- ABMD Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
SC TO-C Filing
Abiomed (ABMD) SC TO-CInformation about tender offer
Filed: 1 Nov 22, 5:25pm
Exhibit 99.4
LinkedIn:
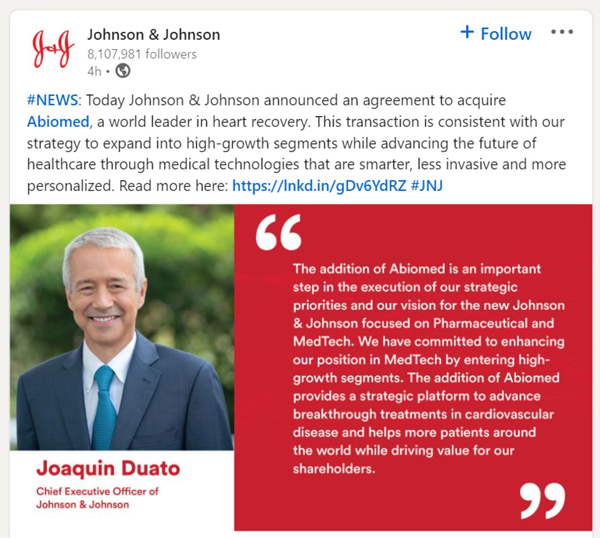
Linkedln: 8.107,981 followers 4h + Follow NEWS: Today Johnson & Johnson announced an agreement to acquire Abiomed, a world leader in heart recovery. This transaction is consistent with our strategy to expand into high-growth segments while advancing the future of healthcare through medical technologies that are smarter, less invasive and more personalized. Read more here: https://lnkd.in/gDv6YdRZ #JNJ “The addition of Abioed is an important step I the execution of our strategic priorities and our vision for the new Johnson & Johnson focused on Pharmaceutical and MedTech. We have committed to enhancing our position in MedTech by entering high-growth segments. The addition of Abiomed provides a strategic platform to advance breakthrough treatments in cardiovascular disease and helps more patients around the world while driving value for our shareholders. Joaquin Duato Chief Executive Officer of Johnson & Johnson
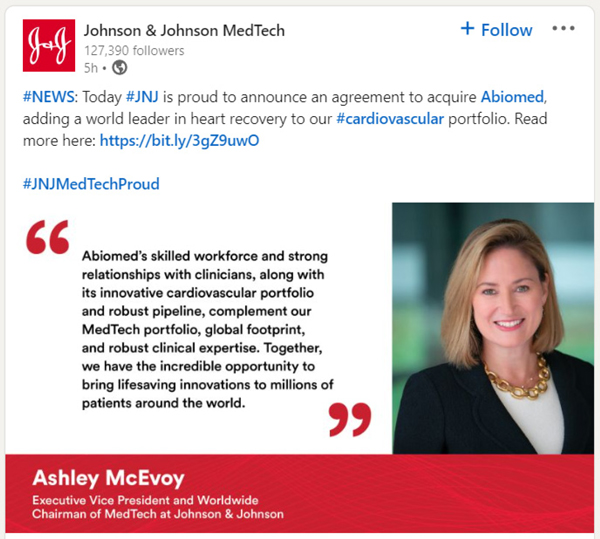
Johnson & Johnson MedTech + Follow 127,390 followers 5h. #NEWS: Today #JNJ is proud to announce an agreement to acquire Abiomed, adding a world leader in heart recovery to our #cardiovascular portfolio. Read more here: https://bit.ly/3gZ9uwO #JNJMedtechProud “Abiomed’s skilled workforce and strong relationships with clinicians, along with its innovative cardiovascular portfolio and robust pipeline, complement our MedTech portfolio, global footprint, and robust clinical expertise. Together, we have the incredible opportunity to bring lifesaving innovations to millions of patients around the world.” Ashley McEvoy Executive Vice president and Worldwide Chairman of MedTech at Johnson & Johnson

Joaquin Duato in . 3++ Follow Today we announced our plans to acquire Abiomed, a world leader in breakthrough heart, lung and kidney support technologies. The addition of Abiomed to our portfolio is an important step in the execution of our strategic priorities and our vision for the new Johnson & Johnson focused on Pharmaceuticals and MedTech. We have committed to enhancing our position in MedTech by entering high growth segments. The addition of Abiomed provides a strategic platform to advance breakthrough treatments in cardiovascular disease and helps more patients around the world while driving value for our shareholders. From everyone here at Johnson & Johnson, we look forward to welcoming the Abiomed team. https://lnkd.in/gDv6YdRZ #MyCompany Joaquin Duato Chief Executive Officer of Johnson & Johnson “ The addition of Abiomed is an important step in the execution of our strategic priorities and our vision for the new Johnson & Johnson focused on Pharmaceutical and MedTech. We have committed to enhancing our position in MedTech by entering high growth segments. The addition of Abiomed provides a strategic platform to advance breakthrough treatments in cardiovascular disease and helps more patients around the world while driving value for our shareholders. “

