eligible for the RMAT designation if it is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the product has the potential to address unmet medical needs for such a disease or condition. The RMAT designation provides access to a
streamlined approval process for regenerative medicine technologies and informal meetings with the FDA in support of the BLA for Avance Nerve Graft, as appropriate. FDA can withdraw the RMAT designation if the designation criteria are no longer met. Notwithstanding the RMAT designation, on November 1, 2024, the FDA notified us it assigned our application a standard review classification.
Section 505(d) of the U.S. Federal Food, Drug and Cosmetic Act requires the FDA to find "substantial evidence" of a new biologic product effectiveness before it can be marketed. The FDA normally requires two adequately designed and well-controlled clinical investigations for the new biologic product. However, through a series of FDA Guidance for Industry documents including the July 1998 guidance "Guideline for the Format and Content of the Clinical and Statistical Sections of an Application" and, more recently, the September 2023 guidance "Demonstrating Substantial Evidence of Effectiveness with One Adequate and Well-Controlled Clinical Investigation and Confirmatory Evidence", the FDA has accepted a single adequate and well-controlled clinical investigation with supporting evidence through preclinical studies and literature to approve new biologic products. For Avance Nerve Graft, in the 2010 Enforcement Discretion letter, the FDA only required Axogen to perform a single phase 3 clinical study. It is Axogen's belief from the guidance documents and communications with the FDA, the phase 3 RECON clinical study as a single adequate and well-controlled clinical investigation with preclinical data, and literature and confirmatory data from the RANGER Registry will support Avance Nerve Graft's approval as a biologic product.
In 2024, we continued to maintain a collaborative dialogue with the FDA and engaged in a pre-BLA meeting in the first quarter. In the third quarter of 2024, we completed our rolling BLA submission, and on November 1, 2024, the FDA notified us that our application was sufficiently complete to permit substantive review and assigned a review goal date of September 5, 2025. The FDA also notified us that, as of November 1, 2024, it was not planning to hold an advisory committee meeting to discuss our application. The FDA will review our regulatory compliance with GMP regulations during the pre-license inspection and Good Clinical Practices as part of the BLA review. If the FDA does not find us to be in compliance, the BLA might not be approved or approval could be delayed. If the FDA does not find the evidence submitted in the BLA supportive, the BLA might not be approved or approval could be delayed, or the FDA may approve a narrower indication than the present use of Avance Nerve Graft, may impose other limitations, or may require post-approval commitments or studies .
We believe that biologic licensing, which typically entails multiple clinical trials and takes many years, would be required for any future competitive peripheral nerve allograft. The FDA provided updated guidance, "Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use" in November 2017, which it revised in July 2020. The guidance clarified the FDA's position that any processing that alters the biological characteristics of peripheral nerve tissue would be considered more than minimal manipulation, and therefore require a BLA prior to marketing.
Clinical Trials
Clinical trials are a category of clinical research designed to evaluate and test new interventions, medications, or procedures. Clinical trials are often conducted in four phases. The trials at each phase have a different purpose and help answer different questions.
•Phase I trials test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.
•In Phase II trials, the experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
•In Phase III trials, the experimental study drug or treatment is given to large groups of people. Researchers aim to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug or treatment to be used safely.
•Phase IV trials, also known as post-marketing studies, are conducted after a treatment is approved for use by the FDA and provide additional information including the treatment or drug’s risks, benefits, and best use.
Clinical trials are required to support a BLA or PMA and are sometimes required for 510(k) clearance or de novo classification. Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators. Clinical trials are conducted under strict requirements to ensure the protection of human subjects participating in the trial and under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring and safety, and the effectiveness criteria to be evaluated. Clinical trials for biological products require the submission and FDA acceptance of an IND and clinical trials for medical devices require the submission and FDA approval of an Investigational Device Exemption ("IDE") application unless the device regulations provide for an exemption from the IDE
requirement. Clinical trials for significant risk devices may not begin until the IDE is approved by the FDA and the IRB overseeing the particular clinical trial. If the product is considered a non-significant risk device under FDA regulations, the trial must only be approved by an IRB prior to its initiation. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND or IDE, for significant risk devices. In addition, for these studies, an IRB at each site at which the study is conducted must approve the protocol, subject consent form and any amendments for each site at which the study is conducted. All research subjects must be informed, among other things, about the risks and benefits of the investigational product and provide their informed consent in writing.
Clinical trials under an IND typically are conducted in three sequential phases, but the phases may overlap or be combined. In our case, we believe that the phase 3 clinical trial study for Avance Nerve Graft represents the only prospective clinical data that will be required to evaluate safety and effectiveness. Phase 3 clinical trials usually further evaluate clinical efficacy and test further for safety in an expanded patient population. Phase 3 clinical trials usually involve comparison with placebo, standard treatments, or other comparators. Usually, multiple well-controlled large phase 3 or pivotal clinical trials demonstrating safety and efficacy are required to support a BLA. These trials are intended to establish the overall risk-benefit profile of the product and provide an adequate basis for physician labeling. Clinical testing may not be completed successfully within any specified period, if at all. Furthermore, we or the FDA may suspend or terminate a clinical trial at any time on various grounds, including a finding that the subjects are exposed to an unacceptable health risk, have experienced a serious and unexpected adverse event, or that continued use in an investigational setting may be unethical. Similarly, an IRB can suspend or terminate approval of research, for example, if the research is not being conducted in accordance with the IRB’s requirements or if the research has been associated with unexpected serious harm to patients. Additionally clinical data obtained from the observational study, RANGER, will be provided as supportive safety data and confirmatory data for Avance Nerve Graft.
Our Clinical Trials
We have an active clinical research program to gather data on our product portfolio. We have completed five clinical studies, are performing four ongoing clinical studies, and have plans to initiate further clinical studies. The ongoing studies are:
•“A Multicenter Retrospective Study of Avance Nerve Graft Utilization, Evaluations, and Outcomes in Peripheral Nerve Injury Repair" (“RANGER”) parent protocol and its Addendum 1 arm, "A Matched Autograft and Tube Conduit Case Control Cohort Arm of RANGER" ("MATCH"). Enrollment, follow-up, and analysis has been completed with reporting and close-out activities underway;
•"Breast Neurotization Outcomes for Women: A Registry Study of Recovery Outcomes, Quality of Life and Patient Satisfaction in Post-Mastectomy Autologous Breast Reconstruction" ("Sensation-NOW"). Enrollment is ongoing;
•"Nerve Protection Evaluation: Revision Cubital Tunnel Syndrome Decompression" ("COVERED"). Enrollment is ongoing; and
•"Tolerability and Feasibility Pilot Clinical Study of a Large-Diameter Nerve Cap for Protecting and Preserving Terminated Nerve Ends" ("REPOSE-XL"). Enrollment is ongoing.
Our completed studies are "A Multicenter, Prospective and Subject Blinded Comparative Study of Axoguard Nerve Cap and Neurectomy for the Treatment of Symptomatic Neuroma and Prevention of Recurrent End-Neuroma Pain" ("REPOSE")", "A Multicenter, Prospective, Randomized, Patient and Evaluator Blinded Comparative Study of Nerve Cuffs and Avance Nerve Graft Evaluating Recovery Outcomes for the Repair of Nerve Discontinuities" (“RECON”)", “A Multicenter, Prospective, Randomized, Comparative Study of Hollow Nerve Conduit and Avance Nerve Graft Evaluation Recovery Outcomes of the Nerve Repair in the Hand" (“CHANGE”) published by Means et al, a pilot study to evaluate the use of Avance Nerve Graft in the reconstruction of nerves following prostatectomy, and "Registry of Avive Soft Tissue Membrane Utilization in Selected Applications of Acute Trauma of the Upper Extremity" ("ASSIST"). As Avive Soft Tissue Membrane is no longer on the market, the registry closed with no planned analysis.
In addition to these clinical research programs, we are developing additional clinical trials in peripheral nerve repair, including nipple areolar complex neurotization, urology, protection, and pain.
Clinical trials are subject to extensive recordkeeping and reporting requirements. Our clinical trials must be conducted under the oversight of an IRB for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to, those relating to Good Clinical Practices. We are also required to obtain the patients’ written, informed consent in a form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not
adequately demonstrate the safety and efficacy of the biological product or device, or may otherwise not be sufficient to obtain FDA approval to market the product in the U.S. Similarly, in the E.U., the clinical study for a medicine product must be authorized by the Competent Authority in each Member State where the clinical trial is to be conducted and must receive a favorable opinion from an ethics committee. See "Risk Factors - Clinical trials can be long, expensive and results are ultimately uncertain, which could jeopardize our ability to obtain regulatory approval and continue to market our Avance Nerve Graft product."
RANGER
The RANGER study is an observational study and is a utilization registry of Avance Nerve Graft. As of December 31, 2023, eleven publications and more than 70 scientific conference presentations have been generated to date from the study. RANGER is designed to allow up to 2,500 subjects. An additional 500 subjects are allowed to be enrolled in Addendum 1, MATCH, and 2,000 enrolled in Addendum 2, Sensation-NOW. Sensation-NOW is a clinical study cohort, currently enrolling, designed to assess breast sensation following reconstruction with or without neurotization. We resumed enrollment in 2021 at select centers after pausing enrollment due to COVID-19 in 2020. The follow-up for the RANGER study is standard of care with a target of up to 36 months post peripheral nerve repair.
The RANGER study database is also utilized to monitor different nerve repair techniques. As part of this, we utilize the database to support additional regulatory submissions for the Axoguard products.
We have worked with leading institutions, researchers, and surgeons to support innovation in the field of surgical peripheral nerve repair. We believe that RANGER is currently the largest multi-center observational clinical study conducted in peripheral nerve gap repair. Various reviewers of the RANGER study have found Avance Nerve Graft nerve repairs resulted in meaningful motor and sensory recovery and reduced pain following neuroma excision and reconstruction with no safety concerns identified.
RECON
The RECON study is a prospective, randomized, controlled, patient and evaluator blinded, comparative study of Avance Nerve Graft and Collagen Nerve Cuffs (manufactured conduits) in the repair of peripheral nerve transections in digital nerves with gaps of 5 to 25mm. The study is designed to assess the outcomes of peripheral nerve repair in approximately 170 subjects in up to 20 centers. Subjects were intraoperatively randomized in a 1:1 ratio after stratification by length of the nerve injury by gap length into short gap (5-14mm) and long gap (15-25mm) categories. The primary objective of the study is to evaluate the safety and efficacy of Avance Nerve Graft for non-inferiority and if met, superiority, of static two-point discrimination, a measure of sensory function, at twelve months as compared to nerve cuffs. Given the pooled standard deviation assumptions and a non-inferiority margin of 2mm, approximately 88 patients per treatment group are required to assess non-inferiority with at least 83% power. In addition to non-inferiority, a minimum treatment effect is required to be demonstrated. Based on an agreement with the FDA in the original protocol and an independent statistical analysis of the pooled standard deviation, the number of subjects was increased to 220 in up to 25 centers. Subjects were followed over the course of 12 months (based on the agreed-upon protocol, subjects have up to an additional three months to complete trial requirements) to assess safety and efficacy outcomes with assessments performed at various defined intervals up to 12 months. The study completed subject enrollment in July 2020. Subject follow-up was completed in August 2021 with topline study data read-out completed during the second quarter of 2022. Topline results showed that this pivotal study met its primary endpoint for the return of nerve function as measured by static two-point discrimination. It also demonstrated that the safety profile was consistent with previously published data. RECON results demonstrated statistical superiority for return of sensory function, as measured by static two-point discrimination, as compared to conduits in gaps greater than 12 mm (p-value <0.05). Avance demonstrated statistical superiority for time to recover of static two-point discrimination over conduits in nerve gaps greater than 10mm (p-value <0.05). The data in this study supported our BLA submission which was completed in September 2024 and was accepted by the FDA for substantive review on November 1, 2024.
REPOSE
We are conducting a multicenter, prospective, randomized, and subject blinded study of Axoguard Nerve Cap as compared to neurectomy for the treatment of systematic neuroma. REPOSE is a two-phase study comparing standard neurectomy to Axoguard Nerve Cap, which leverages our chambered technology to aid in the management of symptomatic neuromas. The first phase, a non-randomized pilot, has completed enrollment and one-year follow-up. The second phase, a prospective, randomized controlled study, completed enrollment in 2022. Overall enrollment is designed to target 101 subjects with 15 in the first pilot phase followed by up to 86 in the randomized, comparative phase. The study assessed pain scores, quality of life, neuroma recurrence, and health outcomes over a 12-month follow-up period. Subject follow-up was completed in the third quarter of 2023 with topline analysis reported in January 2024.
REPOSE XL
REPOSE-XL is a prospective, multi-center clinical pilot study evaluating the tolerability and feasibility of the Axoguard Large-Diameter Nerve Cap (sizes 5-7 mm) for protecting and preserving terminated nerve endings after limb trauma or amputation when immediate attention to the nerve injuries is not possible. Enrollment in REPOSE-XL started in 2022 and is underway.
COVERED
COVERED is a prospective, multi-center clinical case series evaluating HA+ in first revision cubital tunnel decompression. Enrollment in COVERED started in the fourth quarter of 2023.
Post-Market Regulatory Requirements
There are numerous regulatory requirements that apply after a product is cleared or approved. For medical devices, these include, but are not limited to the FDA’s regulations for device labeling (21 CFR Part 801), medical device reporting (21 CFR Part 803), reporting of corrections and removals (21 CFR Part 806), establishment of registration and device listing requirements (21 CFR Part 807), and compliance with the QSR per 21 CFR Part 820. Distribution of medical devices is also subject to license/registration requirements in some states. For tissue and biological products, the regulatory requirements include: the FDA’s registration and listing requirements, donor eligibility requirements and compliance with GTP in 21 CFR Part 1271 for human tissue products, compliance with the FDA’s cGMP in 21 CFR Parts 210, 211, and 600 for licensed biological products, and post-market BLA requirements (21 CFR Part 601), including The Drug Supply Chain Security Act. Among other things, these regulations require manufacturers, including third party manufacturers to:
•Follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the manufacturing process;
•Comply with labeling regulations and FDA prohibitions against the false or misleading promotion or the promotion of products for uncleared, unapproved or off-label uses, or indications;
•Comply with requirements to obtain clearance or approval for certain changes affecting the product, including changes to the product’s manufacturing, labeling, or intended use;
•Report to the FDA certain adverse events, adverse reactions, and deviations;
•Comply with post-approval restrictions or conditions, including post-approval study commitments and post-market safety and annual reporting requirements;
•Follow post-market surveillance regulations that may apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; and
•Follow requirements to issue notices of correction or removal, or conduct market withdrawals, or recalls where quality or other issues arise.
Safety Reporting and other Periodic Reporting
The Medical Device Reporting regulation 21 CFR Part 803 contains mandatory requirements for manufacturers, importers, and device user facilities to report certain device-related adverse events and product problems to the FDA.
In addition to the FDA, the advertising and promotion of medical products are also regulated by the Federal Trade Commission and in some instances by state regulatory and enforcement authorities. Recently, some promotional activities for FDA-regulated products have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the Federal Lanham Act and similar state laws, competitors, and others can initiate litigation relating to advertising claims.
Facilities Listing and Registrations
All of our facilities are properly registered with the FDA as tissue or medical device establishments. The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine compliance with the GTP, cGMP, and other regulations, and these inspections may also include suppliers' manufacturing facilities.
Failure by us or our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other federal or state authorities, which may include any of the following sanctions, among others:
•Warning letters, fines, injunctions, consent decrees and civil penalties;
•Customer notifications, repair, replacement, refunds, recall or seizure of our products;
•Operating restrictions, partial suspension, or total shutdown of production;
•Suspension or termination of our clinical trials;
•Refusing our 510(k), de novo classification request, PMA or BLA for new products, new intended uses, or modifications to existing products;
•Withdrawing or suspending pre-market approvals that have already been granted; and
•Criminal prosecution.
Education Grants, U.S. Anti-kickback, False Claims and Other Healthcare Fraud and Abuse Laws
Educational Grants
A medical product manufacturer may provide financial or in-kind support, including support by way of grants, to third parties for the purpose of conducting medical educational activities. If these supported activities are considered by the FDA to be independent of the manufacturer, then the activities fall outside the FDA restrictions on promotion to which the manufacturer is subject.
We seek to ensure that the educational activities we support through our grants program are in accordance with the appropriate criteria for independent educational activities. However, we cannot provide assurance that the FDA or other government authorities would view the programs supported as being independent.
Fraud, Abuse and False Claims
We are directly and indirectly subject to various federal and state laws governing relationships with healthcare providers and pertaining to healthcare fraud and abuse, including anti-kickback laws. In particular, the U.S. Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. Penalties for violations could include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid, and other federal healthcare programs. In implementing the statute, the Office of Inspector General of the U.S. Department of Health and Human Services (“OIG”) has issued a series of regulations, known as “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute for activities that fit within a safe harbor. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable element of a safe harbor may result in increased scrutiny by government enforcement authorities, such as the OIG, and may be “at risk” activities unless a favorable advisory opinion is obtained from the OIG.
The federal False Claims Act (“FCA”) imposes civil liability on any person or entity that submits, or causes the submission of, a false or fraudulent claim to the U.S. government. Damages under the FCA can be significant and consist of the imposition of fines and penalties. The FCA also allows a private individual or entity with knowledge of past or present fraud against the federal government to sue on behalf of the government to recover the civil penalties and treble damages. The U.S. Department of Justice ("DOJ") has previously alleged that the marketing and promotional practices of pharmaceutical and medical device manufacturers including the off-label promotion of products or the payment of prohibited kickbacks to doctors violated the FCA resulting in the submission of improper claims to federal and state healthcare entitlement programs such as Medicaid.
AdvaMed is one of the primary voluntary U.S. trade associations for medical device manufacturers. PhRMA is another trade association focused on the pharmaceutical industry. These associations have established guidelines and protocols for medical device and pharmaceutical manufacturers, respectively, in their relationships with healthcare professionals on matters, including research and development, product training and education, grants and charitable contributions, support of third-party educational conferences, and consulting arrangements. Adoption of the AdvaMed or PhRMA Codes by a medical device
manufacturer is voluntary, and while the OIG and other federal and state healthcare regulatory agencies encourage its adoption, they do not view adoption of these codes as proof of compliance with applicable laws. Key to the underlying principles of the AdvaMed and PhRMA Codes is the need to focus the relationships between manufacturers and healthcare professionals on matters of training, education and scientific research, and limit payments between manufacturers and healthcare professionals to fair market value for legitimate services provided and payment of modest meal, travel, and other expenses for a healthcare professional under limited circumstances. We have incorporated these principles into our relationships with healthcare professionals under our consulting agreements, payment of travel and lodging expenses, research and educational grant procedures and sponsorship of third-party conferences. In addition, we have conducted and will continue to conduct training sessions on these principles. Finally, the Sunshine Act, as defined below, imposes additional reporting and disclosure requirements on us for any “transfer of value” made or distributed to physicians and teaching hospitals, as well as reporting of certain physician ownership interests. We cannot provide any assurance that regulatory, or enforcement authorities will view our relationships with physicians or policies as being in compliance with applicable regulations and laws.
Regulation Outside of the U.S.
Distribution and sales of medical products outside of the U.S. are subject to foreign governmental regulations that vary substantially from country to country.
There are restrictions under U.S. law on the export of medical devices and biological products that cannot be legally distributed in the U.S. The FDA has set forth certain requirements for the export of devices outside of the U.S. depending on the class of device and its FDA approval. We currently believe we comply with applicable regulations when exporting our products and we intend to continue such compliance in the event there are any regulatory changes regarding its products in the U.S.
The European Medicines Agency ("EMA" is the decentralized body of the E.U., located in Amsterdam in the Netherlands. It is responsible for the scientific evaluation, supervision, and safety monitoring of medicines for human and veterinary use in the E.U. The EMA serves the E.U. and three countries from the European Economic Area—Iceland, Norway, and Liechtenstein. The E.U. has adopted numerous directives, regulations, and promulgated voluntary standards regulating the design, manufacture and labeling of, and clinical trials and adverse event reporting for medicinal products including medical devices. Devices that comply with the requirements of a relevant regulation or directive will be entitled to bear CE marking, indicating that the device conforms to the essential requirements of the applicable regulation and directives and can be commercially distributed throughout the member states of the E.U. and other countries that comply. The method for assessing conformity varies depending on the type and class of the device, but normally involves an assessment by the manufacturer and a third-party assessment by a notified body, an independent and neutral institution appointed by a country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. Such an assessment is required for a manufacturer to commercially distribute the product throughout these countries. In the second quarter of 2014, Axogen’s Quality System became accredited to International Organization for Standardization ("ISO") 13485 for Receipt, Handling, Storage and Distribution of Axoguard Nerve Connector and Axoguard Nerve Protector. We intend to maintain this accreditation on an ongoing basis.
Evergen is responsible for all regulatory filings for the Axoguard Nerve Connector and Axoguard Nerve Protector products, including international registrations. We provide the countries for Evergen to register with, and Evergen prepares and submits the product filing documentation to the Ministry of Health (“MOH”) for the country. Each country or region has its own regulations, and the documentation required for submission varies. It typically takes less than nine months from the initiation of the project to obtain clearance in a given country or region. To date, the Axoguard Nerve Connector and Axoguard Nerve Protector product lines were registered in May 2013 in Canada for distribution and in April 2013 the product lines were awarded the CE Mark allowing distribution into the E.U. and other countries that accept the CE Mark. Evergen received the renewal of the CE Mark for Axoguard Nerve Connector and Axoguard Nerve Protector in May 2021.
In addition, the new European Medical Device Regulation 2017/745 (“E.U. MDR”) passed in the European Parliament on April 5, 2017, and went into effect on May 25, 2017. The E.U. MDR is an extensive reform of the rules governing the medical device industry in Europe. Under this regulation, manufacturers had through May 2021 to comply with a broad set of new rules for almost every kind of medical device. The E.U. MDR requires changes in the clinical evidence required for medical devices, post-market clinical follow-up evidence, annual reporting of safety information for Class IIb and Class III products, and bi-annual reporting for Class IIa products, Unique Device Identification (“UDI”) for all products, submission of core data elements to a European UDI database prior to placement of a device on the market, reclassification of medical devices, and multiple other labeling changes.
While eight years have passed since the adoption of E.U. MDR, the E.U. MDR's transitional provisions were amended in 2023 to give manufacturers and notified bodies more time to conduct the necessary conformity assessment procedures and to avert shortages of devices needed for the E.U. healthcare systems. European Regulation 2023/607 extended the transitional
period according to the risk class of the legacy device until December 2027 or December 2028 if certain requirements are fulfilled (i.e., agreement with a notified body for conformity assessment in place, or a competent authority has granted a derogation from the conformity assessment procedure). European Regulation 2022/112 extended the transitional period for in vitro medical devices according to the risk class of the legacy device until May 2025 for devices already certified by a notified body under the Directive and class D devices, until May 2026 for class C devices and until May 2027 for class B and A sterile devices. Overall, medical device companies can continue to expect longer lead times to obtain product conformity assessments and registrations (i.e., CE Mark Certification) in the E.U. and a substantially costlier pathway to compliance in the E.U.
Evergen is responsible for registering and attaining MDR conformity for Axoguard Nerve Connector and Axoguard Nerve Protector in the E.U. As distributor of these two products in the E.U., we are not yet able to fully determine the costs of complying with these regulations, how the E.U. will continue interpreting and enforcing them, what the timelines for approvals of products will be and the overall effect of the E.U. MDR on the marketplace. Given the significant additional pre-market and post-market requirements imposed by the E.U. MDR, the overall impact of these new rules could have a material, adverse effect on our international revenue and expenses.
The UK left the E.U. in January 2020. We register our human tissue products in each individual E.U. country and our distributor in the UK has import authority for our human tissue product. It is expected that licensed UK establishments that import or export tissues or cells will need written agreements with the relevant E.U. licensed establishments to continue importing and exporting with the E.U. As we ship directly to the UK from the U.S., we did not experience and do not expect delays in shipment of human tissue products into the UK. Further, the RANGER clinical trial being performed at select hospitals in the UK was not affected by Brexit (defined below in "Risk Factors - Regulation Outside of the U.S.") as long as the products continue to come directly from the U.S. Beginning in January 2021, new changes became effective as the transition period for the UK’s exit from the E.U. ended. Specifically, all medical devices placed into the UK market had to be registered, subject to applicable grace periods, with the Medicines and Healthcare products Regulatory Agency ("MHRA"), will need to appoint a UK Responsible Person, and comply with additional product marking and conformity assessment requirements. Medical devices must be registered with the MHRA if they are being placed in the UK market after May 1, 2021. Evergen is responsible for appointing the UK Responsible Person and registering Axoguard Nerve Connector and Axoguard Nerve Protector in the UK.
Tissue products are not currently regulated under the CE Mark
We are responsible for all regulatory filings for Avance Nerve Graft. To obtain international approvals, we prepare the product filing documentation and submit this documentation to the MOH for a country.
Although some standards of harmonization exist, each country in which we conduct business has its own specific regulatory requirements, which are dynamic in nature and continually changing. We procure and process our tissue for the Avance Nerve Graft in the U.S. and market the Avance Nerve Graft in Canada, the UK, and certain other countries under compliance with the individual country regulations. We conduct a regulatory review at the time of submission of the product dossier. This involves reviewing the appropriate MOH regulations, discussion with in-country distributors and use of consultants. It typically takes less than nine months from the initiation of the product to develop a product dossier (specific for that country), submission of the documentation and MOH review of the product filing. While we believe that we are in compliance with all existing pertinent international and domestic laws and regulations, there can be no assurance that changes in governmental administrations and regulations will not negatively impact our operations. The FDA and international regulatory bodies conduct periodic compliance inspections of our U.S. processing facilities. Axogen's processing and distribution locations are properly registered with CBER as tissue establishments. In 2023, AATB re-accredited Axogen for compliance to the AATB standards for tissue banking for all our facilities. Additionally, our facilities are appropriately licensed in the states of Florida, New York, California, Maryland, Delaware, Oregon, and Illinois as tissue establishments. We believe that worldwide regulation of tissue products is likely to intensify as the international regulatory community focuses on the growing demand for these implant products and the attendant safety and efficacy issues of recipients. Changes in governing laws and regulations could have a material adverse effect on our financial condition and results of operations. Our management further believes that it can help to mitigate this exposure by continuing to work closely with government and industry regulators.
Environmental
As a biotech company of our size, we believe our impact on the environment is modest. However, we are continuously evaluating how we can be the best possible stewards of the environment, and follow local, state, and federal environmental regulations. We are taking steps in our operations and facilities to positively impact the environment wherever possible.
Our products, as well as the chemicals used in processing these products, are handled and disposed of in accordance with country-specific, federal, state, and local environmental regulations. Since 2007, we have used outside third parties to perform all biohazard waste disposal.
We contract with independent, third parties to perform sterilization of our allografts. Because of the engagement of a third party to perform irradiation services, the requirements for compliance with radiation hazardous waste do not apply, and therefore we do not anticipate that this engagement will have any material adverse effect upon our capital expenditures, results of operations or financial condition. However, we are responsible for assuring that the service is performed in accordance with applicable regulations. Although we believe we are in compliance with all applicable environmental regulations, the failure to fully comply with any such regulations could result in the imposition of penalties, fines or sanctions that could have a material adverse effect on our business.
Human Capital
As of December 31, 2024, we had 452 total employees, including approximately one part-time employee and 451 full-time employees. Of these employees, 158 work in sales and marketing, 122 work in corporate, 35 work in research and development and 137 work in operations. Approximately 46% of our employees were female and 54% were male. As of the date of this Annual Report on Form 10-K we have not had a work stoppage, and no employees are represented by a labor union.
We believe in creating and maintaining a culture that encourages and rewards honesty, openness, and passionate debate among our employees, respect is the foundation for communication and action, and patient safety is our first priority. We are committed to fostering an inclusive culture. Our corporate values support honest and open communication, mutual support, collaboration, passionate debate, empowerment, and respect. Our Equal Employment Policy includes specific training on preventing discrimination and harassment. We are committed to advertising our opportunities on each state's job boards in order to reach an increasingly diverse population of candidates, and we conduct routine audits of our existing job postings, advertisements and candidate communications for gender coding, and update any gender specific language to gender neutral language. Additionally, we have a policy that supports employees who are veterans that participate in Honors Guards, who are selected from partnerships with veteran organizations and participating companies and attend by invitation, and military funerals, the Honor Guards. Further, some of our recruitment efforts are to engage with the next generation of scientists and engineers through targeted awareness and internship programs. We work with Women in Life Sciences, Society for Asian Scientists and Engineers, Society of Women Engineers, and BioFlorida to educate students and professionals about career opportunities available at our Company.
We strive to offer benefits plans that are viewed as attractive and beneficial to employees. In 2024 we maintained our offerings of diverse medical coverage that includes a consumer driven health plan ("CDHP") with a health savings account and the option of a limited flexible spending account for qualifying dental and vision expenses for those employees who select the CDHP. We continue to offer two options for dental (high or low preferred provider organization) plans and options for employees to select from two different vision plan providers, enabling employees to select the option that best fits their needs and includes their preferred physicians. The short-term disability offering pays 100% of the employee’s bi-weekly earnings for the first eight weeks of disability and 66.67% for the remaining disability period, up to twelve weeks. Axogen also offers supplemental benefits in the form of accidental insurance and critical illness insurance designed to help provide financial protection against expenses associated with accidents or illness not covered by medical insurance.
Employee safety is critical to our operations, and we follow Occupational Safety and Health Administration 29 CFR 1910, and use a series of company-wide policies, trainings, and procedures to protect all employees’ health and safety. We utilize an Environmental Health and Safety committee that meets monthly to analyze potential issues, review any incident data, and implement necessary process or procedural changes that can minimize the work-related injuries and occupational exposure to chemicals, biohazards, or illnesses, and eliminate any potential from serious injuries and fatalities.
The Compensation Committee of our Board of Directors has oversight over human capital management.
Available Information
Our website address is http://www.axogeninc.com. We have included our website address as an inactive textual reference only. We make available, free of charge through our website, our annual reports on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file, or furnish such material to the SEC. We also similarly make available, free of charge on our website, the reports filed with the SEC by our executive officers, directors and 10% shareholders pursuant to Section 16 under the Exchange Act as soon as reasonably practicable after copies of those filings
are provided to us by those persons. Reference to our website, or any other website, does not constitute incorporation by reference of the information contained on the site and should not be considered part of this Annual Report on Form 10-K.
Item 1A. RISK FACTOR SUMMARY
Below is a summary of our risk factors. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” and should be carefully considered, together with other information in this Form 10-K and our other filings with the SEC before making an investment decision regarding our common stock.
Risks Related to Our Business and Strategy
•Approximately 60% of our total revenues are from sales of Avance Nerve Graft and any adverse decision from the FDA would negatively impact our operations and financial condition.
•Our revenue growth depends on our ability to increase distribution and sales to existing customers and develop new customers, domestically and abroad, and there can be no assurance that these efforts will result in significant increases in sales.
•Our revenue depends on a limited number of products.
•We are highly dependent on the continued availability of our facilities and could be harmed if we continue to experience operating challenges with our APC Facility or if any of our facilities are unavailable for any prolonged period of time.
•There may be significant fluctuations in our operating results.
•Macroeconomic trends, such as the inflationary pressure, and political instability could continue to have a material adverse effect on our ability to operate, results of operations, financial condition, liquidity, and capital investments.
•Our operating results could be adversely impacted if we are unable to effectively manage and sustain our future growth or scale our operations.
•We have a history of net losses and have not consistently experienced positive cash flow from operations, and our ability to achieve consistent positive cash flow will depend on increasing revenue from distribution of our products, which may not be achievable.
•Failure to successfully manage the transition associated with the recent management changes could have an adverse impact on our business.
•Loss of key members of management, who we need to succeed, could adversely affect our business.
•Our success will be dependent on continued acceptance of our products by the medical community.
•Delays, interruptions, or the cessation of production by our third-party suppliers, including products supplied by single suppliers, of important materials may prevent or delay our ability to manufacture or process the final products.
•Technological change and competition for newly developed products could reduce demand for our products.
•We may not be successful in our efforts to build a pipeline of additional product candidates.
•We must maintain high quality processing of our products.
•Our revenue depends upon prompt and adequate reimbursement from public and private insurers and national health systems.
•Negative publicity concerning methods of donating human tissue and screening of donated tissue may reduce demand for our products and negatively impact the supply of available donor tissue.
•The failure of third parties to perform many necessary services for the commercialization of our products, including services related to recovery/acquisition, sterilization, distribution, and transportation, would impair our ability to meet commercial demand.
•We are dependent on our relationships with independent agencies to generate a material portion of our revenue.
•If we do not manage product inventory in an effective and efficient manner, it could adversely affect profitability.
•We may be unsuccessful in commercializing our products outside the U.S.
•We may seek to expand our business in ways that could result in diversion of resources and extra expenses.
•We may be subject to future product liability litigation, which could be expensive, and our insurance coverage may not be adequate.
•We are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by geopolitical instability and tensions, Russia's ongoing invasion of Ukraine and illegal annexation of Ukrainian territories, and record inflation and could materially and adversely affect our business, financial condition and results of operations.
