- ABMD Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Abiomed (ABMD) 8-KOther Events
Filed: 7 Dec 12, 12:00am
Abiomed Presentation to FDA 515i Panel Classification Determination for Non-roller Type CPB Pumps December 6, 2012 David Weber, PhD Product and Regulatory Operations Abiomed Exhibit 99.2 |
Abiomed • U.S. Medical Device firm • Headquartered in Danvers, Massachusetts • 450 employees • Expertise in pumping blood for over 30 years • Products & Clearance/Approvals: – Extracorporeal Ventricular Assist Devices (1 st VADs in US); PMA for Heart Recovery, 1992 – Total Artificial Heart; HDE, 2007 – IABP; 510(k) Clearance, 2007 – Impella Microaxial Pumps; First 510(k) Clearance, 2008 2 |
• Considerations for classification of Impella for durations of support up to 6 hours • Discussion of Supporting Valid Scientific Evidence • Controls & Concluding Recommendations • Q&A 3 David Weber, PhD Abiomed Jeffrey Popma, MD Beth Israel Deaconess Medical Center, Boston David Weber, PhD William O’Neill, MD Henry Ford Hospital, Detroit Outline |
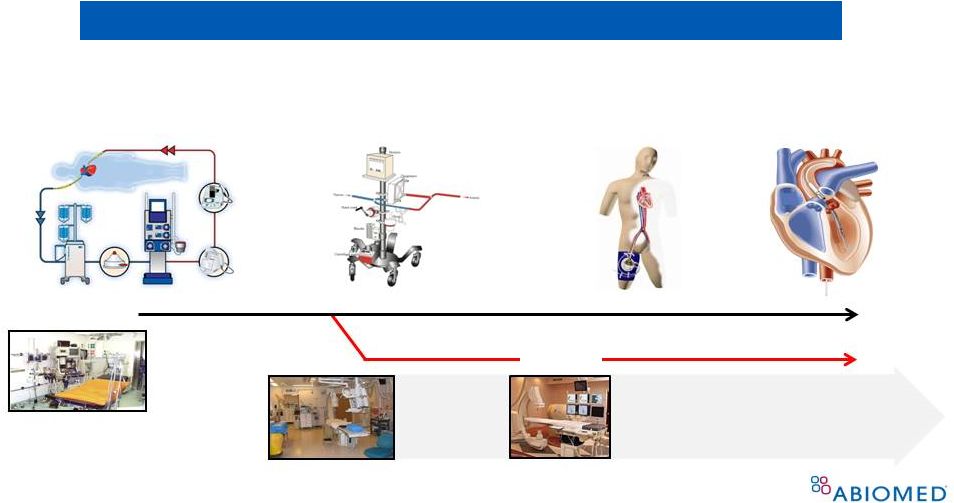 Evolution of Nonroller-Type CPB Pumps 4 Expansion of “On Pump” Concept to Cath Lab, EP lab Surgical Cardiopulmonary Bypass (centrifugal) Percutaneous Cardiac Support (centrifugal) Percutaneous Cardiac Support (micro-axial) Percutaneous Cardiopulmonary Support (CPS) (centrifugal) 1960s 1980s Surgery suite Cath Lab • Reduced size • Reduced invasiveness • Faster set up EP Lab |
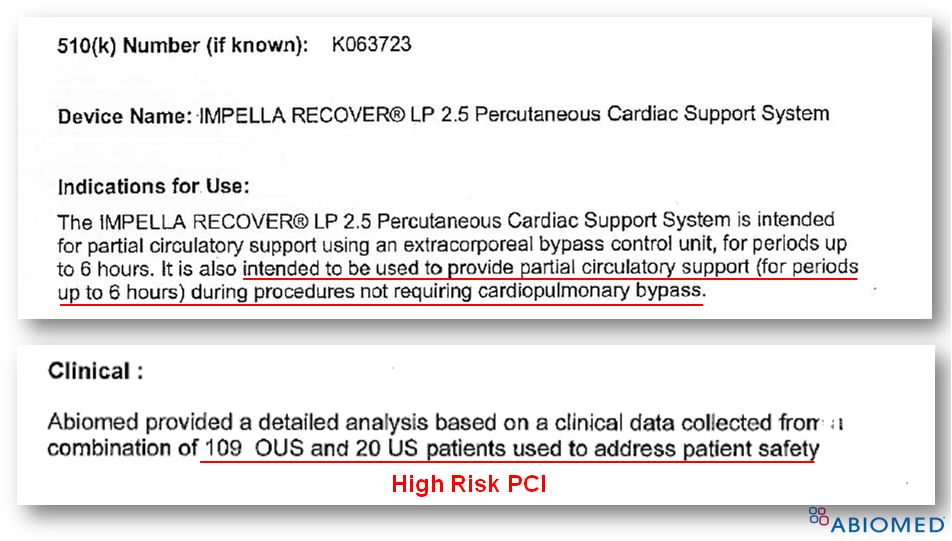
 FDA Clearance for Impella ® 2.5 (K063723) 5 Supporting Clinical Data • Total of 129 patients • 100% from patients provided partial circulatory support ( < 6 hrs) during high risk PCI procedures (n=20 from FDA Safety Study 1 , n=109 from EU registry) • 510(k) Summary for K063723: “Abiomed provided a detailed analysis based on clinical data from a combination of 109 OUS and 20 US patients to address patient safety” 1. Dixon, et al., JACC, 2009 |
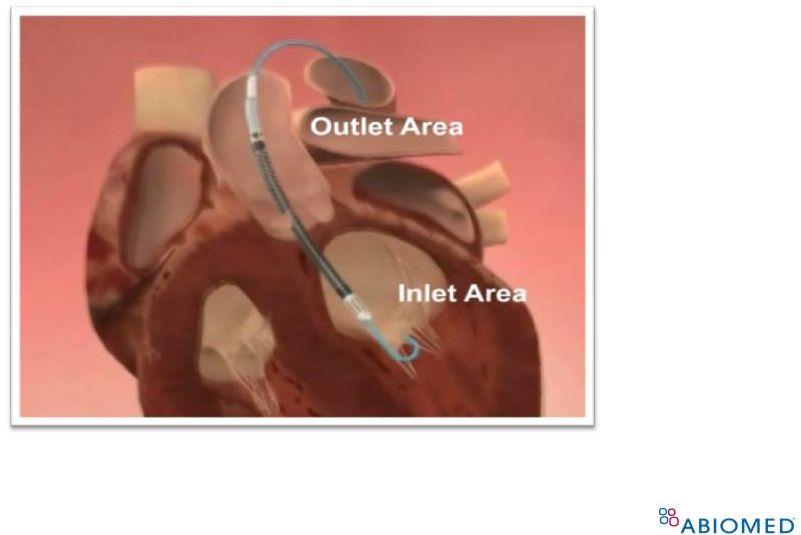 6 Since its 510(k) clearance in 2008, > 15,000 implants have been performed, ~12,000 in the U.S. Impella ® Device |
Impella ® Use in United States Since Clearance For use up to 6 hours, 90% of Impella use is for temporary ventricular support in the setting of HRPCI • Median duration of support during HRPCI is 60-80mins 1,2 • Majority of patients are surgical turn-downs 3 7 1. 60 mins median support duration, US Registry (Maini et al., CCI, 2012) 2. 78 mins median support duration, PROTECT II Study (O’Neill et al., Circulation, 2012) 3. 64% deemed inoperable by site surgical consultants in PROTECT II (O’Neill et al., Circulation, 2012) |
 8 Sufficient Evidence on Impella ® to Identify Risks and Benefits 193 peer-reviewed publications since 1994 124 publications Meet Definition of “Valid Scientific Evidence” 57 pertain to use 6 hours 21 CFR Section 860.7(c)(2): “Valid Scientific Evidence” – Well-controlled investigations – Partially controlled studies – Studies or objective trials without matched controls – Well-documented case histories – Reports of significant human experience |
