As filed with the Securities and Exchange Commission on July 16, 2003
Registration No. 333-
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
APOGENT TECHNOLOGIES INC.
(Exact name of registrant as specified in its charter)
| Wisconsin | | 3843 | | 22-2849508 |
(State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification No.) |
AND ITS GUARANTOR SUBSIDIARIES
Delaware | | Apogent Finance Company | | 02-0522134 |
Delaware | | Apogent Holding Company | | 02-0688113 |
Delaware | | Apogent Service Corporation | | 32-0056654 |
Delaware | | Apogent Transition Corp. | | 13-3326805 |
California | | Applied Biotech, Inc. | | 33-0447325 |
Delaware | | Barnstead Thermolyne Corporation | | 13-3326802 |
Delaware | | BT Canada Holdings Inc. | | 02-0523030 |
Alabama | | Capitol Vial, Inc. | | 63-1091273 |
Wisconsin | | Chase Scientific Glass, Inc. | | 62-1711339 |
Wisconsin | | Consolidated Technologies, Inc. | | 74-2951231 |
Delaware | | Erie Scientific Company | | 13-3326819 |
Delaware | | Erie Scientific Company of Puerto Rico | | 22-2855227 |
Delaware | | Erie UK Holding Company | | 02-0523659 |
Wisconsin | | Ever Ready Thermometer Co., Inc. | | 22-3329530 |
California | | Forefront Diagnostics, Inc. | | 33-0733551 |
New York | | Genevac Inc. | | 13-3614495 |
Delaware | | G&P Labware Holdings Inc. | | 02-0528748 |
Delaware | | Lab-Line Instruments, Inc. | | 36-2160341 |
California | | Lab Vision Corporation | | 94-3204455 |
Delaware | | Marsh Bio Products, Inc. | | 03-0418855 |
Delaware | | Matrix Technologies Corporation | | 04-2876817 |
Delaware | | Metavac LLC | | 02-0530733 |
Delaware | | Microgenics Corporation | | 68-0148167 |
California | | Molecular BioProducts, Inc. | | 95-3244122 |
Delaware | | Nalge Nunc International Corporation | | 13-3326816 |
Wisconsin | | National Scientific Company | | 58-2315507 |
Connecticut | | The Naugatuck Glass Company | | 06-0465440 |
California | | Neomarkers, Inc. | | 94-3223858 |
Wisconsin | | NERL Diagnostics Corporation | | 05-0486109 |
Wisconsin | | Owl Separation Systems, Inc. | | 39-1915146 |
Wisconsin | | Remel Inc. | | 74-2826694 |
Wisconsin | | Richard-Allan Scientific Company | | 38-3235594 |
California | | Robbins Scientific Corporation | | 94-2456711 |
Delaware | | Samco Scientific Corporation | | 95-3145731 |
Delaware | | Separation Technology, Inc. | | 93-0968130 |
Delaware | | Seradyn Inc. | | 02-0530147 |
| | |
(State or other jurisdiction of incorporation or organization) | | (Exact name of Guarantor as specified in its Charter) | | (I.R.S. Employer Identification Number) |
30 Penhallow Street Portsmouth, New Hampshire 03801 (603) 433-6131 | | MICHAEL K. BRESSON Executive Vice President—General Counsel and Secretary Apogent Technologies Inc. 30 Penhallow Street Portsmouth, New Hampshire 03801 (603) 433-6131 |
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices) | | (Name, address, including zip code, and telephone number, including area code of agent for service) |
Copies of all communications, including all communications sent to the agent should be sent to:
BRUCE C. DAVIDSON
JOSEPH D. MASTERSON
Quarles & Brady LLP
411 East Wisconsin Avenue
Milwaukee, WI 53202
(414) 277-5000
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. ¨
If this Form is filed t register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
CALCULATION OF REGISTRATION FEE
Title of each class of securities to be registered | | Amount to be registered(1) | | Proposed maximum offering price per unit | | Proposed maximum aggregate offering price | | Amount of registration fee(1) |
|
Series B 6 1/2% Senior Subordinated Notes Due 2013 | | $250,000,000 principal amount | | 100% | | $250,000,000(2) | | $20,225 |
|
Guarantees of each of the Guarantor Subsidiaries | | (2) | | (3) | | (3) | | None(3) |
| (1) | Fee based upon $80.90 per $1 million of aggregate offering amount. |
| (2) | The Series B 6 1/2% Senior Subordinated Notes Due 2013 (the “Exchange Notes”) will be guaranteed by each of the Guarantor Subsidiaries. |
| (3) | No additional consideration will be paid by the recipients of the Exchange Notes for the Guarantees. Pursuant to Rule 457(n), no separate fee is payable for the Guarantees. |
The registrants hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrants shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PROSPECTUS
SUBJECT TO COMPLETION DATED JULY 16, 2003

Apogent Technologies Inc.
OFFER TO EXCHANGE UP TO $250,000,000 IN PRINCIPAL AMOUNT OF ITS SERIES B 6 1/2% SENIOR SUBORDINATED NOTES DUE 2013 FOR ANY AND ALL OF ITS OUTSTANDING $250,000,000 IN PRINCIPAL AMOUNT OF 6 1/2% SENIOR SUBORDINATED NOTES DUE 2013
THE EXCHANGE OFFER WILL EXPIRE AT 5:00 P.M. NEW YORK CITY TIME ON , 2003, UNLESS EXTENDED.
Apogent Technologies Inc. (“Apogent” or the “Company”) is offering the Series B 6 1/2% Senior Subordinated Notes due 2013 (the “Exchange Notes”). We are offering to exchange (the “Exchange Offer”) up to $250,000,000 in aggregate principal amount of Exchange Notes for $250,000,000 aggregate principal amount of our outstanding 6 1/2% Senior Subordinated Notes due 2013 (the “Original Notes”). We sometimes refer to the Original Notes and the Exchange Notes collectively as the “notes.”
The terms of the Exchange Notes are substantially identical in all respects (including principal amount, interest rate and maturity) to the terms of the Original Notes for which they may be exchanged pursuant to the Exchange Offer, except that the Exchange Notes will be freely transferable by holders thereof (other than as described herein), are issued free of any covenant restricting transfer absent registration and will not have the right to receive liquidated damages in the event of a failure to timely register the Exchange Notes. The Exchange Notes will evidence the same debt as the Original Notes and contain terms that are substantially identical as the terms of the Original Notes. For a description of the terms of the notes, see “Description of Notes.” There will be no cash proceeds to Apogent from the Exchange Offer.
The Exchange Notes will bear interest from the most recent date to which interest has been paid on the Original Notes, or if no interest has been paid on the Original Notes, from June 2, 2003. Holders whose Original Notes are accepted for exchange will not receive any payment in respect of interest on the Original Notes otherwise payable on any interest payment date the record date for which occurs on or after consummation of the Exchange Offer. See “The Exchange Offer—Terms of the Exchange Offer.”
Like the Original Notes, the Exchange Notes will mature on May 15, 2013. Interest is fixed at an annual rate of 6 1/2% and is payable on May 15 and November 15 of each year, beginning on November 15, 2003. Interest will accrue from June 2, 2003. We may redeem some or all of the notes at any time on or after May 15, 2008. Prior to May 15, 2006, we may redeem up to 35% of the aggregate principal amount of the notes with the net cash proceeds of certain equity offerings. Redemption prices are set forth under “Description of Notes—Optional Redemption.” There is no sinking fund for the notes.
The notes will be Apogent Technologies Inc.’s general unsecured obligations and will be guaranteed on a senior subordinated basis by our domestic subsidiaries that have guaranteed, and our subsidiaries that will in the future guarantee, obligations under our revolving credit facility. The notes and the guarantees will rank junior in right of payment to all of Apogent Technologies Inc.’s and each subsidiary guarantor’s existing and future senior debt and will rankpari passu in right of payment with all of Apogent Technologies Inc.’s and each subsidiary guarantor’s senior subordinated debt.
The Original Notes were sold on June 2, 2003, in a transaction that was not registered under the Securities Act. Accordingly, the Original Notes may not be offered or sold within the United States or to U.S. persons, except to qualified institutional buyers in reliance on the exemption from registration provided by Rule 144A and to certain persons in offshore transactions in reliance on Regulation S. We are offering the Exchange Notes to satisfy our obligations under the registration rights agreement relating to the Original Notes. See “The Exchange Offer—Purposes and Effects of the Exchange Offer.”
EACH BROKER-DEALER THAT RECEIVES EXCHANGE NOTES FOR ITS OWN ACCOUNT PURSUANT TO THE EXCHANGE OFFER MUST ACKNOWLEDGE THAT IT WILL DELIVER A PROSPECTUS IN CONNECTION WITH ANY RESALE OF THE EXCHANGE NOTES. THE LETTER OF TRANSMITTAL
STATES THAT BY SO ACKNOWLEDGING AND BY DELIVERING A PROSPECTUS, A BROKER-DEALER WILL NOT BE DEEMED TO ADMIT THAT IT IS AN “UNDERWRITER” WITHIN THE MEANING OF THE SECURITIES ACT. THIS PROSPECTUS, AS IT MAY BE AMENDED OR SUPPLEMENTED FROM TIME TO TIME, MAY BE USED BY A BROKER-DEALER IN CONNECTION WITH ANY RESALES OF EXCHANGE NOTES RECEIVED IN EXCHANGE FOR ORIGINAL NOTES WHERE THE ORIGINAL NOTES WERE ACQUIRED BY THE BROKER-DEALER AS A RESULT OF MARKET-MAKING ACTIVITIES OR OTHER TRADING ACTIVITIES. APOGENT HAS AGREED THAT, FOR A PERIOD OF 180 DAYS AFTER THE EXPIRATION DATE (AS DEFINED HEREIN), IT WILL MAKE THIS PROSPECTUS AVAILABLE TO ANY BROKER-DEALER FOR USE IN CONNECTION WITH ANY SUCH RESALE. SEE “PLAN OF DISTRIBUTION.”
The notes are not listed and will not be listed on any national securities exchange.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed on the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2003.
In making your investment decision, you should rely only on the information contained in this prospectus. We have not, and the initial purchasers have not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. You should assume that the information appearing in this prospectus is accurate as of the date on the front cover of this prospectus only. Our business, financial condition, results of operations and prospects may have changed since that date. Neither the delivery of this prospectus nor any sale made hereunder shall under any circumstances imply that the information herein is correct as of any date subsequent to the date on the cover of this prospectus.
TABLE OF CONTENTS
Apogent Technologies Inc. is a Wisconsin corporation. Our principal executive offices are located at 30 Penhallow Street, Portsmouth, New Hampshire 03801, and our telephone number at that address is (603) 433-6131. Our World Wide Web site address ishttp://www.apogent.com. The information in our website is not part of this prospectus.
This prospectus has been prepared based on information provided by us and by other sources that we believe are reliable. This prospectus summarizes certain documents and other information in a manner we believe to be accurate, but we refer you to the actual documents for a more complete understanding of what we discuss in this prospectus. You must rely on your own examination of our company and the terms of the offering and the notes, including the merits and risks involved.
We are not making any representation to you regarding the legality of an investment in the notes by you under any legal investment or similar laws or regulations. You should not consider any information in this prospectus to be legal, business, tax or other advice. You should consult your own attorney, business advisor and tax advisor for legal, business and tax advice regarding an investment in the notes.
The distribution of this prospectus and the offer and sale of the notes may be restricted by law in certain jurisdictions. Persons in whose possession this prospectus or any of the notes is delivered must inform themselves about, and observe, any such restrictions.
This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any of the notes to any person in any jurisdiction where it is unlawful to make such an offer or solicitation.
We expect that the notes sold pursuant to this prospectus will be represented by one or more global notes, which will be deposited with, or on behalf of, The Depository Trust Company and registered in the name of Cede
i
& Co. Beneficial interests in the global notes representing the notes will be shown on, and transfers thereof will be effected through, records maintained by The Depository Trust Company and its participants. After the initial issuance of the global notes, notes in certificated form will be issued in exchange for the global notes only under limited circumstances on the terms set forth in the indenture.
NOTICE TO NEW HAMPSHIRE RESIDENTS
NEITHER THE FACT THAT A REGISTRATION STATEMENT OR AN APPLICATION FOR A LICENSE HAS BEEN FILED UNDER RSA 421-B WITH THE STATE OF NEW HAMPSHIRE NOR THE FACT THAT A SECURITY IS EFFECTIVELY REGISTERED OR A PERSON IS LICENSED IN THE STATE OF NEW HAMPSHIRE CONSTITUTES A FINDING BY THE SECRETARY OF STATE THAT ANY DOCUMENT FILED UNDER RSA 421-B IS TRUE, COMPLETE AND NOT MISLEADING. NEITHER ANY SUCH FACT NOR THE FACT THAT AN EXEMPTION OR EXCEPTION IS AVAILABLE FOR A SECURITY OR A TRANSACTION MEANS THAT THE SECRETARY OF STATE HAS PASSED IN ANY WAY UPON THE MERITS OR QUALIFICATIONS OF, OR RECOMMENDED OR GIVEN APPROVAL TO, ANY PERSON, SECURITY OR TRANSACTION. IT IS UNLAWFUL TO MAKE, OR CAUSE TO BE MADE, TO ANY PROSPECTIVE PURCHASER, CUSTOMER OR CLIENT ANY REPRESENTATION INCONSISTENT WITH THE PROVISIONS OF THIS PARAGRAPH.
FORWARD-LOOKING STATEMENTS
All statements other than statements of historical facts included in this prospectus, including, without limitation, statements under the captions “Risk Factors,” “Use of Proceeds,” and “Business,” are or could be deemed to be forward-looking statements. Words such as “anticipate,” “believe,” “continue,” “estimate,” “expect,” “goal,” “objective,” “outlook” and similar expressions signify forward-looking statements. These statements are based upon our expectations at the time we made them and are subject to risks and uncertainties, many of which are beyond our control, that could cause actual results to differ materially from those contemplated in the forward-looking statements. In addition to the assumptions and other factors referred to specifically in connection with forward-looking statements, factors that could cause our actual results to differ materially include the factors described under the caption “Cautionary Factors” in this prospectus and in our SEC filings incorporated by reference into this prospectus. The risks and uncertainties so identified are not the only ones facing our company. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial also may adversely affect us. Should any risks and uncertainties develop into actual events, these developments could have material adverse effects on our business, financial condition and results of operations. For these reasons, we caution you not to place undue reliance on our forward-looking statements.
INDUSTRY AND MARKET DATA
In this prospectus we rely on and refer to information and statistics regarding Apogent’s markets and our market share in the sectors in which we compete. We obtained this information and statistics from various third-party sources, discussions with our customers and our own internal estimates. We believe that these sources and estimates are reliable, but have not independently verified them and cannot guarantee their accuracy or completeness.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and special reports, proxy statements, and other documents with the Securities and Exchange Commission. Our SEC filings are available to the public at the SEC’s website athttp://www.sec.gov.
ii
You may also read and copy any document we file with the SEC at the public reference rooms maintained by the Commission at Judiciary Plaza, Room 1024, 450 Fifth Street, N.W., Washington, D.C. 20549. In addition, you may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 450 Fifth Street, N.W., Room 1024, Washington, D.C. 20549. You may obtain information regarding the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
In addition, because our common stock is listed on the New York Stock Exchange (ticker symbol “AOT”), you may read our reports, proxy statement, and other documents at the offices of the New York Stock Exchange at 20 Broad Street, New York, New York 10005.
INCORPORATION BY REFERENCE
The SEC allows us to “incorporate by reference” information in documents that we file with it. We have elected to use this procedure in connection with this prospectus, which means that we can disclose important information by referring you to documents that contain that information, and the information incorporated by reference is considered part of this prospectus. Information that we file later with the SEC is automatically incorporated by reference and will update and supersede the previously filed information. Note that we filed our documents under the name Sybron International Corporation until our name was formally changed to Apogent Technologies Inc. on January 31, 2001, so you may need to look under both names to find all information that is of interest to you. We incorporate by reference into this prospectus:
| | �� | our annual report on Form 10-K for the fiscal year ended September 30, 2002; |
| | • | our quarterly reports on Form 10-Q for the quarters ended December 31, 2002 and March 31, 2003; |
| | • | our current reports on Form 8-K or 8-K/A dated as of December 18, 2002, January 6, 2003, April 22, 2003, May 13, 2003, May 22, 2003, and June 2, 2003; and |
| | • | any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934 until we sell all of the securities offered by this prospectus or terminate the offering. |
You may request copies of these filings, at no cost, by writing or calling us at Apogent Technologies Inc., Attn: Investor Relations, 30 Penhallow Street, Portsmouth, New Hampshire 03801, (603) 433-6131.
We maintain an Internet web site athttp://www.apogent.com. Our web site and information at that site, or connected to that site, are not incorporated into this prospectus.
iii
PROSPECTUS SUMMARY
This summary highlights some information from this prospectus, but it may not contain all of the information that is important to you. You should read this summary together with the entire prospectus, especially “Risk Factors” beginning on p. . For more complete information about Apogent, its business, its financial statements and related matters, you should refer to the documents mentioned in the section of this prospectus entitled “Incorporation by Reference.” For a more complete understanding of the notes, please refer to the section of this document entitled “Description of Notes.” Unless the context otherwise requires, all references to “Apogent,” “us,” “we,” “our,” and, “our company” refer to Apogent Technologies Inc., the issuer of the notes, and its subsidiaries. Our fiscal year ends on September 30.
In the second quarter of our 2003 fiscal year, we made the decision to exit two of our businesses, Applied Biotech, Inc. and BioRobotics Group Ltd., which have been reclassified as discontinued operations. All historical financial information in this prospectus has been restated to reflect this reclassification and, accordingly, the results of operations of these businesses are not included in net sales, cost of sales, or other components of operating income but, instead, are included in discontinued operations.
Apogent Technologies Inc.
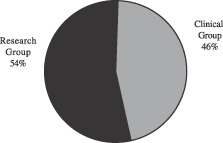
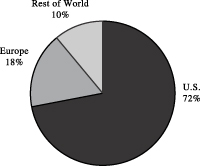
Apogent is a leading global developer, manufacturer, and marketer of value-added consumable products and equipment for the clinical and research industries. We manufacture and sell approximately 10,000 different consumable and non-consumable products. Consumable products, which are ordered on a recurring basis by our customers and end-users, represented over 80% of our net sales for the twelve months ended March 31, 2003. We are organized into two business segments, the Clinical Group and the Research Group, which serve our customers involved in clinical and research activities globally. Our Clinical Group develops and manufactures a full line of clinical and commercial laboratory products, and our Research Group develops and manufactures a full line of research and life science products. We reach our customers and end-users through a network of independent distributors and through our direct sales force. We manufacture most of the products we sell in our 62 manufacturing facilities. We have approximately 7,200 employees in over 125 facilities worldwide. Our customers include distributors, pharmaceutical and biotechnology companies, clinical, academic, governmental, research, and industrial laboratories, and OEMs. Approximately 72% of our consolidated net sales in fiscal 2002 were to customers in the U.S. and the remainder was to customers, primarily in Europe and Japan. For the twelve-month period ended March 31, 2003, we generated net sales of $1,073.3 million and net cash provided by operating activities of $165.6 million.
We focus on developing and manufacturing products that are industry staples and that are used in the majority of clinical and research laboratories. Most of our products have established brand names, many of which have been in existence for decades. We believe industry professionals demand our products because of our reputation for quality and the essential role that our products play in the research and development and diagnostic activities of our end-users.
In December 2000, we spun off our dental business by way of a pro rata distribution to our shareholders of all of the outstanding common stock and related preferred stock purchase rights of Sybron Dental Specialties, Inc. As a result of the spin-off, Sybron Dental Specialties became an independent public company operating what was our dental business. We changed our name from Sybron International Corporation to Apogent Technologies Inc. in 2001. Our financial statements in this prospectus reflect the dental business as a discontinued operation.
1
The following charts show our net sales for the twelve months ended March 31, 2003 by business segment and geographic area.
| Net Sales by Business Segment | | Net Sales by Geography |
 | |  |
Clinical Group
Our Clinical Group manufactures and sells products primarily to clinical and commercial laboratories and to scientific research and industrial customers. These products are used in a number of diagnostic applications, including specimen collection, specimen transportation, drug testing, therapeutic drug monitoring, infectious disease detection, and glucose tolerance testing. Other applications include anatomical pathology (histology and cytology) and immunohistochemistry, with an emphasis on cancer applications. Clinical Group products include:
| | • | microscope slides, cover glass, and glass tubes and vials; |
| | • | histology and immunochemistry instrumentation; |
| | • | sample vials used in diagnostic testing; |
| | • | diagnostic reagents; and |
| | • | other products used in detecting causes of various infectious diseases, conditions, and therapeutic drugs or drugs of abuse. |
For the twelve-month period ended March 31, 2003, our Clinical Group generated net sales of $496.9 million and operating income of $124.7 million.
Research Group
Our Research Group manufactures, distributes, and sells products primarily to the research and clinical life sciences industries. Applications of these products include general everyday laboratory uses as well as genomics, proteomics, high-throughput screening for drug discovery, combinatorial chemistry, cell culture, filtration, and liquid handling. In addition, this segment manufactures basic laboratory equipment needed by medical, pharmaceutical, and scientific laboratories. Research Group products include:
| | • | reusable plastic and glass products (e.g., bottles, carboys, graduated ware, beakers, flasks, and plastic bottles for consumer use); |
| | • | disposable plastic and glass products; |
| | • | products for critical packaging applications; |
2
| | • | environmental and safety containers; |
| | • | liquid handling automation products; |
| | • | autosampler vials and seals used in chromatography analysis; |
| | • | various consumable products for use in applications of cell culture, filtration, molecular biology, cryopreservation, immunology, electrophoresis, liquid handling, genomics, and high-throughput screening for pharmaceutical drug discovery; and |
| | • | heating, cooling, shaking, stirring, mixing, and temperature control instruments. |
For the twelve-month period ended March 31, 2003, our Research Group generated net sales of $576.4 million and operating income of $123.6 million.
Industry
We believe the size of the global life sciences and laboratory products supply markets that we address is approximately $35.0 billion. There are a number of trends in the laboratory supply business that we believe will support continuing growth in the overall market, including:
| | • | growing number of in vitro diagnostic tests per person; |
| | • | increasing rates of cancer; |
| | • | increasing R&D budgets at pharmaceutical companies; |
| | • | growth in large-scale cell culture products; and |
| | • | increasing economic development in emerging market countries. |
Competitive Strengths
Our competitive strengths include:
| | • | Recurring revenue from sale of consumables. Sales of consumable products ordered on a recurring basis by our customers and end-users account for a substantial portion of our net sales. For the twelve months ended March 31, 2003, consumable products sales represented over 80% of our net sales. We supply a significant portion of the consumables used in specimen examinations by pathologists and our products are used in the majority of non-discretionary physician-prescribed clinical applications. We believe our long-standing relationships with loyal customers who purchase our consumable products provide a stable and predictable recurring revenue stream. |
| | • | Strong cash flow generation. We generate substantial cash flow from operations and our manufacturing operations require relatively modest investment. During the twelve months ended March 31, 2003, we generated cash flow from operations of $165.6 million and spent $69.1 million on capital expenditures. Our cash flow provides us with a high degree of flexibility to fund our internal and acquisition growth initiatives and to service our balance sheet obligations. |
| | • | Diverse product mix. We manufacture, distribute, and sell a unique mix of products, including high-margin industry staples and products in new, higher growth markets. Industry staples are composed largely of routine disposable lab supplies and consumable products. Higher growth markets include life sciences products used in diagnostic, biotechnology, and pharmaceutical laboratories. |
| | • | Leading market positions and industry leading brands. We have well established brands in both our Clinical Group and our Research Group. Key brands in our Clinical Group include Remel®, Erie Scientific®, Chase Scientific™, Richard-Allan Scientific®, and Microgenics®. Key brands in our Research Group include Nalgene®, Nunc®, Barnstead International™, Molecular BioProducts™, Matrix®, and ABgene™. We believe we have leading positions in the majority of our key product lines. |
3
| | • | Long established customer relationships. We have long-standing relationships with our core customers, particularly our three primary laboratory distributors with whom we have conducted business for an average of 30 years, and with our key OEMs. |
| | • | Strong and experienced management team. We have assembled an experienced and successful management team. On average, each member of our senior executive management team has over 20 years of relevant industry experience. Our management has demonstrated a strong capability for growing our business and successfully managing our balance sheet and cash flows. |
Business Strategy
Our goal is to consistently grow our worldwide market presence, net sales, earnings, and cash flows. Annual revenue growth in fiscal 2002 was $89.1 million, or 9.5% over the prior year, and in the first six months of fiscal 2003 was $45.4 million or 9.3% over the comparable prior year period. Key elements of our strategy continue to be:
| | • | Maintain prudent capital structure. We consider our ability to manage our balance sheet effectively to be a core competence. Strategic investments which require an outlay of capital are put through a rigorous review process to ensure the investment provides an acceptable cash return which exceeds our cost of capital. We maintain a capital structure which provides us with the financial flexibility to compete effectively in our core markets. |
| | • | Develop profitable new products. We consistently strive to develop and introduce new products that contribute to sales, earnings, and cash flows. These products include new offerings and improvements of our existing products. We are especially focused on developing new products for our life sciences research, and clinical diagnostics customers. |
| | • | Increase sales to existing and new customers. We seek to leverage our strong market presence and excellent customer and distributor relationships into increased sales to current customers and sales to new customers. We believe that our extensive product offering is conducive to cross-selling products to existing customers. This broad product offering is also conducive to negotiating favorable terms with our distributors. |
| | • | Improve operating efficiencies. We are focused on improving our operating efficiencies through vertical integration, streamlined manufacturing techniques, better product sourcing, and the sharing of technology and best practices across our company. We believe that our focus on efficiencies improves our gross margins while maintaining or improving the quality of our products and increases customer satisfaction. |
| | • | Make strategic acquisitions. Our acquisition program has been and continues to be focused on adding complementary products and technologies that enhance our market position. Our operating subsidiaries generally have been able to use their existing channels to market our acquired products. We have a rigorous process of candidate identification, due diligence, and integration designed to mitigate acquisition risk. Acquired businesses are converted to our standard financial reporting system. In most cases, we retain the senior management of acquired businesses and have an integration plan and budget in place at the time the acquisition closes. We presently intend to reduce our emphasis on our acquisition strategy and focus on steadily increasing our earnings and cash flows through the development of new products, increasing sales to existing and new customers, and focusing on improving efficiencies. |
The Recapitalization
On April 23, 2003, we initiated a tender offer for up to 15.0 million of our common shares, representing approximately 15% of our outstanding shares, pursuant to a modified Dutch auction tender offer at a price of not
4
less than $15.00 or more than $17.50 per share. We entered into an amendment of our revolving credit facility which allowed us to consummate the tender offer. The tender offer expired May 21, 2003. We purchased approximately 6.0 million common shares in the tender offer at $17.50 per share, or a total cost of $105.4 million. We used approximately 42% the proceeds of the Original Notes offering to finance our purchase of shares in the tender offer. We have used or expect to use the remaining net proceeds of the Original Notes offering to repurchase shares of our common stock in the open market or otherwise, to repay the outstanding balance under our revolving credit facility, and for general corporate purposes.
Recent Developments
We recently realigned our lines of business for financial reporting purposes. Our three former business segments (Clinical Diagnostics, Labware and Life Sciences, and Laboratory Equipment) have been reclassified into two business segments: Clinical Group and Research Group. The Clinical Group business segment is the former Clinical Diagnostics business segment. The Research Group business segment is composed of the former Labware and Life Sciences and Laboratory Equipment business segments. All financial data presented in this prospectus reflect this change in reporting business segments.
During the second quarter of our 2003 fiscal year, we made the decision to exit two of our businesses: the rapid diagnostic test business (on-site rapid tests in the detection of pregnancy, drugs of abuse, and infectious diseases) as conducted by our Applied Biotech, Inc. subsidiary; and the manufacture and sale of automated microarray instrumentation for the genomics market as conducted by our BioRobotics Group subsidiary. This decision was based in part on our ongoing strategy of strengthening the market positions of our leading brands and focusing on sales of our consumable laboratory products that have more stable growth expectations.
On April 23, 2003, we initiated the tender offer relating to our recapitalization, which expired at midnight, New York City time, on May 21, 2003. On April 23, 2003, in response to the announcement of our tender offer and the offering of the Original Notes, Standard & Poor’s Ratings Services lowered our corporate credit and senior unsecured debt ratings to “BBB-” from “BBB.” In addition, on May 9, 2003, Moody’s Investors Service downgraded our revolving credit facility, senior notes, and senior convertible contingent notes from a rating of Baa3 to Ba1 and assigned a rating of Ba2 to the notes offered in this prospectus. On May 13, 2003, Standard & Poor’s assigned a rating of BB+ to the notes. Under the terms of the indenture which governs the notes, we are subject to certain restrictive covenants until the notes are rated investment grade. See “Description of Notes—Certain Covenants.”
5
The Exchange Offer
Purpose of Exchange Offer | | We sold the Original Notes in a private offering to certain accredited institutions through Lehman Brothers, Credit Suisse First Boston, JPMorgan, ABN AMRO Incorporated, Banc of America Securities LLC, Banc One Capital Markets, Inc., Comerica Securities, Fleet Securities, Inc., Goldman, Sachs & Co., Robert W. Baird & Co., Scotia Capital, SunTrust Robinson Humphrey, The Royal Bank of Scotland, and Wachovia Securities (the “initial purchasers”). In connection with that offering, we executed and delivered for the benefit of holders of the Original Notes a registration rights agreement, which is an exhibit to the registration statement of which this prospectus is a part, providing for, among other things, the Exchange Offer. The Exchange Offer is intended to make the Exchange Notes freely transferable by the holders thereof without registration or any prospectus delivery requirements under the Securities Act, except that a “dealer” or any “affiliate” of a “dealer” (as those terms are defined under the Securities Act) who exchanges Original Notes held for its own account (a “Restricted Holder”) will be required to deliver copies of this prospectus in connection with any resale of the Exchange Notes issued in exchange for those Original Notes. See “The Exchange Offer—Purposes and Effects of the Exchange Offer” and “Plan of Distribution.” |
| |
The Exchange Offer | | We are offering to exchange pursuant to the Exchange Offer up to $250 million aggregate principal amount of our new Series B
6½% Senior Subordinated Notes Due 2013 (the “Exchange Notes”) for $250 million aggregate principal amount of our outstanding 6 ½% Senior Subordinated Notes Due 2013 (the “Original Notes”). We sometimes refer to the Original Notes and the Exchange Notes collectively as the “notes.” The terms of the Exchange Notes are substantially identical in all respects (including principal amount, interest rate and maturity) to the terms of the Original Notes, except that the Exchange Notes are freely transferable by the holders thereof (other than as provided herein), and are not subject to any covenant regarding registration under the Securities Act. See “The Exchange Offer—Terms of the Exchange Offer” and “The Exchange Offer—Procedures for Tendering.” The Exchange Offer is not conditioned upon any minimum aggregate principal amount of Original Notes being tendered for exchange. |
| |
Expiration Date | | The Exchange Offer will expire at 5:00 p.m., New York City time on , 2003, unless extended (the “Expiration Date”). |
| |
Conditions of the Exchange Offer | | Our obligation to consummate the Exchange Offer is subject to certain conditions. We will not be required to accept for exchange any Original Notes tendered and may terminate the Exchange Offer before acceptance of any Original Notes if, among other |
6
| | | things, legal actions or proceedings are instituted that challenge or seek to prohibit the exchange or there shall have been proposed, adopted or enacted any law, statute or regulation materially affecting the benefits of the Exchange Offer. See “The Exchange Offer—Conditions of the Exchange Offer.” We reserve the right to terminate or amend the Exchange Offer at any time prior to the Expiration Date upon the occurrence of any of the conditions. |
| |
Procedures for Tendering Original Notes | | To accept the Exchange Offer, you must complete, sign and date the Letter of Transmittal, or a facsimile of it, in accordance with the instructions in this prospectus and contained in the Letter of Transmittal, and mail or otherwise deliver the Letter of Transmittal or facsimile, together with the Original Notes and any other required documentation, to the exchange agent (the “Exchange Agent”) at the address set forth therein. Physical delivery of the Original Notes is not required if a confirmation of a book-entry transfer of the Original Notes to the Exchange Agent’s account at The Depository Trust Company (“DTC” or the “Depository”) is timely delivered. By executing the Letter of Transmittal, you will represent to us that: • you are acquiring the Exchange Notes in the ordinary course of business, • you are not engaged in, do not intend to engage in, and have no arrangement or understanding with any person to participate in, the distribution of the Original Notes or the Exchange Notes within the meaning of the Securities Act, and • you are not an “affiliate” of Apogent as defined under the Securities Act, or if you are an affiliate, that you will comply with the registration and prospectus delivery requirements of the Securities Act, to the extent applicable. In addition, each broker or dealer that receives Exchange Notes for its own account in exchange for any Original Notes that were acquired by the broker or dealer as a result of market-making activities or other trading activities must acknowledge that it will deliver a prospectus in connection with any resale of the Exchange Notes. See “The Exchange Offer—Procedures for Tendering” and “Plan of Distribution.” |
| |
Special Procedures for Beneficial Owners | | If you are a beneficial owner whose Original Notes are registered in the name of a broker dealer, commercial bank, trust company or other nominee and you wish to tender, you should contact the registered holder promptly and instruct the registered holder to tender on your behalf. If the Original Notes are in certificated |
7
| | | form and you are a beneficial owner who wishes to tender on the registered holder’s behalf, prior to completing and executing the Letter of Transmittal and delivering the Original Notes, you must either make appropriate arrangements to register ownership of the Original Notes in your name or obtain a properly completed bond power from the registered holder. The transfer of registered ownership may take considerable time. See “The Exchange Offer—Procedures for Tendering.” |
| |
Guaranteed Delivery Procedures | | If the Original Notes are in certificated form and you wish to tender your Original Notes in the Exchange Offer and your Original Notes are not immediately available for delivery, you may still tender your shares by completing, signing and delivering the Letter of Transmittal and any other documents required by the Letter of Transmittal to the Exchange Agent prior to the Expiration Date and tendering your Original Notes according to the guaranteed delivery procedures set forth in “The Exchange Offer—Guaranteed Delivery Procedures.” |
| |
Withdrawal Rights | | You may withdraw your tenders at any time prior to 5:00 p.m. New York City time on the Expiration Date. See “The Exchange Offer—Withdrawal of Tenders.” |
| |
Acceptance of Original Notes and Delivery of Exchange Notes | | We will accept for exchange any and all Original Notes that are properly tendered to the Exchange Agent prior to 5:00 p.m. New York City time on the Expiration Date. The Exchange Notes issued pursuant to the Exchange Offer will be delivered promptly following the Expiration Date. See “The Exchange Offer—Terms of the Exchange Offer.” |
| |
Exchange Agent | | The Bank of New York, New York, New York, is serving as the Exchange Agent in connection with the Exchange Offer. See “The Exchange Offer—Exchange Agent.” |
| |
Effect on Holders of the Original Notes | | As a result of making this Exchange Offer, and upon acceptance for exchange of all validly tendered Original Notes pursuant to the terms of this Exchange Offer, we will have fulfilled one of our obligations contained in the registration rights agreement and, accordingly, there will be no registration default and therefore no liquidated damages with respect to the Exchange Offer on the Original Notes pursuant to the registration rights agreement. Holders of Original Notes who do not tender their Original Notes will continue to be entitled to all the rights and limitations applicable thereto under the indenture dated as of June 2, 2003, among Apogent Technologies Inc., the Guarantors, and The Bank of New York, as trustee (the “Trustee”) relating to the Original Notes and the Exchange Notes (the “indenture”), except for any rights under the indenture or the registration’ rights agreement which by their terms terminate or cease to have further |
8
| | | effectiveness as a result of the making of, and the acceptance for exchange of all validly tendered Original Notes pursuant to, the Exchange Offer. All Original Notes that remain outstanding will continue to be subject to the restrictions on transfer provided for in the Original Notes and the indenture. To the extent that Original Notes are tendered and accepted in the Exchange Offer, the trading market for Original Notes could be adversely affected. |
| |
Use of Proceeds | | There will be no cash proceeds to Apogent from the exchange pursuant to the Exchange Offer. |
Terms of the Notes
The Exchange Offer applies to the entire outstanding $250 million principal amount of Original Notes. The terms of the Exchange Notes are identical in all material respects to the Original Notes, except for certain transfer restrictions and registration and other rights relating to the exchange of the Original Notes for Exchange Notes. The Exchange Notes will evidence the same debt as the Original Notes and will governed by the same indenture under which the Original Notes were issued. See “Description of Notes.”
Issuer | | Apogent Technologies Inc. |
| |
Securities Offered | | $250,000,000 aggregate principal amount of Series B 6½% Senior Subordinated Notes due 2013. |
| |
Maturity Date | | May 15, 2013. |
| |
Interest Payment Dates | | May 15 and November 15 of each year, commencing on November 15, 2003. |
| |
Optional Redemption | | We may redeem the notes in whole or in part at any time on or after May 15, 2008, at the redemption prices described under “Description of Notes—Optional Redemption.” Prior to May 15, 2006, we may redeem up to 35% of the aggregate principal amount of the notes (including additional notes, if any) with the net cash proceeds of qualified equity offerings. |
| |
Change of Control | | If we experience specific kinds of changes of control, we must offer to repurchase the notes at 101% of their principal amount, plus accrued and unpaid interest, if any, to the date of repurchase. For more details, see “Description of Notes—Repurchase at the Option of Holders—Change of Control.” |
| |
Guarantees | | All payments with respect to the notes, including principal and interest, will be fully and unconditionally guaranteed, jointly and severally, on a senior subordinated basis by all of our current and future domestic subsidiaries that have guaranteed, and will in the future guarantee, obligations under our revolving credit facility, subject to their release in certain instances described under “Description of Notes—Brief Description of the Notes and the |
9
| | | Guarantees—The Guarantees.” Each guarantee of the notes: • is a general unsecured obligation of the guarantor; • is subordinated in right of payment to all existing and future senior debt of that guarantor; and • ispari passu in right of payment with any future senior subordinated indebtedness of that guarantor. Apogent Technologies Inc. and our material domestic subsidiary guarantors owned 77% of our assets at September 30, 2002 and generated 85% of our income from continuing operations for the fiscal year ended September 30, 2002. |
| |
Ranking | | The notes will be our unsecured senior subordinated obligations. Accordingly, they will rank: • subordinate in right of payment to all of our and our subsidiary guarantors’ existing and future senior indebtedness, including our obligations under our revolving credit facility, 8% senior notes due 2011 and 2.25% Senior Convertible Contingent Debt SecuritiesSM (CODESSM) due 2021, in each case issued by us and guaranteed by certain of our subsidiary guarantors; • effectively subordinate to all of the existing and future indebtedness and other liabilities of our non-guarantor subsidiaries (other than indebtedness and other liabilities owed to us); • pari passu in right of payment to our and our subsidiary guarantors’ future senior subordinated indebtedness; and • senior in right of payment to our and our subsidiary guarantors’ future subordinated indebtedness. On March 31, 2003, after giving pro forma effect to the sale of the Original Notes and the recapitalization, we and our subsidiaries would have had total indebtedness of $923.7 million (of which $250.0 million would have consisted of the notes and the balance would have consisted of our senior indebtedness). Neither we nor our subsidiary guarantors currently have any indebtedness that is expressly subordinated to the notes. |
| |
Certain Covenants | | The notes contain limitations on, among other things: • the payment of dividends and other distributions with respect to our capital stock and the purchase, redemption, or retirement of our capital stock; • our ability to incur additional indebtedness and issue preferred stock; • the right of restricted subsidiaries to make certain payments and distributions; |
10
| | | • asset sales; • transactions with affiliates; • the incurrence of liens; • engaging in certain business activities; and • certain mergers or consolidations and transfers of assets. In the event that the notes are assigned a rating of Baa3 or better by Moody’s Investor Service and BBB- or better by Standard and Poor’s Rating Group, Inc., and no event of default has occurred and is continuing, certain covenants in the indenture will be terminated. For more details, see the section “Description of Notes—Certain Covenants.” |
| |
Exchange Offer, Registration Rights | | In connection with the Original Notes offering, under the registration rights agreement between us, our subsidiary guarantors, and the initial purchasers of the notes entered into on June 2, 2003, we have agreed to: • file with the SEC this registration statement offering to exchange (the “Registered Exchange Offer”) the notes for new notes having substantially identical terms (the “Exchange Notes”) (except that the Exchange Notes will not contain terms with respect to transfer restrictions) within 90 days after the issue date of the Original Notes; and • use our best efforts to cause this registration statement to become effective under the Securities Act within 150 days after the issue date of the Original Notes. Under certain circumstances, in lieu of a Registered Exchange Offer, we have agreed to file a shelf registration statement with respect to the notes and to use our best efforts to keep the shelf registration statement effective for at least two years after its effective date. If we do not comply with these registration obligations, then we will be required to pay liquidated damages to holders of the notes under certain circumstances. See “Description of Notes—Registration Rights; Liquidated Damages.” |
Risk Factors
Prospective purchasers of the notes should carefully consider all information contained in this prospectus, including the risk factors beginning on page 13.
11
Summary Consolidated Financial Data
The following table presents our summary consolidated financial information for the six month periods ended March 31, 2003 and 2002, and the years ended September 30, 2002, 2001, and 2000. The financial information for the six months ended March 31, 2003 and 2002 is derived from our unaudited financial statements which, in the opinion of our management, contain all adjustments necessary for a fair presentation of this information. The financial information for the six months ended March 31, 2003 is not necessarily indicative of the results expected for the full year. The financial information for each of the years ended September 30, 2002, 2001, and 2000 is derived from our audited financial statements. The financial information set forth below should be read in conjunction with our consolidated financial statements and the related notes, “Selected Consolidated Financial Information” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” all included elsewhere in this prospectus.
| | | Twelve Months Ended March 31, 2003
| | | Six Months Ended March 31,
| | | Year Ended September 30,
| |
| | | 2003
| | | 2002
| | | 2002
| | | 2001
| | | 2000
| |
| | | (unaudited) | | | (unaudited) | | | | | | | | | | |
| | | (dollar amounts in thousands) | |
Consolidated Statement of Income Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Net sales | | $ | 1,073,287 | | | $ | 534,436 | | | $ | 489,062 | | | $ | 1,027,913 | | | $ | 938,819 | | | $ | 843,567 | |
Total cost of sales | | | 550,266 | | | | 278,366 | | | | 250,119 | | | | 522,019 | | | | 471,318 | | | | 426,004 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | 523,021 | | | | 256,070 | | | | 238,943 | | | | 505,894 | | | | 467,501 | | | | 417,563 | |
Total selling, general and administrative expenses | | | 274,690 | | | | 140,272 | | | | 123,536 | | | | 257,954 | | | | 256,485 | | | | 234,018 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | 248,331 | | | | 115,798 | | | | 115,407 | | | | 247,940 | | | | 211,016 | | | | 183,545 | |
Interest expense | | | 40,951 | | | | 20,792 | | | | 20,578 | | | | 40,737 | | | | 48,820 | | | | 49,584 | |
Net income (loss) | | $ | 44,580 | | | $ | (27,649 | ) | | $ | 48,920 | | | $ | 121,149 | | | $ | 95,941 | | | $ | 128,321 | |
| | | | | | |
Other Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Gross margin | | | 48.7 | % | | | 47.9 | % | | | 48.9 | % | | | 49.2 | % | | | 49.8 | % | | | 49.5 | % |
Depreciation and amortization | | $ | 60,778 | | | $ | 30,935 | | | $ | 25,571 | | | $ | 55,414 | | | $ | 73,348 | | | $ | 62,901 | |
Expenditures for property, plant and equipment | | | 69,091 | | | | 22,560 | | | | 17,099 | | | | 63,630 | | | | 50,120 | | | | 41,324 | |
Net cash provided by operating activities | | | 165,636 | | | | 56,964 | | | | 83,478 | | | | 192,150 | | | | 179,393 | | | | 115,375 | |
Ratio of earnings to fixed charges(1) | | | 5.1x | | | | 4.8x | | | | 4.9x | | | | 5.2x | | | | 4.2x | | | | 3.5x | |
Consolidated Balance Sheet Data (at end of period): | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | | | | | $ | 17,406 | | | $ | 42,415 | | | $ | 16,327 | | | $ | 9,192 | | | $ | 12,411 | |
Working capital | | | | | | | 293,692 | | | | 191,919 | | | | 230,125 | | | | 150,371 | | | | 269,396 | |
Total assets | | | | | | | 1,984,730 | | | | 1,981,662 | | | | 2,036,085 | | | | 1,828,080 | | | | 1,792,364 | |
Total liabilities | | | | | | | 1,089,463 | | | | 1,099,555 | | | | 1,060,947 | | | | 989,590 | | | | 1,042,848 | |
Shareholders’ equity | | | | | | $ | 895,267 | | | $ | 882,107 | | | $ | 975,138 | | | $ | 838,490 | | | $ | 749,516 | |
| (1) | The ratio of earnings to fixed charges is computed by dividing: |
| | • | income from continuing operations before income taxes and extraordinary item, plus fixed charges, by |
Fixed charges consist of interest expense, amortization of deferred financing fees, and an estimate of interest within rental expense.
The unaudited pro forma ratio of earnings to fixed charges for the year ended September 30, 2002 and the six months ended March 31, 2003 was 4.2 and 3.9, respectively. This ratio has been prepared assuming that the sale of the notes was completed on October 1, 2001.
12
RISK FACTORS
You should carefully consider the following risk factors, in addition to the other information presented in this prospectus and the documents incorporated by reference into this prospectus, in evaluating us, our business and an investment in the notes. Any of the following risks, as well as other risks and uncertainties, could harm our business and financial results and cause the value of the notes to decline, which in turn could cause you to lose all or part of your investment. The risks below are not the only ones facing our company. Additional risks not currently known to us or that we currently deem immaterial also may impair our business.
Risks Relating to the Exchange Offer
You must carefully follow the required procedures in order to exchange your Original Notes.
The Exchange Notes will be issued in exchange for Original Notes only after timely receipt by the Exchange Agent of a duly executed letter of transmittal and all other required documents. Therefore, if you wish to tender your Original Notes, you must allow sufficient time to ensure timely delivery. Neither the Exchange Agent nor we have any duty to notify you of defects or irregularities with respect to tenders of Original Notes for exchange. Any holder of Original Notes who tenders in the Exchange Offer for the purpose of participating in a distribution of the Exchange Notes will be required to comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale transaction. Each broker or dealer that receives Exchange Notes for its own account in exchange for Original Notes that were acquired in market-making or other trading activities must acknowledge that it will deliver a prospectus in connection with any resale of the Exchange Notes. See “Plan of Distribution.”
If you do not exchange Original Notes for Exchange Notes, transfer restrictions will continue and trading of the Original Notes may be adversely affected.
The Original Notes have not been registered under the Securities Act and are subject to substantial restrictions on transfer. Original Notes that are not tendered for exchange for Exchange Notes or are tendered but are not accepted will, following completion of the Exchange Offer, continue to be subject to existing restrictions upon transfers. We do not currently expect to register the Original Notes under the Securities Act. To the extent that Original Notes are tendered and accepted in the Exchange Offer, the trading market for Original Notes could be adversely affected. See “The Exchange Offer—Consequences of Failure to Exchange.”
Risks Relating to Our Company
Our ability to service our indebtedness depends on our receipt of funds from our subsidiaries. Restrictions on their ability to loan or distribute funds to us could adversely affect our ability to service our indebtedness.
We are organized as a holding company, with all of our net sales generated through our subsidiaries. Consequently, our operating cash flow and ability to service indebtedness depend in part upon the operating cash flow of our subsidiaries, including our foreign subsidiaries, and the payment of funds by them to us in the form of loans, dividends or otherwise. Their ability to pay dividends and make loans, advances and other payments to us depends upon any statutory or other contractual restrictions that apply, which may include requirements to maintain minimum levels of working capital and other assets.
A significant portion of our revenue is generated from foreign activities; changes in exchange rates and other changes in foreign economies could have an adverse effect on our business.
We have significant operations outside the United States. Approximately 22% of our net sales and 6% of our operating cash flow for 2002 were from foreign subsidiaries. We are therefore subject to risks affecting our
13
international operations, including relevant foreign currency exchange rates, which can affect the cost of our products or the ability to sell our products in foreign markets, and the value in U.S. dollars of sales made in foreign currencies. Our net sales were increased by $2.9 million in 2002 and reduced by $10.1 million in 2001 by the impact of currency fluctuations. Other factors include our ability to obtain effective hedges against fluctuations in currency exchange rates; foreign trade, monetary and fiscal policies; laws, regulations and other activities of foreign governments, agencies, and similar organizations; risks associated with having major manufacturing facilities located in countries that have historically been less stable than the United States in several respects, including fiscal and political stability; and risks associated with an economic downturn in other countries.
In addition, world events can increase the volatility of the currency markets, and such volatility could affect our financial results. In particular, the September 11, 2001 attacks in New York and Washington, D.C. disrupted commerce throughout the United States and other parts of the world. The continued threat of similar attacks throughout the world and military action taken and to be taken by the United States and other nations in Iraq and elsewhere, as well as the threat of military confrontation on the Korean peninsula, may cause significant disruption to commerce throughout the world. In addition, severe acute respiratory syndrome (SARS) has caused disruption in commerce in East Asia and may cause significant disruption to commerce throughout the world. To the extent that such disruptions further slow the global economy or, more particularly, result in delays or cancellation of purchase orders, our business and results of operations could be materially and adversely affected. We are unable to predict whether the threat of new epidemics or attacks or the responses thereto will result in any long-term commercial disruptions or if such activities or responses will have a long-term material adverse effect on our business, results of operations, or financial condition.
The operating and financial restrictions imposed by our debt agreements, including our revolving credit facility and the indenture relating to the notes, limit our ability to finance operations and capital needs and engage in other business activities.
Our debt agreements contain covenants that restrict our ability to:
| | • | incur additional indebtedness (including guarantees); |
| | • | pay dividends and make other restricted payments; |
| | • | issue some types of preferred stock; |
| | • | enter into sale and leaseback transactions; |
| | • | make loans and investments; |
| | • | enter into new lines of business; |
| | • | enter into some leases; and |
| | • | engage in some transactions with affiliates. |
In addition, our credit facilities require us to comply with specified financial covenants including minimum interest coverage ratios, maximum leverage ratios, and minimum net worth requirements.
Our ability to meet these covenants and requirements in the future may be affected by events beyond our control, including prevailing economic, financial, and industry conditions. Our breach or failure to comply with any of these covenants could result in a default under our credit facilities or the indenture governing the notes. If we default under our credit facilities, the lenders could cease to make further extensions of credit, cause all of our
14
outstanding balances under these credit facilities to become due and payable, require us to apply all of our available cash to repay the indebtedness under these credit facilities, prevent us from making debt service payments on any other indebtedness we owe and/or proceed against the collateral granted to them to secure repayment of those amounts. If a default under the indenture occurs, the holders of the notes could elect to declare the notes immediately due and payable. If the indebtedness under our credit facilities or the notes is accelerated, we may not have sufficient assets to repay amounts due under these existing debt agreements or on other debt securities then outstanding. We also may amend the provisions and limitations of our credit facilities from time to time in a manner that could adversely affect you, as a holder of the notes, without your consent. See “Description of Other Indebtedness” and “Description of Notes.”
Our failure to keep pace with the technological demands of our customers or with the products and services offered by our competitors could significantly harm our business.
Some of the industries we serve are characterized by rapid technological changes and new product introductions. Some of our competitors may invest more heavily in research or product development than we do. Successful new product offerings depend upon a number of factors, including our ability to:
| | • | accurately anticipate customer needs; |
| | • | innovate and develop new technologies and applications; |
| | • | successfully commercialize new products in a timely manner; |
| | • | price our products competitively and manufacture and deliver our products in sufficient volumes and on time; and |
| | • | differentiate our offerings from those of our competitors. |
If we do not introduce new products in a timely manner and make enhancements to meet the changing needs of our customers, some of our products could become obsolete over time, in which case our customer relationships, revenue, and operating results would suffer.
Our operating results may suffer if the industries into which we sell our products are in downward cycles.
Some of the industries and markets into which we sell our products are cyclical. Any further downturn in our customers’ markets or in general economic conditions could result in reduced demand for our products and could harm our business.
Acquisitions have been an important part of our growth strategy; failure to successfully integrate acquisitions could adversely impact our results.
A significant portion of our growth over the past several years has been achieved through our acquisition program. Although we intend to reduce our emphasis on acquisitions, our ability to grow our business through acquisitions is subject to various factors including the cost of capital, the availability of suitable acquisition candidates at reasonable prices, competition for appropriate acquisition candidates, our ability to realize the synergies expected to result from acquisitions, our ability to retain key personnel in connection with acquisitions, and the ability of our existing personnel to efficiently handle increased transitional responsibilities resulting from acquisitions.
We may incur restructuring or impairment charges that would reduce our earnings.
We have in the past and may in the future restructure some of our operations. In such circumstances, we may take actions that would result in a charge and reduce our earnings, including as a result of our inability to dispose of discontinued operations or risks associated with discontinued operations. These restructurings have or may be undertaken to realign our subsidiaries, eliminate duplicative functions, rationalize our operating facilities
15
and products, and reduce our staff. These restructurings may be implemented to improve the operations of recently acquired subsidiaries as well as subsidiaries that have been part of our operations for many years. For a discussion of our recent restructuring activities, see “Business—Restructuring.” Additionally, on October 1, 2001 we adopted Statement of Financial Accounting Standards (SFAS) No. 142, “Goodwill and Other Intangible Assets,” which requires that goodwill and intangible assets that have an indefinite useful life be tested at least annually for impairment. We carry a very significant amount of goodwill and intangible assets and SFAS No. 142 requires us to perform an annual assessment for possible impairment.
We rely heavily upon sales to key distributors and original equipment manufacturers, and could lose sales if any of them stop doing business with us.
Our three most significant distributors represent a significant portion of our revenues. For example, sales to Fisher Scientific, VWR, and Allegiance Corporation accounted for approximately 14%, 11%, and 7% of revenues in fiscal 2002, respectively. Our reliance on major independent distributors for a substantial portion of our sales subjects our sales performance to volatility in demand from distributors. We can experience volatility when distributors merge or consolidate, when inventories are not managed to end-user demand, or when distributors experience softness in their sales. We rely primarily upon the long-standing and mutually beneficial nature of our relationships with our key distributors, rather than on contractual rights, to protect these relationships. Volatility in end-user demand can also arise with large OEM and private label customers to whom we sell directly, particularly when our customers fail to manage inventories to end-user demand, discontinue product lines, or switch business to other manufacturers. Sales to our OEM customers are sometimes unpredictable and wide variances sometimes occur quarter to quarter.
We rely heavily on manufacturing operations to produce the products we sell, and we could be injured by disruptions of our manufacturing operations.
We rely upon our manufacturing operations to produce most of the products we sell. Any significant disruption of those operations for any reason, such as strikes, labor disputes, or other labor unrest, power interruptions, fire, war, or other force majuere, could adversely affect our sales and customer relationships and therefore adversely affect our business. In particular, nearly all of the white glass used in our Clinical Group’s worldwide manufacturing operations is produced in our glass manufacturing facility in Switzerland. Disruption in this supply can result from delays encountered in connection with the periodic rebuilding of the sheet glass furnace or furnace malfunctions. Although most of our raw materials are available from a number of potential suppliers, our operations also depend upon our ability to obtain raw materials at reasonable prices.