•Changes in U.S. trade policy, threats of international tariffs, and changes to the U.S. political landscape may adversely affect our business, results of operations, financial condition, and prospects.
•Our results of operations could be negatively affected by potential fluctuations in foreign currency exchange rates.
•Our failure to protect our technology systems and comply with data protection laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our business, results of operations, financial condition, and prospects.
•We are dependent on internal information and telecommunications systems, and any failure of these systems, including system security breaches, data protection breaches or other cybersecurity attacks, may negatively impact our business and results of operations.
•Our business and financial performance could be adversely affected, directly or indirectly, by natural or man-made disasters or other similar events.
•Changes in the tax code could have a material adverse effect on our results of operations, financial condition, liquidity, and capital investments.
•We may have exposure to additional tax liabilities as a result of our foreign operations.
•Our business and stock price may be adversely affected if our internal controls are not effective.
•We incur costs as a result of operating as a public company, and our management is required to devote substantial time to compliance initiatives.
•Our business, results of operations, financial condition, and prospects could be adversely affected, directly or indirectly, by the effects of a focus on environmental, social and governance issues.
Risks Related to the Regulatory Environment in which We Operate
•Our Avance Nerve Graft product is currently distributed pursuant to enforcement discretion and a transition plan with the FDA; however, we completed the rolling BLA submission for Avance Nerve Graft in September of 2024. If the FDA does not approve our BLA, approves a narrower indication than Avance Nerve Graft’s current use or otherwise limits use of our Avance Nerve Graft product, it would have a significant impact on our revenues and thus would have a material adverse effect on us.
•Even if we obtain regulatory approval for Avance Nerve Graft, we will remain subject to ongoing and new regulatory requirements. Maintaining compliance with ongoing regulatory requirements may result in significant additional expense to us, and any failure to maintain such compliance could subject us to penalties and cause our business to suffer.
•Our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly and could result in negative effects on our business.
•Failure to obtain regulatory and pricing approvals in foreign jurisdictions after BLA approval for Avance Nerve Graft or our other products could delay or prevent commercialization of our products abroad.
•The use, misuse or off-label use of our products may harm our reputation and the reputation of our products, which could result in injuries leading to product liability suits, and could be costly to our business, and/or result in FDA sanctions.
•U.S. governmental regulation could restrict the use of our Avance Nerve Graft product, restrict our procurement of tissue or increase costs.
•Failure to obtain regulatory or other approvals from certain states in which we operate after BLA approval for Avance Nerve Graft could delay, hinder, or prevent commercialization of our products.
•BLA approval for Avance Nerve Graft could result in different protocol to hospitals access for the product, as well as different reimbursement protocols, both of which may negatively affect surgeons’ access to, revenues derived from and profitability of Avance Nerve Graft.
•Our Axoguard products are subject to FDA and international regulatory requirements.
•Our operations must comply with FDA and other governmental requirements.
•Our business is subject to continuing compliance with standards set by various accreditation and registration bodies, which is costly, and loss of accreditation or registration could result in negative effects on our business.
•Defective products could lead to recall or other negative business conditions.
•Clinical trials can be long and expensive, and results are ultimately uncertain.
•We rely on third parties to conduct our clinical trials, and they may not perform as contractually required or expected.
•Healthcare law and policy changes may have a material adverse effect on us.
•We could be subject to civil or criminal penalties if we are found to have violated laws protecting the confidentiality of health information, which could increase our liabilities and harm our reputation or our business.
Risks Related to Our Intellectual Property
•Failure to protect or maintain our IP rights could result in costly and time-consuming litigation and our loss of any potential competitive advantage.
•Future protection for our proprietary rights is uncertain and may impact our ability to successfully compete in our industry.
•The patent protection for our products may expire before we are able to maximize their commercial value, which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue.
•Others may claim an ownership interest in our IP or claim that we infringe on their IP rights, which could expose us to litigation and have a significant adverse effect on our prospects.
•Our trademarks are valuable, and our business may be adversely affected if trademarks are not adequately protected.
•Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Risks Related to Financing Our Business
•Our credit facility and payment obligations under the Revenue Participation Agreement with TPC Investments II LP and Argo SA LLC, each affiliates of Oberland Capital (collectively, “Oberland Capital”), contains operating and financial covenants that restrict our business and financing activities, require cash payments over an extended period of time and are subject to acceleration in specified circumstances, which may result in Oberland Capital taking possession and disposing of any collateral.
•We may need to raise additional funds to finance our future capital or operating needs, which could have adverse impacts on our business, results of operations, and the interests of our shareholders.
Risks Related to Our Common Stock
•An active trading market in our common stock may not be maintained.
•The price of our common stock could be volatile due to a number of factors, which could lead to losses by investors and costly securities litigation.
•We may fail to meet our publicly announced guidance or other expectations about our business and future operating results, which could cause a decline in our stock price.
•We do not anticipate paying any cash dividends in the foreseeable future.
•Anti-takeover provisions in Minnesota law may deter acquisition bids for us that you might consider favorable.
•Our management has broad discretion in the use and placement of our cash and cash equivalents and, despite management’s efforts, cash and cash equivalents may be used in a manner that does not increase the value of shareholders’ investments or placed in otherwise reputable financial institutions that fail.
ITEM 1A. RISK FACTORS
Our business involves a number of risks, some of which are beyond our control. The risk and uncertainties described below are not the only ones we face. Set forth below is a discussion of the risks and uncertainties that management believes to be material to us and could adversely affect our business, financial condition, results of operations, cash flows, growth prospects and the trading price of our common stock.
Risks Related to Our Business and Strategy
Approximately 60% of our total revenues are from sales of Avance Nerve Graft and any adverse decision from the FDA would negatively impact our operations and financial condition.
Approximately 60% of our total revenues are from sales of Avance Nerve Graft, which the FDA considers to be a biological product subject to BLA approval requirements. The product is currently distributed pursuant to a transition plan with the FDA as we await FDA’s decision on the BLA we submitted in September 2024. Any change in position by the FDA regarding its use of enforcement discretion regarding the sale of Avance Nerve Graft in the interim prior to FDA’s decision on the BLA, or if the BLA is not approved, or in the process of BLA approval, our indications for Avance Nerve Graft are narrowed or its use is curtailed in any other way, any such developments would have a material negative impact on our revenues and our operations. For additional information see: “Risk Factors – Our Avance Nerve Graft product is currently distributed pursuant to a transition plan with the FDA; we completed the rolling BLA submission for the Avance Nerve Graft in the third quarter of 2024 and are waiting for FDA approval of our BLA. If the FDA places limits on use of our Avance Nerve Graft product it would have a significant impact on our revenues and thus would have a material adverse effect on us.”
Our revenue growth depends on our ability to increase distribution and sales to existing customers and develop new customers, domestically and abroad, and there can be no assurance that these efforts will result in significant increases in sales.
Beginning in 2020, we adjusted our commercial strategy to focus on deeper penetration of our existing surgeon customers through the development of long-term users of our algorithm of nerve repair in our largest market opportunity of extremity trauma. We believe that near-term growth can be supported first through expanded productivity of our existing sales force with existing accounts and second by adding additional customers. We expect the number of direct sales professionals to increase over time. Additionally, we believe that we have successfully utilized a hybrid commercial approach that includes the use of independent agencies in more remote geographies to provide appropriate local support for customers, without the travel time required of a direct sales representative. We may also need to establish a regional distribution center or centers at some point in the future to account for growth. The incurrence of these expenses may impact our operating results, and there can be no assurance of their effectiveness. If we are unable to increase sales to existing customers and attract new customers, and develop our sales force, there could be a material adverse impact on our business, results of operations, financial condition, and prospects. Additionally, our growth margin is dependent on maintaining a diversified demand mix. If demand only grows in one use application, it could negatively impact gross margin. We are focusing on creating balanced revenue growth and yield improvements in product processing, but we may be unable to do so.
Our revenue depends on a limited number of products.
Substantially all of our revenue is currently derived from six products, Avance Nerve Graft, Axoguard Nerve Protector, Axoguard Nerve Connector, Axoguard HA + Nerve Protector, Axoguard Nerve Cap, and Avive+ Soft Tissue Matrix for the treatment of peripheral nerve damage. Of these six products, Avance Nerve Graft represents approximately 60% of our total revenue. Any disruption in our ability to generate revenue from the processing, distribution, and sale of products, especially Avance Nerve Graft, will have a material adverse impact on our business, results of operations, financial condition, and prospects.
Axoguard Nerve Connector and Axoguard Nerve Protector are only available through a distribution agreement with Evergen. The Distribution Agreement terminates on December 31, 2030. However, there are conditions for continuation of the agreement, including payment terms and minimum purchase requirements, that if breached could result in an earlier termination of the agreement. Through mutual agreement, the parties have not established such minimums and to date have not enforced such minimum purchase provision. Additionally, in the event that Evergen were to enforce minimum purchase quantities and we fail to reach an agreement as to such minimums, Evergen could terminate the agreement if we fail to generate commercially reasonable sales of Axoguard Nerve Connector and Axogen Nerve Protector as measured by sales similar to a competitive product at the same stage in its commercial launch as verified by a mutually acceptable third party. We distribute Axoguard Nerve Connector and Axoguard Nerve Protector for Evergen, and Evergen is the contract manufacturer for our HA+ and Axoguard Nerve Cap products. Although we believe we could develop or obtain products that would replace the Axoguard
products obtained through the Evergen agreements, the loss of the ability to sell the Axoguard products could have a material adverse effect on our business, results of operations, financial condition, and prospects.
We are highly dependent on the continued availability of our facilities and could be harmed if we continue to experience operating challenges with our APC Facility or if any of our facilities are unavailable for any prolonged period of time.
We have completed renovation of our APC Facility and transferred the Avance Nerve Graft tissue processing and packaging to the APC Facility but expect to continue to rely on the Solvita facility for the processing of Avive+ Soft Tissue Matrix. We have experienced unanticipated operating challenges as we commenced processing operations at the APC Facility. Such challenges have negatively impacted our gross margins. Such challenges, if prolonged, could also cause a significant disruption in service to our customers if we were to lose, even temporarily, the availability of our production or distribution facilities. If we are not able to comply with the applicable regulatory requirements or produce product that meets our requirements and specifications, it could delay or disrupt our BLA approval and we will be subject to the same risks that we would be subject to should third parties be unable to comply with the applicable regulatory requirements or produce product meeting our requirements or specifications, as described above. If we continue to experience operating challenges or fail to achieve the operating efficiencies that we anticipate, our business, results of operations, financial condition, and prospects could be adversely impacted.
In operating our new processing facility, we have been forced to devote greater resources and management time than anticipated, particularly in areas relating to operations, quality, raw material supply, regulatory, facilities and information technology. We also have experienced unanticipated employee turnover at our APC Facility. If turnover at the APC Facility or any other facility is higher than anticipated, we may not be able to effectively manage our ongoing processing operations and we may not achieve the operating efficiencies that we anticipate from the APC Facility, which may negatively affect our business, results of operations, financial condition, and prospects.
Any failure in the physical infrastructure of our facilities, including the APC Facility and the facility we lease from Solvita, could lead to significant costs and disruptions that could reduce our revenue and harm both our business reputation and financial results. Any natural or man-made event that impacts our ability to utilize our facilities could have a material impact on our business, results of operations, financial condition, and prospects. Although we have business interruption insurance that would cover certain costs in instances other than service agreement termination, it may not cover all costs nor help to regain our standing in the market. In addition, we may plan to expand the APC facility or open additional office, lab or distributions space in the future, and our ability to license, renovate, rebuild, or find acceptable service facilities takes a considerable amount of time and expense.
There may be significant fluctuations in our operating results.
Significant quarterly fluctuations in our results of operations may be caused by, among other factors, our volume of revenue, seasonal changes in nerve repair activity, timing of sales force expansion, unforeseen restrictions on our ability to access healthcare providers such as inflationary pressures, competitive factors and general economic conditions. There can be no assurance that the level of revenue and profit, if any, we achieve in any particular fiscal period, will not be significantly lower than in other comparable fiscal periods. Our expense levels are based, in part, on our expectations as to future revenue. As a result, if future revenue is below expectations, net income or loss may be disproportionately affected by a reduction in revenue, as any corresponding reduction in expenses may not be proportionate to the reduction in revenue.
Macroeconomic trends, such as the inflationary pressure, and political instability could continue to have a material adverse effect on our ability to operate, results of operations, financial condition, liquidity, and capital investments.
We continue to actively monitor the impact of various macroeconomic trends, including high rates of inflation, interest rates, increasing labor costs, supply chain disruptions, labor shortages and, geopolitical instability on our business. We have experienced increased costs consistent with rising interest rates, and inflationary pressures. At this time, we cannot predict the specific extent, duration or full impact of inflationary conditions, supply chain disruptions, geopolitical instability will have on our ongoing and planned clinical trials, our ability to operate, results of operations, financial condition, liquidity, and capital investments.
Economic conditions, such as rising inflation, higher interest rates, increasing labor costs, supply chain pressures, changes in regulatory laws and monetary exchange rates, and government fiscal policies, can also have a significant effect on the cost of operations, including the cost of materials and labor, as well as the interest on our debt. Moreover, negative macroeconomic conditions could adversely impact our ability to obtain financing in the future on terms acceptable to us, or at all. In addition, the geopolitical instability and related sanctions could continue to have significant ramifications including volatility in the U.S. and global financial markets.
Our operating results could be adversely impacted if we are unable to effectively manage and sustain our future growth or scale our operations.
There can be no assurance that we will be able to manage our future growth efficiently or profitably. Our business is unproven on a large scale, and actual revenue and operating margins, or revenue and margin growth, may be less than expected. If we are unable to scale our production capabilities efficiently or maintain pricing without significant discounting, we may fail to achieve expected operating margins, which would have a material and adverse effect on our operating results. Growth may also stress our ability to adequately manage our operations, quality of products, safety, and regulatory compliance. Failure to implement necessary procedures, equipment, or processes or to hire the necessary personnel in a timely and effective manner could result in higher costs or an inability to meet market demand and could have a material adverse impact on our business, results of operations, financial condition, and prospects. Additionally, our future growth will increase the demands placed on our third-party suppliers, and there is no guarantee that our suppliers will be able to support our anticipated growth. If growth significantly decreases, it will negatively impact our cash reserves, and we may be required to obtain additional financing, which may increase indebtedness or result in dilution to shareholders. Further, there can be no assurance that we would be able to obtain additional financing on acceptable terms, if at all.
We have a history of net losses and have not consistently experienced positive cash flow from operations, and our ability to achieve consistent positive cash flow will depend on increasing revenue from distribution of our products, which may not be achievable.
We have historically incurred net losses and operated with negative cash flow from our operations and may continue to incur losses and operate with negative cash flow from operations for the foreseeable future. We have incurred net losses of $10.0 million, $21.7 million, and $28.9 million for the years ended December 31, 2024, 2023 and 2022, respectively. As of December 31, 2024, we had an accumulated deficit of approximately $291.3 million. If revenue does not increase as anticipated, then we will continue to incur net losses and experience negative cash flows and adverse operating conditions. As our debt obligations mature or if our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures, sell assets, seek additional capital, or restructure or refinance our indebtedness. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. If we raise funds by selling additional equity, such sale would result in dilution to our shareholders. There is no assurance that if we are required to secure funding, we can do so on terms acceptable to us, or at all.
Failure to successfully manage the transition associated with the recent management changes could have an adverse impact on our business.
Michael Dale was appointed our Chief Executive Officer in August 2024. There have been other retirements and replacements, including our Chief Research and Development Officer, and our Vice President of Operations. Leadership transitions can be inherently difficult to manage and competition for qualified executives can be intense, and there are a limited number of people with the requisite knowledge and experience. The transitions may cause additional disruption to our business due to, among other things, diverting management’s attention away from the Company’s financial and operational goals or causing a deterioration in morale. During the transition period there may be uncertainty among investors, customers, and other third parties, concerning our future direction and performance.
Loss of key members of management, who we need to succeed, could adversely affect our business.
Our future success depends on the continued efforts of the members of our executive management team. Competition for experienced management personnel in the healthcare industry is intense. If one or more of our executives or other key personnel are unable or unwilling to continue in their present positions, or if we are unable to attract and retain high quality executives or key personnel in the future, our business, results of operations, financial conditions, and prospects may be adversely affected.
Our success will be dependent on continued acceptance of our products by the medical community.
Our success is dependent on continued acceptance of our products by the medical community, which will depend on our ability to demonstrate that our products are an attractive alternative to existing or new nerve reconstruction treatment options, including both surgical techniques and products. Our ability to do so will depend on surgeons’ evaluations of clinical safety, efficacy, ease of use, reliability, and cost-effectiveness, including insurance reimbursement, of our nerve repair products. For example, although our Avance Nerve Graft follows stringent safety standards, including sterilization by gamma irradiation, we believe that a small portion of the medical community has lingering concerns over the risk of disease transmission through the use of allografts in general. If the medical community and patients do not ultimately accept our products as safe and effective or
we are unable to raise awareness of our products and processes, our ability to sell the products may be materially and adversely affected, and our business, results of operations, financial condition, and prospects may be adversely affected.
Delays, interruptions, or the cessation of production by our third-party suppliers, including products supplied by single suppliers, of important materials may prevent or delay our ability to manufacture or process the final products.
Most of the raw materials used in the process for Avance Nerve Graft are available from more than one supplier. However, there are materials within the manufacturing and production process that come from single suppliers, some of which are outside of the U.S., or certain supplies which may be difficult to procure due to supply chain shortages or changes in global trade regulations. Macroeconomic factors could cause disruptions in the supply chain and impair our ability to obtain the materials needed for our product line.
We do not have written contracts that guarantee supply with our suppliers, and at any time they could stop supplying our orders. FDA review of a new supplier may be required if these materials become unavailable from our current suppliers. Although there may be other suppliers that have equivalent materials that would be available to us, if FDA review is required, it could take several months or years to obtain, if approval is able to be obtained at all. Any delay, interruption, or cessation of production by our third-party suppliers of important materials, or any delay in qualifying new materials, if necessary, would prevent or delay our ability to manufacture products. We are working on identifying and contracting with additional suppliers to reduce our dependence on single source suppliers and service providers.
In addition, an uncorrected impurity, a supplier’s variation in a raw material or testing, either unknown to us or incompatible with our manufacturing process, or any other problem with our materials, testing or components, would prevent or delay our ability to process tissue. These delays may limit our ability to meet demand for our products and delay our clinical trials, which would have a material adverse impact on our business, results of operations, financial condition, and prospects.
Technological change and competition for newly developed products could reduce demand for our products.
The medical technology industry is intensely competitive. We compete with both U.S. and international entities that engage in the development and production of medical technologies and processes, including:
•biotechnology, orthopedic, pharmaceutical, biomaterial, chemical, and other companies;
•academic and scientific institutions; and
•public and private research organizations.
Our products compete with autograft, hollow-tube conduits, commercially available wraps, and amnion products, as well as with alternative medical procedures. For the foreseeable future, we believe a significant number of surgeons will continue to choose to perform autograft procedures when feasible, despite the necessity of performing a second operation and its drawbacks. In addition, many members of the medical community will continue to prefer the use of hollow-tube conduits due in part to their familiarity with these products and the procedures required for their use. Also, steady improvements have been made in synthetic human tissue substitutes, which could compete with our products in the future. Unlike allografts, synthetic tissue technologies are not dependent on the availability of human or animal tissue. Although our growth strategy contemplates the introduction of new technologies, the development of these technologies is a complex and uncertain process, which require a high level of innovation, as well as the ability to accurately predict future technology and market trends. We may not be able to respond effectively to technological changes and emerging industry standards, or to successfully identify, develop or support new technologies or enhancements to existing products in a timely and cost-effective manner, if at all. There can be no assurance that in the future our competitors will not develop products that have superior performance or are less expensive relative to our products, rendering our products obsolete or noncompetitive. Due to our resource allocation, size, and relatively early stage, we may face competitive challenges from new products or existing products and barriers that are difficult to overcome and could negatively impact our growth.
We may not be successful in our efforts to build a pipeline of additional product candidates.
We may not be able to continue to identify and develop new product candidates in addition to our current pipeline. Even if we are successful in continuing to build our pipeline, the potential product candidates that we identify may require extensive resources to development, may not be suitable for clinical development or may not achieve market acceptance. Failure or delay in development of new products could damage the reputation of our R&D capabilities and our reputation in the market as being a leader in the tissue nerve repair space. Furthermore, if we do not successfully develop and commercialize product candidates based upon our approach, we will not be able to obtain product revenue in future periods, which likely would result in significant harm to our financial position and adversely affect our stock price.
We must maintain high quality processing of our products.
Our Avance Nerve Graft is processed through our Avance Method, which requires careful calibration and precise, high-quality processing and manufacturing. Achieving precision and quality control requires skill and diligence by our personnel. If we fail to achieve and maintain these high levels of quality control and processing standards, including avoidance of processing errors, defects, or product failures, we could experience recalls or withdrawals of our product, delays in delivery, cost overruns or other problems that would adversely affect our business. We cannot completely eliminate the risk of errors, defects or failures and could experience quality system issues where corrective actions must be taken. In addition, we may experience difficulties in scaling-up processing of our Avance product, including problems related to yields, quality control and assurance, tissue availability, adequacy of control policies and procedures, and lack of skilled personnel. If we are unable to process and produce our human tissue products on a timely basis, at acceptable quality and costs, and in sufficient quantities, or if we experience unanticipated technological problems or delays in production, our business, results of operations, financial condition, and prospects would be adversely affected.
Our revenue depends upon prompt and adequate reimbursement from public and private insurers and national health systems.
Political, societal, economic, and regulatory influences are fundamentally changing the U.S. healthcare industry. The ability of a hospital or an ambulatory surgery center to pay fees for our products depends in part on the availability of adequate coverage and reimbursement from third-party payors for our products specifically, the procedures associated with the use of our products, or both. Providers that purchase our products generally rely on third-party payors to reimburse all or part of the costs and fees associated with the procedures performed with our products or the products themselves. Therefore, adequate coverage and reimbursement from third-party payors, including government payors such as Medicare and Medicaid, are important for obtaining product acceptance and widespread adoption in the marketplace.
When our products are used in the operating room of a hospital, they are currently commonly treated as general supplies utilized in surgery, and the cost is currently included in payment to the facility for the procedure. When Avance Nerve Graft and Axoguard Connector are used in an outpatient setting where the nerve repair is the primary reason for the procedure, facilities currently may use a Category I CPT code to facilitate payment.
In January 2018, the American Medical Association created a Category I CPT code (64912) specific to nerve repair with nerve allograft (Avance Nerve Graft) and a separate code (+64913) for each additional strand of allograft used in a procedure. Category I CPT codes are used by providers to facilitate payment to the provider (either hospital or ambulatory surgery center) for outpatient procedures. Additionally, Category I CPT codes are used to facilitate payment to the surgeon, for both time spent in outpatient and inpatient procedures. Prior to January 2018, there was no designated Category I CPT code for nerve repair cases that included nerve allograft. The Category I CPT code specific to nerve repair with nerve allograft, has allowed for nerve allograft repair cases to be uniquely identified in the Medicare claims data. This in turn allowed CMS visibility to nerve allograft nerve procedure costs, and thereby confirm that nerve allograft qualified as a device intensive procedure.
Another important change in nerve repair reimbursement occurred in January 2020, when most direct repair procedures were moved from the higher paying level 2 nerve repair Ambulatory Payment Category 5432 to the lower paying level 1 Ambulatory Payment Category 5431, thus aligning payment rates more consistently with the lesser costs of a direct repair.
As a result of the allograft device intensive status and direct repair Ambulatory Payment Category realignment, CMS reimbursement rates for nerve repair in the outpatient setting have changed significantly during the last two years. With the new 2024 CMS reimbursement rates for nerve repair in the outpatient setting that became effective January 1st, reimbursement for procedures using Avance Nerve Graft have increased 40% in hospital outpatient centers and 138% in ambulatory surgery centers since 2019. During this same timeframe, reimbursement rates for procedures involving conduits and connectors also increased 40% in hospital outpatient centers and 70% in ambulatory surgery centers. While Medicare patients represent a relatively small percentage of trauma cases, CMS’ direction often influences commercial payor policies and payments.
The process for securing coding for a product or procedure is separate from the process of securing coverage and establishing a reimbursement payment rate. In the U.S., coverage and reimbursement for medical devices varies among payors. In addition, payors review coverage policies on an ongoing basis and can change or deny coverage for these new products and procedures without notice. We estimate that commercial payors covering a significant number of U.S. covered lives have legacy non-coverage policies relating to our Avance Nerve Graft and our Axoguard Product Line, designating these products investigational or experimental. Some commercial payors do not currently cover or reimburse our products because they have determined insufficient evidence of favorable clinical outcomes is available. Although some payors consider Avance Nerve Graft and our Axoguard Product Line investigational or experimental at this time, these payors may in the future determine sufficient evidence has been developed to cover and reimburse our products and related procedures. In partnership with
healthcare providers, we are working actively to reverse these non-coverage decisions and have been successful with several regional plans. However, we cannot provide assurance that we will continue to be successful in these efforts. If we are not successful in reversing existing non-coverage policies, or if other third-party payors issue similar policies, this could have a material adverse effect on our business and operations. Further, third-party payors who currently cover and reimburse customers for procedures using our products may in the future choose to decrease current levels of reimbursement or eliminate reimbursement altogether, which would cause our business to suffer.
The amount of reimbursement received by our customers from third-party payors is dependent generally on fee schedules established by these payors for the existing CPT codes. For governmental payors, such as Medicare and Medicaid, the fee schedule amount is determined by statutory and regulatory formulas as previously discussed. For commercial payors, the reimbursement amount generally is dependent upon the specific contract terms between the provider and payor. We cannot provide assurance that government or commercial payors will continue to reimburse for procedures with our products using the existing codes, nor can we provide assurance that the payment rates will be adequate. If providers and physicians are unable to obtain reimbursement for the procedure at adequate levels when use of our products is included, this could have a material adverse effect on our business and operations. Hospitals and ambulatory surgery centers may not purchase our products if they do not receive payment sufficient to cover the cost of our products and related procedures. In addition, in the event that the current coding and/or payment methodology for these procedures changes, this could have a material effect on our business, results of operations, financial condition, and prospects.
Additionally, healthcare law and policy changes may have a material adverse effect on our revenues. See: “Risk Factors – Healthcare law and policy changes may have a material adverse effect on us.”
Negative publicity concerning methods of donating human tissue and screening of donated tissue may reduce demand for our products and negatively impact the supply of available donor tissue.
We are highly dependent on our ability to recover human peripheral nerve tissue from tissue donors for our Avance Nerve Graft product. The availability of acceptable donors is relatively limited, and this availability is impacted by regulatory changes, general public opinion of the donation process, and our reputation for handling the donation process. Media reports or other negative publicity concerning both improper methods of tissue recovery from donors and disease transmission from donated tissue, including bones and tendons, may limit widespread acceptance of our Avance Nerve Graft. Unfavorable reports of improper or illegal tissue recovery practices, both in the U.S. and internationally, as well as incidents of improperly processed tissue leading to transmission of disease, may broadly affect the rate of future tissue donation and market acceptance of allograft technologies and donated tissue use. Potential patients may not be able to distinguish our products, technologies, and tissue recovery and processing procedures from others engaged in tissue recovery. In addition, unfavorable reports could make families of our potential donors or donors themselves from whom we are required to obtain consent before processing tissue reluctant to agree to donate tissue to for-profit tissue processors. Any disruption in the supply caused by these publicity issues could have a material impact for our business, results of operations, financial condition, and prospects.
The failure of third parties to perform many necessary services for the commercialization of our products, including services related to recovery/acquisition, sterilization, distribution, and transportation, would impair our ability to meet commercial demand.
We rely upon third parties for certain recovery/acquisition, sterilization, distribution, and transportation services for our products. For example, the Avance Nerve Graft processing consists of several steps, and we use a number of recovery and/or acquisition agencies to supply the human tissue needed for these products. While we believe our current contracts and the ability to enter into future contracts will provide us with the tissues required for the products, we cannot be sure that we will be able to obtain the tissue that we need in the future. Disruptions in the tissue supply may adversely impact both tissue products and our overall business. If any of the third parties that we rely upon in our recovery/acquisition, distribution or transportation process fail to comply with applicable laws and regulations, fail to meet expected deadlines, or otherwise do not carry out their contractual duties, experience delays due to macroeconomic factors, or encounter physical damage or natural disaster at their facilities, our ability to deliver product to meet commercial demand may be significantly impaired, which could have a material adverse impact on our business, results of operations, financial condition or prospects.
We are dependent on our relationships with independent agencies to generate a material portion of our revenue.
We derive material revenue through our relationships with independent agencies. In 2024, approximately 10% of global product revenue was generated through independent agencies. If certain agency relationships were terminated or discontinued for any reason, it could adversely affect our ability to generate revenue and profit. If we require additional agencies, we may not be able to find additional agencies who will agree to market and distribute our products on commercially reasonable terms, if at all. If we are unable to establish new agency relationships or renew certain current distribution agreements on commercially
acceptable terms, our business, results of operations, financial condition, and prospects could be materially and adversely impacted.
If we do not manage product inventory in an effective and efficient manner, it could adversely affect profitability.
Many factors affect the efficient use and planning of product inventory, such as our ability to predict demand for donor tissue, prepare manufacturing to meet that demand and product mix and handle product expiration. We may be unable to manage our inventory efficiently, keep inventory within expected budget goals, keep our work-in-process inventory on hand or manage it efficiently, control expired product or keep sufficient product on hand to meet demand. Finally, we can provide no assurance that we can keep inventory costs within our target levels, particularly in light of overall cost increases due to global inflation. Failure to do so may materially and adversely impact our business, results of operations, financial condition, and prospects.
We may be unsuccessful in commercializing our products outside the U.S.
To date, we have focused our commercialization efforts in the U.S., except for minor revenue in certain international countries. We intend to expand distribution and sales outside the U.S. and will need to comply with applicable foreign regulatory requirements, including obtaining the requisite approvals to do so. The regulatory environment for our portfolio of products is complex. Avance Nerve Graft is distributed in Canada, the UK, and certain other countries. We received approval to distribute Avance Nerve Graft in Germany in December 2019. Avance Nerve Graft use in Spain currently requires approval for each case to be approved by tissue authorities under an alternative therapy designation. The Axoguard Nerve Connector and Axoguard Nerve Protector CE Mark has been renewed as of May 2021 by Evergen.
Further, we will need to either enter into distribution agreements with third parties or develop a direct sales force in international markets. If we do not obtain adequate levels of reimbursement from third-party payers outside of the U.S., we may be unable to develop and grow our revenue internationally. Outside of the U.S., reimbursement systems vary significantly by country. Many ex-U.S. markets have government-managed healthcare systems that govern reimbursement for medical devices, implants, and procedures. Some ex-U.S. reimbursement systems provide for limited payments in a given period and therefore result in extended payment periods. If we are unable to successfully commercialize our products internationally, our long-term growth prospects may be limited.
We may seek to expand our business in ways that could result in diversion of resources and extra expenses.
We may in the future pursue acquisitions of businesses, products and technologies, establish joint venture arrangements, or make minority equity investments to expand our business. We are unable to predict whether or when any prospective acquisition, equity investment or joint venture will be completed. The process of negotiating potential acquisitions, joint ventures or equity investments, as well as the integration of acquired or jointly developed businesses, technologies or products may be prolonged due to unforeseen difficulties and may require a disproportionate amount of our resources and management’s attention. We cannot assure you that we will be able to successfully identify suitable acquisition or investment candidates, complete acquisitions or investments, or integrate acquired businesses or joint ventures with our operations. If we were to make any acquisition or investment or enter into a joint venture, we may not receive the intended benefits of the acquisition, investment or joint venture or such an acquisition, investment or joint venture may not achieve comparable levels of revenues, profitability or productivity as our existing business or otherwise perform as expected. The occurrence of any of these events could harm our business, financial condition or results of operations. Future acquisitions, investments or joint ventures may require substantial capital resources, which may require us to seek additional debt or equity financing.
Future acquisitions, joint ventures or minority equity investments by us could result in the following, any of which could seriously harm our results of operations or the price of our stock:
•issuance of equity securities that would dilute our current shareholders’ percentages of ownership;
•large one-time write-offs or equity investment impairment write-offs;
•incurrence of debt and contingent liabilities;
•difficulties in the assimilation and integration of operations, personnel, technologies, products and information systems of the acquired companies;
•inability to realize cost efficiencies or synergies, thereby incurring higher operating expenditures as a result of the acquisition;
•diversion of management’s attention from other business concerns;
•contractual disputes;
•risks of entering geographic and business markets in which we have no or only limited prior experience; and
•potential loss of key employees of acquired organizations.
We may be subject to future product liability litigation, which could be expensive, and our insurance coverage may not be adequate.
Although we are not currently subject to any product liability proceedings and have no provision for product liability disbursements, we may incur material liabilities relating to product liability claims in the future, including product liability claims arising out of the usage of our products. Although we currently carry product liability insurance in an amount, we believe is consistent with industry averages, our insurance coverage and any provision we may maintain in the future for product related liabilities may not be adequate and our business, results of operations, financial conditions, and prospects could suffer material adverse consequences.
