
APONVIE™ (HTX-019) FDA Approval September 19, 2022 Exhibit 99.2

This presentation contains "forward-looking statements" as defined by the Private Securities Litigation Reform Act of 1995. We caution investors that forward-looking statements are based on management’s expectations and assumptions as of the date of this presentation, and involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. These risks and uncertainties include, but are not limited to, those associated with: the timing of the commercial launch of APONVIE; the potential market opportunity for APONVIE; the extent of the impact of the ongoing Coronavirus Disease 2019 (COVID-19) pandemic on our business; and other risks and uncertainties identified in the Company's filings with the Securities and Exchange Commission. Forward-looking statements reflect our analysis only on their stated date, and we take no obligation to update or revise these statements except as may be required by law. Forward-Looking Statements
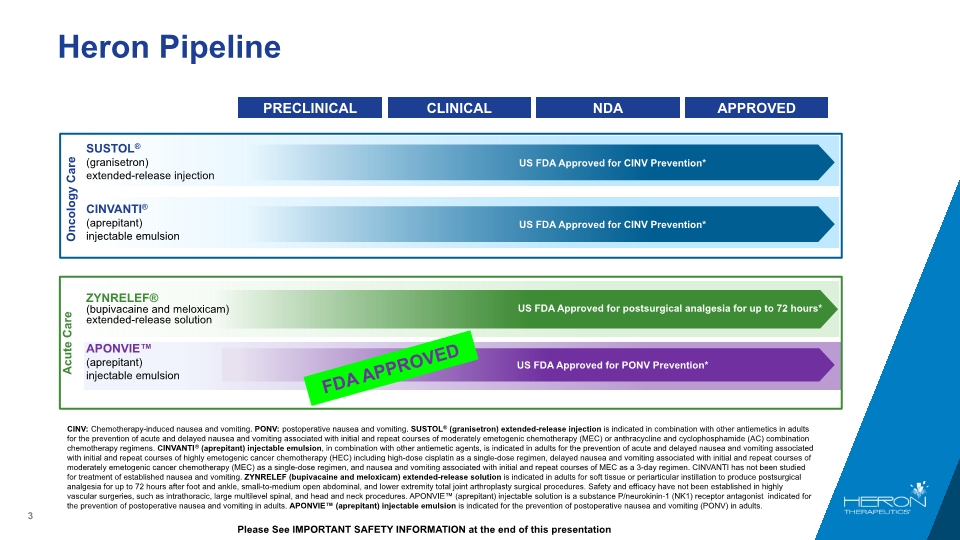
Heron Pipeline US FDA Approved for postsurgical analgesia for up to 72 hours* ZYNRELEF® (bupivacaine and meloxicam) extended-release solution Acute Care CINV: Chemotherapy-induced nausea and vomiting. PONV: postoperative nausea and vomiting. SUSTOL® (granisetron) extended-release injection is indicated in combination with other antiemetics in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline and cyclophosphamide (AC) combination chemotherapy regimens. CINVANTI® (aprepitant) injectable emulsion, in combination with other antiemetic agents, is indicated in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin as a single-dose regimen, delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC) as