The success of many of our products depends on the effectiveness of our patents, trademarks, and licenses to defend our intellectual property rights. If we fail to adequately defend our intellectual property rights, competitors may produce and market products similar to ours.
Our success with many of our products depends, in part, on our ability to protect our current and future innovative products and to defend our intellectual property rights. Our subsidiaries’ products are sold under a variety of trademarks and trade names. They own or license all of the trademarks and trade names we believe to be material to the operation of their businesses. We also rely upon a combination of non-disclosure and other contractual agreements and trade secret, copyright, patent, and trademark laws to protect our intellectual property rights. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. If we fail to adequately protect our intellectual property, competitors may manufacture and market products similar to ours.
We are subject to risk of product liability and other litigation which could adversely affect our business.
We are subject to the risks of claims involving our products (including those of businesses we no longer own) and other legal and administrative proceedings, including the expense of investigating, litigating, and
16
settling any claims. Although we currently maintain insurance against some of these risks, uninsured losses, which may be material, could occur.
Our business is subject to regulatory risks, and changes in regulation could adversely affect our business.
Our ability to continue manufacturing and selling those of our products that are subject to regulation by the United States Food and Drug Administration or other domestic or foreign governments or agencies is subject to a number of risks. In the future, some of our products may be affected by the passage of stricter laws or regulations, reclassification of our products into categories subject to more stringent requirements, or the withdrawal of approvals needed to sell one or more of our products. Additionally, violations of any environmental, health and safety laws or regulations, or the release of toxic or hazardous materials used in our operations into the environment could expose us to significant liability. Similarly, third party lawsuits relating to environmental and workplace safety issues could result in substantial liability.
Demand for and pricing of some of our products are affected by general levels of insurance and reimbursement and changes in the levels of insurance or reimbursement could affect the demand for such products or the prices we are able to charge.
The demand for and pricing of some of our products can be affected by changing levels of public and private health care budgets, including reimbursement by private or governmental insurance programs.
We could be harmed by the loss of key management.
The success of our operations depends in significant part upon the experience and expertise of our management team, both within Apogent and in our operating subsidiaries. Any loss of these key personnel could harm our business.
Our separation from Sybron Dental Specialties poses a potential tax sharing and indemnification risk.
In December 2000, we spun off our dental businesses which are now owned by Sybron Dental Specialties. We and Sybron Dental Specialties each agreed to indemnify the other with respect to certain indebtedness, liabilities, and obligations, including potential tax liabilities if future transactions change the tax treatment of the spin-off. Our ability to collect on these indemnities from Sybron Dental Specialties, if applicable, depends upon the future financial strength of Sybron Dental Specialties.
Our business is subject to quarterly variations in operating results due to factors outside of our control.
Our business is subject to quarterly variation in operating results caused by a number of factors, including business and industry conditions, timing of acquisitions, distribution and OEM customer issues, and other factors listed here. All these factors make it difficult to predict operating results for any particular period.
Risks Relating to the Notes
Our substantial indebtedness could adversely affect our financial health and prevent us from fulfilling our obligations under these notes.
We have now and, after the offering, will continue to have a significant amount of indebtedness. On March 31, 2003, after giving pro forma effect to the sale of the Original Notes and the recapitalization, we would have had total indebtedness of $923.7 million (of which $250.0 million would have consisted of the notes and the balance would have consisted of senior debt).
17
Our substantial indebtedness could have important consequences to you. For example, it could:
| | • | make it more difficult for us to satisfy our obligations with respect to these notes; |
| | • | increase our vulnerability to general adverse economic and industry conditions; |
| | • | reduce the availability of our cash flow to fund working capital, capital expenditures, research and development efforts, program investment efforts and other general corporate needs; |
| | • | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| | • | place us at a competitive disadvantage compared to our competitors with less debt; |
| | • | expose us to the risk of increased interest rates because some of our debt bears interest at variable rates; and |
| | • | limit our ability to borrow additional funds. |
Any default under the agreements governing our indebtedness, including a default under our revolving credit facility that is not waived by the required lenders, and the remedies sought by the holders of such indebtedness could make us unable to pay principal, premium, if any, and interest on the notes and substantially decrease the market value of the notes.
Despite current indebtedness levels, we and our subsidiaries may still be able to incur substantially more debt. This could further exacerbate the risks associated with our substantial leverage.
We and our subsidiaries may be able to incur substantial additional indebtedness in the future. The terms of the indenture do not fully prohibit us or our subsidiaries from doing so. Assuming we had completed the recapitalization on March 31, 2003, our revolving credit facility would have permitted additional borrowings of up to $470.8 million after the sale of the Original Notes and all of those borrowings would rank senior to the notes. If new debt is added to our and our subsidiaries’ current debt levels, the related risks that we and they now face would increase.
To service our indebtedness, we will require a significant amount of cash. Our ability to generate cash depends on many factors beyond our control.
Our ability to make payments on and to refinance our indebtedness, including the notes, and to fund planned capital expenditures, program investment efforts, and research and development efforts will depend on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory, and other factors that are beyond our control.
Based on our current level of operations, we believe our cash flow from operations, available cash and available borrowings under our revolving credit facility will be adequate to meet our liquidity needs for the foreseeable future.
Nevertheless, we cannot assure you that our business will generate sufficient cash flow from operations, or that future borrowings will be available to us under our revolving credit facility to enable us to pay our indebtedness or to fund other liquidity needs. Our existing $325 million 8% senior notes mature in April 2011 and our existing $300 million 2.25% Senior Convertible Contingent Debt SecuritiesSM may be put to us for redemption as early as October 2004. We may need to refinance all or a portion of our indebtedness, including the notes, on or before maturity. We cannot assure you that we will be able to refinance any of our indebtedness on commercially reasonable terms or at all.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures, sell assets, seek additional capital, or seek to restructure or
18
refinance our indebtedness, including the notes. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to sell material assets or operations to attempt to meet our debt service and other obligations. The revolving credit facility and the indenture under which Original Notes have been and the Exchange Notes will be issued restrict our ability to sell assets and use the proceeds from the sales. We may not be able to consummate those sales or to obtain the proceeds which we could realize from them and these proceeds may not be adequate to meet any debt service obligations then due.
Your right to receive payments on the notes and guarantees is unsecured and subordinate to our and our subsidiary guarantors’ existing and future secured and senior indebtedness.
The Original Notes are and the Exchange Notes will be general unsecured senior subordinated obligations of us and our subsidiary guarantors, effectively junior to any secured debt that we and our subsidiary guarantors have and may have in the future to the extent of the value of the assets securing that debt. Payment on the notes and the guarantees will be subordinated in right of payment to all of our and our subsidiary guarantors’ senior debt.
As a result, upon any distribution to our creditors in a bankruptcy, liquidation or reorganization or similar proceeding relating to us or our property, the holders of our senior debt will be entitled to be paid in full and before any payment may be made on the notes. In addition, all payments on the notes will be blocked in the event of a payment default on senior debt and may be blocked for up to 179 of 360 consecutive days in the event of certain non-payment defaults of senior debt.
In the event of a bankruptcy, liquidation or reorganization or similar proceeding related to us, holders of the notes will participate with trade creditors and all other holders of our subordinated indebtedness in the assets remaining after we have paid all of our senior debt. However, because the indenture requires that amounts otherwise payable to holders of the notes in a bankruptcy or similar proceeding be paid to holders of senior debt instead, holders of the notes may receive less, ratably, than holders of trade payables in any such proceeding. In any of these cases, we may not have sufficient funds to pay all of our creditors and holders of notes may receive less, ratably, than the holders of our senior debt and other claimants.
Assuming we had completed the recapitalization on March 31, 2003, the Original Notes and the Exchange Notes would have been subordinated to $673.7 million of senior debt and approximately $470.8 million would have been available for borrowing as additional senior debt under our revolving credit facility.
Not all of our subsidiaries are guarantors, and your claims will be effectively subordinated to all of the creditors of our non-guarantor subsidiaries.
Many, but not all, of our direct and indirect subsidiaries will guarantee the notes. In the event of a bankruptcy, liquidation or reorganization of any of our non-guarantor subsidiaries, holders of their indebtedness and their trade creditors will generally be entitled to payment of their claims from the assets of those non-guarantor subsidiaries before any assets of the non-guarantor subsidiaries are made available for distribution to us. Assuming the recapitalization and the sale of the Original Notes were completed on March 31, 2003, the Original Notes and the Exchange Notes would have been effectively junior to $47.1 million of indebtedness and other liabilities (including trade payables) of these non-guarantor subsidiaries. The non-guarantor subsidiaries generated 22% of our net sales, generated operating income of $36.6 million, and generated cash provided by operating activities of $11.2 million for the year ended September 30, 2002. The non-guarantor subsidiaries held 20% of our consolidated total assets as of September 30, 2002.
The guarantees may be unenforceable due to fraudulent conveyance statutes. Accordingly, you could have no claim against the guarantors.
Our obligations under the notes have been and will be guaranteed on a general unsecured senior subordinated basis by our subsidiary guarantors. The guarantees may be subject to review under U.S. federal
19
bankruptcy law and comparable provisions of state fraudulent conveyance laws in bankruptcy or similar proceeding. Although laws differ among various jurisdictions, a court could, under fraudulent conveyance laws, further subordinate or avoid the guarantees if it found that the guarantees were incurred with actual intent to hinder, delay, or defraud creditors, or the guarantor was a defendant in an action for money damages or had a judgment for money damages docketed against it if, in either case, after final judgment, the judgment is unsatisfied, or the guarantor did not receive fair consideration or reasonably equivalent value for the guarantees and that the guarantor:
| | • | was insolvent or rendered insolvent by reason of the issuance of the guarantees; |
| | • | was engaged or was about to engage in a business or transaction for which the remaining assets of the guarantor constituted unreasonably small capital; or |
| | • | intended to incur, or believed that it would incur, debts beyond its ability to pay debts as they matured. |
If a court voided a guarantee by one or more of our subsidiaries as the result of a fraudulent conveyance, or held it unenforceable for any other reason, holders of the notes would cease to have a claim against the subsidiary based on the guarantee and would be creditors only of Apogent and any guarantor whose guarantee was not similarly held unenforceable.
We cannot assure you that a court would conclude that the notes and the subsidiary guarantees issued concurrently with the notes are incurred for proper purposes and in good faith. We also cannot assure you that a court would conclude that, after giving effect to indebtedness incurred in connection with the issuance of the notes and the issuance of the subsidiary guarantees, Apogent and the subsidiary guarantors are solvent and will continue to be solvent, will have sufficient capital for carrying their respective businesses and will be able to pay their debts as they become absolute and mature.
We may not be able to repurchase the notes upon a change of control.
Upon a “change of control,” as defined in the indenture, we will be required under certain circumstances to make an offer to repurchase the notes at a price equal to 101% of their principal amount, together with any accrued and unpaid interest and liquidated damages to the date of repurchase. If a change of control were to occur, there can be no assurance that we would have sufficient funds to pay the purchase price for all of the notes that we might be required to purchase. Our failure to purchase, or give notice of purchase of, the notes would be a default under the indenture, which would in turn be a default under our revolving credit facility and other long-term debt. In addition, a change of control may constitute an event of default under our revolving credit facility and other long-term debt and the terms of our revolving credit facility presently would prohibit any repurchase of the notes. A default under our revolving credit facility would result in an event of default under the indenture governing the notes if the lenders were to accelerate the debt under our revolving credit facility. If the foregoing occurs, we may not have enough assets to satisfy all obligations under our revolving credit facility and the indenture related to the notes.
If an active trading market does not develop for these notes you may not be able to resell them.
There is currently no public market for the Original Notes. The Exchange Notes are a new issue of securities with no established trading market. We do not intend to apply for listing of the notes on any securities exchange. We have been informed by the initial purchasers that they currently intend to make a market in the notes. However, the initial purchasers may cease their market-making at any time without notice. Any such market-making will be subject to the limitations imposed by the Securities Act and the Exchange Act and may be limited during the Exchange Offer. The Original Notes are eligible for trading in the PORTAL Market of the National Association of Securities Dealers, Inc., but the Exchange Notes will not be eligible for trading in that market. The Original Notes have not been registered under the Securities Act or any state securities laws and, unless registered, may not be sold except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act and applicable state securities laws.
20
We cannot assure you that an active trading market will develop for the notes. If no active trading market develops, you may not be able to resell your notes at their fair market value or at all. Future trading prices of the notes will depend on many factors, including, among other things:
| | • | our ability to effect the Exchange Offer; |
| | • | prevailing interest rates; |
| | • | our operating results; and |
| | • | the market for similar securities. |
21
THE EXCHANGE OFFER
Purposes and Effects of the Exchange Offer
We sold the Original Notes on June 2, 2003 to the initial purchasers, who resold the Original Notes to “qualified institutional buyers” (as defined in Rule 144A under the Securities Act) in a private offering and outside the United States to non-U.S. investors in reliance upon Regulation S under the Securities Act. In connection with the sale of the Original Notes, we and the initial purchasers entered into a registration rights agreement dated as of June 2, 2003 (the “registration rights agreement”) pursuant to which we agreed to file with the SEC a registration statement (the “Registered Exchange Offer Registration Statement”) with respect to an offer to exchange the Original Notes for Exchange Notes within 90 days following the issuance of the Original Notes (the “Issue Date”). In addition, we agreed to use our best efforts to cause the Exchange Offer Registration Statement to become effective under the Securities Act within 150 days after the Issue Date and to offer the Exchange Notes pursuant to the Exchange Offer. A copy of the registration rights agreement has been filed as an exhibit to the registration statement of which this prospectus is a part.
This prospectus is a part of the Exchange Offer Registration Statement that we have filed with the SEC. The Exchange Offer is being made pursuant to the registration rights agreement to satisfy our obligations thereunder. You are a “holder” with respect to the Exchange Offer if your Original Notes are registered in your name on Apogent’s books or if you have obtained a properly completed bond power from the registered holder, or any person whose Original Notes are held of record by the Depository. Upon completion of the Exchange Offer we generally will not be required to file any registration statement to register any outstanding Original Notes. If you do not tender your Original Notes or your Original Notes are tendered but not accepted, you generally will have to rely on exemptions to registration requirements under the securities laws, including the Securities Act, if you wish to sell your Original Notes.
Based on interpretations by the staff of the SEC set forth in no-action letters issued to third parties unrelated to Apogent, we believe that you may offer for resale, resell or otherwise transfer the Exchange Notes issued to you, unless you are an “affiliate” of Apogent within the meaning of Rule 405 under the Securities Act and except as set forth in the next paragraph, without compliance with the registration and prospectus delivery provisions of the Securities Act, provided that you acquire the Exchange Notes in the ordinary course of business and you are not engaged, do not intend to engage, and have no arrangement or understanding with any person to engage, in the distribution of the Exchange Notes.
If you participate in the Exchange Offer for the purpose of distributing securities in a manner not permitted by the SEC’s interpretation, (a) the position of the staff of the SEC enunciated in the interpretive letters is inapplicable to you and (b) you are required to comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale transaction. Each broker-dealer that receives Exchange Notes for its own account as a result of market-making activities or other trading activities must acknowledge that it will deliver a prospectus in connection with any resale of the Exchange Notes. See “Plan of Distribution.”
The Exchange Offer is not being made to you in any jurisdiction, nor may you participate in the Exchange Offer in any jurisdiction, in which the Exchange Offer or the acceptance thereof would not be in compliance with the securities laws of that jurisdiction. Prior to the Exchange Offer, however, we will use our best efforts to register or qualify the Exchange Notes for offer and sale under the securities or laws of any jurisdictions necessary to permit completion of the Exchange Offer and do any and all other acts or things necessary or advisable to enable the offer and sale of the Exchange Notes in those jurisdictions.
Terms of the Exchange Offer
Upon the terms and subject to the conditions set forth in this prospectus and in the accompanying Letter of Transmittal, we will accept any and all Original Notes validly tendered prior to 5:00 p.m., New York City time,
22
on the Expiration Date (as defined). We will issue up to $250 million aggregate principal amount of Exchange Notes in exchange for a like principal amount of outstanding Original Notes that are validly tendered and accepted in the Exchange Offer. Subject to the conditions of the Exchange Offer described below, we will accept any and all Original Notes that are validly tendered. You may tender some or all of your Original Notes pursuant to the Exchange Offer.
The Exchange Offer is not conditioned upon any number of Original Notes being tendered.
The form and terms of the Exchange Notes will be the same in all material respects as the form and terms of Original Notes, except that the Exchange Notes will be registered under the Securities Act and hence will not bear legends restricting their transfer. The Exchange Notes will not represent additional indebtedness of Apogent and will be entitled to the benefits of the indenture, which is the same indenture under which the Original Notes were issued.
Interest on the Exchange Notes will accrue from the most recent date to which interest has been paid on the Original Notes or, if no interest has been paid, from June 2, 2003. Accordingly, registered holders of Exchange Notes on the relevant record date for the first interest payment date following the consummation of the Exchange Offer will receive interest accruing from the most recent date to which interest has been paid or, if no interest has been paid, from June 2, 2003. Original Notes accepted for exchange will cease to accrue interest from and after the date the Exchange Offer closes. If your Original Notes are accepted for exchange, you will not receive any payment in respect of interest on the Original Notes otherwise payable on any interest payment date the record date for which occurs on or after completion of the Exchange Offer.
You do not have any appraisal or dissenters’ rights under the indenture in connection with the Exchange Offer. We intend to conduct the Exchange Offer in accordance with the provisions of the registration rights agreement. If you do not tender for exchange or if your tender is not accepted, the Original Notes will remain outstanding and you will be entitled to the benefits of the indenture, but generally will not be entitled to any registration rights under the registration rights agreement.
We will be deemed to have accepted validly tendered Original Notes when, as and if we have given oral or written notice of acceptance to the Exchange Agent for the Exchange Offer. The Exchange Agent will act as agent for the tendering holders for the purpose of receiving the Exchange Notes from Apogent.
If any tendered Original Notes not accepted for exchange because of an invalid tender, the occurrence of certain other events set forth herein or otherwise, we will return the certificates (if any) for the unaccepted Original Notes to the tendering holder of that note, without expense, as promptly as practicable after the Expiration Date.
If you tender your Original Notes in the Exchange Offer, you will not be required to pay brokerage commissions or fees or, subject to the instructions in the Letters of Transmittal, transfer taxes with respect to the exchange of Original Notes pursuant to the Exchange Offer. Apogent will pay all charges and expenses, other than certain applicable taxes described below, in connection with the Exchange Offer. See “Fees and Expenses.”
Conditions of the Exchange Offer
Notwithstanding any other term of the Exchange Offer, Apogent will not be required to accept for exchange any Original Notes tender for Exchange Notes and may terminate or amend the Exchange Offer as provided herein before the acceptance of any Original Notes, if any of the following conditions exist:
(a) any action or proceeding is instituted or threatened in any court or by or before any governmental agency or regulatory authority with respect to the Exchange Offer which, in Apogent’s judgment, could reasonably be expected to materially impair our ability to proceed with the Exchange Offer or have a material adverse effect on the contemplated benefits of the Exchange Offer; or
23
(b) there shall have been proposed, adopted or enacted any law, statute, rule, regulation or order which, in Apogent’s judgment, could reasonably be expected to materially impair our ability to proceed with the Exchange Offer or have a materially adverse effect on the contemplated benefits of the Exchange Offer.
The foregoing conditions are for Apogent’s sole benefit and may be asserted regardless of the circumstances giving rise to the conditions or may be waived by us in whole or in part at any time and from time to time. If we waive or amend the foregoing conditions, we will, if required by applicable law, extend the Exchange Offer for a minimum of five business days from the date that we first give notice, by public announcement or otherwise, of such waiver of amendment, if the Exchange Offer would otherwise expire within that five business-day period. Any determination by Apogent concerning the events described above will be final and binding upon all parties.
Expiration Date; Extension; Termination; Amendments
The Exchange Offer will expire at 5:00 p.m., New York City time, on , 2003, unless extended (the “Expiration Date”). We reserve the right to extend the Exchange Offer at our discretion, in which event the term “Expiration Date” shall mean the time and date on which the Exchange Offer as so extended shall expire. We will notify the Exchange Agent of any extension by oral or written notice and will make a public announcement thereof, each prior to 9:00 a.m., New York City time, on the next business day after the previously scheduled Expiration Date.
We reserve the right, in our sole discretion, to:
| | • | delay accepting for exchange any Original Notes for any Exchange Notes or to extend or terminate the Exchange Offer and not accept for exchange any Original Notes for any Exchange Notes if any of the events set forth under the caption “Conditions of the Exchange Offer” shall have occurred and shall not have been waived by giving oral or written notice of the delay or termination to the Exchange Agent, or |
| | • | amend the terms of the Exchange Offer in any manner. |
Any delay in acceptance for exchange, extension or amendment will be followed as promptly as practicable by a public announcement of the delay. If we amend the Exchange Offer in a manner we determine constitutes a material change, we will promptly disclose the amendment in a manner reasonably calculated to inform the holders of Exchange Notes of the amendment and we will extend the Exchange Offer for a period of five to ten business days, depending upon the significance of the amendment and the manner of disclosure to the holders of the Exchange Notes, if the Exchange Offer would otherwise expire during that five to ten business-day period. The rights we have reserved in this paragraph are in addition to our rights set forth under the caption “Conditions of the Exchange Offer.”
Procedures For Tendering
Only a holder of Original Notes may tender them in the Exchange Offer. To tender in the Exchange Offer, you must complete, sign and date the Letter of Transmittal, or a facsimile of it, have the signatures thereon guaranteed if required by the Letter of Transmittal, and mail or otherwise deliver the Letter of Transmittal or the facsimile, together with the Original Notes (unless such tender is being effected pursuant to the procedure for book-entry transfer described below) and any other required documents, to the Exchange Agent prior to 5:00 p.m., New York City time, on the Expiration Date.
Any financial institution that is a participant in the Depositary’s Book-Entry Transfer Facility system may make book-entry delivery of the Original Notes by causing the Depositary to transfer the Original Notes into the Exchange Agent’s account in accordance with the Depositary’s procedure for transfer. Although delivery of Original Notes may be effected through book-entry transfer into the Exchange Agent’s account at the Depositary, the Letter of Transmittal (or facsimile thereof), with any required signature guarantees and any other required documents, must, in any case, be transmitted to and received or confirmed by the Exchange Agent at its
24
addresses set forth in “Exchange Agent” below prior to 5:00 p.m., New York City time, on the Expiration Date.
Delivery of documents to the Depositary in accordance with its procedures does not constitute delivery to the Exchange Agent.
If you tender an Original Note, and do not validly withdraw your tender, your actions will constitute an agreement with us in accordance with the terms and subject to the conditions set forth in this prospectus and in the Letter of Transmittal.
The method of delivery of your Original Notes and the Letter of Transmittal and all other required documents to the Exchange Agent is at your election and risk. Instead of delivery by mail, we recommend that you use an overnight or hand delivery service. In all cases, you should allow sufficient time to assure delivery to the Exchange Agent before the Expiration Date. No Letter of Transmittal or Original Note should be sent to Apogent; instead, they should be sent to the Exchange Agent. You may request that your broker, dealer, commercial bank, trust company or nominee effect the tender for you.
Signatures on a Letter of Transmittal or a notice of withdrawal, as the case may be, must be guaranteed by an Eligible Institution (as defined below) unless the Original Notes are being tendered (a) by a registered holder who has not completed the box entitled “Special Issuance Instructions” or “Special Delivery Instructions” on the Letter of Transmittal, or (b) for the account of an Eligible Institution. If signatures on a Letter of Transmittal or a notice of withdrawal, as the case may be, are required to be guaranteed, the guarantee must be by a member of a signature guarantee program within the meaning of Rule 17Ad-15 under the Exchange Act (an “Eligible Institution”).
If the Letter of Transmittal or any Original Notes or bond powers are signed by trustees, executors, administrators, guardians, attorneys-in-fact, officers of corporations or others acting in a fiduciary or representative capacity, those persons should so indicate when signing, and unless waived by Apogent, evidence satisfactory to Apogent of their authority to act must be submitted with the Letter of Transmittal.
We will determine, in our sole discretion, all questions as to the validity, form, eligibility (including time of receipt) and acceptance and withdrawal of tendered Original Notes. Our determination will be final and binding. We reserve the absolute right to reject any and all Original Notes not properly tendered or any Original Notes our acceptance of which would, in the opinion of our counsel, be unlawful. We also reserve the right to waive any defects, irregularities or conditions of tender as to particular Original Notes. Apogent’s interpretation of the terms and conditions of the Exchange Offer (including the instructions in the Letter of Transmittal) will be final and binding on all parties.
Unless waived, you must cure any defects or irregularities in connection with tenders of your Original Notes within a time period we will determine. Although we intend to request that the Exchange Agent notify you of defects or irregularities with respect to your tender of Original Notes, neither Apogent, the Exchange Agent nor any other person will incur any liability for failure to give you any notification. Tenders of Original Notes will not be deemed to have been made until any defects or irregularities have been cured or waived. Any Original Notes received by the Exchange Agent that are not properly tendered and as to which the defects or irregularities have not been cured or waived will be returned by the Exchange Agent to the tendering holders, unless otherwise provided in the Letter of Transmittal, as soon as practicable following the Expiration Date.
In addition, we reserve the right in our sole discretion (subject to the limitations contained in the indenture) (a) to purchase or make offers for any Original Notes that remain outstanding subsequent to the Expiration Date and (b) to the extent permitted by applicable law, to purchase Original Notes in the open market, in privately negotiated transactions or otherwise. The terms of any purchases or offers could differ from the terms of the Exchange Offer.
By tendering, you will be representing to Apogent, among other things, that:
(1) you are obtaining the Exchange Notes in the ordinary course of business whether or not you are the holder,
25
(2) you are not engaged in, do not intend to engage in, and do not have an arrangement or understanding with any person to participate in the distribution of the Exchange Notes, and
(3) you are not an “affiliate,” as defined in Rule 405 under the Securities Act, of Apogent or the guarantor subsidiaries or, if you are an affiliate of Apogent or the guarantor subsidiaries, that you will comply with the registration and prospectus delivery requirements of the Securities Act, to the extent applicable.
If you are a broker-dealer that will receive Exchange Notes for your own account in exchange for Original Notes that were acquired as a result of market-making activities or other trading activities, you must acknowledge that you will deliver a prospectus in connection with any resale of the Exchange Notes.
Guaranteed Delivery Procedures
If you wish to tender your Original Notes and (1) your Original Notes are not immediately available, or (2) you cannot deliver your Original Notes and other required documents to the Exchange Agent, or cannot complete the procedure for book-entry transfer prior to the Expiration Date, you may effect a tender if:
(a) you make a tender through an Eligible Institution;
(b) prior to the Expiration Date, the Exchange Agent receives from the Eligible Institution a properly completed and duly executed Notice of Guaranteed Delivery (by facsimile transmission, mail or hand delivery) setting forth your name and address, the certificate number(s) of the Original Notes (if available) and the principal amount of Original Notes tendered together with a duly executed Letter of Transmittal (or a facsimile thereof), stating that the tender is being made thereby and guaranteeing that, within three business days after the Expiration Date, the certificate(s) representing the Original Notes to be tendered in proper form for transfer (or a confirmation of a book-entry transfer into the Exchange Agent’s account at the Depositary of Original Notes delivered electronically) and any other documents required by the Letter of Transmittal will be deposited by the Eligible Institution with the Exchange Agent; and
(c) the certificate(s) representing all tendered Original Notes in proper form for transfer (or confirmation of a book-entry transfer into the Exchange Agent’s account at the Depositary of Original Notes delivered electronically) and all other documents required by the Letter of Transmittal are received by the Exchange Agent within three business days after the Expiration Date.
Upon request to the Exchange Agent, you will be sent a Notice of Guaranteed Delivery if you wish to tender your Original Notes according to the guaranteed delivery procedures set forth above.
Withdrawal of Tenders
Except as otherwise provided herein, you may withdraw any tenders of Original Notes at any time prior to 5:00 p.m., New York City time, on the Expiration Date, unless previously accepted for exchange.
For your withdrawal to be effective, the Exchange Agent must receive a written or facsimile transmission notice of withdrawal at its address set forth herein prior to 5:00 p.m., New York City time, on the Expiration Date, and prior to acceptance for exchange thereof by Apogent. Any notice of withdrawal must:
(a) specify the name of the person having deposited the Original Notes to be withdrawn (the “Depositor”),
(b) identify the Original Notes to be withdrawn (including the certificate number or numbers, if applicable, and principal amount of the Original Notes),
(c) be signed by the Depositor in the same manner as the original signature on the Letter of Transmittal by which the Original Notes were tendered (including any required signature guarantees) or be accompanied
26
by documents of transfer sufficient to have the Trustee with respect to the Original Notes register the transfer of the Original Notes into the name of the person withdrawing the tender, and
(d) specify the name in which any Original Notes are to be registered, if different from that of the Depositor.
We will determine all questions as to the validity, form and eligibility (including time of receipt) of withdrawal notices. This determination shall be final and binding on all parties. Any Original Notes so withdrawn will be deemed not to have been validly tendered for purposes of the Exchange Offer and no Exchange Notes will be issued with respect to them unless the Original Notes so withdrawn are validly re-tendered. Any Original Notes which have been tendered but which are not accepted for exchange or which are withdrawn will be returned to you, without cost, as soon as practicable after withdrawal, rejection of tender or termination of the Exchange Offer. You may re-tender properly withdrawn Original Notes by following one of the procedures described above under “Procedures for Tender” at any time prior to the Expiration Date.
Fees and Expenses
The expenses of soliciting tenders pursuant to the Exchange Offer will be borne by Apogent. The principal solicitation for tenders pursuant to the Exchange Offer is being made by mail; however, additional solicitation may be made by telegraph, telephone or in person by officers and regular employees of Apogent and its affiliates.
Apogent has not retained any dealer-manager in connection with the Exchange Offer and will not make any payments to brokers, dealers or others soliciting acceptances of the Exchange Offer. However, Apogent will pay the Exchange Agent reasonable and customary fees for its services and will reimburse it for its reasonable out-of-pocket expenses in connection therewith. Apogent may also pay brokerage houses and other custodians, nominees and fiduciaries the reasonable out-of-pocket expenses incurred by them in forwarding copies of this prospectus, Letters of Transmittal and related documents to the beneficial owners of the Original Notes and in handling or forwarding tenders for exchange. Apogent will pay the other expenses to be incurred in connection with the Exchange Offer, including fees and expenses of the Trustee, accounting and legal fees and printing costs.
Apogent will pay all transfer taxes, if any, applicable to the exchange of Original Notes pursuant to the Exchange Offer. If, however, certificates representing Exchange Notes or Original Notes for principal amounts not tendered or accepted for exchange are to be delivered to, or are to be issued in the name of, any person other than the registered holder of the Original Notes tendered, or if tendered Original Notes are registered in the name of any person other than the person signing the Letter of Transmittal, or if a transfer tax is imposed for any reason other than the exchange of Original Notes pursuant to the Exchange Offer, then the amount of any transfer taxes (whether imposed on the registered holder or any other persons) will be payable by the tendering holder. If satisfactory evidence of payment of any taxes or exemption therefrom is not submitted with the Letter of Transmittal, the amount of any transfer taxes will be billed directly to the tendering holder.
Resale of Exchange Notes
Based on an interpretation by the staff of the SEC set forth in no-action letters issued to third parties, we believe that, unless you are a broker-dealer or an affiliate of Apogent or a guarantor subsidiary, you may offer for resale, resell or otherwise transfer the Exchange Notes issued to you pursuant to the Exchange Offer without compliance with the registration and prospectus delivery provisions of the Securities Act, provided that you acquire the Exchange Notes in the ordinary course business and you do not intend to participate and have no arrangement or understanding with any person to participate in the distribution of the Exchange Notes. If you tender in the Exchange Offer with the intention to participate, or for the purpose of participating, in a distribution of the Exchange Notes, you may not rely on the position of the staff of the SEC enunciated inExxon Capital Holdings Corporation (available May 13, 1988) andMorgan Stanley & Co., Incorporated (available June 5, 1991), or similar no-action letters, but rather must comply with the registration and prospectus delivery
27
requirements of the Securities Act in connection with any resale transaction. In addition, any such resale transaction should be covered by an effective registration statement containing the selling security holders information required by Item 507 of Regulation S-K of the Securities Act. Each broker-dealer that receives Exchange Notes for its own account in exchange for Original Notes, where the Original Notes were acquired by the broker-dealer as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of the Exchange Notes. See “Plan of Distribution.”
By tendering in the Exchange Offer, you will represent to Apogent that, among other things:
(1) you are obtaining the Exchange Notes in the ordinary course of business,
(2) you do not have an arrangement or understanding with any person to participate in the distribution of the Exchange Notes, and
(3) you acknowledge that if you participate in the Exchange Offer for the purpose of distributing the Exchange Notes,
(a) you must, in the absence of an exemption therefrom, comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale of the Exchange Notes and cannot rely on the no-action letters referenced above, and
(b) your failure to comply with those requirements could result in you incurring liability under the Securities Act for which your are not indemnified by Apogent.
Further, by tendering in the Exchange Offer, each holder that may be deemed an “affiliate” (as defined under Rule 405 of the Securities Act) of Apogent or any subsidiary guarantor will represent to Apogent that the holder understands and acknowledges that the Exchange Notes may not be offered for resale, resold or otherwise transferred by that holder without registration under the Securities Act or an exemption therefrom.
As set forth above, affiliates of Apogent are not entitled to rely on the foregoing interpretations of the staff of the SEC with respect to resales of the Exchange Notes without compliance with the registration and prospectus delivery requirements of the Securities Act.
Consequences of Failure to Exchange
As a result of making of this Exchange Offer, Apogent will have fulfilled one of its obligations under the registration rights agreement. You generally will not have any further registration rights under the registration rights agreement or otherwise if you do not tender your Original Notes. Accordingly, if you do not exchange your Original Notes for Exchange Notes, you will continue to hold your Original Notes and will be entitled to all the rights and limitations applicable thereto under the indenture, except to the extent of those rights or limitations that, by their terms, terminate or cease to have further effectiveness as a result of the Exchange Offer (including the right to receive additional payments, under certain circumstances, as Liquidated Damages).
The Original Notes that are not exchanged for Exchange Notes pursuant to the Exchange Offer will remain restricted securities. Accordingly, you may only resell the Original Notes
(a) to Apogent (upon redemption thereof or otherwise),
(b) pursuant to an effective registration statement under the Securities Act,
(c) so long as the Original Notes are eligible for resale pursuant to Rule 144A, to a qualified institutional buyer within the meaning of Rule 144A under the Securities Act in a transaction meeting the requirements of 144A,
(d) outside the United States to a foreign person pursuant to the exemption from the registration requirements of the Securities Act provided by Regulation S thereunder,
28
(e) to an institutional accredited investor that, prior to such transfer, furnishes to the Trustee a signed letter containing certain representations and agreements relating to the restrictions on transfer of the Original Notes evidenced thereby (the form of which letter can be obtained from the Trustee), or
(f) pursuant to another available exemption from the registration requirements of the Securities Act,
in each case in accordance with any applicable securities laws of any state of the United States.
Accordingly, if any Original Notes are tendered and accepted in the Exchange Offer, the trading market for the untendered Original Notes could be adversely affected. See “Termination of Certain Rights.”
Termination of Certain Rights
You will not be entitled to certain rights under the registration rights agreement following the completion of the Exchange Offer. The rights that generally will terminate are the rights (a) to have Apogent file with the SEC and use its best efforts to have declared effective a shelf registration statement to cover resales of the Original Notes by the holders thereof and (b) to receive additional payments as Liquidated Damages if the registration statement of which this prospectus is a part or the shelf registration statement are not filed with, or declared effective by, the SEC within certain specified time periods or the Exchange Offer is not consummated within a specified time period.
Other
Participation in the Exchange Offer is voluntary and you should carefully consider whether to accept. You are urged to consult your financial and tax advisors in making your decision on what action to take.
No person has been authorized to give any information or to make any representations in connection with the Exchange Offer other than those contained in this prospectus. If given or made, that information or those representations should not be relied upon as having been authorized by us. Neither the delivery of this prospectus nor any exchange made pursuant to the Exchange Offer will, under any circumstances, create any implication that there has been no change in the affairs of Apogent or its subsidiaries since the respective dates as of which the information contained in this prospectus is given. The Exchange Offer is not being made to (nor will tender be accepted from or on behalf of) holders of Original Notes in any jurisdiction in which the making of the Exchange Offer or the acceptance thereof would not be in compliance with the laws of such jurisdiction. However, Apogent intends to take any action it deems necessary to make the Exchange Offer in any jurisdiction and to extend the Exchange Offer to holders of Original Notes in that jurisdiction.
Apogent may in the future seek to acquire Original Notes or Exchange Notes in open market or privately negotiated transactions, through subsequent exchange offers or otherwise. We have no present plans to acquire any Original Notes that are not tendered in the Exchange Offer or to file a registration statement to permit resales of any Original Notes except to the extent that we may be required to do so under the registration rights agreement.
Accounting Treatment
The Exchange Notes will be recorded at the same carrying value as the Original Notes, as reflected in our accounting records on the date of the exchange. Accordingly, we will not recognize any gain or loss for accounting purposes upon the completion of the Exchange Offer. The expenses of the Exchange Offer will be amortized over the term of the Exchange Notes under generally accepted accounting principles.
29
Exchange Agent
The Bank of New York, New York, New York has been appointed as Exchange Agent for the Exchange Offer. All correspondence in connection with the Exchange Offer and the Letter of Transmittal should be addressed to the Exchange Agent, as follows:
By Hand or Overnight Courier/Mail: | | Facsimile Transmission: |
| |
The Bank of New York | | (212) 815-6339 |
Corporate Trust Services Window | | (For Eligible Institutions Only) |
101 Barclay Street, Ground Level | | |
New York, NY 10286 | | Confirm by Telephone: |
Attn: Mr. Duong Nguyen | | (212) 815-3687 |
| |
By Registered or Certified Mail: | | |
| |
The Bank of New York | | |
Reorganization Unit | | |
101 Barclay Street, 7E | | |
New York, NY 10286 | | |
Attn: Mr. Duong Nguyen | | |
Requests for additional copies of this prospectus or the Letter of Transmittal should be directed to the Exchange Agent.
30
USE OF PROCEEDS
The Exchange Offer is intended to satisfy certain of our obligations under the registration rights agreement. We will not receive any cash proceeds from the issuance of the Exchange Notes offered in this prospectus. In consideration for issuing the Exchange Notes contemplated by this prospectus, we will receive the Original Notes in like principal amount, the form and terms of which are substantially the same as the form and terms of the Exchange Notes (which replace the Original Notes, except as otherwise described herein, and which represent the same indebtedness). The Original Notes surrendered in exchange for the Exchange Notes will be retired and canceled and cannot be reissued. Accordingly, the issuance of the Exchange Notes will not result in any increase or decrease in our indebtedness.
We received approximately $240.3 million of net proceeds from the offering of the Original Notes (after deducting the discounts, fees and offering expenses). We used or intend to use the net proceeds from that offering to repurchase approximately 6.0 million of our common shares pursuant to a modified Dutch auction tender offer at a price of $17.50 per share, to repay the outstanding balance under our revolving credit facility, to repurchase our common shares in the open market or otherwise, and for general corporate purposes.
RATIO OF EARNINGS TO FIXED CHARGES
The following table sets forth our historical ratio of earnings to fixed charges for Apogent for the periods indicated:
| | | Years ended September 30,
| | Six months ended March 31,
|
| | | 2002
| | 2001
| | 2000
| | 1999
| | 1998
| | 2003
| | 2002
|
| | | | | | | | | | | (unaudited) | | (unaudited) |
Ratio of earnings to fixed charges(1) | | 5.2x | | 4.2x | | 3.5x | | 3.7x | | 3.3x | | 4.8x | | 4.9x |
| (1) | The ratio of earnings to fixed charges is computed by dividing: |
| | • | income from continuing operations before income taxes and extraordinary item plus fixed charges, by |
Fixed charges consist of interest expense, amortization of deferred financing fees and an estimate of interest within rental expense.
The unaudited pro forma ratio of earnings to fixed charges for the year ended September 30, 2002 and the six months ended March 31, 2003 was 4.2 and 3.9, respectively. This ratio has been prepared assuming that the sale of the notes was completed on October 1, 2001.
31
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2003, on an actual basis and as adjusted to reflect the recapitalization, including the issuance of the Original Notes. This table should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical financial statements and the related notes included elsewhere in this prospectus.
| | | As of March 31, 2003
| |
| | | Actual
| | | As Adjusted(1)
| |
| | | (unaudited) (in millions) | |
Cash and cash equivalents | | $ | 17.4 | | | $ | 112.3 | |
| | |
|
|
| |
|
|
|
Total debt: | | | | | | | | |
Revolving credit facility | | $ | 62.7 | | | $ | 22.7 | |
8% senior notes, net of discount | | | 323.7 | | | | 323.7 | |
2.25% senior convertible contingent debt | | | 300.0 | | | | 300.0 | |
Sellers’ notes | | | 1.6 | | | | 1.6 | |
Sale/leaseback obligation | | | 11.2 | | | | 11.2 | |
Obligations under capital leases and other | | | 14.5 | | | | 14.5 | |
6 1/2% senior subordinated notes | | | — | | | | 250.0 | |
| | |
|
|
| |
|
|
|
Total debt | | $ | 713.7 | | | $ | 923.7 | |
Shareholders’ equity: | | | | | | | | |
Preferred stock, $.01 par value | | | — | | | | — | |
Common stock, $.01 par value | | | 1.1 | | | | 1.0 | |
Equity rights, 50 rights at $1.09 per right | | | — | | | | — | |
Additional paid-in capital | | | 270.6 | | | | 270.6 | |
Retained earnings | | | 721.1 | | | | 721.1 | |
Accumulated other comprehensive loss | | | (13.5 | ) | | | (13.5 | ) |
Treasury common stock, 4,824,595 shares and 10,846,109 shares, as adjusted, at cost | | | (84.0 | ) | | | (189.3 | ) |
| | |
|
|
| |
|
|
|
Total shareholders’ equity | | | 895.3 | | | | 789.9 | |
| | |
|
|
| |
|
|
|
Total capitalization | | $ | 1,609.0 | | | $ | 1,713.6 | |
| | |
|
|
| |
|
|
|
| (1) | Reflects the receipt and application of the net proceeds from the sale of the Original Notes described under “Use of Proceeds.” |
32
SELECTED CONSOLIDATED FINANCIAL DATA
The following table presents our selected historical consolidated financial information. The financial information for the six month periods ended March 31, 2003 and 2002 is derived from our unaudited financial statements which, in the opinion of our management, contain all adjustments necessary for a fair presentation of this information. The financial information for the six months ended March 31, 2003 is not necessarily indicative of the results expected for the full year. The financial information for each of the years in the five year period ended September 30, 2002 is derived from our audited financial statements. The financial information set forth below should be read in conjunction with our consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” all included elsewhere in this prospectus.
| | | Six Months Ended March 31,
| | | Year Ended September 30,
| |
| | | 2003
| | | 2002
| | | 2002
| | | 2001
| | | 2000
| | | 1999(1)
| | | 1998(1)
| |
| | | (unaudited) | | | (dollar amounts in thousands) | | | (unaudited) | |
Consolidated Statement of Income Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net sales | | $ | 534,436 | | | $ | 489,062 | | | $ | 1,027,913 | | | $ | 938,819 | | | $ | 843,567 | | | $ | 696,621 | | | $ | 562,499 | |
Total cost of sales | | | 278,366 | | | | 250,119 | | | | 522,019 | | | | 471,318 | | | | 426,004 | | | | 357,840 | | | | 288,186 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | 256,070 | | | | 238,943 | | | | 505,894 | | | | 467,501 | | | | 417,563 | | | | 338,781 | | | | 274,313 | |
Total selling, general and administrative expenses | | | 140,272 | | | | 123,536 | | | | 257,954 | | | | 256,485 | | | | 234,018 | | | | 177,710 | | | | 152,918 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | 115,798 | | | | 115,407 | | | | 247,940 | | | | 211,016 | | | | 183,545 | | | | 161,071 | | | | 121,395 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | (20,792 | ) | | | (20,578 | ) | | | (40,737 | ) | | | (48,820 | ) | | | (49,584 | ) | | | (41,228 | ) | | | (35,270 | ) |
Amortization of deferred financing Fees | | | (1,803 | ) | | | (1,741 | ) | | | (3,461 | ) | | | (472 | ) | | | (521 | ) | | | (224 | ) | | | (151 | ) |
Other, net | | | 780 | | | | 2,309 | | | | 1,569 | | | | 5,152 | | | | 1,319 | | | | (286 | ) | | | (185 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations before income taxes and extraordinary item | | | 93,983 | | | | 95,397 | | | | 205,311 | | | | 166,876 | | | | 134,759 | | | | 119,333 | | | | 85,789 | |
Income taxes | | | 34,304 | | | | 34,915 | | | | 75,144 | | | | 65,472 | | | | 53,775 | | | | 46,757 | | | | 34,714 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations before extraordinary item | | | 59,679 | | | | 60,482 | | | | 130,167 | | | | 101,404 | | | | 80,984 | | | | 72,576 | | | | 51,075 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Discontinued operations (net of income tax expense (benefit)) | | | (87,328 | ) | | | (11,562 | ) | | | (9,018 | ) | | | (3,357 | ) | | | 47,337 | | | | 52,800 | | | | 24,968 | |
Income before extraordinary item | | | (27,649 | ) | | | 48,920 | | | | 121,149 | | | | 98,047 | | | | 128,321 | | | | 125,376 | | | | 76,043 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Extraordinary item (net of tax benefit) | | | — | | | | — | | | | — | | | | (2,106 | ) | | | — | | | | 17,171 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income | | $ | (27,649 | ) | | $ | 48,920 | | | $ | 121,149 | | | $ | 95,941 | | | $ | 128,321 | | | $ | 142,547 | | | $ | 76,043 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Other Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross margin | | | 47.9 | % | | | 48.9 | % | | | 49.2 | % | | | 49.8 | % | | | 49.5 | % | | | 48.6 | % | | | 48.8 | % |
Depreciation and amortization | | $ | 30,935 | | | $ | 25,571 | | | $ | 55,414 | | | $ | 73,348 | | | $ | 62,901 | | | $ | 47,054 | | | $ | 38,830 | |
Expenditures for property, plant and equipment | | | 22,560 | | | | 17,099 | | | | 63,630 | | | | 50,120 | | | | 41,324 | | | | 29,566 | | | | 32,797 | |
Net cash provided by operating activities | | | 56,964 | | | | 83,478 | | | | 192,150 | | | | 179,393 | | | | 115,375 | | | | 114,498 | | | | 104,655 | |
Ratio of earnings to fixed charges(2) | | | 4.8x | | | | 4.9x | | | | 5.2x | | | | 4.2x | | | | 3.5x | | | | 3.7x | | | | 3.3x | |
| | | | | | | |
Consolidated Balance Sheet Data (at period end): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 17,406 | | | $ | 42,415 | | | $ | 16,327 | | | $ | 9,192 | | | $ | 12,411 | | | $ | 12,401 | | | | | |
Working capital | | | 293,692 | | | | 191,919 | | | | 230,125 | | | | 150,371 | | | | 269,396 | | | | 268,149 | | | | | |
Total assets | | | 1,984,730 | | | | 1,981,662 | | | | 2,036,085 | | | | 1,828,080 | | | | 1,792,364 | | | | 1,539,975 | | | | | |
Total liabilities | | | 1,089,463 | | | | 1,099,555 | | | | 1,060,947 | | | | 989,590 | | | | 1,042,848 | | | | 914,631 | | | | | |
Shareholders’ equity | | $ | 895,267 | | | $ | 882,107 | | | $ | 975,138 | | | $ | 838,490 | | | $ | 749,516 | | | $ | 625,344 | | | | | |
33
| (1) | The financial information for fiscal years 1998 and 1999 have been restated, on an unaudited basis, to reflect the reclassification of Applied Biotech and BioRobotics Group as discontinued operations. See Note 5 to consolidated financial statements included elsewhere in this prospectus. |
| (2) | The ratio of earnings to fixed charges is computed by dividing: |
| | • | income from continuing operations before income taxes and extraordinary item plus fixed charges, by |
Fixed charges consist of interest expense, amortization of deferred financing fees and an estimate of interest within rental expense.
The unaudited pro forma ratio of earnings to fixed charges for the year ended September 30, 2002 and the six months ended March 31, 2003 was 4.2 and 3.9, respectively. This ratio has been prepared assuming that the sale of the notes was completed on October 1, 2001.
34
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This prospectus contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in forward-looking statements for many reasons, including the risks described in “Risk Factors” and elsewhere in this prospectus. You should read the following discussion with the sections of this prospectus titled “Selected Consolidated Financial Data” and our financial statements and related notes included elsewhere in this prospectus. Our fiscal year ends on September 30.
General
We recently realigned our lines of business for financial reporting purposes. Our three former business segments (Clinical Diagnostics, Labware and Life Sciences, and Laboratory Equipment) have been reclassified into two business segments: Clinical Group and Research Group. The Clinical Group business segment is the former Clinical Diagnostics business segment. The Research Group business segment is composed of the former Labware and Life Sciences and Laboratory Equipment business segments.
Our operating subsidiaries are engaged primarily in the manufacture and sale of laboratory products in the United States and other countries. Our fiscal year ends on September 30.
During March 2003, we made the decision to dispose of two of our businesses: the rapid diagnostic test business (on-site rapid tests used in the detection of pregnancy, drugs of abuse, and infectious diseases) as conducted by our Applied Biotech subsidiary; and the manufacture and sale of automated microarray instrumentation for the genomics market as conducted by our BioRobotics Group subsidiary. The decision was based in part on our ongoing strategy of strengthening the market positions of our leading brands and focusing on sales of our consumable laboratory products that have more stable growth expectations. As a result, these businesses no longer met our strategic requirements.
During March 2002, we made the decision to dispose of our vacuum deposition chamber business, Vacuum Process Technology. The decision was made following a slow-down in the telecommunications industry, in which Vacuum Process Technology targeted a majority of its products, and as a result, the business no longer met our strategic requirements. During the second quarter of fiscal 2003, we completed the sale of Vacuum Process Technology.
On December 11, 2000, Apogent, then known as Sybron International Corporation, completed the spin-off of its dental business as a separate publicly traded company. The spin-off was effected by way of a pro rata distribution of all the outstanding common stock and related preferred stock purchase rights of Sybron Dental Specialties to Apogent’s shareholders. Sybron Dental Specialties is now an independent public company operating what was Sybron’s dental business. As a result of the spin-off, all historical financial data relating to the operations of Sybron Dental Specialties and its affiliates prior to the spin-off have been reclassified to discontinued operations.
Impact of Recently Issued Accounting Standards
We adopted SFAS No. 142, “Goodwill and Other Intangible Assets”, on October 1, 2001. SFAS No. 142 requires that all goodwill and intangible assets with indefinite useful lives will no longer be amortized, but instead tested for impairment at least annually. We have performed our initial impairment tests as well as our initial annual impairment test and the results indicate no circumstances of impaired goodwill.
35
The following table reconciles reported amounts to that which would have been reported if the current method of accounting was used for the fiscal years ended September 30, 2001, 2000, 1999, and 1998:
| | | Year Ended September 30,
|
| | | 2001
| | 2000
| | 1999
| | 1998
|
Income before extraordinary items: | | | | | | | | | | | | |
Reported income before extraordinary items | | $ | 98,047 | | $ | 128,321 | | $ | 125,376 | | $ | 76,043 |
Add back: goodwill amortization, net of tax | | | 22,363 | | | 19,605 | | | 12,813 | | | 11,095 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted income before extraordinary items | | $ | 120,410 | | $ | 147,926 | | $ | 138,189 | | $ | 87,138 |
| | |
|
| |
|
| |
|
| |
|
|
Net income: | | | | | | | | | | | | |
Reported net income | | $ | 95,941 | | $ | 128,321 | | $ | 142,547 | | $ | 76,043 |
Add back: goodwill amortization, net of tax | | | 22,363 | | | 19,605 | | | 12,813 | | | 11,095 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted net income | | $ | 118,304 | | $ | 147,926 | | $ | 155,360 | | $ | 87,138 |
| | |
|
| |
|
| |
|
| |
|
|
Basic earnings per common share: | | | | | | | | | | | | |
Reported earnings per share | | $ | 0.91 | | $ | 1.23 | | $ | 1.38 | | $ | 0.74 |
Add back: goodwill amortization, net of tax | | | 0.21 | | | 0.19 | | | 0.12 | | | 0.11 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted basic earnings per common share | | $ | 1.12 | | $ | 1.42 | | $ | 1.50 | | $ | 0.85 |
| | |
|
| |
|
| |
|
| |
|
|
Diluted earnings per common share: | | | | | | | | | | | | |
Reported fully diluted earnings per share | | $ | 0.89 | | $ | 1.20 | | $ | 1.34 | | $ | 0.72 |
Add back: goodwill amortization, net of tax | | | 0.21 | | | 0.18 | | | 0.12 | | | 0.10 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted diluted earnings per common share | | $ | 1.10 | | $ | 1.38 | | $ | 1.46 | | $ | 0.82 |
| | |
|
| |
|
| |
|
| |
|
|
On October 3, 2001, FASB issued SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets,” that replaced SFAS No. 121, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets To Be Disposed Of.” The primary objectives of this project were to develop one accounting model, based on the framework established in SFAS No. 121, for long-lived assets to be disposed of by sale and to address significant implementation issues. The accounting model for long-lived assets to be disposed of by sale applies to all long-lived assets, including discontinued operations, and replaces the provisions of APB Opinion No. 30, Reporting Results of Operations-Reporting the Effects of Disposal of a Segment of a Business, for the disposal of segments of a business. SFAS No. 144 requires that those long-lived assets be measured at the lower of carrying amount or fair value less cost to sell, whether reported in continuing operations or in discontinued operations. Therefore, discontinued operations will no longer be measured at net realizable value or include amounts for operating losses that have not yet occurred. The provisions of SFAS No. 144 applied to us effective October 1, 2001. We accounted for the disposal of Vacuum Process Technology consistent with this statement.