We are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by geopolitical instability and tensions, Russia's ongoing invasion of Ukraine and illegal annexation of Ukrainian territories, and the war in the Middle East between the state of Israel and the Islamic Republic of Iran and its proxy terror organizations could materially and adversely affect our business, financial condition and results of operations.
We are exposed to the risk of changes in social, geopolitical, legal, and economic conditions. The global economy has been, and may continue to be, negatively impacted by Russia’s invasion of Ukraine and illegal annexation of Ukrainian territories. The negative impacts arising from the war and sanctions and export restrictions imposed by various countries, including those imposed by Russia, may include reduced consumer demand, supply chain disruptions, increased cybersecurity risks, and increased costs for transportation, energy, and raw materials. Additionally, further escalation of trade tensions between the U.S. and China, escalation of tensions between China and Taiwan, further escalation in the conflict between the State of Israel, and the Islamic Republic of Iran and its proxy terror organizations including Hamas, Hezbollah and the Houthis, as well as further escalation of tensions between the State of Israel and various countries or terror organizations in the Middle East and North Africa, further escalation of tensions between the Houthis and the U.S. led multinational force stationed in the Middle East as well as broader involvement of the U.S. in the conflict in the Middle East, could result in a global economic slowdown and long-term changes to global trade. Although we do not have material operations in Russia, Ukraine, China, Taiwan, Israel, or other countries in the Middle East and North Africa, further escalation of geopolitical tensions could have a broader impact that expands into other markets where we have material operations, which may adversely affect our business, financial condition and results of operations.
Further, changes in domestic and global economic conditions, supply chain disruptions, labor shortages, as well as other stimulus and spending programs, have led to higher inflation, which is likely to lead to increased costs and may cause changes in fiscal and monetary policy. Additionally, our ability to access capital markets and other funding sources in the future may not be available on commercially reasonable terms, if at all. Impacts from inflationary pressures, such as increasing costs for research and development of our products, administrative and other costs of doing business, could adversely affect our business, financial condition and results of operations.
Additionally, our customers could experience financial and operational pressures as a result of labor shortages, the supply chain disruptions, and increased inflation, which could impact their ability to access capital markets and other funding sources, increase cost of funding, or impede their ability to comply with debt covenants, which in turn could impede their ability to provide patient care, conduct further research and development, marketing and commercialization efforts, or impact their profitability. To the extent that our customers continue to face such financial pressures, it could impact their willingness to spend on our products and services, which could adversely affect our business, financial condition and results of operations.
Although, to date, our business has not been materially impacted by Russia's ongoing invasion of Ukraine and illegal annexation of Ukrainian territories, geopolitical tensions between China and the U.S. geopolitical tensions between China and Taiwan, the escalation of the conflict between the State of Israel and Hamas, or record inflation, it is impossible to predict the extent to which our operations could be impacted in the short and long term, or the ways in which such matters may impact our business.
Changes in U.S. trade policy, threats of international tariffs, and changes to the U.S. political landscape may adversely affect our business, results of operations, financial condition, and prospects.
Rising threats of international tariffs, including tariffs applied to goods traded between the U.S. and China, could materially and adversely affect our business, results of operations, financial condition, and prospects. Over the past several years, legislative and executive action from U.S. and foreign leaders has led to both threats of and the imposition of tariffs on certain materials and products. The U.S. and China imposed tariffs or announced proposed tariffs to be applied in the future to certain of each other’s exports. Changes in political conditions in China and changes in the state of China-U.S. relations, including the
current trade tensions, are difficult to predict and could adversely affect our operations or financial condition. We cannot predict the extent to which the U.S. or other countries will impose quotas, duties, tariffs, taxes or other similar restrictions upon the import or export of our products in the future, nor can we predict future trade policy or the terms of any renegotiated trade agreements and their impact on our business. The adoption and expansion of trade restrictions, the occurrence of a trade war, or other governmental action related to tariffs or trade agreements or policies has the potential to adversely impact demand for our products, our costs, our customers, our suppliers, and the U.S. economy, which in turn could have a material adverse effect on our business, results of operations, financial condition, and prospects.
Our results of operations could be negatively affected by potential fluctuations in foreign currency exchange rates.
We are exposed to the effects of changes in foreign currency exchange rates. We are exposed to the risk of an increase or decrease in the value of the foreign currencies relative to the U.S. Dollar, which could increase the value of our expenses and decrease the value of our revenue when measured in U.S. Dollars. As a result, our results of operation may be influenced by the effects of future exchange rate fluctuations and such effects may have an adverse impact on our common stock price. Changes in the relative values of currencies occur regularly and, in some instances, could materially adversely affect our business, results of operations, financial condition or prospects.
Our failure to protect our technology systems and comply with data protection laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our business, results of operations, financial condition, and prospects.
We rely on information technology systems, including technology from third-party vendors, to process, transmit and store electronic information in our day-to-day operations. Similar to other companies, the size and complexity of our information technology systems makes them vulnerable to a cyberattack, malicious intrusion, breakdown, destruction, loss of data privacy, or other significant disruption. Our information systems require an ongoing commitment of resources to maintain, protect and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards and the increasing need to protect patient and customer information. We expend significant resources to comply with applicable data privacy and security laws and regulations (together with applicable industry standards) and minimize the risk of security breaches, including deploying additional personnel and protection technologies, training employees annually, and engaging third-party experts and contractors. Significant and increasing investments of time and resources by management and Board have been, and will continue to be, required to anticipate and address cybersecurity risks and incidents. However, given that the techniques used to obtain unauthorized access or to sabotage systems change frequently, and often are not identified until they are launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures in time to stop a cyber incident. Any failure by us to maintain or protect our information technology systems and data integrity could result in the unauthorized access to patient data and personally identifiable information, theft of IP or other misappropriation of assets, or otherwise compromise our confidential or proprietary information and disrupt our operations. Cyberattacks, intrusions, or other breaches could adversely impact our business, results of operations, financial condition, and prospects and potentially subject us to fines and penalties.
In the U.S., federal and state privacy and security laws require certain of our operations to protect the confidentiality of personal information, including patient medical records and other health information. Limiting and/or restricting the use of certain personal data and information, as well as added transparency obligations to data subjects is becoming an increasing focus as evidenced by the implementation of the California Consumer Privacy Act which became effective on January 1, 2020. In Europe, E.U. member states and other foreign jurisdictions, including Switzerland, have adopted data protection laws and regulations which impose significant compliance obligations. Moreover, the collection and use of personal health data in the E.U. is governed by the E.U. General Data Protection Regulation (“GDPR”). The GDPR imposes several requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, the security and confidentiality of the personal data, data breach notification and the use of third-party processors in connection with the processing of personal data. The GDPR also imposes strict rules on the transfer of personal data out of the E.U. to the U.S., provides an enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to 4% of the annual global revenue of the noncompliant company. The recent implementation of the GDPR has increased our responsibility and liability in relation to personal data that we process, including in clinical trials, and we may in the future be required to put in place additional mechanisms to ensure compliance with the GDPR, which could divert management’s attention and increase our cost of doing business.
Additionally, we expect that there will be other proposed laws, regulations and industry standards relating to privacy and data protection in the U.S., the E.U. and other jurisdictions, and we cannot determine the impact such future laws, regulations and standards may have on our business results of operations, financial condition, and prospects.
We are dependent on internal information and telecommunications systems, and any failure of these systems, including system security breaches, data protection breaches or other cybersecurity attacks, may negatively impact our business and results of operations.
Cyberattacks and other tactics designed to gain access to and exploit sensitive information by breaching mission critical systems of large organizations are constantly evolving and have been increasing in sophistication in recent years. High profile security breaches leading to unauthorized release of sensitive information have occurred with increasing frequency at a number of major U.S. companies, despite widespread recognition of the cyberattack threat and improved data protection methods. While to date we have not experienced a significant data loss, significant compromise or any material financial losses related to cybersecurity attacks, our systems, those of our customers, and those of our third-party service providers are under constant threat. Cybercrime, including phishing, social engineering, attempts to overload our servers with denial-of-service attacks, or similar disruptions from unauthorized access to our systems, could cause us critical data loss or the disclosure or use of personal or other confidential information. Outside parties may attempt to fraudulently induce employees to disclose personally identifiable information or other confidential information which could expose us to a risk of loss or misuse of this information. Although we incur significant expenses to minimize the risk of security breaches, given that the techniques used to obtain unauthorized access or to sabotage systems change frequently, and often are not identified until they are launched against a target, we may be unable to anticipate these techniques of implement adequate preventive measures in time to stop or effectively mitigate a cyber incident.
We are dependent on internal information and telecommunications systems, and we are vulnerable to failure of these systems, including through system security breaches, data protection breaches or other cybersecurity attacks. If these events occur, the unauthorized disclosure, loss or unavailability of data and disruption to our business may have a material adverse effect on our reputation and harm our relationships with vendors and customers. Additionally, these events may lead to financial losses from remedial actions, or potential liability from fines, including in relation to noncompliance with the GDPR, as well as possible litigation and punitive damages. Failures of our internal information or telecommunications systems may prevent us from taking customer orders, shipping products and billing customers. Sales may also be impacted if our customers are unable to access our pricing and product availability information. The occurrence of any of these events could have a material adverse impact on our business and results of operations.
Our business and financial performance could be adversely affected, directly or indirectly, by natural or man-made disasters or other similar events.
Neither the occurrence nor the potential impact of risks such as earthquakes, hurricanes, floods and other natural disasters, fire, power shortages, geopolitical unrest, war, terrorist attacks and other hostile acts, epidemics or pandemics, international hostilities or other criminal activities and other events beyond our control and the control of the third parties on which we depend can be predicted. However, these occurrences could impact us directly as a result of damage to our facilities or by preventing us from conducting our business in the ordinary course, or indirectly as a result of their impact on our customers, suppliers, or other counterparties. We could also suffer adverse consequences to the extent that these disasters affect the financial markets or the economy in general or in any particular region.
Additionally, climate change could present immediate and long-term risks to our industry and our customers. The potential for increased severe weather events could have a material adverse effect on our operations and infrastructure or the operations and infrastructure of our suppliers. In addition, the effects of climate change could include long-term changes in temperature levels and water availability, increased energy costs, and increased supply costs impacted by those increasing energy costs.
Our ability to mitigate the adverse consequences of such occurrences is in part dependent on the quality of our resiliency planning, and our ability, if any, to anticipate the nature of any such event that occurs. The adverse impact of natural or man-made disasters also could be increased to the extent that there is a lack of preparedness on the part of national or regional emergency responders or on the part of other organizations and businesses that we deal with, particularly those that we depend upon but have no control over.
Changes in the tax code could have a material adverse effect on our results of operations, financial condition, liquidity, and capital investments.
In recent years, political discourse has centered on potential changes in tax laws or tax rulings. Certain of these changes could negatively affect our financial condition. In addition, our ability to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future tax payments may be limited by provisions of the Internal Revenue Code, and it is possible that certain transactions or a combination of certain transactions may result in material additional limitations on our ability to use our net operating loss and tax credit carryforwards.
We may have exposure to additional tax liabilities as a result of our foreign operations.
We are subject to income taxes in the U.S. and various foreign jurisdictions. We have operations in Canada, Germany, UK, Spain, and several other European, Asian, and Latin American countries. Significant judgment is required in determining our worldwide provision for income taxes and other tax liabilities. In the ordinary course of a global business, there are many intercompany transactions and calculations where the ultimate tax determination is uncertain. We are regularly under audit by tax authorities. Our intercompany transfer pricing may be reviewed by the U.S. Internal Revenue Service and by foreign tax jurisdictions. Although we believe that our tax estimates are reasonable, due to the complexity of our corporate structure, the multiple intercompany transactions and the various tax regimes, we cannot assure you that a tax audit or tax dispute to which we may be subject will result in a favorable outcome for us. If taxing authorities do not accept our tax positions and impose higher tax rates on our foreign operations, our overall tax expenses could increase.
Our business and stock price may be adversely affected if our internal controls are not effective.
Section 404 of the Sarbanes-Oxley Act of 2002 requires that public companies conduct a comprehensive evaluation of their internal control over financial reporting. To comply with this statute, each year we are required to document and test our internal control over financial reporting and our management is required to assess and issue a report concerning it.
Although we have systems in place to strengthen our internal control over financial reporting, we cannot assure you that we will not discover material weaknesses in the future or that no material weakness will result from any difficulties, errors, delays, or disruptions while we implement and transition to new internal systems. The existence of one or more material weaknesses could result in errors in our financial statements, and substantial costs and resources may be required to rectify these or other internal control deficiencies. If we cannot produce reliable financial reports, investors could lose confidence in our reported financial information, the market price of our common stock could decline significantly, we may be unable to obtain additional financing to operate and expand our business and our business, results of operations, financial condition, and prospects could be adversely impacted.
We incur costs as a result of operating as a public company, and our management is required to devote substantial time to compliance initiatives.
As a public company, we incur legal, accounting, and other expenses to comply with relevant securities laws and regulations, including without limitation, the requirement of establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management devotes substantial time and financial resources to these compliance initiatives. Failure to comply with public company requirements could have a material adverse effect on our business. In addition, activity by shareholders or others that bring into question aspects of our business, financial reporting, or management’s integrity, whether based on facts, beliefs or baseless and contrived for individual economic gain, can have a negative impact on the price of our stock and can result in substantial time and financial resources being expended to address the situation.
Our business, results of operations, financial condition, and prospects could be adversely affected, directly or indirectly, by the effects of a focus on environmental, social and governance issues.
In recent years, shareholders have focused on environmental, social and governance ("ESG") issues, including how companies are addressing climate change, diversity, and human rights, among other ESG-related issues. Our failure to comply with stakeholder expectations and standards regarding ESG issues, which are still evolving and can vary considerably, or the perception that we have not responded appropriately to ESG-related issues, could result in reputational harm, and could have an adverse effect on our business, results of operations, financial condition, and prospects.
The cost of mitigating or responding to ESG issues could be significant; however, these costs are too uncertain to predict. In addition, the approaches taken by the U.S. or foreign governments to regulate ESG issues, which may include legislative or regulatory changes, and new reporting requirements, could adversely impact our business, results of operations, financial condition, and prospects, and are too uncertain to predict.
Risks Related to the Regulatory Environment in which We Operate
Our Avance Nerve Graft product is currently distributed pursuant to enforcement discretion and a transition plan with the FDA; however, we completed the rolling BLA submission for Avance Nerve Graft in September of 2024. If the FDA does not approve our BLA, approves a narrower indication than Avance Nerve Graft’s current use or otherwise limits use of our Avance Nerve Graft product it would have a significant impact on our revenues and thus would have a material adverse effect on us.
The FDA considers our Avance Nerve Graft product to be a biological product, subject to BLA approval requirements. Although the Avance Nerve Graft product has not yet been approved by the FDA through a BLA, it is currently distributed under the controls applicable to an HCT/P regulated under section 361 of the PHS Act and 21 CFR Part 1271 of FDA’s regulations, subject to FDA’s enforcement discretion and our compliance with a transition plan established by the FDA. See “Business — Government Regulations — U.S. Government Regulation Overview.” We have continued to communicate with CBER since the acceptance of the transition plan on clinical trial design, pre-clinical studies, CMC for Avance Nerve Graft, and other issues related to the effective IND. Subject to the FDA’s enforcement discretion, we can commercially distribute Avance Nerve Graft until the FDA makes a final determination on an Avance Nerve Graft BLA submission, assuming we remain in compliance with the transition plan and exercise due diligence in executing the transition plan. In the event that the FDA becomes dissatisfied with our progress or actions with respect to the transition plan or the FDA changes its position for any reason regarding its use of enforcement discretion to permit us to distribute the Avance Nerve Graft product in accordance with the transition plan, we would no longer be able to distribute Avance Nerve Graft, which would have a material adverse effect on our operations and financial viability. In addition, if we do not meet the conditions of the transition plan, or fail to comply with applicable regulatory requirements, the FDA could impose civil penalties, including fines, product seizures, injunctions, or product recalls and, in certain cases, criminal sanctions. We completed the rolling BLA submission for the Avance Nerve Graft in September 2024. If the FDA does not approve the BLA, narrows the Avance Nerve Graft indication, takes negative action on the BLA, or limits the use of our Avance Nerve Graft product for any other reason, our operations and financial viability would be significantly negatively impacted as we may no longer be able to distribute our Avance Nerve Graft product or the demand for the Avance Nerve Graft product could drop due to limitations on use.
These consequences also would have a material adverse effect on our operations and financial viability. Additionally, approximately 60% of our total revenues are from sales of Avance Nerve Graft, any change in position by the FDA regarding its use of enforcement discretion to permit the sale of Avance Nerve Graft or a negative action on the BLA could have a material negative impact on our revenues and our operations. For additional information see: “Risk Factors - Approximately 60% of our total revenues are from sales of Avance Nerve Graft.”
Even if we obtain regulatory approval for Avance Nerve Graft, we will remain subject to ongoing regulatory requirements. Maintaining compliance with ongoing regulatory requirements may result in significant additional expense to us, and any failure to maintain such compliance could subject us to penalties and cause our business to suffer.
Even if Avance Nerve Graft receives BLA approval, we will be subject to ongoing regulatory requirements with respect to manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing clinical trials, if any, and submission of safety, efficacy and other post-approval information, including both federal and state requirements in the U.S. and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to continuously comply with FDA and comparable foreign regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to cGMP regulations and corresponding foreign regulatory manufacturing requirements. As such, we and our CMOs will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any BLA or other marketing authorization application.
If we are not able to comply with post-approval regulatory requirements, we could have marketing approval for Avance Nerve Graft withdrawn by regulatory authorities and our ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Thus, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
In addition, later discovery of previously unknown problems with Avance Nerve Graft or problems with the facility where the product is manufactured, or failure to comply with applicable regulatory requirements may result in a variety of risks. For example, a regulatory agency or enforcement authority may, among other things:
•impose restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
•impose requirements to conduct post-marketing studies or clinical trials;
•issue warning or untitled letters if the regulator is the FDA, or comparable notice of violations from foreign regulatory authorities;
•issue consent decrees, injunctions or impose civil or criminal penalties;
•require the payment of fines, restitution or disgorgement of profits or revenues;
•suspend or withdraw regulatory approval;
•suspend any of our ongoing clinical trials;
•refuse to approve pending applications or supplements to approved applications submitted by us;
•impose restrictions on our operations, including closing our or our CMOs’ manufacturing or analytical testing facilities; or
•require product seizure or detention, recalls or refuse to permit the import or export of products.
Any government investigation of alleged violations of law would require us to expend significant time and resources in response and could generate adverse publicity.
Our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly and could result in negative effects on our business.
We are subject to extensive regulation by foreign and domestic government entities, including compliance with regulations governing appropriate relationships with healthcare professionals, such as physicians, hospitals, and those to whom and through whom we may market our products. We are subject to various federal, state, and territorial laws in the U.S. and other jurisdictions in which we conduct business. These include, for example, anti-kickback laws, false claims laws, healthcare fraud, waste, and abuse laws, and anti-bribery laws such as the U.S. Foreign Corrupt Practices Act. Violations of these laws can be punishable by criminal and/or civil sanctions, including, in some instances, fines, imprisonment and, within the U.S., exclusion from participation in government healthcare programs, including Medicare, Medicaid, and Veterans Administration health programs. These laws are administered and enforced by, among others, the DOJ, which issued new compliance guidance in 2020, the Office of Inspector General of the Department of Health and Human Services, state attorneys general, and their respective counterparts in the applicable foreign jurisdictions in which we conduct business. Many of these agencies have increased their enforcement activities with respect to drug and medical device manufacturers in recent years. There can also be changes to the regulations by foreign and domestic government entities that require us to update or upgrade business processes or to perform additional validation activities for product or processes. Compliance with such changes can be costly to implement or result in non-compliance, thus restricting the ability to distribute tissue or sell products, which could have a material adverse effect on our business, results of operations, financial condition, and prospects.
Our products are also subject to regulation by the FDA in the U.S. The FDA regulates the development, pre-clinical and clinical testing, requirements for commercial marketing and distribution, manufacturing and quality, safety, labeling, and promotion of medical products including human cells, tissues and cellular and tissue-based products (HCT/Ps), medical devices, and biological products. The FDA requires the pre-market approval of a biological product, like Avance Nerve Graft, through a BLA, prior to marketing. Although the Avance Nerve Graft product has not yet been approved by the FDA through a BLA, the FDA outlined a transition plan subject to FDA enforcement discretion, that we: (i) transition to compliance with section 501(a)(2)(B) of the FD&C Act, the cGMP regulations in 21 CFR Parts 210 and 211 and the applicable regulations and standards in 21 CFR Parts 600-610 prior to initiation of a phase 3 clinical trial designed to demonstrate the safety, purity, and potency of Avance Nerve Graft; (ii) conduct a phase 3 clinical trial to demonstrate safety, purity, and potency of Avance Nerve Graft under an SPA; (iii) continue to comply with the requirements of 21 CFR Part 1271; and (iv) exercise due diligence in executing the transition plan. See “Business — Government Regulations — U.S. Government Regulation Overview.”
The FDA also regulates medical devices, for example the Axoguard products, and generally requires them to be cleared through the 510(k) pre-market notification process prior to marketing or through other pre-market approval processes. The FDA’s pre-market review process for new and modified existing devices that precedes product marketing can be time consuming and expensive. Some of the future products and enhancements to such products that we expect to develop, and market may require marketing clearance or approval from the FDA.
There can be no assurance, however, that clearance or approval will be granted with respect to any of our medical device products or enhancements of marketed products or that our Avance Nerve Graft will meet FDA’s requirements for continued marketing and transition to a BLA or ultimately an approved BLA. FDA review of our devices or biological products may encounter significant delays during FDA’s pre-market review process that would adversely affect our ability to market our products or enhancements. In addition, there can be no assurance that our products, including the Avance Nerve Graft, or enhancements will not be subject to a lengthy and expensive approval process with the FDA. Moreover, the FDA could decide to revoke its enforcement discretion or change the terms of enforcement discretion for Avance Nerve Graft at any time. In addition, any products regulated solely under Section 361 of the PHS Act are a product category under close scrutiny by FDA for compliance with the regulatory requirements and potentially subject to regulatory change in the future. Failure to comply with applicable regulatory requirements could expose us to potential compliance actions by FDA or other regulators and could risk the commercial availability of the product.
It is possible that if regulatory clearances or approvals to market a product are obtained from the FDA, the clearances or approvals may contain limitations on the indicated uses of such product and other uses may be prohibited, as well as require
continuing regulatory review. Product approvals by the FDA can also be withdrawn due to failure to comply with regulatory standards or the occurrence of unforeseen problems following initial approval. The FDA may require post marketing clinical studies or other activities that may add cost or limit marketing of the product. Furthermore, the FDA could limit or prevent the distribution of our products, and the FDA has the authority to require the recall of such products. FDA regulations depend heavily on administrative interpretation, and there can be no assurance that future interpretations made by the FDA or other regulatory bodies will not adversely affect our business, results of operations, financial condition, and prospects. We, and our facilities, may be inspected by the FDA from time to time to determine compliance with various regulations relating to specifications, development, documentation, validation, testing, manufacturing, quality control and product labeling. A determination that we are in violation of such regulations could lead to imposition of civil penalties, including fines, product recalls or product seizures and, in certain cases, criminal sanctions.
Failure to obtain regulatory and pricing approvals in foreign jurisdictions after BLA approval for Avance Nerve Graft or our other products could delay or prevent commercialization of our products abroad.
Distribution of our human tissue products outside the U.S. are subject to foreign regulatory requirements that vary from country to country. In the E.U., human tissue regulations, if applicable, differ from one E.U. member state to the next. Because of the absence of a fully harmonized regulatory framework and the proposed regulation for advanced therapy medicinal products in the E.U., as well as for other countries, the approval process for human derived cell or tissue based medical products may be extensive, lengthy, expensive, and unpredictable. Our products are subject to E.U. member states’ regulations that govern the donation, procurement, testing, coding, traceability, processing, preservation, storage, and distribution of human tissues and cells and cellular or tissue-based products. In addition, some E.U. member states have their own tissue banking regulations. The inability to meet foreign regulatory requirements could materially affect our future growth and compliance with such requirements could place a significant financial burden on us. As a result of Brexit, we cannot be sure what changes could occur or what the cost of regulatory compliance with the UK would be. Accordingly, the cost of regulatory compliance for sales outside the U.S. can be significant and time consuming.
As a result of our BLA approval for Avance Nerve Graft, we must obtain separate regulatory approvals and comply with numerous and varying foreign regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval abroad may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval and additional risks associated with requirements particular to those foreign jurisdictions where we will seek regulatory approval of our products. We may not obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries. We and our collaborators may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market outside the U.S. The failure to obtain these approvals could materially adversely affect our business, financial condition and results of operations.
Finally, regulatory expectations in foreign jurisdictions are subject to constant change. There can be no assurance that we can meet the requirements of future regulations and guidance or that compliance with current regulations and guidance assures future capability to distribute and sell our products.
The use, misuse or off-label use of our products may harm our reputation and the reputation of our products, which could result in injuries leading to product liability suits, and could be costly to our business, and/or result in FDA sanctions.
If our products are misused or used for off-label purposes, our reputation and our product’s reputation may suffer, injuries could occur, which may lead to product liability litigation, or we may be subject to FDA sanctions if we are deemed to have engaged in off-label promotion. We are seeking a biologics license through the BLA process for specific uses of Avance Nerve Graft. Our promotional materials and training methods must comply with FDA requirements and other applicable laws and regulations, including the prohibition against off-label promotion. Our promotion of the Axoguard products, which are regulated as medical devices, also must comply with FDA’s requirements, and must only use labeling that is consistent with the specific indication(s) for use included in the FDA substantial equivalence order that results in marketing the devices. The FDA does not restrict or regulate a physician’s use of a medical product within the practice of medicine, and we cannot prevent a physician from using our products for an off-label use. However, the FD&C Act and the FDA’s regulations restrict the kind of promotional communications that may be made about our products, and if the FDA determines that our promotional or training materials constitute the unlawful promotion of an off-label use, it could request that we modify training or promotional materials and/or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, civil money penalties, seizure, injunction or criminal fines, and penalties. Other federal, state, or foreign governmental authorities might also take action if they consider our promotion or training materials to constitute promotion of an uncleared or unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting
false claims for reimbursement, or exclusion from participation in federal health programs. In that event, our reputation could be damaged, and our products’ use in the marketplace could be impaired.
There may be increased risk of injury if physicians or others attempt to use our products off-label. Furthermore, the use of our product for indications other than those for which our products have been approved, cleared, or licensed by the FDA may not effectively treat the conditions not referenced in product indications, which could harm our reputation in the marketplace among physicians and patients. Physicians may also misuse our products or use improper techniques if they are not adequately trained in the particular use, potentially leading to injury and an increased risk of product liability litigation. Product liability claims are expensive to defend and could divert management’s attention from our primary business and result in substantial damage awards against us. Any of these events could harm our business, results of operations, financial condition, and prospects.
U.S. governmental regulation could restrict the use of our Avance Nerve Graft product, restrict our procurement of tissue or increase costs.
In addition to the FDA requirements for biological products, Avance Nerve Graft will continue to be subject to various requirements for human tissue under 21 CFR Part 1271. Human tissues intended for transplantation have been regulated by the FDA since 1993. In May 2005, three new comprehensive regulations went into effect that address manufacturing activities associated with HCT/P. The first regulation requires companies that produce and distribute HCT/Ps register with the FDA. The second regulation provides criteria that must be met for donors to be eligible to donate tissues and is referred to as the “Donor Eligibility” rule. The third regulation governs the processing and distribution of the tissues and is often referred to as the “Current Good Tissue Practices” rule. The Current Good Tissue Practices rule covers all stages of allograft processing, from procurement of tissue to distribution of final allografts. Together, the three basic requirements of 21 CFR Part 1271 are designed to ensure that sound, high quality practices are followed to reduce the risk of tissue contamination and of communicable disease transmission to recipients. These regulations increased regulatory scrutiny within the industry in which we operate and have led to increased enforcement actions, which affects the conduct of our business. In addition, guidance was issued by the FDA in November 2017 and revised in July 2020 on Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use, which could have potential implications on future HCT/P products being evaluated by us.
Additional regulations or guidance documents may be implemented by the FDA in the future. These changes may impose new documentation requirements, process changes or testing that could increase costs, and regulatory burden. See “Business — Government Regulations.” These regulations can also increase the cost of tissue recovery activities. Finally, Avance Nerve Graft is subject to certain state and local regulations, as well as compliance with the standards of the tissue bank industry’s accrediting organization, the AATB.
The procurement and transplantation of allograft nerve tissue is also subject to federal law pursuant to the National Organ Transplant Act (“NOTA”), a criminal statute that prohibits the purchase and sale of human organs used in human transplantation, including nerve and related tissue, for “valuable consideration.” NOTA only permits reasonable payments associated with the removal, transportation, processing, preservation, quality control, implantation, and storage of human nerve tissue. We make payments to certain of our clients and tissue banks for their services related to recovering allograft nerve and umbilical cord tissue on our behalf. If NOTA is interpreted or enforced in a manner that prevents us from receiving payment for services we render or prevents us from paying tissue banks or certain of our clients for the services they render for us, our business, results of operations, financial condition, and prospects could be materially and adversely affected.
We have engaged, through marketing employees, independent sales agents and sales representatives, in ongoing efforts designed to educate the medical community as to our products’ benefits, and we intend to continue our educational activities. Although we believe that NOTA permits payments in connection with these educational efforts as reasonable payments associated with the processing, transportation and implantation of our products, payments in connection with such education efforts are not exempt from NOTA’s restrictions and our inability to make such payments in connection with these education efforts may prevent us from paying our sales representatives and could adversely affect our business, results of operations, financial condition, and prospects. No federal agency or court has determined whether NOTA is, or will be, applicable to every allograft nerve tissue-based material that our processing technologies may generate. Assuming that NOTA applies to our processing of allograft nerve and umbilical cord tissue, we believe that we comply with NOTA, but there can be no assurance that more restrictive interpretations of, or amendments to, NOTA will not be adopted in the future, which would call into question one or more aspects of our method of operations.
Failure to obtain regulatory or other approvals from certain states in which we operate after BLA approval for Avance Nerve Graft could delay, hinder, or prevent commercialization of our products.
We are subject to regulations of state agencies which have statutes covering tissue banking. Regulations issued by Florida, New York, California, and Maryland, among other states, are particularly relevant to our business. Most states do not currently have tissue banking regulations. However, incidents of allograft related issues in the industry may stimulate the development of regulation in other states. It is possible that third parties may make allegations against us or against donor recovery groups or tissue banks about non-compliance with applicable FDA regulations or other relevant statutes or regulations. Allegations like these could cause regulators or other authorities to take investigative or other action or could cause negative publicity for our business and the industry in which we operate.
As a result of our BLA approval for Avance Nerve Graft, we must obtain separate regulatory approvals and comply with numerous and varying state regulatory and licensing requirements. The approval procedures vary among states and can involve additional testing, supplying additional information, or filing state specific applications. The time required to obtain state approval may differ from that required to obtain FDA approval. The state approval processes may include all of the risks associated with obtaining FDA approval and additional risks associated with requirements particular to those states where we will seek approval to manufacture and/or distribute our products. We may not obtain state approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by state authorities, and approval by one state authority does not ensure approval by authorities in other states. The failure to obtain these approvals could materially adversely affect our business, financial condition and results of operations.
Finally, state regulatory expectations in are subject to constant change. There can be no assurance that we can meet the requirements of future regulations and guidance or that compliance with current regulations and guidance assures future capability to distribute and sell our products.
BLA approval for Avance Nerve Graft could result in different protocol to hospitals access for the product, as well as different reimbursement protocols, both of which may negatively affect surgeons’ access to, revenues derived from and profitability of Avance Nerve Graft.
BLA approval for Avance Nerve Graft could result in a different characterization of the product which could result in different protocols for hospital access of the product. Avance Nerve Graft may be subject to new or additional pharmacy protocols of hospitals even though our products have had access to such hospitals for an extended period. If this occurs, the availability of Avance Nerve Graft may be subject to the discretion of pharmacy leadership who are subject to pricing pressures and other cost-containment measures in deciding whether to stock Avance Nerve Graft in their pharmacies. In addition, a different characterization of Avance Nerve Graft after BLA approval may result in different reimbursement protocols which could reduce the amount of approved reimbursement for our products, deny coverage altogether, or impose new requirements to justify our prices. Any such result could reduce a surgeon’s access to Avance Nerve Graft which would have an adverse impact on the revenues and profitability derived from Avance Nerve Graft.
Our Axoguard products are subject to FDA and international regulatory requirements.
Our Axoguard Product Line is regulated as a medical device in the U.S. and international countries where we market Axoguard products. In the U.S., the Axoguard Product Line is regulated under the FD&C Act and subject to pre-market notification and clearance requirements under section 510(k) of the FD&C Act, 21 CFR Part 820 QSR and other FDA regulations. In the rest of the world, each region (such as the E.U.) or country has their independent international regulations such as the Medical Device Regulations (CE Mark) in Europe, UK MHRA, and Taiwan Pharmaceutical Affairs Act.
