UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
(Mark One)
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For fiscal year ended March 31, 2016
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-09585
ABIOMED, Inc.
(Exact Name of Registrant as Specified in Its Charter)
Delaware | | 04-2743260 |
(State or Other Jurisdiction of Incorporation or Organization) | | (I.R.S. Employer Identification No.) |
| | |
22 Cherry Hill Drive Danvers, Massachusetts | | 01923 |
(Address of Principal Executive Offices) | | (Zip Code) |
(978) 646-1400
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | | Name of Each Exchange on Which Registered |
Common Stock, $.01 par value | | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Rule 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | x | | | Accelerated filer | o |
Non-accelerated filer | o | | (Do not check if a smaller reporting company) | Smaller reporting company | o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s common stock as of September 30, 2015, held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold as of such date was $3,934,644,424. As of May 12, 2016, 42,735,136 shares of the registrant’s common stock, $.01 par value, were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive Proxy Statement for Abiomed, Inc.’s 2016 Annual Meeting of Stockholders, which is scheduled to be filed within 120 days after the end of Abiomed, Inc.’s fiscal year, are incorporated by reference into Part III (Items 10, 11, 12, 13 and 14) of this Form 10-K.
TABLE OF CONTENTS
NOTE REGARDING TRADEMARKS
ABIOMED, ABIOCOR, IMPELLA, IMPELLA CP, IMPELLA RP, and BVS 5000 are trademarks of ABIOMED, Inc., and are registered in the U.S. and certain foreign countries. BVS is a trademark of ABIOMED, Inc. and is registered in the U.S. AB5000, IMPELLA 2.5, IMPELLA 5.0, IMPELLA LD and cVAD REGISTRY are trademarks of ABIOMED, Inc. RECOVER is a trademark of Abiomed Europe GmbH, a subsidiary of ABIOMED, Inc., and is registered in certain foreign countries.
NOTE REGARDING COMPANY REFERENCES
Throughout this report on Form 10-K (the “Report”), “Abiomed, Inc.,” the “Company,” “we,” “us” and “our” refer to ABIOMED, Inc. and its consolidated subsidiaries.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report, including the documents incorporated by reference in this report, includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements may be accompanied by such words as “anticipate,” “believe,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “potential,” “project,” “target,” “will” and other words and terms of similar meaning. We have based these forward-looking statements on what we believe are our reasonable current expectations and projections about future events. Each forward-looking statement in this report is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Forward-looking statements in these documents include, but are not necessarily limited to, those relating to:
| · | our expectations with respect to submissions to and approvals from regulatory bodies that the application for the Impella 2.5™ and Impella 5.0™ in Japan will receive regulatory approval during calendar 2016; |
| · | the ability of patients and other customers using our products to obtain reimbursement of their medical expenses by government healthcare programs and private insurers including potential changes to current government and private insurers’ reimbursements; |
| · | other competing therapies that may in the future be available to heart failure patients; |
| · | the development of new and existing products and anticipated costs, including research and development, sales and marketing, manufacturing and training costs associated with product development; |
| · | our plans to potentially acquire new businesses or technologies; |
| · | the potential markets that exist or could develop for our products and products under development; |
| · | our business strategy, and commercial plans for our products, including our expansion into new markets such as Japan; |
| · | our revenue growth expectations, our level of operating expenses and our goal of maintaining profitability; |
| · | expected capital expenditures for the fiscal year ending March 31, 2017; |
| · | demand for and expected shipments of our products; |
| · | possible shifts in the revenue mix associated with our products; |
| · | our ability to increase revenues from our Impella® line of products and the sufficiency of revenues, profits and cash flows to fund future operations; |
| · | future actions related to results of ongoing investigations and litigation, and expenditures or costs related thereto; |
| · | plans with respect to clinical trials and registries; and |
| · | the sufficiency of our liquidity and capital resources. |
Factors that could cause actual results or conditions to differ from those anticipated by these and other forward-looking statements include our inability to predict the outcome of investigations and litigation and associated expenses; possible delays in our research and development programs; our ability to obtain regulatory approvals and market our products, and uncertainties related to regulatory processes; greater government scrutiny and regulation of the medical device industry and our ability to respond to changing laws and regulations affecting our industry and changing enforcement practices related thereto; the inability to manufacture products in commercial quantities at an acceptable costs, the acceptance by physicians and hospitals of our products; the impact of competitive products and pricing; uncertainties associated with future capital needs and the risks identified under “Risk Factors” section set forth in Item 1A of Part I and elsewhere in this report, as well as other information we file with the Securities and Exchange Commission, or SEC. Readers are cautioned not to place undue reliance on any forward-looking statements contained in this report, which speak only as of the date of this report. We do not undertake any obligation to update or revise these forward-looking statements whether as a result of new information, future events or otherwise, unless otherwise required by law. Our business is subject to substantial risks and uncertainties, including those referenced above. Investors, potential investors, and others should give careful consideration to these risks and uncertainties.
PART I
Overview
We are a leading provider of temporary mechanical circulatory support devices and we offer a continuum of care to heart failure patients. We develop, manufacture and market proprietary products that are designed to enable the heart to rest, heal and recover by improving blood flow to the coronary arteries and end-organs and/or temporarily performing the pumping function of the heart. Our products are used in the cardiac catheterization lab, or cath lab, by interventional cardiologists, the electrophysiology lab, the hybrid lab and in the heart surgery suite by heart surgeons. A physician may use our devices for patients who are in need of hemodynamic support prophylactically or emergently before, during or after angioplasty or heart surgery procedures. We believe that heart recovery is the optimal clinical outcome for patients experiencing heart failure because it enables patients to go home with their own native heart and restores their quality of life. In addition, we believe that for the care of such patients, heart recovery is the most cost-effective solution for the healthcare system.
Our strategic focus and the driver of the majority of our revenue growth is the market penetration of our family of Impella® products. The Impella product portfolio, which includes the Impella 2.5™, Impella CP®, Impella RP®, Impella LD™ and Impella 5.0™, has supported thousands of patients in the U.S. We expect that most of our product and service revenues in the near future will be from our Impella products. Revenues from our non-Impella products, largely focused on the heart surgery suite, have been decreasing over the past several years as we have strategically shifted our sales and marketing efforts towards our Impella products and the cath lab.
In March 2015, we received the first of two PMA approvals from the FDA for the Impella 2.5 product, for its use during elective and urgent high-risk PCI procedures. With this PMA indication, the Impella 2.5 product is the first hemodynamic support device proven safe and effective by the FDA indicated for use during high-risk PCI procedures. In August 2015, we submitted a PMA supplement requesting to expand our current Impella 2.5 PMA approval to include additional indications for the Impella 2.5 product and also to include all of our other Impella devices (Impella CP, Impella 5.0 and Impella LD) that support the left side of the heart. These submissions are for a set of indications related to the use of the Impella devices in patients suffering cardiogenic shock following acute myocardial infarction or cardiac surgery and for a longer duration of support. In April 2016, the FDA approved the PMA supplement for our Impella 2.5, Impella CP, Impella 5.0and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock. In this setting, the Impella heart pumps stabilize the patient’s hemodynamics, unload the left ventricle, perfuse the end organs and allow for recovery of the native heart.
We expect to continue to make additional PMA supplement submissions for our Impella suite of products for additional indications.
Our Impella 2.5, Impella 5.0, Impella LD, Impella CP and Impella RP products also have CE Mark approval and Health Canada approval which allows us to market these devices in the European Union and Canada. We have submitted an application for the Impella 2.5 and Impella 5.0 devices in Japan and we are hopeful of receiving regulatory approval in calendar 2016.
Corporate Background
Our Company was founded in 1981 and we are currently incorporated in Delaware and trade on the NASDAQ Global Select Market under the ticker symbol ABMD.
Our principal executive offices are located at 22 Cherry Hill Drive, Danvers, Massachusetts 01923. Our telephone number is (978) 646-1400. We make available, free of charge on our website located at www.abiomed.com, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after filing such reports with the SEC. ABIOMED, Inc. has a Code of Conduct and Compliance Policy that applies to all of its directors, officers, and employees. A paper copy of this document may be obtained free of charge by writing to the Company’s Chief Compliance Officer at our principal executive office or by email at ir@abiomed.com. Our audit committee, governance and nominating committee and compensation committee charters are also posted on our website. The contents of our website are not incorporated by reference into this report. In addition, the public may read and copy any materials we file or furnish with the SEC, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 or may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Moreover, the SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding reports that we file or furnish electronically with the SEC at www.sec.gov.
1
Our Products
Impella 2.5™
The Impella 2.5 catheter is a percutaneous micro heart pump with an integrated motor and sensors. The device is designed primarily for use by interventional cardiologists to support patients in the cath lab who may require assistance to maintain their circulation. The Impella 2.5 catheter can be quickly inserted via the femoral artery to reach the left ventricle of the heart where it is directly deployed to draw blood out of the ventricle and deliver it to the circulatory system. This function is intended to reduce ventricular work and provide flow to vital organs. The Impella 2.5 is introduced with normal interventional cardiology procedures and can pump up to 2.5 liters of blood per minute.
The Impella 2.5 product received 510(k) clearance in June 2008 from the FDA for partial circulatory support for up to six hours. In March 2015, we received the first of two PMA approvals from the FDA for the Impella 2.5 product, for its use during elective and urgent high-risk PCI procedures. With this PMA indication, the Impella 2.5 product became the first hemodynamic support device proven safe and effective by the FDA for use during high-risk PCI procedures. Under this first PMA approval, the Impella 2.5 is a temporary (up to six hours) ventricular support device indicated for use during high-risk PCI performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high-risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 device in these patients may prevent hemodynamic instability that may occur during planned temporary coronary occlusions and may reduce periprocedural and post-procedural adverse events. The product labeling allows for the clinical decision to leave the Impella 2.5 product in place beyond the intended duration of up to six hours due to unforeseen circumstances. Pursuant to our PMA approval, we are conducting a single-arm, post-approval study on the Impella 2.5 product, collecting data on high-risk PCI patients. The study is a prospective, multi-center study comprised of 369 patients from up to 70 sites supported with the Impella 2.5 system. The Impella 2.5 device has CE Mark approval in Europe for up to five days of use and is approved for use in over 40 countries.
In August 2015, we submitted a PMA supplement requesting to expand our current Impella 2.5 PMA approval to include additional indications for the Impella 2.5 product and also to include our other Impella devices (Impella CP, Impella 5.0 and Impella LD) that support the left side of the heart. These submissions are for a set of indications related to the use of the Impella devices in patients suffering cardiogenic shock following acute myocardial infarction or cardiac surgery and for a longer duration of support. In April 2016, the FDA approved the PMA supplement for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock. In this setting, the Impella heart pumps stabilize the patient’s hemodynamics, unload the left ventricle, perfuse the end organs and allow for recovery of the native heart.
The data submitted to the FDA in support of the PMA supplement included an analysis of 415 patients from the RECOVER 1 study and the U.S. Impella registry (cVAD Registry™), as well as a literature review using the Impella products in 692 patients from 17 clinical studies. A safety analysis reviewed over 24,000 Impella patients who had used an Impella device, as documented in the FDA medical device reporting, or MDR, database, which draws from seven years of experience using the Impella products in the U.S. We believe this is the most comprehensive review ever submitted to the FDA for circulatory support in the cardiogenic shock population.
A November 2011 update to the American College of Cardiology Foundation (ACCF) /American Heart Association (AHA) Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions Guidelines for Percutaneous Coronary Intervention, for the first time, included Impella devices in both the emergent and prophylactic hemodynamic support settings. In addition, a December 2012 update to the AHA’s Recommendations for the Use of Mechanical Circulatory Support: Device Strategies and Patient Selection recommended Impella devices for use in mechanical circulatory support; a December 2012 update to the ACCF / AHA Guidelines for the Management of ST-Elevation Myocardial Infarction , or “STEMI,” included the Impella 2.5 device for use in patients requiring urgent coronary artery bypass grafting with STEMI and in treatment of patients with cardiogenic shock complications after STEMI. A January 2013 update to the International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support included Impella devices for the first time for patients with multi-organ failure. In addition, Impella devices were included in a January 2013 update to the ACCF / AHA Task Force on Practice Guidelines for the Management of ST-Elevation Myocardial Infarction and a September 2014 AHA / the American College of Cardiology Task Force on Practice Guidelines for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes.
The Impella 2.5 device has CE Mark approval in Europe for up to five days of use. Impella 2.5 also has Health Canada approval which allows us to market the device in Canada. We have submitted an application for the Impella 2.5 in Japan and we are hopeful of receiving regulatory approval in calendar 2016.
2
Impella CP®
In September 2012, we announced that the Impella CP received 510(k) clearance from the FDA. The Impella CP provides blood flow of approximately one liter more per minute than the Impella 2.5 and is primarily used by either interventional cardiologists to support patients in the cath lab or by surgeons in the heart surgery suite.
In August 2015, we submitted a PMA supplement requesting to expand our current Impella 2.5 PMA approval to include additional indications for the Impella 2.5 product and also to include our other Impella devices (Impella CP, Impella 5.0 and Impella LD) that support the left side of the heart. These submissions are for a set of indications related to the use of the Impella devices in patients suffering cardiogenic shock following acute myocardial infarction or cardiac surgery and for a longer duration of support. In April 2016, the FDA approved the PMA supplement for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock. In this setting, the Impella heart pumps stabilize the patient’s hemodynamics, unload the left ventricle, perfuse the end organs and allow for recovery of the native heart.
Pursuant to the April 2016 PMA approval, the Impella 2.5, Impella CP, Impella 5.0 and Impella LD catheters, in conjunction with the Automated Impella Controller, are temporary ventricular support devices intended for short term use (≤ 4 days for the Impella 2.5 and Impella CP, and ≤ 6 days for the Impella 5.0 and LD) and indicated for the treatment of ongoing cardiogenic shock that occurs immediately (< 48 hours) following acute myocardial infarction or open heart surgery as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures. The intent of the Impella system therapy is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function. Optimal medical management and convention treatment measures include volume loading and use of pressors and inotropes, with or without an intraortic ballon pump.
We expect to continue to make additional PMA supplement submissions for our Impella suite of products for additional marketing indications. This would include expanding the current PMA approval that we have for the Impella 2.5 product to the Impella CP product for elective and urgent high-risk PCI procedures.
Impella 5.0™ and Impella LD™
The Impella 5.0 and Impella LD are percutaneous micro heart pumps with integrated motors and sensors for use primarily in the heart surgery suite. These devices are designed to support patients who require higher levels of circulatory support as compared to the Impella 2.5.
The Impella 5.0 pump can be inserted into the left ventricle via femoral cut down or through the axillary artery. The Impella 5.0 pump is passed into the ascending aorta, across the valve and into the left ventricle. The Impella LD pump is similar to the Impella 5.0 pump, but is implanted directly into the ascending aorta through an aortic graft. Both of these procedures are normally performed with the assistance of heart surgeons in the surgery suite. The Impella 5.0 and Impella LD products can pump up to five liters of blood per minute, providing full circulatory support.
The Impella 5.0 and Impella LD devices originally received 510(k) clearance in April 2009, for circulatory support for up to six hours. In August 2015, we submitted a PMA supplement requesting to expand our current Impella 2.5 PMA approval to include additional indications for the Impella 2.5 product and also to include our other Impella devices (Impella CP, Impella 5.0 and Impella LD) that support the left side of the heart. These submissions were for a set of indications related to the use of the Impella devices in patients suffering cardiogenic shock following acute myocardial infarction or cardiac surgery and for a longer duration of support. In April 2016, the FDA approved the PMA supplement for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock. In this setting, the Impella heart pumps stabilize the patient’s hemodynamics, unload the left ventricle, perfuse the end organs and allow for recovery of the native heart.
Pursuant to the April 2016 PMA approval, the Impella 2.5, Impella CP, Impella 5.0 and Impella LD catheters, in conjunction with the Automated Impella Controller, are temporary ventricular support devices intended for short term use (≤ 4 days for the Impella 2.5 and Impella CP, and ≤ 6 days for the Impella 5.0 and LD) and indicated for the treatment of ongoing cardiogenic shock that occurs immediately (< 48 hours) following acute myocardial infarction or open heart surgery as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures. The intent of the Impella system therapy is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function. Optimal medical management and convention treatment measures include volume loading and use of pressors and inotropes, with or without IABP.
We expect to continue to make additional PMA supplement submissions for our Impella suite of products for additional clinical indications.
3
The Impella 5.0 and Impella LD devices have CE Mark approval in Europe for up to ten days’ duration and are approved for use in over 40 countries. We have submitted an application for the Impella 5.0 in Japan and we are hopeful of receiving regulatory approval in calendar 2016.
Impella RP®
The Impella RP is a percutaneous catheter-based axial flow pump that is designed to allow greater than four liters of flow per minute and is intended to provide the flow and pressure needed to compensate for right side heart failure. The Impella RP is the first percutaneous single access heart pump designed for right heart support to receive FDA approval. The Impella RP device is approved to provide support of the right heart during times of acute failure for certain patients who have received a left ventricle assist device or have suffered heart failure due to acute myocardial infarction, or AMI, or a failed heart transplant.
In November 2012, the Impella RP product received U.S. investigational device exemption, or IDE, approval from the FDA for use in RECOVER RIGHT, a pivotal clinical study in the U.S. In March 2014, we completed enrollment of 30 patients that presented signs of right side heart failure, required hemodynamic support, and were capable of being treated in the catheterization lab or cardiac surgery suite. The study collected safety and effectiveness data on the percutaneous use of the Impella RP product and was submitted to the FDA in support of a Humanitarian Device Exemption, or “HDE,” submission. An HDE is similar to a PMA application but is intended for patient populations of 4,000 or less per year in the U.S. and is subject to certain profit and use restrictions. An HDE approval requires demonstration of the safety and probable benefit of the product, which is a lower standard than is applied to a PMA. In order to receive an HDE, there must be no comparable devices approved under a PMA that are available to treat the targeted population. An approved HDE authorizes sales of the device to any hospital after review and approval by the hospital’s Institutional Review Board.
In January 2015, we received FDA approval for the Impella RP product under an HDE. As part of the HDE approval, we are required to conduct two post approval studies for the Impella RP product. One includes an adult patient population of 30 patients and the other, a pediatric patient population for a maximum of 15 patients. These studies will be conducted to monitor the post-market safety and probable benefit of the Impella RP device. Both studies will be single-arm multicenter studies that will follow the respective patients at 30 and 180 days post device explant. We have completed 18 patients to date on the adult patient population study and we expect to complete this study in fiscal 2017. In April 2014, the Impella RP product received CE Mark approval which allows for commercial sales of Impella RP in the European Union and other countries that require a CE Mark approval for commercial sales.
AB5000™
We manufacture and sell the AB5000 Circulatory Support System for the temporary support of acute heart failure patients in profound shock, including patients suffering from cardiogenic shock after a heart attack, post-cardiotomy cardiogenic shock, or myocarditis. The AB5000 device was approved by the FDA in 2003. We believe the AB5000 is the only commercially available cardiac assist device that is approved by the FDA for all indications where heart recovery is the desired outcome, including patients who have undergone successful cardiac surgery and subsequently develop low cardiac output, or patients who suffer from acute cardiac disorders leading to hemodynamic instability. Revenues from the AB5000 device have been declining in recent years and we expect the AB5000 to be a smaller part of our business in the future as we focus our efforts on the Impella family of products.
ECP
In July 2014, we acquired all of the issued shares of ECP Entwicklungsgesellschaft mbH, or ECP, a German limited liability company, for $13.0 million in cash, with additional potential payments up to a maximum of $15.0 million based on the achievement of certain technical, regulatory and commercial milestones. In connection with our acquisition of ECP, ECP acquired all of the issued shares of AIS GmbH Aachen Innovative Solutions, or AIS, a German limited liability company, for $2.8 million in cash which was provided by us. AIS, based in Aachen, Germany, holds certain intellectual property useful to ECP’s business, and, prior to being acquired by ECP, had licensed such intellectual property to ECP.
ECP, based in Berlin, Germany, is engaged in research, development, prototyping and the pre-serial production of a percutaneous expandable catheter pump which increases blood circulation from the heart with an external drive shaft. The ECP pump is designed for blood flow of >3 liters/minute. It is intended to be delivered on the standard Impella 9 Fr catheter and will include an 18 Fr expandable inflow in the left ventricle with a smooth membrane crossing the left ventricle. The ECP pump is still in early stages of research and development and has not been approved for commercial use or sale.
4
Summary of Recent Financial Performance
For fiscal 2016, we recognized net income of $38.1 million, or $0.90 per basic share and $0.85 per diluted share, compared to $113.7 million, or $2.80 per basic share and $2.65 per diluted share for the prior fiscal year. The decrease in our net income for fiscal 2016 was due to an increase in income tax provision for fiscal 2016. Our net income for fiscal 2015 included an income tax benefit of $84.9 million, primarily due to the release of our valuation allowance on certain of our deferred tax assets. In fiscal 2016, we recorded income tax expense of $27.7 million on income before taxes of $65.8 million.
Income from operations for fiscal 2016 increased by $36.4 million, or 127%, to $65.1 million in fiscal 2016 from $28.7 million in fiscal 2015. This resulted in operating margin increasing to 19.8% in fiscal 2016 from 12.4% in fiscal 2015. The increase in income from operations and operating margin for fiscal 2016 was driven primarily by higher Impella product revenue due to greater utilization of our Impella products in the U.S. and Europe and increased gross margins due primarily to higher manufacturing product volume and improved efficiencies in manufacturing production.
Our Markets
According to the AHA, Heart Disease and Stroke Statistics 2016 Update Report, coronary heart disease, or CHD, causes approximately one of every seven deaths in the U.S. CHD is a condition of the coronary arteries that causes reduced blood flow and insufficient oxygen delivery to the affected portion of the heart. CHD leads to acute myocardial infarction, or AMI, commonly known as a heart attack, which may lead to heart failure, a condition in which the heart is unable to pump enough blood to the body’s major organs. In 2013, CHD mortality was approximately 370,000 Americans. Each year, an estimated 660,000 Americans have a new coronary attack (defined as first hospitalized myocardial infarction or coronary heart disease death) and approximately 305,000 have a recurrent attack. It is estimated that an additional 150,000 “silent” first myocardial infarctions occur each year.
A broad spectrum of therapies exists for the treatment of patients in early stages of CHD. Angioplasty procedures and stents are commonly used in the cath lab to restore and increase blood flow to the heart. These treatments are often successful in slowing the progression of heart disease, extending life, and/or improving the quality of life for some period of time. Patients presenting with acute cardiac injuries potentially have recoverable hearts. Treatment for these patients in pre-shock in the cath lab is primarily focused on hemodynamic stabilization. Acute heart failure patients in profound shock typically require treatment in the surgery suite. These are patients suffering from cardiogenic shock after a heart attack, post-cardiotomy cardiogenic shock or myocarditis complicated with cardiogenic shock. Chronic heart failure patients have hearts that are unlikely to be recoverable due to left and/or right-side heart failure and their conditions cause their hearts to fail over time. Limited therapies exist today for patients with severe, end-stage, or chronic heart failure.
In more severe cases of heart failure, patients are sent directly to the surgery suite for coronary bypass or valve replacement surgery. The most severe acute heart failure patients are in profound cardiogenic shock, including those suffering from myocarditis (a viral attack of the heart), or from those suffering from an impaired ability of the heart to pump blood after a heart attack or heart surgery. According to a 2008 AHA Circulation report, Contemporary Reviews in Cardiovascular Medicine: Cardiogenic Shock, approximately 5 to 8% of the patients who are hospitalized for a heart attack suffer from cardiogenic shock and approximately 50% of those patients die. These patients typically require treatments involving the use of mechanical circulatory support devices that provide increased blood flow and reduce the stress on the heart. Many less severe patients in the cath lab could also benefit from circulatory support devices or other clinical treatment, which could potentially prevent them from entering into profound shock.
There are a few primary types of devices used in the cath lab and surgery suite in the U.S. for circulatory support for pre-shock and profound shock patients: intra-aortic balloons, or IABs, percutaneous assist devices, and surgical ventricular assist devices, or VADs.
An IAB is an inflatable balloon inserted via a catheter into a patient’s circulatory system and is inflated and deflated in the aorta. This is used as an initial line of therapy in the cath lab or the surgery suite for patients with diminished heart function. However, IABs typically provide only limited enhancement and depend on the patient’s own heart to generate the majority of the patient’s blood flow. In addition, IABs are often required to be used in conjunction with inotropes or other drugs to stimulate heart muscle ejection. The use of these drugs, however, increases the risk of mortality. Further, the clinical efficacy of IABs has recently come into question due to the conclusions of the randomized, prospective, open-label, multicenter “SHOCK II” Trial. The conclusion of the trial was that the use of IAB counterpulsation did not significantly reduce 30-day mortality in patients with cardiogenic shock complicating acute myocardial infarction for whom an early revascularization strategy was planned. Further, IABs have limited effectiveness in patients that are arrhythmic and/or in cardiogenic shock and published reports have indicated that IABs do not reduce mortality for patients in cardiogenic shock.
Percutaneous assist devices and VADs are mechanical devices that help the failing heart pump blood or take over the pumping function of the failing heart. Historically, VADs have been highly invasive and require implantation in the surgery suite. Percutaneous
5
assist devices allow for less invasive placement and removal, and can be done through a small puncture in the leg in the cath lab, electrophysiology lab, or operating room. The use of surgically placed VADs generally falls into three sub-categories: recovery, bridge-to-transplant and destination therapy.
Recovery VADs are designed to enable the patient’s heart to rest and potentially recover so that the patient can return home with his or her own heart. Because recovery is the goal, these devices are designed to minimize damage to heart tissue and are removed once the patient’s heart has recovered. If possible, recovery of a patient’s heart is generally preferred to transplantation or prolonged device implantation, both of which have significant side effects for the patient and increase the risk of mortality. We believe heart recovery is a preferred clinical outcome for patients, since it generally lowers the overall relative cost to the healthcare system versus alternative therapies and treatment paths that may require multiple surgeries, lengthy or repeated hospital stays, chronic therapeutic and immunosuppressant drugs and other related healthcare costs.
Research and Product Development
Since our founding in 1981, we have gained substantial expertise in circulatory support through the development of many product platforms to support heart patients. This includes our Impella platform and AB5000 system that we currently market and other technologies that we previously supported, such as our BVS system and AbioCor program. We also continue to work on developing new technologies as well, such as the ECP development program. Our current strategy is to develop a complete portfolio of products for partial and full circulatory support to treat acute heart failure patients. We intend to continue to use this experience to develop additional circulatory support products. Our research and development efforts are focused on developing a broader portfolio of products across the continuum of care in heart recovery, primarily focused in the area of circulatory care. In addition, we have a number of new products at various stages of development some of which integrate the Impella technology platform.
As of March 31, 2016, our research and development staff consisted of 152 full-time employees. We expended $49.8 million, $36.0 million and $30.7 million on research and development in fiscal years 2016, 2015 and 2014, respectively. Our research and development expenditures include costs related to clinical trials, including ongoing clinical studies for our Impella products.
Sales, Clinical Support, Marketing and Field Service
As of March 31, 2016, our worldwide sales, clinical support, marketing and field service teams included 303 full-time employees, 266 of whom are in the U.S. and Canada and 37 of whom are in Europe and Japan. In recent years, we have significantly increased the number of our direct sales and clinical support personnel in the U.S and Europe.
Our clinical support personnel consist primarily of registered nurses and other personnel with considerable experience in either the surgery suite or the cath lab, and they play a critical role in training current and prospective customers in the use of our products.
International sales (sales outside the U.S., primarily in Europe) accounted for 8%, 10% and 9% of total product revenue during fiscal years 2016, 2015 and 2014, respectively.
Manufacturing
We manufacture our products in Danvers, Massachusetts and Aachen, Germany. Our Aachen facility performs final assembly and manufactures most of our Impella disposable products, including the Impella 2.5, Impella 5.0, Impella LD, Impella CP and Impella RP devices. Our Danvers facility manufactures certain Impella subsystems and accessories, including our Automated Impella Console, or AIC, our console for our Impella products. Beginning in fiscal 2015, we started producing the Impella CP product in our Danvers facility. In addition, we rely on third-party suppliers to provide us with components used in our existing products and products under development. For example, we outsource some of the manufacturing for components and circuit cards within our consoles.
We believe our existing manufacturing facilities give us the necessary physical capacity to produce sufficient quantities of products to meet anticipated demand for at least the next twelve months based on our current revenue forecast. We have recently expanded our Impella manufacturing capacity in both our Aachen and Danvers facilities to support the growing demand for our Impella products. We expect to continue to expand our Impella manufacturing capacity as we support expected increasing sale of our Impella products. Our U.S. and German manufacturing facilities are certified by the International Organization for Standardization, or ISO, and operate under the FDA’s good manufacturing practice requirements for medical devices set forth in the Quality System Regulation, or QSR.
6
Intellectual Property
We have developed significant know-how and proprietary technology, upon which our business depends. To protect our know-how and proprietary technology, we rely on trade secret laws, patents, copyrights, trademarks, and confidentiality agreements and contracts. However, these methods afford only limited protection. Others may independently develop substantially equivalent proprietary information or technology, gain access to our trade secrets or disclose or use such secrets or technology without our approval.
A substantial portion of our intellectual property rights relating to the Impella products, AB5000, and other products under development, such as ECP, is in the form of trade secrets, rather than patents. We protect our trade secrets and proprietary knowledge in part through confidentiality agreements with employees, consultants and other parties. We cannot assure you that our trade secrets will not become known to or be independently developed by our competitors.
We own or have rights to numerous U.S. and foreign patents. Our U.S. patents have expiration dates ranging from 2016 to 2032 and our foreign patents have expiration dates ranging from 2016 to 2033. We also own or have rights to certain pending U.S. and foreign patent applications. We believe patents will issue pursuant to such applications, but cannot guarantee it. Moreover, neither the timing of any issuance, the scope of protection, nor the actual issue date of these pending applications can be forecasted with precision. Where we have licensed patent rights from third parties, we are generally required to pay royalties.
Our patents may not provide us with competitive advantages. Our pending or future patent applications may not be issued. Others may hold or obtain patents that cover aspects or uses of our innovations. The patents of others may render our patents obsolete, limit our ability to patent or practice our innovations, or otherwise have an adverse effect on our ability to conduct business. Because foreign patents may afford less protection than U.S. patents, they may not adequately protect our technology.
The medical device industry is characterized by a large number of patents and by frequent and substantial intellectual property litigation. Our products and technologies could infringe on the proprietary rights of third parties. If third parties successfully assert infringement or other claims against us, we may not be able to sell our products or we may have to pay significant damages and ongoing royalties. In addition, patent or intellectual property disputes or litigation may be costly, result in product development delays, or divert the efforts and attention of our management and technical personnel. If any such disputes or litigation arise, we may seek to enter into a royalty or licensing arrangement. However, such an arrangement may not be available on commercially acceptable terms, if at all. We may decide, in the alternative, to litigate the claims or seek to design around the patented or otherwise protected proprietary technology, which may also be costly and time consuming.
The U.S. government may obtain certain rights to use or disclose technical data developed under government contracts that supported the development of some of our products. We retain the right to obtain patents on any inventions developed under those contracts, provided we follow prescribed procedures and are subject to a non-exclusive, non-transferable, royalty-free license to the U.S. government.
Competition
Competition among providers of treatments for the failing heart is intense and subject to rapid technological change and evolving industry requirements and standards. We compete with companies that have substantially greater or broader financial, product development, sales and marketing resources and experience than we do. Other advances in medical technology, biotechnology and pharmaceuticals may reduce the size of the potential markets for our products or render those products obsolete. Among our medical device competitors are Getinge (Maquet Cardiovascular), Teleflex Inc., Abbott Laboratories, St. Jude Medical, Inc., HeartWare International Inc., Terumo Heart, Inc. and CardiacAssist Inc.
Our customers are hospitals that have limited budgets. As a result, our products compete against a broad range of medical devices and other therapies for these limited funds. Our continued success will depend in large part upon our ability to enhance our existing products, develop new products to meet regulatory and customer requirements, and achieve market acceptance. We believe that important competitive factors with respect to the development and commercialization of our products include the relative speed with which we can develop products, establish clinical utility, complete clinical trials and regulatory approval processes, obtain and protect reimbursement, maintain cost effectiveness for our products, and supply commercial quantities of our product to meet customer demand.
Third-Party Reimbursement
Our products and services are generally purchased by healthcare institutions that rely on third-party payers to cover and reimburse the costs of related patient care. In the U.S., as well as in many foreign countries, government-funded or private insurance
7
programs pay the cost of a significant portion of a patient’s medical expenses. No uniform policy of coverage or reimbursement for medical technology exists among all these payers. Therefore, coverage and reimbursement can differ significantly from payer to payer and by jurisdiction.
Third-party payers may include government healthcare programs such as Medicare or Medicaid, private insurers or managed care organizations. The Centers for Medicare & Medicaid Services, or CMS, is responsible for administering the Medicare program in the U.S. and, along with its contractors, establishes coverage and reimbursement policies for the Medicare program. Medicare’s coverage and reimbursement policies are particularly significant to our business because a large percentage of the population for which our products are intended includes elderly individuals who are Medicare beneficiaries. In addition, private payers often follow the coverage and reimbursement policies of Medicare. We cannot assure that government or private third-party payers will continue to cover and reimburse the procedures using our products in whole or in part in the future or that payment rates will be adequate.