a single-dose regimen, and nausea and vomiting associated with initial and repeat courses of MEC as a 3-day regimen. CINVANTI has not been studied for treatment of established nausea and vomiting. ZYNRELEF (bupivacaine and meloxicam) extended-release solution is indicated in adults for soft tissue or periarticular instillation to produce postsurgical analgesia for up to 72 hours after foot and ankle, small-to-medium open abdominal, and lower extremity total joint arthroplasty surgical procedures. Safety and efficacy have not been established in highly vascular surgeries, such as intrathoracic, large multilevel spinal, and head and neck procedures. APONVIE™ (aprepitant) injectable solution is a substance P/neurokinin-1 (NK1) receptor antagonist indicated for the prevention of postoperative nausea and vomiting in adults. APONVIE™ (aprepitant) injectable emulsion is indicated for the prevention of postoperative nausea and vomiting (PONV) in adults. Please See IMPORTANT SAFETY INFORMATION at the end of this presentation SUSTOL® (granisetron) extended-release injection Oncology Care US FDA Approved for CINV Prevention* APONVIE™ (aprepitant) injectable emulsion FDA APPROVED

Is Heron’s 4th Drug Approval and an Ideal Strategic Fit INDICATIONS AND USAGE APONVIE is indicated for the prevention of postoperative nausea and vomiting (PONV) in adults. Limitations of Use APONVIE has not been studied for the treatment of established nausea and vomiting. The recommended dose in adults of APONVIE is 32 mg (4.4 ml) administered as a 30 second intravenous injection prior to induction of anesthesia. APONVIE will be available as a ready-to-use 32 mg single-dose vial. Please See IMPORTANT SAFETY INFORMATION at the end of this presentation
