- TEVA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
DEF 14A Filing
Teva Pharmaceutical Industries Limited (TEVA) DEF 14ADefinitive proxy
Filed: 16 Apr 24, 4:10pm
| ☐ | Preliminary Proxy Statement | ☐ | Confidential, For Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | |||
| ☒ | Definitive Proxy Statement | |||||
| ☐ | Definitive Additional Materials | |||||
| ☐ | Soliciting Material under §240.14a-12 |
| ☒ | No fee required. | |
| ☐ | Fee paid previously with preliminary materials. | |
| ☐ | Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and0-11. | |

Notice of 2024 Annual Meeting of Shareholders & Proxy Statement

Dr. Sol J. Barer Chairman of the Board of Directors “I am excited to continue working closely with my fellow Board members, our President and CEO, Richard Francis, our talented management team and dedicated employees. In 2024, we expect to see continued progress across our key innovative growth initiatives, while also executing on our high-value, complex generics business with new product launches, and achieving exciting clinical milestones for our late-stage pipeline assets.” To Our Valued Shareholders, On behalf of my fellow Directors, I want to thank you for your trust and investment in Teva. Our teams across the world are committed to our mission of advancing scientific innovation to help improve health outcomes for millions of patients around the world every day. We believe that everyone, everywhere should have access to quality medicines and this drives our growth priorities and strategic focus. 2023 Highlights and Pivot to Growth Strategy In May 2023, under the leadership of our new President and CEO Richard Francis, we introduced our new Pivot to Growth strategy, which is based on four key pillars: (i) delivering on our growth engines; (ii) stepping up innovation; (iii) sustaining our generics medicines powerhouse; and (iv) focusing our business. In 2023, Teva delivered strong financial and operating results, driven by continued growth of our innovative, biosimilar and generics medicines. Our new strategy is gaining momentum, signified by the following achievements: – Our key innovative medicines, AUSTEDO® and AJOVY®, continued to show promise for sustainable growth, with AUSTEDO revenues growing to $1.225 billion in 2023, and AJOVY now launched in most of the European countries and in Japan, with plans to launch in additional markets. – Our generics business continues to stabilize and has returned back to revenue growth. As part of the strategy, we entered into collaborations to accelerate our late-stage pipeline assets, and in January 2024, we announced that we intend to divest our API business to focus on our core business strengths and capital allocation towards growth engines and innovation. An Independent, Diverse and Experienced Board To effectively oversee Teva’s return to growth, we continue to have an active and ongoing Board refreshment process, as a result of which, we have added three new directors over the course of the last four years. This includes Prof. Varda Shalev, who joined the Board in September 2023 and enhances our Board’s expertise in science, medical informatics and health technology.

Last year, we adopted the Board’s Inclusion and Diversity statement, maintaining a Board composition that aligns with Teva’s diverse customer and employee base. We are therefore pleased with the nomination of Rosemary A. Crane, Gerald M. Lieberman, Prof. Ronit Satchi-Fainaro and Prof. Varda Shalev to continue to serve and contribute as members on our Board. We believe all nominees will complement the balance of our variety of backgrounds, qualifications, skills and experience on the Board to create new opportunities for Teva and drive long-term shareholder value. Sustainability - building a Healthy Future Increasing access to medicines is fundamental to our mission and we collaborate with partner organizations to get medicines to vulnerable populations who wouldn’t otherwise be able to obtain them. By the end of 2023, we had launched 7 of the 8 access programs we had committed to do so by 2025, with two new programs launched in 2023 in Chile and Spain. We also made strong progress on our 2025 access to medicine related Sustainability-Linked Bond commitments with respect to number of regulatory submissions made and supply of product volumes of essential medicines in low- and -middle income countries. As part of our commitment to sustainable growth, we have adopted many best-in-class governance practices and policies, including sustainable goals that improve our operational efficiency and advance our mission. Our renewed sustainability strategy Healthy Future includes new or enhanced commitments across six focus areas, including achieving net-zero green-house-gas emissions across our operations and value chain by 2045 and new targets focused on health systems strengthening and capacity building as well as patient centricity in clinical trials, amongst others. At Teva, we believe that inclusive, diverse workplace with equal opportunities drives innovation and supports growth. Continued dialogue with our shareholders I was pleased to have the opportunity to meet and speak directly with several of our investors, while our management team engaged with others. Our discussions focused on Teva’s Pivot to Growth strategy, our executive compensation program, our human capital management and our Healthy Future sustainability strategy. We look forward to continuing this important dialogue, as shareholder perspectives provide a critical input into the Board’s deliberations as we strive to grow Teva in a responsible and inclusive way. Looking ahead to 2024 As we look ahead, we remain focused on continued value creation, which we expect to be powered by our ongoing momentum. We are also doing so in the face of great adversity in our headquarters of Israel, and we remain deeply committed to our colleagues and their families as they continue to navigate the uncertainties of the war. I am excited to continue working closely with my fellow Board members, our President and CEO, Richard Francis, our talented management team and dedicated employees. In 2024, we expect to see continued progress across our key innovative growth initiatives, while also executing on our high-value, complex generics business with new product launches, and achieving exciting clinical milestones for our late-stage pipeline assets. On behalf of the entire Board, I would like to thank you for your continued confidence in Teva and your support. Thank you, Dr. Sol J. Barer Chairman of the Board of Directors April 16, 2024

Notice of 2024 Annual Meeting of Shareholders

The 2024 Annual Meeting of Shareholders (the “Annual Meeting”) of Teva Pharmaceutical Industries Limited (“we,” “us,” “our,” “Teva” or the “Company”) will be conducted in a virtual format through an online meeting platform. For further information, see “Questions and Answers” below.
In addition, shareholders will consider Teva’s annual consolidated financial statements for the year ended December 31, 2023.
Teva encourages all of its shareholders to review its annual report (“Annual Report”) on Form 10-K for the year ended December 31, 2023.
Only holders of ordinary shares (or American Depositary Shares representing such ordinary shares) of record at the close of business on April 30, 2024, will be entitled to vote at the Annual Meeting. Two holders of ordinary shares who are present at the Annual Meeting, in person or by proxy or represented by their authorized persons, and who hold in the aggregate twenty-five percent or more of such ordinary shares, shall constitute a legal quorum. Should no legal quorum be present one-half hour after the scheduled time, the Annual Meeting shall be adjourned to one week from that day, at the same time and place.
By Order of the Board of Directors,

Dov Bergwerk
Senior Vice President,
Company Secretary
April 16, 2024
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting to be Held on June 6, 2024
The accompanying Proxy Statement and our Annual Report are available at www.tevapharm.com/2024proxymaterials. We expect the proxy materials to be mailed and/or made available on or before April 19, 2024.
Table of Contents
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | i | |
Cautionary Note Regarding Forward-Looking Statements
This proxy statement contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are based on management’s current beliefs and expectations and are subject to substantial risks and uncertainties, both known and unknown, that could cause our future results, performance or achievements to differ significantly from that expressed or implied by such forward-looking statements. You can identify these forward-looking statements by the use of words such as “should,” “expect,” “anticipate,” “estimate,” “target,” “may,” “project,” “guidance,” “intend,” “plan,” “believe” and other words and terms of similar meaning and expression in connection with any discussion of future operating or financial performance. Risks that could cause actual results to differ from those expressed or implied in such forward-looking statements are set forth in our Annual Report on Form 10-K for the year ended December 31, 2023, including in the section captioned “Risk Factors,” and other documents we may subsequently file with the Securities and Exchange Commission. Forward-looking statements speak only as of the date on which they are made, and we assume no obligation to update or revise any forward-looking statements or other information contained herein, whether as a result of new information, future events or otherwise. You are cautioned not to put undue reliance on these forward-looking statements.
| ii | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
2023 Overview
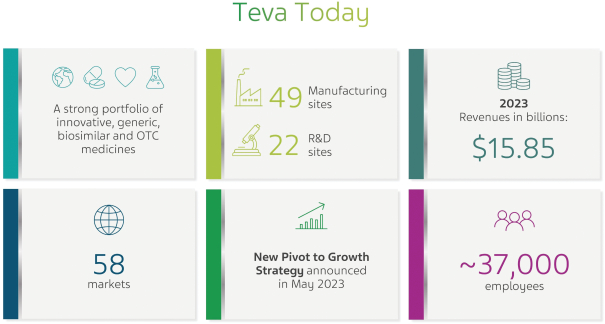
Teva is a global pharmaceutical leader with a category-defying portfolio, harnessing our generics expertise and stepping up innovation to continue the momentum behind the discovery, delivery, and expanded development of modern medicine. For over 120 years, Teva’s commitment to bettering health has never wavered. Today, the Company’s global network of capabilities enables its approximately 37,000 employees across 58 markets to push the boundaries of scientific innovation and deliver quality medicines to help improve health outcomes of millions of patients every day.
Our Pivot to Growth Strategy is Delivering
In May 2023, we introduced our new “Pivot to Growth” strategy, which is based on four key pillars: (i) delivering on our growth engines, mainly AUSTEDO®, AJOVY®, UZEDY® and our late-stage pipeline of biosimilars; (ii) stepping up innovation through delivering on our late-stage innovative pipeline assets as well as building up our early-stage pipeline organically and potentially through business development activities; (iii) sustaining our generics medicines powerhouse with a global commercial footprint, focused portfolio, pipeline and manufacturing footprint; and (iv) focusing our business by optimizing our portfolio and global manufacturing footprint to enable strategic capital deployment to accelerate our near and long-term growth engines and reorganizing certain of our business units to a more optimal structure, while also reorganizing key business units to enhance operational efficiency.

| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 1 | |
2023 Financial Results
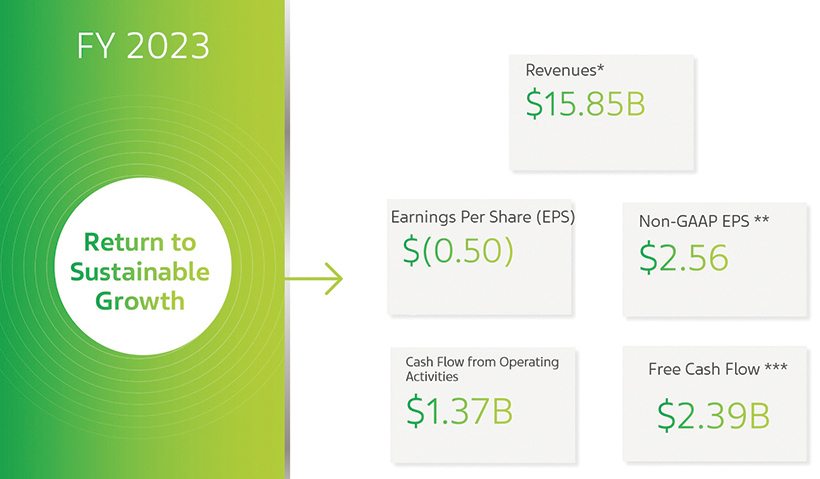
| * | 2023 financial data include the impact from an upfront payment received in connection with the collaboration on our anti-TL1A asset. |
| ** | For a reconciliation of non-GAAP EPS to GAAP EPS, see Appendix A hereto. |
| *** | Free cash flow includes cash flow from operating activities, beneficial interest collected in exchange for securitized accounts receivables, proceeds from divestitures of businesses and other assets, net of cash used for capital investment. For a reconciliation of free cash flow to cash flow from operating activities, see Appendix A hereto. |
| 2 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Key Products and Pipeline Updates
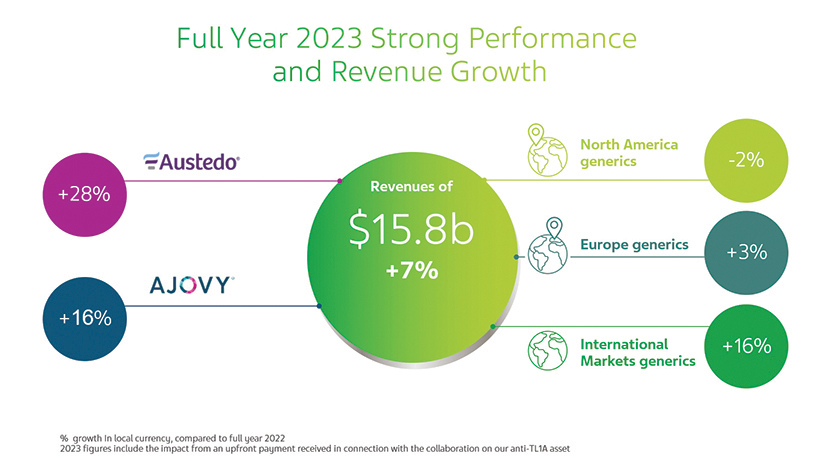
AUSTEDO (deutetrabenazine) Continued strong growth in the U.S. with revenues in 2023 increasing by 27% compared to 2022; launched in China and Israel in 2021 and in Brazil in 2022.
AUSTEDO XR (deutetrabenazine) extended-release tablets was approved by the FDA in February 2023, and became commercially available in the U.S. in May 2023. AUSTEDO XR is a new once-daily formulation, additional to the currently marketed twice-daily AUSTEDO.
|
AJOVY (fremanezumab-vfrm) injection: continued growth in all regions; 24.5% U.S. market share in terms of prescriptions in 2023; by the end of 2023, launched in most European countries and in certain countries in our International Markets, such as Japan, Australia, Israel, South Korea, Brazil and others; auto-injector launched in the U.S. and Canada.
|
UZEDY (risperidone) extended-release injectable suspension was approved by the FDA in April 2023 for the treatment of schizophrenia in adults and launched in the U.S. in May 2023; UZEDY is a subcutaneous, long-acting formulation of risperidone.
| ||||||
Generics Business Continued maintaining our top-three leadership position in many countries, including the U.S. and some key European markets; continued optimizing our global generics portfolio, with a focus on high-value generics; 2023 revenues in Europe and International Markets increased in local currency terms, compared to 2022.
|
Biosimilars Robust biosimilar pipeline, including 16 programs, of which seven are in-house and nine programs are in collaboration with strategic partners; five expected to launch by 2027. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 3 | |
Late-stage Pipeline
Olanzapine LAI (TEV-‘749) Potential to be the first long-acting olanzapine with favorable safety profile; Phase 3 trial results expected in the second half of 2024; in November 2023, we entered into a funding agreement with Royalty Pharma plc. to further accelerate the clinical research program for olanzapine LAI.
|
ICS/SABA (TEV-‘248) Currently in Phase 3 trial; potential to be first ICS/SABA for adult and pediatric indications; on April 1, 2024, we announced that we entered into a clinical collaboration agreement with Launch Therapeutics, Inc., and into a development funding agreement with Abingworth, to further accelerate the development of ICS-SABA (TEV-‘248).
|
Anti-TL1A (TEV-‘574) Potential to be best-in-class for proven TL1 mechanism in ulcerative colitis and Crohn’s disease; initial Phase 2 trial results expected in the second half of 2024; in October 2023, we entered into an exclusive collaboration with Sanofi to co-develop and co-commercialize our anti-TL1A asset.
| ||||||
Continuing to Reduce our Net Debt
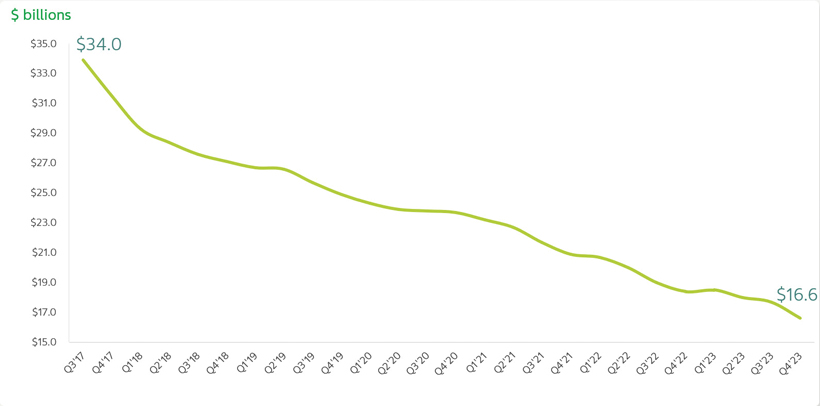
As of December 31, 2023, our total debt was $19,833 million, compared to $21,212 million as of December 31, 2022. As of December 31, 2023, our net debt was $16.6 billion compared to $18.4 billion as of December 31, 2022. In the past seven years, we have reduced our net debt by more than $17 billion.
| 4 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Board and Corporate Governance Highlights
Our Board of Directors (the “Board of Directors” or the “Board”) continually evaluates Teva’s corporate governance policies and practices, focusing on ensuring effective oversight of Teva’s business and management. We have established a strong and effective framework to monitor the risks of our business.
Board and Corporate Governance at a Glance
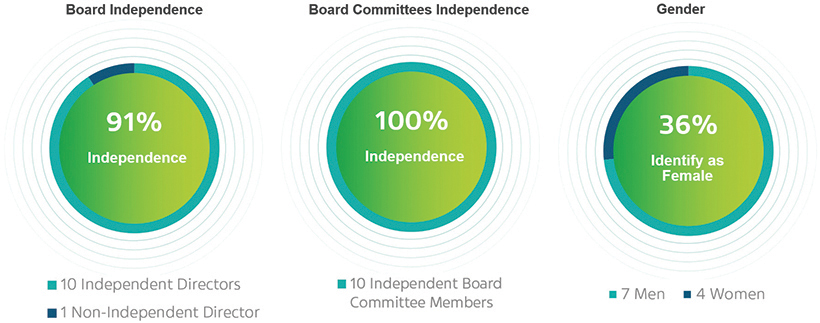
| - | Board refreshment and succession planning—three new directors appointed over the last four years |
| - | 10 out of 11 directors are independent |
| - | All members of our committees are independent |
| - | 36% of directors identify as female |
| - | Annual Board and committees evaluation process |
| - | In March 2023, the Board approved and adopted the “Board’s Inclusion and Diversity Statement” as part of the Company’s Statement of Corporate Governance Principles |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 5 | |
Board and Corporate Governance Highlights

Director Alignment with Shareholder Interests
| - | In 2023, the Board of Directors held 8 meetings with an average attendance rate of 98% |
| - | We maintain director stock ownership guidelines requiring stock ownership of five times the annual cash fee (excluding committees fees) paid to directors, which must be achieved within a certain timeframe |
| 6 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Board and Corporate Governance Highlights
Shareholder Engagement
| - | Active shareholder engagement efforts, led by our Chairman of the Board in late 2023 and early 2024. We invited shareholders to share their concerns regarding our executive compensation program as well as potential compensation arrangements tied to our Pivot to Growth strategy, our human capital management and sustainable growth strategy |
Our Sustainability Priorities and Accomplishments
| - | In 2023, Teva introduced our renewed sustainability strategy, “Healthy Future”—a continuation of our environmental, social and governance (“ESG”) journey |
| - | With findings from our 2023 materiality assessment and additional inputs, we defined eleven priority areas with the greatest potential for Teva to make an impact or to impact Teva, of which we have focused on the six we believe require the most attention to advance sustainability at Teva and create long-term value |
| - | Expanding upon our validated, Science Based Targets initiative (“SBTi”) near-term Scope 1, 2 and 3 greenhouse gas (“GHG”) emissions targets, we have stated our intention to achieve net zero emissions across our operations and value chain by 2045 |
| - | In March 2023, Teva issued $2.49 billion (equivalent) in aggregate principal amount of sustainability-linked bonds (“SLB”) |
| - | Our 2023 ‘Healthy Future’ sustainability report (expected to be published in May 2024) will outline our 2023 progress in each of our priority areas, while our ‘Healthy Future Disclosures’ report will include our actions and performance across a broader range of sustainability topics that we manage. We seek to continue and align our sustainability reporting with relevant Global Reporting Initiative (“GRI”) and Sustainability Accounting Standards Board (“SASB”) standards and the Task Force on Climate-Related Financial Disclosures (“TCFD”) framework |
| - | In 2023, we further tied executive compensation to sustainability goals; 25% of the annual cash bonus is based on individual performance against pre-established goals. As part of this component, there were goals tied to sustainability for all of our executives; goals were related to our sustainability strategy and delivering on our SLB and external commitments—access to medicines, regulatory submissions in low-middle income countries (“LMICs”) and reduction of Scope 1, 2 and 3 GHG emissions, among others |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 7 | |
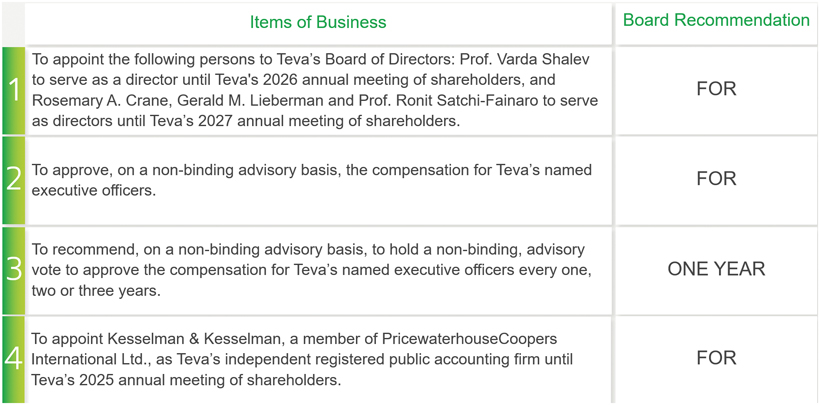
Proposal 1: Election of Directors
The election of highly qualified directors is fundamental to the Board’s successful oversight of Teva’s strategy and risks. We seek directors who add diverse perspectives, possess a variety of skills and provide global pharmaceutical experience and other qualifications. In March 2023, the Board approved and adopted the “Board’s Inclusion and Diversity Statement” (“Board I&D Statement”) as part of the Company’s Statement of Corporate Governance Principles, which is available on our website. The Board’s vision, as reflected in the Board I&D Statement, is that the Board is a reflection of the cultural and geographical breadth of our business, and therefore we should continuously strive to align the composition and diversity of our Board with the size and geographical spread of Teva, its broad product portfolio, history and culture. Additionally, a diverse Board with a range of views, skills and expertise enhances decision-making, which is beneficial to the Company’s success in the interests of generating value for all Teva’s stakeholders. The Board believes, as stipulated in the Board I&D Statement, that Teva’s directors should ultimately be selected based on wide-ranging experiences, backgrounds, skills, knowledge, and insight, while also considering the gender, ethnicity, nationality, and cultural diversity of its members. The process of selecting directors is based on objective criteria without discrimination and focuses on a candidate’s ability to perform as a director.
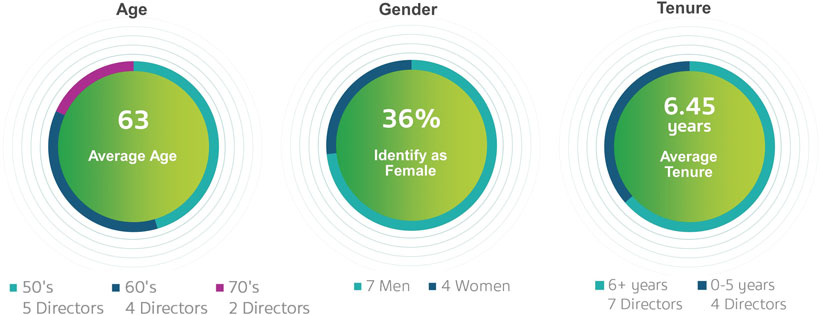
Currently, and subject to the approval by shareholders of this Proposal 1, the average tenure of our directors is 6.45 years of service, the average age is 63 and we have four directors who identify as female out of 11 members serving on our Board of Directors.
Dr. Barer, our Chairman of the Board, is an independent director under NYSE regulations. Richard D. Francis, our President and Chief Executive Officer (the “President and CEO”) serves on the Board, which facilitates collaboration between the Board of Directors and management. Corporate governance remains a high priority and we continue to evaluate the size, composition and average tenure of directors on the Board to ensure that it maintains dynamic and exceptionally qualified leadership.
Our directors are generally elected in three classes for terms of approximately three years. Due to the complexity of our businesses and our extensive global activities, we value the insight and familiarity with our operations that a director is able to develop over his or her service on the Board of Directors. Following the recommendation of our Corporate Governance and Nominating Committee, the Board of Directors recommends that shareholders approve the appointment of Prof. Varda Shalev to serve as a director until Teva’s 2026 annual meeting of shareholders, and Rosemary A. Crane, Gerald M. Lieberman and Prof. Ronit Satchi-Fainaro to serve as directors until our 2027 annual meeting of shareholders, to maintain as equal as possible classes of directors per each term. All nominees are currently members of the Board of Directors and qualify as independent directors under NYSE regulations.
In accordance with the Israeli Companies law, 5759-1999 (as amended from time to time, the “Israeli Companies Law”), all nominees for election as directors at the Annual Meeting have declared in writing that they possess the requisite skills and expertise, as well as sufficient time, to perform their duties as directors.

| 8 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Proposal 1: Election of Directors
Directors
The following table sets forth information regarding the directors and director nominees of Teva, as of April 1, 2024:
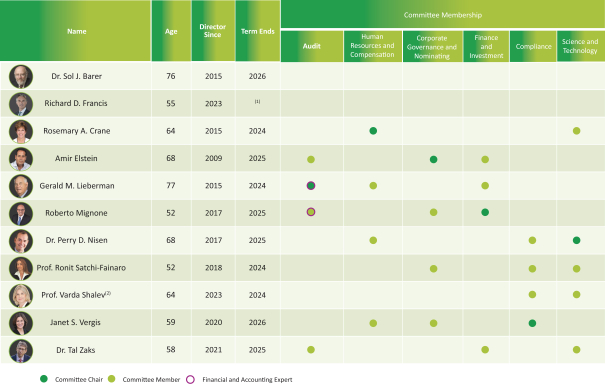
| (1) | Mr. Francis’s term ends contemporaneously with his term as President and CEO. |
| (2) | Prof. Shalev was appointed effective September 2023 by the Board to serve as a director until the Annual Meeting, where her nomination will be presented to shareholders for approval. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 9 | |
Proposal 1: Election of Directors
Persons Being Considered for Election at the Annual Meeting
Rosemary A. Crane Independent Director
Committees: – Human Resources and Compensation (Chair) – Science and Technology | Ms. Crane joined the Board of Directors in 2015. Ms. Crane served as President and Chief Executive Officer of MELA Sciences, Inc. from 2013 to 2014. Ms. Crane was Head of Commercialization and a partner at Appletree Partners from 2011 to 2013. From 2008 to 2011, she served as President and Chief Executive Officer of Epocrates Inc. Ms. Crane served in various senior executive positions at Johnson & Johnson from 2002 to 2008, including as Group Chairman, OTC & Nutritional Group from 2006 to 2008, as Group Chairman, Consumer, Specialty Pharmaceuticals and Nutritionals from 2004 to 2006, and as Executive Vice President of Global Marketing for the Pharmaceutical Group from 2002 to 2004. Prior to that, she held various positions at Bristol-Myers Squibb from 1982 to 2002, including as President of U.S. Primary Care from 2000 to 2002 and as President of Global Marketing and Consumer Products from 1998 to 2000. Ms. Crane has served on the board of directors of Tarsus Pharmaceuticals, Inc. since August 2021 and on the board of directors of Certara, Inc. since July 2022. From 2018 to 2023, she served on the board of directors of Catalent Pharma Solutions, Inc., from 2015 to 2019, she served as Vice Chairman of the Board of Zealand Pharma A/S and from 2017 to 2019, she served on the board of directors of Edge Therapeutics. Ms. Crane received a B.A. in communications and English from the State University of New York and an M.B.A. from Kent State University.
| |
Qualifications:
With over 30 years of experience in commercialization and business operations, primarily in the pharmaceutical and healthcare industries, and more than 25 years of therapeutic and consumer drug launch expertise, Ms. Crane provides broad experience and knowledge of the global pharmaceutical business and industry.
|
Gerald M. Lieberman Independent Director
Committees: – Audit (Chair) – Human Resources and Compensation – Finance and Investment | Mr. Lieberman joined the Board of Directors in 2015. Mr. Lieberman is currently a special advisor at Reverence Capital Partners, a private investment firm focused on the middle-market financial services industry. From 2000 to 2009, Mr. Lieberman was an executive at AllianceBernstein L.P., where he served as President and Chief Operating Officer from 2004 to 2009, as Chief Operating Officer from 2003 to 2004 and as Executive Vice President, Finance and Operations from 2000 to 2003. From 1998 to 2000, he served as Senior Vice President, Finance and Administration at Sanford C. Bernstein & Co., Inc., until it was acquired by Alliance Capital in 2000, forming AllianceBernstein L.P. Prior to that, he served in various executive positions at Fidelity Investments and at Citicorp. Prior to joining Citicorp, he was a certified public accountant with Arthur Andersen. Mr. Lieberman has served as Chairman of the board of directors of EnteraBio Ltd. since 2018. He previously served on the board of directors of Forest Laboratories, LLC from 2011 to 2014, Computershare Ltd. from 2010 to 2012 and AllianceBernstein L.P. from 2004 to 2009. Mr. Lieberman received a B.S. Beta Gamma Sigma with honors in business from the University of Connecticut.
| |
Qualifications:
With his many years of experience as an executive in leading financial services companies and capital markets, including his knowledge and experience in human capital development, succession planning and compensation, Mr. Lieberman provides finance, risk management, operating and human capital expertise for large, complex organizations.
|
| 10 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Proposal 1: Election of Directors
Prof. Ronit Satchi-Fainaro Independent Director
Committees: – Science and Technology – Compliance – Corporate Governance and Nominating | Prof. Satchi-Fainaro joined the Board of Directors in 2018. Prof. Satchi-Fainaro is a Full Professor at Tel Aviv University, where she has served as Head of the Cancer Research and Nanomedicine Laboratory since 2006, Chair of the Department of Physiology and Pharmacology at the Sackler Faculty of Medicine since 2014, The Kurt and Herman Lion Chair in Nanosciences and Nanotechnologies since 2017, Director of the Cancer Biology Research Center since 2020 and a member of the Preclinical Dean’s Committee since 2015. In 2003, she was appointed Instructor in Surgery at Children’s Hospital in Boston and Harvard Medical School, where she has been a Visiting Professor since 2005. Prof. Satchi-Fainaro also serves as a consultant to several biotech and pharmaceutical companies, and is a member of the scientific advisory board of the Blavatnik Center for Drug Discovery, The Israel Cancer Association and Vall d’Hebron University Hospital Foundation—Research Institute. Since December 2023 she is a member of the CAS Life Sciences Advisory Board, a division of the American Chemical Society. She is also a member of several editorial boards of scientific journals. Prof. Satchi-Fainaro received a B.Pharm. from the Hebrew University in Jerusalem in 1995 and a Ph.D. in Polymer Chemistry and Cancer Nanomedicine from the University of London in 1999. She spent two years as a postdoctoral research fellow on biochemistry and protein delivery at Tel Aviv University and two years as a postdoctoral research fellow on vascular and cancer biology at Harvard University and Children’s Hospital in Boston.
| |
Qualifications:
With extensive experience in clinical medicine and multidisciplinary research, Prof. Satchi-Fainaro provides the Board with in-depth knowledge of medicine and science, combined with an expertise in the pharmaceutical industry and experience in academia.
|
Prof. Varda Shalev Independent Director
Committees: – Compliance – Science and Technology | Prof. Varda Shalev joined the Board of Directors in September 2023. Prof. Shalev has been a Managing Partner at Team8 Health since 2022, and a Co-Founder and Advisory Board Member at Alike.Health since 2020. She also serves as a Full Professor of Epidemiology at the Tel Aviv University School of Public Health. In the past two decades, Prof. Shalev served in various senior executive roles at Maccabi Health Services, including most recently until 2020 as Head of the Research and Innovation Institute. Prof. Shalev has served on the board of directors of BATM Advanced Communications Ltd. since 2018. From 2021 to 2023, she served on the board of directors of Pluristem Therapeutics, Inc., and from 2022 to 2023 on the board of directors of Novolog Pharma Up 1996 Ltd. Prof. Shalev received her M.D. from the School of Medicine at Ben-Gurion University of the Negev, a Master of Public Health Administration from Clark University in the United States, and completed her post-doctoral fellowship in Medical Informatics at Johns Hopkins University.
| |
Qualifications:
With over 30 years of hands-on experience in primary care, epidemiology, medical informatics, digital health investing, and medical research, Prof. Varda Shalev brings unique insights, expertise, and experience in healthcare technologies and innovation to the Board.
|
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 11 | |
Proposal 1: Election of Directors
Continuing Directors
Dr. Sol J. Barer Chairman of the Board Independent Director | Dr. Barer became Chairman of the Board of Directors in 2017, after joining Teva’s Board of Directors in January 2015. Dr. Barer is Managing Partner at SJ Barer Consulting. He also serves as an advisor to the Israel Biotech Fund. From 1987 to 2011, he served in top leadership roles at Celgene Corporation, including as Executive Chairman from 2010 to 2011, Chairman and CEO from 2007 to 2010, CEO from 2006 to 2010, President and Chief Operating Officer from 1994 to 2006 and President from 1993 to 1994. Prior to that, he was a founder of the biotechnology group at the chemical company Celanese Corporation, which was later spun off as Celgene. Dr. Barer has served as Chairman of the board of directors of NexImmune, Inc. since 2019, which became a public company in February 2021. From 2011 to 2023, he served on as lead independent director on the board of directors of Contrafect, from 2020 to 2021, on the Board of Directors of Cerecor, Inc. (formerly Aevi Genomic Medicine, Inc.), from 2013 to 2019 he served as Chairman of the Board of Edge Therapeutics, from 2011 to 2016 he served on the board of Aegerion Pharmaceuticals, from 2009 to 2017 on the board of Amicus Therapeutics, and from 2011 to 2017 he served as Chairman of the Board of InspireMD. Dr. Barer is Founding Chair of the Center for Innovation and Discovery at the Hackensack Meridian Medical School. He received his Ph.D. in organic and physical chemistry from Rutgers University and his B.S. in chemistry from Brooklyn College of the City University of New York.
| |
Qualifications:
With his long career as a senior pharmaceutical executive and leadership roles in various biopharmaceutical companies, Dr. Barer provides broad and experienced knowledge of complex global pharmaceutical business and industry and global regulatory regimes, as well as extensive scientific expertise.
|
Richard D. Francis Director and President and Chief Executive Officer | Mr. Francis became Teva’s President and Chief Executive Officer and a member of the Board of Directors in January 2023. Prior to joining Teva, Mr. Francis served as the Chief Executive Officer of Purespring Therapeutics, a pioneering gene therapy company focused on transforming the treatment of kidney diseases, from 2021 to 2022, and as the Chief Executive Officer of Forcefield Therapeutics, a pioneer of best-in-class therapeutics to protect heart function, from 2021 to 2022. He also served as an operating partner for Syncona Investment Management Limited since 2021. From 2014 to 2019, Mr. Francis served as Chief Executive Officer of Sandoz and a member of the executive team of Novartis. Prior to his role at Sandoz, Mr. Francis was a senior executive at Biogen for 13 years, where he held a number of senior roles, including leading Biogen’s U.S. business. Mr. Francis also serves as a member of the board of directors of Mettler-Toledo International Inc. since 2016. He holds a Bachelor of Arts in Economics from The Manchester Metropolitan University.
| |
Qualifications:
With over two and a half decades of leadership positions in various pharmaceutical companies and a proven track-record in the pharmaceutical, biotech and generics sectors, Mr. Francis provides an entrepreneurial, pragmatic and unique global perspective on the healthcare and pharmaceutical industries.
|
| 12 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Proposal 1: Election of Directors
Amir Elstein Independent Director
Committees: – Corporate Governance and Nominating (Chair) – Audit – Finance and Investment | Mr. Elstein rejoined the Board of Directors in 2009. From January 2014 to July 2014, he served as Vice Chairman of the Board of Directors of Teva. Mr. Elstein has served as Chairman of the Board of Tower Semiconductor Ltd. since 2009 and Chairman of the Israel Democracy Institute since 2012. Mr. Elstein also serves as Chairman or as a member of the board of directors of several academic, scientific, educational, social and cultural institutions. Mr. Elstein served as Chairman of the Board of Governors of the Jerusalem College of Engineering from 2009 to 2018 and as Chairman of the Board of Directors of Israel Corporation from 2010 to 2013. From 2004 to 2008, Mr. Elstein was a member of Teva’s senior management, most recently as Executive Vice President, Global Pharmaceutical Resources. From 1995 to 2004, Mr. Elstein served on Teva’s Board of Directors. Prior to joining Teva as an executive in 2004, Mr. Elstein held a number of executive positions at Intel Corporation, most recently as General Manager of Intel Electronics Ltd., an Israeli subsidiary of Intel Corporation. Mr. Elstein received a B.Sc. in physics and mathematics from the Hebrew University in Jerusalem, an M.Sc. in solid state physics from the Hebrew University and a diploma in senior business management from the Hebrew University.
| |
Qualifications:
With leadership positions in various international corporations, including his experience as chairman of international public companies and his service as an executive officer at Teva and other companies, Mr. Elstein provides global business management and pharmaceutical expertise.
|
Roberto A. Mignone Independent Director
Committees: – Finance and Investment (Chair) – Audit – Corporate Governance and Nominating | Mr. Mignone joined the Board of Directors in 2017. Mr. Mignone is the Founder and Managing Partner of Bridger Management LLC, a multi-billion-dollar investment management firm founded in 2000 and specializing in long-term equity strategies. Since inception, Bridger Management has focused on the healthcare sector and has developed considerable research expertise in support of its investments. In addition to healthcare, Bridger Management invests in global consumer, technology and financial services companies. Prior to Bridger Management, Mr. Mignone co-founded and served as a partner of Blue Ridge Capital LLC from 1996 to 2000, an investment management firm specialized in health care, technology, media, telecommunications and financial services. Mr. Mignone serves as a co-Vice Chairman and member of the Finance Committee and Nominating Committee of the New York University Langone Medical Center. He received a Bachelor of Arts degree in Classics from Harvard College and an M.B.A. from Harvard Business School.
| |
Qualifications:
With his long career as a global investment professional focused on healthcare, Mr. Mignone provides the Board with finance and management expertise with respect to large, complex pharmaceutical organizations.
|
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 13 | |
Proposal 1: Election of Directors
Dr. Perry D. Nisen Independent Director
Committees: – Science and Technology (Chair) – Compliance – Human Resources and Compensation | Dr. Nisen joined the Board of Directors in 2017. In July 2021, he became Chief Executive Officer of Quanta Therapeutics Inc., a privately held biotechnology company. In 2018, he joined Soffinova Investments as Executive Partner, Private Equity and transitioned in January 2021 to a consultant role. From 2014 to 2017, Dr. Nisen served as Chief Executive Officer and the Donald Bren Chief Executive Chair of Sanford Burnham Prebys Medical Discovery Institute. From 2004 to 2014, Dr. Nisen held various roles at GlaxoSmithKline, most recently as Senior Vice President, Science and Innovation. From 1997 to 2004, Dr. Nisen served as Divisional Vice President, Global Oncology Development, and as Divisional Vice President, Cancer Research, at Abbott Laboratories. Previously, he was the Lowe Foundation Professor of Neuro-Oncology at the University of Texas Southwestern Medical Center. From 2016 to 2017, Dr. Nisen served as a director of Mirna Therapeutics, Inc. He received a B.S. from Stanford University, a Master’s degree in molecular biology, and an M.D. and PhD from Albert Einstein College of Medicine.
| |
Qualifications:
With extensive experience in medical research and development and management positions in leading pharmaceutical companies, Dr. Nisen provides a unique perspective on business and R&D activities.
|
Janet S. Vergis Independent Director
Committees: – Compliance (Chair) – Human Resources and Compensation – Corporate Governance and Nominating | Ms. Vergis joined the Board of Directors in 2020. She served as a retained executive advisor to various private equity firms from 2013 to 2019. From 2011 to 2012, she served as the Chief Executive Officer of OraPharma, Inc., a specialty pharmaceutical company. From 2004 to 2009, she served as President of Janssen Pharmaceuticals LP, McNeil Pediatrics, Inc. and Ortho-McNeil Neurologics, Inc., subsidiaries of Johnson & Johnson. Ms. Vergis contributed to a number of Johnson & Johnson companies during her career, holding positions of increasing responsibility in research and development, new product development, sales and marketing. Ms. Vergis has served on the board of directors of Church and Dwight Co., Inc. since 2014, Dentsply-Sirona, Inc. since 2019 and SGS SA since March 2021. She previously served on the board of directors of MedDay Pharmaceuticals from 2016 to 2021, Amneal Pharmaceutical from 2015 to 2019, as well as Lumara Health from 2013 to 2014 and OraPharma, Inc. from 2011 to 2012. Ms. Vergis received a Bachelor of Science in Biology and a Master’s of Science in Physiology from The Pennsylvania State University.
| |
Qualifications:
With over 30 years of experience in various fields of the healthcare industry, including research and development, new product development, sales, and various executive roles, as well as her experience as a board member of public pharmaceutical companies, Ms. Vergis provides the Board with broad global business experience in the pharmaceutical industry.
|
| 14 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Proposal 1: Election of Directors
Dr. Tal Zaks Independent Director
Committees: – Audit – Finance and Investment – Science and Technology | Dr. Zaks joined the Board of Directors in 2021. Dr. Zaks, M.D., Ph.D., is a partner at OrbiMed Advisors LLC since November 2021. From 2015 to September 2021, he served as Chief Medical Officer of Moderna, Inc. From 2010 to 2015, he held senior development positions at Sanofi, including Senior Vice President and Head of Global Oncology. From 2008 to 2010, he served as Vice President of Clinical Research, Oncology at Cephalon. From 2004 to 2008, he served as Director, Clinical Development and Translational Medicine at GlaxoSmithKline. From 1996-1999, he was a Postdoctoral Fellow at the National Cancer Institute. Dr. Zaks has also served as an adjunct Associate Professor of Medicine at the University of Pennsylvania since 2004 and as an adjunct Associate Professor of Medicine at Tufts Medical Center since 2017. From 2016 to 2023, Dr. Zaks served on the Board of Directors of Adaptimmune Therapeutics plc. Dr. Zaks received his M.D. and Ph.D. from Ben Gurion University in Israel, conducted post-doctoral research at the U.S. National Institutes of Health and completed clinical training in internal medicine at Temple University Hospital, followed by a fellowship in medical oncology at the University of Pennsylvania. He has also been awarded Ph.D. honoris causa from Bar-Ilan University.
| |
Qualifications:
With a unique combination of medical training, broad academic knowledge and executive experience in the biopharmaceutical industry, Dr. Zaks’s insights and experience in biopharmaceutical development, through his executive and non-executive roles, provide the Board with a broad scientific perspective and understanding of pharmaceutical product development, science and technology.
|
Family Relationships
There are no family relationships among any of our executive officers, directors or director nominees.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 15 | |
Corporate Governance and Director Compensation
Pursuant to our Articles of Association, the Board of Directors must consist of three to 18 directors (which includes our President and CEO, if serving as a member of the Board, and two statutory independent directors, if such are appointed in accordance with the Israeli Companies Law, and in the event the CEO and/or the statutory independent directors are not appointed to the Board of Directors, such maximum number of directors shall be reduced accordingly). Our Board of Directors consists of 11 persons, including our President and CEO. The Board of Directors has determined that all of the directors are independent, except for Richard D. Francis, our President and CEO.