We distribute Axoguard Nerve Connector and Axoguard Nerve Protector products for Evergen, and Evergen is responsible for the regulatory compliance of these products. In the U.S., Evergen has obtained a 510(k) pre-market clearance for Axoguard Nerve Connector from the FDA for porcine (pig) small intestine submucosa for the repair of peripheral nerve transections where gap closure can be achieved by flexion of the extremity. Evergen has also obtained a 510(k) pre-market clearance for Axoguard Nerve Protector for the repair of peripheral nerve damage in which there is no gap or where a gap closure is achieved by flexion of the extremity. In countries where Axoguard is marketed, Evergen has obtained regulatory clearance with the same indications except for Europe and the UK. For the CE Mark, the Axoguard Nerve Protector indication is the same; however, for Axoguard Nerve Connector, the indication is more specific - “The Axoguard Nerve Connector is indicated for the repair of peripheral nerve discontinuities with gaps up to 5 mm.”
We are the authorization holder of the Axoguard Nerve Cap and HA+. We have obtained 510(k) pre-market clearance for Axoguard Nerve Cap, indicated to protect a peripheral nerve end and to separate the nerve from surrounding environment to reduce the development of symptomatic or painful neuroma. We have obtained two 510(k) pre-market clearances for HA+. The first 510(k) K223640 was cleared on April 7, 2023, indicated for the management and protection of peripheral nerve injuries where there is no gap. The second 510(k) K231708 was cleared on October 12, 2023, expanding the indication to the management and protection of peripheral nerve injuries where there is no gap, or following closure of the gap.
If we or Evergen fail to comply with applicable regulatory requirements, the regulatory bodies in each country could deny or withdraw regulatory clearance/approval for the Axoguard products, or impose civil penalties, including fines, product seizures or product recalls and, in certain cases, criminal sanctions.
Our operations must comply with FDA and other governmental requirements.
Our operations require us to comply with the FDA’s and other governmental authorities’ laws and regulations on topics including the manufacture and production and sales and marketing of medical products, and compliance efforts related to such laws is costly, and failure to comply could subject us to enforcement action. See “Business — Government Regulations — Education Grants, U.S. Anti-kickback, False Claims and Other Healthcare Fraud and Abuse Laws — Fraud, Abuse and False Claims." Enforcement actions could impair our ability to produce products in a cost-effective and timely manner to meet customer demands. We may also be required to bear other costs or take other actions that may have an adverse impact on our future revenue and our ability to generate profits. Furthermore, our key material suppliers, licensors and or other contractors may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce products on a timely basis and in the required quantities, if at all.
Healthcare providers and facilities, and third-party payors, often play a primary role in the recommendation and prescription of any currently marketed products and product candidates for which we may obtain marketing approval. Our current and future arrangements with healthcare providers and facilities, third-party payors and customers, and our sales, marketing, and educational activities, may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations (at the federal and state level) that may constrain our business or financial arrangements and relationships through which we market, sell, and distribute our products for which we obtain marketing approval. In addition, our operations are also subject to various federal and state fraud and abuse, and payment transparency laws and regulations.
Payments made to physicians and other healthcare providers, and other financial interests, have been the subject of a range of federal and state laws. The federal physician payment transparency requirements, sometimes referred to as the Physician Payments Sunshine Act ("the Sunshine Act"), was created under the Affordable Care Act. The Sunshine Act, among other things, imposes reporting requirements on drug manufacturers for payments or other transfers of value made by them to physicians and teaching hospitals, as well as ownership and investment interests held by physicians, other healthcare providers, including physician assistants, nurse practitioners, and other mid-level healthcare practitioners, and their immediate family members. Reporting relative to these mid-level practitioners began in 2022 for payments or other transfers of value in 2021, which could increase the likelihood of a mistake in submission or failure to submit the required information by that group. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an additional aggregate of $1 million per year for “knowing failures,” for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission. Additionally, certain states also mandate implementation of compliance programs, impose restrictions on marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to physicians and other HCPs.
In addition to the federal fraud, waste, and abuse laws noted, there are analogous state laws and regulations, such as state anti-kickback and false claims laws, and other state laws addressing the medical product and healthcare industries, which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and in some cases may apply regardless of payor, i.e., even if reimbursement is not available. Some state laws require pharmaceutical or device companies to comply with the industry's voluntary compliance guidelines (the PhRMA Code and AdvaMed Code) and the relevant compliance program guidance promulgated by the OIG in addition to other requirements, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Finally, regulatory expectations in the U.S. are subject to constant change. There can be no assurance that we can meet the requirements of future regulations and guidance or that compliance with current regulations and guidance assures future capability to distribute and sell our products.
Our business is subject to continuing compliance with standards set by various accreditation and registration bodies, which is costly, and loss of accreditation or registration could result in negative effects on our business.
We are subject to accreditation such as that by the AATB and as a National Association of Boards of Pharmacy accredited drug distributor. We have registration requirements such as that with ISO 13485 registration bodies. These accreditations and registration standards can affect distribution and sale of our products on a state-by-state basis, within the U.S. and also affects distribution and sale of our products outside of the U.S. The loss of accreditation or registration could keep us from selling and distributing our products, which may have negative effects on our business, results of operations, financial condition, and prospects.
Defective products could lead to recall or other negative business conditions.
If our products are defective or otherwise pose safety risks, the FDA could require their recall, or we may initiate a voluntary recall of our products. The FDA may require recall of a marketed medical device product, such as the Axoguard products, in the event that it determines the medical device presents a reasonable probability of serious adverse health consequences or death. However, most device recalls do not rise to this level of health significance and result from voluntary action. The FDA has authority to recall biological products when a batch, lot or other quantity of the product presents an imminent or substantial hazard to the public health. However, in such circumstances, the FDA usually initially requests voluntary recalls of biological products, such as the Avance Nerve Graft. If a company does not comply with an FDA request for a recall, the FDA can order one under the above-referenced circumstances or take other enforcement actions, such as product seizure. In addition, manufacturers may, on their own initiative, recall a product to remove or correct a deficiency or to remedy a violation of the FD&C Act that may pose a risk to health. A government-mandated, government-requested, or voluntary recall could occur as a result of an unacceptable risk to health, reports of safety issues, failures, manufacturing errors, design or labeling defects or other deficiencies, and issues. Recalls and other field corrections for any of our products would divert managerial and financial resources and have an adverse effect on our business, results of operations, financial condition, and prospects. A recall could adversely impact our reputation with customers and our sales. If the FDA were to disagree with our internal determinations and decision making relative to potential recalls (including corrections and removal), we could be subject to further regulatory or enforcement action against.
If our products cause or contribute to a death, a serious injury, or any adverse reaction involving a communicable disease, or malfunction in certain ways, we will be subject to reporting regulations, which can result in voluntary corrective actions or agency enforcement actions. See “Business — Regulation — Education Grants, U.S. Anti-kickback, False Claims and Other Healthcare Fraud and Abuse Laws.” If we fail to report these events to the FDA within the required timeframes, or at all, the FDA could take regulatory or enforcement action against us. Any adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall, or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, would require the dedication of time and capital, distract management from operating our business, and may adversely impact our reputation, business, results of operations, financial condition, and prospects.
Clinical trials can be long and expensive, and results are ultimately uncertain.
We were required to perform a clinical trial for our Avance Nerve Graft under FDA’s statutory requirements to obtain approval of a BLA for the product. This trial is subject to FDA approval and there is a risk that the FDA may not agree that the data supports the conclusions of the study which could jeopardize our ability to obtain regulatory approval and continue to market our Avance Nerve Graft product.
The results of pre-clinical studies do not necessarily predict future clinical trial results and predecessor clinical trial results may not be repeated in subsequent clinical trials. Additionally, the FDA may disagree with our interpretation of the data from our pre-clinical studies and clinical trials and may require the company to pursue additional pre-clinical studies or clinical trials, or not approve our BLA. If we are unable to demonstrate the safety, purity and potency of our product through our clinical trials, we will be unable to obtain regulatory approval to market the Avance Nerve Graft, and we will not be able to continue to provide it.
We completed the rolling BLA submission for the Avance Nerve Graft in September 2024. The FDA could take negative action on the BLA or could approve the BLA but restrict the Avance Nerve Graft labeling if the FDA does not agree that the data is sufficiently supportive of the application. Restrictions to our labeling could have an adverse effect on Avance Nerve Graft commercialization.
We rely on third parties to conduct our clinical trials, and they may not perform as contractually required or expected.
We rely on third parties, such as contract research organizations (“CROs”), medical institutions, clinical investigators, and contract laboratories to conduct our clinical trials and certain nonclinical studies. We and our CROs are required to comply with all applicable regulations governing clinical research, including Good Clinical Practices. The FDA enforces these regulations through periodic inspections of trial sponsors, principal investigators, CROs and trial sites. If we or our CROs fail to comply with applicable FDA regulations, the data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving our applications. We cannot be certain that, upon inspection, the FDA and similar foreign regulatory authorities will determine that our clinical trial complies or complied with clinical trial regulations, including GCP. In addition, our clinical trial must be conducted with product produced under applicable GCP regulations. Failure to comply with the clinical trial regulations, including GCP, may require us to repeat clinical trials, which would delay the regulatory approval process. Further, if these third parties do not successfully carry out their contractual duties or regulatory
obligations or meet expected deadlines, need to be replaced, or the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our non-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we would not be able to obtain regulatory approval for our products on a timely basis, if at all, and our business, results of operations, financial condition, and prospects would be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
Healthcare law and policy changes may have a material adverse effect on us.
In the U.S. there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities, and affect our ability, or the ability of our collaborators, to profitably sell any products for which we obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we, or our collaborators, may receive for any approved products.
In the future, there may continue to be additional proposals relating to the reform of the U.S. healthcare system. Future legislation, federal agency regulations and Presidential Executive Orders may impact the healthcare system in ways important to our business. Adoption of certain proposals could limit the prices we are able to charge for our products or the amounts of reimbursement available for our products and could also limit the acceptance and availability of our products. The adoption of some or all of these proposals could have a material adverse effect on our business, results of operations, financial condition, and prospects.
Additionally, initiatives sponsored by government agencies, legislative bodies, and the private sector in the U.S. and elsewhere to limit the growth of healthcare costs, especially for drugs and biologics, including price regulation and policies regarding generic drugs and biosimilars, are ongoing in markets where we do business. For example, on August 16, 2022, the Inflation Reduction Act of 2022 (“IRA”) was signed into law. The IRA includes several provisions to lower prescription drug costs for people with Medicare and reduce drug spending by the federal government, including allowing Medicare to negotiate prices for certain prescription drugs, requiring drug manufacturers to pay a rebate to the federal government if prices for single-source drugs and biologics covered under Medicare Part B and nearly all covered drugs under Part D increase faster than the rate of inflation based on the consumer price index for all urban consumers, and limiting out of pocket spending for Medicare Part D enrollees. Implementation of the drug price negotiation provisions of the IRA began in 2023 and will continue to be implemented over the next several years. Multiple pharmaceutical manufacturers have challenged the law in court, largely on constitutional grounds. These suits will continue through 2024, and the ultimate effects of such legal challenges are unclear. At this time, we continue to evaluate the effect of the IRA on our business operations and financial condition and results as the full impact of the IRA remains uncertain. Additionally, on October 14, 2022, President Biden signed Executive Order 14087 on “Lowering Prescription Drug Costs for Americans.” The Executive Order specifically requests that the Center for Medicare and Medicaid Innovation consider “models that may lead to lower cost sharing for commonly used drugs and support value-based payment that supports high-quality care.” Continued government efforts to lower healthcare costs would affect our market materially. We could experience an adverse impact on operating results due to increased pricing pressure in the U.S. and in other markets. Governments, hospitals, pharmacy benefit managers, and other third-party payors could reduce the amount of approved reimbursement for our products, deny coverage altogether, or impose new requirements on manufacturers to justify their prices. Reductions in reimbursement levels or coverage or other cost-containment measures could unfavorably affect our future operating results.
We could be subject to civil or criminal penalties if we are found to have violated laws protecting the confidentiality of health information, which could increase our liabilities and harm our reputation or our business.
There are a number of federal and state laws protecting the confidentiality of certain health information and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated privacy rules under the Health Insurance Portability and Accountability Act (“HIPAA”). These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. If we are found to be in violation of the privacy rules under HIPAA, we could be subject to civil or criminal penalties, which could increase our liabilities, harm our reputation, and have a material adverse effect on our business, results of operations, financial condition, and prospects.
Risks Related to Our IP
Failure to protect or maintain our IP rights could result in costly and time-consuming litigation and our loss of any potential competitive advantage.
Our success will depend, to a large extent, on our ability to successfully obtain and maintain patents, prevent misappropriation or infringement of IP, maintain trade secret protection, and conduct operations without violating or infringing on the IP rights of third parties. See “Business — Intellectual Property.” There can be no assurance that our patented and patent-pending technologies will provide us with a competitive advantage, that we will be able to develop or acquire additional technology that is patentable, or that third parties will not develop and offer technologies which are similar to ours. Moreover, we can provide no assurance that confidentiality agreements with our employees, consultants and other parties, agreements to protect trade secrets or similar agreements intended to protect unpatented technology or prevent unauthorized use, disclosure, or misappropriation will not be breached by those third parties. IP litigation is extremely expensive and time-consuming, and it is often difficult to predict the outcome of such litigation. A failure by us to protect our IP, or a breach by third parties of agreements aimed at protecting our IP, could have a materially adverse effect on our business, results of operations, financial condition, and prospects.
Future protection for our proprietary rights is uncertain and may impact our ability to successfully compete in our industry.
The degree of future protection for our proprietary rights is uncertain. We cannot ensure that:
•We, or our licensors, were the first to make the inventions covered by each of our patents;
•We, or our licensors, were the first to file patent applications for these inventions;
•Others will not independently develop similar or alternative technologies or duplicate any of our technologies;
•Any of our pending patent applications will result in issued patents;
•Any of our issued patents or those of our licensors are valid and enforceable;
•Any patents issued to us, or our collaborators will provide any competitive advantages or will not be challenged by third parties;
•We will develop additional proprietary technologies that are patentable;
•The patents of others will not have a material adverse effect on our business rights; or
•The measures we rely on to protect our IP underlying our products are adequate to prevent third parties from using, disclosing, or misappropriating that IP, all of which could harm our ability to compete in the market.
Our commercial success depends in part on our ability and the ability of our collaborators and licensors to avoid infringing patents and proprietary rights of third parties, which could expose us or our collaborators and licensors to litigation or commercially unfavorable licensing arrangements. Third parties may accuse us or collaborators and licensors of employing their proprietary technology without authorization in our products, or in the materials or processes used to make our products. Any legal action against our collaborators, licensors or those claiming damages and/or seeking to enjoin our commercial activities relating to the affected products, materials and processes could, in addition to subjecting us to potential liability for damages, require us or our collaborators and licensors to obtain a license to continue to utilize the affected materials or processes or to manufacture or market the affected products. We cannot predict whether we or our collaborators and licensors would prevail in any of these actions or whether any license required under any of these patents would be made available on commercially reasonable terms, if at all. If we were unable to obtain such a license, we and our collaborators and licensors may be unable to continue to utilize the affected materials or processes, or manufacture or market the affected products, or we may be obligated by a court to pay substantial royalties and/or other damages to the patent holder. Even if we were able to obtain such a license, the terms of such a license could substantially reduce the commercial value of the affected product or products and impair our prospects for profitability. Accordingly, we cannot predict whether, or to what extent, the commercial value of the affected product or products or our prospects for profitability may be harmed as a result of any of the liabilities discussed above. Furthermore, infringement and other IP claims, with or without merit, can be expensive and time-consuming to litigate and can divert management’s attention from our core business. We and our collaborators and licensors may be unable to obtain and enforce IP rights to adequately protect our products and related IP, which could materially and adversely impact our business, results of operations, financial condition, or prospects.
The patent protection for our products may expire before we are able to maximize their commercial value, which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue.
The patents for our commercialized products and products in development have varying expiration dates and, when these patents expire, we may be subject to increased competition, and we may not be able to recover our development costs. For example, the material U.S. patents covering the formulations used in our Axoguard Product Line, which are held by Evergen, have expired. Expiration of these patents could adversely affect our ability to successfully execute our business strategy to maximize the value of Axoguard products and could materially and adversely impact our business, results of operations, financial condition, and prospects.
Others may claim an ownership interest in our IP or claim that we infringe on their IP rights, which could expose us to litigation and have a significant adverse effect on our prospects.
A third party may claim an ownership interest in one or more of our patents or other IP. While we believe we own the right, title, and interest in the patents for which we or our licensors have applied and our other IP (including that which is licensed from third parties) and is presently unaware of any claims or assertions by third parties with respect to our patents or IP, we cannot guarantee that a third party will not assert a claim or an interest in any of such patents or IP.
Also, a third party may bring legal actions against us claiming we infringed their IP rights and seek monetary damages and/or enjoin clinical testing, manufacturing, and marketing of the affected product or products. There are many issued patents and pending patent applications in the U.S. and in other jurisdictions, owned by third parties, potentially covering various medical devices and biological products. There may be patents owned by third parties that we are currently unaware of, with issued claims that cover one or more of our current or future products or use or manufacture of those products. Since patents may take many years to issue, there may be pending patent applications owned by third parties that may lead to issued claims that cover one or more of our current or future products or use or manufacture of those products.
If we become involved in any litigation, it could consume a substantial portion of our resources and cause a significant diversion of effort by our technical and management personnel. If any of these actions were successful, in addition to any potential liability for damages, we could be required to obtain a license to continue to manufacture or market the affected product, in which case we may be required to pay substantial royalties or grant cross-licenses to our patents. We cannot, however, assure that any such license will be available on acceptable terms, if at all. Ultimately, we could be prevented from commercializing a product or be forced to cease some aspect of our business operations as a result of claims of patent infringement or violation of other IP rights, which could have a material and adverse effect on our business, results of operations, financial condition, and prospects. Further, the outcome of IP litigation is subject to uncertainties that cannot be adequately quantified in advance, including the demeanor and credibility of witnesses and the identity of the adverse party. This is especially true in IP cases that may turn on the testimony of experts as to technical facts or the scope or meaning of patent claims upon which experts may reasonably disagree.
Our trademarks are valuable, and our business may be adversely affected if trademarks are not adequately protected.
In the U.S. and other countries, we currently hold trademark registrations and have trademark applications pending, any of which may be the subject of a governmental or third-party objection, which could prevent the maintenance or issuance of the same. As our products mature, our reliance on our trademarks to protect our brand, increase our name recognition and, in part, differentiate us from our competitors increases. As a result, if our trademark applications are not successful and if we are unable to prevent third parties from adopting, registering, or using trademarks, including trade dress, that infringe, dilute, or otherwise violate our trademark rights, our business, results of operations, financial condition, and prospects could be materially adversely affected.
Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
New legislation or court precedent on patent law in the U.S. and in other jurisdictions may increase the uncertainties and costs for us to obtain and enforce patent claims broad enough to exclude others from making, using, or selling our current and future products. These changes in the patent law may also increase the uncertainties associated with the potential third party patent infringement claims against our current and future products. Depending on decisions by the U.S. Congress, the U.S. federal courts, the USPTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways to weaken our ability to obtain and enforce patent rights relevant to our products, and/or our ability to defend our business against third party infringement claims in the future.
Risks Related to Financing Our Business
Our credit facility and payment obligations under the Revenue Participation Agreement with Oberland Capital contain operating and financial covenants that restrict our business and financing activities, require cash payments over an
extended period of time and are subject to acceleration in specified circumstances, which may result in Oberland Capital taking possession and disposing of any collateral.
Our credit facility with Oberland Capital contains restrictions that limit our flexibility in operating our business. Under the terms of the credit facility, we must maintain, and cause our subsidiaries to maintain, certain covenants, including with respect to limitations on new indebtedness, restrictions on the payment of dividends and maintenance of revenue levels. Our credit facility is collateralized by all of our assets including, among other things, our IP.
If we breach certain of our debt covenants and are unable to cure such breach, revert to the provided liquidity covenant or are not granted waivers in relation to such breach, it may constitute an event of default under the credit facility, giving Oberland Capital the right to require us to repay the then-outstanding debt immediately. If we are unable to pay the outstanding debt immediately, Oberland Capital could, among other things, foreclose on the collateral granted to them to collateralize such indebtedness. A breach of the covenants contained in the credit facility documents and the acceleration of its repayment obligations by Oberland Capital could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In connection with the credit facility, we entered into a Revenue Participation Agreement (“RPA”) with Oberland Capital. Pursuant to the RPA, we agreed to pay an additional quarterly royalty payment as a percentage of our net revenue, up to $70 million in any given fiscal year, subject to certain limitations set forth therein, during the period commencing on the later of (i) April 1, 2021 and (ii) the date of funding of a loan under the credit facility and ending on the date upon which all amounts owed under the Term Loan Agreement have been paid in full. Payments commenced on September 30, 2021, with the royalty structure resulting in approximately 1.5% per year of additional payments on the outstanding principal amount of the loans.
The credit facility and RPA could have important negative consequences to the holders of our securities. For example, a portion of our cash flow from operations will be needed to make payments to Oberland Capital and will not be available to fund future operations. Additionally, we may have increased vulnerability to adverse general economic and industry conditions. Payment requirements under the credit facility and RPA will increase our cash outflows. Additionally, the credit facility and RPA contain complex provisions, which, if interpreted differently, could materially increase the amount of the payments due to Oberland Capital. Our future operating performance is subject to market conditions and business factors that are beyond our control. If our cash inflows and capital resources are insufficient to allow us to make required payments, we may have to reduce or delay capital expenditures, sell assets, or seek additional capital. If we raise funds by selling additional equity, such sale would result in dilution to our shareholders. There is no assurance that if we are required to secure funding, we can do so on terms acceptable to us, or at all.
We may need to raise additional funds to finance our future capital or operating needs, which could have adverse impacts on our business, results of operations and the interests of our shareholders.
We may need to seek to raise funds through the issuance of public or private debt or the sale of equity to achieve our business strategy. If we raise funds, this could dilute the interests of our shareholders. Moreover, the availability of additional capital, whether debt or equity from private capital sources (including banks) or the public capital markets, fluctuates as our financial condition and industry or market conditions in general change. There may be times when the private capital markets and the public debt or equity markets lack sufficient liquidity or when our securities cannot be sold at attractive prices, in which case we would not be able to access capital from these sources on favorable terms, if at all. We can give no assurance as to the terms or availability of additional capital.
Risks Related to Our Common Stock
An active trading market in our common stock may not be maintained.
The trading market in our common stock has been volatile. The quotation of our common stock on The Nasdaq Capital Market does not assure that a meaningful, consistent, and liquid trading market will exist. We cannot predict whether an active market for our common stock will be maintained in the future. An absence of an active trading market could adversely affect our shareholders’ ability to sell our common stock at current market prices in short time periods, or possibly at all. Additionally, market visibility for our common stock may be limited and such lack of visibility may have a depressive effect on the market price for our common stock. As of December 31, 2024, approximately 34.3% of our outstanding shares of common stock were held by our officers, directors, beneficial owners of 5% or more of our securities and their respective affiliates, which adversely affects the liquidity of the trading market for our common stock, in as much as federal securities laws restrict sales of our shares by these shareholders. If our affiliates continue to hold their shares of common stock, there will be limited trading volume in our common stock, which may make it more difficult for investors to sell their shares or increase the volatility of our stock price.
The price of our common stock could be volatile due to a number of factors, which could lead to losses by investors and costly securities litigation.
Our common stock is listed on The Nasdaq Capital Market under the symbol “AXGN.” The stock market in general, and the market for medical technology companies in particular, have experienced and could in the future experience volatility that has often been unrelated to the operating performance of particular companies. The trading price of our common stock has experienced volatility and is likely to continue to be volatile in response to a number of factors including, without limitation, the following:
•Fluctuations in price and volume due to investor speculation, including short sales, social media speculation and other factors that may not be tied to our financial performance;
•Our performance in the execution of our business plan;
•Financial viability;
•Actual or anticipated variations in our operating results;
•Announcements of developments by us or our competitors;
•Market conditions in our industry;
•Announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
•Adoption of new accounting standards affecting our industry;
•Additions or departures of key personnel;
•Introduction of new products by us or our competitors;
•Sales of our common stock or other securities in the open market;
•Regulatory developments in both the U.S. and foreign countries;
•Performance of products sold and advertised by licensees in the marketplace;
•Economic and other external factors;
•Period-to-period fluctuations in financial results; and
•Other events or factors, including the other factors described in this “Risk Factors” section, many of which are beyond our control.
The stock market is subject to significant price and volume fluctuations. Such fluctuations have and could expose us to securities class action litigation, which could adversely impact our business, results of operations, financial condition, and prospects.
We may fail to meet our publicly announced guidance or other expectations about our business and future operating results, which could cause a decline in our stock price.
We provide financial guidance about our business and future operating results. In developing this guidance, our management makes certain assumptions and judgments about our future operating performance, including projected hiring of sales professionals, continued increase of our market share, and continued stability of the macro-economic environment in our key markets. Furthermore, analysts and investors may develop and publish their own projections of our business, which may form a consensus about our future performance. Our business results may vary significantly from such guidance or that consensus due to a number of factors, many of which are outside of our control, and which could adversely affect our operations and operating results. Furthermore, if we make downward revisions of our previously announced guidance, or if our publicly announced guidance of future operating results fails to meet expectations of securities analysts, investors, or other interested parties, the market price of our common stock could decline.
We do not anticipate paying any cash dividends in the foreseeable future.
The operation and expansion of our business will continue to require funding. We do not anticipate that we will pay any cash dividends on our common stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and will depend upon results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law, and other factors our board of directors deems relevant. Accordingly, if any investor purchases shares of common stock, realization of a gain on such investment will depend on the appreciation of the price of our common stock, which may never occur.
Anti-takeover provisions in Minnesota law may deter acquisition bids for us that you might consider favorable.
We are governed by the provisions of Sections 302A.671, 302A.673 and 302A.675 of the Minnesota Business Corporation Act (the “MBCA”). These provisions may discourage a negotiated acquisition or unsolicited takeover of us and deprive our shareholders of an opportunity to sell their common stock at a premium over the market price.
In general, Section 302A.671 of the MBCA provides that a corporation’s shares acquired in a control share acquisition have no voting rights unless voting rights are approved in a prescribed manner. A “control share acquisition” is a direct or indirect acquisition of beneficial ownership of shares that would, when added to all other shares beneficially owned by the acquiring person, entitle the acquiring person to have voting power of 20% or more in the election of directors.
In general, Section 302A.673 of the MBCA prohibits a public Minnesota corporation from engaging in a business combination with an interested shareholder for a period of four years after the date of the transaction in which the person became an interested shareholder, unless the business combination is approved in a prescribed manner. The term “business combination” includes mergers, asset sales, and other transactions resulting in a financial benefit to the interested shareholder. An “interested shareholder” is a person who is the beneficial owner, directly or indirectly, of 10% or more of a corporation’s voting stock or who is an affiliate or associate of the corporation, and who, at any time within four years before the date in question, was the beneficial owner, directly or indirectly, of 10% or more of the corporation’s voting stock. Section 302A.673 does not apply if a committee of our Board of Directors consisting of all of its disinterested directors (excluding current and former officers) approves the proposed transaction or the interested shareholder’s acquisition of shares before the interested shareholder becomes an interested shareholder.
If a tender offer is made for our common stock, Section 302A.675 of the MBCA precludes the offeror from acquiring additional shares of stock (including in acquisitions pursuant to mergers, consolidations, or statutory share exchanges) within two years following the completion of the tender offer, unless shareholders selling their shares in the later acquisition are given the opportunity to sell their shares on terms that are substantially the same as those contained in the earlier tender offer. Section 302A.675 does not apply if a committee of our Board of Directors consisting of all of its disinterested directors (excluding its current and former officers) approves the proposed acquisition before any shares are acquired pursuant to the earlier tender offer.
We are subject to legal proceedings from time to time. Legal proceedings, if decided adversely to or settled by us, and not covered by insurance, could result in liability material to our financial condition, results of operations or cash flows. Likewise, regardless of outcome, legal proceedings could result in substantial costs and expenses, affect the availability or cost of some of our insurance coverage and significantly divert the attention of our management. There can be no assurance that we will be able to prevail in, or achieve a favorable settlement of, any pending or future legal proceedings to which we become subject. Even claims without merit could subject us to adverse publicity and require us to incur significant legal fees.
Our management has broad discretion in the use and placement of our cash and cash equivalents and, despite management’s efforts, cash and cash equivalents may be used in a manner that does not increase the value of shareholders’ investments or placed in otherwise reputable financial institutions that fail.
Our management has broad discretion in the use and placement of our cash and cash equivalents, and investors must rely on the judgment of management regarding the use and placement of such cash and cash equivalents. Management may invest our cash and cash equivalents in short-term or long-term, investment-grade, interest-bearing securities. These investments may not yield favorable returns to shareholders. If we do not invest or apply our cash and cash equivalents in ways that enhance shareholder value, we may fail to achieve expected financial results, which could cause our stock price to decline. Furthermore, the most reputable financial institutions may fail. Despite the judgment of management regarding the placement of cash and cash equivalents in deemed reputable financial institutions, events outside of our control could occur, the result of which could result in us not having access to our cash and cash equivalents.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
Cybersecurity represents an important component of the Company’s overall approach to risk management. The Company’s cybersecurity policies, standards and practices are integrated into the Company’s enterprise risk management approach, and cybersecurity risks are one of the enterprise risks that are subject to oversight by the Company’s Board of Directors (the “Board”). The Company's cybersecurity standards and practices follow industry trends, which align with frameworks established by the Center for Internet Security. The Company approaches cybersecurity threats through a cross-functional approach which endeavors to: (i) identify, prevent and mitigate cybersecurity threats to the Company; (ii) preserve the
confidentiality, security and availability of the information that we collect and store to use in our business; (iii) protecting the Company’s IP; (iv) maintaining the confidence of our customers, clients and business partners; and (v) providing appropriate public disclosure of cybersecurity risks and incidents when required.
Risk Management and Strategy
The Company’s cybersecurity program focuses on the following areas:
a.Vigilance: The Company maintains cybersecurity threat operations with the goal of identifying, preventing and mitigating cybersecurity threats and responding to cybersecurity incidents in accordance with our established incident response and recovery plans.
b.Systems Safeguards: The Company deploys system safeguards that are designed to protect the Company’s information systems from cybersecurity threats, including firewalls, intrusion prevention and detection systems, anti-malware functionality and access controls, which are evaluated and improved through ongoing vulnerability assessments and cybersecurity threat intelligence.
c.Collaboration: The Company utilizes collaboration mechanisms established with public and private entities, including intelligence and enforcement agencies, industry groups and third-party service providers, to identify, assess and respond to cybersecurity risks.
d.Third-Party Risk Management: The Company endeavors to identify and oversee cybersecurity risks presented by third parties, as well as the systems of third parties that could adversely impact our business in the event of a cybersecurity incident affecting those third-party systems.
e.Training: The Company provides periodic training for personnel regarding cybersecurity threats, which reinforces the Company’s information security policies, standards and practices.
f.Incident Response and Recovery Planning: The Company has established and maintains incident response and recovery plans that address the Company’s response to a cybersecurity incident and the recovery from a cybersecurity incident, and such plans are tested and evaluated periodically.
g.Communication, Coordination and Disclosure: The Company utilizes a cross-functional approach to address the risk from cybersecurity threats, involving management personnel from the Company’s technology, operations, legal, risk management and other key business functions, as well as the members of the Board and the Audit Committee of the Board in an ongoing dialogue regarding cybersecurity threats and incidents, while also implementing controls and procedures for the escalation of cybersecurity incidents pursuant to established thresholds so that decisions regarding the disclosure and reporting of such incidents can be made by management in a timely manner.
h.Governance: The Board’s oversight over the effectiveness of the Company's cybersecurity risk management is supported by the Audit Committee, which regularly interacts with the Company’s Vice President of Information Technology, Security and Business Intelligence and other members of the cyber team and management.
The Company manages risks from cybersecurity threats through the assessment and testing of the Company’s processes and practices focused on evaluating the effectiveness of our cybersecurity measures. The Company engages third parties as appropriate to perform assessments of our cybersecurity measures. The results of such assessments and reviews are reported to the Audit Committee and the Board, and the Company adjusts its cybersecurity policies, standards, processes and practices as necessary based on the information provided by the assessments, audits and reviews.
Governance
The Board, in coordination with the Audit Committee, oversees the effectiveness of management of risks from cybersecurity threats, including the policies, standards, processes and practices that the Company’s management implements to address risks from cybersecurity threats. The Board and the Audit Committee each receive regular presentations and reports on cybersecurity risks, which address a wide range of topics including, for example, recent developments, evolving standards, vulnerability assessments, third-party reviews, the threat environment, technological trends and information security considerations arising with respect to the Company’s peers. The Board and the Audit Committee also receive prompt and timely information regarding any cybersecurity incident that meets established reporting thresholds, as well as ongoing updates regarding such incident until it has been addressed. On a regular basis, the Board and the Audit Committee discuss the Company’s approach to cybersecurity risk management with the Company’s cyber team and senior leadership team.