Medicare payment may be made, in appropriate cases, for procedures performed in the in-patient hospital setting using our technology. Medicare generally reimburses healthcare institutions in which the procedures are performed based upon prospectively determined amounts. For hospital in-patient stays, the prospective payment generally is determined by the patient’s condition and other patient data and procedures performed during the in-patient stay, using a classification system known as International Classification of Diseases, or ICD, and medical severity diagnosis-related groups, or MS DRGs. Prospective rates are adjusted for, among other things, regional differences, co-morbidity and complications. Hospitals performing in-patient procedures using our devices generally do not receive separate Medicare reimbursement for the specific costs of purchasing or implanting our products. Rather, reimbursement for these costs is bundled with the MS DRG-based payments made to hospitals for the procedures during which our devices are implanted, removed, repaired or replaced. Because prospective payments are based on predetermined rates and may be less than a hospital’s actual costs in furnishing care, hospitals have incentives to lower their in-patient operating costs by utilizing products, devices and supplies that will reduce the length of in-patient stays, decrease labor or otherwise lower their costs.
The Impella 2.5 heart pump is supported by clinical guidelines and has been eligible to be reimbursed in the U.S. by the CMS under ICD-9-CM code 37.68 since 2008 for multiple indications, including high-risk PCI. Medicare transitioned from ICD-9 to a new system, ICD-10, in October 2015. In July 2015, CMS reconfirmed Impella reimbursement levels and confirmed that the existing Impella MS-DRG mapping will remain unchanged in the transition from ICD-9 to ICD-10 in October 2015. CMS has stated that the transition to ICD-10 codes was intended to provide more descriptive information about procedures used to deliver care to patients, and is not a mechanism for remapping DRGs or changing payment. Recently CMS updated their description for Impella to use 5A02(1,2)1D: “Assistance with Cardiac Output Using Impeller Pump,” which continues to map to DRG 216-221. We believe this is an accurate description/DRG assignment and do not expect changes. However future updates before and after implementation are possible.
Coverage and reimbursements for procedures to implant, remove, replace or repair our products are generally established in the U.S. market. For instance, Medicare covers the use of LVADs when used for support of blood circulation post-cardiotomy, as a temporary life-support system until a human heart becomes available for transplant, or as destination therapy for patients who require permanent mechanical cardiac support, when the use is consistent with FDA approval and FDA-approved labeling instructions, as applicable. Coverage and reimbursements for procedures to implant the Impella 2.5, Impella CP, Impella 5.0 and Impella LD devices are also established for in-hospital use by Medicare including ICD-10 for procedures and MS DRG coding. Actual coverage and payment may vary by local Medicare fiscal intermediary or third-party insurer. Our Impella products are also covered by commercial and/or Medicare plans of many third-party insurers including Aetna, Humana, Cigna, HCSC Blue Cross Blue Shield, and United Healthcare.
In addition to payments to hospitals for procedures using our technology, Medicare makes separate payments to physicians for their professional services when they perform surgeries to implant, remove, replace or repair our devices or when they perform percutaneous insertion and removal of Impella. Physicians generally bill for such services using a coding system known as Current Procedural Terminology, or CPT, codes. Physician services performed in connection with the implantation, removal, replacement or repair of our approved products are billed using a variety of CPT codes. Generally, Medicare payment levels for physician services are based on the Medicare Physician Fee Schedule and are revised annually by CMS.
In general, third-party reimbursement programs in the U.S. and abroad, whether government-funded or commercially insured, are developing a variety of increasingly sophisticated methods of controlling healthcare costs, including prospective reimbursement and capitation programs, group purchasing, reducing benefit coverage, requiring second opinions prior to major surgery, negotiating reductions to charges on patient bills, promoting healthier lifestyle initiatives and exploring more cost-effective methods of delivering healthcare. These types of cost-containment programs, as well as legislative or regulatory changes to reimbursement policies, could limit the amount which healthcare providers may be willing to pay for our medical devices.
8
Government Regulation
The healthcare industry, and thus our business, is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. In addition, these laws and their interpretations are subject to change.
Premarket Regulation
In the U.S., the FDA strictly regulates medical devices under the authority of the Federal Food, Drug and Cosmetic Act, or FFDCA, and its regulations. The FDA classifies U.S. medical devices into one of three classes (Class I, II or III) based on the statutory framework described in the FFDCA. Class III devices are typically life-sustaining, life-supporting or implantable devices, or new devices that have not been found to be substantially equivalent to legally marketed devices. Class III devices must generally receive PMA approval by the FDA before they may be marketed.
The PMA approval pathway requires that the applicant demonstrate to the FDA’s satisfaction, based on valid scientific evidence, that there is a reasonable assurance of the safety and effectiveness of the device for its intended use. During the PMA process, the FDA examines detailed data to assess the safety and effectiveness of the device. This information includes design, development, manufacture, labeling, advertising, preclinical testing and clinical study data. Prior to approving a PMA, the FDA may conduct an inspection of the manufacturing facilities and the clinical sites where supporting studies were conducted. The facility inspection evaluates the company’s compliance with the QSR. An inspection of clinical sites evaluates compliance with good clinical practice standards, including, for studies conducted under an investigational device exemption, or IDE, the requirements of FDA’s IDE regulations. Typically, the FDA will convene an advisory panel meeting to review the data presented in the PMA. The panel’s recommendation is given substantial weight, but is not binding on the agency. By regulation, the FDA has 180 days to review a PMA application not requiring an advisory panel meeting, and 320 days to review a PMA application that does require an advisory panel meeting. While the FDA has approved PMA applications within the allotted time period, reviews can occur over a significantly protracted period, usually 18 to 36 months, but sometimes longer. Upon completion of its review, FDA will either approve or deny the PMA.
If the FDA’s evaluation is favorable, the PMA is approved and the device may be marketed in the U.S. The FDA may approve a PMA with post-approval conditions intended to ensure the safety and effectiveness of the device including, among other things, restrictions on labeling and promotion, and post-market collection of clinical data. Failure to comply with the conditions of approval can result in material adverse enforcement action, including the loss or withdrawal of the PMA approval. Even after approval of a PMA, a new PMA or PMA supplement is required in the event of a modification to the device, its labeling or its manufacturing process. Even if a device receives PMA approval, the FDA may include significant limitations on the indicated uses for which a device may be marketed. FDA enforcement policy prohibits the promotion of approved medical devices for unapproved uses. In addition, product approvals can be withdrawn for failure to comply with regulatory requirements or the occurrence of unforeseen problems following initial marketing.
Certain Class III devices that were on the market before May 28, 1976, known as pre-amendment Class III devices, and devices that were determined to be substantially equivalent to them, have been allowed to be brought to market through the 510(k) process until the FDA, by regulation, calls for PMA applications for those devices types. The Impella 2.5, Impella CP, Impella 5.0 and Impella LD devices all received 510(k) clearance based on substantial equivalence to a pre-amendment Class III device.
In 2009, the FDA began a process referred to as the “515 Program Initiative” to complete the process of requiring PMAs for Class III. This initiative requires FDA either to down-classify, or to require PMAs, for the remaining pre-amendment Class III device types for which FDA had not yet called for PMAs. Class I and II devices are generally considered to be lower risk than Class III devices and require either clearance through the FDA’s 510(k) premarket notification process (as is the case of most Class II devices) or do not require any premarket review or notification (as is the case for nearly all Class I devices). Class III devices, however, are typically higher risk or novel and require approval through a PMA, which is a more costly and uncertain premarket pathway than the 510(k) premarket notification process.
In December 2012, as part of the FDA’s 515 Program Initiative, an FDA panel voted to recommend continuation of Class III status for temporary ventricular support devices within the non-roller type cardiopulmonary bypass blood pumps category, which includes our Impella products. The panel’s recommendation of Class III for this category of device is consistent with the current Class III designation for these device types. The FDA accepted the panel’s recommendation and, in June 2015, issued a final order requiring PMAs for this device type. We worked with the FDA on a PMA application for our Impella 2.5 pump for use during high-risk PCI procedures. Under the 515 Program Initiative, we are permitted to continue to market our Impella products pursuant to the 510(k) clearance for a sufficient time to allow for the submission and review of PMA applications relating to our Impella products.
9
In March 2015, we received a PMA approval from the FDA for our Impella 2.5 product, during elective and urgent high-risk PCI procedures. The Impella 2.5, is the first hemodynamic support device to receive a PMA indication for use during high-risk PCI procedures, demonstrating its safety and effectiveness for this complex patient population. Under the PMA, the Impella 2.5 is approved as a temporary (up to six hours) ventricular support device indicated for use during high-risk PCI performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high-risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 device in these patients may prevent hemodynamic instability that may occur during planned temporary coronary occlusions and may reduce periprocedural and post-procedural adverse events. The approved product labeling allows for a clinical decision to leave Impella 2.5 in place beyond the intended duration of up to six hours in the event of unforeseen circumstances. Per our PMA approval, we are conducting a single-arm, post-approval study on the Impella 2.5, collecting data on high-risk PCI patients. The study is a prospective, multi-center study comprised of 369 patients from up to 70 sites supported with the Impella 2.5 system. The Impella 2.5 device has CE Mark approval in Europe for up to five days of use and is approved for use in over 40 countries.
In August 2015, we submitted a PMA supplement to the FDA requesting to expand our current Impella 2.5 PMA approval to include additional indications for Impella 2.5 and all of our other Impella devices (Impella CP, Impella 5.0 and Impella LD) that support the left side of the heart. These submissions are for a set of indications related to the use of the Impella devices in patients suffering cardiogenic shock following acute myocardial infarction or cardiac surgery and for a longer duration of support. In April 2016, the FDA approved the PMA supplement for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock. In this setting, the Impella heart pumps stabilize the patient’s hemodynamics, unload the left ventricle, perfuse the end organs and allow for recovery of the native heart. The post approval data collection and reporting requirement is using utilizing the cVAD Registry™ (see below).
In addition to the U.S. clinical trial data, the Impella 2.5 PMA submission included clinical and scientific supporting evidence from more than 215 publications, covering 1,638 Impella 2.5 patients. The submission included a MDR analysis from 13,981 Impella 2.5 patients. In addition to clinical trial data from studies known as PROTECT I and PROTECT II, further data was provided in the submission from 637 high-risk patients enrolled in the U.S. Impella Registry, or cVAD Registry. The cVAD Registry is an ongoing multicenter, observational retrospective registry that includes 49 centers, collecting data on the Impella 2.5, Impella 5.0, and Impella CP devices. The data collection from the registry includes Institutional Review Board, or IRB, approval, complete data monitoring and Clinical Events Committee adjudication.
When clinical trials of a device are required in order to obtain FDA approval, the sponsor of the trial is generally required to file an IDE application before commencing the trials. The FDA reviews and must approve an IDE before a clinical study may begin in the U.S. In addition, the clinical study must be approved by an Institutional Review Board, or IRB, for each clinical site. The FDA, an IRB, or we may suspend a clinical trial at any time for various reasons, including if information emerges suggesting that the subjects are being exposed to an unacceptable health risk. All clinical studies of investigational devices must be conducted in compliance with FDA requirements. Following the completion of a study, the data from the study must be collected, analyzed and presented in an appropriate submission to the FDA, either as a reported submitted to the IDE file or in a marketing application such as a PMA.
In addition, certain medical devices can be approved by the FDA in the U.S. under an HDE rather than a PMA. In order for a device to be eligible for an HDE, there must be a qualifying target patient population of less than 4,000 patients per year for which there is no other comparable device available to treat the condition. The FDA must agree that a device meets these criteria before it can be approved under an HDE. FDA approval of an HDE also requires demonstration that the device is safe for its intended application, that it is potentially effective, and that the probable benefits outweigh the associated risks. If another device receives approval through the PMA process that addresses the same patient population as the HDE device, the HDE device may need to be withdrawn from the U.S. market. An approved HDE authorizes sales of the device to any hospital after review and approval by the hospital’s IRB. Proposed modifications to approved HDE devices, like modifications to approved PMA devices, require FDA approval through a new HDE application or an HDE supplement.
We received FDA approval for the Impella RP heart pump under an HDE in January 2015for providing circulatory assistance for up to 14 days in patients who develop acute right heart failure or decompensation after left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella RP is the first percutaneous single access heart pump designed for right heart support to receive FDA approval.
Our AB5000 system is approved by the FDA for use in patients who have undergone successful cardiac surgery and subsequently develop low cardiac output, or patients who suffer from acute cardiac disorders leading to hemodynamic instability. The intent of therapy with this device is to provide circulatory support, restore normal hemodynamics, reduce ventricular work, and allow the heart time to recover adequate mechanical function. In April and September 2003, the AB5000 Circulatory Support System Console and, the AB5000 VAD, respectively, were approved under PMA supplements. Our Impella 2.5 device received 510(k) clearance in June 2008 for partial circulatory support for up to six hours. We received FDA 510(k) clearance of our Impella 5.0 and
10
Impella LD devices in April 2009 for circulatory support for up to six hours. Our AB Portable Driver received FDA approval under a PMA supplement in March 2009. All of these products have CE Marks allowing distribution within the European Union. In September 2012, the Impella CP device received 510(k) clearance from the FDA for up to six hours of partial circulatory support using an extracorporeal bypass control unit. In March 2015, we received a PMA from the FDA for Impella 2.5 device, during elective and urgent high-risk PCI procedures. In April 2016, the FDA approved the PMA supplement for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock. The Impella CP device received CE Mark approval to market the device in the European Union in April 2012 and received Health Canada approval to market the device in Canada in June 2012. In April 2014, the Impella RP device received CE Mark approval, which allows for commercial sales of Impella RP in the EU and other countries that accept a CE Mark as a form of marketing authorization.
Postmarket Regulation
The medical devices that we manufacture and distribute pursuant to FDA regulatory clearances or approvals by the FDA and other countries’ regulatory authorities are subject to continuing regulation by those agencies. The FDA reviews design, manufacturing, and distribution practices, labeling and record keeping, and manufacturers’ required reports of adverse experience and other information to identify potential problems with marketed medical devices. Among other FDA requirements, we must comply with the FDA’s good manufacturing practice regulations for medical devices, known as QSR. These regulations govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging and servicing of all finished medical devices intended for human use. We must also comply with MDR, which require us to report to the FDA any incident in any of our products that may have caused or contributed to a death or serious injury, including medical intervention to prevent a death or serious injury, or in which any of our products malfunctioned and, if such malfunction were to recur, would be likely to cause or contribute to a death or serious injury. Labeling, advertising, and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Current FDA enforcement policy prohibits the marketing of approved medical devices for unapproved uses. We are subject to routine inspection by the FDA for compliance with the QSR and MDR requirements, as well as other applicable regulations. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could ban such medical devices, detain or seize adulterated or misbranded medical devices, order a recall, repair, replacement, or refund of such devices, and require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health. The FDA may also impose operating restrictions, enjoin and restrain certain violations of applicable law pertaining to medical devices, and assess civil or criminal fines and penalties against our officers, employees, or us. The FDA may also recommend prosecution to the U.S. Department of Justice. Regulatory authorities outside the United States enforce similar laws and regulations within their respective jurisdictions.
The FDA and other regulatory agencies actively enforce regulations prohibiting promotion of off-label uses and the promotion of products for which marketing clearance has not been obtained. If the FDA or another regulatory agency determines that our promotional materials or training constitutes promotion of an unapproved use, it could request that we modify our training or promotional materials or subject us to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and criminal penalties. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged in off-label promotion.
In June 2011, we received a warning letter from the FDA stating that some of our promotional materials marketed the Impella 2.5 for uses that had not been approved by the FDA. We cooperated with the FDA and made changes to our promotional materials in response to the warning letter. However, in April 2012, we received a follow up letter from the FDA stating that some of our promotional materials continued to market the Impella 2.5 device in ways that are not compliant with FDA regulations. After additional action by us, we received a close-out letter in February 2013 from the FDA with respect to this matter, which noted that the FDA’s Office of Compliance had completed its review of the corrective actions we had taken in response to the warning letter and that the concerns cited appeared to have been addressed.
On October 26, 2012, we were informed that the Department of Justice, United States Attorney’s Office for the District of Columbia was conducting an investigation (“Marketing and Labeling Investigation”) focused on our marketing and labeling of the Impella 2.5 heart pump. On October 31, 2012, we accepted service of a subpoena related to this investigation seeking documents and other materials related to the Impella 2.5. We cooperated fully with the Marketing and Labeling Investigation, and on June 29, 2015, we received confirmation that the Department of Justice had closed the Marketing and Labeling Investigation without taking enforcement action.
11
On April 25, 2014, we received a subpoena from the Boston regional office of the United States Department of Health and Human Services, or HHS, Office of Inspector General requesting materials relevant to our reimbursement of expenses and remuneration to healthcare providers for a six month period from July 2012 through December 2012 in connection with a civil investigation under the False Claims Act (the “FCA Investigation”). We submitted the requested documents to HHS and believe that we substantially complied with the subpoena. On November 6, 2014, we received notice from the Department of Justice, United States Attorney’s Office for the District of Massachusetts in the form of a Civil Investigative Demand (“CID”) requesting additional materials relating to this matter for the time period of January 1, 2012 through December 31, 2013. We have responded to the additional requests for information contained in the CID, and are in the process of responding to other informal requests. We intend to continue to cooperate with the U.S. Attorney’s Office in connection with the FCA Investigation.
The FDA can require post-market surveillance, or PMS, for significant risk devices, such as VADs and our medical devices, that require ongoing collection, analysis, and periodic submission to the FDA of clinical data during commercialization over a period of up to several years. The PMS data collection requirements are often burdensome and expensive and have an effect on the PMA approval status. The failure to comply with the FDA’s regulations can result in enforcement action, including seizure of products, injunction, prosecution, civil fines and penalties, recall and/or suspension of FDA approval. The export of devices such as ours is also subject to regulation in certain instances.
The FDA, in cooperation with U.S. Customs and Border Protection, or CBP, administers controls over the import and export of medical devices into and out of the U.S. International sales of our medical devices that have not received FDA approval are therefore subject to FDA export requirements. The CBP imposes its own regulatory requirements on the import of medical devices, including inspection and possible sanctions for noncompliance.
Fraud and Abuse Laws
Our business is regulated by laws pertaining to healthcare fraud and abuse including anti-kickback laws and false claims laws. Violations of these laws are punishable by significant criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, such as Medicare and Medicaid. Because of the far-reaching nature of these laws, we may be required to alter one or more of our practices to be in compliance with these laws. Evolving interpretations of current laws, or the adoption of new laws or regulations, could adversely affect our arrangements with customers and physicians. In addition, any violation of these laws or regulations could have a material adverse effect on our financial condition and results of operations.
Anti-Kickback Statute
Subject to a number of statutory exceptions, the federal healthcare programs Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual for, or the furnishing, recommending, purchasing, leasing, ordering, or arranging for, a good or service for which payment may be made under a federal healthcare program such as Medicare and Medicaid. The term “remuneration” has been broadly interpreted to include anything of value, including payments to physicians or other providers, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, waiver of payments, and providing anything of value at less than fair market value. The Office of the Inspector General of the U.S. Department of Health and Human Services, or the OIG, and the U.S. Department of Justice are responsible for enforcing the federal healthcare programs Anti-Kickback Statute and the OIG is primarily responsible for identifying fraud and abuse activities affecting government healthcare programs.
Penalties for violating the federal healthcare programs Anti-Kickback Statute include substantial criminal fines and/or imprisonment, substantial civil fines and possible exclusion from participation in federal healthcare programs such as Medicare and Medicaid. Many states have adopted prohibitions similar to the federal healthcare programs Anti-Kickback Statute, some of which apply to the referral of patients for healthcare services reimbursed by any source, not only by the Medicare and Medicaid programs and do not include comparable exceptions to those provided by the federal healthcare programs Anti-Kickback Statute.
The OIG has issued safe harbor regulations that identify activities and business relationships that are deemed safe from prosecution under the federal healthcare programs Anti-Kickback Statute. There are safe harbors for various types of arrangements, including certain investment interests, leases, personal service arrangements, discounts and management contracts. The failure of a particular activity to comply with all requirements of an applicable safe harbor regulation does not mean that the activity violates the federal healthcare programs Anti-Kickback Statute or that prosecution will be pursued. However, activities and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG.
In recent years, the federal government and several states have enacted legislation requiring biotechnology, pharmaceutical and medical device companies to establish marketing compliance programs and file periodic reports on sales, marketing, and other
12
activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and available guidance is limited. We could face enforcement action, fines and other penalties and could receive adverse publicity, all of which could harm our business, if it is alleged that we have failed to fully comply with such laws and regulations. Similarly, if the physicians or other providers or entities that we do business with are found to have not complied with applicable laws, they may be subject to sanctions, which could also have a negative impact on our business.
Federal False Claims Act
The federal False Claims Act prohibits knowingly filing or causing the filing of a false claim or the knowing use of false statements to obtain payment from the federal government. A claim that is filed pursuant to an unlawful kickback may be a false claim under this law and, in a number of cases, manufacturers of medical products have entered into settlements of False Claims Act allegations that their financial relationships with customers “caused” these customers to submit false claims. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus mandatory civil penalties for each separate false claim. Private individuals can file suits under the False Claims Act on behalf of the government. These lawsuits are known as “qui tam” actions, and the individuals bringing such suits, sometimes known as “relators” or, more commonly, “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. In addition, certain states have enacted laws modeled after the federal False Claims Act. Qui tam actions have increased significantly in recent years, causing greater numbers of healthcare companies to have to defend a false claim action, pay fines or be excluded from Medicare, Medicaid or other federal or state healthcare programs as a result of an investigation arising out of such action.
HIPAA
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payers. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government-sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment.
HIPAA also protects the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses and their business associates. HIPAA restricts the use and disclosure of patient health information, including patient records. Although we believe that HIPAA does not apply to us directly, most of our customers have significant obligations under HIPAA, and we intend to cooperate with our customers and others to ensure compliance with HIPAA with respect to patient information that comes into our possession. Failure to comply with HIPAA obligations can result in civil fines and/or criminal penalties. Some states have also enacted rigorous laws or regulations protecting the security and privacy of patient information. If we fail to comply with these laws and regulations, we could face additional sanctions.
Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act
In March 2010, the U.S. Congress enacted the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act, or together, the Affordable Care Act. The law includes provisions that, among other things, reduce or limit Medicare reimbursement, require all individuals to have health insurance (with limited exceptions) and impose increased taxes. Specifically, the law requires the medical device industry to subsidize healthcare reform in the form of a medical device excise tax on United States sales of most medical devices beginning in 2013. We began paying the medical device excise tax in January 2013.
In December 2015, the Protecting Americans from Tax Hikes Act of 2015 (“PATH Act”) was implemented, which suspended the medical device excise tax implemented as part of the Affordable Care Act for a two-year period through December 31, 2017. We expect the suspension to have a positive impact on our operating expenses for fiscal years 2017 and 2018. Additionally, the PATH Act permanently extended the research and development tax credit.
The Affordable Care Act also includes provisions known as the Physician Payments Sunshine Act, or PPSA, which requires manufacturers of drugs, biologics, devices and medical supplies covered under Medicare and Medicaid to record any transfers of value to physicians and teaching hospitals and to report this data to CMS, for subsequent public disclosure. Similar reporting requirements have also been enacted in several states, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with healthcare professionals. Particularly, some states such as Massachusetts and Vermont impose an outright ban on certain gifts to physicians. Failure to report appropriate data may result in civil or criminal fines and/or penalties. We have reported the information as required by the PPSA from the August 1, 2013 effective date through December 31, 2015.
13
Additionally, the compliance environment is changing, with more states, such as California, Connecticut, Nevada and Massachusetts, mandating implementation of compliance programs, compliance with industry ethics codes, and spending limits, and other states, such as Vermont, requiring reporting to state governments of gifts, compensation and other remuneration to physicians. The shifting regulatory environment, along with the requirement to comply in multiple jurisdictions with different compliance and reporting requirements, increases the possibility that a company may run afoul of one or more laws.
International Regulation
We are also subject to regulation in each of the foreign countries in which we sell our products. Many of the regulations applicable to our products in these countries are similar to those of the FDA. The European Union requires that our medical devices comply with the Medical Device Directive or the Active Implantable Medical Device Directive, which includes quality system and CE certification requirements. To obtain a CE Mark in the European Union, defined products must meet minimum standards of safety and quality (i.e., the essential requirements) and then undergo an appropriate conformity assessment procedure. A Notified Body assesses the quality management systems of the manufacturer and verifies the conformity of devices to the essential and other requirements within the Medical Device Directive. In the European Union, we are also required to maintain certain ISO certifications in order to sell our products. Our Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, AB5000, and Portable Driver are all approved under CE Mark and are available for sale in the European Union. We are also subject to regulations and periodic review from various regulatory bodies in Canada, Japan and other countries where we sell our products. Lack of regulatory compliance in any of these jurisdictions could limit our ability to distribute products in these countries.
Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally prohibit companies and their intermediaries from making improper payments to foreign government officials for the purpose of obtaining or retaining business. Many of our customer relationships outside of the U.S. are, either directly or indirectly, with governmental entities and employees (such as physicians) and are therefore subject to various anti-bribery laws. Although our corporate policies mandate compliance with these anti-bribery laws, we do sell to certain customers in many parts of the world that have experienced governmental corruption to some degree, and in certain circumstances strict compliance with anti-bribery laws may conflict with local customs and practices. Our internal control policies and procedures may not always protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our business, results of operations and financial condition.
Other Regulations
We are also subject to various international, federal, state and local laws and regulations relating to such matters as safe working conditions, laboratory and manufacturing practices and the use, handling and disposal of hazardous or potentially hazardous substances used in connection with our research and development and manufacturing activities. Specifically, the manufacture of our biomaterials is subject to compliance with federal environmental regulations and by various state and local agencies. Although we believe we are in compliance with these laws and regulations in all material respects, we cannot provide assurance that we will not be required to incur significant costs to comply with these and other laws or regulations in the future.
Seasonality
Our quarterly net sales are influenced by many factors, including new product introductions, acquisitions, regulatory approvals, patient and physician holiday schedules, and other factors. Net sales in the first half of our fiscal year are typically lower than the second half of our fiscal year due to the seasonality of the U.S. and European markets, where summer vacation schedules normally result in fewer medical procedures.
Employees
As of March 31, 2016, we had 747 full-time employees, including:
| · | 152 in product engineering, research and development, clinical development and regulatory; |
| · | 303 in sales, clinical support, marketing, field service and related support; |
| · | 223 in manufacturing; and |
| · | 69 in general and administration. |
14
We routinely enter into contractual agreements with our employees, which typically include confidentiality and non-competition commitments. Our employees are not represented by unions. We consider our employee relations to be good. If we were unable to attract and retain qualified personnel in the future, our operations could be negatively impacted.
Investing in our common stock involves a high degree of risk. Before making an investment decision, you should carefully consider these risks as well as the other information we include or incorporate by reference in this report, including our consolidated financial statements and the related notes. The risks and uncertainties we have described are not the only ones we face. If any of these risks materializes, the trading price of our common stock could fall and you could lose all or part of your investment.
This section includes or refers to forward-looking statements. You should read the explanation of the qualifications and limitations of such forward-looking statements discussed at the beginning of the report.
Risks Related to Our Business
If we fail to obtain and maintain necessary governmental approvals for our products and indications, we may be unable to market and sell our products in certain jurisdictions.
Medical devices such as ours are extensively regulated by the FDA in the U.S. and by other federal, state, local and foreign authorities. Governmental regulations relate to the testing, development, manufacturing, labeling, design, sale, promotion, distribution, importing, exporting and shipping of our products. In the U.S., before we can market a new medical device, or a new use of, or claim for, or significant modification to, an existing product, we must generally first receive PMA approval from the FDA. This process can be expensive and lengthy and entail significant expenses, primarily related to clinical trials. It generally takes between one to three years to receive approval, or even longer, from the time the PMA application is submitted to the FDA. Regulatory clearances or approvals, either foreign or domestic, may not be granted on a timely basis, if at all. If we are unable to obtain regulatory approvals or clearances for use of our products under development, or if the patient populations for which they are approved are not sufficiently broad, the commercial success of these products could be limited. The FDA may also limit the claims that we can make about our products. Our medical devices are now subject to the PMA and HDE processes. In December 2012, as part of the FDA’s 515 Program Initiative, an FDA panel voted to recommend continuation of Class III status for temporary ventricular support devices within the non-roller type cardiopulmonary bypass blood pumps category, which includes our Impella products. PMA or HDE approval requires that any significant modifications to the design, materials, or intended use of those devices require FDA approval through PMA or HDE supplemental applications, and the devices will be subject to more burdensome regulatory reporting requirements than they had been as 510(k) cleared devices.
If we do not receive FDA approval or clearance for one or more of our products, we will be unable to market and sell those products in the U.S., which would have a material adverse effect on our operations and prospects.
We intend to market our products in international markets, including the European Union, Canada, and Japan. Approval processes differ among those jurisdictions and approval in the U.S. or any other single jurisdiction does not guarantee approval in any other jurisdiction. Obtaining foreign approvals could involve significant delays, difficulties and costs for us and could require additional clinical trials.
If the FDA or another regulatory or enforcement agency determines that we have promoted off-label use of our products, we may be subject to various penalties, including civil or criminal penalties.
The FDA, the U.S. Department of Justice, the Office of the Inspector General of the Department of Health and Human Services, and other regulatory or enforcement agencies actively enforce regulations prohibiting promotion of medical devices for unapproved uses. If any such agency determines that our promotional materials or training constitutes promotion of an unapproved use, it could request that we modify our training or promotional materials or subject us to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and criminal penalties. Although our policy is to refrain from statements that could be considered off-label promotion of our products, such agencies could disagree and conclude that we have engaged in off-label promotion.
On October 26, 2012, we were informed that the Department of Justice, United States Attorney’s Office for the District of Columbia was conducting an investigation (“Marketing and Labeling Investigation”) focused on our marketing and labeling of the Impella 2.5 heart pump. On October 31, 2012, we accepted service of a subpoena related to this investigation seeking documents and other materials related to the Impella 2.5 device. We cooperated fully with the Marketing and Labeling Investigation, and on June 29,
15
2015, we received confirmation that the Department of Justice had closed the Marketing and Labeling Investigation without taking enforcement action.
We may not be able to resolve these matters, or any similar matters that may come up in the future, without incurring penalties or facing significant consequences. Even if we are successful in resolving this matter without incurring penalties, responding to the subpoena has resulted and in the future could result in substantial costs and could significantly and adversely impact our reputation and divert management’s attention and resources, which could have a material adverse effect on our business, operating results, financial condition and ability to finance our operations.
Off-label use of our products may result in injuries that lead to product liability suits, which could be costly to our business.
The use of our products outside the indications cleared for use, or “off-label use,” may increase the risk of injury to patients. Clinicians may use our products for off-label uses, as the FDA does not restrict or regulate a clinician’s choice of treatment within the practice of medicine. Off-label use of our products may increase the risk of product liability claims against us. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us.
We have historically been named as a party to purported stockholder class actions and a derivative action, and we may be named in additional litigation in the future, which may require significant management time and attention, and result in significant legal expenses and may result in an unfavorable outcome, which could have a material adverse effect on our business, operating results and financial condition.
On June 10, 2014, the U.S. Court of Appeals for the First Circuit, or the First Circuit, affirmed the dismissal by the U.S. District Court for the District of Massachusetts, or the District Court, of a previously disclosed complaint brought by an alleged stockholder as a derivative action instituted on our behalf against each of our directors. The complaint alleged that the directors breached their fiduciary duties to us and our stockholders in connection with disclosures related to our marketing and labeling of our Impella 2.5 product and sought damages in an unspecified amount. On February 6, 2015, the First Circuit, affirmed the dismissal by the District Court of a previously disclosed complaint brought by alleged purchasers of our common stock, on behalf of themselves and persons or entities that purchased or acquired our common stock between August 5, 2011 and October 31, 2012. The complaint related to two previously reported complaints that were filed on November 16 and 19, 2012 and alleged that we and certain of our officers violated federal securities laws in connection with disclosures related to our marketing and labeling of the Impella 2.5 product and sought damages in an unspecified amount. We do not expect any further activity related to this matter or the derivative action.
We may continue to be the subject of class action or derivative actions, which we intend to defend vigorously. We cannot be assured, however, that we will be successful. We may have to pay damage awards, indemnify our officers and directors from damage awards that may be entered against them or otherwise may enter into settlement arrangements in connection with such claims. Any such payments or settlement arrangements could have material adverse effects on our business, operating results or financial condition. Even if the plaintiffs’ claims are not successful, defending litigation could result in substantial costs and significantly and adversely impact our reputation and divert management’s attention and resources, which could have a material adverse effect on our business, operating results, financial condition and ability to finance our operations.
Our current and planned clinical trials may not begin on time, or at all, and may not be completed on schedule, or at all.
In order to obtain PMA approval and in some cases, 510(k) clearance, we may be required to conduct well-controlled clinical trials designed to test the safety and effectiveness of the product. In order to conduct clinical studies, we must receive an investigational device exemption, or IDE, in effect for each investigational device. An IDE allows us to use an investigational device in a clinical trial to collect data on safety and effectiveness that will support an application for PMA or 510(k) clearance from the FDA.