 9 Largest Studies Supporting Reasonable N = 564 patients, 245 in FDA-approved protocol EU Registry US Registry IDE Safety Study IDE Randomized Study Study name Europella USpella PROTECT I PROTECT II Period 2004 - 2007 2009 - 2010 2006 -2007 2007- 2010 Tot. Patients HR PCI , N=144 HR PCI , N=175 HR PCI, N=20 HR PCI, N=225 (Impella) Tot. Centers 10 18 7 112 Publication JACC, 2009 CCI, 2012 JACC, 2009 Circulation, 2012 Conclusion “This large multicenter registry supports the safety, feasibility, and potential usefulness of hemodynamic support with Impella 2.5 in HR PCI.” “The use of Impella 2.5 in HR PCI appeared feasible and safe in the real-world setting.” “The Impella 2.5 system is safe, easy to implant, and provides excellent hemodynamic support during HR PCI.” “The 30 - day incidence of MAE was not different for patients with IABP or Impella 2.5 hemodynamic support. However, trends for improved outcomes were observed for Impella 2.5 supported patients at 90-days.” Assurance of Safety & Effectiveness |
 10 FDA 515i Panel Classification Determination FDA 515i Panel Classification Determination for Non-roller Type CPB Pumps for Non-roller Type CPB Pumps Clinical Evidence Clinical Evidence December 6, 2012 December 6, 2012 Jeffrey J. Popma, MD Jeffrey J. Popma, MD Director, Interventional Cardiology, BIDMC Director, Interventional Cardiology, BIDMC Professor of Medicine, Harvard Medical School Professor of Medicine, Harvard Medical School Disclosures: Research Grant, Advisory Board (1), Travel Disclosures: Research Grant, Advisory Board (1), Travel |
 Clinical Summary: Objectives-I • FDA Executive Summary identified 5 studies using the Impella 2.5 device, comprising 231 patients undergoing HR-PCI. We would request that the Panel also consider additional analyses published after the 2011 ACC/AHA/SCAI guidelines not included in the FDA Summary. 11 • O’Neill et al The PROTECT II study. Circ 2012;126(14):1717-27. - Dangas et al (abstr). J Am Coll Cardiol 2012:60:B21 - Popma et al. (abstr), J Am Coll Cardiol 2012:59:A372 • The USpella Registry. CCI 2012;80(5):717-25. |
 12 Clinical Summary: Objectives-II • FDA Executive Summary provides a preliminary overview of the primary endpoint of the PROTECT-II study as described by the DSMB. We would request that the Advisory Panel also consider: - PROTECT-II Pre-defined analysis population (PP) - PROTECT-II: Four pre-defined subgroup analyses - Other Registries and post-hoc analyses that describe the totality of clinical evidence supporting the reasonable safety and efficacy of the Impella 2.5 |
   Impella 2.5 Clinical Evidence Base (N=799*) Impella 2.5 Clinical Evidence Base (N=799*) Three Prospective Clinical Trials Design Type Used in FDA Submissions Number of Patients Published in PROTECT I 1 Safety Feasibility Yes 20 JACC Interv. 2009 PROTECT II 2 Randomized Yes 452 (225) Circulation 2012 Cardio ByPass 3 Randomized No 199 (105) EJCTS 2002 Two Post Market High Risk PCI Registries Euro Registry 4 Multicenter Open Yes 144 JACC 2009 U.S. Registry 5 Multicenter Open No 175 CCI 2012 * Does not include control arm patients for randomized trials 13 |
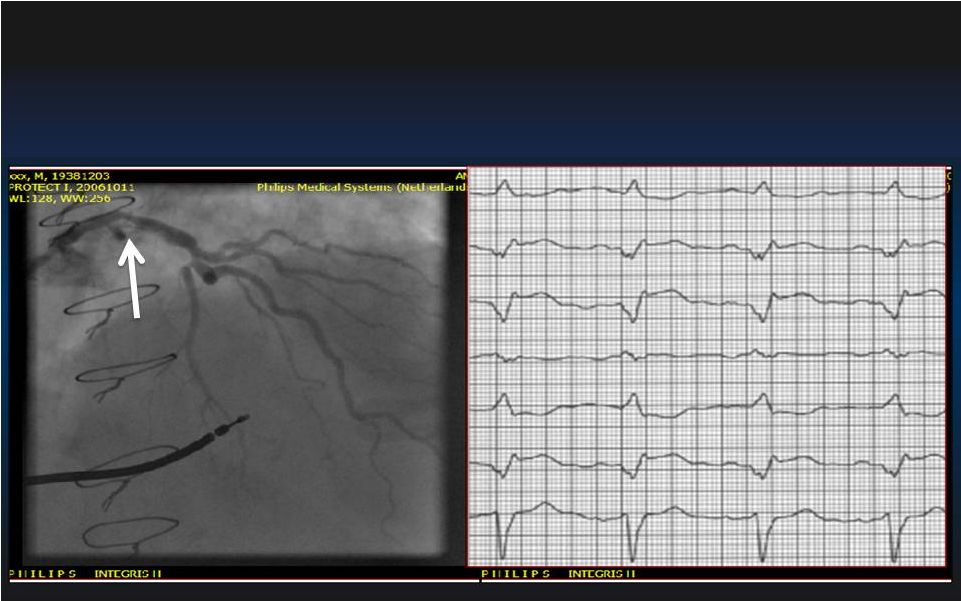
 • In cardiogenic shock - Improvement in cardiac index (ISAR-SHOCK) 1 0.49±0.46 L/min/M 2 v. 0.11±0.31 L/min/M 2 with IABP (P < 0.02) - Increase in mean arterial pressure (USPella) 2 83±17 mmHg v. 59±15 with control-IABP (P < 0.0001) • In Elective High Risk PCI Improved Hemodynamics with Impella 2.5 1 Seyfarth et al. JACC. 2008;42:1594-8; 2 USpella Registry. Transcatheter Therapeutics 2011 3 O’Neill, et al., Circulation. 2012;126:1717-1727 14 - Preserved cardiac power output with Impella v. IABP -4.2±24 v. -14.2±27 with IABP (P=0.001) |
 15 Pre-PCI During PCI Inoperable High-Risk PCI: Hemodynamic Stability with Impella 2.5 Left Main – Left Main – Sole Remaining Vessel Sole Remaining Vessel LVEF < 35% LVEF < 35% |