The Company’s Vice President of Information Technology, Security and Business Intelligence is the member of the Company’s management that is principally responsible for overseeing the Company’s cybersecurity risk management program, in partnership with other business leaders across the Company. The Vice President of Information Technology, Security and Business Intelligence works in coordination with senior leadership, which includes our Chief Executive Officer, Chief Financial Officer, and General Counsel. The Company’s Vice President of Information Technology, Security and Business Intelligence has served in various roles in information technology and information security for over 18 years, including Biogen, AstraZeneca, Iron Mountain, and consulting roles at Charles River Labs, Sunovion Pharmaceuticals, Agero and DentaQuest. The Vice President of Information Technology, Security and Business Intelligence holds a master's degree in business administration from Boston University, a master’s degree in electrical and computer engineering from Utah State University, and a bachelor’s degree in electronics engineering from Mumbai University. The Company’s Information Technology team has specific professional certifications and has over 10 years of experience with managing risks arising from cybersecurity threats.
The Company’s Vice President of Information Technology, Security and Business Intelligence, in coordination with senior leadership, works collaboratively across the Company to implement a program designed to protect the Company’s information systems from cybersecurity threats and to promptly respond to any cybersecurity incidents. To facilitate the success of this program, multidisciplinary teams throughout the Company are deployed to address cybersecurity threats and to respond to cybersecurity incidents in accordance with the Company’s policy as it relates to the incident, management response and recovery plan. Through the ongoing communications from these teams, the Vice President of Information Technology, Security and Business Intelligence and senior leadership monitor the prevention, detection, mitigation and remediation of cybersecurity incidents in real time, and report such incidents to the Audit Committee when appropriate.
Cybersecurity threats, resulting from any previous cybersecurity incidents, have not materially affected or are not reasonably likely to affect the Company, including its business strategy, results of operations, or financial condition.
ITEM 2. PROPERTIES
| | | | | | | | | | | | | | |
| Our material physical properties consisted of the following as of December 31, 2024: | |
| | | | |
| Location | General Character | Total Square Feet | Square Feet Utilized | Expiration |
| | | | |
Alachua, Florida (1) | Headquarters - General office, warehousing and distribution | 19,000 | 19,000 | October 31, 2026 |
Tampa, Florida (1) | Headquarters - General office, medical laboratory, and meeting space | 75,000 | 50,000 | October 31, 2034 |
Burleson, Texas (1) | Facility - Raw material and finished goods warehousing and distribution | 15,000 | 15,000 | April 30, 2027 |
| 10,000 | 5,000 | September 30, 2027 |
Vandalia, Ohio (2) | Facility - Clean-room, manufacturing, warehousing, and office space | 107,000 | 84,000 | N/A |
Dayton, Ohio (3)(4) | Facility - Clean-room, manufacturing, warehousing, and office space | Varies | Varies | December 31, 2026 |
| | | | | | | | | | | | | | | | | |
| (1) | Property is encumbered by a lease agreement and is collateral to our Credit Facility. |
| (2) | Property is collateral to our Credit Facility. |
| (3) | Property is encumbered by our Solvita Agreement as an embedded lease. | | |
| (4) | Total square feet and utilization varies each month for the use of Solvita's clean room, manufacturing, warehousing, and office space in accordance with the Solvita Agreement. |
We believe that our facilities will be sufficient to operate our business for the next 12 months and that current lease obligations will not change materially.
ITEM 3. LEGAL PROCEEDINGS
Information required by this item is set forth in Note 15 - Commitments and Contingencies of the Notes to Consolidated Financial Statements in this Annual Report on Form 10-K and is incorporated herein by reference.
ITEM 4. MINE SAFETY DISCLOSURES
None.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is traded on the Nasdaq Capital Market under the symbol “AXGN.” On February 19, 2025, the last reported closing sale price of our common stock on the Nasdaq Capital Market was $18.68 per share.
Shareholders
As of February 19, 2025, we had 44,343,785 shares of common stock outstanding, and approximately 218 common shareholders of record, based upon information received from our stock transfer agent. However, this number does not include beneficial owners whose shares were held of record by nominees or broker dealers. We estimate that there are approximately 11,964 individual owners. Additional information called for by this item is incorporated herein by reference to the following sections of this Report: Note 11 - Stock-Based Compensation of the Notes to Consolidated Financial Statements included in Item 8; and Part III, Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters – Equity Compensation Plan Information”.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not repurchase any of our securities in the fourth quarter of 2024.
Recent Sales of Unregistered Securities
We had no sales of unregistered securities in 2024.
Securities Authorized for Issuance Under Equity Compensation Plans
See Part III, Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Dividends
We have never declared or paid and do not anticipate paying or declaring a cash dividend on our common stock. We intend to retain any earnings to finance the growth and development of our business. Our Board of Directors may declare dividends at its discretion.
Performance Graph
The following graph compares the cumulative total shareholder return on our common stock for the period from December 31, 2019 to December 31, 2024 with (i) the Nasdaq Stock Market Biotechnology Index and (ii) the Nasdaq Stock Market Composite Index. The graph assumes an investment of $100 in our common stock and the respective indices for the period of December 31, 2019 to December 31, 2024. The comparisons set forth in the graph are provided pursuant to SEC rules and are not intended to forecast or be indicative of the future performance of our common stock or either of the included indices. The performance graph shall not be deemed incorporated by reference by any general statement incorporating by reference this annual report into any filing under the Securities Act of 1933, as amended, or the Exchange Act of 1934, as amended, except to the extent we specifically incorporate this information by reference and shall not otherwise be deemed filed under such acts.
ITEM 6. [RESERVED]
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following information should be read in conjunction with our consolidated financial statements and the notes thereto contained in Item 8 of Part II in this Form 10-K, “Forward-Looking Statements” contained in Part 1 of this Form 10-K, “Risk Factors” contained in Item 1A of this Form 10-K, and the other information appearing elsewhere in, or incorporated by reference into, this Form 10-K. Dollar amounts referenced in this Item 7 are in thousands, except per share amounts.
Financial information for prior periods has been reclassified to reflect the retrospective application of voluntary changes in the Company’s accounting policy for shipping and handling costs and changes to certain allocated corporate costs, as discussed under “Note 2 – Summary of Significant Accounting Policies” of the Notes to Consolidated Financial Statements in this Form 10-K.
Unless the context otherwise requires, all references in this report to “Axogen,” the “Company,” “we,” “us” and “our” refer to Axogen, Inc., and its wholly owned subsidiaries Axogen Corporation (“AC”), Axogen Processing Corporation, Axogen Europe GmbH and Axogen Germany GmbH.
Overview
We are the leading company focused specifically on the science, development, and commercialization of technologies for peripheral nerve regeneration and repair. We are passionate about providing the opportunity to restore nerve function and quality of life for patients with peripheral nerve injuries. We provide innovative, clinically proven, and economically effective repair solutions for surgeons and healthcare providers. Peripheral nerves provide the pathways for both motor and sensory signals throughout the body. Every day, people suffer traumatic injuries or undergo surgical procedures that impact the function of their peripheral nerves. Physical damage to a peripheral nerve or the inability to properly reconnect peripheral nerves can result in the loss of muscle or organ function, the loss of sensory feeling, or the initiation of pain.
Product Portfolio
Our platform for peripheral nerve repair features a comprehensive portfolio of products, including:
•Avance® Nerve Graft, a biologically active off-the-shelf processed human nerve allograft for bridging severed peripheral nerves without the comorbidities associated with a second surgical site.
•Axoguard Nerve Connector®, a porcine (pig) submucosa extracellular matrix ("ECM") coaptation aid for tensionless repair of severed peripheral nerves.
•Axoguard Nerve Protector®, a porcine submucosa ECM product used to wrap and protect damaged peripheral nerves and reinforce the nerve reconstruction while preventing soft tissue attachments.
•Axoguard HA+ Nerve Protector™, is comprised of a processed porcine submucosa ECM base layer with a hyaluronate-alginate gel coating designed to provide short- and long-term protection for peripheral nerve injuries. The gel layer facilitates enhanced nerve gliding to aid in minimizing soft tissue attachments, while the base layer is remodeled into a long-term protective tissue layer.
•Axoguard Nerve Cap®, a porcine submucosa ECM product used to protect a peripheral nerve end and separate the nerve from the surrounding environment to reduce the development of symptomatic or painful neuroma.
•Avive+ Soft Tissue Matrix™, a multi-layer amniotic membrane allograft used to protect and separate tissues in the surgical bed during the critical phase of tissue healing.
Our portfolio of products is currently available in the United States ("U.S."), and 19 other countries including Canada, Germany, United Kingdom, Spain and several other European, Asian and Latin American countries.
We derive substantially all of our revenues from sales of our nerve repair products to customers in the U.S.
On June 24, 2024, we announced the launch of Avive+ Soft Tissue Matrix. Avive+ Soft Tissue Matrix is processed and distributed in accordance with U.S. Food and Drug Administration ("FDA") requirements for Human Cellular and Tissue-based Products under the Code of Federal Regulations ("CFR") Title 21 Part 1271 regulations and U.S. Public Health Service Act regulations as a Section 361 human tissue product. Products regulated solely under Section 361 of the Public Health Service Act are a product category under close scrutiny by the FDA for compliance with the regulatory requirements and potentially
subject to regulatory change in the future. Failure to comply with applicable regulatory requirements could expose us to potential compliance actions by the FDA or state regulators and could risk the commercial availability of the product.
Our strategy remains focused on deepening our presence in high-potential accounts, specifically Level 1 trauma centers and academic-affiliated hospitals with a high number of trained microsurgeons. We will drive growth in these accounts through targeted expansion of nerve repair indications and driving deeper adoption of our nerve repair algorithm across multiple surgical specialties.
Summary of Operational and Business Highlights
•We completed the Biologics License Application ("BLA") submission for Avance Nerve Graft on September 6, 2024. On November 1, 2024, the FDA informed us that they had accepted the BLA for filing and assigned a Prescription Drug User Fee Act goal date of September 5, 2025. The FDA further indicated that it does not currently plan to hold an advisory committee meeting for the application.
•Revenue was $187,338 for the year ended December 31, 2024, an increase of $28,326, or 17.8%, compared to the year ended December 31, 2023.
•Gross profit was $141,977 for the year ended December 31, 2024, an increase of $20,108, or 16.5%, compared to the year ended December 31, 2023.
Results of Operations
Comparison of the Years Ended December 31, 2024 and 2023
The following table sets forth, for the periods indicated, our results of operations expressed as dollar amounts and as percentages of total revenue:
| | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 |
| Amount | | % of Revenue | | Amount | | % of Revenue |
| (dollars in thousands) |
Revenues | $ | 187,338 | | | 100.0 | % | | $ | 159,012 | | | 100.0 | % |
| Cost of goods sold | 45,361 | | | 24.2 | | | 37,143 | | | 23.4 | |
| Gross profit | 141,977 | | | 75.8 | | | 121,869 | | | 76.6 | |
| Costs and expenses: | | | | | | | |
| Sales and marketing | 78,461 | | | 41.9 | | | 77,580 | | | 48.8 | |
| Research and development | 27,767 | | | 14.8 | | | 27,339 | | | 17.2 | |
| General and administrative | 39,036 | | | 20.8 | | | 38,412 | | | 24.2 | |
| Total costs and expenses | 145,264 | | | 77.5 | | | 143,331 | | | 90.1 | |
| Loss from operations | (3,287) | | | (1.8) | | | (21,462) | | | (13.5) | |
| Other (expense) income: | | | | | | | |
| Investment income | 1,141 | | | 0.6 | | | 1,487 | | | 0.9 | |
| | | | | | | |
| Interest expense | (8,206) | | | (4.4) | | | (2,835) | | | (1.8) | |
| Change in fair value of derivatives | 587 | | | 0.3 | | | 1,531 | | | 1.0 | |
| Other expense | (199) | | | (0.1) | | | (437) | | | (0.3) | |
| Total other (expense) income, net | (6,677) | | | (3.7) | | | (254) | | | (0.2) | |
| Net loss | $ | (9,964) | | | (5.3) | % | | $ | (21,716) | | | (13.7) | % |
Revenues
Revenues for the year ended December 31, 2024 increased $28,326, or 17.8%, to $187,338, as compared to $159,012 for the year ended December 31, 2023. Revenue growth was driven by an increase in unit volume of approximately 9.0%, as well as the net impact of changes in product mix and price of approximately 5.5% and 3.3%, respectively.
Gross Profit
Gross profit for the year ended December 31, 2024 increased $20,108, or 16.5%, to $141,977, as compared to $121,869 for the year ended December 31, 2023. Gross margin as a percentage of revenue decreased to 75.8% for the year ended December 31, 2024, as compared to 76.6% for the year ended December 31, 2023. The decrease in gross margin was due to the change in our product mix.
Costs and Expenses
Total costs and expenses increased $1,933, or 1.3%, to $145,264 for the year ended December 31, 2024, as compared to $143,331 for the year ended December 31, 2023. The increase in total operating costs was primarily attributable to the following: (i) $9,745 in compensation costs; (ii) $1,288 in professional services fees; and (iii) $308 in net other costs, partially offset by decreases of (i) $3,593 in research and development project costs; (ii) $3,114 in royalty expenses; (iii) $1,621 in marketing program costs; and (iv) $218 in travel costs.
Sales and marketing costs and expenses increased $881, or 1.1%, to $78,461 for the year ended December 31, 2024, as compared to $77,580 for the year ended December 31, 2023. The increase in sales and marketing costs and expenses was due to the following: (i) $6,028 in compensation costs and (ii) $311 in occupancy related costs, partially offset by decreases of (i) $3,114 in royalty expenses; (ii) $1,621 in marketing program costs; (iii) $173 in travel costs; (iv) $153 in professional services fees; and (v) $396 in net other costs.
Research and development costs and expenses increased $428, or 1.6%, to $27,767 for the year ended December 31, 2024, as compared to $27,339 for the year ended December 31, 2023. The increase was primarily due to product development and clinical expenses. Product development costs include spending for a number of specific programs, including the non-clinical expenses related to the BLA for Avance Nerve Graft. Product development costs and expenses represented approximately 53% and 60% of total research and development costs and expenses for the years ended December 31, 2024 and 2023, respectively. Clinical trial costs and expenses represented approximately 47% and 40% of total research and development costs and expenses for the years ended December 31, 2024 and 2023, respectively. The increase in research and development costs and expenses was due to the following: (i) $3,027 in compensation costs and (ii) $1,814 in professional services fees, partially offset by decreases of (i) $3,593 in research and development project costs; (ii) $414 in occupancy related costs; (iii) $173 in travel costs; and (iv) $97 in other costs to support these clinical and non-clinical expenses.
General and administrative costs and expenses increased $624, or 1.6%, to $39,036 for the year ended December 31, 2024, as compared to $38,412 for the year ended December 31, 2023. The increase was primarily due to (i) $921 in bad debt expense and (ii) $254 in occupancy related costs; partially offset by decreases of (i) $373 in professional services fees and (ii) $187 in compensation costs.
Other Expense
Total other expense increased $6,423, or 2528.7%, to $6,677 for the year ended December 31, 2024, as compared to $254 for the year ended December 31, 2023. The increase was primarily due to the increase in interest expense of $5,372 which was a result of no longer capitalizing interest due to the completion of the Axogen Processing Center facility (the "APC Facility") in 2023. In connection with our credit facility with Oberland Capital ("Credit Facility"), we recognized total interest charges of $8,101 and $8,083 for the years ended December 31, 2024 and 2023, respectively, and of the total interest charges, we capitalized to the construction of the APC Facility interest charges of $— and $5,285 for the years ended December 31, 2024 and 2023, respectively. The decrease in investment income was primarily related to the Federal Reserve decreasing interest rates 100 basis points throughout 2024.
Income Taxes
We had no federal income tax expense or benefit for the years ended December 31, 2024 and 2023 due to the incurrence of net operating losses in both years, the benefits of which have a full valuation allowance. From time to time, we receive notices of examination of prior tax filings from federal and state authorities. The Internal Revenue Service is currently examining the Company's 2021 federal income tax return. We do not believe that there are any additional tax expenses or benefits currently available.
Comparison of the Years Ended December 31, 2023 and 2022
The following table sets forth, for the periods indicated, our results of operations expressed as dollar amounts and as percentages of total revenue:
| | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 |
| Amount | | % of Revenue | | Amount | | % of Revenue |
| (dollars in thousands) |
Revenues | $ | 159,012 | | | 100.0 | % | | $ | 138,584 | | | 100.0 | % |
| Cost of goods sold | 37,143 | | | 23.4 | | | 29,775 | | | 21.5 | |
| Gross profit | 121,869 | | | 76.6 | | | 108,809 | | | 78.5 | |
| Costs and expenses: | | | | | | | |
| Sales and marketing | 77,580 | | | 48.8 | | | 71,983 | | | 51.9 | |
| Research and development | 27,339 | | | 17.2 | | | 25,627 | | | 18.5 | |
| General and administrative | 38,412 | | | 24.2 | | | 40,906 | | | 29.5 | |
| Total costs and expenses | 143,331 | | | 90.1 | | | 138,516 | | | 100.0 | |
| Loss from operations | (21,462) | | | (13.5) | | | (29,707) | | | (21.4) | |
| Other (expense) income: | | | | | | | |
| Investment income | 1,487 | | | 0.9 | | | 569 | | | 0.4 | |
| | | | | | | |
| Interest expense | (2,835) | | | (1.8) | | | (624) | | | (0.5) | |
| Change in fair value of derivatives | 1,531 | | | 1.0 | | | 1,044 | | | 0.8 | |
| Other expense | (437) | | | (0.3) | | | (230) | | | (0.2) | |
| Total other (expense) income, net | (254) | | | (0.2) | | | 759 | | | 0.5 | |
| Net loss | $ | (21,716) | | | (13.7) | % | | $ | (28,948) | | | (20.9) | % |
Revenues
Revenues for the year ended December 31, 2023 increased $20,428, or 14.7%, to $159,012, as compared to $138,584 for the year ended December 31, 2022. Revenue growth was driven by an increase in unit volume of approximately 8.6%, as well as the net impact of changes in price and product mix of approximately 3.7% and 2.5%, respectively. The unit volume increase was attributed to growth in our core and active accounts. As of December 31, 2023, we had 1,006 active accounts, an increase of 3.9% from 968 at December 31, 2022 and 376 core accounts, an increase of 13.3% from 332 at December 31, 2022.
Gross Profit
Gross profit for the year ended December 31, 2023 increased $13,060, or 12.0%, to $121,869, as compared to $108,809 for the year ended December 31, 2022. Gross margin as a percentage of revenue decreased to 76.6% for the year ended December 31, 2023, as compared to 78.5% for the year ended December 31, 2022.
Costs and Expenses
Total costs and expenses increased $4,815, or 3.5%, to $143,331 for the year ended December 31, 2023, as compared to $138,516 for the year ended December 31, 2022. The increase in total operating costs and expenses was due to the following: (i) $5,469 in compensation costs; (ii) $1,243 in marketing program costs; (iii) $1,156 in professional services fees; (iv) $852 in travel costs; and (v) $326 in occupancy related costs; partially offset by decreases of (i) $1,190 in research and development projects and (ii) $3,041 of other general business costs, including insurance expenses of $1,033, bad debt recovery of $883, merchant fees of $727, and other items administrative in nature of $398.
Sales and marketing costs and expenses increased $5,597, or 7.8%, to $77,580 for the year ended December 31, 2023, as compared to $71,983 for the year ended December 31, 2022. The increase in sales and marketing costs and expenses was due to the following: (i) $2,497 in compensation costs; (ii) $1,243 in marketing program costs; (iii) $831 in travel costs; (iv) $625 in professional services fees; (v) other items administrative in nature of $251 and (vi) $150 in occupancy related costs.
Research and development costs and expenses increased $1,712, or 6.7%, to $27,339 for the year ended December 31, 2023, as compared to $25,627 for the year ended December 31, 2022. The increase in research and development costs and expenses was primarily due to product development and clinical expenses. Product development costs include spending for a number of specific programs, including the non-clinical expenses related to the BLA for Avance Nerve Graft. Product development costs and expenses represented approximately 60% and 52% of total research and development costs and expenses for the years ended December 31, 2023 and 2022, respectively. Clinical trial costs and expenses represented approximately 40% and 48% of total research and development costs and expenses for the years ended December 31, 2023 and 2022, respectively. The increase in research and development costs and expenses was due to the following: (i) $2,171 in compensation costs; (ii) $331 in professional services fees; (iii) $287 in occupancy related costs; and (iv) $98 in travel costs to support these clinical and non-clinical expenses, partially offset by a decrease of $1,190 in research and development projects.
General and administrative costs and expenses decreased $2,494, or 6.1%, to $38,412 for the year ended December 31, 2023, as compared to $40,906 for the year ended December 31, 2022. The decrease in general and administrative costs and expenses was primarily due to the following: (i) $1,033 in insurance expenses, (ii) $883 in bad debt recovery, (iii) $727 in merchant fees, (iv) $330 in other services, (v) $205 in licenses and fees; (vi) other items administrative in nature of $128; (vi) $112 in occupancy related costs; and (vii) $78 in travel costs, partially offset by increases of (i) $801 in compensation costs and (ii) $201 in professional services fees.
Other (Expense) Income
Total other expense increased $1,013, or 133.5%, to expense of $254 for the year ended December 31, 2023, as compared to income of $759 for the year ended December 31, 2022. The increase was primarily due to the increase in interest expense of $2,211 and other expenses of $208, partially offset by the increase in investment income of $918 and the fair value of the derivative liability of $488. In connection with the Credit Facility, we recognized total interest charges of $8,083 and $6,721 for the years ended December 31, 2023 and 2022, respectively, and of this interest we capitalized to the construction of the APC Facility, interest charges of $5,285 and $6,155 for the years ended December 31, 2023 and 2022, respectively. The increase in investment income was primarily related to the Federal Reserve raising interest rates 100 basis points throughout 2023.
Income Taxes
We had no federal income tax expense or benefit for the years ended December 31, 2023 and 2022 due to the incurrence of net operating losses in both years, the benefits of which have been fully reserved. We do not believe that there are any additional tax expenses or benefits currently available.
Liquidity and Capital Resources
As of December 31, 2024, our principal sources of liquidity were our cash and cash equivalents and investments totaling $33,482. Our cash equivalent is comprised of a money market mutual fund and our investments are comprised of U.S. Treasuries. Our cash and cash equivalents and investments increased $2,458 to $33,482 from $31,024 at December 31, 2023, due to a reduction in capital expenditures and general operating activities. On December 31, 2024 and 2023, our current assets exceeded our current assets liabilities by $68,607 and $57,574, respectively. Based on current estimates, we believe that our existing cash and cash equivalents and investments, as well as cash provided by sales of our products will allow us to fund our operations through at least the next twelve months from the date of issuance of the accompanying financial statements.
Cash Flow Information
The following table presents a summary of our cash flows from operating, investing and financing activities:
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | | 2024 | | 2023 |
| Net cash provided by (used in): | | | | |
| Operating activities | | $ | 4,535 | | | $ | (5,716) | |
| Investing activities | | (10,297) | | | 19,253 | |
| Financing activities | | 2,290 | | | 1,954 | |
| Net (decrease) increase in cash and cash equivalents | | $ | (3,472) | | | $ | 15,491 | |
Net Cash Provided By (Used In) Operating Activities
Net cash provided by operating activities was $4,535 compared to net cash used in operating activities of $5,716 for the years ended December 31, 2024 and 2023, respectively. The favorable change in net cash provided by operating activities of $10,251 was due to the decrease in net loss of $11,751 and the favorable change in noncash accounts of $6,093, partially offset by an unfavorable change in working capital of $7,594.
A discussion of net cash used in operating activities during the year ended December 31, 2022 can be found in Item 7 of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the Securities and Exchange Commission ("SEC") on March 5, 2024.
Net Cash (Used In) Provided By Investing Activities
Net cash used in investing activities was $10,297 compared to net cash provided by investing activities of $19,253 for the years ended December 31, 2024 and 2023, respectively. The unfavorable change in net cash used in investing activities of $29,550 was due to the decrease in proceeds from the sale of investments, net of investment purchases, totaling $39,944, partially offset by a net decrease in capital expenditures of $10,771, primarily related to the renovation of the APC Facility completed in 2023.
A discussion of net cash used in investing activities during the year ended December 31, 2022 can be found in Item 7 of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 5, 2024.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $2,290 as compared to $1,954 for the years ended December 31, 2024 and 2023, respectively, an increase of $335, or 17%. The increase in net cash provided by financing activities was primarily due to an increase of $336 in proceeds from the exercise of stock options and Employee Stock Purchase Plan (“ESPP”) purchases year-over-year.
A discussion of net cash provided by financing activities during the year ended December 31, 2022 can be found in Item 7 of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 5, 2024.
Sources of Capital
Our expected future capital requirements may depend on many factors including expanding our customer base and sales force and timing and extent of spending in obtaining regulatory approval and introduction of new products. Additional sources of liquidity available to us include issuance of additional equity securities through public or private equity offerings, debt financings or from other sources. The sale of additional equity may result in dilution to our shareholders. There is no assurance that we will be able to secure funding on terms acceptable to us, or at all. The increasing need for capital could also make it more difficult to obtain funding through either equity or debt. Should additional capital not become available to us as needed, we may be required to take certain actions, such as slowing sales and marketing expansion, delaying regulatory approvals, or reducing headcount.
Credit Facilities
As of December 31, 2024, we had $50,000 outstanding in indebtedness under a credit facility; $35,000 maturing on June 30, 2027 and $15,000 maturing on June 30, 2028. Quarterly interest only and revenue participation payments are due through each of the maturity dates. Interest is calculated as 7.5% plus the greater of the forward-looking term rate based on the secured overnight financing rate as set by the Federal Reserve Bank of New York plus 0.10% ("Adjusted SOFR") or 2.0% (12.19% as of December 31, 2024). Revenue participation payments are calculated as a percentage of our net revenues, up to $70,000 in any given year, adding approximately 1.5% per year of additional interest payments on the outstanding indebtedness. Upon each maturity date or upon such date earlier repayment occurs, we will repay the principal balance and provide a make-whole payment calculated to generate an internal rate of return to the lender equal to 11.5%, less the total of all quarterly interest and revenue participation payments previously paid. See Note 9 - Long-Term Debt, Net of Debt Discount and Financing Fees and Note 15 - Commitments and Contingencies in the Notes to the Consolidated Financial Statements Part II, Item 8 of this Form 10-K.
Contractual Obligations and Commitments
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Contractual Obligations | | 2025 | | 2026-2027 | | 2028-2029 | | Thereafter | | Total |
Credit Facility Principal(1) | | $ | — | | | $ | 35,000 | | | $ | 15,000 | | | $ | — | | | $ | 50,000 | |
Credit Facility Interest(1)(2) | | 6,095 | | | 10,057 | | | 914 | | | — | | | 17,066 | |
Credit Facility Revenue Participation(1) | | 756 | | | 1,512 | | | 231 | | | — | | | 2,499 | |
| | | | | | | | | | |
Operating and financing lease obligations(3) | | 4,148 | | | 7,404 | | | 6,306 | | | 15,385 | | | 33,243 | |
Transition and separation obligations to former CEO(4) | | 1,164 | | | 18 | | | — | | | — | | | 1,182 | |
Insurance financing agreement(5) | | 1,304 | | | — | | | — | | | — | | | 1,304 | |
Separation obligation to former employee(6) | | 843 | | | 17 | | | — | | | — | | | 860 | |
| | | | | | | | | | |
| | $ | 14,310 | | | $ | 54,008 | | | $ | 22,451 | | | $ | 15,385 | | | $ | 106,154 | |
(1) See Note 9 - Long-Term Debt, Net of Debt Discount and Financing Fees and Note 15 - Commitments and Contingencies in Part II, Item 8 of this Form 10-K.
(2) Calculated at 12.19%; the interest rate as of December 31, 2024.
(3) See Note 8 - Leases in Part II, Item 8 of this Form 10-K.
(4) Includes former CEO's 2024 bonus to be paid in March of 2025, senior advisory fees through May 2025, eighteen months of Consolidated Omnibus Budget Reconciliation Act ("COBRA") payments and related employer paid taxes. See Note 15 - Commitments and Contingencies in Part II, Item 8 of this Form 10-K.
(5) See Note 15 - Commitments and Contingencies in Part II, Item 8 of this Form 10-K.
(6) Includes severance, accrued paid time off, twenty-four months of COBRA payments and related employer paid taxes payable to a former employee. See Note 15 - Commitments and Contingencies in Part II, Item 8 of this Form 10-K.
See Note 8 - Leases, Note 9 - Long-Term Debt, Net of Debt Discount and Financing Fees, and Note 15 - Commitments and Contingencies in the Notes to the Consolidated Financial Statements in Part II, Item 8 of this Form 10-K, for further information.
Critical Accounting Estimates
In preparing our financial statements in accordance with generally accepted accounting principles, there are certain accounting policies, which may require substantial judgment or estimation in their application. We believe these accounting policies and the others set forth in Note 2 - Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements in Part II, Item 8 of this Form 10-K are critical to understanding our results of operations and financial condition. Actual results could differ from our estimates and assumptions, and any such differences could be material to our results of operations and financial condition.
Inventories
Description
Inventories consist of purchased materials, direct labor and manufacturing overhead, and are stated at the lower of cost or net realizable value, as determined by the first-in, first-out method.
Judgments and Uncertainties
We maintain reserves for excess and obsolete inventory resulting from the potential inability to sell certain products in excess of current carrying cost. We make estimates regarding the future recoverability of the costs of these products and record provisions based on historical experience, expiration dates and expected future trends.
Sensitivity of Estimate to Change
As of December 31, 2024, we have reserved $1,630 for potential losses relating to inventory. If our actual product life cycles, product demand or acceptance of new product introductions are less favorable than projected by management, additional inventory write downs may be required, which could unfavorably affect future operating results.
Derivative Instruments
Description
We review debt instruments to determine whether there are embedded derivative instruments, which are required to be bifurcated and accounted for separately as a derivative financial instrument. Embedded derivatives that are not clearly and closely related to the debt host are bifurcated and recognized at fair value on the Consolidated Balance Sheets with changes in fair value recognized as either a gain or loss on the Consolidated Statements of Operations for each reporting period.
Judgements and Uncertainties
The fair value of embedded derivatives is measured based on equity markets and interest rates, as well as an estimate of our nonperformance risk adjustment. This estimate includes an option adjusted spread and an estimate of our discount rate.
Sensitivity of Estimate to Change
As of December 31, 2024, we recorded a derivative liability of $2,400. However, if the discount rate were to change by 1%, it would have a less than $50 effect on our derivative liability fair value.
Stock-Based Compensation
Description
Stock-based compensation is in the form of stock options, restricted stock units ("RSU") and performance stock units ("PSU") granted to employees and directors. Stock-based compensation expense is based on the fair value of the stock options, RSUs and PSUs.
Judgments and Uncertainties
We estimate the grant date fair value of each stock option award on the date of grant using a multiple-point Black-Scholes option-pricing model. In addition, we estimate the grant date fair value of stock options granted to employees at a premium price based on market conditions, such as the trading price of our common stock, using a Monte Carlo simulation option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods.
The fair value of the PSU grants tied to revenue goals (the "Revenue PSUs") is determined based on the fair value of our common stock on the date of grant and our estimate of achieving the applicable revenue goals. Compensation expense for the Revenue PSUs is recorded as the milestones are achieved. The number of shares delivered to recipients and the related compensation cost recognized as an expense will be based on the actual performance metrics as set forth in the applicable Revenue PSU award agreement. The amount actually awarded will be based upon achievement of the performance measures. Expectations related to the achievement of the revenue goals associated with the Revenue PSU grants is assessed at each reporting period and is used to determine whether any of the Revenue PSU grants are expected to vest. If the performance-based milestones related to the Revenue PSU grants are not met or not expected to be met, any compensation expense recognized associated with such grants is reversed.
The estimated grant date fair value of the Total Shareholder Return PSUs ("TSR PSUs") is calculated by using a Monte Carlo simulation. The number of TSR PSUs that will be earned is based upon the achievement of stock price targets ("Price Targets") over the measurement period. Compensation expense is recognized regardless if the Price Targets are satisfied. Compensation expense will be reversed if an employee's employment is terminated prior to satisfying the requisite service period.
We recognize compensation expense related to the ESPP based on the estimated fair value of the options on the date of grant. We estimate the grant date fair value using a Black-Scholes option pricing model for each purchase period. The grant date fair value is expensed on a straight-line basis over the offering period.
The determination of fair value using option-pricing models, as indicated above, is affected by our stock price, as well as assumptions regarding several subjective variables. These variables include, but are not limited to, our expected stock price volatility over the expected term of the awards. We determine the expected term of each award giving consideration to the contractual terms, vesting schedules, and post-vesting forfeitures. We use the risk-free interest rate on the implied yield available on U.S. Treasury issues with an equivalent remaining term approximately equal to the expected term of the award. The fair value of the Revenue PSUs also includes our estimates of achieving the applicable revenue goals. The value of the
portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in our Consolidated Statements of Operations. If factors change and different assumptions are used, stock-based compensation expense could be materially different in the future.