36
Effective September 30, 2002, we adopted the Emerging Issues Task Force (EITF) Issue No. 00-10,Accounting for Shipping and Handling Fees and Costs, which requires all amounts charged to customers for shipping and handling to be classified as sales revenues. Accordingly, all historical sales revenue amounts have been adjusted to reflect these charges. The costs related to shipping and handling are classified as a selling expense in selling, general and administrative expense. The following table reconciles historically reported amounts to those adjusted in accordance with EITF 00-10:
| | | Year Ended September 30,(1)
| |
| | | 2001
| | | 2000
| | | 1999
| | | 1998
| |
Net sales | | | | | | | | | | | | | | | | |
Reported net sales | | $ | 926,072 | | | $ | 833,797 | | | $ | 687,663 | | | $ | 553,958 | |
Reclassification of freight income | | | 12,747 | | | | 9,770 | | | | 8,958 | | | | 8,541 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Adjusted net sales | | $ | 938,819 | | | $ | 843,567 | | | $ | 696,621 | | | $ | 562,499 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Cost of products sold | | | | | | | | | | | | | | | | |
Reported cost of products sold | | $ | 474,324 | | | $ | 428,655 | | | $ | 358,897 | | | $ | 288,810 | |
Reclassification of freight costs | | | (3,006 | ) | | | (2,651 | ) | | | (1,057 | ) | | | (624 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Adjusted cost of products sold | | $ | 471,318 | | | $ | 426,004 | | | $ | 357,840 | | | $ | 288,186 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Selling, general and administrative expenses | | | | | | | | | | | | | | | | |
Reported selling, general and administrative expenses | | $ | 240,732 | | | $ | 221,597 | | | $ | 167,695 | | | $ | 143,753 | |
Reclassification of freight income and expense | | | 15,753 | | | | 12,421 | | | | 10,015 | | | | 9,165 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Adjusted selling, general, and administrative expenses | | $ | 256,485 | | | $ | 234,018 | | | $ | 177,710 | | | $ | 152,918 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| (1) | Historical amounts have been adjusted to reflect the disposal of Applied Biotech, BioRobotics Group, and Vacuum Process Technology. |
Results of Operations
Six Months Ended March 31, 2003 Compared to the Six Months Ended March 31, 2002
Net Sales
| | | Six Months Ended March 31,
| | Dollar Change
| | Percent Change
| |
| | | 2003
| | 2002
| | |
| | | (dollars in thousands) | | | |
Net Sales | | | | | | | | | | | | |
Clinical Group | | $ | 251,930 | | $ | 228,383 | | $ | 23,547 | | 10.3 | % |
Research Group | | | 282,506 | | | 260,679 | | | 21,827 | | 8.4 | % |
| | |
|
| |
|
| |
|
| | | |
Total Net Sales | | $ | 534,436 | | $ | 489,062 | | $ | 45,374 | | 9.3 | % |
| | |
|
| |
|
| |
|
| | | |
Overall Company. Net sales for the six months ended March 31, 2003 were $534.4 million, an increase of $45.4 million or 9.3% over the same period of fiscal 2002.
Clinical Group. Increased net sales in the Clinical Group resulted primarily from: (a) net sales of products of acquired companies (approximately $12.6 million), (b) net sales of existing products (approximately $8.5 million), (c) foreign currency fluctuations (approximately $4.2 million), and (d) increased net sales of new products developed by us (approximately $2.9 million). Net sales were partially reduced by price reductions (approximately $4.7 million).
Research Group. Increased net sales in the Research Group resulted primarily from: (a) increased net sales of new products developed by us (approximately $9.4 million), (b) net sales of products of acquired companies
37
(approximately $9.2 million), (c) foreign currency fluctuations (approximately $7.0 million), and (d) price increases (approximately $1.5 million). Net sales were partially reduced by a decrease in net sales of existing products (approximately $5.3 million).
Gross Profit
| | | Six Months Ended March 31,
| | | Dollar Change
| | Percent Change
| |
| | | 2003
| | Percent of Sales
| | | 2002
| | Percent of Sales
| | | |
| | | (dollars in thousands) | | | | | | |
Gross Profit | | | | | | | | | | | | | | | | | | |
Clinical Group | | $ | 117,867 | | 46.8 | % | | $ | 110,235 | | 48.3 | % | | $ | 7,632 | | 6.9 | % |
Research Group | | | 138,203 | | 48.9 | % | | | 128,708 | | 49.4 | % | | | 9,495 | | 7.4 | % |
| | |
|
| | | | |
|
| | | | |
|
| | | |
Total Gross Profit | | $ | 256,070 | | 47.9 | % | | $ | 238,943 | | 48.9 | % | | $ | 17,127 | | 7.2 | % |
| | |
|
| | | | |
|
| | | | |
|
| | | |
Overall Company. Gross profit for the six months ended March 31, 2003 was $256.1 million, or 47.9% of net sales, as compared to $238.9 million, or 48.9% of net sales, for the same period of fiscal 2002.
Clinical Group. Increased gross profit in the Clinical Group resulted primarily from: (a) the effects of acquired companies (approximately $5.5 million), (b) increased volume (approximately $3.9 million), (c) product mix (approximately $2.2 million), (d) foreign currency fluctuations (approximately $1.6 million), (e) the effects of new products (approximately $0.9 million), and (f) decreased restructuring charges (approximately $0.3 million). Gross profit was partially reduced by: (a) price reductions (approximately $4.7 million), (b) increased manufacturing overhead (approximately $1.3 million), and (c) higher inventory write-downs (approximately $0.8 million).
Research Group. Increased gross profit in the Research Group resulted primarily from: (a) the effects of new products (approximately $5.2 million), (b) the effects of acquired companies (approximately $3.7 million), (c) foreign currency fluctuations (approximately $3.1 million), (d) decreased restructuring charges (approximately $2.8 million), (e) price increases (approximately $1.5 million), and (f) the effects of inventory write-downs (approximately $0.4 million). Gross profit was slightly reduced by: (a) increased manufacturing overhead (approximately $3.4 million), (b) decreased volume (approximately $2.4 million), and (c) product mix (approximately $1.4 million).
Selling, General and Administrative Expenses
| | | Six Months Ended
March 31,
| | Dollar
Change
| | Percent
Change
| |
| | | 2003
| | 2002
| | |
| | | (dollars in thousands) | | | |
Selling, General and Administrative Expenses | | | | | | | | | | | | |
Clinical Group | | $ | 58,288 | | $ | 51,978 | | $ | 6,310 | | 12.1 | % |
Research Group | | | 81,984 | | | 71,558 | | | 10,426 | | 14.6 | % |
| | |
|
| |
|
| |
|
| | | |
Total Selling, General and Administrative Expenses | | $ | 140,272 | | $ | 123,536 | | $ | 16,736 | | 13.5 | % |
| | |
|
| |
|
| |
|
| | | |
Overall Company. Selling, general and administrative expenses for the six months ended March 31, 2003 were $140.3 million, an increase of $16.7 million, or 13.5%, over the same period of fiscal 2002.
Clinical Group. Increased selling, general and administrative expenses in the Clinical Group resulted primarily from: (a) expenses of acquired businesses (approximately $2.3 million), (b) general and administrative expenses (approximately $1.5 million), (c) foreign currency fluctuations (approximately $1.1 million),
38
(d) increased amortization expense (approximately $0.9 million), (e) marketing expenses (approximately $0.8 million), and (f) research and development expenses (approximately $0.3 million). Selling, general and administrative expenses were partially decreased by lower restructuring expenses (approximately $0.7 million).
Research Group. Increased selling, general and administrative expenses in the Research Group resulted primarily from: (a) expenses of acquired businesses (approximately $3.2 million), (b) foreign currency fluctuations (approximately $3.0 million), (c) general and administrative expenses (approximately $3.0 million), (d) marketing expenses (approximately $1.1 million), (e) research and development expenses (approximately $0.3 million), and (f) increased amortization expense (approximately $0.3 million). Selling, general and administrative expenses were partially decreased by lower restructuring expenses (approximately $0.5 million).
Operating Income
| | | Six Months Ended
March 31,
| | Dollar
Change
| | | Percent
Change
| |
| | | 2003
| | 2002
| | |
| | | (dollars in thousands) | | | | |
Operating Income | | | | | | | | | | | | | |
Clinical Group | | $ | 59,579 | | $ | 58,257 | | $ | 1,322 | | | 2.3 | % |
Research Group | | | 56,219 | | | 57,150 | | | (931 | ) | | -1.6 | % |
| | |
|
| |
|
| |
|
|
| | | |
Total Operating Income | | $ | 115,798 | | $ | 115,407 | | $ | 391 | | | 0.3 | % |
| | |
|
| |
|
| |
|
|
| | | |
Operating income for the six months ended March 31, 2003 was $115.8 million, an increase of $0.4 million, or 0.3%, compared to $115.4 million for the same period of fiscal 2002.
Interest Expense
Interest expense was $20.8 million for the six months ended March 31, 2003, as compared to $20.6 million for the same period of fiscal 2002.
Other Income
Other income was $0.8 million for the six months ended March 31, 2003, as compared to other income of $2.3 million in the same period of fiscal 2002. This decrease of $1.5 million is primarily due to the reduction of income from an equity investment in a joint venture.
Income Taxes
Taxes on income from continuing operations for the six months ended March 31, 2003 were $34.3 million, a decrease of $0.6 million from the same period of fiscal 2002. This decrease resulted primarily from a decrease in taxable income.
Income from Continuing Operations
Income from continuing operations for the six months ended March 31, 2003 was $59.7 million, a decrease of $0.8 million, or 1.3%, from $60.5 million for the same period of fiscal 2002.
Discontinued Operations
The loss from the discontinued operations, net of tax, for the six months ended March 31, 2003 was $87.3 million as compared to a loss of $11.6 million for the same period of fiscal 2002. The loss from discontinued
39
operations consisted of a $85.9 million write-down of net assets to their estimated fair value less costs to sell related to the discontinuance of Applied Biotech and BioRobotics Group and a $2.8 million additional charge related to the disposal of Vacuum Process Technology. These amounts were offset by $1.5 million of income from the operations of discontinued businesses.
Net Income
Net loss for the six months ended March 31, 2003 was $27.6 million, as compared to net income of $48.9 million for the same period of fiscal 2002.
Year Ended September 30, 2002 Compared to the Year Ended September 30, 2001
General
Net sales for 2002 were $1,027.9 million, an increase of $89.1 million or 9.5% over 2001. We continued to see a decline in existing product sales within our Laboratory Equipment and Life Science instrument business, included in the Research Group and we expect this weakness to continue.
Gross profit for 2002 was $505.9 million, representing an increase of $38.4 million over 2001. Although the Clinical Group experienced an increase in gross margin due to price increases, certain subsidiaries within this segment that market rapid diagnostic testing products experienced downward pricing pressure and we expect this weakness to continue.
Selling, general and administrative expenses for 2002 were $258.0 million as compared to $256.5 million in 2001. Had we adopted SFAS No. 142 as of October 1, 2000, selling, general and administrative expenses would have decreased by $28.1 million for 2001.
Operating income and net income for 2002 were $247.9 million and $121.1 million, respectively, compared to $211.0 million and $95.9 million for 2001. Had we adopted SFAS No. 142 as of October 1, 2000, operating income and net income for 2001 would have been $232.6 million and $118.3 million, respectively.
Net Sales
| | | Fiscal
2002
| | Fiscal
2001
| | Dollar
Change
| | Percent
Change
| |
| | | (in thousands) | |
Net Sales | | | | | | | | | | | | |
Clinical Group | | $ | 473,388 | | $ | 431,193 | | $ | 42,195 | | 9.8 | % |
Research Group | | | 554,525 | | | 507,626 | | | 46,899 | | 9.2 | % |
| | |
|
| |
|
| |
|
| | | |
Total Net Sales | | $ | 1,027,913 | | $ | 938,819 | | $ | 89,094 | | 9.5 | % |
| | |
|
| |
|
| |
|
| | | |
Overall Company. Net sales for the year ended September 30, 2002 increased by $89.1 million or 9.5% over fiscal 2001.
Clinical Group. Increased net sales in the Clinical Group resulted primarily from: (a) net sales of products of acquired companies (approximately $28.5 million), (b) price increases (approximately $13.6 million), (c) increased net sales of new products developed by us (approximately $5.3 million), and (d) foreign currency fluctuations (approximately $1.5 million). Net sales were partially reduced by a decrease in net sales of existing products (approximately $6.7 million).
Research Group. Increased net sales in the Research Group resulted primarily from: (a) net sales of products of acquired companies (approximately $41.6 million), (b) increased net sales of new products developed
40
by us (approximately $11.6 million), (c) price increases (approximately $5.0 million), and (d) foreign currency fluctuations (approximately $1.4 million). Net sales were partially reduced by a decrease in net sales of existing products (approximately $12.7 million).
Gross Profit
| | | Fiscal
2002
| | Percent
of Sales
| | | Fiscal
2001
| | Percent
of Sales
| | | Dollar
Change
| | Percent
Change
| |
| | | (in thousands) | |
Gross Profit | | | | | | | | | | | | | | | | | | |
Clinical Group | | $ | 228,647 | | 48.3 | % | | $ | 214,250 | | 49.7 | % | | $ | 14,397 | | 6.7 | % |
Research Group | | | 277,247 | | 50.0 | % | | | 253,251 | | 49.9 | % | | | 23,996 | | 9.5 | % |
| | |
|
| | | | |
|
| | | | |
|
| | | |
Total Gross Profit | | $ | 505,894 | | 49.2 | % | | $ | 467,501 | | 49.8 | % | | $ | 38,393 | | 8.2 | % |
| | |
|
| | | | |
|
| | | | |
|
| | | |
Overall Company. Gross profit for the year ended September 30, 2002 increased by $38.4 million or 8.2% over fiscal 2001.
Clinical Group. Increased gross profit in the Clinical Group resulted primarily from: (a) price increases (approximately $13.1 million), (b) the effects of acquired companies (approximately $10.6 million), (c) lower inventory write-downs (approximately $4.7 million), (d) product mix (approximately $2.4 million), (e) the effects of new products (approximately $2.3 million), and (f) foreign currency fluctuations (approximately $0.4 million). Gross profit was partially reduced by: (a) increased manufacturing overhead (approximately $12.9 million), (b) decreased volume (approximately $4.5 million), and (c) the 2002 Special Charges (defined below under “Special Charges”) (approximately $1.7 million).
Research Group. Increased gross profit in the Research Group resulted primarily from: (a) the effects of acquired companies (approximately $13.9 million), (b) product mix (approximately $10.2 million), (c) the effects of new products (approximately $5.3 million), (d) price increases (approximately $4.7 million), (e) foreign currency fluctuations (approximately $0.7 million), and (f) lower inventory write-downs (approximately $0.6 million). Gross profit was partially reduced by: (a) decreased volume (approximately $6.2 million), (b) the 2002 Special Charges (approximately $3.5 million), and (c) increased manufacturing overhead (approximately $1.7 million).
Selling, General and Administrative Expenses
Effective October 1, 2001, we changed the method used to allocate corporate office expenses to the business segments. This change ensures that all corporate office expenses are allocated to the business segments, and better aligns our segment reporting with the manner in which we are managed. All historical information relating to the fiscal years ended September 30, 2001 and 2000 has been restated to reflect this change.
| | | Fiscal
2002
| | Fiscal
2001
| | Dollar
Change
| | | Percent
Change
| |
| | | (in thousands) | |
Selling, General and Administrative Expenses | | | | | | | | | | | | | |
Clinical Group | | $ | 105,261 | | $ | 110,720 | | $ | (5,459 | ) | | (4.9 | )% |
Research Group | | | 152,693 | | | 145,765 | | | 6,928 | | | 4.8 | % |
| | |
|
| |
|
| |
|
|
| | | |
Total Selling, General and Administrative Expenses | | $ | 257,954 | | $ | 256,485 | | $ | 1,469 | | | 0.6 | % |
| | |
|
| |
|
| |
|
|
| | | |
Overall Company. Selling, general and administrative expenses for the year ended September 30, 2002 increased by $1.5 million or 0.6% from fiscal 2001. Adoption of SFAS No. 142 would have decreased selling, general and administrative expenses by $28.1 million for the year ended September 30, 2001 had it been effective at the time.
41
Clinical Group. Decreased selling, general and administrative expenses in the Clinical Group resulted primarily from a decrease in amortization expense as a result of the implementation of SFAS No. 142 (approximately $14.0 million). This decrease in selling, general and administrative expenses was partially offset by: (a) expenses of acquired businesses (approximately $2.5 million), (b) marketing expenses (approximately $2.4 million), (c) general and administrative expenses (approximately $1.5 million), (d) research and development expenses (approximately $1.4 million), (e) the 2002 Special Charges (approximately $0.4 million), and (f) foreign currency fluctuations (approximately $0.3 million).
Research Group. Increased selling, general and administrative expenses in the Research Group resulted primarily from: (a) expenses of acquired businesses (approximately $9.8 million), (b) marketing expenses (approximately $6.4 million), (c) research and development expense (approximately $1.6 million), (d) foreign currency fluctuations (approximately $0.9 million), (e) general and administrative expenses (approximately $0.5 million), and (f) the 2002 Special Charges (approximately $0.2 million). Selling, general and administrative expenses were partially reduced by decreased amortization expense as a result of SFAS No. 142 (approximately $12.5 million).
Operating Income
| | | Fiscal
2002
| | Fiscal
2001
| | Dollar
Change
| | Percent
Change
| |
| | | (in thousands) | |
Operating Income | | | | | | | | | | | | |
Clinical Group | | $ | 123,386 | | $ | 103,530 | | $ | 19,856 | | 19.2 | % |
Research Group | | | 124,554 | | | 107,486 | | | 17,068 | | 15.9 | % |
| | |
|
| |
|
| |
|
| | | |
Total Operating Income | | $ | 247,940 | | $ | 211,016 | | $ | 36,924 | | 17.5 | % |
| | |
|
| |
|
| |
|
| | | |
Operating income for 2002 increased by $36.9 million over 2001. Adoption of SFAS No. 142 would have increased operating income to $232.6 million for 2001, had it been effective at the time.
Interest Expense
Interest expense was $40.7 million for 2002 as compared to $48.8 million for 2001. This decrease was due primarily to lower effective interest rates for 2002 as compared to 2001.
Other Income
Other income for 2002 was $1.6 million, a decrease of $3.6 million as compared to 2001. Other income for 2002 was made up of income from an equity investment in a joint venture of $3.4 million offset in part by a loss on a sale of an asset of $1.9 million.
Income Taxes
Taxes on income from continuing operations for 2002 were $75.1 million, an increase of $9.7 million from 2001. The increase resulted primarily from increased taxable earnings offset in part by lower effective tax rates resulting from the implementation of SFAS No. 142.
Income from Continuing Operations Before Extraordinary Items
Income from continuing operations was $130.2 million for 2002 as compared to $101.4 million in 2001. Adoption of SFAS No. 142 would have increased income from continuing operations to $123.0 million for 2001, had it been effective at the time.
42
Discontinued Operations
The loss from discontinued operations for 2002 of $9.0 million (net of income tax benefit of $5.2 million) was a result of net income from Applied Biotech of $6.4 million offset by losses from BioRobotics Group of $1.4 million and Vacuum Process Technology of $0.8 million and an estimated loss on sale of Vacuum Process Technology of $13.2 million. For the 2001 period, the loss from discontinued operations was a result of a loss from the spin-off of $11.8 million, offset by operating income from Applied Biotech of $7.0 million, Vacuum Process Technology of $1.0 million, and BioRobotics Group of $0.4 million.
Net Income
Net income was $121.1 million for 2002 as compared to $95.9 million for 2001. Adoption of SFAS No. 142 would have increased net income to $118.3 million for 2001, had it been effective at the time.
Depreciation and Amortization
Depreciation and amortization expense is allocated among cost of sales, selling, general and administrative expenses, and other expenses. Depreciation expense and amortization expense decreased $17.9 million for 2002 due to the adoption of SFAS No. 142, offset in part by additional depreciation and amortization from goodwill and intangibles recorded from the various acquisitions as well as routine operating capital expenditures. Adoption of SFAS No. 142 would have decreased amortization expense by $28.1 million for 2001, had it been effective at the time.
Year Ended September 30, 2001 Compared to the Year Ended September 30, 2000
Net Sales
| | | Fiscal
2001
| | Fiscal
2000
| | Dollar
Change
| | Percent
Change
| |
| | | (in thousands) | |
Net Sales | | | | | | | | | | | | |
Clinical Group | | $ | 431,193 | | $ | 390,097 | | $ | 41,096 | | 10.5 | % |
Research Group | | | 507,626 | | | 453,470 | | | 54,156 | | 11.9 | % |
| | |
|
| |
|
| |
|
| | | |
Total Net Sales | | $ | 938,819 | | $ | 843,567 | | $ | 95,252 | | 11.3 | % |
| | |
|
| |
|
| |
|
| | | |
Overall Company. Net sales for the year ended September 30, 2001 increased by $95.3 million or 11.3% over fiscal 2000.
Clinical Group. Increased net sales in the Clinical Group resulted primarily from: (a) net sales of products of acquired companies (approximately $29.2 million), (b) increased net sales of existing products (approximately $11.0 million), and (c) increased net sales of new products developed by us (approximately $5.8 million). Net sales were partially reduced by: (a) foreign currency fluctuations (approximately $3.2 million) and (b) price decreases (approximately $1.7 million).
Research Group. Increased net sales in the Research Group resulted primarily from: (a) net sales of products of acquired companies (approximately $21.7 million), (b) increased net sales of new products developed by us (approximately $21.5 million), (c) increased net sales of existing products (approximately $9.8 million), and (d) price increases (approximately $8.0 million). Net sales were partially reduced by foreign currency fluctuations (approximately $6.8 million).
43
Gross Profit
| | | Fiscal
2001
| | Percent
of Sales
| | | Fiscal
2000
| | Percent
of Sales
| | | Dollar
Change
| | Percent
Change
| |
| | | (in thousands) | |
Gross Profit | | | | | | | | | | | | | | | | | | |
Clinical Group | | $ | 214,250 | | 49.7 | % | | $ | 190,756 | | 48.9 | % | | $ | 23,494 | | 12.3 | % |
Research Group | | | 253,252 | | 49.9 | % | | | 226,807 | | 50.0 | % | | | 26,444 | | 11.7 | % |
| | |
|
| | | | |
|
| | | | |
|
| | | |
Total Gross Profit | | $ | 467,502 | | 49.8 | % | | $ | 417,563 | | 49.5 | % | | $ | 49,938 | | 12.0 | % |
| | |
|
| | | | |
|
| | | | |
|
| | | |
Overall Company. Gross profit for the year ended September 30, 2001 increased by $49.9 million or 12.0% over fiscal 2000.
Clinical Group. Increased gross profit in the Clinical Group resulted primarily from: (a) the effects of acquired companies (approximately $17.2 million), (b) product mix (approximately $7.5 million), (c) the effects of new products (approximately $3.1 million), (d) the 2000 Special Charges (as defined below under “Special Charges”) (approximately $2.4 million), and (e) increased volume (approximately $1.8 million). Gross profit was partially reduced by: (a) inventory adjustments (approximately $3.7 million), (b) increased manufacturing overhead (approximately $2.0 million), (c) price decreases (approximately $1.7 million), and (d) foreign currency fluctuations (approximately $1.1 million).
Research Group. Increased gross profit in the Research Group resulted primarily from: (a) the effects of new products (approximately $11.2 million), (b) the effects of acquired companies (approximately $8.5 million), (c) price increases (approximately $8.2 million), (d) product mix (approximately $6.6 million), (e) the 2000 Special Charges (approximately $1.9 million), and (f) increased volume (approximately $0.1 million). Gross profit was partially reduced by: (a) increased manufacturing overhead (approximately $4.6 million), (b) foreign currency fluctuations (approximately $3.9 million), and (c) inventory adjustments (approximately $1.6 million).
Selling, General and Administrative Expenses
| | | Fiscal
2001
| | Fiscal
2000
| | Dollar
Change
| | Percent
Change
| |
| | | (in thousands) | |
Selling, General and Administrative Expenses | | | | | | | | | | | | |
Clinical Group | | $ | 110,720 | | $ | 102,026 | | $ | 8,694 | | 8.5 | % |
Research Group | | | 145,765 | | | 131,992 | | | 13,773 | | 10.4 | % |
| | |
|
| |
|
| |
|
| | | |
Total Selling, General and Administrative Expenses | | $ | 256,485 | | $ | 234,018 | | $ | 22,467 | | 9.6 | % |
| | |
|
| |
|
| |
|
| | | |
Overall Company. Selling, general and administrative expenses for the year ended September 30, 2001 increased by $22.5 million or 9.6% from fiscal 2000.
Clinical Group. Increased selling, general and administrative expenses in the Clinical Group resulted primarily from: (a) marketing expenses (approximately $4.7 million), (b) increased amortization of intangibles primarily as a result of acquisitions (approximately $3.0 million), (c) acquired businesses (approximately $3.0 million), and (d) general and administrative expenses (approximately $1.4 million). Selling, general and administrative expenses were partially reduced by: (a) the 2000 Special Charges (approximately $2.2 million), (b) foreign currency fluctuations (approximately $0.7 million), and (c) research and development expenses (approximately $0.5 million).
Research Group. Increased selling, general and administrative expenses in the Research Group resulted primarily from: (a) acquired businesses (approximately $9.6 million), (b) marketing expenses (approximately $3.3 million), (c) research and development expense (approximately $1.4 million), (d) general and administrative
44
expenses (approximately $1.0 million), and (e) increased amortization of intangibles primarily as a result of acquisitions (approximately $0.9 million). Selling, general and administrative expenses were partially reduced by: (a) the 2000 Special Charges (approximately $2.1 million), and (b) foreign currency fluctuations (approximately $0.4 million).
Corporate Office. Decreased general and administrative expenses at the corporate office resulted primarily from: (a) the 2000 Special Charges (approximately $1.7 million), and (b) a decrease in expenses as a result of the closure of our Milwaukee, Wisconsin corporate office (approximately $1.6 million).
Operating Income
| | | Fiscal
2001
| | Fiscal
2000
| | Dollar
Change
| | Percent
Change
| |
| | | (in thousands) | |
Operating Income | | | | | | | | | | | | |
Clinical Group | | $ | 103,530 | | $ | 88,730 | | $ | 14,800 | | 16.7 | % |
Research Group | | | 107,486 | | | 94,815 | | | 12,671 | | 13.4 | % |
| | |
|
| |
|
| |
|
| | | |
Total Operating Income | | $ | 211,016 | | $ | 183,545 | | $ | 27,471 | | 15.0 | % |
| | |
|
| |
|
| |
|
| | | |
As a result of the foregoing, operating income for the year ended September 30, 2001 increased by $27.5 million or 15.0% over fiscal 2000.
Interest Expense
Interest expense was $48.8 million for 2001 compared to $49.6 million in 2000.
Other Income
Other income for 2001 was $5.2 million, an increase of $3.9 million over 2000. The increase resulted primarily from the gain on the sale of assets of $4.1 million during the second quarter of fiscal 2001.
Income Taxes
Taxes on income from continuing operations for 2001 were $65.5 million, an increase of $11.7 million from 2000. The increase resulted primarily from increased taxable earnings.
Income from Continuing Operations Before Extraordinary Items
As a result of the foregoing, for 2001, income from continuing operations was $101.4 million as compared to $81.0 million in 2000.
Discontinued Operations
Losses from discontinued operations were $3.4 million (net of income tax of $5.8 million) for 2001, as compared to income of $47.3 million (net of income tax of $32.2 million) in 2000. The 2001 loss from discontinued operations was a result of transaction expenses relating to the Spin-Off of approximately $12.4 million offset by the operating income from Sybron Dental Specialties (through December 11, 2000) of $0.6 million, operating income from Applied Biotech of $7.0 million, Vacuum Process Technology of $1.0 million, and BioRobotics Group of $0.4 million.
45
Extraordinary Items
As a result of the December 2000 debt refinancing and the April 2001 issuance of our 8% senior notes due 2011, we wrote off deferred financing costs of approximately $3.5 million that related to prior debt agreements. This was recorded as an extraordinary item of $2.1 million, net of income taxes.
Net Income
As a result of the foregoing, we had net income of $95.9 million for 2001, as compared to net income of $128.3 million for 2000.
Depreciation and Amortization
Depreciation and amortization expense is allocated among cost of sales, selling, general and administrative expenses, and other expenses. Depreciation expense and amortization expense increased $10.4 million for 2001 due to additional depreciation and amortization from goodwill and intangibles recorded from the various acquisitions as well as routine operating capital expenditures.
Acquisitions
Company
| | Approximate
Annual Sales
Prior to
Acquisition
| | Acquisition Date
| | Description
|
| | | (in thousands) | | | | |
| | | |
2003 Acquisitions | | | | | | | |
| | | |
Clinical Group: | | | | | | | |
| | | |
PathoDx Product Line of Diagnostic Products Corporation | | $ | 4,880 | | July 2003 | | Manufacturer of immunofluorescent and latex agglutination diagnostic test kits for use in clinical laboratories. |
| | | |
NeoMarkers, Inc. | | $ | 4,000 | | October 2002 | | Developer and manufacturer of antibodies, reagents, and related products for life sciences research applications. |
| | | |
Opus Diagnostics Inc. | | $ | 2,100 | | October 2002 | | Developer and distributor of diagnostic assays, controls, and calibrators for therapeutic drug monitoring. |
| | | |
Research Group: | | | | | | | |
| | | |
Tempyrox Company, Inc. | | $ | 582 | | January 2003 | | Developer and manufacturer of high-temperature cleaning ovens for industrial and laboratory applications. |
| | | |
2002 Acquisitions | | | | | | | |
| | | |
Clinical Group: | | | | | | | |
| | | |
Forefront Diagnostics, Inc. | | $ | 6,300 | | November 2001 | | Manufacturer of rapid diagnostic test kits for the detection of drugs of abuse. |
| | | |
Separation Technology, Inc. | | $ | 3,200 | | January 2002 | | Manufacturer and designer of tabletop microhematocrit centrifuge systems and related consumables for blood, serum, and plasma separation. |
46
Company
| | Approximate
Annual Sales
Prior to
Acquisition
| | Acquisition Date
| | Description
|
| | | (in thousands) | | | | |
| | | |
Capitol Vial, Inc. | | $ | 27,300 | | February 2002 | | Manufacturer and developer of patented, flip-top, leak-proof plastic vials and related process equipment for sample collection and processing. |
| | | |
Mirror Product Line of SMC Manufacturing | | $ | 600 | | May 2002 | | Manufacturer of automotive vanity mirror products. |
| | | |
Research Group: | | | | | | | |
| | | |
Chromacol Limited, Epsom Glass Limited, and Amchro, Inc. (the “Chromacol Group”) | | $ | 9,900 | | October 2001 | | Manufacturers and distributors of chromatography vials and related products. |
| | | |
Research Group: | | | | | | | |
| | | |
Barden Engineering | | $ | 600 | | October 2001 | | Manufacturer of industrial tooling. |
| | | |
Cosmotec Company, Ltd. | | $ | 5,500 | | October 2001 | | Manufacturer of high-throughput liquid dispensing instrumentation. |
| | | |
Marsh Bio Products, Inc. | | $ | 17,100 | | April 2002 | | Distributor of laboratory equipment and consumables. |
| | | |
TFO, Incorporated | | $ | 1,700 | | May 2002 | | Manufacturer of hydration devices for consumer use. |
| | | |
2001 Acquisitions | | | | | | | |
| | | |
Clinical Group: | | | | | | | |
| | | |
Vacuum Process Technology, Inc. | | $ | 3,977 | | November 2000 | | Designer and manufacturer of precision film optical coating equipment used to manufacture optically motivated product for a variety of markets. |
| | | |
Disposable Glass Culture Tube Business of Kimble Glass Inc. | | $ | 5,800 | | April 2001 | | Manufacturer of disposable glass culture tubes used in a variety of general laboratory applications, including blood collection, blood banking, urinalysis, and certain cell culture procedures. |
| | | |
Innovative Diagnostics, Inc. | | $ | 1,300 | | July 2001 | | Distributor of clinical chemistry controls. |
| | | |
Disposable Glass Pasteur Pipette and Perfume Sampler Vial Product Line of Kimble Glass Inc. | | $ | 2,000 | | August 2001 | | Manufacturer of disposable glass Pasteur pipette and perfume sampler products. |
| | | |
Latex Agglutination Product Line of Medtek Diagnostics LLC | | $ | 220 | | July 2001 | | Manufacturer of latex agglutination products. |
| | | |
Daniel Mirror Company | | $ | 6,800 | | September 2001 | | Manufacturer of specialized “cut to order” mirrors. |
47
Company
| | Approximate
Annual Sales
Prior to
Acquisition
| | Acquisition
Date
| | Description
|
| | | (in thousands) | | | | |
| | | |
Research Group: | | | | | | | |
| | | |
BioRobotics Group Ltd. | | $ | 10,500 | | March 2001 | | Designer and manufacturer of automated microarray instrumentation solutions used in functional genomics. |
| | | |
Advanced Biotechnologies Ltd. | | $ | 21,500 | | April 2001 | | Manufacturer of a comprehensive range of molecular biology reagents and special plastic consumables for the life sciences market. |
| | | |
Mosaic Technologies Inc. | | $ | 1,400 | | July 2001 | | Developer and manufacturer of solid phase DNA amplification technology. |
| | | |
Chromatography Vial Product Line of Kimble Glass Inc. | | $ | 7,200 | | August 2001 | | Manufacturer of chromatography vial products including vial inserts and accessories. |
Special Charges
During fiscal 2003, we recorded restructuring charges of approximately $0.3 million (approximately $0.2 million net of tax) for the consolidation of certain facilities and discontinuance of certain product lines due to product rationalizations. The restructuring charges were classified as components of selling, general and administrative expenses. The approximately $0.3 million related to severance associated with non-production employees. Additional restructuring charges of approximately $8.8 million related to these restructuring actions will be recorded during the balance of fiscal 2003.
During fiscal 2002, we recorded a restructuring charge of approximately $7.2 million (approximately $4.4 million net of tax) for the consolidation of certain facilities and discontinuance of certain product lines due to product rationalizations. The restructuring charge was classified as components of cost of sales and selling, general and administrative expenses. The cost of sales component of approximately $5.6 million related to the write-off of inventory, write-offs of fixed assets, certain lease terminations, and severance associated with employees in production activities. The selling, general and administrative component of approximately $1.7 million related to severance associated with non-production employees as well certain lease terminations and other shutdown costs. These charges are referred to as the “2002 Special Charges.” In addition, during the year we recorded a credit of $0.4 million related to the reversal of an unused reserve from the 2000 Special Charge. This credit was classified as a reduction of selling, general, and administrative expenses.
Activity related to the 2002 Special Charges and its components are as follows (dollars in thousands):
| | | Severance(1)
| | | Inventory(2)
| | | Fixed
Assets(2)
| | | Facility
Closure Costs(3)
| | | Other
| | | Total
| |
Fiscal 2002 restructuring charge | | $ | 1,466 | | | $ | 3,709 | | | $ | 353 | | | $ | 1,409 | | | $ | 155 | | | $ | 7,092 | |
Fiscal 2002 cash payments | | | (989 | ) | | | — | | | | — | | | | (682 | ) | | | — | | | | (1,671 | ) |
Fiscal 2002 non-cash charges | | | — | | | | (3,709 | ) | | | (353 | ) | | | — | | | | (155 | ) | | | (4,217 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
September 30, 2002 balance | | $ | 477 | | | $ | — | | | $ | — | | | $ | 727 | | | $ | — | | | $ | 1,204 | |
Fiscal 2003 cash payments(4) | | | (477 | ) | | | — | | | | — | | | | (185 | ) | | | — | | | | (662 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
March 31, 2003 balance | | $ | — | | | $ | — | | | $ | — | | | $ | 542 | | | $ | — | | | $ | 542 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| (1) | Amount represents severance and termination costs for 126 terminated employees (primarily sales, marketing and manufacturing personnel). |
48
| (2) | Amount represents write-offs of inventory and fixed assets associated with discontinued product lines. |
| (3) | Amount represents lease payments and other facility closure costs on exited operations. |
| (4) | Through March 31, 2003. |
Our results for 2001 include a charge of approximately $0.6 million (approximately $0.4 million after tax) relating to adjustments made to the 2000 restructuring reserve (discussed below), consisting of additional severance. All historical financial data relating to Sybron Dental Specialties and its affiliates have been reclassified to discontinued operations.
Our results for 2000 include charges of approximately $11.3 million (approximately $7.5 million after tax) with respect to the restructuring of various parts of our business. These charges relate primarily to restructured staffing (approximately $5.5 million), operating location rationalization (approximately $2.7 million), product rationalization (approximately $2.1 million), and a tax expense from the restructuring of our U.K. operations (approximately $1.0 million). These charges are referred to as the “2000 Special Charges.” Savings were projected to result from: (a) reduced salaries and related expenses as a result of consolidating our CASCO operations with our Microgenics operation, a reduction of workforce at NNI’s Naperville facility, and the elimination of corporate personnel in Milwaukee (approximately $5.6 million); (b) the consolidation of several facilities, including those of CASCO, NNI Biotech, and Nunc U.S. (approximately $0.8 million); and (c) the elimination of product lines that are either duplicative or no longer meet management’s profitability expectations (approximately $0.2 million). We do not anticipate, and have not experienced to date, significant offsets to savings in either increased expenses or reduced revenues.
Activity related to the 2000 Special Charges and its components are as follows (dollars in thousands):
| | | Severance(1)
| | Inventory(2)
| | Fixed
Assets(2)
| | Lease
Commitments(3)
| | Shut-down
Costs(3)
| | Tax(4)
| | Other
| | Total
|
| | | (in thousands) |
2000 Restructuring charge | | $ | 5,500 | | $ | 2,100 | | $ | 1,000 | | $ | 500 | | $ | 300 | | $ | 1,000 | | $ | 900 | | $ | 11,300 |
2000 Cash payments | | | 1,100 | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,100 |
2000 Non-cash charges | | | — | | | 2,100 | | | 1,000 | | | — | | | — | | | — | | | 800 | | | 3,900 |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
September 30, 2000 balance | | $ | 4,400 | | $ | — | | $ | — | | $ | 500 | | $ | 300 | | $ | 1,000 | | $ | 100 | | $ | 6,300 |
Adjustments(5) | | | 600 | | | — | | | — | | | — | | | — | | | — | | | — | | | 600 |
2001 Cash payments | | | 3,800 | | | — | | | — | | | 200 | | | 200 | | | 1,000 | | | — | | | 5,200 |
2001 Non-cash charges | | | — | | | — | | | — | | | — | | | 100 | | | — | | | 100 | | | 200 |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
September 30, 2001 balance | | $ | 1,200 | | $ | — | | $ | — | | $ | 300 | | $ | — | | $ | — | | $ | — | | $ | 1,500 |
2002 Cash payments | | | 850 | | | — | | | — | | | 300 | | | — | | | — | | | — | | | 1,150 |
2002 Non-cash credit | | | 350 | | | — | | | — | | | — | | | — | | | — | | | — | | | 350 |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
September 30, 2002 balance | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
| (1) | Amount represents severance and termination costs for 151 terminated employees (primarily sales, marketing, and corporate personnel). As of September 30, 2002, all employees have been terminated as a result of the restructuring plan. |
| (2) | Amount represents write-offs of inventory and fixed assets associated with discontinued product lines. |
| (3) | Amount represents lease payments and shut down costs on exited facilities. |
| (4) | Amount represents income tax expense associated with the restructuring of our U.K. facilities. |
| (5) | Amount represents an increase in the severance costs for 16 employees (primarily corporate personnel). These employees are included in the total 151 terminated employees referenced above. |
49
Inflation
We do not believe that inflation has had a material impact on net sales or income during any of the periods presented above. There can be no assurance, however, that our business will not be affected by inflation in the future.
International Operations
Our U.S. subsidiaries hold approximately 77% of our assets and generated approximately 85% of our income from continuing operations for the fiscal year ended September 30, 2002, with the balance attributable to our foreign subsidiaries. Portions of our sales, income, and cash flows from both domestic and foreign subsidiaries are derived internationally. The financial position and the results of operations from substantially all of our international operations, other than most U.S. export sales, are measured using the local currency of the countries in which such operations are conducted and are then translated into U.S. dollars. While the reported income of foreign subsidiaries will be impacted by a weakening or strengthening of the U.S. dollar in relation to a particular local currency, the effects of foreign currency fluctuations are partially mitigated by the fact that manufacturing costs and other expenses of foreign subsidiaries are generally incurred in the same currencies in which sales are generated. Such effects of foreign currency fluctuations are also mitigated by the fact that such subsidiaries’ operations are conducted in numerous foreign countries and, therefore, in numerous foreign currencies. In addition, our U.S. export sales may be impacted by foreign currency fluctuations relative to the value of the U.S. dollar as foreign customers may adjust their level of purchases upward or downward according to the weakness or strength of their respective currencies versus the U.S. dollar.
From time to time we may employ currency hedges to mitigate the effect of foreign currency fluctuations. If currency hedges are not employed, we may be exposed to earnings volatility as a result of foreign currency fluctuations.
The following table sets forth our domestic sales and sales outside the United States for the six months ended March 31, 2003 and 2002 respectively, and in fiscal 2002, 2001, and 2000, respectively.
| | | Six Months Ended
March 31,
| | Fiscal Year Ended
September 30,
|
| | | 2003
| | 2002
| | 2002
| | 2001
| | 2000
|
| | | (unaudited) | | | | | | |
| | | (in thousands) |
Domestic net sales | | $ | 380,097 | | $ | 348,223 | | $ | 736,839 | | $ | 694,003 | | $ | 639,081 |
International net sales | | | 154,339 | | | 140,839 | | | 291,074 | | | 244,816 | | | 204,486 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total net sales | | $ | 534,436 | | $ | 489,062 | | $ | 1,027,913 | | $ | 938,819 | | $ | 843,567 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Liquidity and Capital Resources
Our capital requirements arise principally from indebtedness incurred in connection with our working capital needs, primarily related to inventory and accounts receivable, our capital expenditures, primarily related to purchases of machinery and molds, our common stock repurchase program, the purchase of businesses and product lines in execution of our acquisition strategy, the periodic expansion and/or acquisition of physical facilities, and our obligation to pay rent under the sale/leaseback facility.
Net cash provided by operating activities was $57.0 million for the six months ended March 31, 2003 as compared to $83.5 million for the same period in fiscal 2002. The cash outflow resulting from the net change in working capital, net of the effects of acquisitions and divestitures, was $27.6 million for the first six months of fiscal 2003. This cash outflow was primarily the result of increases in accounts receivable (approximately $1.2 million), inventories (approximately $8.9 million), and prepaid expenses and other current assets (approximately $3.4 million) and decreases in accounts payable (approximately $5.6 million), income taxes payable
50
(approximately $4.1 million), accrued payroll and employee benefits (approximately $2.7 million), accrued interest (approximately $0.4 million), and the restructuring reserve (approximately $0.7 million) and net changes in other assets and liabilities (approximately $3.8 million). The cash outflows were partially offset by a decrease in other current liabilities (approximately $3.0 million).
Net cash used in investing activities was $42.0 million for the six months ended March 31, 2003, as compared to $130.6 million for the same period in fiscal 2002. Net cash used in investing activities for the six months ended March 31, 2003 primarily reflects the net payment for businesses acquired of $21.6 million, capital expenditures of $22.6 million and the receipt of $1.5 million from an equity investment in a joint venture. Net cash used in investing activities for the six months ended March 31, 2002 primarily reflects payment for businesses acquired of $113.8 million and capital expenditures of $17.1 million. We have no current material commitments for capital expenditures but we do expect to incur approximately $65.0 million in purchases during fiscal year 2003.
Net cash used in financing activities was $24.8 million for the six months ended March 31, 2003, as compared to cash provided by financing activities of $87.7 million for the same period in fiscal 2002. The net cash used in financing activities for the six months ended March 31, 2003 primarily resulted from $69.3 million in treasury stock purchases and the repayment of $23.4 million of sellers notes offset by $62.7 million in net proceeds from our revolving credit facility. The net cash provided by financing activities for the six months ended March 31, 2002 was primarily related to proceeds from our CODES offering of $300.0 million (see below) and revolving credit facility of $179.5 million. These amounts were partially offset by payments made on the revolving credit facility of $388.0 million. Financing fees of $8.0 million were paid in connection with the CODES offering.
Approximately $192.2 million of cash was generated from operating activities in 2002, an increase of $12.8 million or 7% from 2001. Non-cash depreciation and amortization charged against net income decreased approximately $17.9 million for 2002 primarily as a result of the adoption of SFAS No. 142. The cash outflow resulting from the increase in working capital, net of the effects of acquisitions and divestitures, was $23.6 million for 2002, or an increase in cash outflow of $24.2 million, as compared to $0.6 million in cash inflow for 2001. These changes are set forth in detail in the Consolidated Statements of Cash Flows. The increase in working capital accounts throughout 2002 is attributable to the higher level of business activity as reflected in our increased sales.
Investing activities in fiscal 2002 used approximately $198.3 million of cash. This outflow was due primarily to cash used for acquisitions of $139.7 million. Capital expenditures were $63.6 million for fiscal 2002, compared to $50.1 million in fiscal 2001.
On April 4, 2001, we issued $325.0 million of unsecured senior notes in a private placement with Registration Rights, and in August 2001, we completed a registered exchange of the privately placed notes for similar notes that had been registered with the Commission. The notes were offered at a discount of approximately $1.5 million. They will mature on April 1, 2011. Interest is fixed at an annual rate of 8% and is payable on April 1 and October 1 of each year, beginning on October 1, 2001. The notes are redeemable by us at any time in whole, or from time to time in part, at a price equal to the greater of (i) 100% of the principal amount of the notes to be redeemed or (ii) the sum of the present values of the remaining scheduled payments of principal and interest thereon (exclusive of interest accrued to the date of redemption) discounted to the date of redemption on a semiannual basis at the applicable Treasury Yield (as defined in the bond agreement) plus 35 basis points, plus accrued interest to the date of redemption. The notes are guaranteed by our material U.S. subsidiaries, which also guarantee our obligations under our revolving credit facility.
On October 10, 2001, we issued $300.0 million of senior convertible contingent debt securities (CODES). The CODES have a fixed interest rate of 2.25% per annum. Interest is payable on April 15 and October 15 of each year. We also must pay contingent interest during any six-month period if the average trading price of the
51
CODES during a specified period of five trading days preceding the relevant six-month period is above specified levels. The CODES will mature on October 15, 2021. The CODES are convertible, subject to certain conditions (based on specified factors, including but not limited to the sale price of our common stock, trading prices of the CODES, maintenance of our credit ratings, and the occurrence of specified corporate transactions, including certain repurchases of our common stock), into our common stock, currently at a price of approximately $30.49 per share. We may redeem some or all of the CODES on or after October 20, 2004. The holders may require us to purchase all or a portion of their CODES on October 20, 2004 and on October 15, 2006, 2011, and 2016, or, subject to specified exceptions, upon a change of control event. Certain of our U.S. subsidiaries guarantee our obligations under the CODES.
We maintain a $500 million revolving credit facility. At March 31, 2003, the outstanding balance under this facility was $62.7 million and $430.8 million was available.
During our fiscal quarter ended September 30, 2002, we purchased approximately one million shares of our common stock on the open market. During our current fiscal year we have purchased approximately 4.1 million additional shares on the open market. The purchase price for these open market purchases during our current fiscal year has totaled approximately $69.3 million, or an average of approximately $16.95 per share. Our board of directors has authorized the repurchase of approximately 6.9 million additional shares on the open market during the period ending October 1, 2005. Shares will be repurchased at times and prices deemed appropriate to us. We will use cash generated from operations as well as available credit facilities in order to finance the repurchase of these shares.
Consistent with the strategy to repurchase our shares, on April 23, 2003, we filed a Tender Offer Statement on Schedule TO, which represented the offer to purchase, through a modified Dutch tender auction, up to 15.0 million shares of our common stock (including associated preferred stock purchase rights), representing approximately 15% of our outstanding common shares as of April 22, 2003. The tender offer expired on May 21, 2003. We repurchased 6.0 million of our common shares in the tender offer at $17.50 per share, or $105.4 million in total.
On May 2, 2003, we entered into an amendment of our revolving credit facility which allowed us to consummate the tender offer.
On June 2, 2003, the Company issued the $250 million Original Notes in a private placement with registration rights. The Company has used or intends to use the proceeds from the issuance to repay $40.0 million of its revolving credit facility, to repurchase shares of its common stock pursuant to the Dutch tender auction, to repurchase shares on the open market and otherwise, and for general corporate purposes. The Original Notes will mature on May 15, 2013. Interest is fixed at an annual rate of 6½% and is payable on May 15 and November 15 of each year, beginning on November 15, 2003. Interest accrues from June 2, 2003. Up to 35% of the aggregate principal amount of the Original Notes are redeemable by the Company, in whole or in part, at any time prior to May 15, 2006, at a redemption price of 106.50% of the principal amount with the cash proceeds of certain equity offerings. After May 15, 2008, the Original Notes are redeemable at 103.250%, 102.167%, 101.083%, and 100.000% if redeemed during the twelve-month period beginning on May 15 of 2008, 2009, 2010, and 2011 and thereafter, respectively.
The 6½% senior subordinated notes, the CODES, 8% senior notes, and the revolving credit facility all contain certain cross default provisions. The revolving credit facility includes financial and operating covenants which, if not met, could result in acceleration of payments on outstanding balances. In addition, the 6½% senior subordinated notes, the CODES, 8% senior notes, and/or the revolving credit facility contain covenants which, among other things, restrict investments, loans, and advances, require that we maintain certain financial ratios, require that we maintain certain credit ratings, restrict our ability, and the ability of our subsidiaries, to create or permit liens, or to pay dividends or make other restricted payments (as defined), and limit our incurrence of additional indebtedness. We are not aware of any violations of these covenants and do not anticipate any such violations in light of current business conditions.
52
We believe that loans under our revolving credit facility, proceeds from the sale of the notes, and currently available cash and short term investments, along with cash generated from operations, will be sufficient to finance our working capital needs, as well as our capital expenditures, special charges, and business development needs.
Although we currently do not have specific plans, we may, in the future, depending on business and market conditions, refinance or replace all or a portion of the cash used to purchase shares with proceeds from the sale of debt or equity securities or such other financing as we deem appropriate.
Application of Critical Accounting Policies
The preparation of our financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues, and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, management evaluates these estimates and judgments. Certain of our accounting policies represent a selection among acceptable alternatives under GAAP; however, management believes the policies used best represent the underlying transactions reflected in the financial statements. We believe the following critical accounting policies affect our more significant estimates and judgments used in the preparation of our consolidated financial statements.
We recognize revenue upon shipment of products when persuasive evidence of a sales arrangement exists, the price to the buyer is fixed and determinable, and collectibility of the sales price is reasonably assured. Large portions of our sales are sold through distributors. Revenues associated with sales to distributors are also recognized upon shipment of products when all risks and rewards of ownership of the product have passed.
In connection with transitional and annual impairment tests for SFAS No. 142, fair market valuations are performed for each of the reporting units. These valuations require certain assumptions from management regarding future operating performance as well as various industry trends. Fluctuations in these assumptions could have a material impact on the values ascribed to the reporting units and could result in an indication of impairment. These assumptions include, but are not limited to, estimated future cash flows, estimated sales growth, and weighted average cost of capital for each of the reporting units. In order to make informed assumptions, management relies on certain public information and statistical and industry information as well as internal forecasts to determine the various assumptions. In the event that industry, general economic, and company trends change, these assumptions will change and impact the calculated fair market values.
Through our acquisition program, we have accumulated a large number of intangible assets. The allocation of purchase price premiums to intangible assets, tangible assets, and goodwill involves estimates based on fair value assumptions. Estimated lives assigned to the tangible and intangible assets acquired in a business purchase also involve the use of estimates. These matters are determined subject to judgments and estimates that are inherently uncertain, and different amounts could be reported using different methodologies. Management uses its best estimate in determining the appropriate values and estimated lives to reflect in the financial statements, using historical experience, market data, and all other available information. In most instances, we use outside valuation firms to recommend purchase price allocations and estimated lives after an acquisition is completed.
We have three defined benefit pension plans covering a significant number of domestic employees. Accounting for these plans requires the use of actuarial assumptions, including estimates of the expected long-term rate of return on assets and discount rates. In order to make informed assumptions, management relies on outside actuarial firms as well as public market data, and general economic information. If changes in any of these assumptions occur, they may materially affect certain amounts reported on our balance sheet. In particular, a decrease in the expected long-term rate of return on plan assets could result in an increase in our pension expense, lowering our net equity.
53
We record a valuation allowance to reduce our deferred tax assets to an amount that management estimates is more likely than not to be realized. While management has considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, should management determine that it would be able to realize our deferred assets in the future in excess of the net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made. However, should management determine that it would not be able to realize all or part of the net deferred tax asset in the future, an adjustment to the deferred tax asset would be charged to income in the period such a determination was made.
Off-Balance Sheet Arrangement
We hold a minority interest in an unconsolidated joint venture that is accounted for as an equity investment. We own less than 50% of the underlying joint venture. As of March 31, 2003, the equity investment in this entity, included in “Other Assets,” was approximately $8.3 million. Net income from the joint venture for the six months ended March 31, 2003 was $0.9 million, and is included in “Other Income.” The joint venture is limited to the extent it can incur any debt other than trade payables arising out of its business activities and it does not hold any assets other than inventory and trade receivables. As of March 31, 2003, the joint venture did not have any debt, other than trade payables arising out of its business activities.
Disclosures About Contractual Obligations and Commercial Commitments
In our day-to-day business activities, we incur certain obligations and commitments to make future payments under contracts such as debt and lease agreements. Maturities of these obligations are set forth in the following table as of March 31, 2003 (in millions):
| | | | | Payments Due by Period
|
Contractual Obligations
| | Total
| | Less than 1 Year
| | 1 – 3 Years
| | 4 – 5 Years
| | After 5
Years
|
Long-term debt | | $ | 713.2 | | $ | 16.2 | | $ | 373.3 | | $ | — | | $ | 323.7 |
Capital lease obligations | | | 0.5 | | | 0.2 | | | 0.3 | | | — | | | — |
Operating leases | | | 70.1 | | | 12.3 | | | 19.8 | | | 14.7 | | | 23.3 |
Unconditional purchase obligations | | | 15.6 | | | 14.2 | | | 1.4 | | | — | | | — |
Other long-term obligations | | | — | | | — | | | — | | | — | | | — |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total contractual cash obligations | | $ | 799.4 | | $ | 42.9 | | $ | 394.8 | | $ | 14.7 | | $ | 347.0 |
| | | Total
Amounts
Committed
| | | Amount of Commitment Expiration Per Period
|
Other Commercial Commitments
| | | Less Than
1 Year
| | 1 – 3 Years
| | 4 – 5 Years
| | Over 5
Years
|
Lines of Credit | | $ | — | | | $ | — | | $ | — | | $ | — | | $ | — |
Standby Letters of Credit | | | 6.5 | | | | 6.5 | | | — | | | — | | | — |
Guarantees | | | — | (1) | | | — | | | — | | | — | | | — |
Standby Repurchase Obligations | | | — | | | | — | | | — | | | — | | | — |
Other Commercial Commitments | | | — | | | | — | | | — | | | — | | | — |
| | |
|
|
| |
|
| |
|
| |
|
| |
|
|
Total Commercial Commitments | | $ | 6.5 | | | $ | 6.5 | | $ | — | | $ | — | | $ | — |
| (1) | Certain of our domestic subsidiaries are guarantors under our revolving credit facility, 8% senior notes, and CODES. These amounts are included under Contractual Obligations above as long-term debt. |
54
BUSINESS
General
Apogent Technologies Inc. is a Wisconsin corporation, incorporated in 1993 to be the successor by merger in January 1994 to Sybron Corporation, a Delaware corporation. The merger was accomplished to change our corporate domicile from Delaware to Wisconsin. We changed our name from Sybron International Corporation to Apogent Technologies Inc. in January 2001.
Our operating subsidiaries are engaged primarily in the manufacture and sale of laboratory products in the United States and other countries. Our operations are divided into two reporting business segments: the Clinical Group and the Research Group.