Ashley McEvoy • 3rd+ Executive Vice President, Worldwide Chairman, MedTech, Jo… 4h . ® +Follow Today is an exciting day. We announced this morning that we will acquire Abiomed, broadening our position as a cardiovascular innovator, accelerating near- and long-term performance, but most importantly - giving us the incredible opportunity to bring lifesaving innovation to millions of patients around the world. Our purpose is clear. I’m so proud of the Johnson & Johnson MedTech and Abiomed teams and look forward to the future. https://bit.ly/3zzuMrc Johnson & Johnson #mycompany #JNJMedTechProud Johnson & Johnson Johnson & Johnson to Acquire Abiomed
Twitter:
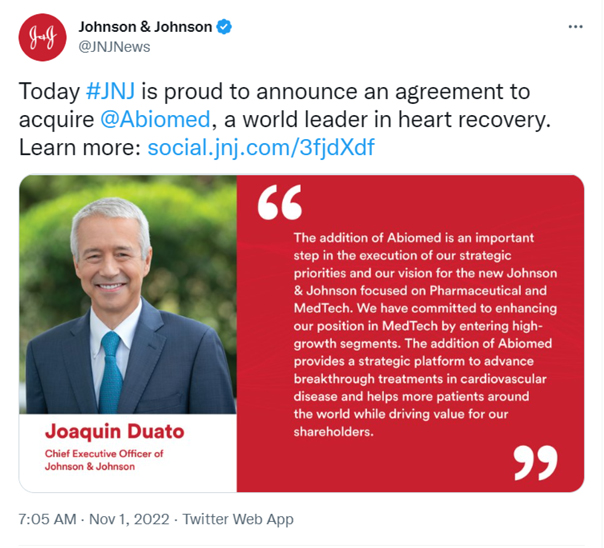
Twitter: Johnson & Johnson @JNJNEWS Today #JNJ is proud to announce an agreement to acquire @abiomed, a world leader in heart recovery. Learn more: social.jnj.com/3fjdXdf “The addition of Abiomed is an important step in the execution of our strategic priorities and our vision for the new Johnson & Johnson focused on Pharmaceutical and MedTech. We have committed to enhancing our position in MedTech by entering high-growth segments. The addition of Abiomed provides a strategic platform to advance breakthrough treatments in cardiovascular disease and helps more patients around the world while driving value for our shareholders.’’ Joaquin Duato Chief Executive Officer of Johnson & Johnson 7:05 AM. Nov 1, 2022. Twitter Web APp
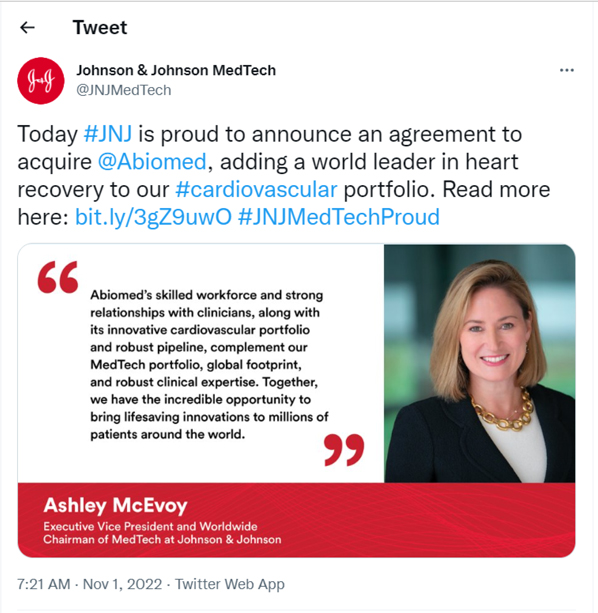
Johnson & Johnson MedTech@JNJMedTechToday #JNJ is proud to announce an agreement to acquire @Abiomed, adding a world leader in heart recovery to our #cardiovascular portfolio. Read more here: bit.ly/3gZ9uwO #JNJMedTechProud”Abiomed’s skilled workforce and strong relationships with clinicians, along with its innovative cardiovascular portfolio and robust pipeline, complement our MedTech portfolio, global footprint, and robust clinical expertise. Together, we have the incredible opportunity to bring lifesaving innovations to millions of patients around the world.” Ashley McEvoy Executive Vice President and Worldwide Chairman of MedTech at Johnson & Johnson7:21 AM . Nov1, 2022.Twitter Web App
Cautionary Statement Regarding Forward-Looking Statements
This communication contains forward-looking statements regarding the potential acquisition of ABIOMED. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of ABIOMED or Johnson & Johnson. Risks and uncertainties include, but are not limited to: the risk that the closing conditions for the acquisition will not be satisfied, including the risk that clearance under the Hart-Scott-Rodino Antitrust Improvements Act or other applicable antitrust laws will not be obtained; uncertainty as to the percentage of ABIOMED stockholders that will support the proposed transaction and tender their outstanding shares of common stock of ABIOMED in the Offer; the possibility that the transaction will not be completed in the expected timeframe or at all; potential adverse effects to the businesses of Johnson & Johnson or ABIOMED during the pendency of the transaction, such as employee departures or distraction of management from business operations; the risk of stockholder litigation relating to the transaction, including resulting expense or delay; the potential that the expected benefits and opportunities of the acquisition, if completed, may not be realized or may take longer to realize than expected; challenges inherent in product