Conducting clinical trials is a long, expensive and uncertain process that is subject to delays and failure at any stage. Clinical trials can take months or years to complete. The commencement or completion of any of our clinical trials may be delayed or halted for numerous reasons, including:
| · | the FDA may not approve a clinical trial protocol or a clinical trial, or may place a clinical trial on hold; |
| · | subjects may not enroll in clinical trials at the rate we expect and/or subjects may not be followed-up on at the rate we expect; |
| · | subjects may experience adverse side effects or events related or unrelated to our products; |
16
| · | third-party clinical investigators may not perform our clinical trials on our anticipated schedule or consistent with the clinical trial protocol and good clinical practices, or other third-party organizations may not perform data collection and analysis in a timely or accurate manner; |
| · | the interim results of any of our clinical trials may be inconclusive or negative; |
| · | regulatory inspections of our clinical trials may require us to undertake corrective action or suspend or terminate our clinical trials if investigators find us not to be in compliance with regulatory requirements; |
| · | availability of our devices may have the effect of slowing down the progress of related clinical trials since physicians can use our devices commercially outside of the trials; |
| · | our manufacturing process may not produce finished products that conform to design and performance specifications expected with the clinical trial; or |
| · | governmental regulations or administrative actions may change and impose new requirements, particularly with respect to reimbursement for products used in clinical trials. |
The results of pre-clinical studies do not necessarily predict future clinical trial results and previous clinical trial results may not be repeated in subsequent clinical trials. We may suffer delays and cost overruns, and terminate manufacturing of certain of our products despite achieving promising results in pre-clinical testing or early clinical testing. In addition, the data obtained from clinical trials may be inadequate to support approval or clearance of a submission. The FDA may disagree with our interpretation of the data from our clinical trials, or may find the clinical trial design, conduct or results inadequate to demonstrate the safety and effectiveness of the product candidate. The FDA may also require us to conduct additional pre-clinical studies or clinical trials which could further delay approval of our products. The FDA or other international regulatory agencies will require post-market studies which can be burdensome and expensive.
In March 2015, we received PMA approval from the FDA for our Impella 2.5 device during elective and urgent high-risk PCI procedures. The Impella 2.5 is the first hemodynamic support device to receive a PMA indication for use during high-risk PCI procedures, demonstrating its safety and effectiveness for this complex patient population. Under this PMA, the Impella 2.5 is approved for temporary (up to six hours) ventricular support device indicated for use during high-risk PCI performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high-risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 device in these patients may prevent hemodynamic instability that may occur during planned temporary coronary occlusions and may reduce periprocedural and post-procedural adverse events. The approved product labeling allows for a clinical decision to leave the Impella 2.5 device in place beyond the intended duration of up to six hours due to unforeseen circumstances. Per our PMA approval, we will conduct a single-arm, post-approval study on the Impella 2.5 device, collecting data on high-risk PCI patients. The study will be a prospective, multi-center study comprised of 369 patients from 70 sites supported with the Impella 2.5 system. The Impella 2.5 device has CE Mark approval in Europe for up to five days of use and is approved for use in over 40 countries.
In August 2015, we submitted a PMA supplement requesting to expand our current Impella 2.5 PMA approval to include additional indications for the Impella 2.5 product and also to include all of our other Impella devices (Impella CP, Impella 5.0 and Impella LD) that support the left side of the heart. These submissions are for a set of indications related to the use of the Impella devices in patients suffering cardiogenic shock following acute myocardial infarction or cardiac surgery and for a longer duration of support. In April 2016, the FDA approved the PMA supplement for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock. In this setting, the Impella heart pumps stabilize the patient’s hemodynamics, unload the left ventricle, perfuse the end organs and allow for recovery of the native heart.
If we are unable to receive FDA approval of an IDE to conduct clinical trials or the trials are halted by the FDA or others or if we are unsuccessful in receiving FDA approval of a product candidate, we would not be able to sell or promote the product candidate in the U.S., which could seriously harm our business. Moreover, we face similar risks in each jurisdiction in which we sell or propose to sell our products. If we make modifications to a product, whether in response to results of clinical testing or otherwise, we could be required to start our clinical trials over, which could cause serious delays that would adversely affect our results of operations. Even modest changes to certain components of our products could result in months or years of additional clinical trials.
Our products are subject to extensive regulatory requirements, including continuing regulatory review, which could affect the manufacturing and marketing of our products.
The FDA and other regulatory agencies continue to review products even after they have received initial approval. If and when the FDA or another regulatory agency clears or approves our products under development, the manufacture and marketing of these products will be subject to continuing regulation, post approval clinical studies, including compliance with the FDA’s adverse event
17
reporting requirements, prohibitions on promoting a product for unapproved uses, and Quality System Regulation, or QSR, requirements, which obligate manufacturers, including third-party and contract manufacturers, to adhere to stringent design, testing, control, documentation and other quality assurance procedures during the design and manufacture of a device.
Any modification to an FDA approved device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a supplemental PMA or HDE approval. The FDA requires each manufacturer to determine in the first instance whether a modification requires approval, but the FDA may review and potentially disagree with any such decision. Modifications of this type are common with new products. We anticipate that the first generation of each of our products will undergo a number of changes, refinements, enhancements and improvements over time. If the FDA requires us to seek approval for modification of a previously cleared product for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval and we may be subject to significant regulatory fines or penalties, which could have a material adverse effect on our financial results and competitive position. We also cannot assure you that we will be successful in obtaining clearances or approvals for our modifications, if required. We and our third-party suppliers of product components are also subject to inspection and market surveillance by the FDA and other regulatory agencies for QSR and our regulatory other requirements, the interpretation of which can change. Compliance with QSR and similar legal requirements can be difficult and expensive. Enforcement actions resulting from failure to comply with government requirements could result in fines, suspensions of approvals or clearances, recalls or seizure of products, operating restrictions or shutdown, and criminal prosecutions that could adversely affect the manufacture and marketing of our products. The FDA or another regulatory agency could withdraw a previously approved product from the market upon receipt of newly discovered information, including a failure to comply with regulatory requirements, the occurrence of unanticipated safety problems of other defects in products following approval, or other reasons, which could adversely affect our operating results.
Even after receiving regulatory clearance or approval, our products may be subject to product recalls which could harm our reputation and divert our managerial and financial resources.
The FDA and similar governmental authorities in other countries have the authority to order mandatory recall of our products or order their removal from the market if the government finds that our products might cause adverse health consequences or death. A government-mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors by us or our suppliers or design defects, including labeling defects, or unanticipated safety problems. We have in the past initiated voluntary recalls of some of our products and we could do so in the future. Any recall of our products may harm our reputation with customers and divert managerial and financial resources.
We depend on third-party reimbursement to our customers for market acceptance of our products. If third-party payers fail to provide coverage and appropriate levels of reimbursement for purchase and use of our products, our sales and profitability would be adversely affected.
Sales of medical devices largely depend on the reimbursement of patients’ medical expenses by government healthcare programs and private health insurers. Without the financial support of government reimbursement or third-party insurers’ payments for patient care, the market for our products will be limited. Medical products and devices incorporating new technologies are closely examined by governments and private insurers to determine whether the products and devices will be covered by reimbursement, and if so, the level of reimbursement which may apply.
We cannot be sure that additional third-party payers will cover and/or adequately reimburse use of our products or other products under development, to enable us to sell them at profitable prices.
In addition, third-party payers increasingly are requiring evidence that medical devices are cost-effective and if we are unable to meet this requirement, the third-party payer may not reimburse the use of our products, which could reduce sales of our products to healthcare providers who depend upon reimbursement for payment. We also cannot be sure that third-party payers will continue the current levels of reimbursement to physicians and medical centers for use of our products. Any reduction in the amount of this reimbursement could harm our business.
Changes in healthcare reimbursement systems in the U.S. and abroad could reduce our revenues and profitability.
In March 2010, the federal government enacted healthcare reform legislation. The legislation has made changes to the manner in which many healthcare services are provided and paid for in the U.S. These changes may impact reimbursement for healthcare services, including reimbursement to hospitals and physicians. States may also enact further legislation that impacts Medicaid payments to hospitals and physicians. In addition, CMS, the federal agency responsible for administering the Medicare program in the U.S., has established payment levels for hospitals and physicians in line with the legislation, which can increase or decrease payment to such entities.
18
In general, such healthcare reforms put greater responsibility for controlling healthcare costs on providers, which may have the effect of causing providers to control costs more closely. The healthcare reform legislation and any future legislative, regulatory and reimbursement initiatives or changes to the reimbursement for our products could adversely affect demand for our products and have a material adverse impact on our revenues. Our business and results of operations could therefore be adversely affected by the healthcare reform legislation as well as future healthcare reform or regulatory actions.
Internationally, medical reimbursement systems vary significantly from country to country, with some countries limiting medical centers spending through fixed budgets, regardless of levels of patient treatment, and other countries requiring application for, and approval of, government or third-party reimbursement. Even if we succeed in bringing our new products to market, uncertainties regarding future healthcare policy, legislation and regulation, as well as private market practices, could affect our ability to sell our products in commercially acceptable quantities at profitable prices.
We must comply with healthcare “fraud and abuse” laws, and we could face substantial penalties for non-compliance and be excluded from government healthcare programs, which would adversely affect our business, financial condition and results of operations.
Certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights may be applicable to our business. We may be subject to healthcare fraud and abuse regulation and patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| · | The federal healthcare program Anti-Kickback Statute, which prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, to induce (i) the referral of an individual, for an item or service, or (ii) the recommending, purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; |
| · | The federal False Claims Act, which prohibits, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent, including claims made pursuant to an unlawful kickback, and which may apply to entities like us that promote medical devices, provide medical device management services and may provide coding and billing advice to customers; |
| · | The Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| · | State law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ in significant ways from state to state and often are not preempted by HIPAA, thus complicating compliance efforts. |
Additionally, the compliance environment is changing, with more states, such as California, Connecticut, Nevada and Massachusetts, mandating implementation of compliance programs, compliance with industry ethics codes, and spending limits, and other states, such as Vermont, requiring reporting to state governments of gifts, compensation and other remuneration to physicians. The Physician Payments Sunshine Act, or PPSA, which was signed into law on March 23, 2010, requires U.S. manufacturers of drug, device, biologics, and medical supplies covered under Medicare, Medicaid, or State Children’s Health Insurance Program, or SCHIP, to report payments made to physicians and teaching hospitals on an annual basis to the government. These laws all provide for penalties for non-compliance. The shifting regulatory environment, along with the requirement to comply with multiple jurisdictions with different and difficult compliance and reporting requirements, increases the possibility that we may run afoul of one or more laws. The costs to comply with these regulatory requirements are becoming more expensive and will also impact our profitability.
Many of these requirements are new and their application is uncertain, and regulatory guidance is limited. We could face enforcement action, fines and other penalties and could receive adverse publicity, all of which could harm our business, if it is alleged that we have failed to fully comply with such laws and regulations. Similarly, if the physicians or other providers or entities that we do business with are found not to comply with applicable laws, they may be subject to sanctions, which could also have a negative impact on our business.
19
On April 25, 2014, we received a subpoena from the Boston regional office of the United States Department of Health and Human Services, or HHS, Office of Inspector General requesting materials relevant to our reimbursement of expenses and remuneration to healthcare providers for a six month period from July 2012 through December 2012 in connection with the FCA Investigation. We submitted the requested documents to HHS and believe that we substantially complied with the subpoena. On November 6, 2014, we received notice from the Department of Justice, United States Attorney’s Office for the District of Massachusetts in the form of a CID requesting additional materials relating to this matter for the time period of January 1, 2012 through December 31, 2013. We have responded to the additional requests for information contained in the CID, and are in the process of responding to other informal requests. We intend to continue to cooperate with the U.S. Attorney’s Office in connection with the FCA Investigation.
We depend on Impella® products for a significant portion of our revenues.
We derive, and expect to continue to derive in the near future, almost all of our revenues from sales of our Impella products. While we cannot fully predict what level of revenues our Impella products will generate, we anticipate that Impella product sales will continue to account for a significant portion of our revenues in the foreseeable future. Implementation of our business strategy depends on continued sales of our Impella products. Our ability to generate sales of our Impella products may be impaired by the factors described below:
| · | our failure to obtain approvals from the FDA and foreign regulatory authorities or to comply with government regulations, or the withdrawal of market clearance or the taking of other enforcement actions; |
| · | lack of acceptance or continued acceptance by physicians; |
| · | our reliance on specialized suppliers for certain components and materials; |
| · | manufacturing or quality control problems; |
| · | our inability to protect our proprietary technologies or an infringement of others’ patents; |
| · | the loss of a distributor or a distributor’s failure to perform its obligations; |
| · | our failure to compete successfully against our existing or potential competitors; |
| · | additional risks associated with selling in international markets; |
| · | long and variable sales and deployment cycles; |
| · | failure by third-party payers to provide appropriate levels of reimbursement for hospitals and physicians using our products; |
| · | our failure to comply with federal and state regulations; and |
| · | product liability claims. |
If we fail to compete successfully against our existing or potential competitors, our sales or operating results may be harmed.
Competition from other companies offering circulatory care products is intense and subject to rapid technological change and evolving industry requirements and standards. We compete with companies that have substantially greater or broader financial, product development, sales and marketing resources and experience than we do. Our ability to compete effectively depends upon our ability to distinguish our company and our products from our competitors and their products. Factors affecting our competitive position include:
| · | the availability of other products and procedures that are technically equivalent or superior to our products, and which may be sold at lower prices; |
| · | product performance and design; |
| · | sales, marketing and distribution capabilities; |
| · | comparable clinical outcomes; |
| · | success and timing of new product development and introductions; |
| · | physician acceptance of our products; |
20
| · | penetration into existing and new geographic markets; and |
| · | intellectual property protection. |
Our customers are primarily hospitals that have limited budgets. As a result, our products compete against a broad range of medical devices and other therapies for these limited funds. Our success will depend in large part upon our ability to enhance our existing products, to develop new products to meet regulatory and customer requirements and to achieve market acceptance for our products. We believe that important competitive factors with respect to the development and commercialization of our products include the relative speed with which we can develop products, establish clinical utility, complete clinical trials and regulatory approval processes, obtain and protect reimbursement, maintain cost effectiveness for our products, and supply commercial quantities of our products to our customers.
Advances in medical technology, biotechnology and pharmaceuticals may reduce the size of the potential markets for our products or render our products obsolete. We are aware of other heart replacement device research efforts in the U.S., Canada, Europe and Japan. In addition, there are a number of companies; including Getinge, Abbott Laboratories, St. Jude Medical, Inc., CardiacAssist, HeartWare International, Terumo Heart and several early-stage companies, that are developing heart assist products, including implantable left ventricular assist devices and miniaturized rotary ventricular assist devices.
If we do not effectively manage our growth, we may be unable to successfully develop, market and sell our products.
Our future revenue and operating results will depend on our ability to manage the anticipated growth of our business. We have experienced significant growth in recent years in the scope of our operations and we have increased our employee headcount. This growth has placed significant demands on our management as well as our financial and operations resources. In order to achieve our business objectives, we will need to continue to grow. However, continued growth presents numerous challenges, including:
| · | developing our global sales and marketing infrastructure and capabilities; |
| · | expanding manufacturing capacity, maintaining quality and increasing production; |
| · | increasing our foreign and domestic regulatory compliance capabilities; |
| · | implementing appropriate operational, financial and IT systems and internal controls; |
| · | identifying, attracting and retaining qualified personnel, particularly experienced clinical staff; and |
| · | hiring, training, managing and supervising our personnel worldwide. |
Any failure to manage our growth effectively could impede our ability to successfully develop, market and sell our products, which could seriously harm our business.
The demand for some of our products and products under development is unproven, and we may be unable to successfully commercialize our products.
Our products and products under development may not enjoy commercial acceptance or success, which could adversely affect our business and operational results. We need to create markets for our Impella products and other existing products, as well as other new or future products, including achieving market acceptance among physicians, hospitals, patients and third-party payers. In particular, we need to gain acceptance of our Impella products among interventional cardiologists. The obstacles we will face in trying to create successful commercial markets for our products include:
| · | limitations inherent in first-generation devices, and our potential inability to develop successive improvements, including increases in service life and improvements in the ease of use of our products; |
| · | introduction by other companies of new treatments, products and technologies that compete with our products; |
| · | timing and amount of reimbursement for these products, if any, by third-party payers; |
| · | potential reluctance of clinicians to obtain adequate training to use our products; |
| · | cost of our products; and |
| · | potential reluctance of physicians, patients and society as a whole to accept medical devices that replace or assist the heart and risk of mechanical failure inherent in such devices. |
21
Our future success depends in part on the development of new circulatory assist products, and our development efforts may not be successful.
We are devoting most of our research and development and regulatory efforts, and significant financial resources, to the development of our Impella products and product extensions of existing commercial products and new products. In July 2014, we acquired ECP, a German company engaged in the research, development, prototyping and pre-serial production of a percutaneous expandable catheter pump which increases blood circulation from the heart with an external drive shaft. The development of new products and product extensions presents enormous challenges in a variety of areas, including blood compatible surfaces, blood compatible flow, manufacturing techniques, pumping mechanisms, physiological control, energy transfer, anatomical fit and surgical techniques. We may be unable to overcome all of these challenges, which could adversely affect our results of operations and prospects and limit our ability to bring new products to market.
The commercial success of our products will require acceptance by surgeons and interventional cardiologists, a limited number of whom have significant influence over medical device selection and purchasing decisions.
We may achieve our business objectives only if our products are accepted and recommended by leading cardiovascular surgeons and interventional cardiologists, whose decisions are likely to be based on a determination by these clinicians that our products are safe and cost-effective and represent acceptable methods of treatment. Although we have developed relationships with leading cardiac surgeons, the commercial success of Impella and our other products will require that we also develop relationships with leading interventional cardiologists in cath labs. We cannot assure you that we can maintain our existing relationships and arrangements or that we can establish new relationships in support of our products. If cardiovascular surgeons and interventional cardiologists do not consider our products to be adequate for the treatment of our target cardiac patient population or if a sufficient number of these clinicians recommend and use competing products, it would seriously harm our business.
Expansion into hospital cardiac centers that have not historically used our products may incur long sales and training cycles that may cause our product sales and operating results to vary significantly from quarter-to-quarter.
Our products have lengthy sales cycles and we may incur substantial sales and marketing expenses and expend significant effort without making a sale. Even after making the decision to purchase our Impella products, our customers often deploy our products slowly or infrequently. In addition, cardiac centers that buy the majority of our products are usually led by cardiac surgeons who are heavily recruited by competing hospitals or by hospitals looking to increase their profiles. When one of these surgeons moves to a new hospital we sometimes experience a temporary but significant reduction in purchases by the hospital from which the physician has departed while it replaces the lead physician supporting our Impella products. As a result, our product sales and operating results may vary significantly from quarter to quarter. In addition, product purchases often lag initial expressions of interest in our product by new centers as training of the products and internal hospital administrative procedures are typically required prior to the initial implant procedures.
The training required for clinicians to use our products could reduce the market acceptance of our products and reduce our revenue.
Clinicians must be trained to use our products proficiently. It is critical to the success of our business that we ensure that there are a sufficient number of clinicians familiar with, trained on and proficient in the use of our products. Convincing clinicians to dedicate the time and energy necessary to obtain adequate training in the use of our products is challenging and we may not be successful in these efforts. If clinicians are not properly trained, they may misuse or ineffectively use our products. Any improper use of our products may result in unsatisfactory outcomes, patient injury, negative publicity or lawsuits against us, any of which could harm our reputation and affect future product sales. Furthermore, our inability to educate and train clinicians to use our products may lead to lower demand for our products.
If we are unable to develop additional, high-quality manufacturing capacity, our growth may be limited and our business could be seriously harmed.
To be successful, we believe we will need to increase our manufacturing capacity. We do not have experience in manufacturing our Impella products in the commercial quantities that might be required to meet potential demand, nor do we have experience manufacturing our other products in large quantities. We may encounter difficulties in scaling up manufacturing of our products, including problems related to product yields, quality control and assurance, component and service availability, dependable sources of supply, adequacy of internal control policies and procedures and lack of skilled personnel. If we cannot hire, train and retain enough experienced and capable scientific and technical workers, we may not be able to manufacture sufficient quantities of our existing or future products on-time and at an acceptable cost, which could limit market acceptance of our products or otherwise damage our business. In order for our manufacturing to meet the expected demand for our Impella products, we have been implementing process
22
improvements on the Impella production line at our manufacturing facilities in Aachen, Germany and Danvers, Massachusetts to increase the output that we can produce at the facility. In addition to programs designed to further increase yield and capacity levels, we have expanded manufacturing employment in Aachen and Danvers and have increased manufacturing floor space in Danvers and Aachen. We have relocated selected Impella sub-assembly production to our manufacturing facility in Danvers, Massachusetts and with third party suppliers and we began production of the Impella CP device in Danvers to support manufacturing at our main Impella production facility in Aachen. We continue to work on initiatives to expand our Impella manufacturing capacity in both Aachen and Danvers. We are also working with our existing suppliers and new suppliers to ensure we are able to have sufficient inventory and sub assembly parts as we increase our manufacturing capability to support growing demand. We are also working on process improvements, such as certain automation techniques, to allow us to manufacture our products more efficiently. If we are unable to implement these process improvements on a timely basis, it could inhibit our revenue growth.
Any failure to achieve and maintain the high manufacturing standards that our products require may seriously harm our business.
Our products require precise, high-quality manufacturing. Achieving precision and quality control requires skill and diligence by our personnel as well as our vendors. Any failure to achieve and maintain these high manufacturing standards, including the incidence of manufacturing errors, design defects or component failures could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously hurt our business. Despite our very high manufacturing standards, we cannot completely eliminate the risk of errors, defects or failures. If we or our vendors are unable to manufacture our products in accordance with necessary quality standards, or if we are unable to procure additional high-quality manufacturing facilities, our business and results of operations may be negatively affected.
If we cannot attract and retain key management, scientific, sales and other personnel we need, we will not be successful.
We depend heavily on the contributions of the principal members of our business, financial, technical, sales and support, regulatory and clinical, operating, manufacturing and administrative management and staff, many of whom would be difficult to replace. Our key personnel include our senior officers, many of whom have very specialized scientific, medical or operational knowledge. The loss of the service of any of the key members of our senior management team may significantly delay or prevent our achievement of our business objectives. Our ability to attract and retain qualified personnel, consultants and advisors is critical to our success. For example, many of the members of our clinical staff are registered nurses with experience in the surgery suite or cath lab, of which only a limited number of whom seek employment with a company like ours. Competition for skilled and experienced personnel in the medical devices industry is intense. We face competition for skilled and experienced management, scientific, clinical, engineering and sales personnel from numerous medical device and life sciences companies, universities, governmental entities and other research institutions. If we lose the services of any of the principal members of our management and staff, or if we are unable to attract and retain qualified personnel in the future, especially scientific and sales personnel, our business could be adversely affected.
If our suppliers cannot provide the components we require, our ability to manufacture our products could be harmed.
We rely on third-party suppliers to provide us with some components used in our existing products and products under development. For example, we outsource the manufacturing of most of our consoles other than final assembly and testing and the sterilization process for our products. Relying on third-party suppliers makes us vulnerable to component part failures or obsolescence and to interruptions in supply, either of which could impair our ability to conduct clinical tests or to ship our products to our customers on a timely basis. Using third-party vendors makes it difficult and sometimes impossible for us to test fully certain components, such as components on circuit boards, maintain quality control, manage inventory and production schedules and control production costs. Manufacturers of our product components may be required to comply with the FDA or other regulatory manufacturing regulations and to satisfy regulatory inspections in connection with the manufacture of the components. Any failure by a supplier to comply with applicable requirements could lead to a disruption in supply. Vendor lead times to supply us with ordered components vary significantly and often can exceed six months or more. Both now and as we expand our manufacturing capacity, we cannot be sure that our suppliers will furnish us required components when we need them or be able to provide us inventory materials to support our expected growth in demand for our products. These factors could make it more difficult for us to manufacture our products effectively and efficiently and could adversely impact our results of operations.
Some of our suppliers may be the only source for a particular component, which makes us vulnerable to significant cost increases. We have many foreign suppliers for some of our parts in which we are subject to currency exchange rate volatility. Some of our vendors are small in size and may have difficulty supplying the quantity and quality of materials required for our products as our business grows. Vendors that are the sole source of certain products may decide to limit or eliminate sales of certain components due to product liability or other concerns and we might not be able to find a suitable replacement for those products. Our inventory may run out before we find alternative suppliers and we might be forced to purchase substantial inventory, if available, to last until we are able to qualify an alternate supplier. If we cannot obtain a necessary component, we may need to find, test and obtain regulatory approval or clearance for a replacement component, produce the component ourselves or redesign the related product, which would
23
cause significant delay and could increase our manufacturing costs. Any of these events could adversely impact our results of operations.
General economic and political conditions could have a material adverse effect on our business.
External factors can affect our profitability and financial condition. Such external factors include general domestic and global economic conditions, such as interest rates, foreign currency exchange rates, tax rates and factors affecting global economic stability, and the political environment regarding healthcare in general. While the economic environment has shown some signs of improvement, the strength and timing of any economic recovery remains uncertain, and we cannot predict to what extent the global economic slowdown may negatively impact our business. For example, an increase in interest rates could result in an increase in our borrowing costs and could otherwise restrict our ability to access the capital markets. Negative conditions in the credit and capital markets could impair our ability to access the financial markets for working capital or other funds, and could negatively impact our ability to borrow. Such conditions could result in decreased liquidity and impairments in the carrying value of our investments, and could adversely affect our results of operations and financial condition. These and other conditions could also adversely affect our customers, and may impact their ability or decision to purchase our products or make payments on a timely basis.
We do business with foreign governments outside the United States. A number of these countries, including certain European countries, have experienced deterioration in credit and economic conditions. These conditions have resulted in, and may continue to result in, a reduction in the number of procedures that use our products and an increase in the average length of time that it takes to collect accounts receivable outstanding in these countries.
We may not be successful in expanding our direct sales activities into international markets.
We are seeking to expand our international sales of our products by recruiting direct sales and support teams outside the U.S. Our international operations in Germany, France, Canada, Japan and the United Kingdom are or will be subject to a number of risks, which may vary from the risks we experience in the U.S., including:
| · | the need to obtain regulatory approvals in foreign countries before our products may be sold or used; |
| · | the need to procure reimbursement for our products in each foreign market; |
| · | the generally lower level of reimbursement available in foreign markets relative to the U.S.; |
| · | the requirement to work with distributors or other partners to sell our products; |
| · | limited protection of intellectual property rights; |
| · | difficulty and delays in collecting accounts receivable; |
| · | different income tax and sales tax environments; |
| · | difficulty in supporting patients using our products; |
| · | different payroll, employee benefits and statutory requirements; |
| · | fluctuations in the values of foreign currencies; and |
| · | political and economic instability. |
If we are unable to effectively expand our sales activities in international markets, our results of operations could be negatively impacted.
We rely on distributors to sell our products in some international markets and poor performance by a distributor could reduce our sales and harm our business.
We rely on distributors to market and sell our products in certain parts of Europe, Asia, South America and the Middle East. Many of these distributors have the exclusive right to distribute our products in their territory. We may hire distributors to market our products in additional international markets in the future. Our success in these markets will depend almost entirely upon the efforts of our distributors, over whom we have little or no control. If a distributor does not market and sell our products aggressively and maintain a continued focus on the sale and distribution of our products up to our standards, we could lose sales and impair our ability to compete in that market. We are also subject to credit risk associated with shipments to our distributors and this could negatively impact our financial condition and liquidity in the future.
24
Many of our customer relationships outside of the U.S. are, either directly or indirectly, with governmental entities, and we could be adversely affected by violations of the United States Foreign Corrupt Practices Act and similar worldwide anti-bribery laws outside the U.S.
The U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally prohibit companies and their intermediaries from making improper payments to foreign government officials for the purpose of obtaining or retaining business. Many of our customer relationships outside of the United States are, either directly or indirectly, with governmental entities and employees (such as physicians) and are therefore subject to such anti-bribery laws. Although our corporate policies mandate compliance with these anti- bribery laws, we operate in many parts of the world that have experienced governmental corruption to some degree, and in certain circumstances strict compliance with anti-bribery laws may conflict with local customs and practices. Our internal control policies and procedures may not always protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our business, results of operations and financial condition.
We have incurred losses in previous periods and it is possible that we may incur losses in future periods.
We have recognized net income of approximately $38.1 million, $113.7 million and $7.4 million for the fiscal years ended March 31, 2016, 2015 and 2014, respectively. The profitability we achieved in recent years may not be indicative of our ability to sustain profitability and it is possible that we may incur losses from operations in future periods. Any losses incurred in the future may result primarily from, among other things:
| · | the expansion of our global distribution network; |
| · | investments in new markets such as Japan; |
| · | ongoing product and clinical development; |
| · | costs related to new business development initiatives, such as potential acquisitions of businesses; |
| · | legal expenses related to the FCA Investigation and patent related matters; |
| · | costs associated with hiring additional personnel, performing clinical trials, continuing our research and development relating to our products under development, seeking regulatory approvals and, if we receive these approvals, commencing commercial manufacturing and marketing activities; |
| · | significant expenditures necessary to market and manufacture in commercial quantities our approved circulatory care products; and |
| · | the amount of these expenditures is difficult to forecast accurately and cost overruns may occur. |
Our operating results may fluctuate unpredictably.
Historically, our annual and quarterly operating results have fluctuated widely and we expect these fluctuations to continue. Among the factors that may cause our operating results to fluctuate are:
| · | the timing of customer orders and deliveries; |
| · | competitive changes, such as price changes or new product introductions that we or our competitors may make; |
| · | the timing of regulatory actions, such as product approvals or recalls; |
| · | costs we incur developing and testing our Impella heart pumps and other products; |
| · | costs we incur in anticipation of future sales, such as inventory purchases, expansion of manufacturing facilities, or establishment of international sales offices; |
| · | costs we incur in connection with the class action suits and derivative action that has been filed against us; |
| · | costs we incur in connection with the investigation being conducted by the U.S. Department of Justice; |
| · | additional taxes, such as the Medical Device tax; |
| · | timing of certain marketing programs and events; |
| · | the availability of physicians to use our products, as there are seasonal impacts, due to physician vacation or training events that limit their ability to be in the hospital to perform procedures that involve our products; |
25
| · | impact of any businesses or technologies we may acquire in the future; |
| · | economic conditions in the healthcare industry; and |
| · | efforts by governments, insurance companies and others to contain healthcare costs, including changes to reimbursement policies. |
We believe that period-to-period comparisons of our historical results are not necessarily meaningful, and investors should not rely on them as an indication of our future performance. To the extent we experience the factors described above, our future operating results may not meet the expectations of securities analysts or investors from time to time, which may cause the market price of our common stock to decline.
We may undergo an “ownership change” for U.S. federal income tax purposes, which would limit our ability to utilize net operating losses from prior tax years.
If we undergo an “ownership change” for U.S. federal income tax purposes, our ability to utilize net operating loss carry-forwards from prior years to reduce taxable income in future tax years might be limited by operation of the Internal Revenue Code, either by limiting the amount of net operating losses that can be utilized to offset taxable income in a given year, or in total over the entire carry-forward period. Certain changes in the ownership of our common stock may result in an ownership change sufficient to limit the availability of our net operating losses. We also have net operating loss carry-forwards in other countries outside of the U.S. and our ability to use those losses in the future to offset taxable income could be limited by tax regulations in those countries.
We may not have sufficient funds to develop and commercialize our new products or make acquisitions of desirable companies, products or technologies.
The development, manufacture and sale of any medical device is very expensive and we may require additional funds to make acquisitions of desirable companies, products or technologies. We cannot be sure that we will have the necessary funds to develop and commercialize our new products or acquire companies, products, or technologies, or that additional funds will be available on commercially acceptable terms, if at all. If we are unable to obtain the necessary funding to support these efforts, our business may be adversely affected. We believe we have sufficient liquidity to finance our operations for the next fiscal year. We also may evaluate from time to time other financing alternatives as necessary to fund operations, and any equity or convertible debt financing may involve substantial dilution to our existing stockholders.
We own patents, trademarks, trade secrets, copyrights and other intellectual property and know-how that we believe gives us a competitive advantage. If we cannot protect our intellectual property and develop or otherwise acquire additional intellectual property, competition could force us to lower our prices, which could hurt our profitability.
Our intellectual property rights are and will continue to be a critical component of our success. We rely and expect to continue to rely on a combination of intellectual property, including patent, trademark, copyright, trade secret and domain name protection laws, as well as confidentiality agreements with our employees and others, to protect our intellectual property and proprietary rights. If we fail to obtain and maintain adequate intellectual property protection, we may not be able to prevent third parties from using our proprietary technologies or from marketing products that are very similar or identical to ours.
A substantial portion of our intellectual property rights relating to the Impella products and other products under development is in the form of trade secrets, rather than patents. Unlike patents, trade secrets are only recognized under applicable law if they are kept secret by restricting their disclosure to third parties. We protect our trade secrets and proprietary knowledge in part through confidentiality agreements with employees, consultants and other parties. However, certain consultants and third parties with whom we have business relationships, and to whom in some cases we have disclosed trade secrets and other proprietary knowledge, may also provide services to other parties in the medical device industry, including companies, universities and research organizations that are developing competing products. In addition, some of our former employees who were exposed to certain of our trade secrets and other proprietary knowledge in the course of their employment may seek employment with, and become employed by, our competitors. We cannot be assured that consultants, employees and other third parties with whom we have entered into confidentiality agreements will not breach the terms of such agreements by improperly using or disclosing our trade secrets or other proprietary knowledge, that we will have adequate remedies for any such breach, or that our trade secrets will not become known to or be independently developed by our competitors. The loss of trade secret protection for technologies or know-how relating to our product portfolio and products under development could adversely affect our business and our prospects.
