UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
| | |
| | [X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
or |
| | |
| | [ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 001-16783
VCA Inc.
(Exact name of registrant as specified in its charter)
|
| | |
| Delaware | | 95-4097995 |
(State or other jurisdiction of incorporation or organization) 12401 West Olympic Boulevard, Los Angeles, California (Address of principal executive offices) | | (I.R.S. employer identification no.) 90064-1022 (Zip code) |
| |
Registrant’s telephone number, including area code: (310) 571-6500 Securities registered pursuant to Section 12(b) of the Act: |
|
| | |
| Title of Each Class | | Name of Each Exchange on Which Registered |
| Common Stock, par value $0.001 per share | | Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [X] No [ ]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes [ ] No [X]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
| | | | | | |
| Large accelerated filer [X] | | Accelerated filer [ ] | | Non-accelerated filer [ ] | | Smaller reporting company [ ]
|
| | | | | (Do not check if a smaller reporting company) | | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes [ ]
No [X]
The aggregate market value of the voting common equity held by non-affiliates as of June 30, 2015, was approximately $4.3 billion, computed by reference to the price of $54.41 per share, the price at which the common equity was last sold on such date as reported on the NASDAQ Global Select Market. For purposes of this computation, it is assumed that the shares beneficially held by directors and officers of the registrant would be deemed to be stock held by affiliates. Non-affiliated common stock outstanding at June 30, 2015 was 78,669,691 shares. Total common stock outstanding at February 22, 2016 was 80,765,877 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Parts of the definitive Proxy Statement to be delivered to stockholders in connection with the 2016 Annual Meeting of Stockholders are incorporated by reference into Items 10, 11, 12, 13 and 14 hereof.
VCA Inc. and Subsidiaries
Table of Contents
|
| | |
| | | |
| | | Page |
| | PART I | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | PART II | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | PART III | |
| | |
| | |
| | |
| | |
| | |
| | | |
| | PART IV | |
| | |
| | | |
PART I
Company Overview
We are a leading national animal healthcare company operating in the United States and Canada. We provide veterinary services and diagnostic testing to support veterinary care, we sell diagnostic imaging equipment and other medical technology products and related services to the veterinary market.
Our animal hospitals offer a full range of general medical and surgical services for companion animals, as well as specialized treatments including advanced diagnostic services, internal medicine, oncology, ophthalmology, dermatology and cardiology. In addition, we provide pharmaceutical products and perform a variety of pet wellness programs including health examinations, diagnostic testing, routine vaccinations, spaying, neutering and dental care. Our network of animal hospitals is supported by nearly 3,000 veterinarians and had approximately 9.8 million patient visits in 2015.
Our network of veterinary diagnostic laboratories provides sophisticated testing and consulting services used by veterinarians in the detection, diagnosis, evaluation, monitoring, treatment and prevention of diseases and other conditions affecting animals. Our network of veterinary diagnostic laboratories provides diagnostic testing for over 17,000 clients, which includes standard animal hospitals, large animal practices, universities and other government organizations.
Our Medical Technology business sells digital radiography, ultrasound imaging equipment, and other advanced imaging/diagnostic modalities, in addition provides education and training on the use of that equipment, and provides consulting and mobile imaging services.
Our pet services business primarily franchises a premier provider of pet services including dog day care, overnight boarding, grooming and other ancillary services at specially designed pet care facilities, principally under the trademark Camp Bow Wow®. Camp Bow Wow also operates several corporate-owned facilities.
Our principal executive offices are located at 12401 West Olympic Boulevard, Los Angeles, California. We can be contacted at (310) 571-6500.
Company History
Our company was formed in 1986 as a Delaware corporation and has since established a position in the animal healthcare industry through both internal growth and by acquisitions. By 2011, we operated a total of 541 animal hospitals, 53 laboratories, a supplier of digital radiography and ultrasound imaging equipment and a provider of online communications, professional education and marketing solutions to the veterinary community. Subsequent to 2011, our company continued to grow by adding, additional laboratories, independent animal hospitals, animal hospital chains and other ancillary businesses, the following of which were noteworthy:
| |
| • | On January 31, 2012 we expanded our operations into Canada with an increased investment in Associate Veterinary Clinics (1984) Limited ("AVC"), which operated 44 hospitals in three Canadian provinces as of the acquisition date. |
| |
| • | On August 15, 2014, we acquired Camp Bow Wow Franchising, Inc., formerly known as D.O.G. Enterprises, LLC ("Camp Bow Wow"). On the acquisition date, there were 125 Camp Bow Wow® franchise locations operating in 37 states and one Canadian province. The acquisition has expanded our participation in the dog boarding and day care service segment of the pet health industry. |
On December 31, 2015, our company sold substantially all of the assets of Vetstreet Inc., formerly known as MediMedia Animal Health LLC ("Vetstreet") to a subsidiary of Henry Schein, Inc..
| |
| • | Concurrent with the sale of Vetstreet, we purchased a 19.9% interest in the continuing Vetstreet business. |
Industry Overview
According to American Pet Products Association, Inc’s. (“APPA”) 2015-2016 APPA National Pet Owners Survey, the United States population of companion animals is approximately 185 million, including about 164 million dogs and cats. APPA estimates that approximately $35 billion was spent in the United States on pets in 2015 for veterinary care, supplies, medicine and boarding and grooming. The survey indicated that the ownership of pets is widespread with approximately 80 million, or 65%, of U.S. households owning at least one pet, including companion and other animals. Specifically, 54 million households owned at least one dog and 43 million households owned at least one cat.
We believe that among pet owners there is a growing awareness of pet health and wellness, including the benefits of preventive care and specialized services. As technology continues to migrate from the human healthcare sector into the practice of veterinary medicine, more sophisticated treatments, diagnostic tests and equipment are becoming available to treat companion animals. These new and increasingly complex procedures, diagnostic tests, including laboratory testing and advanced imaging, and pharmaceuticals are gaining wider acceptance as pet owners are exposed to these previously unconsidered treatment programs through their exposure with this technology in human healthcare, and through literature and marketing programs sponsored by large pharmaceutical and pet nutrition companies.
Even as treatments available in veterinary medicine become more complex, prices for veterinary services typically remain a low percentage of a pet owner’s income, facilitating payment at the time of service. Unlike the human healthcare industry, providers of veterinary services are not dependent on third-party payers in order to collect fees. As such, providers of veterinary services typically do not have the problems of extended payment collection cycles or pricing pressures from third-party payers faced by human healthcare providers. Outsourced laboratory testing and diagnostic equipment sales are wholesale businesses that collect payments directly from animal hospitals under standard industry payment terms. Fees for services provided in our animal hospitals are due at the time of service. In 2015, over 99% of our animal hospital services were paid at the time of service. In addition, over the past three fiscal years our bad debt expense has averaged less than 1% of total revenue.
The practice of veterinary medicine is subject to seasonal fluctuation. In particular, demand for veterinary services is significantly higher during the warmer months because pets spend a greater amount of time outdoors, where they are more likely to be injured and are more susceptible to disease and parasites. In addition, use of veterinary services may be affected by levels of infestation of fleas, heartworms and ticks, and the number of daylight hours.
Animal Hospital Industry
Animal healthcare is provided predominately by the veterinarian practicing as a sole practitioner, or as part of a larger group practice or hospital. Veterinarians diagnose and treat animal illnesses and injuries, perform surgeries, provide routine medical exams and prescribe medication. Some veterinarians specialize by type of medicine, such as orthopedics, dentistry, ophthalmology or dermatology. Others focus on a particular type of animal. The principal factors in a pet owner’s decision as to which veterinarian to use include convenient location and hours, personal recommendations, reasonable fees and quality of care.
According to the American Veterinary Medical Association, the U.S. market for veterinary services is highly fragmented with more than 53,000 veterinarians practicing at the end of 2015. We have estimated that there are over 26,000 companion animal hospitals operating at the end of 2015. Although most animal hospitals are single-site, sole-practitioner facilities, we believe veterinarians are gravitating toward larger, multi-doctor animal hospitals that provide state-of-the-art facilities, treatments, methods and pharmaceuticals to enhance the services they can provide their clients.
Well-capitalized animal hospital operators have the opportunity to supplement their internal growth with selective acquisitions. We believe the extremely fragmented animal hospital industry is consolidating due to:
| |
| • | the choice of some owners of animal hospitals to diversify their investment portfolio by selling all or a portion of their investment in the animal hospital; |
| |
| • | the purchasing, marketing and administrative cost advantages that can be realized by a large, multiple location, multi-doctor veterinary provider; |
| |
| • | the cost of financing equipment purchases and upgrading technology necessary for a successful practice; |
| |
| • | the desire of veterinarians to focus on practicing veterinary medicine, rather than spending large portions of their time performing the administrative tasks necessary to operate an animal hospital; and |
| |
| • | the appeal to many veterinarians of the benefits and flexible work schedule that is not typically available to a sole practitioner or single-site provider. |
Diagnostic Laboratory Industry
Veterinarians use laboratory tests to diagnose and monitor illnesses and conditions through the detection of substances in urine, tissue, fecal and blood samples, and other specimens. As is the case with the physician treating a human patient, laboratory diagnostic testing is becoming a routine diagnostic tool used by the veterinarian.
Veterinary laboratory tests are performed primarily at veterinary diagnostic laboratories, universities or at animal hospitals using on-site diagnostic equipment. For certain tests, on-site diagnostic equipment can provide more timely results than outside laboratories, but this in-house testing requires the animal hospital or veterinarian to purchase or lease the equipment, maintain and calibrate the equipment periodically to avoid testing errors, employ trained personnel to operate it and purchase testing supplies. Conversely, veterinary diagnostic laboratories can provide a wider range of tests than generally are available on-site at most animal hospitals and do not require any up-front investment on the part of the animal hospital or veterinarian. Leading veterinary diagnostic laboratories also employ highly trained individuals who specialize in the detection and diagnosis of diseases and thus are a valuable resource for the veterinarian.
Our laboratories offer a broad spectrum of standard and customized tests to the veterinary market, convenient sample pick-up times, rapid test reporting and access to professional consulting services provided by trained specialists. Providing the customer with this level of service at competitive prices requires high throughput volumes due to the operating leverage associated with the laboratory business. As a result, larger laboratories are likely to have a competitive advantage relative to smaller laboratories.
We believe that the outsourced laboratory testing market is an integral segment of the animal healthcare industry as a result of:
| |
| • | the emphasis in veterinary education on diagnostic tests and the trend toward specialization in veterinary medicine, which are causing veterinarians to increasingly rely on tests for more accurate diagnoses; |
| |
| • | the continued technological developments in veterinary medicine, which are increasing the breadth of tests offered; and |
| |
| • | the continued focus on wellness, early detection and monitoring programs in veterinary medicine. |
Business Strategy
Our business strategy is to continue expanding our market leadership in animal healthcare through our Animal Hospital, Laboratory, Medical Technology and Camp Bow Wow operating segments. Key elements to our strategy include:
| |
| • | Capitalizing on our Leading Market Position to Generate Revenue Growth. Our leading market position in the animal hospital, veterinary laboratory and pet services franchising markets positions us to capitalize on favorable growth trends in the animal healthcare industry. In our animal hospitals, we seek to generate revenue growth by capitalizing on the growing emphasis on pet health and wellness. In our laboratories, we seek to generate revenue growth by taking advantage of the growing number of outsourced diagnostic tests, the opportunities to expand the testing that we provide and by increasing our market share. We continually educate veterinarians on new and existing technologies and tests available to diagnose medical conditions. Further, we leverage the knowledge of our specialists by providing veterinarians with extensive client support in utilizing and understanding these diagnostic tests. Our Medical Technology business seeks to leverage our strengths in the broader veterinary markets by introducing technologies, products and services to the veterinary market. We seek to generate revenue growth by increasing our market share and educating veterinarians on better utilization of new and existing technologies. In our Camp Bow Wow business, we provide an all-day, full-service offering, including large outdoor and indoor play areas, spacious cabins, comfortable cots, live webcams and Certified Camp Counselors. More importantly, we provide assurance to owners that their pets will be in a safe, happy and loved environment. Our Home Buddies programs provide dog walking, pet sitting, in-home webcam rentals, pet waste cleanup and other ancillary services. Our Home Buddies programs provide reward-based dog training including obedience classes, private sessions and advanced behavior modification. We use certified trainers to help dogs deal with aggression towards people and other animals, fence fighting, leash reactivity and separation anxiety. We plan to leverage Camp Bow Wow's and VCA's existing customer bases and facilities to cross-promote VCA and Camp Bow Wow services. |
| |
| • | Leveraging Established Infrastructure to Improve Margins. We intend to leverage our established Animal Hospital and Laboratory infrastructure to increase our operating margins. Due to our established networks and the fixed cost nature of our business model, we are able to realize high margins on incremental revenue from Animal Hospital and Laboratory customers. For example, given that our nationwide transportation network servicing our Laboratory customers is a relatively fixed cost, we are able to achieve significantly higher margins on most incremental tests ordered by the same customer when picked up by our couriers at the same time. |
| |
| • | Utilizing Enterprise-Wide Information Systems to Improve Operating Efficiencies. Our Laboratory and the majority of our Animal Hospital operations utilize enterprise-wide management information systems. We believe that these common systems enable us to more effectively manage the key operating metrics that drive our business. With the aid of these systems, we seek to standardize pricing, expand the services we provide and increase volume through targeted marketing programs. |
| |
| • | Pursuing Selected Acquisitions. The fragmentation of the animal hospital industry provides us with significant expansion opportunities in our Animal Hospital segment. Depending upon the attractiveness of the candidates and the strategic fit with our existing operations, we intend to acquire independent animal hospitals each year. Our overall acquisition strategy involves the identification of high-quality practices in both domestic and international markets, where we can create additional value through the services and scale we can provide. Our typical candidate mirrors the profile of our existing animal hospital base. These acquisitions will be used to both expand existing markets and to enter into new geographic areas. In addition, we also evaluate the acquisition of animal hospital chains, laboratories or related businesses if favorable opportunities are presented. We intend primarily to use cash in our acquisitions but, depending on the timing and amount of our acquisitions, we may use stock or debt. |
Business Segments
We report our results of operations through two reportable segments: Animal Hospital and Laboratory. Our Medical Technology and Camp Bow Wow operating segments do not meet the materiality requirements to be presented as separate reportable segments. Accordingly, they are grouped into an “All Other” category.
Information regarding revenue and operating income, attributable to each of our reportable segments, is included in the Segment Results section within Management’s Discussion and Analysis of Financial Condition and Results of Operations, and within Note 15, Lines of Business, of our Notes to Consolidated Financial Statements, which are incorporated herein by reference.
Animal Hospital
At December 31, 2015, we operated or managed 682 animal hospitals serving 41 states and four Canadian provinces. Our Animal Hospital revenue accounted for 79% of total consolidated revenue in 2015, 2014 and 2013, respectively.
Services
In addition to general medical and surgical services, we offer specialized treatments for companion animals, including advanced diagnostic services, internal medicine, oncology, neurology, endocrinology, ophthalmology, dermatology and cardiology. We also provide pharmaceutical products for use in the delivery of treatments by our veterinarians and pet owners. Many of our animal hospitals offer additional services, including grooming, bathing and boarding. We also sell specialty pet products at our animal hospitals, including pet food, vitamins, therapeutic shampoos and conditioners, flea collars and sprays, and other accessory products. During 2014 we launched VCA CareClubTM as the pet healthcare solution for pet owners who want a comprehensive and affordable way to keep their pets as healthy and happy as possible through every stage of their lives. VCA CareClubTM is a membership program that covers all life stages and includes: convenient monthly payments, multiple visits to a VCA hospital each year, veterinarian-recommended vaccines, prevention and early detection tests of serious diseases and routine dental care. We believe this preventative and early detection wellness program offers our customers peace of mind knowing they are doing the best for their pet.
Animal Hospital Network
We seek to provide quality care in clean, attractive facilities that are generally open between 10 to 15 hours per day, six to seven days per week. Our typical animal hospital:
| |
| • | is located in a 4,000 to 6,000 square-foot, freestanding facility in an attractive location; |
| |
| • | has annual revenue between $1 million and $3 million; |
| |
| • | is supported by three to five veterinarians; and |
| |
| • | has an operating history of over 10 years. |
As of December 31, 2015, our network of freestanding, full-service animal hospitals had facilities located in the following states and four Canadian provinces:
|
| | | | | | | | |
| California | | 120 |
| | Oklahoma | | 7 |
|
| Texas* | | 49 |
| | Nevada | | 7 |
|
| Washington* | | 36 |
| | Alaska | | 6 |
|
| New York* | | 36 |
| | Hawaii | | 5 |
|
| Florida | | 31 |
| | New Mexico | | 5 |
|
| Massachusetts | | 28 |
| | New Hampshire* | | 5 |
|
| Pennsylvania | | 25 |
| | Delaware | | 4 |
|
| Colorado | | 21 |
| | Wisconsin | | 4 |
|
| Illinois | | 20 |
| | Tennessee | | 4 |
|
| Georgia | | 20 |
| | South Carolina | | 3 |
|
| Virginia | | 19 |
| | Missouri | | 3 |
|
| Arizona | | 17 |
| | Rhode Island* | | 3 |
|
| Connecticut | | 16 |
| | Nebraska* | | 3 |
|
| Oregon* | | 14 |
| | Utah | | 3 |
|
| New Jersey* | | 13 |
| | Kansas* | | 2 |
|
| Ohio | | 13 |
| | Kentucky | | 2 |
|
| Indiana | | 12 |
| | Louisiana* | | 2 |
|
| North Carolina* | | 12 |
| | Vermont | | 2 |
|
| Michigan* | | 12 |
| | Alabama* | | 1 |
|
| Maryland | | 11 |
| | West Virginia* | | 1 |
|
| Minnesota* | | 9 |
| | | | |
| | | | | | | |
| | | | | | | |
| Ontario, Canada* | | 32 |
| | | | |
| Alberta, Canada* | | 26 |
| | | | |
| British Columbia, Canada* | | 14 |
| | | | |
| Quebec, Canada* | | 4 |
| | | | |
|
| |
| * | States and Canadian provinces with laws, rules and regulations which require that veterinary medical practices be owned by licensed veterinarians and that corporations which are not owned by licensed veterinarians refrain from providing, or holding themselves out as providers of, veterinary medical care. In these states/provinces we do not own the veterinary practices or provide veterinary care but provide management and other administrative services to the independently owned veterinary practices. |
Marketing
We direct our marketing efforts toward increasing the number of annual visits from existing clients through customer education efforts and toward attracting new clients to our network of hospitals through online and offline initiatives. We inform and educate our clients about pet wellness and quality care through mailings of HealthyPet Magazine, which focuses on pet care and wellness. We also market through targeted demographic mailings regarding specific pet health issues and collateral health material made available at each animal hospital. With these internal marketing programs, we seek to leverage our existing customer base by increasing the number and intensity of the services received during each visit. We send reminder notices to increase awareness of the advantages of regular, comprehensive veterinary medical care, including preventive care such as early disease detection exams, vaccinations, dental screening and geriatric care.
Personnel
Our animal hospitals generally employ a staff of between 20 and 30 full-time-equivalent employees, depending upon the facility’s size and customer base. The staff includes administrative and technical support personnel, three to five veterinarians, and a hospital manager who supervises the day-to-day activities of the facility.
We actively recruit qualified veterinarians and technicians and are committed to supporting continuing education for our professional staff. We operate post-graduate teaching programs for veterinarians at 35 of our facilities, which train over 170 veterinarians each year. We believe that these programs enhance our reputation in the veterinary profession and further our ability to continue to recruit the most talented veterinarians.
We seek to establish an environment that supports the veterinarian in the delivery of quality medicine and fosters professional growth through increased patient flow and a diverse case mix, continuing education, state-of-the-art equipment and access to specialists. We believe our animal hospitals offer attractive employment opportunities to veterinarians because of our professional environment, competitive compensation, management opportunities, employee benefits not generally available to a sole practitioner, flexible work schedules that accommodate personal lifestyles and the ability to relocate to different regions of the country.
We have established a medical advisory board to support our operations. Our advisory board, under the direction of our Chief Medical Officer, recommends medical standards for our network of animal hospitals and is comprised of veterinarians recognized for their outstanding knowledge and reputations in the veterinary field. Our advisory board members represent both the different geographic regions in which we operate and the medical specialties practiced by our veterinarians; and three members are faculty members at highly-ranked veterinary colleges. Additionally, our regional medical directors, a group of highly experienced veterinarians, are also closely involved in the development and implementation of our medical programs.
Laboratory
We operate a full-service, veterinary diagnostic laboratory network serving all 50 states and certain areas in Canada. Our Laboratory revenue accounted for 16% of total consolidated revenue in 2015, 2014 and 2013. We service a diverse customer base of over 17,000 clients including animal hospitals we operate.
Services
Our diagnostic spectrum includes over 300 different tests in the area of chemistry, pathology, endocrinology, serology, hematology and microbiology, as well as tests specific to particular diseases.
Although modified to address the particular requirements of the species tested, the tests performed in our veterinary laboratories are similar to those performed in human clinical laboratories and utilize similar laboratory equipment and technologies. We believe that the growing concern for animal health, combined with the movement of veterinary medicine toward increasing specialization, may result in the migration of additional areas of human testing into the veterinary field.
Given the recent advancements in veterinary medical technology and the increased breadth and depth of knowledge required for the practice of veterinary medicine, many veterinarians solicit the knowledge and experience of our specialists to interpret test results to aid in the diagnosis of illnesses and to suggest possible treatment alternatives. Our diagnostic experts include veterinarians, chemists and other scientists with expertise in pathology, internal medicine, oncology, cardiology, dermatology, neurology and endocrinology. Because of our specialists involvement, we believe the quality of our service further distinguishes our laboratory services as a premiere service provider.
Laboratory Network
At December 31, 2015, we operated 60 veterinary diagnostic laboratories. Our laboratory network includes:
| |
| • | primary hubs that are open 24 hours per day and offer a full-testing menu; |
| |
| • | secondary laboratories that are open 24 hours per day and offer a wide-testing menu servicing large metropolitan areas; and |
| |
| • | short-term assessment and treatment (“STAT”) laboratories that service other locations with demand sufficient to warrant nearby laboratory facilities and are open primarily during daytime hours. |
We connect our laboratories to our customers with what we believe is the industry’s largest transportation network, picking up requisitions daily through an extensive network of independent drivers and couriers. Customers outside our transportation network use FedEx to send specimens to our laboratory just outside of Memphis, Tennessee, which permits rapid and cost-efficient testing because of the proximity to the primary sorting facility of FedEx.
In 2015, we derived approximately 87.4% of our Laboratory revenue from major metropolitan areas, where we offer twice-a-day pick-up service and same-day results. In addition, in these areas we generally offer to report results within three hours of pick-up. Outside of these areas, we typically provide test results to veterinarians before 8:00 a.m. the day following pick-up.
Sales, Marketing and Client Service
Our full-time sales and field-service representatives market laboratory services and maintain relationships with existing customers. Our sales force is compensated via salary plus commission and organized along geographic regions. We support our sales efforts by strengthening our industry-leading team of specialists, developing marketing literature, attending trade shows, participating in trade associations and providing educational services to veterinarians. Our client-service representatives respond to customer inquiries, provide test results and, when appropriate, introduce the customer to other services offered by the laboratory.
Personnel
Each of our primary and secondary laboratory locations includes a manager, supervisors for each department and personnel for laboratory testing. In addition, we employ or contract with specialists to interpret test results to assist veterinarians in the diagnosis of illnesses and to suggest possible treatment alternatives.
We actively recruit qualified personnel and are committed to supporting continuing education for our professional staff. We have internal training programs for routine testing procedures to improve the skill level of our technicians and to improve the overall capacity of our existing staff. We sponsor various residents and certain other educational programs. These programs serve to build awareness of our company with students, who may seek employment with our company following graduation.
Systems
We use an enterprise-wide management information system to support our Animal Hospital operations. In addition, a majority of our animal hospitals utilize consistent patient accounting/point-of-sale software and we are able to track performance of hospitals on a per-service, per-veterinarian and per-client basis.
We use an enterprise-wide management information system to support our veterinary laboratories. All of our financial data, customer records and laboratory results are stored in computer databases. Laboratory technicians and specialists are able to electronically access test results from remote testing sites. Our software gathers data in a data warehouse enabling us to provide expedient results via fax or through our Internet online resulting system.
Competition
The companion animal healthcare industry is highly competitive and subject to continual change in the manner in which services are delivered and providers are selected. We believe that the primary factors influencing a customer’s selection of an animal hospital are convenient location and hours, personal recommendations, reasonable fees and quality of care. Our primary competitors for our animal hospitals in most markets are individual practitioners or small, regional multi-clinic practices. In addition, some national companies in the pet care industry such as Banfield Pet Hospitals and National Veterinary Associates, are developing networks of animal hospitals in markets that include our animal hospitals. We also compete with sellers of pet-related products and diagnostic services delivered via the Internet.
Among veterinary diagnostic laboratories, we believe that quality, price, specialist support and the time required to deliver results are the major competitive factors. There are many clinical laboratories that provide a broad range of diagnostic testing services in the same markets serviced by us, and we also face competition from several providers of on-site diagnostic equipment that allows veterinarians to perform various testing. Our principal competitor in most geographic locations in the United States is IDEXX Laboratories.
Our Medical Technology business is a leader in the market for medical imaging equipment in the animal healthcare industry. Our primary competitors are companies that are much larger than us and have substantially greater capital, manufacturing, marketing and research and development resources than we do, including companies such as Siemens, Philips and Canon. The success of our Medical Technology business, in part, is due to its focus on the veterinary market, which allows us to differentiate our products and services to meet the unique needs of this market. If this market receives more focused attention from these larger competitors, we may find it difficult to compete and as a result our business, financial condition and results of operations could be adversely and negatively impacted.
Government Regulation
Certain states and provinces have laws, rules and regulations which require that veterinary medical practices be either wholly-owned or majority-owned by licensed veterinarians and that corporations which are not wholly-owned or majority-owned by licensed veterinarians refrain from providing, or holding themselves out as providers of, veterinary medical care. In these states and provinces, we provide management and other administrative services to veterinary practices rather than owning such practices or providing such care.
At December 31, 2015, we provided management and administrative services to 198 animal hospitals in 15 states, and 76 animal hospitals in four Canadian provinces. In some cases, in addition to providing management and administrative services we may lease the veterinary facility and equipment to the veterinary practice. We consolidate these veterinary practices for financial reporting purposes. Although we have structured our operations to comply with our understanding of the veterinary medicine laws of each state and province in which we operate, interpretive legal precedent and regulatory guidance varies by jurisdiction and is often sparse and not fully developed. A determination that we are in violation of applicable restrictions on the practice of veterinary medicine in any jurisdiction in which we operate could have a material adverse effect on our operations, particularly if we were unable to restructure our operations to comply with the requirements of that jurisdiction.
In addition, all of the states in which we operate impose various registration requirements. To fulfill these requirements, we have registered each of our facilities with appropriate governmental agencies and, where required, have appointed a licensed veterinarian to act on behalf of each facility. All veterinarians practicing in our animal hospitals are required to maintain valid state licenses to practice.
Our acquisitions may be subject to pre-merger or post-merger review by governmental authorities for anti-trust and other legal compliance. Adverse regulatory action could negatively affect our operations through the assessment of fines or penalties against us or the possible requirement of divestiture of one or more of our operations.
Employees
At December 31, 2015 we employed or managed on behalf of the professional corporations to which we provide services approximately 12,700 full-time-equivalent employees. At that date, none of these employees were a party to a collective bargaining agreement.
Availability of Our Reports Filed with the Securities and Exchange Commission (“SEC”)
We maintain a website with the address http://investor.vca.com. We are not including the information contained on our website as a part of, or incorporating it by reference into, this annual report on Form 10-K. We make available free of charge through our website our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, and amendments to these reports, as soon as reasonably practicable after we electronically file that material with, or furnish that material to, the SEC.
The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Copies of our reports filed electronically with the SEC may be accessed on the SEC’s website www.sec.gov. The public may also read and copy any materials filed with the SEC at the SEC’s Public Reference Room at 100 F Street NE, Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at (800) SEC-0330.
Our future operating results involve a number of risks and uncertainties. Actual events or results may differ materially from those discussed in this report. Any of the following risks could have a material adverse effect on our business, operating results and financial condition and cause the trading price of our common stock to decline.
Changes in the demand for our products and services could negatively affect our operating results.
We believe that the frequency of visits to our animal hospitals is impacted by consumer spending habits, which are affected by a number of factors, including among other things, prevailing economic conditions, levels of employment, salaries and wage rates, consumer confidence and consumer perception of economic conditions. The United States and Canadian economies have undergone and may in the future undergo, significant volatility and disruption. Business and financial disruptions in the United States' and Canadian economies could have a material adverse effect on the demand for our services. In addition, economic concerns may cause some pet owners to elect to skip or defer visits to veterinary hospitals, affect their willingness to approve certain diagnostic tests, comply with a treatment plan, forgo expensive treatment options, defer treatment for their pets altogether or even own a pet. Additionally, the frequency of visits to our animal hospitals and the number of diagnostic tests performed by our laboratories may be negatively impacted as a result of preventative care and better pet nutrition. Demand for vaccinations will be impacted in the future as protocols for vaccinations change. Our veterinarians establish their own vaccine protocols. Some of our veterinarians have changed their protocols and others may change their protocols in light of recent and/or future literature. The demand for our products and services may decline as a result of the eradication or substantial declines in the prevalence of certain diseases. Also, many pet-related products traditionally sold at animal hospitals have become more widely available in retail stores and other channels of distribution, including the Internet, resulting in a decline in demand for these products at our animal hospitals. All of the foregoing may result in a decrease in sales of our products and services, which could have a material adverse effect on our business, financial position, results of operations, and cash flows.
The significant competition in the companion animal healthcare industry could result in a decrease in our prices, an increase in our acquisition costs, or a loss of market share, any of which materially affect our revenue and profitability.
The companion animal healthcare industry is highly competitive with few barriers to entry and we expect that competition may become even more intense in the future. New competitors have been entering our market and may continue to do so and existing competitors may introduce new and competitive products and services. Some of our competitors or potential competitors may seek to differentiate themselves by offering similar products or services at lower prices or bundled product and service offerings through co-marketing arrangements. To compete successfully, we may be required to reduce prices, increase our acquisition and operating costs or take other measures that could have a material adverse effect on our business, financial condition or results of operations, margins and cash flow. In addition, if we are unable to compete successfully, we may lose market share.
A significant component of our annual growth strategy includes the acquisition of independent animal hospitals. The competition for animal hospital acquisitions from national and regional multi-clinic companies may cause us to increase the amount we pay to acquire additional animal hospitals and may result in fewer acquisitions than anticipated by our growth strategy. If we are unable to acquire our forecasted number of animal hospitals in any year, or if our acquisition costs increase, we may be unable to effectively implement our growth strategy and realize anticipated increases in Animal Hospital revenue and operating income.
Some national companies in the pet care industry, including the operators of super-stores, are developing networks of animal hospitals in markets that include our animal hospitals; this may cause us to reduce prices to remain competitive. Reducing prices may have a material adverse effect on our Animal Hospital revenue, operating income and margins, alternatively not reducing prices may cause us to lose market share.
We compete with clinical laboratory companies in the same markets we service. Some of these companies have acquired additional laboratories in the markets in which we operate and may continue their expansion, and aggressively “bundle” their products and services to compete with us. Increased competition may materially adversely affect our Laboratory revenue and margins. Several national companies develop and sell on-site diagnostic equipment that allows veterinarians to perform their own laboratory tests. Growth of the on-site diagnostic testing market may have a material adverse effect on our Laboratory revenue and operating income.
Our Medical Technology business is a leader in the market for medical imaging equipment in the animal healthcare industry. Our primary competitors are companies that are much larger than us and have substantially greater capital, manufacturing, marketing and research and development resources than we do, including companies such as Siemens, Philips and Canon. The success of our Medical Technology business, in part, is due to its focus on the veterinary market, which allows us to differentiate our products and services to meet the unique needs of this market. If this market receives more focused attention from these larger competitors, we may find it difficult to compete and as a result our business, financial condition and results of operations could be adversely and negatively impacted.
If we are unable to effectively execute our growth strategy, it may have a negative impact on our growth and profitability.
Our ability to maintain or enhance our growth rates and profitability depends in part on our ability to increase our revenue and operating income through a balanced program of organic growth initiatives and selective acquisitions of established animal hospitals, laboratories and related businesses. If we are not able to implement or effectively execute on this strategy, our rate of growth or profitability may be negatively impacted. We currently have implemented several key initiatives in order to enhance our organic growth rates such as our Client Experience program, our Veterinarian Communication training and our VCA CareClubTM wellness plans. Our Animal Hospital same-store revenue, adjusted for differences in business days, has fluctuated between a decline of 0.7% and growth of 6.1% for 2011 through 2015. Our Laboratory internal revenue growth, adjusted for differences in billing days, has fluctuated between 2.0% and 6.9% over the same years. Our internal growth may continue to fluctuate and may be below our historical rates. Any reduction in the rate of our internal growth may cause our revenue and operating income to decrease. Investors should not assume that our historical growth rates are reliable indicators of results in future periods.
Due to the fixed cost nature of our business, fluctuations in our revenue could adversely affect our gross profit, operating income and margins.
A substantial portion of our expense, particularly rent and personnel costs, are fixed and are based in part on expectations of revenue. We may be unable to reduce spending in a timely manner to compensate for any significant fluctuations in our revenue. Accordingly, shortfalls in revenue may materially adversely affect our gross profit, operating income and margins.
We may experience difficulties hiring skilled veterinarians due to shortages that could disrupt our business.
From time to time we experience shortages of skilled veterinarians in some specialties and some regional markets in which we operate animal hospitals which may require us to enhance wages and benefits to recruit and retain enough qualified veterinarians to adequately staff our animal hospitals. If our labor costs increase, we may not be able to raise our rates for our products and services to offset these increased costs. Our failure to recruit and retain qualified veterinarians, or to control our labor costs, could have a material adverse effect on our business, financial position, results of operations, and cash flows.
Any failure in our information technology systems, disruption in our transportation network or failure to receive supplies could significantly increase testing turn-around time, reduce our production capacity and otherwise disrupt our operations.
Our Laboratory operations depend on the continued and uninterrupted performance of our information technology systems and transportation network, including third-party overnight delivery services on which we rely. Sustained system failures or interruption in our transportation network could disrupt our ability to process laboratory requisitions, perform testing, provide test results in a timely manner and/or bill the appropriate party. We could lose customers and revenue as a result of a system or transportation network failure. In addition, any change in government regulation related to transportation samples or specimens could also have an impact on our business.
Our computer systems are vulnerable to damage or interruption from a variety of sources, including telecommunications failures, electricity brownouts or blackouts, malicious human acts and natural disasters. Moreover, despite network security measures, our servers are potentially vulnerable to digital break-ins, computer viruses and similar disruptive problems. Despite the precautions we have taken, unanticipated problems affecting our systems could cause interruptions in our information technology systems. Our insurance policies may not adequately compensate us for any losses that may occur due to any failures in our systems.
Our Laboratory operations depend on a limited number of employees to upgrade and maintain its customized computer systems. If we were to lose the services of some or all of these employees, it may be time-consuming for new employees to become familiar with our systems, and we may experience disruptions in service during these periods.
Our operations depend, in some cases, on the ability of single source suppliers or a limited number of suppliers, to deliver products and supplies on a timely basis. Some of these suppliers are smaller companies with limited capital resources and some of the products that we purchase from these suppliers are proprietary, and, therefore, cannot be readily or easily replaced by alternative suppliers. We have in the past experienced, and may in the future experience, shortages of or difficulties in acquiring products and/or supplies in the quantities and of the quality needed. Shortages in the availability of products and/or supplies for an extended period of time will disrupt our ability to deliver products and provide services in a timely manner, could result in the loss of customers, and could have a material adverse impact on our results of operations.
Difficulties integrating new acquisitions may impose increased costs, loss of customers and a decline in operating margins and profitability and other risks that we may not anticipate.
