
| Nasdaq: ATSI |

| SAFE-HARBOR THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT ARE SUBJECT TO NUMEROUS RISKS AND UNCERTAINTIES. SEE OUR SECURITIES AND EXCHANGE COMMISSION FILINGS FOR A DESCRIPTION OF MANY OF THE RISKS AND UNCERTAINTIES. THIS PRESENTATION HAS BEEN PREPARED SOLELY BY US AND IS SOLELY OUR RESPONSIBILITY. THIS PRESENTATION DOES NOT PURPORT TO BE COMPREHENSIVE OR TO CONTAIN ALL THE INFORMATION THAT YOU MAY DESIRE IN EVALUATING AN INVESTMENT IN OUR SHARES. YOU SHOULD NOT CONSTRUE THE CONTENTS OF THIS PRESENTATION AS INVESTMENT, TAX OR LEGAL ADVICE. THIS PRESENTATION DOES NOT CONSTITUTE AN OFFER TO SELL OR A SOLICITATION TO BUY ANY OF OUR SHARES IN ANY STATE OR OTHER JURISDICTION OR TO ANY PERSON IF SUCH AN OFFER OR SOLICITATION IS UNLAWFUL OR UNAUTHORIZED. |

| FULFILLING THE MISSION and |

| MISSION Dedicated to creating a leading cardiac surgery platform by continuously "ADVANCING THE STANDARD" through collaboration with customers to provide superior products that improve quality of life for patients. |

| ATS MEDICAL OVERVIEW Founded in September 1990 To design, develop and establish the ATS Open Pivot(r) heart valve as the standard of care for patients requiring a mechanical heart valve Headquartered in Minneapolis, MN Strategy Objective Create leadership position in cardiac surgery through distribution and acquisition of differentiated new technology The ATS Open Pivot(r) Heart Valve |

| GROWTH OBJECTIVES TO ATTAIN LEADERSHIP Mechanical Valve Market Share Capture Attain 25%+ market share Continued distribution expansion & development Next generation driven R&D Lower Cost of Goods 50% reduction by end 2006 Move to self manufactured carbon components New Business Development Growth & leverage through acquisition & partnership Tissue heart valves Surgical arrhythmia ablation Allograft tissue services Surgical tools & valve repair Blood auto-transfusion |

| KEY MILESTONES TOWARDS LEADERSHIP New management team assembled Global sales and marketing operations established for commercialization of ATS Open Pivot(r) heart valve Made first strategic investment in PARSUS(tm) auto transfusion blood filtration technology Entered strategic distribution partnership with Cryocath Technologies for Surgical ablation of cardiac arrhythmias, and Regeneration Technologies for cardiovascular allograft tissue Entered exclusive partnership with privately held Genesee BioMedical, Inc. based in Denver, Colorado to develop and manufacture a line of heart valve annuloplasty rings Acquisition of 3F Therapeutics to complete corporate vision to become a leader in cardiovascular surgery. 3F brings advanced tissue heart valves and less invasive surgical technologies to ATS Medicals heart valve therapy portfolio 2002 2003 2004 2005 2006 |

| CARDIAC SURGERY MARKET OVERVIEW CARDIAC SURGERY MARKET OVERVIEW $2.5 Billion in Market Opportunity |

| HEART VALVE THERAPY MARKET 2006 Estimated MHV THV REPAIR OUS 161441 158587 71660 MHV THV REPAIR North America 406.4 661 128 Revenue $1.186 billion Procedures 391,687 units (5-7% Growth) Source: Piper Jaffray Equity Research |