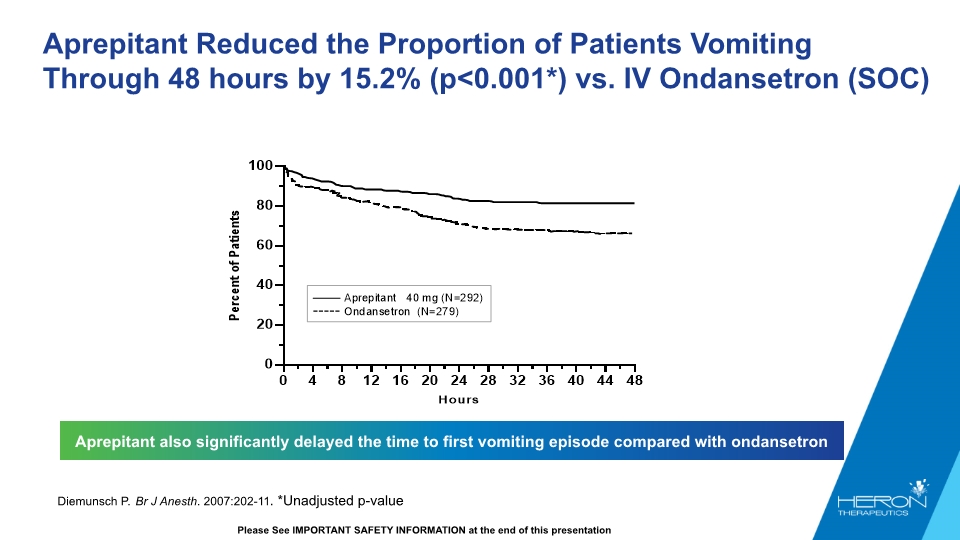
Aprepitant Reduced the Proportion of Patients Vomiting Through 48 hours by 15.2% (p<0.001*) vs. IV Ondansetron (SOC) Aprepitant also significantly delayed the time to first vomiting episode compared with ondansetron Diemunsch P. Br J Anesth. 2007:202-11. *Unadjusted p-value Please See IMPORTANT SAFETY INFORMATION at the end of this presentation
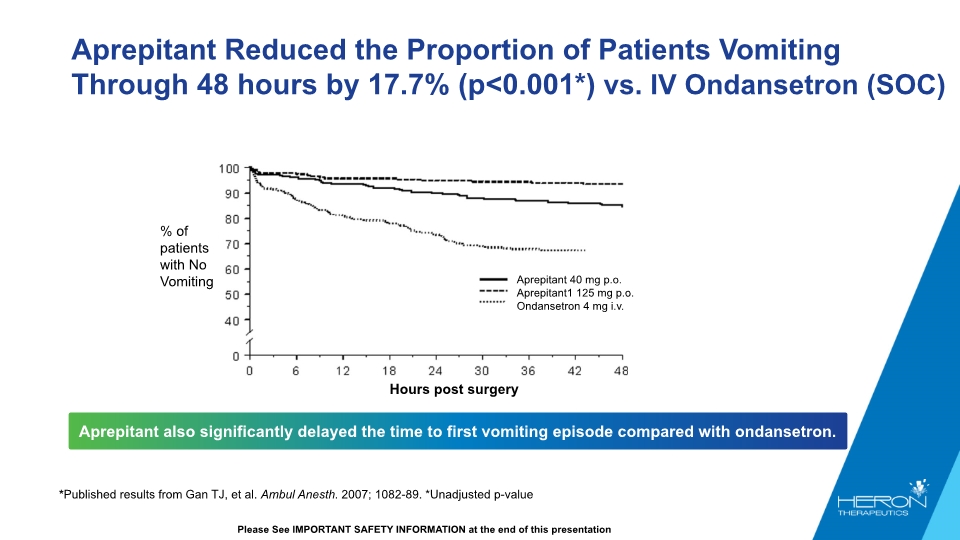
Aprepitant Reduced the Proportion of Patients Vomiting Through 48 hours by 17.7% (p<0.001*) vs. IV Ondansetron (SOC) Aprepitant also significantly delayed the time to first vomiting episode compared with ondansetron. *Published results from Gan TJ, et al. Ambul Anesth. 2007; 1082-89. *Unadjusted p-value Please See IMPORTANT SAFETY INFORMATION at the end of this presentation
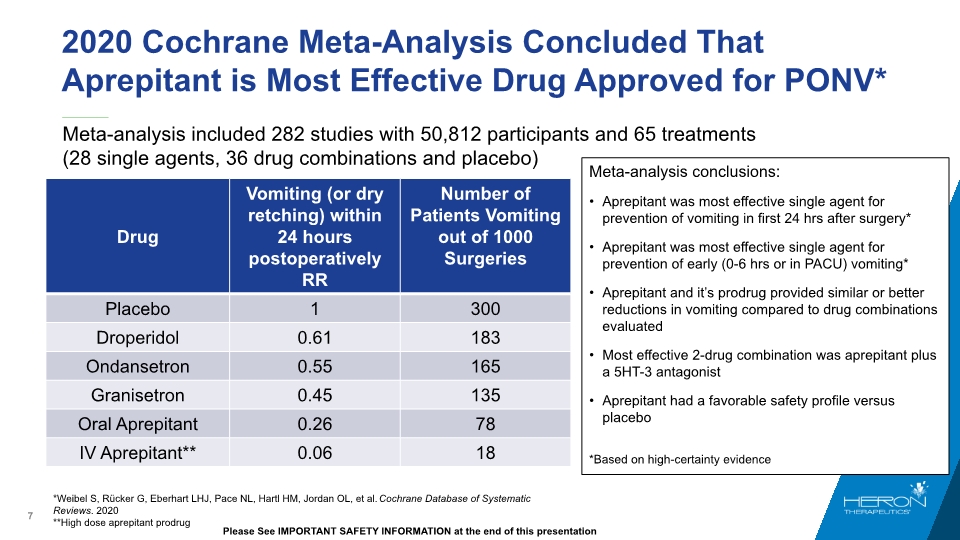
Meta-analysis included 282 studies with 50,812 participants and 65 treatments (28 single agents, 36 drug combinations and placebo) 2020 Cochrane Meta-Analysis Concluded That Aprepitant is Most Effective Drug Approved for PONV* *Weibel S, Rücker G, Eberhart LHJ, Pace NL, Hartl HM, Jordan OL, et al. Cochrane Database of Systematic Reviews. 2020 **High dose aprepitant prodrug Meta-analysis conclusions: Aprepitant was most effective single agent for prevention of vomiting in first 24 hrs after surgery* Aprepitant was most effective single agent for prevention of early (0-6 hrs or in PACU) vomiting* Aprepitant and it’s prodrug provided similar or better reductions in vomiting compared to drug combinations evaluated Most effective 2-drug combination was aprepitant plus a 5HT-3 antagonist Aprepitant had a favorable safety profile versus placebo *Based on high-certainty evidence