We currently maintain a policy to have at least two directors qualify as financial and accounting experts under Israeli law. Accordingly, the Board of Directors has determined that Gerald M. Lieberman and Roberto A. Mignone are financial and accounting experts under such criteria.
Our directors are generally entitled to review and retain copies of our documentation and examine our assets, as required to perform their duties as directors and to receive assistance, in special cases, from outside experts at our expense.
Board Diversity and Skills*
Over the course of 2023, inclusion and diversity was a point of emphasis for our Board and our management team. The Board I&D Statement, which was adopted by the Board in March 2023, emphasizes that:
| - | Teva will seek to maintain a diverse Board with a range of views, skills and expertise, enhances decision-making, which is beneficial to the Company’s success in the interest of generating value for all Teva’s stakeholders; |
| - | Teva’s directors should ultimately be selected based on wide-ranging experiences, backgrounds, skills, knowledge, and insight with consideration of gender, ethnicity, nationality and cultural diversity of its members; |
| - | The process of selecting directors is based on objective criteria without discrimination and focuses on a candidate’s ability to perform as a director. |
Teva believes that inclusion and diversity are essential to its ability to innovate and grow its business. It is our desire to create and sustain an inclusive and diverse work environment. In its search for suitable candidates, our Board recognizes the value of overall diversity and considers members’ and candidates’ opinions, perspectives, personal and professional experiences, and backgrounds, including gender, race and ethnicity.
| * | Currently, and subject to the approval of shareholders of Proposal 1. |
| 16 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Corporate Governance and Director Compensation
The chart below summarizes the notable skills, qualifications and experience of each of our directors and director nominees (in addition to requisite skills and expertise to perform their duties as directors) and highlights the balanced mix of skills, qualifications and experience of the Board as a whole. These are the same attributes that the Board considers as part of its ongoing director succession planning process. This high-level summary is not intended to be an exhaustive list of each director’s and director nominee’s skills or contributions to the Board.
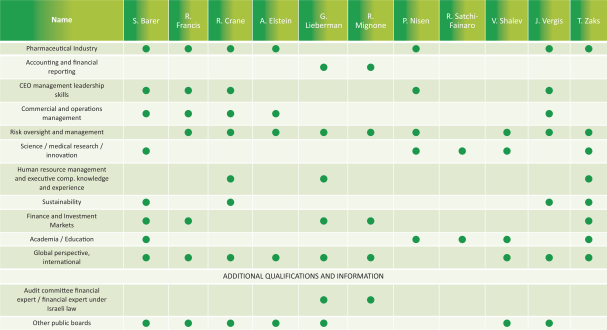
Board Practices
Director Terms and Education. Our directors are generally elected in three classes for terms of approximately three years. Due to the complexity of our businesses and our extensive global activities, we value the insight and familiarity with our operations that a director is able to develop over his or her service on the Board of Directors. Therefore, we believe that extended service on our Board enhances a director’s ability to make significant contributions to Teva, and we do not believe that arbitrary term limits on directors’ service are appropriate. At the same time, it is the policy of the Board that directors should not expect to be renominated automatically.
In recent years, we strengthened our Board of Directors with the addition of new highly qualified and talented directors, adding expertise as well as diversity to our Board of Directors. Currently, and subject to the approval of shareholders of Proposal 1, the average tenure of our directors is 6.45 years of service, the average age is 63 and we have four directors who identify as female out of 11 members serving on our Board of Directors. Our Chairman of the Board is independent under NYSE regulations, and 10 out of 11 of our directors are independent under NYSE regulations. Our only non-independent director is our President and CEO, which facilitates collaboration between the Board of Directors and management. We continue to evaluate the size, composition and tenure on the Board of Directors to ensure it maintains dynamic, exceptionally qualified members.
We provide an orientation program and a continuing education process for our directors, which include business and industry briefings, provision of materials, sessions from leading experts and professionals, meetings with key management and visits to Teva facilities. We evaluate and improve our education and orientation programs to ensure that our directors have the knowledge and background needed for them to best perform their duties. For example, in 2023, the Board held an education session with leading academic experts.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 17 | |
Corporate Governance and Director Compensation
Board Meetings. The Board of Directors holds at least six meetings each year to review significant developments affecting Teva, and to consider matters requiring approval of the Board, with additional meetings scheduled when important matters require Board of Directors action between scheduled meetings. Members of senior management regularly attend Board meetings to report on and discuss their areas of responsibility. Information regarding the number of Board committee meetings and attendance rates for 2023 is presented in the table below under “—Committee Composition and Board and Committee Attendance in 2023.”
Executive Sessions of the Board. Our directors meet in executive session (i.e., without the presence of management, including our President and CEO) generally in connection with each regularly scheduled Board meeting and additionally as needed. Executive sessions are chaired by Dr. Barer, the Chairman of the Board.
Annual Meetings. We do not have a formal policy requiring members of the Board to attend our annual meetings, although all directors are strongly encouraged to attend. Eight directors attended our 2023 annual meeting of shareholders (out of ten directors serving at the time of the 2023 annual meeting).
Board Leadership. The Board of Directors recognizes that one of its key responsibilities is to establish and evaluate an appropriate leadership structure for the Board of Directors so as to provide effective oversight of management. The Board of Directors has separate roles for the Chief Executive Officer and Chairman of the Board of Directors, with Dr. Barer serving as independent Chairman and Mr. Richard D. Francis as President and CEO. Dr. Barer’s long career as a senior pharmaceutical executive and leadership roles in various biopharmaceutical companies, as well as his extensive scientific expertise and knowledge of the global pharmaceutical business, have made him an invaluable resource to both the Board of Directors and the Chief Executive Officer. The Board of Directors has determined that this leadership structure is appropriate for Teva at this time because it ensures that the appropriate level of oversight, independence and responsibility is applied to all Board decisions.
Board of Directors Role in Risk Oversight. Management is responsible for assessing and managing risk, subject to oversight by the Board of Directors. Our annual risk assessment process includes both a top-down review of strategic risks and a bottom-up review of operational risks, which are presented to the Board of Directors. The Board of Directors fulfills its oversight responsibility for risk assessment and management by reviewing risk management policies and the risk appetite of our operations and business strategy, while its committees, as appropriate, monitor and address risks that may be within the scope of a particular committee’s expertise or charter, as described below. Each committee provides regular updates to the full Board regarding its activities.
| - | The Board oversees our risk management policies and risk appetite, including operational risks and risks relating to our business strategy and transactions. Various committees of the Board assist the Board in this oversight responsibility in their respective areas of expertise. |
| - | The Audit Committee assists the Board with the oversight of our financial reporting, independent auditors, internal controls, internal audit function and cybersecurity risks, processes and procedures. It is charged with identifying any flaws in business management and recommending remedies, detecting fraud risks and implementing anti-fraud measures. The Audit Committee further discusses our policies with respect to risk assessment and management regarding financial reporting, cybersecurity risks and other material risks. |
| - | The Compliance Committee oversees our policies and practices for legal, regulatory and internal compliance (other than regarding financial reporting), our strategy regarding sustainability matters and reviews policies and practices that may seriously impact our reputation. |
| - | The Finance and Investment Committee reviews our financial risk management policies, including our investment guidelines, financings and foreign exchange and currency hedging, as well as financial risk of certain transactions. |
| - | The HR and Compensation Committee oversees compensation, retention, succession and other human resources-related issues and risks. |
| 18 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Corporate Governance and Director Compensation
| - | The Science and Technology Committee oversees risks relating to our intellectual property and research and development activities. |
| - | The Corporate Governance and Nominating Committee oversees risks relating to our governance policies and initiatives. |
Cybersecurity Risk Management. The Audit Committee assists the Board with the oversight of cybersecurity risks. As part of its overall risk oversight function, the Audit Committee oversees risks associated with our information systems and technology, including cybersecurity, reviews our cyber risk assessment and management policies and receives updates from our Chief Information Officer and Chief Information Security Officer relating to Teva’s information security and technology risks, including cybersecurity risks. During 2023, the Audit Committee received regular briefings on Teva’s information security and risk management programs, including with respect to cyber security, global cyber threat trends and statistics, risks related to the increasing use of artificial intelligence (“AI”) technologies and AI tools that can assist the Company, as well as on Teva’s related policies, processes and practices in place for managing and mitigating cybersecurity incidents and other technology-related risks. Additionally, during 2023, the Board received dedicated cybersecurity training and performed an exercise tabletop relating to potential cybersecurity risks. Teva’s Chief Information Security Officer leads our cybersecurity risk management program and manages ongoing cybersecurity risk management activities through our information security office, which is responsible for the day-to-day identification, monitoring and management of cybersecurity risks. Teva’s information security office leads our cybersecurity risk management program. We also maintain cyber risk insurance coverage. For more information on our cybersecurity risk management program, see “Item 1C—Cybersecurity” in our Annual Report on Form 10-K for the year ended December 31, 2023.
Director Service Contracts. Except for equity awards that accelerate upon termination, we do not have any contracts with any of our non-employee directors that provide for benefits upon termination of services. Information regarding director compensation can be found under “Non-Employee Director Compensation” below.
Communications with the Board. Shareholders, employees and other interested parties can contact any director or committee of the Board of Directors by writing to them care of Teva Pharmaceutical Industries Ltd., 124 Dvora HaNevi’a Street, Tel Aviv, 6944020, Israel, Attn: Company Secretary or Internal Auditor, or by email to TevaIR@tevapharm.com. Comments or complaints relating to our accounting, internal controls or auditing matters may also be referred to members of the Audit Committee, as well as other appropriate Teva departments. The Board of Directors has adopted a global “whistleblower” policy, which provides employees and others with an anonymous means of communicating with the Audit Committee.
Nominees for Directors. Pursuant to the Israeli Companies Law, a nominee for service as a director must submit a declaration to us, prior to his or her election, specifying that he or she has the requisite qualifications to serve as a director and the ability to devote the appropriate time to performing his or her duties as such and that he or she is not restricted from serving as director under the Israeli Companies Law.
All of our directors, including those nominated for appointment as directors at the Annual Meeting, have provided such declaration. A director who ceases to meet the statutory requirements to serve as a director must notify us to that effect immediately and his or her service as a director will terminate upon submission of such notice.
Our Board of Directors believes that it should be composed of directors with diverse, complementary backgrounds and that directors should, at a minimum, exhibit proven leadership capabilities and possess experience at a high level of responsibility within their chosen fields. When considering a candidate for director, our Corporate Governance and Nominating Committee considers whether the directors, both individually and collectively, can and do provide the experience, judgment, commitment, skills and
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 19 | |
Corporate Governance and Director Compensation
expertise appropriate to lead Teva in the context of its industry. In addition, our Corporate Governance and Nominating Committee considers a nominee’s expected contribution to the diversity of skills, background, experiences and perspectives, as well as whether such nominee could provide added value to any of the committees of the Board of Directors, given the then existing composition of the Board of Directors as a whole. When seeking new candidates, the Corporate Governance and Nominating Committee also considers candidates representing a diversity of backgrounds, perspectives, ethnicities, races, genders, nationalities, and cultures. Our Corporate Governance and Nominating Committee also provides input and guidance regarding the independence of directors, for formal review and approval by our Board of Directors.
When seeking candidates for directorships, our Corporate Governance and Nominating Committee may solicit suggestions from incumbent directors, management, shareholders and others. Additionally, the Board of Directors has in the past used and may continue to use the services of third-party search firms to assist in the identification and analysis of appropriate candidates. After conducting an initial evaluation of a prospective candidate, members of the Board of Directors will interview that candidate if they believe the candidate may be suitable. The Chairman of the Board of Directors may also ask the candidate to meet with certain members of executive management.
If our Corporate Governance and Nominating Committee believes a director should be re-elected or a candidate would be a valuable addition to the Board of Directors, it may recommend to the Board of Directors that candidate’s appointment or re-election, who, in turn, can submit the candidate for consideration by the shareholders.
Pursuant to a recent amendment to regulations promulgated under the Israeli Companies Law providing certain relief to Israeli companies whose shares are listed outside of Israel on certain stock exchanges (including the NYSE) (the “Amended Relief Regulations”) one or more shareholders holding 5% or more of the voting rights of Teva may propose the nomination of a candidate to the Board of Directors. See “Shareholder Proposals for the 2024 Annual Meeting and the 2025 Annual Meeting” below.
Non-Employee Director Compensation
As required by the Israeli Companies Law, we have adopted a Compensation Policy for Executive Officers and Directors (the “Compensation Policy”), which is presented for shareholder approval at least once every three years. Pursuant to the Israeli Companies Law and regulations promulgated thereunder, any arrangement between Teva and a director relating to his or her compensation as a director or other position with Teva must generally be consistent with Teva’s Compensation Policy and approved by the HR and Compensation Committee, the Board and by a simple majority of Teva’s shareholders.
As approved at our 2019 annual general meeting of shareholders, our non-employee director annual compensation program (applicable to all non-employee directors except for the Chairman of the Board) is comprised of:
| (i) | an annual Board membership fee of $130,000 paid in cash; |
| (ii) | additional annual cash fees for service on Board committees: |
| a. | $20,000 per annum to serve as a member of the Audit Committee; and $40,000 per annum to serve as chairperson of the Audit Committee; |
| b. | $15,000 per annum to serve as a member of the HR and Compensation Committee; and $30,000 per annum to serve as chairperson of the HR and Compensation Committee; |
| c. | $20,000 per annum to serve as a member on a special or ad-hoc committee of the Board; and $30,000 to serve as chairperson of such special or ad-hoc committee; and |
| 20 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Corporate Governance and Director Compensation
| d. | $10,000 per annum to serve as a member of any other standing Board committee that is not listed in sub-sections (a)-(b); and $20,000 per annum to serve as chairperson on such committee; and |
| (iii) | an annual equity-based award in the form of restricted share units (“RSUs”) with an approximate aggregate grant date fair value of $160,000 and a one year cliff vesting. |
As approved at our 2019 annual general meeting of shareholders, the annual compensation for the Chairman of the Board is comprised of:
| (i) | an annual Board membership fee of $255,000 paid in cash; |
| (ii) | an annual equity-based award in the form of RSUs with an approximate aggregate grant date fair value of $285,000 and a one year cliff vesting; and |
| (iii) | office and secretarial services at Teva’s offices. |
The Chairman of the Board is not entitled to additional annual cash fees for service on Board Committees.
Fees for Board and committee service are payable over the period of time during which the individual serves as a non-employee director. In the event that a non-employee director serves as a member of the Board during only part of the year, a pro-rated amount of the annual board membership fee and standing committee fees will be paid. In the event of an appointment to the Board between annual meetings of shareholders, the annual equity-based award shall be pro-rated. Upon completion of a non-employee director’s service as a director, other than removal pursuant to a shareholder resolution due to a breach of fiduciary duties, any unvested awards granted to such director by virtue of such position and held by such director will immediately become vested.
We purchase directors’ and officers’ liability insurance for our directors and executive officers, as approved by our shareholders and consistent with the Compensation Policy. In addition, we release our directors from liability and indemnify them to the fullest extent permitted by law and our Articles of Association, and provide them with indemnification and release agreements for this purpose, substantially in the form approved by our shareholders at our 2012 annual meeting.
In addition, Teva reimburses or covers its non-employee directors’ expenses (including travel expenses) incurred in connection with attending meetings of the Board and its committees or in performing other services for Teva in their capacity as non-employee directors, in accordance with Israeli law and the Compensation Policy.
Any director elected to serve as a member of our Board and all continuing non-employee directors will be compensated in the manner described above and will benefit from the insurance, indemnification and release discussed above.
No additional compensation is received for attendance at a Board or committee meeting.
Director Stock Ownership Guidelines
In 2019, we established director stock ownership guidelines requiring ownership of five times the annual cash fee paid to directors for board membership (excluding committees fees), which must be achieved within the later of six years of first becoming subject to these guidelines and January 1, 2025. For more information see “Executive Compensation—Compensation Discussion & Analysis—Stock Ownership Guidelines.”
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 21 | |
Corporate Governance and Director Compensation
2023 Director Compensation
Name | Fees Earned or Paid in Cash ($) (1) | Stock Awards ($) (2) | Total ($) | |||
Dr. Sol J. Barer (3) | 255,000 | 284,993 | 539,993 | |||
Rosemary A. Crane | 170,000 | 159,999 | 329,999 | |||
Amir Elstein | 180,000 | 159,999 | 339,999 | |||
Jean-Michel Halfon (4) | 56,658 | — | 56,658 | |||
Gerald M. Lieberman | 195,000 | 159,999 | 354,999 | |||
Roberto A. Mignone | 180,000 | 159,999 | 339,999 | |||
Dr. Perry D. Nisen | 167,870 | 159,999 | 327,869 | |||
Nechemia (Chemi) J. Peres (5) | 71,024 | — | 71,024 | |||
Prof. Ronit Satchi-Fainaro | 155,247 | 159,999 | 315,246 | |||
Prof. Varda Shalev (6) | 50,000 | 126,660 | 176,660 | |||
Janet S. Vergis | 165,459 | 159,999 | 325,458 | |||
Tal Zaks | 170,000 | 159,999 | 329,999 | |||
| (1) | The amounts shown include the paid cash portion of the annual fee for the Chairman of the Board and Board membership fees and committee service fees for other non-employee directors. |
| (2) | In June 2023, each non-employee director serving at that time was granted 21,108 RSUs, and the Chairman of the Board was granted 37,598 RSUs, based on the grant date fair value of a share of $7.58. Non-employee directors that join between annual general meetings are eligible for an equity grant value that is pro-rated in an amount equal to the difference between (i) an annual grant of $160,000 (for non-employee directors other than the chairman) and (ii) the product of (x) an annual grant ($160,000) divided by 12 and (y) the number of months (including partial months) in the period between the last annual meeting of shareholders and the date of such appointment. Accordingly, in September 2023, Prof. Varda Shalev was granted 12,872 RSUs based on the grant date fair value of a share of $9.84. The amounts shown in the Stock Awards column represent the aggregate grant date fair values of RSUs computed in accordance with FASB Accounting Standards Codification Topic 718 (“Topic 718”). Valuations of RSUs were determined based on the fair market value of a Teva share on the grant date, less the net present value of dividends, as relevant. For information regarding assumptions, factors and methodologies used in our computations pursuant to Topic 718, see note 14b. to our consolidated financial statements set forth in our Annual Report on Form 10-K for the year ended December 31, 2023. These RSUs vest one year from the grant date. As of December 31, 2023, the aggregate number of unvested RSUs held by each current non-employee director was as follows: Dr. Sol J. Barer: 37,598; Rosemary A. Crane: 21,108; Amir Elstein: 21,108; Gerald M. Lieberman: 21,108; Roberto A. Mignone: 21,108; Dr. Perry D. Nisen: 21,108; Prof. Ronit Satchi-Fainaro: 21,108; Prof. Varda Shalev: 12,872; Janet S. Vergis: 21,108; and Tal Zaks: 21,108. Upon completion or termination of a non-employee director’s service as a director, other than removal pursuant to a shareholder resolution due to a breach of fiduciary duties, any unvested awards granted to such director in virtue of such position and held by such director will immediately become vested. In 2023, Jean-Michel Halfon and Nechemia (Chemi) J. Peres received accelerated vesting of equity in connection with their completion of Board service. |
| (3) | During his service as Chairman of the Board, Dr. Barer is entitled to an annual fee of $255,000 and an annual equity-based award with an approximate grant date fair value of $285,000. |
| (4) | Jean-Michel Halfon stepped down from the Board in May 2023. |
| (5) | Nechemia (Chemi) J. Peres’s term on the Board ended in June 2023. |
| (6) | Prof. Varda Shalev was appointed to the Board effective September 1, 2023. |
Mr. Richard D. Francis was not and will not be entitled to any compensation in his capacity as a member of the Board or any committee thereof.
| 22 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Corporate Governance and Director Compensation
Committees of the Board
Our Articles of Association provide that the Board of Directors may delegate its powers to one or more committees as it deems appropriate to the extent such delegation is permitted under the Israeli Companies Law. The Board of Directors has appointed the standing committees listed below, as well as ad-hoc committees appointed from time to time for specific purposes determined by the Board.
We have adopted charters for all of our standing committees, formalizing the committees’ procedures and duties. These committee charters are available on our website at www.tevapharm.com.
Committee Composition and Board and Committee Attendance in 2023
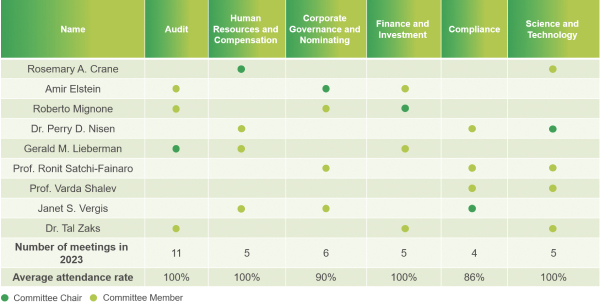
In 2023, our Board of Directors met 8 times with an average attendance rate of 98%, and each of our current directors attended 100% of the meetings of the Board and Board committees on which he or she served.
Audit Committee
The Israeli Companies Law requires publicly held Israeli companies to appoint an audit committee. As a NYSE-listed company, Teva’s Audit Committee must be comprised solely of independent directors, as defined by the SEC and NYSE regulations.
The responsibilities of our Audit Committee include, among others: (a) identifying flaws in the management of our business and making recommendations to the Board of Directors as to how to correct them and providing for arrangements regarding employee complaints with respect thereto; (b) making determinations and considering providing approvals concerning certain related party transactions and certain actions involving conflicts of interest; (c) reviewing the internal auditor’s performance and approving the internal audit work program and examining our internal control structure and processes; (d) examining the independent auditor’s scope of work and fees; and (e) providing for arrangements regarding employee complaints regarding questionable accounting or auditing matters and monitoring compliance with and investigating alleged violations and enforcing provisions of Teva’s Code of Conduct. Furthermore, the Audit Committee discusses the financial statements and the disclosure under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” (the “MD&A”) and presents to the Board of Directors its recommendations with respect to the proposed financial statements and MD&A.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 23 | |
Corporate Governance and Director Compensation
In accordance with the Sarbanes-Oxley Act and NYSE requirements, the Audit Committee is directly responsible for the appointment, compensation and oversight of the work of our independent auditors. In addition, the Audit Committee is responsible for assisting the Board of Directors in monitoring our financial statements, the effectiveness of our internal controls and our compliance with legal and regulatory requirements. The Audit Committee also discusses our policies with respect to risk assessment and risk management regarding financial reporting and risks that may be material to us and major legislative and regulatory developments that could materially impact Teva’s contingent liabilities and risks.
The Audit Committee charter sets forth the scope of the committee’s responsibilities, including its structure, processes and membership requirements; the committee’s purpose; its specific responsibilities and authority with respect to, among others, registered public accounting firms; complaints relating to accounting, internal accounting controls or auditing matters; and its authority to engage advisors as determined by the Audit Committee.
The Audit Committee also reviews and receives briefings concerning Teva’s information security and technology risks, including cybersecurity, and is briefed on Teva’s information security and risk management programs, policies, practices and processes. Teva’s information security office leads our cybersecurity risk management program.
All of the Audit Committee members have been determined to be independent as defined by SEC and NYSE regulations.
The Board of Directors has determined that, of the directors on this committee, Gerald M. Lieberman (chair) and Roberto A. Mignone are “audit committee financial experts” as defined by applicable SEC regulations.
Human Resources and Compensation Committee
The Israeli Companies Law requires publicly held Israeli companies to appoint a compensation committee. As a NYSE-listed company, Teva’s HR and Compensation Committee must be comprised solely of independent directors, as defined by the SEC and NYSE regulations.
The HR and Compensation Committee is responsible for establishing annual and long-term performance goals and objectives for our executive officers, as well as reviewing our compensation philosophy and policies (including our Compensation Policy).
The HR and Compensation Committee is responsible for reviewing plans for the succession of our chief executive officer and other senior members of executive management.
The HR and Compensation Committee also evaluates the performance of our chief executive officer and other executive officers, makes recommendations to the Board of Directors regarding the compensation of our executive officers and directors, reviews any organizational restructuring pertaining to the roles, responsibilities and selection of executive officers and oversees our labor practices.
All of the HR and Compensation Committee members have been determined to be independent as defined by SEC and NYSE regulations.
Corporate Governance and Nominating Committee
The NYSE Listed Company Manual requires publicly listed companies to appoint a corporate governance / nominating committee composed entirely of independent directors, as defined by NYSE regulations.
| 24 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Corporate Governance and Director Compensation
The role of our Corporate Governance and Nominating Committee is to (i) identify individuals who are qualified to become directors; (ii) recommend to the Board of Directors director nominees for each annual meeting of shareholders; and (iii) assist the Board of Directors in establishing and reviewing Teva’s statement of corporate governance principles and promoting good corporate governance in Teva.
All of the Corporate Governance and Nominating Committee members have been determined to be independent as defined by NYSE regulations.
Finance and Investment Committee
The role of our Finance and Investment Committee is to assist the Board of Directors in fulfilling its responsibilities with respect to our financial and investment strategies and policies, including determining policies on these matters and monitoring implementation. It is also authorized to approve certain financial transactions (such as material loans and other financing arrangements), review our financial risk management policies and evaluate the execution, financial results and integration of Teva’s completed acquisitions, as well as various other finance-related matters, including our global tax structure and allocation policies. According to the committee’s charter, at least one of the committee’s members must be qualified as a financial and accounting expert under SEC regulations and/or the Israeli Companies Law.
The Board of Directors has determined that, of the directors on this committee, Gerald M. Lieberman and Roberto A. Mignone (chair) are financial and accounting experts under Israeli law.
A majority of committee members must be determined to be independent as defined by NYSE regulations.
Compliance Committee
The role of our Compliance Committee is to oversee our: (i) policies and practices for complying with laws, regulations and internal procedures; (ii) policies and practices regarding issues that have the potential to seriously impact our business and reputation; (iii) global public policy positions; (iv) strategy and governance of sustainability matters and to advise the Board on sustainability matters; and (v) implementation of our culture of integrity.
A majority of committee members must be determined to be independent as defined by NYSE regulations. The chairperson of the Audit Committee shall be invited by the committee chairperson to participate in the Compliance Committee, as deemed relevant to the committee’s agenda.
Science and Technology Committee
The Science and Technology Committee oversees our overall strategic direction and investment in research and development and technological and scientific initiatives. As part of this responsibility, it reviews scientific and R&D strategy and priorities, scientific aspects of business development activities and technological trends. It assists the Board of Directors in risk management oversight relating to R&D and our intellectual property, advises on our intellectual property strategy, reviews new technology in which Teva is, or is considering, investing and reviews the efficacy and safety profile of new pharmaceuticals.
All of the committee members must be determined to have scientific, medical or other related expertise. A majority of committee members must be determined to be independent as defined by NYSE regulations.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 25 | |
Corporate Governance and Director Compensation
Code of Business Conduct
Teva has adopted a code of business conduct applicable to its directors, executive officers, and all other employees. A copy of the code is available to every Teva employee on Teva’s internet site, upon request to its human resources department, and to investors and others on Teva’s website at www.tevapharm.com or by contacting Teva’s investor relations department, legal department, compliance department or the
internal auditor. If we make any amendment or grant any waiver to this code that applies to our chief executive officer, chief compliance officer, chief financial officer, chief accounting officer or controller, or persons performing similar functions, and that relates to an element of the SEC’s “code of ethics” definition, then we will disclose the nature of the amendment or waiver on Teva’s website. The Board of Directors has approved a whistleblower policy which functions in coordination with Teva’s code of business conduct and provides an anonymous means for employees and others to communicate with various departments of Teva, including the Audit Committee. Teva has also implemented a training program for new and existing employees concerning the code of business conduct and whistleblower policy.
Principles of Corporate Governance
We have adopted a set of corporate governance principles, which is available on our website at www.tevapharm.com. We place great emphasis on maintaining high standards of corporate governance and continuously evaluate and seek to improve our governance standards. These efforts are expressed in our corporate governance principles, our committee charters and the policies of our Board of Directors. Teva is in compliance with all corporate governance standards currently applicable to Teva under Israeli and U.S. laws, SEC regulations and NYSE listing standards. In March 2023, the Board approved and adopted the “Board’s Inclusion and Diversity Statement” as part of the Company’s Statement of Corporate Governance Principles.
Insider Trading Policy
Our directors, executive officers and employees, as well as their immediate family members, persons living in their home and entities controlled by any of the foregoing persons are subject to Teva’s insider trading policy (the “Policy”). The Policy prohibits insider trading and certain speculative transactions (including short sales, buying put and selling call options and other hedging or derivative transactions in Teva’s securities), and establishes a regular blackout period schedule during which directors, executive officers and certain employees may not trade in Teva’s securities. In addition, the Policy establishes pre-clearance procedures that directors and executive officers must observe prior to effecting any transaction in Teva’s securities. The Policy applies not only to Teva’s ADSs and ordinary shares, but also to its debt securities and other securities for which Teva securities serve as underlying assets.
Board Evaluation Process
Our Board of Directors is committed to continuous improvement and recognizes the fundamental role a robust Board of Directors and committee evaluation process play in ensuring that our Board of Directors maintains optimal composition and functions effectively.
In the annual self-evaluation process, the members of the Board of Directors conduct a confidential oral assessment of the performance, risk oversight and composition of the Board and any committees of which he or she is a member with the Company Secretary. As part of the evaluation process, the Board of Directors, in conjunction with the Corporate Governance and Nominating Committee, reviews the effectiveness and overall composition of the Board of Directors, including director tenure, board leadership structure, diversity and skill sets, the quality and scope of the materials distributed in advance of meetings and the Board’s access to Company executives and operations, to ensure the Board of Directors serves the best interests of shareholders and positions the Company for future
| 26 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Corporate Governance and Director Compensation
success. The results of the oral assessments are then communicated back to each committee, committee chair and the entire Board of Directors during an executive session. After the evaluations, each committee, committee chair and the entire Board of Directors and management work to improve upon any issues presented during the evaluation process and to identify opportunities that may lead to further improvement. The Corporate Governance and Nominating Committee also uses this process to assess and determine the characteristics and skills required of prospective candidates for election to the Board of Directors.
Self-evaluation items requiring follow-up and execution are monitored by the Board and each of the committees. While this formal self-evaluation is conducted on an annual basis, the evaluation process is an ongoing process throughout the year, during which Directors continuously share their perspectives, feedback, and suggestions.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 27 | |
Shareholder Engagement
In late 2023 and early 2024, the Board and senior management conducted discussions with shareholders, as part of our regular, ongoing, annual commitment to strong corporate governance and continuous dialogue with our stakeholders, which we believe enables us to better understand their perspectives.
As part of this outreach, we contacted our top 30 institutional shareholders, representing more than 46% of our outstanding shares (after which the concentration of shareholders declines meaningfully). Despite there being a meaningful portion of our shareholder base that does not generally engage, we ultimately spoke with shareholders representing approximately 20% of our outstanding shares, while a further 16% briefly engaged to inform us that they did not view an in-depth discussion as necessary. Our Chairman of the Board, the Chair of our HR and Compensation Committee, the Chair of our Compliance Committee, as well as senior management with responsibility for investor relations, corporate governance, sustainability and executive compensation participated in relevant discussions with shareholders.
We invited shareholders to share their concerns regarding our executive compensation program, discuss potential actions or commitments that might reduce their concerns with our compensation structure and/or outcomes, and review our human capital management and sustainable growth strategy. We also sought their reactions to potential compensation alternatives tied to our Pivot to Growth strategy.
Feedback from our shareholders was shared and discussed with the HR and Compensation Committee, the Corporate Governance and Nominating Committee, the Compliance Committee and the Board.
Subsequent to the shareholder engagement, we also engaged with the research teams at proxy advisory firms Institutional Shareholder Services Inc. and Glass Lewis & Co., to share the feedback and to hear their input regarding our programs.
The Board of Directors and management continue to engage regularly in dialogue with many of the Company’s largest shareholders, and the HR and Compensation Committee will continue to consider the shareholder-approved Compensation Policy, shareholder feedback and the results of the advisory vote on executive compensation in connection with its determinations of executive compensation. Through our shareholder outreach, we have established important feedback channels that provide a valuable way to receive ongoing input from our shareholders.
See also “Executive Compensation—Compensation Discussion & Analysis—2023 Say-on-Pay Vote and Shareholder Engagement.”
Human Capital Management
Our employees are the heart of our Company. In the highly competitive pharmaceutical industry, it is imperative that we attract, develop and retain top talent on an ongoing basis. To do this, we seek to make Teva an inclusive, diverse and safe workplace, with meaningful compensation, benefits and wellbeing programs, and we offer training and leadership development programs that foster career growth.
Our HR and Compensation Committee, Compliance Committee and Board play key roles in overseeing culture and talent at Teva and devote time throughout the year to human capital strategy and execution.
The Board and executive management view inclusion and diversity as essential to our ability to innovate and grow our business, leveraging our diverse workforce to deliver on business excellence and innovation. We strive to create and sustain an inclusive and diverse work environment.
We foster an inclusive work environment that allows all people to express themselves and realize their full potential. Our Inclusion and Diversity (“I&D”) framework, governed by our I&D global team, provides a
| 28 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Human Capital Management
foundation for embedding I&D across our business. Our dedicated global I&D lead is responsible for the execution of the global I&D framework, including strategy and initiatives, partnerships and alignment of activities across regions and business units. In addition, we support recruitment, development and retention of individuals with diverse backgrounds.
As of December 31, 2023, Teva’s workforce consisted of 37,851 employees. We have employees in 58 countries around the world, representing a wide range of nationalities. Employees identifying as female represent 47% of our global employee population, 49% of managers and 29% of senior management as of December 31, 2023.
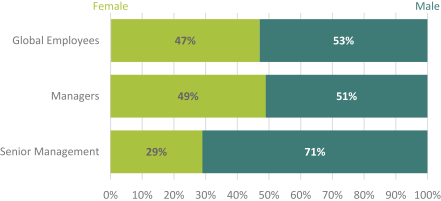
We seek to support our inclusive and diverse culture through employee resource groups, partnerships and training, among other things. For instance, in the U.S., the Teva Employee Resource Group Network represents ten distinct ERGs, which have a key role in creating a culture of inclusion and bringing together employees with shared characteristics and life experiences to foster opportunities for networking, mentoring, collaboration, community outreach, career development, leadership training and cultural exchanges. In Israel, we partner with several providers, including the Israeli Center for Supported Employment and the National Institute of Neuropsychological Rehabilitation, to ensure our opportunities are available to all and help identify suitable positions for people with different abilities. In addition, we provided mandatory training for all employees and managers globally on fostering inclusive behavior and psychological safety and we include an inclusive leadership module in all Teva global leadership development programs. The health and safety of our employees is critical to our ability to supply medicines to our patients. Our Environment, Health, Safety and Sustainability Policy and global Environment Health and Safety Management System guide our employee health and safety practices. We have implemented this system, which often exceeds regulatory requirements, to provide a global standard of care.