| TISSUE & MECHANICAL VALVE PROCEDURES 2006 Estimated MHV THV OUS 136827 83034 MHV THV North America 24614 75552 USA 100,166 Units (2-3% Growth) International 219,862 Units (5-10% Growth) Source: Piper Jaffray Equity Research Pop. Growth Healthcare Access AGE > 65 Source: Piper Jaffray Equity Research |
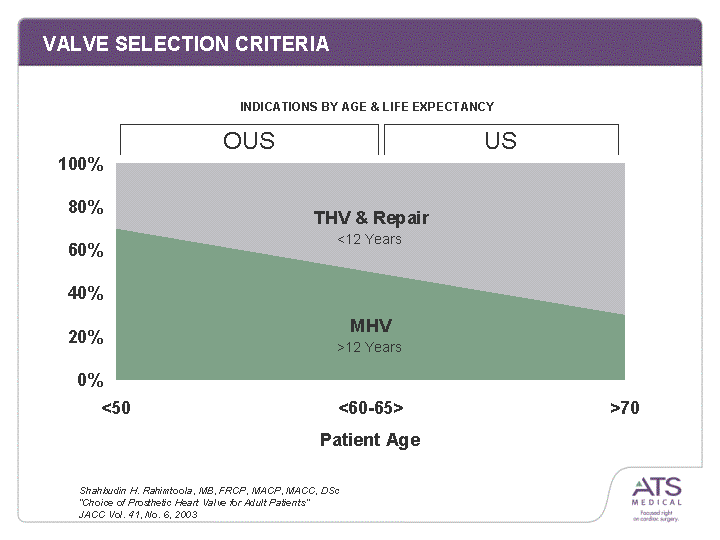
| VALVE SELECTION CRITERIA <50 <60-65> >70 MHV 0.7 0.5 0.3 THV & Repair 0.3 0.5 0.7 OUS US Shahbudin H. Rahimtoola, MB, FRCP, MACP, MACC, DSc "Choice of Prosthetic Heart Valve for Adult Patients" JACC Vol. 41, No. 6, 2003 <12 Years >12 Years INDICATIONS BY AGE & LIFE EXPECTANCY |
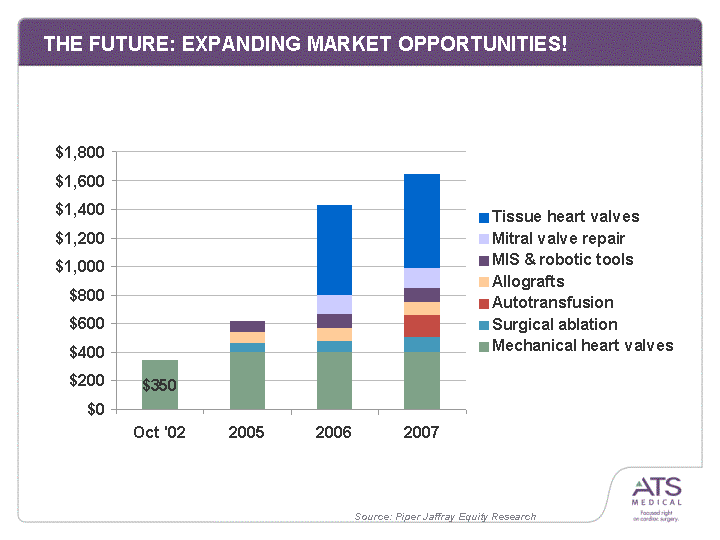
| THE FUTURE: EXPANDING MARKET OPPORTUNITIES! Oct '02 2005 2006 2007 Mechanical heart valves 350 406 400 406 Surgical ablation 61 85 100 Autotransfusion 0 0 160 Allografts 75 85 85 MIS & robotic tools 80 100 100 Mitral valve repair 128 138 Tissue heart valves 630 660 Source: Piper Jaffray Equity Research |