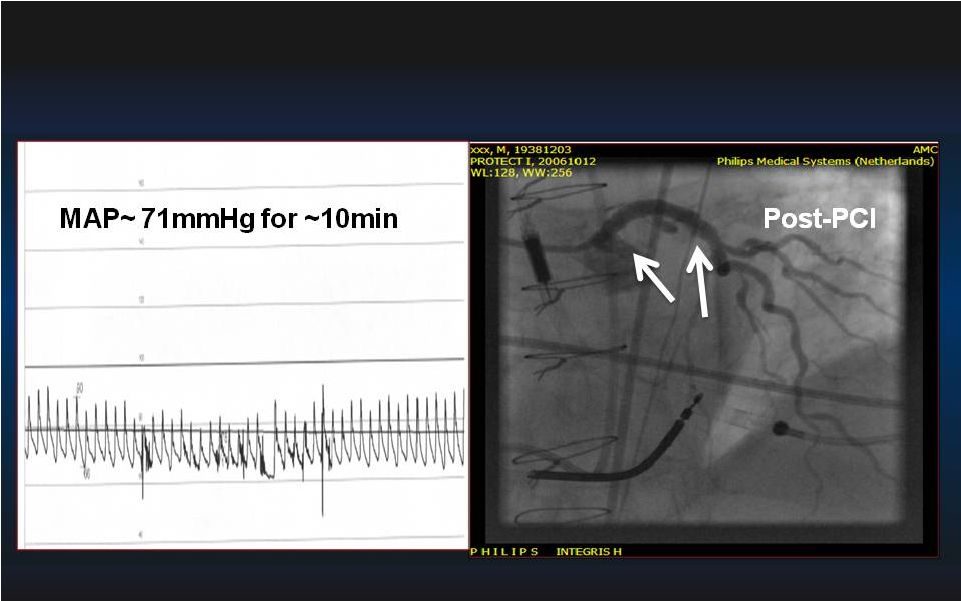 16 Inoperable High-Risk PCI: Hemodynamic Stability with Impella 2.5 60 80 |
   17 Safety: Risks For Temp Ventricular Support Safety: Risks For Temp Ventricular Support Adapted from FDA Executive Summary: References in Appendix Potential Risks (use < 6 hrs) Reasonable Assurance to Support Safety Alteration in Blood Composition Large prospective RCT (N=199 patients) showed favorable results of Impella compared to CPB in OPCABG with respect to blood interaction 3 Inadequate Tissue Perfusion Prospective RCT showed superior hemodynamic support and better tissue perfusion with Impella vs IABP 6 Embolism Stroke = 0.5% 1,2,4,5 Non-CNS thromboembolism = 0.8% 3 Duration of Use Median ranges from 60 [45;127] to 78 [52,113] min. 1,2,4,5 Fluid Leakage Access site bleeding req. transfusion = 3.6% 1,3,7 Device leakage malfunction = 0.2% 1,3 |
 References in Appendix Safety: Risks For Temp Ventricular Support Potential Risk (use < 6 hrs) Reasonable Assurance to Support Safety Adverse Tissue Reaction Two Large prospective RCT showed no evidence of adverse tissue 3 or blood 5 reaction compared to IABP 3 or CPB 5 respectively Infection 3.2% 1,2,4,5 Structural / Tissue Damage 0% in 3 FDA trials and registries (N=564) 1,2,3,5,6,7 0.06% including all Impella literature in PubMed (1/1697 patients in 197 publications) Local Heat Generation Stroke= 0.5% Non-CNS Thromboembolism (PE, DVT) = 0.8% Flow Dynamics Hemolysis rate = 0.8% 1,3,6,7 RCT showed no complement activation and lower inflammatory response compared to CPB 1,3 18 |
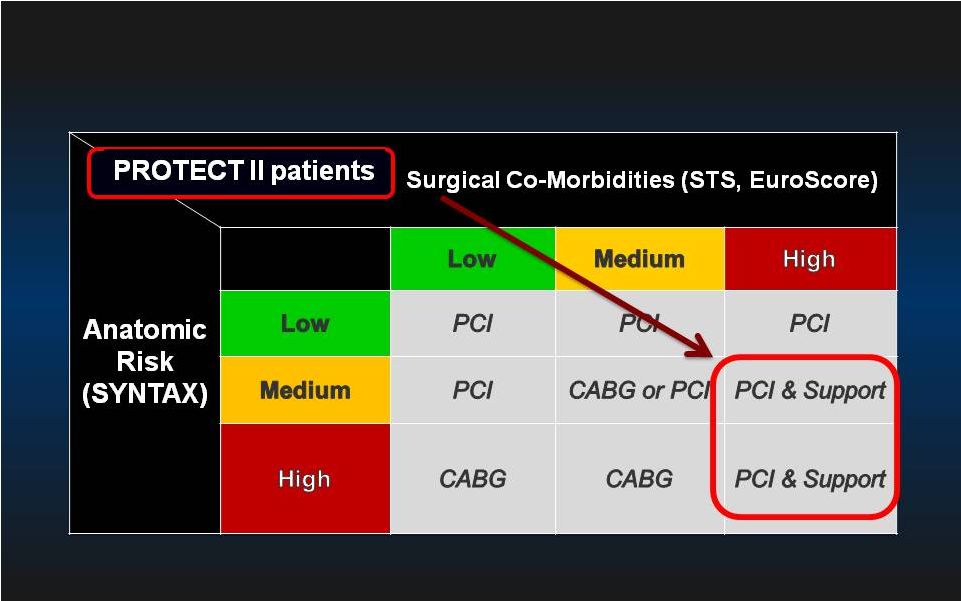 CABG is Preferred in Patients with Complex Anatomy and Less Than High Surgical Risk 19 |
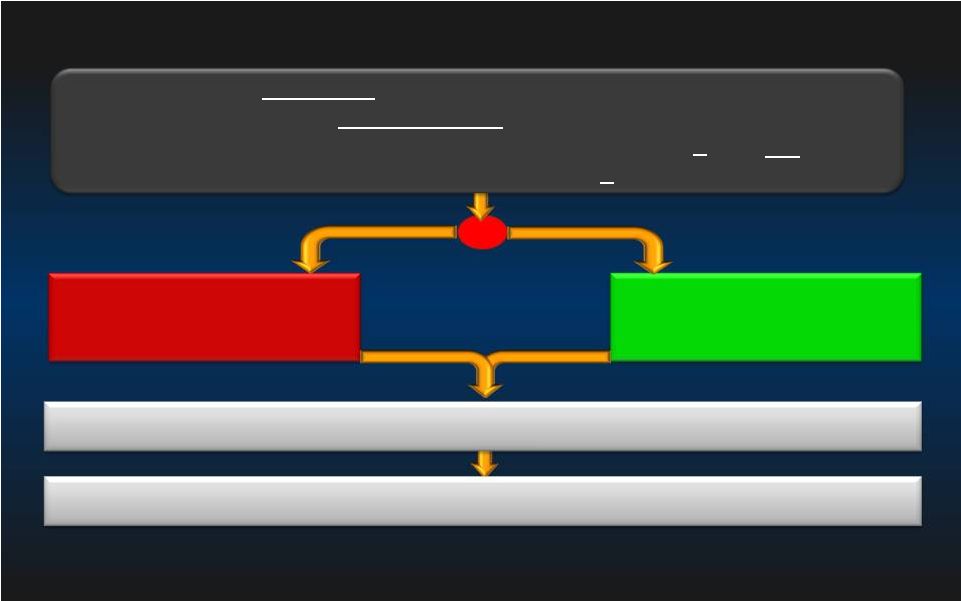 PROTECT II Trial Design IMPELLA 2.5 + PCI IABP + PCI Primary Endpoint = 30-day Composite MAE* rate 1:1 R Patients Requiring Prophylactic Hemodynamic Support During Non-Emergent High Risk PCI on Unprotected LM/Last Patent Conduit and LVEF 35% OR 3 Vessel Disease and LVEF 30% Follow-up of the Composite MAE* rate at 90 days 20 < < *Major Adverse Events (MAE) : Death, MI (>3xULN CK-MB or Troponin) , Stroke/TIA, Repeat Revasc, Cardiac or Vascular Operation of Vasc. Operation for limb ischemia, Acute Renal Dysfunction, Increase in Aortic insufficiency, Severe Hypotension, CPR/VT, Angio Failure |
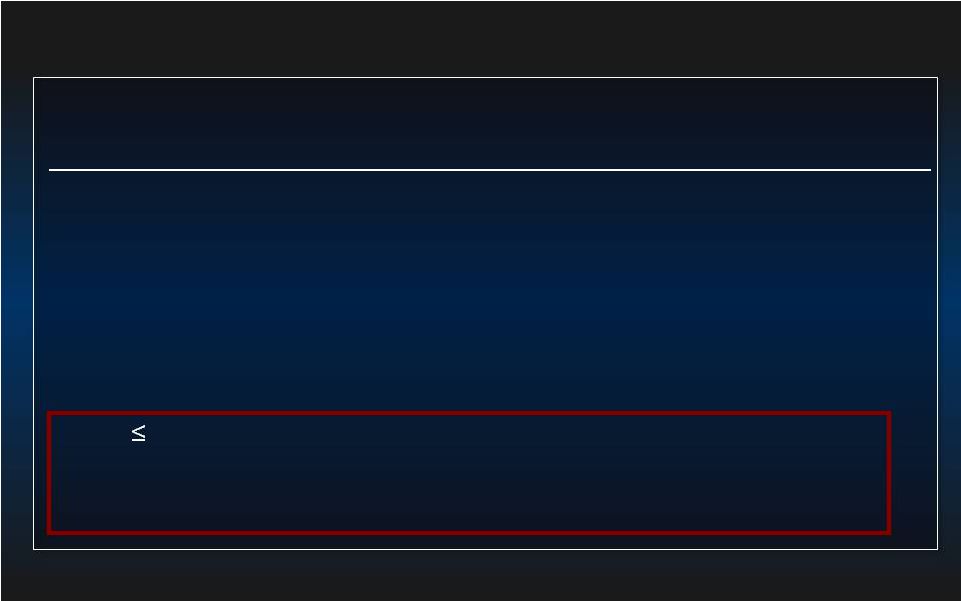 21 Risk Assessment: SYNTAX vs PROTECT-II 1 2 SYNTAX (n=903) PROTECT II (n=448) Age (Mean±SD) 65±10 67±11 Diabetes (%) 26 52 Prior MI (%) 32 68 CHF (%) 4 87 Prior PCI (%) 0 39 Prior CABG (%) 0 33 LVEF 30% (%) 1.3 92 Not Surgical Candidate (%) 0 64 Euroscore (Mean±SD) 4±3 18±18 1 PCI arm in SYNTAX trial, Serruys et al. N Engl J Med. 2009; 2 O’Neill et al. Circulation 2012 |
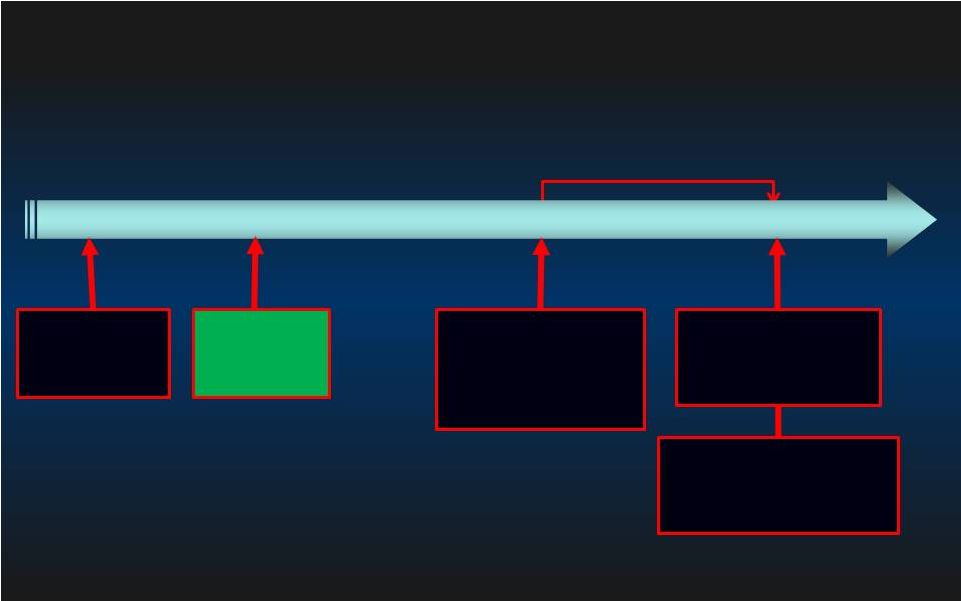 PROTECT II Enrollment & Milestones 22 Initial Initial Goal: 654 pts Goal: 654 pts Time for data collection Time for data collection for interim analysis for interim analysis 2008 2010 2009 2011 + 125 Patients + 125 Patients 11/27/2007 11/27/2007 PROTECT II PROTECT II 1st 1st st Patient Patient 6/2/2008 510(k) Clearance 2/26/2010 2/26/2010 50% Enrollment 50% Enrollment Achieved Achieved (N=327) (N=327) 12/6/2010 12/6/2010 69% Enrollment 69% Enrollment (N=452) (N=452) 12/6/2010 12/6/2010 Trial Halted for Futility Trial Halted for Futility Determination* Determination* *PROTECT *PROTECT II II protocol protocol Stopping Stopping rule rule for for futility futility = = power at interim analysis <40%. Conditional |