Sensitivity of Estimate of Change
As of December 31, 2024, if we determine that the unvested PSUs outstanding were in a pay-out range of 150%, our stock-based compensation expense would increase by $616.
Recent Accounting Pronouncements
See Note 2 - Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements, Part II Item 8 of this Form 10-K for further information.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
We are subject to interest rate risk from exposure to changes in interest rates based upon our investing and cash management activities. For our cash equivalents and investments, a change in interest rates affects the amount of interest income that can be earned.
We have not entered into derivative transactions related to cash and cash equivalents. We do not expect changes in interest rates to have a material adverse effect on our income or our cash flows in 2025. However, we give no assurance that interest rates will not significantly change in the future.
We also have interest rate exposure as a result of the Credit Facility. As of December 31, 2024, the outstanding principal amount of our loans under the Credit Facility was $50,000. Interest on our loans under the Credit Facility is payable quarterly during the term of the loans and is calculated as 7.5% plus the greater of Adjusted SOFR or 2.0% (12.19% as of December 31, 2024); provided that the interest rate shall never be less than 9.5%. Changes in the Adjusted SOFR rate may therefore affect our interest expense associated with the loans. An increase of 100 basis points in interest rates would increase expense by approximately $500 annually based on the amounts currently outstanding and would not materially affect our results of operations.
Credit Risk
Financial instruments that potentially subject us to credit risk consist of cash and cash equivalent balances, investments in U.S. Treasuries and accounts receivable. Certain of our cash and cash equivalents balances exceed Federal Deposit Insurance Corporation ("FDIC") insured limits or are invested in money market accounts with investment banks that are not FDIC-insured. As of December 31, 2024, $27,054 of the cash and cash equivalents balance was in excess of FDIC limits.
We invest our cash primarily in money market accounts and in U.S. Treasuries. We believe our cash is invested in a conservative manner, with cash preservation being the primary investment objective.
With respect to accounts receivable, we perform credit evaluations of our customers and do not require collateral. There have been no material losses on accounts receivable. Concentrations of credit risk with respect to accounts receivable are limited because a large number of geographically diverse customers make up our customer base, thus spreading the trade credit risk. We also control credit risk through credit approvals and monitoring procedures.
Foreign Currency Exchange Risk
The value of the U.S. dollar compared to the foreign currencies of the countries where we distribute our products has little to no effect on our financial results. In our international markets, we distribute our products and services to independent distributors who, in turn, distribute and market to medical clinics. The revenue from the distribution of our products in our international markets through independent distributors is denominated in U.S. dollars. As a result, we have minimal exposure related to foreign exchange rate fluctuations. Our portfolio of products is currently available in the U.S. and 19 other countries, including Canada, Germany, United Kingdom, Spain and several other European, Asian and Latin American countries.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
CONTENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders and the Board of Directors of Axogen, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Axogen, Inc and subsidiaries (the "Company") as of December 31, 2024 and 2023, the related consolidated statements of operations, shareholders’ equity, and cash flows, for each of the three years in the period ended December 31, 2024, and the related notes and the schedule listed in the Index at Item 15(a) (collectively referred to as the "financial statements"). We also have audited the Company’s internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023 and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
Basis for Opinions
The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on these financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Inventory – Excess and Obsolete (E&O) Inventory — Refer to Notes 2 and 3 to the financial statements
Critical Audit Matter Description
Inventories, consisting of materials, direct labor and manufacturing overhead, are stated at the lower of cost or net realizable value. At each balance sheet date, the Company evaluates inventories for excess quantities, obsolescence or shelf-life.
The Company monitors the shelf life of its products and historical expiration and spoilage trends, and reserves for inventory based on the estimated amount of inventory that will not be distributed before expiration or spoilage. To estimate the amount of inventory that will expire or spoil prior to being distributed, the Company reviews inventory quantities on hand, historical and projected distribution levels, historical expiration trends, and historical spoilage trends. The Company’s calculation of the amount of inventory that will expire prior to distribution has three components: 1) a spoilage-based component that compares historical spoilage rates to inventory quantities on hand 2) a demand or consumption-based component that compares projected distribution to inventory quantities on hand; and 3) an expiring inventory component that assesses the risk related to inventory that is near expiration.
Given the significant judgments associated with evaluating the valuation of E&O inventory, auditing the reasonableness of management’s estimates and assumptions involved especially subjective judgment and an increased extent of effort.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the Company’s valuation of E&O inventory included the following, among others:
•We tested the design, implementation and operating effectiveness of controls over the E&O inventory valuation.
•We evaluated management’s ability to accurately forecast E&O inventory by comparing the historical inventory reserve estimates to subsequent inventory destructions and expirations.
•We evaluated the reasonableness of the Company’s E&O valuation methodology and tested the mathematical accuracy of the calculation.
•We tested the accuracy and completeness of the underlying data used in the calculation of the Company’s expiring inventory and spoilage models.
•We made inquiries of the Company’s employees outside of the accounting department and evaluated other areas of the audit to identify business, product, or industry changes that may impact the inputs in the inventory valuation calculation.
/s/ Deloitte & Touche LLP
Tampa, Florida
February 26, 2025
We have served as the Company's auditor since 2018.
AXOGEN, INC.
CONSOLIDATED BALANCE SHEETS
December 31, 2024 and 2023
(In Thousands, Except Share and Per Share Amounts)
| | | | | | | | | | | |
| 2024 | | 2023 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 27,554 | | | $ | 31,024 | |
| Restricted cash | 6,000 | | | 6,002 | |
| Investments | 5,928 | | | — | |
| Accounts receivable, net of allowance for doubtful accounts of $788 and $337, respectively | 24,105 | | | 25,147 | |
| Inventory | 33,183 | | | 23,020 | |
| Prepaid expenses and other | 2,447 | | | 2,811 | |
| Total current assets | 99,217 | | | 88,004 | |
| Property and equipment, net | 84,667 | | | 88,730 | |
| Operating lease right-of-use assets | 14,265 | | | 15,562 | |
| | | |
| Intangible assets, net | 5,579 | | | 4,531 | |
| Total assets | $ | 203,728 | | | $ | 196,827 | |
| | | |
| Liabilities and shareholders’ equity | | | |
| Current liabilities: | | | |
| Accounts payable and accrued expenses | $ | 28,641 | | | $ | 28,883 | |
| Current maturities of long-term lease obligations | 1,969 | | | 1,547 | |
| Total current liabilities | 30,610 | | | 30,430 | |
| | | |
| Long-term debt, net of debt discount and financing fees | 47,496 | | | 46,603 | |
| Long-term lease obligations | 19,221 | | | 21,142 | |
| Debt derivative liabilities | 2,400 | | | 2,987 | |
| Other long-term liabilities | 94 | | | — | |
| Total liabilities | 99,821 | | | 101,162 | |
| | | |
| Commitments and contingencies - see Note 15 | | | |
| | | |
| Shareholders’ equity: | | | |
| Common stock, $0.01 par value per share; 100,000,000 shares authorized; 44,148,836 and 43,124,496 shares issued and outstanding | 441 | | | 431 | |
| Additional paid-in capital | 394,726 | | | 376,530 | |
| Accumulated deficit | (291,260) | | | (281,296) | |
| Total shareholders’ equity | 103,907 | | | 95,665 | |
| Total liabilities and shareholders’ equity | $ | 203,728 | | | $ | 196,827 | |
The accompanying notes are an integral part of these consolidated financial statements.
AXOGEN, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
Years ended December 31, 2024, 2023 and 2022
(In Thousands, Except Share and Per Share Amounts)
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Revenues | $ | 187,338 | | | $ | 159,012 | | | $ | 138,584 | |
| Cost of goods sold | 45,361 | | | 37,143 | | | 29,775 | |
| Gross profit | 141,977 | | | 121,869 | | | 108,809 | |
| Costs and expenses: | | | | | |
| Sales and marketing | 78,461 | | | 77,580 | | | 71,983 | |
| Research and development | 27,767 | | | 27,339 | | | 25,627 | |
| General and administrative | 39,036 | | | 38,412 | | | 40,906 | |
| Total costs and expenses | 145,264 | | | 143,331 | | | 138,516 | |
| Loss from operations | (3,287) | | | (21,462) | | | (29,707) | |
| Other (expense) income: | | | | | |
| Investment income | 1,141 | | | 1,487 | | | 569 | |
| | | | | |
| Interest expense | (8,206) | | | (2,835) | | | (624) | |
| Change in fair value of derivatives | 587 | | | 1,531 | | | 1,044 | |
| | | | | |
| Other expense | (199) | | | (437) | | | (230) | |
| Total other (expense) income, net | (6,677) | | | (254) | | | 759 | |
| Net loss | $ | (9,964) | | | $ | (21,716) | | | $ | (28,948) | |
| | | | | |
| Weighted average common shares outstanding — basic and diluted | 44,257,754 | | | 42,878,543 | | | 42,083,125 | |
| Loss per common share — basic and diluted | $ | (0.23) | | | $ | (0.51) | | | $ | (0.69) | |
The accompanying notes are an integral part of these consolidated financial statements.
AXOGEN, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
Years ended December 31, 2024, 2023 and 2022
(In Thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional
Paid-in
Capital | | Accumulated
Deficit | | Total
Shareholders’
Equity |
| Shares | | Amount | | | |
| Balance, December 31, 2021 | 41,737 | | | $ | 417 | | | $ | 342,765 | | | $ | (230,632) | | | $ | 112,550 | |
| | | | | | | | | |
| Stock-based compensation | — | | | — | | | 15,591 | | | — | | | 15,591 | |
| Issuance of restricted and performance stock units | 343 | | | 3 | | | (3) | | | — | | | — | |
| Exercise of stock options and employee stock purchase plan | 365 | | | 4 | | | 1,802 | | | — | | | 1,806 | |
| | | | | | | | | |
| Net loss | — | | | — | | | — | | | (28,948) | | | (28,948) | |
| | | | | | | | | |
| Balance, December 31, 2022 | 42,445 | | | 424 | | | 360,155 | | | (259,580) | | | 100,999 | |
| | | | | | | | | |
| Stock-based compensation | — | | | — | | | 14,418 | | | — | | | 14,418 | |
| Issuance of restricted and performance stock units | 369 | | | 4 | | | (4) | | | — | | | — | |
| | | | | | | | | |
| Exercise of stock options and employee stock purchase plan | 310 | | | 3 | | | 1,961 | | | — | | | 1,964 | |
| | | | | | | | | |
| Net loss | — | | | — | | | — | | | (21,716) | | | (21,716) | |
| | | | | | | | | |
| Balance, December 31, 2023 | 43,124 | | | 431 | | | 376,530 | | | (281,296) | | | 95,665 | |
| | | | | | | | | |
| Stock-based compensation | — | | | — | | | 15,906 | | | — | | | 15,906 | |
| Issuance of restricted and performance stock units | 713 | | | 7 | | | (7) | | | — | | | — | |
| | | | | | | | | |
| Exercise of stock options and employee stock purchase plan | 312 | | | 3 | | | 2,297 | | | — | | | 2,300 | |
| | | | | | | | | |
| Net loss | — | | | — | | | — | | | (9,964) | | | (9,964) | |
| | | | | | | | | |
| Balance, December 31, 2024 | 44,149 | | | $ | 441 | | | $ | 394,726 | | | $ | (291,260) | | | $ | 103,907 | |
The accompanying notes are an integral part of these consolidated financial statements.
AXOGEN, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years ended December 31, 2024, 2023 and 2022
(In Thousands) | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (9,964) | | | $ | (21,716) | | | $ | (28,948) | |
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | |
| Depreciation | 6,467 | | | 4,218 | | | 2,827 | |
| Amortization of right-of-use assets | 1,103 | | | 1,062 | | | 1,761 | |
| Amortization of intangible assets | 267 | | | 273 | | | 265 | |
| | | | | |
| Amortization of debt discount and deferred financing fees | 893 | | | 891 | | | 891 | |
| Loss on disposal of equipment | — | | | 56 | | | — | |
| | | | | |
| Provision for (recovery of) bad debts | 650 | | | (271) | | | 612 | |
| | | | | |
| Investment gains | (155) | | | (666) | | | (228) | |
| Change in fair value of derivatives | (587) | | | (1,531) | | | (1,044) | |
| Stock-based compensation | 15,906 | | | 14,418 | | | 15,591 | |
| Change in operating assets and liabilities: | | | | | |
| Accounts receivable | 392 | | | (2,691) | | | (4,639) | |
| Inventory | (10,163) | | | (4,115) | | | (1,887) | |
| Prepaid expenses and other | 784 | | | (867) | | | (84) | |
| Accounts payable and accrued expenses | 125 | | | 6,509 | | | 660 | |
| Operating lease obligations | (1,603) | | | (1,269) | | | (1,841) | |
| Cash paid for interest portion of finance leases | (4) | | | (3) | | | (2) | |
| Contract and other liabilities | 424 | | | (14) | | | — | |
| Net cash provided by (used in) operating activities | 4,535 | | | (5,716) | | | (16,066) | |
| | | | | |
| Cash flows from investing activities: | | | | | |
| Purchase of property and equipment | (3,101) | | | (13,872) | | | (20,078) | |
| | | | | |
| Purchase of investments | (5,773) | | | (10,203) | | | (39,247) | |
| Proceeds from sale of investments | — | | | 44,374 | | | 57,300 | |
| Cash payments for intangible assets | (1,423) | | | (1,046) | | | (1,175) | |
| Net cash provided by (used in) investing activities | (10,297) | | | 19,253 | | | (3,200) | |
| | | | | |
| Cash flows from financing activities: | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Cash paid for debt portion of finance leases | (10) | | | (10) | | | (12) | |
| Proceeds from exercise of stock options and ESPP stock purchases | 2,300 | | | 1,964 | | | 1,806 | |
| | | | | |
| Net cash provided by financing activities | 2,290 | | | 1,954 | | | 1,794 | |
| Net increase (decrease) in cash, cash equivalents, and restricted cash | (3,472) | | | 15,491 | | | (17,472) | |
| Cash, cash equivalents, and restricted cash, beginning of period | 37,026 | | | 21,535 | | | 39,007 | |
| Cash, cash equivalents, and restricted cash, end of period | $ | 33,554 | | | $ | 37,026 | | | $ | 21,535 | |
| | | | | | | | | | | | | | | | | |
| | | | | |
| Supplemental disclosures of cash flow activity: | | | | | |
| Cash paid for interest, net of capitalized interest | $ | 7,301 | | | $ | 1,944 | | | $ | — | |
| Supplemental disclosure of non-cash investing and financing activities: | | | | | |
| Acquisition of fixed assets in accounts payable and accrued expenses | $ | 114 | | | $ | 704 | | | $ | 866 | |
| | | | | |
| | | | | |
| Obtaining a right-of-use asset in exchange for a lease liability | $ | 21 | | | $ | 2,298 | | | $ | 1,018 | |
| | | | | |
| Acquisition of intangible assets in accounts payable and accrued expenses | $ | 299 | | | $ | 407 | | | $ | 299 | |
The accompanying notes are an integral part of these consolidated financial statements.
AXOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024, 2023 and 2022
(In thousands. except shares and per share amounts)
1.Nature of Business
Axogen, Inc. (together with its wholly-owned subsidiaries, the “Company”) was incorporated in Minnesota. The Company's business is focused on the science, development and commercialization of the technologies used for peripheral nerve regeneration and repair. The Company's products include Avance® Nerve Graft, Axoguard Nerve Connector®, Axoguard Nerve Protector®, Axoguard HA+ Nerve Protector™, Axoguard Nerve Cap®, and Avive+ Soft Tissue Matrix™. The Company is headquartered in Florida. The Company has processing, warehousing, and distribution facilities in Ohio and Texas.
The Company manages its operations as a single operating segment. Substantially all of the Company's assets are maintained in the United States ("U.S."). The Company derives substantially all of its revenues from sales to customers in the U.S.
2. Summary of Significant Accounting Policies
Reclassifications
Certain reclassifications have been made to the prior period financial information to conform to the presentation used in the Consolidated Statement of Operations for the year ended December 31, 2024.
Effective in the first quarter of 2024, the Company voluntarily changed its accounting policy for shipping and handling costs. Under the new accounting policy, these costs are included in Cost of goods sold, whereas they were previously included in Sales and marketing costs and expenses. Including these expenses in Cost of goods sold better aligns these costs with the related revenue in the gross profit calculation. Although the prior method of accounting continues to be an accepted alternative, the new accounting policy is more widely used in the industry and provides improved comparability of the Company's financial statements to its peers. This change in accounting policy has been applied retrospectively. The Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 have been reclassified to reflect this change in accounting policy. The impact of this reclassification was an increase of $5,632 and $5,549 to Cost of goods sold for the years ended December 31, 2023 and 2022, respectively, and a corresponding decrease to Sales and marketing costs and expenses in the same periods.
Effective in the first quarter of 2024, the Company also ceased allocating certain costs to and from certain departments. Previously such costs had been allocated based on the Company’s estimate of the proportionate share of total expense to Cost of goods sold, Sales and marketing costs and expenses, Research and development costs and expenses, and General and administrative costs and expenses. The Company determined that these changes would better reflect industry practice and provide more meaningful information, as well as increased transparency of its operations. This change in presentation has been applied retrospectively. The Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 have been reclassified to reflect this change. Accordingly, $3,470 and $4,148 were reclassified to General and administrative costs and expenses and $374 and $79 were reclassified to Cost of goods sold, of which $2,849 and $2,696 were previously included in Sales and marketing costs and expenses and $995 and $1,531 were previously included in Research and development costs and expenses for the years ended December 31, 2023 and 2022, respectively, on the Consolidated Statements of Operations.
These reclassifications had no impact on net revenue, loss from operations, net loss, or loss per common share for prior periods and do not represent a restatement of the Company's previously issued consolidated financial statements.
Basis of Presentation
The accompanying consolidated financial statements of the Company are prepared in accordance with accounting principles generally accepted in the United States of America ("U.S. GAAP"). All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The significant estimates affecting the amounts reported or disclosed in the consolidated financial statements include the realizable value of inventories, the valuation of stock-based compensation, the valuation of derivative instruments, and the fair
value of debt instruments. Other estimates that affect the amounts reported or disclosed in the consolidated financial statements include the allowance for doubtful accounts, the useful life and recoverability of long-lived assets, incremental borrowing rates for operating leases, and accounting for income taxes, including the realizability of deferred tax assets and the related valuation allowance. The Company bases its estimates on historical and anticipated results, trends, and various other assumptions that management believes are reasonable under the circumstances, including assumptions as to future events. Actual results may differ from those estimates.
Risk and Uncertainties
The Company is dependent on its suppliers, including single source suppliers, some of which are outside of the U.S., and the inability of these suppliers to deliver necessary components of their products in a timely manner at prices, quality levels and volumes acceptable to the Company, or the Company's inability to effectively manage these components from its suppliers, could have a material adverse effect on the Company's business, financial condition and operating results.
Cash and Cash Equivalents
Cash and cash equivalents consist of short-term, highly liquid investments with original maturities of three months or less from the date of acquisition. Certain of the Company's cash and cash equivalents balances exceed Federal Deposit Insurance Corporation ("FDIC") insured limits or are invested in money market accounts with investment banks that are not FDIC-insured. The Company places its cash and cash equivalents in what they believe to be credit-worthy financial institutions. As of December 31, 2024, $27,054 of the cash and cash equivalents balance was in excess of FDIC limits.
Restricted Cash
Amounts included in restricted cash represent those required to be set aside to meet contractual terms of a lease agreement held by the Company. See Note 9 - Long-Term Debt, Net of Debt Discount and Financing Fees- Other Credit Facilities.
The following table provides a reconciliation of cash and cash equivalents, and restricted cash reported on the Consolidated Balance Sheets that sum to the total of the same reported on the Consolidated Statements of Cash Flows:
| | | | | | | | | | | |
| (in thousands) | December 31,
2024 | | December 31,
2023 |
| Cash and cash equivalents | $ | 27,554 | | | $ | 31,024 | |
| Restricted cash | 6,000 | | | 6,002 | |
| Total cash and cash equivalents, and restricted cash shown on the Consolidated Statements of Cash Flows | $ | 33,554 | | | $ | 37,026 | |
Investments
Investments, consisting of U.S. Treasuries, are classified as available-for-sale and have maturities less than one year as of December 31, 2024. Investments are carried at fair value based upon quoted market prices. The Company elected the fair value option ("FVO") for all of its available-for-sale investments. The FVO election results in all changes in unrealized gains and losses being included in Investment income on the Consolidated Statements of Operations.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at invoiced amounts and do not bear interest. The Company grants credit to customers in the normal course of business, but generally does not require collateral or other security to support its receivables.
An allowance for doubtful accounts is established for estimated uncollectible receivables based on the Company's assessment of the collectability of customer accounts. The Company recognizes the provision in General and administrative costs and expenses on the Consolidated Statements of Operations. In determining the amount of the allowance, the Company considers aging of account balances, historical credit losses, customer-specific information, the current economic environment, supportable forecasts, and other relevant factors. Uncollectible receivables are written off against the allowance for doubtful accounts when all attempts to collect the receivable have been exhausted.
Concentration Risk
Credit Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash and cash equivalents, which are held at major financial institutions, and trade receivables.
The Company's products are sold on an uncollateralized basis and on credit terms.
None of the Company's customers accounted for 10% or more of the consolidated revenues or accounts receivable during the years ended December 31, 2024, 2023 and 2022.
Inventory
Inventory, consisting of materials, direct labor, and manufacturing overhead, are stated at the lower of cost or net realizable value. At each balance sheet date, the Company evaluates inventory for excess quantities, obsolescence or shelf life.
The Company monitors the shelf life of its products and historical expiration and spoilage trends, and reserves for inventory based on the estimated amount of inventory that will not be distributed before expiration or spoilage. To estimate the amount of inventory that will expire or spoil prior to being distributed, the Company reviews inventory quantities on hand, historical and projected distribution levels, historical expiration trends, and historical spoilage trends. The Company’s calculation of the amount of inventory that will expire prior to distribution has three components: (i) a spoilage-based component that compares historical spoilage rates to inventory quantities on hand, (ii) a demand or consumption-based component that compares projected distribution to inventory quantities on hand; and (iii) an expiring inventory component that assesses the risk related to inventory that is near expiration. The Company’s model assumes that inventory will be distributed on a first-in-first-out basis. Due to the nature of the inventory (surgical implants with expiration dates) and the fact that a significant portion of the Company’s inventory is at medical facility consignment locations, estimating the amount of spoilage, the amount of inventory that will expire, and the amount of inventory that should be reserved for involves significant judgments and estimates.
Property and Equipment, Net
Property and equipment, net are stated at historical cost less accumulated depreciation and amortization. Additions and improvements that extend the lives of the assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. Leasehold improvements are amortized on a straight-line basis over the shorter of the asset’s estimated useful life or the remaining lease term. Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets ranging from three to thirty-nine years.
Gains or losses on the disposition of property and equipment are recorded in the period incurred and recorded in General and administrative costs and expenses on the Consolidated Statements of Operations.
Capitalized Interest
The interest cost on capital projects, including facility build-outs, is capitalized and included in the cost of the project. Capitalization begins with the first expenditure for the project and continues until the project is substantially complete and ready for its intended use. For the years ended December 31, 2024 and 2023, the Company capitalized $— and $5,285, respectively, of interest expense into property and equipment.
Impairment of Long-Lived Assets
The Company analyzes long-lived assets (asset groups), including property and equipment and definite-lived intangible assets, for impairment whenever events or changes in circumstances indicate that the carrying amounts may not be recoverable. An impairment is recognized when the estimated undiscounted cash flows generated by those assets is less than the carrying amounts of such assets. If it is determined that a long-lived asset (asset groups) is not recoverable, an impairment loss would be calculated based on the excess of the carrying value of the long-lived asset (asset groups) over the fair value of the long-lived asset (asset groups). There have been no impairments of long-lived assets during the years ended December 31, 2024, 2023 and 2022.
Indefinite-lived intangible assets are not subject to amortization, however, annually in the third quarter or whenever an event occurs or circumstances indicate that the indefinite-lived intangible assets may be impaired, the Company evaluates qualitative factors to determine whether it is more likely or not that the fair value of the indefinite lived asset is less than its
carrying amount. The Company's qualitative evaluation includes an assessment of factors, including specific operating results as well as industry, market and general economic conditions. The Company may elect to bypass this qualitative evaluation and perform a quantitative test.
Intangible Assets, Net
Intangible assets are recorded at cost and include patents and patent application costs, licenses, and trademarks. Intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives and reported net of accumulated amortization. Amortization expense is recorded in General and administrative costs and expenses on the Consolidated Statements of Operations. The useful lives of intangible assets are as follows:
•License agreements(1): 17 to 20 years
•Patents: up to 20 years
•Trademarks: indefinite lived
(1) The Company pays royalty fees based on net sales of the licensed products, which are recorded in Sales and marketing costs and expenses on the Consolidated Statements of Operations. The license agreements expired during 2023. See Note 5 - Intangible Assets, Net.
Global Nerve Foundation
Periodically, the Company may make contributions to the Global Nerve Foundation ("GNF"), a related party, due to certain executives of the Company being members of GNF's board of directors. The GNF was incorporated in 2021 exclusively for charitable, educational, and scientific purposes and qualifies under Internal Revenue Code 501(c)(3) as an exempt private foundation. Under its charter, the GNF engages in activities that focus on improving the awareness and care of patients with peripheral nerve injuries through grants, contributions and other appropriate means. The GNF is a separate legal entity and is not a subsidiary of the Company; therefore, its results are not included in the accompanying consolidated financial statements.
On July 20, 2024, the Company entered into a Qualified Founding Partner Agreement ("Agreement") which grants certain benefits to the Company related to the GNF's programming and marketing. The Agreement terminates on December 31, 2031. The Company may terminate the Agreement early by giving notice of termination between January 2 and January 31 in any calendar year; however, if the Company terminates the Agreement early, it will be required to make the next two payments per the payment schedule in a total amount not to exceed $100. Per the terms of the Agreement, the Company contributed $100, $— and $700 to the GNF during the years ended December 31, 2024, 2023 and 2022, respectively, and will contribute $175 in 2025. These contributions were recorded in Sales and marketing costs and expenses on the Consolidated Statements of Operations.
Fair Value
The Company uses fair value measurements to record fair value adjustments to certain assets and liabilities and to determine fair value disclosures. Cash equivalents, investments, and derivative instruments are recorded at fair value on a recurring basis. Fair value is defined as the price that would be received upon the sale of an asset or paid to transfer a liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy defines a three-level valuation hierarchy for classification and disclosure of fair value measurements as follows:
Level 1 – Quoted prices in active markets for identical assets or liabilities.
Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Derivative Instruments
The Company reviews its debt instruments in determining whether there are embedded derivative instruments, which are required to be bifurcated and accounted for separately as a derivative financial instrument. Embedded derivatives that are not
clearly and closely related to the debt host are bifurcated and recognized at fair value on the Consolidated Balance Sheets with changes in fair value recognized as either a gain or loss on the Consolidated Statement of Operations for each reporting period. The fair value of embedded derivatives is measured based on equity markets and interest rates, as well as an estimate of the Company's nonperformance risk adjustment. This estimate includes an option adjusted spread and an estimate of the Company's risk-free rate.
Leases
The Company determines if a contract contains a lease at the inception date and determines the lease classification, recognition, and measurement at commencement date. All operating lease commitments with a lease term greater than twelve months are recognized as right-of-use assets and obligations on a discounted basis on the Consolidated Balance Sheets. Leases with an initial term of twelve months or less are not recorded on the Consolidated Balance Sheets.
The Company classifies a lease based on whether the arrangement is effectively a purchase of the underlying asset. Leases that transfer the control of the underlying asset are classified as finance leases and all others are classified as operating leases. Interest and amortization expense are recognized for operating leases on a straight-line basis. If a change to the lease term leads to a reassessment of the lease classification and remeasurement, assumptions such as the discount rate and variable rents based on a rate or index will be updated as of the remeasurement date. If an arrangement is modified, the Company will reassess whether the arrangement contains a lease. Any subsequent changes in lease payments are recognized when incurred, unless the change requires a remeasurement of the lease liability.
Certain of the Company's leases include options for the Company to extend the lease term. The exercise of a lease renewal option is generally at the Company's sole discretion. Certain of the Company's lease agreements include provisions for the Company to reimburse the lessor for common area maintenance, real estate taxes, and insurance, which the Company accounts for as variable lease costs. The Company's lease agreements do not contain any material residual value guarantees or material restrictive covenants.
The Company accounts for its sublease income on a net basis, recording the sublease income with the operating lease expense on its Consolidated Statements of Operations.
Revenue Recognition
The Company enters into contracts to sell and distribute products and services to hospitals and surgical facilities for use in caring for patients with peripheral nerve damage or transection. Revenue is recognized when the Company transfers control of the products and services to the Company’s customers when the product is shipped or when it is delivered to the customer depending on the agreement. Products are primarily transferred to customer at a point in time.
A portion of the Company's product revenue is generated from consigned inventory maintained at hospitals and independent sales agencies, and also from inventory physically held by field sales representatives. For these types of product sales, the Company retains control until the product has been used or implanted, at which time revenue is recognized.
In the case of products or services sold to a customer under a distribution or purchase agreement, the customers are granted exclusive distribution rights to sell the implants internationally in a territory defined by the contract. These international distributor agreements contain provisions that allow the Company to terminate the distribution agreement with the distributor, and upon termination, the right to repurchase inventory from the distributor at the distributor’s cost. The Company has determined that its contractual rights to repurchase distributor inventory upon termination of the distributor agreement are not substantive and do not impact the timing of when control transfers; and therefore, the Company has determined it is appropriate to recognize revenue when: (i) the product is shipped via common carrier; or (ii) the product is delivered to the customer or distributor, depending on the terms of the agreement. Determining the timing of revenue recognition for such contracts is subject to judgment because an evaluation must be made regarding the distributor’s ability to direct the use of, and obtain substantially all of the remaining benefits from, the implants received from the Company. Changes in these assessments could have an impact on the timing of revenue recognition from sales to distributors. The Company accounts for shipping and handling activities as a fulfillment cost rather than a separate performance obligation. Amounts billed to customers for shipping and handling are included as part of the transaction price and recognized as revenue when control of the underlying products is transferred to the customer.
The Company operates in a single reportable segment of peripheral nerve repair, offers similar products to its customers, and enters into consistently structured arrangements with similar types of customers. As such, the Company does not disaggregate revenue from contracts with customers as the nature, amount, timing, and uncertainty of revenue and cash flows does not materially differ within and among the contracts with customers.
The contract with the customer states the final terms of the sale, including the description, quantity, and price of each implant distributed. The payment terms and conditions in the Company’s contracts vary; however, as a common business practice, payment terms are typically due in full within 30 days of delivery. Since the customer agrees to a stated price in the contract that does not vary over the contract term, the contracts do not contain any material types of variable consideration, and contractual rights of return are not material. The Company has several contracts with distributors in international markets that include consideration paid to the customer in exchange for distinct marketing and other services. The Company records such consideration paid to the customer as a reduction to revenue from the contracts with those distributor customers, which totaled $1,271, $1,056 and $856 for the years ended December 31, 2024, 2023 and 2022, respectively.
Government Assistance
As there is no authoritative guidance under U.S. GAAP for accounting for grants to for-profit business entities, the Company accounts for the grants by analogy to International Accounting Standards 20 Accounting for Government Grants and Disclosure of Government Assistance. Government assistance and grants are recognized when there is reasonable assurance that the Company has met the requirements of the assistance and there is reasonable assurance that the grant will be received. The Company received government grants of $717, $393 and $158 during the years ended December 31, 2024, 2023 and 2022, respectively.
Government grants totaling $300, $393 and $158 were recorded as an offset to Research and development costs and expenses on the Consolidated Statements of Operations during the years ended December 31, 2024, 2023 and 2022, respectively. Government assistance totaling $87 was recorded as an offset to General and administrative costs and expenses on the Consolidated Statement of Operations during the year ended December 31, 2024 and none were offset in 2023 and 2022. Deferred grants totaling $330 were recorded in Accounts payable and accrued expenses as of December 31, 2024 on the Consolidated Balance Sheet.
Cost of Goods Sold
Cost of goods sold includes materials, direct labor, and manufacturing overhead costs related to each product sold or produced, including processing, quality assurance labor, scrap, and inbound freight costs, as well as facility, warehousing and overhead supporting the Company's manufacturing operations. All of the Company's manufacturing costs are included in Cost of goods sold on the Consolidated Statements of Operations.
Research and Development Costs and Expenses
Research and development costs and expenses are charged to expense as incurred. Costs of research and development activities relate to product development, clinical trial expenses, and technical support of products. Costs primarily consist of salaries, wages, consulting fees, and depreciation and maintenance of research facilities and equipment.
Shipping and Handling
All shipping and handling costs, including facility and warehousing overhead, directly related to bringing the Company’s products to their final selling destination are included in Cost of goods sold on the Consolidated Statements of Operations. The Company has elected to account for shipping and handling costs for products shipped to customers as a fulfillment activity as the costs are incurred as part of the transfer of the goods to the customer in accordance with Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") 606. See Reclassifications above.