Business Segments
We are a leading developer and manufacturer of products for the clinical and research industries. We are organized into two business segments, the Clinical Group and the Research Group, to serve our customers in these industries. During the quarter ended March 31, 2003, we changed our reporting business segments by combining the laboratory equipment and the labware life sciences segments into a new segment, the Research Group. This change aligns our financial reporting with the management of our operational activity. In addition, we renamed our clinical diagnostics segment as the Clinical Group. All historical financial information for the six-month periods ended March 31, 2003 and 2002 and for each year in the five-year period ended September 30, 2002, have been restated to reflect this change.
Our subsidiaries manufacture most of the products we sell in our approximately 60 manufacturing facilities. We have over 125 facilities worldwide. Our customers include distributors, pharmaceutical and biotechnology companies, clinical, research and industrial laboratories, OEMs, and others. Approximately 72% of our consolidated net sales in fiscal 2002 were generated from sales transactions with customers within the U.S. and the remainder was generated internationally, mostly from Europe and Japan.
The end-users of our products include scientists and lab technicians in the fields of life science research, clinical diagnostics, and basic scientific research. These individuals typically work at pharmaceutical companies, other types of manufacturing companies, hospitals, scientific research organizations, academic and government institutions, and clinical reference laboratories.
Clinical Group
Our Clinical Group manufactures and sells products primarily to clinical and commercial laboratories and to scientific research and industrial customers. These products are used in a number of diagnostic applications, including specimen collection, specimen transportation, drug testing, therapeutic drug monitoring, infectious disease detection, and glucose tolerance testing. Other applications include anatomical pathology (histology and cytology) and immunohistochemistry, with an emphasis on cancer applications. Clinical Group products include:
| | • | microscope slides, cover glass, and glass tubes and vials; |
| | • | histology and immunochemistry instrumentation; |
| | • | sample vials used in diagnostic testing; |
| | • | diagnostic reagents; and |
| | • | other products used in detecting causes of various infectious diseases, conditions, and therapeutic drugs or drugs of abuse. |
55
Our primary U.S. and foreign subsidiaries in this business segment include:
Capitol Vial, Inc.
Chase Scientific Glass, Inc.
Erie Electroverre S.A.
Erie Scientific Company
Gerhard Menzel Glasbearbeitungswerk GmbH & Co. K.G.
Lab Vision Corporation
Microgenics Corporation
Microm International GmbH
Neomarkers, Inc.
The Naugatuck Glass Company
Remel Inc.
Richard-Allan Scientific Company
Samco Scientific Corporation
Seradyn Inc.
During fiscal 2002, we acquired: Separation Technology, a manufacturer of tabletop microhematocrit centrifuge systems; Capitol Vial, a manufacturer of plastic vials and related products for sample collection and processing; and the automotive vanity mirror business of SMC Manufacturing.
During the six months ended March 31, 2003 we acquired: NeoMarkers, Inc. (developer and manufacturer of antibodies, reagents and related products for life science research applications); and Opus Diagnostics Inc. (developer and distributor of diagnostic assays, controls and calibrators for therapeutic drug monitoring).
The Clinical Group accounted for approximately 46% of our consolidated net sales in 2002, 2001, and 2000. For the fiscal years 2002, 2001, and 2000, net sales for this segment grew 10%, 11%, and 21%, respectively, compared to the prior year.
Research Group
Our Research Group manufactures, distributes, and sells products primarily to the research and clinical life sciences industries. Applications of these products include general everyday laboratory uses as well as genomics, proteomics, high-throughput screening for drug discovery, combinatorial chemistry, cell culture, filtration, and liquid handling. In addition, this segment manufactures, distributes, and sells basic laboratory equipment used by medical, pharmaceutical, and scientific laboratories. Research Group products include:
| | • | reusable plastic and glass products (e.g., bottles, carboys, graduated ware, beakers, flasks, and plastic bottles for consumer use); |
| | • | disposable plastic and glass products; |
| | • | products for critical packaging applications; |
| | • | environmental and safety containers; |
| | • | liquid handling automation products; |
| | • | autosampler vials and seals used in chromatography analysis; |
| | • | various consumable products for use in applications of cell culture, filtration, molecular biology, cryopreservation, immunology, electrophoresis, liquid handling, genomics, and high-throughput screening for pharmaceutical drug discovery; and |
| | • | heating, cooling, shaking, stirring, mixing, and temperature control instruments. |
56
Our primary U.S. and foreign subsidiaries in this business segment include:
Advanced Biotechnologies Ltd.
Chromacol Limited
Marsh Bio Products, Inc.
Matrix Technologies Corporation
Molecular BioProducts, Inc.
Nalge Nunc International Corporation
Nalge Nunc International K.K.
National Scientific Company
Nunc A/S
Barnstead Thermolyne Corporation
Electrothermal Engineering, Ltd.
Genevac Ltd.
Lab-Line Instruments, Inc.
In addition, we participate as a minority owner of a sales and marketing joint venture with Kimble Glass, Inc. that, through Nalge Nunc International’s marketing and sales efforts, markets and sells Kimble/Kontes reusable, disposable and specialty glassware for the laboratory.
During fiscal 2002, we acquired: Marsh Bio Products, a distributor of laboratory equipment and consumables, including products manufactured at our U.K. Advanced Biotechnologies Ltd. facility; TFO, Incorporated, a manufacturer of consumer hydration products for athletic, outdoor and other uses; Chromocal/Epsom, a manufacturer and distributor of chromatography vials and related products; Barden Engineering, an industrial tool manufacturer for our ABgene division; and Cosmotec Company, a manufacturer of high-throughput liquid dispensing instrumentation.
During the six months ended March 31, 2003, we acquired Tempyrox, Inc., a developer and manufacturer of high-temperature cleaning ovens for industrial and laboratory applications.
The Research Group business segment accounted for approximately 54% of our net sales in 2002, 2001, and 2000, with sales growing 12%, 9%, and 21%, respectively, compared to the prior year.
Discontinued Operations
During March 2002, we made the decision to dispose of our vacuum deposition chamber business, Vacuum Process Technology. The decision was made following a slow-down in the telecommunications industry, in which Vacuum Process Technology targeted a majority of its products, and as a result, the business no longer met our strategic requirements. In the second quarter of fiscal 2002, in connection with the discontinuance of this business, we incurred a charge of $13.2 million, net of income tax benefit of $7.6 million, related to the write-down of net assets to their estimated fair value less costs to sell. The decision to sell Vacuum Process Technology represents a disposal of long-lived assets and disposal group under SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.” Accordingly, results of this business have been classified as discontinued operations, and prior periods have been restated. During the second quarter of fiscal 2003, we completed the sale of Vacuum Process Technology and recorded an additional charge of $2.8 million, net of income tax benefit of $1.6 million. For business reporting purposes, Vacuum Process Technology was previously classified in the Clinical Group segment.
During March 2003, we made the decision to dispose of two businesses: the rapid diagnostic test business (on-site rapid tests used in the detection of pregnancy, drugs of abuse and infectious diseases) as conducted by our Applied Biotech subsidiary; and the manufacture and sale of automated microarray instrumentation for the genomics market as conducted by our BioRobotics Group subsidiary. The decision was based in part on our
57
ongoing strategy of strengthening the market positions of our leading brands and focusing on sales of our consumable laboratory products that have more stable growth expectations. As a result, these businesses no longer met our strategic requirements. In the second quarter of fiscal 2003, in connection with the discontinuance of these businesses, we incurred a charge of approximately $85.9 million, net of income tax benefit of $21.6 million, related to the write-down of net assets to their estimated fair value less costs to sell. The decision to sell these companies represented a disposal of long-lived assets and disposal group under SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.” Accordingly, results of these businesses have been classified as discontinued operations, and prior periods have been restated to reflect this reclassification. We are actively pursuing the sale of these businesses and anticipate disposal within the next twelve months. In the event we ultimately dispose of Applied Biotech and BioRobotics Group for an amount less than the carrying value of the businesses, an additional charge will be recognized upon disposal. For business reporting purposes, Applied Biotech was previously classified in the Clinical Group segment and BioRobotics Group was classified in the Research Group segment.
In December 2000, we spun off our dental business by way of a pro rata distribution to our shareholders of all the outstanding common stock and related preferred stock purchase rights of Sybron Dental Specialties. As a result of the spin-off, Sybron Dental Specialties has been accounted for as a discontinued operation.
Certain Financial Information
The following table sets forth our net sales by segment.
| | | Six Months Ended March 31,
| | Years Ended September 30,
|
| | | 2003
| | 2002
| | 2002
| | 2001
| | 2000
|
| | | (unaudited) | | | | | | |
| | | (in thousands) |
Clinical Group | | $ | 251,930 | | $ | 228,383 | | $ | 473,388 | | $ | 431,193 | | $ | 390,097 |
Research Group | | | 282,506 | | | 260,679 | | | 554,525 | | | 507,626 | | | 453,470 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total Net Sales | | $ | 534,436 | | $ | 489,062 | | $ | 1,027,913 | | $ | 938,819 | | $ | 843,567 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
We have included other financial information about our business segments and foreign operations in Note 15 to our consolidated financial statements.
New Products
During fiscal 2002, we introduced a number of new products that contributed approximately $16.9 million in net sales. No single new product or group of related products was material to our business or either of our business segments.
Business Strategy
Our goal is to consistently grow our worldwide market presence, net sales, earnings, and cash flows. Annual revenue growth in fiscal 2002 was $89.1 million, or 9.5% over the prior year, and in the first six months of fiscal 2003 was $45.4 million or 9.3% over the comparable prior year period. Key elements of our strategy continue to be:
Maintain prudent capital structure. We consider our ability to manage our balance sheet effectively to be a core competence. Strategic investments which require an outlay of capital are put through a rigorous review process to ensure the investment provides an acceptable cash return which exceeds our cost of capital. We maintain a capital structure which provides us with the financial flexibility to compete effectively in our core markets.
58
Develop profitable new products. We consistently strive to develop and introduce new products that contribute to sales, earnings, and cash flows. These products include new offerings and improvements of our existing products. We are especially focused on developing new products for our life sciences research and clinical diagnostics customers.
Increase sales to existing and new customers. We seek to leverage our strong market presence and excellent customer and distributor relationships into increased sales to current customers and sales to new customers. We believe that our extensive product offering is conducive to cross-selling products to existing customers. This broad product offering is also conducive to negotiating favorable terms with our distributors.
Improve operating efficiencies. We are focused on improving our operating efficiencies through vertical integration, streamlined manufacturing techniques, better product sourcing, and the sharing of technology and best practices across our company. We believe that our focus on efficiencies improves our gross margins while maintaining or improving the quality of our products and increasing customer satisfaction.
Make strategic acquisitions. Our acquisition program has been and continues to be focused on adding complementary products and technologies that enhance our market position. Our operating subsidiaries generally have been able to use their existing channels to market our acquired products. We have a rigorous process of candidate identification, due diligence, and integration designed to mitigate acquisition risk. Acquired businesses are converted to our standard financial reporting system. In most cases, we retain the senior management of acquired businesses and have an integration plan and budget in place at the time the acquisition closes. We presently intend to reduce our emphasis on our acquisition strategy and focus on steadily increasing our earnings and cash flows through the development of new products, increasing sales to existing and new customers, and focusing on improving efficiencies.
Sales, Marketing, and Distribution
Industrial, academic, clinical, governmental, pharmaceutical, and biotechnology laboratories are existing and potential customers for our products. Our products reach these customers in several ways. Our Research Group business segment sells primarily through distributors, although some of our companies in this segment, such as Matrix Technologies and Marsh Bio Products, have direct sales forces and sell directly to end-users. Sales from our Clinical Group businesses are made both directly and through distribution, depending on the type of product and/or the type of end-user. For example, Richard-Allan and Microm International sell directly to end-users, the microbiology products of our Remel subsidiary are primarily sold directly to end-users, and the drugs of abuse testing products of Microgenics are primarily sold to OEMs of immunoassay analyzers. Most of our subsidiaries maintain their own sales forces, whether they sell directly to end-users, through distribution, or otherwise.
Our net sales performance is affected by short-term volatility in demand from distributors. We also experience volatility in demand when distributors merge or consolidate, when distributors do not manage their inventories to end-user demand and when distributors otherwise experience softness in sales.
Our major distributors offer a wide variety of supplies, apparatus and instruments for the laboratory, primarily through catalogs and through e-commerce web sites. End-users rely heavily on these catalogs and web sites in identifying suitable products and making purchase decisions, and the prominence of and the number of product items listed for a particular vendor are critical marketing variables. We believe the number of our products offered by the major distributors is among the highest of any of our competitors. Also, the major distributors often have contracts with large end-users or purchasing organizations to supply such users or organizations with a broad array of laboratory products and supplies. We believe that our ability to manufacture and supply a broad range of products can help distributors be more efficient in these situations.
59
Our three major distributors (primarily domestic), Fisher Scientific, VWR, and Allegiance Corporation, accounted in the aggregate for approximately 27% of our Clinical Group sales and 36% of our Research Group sales in fiscal 2002. See Notes 2 and 15 to our consolidated financial statements. The loss of any one of these major laboratory distributors could have a material adverse effect on our business. Only a few of our subsidiaries have written contractual relationships with these distributors. However, our subsidiaries have long-standing relationships with them or their predecessors.
Our subsidiaries private label for and sell products to a number of original equipment manufacturers (OEMs). These private label and OEM relationships are most prevalent in our Clinical Group business segment, although subsidiaries in the Research segment also enter into OEM and private label relationships as opportunities arise. Volatility in demand can arise if these customers fail to manage inventories to end-user demand, discontinue product lines, or switch business to other manufacturers.
Geographic Information
Our U.S. subsidiaries held approximately 77% of our assets as of September 30, 2002 and generated approximately 85% of our income from continuing operations (calculated as a percentage of income generated in total by our domestic and foreign subsidiaries and excluding corporate office expenses—See Note 16 to our consolidated financial statements) for the fiscal year ended September 30, 2002, with the balance attributable to our foreign subsidiaries. In addition to an extensive distributor network, our subsidiaries maintain sales offices and manufacturing plants in many international locations. Foreign sales offices are located in the United Kingdom, Japan, Germany, Spain, Hong Kong, Australia, and Switzerland. International manufacturing facilities are located in Denmark, Germany, Switzerland, Hungary, the United Kingdom, Hong Kong, Japan, Mexico, and Puerto Rico.
Domestic and international sales of our products by business segment are as follows:
| | | Six Months Ended
March 31,
| | Fiscal Year Ended September 30,
|
| | | 2003
| | 2002
| | 2002
| | 2001
| | 2000
|
| | | (unaudited) | | | | | | |
| | | (in thousands) |
Clinical Group: | | | | | | | | | | | | | | | |
Domestic | | $ | 197,485 | | $ | 178,791 | | $ | 372,108 | | $ | 345,847 | | $ | 318,279 |
International | | | 54,445 | | | 49,592 | | | 101,280 | | | 85,346 | | | 71,818 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total | | $ | 251,930 | | $ | 228,383 | | $ | 473,388 | | $ | 431,193 | | $ | 390,097 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Research Group: | | | | | | | | | | | | | | | |
Domestic | | $ | 182,612 | | $ | 169,432 | | $ | 364,781 | | $ | 348,156 | | $ | 314,710 |
International | | | 99,894 | | | 91,247 | | | 189,794 | | | 159,470 | | | 138,760 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total | | $ | 282,506 | | $ | 260,679 | | $ | 554,525 | | $ | 507,626 | | $ | 453,470 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
We have included other financial information about our business segments and foreign operations in Note 15 to our consolidated financial statements.
Research and Development
We have a number of research and development programs in our two business segments. We spent approximately $12.6 million, $23.4 million, $19.2 million, and $17.2 million on research and development in the first six months of 2003, and fiscal years 2002, 2001, and 2000, respectively, focused primarily on product development. We have, from time to time, worked on customer-sponsored research and development programs, and expect to continue to do so in the future.
60
Our research and development expenditures by business segment are as follows:
| | | Six Months Ended
March 31,
| | Years Ended September 30,
|
| | | 2003
| | 2002
| | 2002
| | 2001
| | 2000
|
| | | (unaudited) | | | | | | |
| | | (in thousands) |
Clinical Group | | $ | 4,664 | | $ | 4,017 | | $ | 8,340 | | $ | 6,882 | | $ | 6,979 |
Research Group | | | 7,949 | | | 7,393 | | | 15,024 | | | 12,271 | | | 10,199 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total | | $ | 12,613 | | $ | 11,411 | | $ | 23,365 | | $ | 19,153 | | $ | 17,178 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Backlog
The dollar amount of backlog orders that we believe to be firm commitments is not material to our business.
Seasonality
Neither of our business segments is seasonal to a material extent.
Markets; Competition
Our products serve a large number of markets worldwide in which there are numerous competitors. We strive to achieve a leading market share in every market in which we compete, and we believe that our size and breadth of products offered as well as our relationships with our customers provide us with competitive advantages relative to many of our small and mid-sized competitors. The strategies outlined under “Business Strategy” above are key to our ability to stay competitive, although there can be no assurance that we will not encounter increased competition in the future.
We have significant competitors in each of our business segments. Our competitors include product manufacturers, private label resellers and product distributors, a number of whom have substantially greater financial and other resources than ours. Product price, product quality, product brand recognition, customer service, breadth of product lines and convenience for customers are relevant factors to achieving and maintaining our competitive position.
Principal competitors in the Clinical Group business segment include (among others) Shandon (a subsidiary of Thermo Electron Corporation), Leica Microsystems, Sakura Finetek, Knittel Glaser, Surgipath Medical Industries, Inc., Ventana Medical Systems, Dako A.S., Tyco Plastics, Becton Dickinson, Meridian Diagnostics, Dade Behring, Roche Diagnostics, Quidel Corporation, Inverness Medical and Fisher Scientific.
Our principal competitors in the Research Group segment include (among others) Corning Incorporated, Millipore Corporation, Becton Dickinson, Mettler Toledo, Beckman Coulter, and Greiner Holding AG. Fisher Scientific, Corning Incorporated, Millipore Corporation, New Brunswick Scientific Company, Inc., Forma Scientific, Inc., and SPX Corporation.
Employees
We employed approximately 7,200 people at March 31, 2003, of which approximately 5,130 were located in the U.S. As of March 31, 2003, approximately 314 of our U.S. employees were covered by collective bargaining agreements. We believe our relationships with the unions are good, and we expect that, upon expiration, all of the agreements will either be extended or new agreements will be entered into on terms substantially similar to the existing terms. Many of our non-management employees in Europe are subject to national labor contracts, which are negotiated from time to time at the national level between the national labor union and an employers’ council.
61
Once national contracts are set, further negotiation may take place at the local level. Such negotiations may affect local operations. Our Danish subsidiary, Nunc A/S, was closed during the third quarter of 1998 for nine days as the result of the first national strike in Denmark since 1985. After the national strike was settled, Nunc A/S non-management employees struck for two days over local issues. All issues were resolved in a new year to year contract which next expires in 2004. The national industry contract is scheduled for renegotiation in 2004.
Government Regulation
Medical Devices. Certain of our products are medical devices that are subject to regulation by the Food and Drug Administration (FDA) and by the counterpart agencies of the foreign countries where our products are sold. Some of the regulatory requirements of these foreign countries are more stringent than those applicable in the United States. Pursuant to the Federal Food, Drug, and Cosmetic Act (the “FDCA”), the FDA regulates virtually all phases of the manufacture, sale, and distribution of medical devices, including their introduction into interstate commerce and their advertising, labeling, packaging, marketing, distribution, and recordkeeping. Pursuant to the FDCA and FDA regulations, certain facilities of our operating subsidiaries are registered with the FDA as medical device manufacturing establishments, and many of our products are regulated as Class I or Class II medical devices. The FDA regularly inspects these facilities and operations. During fiscal 2002, we did not experience any material FDA issues.
Environmental, Health and Safety. Our operations entail a number of environmentally sensitive production processes. Compliance with environmental laws and regulations, along with regulations relating to workplace safety, is a priority in our businesses. Our domestic facilities are subject to federal, state, and local laws and regulations concerning, among other things, solid and hazardous waste disposal, air emissions and wastewater discharge. Our foreign facilities are subject to local laws and regulations regarding the environment. Our operations are also subject to regulation relating to workplace safety, both in the United States and abroad. During fiscal 2002, we did not make, and don’t anticipate having to make, any material capital expenditures for environmental control facilities.
Patents, Trademarks and Licenses
Our products are sold under a variety of trademarks and trade names. We own or license all of the trademarks and trade names we believe to be material to the operation of our businesses, including the ART®, ABgene™, BARNSTEAD®, CAPITOL VIAL®, COLORFROST®, KIMAX®, KIMBLE®, LAB-LINE®, MARSH BIO PRODUCTS®, NALGE®, NALGENE®, NUNC®, REMEL®, RICHARD-ALLAN SCIENTIFIC®, SUPERFROST® and THERMOLYNE® trademarks, each of which we believe to have widespread name brand recognition in its respective field and all of which we intend to continue to protect. We also own various patents, employ various patented processes, and from time to time acquire licenses from owners of patents. In addition to trade secret, copyright, patent and trademark laws, we rely upon a combination of non-disclosure and other contractual agreements to protect our intellectual property rights. Except for the trademarks referred to above, we do not believe any single patent, trademark or license is material to the operations of our business as a whole.
Raw Materials
We purchase a wide range of raw materials and supplies from a number of suppliers, and except with respect to our supply of white glass, we do not rely on sole sources to any material extent. All of our white glass comes from a single source, our Electroverre, SA facility in Switzerland. In the event that Electroverre could not continue to supply the necessary white glass, we would have to seek alternative sources, which could have a material effect on our Clinical Group segment. We do not foresee any significant difficulty in obtaining necessary materials or supplies.
62
Risks Attendant to Foreign Operations
We conduct our businesses in numerous foreign countries and as a result are subject to risks of fluctuations in exchange rates of various foreign currencies and other risks associated with foreign trade. See, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—International Operations” in this prospectus for further information concerning the possible effects of foreign currency fluctuations and currency hedges intended to mitigate their impact.
Properties
We currently lease or own over 3.5 million square feet worldwide. Typically, each of our subsidiaries maintains its own manufacturing, research and development, warehouse and office space.
The following table sets forth information regarding our principal properties by business segment. Properties with less than 20,000 square feet of building space have been omitted from this table.
Location
| | Building space and use
| | Owned or leased
|
|
| Clinical Group |
Rockwood, Tennessee | | 220,000 sq. ft./manufacturing, warehouse, and office | | owned |
Portsmouth, New Hampshire | | 162,000 sq. ft./manufacturing, warehouse, and office | | leased |
Braunschweig, Germany | | 40,000 sq. ft./manufacturing, warehouse, and office | | owned |
Romont, Switzerland | | 200,000 sq. ft./manufacturing, warehouse, and office | | owned |
Aguadilla, Puerto Rico | | 23,000 sq. ft./manufacturing, warehouse, and office | | leased |
Naugatuck, Connecticut | | 80,000 sq. ft./manufacturing, warehouse, and office | | owned |
Budapest, Hungary | | 28,000 sq. ft./manufacturing, warehouse, and office | | owned |
San Fernando, California | | 77,000 sq. ft./manufacturing, warehouse, and office | | owned |
Meiningen, Germany | | 22,000 sq. ft./manufacturing, warehouse, and office | | owned |
Holtsville, New York | | 30,000 sq. ft./manufacturing, warehouse, and office | | owned |
Baltimore, Maryland | | 21,000 sq. ft./manufacturing and office | | leased |
Kalamazoo, Michigan | | 116,000 sq. ft./manufacturing, warehouse, and office | | leased |
Indianapolis, Indiana | | 49,000 sq. ft./manufacturing and warehouse | | leased |
Lenexa, Kansas | | 116,000 sq. ft./manufacturing and office | | owned |
Lenexa, Kansas | | 63,000 sq. ft./warehouse and office | | leased |
Lake Charles, Louisiana | | 24,000 sq. ft./manufacturing and office | | owned |
Ramsey, Minnesota | | 25,000 sq. ft./manufacturing, warehouse, and office | | leased |
San Diego, California | | 72,000 sq. ft./manufacturing, warehouse, and office | | leased |
Fremont, California | | 109,000 sq. ft./manufacturing, warehouse, and office | | leased |
Austin, Texas | | 26,000 sq. ft/manufacturing, warehouse, and office | | leased |
East Providence, Rhode Island | | 69,000 sq. ft./manufacturing, warehouse, and office | | leased |
Walldorf, Germany | | 24,000 sq. ft./manufacturing | | leased |
Kent, England | | 45,000 sq. ft./manufacturing, warehouse, and office | | leased |
Auburn, Alabama | | 67,000 sq. ft./manufacturing, warehouse, and office | | owned |
Fultonville, New York | | 30,000 sq. ft./manufacturing, warehouse, and office | | owned |
63
Location
| | Building space and use
| | Owned or leased
|
|
| Research Group |
Penfield, New York | | 340,000 sq. ft./manufacturing, warehouse, and office | | leased |
New Castle, Delaware | | 26,000 sq. ft./manufacturing, warehouse, and office | | leased |
Wiesbaden, Germany | | 21,000 sq. ft./warehouse and office | | leased |
Naperville, Illinois | | 103,000 sq. ft./manufacturing, warehouse, and office | | owned |
Roskilde, Denmark | | 151,000 sq. ft./manufacturing, warehouse, and office | | owned |
Ichikawa, Japan | | 38,000 sq. ft./warehouse | | leased |
Hudson, New Hampshire | | 59,000 sq. ft./manufacturing, warehouse, and office | | leased |
Otay, California | | 29,000 sq. ft./warehouse | | leased |
Duluth, Georgia | | 55,000 sq. ft./office and warehouse | | leased |
Tijuana, Mexico | | 87,000 sq. ft./manufacturing, warehouse, and office | | leased |
San Diego, California | | 52,000 sq. ft./manufacturing and office | | leased |
Hereford, England | | 24,000 sq. ft./warehouse and office | | leased |
Portsmouth, New Hampshire | | 27,000 sq. ft./manufacturing, warehouse, and office | | leased |
Sunnyvale, California | | 70,000 sq. ft./manufacturing and office | | leased |
Surrey, England | | 45,000 sq. ft./manufacturing, office, and warehouse | | leased |
Surrey, England | | 43,000 sq. ft./manufacturing, office, and warehouse | | leased |
Rochester, New York | | 24,000 sq. ft./manufacturing, office, and warehouse | | leased |
Dubuque, Iowa | | 266,000 sq. ft./manufacturing and office | | leased |
Melrose Park, Illinois | | 117,000 sq. ft./manufacturing and office | | owned |
West Paterson, New Jersey | | 20,000 sq. ft./manufacturing | | leased |
Southend-on-Sea, England | | 29,000 sq. ft./manufacturing, warehouse, and office | | leased |
Perinton, New York | | 87,000 sq. ft./manufacturing, warehouse, and office | | owned |
Suffolk, England | | 51,000 sq. ft./manufacturing, warehouse, and office | | leased |
|
| Apogent Corporate Headquarters |
Portsmouth, New Hampshire | | 29,000 sq. ft./office | | leased |
We consider our plants and equipment to be well maintained and suitable for their purposes. We have, from time to time, expanded and will continue to expand our facilities as the need arises. We expect to fund such expansions through internally generated funds or borrowings under our credit facilities described in Note 7 to our consolidated financial statements. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources.”
Legal Proceedings
Nalge Nunc International, a subsidiary in our Research Group business segment, has been identified as a potentially responsible party (“PRP”) at the Aqua-Tech site in South Carolina (the “Aqua-Tech Site”) with respect to a previously owned facility. An action has been conducted at the Aqua-Tech Site for the removal of surface contaminants under the supervision of the Environmental Protection Agency (“EPA”) pursuant to the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended (“CERCLA”). Our total expenses (including legal fees) to date have been approximately $165,000. The site has been placed by the EPA on the federal National Priority List under CERCLA, which is a prerequisite to any federally-mandated requirement for long-term remedial work at the site under CERCLA, such as would be involved in soil and groundwater remediation. We are participating with a PRP group composed of approximately 100 parties in an agreement with the EPA to undertake a remedial investigation and feasibility study, which will be used by the EPA to determine what remedy, if any, should be required at the site. A draft remedial investigation was submitted to the EPA in August 1999, and a draft baseline risk assessment was submitted in October 1999. After
64
review of the draft remedial investigation, the EPA requested and obtained additional sampling work from the PRP group. The final remedial investigation was submitted in 2000, and the feasibility study is now expected to be completed in June 2003. Because the study, which involves extensive testing to characterize the existence, extent and nature of any contamination in order to determine potential remedies, has not yet been completed, an estimate of our potential liability cannot be made. Our share of waste allegedly sent to the site is reportedly not more than 1% of the total waste sent; therefore, even though CERCLA does provide for joint and several liability, we believe that any ultimate liability will not have a material adverse effect on our results of operations or financial condition.
Applied Biotech, a subsidiary in our Clinical Group business segment, formerly manufactured and supplied immunoassay pregnancy tests to Warner Lambert Co. (now part of Pfizer Inc.). Warner Lambert sold the tests to retailers who sell them over-the-counter to consumers. Applied Biotech supplied the product to Warner Lambert pursuant to a supply agreement that Warner Lambert claims required Applied Biotech to defend and indemnify Warner Lambert with respect to any liability arising out of claims that the product infringes any patents held by third parties. On January 8, 1999, Conopco, Inc. d/b/a Unipath Diagnostics Company filed a lawsuit against Warner Lambert in the U.S. District Court for the District of New Jersey. The Unipath Diagnostics business, along with this lawsuit, were subsequently sold to Inverness Medical Switzerland GmbH (together with its affiliates, “Inverness”). In the Conopco litigation and in two other cases, Inverness has claimed that the Warner Lambert pregnancy test supplied by ABI infringes certain patents owned by Inverness. Applied Biotech agreed to defend in two of these lawsuits on behalf of Warner Lambert. On June 9, 2003, Inverness announced that it had settled the outstanding cases against Pfizer/Warner Lambert without payment of damages. The parties are in the process of effecting formal dismissals of these actions. We believe that the resolution of these lawsuits will not have a material adverse effect on our results of operations or financial condition. Additionally, on October 15, 2002, Armkel, LLC sued Pfizer in the U.S. District Court for the District of New Jersey for patent infringement with respect to these same pregnancy test products. To date, Applied Biotech has not agreed to defend this lawsuit on behalf of Pfizer. Applied Biotech does not believe that it has an obligation to defend and indemnify Pfizer with respect to this lawsuit. Further, it believes that there are meritorious defenses to the patent claims.
We and our subsidiaries are parties to a number of lawsuits or subject to claims arising out of our respective operations, or the operation of businesses divested since the 1980’s for which certain of our subsidiaries may continue to have legal or contractual liability, including product liability, patent and trademark or other intellectual property infringement, contractual liability, workplace safety, and environmental claims and cases, some of which involve claims for substantial damages. We vigorously defend lawsuits and other claims against us. Based upon our assessment of the cases and claims against us and our experiences with similar cases and claims in the past, we believe that any liabilities which might reasonably result from any of the pending cases and claims would not have a material adverse effect on our results of operations or financial condition. However, there can be no assurance as to this or that we will not be subjected to significant additional claims in the future with respect to our current or former operations which would have such a material adverse effect. See Note 13 to our consolidated financial statements and Risk Factors beginning on page 13 of this prospectus.
65
MANAGEMENT
Directors and Executive Officers of the Registrant
Set forth below is a complete list of the names, ages, and positions(s) of our directors and executive officers. All executive officers hold office at the pleasure of the Board of Directors.
Name
| | Age
| | Position
|
Kenneth F. Yontz | | 58 | | Chairman of the Board |
Frank H. Jellinek, Jr | | 58 | | Director, President and Chief Executive Officer |
William H. Binnie | | 45 | | Director |
Don H. Davis, Jr. | | 63 | | Director |
Christopher L. Doerr | | 53 | | Director |
Stephen R. Hardis | | 68 | | Director |
R. Jeffrey Harris | | 48 | | Director |
Joe L. Roby | | 64 | | Director |
Richard W. Vieser | | 75 | | Director |
Dennis Brown | | 55 | | Chief Financial Officer and Treasurer |
Michael K. Bresson | | 45 | | Executive Vice President—General Counsel and Secretary |
Robert N. Griffin | | 64 | | Vice President, Regulatory Affairs and Quality Assurance |
Gary J. Marmontello | | 58 | | Vice President, Human Resources |
Robert V. Ahlgren | | 50 | | Group President, Research Group |
Mark F. Stuppy | | 49 | | Group President, Clinical Consumables |
Stephen K. Wiatt | | 58 | | Group President, Industrial Glass Operations |
Peter Scheu | | 38 | | Group President, Clinical Diagnostics |
The following sets forth the principal occupations of the executive officers for the periods specified.
Mr. Yontz. Chairman of the Board since December 1987; President and Chief Executive Officer of the Company from October 1987 until December 2000. Director of Viasystems Group, Inc., and Rockwell Automation, Inc. Chairman of the Board of Sybron Dental Specialties, Inc.
Mr. Jellinek. President and Chief Executive Officer since December 2000; President and Chief Executive Officer of Sybron Laboratory Products Corporation (“SLP”) from 1998 to 2000; President of Erie Scientific Company (“Erie”) from 1975 to 1998; has from time to time held general management responsibilities for various businesses of Apogent’s predecessor.
Mr. Binnie. President and Chief Executive Officer of Carlisle Capital Corporation (private investment management) since 1982; Chairman and CEO of Carlisle Plastics, Inc. (manufacturing) from 1984 to 1996.
Mr. Davis. Chairman and Chief Executive Officer of Rockwell Automation, Inc. (provider of industrial automation power, control and information solutions) since February 1998 and October 1997, respectively; President of Rockwell from July 1995 to October 1997. Director of Ciena Corporation and Illinois Tool Works Inc.
Mr. Doerr. Co-Chief Executive Officer of Passage Partners, LLC (a private investment company) since October 2000; President and Co-Chief Executive Officer of Leeson Electric Corporation (a manufacturer of custom and standard industrial purpose electric motors) from December 1989 to September 2000.
Mr. Hardis. Chairman of Axcelis Technologies, Inc. (manufacturing) since July 2000; Chairman of Eaton Corporation (manufacturing) from 1996 through July 2000. Director of: American Greetings Corporation;
66
Lexmark International, Inc.; Marsh & McLennan Companies, Inc.; Nordson Corporation; Progressive Corporation; and STERIS Corporation.
Mr. Harris. Of Counsel to the Company since December 2000; Vice President-General Counsel and Secretary of the Company from January 1988 to December 2000. Director of Playtex Products Inc.
Mr. Roby. Chairman Emeritus and Senior Advisor of Credit Suisse First Boston LLC (“CSFB”) (investment banking), a subsidiary of Credit Suisse Group, since December 2001; Chairman of CSFB from November 2000 to December 2001; President and Chief Executive Officer of Donaldson, Lufkin & Jenrette, Inc. (“DLJ”) from February 1998 to November 2000; Chief Operating Officer of DLJ from November 1995 until February 1998, and President of DLJ from February 1996 to November 2000. Director of Advanced Micro Devices, Inc.
Mr. Vieser. Retired from active employment since 1989; former Chairman of the Board, President and CEO of Lear Siegler, Inc., FL Industries, Inc., and FL Aerospace. Chairman of the Board of Varian Medical Systems, Inc. Director of Harvard Industries, Inc., and Viasystems Group, Inc.
Mr. Brown. Reappointed as Chief Financial Officer and Treasurer of the Company in January, 2003; Financial Consultant to the Company from 2000 to 2003; Chief Financial Officer, Vice President—Finance and Treasurer of the Company from 1993 to 2000; President of Allen-Bradley Europe from March 1990 to January 1993; and Treasurer of The Marmon Group, Inc., from 1987 to 1990. In addition, Mr. Brown has been a Director of Sybron Dental Specialties, Inc. since 2000, and recently was appointed Director and Chairman of the Audit Committee of Merge Technologies Incorporated. Mr. Brown had previously served as a Director of Merge Technologies through 2000.
Mr. Bresson. Executive Vice President—General Counsel and Secretary since December 2000; Group Counsel of SLP from 1998 to 2000; Partner at the law firm of Quarles & Brady LLP from 1990 to 1998.
Mr. Griffin. Vice President, Regulatory Affairs and Quality Assurance since December 2000; Vice President, Regulatory Affairs of SLP from 1998 to 2000; Director of Quality and Safety at Erie from prior to 1996 to 1998.
Mr. Marmontello. Vice President, Human Resources since December 2000; Vice President, Human Resources of SLP from 1997 to 2000; Associate Director for the University System of New Hampshire prior to joining SLP.
Mr. Ahlgren. Group President, Research Group since April 2002; President of Nalge Nunc International Corporation (“NNI”) since November 1999; Senior Vice President—Sales and Marketing of NNI from January 1999 to November 1999. General Manager of International Murex Technologies Corporation from 1994 to 1998.
Mr. Stuppy. Group President, Clinical Consumables since April 2001; President of Erie since January 2001; Executive Vice President, Sales and Marketing, Clinical Products from 2000 to 2001; Executive Vice President of Sales & Marketing at SLP from 1998 to 2000; Vice President of Marketing at Erie from 1986 to 1998.
Mr. Wiatt. Group President, Industrial Glass Operations since April 2001; Executive Vice President, Worldwide Glass Operations from 2000 to 2001; Executive Vice President, Worldwide Glass Operations of SLP from 1998 to 2000; Vice President of Manufacturing at Erie from 1978 to 1998.
Mr. Scheu. Group President, Clinical Diagnostics since September 2000; President of Richard-Allan Scientific Company (“Richard-Allan”) from 1997 to 2000; Executive Vice President of Richard-Allan from 1995 to 1997.
SLP, Erie, NNI, and Richard-Allan are subsidiaries of Apogent.
67
DESCRIPTION OF OTHER INDEBTEDNESS
Revolving Credit Facility
Borrowings under our revolving credit facility mature on December 1, 2005. The revolving credit facility, as amended effective upon consummation of our tender offer, provides for an annual interest rate, equal to, at our option, either (a) ABR (a floating rate-based interest calculation) plus 0% to 0.625% (the “Revolving Loan ABR Margin”) or (b) the Eurodollar Rates plus 0.625% to 1.625% (the “Revolving Loan Eurodollar Rate Margin”). In addition, we have a third option to set the rate by a competitive bid process among the parties to the revolving credit facility. We also pay a facility fee of 0.125% to 0.375% for all commitments from the lenders, whether drawn or undrawn, and a utilization fee of 0.25% per annum if more than 50% of the facility is drawn. The Revolving Loan ABR Margin, the Revolving Loan Eurodollar Rate Margin and the facility fee depend upon our senior, unsecured long-term credit rating from S&P and Moody’s. Based upon our current credit rating, the Revolving Loan ABR Margin, the Revolving Loan Eurodollar Rate Margin, and the facility fee would be 0.25%, 1.25%, and 0.25%, respectively. Our revolving credit facility also provides for a multi-currency sub-facility providing up to $100.0 million in sub-commitments in non-dollar currencies. Terms and conditions on the multi-currency sub-facility are to be agreed upon between us and JPMorgan Chase Bank and the lenders providing funding under such facility. We may not exceed a total of $500.0 million in dollar and non-dollar commitments under this facility. Our revolving credit facility also provides for the issuance of standby letters of credit and commercial letters of credit on behalf of our subsidiaries as required in the ordinary course of business as part of the working capital line. The outstanding balance under the revolving credit facility as of March 31, 2003 was $69.2 million.
Our revolving credit facility contains financial and operating covenants, including, among other things: restrictions on investments, loans and advances; requirements that we maintain certain financial ratios; restrictions on our ability and our subsidiaries’ ability to create or permit liens, or to pay dividends or make other restricted payments (as defined) in excess of $100.0 million plus 50% of our defined consolidated net income for each fiscal quarter ending after September 30, 2000, less any dividends paid or other restricted payments made after September 30, 2000; and limitations on incurrence of additional indebtedness. Our obligations under our revolving credit facility are guaranteed by our material domestic subsidiaries.
8% Senior Notes
On April 4, 2001, we issued $325.0 million of unsecured senior notes in a private placement with Registration Rights, and in August 2001, we completed a registered exchange of the privately placed notes for similar notes that had been registered with the Commission. The notes were offered at a discount of approximately $1.5 million. They will mature on April 1, 2011. Interest is fixed at an annual rate of 8% and is payable on April 1 and October 1 of each year, beginning on October 1, 2001. The notes are redeemable by us at any time in whole, or from time to time in part, at a price equal to the greater of (i) 100% of the principal amount of the notes to be redeemed or (ii) the sum of the present values of the remaining scheduled payments of principal and interest thereon (exclusive of interest accrued to the date of redemption) discounted to the date of redemption on a semiannual basis at the applicable Treasury Yield (as defined in the bond agreement) plus 35 basis points, plus accrued interest to the date of redemption. The notes are guaranteed by our material U.S. subsidiaries, which also guarantee our obligations under our revolving credit facility.
Senior Convertible Contingent Debt
On October 10, 2001, we issued $300.0 million of senior convertible contingent debt securities (CODES). The CODES have a fixed interest rate of 2.25% per annum. Interest is payable on April 15 and October 15 of each year. We also must pay contingent interest during any six-month period if the average trading price of the CODES during a specified period of five trading days preceding the relevant six-month period is above specified levels. No contingent interest is payable during the six-month period from April 15, 2002 to October 14, 2002.
68
The CODES will mature on October 15, 2021. The CODES are convertible, subject to certain conditions (based upon specified factors including but not limited to the sale price of our common stock, trading prices of the CODES, maintenance of our credit ratings, and the occurrence of specified corporate transactions, including certain repurchases of our common stock), into our common stock at a price of approximately $30.49 per share. We may redeem some or all of the CODES on or after October 20, 2004. The holders may require us to purchase all or a portion of their CODES on October 20, 2004 and on October 15, 2006, 2011 and 2016, or subject to specified exceptions, upon a change of control event. Certain of our subsidiaries guarantee our obligations under the CODES.
Sellers’ Notes
In connection with certain acquisitions, we have issued notes payable to the related sellers. The notes bear interest of 5% to 6% and mature at various dates through May 2005. Certain notes are redeemable by the holders, subject to certain time restrictions. The notes are unsecured, however in certain instances, some are guaranteed by our subsidiaries.
Sale/Leaseback
On December 22, 1988, we completed the sale and leaseback (the “Sale/Leaseback”) of our then principal domestic manufacturing and office facilities with an unaffiliated third party. The proceeds of $22.5 million (net of approximately $1.1 million in fees) were used to retire debt. The transaction has been accounted for as a financing for financial statement purposes and as a sale for income tax purposes. The financing obligation is being amortized over the initial 25-year lease term.
We pay all costs of maintenance and repair, insurance, taxes, and all other expenses associated with the properties. In addition, each of the leases is unconditionally guaranteed by us.
The initial term of each lease is 25 years with five five-year renewal options. The initial aggregate annual payments relating to Apogent under the leases were $1.7 million payable monthly in advance. On the fifth anniversary of the leases and every five years thereafter (including renewal terms), the rent is increased by the percentage equal to 75% of the percentage increase in the Consumer Price Index over the preceding five years. The percentage increase to the rent in any five-year period will be capped at 15%. Beginning January 1, 1999 annual payments increased to $2.2 million. The next adjustment will not occur until January 1, 2004.
We have the option to purchase the facilities according to the terms of any bona fide offer received by the lessor from a third party (the “Third Party Offer”) at any time during the term of the leases. The purchase price upon exercise of the option will be an amount equal to the purchase price contained in the Third Party Offer. We also have the option to purchase the facilities, subject to complying with the notice provision in the leases, on any date between June 1, 2008 and May 31, 2009. The purchase price upon the exercise of the option is the greater of the fair market value of the leased premises or the sum of the landlord’s acquisition cost for the leased premises and any prepayment premiums that would be payable under the landlord’s financing for the premises.
In the event of a breach of certain covenants which include, subject to certain exceptions, restrictions on our and our subsidiaries’ incurrence of certain additional indebtedness, payment of dividends or the making of other distributions or the repurchase of our capital stock, or the creation of liens on our respective properties, we must cause each subsidiary to make a rejectable offer to the lessor to purchase its facility. If the lessor accepts the rejectable offer, each subsidiary will pay to the lessor a formula price based upon the lessor’s equity in the property and the lessor’s pre-payment premium to its lender. We may also be obligated to repurchase the property upon the occurrence of certain other events.
Securities Lending Agreement
On September 29, 1999, we purchased a United States Treasury Bond (“Treasury”) with a par value of $50.0 million, an interest rate of 6.15% and a maturity date of August 15, 2029. Concurrent with the purchase of
69
the Treasury, we loaned the security to an unrelated third party for a period of 23 years. In exchange for the loaned Treasury, we received collateral equal to the market value of the Treasury on the date of the loan, and adjusted on a weekly basis. This securities lending transaction is related to our existing lending policy by fixing $50.0 million of its floating rate debt. For a period of five years, we are obligated to pay a rebate on the loaned collateral at an annual fixed rate of 6.478% and are entitled to receive a fee for the loan of the security at a floating rate equal to LIBOR minus 0.75%. Thereafter, we are required to pay the unrelated third party a collateral fee equal to the one-week general collateral rate of interest (as determined weekly in good faith by the unrelated third party, provided that such rate shall not exceed the federal funds rate in effect as of the day of determination plus 0.25%) and we receive all distributions made on or in respect to the Treasury. This transaction is accounted for as a secured borrowing under SFAS No. 140.
70
DESCRIPTION OF NOTES
You can find the definitions of certain terms used in this description of notes under the subheading “Certain Definitions.” In this description, the word “Apogent” refers only to Apogent Technologies Inc., a Wisconsin corporation, and not to any of its subsidiaries.
The Original Notes were issued and the Exchange Notes will be issued under an indenture dated June 2, 2003, among Apogent, the Guarantors, and The Bank of New York, as trustee. The Original Notes were issued in a private transaction that was not subject to the registration requirements of the Securities Act. The terms of the notes include those stated in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939.
The following description is a summary of the material provisions of the indenture and the registration rights agreement. It is not a complete restatement of those agreements. We urge you to read the indenture and the registration rights agreement because they, and not this description, define your rights as a holder of the notes. Some defined terms used in this description but not defined below under “—Certain Definitions” have the meanings assigned to them in the indenture.
The registered holder of a note will be treated as the owner of it for all purposes. Only registered holders will have rights under the indenture.
Brief Description of the Notes and the Guarantees
The Notes
The notes:
| | • | are general unsecured obligations of Apogent; |
| | • | are subordinated in right of payment to all existing and future Senior Debt of Apogent; |
| | • | arepari passu in right of payment with any future senior subordinated Indebtedness of Apogent; and |
| | • | are unconditionally guaranteed by the Guarantors on a senior subordinated basis. |
The Guarantees
The notes are Guaranteed by all of Apogent’s current and future Domestic Subsidiaries that have Guaranteed, and will in the future Guarantee, Obligations under Apogent’s Credit Facility;provided that, upon the release of a Guarantee by a Subsidiary under all then outstanding Credit Facilities and at any time after the termination of certain covenants as provided below under the caption “—Certain Covenants—Termination of Covenants when Notes Rated Investment Grade,” such Subsidiary will be deemed released from all of its Obligations under the indenture and its Guarantee will terminate;provided, further, that in the event that any such Subsidiary thereafter Guarantees any Indebtedness of Apogent under any Credit Facility (or if any released Guarantee under any Credit Facility is reinstated or renewed), then such Subsidiary will Guarantee the notes on the terms and conditions set forth in the indenture. See “—Subsidiary Guarantees.”
Each Guarantee of the notes:
| | • | is a general unsecured obligation of the Guarantor; |
| | • | is subordinated in right of payment to all existing and future Senior Debt of that Guarantor; and |
| | • | ispari passu in right of payment with any future senior subordinated Indebtedness of that Guarantor. |
As of March 31, 2003, after giving pro forma effect to this offering and the recapitalization, Apogent and the Guarantors would have had total Senior Debt of approximately $673.7 million. As indicated above and as
71
discussed in detail below under the caption “—Subordination,” payments on the notes and under these Guarantees will be subordinated to the payment of Senior Debt. The indenture permits us and the Guarantors to incur additional Senior Debt.
Not all of our Subsidiaries guarantee the notes. In the event of a bankruptcy, liquidation or reorganization of any of these Subsidiaries who are not Guarantors, the Subsidiaries who are not Guarantors will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to us. The Guarantors generated 85% of our net sales in the twelve-month period ended March 31, 2003 and held 80% of our consolidated assets as of March 31, 2003. See note 17 to our consolidated financial statements included elsewhere in this prospectus for more detail about the division of our consolidated revenues and assets between our Guarantor and Subsidiaries who are not Guarantors.
As of June 2, 2003, all of Apogent’s Subsidiaries are “Restricted Subsidiaries.” However, under the circumstances described below under the subheading “—Certain Covenants—Designation of Restricted and Unrestricted Subsidiaries,” we are permitted to designate certain of Apogent’s Subsidiaries as “Unrestricted Subsidiaries.” Our Unrestricted Subsidiaries are not subject to many of the restrictive covenants in the indenture. Our Unrestricted Subsidiaries do not Guarantee the notes.
Principal, Maturity and Interest
Apogent issued an aggregate principal amount of $250.0 million Original Notes, which are exchangeable for the Exchange Notes that have substantially identical terms. Apogent may issue additional notes from time to time. Any offering of additional notes is subject to the covenant described below under the caption “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock.” The notes and any additional notes subsequently issued under the indenture will be treated as a single class for all purposes under the indenture, including, without limitation, waivers, amendments, redemptions, and offers to purchase. Apogent issued the notes in denominations of $1,000 and integral multiples of $1,000. The notes will mature on May 15, 2013.
Interest on the notes accrues at the rate of 6 1/2% per annum and is payable semi-annually in arrears on May 15 and November 15, commencing on November 15, 2003. Apogent will make each interest payment to the holders of notes of record on the last day of the month immediately preceding May 15 and November 15.
Interest on the notes accrues from the date of original issuance, June 2, 2003, or, if interest has already been paid, from the date it was most recently paid. Interest is computed on the basis of a 360-day year comprised of twelve 30-day months.
Methods of Receiving Payments on the Notes
If a holder of notes has given wire transfer instructions to Apogent, Apogent will pay all principal, interest and premium and liquidated damages under the registration rights agreement (“Liquidated Damages”), if any, on that holder’s notes in accordance with those instructions. All other payments on notes will be made at the office or agency of the paying agent and registrar within the City and State of New York unless Apogent elects to make interest payments by check mailed to the holders of notes at their address set forth in the register of holders.
Paying Agent and Registrar for the Notes
The trustee will initially act as paying agent and registrar. Apogent may change the paying agent or registrar without prior notice to the holders of the notes, and Apogent or any of its Subsidiaries may act as paying agent or registrar.
Transfer and Exchange
A holder of notes may transfer or exchange notes in accordance with the indenture. The registrar and the trustee may require a holder of notes to furnish appropriate endorsements and transfer documents in connection
72
with a transfer of notes. Holders of notes will be required to pay all taxes due on transfer. Apogent is not required to transfer or exchange any note selected for redemption. Also, Apogent is not required to transfer or exchange any note for a period of 15 days before a selection of notes to be redeemed.
Subsidiary Guarantees
The notes are Guaranteed by each of Apogent’s current and future Domestic Subsidiaries that have Guaranteed, and will in the future Guarantee, Obligations under Apogent’s Credit Facility. These Subsidiary Guarantees are joint and several obligations of the Guarantors. Each Subsidiary Guarantee is subordinated to the prior payment in full of all Senior Debt of that Guarantor. The obligations of each Guarantor under its Subsidiary Guarantee are limited as necessary to prevent that Subsidiary Guarantee from constituting a fraudulent conveyance under applicable law. See “Risk Factors—The Guarantees may be unenforceable due to fraudulent conveyance statutes.”
A Guarantor may not sell or otherwise dispose of all or substantially all of its assets to, or consolidate with or merge with or into (whether or not such Guarantor is the surviving Person), another Person, other than Apogent or another Guarantor, unless:
(1) immediately after giving effect to that transaction, no Default or Event of Default exists; and
(2) either:
(a) the Person acquiring the property in any such sale or disposition or the Person formed by or surviving any such consolidation or merger assumes all the obligations of that Guarantor under the indenture, its Subsidiary Guarantee and the registration rights agreement pursuant to a supplemental indenture satisfactory to the trustee; or
(b) the Net Proceeds of such sale or other disposition are applied in accordance with the applicable provisions of the indenture.
The Subsidiary Guarantee of a Guarantor will be released:
(1) in connection with any sale or other disposition of all or substantially all of the assets of that Guarantor (including by way of merger or consolidation) to a Person that is not (either before or after giving effect to such transaction) a Subsidiary of Apogent, if the sale or other disposition complies with the “Asset Sale” provisions of the indenture; or
(2) in connection with any sale of all of the Capital Stock of a Guarantor to a Person that is not (either before or after giving effect to such transaction) a Subsidiary of Apogent, if the sale complies with the “Asset Sale” provisions of the indenture; or
(3) if Apogent designates any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in accordance with the applicable provisions of the indenture; or
(4) upon the release of a Guarantee by a Subsidiary under all then outstanding Credit Facilities and at any time after the termination of certain covenants as provided below under the caption “—Certain Covenants—Termination of Covenants when Notes Rated Investment Grade”;provided, however, that in the event that any such Subsidiary thereafter Guarantees any Indebtedness of Apogent under any Credit Facility (or if any released Guarantee under any Credit Facility is reinstated or renewed), then such Subsidiary will Guarantee the notes on the terms and conditions set forth in the indenture.
See “—Repurchase at the Option of Holders—Asset Sales.”
Subordination
The payment of principal, interest and premium and Liquidated Damages, if any, on the notes is subordinated to the prior payment in full of all Senior Debt of Apogent, including Senior Debt incurred after June 2, 2003.
73
The holders of Senior Debt are entitled to receive payment in full of all Obligations due in respect of Senior Debt (including interest after the commencement of any bankruptcy proceeding at the rate specified in the applicable Senior Debt) before the holders of notes will be entitled to receive any payment with respect to the notes (except that holders of notes may receive and retain Permitted Junior Securities and payments made from the trust described under “—Legal Defeasance and Covenant Defeasance”), in the event of any distribution to creditors of Apogent:
(1) in a liquidation or dissolution of Apogent;
(2) in a bankruptcy, reorganization, insolvency, receivership or similar proceeding relating to Apogent or its property;
(3) in an assignment for the benefit of creditors; or
(4) in any marshaling of Apogent’s assets and liabilities.
Apogent also may not make any payment in respect of the notes (except in Permitted Junior Securities or from the trust described under “—Legal Defeasance and Covenant Defeasance”) if:
(1) a payment default on Designated Senior Debt occurs and is continuing beyond any applicable grace period; or
(2) any other default occurs and is continuing on any series of Designated Senior Debt that permits holders of that series of Designated Senior Debt to accelerate its maturity and the trustee receives a notice of such default (a “Payment Blockage Notice”) from Apogent or the holders of any Designated Senior Debt.