Our success depends in part on our ability to timely and cost-effectively acquire, and integrate into our business, additional animal hospitals, laboratories and related businesses. In 2015, we acquired 55 animal hospitals. In 2014, we acquired 47 animal hospitals and Camp Bow Wow and in 2013, we acquired 20 animal hospitals and one laboratory. We expect to continue our animal hospital acquisition program and, if presented with favorable opportunities, we may acquire animal hospital chains, laboratories or related businesses. Our expansion into new territories and new business segments creates the risk that we will be unsuccessful in the integration of the acquired businesses that are new to our operations. Any difficulties in the integration process could result in increased expense, loss of customers and a decline in operating margins and profitability. In some cases, we have experienced delays and increased costs in integrating acquired businesses, particularly where we acquire a large number of animal hospitals in a single region at or about the same time. We also could experience delays in converting the systems of acquired businesses into our systems, which could result in increased staff and payroll expense to collect our results as well as delays in reporting our results, both for a particular region and on a consolidated basis. Further, the legal and business environment prevalent in new territories and with respect to new businesses may pose risks that we do not anticipate and materially adversely impact our ability to integrate newly acquired operations. In addition, our field management may spend a greater amount of time integrating these new businesses and less time managing our existing businesses. During these periods, there may be less attention directed to marketing efforts or staffing issues, which could affect our revenue and expense. For all of these reasons, our historical success in integrating acquired businesses is not a reliable indicator of our ability to do so in the future.
The growth in multi-clinic animal hospital organizations and buying consortiums has resulted in the increased dependence in our Laboratory and Medical Technology Divisions on a few large supply contracts, the loss of any one of which could have a material adverse impact on our laboratory business and results of operations.
An increasing percentage of veterinary hospitals in the U.S. are owned by corporations that are in the business of acquiring veterinary hospitals and/or opening new veterinary hospitals nationally or regionally. Major corporate hospital owners in the U.S. include Banfield Pet Hospital and National Veterinary Associates, each of which are currently customers of our laboratories. A similar trend exists in Canada and may in the future also develop in other international markets. Furthermore, an increasing percentage of individually-owned veterinary hospitals in the U.S. are participating in buying consortiums. Corporate owners of veterinary hospitals and buying consortiums often seek to improve profitability by leveraging the buying power they derive from their scale to obtain favorable pricing from suppliers, which could have a negative impact on our results of operations. While we have strong supplier relationships with several corporate hospital groups and buying consortiums, decisions by larger corporate owners and buying consortiums to shift their purchasing of products and services away from us and to a competitor would have a negative impact on our results of operations.
Our business and reputation may be impacted by information technology system failures or network disruptions.
Despite the precautions we have taken, we may be subject to information technology system failures and network disruptions. These may be caused by natural disasters, accidents, power disruptions, telecommunications failures, acts of terrorism or war, computer viruses, physical or electronic break-ins, or similar events or disruptions. System redundancy may be ineffective or inadequate and our disaster recovery planning may not be sufficient for all eventualities. In addition, we rely on third parties for payment processing and secure handling of credit card and other confidential information. Failures or disruptions in their systems could prevent access to our services. System failures and disruptions could also impede the manufacturing and shipping of products, delivery of online services, transactions processing and financial reporting. If any of the above were to occur, our business, financial condition and results of operations could be materially and adversely affected.
We may be subject to loss of sensitive data that could subject us to significant reputational, financial, legal and operational consequences.
Our business requires us to use and store customer, employee and business personally identifiable information. This may include names, addresses, phone numbers, email addresses, contact preferences, tax identification numbers and payment account information. We require user names and passwords in order to access our information technology systems. We also use encryption and authentication technologies to secure the transmission and storage of data. These security measures may be compromised as a result of third-party security breaches, fraud, employee error, malfeasance, faulty password management or other irregularities, and result in persons obtaining unauthorized access to our data or our customers’ accounts. Third parties may attempt to fraudulently induce employees or customers into disclosing user names, passwords, payment information or other sensitive information, which may in turn be used to access our information technology systems.
We devote significant resources to network security, data encryption, and other security measures to protect our systems and data, but these security measures cannot provide absolute security. We may experience a breach of our systems and may be unable to protect sensitive data. Moreover, if fraud or a computer security breach affects our systems or results in the unauthorized release of data, our reputation and brand could be materially damaged and use of our products and services could decrease. As a consequence, we could also be exposed to the risk of direct loss or litigation, governmental investigations and inquiries and possible liability.
Failure to comply with federal, state and international laws and regulations relating to privacy, data protection and consumer protection, or the expansion of current or the enactment of new laws or regulations relating to privacy, data protection and consumer protection, could adversely affect our business and our financial condition.
A variety of federal, state and international laws and regulations govern the collection, use, retention, sharing and security of consumer data. Laws and regulations relating to privacy, data protection and consumer protection are evolving and are subject to potentially differing interpretations.
We strive to comply with all applicable laws, regulations and other legal obligations relating to privacy, data protection and consumer protection, including those relating to the use of data for marketing purposes. It is possible, however, that these requirements may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another or may conflict with other rules or our practices. We cannot guarantee that our practices have complied, comply or will comply fully with all such laws, regulations, requirements and obligations. Any failure, or perceived failure, by us to comply with our posted privacy policies or with any federal, state or international privacy or consumer protection-related laws, industry standards or codes of conduct, regulatory guidance, orders to which we may be subject or other legal obligations relating to privacy or consumer protection could adversely affect our reputation, brand and business, and may result in claims, proceedings or actions against us by governmental entities or others or other liabilities. Any such claim, proceeding or action could hurt our reputation, brand and business, force us to incur significant expenses in defense of such proceedings, distract our management, increase our costs of doing business, result in a loss of customers and suppliers and may result in the imposition of monetary liability. We may also be contractually liable to indemnify and hold harmless third parties from the costs or consequences of non-compliance with any laws, regulations or other legal obligations relating to privacy or consumer protection or any inadvertent or unauthorized use or disclosure of data that we store or handle as part of operating our business. Many foreign countries have adopted similar laws governing privacy. As we expand internationally, we will be subject to these laws and regulations, as well as to foreign intellectual property laws, laws regulating competition and other laws. There can be no assurance that we will not violate any applicable current or future foreign laws and regulations, which could result in fines and other penalties against us, litigation and/or regulatory action, damage our reputation and adversely affect our ability to successfully expand our business internationally, all of which could have a material adverse effect on our business, financial condition and operating results. In addition, various federal, state and foreign legislative and regulatory bodies may expand current laws or regulations, enact new laws or regulations or issue revised rules or guidance regarding privacy, data protection and consumer protection. Any such
changes may force us to incur substantial costs or require us to change our business practices. This could compromise our ability to pursue our growth strategy effectively and may adversely affect our ability to acquire customers or otherwise harm our business, financial condition and results of operations.
The carrying value of our goodwill and other intangible assets could be subject to an impairment write-down.
At December 31, 2015, our consolidated balance sheet reflected $1.5 billion of goodwill and $97.4 million of other intangible assets, constituting a substantial portion of our total assets of $2.5 billion at that date. We expect that the aggregate amount of goodwill and other intangible assets on our consolidated balance sheet will increase as a result of future acquisitions. We continually evaluate whether events or circumstances have occurred that suggest that the fair value of our other intangible assets or each of our reporting units are below their respective carrying values. The determination that the fair value of our intangible assets or one of our reporting units is less than its carrying value would result in an impairment write-down. The impairment write-down would be reflected as an operating expense and could have a material adverse effect on our results of operations during the period in which we recognize the expense.
Our estimated fair values are calculated in accordance with generally accepted accounting principles related to fair value and utilize valuation methods consisting primarily of discounted cash flow techniques, and market comparables, where applicable. These valuation methods involve the use of significant assumptions and estimates such as forecasted growth rates, valuation multiples, the weighted-average cost of capital, and risk premiums, which are based upon the best available market information and are consistent with our long-term strategic plans. We provide no assurance that forecasted growth rates, valuation multiples, and discount rates will not deteriorate or that we will not incur additional impairment charges. We will continue to analyze changes to these assumptions in future periods.
We require a significant amount of cash to service our debt and expand our business as planned.
We have, and will continue to have, a substantial amount of debt. Our substantial amount of debt requires us to dedicate a significant portion of our cash flow from operations to service interest and principal payments on our debt, thereby reducing the funds available for use for working capital, capital expenditures, acquisitions and general corporate purposes.
Our failure to satisfy covenants in our debt instruments will cause a default under those instruments.
In addition to imposing restrictions on our business and operations, our debt instruments include a number of covenants relating to financial ratios and tests. Our ability to comply with these covenants may be affected by events beyond our control, including prevailing economic, financial and industry conditions. The breach of any of these covenants would result in a default under these instruments. An event of default would permit our lenders and other debtholders to declare all amounts borrowed from them to be due and payable, together with accrued and unpaid interest. If we are unable to repay debt to our senior lenders, these lenders and other debtholders could proceed against our assets.
Our debt instruments may adversely affect our ability to run our business.
Our substantial amount of debt, as well as the guarantees of our subsidiaries and the security interests in our assets and those of our subsidiaries, could impair our ability to operate our business effectively and may limit our ability to take advantage of business opportunities. For example, our senior credit facility may:
| |
| • | limit our ability to borrow additional funds or to obtain other financing in the future for working capital, capital expenditures, acquisitions, investments and general corporate purposes; |
| |
| • | limit our ability to dispose of our assets, create liens on our assets or to extend credit; |
| |
| • | make us more vulnerable to economic downturns and reduce our flexibility in responding to changing business and economic conditions; |
| |
| • | limit our flexibility in planning for, or reacting to, changes in our business or industry; |
| |
| • | place us at a competitive disadvantage to our competitors with less debt; and |
| |
| • | restrict our ability to pay dividends, repurchase or redeem our capital stock or debt, or merge or consolidate with another entity. |
The terms of our senior credit facility allow us, under specified conditions, to incur further indebtedness, which would heighten the foregoing risks. If compliance with our debt obligations materially hinders our ability to operate our business and adapt to changing industry conditions, we may lose market share, our revenue may decline and our operating results may suffer.
Any failure by the manufacturers of our medical imaging equipment, failure in our ability to develop functional and cost-effective software for our products, or any product malfunctions could result in a decline in customer purchases and a reduction in our revenue and profitability.
We do not develop or manufacture the medical imaging equipment that we distribute, except for the software component of our digital radiography machines. Our business in large part is dependent upon distribution agreements with the manufacturers of the equipment, the ability of those manufacturers to produce desirable equipment and to keep pace with advances in technology, our ability to develop cost-effective, functional, and user-friendly software for the digital radiography machines, and the overall rate of new development within the industry. If the distribution agreements terminate or are not renewed, if the manufacturers breach their covenants under these agreements, if the equipment manufactured by these manufacturers or our software becomes less competitive or if there is a general decrease in the rate of new development within the industry, demand for our products and services would decrease.
Manufacturing flaws, component failures, design defects, or inadequate disclosure of product-related information could result in an unsafe condition or injury. These problems could result in product liability claims and lawsuits alleging that our products have resulted or could result in an unsafe condition or injury. In addition, a material adverse event involving one of our products could result in reduced market acceptance and demand for all of our products, and could harm our reputation and our ability to market our products in the future. Any of the foregoing problems could disrupt our business and have a material adverse effect on our business, results of operations, financial condition and cash flows.
Our use of self-insurance, self-insured retention and high-deductible insurance programs to cover certain claims for losses suffered and costs or expenses incurred could negatively impact our business upon the occurrence of an uninsured and/or significant event.
We self-insure and use high-retention or high-deductible insurance programs with regard to property risks, general, professional and employment practice liabilities, health benefits, and workers’ compensation. In the event that the frequency of losses we experience increases unexpectedly, the aggregate of those losses could materially increase our liability and adversely affect our cash flows and results of operations. In addition, we have made certain judgments as to the limits on our existing insurance coverage that we believe are in line with industry standards, as well as in light of economic and availability considerations. If we experience losses above these limits it could materially adversely affect our financial and business condition.
If we fail to comply with governmental regulations applicable to our business, various governmental agencies may impose fines, institute litigation or preclude us from operating in certain states.
Certain states and provinces have laws, rules and regulations which require that veterinary medical practices be owned by licensed veterinarians and that corporations which are not owned by licensed veterinarians refrain from providing, or holding themselves out as providers of, veterinary medical care. At December 31, 2015, we provided management and administrative services to 198 animal hospitals in 15 states and 76 animal hospital in four Canadian provinces, under management agreements with these veterinary practices, including 49 practices in Texas, 36 in Washington, and 36 in New York. We may experience difficulty in expanding our operations into other states or provinces with similar laws, rules and regulations. Although we have structured our operations to comply with our understanding of the veterinary medicine laws of each state and province in which we operate, interpretive legal precedent and regulatory guidance varies by jurisdiction and is often sparse and not fully developed. A determination that we are in violation of applicable restrictions on the practice of veterinary medicine in any jurisdiction in which we operate, could have a material adverse effect on us, particularly if we are unable to restructure our operations to comply with the requirements of that jurisdiction.
All of the states in which we operate impose various registration requirements. To fulfill these requirements, we have registered each of our facilities with appropriate governmental agencies and, where required, have appointed a licensed veterinarian to act on behalf of each facility. All veterinarians practicing in our animal hospitals are required to maintain valid state licenses to practice.
Fluctuations in foreign currency exchange rates could adversely affect our operating results.
At December 31, 2015, we operated 76 animal hospitals and four laboratories in Canada. Transactions in those facilities are in Canadian dollars. Any strengthening of the rate of exchange for the U.S. dollar against the Canadian dollar adversely affects our results, as it reduces the dollar value of sales that are made in Canada and reduces the profits on products manufactured or sourced in U.S. dollars and exported to Canada. Approximately 8.2%, 8.2% and 7.3% of our consolidated revenue for each of the years ended December 31, 2015, 2014 and 2013 was derived from our operations in Canada. In the future, we may expand into other international markets. Currency exchange rates fluctuate on a daily basis as a result of a number of factors and cannot easily be predicted. To date, we have not hedged against foreign currency fluctuations; however, we may pursue hedging alternatives in the future. Our business, financial condition, and results of operations could be materially adversely affected by fluctuations in the exchange rate between international currencies and the U.S. dollar.
Our international operations may result in additional market risks, which may harm our business.
As our international operations grow, they may require greater management and financial resources. Internal operations require the integration of personnel with varying cultural and business backgrounds and an understanding of the relevant differences in the cultural, legal and regulatory environments. Our results may be increasingly affected by the risks of our international operations, including:
| |
| • | changes in internal staffing and employment issues, |
| |
| • | failure to understand the local culture and market, and |
| |
| • | the burden of complying with foreign laws, including tax and labor laws and foreign financial accounting standards. |
|
| |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
Our corporate headquarters and principal executive offices are located in Los Angeles, California, in approximately 99,000 square feet of leased space. At February 26, 2016, we leased or owned facilities at 828 other locations that house our animal hospitals, laboratories, Medical Technology business, and Camp Bow Bow business. We own 171 facilities and the remainder are leased. We believe that our real property facilities are adequate for our current needs.
On July 16, 2014, two additional former veterinary assistants filed a purported class action lawsuit against us in the Superior Court of the State of California for the County of Los Angeles, titled La Kimba Bradsbery and Cheri Brakensiek vs. Vicar Operating, Inc., et. al. The lawsuit seeks to assert claims on behalf of current and former veterinary assistants, kennel assistants, and client service representatives employed by us in California, and alleges, among other allegations, that we improperly failed to pay regular and overtime wages, improperly failed to provide proper meal and rest periods, improperly failed to pay reporting time pay, improperly failed to reimburse for certain business-related expenses, and engaged in unfair business practices. The lawsuit seeks damages, statutory penalties, and other relief, including attorneys’ fees and costs.
In September 2014, the court issued an order staying the La Kimba Bradsbery lawsuit, which stay remains in place.
If the stay is lifted, we intend to vigorously defend against the Bradsbery action. At this time, we are unable to estimate the reasonably possible loss or range of possible loss, but do not believe losses, if any, would have a material effect on our results of operations or financial position taken as a whole.
On July 12, 2013, an individual who provided courier services with respect to our laboratory clients in California filed a purported class action lawsuit against us in the Superior Court of the State of California for the County of Santa Clara - San Jose Branch, titled Carlos Lopez vs. Logistics Delivery Solutions, LLC, Antech Diagnostics, Inc., et. al. Logistics Delivery
Solutions, LLC, a co-defendant in the lawsuit, is a company with which Antech has contracted to provide courier services in California. The lawsuit seeks to assert claims on behalf of individuals who were engaged by Logistics Delivery Solutions, LLC to perform such courier services and alleges, among other allegations, that Logistics Delivery Solutions and Antech Diagnostics improperly classified the plaintiffs as independent contractors, improperly failed to pay overtime wages, and improperly failed to provide proper meal periods. The lawsuit seeks damages, statutory penalties, and other relief, including attorneys' fees and costs. The parties have an agreement in principle to settle the action, on a class-wide basis, for an amount not to exceed $1,250,000. Logistics Delivery Solutions, LLC, has agreed to pay half of the claim. Accordingly, as of December 31, 2015, we have accrued the remaining fifty percent. The proposed settlement, when and if it becomes effective, would not be an admission of wrongdoing or acceptance of fault by any of the defendants named in the complaint. Antech Diagnostics and Logistics Delivery Solutions have agreed upon the terms of this proposed settlement to eliminate the uncertainties, risk, distraction and expense associated with protracted litigation. The Court granted preliminary approval of the settlement on November 30, 2015. The proposed settlement remains subject to court approval and class notice administration before it will be effective. The Court is scheduled to hear a motion for final approval of the settlement on March 25, 2016.
On May 12, 2014, an individual client who purchased goods and services from one of our animal hospitals filed a purported class action lawsuit against us in the United States District Court for the Northern District of California, titled Tony M. Graham vs. VCA Antech, Inc. and VCA Animal Hospitals, Inc. The lawsuit seeks to assert claims on behalf of the plaintiff and other individuals who purchased similar goods and services from our animal hospitals and alleges, among other allegations, that we improperly charged such individuals for “biohazard waste management” in connection with the services performed. The lawsuit seeks compensatory and punitive damages in unspecified amounts, and other relief, including attorneys' fees and costs. VCA successfully had the venue transferred to the Southern District of California. This case is in an early procedural stage and we intend to vigorously defend this action. At this time, we are unable to estimate the reasonably possible loss or
range of possible loss, but do not believe losses, if any, would have a material effect on our results of operations or financial position taken as a whole.
In addition to the lawsuits described above, we are party to ordinary routine legal proceedings and claims incidental to our business, but we are not currently a party to any legal proceeding that we believe would have a material adverse effect on our financial position, results of operations, or cash flows.
|
| |
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
|
| |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
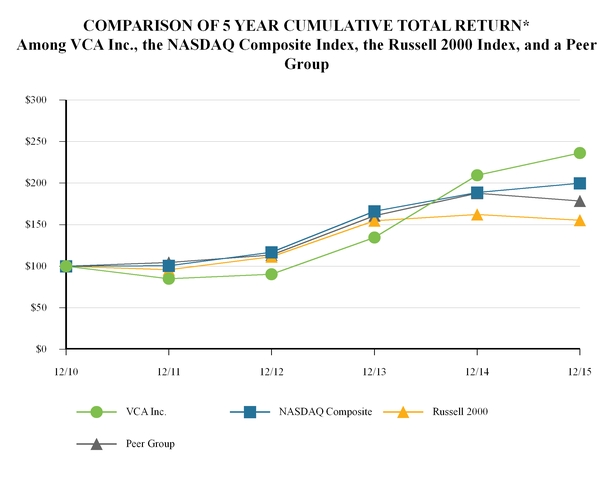
Our common stock trades on the NASDAQ Global Select Market under the symbol “WOOF.” The following table sets forth the range of high and low sales prices per share for our common stock as quoted on the NASDAQ Global Select Market for the periods indicated.
|
| | | | | | | | |
| | | High | | Low |
| Fiscal 2015 by Quarter | | | | |
| Fourth | | $ | 56.86 |
| | $ | 51.88 |
|
| Third | | $ | 62.45 |
| | $ | 51.23 |
|
| Second | | $ | 56.01 |
| | $ | 49.38 |
|
| First | | $ | 54.98 |
| | $ | 47.55 |
|
| Fiscal 2014 by Quarter | | | | |
| Fourth | | $ | 50.14 |
| | $ | 36.80 |
|
| Third | | $ | 42.14 |
| | $ | 34.58 |
|
| Second | | $ | 36.53 |
| | $ | 29.36 |
|
| First | | $ | 35.61 |
| | $ | 30.30 |
|
At February 22, 2016, there were 303 holders of record of our common stock.
The following graph sets forth the percentage change in cumulative total stockholder return on our common stock from December 31, 2010 to December 31, 2015. These periods are compared with the cumulative returns of the NASDAQ Stock Market (U.S. Companies) Index, the Russell 2000 Index, and our Peer Group. The comparison assumes $100 was invested on December 31, 2010 in our common stock and in each of the foregoing indices. The stock price performance on the following graph is not necessarily indicative of future stock price performance.
*$100 invested on 12/31/10 in stock or index, including reinvestment of dividends. Fiscal year ending December 31.
|
| | | | | | | | | | | | | | | | | | |
| | | 12/10 | | 12/11 | | 12/12 | | 12/13 | | 12/14 | | 12/15 |
| VCA Inc. | | 100.00 |
| | 84.80 |
| | 90.38 |
| | 134.65 |
| | 209.40 |
| | 236.15 |
|
| NASDAQ Composite | | 100.00 |
| | 100.53 |
| | 116.92 |
| | 166.19 |
| | 188.78 |
| | 199.95 |
|
| Russell 2000 | | 100.00 |
| | 95.82 |
| | 111.49 |
| | 154.78 |
| | 162.35 |
| | 155.18 |
|
Peer Group (1) | | 100.00 |
| | 104.41 |
| | 113.50 |
| | 160.68 |
| | 187.70 |
| | 178.46 |
|
____________________________
| |
(1) | Our Peer Group includes: C.R. Bard, Inc., Chipotle Mexican Grill, Inc., Chico's FAS, Inc., GNC Holdings, Inc., Guess?, Inc., HealthSouth Corporation, Hologic, Inc., Idexx Laboratories, Inc., Magellan Health Services, Inc., Mednax, Inc. and The Cheesecake Factory Incorporated. |
Dividends
We have not paid cash dividends on our common stock, and we do not anticipate paying cash dividends in the foreseeable future. In addition, our senior credit facility places limitations on our ability to pay cash dividends in respect of our common stock. Specifically, our senior credit facility dated August 27, 2014 prohibits us from declaring, ordering, paying, or setting apart any sum for any dividends or other distributions on account of any shares of any class of stock, other than dividends payable solely in shares of stock to holders of such class of stock. Any future determination as to the payment of dividends on our common stock will be restricted by these limitations, will be at the discretion of our Board of Directors and will depend on our results of operations, financial condition, capital requirements and other factors deemed relevant by the Board of Directors, including the General Corporation Law of the State of Delaware, which provides that dividends are only payable out of surplus or current net profits.
Transactions in Our Equity Securities
For the period covered by this report, we have not engaged in any transactions involving the sale of our unregistered equity securities that were not disclosed in a quarterly report on Form 10-Q or a current report on Form 8-K. We have not engaged in any sales of registered securities for which the use of proceeds is required to be disclosed.
The following table provides information on shares of our common stock we repurchased during the fourth quarter of 2015 (in thousands except average price paid per share data):
|
| | | | | | | | | | | | | | |
| | | | | | | Total Number of | | Approximate Dollar |
| | | | | | | Shares Purchased as | | Value of Shares That |
| | | Total Number | | Average | | Part of Publicly | | May Yet Be Purchased |
| | | of Shares | | Price Paid | | Announced Plan | | Under the Plan |
| Period | | Purchased | | Per Share | | or Program | | or Program |
| (1) | | (2) | | (3) | | (4) | | (4) |
| | | | | | | | | |
| October 1, 2015 to October 31, 2015 | | 30,669 |
| | $ | 54.02 |
| | — |
| | $ | 101,059 |
|
| November 1, 2015 to November 30, 2015 | | 26,760 |
| | $ | 53.48 |
| | — |
| | $ | 101,059 |
|
| December 1, 2015 to December 31, 2015 | | 24,663 |
| | $ | 54.98 |
| | — |
| | $ | 101,059 |
|
| | | 82,092 |
| | $ | 54.13 |
| | — |
| | $ | 101,059 |
|
____________________________
| |
(1) | Information is based on settlement dates of repurchase transactions. |
(2) Consists of shares of our common stock, par value $0.001 per share. Of these shares, none were repurchased in the open market pursuant to a previously-announced share repurchase program (see (4) below). The balance of the repurchases were related to 82,092 shares of common stock surrendered to us by employees to satisfy minimum statutory tax withholding obligations in connection with the vesting of restricted stock. In the table above, these shares were excluded from column (4) as they do not affect the number of shares that may be repurchased under the Share Repurchase Program.
(3) The average price paid for shares repurchased under the Share Repurchase Program excludes commissions paid.
(4) In August 2014, our Board of Directors authorized a repurchase program to purchase up to $400 million in shares of our common stock in open market purchases or negotiated transactions.
|
| |
| ITEM 6. | SELECTED FINANCIAL DATA |
The following table provides our selected consolidated financial data as of and for each of the five-year periods ended December 31, 2015. The income statement, cash flow data, and other financial data for each of the three years ended December 31, 2015, and the balance sheet data as of December 31, 2015 and 2014 has been derived from our financial statements included elsewhere in this Form 10-K. The other periods presented were derived from our financial statements that are not included in this Form 10-K.
The selected financial data presented below is not necessarily indicative of results of future operations and should be read in conjunction with the Management’s Discussion and Analysis of Financial Condition and Results of Operations section and our consolidated financial statements and related notes included elsewhere in this 10-K.
|
| | | | | | | | | | | | | | | | | | | |
| | December 31, |
| | 2015 | | 2014 | | 2013 | | 2012 | | 2011 |
| | (in thousands, except per share amounts) |
| Income Statement Data: | | | | | | | | | |
Animal Hospital revenue (1) | $ | 1,697,870 |
| | $ | 1,514,878 |
| | $ | 1,417,908 |
| | $ | 1,331,314 |
| | $ | 1,150,120 |
|
| Laboratory revenue | 393,900 |
| | 360,396 |
| | 344,831 |
| | 327,801 |
| | 316,797 |
|
| All Other revenue | 126,988 |
| | 115,785 |
| | 112,740 |
| | 112,960 |
| | 80,430 |
|
| Intercompany revenue | (85,083 | ) | | (72,576 | ) | | (72,110 | ) | | (72,433 | ) | | (61,986 | ) |
| Total revenue | 2,133,675 |
| | 1,918,483 |
| | 1,803,369 |
| | 1,699,642 |
| | 1,485,361 |
|
| Direct costs | 1,623,604 |
| | 1,473,842 |
| | 1,393,989 |
| | 1,324,668 |
| | 1,146,904 |
|
| Gross profit | 510,071 |
| | 444,641 |
| | 409,380 |
| | 374,974 |
| | 338,457 |
|
| Selling, general and administrative expense | 183,995 |
| | 171,506 |
| | 157,911 |
| | 157,155 |
| | 121,112 |
|
Impairment of goodwill and other long-lived assets (2) | — |
| | 27,019 |
| | — |
| | 123,573 |
| | 21,310 |
|
| Business interruption insurance gain | (4,523 | ) | | — |
| | — |
| | — |
| | — |
|
| Net loss (gain) on sale of assets | 829 |
| | (1,152 | ) | | 2,455 |
| | 1,310 |
| | 382 |
|
Operating income (2) | 329,770 |
| | 247,268 |
| | 249,014 |
| | 92,936 |
| | 195,653 |
|
| Interest expense, net | 21,076 |
| | 17,779 |
| | 18,549 |
| | 16,552 |
| | 16,884 |
|
| Debt retirement costs | — |
| | 1,709 |
| | — |
| | — |
| | 2,764 |
|
| Business combination adjustment gain | — |
| | — |
| | — |
| | (5,719 | ) | | — |
|
| Other expense (income) | 359 |
| | 219 |
| | 90 |
| | (488 | ) | | 118 |
|
Gain on sale of business (3) | (43,306 | ) | | — |
| | — |
| | — |
| | — |
|
| Income before provision for income taxes | 351,641 |
| | 227,561 |
| | 230,375 |
| | 82,591 |
| | 175,887 |
|
Provision for income taxes (4) | 135,543 |
| | 86,878 |
| | 87,453 |
| | 31,875 |
| | 76,027 |
|
| Net income | 216,098 |
| | 140,683 |
| | 142,922 |
| | 50,716 |
| | 99,860 |
|
| Net income attributable to noncontrolling interests | 5,049 |
| | 5,245 |
| | 5,411 |
| | 5,165 |
| | 4,455 |
|
| Net income attributable to VCA Inc. | $ | 211,049 |
| | $ | 135,438 |
| | $ | 137,511 |
| | $ | 45,551 |
| | $ | 95,405 |
|
| Basic earnings per share | $ | 2.59 |
| | $ | 1.56 |
| | $ | 1.55 |
| | $ | 0.52 |
| | $ | 1.10 |
|
| Diluted earnings per share | $ | 2.56 |
| | $ | 1.54 |
| | $ | 1.53 |
| | $ | 0.51 |
| | $ | 1.09 |
|
| Weighted-average shares outstanding for basic earnings per share | 81,443 |
| | 86,656 |
| | 88,621 |
| | 87,681 |
| | 86,606 |
|
| Weighted-average shares outstanding for diluted earnings per share | 82,414 |
| | 87,825 |
| | 89,663 |
| | 88,671 |
| | 87,394 |
|
|
| | | | | | | | | | | | | | | | | | | | |
| | | December 31, |
| | | 2015 | | 2014 | | 2013 | | 2012 | | 2011 |
| | | (in thousands, except percentages) |
| Other Financial Data: | | | | | | | | | | |
| Consolidated gross margin | | 23.9 | % | | 23.2 | % | | 22.7 | % | | 22.1 | % | | 22.8 | % |
| Animal Hospital gross margin | | 15.6 | % | | 15.2 | % | | 14.7 | % | | 14.2 | % | | 15.6 | % |
| Laboratory gross margin | | 51.2 | % | | 48.8 | % | | 47.5 | % | | 46.3 | % | | 45.4 | % |
| All Other gross margin | | 36.8 | % | | 33.4 | % | | 34.2 | % | | 34.3 | % | | 26.1 | % |
Consolidated operating margin (1)(2) | | 15.5 | % | | 12.9 | % | | 13.8 | % | | 5.5 | % | | 13.2 | % |
| Animal Hospital operating margin | | 13.0 | % | | 12.6 | % | | 12.1 | % | | 11.7 | % | | 13.5 | % |
| Laboratory operating margin | | 41.5 | % | | 39.5 | % | | 38.3 | % | | 37.3 | % | | 36.6 | % |
All Other operating margin (2) | | 13.8 | % | | (17.0 | )% | | 5.0 | % | | (108.2 | )% | | (24.2 | )% |
| Cash Flow Data: | | | | | | | | | | |
| Net cash provided by operating activities | | $ | 317,545 |
| | $ | 270,210 |
| | $ | 256,372 |
| | $ | 237,253 |
| | $ | 191,051 |
|
| Net cash used in investing activities | | $ | (203,652 | ) | | $ | (219,242 | ) | | $ | (126,731 | ) | | $ | (219,258 | ) | | $ | (271,310 | ) |
| Net cash (used in) provided by financing activities | | $ | (95,273 | ) | | $ | (93,765 | ) | | $ | (72,013 | ) | | $ | (13,514 | ) | | $ | 47,004 |
|
| Capital expenditures | | $ | (95,234 | ) | | $ | (72,948 | ) | | $ | (73,270 | ) | | $ | (76,807 | ) | | $ | (63,485 | ) |
| Balance Sheet Data (at period end): | | | | | | | | | | |
| Cash and cash equivalents | | $ | 98,888 |
| | $ | 81,383 |
| | $ | 125,029 |
| | $ | 68,435 |
| | $ | 63,651 |
|
| Goodwill | | $ | 1,517,650 |
| | $ | 1,415,861 |
| | $ | 1,321,234 |
| | $ | 1,281,590 |
| | $ | 1,237,607 |
|
| Total assets | | $ | 2,507,265 |
| | $ | 2,301,689 |
| | $ | 2,199,191 |
| | $ | 2,059,360 |
| | $ | 1,969,058 |
|
| Long-term obligations | | $ | 872,474 |
| | $ | 794,768 |
| | $ | 619,645 |
| | $ | 630,643 |
| | $ | 618,853 |
|
| Total VCA Inc. stockholders’ equity | | $ | 1,244,962 |
| | $ | 1,200,646 |
| | $ | 1,307,416 |
| | $ | 1,183,503 |
| | $ | 1,107,878 |
|
____________________________
| |
(1) | On January 31, 2012, we increased our investment in Associate Veterinary Clinics (1981) LTD ("AVC"), becoming the sole non-veterinary shareholder. Accordingly, we now consolidate their results into our own. At the time of the additional investment, AVC operated 44 animal hospitals. |
| |
(2) | In 2014, our operating income and operating margin were unfavorably impacted by a $27.0 million non-cash impairment charge. The charge was attributable to impairment of goodwill and intangible assets, related to our Vetstreet business, included in our All Other segments category. The charge impacted our 2014 operating margin by 1.4%. |
In 2012, our operating income and operating margin were unfavorably impacted by a $123.6 million non-cash impairment charge. The charge was attributable to impairment of our goodwill and intangible assets, related to our Vetstreet business, included in our All Other segments category. Our operating income was also impacted by a $3.1 million out-of-period adjustment to depreciation expense related to our acquired capital leases. These charges impacted our 2012 consolidated operating margin by 7.5%.
In 2011, our operating income and operating margin were unfavorably impacted by a $21.3 million non-cash goodwill impairment charge, related to our Medical Technology business. The charge impacted our 2011 operating margin by 1.4%.
| |
(3) | On December 31, 2015, we completed the sale of our wholly owned subsidiary, Vetstreet resulting in a gain of $43.3 million. |
| |
(4) | The 2015 provision for income taxes was impacted by the tax effect of the business interruption insurance gain and the gain on sale of our Vetstreet business. |
The 2014, 2012 and 2011 provisions for income taxes were impacted by the tax effect of the impairment charges mentioned above.
|
| |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion should be read in conjunction with our consolidated financial statements provided under Part II, Item 8 of this annual report on Form 10-K. We have included herein statements that constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. We generally identify forward-looking statements in this report using words like “believe,” “intend,” “seek,” “expect,” “estimate,” “may,” “plan,” “should plan,” “project,” “contemplate,” “anticipate,” “predict,” “potential,” “continue,” or similar expressions. You may find some of these statements below and elsewhere in this report. These forward-looking statements are not historical facts and are inherently uncertain and outside of our control. Any or all of our forward-looking statements in this report may turn out to be wrong. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. Many factors mentioned in our discussion in this report will be important in determining future results. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially. Factors that may result in these forward-looking statements in being different than reflected in this report are described throughout this annual report and particularly in “Risk Factors” Part I, Item 1A of this annual report on Form 10-K.
The forward-looking information set forth in this annual report on Form 10-K is as of February 26, 2016, and we undertake no duty to update this information. Shareholders and prospective investors can find information filed with the SEC after February 26, 2016, at our website at http://investor.vca.com or at the SEC’s website at www.sec.gov.
Overview
We are a leading North American animal healthcare company. We provide veterinary services and diagnostic testing services to support veterinary care and we sell diagnostic imaging equipment and other medical technology products and related services to veterinarians. Additionally, we franchise a premier provider of pet services including dog day care, overnight boarding, grooming and other ancillary services at specially designed pet care facilities.