| 3F CORE PRODUCT FOCUS Regulated Clinical Study |

| 3F DESIGN HYPOTHESIS Native heart valves function as if they were simple tubes with sides that collapse when external pressure is applied. i.e. "form follows function" Proof of concept by Finite Element Analysis and in-vitro testing, documented that under the same anatomic constraints and physiologic conditions as the native valve, a tube would assume the form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve form of that native valve Source: James Cox, MD et al. J Thoracic Surg 2005; 130:520-7 |
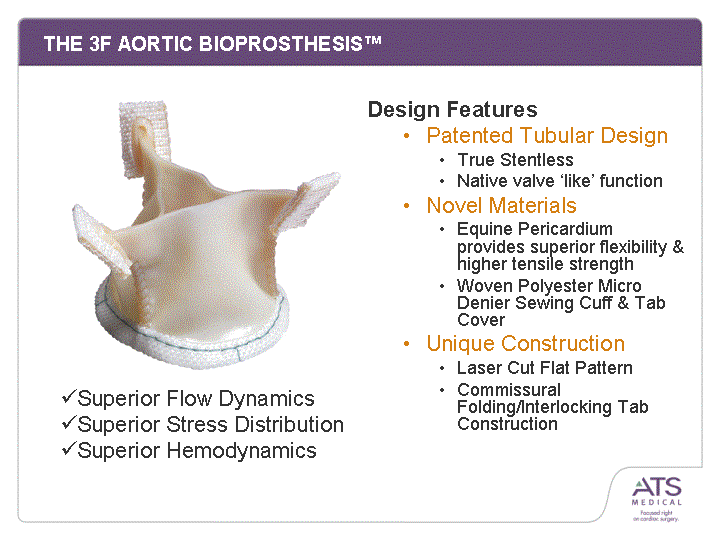
| THE 3F AORTIC BIOPROSTHESIS(tm) Design Features Patented Tubular Design True Stentless Native valve 'like' function Novel Materials Equine Pericardium provides superior flexibility & higher tensile strength Woven Polyester Micro Denier Sewing Cuff & Tab Cover Unique Construction Laser Cut Flat Pattern Commissural Folding/Interlocking Tab Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Construction Superior Flow Dynamics Superior Stress Distribution Superior Hemodynamics |

| Commercially Available Tissue Valves Standard Porcine Supra-Annular Porcine Bovine Pericardial Site of Highest Stress at Closure MARKET OPPORTUNITY: VALVE DURABILITY ...."The average life span of a bioprosthetic valve is 10-12 years."... Thoracic Surgery News. Vol. 2, NO. 1 January/February 2006 The official newspaper of the American Association For Thoracic Surgery |

| Finite Element Analysis of Stress Distribution DURABILITY OF THE 3F AORTIC BIOPROSTHESIS(tm) Commissural Tabs Sewing Rim Source: James Cox, MD et al. J Thoracic Surg 2005; 130:520-7 |
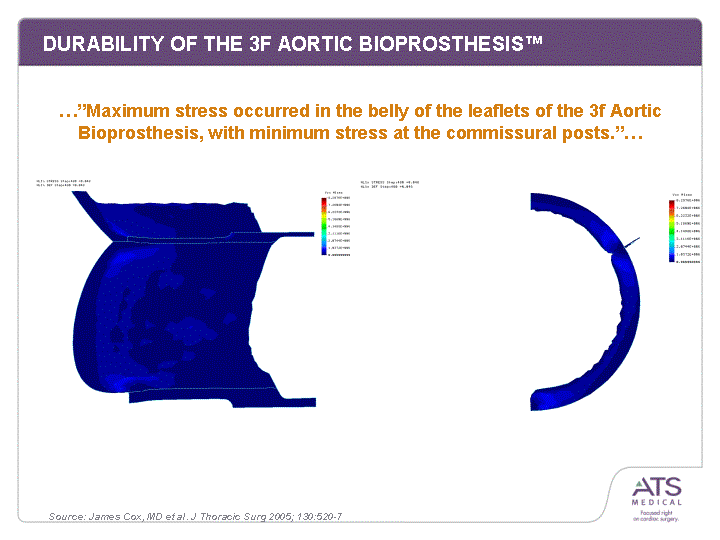
| DURABILITY OF THE 3F AORTIC BIOPROSTHESIS(tm) ...."Maximum stress occurred in the belly of the leaflets of the 3f Aortic Bioprosthesis, with minimum stress at the commissural posts."... Source: James Cox, MD et al. J Thoracic Surg 2005; 130:520-7 |

| DURABILITY OF THE 3F AORTIC BIOPROSTHESIS(tm) Accelerated Wear Test Comparison 3F Aortic Bioprosthesis(tm) St. Jude Toronto SPV(tm) ...."The 3f Aortic Bioprosthesis showed superior performance in comparison with the control valve..." Source: James Cox, MD et al. J Thoracic Surg 2005; 130:520-7 |

| DURABILITY OF THE 3F AORTIC BIOPROSTHESIS(tm) ...."at 200 million cycles, the 19-mm 3f Aortic Bioprosthesis shows little or no evidence of structural failure..." Source: James Cox, MD et al. J Thoracic Surg 2005; 130:520-7 |

| Accelerated Wear Survival Curves DURABILITY OF THE 3F AORTIC BIOPROSTHESIS(tm) Source: James Cox, MD et al. J Thoracic Surg 2005; 130:520-7 |
================================================================================