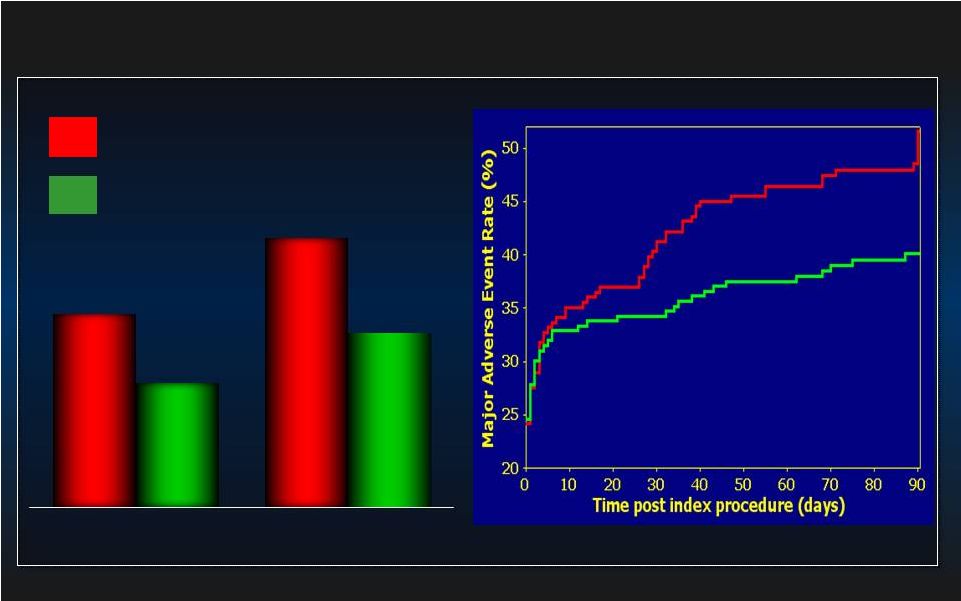
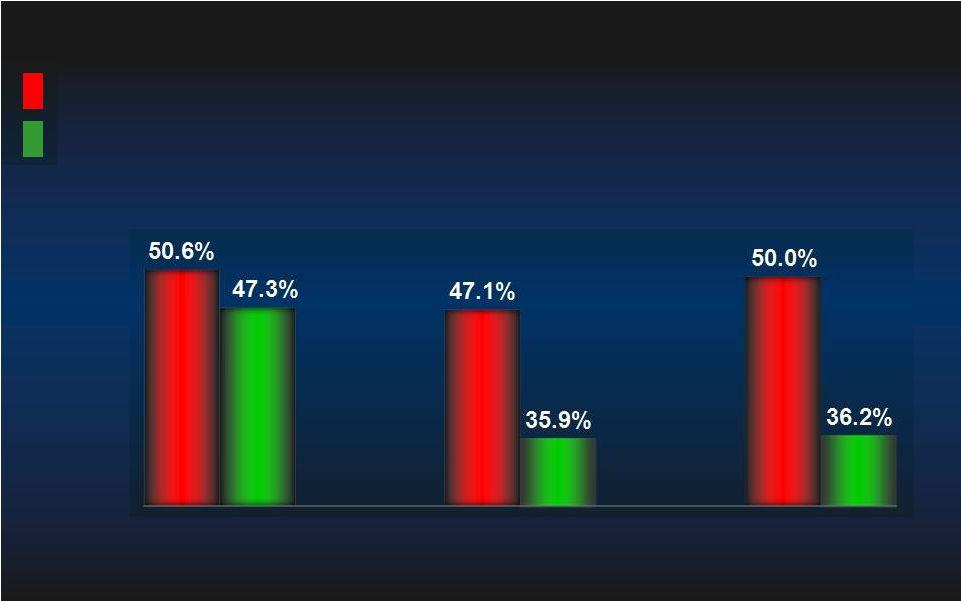
 PROTECT II: ITT MAE (N=448) PROTECT II: ITT MAE (N=448) IABP IABP IMPELLA IMPELLA MAE= Major Adverse Event Rate. MAE= Major Adverse Event Rate. p=0.277 p=0.277 N=225 N=225 N=223 N=223 p=0.066 p=0.066 N=224 N=224 N=219 N=219 Log rank test, p=0.147 Log rank test, p=0.147 IABP IABP IMPELLA IMPELLA 40.1% 49.3% 35.1% 40.6% 30 day MAE 90 day MAE 23 |
 Adverse Events Directly Attributed to the Device (90 days) Impella (N=225) IABP (N=223) Death 0 0 Stroke/TIA 0 0 Myocardial Infarction 0 0 Repeat Revascularization 0 0 Need for Cardiac ,Thorac. or Vasc. Operation 0 1 Acute Renal Dysfunction 0 0 CPR or VT req. Cardioversion 0 0 Increase in Aortic Insufficiency 0 0 Severe Hypotension 0 2 Total 0 3 CEC Adjudicated Adverse Events Directly Related to the Device in PROTECT II 24 |
 25 PROTECT-II: Additional Analyses in SAP PROTECT-II: Additional Analyses in SAP • • Pre-Specified Population Analyses: Per Protocol Pre-Specified Population Analyses: Per Protocol • • Predefined Secondary Analysis: Predefined Secondary Analysis: - - Learning Curve Learning Curve - - Operator-Technique (Rotational Atherectomy) Operator-Technique (Rotational Atherectomy) - - Anatomic Indications (Left Main v. 3 V CAD) Anatomic Indications (Left Main v. 3 V CAD) - - Extreme Surgical Co-Morbidity (STS PROM) Extreme Surgical Co-Morbidity (STS PROM) 25 |
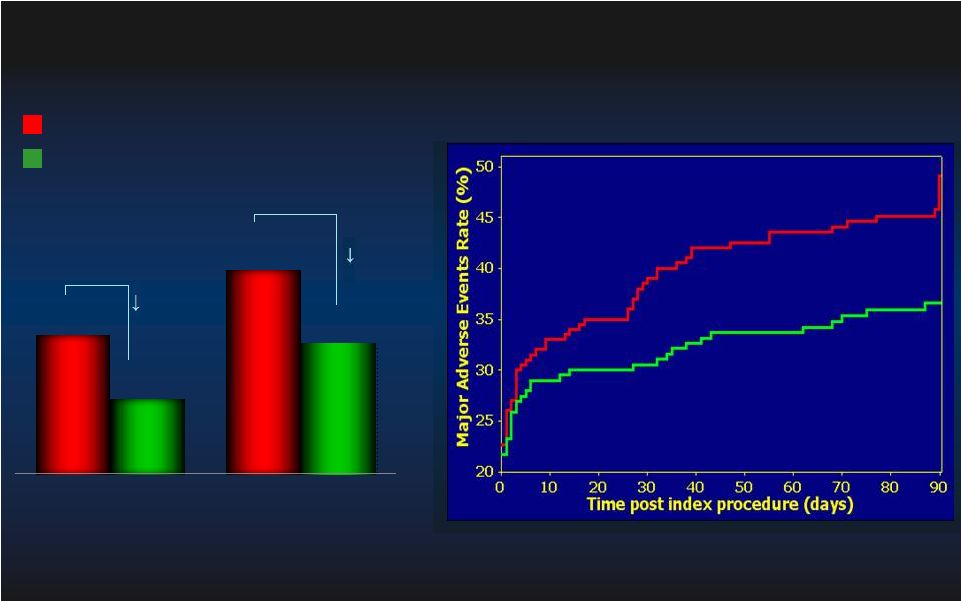
 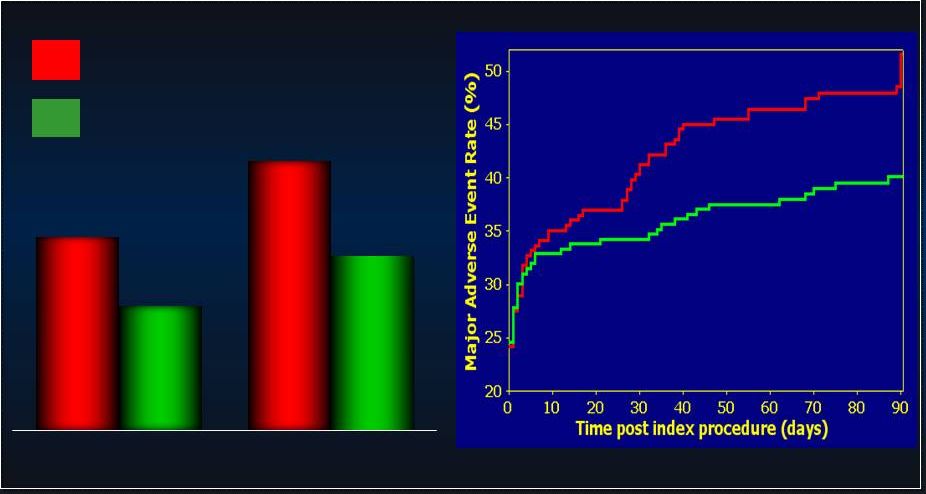 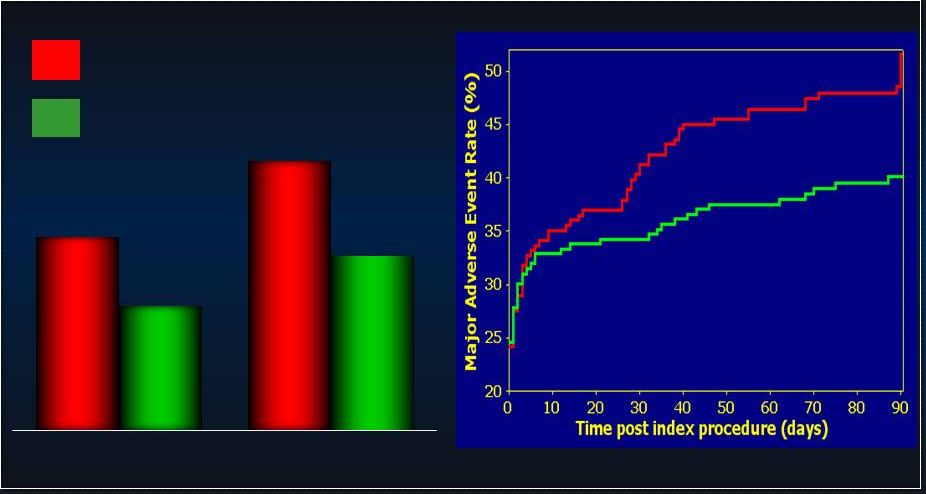 26 PROTECT II: Per Protocol MAE (N=427) PROTECT II: Per Protocol MAE (N=427) p=0.092 p=0.092 N=216 N=216 N=211 N=211 p=0.023 p=0.023 N=215 N=215 N=210 N=210 IABP IABP IMPELLA IMPELLA 42.2% 51.0% 34.3% 40.0% 30 day MAE 90 day MAE Log rank test, p=0.048 Log rank test, p=0.048 IABP IABP IMPELLA IMPELLA |
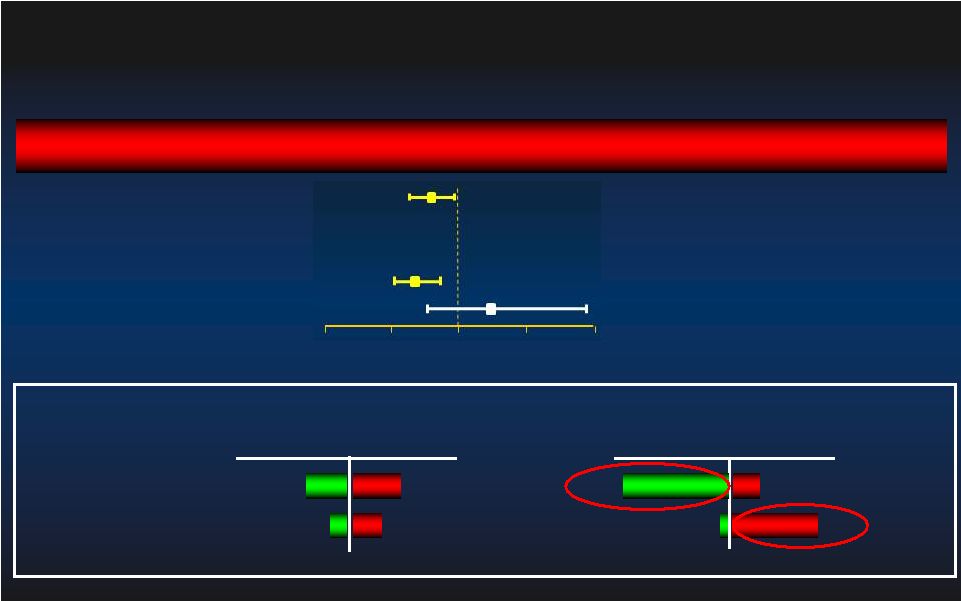
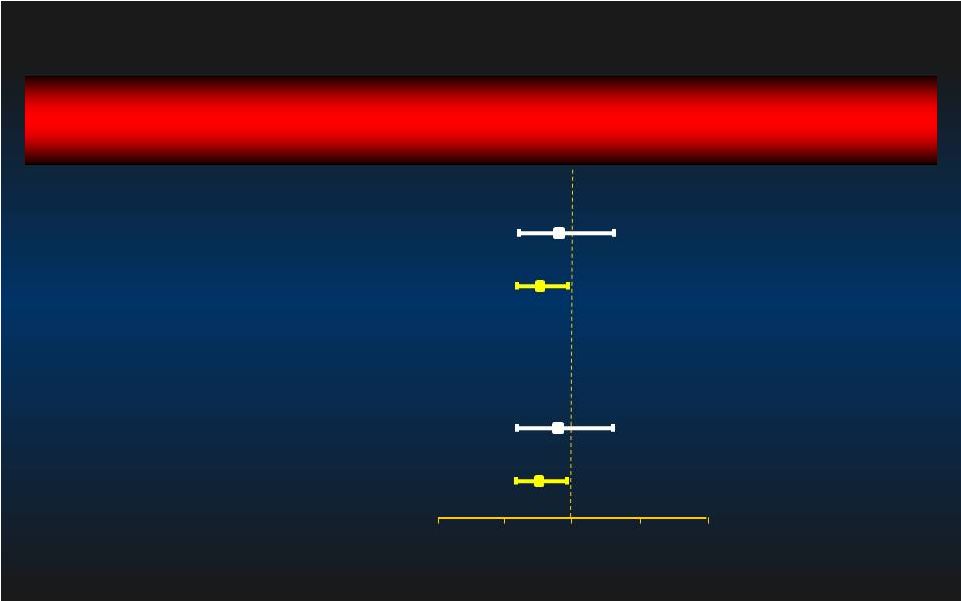 90 day MAE Relative Risk [95% CI] Relative Risk [95% CI] Group p-value 1.02 [0.70, 1.48] 0.936 0.76 [0.59, 0.97] 0.029 0.92 [0.62, 1.38] 0.697 0.74 [0.58, 0.95] 0.016 Learning Curve: 1st Learning Curve: 1st st Patient Excluded Patient Excluded 1 Impella/IABP Patient @ each site (n=119) Intent-to-Treat Impella better IABP better 0.0 0.0 0.5 0.5 1.0 1.0 1.5 1.5 2.0 2.0 Per Protocol After 1 Impella/IABP Pt @ each site (n=324) 1 Impella/IABP Pt @ each site (n=116) After 1 Impella/IABP Pt @ each site (n=309) 27 st st st st |
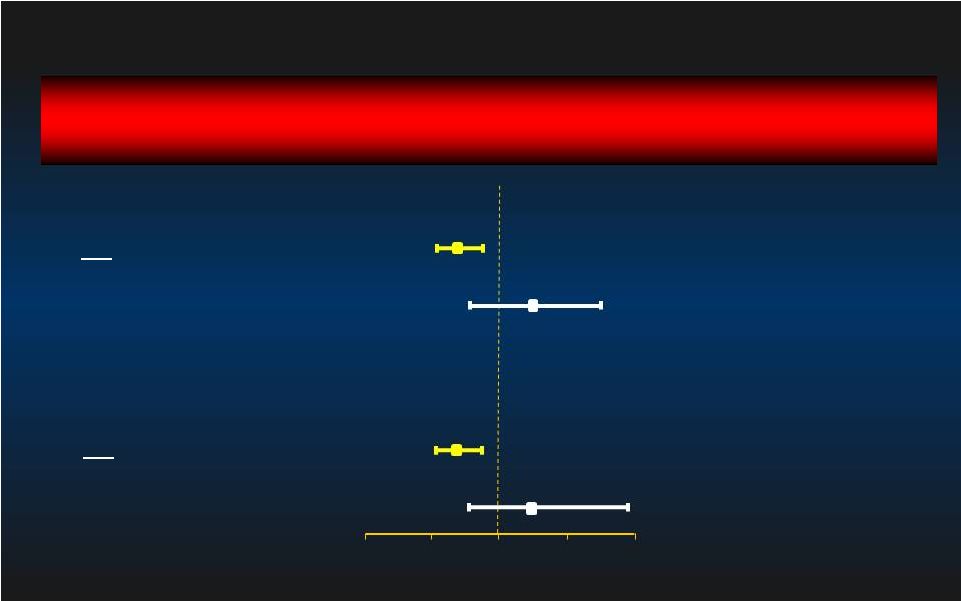 90 day MAE Relative Risk [95% CI] Relative Risk [95% CI] Group p-value 0.75 [0.59, 0.95] 0.014 1.19 [0.75, 1.91] 0.444 0.70 [0.55, 0.89] 0.003 1.19 [0.75, 1.91] 0.444 Rotational Atherectomy: Sub-group Rotational Atherectomy: Sub-group Atherectomy (n=52) No Atherectomy (n=391) Intent-to-Treat Impella better IABP better 0.0 0.0 0.5 0.5 1.0 1.0 1.5 1.5 2.0 2.0 Atherectomy (n=52) No Atherectomy (n=373) Per Protocol 28 |
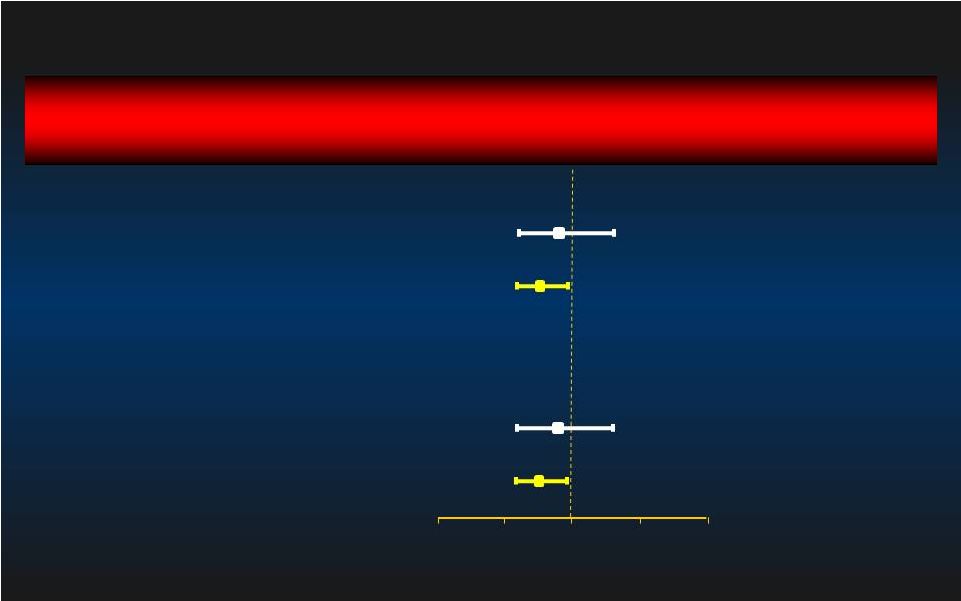 90 day MAE Relative Risk [95% CI] Relative Risk [95% CI] Group p-value 0.88 [0.59, 1.33] 0.552 0.81 [0.63, 1.03] 0.077 0.82 [0.53, 1.25] 0.351 0.78 [0.61, 0.99] 0.039 Anatomy Sub-group: Left Main vs 3VD Anatomy Sub-group: Left Main vs 3VD Left Main / Last Conduit (n=106) Intent-to-Treat Impella better IABP better 0.0 0.0 0.5 0.5 1.0 1.0 1.5 1.5 2.0 2.0 Per Protocol Left Main/ Last conduit (n=101) 3VD (n=324) 3VD (n=337) 29 |