Our reportable segments are as follows:
| |
| • | Our Animal Hospital segment operates the largest network of freestanding, full-service animal hospitals in the nation. Our animal hospitals offer a full range of general medical and surgical services for companion animals. We treat diseases and injuries, offer pharmaceutical and retail products and perform a variety of pet wellness programs, including health examinations, diagnostic testing, routine vaccinations, spaying, neutering and dental care. At December 31, 2015, our animal hospital network consisted of 682 animal hospitals in 41 states and in four Canadian provinces. |
| |
| • | Our Laboratory segment operates the largest network of veterinary diagnostic laboratories in the nation. Our laboratories provide sophisticated testing and consulting services used by veterinarians in the detection, diagnosis, evaluation, monitoring, treatment and prevention of diseases and other conditions affecting animals. At December 31, 2015, our laboratory network consisted of 60 laboratories serving all 50 states and certain areas in Canada. |
Our “All Other” category includes the results of our Medical Technology, Vetstreet and Camp Bow Wow operating segments. Each of these segments did not meet the materiality thresholds to be reported individually as segments.
The practice of veterinary medicine is subject to seasonal fluctuation. In particular, demand for veterinary services is significantly higher during the warmer months because pets spend a greater amount of time outdoors where they are more likely to be injured and are more susceptible to disease and parasites. In addition, use of veterinary services may be affected by levels of flea infestation, heartworms and ticks, and the number of daylight hours.
Use of Supplemental Non-GAAP Financial Measures
In this management's discussion and analysis, we use supplemental measures of our performance, which are derived from our consolidated financial information, but which are not presented in our consolidated financial statements prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). These financial measures, which are considered “Non-GAAP financial measures” under SEC rules, include our Non-GAAP gross profit and our Non-GAAP gross margin on a consolidated basis for our Animal Hospital segment, and the same measures expressed on a same-store basis. Additionally, our Non-GAAP financial measures include our Non-GAAP operating income and Non-GAAP
operating margin on a consolidated basis. Lastly, our Non-GAAP financial measures also include our Non-GAAP consolidated net income and Non-GAAP diluted earnings per share. See Consolidated Results of Operations - Non-GAAP Financial Measures below for information about our use of these Non-GAAP financial measures, including our reasons for including the measures, material limitations with respect to the usefulness of the measures, and a reconciliation of each Non-GAAP financial measure to the most directly comparable GAAP financial measure.
Executive Overview
During the year ended December 31, 2015, we experienced increases in both consolidated revenue and gross profit. The increases were primarily driven by revenue from our acquisitions, as well as organic growth in our Animal Hospital and Laboratory segments. Our Animal Hospital same-store revenue increased 6.1% in 2015 and 2.8%, in 2014. Our Laboratory internal revenue increased 6.9% in 2015 and 4.4% in 2014. Our consolidated operating income increased 33.4% in 2015 and decreased 0.7% in 2014. Our consolidated operating margin increased 260 basis points in 2015 and decreased 90 basis points in 2014. Excluding the impact of the adjustments detailed below under the caption, Operating Income, our Non-GAAP consolidated operating income increased 18.1% and 9.6% in 2015 and 2014, respectively, and our Non-GAAP consolidated operating margin increased 90 basis points and 50 basis points in 2015 and 2014, respectively. The increase in Non-GAAP consolidated operating income was primarily due to improved results from our Animal Hospital and Laboratory business segments.
Share Repurchase Program
In April 2013, our Board of Directors authorized a share repurchase program for up to $125 million of our common shares, which was completed in August 2014. In August 2014, our Board of Directors authorized the continuance of that share repurchase program, authorizing us to repurchase up to an additional $400 million of our common shares. These repurchases may be made from time to time in open market transactions, pursuant to trading plans established in accordance with SEC rules, through privately negotiated transactions, block trades or accelerated share repurchases. The timing and number of shares repurchased will depend on a variety of factors, including price, capital availability, legal requirements and economic and market conditions. The company is not obligated to purchase any shares under the repurchase program, and repurchases may be suspended or discontinued at any time without prior notice. Our share repurchase program has no expiration date. The repurchases have been and will continue to be funded by existing cash balances and by our revolving credit facility. Refer to Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities in Part II of this report.
Sale of Vetstreet
On December 31, 2015 we completed the sale of our Vetstreet business, resulting in a gain of $43.3 million. Concurrent with the sale of substantially all of the assets of Vetstreet, we purchased a 19.9% interest in the continuing Vetstreet business.
Acquisitions
Our annual growth strategy includes the acquisition of independent animal hospitals. We also evaluate the acquisition of animal hospital chains, laboratories or related businesses if favorable opportunities are presented. In 2015, we acquired 55 independent animal hospitals with annualized revenue of $122.0 million.
The following table summarizes the changes in the number of facilities operated by our Animal Hospital and Laboratory segments:
|
| | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Animal Hospitals: | | | | | | |
| Beginning of period | | 643 |
| | 609 |
| | 609 |
|
| Acquisitions | | 55 |
| | 47 |
| | 20 |
|
| Acquisitions, merged | | (7 | ) | | (4 | ) | | (2 | ) |
| Sold, closed or merged | | (9 | ) | | (9 | ) | | (18 | ) |
| End of period | | 682 |
| | 643 |
| | 609 |
|
| Laboratories: | | | | | | |
| Beginning of period | | 59 |
| | 56 |
| | 55 |
|
| Acquisitions | | 1 |
| | — |
| | 1 |
|
| Acquisitions, merged | | (1 | ) | | — |
| | — |
|
| New facilities | | 1 |
| | 3 |
| | — |
|
| End of period | | 60 |
| | 59 |
| | 56 |
|
Critical Accounting Policies and Estimates
We believe that the application of the following accounting policies, which are important to our financial position and results of operations, require significant judgments and estimates on the part of management. For a summary of all of our accounting policies, including the accounting policies discussed below, see Note 2, Summary of Significant Accounting Policies, in our consolidated financial statements of this annual report on Form 10-K.
Revenue
Generally, we recognize revenue when persuasive evidence of a sales arrangement exists, delivery of goods has occurred or services have been rendered, the sales price or fee is fixed or determinable and collectability is reasonably assured. For the Animal Hospital segment, revenue is recognized when services are performed or products are sold. For the Laboratory segment, revenue is recognized when services are performed. For the other segments, revenue is generally recognized when services are provided or delivery of goods has occurred. Our Medical Technology business sells digital radiography imaging equipment bundled with other services in certain instances. Under these arrangements, the sales consideration is allocated at inception to all deliverables using the relative selling price method, whereby any discount in the arrangement is allocated proportionally to each deliverable on the basis of each deliverable's selling price.
Valuation of Goodwill and Other Long Lived Assets
Goodwill
We allocate a significant portion of the purchase price for our acquired businesses to goodwill. Our goodwill represents the excess of the cost of an acquired entity over the net of the amounts assigned to identifiable assets acquired and liabilities assumed. The total amount of our goodwill at December 31, 2015 was $1.5 billion, consisting of $1.4 billion for our Animal Hospital reporting unit, $101.3 million for our Laboratory reporting unit, $8.2 million for our Medical Technology reporting unit and $6.1 million for our Camp Bow Wow reporting unit.
We test our goodwill for impairment annually, or sooner if circumstances indicate impairment may exist, in accordance with goodwill guidance. Our annual impairment testing date is October 31, which allows us time to accurately complete our impairment testing process in order to incorporate the results in our annual financial statements and timely file those statements with the Securities and Exchange Commission (“SEC”).
We have the option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its net book value. If we elect to not use this option, or we determine, using the qualitative method, that it is more likely than not that the fair value of a reporting unit is less than its net book value, we then perform the more detailed two-step impairment test.
In the two-step test, as mentioned above, first we identify potential impairment by comparing the estimated fair value of our reporting units with the carrying value defined as the reporting unit’s net assets, including goodwill. If the estimated fair value of our reporting units is greater than our carrying value, there is no impairment and the second step is not needed.
If we identify a potential impairment in the first step, we then measure the amount of impairment. The amount of the impairment is determined by allocating the estimated fair value of the reporting unit as determined in step one to the reporting unit’s net assets based on fair value as would be done in an acquisition. In this hypothetical purchase price allocation, the residual estimated fair value after allocation to the reporting units’ identifiable net assets is the implied current fair value of goodwill. If the implied current fair value of goodwill is less than the carrying amount of goodwill, goodwill is considered impaired and written down to the implied current fair value with a corresponding charge to earnings. However, if the implied current fair value of goodwill is greater than the carrying amount of goodwill, goodwill is not considered impaired and is not adjusted to the implied current fair value. Determining the fair value of the net assets of our reporting units under this step requires significant estimates.
Our estimated fair values are calculated in accordance with generally accepted accounting principles related to fair value and utilize generally accepted valuation techniques consisting primarily of discounted cash flow techniques and market comparables, where applicable. These valuation methods involve the use of significant assumptions and estimates such as forecasted growth rates, valuation multiples, the weighted-average cost of capital, and risk premiums, which are based upon the best available market information and are consistent with our long-term strategic plans.
Negative changes in our projected cash flows related to variables such as revenue growth rates, margins, or the discount rate could result in a decrease in the estimated fair value of our reporting units and could ultimately result in a substantial goodwill impairment charge. The performance of our reporting units, and in turn the risk of goodwill impairment, is subject to a number of risks and uncertainties, some of which are outside of our control.
2015 Impairment Test
After completing our October 31, 2015 impairment test for each of our reporting units, we concluded that goodwill was not impaired for any of our reporting units. In addition, our Animal Hospital, Laboratory, Medical Technology and Camp Bow Wow reporting units were not at risk of failing step one of the goodwill impairment test. Our Laboratory reporting unit exceeded its carrying value by a substantial margin. We applied a hypothetical ten percent decrease to the fair values of all of our reporting units which would not have triggered additional impairment testing and analysis.
2014 Interim Impairment Review
As a result of an interim impairment review, we determined that goodwill related to our Vetstreet reporting unit was impaired. We determined that a write-down of goodwill and long-lived assets was necessary as Vetstreet's fiscal 2014 actual operating results, cash flow, and projections of future operating results and cash flow, were significantly lower than previously forecasted. Accordingly, we recorded a goodwill impairment charge in our Vetstreet reporting unit of $9.2 million, $6.2 million net of tax, for the quarter ended September 30, 2014.
2014 Qualitative Assessment
As of October 31, 2014, we evaluated our goodwill for impairment using the qualitative method. Based on this analysis, we determined that it was more likely than not that the fair values of each of our reporting units were greater than their net carrying values at that date. As such, we concluded that goodwill was not impaired for any of our reporting units. In making this determination, we considered several factors, including the following: (1) the amount by which the fair value of the Laboratory reporting segment exceeded its carrying value as of October 31, 2013, indicated that there would need to be a substantial deterioration of the business in order for there to be a potential impairment; (2) during the latter half of 2014, two large third-party animal hospital chain transactions occurred at high valuation multiples; (3) the carrying values of our reporting units as of October 31, 2014 compared favorably to the previously calculated fair values as of October 31, 2013; and (4) the pet care industry remained very strong.
2013 Impairment Test
After completing our October 31, 2013 impairment test for each of our reporting units, we concluded that goodwill was not impaired for any of our reporting units. In addition, our Animal Hospital, Laboratory and Medical Technology reporting units were not at risk of failing step one of the goodwill impairment test. Our Laboratory reporting unit exceeded its carrying value by a substantial margin. We applied a hypothetical ten percent decrease to the fair values of all of our reporting units which would not have triggered additional impairment testing and analysis.
The fair value of our Vetstreet reporting unit, exceeded its carrying value by 10%. The carrying amount of goodwill in the Vetstreet reporting unit was $9.2 million. As mentioned in previous filings, Vetstreet had experienced operational delays with the execution of their business strategy, in part due to complexities involved in the migration of their information systems from their former parent to our corporate data center. In early 2013, the migration was completed and the company began the full implementation of their new business strategy. The fair value of the reporting unit for purposes of impairment testing was determined utilizing a revised estimate of future cash flows that reflected a recovery of certain aspects of the Vetstreet business in 2014 and beyond.
Long-lived Assets
In addition to goodwill, we acquire other identifiable intangible assets in our acquisitions, including but not limited to covenants-not-to-compete, client lists, lease related assets and customer relationships. We value these identifiable intangible assets at estimated fair value. Our estimated fair values are based on generally accepted valuation techniques such as market comparables, discounted cash flow techniques or costs to replace. These valuation methods involve the use of significant assumptions such as the timing and amount of future cash flows, risks, appropriate discount rates, and the useful lives of intangible assets.
Subsequent to acquisition, we test our identifiable intangible assets for impairment as part of a broader test for impairment of long-lived assets under the FASB’s accounting guidance whenever events or changes in circumstances indicate that the carrying value may not be recoverable. The recognition and measurement of an impairment loss under the FASB’s accounting guidance also involves a two-step process:
First we identify potential impairment for any asset groups with a triggering event by estimating the aggregate projected undiscounted future cash flows associated with an asset or asset group and compare that amount with the carrying value of those assets. If the aggregate projected cash flow is greater than our carrying amount, there is no impairment and the second step is not needed.
If the estimated aggregate projected undiscounted future cash flows associated with an asset or asset group is less than the carrying value, we then write the assets or asset group down to the estimated fair value with a corresponding charge to earnings. If the estimated fair value is greater than carrying value, there is no adjustment. We may be required to make significant estimates in determining the fair value of some of our assets or asset groups.
There was no impairment charge recorded on our long-lived assets in either 2015 or 2013. In conjunction with our interim impairment review during the quarter ended September 30, 2014, we recorded a long-lived intangible asset impairment charge of $13.1 million, $8.0 million net of tax, related to the aforementioned Vetstreet business. The intangibles consisted of technology, customer relationships, trademarks and certain other contracts. Additionally, we recorded a long-lived tangible asset impairment charge of approximately $4.7 million, $2.8 million net of tax, also related to the aforementioned Vetstreet business.
Income Taxes
We account for income taxes under the FASB’s accounting guidance for income taxes. We record deferred tax liabilities and deferred tax assets, which represent taxes to be settled or recovered in the future. We adjust our deferred tax assets and deferred tax liabilities to reflect changes in tax rates or other statutory tax provisions. Changes in tax rates or other statutory provisions are recognized in the period the enactment occurs.
We make judgments in assessing our ability to realize future benefits from our deferred tax assets, which includes operating loss carryforwards. We believe that our earnings during the periods when the temporary differences become deductible will be sufficient to realize the related future tax benefits. Should we determine that we would not be able to realize all or a portion of our deferred tax assets, an adjustment would be made to the carrying amount through a valuation allowance.
Also, our net deductible temporary differences and tax carryforwards are recorded using the enacted tax rates expected to apply to taxable income in the periods in which the deferred tax liability or asset is expected to be settled or realized. At December 31, 2015, we have a net deferred tax liability of $131.5 million. Should the expected applicable tax rates change in the future, an adjustment to the net deferred tax liability would be credited or charged, as appropriate, to income in the period such determination was made. For example, an increase of 1.0% in our income tax rate would cause us to increase our net deferred tax liability balance by $3.3 million with a corresponding charge to earnings.
We also assess differences between our tax bases, which are more likely than not to be realized, and the as-filed tax bases of certain assets and liabilities. We account for unrecognized tax benefits in accordance with the FASB’s accounting guidance on income taxes, which prescribe a minimum probability threshold that a tax position must meet before a financial statement benefit is recognized. The minimum threshold is defined as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation, based solely on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. We did not have any unrecognized tax benefits on December 31, 2015 and 2014.
Recent Accounting Pronouncements Adopted
On November 20, 2015, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes, requiring all deferred tax assets and liabilities, and any related valuation allowance, to be classified as non-current on the balance sheet. The classification change for all deferred taxes as non-current simplifies entities’ processes as it eliminates the need to separately identify the net current and net non-current deferred tax asset or liability in each jurisdiction and allocate valuation allowances. We elected to adopt the accounting standard in the fourth quarter of 2015. Prior periods in our Consolidated Financial Statements were retrospectively adjusted.
Consolidated Results of Operations
The following table sets forth components of our income statements expressed as a percentage of revenue:
|
| | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Revenue: | | | | | | |
| Animal Hospital | | 79.5 | % | | 79.0 | % | | 78.6 | % |
| Laboratory | | 18.5 |
| | 18.8 |
| | 19.1 |
|
| All Other | | 6.0 |
| | 6.0 |
| | 6.3 |
|
| Intercompany | | (4.0 | ) | | (3.8 | ) | | (4.0 | ) |
| Total revenue | | 100.0 |
| | 100.0 |
| | 100.0 |
|
| Direct costs | | 76.1 |
| | 76.8 |
| | 77.3 |
|
| Gross profit | | 23.9 |
| | 23.2 |
| | 22.7 |
|
| Selling, general and administrative expense | | 8.6 |
| | 9.0 |
| | 8.8 |
|
| Impairment of goodwill and other long-lived assets | | — |
| | 1.4 |
| | — |
|
| Business interruption insurance gain | | (0.2 | ) | | — |
| | — |
|
| Net (gain) loss on sale or disposal of assets | | — |
| | (0.1 | ) | | 0.1 |
|
| Operating income | | 15.5 |
| | 12.9 |
| | 13.8 |
|
| Interest expense, net | | 1.0 |
| | 0.9 |
| | 1.0 |
|
| Debt retirement costs | | — |
| | 0.1 |
| | — |
|
| Gain on sale of business | | (2.0 | ) | | — |
| | — |
|
| Income before provision for income taxes | | 16.5 |
| | 11.9 |
| | 12.8 |
|
| Provision for income taxes | | 6.4 |
| | 4.6 |
| | 4.9 |
|
| Net income | | 10.1 |
| | 7.3 |
| | 7.9 |
|
| Net income attributable to noncontrolling interests | | 0.2 |
| | 0.2 |
| | 0.3 |
|
| Net income attributable to VCA Inc. | | 9.9 | % | | 7.1 | % | | 7.6 | % |
Revenue
The following table summarizes our revenue (in thousands, except percentages):
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | | | |
| | | 2015 | | 2014 | | 2013 | | % Change |
| | | $ | | % of Total | | $ | | % of Total | | $ | | % of Total | | 2015 | | 2014 |
| Animal Hospital | | $ | 1,697,870 |
| | 79.5 | % | | $ | 1,514,878 |
| | 79.0 | % | | $ | 1,417,908 |
| | 78.6 | % | | 12.1 | % | | 6.8 | % |
| Laboratory | | 393,900 |
| | 18.5 | % | | 360,396 |
| | 18.8 | % | | 344,831 |
| | 19.1 | % | | 9.3 | % | | 4.5 | % |
| All Other | | 126,988 |
| | 6.0 | % | | 115,785 |
| | 6.0 | % | | 112,740 |
| | 6.3 | % | | 9.7 | % | | 2.7 | % |
| Intercompany | | (85,083 | ) | | (4.0 | )% | | (72,576 | ) | | (3.8 | )% | | (72,110 | ) | | (4.0 | )% | | (17.2 | )% | | (0.6 | )% |
| Total revenue | | $ | 2,133,675 |
| | 100.0 | % | | $ | 1,918,483 |
| | 100.0 | % | | $ | 1,803,369 |
| | 100.0 | % | | 11.2 | % | | 6.4 | % |
Consolidated revenue increased $215.2 million in 2015, as compared to 2014. The increase was primarily attributable to revenue from animal hospitals acquired since the beginning of the comparable period in the prior year. Excluding the impact of acquisitions, revenue increased $78.6 million, primarily due to organic revenue growth in our Animal Hospital and Laboratory segments. The increase was partially offset by the impact of foreign currency translation and intercompany sales. Our Animal Hospital same-store revenue increased 6.1% in 2015. Our Laboratory internal revenue growth was 6.9% in 2015.
Consolidated revenue increased $115.1 million in 2014, as compared to 2013. The increase was primarily attributable to revenue from animal hospitals acquired since the beginning of the comparable period in the prior year. Excluding the impact of acquisitions, revenue increased $42.8 million, primarily due to organic revenue growth in our Animal Hospital and Laboratory segments. The increase was partially offset by the impact of foreign currency translation and declining revenues in our Vetstreet
business. Our Animal Hospital same-store revenue increased 2.8% in 2014. Our Laboratory internal revenue growth was 4.4% in 2014.
Direct Costs
The following table summarizes our direct costs (in thousands, except percentages):
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | |
| | | 2015 | | 2014 | | 2013 | | % Change |
| | | $ | | % of Revenue | | $ | | % of Revenue | | $ | | % of Revenue | | 2015 | | 2014 |
| Animal Hospital | | $ | 1,433,535 |
| | 84.4 | % | | $ | 1,284,077 |
| | 84.8 | % | | $ | 1,208,781 |
| | 85.3 | % | | 11.6 | % | | 6.2 | % |
| Laboratory | | 192,198 |
| | 48.8 | % | | 184,588 |
| | 51.2 | % | | 180,952 |
| | 52.5 | % | | 4.1 | % | | 2.0 | % |
| All Other | | 80,286 |
| | 63.2 | % | | 77,161 |
| | 66.6 | % | | 74,139 |
| | 65.8 | % | | 4.0 | % | | 4.1 | % |
| Intercompany | | (82,415 | ) | | (3.9 | )% | | (71,984 | ) | | (3.8 | )% | | (69,883 | ) | | (3.9 | )% | | (14.5 | )% | | (3.0 | )% |
| Total direct costs | | $ | 1,623,604 |
| | 76.1 | % | | $ | 1,473,842 |
| | 76.8 | % | | $ | 1,393,989 |
| | 77.3 | % | | 10.2 | % | | 5.7 | % |
Consolidated direct costs increased $149.8 million and $79.9 million for the years ended 2015 and 2014, respectively. The increases were primarily attributable to compensation related costs, supplies and acquisitions, predominately in the animal hospital segment and discussed further under Segment Results.
Gross Profit
The following table summarizes our consolidated gross profit and consolidated Non-GAAP gross profit in dollars and as a percentage of applicable revenue (in thousands, except percentages):
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | | | |
| | | 2015 | | 2014 | | 2013 | | % Change |
| | | $ | | Gross Margin | | $ | | Gross Margin | | $ | | Gross Margin | | 2015 | | 2014 |
| Animal Hospital | | $ | 264,335 |
| | 15.6 | % | | $ | 230,801 |
| | 15.2 | % | | $ | 209,127 |
| | 14.7 | % | | 14.5 | % | | 10.4 | % |
| Laboratory | | 201,702 |
| | 51.2 | % | | 175,808 |
| | 48.8 | % | | 163,879 |
| | 47.5 | % | | 14.7 | % | | 7.3 | % |
| All Other | | 46,702 |
| | 36.8 | % | | 38,624 |
| | 33.4 | % | | 38,601 |
| | 34.2 | % | | 20.9 | % | | 0.1 | % |
| Intercompany | | (2,668 | ) | |
|
| | (592 | ) | |
|
| | (2,227 | ) | |
|
| |
|
| |
|
|
| Consolidated gross profit | | $ | 510,071 |
| | 23.9 | % | | $ | 444,641 |
| | 23.2 | % | | $ | 409,380 |
| | 22.7 | % | | 14.7 | % | | 8.6 | % |
| Impact of inventory adjustment | | — |
| | | | — |
| | | | (2,808 | ) | | | | | | |
| Impact of rent expense adjustment | | — |
| | | | — |
| | | | (1,396 | ) | | | | | | |
| Impact of vacant property adjustment | | — |
| | | | — |
| | | | 2,046 |
| | | | | | |
| Intangible asset amortization associated with acquisitions | | 23,153 |
| | | | 20,780 |
| | | | 20,753 |
| | | | | | |
Non-GAAP consolidated gross profit and Non-GAAP consolidated gross margin (1) | | $ | 533,224 |
| | 25.0 | % | | $ | 465,421 |
| | 24.3 | % | | $ | 427,975 |
| | 23.7 | % | | 14.6 | % | | 8.7 | % |
____________________________
| |
(1) | Non-GAAP consolidated gross profit and Non-GAAP gross margin are not measurements of financial performance prepared in accordance with GAAP. See Non-GAAP Financial Measures below for information about these Non-GAAP financial measures, including our reasons for including the measures, material limitations with respect to the usefulness of the measures, and a reconciliation of each Non-GAAP financial measure to the most directly comparable GAAP financial measure. |
Consolidated gross profit increased $65.4 million in 2015, as compared to 2014. Non-GAAP consolidated gross profit, which excludes the impact of the adjustments detailed in the table above, increased $67.8 million in 2015, as compared to 2014. The increase in Non-GAAP consolidated gross profit was primarily attributable to organic revenue growth and increased gross margins in our Animal Hospital and Laboratory business segments. The increase also included $26.2 million of gross profit related to the acquisitions consummated since the beginning of 2014.
Consolidated gross profit increased $35.3 million in 2014, as compared to 2013. Excluding the impact of the adjustments detailed in the table above, Non-GAAP consolidated gross profit increased $37.4 million in 2014, as compared to 2013. The increase in Non-GAAP consolidated gross profit was primarily attributable to organic revenue growth and increased gross margins at our Animal Hospital and Laboratory business segments. The increase also included $12.9 million of gross profit related to the acquisitions consummated since the beginning of 2013.
Segment Results
Animal Hospital Segment
Revenue
Animal Hospital revenue increased $183.0 million in 2015, as compared to 2014 and $97.0 million in 2014, as compared to 2013. The components of the increases are summarized in the following table (in thousands, except percentages and average price per order):
|
| | | | | | | | | | | | | | | | | | | | | | |
| | | 2015 Comparative Analysis | | 2014 Comparative Analysis |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | % Change | | 2014 | | 2013 | | % Change |
| Animal Hospital Revenue: | | | | | | | | | | | | |
| Same-store facility: | | | | | | | | | | | | |
Orders (1) | | 8,738 |
| | 8,472 |
| | 3.1 | % | | 8,003 |
| | 8,011 |
| | (0.1 | )% |
Average revenue per order (2) | | $ | 177.25 |
| | $ | 172.38 |
| | 2.8 | % | | $ | 177.18 |
| | $ | 172.13 |
| | 2.9 | % |
Same-store revenue (1) | | $ | 1,548,798 |
| | $ | 1,460,363 |
| | 6.1 | % | | $ | 1,417,928 |
| | $ | 1,378,997 |
| | 2.8 | % |
| Foreign currency impact | | (26,408 | ) | | — |
| | | | (10,806 | ) | | — |
| | |
Net acquired revenue (3) | | 175,480 |
| | 54,515 |
| | | | 107,756 |
| | 38,911 |
| | |
| Total | | $ | 1,697,870 |
| | $ | 1,514,878 |
| | 12.1 | % | | $ | 1,514,878 |
| | $ | 1,417,908 |
| | 6.8 | % |
____________________________
| |
(1) | Same-store revenue and orders were calculated using Animal Hospital operating results, adjusted to exclude the operating results for newly acquired animal hospitals that we did not own, as of the beginning of the comparable period in the prior year. Same-store revenue also includes revenue generated by customers referred from our relocated or combined animal hospitals, including those merged upon acquisition. |
| |
(2) | Computed by dividing same-store revenue by same-store orders. The average revenue per order may not calculate exactly due to rounding. |
| |
(3) | Net acquired revenue represents the revenue from those animal hospitals acquired, net of revenue from animal hospitals sold or closed, on or after the beginning of the comparable period in the prior year. Fluctuations in net acquired revenue occur due to the volume, size and timing of acquisitions and dispositions. |
During the year ended December 31, 2015, as compared to the same period in the prior year, our volume of same-store orders increased primarily due to the combination of an overall improvement in the economy during the year and the impact of certain previously implemented initiatives in our animal hospitals.
Our business strategy is to place a greater emphasis on comprehensive wellness visits and advanced medical procedures, which typically generate higher priced orders. For the year ended December 31, 2015, we experienced an increase in both the number of lower priced orders and higher priced orders.
Price increases as well as the mix in year over year growth rates of low to high-priced orders contributed to the overall increase in the average revenue per order. Prices at each of our hospitals are reviewed regularly and adjustments are made based on market considerations, demographics and our costs. These adjustments historically approximated 3% to 6% on most services at the majority of our animal hospitals and are typically implemented in November of each year; however, price increases in 2015 generally ranged between 4% and 6%.
Direct Costs
Animal Hospital direct costs increased $149.5 million for the year ended December 31, 2015, as compared to 2014. The increase was primarily due to an increase in compensation related expenses of $85.5 million, supplies of $26.3 million, rent of $5.6 million, and depreciation and amortization of $5.5 million. The remainder of the increase was due to numerous items, all of which were individually immaterial. The increases in compensation related costs and supplies generally are related to overall revenue growth. The increase in rent and depreciation and amortization is primarily related to acquired animal hospitals.
Animal Hospital direct costs increased $75.3 million for the year ended December 31, 2014, as compared to 2013. The increase was primarily due to an increase in compensation related expenses of $46.6 million, supplies of $13.4 million and depreciation and amortization of $3.4 million. The remainder of the increase was due to numerous items, all of which were individually immaterial. The increases in compensation related costs and supplies generally are related to overall revenue growth. The increase in depreciation and amortization is primarily related to acquired animal hospitals.
Gross Profit
Animal Hospital gross profit is calculated as Animal Hospital revenue less Animal Hospital direct costs. Animal Hospital direct costs comprise all costs of services and products at the animal hospitals including, but not limited to, salaries of veterinarians, technicians and all other animal hospital-based personnel, facilities rent, occupancy costs, supply costs, depreciation and amortization, certain marketing and promotional expenses and costs of goods sold associated with the retail sales of pet food and pet supplies.
The following table summarizes gross profit, gross margin, Non-GAAP gross profit, and Non-GAAP gross margin for our Animal Hospital segment (in thousands, except percentages) and the same measures on a same-store basis:
|
| | | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2015 | | 2014 | | % Change | | 2014 | | 2013 | | % Change |
| Gross profit | $ | 264,335 |
| | $ | 230,801 |
| | 14.5 | % | | $ | 230,801 |
| | $ | 209,127 |
| | 10.4 | % |
| Impact of inventory adjustment | — |
| | — |
| | | | — |
| | (2,808 | ) | | |
| Impact of rent expense adjustment | — |
| | — |
| | | | — |
| | (1,396 | ) | | |
| Impact of vacant property adjustment | — |
| | — |
| | | | — |
| | 2,046 |
| | |
| Intangible asset amortization associated with acquisitions | 18,938 |
| | 16,576 |
| | | | 16,576 |
| | 16,088 |
| | |
Non-GAAP gross profit (1) | $ | 283,273 |
| | $ | 247,377 |
| | 14.5 | % | | $ | 247,377 |
| | $ | 223,057 |
| | 10.9 | % |
| Gross margin | 15.6 | % | | 15.2 | % | | | | 15.2 | % | | 14.7 | % | | |
Non-GAAP gross margin (1) | 16.7 | % | | 16.3 | % | | | | 16.3 | % | | 15.7 | % | | |
| | | | | | | | | | | | |
| Same-store gross profit | 251,625 |
| | 227,844 |
| | 10.4 | % | | 223,044 |
| | 209,173 |
| | 6.6 | % |
| Impact of inventory adjustment | — |
| | — |
| | | | — |
| | (2,757 | ) | | |
| Impact of rent expense adjustment | — |
| | — |
| | | | — |
| | (1,396 | ) | | |
| Impact of vacant property adjustment | — |
| | — |
| | | | — |
| | 1,662 |
| | |
| Intangible asset amortization associated with acquisitions | 12,853 |
| | 15,242 |
| | | | 12,649 |
| | 14,937 |
| | |
Non-GAAP same-store gross profit (1) | $ | 264,478 |
| | $ | 243,086 |
| | 8.8 | % | | $ | 235,693 |
| | $ | 221,619 |
| | 6.4 | % |
| Same-store gross margin | 16.2 | % | | 15.6 | % | | | | 15.7 | % | | 15.2 | % | | |
Non-GAAP same-store gross margin (1) | 17.1 | % | | 16.6 | % | | | | 16.6 | % | | 16.1 | % | | |
____________________________
| |
(1) | Non-GAAP gross profit, Non-GAAP gross margin and the same measures expressed on a same store basis, are not measurements of financial performance prepared in accordance with GAAP. See Non-GAAP Financial Measures below for information about these Non-GAAP financial measures, including our reasons for including the measures, material limitations with respect to the usefulness of the measures, and a reconciliation of each Non-GAAP financial measure to the most directly comparable GAAP financial measure. |
Consolidated Animal Hospital gross profit increased $33.5 million for the year ended December 31, 2015, as compared to 2014. Non-GAAP gross profit, which excludes the impact of intangible asset amortization associated with acquisitions, increased $35.9 million in 2015, as compared to 2014. The increase in Non-GAAP consolidated gross profit was primarily attributable to an increase in Animal Hospital same-store gross profit and an additional $17.3 million of adjusted gross profit from acquired animal hospitals, net of closures.
Consolidated Animal Hospital gross profit increased $21.7 million for the year ended December 31, 2014, as compared to 2013. Non-GAAP gross profit, which excludes the impact of intangible asset amortization associated with acquisitions, increased $24.3 million in 2014, as compared to 2013. The increase in Non-GAAP consolidated gross profit was primarily attributable to an increase in Animal Hospital same-store gross profit and an additional $11.6 million of adjusted gross profit from acquired animal hospitals, net of closures.
Over the last several years, we have acquired a significant number of animal hospitals. Many of these newly acquired animal hospitals had lower gross margins at the time of acquisition than those previously operated by us. We have improved these lower gross margins, in the aggregate, subsequent to the acquisition primarily through cost efficiencies.
Laboratory Segment
The following table summarizes revenue and gross profit for our Laboratory segment (in thousands, except percentages):
|
| | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | % Change |
| | | 2015 | | 2014 | | 2013 | | 2015 | | 2014 |
| Revenue | | $ | 393,900 |
| | $ | 360,396 |
| | $ | 344,831 |
| | 9.3 | % | | 4.5 | % |
| Gross profit | | $ | 201,702 |
| | $ | 175,808 |
| | $ | 163,879 |
| | 14.7 | % | | 7.3 | % |
| Gross margin | | 51.2 | % | | 48.8 | % | | 47.5 | % | | | | |
Laboratory revenue increased $33.5 million in 2015, as compared to 2014 and $15.6 million in 2014, as compared to 2013. The components of the increase in Laboratory revenue are detailed below (in thousands, except percentages and average price per requisition):
|
| | | | | | | | | | | | | | | | | | | | | | |
| | | 2015 Comparative Analysis | | 2014 Comparative Analysis |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | % Change | | 2014 | | 2013 | | % Change |
| Laboratory Revenue: | | | | | | | | | | | | |
| Internal growth: | | | | | | | | | | | | |
Number of requisitions (1) | | 13,105 |
| | 12,794 |
| | 2.4 | % | | 12,785 |
| | 12,883 |
| | (0.8 | )% |
Average revenue per requisition (2) | | $ | 29.40 |
| | $ | 28.17 |
| | 4.4 | % | | $ | 28.15 |
| | $ | 26.77 |
| | 5.2 | % |
Total internal revenue(1) | | $ | 385,258 |
| | $ | 360,396 |
| | 6.9 | % | | $ | 359,903 |
| | $ | 344,831 |
| | 4.4 | % |
| Billing day adjustment | | — |
| | — |
| | | | — |
| | — |
| | |
Acquired revenue (3) | | 8,642 |
| | — |
| | | | 493 |
| | — |
| | |
| Total | | $ | 393,900 |
| | $ | 360,396 |
| | 9.3 | % | | $ | 360,396 |
| | $ | 344,831 |
| | 4.5 | % |
____________________________
| |
(1) | Internal revenue and requisitions were calculated using Laboratory operating results, which are adjusted (i) to exclude the operating results of acquired laboratories that we did not own as of the beginning of the comparable period in the prior year, and (ii) for the impact resulting from any differences in the number of billing days in the comparable period, if applicable. |
| |
(2) | Computed by dividing internal revenue by the number of requisitions. |
| |
(3) | Acquired revenue in both the 2015 and 2014 Comparative Analyses represents the current-year period revenue recognized from our acquired laboratories that we did not own as of the beginning of the comparable period in the prior year. |
The increase in Laboratory revenue for 2015 and 2014 in comparison to the prior year periods was largely due to an increase in average revenue per requisition, primarily as a result of price increases in February 2015 and 2014, respectively, as well as changes in product mix.