As a result of the state of war declared in Israel in October 2023 and the military activity in the region, the health, safety and wellbeing of our Israel-based employees have been a top priority. We provided support through mental health professionals, training for managers, designated support groups, and initiatives to support our employees’ families. In addition, we increased support of an emergency supply of medicines to hospitals, pharmacies and patients and we donated products and are providing other humanitarian aid. Despite the circumstances, we maintained full business continuity with uninterrupted supply of medicines.
We invest in employee career growth and development at Teva. Our talent development programs benefit employees individually by providing them with the resources they need to enhance their professional and management abilities, develop leadership skills and achieve their career aspirations, which in turn helps us to remain competitive in our industry.
In 2023, we introduced a new talent development system based on AI capabilities to match employee skills with development opportunities across the Company.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 29 | |
Human Capital Management
Our Teva Grow program for employees provides development in essential soft skills, success in a global setting and company knowledge. For our managers, we refreshed our development programs to develop the skills, capabilities and mindset required of managers, taking into account our Pivot to Growth strategy.
We focus on succession planning through global and business unit talent review processes that identify and accelerate successors’ readiness to fill senior positions across Teva.
In 2023, we continued to focus on employee wellbeing. In addition to having our second global wellbeing month dedicated to raising awareness of the importance of wellbeing, we leveraged practical tools and local programs to address the physical, financial, social and mental health needs of our employees and their families.
We have been monitoring employee morale during this time in many ways, including by conducting our annual employee survey. Results of the survey show that employee engagement levels have remained high and improved since 2022. Employees feel connected with Teva’s purpose and values, are confident in Teva’s positive impact on society and believe they are treated with respect. In addition, they feel they are able to be themselves at work, they are treated fairly regardless of personal background or characteristics, and that Teva promotes a culture of diversity and inclusiveness.
For further information, see “Item 1—Business— Human Capital Management” in our Annual Report on Form 10-K for the year ended December 31, 2023.
Sustainability at Teva, our Healthy Future strategy
We believe that driving sustainability makes our Company stronger and is essential for continued growth. By doing business responsibly and leveraging our footprint, resources, products and skills to help address global challenges and support our communities, we aim to create long-term value and position Teva to continue providing medicines to the millions of patients around the world who count on us.
In 2023, Teva introduced our renewed sustainability strategy, “Healthy Future”—a continuation of our ESG journey, reflecting Teva’s new leadership, corporate strategy and purpose, as well as an evolution in the external landscape, including stakeholder expectations. Healthy Future encompasses our approach to promoting healthy people, a healthy planet and a healthy business.
In line with our Pivot to Growth strategy, Healthy Future guides us in being (i) stronger—with enhanced action and transparency across topics that are most important to Teva and our stakeholders; (ii) bolder—with a clear focus on key topics that have the greatest impact on our business and on which we make the greatest impact, including ambitious targets; and (iii) simpler—further integrating sustainability into our Company.
We regularly conduct materiality assessments, seeking insights of key stakeholders on the issues they believe are most relevant to our business. With findings from our 2023 materiality assessment and additional inputs, we defined the following eleven priority areas with the greatest potential for Teva to make an impact or to impact Teva: access to medicines and healthcare, patient safety, inclusion and diversity, employee health and safety, climate action and resilience, pharmaceuticals in the environment, ethics and integrity, sustainable procurement, corporate governance, quality manufacturing and risk management.
Within these priority areas, we have focused on the six we believe require the most attention to advance sustainability at Teva and create long-term value, as demonstrated by the accompanying visual. For these, we have ambitious targets to hold us accountable and help track our progress. As illustrated, we recognize the interconnectedness of these topics and the importance of managing them in a coordinated way to contribute to a healthy future.
| 30 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Sustainability at Teva, our Healthy Future strategy
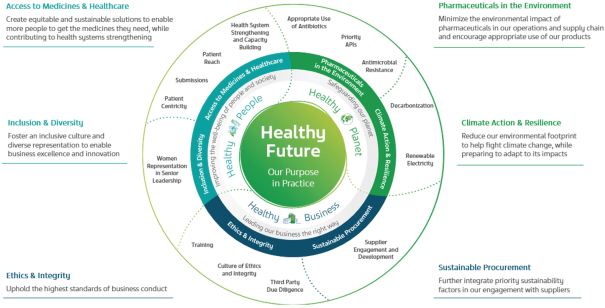
Our 2023 ‘Healthy Future’ (Sustainability) Report (expected to be published in May 2024), will outline our 2023 progress in each of our priority areas, while our ‘Healthy Future Disclosures’ report will include our actions and performance across a broader range of sustainability topics that we manage. We seek to continue and report in alignment with relevant GRI and SASB standards as well as the TCFD framework.
In 2023, we refreshed and simplified our governance model to drive responsibility for Teva’s Healthy Future strategy and related-activities. During 2023, we held dedicated sessions for our Board of Directors, as well as Sustainability Steering Committee meetings and Sustainability Global Forum meetings, which focused on topics such as development and approval of our Healthy Future strategy, new sustainability disclosure regulations and standards, updates and achievements, and our quarterly sustainability dashboard.
All of Teva’s Executive Management members have sustainability goals across various topics (such as GHG emissions and access to medicines) tied to their compensation (annual bonuses). For our six focus areas, we have defined the executives and delivery leads that are accountable for executing on our targets. Our efforts and management approach for our priority areas are guided by taskforces, working groups or committees.
We strengthen our accountability by tying our commitments and progress in priority areas that are material to our business, such as climate action and resilience, and access to medicines and healthcare, to our business strategy through sustainable finance instruments. In March 2023, we issued $2.49 billion of sustainability-linked bonds (“SLB”) following our November 2021 debut $5 billion SLB issuance. This brings Teva’s combined SLB issuance total to approximately $7.5 billion, being the first pharmaceutical company to issue an SLB tied to both access to medicines and environmental targets, reflecting our commitment to sustainability. Our SLBs are linked to the following objectives:
| - | Access to medicines and healthcare: bringing more medicines to LMICs to treat noncommunicable diseases (NCDs) by increasing regulatory submissions and product volume on the World Health Organization Essential Medicines List (“EML”) across six key therapeutic areas; and |
| - | Climate action and resilience: reducing Scope 1 and 2 GHG emissions to support efforts to limit global temperature increase to 1.5oC above pre-industrial levels. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 31 | |
Sustainability at Teva, our Healthy Future strategy
Our SLBs are supplemented by an additional sustainable finance instrument, our March 2022 $1.8 billion Sustainability-Linked Revolving Credit Facility. As has been the case in previous years, Teva plans to obtain external assurance and report on related progress and performance against our SLB KPI’s, which will be published in our 2023 Healthy Future (Sustainability) Report (expected to be published May 2024).
Healthy People
Healthy People reflects how we care for the well-being of our patients and our employees. At Teva, we are all in for better health. Increasing access to medicines is fundamental to improving the health of patients, as well as the success of our business. Our generic medicines offer more affordable options, and our innovative medicines address unmet health needs. Our efforts to create quality and affordable medicines for more people around the world are governed by our Access to Medicines steering committee and guided by Teva’s Position on Access to Medicines.
In 2023, as part of our Healthy Future strategy, we adopted a holistic approach to access to medicines, further addressing financial, geographic, socio-economic and cultural factors. This approach goes beyond our portfolio—with efforts to strengthen healthcare systems and integrate access into clinical trial design. We have two new targets focused on health systems strengthening and capacity building, as well as patient centricity in clinical trials, and we are in the process of defining a patient reach target. With a longstanding legacy in quality generic medicines, we are well-positioned to help more people get the medicines they need, including underserved populations. To reach them, we partner with trusted organizations that know these communities, are familiar with the local health systems, understand patients’ treatment plans and work directly with healthcare providers to implement those plans.
Our SLBs are tied to two 2025 social targets related to access to medicines—(i) increasing the cumulative number of new regulatory submissions in LMICs on the World Health Organization’s EML by 150% (compared to a 2017 to 2020 baseline) between 2022 and 2025; and (ii) increasing product volume by 150% in 2025 (compared to a 2020 baseline). By 2023, we had submitted 46 new regulatory submissions, which represent 61% of our target, and we donated ~800,000 doses eligible to our SLB target.
We seek to weave inclusion and diversity into every aspect of our business—including our workforce and Board of Directors, as well as our clinical trials, supply chain and communications. This drives innovation and enables us to better meet the needs of our patients, while also contributing to the success of our business.
In 2023, as part of our Healthy Future strategy, we restated our aim to achieve gender equality at all levels, with a specific focus on management positions.
Healthy Planet
Healthy Planet reflects how we protect the environment, especially because we recognize the relationship between the health of the planet and the health of people, including the patients we serve each day.
We seek to reduce our carbon footprint to help mitigate climate change, while preparing to adapt to its impacts. Expanding upon our validated, SBTi near-term Scope 1, 2 and 3 GHG emissions targets, we have now stated our intention to achieve net zero emissions across our operations and value chain by 2045. This target reflects our commitment to taking long-term climate action. Additionally, in 2023, we increased the ambition level of our renewable electricity target to 100%, which we aim to achieve across Teva sites by 2035.
| 32 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Sustainability at Teva, our Healthy Future strategy
Our validated near-term SBTi targets are to reduce absolute GHG emissions as follows:
| - | Scope 1 and 2 by 46% by 2030, as compared to 2019 (with an interim 2025 target tied to our SLBs of 25% by 2025). As of the end of 2022, we had reduced these emissions by 24.1%.1 Our 2023 performance will be published in our upcoming Healthy Future (Sustainability) Report (expected to be published in May 2024). |
| - | Scope 3 by 25% by 2030, as compared to 2020. As of the end of 2022, we had reduced these emissions by 12%.1 Our 2023 performance will be published in our upcoming Healthy Future (Sustainability) Report (expected to be published in May 2024). |
We are committed to assessing and mitigating potential environmental and public health impacts of pharmaceutical substances manufactured and used in our operations and by our suppliers. Our Environment, Health and Safety Management System (“EHSMS”) requires our sites to comply with emission and effluent-related regulatory and permit requirements, conditions and limits, and we take a risk-based approach to prevent and control active pharmaceutical ingredients (“APIs”) in our facilities’ wastewater.
Monitoring and delivery in respect of our environmental targets is overseen by our Global Sustainability Taskforce, and our EHSMS is governed by our EHS&S Corporate Committee.
In 2023, we updated our Pharmaceuticals in the Environment targets, which incorporate our commitments around antimicrobial resistance (“AMR”). We are committed to taking a holistic approach to addressing the AMR issue, by tackling it across our value chain. Upstream—in our extended manufacturing supply chain—in our own operations through responsible antibiotic production, and downstream—through healthcare professional and patient education on appropriate antibiotic use.
Healthy Business
Healthy Business reflects how we manage our Company responsibly—holding ourselves to the highest standards and expecting the same of our suppliers.
In 2023, we continued supporting ethical behaviors and deterring non-compliance. Over the last eight years, Teva has strengthened its compliance and ethics program across the globe, with an emphasis on policies, training and support that meet the business in areas related to compliance. Our Compliance Committee oversees our policies and practices for legal, regulatory and internal compliance and is updated on a regular basis by our Chief Compliance Officer. Our Global Compliance & Ethics department is structured to ensure our business partners have a consistent and dedicated partner at all levels of work.
Our Global Compliance and Ethics program encompasses policies, procedures, platforms, and targeted compliance advice to drive ethical behavior. It includes education on compliance guidance and oversight, third-party due diligence management, compliance risk assessment and monitoring, our Code of Conduct, and confidential reporting of misconduct or concerns. We ensure the program’s continuous improvement through benchmarking, surveys, assessments and analyses. We also hold ourselves accountable through our public targets on training, evaluation processes and culture of compliance.
In 2023, we updated our compliance and ethics goals, including:
| - | Train 100%* of targeted active employees assigned to annual ‘Our Way’ Global Compliance & Ethics training campaigns |
| - | Recertify 100%* of active employees on Teva’s Code of Conduct biennially (next due in 2024) |
| 1 | Externally assured emissions reduction data is published in our 2022 ESG Progress Report. |
| * | Teva’s compliance training goals are 95% completion after training campaigns are assigned and 100% year-end completion (percentage calculated as average of all Our Way campaigns, within -1% for employees on leave). |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 33 | |
Sustainability at Teva, our Healthy Future strategy
| - | Meet or exceed the benchmark of high performing** organizations for the 4 comparable questions that appear in Teva’s Organizational Health Survey related to compliance and ethics by 2028 |
| - | Maintain 100% evaluation of all submitted Third Party Representative business partners through Teva’s Third-Party Due Diligence tool (RiskMate) annually |
Progress on the performance of our 2023 compliance and ethics goals, will be described in our 2023 Healthy Future (Sustainability) Report (expected to be published in May 2024).
Teva has a risk-based global compliance training and communications program. To determine compliance and ethics training requirements, Teva evaluates job roles on a compliance-risk basis, and then assigns a general or advanced training curriculum based on that evaluation. In 2023, Teva trained a total of over 31,000 employees on a variety of compliance and ethics topics, with a completion rate of more than 99% of the target training populations. Additionally, Teva assigns all new employees a Code of Conduct training, and conducts a recertification regarding the Code of Conduct every two years for all active employees.
Teva works with more than 43,000 suppliers, which means our impact on people, the planet and our business extends far beyond our own company. Together with our suppliers and through collaborative industry initiatives, we seek to accelerate sustainability performance across our supply chain, including progress toward our environmental targets.
In 2023, as part of the development of our Healthy Future strategy, we revisited our Sustainable Procurement targets, by 2030 we now commit to evaluating 95% of our significant suppliers on sustainability topics, and enhance the sustainability performance of significant suppliers aiming to have 70% of them score over 60 points in EcoVadis by 2030.
Sustainability Governance
Our executive management and Board of Directors provide oversight of our Sustainability activities. Teva’s Board of Directors oversees our Sustainability activities and provides strategic guidance and direction, and the Compliance Committee oversees our Sustainability strategy and receives updates on sustainability matters. Other committees of the Board also receive updates on specific sustainability matters related to their oversight remit, as relevant.
In 2023, our Sustainability Steering Committee which is chaired by our President and CEO and was formed in 2022, continued to meet. This committee provides strategic guidance on a quarterly basis, reviews and approves, as necessary, our global commitments and targets and reviews Teva’s annual Sustainability Progress Report. As part of our Sustainability governance, we have a Sustainability Forum, which brings together the Sustainability leaders from various business units and functions to discuss emerging Sustainability issues, risks and opportunities.
| ** | High performing organization are the average of the top quartile of responding companies. |
| 34 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Sustainability at Teva, our Healthy Future strategy
Teva’s 2023 Sustainability Performance
We continue to enhance Sustainability transparency, which has contributed to further improvements in sustainability rating indices in 2023, as evidenced by the table below:
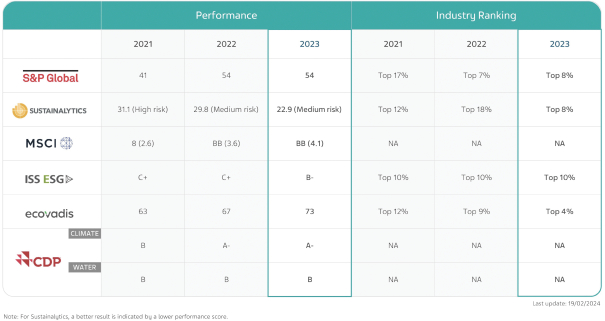
For more details on Teva’s Sustainability performance, please see our 2023 Healthy Future (Sustainability) Report (expected to be published in May 2024). Information on our website is not part of, and is not incorporated into, this Proxy Statement.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 35 | |
Executive Officers
The following table sets forth information regarding our executive officers as of April 1, 2024:
Richard D. Francis
President and Chief Executive Officer | The biography of Richard D. Francis, our President and Chief Executive Officer, and one of our directors, appears under “—Directors” above. | |
Vikki Conway
Acting Head of Global Human Resources | Ms. Conway was appointed as acting Head of Global Human Resources in September 2023. She joined Teva in 2010 and has held various HR leadership roles, most recently as HR business partner in Global R&D and prior to that in Global Marketing. Ms. Conway has provided HR leadership in business transformations and played a pivotal role in several past R&D business development acquisitions. In this current role she oversees Teva’s global human capital strategy, shapes Teva’s culture, and designs best practices and processes. Prior to joining Teva in 2010, she held related leadership roles at Cephalon Pharmaceuticals, Digitas Health and Medical Broadcasting Company. Ms. Conway received her bachelor degree in Psychology from Temple University in Philadelphia, United States. | |
Richard Daniell
Executive Vice President, European Commercial | Mr. Daniell was appointed Executive Vice President, European Commercial in 2017. From 2016 to 2017, he served as President and CEO, Teva Europe Generics. From 2015 to 2016, he served as Chief Integration Officer, leading the integration of the Actavis Generics business into Teva. From 2015 to 2016, he served as Chief Operating Officer, International Markets. From 2011 to 2015, he served as Cluster General Manager, United Kingdom and Ireland. Mr. Daniell received a B.Sc. degree in chemistry from the University of Auckland, New Zealand. | |
| 36 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Officers
Eric Drapé
Executive Vice President, Global Operations | Mr. Drapé was appointed Executive Vice President, Global Operations in 2019. From 2015 to 2019, he served as Teva’s Chief Quality Officer. From 2014 to 2017, he also served as head of Teva’s Biologics Operations, and from 2014 to 2015, he served as Senior Vice President, Technical Operations Steriles, Respiratory and Biologics at Teva. Prior to joining Teva, Mr. Drapé served as Executive Vice President, Technical Operations of Ipsen Pharma and in several leading positions at Novo Nordisk. Mr. Drapé holds a Doctorate degree in Pharmacy and a DESS in Analytical Control of Drugs from the Université Paris XI. He also received his Executive MBA from the Scandinavian International Management Institute in Copenhagen. | |
Christine Fox
Executive Vice President, U.S. Commercial | Ms. Fox was appointed Executive Vice President, U.S. Commercial in November 2023. Ms. Fox brings more than 30 years of healthcare industry experience with leadership roles in sales, marketing, and commercial operations, responsible for teams ranging from 2500+ associates to small, nimble consulting teams. She has a deep understanding of issues affecting the pharmaceutical and biotech sectors, as well as expertise across a range of therapy areas. Prior to joining Teva, she served as President at Novartis Gene Therapies since December 2021. Previously, Chris was vice president and general manager for the Bone, Cardiometabolic and Nephrology business for Amgen. She also served as the general manager of the company’s United Kingdom and Ireland affiliate. Ms. Fox received her bachelor degree in Business Administration, Marketing from the University of Wisconsin in Whitewater, United States. | |
Dr. Angus Grant
Executive Vice President, Business Development | Dr. Grant was appointed Executive Vice President, Business Development on August 1, 2023. Prior to joining Teva, from 2020 to 2023, Dr. Grant was Senior Vice President, Chief Business Executive at BeiGene. From 2018 to 2020, Dr. Grant served as Chief Executive Officer at Dementia Discovery Fund. From 2006 to 2018, he held several executive and senior positions at Celgene, including Corporate Vice President, Business Development. Dr. Grant received his bachelor degree in Biology from the University of Richmond and a Ph.D. in anatomy and immunology from the Medical College of Virginia, and he completed his postdoctoral training at the U.S. National Cancer Institute. | |
Dr. Eric A. Hughes
Executive Vice President, Global R&D and Chief Medical Officer | Dr. Hughes was appointed Executive Vice President, Global R&D and Chief Medical Officer in August 2022. Prior to joining Teva, from 2021 to 2022, Dr. Hughes was Senior Vice President of Clinical Development and Translational Medicine at Vertex Pharmaceuticals. From 2015 to 2021, Dr. Hughes served as Global Development Unit Head for Immunology, Hepatology and Dermatology at Novartis, ultimately responsible for leading all clinical development activities and biostatistician talent across multiple therapeutic areas and for expanding development in China. Additionally, between 2020 and 2021, during the COVID-19 pandemic, Dr. Hughes also served as Co-Chair of the Therapeutics Clinical Working Group for the Accelerating COVID-19 Therapeutic Interventions and Vaccines public-private partnership at the National Institutes of Health. From 2010 to 2015, Dr. Hughes held several executive and senior positions at Bristol Myers-Squibb, including Head of Virology, Fibrotic Diseases, Genetically Defined Diseases, Autoimmunity, and Cardiology Discovery Medicine, Exploratory Clinical & Translational Research. He received his MD and Ph.D from Yale School of Medicine. | |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 37 | |
Executive Officers
Eli Kalif
Executive Vice President, Chief Financial Officer | Mr. Kalif was appointed Executive Vice President, Chief Financial Officer in 2019. From 2001 to 2019 he held various leadership and senior executive finance positions at Flex Ltd., a Nasdaq listed global technology, design and manufacturing service provider. From 2010 to 2019, Mr. Kalif served as Flex Ltd.’s Senior Vice President, Finance, leading its finance organization. From 1996 to 2001, Mr. Kalif worked for Deloitte Israel in various positions as a certified public accountant. Mr. Kalif received his bachelor degree in accounting and economics from the College of Management Academic Studies in Israel and is a Certified Public Accountant. | |
David R. McAvoy
Executive Vice President, Chief Legal Officer | Mr. McAvoy was appointed Executive Vice President, Chief Legal Officer in March 2024. He is an experienced legal and compliance leader with three decades of experience in the biotech and pharmaceutical industry. Most recently from 2019 to 2023, Mr. McAvoy was General Counsel and Chief Compliance Officer at Fresh Tracks Therapeutics, an immunology biotech company. Prior to that, from 2018 to 2019, he served as General Counsel and Chief Compliance Officer at Endocyte, a nuclear medicine oncology company. Previously, he spent over 25 years at Eli Lilly and Company holding numerous global leadership roles in legal, policy and business functions, most recently as General Counsel of Emerging Markets, General Counsel of the Neuroscience Business Unit, and General Counsel of Oncology, GI, Diabetes, and Women’s Health. He received his Bachelor of Arts Degree from the University of Notre Dame, his J.D. from Indiana University Maurer School of Law, and a Master of Science in Environmental Science from Indiana University. | |
Mark Sabag
Executive Vice President, International Markets Commercial | Mr. Sabag was appointed Executive Vice President, International Markets Commercial, in August 2021. Prior to that, he held several executive and senior positions at Teva, including, Executive Vice President, Chief Human Resources Officer and Global Communication and Brand from 2019 to 2021, Executive Vice President, Global Human Resources from 2013 to 2019, Global Deputy Vice President, Human Resources from 2012 to 2013, Vice President, Human Resources for Teva’s International Group and Vice President, Global Human Capital and M&A from 2006 to 2012. Prior to joining Teva, Mr. Sabag held several senior global human resources roles at Intel Corporation. Mr. Sabag received a B.A. in economics and business management from Haifa University. | |
| 38 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
COMPENSATION DISCUSSION AND ANALYSIS
This compensation discussion and analysis (“CD&A”) describes the philosophy, objectives, process, components and additional aspects of our 2023 executive compensation program. This CD&A is intended to be read in conjunction with the tables that immediately follow this section, which provide further historical compensation information for the following named executive officers (“NEOs”):
Name |
Position | |
Richard Francis | President and Chief Executive Officer (“CEO”) | |
Eli Kalif | Executive Vice President, Chief Financial Officer (“CFO”) | |
Richard Daniell | Executive Vice President, European Commercial | |
Eric Drapé | Executive Vice President, Global Operations | |
Mark Sabag | Executive Vice President, International Markets Commercial | |
Quick CD&A Reference Guide
I. BUSINESS AND COMPENSATION OVERVIEW
Compensation Philosophy and Objectives
In order to accomplish our key corporate objectives, we must attract, motivate and retain highly skilled and experienced people to execute our corporate strategy and lead our team. To that end, our executive officer compensation program is designed to:
| (i) | link pay to performance; |
| (ii) | align executive officers’ interests with those of Teva and its shareholders over the long term; |
| (iii) | provide competitive compensation to attract and retain talent; and |
| (iv) | encourage performance without excessive risk. |
“Pivot to Growth” Strategy
In the years leading up to 2023, we stabilized the business and executed a significant operational transformation across our global footprint. For Teva’s next chapter, the Board sought a leader with extensive global, generics and branded experience, who could build on this solid foundation and drive growth. In November 2022, the Board appointed Richard D. Francis, a seasoned pharmaceutical executive with over two and a half decades of experience, as CEO, effective as of January 1, 2023.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 39 | |
Executive Compensation
As he began his tenure, in order to better understand the opportunities and challenges facing Teva, Mr. Francis listened to patients, customers, shareholders and other stakeholders and intently studied Teva’s businesses, products, pipeline and capabilities. Based on this information and after assessing the Company’s strengths and opportunities, Mr. Francis and our executive leadership team developed an incisive and compelling strategy to improve performance and returns. At a May 2023 Investor Day, Mr. Francis and Teva’s executive leadership presented our newly-developed “Pivot to Growth” strategy.
This new Pivot to Growth strategy is based on four key pillars:
| - | Delivering on our growth engines, mainly by further commercializing AUSTEDO, AJOVY, UZEDY and developing our late-stage pipeline of biosimilars; |
| - | Stepping up innovation, through delivering on our late-stage innovative pipeline assets as well as building up our early-stage pipeline organically and potentially through business development activities; |
| - | Sustaining our strong generics medicines franchise, with a global commercial footprint, focused portfolio and pipeline, and excellent manufacturing network; and |
| - | Focusing our business, by optimizing our portfolio and global manufacturing footprint to enable strategic capital deployment to accelerate our near and long-term growth engines, and reorganizing certain of our business units to a more optimal structure, while also reorganizing key business units to enhance operational efficiency. |
As 2023 progressed, we initiated the implementation of our Pivot to Growth strategy, and by the end of the year, we achieved strong growth on our key innovative brands, accelerated our late-stage pipeline assets, and brought our generics business back to growth. More specifically:
| - | In 2023, AUSTEDO grew by 28% and AJOVY grew by 16% compared to 2022, and gained share in many markets. UZEDY is now positioned for growth in 2024 and we made continued progress on launching additional biosimilars in the next four years. |
| - | For our late-stage candidate olanzapine LAI for schizophrenia, we completed enrollment for a Phase 3 trial and expect results in the second half of 2024, and we are collaborating with Royalty Pharma to accelerate clinical research with a funding agreement; another candidate entered clinical trials, and we are collaborating with Sanofi on our anti-TL1A candidate. |
| - | With respect to generics, we are focusing on making sure we have the right portfolio in the market, focusing our pipeline to bring high value products (like complex generics) to drive growth, on time, and optimizing our network of manufacturing facilities. |
| - | In terms of optimizing the portfolio, subsequent to year-end, we announced our intention to divest our active-pharmaceutical ingredient (API) business, a global leader in the small-molecule API industry, which we believe will help us focus our capital allocation on driving growth in our innovative and generics portfolios. |
The roadmap for the Pivot to Growth strategy envisions: in 2023 and 2024, a return to growth based on AUSTEDO, AJOVY and UZEDY; from 2025 to 2027, an acceleration of growth based on launches of new innovative products, growth in biosimilar generics, an optimized generics business and focused business development; and in 2028 and beyond, sustained growth through further launches of innovative products, a sustainable innovative pipeline, additional focused business development and margin expansion.
The strategy also posits that in the future, Teva will be stronger, bolder and simpler: stronger, based on an engine of sustainable generics; bolder, based on additional innovative products; and simpler, with focused and optimized capital allocation.
| 40 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
2023 Select Business Highlights
2023 was a year of significant advances for Teva, a year in which we began to implement our Pivot to Growth strategy, achieved strong growth on our key innovative brands, accelerated our late-stage pipeline assets, and brought our generics business back to growth, as detailed below.
Leadership Transitions
|
| - | Effective January 1, 2023, our Board of Directors appointed Richard D. Francis to become the Company’s President and Chief Executive Officer and a member of the Board. |
| - | In July 2023, Teva appointed Dr. Angus Grant, Ph.D., as Executive Vice President, Business Development. |
| - | In September 2023, Teva appointed Christine (Chris) Fox as Executive Vice President, U.S. Commercial. |
| - | In March 2024, Teva appointed David McAvoy as Executive Vice President, Chief Legal Officer. |
Strategic Developments
|
| - | We developed and initiated the implementation of our Pivot to Growth strategy. Please see “Pivot to Growth Strategy” above. |
| - | Revenues from AUSTEDO for the treatment of chorea associated with Huntington’s disease and tardive dyskinesia continued to grow, with $1.225 billion in U.S. revenues in 2023, an increase of 27% compared to 2022. AUSTEDO XR (deutetrabenazine) extended-release tablets was approved by the FDA on February 17, 2023, and became commercially available in the U.S. in May 2023. |
| - | Revenues from AJOVY for the preventive treatment of migraines in adults continued to grow, with $435 million in global revenues in 2023, an increase of 16% compared to 2022. In North America, revenues were $230 million, an increase of 6% compared to 2022, in Europe revenues were $160 million, an increase of 29% compared to 2022, and in International Markets, revenues were $44 million, an increase of 25%, or 35% in local currency, compared to 2022. |
| - | UZEDY (risperidone) extended-release injectable suspension was approved by the FDA on April 28, 2023 for the treatment of schizophrenia in adults, and launched in the U.S. in May 2023. UZEDY is a subcutaneous, long-acting formulation of risperidone that controls the steady release of risperidone. |
| - | In October 2023, we entered into an exclusive collaboration with Sanofi to co-develop and co-commercialize Teva’s anti-TL1A (TEV-’574) asset, a novel anti-TL1A therapy for the treatment of ulcerative colitis and Crohn’s disease, two types of inflammatory bowel disease, which is currently in Phase 2b clinical trials. Under the terms of the collaboration agreement, Teva received an upfront payment of $500 million in 2023 and Teva may receive up to $1 billion in development and launch milestones in the future. Each company will equally share the development costs globally and net profits and losses in major markets, with other markets subject to a royalty arrangement, and Sanofi will lead the development of the Phase 3 program. Teva will lead commercialization of the product in Europe, Israel and specified other countries, and Sanofi will lead commercialization in North America, Japan, other parts of Asia and the rest of the world. |
| - | In November 2023, we entered into a funding agreement with Royalty Pharma plc. (“Royalty Pharma”) to further accelerate the clinical research program for Teva’s olanzapine LAI (TEV-’749), a once-monthly subcutaneous long-acting injection that is currently in a Phase 3 trial for the treatment of schizophrenia. Under the terms of the funding agreement, Royalty Pharma will provide Teva up to $100 million to fund ongoing development costs, and Royalty Pharma and Teva have a mutual option to increase the total funding amount to $125 million. In exchange and subject to regulatory approval, Teva will pay Royalty Pharma a milestone payment in the amount actually funded by Royalty Pharma, paid over 5 years, in addition to royalties upon commercialization. Teva will continue to lead the development and commercialization of the product globally. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 41 | |
Executive Compensation
| - | In November 2023, we entered into a license agreement with Biolojic Design Ltd. (“Biolojic”), pursuant to which we received exclusive rights to develop, manufacture and commercialize worldwide a BD9 multibody for the potential treatment of Atopic Dermatitis and Asthma. In exchange, we agreed to pay an upfront payment in the amount of $10 million. Biolojic may be eligible to receive additional development and commercial milestones payments of up to approximately $500 million, over the next several years, based on the achievement of certain pre-clinical, clinical and regulatory milestones, with the majority of the payments based on future revenue achievements. |
| - | We reduced our net debt by an additional $1.8 billion to $16.6 billion at the end of 2023. |
Financial Results
|
| - | Our revenues in 2023 were $15,846 million, an increase of 6% in U.S. dollars, or 7% in local currency terms, compared to 2022, mainly driven by an upfront payment received in connection with the collaboration on our anti-TL1A asset mentioned above, higher revenues from generic products in our International Markets and Europe segments and our innovative products AUSTEDO and AJOVY, the sale of certain product rights in our Europe segment, as well as higher revenues from Anda, partially offset by lower revenues from COPAXONE®, API sales to third parties, BENDEKA® and TREANDA®, and generic products in our North America segment. |
| - | Operating income was $433 million in 2023, compared to operating loss of $2,197 million in 2022, mainly due to higher goodwill impairment charges and legal settlements and loss contingencies in 2022. Non-GAAP operating income was $4,361 million in 2023, compared to $4,139 million, in 2022, mainly impacted by lower gross profit margin, partially offset by lower operating expenses as a percentage of revenues. Please see Appendix A hereto for a reconciliation of non-GAAP operating income to GAAP operating income. |
| - | As of December 31, 2023, our debt was $19,833 million, compared to $21,212 million as of December 31, 2022. This decrease was mainly due to $1,646 million senior notes repaid at maturity, partially offset by $302 million of exchange rate fluctuations. Additionally, during the first quarter of 2023, we repurchased $2,506 million aggregate principal amount of notes upon consummation of a cash tender offer, and issued $2,445 million in aggregate principal amount of sustainability-linked senior notes, net of issuance costs. |
| - | Net loss attributable to Teva and diluted loss per share in 2023 were $559 million and $0.50, respectively, compared to net loss attributable to Teva of $2,446 million and diluted loss per share of $2.20 in 2022. Non-GAAP net income attributable to Teva and non-GAAP diluted earnings per share in 2023 were $2,898 million and $2.56, respectively, compared to $2,812 million and $2.52 in 2022. Please see Appendix A hereto for a reconciliation of non-GAAP net income and non-GAAP EPS. |
| - | During 2023, we generated free cash flow of $2,387 million, which we define as comprising $1,368 million in cash flow generated from operating activities, $1,477 million in beneficial interest collected in exchange for securitized accounts receivables (under our EU securitization program) and $68 million proceeds from sale of businesses and long-lived assets, partially offset by $526 million in cash used for capital investments, compared to free cash flow of $2,243 million in 2022. Please see Appendix A hereto for a reconciliation of free cash flow to net cash provided by operating activities. |
Geopolitical Environment. In October 2023, Israel was attacked by a terrorist organization and entered a state of war, which is still ongoing as of the date of this Proxy Statement. The health, safety and wellbeing of our Israel-based employees have been a top priority. We provided support through mental health professionals, training for managers, designated support groups, and initiatives to support our employees’ families. In addition, we increased support of an emergency supply of medicines to hospitals, pharmacies and patients, donated products and are providing other humanitarian aid. Despite the circumstances, we maintained full business continuity with uninterrupted supply of medicines.
| 42 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Sustainability is a Key Business Priority: Healthy Future
Teva leverages our footprint, scale, resources, products and skills to help address global challenges and support our communities, while ensuring our business success so that we can continue providing medicines to the millions of patients around the world who count on us. Healthy Future, Teva’s renewed sustainability strategy, guides our approach on the themes of:
| - | healthy people – improving the well-being of people and society; |
| - | a healthy planet – safeguarding our planet; and |
| - | healthy business – leading our business the right way. |
Aligned with our Pivot to Growth strategy, our sustainability strategy is grounded in being stronger, bolder and simpler, resulting in actions that are thoughtful, intentional and lead to meaningful change. It is our purpose in practice: we are all in for better health.
Teva continued to make steady progress toward its sustainability goals, including on the measures that are part of its unsecured syndicated sustainability-linked revolving credit facility and its sustainability-linked bonds. By end of 2023, we had launched 7 access programs (out of a 2025 target of 8) and made 46 regulatory submissions (61% of the 2025 target). We have distributed over 20.9 million doses of medicine in access programs in low- and middle-income countries. During 2023, we continued to increase or maintain our ESG ratings performance as determined by leading ESG rating agencies (e.g. S&P Global, Sustainalytics, MSCI, ISS ESG and EcoVadis). At the Corporate ESG Awards, we were recognized as the Best Company for Sustainability Reporting in the healthcare sector and we were a finalist for Best Company for Social Responsibility.
Similar to previous years, the CEO and all other executive officers were given objective and measurable goals as part of their individual performance goals that are directly related to our renewed sustainability strategy. In addition, 25% of the CEO’s sign-on PSU award is tied to achievement of goals directly related to the metrics in our Sustainability-Linked Bonds.
Key Aspects of 2023 Executive Compensation
| 1. | 2023 CEO Compensation: Majority Performance-Based (Total and Long-Term Incentive Equity) |
Effective January 1, 2023, the Company appointed Richard D. Francis to serve as President and CEO. Approximately 88% of our CEO’s 2023 compensation was variable and at-risk, with the substantial majority being performance-based. In addition, 70% of our CEO’s annual long-term incentive equity grant was in the form of performance share units (“PSUs”). The PSUs are subject to three-year performance metrics tied to our key goals and there is a relative Total Shareholder Return (“TSR”) modifier (+/-10%). The PSUs will vest, if at all, at the end of the performance period. The other 30% of our CEO’s annual long-term incentive equity grant was in the form of time-based restricted share units (“RSUs”), which vest over four years.
In connection with the transition to a new CEO and as is common when a new CEO is appointed following a longer-tenured CEO, the HR and Compensation Committee considered pay quantum and structures for CEOs at peer companies, advice from its independent compensation consultant and other factors, and set Mr. Francis’ target total annual compensation 12% below the outgoing CEO, with a lower base salary, lower target annual incentive cash value and lower target PSU and RSU grant value. As part of his hiring, he received a sign-on equity award with a value of $10 million, of which 50% was PSUs and 50% was RSUs, along with a $5 million cash sign-on bonus, with a portion of such cash and equity being in replacement of awards foregone at his prior employer to incentivize him to accept the job offer with Teva.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 43 | |
Executive Compensation
2023 Annual CEO Target Total Direct Compensation
Executive
| Principal
| Base Salary
| Target
|
Target
|
Target
| Total (*)
| ||||||
Richard Francis | CEO | $1,600,000 | $2,400,000 | $6,300,000 | $2,700,000 | $13,000,000 | ||||||
% of Total Target Compensation |
| 12% | 18% | 49% | 21% | 100% | ||||||
% of Long-Term Incentive |
|
|
| 70% | 30% |
| ||||||
| (*) | Equity values have been rounded to the nearest $10,000. |
| 2. | 2023 Long-Term Incentives: 70% Performance-Based Equity for CEO, 50% for all other NEOs; High Threshold Performance Level; Three-Year Performance Period |
In addition to 70% of the CEO’s target equity grant in the form of PSUs, similar to 2022, 50% of the value of the target equity grant for our other executive officers is subject to performance-based vesting conditions in the form of PSUs, and the other 50% is in the form of time-based RSUs. This enhances the strong link between pay and performance for our NEOs and the alignment of the interests of the NEOs with those of Teva and its shareholders.
A minimum of 85% of target performance must be achieved in order to earn any PSUs for a particular metric, which is a rigorous and challenging level. The HR and Compensation Committee and the Board established a three-year performance period.
| 3. | Rigorous Targets |
Long-Term Incentives. For the 2021-2023 PSUs, the HR and Compensation Committee and the Board determined that the achievement for the combined three-year performance period was 94% of the target for Net Revenue, resulting in an earning percentage of 70%, and achievement of 93% of the target for Non-GAAP Operating Income, resulting in an earning percentage of 64%. The weighted average of these earning percentages, 67%, was then subject to a relative TSR modifier, which reduced the payout by 18% based on our TSR ranking relative to the TSR peer group, resulting in a modified final earning percentage of 55% of the target number of PSUs.