Income Taxes
The Company uses the asset and liability method to account for income taxes in accordance with the authoritative guidance for income taxes. Under this method, deferred tax assets and liabilities are determined based on future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and tax loss and credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates applied to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that has a greater than 50% likelihood of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs.
The Company identifies and evaluates uncertain tax positions, if any, and recognizes the impact of uncertain tax positions for which there is a less than more-likely-than-not probability of the position being upheld when reviewed by the relevant taxing authority. Such positions are deemed to be unrecognized tax benefits, and a corresponding liability is established on the Consolidated Balance Sheet. The Company has not recognized a liability for uncertain tax positions. If there were an unrecognized tax benefit, the Company would recognize interest accrued related to unrecognized tax benefits in interest expense and penalties in operating expenses.
Stock-Based Compensation
Stock-based compensation is in the form of stock options, restricted stock units ("RSU"), and performance stock units ("PSU") granted to employees and directors. Stock-based compensation expense is based on the fair value of the stock options, RSUs and PSUs.
The Company estimates the fair value of each stock option award on the date of grant using a multiple-point Black-Scholes option-pricing model. In addition, the Company estimates the grant date fair value of stock options granted to employees at a premium price based on market conditions, such as the trading price of the Company’s common stock, using a Monte Carlo simulation option-pricing model.
The Company estimates the fair value of RSU grants based upon the grant date closing market price of the Company’s common stock.
The fair value of the PSU grants is based on the Company’s closing stock price on the grant date and its estimate of achieving the applicable performance target goals. The number of PSUs that will ultimately be earned is based upon the Company’s performance as measured against specified targets over the measurement period. Expectations related to the achievement of performance goals associated with PSU grants is assessed as of each reporting period and is used to determine whether PSU grants are expected to vest. If performance-based milestones related to PSU grants are not met or not expected to be met, any compensation expense recognized associated with such grants is reversed.
The grant date fair value of the Total Shareholder Return PSU ("TSR PSU") grants is calculated using a Monte Carlo simulation. The number of PSUs that will be earned is based upon the achievement of stock price hurdles ("Price Targets") over the measurement period. Compensation expense is recognized regardless if the Price Targets are satisfied. Compensation expense is reversed if an employee's employment is terminated prior to satisfying the requisite service period.
The Company recognizes expense for all stock-based compensation awards, including stock options, RSUs, and PSUs granted to employees eligible for retirement, as defined within the award notice and allowing for continued vesting post-retirement, over the retirement notice period and continuously updates its estimate of expense over the notice period each reporting period if a retirement notice has not been provided.
The Company recognizes compensation expense related to the Employee Stock Purchase Plan (“ESPP”) based on the estimated fair value of the options on the date of grant. The Company estimates the grant date fair value, and the resulting stock-based compensation expense, using a Black-Scholes option pricing model for each purchase period. The grant date fair value is expensed on a straight-line basis over the offering period.
The determination of fair value using option-pricing models, as indicated above, is affected by the Company’s stock price, as well as assumptions regarding several subjective variables. These variables include, but are not limited to, the Company’s expected stock price volatility over the expected term of the awards. The Company determines the expected term of each award giving consideration to the contractual terms, vesting schedules, and post-vesting forfeitures. The Company uses the risk-free interest rate on the implied yield available on U.S. Treasury issues with an equivalent remaining term approximately equal to the expected term of the award. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in the Company’s Consolidated Statements of Operations. The expense is reduced for forfeitures as they occur.
Net Loss Per Share
Basic net loss per common share is computed by dividing reported net loss by the weighted average number of common shares outstanding during the period without consideration of potentially dilutive securities. Diluted net loss per share reflects the potential dilution that could occur if contracts to issue common stock were exercised or converted into common stock of the Company. Diluted net loss per share is the same as basic net loss per common share for all periods presented, since the effect of the potentially dilutive securities are anti-dilutive. Potential dilutive common share equivalents consist of the incremental common shares issuable upon exercise of vested stock options, RSUs, and PSUs.
Recently Issued Accounting Pronouncements
In December 2023, the FASB issued Accounting Standards Update 2023-09 — Income Taxes (Topic 740) — Improvements to Income Tax Disclosures ("ASU 2023-09"). The new guidance provides for disclosure on an annual basis of the following: (i) specific categories in the rate reconciliation and (ii) additional information for reconciling items that meet a quantitative threshold of greater than 5% of the amount computed by multiplying pretax income (or loss) by the applicable statutory income tax rate. The amendment in ASU 2023-09 is effective for annual periods beginning after December 15, 2024, and early adoption is permitted. The Company expects to enhance annual income tax reporting disclosures based on the new requirements.
In November 2024, the FASB issued Accounting Standards Update 2024-03 — Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220-40) — Disaggregation of Income Statement Expenses ("ASU 2024-03"), and in January 2025, the FASB issued Accounting Standards Update No. 2025-01, Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220-40): Clarifying the Effective Date ("ASU 2025-01"). ASU 2024-03 requires additional disclosure of the nature of expenses included in the income statement as well as disclosures about specific types of expenses included in the expense captions presented in the income statement. ASU 2024-03, as clarified by ASU 2025-01, is effective for annual periods beginning after December 15, 2026 and interim reporting periods beginning after December 15, 2027. Both early adoption and retrospective application are permitted. The Company expects to enhance annual expense disclosures based on the new requirements.
Recently Adopted Accounting Pronouncements
In November 2023, the FASB issued Accounting Standards Update 2023-07 — Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures ("ASU 2023-07"). The new guidance requires disclosure on an annual and interim basis of the following: (i) significant segment expenses regularly provided to the chief operating decision maker ("CODM") and a measure of segment profit or loss; (ii) an amount for other segment items by reportable segment and a description of its composition; (iii) all annual disclosures about a reportable segment's profit and loss and assets as currently required by Topic 280; (iv) clarify if the CODM uses more than one measure of a segment's profit or loss in assessing segment performance and deciding how to allocate resources; and (v) disclose title and position of the CODM and how the CODM uses the reported measures. Public entities with a single reportable segment are required to provide all the disclosures required by this amendment. The Company adopted ASU 2023-07 effective December 31, 2024 and the adoption of this accounting standard did not have an impact on the Company's consolidated financial statements. See Note 13 - Segments.
All other Accounting Standards Updates issued and not yet effective as of December 31, 2024, and through the date of this report, were assessed and determined to be either not applicable or are expected to have minimal impact on the Company’s current or future financial position or results of operations.
3.Inventory
Inventory consists of the following:
| | | | | | | | | | | |
| (in thousands) | December 31,
2024 | | December 31,
2023 |
| Finished goods | $ | 27,054 | | | $ | 13,545 | |
| Work in process | 1,325 | | | 2,120 | |
| Raw materials | 4,804 | | | 7,355 | |
| Inventory | $ | 33,183 | | | $ | 23,020 | |
The provision for inventory write-down is as follows for the years ended:
| | | | | | | | | | | | | | | | | |
| December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Provision for inventory write-down | $ | 6,989 | | | $ | 1,939 | | | $ | 1,769 | |
As of December 31, 2024 and 2023, the Company reserved $1,630 and $1,342, respectively, for potential losses relating to inventory.
4.Property and Equipment, Net
Property and equipment, net consist of the following:
| | | | | | | | | | | |
| (in thousands) | December 31,
2024 | | December 31,
2023 |
| Land | $ | 731 | | | $ | 731 | |
| Building | 60,679 | | | 60,679 | |
| Leasehold improvements | 17,977 | | | 15,348 | |
| Processing equipment | 13,950 | | | 13,116 | |
| Furniture and equipment | 9,583 | | | 8,741 | |
| Finance lease right-of-use assets | 159 | | | 138 | |
| Projects in process | 1,499 | | | 3,674 | |
| Property and equipment, at cost | 104,578 | | | 102,427 | |
| Less: accumulated depreciation and amortization | (19,911) | | | (13,697) | |
| Property and equipment, net | $ | 84,667 | | | $ | 88,730 | |
Depreciation expense is as follows for the years ended:
| | | | | | | | | | | | | | | | | |
| December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Depreciation expense | $ | 6,467 | | | $ | 4,218 | | | $ | 2,827 | |
5.Intangible Assets, Net
Intangible assets consist of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2024 | | December 31, 2023 |
| (in thousands) | | Gross Carrying Amount | | Accumulated Amortization | | Net Carrying Amount | | Gross Carrying Amount | | Accumulated Amortization | | Net Carrying Amount |
| Amortizable intangible assets: | | | | | | | | | | | | |
| Patents | | $ | 6,090 | | | $ | (1,073) | | | $ | 5,017 | | | $ | 4,905 | | | $ | (820) | | | $ | 4,085 | |
| License agreements | | — | | | — | | | — | | | 1,101 | | | (1,087) | | | 14 | |
| Total amortizable intangible assets | | 6,090 | | | (1,073) | | | 5,017 | | | 6,006 | | | (1,907) | | | 4,099 | |
| | | | | | | | | | | | |
| Unamortized intangible assets: | | | | | | | | | | | | |
| Trademarks | | 562 | | | — | | | 562 | | | 432 | | | — | | | 432 | |
| Total intangible assets | | $ | 6,652 | | | $ | (1,073) | | | $ | 5,579 | | | $ | 6,438 | | | $ | (1,907) | | | $ | 4,531 | |
The amortization expense is as follows for the years ended:
| | | | | | | | | | | | | | | | | |
| December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Amortization expense | $ | 267 | | | $ | 273 | | | $ | 265 | |
As of December 31, 2024, future amortization of patents and license agreements is as follows:
| | | | | |
| Year Ending December 31, | (in thousands) |
| 2025 | $ | 292 | |
| 2026 | 292 | |
| 2027 | 292 | |
| 2028 | 292 | |
| 2029 | 292 | |
| Thereafter | 3,557 | |
| Total | $ | 5,017 | |
License Agreements
The Company had multiple license agreements with the University of Florida Research Foundation and the University of Texas at Austin (the "License Agreements") in which the Company acquired exclusive worldwide licenses for underlying technology used in repairing and regenerating nerves. The licensed technologies included the rights to issue patents and patents pending in the U.S. and international markets. The License Agreement with the University of Texas expired in September 2023 and the royalty obligations associated with the License Agreement with the University of Florida Research Foundation expired in December 2023.
The Company paid royalty fees ranging from 1% to 3% under the License Agreements based on net sales of licensed products. Also, when the Company paid royalties to more than one licensor for sales of the same product, a royalty stack cap applied, capping total royalties at 3.75%.
Royalty fees included in Sales and marketing costs and expenses on the Consolidated Statements of Operations are as follows for the years ended:
| | | | | | | | | | | | | | | | | |
| December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Royalty fees | $ | — | | | $ | 3,110 | | | $ | 3,103 | |
6.Fair Value Measurement
The following tables represent the Company’s fair value hierarchy for its financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2024 and 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Level 1 | | Level 2 | | Level 3 | | Total |
| December 31, 2024 | | | | | | | |
| Assets: | | | | | | | |
| Money market funds | $ | 19,399 | | | $ | — | | | $ | — | | | $ | 19,399 | |
| U.S. Treasuries | 5,928 | | | — | | | — | | | 5,928 | |
| | | | | | | |
| | | | | | | |
| Total assets | $ | 25,327 | | | $ | — | | | $ | — | | | $ | 25,327 | |
| | | | | | | |
| Liabilities: | | | | | | | |
| | | | | | | |
| Debt derivative liabilities | $ | — | | | $ | — | | | $ | 2,400 | | | $ | 2,400 | |
| Total liabilities | $ | — | | | $ | — | | | $ | 2,400 | | | $ | 2,400 | |
| | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 | Level 1 | | Level 2 | | Level 3 | | Total |
| Assets: | | | | | | | |
| Money market funds | $ | 24,977 | | | $ | — | | | $ | — | | | $ | 24,977 | |
| | | | | | | |
| | | | | | | |
| Total assets | $ | 24,977 | | | $ | — | | | $ | — | | | $ | 24,977 | |
| | | | | | | |
| Liabilities: | | | | | | | |
| | | | | | | |
| Debt derivative liabilities | $ | — | | | $ | — | | | $ | 2,987 | | | $ | 2,987 | |
| Total liabilities | $ | — | | | $ | — | | | $ | 2,987 | | | $ | 2,987 | |
| | | | | | | |
The changes in Level 3 liabilities measured at fair value on a recurring basis are as follows:
| | | | | | | | |
| (in thousands) | | Debt Derivative Liabilities |
| Balance, December 31, 2022 | | $ | 4,518 | |
| | |
| Change in fair value included in net loss | | (1,531) | |
| | |
| Balance, December 31, 2023 | | 2,987 | |
| | |
| Change in fair value included in net loss | | (587) | |
| Balance, December 31, 2024 | | $ | 2,400 | |
There were no changes in the levels or methodology of the measurement of financial assets or liabilities during the years ended December 31, 2024 and 2023.
The fair value of cash, restricted cash, accounts receivable, and accounts payable and accrued expenses approximates the carrying values because of the short-term nature of these instruments. The carrying value and fair value of the credit facility the Company has with Oberland Capital ("Credit Facility") were $47,496 and $51,307 at December 31, 2024, respectively, and $46,603 and $51,486 at December 31, 2023, respectively. See Note 9 - Long-Term Debt, Net of Debt Discount and Financing Fees.
The debt derivative liabilities are measured using a ‘with and without’ valuation model to compare the fair value of each tranche of the Credit Facility including the identified embedded derivative feature and the fair value of a plain vanilla note with the same terms. The fair value of the Credit Facility including the embedded derivative features was determined using a probability-weighted expected return model based on four potential settlement scenarios for the Credit Facility included in the table below. The estimated settlement value of each scenario, which would include any required make-whole payment (see Note 9 - Long-Term Debt, Net of Debt Discount and Financing Fees), is then discounted to present value using a discount rate that is derived based on the initial terms of the Credit Facility at issuance and corroborated utilizing a synthetic credit rating analysis.
The significant inputs that are included in the valuation of the debt derivative liability - first tranche include:
| | | | | | | | | | | | | | | | | |
| December 31, 2024 | | December 31, 2023 |
| Input | | | | | |
| Remaining term (years) | 2.5 years | | | 3.5 years | |
| Maturity date | June 30, 2027 | | | June 30, 2027 | |
| Coupon rate | 9.5% - 13.0% | | | 9.5% - 13.2% | |
| Revenue participation payments | Maximum each year | | | Maximum each year | |
| Discount rate | 12.22% | | 1 | | 12.06 | % | 1 |
| | | | | |
| | | | | |
| Probability of mandatory prepayment after 2024 | 15.0% | | 1 | | 15.0% | | 1 |
| Estimated timing of mandatory prepayment event after 2024 | March 31, 2026 | 1 | | March 31, 2026 | 1 |
| Probability of optional prepayment event | 5.0% | | 1 | | 5.0% | | 1 |
| Estimated timing of optional prepayment event | December 31, 2025 | 1 | | December 31, 2025 | 1 |
Probability of note held-to-maturity 2 | 80.0% | | 1 | | 80.0% | | 1 |
1 Represents a significant unobservable input.
2 See Maturity date in table.
The significant inputs that are included in the valuation of the debt derivative liability - second tranche include:
| | | | | | | | | | | | | | | | | |
| December 31, 2024 | | December 31, 2023 |
| Input | | | | | |
| Remaining term (years) | 3.5 years | | | 4.5 years | |
| Maturity date | June 30, 2028 | | | June 30, 2028 | |
| Coupon rate | 9.5% - 13.0% | | | 9.5% - 13.2% | |
| Revenue participation payments | Maximum each year | | | Maximum each year | |
| Discount rate | 15.48 | % | 1 | | 15.60 | % | 1 |
| | | | | |
| | | | | |
| Probability of mandatory prepayment after 2024 | 15.0% | | 1 | | 15.0% | | 1 |
| Estimated timing of mandatory prepayment event after 2024 | March 31, 2026 | 1 | | March 31, 2026 | 1 |
| Probability of optional prepayment event | 5.0% | | 1 | | 5.0% | | 1 |
| Estimated timing of optional prepayment event | December 31, 2025 | 1 | | December 31, 2025 | 1 |
Probability of note held-to-maturity2 | 80.0 | % | 1 | | 80.0 | % | 1 |
1 Represents a significant unobservable input.
2 See Maturity date in table.
7.Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses consist of the following:
| | | | | | | | | | | |
| (in thousands) | December 31,
2024 | | December 31,
2023 |
| Accounts payable | $ | 8,008 | | | $ | 11,774 | |
| Accrued expenses | 2,050 | | | 3,180 | |
| Accrued compensation | 18,583 | | | 13,929 | |
| Accounts payable and accrued expenses | $ | 28,641 | | | $ | 28,883 | |
8.Leases
The Company leases administrative, manufacturing, research, and distribution facilities through operating leases. Several leases include fixed payments including rent and non-lease components such as common-area or other maintenance costs.
The components of total lease expense are as follows for the years ended:
| | | | | | | | | | | | | | | | | |
| December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Finance lease costs | | | | | |
| Amortization of right-of-use assets | $ | 6 | | | $ | 4 | | | $ | 20 | |
| Interest on lease obligations | 4 | | | 3 | | | 2 | |
| Operating lease costs | | | | | |
| Operating lease costs | 3,664 | | | 3,316 | | | 4,077 | |
| Short-term lease costs | 576 | | | 520 | | | 72 | |
| Variable lease costs | 610 | | | 1,438 | | | 1,241 | |
| Total lease expense | $ | 4,860 | | | $ | 5,282 | | | $ | 5,412 | |
Supplemental balance sheet information related to the operating and financing leases is as follows:
| | | | | | | | | | | |
| (In thousands, except lease term and discount rate) | December 31, 2024 | | December 31, 2023 |
| Operating Leases | | | |
| Right-of-use operating assets | $ | 14,265 | | | $ | 15,562 | |
| Current maturities of long-term lease obligations | $ | 1,960 | | | $ | 1,541 | |
| Long-term lease obligations | $ | 19,191 | | | $ | 21,123 | |
| Financing Leases | | | |
Right-of-use financing leases (1) | $ | 37 | | | $ | 28 | |
| Current maturities of long-term lease obligations | $ | 9 | | | $ | 6 | |
| Long-term lease obligations | $ | 30 | | | $ | 19 | |
| | | |
| Weighted average operating lease term (in years): | 8.8 | | 9.6 |
| Weighted average financing term (in years): | 3.6 | | 6.5 |
| | | |
| Weighted average discount rate - operating leases | 10.95 | % | | 10.99 | % |
| Weighted average discount rate - financing leases | 14.06 | % | | 13.22 | % |
(1) Financing leases are included in Property and equipment, net on the Consolidated Balance Sheets.
Future minimum lease payments under operating and financing leases as of December 31, 2024 are as follows:
| | | | | |
| (In thousands) | |
| Year ending December 31, | |
| 2025 | $ | 4,148 | |
| 2026 | 4,284 | |
| 2027 | 3,120 | |
| 2028 | 3,119 | |
| 2029 | 3,187 | |
| Thereafter | 15,385 | |
| Total | 33,243 | |
| Less: Imputed interest | (12,053) | |
| Total lease liability | 21,190 | |
| Less: Current lease liability | (1,969) | |
| Long-term lease liability | $ | 19,221 | |
Lease modifications
The Company accounts for lease revisions as a lease modification in accordance with FASB ASC 842, Leases, when the modification effectively terminates the existing lease and creates a new lease. No lease modifications were recorded during the years ended December 31, 2024 and 2023.
Sublease Agreement
On May 31, 2024, the Company entered into a sublease agreement to sublease a portion of its headquarters in Tampa, Florida expiring on October 31, 2031. The Company or the sublessor can terminate the sublease agreement after sixty-three months with twelve months written notice. There is no option to extend the sublease agreement. The Company accounts for this sublease in accordance with FASB ASC 842, Leases.
9.Long-Term Debt, Net of Debt Discount and Financing Fees
Long-term debt, net of debt discount and financing fees consists of the following:
| | | | | | | | | | | | | |
| (in thousands) | December 31, 2024 | | December 31, 2023 | | |
| Credit Facility - first tranche | $ | 35,000 | | | $ | 35,000 | | | |
| Credit Facility - second tranche | 15,000 | | | 15,000 | | | |
| Less - unamortized debt discount and deferred financing fees | (2,504) | | | (3,397) | | | |
| Long-term debt, net of debt discount and financing fees | $ | 47,496 | | | $ | 46,603 | | | |
Credit Facility
On June 29, 2023, the Company amended its Credit Facility with Oberland Capital and its affiliates, TPC Investments II LP and Argo LLC (collectively, the "Lender"), to transition the base interest rate from three-month LIBOR to the forward looking term rate based on the secured overnight financing rate as set by the Federal Reserve Bank of New York plus 0.10% ("Adjusted SOFR"). The Company obtained the first tranche of $35,000 at closing on June 30, 2020. On June 30, 2021, the second tranche of $15,000 was drawn down by the Company.
Each tranche under the Credit Facility requires quarterly interest payments for seven years. Interest is calculated as 7.5% plus the greater of Adjusted SOFR or 2.0% (12.19% as of December 31, 2024); provided that the interest rate shall never be less than 9.5%. Each tranche of the Credit Facility has a term of seven years from the date of issuance (with the first tranche issued on June 30, 2020, maturing on June 30, 2027, and the second tranche issued on June 30, 2021, maturing on June 30, 2028). In connection with the Credit Facility, the Company entered into a revenue participation agreement (the “Revenue Participation Agreement”) with the Lender, which provided that, among other things, a quarterly royalty payment as a percentage of the Company’s net revenues up to $70 million in any given year, after April 1, 2021, ending on the date upon
which all amounts owed under the Credit Facility have been paid in full. This structure results in approximately 1.5% per year of additional interest payments on the outstanding loan amount. The Company recorded interest expense for this Revenue Participation Agreement of $756 for each of the years ended December 31, 2024, 2023 and 2022, respectively. The Company pays the quarterly debt interest on the last day of the quarter, and for the years ended December 31, 2024 and 2023, paid $6,475 and $6,436, respectively, to the Lender. The Company capitalized interest of $— and $5,285 for the years ended December 31, 2024 and 2023, respectively, towards the costs to construct and retrofit its Axogen Processing Center facility (the "APC Facility") in Vandalia, Ohio, which was completed during 2023.
The amounts outstanding under the Credit Facility may be accelerated upon certain events, including: (a) required mandatory prepayments upon an asset sale; (b) in the event the Company is subject to (i) any litigation brought by a Governmental Authority (as defined in the Credit Facility) including intervention after litigation is commenced by a Person (as defined in the Credit Facility), or (ii) any final administrative action by a Governmental Authority, in each case arising out of or in connection with any of the Company’s registry studies, payments made to doctors or training activities with respect to healthcare professionals (excluding certain final administrative actions that have been fully and finally resolved by the parties pursuant to a settlement agreement) or (c) upon the occurrence of an event of default (either automatically or at the option of the Lender depending on the nature of the event). In addition, the Company has the right to prepay any amounts outstanding under the Credit Facility. Upon maturity or upon such earlier repayment of the Credit Facility, the Company will repay the principal balance and provide a make-whole payment calculated to generate an internal rate of return to the Lender equal to 11.5%, less the total of all quarterly interest and royalty payments previously paid to the Lender. See Note 15 - Commitments and Contingencies for further information related to the make-whole payment calculation.
Upon the occurrence of an event of default, the interest rate incurred on amounts outstanding under the Credit Facility will be increased by 4%. The Credit Facility includes a financial covenant requiring the Company to achieve certain revenue targets each quarter. As of December 31, 2024, the Company was in compliance with all the covenants. In the event of a failure to meet such covenants, the Company may avoid a default by electing to be subject to a liquidity covenant and meeting all of the obligations required by such covenant. The borrowings under the Credit Facility are secured by substantially all of the assets of the Company.
The debt derivative liabilities are recorded at fair value, with the change in fair value reported in Change in fair value of derivatives on the Consolidated Statements of Operations at each reporting date. See Note 6 - Fair Value Measurement.
Unamortized Debt Discount and Financing Fees
The unamortized debt discount consists of the remaining initial fair values of the embedded derivatives related to the Credit Facility.
The financing fees for the Credit Facility were $642 and recorded as a contra liability to Long-term debt on the Consolidated Balance Sheets.
Amortization of debt discount and deferred financing fees for the years ended December 31, 2024, 2023 and 2022 was $893, $891 and $891, respectively, and recorded in interest expense using the effective interest rate method.
Other Credit Facilities
The Company had restricted cash of $6,000 and $6,002 at December 31, 2024 and 2023, respectively. The December 31, 2024 and 2023 balances both include $6,000, which represents collateral for an irrevocable standby letter of credit.
10.Basic and Diluted Loss per Common Share
The following reflects the net loss attributable to common shareholders and share data used in the basic and diluted earnings per share computations using the two-class method as of:
| | | | | | | | | | | | | | | | | | | | |
| December 31, |
| (in thousands, except share and per share amounts) | | 2024 | | 2023 | | 2022 |
| Numerator: | | | | | | |
| Net loss | | $ | (9,964) | | | $ | (21,716) | | | $ | (28,948) | |
| Denominator: | | | | | | |
| Weighted average common shares outstanding — basic and diluted | | 44,257,754 | | | 42,878,543 | | | 42,083,125 | |
| | | | | | |
| Net loss per common share (basic and diluted) | | $ | (0.23) | | | $ | (0.51) | | | $ | (0.69) | |
| | | | | | |
Anti-dilutive shares excluded from the calculation of diluted earnings per share (1) | | | | | | |
| Stock options | | 167,570 | | | 3,929 | | | 176,281 | |
| Restricted and performance stock units | | 1,770,253 | | | 733,012 | | | 576,010 | |
(1) These common equivalent shares are not included in the diluted per share calculations as they would be dilutive if the Company was in a net income position.
11.Stock-Based Compensation
The Company maintains two stock-based incentive plans: (i) The Axogen, Inc. Third Amended and Restated 2019 Long-Term Incentive Plan ("2019 Plan") which provides incentives through the grants of stock options, non-qualified stock options, PSUs and RSUs to employees, directors and consultants which replaced the Company's 2010 Stock Incentive Plan and (ii) The Axogen 2017 Employee Stock Purchase Plan (“2017 ESPP”).
At the June 5, 2024 Annual Shareholder Meeting, approval was received to increase the number of shares available under the 2019 Plan from 8,000,000 to 10,500,000.
As of December 31, 2024, there were 2,949,495 shares of common stock available for future grant under the 2019 Plan. Additionally, the Company issued 18,700 options, 330,000 RSUs and 600,000 PSUs as inducement grants to certain employees in accordance with Nasdaq Listing Rule 5635(c)(4).
Stock-based compensation expense is included in the following line items on the accompanying Consolidated Statements of Operations for the years ended:
| | | | | | | | | | | | | | | | | | | | |
| December 31, |
| (in thousands) | | 2024 | | 2023 | | 2022 |
| Cost of goods sold | | $ | 1,752 | | | $ | 796 | | | $ | 215 | |
| Sales and marketing | | 3,175 | | | 2,982 | | | 2,341 | |
| Research and development | | 3,417 | | | 3,875 | | | 2,640 | |
| General and administrative | | 7,562 | | | 6,764 | | | 10,395 | |
| Total stock-based compensation expense | | $ | 15,906 | | | $ | 14,418 | | | $ | 15,591 | |
Stock Options
Stock options granted to employees typically vest 50% two years after the grant date and 12.5% every six months thereafter for the remaining two-year period until fully vested after four years. Stock options granted to directors and certain options granted from time to time to certain executive officers vest ratably over three years or 25% per quarter over one year. Options typically have terms ranging from seven to ten years. The Company estimates the fair value of each option award on the date of grant using a multiple-point Black-Scholes option-pricing model. In addition, the Company estimates the grant date fair value of stock options granted to employees at a premium price based on market conditions, such as the trading price of the Company’s common stock, using a Monte Carlo simulation option-pricing model. The value of the portion of the award that is
ultimately expected to vest is recognized as expense over the requisite service periods in the Company’s Consolidated Statements of Operations. The expense is reduced for forfeitures as they occur.
The following weighted-average assumptions were used in the calculation of fair value for stock options granted for the following periods:
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | Year Ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Expected term (in years) | | 5.48 | | 5.40 | | 6.01 |
| Expected volatility | | 65.60 | % | | 59.32 | % | | 61.17 | % |
| Risk free interest rate | | 4.19 | % | | 3.52 | % | | 2.31 | % |
| Expected dividends | | — | % | | — | % | | — | % |
| | | | | | |
The following table summarizes the Company's stock option activity for the year ended December 31, 2024:
| | | | | | | | | | | | | | | | | | | | | | | |
| Options | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Life (Years) | | Aggregate
Intrinsic
Value (in thousands) |
| Outstanding at December 31, 2023 | 4,372,243 | | | $ | 12.87 | | | 6.43 | | $ | 87 | |
Granted (1) | 164,684 | | | $ | 7.35 | | | | | |
Forfeited (2) | (455,144) | | | $ | 16.46 | | | | | |
| Exercised | (154,831) | | | $ | 8.63 | | | | | |
| Outstanding at December 31, 2024 | 3,926,952 | | | $ | 12.39 | | | 4.56 | | $ | 22,692 | |
| | | | | | | |
| Exercisable at December 31, 2024 | 2,574,651 | | | $ | 14.37 | | | 3.53 | | $ | 11,933 | |
(1) Options granted include 18,700 inducement shares in accordance with Nasdaq Listing Rule 5635(c)(4).
(2) Options forfeited include 65,800 inducement shares in accordance with Nasdaq Listing Rule 5635(c)(4).
The weighted-average grant-date fair value of stock options granted during the years ended December 31, 2024, 2023 and 2022 was $4.33, $4.72 and $4.65, respectively.
The total intrinsic value of options exercised for the years ended December 31, 2024, 2023 and 2022 was $2,031, $1,710 and $2,643, respectively.
As of December 31, 2024, there was approximately $2,365 of total unrecognized compensation costs related to unvested stock options. These costs are expected to be recognized over a weighted-average period of 1.5 years.
Restricted Stock Units
RSUs granted to employees have a requisite service period of four years. The RSUs granted to directors and certain RSUs granted from time to time to certain executive officers and vice presidents have a requisite service period of three years, while certain of these RSUs have a requisite service period of one year. The Company estimates the fair value of RSU grants based upon the grant date closing market price of the Company’s common stock. The Company expenses the fair value of RSUs on a straight-line basis over the requisite service period.
The following table summarizes the activity for RSUs for the year ended December 31, 2024:
| | | | | | | | | | | | | | | | | | | | | | | |
| Outstanding Restricted Stock Units |
| Restricted Stock Units | | Weighted
Average Fair Value at Date of Grant per Share | | Weighted Average Remaining Vesting Life (Years) | | Aggregate Intrinsic Value (in thousands) |
| Unvested at December 31, 2023 | 2,335,230 | | | $ | 9.00 | | | 1.39 | | $ | 15,950 | |
Granted (1) | 1,010,671 | | | $ | 8.79 | | | | | |
| Vested and released | (703,060) | | | $ | 9.45 | | | | | |
Forfeited (2) | (284,518) | | | $ | 9.04 | | | | | |
| Unvested at December 31, 2024 | 2,358,323 | | | $ | 8.77 | | | 1.17 | | $ | 38,865 | |
(1) RSUs granted include 330,000 inducement shares in accordance with Nasdaq Listing Rule 5635(c)(4).
(2) RSUs forfeited include 37,500 inducement shares in accordance with Nasdaq Listing Rule 5635(c)(4).
The weighted-average grant-date fair value of RSUs granted during the years ended December 31, 2024, 2023 and 2022 was $8.79, $8.10 and $8.40, respectively.
As of December 31, 2024, there was approximately $11,576 of total unrecognized compensation costs related to unvested restricted stock. These costs are expected to be recognized over a weighted-average period of 2.2 years.
Performance Stock Units
The Company estimates the fair value of PSUs based on its closing stock price at the time of grant and its estimate of achieving the applicable performance target goals and records compensation expense as the milestones are achieved. The number of shares delivered to recipients and the related compensation cost recognized as an expense will be based on the actual performance metrics as set forth in the applicable PSU award agreement. The amount actually awarded will be based upon achievement of the performance measures.
The Company's TSR PSUs generally have a requisite service period of three years and are subject to graded vesting conditions based on goals defined within the award. The grant date fair value of the TSR PSUs is calculated using a Monte Carlo simulation. The Company expenses their fair value over the requisite service period. Over the performance period, the number of shares of common stock that will ultimately vest and be issued and the related compensation expense will be adjusted based upon the Company’s estimate of achieving such performance target.
PSUs issued in 2017 and 2019 tied to the achievement of certain milestones have performance periods through December 31, 2025 and a requisite service period of one year after the milestone achievement date but not sooner than one year after the grant date. PSUs issued in 2018 tied to the achievement of certain milestones have performance periods through January 1, 2025 and a requisite service period of one year after the milestone achievement date but not sooner than one year after the grant date.