 90 day MAE Relative Risk [95% CI] Relative Risk [95% CI] Group p-value 1.08 [0.71, 1.63] 0.733 0.77 [0.61, 0.98] 0.030 1.14 [0.75, 1.71] 0.540 0.71 [0.56, 0.91] 0.006 Excessive Surgical Co-Morbidity Excessive Surgical Co-Morbidity STS 10 (n=73) Intent-to-Treat Impella better IABP better 0.0 0.0 0.5 0.5 1.0 1.0 1.5 1.5 2.0 2.0 Per Protocol STS < 10 (n=370) STS 10 (n=71) STS < 10 (n=354) 30 |
 Ongoing US Impella Registry Ongoing US Impella Registry • Observational open registry for Impella in North America • Sites invited to participate on June 10, 2009 • 949 patients enrolled to-date at 41 centers • Includes all consecutive, unselected patients at each site • Data obtained after IRB approval • Data monitoring against source documentation • Independent CEC for events adjudication (2 Cardiologists + 1 CT Surgeon) • CEC adjudicated events per FDA Impella 2.5 study definitions 31 |
 32 Consistent Results in High-Risk PCI Impella Registries US Registry (175) EU Registry (N=144) 30 day Outcomes (%) Death related to the device All causes of Death Myocardial Infarction Stroke Revascularization Bleeding req. surgery Site bleeding req. transfusion Vascular complications Valve damage Hemolysis 0 4.0 1.1 0.6 0.6 1.7 3.4 4.0 0 0 0 5.5 0 0.7 0 0.7 5.5 4.0 0 0.7 2 1 1 Sjauw et al. J Am Coll Cardiol. 2009;54(25):2430-4. ²Maini et al. Catheter Cardiovasc Interv. 2012;80(5):717-25.; |
 33 Summary Summary • Safety concerns mitigated with review of all available clinical evidence associated with the use of the Impella 2.5 • Substantial improvements in hemodynamics have been demonstrated with Impella 2.5 v. IABP • Examination of the totality of the clinical evidence associated with the use of the Impella 2.5 v. IABP support efficacy in defined patient populations |
 34 Conclusion Conclusion • Comprehensive review of the totality of clinical evidence associated with the use of the Impella 2.5 device for its labeled “< 6 hour” indication supports the safety and efficacy of this device, particularly in patients undergoing high-risk PCI • Ongoing surveillance with a comprehensive National Registry is recommended to understand patient outcomes for these and other indications when applied beyond a randomized study |
 35 References References 1. Dixon SR, Henriques JP, Mauri L, Sjauw K, Civitello A, Kar B, Loyalka P, Resnic FS, Teirstein P, Makkar R, Palacios IF, Collins M, Moses J, Benali K, O'Neill WW. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience.. JACC Cardiovasc Interv. 2009 (2):91-6. 2. Griffith BP, Anderson MB, Samuels LE, Pae WE Jr, Naka Y, Frazier OH.The RECOVER I: A multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J Thorac Cardiovasc Surg. 2012 Mar 9. [Epub ahead of print] 3. O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Dzavík V, Popma J, Douglas PS, Ohman M. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012 ;126(14):1717-27. 4. Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584-8. 5. Meyns B, Autschbach R, Böning A, Konertz W, Matschke K, Schöndube F, Wiebe K, Fischer. Coronary artery bypass grafting supported with intracardiac microaxial pumps versus normothermic cardiopulmonary bypass: a prospective randomized trial. E. Eur J Cardiothorac Surg. 2002;22(1):112-7 6. Sjauw KD, Konorza T, Erbel R, Danna PL, Viecca M, Minden HH, Butter C, Engstrøm T, Hassager C, Machado FP, Pedrazzini G, Wagner DR, Schamberger R, Kerber S, Mathey DG, Schofer J, Engström AE, Henriques JP. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54(25):2430-4. 7. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: The USpella Registry. Maini B, Naidu SS, Mulukutla S, Kleiman N, Schreiber T, Wohns D, Dixon S, Rihal C, Dave R, O'Neill W. Catheter Cardiovasc Interv. 2012;80(5):717-25. 8. ABIOMED IDE G050017- Autopsy reports 2006 and 2007. 9. Dangas et al. Impact of Hemodynamic Support with Impella vs. Intraaortic Balloon Counterpulsation on Prognostically Important Ischemic Endpoints: Results from the PROTECT-II Trial. ., J Am Coll Cardiol 2012:60:B21 10. Popma et al., Impella Improves Clinical Outcomes When Extensive Revascularization is Performed: The PROTECT II Study. ., J Am Coll Cardiol 2012:59:13- ppA372 11. Alasnag MA, Gardi DO, Elder M, Kannam H, Ali F, Petrina M, Kheterpal V, Hout MS, Schreiber TL.Use of the Impella 2.5 for prophylactic circulatory support during elective high-ris11.k percutaneous coronary intervention. Cardiovasc Revasc Med. 2011;12(5):299-303 |
 Special Controls Have Been Identified and Implemented to Mitigate Known Risks 36 1. Extensive testing submitted for 510(k): Non-clinical Performance/Bench testing (41 tests on average per application) Clinical data (Impella 2.5: 129 patients) Testing includes evaluations of structural/tissue damage to heart, local heat generation and flow dynamics 2. Impella Post Market Registry: Local IRB approval, Independent CEC, Corelab 3. Physician and Staff Certification Programs 4. Appropriate caution and warnings added to package insert based on post market data (studies, registries) |
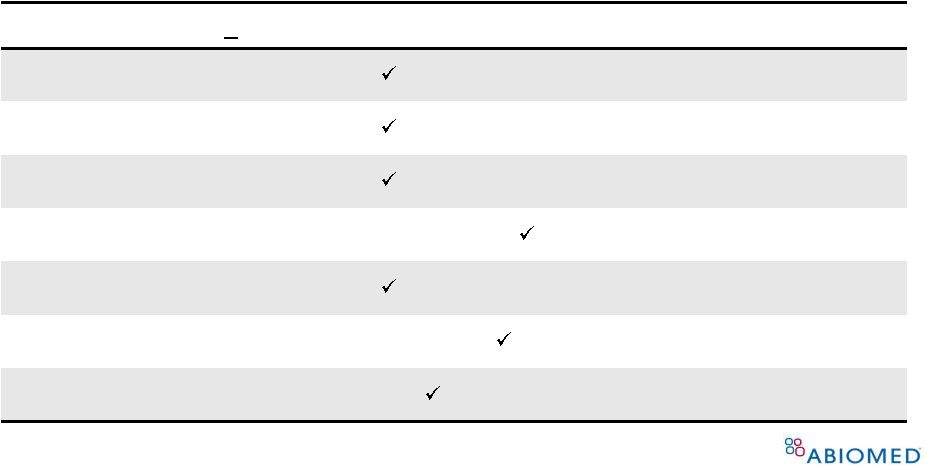 General and Special Controls are In Place to Mitigate Potential Risks to Public Health 37 Potential Risk (use < 6 hrs) Implemented Controls Alteration in blood composition Performance/Bench Testing, Labeling, Registry Inadequate tissue perfusion Performance/Bench Testing, Labeling, Registry Embolism Performance/Bench Testing, Labeling, Registry Duration of use Labeling, Registry Fluid leakage Performance/Bench Testing, Labeling, Registry Adverse tissue reaction Biocompatibility Testing Infection Sterility and Shelf-life testing, Registry |
 General and Special Controls are In Place to Mitigate Potential Risks to Public Health 38 Potential Risk (use < 6 hrs) Implemented Controls Structural/tissue damage to heart Performance/Bench Testing, Labeling, Training, Registry Local heat generation Performance/Bench Testing, Registry Flow dynamics Performance/Bench Testing, Registry |
 • Abiomed Recommendation: Establish an inclusive Prospective National Registry of Percutaneous Circulatory Support • Leveraging current infrastructure of the Impella Registry • On-going registry, in cooperation with FDA and the appropriate professional society, would enhance the current risk mitigation controls for class II regulation of these devices • Combined with existing data, can potentially support future PMA indications 39 Recommendation to Expand US Impella ® Registry |
Impella ® Safety Record Supports Effectiveness of Current Controls • Since 2008, Impella has been used over 15,000 times worldwide and has had no recalls in the United States and 1 recall outside the US which effected a total of 4 units • There have been a total of 49 MDR’s since its introduction for an overall MDR rate of 0.32% • Only 16 of these MDR’s were associated with Adverse Events, for an MDR with AE rate of 0.11% 40 |
 Conclusion 41 |