Annual Cash Incentives. At the beginning of 2023, we established annual cash incentive plan targets for Non-GAAP EPS and Free Cash Flow that were aligned to the middle of the range of our outlook as communicated to investors in February 2023. The 2023 Non-GAAP EPS goal was set at $2.40 and the Free Cash Flow goal was set at $1.96 billion. In setting these goals, the HR and Compensation Committee took into account various factors, including forecasted growth levels for the global economy, for the geographic areas where the Company operates and for the market for generic products, as well as the recognition that goals were being established before our new CEO’s development and implementation of the Pivot to Growth strategy. In addition, the goals were based on the assumptions communicated to investors in February 2023, including:
| - | Anticipated continued decline in COPAXONE revenue from $691 million in 2022 to approximately $500 million in 2023, due to an expected increase in generic competition; |
| - | Anticipated continued increase of AUSTEDO revenue from $971 million in 2022 to approximately $1.2 billion in 2023; and |
| - | Anticipated continued increase of global AJOVY revenue from $377 million in 2022 to approximately $400 million in 2023. |
| 44 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
In light of all of these factors, these goals were considered rigorous, aggressive and challenging, attainable only with strong performance, and took into account the relevant opportunities and risks we were facing.
Downward Modification. For the 2023 annual incentive plan, based on the annual results as reported in the Annual Report on Form 10-K for the year ended December 31, 2023, the Company’s achievement should have been 107% of our 2023 Non-GAAP EPS target, and 122% of our 2023 Free Cash Flow target. However, in October 2023, the Company had announced a collaboration with Sanofi to co-develop a Teva product candidate for the treatment of Ulcerative Colitis and Crohn’s Disease under which Teva received an upfront payment in 2023 of $500 million. The HR and Compensation Committee and the Board determined to modify downward the Company Financial performance achievement, so that the outcome did not result in an unintended windfall, while still recognizing the considerable effort expended to achieve the result and the strategic and financial significance of this collaboration. The HR and Compensation Committee reduced the Company Financial performance achievement to 100%, a reduction of approximately 7% from the performance achievement of Non-GAAP EPS and 22% from the performance achievement of Free Cash Flow determined when the upfront payment was included. Based on this downward adjustment in the performance achievement, along with the determinations of the NEOs’ individual performance achievement (which represented 25% of the target total opportunity), the HR and Compensation Committee and the Board approved an annual incentive plan payout of 108% of target for the CEO and between 50% and 113% of target for the other NEOs.
2023 Say-on-Pay Vote and Shareholder Engagement
Our Board places a high value on incorporating shareholder feedback on our executive compensation program, and actively seeks input, including in connection with contemplated compensation alternatives related to our Pivot to Growth strategy. We regularly engage with investors on a variety of topics, including corporate governance and executive compensation, in the context of driving long-term, responsible growth, as well as sustainability and human capital management. We strive to be responsive to shareholder feedback.
At the 2023 annual meeting of shareholders, our advisory Say-on-Pay proposal regarding the compensation of our NEOs received the support of approximately 49.6% of the votes cast. The Board took this low level of support very seriously and recognized that it signaled concern. In response to this vote, the HR and Compensation Committee and the Board conducted shareholder engagement following the annual meeting to specifically understand shareholders’ perspectives on the 2022 executive compensation program and consider appropriate responsive actions.
Also, shortly before the annual meeting, at a May 2023 Investor Day, Mr. Francis, our new CEO, and Teva’s executive leadership presented our newly-developed “Pivot to Growth” strategy, and began taking steps to implement it. In connection with developing and commencing the execution of the strategy, our HR and Compensation Committee and Board sought to determine effective ways to tie compensation earned by our NEOs under our executive compensation program to the successful execution of our new Pivot to Growth strategy.
As part of this outreach, we contacted our top 30 institutional shareholders, representing more than 46% of the Company’s outstanding shares (after which the concentration of shareholders declines meaningfully). We invited our shareholders to discuss their concerns regarding our executive compensation program and potential actions or commitments that might address their concerns with regard to our compensation structure and/or outcomes, and review our human capital management and sustainable growth strategy. We also sought their reactions to potential compensation alternatives tied to our Pivot to Growth strategy. Despite there being a meaningful portion of our shareholder base that does not generally engage, we ultimately spoke with shareholders representing approximately 20% of the Company’s outstanding shares, while a further 16% briefly engaged to inform us that they did not view an in-depth discussion as necessary. We viewed this level of response as being favorable, within the context that: i) we are a global company organized and headquartered outside the U.S., with shareholders correspondingly located outside the U.S.,
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 45 | |
Executive Compensation
with their own home country engagement approaches and compensation policies, even though we compete for talent globally; and ii) ahead of and in connection with the transition to new leadership and strategy, and as is common during these transitions, there has been a change in our shareholder base, with some shareholders that held positions on last year’s annual meeting record date no longer holding such positions at the time of our engagement, and thus were not part of the engagement about the prior year vote.
Engaging on behalf of the Company were our Chairman of the Board, the Chair of our HR and Compensation Committee, the Chair of our Compliance Committee, as well as senior management with responsibility for investor relations, corporate governance, sustainability and executive compensation. Shareholder feedback was subsequently shared and discussed with the HR and Compensation Committee, the Corporate Governance and Nominating Committee, the Compliance Committee and the full Board.
Following the discussions, the HR and Compensation Committee evaluated the concerns and preferences raised by shareholders, and discussed which actions or compensation program modifications might be appropriate to address concerns while maintaining a robust alignment with our corporate growth strategy. That evaluation was shared with the full Board, which determined to take the specific actions that were directly responsive to our shareholders as further detailed below. The HR and Compensation Committee also took into consideration the input on go-forward compensation vehicles and metrics, incorporating such input into the design of the 2024 executive compensation program. Overall, shareholders were generally supportive of our approach to executive compensation and did not think that any major structural changes were necessary.
Subsequent to the shareholder engagement, we also reached out to and engaged with the research teams at proxy advisory firms Institutional Shareholder Services Inc. and Glass Lewis & Co. to share the feedback and to hear their input regarding our programs.
| What We Heard | Teva’s Response | |
Demonstrate, through fulsome disclosure, that the HR and Compensation Committee has conducted a serious, robust review, considered the shareholders’ experience with the event driving an adjustment, and weighed all implications including the magnitude, context and negative impact when making an adjustment to Company performance for executive compensation purposes, such as an adjustment for amounts relating to opioid litigation settlements. | - The HR and Compensation Committee and the Board have committed to enhancing transparency in connection with the decision-making process for executive compensation.
- If any significant, unique transaction affects reported results, the HR and Compensation Committee will consider the shareholders’ experience, the risks to our commitment to accountability and reflection of performance before making a material adjustment. The Board will demonstrate the robustness of the review process and disclose all factors considered as part of the decision-making process for any use of discretion in the determination of incentive plan payouts.
- For 2023, the HR and Compensation Committee and the Board determined to modify downward the Company Financial Measures performance achievement of the annual cash incentive plan, so that the outcome did not result in an unintended windfall due to the upfront payment from the collaboration with Sanofi. The process in connection with this decision is described herein. | |
| 46 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
| What We Heard | Teva’s Response | |
Align the go-forward goals of the executive compensation program with the new “Pivot to Growth” strategy in order to motivate executive officers to achieve strategic, operational and financial goals that are part of the Pivot to Growth strategy in an effort to increase shareholder value. |
The HR and Compensation Committee and the Board have sought to align the executive compensation program and the incentives contained in the 2024 opportunities and grants with the Pivot to Growth strategy. Beginning in 2024:
For the annual cash incentive:
- 25% of the opportunity will be allocated to Revenue, which is consistent with prioritizing top line growth.
- Greater differentiation will be applied to the individual component, with higher performance needed in order to qualify for the highest individual performance rating.
For the long-term incentives:
- Consistent with the Pivot to Growth strategy of growing the top line and generating cash, the metrics will be long-term Net Revenue growth and Free Cash Flow.
- To underscore the development of a culture of excellence, and consistent with peer practices, the payout curve is increased for maximum performance as compared to 2023.
- Performance on the operational metrics will continue to have a modifier applied to reflect the shareholder experience. An absolute stock price modifier will be applied to the earned percentage of the performance metrics in order to incentivize efforts to increase the Company’s market cap. | |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 47 | |
Executive Compensation
The Board and management continue to regularly engage in dialogue with many of the Company’s largest shareholders, and the HR and Compensation Committee will continue to consider the shareholder-approved Compensation Policy, shareholder feedback, and the results of the advisory vote on executive compensation in connection with its determinations of executive compensation as depicted in our annual shareholder engagement cycle below. Through our shareholder outreach, we have established important feedback channels that provide a valuable way to receive ongoing input from our shareholders.
II. COMPENSATION PHILOSOPHY AND OBJECTIVES
Compensation Philosophy
| - | Pay-for-performance: We aim to incentivize our executive officers by creating a strong link between their performance and compensation. Therefore, a significant portion of the total compensation package provided to our executive officers is based on measures that reflect both our short- and long-term goals and performance, as well as the executive officer’s individual performance and impact on shareholder value. |
| - | Alignment of executive officers’ interests with those of Teva and its shareholders: In order to promote retention and motivate executive officers to focus on our long-term objectives and the performance of Teva’s shares, a significant portion of the compensation packages of our executive officers is granted in the form of equity-based compensation, which creates a direct link between the interests of executive officers and the interests of Teva and its shareholders. By making executive officers into shareholders with a personal stake in the value of Teva, we are motivating them to create, and enabling them to share in, Teva’s growth and success, while also fostering an ownership culture among executive officers. |
| - | Competitive compensation to attract and retain talent: We compete with global companies to attract and retain highly talented professionals with the necessary capabilities to promote creativity, encourage high achievement, manage our complex business and worldwide operations and execute our strategy. The HR and Compensation Committee and the Board reference, among other things, the amounts and structures of the compensation of executive officers in the companies in our peer group in determining competitive pay levels. |
| 48 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
| - | High standards of corporate governance, compliance and risk management: We are committed to transparent and ethical business practices. Maintaining high standards of corporate governance and legal compliance are key factors in our success. This allows us to create long-term value for our shareholders as well as all of our other stakeholders, including employees, customers, suppliers and, above all, patients worldwide. Compensation is structured in a manner that creates an incentive to deliver high performance (both short- and long-term) while taking into account our compliance and risk management philosophy and avoiding undue pressure on executive officers to take excessive risks, thereby encouraging a balanced and effective risk-taking approach. |
Compensation Policy under the Israeli Companies Law
Due to our unique position as an Israeli company with an extensive global footprint, we aim to adopt compensation policies and practices that match those of similar global companies, but we must also comply with applicable Israeli law, including the requirement that Israeli publicly-traded companies adopt a compensation policy which is submitted periodically for shareholder approval and contains certain limits on elements of compensation. Executive compensation decisions must generally be consistent with that policy.
As approved at our 2022 annual meeting of shareholders, and as required by the Israeli Companies Law, we have adopted a Compensation Policy regarding the terms of office and employment of our “Office Holders” (as defined under the Israeli Companies Law, which includes directors, the CEO, other executive officers and any other managers directly subordinate to the CEO), including cash compensation, equity-based awards, releases from liability, indemnification and insurance, severance, and other benefits (the “Terms of Office and Employment”). During 2023, each of our NEOs was an Office Holder within the meaning of the Israeli Companies Law. The Compensation Policy is reviewed from time to time by the HR and Compensation Committee and the Board to ensure its alignment with our compensation philosophy and objectives and to consider its appropriateness for Teva. Under the Israeli Companies law, we are required to submit the compensation policy to shareholders at least once every three years for approval. Please see “—Compensation Policy Requirements” below.
Compensation Governance
The HR and Compensation Committee assesses the effectiveness of our compensation program periodically and reviews risk mitigation and governance matters. We do this by maintaining the following best practices:
What We Do
| ||
Shareholder Engagement |
We reach out to shareholders to understand and address their perceptions and concerns regarding our executive compensation program.
| |
Shareholder-Approved Compensation Policy
|
We must generally comply with the provisions of our shareholder-approved Compensation Policy in all decisions in connection with executive compensation.
| |
Majority Variable Pay
|
The majority of total executive compensation is variable and at-risk, and a meaningful percentage is performance-based.
| |
Balance Short- and Long-Term Compensation
|
The allocation of incentives among the annual incentive plan and the long-term incentive plan does not over-emphasize short-term performance at the expense of achieving long-term goals.
| |
Multiple Performance Metrics in Incentive Programs
|
We use multiple performance metrics in our incentive plans to capture our performance from different perspectives, providing a more complete picture.
| |
Equity for Long-Term Incentives
|
We use PSUs with a three-year performance period and RSUs that vest over four years to motivate long-term performance, align the interests of executive officers and shareholders and provide an incentive for retention.
| |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 49 | |
Executive Compensation
What We Do
| ||
Independent Compensation Consultant
|
Our HR and Compensation Committee has engaged an independent compensation consultant to provide information and advice for use in Committee decision-making.
| |
Peer Data
|
We review compensation benchmark data from peer companies whose industry, revenues, and global footprint share similarities with Teva.
| |
Stock Ownership Guidelines
|
We maintain guidelines for executive officers and directors to maintain meaningful levels of stock ownership.
| |
Clawback
|
We maintain robust clawback policies compliant with SEC and NYSE rules and Israeli law to recoup cash and equity-based incentives paid to executive officers based on erroneously prepared financial statements or other misconduct.
| |
Risk Assessment
|
We conduct an annual risk assessment of our compensation program.
| |
Cap Bonus, Equity Grant Fair Values and PSU Payouts
|
We cap annual cash incentive payouts, annual equity grant date fair values at target, and the number of PSUs that may be earned under an award, pursuant to the Compensation Policy.
| |
Double Trigger Change-in-Control Provisions
|
If there is a change in control, outstanding equity awards will vest only if there is both a change-in-control and a termination of employment (a “double trigger”). A change-in-control alone will not trigger vesting.
| |
What We Don’t Do
| ||
No Dividends on Unearned Awards
|
Under our equity plan, we do not pay dividends or dividend equivalents on shares that a participant has not yet earned or that have not vested.
| |
No Hedging or Pledging of Company Securities
|
We prohibit executive officers and non-employee directors from engaging in hedging, pledging or short sale transactions in Company securities.
| |
No Repricing of Underwater Stock Options
|
Our equity plan does not permit the repricing of stock options where the strike price exceeds the then-current fair market value without shareholder approval.
| |
No Excise Tax Gross-Ups
|
We do not provide excise tax gross-ups in employment agreements.
| |
No Guaranteed Bonuses
|
We do not provide guaranteed performance bonuses to our executive officers.
| |
No Backdating or Discounting Stock Options
|
We do not backdate stock options or provide discounted stock options.
| |
| 50 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
III. COMPENSATION DETERMINATION PROCESS
Key Participants
The roles and responsibilities of all parties involved with the compensation determination process are set forth below:
Participant | Responsibilities | |
Shareholders |
- Approve the Compensation Policy as required under the Israeli Companies Law, including caps for cash incentives and equity, at least once every three years, and any changes thereto
- Cast advisory vote on proposal(s) regarding executive compensation under U.S. law
- Approve any compensation that deviates from the Compensation Policy
- Approve compensation of the CEO
- Approve compensation of directors
- Approve equity plans, material changes to equity plans and share reserve increases
- Provide direct feedback and input to Teva and our Board | |
Board of Directors |
- Evaluate performance of the CEO and executive officers, including the NEOs
- Review and approve (subject to shareholder approval in certain cases):
- Equity plans, material changes to equity plans and share reserve increases
- CEO and executive officer compensation, with input and recommendation from, and prior approval of, the HR and Compensation Committee
- Changes to the Compensation Policy | |
Human Resources and Compensation Committee |
- Consider shareholder feedback and all other factors to help align our executive compensation program with the interests of Teva and our shareholders and long-term value creation
- Review and approve (subject to Board and shareholder approval in certain cases):
- CEO and executive officer compensation, including adjustments to executive officers’ base salaries, cash incentives and equity compensation, as well as other components of compensation, in alignment with the Compensation Policy
- Performance-based metrics and goals under the annual cash incentive plan and PSU plan
- Achievement of performance-based goals under the annual cash incentive plan and associated with PSUs
- Equity plans and awards
- The Compensation Policy and its continued appropriateness (periodically)
- The CD&A and the compensation tables and accompanying narrative descriptions | |
Independent Compensation Consultant |
- Advise the HR and Compensation Committee on various director and executive officer compensation and governance topics, including:
- Compensation Policy, pay philosophy, best practices and market trends
- Selection of peer group companies
- Director and executive officer compensation practices and levels at peer group companies
- Design of annual cash incentive plan, and performance and other equity plans, and awards and grants under each plan
- Stock ownership guidelines
- Review and provide an independent assessment of the data and materials presented by management to the HR and Compensation Committee
- Participate in HR and Compensation Committee meetings as requested | |
CEO |
- Evaluate the performance of other executive officers, including the other NEOs, and recommend adjustments to base salaries, annual cash incentives and long-term equity compensation
- Develop business goals, which are evaluated and incorporated by the HR and Compensation Committee and the Board in the design of our executive officer compensation program | |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 51 | |
Executive Compensation
Role of Independent Compensation Consultant
The HR and Compensation Committee has the authority to retain independent compensation consultants to assist it in the performance of its duties and responsibilities without consulting or obtaining the approval of management of the Company. For 2023, the HR and Compensation Committee retained Semler Brossy Consulting Group LLC (“Semler Brossy”) as its independent compensation consultant. Semler Brossy reported directly to, and was directly accountable to, the HR and Compensation Committee. While the HR and Compensation Committee took into consideration the review and recommendations of this independent advisor when making decisions about the Company’s executive officer and director compensation practices and governance related topics, the HR and Compensation Committee ultimately made its own independent decisions about these matters.
The HR and Compensation Committee assessed the independence of Semler Brossy pursuant to the rules of the SEC and the NYSE. In doing so, the HR and Compensation Committee considered the factors set forth by the SEC and NYSE with respect to a compensation consultant’s independence. The HR and Compensation Committee also considered the nature and amount of work performed for the HR and Compensation Committee and the fees paid for those services in relation to the firm’s total revenues. After these reviews, the HR and Compensation Committee concluded that there were no conflicts of interest, and that Semler Brossy was independent pursuant to SEC and NYSE rules.
In December 2023, the HR and Compensation Committee engaged a new independent compensation consultant, Meridian Compensation Partners, LLC (“Meridian”). Meridian reported directly to the HR and Compensation Committee, and the HR and Compensation Committee had the sole authority to retain, terminate and obtain the advice of Meridian at the Company’s expense. The HR and Compensation Committee assessed the independence of Meridian pursuant to SEC and NYSE rules and concluded that there were no conflicts of interest.
Compensation Peer Group and Peer Selection Process
The HR and Compensation Committee believes that obtaining relevant market and benchmark data is very important to making determinations about executive compensation. This information provides a solid reference point for making decisions and very helpful context even though, relative to other companies, there are differences and unique aspects of the Company.
The HR and Compensation Committee takes into consideration the structure and components of, and the amounts paid under, the executive compensation programs of other, comparable peer companies, as derived from public filings and other sources when making decisions about the structure and component mix of our executive compensation program. The HR and Compensation Committee also considers broader industry practices and our competitors for talent.
The HR and Compensation Committee has developed and maintained a relevant group of peer companies (the “Peer Group”) that represent the companies with which we believe we primarily compete for talent. The HR and Compensation Committee generally use the following criteria when conducting the initial screening of the Peer Group:
| - | Industry: Pharmaceutical sector/subsector; |
| - | Company size: $10 billion to $40 billion of revenues, market capitalization of $10 billion to $160 billion, and a similar number of employees as Teva; and |
| - | Global presence and geography: Global footprint and breadth, with focus on U.S. and European markets; the HR and Compensation Committee made a conscious decision to include both U.S. and non-U.S. companies (in terms of headquarters and country of primary exchange listing) in order to reflect Teva’s international presence and competition in the global talent market. |
| 52 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
In general, the HR and Compensation Committee accords less weight to market capitalization due in part to the number of factors that can influence and cause volatility in the spot price of the common stock of a company. In addition, the HR and Compensation Committee views the consistency and robustness of the peer group over time as being very important.
The Peer Group established for setting 2023 compensation consisted of the following companies:
Company | Headquarters | Revenues (*) ($ in millions) (Fiscal Year | Market Cap (*) October 28, | Employees (*) | ||||||||||
AbbVie, Inc. | United States | $ | 56,197 | $ | 260,957 | 50,000 | ||||||||
Amgen, Inc. | United States | $ | 25,979 | $ | 146,469 | 22,000 | ||||||||
Astellas Pharma, Inc. | Japan | $ | 10,674 | $ | 25,056 | 15,455 | ||||||||
AstraZeneca Plc | United Kingdom | $ | 37,417 | $ | 181,792 | 70,600 | ||||||||
Biogen, Inc. | United States | $ | 10,982 | $ | 40,938 | 9,600 | ||||||||
Bristol-Myers Squibb Co. | United States | $ | 46,385 | $ | 170,535 | 30,000 | ||||||||
Eli Lilly & Co. | United States | $ | 28,318 | $ | 341,968 | 38,815 | ||||||||
Gilead Sciences, Inc. | United States | $ | 27,305 | $ | 99,354 | 14,400 | ||||||||
GlaxoSmithKline Plc | United Kingdom | $ | 46,192 | $ | 66,459 | 100,000 | ||||||||
Merck KGaA | Germany | $ | 22,389 | $ | 72,650 | 61,508 | ||||||||
Novo Nordisk A/S | Denmark | $ | 21,536 | $ | 245,311 | 49,295 | ||||||||
Sanofi | France | $ | 44,552 | $ | 107,357 | 95,442 | ||||||||
Takeda Pharmaceutical Co., Ltd. | Japan | $ | 49,580 | $ | 29,391 | 40,413 | ||||||||
Viatris, Inc. | United States | $ | 17,886 | $ | 12,211 | 37,000 | ||||||||
| (*) | Source: Dow Jones (October 2022). |
|
| Revenues ($ in millions) | Market Cap ($ in millions) | Employees | |||
Teva Pharmaceutical Industries Ltd. Percentile Rank | 13th | 0 | 49th | |||
Median | $27,812 | $103,356 | 44,055 | |||
The HR and Compensation Committee periodically reviews the Peer Group to ensure that the companies included continue to meet the primary selection criteria outlined above. The HR and Compensation Committee decided to keep the same Peer Group as the prior year.
The HR and Compensation Committee and the Board consider data from the Peer Group companies as a reference point in reviewing market pay levels, allocations and practices, but do not tie CEO and executive officer compensation to specific market percentiles. Other factors considered when setting the compensation of our CEO and executive officers include sustained performance, criticality of contributions to Teva, and the executive officer’s role, skills, experience and development. The HR and Compensation Committee retains discretion in determining the nature and extent of the use of Peer Group data.
Internal Considerations. The HR and Compensation Committee also reviews relevant internal ratios between executive officer compensation and the compensation of other employees, specifically the average and median values of other employee compensation, and its potential effect on the Company’s labor relations in connection with the review and approval of compensation to executive officers.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 53 | |
Executive Compensation
IV. COMPONENTS OF OUR COMPENSATION PROGRAM
2023 Components in General
The HR and Compensation Committee, Board and shareholders each participated in the selection of the components of compensation set forth in the chart below to achieve our stated executive compensation program objectives. The majority of the compensation of each executive officer is variable and at-risk and a significant portion is subject to the achievement of performance goals in order to be earned. The HR and Compensation Committee and the Board review all components of the compensation of executive officers in order to verify that each executive officer’s total compensation is consistent with our compensation philosophy and objectives and within the parameters set by our shareholder-approved Compensation Policy.
Element | Description | Additional Detail | ||
Base Salary |
Fixed cash compensation
Determined based on each executive officer’s role, individual skills, experience, performance, external market value and internal equity |
Base salaries are intended to provide stable compensation to executive officers, allow Teva to attract and retain qualified global executive talent and maintain a stable leadership team | ||
Short-Term Incentives: Annual Cash Incentive Opportunities |
Variable cash compensation based on the level of achievement relative to Company and individual performance objectives that are pre-determined annually
Weighted performance against goals must be at least 85% of target and other predetermined thresholds must be met in order for any payout to occur
Cash incentives are capped at a maximum of 200% of target cash award (133% for the CEO) if achievement level is at least 120% of performance goal
Per the Compensation Policy, a target cash award as a percentage of base salary is capped at 100% (150% for the CEO) |
Annual cash incentive opportunities are designed to ensure that our executive officers are incentivized to reach Teva’s annual goals; payout levels are determined based on actual financial and operational results, as well as individual performance | ||
| 54 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Element | Description | Additional Detail | ||
Long-Term Incentives: Annual Equity-Based Compensation |
Variable equity-based compensation
Per the Compensation Policy, the maximum monetary grant value of the annual equity award is $11.0 million at target for the CEO and $4.5 million at target for executive officers
Performance Share Units (“PSUs”): Restricted share units that are earned only upon the attainment of performance goals over the 3-year period
Corporate performance against goals must be at least 85% of target in order for the award to be earned for that metric
Awards are capped at 165% of target number of shares if achievement level is at least 120% of performance goal and relative TSR performance is at the 75th percentile of peers
Restricted Share Units (“RSUs”): Restricted share units that are time-based, with a 4-year vesting period |
Equity-based compensation is used to foster a long-term link between executive officers’ interests and the interests of Teva and its shareholders, as well as to attract, motivate and retain executive officers for the long term
In 2023, the CEO received 70% of the long-term incentive value granted in the form of PSUs and 30% in the form of RSUs; all other executive officers received 50% of the value granted in the form of PSUs and 50% in the form of RSUs | ||
2023 Target Pay Mix
The target pay mix supports the core principles of our executive officer compensation philosophy of compensating for performance and aligning executive officers’ interests with those of Teva and its shareholders, by emphasizing short- and long-term incentives.
The following charts outline the HR and Compensation Committee and the Board’s allocation of annual target total direct compensation payable to the CEO and to other NEOs. The HR and Compensation Committee and the Board allocated compensation among (i) base salary, (ii) short-term annual cash incentive opportunity and (iii) long-term annual equity.
A sizeable majority of target total direct compensation is variable, at-risk pay, consistent with our pay-for-performance philosophy. Specifically, in 2023, 88% of our CEO’s target total direct compensation was at-risk compensation, and 78%, on average, of the target total direct compensation of our other NEOs was at-risk compensation. We consider compensation to be “at risk” if it is subject to performance-based payment or vesting conditions or if its value depends on stock price appreciation.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 55 | |
Executive Compensation
Target Pay Mix
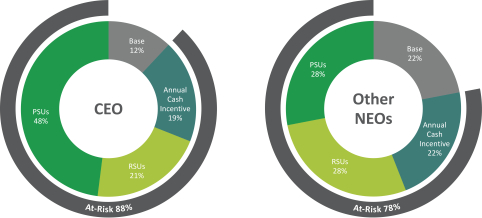
The percentages of target total direct compensation as calculated above are based on the annualized 2023 base salary, the 2023 annual cash incentive compensation opportunity (assuming achievement at the target level), and the grant date fair value of the annual equity grants. Each compensation element is outlined in more detail in the 2023 Summary Compensation Table and 2023 Grants of Plan-Based Awards table.
Base Salary
Base salaries provide stable compensation to executive officers, allow Teva to attract and retain qualified global executive talent and maintain a stable management team. Base salaries vary among executive officers and are individually determined according to each executive officer’s areas of responsibility, role and experience based on a variety of considerations, which may include, among other things, professional background (education, skills, expertise, professional experience and achievements and previous compensation arrangements, as relevant), external competitiveness, job criticality and internal fairness.
The HR and Compensation Committee and the Board established the base salary and other compensation in connection with the hiring of our new CEO, Richard D. Francis. The HR and Compensation Committee adjusted the annual base salary of our CFO, EVP, International Markets Commercial and EVP, Global Operations to bring them more in line with market levels.
Executive | Annualized ($) | Annualized ($)(1) | 2022-2023 % Change | |||||||||
Richard Francis | NA | $ | 1,600,000 | NA | �� | |||||||
Eli Kalif | $ | 799,658 | $ | 800,869 | 0% (2) | |||||||
Richard Daniell | NA | $ | 790,298 | NA (3) | ||||||||
Eric Drapé | $ | 700,723 | $ | 747,485 | 7% (4) | |||||||
Mark Sabag | $ | 744,787 | $ | 691,679 | -7% (2) | |||||||
| (1) | Annualized base salary as of December 31, 2023 converted from local currency, where relevant, using a 2023 annual average exchange rate. |
| (2) | Mr. Kalif and Mr. Sabag are paid in Israeli shekels. The change in salary shown for Mr. Kalif is due to a 10% salary increase and fluctuations in exchange rates, and the change in salary for Mr. Sabag is due to a 2% salary increase and fluctuations in exchange rates. |
| (3) | Mr. Daniell is paid in British pounds. |
| (4) | Mr. Drapé is paid in Euros. The change in salary shown is due to a 4% salary increase and fluctuations in exchange rates. |
| 56 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Annual Cash Incentives
The annual cash incentive component aims to incentivize our executives to reach our annual goals. Annual cash incentives are designed to provide a significant pay-for-performance element of our executive compensation package, as payout eligibility and levels are determined based on actual financial and operational performance, as well as individual performance.
Pursuant to our Compensation Policy, which serves as a shareholder-approved framework, executive officer target annual cash incentive opportunity is capped at 100% of annual base salary, and for the CEO, target annual cash incentive opportunity is capped at 150% of annual base salary. In addition, the maximum annual cash incentive payout cannot exceed 200% of target annual cash incentive.
The 2023 annual cash incentive plan offers incentives to the executive officers to accomplish certain short-term financial results that the HR and Compensation Committee and Board view as key steps in the execution of our overall business strategy, with the intent ultimately of increasing shareholder value.
The amount of the payout, if any, under the annual cash incentive plan is determined as follows:
Eligible Salary
| X |
Target Incentive %
| X |
Overall Performance Factor %
|
=
Annual Cash Incentive Plan Payout
|
Target Opportunities
The HR and Compensation Committee and the Board determine the target annual cash incentive opportunity available to each NEO by taking the individual’s base salary and multiplying it by the individual’s target incentive percentage. The HR and Compensation Committee and the Board did not change the target incentive percentages for our recurring NEOs from 2022 levels.
Executive | 2023 Target Annual Cash Incentive (% of Base Salary) | |
Richard Francis | 150% | |
Eli Kalif | 100% | |
Richard Daniell | 100% | |
Eric Drapé | 100% | |
Mark Sabag | 100% | |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 57 | |
Executive Compensation
Performance Measures
The HR and Compensation Committee and the Board used the same performance measure categories, Company Financial and Individual, and weightings as in 2022:
Category | Weighting | Metric Weighting | ||
Company Financial | 75% | 50% Non-GAAP EPS
| ||
|
| 25% Free Cash Flow
| ||
Individual | 25% | See Below | ||
Company Financial Measures. The HR and Compensation Committee and the Board believe that certain financial measures are key performance indicators of the present and future prospects of our business and key drivers of shareholder value, and selected the following financial measures for use in the annual cash incentive plan:
| - | Non-GAAP Earnings Per Share: This measure of income is calculated as net income attributable to ordinary shareholders divided by the weighted average number of shares outstanding (fully diluted) and represents profitability. It focuses managers on expense control in addition to revenue generation and is viewed as a strong indicator of sustained performance over the short- and long-term. |
| - | Free Cash Flow: This measure, for purposes of the Annual Cash Incentive, is calculated as the cash flow generated from operating activities, cash received for beneficial interest collected in exchange for securitized trade receivables (under our EU securitization program) and proceeds from divestitures of businesses and other assets, net of capital investments. It serves to focus employees on generating cash in the short- and long-term to fund operations and pay debt. It focuses managers on expense control in addition to revenues and on improvement in working capital. |
These performance measures were selected because they focus management on metrics that align with our most critical strategic priorities of generating revenue, controlling expenses and improving profitability, and generating cash and servicing debt, and give a clear line of sight into how achieving operating goals drives Teva performance and generates rewards.
We use non-GAAP measures as our compensation performance metrics, as do many other companies, because they are used by management and our Board, in conjunction with other performance metrics, to evaluate our operational performance, and to prepare and evaluate our work plans and annual budgets. While other qualitative factors and judgment also affect the determination of performance targets that in turn determine the levels of annual compensation, the principal quantitative element in the determination of such compensation are performance targets tied to the work plan, which are based on these non-GAAP measures. The non-GAAP measures we use as the basis of our compensation performance metrics exclude items which management believes are non-recurring and not reflective of the Company’s underlying business performance so that compensation is not disproportionately impacted by factors unrelated to the ongoing operation of our business by management. The adjustments made to arrive at non-GAAP measures enable the metrics to reflect the core business performance of the Company and executive officers for the performance period. Using the non-GAAP measures as the basis for our compensation performance metrics aligns such metrics with the intent of the HR and Compensation Committee in setting business performance-related goals for our executive officers. For more information on the non-GAAP measures we use for our compensation performance metrics and how such non-GAAP measures are calculated, please see the earnings press release furnished with our Current Report on Form 8-K filed with the SEC on January 31, 2024. For a reconciliation of these non-GAAP measures to their most directly comparable financial measures calculated and presented in accordance with GAAP, please see Appendix A hereto.
| 58 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Threshold, Target, and Maximum Company Performance Levels. The HR and Compensation Committee and the Board set the 2023 threshold, target and maximum performance levels at levels that were considered rigorous, aggressive and challenging, attainable only with strong performance, and that took into account the relevant risks and opportunities.
The HR and Compensation Committee developed in conjunction with our 2023 business plan and corresponding incentive plan performance metric targets, which were based on the business plan and were aligned to the middle of the range of our outlook as communicated to investors in February 2023. In setting these goals, the HR and Compensation Committee took into account various factors, including forecasted growth levels for the global economy, for the geographic areas where the Company operates and for the market for generic products, as well as the recognition that goals were being established before our new CEO’s development and implementation of the Pivot to Growth strategy. In addition, the goals were based on key assumptions communicated to investors in February 2023, including:
| - | Anticipated continued decline in COPAXONE revenue from $691 million in 2022 to approximately $500 million in 2023, due to an expected increase in generic competition; |
| - | Anticipated continued increase of AUSTEDO revenue from $971 million in 2022 to approximately $1.2 billion in 2023; and |
| - | Anticipated continued increase of global AJOVY revenue from $377 million in 2022 to approximately $400 million in 2023. |
In light of all of these factors, these goals were considered rigorous, aggressive and challenging, attainable only with strong performance, and took into account the relevant opportunities and risks we were facing.
After setting the targets, the HR and Compensation Committee and the Board also set the threshold and maximum performance levels. To achieve threshold performance, 85% of target performance for each Company performance metric must be attained, which is a rigorous and challenging level of achievement that must be met. An achievement percentage of less than 85% of target for either Non-GAAP EPS or Free Cash Flow would result in no annual cash incentive payment for executive officers. The HR and Compensation Committee and the Board set the maximum level of performance at 120% of target for each Company performance metric, a level that presented a significant challenge requiring exceptionally strong performance. Based on the annual results as reported in the Annual Report on Form 10-K for the year ended December 31, 2023, the Company’s achievement should have been 107% of our 2023 Non-GAAP EPS target, and 122% of our 2023 Free Cash Flow target. However, in October 2023, the Company had announced a collaboration with Sanofi to co-develop a Teva product candidate for the treatment of Ulcerative Colitis and Crohn’s Disease under which Teva received an upfront payment in 2023 of $500 million. The HR and Compensation Committee and the Board determined to modify downward the Company Financial performance achievement, so that the outcome did not result in an unintended windfall, while still recognizing the considerable effort expended to achieve the result and the strategic and financial significance of this collaboration. The HR and Compensation Committee reduced the Company Financial performance achievement to 100%, a reduction of approximately 7% from the performance achievement of Non-GAAP EPS and 22% from the performance achievement of Free Cash Flow determined when the upfront payment was included.
Weighting | Performance Metric | Threshold (85%) | Target (100%) | Maximum (120%) | Actual Results | % Achievement | Downward Adjusted % Achievement | |||||||
50% | Non-GAAP EPS | $2.04 | $2.40 | $2.88 | $2.56 | 107% | 100% | |||||||
25% | Free Cash Flow | $1.66B | $1.96B | $2.35B | $2.39B | 122% | 100% | |||||||
Individual Measures. The remaining 25% of the measures under the 2023 annual cash incentive plan were individual performance measures established by the HR and Compensation Committee and the Board early
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 59 | |
Executive Compensation
in the year. These measures included components specific to the nature of each executive officer’s position and area of responsibility. The HR and Compensation Committee and the Board established measures that it viewed as rigorous and challenging, requiring significant effort to achieve. The HR and Compensation Committee and the Board evaluated performance with respect to the individual measures by determining an individual performance rating and individual performance achievement percentage, which was then used as a component for determining the overall performance factor.