Our business position also depends in part on our ability to maintain and defend our existing patents and obtain, maintain, and defend additional patents and other intellectual property rights. We intend to seek additional patents, but our pending and future patent
26
applications may not result in issued patents or be granted on a timely basis. In addition, issued patents may not contain claims sufficiently broad to protect us against third parties with similar technologies or products or provide us with any competitive advantage, including exclusivity in a particular product area. The scope of our patent claims also may vary between countries, as individual countries have distinctive patent laws. We may be subject to challenges by third parties regarding our intellectual property, including, among others, claims regarding validity, enforceability, scope and effective term. Patent prosecution, related proceedings, and litigation in the U.S. and in other countries may be expensive, time consuming and ultimately unsuccessful. In addition, patents issued by foreign countries may afford less protection than is available under U.S. patent law and may not adequately protect our proprietary information. Our competitors may independently develop proprietary technologies and processes that are the same as or substantially equivalent to ours or design around our patents. Our competition may also hold or obtain intellectual property rights that would threaten our ability to develop or commercialize our product offerings. The expiration of patents on which we rely for protection of key products could diminish our competitive advantage and adversely affect our business and our prospects.
Companies in the medical device industry typically obtain patents and frequently engage in substantial intellectual property litigation. Our products and technologies could infringe on the rights of others. If a third-party successfully asserts a claim for infringement against us, we may be liable for substantial damages, be unable to sell products using that technology, or have to seek a license or redesign the related product. These alternatives may be uneconomical or impossible. Intellectual property litigation could be costly, result in product development delays and divert the efforts and attention of management from our business.
In July and August 2015, Thoratec Corporation (“Thoratec”), acquired by St. Jude Medical, Inc. in October 2015, brought actions in connection with two of our patents relevant to Thoratec’s HeartMate PHP medical device (“PHP”). In those proceedings, which are in the United Kingdom and Germany, Thoratec asserts that the two patents are invalid. In September 2015, we filed counterclaims to the action in Germany asserting that the PHP product infringes the two patents and two other patents owned by us. Both the Germany and United Kingdom proceedings are ongoing.
In December 2015, we received a letter from Maquet Cardiovascular LLC, a subsidiary of the Getinge Group (“Maquet”), and maker of the intra-aortic balloon pump, asserting that our Impella products infringe certain guidewire, lumen and sensor claims of two Maquet patents and one pending patent application that Maquet has filed in the U.S. and elsewhere, and encouraged us to discuss taking a license from Maquet. In January 2016, we responded to Maquet stating that we believed that the cited claims were invalid and that our Impella products did not infringe the cited patents. In May 2016, Maquet sent a second letter notifying us that the pending patent application had been issued as a U.S. patent and repeated their earlier assertion and encouraged us to discuss taking a license from Maquet. On May 19, 2016, we filed suit in Massachusetts District Court against Maquet seeking a declaratory judgment that our Impella products do not infringe Maquet’s cited patent rights.
Product liability claims could damage our reputation and adversely affect our financial results.
The clinical use of medical products, even after regulatory approval, poses an inherent risk of product liability claims. We maintain limited product liability insurance coverage, subject to certain deductibles and exclusions. We cannot be sure that product liability insurance will be available in the future or will be available on acceptable terms or at reasonable costs, or that such insurance will provide us with adequate coverage against potential liabilities. Claims against us, regardless of their merit or potential outcome, may also hurt our ability to obtain physician endorsement of our products or expand our business. As we continue to introduce more products and our products are used more widely, we face an increased risk that a product liability claim will be brought against us.
Some of our products are designed for patients who suffer from late-stage or end-stage heart failure, and many of these patients do not survive, even when supported by our products. There are many factors beyond our control that could result in patient death, including the condition of the patient prior to use of the product, the skill and reliability of physicians and hospital personnel using and monitoring the product and product maintenance by customers. However, the failure of the products we distribute for clinical testing or sale could give rise to product liability claims and negative publicity.
The risk of product liability claims is heightened when we sell products that are intended to support a patient until the end of life. The finite life of our products, as well as complications associated with their use, could give rise to product liability claims whether or not the products have extended or improved the quality of a patient’s life. If we have to pay product liability claims in excess of our insurance coverage, our financial condition will be adversely affected.
Quality problems can result in substantial costs and inventory write-downs.
Government regulations require us to track materials used in the manufacture of our products, so that if a problem is identified in one product it can be traced to other products that may have the same problem. An identified quality problem may require reworking or scrapping related inventory and recalling previous shipments. Because a malfunction in our products can be life-
27
threatening, we may be required to recall and replace, free of charge, products already in the marketplace. Any quality problem could cause us to incur significant expenses, lead to significant write-offs, injure our reputation and harm our business and financial results.
Disruptions of critical information systems or material breaches in the security of our systems could harm our business, customer relations and financial condition.
We rely in part on information technology to store information, interface with customers, maintain financial accuracy, secure our data and accurately produce our financial statements. If our information technology systems do not effectively and securely collect, store, process and report relevant data for the operation of our business, whether due to equipment malfunction or constraints, software deficiencies or human error, our ability to effectively plan, forecast and execute our business plan and comply with applicable laws and regulations would be materially impaired. Any such impairment could have a material adverse effect on our results of operations, financial condition and the timeliness with which we report our operating results.
Our business requires us to use and store customer, vendor, employee and business partner and, in certain instances patient, personally identifiable information. We are subject to various domestic and international privacy and security regulations, including but not limited to HIPAA, which mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent than HIPAA. If we fail to comply with these standards, we could be subject to criminal penalties and civil sanctions.
While we devote significant resources to network security, data encryption and other security measures to protect our systems and data, including our own proprietary information and the confidential and personally identifiable information of our customers, employees, business partners and patients, these measures cannot provide absolute security. The costs to us to eliminate or alleviate network security problems, bugs, viruses, worms, malicious software programs and security vulnerabilities could be significant, and our efforts to address these problems may not be successful, resulting potentially in the theft, loss, destruction or corruption of information we store electronically, as well as unexpected interruptions, delays or cessation of service, any of which could cause harm to our business operations. Moreover, if a computer security breach or cyber-attack affects our systems or results in the unauthorized release of proprietary or personally identifiable information, our reputation could be materially damaged and our operations could be impaired. We would also be exposed to a risk of loss or litigation and potential liability, which could have a material adverse effect on our business, results of operations and financial condition.
If we acquire other companies or businesses, we will be subject to risks that could hurt our business.
We may pursue acquisitions to obtain complementary businesses, products or technologies. Any such acquisition may not produce the revenues, earnings or business synergies that we anticipate and an acquired business, product or technology might not perform as we expect. Our management could spend a significant amount of time, effort and money in identifying, pursuing and completing the acquisition. If we complete an acquisition, we may encounter significant difficulties and incur substantial expenses in integrating the operations and personnel of the acquired company into our operations. In particular, we may lose the services of key employees of the acquired company and we may make changes in management that impair the acquired company’s relationships with employees, vendors and customers. Additionally, we may acquire development-stage companies that are not yet profitable and which require continued investment, which could decrease our future earnings.
Any of these outcomes could prevent us from realizing the anticipated benefits of an acquisition. To pay for an acquisition, we might use stock or cash. Alternatively, we might borrow money from a bank or other lender. If we use stock, our stockholders would experience dilution of their ownership interests. If we use cash or debt financing, our financial liquidity would be reduced.
If we include future milestones as part of the potential purchase price of an acquisition, as we did in connection with our acquisition of ECP in July 2014, then we will have to estimate the value of these milestones each reporting period and any changes underlying these estimates with respect to expected timing or valuation of these milestones could have a volatile impact on our earnings.
Revisions to accounting standards and financial reporting and corporate governance requirements could result in changes to our standard practices and could require a significant expenditure of time, attention and resources, especially by senior management.
We must follow accounting standards and financial reporting and corporate governance requirements and tax laws set by the governing bodies and lawmakers in the U.S. and in other jurisdictions where we do business, as well as NASDAQ. From time to time, these governing bodies and lawmakers implement new and revised rules and laws. These new and revised accounting standards and financial reporting and corporate governance requirements may require changes to our financial statements, the composition of our
28
Board of Directors, the responsibility and manner of operation of various board level committees and the information filed by us with the governing bodies. Our accounting practices that may be affected by changes in the accounting principles are as follows:
| · | accounting for revenue recognition; |
| · | accounting for intangibles—goodwill and other; |
| · | accounting for income taxes; |
| · | accounting for stock-based compensation; |
| · | accounting for leases; and |
| · | accounting for business combinations. |
Implementing changes required by new standards, requirements or laws likely will require a significant expenditure of time, attention and resources. It is impossible to completely predict the impact, if any, on us of future changes to accounting standards and financial reporting and corporate governance requirements.
We use estimates, make judgments and apply certain methods in measuring the progress of our business in determining our financial results and in applying our accounting policies. As these estimates, judgments and methods change, our assessment of the progress of our business and our results of operations could vary.
The methods, estimates and judgments we use in applying our accounting policies have a significant impact on our results of operations. Such methods, estimates and judgments are, by their nature, subject to substantial risks, complexities, uncertainties and assumptions, and factors may arise over time that may lead us to change our methods, estimates and judgments. Changes in any of our assumptions may cause variation in our financial reporting and may adversely affect our reported financial results.
Environmental and health safety laws may result in liabilities, expenses and restrictions on our operations.
Federal, state, local and foreign laws regarding environmental protection, hazardous substances and human health and safety may adversely affect our business. Using hazardous substances in our operations exposes us to the risk of accidental injury, contamination or other liability from the use, storage, importation, handling, or disposal of hazardous materials. If our or our suppliers’ operations result in the contamination of the environment or expose individuals to hazardous substances, we could be liable for damages and fines, and any liability could significantly exceed our insurance coverage and have a material adverse effect on our financial condition. We maintain insurance for certain environmental risks, subject to substantial deductibles; however, we cannot assure you we can continue to maintain this insurance in the future at an acceptable cost or at all. Future changes to environmental and health and safety laws could cause us to incur additional expenses or restrict our operations.
Fluctuations in foreign currency exchange rates could result in declines in our reported sales and results of operations.
Because some of our international sales are denominated in local currencies and not in U.S. dollars, our reported sales and earnings are subject to fluctuations in foreign currency exchange rates, primarily the Euro. At present, we do not hedge our exposure to foreign currency fluctuations. As a result, sales and expenses occurring in the future that are denominated in foreign currencies may be translated into U.S. dollars at less favorable rates, resulting in reduced revenues and earnings.
Risks Related to Our Common Stock
The market price of our common stock is volatile.
The market price of our common stock has fluctuated widely and may continue to do so. For example, from April 1, 2015 to March 31, 2016, the price of our stock ranged from a low of $59.04 per share to a high of $110.68 per share. Many factors could cause the market price of our common stock to rise and fall. Some of these factors are:
| · | variations in our quarterly results of operations; |
| · | status of regulatory approvals for our products; |
| · | introduction of new products by us or our competitors; |
| · | acquisitions or strategic alliances involving us or our competitors; |
29
| · | changes in healthcare policy or third-party reimbursement practices; |
| · | changes in estimates of our performance or recommendations by securities analysts; |
| · | the hiring or departure of key personnel; |
| · | results of clinical trials of our products; |
| · | notice of a recall or other safety issue that impacts the ability for customers to use our products; |
| · | future sales of shares of common stock in the public market; |
| · | the outcome of currently pending litigation and governmental investigations; and |
| · | market conditions in the industry, particularly around reimbursement for our products and the economy as a whole. |
In addition, the stock market in general and the market for shares of medical device companies in particular have experienced extreme price and volume fluctuations in recent years. These fluctuations are often unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the market price of our common stock. When the market price of a company’s stock drops significantly, stockholders often institute securities class action litigation against that company. Any litigation against us could cause us to incur substantial costs, divert the time and attention of our management and other resources, or otherwise harm our business.
The sale of additional shares of our common stock, or the exercise of outstanding options to purchase our common stock, would dilute our stockholders’ ownership interest.
We have issued a substantial number of restricted stock units and options to acquire our common stock and we expect to continue to issue restricted stock units and stock options to our employees and others. If all outstanding stock options were exercised and all outstanding restricted stock units vested, our stockholders would suffer dilution of their ownership interest. In addition, we have issued from time to time, additional shares of our common stock in connection with acquisitions, public offerings, and other activities. Future issuances of our common stock would also result in a dilution of our stockholders’ ownership interest.
The sale of material amounts of common stock could encourage short sales by third parties and depress the price of our common stock. As a result, our stockholders may lose all or part of their investment.
The downward pressure on our stock price caused by the sale of a significant number of shares of our common stock or the perception that such sales could occur by any of our significant stockholders could cause our stock price to decline, thus allowing short sellers of our stock an opportunity to take advantage of any decrease in the value of our stock. The presence of short sellers in our common stock may further depress the price of our common stock.
Our certificate of incorporation and Delaware law could make it more difficult for a third-party to acquire us and may prevent our stockholders from realizing a premium on our stock.
Provisions of our certificate of incorporation and Delaware General Corporation Law may make it more difficult for a third-party to acquire us, even if doing so would allow our stockholders to receive a premium over the prevailing market price of our stock. Those provisions of our certificate of incorporation and Delaware law are intended to encourage potential acquirers to negotiate with us and allow our Board of Directors the opportunity to consider alternative proposals in the interest of maximizing stockholder value. However, such provisions may also discourage acquisition proposals or delay or prevent a change in control which could negatively affect our stock price.
The market value of our common stock could vary significantly based on market perceptions of the status of our development efforts.
The perception of securities analysts regarding our product development efforts could significantly affect our stock price. As a result, the market price of our common stock has and could in the future change substantially when we or our competitors make product announcements. Many factors affecting our stock price are industry related and beyond our control.
30
We have not paid and do not expect to pay dividends and any return on our stockholders’ investment will likely be limited to the value of our common stock.
We have never paid dividends on our common stock and do not anticipate paying dividends on our common stock in the foreseeable future. The payment of dividends on our common stock will depend on our earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on our stockholders’ investment will only occur if our stock price appreciates.
ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
Our headquarters is located at 22 Cherry Hill Drive in Danvers, Massachusetts and consists of approximately 125,560 square feet of space under an operating lease.
The monthly lease payments over the remaining term of the lease are as follows:
| · | $84,914 base rent per month from March 2016 through February 2018; and |
| · | $87,530 base rent per month from March 2018 through February 2021. |
This facility encompasses most of our U.S. operations, including research and development, manufacturing, sales and marketing and general and administrative departments. On December 9, 2015, we entered into a purchase and sale agreement (the “P&S Agreement”) to acquire our existing corporate headquarters space. Pursuant to the P&S Agreement, we expect, among other things and subject to closing conditions, to acquire the real estate commonly known as 18-22 Cherry Hill Drive, located in Danvers, Massachusetts. Subject to the terms and conditions of the P&S Agreement, the purchase price of the property will be $16.5 million. We have entered into two amendments of the P&S Agreement dated January 19, 2016 and April 19, 2016 to extend the due diligence period related to the purchase of the property until July 19, 2016.
Our European headquarters is located in Aachen, Germany and consists of approximately 33,000 square feet of space under an operating lease. In July 2013, we entered into a lease agreement to continue renting our existing space in Aachen, Germany through July 31, 2023. In October 2015, we entered into an amendment to this lease agreement to lease 9,000 square feet of additional space effective July 1, 2015. We also entered into another lease agreement in October 2015 to lease approximately 30,000 square feet of additional space adjacent to our Aachen facility from July 1, 2015 through June 30, 2016. This agreement also provided us with options to extend the lease through July 31, 2033. The lease payments under these agreements are approximately 64,500€ (euro) (approximately U.S. $73,000 at March 31, 2016 exchange rates) per month. The building houses most of the manufacturing operations for the Impella product lines as well as certain research and development functions and the sales, marketing and general and administrative functions for most of our product lines sold in Europe and the Middle East.
We lease a small office in Paris, France, which focuses on the sales and marketing of our product lines sold in France. We also lease a small office in Tokyo, Japan which houses regulatory and training personnel as we prepare for commercial launch in Japan.
We are from time to time involved in various legal actions, the outcomes of which are not within our complete control and may not be known for prolonged periods of time. For a discussion of our material legal proceedings as of March 31, 2016, please see Note 12 to our consolidated financial statements entitled “Commitments and Contingencies,” which is incorporated by reference into this item.
ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
31
PART II
ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Price
Our common stock is traded on the NASDAQ Global Market under the symbol “ABMD.” The following table sets forth the range of high and low sales prices per share of common stock, as reported by the NASDAQ Global Market for our two most recent fiscal years:
| | High | | | Low | |
Fiscal Year Ended March 31, 2016 | | | | | | | | | | |
First Quarter | | $ | | 76.90 | | | $ | | 59.04 | |
Second Quarter | | | | 110.68 | | | | | 64.03 | |
Third Quarter | | | | 99.22 | | | | | 68.25 | |
Fourth Quarter | | | | 95.21 | | | | | 67.81 | |
| | | High | | | | Low | |
Fiscal Year Ended March 31, 2015 | | | | | | | | | | |
First Quarter | | $ | | 26.57 | | | $ | | 20.29 | |
Second Quarter | | | | 26.94 | | | | | 24.19 | |
Third Quarter | | | | 39.02 | | | | | 21.84 | |
Fourth Quarter | | | | 74.70 | | | | | 35.75 | |
Number of Stockholders
As of May 12, 2016, we had approximately 492 holders of record of our common stock and there were approximately 28,160 beneficial holders of our common stock. Many beneficial holders hold their stock through depositories, banks and brokers included as a single holder in the single “street” name of each respective depository, bank, or broker.
Dividends
We have never declared or paid any cash dividends on our common stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We anticipate that we will retain all of our future earnings, if any, to support operations and to finance the growth and development of our business. Our payment of any future dividends will be at the discretion of our board of directors and will depend upon our financial condition, operating results, cash needs and growth plans.
Performance Graph
The following graph compares the yearly change in the cumulative total stockholder return for our last five full fiscal years, based upon the market price of our common stock, with the cumulative total return on a NASDAQ Composite Index (U.S. Companies) and a peer group, the NASDAQ Medical Equipment-SIC Code 3840-3849 Index, which is comprised of medical equipment companies, for that period. The performance graph assumes the investment of $100 on March 31, 2011 in our Common Stock, the NASDAQ Composite Index (U.S. Companies) and the peer group index, and the reinvestment of any and all dividends.
32
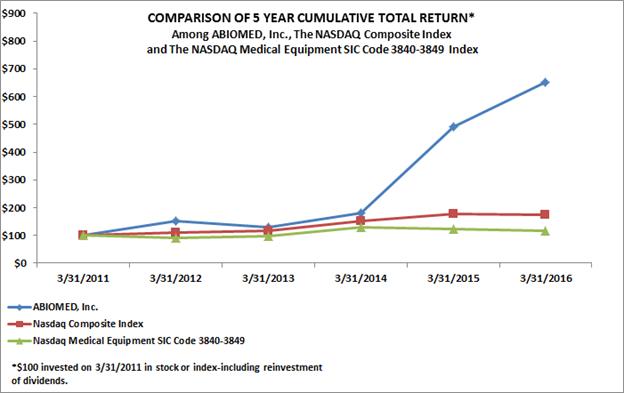
| Cumulative Total Return ($) | |
| 3/31/2011 | | 3/31/2012 | | 3/31/2013 | | 3/31/2014 | | 3/31/2015 | | 3/31/2016 | |
ABIOMED, Inc | | 100 | | | 153 | | | 128 | | | 179 | | | 493 | | | 653 | |
Nasdaq Composite Index | | 100 | | | 111 | | | 117 | | | 151 | | | 176 | | | 175 | |
Nasdaq Medical Equipment SIC Code 3840-3849 | | 100 | | | 90 | | | 99 | | | 131 | | | 123 | | | 115 | |
This graph is not “soliciting material” under Regulation 14A or 14C of the rules promulgated under the Securities Exchange Act of 1934, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any of our filings under the Securities Act of 1933, as amended, or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
Transfer Agent
American Stock Transfer & Trust Company, 59 Maiden Lane, New York, NY 10038, is our stock Transfer Agent.
33
ITEM 6. | SELECTED FINANCIAL DATA |
The financial data included within the tables below should be read in conjunction with our consolidated financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this report and our previously filed Form 10-Ks.
SELECTED CONSOLIDATED FINANCIAL DATA
(In thousands, except per share data)
| | Fiscal Years Ended March 31, | |
| | 2016 | | | 2015 | | | 2014 | | | 2013 | | | 2012 | |
Statement of Operations Data: | (in $000's) | |
Revenue: | | | | | | | | | | | | | | | | | | | | | | | | |
Products | | $ | | 329,520 | | | $ | | 229,950 | | | $ | | 183,280 | | | $ | | 157,614 | | | $ | | 125,286 | |
Funded research and development | | | | 23 | | | | | 361 | | | | | 363 | | | | | 510 | | | | | 1,089 | |
| | | | 329,543 | | | | | 230,311 | | | | | 183,643 | | | | | 158,124 | | | | | 126,375 | |
Costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of product revenue | | | | 50,419 | | | | | 39,945 | | | | | 37,322 | | | | | 31,596 | | | | | 24,507 | |
Research and development | | | | 49,759 | | | | | 35,973 | | | | | 30,707 | | | | | 25,647 | | | | | 27,159 | |
Selling, general and administrative | | | | 164,261 | | | | | 125,727 | | | | | 107,251 | | | | | 84,227 | | | | | 71,711 | |
Amortization of intangible assets | | | | - | | | | | - | | | | | - | | | | | 111 | | | | | 1,478 | |
| | | | 264,439 | | | | | 201,645 | | | | | 175,280 | | | | | 141,581 | | | | | 124,855 | |
Income from operations | | | | 65,104 | | | | | 28,666 | | | | | 8,363 | | | | | 16,543 | | | | | 1,520 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | |
Investment income (expense), net | | | | 395 | | | | | 196 | | | | | 118 | | | | | (7 | ) | | | | (3 | ) |
Gain on settlement of investment | | | | - | | | | | - | | | | | - | | | | | - | | | | | 1,017 | |
Other income (expense), net | | | | 339 | | | | | (97 | ) | | | | 49 | | | | | 326 | | | | | 9 | |
| | | | 734 | | | | | 99 | | | | | 167 | | | | | 319 | | | | | 1,023 | |
Income before income taxes | | | | 65,838 | | | | | 28,765 | | | | | 8,530 | | | | | 16,862 | | | | | 2,543 | |
Income tax provision (benefit) (1) | | | | 27,691 | | | | | (84,923 | ) | | | | 1,179 | | | | | 1,848 | | | | | 1,048 | |
Net income | | $ | | 38,147 | | | $ | | 113,688 | | | $ | | 7,351 | | | $ | | 15,014 | | | $ | | 1,495 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Basic net income per share | | $ | | 0.90 | | | $ | | 2.80 | | | $ | | 0.19 | | | $ | | 0.38 | | | $ | | 0.04 | |
Basic weighted average shares outstanding | | | | 42,204 | | | | | 40,632 | | | | | 39,334 | | | | | 39,113 | | | | | 38,374 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Diluted net income per share | | $ | | 0.85 | | | $ | | 2.65 | | | $ | | 0.18 | | | $ | | 0.37 | | | $ | | 0.04 | |
Diluted weighted average shares outstanding | | | | 44,895 | | | | | 42,858 | | | | | 41,606 | | | | | 41,052 | | | | | 40,172 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents, and short and long term marketable securities | | $ | | 213,053 | | | $ | | 145,954 | | | $ | | 118,340 | | | $ | | 88,113 | | | $ | | 77,223 | |
Working capital (2) | | | | 241,851 | | | | | 145,720 | | | | | 87,555 | | | | | 89,549 | | | | | 88,124 | |
Total assets | | | | 423,931 | | | | | 338,367 | | | | | 205,407 | | | | | 169,999 | | | | | 153,911 | |
Stockholders' equity | | | | 368,775 | | | | | 291,560 | | | | | 168,353 | | | | | 137,080 | | | | | 126,297 | |
| (1) | Income tax benefit for the quarter and year ended March 31, 2015 were impacted by the release of the $101.5 million valuation allowance on certain deferred tax assets. |
| (2) | This reflects a $35.1 million reclassification of current deferred tax assets to long-term deferred tax assets on the March 31, 2015 consolidated balance sheet due to the adoption of ASU No. 2015-17, Income Taxes (Topic 740)—Balance Sheet Classification of Deferred Taxes. This reclassification did not impact working capital at March 31, 2014, 2013 and 2012 due to the full valuation allowance on deferred tax assets for those years. |
34
ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
All statements, trend analysis and other information contained in the following discussion relative to markets for our products and trends in revenue, gross margin and anticipated expense levels, as well as other statements, including words such as “may,” “anticipate,” “believe,” “plan,” “estimate,” “expect,” and “intend” and other similar expressions constitute forward-looking statements. These forward-looking statements are subject to business and economic risks and uncertainties and our actual results of operations may differ materially from those contained in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed under Item 1A Risk Factors as well as other risks and uncertainties referenced in this report.
Overview
We are a leading provider of temporary percutaneous mechanical circulatory support devices and we offer a continuum of care to heart failure patients. We develop, manufacture and market proprietary products that are designed to enable the heart to rest, heal and recover by improving blood flow to the coronary arteries and end-organs and/or temporarily performing the pumping function of the heart. Our products are used in the cardiac catheterization lab, or cath lab, by interventional cardiologists, the electrophysiology lab, the hybrid lab and in the heart surgery suite by heart surgeons. A physician may use our devices for patients who are in need of hemodynamic support prophylactically or emergently before, during or after angioplasty or heart surgery procedures. We believe heart recovery is the optimal clinical outcome for patients experiencing heart failure because it enables patients to go home with their own native heart and restores their quality of life. In addition, we believe that for the care of such patients, heart recovery is the most cost-effective solution for the healthcare system.
Our strategic focus and most of our revenue growth is derived from our family of Impella products. The Impella product portfolio, which includes the Impella 2.5, Impella CP, Impella RP, Impella LD and Impella 5.0, has supported thousands of patients in the U.S. We expect that most of our revenues in the near future will be from our Impella products. Revenues from our non-Impella products, largely focused on the heart surgery suite, have been decreasing over the past several years as we have strategically shifted our sales and marketing efforts towards our Impella products and the cath lab.
Our Impella 2.5, Impella 5.0, Impella LD, Impella CP and Impella RP products also have CE Mark approval and Health Canada approval which allows us to market these devices in the European Union and Canada. We have submitted an application for the Impella 2.5 and Impella 5.0 in Japan and we are hopeful of receiving regulatory approval in the first half of fiscal 2017.
The Impella 2.5 received Pre-Market Approval, or PMA, from the FDA in March 2015 for use during elective and high risk percutaneous coronary intervention, or PCI, procedures. In April 2016, we received a PMA for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD products to provide treatment for ongoing cardiogenic shock. Our Impella RP product was approved by the FDA under a Humanitarian Device Exemption, or HDE, in January 2015, for providing circulatory assistance for up to 14 days in patients who develop acute right heart failure or decompensation after left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery.
In July 2014, we acquired all of the issued shares of ECP, a German limited liability company, for $13.0 million in cash, with additional potential payments up to a maximum of $15.0 million based on the achievement of certain technical, regulatory and commercial milestones. ECP, based in Berlin, Germany, is engaged in research, development, prototyping and the pre-serial production of a percutaneous expandable catheter pump which increases blood circulation from the heart with an external drive shaft.
Summary of Recent Financial Performance
During fiscal 2016, we recognized net income of $38.1 million, or $0.90 per basic share and $0.85 per diluted share, compared to $113.7 million, or $2.80 per basic share and $2.65 per diluted share for the prior fiscal year. Our net income for the year ended March 31, 2015 included an income tax benefit of $84.9 million, primarily due to the release of our valuation allowance on certain of our deferred tax assets in the year ended March 31, 2015. We also had higher Impella product revenue due to greater utilization of our Impella products in the U.S. and Europe in fiscal 2016.
Critical Accounting Policies and Estimates
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States. Preparation of the consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported amounts of revenue and expenses during the reporting period. Actual results could differ from these
35
estimates. The accounting policies we believe are critical in the preparation of our consolidated financial statements relate to revenue recognition, in-process research and development, contingent consideration and income taxes. Our significant accounting policies are more fully described under the heading “Summary of Significant Accounting Policies” in Note 2 to our consolidated financial statements contained elsewhere herein.
Revenue Recognition
We recognize revenue when evidence of an arrangement exists, title has passed (generally upon shipment) or services have been rendered, the selling price is fixed or determinable and collectability is reasonably assured.
Revenue from product sales to customers is recognized when delivery has occurred. All costs related to product sales are recognized at time of delivery. We do not provide for rights of return to customers on our product sales and therefore we do not record a provision for returns.
Maintenance and service support contract revenues are included in product revenue and are recognized ratably over the service contract term. Revenue is recognized as it is earned in limited instances where we rent console medical devices to customers on a month-to-month basis or for a longer specified period of time. Other service revenues are recognized as the services are performed.
In-Process Research and Development
In-process research and development, or IPR&D, assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development projects. IPR&D assets represent the fair value assigned to technologies that we acquire, which at the time of acquisition have not reached technological feasibility and have no alternative future use. During the period that IPR&D assets are considered indefinite-lived, they are tested for impairment on an annual basis, or more frequently if we become aware of any events occurring or changes in circumstances that indicate that the fair value of the IPR&D assets are less than their carrying amounts. If and when development is complete, which generally occurs when we have regulatory approval and are able to commercialize products associated with the IPR&D assets, these assets are then deemed definite-lived and the value of the assets at that time are amortized moving forward based on their estimated useful lives. If development is terminated or abandoned, we may have a full or partial impairment charge related to the IPR&D assets, calculated as the excess of carrying value of the IPR&D assets over fair value.
Contingent Consideration
Contingent consideration is recorded as a liability and measured at fair value using a discounted cash flow model utilizing significant unobservable inputs including the probability of achieving each of the potential milestones and an estimated discount rate associated with the risks of the expected cash flows attributable to the various milestones. Significant increases or decreases in any of the probabilities of success or changes in expected timelines for achievement of any of these milestones could result in a significantly higher or lower fair value of the liability. The fair value of the contingent consideration at each reporting date is updated by reflecting the changes in fair value reflected in our statement of operations. We have a liability of $7.6 million of contingent consideration at March 31, 2016 related to $15.0 million in potential milestone payments, related to the ECP acquisition.
Income Taxes
Our provision for income taxes is composed of a current and a deferred portion. The current income tax provision is calculated as the estimated taxes payable or refundable on tax returns for the current year. The deferred income tax provision is calculated for the estimated future tax effects attributable to temporary differences and net operating loss carryforwards using expected tax rates in effect in the years during which the differences are expected to reverse.
We regularly assess our ability to realize our deferred tax assets. Assessing the realization of deferred tax assets requires significant management judgment. We consider whether a valuation allowance is needed on our deferred tax assets by evaluating all positive and negative evidence relative to our ability to recover deferred tax assets, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies and recent financial results. We determined that there was sufficient positive evidence that most of our federal, state and certain foreign deferred tax assets were more likely than not recoverable as of March 31, 2015. Our conclusion was primarily driven by the receipt of PMA approval for our Impella 2.5 product in March 2015, our history of profits in recent years and our expectation of continuing future profitability. Accordingly, we recorded a $101.5 million reversal of the valuation allowance during the year ended March 31, 2015, primarily related to the Company expecting to be able to use NOL carryforwards in the future in the U.S. and Germany.
36
As of March 31, 2016 and 2015, respectively, the remaining $2.4 million and $2.9 million valuation allowance represents deferred tax assets primarily related to NOL carryforwards in certain foreign jurisdictions. Based on the review of all available evidence, we recorded a valuation allowance to reduce these deferred tax assets to the amount that is more likely than not to be realizable as of March 31, 2016 and 2015.
Recent Accounting Pronouncements
Information regarding recent accounting pronouncements is included in Note 2. “Summary of Significant Accounting Policies” to our consolidated financial statements in this Report.