 For temporary ventricular support up to 6 hours, including support provided during HRPCI, Impella meets the requirements for a class II designation 42 Conclusion Further, we recommend the establishment of a prospective national registry of percutaneous circulatory support as an added special control for class II regulation of current and future devices Sufficient information (valid scientific evidence) exists to identify risks and benefits The risks to health have been identified and quantified Special controls have been currently implemented to mitigate these risks Impella safety record supports effectiveness of current controls |
 Thank you 43 |
44 APPENDIX |
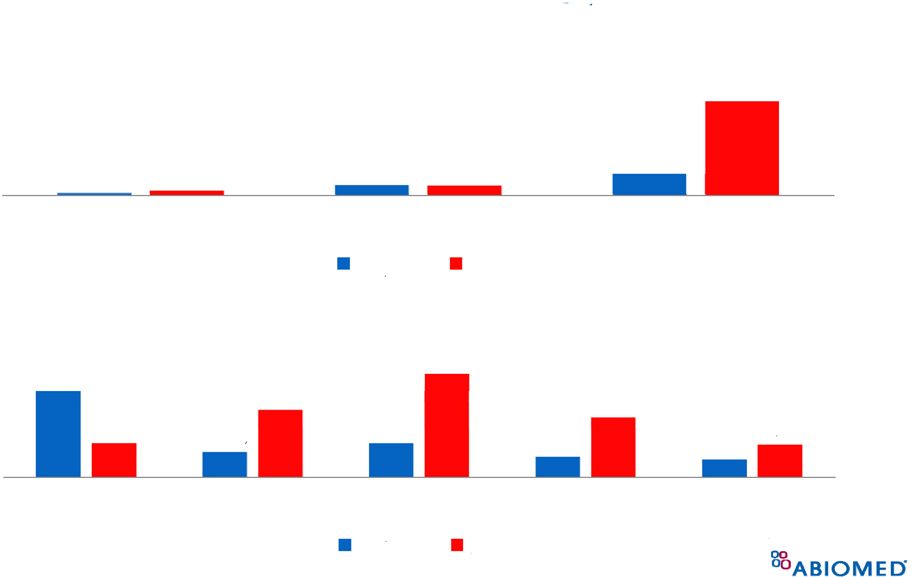 45 (thru 11/15/12) Impella ® MDR Rate Compared to IAB * * Assumes 75,000 IABs used each year 0.01% 0.07% 0.16% 0.03% 0.07% 0.73% Death Injury Malfunction Impella IAB 1.13% 0.32% 0.44% 0.26% 0.23% 0.44% 0.88% 1.36% 0.78% 0.42% 2008 2009 2010 2011 2012 Impella IAB |
 Procedural Characteristics Procedural Characteristics Procedural Characteristics Procedural Characteristics IABP IABP (N=211) (N=211) Impella Impella (N=216) (N=216) p-value p-value Use of Heparin 82.4% 93.5% <0.001 IIb/IIIa Inhibitors 26.5% 13.4% <0.001 Total Contrast Media (cc) 241±115 267±141 0.035 Rotational Atherectomy (RA) 9.0% 14.2% 0.083 Median # of RA Passes/lesion (IQ range) 1 (1-2) 3 (2-5) 0.001 Median # of RA passes/pt (IQ range) 2.0 (2.0-4.0) 5.0 (3.5-8.5) 0.003 Median RA time/lesion (IQ range sec) 40 (20-47) 60 (40-97) 0.004 RA of Left Main Artery 3.1% 8.0% 0.024 Total Support Time (hours) 8.23±21.0 1.86±2.7 <0.001 Discharge from Cath Lab on device 37.7% 5.6% <0.001 |
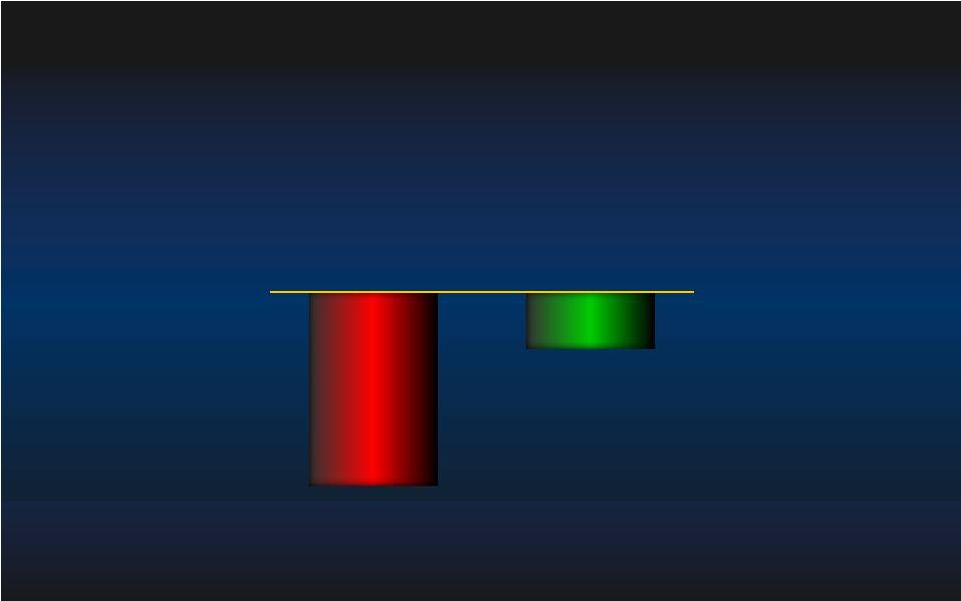 Hemodynamic Support Effectiveness Hemodynamic Support Effectiveness CPO= Cardiac Power Output = Cardiac Output x Mean Arterial Pressure x 0.0022 (Fincke R, Hochman J et al JACC 2004; 44:340-348) Cardiac Power Output Cardiac Power Output Maximal Decrease in CPO on device Support Maximal Decrease in CPO on device Support from Baseline (in x0.01 Watts) from Baseline (in x0.01 Watts) IABP IABP Impella Impella N=138 N=138 N=141 N=141 - 4.2 ± 24 - 14.2 ± 27 p=0.001 p=0.001 CPO data available only for 279 patients (N=138 IABP and N=141 Impella) 47 |
 CoreLab Analysis Shows No Evidence of Damage to Cardiac or Valve Structures Aortic/Mitral valve injury Valve prolapse Chordal rupture Papillary muscle rupture Aortic aneurysm Structural damage to heart chambers or septum 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% Structure PROTECT I (N=20) 1 PROTECT II (N=225) 2 1 Dixon et al. JACC Interv. 2009; 2 O’Neill et al Circulation 2012 |
 49 O’Neill et al 2012 Maini et al 2012 Chandola et al 2012 Dixon et al. 2009 Henriques and al. 2006 Valgimigli and al. 2005 Vlasselaers and al. 2005 Meyns and al. 2003 Siengenthaler and al. 2005 Cantena and al. 2005 Catena and al. 2004 Colombo and al. 2003 Strecker and al. 2004 Jurmann and al. 2004 1.2 hrs 1 hr 2 weeks 1.3 hrs Up to 2 hrs ~ 2.5 hrs 6 days 2-8 days 1-10 days N.A 2-19 days 18 days 6 days 1-7 days 2.5 2.5 5.0 2.5 2.5 2.5 2.5 5.0 5.0 5.0 5.0 5.0 5.0 5.0 225 175 1 20 19 1 1 16 24 1 8 1 1 6 TTE TTE TTE TTE TTE / Angio Angio Serial TEE Serial TEE Serial TEE 3D TTE / 2D TEE Serial TEE Serial TEE Serial TEE Serial TEE No No 1 patient No No No No No No No No No No No No No No No No No No No No No No No No No Study Device n LOS Imaging Modality Aortic Regurgitation Echo findings Trauma / valve LOS=length of support; TEE = Trans-oesophageal echocardiogram Over 330 Cases with Imaging Showed No Device Related Valve Regurgitation or Trauma |