Please See IMPORTANT SAFETY INFORMATION at the end of this presentation Drug Vomiting (or dry retching) within 24 hours postoperatively RR Number of Patients Vomiting out of 1000 Surgeries Placebo 1 300 Droperidol 0.61 183 Ondansetron 0.55 165 Granisetron 0.45 135 Oral Aprepitant 0.26 78 IV Aprepitant** 0.06 18
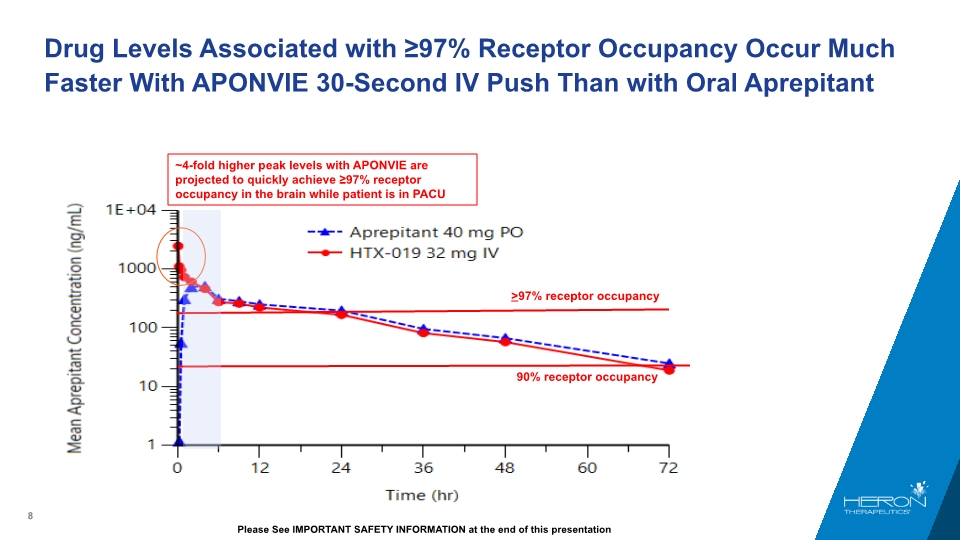
Drug Levels Associated with ≥97% Receptor Occupancy Occur Much Faster With APONVIE 30-Second IV Push Than with Oral Aprepitant >97% receptor occupancy 90% receptor occupancy ~4-fold higher peak levels with APONVIE are projected to quickly achieve ≥97% receptor occupancy in the brain while patient is in PACU Please See IMPORTANT SAFETY INFORMATION at the end of this presentation
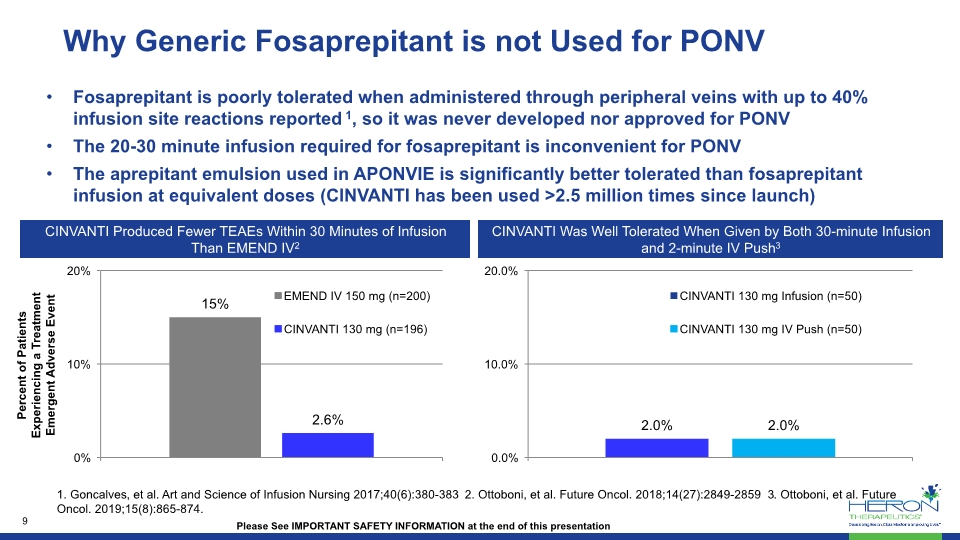
Why Generic Fosaprepitant is not Used for PONV Fosaprepitant is poorly tolerated when administered through peripheral veins with up to 40% infusion site reactions reported 1, so it was never developed nor approved for PONV The 20-30 minute infusion required for fosaprepitant is inconvenient for PONV The aprepitant emulsion used in APONVIE is significantly better tolerated than fosaprepitant infusion at equivalent doses (CINVANTI has been used >2.5 million times since launch) Please See IMPORTANT SAFETY INFORMATION at the end of this presentation CINVANTI Produced Fewer TEAEs Within 30 Minutes of Infusion Than EMEND IV2 CINVANTI Was Well Tolerated When Given by Both 30-minute Infusion and 2-minute IV Push3 Percent of Patients Experiencing a Treatment Emergent Adverse Event 1. Goncalves, et al. Art and Science of Infusion Nursing 2017;40(6):380-383 2. Ottoboni, et al. Future Oncol. 2018;14(27):2849-2859 3. Ottoboni, et al. Future Oncol. 2019;15(8):865-874.