The increase in requisitions in 2015 in comparison to the prior year has been driven by an ongoing trend in veterinary medicine to focus on the importance of laboratory diagnostic testing in the diagnosis, early detection and treatment of diseases, and the migration of certain tests to outside laboratories that have historically been performed in animal hospitals. While these factors have resulted in significant increases in requisitions in the current year, the prior year decline in requisitions was driven by a combination of factors including the economic downturn, slow economic recovery and the effects of increased competition which negatively impacted requisitions in the prior year period.
We derive our laboratory revenue from services provided to over 17,000 independently owned animal hospitals. Shifts in the purchasing habits of any individual animal hospital or small group of animal hospitals is not material to our laboratory revenues. Other companies are developing networks of animal hospitals and shifts in the purchasing habits of these networks have the potential of a greater impact on our laboratory revenues.
Laboratory gross profit is calculated as Laboratory revenue less direct costs. Laboratory direct costs comprise all costs of laboratory services including, but not limited to, salaries of veterinarians, specialists, technicians and other laboratory-based personnel, transportation and delivery costs, facilities rent, occupancy costs, depreciation and amortization and supply costs.
Our Laboratory gross margin increased to 51.2% in 2015, as compared to 48.8% in 2014. The improvement in gross margins is primarily attributable to leverage on labor, lab supplies and transportation costs.
Our Laboratory gross margin increased to 48.8% in 2014, as compared to 47.5% in 2013. The improvement in gross margins is primarily attributable to leverage on labor and transportation costs.
Intercompany Revenue
Laboratory revenue in 2015, 2014 and 2013, included intercompany revenue of $63.1 million, $56.6 million and $54.2 million, respectively, generated by providing laboratory services to our animal hospitals. All Other revenue in 2015, 2014 and 2013, included intercompany revenue of $26.1 million, $19.7 million and $21.1 million, respectively, generated by providing products and services to our animal hospitals and laboratories. For purposes of reviewing the operating performance of our operating segments, all intercompany transactions are generally accounted for as if the transactions were with an independent third party at current market prices. For financial reporting purposes, intercompany transactions are eliminated as part of our consolidation.
Selling, General and Administrative Expense
SG&A is primarily comprised of costs incurred to support each of our business units. These costs typically include compensation related items for our accounting, legal, information technology, marketing, training, and medical operations departments and in addition, other shared costs such as marketing and rent for corporate facilities.
The following table summarizes our selling, general and administrative (“SG&A”) expense in both dollars and as a percentage of applicable revenue (in thousands, except percentages):
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | |
| | | 2015 | | 2014 | | 2013 | | % Change |
| | | $ | | % of Revenue | | $ | | % of Revenue | | $ | | % of Revenue | | 2015 | | 2014 |
| Animal Hospital | | $ | 44,311 |
| | 2.6 | % | | $ | 39,022 |
| | 2.6 | % | | $ | 34,133 |
| | 2.4 | % | | 13.6 | % | | 14.3 | % |
| Laboratory | | 38,075 |
| | 9.7 | % | | 33,550 |
| | 9.3 | % | | 31,915 |
| | 9.3 | % | | 13.5 | % | | 5.1 | % |
| All Other | | 33,569 |
| | 26.4 | % | | 33,456 |
| | 28.9 | % | | 32,941 |
| | 29.2 | % | | 0.3 | % | | 1.6 | % |
| Corporate | | 68,040 |
| | 3.2 | % | | 65,478 |
| | 3.4 | % | | 58,922 |
| | 3.3 | % | | 3.9 | % | | 11.1 | % |
| Total SG&A | | $ | 183,995 |
| | 8.6 | % | | $ | 171,506 |
| | 9.0 | % | | $ | 157,911 |
| | 8.8 | % | | 7.3 | % | | 8.6 | % |
Consolidated SG&A expense increased $12.5 million in 2015, as compared to 2014. The increase in consolidated SG&A in 2015, as compared to 2014, was primarily due to an increase in compensation related expenses at our Corporate, Animal Hospital and Laboratory segments of $0.6 million, $5.3 million and $2.3 million, respectively, related to increased headcount to support our growing operations and to a lesser extent, executive compensation. The increases were partially offset by a decrease in SG&A of $3.6 million at our Vetstreet business due to our cost containment efforts. The remainder of the variance is attributable to several individually immaterial items.
Consolidated SG&A expense increased $13.6 million in 2014, as compared to 2013. The increase in consolidated SG&A in 2014, as compared to 2013, was primarily due to an increase in compensation related expenses at our Corporate, Animal Hospital, Laboratory segments of $5.3 million, $3.6 million and $1.6 million, respectively, related to increased headcount to support our growing operations and to a lesser extent, executive compensation. The increases were partially offset by a decrease in SG&A of $1.8 million at our Vetstreet business due to our cost containment efforts. The remainder of the variance is attributable to several individually immaterial items.
Operating Income
The following table summarizes our consolidated operating income and Non-GAAP consolidated operating income in both dollars and as a percentage of applicable revenue (in thousands, except percentages):
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | |
| | | 2015 | | 2014 | | 2013 | | % Change |
| | | $ | | % of Revenue | | $ | | % of Revenue | | $ | | % of Revenue | | 2015 | | 2014 |
| Animal Hospital | | $ | 220,579 |
| | 13.0 | % | | $ | 190,306 |
| | 12.6 | % | | $ | 172,141 |
| | 12.1 | % | | 15.9 | % | | 10.6 | % |
| Laboratory | | 163,601 |
| | 41.5 | % | | 142,346 |
| | 39.5 | % | | 132,041 |
| | 38.3 | % | | 14.9 | % | | 7.8 | % |
| All Other | | 17,574 |
| | 13.8 | % | | (19,650 | ) | | (17.0 | )% | | 5,653 |
| | 5.0 | % | | 189.4 | % | | (447.6 | )% |
| Corporate | | (69,316 | ) | |
|
| | (65,142 | ) | |
|
| | (58,594 | ) | |
|
| | (6.4 | )% | | (11.2 | )% |
| Eliminations | | (2,668 | ) | |
|
| | (592 | ) | |
|
| | (2,227 | ) | |
|
| | (350.7 | )% | | 73.4 | % |
| Total GAAP consolidated operating income | | $ | 329,770 |
| | 15.5 | % | | $ | 247,268 |
| | 12.9 | % | | $ | 249,014 |
| | 13.8 | % | | 33.4 | % | | (0.7 | )% |
| Business interruption insurance gain | | (4,523 | ) | | | | — |
| | | | — |
| | | | | | |
| Impact of inventory adjustment | | — |
| | | | — |
| | | | (2,808 | ) | | | | | | |
| Impact of rent expense adjustment | | — |
| | | | — |
| | | | (1,396 | ) | | | | | | |
| Impact of vacant property adjustment | | — |
| | | | — |
| | | | 3,804 |
| | | | | | |
| Impact of goodwill and other long-lived assets impairment | | — |
| | | | 27,019 |
| | | | — |
| | | | | | |
| Intangible asset amortization associated with acquisitions | | 23,396 |
| | | | 21,039 |
| | | | 20,934 |
| | | | | | |
Non-GAAP consolidated operating income (1) | | $ | 348,643 |
| | 16.3 | % | | $ | 295,326 |
| | 15.4 | % | | $ | 269,548 |
| | 14.9 | % | | 18.1 | % | | 9.6 | % |
____________________________
| |
(1) | Non-GAAP consolidated operating income and Non-GAAP consolidated operating margin are not measurements of financial performance prepared in accordance with GAAP. See Non-GAAP Financial Measures below for information about these Non-GAAP financial measures, including our reasons for including the measures, material limitations with respect to the usefulness of the measures, and a reconciliation of each Non-GAAP financial measure to the most directly comparable GAAP financial measure. |
Consolidated operating income increased by $82.5 million in 2015, as compared to 2014. Excluding the impact of the adjustments detailed in the above table, consolidated operating income increased by $53.3 million primarily related to the improved results mentioned above in our Animal Hospital and Laboratory segments.
Consolidated operating income decreased by $1.7 million in 2014, as compared to 2013. Excluding the impact of the adjustments detailed in the above table, consolidated operating income increased by $25.8 million in 2014, as compared to 2013 related to the improved results mentioned above in our Animal Hospital and Laboratory segments.
Intangible asset amortization associated with acquisitions
Included in our direct costs is amortization expense related to our acquired intangible assets. At acquisition we assign a fair market value to identifiable intangible assets other than goodwill in our purchase price allocation. These assets include non-contractual customer relationships, covenants not-to-compete, trademarks, contracts and technology. For those identified intangible assets that have finite lives, we amortize those values over the estimated useful lives to direct costs. For the years ended 2015, 2014 and 2013, amortization expense associated with acquisitions was $23.4 million, $21.0 million, and $20.9 million, respectively.
Interest Expense, Net
The following table summarizes our interest expense, net of interest income (in thousands):
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Interest expense: | | | | | | |
| Senior term notes and revolving credit facility | | $ | 13,442 |
| | $ | 10,730 |
| | $ | 11,448 |
|
| Capital leases and other | | 6,024 |
| | 5,830 |
| | 4,834 |
|
| Amortization of debt costs | | 1,742 |
| | 1,391 |
| | 1,245 |
|
Non-GAAP interest expense (1) | | 21,208 |
| | 17,951 |
| | 17,527 |
|
| Rent expense adjustment | | — |
| | — |
| | 1,401 |
|
| Consolidated interest expense | | 21,208 |
| | 17,951 |
| | 18,928 |
|
| Interest income | | (132 | ) | | (172 | ) | | (379 | ) |
| Total consolidated interest expense, net of interest income | | $ | 21,076 |
| | $ | 17,779 |
| | $ | 18,549 |
|
____________________________
| |
(1) | Non-GAAP interest expense is not a measurement of financial performance prepared in accordance with GAAP. See Non-GAAP Financial Measures below for information about this financial measure including our reasons for including the measure and material limitations with respect to the usefulness of this measure. |
Consolidated net interest expense increased $3.3 million in 2015, as compared to 2014. The increase was primarily attributable to an increase in the weighted average debt balance of our senior credit facility, primarily related to an additional $97 million borrowed from our revolving credit facility during the year ended December 31, 2015, partially offset by a slight decline in our weighted average interest rates.
Consolidated net interest expense decreased $0.8 million in 2014, as compared to 2013. Excluding the impact of the adjustment in the table above, Non-GAAP net interest expense increased $0.6 million. The increase in Non-GAAP net interest expense was primarily attributable to new capital leases from animal hospitals acquired in 2014 and increases in amortized debt costs and bank administrative expenses related to the refinance of our senior credit facility. These increases in Non-GAAP net interest expense were partially offset by lower interest expense on our senior term notes. Interest expense on our senior term notes decreased due to a decline in year-over-year weighted average interest rates, which was primarily a result of improved interest rates under our senior credit facility due to more favorable leverage ratios. This effect was partially offset by an increase in the weighted average debt balance under our senior credit facility. See Note 7, Long-Term Obligations, in our consolidated financial statements of this annual report on Form 10-K for a more detailed discussion.
Provision for Income Taxes
The effective tax rate of income attributable to VCA for 2015, 2014 and 2013 was 39.1%, 39.1% and 38.9%, respectively.
The effective tax rate for 2013 was lower than that of 2014 primarily due to a nondeductible charge related to goodwill impairment in 2014.
Inflation
Historically, our operations have not been materially affected by inflation. We cannot assure that our operations will not be affected by inflation in the future.
Non-GAAP Financial Measures
We use Non-GAAP financial measures to supplement the financial information presented on a GAAP basis. We believe that excluding certain items from our GAAP results allows our management to better understand our consolidated financial performance from period to period and in relationship to the operating results of our segments. We also believe that excluding certain items from our GAAP results allows our management to better project our future consolidated financial performance because our forecasts are developed at a level of detail different from that used to prepare GAAP-based financial measures. Moreover, we believe these Non-GAAP financial measures provide investors with useful information to help them evaluate our operating results by facilitating an enhanced understanding of our operating performance, and enabling them to make more meaningful period to period comparisons.
The Non-GAAP financial measures presented in this report include Non-GAAP gross profit and Non-GAAP gross margin, computed on a consolidated basis, for our Animal Hospital segment, and the same measures expressed on a same-store basis. Additionally, our Non-GAAP financial measures include our Non-GAAP operating income and Non-GAAP operating margin computed on a consolidated basis. Lastly, our Non-GAAP financial measures also include our Non-GAAP interest expense, Non-GAAP consolidated net income, and Non-GAAP diluted earnings per share. These financial measures, as defined by us, represent the comparable GAAP measures adjusted to exclude certain charges or credits, as detailed in the tables above and below. In future fiscal periods, we may exclude such items and may incur income and expenses similar to these excluded items. Accordingly, the exclusion of these items and other similar items in our Non-GAAP presentation should not be interpreted as implying that these items are non-recurring, infrequent, or unusual.
There are limitations to the use of the Non-GAAP financial measures presented in this report. Our Non-GAAP financial measures may not be comparable to similarly titled measures of other companies. Other companies, including companies in our industry, may calculate the Non-GAAP financial measures differently than we do, limiting the usefulness of those measures for comparative purposes. In addition, these items can have a material impact on earnings. Our management compensates for the foregoing limitations by relying primarily on our GAAP results and using Non-GAAP financial measures supplementally. The Non-GAAP financial measures are not meant to be considered as indicators of performance in isolation from or as a substitute for consolidated gross profit or gross margin prepared in accordance with GAAP and should be read only in conjunction with financial information presented on a GAAP basis. We have presented reconciliations of each Non-GAAP financial measure to the most comparable GAAP measure for the years ended 2015, 2014 and 2013 and encourage you to review the reconciliations in conjunction with the presentation of the Non-GAAP financial measures for each of the periods included in this report. Refer to the tables above in the gross profit and operating income sections within Part II, Item 7 of this report for a reconciliation of consolidated gross profit to Non-GAAP gross profit and to consolidated operating income to Non-GAAP operating income.
Our Non-GAAP adjustments include the following:
| |
| • | Gain on sale of business - In 2015, we recognized a gain on sale of business of $43.3 million, related to the sale of our majority interest in our Vetstreet business. |
| |
| • | Goodwill and other long-lived assets impairment - In 2014, we recognized a non-cash impairment charge of $27.0 million, related to the write-down of goodwill and other long-lived assets in our Vetstreet business. |
| |
| • | Debt retirement costs - In 2014, we incurred debt retirement costs of $1.7 million related to the refinancing of our senior credit facility |
| |
| • | Inventory adjustment - In 2013, we recorded a non-cash physical inventory adjustment in our Animal Hospital business segment, which resulted in a $2.8 million credit adjustment to direct costs. |
| |
| • | Rent expense adjustment - In 2013, we reclassified an operating lease to a capital lease and accordingly recorded a corresponding credit to rent expense of $1.4 million and an offsetting debit to interest expense for approximately the same amount. This adjustment had an immaterial impact on the consolidated income statement and to net income. |
| |
| • | Vacant property adjustment - In 2013, we vacated properties of two animal hospitals whose operations were consolidated into a newly constructed, newly owned animal hospital. Accordingly, we recorded a write-down to fair value and accrued certain lease related costs totaling $3.8 million. |
| |
| • | Intangible asset amortization associated with acquisitions - Our GAAP net income includes amortization expense related to intangible assets in our acquired businesses. The amortization expense related to our acquired intangible assets can vary significantly depending upon the amount and size of our acquisitions in each period; accordingly, we |
exclude amortization from our GAAP net income, for all periods presented, to provide investors with more comparable operating results.
The following table reconciles our GAAP net income to Non-GAAP net income and calculates our Non-GAAP diluted earnings per share for the adjustments mentioned above:
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| GAAP Net income | | $ | 211,049 |
| | $ | 135,438 |
| | $ | 137,511 |
|
| Impact of goodwill and other long-lived assets impairment | | — |
| | 27,019 |
| | — |
|
| Impact of business interruption gain | | (4,523 | ) | | | | |
| Impact of debt retirement costs | | — |
| | 1,709 |
| | — |
|
| Impact of inventory adjustment | | — |
| | — |
| | (2,808 | ) |
| Impact of vacant property adjustment | | — |
| | — |
| | 3,804 |
|
| Impact of gain on sale of business | | (43,306 | ) | | — |
| | — |
|
| Intangible asset amortization associated with acquisitions | | 23,396 |
| | 21,039 |
| | 20,934 |
|
| Tax expense (benefit) on above adjustments | | 9,564 |
| | (18,882 | ) | | (8,584 | ) |
| Non-GAAP Net income | | $ | 196,180 |
| | $ | 166,323 |
| | $ | 150,857 |
|
| Non-GAAP diluted earnings per share | | $ | 2.38 |
| | $ | 1.89 |
| | $ | 1.68 |
|
| Shares used for computing adjusted diluted earnings per share | | 82,414 |
| | 87,825 |
| | 89,663 |
|
Related Party Transactions
Related Party Vendors
Frank Reddick joined our company as a director in February 2002 and is a partner in the law firm of Akin Gump Strauss Hauer & Feld, LLP (“Akin”). Akin provided legal services to us during 2015, 2014 and 2013. The amount paid by our company to Akin for these legal services was approximately $1.5 million, $0.9 million and $0.4 million in 2015, 2014 and 2013, respectively.
Transactions with VetSource
In 2006, we entered into a pharmacy distribution agreement with Strategic Pharmaceutical Solutions, Inc. (“VetSource”) a start-up pharmacy distribution company. Pursuant to the terms of this agreement we are entitled to one representative on the VetSource Board of Directors. Under the agreement we promote the use of VetSource as the preferred provider of pharmaceutical products to VCA animal hospitals. The agreement has a five-year term and will renew for one year terms unless either party provides written notice of termination to the other party at least 120 days prior to expiration of the then current term. The amount paid by our company to VetSource for pharmaceutical products was $6.0 million, $5.7 million and $13.0 million in 2015, 2014 and 2013, respectively. We own 39.3% of the outstanding preferred stock of VetSource.
On October 24, 2013, we entered into a $1.2 million revolving credit agreement with Strategic Pharmaceutical Solutions, Inc. (“VetSource”) in the form of a promissory note. Our commitment under the revolving credit agreement is to loan up to $0.5 million, equitable to our 39.3% pro rata share in VetSource. As of December 31, 2015, VetSource has no outstanding loans.
Transactions with Vetstreet
On December 31, 2015, our company sold substantially all of the assets of Vetstreet Inc., formerly known as MediMedia Animal Health LLC ("Vetstreet") to a subsidiary of Henry Schein, Inc.. Concurrent with the sale of Vetstreet, we purchased a 19.9% interest in the continuing Vetstreet business for $9.6 million, which will be accounted for under the equity method of accounting for investments in common stock.
Liquidity and Capital Resources
Introduction
We generate cash primarily from (i) payments made by customers for our veterinary services, (ii) payments from animal hospitals and other clients for our laboratory services, (iii) proceeds received from the sale of our imaging equipment and other related services. In addition, in 2013, 2014 and 2015, we generated cash from payments received from participating hospitals for Vetstreet subscriptions and reminder notices. Our business historically has experienced strong liquidity, as fees for services provided in our animal hospitals are due at the time of service and fees for laboratory services are collected under standard industry terms. Our cash disbursements are primarily for payments related to the compensation of our employees, supplies and inventory purchases for our operating segments, occupancy and other administrative costs, interest expense, payments on long-term borrowings, capital expenditures, acquisitions, and share repurchases. Cash outflows fluctuate with the amount and timing of the settlement of these transactions.
We manage our cash, investments and capital structure so that we are able to meet the short-term and long-term obligations of our business while maintaining financial flexibility and liquidity. We forecast, analyze and monitor our cash flows to enable investment and financing within the overall constraints of our financial strategy.
At December 31, 2015, our consolidated cash and cash equivalents totaled $98.9 million, representing a increase of $17.5 million as compared to the prior year. Cash flows generated from operating activities totaled $317.5 million in 2015, representing an increase of $47.3 million as compared to the prior year.
At December 31, 2015, $13.8 million of the $98.9 million of cash and cash equivalents was held by foreign subsidiaries. Our intention is to indefinitely reinvest foreign earnings in our foreign subsidiaries. If these earnings were used to fund domestic operations, they would be subject to additional income taxes upon repatriation.
We have historically funded our working capital requirements, capital expenditures, investments in the acquisition of individual hospitals and laboratories, and other smaller acquisitions primarily from internally-generated cash flows. In the future, we plan to continue to utilize our revolving credit facility to supplement our internally generated cash flows to fund both our acquisition pipeline and our share repurchase program. As of December 31, 2015, we have access to $568 million under our revolving credit facility which allows us to maintain further operating and financial flexibility. See Note 7, Long-Term Obligations, in our consolidated financial statements of this annual report on Form 10-K for a more detailed discussion.
Historically, we have been able to access the capital markets to fund larger acquisitions that could not be funded out of internally generated cash flows. The availability of financing in the form of debt or equity is influenced by many factors including our profitability, operating cash flows, debt levels, debt ratings, contractual restrictions, and market conditions. Although in the past we have been able to obtain financing for material transactions on terms we believe to be reasonable, there is a possibility that we may not be able to obtain financing on favorable terms in the future.
Future Cash Flows
Short-term
We anticipate that our cash on hand and net cash provided by operations and available funds under our revolving credit agreement and incremental facilities will be sufficient to meet our anticipated cash requirements for the next 12 months. If we consummate additional significant acquisitions during this period, we may seek additional debt or equity financing.
In August 2014, our Board of Directors authorized the continuance of our April 2013 share repurchase program. The plan authorizes us to repurchase up to an additional $400 million of our common shares from time to time in open market purchases, pursuant to trading plans established in accordance with SEC rules, through privately negotiated transactions, block trades and accelerated share repurchases. The timing and number of shares repurchased will depend on a variety of factors, including price, capital availability, legal requirements and economic and market conditions. The company is not obligated to purchase any shares under the repurchase program, and repurchases may be suspended or discontinued at any time without prior notice. The repurchases have been and will continue to be funded by existing cash balances and by our revolving credit facility. For the year ended December 31, 2015, we repurchased an aggregate of 2,720,000 shares of common stock for $145.7 million under our plan.
In 2016, we expect to spend approximately $105 million for both property and equipment additions and capital costs necessary to maintain our existing facilities.
Long-term
Our long-term liquidity needs, other than those related to the day-to-day operations of our business, including commitments for operating leases, generally are comprised of scheduled principal and interest payments for our outstanding long-term indebtedness, capital expenditures related to the expansion of our business and acquisitions in accordance with our growth strategy. The scheduled payments on our long-term obligations are included in our contractual obligations table below.
We are unable to project with certainty whether our long-term cash flow from operations will be sufficient to repay our long-term debt when it comes due. If this cash flow is insufficient, we expect that we will need to refinance such indebtedness, amend its terms to extend maturity dates, or issue common stock in our company. Our management cannot make any assurances that such refinancing or amendments, if necessary, will be available on attractive terms, if at all.
Debt Related Covenants
Our senior credit facility contains certain financial covenants pertaining to interest coverage and leverage ratios. As of December 31, 2015, we were in compliance with these covenants, including the two covenant ratios, the interest coverage ratio and the leverage ratio.
At December 31, 2015, we had an interest coverage ratio of 20.57 to 1.00, which was in compliance with the required ratio of no less than 3.00 to 1.00. The senior credit facility defines the interest coverage ratio as that ratio that is calculated on a last 12-month basis by dividing pro forma earnings before interest, taxes, depreciation and amortization, as defined by the agreement (“pro forma earnings”), by consolidated interest expense. Interest expense is defined as total interest expense with respect to all outstanding indebtedness, including commissions, discounts and other fees charged related to letters of credit. Pro forma earnings include 12 months of operating results for businesses acquired during the period.
At December 31, 2015, we had a leverage ratio of 2.07 to 1.00, which was in compliance with the required ratio of no more than 4.25 to 1.00 from June 30, 2015 through December 31, 2015 as defined under the senior credit facility. The senior credit facility defines the leverage ratio as that ratio which is calculated as total debt divided by pro forma earnings.
Historical Cash Flows
The following table summarizes our cash flows (in thousands):
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Cash provided by (used in): | | | | | | |
| Operating activities | | $ | 317,545 |
| | $ | 270,210 |
| | $ | 256,372 |
|
| Investing activities | | (203,652 | ) | | (219,242 | ) | | (126,731 | ) |
| Financing activities | | (95,273 | ) | | (93,765 | ) | | (72,013 | ) |
| Effect of currency exchange rate changes on cash and cash equivalents | | (1,115 | ) | | (849 | ) | | (1,034 | ) |
| Increase (decrease) in cash and cash equivalents | | 17,505 |
| | (43,646 | ) | | 56,594 |
|
| Cash and cash equivalents at beginning of year | | 81,383 |
| | 125,029 |
| | 68,435 |
|
| Cash and cash equivalents at end of year | | $ | 98,888 |
| | $ | 81,383 |
| | $ | 125,029 |
|
Cash Flows from Operating Activities
Net cash provided by operating activities increased $47.3 million in 2015, as compared to 2014. Operating cash flow for the year ended December 31, 2015 included $216.1 million of net income and net non-cash expenses of $113.4 million, partially offset by net cash used as a result of changes in operating assets and liabilities of $12.0 million. The changes in operating assets and liabilities primarily included a $28.7 million increase in trade accounts receivable, and a $9.7 million increase in inventory, prepaid expenses and other assets, partially offset by an $11.3 million increase in accrued payroll and related liabilities, a $10.8 million increase in accounts payable and other accrued liabilities and accrued interest, and a $4.3 million decrease in prepaid income taxes. The increase in trade accounts receivable was primarily due to increased revenues in both our Hospital and Laboratory business segments as compared to the prior-year period. The increase in inventory, prepaid expenses and other assets was primarily due to increased service agreements entered into by our Laboratory segment. The increase is partially offset due to the receipt of an insurance payment related to the May 2014 fire at our Medical Technology business, and the depletion of certain products. The increases in accrued payroll and related liabilities and accounts payable and other accrued liabilities, and the decrease in prepaid income taxes were primarily due to the timing of payment obligations.
Net cash provided by operating activities increased $13.8 million in 2014, as compared to 2013. Operating cash flow for the year ended December 31, 2014 included $140.7 million of net income and net non-cash expenses of $135.0 million, partially offset by net cash used as a result of changes in operating assets and liabilities of $5.5 million. The changes in operating assets and liabilities primarily included a $22.9 million increase in inventory, prepaid expenses and other assets, and a $3.9 million increase in net, trade accounts receivable, primarily offset by an $11.6 million increase in accounts payable and other accrued liabilities and accrued interest, a $6.8 million increase in accrued payroll and related liabilities, and a $3.0 million decrease in prepaid incomes taxes. The increase in inventory, prepaid expenses and other assets is primarily due to increased medical supplies in relation to the acquired hospitals in 2014, along with new product offerings, an increase in the accruals for partner program receivables, the addition of new wellness plan receivables which are collected through equal monthly payments rather than payment in full at the time of services, as well as an $8.0 million receivable from insurance recoveries in connection with the May 2014 fire at our Medical Technology business. The increase in net, trade accounts receivable was primarily due to the addition of new hospitals in 2014, as well as increased Antech revenue. The increases in accounts payable and other accrued liabilities, accrued payroll and related liabilities, and the decrease in prepaid income taxes was primarily due to the timing of payment obligations.
Cash Flows from Investing Activities
The table below presents the components of the changes in investing cash flows (in thousands):
|
| | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | Variance |
| | | 2015 | | 2014 | | 2013 | | 2015 | | 2014 |
| Investing Cash Flows: | | | | | | | | | | |
Acquisition of independent animal hospitals and laboratories (1) | | $ | (151,586 | ) | | $ | (131,821 | ) | | $ | (58,016 | ) | | $ | (19,765 | ) | | $ | (73,805 | ) |
| Acquisition of Camp Bow Wow | | — |
| | (15,686 | ) | | — |
| | 15,686 |
| | (15,686 | ) |
Total business acquisitions, net of cash acquired (1) | | (151,586 | ) | | (147,507 | ) | | (58,016 | ) | | (4,079 | ) | | (89,491 | ) |
Investment in Vetstreet, Inc. (2) | | (9,552 | ) | | — |
| | — |
| | (9,552 | ) | | — |
|
Property and equipment additions (3) | | (95,234 | ) | | (72,948 | ) | | (73,270 | ) | | (22,286 | ) | | 322 |
|
Proceeds from sale or disposal of assets (4) | | 6,762 |
| | 3,904 |
| | 7,096 |
| | 2,858 |
| | (3,192 | ) |
Proceeds from sale of business (5) | | 48,000 |
| | — |
| | — |
| | 48,000 |
| | — |
|
| Other | | (2,042 | ) | | (2,691 | ) | | (2,541 | ) | | 649 |
| | (150 | ) |
| Net cash used in investing activities | | $ | (203,652 | ) | | $ | (219,242 | ) | | $ | (126,731 | ) | | $ | 15,590 |
| | $ | (92,511 | ) |
____________________________
| |
(1) | The number of acquisitions will vary from year-to-year based upon the available pool of suitable candidates. A discussion of our acquisitions is provided above in our Executive Overview. |
| |
(2) | Concurrent with the sale of substantially all of the assets of Vetstreet, we purchased a 19.9% interest in the continuing Vetstreet business. |
| |
(3) | The cash used to acquire property and equipment will vary from year-to-year based on upgrade requirements and expansion of our animal hospitals and laboratory facilities. |
| |
(4) | The proceeds from the sale or disposal of assets in 2014 primarily relate to the insurance recovery received on assets from our Medical Technology business that were destroyed by fire. |
The proceeds from the sale or disposal of assets in 2013 primarily relate to the sale of our previous West Los Angeles location.
| |
(5) | The proceeds from the sale of business in 2015 relate to the sale of our wholly-owned subsidiary, Vetstreet on December 31, 2015. |
Cash Flows from Financing Activities
The table below presents the components of the changes in financing cash flows (in thousands):
|
| | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | Variance |
| | | 2015 | | 2014 | | 2013 | | 2015 | | 2014 |
| Financing Cash Flows: | | | | | | | | | | |
Repayment of long-term obligations (1) | | $ | (35,017 | ) | | $ | (568,011 | ) | | $ | (41,129 | ) | | $ | 532,994 |
| | $ | (526,882 | ) |
Proceeds from the issuance of long-term obligations (1) | | — |
| | 600,000 |
| | — |
| | (600,000 | ) | | 600,000 |
|
Proceeds from revolving credit facility (2) | | 97,000 |
| | 135,000 |
| | — |
| | (38,000 | ) | | 135,000 |
|
Payment of financing costs (1) | | — |
| | (7,987 | ) | | — |
| | 7,987 |
| | (7,987 | ) |
Distributions to noncontrolling interest partners | | (4,962 | ) | | (5,009 | ) | | (4,866 | ) | | 47 |
| | (143 | ) |
Purchase of noncontrolling interests (3) | | (2,500 | ) | | (326 | ) | | (6,581 | ) | | (2,174 | ) | | 6,255 |
|
Proceeds from issuance of common stock under stock incentive plans (4) | | 2,683 |
| | 2,859 |
| | 17,233 |
| | (176 | ) | | (14,374 | ) |
Excess tax benefits from stock based compensation (5) | | 11,089 |
| | 6,241 |
| | 3,446 |
| | 4,848 |
| | 2,795 |
|
Stock repurchases (6) | | (165,607 | ) | | (255,108 | ) | | (39,367 | ) | | 89,501 |
| | (215,741 | ) |
Other (7) | | 2,041 |
| | (1,424 | ) | | (749 | ) | | 3,465 |
| | (675 | ) |
| Net cash used in financing activities | | $ | (95,273 | ) | | $ | (93,765 | ) | | $ | (72,013 | ) | | $ | (1,508 | ) | | $ | (21,752 | ) |
____________________________
| |
(1) | In August 2014, we entered into a new senior credit facility which resulted in the payoff of our previous credit agreement in the amount of $533.2 million, proceeds of $600.0 million from the issuance of new long-term obligations, and payments of $8.0 million related to financing costs to creditors and third parties. See Note 7, Long-term Obligations, for more details on the refinance of our senior credit facility. |
| |
(2) | Our share repurchases were partially funded by our revolving credit facility which declined for the year ended December 31, 2015 and increased for the year ended December 31, 2014. |
| |
(3) | The cash paid to purchase noncontrolling interests will vary based upon differing opportunities and circumstances during each of the respective periods. |
| |
(4) | The proceeds from the issuance of stock under stock option plans decreased in 2014 as there were more exercises in 2013 as options were near expiration. |
| |
(5) | The amount of excess tax benefits varies from period to period based upon the volume of options exercised or number of shares vested, and the related stock price at the time these stock activities occur. |
| |
(6) | The cash paid for stock repurchases includes both the repurchase of our common shares, in accordance with our share repurchase program, and income taxes paid on behalf of employees who elected to settle their tax obligations on vested stock with a portion of their vested stock. |
| |
(7) | In September 2015, we received $2.7 million in proceeds from the sale of a noncontrolling interest. |
Future Contractual Cash Requirements
The following table sets forth the scheduled principal, interest and other contractual cash obligations due by us for each of the years indicated as of December 31, 2015 (in thousands):
|
| | | | | | | | | | | | | | | | | | | | |
| | | Payment due by period |
| | | Total | | Less than 1 year | | 1-3 years | | 3-5 years | | More than 5 years |
| Contractual Obligations: | | | | | | | | | | |
| Long-term debt | | $ | 817,230 |
| | $ | 30,230 |
| | $ | 90,000 |
| | $ | 697,000 |
| | $ | — |
|
| Capital lease obligations | | 55,244 |
| | 3,393 |
| | 6,890 |
| | 6,870 |
| | 38,091 |
|
| Operating leases | | 1,196,248 |
| | 79,332 |
| | 156,474 |
| | 146,705 |
| | 813,737 |
|
| Fixed cash interest expense | | 38,227 |
| | 4,450 |
| | 8,002 |
| | 5,645 |
| | 20,130 |
|
Variable cash interest expense Term A(1) | | 46,692 |
| | 13,609 |
| | 25,445 |
| | 7,638 |
| | — |
|
Purchase obligations(2) | | 29,975 |
| | 14,231 |
| | 12,521 |
| | 3,223 |
| | — |
|
Other long-term liabilities(3) | | 38,822 |
| | 4,267 |
| | 7,759 |
| | 6,395 |
| | 20,401 |
|
Earn-out payments(4) | | 5,855 |
| | 2,983 |
| | 2,572 |
| | 100 |
| | 200 |
|
| | | $ | 2,228,293 |
| | $ | 152,495 |
| | $ | 309,663 |
| | $ | 873,576 |
| | $ | 892,559 |
|
____________________________
| |
(1) | The interest payments on our variable-rate senior term notes and revolving credit facility are based on rates effective as of December 31, 2015. |
| |
(2) | Purchase obligations consist primarily of supply purchase agreements related to our Medical Technology and animal hospital businesses and construction contracts primarily for our animal hospitals. |
| |
(3) | Includes future payments under our SERP, Consulting Agreements and Mandatorily Redeemable Partnership Interests. |
| |
(4) | Represents contractual arrangements whereby additional cash may be paid to former owners of acquired businesses upon attainment of specified performance targets. |
Off-Balance Sheet Financing Arrangements
Other than operating leases, which are included in the Contractual Obligations table listed above as of December 31, 2015, we do not have any off-balance sheet financing arrangements.
Description of Indebtedness
Senior Credit Facility
We pay interest on our senior term notes and revolving credit facility based on the interest rate offered to our administrative agent on, the Eurodollar rate plus the applicable margin determined by reference to the Leverage Ratio in effect from time-to-time, ranging from 1.00% to 2.25% per annum. We pay a commitment fee on our revolving credit facility determined by reference to the Leverage Ratio in effect from time-to-time ranging from 0.25% to 0.45% per annum, as set forth in the table in Note 7, Long-Term Obligations, of this annual report on Form 10-K.