Results of Operations
The following table sets forth certain consolidated statements of operations data for the periods indicated as a percentage of total revenues:
| Fiscal Years Ended March 31, | | |
| 2016 | | | | 2015 | | | | 2014 | | |
Revenue: | | | | | | | | | | | | | | |
Product revenue | | 100.0 | | % | | | 99.8 | | % | | | 99.8 | | % |
Funded research and development | | - | | | | | 0.2 | | | | | 0.2 | | |
Total revenue | | 100.0 | | | | | 100.0 | | | | | 100.0 | | |
| | | | | | | | | | | | | | |
Costs and expenses as a percentage of total revenue: | | | | | | | | | | | | | | |
Cost of product revenue | | 15.3 | | | | | 17.3 | | | | | 20.3 | | |
Research and development | | 15.1 | | | | | 15.6 | | | | | 16.7 | | |
Selling, general and administrative | | 49.8 | | | | | 54.6 | | | | | 58.4 | | |
Total costs and expenses | | 80.2 | | | | | 87.5 | | | | | 95.4 | | |
Income from operations | | 19.8 | | | | | 12.5 | | | | | 4.6 | | |
Other income and income tax provision (benefit) | | 8.2 | | | | | (36.9 | ) | | | | 0.6 | | |
Net income as a percentage of total revenue | | 11.6 | | % | | | 49.4 | | % | | | 4.0 | | % |
Fiscal Years Ended March 31, 2016 and March 31, 2015 (“fiscal 2016” and “fiscal 2015”)
Revenue
Our revenue is comprised of the following:
| | Fiscal Years Ended March 31, | |
| | | 2016 | | | | | 2015 | |
| | (in $000's) | |
Impella product revenue | $ | | 310,138 | | | $ | | 212,665 | |
Service and other revenue | | | 16,588 | | | | | 13,768 | |
Other products | | | 2,794 | | | | | 3,517 | |
Total product revenue | | | 329,520 | | | | | 229,950 | |
Funded research and development | | | 23 | | | | | 361 | |
Total revenue | $ | | 329,543 | | | $ | | 230,311 | |
Impella product revenue encompasses Impella 2.5, Impella CP, Impella 5.0, Impella LD and Impella RP product sales. Service and other revenue represents revenue earned on service maintenance contracts and preventive maintenance calls. Other product revenue includes AB5000 and product accessory revenue.
Total revenue for fiscal 2016 increased $99.2 million, or 43%, to $329.5 million from $230.3 million for fiscal 2015. The increase in total revenues was primarily due to higher Impella product revenue from increased utilization in the U.S., which was attributable to higher sales use of Impella 2.5 as a result of PMA approval for elective and high risk PCI procedures in March 2015 and higher utilization of Impella CP for those interventional cardiologists who prefer higher blood flow.
37
Impella product revenue for fiscal 2016 increased by $97.4 million, or 46%, to $310.1 million from $212.7 million for fiscal 2015. Most of the increase in Impella product revenue was from Impella CP and Impella 2.5 catheter sales in the U.S., as we focus on increasing utilization of our disposable catheter products through continued investment in our field organization and physician training programs. We also experienced an increase in Impella RP revenue after receiving HDE approval in the U.S. in January 2015. We expect Impella product revenues to continue to increase due to our recent PMA approvals in the U.S. for our Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for ongoing cardiogenic shock, continued utilization for high risk PCI procedures, continue our controlled launch of Impella RP in the U.S. and our expansion efforts in Europe, particularly Germany.
Service and other revenue for fiscal 2016 increased by $2.8 million, or 20%, to $16.6 million from $13.8 million for fiscal 2015. The increase in service revenue was primarily due to an increase in preventative maintenance service contracts. We have expanded the use of our Impella AIC consoles to additional sites and placed more consoles at existing higher using sites. Many customers are entering into maintenance service contracts as these AIC consoles are being delivered.
Other product revenue for fiscal 2016 decreased by $0.7 million, or 20%, to $2.8 million from $3.5 million for fiscal 2015. Most of the decrease was due to lower AB sales in the U.S. We expect that AB5000 revenue will continue to decline in fiscal 2017 as we focus our sales efforts in the surgical suite on Impella 5.0, Impella LD and Impella RP and we focus more of our attention on the cath lab.
Costs and Expenses
Cost of Product Revenue
Cost of product revenue for fiscal 2016 increased by $10.5 million, or 26%, to $50.4 million from $39.9 million for fiscal 2015. Gross margin was 85% for fiscal 2016 and 83% for fiscal 2015. The increase in cost of product revenues was related to increased demand for Impella products and higher production volume and costs to support growing demand for our Impella products. Gross margin has been impacted favorably in fiscal 2016 by higher manufacturing production volume, fewer shipments of Impella AIC consoles, improved efficiencies in manufacturing production and favorable foreign currency impact of lower Euro as much of our manufacturing is performed in Germany.
Research and Development Expenses
Research and development expenses for fiscal 2016 increased by $13.8 million, or 38%, to $49.8 million from $36.0 million for fiscal 2015. The increase in research and development expenses was primarily due to product development initiatives on our existing products and new technologies, increased clinical spending primarily related to our cVAD Registry™ and post approval studies, a focus on quality initiatives for our Impella products and a full year of activities related to our ECP purchase that was completed in July 2014.
We expect research and development to increase for fiscal 2017 as we continue to increase clinical spending related to our cVAD Registry™ and incur additional costs as we continue to focus on engineering initiatives to improve our existing products and develop new technologies.
Selling, General and Administrative Expenses
Selling, general and administrative expenses for fiscal 2016 increased by $38.6 million, or 31%, to $164.3 million from $125.7 million for fiscal 2015. The increase in selling, general and administrative expenses was primarily due to the hiring of additional U.S. field sales and clinical personnel, increased spending on marketing initiatives as we continue to educate physicians on the benefits of hemodynamic support after receiving PMA approval for Impella 2.5, higher stock-based compensation expense and higher professional fees to support the growth of our business.
We expect to continue to increase our expenditures on sales and marketing activities, with particular investments in field sales and clinical personnel with cath lab expertise to drive recovery awareness for acute heart failure patients. We also plan to increase our marketing, service and training investments as a result of PMA approvals in the U.S. for Impella 2.5, Impella CP®, Impella 5.0™and Impella LD™ heart pumps and as we expand to new markets outside of the U.S., such as Japan. We also expect to continue to incur significant legal expenses for the foreseeable future related to the FCA Investigation and patent related matters discussed in “Note 12. Commitments and Contingencies—Litigation,” to our consolidated financial statements. We expect that this increase in selling, general and administrative expense will be offset somewhat by the moratorium of the medical device tax in the U.S. for the next two calendar years beginning in January 2016.
38
Income Tax Provision
We recorded an income tax provision of $27.7 million for fiscal 2016, compared to an income tax benefit of $84.9 million for fiscal 2015. The increase in income tax provision for fiscal 2016 was due to the fact that we had a full valuation allowance on most of our federal, state and certain foreign deferred tax assets prior to March 31, 2015, at which time most of the valuation allowance was reversed. The income tax provision for fiscal 2016 was primarily due to the income before taxes of $65.8 million generated in fiscal 2016, primarily in the U.S. and Germany. The income tax benefit in fiscal 2015 was comprised of an $87.1 million deferred tax benefit primarily due to the release of our valuation allowance on certain of our deferred tax assets in the year ended March 31, 2015, partially offset by a current income tax provision of $2.2 million in U.S and Germany.
Net Income
For fiscal 2016, we recognized net income of $38.1 million, or $0.90 per basic share and $0.85 per diluted share, compared to $113.7 million, or $2.80 per basic share and $2.65 per diluted share for fiscal 2015. Our net income for fiscal 2016 was driven primarily to higher Impella product revenue due to greater utilization of our Impella products in the U.S. and Europe, partially offset by the increase in income tax provision for fiscal 2016 due to the fact that we had a full valuation allowance on most of our deferred tax assets prior to March 31, 2015, at which time most of the valuation allowance was reversed. Our net income for fiscal 2015 included an income tax benefit of $84.9 million, primarily due to the release of our valuation allowance on certain of our deferred tax assets.
Fiscal Years Ended March 31, 2015 and March 31, 2014 (“fiscal 2015” and “fiscal 2014”)
Revenue
Our revenue is comprised of the following:
| | Fiscal Years Ended March 31, | |
| | | 2015 | | | | | 2014 | |
| | (in $000's) | |
Impella product revenue | $ | | 212,665 | | | $ | | 166,971 | |
Service and other revenue | | | 13,768 | | | | | 10,944 | |
Other products | | | 3,517 | | | | | 5,365 | |
Total product revenue | | | 229,950 | | | | | 183,280 | |
Funded research and development | | | 361 | | | | | 363 | |
Total revenue | $ | | 230,311 | | | $ | | 183,643 | |
Impella product revenue encompasses Impella 2.5, Impella CP, Impella 5.0, Impella LD and Impella RP product sales. Service and other revenue represents revenue earned on service maintenance contracts and preventive maintenance calls. Other product revenue includes AB5000 and product accessory revenue.
Total revenue for fiscal 2015 increased by $46.7 million, or 25%, to $230.3 million from $183.6 million for fiscal 2014. The increase in total revenue was primarily due to higher Impella revenue due mainly to greater utilization of our products in the U.S.
Impella product revenue for fiscal 2015 increased by $45.7 million, or 27%, to $212.7 million from $167.0 million for fiscal 2014. Most of our increase in Impella revenue was from disposable catheter sales in the U.S., as we focus on increasing utilization of our disposable catheter products through continued investment in our field organization and physician training programs.
Service and other revenue for fiscal 2015 increased by $2.9 million, or 27%, to $13.8 million from $10.9 million for fiscal 2014. The increase in service revenue was primarily due to an increase in preventative maintenance service contracts, as we expand the use of our Impella AIC consoles to additional sites.
Other product revenue for fiscal 2015 decreased by $1.9 million, or 35%, to $3.5 million from $5.4 million for fiscal 2014. The decrease in other revenue was due to a decline in AB5000 disposable sales. We also had no sales of BVS 5000 in fiscal 2015 and we are no longer actively producing or selling that product.
39
Costs and Expenses
Cost of Product Revenue
Cost of product revenue for fiscal 2015 increased by $2.6 million, or 7%, to $39.9 million from $37.3 million for fiscal 2014. Gross margin was 83% for fiscal 2015 and 80% for fiscal 2014. The increase in cost of product revenues was related to increased Impella demand and higher production volume and costs to support growing demand for our Impella products. Gross margin was impacted favorably by higher manufacturing production volume, fewer shipments of AIC consoles, improved efficiencies in manufacturing production and a favorable Euro in the second half of fiscal 2015
Research and Development Expenses
Research and development expenses for fiscal 2015 increased by $5.3 million, or 17%, to $36.0 million from $30.7 million in fiscal 2014. The increase in research and development expenses was primarily due to operating expenses associated with the ECP business in Berlin that we acquired in July 2014 and a $1.5 million up-front license payment made in April 2014 for optical sensor technology.
Selling, General and Administrative Expenses
Selling, general and administrative expenses for fiscal 2015 increased by $18.4 million, or 17%, to $125.7 million from $107.3 million in fiscal 2014. The increase in selling, general and administrative expenses was primarily due to the hiring of additional U.S. field sales and clinical personnel, increased spending on marketing initiatives as we continue to educate physicians on the benefits of hemodynamic support, higher stock-based compensation expense, higher excise taxes associated with the medical device tax in the U.S. and legal and professional expenses related to the ECP acquisition. These amounts were partially offset by lower legal expenses during fiscal 2015.
Income Tax Provision
We recorded an income tax benefit of $84.9 million in fiscal 2015 compared to an income tax expense of $1.2 million in fiscal 2014. The income tax benefit in fiscal 2015 was comprised of an $87.1 million deferred tax benefit primarily due to the release of our valuation allowance on certain of our deferred tax assets in the quarter ended March 31, 2015, partially offset by a current income tax provision of $2.2 million in U.S and Germany. The income tax provision for fiscal 2014 was comprised of income taxes in Germany and income taxes related to the deferred tax liability associated with tax deductible goodwill.
Net Income
During fiscal 2015, we recognized net income of $113.7 million, or $2.80 per basic share and $2.65 per diluted share, compared to $7.4 million, or $0.19 per basic share and $0.18 per diluted share for fiscal 2014. Our net income for fiscal 2015 included an income tax benefit of $84.9 million, primarily due to the release of our valuation allowance on certain of our deferred tax assets. The increase in net income in fiscal 2015 was also due to higher Impella product revenue in the U.S. and Europe.
Liquidity and Capital Resources
At March 31, 2016, our total cash, cash equivalents, and short and long-term marketable securities totaled $213.1 million, an increase of $67.1 million compared to $146.0 million at March 31, 2015. The increase in our cash, cash equivalents, and short and long-term marketable securities was due primarily to positive cash flows from operations in fiscal 2016.
Following is a summary of our cash flow activities:
| | For the Year Ended March 31, | |
| | 2016 | | | 2015 | | | 2014 | |
Net cash provided by operating activities | | $ | 76,795 | | | $ | 43,290 | | | $ | 23,466 | |
Net cash used for investing activities | | | (57,710 | ) | | | (49,863 | ) | | | (22,272 | ) |
Net cash provided by financing activities | | | 7,160 | | | | 9,523 | | | | 9,632 | |
Effect of exchange rate changes on cash | | | (415 | ) | | | (1,465 | ) | | | 639 | |
Net increase in cash and cash equivalents | | $ | 25,830 | | | $ | 1,485 | | | $ | 11,465 | |
40
Cash Provided by Operating Activities
For the year ended March 31, 2016, cash provided by operating activities consisted of net income of $38.1 million, adjustments for non-cash items of $54.2 million and cash used in working capital of $15.6 million. Our net income for fiscal 2016 was driven primarily to higher Impella product revenue due to greater utilization of our Impella products in the U.S. and Europe, partially offset by the increase in income tax provision for fiscal 2016. Adjustments for non-cash items consisted primarily of $29.1 million of stock-based compensation expense, a $22.3 million change in deferred tax provision and $3.3 million of depreciation and amortization of property, plant and equipment. The decrease in cash from changes in working capital included a $10.9 million increase in accounts receivable associated with our higher revenues, a $11.5 million increase in inventory as we build up our inventory safety stock to support growing demand for our Impella products, a $7.4 million increase in accounts payable and accrued expenses due to increase in operating expenses.
For the year ended March 31, 2015, cash provided by operating activities consisted of net income of $113.7 million, less non-cash items of $65.7 million and cash used for working capital of $4.7 million. The increase in net income in fiscal 2015 included a deferred income tax benefit of $87.1 million primarily due to the release of our valuation allowance on certain of our deferred tax assets and higher Impella product revenue due to greater utilization in the U.S. and Europe. Adjustments for non-cash items primarily consisted of $16.5 million of stock-based compensation expense, $2.8 million of depreciation and amortization of long-lived assets, $2.2 million of write-downs of inventory and a $0.5 million change in fair value of contingent consideration. The decrease in cash from changes in working capital included an $8.0 million increase in accounts receivable from higher revenues, a $7.0 million increase in inventory to support growing demand for our Impella products, and a $1.5 million increase in prepaid expenses. These amounts were partially offset by $9.4 million increase in accounts payable and accrued expenses due to increase in operating expenses and an increase in deferred revenue of $2.3 million primarily due to an increase in preventative maintenance service contracts as we expand the use of our Impella AIC consoles to additional sites.
For the year ended March 31, 2014, cash provided by operating activities consisted of net income of $7.4 million, adjustments for non-cash items of $16.6 million and cash used for working capital of $0.5 million. Adjustments for non-cash items primarily consisted of $11.2 million of stock-based compensation expense, $2.5 million of depreciation and amortization of long-lived assets, $2.0 million of write-downs of inventory and a $0.9 million change in deferred tax provision. The decrease in cash from changes in working capital was primarily due to a $2.4 million increase in accounts receivable and prepaid expenses, partially offset by a $1.9 million decrease in accounts payable and accrued expenses mostly related to timing of vendor payments.
Cash Used in Investing Activities
For the year ended March 31, 2016, net cash used for investing activities included $41.3 million in purchases (net of maturities) of marketable securities and $15.6 million for the purchase of property and equipment mostly related to expansion of manufacturing cleanroom capacity and office space in Danvers, Massachusetts and Aachen, Germany as well as investments in upgrading computer software. We also made a $0.8 million investment in a private medical technology company during fiscal 2016.
For the year ended March 31, 2015 , net cash used for investing activities included $26.1 million for the purchase (net of maturities) of marketable securities, $15.7 million for our acquisition of ECP and AIS, $5.2 million for the purchase of property and equipment mostly related to expansion of manufacturing capacity in Danvers, Massachusetts and Aachen, Germany and $2.9 million of investments in private medical technology companies.
For the year ended March 31, 2014, net cash used for investing activities included $18.8 million for the purchase (net of maturities) of marketable securities, $2.8 million for the purchase of property and equipment mostly related to leasehold improvements in Danvers, Massachusetts and Aachen, Germany and $0.8 million of investments in private medical technology companies.
Capital expenditures for fiscal 2017 are estimated to range from $30 to $35 million, including the $16.5 million purchase price for the expected acquisition of the Company’s corporate headquarters in Danvers, Massachusetts. We are also expecting to have additional capital expenditures for manufacturing capacity expansions in both our Danvers, Massachusetts and Aachen, Germany facilities, additional office space, leasehold improvements and software development projects.
Cash Provided by Financing Activities
For the year ended March 31, 2016, net cash provided by financing activities included $9.8 million in proceeds from the exercise of stock options, $1.1 million in proceeds from the issuance of stock under the employee stock purchase plan and $3.6 million in excess tax benefits on stock-based awards. These amounts were partially offset by $7.3 million in payments in lieu of issuance of common stock for payroll withholding taxes upon vesting of certain equity awards.
41
For the year ended March 31, 2015, net cash provided by financing activities included $10.9 million in proceeds from the exercise of stock options, $0.8 million in proceeds from the issuance of stock under the employee stock purchase plan and $0.6 million in excess tax benefits on stock-based awards. These amounts were partially offset by $2.8 million in payments in lieu of issuance of common stock for payroll withholding taxes upon vesting of certain equity awards.
For the year ended March 31, 2014, net cash provided by financing activities included $9.4 million in proceeds from the exercise of stock options and $0.7 million in proceeds from the issuance of stock under the employee stock purchase plan. These amounts were partially offset by $0.4 million in payments in lieu of issuance of common stock for payroll withholding taxes upon vesting of certain equity awards.
Operating Capital and Liquidity Requirements
We believe that our revenue from product sales together with existing resources will be sufficient to fund our operations for at least the next twelve months, exclusive of activities involving any future acquisitions of products or companies that complement or augment our existing line of products.
Our primary liquidity requirements are to fund the expansion of our commercial infrastructure in the U.S., increase our manufacturing capacity, incur additional capital expenditures as we expand our office space and manufacturing capacity in Danvers and Aachen, increase our inventory levels in order to meet growing customer demand for our Impella products, fund new product development initiatives, prepare for commercial launches of Impella products in new markets in the future, such as Japan, increased clinical spending associated with our cVAD Registry, as well as post approval study on Impella 2.5 related to our PMA approval and the Impella RP post approval study, costs of legal fees related to the FCA Investigation and ongoing patent litigation and to provide for general working capital needs. To date, we have primarily funded our operations principally from product sales and through the sale of equity securities.
Our liquidity is influenced by our ability to sell our products in a competitive industry and our customers’ ability to pay for our products. Factors that may affect liquidity include our ability to penetrate the market for our products, maintain or reduce the length of the selling cycle for our products, capital expenditure requirements, investments in collaborative arrangements with other partners, and our ability to collect cash from customers after our products are sold. We also expect to continue to incur legal expenses for the foreseeable future related to the FCA Investigation and ongoing patent litigation. We continue to review our short-term and long-term cash needs on a regular basis. At March 31, 2016, we had no long-term debt outstanding.
Marketable securities at March 31, 2016 consisted of $164.8 million held in funds that invest in U.S. Treasury and government-backed securities. We are not a party to any interest rate swaps, currency hedges or derivative contracts of any type and we currently have no exposure to commercial paper or auction rate securities markets.
Cash and cash equivalents held by our foreign subsidiaries totaled $4.5 million and $3.6 million at March 31, 2016 and March 31, 2015, respectively. Any operating income earned outside the U.S. is intended to be permanently reinvested in foreign jurisdictions. We do not intend or currently foresee a need to repatriate cash and cash equivalents held by our foreign subsidiaries. If we ever do need to repatriate cash to the U.S., we believe that the potential U.S. tax impact to repatriate these funds would be immaterial.
Contractual Obligations and Commercial Commitments
The following table summarizes our contractual obligations at March 31, 2016 and the effects such obligations are expected to have on our liquidity and cash flows in future periods.
| | Payments Due By Fiscal Year (in $000’s) | |
| | | | | Less than 1 Year | | | 1-3 Years | | | 3-5 Years | | | More than 5 Years | |
Operating lease commitments | | $ | | 12,223 | | | $ | | 2,177 | | | $ | | 4,127 | | | $ | | 3,871 | | | $ | | 2,048 | |
Contractual obligations (1) | | | | 150 | | | | | 75 | | | | | 75 | | | | | — | | | | | — | |
Total obligations | | $ | | 12,373 | | | $ | | 2,252 | | | $ | | 4,202 | | | $ | | 3,871 | | | $ | | 2,048 | |
42
(1) | Contractual obligations represent future cash commitments and expected liabilities under agreements with third parties, primarily for research and development activities, such as clinical trials and material purchases for new product testing. We had no long-term debt or capital leases at March 31, 2016. |
On July 1, 2014, we acquired ECP for $13.0 million in cash, with additional potential payouts totaling $15.0 million based on the achievement of certain technical, regulatory and commercial milestones. These milestone payments may be made, at our option, by a combination of cash or our common stock. The above table does not reflect these milestone payments, which may be payable over the next 20 years as the timing and amount of the milestone payments are indeterminate at this time. The maximum amount of the aggregate milestone payments could be $15.0 million. As of March 31, 2016, the fair value of the contingent payments was approximately $7.6 million and is reflected on our consolidated balance sheet.
Our headquarters is located at 22 Cherry Hill Drive in Danvers, Massachusetts and consists of approximately 125,560 square feet of space under an operating lease.
The monthly lease payments over the remaining term of the lease are as follows:
| · | $84,914 base rent per month from March 2016 through February 2018; and |
| · | $87,530 base rent per month from March 2018 through February 2021. |
This facility encompasses most of our U.S. operations, including research and development, manufacturing, sales and marketing and general and administrative departments. On December 9, 2015, we entered into a purchase and sale agreement (the “P&S Agreement”) to acquire our existing corporate headquarters space. Pursuant to the P&S Agreement, we expect, among other things and subject to closing conditions, to acquire the real estate commonly known as 18-22 Cherry Hill Drive, located in Danvers, Massachusetts. Subject to the terms and conditions of the P&S Agreement, the purchase price of the property will be $16.5 million. We have entered into two amendments of the P&S Agreement dated January 19, 2016 and April 19, 2016 to extend the due diligence period related to the purchase of the property until July 19, 2016.
Our European headquarters is located in Aachen, Germany and consists of approximately 33,000 square feet of space under an operating lease. In July 2013, we entered into a lease agreement to continue renting our existing space in Aachen, Germany through July 31, 2023. In October 2015, we entered into an amendment to this lease agreement to lease 9,000 square feet of additional space effective July 1, 2015. We also entered into another lease agreement in October 2015 to lease approximately 30,000 square feet of additional space adjacent to our Aachen facility from July 1, 2015 through June 30, 2016. This agreement also provided us with options to extend the lease through July 31, 2033. The lease payments under these agreements are approximately 64,500€ (euro) (approximately U.S. $73,000 at March 31, 2016 exchange rates) per month. The building houses most of the manufacturing operations for the Impella product lines as well as certain research and development functions and the sales, marketing and general and administrative functions for most of our product lines sold in Europe and the Middle East.
In April 2014, we entered into an exclusive license agreement for the rights to certain optical sensor technologies in the field of cardio-circulatory assist devices. Pursuant to the terms of the license agreement, we made a $1.5 million upfront payment upon execution of the agreement and agreed to make additional payments of up to $4.5 million upon achievement of specified development milestones. The future milestone payment amounts have not been included in the contractual obligations table above due to the uncertainty related to the successful achievement of these milestones.
In November 2015, we entered into an exclusive license agreement for the rights to certain vascular closure device technologies. We made a $0.5 million upfront payment upon execution of the agreement and a milestone payment of $0.6 million in December 2015 and $0.5 million in April 2016. We could make additional payments of up to $2.3 million upon the achievement of certain development milestones.
We are also party to a license agreement related to certain circulatory care device patents and know-how. Under this agreement, we would be obligated to pay up to $3.0 million in cash or stock, if certain development and regulatory milestones are achieved. The amount has not been included in the contractual obligations table above due to the uncertainty related to the successful achievement of these milestones.
We apply the disclosure provisions of Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Guarantees of Indebtedness of Others, to our agreements that contain guarantee or indemnification clauses. These disclosure provisions require that guarantors disclose certain types of guarantees, even if the likelihood of requiring the guarantor’s performance is remote. The following is a description of arrangements in which we are a guarantor.
43
We enter into agreements with other companies in the ordinary course of business, typically with underwriters, contractors, clinical sites and customers that include indemnification provisions. Under these provisions we generally indemnify and hold harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of our activities. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited. We have never incurred any material costs to defend lawsuits or settle claims related to these indemnification agreements. As a result, the estimated fair value of these agreements is minimal and we have no liabilities recorded for these agreements at March 31, 2016.
In our clinical study agreements, we have agreed to indemnify the participating institutions against losses incurred by them for claims related to any personal injury of subjects taking part in the study to the extent they relate to use of our devices in accordance with the clinical study agreement, the protocol for the device and our instructions. The indemnification provisions contained within our clinical study agreements do not generally include limits on the claims. We have never incurred any material costs related to the indemnification provisions contained in our clinical study agreements.
In many sales transactions, we indemnify customers against possible claims of patent infringement caused by our products. The indemnifications contained within sales contracts usually do not include limits on the claims. We have never incurred any material costs to defend lawsuits or settle patent infringement claims related to sales transactions.
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK |
Primary Market Risk Exposures
Our cash, cash equivalents and short-term marketable securities are subject to interest rate risk and will fall in value if market interest rates increase. Marketable securities at March 31, 2016 consisted of $164.8 million held in funds that invest in U.S. Treasury and government-backed securities. If market interest rates were to increase immediately and uniformly by 10 percent from levels at March 31, 2016, we believe the decline in fair market value of our investment portfolio would be immaterial.
Currency Exchange Rates
We have foreign currency exposure to exchange rate fluctuations and particularly with respect to the euro, British pound sterling and Japanese yen. Therefore, our investment in our subsidiaries is sensitive to fluctuations in currency exchange rates. The effect of a change in currency exchange rates on our net investment in international subsidiaries is reflected in the accumulated other comprehensive income component of stockholders’ equity. If rates of exchange for the euro, British pound and Japanese yen were to have depreciated immediately and uniformly by 10% relative to the U.S. dollar from levels at March 31, 2016, the result would have been a reduction of stockholders’ equity of approximately $6.4 million.
Fair Value of Financial Instruments
Our financial instruments consist primarily of cash and cash equivalents, marketable securities, accounts receivable, accounts payable and accrued expenses. The estimated fair values of the financial instruments have been determined by us using available market information and appropriate valuation techniques. Considerable judgment is required, however, to interpret market data to develop the estimates of fair value. Accordingly, the estimates presented are not necessarily indicative of the amounts that we could realize in a current market exchange. The use of different market assumptions and/or estimation methodologies may have a material effect on the estimated fair value amounts.
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The information required by this item is incorporated by reference from the discussion under the heading Part IV, Item 15 of this Annual Report on Form 10-K.
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
44
ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of March 31, 2016. Based on this evaluation, our principal executive officer and principal financial officer concluded that, as of March 31, 2016, these disclosure controls and procedures were effective to provide reasonable assurance that material information required to be disclosed by us, including our consolidated subsidiaries, in reports that we file or submit under the Exchange Act, is recorded, processed, summarized and reported, within the time periods specified in the Commission rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Evaluation of Changes in Internal Control over Financial Reporting
During the fourth quarter of our fiscal year ended March 31, 2016, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we assessed the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our assessment under the framework in Internal Control—Integrated Framework (2013), our management concluded that our internal control over financial reporting was effective as of March 31, 2016.
Important Considerations
Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
45
Deloitte & Touche LLP, an independent registered public accounting firm that audited our financial statements for the fiscal year ended March 31, 2016, included in this annual report, has issued an attestation report on the effectiveness of our internal control over financial reporting. This report is set forth below:
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
ABIOMED, Inc.
Danvers, Massachusetts
We have audited the internal control over financial reporting of ABIOMED, Inc. and subsidiaries (the “Company”) as of March 31, 2016 based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2016, based on the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the year ended March 31, 2016 of the Company and our report dated May 26, 2016 expressed an unqualified opinion on those financial statements.
|
/s/ DELOITTE & TOUCHE LLP |
|
Boston, Massachusetts |
May 26, 2016 |
46
ITEM 9B. | OTHER INFORMATION |
Not applicable.
47
PART III
ITEM 10. | DIRECTOR, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by Item 10 is incorporated by reference from our definitive proxy statement which will be filed no later than 120 days after the close of the fiscal year covered by this Annual Report on Form 10-K.
We have a Code of Conduct and Compliance Policy that applies to all of its directors, officers, and employees. A paper copy of this document may be obtained free of charge by writing to the Company’s Chief Compliance Officer at the Company’s corporate headquarters located at 22 Cherry Hill Drive, Danvers, Massachusetts 01923, or by email at IR@abiomed.com.
ITEM 11. | EXECUTIVE COMPENSATION |
The information required by Item 11 is incorporated by reference from our definitive proxy statement which will be filed no later than 120 days after the close of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCK HOLDER MATTERS |
The information required by Item 12 is incorporated by reference from our definitive proxy statement which will be filed no later than 120 days after the close of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by Item 13 is incorporated by reference from our definitive proxy statement which will be filed no later than 120 days after the close of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by Item 14 is incorporated by reference from our definitive proxy statement which will be filed no later than 120 days after the close of the fiscal year covered by this Annual Report on Form 10-K.
48
PART IV
ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are filed as part of this report:
(1)The financial statements from our Annual Report for our fiscal year ending March 31, 2016 are attached hereto.
(2) Consolidated financial statement schedule
Information is contained within Note 5. “Accounts Receivable” to our consolidated financial statements in this Report.