Payments on the notes may and will be resumed:
(1) in the case of a payment default, upon the date on which such default is cured or waived; and
(2) in the case of a nonpayment default, upon the earlier of the date on which such nonpayment default is cured or waived or 179 days after the date on which the applicable Payment Blockage Notice is received, unless the maturity of any Designated Senior Debt has been accelerated.
No new Payment Blockage Notice may be delivered unless and until:
(1) 360 days have elapsed since the delivery of the immediately prior Payment Blockage Notice; and
(2) all scheduled payments of principal, interest and premium and Liquidated Damages, if any, on the notes that have come due have been paid in full in cash.
No nonpayment default that existed or was continuing on the date of delivery of any Payment Blockage Notice to the trustee will be, or be made, the basis for a subsequent Payment Blockage Notice unless such default has been cured or waived for a period of not less than 80 days.
If the trustee or any holder of the notes receives a payment in respect of the notes (except in Permitted Junior Securities or from the trust described under “—Legal Defeasance and Covenant Defeasance”) when:
(1) the payment is prohibited by these subordination provisions; and
(2) the trustee or the holder of notes has actual knowledge that the payment is prohibited;
the trustee or the holder of notes, as the case may be, will hold the payment in trust for the benefit of the holders of Senior Debt. Upon the proper written request of the holders of Senior Debt, the trustee or the holder of notes, as the case may be, will deliver the amounts in trust to the holders of Senior Debt or their proper representative.
Apogent must promptly notify holders of Senior Debt if payment of the notes is accelerated because of an Event of Default.
As a result of the subordination provisions described above, in the event of a bankruptcy, liquidation or reorganization of Apogent, holders of notes may recover less ratably than creditors of Apogent who are holders
74
of Senior Debt. See “Risk Factors—Your right to receive payments on these notes and Guarantees is subordinated to our senior debt.”
Optional Redemption
At any time prior to May 15, 2006, Apogent may on any one or more occasions redeem up to 35% of the aggregate principal amount of notes (including additional notes, if any) issued under the indenture at a redemption price of 106.50% of the principal amount, plus accrued and unpaid interest and Liquidated Damages, if any, to the redemption date, with the net cash proceeds of one or more Equity Offerings by Apogent,provided that:
(1) at least 65% of the original aggregate principal amount of notes issued under the indenture remains outstanding immediately after the occurrence of such redemption (excluding notes held by Apogent and its Subsidiaries); and
(2) the redemption occurs within 90 days of the date of the closing of such Equity Offering.
Except pursuant to the preceding paragraph, the notes will not be redeemable at Apogent’s option prior to May 15, 2008.
On or after May 15, 2008, Apogent may redeem all or a part of the notes upon not less than 30 nor more than 60 days prior notice, at the redemption prices (expressed as percentages of principal amount) set forth below plus accrued and unpaid interest and Liquidated Damages, if any, on the notes redeemed, to the applicable redemption date, if redeemed during the twelve-month period beginning on May 15 of the years indicated below:
Year
| | Percentage
| |
2008 | | 103.250 | % |
2009 | | 102.167 | % |
2010 | | 101.083 | % |
2011 and thereafter | | 100.000 | % |
Mandatory Redemption
Apogent is not required to make mandatory redemption or sinking fund payments with respect to the notes.
Repurchase at the Option of Holders
Change of Control
If a Change of Control occurs, each holder of notes will have the right to require Apogent to repurchase all or any part (equal to $1,000 or an integral multiple of $1,000) of that holder’s notes pursuant to the offer described below (the “Change of Control Offer”) on the terms set forth in the indenture. In the Change of Control Offer, Apogent will offer a Change of Control Payment in cash equal to 101% of the aggregate principal amount of notes repurchased plus accrued and unpaid interest and Liquidated Damages, if any, on the notes repurchased, to the date of purchase (the “Change of Control Payment”). Within ten days following any Change of Control, Apogent will mail a notice to each holder of notes describing the transaction or transactions that constitute the Change of Control and offering to repurchase notes on the date specified in the notice, which date will be no earlier than 30 days and no later than 60 days from the date such notice is mailed (the “Change of Control Payment Date”), pursuant to the procedures required by the indenture and described in such notice. Apogent will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with the repurchase of the notes as a result of a Change of Control. To the extent that the provisions of any securities laws or regulations conflict with the Change of Control provisions of the indenture, Apogent will comply with the
75
applicable securities laws and regulations and will not be deemed to have breached its obligations under the Change of Control provisions of the indenture by virtue of such conflict.
On the Change of Control Payment Date, Apogent will, to the extent lawful:
(1) accept for payment all notes or portions of notes properly tendered pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the Change of Control Payment in respect of all notes or portions of notes properly tendered; and
(3) deliver or cause to be delivered to the trustee the notes properly accepted together with an officers’ certificate stating the aggregate principal amount of notes or portions of notes being purchased by Apogent.
The paying agent will promptly mail to each holder of notes properly tendered the Change of Control Payment for such notes, and the trustee will promptly authenticate and mail (or cause to be transferred by book entry) to each holder of notes a new note equal in principal amount to any unpurchased portion of the notes surrendered, if any;provided that each new note will be in a principal amount of $1,000 or an integral multiple of $1,000.
Prior to complying with any of the provisions of this “Change of Control” covenant, but in any event within 90 days following a Change of Control, Apogent will either repay all outstanding Senior Debt or obtain the requisite consents, if any, under all agreements governing outstanding Senior Debt to permit the repurchase of notes required by this covenant. Apogent will publicly announce the results of the Change of Control Offer on or as soon as practicable after the Change of Control Payment Date.
The provisions described above that require Apogent to make a Change of Control Offer following a Change of Control will be applicable whether or not any other provisions of the indenture are applicable. Except as described above with respect to a Change of Control, the indenture does not contain provisions that permit the holders of the notes to require that Apogent repurchase or redeem the notes in the event of a takeover, recapitalization or similar transaction.
Apogent will not be required to make a Change of Control Offer upon a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the indenture applicable to a Change of Control Offer made by Apogent and purchases all notes properly tendered and not withdrawn under the Change of Control Offer.
The definition of Change of Control includes a phrase relating to the direct or indirect sale, lease, transfer, conveyance or other disposition of “all or substantially all” of the properties or assets of Apogent and its Subsidiaries taken as a whole. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, the ability of a holder of notes to require Apogent to repurchase its notes as a result of a sale, lease, transfer, conveyance or other disposition of less than all of the assets of Apogent and its Subsidiaries taken as a whole to another Person or group may be uncertain.
Asset Sales
Apogent will not, and will not permit any of its Restricted Subsidiaries to, consummate an Asset Sale unless:
(1) Apogent (or the Restricted Subsidiary, as the case may be) receives consideration at the time of the Asset Sale at least equal to the fair market value of the assets or Equity Interests issued or sold or otherwise disposed of;
(2) the fair market value is determined by Apogent’s Board of Directors and evidenced by a resolution of the Board of Directors set forth in an officers’ certificate delivered to the trustee; and
76
(3) at least 75% of the consideration received in the Asset Sale by Apogent or such Restricted Subsidiary, as the case may be, is in the form of cash. For purposes of this provision, each of the following will be deemed to be cash:
(a) any liabilities, as shown on Apogent’s or such Restricted Subsidiary’s most recent balance sheet, of Apogent or any Subsidiary (other than contingent liabilities and liabilities that are by their terms subordinated to the notes or any Subsidiary Guarantee) that are assumed by the transferee of any such assets pursuant to a customary novation agreement that releases Apogent or such Subsidiary from further liability; and
(b) any securities, notes or other obligations received by Apogent or any such Restricted Subsidiary from such transferee that are converted by Apogent or such Subsidiary into cash within 90 days, to the extent of the cash received in that conversion.
Within 360 days after the receipt of any Net Proceeds from an Asset Sale, Apogent may apply those Net Proceeds at its option:
(1) to repay Senior Debt and, if the Senior Debt repaid is revolving credit Indebtedness, to correspondingly reduce commitments with respect thereto;
(2) to acquire all or substantially all of the assets of, or a majority of the Voting Stock of, another Permitted Business; or
(3) to acquire other long-term assets or property that are used or useful in a Permitted Business.
Pending the final application of any Net Proceeds, Apogent may temporarily reduce revolving credit borrowings or otherwise invest the Net Proceeds in any manner that is not prohibited by the indenture.
Any Net Proceeds from Asset Sales that are not applied or invested as provided in the preceding second paragraph will constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $15.0 million, Apogent will make an Asset Sale Offer to all holders of notes and all holders of other Indebtedness that ispari passu with the notes containing provisions similar to those set forth in the indenture with respect to offers to purchase or redeem with the proceeds of sales of assets to purchase the maximum principal amount of notes and such otherpari passu Indebtedness that may be purchased out of the Excess Proceeds. The offer price in any Asset Sale Offer will be equal to 100% of principal amount plus accrued and unpaid interest and Liquidated Damages, if any, to the date of purchase, and will be payable in cash. If any Excess Proceeds remain after consummation of an Asset Sale Offer, Apogent may use those Excess Proceeds for any purpose not otherwise prohibited by the indenture. If the aggregate principal amount of notes and otherpari passu Indebtedness tendered into such Asset Sale Offer exceeds the amount of Excess Proceeds, the trustee will select the notes and such otherpari passu Indebtedness to be purchased on a pro rata basis. Upon completion of each Asset Sale Offer, the amount of Excess Proceeds will be reset at zero.
Apogent will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with each repurchase of notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the Asset Sale provisions of the indenture, Apogent will comply with the applicable securities laws and regulations and will not be deemed to have breached its obligations under the Asset Sale provisions of the indenture by virtue of such conflict.
The agreements governing Apogent’s outstanding Senior Debt currently prohibit Apogent from purchasing any notes, and also provides that certain change of control or asset sale events with respect to Apogent would constitute a default under these agreements. Any future credit agreements or other agreements relating to Senior Debt to which Apogent becomes a party may contain similar restrictions and provisions. In the event a Change of Control or Asset Sale occurs at a time when Apogent is prohibited from purchasing notes, Apogent could seek
77
the consent of its senior lenders to the purchase of notes or could attempt to refinance the borrowings that contain such prohibition. If Apogent does not obtain such a consent or repay such borrowings, Apogent will remain prohibited from purchasing notes. In such case, Apogent’s failure to purchase tendered notes would constitute an Event of Default under the indenture which would, in turn, constitute a default under such Senior Debt. In such circumstances, the subordination provisions in the indenture would likely restrict payments to the holders of notes.
Selection and Notice
If less than all of the notes are to be redeemed at any time, the trustee will select notes for redemption as follows:
(1) if the notes are listed on any national securities exchange, in compliance with the requirements of the principal national securities exchange on which the notes are listed; or
(2) if the notes are not listed on any national securities exchange, on a pro rata basis, by lot or by such method as the trustee deems fair and appropriate.
No notes of $1,000 or less can be redeemed in part. Notices of redemption will be mailed by first class mail at least 30 but not more than 60 days before the redemption date to each holder of notes to be redeemed at its registered address, except that redemption notices may be mailed more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the notes or a satisfaction and discharge of the indenture. Notices of redemption may not be conditional.
If any note is to be redeemed in part only, the notice of redemption that relates to that note will state the portion of the principal amount of that note that is to be redeemed. A new note in principal amount equal to the unredeemed portion of the original note will be issued in the name of the holder of notes upon cancellation of the original note. Notes called for redemption become due on the date fixed for redemption. On and after the redemption date, interest ceases to accrue on notes or portions of them called for redemption.
Certain Covenants
Termination of Covenants when Notes Rated Investment Grade
In the event that:
(1) the notes have been assigned an Investment Grade rating by both Rating Agencies;
(2) the Investment Grade rating shall not be accompanied by either (i) in the case of S&P, a negative outlook, creditwatch negative or the equivalent thereof or (ii) in the case of Moody’s, a negative outlook, a review for possible downgrade or the equivalent thereof; and
(3) no Default or Event of Default under the indenture has occurred or is continuing,
then, Apogent and its Restricted Subsidiaries will no longer be subject to the following agreements and covenants contained in the indenture:
(a) “—Repurchase at the Option of the Holders—Asset Sales;”
(b) “—Certain Covenants—Restricted Payments;”
(c) “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock;”
(d) “—Certain Covenants—Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries;”
(e) “—Certain Covenants—Designation of Restricted and Unrestricted Subsidiaries;”
(f) “—Certain Covenants—Transactions with Affiliates;”
78
(g) clause 4 of the covenant listed under “—Certain Covenants—Merger, Consolidation or Sale of Assets;” and
(h) “—Certain Covenants—Business Activities.”
A change in the rating of the notes by either Rating Agency shall be deemed to have occurred on the date that such Rating Agency shall have publicly announced the change. There can be no assurance that the notes will ever achieve an Investment Grade rating or that any such rating will be maintained.
Restricted Payments
Apogent will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(1) declare or pay any dividend or make any other payment or distribution on account of Apogent’s or any of its Restricted Subsidiaries’ Equity Interests (including, without limitation, any payment in connection with any merger or consolidation involving Apogent or any of its Restricted Subsidiaries) or to the direct or indirect holders of Apogent’s or any of its Restricted Subsidiaries’ Equity Interests in their capacity as such (other than dividends or distributions payable in Equity Interests (other than Disqualified Stock) of Apogent or payable to Apogent or a Restricted Subsidiary of Apogent);
(2) purchase, redeem or otherwise acquire or retire for value (including, without limitation, in connection with any merger or consolidation involving Apogent) any Equity Interests of Apogent (excluding the maximum number of shares Apogent has offered to purchase pursuant to the Common Stock Tender Offer);
(3) make any payment on or with respect to, or purchase, redeem, defease or otherwise acquire or retire for value any Indebtedness that is subordinated to the notes or the Subsidiary Guarantees, except a payment of interest or principal at the Stated Maturity thereof; or
(4) make any Restricted Investment (all such payments and other actions set forth in these clauses (1) through (4) above being collectively referred to as “Restricted Payments”),
unless, at the time of and after giving effect to such Restricted Payment:
(1) no Default or Event of Default has occurred and is continuing or would occur as a consequence of such Restricted Payment; and
(2) Apogent would, at the time of such Restricted Payment and after giving pro forma effect thereto as if such Restricted Payment had been made at the beginning of the applicable four-quarter period, have been permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described below under the caption “—Incurrence of Indebtedness and Issuance of Preferred Stock;” and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by Apogent and its Restricted Subsidiaries after the date of the indenture (excluding Restricted Payments permitted by clauses (2), (3), (4), (6) and (7) of the next succeeding paragraph), is less than the sum, without duplication, of:
(a) 50% of the Consolidated Net Income of Apogent for the period (taken as one accounting period) from March 31, 2003 to the end of Apogent’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment (or, if such Consolidated Net Income for such period is a deficit, less 100% of such deficit),plus
(b) 100% of the aggregate net cash proceeds received by Apogent since the date of the indenture as a contribution to its common equity capital or from the issue or sale of Equity Interests of Apogent (other than Disqualified Stock) or from the issue or sale of convertible or exchangeable Disqualified Stock or convertible or exchangeable debt securities of Apogent that have been converted into or
79
exchanged for such Equity Interests (other than Equity Interests (or Disqualified Stock or debt securities) sold to a Subsidiary of Apogent),plus
(c) to the extent that any Restricted Investment that was made after the date of the indenture is sold for cash or otherwise liquidated or repaid for cash, the lesser of (i) the cash return of capital with respect to such Restricted Investment (less the cost of disposition, if any) and (ii) the initial amount of such Restricted Investment,plus
(d) to the extent that any Unrestricted Subsidiary of Apogent is redesignated as a Restricted Subsidiary after the date of the indenture, the lesser of (i) the fair market value of Apogent’s Investment in such Subsidiary as of the date of such redesignation or (ii) such fair market value as of the date on which such Subsidiary was originally designated as an Unrestricted Subsidiary.
So long as no Default or Event of Default has occurred and is continuing or would be caused thereby, the preceding provisions will not prohibit:
(1) the payment of any dividend within 60 days after the date of declaration of the dividend, if at the date of declaration the dividend payment would have complied with the provisions of the indenture;
(2) the redemption, repurchase, retirement, defeasance or other acquisition of any subordinated Indebtedness of Apogent or any Guarantor or of any Equity Interests of Apogent in exchange for, or out of the net cash proceeds of the substantially concurrent sale (other than to a Subsidiary of Apogent) of, Equity Interests of Apogent (other than Disqualified Stock);provided that the amount of any such net cash proceeds that are utilized for any such redemption, repurchase, retirement, defeasance or other acquisition will be excluded from clause (3)(b) of the preceding paragraph;
(3) the defeasance, redemption, repurchase or other acquisition of subordinated Indebtedness of Apogent or any Guarantor with the net cash proceeds from an incurrence of Permitted Refinancing Indebtedness;
(4) the payment of any dividend by a Restricted Subsidiary of Apogent to the holders of its Equity Interests on a pro rata basis;
(5) the repurchase, redemption or other acquisition or retirement for value of any Equity Interests of Apogent or any Restricted Subsidiary of Apogent held by any member of Apogent’s (or any of its Restricted Subsidiaries’) management pursuant to any management equity subscription agreement, stock option agreement or similar agreement;provided that the aggregate price paid for all such repurchased, redeemed, acquired or retired Equity Interests may not exceed $5.0 million in any twelve-month period;
(6) repurchases of Equity Interests deemed to occur upon the cashless exercise of stock options; and
(7) other Restricted Payments in an aggregate amount since the date of the indenture not to exceed 7.5% of Consolidated Assets of Apogent at the time of such Restricted Payment (with each such Restricted Payment being valued as of the date made and without regard to subsequent changes in value).
The amount of all Restricted Payments (other than cash) will be the fair market value on the date of the Restricted Payment of the asset(s) or securities proposed to be transferred or issued by Apogent or such Restricted Subsidiary, as the case may be, pursuant to the Restricted Payment. The fair market value of any assets or securities that are required to be valued by this covenant will be determined by the Board of Directors whose resolution with respect thereto will be delivered to the trustee. The Board of Directors’ determination must be based upon an opinion or appraisal issued by an accounting, appraisal or investment banking firm of national standing if the fair market value exceeds $10.0 million. Not later than the date of making any Restricted Payment, Apogent will deliver to the trustee an officers’ certificate stating that such Restricted Payment is permitted and setting forth the basis upon which the calculations required by this “Restricted Payments” covenant were computed, together with a copy of any fairness opinion or appraisal required by the indenture.
80
Incurrence of Indebtedness and Issuance of Preferred Stock
Apogent will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise, with respect to (collectively, “incur”) any Indebtedness (including Acquired Debt), and Apogent will not issue any Disqualified Stock and will not permit any of its Restricted Subsidiaries to issue any shares of preferred stock;provided, however, that (A) Apogent and any Guarantor may incur Indebtedness (including Acquired Debt) or Apogent may issue Disqualified Stock, if the Fixed Charge Coverage Ratio for Apogent’s most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock is issued would have been at least 2.25 to 1 and (B) any Foreign Restricted Subsidiary may incur Indebtedness, Disqualified Stock or preferred stock, if the Fixed Charge Coverage Ratio would have been at least 2.75 to 1, in each case, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred or Disqualified Stock had been issued, as the case may be, at the beginning of such four-quarter period;
The first paragraph of this covenant will not prohibit the incurrence of any of the following items of Indebtedness (collectively, “Permitted Debt”):
(1) the incurrence by Apogent and any Guarantor of additional Indebtedness and letters of credit under one or more Credit Facilities in an aggregate principal amount at any one time outstanding under this clause (1) (with letters of credit being deemed to have a principal amount equal to the maximum potential liability of Apogent and its Subsidiaries thereunder) not to exceed $500.0 million less the aggregate amount of all Net Proceeds of Asset Sales applied to repay any such Indebtedness pursuant to the covenant described above under the caption “—Asset Sales”;
(2) the incurrence by Apogent and its Restricted Subsidiaries of the Existing Indebtedness;
(3) the incurrence by Apogent and the Guarantors of Indebtedness represented by the notes issued and sold in this offering and the related Subsidiary Guarantees to be issued on the date of the indenture and the Exchange Notes and the related Subsidiary Guarantees to be issued pursuant to the registration rights agreement;
(4) the incurrence by Apogent and the Restricted Subsidiaries of Indebtedness represented by Capital Lease Obligations, mortgage financings or purchase money obligations, in each case, incurred for the purpose of financing all or any part of the purchase price or cost of construction or improvement of property, plant or equipment used in the business of Apogent or such Subsidiary, in an aggregate principal amount not to exceed $25.0 million at any time outstanding;
(5) the incurrence by Apogent or any of its Restricted Subsidiaries of Permitted Refinancing Indebtedness in exchange for, or the net proceeds of which are used to refund, refinance or replace Indebtedness (other than intercompany Indebtedness) that was permitted by the indenture to be incurred under the first paragraph of this covenant or clauses (2), (3), (4), (5), or (10) of this paragraph;
(6) the incurrence by Apogent or any of its Restricted Subsidiaries of intercompany Indebtedness between or among Apogent and any of its Restricted Subsidiaries;provided, however, that:
(a) if Apogent or any Guarantor is the obligor on such Indebtedness, such Indebtedness must be expressly subordinated to the prior payment in full in cash of all Obligations with respect to the notes, in the case of Apogent, or the Subsidiary Guarantee, in the case of a Guarantor; and
(b) (i) any subsequent issuance or transfer of Equity Interests that results in any such Indebtedness being held by a Person other than Apogent or a Subsidiary of Apogent or (ii) any sale or other transfer of any such Indebtedness to a Person that is not either Apogent or a Restricted Subsidiary of Apogent; will be deemed, in each case, to constitute an incurrence of such Indebtedness by Apogent or such Subsidiary, as the case may be, that was not permitted by this clause (6);
(7) the incurrence by Apogent or any of its Subsidiaries of Hedging Obligations;
81
(8) the Guarantee by Apogent or any of the Guarantors of Indebtedness of Apogent or any Guarantor of Apogent that was permitted to be incurred by another provision of this covenant;
(9) the incurrence by Apogent’s Unrestricted Subsidiaries of Non-Recourse Debt,provided, however, that if any such Indebtedness ceases to be Non-Recourse Debt of an Unrestricted Subsidiary, such event will be deemed to constitute an incurrence of Indebtedness by a Restricted Subsidiary of Apogent that was not permitted by this clause (9);
(10) the incurrence by Apogent’s Foreign Restricted Subsidiaries of Indebtedness on or after the date of the indenture in an aggregate principal amount not to exceed $30.0 million outstanding at any time; and
(11) the incurrence by Apogent or any of the Guarantors of additional Indebtedness in an aggregate principal amount (or accreted value, as applicable) at any time outstanding, including all Permitted Refinancing Indebtedness incurred to refund, refinance or replace any Indebtedness incurred pursuant to this clause (11), not to exceed $50.0 million.
For purposes of determining compliance with this “Incurrence of Indebtedness and Issuance of Preferred Stock” covenant, in the event that an item of proposed Indebtedness (including Acquired Debt) meets the criteria of more than one of the categories of Permitted Debt described in clauses (1) through (11) above, or is entitled to be incurred pursuant to the first paragraph of this covenant, Apogent will be permitted to classify (or later classify or reclassify in whole or in part in its sole discretion) such item of Indebtedness in any manner that complies with this covenant.
Restrictions on Senior Subordinated Debt
Apogent will not incur, create, issue, assume, guarantee or otherwise become liable for any Indebtedness that is subordinate or junior in right of payment to any Senior Debt of Apogent and senior in any respect in right of payment to the notes. No Guarantor will incur, create, issue, assume, guarantee or otherwise become liable for any Indebtedness that is subordinate or junior in right of payment to the Senior Debt of such Guarantor and senior in any respect in right of payment to such Guarantor’s Subsidiary Guarantee.
Liens
Apogent will not, and will not permit any Restricted Subsidiary to, directly or indirectly, create, incur, assume or suffer to exist any Lien upon any of its property (including Capital Stock of a Restricted Subsidiary), whether owned at the closing of the offering or thereafter acquired, or any interest therein or any income or profits therefrom, securing Indebtedness other than Senior Debt, without effectively providing that all payments due under the indenture and the notes are secured on an equal and ratable basis with the obligations so secured until such time as such obligations are no longer secured by a Lien.
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
Apogent will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create or permit to exist or become effective any consensual encumbrance or restriction on the ability of any Restricted Subsidiary to:
(1) pay dividends or make any other distributions on its Capital Stock to Apogent or any of its Subsidiaries, or with respect to any other interest or participation in, or measured by, its profits, or pay any indebtedness owed to Apogent or any of its Restricted Subsidiaries;
(2) make loans or advances to Apogent or any of its Restricted Subsidiaries; or
(3) transfer any of its properties or assets to Apogent or any of its Restricted Subsidiaries.
82
However, the preceding restrictions will not apply to encumbrances or restrictions existing under or by reason of:
(1) agreements governing Existing Indebtedness and Credit Facilities as in effect on the date of the indenture and any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements, or refinancings of those agreements,providedthat the amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements, or refinancings are no more restrictive, taken as a whole, with respect to such dividend and other payment restrictions than those contained in those agreements on the date of the indenture;
(2) the indenture, the notes, the Exchange Notes, and the Subsidiary Guarantees;
(3) applicable law;
(4) any instrument governing Indebtedness or Capital Stock of a Person acquired by Apogent or any of its Restricted Subsidiaries as in effect at the time of such acquisition (except to the extent such Indebtedness or Capital Stock was incurred in connection with or in contemplation of such acquisition), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person, or the property or assets of the Person, so acquired,providedthat, in the case of Indebtedness, such Indebtedness was permitted by the terms of the indenture to be incurred;
(5) customary non-assignment provisions in leases entered into in the ordinary course of business and consistent with past practices;
(6) purchase money obligations for property acquired in the ordinary course of business that impose restrictions on that property of the nature described in clause (3) of the preceding paragraph;
(7) any agreement for the sale or other disposition of a Restricted Subsidiary that restricts distributions by that Restricted Subsidiary pending its sale or other disposition;
(8) Permitted Refinancing Indebtedness,providedthat the restrictions contained in the agreements governing such Permitted Refinancing Indebtedness are no more restrictive, taken as a whole, than those contained in the agreements governing the Indebtedness being refinanced;
(9) Liens securing Indebtedness otherwise permitted to be incurred under the provisions of the covenant described above under the caption “—Liens” that limit the right of the debtor to dispose of the assets subject to such Liens; and
(10) provisions with respect to the disposition or distribution of assets or property in joint venture agreements, assets sale agreements, stock sale agreements and other similar agreements entered into in the ordinary course of business.
Merger, Consolidation, or Sale of Assets
Apogent may not, directly or indirectly: (1) consolidate or merge with or into another Person (whether or not Apogent is the surviving corporation); or (2) sell, assign, transfer, convey, or otherwise dispose of all or substantially all of the properties or assets of Apogent and its Restricted Subsidiaries taken as a whole, in one or more related transactions, to another Person; unless:
(1) either: (a) Apogent is the surviving corporation; or (b) the Person formed by or surviving any such consolidation or merger (if other than Apogent) or to which such sale, assignment, transfer, conveyance, or other disposition has been made is a corporation organized or existing under the laws of the United States, any state of the United States or the District of Columbia;
(2) the Person formed by or surviving any such consolidation or merger (if other than Apogent) or the Person to which such sale, assignment, transfer, conveyance, or other disposition has been made assumes all the obligations of Apogent under the notes, the indenture and the registration rights agreement pursuant to agreements reasonably satisfactory to the trustee;
83
(3) immediately after such transaction no Default or Event of Default exists; and
(4) Apogent or the Person formed by or surviving any such consolidation or merger (if other than Apogent), or to which such sale, assignment, transfer, conveyance or other disposition has been made will, on the date of such transaction after giving pro forma effect thereto and any related financing transactions as if the same had occurred at the beginning of the applicable four-quarter period, be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described above under the caption “—Incurrence of Indebtedness and Issuance of Preferred Stock.”
In addition, Apogent may not, directly or indirectly, lease all or substantially all of its properties or assets, in one or more related transactions, to any other Person.
Transactions with Affiliates
Apogent will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or Guarantee with, or for the benefit of, any Affiliate (each, an “Affiliate Transaction”), unless:
(1) the Affiliate Transaction is on terms that are no less favorable to Apogent or the relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by Apogent or such Restricted Subsidiary with an unrelated Person; and
(2) Apogent delivers to the trustee:
(a) with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate consideration in excess of $2.5 million, a resolution of the Board of Directors set forth in an officers’ certificate certifying that such Affiliate Transaction complies with this covenant and that such Affiliate Transaction has been approved by a majority of the disinterested members of the Board of Directors; and
(b) with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate consideration in excess of $10.0 million, an opinion as to the fairness to the holders of such Affiliate Transaction from a financial point of view issued by an accounting, appraisal or investment banking firm of national standing.
The following items will not be deemed to be Affiliate Transactions and, therefore, will not be subject to the provisions of the prior paragraph:
(1) any employment agreement entered into by Apogent or any of its Restricted Subsidiaries in the ordinary course of business of Apogent or such Restricted Subsidiary;
(2) transactions between or among Apogent and/or its Restricted Subsidiaries;
(3) transactions with a Person that is an Affiliate of Apogent solely because Apogent owns an Equity Interest in, or controls, such Person;
(4) payment of reasonable directors fees to Persons who are not otherwise Affiliates of Apogent;
(5) sales of Equity Interests (other than Disqualified Stock) to Affiliates of Apogent; and
(6) Restricted Payments that are permitted by the provisions of the indenture described above under the caption “—Restricted Payments.”
Designation of Restricted and Unrestricted Subsidiaries
The Board of Directors may designate any Restricted Subsidiary to be an Unrestricted Subsidiary if that designation would not cause a Default. If a Restricted Subsidiary is designated as an Unrestricted Subsidiary, the
84
aggregate fair market value of all outstanding Investments owned by Apogent and its Restricted Subsidiaries in the Subsidiary properly designated will be deemed to be an Investment made as of the time of the designation and will reduce the amount available for Restricted Payments under the first paragraph of the covenant described above under the caption “—Restricted Payments” or Permitted Investments, as determined by Apogent. That designation will only be permitted if the Investment would be permitted at that time and if the Restricted Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. The Board of Directors may redesignate any Unrestricted Subsidiary to be a Restricted Subsidiary if the redesignation would not cause a Default.
Additional Subsidiary Guarantees
If Apogent or any of its Restricted Subsidiaries acquires or creates another Domestic Subsidiary or if any existing or newly acquired or created Subsidiary of Apogent or any of its Subsidiaries that is not already a Guarantor Guarantees Obligations under Apogent’s Credit Facility after the date of the indenture, then that existing or newly acquired or created Subsidiary will become a Guarantor and execute a supplemental indenture and deliver an opinion of counsel satisfactory to the trustee within 10 Business Days of the date on which it Guaranteed such Credit Facility or was acquired or created;provided, however, that the foregoing shall not apply to subsidiaries that have properly been designated as Unrestricted Subsidiaries in accordance with the indenture for so long as they continue to constitute Unrestricted Subsidiaries.
Notwithstanding the foregoing, at any time after the termination of certain covenants as provided under the caption “—Certain Covenants—Termination of Covenants when Notes Rated Investment Grade,” no existing or newly acquired or created Subsidiary that is not already a Guarantor will be required to execute a supplemental indenture unless such Subsidiary Guarantees Indebtedness of Apogent under the Credit Facility. However, any Subsidiary that Guarantees any Indebtedness of Apogent under the Credit Facility will become a Guarantor.
Business Activities
Apogent will not, and will not permit any Restricted Subsidiary to, engage in any business other than Permitted Businesses, except to such extent as would not be material to Apogent and its Subsidiaries taken as a whole.
Payments for Consent
Apogent will not, and will not permit any of its Subsidiaries to, directly or indirectly, pay or cause to be paid any consideration to or for the benefit of any holder of notes for or as an inducement to any consent, waiver or amendment of any of the terms or provisions of the indenture or the notes unless such consideration is offered to be paid and is paid to all holders of the notes that consent, waive or agree to amend in the time frame set forth in the solicitation documents relating to such consent, waiver or agreement.
Reports
If Apogent ceases to be subject to the reporting requirements of the Exchange Act, so long as any notes are outstanding, Apogent will furnish to the holders of notes, within the time periods specified in the Commission’s rules and regulations:
(1) all quarterly and annual financial information that would be required to be contained in a filing with the Commission on Forms 10-Q and 10-K if Apogent were required to file such Forms, including a “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and, with respect to the annual information only, a report on the annual financial statements by Apogent’s certified independent accountants; and
(2) all current reports that would be required to be filed with the Commission on Form 8-K if Apogent were required to file such reports.
If Apogent has designated any of its Subsidiaries as Unrestricted Subsidiaries, then the quarterly and annual financial information required by the preceding paragraph will include a reasonably detailed presentation, either
85
on the face of the financial statements or in the footnotes thereto, and in Management’s Discussion and Analysis of Financial Condition and Results of Operations, of the financial condition and results of operations of Apogent and its Restricted Subsidiaries separate from the financial condition and results of operations of the Unrestricted Subsidiaries of Apogent.
Events of Default and Remedies
Each of the following is an Event of Default:
(1) default for 30 days in the payment when due of interest on, or Liquidated Damages with respect to, the notes whether or not prohibited by the subordination provisions of the indenture;
(2) default in payment when due of the principal of, or premium, if any, on the notes, whether or not prohibited by the subordination provisions of the indenture;
(3) failure by Apogent or any of its Restricted Subsidiaries to comply with the provisions described under the captions “—Repurchase at the Option of Holders—Asset Sales”, “—Repurchase at the Option of Holders—Change of Control” or “—Certain Covenants—Merger, Consolidation or Sale of Assets;”
(4) failure by Apogent or any of its Restricted Subsidiaries for 30 days after notice to comply with the provisions described under the captions “—Certain Covenants—Restricted Payments” or “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock”
(5) failure by Apogent or any of its Restricted Subsidiaries for 60 days after notice to comply with any of the other agreements in the indenture;
(6) default under any mortgage, indenture or instrument under which there may be issued or by which there may be secured or evidenced any Indebtedness for money borrowed by Apogent or any of its Restricted Subsidiaries (or the payment of which is Guaranteed by Apogent or any of its Restricted Subsidiaries) whether such Indebtedness or Guarantee now exists, or is created after the date of the indenture, if that default:
(a) is caused by a failure to pay principal of, or interest or premium, if any, on such Indebtedness prior to the expiration of the grace period provided in such Indebtedness on the date of such default (a “Payment Default”); or
(b) results in the acceleration of such Indebtedness prior to its express maturity,
and, in each case, the principal amount of any such Indebtedness, together with the principal amount of any other such Indebtedness under which there has been a Payment Default or the maturity of which has been so accelerated, aggregates $20.0 million or more;
(7) failure by Apogent or any of its Subsidiaries to pay final judgments aggregating in excess of $20.0 million, which judgments are not paid, discharged or stayed for a period of 60 days;
(8) except as permitted by the indenture, any Subsidiary Guarantee shall be held in any judicial proceeding to be unenforceable or invalid or shall cease for any reason to be in full force and effect or any Guarantor, or any Person acting on behalf of any Guarantor, shall deny or disaffirm its obligations under its Subsidiary Guarantee; and
(9) certain events of bankruptcy or insolvency described in the indenture with respect to Apogent or any of its Restricted Subsidiaries.
In the case of an Event of Default arising from certain events of bankruptcy or insolvency, with respect to Apogent, any Subsidiary that is a Significant Subsidiary or any group of Subsidiaries that, taken together, would constitute a Significant Subsidiary, all outstanding notes will become due and payable immediately without further action or notice.
86
If any other Event of Default occurs and is continuing, the trustee or the holders of at least 25% in principal amount of the then outstanding notes may declare all the notes to be due and payable immediately; provided, however, that so long as any Designated Senior Debt is outstanding, such declaration shall not become effective until the earlier of:
(1) the day which is five business days after receipt by the Representatives of Designated Senior Debt of such notice of acceleration; or
(2) the date of the acceleration of any Designated Senior Debt.
Holders of the notes may not enforce the indenture or the notes except as provided in the indenture. Subject to certain limitations, holders of a majority in principal amount of the then outstanding notes may direct the trustee in its exercise of any trust or power. The trustee may withhold from holders of the notes notice of any continuing Default or Event of Default if it determines that withholding notice is in their interest, except a Default or Event of Default relating to the payment of principal or interest or Liquidated Damages.
The holders of a majority in aggregate principal amount of the notes then outstanding by notice to the trustee may on behalf of the holders of all of the notes waive any existing Default or Event of Default and its consequences under the indenture except a continuing Default or Event of Default in the payment of interest or Liquidated Damages on, or the principal of, the notes.
In the case of any Event of Default occurring by reason of any willful action or inaction taken or not taken by or on behalf of Apogent with the intention of avoiding payment of the premium that Apogent would have had to pay if Apogent then had elected to redeem the notes pursuant to the optional redemption provisions of the indenture, an equivalent premium will also become and be immediately due and payable to the extent permitted by law upon the acceleration of the notes. If an Event of Default occurs prior to May 15, 2008, by reason of any willful action (or inaction) taken (or not taken) by or on behalf of Apogent with the intention of avoiding the prohibition on redemption of the notes prior to May 15, 2008, then the premium specified in the indenture will also become immediately due and payable to the extent permitted by law upon the acceleration of the notes.
Apogent is required to deliver to the trustee annually a statement regarding compliance with the indenture. Upon becoming aware of any Default or Event of Default, Apogent is required to deliver to the trustee a statement specifying such Default or Event of Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of Apogent or any Guarantor, as such, will have any liability for any obligations of Apogent or the Guarantors under the notes, the indenture, the Subsidiary Guarantees, or for any claim based on, in respect of, or by reason of, such obligations or their creation. Each holder of notes by accepting a note waives and releases all such liability. The waiver and release are part of the consideration for issuance of the notes. The waiver may not be effective to waive liabilities under the federal securities laws.
Legal Defeasance and Covenant Defeasance
Apogent may, at its option and at any time, elect to have all of its obligations discharged with respect to the outstanding notes and all obligations of the Guarantors discharged with respect to their Subsidiary Guarantees (“Legal Defeasance”) except for:
(1) the rights of holders of outstanding notes to receive payments in respect of the principal of, or interest or premium and Liquidated Damages, if any, on such notes when such payments are due from the trust referred to below;
(2) Apogent’s obligations with respect to the notes concerning issuing temporary notes, registration of notes, mutilated, destroyed, lost or stolen notes and the maintenance of an office or agency for payment and money for security payments held in trust;
87
(3) the rights, powers, trusts, duties and immunities of the trustee, and Apogent’s and the Guarantor’s obligations in connection therewith; and
(4) the Legal Defeasance provisions of the indenture.
In addition, Apogent may, at its option and at any time, elect to have the obligations of Apogent and the Guarantors released with respect to certain covenants that are described in the indenture (“Covenant Defeasance”) and thereafter any omission to comply with those covenants will not constitute a Default or Event of Default with respect to the notes. In the event Covenant Defeasance occurs, certain events (not including non-payment, bankruptcy, receivership, rehabilitation and insolvency events) described under “—Events of Default and Remedies” will no longer constitute an Event of Default with respect to the notes.
In order to exercise either Legal Defeasance or Covenant Defeasance:
(1) Apogent must irrevocably deposit with the trustee, in trust, for the benefit of the holders of the notes, cash in U.S. dollars, non-callable Government Securities, or a combination of cash in U.S. dollars and non-callable Government Securities, in amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, or interest and premium and Liquidated Damages, if any, on the outstanding notes on the stated maturity or on the applicable redemption date, as the case may be, and Apogent must specify whether the notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, Apogent has delivered to the trustee an opinion of counsel reasonably acceptable to the trustee confirming that (a) Apogent has received from, or there has been published by, the Internal Revenue Service a ruling or (b) since the date of the indenture, there has been a change in the applicable federal income tax law, in either case to the effect that, and based thereon such opinion of counsel will confirm that, the holders of the outstanding notes will not recognize income, gain or loss for federal income tax purposes as a result of such Legal Defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, Apogent has delivered to the trustee an opinion of counsel reasonably acceptable to the trustee confirming that the holders of the outstanding notes will not recognize income, gain or loss for federal income tax purposes as a result of such Covenant Defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default or Event of Default has occurred and is continuing on the date of such deposit (other than a Default or Event of Default resulting from the borrowing of funds to be applied to such deposit);
(5) such Legal Defeasance or Covenant Defeasance will not result in a breach or violation of, or constitute a default under any material agreement or instrument (other than the indenture) to which Apogent or any of its Subsidiaries is a party or by which Apogent or any of its Subsidiaries is bound;
(6) Apogent must deliver to the trustee an officers’ certificate stating that the deposit was not made by Apogent with the intent of preferring the holders of notes over the other creditors of Apogent or with the intent of defeating, hindering, delaying or defrauding any other creditors of Apogent or others; and
(7) Apogent must deliver to the trustee an officers’ certificate and an opinion of counsel, each stating that all conditions precedent relating to the Legal Defeasance or the Covenant Defeasance have been complied with.
Amendment, Supplement, and Waiver
Except as provided in the next two succeeding paragraphs, the indenture or the notes may be amended or supplemented with the consent of the holders of at least a majority in principal amount of the notes (including
88
additional notes, if any) then outstanding (including, without limitation, consents obtained in connection with a purchase of, or tender offer or exchange offer for, notes), and any existing default or compliance with any provision of the indenture, the Subsidiary Guarantees or the notes may be waived with the consent of the holders of a majority in principal amount of the then outstanding notes (including, without limitation, consents obtained in connection with a purchase of, or tender offer or exchange offer for, notes).
Without the consent of each holder of notes affected, an amendment or waiver may not (with respect to any notes held by a non-consenting holder):
(1) reduce the principal amount of notes whose holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed maturity of any note or alter or waive the provisions with respect to the redemption of the notes (other than provisions relating to the covenants described above under the caption “—Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest, including default interest, on any note;
(4) waive a Default or Event of Default in the payment of principal of, or interest or premium, or Liquidated Damages, if any, on the notes (except a rescission of acceleration of the notes by the holders of at least a majority in aggregate principal amount of the notes then outstanding and a waiver of the payment default that resulted from such acceleration);
(5) make any note payable in money other than that stated in the notes;
(6) make any change in the provisions of the indenture relating to waivers of past Defaults or the rights of holders of notes to receive payments of principal of, or interest or premium or Liquidated Damages, if any, on the notes;
(7) waive a redemption payment with respect to any note (including a payment required by one of the covenants described above under the caption “—Repurchase at the Option of Holders”);
(8) release any Guarantor from any of its obligations under its Subsidiary Guarantee or the indenture, except in accordance with the terms of the indenture; or
(9) make any change in the preceding amendment and waiver provisions.
In addition, any amendment to, or waiver of, the provisions of the indenture relating to subordination that adversely affects the rights of the holders of the notes will require the consent of the holders of at least 75% in aggregate principal amount of notes then outstanding.
Notwithstanding the preceding, without the consent of any holder of notes, Apogent, the Guarantors, and the trustee may amend or supplement the indenture, the Subsidiary Guarantees or the notes:
(1) to cure any ambiguity, defect or inconsistency;
(2) to provide for uncertificated notes in addition to or in place of certificated notes;
(3) to provide for the assumption of Apogent’s or a Guarantor’s Obligations to holders of notes in the case of a merger or consolidation or sale of all or substantially all of Apogent’s assets;
(4) to make any change that would provide any additional rights or benefits to the holders of notes or that does not adversely affect the legal rights under the indenture of any such holder of notes;
(5) to comply with requirements of the Commission in order to effect or maintain the qualification of the indenture under the Trust Indenture Act;
(6) to provide for the issuance of additional notes in accordance with the limitations set forth in the indenture; or
(7) to allow any Guarantor to execute a supplemental indenture and/or a Subsidiary Guarantee with respect to the notes.
89
Satisfaction and Discharge
The indenture will be discharged and will cease to be of further effect as to all notes issued thereunder, when:
(1) either:
(a) all notes that have been authenticated, except lost, stolen or destroyed notes that have been replaced or paid and notes for whose payment money has been deposited in trust and thereafter repaid to Apogent, have been delivered to the trustee for cancellation; or
(b) all notes that have not been delivered to the trustee for cancellation have become due and payable by reason of the mailing of a notice of redemption or otherwise or will become due and payable within one year and Apogent or any Guarantor has irrevocably deposited or caused to be deposited with the trustee as trust funds in trust solely for the benefit of the holders of notes, cash in U.S. dollars, non-callable Government Securities, or a combination of cash in U.S. dollars and non-callable Government Securities, in amounts as will be sufficient without consideration of any reinvestment of interest, to pay and discharge the entire indebtedness on the notes not delivered to the trustee for cancellation for principal, premium and Liquidated Damages, if any, and accrued interest to the date of maturity or redemption;
(2) no Default or Event of Default has occurred and is continuing on the date of the deposit or will occur as a result of the deposit and the deposit will not result in a breach or violation of, or constitute a default under, any other instrument to which Apogent or any Guarantor is a party or by which Apogent or any Guarantor is bound;
(3) Apogent or any Guarantor has paid or caused to be paid all sums payable by it under the indenture; and
(4) Apogent has delivered irrevocable instructions to the trustee under the indenture to apply the deposited money toward the payment of the notes at maturity or the redemption date, as the case may be.
In addition, Apogent must deliver an officers’ certificate and an opinion of counsel to the trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Concerning the Trustee
If the trustee becomes a creditor of Apogent or any Guarantor, the indenture limits its right to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The trustee will be permitted to engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the Commission for permission to continue or resign.
The holders of a majority in principal amount of the then outstanding notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the trustee, subject to certain exceptions. The indenture provides that in case an Event of Default occurs and is continuing, the trustee will be required, in the exercise of its power, to use the degree of care of a prudent man in the conduct of his own affairs. Subject to such provisions, the trustee will be under no obligation to exercise any of its rights or powers under the indenture at the request of any holder of notes, unless such holder has offered to the trustee security and indemnity satisfactory to it against any loss, liability or expense.
Anyone who receives this offering prospectus may obtain a copy of the indenture and registration rights agreement without charge by writing to Apogent Technologies Inc., 30 Penhallow Street, Portsmouth, NH 03801, Attention: General Counsel.
90
Book-Entry, Delivery, and Form
Except as set forth below, the Original Notes are and the Exchange Notes will be represented by Global Notes registered in the name of Cede & Co. as nominee for the Depository Trust Company (“DTC”). The Global Notes may be transferred, in whole and not in part, only to another nominee of DTC or to a successor of DTC or its nominee. Beneficial interests in the Global Notes may not be exchanged for notes in certificated form except in the limited circumstances described below. See “—Exchange of Global Notes for Certificated Notes.” Except in the limited circumstances described below, owners of beneficial interests in the Global Notes will not be entitled to receive physical delivery of notes in certificated form.
Depository Procedures
The following description of the operations and procedures of DTC, Euroclear and Clearstream are provided solely as a matter of convenience. These operations and procedures are solely within the control of the respective settlement systems and are subject to changes by them. Apogent takes no responsibility for these operations and procedures and urges investors to contact the system or their participants directly to discuss these matters.
DTC has advised Apogent that DTC is a limited-purpose trust company created to hold securities for its participating organizations (collectively, the “Participants”) and to facilitate the clearance and settlement of transactions in those securities between Participants through electronic book-entry changes in accounts of its Participants. The Participants include securities brokers and dealers (including the Initial purchasers), banks, trust companies, clearing corporations and certain other organizations. Access to DTC’s system is also available to other entities such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a Participant, either directly or indirectly (collectively, the “Indirect Participants”). Persons who are not Participants may beneficially own securities held by or on behalf of DTC only through the Participants or the Indirect Participants. The ownership interests in, and transfers of ownership interests in, each security held by or on behalf of DTC are recorded on the records of the Participants and Indirect Participants.
DTC has also advised Apogent that, pursuant to procedures established by it:
(1) upon deposit of the Global Notes, DTC will credit the accounts of Participants designated by the Initial purchasers with portions of the principal amount of the Global Notes; and
(2) ownership of these interests in the Global Notes will be shown on, and the transfer of ownership of these interests will be effected only through, records maintained by DTC (with respect to the Participants) or by the Participants and the Indirect Participants (with respect to other owners of beneficial interest in the Global Notes).
Investors in the Global Notes who are Participants in DTC’s system may hold their interests therein directly through DTC. Investors in the Global Notes who are not Participants may hold their interests therein indirectly through organizations (including Euroclear and Clearstream) which are Participants in such system. All interests in a Global Note, including those held through Euroclear System (“Euroclear”) and Clearstream Banking S.A. (“Clearstream”) (as indirect participants in DTC), may be subject to the procedures and requirements of DTC. Those interests held through Euroclear or Clearstream may also be subject to the procedures and requirements of such systems. The laws of some states require that certain Persons take physical delivery in definitive form of securities that they own. Consequently, the ability to transfer beneficial interests in a Global Note to such Persons will be limited to that extent. Because DTC can act only on behalf of Participants, which in turn act on behalf of Indirect Participants, the ability of a Person having beneficial interests in a Global Note to pledge such interests to Persons that do not participate in the DTC system, or otherwise take actions in respect of such interests, may be affected by the lack of a physical certificate evidencing such interests.
Except as described below, owners of interest in the Global Notes will not have notes registered in their names, will not receive physical delivery of notes in certificated form and will not be considered the registered owners or “holders” thereof under the indenture for any purpose.
91
Payments in respect of the principal of, and interest and premium and Liquidated Damages, if any, on a Global Note registered in the name of DTC or its nominee will be payable to DTC in its capacity as the registered holder of the notes under the indenture. Under the terms of the indenture, Apogent and the trustee will treat the Persons in whose names the notes, including the Global Notes, are registered as the owners of the notes for the purpose of receiving payments and for all other purposes. Consequently, neither Apogent, the trustee nor any agent of Apogent or the trustee has or will have any responsibility or liability for:
(1) any aspect of DTC’s records or any Participant’s or Indirect Participant’s records relating to or payments made on account of beneficial ownership interest in the Global Notes or for maintaining, supervising or reviewing any of DTC’s records or any Participant’s or Indirect Participant’s records relating to the beneficial ownership interests in the Global Notes; or
(2) any other matter relating to the actions and practices of DTC or any of its Participants or Indirect Participants.
DTC has advised Apogent that its current practice, upon receipt of any payment in respect of securities such as the notes (including principal and interest), is to credit the accounts of the relevant Participants with the payment on the payment date unless DTC has reason to believe it will not receive payment on such payment date. Each relevant Participant is credited with an amount proportionate to its beneficial ownership of an interest in the principal amount of the relevant security as shown on the records of DTC. Payments by the Participants and the Indirect Participants to the beneficial owners of notes will be governed by standing instructions and customary practices and will be the responsibility of the Participants or the Indirect Participants and will not be the responsibility of DTC, the trustee or Apogent. Neither Apogent nor the trustee will be liable for any delay by DTC or any of its Participants in identifying the beneficial owners of the notes, and Apogent and the trustee may conclusively rely on and will be protected in relying on instructions from DTC or its nominee for all purposes.
Subject to the transfer restrictions set forth under “Notice to Investors,” transfers between Participants in DTC will be effected in accordance with DTC’s procedures, and will be settled in same-day funds, and transfers between participants in Euroclear and Clearstream will be effected in accordance with their respective rules and operating procedures.
Subject to compliance with the transfer restrictions applicable to the notes described herein, cross-market transfers between the Participants in DTC, on the one hand, and Euroclear or Clearstream participants, on the other hand, will be effected through DTC in accordance with DTC’s rules on behalf of Euroclear or Clearstream, as the case may be, by its respective depositary; however, such cross-market transactions will require delivery of instructions to Euroclear or Clearstream, as the case may be, by the counterparty in such system in accordance with the rules and procedures and within the established deadlines (Brussels time) of such system. Euroclear or Clearstream, as the case may be, will, if the transaction meets its settlement requirements, deliver instructions to its respective depositary to take action to effect final settlement on its behalf by delivering or receiving interests in the relevant Global Note in DTC, and making or receiving payment in accordance with normal procedures for same-day funds settlement applicable to DTC. Euroclear participants and Clearstream participants may not deliver instructions directly to the depositories for Euroclear or Clearstream.
DTC has advised Apogent that it will take any action permitted to be taken by a holder of notes only at the direction of one or more Participants to whose account DTC has credited the interests in the Global Notes and only in respect of such portion of the aggregate principal amount of the notes as to which such Participant or Participants has or have given such direction. However, if there is an Event of Default under the notes, DTC reserves the right to exchange the Global Notes for legended notes in certificated form, and to distribute such notes to its Participants.
Although DTC, Euroclear and Clearstream have agreed to the foregoing procedures to facilitate transfers of interests in the Global Notes among participants in DTC, Euroclear and Clearstream, they are under no obligation
92
to perform or to continue to perform such procedures, and may discontinue such procedures at any time. Neither Apogent nor the trustee nor any of their respective agents will have any responsibility for the performance by DTC, Euroclear or Clearstream or their respective participants or indirect participants of their respective obligations under the rules and procedures governing their operations.
Exchange of Global Notes for Certificated Notes
A Global Note is exchangeable for definitive notes in registered certificated form (“Certificated Notes”) if:
(1) DTC (a) notifies Apogent that it is unwilling or unable to continue as depositary for the Global Notes and Apogent fails to appoint a successor depositary or (b) has ceased to be a clearing agency registered under the Exchange Act;
(2) Apogent, at its option, notifies the trustee in writing that it elects to cause the issuance of the Certificated Notes; or
(3) there has occurred and is continuing a Default or Event of Default with respect to the notes.
In addition, beneficial interests in a Global Note may be exchanged for Certificated Notes upon prior written notice given to the trustee by or on behalf of DTC in accordance with the indenture. In all cases, Certificated Notes delivered in exchange for any Global Note or beneficial interests in Global Notes will be registered in the names, and issued in any approved denominations, requested by or on behalf of the depositary (in accordance with its customary procedures) and will bear the applicable restrictive legend referred to in “Notice to Investors,” unless that legend is not required by applicable law.
Exchange of Certificated Notes for Global Notes
Certificated Notes may not be exchanged for beneficial interests in any Global Note unless the transferor first delivers to the trustee a written certificate (in the form provided in the indenture) to the effect that such transfer will comply with the appropriate transfer restrictions applicable to such notes. See “Notice to Investors.”
Same Day Settlement and Payment
Apogent will make payments in respect of the notes represented by the Global Notes (including principal, premium, if any, interest and Liquidated Damages, if any) by wire transfer of immediately available funds to the accounts specified by the Global Note Holder. Apogent will make all payments of principal, interest and premium and Liquidated Damages, if any, with respect to Certificated Notes by wire transfer of immediately available funds to the accounts specified by the holders of the Certificated Notes or, if no such account is specified, by mailing a check to each such holder’s registered address. The notes represented by the Global Notes are expected to trade in DTC’s Same-Day Funds Settlement System, and any permitted secondary market trading activity in such notes will, therefore, be required by DTC to be settled in immediately available funds. Apogent expects that secondary trading in any Certificated Notes will also be settled in immediately available funds.
Because of time zone differences, the securities account of a Euroclear or Clearstream participant purchasing an interest in a Global Note from a Participant in DTC will be credited, and any such crediting will be reported to the relevant Euroclear or Clearstream participant, during the securities settlement processing day (which must be a business day for Euroclear and Clearstream) immediately following the settlement date of DTC. DTC has advised Apogent that cash received in Euroclear or Clearstream as a result of sales of interests in a Global Note by or through a Euroclear or Clearstream participant to a Participant in DTC will be received with value on the settlement date of DTC but will be available in the relevant Euroclear or Clearstream cash account only as of the business day for Euroclear or Clearstream following DTC’s settlement date.