At December 31, 2015, we had $585.0 million in principal outstanding under our senior term notes and $232.0 million in borrowings outstanding under our revolving credit facility.
The senior term notes and the revolving credit facility mature in August 2019.
Other Debt and Capital Lease Obligations
At December 31, 2015, we had a seller note secured by assets of a certain animal hospital, capital leases, and other debt that consisted of $3.6 million and $51.9 million included in the current portion and non-current portion of long-term debt, respectively. Our seller note matures in 2016 and has an interest rate of 10.0%. Our capital leases and other debt have various maturities through 2042 and various interest rates ranging from 1.9% to 15.0%.
|
| |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rate Risk
At December 31, 2015, under our senior credit facility we had borrowings of $585.0 million in senior term notes and $232.0 million in borrowings outstanding under our Revolving Credit Facility with fluctuating interest rates based on market benchmarks such as the Eurodollar rate. Changes in interest rates could adversely affect the market value of our variable-rate debt. To mitigate our exposure to increasing interest rates we have historically entered into interest rate swap agreements that effectively convert a certain amount of our variable-rate debt to fixed-rate debt. Given the fact that interest rates have been declining for the past few years, as of December 31, 2015, we had no interest rate swap agreements. In the future, we may enter into interest rate strategies to hedge against the risk of increasing interest rates as well as to maintain an appropriate mix of fixed-rate and variable-rate debt. However, we have not yet determined what those strategies will be or their possible impact.
If the Eurodollar rate increases or decreases 1% from December 31, 2015, the additional annual interest expense or savings would amount to $8.1 million.
Foreign Currency Risk
Growth in our Canadian operations will incrementally increase our exposure to foreign currency fluctuations as well as other risks typical of international operations that could impact our business, including, but not limited to, differing economic conditions, changes in political climate, differing tax structures and other regulations and restrictions. Foreign exchange rate fluctuations may adversely impact our consolidated results of operations as exchange rate fluctuations on transactions denominated in currencies other than our functional currencies result in gains and losses which will be reflected in our consolidated income statement. To the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions will result in increased net revenues and operating expenses. Conversely, our net revenues and operating expenses will decrease when the U.S. dollar strengthens against foreign currencies.
Transaction Exposure
We do not currently hedge our foreign currency exposure.
Translation Exposure
Foreign exchange rate fluctuations may adversely impact our consolidated financial position as the assets and liabilities of our foreign operations are translated into U.S. dollars in preparing the consolidated balance sheets. These gains and losses are recognized as an adjustment to stockholders' equity which is reflected in our balance sheet under accumulated other comprehensive income (loss).
|
| |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
VCA Inc. and Subsidiaries
Index to Consolidated Financial Statements
MANAGEMENT’S ANNUAL REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our consolidated financial statements for external reporting purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management has carried out an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our internal control over financial reporting as of December 31, 2015. In performing this evaluation, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control — Integrated Framework (2013). Based on our assessment of internal control over financial reporting, our management has concluded that, as of December 31, 2015, our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
The effectiveness of our internal control over financial reporting as of December 31, 2015 has been audited by KPMG LLP, an independent registered public accounting firm, as stated in their report, which is included below.
February 26, 2016
|
| |
| | |
/s/ ROBERT L. ANTIN | |
Robert L. Antin Chairman of the Board, President and Chief Executive Officer | |
|
| |
| | |
/s/ TOMAS W. FULLER | |
Tomas W. Fuller Chief Financial Officer, Principal Accounting Officer, Vice President and Secretary | |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
VCA Inc.:
We have audited the accompanying consolidated balance sheets of VCA Inc. and subsidiaries as of December 31, 2015 and 2014, and the related consolidated statements of income, comprehensive income, stockholders’ equity and cash flows for each of the years in the three-year period ended December 31, 2015. In connection with our audits of the consolidated financial statements, we also have audited financial statement schedules I and II. These consolidated financial statements and financial statement schedules are the responsibility of the company’s management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedules based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of VCA Inc. and subsidiaries as of December 31, 2015 and 2014, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2015, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the related financial statement schedules, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the internal control over financial reporting of VCA Inc. as of December 31, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated February 26, 2016 expressed an unqualified opinion on the effectiveness of the internal control over financial reporting of VCA Inc.
/s/ KPMG LLP
Los Angeles, California
February 26, 2016
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
VCA Inc.:
We have audited VCA Inc.'s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). VCA Inc.'s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, VCA Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of VCA Inc. and subsidiaries as of December 31, 2015 and 2014, and the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2015, and the related financial statement schedules, and our report dated February 26, 2016, expressed an unqualified opinion on those consolidated financial statements and financial statement schedules.
/s/ KPMG LLP
Los Angeles, California
February 26, 2016
VCA Inc. and Subsidiaries
Consolidated Balance Sheets
(In thousands, except par value)
|
| | | | | | | | |
| | | December 31, |
| | | 2015 | | 2014 |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 98,888 |
| | $ | 81,383 |
|
| Trade accounts receivable, less allowance for uncollectible accounts of $21,775 and $19,846 at December 31, 2015 and 2014, respectively | | 76,634 |
| | 60,482 |
|
| Inventory | | 51,523 |
| | 56,050 |
|
| Prepaid expenses and other | | 30,521 |
| | 36,924 |
|
| Prepaid income taxes | | 24,598 |
| | 18,277 |
|
| Total current assets | | 282,164 |
| | 253,116 |
|
| Property and equipment, net | | 507,753 |
| | 468,041 |
|
| Goodwill | | 1,517,650 |
| | 1,415,861 |
|
| Other intangible assets, net | | 97,377 |
| | 88,175 |
|
| Notes receivable | | 2,194 |
| | 2,807 |
|
| Deferred financing costs, net | | 6,133 |
| | 7,874 |
|
| Other | | 93,994 |
| | 65,815 |
|
| Total assets | | $ | 2,507,265 |
| | $ | 2,301,689 |
|
| Liabilities and Equity | | | | |
| Current liabilities: | | | | |
| Current portion of long-term debt | | $ | 33,623 |
| | $ | 19,356 |
|
| Accounts payable | | 52,337 |
| | 46,284 |
|
| Accrued payroll and related liabilities | | 75,519 |
| | 64,359 |
|
| Other accrued liabilities | | 70,828 |
| | 67,219 |
|
| Total current liabilities | | 232,307 |
| | 197,218 |
|
| Long-term debt, less current portion | | 838,851 |
| | 775,412 |
|
| Deferred income taxes | | 131,478 |
| | 73,171 |
|
| Other liabilities | | 36,084 |
| | 33,190 |
|
| Total liabilities | | 1,238,720 |
| | 1,078,991 |
|
| Commitments and contingencies | |
| |
|
| Redeemable noncontrolling interests | | 11,511 |
| | 11,077 |
|
| Preferred stock, par value $0.001, 11,000 shares authorized, none outstanding | | — |
| | — |
|
| VCA Inc. stockholders’ equity: | | | | |
| Common stock, par value $0.001, 175,000 shares authorized, 80,764 and 82,937 shares outstanding as of December 31, 2015 and 2014, respectively | | 81 |
| | 83 |
|
| Additional paid-in capital | | 19,708 |
| | 155,802 |
|
| Retained earnings | | 1,275,207 |
| | 1,064,158 |
|
| Accumulated other comprehensive loss | | (50,034 | ) | | (19,397 | ) |
| Total VCA Inc. stockholders’ equity | | 1,244,962 |
| | 1,200,646 |
|
| Noncontrolling interests | | 12,072 |
| | 10,975 |
|
| Total equity | | 1,257,034 |
| | 1,211,621 |
|
| Total liabilities and equity | | $ | 2,507,265 |
| | $ | 2,301,689 |
|
The accompanying notes are an integral part of these consolidated financial statements.
VCA Inc. and Subsidiaries
Consolidated Statements of Income
(In thousands, except per share amounts)
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Revenue | | $ | 2,133,675 |
| | $ | 1,918,483 |
| | $ | 1,803,369 |
|
| Direct costs | | 1,623,604 |
| | 1,473,842 |
| | 1,393,989 |
|
| Gross profit | | 510,071 |
| | 444,641 |
| | 409,380 |
|
| Selling, general and administrative expense | | 183,995 |
| | 171,506 |
| | 157,911 |
|
| Impairment of goodwill and other long-lived assets | | — |
| | 27,019 |
| | — |
|
| Business interruption insurance gain | | (4,523 | ) | | — |
| | — |
|
| Net loss (gain) on sale or disposal of assets | | 829 |
| | (1,152 | ) | | 2,455 |
|
| Operating income | | 329,770 |
| | 247,268 |
| | 249,014 |
|
| Interest expense | | 21,208 |
| | 17,951 |
| | 18,928 |
|
| Interest income | | (132 | ) | | (172 | ) | | (379 | ) |
| Debt retirement costs | | — |
| | 1,709 |
| | — |
|
| Other expense | | 359 |
| | 219 |
| | 90 |
|
| Gain on sale of business | | (43,306 | ) | | — |
| | — |
|
| Income before provision for income taxes | | 351,641 |
| | 227,561 |
| | 230,375 |
|
| Provision for income taxes | | 135,543 |
| | 86,878 |
| | 87,453 |
|
| Net income | | 216,098 |
| | 140,683 |
| | 142,922 |
|
| Net income attributable to noncontrolling interests | | 5,049 |
| | 5,245 |
| | 5,411 |
|
| Net income attributable to VCA Inc. | | $ | 211,049 |
| | $ | 135,438 |
| | $ | 137,511 |
|
| Basic earnings per share | | $ | 2.59 |
| | $ | 1.56 |
| | $ | 1.55 |
|
| Diluted earnings per share | | $ | 2.56 |
| | $ | 1.54 |
| | $ | 1.53 |
|
| Weighted-average shares outstanding for basic earnings per share | | 81,443 |
| | 86,656 |
| | 88,621 |
|
| Weighted-average shares outstanding for diluted earnings per share | | 82,414 |
| | 87,825 |
| | 89,663 |
|
The accompanying notes are an integral part of these consolidated financial statements.
VCA Inc. and Subsidiaries
Consolidated Statements of Comprehensive Income
(In thousands)
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
Net income (1) | | $ | 216,098 |
| | $ | 140,683 |
| | $ | 142,922 |
|
| Other comprehensive income: | | | | | | |
| Foreign currency translation adjustments | | (31,599 | ) | | (13,963 | ) | | (7,999 | ) |
| Other comprehensive loss | | (31,599 | ) | | (13,963 | ) | | (7,999 | ) |
| Total comprehensive income | | 184,499 |
| | 126,720 |
| | 134,923 |
|
Comprehensive income attributable to noncontrolling interests (1) | | 4,087 |
| | 4,489 |
| | 5,449 |
|
| Comprehensive income attributable to VCA Inc. | | $ | 180,412 |
| | $ | 122,231 |
| | $ | 129,474 |
|
____________________________
| |
(1) | Includes $2.4 million, $2.4 million and $3.3 million for 2015, 2014 and 2013, respectively, related to redeemable and mandatorily redeemable noncontrolling interests. |
The accompanying notes are an integral part of these consolidated financial statements.
VCA Inc. and Subsidiaries
Consolidated Statements of Stockholders' Equity
(In thousands)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional Paid-In Capital | | Retained Earnings | | Accumulated Other Comprehensive Income (Loss) | | Noncontrolling Interests | | Total |
| | | Shares | | Amount | |
| Balances, December 31, 2012 | | 88,372 |
| | $ | 88 |
| | $ | 390,359 |
| | $ | 791,209 |
| | $ | 1,847 |
| | $ | 10,890 |
| | $ | 1,194,393 |
|
| Net income (excludes $1,233 and $2,003 related to redeemable and mandatorily redeemable noncontrolling interests, respectively) | | — |
| | — |
| | — |
| | 137,511 |
| | — |
| | 2,175 |
| | 139,686 |
|
| Other comprehensive loss (excludes $44 related to mandatorily redeemable noncontrolling interests) | | — |
| | — |
| | — |
| | — |
| | (8,037 | ) | | (6 | ) | | (8,043 | ) |
| Formation of noncontrolling interest | | — |
| | — |
| | | | — |
| | — |
| | 3,336 |
| | 3,336 |
|
| Distribution to noncontrolling interests | | — |
| | — |
| | — |
| | — |
| | — |
| | (1,886 | ) | | (1,886 | ) |
| Purchase of noncontrolling interests | | — |
| | — |
| | (785 | ) | | — |
| | — |
| | (4,309 | ) | | (5,094 | ) |
| Share-based compensation | | — |
| | — |
| | 14,104 |
| | — |
| | — |
| | — |
| | 14,104 |
|
| Issuance of common stock under stock incentive plans | | 1,559 |
| | 2 |
| | 17,231 |
| | — |
| | — |
| | — |
| | 17,233 |
|
| Stock repurchases | | (1,423 | ) | | (1 | ) | | (39,366 | ) | | — |
| | — |
| | — |
| | (39,367 | ) |
| Excess tax benefit from stock based compensation | | — |
| | — |
| | 3,446 |
| | — |
| | — |
| | — |
| | 3,446 |
|
| Tax shortfall and other from stock options and awards | | — |
| | — |
| | (268 | ) | | — |
| | — |
| | — |
| | (268 | ) |
| Other | | — |
| | — |
| | 76 |
| | — |
| | — |
| | — |
| | 76 |
|
| Balances, December 31, 2013 | | 88,508 |
| | 89 |
| | 384,797 |
| | 928,720 |
| | (6,190 | ) | | 10,200 |
| | 1,317,616 |
|
| Net income (excludes $1,210 and $1,625 related to redeemable and mandatorily redeemable noncontrolling interests, respectively) | | — |
| | — |
| | — |
| | 135,438 |
| | — |
| | 2,410 |
| | 137,848 |
|
| Other comprehensive loss (excludes $469 related to mandatorily redeemable noncontrolling interests) | | — |
| | — |
| | — |
| | — |
| | (13,207 | ) | | (287 | ) | | (13,494 | ) |
| Formation of noncontrolling interest | | — |
| | — |
| | — |
| | — |
| | — |
| | 933 |
| | 933 |
|
| Distribution to noncontrolling interests | | — |
| | — |
| | — |
| | — |
| | — |
| | (2,281 | ) | | (2,281 | ) |
| Purchase of noncontrolling interests | | — |
| | — |
| | 30 |
| | — |
| | — |
| | — |
| | 30 |
|
| Share-based compensation | | — |
| | — |
| | 17,200 |
| | — |
| | — |
| | — |
| | 17,200 |
|
| Issuance of common stock under stock incentive plans | | 881 |
| | — |
| | 2,859 |
| | — |
| | — |
| | — |
| | 2,859 |
|
| Stock repurchases | | (6,452 | ) | | (6 | ) | | (255,102 | ) | | — |
| | — |
| | — |
| | (255,108 | ) |
| Excess tax benefit from stock based compensation | | — |
| | — |
| | 6,241 |
| | — |
| | — |
| | — |
| | 6,241 |
|
| Tax shortfall and other from stock options and awards | | — |
| | — |
| | (223 | ) | | — |
| | — |
| | — |
| | (223 | ) |
| Balances, December 31, 2014 | | 82,937 |
| | 83 |
| | 155,802 |
| | 1,064,158 |
| | (19,397 | ) | | 10,975 |
| | 1,211,621 |
|
| Net income (excludes $958 and $1,447 related to redeemable and mandatorily redeemable noncontrolling interests, respectively) | | — |
| | — |
| | — |
| | 211,049 |
| | — |
| | 2,644 |
| | 213,693 |
|
| Other comprehensive loss (excludes $440 related to mandatorily redeemable noncontrolling interests) | | — |
| | — |
| | — |
| | — |
| | (30,637 | ) | | (522 | ) | | (31,159 | ) |
| Formation of noncontrolling interests | | — |
| | — |
| | — |
| | — |
| | — |
| | 1,916 |
| | 1,916 |
|
| Distribution to noncontrolling interests | | — |
| | — |
| | — |
| | — |
| | — |
| | (2,468 | ) | | (2,468 | ) |
| Purchase of noncontrolling interests | | — |
| | — |
| | (217 | ) | | — |
| | — |
| | (473 | ) | | (690 | ) |
| Share-based compensation | | — |
| | — |
| | 16,264 |
| | — |
| | — |
| | — |
| | 16,264 |
|
| Issuance of common stock under stock incentive plans | | 911 |
| | 1 |
| | 2,682 |
| | — |
| | — |
| | — |
| | 2,683 |
|
| Stock repurchases | | (3,084 | ) | | (3 | ) | | (165,604 | ) | | — |
| | — |
| | — |
| | (165,607 | ) |
| Excess tax benefit from stock based compensation | | — |
| | — |
| | 11,089 |
| | — |
| | — |
| | — |
| | 11,089 |
|
| Tax shortfall and other from stock options and awards | | — |
| | — |
| | (308 | ) | | — |
| | — |
| | — |
| | (308 | ) |
| Balances, December 31, 2015 | | 80,764 |
| | $ | 81 |
| | $ | 19,708 |
| | $ | 1,275,207 |
| | $ | (50,034 | ) | | $ | 12,072 |
| | $ | 1,257,034 |
|
The accompanying notes are an integral part of these consolidated financial statements.
VCA Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Cash flows from operating activities: | | | | | | |
| Net income | | $ | 216,098 |
| | $ | 140,683 |
| | $ | 142,922 |
|
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | |
| Impairment of goodwill and other long-lived assets | | — |
| | 27,019 |
| | — |
|
| Gain on sale of business | | (43,306 | ) | | — |
| | — |
|
| Depreciation and amortization | | 81,688 |
| | 79,427 |
| | 77,409 |
|
| Amortization of debt issue costs | | 1,741 |
| | 1,391 |
| | 1,245 |
|
| Provision for uncollectible accounts | | 8,401 |
| | 6,248 |
| | 7,360 |
|
| Debt retirement costs | | — |
| | 1,709 |
| | — |
|
| Net loss (gain) on sale or disposal of assets | | 829 |
| | (1,152 | ) | | 2,455 |
|
| Share-based compensation | | 16,264 |
| | 17,200 |
| | 14,104 |
|
| Deferred income taxes | | 56,722 |
| | 8,853 |
| | 18,064 |
|
| Excess tax benefits from stock based compensation | | (11,089 | ) | | (6,241 | ) | | (3,446 | ) |
| Other | | 2,159 |
| | 531 |
| | (377 | ) |
| Changes in operating assets and liabilities: | | | | | | |
| Trade accounts receivable | | (28,720 | ) | | (3,900 | ) | | (11,048 | ) |
| Inventory, prepaid expenses and other assets | | (9,716 | ) | | (22,897 | ) | | (7,134 | ) |
| Accounts payable and other accrued liabilities | | 10,812 |
| | 11,597 |
| | 557 |
|
| Accrued payroll and related liabilities | | 11,323 |
| | 6,782 |
| | 6,502 |
|
| Income taxes | | 4,339 |
| | 2,960 |
| | 7,759 |
|
| Net cash provided by operating activities | | 317,545 |
| | 270,210 |
| | 256,372 |
|
| Cash flows from investing activities: | | | | | | |
| Business acquisitions, net of cash acquired | | (151,586 | ) | | (147,507 | ) | | (58,016 | ) |
| Investment in Vetstreet Inc. | | (9,552 | ) | | — |
| | — |
|
| Property and equipment additions | | (95,234 | ) | | (72,948 | ) | | (73,270 | ) |
| Proceeds from sale or disposal of assets | | 6,762 |
| | 3,904 |
| | 7,096 |
|
| Proceeds from sale of business | | 48,000 |
| | — |
| | — |
|
| Other | | (2,042 | ) | | (2,691 | ) | | (2,541 | ) |
| Net cash used in investing activities | | (203,652 | ) | | (219,242 | ) | | (126,731 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
VCA Inc. and Subsidiaries
Consolidated Statements of Cash Flows - (Continued)
(In thousands)
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Cash flows from financing activities: | | | | | | |
| Repayment of long-term obligations | | (35,017 | ) | | (568,011 | ) | | (41,129 | ) |
| Proceeds from the issuance of long-term obligations | | — |
| | 600,000 |
| | — |
|
| Proceeds from revolving credit facility | | 97,000 |
| | 135,000 |
| | — |
|
| Payment of financing costs | | — |
| | (7,987 | ) | | — |
|
| Distributions to noncontrolling interest partners | | (4,962 | ) | | (5,009 | ) | | (4,866 | ) |
| Purchase of noncontrolling interests | | (2,500 | ) | | (326 | ) | | (6,581 | ) |
| Proceeds from issuance of common stock under stock incentive plans | | 2,683 |
| | 2,859 |
| | 17,233 |
|
| Excess tax benefits from stock based compensation | | 11,089 |
| | 6,241 |
| | 3,446 |
|
| Stock repurchases | | (165,607 | ) | | (255,108 | ) | | (39,367 | ) |
| Other | | 2,041 |
| | (1,424 | ) | | (749 | ) |
| Net cash used in financing activities | | (95,273 | ) | | (93,765 | ) | | (72,013 | ) |
| Effect of currency exchange rate changes on cash and cash equivalents | | (1,115 | ) | | (849 | ) | | (1,034 | ) |
| Increase (decrease) in cash and cash equivalents | | 17,505 |
| | (43,646 | ) | | 56,594 |
|
| Cash and cash equivalents at beginning of year | | 81,383 |
| | 125,029 |
| | 68,435 |
|
| Cash and cash equivalents at end of year | | $ | 98,888 |
| | $ | 81,383 |
| | $ | 125,029 |
|
| | | | | | | |
| Supplemental disclosures of cash flow information: | | | | | | |
| Interest paid | | $ | 18,517 |
| | $ | 15,049 |
| | $ | 16,422 |
|
| Income taxes paid | | $ | 74,315 |
| | $ | 75,098 |
| | $ | 61,825 |
|
| | | | | | | |
| Supplemental schedule of non-cash investing and financing activities: | | | | | | |
| Capital lease additions | | $ | — |
| | $ | — |
| | $ | 21,668 |
|
| Detail of Vetstreet sale: | | | | | | |
| Accounts receivable, net | | $ | (4,480 | ) | | | | |
| Prepaid and other | | (1,300 | ) | | | | |
| Fixed assets, net | | (487 | ) | | | | |
| Intangibles | | (2,567 | ) | | | | |
| Liabilities | | 4,140 |
| | | | |
| | | $ | (4,694 | ) | | | | |
| | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Our company, VCA Inc. (“VCA”) is a Delaware corporation formed in 1986 and is based in Los Angeles, California. We are an animal healthcare company with the following five operating segments: animal hospitals (“Animal Hospital”), veterinary diagnostic laboratories (“Laboratory”), veterinary medical technology (“Medical Technology”), Vetstreet and Camp Bow Wow Franchising, Inc. (f/k/a D.O.G. Enterprises, LLC) ("Camp Bow Wow").
Our animal hospitals offer a full range of general medical and surgical services for companion animals. Our animal hospitals treat diseases and injuries, provide pharmaceutical products and perform a variety of pet-wellness programs, including health examinations, diagnostic testing, vaccinations, spaying, neutering and dental care. At December 31, 2015, we operated or managed 682 animal hospitals throughout 41 states and four Canadian provinces.
We operate a full-service veterinary diagnostic laboratory network serving all 50 states and certain areas in Canada. Our laboratory network provides sophisticated testing and consulting services used by veterinarians in the detection, diagnosis, evaluation, monitoring, treatment and prevention of diseases and other conditions affecting animals. At December 31, 2015, we operated 60 laboratories of various sizes located strategically throughout the United States and Canada.
Our Medical Technology business sells digital radiography and ultrasound imaging equipment, provides education and training on the use of that equipment, provides consulting and mobile imaging services, and sells software and ancillary services to the veterinary market.
Our Vetstreet business provides several different services to the veterinary community including, online communications, professional education, marketing solutions and a home delivery platform for independent animal hospitals. On December 31, 2015 we completed the sale of our Vetstreet business, resulting in a gain of $43.3 million. Concurrent with the sale of substantially all of the assets of the company we purchased a 19.9% interest.
Our Camp Bow Wow business franchises a premier provider of pet services including dog day care, overnight boarding, grooming and other ancillary services at specially designed pet care facilities, principally under the trademark Camp Bow Wow®. As of December 31, 2015, there were 126 Camp Bow Wow® franchise locations operating in 34 states and one Canadian province.
|
| |
| 2. | Summary of Significant Accounting Policies |
a. Principles of Consolidation
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States ("GAAP"), and include the accounts of our parent company, all majority-owned subsidiaries where we have control, certain fifty-percent owned subsidiaries where we possess the power to direct or cause the direction of management and policies and certain veterinary medical groups to which we provide services as discussed below. We have eliminated all intercompany transactions and balances in consolidation.
We provide management and other administrative services to certain veterinary practices in states and Canadian provinces with laws, rules and regulations which require that veterinary medical practices be owned by licensed veterinarians and that corporations which are not owned by licensed veterinarians refrain from providing, or holding themselves out as providers of, veterinary medical care. In these states and Canadian provinces, we provide management and other administrative services to the veterinary medical practices. At December 31, 2015, we operated 198 animal hospitals in 15 of these states and 76 animal hospitals in four Canadian provinces, under management agreements with these veterinary practices. Pursuant to the management agreements, the veterinary medical practices are each solely responsible for all aspects of the practice of veterinary medicine, as defined by their respective state or province.
We have determined that the veterinary medical practices are variable interest entities as defined by the Financial Accounting Standards Board (“FASB”), and that we have a variable interest in those entities through our management agreements. We also determined that our variable interests in these veterinary medical practices, in aggregate with the variable interests held by our related parties, provide us with the power to direct the activities of these practices that most significantly impact their economic performance and obligate us to absorb losses that could potentially be significant or the right to receive
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
benefits from the veterinary medical practices that could potentially be significant. Based on these determinations, we consolidated the veterinary medical practices in our consolidated financial statements.
b. Foreign Currency Translation
The functional currency of our Canadian subsidiaries is their local currency. Assets and liabilities are translated into U.S. dollars at the exchange rate at the balance sheet date, whereas revenues and expenses are translated into U.S. dollars at the average exchange rate for the reporting period.
Translation adjustments are included in accumulated other comprehensive income (loss) and realized transaction gains and losses are recorded in the results of operations.
c. Use of Estimates in Preparation of Financial Statements
The preparation of our consolidated financial statements in conformity with GAAP requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and contingent liabilities at the date of our consolidated financial statements and our reported amounts of revenue and expense during the reporting period. Actual results could differ from our estimates. Amounts subject to significant judgment and estimates include, but are not limited to, collectability of receivables, cash flows used in the evaluation of impairment of goodwill, cash flows used in the evaluation of impairment of long-lived assets, valuation allowance on deferred tax assets, estimated redemption value of mandatorily redeemable partnership interests and inputs used for computing stock-based compensation.
d. Revenue and Related Cost Recognition
General
We recognize revenue, barring other facts, when the following revenue recognition criteria are met:
| |
| • | persuasive evidence of a sales arrangement exists; |
| |
| • | delivery of goods has occurred or services have been rendered; |
| |
| • | the sales price or fee is fixed or determinable; and |
| |
| • | collectability is reasonably assured. |
Revenue is reported net of sales discounts and excludes sales taxes.
We generally recognize revenue and costs as follows:
| |
| • | For non-contractual services provided by our business units, at the time services are rendered. |
| |
| • | For the sale of merchandise, when delivery of the goods has occurred. |
| |
| • | For services under defined support and maintenance contracts, on a straight-line basis over the contractual period, recognizing costs as incurred; these services include, but are not limited to, technical support, when-and-if available product updates for software and extended warranty coverage. |
| |
| • | For the sale of our digital radiography imaging equipment and ultrasound imaging equipment sold on a standalone basis, at the time title and risk of loss transfers to the customer, which is generally upon delivery or upon installation and customer acceptance if required per the sale arrangement. |
| |
| • | For the sale of reminder cards, when shipment has occurred. |
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
| |
| • | For revenue related to bundled products and services, sales arrangement consideration is allocated at the inception of the arrangement to all deliverables using the relative selling price method, whereby any discount in the arrangement is allocated proportionally to each deliverable on the basis of each deliverable’s selling price. |
Deferred Revenue
We defer revenue for certain transactions as follows:
| |
| • | We defer revenue for pre-paid services such as our veterinarian wellness, consulting, marketing and education services and recognize that revenue on a straight-line basis over the contract period or as the services are provided depending on the nature of the service. |
| |
| • | We defer revenue for services provided as part of the purchase of equipment and software and recognize that revenue on a straight-line basis over the service period. |
As a result of these policies, we have deferred revenue and costs at December 31, 2015 and 2014 consisting of the following (in thousands):
|
| | | | | | | | |
| | | 2015 | | 2014 |
Deferred equipment revenue(1) | | $ | 753 |
| | $ | 883 |
|
| Deferred fixed-priced support or maintenance contract revenue | | 3,811 |
| | 4,265 |
|
Other deferred revenue(2) | | 10,083 |
| | 9,190 |
|
| Total deferred revenue | | 14,647 |
| | 14,338 |
|
| Less current portion included in other accrued liabilities | | 14,647 |
| | 14,304 |
|
| Long-term portion of deferred revenue included in other liabilities | | $ | — |
| | $ | 34 |
|
| Current portion of deferred costs included in prepaid expenses and other | | $ | 1,172 |
| | $ | 866 |
|
| Long-term portion of deferred costs included in other assets | | (72 | ) | | 1,126 |
|
Total deferred costs(3) | | $ | 1,100 |
| | $ | 1,992 |
|
____________________________
| |
(1) | Represents amounts received for sales arrangements that include equipment, hardware, software and services. |
| |
(2) | Represents amounts received in advance for services. |
| |
(3) | Represents costs related to warranties, equipment and hardware included in deferred equipment revenue. |
Customer Loyalty Programs
We record reductions to revenue related to customer incentive programs, which include various forms of cash consideration. Incentives may be provided in the form of credits, coupons or loans and are earned by clients upon entering into an agreement to purchase products or services in future periods while maintaining defined volume purchase or utilization levels. These incentives are capitalized and recognized as a reduction to revenue over the term of the customer agreement. We monitor customer purchases over the term of their agreement to assess the realizability of our capitalized customer acquisition costs. For the years ended December 31, 2015, 2014 and 2013, we did not have any impaired customer acquisition costs.
e. Direct Costs
Direct costs are comprised of all service and product costs, including but not limited to, salaries of veterinarians, technicians and other hospital-based, laboratory-based personnel, and content-development personnel, transportation and delivery costs, facilities rent, occupancy costs, supply costs, depreciation and amortization, certain marketing and promotional expenses and costs of goods sold.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
f. Cash and Cash Equivalents
We consider only highly liquid investments with original maturities of less than 90 days to be cash equivalents. We maintain balances in our bank accounts that are in excess of FDIC insured levels.
g. Inventory
Our inventory consists primarily of finished goods and includes imaging equipment, pet food and products and medical supplies. It is valued at the lower of cost or market using the first-in, first-out method and is adjusted for estimated obsolescence and written down to net realizable value based upon estimates of future demand, technology developments and market conditions.
h. Property and Equipment
Property and equipment is recorded at cost. Equipment held under capital leases is recorded at the lower of the present value of the minimum lease payments or the fair value of the equipment at the beginning of the lease term.
We develop and implement new software to be used internally, or enhance our existing internal software. We develop the software using our own employees and/or outside consultants. We capitalize software development costs when application development begins, it is probable that the project will be completed, and the software will be used as intended. We expense costs associated with preliminary project stage activities, training, maintenance and all other post implementation stage activities as we incur these costs. The capitalized costs are amortized over the expected useful lives of the software. Costs related to upgrades or enhancements of existing systems are capitalized if the modifications result in additional functionality.
Depreciation and amortization are recognized on the straight-line method over the following estimated useful lives:
|
| |
| Buildings and improvements | 5 to 40 years |
| Leasehold improvements | Lesser of lease term or 15 years |
| Furniture and equipment | 3 to 10 years |
| Software | 3 to 10 years |
| Equipment held under capital leases | 5 to 10 years |
Depreciation and amortization expense, including the amortization of property under capital leases, in 2015, 2014 and 2013 was $58.3 million, $58.4 million and $56.5 million, respectively.
Property and equipment at December 31, 2015 and 2014 consisted of (in thousands):
|
| | | | | | | | |
| | | 2015 | | 2014 |
| Land | | $ | 72,651 |
| | $ | 64,495 |
|
| Building and improvements | | 156,417 |
| | 152,812 |
|
| Leasehold improvements | | 213,272 |
| | 193,274 |
|
| Furniture and equipment | | 325,244 |
| | 296,789 |
|
| Software | | 46,366 |
| | 39,987 |
|
| Buildings held under capital leases | | 58,984 |
| | 59,555 |
|
| Equipment held under capital leases | | 55 |
| | 875 |
|
| Construction in progress | | 22,230 |
| | 12,466 |
|
| Total property and equipment | | 895,219 |
| | 820,253 |
|
| Less — accumulated depreciation and amortization | | (387,466 | ) | | (352,212 | ) |
| Total property and equipment, net | | $ | 507,753 |
| | $ | 468,041 |
|
Accumulated amortization on buildings and equipment held under capital leases amounted to $19.1 million and $17.0 million at December 31, 2015 and 2014, respectively.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
i. Operating Leases
Most of our facilities are under operating leases. The minimum lease payments, including predetermined fixed escalations of the minimum rent, are recognized as rent expense on a straight-line basis over the lease term as defined in the FASB’s accounting guidance pertaining to leases. The lease term includes contractual renewal options that are reasonably assured based on significant leasehold improvements acquired. Any leasehold improvement incentives paid to us by a landlord are recorded as a reduction of rent expense over the lease term.
j. Goodwill
Goodwill represents the excess of the consideration transferred over the net of the fair value of identifiable assets acquired and liabilities assumed in a business combination.
Impairment testing for goodwill is performed at the reporting unit level. A reporting unit is an operating segment or one level below an operating segment (also known as a component). In accordance with the FASB’s accounting guidance pertaining to goodwill and other intangibles, we have determined that we have five reporting units: Animal Hospital, Laboratory, Medical Technology, Vetstreet and Camp Bow Wow. Annually, or sooner if circumstances indicate an impairment may exist, we estimate the fair value of each of our reporting units and compare their estimated fair value against the net book value of those reporting units to determine if our goodwill is impaired.
The recognition and measurement of a goodwill impairment loss involves either a qualitative assessment of the fair value of each reporting unit or a more detailed quantitative two-step process. We have the option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its net book value. If we elect to not use this option, or we determine, using the qualitative method, that it is more likely than not that the fair value of a reporting unit is less than its net book value, we then perform the more detailed two-step impairment test. Step one of the two-step test compares the fair value of the reporting unit to its carrying value. If the carrying value exceeds the fair value, there is a potential impairment and step two must be performed. Step two compares the carrying value of the reporting unit’s goodwill to its implied fair value (i.e., the fair value of the reporting unit less the fair value of the unit’s assets and liabilities, including identifiable intangible assets). If the carrying value of goodwill exceeds its implied fair value, the excess is required to be recorded as an impairment.
Our estimated reporting unit fair values are calculated using valuation methods consisting primarily of discounted cash flow techniques, and market comparables, where applicable. These valuation methods involve the use of significant assumptions and estimates such as forecasted growth rates, valuation multiples, the weighted-average cost of capital, and risk premiums, which are based upon the best available market information and are consistent with our long-term strategic plans. Negative changes in our projected cash flows related to variables such as revenue growth rates, margins, or the discount rate could result in a decrease in the estimated fair value of our reporting units and could ultimately result in a substantial goodwill impairment charge. The performance of our reporting units, and in turn the risk of goodwill impairment, is subject to a number of risks and uncertainties, some of which are outside of our control.
Consumer spending habits for our business are affected by, among other things, prevailing economic conditions, levels of employment, salaries and wage rates, consumer confidence and consumer perception of economic conditions. We believe these factors have and may continue to impact consumer spending for our products and services. Deterioration in consumer spending habits for our business would negatively impact the value of our reporting units and could result in additional goodwill impairment. Any potential impairment charge could be material and would be reflected as expense in our consolidated statements of income. We provide no assurance that forecasted growth rates, valuation multiples, and discount
rates will not deteriorate in the near term. We will continue to analyze changes to these assumptions in future periods.