Postoperative Nausea and Vomiting (PONV) Market Opportunity Please see IMPORTANT SAFETY INFORMATION at the end of this presentation

Large market potential Addressable market = 69M procedures* Significant Unmet Need Convenient, more effective and longer lasting treatments are needed Synergies with Heron commercial organization Majority of same ZYNRELEF target accounts and audiences (ASA) Existing positive experience with CINVANTI at major hospitals/IDNs APONVIE – The Next Big Opportunity at Heron Brand Name Conveys “Aprepitant for PONV” Source: DRG / Clarivate PONV Demand Study (Dec. 2021) * 2023 Procedure projections Please see IMPORTANT SAFETY INFORMATION at the end of this presentation
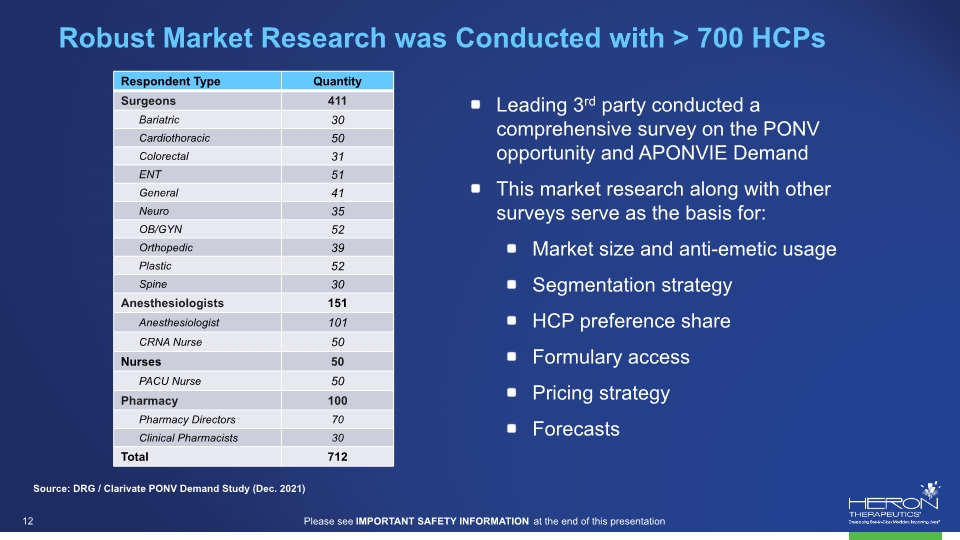
Robust Market Research was Conducted with > 700 HCPs Leading 3rd party conducted a comprehensive survey on the PONV opportunity and APONVIE Demand This market research along with other surveys serve as the basis for: Market size and anti-emetic usage Segmentation strategy HCP preference share Formulary access Pricing strategy Forecasts Source: DRG / Clarivate PONV Demand Study (Dec. 2021) Respondent Type Quantity Surgeons 411 Bariatric 30 Cardiothoracic 50 Colorectal 31 ENT 51 General 41 Neuro 35 OB/GYN 52 Orthopedic 39 Plastic 52 Spine 30 Anesthesiologists 151 Anesthesiologist 101 CRNA Nurse 50 Nurses 50 PACU Nurse 50 Pharmacy 100 Pharmacy Directors 70 Clinical Pharmacists 30 Total 712 projections Please see IMPORTANT SAFETY INFORMATION at the end of this presentation
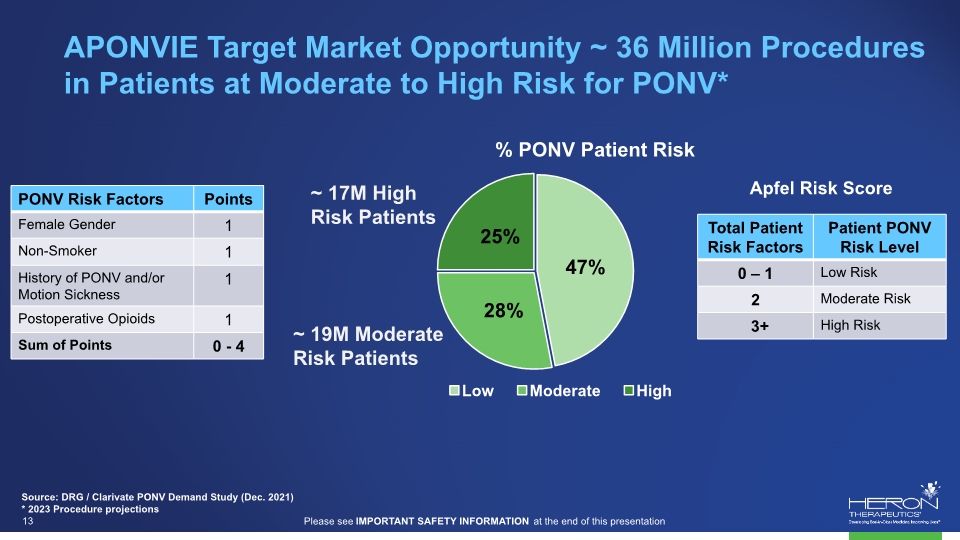
APONVIE Target Market Opportunity ~ 36 Million Procedures in Patients at Moderate to High Risk for PONV* Source: DRG / Clarivate PONV Demand Study (Dec. 2021) * 2023 Procedure projections Apfel Risk Score ~ 17M High Risk Patients % PONV Patient Risk ~ 19M Moderate Risk Patients Low Moderate High 25% 47% 28% PONV Risk Factors Points Female Gender 1 Non-Smoker 1 History of PONV and/or Motion Sickness 1 Postoperative Opioids 1 Sum of Points 0 – 4 Total Patient Risk Factors Patient PONV Risk Level 0 – 1 Low Risk 2 Moderate Risk 3+ High Risk projections Please see IMPORTANT SAFETY INFORMATION at the end of this presentation
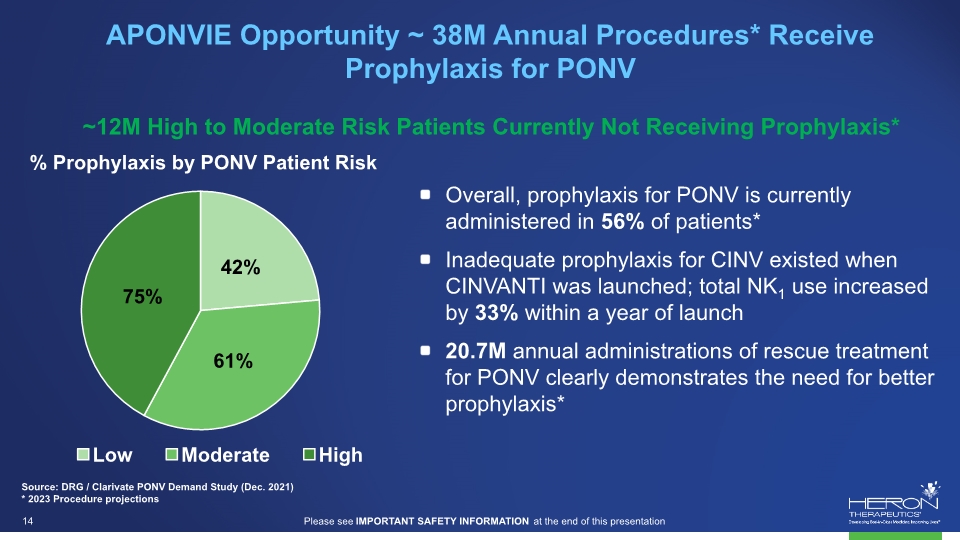