(3) Exhibits
EXHIBIT INDEX
Exhibit No. | | Description | | Filed with this Form 10-K | | Incorporated by Reference |
| | | Form | | Filing Date | | Exhibit No. |
| | | | | | | | | | |
2.1 | | Share Purchase Agreement for the acquisition of Impella Cardio Systems AG, dated April 26, 2005. | | | | 8-K (File No. 001-09585) | | May 16, 2005 | | 2.1 |
| | | | | | | | | | |
2.2 | | Agreement on the Sale and Transfer of all shares in ECP Entwicklungsgellschaft mbH | | | | 8-K (File No. 001-09585) | | July 7, 2014 | | 2.1 |
| | | | | | | | | | |
2.3 | | Agreement on the Sale and Transfer of all shares in AIS GmbH Aachen Innovative Solutions | | | | 8-K (File No. 001-09585) | | July 7, 2014 | | 2.2 |
| | | | | | | | | | |
3.1 | | Restated Certificate of Incorporation. | | | | S-3 | | September 29, 1997 | | 3.1 |
3.2 | | Restated By-Laws, as amended. | | | | 10-K (File No. 001-09585) | | May 27, 2004 | | 3.2 |
| | | | | | | | | | |
3.3* | | Certificate of Designations of Series A Junior Participating Preferred Stock—filed as Exhibit 3.3 to the 1997 Registration Statement. | | | | S-3 | | September 29, 1997 | | 3.3 |
| | | | | | | | | | |
3.4 | | Amendment to the Company’s Restated Certificate of Incorporation to increase the authorized shares of common stock from 25,000,000 to 100,000,000. | | | | 8-K (File No. 001-09585) | | March 21, 2007 | | 3.4 |
| | | | | | | | | | |
4.1 | | Specimen Certificate of common stock. | | | | S-1 | | June 5, 1987 | | 4.1 |
10.1* | | Form of Indemnification Agreement for Directors and Officers. | | | | S-1 | | June 5, 1987 | | 10.13 |
| | | | | | | | | | |
10.2* | | Amendment to 1992 Combination Stock Option Plan. | | | | 10-Q (File No. 001-09585) | | October 14, 1997 | | 10.2 |
| | | | | | | | | | |
10.3* | | 1988 Employee Stock Purchase Plan, as amended. | | | | 10-Q (File No. 001-09585) | | February 8, 2005 | | 10.11 |
49
Exhibit No. | | Description | | Filed with this Form 10-K | | Incorporated by Reference |
| | | Form | | Filing Date | | Exhibit No. |
| | | | | | | | | | |
10.4* | | 1989 Non-Qualified Stock Option Plan for Non-Employee Directors. | | | | 10-Q (File No. 001-09585) | | October 27, 1995 | | 10.1 |
| | | | | | | | | | |
10.5* | | 1998 Equity Incentive Plan. | | | | 10-Q/A (File No. 001-09585) | | January 8, 1999 | | 10 |
| | | | | | | | | | |
10.6* | | 2000 Stock Incentive Plan Agreement, as amended. | | | | Sch. 14A (File No. 001-09585) | | July 15, 2005 | | Appendix A |
| | | | | | | | | | |
10.7* | | Form of Abiomed, Inc. Non-Statutory Stock Option Agreement for the 2000 Stock Incentive Plan for Directors. | | | | 10-Q (File No. 001-09585) | | February 9, 2006 | | 10.16 |
| | | | | | | | | | |
10.8* | | Form of Abiomed, Inc. Non-Statutory Stock Option Agreement for the 2000 Stock Incentive Plan for Employees or Consultants. | | | | 10-Q (File No. 001-09585) | | February 9, 2006 | | 10.17 |
| | | | | | | | | | |
10.9* | | Fourth Amended and Restated 2008 Stock Incentive Plan. | | | | 10-K (File No. 001-09585) | | May 28, 2015 | | 10.29 |
| | | | | | | | | | |
10.10* | | Form of Non-Statutory Stock Option Agreement for Employees and Consultants under 2008 Stock Incentive Plan. | | | | 8-K (File No. 001-09585) | | August 18, 2008 | | 10.1 |
| | | | | | | | | | |
10.11* | | Form of Non-Statutory Stock Option Agreement for Non-Employee Directors under 2008 Stock Incentive Plan. | | | | 8-K (File No. 001-09585) | | August 18, 2008 | | 10.2 |
| | | | | | | | | | |
10.12* | | Form of Restricted Stock Agreement under 2008 Stock Incentive Plan. | | | | 8-K (File No. 001-09585) | | August 18, 2008 | | 10.3 |
| | | | | | | | | | |
10.13* | | 2015 Omnibus Incentive Plan. | | | | Sch. 14A (File No. 001-09585) | | July 2, 2015 | | Appendix A |
10.14* | | Form of TSR Award (Performance and Time-Based RSU). | | | | 10-Q (File No. 001-09585) | | August 6, 2016 | | 10.4 |
10.15* | | Form of Non-Employee Director Time-Based RSU Agreement under the 2015 Omnibus Incentive Plan. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.4 |
| | | | | | | | | | |
10.16* | | Form of Employee Time-Based RSU Agreement under the 2015 Omnibus Incentive Plan. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.3 |
10.17* | | Form of Non-Employee Director Time-Based RSU Agreement under the 2015 Omnibus Incentive Plan. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.4 |
10.18* | | Form of Performance-Based RSU Agreement under the 2015 Omnibus Incentive Plan. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.5 |
10.19* | | Form of Employee Time-Based Option Agreement under the 2015 Omnibus Incentive Plan. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.6 |
10.20* | | Form of Non-Employee Director Time-Based Option Agreement. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.7 |
| | | | | | | | | | |
50
Exhibit No. | | Description | | Filed with this Form 10-K | | Incorporated by Reference |
| | | Form | | Filing Date | | Exhibit No. |
10.21* | | Employment Agreement of Michael R. Minogue dated April 5, 2004 (including Change in Control Agreement). | | | | 10-Q (File No. 001-09585) | | August 9, 2004 | | 10.10 |
| | | | | | | | | | |
10.22* | | Amendment to Employment Agreement with Michael R. Minogue dated December 31, 2008. | | | | 10-Q (File No. 001-09585) | | February 9, 2009 | | 10.1 |
| | | | | | | | | | |
10.23* | | Amendment to Change in Control Agreement with Michael R. Minogue dated December 31, 2008. | | | | 10-Q (File No. 001-09585) | | February 9, 2009 | | 10.1 |
| | | | | | | | | | |
10.24* | | Inducement stock option granted to Michael R. Minogue dated April 5, 2004. | | | | 10-Q (File No. 001-09585) | | August 9, 2004 | | 10.11 |
| | | | | | | | | | |
10.25* | | Restricted Stock Agreement between Abiomed, Inc. and Michael R. Minogue. | | | | 10-Q (File No. 001-09585) | | October 9, 2005 | | 10.15 |
| | | | | | | | | | |
10.26* | | Offer Letter with Robert L. Bowen dated December 15, 2008. | | | | 8-K (File No. 001-09585) | | December 22, 2008 | | 99.2 |
| | | | | | | | | | |
10.27* | | Offer letter with David Weber dated April 23, 2007. | | | | 10-Q (File No. 001-09585) | | August 9, 2007 | | 10.1 |
| | | | | | | | | | |
10.28* | | Offer letter with Michael J. Tomsicek dated May 26, 2015. | | | | 10-Q (File No. 001-09585) | | August 6, 2015 | | 10.2 |
| | | | | | | | | | |
10.29* | | Retirement Agreement with Robert L. Bowen dated May 26, 2015. | | | | 10-Q (File No. 001-09585) | | August 6, 2015 | | 10.3 |
| | | | | | | | | | |
10.30* | | Summary of Executive Compensation. | | X | | | | | | |
| | | | | | | | | | |
10.31* | | Form of Employment, Nondisclosure and Non- Competition Agreement. | | | | 10-K (File No. 001-09585) | | June 14, 2006 | | 10.20 |
| | | | | | | | | | |
10.32 | | Lease agreement dated July 29, 2013 for the facility located in Aachen, Germany. | | | | 10-Q (File No. 001-09585) | | November 8, 2013 | | 10.1 |
| | | | | | | | | | |
10.33 | | Lease agreement for additional commercial space dated October 19, 2015 for the facility located in Aachen, Germany. | | | | 10-Q (File No. 001-09585) | | November 4, 2015 | | 10.1 |
| | | | | | | | | | |
10.34 | | Supplemental contract no. 1 dated October 19, 2015, to the lease agreement dated July 29, 2013 for the facility located in Aachen, Germany. | | | | 10-Q (File No. 001-09585) | | November 4, 2015 | | 10.2 |
| | | | | | | | | | |
10.34 | | Amended and Restated Lease dated as of February 24, 2014 between Abiomed, Inc. and Leo C. Thibeault, Jr., Trustee of The Thibeault Nominee Trust. | | | | 10-K (File No. 001-09585) | | May 28, 2014 | | 10.27 |
| | | | | | | | | | |
10.36 | | Amended Lease dated as of April 30, 2015 between Abiomed, Inc. and Leo C. Thibeault, Jr., Trustee of The Thibeault Nominee Trust. | | | | 10-K (File No. 001-09585) | | May 28, 2015 | | 10.29 |
| | | | | | | | | | |
10.37* | | Form of Change of Control Agreement. | | | | 8-K (File No. 001-09585) | | August 18, 2008 | | 10.4 |
| | | | | | | | | | |
10.38 | | Purchase and Sale Agreement dated as of December 9, 2015 between Abiomed, Inc. and Thibeault Nominee Trust. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.1 |
| | | | | | | | | | |
51
Exhibit No. | | Description | | Filed with this Form 10-K | | Incorporated by Reference |
| | | Form | | Filing Date | | Exhibit No. |
10.39 | | First Amendment to Purchase and Sale Agreement dated as of January 19, 2016 between Abiomed, Inc. and Thibeault Nominee Trust. | | | | 10-Q (File No. 001-09585) | | February 5, 2016 | | 10.2 |
10.40 | | Amended Lease dated as of March 23, 2016 between Abiomed, Inc. and Leo C. Thibeault, Jr., Trustee of The Thibeault Nominee Trust. | | X | | | | | | |
10.41 | | Second Amendment to Purchase and Sale Agreement dated as of April 19, 2016 between Abiomed, Inc. and Thibeault Nominee Trust. | | X | | | | | | |
| | | | | | | | | | |
11.1 | | Statement regarding computation of Per Share Earnings (see Note 2, Notes to Consolidated Financial Statements). | | X | | | | | | |
| | | | | | | | | | |
21.1 | | Subsidiaries of the Registrant. | | X | | | | | | |
| | | | | | | | | | |
23.1 | | Consent of Deloitte & Touche LLP, independent registered public accounting firm. | | X | | | | | | |
| | | | | | | | | | |
31.1 | | Rule 13a—14(a)/15d—14(a) certification of principal executive officer. | | X | | | | | | |
| | | | | | | | | | |
31.2 | | Rule 13a—14(a)/15d—14(a) certification of principal accounting officer. | | X | | | | | | |
| | | | | | | | | | |
32.1 | | Section 1350 certification. | | X | | | | | | |
| | | | | | | | | | |
101 | | The following financial information from the ABIOMED, Inc. Annual Report on Form 10-K for the fiscal year ended March 31, 2016, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets as of March 31, 2016 and 2015; (ii) Consolidated Statements of Operations for the fiscal years ended March 31, 2016, 2015 and 2014; (iii) Consolidated Statements of Comprehensive Income for the fiscal years ended March 31, 2016, 2015 and 2014; (iv) Consolidated Statements of Stockholders’ Equity for the fiscal years ended March 2016, 2015 and 2014; (v) Consolidated Statements of Cash Flows for the fiscal years ended March 31, 2016, 2015 and 2014; and (vi) Notes to Consolidated Financial Statements. | | X | | | | | | |
* | Management contract or compensatory plan. |
52
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | ABIOMED, Inc. |
| | | | |
Dated: May 26, 2016 | | By | | /s/ MICHAEL J. TOMSICEK |
| | | | Michael J. Tomsicek |
| | | | Vice President, Chief Financial Officer |
| | | | (Principal Financial Officer and |
| | | | Principal Accounting Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
SIGNATURE | | TITLE | | DATE |
| | | | |
/S/ MICHAEL R. MINOGUE | | President, Chief Executive Officer, President and Chairman | | May 26, 2016 |
Michael R. Minogue | | (Principal Executive Officer) | | |
| | | | |
/S/ MICHAEL J. TOMSICEK | | Vice President, | | May 26, 2016 |
Michael J. Tomsicek | | Chief Financial Officer (Principal Financial | | |
| | Officer and Principal Accounting Officer) | | |
| | | | |
/S/ W. GERALD AUSTEN | | Director | | May 26, 2016 |
W. Gerald Austen | | | | |
| | | | |
/S/ DOROTHY E. PUHY | | Director | | May 26, 2016 |
Dorothy E. Puhy | | | | |
| | | | |
/S/ JEANNINE M. RIVET | | Director | | May 26, 2016 |
Jeannine M. Rivet | | | | |
| | | | |
/S/ ERIC ROSE | | Director | | May 26, 2016 |
Eric Rose | | | | |
| | | | |
/S/ MARTIN P. SUTTER | | Director | | May 26, 2016 |
Martin P. Sutter | | | | |
| | | | |
/S/ HENRI A. TERMEER | | Director | | May 26, 2016 |
Henri A. Termeer | | | | |
| | | | |
/S/ PAUL THOMAS | | Director | | May 26, 2016 |
Paul Thomas | | | | |
| | | | |
/S/ CHRIS VAN GORDER | | Director | | May 26, 2016 |
Chris Van Gorder | | | | |
53
ABIOMED, INC.
Consolidated Financial Statements
Index
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
ABIOMED, Inc.
Danvers, Massachusetts
We have audited the accompanying consolidated balance sheets of ABIOMED, Inc. and subsidiaries (the “Company”) as of March 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended March 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of ABIOMED, Inc. and subsidiaries as of March 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended March 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of March 31, 2016, based on the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated May 26, 2016 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ DELOITTE & TOUCHE LLP |
|
Boston, Massachusetts |
May 26, 2016 |
F-2
ABIOMED, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
(in thousands, except share data)
| | March 31, 2016 | | | March 31, 2015 | |
| | | | | | | | |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 48,231 | | | $ | 22,401 | |
Short-term marketable securities | | | 163,822 | | | | 109,557 | |
Accounts receivable, net | | | 42,821 | | | | 31,828 | |
Inventories | | | 26,740 | | | | 16,774 | |
Prepaid expenses and other current assets | | | 6,778 | | | | 4,479 | |
Total current assets | | | 288,392 | | | | 185,039 | |
Long-term marketable securities | | | 1,000 | | | | 13,996 | |
Property and equipment, net | | | 23,184 | | | | 9,127 | |
Goodwill | | | 33,003 | | | | 31,534 | |
In-process research and development | | | 15,396 | | | | 14,711 | |
Long-term deferred tax assets, net | | | 58,534 | | | | 80,306 | |
Other assets | | | 4,422 | | | | 3,654 | |
Total assets | | $ | 423,931 | | | $ | 338,367 | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 9,381 | | | $ | 10,389 | |
Accrued expenses | | | 28,382 | | | | 21,894 | |
Deferred revenue | | | 8,778 | | | | 7,036 | |
Total current liabilities | | | 46,541 | | | | 39,319 | |
Other long-term liabilities | | | 220 | | | | 183 | |
Contingent consideration | | | 7,563 | | | | 6,510 | |
Long-term deferred tax liabilities | | | 832 | | | | 795 | |
Total liabilities | | | 55,156 | | | | 46,807 | |
Commitments and contingencies (Note 12) | | | | | | | | |
Stockholders' equity: | | | | | | | | |
Class B Preferred Stock, $.01 par value | | | — | | | | — | |
Authorized - 1,000,000 shares; Issued and outstanding - none | | | | | | | | |
Common stock, $.01 par value | | | 426 | | | | 413 | |
Authorized - 100,000,000 shares; Issued - 43,973,119 shares at March 31, 2016 and 42,618,717 shares at March 31, 2015; | | | | | | | | |
Outstanding - 42,596,228 shares at March 31, 2016 and 41,335,773 shares at March 31, 2015 | | | | | | | | |
Additional paid in capital | | | 508,624 | | | | 465,046 | |
Accumulated deficit | | | (99,075 | ) | | | (137,222 | ) |
Treasury stock at cost - 1,376,891 shares at March 31, 2016 and 1,282,944 shares at March 31, 2015 | | | (26,660 | ) | | | (19,347 | ) |
Accumulated other comprehensive loss | | | (14,540 | ) | | | (17,330 | ) |
Total stockholders' equity | | | 368,775 | | | | 291,560 | |
Total liabilities and stockholders' equity | | $ | 423,931 | | | $ | 338,367 | |
The accompanying notes are an integral part of the consolidated financial statements.
F-3
ABIOMED, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
(in thousands, except per share data)
| Fiscal Years Ended March 31, | |
| 2016 | | | 2015 | | | 2014 | |
Revenue: | | | | | | | | | | | | | | |
Product revenue | $ | | 329,520 | | | $ | | 229,950 | | | $ | | 183,280 | |
Funded research and development | | | 23 | | | | | 361 | | | | | 363 | |
| | | 329,543 | | | | | 230,311 | | | | | 183,643 | |
Costs and expenses: | | | | | | | | | | | | | | |
Cost of product revenue | | | 50,419 | | | | | 39,945 | | | | | 37,322 | |
Research and development | | | 49,759 | | | | | 35,973 | | | | | 30,707 | |
Selling, general and administrative | | | 164,261 | | | | | 125,727 | | | | | 107,251 | |
| | | 264,439 | | | | | 201,645 | | | | | 175,280 | |
Income from operations | | | 65,104 | | | | | 28,666 | | | | | 8,363 | |
Other income: | | | | | | | | | | | | | | |
Investment income, net | | | 395 | | | | | 196 | | | | | 118 | |
Other income (expense), net | | | 339 | | | | | (97 | ) | | | | 49 | |
| | | 734 | | | | | 99 | | | | | 167 | |
Income before income taxes | | | 65,838 | | | | | 28,765 | | | | | 8,530 | |
Income tax provision (benefit) | | | 27,691 | | | | | (84,923 | ) | | | | 1,179 | |
Net income | $ | | 38,147 | | | $ | | 113,688 | | | $ | | 7,351 | |
| | | | | | | | | | | | | | |
Basic net income per share | $ | | 0.90 | | | $ | | 2.80 | | | $ | | 0.19 | |
Basic weighted average shares outstanding | | | 42,204 | | | | | 40,632 | | | | | 39,334 | |
| | | | | | | | | | | | | | |
Diluted net income per share | $ | | 0.85 | | | $ | | 2.65 | | | $ | | 0.18 | |
Diluted weighted average shares outstanding | | | 44,895 | | | | | 42,858 | | | | | 41,606 | |
The accompanying notes are an integral part of the consolidated financial statements.
F-4
ABIOMED, INC. AND SUBSIDIARIES
Consolidated Statements of Comprehensive Income
(in thousands)
| | Fiscal Years Ended March 31, | |
| | 2016 | | | 2015 | | | 2014 | |
Net income | | $ | 38,147 | | | $ | 113,688 | | | $ | 7,351 | |
| | | | | | | | | | | | |
Other comprehensive income (loss): | | | | | | | | | | | | |
Foreign currency translation gains (losses) | | | 2,724 | | | | (16,613 | ) | | | 3,025 | |
Net unrealized gain (losses) on marketable securities | | | 66 | | | | 13 | | | | (18 | ) |
Other comprehensive income (loss) | | | 2,790 | | | | (16,600 | ) | | | 3,007 | |
| | | | | | | | | | | | |
Comprehensive income | | $ | 40,937 | | | $ | 97,088 | | | $ | 10,358 | |
The accompanying notes are an integral part of the consolidated financial statements.
F-5
ABIOMED, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders’ Equity
(dollars in thousands)
| | Common Stock | | | Treasury Stock | | | | | | | | | | | | | | | | | |
| | Number of shares | | | Par value | | | Number of shares | | | Amount | | | Additional Paid in Capital | | | Accumulated Deficit | | | Accumulated Other Comprehensive Income (Loss) | | | Total Stockholders' Equity | |
Balance, April 1, 2013 | | | 38,601,384 | | $ | | 397 | | | | 1,186,999 | | $ | | (16,129 | ) | $ | | 414,810 | | $ | | (258,261 | ) | $ | | (3,737 | ) | $ | | 137,080 | |
Restricted stock issued | | | 254,991 | | | | 3 | | | | - | | | | - | | | | (3 | ) | | | - | | | | - | | | | - | |
Stock options exercised | | | 1,029,024 | | | | 11 | | | | - | | | | - | | | | 9,349 | | | | - | | | | - | | | | 9,360 | |
Stock issued under employee stock purchase plan | | | 43,779 | | | | - | | | | - | | | | - | | | | 697 | | | | - | | | | - | | | | 697 | |
Stock issued to directors | | | 6,518 | | | | - | | | | - | | | | - | | | | 65 | | | | - | | | | - | | | | 65 | |
Return of common stock to pay withholding taxes on restricted stock | | | (19,368 | ) | | | - | | | | 19,368 | | | | (425 | ) | | | - | | | | - | | | | - | | | | (425 | ) |
Stock compensation expense | | | - | | | | - | | | | - | | | | - | | | | 11,218 | | | | - | | | | - | | | | 11,218 | |
Other comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 3,007 | | | | 3,007 | |
Net income | | | - | | | | - | | | | - | | | | - | | | | - | | | | 7,351 | | | | - | | | | 7,351 | |
Balance, March 31, 2014 | | | 39,916,328 | | $ | | 411 | | | | 1,206,367 | | $ | | (16,554 | ) | $ | | 436,136 | | $ | | (250,910 | ) | $ | | (730 | ) | $ | | 168,353 | |
Restricted stock issued | | | 543,420 | | | | 5 | | | | - | | | | - | | | | (5 | ) | | | - | | | | - | | | | - | |
Stock options exercised | | | 911,553 | | | | 9 | | | | - | | | | - | | | | 10,918 | | | | - | | | | - | | | | 10,927 | |
Stock issued under employee stock purchase plan | | | 39,095 | | | | - | | | | - | | | | - | | | | 795 | | | | - | | | | - | | | | 795 | |
Stock issued to directors | | | 1,954 | | | | - | | | | - | | | | - | | | | 76 | | | | - | | | | - | | | | 76 | |
Return of common stock to pay withholding taxes on restricted stock | | | (76,577 | ) | | | (12 | ) | | | 76,577 | | | | (2,793 | ) | | | - | | | | - | | | | - | | | | (2,805 | ) |
Stock compensation expense | | | - | | | | - | | | | - | | | | - | | | | 16,520 | | | | - | | | | - | | | | 16,520 | |
Excess tax benefit from stock-based awards | | | - | | | | - | | | | - | | | | - | | | | 606 | | | | - | | | | - | | | | 606 | |
Other comprehensive loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (16,600 | ) | | | (16,600 | ) |
Net income | | | - | | | | - | | | | - | | | | - | | | | - | | | | 113,688 | | | | - | | | | 113,688 | |
Balance, March 31, 2015 | | | 41,335,773 | | $ | | 413 | | | | 1,282,944 | | $ | | (19,347 | ) | $ | | 465,046 | | $ | | (137,222 | ) | $ | | (17,330 | ) | $ | | 291,560 | |
Restricted stock issued | | | 507,471 | | | | 5 | | | | - | | | | - | | | | (5 | ) | | | - | | | | - | | | | - | |
Stock options exercised | | | 829,385 | | | | 8 | | | | - | | | | - | | | | 9,763 | | | | - | | | | - | | | | 9,771 | |
Stock issued under employee stock purchase plan | | | 16,772 | | | | - | | | | - | | | | - | | | | 1,135 | | | | - | | | | - | | | | 1,135 | |
Stock issued to directors | | | 774 | | | | - | | | | - | | | | - | | | | 65 | | | | - | | | | - | | | | 65 | |
Return of common stock to pay withholding taxes on restricted stock | | | (93,947 | ) | | | - | | | | 93,947 | | | | (7,313 | ) | | | - | | | | - | | | | - | | | | (7,313 | ) |
Stock compensation expense | | | - | | | | - | | | | - | | | | - | | | | 29,053 | | | | - | | | | - | | | | 29,053 | |
Excess tax benefit from stock-based awards | | | - | | | | - | | | | - | | | | - | | | | 3,567 | | | | - | | | | - | | | | 3,567 | |
Other comprehensive income | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 2,790 | | | | 2,790 | |
Net income | | | - | | | | - | | | | - | | | | - | | | | - | | | | 38,147 | | | | - | | | | 38,147 | |
Balance, March 31, 2016 | | | 42,596,228 | | $ | | 426 | | | | 1,376,891 | | $ | | (26,660 | ) | $ | | 508,624 | | $ | | (99,075 | ) | $ | | (14,540 | ) | $ | | 368,775 | |
The accompanying notes are an integral part of the consolidated financial statements.
F-6
ABIOMED, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
(in thousands)
| | Fiscal Years Ended March 31, | |
| | 2016 | | | 2015 | | | 2014 | |
Operating activities: | | | | | | | | | | | | |
Net income | | $ | 38,147 | | | $ | 113,688 | | | $ | 7,351 | |
Adjustments required to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 3,277 | | | | 2,770 | | | | 2,508 | |
Bad debt expense | | | 42 | | | | (5 | ) | | | 47 | |
Stock-based compensation | | | 29,053 | | | | 16,520 | | | | 11,218 | |
Write-down of inventory | | | 2,094 | | | | 2,231 | | | | 2,012 | |
Excess tax benefit from stock-based awards | | | (3,567 | ) | | | (606 | ) | | | — | |
Deferred tax provision (benefit) | | | 22,296 | | | | (87,094 | ) | | | 860 | |
Change in fair value of contingent consideration | | | 1,053 | | | | 510 | | | | — | |
Changes in assets and liabilities: | | | | | | | | | | | | |
Accounts receivable | | | (10,930 | ) | | | (7,970 | ) | | | (1,312 | ) |
Inventories | | | (11,473 | ) | | | (6,967 | ) | | | (622 | ) |
Prepaid expenses and other assets | | | (2,290 | ) | | | (1,479 | ) | | | (1,039 | ) |
Accounts payable | | | (2,645 | ) | | | 3,372 | | | | (54 | ) |
Accrued expenses and other liabilities | | | 10,020 | | | | 6,011 | | | | 1,938 | |
Deferred revenue | | | 1,718 | | | | 2,309 | | | | 559 | |
Net cash provided by operating activities | | | 76,795 | | | | 43,290 | | | | 23,466 | |
Investing activities: | | | | | | | | | | | | |
Purchases of marketable securities | | | (260,975 | ) | | | (97,658 | ) | | | (87,026 | ) |
Proceeds from the sale and maturity of marketable securities | | | 219,639 | | | | 71,530 | | | | 68,265 | |
Acquisition of ECP and AIS, net of cash assumed | | | — | | | | (15,697 | ) | | | — | |
Purchase of other investment | | | (750 | ) | | | (2,850 | ) | | | (750 | ) |
Purchases of property and equipment | | | (15,624 | ) | | | (5,188 | ) | | | (2,761 | ) |
Net cash used for investing activities | | | (57,710 | ) | | | (49,863 | ) | | | (22,272 | ) |
Financing activities: | | | | | | | | | | | | |
Proceeds from the exercise of stock options | | | 9,771 | | | | 10,927 | | | | 9,360 | |
Excess tax benefit from stock-based awards | | | 3,567 | | | | 606 | | | | — | |
Taxes paid related to net share settlement upon vesting of stock awards | | | (7,313 | ) | | | (2,805 | ) | | | (425 | ) |
Proceeds from the issuance of stock under employee stock purchase plan | | | 1,135 | | | | 795 | | | | 697 | |
Net cash provided by financing activities | | | 7,160 | | | | 9,523 | | | | 9,632 | |
Effect of exchange rate changes on cash | | | (415 | ) | | | (1,465 | ) | | | 639 | |
Net increase in cash and cash equivalents | | | 25,830 | | | | 1,485 | | | | 11,465 | |
Cash and cash equivalents at beginning of year | | | 22,401 | | | | 20,916 | | | | 9,451 | |
Cash and cash equivalents at end of year | | $ | 48,231 | | | $ | 22,401 | | | $ | 20,916 | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
Cash paid for income taxes | | $ | 848 | | | $ | 1,215 | | | $ | 1,324 | |
Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | | | | | |
Property and equipment in accounts payable and accrued expenses | | | 1,797 | | | | 193 | | | | 60 | |
Contingent consideration related to acquisition of ECP | | | — | | | | 6,000 | | | | — | |
The accompanying notes are an integral part of the consolidated financial statements.
F-7
ABIOMED, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
(Dollars in thousands, except per share data)
Note 1. Nature of Operations
Abiomed, Inc. (the “Company” or “Abiomed”) is a provider of mechanical circulatory support devices and offers a continuum of care to heart failure patients. The Company develops, manufactures and markets proprietary products that are designed to enable the heart to rest, heal and recover by improving blood flow and/or performing the pumping function of the heart. The Company’s products are used in the cardiac catheterization lab, or cath lab, by interventional cardiologists and in the heart surgery suite by heart surgeons for patients who are in need of hemodynamic support prophylactically or emergently before, during or after angioplasty or heart surgery procedures.
Note 2. Summary of Significant Accounting Policies
The accompanying consolidated financial statements reflect the application of certain significant accounting policies described below.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles of the United States of America, or GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. The Company bases its estimates on historical experience and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. On an ongoing basis, the Company evaluates its estimates, including those related to revenue recognition, collectability of receivables, realizability of inventory, goodwill, intangible and long-lived assets, accrued expenses, stock-based compensation, income taxes including deferred tax assets and liabilities, contingencies and litigation. Provisions for depreciation are based on their estimated useful lives using the straight-line method. Some of these estimates can be subjective and complex and, consequently, actual results may differ from these estimates under different assumptions or conditions.
Cash Equivalents and Marketable Securities
The Company classifies any marketable security with a maturity date of 90 days or less at the time of purchase as a cash equivalent. Cash equivalents are carried on the balance sheet at fair market value.
The Company classifies any security with a maturity date of greater than 90 days at the time of purchase as marketable securities and classifies marketable securities with a maturity date of greater than one year from the balance sheet date as long-term marketable securities. Securities that the Company has the positive intent and ability to hold to maturity are reported at amortized cost and classified as held-to-maturity securities. If the Company does not have the intent and ability to hold a security to maturity, it reports the investment as available-for-sale securities. The Company reports available-for-sale securities at fair value, and includes unrealized gains and, to the extent deemed temporary, unrealized losses in stockholders’ equity. If any adjustment to fair value reflects a decline in the value of the investment, the Company considers available evidence to evaluate whether the decline is “other than temporary” and, if so, marks the security to market through a charge to unrealized loss on short-term marketable securities in the consolidated statements of operations.
Major Customers and Concentrations of Credit Risk
The Company primarily sells its products to hospitals and distributors. No customer accounted for more than 10% of total product revenues in fiscal years ended March 31, 2016, 2015 or 2014. No individual customer had an accounts receivable balance greater than 10% of total accounts receivable at March 31, 2016 and 2015.
F-8
Credit is extended based on an evaluation of a customer’s financial condition and generally collateral is not required. To date, credit losses have not been significant and the Company maintains an allowance for doubtful accounts based on its assessment of the collectability of accounts receivable. Receivables are geographically dispersed, primarily throughout the U.S., as well as in Europe and other foreign countries where formal distributor agreements exist.
Financial instruments which potentially subject the Company to a concentration of credit risk consist of cash, cash equivalents, short and long-term marketable securities and accounts receivable. Management mitigates credit risk by limiting the investment type and maturity to securities that preserve capital, maintain liquidity and have a high credit quality.
Financial Instruments
The Company’s financial instruments are comprised of cash and cash equivalents, accounts receivable, accounts payable and accrued expenses, the carrying amounts of which approximate fair market value as they are highly liquid and primarily short term in nature.
Inventories
Inventories are stated at the lower of cost or market. Cost is based on the first in, first out method. The Company regularly reviews inventory quantities on hand and writes down to its net realizable value any inventory that it believes to be impaired. Management considers forecast demand in relation to the inventory on hand, competitiveness of product offerings, market conditions and product life cycles when determining excess and obsolescence and net realizable value adjustments. Once inventory is written down and a new cost basis is established, it is not written back up if demand increases.
Property and Equipment
Property and equipment is recorded at cost less accumulated depreciation. Depreciation is computed using the straight line method based on estimated useful lives of three to five years for machinery and equipment, computer software, and furniture and fixtures. Leasehold improvements are amortized using the straight-line method over the shorter of the lease term or the estimated useful lives of the related assets. Expenditures for maintenance and repairs are expensed as incurred. Upon retirement or other disposition of assets, the costs and related accumulated depreciation are eliminated from the accounts and the resulting gain or loss is reflected in operating expenses.
Property and equipment is reviewed for impairment losses whenever events or changes in circumstances indicate the carrying amount may not be recoverable. An impairment loss would be recognized based on the amount by which the carrying value of the asset or asset group exceeds its fair value. Fair value is determined primarily using the estimated future cash flows associated with the asset or asset group under review discounted at a rate commensurate with the risk involved and other valuation techniques.
Goodwill
Goodwill is recorded when consideration for an acquisition exceeds the fair value of the net tangible and intangible assets acquired. Goodwill is not amortized, instead the Company evaluates goodwill for impairment at least annually at October 31, as well as whenever events or changes in circumstances suggest that the carrying amount may not be recoverable.
Goodwill impairment assessments are performed at the reporting unit level. The goodwill test involves a two-step process. The first step is a comparison of the reporting unit’s fair value to its carrying value. If the reporting unit’s fair value exceeds its carrying value, no further procedures are required. However, if the reporting unit’s fair value is less than the carrying value, an impairment of goodwill may exist, requiring a second step to measure the amount of impairment loss. If the implied fair value of goodwill is less than the recorded goodwill, an impairment charge is recorded for the difference.
In applying the goodwill impairment test, the Company may assess qualitative factors to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying value (“Step 0”). Qualitative factors may include, but are not limited to, macroeconomic conditions, industry conditions, the competitive environment, changes in the market for our products and services, regulatory and political developments, cost factors, and entity specific factors such as strategies and overall financial performance. If, after assessing these qualitative factors, the Company determines it is not more likely than not that the fair value of a reporting unit is less than its carry amount, then performing the two-step impairment test is unnecessary.
The goodwill impairment test is performed at the reporting unit level by comparing the reporting unit’s carrying amount, including goodwill, to the fair value of the reporting unit. The Company estimates the fair value of its single reporting unit using a combination of the income approach and the market approach. The income approach incorporates the use of a discounted cash flow
F-9
method in which the estimated future cash flows and terminal values for the reporting unit is discounted to a present value using an appropriate discount rate. Cash flow projections are based on management’s estimates of economic and market conditions which drive key assumptions of revenue growth rates, operating margins, cash flows, capital expenditures and working capital requirements. The discount rate is based on the specific risk characteristics of the reporting unit and its underlying forecast. The market approach estimates fair value by comparing publicly traded companies with similar operating and investment characteristics as the reporting unit. The fair values determined by the market approach and income approach, are weighted to determine the fair value for the reporting unit based primarily on the similarity of the operating and investment characteristics of the reporting unit to the comparable publicly traded companies used in the market approach.
In-Process Research and Development
In-process research and development, or IPR&D, assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development projects. IPR&D assets represent the fair value assigned to technologies that are acquired, which at the time of acquisition have not reached technological feasibility and have no alternative future use. During the period that the IPR&D assets are considered indefinite-lived, they are tested for impairment on an annual basis, or more frequently if the Company becomes aware of any events occurring or changes in circumstances that indicate that the fair value of the IPR&D assets are less than their carrying amounts. If and when development is complete, which generally occurs upon regulatory approval and are able to commercialize products associated with the IPR&D assets, these assets are then deemed definite-lived and are amortized based on their estimated useful lives at that point in time. If development is terminated or abandoned, the Company may have a full or partial impairment charge related to the IPR&D assets, calculated as the excess of carrying value of the IPR&D assets over fair value.
Contingent Consideration
Contingent consideration is recorded as a liability and is the estimate of the fair value of potential milestone payments related to business acquisitions. Contingent consideration is measured at fair value using a discounted cash flow model utilizing significant unobservable inputs including the probability of achieving each of the potential milestones and an estimated discount rate associated with the risks of the expected cash flows attributable to the various milestones. Significant increases or decreases in any of the probabilities of success or changes in expected timelines for achievement of any of these milestones could result in a significantly higher or lower fair value of the liability. The fair value of the contingent consideration at each reporting date is updated by reflecting the changes in fair value reflected within research and development expenses in our statement of operations.