PSUs issued in 2024 tied to the achievement of certain milestones have performance periods through December 31, 2025 and requisite service periods through the date of the milestone achievement but not sooner than one year after the grant date. The Company expenses the fair value upon the achievement of such milestone and subsequent requisite period.
The following table summarizes the activity for PSUs for the year ended December 31, 2024:
| | | | | | | | | | | | | | | | | | | | | | | |
| Outstanding Performance Stock Units |
| Performance Stock Units | | Weighted
Average Fair Value at Date of Grant per Share | | Weighted Average Remaining Vesting Life (Years) | | Aggregate Intrinsic Value (in thousands) |
| Outstanding as of December 31, 2023 | 1,257,412 | | | $ | 11.69 | | | 1.76 | | $ | 8,588 | |
Granted (1) | 1,904,668 | | | $ | 9.11 | | | | | |
| Released | (9,681) | | | $ | 25.14 | | | | | |
| Forfeited | (190,107) | | | $ | 14.05 | | | | | |
| Outstanding as of December 31, 2024 | 2,962,292 | | | $ | 9.84 | | | 1.64 | | $ | 48,819 | |
| Vested and deferred | 728,749 | | | $ | 8.24 | | | — | | | $ | 12,010 | |
(1) PSUs granted include 600,000 inducement shares in accordance with Nasdaq Listing Rule 5635(c)(4).
The weighted-average grant-date fair value of PSUs granted during the years ended December 31, 2024, 2023 and 2022 was $9.11, $8.29 and $8.23, respectively.
As of December 31, 2024, there was approximately $8,826 of total unrecognized compensation costs related to unvested PSU awards, excluding the PSU awards for milestones not achieved described in the paragraph below. These costs are expected to be recognized over a weighted-average period of 1.7 years.
During 2017, 2018 and 2019, the Company issued PSU awards to certain employees related to their work on the Company’s Biologics License Application ("BLA"). The number of shares was allocated to certain milestones related to the BLA submission to and approval by the FDA. The performance measure is based upon achieving each of the specific milestones and will vest 50% upon achieving each of the milestones and 50% one year later. As of December 31, 2024, 10% of the awards issued related to achieving the BLA submission milestone; 5% vested upon submission of the BLA during the third quarter of 2024 and 5% will vest in the third quarter of 2025. During 2024, the Company issued PSU awards to certain officers and employees related to their work on the Company’s BLA. The number of shares was allocated to certain milestones related to the BLA submission to and approval by the FDA. The performance measure is based upon achieving each of the specific milestones and will vest upon achieving each of the milestones but not sooner than one year after the grant date. As of December 31, 2024, 10% of the awards issued related to achieving the BLA submission milestone and will vest one year after the respective grant dates. The Company recognizes expense on these milestones upon the achievement. As of December 31, 2024, there was approximately $10,187 of total unrecognized compensation costs related to unvested milestones.
During 2022, the Company issued PSU awards to certain officers and employees tied to revenue from 2022 to 2024 with a pay-out range from 0% to 150% upon achievement of specific revenue targets. These awards were achieved at 99.2%, vested in the fourth quarter of 2024 and will be released in the first quarter of 2025.
During 2023, the Company issued PSU awards to certain officers and employees tied to revenue from 2023 to 2025 with a pay-out range from 0% to 150% upon achievement of specific revenue targets.
During 2024, the Company issued TSR PSU awards to certain officers and employees tied to the Company's share price targets with a pay-out range from 0% to 200% upon achievement of specific average share prices over a 30 day trading period immediately preceding the end of a performance period of February 2, 2024 through February 22, 2027.
Employee Stock Purchase Plan
The 2017 ESPP allows eligible employees to acquire shares of the Company's common stock through payroll deductions at a discount to market price (currently 15.0%) of the lesser of the closing price of the Company’s common stock on the first day or last day of the offering period. The offering period is currently 6 months. Participants may not purchase more than $25 or 3,000 shares of the Company’s common stock in a calendar year through the ESPP. The Company estimates the grant date fair value, and the resulting stock-based compensation expense, using a Black-Scholes option pricing model for each purchase period. Stock-based compensation expense related to the 2017 ESPP, included in total stock-based compensation expense, was $371, $333 and $844 for the years ended December 31, 2024, 2023 and 2022, respectively.
The following are the weighted average assumptions used in the valuation of ESPP options for the years ended December 31:
| | | | | | | | | | | | | | | | | | | | |
| | December 31, |
| | |
| | 2024 | | 2023 | | 2022 |
| Expected term (in years) | | 0.5 | | 0.5 | | 0.5 |
| Expected volatility | | 70.2% | | | 53.6% | | | 66.5% | |
| Risk-free interest rate | | 5.3% | | | 5.1% | | | 1.1% | |
| Expected dividends | | —% | | | —% | | | —% | |
The weighted-average grant-date fair value of ESPP options during the years ended December 31, 2024, 2023 and 2022 was $2.34, $2.84 and $2.87, respectively.
12.Income Taxes
Deferred income taxes are accounted for using the balance sheet approach, which requires recognition of deferred tax assets and liabilities for the expected future consequences of temporary differences between the financial reporting basis and the tax basis of assets and liabilities, as measured by enacted state and federal tax rates. Deferred tax assets and deferred tax liabilities are as follows:
| | | | | | | | | | | |
| (in thousands) | December 31,
2024 | | December 31,
2023 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 37,323 | | | $ | 41,454 | |
| Inventory write-down | 423 | | | 347 | |
| | | |
| | | |
| | | |
| Allowance for doubtful accounts | 204 | | | 87 | |
| Lease obligations | 5,489 | | | 5,867 | |
| Stock-based compensation | 7,371 | | | 6,084 | |
| Capitalized research and development costs | 14,785 | | | 11,530 | |
| Debt derivative liabilities | 622 | | | 772 | |
| Charitable contributions | 31 | | | 3 | |
| Accrued compensation | 49 | | | 56 | |
| Total deferred tax assets | 66,297 | | | 66,200 | |
| Deferred tax liabilities: | | | |
| Depreciation | (949) | | | (978) | |
| Amortization | (99) | | | (51) | |
| Right-of-use assets | (3,708) | | | (4,031) | |
| Contract liabilities | (86) | | | — | |
| Total deferred tax liabilities | (4,842) | | | (5,060) | |
| Net deferred tax assets | 61,455 | | | 61,140 | |
| Valuation allowance | $ | (61,455) | | | $ | (61,140) | |
A valuation allowance is provided to reduce the deferred tax assets reported if, based on the weight of the evidence, it is more-likely-than-not that a portion or none of the deferred tax assets will be realized. As of December 31, 2024 and 2023, management assessed the realizability of deferred tax assets. After consideration of all the evidence, including reversal of deferred tax liabilities, future taxable income and other factors, management determined that a full valuation allowance was necessary as of December 31, 2024 and 2023. The valuation allowance increased by $315 and $2,831 during 2024 and 2023, respectively, primarily as a result of the increase in capitalized research and development costs for tax purposes.
The Company adopted Section 174 of the Tax Cuts and Jobs Act of 2017 ("TCJA") which requires taxpayers to capitalize and amortize research and development expenditures for the tax years beginning after December 31, 2021. This rule became
effective for the Company during 2022 and resulted in capitalized research and development costs of $3,255 being recorded to deferred tax assets as of December 31, 2024.
The difference between the financial statement income tax benefit and the income tax benefit using statutory rates is primarily due to the valuation allowance and non-deductible permanent items such as meals, entertainment and equity compensation. The Company’s effective income tax rate differs from the statutory federal income tax rate as follows:
| | | | | | | | | | | | | | | | | |
| December 31, |
| 2024 | | 2023 | | 2022 |
| Federal tax rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State taxes - net of Federal benefit | 1.0 | | | 1.5 | | | 4.1 | |
| Permanent items and other deductions | (16.1) | | | (8.7) | | | (7.1) | |
| Other | (2.7) | | | (0.8) | | | (0.5) | |
| Valuation allowance | (3.2) | | | (13.0) | | | (17.5) | |
| Effective income tax rate | — | % | | — | % | | — | % |
As of December 31, 2024, the Company had tax-effected net operating loss carryforwards of $37,323 to offset future taxable income. The TCJA enacted significant changes to net operating loss ("NOL") utilization. NOLs generated after January 1, 2018 limit the NOL utilization to 80% of taxable income. The remaining 20% is carried forward to subsequent years. Net operating losses incurred in tax years beginning on or after January 1, 2018 are carried forward indefinitely. Net operating losses incurred in tax years prior to January 1, 2018 are subject to a twenty-year carryforward before expiring. A portion of the net operating loss carryforwards may expire due to limitations imposed by Section 382 of the Internal Revenue Code. Future utilization of the available net operating loss carryforwards may be limited under Internal Revenue Code Section 382 as a result of changes in ownership.
The Company files U.S. federal and state income tax returns in jurisdictions with varying statutes of limitations. In the normal course of business, the Company is subject to examination by taxing authorities throughout the U.S. These examinations could include examining the timing and amount of deductions, the allocation of income among various tax jurisdictions and compliance with federal, state, and local laws. The Company’s remaining open tax years subject to examination by federal tax authorities include the years ended December 31, 2021 through 2024. The Internal Revenue Service is currently examining the Company's 2021 federal income tax return. The Company's remaining open tax years subject to examination by state and foreign tax authorities include the years ended December 31, 2020 through 2024. However, for tax years 2004 through 2017, federal and state taxing authorities may examine and adjust loss carryforwards in the years in which those loss carryforwards are ultimately utilized.
Legislation enacted in 2018, titled the Tax Cuts and Jobs Act of 2017, subjects a U.S. shareholder to tax on global intangible low-taxed income (“GILTI”) earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740, No. 5, Accounting for Global Intangible Low-Taxed Income, states that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense only. The Company has elected to account for GILTI in the year the tax is incurred.
The Company has no recorded income tax expense or income tax benefit for the years ended December 31, 2024, 2023 and 2022 due to the generation of net operating losses. The Company is in a three-year cumulative loss and net deferred tax asset position, the benefits of which have been fully reserved as of December 31, 2024. The Company does not believe there are any additional tax refund opportunities currently available.
13. Segments
The Company determines its operating segments in accordance with FASB ASC 280, Segment Reporting ("ASC 280"). FASB ASC 280 defines operating segments as components where discrete financial information is regularly reviewed by the chief operating decision maker (“CODM”), which for the Company is the Chief Executive Officer (“CEO”), to determine resource allocation and assess performance. As such, based on the way the CODM monitors and makes decisions affecting operations, the Company has concluded that it has one operating and reportable segment. The CODM is regularly provided with only the consolidated expenses as noted on the face of the Consolidated Statements of Operations. As the Company has only one operating segment and is managed on a consolidated basis, the measure of profit or loss is consolidated net income or loss. The metrics are used to review operating trends, to perform analytical comparisons between periods and to monitor budget to actual variances. See the Consolidated Statements of Operations.
14. Retirement Plan
The Company sponsors the Axogen 401(k) plan (the "401(k) Plan"), a defined contribution plan covering substantially all employees of the Company. All full-time employees who have attained the age of 18 are eligible to participate in the 401(k) Plan. Eligibility is immediate upon employment and enrollment is available any time during employment. Participating employees may make annual pretax contributions to their accounts up to a maximum amount as limited by law. The 401(k) Plan requires the Company to make 100% matching contributions on up to 3% of the employee’s annual salary and 50% matching contributions on up to the next 2% of the employee’s annual salary as long as the employee participates in the 401(k) Plan. Both employee contributions and Company contributions vest immediately. Employer contributions to the 401(k) Plan were $1,669, $1,612 and $1,409 for the years ended December 31, 2024, 2023 and 2022, respectively.
15. Commitments and Contingencies
Service Agreements
The Company pays Community Blood Services (doing business as Solvita) ("Solvita") a facility fee for the use of clean-rooms, manufacturing, storage, and office space and for services in support of its tissue processing including for routine sterilization of daily supplies, providing disposable supplies and microbial services, and office support pursuant to a License and Services Agreement, as amended (the "Solvita Agreement"). Pursuant to the Solvita Agreement, the Company recorded expenses of $910, $2,327 and $2,278 for the years ended December 31, 2024, 2023 and 2022, respectively, in Cost of goods sold. The Solvita Agreement was amended on December 21, 2023, extending the term through December 31, 2026. The Solvita Agreement may be terminated by either party by providing an eighteen month written notice. While the Company ended its utilization of Solvita for Avance Nerve Graft in the fourth quarter of 2023, the Company continues to utilize Solvita for processing and packaging of Avive+ Soft Tissue Matrix.
In December 2011, the Company entered into a Master Services Agreement for Clinical Research and Related Services. The Company was required to pay $151 upon execution of this agreement and the remainder monthly based on activities associated with the execution of the Company's phase 3 pivotal clinical trial to support the BLA for Avance Nerve Graft. Payments made under this agreement were $—, $191 and $1,254 for the years ended December 31, 2024, 2023 and 2022, respectively. The Master Services Agreement for Clinical Research and Related Services was completed in 2023.
Distribution and Supply Agreements
In August 2008, the Company entered into an exclusive distribution agreement with Cook Biotech Incorporated (acquired on January 31, 2024 by RTI Surgical, Inc. and rebranded on December 17, 2024 as Evergen) ("Evergen"), to distribute the Axoguard Nerve Connector and Axoguard Nerve Protector products worldwide and the parties subsequently amended the agreement on August 4, 2023. The distribution agreement expires on December 31, 2030. The distribution agreement establishes a formula for the transfer cost of the Axoguard Nerve Connector and Axoguard Nerve Protector products and requires certain minimum purchases by the Company, although, through mutual agreement, the parties have not established such minimums; and, to date, have not enforced such provision. Under the distribution agreement, the Company provides purchase orders to Evergen, and Evergen fulfills the purchase orders. The distribution agreement allows for termination provisions for both parties. The loss of the ability to sell the Axoguard Nerve Connector and Axoguard Nerve Protector products could have a material adverse effect on the Company's business until other replacement products would be available.
In June 2017, the Company entered into the Nerve End Cap Supply Agreement (the "Supply Agreement") with Evergen whereby Evergen is the exclusive contract manufacturer of the Axoguard Nerve Cap, and the parties subsequently amended the agreement on August 4, 2023. The Supply Agreement expires on December 31, 2030. The Supply Agreement establishes the terms and conditions in which Evergen will manufacture the product for the Company. Under the Supply Agreement, the Company provides purchase orders to Evergen and Evergen fulfills the purchase orders. The Supply Agreement allows for termination provisions for both parties. The loss of the ability to sell the Axoguard Nerve Cap product could have a material adverse effect on the Company's business until other replacement products would be available.
In May 2023, the Company entered into a Supply and Manufacturing Agreement ("HA+ Supply Agreement") with Evergen whereby Evergen is the exclusive contract manufacturer of the Axoguard HA + Nerve Protector. The HA+ Supply Agreement expires on July 1, 2030. The HA+ Supply Agreement establishes the terms and condition in which Evergen will manufacture, package, label and deliver the product to the Company. Under the HA+ Supply Agreement, the Company provides purchase orders to Evergen, and Evergen fulfills the purchase orders. The HA+ Supply Agreement allows for termination provisions for both parties. The loss of the ability to sell the Axoguard HA + Nerve Protector product could have a material adverse effect on the Company's business until other replacement products would be available.
Insurance Financing Agreement
The Company finances certain of its commercial insurance policies. The Company entered into an Insurance Financing Agreement on December 31, 2024. Outstanding payments owed under the Insurance Financing Agreement are included in Prepaid expenses and other on the Consolidated Balance Sheets. The amounts owed under the Insurance Financing Agreement were $1,255 and $— as of December 31, 2024 and 2023, respectively.
Processing Facilities
The Company is highly dependent on the continued availability of its processing facilities at its APC Facility in Vandalia, Ohio and the facility it leases from Solvita in Dayton, Ohio and could be harmed if the physical infrastructure of these facilities is unavailable for any prolonged period of time.
Certain Economic Development Grants
The Company obtained certain economic development grants from state and local authorities totaling up to $2,685 including $1,250 of cash grants to offset costs to acquire and develop the APC Facility. Certain of these economic development grants were subject to fixed asset investments and job creation milestones by December 31, 2024, and have clawback clauses if the Company does not meet the job creation milestones. The Company has not met certain job creation milestones and has requested a reduction or waiver of clawbacks or extensions from the grant authorities to extend the job creation milestones evaluation date from December 31, 2024 and the expiration date to December 31, 2026. Certain grant authorities have not approved the Company's request. The Company is continuing discussions with these grant authorities regarding the evaluation, expiration and clawbacks of the job creation milestones and expects to receive a response during 2025. The Company could be obligated to pay back up to approximately $950 as of December 31, 2024 related to these grants. As of December 31, 2024, the Company had received $1,188 in cash grants related to these economic development grants during the years ended 2021 and 2020.
Fair Value of the Debt Derivative Liabilities
The fair value of the debt derivative liabilities is $2,400 as of December 31, 2024. The fair value of the debt derivative liabilities was determined using a probability-weighted expected return model based upon four potential settlement scenarios for the Credit Facility which are described in Note 2 - Summary of Significant Accounting Policies – Derivative Instruments. The estimated settlement value of each scenario includes any required make-whole payment, see Note 9 - Long-Term Debt, Net of Debt Discount and Financing Fees, and then discounted to present value using a discount rate that is derived based upon the initial terms of the Credit Facility at issuance and corroborated utilizing a synthetic rating analysis. The calculated fair values under the four scenarios are then compared to the fair value of a plain vanilla note, with the difference reflecting the fair value of the debt derivative liabilities. The Company estimated the make-whole payments required under each scenario according to the terms of the Credit Facility to generate an internal rate of return equal to 11.5% through the scheduled maturity dates, less the total of all quarterly interest and royalty payments previously paid to the Lender. The calculation utilized the XIRR function in Microsoft Excel as required by the Credit Facility. If the debt is not prepaid but instead is held to its scheduled maturities, the Company’s estimate of the make-whole payment for the first and second tranches of the Credit Facility due on June 30, 2027 and June 30, 2028, respectively, are approximately zero. The Company has consistently applied this approach since the inception of the debt agreement on June 30, 2020.
The Company has become aware that the Lender may have an alternative interpretation of the calculation of the make-whole payments that the Company believes does not properly utilize the same methodology utilized by the XIRR function in Microsoft Excel as described in the Credit Facility. The Company estimates the top end of the range of the make-whole payments if the debt is held to its scheduled maturities under an alternative interpretation to be approximately $8,000 for the first tranche of the Credit Facility due on June 30, 2027, and approximately $3,000 for the second tranche of the Credit Facility due on June 30, 2028. Further, if the debt is prepaid prior to the scheduled maturity dates and subject to the alternative interpretation, the make-whole payments would be larger than the amounts herein.
Other Commitments
Certain executive officers of the Company are parties to employment contracts. Such contracts have severance payments for certain conditions including change of control. On January 24, 2025, the Company entered into a separation agreement with a former vice president which will result in payments of $843 and $17 in 2025 and 2026, respectively, which includes severance, accrued paid time off, twenty-four months of Consolidated Omnibus Budget Reconciliation Act ("COBRA") payments and related employer paid taxes.
The Company also entered into a Transition and Separation Agreement with its former CEO on January 4, 2024, which results in payments of $1,164 and $18 in 2025 and 2026, respectively, which includes the 2024 bonus to be paid in March of 2025, senior advisory fees, eighteen months of COBRA payments and related employer paid taxes.
Legal Proceedings
The Company is and may be subject to various claims, lawsuits, and proceedings in the ordinary course of the Company's business. Such matters are subject to many uncertainties and outcomes are not predictable with assurance. While there can be no assurances as to the ultimate outcome of any legal proceeding or other loss contingency involving the Company. In the opinion of management, such claims are either adequately covered by insurance or otherwise indemnified, or are not expected, individually or in the aggregate, to result in a material, adverse effect on the Company's financial condition, results of operations or cash flows. However, it is possible that the Company's results of operations, financial position and cash flows in a particular period could be materially affected by these contingencies.
16. Quarterly Results of Operations (Unaudited)
The Company's quarterly results of operations for the years ended December 31, 2024 and 2023 were previously disclosed in the Company's Quarterly Reports on Form 10-Q and filed with the Securities and Exchange Commission on May 2, 2024, August 8, 2024 and November 7, 2024. The 2023 quarterly results of operations as reclassified were previously disclosed in the Company's Current Report on Form 8-K filed on May 2, 2024.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain “disclosure controls and procedures” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are designed to ensure that information required to be disclosed by us in reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, and Board of Directors, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognizes that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable assurance of achieving the desired objectives, and we necessarily are required to apply our judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures.
Our management, including our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2024, and concluded that our disclosure controls and procedures were effective.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the three months ended December 31, 2024, that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting (as defined in Rules 13a-15(d) or 15d-15(f) of the Exchange Act).
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Our internal control system is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP and includes those policies and procedures that:
•Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
•Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
•Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the consolidated financial statements.
Because of inherent limitations, a system of internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate due to a change in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management, including our principal executive officer and principal financial officer, evaluated the effectiveness of the design and operation of our internal control over financial reporting as of December 31, 2024. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013). Based on their evaluation, the principal executive officer and principal financial officer concluded that our internal controls over financial reporting were effective.
Our independent registered public accounting firm, Deloitte & Touche LLP, who audited the consolidated financial statements included in this Annual Report on Form 10-K, has issued an attestation report on the effectiveness of management's internal control over financial reporting as of December 31, 2024.
ITEM 9B. OTHER INFORMATION
During the three months ended December 31, 2024, none of our directors or officers adopted, modified or terminated a
“Rule 10b5-1 trading arrangement” or a “non-Rule 10b5-1 trading arrangement” as such terms are defined under Item 408 of
Regulation S-K.
During the three months ended December 31, 2024, the Company did not adopt, modify or terminate a “Rule 10b5-1
trading arrangement” or a “non-Rule 10b5-1 trading arrangement” as such terms are defined under Item 408 of Regulation S-K.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Information required by this item concerning our directors will be set forth under the caption “Election of Directors” in our definitive proxy statement for our 2025 annual meeting, which will be filed no later than 120 days after December 31, 2024, and is incorporated herein by reference.
If applicable, information required by this item concerning compliance with Section 16(a) of the Exchange Act, as amended, will be set forth under the caption "Security Ownership of Certain Beneficial Owners and Management — Delinquent Section 16(a) Reports” in our definitive proxy statement for our 2025 annual meeting, and is incorporated herein by reference.
Information required by this item concerning our audit committee, our audit committee financial expert and any material changes to the way in which security holders may recommend nominees to our Board of Directors will be set forth under the caption “Corporate Governance” in our definitive proxy statement for our 2025 annual meeting and is incorporated herein by reference.
The Board of Directors adopted a Code of Business Conduct and Ethics, which is posted on our website https://ir.axogeninc.com/governance-docs, that is applicable to all employees and directors. We will provide copies of our Code of Business Conduct and Ethics without charge upon request. To obtain a copy, please visit our website or send your written request to Investors Relations, 13631 Progress Blvd., Suite 400, Alachua, FL 32615. With respect to any amendments or waivers of this Code of Business Conduct and Ethics (to the extent applicable to our chief executive officer, principal accounting officer or controller, or persons performing similar functions) we intend to either post such amendments or waivers on our website or disclose such amendments or waivers pursuant to a Current Report on Form 8-K.
ITEM 11. EXECUTIVE COMPENSATION.
Information required by this item will be set forth under the caption “Executive Compensation” in our definitive proxy statement for our 2025 annual meeting, which will be filed no later than 120 days after December 31, 2024, and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Information required by this item concerning ownership will be set forth under the caption “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in our definitive proxy statement for our 2025 annual meeting, which will be filed no later than 120 days after December 31, 2024, and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Information required by this item concerning ownership will be set forth under the caption “Corporate Governance — Director Independence” and “Certain Relationships and Related Transactions” in our definitive proxy statement for our 2025 annual meeting, which will be filed no later than 120 days after December 31, 2024, and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Information required by this item concerning ownership will be set forth under the caption “Ratification of Appointment of Independent Registered Public Accounting Firm” in our definitive proxy statement for our 2025 annual meeting, which will be filed no later than 120 days after December 31, 2024, and is incorporated herein by reference.
PART IV
Schedule II – Valuation and Qualifying Accounts
AXOGEN, INC.
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS
THREE YEARS ENDED DECEMBER 31, 2024, 2023 AND 2022
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Balance at Beginning of Year | | Additions | | Deductions (Charge-offs) | | Balance at End of Year |
| Allowance for doubtful accounts | | | | | | | |
| 2022 | $ | 276 | | | $ | 612 | | | $ | (238) | | | $ | 650 | |
| 2023 | $ | 650 | | | $ | — | | | $ | (313) | | | $ | 337 | |
| 2024 | $ | 337 | | | $ | 650 | | | $ | (199) | | | $ | 788 | |
| | | | | | | |
| Valuation allowance for deferred tax assets | | | | | | | |
| 2022 | $ | 53,251 | | | $ | 5,058 | | | $ | — | | | $ | 58,309 | |
| 2023 | $ | 58,309 | | | $ | 2,831 | | | $ | — | | | $ | 61,140 | |
| 2024 | $ | 61,140 | | | $ | 315 | | | $ | — | | | $ | 61,455 | |
| | | | | | | |
| Valuation for inventory reserves | | | | | | | |
| 2022 | $ | 2,576 | | | $ | 1,769 | | | $ | (2,410) | | | $ | 1,935 | |
| 2023 | $ | 1,935 | | | $ | 1,939 | | | $ | (2,533) | | | $ | 1,341 | |
| 2024 | $ | 1,341 | | | $ | 6,989 | | | $ | (6,700) | | | $ | 1,630 | |
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)Financial Statements and Financial Statement Schedules
The financial statements required by Item 15(a) are filed in Part II Item 8 of this Annual Report on Form 10-K. Schedules not included have been omitted because they are not applicable or because the required information is included in the Consolidated Financial Statements and notes thereto.
(b)Exhibits
The following exhibits are included in this Annual Report on Form 10-K or incorporated by reference in the Form 10-K.
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| 3.1 | | |
| | |
| 3.2 | | |
| | |
| 4.1 | | |
| | |
| *10.2.1 | | |
| | |
| 10.2.2 | | |
| | |
| 10.2.3 | | |
| | |
| *10.3 | | |
| | |
| *10.4.1 | | |
| | |
| *10.4.2 | | |
| | |
| *10.5.1 | | |
| | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| 10.5.2 | | |
| | |
| 10.5.3 | | |
| 10.5.4 | | |
| | |
| 10.6.1 | | |
| | |
| 10.6.2 | | |
| | |
| 10.6.3 | | |
| | |
| 10.6.4 | | |
| | |
| 10.6.5 | | |
| | |
| 10.6.6 | | |
| | |
| 10.6.7 | | |
| | |
| 10.6.8 | | |
| | |
| 10.6.9 | | |
| | |
| **10.7 | | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| **10.8.1 | | |
| | |
| **10.8.2 | | |
| | |
| 10.9.1 | | |
| | |
| 10.9.2 | | |
| | |
| 10.9.3 | | |
| | |
| 10.9.4 | | |
| | |
| 10.9.5 | | |
| | |
| 10.9.6 | | |
| | |
| 10.9.7 | | |
| | |
| 10.9.8 | | |
| | |
| 10.10.1 | | |
| | |
| 10.10.2 | | |
| | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| 10.10.3 | | |
| | |
| 10.10.4 | | |
| | |
| 10.10.5 | | |
| | |
| 10.11 | | |
| | |
| *10.12 | | |
| | |
| **10.13 | | Retention Stock Unit Award Agreement, dated December 29, 2016, by and between Axogen, Inc. and Karen Zaderej, pursuant to Axogen, Inc. 2010 Stock Incentive Plan, as amended and restated as of March 23, 2016 (incorporated by reference to Exhibit 10.24 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2016, filed on March 1, 2017). |
| | |
| **10.14 | | |
| | |
| **10.15 | | |
| | |
| 10.16 | | |
| | |
| | |
| 10.17 | | |
| | |
| 10.18.1 | | |
| | |
| 10.18.2 | | |
| | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| **10.19 | | |
| | |
| **10.20 | | |
| | |
| 10.21 | | |
| | |
| 10.22.1 | | |
| | |
| 10.22.2 | | |
| | |
| 10.22.3 | | |
| | |
| **10.23 | | |
| | |
| **10.24 | | |
| | |
| 10.25 | | |
| | |
| **10.26 | | |
| | |
| ***10.27.1 | | |
| | |
| 10.27.2 | | |
| | |
| 10.27.3 | | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| 10.28.1 | | |
| | |
| 10.28.2 | | |
| | |
| 10.29 | | |
| | |
| 10.30 | | |
| | |
| 10.31 | | | |
| | |
| **10.32 | | |
| | |
| **10.33 | | |
| | |
| **10.34 | | |
| | |
| 10.35.1 | | |
| | |
| 10.35.2 | | |
| | |
| **10.36 | |
|
| | |
| **10.37 | | |
| | |
| **10.38 | | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| **10.39 | | |
| | |
| **10.40 | | |
| | |
| 10.41 | | |
| | |
| **10.52 | | |
| | |
| **10.53 | | |
| | |
| **†10.54 | | |
| | |
| **†10.55 | | |
| | |
| **10.56.1 | | |
| | |
| **10.56.2 | | |
| | |
| **10.57 | | |
| | |
| **10.58.1 | | |
| | |
| **10.58.2 | | |
| | |
| **10.59 | | |
| | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| **10.60.1 | | |
| | |
| **10.60.2 | | |
| | |
| **10.61 | | |
| | |
| **10.62 | | |
| | |
| **10.63 | | |
| | |
| **10.64 | | |
| | |
| **10.65 | | |
| | |
| **10.66 | | |
| | |
| **10.67 | | |
| | |
| +19.1 | | |
| | |
| +21.1 | | |
| | |
| +23.1 | | |
| | |
| ++24.1 | | Power of Attorney. |
| | |
| +31.1 | | |
| | |
| +31.2 | | |
| | |
| | | | | | | | |
| Exhibit Number | | Description |
| | |
| +++32.1 | | |
| | |
| 97 | | |
| | |
| 101 | | Inline XBRL Document Set for the consolidated financial statements and accompanying notes in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K. |
| | |
| +101.INS | | XBRL Instance Document – The instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| | |
| +101.SCH | | Inline XBRL Taxonomy Extension Schema Document. |
| | |
| +101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| | |
| +101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| | |
| +101.LAB | | Inline XBRL Extension Labels Linkbase. |
| | |
| +101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| | |
| 104 | | Inline XBRL for the cover page of this Annual Report on Form 10-K, included in the Exhibit 101 Inline XBRL Document Set. |
_______________________________
| | | | | |
| * | Confidential treatment has been granted for portions of this Exhibit pursuant to Rule 24b-2 under the Securities Exchange Act of 1934 as amended. The confidential portions have been deleted and filed separately with the U.S. Securities and Exchange Commission. |
| |
| ** | Management contract or compensatory plan or arrangement. |
| |
| *** | Confidential treatment has been requested as to certain portions, which portions have been omitted and filed separately with the Securities and Exchange Commission. |
| |
| + | Filed herewith. |
| |
| ++ | Included on signature page. |
| |
| +++ | Furnished herewith. |
| |
| † | Portions of this exhibit have been omitted pursuant to Item 601(b)(10) of Regulation S-K because the information is not material and is the type of information that the Company treats as private or confidential. The Company agrees to furnish an unredacted copy of this exhibit on a supplemental basis to the SEC or its staff upon request. |
ITEM 16. Form 10-K Summary
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| AXOGEN, INC |
| |
| /s/ Michael J. Dale |
| Michael J. Dale |
| Chief Executive Officer, President and Director |
| February 26, 2025 |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Michael J. Dale (with full power to act alone), as his or her true and lawful attorney-in-fact and agent, with full powers of substitution and re-substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to the Annual Report on Form 10-K of Axogen, Inc., and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or their substitute or substitutes, lawfully do or cause to be done by virtue hereof.
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | |
| /s/ Michael J. Dale | | February 26, 2025 |
Michael J. Dale, Chief Executive Officer, President and Director (Principal Executive Officer) | | |
| | |
| /s/ Nir Naor | | February 26, 2025 |
Nir Naor, Chief Financial Officer
(Principal Financial and Accounting Officer) | | |
| | |
| /s/ William Burke | | February 26, 2025 |
William Burke Director | | |
| | |
| | |
| | |
| | |
| /s/ John H. Johnson | | February 26, 2025 |
John H. Johnson Director | | |
| | |
| /s/ Adam M. Levine | | February 26, 2025 |
Alan M. Levine
Director | | |
| | |
| /s/ Guido J. Neels | | February 26, 2025 |
Guido J. Neels
Director | | |
| | |
| /s/ Paul G. Thomas | | February 26, 2025 |
Paul G. Thomas
Chairman of the Board | | |
| | |
| /s/ Joseph A. Tyndall | | February 26, 2025 |
Joseph A. Tyndall Director | | |
| | |
| /s/ Kathy Weiler | | February 26, 2025 |
Kathy Weiler
Director | | |
| | |
| /s/ Amy Wendell | | February 26, 2025 |
Amy Wendell
Director | | |