We adopted the end of October as our annual impairment testing date although, as mentioned above, we test our reporting units sooner if an event or circumstances change that would more than likely than not reduce the fair value of a reporting unit below its carrying value.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
As of October 31, 2015, we evaluated our goodwill for impairment. Based on this analysis, we concluded that goodwill was not impaired for any of our reporting units. As of December 31, 2015, no events or changes in circumstances occurred that would have triggered the need for an additional impairment review of goodwill.
k. Other Intangible Assets
In addition to goodwill, we have amortizable intangible assets at December 31, 2015 and 2014, as follows (in thousands):
|
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2015 | | 2014 |
| | | Gross Carrying Amount | | Accumulated Amortization | | Net Carrying Amount | | Gross Carrying Amount | | Accumulated Amortization | | Net Carrying Amount |
| Non-contractual customer relationships | | $ | 116,082 |
| | $ | (48,821 | ) | | $ | 67,261 |
| | $ | 101,056 |
| | $ | (45,295 | ) | | $ | 55,761 |
|
| Covenants not-to-compete | | 12,435 |
| | (4,779 | ) | | 7,656 |
| | 10,093 |
| | (4,422 | ) | | 5,671 |
|
| Favorable lease asset | | 9,441 |
| | (5,440 | ) | | 4,001 |
| | 9,576 |
| | (4,962 | ) | | 4,614 |
|
| Technology | | 1,377 |
| | (589 | ) | | 788 |
| | 1,627 |
| | (414 | ) | | 1,213 |
|
| Trademarks | | 11,591 |
| | (4,086 | ) | | 7,505 |
| | 13,503 |
| | (4,015 | ) | | 9,488 |
|
| Contracts | | — |
| | — |
| | — |
| | 100 |
| | (11 | ) | | 89 |
|
| Franchise rights | | 11,730 |
| | (1,564 | ) | | 10,166 |
| | 11,730 |
| | (391 | ) | | 11,339 |
|
| Total | | $ | 162,656 |
| | $ | (65,279 | ) | | $ | 97,377 |
| | $ | 147,685 |
| | $ | (59,510 | ) | | $ | 88,175 |
|
The recoverability of the carrying values of all intangible assets with finite lives are re-evaluated when events or changes in circumstances indicate an asset's value may be impaired. We perform a quarterly review of identified intangible assets to determine if facts and circumstances indicate that the useful life is shorter than we had originally estimated or that the carrying amount of assets may not be recoverable. If such facts and circumstances exist, we assess recoverability by comparing the projected undiscounted net cash flows associated with the related asset or group of assets over their remaining lives against their respective carrying amounts. Impairments, if any, are based on the excess of the carrying amount over the fair value of those assets. If the useful life is shorter than originally estimated, we accelerate the rate of amortization and amortize the remaining carrying value over the new shorter useful life.
In 2014, we recorded a $13.1 million intangible asset impairment charge related to non-contractual customer relationships, technology, trademarks and contracts related to Vetstreet. Our determination that the fair value of the intangible assets was less than carrying value was based upon changes in our estimate of forecasted cash flows. These forecasted cash flow changes were related to the less than anticipated positive impact of new product offerings outlined in an operational and financial plan established in 2013 and the negative impact of the overall competitive environment, discussed in further detail below in Note 5, Goodwill. The fair values of the impaired intangibles were calculated utilizing valuation methods consisting primarily of discounted cash flow techniques, and market comparables, where applicable. The impairment is included under the caption "Impairment of goodwill and other long-lived assets" in our consolidated income statement.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
Amortization is recognized on the straight-line method over the following estimated useful lives:
|
| | |
| Non-contractual hospital customer relationships | | 5 years |
| Non-contractual laboratory customer relationships | | 5 to 25 years |
| All other non-contractual customer relationships | | 5 to 7 years |
| Covenants not-to-compete | | 3 to 25 years |
| Favorable lease asset | | 4 to 27 years |
| Technology | | 4 to 10 years |
| Trademarks | | 2 to 10 years |
The following table summarizes our aggregate amortization expense related to other intangible assets (in thousands):
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Aggregate amortization expense | | $ | 23,395 |
| | $ | 21,039 |
| | $ | 20,934 |
|
The estimated amortization expense related to intangible assets for each of the five succeeding years and thereafter at December 31, 2015 is as follows (in thousands):
|
| | | |
| Definite-lived intangible assets: | |
| 2016 | $ | 22,841 |
|
| 2017 | 17,302 |
|
| 2018 | 14,006 |
|
| 2019 | 11,127 |
|
| 2020 | 6,390 |
|
| Thereafter | 24,671 |
|
| Total | $ | 96,337 |
|
| Indefinite-lived intangible assets: | |
| Trademarks | 1,040 |
|
| Total intangible assets | $ | 97,377 |
|
l. Income Taxes
We account for income taxes under the FASB’s accounting guidance on income taxes. In accordance with the guidance, we record deferred tax liabilities and deferred tax assets, which represent taxes to be recovered or settled in the future. We adjust our deferred tax assets and deferred tax liabilities to reflect changes in tax rates or other statutory tax provisions. We make judgments in assessing our ability to realize future benefits from our deferred tax assets, which include operating and capital loss carryforwards. As such, we have a valuation allowance to reduce our deferred tax assets for the portion we believe will not be realized. Changes in tax rates or other statutory provisions are recognized in the period the change occurs. We also assess differences between our probable tax bases and the as-filed tax bases of certain assets and liabilities.
We account for unrecognized tax benefits also in accordance with the FASB’s accounting guidance on income taxes which prescribe a minimum probability threshold that a tax position must meet before a financial statement benefit is recognized. The minimum threshold is defined as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation, based solely on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. We did not have any unrecognized tax benefits at December 31, 2015 and 2014.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
m. Notes Receivable
Notes receivable are financial instruments issued in the normal course of business and are not market traded. The amounts recorded approximate fair value and are shown net of valuation allowances. There were no valuation allowances recorded as of December 31, 2015 and December 31, 2014. The notes bear interest at rates varying from 2.6% to 7.5% per annum.
n. Deferred Financing Costs
Deferred financing costs are amortized using the effective interest method over the life of the related debt. Amortization of deferred financing costs was $1.7 million and $1.4 million for the periods ending December 31, 2015 and 2014, respectively.
o. Fair Value of Financial Instruments and Concentration of Risk
The carrying amount reported in our consolidated balance sheets for cash, cash equivalents, trade accounts receivable, accounts payable and accrued liabilities approximates fair value because of the immediate or short-term maturity of these financial instruments. Our policy is to place our cash and cash equivalents in highly-rated financial instruments and institutions,which we believe mitigates our credit risk. Concentration of credit risk with respect to accounts receivable is limited due to the diversity of our customer base. We routinely review the collection of our accounts receivable and maintain an allowance for potential credit losses, but historically have not experienced any significant losses related to an individual customer or groups of customers in a geographic area.
Our operations depend, in some cases, on the ability of single source suppliers or a limited number of suppliers, to deliver products and supplies on a timely basis. We have in the past experienced, and may in the future experience, shortages of or difficulties in acquiring products and supplies in the quantities and of the quality needed. Shortages in the availability of products and supplies for an extended period of time could have a negative impact on our operating results.
p. Marketing and Advertising
Marketing and advertising costs are expensed as incurred. Total marketing and advertising expense included in direct costs amounted to $25.5 million, $23.9 million and $25.4 million for 2015, 2014 and 2013, respectively. Total marketing and advertising expense included in selling, general and administrative expense amounted to $7.5 million, $6.8 million and $5.9 million for 2015, 2014 and 2013, respectively.
q. Insurance and Self-Insurance
We use a combination of insurance and self-insurance with high-retention or high-deductible provisions for a number of risks, including workers’ compensation, general liability, property insurance and our group health insurance benefits.
Liabilities associated with these risks are estimated based on an undiscounted basis by considering historical claims experience, demographic factors, severity factors and other actuarial assumptions.
r. Product Warranties
We accrue the cost of basic product warranties included with the sale of our digital radiography imaging equipment and our ultrasound imaging equipment at the time we sell these units to our customers. Our warranty costs are primarily for our assistance in helping our customers resolve issues with the warranties they have with the original equipment manufacturers. We estimate our warranty costs based on historical warranty claim experience. Accrued warranty costs at December 31, 2015 were$0.05 million. There were no accrued warranty costs at December 31, 2014.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
s. Calculation of Earnings per Share
Basic earnings per share is calculated by dividing net income by the weighted-average number of shares outstanding during the period. Diluted earnings per share is calculated by dividing net income by the weighted-average number of common shares outstanding after giving effect to all potentially dilutive common shares outstanding during the period. Basic and diluted earnings per share were calculated as follows (in thousands, except per share amounts):
|
| | | | | | | | | | | | |
| | | For Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Net income attributable to VCA Inc. | | $ | 211,049 |
| | $ | 135,438 |
| | $ | 137,511 |
|
| Weighted average common shares outstanding: | | | | | | |
| Basic | | 81,443 |
| | 86,656 |
| | 88,621 |
|
| Effect of dilutive potential common stock: | | | | | | |
| Stock options | | 330 |
| | 302 |
| | 305 |
|
| Non-vested shares and units | | 641 |
| | 867 |
| | 737 |
|
| Diluted | | 82,414 |
| | 87,825 |
| | 89,663 |
|
| Basic earnings per common share | | $ | 2.59 |
| | $ | 1.56 |
| | $ | 1.55 |
|
| Diluted earnings per common share | | $ | 2.56 |
| | $ | 1.54 |
| | $ | 1.53 |
|
For the years ended December 31, 2015, 2014 and 2013, potential common shares of 55,351, 31,668 and 43,300, respectively, were excluded from the computation of diluted earnings per share because their inclusion would have had an anti-dilutive effect.
t. Share-Based Compensation
We account for share-based compensation in accordance with FASB’s accounting guidance for stock compensation. Accordingly, we measure the cost of share-based payments based on the grant-date fair value of the equity instruments and recognize the cost over the requisite service period, which is typically the vesting period. Our company’s share-based employee compensation plans are described further in Note 10, Share-Based Compensation.
u. Acquisitions
We account for acquisitions based upon the provisions of the FASB’s accounting guidance on business combinations. Accordingly, acquisitions are accounted for at fair value under the acquisition method of accounting. Acquisition costs are expensed as incurred; noncontrolling interests are valued at fair value at the acquisition date; and changes in deferred tax asset valuation allowances and income tax uncertainties after the acquisition date generally affect income tax expense.
v. Litigation
We are party to various claims and lawsuits arising in the normal course of business. We closely monitor these claims and lawsuits and frequently consult with our legal counsel to determine whether they may, when resolved, have a material adverse effect on our financial position or results of operations and accrue and/or disclose loss contingencies as appropriate.
w. Recent Accounting Pronouncements
In November 2015, the FASB issued Accounting Standards Update (ASU) 2015-17, “Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes”, that now requires deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. This Update amends current GAAP that requires an entity to separate deferred income tax liabilities and assets into current and noncurrent amounts in a classified statement of financial position, simplifying the presentation and aligning it with the International Financial Reporting Standards (IFRS). This ASU is effective for fiscal
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
years beginning after December 15, 2016, including interim periods within those fiscal years, with early adoption permitted.
We elected to retrospectively adopt the accounting standard in the fourth quarter of 2015.
In September 2015, the FASB issued Accounting Standards Update (ASU) 2015-16, “Business Combinations (Topic 805): Simplifying the Accounting for Measurement-Period Adjustments” , which amends the requirement for the acquirer in a business combination to retrospectively adjust the provisional amounts recognized at the acquisition date with a corresponding adjustment to goodwill and revise comparative information for prior year periods presented. This update simplifies the accounting for the adjustments to provisional amounts by eliminating the requirement to retrospectively account for those adjustments. The adjustments to provisional amounts identified during the measurement period shall now be recognized in the reporting period in which the adjustment amounts are determined. This ASU is effective for fiscal years beginning after December 15, 2015, including interim periods within those fiscal years, with early adoption permitted. We do not expect this adoption to have a significant impact on our consolidated financial statements.
In August 2015, the FASB issued Accounting Standards Update (ASU) 2015-15, “Interest-Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line of Credit Arrangements”, given that the authoritative guidance within ASU 2015-03 for debt issuance costs does not address presentation or subsequent measurement of debt issuance costs related to line-of-credit arrangements. The SEC staff would not object to an entity deferring and presenting debt issuance costs as an asset and subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. We do not expect this adoption to have a significant impact on our consolidated financial statements.
In July 2015, the FASB approved the deferral of the effective date of the new revenue recognition standard ASU 2014-09 by one year. This ASU is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years.
In July 2015, the FASB issued Accounting Standards Update (ASU) 2015-11, “Inventory (Topic 330): “Simplifying the Measurement of Inventory,” which amends the guidelines for the measurement of inventory. The amendments in this ASU do not apply to inventory that is measured using last-in, first-out (LIFO) or the retail inventory method, but do apply to all other inventory, which includes inventory that is measured using first-in, first-out (FIFO) or average cost. Under the amendments, an entity should measure inventory at the lower of cost and net realizable value. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and
transportation. This ASU is effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The amendments in this ASU should be applied prospectively with earlier application permitted. We will further study the implications of this statement in order to evaluate the expected impact on the consolidated financial statements.
In April 2015, the FASB issued ASU 2015-03 - “Interest - Imputation of Interest (Subtopic 2015-03): Simplifying the Presentation of Debt Issuance Costs” which requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of the related debt liability instead of being presented as an asset, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by ASU 2015-03. This ASU is effective for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years and is to be implemented retrospectively. Early adoption is permitted for financial statements that have not been previously issued. Adoption of the new guidance will only affect the presentation of our consolidated balance sheets and will not have a significant impact on our consolidated financial statements.
In February 2015, the FASB issued ASU 2015-02, “Consolidation (Topic 810): Amendments to the Consolidation Analysis.” The amendments in this update affect reporting entities that are required to evaluate whether they should consolidate certain legal entities. All legal entities are subject to reevaluation under the revised consolidation model. Specifically, the amendments (i) modify the evaluation of whether limited partnerships and similar legal entities are variable interest entities (VIEs) or voting interest entities, (ii) eliminate the presumption that a general partner should consolidate a limited partnership, (iii) affect the consolidation analysis of reporting entities that are involved with VIEs, particularly those that have fee arrangements and related party relationships, and (iv) provide a scope exception from consolidation guidance for reporting entities with interests in certain legal entities. This ASU is effective for public business entities for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2015. Early adoption is permitted, including
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 2. | Summary of Significant Accounting Policies, continued |
adoption in an interim period. We do not expect this adoption to have a significant impact on our consolidated financial statements.
x. Corrections
During 2013, we recorded a $2.8 million immaterial out-of-period non-cash physical inventory adjustment in our Animal Hospital business segment which resulted in a debit to inventory and a credit to direct costs.
We have analyzed the impact of this item and concluded that the adjustment would not be material to any individual period, taking into account the requirements of the Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin No. 108, Considering the Effects of Prior Year Misstatements in the Current Year Financial Statements (“SAB 108”). In accordance with the relevant guidance, we evaluated the materiality of errors from a quantitative and qualitative perspective. Based on such evaluation, we concluded that correcting the errors would not have had a material impact on any individual prior period presented in the 2015 Form 10-K nor would it have affected the trend of financial results. As provided by SAB 108, the error correction did not require the restatement of the consolidated financial statements for prior periods.
y. Reclassifications
Certain reclassifications have been made herein to prior year balances to conform to the 2015 financial statement presentation.
|
| |
| 3. | Related Party Transactions |
a. Related Party Vendors
Frank Reddick joined our company as a director in February 2002 and is a partner in the law firm of Akin Gump Strauss Hauer & Feld, LLP (“Akin”). Akin provided legal services to us during 2015, 2014 and 2013. The amount paid by our company to Akin for these legal services was approximately $1.5 million, $0.9 million and $0.4 million in 2015, 2014 and 2013, respectively.
b. Transactions with VetSource
In 2006, we entered into a pharmacy distribution agreement with Strategic Pharmaceutical Solutions, Inc. (“VetSource”) a start-up pharmacy distribution company. Pursuant to the terms of this agreement we are entitled to one representative on the VetSource Board of Directors. Under the agreement we promote the use of VetSource as the preferred provider of pharmaceutical products to VCA animal hospitals. The agreement has a five-year term and will renew for one year terms unless either party provides written notice of termination to the other party at least 120 days prior to expiration of the then current term. The amount paid by our company to VetSource for pharmaceutical products was $6.0 million, $5.7 million and $13.0 million in 2015, 2014 and 2013, respectively. We own 39.3% of the outstanding preferred stock of VetSource.
On October 24, 2013, we entered into a $1.2 million revolving credit agreement with VetSource in the form of a promissory note. Our commitment under the revolving credit agreement is to loan up to $0.5 million, equitable to our 39.3% pro rata share in VetSource. As of December 31, 2015, we have no outstanding loan to VetSource.
c. Transactions with Vetstreet
On December 31, 2015, our company sold substantially all of the assets of Vetstreet Inc., formerly known as MediMedia Animal Health LLC ("Vetstreet") to a subsidiary of Henry Schein, Inc.. Concurrent with the sale of Vetstreet, we purchased a 19.9% interest in the continuing Vetstreet business for $9.6 million, which will be accounted for under the equity method of accounting for investments in common stock.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
Our acquisition strategy includes the acquisition of animal hospitals, animal hospital chains, laboratories or related businesses. In accordance with that strategy, we acquired the following:
|
| | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Animal Hospitals: | | | | | | |
| Acquisitions | | 55 |
| | 47 |
| | 20 |
|
| Acquisitions, merged | | (7 | ) | | (4 | ) | | (2 | ) |
| Sold, closed or merged | | (9 | ) | | (9 | ) | | (18 | ) |
| Net increase | | 39 |
| | 34 |
| | — |
|
| Laboratories: | | | | | | |
| Acquisitions | | 1 |
| | — |
| | 1 |
|
| Acquisitions, merged | | (1 | ) | | — |
| | — |
|
| New facilities | | 1 |
| | 3 |
| | — |
|
| Net increase | | 1 |
| | 3 |
| | 1 |
|
Animal Hospital and Laboratory Acquisitions
The purchase price allocations for some of the 2015 animal hospital acquisitions included in the table below are preliminary; however, adjustments, if any, are not expected to be material. The measurement periods for purchase price allocations do not exceed 12 months from the acquisition date. The following table summarizes the aggregate consideration for our acquired independent animal hospitals and the Abaxis Veterinary Reference Laboratory ("AVRL") and the allocation of the purchase price (in thousands):
|
| | | | | | | | | | | | |
| | | For Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Consideration: | | | | | | |
| Cash, net of cash acquired | | $ | 147,121 |
| | $ | 122,803 |
| | $ | 52,688 |
|
| Assumed debt | | 16,885 |
| | 7,426 |
| | 2,360 |
|
| Holdbacks | | 5,040 |
| | 3,000 |
| | 1,092 |
|
| Earn-outs | | 1,671 |
| | 2,037 |
| | 1,285 |
|
| Fair value of total consideration transferred | | $ | 170,717 |
| | $ | 135,266 |
| | $ | 57,425 |
|
| Allocation of the Purchase Price: | | | | | | |
| Tangible assets | | $ | 11,482 |
| | $ | 5,902 |
| | $ | 14,779 |
|
Identifiable intangible assets (1) | | 41,216 |
| | 22,964 |
| | 15,001 |
|
Goodwill (2) | | 124,198 |
| | 110,234 |
| | 45,665 |
|
| Notes payable and other liabilities assumed | | (2,206 | ) | | (115 | ) | | (11,084 | ) |
| Fair value of assets acquired and liabilities assumed | | $ | 174,690 |
| | $ | 138,985 |
| | $ | 64,361 |
|
| Noncontrolling interest | | (2,675 | ) | | (1,705 | ) | | (6,936 | ) |
| Notes receivable from shareholder for formation of noncontrolling interest | | (1,298 | ) | | �� |
| | — |
|
| Fair value of pre-existing investment | | — |
| | (2,014 | ) | | — |
|
| Total | | $ | 170,717 |
| | $ | 135,266 |
| | $ | 57,425 |
|
| |
(1) | Identifiable intangible assets include customer relationships, trademarks and covenants-not-to-compete. The weighted-average amortization period for the total identifiable intangible assets is approximately eleven years. The weighted-average amortization period for customer relationships, trademarks and covenants-not-to-compete is approximately twelve, nine and five years, respectively. |
| |
(2) | We expect that $101.6 million, $67.2 million and $15.0 million of the goodwill recorded in 2015, 2014 and 2013, respectively, will be fully deductible for income tax purposes. |
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 4. | Acquisitions, continued |
Included in the table above is Antech Diagnostics, Inc.'s March 31, 2015 acquisition of AVRL for total consideration of $21.0 million. The purchase price allocation has been finalized during the quarter ended December 31, 2015.
2014 Camp Bow Wow
On August 15, 2014, we acquired 100% of D.O.G. Enterprises, LLC for $17.0 million in cash and contingent consideration of up to $3.0 million that may be earned over the next three years. Camp Bow Wow primarily franchises a premier provider of pet services including dog day care, overnight boarding, grooming and other ancillary services at specially designed pet care facilities, principally under the service mark Camp Bow Wow®. As of December 31, 2015, there were 126 Camp Bow Wow® franchise locations operating in 34 states and one Canadian province.
The following table summarizes the total purchase price and the final allocation of the purchase price (in thousands):
|
| | | |
| Consideration: | |
| Cash, net of cash acquired | $ | 15,174 |
|
| Assumed debt | 323 |
|
| Holdbacks | 1,500 |
|
| Earn-outs | 760 |
|
| Fair value of total consideration transferred | $ | 17,757 |
|
| | |
| Allocation of the Purchase Price: | |
| Tangible assets | $ | 637 |
|
Identifiable intangible assets (1) | 13,420 |
|
Goodwill (2) | 4,219 |
|
| Other liabilities assumed | (519 | ) |
| Total | $ | 17,757 |
|
| |
(1) | Identifiable intangible assets primarily include franchise rights, trademarks, covenants-not-to-compete and existing technology. The weighted-average amortization period for the total identifiable intangible assets is approximately ten years. The weighted-average amortization periods for franchise rights, covenants and existing technology is approximately ten, three and four years, respectively. The trademarks have an indefinite life and will be assessed annually for impairment. |
| |
(2) | As of December 31, 2015, we expect that the full amount of goodwill recorded for this acquisition will be deductible for income tax purposes. |
Pro Forma Information (unaudited)
The following unaudited pro forma financial information for the years ended December 31, 2015 and 2014 presents, (i) the actual results of operations of our 2015 acquisitions and (ii) the combined results of operations for our company and our 2015 acquisitions as if those acquisitions had been completed on January 1, 2014, the first day of the comparable prior annual reporting period. The pro forma financial information considers principally (i) our company’s financial results, (ii) the unaudited historical financial results of our acquisitions, and (iii) select pro forma adjustments to the historical financial results of our acquisitions. Such pro forma adjustments represent principally estimates of (i) the impact of the hypothetical amortization of acquired intangible assets, (ii) the recognition of fair value adjustments relating to tangible assets, (iii) adjustments reflecting the new capital structure, including additional financing or repayments of debt as part of the acquisitions and (iv) the tax effects of the acquisitions and related adjustments as if those acquisitions had been completed on January 1, 2014. The unaudited pro forma financial information is not necessarily indicative of what our consolidated results of operations would have been had we completed the acquisition at the beginning of the comparable prior annual reporting period.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 4. | Acquisitions, continued |
In addition, the unaudited pro forma financial information does not attempt to project the future results of operations of our company:
|
| | | | | | | | |
| | | Revenue | | Net Income |
| | | (Unaudited) |
| (In thousands): | | | | |
| Actual from acquisition date to December 31, 2015 | | $ | 57,915 |
| | $ | 6,663 |
|
2015 supplemental pro forma from January 1, 2015 to December 31, 2015 (1) | | $ | 2,215,252 |
| | $ | 217,421 |
|
2014 supplemental pro forma from January 1, 2014 to December 31, 2014 (1) | | $ | 2,124,447 |
| | $ | 150,149 |
|
| |
(1) | 2015 supplemental pro forma net income was adjusted to exclude $0.1 million of acquisition-related costs incurred in 2015. 2014 supplemental pro forma net income was adjusted to include these charges. |
The following table presents the changes in the carrying amount of our goodwill for 2015 and 2014 (in thousands):
|
| | | | | | | | | | | | | | | | |
| | | Animal Hospital | | Laboratory | | All Other | | Total |
| Balance as of December 31, 2013 | | | | | | | | |
| Goodwill | | $ | 1,206,213 |
| | $ | 97,556 |
| | $ | 138,276 |
| | $ | 1,442,045 |
|
| Accumulated impairment losses | | — |
| | — |
| | (120,811 | ) | | (120,811 | ) |
| Subtotal | | 1,206,213 |
| | 97,556 |
| | 17,465 |
| | 1,321,234 |
|
| Goodwill acquired | | 110,207 |
| | 27 |
| | 4,701 |
| | 114,935 |
|
| Goodwill impairment | | — |
| | — |
| | (9,246 | ) | | (9,246 | ) |
| Foreign translation adjustment | | (9,722 | ) | | (48 | ) | | — |
| | (9,770 | ) |
Other (1) | | (1,140 | ) | | — |
| | (152 | ) | | (1,292 | ) |
| Balance as of December 31, 2014 | | | | | | | | |
| Goodwill | | 1,305,558 |
| | 97,535 |
| | 142,825 |
| | 1,545,918 |
|
| Accumulated impairment losses | | — |
| | — |
| | (130,057 | ) | | (130,057 | ) |
| Subtotal | | 1,305,558 |
| | 97,535 |
| | 12,768 |
| | 1,415,861 |
|
| Goodwill acquired | | 120,374 |
| | 3,824 |
| | 1,507 |
| | 125,705 |
|
| Goodwill impairment | | — |
| | — |
| | — |
| | — |
|
| Foreign translation adjustment | | (22,441 | ) | | (86 | ) | | — |
| | (22,527 | ) |
Other (1) | | (1,385 | ) | | (4 | ) | | — |
| | (1,389 | ) |
| Balance as of December 31, 2015 | |
|
| |
|
| |
|
| |
|
|
| Goodwill | | 1,402,106 |
| | 101,269 |
| | 144,332 |
| | 1,647,707 |
|
| Accumulated impairment losses | | — |
| | — |
| | (130,057 | ) | | (130,057 | ) |
| Subtotal | | $ | 1,402,106 |
| | $ | 101,269 |
| | $ | 14,275 |
| | $ | 1,517,650 |
|
| |
(1) | In 2015 and 2014, "Other" primarily includes write-offs related to the sale of animal hospitals partially offset by measurement period adjustments. Additionally, 2014 includes an immaterial write-off related to the sale of a Camp Bow Wow camp. |
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
Goodwill Impairment Charges
2014 Charge
Impairment testing for goodwill is performed at least annually at the reporting unit level. A reporting unit is an operating segment or one level below an operating segment (also known as a component). We perform our annual impairment test as of October 31st. Goodwill is also tested for impairment between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying value. We have the option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its net book value. If we elect to not use this option, or we determine, using the qualitative method, that it is more likely than not that the fair value of a reporting unit is less than its net book value, we then perform the more detailed two-step impairment test.
Step one compares the fair value of the reporting unit to its carrying value including goodwill. If the carrying value exceeds the fair value, there is a potential impairment and step two must be performed. Step two compares the carrying value of the reporting unit's goodwill to its implied fair value (i.e., the fair value of the reporting unit less the fair value of the unit's assets and liabilities, including identifiable intangible assets). If the carrying value of goodwill exceeds its implied fair value, the excess is recorded as an impairment.
We calculate the implied fair value of the Vetstreet reporting unit utilizing the income approach. The income approach is based on a discounted cash flow analysis and calculates the fair value of the reporting unit by estimating the after-tax cash flows attributable to the reporting unit and then discounting the after-tax cash flows to a present value, using a weighted average cost of capital ("WACC"). The WACC utilized in our analysis using the income approach was 14.0%. The WACC is an estimate of the overall after-tax rate of return required for equity and debt holders of a business enterprise. The reporting unit's cost of equity and debt was developed based on data and factors relevant to the economy, the industry and the reporting unit. The cost of equity was estimated using the capital asset pricing model ("CAPM"). The CAPM uses a risk-free rate of return and an appropriate market risk premium for equity investments and the specific risks of the investment. The analysis also included comparisons to a group of guideline companies engaged in the same or similar businesses. The cost of debt was estimated using the current after-tax average borrowing cost that a market participant would expect to pay to obtain its debt financing assuming a target capital structure.
The fair value estimates used in the goodwill impairment analysis for the Vetstreet reporting unit required significant judgment. The company's fair value estimates for purposes of determining the goodwill impairment charge are considered Level 3 fair value measurements.
We based our fair value estimates on assumptions that we believe to be reasonable but that are inherently uncertain, including estimates of future revenues and operating margins and assumptions about the overall economic climate and the competitive environment for our business.
With respect to our Vetstreet reporting unit, during 2013 we established a Fiscal 2014 Operating and Financial Performance - Turnaround Plan. The Plan anticipated the launch of numerous product enhancements designed to restore our competitive advantage in the marketplace. Although certain of these product enhancements were delivered in a timely fashion, others were not. In addition, increasing competition created the need for additional product enhancements to those already planned. Given the less than anticipated positive impact of new product offerings combined with the negative impact of increased competition, we determined that a triggering event had occurred with respect to goodwill and long-lived assets of our Vetstreet reporting unit. Accordingly, we established revised multi-year projections and performed an interim test of Vetstreet’s recorded goodwill and long-lived assets for impairment in the third quarter of 2014, prior to our annual October 31, 2014 test. As a result of our interim impairment review, we determined that the carrying value of the Vetstreet reporting unit including goodwill exceeded the fair value of the reporting unit, requiring us to perform step two of the goodwill impairment test to measure the amount of impairment loss, if any.
In performing step two of the goodwill impairment test, we compared the implied fair value of the reporting unit's goodwill to its carrying value. As the carrying value of Vetstreet's goodwill exceeded its implied fair value, we recognized a non-cash, goodwill impairment charge of $9.2 million, representing the entire balance of Vetstreet's goodwill. The impairment
charge was recognized during the third quarter ended September 30, 2014. This charge had no impact on our cash flows or our compliance with debt covenants.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
As of October 31, 2014, we evaluated our goodwill for impairment using the qualitative method. Based on this analysis, we determined that it was more likely than not that the fair values of each of our reporting units were greater than their net carrying values at that date. As such, we concluded that goodwill was not impaired for any of our reporting units. As of December 31, 2014, no events or changes in circumstances occurred that would have triggered the need for an additional impairment review of goodwill.
|
| |
| 6. | Other Accrued Liabilities |
Other accrued liabilities consisted of the following (in thousands):
|
| | | | | | | | |
| | | As of December 31, |
| | | 2015 | | 2014 |
| Deferred revenue | | $ | 14,647 |
| | $ | 14,304 |
|
| Accrued health insurance | | 4,952 |
| | 5,194 |
|
| Deferred rent | | 4,791 |
| | 4,535 |
|
| Accrued other insurance | | 5,013 |
| | 4,381 |
|
Miscellaneous accrued taxes (1) | | 3,317 |
| | 3,025 |
|
| Accrued accounting and legal fees | | 2,697 |
| | 2,900 |
|
| Accrued workers' compensation | | 3,212 |
| | 2,781 |
|
| Holdbacks and earn-outs | | 9,959 |
| | 7,878 |
|
| Customer deposits | | 2,971 |
| | 2,229 |
|
| Accrued consulting fees | | — |
| | 3,172 |
|
| Accrued lease payments | | 1,536 |
| | 1,657 |
|
| Other | | 17,733 |
| | 15,163 |
|
| | | $ | 70,828 |
| | $ | 67,219 |
|
____________________________
(1) Includes property, sales, and use taxes.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
Long-term obligations consisted of the following at December 31, 2015 and 2014 (in thousands):
|
| | | | | | | | | | |
| | | | | 2015 | | 2014 |
| Senior term notes | | Notes payable, maturing in 2019, secured by assets, variable interest rate (1.92% and 1.67% at 2015 and 2014, respectively) | | 585,000 |
| | 600,000 |
|
| Revolving credit | | Revolving line of credit, maturing in 2019, secured by assets, variable interest rate (1.92% at 2015 and 1.67% at 2014) | | 232,000 |
| | 135,000 |
|
| Secured seller notes | | Notes payable matures in 2016, secured by assets and stock of certain subsidiaries, with interest rate of 10.0% | | 230 |
| | 230 |
|
| | | Total debt obligations | | 817,230 |
| | 735,230 |
|
| | | Capital lease obligations and other debt | | 55,244 |
| | 59,538 |
|
| | | | | 872,474 |
| | 794,768 |
|
| | | Less — current portion | | (33,623 | ) | | (19,356 | ) |
| | | | | $ | 838,851 |
| | $ | 775,412 |
|
The annual aggregate scheduled maturities of our long-term obligations for the five years subsequent to December 31, 2015 are presented below (in thousands):
|
| | | | | | | | | | | | |
| | | Debt Obligations | | Capital Lease Obligations | | Total |
| 2016 | | 30,230 |
| | 3,393 |
| | 33,623 |
|
| 2017 | | 37,500 |
| | 3,303 |
| | 40,803 |
|
| 2018 | | 52,500 |
| | 3,587 |
| | 56,087 |
|
| 2019 | | 697,000 |
| | 3,682 |
| | 700,682 |
|
| 2020 | | — |
| | 3,188 |
| | 3,188 |
|
| Thereafter | | — |
| | 38,091 |
| | 38,091 |
|
| Total | | $ | 817,230 |
| | $ | 55,244 |
| | $ | 872,474 |
|
Senior Credit Facility
In August 2010, we entered into a senior credit facility with various lenders for $600 million of senior secured credit facilities with Bank of America, N.A. as the syndication agent and Wells Fargo Bank, N.A. as the administrative agent, collateral agent, issuing bank and swing line lender. At the time of entering into the senior credit facility, it included $500 million of senior term notes and $100 million revolving credit facility. In connection with this transaction, we paid financing costs in the amount of $9.1 million, of which $2.1 million, or $1.3 million after tax were expensed as part of net income, the remainder was capitalized as deferred financing costs.
In August 2011, we amended and restated our existing senior credit facility to allow for additional senior term notes in the amount of $100 million and an additional $25 million aggregate principal amount of revolving commitments. Bank of
America, N.A. and JP Morgan Chase Bank, N.A. were co-syndication agents for the amended senior credit facility, while Wells Fargo, N.A. remained the administrative agent, collateral agent, issuing bank and swing line lender. The amended senior credit facility called for $581.3 million in senior term notes and a $125 million revolving credit facility. The funds borrowed from the additional senior term notes were used to repay in full, amounts borrowed in connection with the acquisition of Vetstreet on August 9, 2011. In connection with the amendment we incurred $2.9 million in financing costs, of which approximately $865,000 were expensed as part of net income and approximately $2.0 million were capitalized as deferred financing costs. In addition, we expensed $1.1 million of previously capitalized deferred financing costs associated with lenders who exited the syndicate on the amendment date or those that were determined to be extinguished.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 7. | Long-Term Obligations, continued |
On January 25, 2012, we executed an amendment (the "First Amendment") to our Amended and Restated Credit and Guaranty Agreement entered into as of August 16, 2011 (our "Senior Credit Facility"). On January 24, 2012, we issued new term loans in the aggregate principal amount of $50.0 million. The First Amendment replenished the aggregate principal amount of uncommitted incremental facilities by $50.0 million, permitting us to request up to an aggregate principal amount of $100.0 million in uncommitted incremental facilities. The funds borrowed from the Incremental Facility were used to fully repay amounts borrowed to fund an additional investment in AVC on February 1, 2012. In connection with the First Amendment we incurred $122,000 in financing costs, of which approximately $47,000 were expensed as part of net income and $75,000 were capitalized as deferred financing costs.