APONVIE Opportunity ~ 38M Annual Procedures* Receive Prophylaxis for PONV ~12M High to Moderate Risk Patients Currently Not Receiving Prophylaxis* Source: DRG / Clarivate PONV Demand Study (Dec. 2021) * 2023 Procedure projections Overall, prophylaxis for PONV is currently administered in 56% of patients* Inadequate prophylaxis for CINV existed when CINVANTI was launched; total NK1 use increased by 33% within a year of launch 20.7M annual administrations of rescue treatment for PONV clearly demonstrates the need for better prophylaxis* % Prophylaxis by PONV Patient Risk 75% 42% 61% Low Moderate High projections Please see IMPORTANT SAFETY INFORMATION at the end of this presentation

“In this iteration of the PONV guideline, one of the major changes is that we now recommend the use of multimodal prophylaxis in patients with one or more risk factors”. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting1 1Gan TJ, Belani KG, Bergese S, et al. Anesth Analg. 2020;131(2):411-448. Please see IMPORTANT SAFETY INFORMATION at the end of this presentation

APONVIE Key Attributes One Push to Prevent PONV References: 1. Diemunsch P, Apfel C, Gan TJ, et al. Preventing postoperative nausea and vomiting: post hoc analysis of pooled data from two randomized active-controlled trials of aprepitant. Curr Med Res Opin. 2007;23(10):2559-2565. doi:10.1185/030079907X233115. 2. Gan TJ, Apfel CC, Kovac A, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104(5):1082-1089. doi:10.1213/01.ane.0000263277.35140.a3. 3. APONVIE [package insert]. San Diego, CA: Heron Therapeutics Inc; 2022. 4. EMEND [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2019. 5. Data on file. Summary of clinical pharmacology studies. San Diego, CA: Heron Therapeutics Inc; 2021. 6. Van Laere K, De Hoon J, Bormans G, et al. Equivalent dynamic human brain NK1-receptor occupancy following single-dose i.v. fosaprepitant vs. oral aprepitant as assessed by PET imaging. Clin Pharmacol Ther. 2012;92(2):243-250. doi:10.1038/clpt.2012.62. Please see IMPORTANT SAFETY INFORMATION at the end of this presentation
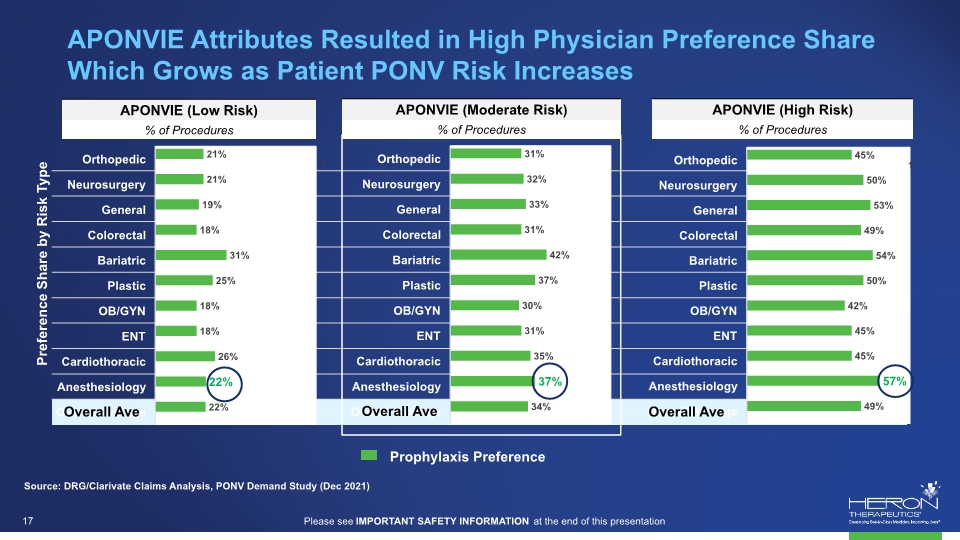
Source: DRG/Clarivate Claims Analysis, PONV Demand Study (Dec 2021) Preference Share by Risk Type APONVIE Attributes Resulted in High Physician Preference Share Which Grows as Patient PONV Risk Increases Overall Ave Overall Ave Overall Ave Please see IMPORTANT SAFETY INFORMATION at the end of this presentation 17 Aponvie (low risk) %of procedures aponvie (moderate risk) % of procedures aponvie (high risk) % of procedures Orthopedic 21% 31% 45% Neurosurgery 21% 32% 50% General 19% 33% 53% Colorectal 18% 31% 49% Bariatric 31% 42% 54% Plastic 25% 37% 50% Ob/gyn 18% 30% 42% Ent 18% 31% 45% Cardiothoracic 26% 35% 45% Anesthesiology 22% 37% 57% 22% 34% 49% Prophylaxis Preference