Registration Rights; Liquidated Damages
The following description is a summary of the material provisions of the registration rights agreement. It does not restate that agreement in its entirety. We urge you to read the registration rights agreement in its entirety
93
because it, and not this description, defines your registration rights as a holder of these notes. See “—Where You Can Find More Information.”
Apogent, the Guarantors and the Initial purchasers entered into the registration rights agreement on June 2, 2003. Pursuant to the registration rights agreement, Apogent and the Guarantors agreed to file with the Commission the Exchange Offer Registration Statement on the appropriate form under the Securities Act with respect to the Exchange Notes. Upon the effectiveness of the Exchange Offer Registration Statement, Apogent and the Guarantors will offer to the holders of Transfer Restricted Securities pursuant to the Exchange Offer who are able to make certain representations the opportunity to exchange their Transfer Restricted Securities for Exchange Notes.
If:
(1) Apogent and the Guarantors are not
(a) required to file the Exchange Offer Registration Statement; or
(b) permitted to consummate the Exchange Offer because the Exchange Offer is not permitted by applicable law or Commission policy; or
(2) any holder of Transfer Restricted Securities notifies Apogent prior to the 20th day following consummation of the Exchange Offer that:
(a) it is prohibited by law or Commission policy from participating in the Exchange Offer; or
(b) that it may not resell the Exchange Notes acquired by it in the Exchange Offer to the public without delivering a prospectus and the prospectus contained in the Exchange Offer Registration Statement is not appropriate or available for such resales; or
(c) that it is a broker-dealer and owns notes acquired directly from Apogent or an affiliate of Apogent,
Apogent and the Guarantors will file with the Commission a shelf registration statement (“Shelf Registration Statement”) to cover resales of the notes by the holders of the notes who satisfy certain conditions relating to the provision of information in connection with the Shelf Registration Statement.
Apogent and the Guarantors have agreed to use their best efforts to cause the applicable registration statement to be declared effective as promptly as possible by the Commission.
For purposes of the preceding, “Transfer Restricted Securities” means each note until:
(1) the date on which such note has been exchanged by a Person other than a broker-dealer for an Exchange Note in the Exchange Offer;
(2) following the exchange by a broker-dealer in the Exchange Offer of a note for an Exchange Note, the date on which such Exchange Note is sold to a purchaser who receives from such broker-dealer on or prior to the date of such sale a copy of the prospectus contained in the Exchange Offer Registration Statement;
(3) the date on which such note has been effectively registered under the Securities Act and disposed of in accordance with the Shelf Registration Statement; or
(4) the date on which such note is distributed to the public pursuant to Rule 144 under the Securities Act.
The registration rights agreement provides that:
(1) Apogent and the Guarantors will file an Exchange Offer Registration Statement with the Commission on or prior to 90 days after the closing of the sale of the Original Notes;
94
(2) Apogent and the Guarantors will use their best efforts to have the Exchange Offer Registration Statement declared effective by the Commission on or prior to 150 days after the closing of the sale of the Original Notes;
(3) unless the Exchange Offer would not be permitted by applicable law or Commission policy, Apogent and the Guarantors will
(a) commence the Exchange Offer; and
(b) use their best efforts to issue on or prior to 30 business days, or longer, if required by the federal securities laws, after the date on which the Exchange Offer Registration Statement was declared effective by the Commission, Exchange Notes in exchange for all notes tendered prior thereto in the Exchange Offer; and
(4) if obligated to file the Shelf Registration Statement, Apogent and the Guarantors will use their best efforts to file the Shelf Registration Statement with the Commission on or prior to 30 days after such filing obligation arises and to cause the Shelf Registration to be declared effective by the Commission on or prior to 90 days after such obligation arises.
If:
(1) Apogent and the Guarantors fail to file any of the registration statements required by the registration rights agreement on or before the date specified for such filing; or
(2) any of such registration statements is not declared effective by the Commission on or prior to the date specified for such effectiveness (the “Effectiveness Target Date”); or
(3) Apogent and the Guarantors fail to consummate the Exchange Offer within 30 business days of the Effectiveness Target Date with respect to the Exchange Offer Registration Statement; or
(4) the Shelf Registration Statement or the Exchange Offer Registration Statement is declared effective but thereafter ceases to be effective or usable in connection with resales of Transfer Restricted Securities during the periods specified in the registration rights agreement (each such event referred to in clauses (1) through (4) above, a “Registration Default”),
then Apogent and the Guarantors will pay Liquidated Damages to each holder of notes, with respect to the first 90-day period immediately following the occurrence of the first Registration Default in an amount equal to $.05 per week per $1,000 principal amount of notes held by such holder.
The amount of the Liquidated Damages will increase by an additional $.05 per week per $1,000 principal amount of notes with respect to each subsequent 90-day period until all Registration Defaults have been cured, up to a maximum amount of Liquidated Damages for all Registration Defaults of $.50 per week per $1,000 principal amount of notes.
All accrued Liquidated Damages will be paid by Apogent and the Guarantors on each Damages Payment Date to the Global Note Holder by wire transfer of immediately available funds or by federal funds check and to holders of Certificated Notes by wire transfer to the accounts specified by them or by mailing checks to their registered addresses if no such accounts have been specified.
Following the cure of all Registration Defaults, the accrual of Liquidated Damages will cease.
The registration statement of which this prospectus is a part is the Exchange Offer Registration Statement described above, and the Exchange Offer described in this prospectus is the Exchange Offer described above. For each Original Note surrendered to us pursuant to the Exchange Offer, we will issue to the holder an Exchange Note having a principal amount equal to that of the surrendered Original Note. Interest on the Exchange Note will accrue from the last interest payment date in which interest was paid on the Original Note surrendered in exchange therefore, or if no interest has been paid on that Original Note, from the date interest began to accrue on that Original Note.
95
Holders of notes will be required to make certain representations to Apogent (as described in this prospectus and the registration rights agreement) in order to participate in the Exchange Offer and will be required to deliver certain information to be used in connection with the Shelf Registration Statement and to provide comments on the Shelf Registration Statement within the time periods set forth in the registration rights agreement in order to have their notes included in the Shelf Registration Statement and benefit from the provisions regarding Liquidated Damages set forth above. By acquiring Transfer Restricted Securities, a holder will be deemed to have agreed to indemnify Apogent and the Guarantors against certain losses arising out of information furnished by such holder in writing for inclusion in any Shelf Registration Statement. Holders of notes will also be required to suspend their use of this prospectus under certain circumstances upon receipt of written notice to that effect from Apogent.
Certain Definitions
Set forth below are certain defined terms used in the indenture. Reference is made to the indenture for a full disclosure of all such terms, as well as any other capitalized terms used herein for which no definition is provided.
“Acquired Debt” means, with respect to any specified Person:
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Subsidiary of such specified Person, whether or not such Indebtedness is incurred in connection with, or in contemplation of, such other Person merging with or into, or becoming a Subsidiary of, such specified Person; and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control,” as used with respect to any Person, means the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise. For purposes of this definition, the terms “controlling,” “controlled by” and “under common control with” have correlative meanings.
“Asset Sale” means:
(1) the sale, lease, conveyance or other disposition of any assets or rights, other than sales of inventory in the ordinary course of business consistent with past practices;provided that the sale, conveyance or other disposition of all or substantially all of the assets of Apogent and its Restricted Subsidiaries taken as a whole will be governed by the provisions of the indenture described above under the caption “—Repurchase at the Option of Holders—Change of Control” and/or the provisions described above under the caption “—Certain Covenants—Merger, Consolidation or Sale of Assets” and not by the provisions of the Asset Sale covenant; and
(2) the issuance of Equity Interests in any of Apogent’s Restricted Subsidiaries or the sale of Equity Interests in any of its Subsidiaries.
Notwithstanding the preceding, the following items will not be deemed to be Asset Sales:
(1) any single transaction or series of related transactions that involves assets having a fair market value of less than $5.0 million;
(2) a transfer of assets between or among Apogent and its Restricted Subsidiaries,
(3) an issuance of Equity Interests by a Restricted Subsidiary to Apogent or to another Restricted Subsidiary;
(4) the sale or lease of equipment, inventory, accounts receivable or other assets in the ordinary course of business;
96
(5) the sale or other disposition of cash or Cash Equivalents;
(6) a Restricted Payment or Permitted Investment that is permitted by the covenant described above under the caption “—Certain Covenants—Restricted Payments”;
(7) the sale, exchange or disposition of obsolete assets not integral to any Permitted Business;
(8) the sale, lease, conveyance, or other disposition of any property or assets recorded on the balance sheet as of March 31, 2003 of Apogent as assets of discontinued operations available for sale;
(9) the licensing or sublicensing of intellectual property or other general intangibles and licenses, leases or subleases of other property;
(10) dispositions of receivables in connection with the compromise, settlement or collection thereof in the ordinary course of business or in bankruptcy or similar proceedings and exclusive of factoring or similar arrangements; and
(11) foreclosure on assets.
“Attributable Debt” in respect of a sale and leaseback transaction means, at the time of determination, the present value of the obligation of the lessee for net rental payments during the remaining term of the lease included in such sale and leaseback transaction including any period for which such lease has been extended or may, at the option of the lessor, be extended. Such present value shall be calculated using a discount rate equal to the rate of interest implicit in such transaction, determined in accordance with GAAP.
“Beneficial Owner” has the meaning assigned to such term in Rule 13d-3 and Rule 13d-5 under the Exchange Act, except that in calculating the beneficial ownership of any particular “person” (as that term is used in Section 13(d)(3) of the Exchange Act), such “person” will be deemed to have beneficial ownership of all securities that such “person” has the right to acquire by conversion or exercise of other securities, whether such right is currently exercisable or is exercisable only upon the occurrence of a subsequent condition. The terms “Beneficially Owns” and “Beneficially Owned” have a corresponding meaning.
“Board of Directors” means:
(1) with respect to a corporation, the board of directors of the corporation;
(2) with respect to a partnership, the Board of Directors of the general partner of the partnership; and
(3) with respect to any other Person, the board or committee of such Person serving a similar function.
“Board Resolution” means a resolution duly adopted by the Board of Directors, a copy of which, certified by the Secretary or an Assistant Secretary of Apogent to be in full force and effect on the date of such certification, shall have been delivered to the trustee.
“Capital Lease Obligation” means, at the time any determination is to be made, the amount of the liability in respect of a capital lease that would at that time be required to be capitalized on a balance sheet in accordance with GAAP.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person.
97
“Cash Equivalents” means:
(1) United States dollars;
(2) securities issued or directly and fully Guaranteed or insured by the United States government or any agency or instrumentality of the United States government (provided that the full faith and credit of the United States is pledged in support of those securities) having maturities of not more than six months from the date of acquisition;
(3) certificates of deposit and eurodollar time deposits with maturities of six months or less from the date of acquisition, bankers’ acceptances with maturities not exceeding six months and overnight bank deposits, in each case, with any lender party to the Credit Agreement or with any domestic commercial bank having capital and surplus in excess of $500.0 million and a Thomson Bank Watch Rating of “B” or better;
(4) repurchase obligations with a term of not more than seven days for underlying securities of the types described in clauses (2) and (3) above entered into with any financial institution meeting the qualifications specified in clause (3) above;
(5) commercial paper having the highest rating obtainable from Moody’s or S&P’s Rating Services and in each case maturing within six months after the date of acquisition; and
(6) money market funds at least 95% of the assets of which constitute Cash Equivalents of the kinds described in clauses (1) through (5) of this definition.
“Change of Control” means the occurrence of any of the following:
(1) the direct or indirect sale, transfer, conveyance or other disposition (other than by way of merger or consolidation), in one or a series of related transactions, of all or substantially all of the properties or assets of Apogent and its Restricted Subsidiaries taken as a whole to any “person” (as that term is used in Section 13(d)(3) of the Exchange Act);
(2) the adoption of a plan relating to the liquidation or dissolution of Apogent;
(3) the consummation of any transaction (including, without limitation, any merger or consolidation) the result of which is that any “person” (as defined above) becomes the Beneficial Owner, directly or indirectly, of more than 50% of the Voting Stock of Apogent, measured by voting power rather than number of shares;
(4) the first day on which a majority of the members of the Board of Directors of Apogent are not Continuing Directors; or
(5) Apogent consolidates with, or merges with or into, any Person, or any Person consolidates with, or merges with or into, Apogent, in any such event pursuant to a transaction in which any of the outstanding Voting Stock of Apogent or such other Person is converted into or exchanged for cash, securities or other property, other than any such transaction where the Voting Stock of Apogent outstanding immediately prior to such transaction is converted into or exchanged for Voting Stock (other than Disqualified Stock) of the surviving or transferee Person constituting a majority of the outstanding shares of such Voting Stock of such surviving or transferee Person (immediately after giving effect to such issuance).
“Commission” means Securities and Exchange Commission.
“Common Stock Tender Offer” means the tender offer for shares of Apogent’s common stock and associated preferred stock purchase rights commenced by Apogent on April 23, 2003.
“Consolidated Assets” of any Person as of any date means the total assets of such Person and its Restricted Subsidiaries on a consolidated basis at such date, as determined in accordance with GAAP.
98
“Consolidated Cash Flow” means, with respect to any specified Person for any period, the Consolidated Net Income of such Person for such periodplus:
(1) an amount equal to any extraordinary loss plus any net loss realized by such Person or any of its Subsidiaries in connection with an Asset Sale, to the extent such losses were deducted in computing such Consolidated Net Income;plus
(2) provision for taxes based on income or profits of such Person and its Restricted Subsidiaries for such period, to the extent that such provision for taxes was deducted in computing such Consolidated Net Income;plus
(3) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, whether paid or accrued and whether or not capitalized (including, without limitation, amortization of debt issuance costs and original issue discount, non-cash interest payments, the interest component of any deferred payment obligations, the interest component of all payments associated with Capital Lease Obligations, imputed interest with respect to Attributable Debt, commissions, discounts and other fees and charges incurred in respect of letter of credit or bankers’ acceptance financings, and net of the effect of all payments made or received pursuant to Hedging Obligations), to the extent that any such expense was deducted in computing such Consolidated Net Income;plus
(4) depreciation, amortization (including amortization of goodwill and other intangibles but excluding amortization of prepaid cash expenses that were paid in a prior period) and other non-cash expenses (excluding any such non-cash expense to the extent that it represents an accrual of or reserve for cash expenses in any future period or amortization of a prepaid cash expense that was paid in a prior period) of such Person and its Subsidiaries for such period to the extent that such depreciation, amortization and other non-cash expenses were deducted in computing such Consolidated Net Income;minus
(5) non-cash items increasing such Consolidated Net Income for such period, other than the accrual of revenue in the ordinary course of business,
in each case, on a consolidated basis and determined in accordance with GAAP.
“Consolidated Net Income” means, with respect to any specified Person for any period, the aggregate of the Net Income of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, determined in accordance with GAAP;provided that:
(1) the Net Income (but not loss) of any Person that is not a Restricted Subsidiary or that is accounted for by the equity method of accounting will be included only to the extent of the amount of dividends or distributions paid in cash to the specified Person or a Restricted Subsidiary of the Person;
(2) the Net Income of any Restricted Subsidiary will be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of that Net Income is not at the date of determination permitted without any prior governmental approval (that has not been obtained) or, directly or indirectly, by operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule or governmental regulation applicable to that Restricted Subsidiary or its stockholders;
(3) the Net Income of any Person acquired in a pooling of interests transaction for any period prior to the date of such acquisition will be excluded; and
(4) the cumulative effect of a change in accounting principles will be excluded.
“Consolidated Net Worth” of any Person as of any date means the stockholders’ equity (including any preferred stock that is classified as equity under GAAP, other than Disqualified Stock) of such Person and its Restricted Subsidiaries (excluding any equity adjustment for foreign currency translation for any period subsequent to the date of the indenture) on a consolidated basis at such date, as determined in accordance with GAAP.
99
“Continuing Directors” means, as of any date of determination, any member of the Board of Directors of Apogent who:
(1) was a member of such Board of Directors on the date of the indenture (June 2, 2003); or
(2) was nominated for election or elected to such Board of Directors with the approval of a majority of the Continuing Directors who were members of such Board of Directors at the time of such nomination or election.
“Credit Agreement” means that certain Credit Agreement, dated as of December 1, 2000, by and among Apogent and the several banks and other financial institutions from time to time parties thereto (as the same may be further amended, supplemented or otherwise modified), providing for up to $500.0 million of revolving credit borrowings, including any related notes, Guarantees, collateral documents, instruments and agreements executed in connection therewith, and in each case as amended, modified, renewed, refunded, replaced or refinanced from time to time.
“Credit Facilities” means one or more debt facilities or agreements (including, without limitation, the Credit Agreement) or commercial paper facilities, in each case with banks or other institutional lenders or investors providing for revolving credit loans, term loans, receivables financing (including through the sale of receivables to such lenders or to special purpose entities formed to borrow from such lenders against such receivables) or letters of credit, in each case, as amended, restated, modified, renewed, refunded, replaced or refinanced (including any agreement to extend the maturity thereof and adding additional borrowers or guarantors) in whole or in part from time to time under the same or any other agent, lender or group of lenders and including increasing the amount of available borrowings thereunder, provided that such increase is permitted by the “—Incurrence of Indebtedness and Issuance of Preferred Stock” covenant above.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Senior Debt” means:
(1) any Indebtedness outstanding under the Credit Agreement; and
(2) after payment in full of all Obligations under the Credit Agreement, any other Senior Debt permitted under the indenture the principal amount of which is $25.0 million or more and that has been designated by Apogent as “Designated Senior Debt.”
“Disqualified Stock” means any Capital Stock that, by its terms (or by the terms of any security into which it is convertible, or for which it is exchangeable, in each case at the option of the holder of the Capital Stock), or upon the happening of any event, matures or is mandatorily redeemable, pursuant to a sinking fund obligation or otherwise, or redeemable at the option of the holder of the Capital Stock, in whole or in part, on or prior to the date that is 91 days after the date on which the notes mature. Notwithstanding the preceding sentence, any Capital Stock that would constitute Disqualified Stock solely because the holders of the Capital Stock have the right to require Apogent to repurchase such Capital Stock upon the occurrence of a change of control or an asset sale will not constitute Disqualified Stock if the terms of such Capital Stock provide that Apogent may not repurchase or redeem any such Capital Stock pursuant to such provisions unless such repurchase or redemption complies with the covenant described above under the caption “—Certain Covenants—Restricted Payments.”
“Domestic Subsidiary” means any Restricted Subsidiary of Apogent that was formed under the laws of the United States or any state of the United States or the District of Columbia and that Guarantees or otherwise provides direct credit support for any Indebtedness of Apogent.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock (but excluding any debt security that is convertible into, or exchangeable for, Capital Stock).
100
“Equity Offering” means any public or private sale of Capital Stock (other than Disqualified Stock) made for cash on a primary basis by Apogent after the date of the indenture.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Exchange Notes” means the exchange notes to be issued in exchange for the offered notes having substantially identical terms as the offered notes (except that the exchange notes will not contain terms with respect to transfer restrictions) pursuant to a registered exchange offer as provided in the registration rights agreement. See “Registration Rights; Liquidated Damages.”
“Existing Indebtedness” means any Indebtedness of Apogent and its Restricted Subsidiaries (other than Indebtedness under the Credit Agreement) in existence on the date of the indenture, until such amounts are repaid.
“Fixed Charges” means, with respect to any specified Person for any period, the sum, without duplication, of:
(1) the consolidated interest expense of such Person and its Restricted Subsidiaries for such period, whether paid or accrued, including, without limitation, amortization of debt issuance costs and original issue discount, non-cash interest payments, the interest component of any deferred payment obligations, the interest component of all payments associated with Capital Lease Obligations, imputed interest with respect to Attributable Debt, commissions, discounts and other fees and charges incurred in respect of letter of credit or bankers’ acceptance financings, and net of the effect of all payments made or received pursuant to Hedging Obligations;plus
(2) the consolidated interest of such Person and its Restricted Subsidiaries that was capitalized during such period;plus
(3) any interest expense on Indebtedness of another Person that is Guaranteed by such Person or one of its Restricted Subsidiaries or secured by a Lien on assets of such Person or one of its Restricted Subsidiaries, whether or not such Guarantee or Lien is called upon;plus
(4) the product of (a) all dividends, whether paid or accrued and whether or not in cash, on any series of preferred stock of such Person or any of its Restricted Subsidiaries, other than dividends on Equity Interests payable solely in Equity Interests of Apogent (other than Disqualified Stock) or to Apogent or a Restricted Subsidiary of Apogent, times (b) a fraction, the numerator of which is one and the denominator of which is one minus the then current combined federal, state and local statutory tax rate of such Person, expressed as a decimal,
in each case, on a consolidated basis and in accordance with GAAP.
“Fixed Charge Coverage Ratio” means with respect to any specified Person for any period, the ratio of the Consolidated Cash Flow of such Person for such period to the Fixed Charges of such Person for such period. In the event that the specified Person or any of its Subsidiaries incurs, assumes, Guarantees, repays, repurchases or redeems any Indebtedness (other than ordinary working capital borrowings) or issues, repurchases or redeems preferred stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated and on or prior to the date on which the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Calculation Date”), then the Fixed Charge Coverage Ratio will be calculated giving pro forma effect to such incurrence, assumption, Guarantee, repayment, repurchase or redemption of Indebtedness, or such issuance, repurchase or redemption of preferred stock, and the use of the proceeds therefrom as if the same had occurred at the beginning of the applicable four-quarter reference period.
In addition, for purposes of calculating the Fixed Charge Coverage Ratio:
(1) acquisitions that have been made by the specified Person or any of its Restricted Subsidiaries, including through mergers or consolidations and including any related financing transactions, during the four-quarter reference period or subsequent to such reference period and on or prior to the Calculation Date
101
will be given pro forma effect as if they had occurred on the first day of the four-quarter reference period and Consolidated Cash Flow for such reference period will be calculated on a pro forma basis in accordance with Regulation S-X under the Securities Act, but without giving effect to clause (3) of the proviso set forth in the definition of Consolidated Net Income;
(2) the Consolidated Cash Flow attributable to discontinued operations, as determined in accordance with GAAP, and operations or businesses disposed of prior to the Calculation Date, will be excluded; and
(3) the Fixed Charges attributable to discontinued operations, as determined in accordance with GAAP, and operations or businesses disposed of prior to the Calculation Date, will be excluded, but only to the extent that the obligations giving rise to such Fixed Charges will not be obligations of the specified Person or any of its Restricted Subsidiaries following the Calculation Date.
“Foreign Restricted Subsidiary” means any Restricted Subsidiary that is not a Domestic Restricted Subsidiary.
“GAAP” means generally accepted accounting principles set forth in the opinions and pronouncements of the Accounting Principles Board of the American Institute of Certified Public Accountants and statements and pronouncements of the Financial Accounting Standards Board or in such other statements by such other entity as have been approved by a significant segment of the accounting profession, as in effect from time to time;provided, however, that any change in GAAP that would cause Apogent or a Restricted Subsidiary to record an existing item as a liability upon that entity’s balance sheet, which item was not previously required by GAAP to be so recorded, shall not constitute an incurrence of Indebtedness for purposes of the indenture.
“Guarantee” means a guarantee other than by endorsement of negotiable instruments for collection in the ordinary course of business, direct or indirect, in any manner including, without limitation, by way of a pledge of assets or through letters of credit or reimbursement agreements in respect thereof, of all or any part of any Indebtedness.
“Guarantors” means:
(1) each Subsidiary of Apogent that has guaranteed Obligations under the Credit Agreement in existence on the date of the indenture; and
(2) any other Subsidiary that will in the future guarantee Obligations under the Credit Agreement and that executes a Subsidiary Guarantee or supplemental indenture in accordance with the provisions of the indenture;
and their respective successors and assigns.
“Hedging Obligations” means, with respect to any specified Person, the obligations of such Person incurred in the normal course of business and consistent with past practices and not for speculative purposes under:
(1) interest rate swap agreements, interest rate cap agreements and interest rate collar agreements;
(2) foreign exchange contracts and currency protection agreements entered into with one of more financial institutions is designed to protect the person or entity entering into the agreement against fluctuations in interest rates or currency exchanges rates with respect to Indebtedness incurred and not for purposes of speculation; and
(3) other agreements or arrangements designed to protect such person against fluctuations in interest rates or currency exchange rates.
“Indebtedness” means, with respect to any specified Person, any indebtedness of such Person, whether or not contingent:
(1) in respect of borrowed money;
102
(2) evidenced by bonds, notes, debentures or similar instruments or letters of credit (or reimbursement agreements in respect thereof);
(3) in respect of banker’s acceptances;
(4) representing Capital Lease Obligations;
(5) representing the balance deferred and unpaid of the purchase price of any property, except any such balance that constitutes an accrued expense or trade payable; or
(6) representing any Hedging Obligations,
if and to the extent any of the preceding items (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet of the specified Person prepared in accordance with GAAP. In addition, the term “Indebtedness” includes all Indebtedness of others secured by a Lien on any asset of the specified Person (whether or not such Indebtedness is assumed by the specified Person) and, to the extent not otherwise included, the Guarantee by the specified Person of any indebtedness of any other Person.
The amount of any Indebtedness outstanding as of any date will be:
(1) the accreted value of the Indebtedness, in the case of any Indebtedness issued with original issue discount; and
(2) the principal amount of the Indebtedness, together with any interest on the Indebtedness that is more than 30 days past due, in the case of any other Indebtedness.
“Investment Grade” means:
(1) with respect to S&P, any of the rating categories from and including AAA to and including BBB-; and
(2) with respect to Moody’s, any of the rating categories from and including Aaa to and including Baa3.
“Investments” means, with respect to any Person, all direct or indirect investments by such Person in other Persons (including Affiliates) in the forms of loans (including Guarantees or other obligations), advances or capital contributions (excluding commission, travel and similar advances to officers and employees made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities, together with all items that are or would be classified as investments on a balance sheet prepared in accordance with GAAP. If Apogent or any Subsidiary of Apogent sells or otherwise disposes of any Equity Interests of any direct or indirect Subsidiary of Apogent such that, after giving effect to any such sale or disposition, such Person is no longer a Subsidiary of Apogent, Apogent will be deemed to have made an Investment on the date of any such sale or disposition equal to the fair market value of the Equity Interests of such Subsidiary not sold or disposed of in an amount determined as provided in the final paragraph of the covenant described above under the caption “—Certain Covenants—Restricted Payments.” The acquisition by Apogent or any Subsidiary of Apogent of a Person that holds an Investment in a third Person will be deemed to be an Investment by Apogent or such Subsidiary in such third Person in an amount equal to the fair market value of the Investment held by the acquired Person in such third Person in an amount determined as provided in the final paragraph of the covenant described above under the caption “—Certain Covenants—Restricted Payments.”
“Lien” means, with respect to any asset, any mortgage, lien, pledge, charge, security interest or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction.
“Moody’s” means Moody’s Investors Service, Inc. or any successor to the rating agency business thereof.
103
“Net Income” means, with respect to any specified Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of preferred stock dividends, excluding, however:
(1) any gain (but not loss), together with any related provision for taxes on such gain (but not loss), realized in connection with: (a) any Asset Sale; or (b) the disposition of any securities by such Person or any of its Subsidiaries or the extinguishment of any Indebtedness of such Person or any of its Subsidiaries; and
(2) any extraordinary gain (but not loss), together with any related provision for taxes on such extraordinary gain (but not loss).
“Net Proceeds” means the aggregate cash proceeds received by Apogent or any of its Restricted Subsidiaries in respect of any Asset Sale (including, without limitation, any cash received upon the sale or other disposition of any non-cash consideration received in any Asset Sale), net of the direct costs relating to such Asset Sale, including, without limitation, legal, accounting and investment banking fees, and sales commissions, and any relocation expenses incurred as a result of the Asset Sale, taxes paid or payable as a result of the Asset Sale, in each case, after taking into account any available tax credits or deductions and any tax sharing arrangements, and amounts required to be applied to the repayment of Indebtedness, other than Senior Debt secured by a Lien on the asset or assets that were the subject of such Asset Sale and any reserve for adjustment in respect of the sale price of such asset or assets established in accordance with GAAP.
“Non-Recourse Debt” means Indebtedness:
(1) as to which neither Apogent nor any of its Restricted Subsidiaries (a) provides credit support of any kind (including any undertaking, agreement or instrument that would constitute Indebtedness), (b) is directly or indirectly liable as a guarantor or otherwise, or (c) constitutes the lender;
(2) no default with respect to which (including any rights that the holders of the Indebtedness may have to take enforcement action against an Unrestricted Subsidiary) would permit upon notice, lapse of time or both any holder of any other Indebtedness (other than the notes) of Apogent or any of its Restricted Subsidiaries to declare a default on such other Indebtedness or cause the payment of the Indebtedness to be accelerated or payable prior to its stated maturity; and
(3) as to which the lenders have been notified in writing that they will not have any recourse to the stock or assets of Apogent or any of its Restricted Subsidiaries.
“Obligations” means any principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities payable under the documentation governing any Indebtedness.
“Permitted Business” means the lines of business conducted by Apogent and its Restricted Subsidiaries on the date hereof and any business incidental or reasonably related thereto or which is a reasonable extension thereof as determined in good faith by Apogent’s Board of Directors.
“Permitted Investments” means:
(1) any Investment in Apogent or in a Restricted Subsidiary of Apogent;
(2) any Investment in Cash Equivalents;
(3) any Investment by Apogent or any Subsidiary of Apogent in a Person, if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary of Apogent; or
(b) such Person is merged, consolidated or amalgamated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, Apogent or a Restricted Subsidiary of Apogent;
(4) any advances and extensions of credit in the nature of accounts receivable arising from the sale or lease of goods or services or the licensing of property in the ordinary course of business;
104
(5) any Investment made as a result of the receipt of non-cash consideration from an Asset Sale that was made pursuant to and in compliance with the covenant described above under the caption ”—Repurchase at the Option of Holders—Asset Sales”;
(6) any acquisition of assets solely in exchange for the issuance of Equity Interests (other than Disqualified Stock) of Apogent;
(7) any Investments received in compromise of obligations of such persons incurred in the ordinary course of business with trade creditors or customers that were incurred in the ordinary course of business, including pursuant to any plan of reorganization or similar arrangement upon the bankruptcy or insolvency of any trade creditor or customer;
(8) Hedging Obligations permitted to be incurred under the “Incurrence of Indebtedness and Issuance of Preferred Stock” covenant; and
(9) other Investments in any Person having an aggregate fair market value (measured on the date each such Investment was made and without giving effect to subsequent changes in value), when taken together with all other Investments made pursuant to this clause (9) that are at the time outstanding (with each such Investment being valued as of the date it was made and without regard to subsequent changes in value), not to exceed the greater of (a) $35.0 million or (b) 2.5% of Consolidated Net Worth of Apogent at the time of such Investment.
“Permitted Junior Securities” means:
(1) Equity Interests in Apogent or any Guarantor; or
(2) debt securities that are subordinated to all Senior Debt and any debt securities issued in exchange for Senior Debt to substantially the same extent as, or to a greater extent than, the notes and the Subsidiary Guarantees are subordinated to Senior Debt under the indenture.
“Permitted Refinancing Indebtedness” means any Indebtedness of Apogent or any of its Subsidiaries issued in exchange for, or the net proceeds of which are used to extend, refinance, renew, replace, defease or refund other Indebtedness of Apogent or any of its Subsidiaries (other than intercompany Indebtedness);providedthat:
(1) the principal amount (or accreted value, if applicable) of such Permitted Refinancing Indebtedness does not exceed the principal amount (or accreted value, if applicable) of the Indebtedness extended, refinanced, renewed, replaced, defeased or refunded (plus all accrued interest on the Indebtedness and the amount of all expenses and premiums incurred in connection therewith);
(2) such Permitted Refinancing Indebtedness has a final maturity date later than the final maturity date of, and has a Weighted Average Life to Maturity equal to or greater than the Weighted Average Life to Maturity of, the Indebtedness being extended, refinanced, renewed, replaced, defeased or refunded;
(3) if the Indebtedness being extended, refinanced, renewed, replaced, defeased or refunded is subordinated in right of payment to the notes, such Permitted Refinancing Indebtedness has a final maturity date later than the final maturity date of, and is subordinated in right of payment to, the notes on terms at least as favorable to the holders of notes as those contained in the documentation governing the Indebtedness being extended, refinanced, renewed, replaced, defeased or refunded; and
(4) such Indebtedness is incurred either by Apogent or by the Subsidiary who is the obligor on the Indebtedness being extended, refinanced, renewed, replaced, defeased or refunded.
“Person” means any individual, corporation, partnership, joint venture, association, joint-stock company, trust, unincorporated organization, limited liability company or government or other entity.
“Rating Agency” means S&P or Moody’s, or if S&P or Moody’s or both shall not make a rating agency on the notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be,
105
selected by Apogent (as certified by the Board of Directors) which shall be substituted for S&P or Moody’s or both, as the case may be.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” of a Person means any Subsidiary of the referent Person that is not an Unrestricted Subsidiary.
“Senior Debt” means:
(1) all Indebtedness of Apogent or any Guarantor outstanding under Credit Facilities and all Hedging Obligations with respect thereto;
(2) any other Indebtedness of Apogent or any Guarantor permitted to be incurred under the terms of the indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is on a parity with or subordinated in right of payment to the notes or any Subsidiary Guarantee; and
(3) all Obligations with respect to the items listed in the preceding clauses (1) and (2).
Notwithstanding anything to the contrary in the preceding, Senior Debt will not include:
(1) any liability for federal, state, local or other taxes owed or owing by Apogent;
(2) any intercompany Indebtedness of Apogent or any of its Subsidiaries to Apogent or any of its Affiliates;
(3) any trade payables; or
(4) the portion of any Indebtedness that is incurred in violation of the indenture.
“Significant Subsidiary” means any Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such Regulation is in effect on the date hereof.
“S&P” means Standard & Poor’s Rating Group, Inc., or any successor to the rating agency business thereof.
“Stated Maturity” means, with respect to any installment of interest or principal on any series of Indebtedness, the date on which the payment of interest or principal was scheduled to be paid in the original documentation governing such Indebtedness, and will not include any contingent obligations to repay, redeem or repurchase any such interest or principal prior to the date originally scheduled for the payment thereof.
“Subsidiary” means, with respect to any specified Person:
(1) any corporation, association or other business entity of which more than 50% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees of the corporation, association or other business entity is at the time owned or controlled, directly or indirectly, by that Person or one or more of the other Subsidiaries of that Person (or a combination thereof); and
(2) any partnership (a) the sole general partner or the managing general partner of which is such Person or a Subsidiary of such Person or (b) the only general partners of which are that Person or one or more Subsidiaries of that Person (or any combination thereof).
“Subsidiary Guarantee” means any Guarantee of the notes to be executed by any Subsidiary of Apogent pursuant to the covenants described above under “—Subsidiary Guarantees” and “—Additional Subsidiary Guarantees.”
106
“Unrestricted Subsidiary” means any Subsidiary of Apogent that is designated by the Board of Directors as an Unrestricted Subsidiary pursuant to a Board Resolution, but only to the extent that such Subsidiary:
(1) has no Indebtedness other than Non-Recourse Debt;
(2) is not party to any agreement, contract, arrangement or understanding with Apogent or any Restricted Subsidiary of Apogent unless the terms of any such agreement, contract, arrangement or understanding are no less favorable to Apogent or such Restricted Subsidiary than those that might be obtained at the time from Persons who are not Affiliates of Apogent;
(3) is a Person with respect to which neither Apogent nor any of its Restricted Subsidiaries has any direct or indirect obligation (a) to subscribe for additional Equity Interests or (b) to maintain or preserve such Person’s financial condition or to cause such Person to achieve any specified levels of operating results;
(4) has not guaranteed or otherwise directly or indirectly provided credit support for any Indebtedness of Apogent or any of its Restricted Subsidiaries; and
(5) has at least one director on its Board of Directors that is not a director or executive officer of Apogent or any of its Restricted Subsidiaries and has at least one executive officer that is not a director or executive officer of Apogent or any of its Restricted Subsidiaries.
Any designation of a Subsidiary of Apogent as an Unrestricted Subsidiary will be evidenced to the trustee by filing with the trustee a certified copy of the Board Resolution giving effect to such designation and an officers’ certificate certifying that such designation complied with the preceding conditions and was permitted by the covenant described above under the caption “—Certain Covenants—Restricted Payments.” If, at any time, any Unrestricted Subsidiary would fail to meet the preceding requirements as an Unrestricted Subsidiary, it will thereafter cease to be an Unrestricted Subsidiary for purposes of the indenture and any Indebtedness of such Subsidiary will be deemed to be incurred by a Restricted Subsidiary of Apogent as of such date and, if such Indebtedness is not permitted to be incurred as of such date under the covenant described under the caption “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock,” Apogent will be in default of such covenant. The Board of Directors of Apogent may at any time designate any Unrestricted Subsidiary to be a Restricted Subsidiary;provided that such designation will be deemed to be an incurrence of Indebtedness by a Restricted Subsidiary of Apogent of any outstanding Indebtedness of such Unrestricted Subsidiary and such designation will only be permitted if (1) such Indebtedness is permitted under the covenant described under the caption “—Certain Covenants—Incurrence of Indebtedness and Issuance of Preferred Stock,” calculated on a pro forma basis as if such designation had occurred at the beginning of the four-quarter reference period; and (2) no Default or Event of Default would be in existence following such designation.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the Board of Directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness at any date, the number of years obtained by dividing:
(1) the sum of the products obtained by multiplying (a) the amount of each then remaining installment, sinking fund, serial maturity or other required payments of principal, including payment at final maturity, in respect of the Indebtedness, by (b) the number of years (calculated to the nearest one-twelfth) that will elapse between such date and the making of such payment; by
(2) the then outstanding principal amount of such Indebtedness.
107
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of the material U.S. federal income tax consequences of the acquisition, ownership, disposition and retirement of notes by a holder thereof. This summary only applies to notes held as capital assets (generally, property held for investment) within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”). Except as set forth below, this summary does not address all of the tax consequences that may be relevant to a particular holder and it is not intended to be applicable to holders that are subject to special tax rules, such as financial institutions, insurance companies, real estate investment trusts, regulated investment companies, grantor trusts, U.S. expatriates, partnerships or other pass-through entities, tax-exempt organizations or dealers or traders in securities or currencies, or to holders that will hold a note as part of a position in a straddle or as part of a hedging, conversion or integrated transaction for U.S. federal income tax purposes or that have a functional currency other than the U.S. dollar. Moreover, except as set forth below, this summary does not address the U.S. federal estate and gift tax law, the tax laws of any state, local or foreign government or alternative minimum tax consequences of the acquisition, ownership, disposition or retirement of notes. Each prospective purchaser should consult its tax advisor with respect to the U.S. federal, state, local and foreign tax consequences of acquiring, holding and disposing of notes.
This summary is based on the Code, as amended, existing and proposed U.S. Treasury Regulations, administrative pronouncements and judicial decisions, each as available and in effect on the date hereof. All of the foregoing are subject to change, possibly with retroactive effect, or differing interpretations which could affect the tax consequences described herein.
WE URGE PROSPECTIVE INVESTORS TO CONSULT THEIR TAX ADVISORS REGARDING THE U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE ACQUISITION, OWNERSHIP, DISPOSITION AND RETIREMENT OF THE NOTES IN LIGHT OF THEIR PARTICULAR SITUATIONS, AS WELL AS ANY TAX CONSEQUENCES THAT MAY ARISE UNDER THE LAWS OF ANY FOREIGN, STATE, LOCAL OR OTHER TAXING JURISDICTION.
For purposes of this summary, a U.S. Holder is a beneficial owner of notes who for U.S. federal income tax purposes is (i) a citizen or resident (or is treated as a resident for U.S. federal income tax purposes) of the United States; (ii) a corporation, partnership or other entity created or organized in or under the laws of the United States or any State thereof, including the District of Columbia; (iii) an estate the income of which is subject to U.S. federal income taxation regardless of its source; or (iv) a trust (1) that validly elects to be treated as a U.S. person for U.S. federal income tax purposes or (2) (a) the administration over which a U.S. court can exercise primary supervision and (b) all of the substantial decisions of which one or more U.S. persons have the authority to control. A Non-U.S. Holder is a beneficial owner of notes other than a U.S. Holder.
If a partnership (or any other entity treated as a partnership for U.S. federal income tax purposes) holds the notes, the tax treatment of a partner in such partnership will generally depend on the status of the partner and the activities of the partnership. Such partner should consult its own tax advisor as to its consequences.
U.S. Holders
Interest
Except as set forth below, interest paid on a note generally will be includible in a U.S. Holder’s gross income as ordinary interest income at the time it is paid or accrued in accordance with the U.S. Holder’s usual method of tax accounting for U.S. federal income tax purposes.
As more fully described under “Description of Notes—Registration Rights; Liquidated Damages,” we may be required to pay Liquidated Damages to a U.S. Holder of a note. Although the matter is not free from doubt, we intend to take the position that a U.S. Holder of a note should be required to report any Liquidated Damages as
108
ordinary income for U.S. federal income tax purposes at the time the payment of the Liquidated Damages accrues or is received in accordance with such U.S. Holder’s usual method of accounting. It is possible, however, that the Internal Revenue Service may take a different position, in which case the timing and amount of income may be different.
Market Discount
A note sold on a secondary market after its original issue for a price lower than its stated redemption price at maturity is generally said to be acquired at market discount. Section 1278 of the Code defines “market discount” as the excess, if any, of the stated redemption price at maturity of the note, over the purchaser’s initial adjusted basis of the note. If, however, the market discount with respect to a note is less than ¼ of one percent (.0025) of the stated redemption price at maturity of the note multiplied by the number of complete years to maturity from the date the subsequent purchaser has acquired the note, then the market discount is considered to be zero. Notes acquired by holders at original issue and notes maturing not more than one year from the date of issue are not subject to the market discount rules.
Gain on the sale, redemption or retirement of a note, including full or partial redemption thereof, having “market discount” will be treated as interest income to the extent the gain does not exceed the accrued market discount on the note at the time of the disposition. A holder may elect to include market discount in taxable income for the taxable years to which it is attributable. The amount included is treated as interest income. If this election is made, the rule requiring interest income treatment of all or a portion of the gain upon disposition is inapplicable. Once the election is made to include market discount in income currently, it cannot be revoked without the consent of the Internal Revenue Service. The election applies to all market discount notes acquired by the holder on or after the first day of the first taxable year to which such election applies.
Sale, Exchange or Retirement of Notes
A U.S. Holder’s adjusted tax basis in a note generally will equal the cost of the note to such U.S. Holder. Upon the sale, exchange (other than the exchange of Original Notes for Exchange Notes to which different rules are expected to apply as discussed below) or retirement of a note, a U.S. Holder will recognize taxable gain or loss equal to the difference, if any, between the amount realized on the sale, exchange or retirement (less an amount equal to the accrued but unpaid interest which will be taxable as ordinary income) and such U.S. Holder’s adjusted tax basis in the note. Any such gain or loss generally will be capital gain or loss. In the case of a noncorporate U.S. Holder, capital gains derived in respect of a note that is held as a capital asset and that is held for more than one year are eligible for reduced income tax rates. The deductibility of capital losses is subject to limitations.
A U.S. Holder will recognize no gain or loss on the exchange of an Original Note for an Exchange Note. The Exchange Notes will be treated for federal income tax purposes as a continuation of the Original Notes. Consequently, (i) the holding period of the Exchange Note will include the holding period of the Original Note exchanged therefor, and (ii) the adjusted tax basis of the Exchange Note should be the same as the adjusted tax basis of the Original Note exchanged therefor immediately before the exchange.
Non-U.S. Holders
Interest
Subject to the discussion below under the heading “U.S. Backup Withholding and Information Reporting,” payments of principal of, and interest on, a note to a Non-U.S. Holder, other than (i) a controlled foreign corporation, as such term is defined in Section 957 of the Code, which is related to us, directly or indirectly, through stock ownership, (ii) a person owning, actually or constructively, securities representing at least 10% of the total combined outstanding voting power of all classes of our voting stock and (iii) banks which acquire such
109
note in consideration of an extension of credit made pursuant to a loan agreement entered into in the ordinary course of business, will not be subject to any U.S. withholding tax provided that the beneficial owner of the note provides certification completed in compliance with applicable statutory and regulatory requirements, which requirements are discussed below under the heading “U.S. Backup Withholding and Information Reporting,” or an exemption is otherwise established.
If a Non-U.S. Holder cannot satisfy the requirements above, payments of interest made to a Non-U.S. Holder will be subject to a U.S. withholding tax equal to 30% of the gross payments made to the Non-U.S. Holder unless the Non-U.S. Holder provides us or our paying agent, as the case may be, with a properly executed (1) IRS Form W-8BEN claiming an exemption from or reduction in withholding under the benefit of an applicable income tax treaty or (2) IRS Form W-8ECI stating that interest paid on the note is not subject to withholding tax because it is effectively connected with the beneficial owner’s conduct of a trade or business in the United States. Alternative documentation may be applicable in certain situations.
If a Non-U.S. Holder is engaged in a trade or business in the United States and interest on a note is effectively connected with the conduct of such trade or business, the Non-U.S. Holder, although exempt from withholding as discussed above (provided the certification requirements described above are satisfied), will be subject to U.S. federal income tax on such interest on a net income basis in the same manner as if the Non-U.S. Holder were a U.S. Holder. In addition, if such Non-U.S. Holder is a foreign corporation, it may be subject to a branch profits tax equal to 30% (or lesser rate under an applicable income tax treaty) of such amount, subject to adjustments.
Sale, Exchange or Other Disposition of Notes
Subject to the discussion below under the heading “U.S. Backup Withholding and Information Reporting,” any gain realized by a Non-U.S. Holder upon the sale, exchange (other than the exchange of Original Notes for Exchange Notes in which no gain or loss will be recognized by a Non-U.S. Holder) or retirement of a note generally will not be subject to U.S. federal income tax or withholding tax, unless (i) such gain is effectively connected with the conduct by such Non-U.S. Holder of a trade or business in the United States or (ii) in the case of any gain realized by an individual Non-U.S. Holder, such Non-U.S. Holder is present in the United States for 183 days or more in the taxable year of such sale, exchange or disposition and certain other conditions are met. Special rules may apply upon the sale, exchange or disposition of a note to certain Non-U.S. Holders, such as “controlled foreign corporations,” “passive foreign investment companies,” “foreign personal holding companies” and certain expatriates, that are subject to special treatment under the Code. Such entities and individuals should consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them.
As more fully described under “Description of Notes—Registration Rights; Liquidated Damages,” upon the occurrence of certain enumerated events we may be required to pay Liquidation Damages to you. It is possible that such payments might be subject to U.S. federal withholding tax.
U.S. Federal Estate Taxes
A note that is held by an individual who at the time of death is not a citizen or resident (as specially defined for United States federal estate tax purposes) of the United States will not generally be subject to U.S. federal estate tax as a result of such individual’s death, provided that such individual is not a shareholder owning actually or constructively 10% or more of the total combined voting power of all classes of our stock entitled to vote and, at the time of such individual’s death, payments of interest with respect to such note would not have been effectively connected with the conduct by such individual of a trade or business in the United States.
U.S. Backup Withholding and Information Reporting
Information reporting requirements will apply to certain payments of principal of, and interest on, an obligation and to proceeds of the sale, exchange or retirement of an obligation, to certain U.S. Holders. This
110
obligation, however, does not apply with respect to certain U.S. Holders including, corporations, tax-exempt organizations, qualified pension and profit sharing trusts and individual retirement accounts. A U.S. backup withholding tax will apply to such payments if a U.S. Holder fails to provide a taxpayer identification number or certification of other tax-exempt status or fails to report in full dividend and interest income.
Information reporting will generally apply to payments of interest on a note to a Non-U.S. Holder and the amount of tax, if any, withheld with respect to such payments. Copies of the information returns reporting such interest payments and any withholding may also be made available to the tax authorities in the country in which the Non-U.S. Holder resides under the provisions of an applicable income tax treaty. Payments of principal and interest on any notes to Non-U.S. Holders will not be subject to any U.S. backup withholding tax if the beneficial owner of the note (or a financial institution holding the note on behalf of the beneficial owner in the ordinary course of its trade or business) provides an appropriate certification to the payor and the payor does not have actual knowledge or reason to know, that the certification is incorrect. Payments of principal and interest on notes not excluded from U.S. backup withholding tax discussed above generally will be subject to United States withholding tax, currently 28%, except where an applicable United States income tax treaty provides for the reduction or elimination of such withholding tax.
In addition, information reporting and, depending on the circumstances, backup withholding, will apply to the proceeds of the sale of a note within the United States or conducted through United States-related financial intermediaries unless the beneficial owner provides the payor with an appropriate certification as to its non-U.S. status and the payor does not have actual knowledge or reason to know that the certification is incorrect.
Any amounts withheld under the backup withholding rules will be allowed as a refund or credit against a holder’s U.S. federal income tax liability provided the required information if furnished to the Internal Revenue Service.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSEQUENCES RELATING TO THE ACQUISITION, OWNERSHIP, AND RETIREMENT OF THE NOTES. PROSPECTIVE PURCHASERS OF NOTES SHOULD CONSULT THEIR OWN TAX ADVISORS CONCERNING THE TAX CONSEQUENCES OF THEIR PARTICULAR SITUATIONS.
111
PLAN OF DISTRIBUTION
Each broker-dealer that receives Exchange Notes for its own account pursuant to the Exchange Offer must acknowledge that it will deliver a prospectus in connection with any resale of such Exchange Notes. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of Exchange Notes received in exchange for Original Notes where such Original Notes were acquired as a result of market-making activities or other trading activities. Apogent has agreed that, for a period of 180 days after the Expiration Date, it will make this prospectus, as amended or supplemented, available to any broker-dealer for use in connection with any such resale.
Apogent will not receive any proceeds from any sale of Exchange Notes by broker-dealers. Exchange Notes received by broker-dealers for their own account pursuant to the Exchange Offer may be sold from time to time in one or more transactions in the over-the-counter market, in negotiated transactions, through the writing of options on the Exchange Notes or a combination of such methods of resale, at market prices prevailing at the time of resale, at prices related to such prevailing market prices or at negotiated prices. Any such resale may be made directly to purchasers or to or through brokers or dealers who may receive compensation in the form of commissions or concessions from any such broker-dealer or the purchasers of any such Exchange Notes. Any broker-dealer that resells Exchange Notes that were received by it for its own account pursuant to the Exchange Offer and any broker or dealer that participates in a distribution of such Exchange Notes may be deemed to be an “underwriter” within the meaning of the Securities Act and any profit on any such resale of Exchange Notes and any commission or concessions received by any such persons may be deemed to be underwriting compensation under the Securities Act. The Letter of Transmittal states that, by acknowledging that it will deliver and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act.
For a period of 180 days after the Expiration Date Apogent will promptly send additional copies of this prospectus and any amendment or supplement to this Prospectus to any broker-dealer that requests such documents in the Letter of Transmittal. Apogent has agreed to pay all expenses incident to the Exchange Offer (including the expenses of one counsel for the holders of the Original Notes and the Exchange Notes) other than commissions or concessions of any broker-dealers and will indemnify the holders of the Original Notes and the Exchange Notes (including any broker-dealers) against certain liabilities, including liabilities under the Securities Act.
LEGAL MATTERS
The validity of the notes offered hereby will be passed upon for us by Quarles & Brady LLP, Milwaukee, Wisconsin.
EXPERTS
The consolidated financial statements of Apogent Technologies Inc. as of September 30, 2002 and 2001 and for the three years in the period ended September 30, 2002, have been included herein and in the registration statement in reliance upon the report of KPMG LLP, independent auditors, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the September 30, 2002, financial statements refers to the adoption of Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets.”
The statements of The Dairy and Drugs of Abuse Testing Business of CV Holdings, LLC and CV Leasing LLC, included in the Current Report on Form 8-K of Apogent Technologies Inc. dated December 18, 2002, as amended, incorporated by reference in this prospectus and registration statement have been audited by Ernst & Young LLP, independent auditors, as set forth in their report thereon. Such statements are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
112
Index to Consolidated Financial Statements
Apogent Technologies Inc. and Subsidiaries
| | | Page
|
Independent Auditors’ Report | | F-2 |
| |
Consolidated Balance Sheets as of March 31, 2003 (unaudited) and September 30, 2002 and 2001 | | F-3 |
| |
Consolidated Statements of Operations for the six months ended March 31, 2003 and 2002 (unaudited) and for the years ended September 30, 2002, 2001, and 2000 | | F-4 |
| |
Consolidated Statements of Shareholders’ Equity for the six months ended March 31, 2003 (unaudited) and for the years ended September 30, 2002, 2001, and 2000 | | F-5 |
| |
Consolidated Statements of Cash Flows for the six months ended March 31, 2003 and 2002 (unaudited) and for the years ended September 30, 2002, 2001, and 2000 | | F-6 |
| |
Notes to Consolidated Financial Statements | | F-7 |
F-1
INDEPENDENT AUDITORS’ REPORT
The Board of Directors
Apogent Technologies Inc. and Subsidiaries:
We have audited the accompanying consolidated balance sheets of Apogent Technologies Inc. and subsidiaries as of September 30, 2002 and 2001, and the related consolidated statements of income, shareholders’ equity and cash flows for each of the years in the three-year period ended September 30, 2002. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Apogent Technologies Inc. and subsidiaries as of September 30, 2002 and 2001, and the results of their operations and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 7 to the consolidated financial statements, effective October 1, 2001, the Company adopted the provisions of Statement of Financial Accounting Standards No. 142 “Goodwill and Other Intangible Assets.”