On August 27, 2014, we entered into a new senior credit facility with various lenders for $1.4 billion of senior secured credit facilities with Bank of America, N.A., as the administrative agent, swingline lender and Letter of Credit issuer, and JPMorgan Chase Bank, N.A. and Suntrust Bank as co-syndication agents. This senior credit facility replaced our previous senior credit facility which provided for $534 million of term notes and a $125 million revolving credit facility. The new senior credit facility provided for $600 million of senior secured term notes and an $800 million senior secured revolving facility, which may be used to borrow, on a same-day notice under a swing line, the lesser of $25 million and the aggregate unused amount of the revolving credit facility then in effect. In addition to refinancing all outstanding amounts under our previous credit agreement, borrowings under our new senior credit facility may be used for general corporate purchases, including permitted share repurchases.
In connection with the new senior credit facility, we incurred $8.0 million in financing costs, of which approximately $6.5 million were capitalized as deferred financing costs and $1.5 million were expensed as part of net income. In addition, we expensed $0.2 million of previously capitalized deferred financing costs associated with lenders under our previous senior credit facility who are not lenders under our new senior credit facility.
As of December 31, 2015, we have borrowed $232.0 million from our revolving credit facility to fund our acquisition pipeline and for repurchases under our existing $400 million share repurchase authorization.
Interest Rate. In general, borrowings under the senior credit facility (including swing line borrowings) bear interest, at our option, on either:
| |
| • | the base rate (as defined below) plus the applicable margin of 0.50% (Pricing Tier 4, see table below) per annum; or |
| |
| • | the Eurodollar rate (as defined below), plus a margin of 1.50% (Pricing Tier 4, see table below) per annum |
Each of the aforementioned margins remain applicable until the date of delivery of the compliance certificate and the financial statements, for the period ended December 31, 2015, at which time the applicable margin will be determined by reference to the leverage ratio in effect from time to time as set forth in the following table:
|
| | | | | | | | | | | |
| Pricing Tier | | Consolidated Leverage Ratio | | Applicable Margin for Eurodollar Loans/Letter of Credit Fees | | Applicable Margin for Base Rate Loans | | Commitment Fee |
| 1 | | ≥ 4.00:1.00 | | 2.25 | % | | 1.25 | % | | 0.45 | % |
| 2 | | < 4.00:1.00 and ≥ 3.25:1.00 | | 2.00 | % | | 1.00 | % | | 0.40 | % |
| 3 | | < 3.25:1.00 and ≥ 2.50:1.00 | | 1.75 | % | | 0.75 | % | | 0.35 | % |
| 4 | | < 2.50:1.00 and ≥ 1.75:1.00 | | 1.50 | % | | 0.50 | % | | 0.30 | % |
| 5 | | < 1.75:1.00 and ≥ 1.00:1.00 | | 1.25 | % | | 0.25 | % | | 0.25 | % |
| 6 | | < 1.00:1.00 | | 1.00 | % | | — | % | | 0.25 | % |
The base rate for the senior term notes is a rate per annum equal to the highest of the (a) Federal Funds Rate plus 0.5%, (b) Bank of America, N.A.'s ("Bank of America") prime rate in effect on such day, and (c) the Eurodollar rate plus 1.0%. The Eurodollar rate is defined as the rate per annum equal to the London Interbank Offered Rate ("LIBOR"), or a comparable or successor rate which is approved by Bank of America.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 7. | Long-Term Obligations, continued |
Maturity and Principal Payments. The senior term notes mature on August 27, 2019. Principal payments on the senior term notes of $7.5 million are due each calendar quarter from March 31, 2016 to and including June 30, 2017, $11.3 million are due each calendar quarter from September 30, 2017 to and including June 30, 2018 and $15.0 million are due each calendar quarter thereafter with a final payment of the outstanding principal balance due upon maturity.
The revolving credit facility has a per annum commitment fee determined by reference to the Leverage Ratio in effect from time to time as set forth in the table above and is applied to the unused portion of the commitment. The revolving credit facility matures on August 27, 2019. Principal payments on the revolving credit facility are made at our discretion with the entire unpaid amount due at maturity. At December 31, 2015, we had borrowings of $232.0 million under our revolving credit facility.
The following table sets forth the scheduled principal payments for the senior credit facility (in thousands):
|
| | | | | | | | | | | | | | | | |
| | | 2016 | | 2017 | | 2018 | | 2019 |
| Senior term notes | | $ | 30,000 |
| | $ | 37,500 |
| | $ | 52,500 |
| | $ | 465,000 |
|
| Revolving loans | | — |
| | — |
| | — |
| | 232,000 |
|
| | | $ | 30,000 |
| | $ | 37,500 |
| | $ | 52,500 |
| | $ | 697,000 |
|
Guarantees and Security. We and each of our wholly-owned domestic subsidiaries guarantee the outstanding indebtedness under the senior credit facility. Any borrowings, along with the guarantees of the domestic subsidiaries, are further secured by a pledge of substantially all of our consolidated assets, including 65% of the voting equity and 100% of the non-voting equity interest in each of our foreign subsidiaries.
Debt Covenants. The senior credit facility contains certain financial covenants pertaining to interest coverage and leverage ratios. In addition, the senior credit facility has restrictions pertaining to the payment of cash dividends on all classes of stock. At December 31, 2015, we had a interest coverage ratio of 20.57 to 1.00, which was in compliance with the required ratio of no less than 3.00 to 1.00, and a leverage ratio of 2.07 to 1.00, which was in compliance with the required ratio of no more than 4.25 to 1.00.
Current fair value accounting guidance includes a hierarchy that is intended to increase consistency and comparability in fair value measurements and related disclosures. The fair value hierarchy is based on inputs to valuation techniques that are used to measure fair value that are either observable or unobservable. Observable inputs reflect assumptions market participants would use in pricing an asset or liability based on market data obtained from independent sources while unobservable inputs reflect a reporting entity’s pricing based upon their own market assumptions. The current guidance establishes a three-tiered fair value hierarchy which prioritizes the inputs used in measuring fair value as follows:
| |
| • | Level 1. Observable inputs such as quoted prices in active markets; |
| |
| • | Level 2. Inputs, other than quoted prices, that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active; and |
| |
| • | Level 3. Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
Non-Recurring Financial Measurements
Non-financial assets such as property, plant and equipment, land, goodwill and intangible assets are subject to non-recurring fair value measurements if they are deemed to be impaired. The impairment models used for nonfinancial assets depend on the type of asset and are accounted for in accordance with FASB’s guidance on fair value measurement.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
During the quarter ended September 30, 2014, the entire balance of $9.2 million of our Vetstreet goodwill was written off in an impairment charge which was included in earnings in the period. Additionally, during the quarter ended September 30, 2014, our Vetstreet long-lived assets were written down to their estimated fair value resulting in an impairment charge of $17.8 million, which was included in earnings in the period. Our Vetstreet long-lived assets balance as of December 31, 2014 was $4.8 million. Both the implied fair value of goodwill and the estimated fair value of long-lived assets were calculated using Level 3 inputs.
Fair Value of Financial Instruments
The FASB accounting guidance requires disclosure of fair value information about financial instruments, whether or not recognized in the accompanying consolidated balance sheets. Fair value as defined by the guidance is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value estimates of financial instruments are not necessarily indicative of the amounts we might pay or receive in actual market transactions. The use of different market assumptions and/or estimation methodologies may have a material effect on the estimated fair value amounts.
Cash and Cash Equivalents. These balances include cash and cash equivalents with maturities of less than three months. The carrying amount approximates fair value due to the short-term maturities of these instruments.
Receivables, Less Allowance for Doubtful Accounts, Accounts Payable and Certain Other Accrued Liabilities. Due to their short-term nature, fair value approximates carrying value.
Long-Term Debt. The fair value of debt at December 31, 2015 and December 31, 2014 is based upon the ask price quoted from an external source, which is considered a Level 2 input.
The following table reflects the carrying value and fair value of our variable-rate long-term debt (in thousands):
|
| | | | | | | | | | | | | | | | |
| | | As of December 31, |
| | | 2015 | | 2014 |
| | | Carrying Value | | Fair Value | | Carrying Value | | Fair Value |
| Variable-rate long-term debt | | $ | 817,000 |
| | $ | 817,000 |
| | $ | 735,000 |
| | $ | 735,000 |
|
|
| |
| 9. | Dividends and Share Repurchase Program |
Dividends
We have not paid cash dividends on our common stock and we do not anticipate paying cash dividends in the foreseeable future. In addition, our New Senior Credit Facility places limitations on our ability to pay cash dividends or make other distributions in respect of our common stock. Specifically, our New Senior Credit Facility dated August 27, 2014 prohibits, at various intervals during the term of the New Senior Credit Facility, the payment of cash dividends if specified leverage ratios are exceeded and liquidity and net income thresholds fail to be met after giving effect to the payment of the proposed cash dividends. Any future determination as to the payment of dividends will depend on our results of operations, financial condition, capital requirements and other factors deemed relevant by our Board of Directors, including the General Corporation Law of the State of Delaware, which provides that dividends are only payable out of surplus or current net profits.
Share Repurchase Program
In April 2013, our Board of Directors authorized a share repurchase for up to $125 million of our common shares, which was completed in August 2014. In August 2014, our Board of Directors authorized the continuance of that share repurchase program, authorizing us to repurchase up to an additional $400 million of our common shares. These repurchases may be made from time to time through various methods, including open market transactions, block trades, accelerated share repurchases, privately negotiated transactions or otherwise and may be effected through Rule 10b5-1 and Rule 10b-18 plans. The timing and number of shares repurchased will depend on a variety of factors, including price, capital availability, legal requirements and economic and market conditions. The company is not obligated to purchase any shares under the repurchase program, and
|
| |
| 9. | Dividends and Share Repurchase Program, continued |
repurchases may be suspended or discontinued at any time without prior notice. The repurchases have been and will continue to be funded by existing cash balances and by our revolving credit facility.
|
| |
| 10. | Share-Based Compensation |
Stock Incentive Plans
At December 31, 2015, there were stock options, non-vested shares and restricted stock units outstanding under our existing stock incentive plans. At December 31, 2015, we maintained two plans: the 2006 Equity Incentive Plan (“2006 Plan”) and the 2015 Equity Incentive Plan ("2015 Plan"). Pursuant to the 2015 Plan, new options and other stock awards may only be granted under the 2015 Plan. In addition, any shares that are forfeited, cancelled, expired or settled in cash under the 2006 Plan will not be available for issuance under the 2006 Plan nor the 2015 Plan. The number of shares of common stock remaining available for future issuance under the 2015 Plan may increase by any shares of common stock underlying prior outstanding options that expire, are forfeited, canceled or terminate for any reason without having been exercised in full. The number of shares available for issuance at December 31, 2015 was 3,120,750. Outstanding options, non-vested shares and restricted stock units granted under our plans typically vest over periods that range from three to six years, and outstanding options typically expire between five and ten years from the date of grant.
Stock Options
A summary of our stock option activity for 2015 and 2014 is as follows (in thousands, except weighted-average exercise price and weighted-average remaining contractual term):
|
| | | | | | | | | | | | | |
| | | Stock Options | | Weighted- Average Exercise Price | | Weighted- Average Remaining Contractual Term (Years) | | Aggregate Intrinsic Value |
| Outstanding at December 31, 2013 | | 1,031 |
| | $ | 17.28 |
| | | | |
| Granted | | — |
| | $ | — |
| | | | |
| Exercised | | (177 | ) | | $ | 16.12 |
| | | | |
| Expired | | — |
| | $ | — |
| | | | |
| Forfeited/Canceled | | (9 | ) | | $ | 15.98 |
| | | | |
| Outstanding at December 31, 2014 | | 845 |
| | $ | 17.54 |
| | 2.7 | | $ | 26,400 |
|
| Granted | | — |
| | $ | — |
| | | | |
| Exercised | | (166 | ) | | $ | 16.13 |
| | | | |
| Expired | | — |
| | $ | — |
| | | | |
| Forfeited/Canceled | | (11 | ) | | $ | 15.98 |
| | | | |
| Outstanding at December 31, 2015 | | 668 |
| | $ | 17.91 |
| | 1.7 | | $ | 24,768 |
|
| Exercisable at December 31, 2015 | | 552 |
| | $ | 17.70 |
| | 1.7 | | $ | 20,601 |
|
| Vested and expected to vest at December 31, 2015 | | 668 |
| | $ | 17.91 |
| | 1.7 | | $ | 24,768 |
|
There were no stock options granted during 2015, 2014 and 2013. The aggregate intrinsic value of our stock options exercised during 2015, 2014 and 2013 was $6.4 million, $4.6 million and $11.4 million, respectively. The actual tax benefit realized on options exercised during 2015, 2014 and 2013 was $2.5 million, $1.8 million and $4.5 million, respectively. The total fair value of options vested during 2015, 2014 and 2013 was $1.8 million, $1.8 million and $1.8 million, respectively.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 10. | Share-Based Compensation, continued |
The following table summarizes information about the options outstanding at December 31, 2015 (in thousands, except per share amounts and the weighted-average remaining contractual life):
|
| | | | | | | | | | |
| Options Outstanding | | Options Exercisable |
| Exercise Price | | Outstanding | | Weighted-Avg. Remaining Contractual Life | | Weighted-Avg. Exercise Price | | Exercisable | | Weighted-Avg. Exercise Price |
| $15.98 - $18.94 | | 667,851 | | 1.7 | | $17.91 | | 552,294 | | $17.70 |
At December 31, 2015, there was $0.4 million of total unrecognized compensation cost related to our stock options. This cost is expected to be recognized over a weighted-average period of 0.6 years.
Calculation of Fair Value
The fair value of our options is estimated on the date of grant using the Black-Scholes option pricing model. We amortize the fair value of our options on a straight-line basis, net of estimated forfeitures, over the requisite service period. There were no options granted during 2015, 2014 and 2013.
We use historical data to estimate pre-vesting option forfeitures. We recognize share-based compensation only for those awards that we expect to vest.
The compensation cost charged against income for stock options was $1.3 million, $1.6 million and $1.8 million for 2015, 2014 and 2013, respectively. The corresponding income tax benefit recognized in the income statement was $0.5 million, $0.6 million and $0.7 million for 2015, 2014 and 2013, respectively.
Non-Vested Shares
Additionally, under the 2006 and 2015 Equity Incentive Plans, we have issued non-vested stock awards in our common stock to certain employees and members of our Board of Directors. The outstanding non-vested stock awards to employees and executives generally vest in three or four equal annual installments starting with the first anniversary of the grant date. The non-vested stock awards to members of our Board of Directors generally vest in equal annual installments over three years from the date of grant. A summary of our non-vested stock activity for 2015 and 2014 is as follows (in thousands, except weighted-average fair value per share):
|
| | | | | | | |
| | | Shares | | Grant Date Weighted- Average Fair Value Per Share |
| Outstanding at December 31, 2013 | | 1,183 |
| | $ | 21.56 |
|
| Granted | | 172 |
| | $ | 40.21 |
|
| Vested | | (512 | ) | | $ | 21.03 |
|
| Forfeited/Canceled | | (21 | ) | | $ | 22.40 |
|
| Outstanding at December 31, 2014 | | 822 |
| | $ | 25.77 |
|
| Granted | | 131 |
| | $ | 53.76 |
|
| Vested | | (484 | ) | | $ | 22.40 |
|
| Forfeited/Canceled | | (52 | ) | | $ | 26.77 |
|
| Outstanding at December 31, 2015 | | 417 |
| | $ | 38.28 |
|
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 10. | Share-Based Compensation, continued |
During 2015, we granted 130,900 shares of non-vested common stock. Of these awards, 125,008 shares were granted to employees and 5,892 shares were granted to our non-employee directors. The awards to employees will vest in equal annual installments over four years. The shares awarded to our non-employee directors will vest in three equal annual installments.
Total compensation expense related to non-vested stock awards was $6.0 million, $7.5 million and $8.8 million in 2015, 2014 and 2013, respectively. The corresponding income tax benefit recognized in the income statement was $2.3 million, $2.9 million and $3.4 million for 2015, 2014 and 2013, respectively. As of December 31, 2015, there was $15.1 million of unrecognized compensation cost related to these non-vested shares that will be recognized over a weighted-average period of 3.0 years.
Restricted Stock Units
Pursuant to the terms of the 2006 and 2015 Equity Incentive Plans, we have also granted performance-based restricted stock units ("RSUs") to our executive officers representing the right to receive one share of common stock. These RSUs will be earned upon achievement of the applicable performance criteria during a three year performance period from the date of grant and in accordance with the specific equity performance award agreement they were issued from. Assuming achievement of the required performance conditions and continued service through each vesting date, these awards will generally vest in equal annual installments over four years from the date of grant.
A summary of our RSU award activities for 2015 and 2014 is as follows (in thousands, except weighted-average fair value per share):
|
| | | | | | | |
| | | Units | | Grant Date Weighted- Average Fair Value Per Unit |
| Outstanding at December 31, 2013 | | 562 |
| | $ | 23.54 |
|
| Granted | | 253 |
| | $ | 39.05 |
|
| Vested/Paid out | | (192 | ) | | $ | 21.04 |
|
Performance Share Units Adjustment (1) | | 62 |
| | $ | 18.94 |
|
| Forfeited/Canceled | | — |
| | $ | — |
|
| Outstanding at December 31, 2014 | | 685 |
| | $ | 29.60 |
|
| Granted | | 250 |
| | $ | 50.37 |
|
| Vested/Paid out | | (261 | ) | | $ | 26.15 |
|
Performance Share Units Adjustment (1) | | 73 |
| | $ | 27.78 |
|
| Forfeited/Canceled | | — |
| | $ | — |
|
| Outstanding at December 31, 2015 | | 747 |
| | $ | 37.91 |
|
____________________________
| |
(1) | The Performance Share Units Adjustment represent the difference of restricted stock units earned above or below the units that were initially granted and what were actually earned at the end of each performance period. |
Share-based compensation expense related to performance-based restricted stock units reflects the estimated probable outcome at each performance period. Upon completion of the performance period, the actual number of restricted stock units earned are determined and are subject to further vesting. Total compensation cost charged against income related to RSU awards was $9.0 million, $8.1 million and $3.5 million for 2015, 2014 and 2013, respectively. The corresponding income tax benefit recognized in the income statement was $3.5 million, $3.2 million and $1.4 million for 2015, 2014 and 2013 , respectively. As of December 31, 2015, there was $14.6 million of total unrecognized compensation cost related to our RSU awards. This cost is expected to be recognized over a weighted-average period of over 3.2 years .
There were 249,665 performance based RSUs granted to our executive officers in 2015. The RSUs earned upon achievement of performance goals are further subject to vesting where an aggregate of 25% of RSUs earned will vest in four equal annual installments starting on the applicable anniversary date.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 11. | Commitments and Contingencies |
a. Leases
We operate many of our animal hospitals from premises that are leased under operating leases with terms, including renewal options, ranging from five to 35 years. Certain leases include fair-value purchase options that can be exercised at our discretion at various times within the lease terms.
The future minimum lease payments on operating leases at December 31, 2015 are as follows (in thousands):
|
| | | |
| | |
| 2016 | $ | 79,332 |
|
| 2017 | 79,004 |
|
| 2018 | 77,470 |
|
| 2019 | 74,608 |
|
| 2020 | 72,097 |
|
| Thereafter | 813,737 |
|
| Total | $ | 1,196,248 |
|
| | |
Rent expense totaled $76.7 million, $69.7 million and $67.6 million in 2015, 2014 and 2013, respectively. Rental income totaled $1.1 million, $1.2 million and $1.0 million in 2015, 2014 and 2013, respectively.
b. Purchase Commitments
Under the terms of certain purchase agreements, we have aggregate commitments to purchase approximately $30.0 million of products and services through 2019.
c. Earn-out Payments
We have contractual arrangements in connection with certain acquisitions, whereby additional cash may be paid to former owners of acquired companies upon fulfillment of specified financial criteria as set forth in the respective agreements. The amount to be paid cannot be determined until the earn-out periods have expired. If the specified financial criteria are satisfied, we will be obligated to pay an additional $5.9 million.
In accordance with business combination accounting guidance, contingent consideration, such as earn-outs, are recognized as part of the consideration transferred on the acquisition date. A liability is initially recorded based upon its acquisition date fair value. The changes in fair value are recognized in earnings where applicable at each reporting period. The fair value is determined using a contractually stated formula using either a multiple of revenue or Earnings Before Interest, Tax, Depreciation and Amortization ("EBITDA"). The formulas used to determine the estimated fair value are level 3 inputs. The changes in fair value were immaterial to our consolidated financial statements taken as a whole. We recorded $3.5 million and $3.2 million in earnout liabilities as of December 31, 2015 and 2014, respectively, which are included in other accrued liabilities in our consolidated balance sheets.
d. Holdbacks
In connection with certain acquisitions, we withheld a portion of the purchase price, or the holdback, as security for indemnification obligations of the sellers under the acquisition agreement. The amounts withheld are typically payable within a 12-month period. The total outstanding holdbacks at December 31, 2015 and 2014 were $6.5 million and $4.7 million, respectively, and are included in other accrued liabilities.
We paid $3.2 million, $0.7 million and $2.4 million in 2015, 2014 and 2013, respectively, to sellers for the unused portion of holdbacks.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 11. | Commitments and Contingencies, continued |
e. Legal Proceedings
On July 16, 2014, two additional former veterinary assistants filed a purported class action lawsuit against us in the Superior Court of the State of California for the County of Los Angeles, titled La Kimba Bradsbery and Cheri Brakensiek vs. Vicar Operating, Inc., et. al. The lawsuit seeks to assert claims on behalf of current and former veterinary assistants, kennel assistants, and client service representatives employed by us in California, and alleges, among other allegations, that we improperly failed to pay regular and overtime wages, improperly failed to provide proper meal and rest periods, improperly failed to pay reporting time pay, improperly failed to reimburse for certain business-related expenses, and engaged in unfair business practices. The lawsuit seeks damages, statutory penalties, and other relief, including attorneys’ fees and costs.
In September 2014, the court issued an order staying the La Kimba Bradsbery lawsuit, which stay remains in place.
If the stay is lifted, we intend to vigorously defend against the Bradsbery action. At this time, we are unable to estimate the reasonably possible loss or range of possible loss, but do not believe losses, if any, would have a material effect on our results of operations or financial position taken as a whole.
On July 12, 2013, an individual who provided courier services with respect to our laboratory clients in California filed a purported class action lawsuit against us in the Superior Court of the State of California for the County of Santa Clara - San Jose Branch, titled Carlos Lopez vs. Logistics Delivery Solutions, LLC, Antech Diagnostics, Inc., et. al. Logistics Delivery Solutions, LLC, a co-defendant in the lawsuit, is a company with which Antech has contracted to provide courier services in California. The lawsuit seeks to assert claims on behalf of individuals who were engaged by Logistics Delivery Solutions, LLC to perform such courier services and alleges, among other allegations, that Logistics Delivery Solutions and Antech Diagnostics improperly classified the plaintiffs as independent contractors, improperly failed to pay overtime wages, and improperly failed to provide proper meal periods. The lawsuit seeks damages, statutory penalties, and other relief, including attorneys' fees and costs. The parties have an agreement in principle to settle the action, on a class-wide basis, for an amount not to exceed $1,250,000. Logistics Delivery Solutions, LLC, has agreed to pay half of the claim. Accordingly, as of December 31, 2015, we have accrued the remaining fifty. The proposed settlement, when and if it becomes effective, would not be an admission of wrongdoing or acceptance of fault by any of the defendants named in the complaint. Antech Diagnostics and Logistics Delivery Solutions have agreed upon the terms of this proposed settlement to eliminate the uncertainties, risk, distraction and expense associated with protracted litigation. The Court granted preliminary approval of the settlement on November 30, 2015. The proposed settlement remains subject to court approval and class notice administration before it will be effective. The Court is scheduled to hear a motion for final approval of the settlement on March 25, 2016.
On May 12, 2014, an individual client who purchased goods and services from one of our animal hospitals filed a purported class action lawsuit against us in the United States District Court for the Northern District of California, titled Tony M. Graham vs. VCA Antech, Inc. and VCA Animal Hospitals, Inc. The lawsuit seeks to assert claims on behalf of the plaintiff and other individuals who purchased similar goods and services from our animal hospitals and alleges, among other allegations, that we improperly charged such individuals for “biohazard waste management” in connection with the services performed. The lawsuit seeks compensatory and punitive damages in unspecified amounts, and other relief, including attorneys' fees and costs. VCA successfully had the venue transferred to the Southern District of California. This case is in an early procedural stage and we intend to vigorously defend this action. At this time, we are unable to estimate the reasonably possible loss or range of possible loss, but do not believe losses, if any, would have a material effect on our results of operations or financial position taken as a whole.
In addition to the lawsuits described above, we are party to ordinary routine legal proceedings and claims incidental to our business, but we are not currently a party to any legal proceeding that we believe would have a material adverse effect on our financial position, results of operations, or cash flows.
f. Other Contingencies
On May 14, 2014, the headquarters of our Medical Technology business in Carlsbad, California was severely damaged by wildfires. There were no injuries to personnel. However, the fire caused severe damage to a substantial portion of the facility. We have worked diligently to satisfy customer requirements and to prevent supply disruptions. We maintain standard
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 11. | Commitments and Contingencies, continued |
insurance coverage for both property damage and business interruption losses. For the year ended December 31, 2014, we recorded approximately $18.9 million in estimated losses in connection with this event, primarily associated with property damage. This amount is included in operating expenses in our consolidated income statements, offset by the related insurance recovery of $20.0 million, resulting in a gain of $1.1 million. For the year ended December 31, 2014 we recorded receivables of $8.0 million from the insurance recoveries. During the year ended December 31, 2015 we recorded a gain on insurance proceeds of $4.5 million.
The provision for income taxes is comprised of the following (in thousands):
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Federal: | | | | | | |
| Current | | $ | 59,792 |
| | $ | 61,688 |
| | $ | 54,915 |
|
| Deferred | | 48,962 |
| | 8,394 |
| | 16,150 |
|
| | | 108,754 |
| | 70,082 |
| | 71,065 |
|
| State: | |
| |
| |
|
| Current | | 13,584 |
| | 12,965 |
| | 11,156 |
|
| Deferred | | 9,184 |
| | 1,013 |
| | 3,017 |
|
| | | 22,768 |
| | 13,978 |
| | 14,173 |
|
| Foreign: | | | | | | |
| Current | | 5,445 |
| | 3,372 |
| | 3,318 |
|
| Deferred | | (1,424 | ) | | (554 | ) | | (1,103 | ) |
| | | 4,021 |
| | 2,818 |
| | 2,215 |
|
| | | $ | 135,543 |
| | $ | 86,878 |
| | $ | 87,453 |
|
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
12. | Income Taxes, continued |
The net deferred income tax assets (liabilities) at December 31, 2015 and 2014 are comprised of the following (in thousands):
|
| | | | | | | | |
| | | December 31, |
| | | 2015 | | 2014 |
| Accounts receivable | | $ | 8,754 |
| | $ | 7,977 |
|
| State taxes | | 5,482 |
| | 2,632 |
|
| Other liabilities and reserves | | 24,947 |
| | 21,878 |
|
| Other assets | | 34,731 |
| | 33,606 |
|
| Inventory | | 1,360 |
| | 1,271 |
|
| Net operating loss carryforwards | | 26,256 |
| | 27,610 |
|
| Share-based compensation | | 5,415 |
| | 6,615 |
|
| Intangible assets | | (201,350 | ) | | (138,214 | ) |
| Property and equipment | | (24,419 | ) | | (22,753 | ) |
| Unrealized loss on investments | | — |
| | (3 | ) |
| Valuation allowance | | (12,654 | ) | | (13,790 | ) |
| Total non-current deferred income tax liabilities | | $ | (131,478 | ) | | $ | (73,171 | ) |
At December 31, 2015, we had Federal net operating loss (“NOL”) carryforwards of approximately $61.9 million, comprised mainly of acquired NOL carryforwards. These NOLs expire at various dates through 2035. The utilization of NOL carryforwards to reduce taxable income is subject to certain statutory limitations. Events that cause such a limitation include, but are not limited to, a cumulative ownership change of more than 50% over a three-year period. We believe that some of our acquisitions caused such a change of ownership and, accordingly, utilization of the NOL carryforwards may be limited in future years. Accordingly, the valuation allowance is principally related to subsidiaries’ NOL carryforwards. We believe that it is more likely than not that the benefit from the remaining net deferred tax assets will be realizable.
On November 20, 2015, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes, requiring all deferred tax assets and liabilities, and any related valuation allowance, to be classified as non-current on the balance sheet. We elected to retrospectively adopt the accounting standard in the fourth quarter of 2015. As a result, total deferred tax assets of $30.3 million were reclassified to partially offset and be presented in a single non-current deferred income tax liability amount.
Taxes on Foreign Income
As of December 31, 2015, unremitted earnings of subsidiaries outside of the United States were approximately $50.4 million on which no United States taxes had been provided. Our intention is to indefinitely reinvest these earnings outside the United States. Since these unremitted earnings have been permanently reinvested, deferred taxes were not provided on these unremitted earnings. The amount of unrecognized deferred taxes totaled $7.6 million with respect to these unremitted earnings.
Our effective tax rate was 39.1%, 39.1% and 38.9% in 2015, 2014 and 2013, respectively.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
12. | Income Taxes, continued |
A reconciliation of the provision for income taxes to the amount computed at the Federal statutory rate is as follows:
|
| | | | | | | | | |
| | | For Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Federal income tax at statutory rate | | 35.0 | % | | 35.0 | % | | 35.0 | % |
| State taxes, net of Federal benefit | | 4.3 |
| | 4.1 |
| | 4.2 |
|
| Goodwill impairment | | 0.3 |
| | 0.2 |
| | — |
|
| Foreign rate differential | | (0.4 | ) | | (0.4 | ) | | (0.4 | ) |
| Miscellaneous | | (0.1 | ) | | 0.2 |
| | 0.1 |
|
| | | 39.1 | % | | 39.1 | % | | 38.9 | % |
We are regularly audited by federal and state tax authorities. The statute is generally open for four years for state audits.
|
| |
| 13. | Noncontrolling Interests |
We own some of our animal hospitals in partnerships with noncontrolling interest holders. We consolidate our partnerships in our consolidated financial statements because our ownership interest in these partnerships is equal to or greater than 50.1% and we control these entities. We record noncontrolling interest in income of subsidiaries equal to our partners’ percentage ownership of the partnerships’ income. We also record changes in the redemption value of our redeemable noncontrolling interests in net income attributable to noncontrolling interests in our consolidated income statements. Noncontrolling interest in income of subsidiaries was $5.0 million, $5.2 million and $5.4 million in 2015, 2014 and 2013, respectively. In addition, we reflect our noncontrolling partners’ cumulative share in the equity of the respective partnerships as either noncontrolling interests in equity, mandatorily redeemable noncontrolling interests in other liabilities, or redeemable noncontrolling interests in temporary equity (mezzanine) in our consolidated balance sheets.
a. Mandatorily Redeemable Noncontrolling Interests
The terms of some of our partnership agreements require us to purchase the partner’s equity in the partnership in the event of the partner’s death. We report these redeemable noncontrolling interests at their estimated redemption value, which approximates fair value and classify them as liabilities due to the certainty of the related event. Estimated redemption value is determined using either a contractually stated formula or a discounted cash flow technique, both of which are used as an approximation of fair value. The discounted cash flow inputs used to determine the redemption value are level 3 and include forecasted growth rates, valuation multiples, and the weighted average cost of capital. We recognize changes in the obligation as an interest cost in the consolidated income statement.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 13. | Noncontrolling Interests, continued |
The following table provides a summary of mandatorily redeemable noncontrolling interests included in other liabilities in our consolidated balance sheets (in thousands): |
| | | | | | | |
| | Income Statement Impact | | Mandatorily Redeemable Noncontrolling Interests |
| Balance as of December 31, 2012 | | | $ | 11,047 |
|
| Noncontrolling interest expense | 2,003 |
| | |
| Redemption value change | 74 |
| | 2,077 |
|
| Formation of noncontrolling interests | | | 80 |
|
| Purchase of noncontrolling interests | | | (1,703 | ) |
| Dissolution of noncontrolling interests | | | (357 | ) |
| Distribution to noncontrolling interests | | | (1,833 | ) |
| Currency translation adjustment | | | 44 |
|
| Balance as of December 31, 2013 | | | $ | 9,355 |
|
| | | | |
| Noncontrolling interest expense | $ | 1,625 |
| | |
| Redemption value change | 312 |
| | 1,937 |
|
| Distribution to noncontrolling interests | | | (1,418 | ) |
| Currency translation adjustment | | | (469 | ) |
| Balance as of December 31, 2014 | | | $ | 9,405 |
|
| | | | |
| Noncontrolling interest expense | $ | 1,447 |
| | |
| Redemption value change | 229 |
| | 1,676 |
|
| Distribution to noncontrolling interests | | | (1,250 | ) |
| Purchase of noncontrolling interests | | | (803 | ) |
| Currency translation adjustment | | | (440 | ) |
| Balance as of December 31, 2015 | | | $ | 8,588 |
|
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 13. | Noncontrolling Interests, continued |
b. Redeemable Noncontrolling Interests
The following table provides a summary of redeemable noncontrolling interests (in thousands):
|
| | | | | | | | |
| | | Income Statement Impact | | Redeemable Noncontrolling Interests |
| Balance as of December 31, 2012 | | | | $ | 6,991 |
|
| Noncontrolling interest | | $ | 1,146 |
| | |
| Redemption value change | | 87 |
| | 1,233 |
|
| Formation of noncontrolling interests | | | | 3,601 |
|
| Distribution to noncontrolling interests | | | | (1,147 | ) |
| Balance as of December 31, 2013 | | | | 10,678 |
|
| | | | | |
| Noncontrolling interest | | $ | 1,226 |
| | |
| Redemption value change | | (16 | ) | | 1,210 |
|
| Formation of noncontrolling interests | | | | 855 |
|
| Purchase of noncontrolling interests | | | | (356 | ) |
| Distribution of noncontrolling interests | | | | (1,310 | ) |
| Balance as of December 31, 2014 | | | | 11,077 |
|
| | | | | |
| Noncontrolling interest | | $ | 958 |
| | |
| Redemption value change | | — |
| | 958 |
|
| Formation of noncontrolling interests | | | | 2,062 |
|
| Purchase of noncontrolling interests | | | | (1,007 | ) |
| Distribution of noncontrolling interests | | | | (1,579 | ) |
| Balance as of December 31, 2015 | | | | $ | 11,511 |
|
In 1992, we established a voluntary retirement plan under Section 401(k) of the Internal Revenue Code. The plan covers all employees within one month of employment with our company and provides the annual matching contributions by us at the discretion of our Board of Directors. Our expense for matching contributions to our voluntary retirement plan approximated $2.1 million, $1.7 million and $1.6 million in 2015, 2014 and 2013, respectively.