 50 Results of a Randomized Study Supports Results of a Randomized Study Supports Blood Compatibility of IMPELLA Blood Compatibility of IMPELLA ® ® Leucocytes (10x9/l) Granulocytes (%) Eosinophilic leucocytes (%) Basophilic leucytes (%) Monocytes (%) Lymphocytes (%) Platelets (10x9/l) Antithrombin III (%) D-Dimers (ug/dl) Granulocyte elastase (ug/l) Complement C3 (g/l) IMPELLA (n=105) 12.3 ± 4.4 78 ± 8.1 1.48 ± 0.98 0.65 ± 0.41 5.31 ± 2.56 14 ± 7.5 144 ± 54 60 ± 15 0.75 ± 0.65 150 ± 126 0.65 ± 0.2 Cardio-Pulmonary Bypass p-value 11.3 ± 4.4 78 ± 6.1 1.26 ± 0.97 0.65 ± 0.41 5.1 ± 2.72 13 ± 6.8 163 ± 50 68 ± 15 1.06 ± 2.2 259 ± 195 0.73 ± 0.2 N.S N.S N.S N.S N.S N.S N.S 0.0007 N.S 0.00001 0.008 Meyns and al. Eur. J. Cardio-thoracic Surgery 22 (2002): 112-117 (n=94) |
 51 MAE= Major Adverse Event Rate MAE= Major Adverse Event Rate N=91 N=91 N=85 N=85 N=64 N=64 N=68 N=68 N=69 N=69 N=66 N=66 Study Device Learning Curve Effect Study Device Learning Curve Effect 90day MAE Outcome 90day MAE Outcome Intent-to-Treat Intent-to-Treat IABP IABP IMPELLA IMPELLA 2008 2009 2010 |
 52 PROTECT II MAE Outcome PROTECT II MAE Outcome Pre-specified High Risk PCI Without Atherectomy Group Pre-specified High Risk PCI Without Atherectomy Group MAE= Major Adverse Event Rate MAE= Major Adverse Event Rate 39.6% 48.7% 30.6% 38.5% 30 day MAE 90 day MAE 21% MAE p=0.014 p=0.014 N=192 N=192 N=202 N=202 p=0.06 p=0.06 N=193 N=193 N=203 N=203 23% MAE IMPELLA IMPELLA IABP IABP Log rank test, p=0.03 Log rank test, p=0.03 Intent-to-Treat (N=396) Intent-to-Treat (N=396) IABP IABP IMPELLA IMPELLA |
 90 day MAE Relative Risk [95% CI] Relative Risk [95% CI] Group p-value Interaction p-value 0.79 [0.64, 0.97] 0.023 0.70 [0.55, 0.89] 0.003 1.25 [0.75, 1.91] 0.444 Confounder: Use of Atherectomy Confounder: Use of Atherectomy Pre-Specified Sub-group Analysis Pre-Specified Sub-group Analysis With Atherectomy (n=52, 12%) Without Atherectomy (n=373, 88%) PCI Procedure Overall – Per Protocol (n=425) Impella better IABP better 0.08 MAE = Major Adverse Event ; Per Protocol (PP)= Patients that met MAE = Major Adverse Event ; Per Protocol (PP)= Patients that met all incl./ excl. criteria. all incl./ excl. criteria. Without Atherectomy Without Atherectomy MI (>3x ULN) MI (>3x ULN) Repeat Revascularization Repeat Revascularization 14.8% 14.8% 6.6% 6.6% 17.4% 17.4% 10.5% 10.5% IMPELLA IMPELLA IABP IABP 37.5% 37.5% 3.1% 3.1% 10.0% 10.0% With Atherectomy With Atherectomy IMPELLA IMPELLA IABP IABP (p=0.006) (p=0.006) (p=0.03) (p=0.03) (p=0.492) (p=0.492) (p=0.171) (p=0.171) 30.0% 30.0% 0.0 0.0 0.5 0.5 1.0 1.0 1.5 1.5 2.0 2.0 |
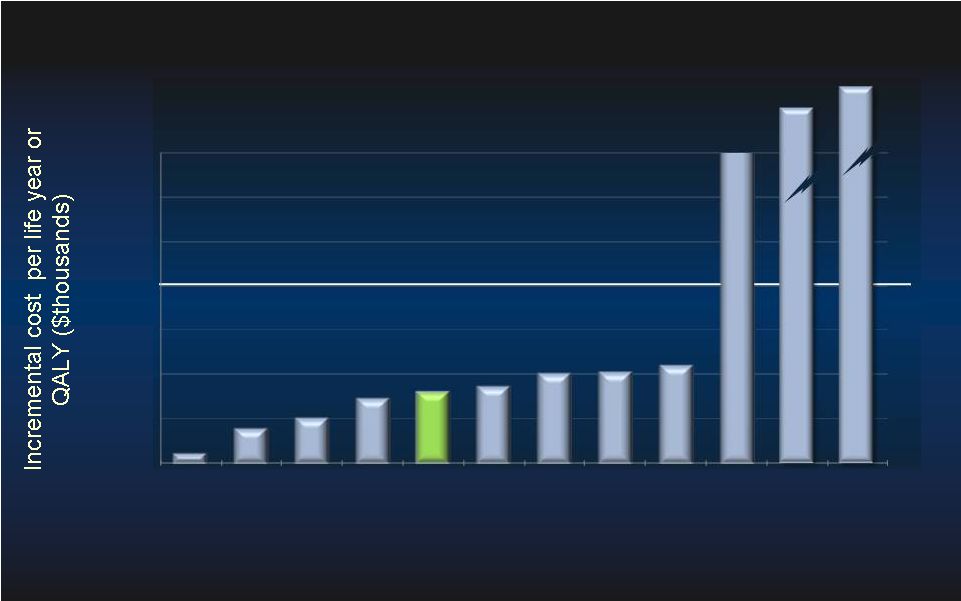 54 1. Earnshaw, et al. Arch. of Intern Med., 2011. 2. Feldman, et al. JACC, 2005. COMPANION 3. Choudhry, et al. JACC, 2011. JUNIPER 4. Chen, et al. ISPOR, 2009.CHARISMA <$100,000 Threshold 9. Winkelmayer, et al. Medical Decision Making, 2002. 10. Moore, et al. BMC Health Services Research 2009 11. Slaughter, et al. AHA, 2011. 12. Russo, et al. ACC 2010. 5. Maini, et al. TCT, 2011 PROTECT II 6. Feldman, et al. JACC, 2005. COMPANION 7. Reynolds, et al. ACC, 2011. 8. Reynolds, et al. Circ Arrhythm Electrophysiol, 2009 $274k $199k $39,367 $39,367 Comparative Cost-Effectiveness Studies Comparative Cost-Effectiveness Studies $0 $25 $50 $75 $100 $125 $150 $175 Aspirin MI CRT-P C-reactive Protein Clopidogrel Impella PROTECT II CRT-D TAVI (LYG) AF ablation Dialysis (LYG) MRI v. Mammo LVAD 1 DT(LYG) LVAD 2 DT(LYG) |