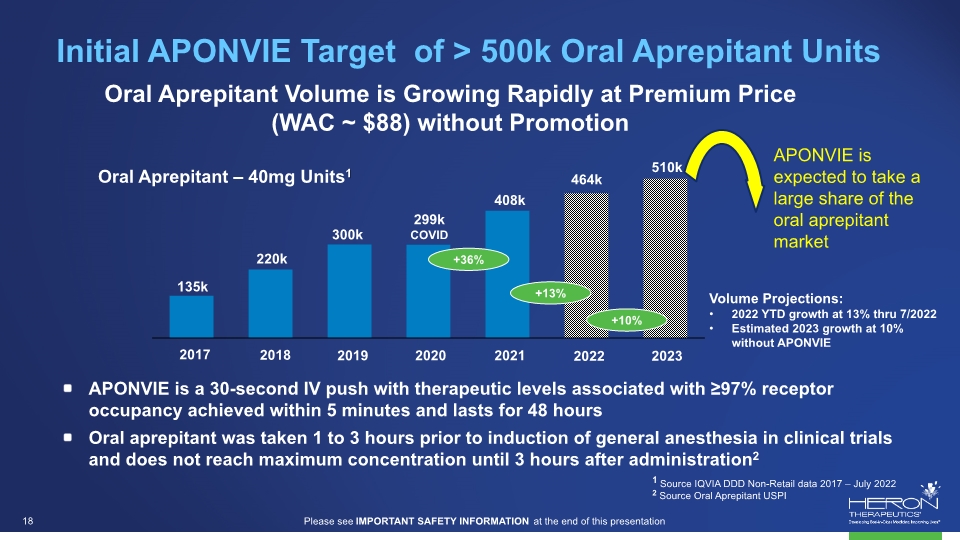
APONVIE is a 30-second IV push with therapeutic levels associated with ≥97% receptor occupancy achieved within 5 minutes and lasts for 48 hours Oral aprepitant was taken 1 to 3 hours prior to induction of general anesthesia in clinical trials and does not reach maximum concentration until 3 hours after administration2 Initial APONVIE Target of > 500k Oral Aprepitant Units 1 Source IQVIA DDD Non-Retail data 2017 – July 2022 2 Source Oral Aprepitant USPI 135k 2017 2022 2018 2020 2019 2021 2023 220k 300k 299k COVID 408k 464k 510k Oral Aprepitant – 40mg Units1 Volume Projections: 2022 YTD growth at 13% thru 7/2022 Estimated 2023 growth at 10% without APONVIE +10% +13% +36% Oral Aprepitant Volume is Growing Rapidly at Premium Price (WAC ~ $88) without Promotion APONVIE is expected to take a large share of the oral aprepitant market Please see IMPORTANT SAFETY INFORMATION at the end of this presentation 18

PONV is a large market opportunity with 36 million annual procedures in patients at moderate to high risk for PONV and ~12M high to moderate risk patients currently not receiving prophylaxis Significant Advantages with Unmet Needs Aprepitant is the most effective approved antiemetic, alone or in combination, for vomiting prevention1 Administered via a single 30-second IV push with rapid onset of action Safety profile comparable to ondansetron without QT prolongation Synergies with Heron commercial organization Rapid uptake converting oral aprepitant business based on more convenient IV formulation with much less variable absorption and faster onset of action Leverage existing CINVANTI manufacturing capabilities to meet COGS target Is the Ideal Strategic Fit for Heron 1. Weibel S, Rücker G, Eberhart LHJ, Pace NL, Hartl HM, Jordan OL, et al. Cochrane Database of Systematic Reviews. 2020 Please see IMPORTANT SAFETY INFORMATION at the end of this presentation 19

Establish Heron as a Leader in Acute Care References: 1. ZYNRELEF [package insert]. San Diego, CA: Heron Therapeutics Inc; 2021. 2. Viscusi E, Gimbel JS, Pollack RA, et al. Reg Anesth Pain Med. 2019;44(7):700-706. 3. Viscusi E, Minkowitz H, Winkle P, et al. Hernia. 2019;23(6):1071-1080. 4. APONVIE (package insert) The first and only extended-release, dual-acting local anesthetic (DALA), keeping more patients out of severe pain and opioid-free for 72 hours after surgery1-3 Portfolio of Two Best-in-Class Products Addressing Significant Unmet Needs The first and only IV NK1 approved with a rapid onset of action and demonstrated superiority to the standard-of-care for prevention of vomiting for 48 hours after surgery4 Please see IMPORTANT SAFETY INFORMATION at the end of this presentation 20