Our Animal Hospital and Laboratory business segments are each considered reportable segments in accordance with the FASB's guidance related to Segment Reporting. Our Animal Hospital segment provides veterinary services for companion animals and sells related retail and pharmaceutical products. Our Laboratory segment provides diagnostic laboratory testing services for veterinarians, both associated with our animal hospitals and those independent of us. Our other operating segments included in the “All Other” category in the following tables are our Medical Technology business, which sells digital radiography and ultrasound imaging equipment, related computer hardware, software and ancillary services to the veterinary market, our Vetstreet business, which provides online and printed communications, professional education, marketing solutions to the veterinary community and an ecommerce platform for independent animal hospitals, and our Camp Bow Wow business, which primarily franchises a premier provider of pet services including dog day care, overnight boarding, grooming and other ancillary services at specially designed pet care facilities. These operating segments do not meet the quantitative requirements for reportable segments. Our operating segments are strategic business units that have different services, products and/or functions. The segments are managed separately because each is a distinct and different business venture with unique challenges, risks and rewards. We also operate a corporate office that provides general and administrative support services for our other segments.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 15. | Lines of Business, continued |
The accounting policies of our segments are the same as those described in the summary of significant accounting policies included in Note 2, Summary of Significant Accounting Policies. We evaluate the performance of our segments based on gross profit and operating income. For purposes of reviewing the operating performance of our segments, all intercompany sales and purchases are generally accounted for as if they were transactions with independent third parties at current market prices.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 15. | Lines of Business, continued |
The following is a summary of certain financial data for each of our segments (in thousands): |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Animal Hospital | | Laboratory | | All Other | | Corporate | | Eliminations | | Total |
| 2015 | | | | | | | | | | | | |
| External revenue | | $ | 1,697,870 |
| | $ | 330,828 |
| | $ | 100,843 |
| | $ | — |
| | $ | 4,134 |
| | $ | 2,133,675 |
|
| Intercompany revenue | | — |
| | 63,072 |
| | 26,145 |
| | — |
| | (89,217 | ) | | — |
|
| Total revenue | | 1,697,870 |
| | 393,900 |
| | 126,988 |
| | — |
| | (85,083 | ) | | 2,133,675 |
|
| Direct costs | | 1,433,535 |
| | 192,198 |
| | 80,286 |
| | — |
| | (82,415 | ) | | 1,623,604 |
|
| Gross profit | | 264,335 |
| | 201,702 |
| | 46,702 |
| | — |
| | (2,668 | ) | | 510,071 |
|
| Selling, general and administrative expense | | 44,311 |
| | 38,075 |
| | 33,569 |
| | 68,040 |
| | — |
| | 183,995 |
|
| Operating income (loss) before charges | | 220,024 |
| | 163,627 |
| | 13,133 |
| | (68,040 | ) | | (2,668 | ) | | 326,076 |
|
| Business interruption insurance gain | | — |
| | — |
| | (4,523 | ) | | — |
| | — |
| | (4,523 | ) |
| Net (gain) loss on sale or disposal of assets | | (555 | ) | | 26 |
| | 82 |
| | 1,276 |
| | — |
| | 829 |
|
| Operating income (loss) | | $ | 220,579 |
| | $ | 163,601 |
| | $ | 17,574 |
| | $ | (69,316 | ) | | $ | (2,668 | ) | | $ | 329,770 |
|
| Depreciation and amortization | | $ | 66,129 |
| | $ | 10,726 |
| | $ | 4,640 |
| | $ | 2,349 |
| | $ | (2,156 | ) | | $ | 81,688 |
|
| Property and equipment additions | | $ | 71,155 |
| | $ | 17,418 |
| | $ | 1,849 |
| | $ | 8,319 |
| | $ | (3,507 | ) | | $ | 95,234 |
|
| Total assets at December 31, 2015 | | $ | 2,186,959 |
| | $ | 306,296 |
| | $ | 73,491 |
| | $ | 477,245 |
| | $ | (536,726 | ) | | $ | 2,507,265 |
|
| 2014 | | | | | | | | | | | | |
| External revenue | | $ | 1,514,878 |
| | $ | 303,798 |
| | $ | 96,053 |
| | $ | — |
| | $ | 3,754 |
| | $ | 1,918,483 |
|
| Intercompany revenue | | — |
| | 56,598 |
| | 19,732 |
| | — |
| | (76,330 | ) | | — |
|
| Total revenue | | 1,514,878 |
| | 360,396 |
| | 115,785 |
| | — |
| | (72,576 | ) | | 1,918,483 |
|
| Direct costs | | 1,284,077 |
| | 184,588 |
| | 77,161 |
| | — |
| | (71,984 | ) | | 1,473,842 |
|
| Gross profit | | 230,801 |
| | 175,808 |
| | 38,624 |
| | — |
| | (592 | ) | | 444,641 |
|
| Selling, general and administrative expense | | 39,022 |
| | 33,550 |
| | 33,456 |
| | 65,478 |
| | — |
| | 171,506 |
|
| Operating income (loss) before charges | | 191,779 |
| | 142,258 |
| | 5,168 |
| | (65,478 | ) | | (592 | ) | | 273,135 |
|
| Impairment of goodwill and other long-lived assets | | — |
| | — |
| | 27,019 |
| | — |
| | — |
| | 27,019 |
|
| Net loss (gain) on sale or disposal of assets | | 1,473 |
| | (88 | ) | | (2,201 | ) | | (336 | ) | | — |
| | (1,152 | ) |
| Operating income (loss) | | $ | 190,306 |
| | $ | 142,346 |
| | $ | (19,650 | ) | | $ | (65,142 | ) | | $ | (592 | ) | | $ | 247,268 |
|
| Depreciation and amortization | | $ | 61,031 |
| | $ | 10,444 |
| | $ | 6,940 |
| | $ | 2,943 |
| | $ | (1,931 | ) | | $ | 79,427 |
|
| Property and equipment additions | | $ | 55,814 |
| | $ | 8,306 |
| | $ | 4,912 |
| | $ | 6,649 |
| | $ | (2,733 | ) | | $ | 72,948 |
|
| Total assets at December 31, 2014 | | $ | 2,021,725 |
| | $ | 258,550 |
| | $ | 89,596 |
| | $ | 240,083 |
| | $ | (308,265 | ) | | $ | 2,301,689 |
|
| 2013 | | | | | | | | | | | | |
| External revenue | | $ | 1,417,908 |
| | $ | 290,583 |
| | $ | 91,686 |
| | $ | — |
| | $ | 3,192 |
| | $ | 1,803,369 |
|
| Intercompany revenue | | — |
| | 54,248 |
| | 21,054 |
| | — |
| | (75,302 | ) | | — |
|
| Total revenue | | 1,417,908 |
| | 344,831 |
| | 112,740 |
| | — |
| | (72,110 | ) | | 1,803,369 |
|
| Direct costs | | 1,208,781 |
| | 180,952 |
| | 74,139 |
| | — |
| | (69,883 | ) | | 1,393,989 |
|
| Gross profit | | 209,127 |
| | 163,879 |
| | 38,601 |
| | — |
| | (2,227 | ) | | 409,380 |
|
| Selling, general and administrative expense | | 34,133 |
| | 31,915 |
| | 32,941 |
| | 58,922 |
| | — |
| | 157,911 |
|
| Operating income (loss) before charges | | 174,994 |
| | 131,964 |
| | 5,660 |
| | (58,922 | ) | | (2,227 | ) | | 251,469 |
|
| Net loss (gain) on sale or disposal of assets | | 2,853 |
| | (77 | ) | | 7 |
| | (328 | ) | | — |
| | 2,455 |
|
| Operating income (loss) | | $ | 172,141 |
| | $ | 132,041 |
| | $ | 5,653 |
| | $ | (58,594 | ) | | $ | (2,227 | ) | | $ | 249,014 |
|
| Depreciation and amortization | | $ | 57,748 |
| | $ | 10,251 |
| | $ | 7,909 |
| | $ | 3,284 |
| | $ | (1,783 | ) | | $ | 77,409 |
|
| Property and equipment additions | | $ | 54,116 |
| | $ | 11,148 |
| | $ | 4,288 |
| | $ | 5,908 |
| | $ | (2,190 | ) | | $ | 73,270 |
|
| Total assets at December 31, 2013 | | $ | 1,844,235 |
| | $ | 248,282 |
| | $ | 96,245 |
| | $ | 48,246 |
| | $ | (37,817 | ) | | $ | 2,199,191 |
|
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 15. | Lines of Business, continued |
The table below lists our revenue by geographic area for fiscal 2015, 2014 and 2013 (in thousands):
|
| | | | | | | | | | | |
| | 2015 | | 2014 | | 2013 |
| Revenue* | | | | | |
| United States | $ | 1,957,842 |
| | $ | 1,760,946 |
| | $ | 1,671,170 |
|
| Canada | 175,833 |
| | 157,537 |
| | 132,199 |
|
| Total | $ | 2,133,675 |
| | $ | 1,918,483 |
| | $ | 1,803,369 |
|
____________________________
*Revenues are determined based on the location of the customer. Long-lived assets were not disclosed, as the amounts attributable to the Canadian operations are immaterial, as compared to total consolidated long-lived assets.
|
| |
| 16. | Selected Quarterly Financial Data (Unaudited) |
Quarterly Results
The following table sets forth selected unaudited quarterly results for the eight quarters commencing January 1, 2014 and ending December 31, 2015 (in thousands):
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2015 Quarter Ended | | 2014 Quarter Ended |
| | | Dec. 31 (1) | | Sept. 30 (2) | | Jun. 30 | | Mar. 31 | | Dec. 31 | | Sept. 30 (3) | | Jun. 30 | | Mar. 31 |
| Revenue | | $ | 533,720 |
| | $ | 551,717 |
| | $ | 548,785 |
| | $ | 449,453 |
| | $ | 479,927 |
| | $ | 499,577 |
| | $ | 489,472 |
| | $ | 449,507 |
|
| Gross profit | | $ | 117,696 |
| | $ | 137,666 |
| | $ | 140,847 |
| | $ | 113,862 |
| | $ | 99,018 |
| | $ | 123,757 |
| | $ | 120,415 |
| | $ | 101,451 |
|
| Operating income | | $ | 66,381 |
| | $ | 97,079 |
| | $ | 97,181 |
| | $ | 69,129 |
| | $ | 52,654 |
| | $ | 53,476 |
| | $ | 79,906 |
| | $ | 61,232 |
|
| Net income | | $ | 64,154 |
| | $ | 56,468 |
| | $ | 55,923 |
| | $ | 39,553 |
| | $ | 29,909 |
| | $ | 28,951 |
| | $ | 46,908 |
| | $ | 34,915 |
|
| Net income attributable to VCA Inc. | | $ | 63,595 |
| | $ | 54,854 |
| | $ | 54,299 |
| | $ | 38,301 |
| | $ | 28,359 |
| | $ | 27,452 |
| | $ | 45,584 |
| | $ | 34,043 |
|
| Basic earnings per common share | | $ | 0.79 |
| | $ | 0.68 |
| | $ | 0.66 |
| | $ | 0.47 |
| | $ | 0.34 |
| | $ | 0.32 |
| | $ | 0.52 |
| | $ | 0.39 |
|
| Diluted earnings per common share | | $ | 0.78 |
| | $ | 0.67 |
| | $ | 0.65 |
| | $ | 0.46 |
| | $ | 0.33 |
| | $ | 0.31 |
| | $ | 0.51 |
| | $ | 0.38 |
|
____________________________
| |
(1) | Included in the 2015 fourth quarter is a gain of $43.3 million, $26.4 million net of tax, or $0.32 per diluted common share related to the sale of our Vetstreet business. |
| |
(2) | Included in the 2015 third quarter is business interruption proceeds of $4.5 million, $2.8 million net of tax, or $0.03 per diluted common share. |
| |
(3) | Included in the 2014 third quarter is a non-cash impairment charge of $27.0 million, or $0.20 per diluted share. The charge is primarily related to our Vetstreet reporting unit, see Note 5, Goodwill. |
Additionally, the 2014 third quarter net income includes $1.7 million, or $0.01 per diluted share related to costs incurred in conjunction with the new senior credit facility we entered into during that period.
VCA Inc. and Subsidiaries
Notes to Consolidated Financial Statements (Continued)
|
| |
| 16. | Selected Quarterly Financial Data (Unaudited), continued |
Although not readily detectable because of the impact of acquisitions, our operations are subject to seasonal fluctuation. In particular, our Animal Hospital and Laboratory revenue historically has been greater in the second and third quarters than in the first and fourth quarters.
The demand for our veterinary services is significantly higher during warmer months because pets spend a greater amount of time outdoors, where they are more likely to be injured and are more susceptible to disease and parasites. In addition, use of veterinary services may be affected by levels of infestation of fleas, heartworms and ticks, and the number of daylight hours. A substantial portion of our costs for our veterinary services are fixed and do not vary with the level of demand. Consequently, our operating income and operating margins generally have been higher for the second and third quarters than that experienced in the first and fourth quarters.
Subsequent to December 31, 2015, we acquired 16 hospitals for $109.3 million cash, with acquired revenues of $62.8 million. In connection with two of the hospital acquisitions, we purchased real estate for $8.4 million cash.
During February 2016, we borrowed $50 million from our revolving credit facility to fund our acquisition pipeline.
VCA Inc. and Subsidiaries
Schedule I — Condensed Financial Information of Registrant
VCA Inc. (Parent Company)
Condensed Balance Sheets
(In thousands)
|
| | | | | | | | |
| | | December 31, |
| | | 2015 | | 2014 |
| Assets: | | | | |
| Investment in subsidiaries | | $ | 1,510,723 |
| | $ | 1,330,311 |
|
| Intercompany payable | | (265,761 | ) | | (129,665 | ) |
| Total assets | | $ | 1,244,962 |
| | $ | 1,200,646 |
|
| Stockholders’ equity: | | | | |
| Common stock | | $ | 81 |
| | $ | 83 |
|
| Additional paid-in capital | | 19,708 |
| | 155,802 |
|
| Retained earnings | | 1,275,207 |
| | 1,064,158 |
|
| Accumulated other comprehensive loss | | (50,034 | ) | | (19,397 | ) |
| Total stockholders’ equity | | $ | 1,244,962 |
| | $ | 1,200,646 |
|
The accompanying notes are an integral part of these condensed financial statements.
See accompanying Report of Independent Registered Public Accounting Firm.
VCA Inc. and Subsidiaries
Schedule I — Condensed Financial Information of Registrant — (Continued)
VCA Inc. (Parent Company)
Condensed Statements of Income
(In thousands)
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Revenue | | $ | — |
| | $ | — |
| | $ | — |
|
| Direct costs | | — |
| | — |
| | — |
|
| Gross profit | | — |
| | — |
| | — |
|
| Selling, general and administrative expense | | — |
| | — |
| | — |
|
| Loss on sale of assets | | — |
| | — |
| | — |
|
| Operating income | | — |
| | — |
| | — |
|
| Interest income, net | | — |
| | — |
| | — |
|
| Equity interest in income of subsidiaries | | 211,049 |
| | 135,438 |
| | 137,511 |
|
| Income before provision for income taxes | | 211,049 |
| | 135,438 |
| | 137,511 |
|
| Provision for income taxes | | — |
| | — |
| | — |
|
| Net income | | 211,049 |
| | 135,438 |
| | 137,511 |
|
| Net income attributable to noncontrolling interests | | — |
| | — |
| | — |
|
| Net income attributable to VCA Inc. | | $ | 211,049 |
| | $ | 135,438 |
| | $ | 137,511 |
|
The accompanying notes are an integral part of these condensed financial statements.
See accompanying Report of Independent Registered Public Accounting Firm.
VCA Inc. and Subsidiaries
Schedule I — Condensed Financial Information of Registrant — (Continued)
VCA Inc. (Parent Company)
Condensed Statements of Cash Flows
(In thousands)
|
| | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Cash flows from operating activities: | | | | | | |
| Net income | | $ | 211,049 |
| | $ | 135,438 |
| | $ | 137,511 |
|
| Adjustments to reconcile net income to net cash used in operating activities: | | | | | | |
| Equity interest in earnings of subsidiaries | | (211,049 | ) | | (135,438 | ) | | (137,511 | ) |
| Net cash used in operating activities | | — |
| | — |
| | — |
|
| Cash flows used in investing activities: | | | | | | |
| Decrease in intercompany receivable | | (2,683 | ) | | (2,859 | ) | | (17,233 | ) |
| Net cash used in investing activities | | (2,683 | ) | | (2,859 | ) | | (17,233 | ) |
| Cash flows provided by financing activities: | | | | | | |
| Proceeds from issuance of common stock under stock option plans | | 2,683 |
| | 2,859 |
| | 17,233 |
|
| Net cash provided by financing activities | | 2,683 |
| | 2,859 |
| | 17,233 |
|
| Increase (decrease) in cash and cash equivalents | | — |
| | — |
| | — |
|
| Cash and cash equivalents at beginning of year | | — |
| | — |
| | — |
|
| Cash and cash equivalents at end of year | | $ | — |
| | $ | — |
| | $ | — |
|
The accompanying notes are an integral part of these condensed financial statements.
See accompanying Report of Independent Registered Public Accounting Firm.
VCA Inc. and Subsidiaries
Schedule I — Condensed Financial Information of Registrant — (Continued)
VCA Inc. (Parent Company)
Notes to Condensed Financial Statements
Note 1. Guarantees
The borrowings under the new senior credit facility are guaranteed by VCA Inc. (“VCA”) and its wholly-owned subsidiaries. Vicar Operating, Inc. (“Vicar”), a wholly-owned subsidiary of VCA, may borrow up to $800 million under a revolving line of credit under the new senior credit facility. VCA’s guarantee under the new senior credit facility is secured by a pledge of substantially all of our consolidated assets, including 65% of the voting equity and 100% of the non-voting equity interest in each of our foreign subsidiaries.
See Note 7, Long-Term Obligations, in our accompanying consolidated financial statements of this annual report on Form 10-K for a five-year schedule of debt maturities.
Note 2. Dividends from Subsidiaries
The senior credit facility has restrictions on the ability of Vicar and its consolidated subsidiaries to transfer assets in the form of cash, dividends, loans or advances to VCA. In 2015, 2014 and 2013, VCA did not receive any cash dividends from its consolidated subsidiaries.
See accompanying Report of Independent Registered Public Accounting Firm.
VCA Inc. and Subsidiaries
Schedule II — Valuation and Qualifying Accounts
(In thousands)
|
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Additions | | | | | | |
| | | Balance at Beginning of Period | | Charged to Costs and Expenses | | Charged to Other Accounts | | Write-offs | | Other(1) | | Balance at End of Period |
| Year ended December 31, 2015 | | | | | | | | | | | | |
Allowance for uncollectible accounts(2) | | $ | 19,846 |
| | $ | 8,401 |
| | $ | — |
| | $ | (6,098 | ) | | $ | (374 | ) | | $ | 21,775 |
|
| Year ended December 31, 2014 | | | | | | | | | | | | |
Allowance for uncollectible accounts(2) | | $ | 17,702 |
| | $ | 6,248 |
| | $ | — |
| | $ | (6,129 | ) | | $ | 2,025 |
| | $ | 19,846 |
|
| Year ended December 31, 2013 | | | | | | | | | | | | |
Allowance for uncollectible accounts(2) | | $ | 16,546 |
| | $ | 7,360 |
| | $ | — |
| | $ | (6,325 | ) | | $ | 121 |
| | $ | 17,702 |
|
____________________________
| |
(1) | “Other” changes in the allowance for uncollectible accounts include allowances acquired with animal hospitals and laboratory acquisitions. |
| |
(2) | Balance includes allowance for trade accounts receivable and notes receivable. |
See accompanying Report of Independent Registered Public Accounting Firm.
|
| |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
|
| |
| ITEM 9A. | CONTROLS AND PROCEDURES |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
We carried out an evaluation required by the Securities Exchange Act of 1934, as amended (the “1934 Act”), under the supervision and with the participation of our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rule 13a-15(e) of the 1934 Act, as of December 31, 2015. Based on this evaluation, our chief executive officer and chief financial officer concluded that, as of December 31, 2015, our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the 1934 Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and to provide reasonable assurance that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosures.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management does not expect that our internal control over financial reporting will prevent all error and all fraud. Internal controls over financial reporting, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the internal control over financial reporting are met. Further, the design of internal controls over financial reporting must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all internal controls over financial reporting, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple errors or mistakes. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective internal control over financial reporting, misstatements due to error or fraud may occur and not be detected.
Our management’s report on internal control over financial reporting, and the related report of our independent public accounting firm, are included in Item 8 of this annual report on Form 10-K under Management’s Annual Report on Internal Control Over Financial Reporting and Report of Independent Registered Public Accounting Firm on Internal Control Over Financial Reporting, respectively, and are incorporated by reference.
Changes in Internal Control Over Financial Reporting
During our most recent fiscal quarter, there were no changes in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
|
| |
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
|
| |
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information regarding our directors and executive officers will appear in the proxy statement for the 2016 Annual Meeting of Stockholders and is incorporated herein by this reference.
|
| |
| ITEM 11. | EXECUTIVE COMPENSATION |
Information regarding executive compensation will appear in the proxy statement for the 2016 Annual Meeting of Stockholders and is incorporated herein by this reference.
|
| |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information regarding security ownership of certain beneficial owners and management and related stockholder matters will appear in the proxy statement for the 2016 Annual Meeting of Stockholders and is incorporated herein by this reference.
|
| |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Information regarding certain relationships and related transactions will appear in the proxy statement for the 2016 Annual Meeting of Stockholders and is incorporated herein by this reference.
|
| |
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Information regarding principal accountant fees and services will appear in the proxy statement for the 2016 Annual Meeting of Stockholders and is incorporated herein by this reference.
PART IV
|
| |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| |
| (1) | FINANCIAL STATEMENTS — See Item 8 of this annual report on Form 10-K. |
REPORTS OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM — See Item 8 of this annual report on Form 10-K.
| |
| (2) | SCHEDULE I — CONDENSED FINANCIAL INFORMATION — See Item 8 of this annual report on Form 10-K. |
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS — See Item 8 of this annual report on Form 10-K.
All other schedules have been omitted because they are not applicable or not required, or the information is included in the Consolidated Financial Statements or Notes thereto.
| |
| (3) | EXHIBITS — See Exhibit Index attached to this annual report on Form 10-K. |
List of Exhibits
|
| | |
| | | |
| Number | | Exhibit Description |
| 3.1 | | Amended and Restated Certificate of Incorporation of Registrant. Incorporated by reference to Exhibit 3.1 to the Registrant’s annual report on Form 10-K filed March 29, 2002. |
| 3.2 | | Certificate of Amendment to the Certificate of Incorporation of Registrant. Incorporated by reference to Exhibit 3.1 to the Registrant’s current report on Form 8-K filed July 16, 2004. |
| 3.3 | | Certificate of Correction to the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Registrant. Incorporated by reference to Exhibit 3.2 to the Registrant’s current report on Form 8-K filed July 16, 2004. |
| 3.4 | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of Registrant. Incorporated by reference to Exhibit 3.1 to the Registrant’s current report on Form 8-K filed June 5, 2014. |
| 3.5 | | Third Amended and Restated Bylaws of Registrant. Incorporated by reference to Exhibit 3.1 to the Registrant’s current report on Form 8-K filed February 14, 2013. |
| 3.6 | | Amendment to the Third Amended and Restated Bylaws of Registrant. Incorporated by reference to Exhibit 3.2 to the Registrant’s current report on Form 8-K filed June 5, 2014. |
| 3.7 | | Amendment to the Third Amended and Restated Bylaws of Registrant. Incorporated by reference to Exhibit 3.1 to the Registrant’s current report on Form 8-K filed October 29, 2015. |
| 4.1 | | Specimen Certificate for shares of common stock of Registrant. Incorporated by reference to Exhibit 4.9 to Amendment No. 3 to the Registrant’s registration statement on Form S-1 filed November 16, 2001. |
| 10.1 | | Credit Agreement, dated as of August 27, 2014, by and among the Vicar Operating, Inc., as borrower, VCA Inc., as guarantor, certain subsidiaries of VCA party thereto, as guarantors, the lenders party thereto, Bank of America, N.A., as administrative agent, swing line lender and L/C issuer, JPMorgan Chase Bank, N.A., and Suntrust Bank as co-syndication agents. Incorporated by reference to Exhibit 10.1 to the Registrant’s current report on Form 8-K filed August 28, 2014. |
| 10.2* | | Employment Agreement, dated as of November 27, 2001, by and between VCA Inc. and Robert L. Antin. Incorporated by reference to Exhibit 10.5 to the registration statement of Vicar Operating, Inc., on Form S-4 filed February 1, 2002. |
| 10.3* | | Employment Agreement, dated as of November 27, 2001, by and between VCA Inc. and Arthur J. Antin. Incorporated by reference to Exhibit 10.6 to the registration statement of Vicar Operating, Inc., on Form S-4 filed February 1, 2002. |
| 10.4* | | Employment Agreement, dated as of November 27, 2001, by and between VCA Inc. and Tomas W. Fuller. Incorporated by reference to Exhibit 10.7 to the registration statement of Vicar Operating, Inc., on Form S-4 filed February 1, 2002. |
| 10.5* | | Letter Agreement, dated as of March 9, 2004, by and between VCA Inc. and Robert L. Antin. Incorporated by reference to Exhibit 10.20 to the Registrant’s annual report on Form 10-K filed March 12, 2004. |
| 10.6* | | Letter Agreement, dated as of March 9, 2004, by and between VCA Inc. and Arthur J. Antin. Incorporated by reference to Exhibit 10.21 to the Registrant’s annual report on Form 10-K filed March 12, 2004. |
| 10.7* | | Letter Agreement, dated as of March 9, 2004, by and between VCA Inc. and Tomas W. Fuller. Incorporated by reference to Exhibit 10.22 to the Registrant’s annual report on Form 10-K filed March 12, 2004. |
| 10.8* | | VCA Inc. 2007 Annual Cash Incentive Plan. Incorporated by reference to Appendix A to the Registrant's proxy statement on Schedule 14A filed on March 6, 2015. |
| 10.9* | | Post-Retirement Medical Benefits Coverage Agreement dated as of December 27, 2007, by and between VCA Inc. and Robert L. Antin. Incorporated by reference to Exhibit 10.15 to the Registrant’s annual report on Form 10-K filed February 29, 2008. |
| 10.10* | | Post-Retirement Medical Benefits Coverage Agreement dated as of December 27, 2007, by and between VCA Inc. and Arthur J. Antin. Incorporated by reference to Exhibit 10.16 to the Registrant’s annual report on Form 10-K filed February 29, 2008. |
| 10.11* | | Post-Retirement Medical Benefits Coverage Agreement dated as of December 27, 2007, by and between VCA Inc. and Neil Tauber. Incorporated by reference to Exhibit 10.17 to the Registrant’s annual report on Form 10-K filed February 29, 2008. |
| 10.12* | | Post-Retirement Medical Benefits Coverage Agreement dated as of December 27, 2007, by and between VCA Inc. and Tomas W. Fuller. Incorporated by reference to Exhibit 10.18 to the Registrant’s annual report on Form 10-K filed February 29, 2008. |
| 10.13* | | Letter Agreement, dated as of April 15, 2008, by and between VCA Inc. and Neil Tauber. Incorporated by reference to Exhibit 10.1 to the Registrant's current report on Form 8-K filed April 28, 2008. |
| 10.14* | | Second Amendment to Amended and Restated Employment Agreement, effective January 1, 2009, by and between VCA Inc. and Robert L. Antin. Incorporated by reference to Exhibit 10.18 to the Registrant’s annual report on Form 10-K filed February 26, 2010. |
|
| | |
| Number | | Exhibit Description |
| 10.15* | | Second Amendment to Amended and Restated Employment Agreement, effective January 1, 2009, by and between VCA Inc. and Arthur J. Antin. Incorporated by reference to Exhibit 10.19 to the Registrant’s annual report on Form 10-K filed February 26, 2010. |
| 10.16* | | Second Amendment to Amended and Restated Employment Agreement, effective January 1, 2009, by and between VCA Inc. and Tomas W. Fuller. Incorporated by reference to Exhibit 10.20 to the Registrant’s annual report on Form 10-K filed February 26, 2010. |
| 10.17* | | Supplemental Executive Retirement Program Agreement, effective as of June 28, 2010, by and between VCA Inc. and Robert L. Antin. Incorporated by reference to Exhibit 10.1 to the Registrant’s current report on Form 8-K dated July 7, 2010. |
| 10.18* | | Supplemental Executive Retirement Program Agreement, effective as of June 28, 2010, by and between VCA Inc. and Arthur J. Antin. Incorporated by reference to Exhibit 10.2 to the Registrant’s current report on Form 8-K dated July 7, 2010. |
| 10.19* | | Supplemental Executive Retirement Program Agreement, effective as of June 28, 2010, by and between VCA Inc. and Neil Tauber. Incorporated by reference to Exhibit 10.3 to the Registrant’s current report on Form 8-K dated July 7, 2010. |
| 10.20* | | Supplemental Executive Retirement Program Agreement, effective as of June 28, 2010, by and between VCA Inc. and Tomas W. Fuller. Incorporated by reference to Exhibit 10.4 to the Registrant’s current report on Form 8-K dated July 7, 2010. |
| 10.21* | | Consulting Agreement, effective as of June 28, 2010, by and between VCA Inc. and Robert L. Antin. Incorporated by reference to Exhibit 10.5 to the Registrant’s current report on Form 8-K dated July 7, 2010. |
| 10.22* | | Consulting Agreement, effective as of June 28, 2010, by and between VCA Inc. and Arthur J. Antin. Incorporated by reference to Exhibit 10.6 to the Registrant’s current report on Form 8-K dated July 7, 2010. |
| 10.23* | | Letter Agreement, dated as of August 18, 2011, by and between VCA Inc. and Josh Drake. Incorporated by reference to Exhibit 10.1 to the Registrant's current report on Form 8-K filed August 22, 2011. |
| 10.24* | | Summary of Executive Officer Compensation. |
| 10.25* | | Summary of Board of Directors Compensation. Incorporated by reference to Exhibit 10.23 to the Registrant’s annual report on Form 10-K filed February 26, 2010. |
| 10.26 | | VCA Inc. 2006 Equity Incentive Plan, as amended on May 22, 2006. Incorporated by reference to Exhibit 4.5 to the Registrant’s registration statement on Form S-8 filed on December 15, 2006. |
| 10.27 | | Stock Option Agreement for VCA Inc. 2006 Equity Incentive Plan. Incorporated by reference to Exhibit 4.6 to the Registrant’s registration statement on Form S-8 filed on December 15, 2006. |
| 10.28 | | Restricted Stock Award Agreement for VCA Inc. 2006 Equity Incentive Plan. Incorporated by reference to Exhibit 4.7 to the Registrant’s registration statement on Form S-8 filed on December 15, 2006. |
| 10.29 | | Restricted Stock Unit Agreement for VCA Inc. 2006 Equity Incentive Plan. Incorporated by reference to Exhibit 10.29 to the Registrant’s annual report on Form 10-K filed February 26, 2010. |
| 10.30 | | VCA Inc. 2015 Equity Incentive Plan, effective March 1, 2015. Incorporated by reference to Exhibit 4.7 to the Registrant’s registration statement on Form S-8 filed on May 8, 2015. |
| 10.31 | | Stock Option Agreement for VCA Inc. 2015 Equity Incentive Plan. Incorporated by reference to Exhibit 4.8 to the Registrant’s registration statement on Form S-8 filed on May 8, 2015. |
| 10.32 | | Restricted Stock Award Agreement for VCA Inc. 2015 Equity Incentive Plan. Incorporated by reference to Exhibit 4.9 to the Registrant’s registration statement on Form S-8 filed on May 8, 2015. |
| 10.33 | | Restricted Stock Unit Agreement for VCA Inc. 2015 Equity Incentive Plan. Incorporated by reference to Exhibit 4.10 to the Registrant’s registration statement on Form S-8 filed on May 8, 2015. |
| 10.34 | | Corporate Headquarters Lease, dated as of January 1, 1999, by and between VCA Inc. and Werner Wolfen, Michael Dunitz, Nancy Bruch, Dorothy A. Dunitz, Harvey Rosenberg and Judy Rosenberg (Landlords). Incorporated by reference to Exhibit 10.11 to Amendment No. 1 to the Registrant’s registration statement on Form S-1 filed October 15, 2001. |
| 10.35 | | First Amendment to Standard Industrial/Commercial Single-Tenant Lease - Net, dated as of March 11, 1999, by and between VCA Inc. and Werner Wolfen, Michael Dunitz, Nancy Bruch, Dorothy A. Duntz, Harvey Rosenberg and Judy Rosenberg (Landlords). Incorporated by reference to Exhibit 10.33 to the Registrant’s annual report on Form 10-K filed February 28, 2014. |
| 10.36 | | Second Amendment to Lease, dated as of January 16, 2013, by and between VCA Inc. and Werner Wolfen, Michael Dunitz, Nancy Bruch, Dorothy A. Dunitz and Judy Rosenberg (Landlords). Incorporated by reference to Exhibit 10.1 to the Registrant’s quarterly report on Form 10-Q filed May 10, 2013. |
| 10.37 | | Third Amendment to Lease, dated as of December 23, 2014, by and between VCA Inc. and Werner Wolfen, Michael Dunitz, Nancy Bruch, Dorothy A. Dunitz and Judy Rosenberg (Landlords). Incorporated by reference to Exhibit 10.35 to the Registrant’s annual report on Form 10-K filed February 28, 2014. |
| 10.38 | | Fourth Amendment to Lease, dated as of February 22, 2016, by and between VCA Inc. and Werner Wolfen, Michael Dunitz, Nancy Bruch, Dorothy A. Dunitz and Judy Rosenberg (Landlords). |
|
| | |
| Number | | Exhibit Description |
| 10.39 | | Corporate Headquarters Lease, dated as of June 9, 2004, by and between VCA Inc. and Martin Shephard, Trustee of the Shephard Family Trust of 1998 (Lessor). Incorporated by reference to Exhibit 10.21 to the Registrant’s annual report on Form 10-K filed March 14, 2006. |
| 10.40 | | Corporate Headquarters Lease, dated as of February 28, 2015, by and between VCA Inc. and Martin Shephard, Trustee of the Shephard Family Trust of 1998 (Lessor). Incorporated by reference to Exhibit 10.1 to the Registrant’s quarterly report on Form 10-Q filed on May 8, 2015. |
| 10.41 | | Form of Indemnification Agreement. Incorporated by reference to Exhibit 10.13 to the Registrant’s registration statement on Form S-1 filed August 9, 2001. |
| 14.1 | | Code of Conduct and Business Ethics of the Registrant. Incorporated by reference to Exhibit 14.1 to the Registrant’s annual report on Form 10-K filed March 12, 2004. |
| 21.1 | | Subsidiaries of Registrant. |
| 23.1 | | Consent of Independent Registered Public Accounting Firm. |
| 24.1 | | Power of Attorney (included in signature page). |
| 31.1 | | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 31.2 | | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 32.1 | | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| 101.INS | | XBRL Instance Document |
| 101.SCH | | XBRL Taxonomy Extension Schema Document |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase |
| 101.DEF | | XBRL Taxonomy Definition Linkbase |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase |
____________________
|
| |
| * | Management contract or compensatory plan or arrangement. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on February 26, 2016.
|
| | |
| | | |
| | VCA Inc. |
| | | |
| | By: | /s/ TOMAS W. FULLER |
| | | Tomas W. Fuller |
| | | Chief Financial Officer, Principal Accounting Officer, |
| | | Vice President and Secretary |
KNOWN BY ALL PERSONS THESE PRESENTS, that each person whose signature appears below constitutes and appoints Robert L. Antin and Tomas W. Fuller, or any one of them, their attorneys-in-fact and agents with full power of substitution and re-substitution, for him and his name, place and stead, in any and all capacities, to sign any or all amendments to this annual report on Form 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the foregoing, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
|
| | | | |
| | | | | |
| Signature | | Title | | Date |
| | | |
| /s/ Robert L. Antin | | Chairman of the Board, President and | | February 26, 2016 |
| Robert L. Antin | Chief Executive Officer | |
| | | | | |
| /s/ Tomas W. Fuller | | Chief Financial Officer, Principal | | February 26, 2016 |
| Tomas W. Fuller | | Accounting Officer, Vice President | |
| | | and Secretary | | |
| | | | | |
| /s/ John M. Baumer | | Director | | February 26, 2016 |
| John M. Baumer | | | | |
| | | |
| /s/ John Heil | | Director | | February 26, 2016 |
| John Heil | | |
| | | | | |
| /s/ Frank Reddick | | Director | | February 26, 2016 |
| Frank Reddick | | |
| | | | | |
| /s/ John B. Chickering, Jr. | | Director | | February 26, 2016 |
| John B. Chickering, Jr. | | | | |
|
| | | | | |
| *By: | | | Director | | |
| | Attorney-in-Fact | | | | |