research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new products; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; economic conditions, including currency exchange and interest rate fluctuations; the risks associated with global operations; competition, including technological advances, new products and patents attained by competitors; challenges to patents; changes to applicable laws and regulations, including tax laws and global health care reforms; adverse litigation or government action; changes in behavior and spending patterns or financial distress of purchasers of health care services and products; and trends toward health care cost containment. In addition, if and when the transaction is consummated, there will be risks and uncertainties related to the ability of the Johnson & Johnson family of companies to successfully integrate the products and employees/operations and clinical work of ABIOMED, as well as the ability to ensure continued performance or market growth of ABIOMED’s products. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended January 2, 2022, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q, and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.
Additional Information
The tender offer described in this communication has not yet commenced, and this communication is neither an offer to purchase nor a solicitation of an offer to sell securities. At the time the tender offer is commenced, Johnson & Johnson will cause Merger Sub to file a tender offer statement on Schedule TO with the U.S. Securities and Exchange Commission (“SEC”). Investors and ABIOMED security holders are strongly advised to read the tender offer statement (including an offer to purchase, letter of transmittal and related tender offer documents) that will be filed by Johnson & Johnson with the SEC and the related solicitation/recommendation statement on Schedule 14D-9 that will be filed by ABIOMED with the SEC, when they become available, because they will contain important information. These documents will be available at no charge on the SEC’s website at www.sec.gov. In addition, a copy of the offer to purchase, letter of transmittal and certain other related tender offer documents (once they become available) may be obtained free of charge by directing a request to Johnson & Johnson, Office of the Corporate Secretary, One Johnson & Johnson Plaza, New Brunswick, NJ 08933, Attn: Corporate Secretary’s Office. A copy of the solicitation/recommendation statement on Schedule 14D-9 (once it becomes available) also may be obtained free of charge from ABIOMED under the “Investors” section of ABIOMED’ website at https://investors.abiomed.com.