APONVIE should not be used: if you are allergic to aprepitant or any of the ingredients in APONVIE if you are taking pimozide APONVIE may cause serious side effects. Tell your doctor or nurse right away if you have any of these signs or symptoms of an allergic reaction: trouble breathing or swallowing, shortness of breath or wheezing swelling of your eyes, face, tongue, or throat flushing or redness of your face or skin hives, rash, or itching dizziness, a rapid or weak heartbeat, or you feel faint APONVIE may affect how other medicines work. Other medicines may affect how APONVIE works. Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements. If you take the blood-thinner medicine warfarin, your doctor may do blood tests after you receive APONVIE to check your blood clotting. Important Safety Information for Patients Please see IMPORTANT SAFETY INFORMATION at the end of this presentation 21

Women who use birth control medicines containing hormones to prevent pregnancy (birth control pills, skin patches, implants, and certain IUDs) should also use back-up methods of birth control (such as condoms and spermicides) for 1 month after receiving APONVIE. Before you receive APONVIE, tell your doctor if you are pregnant or plan to become pregnant. APONVIE contains alcohol and may harm your unborn baby. Before you receive APONVIE, tell your doctor if you are breast-feeding or plan to breastfeed because it is likely APONVIE passes into your milk, and it is not known if it can harm your baby. You and your doctor should decide if you will receive APONVIE, if breast-feeding. The most common side effects of APONVIE are constipation, low blood pressure, tiredness, and headache. Talk to your healthcare provider for medical advice about side effects. Report side effects to Heron at 1-844-437-6611 or to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. The information provided here is not comprehensive. Please see full Prescribing Information. Important Safety Information for Patients (cont.) Please see IMPORTANT SAFETY INFORMATION at the end of this presentation 22





