Executive | Individual Goals | Key Performance Accomplishments | % Achievement | |||
Richard Francis | - Developing and beginning to implement a corporate growth strategy - Achieving global sales targets - Driving innovative product growth - Achieving Sustainability targets related to reduction in absolute Scope 1, 2 and 3 Greenhouse Gas emissions; developing, launching, and expanding access to medicine programs; completing new regulatory submissions in low- and middle-income countries of Teva products on the World Health Organization Essential Medicines List across several therapeutic areas; and increasing ESG rating scores
| - Led the formulation of our “Pivot to Growth” strategy, which was presented at a May 2023 Investor Day - Increased global revenues by 6% in U.S. dollars, or 7% in local currency terms compared to 2022 - Grew AUSTEDO by 28% and AJOVY by 16% compared to 2022 - Launched UZEDY in the U.S. in May 2023 - Collaborated with Royalty Pharma to accelerate clinical research with a funding agreement - Collaborated with Sanofi on our anti-TL1A asset; Teva received an upfront payment of $500 million in 2023. - Continued to build out management team with appointment of two new executive officers in 2023 - By end of 2023, launched 7 access programs (out of a 2025 target of 8) and made 46 regulatory submissions (61% of the 2025 target); continued to increase or maintain our ESG ratings performance
| 120% | |||
Eli Kalif | - Supporting the new CEO in developing and beginning to implement a corporate growth strategy - Achieving financial targets - Achieving employee engagement and retention targets - Achieving Sustainability targets related to Sustainability-Linked Bonds and external commitments - Achieving other strategic initiatives
| - Collaborated in the development of our “Pivot to Growth” strategy, which was presented at a May 2023 Investor Day - Generated free cash flow of $2,387 million, a 6% increase compared to 2022 - Non-GAAP operating income was $4,361 million, a 5% increase compared to 2022 - Non-GAAP diluted earnings per share were $2.56, a 2% increase compared to 2022 - Continued to make steady progress toward sustainability goals
| 110% | |||
| 60 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Executive | Individual Goals | Key Performance Accomplishments | % Achievement | |||
Richard Daniell | - Supporting the new CEO in developing and beginning to implement a corporate growth strategy - Achieving regional sales targets and sales targets for new innovative and generic products - Achieving employee engagement and retention targets - Achieving Sustainability targets related to developing and launching an access to medicines program - Achieving other strategic initiatives
| - Collaborated in the development of our “Pivot to Growth” strategy, which was presented at a May 2023 Investor Day - Increased Europe revenues by 7% in U.S. dollars, or 5% in local currency terms compared to 2022 - Grew AJOVY in Europe by 29% in U.S. dollars, or 27% in local currency terms compared to 2022 - Continued to make steady progress toward sustainability goals
| 97% | |||
Eric Drapé | - Supporting the new CEO in developing and beginning to implement a corporate growth strategy - Achieving targets related to quality, environmental, health and safety, and customer service - Achieving global gross margin and business unit targets - Achieving employee engagement and retention targets - Achieving Sustainability targets related to reduction in absolute scope 1, 2 and 3 Greenhouse Gas emissions and mitigation for antimicrobial resistance discharges
| - Collaborated in the development of our “Pivot to Growth” strategy, which was presented at a May 2023 Investor Day - Continued the implementation of our global Environment Health and Safety management system in all countries where we operate - Completed numerous inspections by various regulatory agencies of our finished dosage pharmaceutical and API plants and actively engaged in discussions with authorities to mitigate drug shortages; participated in several industry-wide task forces - Continued to promote climate change mitigation and adaptation strategy according to international standards
| 60% | |||
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 61 | |
Executive Compensation
Executive | Individual Goals | Key Performance Accomplishments | % Achievement | |||
Mark Sabag | - Supporting the new CEO in developing and beginning to implement a corporate growth strategy - Achieving regional sales targets and sales targets for new innovative and generic products - Achieving employee engagement and retention targets - Achieving Sustainability targets related to developing, launching, and expanding access to medicine programs, and completing new regulatory submissions in low- and middle-income countries of Teva products on the World Health Organization Essential Medicines List across several therapeutic areas - Achieving other strategic initiatives
| - Collaborated in the development of our “Pivot to Growth” strategy, which was presented at a May 2023 Investor Day - Increased International Markets revenues by 3% in U.S. dollars, or 16% in local currency terms compared to 2022 - Grew AJOVY in International Markets by 25% in U.S. dollars, or 35% in local currency terms, compared to 2022 - Despite the war in Israel, maintained full business continuity with uninterrupted supply of medicines - Continued to make steady progress toward sustainability goals
| 110% | |||
Compliance Modifier. Strong individual goal performance by the CEO and NEOs, as measured by the various components, is fully rewarded only if there are no substantial compliance events. Individual goals performance achievement may be decreased by up to 100% if there is a substantial compliance event.
Embedding Sustainability in Individual Measures. The HR and Compensation Committee and the Board set the individual goals for executive officers to ensure greater alignment with key strategic priorities. In addition to continuing to include items that align with other strategic objectives, a continuing area of focus was sustainability. In recent years, investors and other stakeholders have brought sustainability issues to the fore. The CEO and all other executive officers were given objective and measurable goals directly related to our renewed sustainability strategy including reduction in absolute Scope 1, 2 and 3 Greenhouse Gas emissions; developing, launching, and expanding access to medicine programs; completing new regulatory submissions in low- and middle-income countries of Teva products on the World Health Organization Essential Medicines List across several therapeutic areas; compliance and ethics, inclusion and diversity, and other objectives.
| 62 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Overall Performance Factor and Payout Calculation
Overall Performance Factor. The HR and Compensation Committee and the Board calculated an overall performance factor for each executive officer by taking the weighted average of the achievement percentages of the Company financial measures and the individual measures. Specifically, the HR and Compensation Committee takes the non-GAAP EPS achievement percentage times the weight allocated to that measure, the Free Cash Flow achievement percentage times the weight allocated to that measure, and the individual measure achievement percentage for each NEO and the weight allocated to the individual measure, and determines the overall weighted average achievement percentage. As a last step, that overall weighted average achievement percentage is then converted to an overall performance factor based on the payout curve set forth in the table below.
Weighted Average Level of Achievement of Objectives | Overall Performance Achievement % (1) | Overall Payout Performance Factor: Potential Annual Cash Incentive | ||
Below Threshold | Below 85% | 0% (no annual cash incentive payment) | ||
Threshold | 85% | 25% | ||
Target | 100% | 100% | ||
Maximum Cash Incentive | 120% | 200% (133% for CEO) | ||
| (1) | Payouts are determined linearly based on a straight-line interpolation of the applicable payout range (i.e., for the CEO 5.0% for each percentile change in weighted average performance between threshold and target and 1.7% for each percentile change in performance between target and maximum, and for other executive officers, 5% for each percentile change in weighted average performance between threshold and maximum). No additional payout is made for weighted average performance achievement in excess of 120%. |
The HR and Compensation Committee and the Board reviewed Company financial and individual performance against objectives in order to make determinations regarding whether any payouts were due under our annual incentive plan. The table below sets forth the calculation of the overall performance achievement percentage and performance factor for our CEO and other NEOs:
Executive | Non-GAAP EPS % Achievement (50% weighting) | Free Cash Flow (25% weighting) | Individual % Achievement (25% weighting) | Overall Weighted Avg | Overall Factor% | |||||||||||||||
Richard Francis | 100% | 100% | 120% | 105% | 108% | |||||||||||||||
Eli Kalif | 100% | 100% | 110% | 103% | 113% | |||||||||||||||
Richard Daniell | 100% | 100% | 97% | 99% | 96% | |||||||||||||||
Eric Drapé | 100% | 100% | 60% | 90% | 50% | |||||||||||||||
Mark Sabag | 100% | 100% | 110% | 103% | 113% | |||||||||||||||
The HR and Compensation Committee and the Board then took the target award opportunity and applied the overall payout performance factor to determine the total 2023 annual incentive plan payout for the CEO and each NEO.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 63 | |
Executive Compensation
This amount is reflected in the “Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table presented below under the “Additional Compensation Information” section.
Executive | Eligible Base ($) (1) | Target Annual Cash Incentive (% of Base Salary) | Target Award ($) | Overall Payout Performance Factor (2) | Payout ($) (3) | |||||||||||||||
Richard Francis | $ | 1,600,000 | 150 | % | $ | 2,400,000 | 108 | % | $ | 2,600,000 | ||||||||||
Eli Kalif | $ | 781,918 | 100 | % | $ | 781,918 | 113 | % | $ | 880,504 | ||||||||||
Richard Daniell | $ | 772,754 | 100 | % | $ | 772,754 | 96 | % | $ | 743,372 | ||||||||||
Eric Drapé | $ | 740,353 | 100 | % | $ | 740,353 | 50 | % | $ | 370,147 | ||||||||||
Mark Sabag | $ | 688,147 | 100 | % | $ | 688,147 | 113 | % | $ | 774,323 | ||||||||||
| (1) | Eligible base salary is generally defined as actual base salary earned during the year. |
| (2) | Percentages have been rounded to the nearest whole percentage. |
| (3) | Annual cash incentive payout amount is calculated in the currency in which the amount is paid and converted to U.S. dollars. |
Long-Term Incentive Equity-Based Compensation
The third and largest main component of the executive compensation program is long-term equity incentives.
Equity-based compensation is intended to incentivize and reward for future long-term performance, as reflected by the market price of our shares and/or other performance criteria, and is used to foster a long-term link between executive officers’ interests and the interests of Teva and its shareholders. Equity-based compensation is also intended to attract, motivate and retain executive officers for the long term by (i) providing them with a meaningful interest in Teva’s share performance; (ii) linking equity-based compensation to potential and sustained performance; and (iii) spreading benefits over a longer performance cycle through the vesting period mechanism.
Pursuant to our Compensation Policy, which serves as a shareholder-approved framework, the minimum full vesting period of all equity-based awards is three years from the date of grant (partial vesting can occur before), the maximum monetary grant date fair value of annual equity-based awards granted to the CEO cannot exceed $11 million at target and to any other executive officer $4.5 million at target, and the maximum number of shares settled for a performance-based equity award shall not exceed 250% of the target number of shares granted.
| 64 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Equity Vehicles and Mix
The HR and Compensation Committee and the Board used the equity vehicles and mix set forth in the following table:
Type of Long- Vehicle | Proportion of Long- Term Incentive Grant | Vesting Schedule | Performance
| Rationale for Use of Performance Metrics | ||||
Performance Share Units (PSUs) | 70% for CEO; 50% for other executive officers | Three-year cliff vesting | 2023-2025 Non-GAAP Operating Income (50%) | Leading indicator of profitability, expense control and sustained long-term performance
| ||||
| 2023-2025 Net Revenue from Innovative Products (25%) | In alignment with Pivot to Growth strategy, this is a primary measure of top line growth from patented, proprietary Teva products, to increase specific focus on increasing this aspect of the business
| |||||||
| 2023-2025 Net Revenue from All Other Products (25%) | In alignment with Pivot to Growth strategy, this is a primary measure of top line growth from generic, biosimilar and other Teva products
| |||||||
| 2023-2025 Relative TSR (Modifier) | Strong performance, as measured by the other three operating metrics, is fully rewarded only if it also results in above-median shareholder returns. TSR ties executive officer compensation to shareholder value creation and aligns the interests and experience of executive officers with those of Teva and its shareholders. Relative TSR filters out macroeconomic and other factors that are not within management’s control. The relative TSR modifier for the 2023 award decreases or increases the average earning percentage by up to 10%.
| |||||||
Restricted Share Units (RSUs) | 30% for CEO; 50% for other executive officers |
Four equal tranches vesting on the first, second, third and fourth anniversaries of the date of grant | N/A | N/A | ||||
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 65 | |
Executive Compensation
The HR and Compensation Committee and the Board have structured the mix of equity vehicles and the relative weight assigned to each type to motivate performance against long-term targets and stock price appreciation over the long term and to encourage ownership and retention while aligning executive officers’ interests with those of our shareholders. The RSUs are complementary to the PSUs because they have upside potential but deliver some value even during periods of market or stock price underperformance, providing a retention incentive and reinforcing an ownership culture and commitment to Teva.
Performance Metrics
Financial Measures
All metrics give recipients a clear line of sight into how achieving operating goals drives Teva performance and generates rewards.
For purposes of the PSU performance measures:
| 1) | Non-GAAP Operating Income is defined as consolidated operating income (loss) excluding goodwill impairment; legal settlements and loss contingencies; amortization of purchased intangible assets; intangible assets impairment; other asset impairments, restructuring and other items; and other unallocated items. For a reconciliation of Non-GAAP Operating Income to the most directly comparable financial measure calculated and presented in accordance with GAAP, please see Appendix A hereto. |
| 2) | Net Revenue from Innovative Products is defined as GAAP Net Revenue as reported in the financial statements included in the Company’s Annual Report on Form 10-K for the applicable years for products in our innovative portfolio. |
| 3) | Net Revenue from All Other Products is defined as GAAP Net Revenue from as reported in the financial statements included in the Company’s Annual Report on Form 10-K for the applicable years, excluding products in our innovative portfolio. |
Before the conclusion of the three-year performance period, we do not publicly disclose our specific performance measure targets and the corresponding minimums and maximums because of the potential for competitive harm from such disclosure. These measures are competitively sensitive and would reveal information about our view of our anticipated trajectory, which is not otherwise public. The HR and Compensation Committee and the Board believe that they have set performance goals at rigorous and challenging levels so as to require significant effort and achievement by our executive officers to be attained, and that such goals have been established in light of our internal forecast as well as the macroeconomic and industry environments. After the end of the performance period, the targets and achievement relative to such targets will be disclosed.
Target, Threshold and Maximum Performance Levels. Similar to the annual cash incentive plan, for the PSUs, the HR and Compensation Committee and the Board defined payout levels representing the number of PSUs to be earned by executive officers based on the level of actual performance relative to the three-year targets.
| 66 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
For each of the three measures, Non-GAAP Operating Income, Net Revenue from Innovative Products and Net Revenue from All Other Products, the HR and Compensation Committee determines the Company’s performance achievement percentage for the three-year period. The performance achievement percentage is then converted to an earning percentage as set forth below.
Level of Achievement of Objectives (*) | Performance Achievement % | Earning Percentage | ||||||||
Below Threshold | Below 85% | 0% | ||||||||
Threshold | 85% | 62.5% | ||||||||
Target | 100% | 100% | ||||||||
Maximum | 120% | 150% | ||||||||
| (*) | Linear interpolation will be used to determine the applicable earning percentage between levels. |
The HR and Compensation Committee then calculates the weighted average of the earning percentages for the three performance measures.
Relative TSR Modifier. The HR and Compensation Committee and the Board view the inclusion of Total Shareholder Return as critical because it ties executive officer compensation to the shareholder experience and the creation of shareholder value, and it aligns the interests of executive officers with those of Teva and its shareholders. By measuring our stock performance relative to peers, it mitigates the impact of macroeconomic factors, both positive and negative, that affect the industry and/or stock price performance and are beyond the control of management, and it provides rewards that are more directly aligned with performance through different economic cycles.
The average of the earning percentages is multiplied by a modifier that has been determined based on our relative TSR performance for the three-year period as set forth below. The Company’s TSR is ranked relative to our Peer Group; the HR and Compensation Committee and the Board believe that the Peer Group is an appropriate comparator group that is broadly representative in terms of its size, industry, geographic footprint and employee base. The HR and Compensation Committee and the Board tightened the range of the modifier in 2023 to a range of 90% to 110% to reflect a reasonable range of modification and to appropriately balance the various PSU performance elements. See “—III. Compensation Determination Process—Compensation Peer Group and Peer Selection Process” for a list of the peer group companies used for this purpose.
Level of Achievement of Relative TSR (*) | Relative TSR Ranking | Modifier | ||
Threshold | Up to 25th percentile | 90% (i.e., 10% less than unmodified average of the earning percentages) | ||
Target | 50th percentile | 100% | ||
Maximum | 75th percentile or above | 110% | ||
| (*) | Linear interpolation will be used to determine the applicable modifier. |
The product of (1) the average of the earning percentages and (2) the relative TSR modifier is multiplied by the target number of PSUs granted to each of the executive officers to determine the final number of PSUs earned by each individual. For example, if the Company’s TSR rank is less than or equal to the 25th percentile, then the average of the earning percentages is multiplied by 90% (equivalent to a reduction of 10%) to determine the final performance factor and then multiplied by the target number of PSUs to determine the final number of earned PSUs. If the Company’s TSR rank is at the 75th percentile or above,
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 67 | |
Executive Compensation
then the average of the earning percentages is multiplied by 110% (equivalent to an increase of 10%) and then incorporated into the calculation.
Average Earning Percentage
| X |
Relative TSR Modifier (90-110%)
| X |
Target Number of PSUs
|
=
Number of Earned PSUs
|
Ultimately, the HR and Compensation Committee approves and presents the performance achievement percentages, the calculation of the average earning percentage and the TSR modifier, and the determination of the number of PSUs earned by each executive officer to the Board for its review and approval.
2023 Grants of PSUs and RSUs
In making determinations about 2023 long-term equity incentive grants to executive officers, the HR and Compensation Committee and the Board considered, among other things: sustained performance; criticality of contributions to Teva; comparison against our Peer Group; the executive officer’s role, skills, experience and development; internal fairness among executive officers; current value of prior granted equity and pay mix. The portion of executive officer compensation that is composed of these equity vehicles is “at risk” and directly aligned with shareholder value creation.
The following table sets forth the 2023 annual grant date fair values at target approved by the HR and Compensation Committee and the Board for the CEO and the other NEOs.
Executive | PSUs ($) (*) | RSUs ($) (*) | Total ($) (*) | ||||||||||||
Richard Francis | $ | 6,300,000 | $ | 2,700,000 | $ | 9,000,000 | |||||||||
Eli Kalif | $ | 1,000,000 | $ | 1,000,000 | $ | 2,000,000 | |||||||||
Richard Daniell | $ | 1,000,000 | $ | 1,000,000 | $ | 2,000,000 | |||||||||
Eric Drapé | $ | 1,000,000 | $ | 1,000,000 | $ | 2,000,000 | |||||||||
Mark Sabag | $ | 1,000,000 | $ | 1,000,000 | $ | 2,000,000 | |||||||||
| (*) | Equity values have been rounded to the nearest $10,000. |
Consistent with historical practice, the dollar value allocated to PSUs was converted to a number of units, based on the grant date fair value as determined in accordance with the Financial Accounting Standards Board Accounting Standards Codification Topic 718. The dollar amount allocated to RSUs was converted to a number of shares using the fair market value on the grant date.
| 68 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
2021-2023 Performance Share Unit Payout
In 2021, the HR and Compensation Committee and the Board selected Net Revenue and Non-GAAP Operating Income as metrics in the PSUs and granted PSUs with performance-based vesting requirements for the three-year performance period of 2021-2023. In connection with the 2021 PSU grants, the number of PSUs earned by the NEOs who were executive officers at the time the grants were made has been determined in two steps as follows.
In step one, there were two Company financial performance measures, 2021-2023 Non-GAAP Operating Income and 2021-2023 Net Revenue, each of which was weighted 50%. For each of these two measures, the HR and Compensation Committee and the Board determined the Company’s performance achievement percentage for the three-year period. The performance achievement percentage was then converted to an earning percentage based on the following:
Level of Achievement of Objectives (*) | % Achievement of Target | Earning Percentage | ||
Below Threshold | Below 85% | 0% | ||
Threshold | 85% | 25% | ||
Target | 100% | 100% | ||
Maximum | 120% | 200% | ||
| (*) | Linear interpolation was used to determine the applicable earning percentage between levels. |
The HR and Compensation Committee and the Board then calculated the average of the earning percentages for the two performance measures.
In step two, this average of the earning percentages was multiplied by a modifier that was determined based on our TSR performance relative to the companies included in the peer group used as a reference point for 2021 compensation decisions for the three-year period, as set forth below.
Level of Achievement of Relative TSR (*) | Relative TSR Ranking | Modifier | ||
Threshold | Up to 25th percentile | 80% (i.e., 20% less than unmodified average of the earning percentages) | ||
Target | 50th percentile | 100% | ||
Maximum | 75th percentile | 120% | ||
| (*) | Linear interpolation was used to determine the applicable modifier. |
The product of (1) the average of the earning percentages and (2) the relative TSR modifier was multiplied by the target number of PSUs granted to the NEOs, to determine the final number of PSUs earned by each individual, except that the number of PSUs to be issued could not exceed 240% of the target number of PSUs.
The HR and Compensation Committee and the Board approved an average earning percentage of 67% which was then subject to a relative TSR modifier, which adjusted the earning percentage to be 82% of the actual earning percentage, resulting in a modified earning percentage of 55%.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 69 | |
Executive Compensation
Weighting | Performance Metric | Threshold (85%) ($B) (*) | Target (100%) ($B) (*) | Maximum (120%) ($B) (*) | Actual Results ($B) | % Achievement | Earning % | |||||||
| 50% | Non-GAAP Operating Income | 11,807 | 13,890 | 16,668 | 12,901 | 93% | 64% | |||||||
| 50% | Net Revenue | 42,199 | 49,646 | 59,575 | 46,649 | 94% | 70% | |||||||
| Weighted Average: |
|
|
|
|
|
| 67% | |||||||
Relative TSR Modifier (-18%, reflecting 27th percentile) |
|
|
| 82% | ||||||||||
Final Earning % |
|
|
|
| 55% | |||||||||
| (*) | We applied actual foreign exchange rates throughout the period when determining performance achievement. |
Based on this outcome, the NEOs earned Teva PSUs in respect of their 2021-2023 PSU awards as follows (our 2023 CEO had not joined Teva in 2021 when these PSUs were granted):
Executive | Target Award (# of PSUs) | Final Earning Percentage (*) | Final (# of PSUs) | |||||||||
Eli Kalif | 95,057 | 55% | 52,320 | |||||||||
Richard Daniell | 100,337 | 55% | 55,226 | |||||||||
Eric Drapé | 100,337 | 55% | 55,226 | |||||||||
Mark Sabag | 105,618 | 55% | 58,133 | |||||||||
| (*) | Percentages have been rounded to the nearest whole percentage. |
RSU Grants to Two NEOs
In certain specific circumstances and on rare occasion, the HR and Compensation Committee and the Board may award executive officers supplemental cash or equity grants as an important way to meet the Company’s needs for a specific purpose or during a specific period. In the global pharmaceutical industry, there is heavy competition for executive officers with the skills and experience needed to execute our strategy and lead our team, especially during the critical planning and initial phases of execution of the Pivot to Growth strategy adopted in 2023. Various factors, including geopolitical circumstances, pharmaceutical industry market dynamics, inflation and the employment market have made the situation even more challenging. In addition, our executive officers are desirable as candidates for employment with other organizations, and they receive substantial ongoing interest from other companies. Attracting and retaining executive talent of the caliber of our leaders, and maintaining a stable, consistent team, particularly during a period of leadership transition, is both extremely challenging and vitally important. Our ability to execute our strategy, our future success and the promotion and protection of the interests of our shareholders depend to a great extent on our continuing ability to retain highly qualified and skilled employees. In light of these factors, the HR and Compensation Committee and the Board determined that it was in the best interest of the Company and our shareholders to make certain supplemental RSU grants to Eli Kalif with a fair value of $1.0 million and Richard Daniell with a fair value of $1.5 million, vesting over four years.
| 70 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Leadership Transitions Effective in 2023
Appointment of Mr. Richard D. Francis as President and CEO
As previously disclosed in our 2023 Proxy Statement, as Mr. Schultz’s agreement was due to expire in November 2023, in 2022 our Board led a global search process (with the assistance of a search firm) to find our next President and CEO. In November 2022, the Board successfully completed this process when it appointed Mr. Francis to the position effective as of January 1, 2023, and he joined the Board at that time. Under Mr. Schultz’s leadership, we stabilized the business and executed a significant operational transformation across our international footprint. In its search, the Board sought a leader with extensive global pharmaceutical experience and both generic and branded experience, who could build on this solid foundation and drive growth.
The terms of the employment agreement with Mr. Francis were negotiated in order to induce him to accept the Board’s offer to become our President and CEO, and to render services from the Company’s principal offices in Israel. The Board was mindful of the critical importance of returning to growth, the fierce competition for talented executives in the pharmaceutical industry and the extreme pressure on, and vast duties of, the leader of an international organization of the size and complexity of Teva in approving the components of the compensation package, including the annual base salary, target annual incentive, annual equity grants, and one-time inducement equity grants and cash awards to Mr. Francis. Accordingly, the Board, with the assistance of its independent compensation consultant, developed a compensation package that considered pay structures for CEOs at peer companies, and which includes pay that depends on long-term Company performance as well as the opportunity to accumulate a significant ownership interest in Teva upon the creation of sustained shareholder value. In light of all of these factors, we entered into an employment agreement with Mr. Francis which provides for an initial employment term of three years, subject to automatic renewal for subsequent one-year periods unless either party gives at least 180-day advance notice of non-renewal or termination.
Under the Employment Agreement, Mr. Francis receives an annual base salary of $1.6 million, a performance-based target annual cash incentive opportunity equal to 150% of his annual base salary (and a maximum opportunity of 200% of his annual base salary), annual long-term equity incentives with a total target grant date fair value of $9 million with vesting terms similar to other senior executive officers, 70% of which are performance-based and in the form of PSUs and 30% of which are in the form of RSUs, and the same employee benefits as are provided to similarly situated senior executives of the Company.
Upon commencing employment, in light of forfeited compensation opportunities at his prior employer and to incentivize him to join the Company, Mr. Francis received (a) the following sign-on equity awards: (i) an RSU award with a grant date fair value of $5 million, which will vest in three equal installments on the first, second and third anniversaries of the grant date, subject to his continued employment through the applicable vesting dates; and (ii) a PSU award with a target grant date fair value of $5 million, which will be earned based on the achievement of stretch performance goals as described below, and (b) a sign-on cash award of $5 million, which will vest and be paid in three installments, with the first installment, equal to $1,500,000, paid on the first business day following the date that Mr. Francis commenced employment with the Company (the “Effective Date”), the second installment, equal to $1,500,000, paid on the first business day following the first anniversary of the Effective Date, and the third installment, equal to $2,000,000, to be paid on the first business day following the third anniversary of the Effective Date, in each case subject to his continued employment through the applicable vesting date. In connection with rendering services from the Company’s principal offices in Israel, Mr. Francis also receives certain housing and travel reimbursements, annual allowance for financial and tax advisory fees, and certain other relocation benefits in accordance with the Company’s policies. The Employment Agreement also provided for a one-time legal fee reimbursement.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 71 | |
Executive Compensation
The PSU award will be earned based on achievement over the 2023-2025 performance period relative to four performance goals, each weighted equally: 1) Non-GAAP Operating Margin; 2) Net Debt reduction; 3) Objectives related to Teva’s Sustainability-Linked Bonds, including greenhouse gas emissions reductions, regulatory submissions of medicines on the WHO Essential Medicines List in low and middle income countries, and increases in access to medicines program product volume; and 4) Revenue growth of a specified compound annual growth.
The HR and Compensation Committee and the Board will determine the Company’s performance achievement percentage for the three-year performance period. For three of these four measures (non-GAAP operating margin, net debt reduction, and revenue growth), the performance achievement percentage will then be converted to an earning percentage based on the following:
Level of Achievement of Objectives (*) | % Achievement of Target | Earning Percentage | ||||||||
Below Threshold | Below 85% | 0% | ||||||||
Threshold | 85% | 85% | ||||||||
Target | 100% | 100% | ||||||||
Maximum | 120% or above | 120% | ||||||||
| (*) | Linear interpolation was used to determine the applicable earning percentage between levels. |
For the objectives related to the Sustainability-Linked Bonds, achievement must be 100% to earn any payout for that category of goals. Achievement above 100% will be converted to an earning percentage based on the table above. The HR and Compensation Committee and the Board will then calculate the weighted average of the earning percentages for the four performance measures. The number of PSUs earned will be determined by multiplying the target number of PSUs granted by the PSU earning percentage. Any PSUs that do not become earned PSUs based on performance at the end of the PSU performance period shall immediately be forfeited.
In connection with the transition to Mr. Francis as CEO the HR and Compensation Committee set his target total annual compensation 12% below the outgoing CEO, with a lower base salary, lower target annual incentive cash value and lower target PSU and RSU grant value. A portion of the sign-on cash and equity were in replacement of awards foregone at his prior employer.
Supplemental Non-GAAP Income Data
We utilize certain non-GAAP financial measures to evaluate performance, in conjunction with other performance metrics. The following are examples of how we utilize the non-GAAP measures:
| - | our executives and Board use non-GAAP measures to evaluate our operational performance, to prepare and evaluate our work plans and annual budgets, and ultimately to evaluate the performance of our executives; |
| - | our annual budgets are prepared on a non-GAAP basis; and |
| - | senior executive officers’ annual compensation is derived, in part, using these non-GAAP measures. While qualitative factors and judgment also affect annual cash incentives, the principal quantitative elements in the determination of the annual cash incentives are the various performance targets tied to the work plan. |
Non-GAAP financial measures have no standardized meaning and accordingly have limitations in their usefulness to investors. We provide this non-GAAP data because our executives believe that the data provide useful information to investors. However, investors are cautioned that, unlike financial measures prepared in accordance with U.S. GAAP, non-GAAP measures may not be comparable with the calculation of similar measures for other companies.
| 72 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
For the definitions of non-GAAP financial measures and a reconciliation of these items to the most directly comparable financial measures calculated and presented in accordance with GAAP, reference is made to the section captioned “Non-GAAP Net Income and Non-GAAP EPS Data” in the Company’s Form 10-K for the year ended December 31, 2023 and the reconciliations provided in Appendix A hereto.
Other Elements of Compensation
We generally provide to our executive officers the same benefits that are provided to all employees, including certain health and welfare benefits and other benefits. In addition, our executive officers are provided with certain additional benefits, intended to be competitive with the practices of companies in our Peer Group.
Health and Welfare Benefits
We offer health and welfare benefits to all eligible employees, including all executive officers, which are tailored to each location’s competitive market. Health and welfare benefits may include medical, dental, prescription drug, vision, life insurance, accidental death and dismemberment, short- and long-term disability coverage and an employee assistance program.
Retirement and Other Local Benefits
Israel
Israeli law generally requires severance pay equal to one month’s salary for each year of employment upon the termination of an employee’s employment due to retirement, death, termination without cause and other circumstances as defined under Israeli law. We make monthly contributions on behalf of our executive officers on Israel payroll to pension plans. These funds provide a combination of pension allowance and/or insurance and severance pay benefits to the executive officers. We contribute 7.5% of an NEO’s monthly salary to the pension component (including disability insurance) and 8.33% of the NEO’s monthly salary to the severance component, and the employee contributes an amount between 6% and 7% of the monthly salary to the pension component. The contributions to the severance component are on account of Teva’s obligation to pay severance upon termination as referenced above. Our CEO and EVP, Global Operations are entitled to similar contributions on behalf of the Company as a pension contribution and on account of severance. Accordingly, a substantial part of our statutory severance obligation is covered by these monthly contributions.
Generally, in addition, our NEOs on Israel payroll (excluding those on relocation), like all of our employees in the country, are entitled to participate in a study fund plan, pursuant to which each employee who participates in the plan contributes an amount equal to 2.5% of his or her monthly salary to the study fund and Teva contributes to this fund or pays 7.5% of his or her monthly salary.
Furthermore, as is very common and accepted market practice in Israel, we provide eligible employees, including our NEOs on Israel payroll, with a car or car allowance. This is the norm in Israel and this benefit is provided to a broad range of our employees. This benefit is similarly provided in other countries where it is typical market practice.
North America
Our North American subsidiaries mainly provide various defined contribution plans for the benefit of their employees. Under these plans, contributions are based on specified percentages of pay. In addition, Teva USA offers a supplemental deferred compensation plan to eligible employees. The plan is a nonqualified plan which is intended to work as a complement to the qualified 401(k) Retirement Savings Plan. The plan has been designed to address the “retirement gap” that many senior level employees face, primarily due to IRS-imposed limits on qualified plans and IRAs.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 73 | |
Executive Compensation
Expatriate Benefits / International Assignment and Relocation Benefits
Teva provides benefits to our employees who either accept an expatriate assignment or relocate internationally. The benefits are designed to provide ongoing assignment management and physical relocation support services. These benefits can vary depending on the nature of the assignment or relocation, but generally include a housing allowance, transportation support, a cost of living allowance (where applicable), home leave, global health insurance, and Company-paid education for approved dependents in locations where public education is not suitable. Additionally, we provide tax preparation and tax support services, dependent on the nature of the assignment (e.g., tax equalization for home-based assignments or tax gross up of relocation benefits and ongoing assignment allowances for host-based assignments), as well as immigration services to manage compliance within all global jurisdictions. The purpose of these benefits is to keep our expatriate employees “economically neutral” for the costs associated with living and working outside their home country, with the goal that they are not financially advantaged or disadvantaged as a result of relocating to another country and incurring associated taxes.
Details regarding benefits and perquisites specific to each NEO can be found in the footnotes to the 2023 Summary Compensation Table set forth below under “Additional Compensation Information.”
V. ADDITIONAL COMPENSATION POLICIES AND PRACTICES
Stock Ownership Guidelines
Teva and its shareholders are best served by executives and directors that manage the business with a long-term perspective. Therefore, we adopted stock ownership guidelines, as we believe stock ownership is an important tool to strengthen the alignment of interests among our executive officers and directors and our shareholders, to reinforce executive officers’ commitment to Teva and to demonstrate Teva’s commitment to sound corporate governance.
The policy provides that Teva expects the applicable level of stock ownership to be satisfied by our executive officers within five years, and by our directors within six years, of the later of the date the guidelines were adopted or the date of appointment as an executive officer or director. If an executive officer’s holding requirement increases because of a change in annual base salary, or if a director’s holding requirement increases because of a change in annual cash retainer, the executive officer is expected to achieve the higher holding requirement within one year, and the director within two years, of the date of the increase.
The HR and Compensation Committee receives periodic reports of the ownership achieved by each executive officer and director. For purposes of determining compliance with the guidelines, the value of an executive officer or director’s share holdings is based on the closing price of Teva’s American Depositary Shares reported on the principal U.S. national securities exchange on which the shares are listed (currently the NYSE) on the last trading day of the year.
The ownership guidelines are as follows:
Current Position | Multiple of Base Salary/Retainer | |
CEO | 6x | |
All other executive officers | 3x | |
Directors | 5x annual cash fee(*) | |
| (*) | Excluding committee fees. |
| 74 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
The value of all of the following types of Teva shares or stock options owned by, or granted to, an executive officer or director qualifies toward the attainment of the target multiple of pay:
| - | shares owned outright by the executive officer or director or jointly with, or separately by, his or her immediate family members residing in the same household; |
| - | shares held in a grantor trust or under a similar arrangement for the economic benefit of the executive officer or director or his or her immediate family members residing in the same household; |
| - | shares held in any Teva retirement plan; |
| - | unvested time-based restricted shares and RSUs; and |
| - | the intrinsic value of vested but unexercised in-the-money stock options. |
At the last measurement date, all NEOs are in compliance with our stock ownership guidelines, or fall within the period to attain the guideline level of stock ownership.
Clawback Policy
In addition, the Company has adopted a Clawback Policy that complies with the NYSE’s new clawback listing standards, Section 10D of the Exchange Act and the rules promulgated thereunder (the “Clawback Policy”). In the event that we are required to prepare an accounting restatement of our financial statements due to our material noncompliance with any financial reporting requirement under the securities laws, the Clawback Policy requires that covered executives must reimburse us, or forfeit, the amount of any excess incentive-based compensation received by such covered executive during the three completed fiscal years immediately preceding the date on which we are required to prepare the restatement. Executives covered by the Clawback Policy include our current and former executive officers, as determined by the HR and Compensation Committee in accordance with Section 10D of the Exchange Act and the NYSE listing standards, and such other senior executives or employees who may from time to time be deemed subject to the Clawback Policy by the HR and Compensation Committee.
Incentive-based compensation subject to the Clawback Policy includes any compensation that is granted, earned or vested based wholly or in part on the attainment of a financial reporting measure. The amount subject to recovery is the excess of the incentive-based compensation received based on the erroneous data over the incentive-based compensation that would have been received had it been based on the restated results, and is computed without regard to any taxes paid. The Clawback Policy will only apply to incentive-based compensation received on or after the effective date of Section 303A.14 of the NYSE Listed Company Manual.
Without derogating from and in addition to the above Clawback Policy, pursuant to the Company’s required, shareholder-approved Compensation Policy, (i) executive officers are required to return any compensation paid to them on the basis of results included in financial statements that turned out to be erroneous and that were subsequently restated, during the three-year period following filing thereof, and (ii) in the event that it is discovered that an executive officer engaged in conduct that resulted in a material inaccuracy in Teva’s financial statements or caused severe financial or reputational damage to Teva, or in the event that it is discovered that an executive officer breached confidentiality and/or non-compete obligations to Teva (as determined by the Company), the Company has broad remedial and disciplinary authority, depending on the facts and circumstances of each specific event[, including, termination of employment, offset to, or cancellation of, outstanding grants or opportunities affected by such actions.
In connection with the preparation of our 2023 financial statements, we identified errors in a single contingent consideration liability and related expenses in connection with estimated future royalty payments, along with corresponding deferred tax adjustments, that aggregated into an understatement of the contingent consideration liability. Please see note 1b to our consolidated financial statements as of and
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 75 | |
Executive Compensation
for the year ended December 31, 2023. We evaluated the errors, individually and in the aggregate, considering both qualitative and quantitative factors, and concluded that these errors did not have a material impact on any of the prior periods. However, the aggregate amount of the prior period errors in 2022 would have been material to the consolidated financial statements for fiscal year 2023. Therefore, the Company has made revisions to line items impacted by these errors in its financial statements as of and for the year ended December 31, 2022 and for the quarterly and year-to-date periods ended June 30, September 30, and December 31, 2022, and March 31, June 30 and September 30, 2023. We evaluated this revision and concluded that the change did not affect any financial performance measure in any of our incentive programs, and thus there was no erroneous compensation and no clawback was applicable.
Anti-Hedging and Anti-Pledging Policies
Our directors and executive officers are prohibited from hedging their equity-based awards and any other Teva securities held by them (whether they are subject to transfer restrictions or not), such as purchasing or selling options on Teva securities, purchasing or selling puts, calls, straddles, equity swaps or other derivative securities linked to Teva’s securities, or engaging in “short” sales on Teva securities. This policy applies to each director and each executive officer until one year after the director’s or executive officer’s termination or retirement. Our employees are also strongly discouraged from participating in any transaction in which profit is earned through a decline in value of Teva securities, such as short sales or hedges. Directors and executive officers are prohibited from pledging or using their equity-based awards and any other Teva securities held by them (whether they are subject to transfer restrictions or not) as collateral for loans.
Risk Considerations and Assessment
Our compensation elements are designed to include mechanisms that reduce incentives to expose Teva to imprudent risks that may harm the Company or our shareholders in the short- and long-term. This is achieved by using tools such as (i) placing maximum limits on short- and long-term incentives; (ii) measuring performance using key performance indicators that are designed to reduce incentives to take excessive risks; (iii) using compensation vehicles with multiple performance measures; (iv) granting a mix of equity-based compensation types that have long-term vesting schedules, which tie the awards to a longer performance cycle; (v) requiring clawback of compensation payments in certain circumstances; and (vi) aligning executive officer interests with Teva and shareholders through meaningful stock ownership guidelines.
While the Board and the Audit Committee have overall responsibility for risk oversight, each of the standing committees of the Board regularly assesses risk in its area of oversight in connection with executing its responsibilities. Thus, the HR and Compensation Committee assesses the potential risks arising from our compensation program, policies and practices. The HR and Compensation Committee coordinates with our legal, human resources and other departments, considers shareholder feedback and interests and consults with its compensation consultant. The HR and Compensation Committee reviewed and discussed the assessment for 2023. The HR and Compensation Committee determined that our compensation program, policies and practices do not create risks that are reasonably likely to have a material adverse effect on Teva.
Compensation Policy Requirements
Pursuant to the Israeli Companies Law, arrangements between Teva and its Office Holders must generally be consistent with the Compensation Policy. However, under certain circumstances, we may approve an arrangement that is not consistent with the Compensation Policy, if the arrangement is approved by a majority of our shareholders who participate and vote, provided that (i) the majority includes a majority of the votes cast by shareholders who participate and vote (abstentions are disregarded) who (A) are not controlling shareholders and (B) do not have a personal interest in the matter, or (ii) the votes cast against
| 76 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
the arrangement by shareholders who are not controlling shareholders and who do not have a personal interest in the matter who participate and vote constitute two percent or less of the voting power of the Company (a “disinterested majority”).
In addition, pursuant to the Israeli Companies Law, the Terms of Office and Employment of Office Holders generally require the approval of the HR and Compensation Committee and the Board. The Terms of Office and Employment as applicable to directors, including with respect to other positions in the Company, further require the approval of the shareholders by a simple majority. The Terms of Office and Employment with respect to a CEO (who is not a director) generally require the approval of the shareholders by the disinterested majority referenced in the immediately preceding paragraph. Pursuant to regulations promulgated under the Israeli Companies Law, shareholder approval is not required with respect to Terms of Office and Employment granted to a director or a CEO for the period following his or her appointment until the next general meeting of shareholders, provided these terms are (i) approved by the HR and Compensation Committee and the Board, (ii) consistent with the Compensation Policy and (iii) on similar or less favorable terms than those of the person’s predecessor. In addition, under certain circumstances, shareholder approval is not required with respect to the Terms of Office and Employment of a candidate for CEO if the HR and Compensation Committee determines that the engagement will be frustrated if the approval is pursued, provided that the terms are consistent with the Compensation Policy. This provision was followed in the recruitment of our President and CEO, Richard D. Francis.