Accrued Expenses
As part of the process of preparing its financial statements, the Company is required to estimate accrued expenses. This process includes identifying services that third parties have performed and estimating the level of service performed and the associated cost incurred on these services as of each balance sheet date in its financial statements. Examples of estimated accrued expenses include contract service fees, such as amounts due to clinical research organizations, investigators in conjunction with clinical trials, professional service fees, such as attorneys and accountants, and third party expenses relating to marketing efforts associated with commercialization of the Company’s product and product candidates. Accrued expenses also include estimates for payroll costs, such as bonuses and commissions. In the event that the Company does not identify certain costs that have been incurred or it under or over-estimates the level of services or the costs of such services, reported expenses for a reporting period could be overstated or understated. The dates in which certain services commence and end, the level of services performed on or before a given date and the cost of services is often subject to the Company’s judgment. The Company makes these judgments and estimates based upon known facts and circumstances.
Revenue Recognition
The Company recognizes revenue when evidence of an arrangement exists, title has passed or services have been rendered, the selling price is fixed or determinable and collectability is reasonably assured.
Revenue from product sales to customers is recognized when delivery has occurred. All costs related to product sales are recognized at time of delivery. The Company does not provide for rights of return to customers on product sales and therefore does not record a provision for returns.
Maintenance and service support contract revenues are included in product sales and are recognized ratably over the term of the service contracts. Revenue is recognized as earned in limited instances where the Company rents its console medical devices on a month-to-month basis or for a longer specified period of time to customers.
F-10
Government-sponsored research and development contracts and grants generally provide for payment on a cost-plus-fixed-fee basis. Revenues from these contracts and grants are recognized as work is performed, provided the government has appropriated sufficient funds for the work. Under contracts in which the Company elects to spend significantly more on the development project during the term of the contract than the total contract amount, the Company prospectively recognizes revenue on such contracts ratably over the term of the contract as related research and development costs are incurred.
Product Warranty
The Company generally provides a one-year warranty for certain products sold in which estimated contractual warranty obligations are recorded as an expense at the time of shipment and are included in accrued expenses in the accompanying consolidated balance sheets. The Company’s products are subject to regulatory and quality standards. Future warranty costs are estimated based on historical product performance rates and related costs to repair given products. The accounting estimate related to product warranty expense involves judgment in determining future estimated warranty costs. Should actual performance rates or repair costs differ from estimates, revisions to the estimated warranty liability would be required.
Accumulated Other Comprehensive Income (Loss)
Comprehensive income (loss) is comprised of net income, plus all changes in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources, including any foreign currency translation adjustments. These changes in equity are recorded as adjustments to accumulated other comprehensive income (loss) in the Company’s consolidated balance sheet. The components of accumulated other comprehensive income (loss) consist primarily of foreign currency translation adjustments. There were no reclassifications out of accumulated other comprehensive income (loss) during the fiscal years ended March 31, 2016, 2015 and 2014.
Translation of Foreign Currencies
The functional currency of the Company’s foreign subsidiaries is their local currency. The assets and liabilities of the Company’s foreign subsidiaries are translated into U.S. dollars at exchange rates in effect at the balance sheet date. Income and expense items are translated at the average exchange rates prevailing during the period. The cumulative translation effect for subsidiaries using a functional currency other than the U.S. dollar is included in accumulated other comprehensive income or loss as a separate component of stockholders’ equity.
The Company’s intercompany accounts are denominated in the functional currency of the foreign subsidiary. Gains and losses resulting from the remeasurement of intercompany receivables that the Company considers to be of a long-term investment nature are recorded in accumulated other comprehensive income or loss as a separate component of stockholders’ equity, while gains and losses resulting from the remeasurement of intercompany receivables from those foreign subsidiaries for which the Company anticipates settlement in the foreseeable future are recorded in the consolidated statement of operations. The net foreign currency translation gains and losses recorded in the consolidated statements of operations for the fiscal years ended March 31, 2016, 2015 and 2014 were not significant.
F-11
Net Income Per Share
Basic net income per share is computed by dividing net income by the weighted average number of common shares outstanding during the fiscal year. Diluted net income per share is computed using the treasury stock method by dividing net income by the weighted average number of dilutive common shares outstanding during the fiscal year. Diluted shares outstanding is calculated by adding to the weighted average shares outstanding any potential dilutive securities outstanding for the fiscal year. Potential dilutive securities include stock options, restricted stock awards, restricted stock units, performance-based awards and shares to be purchased under the employee stock purchase plan. In fiscal years when a net loss is reported, all common stock equivalents are excluded from the calculation because they would have an anti-dilutive effect, meaning the loss per share would be reduced. Therefore, in periods when a loss is reported basic and dilutive loss per share are the same.
| Fiscal Years Ended March 31, | |
| 2016 | | | 2015 | | | 2014 | |
Basic Net Income Per Share | | | | | | | | | | | | | | |
Net income | $ | | 38,147 | | | $ | | 113,688 | | | $ | | 7,351 | |
| | | | | | | | | | | | | | |
Weighted average shares used in computing basic net income per share | | | 42,204 | | | | | 40,632 | | | | | 39,334 | |
| | | | | | | | | | | | | | |
Net income per share - basic | $ | | 0.90 | | | $ | | 2.80 | | | $ | | 0.19 | |
| Fiscal Years Ended March 31, | |
| 2016 | | | 2015 | | | 2014 | |
Diluted Net Income Per Share | | | | | | | | | | | | | | |
Net income | $ | | 38,147 | | | $ | | 113,688 | | | $ | | 7,351 | |
| | | | | | | | | | | | | | |
Weighted average shares used in computing basic net income per share | | | 42,204 | | | | | 40,632 | | | | | 39,334 | |
Effect of dilutive securities | | | 2,691 | | | | | 2,226 | | | | | 2,272 | |
Weighted average shares used in computing diluted net income per share | | | 44,895 | | | | | 42,858 | | | | | 41,606 | |
| | | | | | | | | | | | | | |
Net income per share - diluted | $ | | 0.85 | | | $ | | 2.65 | | | $ | | 0.18 | |
For the fiscal years ended March 31, 2016, 2015 and 2014, approximately 62,000, 2,000 and 94,000 shares of common stock underlying outstanding securities primarily related to out-of-the-money stock options and performance-based awards where milestones were not met were not included in the computation of diluted earnings per share because their inclusion would be anti-dilutive.
Stock-Based Compensation
The Company’s stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as an expense over the requisite service period, and includes an estimate of awards that will be forfeited.
The fair value of stock option grants is estimated using the Black-Scholes option pricing model. Use of the valuation model requires management to make certain assumptions with respect to selected model inputs. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for a term consistent with the expected life of the stock options. Volatility assumptions are calculated based on historical volatility of the Company’s stock. The Company estimates the expected term of options based on historical exercise experience and estimates of future exercises of unexercised options. In addition, an expected dividend yield of zero is used in the option valuation model because the Company does not pay dividends and does not expect to pay any cash dividends in the foreseeable future. Forfeitures are estimated based on an analysis of actual forfeitures, adjusted to the extent historical forfeitures may not be indicative of expected forfeitures in the future.
For awards with service conditions only, the Company recognizes compensation cost on a straight-line basis over the requisite service period. For awards with service and performance conditions, the Company recognizes compensation costs using the graded vesting method over the requisite service period. Estimates of stock-based compensation expense for an award with performance conditions are based on the probable outcome of the performance conditions. The cumulative effect of changes in the probability
F-12
outcomes are recorded in the period in which the changes occur. For awards with market-based conditions, the Company uses a Monte Carlo simulation model to estimate that the grant-date fair value. The fair value related to market-based awards are recorded as stock-based compensation expense over the vesting period regardless of whether the market condition is achieved or not.
Income Taxes
The Company’s provision for income taxes is comprised of a current and a deferred provision. The current income tax provision is calculated as the estimated taxes payable or refundable on income tax returns for the current fiscal year. The deferred income tax provision is calculated for the estimated future income tax effects attributable to temporary differences and carryforwards using expected tax rates in effect in the years during which the differences are expected to reverse.
Deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each fiscal year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to impact taxable income.
The Company regularly assesses its ability to realize its deferred tax assets. Assessing the realization of deferred tax assets requires significant management judgment. Valuation allowances are established when necessary to reduce net deferred tax assets to the amount that is more likely than not to be realized.
The Company recognizes and measures uncertain tax positions using a two-step approach. The first step is to evaluate the tax position for recognition by determining if, based on the technical merits, it is more likely than not that the position will be sustained upon audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit at the largest amount that is more than 50% likely of being realized upon ultimate settlement. The Company reevaluates these uncertain tax positions on a quarterly basis. This evaluation is based on factors including, but not limited to, changes in facts or circumstances, changes in tax laws, effectively settled issues under audit and new audit activity. Any changes in these factors could result in the recognition of a tax benefit or an additional charge to the tax provision. When applicable, the Company accrues for the effects of uncertain tax positions and the related potential penalties and interest through income tax expense. As of March 31, 2016, the Company has no material uncertain tax positions and no interest and penalties have been recognized to date.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers to provide updated guidance on revenue recognition. ASU 2014-09 requires a company to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In doing so, companies may need to use more judgment and make more estimates than under today’s guidance. These may include identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. ASU 2014-09 will become effective for the Company beginning in fiscal 2019 under either full or modified retrospective adoption, with early adoption permitted as of the original effective date of ASU 2014-09. The Company is currently evaluating the impact of adopting ASU 2014-09 on its consolidated financial statements.
In July 2015, the FASB issued ASU 2015-11, Inventory (Topic 330): Simplifying the Measurement of Inventory, which applies to inventory that is measured using first-in, first-out or average cost methods. Under the updated guidance, an entity should measure inventory that is within scope at the lower of cost and net realizable value, which is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. Subsequent measurement is unchanged for inventory that is measured using last-in, last-out. ASU-2015-11 is effective for annual and interim periods beginning after December 15, 2016, and should be applied prospectively with early adoption permitted at the beginning of an interim or annual reporting period. The Company does not expect the adoption of ASU 2015-11 to have a material impact on its consolidated financial statements.
In September 2015, the FASB issued ASU 2015-16, Business Combinations (Topic 805)—Simplifying the Accounting for Measurement-Period Adjustments. The amendments in this update require that an acquirer recognizes adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. The amendments in this update require an entity to present separately on the face of the income statement or disclose in the notes the portion of the amount recorded in current-period earnings by line item that would have been recorded in previous reporting periods if the adjustment to the provisional amounts had been recognized as of the acquisition date. For public business entities, the amendments in this update are effective for fiscal years beginning after December 15, 2015, including interim periods within those fiscal years. For all other entities, the amendments in this update are effective for fiscal years beginning after December 15, 2016, and interim periods
F-13
within fiscal years beginning after December 15, 2017. The Company does not expect the adoption of ASU 2015-16 to have a material impact on its consolidated financial statements.
In November 2015, the FASB issued ASU No. 2015-17, Income Taxes (Topic 740)—Balance Sheet Classification of Deferred Taxes. This ASU requires an entity to classify deferred income tax assets and liabilities as noncurrent on the entity’s classified statement of financial position. This amendment eliminates the current requirement to classify deferred tax assets and liabilities as either current or noncurrent on the entity’s balance sheet. This amendment may be applied either prospectively to all deferred tax liabilities and assets or retrospectively to all periods presented. If applied prospectively, the entity should disclose in the first interim and first annual period of change, the nature of and the reason for the change in accounting principle and a statement that prior periods were not retrospectively adjusted. If applied retrospectively, the entity should disclose in the first interim and first annual period of change, the nature of and reason for the change in accounting principle and quantitative information about the effects of the accounting change on prior periods. ASU 2015-17 is effective for fiscal years beginning after December 15, 2016, and for interim periods within those fiscal years. Earlier application is permitted as of the beginning of an interim or annual reporting period. The Company early adopted ASU 2015-17 and has applied the guidance retrospectively to all periods presented. The impact on the March 31, 2015 balance sheet was a reclassification of $35.1 million from current deferred tax assets to long-term deferred tax assets. Adoption of this standard did not impact the Company’s results of operations, retained earnings, or cash flows in the current or previous interim and annual reporting periods.
In February 2016, the FASB issued ASU 2016-02, Leases. This guidance requires an entity to recognize lease liabilities and a right-of-use asset for all leases on the balance sheet and to disclose key information about the entity's leasing arrangements. ASU 2016-02 is effective for annual reporting periods beginning after December 15, 2018, including interim periods within that reporting period, with earlier adoption permitted. ASU 2016-02 must be adopted using a modified retrospective approach for all leases existing at, or entered into after the date of initial adoption, with an option to elect to use certain transition relief. The Company is currently evaluating the impact of adopting ASU 2016-02 on its consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, Compensation-Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting, which is intended to simplify several aspects of the accounting for share-based payment transactions, including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as classification in the statement of cash flows. ASU 2016-09 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2016. Early adoption is permitted. The Company is currently evaluating the impact of adopting ASU 2016-09 on its consolidated financial statements.
Note 3. Acquisitions
Acquisition of ECP Entwicklungsgesellschaft mbH
On July 1, 2014, the Company entered into a share purchase agreement with its wholly owned German subsidiary, Abiomed Europe GmbH (“Abiomed Europe”) and Syscore GmbH (“Syscore”), a limited liability company located in Berlin, Germany, providing for the Company’s acquisition of all of the share capital of ECP Entwicklungsgesellschaft mbH (“ECP”), a limited liability company incorporated in Germany. ECP is engaged in research, development, prototyping and the production of a percutaneous expandable catheter pump which increases blood circulation from the heart with an external drive shaft. The Company’s acquisition of ECP closed on July 1, 2014.
F-14
The Company acquired ECP for $13.0 million in cash, with additional potential payouts totaling $15.0 million payable to Syscore based on the achievement of certain technical, regulatory and commercial milestones. These milestone payments may be made, at the Company’s option, by a combination of cash or the Company’s common stock. With respect to such milestone payments, the share purchase agreement provides:
| · | that, upon the earlier of (i) the Company’s receipt of European CE Marking approval relating to the sale of an expandable device based on certain patent rights acquired from ECP, or (ii) the Company’s bringing of a successful claim against a third party competitor (or reaching an economically equivalent settlement) for the infringement of certain patent rights acquired from ECP, it will pay Syscore an additional $7.0 million (provided that if such claim or settlement does not prohibit the third party competitor’s further marketing, production, sale, distribution, lease or use of any violating or infringing products, but only awards monetary damages to the Company or to Abiomed Europe, the amount payable to Syscore shall be limited to the lower of the amount of aggregate damages received and $7.0 million); and |
| · | that, upon the first to occur of (i) the Company’s successful commercialization of one or more rotatable and expandable devices based on certain patent rights acquired from ECP, where such devices achieve aggregate worldwide revenues of $125.0 million, including the revenues of third-party licensees, or (ii) the Company’s sale of (A) ECP, (B) all or substantially all of ECP’s assets, or (C) certain of ECP’s patent rights, the Company will pay to Syscore the lesser of (x) one-half of the profits earned from such sale described in the foregoing item (ii), after accounting for the costs of acquiring and operating ECP, or (y) $15.0 million (less any previous milestone payment). |
ECP’s Acquisition of AIS GmbH Aachen Innovative Solutions
In connection with the Company’s acquisition of ECP, ECP acquired all of the share capital of AIS GmbH Aachen Innovative Solutions (“AIS”), a limited liability company incorporated in Germany, pursuant to a share purchase agreement dated as of June 30, 2014, by and among ECP and AIS’s four individual shareholders. AIS, based in Aachen, Germany, holds certain intellectual property useful to ECP’s business, and, prior to being acquired by ECP, had licensed such intellectual property to ECP.
The purchase price for the acquisition of AIS’s share capital was approximately $2.8 million in cash, which was provided by the Company, and the acquisition closed immediately prior to Abiomed Europe’s acquisition of ECP. The share purchase agreement contains representations, warranties and closing conditions customary for transactions of its size and nature.
Purchase Price Allocation
The acquisition of ECP and AIS was accounted for as a business combination. The purchase price for the acquisition has been allocated to the assets acquired and liabilities assumed based on their estimated fair values.
The acquisition-date fair value of the consideration transferred is as follows (in thousands):
| | Total Acquisition Date Fair Value | |
Cash consideration | | $ | 15,750 | |
Contingent consideration | | | 6,000 | |
Total consideration transferred | | $ | 21,750 | |
F-15
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed on July 1, 2014, the date of acquisition (in thousands):
Acquired assets: | | | | |
Cash and cash equivalents | | $ | 53 | |
Accounts receivable | | | 25 | |
Property and equipment | | | 619 | |
In-process research and development | | | 18,500 | |
Goodwill | | | 1,964 | |
Long-term deferred tax assets | | | 1,874 | |
Other assets acquired | | | 141 | |
Total assets acquired | | | 23,176 | |
Liabilities assumed: | | | | |
Accounts payable | | | 295 | |
Accrued liabilities | | | 131 | |
Long-term deferred tax liabilities | | | 1,000 | |
Total liabilities assumed | | | 1,426 | |
| | | | |
Net assets acquired | | $ | 21,750 | |
In-process research and development assets (“IPR&D”) is principally the estimated fair value of the ECP and AIS technology which had not reached commercial technological feasibility nor had alternative future use at the time of the acquisition and therefore the Company has considered as IPR&D, with assigned values to be allocated among the various IPR&D assets acquired.
Goodwill is calculated as the difference between the acquisition-date fair value of the consideration transferred and the fair values of the assets acquired and liabilities assumed. The goodwill resulting from these acquisitions arises largely from synergies expected from combining the operations of ECP and AIS with the Company’s existing operations. The goodwill is not deductible for income tax purposes.
All legal, consulting and other costs related to the acquisition, aggregating approximately $1.1 million, have been expensed as incurred and are included in selling, general and administrative expenses in the Company’s consolidated statements of operations. The results of operations for ECP and AIS are included in the Company’s consolidated statements of operations for the period from the July 1, 2014 acquisition date to March 31, 2016. The Company has no material external revenues and incurred $3.7 million and $2.3 million in net losses associated with the operations of ECP and AIS acquisitions in the fiscal years ended March 31, 2016 and 2015, respectively.
The following unaudited pro forma information presents the combined results of operations for the fiscal years ended March 31, 2015 and 2014, as if the Company had completed the ECP and AIS acquisitions at the beginning of fiscal 2014. The pro forma financial information is provided for comparative purposes only and is not necessarily indicative of what actual results would have been had the acquisition occurred on the date indicated, nor does it give effect to synergies, cost savings, fair market value adjustments, immaterial amortization expense and other changes expected to result from the acquisition. Accordingly, the pro forma financial results do not purport to be indicative of consolidated results of operations as of the date hereof, for any period ended on the date hereof, or for any other future date or period. The pro forma consolidated financial information has been calculated after applying the Company’s accounting policies and includes adjustments for transaction-related costs, to eliminate revenues earned by AIS from ECP and expenses paid by ECP to AIS associated with a license agreement between the two parties, interest expense incurred by ECP related to bank loans accounted as if the repayment of ECP debt had occurred on April 1, 2013 and was not outstanding during the periods, and income tax provision of AIS due to the elimination of revenue on the license agreement with ECP.
| | Fiscal Years Ended March 31, | |
| | | 2015 | | | | 2014 | |
| | (in $000's) | |
Revenue | | $ | 230,323 | | | $ | 183,689 | |
Income before income tax provision | | | 28,871 | | | | 4,802 | |
Net income | | | 113,794 | | | | 3,623 | |
F-16
Note 4. Marketable Securities and Fair Value Measurements
Marketable Securities
The Company’s marketable securities are classified as available-for-sale securities and, accordingly, are recorded at fair value. The difference between amortized cost and fair value is reported as a component of other comprehensive income (loss).
The Company’s marketable securities at March 31, 2016 and 2015 are classified on the balance sheet as follows:
| | March 31, 2016 | | | March 31, 2015 | |
| | (in $000's) | |
Short-term marketable securities (within one year to maturity) | | $ | 163,822 | | | $ | 109,557 | |
Long-term marketable securities (one to five years to maturity) | | | 1,000 | | | | 13,996 | |
| | $ | 164,822 | | | $ | 123,553 | |
The Company’s marketable securities at March 31, 2016 and 2015 are invested in the following:
| | | | | | | | | | | | | | | | |
| | Amortized | | | Gross Unrealized | | | Gross Unrealized | | | Fair Market | |
| | Cost | | | Gains | | | Losses | | | Value | |
| | (in $000's) | |
March 31, 2016: | | | | | | | | | | | | | | | | |
US Treasury mutual fund securities | | $ | 45,635 | | | $ | 21 | | | $ | — | | | $ | 45,656 | |
Short-term government-backed securities | | | 118,125 | | | | 45 | | | | (4 | ) | | | 118,166 | |
Long-term government-backed securities | | | 999 | | | | 1 | | | | — | | | | 1,000 | |
| | $ | 164,759 | | | $ | 67 | | | $ | (4 | ) | | $ | 164,822 | |
| | | | | | | | | | | | | | | | |
| | Amortized | | | Gross Unrealized | | | Gross Unrealized | | | Fair Market | |
| | Cost | | | Gains | | | Losses | | | Value | |
| | (in $000's) | |
March 31, 2015: | | | | | | | | | | | | | | | | |
US Treasury mutual fund securities | | $ | 19,487 | | | $ | — | | | $ | — | | | $ | 19,487 | |
Short-term government-backed securities | | | 90,070 | | | | 9 | | | | (9 | ) | | | 90,070 | |
Long-term government-backed securities | | | 13,999 | | | | 2 | | | | (5 | ) | | | 13,996 | |
| | $ | 123,556 | | | $ | 11 | | | $ | (14 | ) | | $ | 123,553 | |
Fair Value Hierarchy
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three categories:
Level 1: Quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market based inputs or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs that are not corroborated by market data.
Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities.
Level 2 includes financial instruments that are valued using models or other valuation methodologies. These models are primarily industry-standard models that consider various assumptions, including time value, yield curve, volatility factors, prepayment speeds, default rates, loss severity, current market and contractual prices for the underlying financial instruments, as well as other relevant economic measures. Substantially all of these assumptions are observable in the marketplace, can be derived from observable data or are supported by observable levels at which transactions are executed in the marketplace.
F-17
Level 3 is comprised of unobservable inputs that are supported by little or no market activity. Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable.
The following table presents the Company’s fair value hierarchy for its financial instruments measured at fair value as of March 31, 2016 and 2015:
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
March 31, 2016: | | (in $000's) | |
Assets | | | | | | | | | | | | | | | | |
U.S. Treasury mutual fund securities | | $ | — | | | $ | 45,656 | | | $ | — | | | $ | 45,656 | |
Short-term government-backed securities | | | — | | | | 118,166 | | | | — | | | | 118,166 | |
Long-term government-backed securities | | | — | | | | 1,000 | | | | — | | | | 1,000 | |
Liabilities | | | | | | | | | | | | | | | | |
Contingent consideration | | | — | | | | — | | | | 7,563 | | | | 7,563 | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
March 31, 2015: | | (in $000's) | |
Assets | | | | | | | | | | | | | | | | |
U.S. Treasury mutual fund securities | | $ | — | | | $ | 19,487 | | | $ | — | | | $ | 19,487 | |
Short-term government-backed securities | | | — | | | | 90,070 | | | | — | | | | 90,070 | |
Long-term government-backed securities | | | — | | | | 13,996 | | | | — | | | | 13,996 | |
Liabilities | | | | | | | | | | | | | | | | |
Contingent consideration | | | — | | | | — | | | | 6,510 | | | | 6,510 | |
The Company has determined that the estimated fair value of its investments in U.S. Treasury mutual fund securities, short-term government-backed securities and long-term government-backed securities are reported as Level 2 financial assets as they are not exchange-traded instruments.
The Company’s financial liabilities consisted of contingent consideration potentially payable to former ECP shareholders related to the acquisition of ECP in July 2014. This liability is reported as Level 3 as estimated fair value of the contingent consideration related to the acquisition of the ECP requires significant management judgment or estimation and is calculated using the income approach, using various revenue and cost assumptions and applying a probability to each outcome.
The following table summarizes the change in fair value of the contingent consideration for the fiscal years ended March 31, 2016 and 2015:
| | Fiscal Years Ended March 31, | |
| | | 2016 | | | | 2015 | |
| | (in $000's) | |
Level 3 liabilities, beginning balance | | $ | 6,510 | | | $ | — | |
Additions | | | — | | | | 6,000 | |
Payments | | | — | | | | — | |
Change in fair value | | | 1,053 | | | | 510 | |
Level 3 liabilities, ending balance | | $ | 7,563 | | | $ | 6,510 | |
The change in fair value of the contingent consideration of $1.1 million and $0.5 million for the fiscal years ended March 31, 2016 and 2015, respectively, was primarily due to an increase in fair value caused by the effect of the passage of time on the fair value measurement of milestones related to the ECP acquisition and continued progress on the development of the underlying technology. Adjustments associated with the change in fair value of contingent consideration are included in research and development expenses in the Company’s consolidated statements of operations.
F-18
The following table presents quantitative information about the inputs and valuation methodologies used for the Company’s fair value measurements as of March 31, 2016 classified in Level 3:
| | Fair Value at | | | | | | | Weighted Average |
| | March 31, 2016 | | | | | Significant | | (range, if |
| | (in $000's) | | | Valuation Methodology | | Unobservable Input | | applicable) |
Contingent consideration | | $ | 7,563 | | | Probability weighted income approach | | Milestone dates | | 2018 to 2021 |
| | | | | | | | Discount rate | | 8% to 12% |
| | | | | | | | Probability of occurrence | | Probability adjusted level of 40% for the base case scenario and 5% to 30% for various upside and downside scenarios |
Other Investments
The Company periodically makes investments in private medical device companies that focus on heart failure and heart pump technologies. In July 2015, the Company invested $0.8 million for its participation in a preferred stock offering of a private medical technology company. The aggregate carrying amount of the Company’s other investments was $4.4 million and $3.6 million at March 31, 2016 and 2015, respectively, and is classified within other assets in the consolidated balance sheets. These investments are accounted for using the cost method and are measured at fair value only if there are identified events or changes in circumstances that may have a significant adverse effect on the fair value of these investments.
Note 5. Accounts Receivable
The components of accounts receivable are as follows:
| | March 31, 2016 | | | March 31, 2015 | |
| | (in $000's) | |
Trade receivables | | $ | 42,945 | | | $ | 32,005 | |
Allowance for doubtful accounts | | | (124 | ) | | | (177 | ) |
| | $ | 42,821 | | | $ | 31,828 | |
The following table summarizes activity in the Company's allowance for doubtful accounts:
| | Fiscal Years Ended March 31, | |
| | | 2016 | | | | 2015 | | | | 2014 | |
| | (in $000's) | |
Balance at beginning of year | | $ | 177 | | | $ | 185 | | | $ | 136 | |
Additions | | | 42 | | | | 115 | | | | 81 | |
Deductions | | | (95 | ) | | | (123 | ) | | | (32 | ) |
Balance at end of year | | $ | 124 | | | $ | 177 | | | $ | 185 | |
Note 6. Inventories
The components of inventories are as follows:
| | March 31, 2016 | | | March 31, 2015 | |
| | (in $000's) | |
Raw materials and supplies | | $ | 7,993 | | | $ | 7,417 | |
Work-in-progress | | | 13,147 | | | | 6,466 | |
Finished goods | | | 5,600 | | | | 2,891 | |
| | $ | 26,740 | | | $ | 16,774 | |
F-19
The Company’s inventories relate to its circulatory care product lines, primarily the Impella® and AB5000™ product platforms. Finished goods and work-in-process inventories consist of direct material, labor and overhead.
Note 7. Property and Equipment
The components of property and equipment are as follows:
| | March 31, 2016 | | | March 31, 2015 | |
| | (in $000's) | |
Machinery and equipment | | $ | 25,211 | | | $ | 19,335 | |
Furniture and fixtures | | | 1,510 | | | | 1,000 | |
Leasehold improvements | | | 11,833 | | | | 2,874 | |
Construction in progress | | | 3,712 | | | | 1,685 | |
Total cost | | | 42,266 | | | | 24,894 | |
Less accumulated depreciation | | | (19,082 | ) | | | (15,767 | ) |
| | $ | 23,184 | | | $ | 9,127 | |
Depreciation expense related to property and equipment was $3.3 million, $2.7 million, and $2.4 million for the fiscal years ending March 31, 2016, 2015 and 2014, respectively.
Note 8. Goodwill and In-Process Research and Development
The carrying amount of goodwill at March 31, 2016 and 2015 was $33.0 million and $31.5 million, respectively, and has been recorded in connection with the Company’s acquisition of Impella Cardiosystems AG, or Impella, in May 2005 and ECP and AIS in July 2014. The goodwill activity is as follows:
| | (in $000's) | |
Balance at March 31, 2014 | | $ | 37,990 | |
Additions | | | 1,964 | |
Foreign currency translation impact | | | (8,420 | ) |
Balance at March 31, 2015 | | $ | 31,534 | |
Foreign currency translation impact | | | 1,469 | |
Balance at March 31, 2016 | | $ | 33,003 | |
The Company has no accumulated impairment losses on goodwill. The Company performed a Step 0 qualitative assessment during the annual impairment review for fiscal 2016 as of October 31, 2015 and concluded that it is not more likely than not that the fair value of the Company’s single reporting unit is less than its carrying amount. Therefore, the two-step goodwill impairment test for the reporting unit was not necessary in fiscal 2016.
As described in Note 3. “Acquisitions,” in July 2014, the Company acquired ECP and AIS and recorded $18.5 million of IPR&D. The estimated fair value of the IPR&D was determined using a probability-weighted income approach, which discounts expected future cash flows to present value. The projected cash flows from the expandable catheter pump technology were based on certain key assumptions, including estimates of future revenue and expenses, taking into account the stage of development of the technology at the acquisition date and the time and resources needed to complete development. The Company used a discount rate of 22.5% and cash flows that have been probability adjusted to reflect the risks of product commercialization, which the Company believes are appropriate and representative of market participant assumptions.
F-20
The carrying value of the Company’s IPR&D assets and the change in the balance for the fiscal years ended March 31, 2016 and 2015 is as follows:
| | (in $000's) | |
Balance at March 31, 2014 | | $ | — | |
Additions | | | 18,500 | |
Foreign currency translation impact | | | (3,789 | ) |
Balance at March 31, 2015 | | $ | 14,711 | |
Foreign currency translation impact | | | 685 | |
Balance at March 31, 2016 | | $ | 15,396 | |
The Company tests IPR&D for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the IPR&D intangible asset is less than its carrying amount. The Company performed its annual impairment review for fiscal 2016 as of October 31, 2015 and concluded that it is not more likely than not that the fair value of the IPR&D is less than its carrying amount.
Note 9. Stockholders’ Equity
Class B Preferred Stock
The Company has authorized 1,000,000 shares of Class B Preferred Stock, $.01 par value, of which the Board of Directors can set the designation, rights and privileges. No shares of Class B Preferred Stock have been issued or are outstanding.
Note 10. Stock Award Plans and Stock-Based Compensation
Stock Award Plans
The Company grants stock options and restricted stock awards to employees and others. All outstanding stock options of the Company as of March 31, 2016 were granted with an exercise price equal to the fair market value on the date of grant. Outstanding stock options, if not exercised, expire 10 years from the date of grant.
2015 Stock Incentive Plan
The Company’s 2015 Stock Incentive Plan (the “2015 Plan”) authorizes the grant of a variety of equity awards to the Company’s officers, directors, employees, consultants and advisers, including awards of unrestricted and restricted stock, restricted stock units, incentive and nonqualified stock options to purchase shares of common stock, performance share awards and stock appreciation rights. The 2015 Plan provides that options may only be granted at the current market value on the date of grant. Each share of stock issued pursuant to a stock option or stock appreciation right counts as one share against the maximum number of shares issuable under the 2015 Plan, while each share of stock issued pursuant to any other type of award counts as 1.7 shares against the maximum number of shares issuable under the 2015 Plan. The Company’s policy for issuing shares upon exercise of stock options or the vesting of its restricted stock awards and restricted stock units is to issue shares of common stock at the time of exercise or conversion. At March 31, 2016, a total of approximately 1,988,000 shares were available for future issuance under the 2015 Plan.
2008 Stock Incentive Plan
The Company’s 2008 Stock Incentive Plan (the “2008 Plan”) authorizes the grant of a variety of equity awards to the Company’s officers, directors, employees, consultants and advisers, including awards of unrestricted and restricted stock, restricted stock units, incentive and nonqualified stock options to purchase shares of common stock, performance share awards and stock appreciation rights. The 2008 Plan provides that options may only be granted at the current market value on the date of grant. Each share of stock issued pursuant to a stock option or stock appreciation right counts as one share against the maximum number of shares issuable under the 2008 Plan, while each share of stock issued pursuant to any other type of award counts as 1.58 shares against the maximum number of shares issuable under the 2008 Plan for grants made on or after August 11, 2010 (and as 1.5 shares for grants made prior to that date). The Company’s policy for issuing shares upon exercise of stock options or the vesting of its restricted stock awards and restricted stock units is to issue shares of common stock at the time of exercise or conversion. At March 31, 2016, a total of approximately 648,000 shares were available for future issuance under the 2008 Plan.