| MARKET OPPORTUNITY: HEMODYNAMICS 3F's True 'Stentless' design provides a superior ratio of flow area to valve mounting area Lower pressure gradients Higher effective orifice area Better coronary filling Greater Flow Area |
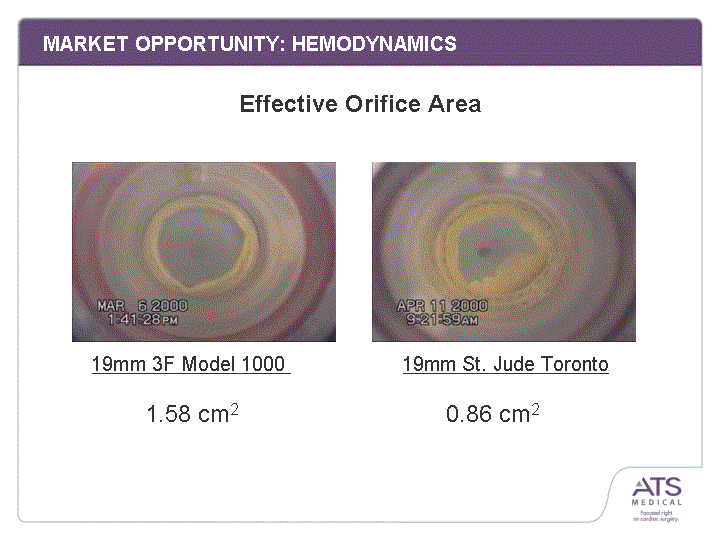
| MARKET OPPORTUNITY: HEMODYNAMICS 19mm 3F Model 1000 19mm St. Jude Toronto 1.58 cm2 0.86 cm2 Effective Orifice Area |
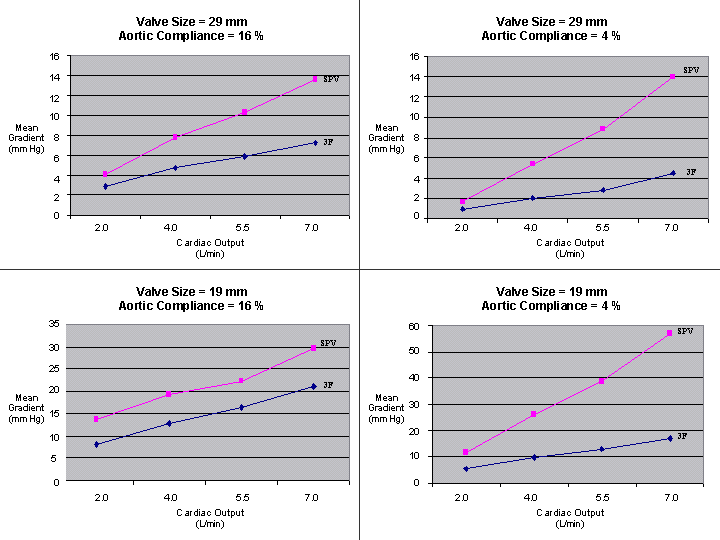
| Valve Size = 29 mm Aortic Compliance = 16 % |
| Valve Size = 29 mm Aortic Compliance = 4 % |
| Valve Size = 19 mm Aortic Compliance = 16 % |
| Valve Size = 19 mm Aortic Compliance = 4 % |

| Valve Size = 29 mm Aortic Compliance = 16 % |
| Valve Size = 29 mm Aortic Compliance = 4 % |
| Valve Size = 19 mm Aortic Compliance = 16 % |
| Valve Size = 19 mm Aortic Compliance = 4 % |

| 3F ENABLE AORTIC HEART VALVE(tm) Features 3F Aortic Bioprosthesis(tm) Nitinol Stent Benefits Nitinol stent expands to fit annulus- eliminating conventional suturing (20- 40) One suture implantation reduces cross-clamp & bypass time 2 Min vs. 30+ Min. Enables true Key-hole, less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery less invasive surgery |
| 3F ENABLE AORTIC HEART VALVE(tm) |