Under certain circumstances, if the Terms of Office and Employment of Office Holders who are not directors are not approved by the shareholders, where such approval is required, the HR and Compensation Committee and the Board may nonetheless approve such terms. In addition, non-material amendments of the Terms of Office and Employment of Office Holders who are not directors may be approved by the HR and Compensation Committee only and non-material amendments of the Terms of Office and Employment of Office Holders who are not directors and excluding the CEO may be approved by the CEO only, provided such approvals are permitted under the Compensation Policy and consistent therewith.
Accordingly, pursuant to our Compensation Policy, our CEO is authorized to approve changes in terms for any other executive officer with respect to any calendar year, provided that it does not exceed the value of such executive officer’s one-month base salary, unless determined otherwise by the HR and Compensation Committee and the Board.
Accounting Considerations
We follow Financial Accounting Standards Board ASC Topic 718 for our equity-based compensation awards. In accordance with ASC Topic 718, equity-based compensation cost is measured at the grant date, or with respect to performance-based awards, the service inception date, based on the estimated fair value of the awards using a variety of assumptions. This calculation is performed for accounting purposes and, as applicable, reported in the compensation tables, even though recipients may never realize any value from their awards. We record this expense on an ongoing basis over the requisite employee service period. Accounting rules also require us to record cash compensation as an expense at the time the obligation is incurred.
HR and Compensation Committee Report
The HR and Compensation Committee has reviewed and discussed this “Compensation Discussion and Analysis” section of this Proxy Statement with our executives. Based upon this review and discussions, the HR and Compensation Committee recommended to the Board that the “Compensation Discussion and Analysis” be included in this Proxy Statement.
This HR and Compensation Committee Report shall not be deemed to be incorporated by reference into any filing made by the Company under the Securities Act of 1933, as amended (the “Securities Act”) or
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 77 | |
Executive Compensation
the U.S. Securities Exchange Act of 1934, as amended, notwithstanding any general statement contained in any such filing incorporating this proxy statement by reference, except to the extent the Company incorporates such Report by specific reference.
Members of the HR and Compensation Committee:
Rosemary A. Crane, Chair
Gerald M. Lieberman
Dr. Perry D. Nisen
Janet S. Vergis
| 78 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
ADDITIONAL COMPENSATION INFORMATION
2023 Summary Compensation Table
Name and Principal Position | Year | Salary ($) (1) | Bonus ($) (2) | Stock Awards ($) (3) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) (4) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) (5) | Total ($) | ||||||||||||||||||||||||||||||||||||
Richard Francis President and Chief Executive Officer | 2023 | 1,600,000 | 1,500,000 | 18,999,993 | (6) | 0 | 2,600,000 | 0 | 1,006,887 | 25,706,880 | |||||||||||||||||||||||||||||||||||
Eli Kalif Executive Vice President, Chief Financial Officer | 2023 | 781,918 | 0 | 2,999,984 | 0 | 880,504 | 0 | 281,426 | 4,943,832 | ||||||||||||||||||||||||||||||||||||
| 2022 | 780,603 | 0 | 1,999,995 | 0 | 943,877 | 0 | 290,142 | 4,014,617 | |||||||||||||||||||||||||||||||||||||
| 2021 | 747,175 | 0 | 1,799,996 | 0 | 715,340 | 0 | 282,472 | 3,544,983 | |||||||||||||||||||||||||||||||||||||
Richard Daniell Executive Vice President, European Commercial | 2023 | 772,754 | 0 | 3,499,986 | 0 | 743,372 | 0 | 148,051 | 5,164,163 | ||||||||||||||||||||||||||||||||||||
Eric Drapé Executive Vice President, Global Operations | 2023 | 740,353 | 0 | 1,999,990 | 0 | 370,147 | 0 | 1,143,590 | 4,254,080 | ||||||||||||||||||||||||||||||||||||
| 2022 | 695,279 | 0 | 1,999,995 | 0 | 709,731 | 0 | 501,828 | 3,906,833 | |||||||||||||||||||||||||||||||||||||
| 2021 | 756,108 | 0 | 1,899,989 | 0 | 724,106 | 0 | 432,056 | 3,812,259 | |||||||||||||||||||||||||||||||||||||
Mark Sabag Executive Vice President, International Markets Commercial | 2023 | 688,147 | 0 | 1,999,990 | 0 | 774,323 | 0 | 266,674 | 3,729,134 | ||||||||||||||||||||||||||||||||||||
| 2022 | 744,787 | 0 | 1,999,995 | 0 | 899,554 | 0 | 279,597 | 3,923,933 | |||||||||||||||||||||||||||||||||||||
| 2021 | 765,294 | 0 | 1,999,991 | 0 | 732,686 | 0 | 290,770 | 3,788,741 | |||||||||||||||||||||||||||||||||||||
Salary
| (1) | The Company paid the salaries of Mr. Kalif and Mr. Sabag in Israeli shekels. The U.S. dollar amounts in the table above for Mr. Kalif and Mr. Sabag were converted from Israeli shekels using a 2023 monthly average exchange rate for the month of each salary payment, ranging from 3.45 to 3.97 shekels per U.S. dollar. The Company paid the salary of Mr. Drapé in euros. The U.S. dollar amount in the table above for Mr. Drapé was converted from euros using a 2023 monthly average exchange rate for the month of each salary payment, ranging from 0.90 to 0.95 euros per U.S. dollar. The Company paid the salary of Mr. Daniell in British pounds. The U.S. dollar amount in the table above for Mr. Daniell was converted from British pounds using a 2023 monthly average exchange rate for the month of each salary payment, ranging from 0.78 to 0.82 British pounds per U.S. dollar. The reduction in base salary shown for Mr. Sabag in 2022 and 2023 and Mr. Drapé in 2022 are due to fluctuations in exchange rates. |
Bonus
| (2) | In connection with Mr. Francis’ hiring, Mr. Francis received the first of three tranches of his sign-on cash award of $5,000,000, with such first tranche in the amount of $1,500,000 being paid on the first business day following the date that Mr. Francis commenced employment in 2023. For more information about the sign-on cash award (i.e., vesting conditions, payment schedule, etc.), see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Leadership Transitions Effective in 2023—Appointment of Mr. Richard D. Francis as President and CEO” above. |
Stock Awards
| (3) | The amounts shown in the Stock Awards column represent the aggregate grant date fair value of the PSUs and RSUs awarded to our NEOs, computed in accordance with Topic 718. For information regarding assumptions, factors and methodologies used in our computations pursuant to Topic 718, see note 14b. to our consolidated financial statements set forth in our Annual Report on Form 10-K for the year ended December 31, 2023. For more information on these and other share awards granted during 2023, see the table entitled “2023 Grants of Plan-Based Awards” and related narrative and footnotes. |
The grant date fair value of PSUs included above is determined based upon achievement of performance at the “target” level, which is the probable outcome of the performance metrics associated with each award of PSUs. If performance were to be achieved at “maximum” level, the grant date fair value of the 2023 PSU awards as of the respective grant dates would have been as follows: Mr. Francis: $16,395,011; Mr. Kalif: $1,650,001; Mr. Daniell: $1,650,001; Mr. Sabag: $1,650,001; and Mr. Drapé: $1,650,001.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 79 | |
Executive Compensation
Non-Equity Incentive Awards
| (4) | The amounts shown in the Non-Equity Incentive Plan Compensation column are comprised of amounts paid in respect of the 2023 annual cash incentive plan, as determined by the HR and Compensation Committee and the Board in accordance with the terms of such plan and the awards thereunder. Payments pursuant to the 2023 annual cash incentive plan were made in early 2024, immediately following the year in which they were earned. The Company paid the amounts reported in 2023 for Mr. Kalif and Mr. Sabag in Israeli shekels. The 2023 U.S. dollar amounts in the table above were converted from Israeli shekels using a 2023 annual average exchange rate of 3.68 shekels per U.S. dollar. The Company paid the amount reported in 2023 for Mr. Drapé in euros. The 2023 U.S. dollar amount in the table above was converted from euros using a 2023 annual average exchange rate of 0.92 euros per U.S. dollar. The Company paid the amount reported in 2023 for Mr. Daniell in British pounds. The 2023 U.S. dollar amount in the table above was converted from British pounds using a 2023 annual average exchange rate of 0.80 British pounds per U.S. dollar. |
All Other Compensation
| (5) |
Name | Defined (a) | Automobile (b) | Housing (c) | Tax (d) | Other (e) | Total ($) | ||||||||||||||||||
Richard Francis (*) | 252,988 | 43,681 | 205,585 | 317,853 | 186,780 | 1,006,887 | ||||||||||||||||||
Eli Kalif (*) | 123,889 | 49,113 | — | 45,413 | 63,011 | 281,426 | ||||||||||||||||||
Richard Daniell (*) | 68,698 | 13,456 | 57,607 | — | 8,290 | 148,051 | ||||||||||||||||||
Eric Drapé (*) | 194,952 | 46,111 | 86,070 | 811,804 | 4,653 | 1,143,590 | ||||||||||||||||||
Mark Sabag (*) | 108,932 | 51,327 | — | 46,866 | 59,549 | 266,674 | ||||||||||||||||||
| (*) | The U.S. dollar amounts in the table above were converted from local currency, where needed, using the relevant 2023 monthly average exchange rates of 3.45 to 3.97 Israeli shekels per U.S. dollar, 0.90 to 0.95 euros per U.S. dollar and 0.78 to 0.82 British pounds per U.S. dollar. |
| (a) | Amounts disclosed in this column reflect the Company’s contributions and/or payments related to tax-qualified retirement plans, taxable non-qualified retirement arrangements (in lieu of statutory schemes), and Israeli pension arrangements, which include pension and severance contributions, pursuant to Israeli law. For more information, see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Other Elements of Compensation—Retirement and Other Local Benefits.” |
| (b) | Amounts disclosed in this column reflect automobile allowances, participation in the Company’s car lease program, and/or reimbursement of automobile expenses, which is very common and market practice in Israel and provided to a broad range of employees. |
| (c) | Amounts disclosed in this column reflect expenses related to relocation such as housing accommodation costs for Mr. Francis ($54,904), Mr. Drapé ($67,571) and Mr. Daniell ($42,186), travel costs for Mr. Francis ($146,249), tax services and other relocation costs (all). For more information, see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Other Elements of Compensation—Expatriate Benefits/International Assignment and Relocation Benefits” above. |
| (d) | Amounts disclosed in this column reflect tax gross-ups paid to our NEOs as follows: Mr. Francis—gross-ups are provided for the income associated with accommodation in Israel, travel costs associated with travel allowance, other items related to his relocation (paid in accordance with Teva’s relocation policy), legal and tax consulting fee reimbursements, and costs associated with the Company-leased automobile and/or automobile allowance and cell phone; Mr. Kalif and Mr. Sabag—gross-ups are provided for costs associated with the Company-leased automobile and/or automobile allowance and cell phone; Mr. Drapé —gross-ups are provided for the income associated with accommodation in Israel, payments for French social security contributions, tax equalization and other items related to his relocation to Israel (paid in accordance with Teva’s relocation policy), costs associated with the Company-leased automobile and/or automobile allowance and cell phone. In addition, gross-ups are provided to all applicable NEOs for miscellaneous fringe benefits, as are generally provided to other eligible employees in their respective countries. |
| (e) | Amounts disclosed in this column reflect legal and tax consulting fee reimbursements for Mr. Francis ($182,246), study fund contributions or payments for Mr. Kalif ($58,644 ) and Mr. Sabag ($51,611), as provided to all Israel-based employees (excluding those on relocation), life insurance premium payments made by the Company on behalf of the NEOs, and miscellaneous cash and other fringe benefits provided generally to all eligible employees in applicable countries. |
| (6) | In addition to an annual equity grant with a target grant date fair value of $9 million (70% of which was in the form of PSUs and 30% in the form of RSUs), in connection with Mr. Francis’ recruitment and hiring, in light of forfeited compensation opportunities at his prior employer and to incentivize him to join the Company, Mr. Francis received a PSU award with a target grant date fair value of $5 million, which will be earned based on the achievement of stretch performance goals as described above, and an RSU |
| 80 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
| award with a grant date fair value of $5 million, which will vest in three equal installments on the first, second and third anniversaries of the grant date, subject to his continued employment through the applicable vesting dates. For more information about these equity grants, see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Leadership Transitions Effective in 2023—Appointment of Mr. Richard D. Francis as President and CEO” above. |
Employment Agreements
We have entered into employment agreements with all of our NEOs. The employment agreements provide for, among other things, the term of employment, the position and duties, the compensation and benefits payable during the term of the agreement and certain restrictive covenants. The agreements also set forth the terms in the event that the NEO’s employment is terminated under various conditions. The material provisions pertaining to termination of employment of the NEOs are set forth below under “—2023 Potential Payments Upon Termination or Change in Control.”
Richard D. Francis
On November 21, 2022, we entered into an employment agreement with Mr. Francis to serve as our President and CEO commencing January 1, 2023. He is eligible for benefit plans provided to similarly situated executive officers, including medical, dental, group life and other programs, pension and severance amounts according to Israeli law, relocation benefits in accordance with our policy, up to $12,000 per month for reimbursement of housing (including hotel), utilities, other accommodation expenses in Israel, and air travel (grossed-up for applicable taxes), up to $100,000 per year for reimbursement of personal travel expenses for himself and his family (grossed-up for applicable taxes), up to $15,000 per year for reimbursement of tax and financial advisory support, tax protection benefits, and a car benefit. He was also eligible for a one-time legal fee reimbursement. For a summary of the material terms of Mr. Francis’ employment agreement, see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Leadership Transitions Effective in 2023—Appointment of Mr. Richard D. Francis as President and CEO” above. The agreement also contains a noncompetition covenant for 12 months after the term of the agreement, a nonsolicitation covenant for 24 months after the term of the agreement, nondisclosure and nondisparagement covenants and assignment of inventions.
Eli Kalif
On November 6, 2019, we entered into an employment agreement with Mr. Kalif. The agreement provides that Mr. Kalif will be employed as Executive Vice President and CFO, until his death, disability, termination with or without cause or resignation with or without good reason. The agreement provided for an initial annual base salary of 2,343,200 Israeli shekels (approximately $636,436 using a 2023 average monthly exchange rate of 3.68 shekels per U.S. dollar).
Mr. Kalif is eligible to be considered for an annual cash incentive with a target of 100% of his then-current base salary, and for equity-based awards under our equity compensation plan. Under the agreement, Mr. Kalif is also provided with a company or leased car (grossed-up for applicable taxes), certain pension and severance fund contributions pursuant to Israeli law (by both the Company and Mr. Kalif), and group life insurance and other benefits customary for executives in Israel.
In addition, Mr. Kalif received a sign-on equity award in February 2020 in the form of restricted stock units with a grant date fair value of $250,000 in consideration of certain equity grants with Mr. Kalif’s prior employer that were forfeited upon his resignation. These RSUs vested in three equal installments on the second, third, and fourth anniversaries of the grant date, which were subject to his continued employment through the applicable vesting dates.
The agreement also contains noncompetition and nonsolicitation covenants for 6 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
Richard Daniell
On September 25, 2018, we entered into an executive employment agreement with Mr. Daniell. The agreement provides that Mr. Daniell will serve as Executive Vice President, European Commercial until his
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 81 | |
Executive Compensation
death, disability, termination with or without cause or resignation. The agreement provided for an initial annual base salary of 429,400 British pounds (approximately $533,911 using a 2023 average monthly exchange rate of 0.80 British pounds per U.S. dollar).
Mr. Daniell is eligible to be considered for an annual cash incentive and for equity-based awards under our equity compensation plan. He is eligible for benefit plans provided to similarly situated executive officers, including medical, disability, dental, life, pension and other programs. Under the agreement, Mr. Daniell is also provided with a car allowance. In conjunction with Mr. Daniell’s commuter assignment to the Netherlands, he is entitled to commuter benefits in accordance with the terms of our international commuter assignment policy. He is entitled to a housing allowance of up to 3,100 euro per month ($3,352 using a 2023 average monthly exchange rate of 0.92 euro per U.S. dollar).
The agreement also contains noncompetition and nonsolicitation covenants for 12 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
Eric Drapé
On March 12, 2020, we entered into an executive employment agreement with Mr. Drapé. The agreement provides that Mr. Drapé will serve as Executive Vice President, Teva Global Operations until his death, disability, termination with or without cause or resignation with or without good reason. The agreement provided for an initial annual base salary of 620,500 euros (approximately $670,931 using a 2023 average monthly exchange rate of 0.92 euros per U.S. dollar).
Mr. Drapé is eligible to be considered for an annual cash incentive with a target of 100% of his then-current base salary, and for equity-based awards under our equity compensation plan. Mr. Drapé is eligible for benefit plans provided to similarly situated executive officers, including medical, dental, group life and other programs, and pension and severance contributions pursuant to Israeli law, of which the pension portion and any supplement thereof is provided to Mr. Drapé as reimbursement of an amount equal to the required monthly French contribution, to be paid by Mr. Drapé to French social security to enable continued coverage. Under the agreement, Mr. Drapé is also provided with a company or leased car (grossed-up for applicable taxes). In conjunction with Mr. Drapé’s relocation to Israel, he is entitled to relocation benefits in accordance with the terms of our relocation policy. He is entitled to a housing allowance of up to 25,000 Israeli shekels per month ($6,790 using a 2023 average monthly exchange rate of 3.68 shekels per U.S. dollar).
The agreement also contains noncompetition and nonsolicitation covenants for 6 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
Mark Sabag
On December 22, 2013, we entered into an executive employment agreement with Mr. Sabag. The agreement provides that Mr. Sabag will serve as Group Executive Vice President, Human Resources until his death, disability, aged retirement, termination with or without cause or resignation with or without good reason. The agreement provides for an initial annual base salary of 1,518,000 Israeli shekels (approximately $412,303 using a 2023 average monthly exchange rate of 3.68 shekels per U.S. dollar).
Mr. Sabag is eligible to be considered for an annual cash incentive and for equity-based awards under our equity compensation plan. Mr. Sabag is also provided with a company or leased car (grossed-up for applicable taxes), certain pension and severance fund contributions pursuant to Israeli law (by both the Company and Mr. Sabag), and group life insurance and other benefits customary for executives in Israel.
The agreement also contains a noncompetition covenant for 12 months after termination, nondisclosure and nondisparagement covenants and an assignment of inventions.
Effective as of August 15, 2021, we appointed Mr. Sabag to the position of Executive Vice President, International Markets Commercial. His employment remains subject to the terms of his employment agreement.
| 82 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
2023 Grants of Plan-Based Awards
| Estimated Future Payouts Under Non-Equity Incentive Plan Awards (1) | Estimated Future Payouts Under Equity Incentive Plan Awards (2) | |||||||||||||||||||||||||||||||||||||||||
Name | Approval Date | Grant Date | Award Type | Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | All (#) (3) | Grant ($) | |||||||||||||||||||||||||||||||
Richard Francis |
|
|
|
|
|
| Annual Incentive | 600,000 | 2,400,000 | 3,200,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 2/7/2023 | 2/15/2023 | PSU (4) |
|
|
|
|
|
|
|
|
| 478,230 | 538,851 | 646,622 |
|
|
| 4,999,998 | |||||||||||||||||||||||
| 2/7/2023 | 2/15/2023 | RSU (4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 484,966 | 4,999,999 | |||||||||||||||||||
| 2/7/2023 | 3/3/2023 | PSU |
|
|
|
|
|
|
|
|
| 392,182 | 697,211 | 1,150,399 |
|
|
| 6,299,999 | |||||||||||||||||||||||
| 2/7/2023 | 3/3/2023 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 268,924 | 2,699,997 | |||||||||||||||||||
Eli Kalif |
|
|
|
|
|
| Annual Incentive | 195,479 | 781,918 | 1,563,836 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 2/7/2023 | 3/3/2023 | PSU |
|
|
|
|
|
|
|
|
| 62,251 | 110,668 | 182,603 |
|
|
| 999,996 | |||||||||||||||||||||||
| 2/7/2023 | 3/3/2023 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 99,601 | 999,994 | |||||||||||||||||||
| 2/7/2023 | 3/3/2023 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 99,601 | 999,994 | |||||||||||||||||||
Richard Daniell |
|
|
|
|
|
| Annual Incentive | 193,188 | 772,754 | 1,545,508 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 2/7/2023 | 3/3/2023 | PSU |
|
|
|
|
|
|
|
|
| 62,251 | 110,668 | 182,603 |
|
|
| 999,996 | |||||||||||||||||||||||
| 2/7/2023 | 3/3/2023 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 99,601 | 999,994 | |||||||||||||||||||
| 2/7/2023 | 3/3/2023 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 149,402 | 1,499,996 | |||||||||||||||||||
Eric Drapé |
|
|
|
|
|
| Annual Incentive | 185,088 | 740,353 | 1,480,707 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 2/7/2023 | 3/3/2023 | PSU |
|
|
|
|
|
|
|
|
| 62,251 | 110,668 | 182,603 |
|
|
| 999,996 | |||||||||||||||||||||||
| 2/7/2023 | 3/3/2023 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 99,601 | 999,994 | |||||||||||||||||||
Mark Sabag |
|
|
|
|
|
| Annual Incentive | 172,037 | 688,147 | 1,376,295 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 2/7/2023 | 3/3/2023 | PSU |
|
|
|
|
|
|
|
|
| 62,251 | 110,668 | 182,603 |
|
|
| 999,996 | |||||||||||||||||||||||
|
| 2/7/2023 | 3/3/2023 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 99,601 | 999,994 | |||||||||||||||||||
Annual Incentive Plan
| (1) | The amounts disclosed in these columns reflect the threshold, target and maximum annual cash incentive opportunities for 2023 under the executive officer annual incentive plan. Actual amounts earned, as determined in the first quarter of 2024, are reflected in the 2023 Summary Compensation Table in the Non-Equity Incentive Plan Compensation column. For more information on the 2023 Annual Cash Incentive Plan awards, including the applicable performance metrics, please see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Annual Cash Incentives.” |
Performance Share Units (PSUs)
| (2) | Amounts disclosed in these columns reflect the potential threshold, target and maximum number of PSUs that may be earned in respect of the PSUs awarded in 2023 to each NEO. Actual amounts earned are based on the level of achievement relative to three financial goals (i.e. Non-GAAP Operating Income, Net Revenue from Innovative Products and Net Revenue from All Other Products), as modified by a relative TSR modifier, for the 2023-2025 performance period. Results will be certified by the HR and Compensation Committee and the Board in the first quarter of 2026. For more information on the 2023 Long-Term Incentive Plan awards, including the applicable performance metrics, please see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Long-Term Incentive Equity-Based Compensation—Performance Metrics.” Valuations of PSUs disclosed in this table were determined based on the fair market value of a Teva share on the grant date, less the net present value of dividends, as relevant, and then applying a discount factor. |
Restricted Share Units (RSUs)
| (3) | Amounts disclosed in this column reflect the number of RSUs granted to our NEOs in 2023. The RSUs granted as part of the executive officer annual equity grant vest in equal annual installments on the first, second, third and fourth anniversaries of the grant date. The Company granted Mr. Kalif and Mr. Daniell a one-time grant of RSUs, which will vest in equal annual installments on the first, second, third and fourth anniversaries of the grant date. Valuations of RSUs were determined based on the fair market value of a Teva share on the grant date, less the net present value of dividends, as relevant. For more information, see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Long-Term Incentive Equity-Based Compensation—RSU Grants to Two NEOs” above. |
Sign on Grants to Mr. Francis of PSUs and RSUs
| (4) | Amounts disclosed in these rows reflect sign-on PSUs and RSUs granted to Mr. Francis. Pursuant to his employment agreement, Mr. Francis received a sign-on PSU grant with a three-year, 2023-2025 performance period. Actual amounts earned are based on the level of achievement relative to goals related to the Company’s key strategic priorities of returning to growth, optimizing global operations, reducing debt and delivering on our Sustainability-Linked Bond commitments, which are each weighted equally, with no requirement that such earned amounts be modified by a relative TSR modifier. Results will be certified by the HR and Compensation Committee and the Board in the first quarter of 2026. The PSUs vest on the third anniversary of the grant date and the RSUs vest on the first, second and third anniversaries of the grant date. For more information on the sign-on grants, please see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Leadership Transitions Effective in 2023—Appointment of Mr. Richard D. Francis as President and CEO” above. Valuations of PSUs disclosed in this table were determined based on the fair market value of a Teva share on the grant date, less the net present value of dividends, as relevant, and then applying a discount factor. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 83 | |
Executive Compensation
2023 Outstanding Equity Awards at Fiscal Year-End
|
|
|
| Option Awards | Stock Awards |
| |||||||||||||||||||||||||||||||||
Name | Award Type | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable (1) | Number of Securities Underlying Unexercised Options (#) Unexercisable (2) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Shares That Have Not Vested (#) (3) | Market Value of Shares or Units of Shares That Have Not Vested ($) (4) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (5) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That have Not Vested ($) (6) | Vesting Schedule (7) | |||||||||||||||||||||||||||
Richard Francis | RSUs | 2/15/2023 |
|
|
|
|
|
|
|
|
|
|
|
| 484,966 | 5,063,045 |
|
|
|
|
|
| 33% in 2024, 2025 and 2026 | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
| 268,924 | 2,807,567 |
|
|
|
|
|
| 25% in 2024, 2025, 2026 and in 2027 | |||||||||||||||
| PSUs | 2/15/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 478,230 | 4,992,724 | 100% in 2026, subject to performance | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 435,757 | 4,549,302 | 100% in 2026, subject to performance | |||||||||||||||
Eli Kalif | RSUs | 2/13/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 6,630 | 69,217 |
|
|
|
|
|
| 33% in 2022, 2023 and 2024 | |||||||||||||||
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 18,430 | 192,409 |
|
|
|
|
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||
|
| 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 42,777 | 446,592 |
|
|
|
|
|
| 25% in 2022, 2023, 2024, and 2025 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
| 100,536 | 1,049,596 |
|
|
|
|
|
| 25% in 2023, 2024, 2025, and 2026 | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
| 199,202 | 2,079,669 |
|
|
|
|
|
| 25% in 2024, 2025, 2026 and 2027 | |||||||||||||||
| PSUs | 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 52,320 | 546,221 |
|
|
|
|
|
| 100% in 2024 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 148,942 | 1,554,954 | 100% in 2025, subject to performance | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 69,168 | 722,109 | 100% in 2026, subject to performance | |||||||||||||||
Richard Daniell | Options | 3/12/2014 | 12,502 |
|
|
| 48.76 | 3/12/2024 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 3/12/2015 | 15,001 |
|
|
| 60.21 | 3/12/2025 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 3/17/2016 | 27,504 |
|
|
| 53.50 | 3/17/2026 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 9/9/2016 | 14,571 |
|
|
| 50.21 | 9/9/2026 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 3/3/2017 | 45,003 |
|
|
| 34.70 | 3/3/2027 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 9/18/2017 | 15,011 |
|
|
| 16.99 | 9/18/2027 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/9/2018 | 100,457 |
|
|
| 18.61 | 2/9/2028 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
| RSUs | 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 20,599 | 215,054 |
|
|
|
|
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||
|
| 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 45,152 | 471,387 |
|
|
|
|
|
| 25% in 2022, 2023, 2024, and 2025 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
| 100,536 | 1,049,596 |
|
|
|
|
|
| 25% in 2023, 2024, 2025, and 2026 | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
| 249,003 | 2,599,591 |
|
|
|
|
|
| 25% in 2024, 2025, 2026 and 2027 | |||||||||||||||
| PSUs | 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 55,226 | 576,559 |
|
|
|
|
|
| 100% in 2024 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 148,942 | 1,554,954 | 100% in 2025, subject to performance | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 69,168 | 722,109 | 100% in 2026, subject to performance | |||||||||||||||
Eric Drapé | Options | 3/12/2014 | 15,002 |
|
|
| 48.76 | 3/12/2024 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/12/2015 | 54,623 |
|
|
| 57.35 | 2/12/2025 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/12/2016 | 54,950 |
|
|
| 55.75 | 2/12/2026 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/14/2017 | 62,364 |
|
|
| 34.90 | 2/14/2027 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 9/18/2017 | 33,339 |
|
|
| 16.99 | 9/18/2027 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/9/2018 | 50,231 |
|
|
| 18.61 | 2/9/2028 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
| RSUs | 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 19,515 | 203,737 |
|
|
|
|
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||
|
| 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 45,152 | 471,387 |
|
|
|
|
|
| 25% in 2022, 2023, 2024, and 2025 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
| 100,536 | 1,049,596 |
|
|
|
|
|
| 25% in 2023, 2024, 2025, and 2026 | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
| 99,601 | 1,039,834 |
|
|
|
|
|
| 25% in 2024, 2025, 2026 and 2027 | |||||||||||||||
| PSUs | 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 55,226 | 576,559 |
|
|
|
|
|
| 100% in 2024 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 148,942 | 1,554,954 | 100% in 2025, subject to performance | |||||||||||||||
|
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 69,168 | 722,109 | 100% in 2026, subject to performance | |||||||||||||||
| 84 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
|
|
|
| Option Awards | Stock Awards |
| |||||||||||||||||||||||||||||||||
Name | Award Type | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable (1) | Number of Securities Underlying Unexercised Options (#) Unexercisable (2) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Shares That Have Not Vested (#) (3) | Market Value of Shares or Units of Shares That Have Not Vested ($) (4) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (5) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That have Not Vested ($) (6) | Vesting Schedule (7) | |||||||||||||||||||||||||||
Mark Sabag | Options | 3/12/2014 | 73,933 |
|
|
| 48.76 | 3/12/2024 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/12/2015 | 67,035 |
|
|
| 57.35 | 2/12/2025 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/12/2016 | 64,940 |
|
|
| 55.75 | 2/12/2026 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/14/2017 | 90,710 |
|
|
| 34.90 | 2/14/2027 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
|
| 2/9/2018 | 118,724 |
|
|
| 18.61 | 2/9/2028 |
|
|
|
|
|
|
|
|
|
|
|
| Vested | |||||||||||||||||
| RSUs | 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 20,599 | 215,054 |
|
|
|
|
|
| 25% in 2021, 2022, 2023, and 2024 | |||||||||||||||
|
| 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 47,529 | 496,203 |
|
|
|
|
|
| 25% in 2022, 2023, 2024, and 2025 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
| 100,536 | 1,049,596 |
|
|
|
|
|
| 25% in 2023, 2024, 2025, and 2026 | |||||||||||||||
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
| 99,601 | 1,039,834 |
|
|
|
|
|
| 25% in 2024, 2025, 2026 and 2027 | |||||||||||||||
| PSUs | 3/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
| 58,133 | 606,909 |
|
|
|
|
|
| 100% in 2024 | |||||||||||||||
|
| 3/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 148,942 | 1,554,954 | 100% in 2025, subject to performance | |||||||||||||||
|
|
| 3/3/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 69,168 | 722,109 | 100% in 2026, subject to performance | |||||||||||||||
| (1) | Amounts disclosed in this column reflect the number of options granted to our NEOs that were subject to time-based vesting and have vested. The options generally expire ten years from the date of grant and have an exercise price of no less than 100% of the fair market value of a Teva share on the date of grant. See “2023 Potential Payments Upon Termination or Change in Control” for information on the treatment of options upon retirement, death, disability, termination or change in control. |
| (2) | Amounts disclosed in this column reflect the number of options granted to our NEOs that were subject to time-based vesting that had not vested as of December 31, 2023. |
| (3) | Amounts disclosed in this column reflect the number of unvested RSUs granted that were subject to time-based vesting and unvested PSUs granted for the 2021-2023 performance period. The number of PSUs reported in this column reflects the PSUs vested in March 2024 for the 2021-2023 performance period at their actual payout percentage equal to 55% of target performance. As of December 31, 2023, the relevant performance period had been completed and in January 2024 the HR and Compensation Committee and Board determined the performance results and the awards fully vested thereafter. See “2023 Potential Payments Upon Termination or Change in Control” for information on the treatment of RSUs and PSUs upon retirement, death, disability, termination or change in control. |
| (4) | Amounts disclosed in this column reflect the market value of the RSUs and PSUs reported in the preceding column using the closing price of a Teva share as reported on the NYSE on December 29, 2023, the last trading day of the year, multiplied by the number of shares underlying each award. This column does not include the value of dividends paid on our ordinary shares during the performance period as no dividends accrue on unvested RSUs and PSUs. |
| (5) | Amounts disclosed in this column reflect the number of unearned and unvested PSUs held by our NEOs, based on achievement of all applicable performance goals at target level for the open performance cycle ending in 2024 and at threshold level for the open performance cycle ending in 2025. PSUs generally vest following completion of the year indicated and following the date on which the HR and Compensation Committee and Board certify whether the performance conditions have been achieved. The actual number of PSUs that will be earned in respect of these unvested awards, if any, will be determined at the end of each performance cycle and might be less or more than the number shown in this column. For more information, see “2023 Potential Payments Upon Termination or Change in Control” for information on the treatment of PSUs upon retirement, death, disability, termination or change in control. |
| (6) | Amounts disclosed in this column reflect the market value of the unvested PSUs held by our NEOs and reported in the preceding column using the closing price of a Teva share as reported on the NYSE on December 29, 2023, the last trading day of the year, multiplied by the number of shares underlying each award. This column does not include the value of dividends paid on our ordinary shares during the performance period as no dividends accrue on unvested PSUs. |
| (7) | This column discloses the vesting dates of outstanding awards held by our NEOs as of December 31, 2023, which generally occur on the relevant anniversary of the date of grant. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 85 | |
Executive Compensation
2023 Option Exercises and Stock Vested
The table below shows the number of shares each of our NEOs acquired and the values they realized upon the vesting of PSUs and RSUs during 2023. Values are shown before payment of any applicable withholding taxes or brokerage commissions. There were no stock options exercised by the NEOs in 2023.
|
| Stock Awards | |||||||
Name | Number of on Vesting (#) (1) | Value Vesting ($) (2) | ||||||
Eli Kalif | 124,911 | 1,240,453 | ||||||
Richard Daniell | 142,849 | 1,415,634 | ||||||
Eric Drapé | 135,140 | 1,339,237 | ||||||
Mark Sabag | 144,037 | 1,427,407 | ||||||
| (1) | Amounts disclosed in this column reflect the total number of PSUs and RSUs that vested during 2023. |
| (2) | Amounts disclosed in this column reflect the total dollar value realized upon vesting of the PSUs and RSUs, as calculated based on the price of a Teva share on the vesting date, multiplied by the total number of shares underlying each award. |
2023 Pension Benefits
None of our NEOs participate in or have accrued benefits under qualified or non-qualified defined benefit plans sponsored by us.
2023 Nonqualified Deferred Compensation
None of our NEOs participate in a nonqualified deferred compensation plan sponsored by us.
2023 Potential Payments Upon Termination or Change in Control
In connection with any termination of employment, including if there is a termination in connection with a change in control of the Company, our NEOs would be eligible to receive certain payments, benefits and treatment of the various forms of equity that the NEO holds (provided, in some cases, that certain conditions are met).
The amounts that the NEOs would receive are set forth below for the following types of termination of employment: termination for cause, death, disability, retirement, termination without cause, resignation for good reason, resignation without good reason and a termination in connection with a change in control of the Company.
In accordance with SEC rules, we have used certain assumptions in determining the amounts shown. We have assumed that the termination of employment or change in control occurred on December 29, 2023, the last business day of the year, and that the value of a Teva share on that day was $10.44, the closing price on the NYSE on December 29, 2023, the last trading day of 2023.
Under these SEC rules, the potential payments upon termination do not include certain distributions or benefits which are not enhanced or accelerated by a qualifying termination of employment or change in control. These payments and benefits are referred to as “vested benefits” and include:
| - | Amounts payable when employment terminates under programs generally applicable to the Company’s salaried employees; and |
| - | Vested benefits accrued under the 401(k) and pension plans. |
| 86 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Richard D. Francis
Mr. Francis’ employment terms generally require the Company and Mr. Francis to provide 180 days’ notice of termination of employment, other than in connection with termination for cause, death or disability. We may waive Mr. Francis’ services during the notice of termination period or any part thereof, or accelerate the termination date upon mutual agreement, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
Mr. Francis’ employment terms provide that in connection with his termination of employment, Mr. Francis will be entitled to receive payments associated with termination as required pursuant to applicable Israeli law and certain accrued obligations. Upon termination by the Company without cause or by Mr. Francis with good reason, Mr. Francis will generally be entitled to receive cash severance, together with severance amounts accumulated in his severance account, equal to the product of twenty-four times his monthly base salary (or the minimum amount required under applicable law, if greater). Mr. Francis is also entitled to receive an amount equal to twelve times his monthly base salary, in consideration for, and conditioned upon, his undertaking not to compete with Teva for one year following termination of employment and other restrictive covenants (i.e. confidentiality, non-disparagement and non-solicitation covenants), and his compliance with such undertaking, which amount would be paid in connection with terminations other than in the event of his termination by the Company for cause or his death. Upon his termination due to death, disability, termination without cause, resignation with good reason, and upon non-renewal, Mr. Francis will receive a prorated annual bonus for the year in which the date of termination occurs. In the event that his employment is terminated by the Company without cause or by Mr. Francis with good reason within one year following a change in control and as a result thereof, Mr. Francis will be entitled to an additional lump sum cash payment equal to his current annual salary.
Upon his termination due to death, disability, termination without cause and resignation with good reason, Mr. Francis will receive vesting of any portion of his sign-on cash award and sign-on RSU award that is unvested on the date of termination, and continued vesting of his sign-on PSU awards (which will ultimately be settled based on actual performance through the end of the applicable three-year performance period). Upon his termination due to death or disability, Mr. Francis will receive accelerated vesting of any portion of his annual equity awards that are unvested on the date of termination, pursuant to the 2020 Long-Term Equity-Based Incentive Plan.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, in the event that he breaches any of his restrictive covenants. In addition, in the event of continuous and willful material breach of any of his restrictive covenants, the Company shall be entitled to a repayment of such termination payments, including forfeiture of any post-termination equity vesting.
Eli Kalif
Mr. Kalif’s employment terms generally require the Company and Mr. Kalif to provide six months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Mr. Kalif’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
Mr. Kalif’s employment terms provide that in connection with his termination of employment, Mr. Kalif will be entitled to receive payments associated with termination as required pursuant to applicable Israeli law and certain accrued obligations. Upon termination by the Company without cause or by Mr. Kalif with good reason, Mr. Kalif will generally be entitled to receive cash severance, together with severance amounts accumulated in his severance account, equal to twice his monthly base salary multiplied by the number of years of employment, up to a maximum payment of 18 times his monthly base salary (or the minimum
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 87 | |
Executive Compensation
amount required under applicable law, if greater). In the event that his employment is terminated by the Company without cause within one year following a change in control event (as such term is defined in the Compensation Policy as in effect on the date hereof), Mr. Kalif will be entitled to an additional lump sum cash payment equal to $1.5 million.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, and Mr. Kalif shall repay Teva any such payments or benefits provided, in the event that he breaches any of his restrictive covenants, including an undertaking not to compete with Teva for six months following termination.