F-21
Stock-Based Compensation
The following table summarizes stock-based compensation expense by financial statement line item in the Company’s consolidated statements of operations for the fiscal years ended March 31, 2016, 2015 and 2014:
| | Fiscal Years Ended March 31, | |
| | | 2016 | | | | 2015 | | | | 2014 | |
| | (in $000's) | |
Cost of product revenue | | $ | 895 | | | $ | 665 | | | $ | 614 | |
Research and development | | | 3,950 | | | | 3,205 | | | | 2,347 | |
Selling, general and administrative | | | 24,208 | | | | 12,650 | | | | 8,257 | |
| | $ | 29,053 | | | $ | 16,520 | | | $ | 11,218 | |
The components of stock-based compensation for the fiscal years ended March 31, 2016, 2015 and 2014 were as follows:
| | Fiscal Years Ended March 31, | |
| | | 2016 | | | | 2015 | | | | 2014 | |
| | (in $000's) | |
Restricted stock units and restricted stock | | $ | 23,708 | | | $ | 13,539 | | | $ | 8,322 | |
Stock options | | | 4,866 | | | | 2,708 | | | | 2,679 | |
Employee stock purchase plan | | | 479 | | | | 273 | | | | 217 | |
| | $ | 29,053 | | | $ | 16,520 | | | $ | 11,218 | |
The Company’s former Chief Financial Officer retired effective July 31, 2015 and currently serves as a consultant to the Company through July 31, 2017. In connection with the former Chief Financial Officer’s retirement agreement, his unvested options and restricted stock units were modified such that they will continue to vest and he will be permitted to exercise any vested options until July 31, 2017, including any options that vest after his retirement date, other than such options that expire on the tenth anniversary of the grant date. As a result of the modification, the Company recorded $2.5 million in stock compensation expense, which is recorded in selling, general and administrative expenses for the year ended March 31, 2016.
In June 2015, the Company’s Board of Directors adopted a non-employee director retirement policy that provides for the accelerated vesting of all stock options, restricted stock units and other equity awards held by a non-employee director if he or she permanently ceases his or her service on the Company’s Board of Directors by reason of death, disability, or the non-employee director’s retirement following at least five years of service and so long as his or her age plus service equals or exceeds 65. This retirement policy accelerated the recognition of stock-based compensation because the outstanding unvested restricted stock units held by retirement eligible non-employee directors are able to vest at their decision to retire. The Company recorded $1.4 million in stock compensation expense related to this accelerated vesting, which is recorded in selling, general and administrative expenses for the year ended March 31, 2016.
In August 2015, the Company approved the annual equity award grant to non-employee directors in the form of restricted stock units covering 3,900 shares of the Company’s common stock, which vest on the earlier of: (a) the one year anniversary of the grant date; or (b) the next annual meeting of stockholders. In conjunction with the Company’s non-employee director retirement policy, the stock compensation expense for awards to retirement eligible non-employee directors was fully recognized upon grant. The Company recorded $2.0 million in stock compensation expense, which is recorded in selling, general and administrative expenses for the year ended March 31, 2016.
F-22
Stock Options
The following table summarized stock option activity for the year ended March 31, 2016:
| | | | | | | | | | Weighted | | | | | |
| | | | | | Weighted | | | Average | | | Aggregate | |
| | | | | | Average | | | Remaining | | | Intrinsic | |
| | Options | | | Exercise | | | Contractual | | | Value | |
| | (in thousands) | | | Price | | | Term (years) | | | (in thousands) | |
Outstanding at beginning of year | | | 2,892 | | | $ | 14.72 | | | | 5.18 | | | | | |
Granted | | | 183 | | | | 72.67 | | | | | | | | | |
Exercised | | | (829 | ) | | | 11.78 | | | | | | | | | |
Cancelled and expired | | | (2 | ) | | | 16.02 | | | | | | | | | |
Outstanding at end of year | | | 2,244 | | | $ | 20.55 | | | | 5.19 | | | $ | 166,722 | |
Exercisable at end of year | | | 1,594 | | | $ | 13.84 | | | | 4.05 | | | $ | 129,040 | |
Options vested and expected to vest at end of year | | | 2,182 | | | $ | 20.07 | | | | 5.11 | | | $ | 163,162 | |
The remaining unrecognized stock-based compensation expense for unvested stock option awards at March 31, 2016 was approximately $6.0 million, net of forfeitures, and the weighted-average period over which this cost will be recognized is 2.4 years.
The aggregate intrinsic value of options exercised for fiscal years 2016, 2015 and 2014 was $58.6 million, $20.0 million and $16.3 million, respectively. The total cash received as a result of employee stock option exercises during the fiscal years ended March 31, 2016, 2015 and 2014 was approximately $9.8 million, $10.9 million and $9.4 million, respectively. The total fair value of options vested in fiscal years 2016, 2015 and 2014 was $2.6 million, $2.6 million and $2.5 million, respectively.
The Company estimates the fair value of each stock option granted at the grant date using the Black-Scholes option valuation model. The weighted average grant-date fair values and weighted average assumptions used in the calculation of fair value of options granted during the fiscal years ended March 31, 2016, 2015 and 2014 was as follows:
| | Fiscal Years Ended March 31, | |
| | | 2016 | | | | 2015 | | | | 2014 | |
Valuation assumptions: | | | | | | | | | | | | |
Weighted average grant-date fair value | | $ | 29.57 | | | $ | 9.29 | | | $ | 9.85 | |
Risk-free interest rate | | | 1.55 | % | | | 1.60 | % | | | 0.94 | % |
Expected option life (years) | | | 4.15 | | | | 4.19 | | | | 4.25 | |
Expected volatility | | | 49.7 | % | | | 49.3 | % | | | 51.7 | % |
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for a term consistent with the expected life of the stock options. Volatility assumptions are calculated based on the historical volatility of the Company’s stock and adjustments for factors not reflected in historical volatility that may be more indicative of future volatility. The Company estimates the expected term of options based on historical exercise experience and estimates of future exercises of unexercised options. An expected dividend yield of zero is used in the option valuation model because the Company does not pay cash dividends and does not expect to pay any cash dividends in the foreseeable future. The Company estimates forfeitures based on an analysis of actual historical forfeitures, adjusted to the extent historic forfeitures may not be indicative of expected forfeitures in the future.
F-23
Restricted Stock and Restricted Stock Units
The following table summarizes restricted stock and restricted stock unit activity for the fiscal year ended March 31, 2016:
| | Number of Shares | | | Weighted Average Grant Date Fair Value | |
| | (in thousands) | | | (per share) | |
Restricted stock and restricted stock units at beginning of year | | | 1,160 | | | $ | 21.90 | |
Granted | | | 696 | | | $ | 87.45 | |
Vested | | | (543 | ) | | $ | 22.52 | |
Forfeited | | | (50 | ) | | $ | 17.11 | |
Restricted stock and restricted stock units at end of year | | | 1,263 | | | $ | 57.95 | |
The remaining unrecognized compensation expense for outstanding restricted stock and restricted stock units, including performance-based awards, as of March 31, 2016 was $27.5 million and the weighted-average period over which this cost will be recognized is 2.3 years.
The weighted average grant-date fair value for restricted stock and restricted stock units granted during the fiscal years ended March 31, 2016, 2015 and 2014 was $87.45, $22.07 and $23.34 per share, respectively. The total fair value of restricted stock and restricted stock units vested in fiscal years 2016, 2015 and 2014 was $39.6 million, $11.2 million and $6.0 million, respectively.
Performance and Market-Based Awards
Restricted stock units include certain awards that vest subject to certain performance and market-based criteria. The remaining unrecognized compensation expense for outstanding performance and market-based restricted stock units as of March 31, 2016 was $17.2 million and the weighted-average period over which this cost will be recognized is 2.4 years.
Performance-Based Awards
In May 2015, performance-based awards of restricted stock units for the potential issuance of 183,940 shares of common stock were issued to certain executive officers and employees, all of which vest upon achievement of prescribed service milestones by the award recipients and performance milestones by the Company. The Company met the prescribed performance milestones in fiscal 2016 such that the remaining outstanding 183,940 shares of common stock as of March 31, 2016 will vest subject to service requirements for vesting for these employees and stock-based compensation expense is being recognized accordingly over the employee’s service term. As of March 31, 2016, the Company is recognizing compensation expense based on the probable outcome related to the prescribed performance targets on the outstanding awards.
In May 2014, performance-based awards of restricted stock units for the potential issuance of 379,752 shares of common stock were issued to certain executive officers and employees, all of which vest upon achievement of prescribed service milestones by the award recipients and performance milestones by the Company. The Company met the prescribed performance milestones in fiscal 2015. As of March 31, 2016, approximately 123,000 shares of common stock underlying restricted stock units remain unvested and such restricted stock units will vest subject to service requirements for vesting for these employees.
In May 2013, performance-based awards of restricted stock units for the potential issuance of 268,988 shares of common stock were issued to certain executive officers and employees, all of which vest upon achievement of prescribed service milestones by the award recipients and performance milestones by the Company. The Company met the prescribed performance milestones in fiscal 2014. As of March 31, 2016, approximately 70,000 shares of common stock underlying restricted stock units remain unvested and such restricted stock units will vest subject to service requirements for vesting for these employees.
In June 2011, performance-based awards of restricted stock units for the potential issuance of 100,000 shares of common stock was issued to a certain senior executive officer of the Company that would vest upon achievement of prescribed service milestones by the award recipient and performance milestones by the Company. As of March 31, 2016, the Company has met the prescribed milestones for 50,000 shares of this award. The Company modified the performance condition on the 50,000 remaining restricted stock units that were related to this performance award in March 2014 and December 2015, all of which will vest upon achievement of a prescribed service milestone by the award recipients and a performance milestone by the Company. The Company recorded $0.5 million in stock compensation expense related to this accounting modification, which is recorded in selling, general and administrative
F-24
expenses for the year ended March 31, 2016. The Company believes that it is probable that the prescribed performance milestones will be met and the compensation expense is being recognized accordingly.
Market-Based Awards
In June 2015, the Company awarded certain executive officers a total of up to 322,980 market-based restricted share units. These restricted stock units will vest and result in the issuance of common stock based on continuing employment and the relative ranking of the total shareholder return (“TSR”) of the Company’s common stock in relation to the TSR of the component companies in the S&P Health Care Equipment Select Industry Index over a three-year performance period based on a comparison of average closing stock prices between June 2015 and June 2018. The actual number of market-based restricted stock units that may be earned can range from 0% to 300% of the target number of shares. One-half of the market-based restricted stock units earned will vest in June 2018 and the remaining restricted stock units will vest one year thereafter provided the executive officers are still employed with the Company.
The Company used a Monte Carlo simulation model to estimate that the grant-date fair value of the restricted stock units. The fair value related to the restricted stock units is being recorded as stock compensation expense over the period from date of grant to June 2019 regardless of the actual TSR outcome achieved.
The table below sets forth the assumptions used to value the market-based awards and the estimated grant-date fair value:
Risk-free interest rate | | | 1.10 | % |
Dividend yield | | | 0 | % |
Remaining performance period (years) | | | 2.21 | |
Expected volatility | | | 47.2 | % |
Estimated grant date fair value (per share) | | $ | 107.10 | |
Target performance (number of shares) | | | 107,660 | |
Employee Stock Purchase Plan
The Company has an employee stock purchase plan, or ESPP. Under the ESPP, eligible employees, including officers and directors, who have completed at least three months of employment with the Company or its subsidiaries who elect to participate in the purchase plan instruct the Company to withhold a specified amount of the employee’s income each payroll period during a six-month payment period (the periods April 1—September 30 and October 1—March 31). On the last business day of each six-month payment period, the amount withheld is used to purchase shares of the Company’s common stock at an exercise price equal to 85% of the lower of its market price on the first business day or the last business day of the payment period. The Company recognized compensation expense of $0.5 million, $0.3 million and $0.2 million for the fiscal years ended March 31, 2016, 2015 and 2014, respectively, related to the ESPP.
F-25
Note 11. Income Taxes
The components of the Company’s income tax provision (benefit) for the fiscal years ended March 31, 2016, 2015 and 2014 are as follows:
| | 2016 | | | 2015 | | | 2014 | |
| | | | | | (in $000's) | | | | | |
Income before provision for income taxes: | | | | | | | | | | | | |
United States | $ | | 54,406 | | $ | | 22,243 | | $ | | 4,267 | |
Foreign | | | 11,432 | | | | 6,522 | | | | 4,263 | |
Income before income taxes | $ | | 65,838 | | $ | | 28,765 | | $ | | 8,530 | |
| | | | | | | | | | | | |
Current tax expense (benefit): | | | | | | | | | | | | |
Federal | $ | | 1,690 | | $ | | 464 | | $ | | (100 | ) |
State | | | 2,113 | | | | 424 | | | | (106 | ) |
Foreign | | | 1,592 | | | | 1,283 | | | | 525 | |
| | | 5,395 | | | | 2,171 | | | | 319 | |
Deferred tax expense (benefit): | | | | | | | | | | | | |
Federal | | | 18,769 | | | | (66,140 | ) | | | 825 | |
State | | | 1,284 | | | | (13,430 | ) | | | 35 | |
Foreign | | | 2,243 | | | | (7,524 | ) | | | - | |
| | | 22,296 | | | | (87,094 | ) | | | 860 | |
Total income tax provision (benefit) | $ | | 27,691 | | $ | | (84,923 | ) | $ | | 1,179 | |
F-26
The components of the Company’s net deferred taxes were as follows:
| March 31, | |
| 2016 | | | 2015 | |
| (in $000's) | |
Deferred tax assets | | | | | | | | | |
NOL carryforwards and tax credit carryforwards | $ | | 34,305 | | | $ | | 60,081 | |
Stock-based compensation | | | 14,879 | | | | | 10,568 | |
Nondeductible reserves and accruals | | | 8,550 | | | | | 7,573 | |
Amortizable intangibles other than goodwill | | | 2,420 | | | | | 2,993 | |
Capitalized research and development | | | 442 | | | | | 1,597 | |
Foreign NOL carryforwards | | | 17,635 | | | | | 19,617 | |
Deferred revenue | | | 3,351 | | | | | 2,669 | |
Depreciation | | | 353 | | | | | 276 | |
Other, net | | | 1,802 | | | | | 1,298 | |
| 83,737 | | | 106,672 | |
Deferred tax liabilities | | | | | | | | | |
Indefinite lived intangibles | | | (8,480 | ) | | | | (7,530 | ) |
In-process research and development | | | (4,649 | ) | | | | (4,443 | ) |
Domestic deferred tax liability on foreign NOL carryforwards | | | (10,488 | ) | | | | (12,276 | ) |
| | | (23,617 | ) | | | | (24,249 | ) |
| | | | | | | | | |
Net deferred tax assets | | | 60,120 | | | | | 82,423 | |
Valuation allowance | | | (2,418 | ) | | | | (2,912 | ) |
Net deferred tax assets | $ | | 57,702 | | | $ | | 79,511 | |
| | | | | | | | | |
Reported as: | | | | | | | | | |
Long-term deferred tax assets, net | | | 58,534 | | | | | 80,306 | |
Long-term deferred tax liabilities | | | (832 | ) | | | | (795 | ) |
Net deferred tax assets | $ | | 57,702 | | | $ | | 79,511 | |
As disclosed in Note 2. “Summary of Significant Accounting Policies,” The Company early adopted ASU No. 2015-17 and has applied the guidance retrospectively to all periods presented. The impact on the March 31, 2015 balance sheet was a reclassification of $35.1 million from current deferred tax assets to long-term deferred tax assets. Adoption of this standard did not impact the Company’s results of operations, retained earnings, or cash flows in the current or previous interim and annual reporting periods.
A reconciliation of the federal statutory income tax rate to the Company’s effective income tax rate is as follows for the fiscal years ended March 31, 2016, 2015, and 2014:
| | 2016 | | | 2015 | | | 2014 | | |
Statutory income tax rate | | | 35.0 | | % | | 35.0 | | % | | 34.0 | | % |
Increase (decrease) resulting from: | | | | | | | | | | | | | |
Change in valuation allowance | | | 0.7 | | | | (342.8 | ) | | | (53.7 | ) | |
Credits | | | (4.1 | ) | | | (1.9 | ) | | | (20.1 | ) | |
Foreign taxes | | | 2.5 | | | | 4.5 | | | | - | | |
State taxes, net | | | 3.7 | | | | 4.0 | | | | 12.9 | | |
Permanent differences | | | 3.0 | | | | 3.9 | | | | 0.4 | | |
Stock based compensation | | | 0.3 | | | | 0.3 | | | | 0.9 | | |
Rate differential on foreign operations | | | - | | | | 0.2 | | | | 31.1 | | |
Other | | | 1.0 | | | | 1.6 | | | | 8.4 | | |
Effective tax rate | | | 42.1 | | % | | (295.2 | ) | % | | 13.9 | | % |
F-27
The Company regularly assesses its ability to realize its deferred tax assets. Assessing the realization of deferred tax assets requires significant management judgment. In determining whether its deferred tax assets are more likely than not realizable, the Company evaluates all available positive and negative evidence, and weights the evidence based on its objectivity.
During the fiscal year ended March 31, 2015, the Company determined based on its consideration of the weight of positive and negative evidence that there was sufficient positive evidence that most of its federal, state and certain foreign deferred tax assets are more likely than not recoverable as of March 31, 2015. The Company’s conclusion was primarily driven by the receipt of PMA approval for our Impella 2.5™ product in March 2015, our history of profits in recent years and our expectation of continuing future profitability. Accordingly, the Company recorded a $101.5 million reversal of the valuation allowance in the quarter ended March 31, 2015, primarily related to the Company expecting to be able to use NOL carryforwards in the future in the U.S. and Germany.
As of March 31, 2016 and 2015, respectively, the Company recorded a valuation allowance of $2.4 million and $2.9 million which represents deferred tax assets related to NOL carryforwards in certain foreign jurisdictions in which the Company has had limited history of profitability. Based on the review of all available evidence, the Company recorded a valuation allowance to reduce these deferred tax assets to the amount that is more likely than not to be realizable as of March 31, 2016 and 2015.
Changes in the valuation allowance for deferred tax assets during the fiscal years ended March 31, 2016, 2015 and 2014 were as follows:
| | 2016 | | | 2015 | | | 2014 | |
| | (in $000's) | |
Valuation allowance as of beginning of year | $ | | 2,912 | | $ | | 102,093 | | $ | | 106,670 | |
Decreases recorded as benefit to income tax provision | | | (1,171 | ) | | | (101,468 | ) | | | (4,577 | ) |
Increases due to foreign net operating loss in certain foreign jurisdictions | | | 677 | | | | 2,287 | | | | - | |
Valuation allowance as of end of year | $ | | 2,418 | | $ | | 2,912 | | $ | | 102,093 | |
At March 31, 2016, the Company had federal net operating loss carryforwards, or NOLs, of approximately $173.2 million which expire in varying years from fiscal 2018 through fiscal 2034. At March 31, 2016, the Company had German and French NOLs of approximately $25.0 million and $2.4 million, respectively, which do not expire. In addition, at March 31, 2016, the Company had federal and state research and development credit carryforwards of approximately $12.4 million and $8.0 million, respectively, which expire in varying years from fiscal 2017 through fiscal 2036.
Of the total amount of available federal NOLs, $142.0 million relates to stock-based compensation tax deductions in excess of stock-based compensation expense for financial reporting purposes (“excess tax benefits”). Excess tax benefits are realized when they reduce income taxes payable, as determined using a “with and without” method, and are credited to additional paid-in capital rather than as a reduction of the income tax provision. During the year ended March 31, 2016, the Company realized excess tax benefits from federal and state tax deductions of $3.6 million which were credited to additional paid-in capital.
The Company and its subsidiaries are subject to U.S. federal income tax, as well as income tax of multiple state and foreign jurisdictions. Fiscal years 2012 through 2016 remain open to examination in Germany. All tax years remain subject to examination by the Internal Revenue Service and state tax authorities, because the Company has net operating loss and tax credit carryforwards which may be utilized in future years to offset taxable income, those years may also be subject to review by relevant taxing authorities if the carryforwards are utilized.
Note 12. Commitments and Contingencies
Commitments
The following is a description of the Company’s significant arrangements in which the Company is a guarantor.
Indemnifications—In many sales transactions, the Company indemnifies customers against possible claims of patent infringement caused by the Company’s products. The indemnifications contained within sales contracts usually do not include limits on the claims. The Company has never incurred any material costs to defend lawsuits or settle patent infringement claims related to sales transactions.
F-28
The Company enters into agreements with other companies in the ordinary course of business, typically with underwriters, contractors, clinical sites and customers that include indemnification provisions. Under these provisions the Company generally indemnifies and holds harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of its activities. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments the Company could be required to make under these indemnification provisions is unlimited. The Company has never incurred any material costs to defend lawsuits or settle claims related to these indemnification agreements. As a result, the estimated fair value of these agreements is immaterial. Accordingly, the Company has no liabilities recorded for these agreements as of March 31, 2016.
Clinical study agreements—In the Company’s clinical study agreements, the Company has agreed to indemnify the participating institutions against losses incurred by them for claims related to any personal injury of subjects taking part in the study to the extent they relate to uses of the Company’s devices in accordance with the clinical study agreement, the protocol for the device and the Company’s instructions. The indemnification provisions contained within the Company’s clinical study agreements do not generally include limits on the claims. The Company has never incurred any material costs related to the indemnification provisions contained in its clinical study agreements.
Facilities leases— The Company’s headquarters is located at 22 Cherry Hill Drive in Danvers, Massachusetts and consists of approximately 125,560 square feet of space under an operating lease.
The monthly lease payments over the remaining term of the lease are as follows:
| · | $84,914 base rent per month from March 2016 through February 2018; and |
| · | $87,530 base rent per month from March 2018 through February 2021. |
This facility encompasses most of the Company’s U.S. operations, including research and development, manufacturing, sales and marketing and general and administrative departments. On December 9, 2015, the Company entered into a purchase and sale agreement (the “P&S Agreement”) to acquire its existing corporate headquarters space. Pursuant to the P&S Agreement, the Company expects, among other things and subject to closing conditions, to acquire the real estate commonly known as 18-22 Cherry Hill Drive, located in Danvers, Massachusetts. Subject to the terms and conditions of the P&S Agreement, the purchase price of the property will be $16.5 million. The Company has entered into two amendments of the P&S Agreement dated January 19, 2016 and April 19, 2016 to extend the due diligence period related to the purchase of the property until July 19, 2016.
The Company’s European headquarters is located in Aachen, Germany and consists of approximately 33,000 square feet of space under an operating lease. In July 2013, the Company entered into a lease agreement to continue renting its existing space in Aachen, Germany through July 31, 2023. In October 2015, the Company entered into an amendment to this lease agreement to lease 9,000 square feet of additional space effective July 1, 2015. The Company also entered into another lease agreement in October 2015 to lease approximately 30,000 square feet of additional space adjacent to its Aachen facility from July 1, 2015 through June 30, 2016. This agreement also provided the Company with options to extend the lease through July 31, 2033. The lease payments under these agreements are approximately 64,500€ (euro) (approximately U.S. $73,000 at March 31, 2016 exchange rates) per month. The building houses most of the manufacturing operations for the Impella® product lines as well as certain research and development functions and the sales, marketing and general and administrative functions for most of its product lines sold in Europe and the Middle East.
Total rent expense for the Company’s operating leases included in the accompanying consolidated statements of operations approximated $2.4 million, $1.9 million and $1.5 million for the fiscal years ended March 31, 2016, 2015 and 2014, respectively.
Future minimum lease payments under non-cancelable operating leases and contractual obligations as of March 31, 2016 are approximately as follows:
Fiscal Years Ending March 31, | | | | | | | | | | |
| | | | | | | | | | | (in $000s) | |
2017 | | | | | | | | | | | $ | | 2,252 | |
2018 | | | | | | | | | | | | | 2,185 | |
2019 | | | | | | | | | | | | | 2,017 | |
2020 | | | | | | | | | | | | | 1,979 | |
2021 | | | | | | | | | | | | | 1,892 | |
Thereafter | | | | | | | | | | | | | 2,048 | |
Total future minimum lease payments | | | | | | | | | | | $ | | 12,373 | |
F-29
License agreements—In April 2014, the Company entered into an exclusive license agreement for the rights to certain optical sensor technologies in the field of cardio-circulatory assist devices. Under the agreement, the Company made a $1.5 million upfront payment upon execution of the agreement and agreed to make additional payments of up to $4.5 million upon achievement of development milestones.
In November 2015, the Company entered into an exclusive license agreement for the rights to certain vascular closure device technologies. The Company made a $0.5 million upfront payment upon execution of the agreement and a milestone payment of $0.6 million in December 2015 and $0.5 million in April 2016. The Company could make additional payments of up to $2.0 million upon the achievement of certain development milestones.
The Company is also party to a license agreement related to certain circulatory care device patents and know-how. Under this agreement, the Company would be obligated to pay up to $3.0 million in cash or stock, if certain development and regulatory milestones are achieved.
Contingencies
From time to time, the Company is involved in legal and administrative proceedings and claims of various types. In some actions, the claimants seek damages, as well as other relief, which, if granted, would require significant expenditures. The Company records a liability in its consolidated financial statements for these matters when a loss is known or considered probable and the amount can be reasonably estimated. The Company reviews these estimates each accounting period as additional information is known and adjusts the loss provision when appropriate. If a matter is both probable to result in liability and the amounts of loss can be reasonably estimated, the Company estimates and discloses the possible loss or range of loss. If the loss is not probable or cannot be reasonably estimated, a liability is not recorded in its consolidated financial statements.
On October 26, 2012, the Company was informed that the Department of Justice, United States Attorney’s Office for the District of Columbia was conducting an investigation (“Marketing and Labeling Investigation”) focused on the Company’s marketing and labeling of the Impella 2.5™ heart pump. On October 31, 2012, the Company accepted service of a subpoena related to this investigation seeking documents and other materials related to the Impella 2.5. The Company cooperated fully with the Marketing and Labeling Investigation, and on June 29, 2015, the Company received confirmation that the Department of Justice had closed the Marketing and Labeling Investigation without taking enforcement action.
On April 25, 2014, the Company received a subpoena from the Boston regional office of the United States Department of Health and Human Services, or HHS, Office of Inspector General requesting materials relevant to the Company’s reimbursement of expenses and remuneration to healthcare providers for a six month period from July 2012 through December 2012 in connection with a civil investigation under the False Claims Act (the “FCA Investigation”). The Company submitted the requested documents to HHS and believes that it substantially complied with the subpoena. On November 6, 2014, the Company received notice from the Department of Justice, United States Attorney’s Office for the District of Massachusetts in the form of a Civil Investigative Demand (“CID”) requesting additional materials relating to this matter for the time period of January 1, 2012 through December 31, 2013. The Company has responded to the additional requests for information contained in the CID, and is in the process of responding to other informal requests. The Company intends to continue to cooperate with the U.S. Attorney’s Office in connection with the FCA Investigation.
In July and August 2015, Thoratec Corporation (“Thoratec”), acquired by St. Jude Medical, Inc. in October 2015, brought actions in connection with two Company patents relevant to Thoratec’s HeartMate PHP medical device (“PHP”). In those proceedings, which are in the United Kingdom and Germany, Thoratec asserts that the two patents are invalid. In September 2015, the Company filed counterclaims in the action in Germany asserting that the PHP product infringes the two patents and a third patent owned by the Company. Both the Germany and United Kingdom proceedings are ongoing.
In December 2015, the Company received a letter from Maquet Cardiovascular LLC, a subsidiary of the Getinge Group (“Maquet”), and maker of the intra-aortic balloon pump, asserting that Abiomed’s Impella products infringe certain guidewire, lumen and sensor claims of two Maquet patents and Maquet filed one pending patent application that Maquet has filed in the U.S. and elsewhere, and encouraged the Company to discuss taking a license from Maquet. In January 2016, the Company responded to Maquet stating that it believed that the cited claims were invalid and that its Impella products did not infringe the cited patents. In May 2016, Maquet sent a second letter to Abiomed notifying the Company that the pending patent application had been issued as a U.S. patent and repeated their earlier assertion and encouraged the Company to discuss taking a license from Maquet. On May 19, 2016, the Company filed suit in Massachusetts District Court against Maquet seeking a declaratory judgment that Abiomed’s Impella products do not infringe Maquet’s cited patent rights.
F-30
The Company is unable to estimate a potential liability with respect to the legal matters noted above. There are numerous factors that make it difficult to meaningfully estimate possible loss or range of loss at this stage of the legal proceedings, including that the FCA Investigation and patent disputes with Thoratec and Maquet remain in relatively early stages, there are significant factual and legal issues to be resolved and information obtained or rulings made during any potential lawsuits or investigations could affect the methodology for calculation. Therefore, the Company is unable at this time to estimate a possible loss or range of possible loss, and no adjustment has been made to the financial statements to reflect the outcome of these uncertainties.
Note 13. Accrued Expenses
Accrued expenses consisted of the following:
| | March 31, 2016 | | | March 31, 2015 | |
| | (in $000's) | |
Employee compensation | | $ | 18,359 | | | $ | 15,978 | |
Sales and income taxes | | | 2,527 | | | | 1,506 | |
Professional, legal and accounting fees | | | 1,764 | | | | 710 | |
Research and development | | | 1,587 | | | | 1,744 | |
Marketing | | | 1,146 | | | | - | |
Warranty | | | 998 | | | | 1,103 | |
Other | | | 2,001 | | | | 853 | |
| | $ | 28,382 | | | $ | 21,894 | |
Accrued employee compensation consists primarily of accrued bonuses, accrued commissions and accrued employee benefits at March 31, 2016 and 2015.
Note 14. Segment and Enterprise Wide Disclosures
The Company operates in one business segment—the research, development and sale of medical devices to assist or replace the pumping function of the failing heart. The Company’s chief operating decision maker (determined to be the Chief Executive Officer) does not manage any part of the Company separately, and the allocation of resources and assessment of performance are based on the Company’s consolidated operating results. International sales (sales outside the U.S. and primarily in Europe) accounted for 8%, 10% and 9% of total product revenue during the fiscal years ended March 31, 2016, 2015 and 2014, respectively. As of March 31, 2016 and 2015, most of the Company’s long-lived assets are located in the U.S. except for $5.9 million and $3.8 million at March 31, 2016 and 2015, respectively, which are located primarily in Germany.
Note 15. Quarterly Results of Operation (Unaudited)
The following is a summary of the Company’s unaudited quarterly results of operations for the fiscal years ending March 31, 2016 and 2015:
| | Fiscal Year Ended March 31, 2016 | |
| | 1st Quarter | | | 2nd Quarter | | | 3rd Quarter | | | 4th Quarter | | | Total Year | |
| | (in $000's) | |
Total revenues | | $ | | 73,432 | | | $ | | 76,359 | | | $ | | 85,795 | | | $ | | 93,957 | | | $ | | 329,543 | |
Cost of product revenue | | | | 10,868 | | | | | 12,144 | | | | | 12,744 | | | | | 14,663 | | | | | 50,419 | |
Other operating expenses | | | | 47,533 | | | | | 51,398 | | | | | 55,608 | | | | | 59,481 | | | | | 214,020 | |
Other income, net | | | | 116 | | | | | 149 | | | | | 55 | | | | | 414 | | | | | 734 | |
Income before income taxes | | | | 15,147 | | | | | 12,966 | | | | | 17,498 | | | | | 20,227 | | | | | 65,838 | |
Income tax provision | | | | 6,288 | | | | | 5,231 | | | | | 6,943 | | | | | 9,229 | | | | | 27,691 | |
Net income | | $ | | 8,859 | | | $ | | 7,735 | | | $ | | 10,555 | | | $ | | 10,998 | | | $ | | 38,147 | |
Basic net income per share | | $ | | 0.21 | | | $ | | 0.18 | | | $ | | 0.25 | | | $ | | 0.26 | | | $ | | 0.90 | |
Diluted net income per share | | $ | | 0.20 | | | $ | | 0.17 | | | $ | | 0.23 | | | $ | | 0.24 | | | $ | | 0.85 | |
F-31
| | Fiscal Year Ended March 31, 2015 | |
| | 1st Quarter | | | 2nd Quarter | | | 3rd Quarter | | | 4th Quarter | | | Total Year | |
| | (in $000's) | |
Total revenues | $ | | 48,811 | | | $ | | 51,938 | | | $ | | 62,005 | | | $ | | 67,557 | | | $ | | 230,311 | |
Cost of product revenue | | | | 9,689 | | | | | 9,612 | | | | | 9,838 | | | | | 10,806 | | | | | 39,945 | |
Other operating expenses | | | | 40,660 | | | | | 38,148 | | | | | 38,504 | | | | | 44,388 | | | | | 161,700 | |
Other income (expense), net | | | | 55 | | | | | (3 | ) | | | | 38 | | | | | 9 | | | | | 99 | |
Income (loss) before income taxes | | | | (1,483 | ) | | | | 4,175 | | | | | 13,701 | | | | | 12,372 | | | | | 28,765 | |
Income tax (benefit) provision (1) | | | | 226 | | | | | 336 | | | | | 1,017 | | | | | (86,502 | ) | | | | (84,923 | ) |
Net income (loss) | | $ | | (1,709 | ) | | $ | | 3,839 | | | $ | | 12,684 | | | $ | | 98,874 | | | $ | | 113,688 | |
Basic net income (loss) per share | | $ | | (0.04 | ) | | $ | | 0.09 | | | $ | | 0.31 | | | $ | | 2.40 | | | $ | | 2.80 | |
Diluted net income (loss) per share | | $ | | (0.04 | ) | | $ | | 0.09 | | | $ | | 0.30 | | | $ | | 2.24 | | | $ | | 2.65 | |
(1) | Income tax benefit for the quarter and year ended March 31, 2015 were impacted by the release of the $101.5 million valuation allowance on certain deferred tax assets. |
F-32