| 3F ENABLE AORTIC HEART VALVE(tm) |

| 3F ENABLE AORTIC HEART VALVE(tm) Valve Size 19 20 21 22 23 ATS AP 11.4 10 Perimount 22 18 16 Mosaic 14.7 12.9 3f Enable 8.3 ATS AP: Jazayeri S, et al. J Heart Valve Dis. 2003; 12(2):628.634. Perimount: Khan S, et al. Ann Thorac Surg. 2000;69:531-535. Mosaic: Medtronic, Inc., Pre-Market Approval Application - Summary of Safety and Effectiveness: 2000. Enable: Sadowski J, et al. SHVD 2005 |

| Balloon/Catheter Delivery Leaves Native Valve Annealed 316L Stainless Stent Uses Aortic Bioprosthesis(tm) Valve Design Surgical Access Beating Heart Sub Xyphoid / Apical LV or Others Possible 3F ENTRATA AORTIC VALVE SYSTEM(tm) Features 3F Aortic Bioprosthesis(tm) Annealed 316L Stainless Stent SCAPUS(tm)Delivery System Leaves Native Valve Benefits Beating Heart delivery opens treatment to in-operable patients Sub Xyphoid / Apical LV approach is more direct, offering greater feel, control, safety Precedent for apical approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery approach in surgery |
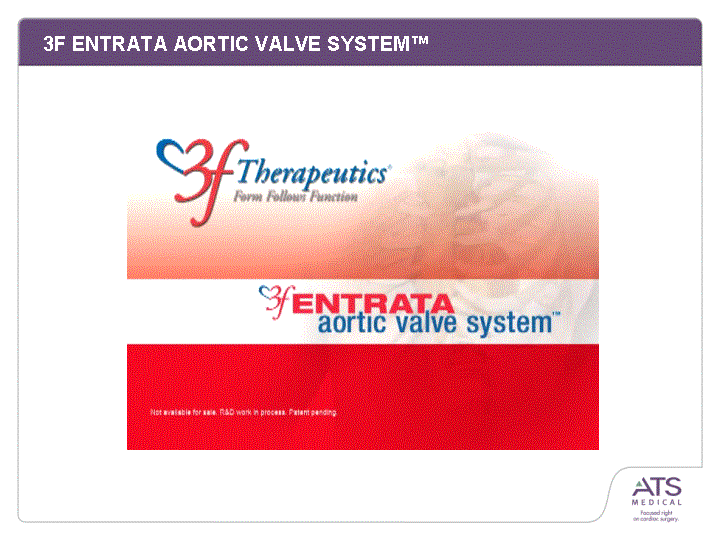
| Balloon/Catheter Delivery Leaves Native Valve Annealed 316L Stainless Stent Uses Aortic Bioprosthesis(tm) Valve Design Surgical Access Beating Heart Sub Xyphoid / Apical LV or Others Possible 3F ENTRATA AORTIC VALVE SYSTEM(tm) |

| COMPARISION OF BEATING HEART TECHNIQUE Similar Any beating heart, closed chest approach will require in-direct imaging for placement and outcome assessment Different Catheter 3F Scapus(tm) Bendable Resists Bending Twistable Resists Twisting Procedures through vasculature Trans-Apical & Trans-Heart Flexible Material Rigid Material Typically >50cm Typically <50cm Requires guidewire Does not require guidewire |