Richard Daniell
Mr. Daniell’s employment terms generally require the Company and Mr. Daniell to provide six months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Mr. Daniell’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
Mr. Daniell’s employment terms provide that in connection with his termination of employment, Mr. Daniell will be entitled to receive payments associated with termination as required pursuant to applicable law and certain accrued obligations. Upon termination by the Company without cause, Mr. Daniell will generally be entitled to receive cash severance based on a determined calculation as detailed in his agreement, capped at the product of 12 times his monthly base salary, which he currently meets. Mr. Daniell is also entitled to receive an amount equal to twelve times his monthly base salary, in consideration for, and conditioned upon, his undertaking not to compete with Teva for twelve months following termination and other restrictive covenants (i.e. confidentiality, non-disparagement and non-solicitation covenants), and his compliance with such undertaking. In the event that his employment is terminated by the Company without cause within one year following certain mergers and as a result thereof, Mr. Daniell will be entitled to an additional lump sum cash payment of $1.5 million.
Because Mr. Daniell meets the requirements for a qualifying retirement and termination under the Company’s policy pursuant to its 2015 and 2020 Long-Term Equity-Based Incentive Plan, if he is terminated without cause and the current retirement policy is in effect, he will be entitled to full continued vesting of his outstanding awards.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a settlement agreement, and shall immediately terminate without further obligation of Teva, and Mr. Daniell shall repay Teva any such payments or benefits provided, in the event that he breaches his restrictive covenants.
Eric Drapé
Mr. Drapé’s employment terms generally require the Company and Mr. Drapé to provide six months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Mr. Drapé’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
Mr. Drapé’s employment terms provide that in connection with his termination of employment, Mr. Drapé will be entitled to receive payments associated with termination as required pursuant to applicable Israeli law and certain accrued obligations. Upon termination by the Company without cause or by Mr. Drapé with good reason, Mr. Drapé will generally be entitled to receive cash severance, together with severance
| 88 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
amounts accumulated in his severance account, equal to the product of 15 times his monthly base salary (or the minimum amount required under applicable law, if greater). Mr. Drapé is also entitled to receive an amount equal to three times his monthly base salary, in consideration for, and conditioned upon, his undertaking not to compete with Teva for six months following termination and other restrictive covenants (i.e. confidentiality, non-disparagement and non-solicitation covenants), and his compliance with such undertaking, which amount would be paid in connection with terminations other than in the event of his termination by the Company for cause, his death or disability. In the event that his employment is terminated by the Company without cause within one year following a change in control event (as such term is defined in the Compensation Policy as in effect on the date hereof), Mr. Drapé will be entitled to an additional lump sum cash payment equal to $1.5 million.
Mr. Drapé is also entitled to full continued vesting of equity-based awards following termination without cause, or upon retirement on or after December 31, 2024.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, and Mr. Drapé shall repay Teva any such payments or benefits provided, in the event that he breaches any of his restrictive covenants.
Mark Sabag
Mr. Sabag’s employment terms generally require the Company and Mr. Sabag to provide nine months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Mr. Sabag’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period. Mr. Sabag’s employment terms provide that in connection with his termination of employment, Mr. Sabag will be entitled to receive payments associated with termination as required pursuant to applicable Israeli law. In the event of retirement to pension at the statutory age, termination due to death or disability, termination without cause, or resignation for good reason, Mr. Sabag will be entitled to a make-up payment equal to his then monthly base salary multiplied by the number of his years of service, that together with severance amounts accumulated in his pension insurance fund account cannot exceed twice his then monthly base salary multiplied by the number of his years of service. In the event of a resignation without good reason, the make-up payment will be equal to half his then monthly base salary multiplied by the number of his years of service, that together with severance amounts accumulated in his pension insurance fund account cannot exceed 1.5 times his then monthly base salary multiplied by the number of his years of service.
Mr. Sabag is also entitled to receive an amount equal to 12 times his monthly base salary, in consideration for and conditioned upon his undertaking not to compete with Teva for one year following termination. In the event of a material breach, payment will cease and the Company will be entitled to reclaim amounts already paid. This amount would not be paid upon termination upon death and the Company has the sole discretion to determine if it is paid upon termination for cause. In the event that his employment is terminated without cause within one year following certain mergers and as a result thereof, Mr. Sabag will be entitled to an additional lump sum payment of $1.5 million.
Mr. Sabag is also entitled to continued vesting of equity-based awards for 24 months following termination without cause. In addition, in the event that his employment is terminated without cause within one year following certain mergers and as a result thereof, Mr. Sabag will be entitled to accelerated vesting of unvested equity upon termination.
Potential Payments Upon Termination or Change In Control
The following tables summarize the payments the NEOs would receive upon termination and completion of the required notice period at December 29, 2023, the last business day of the year. The U.S. dollar
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 89 | |
Executive Compensation
amounts in the tables below were converted from local currency, where needed, using the December monthly average exchange rate of 3.67 Israeli shekels per U.S. dollar, 0.92 euros per U.S. dollar and 0.79 British pounds per U.S. dollar.
Payments Resulting From Termination without Cause or Resignation with Good Reason
Category | Richard D. Francis | Eli Kalif | Richard Daniell | Eric Drapé | Mark Sabag | |||||||||||||||
Severance payments (1) | 3,066,554 | 296,826 | 804,753 | 726,648 | 972,136 | |||||||||||||||
Non-compete payments (2) | 1,600,000 | 0 | 804,753 | 188,592 | 694,206 | |||||||||||||||
Accrued vacation | 0 | 179,654 | 12,381 | 93,603 | 202,006 | |||||||||||||||
Health benefits continuation | 0 | 0 | 930 | 0 | 0 | |||||||||||||||
Sign-on cash bonus acceleration (3) | 3,500,000 | 0 | 0 | 0 | 0 | |||||||||||||||
Post-termination equity vesting (4)(5) | 10,688,649 | 0 | 7,622,515 | 6,051,442 | 4,092,762 | |||||||||||||||
Total amount without change in control | $ | 18,855,203 | $ | 476,480 | $ | 9,245,332 | $ | 7,060,285 | $ | 5,961,110 | ||||||||||
Post-change in control cash termination payment (6) | 1,600,000 | 1,500,000 | 1,500,000 | 1,500,000 | 1,500,000 | |||||||||||||||
Additional post-change in control equity acceleration (7) | 0 | 0 | 0 | 0 | 2,025,162 | |||||||||||||||
Total amount with change in control | $ | 20,455,203 | $ | 1,976,480 | $ | 10,745,332 | $ | 8,560,285 | $ | 9,486,272 | ||||||||||
| (1) | In addition to the amounts reported above, Mr. Francis would receive $133,445, Mr. Kalif would receive $243,075, Mr. Drapé would receive $216,312, and Mr. Sabag would receive $1,036,307, which amounts relate to required contributions already made by the Company to arrangements set forth in their respective employment agreements and held in severance accounts on their behalf. For Mr. Sabag, the severance amount in the table would also be payable upon retirement to pension at the statutory age, or termination due to death or disability. Upon resignation without good reason, Mr. Sabag would be entitled to a severance payment amount of $470,025 in addition to the amount accumulated in his severance accounts. |
| (2) | The non-compete payment would be paid, assuming compliance with the non-compete covenant, in connection with terminations other than termination by the Company for cause or death for Mr. Francis and termination by the Company for cause, death, or disability for Mr. Drapé. For Mr. Sabag, the non-compete payment is also paid upon retirement to pension at the statutory age, termination due to disability, or resignation without good reason, and the Company has the sole discretion to determine if it is paid upon termination for cause. |
| (3) | For Mr. Francis, the sign-on cash bonus acceleration also applies upon termination due to death or disability. |
| (4) | Amounts reported are based on the price of a Teva share on December 29, 2023, the last trading day of 2023 ($10.44) and, with respect to PSUs, target performance, except for 2021-2023 PSUs, for which actual performance was used. |
| (5) | For Mr. Daniell, Mr. Sabag, and Mr. Drapé, the equity vesting does not apply to resignation with good reason. |
| (6) | For Mr. Daniell and Mr. Sabag, change in control is defined as certain mergers of the Company as set forth in their respective employment agreements followed by a termination without cause. For Mr. Francis, Mr. Kalif and Mr. Drapé, change in control is defined in the Compensation Policy as in effect on the date thereof. |
| (7) | Mr. Sabag’s employment agreement provides for equity acceleration upon a post-merger involuntary termination without cause, which is the only change in control trigger for this equity acceleration. Amounts reported are based on the end of year stock price ($10.44) and, with respect to PSUs, target performance. The amount reported would be in addition to the amount reported under post-termination equity vesting. |
| 90 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Executive Compensation
Accelerated Equity Vesting Upon Death or Disability
Under our 2015 and 2020 Long-Term Equity-Based Incentive Plans, upon death or disability, (i) performance awards, such as PSUs, will immediately vest and pay out based on the target level of performance as of the date of termination, (ii) RSUs will immediately be vested and settled and (iii) options will immediately vest and remain exercisable through the original expiration date.
Category | Richard D. Francis | Eli Kalif | Richard Daniell | Eric Drapé | Mark Sabag | |||||||||||||||
Value (1) | $ | 20,775,099 | $ | 7,094,032 | $ | 7,622,515 | $ | 6,051,442 | $ | 6,117,924 | ||||||||||
| (1) | Amounts reported are based on the price of a Teva share on December 29, 2023, the last trading day of 2023 ($10.44) and, with respect to PSUs, target performance, except for 2021-2023 PSUs, for which actual performance was used. |
2023 Pay Ratio
We have estimated the compensation of the 2023 median employee to be $64,562. The annual total compensation of our CEO was $25,706,880. The ratio of the annual total compensation of our CEO to the annual total compensation of our median employee was 398 to 1. We note that a substantial portion of our CEO’s total compensation for 2023 was the $10 million sign-on equity awards and the first tranche of his sign-on cash award in the amount of $1.5 million that he received in accordance with the terms of his employment agreement. Excluding these awards, the ratio would have been 220 to 1.
We selected December 31, 2023, as the date upon which we would identify the “median employee” and included employees from all relevant countries. Earnings of our employees outside the U.S. were converted to U.S. dollars using the 2023 annual average exchange rates.
To identify the “median employee,” we utilized the annualized 2023 base salary and target annual cash incentive for our consistently applied compensation measure because we believe that this measure reasonably reflects the annual compensation of our employees.
Using this measure, we identified our “median employee” who is a full-time, salaried employee located in Israel. We totaled all of the elements of the employee’s compensation for 2023 in the same manner as the CEO and in accordance with SEC Summary Compensation Table disclosure requirements, which resulted in an annual total compensation of $64,562, of which $33,276 is base salary, $5,103 is non-equity incentive compensation, and $26,183 is comprised of Company contributions to a pension fund, as is required by Israeli law, other compensation such as overtime pay and other cash allowances, and Company contributions to a study fund, as is common practice for Israel-based employees of the Company.
Because the SEC rules for identifying the median of the annual total compensation of our employees and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, which may include applying certain exclusions, and making reasonable estimates and assumptions that reflect their employee populations and compensation practices, the pay ratio reported by other companies may not be comparable to the pay ratio for our Company, as other companies have office headquarters in different countries, have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their respective pay ratios.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 91 | |
Year | Summary Compensation Table Total for PEO 1 ($) (1) | Summary Compensation Table Total for PEO 2 ($) (1) | Compensation Actually Paid to PEO 1 ($) (1)(2)(3) | Compensation Actually Paid to PEO 2 ($) (1)(2)(3) | Average Summary Compensation Table Total for Non-PEO NEOs ($) (1) | Average Compensation Actually Paid to Non-PEO NEOs ($) (1)(2)(3) | Value of Initial Fixed $100 Investment based on: (4) | Net Income ($ Millions) | Non-GAAP Operating Income ($ Millions) (5) | |||||||||||||||||||||||||||||||
TSR ($) | Peer Group TSR ($) | |||||||||||||||||||||||||||||||||||||||
(a) | (b) | (b) | (c) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | ||||||||||||||||||||||||||||||
| 2023 | 25,706,880 | 26,191,537 | 4,522,802 | 4,825,132 | 106.53 | 124.97 | (615 | ) | 4,361 | |||||||||||||||||||||||||||||||
| 2022 | 15,529,138 | 17,214,057 | 3,977,166 | 4,426,020 | 93.06 | 123.43 | (2,499 | ) 6 | 4,139 | |||||||||||||||||||||||||||||||
| 2021 | 14,681,772 | 7,570,056 | 3,740,840 | 3,075,466 | 81.73 | 129.31 | 456 | 4,401 | ||||||||||||||||||||||||||||||||
| 2020 | 15,724,518 | 10,866,321 | 3,997,258 | 3,783,167 | 98.47 | 114.32 | (4,099 | ) | 4,388 | |||||||||||||||||||||||||||||||
| (1) | Kåre Schultz (PEO 1) was our PEO for the years 2020 through 2022. Richard Francis (PEO 2) was the CEO in 2023. The individuals comprising theNon-PEO NEOs for each year presented are listed below. |
2020 | 2021 | 2022 | 2023 | |||
Eli Kalif | Eli Kalif | Eli Kalif | Eli Kalif | |||
Dr. Hafrun Fridriksdottir | Dr. Sven Dethlefs | Dr. Sven Dethlefs | Richard Daniell | |||
Brendan O’Grady | Eric Drapé | Mark Sabag | Eric Drapé | |||
Eric Drapé | Mark Sabag | Eric Drapé | Mark Sabag | |||
| (2) | The amounts shown for Compensation Actually Paid have been calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually earned, realized, or received by the Company’s NEOs. These amounts reflect the Summary Compensation Table Total with certain adjustments as described in footnote 3 below. |
| (3) | Compensation Actually Paid reflects the exclusions and inclusions of certain amounts for the PEO and the Non-PEO NEOs as set forth below. Equity values are calculated in accordance with FASB ASC Topic718 . Amounts in the Exclusion of Stock Awards column are the totals from the Stock Awards columns set forth in the Summary Compensation Table. |
Year | Summary Compensation Table Total for PEO 2 ($) | Exclusion of Stock Awards and Option Awards for PEO 2 ($) | Inclusion of Equity Values for PEO 2 ($) | Compensation Actually Paid to PEO 2 ($) | ||||||||||||
| 2023 | 25,706,880 | (18,999,993 | ) | 19,484,650 | 26,191,537 | |||||||||||
Year | Average Summary Compensation Table Total for Non-PEO NEOs ($) | Average Exclusion of Stock Awards and Option Awards for Non-PEO NEOs($) | Average Inclusion of Equity Values for Non-PEO NEOs($) | Average Compensation Actually Paid to Non-PEO NEOs($) | ||||||||||||
| 2023 | 4,522,802 | (2,624,988 | ) | 2,927,317 | 4,825,132 | |||||||||||
| 92 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Year | Year-End FairValue of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for PEO 2 ($) | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for PEO 2 ($) | Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for PEO 2 ($) | Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for PEO 2 ($) | Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for PEO 2 ($) | Value of Dividends or Other Earnings Paid on Equity Awards Not Otherwise Included for PEO 2 ($) | Total - Inclusion of Equity Values for PEO 2 ($) | |||||||||||||||||||||
| 2023 | 19,484,650 | 0 | 0 | 0 | 0 | 0 | 19,484,650 | |||||||||||||||||||||
Year | Average Year- End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for Non-PEO NEOs ($) | Average Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for Non-PEO NEOs ($) | Average Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for Non-PEO NEOs ($) | Average Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for Non-PEO NEOs ($) | Average Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for Non-PEO NEOs ($) | Average Value of Dividends or Other Earnings Paid on Equity Awards Not Otherwise Included for Non-PEO NEOs ($) | Total - Average Inclusion of Equity Values for Non-PEO NEOs ($) | |||||||||||||||||||||
| 2023 | 2,729,569 | 92,894 | 0 | 104,854 | 0 | 0 | 2,927,317 | |||||||||||||||||||||
| (4) | The Peer Group TSR set forth in this table utilizes the Dow Jones U.S. Select Pharmaceuticals Index, which we also utilize in the stock performance graph required by Item 201(e) of Regulation S-K included in our Annual Report for the year ended December 31, 2023. The comparison assumes $100 was invested for the period starting December 31, 2019, through the end of the listed year in the Company and in the Dow Jones U.S. Select Pharmaceuticals Total Return Index, respectively. Historical stock performance is not necessarily indicative of future stock performance. |
| (5) | We determined Non-GAAP Operating Income to be the most important financial performance measure used to link Company performance to Compensation Actually Paid to our PEO andNon-PEO NEOs in 2023. Please see “Executive Compensation—Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Long-Term Incentive Equity-Based Compensation” above for a description of howNon-GAAP Operating Income is determined. This performance measure may not have been the most important financial performance measure for years 2022, 2021 and 2020 and we may determine a different financial performance measure to be the most important financial performance measure in future years. |
| (6) | The amount set forth for Net Income in 2022 has been updated in connection with a revision made in 2023. Please see note 1b to the consolidated financial statements included in the Annual Report on Form 10-K for the year ended December 31, 2023. |
Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 93 | |
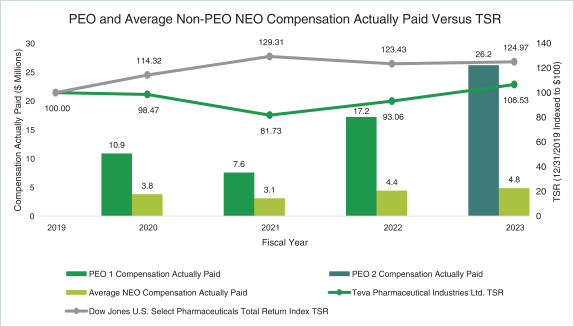
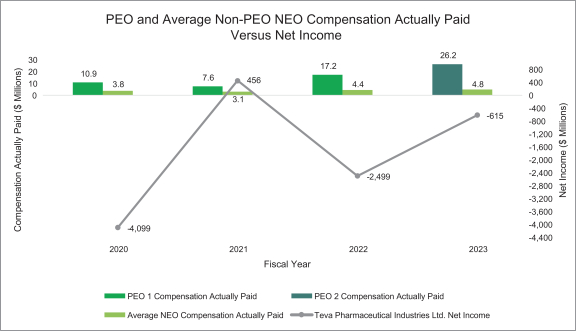
| 94 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
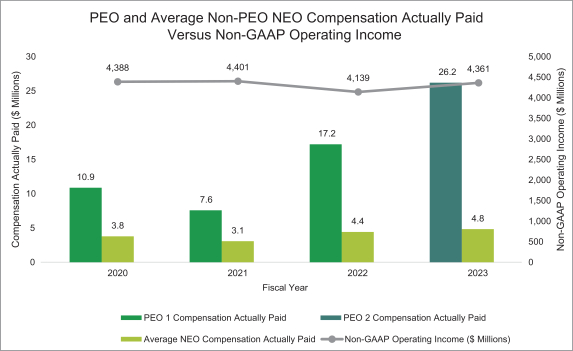
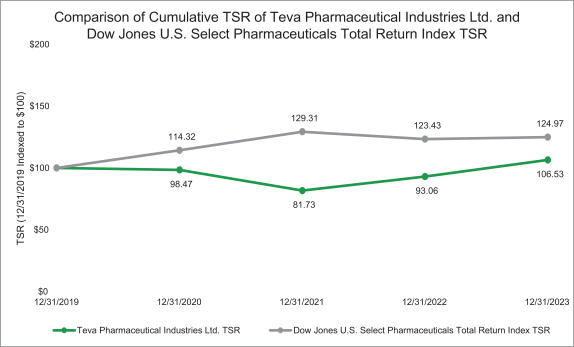
Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 95 | |
Non-GAAP Operating IncomeNet Revenue from Innovative Products Net Revenue from All Other Products Relative TSR Non-GAAP Earnings Per Share |
| Free Cash Flow |
| 96 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
HR and Compensation Committee Interlocks and Insider Participation
The HR and Compensation Committee currently consists of Rosemary A. Crane (chair), Gerald M. Lieberman, Dr. Perry Nisen and Janet S. Vergis. During fiscal year 2023, no member of the HR and Compensation Committee was an employee, officer or former officer of Teva or any of its subsidiaries. During fiscal year 2023, no member of the HR and Compensation Committee had a relationship that must be described under the SEC rules relating to disclosure of related party transactions. During fiscal year 2023, none of our executive officers served on the board of directors or compensation committee of any entity that had one or more of its executive officers serving on Teva’s Board of Directors or HR and Compensation Committee.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 97 | |
Proposal 2: Advisory Vote on Compensation of Named Executive Officers
As required by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”) and Schedule 14A of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), we are providing our shareholders with the opportunity to approve, by advisory vote, the compensation of our NEOs, as disclosed in this Proxy Statement in accordance with the rules of the SEC.
This proposal, commonly referred to as the “say-on-pay” vote, gives our shareholders the opportunity to express their views on the compensation of our NEOs. This vote is not intended to address any specific item of compensation or any specific NEOs, but rather the overall compensation of our NEOs and our executive compensation philosophy, objectives and program, as described in this Proxy Statement. Accordingly, we ask our shareholders to approve the compensation of our NEOs, as disclosed pursuant to Item 402 of Regulation S-K of the Exchange Act in the section entitled “Executive Compensation” of this Proxy Statement, including the CD&A, the compensation tables and the related narrative disclosure, by casting a non-binding advisory vote “FOR” the following resolution:
“RESOLVED, that the shareholders of Teva Pharmaceutical Industries Limited approve, on a non-binding advisory basis, the compensation of its named executive officers, as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion.”
As an advisory vote, the result will not be binding on the Board or the HR and Compensation Committee. The say-on-pay vote will, however, provide us with important feedback from our shareholders about our executive compensation philosophy, objectives and program. Our Board and the HR and Compensation Committee value the opinions of our shareholders and expect to take into account the outcome of the vote when considering future executive compensation decisions and when evaluating our executive compensation program. Following our 2024 Annual Meeting, the next advisory vote on named executive officer compensation is expected to occur at the 2025 annual meeting, unless the Board of Directors modifies its policy on the frequency of holding such advisory votes.

| 98 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Proposal 3: Advisory Vote on Frequency of Advisory Vote on Compensation of Named Executive Officers
The Dodd-Frank Act and Schedule 14A of the Exchange Act also enable our shareholders to indicate, at least once every six years, how frequently we should seek an advisory vote on named executive officer compensation, such as Proposal 2 above. By voting on this Proposal 3, shareholders may indicate whether they would prefer an advisory vote on named executive compensation once every one, two or three years.
After careful consideration, our Board has determined that an advisory say-on-pay vote should be held annually. Our Board believes that holding a say-on-pay vote annually is the most appropriate option because it will give us more frequent feedback from our shareholders on our executive compensation philosophy, objectives and program, as well as the compensation paid to our named executive officers.
For the reasons discussed above, our Board recommends that you vote for once every “1 Year” as the frequency of future say-on-pay votes.
You may cast your advisory vote on the frequency of future say-on-pay votes by choosing the option of “1 Year,” “2 Years” or “3 Years,” or you may abstain from voting on this Proposal 3. The option of “1 Year,” “2 Years” or “3 Years” that receives the highest number of ordinary shares participating and voting at the Annual Meeting, in person or by proxy or through their representatives, will be deemed to be the frequency of future say-on-pay votes recommended by our shareholders. Abstentions will have no effect on this proposal.
While our Board believes that its recommendation is appropriate at this time, shareholders are not voting to approve or disapprove the recommendation, but are instead asked to recommend, on a non-binding advisory basis, whether the say-on-pay vote should be held once every one, two or three years.
Our Board and our HR and Compensation Committee value the opinions of our shareholders on this matter and, to the extent there is any significant vote in favor of one time period over another, will take into account the outcome of this vote when making decisions regarding the frequency of holding future say-on-pay votes. However, because this vote is advisory and not binding on Teva or the Board in any way, the Board may decide that it is in the best interests of our shareholders and Teva to hold a say-on-pay vote more or less frequently than the option recommended by our shareholders.

| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 99 | |
Proposal 4: Appointment of Independent Registered Public Accounting Firm
The Audit Committee recommends that, as required under Israeli law, shareholders appoint Kesselman & Kesselman, an independent registered public accounting firm in Israel and a member of PricewaterhouseCoopers International Limited (“PwC”), as Teva’s independent registered public accounting firm until Teva’s 2025 annual meeting of shareholders. PwC has been our independent registered public accounting firm since at least 1976.
Pursuant to Teva’s Articles of Association, the Board of Directors is authorized to determine the remuneration of Teva’s independent registered public accounting firm.
Representatives of PwC are expected to be present at the Annual Meeting and will also be available to respond to questions from shareholders. They also will have the opportunity to make a statement if they desire to do so.
Audit Committee Report
The Audit Committee has reviewed and discussed with management Teva’s audited consolidated financial statements as of and for the year ended December 31, 2023.
The Audit Committee has also discussed with Kesselman & Kesselman the matters required to be discussed by the Public Company Accounting Oversight Board (“PCAOB”) Auditing Standard No. 1301, Communications with Audit Committees.
The Audit Committee has received and reviewed the written disclosures and the letter from Kesselman & Kesselman required by the applicable requirements of the PCAOB regarding Kesselman & Kesselman’s communication with the Audit Committee concerning independence and has discussed with Kesselman & Kesselman their independence.
Based on the reviews and discussions referred to above, the Audit Committee recommended to the Board of Directors that the audited consolidated financial statements referred to above be included in Teva’s Annual Report on Form 10-K for the year ended December 31, 2023 for filing with the SEC.
Audit Committee of the Board of Directors
Gerald M. Lieberman, Chair
Amir Elstein
Roberto A. Mignone
Dr. Tal Zaks
Policy on Pre-Approval of Audit and Non-Audit Services of Independent Auditors
Teva’s Audit Committee is responsible for overseeing the work of its independent auditors. The Audit Committee’s policy is to pre-approve all audit and non-audit services provided by PwC and other members of PricewaterhouseCoopers International Limited. These services may include audit services, audit-related services, tax services and other services, as further described below. The Audit Committee sets forth the basis for its pre-approval in detail, listing the particular services or categories of services which are pre-approved, and setting forth a specific budget for such services. Other services are approved by the Audit Committee on an individual basis. Once services have been pre-approved, PwC and management then report to the Audit Committee on a periodic basis regarding the extent of services actually provided in accordance with the applicable pre-approval, and regarding the fees for the services performed. Such fees for 2023 and 2022 were pre-approved by the Audit Committee in accordance with these procedures.
| 100 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Proposal 4: Appointment of Independent Registered Public Accounting Firm
Principal Accountant Fees and Services
Teva incurred the following fees for professional services rendered by Kesselman & Kesselman and other PwC member firms, for the years ended December 31, 2023 and 2022:
|
| 2023 | 2022 | ||||||
|
| (U.S. $ in thousands) | |||||||
Audit fees | $ | 15,472 | $ | 13,152 | ||||
Audit-related fees | 109 | 331 | ||||||
Tax fees | 2,021 | 2,693 | ||||||
All other fees | 200 | 68 | ||||||
Total | $ | 17,802 | $ | 16,244 | ||||
The audit fees for the years ended December 31, 2023 and 2022 included professional services rendered for the integrated audit of Teva’s annual consolidated financial statements and internal control over financial reporting as of December 31, 2023 and 2022, review of consolidated quarterly financial statements, statutory audits of Teva and its subsidiaries, review of securitization and other management actions, issuance of comfort letters, consents and assistance with review of documents filed with the SEC and other services that can only be provided by the independent auditor.
The audit-related fees for the years ended December 31, 2023 and 2022 included accounting consultations, employee benefit plan audits, agreed upon procedures that are not required by statute or regulation and consultations concerning financial accounting and reporting standards.
Tax fees for the years ended December 31, 2023 and 2022 included services related to tax compliance including the preparation of tax returns and claims for refund, tax planning and tax advice including assistance with tax audits and appeals, advice related to mergers and acquisitions, tax services for employee benefit plans and assistance with respect to requests for rulings from tax authorities.
All other fees for the years ended December 31, 2023 and 2022 were mainly for providing benchmarking data, as well as for license fees for the use of accounting research tools and training regarding general financial reporting developments.
The Audit Committee believes that the provision of all non-audit services rendered is compatible with maintaining PwC’s independence.

| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 101 | |
Presentation of 2023 Financial Statements
The Board of Directors has approved, and is presenting to shareholders for receipt and consideration at the Annual Meeting, Teva’s annual consolidated financial statements for the year ended December 31, 2023, which are included in Teva’s Annual Report on Form 10-K for the year ended December 31, 2023, available on Teva’s website at www.tevapharm.com.
| 102 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Security Ownership
The following table describes, as of April 1, 2024, the beneficial ownership of Teva ordinary shares (and ADSs representing ordinary shares) by:
| - | each of our NEOs; |
| - | each of our directors and director nominees; and |
| - | all of our directors and executive officers as a group. |
As of April 1, 2024, there were no beneficial owners of more than 5% of Teva’s outstanding ordinary shares.
Beneficial Owner | Ordinary Shares Beneficially Owned*** | Percent of Ordinary Shares Outstanding**** | ||||||
Named Executive Officers, Directors and Director Nominees:* |
|
|
|
|
|
| ||
Dr. Sol J. Barer | 371,516 | * | * | |||||
Richard D. Francis | 123,295 | * | * | |||||
Rosemary A. Crane | 82,507 | * | * | |||||
Amir Elstein | 2,073,223 | * | * | |||||
Gerald M. Lieberman | 84,917 | * | * | |||||
Roberto A. Mignone | 1,574,575 | (1) | * | * | ||||
Dr. Perry D. Nisen | 74,575 | * | * | |||||
Prof. Ronit Satchi-Fainaro | 67,169 | * | * | |||||
Prof. Varda Shalev | — | * | * | |||||
Janet S. Vergis | 42,957 | * | * | |||||
Dr. Tal Zaks | 28,032 | * | * | |||||
Eli Kalif | 203,691 | * | * | |||||
Mark Sabag | 723,999 | * | * | |||||
Richard Daniell | 316,490 | * | * | |||||
Eric Drapé | 401,422 | * | * | |||||
All directors and executive officers as a group (20 persons) | 6,239,671 | * | * | |||||
| * | The address of each named executive officer and director is c/o Teva Pharmaceutical Industries Limited, 124 Dvora HaNevi’a Street, Tel Aviv, 6944020, Israel. |
| ** | Represents less than 1%. |
| *** | For purposes of this table, “beneficial ownership” is determined in accordance with Rule 13d-3 under the Exchange Act pursuant to which a person or group of persons is deemed to have “beneficial ownership” of any ordinary shares with respect to which such person has (or has the right to acquire within 60 days) sole or shared voting power or investment power. |
| **** | Percentage of beneficial ownership is based on 1,132,640,597 ordinary shares outstanding at April 1, 2024. |
| (1) | 1,500,000 ordinary shares are held of record by Swiftcurrent Partners, L.P. and Swiftcurrent Offshore Master, Ltd. Bridger Management, LLC is the investment adviser to these funds and Mr. Mignone is the manager of Bridger Management, LLC. Mr. Mignone disclaims beneficial ownership of the 1,500,000 ordinary shares held of record by these funds, except to the extent of his indirect pecuniary interest therein. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 103 | |
Securities Authorized for Issuance under Equity Compensation Plans
The following table sets forth, as of December 31, 2023, certain information related to our equity compensation plans:
Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted- Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) (c) | |||||||||
Equity compensation plans approved by security holders |
|
|
|
|
|
|
|
|
| |||
2020 Long-Term Equity-Based | 34,054,152 | — | 65,803,586 | (1) | ||||||||
2015 Long-Term Equity-Based | 18,950,491 | $ | 31.40 | — | (2) | |||||||
2010 Long-Term Equity-Based | 5,362,250 | $ | 54.64 | — | (2) | |||||||
2008 Employee Stock Purchase Plan For U.S. Employees (“ESPP”) | — | — | 1,294,135 | (3) | ||||||||
Equity compensation plans not approved by security holders | — | — | — | |||||||||
Total | 58,366,893 | $ | 36.89 | 67,097,722 | (1) | |||||||
| (1) | Includes awards that were cancelled or forfeited under the 2010 and 2015 Long-Term Equity-Based Incentive Plans. |
| (2) | This plan expired and no future grants are available thereunder. |
| (3) | A total of 8,500,000 shares have been authorized for purchase at a discount under the plan. |
| 104 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Related Party Transactions
Certain Relationships and Related Party Transactions
In November 2019, Teva entered into two collaborative research agreements with Tel Aviv University pursuant to which Teva provided funding in the amounts of €250,000 and $94,500, respectively, and worked with the Tel Aviv University scientists to advance cancer and brain studies. Following finalization of the research plans, in May 2021, Teva entered into two continuation research agreements (of the two collaborative research agreements entered into in November 2019) with Tel Aviv University, to conduct additional and further research plans, to which Teva provided funding in the amount of NIS 1,238,490 and NIS 313,275, respectively. In November 2022 and November 2023, Teva extended these two continuation research agreements with Tel Aviv University for an additional one-year period per each extension and in a total amount of $600,000 per year. Prof. Ronit Satchi-Fainaro, a member of our Board of Directors, has been a professor at Tel Aviv University since 2015. Prof. Ronit Satchi-Fainaro holds various other positions at Tel Aviv University, including Head of the Cancer Research and Nanomedicine Laboratory since 2006, Chair of the Department of Physiology and Pharmacology at the Sackler Faculty of Medicine since 2014, The Kurt and Herman Lion Chair in Nanosciences and Nanotechnologies since 2017, Director of the Cancer Biology Research Center since 2020 and a member of the Preclinical Dean’s Committee since 2015.
The related party transaction described above was reviewed and approved in accordance with the provisions of the Israeli Companies Law, Teva’s Articles of Association and Teva’s written policy regarding transactions with related parties (the “Related Party Transactions Policy”), as described below. Any extensions to these agreements will also be reviewed accordingly.
Approval of Related Party Transactions
The Israeli Companies Law requires that an “office holder” (as defined in the Israeli Companies Law) of a company promptly disclose any personal interest that he or she may have and all related material information known to him or her, in connection with any existing or proposed transaction of the company. Each of our directors and executive officers is an ‘office holder’ under the Israeli Companies Law and Teva’s Related Party Transactions Policy. Teva’s Related Party Transactions Policy also applies to any director nominees (“Director Nominees”), any holders of 5% or more of Teva’s outstanding share capital or voting rights (“5% Holders” and collectively with Teva’s office holders and Director Nominees, the “Covered Persons”) and with respect to transactions in which a Covered Person has a direct or indirect personal interest, including a personal interest of a relative of such Covered Person and a personal interest of an entity in which such Covered Person or a relative of such Covered Person is an interested party (as defined in the Israeli Companies Law).
Pursuant to the Israeli Companies Law and Teva’s Related Party Transactions Policy, the Audit Committee shall determine whether any transaction with a Covered Person in which the Covered Person has a personal interest (other than, with respect to a Covered Person who is an office holder, such office holder’s Terms of Office and Employment, see “Executive Compensation—Compensation Discussion and Analysis—Compensation-Related Requirements of the Israeli Companies Law”) is an “extraordinary transaction” (defined as a transaction not in the ordinary course of business, not on market terms or likely to have a material impact on the company’s profitability, assets or liabilities).
Pursuant to the Israeli Companies Law, the Articles of Association and Teva’s Related Party Transactions Policy, in the event that the Audit Committee determines that the transaction is an extraordinary transaction, Audit Committee and Board approval are required and, in some circumstances, shareholder approval may also be required; if however, it is determined that the transaction is not an extraordinary transaction, the transaction will not require Board or shareholder approval. A related party transaction may only be approved if it is determined to be in the best interests of Teva. The Company should normally not commit to a Related Party Transaction for a term of more than three years without the right to review and re-negotiate its terms and provisions at least once every three years.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 105 | |
Related Party Transactions
A person with a personal interest in the matter generally may not be present at meetings of the Board or certain committees where the matter is being considered and, if a member of the Board or a committee, may generally not vote on the matter.
Transactions with Controlling Shareholders
Under Israeli law, extraordinary transactions with a controlling shareholder, or in which the controlling shareholder has a personal interest, and any engagement with a controlling shareholder, or a controlling shareholder’s relative, with respect to the provision of services to the company or their Terms of Office and Employment as an office holder or their employment, if they are not an office holder, generally require the approval of the Audit Committee (or with respect to Terms of Office and Employment, the HR and Compensation Committee), the Board of Directors and the shareholders. If required, shareholder approval must include (i) at least a majority of the shareholders who do not have a personal interest in the transaction and are present and voting at the meeting (abstentions are disregarded), or, alternatively, that (ii) the total shareholdings of the disinterested shareholders who vote against the transaction do not represent more than two percent of the voting rights in the company. Transactions for a period of more than three years generally need to be brought for approval in accordance with the above procedures every three years. A shareholder who holds 25% or more of the voting rights in a company is considered a controlling shareholder for these purposes if no other shareholder holds more than 50% of the voting rights. If two or more shareholders are interested parties in the same transaction, their shareholdings are combined for the purposes of calculating percentages.
Change of Control
Subject to certain exceptions, the Israeli Companies Law generally requires that a merger between two Israeli companies be approved by both the board of directors and by the shareholders of each of the merging companies by a simple majority (unless a higher majority is required by the articles of association).
Furthermore, the Israeli Companies Law generally requires that an acquisition of shares in a public company be made by means of a tender offer if as a result of the acquisition the purchaser would hold 25% or more of the voting rights of the company if there is no other holder of 25% or more of the company’s voting rights, or more than 45% of the voting rights of the company if there is no other holder of more than 45% of the company’s voting rights. The Israeli Companies Law generally further requires that such offer be consummated only if at least 5% of the company’s voting rights will be acquired, and that subject to certain exceptions, the majority of the offerees who responded to the offer accepted the offer. Notwithstanding the above, the Amended Relief Regulations provide certain Israeli Companies whose shares are traded on a stock exchange in Israel and outside of Israel (dual-listed) an exemption from the above limitations, but only in circumstances where the law of the foreign country in which such company is traded, restricts the acquisition of control by a certain percentage of the company or if the acquisition of control by a certain percentage of the company obligate the purchaser to provide a tender offer also to shareholders from the public. Therefore, currently this relief does not apply to companies traded on NYSE.
| 106 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Shareholder Proposals for the 2024 Annual Meeting and the 2025 Annual Meeting
Under Israeli law, one or more shareholders holding 1% or more of the voting rights of Teva may propose to include any matter appropriate for deliberation at a shareholders meeting to be included on the agenda of a shareholders meeting, provided however, that under the Amended Relief Regulations, only one or more shareholders holding 5% or more of the voting rights of Teva may propose the nomination or removal of a candidate to the Board of Directors (which will be brought for consideration by Teva’s Corporate Governance and Nominating Committee). All proposals shall be made by submitting such proposal within seven days of publication of Teva’s notice with respect to its general meeting of shareholders, unless Teva publishes a preliminary notice at least twenty-one days prior to a publication of the notice of the meeting, stating its intention to convene such meeting and the agenda thereof, in which case the shareholder proposal should be submitted within fourteen days of such preliminary notice. Accordingly, any one or more shareholders holding 5% or more of the voting rights of Teva or 1% or more of the voting rights of Teva, may request to include a proposal on the nomination or removal of a candidate to the Board of Directors, or for any other matter on the agenda of the Annual Meeting, respectively, all by submitting such proposal in writing to Teva no later than April 24, 2024, at its executive offices located at 124 Dvora HaNevi’a Street, Tel Aviv, 6944020, Israel, Attn: Dov Bergwerk, Company Secretary.