KPMG LLP
Boston, Massachusetts
November 11, 2002, except as to the second paragraph of note 1, and as to notes 1(r), 2, 4, 5, 7 and 15,
which are as of March 25, 2003
F-2
APOGENT TECHNOLOGIES INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(In thousands except share and per share data)
| | | March 31,
| | | September 30,
| |
| | | 2003
| | | 2002
| | | 2001
| |
| | | (unaudited) | | | | | | | |
ASSETS | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 17,406 | | | $ | 16,327 | | | $ | 9,192 | |
Accounts receivable (less allowance for doubtful accounts of $4,288, $5,723 and $3,975, respectively) | | | 176,941 | | | | 186,950 | | | | 183,278 | |
Inventories | | | 207,308 | | | | 203,997 | | | | 167,436 | |
Deferred income taxes | | | 14,127 | | | | 14,127 | | | | 12,135 | |
Prepaid expenses and other current assets | | | 18,442 | | | | 19,689 | | | | 20,985 | |
Assets of discontinued operations-held for sale | | | 52,404 | | | | 5,436 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total current assets | | | 486,628 | | | | 446,526 | | | | 393,026 | |
Available for sale security | | | 58,579 | | | | 60,183 | | | | 55,072 | |
Property, plant and equipment, net | | | 266,289 | | | | 270,893 | | | | 223,687 | |
Intangible assets, net | | | 1,154,949 | | | | 1,243,113 | | | | 1,140,334 | |
Other assets | | | 18,285 | | | | 15,370 | | | | 15,961 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total assets | | $ | 1,984,730 | | | $ | 2,036,085 | | | $ | 1,828,080 | |
| | |
|
|
| |
|
|
| |
|
|
|
| | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | |
Short-term debt and overdrafts | | $ | 14,026 | | | $ | 10,640 | | | $ | 9,576 | |
Current portion of long-term debt | | | 2,207 | | | | 25,352 | | | | 64,066 | |
Accounts payable | | | 46,281 | | | | 53,779 | | | | 53,822 | |
Income taxes payable | | | 49,332 | | | | 53,064 | | | | 38,747 | |
Accrued payroll and employee benefits | | | 29,243 | | | | 32,009 | | | | 33,236 | |
Accrued interest | | | 16,183 | | | | 16,630 | | | | 15,292 | |
Restructuring reserve | | | 874 | | | | 1,548 | | | | 1,552 | |
Other current liabilities | | | 29,452 | | | | 23,074 | | | | 26,364 | |
Liabilities of discontinued operations | | | 5,338 | | | | 305 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total current liabilities | | | 192,936 | | | | 216,401 | | | | 242,655 | |
Long-term debt, less current portion | | | 697,520 | | | | 635,020 | | | | 583,788 | |
Securities lending agreement | | | 58,579 | | | | 60,183 | | | | 55,072 | |
Deferred income taxes | | | 123,400 | | | | 132,100 | | | | 101,073 | |
Other liabilities | | | 17,028 | | | | 17,243 | | | | 7,002 | |
Commitments and contingent liabilities | | | — | | | | — | | | | — | |
| | | |
Shareholders’ equity: | | | | | | | | | | | | |
Preferred stock, $0.01 par value; authorized 20,000,000 shares | | | — | | | | — | | | | — | |
Common stock, $0.01 par value; authorized 250,000,000 shares issued 107,019,535, 106,976,877 and 105,875,768 shares, respectively; outstanding 102,194,940, 105,967,853
and 105,875,548 shares, respectively | | | 1,067 | | | | 1,070 | | | | 1,059 | |
Equity rights, 50 rights at $1.09 per right | | | — | | | | — | | | | — | |
Additional paid-in capital | | | 270,627 | | | | 271,682 | | | | 254,637 | |
Retained earnings | | | 721,142 | | | | 748,791 | | | | 627,642 | |
Accumulated other comprehensive loss | | | (13,574 | ) | | | (26,419 | ) | | | (44,848 | ) |
Treasury common stock, 4,824,595, 1,009,024 and 220 shares, at cost | | | (83,995 | ) | | | (19,986 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total shareholders’ equity | | | 895,267 | | | | 975,138 | | | | 838,490 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total liabilities and shareholders’ equity | | $ | 1,984,730 | | | $ | 2,036,085 | | | $ | 1,828,080 | |
| | |
|
|
| |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements
F-3
APOGENT TECHNOLOGIES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands except per share data)
| | | Six Months Ended
March 31,
| | | Year Ended September 30,
| |
| | | 2003
| | | 2002
| | | 2002
| | | 2001
| | | 2000
| |
| | | (unaudited) | | | | | | | | | | |
Net sales | | $ | 534,436 | | | $ | 489,062 | | | $ | 1,027,913 | | | $ | 938,819 | | | $ | 843,567 | |
Cost of sales: | | | | | | | | | | | | | | | | | | | | |
Cost of products sold | | | 278,366 | | | | 246,446 | | | | 516,416 | | | | 471,318 | | | | 421,618 | |
Restructuring charges | | | — | | | | 3,673 | | | | 5,603 | | | | — | | | | 4,386 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total cost of sales | | | 278,366 | | | | 250,119 | | | | 522,019 | | | | 471,318 | | | | 426,004 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | 256,070 | | | | 238,943 | | | | 505,894 | | | | 467,501 | | | | 417,563 | |
Selling, general and administrative expenses | | | 139,940 | | | | 121,922 | | | | 256,692 | | | | 255,902 | | | | 228,178 | |
Restructuring charges | | | 332 | | | | 1,614 | | | | 1,262 | | | | 583 | | | | 5,840 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total selling, general and administrative expenses | | | 140,272 | | | | 123,536 | | | | 257,954 | | | | 256,485 | | | | 234,018 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | 115,798 | | | | 115,407 | | | | 247,940 | | | | 211,016 | | | | 183,545 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | (20,792 | ) | | | (20,578 | ) | | | (40,737 | ) | | | (48,820 | ) | | | (49,584 | ) |
Amortization of deferred financing fees | | | (1,803 | ) | | | (1,741 | ) | | | (3,461 | ) | | | (472 | ) | | | (521 | ) |
Other, net | | | 780 | | | | 2,309 | | | | 1,569 | | | | 5,152 | | | | 1,319 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations before income taxes and extraordinary item | | | 93,983 | | | | 95,397 | | | | 205,311 | | | | 166,876 | | | | 134,759 | |
Income taxes | | | 34,304 | | | | 34,915 | | | | 75,144 | | | | 65,472 | | | | 53,775 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations before extraordinary item | | | 59,679 | | | | 60,482 | | | | 130,167 | | | | 101,404 | | | | 80,984 | |
Discontinued operations, net of income tax | | | (87,328 | ) | | | (11,562 | ) | | | (9,018 | ) | | | (3,357 | ) | | | 47,337 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income before extraordinary item | | | (27,649 | ) | | | 48,920 | | | | 121,149 | | | | 98,047 | | | | 128,321 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Extraordinary item (net of income tax benefit
of $1,359) | | | — | | | | — | | | | — | | | | (2,106 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income | | $ | (27,649 | ) | | $ | 48,920 | | | $ | 121,149 | | | $ | 95,941 | | | $ | 128,321 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Basic earnings per common share from continuing operations | | $ | 0.57 | | | $ | 0.57 | | | $ | 1.22 | | | $ | 0.96 | | | $ | 0.77 | |
Discontinued operations | | | (0.83 | ) | | | (0.11 | ) | | | (0.08 | ) | | | (0.03 | ) | | | 0.45 | |
Extraordinary item | | | — | | | | — | | | | — | | | | (0.02 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Basic earnings per common share | | $ | (0.26 | ) | | $ | 0.46 | | | $ | 1.14 | | | $ | 0.91 | | | $ | 1.23 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Diluted earning per common share from continuing operations | | $ | 0.56 | | | $ | 0.55 | | | $ | 1.20 | | | $ | 0.94 | | | $ | 0.76 | |
Discontinued operations | | | (0.82 | ) | | | (0.11 | ) | | | (0.08 | ) | | | (0.03 | ) | | | 0.44 | |
Extraordinary item | | | — | | | | — | | | | — | | | | (0.02 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Diluted earnings per common share | | $ | (0.26 | ) | | $ | 0.45 | | | $ | 1.11 | | | $ | 0.89 | | | $ | 1.20 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Weighted average basic shares outstanding | | | 104,863 | | | | 106,270 | | | | 106,467 | | | | 105,517 | | | | 104,570 | |
Weighted average diluted shares outstanding | | | 105,920 | | | | 108,996 | | | | 108,656 | | | | 108,072 | | | | 106,803 | |
See accompanying notes to consolidated financial statements
F-4
APOGENT TECHNOLOGIES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(In thousands)
| | | Common
Stock
| | | Equity
Rights
| | Additional
Paid-In
Capital
| | | Retained
Earnings
| | | Accumulated
Other
Comprehensive
Income (loss)
| | | Treasury
Common
Stock
| | | Total
Shareholders’
Equity
| |
Balance at September 30, 1999 | | $ | 1,040 | | | $ | — | | $ | 251,251 | | | $ | 403,380 | | | $ | (30,327 | ) | | | — | | | $ | 625,344 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | — | | | | — | | | — | | | | 128,321 | | | | — | | | | — | | | | 128,321 | |
Translation adjustment | | | — | | | | — | | | — | | | | — | | | | (26,773 | ) | | | — | | | | (26,773 | ) |
Unrealized gain on security available for sale | | | — | | | | — | | | — | | | | — | | | | 2,124 | | | | — | | | | 2,124 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total comprehensive income | | | — | | | | — | | | — | | | | 128,321 | | | | (24,649 | ) | | | — | | | | 103,672 | |
Shares issued in connection with stock options | | | 12 | | | | — | | | 12,587 | | | | — | | | | — | | | | — | | | | 12,599 | |
Tax benefit related to stock options | | | — | | | | — | | | 7,901 | | | | — | | | | — | | | | — | | | | 7,901 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at September 30, 2000 | | | 1,052 | | | | — | | | 271,739 | | | | 531,701 | | | | (54,976 | ) | | | — | | | | 749,516 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cumulative effect of accounting change for cash flow hedge, net of tax effect of $1,687 | | | — | | | | — | | | — | | | | — | | | | 2,530 | | | | — | | | | 2,530 | |
Net income | | | — | | | | — | | | — | | | | 95,941 | | | | — | | | | — | | | | 95,941 | |
Translation adjustment | | | — | | | | — | | | — | | | | — | | | | (3,611 | ) | | | — | | | | (3,611 | ) |
Adjustment to interest rate swap agreements upon sale, net of tax benefit of $984 | | | — | | | | — | | | — | | | | — | | | | (1,475 | ) | | | — | | | | (1,475 | ) |
Amortization of gain on sale of interest rate swaps, net of tax benefit of $413 | | | — | | | | — | | | — | | | | — | | | | (619 | ) | | | — | | | | (619 | ) |
Unrealized gain on security available for sale, net of tax effect of $251 | | | — | | | | — | | | — | | | | — | | | | 377 | | | | | | | | 377 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total comprehensive income | | | — | | | | — | | | — | | | | 95,941 | | | | (2,798 | ) | | | — | | | | 93,143 | |
Shares issued in connection with stock options | | | 7 | | | | — | | | 6,624 | | | | — | | | | — | | | | — | | | | 6,631 | |
Tax benefit related to stock options | | | — | | | | — | | | 3,301 | | | | — | | | | — | | | | — | | | | 3,301 | |
Distribution of the equity of Sybron Dental Specialties, Inc. on December 11, 2000, net of dividends of $142,880 | | | — | | | | — | | | (27,027 | ) | | | — | | | | 12,926 | | | | — | | | | (14,101 | ) |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at September 30, 2001 | | | 1,059 | | | | — | | | 254,637 | | | | 627,642 | | | | (44,848 | ) | | | — | | | | 838,490 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | — | | | | — | | | — | | | | 121,149 | | | | — | | | | — | | | | 121,149 | |
Translation adjustment | | | — | | | | — | | | — | | | | — | | | | 22,247 | | | | — | | | | 22,247 | |
Adjustment to minimum pension liability, net of tax of $4,120 | | | — | | | | — | | | — | | | | — | | | | (6,445 | ) | | | — | | | | (6,445 | ) |
Amortization of gain on sale of interest rate swaps, net of tax benefit of $292 | | | — | | | | — | | | — | | | | — | | | | (440 | ) | | | — | | | | (440 | ) |
Unrealized loss on security available for sale, net of tax of $2,044 | | | — | | | | — | | | — | | | | — | | | | 3,067 | | | | — | | | | 3,067 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total comprehensive income | | | — | | | | — | | | — | | | | 121,149 | | | | 18,429 | | | | — | | | | 139,578 | |
Treasury shares purchased | | | — | | | | — | | | — | | | | — | | | | — | | | | (19,986 | ) | | | (19,986 | ) |
Shares issued in connection with stock options | | | 11 | | | | — | | | 10,282 | | | | — | | | | — | | | | — | | | | 10,293 | |
Tax benefit related to stock options | | | — | | | | — | | | 6,763 | | | | — | | | | — | | | | — | | | | 6,763 | |
Final true-up of dividend to Sybron Dental Specialties relating to deferred income taxes | | | — | | | | — | | | 919 | | | | — | | | | — | | | | — | | | | 919 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at September 30, 2002 | | | 1,070 | | | | — | | | 271,682 | | | | 748,791 | | | | (26,419 | ) | | | (19,986 | ) | | | 975,138 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | — | | | | — | | | — | | | | (27,649 | ) | | | — | | | | — | | | | (27,649 | ) |
Translation adjustment | | | — | | | | — | | | — | | | | — | | | | 13,807 | | | | — | | | | 13,807 | |
Unrealized loss on security available for sale, net of tax of $642 | | | — | | | | — | | | — | | | | — | | | | (962 | ) | | | — | | | | (962 | ) |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total comprehensive income (loss) | | | — | | | | — | | | — | | | | (27,649 | ) | | | 12,845 | | | | — | | | | (14,804 | ) |
Treasury shares purchased | | | (3 | ) | | | — | | | — | | | | — | | | | — | | | | (69,325 | ) | | | (69,328 | ) |
Shares issued in connection with stock options | | | — | | | | — | | | (2,971 | ) | | | — | | | | — | | | | 5,316 | | | | 2,345 | |
Shares issued in connection with employee stock purchase program | | | — | | | | — | | | 927 | | | | — | | | | — | | | | — | | | | 927 | |
Tax benefit related to stock options | | | — | | | | — | | | 989 | | | | — | | | | — | | | | — | | | | 989 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at March 31, 2003 (unaudited) | | $ | 1,067 | | | $ | — | | $ | 270,627 | | | $ | 721,142 | | | $ | (13,574 | ) | | $ | (83,995 | ) | | $ | 895,267 | |
| | |
|
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements
F-5
APOGENT TECHNOLOGIES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | Six Months Ended
March 31,
| | | Year Ended September 30,
| |
| | | (unaudited) | | | | | | | | | | |
| | | 2003
| | | 2002
| | | 2002
| | | 2001
| | | 2000
| |
Cash flows from operating activities: | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (27,649 | ) | | $ | 48,920 | | | $ | 121,149 | | | $ | 95,942 | | | $ | 128,321 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | | | | | | | | | |
Discontinued operations | | | 87,328 | | | | 11,562 | | | | 9,018 | | | | 3,357 | | | | (47,337 | ) |
Depreciation | | | 21,376 | | | | 18,472 | | | | 38,354 | | | | 32,809 | | | | 29,009 | |
Amortization | | | 9,559 | | | | 7,099 | | | | 17,060 | | | | 40,539 | | | | 33,892 | |
Gain (loss) on sale of property, plant and equipment | | | 99 | | | | 111 | | | | 1,859 | | | | (4,784 | ) | | | 63 | |
Provision for losses on doubtful accounts | | | (525 | ) | | | 48 | | | | 924 | | | | 129 | | | | 474 | |
Inventory provisions | | | 1,044 | | | | (2,352 | ) | | | 1,826 | | | | 4,751 | | | | (1,000 | ) |
Deferred income taxes | | | (6,667 | ) | | | 7,300 | | | | 24,753 | | | | 3,954 | | | | 10,258 | |
Extraordinary item | | | — | | | | — | | | | — | | | | 2,106 | | | | — | |
Changes in assets and liabilities, net of effects of businesses acquired: | | | | | | | | | | | | | | | | | | | | |
(Increase) decrease in accounts receivable | | | (1,213 | ) | | | (888 | ) | | | 9,434 | | | | (133 | ) | | | (13,230 | ) |
Increase in inventories | | | (8,941 | ) | | | (14,420 | ) | | | (26,446 | ) | | | (27,117 | ) | | | (5,499 | ) |
(Increase) decrease in prepaid expenses and other current assets | | | (3,365 | ) | | | 3,158 | | | | (832 | ) | | | (4,621 | ) | | | (2,177 | ) |
Decrease in accounts payable | | | (5,453 | ) | | | (6,938 | ) | | | (1,759 | ) | | | (3,300 | ) | | | (915 | ) |
Increase (decrease) in income taxes payable | | | (4,055 | ) | | | 12,148 | | | | (2,448 | ) | | | 23,849 | | | | (1,768 | ) |
Increase (decrease) in accrued payroll and employee benefits | | | (2,667 | ) | | | (4,288 | ) | | | (1,724 | ) | | | 983 | | | | (5,041 | ) |
Increase (decrease) in accrued interest expense | | | (448 | ) | | | 2,138 | | | | 1,337 | | | | 2 | | | | — | |
Increase (decrease) in restructuring reserve | | | (674 | ) | | | 3,183 | | | | (118 | ) | | | (5,164 | ) | | | 1,744 | |
Increase (decrease) in other current liabilities | | | 3,016 | | | | 547 | | | | (10,211 | ) | | | 15,690 | | | | (5,046 | ) |
Net change in other assets and liabilities | | | (3,801 | ) | | | (2,322 | ) | | | 9,974 | | | | 401 | | | | (6,373 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net cash provided by operating activities | | | 56,964 | | | | 83,478 | | | | 192,150 | | | | 179,393 | | | | 115,375 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | (22,560 | ) | | | (17,099 | ) | | | (63,630 | ) | | | (50,120 | ) | | | (41,324 | ) |
Proceeds from sales of property, plant and equipment | | | 558 | | | | 268 | | | | 5,007 | | | | 12,457 | | | | 924 | |
Net payment for businesses acquired | | | (21,567 | ) | | | (113,780 | ) | | | (139,735 | ) | | | (163,519 | ) | | | (207,153 | ) |
Dividends received from Sybron Dental Specialties | | | — | | | | — | | | | — | | | | 67,900 | | | | 58,512 | |
Capital contributions paid to Sybron Dental Specialties | | | — | | | | — | | | | — | | | | (4,623 | ) | | | (21,399 | ) |
Net change in advances and loans to Sybron Dental Specialties | | | — | | | | — | | | | — | | | | (2,782 | ) | | | 20,985 | |
Distribution of the net equity of Sybron Dental Specialties | | | — | | | | — | | | | — | | | | (14,101 | ) | | | — | |
Other investing activities | | | 1,546 | | | | — | | | | — | | | | — | | | | (2,600 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net cash used in investing activities | | | (42,023 | ) | | | (130,611 | ) | | | (198,358 | ) | | | (154,788 | ) | | | (192,055 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | | |
Proceeds from revolving credit facility | | | 323,100 | | | | 179,500 | | | | 358,500 | | | | 454,560 | | | | 332,640 | |
Principal payments on revolving credit facility | | | (260,400 | ) | | | (388,000 | ) | | | (567,000 | ) | | | (502,460 | ) | | | (274,320 | ) |
Proceeds from long-term debt | | | — | | | | 300,000 | | | | 300,000 | | | | 703,448 | | | | — | |
Principal payments on long-term debt | | | (23,408 | ) | | | — | | | | (79,089 | ) | | | (681,854 | ) | | | (450 | ) |
Financing fees paid | | | — | | | | (8,017 | ) | | | (8,259 | ) | | | (6,721 | ) | | | — | |
Purchase of treasury stock | | | (69,328 | ) | | | — | | | | (19,986 | ) | | | — | | | | — | |
Proceeds from the exercise of stock options | | | 2,345 | | | | 4,790 | | | | 10,293 | | | | 6,631 | | | | 12,599 | |
Proceeds from employee stock purchase program | | | 927 | | | | — | | | | — | | | | — | | | | — | |
Other financing activities | | | 1,958 | | | | (560 | ) | | | 6,175 | | | | (4,118 | ) | | | 7,190 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | (24,806 | ) | | | 87,713 | | | | 634 | | | | (30,514 | ) | | | 77,659 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Effect of exchange rate changes on cash and cash equivalents | | | 10,944 | | | | (7,499 | ) | | | 12,709 | | | | 2,690 | | | | (969 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net increase (decrease) in cash and cash equivalents | | | 1,079 | | | | 33,081 | | | | 7,135 | | | | (3,219 | ) | | | 10 | |
Cash and cash equivalents at beginning of period | | | 16,327 | | | | 9,335 | | | | 9,192 | | | | 12,411 | | | | 12,401 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Cash and cash equivalents at end of period | | $ | 17,406 | | | $ | 42,416 | | | $ | 16,327 | | | $ | 9,192 | | | $ | 12,411 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Supplemental disclosures of cash flow information: | | | | | | | | | | | | | | | | | | | | |
Cash paid during the period for: | | | | | | | | | | | | | | | | | | | | |
Interest | | $ | 20,987 | | | $ | 17,819 | | | $ | 39,691 | | | $ | 37,132 | | | $ | 55,833 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income taxes | | $ | 37,325 | | | $ | 19,105 | | | $ | 39,513 | | | $ | 43,070 | | | $ | 42,412 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Capital lease obligations incurred | | $ | 68 | | | $ | 117 | | | $ | 334 | | | $ | 104 | | | $ | 25 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements
F-6
APOGENT TECHNOLOGIES INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands except share and per share data)
1. Summary of Significant Accounting Policies
The subsidiaries of Apogent are leading manufacturers of value-added products for the laboratory market in the United States and abroad.
On March 25, 2003 the Company made the decision to dispose of two of its businesses: the rapid diagnostic test business (on-site rapid tests used in the detection of pregnancy, drugs, of abuse, and infectious diseases) as conducted by our Applied Biotech, Inc. subsidiary; and the manufacture and sale of automated microarray instrumentation for the genomics market as conducted by our BioRobotics Group Ltd. subsidiary. In addition, in March 2003, the Company realigned its lines of business for financial reporting purposes. The three former business segments of the Company (clinical diagnostics, labware and life sciences, and laboratory equipment) have been reclassifed into two business segments: clinical group and research group. The clinical group business segment is the former clinical diagnostics business segment. The research group business segment is composed of the former labware and life sciences and laboratory equipment business segments. All financial information presented herein has been restated as to reflect the discontinuance of these businesses and this business segment realignment.
On November 8, 2000, Sybron International Corporation (which subsequently changed its name to Apogent Technologies Inc.) announced that it had declared a pro rata distribution to its shareholders of the common stock and related preferred stock purchase rights of Sybron Dental Specialties, Inc. (formerly known as SDS Holding Co.) (the “Spin-Off”). On December 11, 2000, shareholders of record as of November 30, 2000 received one share of Sybron Dental Specialties, Inc. common stock for every three shares of Sybron International common stock they owned as of the record date. Sybron Dental Specialties, Inc. owns all of the outstanding stock of Sybron Dental Management, Inc., formerly named Sybron Dental Specialties, Inc. Prior to the Spin-Off, Sybron Dental Management, Inc. was a direct wholly-owned subsidiary of the Company and operated the Company’s dental business. Immediately prior to the Spin-Off, the Company contributed all of the stock of Sybron Dental Management, Inc. to Sybron Dental Specialties, Inc. As used in these Notes to the Consolidated Financial Statements, the term “SDS” means Sybron Dental Management, Inc. (formerly known as Sybron Dental Specialties, Inc.) for the periods prior to the Spin-Off, and Sybron Dental Specialties, Inc. (formerly known as SDS Holding Co.) for periods after the Spin-Off.
On January 31, 2001, the name of the Company was changed from Sybron International Corporation to Apogent Technologies Inc.
(a) Principles of Consolidation and Fiscal Year End
The consolidated financial statements reflect the accounts of Apogent Technologies Inc. and its subsidiaries. The term “Company” or “Apogent” as used herein refers to Apogent Technologies Inc. and its subsidiaries and their respective predecessors, unless the context otherwise requires. All significant intercompany balances and transactions have been eliminated. The Company’s fiscal year ends on September 30. The fiscal years ended September 30, 2002, 2001, and 2000 are hereinafter referred to as “2002”, “2001”, and “2000”, respectively. Dollar references throughout these footnotes are in thousands, except per share amounts or as otherwise indicated.
The financial information as of March 31, 2003 and for the six month periods ended March 31, 2003 and March 31, 2002 is unaudited. In the opinion of management, all adjustments and note disclosures that are necessary for fair statement of the results for the periods presented have been included, and are of normal recurring nature.
F-7
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
On March 25, 2003, the Company made the decision to dispose of two its businesses: the rapid diagnostic test business (on-site rapid tests used in the detection of pregnancy, drugs of abuse, and infectious diseases) as conducted by our Applied Biotech subsidiary; and the manufacture and sale of automated microarray instrumentation for the genomics market as conducted by our BioRobotics Group subsidiary. During March 2002, we made the decision to dispose of our vacuum deposition chamber business, Vacuum Process Technology. On December 11, 2000, Apogent, then known as Sybron International Corporation, completed the spin-off (“Spin-Off”) of its dental business as a separate publicly traded company. The results of operations of Vacuum Process Technology and SDS have been presented as discontinued operations in all years presented herein.
(b) Cash Equivalents
For purposes of reporting cash flows, cash and cash equivalents include investments in debt obligations with original maturities of three months or less.
(c) Inventories
Inventories are stated at the lower of cost or market. Elements of cost included in inventories are: raw materials, direct labor, and manufacturing overhead (which includes indirect labor, fringe benefits, consumable supplies, depreciation of production equipment, and tooling). Certain domestic inventories of approximately $51,777 and $57,698 at September 30, 2002 and 2001, respectively, are valued on the last-in, first-out (LIFO) method. The remaining inventories are valued on the first-in, first-out (FIFO) method.
(d) Securities
When securities are purchased they are classified as held-to-maturity, available for sale, or trading securities. Held to maturity securities are those that the Company has the positive intent and ability to hold until maturity. Trading securities are those purchased and held with the intent to sell in the near term. Available for sale securities include debt securities that are held for an indefinite period but are neither held to maturity nor trading securities. At March 31, 2003 and at September 30, 2002 and 2001, the Company held a U.S. Treasury Bond classified as an available for sale security. Available for sale securities are reported at fair market value. Unrealized gains and losses for this security are included in comprehensive income as a separate component of shareholders’ equity.
(e) Property, Plant and Equipment
Property, plant and equipment are stated at cost less accumulated depreciation. Depreciation is provided over the estimated useful lives of depreciable assets (5 to 45 years for land improvements, buildings and building improvements, and 3 to 12 years for machinery and equipment) using the straight-line method. The Company assesses the recoverability of assets by comparing the carrying amount of an asset to future net cash flows expected to be generated by that asset. If such assets are considered impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair market value of the assets.
(f) Intangible Assets
Intangible assets include both goodwill and amortizable intangible assets. As of September 30, 2002, the Company had no unamortizable intangible assets except goodwill. Amortizable intangible assets (those intangible assets with definite estimated useful lives) are recorded at cost and are amortized, using the straight-line method, over their estimated useful lives. Proprietary technology, trademarks, patents, licenses, drawings,
F-8
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
non-compete agreements, and other intangibles are amortized over 4 to 18 years, 5 to 40 years, 3 to 20 years, 5 to 40 years, 8 to 30 years, 3 to 10 years, and 1 to 40 years, respectively. The Company assesses the recoverability of its amortizable intangible assets in accordance with Statement of Financial Accounting Standards (SFAS) No. 144 by determining whether the amortization of the asset balance over its remaining life can be recovered through projected undiscounted future cash flows of the acquired businesses. If projected future cash flows indicate that unamortized asset will not be recovered, an adjustment would be made to reduce the net asset to fair value. Cash flow projections are based on trends of historical performance and management’s estimate of future performance, giving consideration to existing and anticipated competitive and economic conditions. In accordance with SFAS No. 142, the Company tests goodwill for impairment on an annual basis by comparing the fair value of its reporting units to their fair value. During the six months ended March 31, 2003 the Company included approximately $101 million in goodwill and intangibles in the calculation of the estimated loss on the sales of Applied Biotech and BioRobotics Group. During fiscal 2002 the Company included approximately $21 million in goodwill and intangibles in the calculation of the estimated loss on sale of Vacuum Process Technology.
(g) Revenue Recognition
The Company recognizes revenue upon shipment of products when persuasive evidence of a sales arrangement exists, the price to the buyer is fixed and determinable, and collectibility of the sales price is reasonably assured. Large portions of the Company’s sales are sold through distributors. Revenues associated with sales to distributors are also recognized upon shipment of products when all risks and rewards of ownership of the product are passed.
(h) Income Taxes
Income taxes are accounted for under the asset and liability method wherein deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income or other comprehensive income in the period that includes the enactment date.
(i) Research and Development Costs
Research and development costs are charged to selling, general and administrative expenses in the year they are incurred. Research and development costs for the six months ended March 31, 2003 and 2002 and for fiscal years ended 2002, 2001, and 2000 were approximately $12,613, $11,411, $23,365, $19,153, and $17,178, respectively.
(j) Foreign Currency Translation
The functional currency for the Company’s foreign operations is the applicable local currency. The translation from the applicable foreign currencies to U.S. dollars is performed for balance sheet accounts using current exchange rates in effect at the balance sheet date and for revenue and expense accounts using a weighted average exchange rate during the period. The gains or losses, net of applicable deferred income taxes, resulting from such translations are included in shareholders’ equity. Gains and losses resulting from foreign currency transactions are included in net income. Foreign currency transaction gains for 2002, 2001, and 2000 were approximately $354, $177, and $1,306, respectively.
F-9
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
(k) Pensions
The Company and its subsidiaries have various pension plans covering substantially all employees. U.S. pension obligations are funded by payments to pension fund trusts. Other foreign pensions are funded as expenses are incurred. The Company’s policy with respect to its defined benefit plans is generally to fund the minimum amount required under the Employee Retirement Income Security Act of 1974, as amended, for plans subject thereto.
(l) Earnings Per Common Share
Basic earnings per common share is calculated by dividing net income by the weighted average number of common shares outstanding in the period presented. Diluted earnings per common share is calculated by dividing net income by the weighted average number of common shares outstanding plus the dilutive effects of potential common shares outstanding during the period. A reconciliation of shares used in calculating basic and diluted earnings per share follows:
| | | Six Months Ended
March 31,
| | Year Ended September 30,
|
| | | 2003
| | 2002
| | 2002
| | 2001
| | 2000
|
Basic | | 104,863 | | 106,270 | | 106,467 | | 105,517 | | 104,570 |
Effect of assumed conversion of employee stock options | | 1,057 | | 2,726 | | 2,189 | | 2,555 | | 2,233 |
| | |
| |
| |
| |
| |
|
Diluted | | 105,920 | | 108,996 | | 108,656 | | 108,072 | | 106,803 |
| | |
| |
| |
| |
| |
|
Options to purchase 9,381,510 shares of common stock at prices ranging from $17.97 to $25.10 per share were outstanding during a portion of the six months ended March 31, 2003 but were not included in the computation of diluted earnings per share because the options’ exercise price was greater than the average market price of the common shares.
Options to purchase 1,319,822 shares of common stock at prices ranging from $23.79 to $25.10 per share were outstanding during a portion of 2002 but were not included in the computation of diluted earnings per share because the options’ exercise price was greater than the average market price of the common shares.
Options to purchase 4,504,840 shares of common stock at prices ranging from $23.79 to $25.10 per share were outstanding during a portion of 2002 but were not included in the computation of diluted earnings per share because the options’ exercise price was greater than the average market price of the common shares. The options, which expire in fiscal 2012, were still outstanding at the end of fiscal year 2002.
Options to purchase 1,568,845 shares of common stock at prices ranging from $22.24 to $24.51 per share were outstanding during a portion of 2001 but were not included in the computation of diluted earnings per share because the options’ exercise price was greater than the average market price of the common shares. The options, which expire in fiscal 2011, were still outstanding at the end of fiscal year 2001.
Options to purchase 904,844 shares of common stock at prices ranging from $25.31 to $32.00 per share were outstanding during a portion of 2000 but were not included in the computation of diluted earnings per share because the options’ exercise price was greater than the average market price of the common shares. The options, which expire in fiscal 2010, were still outstanding at the end of fiscal year 2000.
(m) Deferred Financing Fees
Deferred financing fees are capitalized and amortized as a separate component of other income over the life of the related debt agreements.
F-10
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
(n) Advertising Costs
Advertising costs included in selling, general and administrative expenses are expensed as incurred and for the six months ended March 31, 2003 and 2002 and for fiscal years ended 2002, 2001, and 2000 were approximately $2,799, $2,331, $6,626, $4,635 and $5,093, respectively.
(o) Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
(p) Derivative Financial Instruments
In the normal course of business, we manage risks associated with foreign exchange and interest rates through a variety of strategies, including the use of hedging transactions, executed in accordance with our policies. Our hedging transactions include, but are not limited to, the use of derivative instruments. As a matter of policy, we do not use derivative instruments unless there is an underlying exposure. Any change in the value of our derivative instruments would be substantially offset by an opposite change in the value of the underlying hedged items. We do not use derivative instruments for trading or speculative purposes.
The Company uses interest rate swaps, from time to time, to manage its interest rate risk. The net amounts to be paid or received under interest rate swap agreements designated as hedges are accrued as interest rates change and are recognized over the life of the swap agreements, as an adjustment to interest expense from the underlying debt to which the swap is designated. The related amounts payable to, or receivable from, the counterparties are included in other current assets or other current liabilities.
The Company, from time to time, enters into foreign currency options to hedge the exposure from adverse changes in foreign currency rates. The purpose of the Company’s foreign currency hedging activities is to protect against risk that eventual cash flows from foreign activities will be adversely affected by changes in exchange rates and the effect of related changes on payments on long-term debt denominated in foreign currencies. Recognized and unrecognized gains or losses on foreign currency contracts entered into to hedge long-term debt are recorded as “other income”. The Company has not entered into any foreign currency options to hedge against exposure from operations in fiscal 2002.
On October 1, 2000, the Company adopted Financial Accounting Standard Board Opinions No. 133, as modified by FASB Opinion No. 138. These standards establish accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts, and hedging activities. They require the recognition of all derivative instruments as assets or liabilities in the balance sheet at fair value. The accounting treatment of changes in fair value is dependent upon whether or not a derivative instrument is designated as a hedge and if so, the type of hedge. For derivatives designated as a cash flow hedge, changes in fair value are recognized in other comprehensive income until the hedged item is recognized in earnings. At October 1, 2000, the Company had no freestanding derivatives in place other than interest rate swaps used to hedge variable rate long-term debt and had no material embedded derivatives. The interest rate swaps meet the criteria for cash flow hedge accounting. As a result, the swaps are recorded on the balance sheet as an asset at fair value with the corresponding gain or loss recorded in other comprehensive income beginning October 1, 2000. The impact on other comprehensive income upon adoption of the standard was an unrealized gain, net of tax, of approximately $2,530.
F-11
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
(q) Environmental Expenditures
Environmental expenditures that relate to current ongoing operations or to conditions caused by past operations are expensed. The Company determines its liability on a site-by-site basis and records a liability at the time when the liability is probable and can be reasonably estimated. The estimated liability is not reduced for possible recoveries from insurance carriers.
(r) Reclassifications
Certain reclassifications to prior year balances have been made to conform with current year presentations.
Effective September 30, 2002, the Company adopted the Emerging Issues Task Force (EITF) Issue No. 00-10,Accounting for Shipping and Handling Fees and Costs, which requires all amounts charged to customers for shipping and handling to be classified as sales revenues. Accordingly, all historical sales revenue amounts have been adjusted to reflect these charges. The costs related to shipping and handling are classified as a selling expense in selling, general and administrative expense. The following table reconciles historically reported amounts to those adjusted in accordance with EITF 00-10:
| | | Year Ended September 30,(a)
| |
| | | 2001
| | | 2000
| | | 1999
| | | 1998
| |
Net sales | | | | | | | | | | | | | | | | |
Reported net sales | | $ | 926,072 | | | $ | 833,797 | | | $ | 687,663 | | | $ | 553,958 | |
Reclassification of freight income | | | 12,747 | | | | 9,770 | | | | 8,958 | | | | 8,541 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Adjusted net sales | | $ | 938,819 | | | $ | 843,567 | | | $ | 696,621 | | | $ | 562,499 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Cost of products sold | | | | | | | | | | | | | | | | |
Reported cost of products sold | | $ | 474,324 | | | $ | 428,655 | | | $ | 358,897 | | | $ | 288,810 | |
Reclassification of freight costs | | | (3,006 | ) | | | (2,651 | ) | | | (1,057 | ) | | | (624 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Adjusted cost of products sold | | $ | 471,318 | | | $ | 426,004 | | | $ | 357,840 | | | $ | 288,186 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Selling, general and administrative expenses | | | | | | | | | | | | | | | | |
Reported selling, general and administrative expenses | | $ | 240,732 | | | $ | 221,597 | | | $ | 167,695 | | | $ | 143,753 | |
Reclassification of freight income and expense | | | 15,753 | | | | 12,421 | | | | 10,015 | | | | 9,165 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Adjusted selling, general, and administrative expenses | | $ | 256,485 | | | $ | 234,018 | | | $ | 177,710 | | | $ | 152,918 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| (a) | Historical amounts have been adjusted to reflect the disposal of Applied Biotech, BioRobotics Group, and Vacuum Process Technology. |
2. Business and Credit Concentrations
Many of the Company’s products are sold through major distributors. Two of these distributors accounted for 14% and 11%, respectively, of the Company’s net sales for 2002, 13% and 10%, respectively, of the Company’s net sales in 2001, and 14% and 9%, respectively, of the Company’s net sales in 2000. Accounts receivable from these distributors comprised approximately 24% and 10%, respectively, of the outstanding consolidated accounts receivable balances at September 30, 2002 and approximately 10.6% and 10.7%, respectively, of the outstanding consolidated accounts receivable balances at September 30, 2001.
F-12
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
3. Inventories
Inventories at March 31, 2003 and September 30, 2002 and 2001 consisted of the following:
| | | March 31, 2003
| | September 30,
|
| | | | 2002
| | 2001
|
| | | (unaudited) | | | | |
Raw materials and supplies | | $ | 69,129 | | $ | 74,293 | | $ | 56,660 |
Work in process | | | 20,647 | | | 19,400 | | | 25,974 |
Finished goods | | | 117,532 | | | 110,304 | | | 84,802 |
| | |
|
| |
|
| |
|
|
| | | $ | 207,308 | | $ | 203,997 | | $ | 167,436 |
| | |
|
| |
|
| |
|
|
The Company uses the last-in, first-out (LIFO) method to value inventory at certain subsidiaries. Inventories would have been $6,674 and $7,573 higher at September 30, 2002 and 2001, respectively, if the first-in, first-out (FIFO) method had been used.
During 2000, quantities of inventory valued on a LIFO basis were consumed. This resulted in the liquidation of LIFO inventories valued at lower prevailing costs when such LIFO quantities were originally acquired in prior years. If these LIFO quantities had not been consumed, but replenished with the quantities valued at current costs, net income in 2000 would have been decreased by approximately $125 and would have had no impact on either basic or diluted earnings per share. No such events occurred in 2002 and 2001.
4. Income Taxes
Total income tax expense (benefit) for the years ended September 30, 2002, 2001, and 2000 is allocated as follows:
| | | 2002
| | | 2001
| | | 2000
| |
Income from continuing operations | | $ | 75,144 | | | $ | 65,472 | | | $ | 53,775 | |
Extraordinary items | | | — | | | | (1,359 | ) | | | — | |
Discontinued operations | | | (5,199 | ) | | | 5,831 | | | | 32,165 | |
Shareholders’ equity for unrealized gain on security available for sale | | | 2,044 | | | | 251 | | | | (1,420 | ) |
Shareholders’ equity for cumulative effect of accounting changes for cash flow hedge | | | — | | | | 1,687 | | | | — | |
Shareholders’ equity for interest rate swap agreements | | | (292 | ) | | | (1,397 | ) | | | — | |
Shareholders’ equity for pension | | | (4,120 | ) | | | | | | | | |
Shareholders’ equity for compensation expense for tax purposes in excess of amounts recognized for financial reporting purposes | | | (6,763 | ) | | | (3,301 | ) | | | (7,901 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
| | | $ | 60,814 | | | $ | 67,184 | | | $ | 76,619 | |
| | |
|
|
| |
|
|
| |
|
|
|
F-13
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Income tax expense (benefit) attributable to income from continuing operations consists of:
| | | Current
| | Deferred
| | | Total
|
Year ended September 30, 2002: | | | | | | | | | | |
U.S., state and local | | $ | 54,635 | | $ | 11,068 | | | $ | 65,703 |
Foreign | | | 10,459 | | | (1,018 | ) | | | 9,441 |
| | |
|
| |
|
|
| |
|
|
| | | $ | 65,094 | | $ | 10,050 | | | $ | 75,144 |
| | |
|
| |
|
|
| |
|
|
Year ended September 30, 2001: | | | | | | | | | | |
U.S., state and local | | $ | 52,258 | | $ | 3,296 | | | $ | 55,554 |
Foreign | | | 9,260 | | | 658 | | | | 9,918 |
| | |
|
| |
|
|
| |
|
|
| | | $ | 61,518 | | $ | 3,954 | | | $ | 65,472 |
| | |
|
| |
|
|
| |
|
|
Year ended September 30, 2000: | | | | | | | | | | |
U.S., state and local | | $ | 38,838 | | $ | 8,401 | | | $ | 47,239 |
Foreign | | | 4,679 | | | 1,857 | | | | 6,536 |
| | |
|
| |
|
|
| |
|
|
| | | $ | 43,517 | | $ | 10,258 | | | $ | 53,775 |
| | |
|
| |
|
|
| |
|
|
The domestic and foreign components of income from continuing operations before income taxes, discontinued operations, and extraordinary items are as follows:
| | | 2002
| | 2001
| | 2000
|
United States | | $ | 168,672 | | $ | 137,261 | | $ | 113,856 |
Foreign | | | 36,638 | | | 29,634 | | | 20,903 |
| | |
|
| |
|
| |
|
|
Income before income taxes, discontinued operations, and extraordinary items | | $ | 205,310 | | $ | 166,895 | | $ | 134,759 |
| | |
|
| |
|
| |
|
|
Income tax expense attributable to income from continuing operations was $75,144, $65,472, and $53,775 and in 2002, 2001, and 2000, respectively, and differed from the amounts computed by applying the U.S. Federal income tax rate of 35 percent to income from continuing operations before income taxes, discontinued operations, and extraordinary items in 2002, 2001, and 2000 as a result of the following:
| | | 2002
| | | 2001
| | | 2000
| |
Computed “expected” tax expense | | $ | 71,859 | | | $ | 58,413 | | | $ | 47,166 | |
Increase (reduction) in income taxes resulting from: | | | | | | | | | | | | |
Change in beginning of year valuation allowance for deferred tax assets allocated to income tax expense | | | — | | | | — | | | | (21 | ) |
Amortization of goodwill | | | — | | | | 4,082 | | | | 3,747 | |
State and local income taxes, net of Federal income tax benefit | | | 7,205 | | | | 6,225 | | | | 3,898 | |
Foreign income taxed at rates higher than U.S. Federal Income | | | (2,650 | ) | | | (860 | ) | | | (836 | ) |
Foreign tax credits utilized in excess of U.S. tax on foreign earnings | | | — | | | | — | | | | 205 | |
Other, net | | | (1,270 | ) | | | (2,388 | ) | | | (384 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
| | | $ | 75,144 | | | $ | 65,472 | | | $ | 53,775 | |
| | |
|
|
| |
|
|
| |
|
|
|
F-14
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
The significant components of deferred income tax benefit attributable to income from continuing operations for 2002, 2001, and 2000 are as follows:
| | | 2002
| | 2001
| | 2000
|
Deferred tax (benefit)/expense (exclusive of the effects of other components listed below) | | | 7,625 | | $ | 3,651 | | $ | 10,038 |
Increase in the valuation allowance for deferred tax assets | | | 2,425 | | | 303 | | | 220 |
| | |
|
| |
|
| |
|
|
| | | $ | 10,050 | | $ | 3,954 | | $ | 10,258 |
| | |
|
| |
|
| |
|
|
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities at September 30, 2002 and 2001 are presented below.
| | | 2002
| | | 2001
| |
Deferred tax assets: | | | | | | | | |
Inventories | | $ | 5,635 | | | $ | 5,143 | |
Compensation | | | 2,929 | | | | 2,369 | |
Sale/Leaseback | | | 4,424 | | | | 4,427 | |
Employee benefits | | | 1,032 | | | | 826 | |
Net operating loss carryforwards | | | 3,839 | | | | 1,414 | |
Pension | | | 5,749 | | | | — | |
Warranty and other accruals | | | 5,327 | | | | 6,428 | |
| | |
|
|
| |
|
|
|
Total gross deferred tax assets | | | 28,935 | | | | 20,607 | |
Less valuation allowance | | | (3,839 | ) | | | (1,414 | ) |
| | |
|
|
| |
|
|
|
Net deferred tax assets | | | 25,096 | | | | 19,193 | |
| | |
|
|
| |
|
|
|
Deferred tax liabilities: | | | | | | | | |
Depreciation | | | (16,918 | ) | | | (16,338 | ) |
Purchase accounting | | | (118,908 | ) | | | (88,818 | ) |
Unrealized appreciation on securities available for sale | | | (4,073 | ) | | | (2,029 | ) |
Other | | | (3,170 | ) | | | (946 | ) |
| | |
|
|
| |
|
|
|
Total deferred tax liabilities | | | (143,069 | ) | | | (108,131 | ) |
| | |
|
|
| |
|
|
|
Net deferred tax liability | | $ | (117,973 | ) | | $ | (88,938 | ) |
| | |
|
|
| |
|
|
|
The change in the net deferred tax liability contains $21,353 and $2,044 of deferred tax liabilities related to acquisitions and the unrealized appreciation on securities available-for-sale, a reduction of $292 related to the amortization of gain on sale of interest rate swaps and $4,120 of deferred tax assets related to pensions. The valuation allowance for deferred tax assets as of October 1, 2000 was $1,111. The net change in the total valuation allowance for the years ended September 30, 2002 and 2001 was an increase of $2,425 and $303 respectively. The valuation allowance relates primarily to net operating loss carryforwards in certain foreign jurisdictions and U.S. states, in which there is a history of pre-tax accounting losses and foreign tax credit carryforwards. Management is unable to conclude that there will be pre-tax accounting income in those jurisdictions in the near term. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities and projected future taxable income in making this assessment.
F-15
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
At September 30, 2002, the Company has an aggregate of $991 of foreign net operating loss carry forwards from certain foreign jurisdictions, the majority of which have no expiration. The Company has an aggregate of $24,507 of various state net operating losses that expire between 2006 and 2018. At September 30, 2002 the Company has $2,195 of foreign tax credit carryforwards that expire in 2007.
Accumulated earnings of foreign subsidiaries at September 30, 2002, 2001, and 2000 of approximately $51,000, $25,000, and $11,000, respectively, have been reinvested in the business and no provision for income taxes has been made for the repatriation of these earnings.
5. Acquisitions and Divestitures
The Company has completed 32 acquisitions, discontinued three business and spun off one business since the beginning of 2000. The acquired companies are all engaged in businesses that are similar to the Company’s existing businesses. The divested company was engaged in the business of vacuum process technologies. The spun off company was engaged in the dental business.
2003
During the six months ended March 31, 2003, the Company completed three acquisitions for cash. The aggregate purchase price for these acquisitions, net of cash acquired, was approximately $21.6 million. None of the acquisitions was considered individually significant. The total goodwill and identifiable intangibles assets for the acquired companies was approximately $26.7 million (allocated approximately $13.0 million to goodwill and $13.7 million to amortizable intangible assets). The intangible assets will be amortized over their expected lives ranging from 5 to 20 years. The following table outlines the sales, operating income and total assets for the most recent available twelve-month period prior to each cash acquisition.
Business Segment
Company Acquired
| | Acquisition Date
| | Sales
| | Operating
Income
| | Total
Assets
| | Type of
Acquisition
|
| | | | | (in thousands) |
Clinical Group: | | | | | | | | | | | | | |
NeoMarkers, Inc. | | October 2002 | | $ | 4,000 | | $ | 1,900 | | $ | 1,900 | | Stock |
Opus Diagnostics Inc. | | October 2002 | | | 2,100 | | | 1,200 | | | 600 | | Asset |
| | | | | |
Research Group: | | | | | | | | | | | | | |
Tempyrox Company, Inc. | | January 2003 | | | 582 | | | 24 | | | 181 | | Asset |
F-16
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
2002
During 2002, the Company completed nine acquisitions for cash. The aggregate purchase price for these acquisitions, net of cash acquired, was approximately $141 million. None of the acquisitions were considered individually significant. The total goodwill and identifiable intangibles assets for the acquired companies was approximately $111 million. The intangible assets will be amortized over their expected lives ranging from 3 to 20 years. The following table outlines the sales, operating income and total assets for the most recent available twelve-month period prior to each cash acquisition:
Business Segment
Company Acquired
| | Acquisition Date
| | Sales
| | Operating
Income
| | Total
Assets
| | Type of
Acquisition
|
| | | | | (in thousands) |
Clinical Group: | | | | | | | | | | | | | |
Forefront Diagnostics, Inc. | | November 2001 | | $ | 6,300 | | $ | 1,700 | | $ | 9,900 | | Stock |
Separation Technology, Inc. | | January 2002 | | | 3,200 | | | 1,000 | | | 3,000 | | Stock |
Capitol Vial, Inc. | | February 2002 | | | 27,000 | | | 9,600 | | | 26,200 | | Stock |
Mirror Product Line of SMC Manufacturing | | May 2002 | | | 600 | | | 200 | | | 10 | | Asset |
| | | | | |
Research Group: | | | | | | | | | | | | | |
Chromacol Limited, Epsom Glass Industries Limited, and Amchro Inc. | | October 2001 | | | 9,900 | | | 350 | | | 5,080 | | Stock |
Barden Engineering | | October 2001 | | | 600 | | | 130 | | | 540 | | Asset |
Cosmotec Co. Ltd. | | October 2001 | | | 5,500 | | | 2,500 | | | 2,600 | | Stock |
Marsh Bio Products, Inc. | | April 2002 | | | 17,000 | | | 1,800 | | | 4,700 | | Asset |
TFO, Incorporated | | May 2002 | | | 1,700 | | | 160 | | | 850 | | Asset |
The following pro forma financial information presents the combined results of operations of the Company and the purchased businesses referred above as if the 2002 acquisitions had occurred as of October 1, 2000, after giving effect to certain adjustments including amortization of intangible assets, additional depreciation expense, increased interest expense on debt related to the acquisition and related tax effects. The pro forma information does not necessarily reflect the results of operations that would have occurred had the Company and the purchased companies listed above constituted a single entity during such periods.
| | | 2002
| | 2001
|
Net sales | | $ | 1,102,105 | | $ | 1,049,776 |
Income before extraordinary item | | | 126,347 | | | 107,662 |
Net income | | | 126,347 | | | 105,556 |
Basic earnings per common share | | | 1.19 | | | 1.00 |
Diluted earnings per common share | | | 1.16 | | | 0.98 |
2001
During 2001, the Company completed ten acquisitions, eight for all cash and two for cash and notes in the amount of $79,885. The aggregate cash price of the acquisitions (none of which individually or aggregated was significant) was approximately $158 million. The results of these acquisitions were included as of the date they were acquired. The total goodwill and intangibles for the acquired companies was approximately $153 million. Intangible assets with a definite life will be amortized over 3 to 40 years. The following table outlines unaudited sales and operating income for the most recent date prior to the acquisition, and unaudited total assets at the most recent available date prior to acquisition, for each of the acquired companies. The type of acquisition refers to whether the Company purchased assets or the stock of the acquired companies.
F-17
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Business Segment Company Acquired
| | Date
| | Sales
| | Operating
Income
| | | Total
Assets
| | Type of
Acquisition
|
| | | | | (in thousands) |
Clinical Group: | | | | | | | | | | | | | | |
Vacuum Process Technology, Inc. | | November 2000 | | $ | 3,977 | | $ | (19 | ) | | $ | 1,097 | | Asset |
Disposable Glass Culture Tube Business
of Kimble Glass Inc. | | April 2001 | | | 5,800 | | | 331 | | | | — | | Asset |
Innovative Diagnostics, Inc. | | July 2001 | | | 1,300 | | | 163 | | | | — | | Asset |
Disposable Glass Pasteur Pipette and
Perfume Sampler Vial Product Line of Kimble Glass Inc. | | August 2001 | | | 2,000 | | | 400 | | | | — | | Asset |
Latex Agglutination Product Line of Medtek Diagnostics LLC. | | July 2001 | | | 220 | | | 150 | | | | — | | Asset |
Daniel Mirror Company | | September 2001 | | | 6,800 | | | 2,000 | | | | — | | Asset |
Research Group: | | | | | | | | | | | | | | |
BioRobotics Group Ltd. | | March 2001 | | | 10,500 | | | 2,500 | | | | 4,592 | | Stock |
Advanced Biotechnologies Ltd. | | April 2001 | | | 21,500 | | | 6,700 | | | | 14,265 | | Stock |
Mosaic Technologies Inc. | | July 2001 | | | 1,400 | | | (747 | ) | | | — | | Asset |
Chromatography Vial Product Line of
Kimble Glass Inc. | | August 2001 | | | 7,200 | | | 1,300 | | | | — | | Asset |
2000
During 2000, the Company completed ten acquisitions, nine for all cash and one for cash and notes in the amount of $30,600. The aggregate cash price of the acquisitions (none of which individually or aggregated was significant) was $206,900. The Company was subject to future purchase price adjustments based upon earnout provisions under one of the purchase and sale agreements. Such earnout provision has a maximum payout of $6,000. The earnout provision is subject to the achievement of certain financial goals and is not contingent upon employment. The entire earnout was paid in fiscal 2001 and is accounted for as additional goodwill. All acquisitions were accounted for as purchases. The results of the acquisitions were included as of the date they were acquired. The total goodwill for the acquired companies was approximately $205,100. The following table outlines unaudited sales and operating income for the most recent date prior to the acquisition, and unaudited total assets at the most recent available date prior to acquisition, for each of the acquired companies.
Business Segment
Company Acquired
| | Date
| | Sales
| | Operating
Income
| | Total
Assets
| | Type of
Acquisition
|
| | | | | (in thousands) |
Clinical Group: | | | | | | | | | | | | | |
Microm Laborgoräte GmbH | | October 1999 | | $ | 20,676 | | $ | 1,112 | | $ | 7,907 | | Asset |
Lab Vision Corporation | | August 2000 | | | 7,487 | | | 1,146 | | | 4,426 | | Stock |
Consolidated Technologies, Inc | | March 2000 | | | 7,782 | | | 2,888 | | | 6,100 | | Asset |
Murex bacteriology latex agglutination product line of Abbott Laboratories | | August 2000 | | | 20,461 | | | 10,026 | | | N/A | | Asset |
The thyroid and coagulation product line of Axis Shield | | September 2000 | | | 4,507 | | | 2,174 | | | N/A | | Asset |
F-18
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Business Segment
Company Acquired
| | Date
| | Sales
| | Operating
Income
| | | Total
Assets
| | Type of
Acquisition
|
| | | | | (in thousands) |
| | | | | |
Research Group: | | | | | | | | | | | |
Robbins Scientific Corporation | | October 1999 | | 19,601 | | 4,088 | | | 9,876 | | Stock |
Versi Dry® product line of National Packaging Services Corporation | | February 2000 | | 2,494 | | 1,300 | | | — | | Asset |
Sun International | | February 2000 | | 5,818 | | (270 | ) | | 2,269 | | Asset |
Genevac Limited | | May 2000 | | 10,624 | | 1,082 | | | 4,165 | | Stock |
Genevac Inc | | May 2000 | | 1,626 | | — | | | N/A | | Stock |
Discontinued Operations:
Divestitures
On March 25, 2003, the Company made the decision to dispose of two of its businesses: the rapid diagnostic test business (on-site rapid tests used in the detection of pregnancy, drugs of abuse, and infectious diseases) as conducted by our Applied Biotech subsidiary; and the manufacture and sale of automated microarray instrumentation for the genomics market as conducted by our BioRobotics Group subsidiary. The decision was based in part on the Company’s ongoing strategy of strengthening the market positions of our leading brands and focusing on sales of our consumable laboratory products that have more stable growth expectations. As a result, these businesses no longer met the Company’s strategic requirements. In the second quarter of fiscal 2003, in connection with the discontinuance of these businesses, we incurred a charge of approximately $85,900, net of income tax benefit of $21,600, related to the write-down of net assets to their estimated fair value less costs to sell. The decision to sell these companies represented a disposal of long-lived assets and disposal group under SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.” Accordingly, results of these businesses have been classified as discontinued operations, and prior periods have been restated to reflect this reclassification. The Company is actively pursuing the sale of these businesses and anticipates disposal within the next twelve months. In the event the Company ultimately disposes of Applied Biotech and BioRobotics Group for an amount less than the carrying value of the businesses, additional charges will be recognized upon disposal. For business reporting purposes, Applied Biotech was previously classified in the clinical group business segment, and BioRobotics Group was classified in the research group business segment.