| COMPARISION OF BEATING HEART TECHNIQUE |
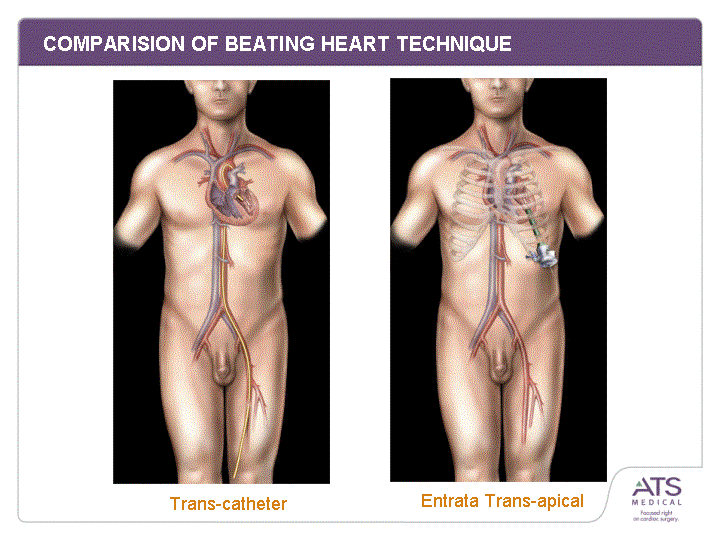
| COMPARISION OF BEATING HEART TECHNIQUE Trans-catheter Entrata Trans-apical |
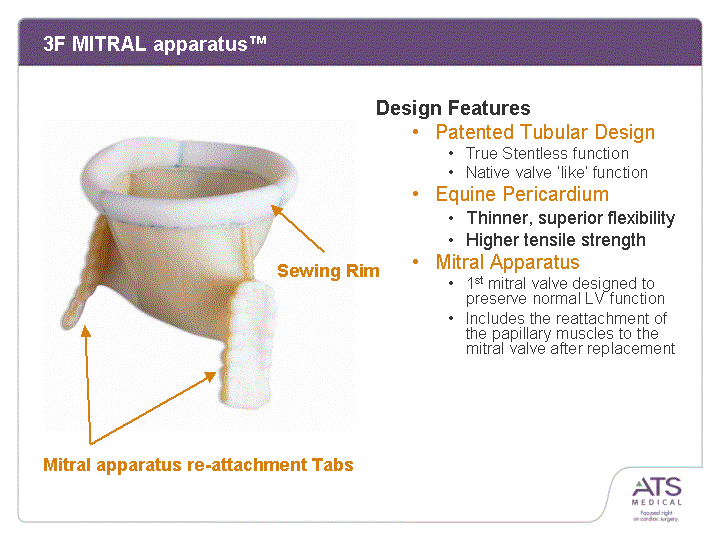
| 3F MITRAL apparatus(tm) Design Features Patented Tubular Design True Stentless function Native valve 'like' function Equine Pericardium Thinner, superior flexibility Higher tensile strength Mitral Apparatus 1st mitral valve designed to preserve normal LV function Includes the reattachment of the papillary muscles to the mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement mitral valve after replacement Mitral apparatus re-attachment Tabs Sewing Rim |

| 3F ENDURANCE Valvestent SYSTEM(tm) System Objective To mitigate the effects of TR regurgitation and backward heart failure by Trans-Catheter Implantation of Valves in IVC and SVC. Clinical Conditions addressed: Congestive Heart Failure Ascites Cardiac Cirrhosis (Hepatic Failure) Peripheral Edema Renal Dysfunction |

| 3F ENDURANCE Valvestent SYSTEM(tm) Superior Vena Cava Inferior Vena Cava |

| 3F ENDURANCE Valvestent SYSTEM(tm) |

| 3F TECHNOLOGY SUMMARY 1. 3f Aortic Bioprosthesis(tm)platform product - Superior performance in-vitro - Superior performance thus far clinically 2. Single Suture Enable Aortic Valve - Excellent clinical performance - No competition - Potentially the largest market 3. Trans-Apical Entrata Valve - Unique market expansion opportunity 4. Mitral Valve - - Large potential market in field of heart failure |

| AFTER 3 YR'S: INVESTMENT CONSIDERATIONS Focus- pure-play cardiac surgery One customer-one distribution platform-clear vision Management- proven ability to execute Net market share taker in mechanical heart valves Net market share taker in new distributed products Surgical ablation, Cardiovascular allografts Successful move to self-manufactured carbon providing significant reductions in COGS Technology- the most differentiated pipeline in surgery 06' introduction of Simulus(tm) heart valve repair products 07' introduction of PARSUS(tm) blood filtration technology 06' -09' 3f Therapeutics tissue heart valves Strong patent positions in all market segments |

| EXECUTION PROOF POINTS: REVENUE GROWTH 2002 2003 2004 2005 13.3 18.5 28 34.64 24% Annual Growth |

| WORLDWIDE MECHANICAL VALVE SHARE GROWTH Source: Piper Jaffray Equity Research Other ATS East 86 14 Other ATS East 99.2 0.8 30% CAGR Growth! October 2002 December 2005 |

| THE ATS OPEN PIVOT(r) HEART VALVE Clinical experience 100,000+ Implants 14 Yr clinical record 42 publications Proven performance Zero structural failures! 30% lower risk of stroke 50% less anticoagulation required Quiet function The ATS Open Pivot(r) Heart Valve |