Any such shareholder proposal must comply with the requirements of applicable law and Teva’s Articles of Association. The requirements under Teva’s Articles of Association include providing information such as: (i) the number of ordinary shares held by the proposing shareholder, directly or indirectly, and, if any such ordinary shares are held indirectly, an explanation of how they are held and by whom; (ii) the shareholder’s purpose in making the request; and (iii) any agreements, arrangements, understandings or relationships between the shareholder and any other person with respect to any securities of Teva or the subject matter of the request. If the proposal is to nominate a candidate for election to the Board of Directors, the proposing shareholder must also provide (a) a declaration signed by the nominee and any other information required under the Israeli Companies Law, (b) additional information in respect of the nominee as would be required in response to the applicable law, regulation and/or stock exchange rules, including disclosure requirements in Israel and/or abroad, (c) a representation made by the nominee of whether the nominee meets the objective criteria for independence under any applicable law, regulation or stock exchange rules, in Israel or abroad, and if not, then an explanation of why not, and (d) details of all relationships and understandings between the proposing shareholder and the nominee.
In addition to satisfying the requirements of Teva’s Articles of Association, to comply with the requirements set forth in the Exchange Act, (i) shareholders who intend to solicit proxies in support of director nominees, other than the Board’s nominees, in connection with Teva’s 2025 annual general meeting of shareholders must provide written notice to the Company’s Secretary that sets forth all the information required by Rule 14a-19 of the Exchange Act, postmarked or transmitted electronically to the Company at the Company’s executive offices located at 124 Dvora HaNevi’a Street, Tel Aviv, 6944020, Israel, Attn: Dov Bergwerk, Company Secretary, no later than April 7, 2025, and (ii) shareholders who seek to include other shareholder proposals in the proxy statement and proxy card for Teva’s 2025 annual general meeting of shareholders pursuant to Rule 14a-8 must provide written notice postmarked to the Company at the Company’s executive offices, on or before December 20, 2024 and must comply with Rule 14a-8.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 107 | |
Incorporation by Reference
In accordance with SEC rules, notwithstanding anything to the contrary set forth in any of our previous or future filings under the Securities Act or the Exchange Act that might incorporate this Proxy Statement or future filings made by Teva under those statutes, the information included under the caption “HR and Compensation Committee Report” and those portions of the information included under the caption “Audit Committee Report” required by the SEC’s rules to be included therein shall not be deemed to be “soliciting material” or “filed” with the SEC and shall not be deemed incorporated by reference into any of those prior filings or into any future filings made by Teva under those statutes, except to the extent we specifically incorporate these items by reference.
Householding of Proxy Materials
Some banks, brokers and other nominee record holders may participate in the practice of “householding” proxy statements. This means that only one copy of the proxy materials may have been sent to multiple shareholders in your household. Teva will promptly deliver a separate copy of the proxy statement, as well as its Annual Report, to you if you write or call the Company at: TevaIR@tevapharm.com or mailing address: Teva Pharmaceutical Industries Ltd., 124 Dvora HaNevi’a Street, Tel Aviv, 6944020, Israel, Attn: Investor Relations, or phone: +972 (3) 914-8262. If you want to receive copies of Teva’s proxy statement in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker or other nominee record holder, or you may contact us at the above address and phone numbers.
| 108 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Questions and Answers about the Annual Meeting
The Meeting
When and how will the Annual Meeting be held?
The Annual Meeting will be conducted in a virtual format through an online meeting platform, on Thursday, June 6, 2024, at 4:00 p.m. Israel time / 9:00 a.m. Eastern time. Shareholders will not be able to physically attend the Annual Meeting.
For additional information on how to vote and participate at the Annual Meeting see below under “Participating and Voting.”
Who may attend the Annual Meeting?
Attendance at the Annual Meeting, including any adjournments or postponements thereof, will be limited to holders of record as of the close of business on April 30, 2024 (the “Record Date”) who hold ordinary shares or American Depositary Shares (“ADSs”), directly in their own name, and beneficial owners who hold ordinary shares or ADSs through a broker, bank or other nominee rather than directly in their own name, and each of their legal proxy holders or their authorized persons.
What is a quorum for the Annual Meeting?
A minimum of two holders of ordinary shares (or ADSs representing such ordinary shares) who are present at the Annual Meeting, in person or by proxy or represented by their authorized persons, and who hold in the aggregate twenty-five percent or more of such ordinary shares (or ADSs representing such ordinary shares), will constitute a legal quorum. At the close of business on April 1, 2024, 1,132,640,597 ordinary shares were outstanding and entitled to vote. Ordinary shares held in treasury will not be included in the calculation to determine if a quorum is present. Abstentions and broker non-votes will be considered present and entitled to vote for the purpose of determining the presence of a quorum. Should no legal quorum be present one-half hour after the scheduled time, the Annual Meeting will be adjourned to one week from that day, at the same time and in a virtual format. Should such legal quorum not be present one-half hour after the time set for the Annual Meeting, as adjourned, any two holders of ordinary shares present, in person or by proxy, who jointly hold twenty percent or more of such ordinary shares (or ADSs representing ordinary shares) will then constitute a legal quorum.
Who may vote at the Annual Meeting?
Ordinary Shares
Holders of record of ordinary shares as of the Record Date may vote at the Annual Meeting.
Beneficial owners who hold ordinary shares through a nominee company pursuant to Section 177(1) of the Israeli Companies Law, as of the Record Date (a “Non-Registered Holder”), rather than directly in their own name, have the right to direct their broker, bank or other nominee how to vote using the instructions provided by the broker, bank or other nominee, but may not vote their shares at the Annual Meeting unless they obtain a proof of share ownership as of the Record Date (“Proof of Ownership”), which must be approved by a member of the Tel Aviv Stock Exchange (“TASE”).
ADSs
As an ADS holder, you will not be entitled to vote at the Annual Meeting through the online meeting platform. To the extent you provide the Depositary (as defined below) or your broker, bank or other nominee, as applicable, with voting instructions, the Depositary has advised us that it will vote the ordinary shares underlying your ADSs in accordance with your instructions.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 109 | |
Questions and Answers about the Annual Meeting
You also may exercise the right to vote the ordinary shares underlying your ADSs by surrendering your ADSs to Citibank, N.A., as depositary for the ADSs (the “Depositary”) for cancellation and withdrawal of the corresponding ordinary shares pursuant to the terms described in the Second Amended and Restated Deposit Agreement (the “Deposit Agreement”), dated as of December 4, 2018, by and among the Company, the Depositary, and the holders and beneficial owners of ADSs. In order to be able to vote at the Annual Meeting, you must complete the ADS cancellation process and become a holder of the corresponding ordinary shares by the Record Date. However, it is possible that you may not have sufficient time to withdraw your ordinary shares and vote them at the upcoming Annual Meeting as a holder of record of ordinary shares as of the Record Date. Holders of ADSs may incur additional costs associated with the ADS cancellation process.
The Israeli Companies Law does not permit shareholders of public companies to act by written consent.
Participating and Voting
How can I access the online meeting platform and vote my ordinary shares or ADSs?
To access the Annual Meeting, holders of Teva’s ADSs and ordinary shares should visit meetnow.global/MS7KDRJ, and when prompted, enter their 15-digit control number (if holders of ADSs) or the control number received from the Company upon their registration (for holders of ordinary shares).
Your vote is very important and we encourage you to vote your shares and submit your proxy regardless of whether or not you plan to attend the Annual Meeting. Each issued and outstanding ordinary share (or ADS representing an ordinary share) shall entitle its holder to one vote on each matter properly submitted at the Annual Meeting. Ordinary shares held in treasury by Teva do not entitle Teva to vote in respect thereof at the Annual Meeting.
Ordinary Shares
Record holders of ordinary shares: If you are the record holder of ordinary shares as of the Record Date, you have the right to (i) vote at the Annual Meeting through the online meeting platform, (ii) vote by submitting your proxy card by mail or by email to TevaAGM2024@tevapharm.com, or (iii) grant your voting proxy to an authorized person.
If you choose to submit your proxy card by mail or by email to TevaAGM2024@tevapharm.com, mark the enclosed proxy card in accordance with the instructions, date, sign and return it to Teva. To be taken into account, your proxy card must be received by Teva, by 4:00 p.m., Israel time, on June 3, 2024, unless determined otherwise by the chairman of the Annual Meeting.
In order to access the Annual Meeting through the online meeting platform, record holders of ordinary shares must register in advance. To register, record holders of ordinary shares must submit their registration request via email to Teva at TevaAGM2024@tevapharm.com, with their name and identification number. Requests for registration must be received no later than June 4, 2024 at 3:00 p.m., Israel time.
Record holders of ordinary shares will then receive a confirmation of registration from the Company along with a control number to access the Annual Meeting. The control number will consist of the holder’s Israeli identification number or Israeli company registration number as it appears in Teva’s share register.
Record holders of ordinary shares will also be able to ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting with their name and control number.
| 110 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Questions and Answers about the Annual Meeting
If you appoint another person to act as your authorized proxy, such proxy must be submitted in writing by mail or by email to TevaAGM2024@tevapharm.com (in a form approved by the Company Secretary) from the record holder of ordinary shares, including such record holder’s Israeli identification number or company registration number, along with the authorized proxy’s name, Israeli identification number, proof of identification and email address, and must be received by Teva by 4:00 p.m., Israel time, on June 3, 2024, unless determined otherwise by the chairman of the Annual Meeting. The submission of a proxy by the aforementioned deadline will also serve to register the authorized proxy in advance in order to participate in the Annual Meeting, vote your shares and ask questions through the online meeting platform. Such authorized proxy will then be able to use their Israeli identification number as it appears on the proof of Israeli identification provided to Teva as the control number to access the online meeting platform.
Non-registered holders of ordinary shares: If you are a Non-Registered Holder, you may (i) direct your broker, bank or other nominee how to vote using the instructions provided by such broker, bank or other nominee; (ii) vote through the electronic voting system of the Israeli Securities Authority at least a day prior to the Annual Meeting (i.e., before 4:00 p.m., Israel time, on June 5, 2024); or (iii) if you obtain a Proof of Ownership as detailed above, submit your vote (a) by submitting your proxy card by mail or by email to TevaAGM2024@tevapharm.com, together with the Proof of Ownership, by 4:00 p.m., Israel time, on June 3, 2024, unless determined otherwise by the chairman of the Annual Meeting; or (b) vote at the Annual Meeting through the online meeting platform.
In order to access the Annual Meeting through the online meeting platform, Non-Registered Holders of ordinary shares must register in advance. To register, Non-Registered Holders of ordinary shares must submit the Proof of Ownership (including Israeli identification number or company registration number) reflecting the number of ordinary shares beneficially owned as of the Record Date, along with their name and email address, to Teva at TevaAGM2024@tevapharm.com. Requests for registration must be received no later than June 4, 2024 at 3:00 p.m., Israel time.
Non-Registered Holders of ordinary shares will then receive a confirmation of registration from the Company along with a control number to access the Annual Meeting. The control number will consist of the holder’s Israeli identification number or Israeli company registration number as it appears on the Proof of Ownership provided to Teva. Non-Registered Holders will then be able to use such control number to access the Annual Meeting through the online meeting platform.
Non-Registered Holders of ordinary shares will also be able to ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting with their name and control number.
If a Non-Registered Holder would like to appoint another person to act as his/her authorized proxy, such proxy must be submitted to Teva along with the Proof of Ownership, following the instructions set forth above.
ADSs
The Deposit Agreement sets out the rights of ADS holders as well as the rights and obligations of the Depositary. Each ADS represents the right to receive one ordinary share deposited with Citibank Tel Aviv, as custodian for the Depositary under the Deposit Agreement or any successor custodian.
Record holders of ADSs: If you are a record holder of ADSs as of the Record Date, you will receive instructions from the Depositary for the ordinary shares underlying your ADSs to be voted. If you held ADSs directly as of the Record Date, you have the right to instruct the Depositary how to vote. So long as the Depositary receives your voting instructions by 8:00 a.m., Eastern time, on June 5, 2024, it will, to the extent practicable and subject to Israeli law and the terms of the Deposit Agreement, vote the underlying ordinary shares as you instruct.
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 111 | |
Questions and Answers about the Annual Meeting
Record holders of ADSs as of the close of the Record Date will be able to access the Annual Meeting and ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting; however, they will not be able to vote through the online meeting platform.
To access the Annual Meeting through the online meeting platform, record holders of ADSs should enter the 15-digit control number on the proxy card or Notice of Internet Availability of Proxy Materials received.
Beneficial owners of ADSs that are registered in the name of a broker, bank or other agent: If you beneficially own ADSs as of the Record Date through a broker, bank or other nominee, such intermediary will provide you instructions on how you may vote the ordinary shares underlying your ADSs. Please check with your broker, bank or other nominee, as applicable, and carefully follow the voting procedures provided to you.
To access the Annual Meeting and ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting, beneficial holders of ADSs that are registered in the name of a broker, bank or other agent must register in advance; however, they will not be able to vote through the online meeting platform.
To register, beneficial holders of ADSs must submit proof of ownership reflecting the number of ADSs beneficially owned as of the Record Date, along with their name and email address, to Computershare at legalproxy@computershare.com. Requests for registration must be labeled as “TEVA MEETING REQUEST” and must be received no later than Wednesday, June 4, 2024 at 8:00 a.m., Eastern time. Beneficial holders of ADSs will then receive a confirmation of registration with a 15-digit control number by email from Computershare. Beneficial holders of ADSs will then be able to use such control number to access the Annual Meeting through the online meeting platform.
How will my ordinary shares or ADSs be voted if I do not vote?
Ordinary Shares
If you hold ordinary shares and do not (i) vote at the Annual Meeting through the online meeting platform, (ii) vote by submitting your proxy card by mail or by email, (iii) grant your voting proxy to an authorized person or (iv) as a Non-Registered Holder, vote by submitting your proxy card and Proof of Ownership by mail, by email or through the electronic voting system of the Israeli Securities Authority, your ordinary shares will not be counted as votes cast and will have no effect on the outcome of the vote with respect to any matter.
ADSs
If you are a record holder of ADSs and do not instruct the Depositary how to vote the ordinary shares underlying your ADSs, the ordinary shares underlying your ADSs will not be counted as votes cast and will have no effect on the outcome of the vote with respect to any matter.
If you are a beneficial owner whose ADSs are held of record by a broker, your broker has “discretionary voting” authority under the New York Stock Exchange (“NYSE”) rules to vote the shares represented by your ADSs on “routine” matters, such as the ratification of appointment of Kesselman & Kesselman, an independent registered public accounting firm in Israel and a member of PricewaterhouseCoopers International Ltd., as our independent registered public accounting firm, even if the broker does not receive voting instructions from you. However, your broker does not have discretionary authority absent specific instructions from you to vote on the following “non-routine” matters: the election of directors, the advisory vote on the compensation of our named executive officers, and the advisory vote on whether the vote on the compensation of our named executive officers should be held once every one, two or three years, in which case a broker non-vote will occur and the shares represented by your ADSs will not be voted on these matters.
| 112 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Questions and Answers about the Annual Meeting
What are the voting requirements to elect the directors and to approve each of the proposals discussed in this Proxy Statement?
Each proposal requires a simple majority for approval, with a simple majority requiring the affirmative vote of the holders of a majority of Teva’s ordinary shares participating and voting at the Annual Meeting, in person or by proxy or through their representatives. Abstentions and broker non-votes are disregarded. Broker discretionary voting is permitted only for Proposal 4.
Under the terms of the Deposit Agreement, the Depositary shall endeavor (insofar as is practicable and in accordance with our Articles of Association) to vote or cause to be voted the number of ordinary shares represented by ADSs in accordance with the instructions provided by the holders of ADSs to the Depositary by the deadline set. If instructions are not received by the Depositary by the deadline, the ordinary shares represented by such uninstructed ADSs shall not be voted at the Annual Meeting. If instructions are signed and timely returned to the Depositary, but no specific voting instruction is marked for a proposal, the holder shall be deemed to have directed the Depositary to give voting instructions “FOR” the unmarked proposal.
Can I change my vote?
Ordinary Shares
If you hold ordinary shares of record and submit your proxy card to vote by mail, by email, or appoint a proxy in advance of the Annual Meeting, you may change your vote by delivering a valid later-dated proxy within the time limitations set forth above, or by voting at the Annual Meeting through the online meeting platform.
If you are a Non-Registered Holder of ordinary shares and vote through the electronic voting system of the Israeli Securities Authority, you may revoke your vote through such voting system before 4:00 p.m., Israel time, on June 5, 2024, or by voting at the Annual Meeting through the online meeting platform. If you are a Non-Registered Holder and submit your proxy card to vote by mail, by email, or appoint an authorized proxy in advance of the Annual Meeting, you may change your vote by delivering a valid later-dated proxy within the time limitations set forth above, or by voting at the Annual Meeting, subject to the instructions set forth above.
Attendance at the Annual Meeting will not cause your previous vote to be revoked, unless you specifically so request as detailed above.
ADSs
If you are the record owner of ADSs, you must follow the instructions provided by the Depositary in order to change your vote. If you hold your ADSs through a broker, bank or other nominee, you must follow the instructions provided by your broker, bank or other nominee, in order to change your vote. The last instructions you submit prior to the deadline indicated by the Depositary or the broker, bank or other nominee, as applicable, will be used to instruct the Depositary how to vote the ordinary shares underlying your ADSs. Attendance at the Annual Meeting will not cause your previous vote to be revoked.
Additional information for holders of ordinary shares and ADSs on how to participate at the virtual Annual Meeting
| - | To access the Annual Meeting through the online meeting platform go to meetnow.global/MS7KDRJ and enter your 15-digit control number (if holders of ADSs) or the control number received from the Company upon your registration (for holders of ordinary shares) beginning on Thursday, June 6, 2024 at 3:45 p.m., Israel time, 8:45 a.m., Eastern time. |
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 113 | |
Questions and Answers about the Annual Meeting
| - | To submit a question, visit meetnow.global/MS7KDRJ within 24 hours prior to or throughout the Annual Meeting with your control number (for holders of ADSs) or Israeli identification number or the control number received from the Company upon your registration (for holders of ordinary shares) and click on the messages icon to submit your question. |
| - | If you encounter any technical difficulties with the online meeting platform on the date of the Annual Meeting, please call the support team at the numbers listed on the log-in screen, technical support will be available during this time and will remain available until the Annual Meeting has ended. |
| - | Whether or not you plan to attend the Annual Meeting, we urge you to vote and submit your proxy in advance by one of the methods described above and in the other proxy materials for the Annual Meeting. |
| - | No recording of the Annual Meeting is allowed, including audio and video recording. |
Proxy Materials
Why did I receive a “Notice of Internet Availability of Proxy Materials” but no proxy materials?
We distribute our Notice of Annual Meeting of Shareholders, Proxy Statement and Annual Report (collectively, the “proxy materials”) to certain shareholders via the Internet under the “Notice and Access” approach permitted by rules of the SEC. This approach conserves natural resources and reduces our distribution costs, while providing a timely and convenient method of accessing the materials and voting. On or by April 19, 2024, we expect to have mailed a “Notice of Internet Availability of Proxy Materials” to participating shareholders containing instructions on how to access the proxy materials on the Internet.
Can I access the proxy materials on the Internet?
The proxy materials are available on our website at www.tevapharm.com/2024proxymaterials. Information on our website is not part of the proxy materials and is not incorporated into the proxy statement by reference. Record owners of our ADSs may also access the proxy materials at www.investorvote.com/teva by following the instructions provided by the Depositary. Beneficial owners of our ADSs may also access the proxy materials at www.proxyvote.com by following the instructions provided by their broker, bank or other nominee. Instead of receiving future proxy statements and accompanying materials by mail, most shareholders and ADS holders can elect to receive an e-mail that will provide electronic links to them. Opting to receive your proxy materials online will conserve natural resources and will save us the cost of producing documents and mailing them to you. The proxy materials are also available through Teva’s public filing on MAGNA (the Israeli Securities Authority’s electronic filing system) at www.magna.isa.gov.il, on the TASE’s website at www.maya.tase.co.il, or on the SEC’s website at www.sec.gov.
How do I request paper copies of the proxy materials at no charge?
You may contact Investor Relations by sending an email to TevaIR@tevapharm.com, or you may contact our proxy solicitor, Innisfree M&A Incorporated at 501 Madison Avenue, 19th Floor, New York, NY, 10022, or by calling (877) 825-8964 (toll-free for shareholders from the U.S. and Canada), or (412) 232-3651 (from other countries) by May 16, 2024.
If you are a record owner of ADSs, you may request proxy materials at www.investorvote.com/teva, by calling toll-free within the U.S. at (866) 641-4276, or by sending an email to investorvote@computershare.com, by May 16, 2024 and following the instructions provided by the Depositary.
If you are a beneficial owner of ADSs, you may request proxy materials by following the instructions at www.proxyvote.com, or by calling toll free within the U.S. at (800) 579-1639, or by sending an email to sendmaterial@proxyvote.com, by May 16, 2024 and following the instructions provided by your broker, bank or other nominee.
| 114 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Questions and Answers about the Annual Meeting
Other Questions
Could other matters be decided at the Annual Meeting?
The only items of business that our Board of Directors intends to present at the Annual Meeting are set forth in this Proxy Statement. As of the date of this Proxy Statement, no shareholder has advised us of the intent to present any other matter, and we are not aware of any other matter to be presented at the Annual Meeting. For more information, please see “Shareholder Proposals for the 2024 Annual Meeting and the 2025 Annual Meeting” above.
Who will pay for the cost of this proxy solicitation?
Teva will bear the entire cost of solicitation of proxies, including preparation, assembly, printing, and mailing of this Proxy Statement, the voting instruction card and any additional information furnished to shareholders. Teva may reimburse brokerage firms and other persons representing beneficial owners of ordinary shares or ADSs for reasonable expenses incurred by them in forwarding proxy soliciting materials to such beneficial owners. We retained Innisfree M&A Incorporated to assist with the solicitation of proxies for a fee in the amount of $25,000, plus reimbursable expenses. In addition to solicitation by mail, certain of our directors, officers and regular employees, without additional remuneration, may solicit proxies by telephone, facsimile or personal contact.
Who can I contact if I require further assistance?
If you need assistance in submitting your proxy or have questions regarding the Annual Meeting, please contact our Investor Relations department by email at TevaIR@tevapharm.com or by mail at Teva Pharmaceutical Industries Ltd., 124 Dvora HaNevi’a Street, Tel Aviv, 6944020 Israel, attention: Investor Relations, or by telephone at +972-3-914-8262. You may also contact our proxy solicitor, Innisfree M&A Incorporated at 501 Madison Avenue, 19th Floor, New York NY 10022 or by or by calling (877) 825-8964 (toll-free for shareholders from the U.S. and Canada) or (412) 232-3651 (from other countries). Banks and brokers may call (212) 750-5833.
* * *
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 115 | |
Appendix A
Reconciliations of GAAP Reported to Non-GAAP Financial Information
This Proxy Statement contains certain financial information that differs from what is reported under accounting principles generally accepted in the United States (“GAAP”). These non-GAAP financial measures include non-GAAP net income, non-GAAP earnings per share (“EPS”), non-GAAP operating income, non-GAAP operating margin and free cash flow. We present these non-GAAP financial measures as management believes that such data provide useful information to investors because they are used by management and our Board of Directors, in conjunction with other performance metrics, to evaluate our operational performance, to prepare and evaluate our work plans and annual budgets and ultimately to evaluate the performance of management, including annual compensation. While other qualitative factors and judgment also affect annual compensation, the principal quantitative element in the determination of such compensation are performance targets tied to the work plan, which are based on these non-GAAP measures.
Non-GAAP financial measures have no standardized meaning and accordingly have limitations in their usefulness to investors. Investors are cautioned that, unlike financial measures prepared in accordance with U.S. GAAP, non-GAAP measures may not be comparable with the calculation of similar measures for other companies. These non-GAAP financial measures are presented solely to permit investors to more fully understand how management assesses our performance. The limitations of using non-GAAP financial measures as performance measures are that they provide a view of our results of operations without including all events during a period and may not provide a comparable view of our performance to other companies in the pharmaceutical industry. Investors should consider our non-GAAP financial measures in addition to, and not as replacements for, or superior to, measures of financial performance prepared in accordance with GAAP.
Non-GAAP Net Income and Non-GAAP EPS
The following table presents our non-GAAP net income and non-GAAP EPS for the years ended December 31, 2023 and 2022, as well as reconciliations of each measure to their nearest GAAP equivalents:
| Year ended December 31, | ||||||||||||
($ in millions except per share amounts)
| 2023 | 2022 | ||||||||||
Net income (loss) attributable to Teva (1) | ($ | ) | (559 | ) | (2,446 | ) | ||||||
Increase (decrease) for excluded items: |
|
|
|
|
|
|
|
|
| |||
Amortization of purchased intangible assets |
|
|
| 616 | 732 | |||||||
Legal settlements and loss contingencies (2) |
|
|
| 1,043 | 2,082 | |||||||
Goodwill impairment |
|
|
| 700 | 2,045 | |||||||
Impairment of long-lived assets |
|
|
| 378 | 402 | |||||||
Restructuring costs |
|
|
| 111 | 146 | |||||||
Costs related to regulatory actions taken in facilities |
|
|
| 4 | 7 | |||||||
Equity compensation |
|
|
| 121 | 124 | |||||||
Contingent consideration (1)(3) |
|
|
| 548 | 261 | |||||||
Gain on sale of business |
|
|
| (3 | ) | (47 | ) | |||||
Accelerated depreciation |
|
|
| 80 | 117 | |||||||
Financial expenses |
|
|
| 66 | 61 | |||||||
Share in profits (losses) of associated companies - net |
|
|
| — | (22 | ) | ||||||
Items attributable to non-controlling interests |
|
|
| (92 | ) | (96 | ) | |||||
Other non-GAAP items (4) |
|
|
| 330 | 465 | |||||||
Corresponding tax effects and unusual tax items (1)(5) |
|
|
| (446 | ) | (1,021 | ) | |||||
Non-GAAP net income attributable to Teva | ($ | ) | 2,898 | 2,812 | ||||||||
Non-GAAP tax rate (6) |
|
|
| 13.0 | % | 11.7 | % | |||||
GAAP diluted earnings (loss) per share attributable to Teva | ($ | ) | (0.50 | ) | (2.20 | ) | ||||||
EPS difference (7) |
|
|
| 3.06 | 4.73 | |||||||
Non-GAAP diluted EPS attributable to Teva (7) | ($ | ) | 2.56 | 2.52 | ||||||||
Non-GAAP average number of shares (in millions) (7)
|
|
|
| 1,131 | 1,115 | |||||||
| 116 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |
Appendix A
| (1) | The data presented for the prior period have been revised to reflect a revision in the presentation of these items in the consolidated financial statements. For additional information see note 1b to our consolidated financial statements included in our 2023 Annual Report on Form 10-K. |
| (2) | Expenses in 2023 were mainly related to an estimated provision for the U.S. DOJ patient assistance program litigation, an update to the estimated settlement provision for the opioid cases, the provision for the settlement of the U.S. DOJ criminal antitrust charges on the marketing and pricing of certain Teva USA generic products, and the provision for the settlement of the reverse-payment antitrust litigation over certain HIV medicines. |
| (3) | For the year ended December 31, 2023, contingent consideration expenses primarily related to $422 million in changes in the estimated future royalty payments to be paid by Teva to Allergan PLC and $132 million in changes in the estimated future royalty payments to be paid by Teva to Eagle Pharmaceuticals, Inc. For the year ended December 31, 2022, contingent consideration expenses primary related to $240 million in changes in the estimated future royalty payments to be paid by Teva to Allergan PLC and $21 million in changes in the estimated future royalty payments to be paid by Teva to Eagle Pharmaceuticals, Inc. |
| (4) | Other non-GAAP items include other exceptional items that we believe are sufficiently large that their exclusion is important to facilitate an understanding of trends in our financial results, primarily related to the rationalization of our plants, certain inventory write-offs, material litigation fees and other unusual events. For the year ended December 31, 2023, other non-GAAP items primarily consisted of $107 million of litigation fees mainly attributed to fees related to the settlement of Teva’s opioids litigation, the settlement of the U.S. DOJ’s antitrust charges against Teva on the marketing and pricing of certain Teva USA generic products; and $154 million of costs relating to our plant rationalization efforts, a portion of which consists of costs related to plants used in our API business that we intend to divest. For the year ended December 31, 2022, other non-GAAP items primarily consisted of $114 million of litigation fees related to the settlement of Teva’s opioids litigation and the settlement of the U.S. DOJ’s antitrust charges against Teva on the marketing and pricing of certain Teva USA generic products; $179 million of costs relating to Teva’s plant rationalization efforts; and $122 million of expenses related to a specific product that Teva decided to divest, consisting primarily of a write-off of raw materials purchased to produce the product and penalties related to Teva’s exit from a contract related to the product. |
| (5) | Amount for the year ended December 31, 2022 included $436 million representing a portion of the realization of a loss related to an investment in one of our U.S. subsidiaries as well as $585 million corresponding tax effects on non-GAAP items. |
| (6) | Non-GAAP tax rate is tax expenses (benefit) excluding the impact of non-GAAP tax adjustments presented above as a percentage of income (loss) before income taxes excluding the impact of non-GAAP adjustments presented above. |
| (7) | EPS difference and diluted non-GAAP EPS are calculated by dividing our non-GAAP net income attributable to Teva by our non-GAAP diluted weighted average number of shares. |
Non-GAAP Operating Income (Loss) and Non-GAAP Operating Margin
The following table presents our non-GAAP operating income (loss) and non-GAAP operating margin for the years ended December 31, 2023 and 2022, as well as reconciliations of each measure to its nearest GAAP equivalent:
| Year ended December 31, | ||||||||
| ($ in millions) | 2023 | 2022 | ||||||
GAAP operating income (loss) (1) | $ | 433 | (2,197 | ) | ||||
GAAP operating margin (1) | 2.7 | % | (14.7 | %) | ||||
Increase (decrease) for excluded items: |
|
|
|
|
|
| ||
Amortization of purchased intangible assets | 616 | 732 | ||||||
Legal settlements and loss contingencies (2) | 1,043 | 2,082 | ||||||
Goodwill impairment | 700 | 2,045 | ||||||
Impairment of long-lived assets | 378 | 402 | ||||||
Restructuring costs | 111 | 146 | ||||||
Costs related to regulatory actions taken in facilities | 4 | 7 | ||||||
Equity compensation | 121 | 124 | ||||||
Contingent consideration (1)(3) | 548 | 261 | ||||||
Gain on sale of business | (3 | ) | (47 | ) | ||||
Accelerated depreciation | 80 | 117 | ||||||
Other non-GAAP items (4) | 330 | 465 | ||||||
Non-GAAP operating income (loss) | 4,361 | 4,139 | ||||||
Non-GAAP operating margin (5) | 27.5 | % | 27.7 | % | ||||
| Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | 117 | |
Appendix A
| (1) | The data presented for prior periods have been revised to reflect a revision in the presentation of these items in the consolidated financial statements included in our 2023 Annual Report on Form 10-K. See note 1b to the consolidated financial statements included in our 2023 Annual Report on Form 10-K for additional information. |
| (2) | Expenses in 2023 were mainly related to an estimated provision for the U.S. DOJ patient assistance program litigation, an update to the estimated settlement provision for the opioid cases, the provision for the settlement of the U.S. DOJ criminal antitrust charges on the marketing and pricing of certain Teva USA generic products, and the provision for the settlement of the reverse-payment antitrust litigation over certain HIV medicines. |
| (3) | In the year ended December 31, 2023, contingent consideration expenses primarily related to $422 million in changes in the estimated future royalty payments to be paid by Teva to Allergan PLC and $132 million in changes in the estimated future royalty payments to be paid by Teva to Eagle Pharmaceuticals, Inc. For the year ended December 31, 2022, contingent consideration expenses primary related to $240 million in changes in the estimated future royalty payments to be paid by Teva to Allergan PLC and $21 million in changes in the estimated future royalty payments to be paid by Teva to Eagle Pharmaceuticals, Inc. |
| (4) | Other non-GAAP items include other exceptional items that we believe are sufficiently large that their exclusion is important to facilitate an understanding of trends in our financial results, primarily related to the rationalization of our plants, certain inventory write-offs, material litigation fees and other unusual events. For the year ended December 31, 2023, other non-GAAP items primarily consisted of $107 million of litigation fees mainly attributed to fees related to the settlement of Teva’s opioids litigation, the settlement of the U.S. DOJ’s antitrust charges against Teva on the marketing and pricing of certain Teva USA generic products; and $154 million of costs relating to our plant rationalization efforts, a portion of which consists of costs related to plants used in our API business that we intend to divest. For the year ended December 31, 2022, other non-GAAP items primarily consisted of $114 million of litigation fees related to the settlement of Teva’s opioids litigation and the settlement of the U.S. DOJ’s antitrust charges against Teva on the marketing and pricing of certain Teva USA generic products; $179 million of costs relating to Teva’s plant rationalization efforts; and $122 million of expenses related to a specific product that Teva decided to divest, consisting primarily of a write-off of raw materials purchased to produce the product and penalties related to Teva’s exit from a contract related to the product. |
| (5) | Non-GAAP operating margin is Non-GAAP operating income as a percentage of revenues. |
Free Cash Flow
The following table presents our Free Cash Flow for the years ended December 31, 2023 and 2022, as well as reconciliations of Free Cash Flow for such periods to its nearest GAAP equivalent:
| Year ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| ($ in millions) | ||||||||
Net cash provided by operating activities | 1,368 | 1,590 | ||||||
Beneficial interest collected in exchange for securitized trade receivables | 1,477 | 1,140 | ||||||
Purchases of property, plant and equipment and intangible assets | (526 | ) | (548 | ) | ||||
Proceeds from sale of business and long lived assets | 68 | 68 | ||||||
Acquisition of subsidiary, net of cash acquired | — | (7 | ) | |||||
|
|
|
| |||||
Free cash flow | $ | 2,387 | $ | 2,243 | ||||
| 118 | Teva Pharmaceutical Industries Ltd. 2024 Proxy Statement | |

Thursday, June 6, 2024 4:00 p.m. Israel time 9:00 a.m. Eastern time The 2024 Annual Meeting will be conducted in a virtual format only. See inside for further details. If you need assistance or have questions regarding the 2024 Annual Meeting, please contact our Investor Relations department: TevaIR@tevapharm.com Teva Pharmaceutical Industries Ltd. 124 Dvora HaNevi’a Street, Tel Aviv, 6944020 Israel

TEVA PHARMACEUTICAL INDUSTRIES LIMITED (“TEVA”)
2024 ANNUAL GENERAL MEETING OF SHAREHOLDERS TO BE HELD ON JUNE 6, 2024
PROXY CARD
THIS PROXY IS SOLICITED BY THE BOARD OF DIRECTORS OF TEVA
Teva’s Board of Directors recommends that you vote FOR proposals 1, 2 and 4, and vote 1 Year with respect to Proposal 3. If you execute and return this proxy card without indicating any directions with respect to any matter, this proxy card will be voted FOR proposals 1, 2 and 4, and vote ONE YEAR with respect to Proposal 3.
Information in respect of the undersigned:
Shareholder name:
|
| |
Number of identity card or passport (country) or corporation number (country): |
| |
Number of Teva ordinary shares being voted: |
| |
The undersigned hereby constitutes and appoints each of DOV BERGWERK, DIKLA TADMOR, SHIRA ARAN-PORAT and MATAN KIMCHI, acting individually, the true and lawful attorney, agent and proxy of the undersigned, with full power of substitution, to vote with respect to the number of shares set forth above, standing in the name of the undersigned at the close of trading on the Record Date, at the 2024 Annual General Meeting of Shareholders, and at any and all adjournments thereof, with all the power that the undersigned would possess if personally present and especially (but without limiting the general authorization and power hereby given) to vote as instructed on the reverse side.
In order to be counted, a duly executed proxy must be received by Teva by 4:00 p.m., Israel time, on June 3, 2024 (if not revoked prior to such time), unless determined otherwise by the chairman of the meeting, by submitting this proxy card to Teva’s executive offices at 124 Dvora HaNevi’a Street, Tel Aviv, 6944020, Israel to the attention of the Company Secretary or by email to TevaAGM2024@tevapharm.com.
In order to be counted, in addition to this proxy card: (i) shareholders registered in Teva’s shareholder register (Registered Holders) must also provide Teva with a copy of such Registered Holder’s identity card, passport or certificate of incorporation, as the case may be; and (ii) a shareholder registered pursuant to Section 177(1) of the Israeli Companies Law, 5759-1999, through a nominee company (Non-Registered Holders) must also provide Teva with an ownership certificate confirming such Non-Registered Holder’s ownership of Teva’s ordinary shares on the Record Date, which certificate must be approved by a member of the Tel Aviv Stock Exchange, as required by the Israeli Companies Regulations (Proof of Share Ownership for Voting at a General Meeting), 5760-2000. Non-Registered Holders may alternatively submit their votes through the electronic voting system of the Israeli Securities Authority at https//:votes.isa.gov.il.
This proxy card, when properly executed, will be voted in the manner directed herein by the undersigned. Any and all proxies heretofore given are hereby revoked.
(Continued and to be signed on the reverse side)
PLEASE COMPLETE, SIGN, DATE AND RETURN PROMPTLY
Matter on the Agenda: | Please vote by marking “X” in the | |||||||||||||
For | Against | Abstain | ||||||||||||
1. |
ELECTION OF DIRECTORS:
| |||||||||||||
(a) Rosemary A. Crane
| ||||||||||||||
(b) Gerald M. Lieberman
| ||||||||||||||
(c) Prof. Ronit Satchi-Fainaro
| ||||||||||||||
(d) Prof. Varda Shalev
| ||||||||||||||
2. |
TO APPROVE, ON A NON-BINDING ADVISORY BASIS, THE COMPENSATION FOR TEVA’S NAMED EXECUTIVE OFFICERS
| |||||||||||||
3. |
TO RECOMMEND, ON A NON-BINDING ADVISORY BASIS, TO HOLD A NON-BINDING ADVISORY VOTE TO APPROVE THE COMPENSATION FOR TEVA’S NAMED EXECUTIVE OFFICERS EVERY ONE, TWO OR THREE YEARS
| 1 YEAR | 2 YEARS | 3 YEARS | Abstain | |||||||||
| ||||||||||||||
For | Against | Abstain | ||||||||||||
4. |
TO APPOINT KESSELMAN & KESSELMAN, A MEMBER OF PRICEWATERHOUSECOOPERS INTERNATIONAL LTD., AS TEVA’S INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM UNTIL TEVA’S 2025 ANNUAL MEETING OF SHAREHOLDERS
| |||||||||||||
| Signature | Date |