| GLOBAL MECHANICAL VALVE SHARE GROWTH Source: Piper Jaffray Equity Research Other ATS East 86 14 Other ATS East 99.2 0.8 30% CAGR Growth! October 2002 December 2005 |

| 'BEST OF CLASS' DISTRIBUTION Experienced cardiac surgery sales and marketing 40+ Dedicated headcount in US Direct and Agent based 28+ Dedicated headcount OUS Direct sales and management 65+ Exclusive distributors OUS Proven competitiveness Additional OUS 'go-direct' opportunities |
| NEW BUSINESS DEVELOPMENT PROGRESS Objectives Call point leverage Relationship enhancement Revenue diversification Cost structure leverage Mission fulfillment! |

| SURGICAL AFIB MARKET OPPORTUNITY Mitral Aortic CABG $150 Million US Market - -AFIB most common arrhythmia - -Patient pop. to double in next 10 years - -Surgical Ablation Market CAGR of 64% LEFT ATRIAL ABLATION 23,400 30,240 3,575 |

| SURGICAL AFIB MARKET OPPORTUNITY ATS Business Model Global partnership with Cryocath Technologies, Inc. for representation of cryotherapy products for the surgical treatment of cardiac arrhythmias Commission based Agent in US & Distributor OUS Products Highest reported success rate of any energy source Fastest growing surgical ablation provider-100+ new accounts in 2005 Freezes tissue to -160oC in seconds |

| ALLOGRAFT MARKET OPPORTUNITY ATS Business Model Exclusive marketing services agreement for representation Regeneration Technologies Inc. (RTI-CV) cardiovascular allografts Commission on services revenue Products RTI-CV is one of the largest processors of allograft tissue in the United States Considered the leader in science, safety and innovation in biologics GTP compliant FDA audited AATB certified |

| ALLOGRAFT MARKET OPPORTUNITY Market Opportunity 2005 RTI-CV allograft services revenue was approximately $9 million Market Drivers RTI-CV donor tissue recovery, safety & quality ATS Sales Force CRY 12.2 ARC 0.312 RTI_CV 4.1 LifeNet 9 NWTC 0.83 $75 Million US Market Heart Valves and Grafts |

| MHV THV REPAIR OUS 161441 158587 71660 HEART VALVE REPAIR MARKET OPPORTUNITY Simulus(tm) Annuloplasty Ring Mitral and Tricuspid heart valve repair prosthesis Q1 2006 market launch Market Opportunity Low technology barriers Same 'Call Point' AFIB procedure synergy Source: Piper Jaffray Equity Research $128 Million Market 10+% CAGR ATS Simulus(tm) |

| AUTO-TRANSFUSION MARKET OPPORTUNITY Blood loss during surgery Blood bank issues Cross contamination danger from transfusions Availability & cost Current Centrifuge methods Low efficacy ~50% emboli removal Slow- not real-time High tech support req. High blood cell trauma Clinical Opportunity Improve emboli removal Salvage red blood cell Improve ease of use Clinical Challenge Lipid micro emboli in Brain |
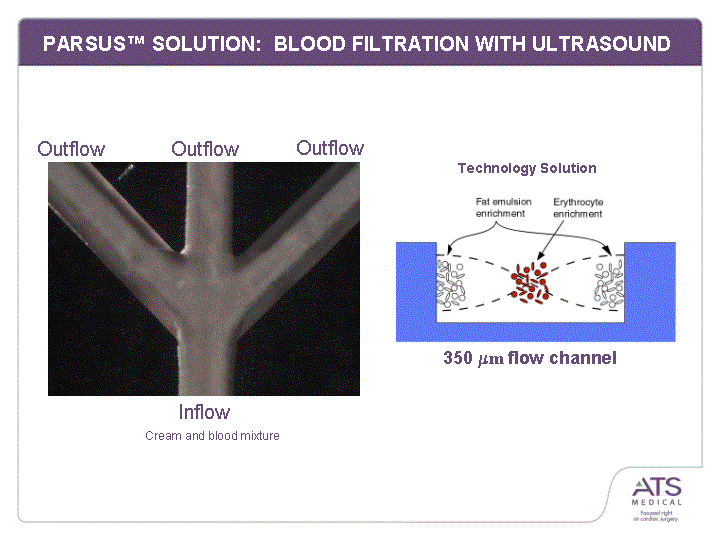
| PARSUS(tm) SOLUTION: BLOOD FILTRATION WITH ULTRASOUND Cream and blood mixture Outflow Outflow Outflow Inflow 350 mm flow channel Technology Solution |

| PARSUS(tm) BENEFITS vs. CENTRIFUGE Ultrasound provides ultra high quality filtration of emboli from blood Fast, easy to use, real-time 94%+ efficacy! Atraumatic to blood cells Centrifuge PARSUS(tm) Technology Solution |

| PARSUS(tm): SYSTEM OVERVIEW $150 Million Cardiac Surgery Market One disposable set sold per cardiac surgery procedure |

| FINANCIAL SUMMARY |
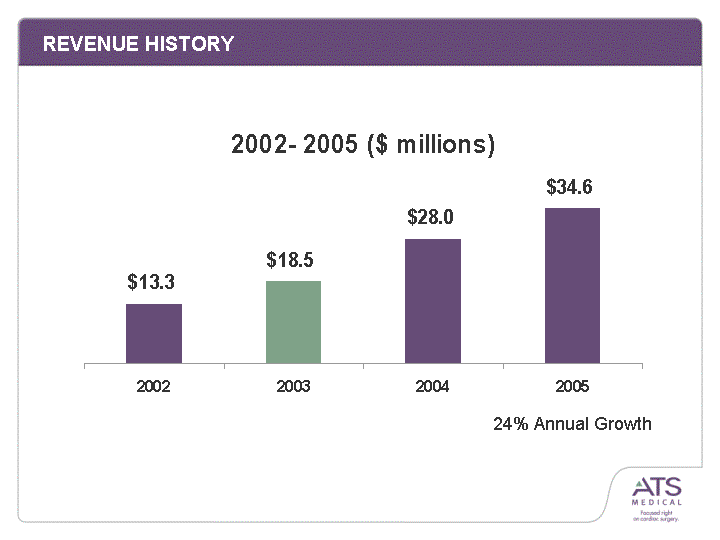
| REVENUE HISTORY 2002 2003 2004 2005 13.3 18.5 28 34.64 24% Annual Growth |

| 2005 SALES GROWTH BY QUARTER 1st Qtr 2nd Qtr 3rd Qtr 4th Qtr 2005 7.06 9.3 8.33 9.93 2004 6.694 7.547 6.547 7.225 37.5% Yr-Yr Growth |

| HIGHLIGHTS 2005 Q4 Performance As compared to prior Q4 to Quarterly sales of $9.9 million, up 37.5% Other considerations ATS Balance Sheet $20+ million in cash $20+ million in paid-for inventories $6 million in borrowing capacity 3F Therapeutics has approximately $10 million in cash as of December 1st 2005 Net Operating Loss Carry Forwards In excess of $80 Million Estimated cash value greater than $30 Million @ current tax rate |

| GROSS MARGIN PROJECTIONS 2004 2005 Future Gross Profit 0.31 0.38 0.67 Key Drivers: Self-manufactured Carbon New products |

| EXECUTIVE TEAM Michael Dale, President & CEO Edwards Laboratories, St. Jude Medical, Cyberonics, & Endocardial Solutions Walter Cuevas, President 3F Operations Medtronic, Allergan, & Edwards Richard Curtis, VP of Mktg & Bus. Development. Pharmacia Deltec, St. Jude Medical, Hill-Rom & Cardinal Health Marc Sportsman, VP of US Sales Shiley, Inc. & St. Jude Medical Terrie Ajamil, VP of International 3M & St. Jude Medical Jack Judd, Chief Financial Officer American Medical Systems & Apogee Enterprises |
| INVESTMENT SUMMARY Superior Products Best of Class, clinically proven, Patent protected Technology- the most differentiated pipeline in surgery Attractive Markets $1.6+ billion in market opportunity $1.1+ billion heart valve therapy market $150+ million surgical blood salvage market $200+ million emerging surgical ablation market Legitimate clinical needs for "better performance" Excellent Fundamentals Proven sales & marketing team Strong revenue growth in all markets Additional platform expansion & leverage opportunities Consistent Business Plan Execution! |