Operating results from Applied Biotech for the six months ended March 31, 2003 and 2002 and the years ended September 30, 2002, 2001, and 2000 were as follows:
| | | Six Months Ended March 31,
| | Years Ended September 30,
|
| | | 2003
| | | 2002
| | 2002
| | 2001
| | 2000
|
| | | (unaudited) | | | | | | |
Net Sales | | $ | 13,841 | | | $ | 17,329 | | $ | 40,954 | | $ | 38,239 | | $ | 30,053 |
Gross Profit | | | 4,261 | | | | 9,234 | | | 18,957 | | | 16,698 | | | 17,124 |
Pretax income (loss) | | | (64,806 | ) | | | 4,316 | | | 10,056 | | | 11,095 | | | 9,566 |
Income tax | | | (11,360 | ) | | | 1,580 | | | 3,673 | | | 4,351 | | | 3,829 |
Net income (loss) | | | (53,447 | ) | | | 2,736 | | | 6,421 | | | 7,035 | | | 5,740 |
F-19
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Operating results from BioRobotics Group for the six months ended March 31, 2003 and 2002 and the years ended September 30, 2002 and 2001 were as follows:
| | | Six Months Ended March 31,
| | | Years Ended September 30,
|
| | | 2003
| | | 2002
| | | 2002
| | | 2001
|
| | | (unaudited) | | | | | | |
Net Sales | | $ | 1,951 | | | $ | 3,038 | | | $ | 5,752 | | | $ | 5,110 |
Gross Profit | | | 921 | | | | 1,706 | | | | 2,931 | | | | 3,798 |
Pretax income (loss) | | | (42,508 | ) | | | (787 | ) | | | (2,091 | ) | | | 1,159 |
Income tax benefit (expense) | | | (12,120 | ) | | | (265 | ) | | | (755 | ) | | | 427 |
Net income (loss) | | | (30,388 | ) | | | (522 | ) | | | (1,366 | ) | | | 442 |
Assets and liabilities of Applied Biotech were as follows:
| | | March 31, 2003
| | September 30,
|
| | | | 2002
| | 2001
|
| | | (unaudited) | | | | |
Current assets | | $ | 16,677 | | $ | 22,844 | | $ | 16,884 |
Property, plant and equipment, net | | | 5,618 | | | 5,496 | | | 3,940 |
Intangible assets | | | 12,919 | | | 74,801 | | | 44,627 |
Total assets | | | 35,214 | | | 103,335 | | | 65,451 |
Current liabilities | | | 4,585 | | | 2,830 | | | 4,278 |
Total liabilities | | | 4,585 | | | 2,830 | | | 4,278 |
Assets and liabilities of BioRobotics Group were as follows:
| | | March 31, 2003
| | September 30,
|
| | | | 2002
| | 2001
|
| | | (unaudited) | | | | |
Current assets | | $ | 15,994 | | $ | 3,617 | | $ | 4,526 |
Property, plant and equipment, net | | | 771 | | | 714 | | | 461 |
Intangible assets | | | 425 | | | 39,711 | | | 41,992 |
Total assets | | | 17,190 | | | 44,042 | | | 48,136 |
Current liabilities | | | 753 | | | 6,174 | | | 1,897 |
Total liabilities | | | 753 | | | 6,174 | | | 1,897 |
During March 2002, we made the decision to dispose of our vacuum deposition chamber business, Vacuum Process Technology, Inc. The decision was made following a slow-down in the telecommunications industry, in which Vacuum Process Technology targeted a majority of its products, and, as a result, the business no longer met the Company’s strategic requirements. In the second quarter of fiscal 2002, in connection with the discontinuance of this business, we incurred a charge of $13,200, net of income tax benefit of $7,600, related to the write-down of net assets to their estimated fair value less costs to sell. During the second quarter of fiscal 2003, the Company completed the sale of Vacuum Process Technology and, as a result, incurred an additional charge of $2,800, net of income tax benefit of $1,600. The decision to sell Vacuum Process Technology represents a disposal of long-lived assets and disposal group under SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.” Accordingly, results of this business have been classified as discontinued operations. For business reporting purposes, Vacuum Process Technology was previously classified in the clinical group business
F-20
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
segment. Operating results from Vacuum Process Technology for the six months ended March 31, 2003 and 2002 and the years ended September 30, 2002 and 2001 were as follows:
| | | Six Months Ended
March 31,
| | | Year Ended
September 30,
| |
| | | 2003
| | | 2002
| | | 2002
| | | 2001
| |
| | | (unaudited) | | | | | | | |
Net Sales | | $ | 884 | | | $ | 1,972 | | | $ | 3,982 | | | $ | 15,372 | |
Gross Profit | | | (803 | ) | | | 300 | | | | 544 | | | | 3,127 | |
Pretax income (loss) | | | (5,042 | ) | | | (944 | ) | | | (1,370 | ) | | | 1,610 | |
Income tax (benefit) expense | | | (1,549 | ) | | | (368 | ) | | | 497 | | | | (620 | ) |
Net income (loss) | | | (3,493 | ) | | | (13,776 | ) | | | (873 | ) | | | 990 | |
Assets and liabilities of Vacuum Process Technology were as follows:
| | | September 30,
|
| | | 2002
| | 2001
|
Current assets | | $ | 3,771 | | $ | 6,296 |
Property, plant and equipment, net | | | 817 | | | 912 |
Intangible assets | | | — | | | 21,023 |
Total assets | | | 5,436 | | | 29,083 |
Current liabilities | | | 305 | | | 2,630 |
Total liabilities | | | 305 | | | 2,630 |
Distribution
On November 8, 2000, the Company announced that it had declared a pro rata distribution (or spin-off) to its shareholders of the common stock and related preferred stock purchase rights of Sybron Dental Specialties, Inc. (the “Spin-Off”). Shareholders of record as of November 30, 2000 received one share of Sybron Dental Specialties, Inc. (“SDS”) common stock for every three shares of Apogent common stock they owned. These consolidated financial statements have reclassified SDS and its affiliates to discontinued operations. On December 11, 2000 the Spin-Off was completed. No proceeds were received by the Company in connection with the Spin-Off. For 2001 the Company has included a net loss of $11,800 from discontinued operations. The net loss included transaction expenses of $12,500 relating to the Spin-Off of SDS. Revenues and net income from SDS through the date of the Spin-Off (December 11, 2000) were $67,400 and $638, respectively, and offset the transaction expenses. Income included in discontinued operations for 2000 and 1999 was $41,597 and $47,844, respectively. SDS issued its own financial statements as of September 30, 2000.
As a result, these consolidated financial statements have classified SDS and its affiliates to discontinued operations. SDS now owns and operates what were formerly the professional dental, orthodontics and infection control products business segments.
F-21
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
6. Property, Plant, and Equipment
Major classifications of property, plant, and equipment at September 30, 2002 and 2001 are as follows:
| | | 2002
| | | 2001
| |
Land and land improvements | | $ | 13,279 | | | $ | 13,067 | |
Buildings and building improvements | | | 119,368 | | | | 102,856 | |
Machinery and equipment | | | 369,996 | | | | 291,564 | |
Construction in progress | | | 22,930 | | | | 18,755 | |
| | |
|
|
| |
|
|
|
| | | | 525,573 | | | | 426,242 | |
Less: Accumulated depreciation | | | (254,680 | ) | | | (202,555 | ) |
| | |
|
|
| |
|
|
|
| | | $ | 270,893 | | | $ | 223,687 | |
| | |
|
|
| |
|
|
|
7. Intangible Assets
The Company adopted SFAS No. 142, “Goodwill and Other Intangible Assets,” on October 1, 2001. SFAS No. 142 requires that all goodwill and intangible assets with indefinite useful lives will no longer be amortized, but instead tested for impairment at least annually. The Company has performed its initial impairment tests as well as its initial annual impairment test and the results indicate no circumstances of impaired goodwill. The following table reconciles reported amounts to that which would have been reported if the current method of accounting was used for the fiscal years ended September 30, 2001, 2000, 1999, and 1998:
| | | Year Ended September 30,
|
| | | 2001
| | 2000
| | 1999
| | 1998
|
Income before extraordinary items: | | | | | | | | | | | | |
Reported income before extraordinary items | | $ | 98,047 | | $ | 128,321 | | $ | 125,376 | | $ | 76,043 |
Add back: goodwill amortization, net of tax | | | 22,363 | | | 19,605 | | | 12,813 | | | 11,095 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted income before extraordinary items | | $ | 120,410 | | $ | 147,926 | | $ | 138,189 | | $ | 87,138 |
| | |
|
| |
|
| |
|
| |
|
|
Net income | | | | | | | | | | | | |
Reported net income | | $ | 95,941 | | $ | 128,321 | | $ | 142,547 | | $ | 76,043 |
Add back: goodwill amortization, net of tax | | | 22,363 | | | 19,605 | | | 12,813 | | | 11,095 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted net income | | $ | 118,304 | | $ | 147,926 | | $ | 155,360 | | $ | 87,138 |
| | |
|
| |
|
| |
|
| |
|
|
Basic earnings per common share: | | | | | | | | | | | | |
Reported earnings per share | | $ | 0.91 | | $ | 1.23 | | $ | 1.38 | | $ | 0.74 |
Add back: goodwill amortization, net of tax | | | 0.21 | | | 0.19 | | | 0.12 | | | 0.11 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted basic earnings per common share | | $ | 1.12 | | $ | 1.42 | | $ | 1.50 | | $ | 0.85 |
| | |
|
| |
|
| |
|
| |
|
|
Diluted earnings per common share: | | | | | | | | | | | | |
Reported fully diluted earnings per share | | $ | 0.89 | | $ | 1.20 | | $ | 1.34 | | $ | 0.72 |
Add back: goodwill amortization, net of tax | | | 0.21 | | | 0.18 | | | 0.12 | | | 0.10 |
| | |
|
| |
|
| |
|
| |
|
|
Adjusted Diluted earnings per common share | | $ | 1.10 | | $ | 1.38 | | $ | 1.46 | | $ | 0.82 |
| | |
|
| |
|
| |
|
| |
|
|
As a result of SFAS No. 142, the Company is no longer amortizing approximately $967,753 of goodwill as of March 31, 2003.
F-22
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Intangible assets are as follows:
| | | March 31,
2003
| | | September 30,
| | | Weighted
Average
Life
|
| | | | 2002
| | | 2001
| | |
| | | (unaudited) | | | | | | | | | |
Amortizable intangible assets | | | | | | | | | | | | | | |
Proprietary technology | | $ | 75,195 | | | $ | 91,571 | | | $ | 109,376 | | | 14.23 |
Trademarks | | | 79,590 | | | | 80,402 | | | | 58,894 | | | 31.09 |
Patents | | | 35,768 | | | | 34,092 | | | | 28,547 | | | 15.08 |
Licenses | | | 11,826 | | | | 17,798 | | | | 10,703 | | | 26.61 |
Drawings | | | 11,774 | | | | 11,754 | | | | 11,486 | | | 19.37 |
Non-compete agreements | | | 12,796 | | | | 16,316 | | | | 13,240 | | | 4.60 |
Other | | | 37,047 | | | | 37,022 | | | | 10,611 | | | 9.35 |
Less: Accumulated amortization | | | (76,800 | ) | | | (75,433 | ) | | | (53,389 | ) | | |
| | |
|
|
| |
|
|
| |
|
|
| | |
Net amortizable intangible assets | | | 187,196 | | | | 213,522 | | | | 189,468 | | | |
Unamortizable intangible assets (goodwill) | | | 967,753 | | | | 1,029,591 | | | | 950,866 | | | |
| | |
|
|
| |
|
|
| |
|
|
| | |
| | | $ | 1,154,949 | | | $ | 1,243,113 | | | $ | 1,140,334 | | | |
| | |
|
|
| |
|
|
| |
|
|
| | |
Intangible assets at March 31, 2003 by business segment were as follows (unaudited):
| | | Clinical
Group
| | | Research
Group
| | | Consolidated
| |
Proprietary technology | | $ | 60,661 | | | $ | 14,534 | | | $ | 75,195 | |
Less: Accumulated amortization | | | (19,034 | ) | | | (5,536 | ) | | | (24,570 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net proprietary technology | | | 41,627 | | | | 8,998 | | | | 50,625 | |
| | |
|
|
| |
|
|
| |
|
|
|
Trademarks | | | 18,448 | | | | 61,142 | | | | 79,591 | |
Less: Accumulated amortization | | | (1,706 | ) | | | (15,484 | ) | | | (17,190 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net trademarks | | | 16,742 | | | | 45,658 | | | | 62,400 | |
| | |
|
|
| |
|
|
| |
|
|
|
Patents | | | 20,103 | | | | 15,665 | | | | 35,768 | |
Less: Accumulated amortization | | | (4,218 | ) | | | (3,745 | ) | | | (7,963 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net patents | | | 15,885 | | | | 11,920 | | | | 27,805 | |
| | |
|
|
| |
|
|
| |
|
|
|
Licenses | | | 9,488 | | | | 2,338 | | | | 11,826 | |
Less: Accumulated amortization | | | (3,065 | ) | | | (229 | ) | | | (3,294 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net licenses | | | 6,423 | | | | 2,109 | | | | 8,532 | |
| | |
|
|
| |
|
|
| |
|
|
|
Drawings | | | — | | | | 11,774 | | | | 11,774 | |
Less: Accumulated amortization | | | — | | | | (6,026 | ) | | | (6,026 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net drawings | | | — | | | | 5,748 | | | | 5,748 | |
| | |
|
|
| |
|
|
| |
|
|
|
Non-compete agreements | | | 5,288 | | | | 7,508 | | | | 12,796 | |
Less: Accumulated amortization | | | (3,046 | ) | | | (4,965 | ) | | | (8,011 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net non-compete agreements | | | 2,243 | | | | 2,543 | | | | 4,786 | |
| | |
|
|
| |
|
|
| |
|
|
|
Other identifiable intangible assets (a) | | | 7,447 | | | | 12,993 | | | | 20,440 | |
Less: Accumulated amortization | | | (563 | ) | | | (2,782 | ) | | | (3,345 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net other identifiable intangibles (a) | | | 6,883 | | | | 10,211 | | | | 17,094 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net amortizable intangible assets (a) | | $ | 89,804 | | | $ | 87,187 | | | $ | 176,991 | |
| | |
|
|
| |
|
|
| |
|
|
|
Excess cost over net asset values acquired (goodwill) | | $ | 510,847 | | | $ | 456,907 | | | $ | 967,754 | |
| | |
|
|
| |
|
|
| |
|
|
|
Unamortizable intangible assets | | | 510,847 | | | | 456,907 | | | | 967,754 | |
| | |
|
|
| |
|
|
| |
|
|
|
F-23
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Note (a): At March 31, 2003, Apogent Corporate Office had $16,607 of amortizable other identifiable intangible assets and $6,401 of related accumulated amortization that was not allocated to any of the business segments.
Amortization expense relating to the existing identifiable intangible assets for each of the next five years (beginning with fiscal 2003) is expected to be $18,372, $17,376, $14,036, $13,032, and $12,506, respectively.
The changes in the carrying amount of goodwill for the six months ended March 31, 2003 and the year ended September 30, 2002 are as follows:
| | | Clinical
Group
| | | Research
Group
| | | Consolidated
| |
Balance at September 30, 2001 | | $ | 397,433 | | | $ | 454,458 | | | $ | 851,891 | |
Goodwill acquired during the year (a) | | | 95,087 | | | | 4,456 | | | | 99,543 | |
Reclassification of customer lists and workforce(b) | | | 73,835 | | | | 25,141 | | | | 98,976 | |
Goodwill written off related to disposal of Vacuum Process Technology | | | (21,023 | ) | | | — | | | | (21,023 | ) |
Reduction in purchase price of prior year acquisition | | | — | | | | (10,635 | ) | | | (10,635 | ) |
Effect of change in foreign currencies | | | 3,707 | | | | 7,132 | | | | 10,839 | |
| | |
|
|
| |
|
|
| |
|
|
|
Balance at September 30, 2002 | | $ | 549,039 | | | $ | 480,552 | | | $ | 1,029,591 | |
| | |
|
|
| |
|
|
| |
|
|
|
Goodwill acquired during the year | | | 13,031 | | | | — | | | | 13,031 | |
Goodwill written off related to disposal of Applied Biotech and BioRobotics Group | | | (54,421 | ) | | | (30,827 | ) | | | (85,248 | ) |
Change in purchase price of prior year acquisitions | | | 3,109 | | | | 155 | | | | 3,264 | |
Effect of change in foreign currencies | | | 88 | | | | 7,027 | | | | 7,115 | |
| | |
|
|
| |
|
|
| |
|
|
|
Balance at March 31, 2003 | | $ | 510,846 | | | $ | 456,907 | | | $ | 967,753 | |
| | |
|
|
| |
|
|
| |
|
|
|
| (a) | Includes effect of final purchase price allocation related to prior year acquisitions. |
| (b) | The Company reclassified certain customer lists amounting to $98,976 to goodwill in accordance with SFAS No. 142. These amounts were determined to be inseparable from the underlying businesses to which they relate. |
8. Long-Term Debt
Long-term debt at September 30, 2002 and 2001 consists of the following:
| | | 2002
| | | 2001
| |
Revolving Credit Facility | | $ | — | | | $ | 208,500 | |
8% Senior Notes, net of discount | | | 323,685 | | | | 323,580 | |
2.25% Senior Convertible Contingent Debt | | | 300,000 | | | | — | |
Sellers’ Notes | | | 24,656 | | | | 103,685 | |
Sale/Leaseback Obligation | | | 11,416 | | | | 11,734 | |
Capital leases and other | | | 615 | | | | 9,931 | |
| | |
|
|
| |
|
|
|
| | | | 660,372 | | | | 657,430 | |
Less: Current portion of long-term debt | | | (25,352 | ) | | | (73,642 | ) |
| | |
|
|
| |
|
|
|
| | | $ | 635,020 | | | $ | 583,788 | |
| | |
|
|
| |
|
|
|
Securities Lending Agreement | | $ | 60,183 | | | $ | 55,072 | |
| | |
|
|
| |
|
|
|
F-24
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Credit Agreements: Until December 11, 2000, the Company and its principal domestic subsidiaries (including certain subsidiaries of SDS) were parties to a credit agreement (as amended, the “Previous Credit Agreement”) with The Chase Manhattan Bank (“Chase”) and certain other lenders providing for a term A loan facility of $300,000 (the “Tranche A Term Loan Facility”), a term B loan facility (the “Tranche B Term Loan Facility”) and a revolving credit facility of up to $600,000 (the “Previous Revolving Credit Facility”). In connection with the Spin-Off, on December 1, 2000, the Company entered into a new credit agreement (the “Credit Agreement”) with Chase and certain other lenders providing for a term loan of $300,000 (the “Term Loan Facility”) and a revolving credit facility of up to $500,000 (the “Revolving Credit Facility” and together with the Term Loan Facility, the “Credit Facilities”). Borrowings under the Credit Facilities are unsecured. On December 11, 2000, the Company borrowed approximately $563,000 under the Credit Facilities and together with funds aggregating $375,000 (approximately $307,100, the amount equal to the outstanding amounts under the Previous Credit Agreement attributable to SDS on December 11, 2000 including accrued interest plus a cash dividend of $67,900 from SDS to the Company), used such funds to repay all of the outstanding amounts under the Previous Credit Agreement (including amounts attributable to SDS and accrued interest) aggregating $938,000.
Revolving Credit Facility: Borrowings under the Revolving Credit Facility mature on December 1, 2005. The Revolving Credit Facility provides for an annual interest rate at the option of the Company, equal to (a) ABR plus 0% to 0.375% (the “Revolving ABR Margin”) or (b) the Eurodollar Rates plus 0.375% to 1.375% (the “Revolving Loan Eurodollar Rate Margin”). In addition, the Company has a third option to set the rate by a competitive bid process among the parties to the Revolving Credit Facility (the “CAF”). The Company also pays a facility fee of 0.125% to 0.375% for all commitments from the lenders, whether drawn, or undrawn and pays a utilization fee of 0.25% per annum if more than 50% of the Revolving Credit Facility is drawn. The Revolving ABR Margin, the Revolving Loan Eurodollar Rate Margin and the facility fee depend upon the Company’s credit rating from S&P and Moody’s. Based upon the Company’s current credit rating, the Revolving ABR Margin, the Revolving Loan Eurodollar Rate Margin and the facility fee would be 0%, 0.8% and 0.2%, respectively. The Revolving Credit Facility also provides for a multi-currency sub-facility providing up to $100,000 in sub-commitments in non-dollar currencies. Terms and conditions on the multi-currency sub-facility are to be agreed upon between the Company and Chase and the lenders providing funding under such facility. The Company may not exceed a total of $500,000 in dollar and non-dollar commitments under this Revolving Credit Facility. The Revolving Credit Facility also provides for the issuance of standby letters of credit and commercial letters of credit on behalf of the Company’s subsidiaries as required in the ordinary course of business as part of the working capital line. There were no outstanding balances under the Revolving Credit Facility as of September 30, 2002.
The Credit Agreement contains financial and operating covenants, including, among other things: restrictions on investments; requirements that the Company maintain certain financial ratios; restrictions on the ability of the Company and its subsidiaries to create or permit liens, or to pay dividends or make other restricted payments (as defined) in excess of $100,000 plus 50% of the defined consolidated net income of the Company for each fiscal quarter ending after September 30, 2000, less any dividends paid or other restricted payments made after September 30, 2000; and limitations on incurrence of additional indebtedness. The Company’s obligations under the Revolving Credit Facility are guaranteed by the Company’s material domestic subsidiaries.
Term Loan Facility: Borrowings under the Term Loan Facility were paid in full from the proceeds of the Senior Notes Offering completed in April 2001.
8% Senior Notes: On April 4, 2001 the Company issued $325,000 of unsecured senior notes in a private placement with exchange and registration rights, and in August 2001 we completed a registered exchange of the
F-25
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
privately placed notes for similar notes that had been registered with the SEC. The notes were offered at a discount of approximately $1,469. They will mature on April 1, 2011. Interest is fixed at an annual rate of 8% and is payable on April 1 and October 1 of each year, beginning on October 1, 2001. The notes are redeemable by the Company at any time in whole, or from time to time in part, at a price equal to the greater of (i) 100% of the principal amount of the notes to be redeemed or (ii) the sum of the present values of the remaining scheduled payments of principal and interest thereon (exclusive of interest accrued to the date of redemption) discounted to the date of redemption on a semiannual basis at the applicable Treasury Yield (as defined in the bond agreement) plus 35 basis points, plus accrued interest to the date of redemption. The Company used the proceeds from the issuance to repay all of its Term Loan Facility ($300 million) and a portion of its Revolving Credit Facility. The notes are guaranteed by the Company’s material U.S. subsidiaries, which also guarantee the Company’s obligations under its Revolving Credit Facility.
Senior Convertible Contingent Debt: On October 10, 2001, the Company issued $300 million of senior convertible contingent debt securities (CODES). The CODES have a fixed interest rate of 2.25% per annum. Interest is payable on April 15 and October 15 of each year. The Company will also pay contingent interest during any six-month period if the average trading price of the CODES during a specified period of five trading days preceding the relevant six-month period is above specified levels. No contingent interest is payable during the six-month period from April 15, 2002 to October 14, 2002. The CODES will mature on October 15, 2021. The CODES are convertible, subject to certain conditions (based upon specified factors including but not limited to the sale price of the Company’s common stock, trading prices of the CODES, maintenance of the Company’s credit ratings, and the occurrence of specified corporate transactions, including certain repurchases of common stock by the Company), into common stock of the Company at a price of approximately $30.49 per share. The Company may redeem some or all of the CODES on or after October 20, 2004. The holders may require the Company to purchase all or a portion of their CODES on October 20, 2004 and on October 15, 2006, 2011 and 2016, or subject to specified exceptions, upon a change of control event. Certain of the Company’s U.S. subsidiaries guarantee the Company’s obligations under the CODES. The proceeds from the issuance were used to pay down the outstanding balance on our Revolving Credit Facility, and for general corporate purposes.
Sellers’ Notes: In connection with certain acquisitions, the Company has issued notes payable to the related sellers. The notes bear interest of 5% to 6% and mature at various dates through July, 2003. Certain notes are redeemable by the holders, subject to certain time restrictions. The notes are unsecured, however in certain instances, some are guaranteed by a subsidiary of the Company.
Sale/Leaseback: On December 22, 1988, the Company completed the sale and leaseback (the “Sale/Leaseback”) of its then principal domestic manufacturing and office facilities with an unaffiliated third party. The proceeds of $22,500 (net of approximately $1,100 in fees) were used to retire debt. The transaction has been accounted for as a financing for financial statement purposes and as a sale for income tax purposes. The financing obligation is being amortized over the initial 25-year lease term.
The Company pays all costs of maintenance and repair, insurance, taxes, and all other expenses associated with the properties. In addition, each of the leases is unconditionally guaranteed by the Company.
The initial term of each lease is 25 years with five five-year renewal options. The initial aggregate annual payments relating to the Company under the leases were $1,727 payable monthly in advance. On the fifth anniversary of the leases and every five years thereafter (including renewal terms), the rent is increased by the percentage equal to 75% of the percentage increase in the Consumer Price Index over the preceding five years. The percentage increase to the rent in any five-year period will be capped at 15%. Beginning January 1, 1999 annual payments increased to $2,176. The next adjustment will not occur until January 1, 2004.
F-26
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
The Company has the option to purchase the facilities according to the terms of any bona fide offer received by the lessor from a third party (the “Third Party Offer”) at any time during the term of the leases. The purchase price upon exercise of the option will be an amount equal to the purchase price contained in the Third Party Offer. The Company also has the option to purchase the facilities, subject to complying with the notice provision in the leases, on any date between June 1, 2008 and May 31, 2009. The purchase price upon the exercise of the option is the greater of the fair market value of the leased premises or the sum of the landlord’s acquisition cost for the leased premises and any prepayment premiums that would be payable under the landlord’s financing for the premises.
In the event of a breach of certain covenants which include, subject to certain exceptions, restrictions on the Company’s and its subsidiaries’ incurrence of certain additional indebtedness, payment of dividends or the making of other distributions or the repurchase of the Company’s capital stock, or the creation of liens on their respective properties, the Company must cause each subsidiary to make a rejectable offer to the lessor to purchase its facility. If the lessor accepts the rejectable offer, each subsidiary will pay to the lessor a formula price based upon the lessor’s equity in the property and the lessor’s pre-payment premium to its lender. The Company may also be obligated to repurchase the property upon the occurrence of certain other events.
Securities Lending Agreement: On September 29, 1999, the Company purchased a United States Treasury Bond (“Treasury”) with a par value of $50,000, an interest rate of 6.15% and a maturity date of August 15, 2029. Concurrent with the purchase of the Treasury, the Company loaned the security to an unrelated third party for a period of 23 years. In exchange for the loaned Treasury, the Company has received collateral equal to the market value of the Treasury on the date of the loan, and adjusted on a weekly basis. This securities lending transaction is related to the Company’s existing lending policy by fixing $50,000 of its floating rate debt. For a period of five years, the Company is obligated to pay a rebate on the loaned collateral at an annual fixed rate of 6.478% and is entitled to receive a fee for the loan of the security at a floating rate equal to LIBOR minus .75%. Thereafter, the Company is required to pay the unrelated third party a collateral fee equal to the one-week general collateral rate of interest (as determined weekly in good faith by the unrelated third party, provided that such rate shall not exceed the federal funds rate in effect as of the day of determination plus .25%) and the Company receives all distributions made on or in respect to the Treasury. This transaction is accounted for as a secured borrowing under SFAS No. 140.
Maturities of Long-Term Debt: As of September 30, 2002, maturities of long-term debt, including capital leases, are as follows:
Fiscal
| | |
2003 | | $ | 25,352 |
2004 | | | 704 |
2005 | | | 300,637 |
2006 | | | 650 |
2007 | | | 754 |
Thereafter | | | 332,275 |
| | |
|
|
| | | $ | 660,372 |
| | |
|
|
F-27
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
9. Lease Commitments
As of September 30, 2002, minimum rentals, excluding rent payments under the Sale/Leaseback described in note 8, under capital and noncancellable operating leases consisting primarily of machinery and equipment, and building leases are:
Fiscal
| | Capital
| | Operating
|
2003 | | $ | 286 | | $ | 12,298 |
2004 | | | 185 | | | 10,458 |
2005 | | | 89 | | | 9,324 |
2006 | | | 5 | | | 7,716 |
2007 | | | — | | | 7,035 |
Thereafter | | | — | | | 23,224 |
| | |
|
| |
|
|
| | | $ | 565 | | $ | 70,055 |
| | | | | |
|
|
Less amounts representing interest | | | 48 | | | |
| | |
|
| | | |
Present value of net minimum lease payments | | | 517 | | | |
Less current portion | | | 255 | | | |
| | |
|
| | | |
Long-term obligations under capital leases | | $ | 262 | | | |
| | |
|
| | | |
Amortization of assets held under capital leases is included with depreciation expense.
Rental expense under operating leases for the six months ended March 31, 2003 and 2002 and for fiscal years ended 2002, 2001, and 2000 was approximately $6,863, $5,792, $13,402, $10,725, and $8,716, respectively.
10. Fair Market Value of Financial Instruments
The carrying amounts of financial instruments approximate fair value due to the short maturity of those instruments except as follows:
8% Senior Notes and 2.25% Senior Convertible Contingent Debt (CODES): The fair values of our issued debt securities were obtained from dealer quotes. The estimated fair market value of the 8% Senior Notes approximated the reported amount as of September 30, 2001. There were no CODES outstanding as of September 30, 2001.
| | | September 30, 2002
| | September 30, 2001
|
| | | Reported
Amount
| | Estimated
Fair Value
| | Reported
Amount
| | Estimated
Fair Value
|
8% Senior Notes | | $ | 323,685 | | $ | 377,000 | | $ | 323,580 | | $ | 351,000 |
2.25% CODES | | | 300,000 | | | 303,000 | | | — | | | — |
Sale/Leaseback: The fair value was determined by estimating the interest rate at which the Company could refinance the Sale/Leaseback given the same maturity period.
| | | September 30, 2002
| | September 30, 2001
|
| | | Reported
Amount
| | Estimated
Fair Value
| | Reported
Amount
| | Estimated
Fair Value
|
Sale Leaseback | | $ | 11,416 | | $ | 10,166 | | $ | 11,734 | | $ | 10,886 |
F-28
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
Foreign Exchange Contracts: The Company enters into foreign exchange hedging contracts to hedge certain sales commitments and loans made to foreign subsidiaries denominated in foreign currencies. The purpose of the Company’s foreign currency-hedging activities is to protect the Company from the risk that the eventual cash flows resulting from foreign activities will be adversely affected by changes in exchange rates. The recognition of gains and losses on contracts entered into to hedge sales commitments are included in net income as an adjustment to net sales. At September 30, 2002 and 2001, the Company had no foreign exchange option contracts with respect to sales commitments. At September 30, 2002, the Company had one foreign exchange option contract relating to loans made to purchase a foreign subsidiary.
Interest Rate Swaps: The Company enters into interest rate swaps to stabilize funding costs by minimizing the effect of potential interest rate increases on floating-rate debt in a rising interest rate environment. Under these agreements, the Company contracts with a counter party to exchange the difference between a fixed rate and a floating rate applied to the notional amount of the swap. Swap contracts are principally between one and five years in duration. The differential to be paid or received on interest rate swap agreements is accrued as interest rates change and is recognized in net income as an adjustment to interest expense. Gains and losses resulting from terminated interest rate swap agreements are deferred and recognized in net income over the shorter term of the remaining contractual life of the swap agreement or the remaining term of the debt underlying the swap agreement. If swap agreements are terminated due to the underlying debt being extinguished, any resulting gain or loss is recognized in net income as an adjustment to interest expense at the time of the termination. As of September 30, 2002, the Company has no interest rate swap agreements.
On December 11, 2000, the Company extinguished the variable rate long-term debt to which the then-existing swaps were designated and as a result the interest rate swaps ceased to be accounted for as hedges. On December 12, 2000, the Company sold the interest rate swaps for an aggregate gain of $1,055, net of tax. Upon the sale of the interest rate swaps, the Company reduced the unrealized gain recorded at October 1, 2000 in other comprehensive income to reflect the fair market value net of tax on the date of sale. Because these interest rate swaps were designated as a hedge against future variable rate interest payments and the extinguished debt, the gain continued to be carried in other comprehensive income and recognized as an adjustment to yield interest expense of the new credit facilities over the remaining term of the interest rate contract. As of September 30, 2002, the gains had been fully amortized. For 2002 and 2001, the Company recognized gains, net of tax of $440 and $619, respectively.
11. Employee Benefit Plans
Pension and Other Postretirement Benefits: The Company has defined benefit pension plans covering approximately 48 percent of its U.S. employees. The benefits are generally based on various formulas, the principal factors of which are years of service and compensation. The Company’s funding policy is to generally make the minimum annual contributions required by applicable regulations. Plan assets are invested primarily in U.S. stocks, bonds and international stocks. In addition to the defined benefit plans, the Company provides certain health care benefits for eligible retired employees, which are funded as costs are incurred. Certain employees who reached the age of 55 prior to January 1, 1996 will become eligible for post-retirement health care only if they reach retirement age while working for the Company. The Company accrues, as current costs, the future lifetime retirement benefits for both qualifying active and retired employees and their dependents. The post-retirement health care plans for subsidiaries of the Company and certain divested operations are generally contributory, with retiree contributions adjusted annually. In 1986, the Company instituted a policy with respect to post-retirement medical premiums whereby the Company’s contributions were frozen at the levels equal to the Company’s contribution on December 31, 1988, except where collective bargaining agreements prohibited such a freeze.
F-29
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
The following assumptions were used in determining the funded status of the Company’s defined benefit plans:
| | | 2002
| | | 2001
| |
Discount rate | | 7.25 | % | | 7.75 | % |
Rate of increase in compensation levels | | 4.0 | % | | 4.0 | % |
Expected long-term rate of return on assets | | 9.5 | % | | 10.0 | % |
The following assumptions were used in determining the accumulated post-retirement benefit obligation of the Company’s post-retirement plans:
| | | 2002
| | | 2001
| |
Discount rate | | 7.25 | % | | 7.75 | % |
Average increase in medical costs | | 10 | %(a) | | 5.5 | % |
| (a) | For measurement purposes, a 10% annual rate of increase in the Company-paid medical premiums for non-frozen groups was assumed for 2002, decreasing gradually to 5% in year 2007 and thereafter. |
| | | Pension Benefits
| | | Other Benefits
| |
| | | 2002
| | | 2001
| | | 2002
| | | 2001
| |
Change in benefit obligations: | | | | | | | | | | | | | | | | |
Obligations at beginning of year | | $ | 63,170 | | | $ | 51,009 | | | $ | 5,792 | | | $ | 4,457 | |
Service cost | | | 2,915 | | | | 2,125 | | | | 15 | | | | 13 | |
Interest cost | | | 4,823 | | | | 3,981 | | | | 418 | | | | 298 | |
Actuarial loss (gain) | | | 7,585 | | | | 8,603 | | | | 2,114 | | | | 2,475 | |
Benefit payments | | | (2,665 | ) | | | (2,548 | ) | | | (1,195 | ) | | | (1,451 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Obligations at end of year | | $ | 75,828 | | | $ | 63,170 | | | $ | 7,144 | | | $ | 5,792 | |
Change in fair value of plan assets: | | | | | | | | | | | | | | | | |
Fair value of plan assets at beginning of year | | $ | 51,801 | | | $ | 50,483 | | | $ | — | | | $ | — | |
Actual return on plan assets | | | (2,249 | ) | | | 1,250 | | | | — | | | | — | |
Employer contributions | | | 210 | | | | 2,692 | | | | 1,195 | | | | — | |
Benefit payments | | | (2,665 | ) | | | (2,624 | ) | | | (1,195 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Fair value of plan assets at end of year | | $ | 47,097 | | | $ | 51,801 | | | $ | — | | | $ | — | |
Funded Status: | | | | | | | | | | | | | | | | |
Funded status at end of year | | $ | (28,731 | ) | | $ | (11,369 | ) | | $ | (7,144 | ) | | $ | (5,792 | ) |
Unrecognized transition (asset) obligation | | | — | | | | (6 | ) | | | — | | | | — | |
Unrecognized prior service cost | | | 123 | | | | 145 | | | | — | | | | — | |
Unrecognized (gain) loss | | | 20,253 | | | | 5,565 | | | | 4,497 | | | | 2,721 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net amount recognized at measurement date | | | (8,355 | ) | | | (5,665 | ) | | | (2,647 | ) | | | (3,071 | ) |
Employer contribution paid after measurement date | | | 2,932 | | | | — | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net amount recognized at end of year | | $ | (5,423 | ) | | $ | (5,665 | ) | | $ | (2,647 | ) | | $ | (3,071 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
F-30
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
The following table provides the amounts recognized in the Company’s consolidated balance sheets:
| | | Pension Benefits
| | | Other Benefits
| |
| | | 2002
| | | 2001
| | | 2002
| | | 2001
| |
Prepaid benefit cost | | $ | — | | | $ | 67 | | | $ | — | | | $ | — | |
Accrued benefit liability | | | (19,042 | ) | | | (5,732 | ) | | | (2,647 | ) | | | (3,071 | ) |
Intangible asset | | | 123 | | | | — | | | | — | | | | — | |
Accumulated other comprehensive income | | | 10,564 | | | | — | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net amount recognized at measurement date | | | (8,355 | ) | | | (5,665 | ) | | | (2,647 | ) | | | (3,071 | ) |
Employer contribution paid after measurement date | | | 2,932 | | | | — | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net amount recognized at September 30, | | $ | (5,423 | ) | | $ | (5,665 | ) | | $ | (2,647 | ) | | $ | (3,071 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
|
The following table provides disclosure of the net periodic benefit cost:
| | | Pension Benefits
| | | Other Benefits
|
| | | 2002
| | | 2001
| | | 2000
| | | 2002
| | 2001
| | 2000
|
Service cost | | $ | 2,915 | | | $ | 2,125 | | | $ | 2,032 | | | $ | 15 | | $ | 13 | | $ | 12 |
Interest cost | | | 4,823 | | | | 4,026 | | | | 3,691 | | | | 419 | | | 298 | | | 399 |
Expected return on plan assets | | | (4,858 | ) | | | (4,940 | ) | | | (4,509 | ) | | | — | | | — | | | — |
Amortization of transition (asset) obligation | | | (6 | ) | | | 12 | | | | 107 | | | | — | | | — | | | — |
Amortization of prior service cost | | | 23 | | | | (1 | ) | | | (52 | ) | | | — | | | — | | | — |
Amortization of net loss (gain) | | | 3 | | | | (110 | ) | | | 65 | | | | 128 | | | — | | | — |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
| |
|
|
Net periodic benefit cost | | $ | 2,900 | | | $ | 1,112 | | | $ | 1,334 | | | $ | 562 | | $ | 311 | | $ | 411 |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
| |
|
|
The projected benefit obligation, accumulated benefit obligation, and fair value of plan assets for the pension plans with accumulated benefit obligations in excess of fair value of plan assets were $74,144, $64,934, and $45,941, respectively, as of September 30, 2002 and $2,907, $1,853, and $0, respectively, as of September 30, 2001.
As a result of the cumulative benefit obligations of the Company’s pension benefit plans exceeding the fair market value of the plans’ assets, the Company has recorded a $6,445 minimum liability, net of tax of $4,120, through a charge to equity during 2002. This charge is reflected as a reduction to other comprehensive income.
An increase of one percentage point in the per capita cost of health care costs associated with the plans for which the Company contributions are not frozen would increase the accumulated post-retirement benefit obligation and service and interest cost components as of September 30, 2002 by approximately $212 and $8, respectively.
Because the majority of the post-retirement plans are remaining liabilities from certain divested operations and more than 85% of the 2002, 2001 and 2000 net periodic post-retirement benefit costs relate to interest costs, the Company has classified such interest costs as interest expense. This results in a non-cash increase in interest expense of approximately $419, $298, and $399 in 2002, 2001, and 2000, respectively.
Savings Plans: Employees in the United States are eligible to participate in contributory savings plans maintained by the Company under Section 401(k) of the Internal Revenue Code of 1986, as amended. Matching contributions made by the Company under the plans, net of forfeitures, were approximately $3,242, 3,079, and $2,770 for 2002, 2001, and 2000, respectively.
F-31
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
12. Restructuring Charges
During fiscal 2003, the Company recorded restructuring charges of approximately $332 (approximately $211 net of tax) for the consolidation of certain facilities and discontinuance of certain product lines due to product rationalizations. The restructuring charges were classified as components of selling, general and administrative expenses. The $332 related to severance associated with non-production employees. Additional restructuring charges of approximately $8.8 million related to these restructuring actions will be recorded during the balance of fiscal 2003.
During fiscal 2002, the Company recorded restructuring charges of approximately $7.2 million (approximately $4.4 million net of tax) for the consolidation of certain facilities and discontinuance of certain product lines due to product rationalizations. The restructuring charges were classified as components of cost of sales and selling, general and administrative expenses. The cost of sales component of approximately $5.6 million related to the write-off of inventory, write-offs of fixed assets, certain lease terminations, and severance associated with employees in production activities. The selling, general and administrative component of approximately $1.3 million related to severance associated with non-production employees as well as certain lease terminations and other shut down costs. These charges are referred to as the “2002 Special Charges”.
Activity related to the 2002 Special Charges and its components are as follows (dollars in thousands):
| | | Severance(a)
| | | Inventory(b)
| | | Fixed Assets(b)
| | | Facility Closure Costs(c)
| | | Other
| | | Total
| |
2002 Restructuring charge | | $ | 1,500 | | | $ | 3,700 | | | $ | 400 | | | $ | 1,400 | | | $ | 200 | | | $ | 7,200 | |
2002 Cash payments | | | (900 | ) | | | — | | | | — | | | | (500 | ) | | | — | | | | (1,400 | ) |
2002 Non-cash charges | | | — | | | | (3,700 | ) | | | (400 | ) | | | — | | | | (200 | ) | | | (4,300 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
September 30, 2002 balance | | $ | 600 | | | $ | — | | | $ | — | | | $ | 900 | | | $ | — | | | $ | 1,500 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| (a) | Amount represents severance and termination costs for 126 terminated employees (primarily sales, marketing and manufacturing personnel). |
| (b) | Amount represents write-offs of inventory and fixed assets associated with discontinued product lines. |
| (c) | Amount represents lease payments and other facility closure costs on exited operations. |
F-32
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
In September 2000, the Company recorded a restructuring charge of approximately $11,300 (approximately $7,500 after tax or $.07 per share on a diluted basis) for the consolidation of certain businesses, product rationalizations, changes in management structure and taxes associated with restructuring U.K. operations. The restructuring charge was classified as components of cost of sales (approximately $4,400 relating to the write-off of inventory, write-offs of fixed assets, certain lease terminations and severance associated with employees in production activities), selling, general and administrative expense of $5,800 and income tax expense of $1,000, related to the Company’s restructuring of its U.K. operations. Restructuring activity since its inception in September 2000 and its components are as follows:
| | | Severance(a)
| | Inventory(b)
| | Fixed
Assets(b)
| | Lease
Commitments(c)
| | Shut- down Costs(c)
| | Tax(d)
| | Other
| | Total
|
| | | (in thousands) |
2000 Restructuring charge | | $ | 5,500 | | $ | 2,100 | | $ | 1,000 | | $ | 500 | | $ | 300 | | $ | 1,000 | | $ | 900 | | $ | 11,300 |
2000 Cash payments | | | 1,100 | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,100 |
2000 Non-cash charges | | | — | | | 2,100 | | | 1,000 | | | — | | | — | | | — | | | 800 | | | 3,900 |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
September 30, 2000 balance | | $ | 4,400 | | $ | — | | $ | — | | $ | 500 | | $ | 300 | | $ | 1,000 | | $ | 100 | | $ | 6,300 |
Adjustments(e) | | | 600 | | | — | | | — | | | — | | | — | | | — | | | — | | | 600 |
2001 Cash payments | | | 3,800 | | | — | | | — | | | 200 | | | 200 | | | 1,000 | | | — | | | 5,200 |
2001 Non-cash charges | | | — | | | — | | | — | | | — | | | 100 | | | — | | | 100 | | | 200 |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
September 30, 2001 balance | | $ | 1,200 | | $ | — | | $ | — | | $ | 300 | | $ | — | | $ | — | | $ | — | | $ | 1,500 |
2002 Cash payments | | | 850 | | | — | | | — | | | 300 | | | — | | | — | | | — | | | 1,150 |
2002 Non-cash credit | | | 350 | | | — | | | — | | | — | | | — | | | — | | | — | | | 350 |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
September 30, 2002 balance | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
| (a) | Amount represents severance and termination costs for 151 terminated employees (primarily sales, marketing and corporate personnel). |
| (b) | Amount represents write-offs of inventory and fixed assets associated with discontinued product lines. |
| (c) | Amount represents lease payments and shut down costs on exited facilities. |
| (d) | Amount represents income tax expense associated with the restructuring of our U.K. facilities. |
| (e) | Amount represents an increase in the severance costs for 16 employees (primarily corporate personnel). These employees are included in the total 151 terminated employees referenced above. |
13. Commitments and Contingent Liabilities
Nalge Nunc International, a subsidiary of Apogent has been identified as a potentially responsible party (“PRP”) at the Aqua-Tech site in South Carolina (the “Aqua-Tech Site”) with respect to a previously owned facility. An action has been conducted at the Aqua-Tech Site for the removal of surface contaminants under the supervision of the Environmental Protection Agency (“EPA”) under the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended (“CERCLA”). Our total expenses (including legal expenses) to date have been approximately $165,000. The site has been placed by the EPA on the federal National Priority List under CERCLA, which is a prerequisite to any federally-mandated requirement for long-term remedial work at the site under CERCLA, such as would be involved in soil and groundwater remediation. We are participating with a PRP group composed of approximately 100 parties in an agreement with the EPA to undertake a remedial investigation and feasibility study, which will be used by the EPA to determine what remedy, if any, should be required at the site. A draft remedial investigation was submitted to the EPA in August 1999, and a draft baseline risk assessment was submitted in October 1999. After review of the draft remedial investigation, the EPA requested and obtained additional sampling work from the PRP group. The final remedial
F-33
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
investigation was submitted in 2000, and the feasibility study is now expected to be completed in June 2003. Because the study, which involves extensive testing to characterize the existence, extent and nature of any contamination in order to determine potential remedies, has not yet been completed, an estimate of our potential liability cannot be made. Our share of waste allegedly sent to the site is reportedly not more than 1% of the total waste sent; therefore, even though CERCLA does provide for joint and several liability, we believe that any ultimate liability will not have a material adverse effect on our results of operations or financial condition.
Applied Biotech, a subsidiary in our clinical group business segment, formerly manufactured and supplied immunoassay pregnancy tests to Warner Lambert Co. (now part of Pfizer Inc.). Warner Lambert sold the tests to retailers who sell them over-the-counter to consumers. Applied Biotech supplied the product to Warner Lambert pursuant to a supply agreement that Warner Lambert claims required Applied Biotech to defend and indemnify Warner Lambert with respect to any liability arising out of claims that the product infringes any patents held by third parties. On January 8, 1999, Conopco, Inc. d/b/a Unipath Diagnostics Company filed a lawsuit against Warner Lambert in the U.S. District Court for the District of New Jersey. The Unipath Diagnostics business, along with this lawsuit, were subsequently sold to Inverness Medical Switzerland GmbH (“Inverness”). Inverness (as Conopco’s successor) claims in the suit that the Warner Lambert pregnancy test supplied by Applied Biotech infringes certain patents owned by Inverness. Applied Biotech agreed to defend the lawsuit on behalf of Warner Lambert. In November 2000, the U.S. District Court granted a motion for summary judgment in favor of Warner Lambert and Applied Biotech, ruling that Applied Biotech’s product does not infringe the patents. The U.S. Court of Appeals vacated the summary judgment and held that the case should be returned to the trial court for further consideration. A Petition for Rehearing has been filed in the U.S. Court of Appeals. We believe, although there can be no assurance that, the resolution of this lawsuit will not have a material adverse effect on our results of operations or financial condition. Additionally, on October 15, 2002, Armkel, LLC sued Pfizer in the U.S. District Court for the District of New Jersey for patent infringement with respect to these same products. To date, Applied Biotech has not agreed to defend this lawsuit on behalf of Pfizer. Applied Biotech does not believe that it has an obligation to defend and indemnify Pfizer with respect to this lawsuit. Further, it believes that there are meritorious defenses to the patent claims.
The Company or its subsidiaries are parties to a number of lawsuits or subject to claims arising out of their respective operations, or the operation of businesses divested since the 1980’s for which certain of the Company’s subsidiaries may continue to have legal or contractual liability, including product liability, patent and trademark or other intellectual property infringement, contractual liability, workplace safety and environmental claims and cases, some of which involve claims for substantial damages. The Company and its subsidiaries vigorously defend lawsuits and other claims against them. Based on our assessment of the cases and claims against us and our experiences with similar cases and claims in the past, the Company believes that any liabilities which might reasonably result from any of the pending cases and claims would not have a material adverse effect on the results of operations or financial condition of the Company. However, there can be no assurance as to this, or that we will not be subjected to significant additional claims in the future with respect to our current or former operations which would have such a material adverse effect. The Company does not reduce legal or contractual liabilities for possible recoveries from insurance companies.
On September 30, 1999, the Company assigned its rights to receive in 2022 a Treasury with a par value of $50,000 to an unrelated third party. The third party has also agreed to assume any obligations for which the security has been pledged.
14. Capital Stock
Stock Option Plans: The Company has six stock option plans. As of September 30, 2002, there were options with respect to 4,986 shares of Common Stock outstanding under the 1988 Stock Option Plan (the “1988 Plan”), and there were no shares available for the granting of options under such plan; there were options with
F-34
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
respect to 20,871 shares of Common Stock outstanding under the 1990 Stock Option Plan (the “1990 Plan”) and there were no shares remaining available for the granting of options under such plan; there were options with respect to 8,479,889 shares of Common Stock outstanding under the Amended and Restated 1993 Long-Term Incentive Plan (the “1993 Plan”) and there were no shares remaining available for the granting of options under such plan; there were options with respect to 2,569,740 shares of Common Stock outstanding under the 2001 Equity Incentive Plan and there were 4,430,260 shares remaining available for granting options under such plan; there were options with respect to 403,893 shares of Common Stock outstanding under the Amended and Restated 1994 Outside Directors’ Stock Option Plan (the “1994 Outside Directors’ Plan”), and there were no shares available for the granting of options under such plan; there were options with respect to 305,426 shares of Common Stock outstanding under the 1999 Outside Directors’ Stock Option Plan (the “1999 Outside Directors’ Plan”), and there were no shares remaining available for the granting of options under such plan.
On December 11, 2000, in connection with the Spin-Off of SDS, certain employees of SDS exchanged 1,320,515 outstanding options to purchase Apogent common stock for 2,331,214 options to purchase Sybron Dental Specialties, Inc. common stock. All remaining stock options (owned by remaining employees and directors of the Company) were adjusted by adjusting the exercise price and the number of shares subject to each such option to reflect the change in market value of the Company’s common stock resulting from the Spin-Off, so that the intrinsic value of the options (the spread between the market value and the exercise price of the option shares) after the Spin-Off was equal to their intrinsic value immediately prior to the Spin-Off. The spread on options for fractional shares resulting from the exchange or adjustment was paid in cash. As a result of these exchanges and adjustments, the number of outstanding employee and director stock options increased by 1,449,749 and the average exercise price decreased by approximately $3.80.
| | | Number of
Shares
| | | Price Per Share
| | Weighted Average
Exercise Price
|
Options outstanding at September 30, 1999 | | 9,027,771 | | | $ 6.06 -$26.75 | | $ | 17.88 |
Granted | | 764,040 | | | 22.00 - 32.00 | | | 24.05 |
Exercised | | (1,167,775 | ) | | 6.06 - 26.75 | | | 10.79 |
Canceled and available for reissue | | (230,378 | ) | | 11.54 - 26.75 | | | 24.13 |
| | |
|
| | | | | |
Options outstanding at September 30, 2000 | | 8,393,658 | | | 6.36 - 32.00 | | | 19.27 |
Effect on outstanding options from Spin-Off of SDS | | 1,449,749 | | | | | | |
Granted | | 953,443 | | | 21.58 - 24.52 | | | 22.69 |
Exercised | | (706,522 | ) | | 5.10 - 21.46 | | | 9.29 |
Canceled and available for reissue | | (151,880 | ) | | 12.31 - 25.67 | | | 20.47 |
| | |
|
| | | | | |
Options outstanding at September 30, 2001 | | 9,938,448 | | | 5.10 - 24.84 | | | 17.10 |
Granted | | 3,111,640 | | | 19.20 - 25.10 | | | 25.01 |
Exercised | | (1,082,688 | ) | | 5.10 - 22.24 | | | 8.15 |
Canceled and available for reissue | | (127,745 | ) | | 18.40 - 25.10 | | | 22.87 |
| | |
|
| | | | | |
Options outstanding at September 30, 2002 | | 11,839,655 | | | 5.10 - 25.10 | | | 19.95 |
| | |
|
| | | | | |
Granted | | 2,601,700 | | | 16.67 - 16.67 | | | 16.67 |
Exercised | | (275,705 | ) | | 5.10 - 19.10 | | | 9.15 |
Canceled and available for reissue | | (201,704 | ) | | 18.40 - 25.10 | | | 23.20 |
| | |
|
| | | | | |
Options outstanding at March 31, 2003 | | 13,963,946 | | | $ 5.10 -$25.10 | | $ | 19.51 |
| | |
|
| | | | | |
Options exercisable at September 30, 2002 | | 6,184,794 | | | $ 5.10 -$25.10 | | $ | 17.32 |
| | |
|
| | | | | |
Options exercisable at March 31, 2003 | | 8,483,540 | | | $ 5.10 -$25.10 | | $ | 18.63 |
| | |
|
| | | | | |
Options available for grant at March 31, 2003 | | 1,929,997 | | | | | | |
| | |
|
| | | | | |
F-35
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
The range of exercise prices for options outstanding at March 31, 2003 was $5.10 to $25.10. The range of exercise prices for options is wide due to the increasing price of the Company’s stock (upon which the exercise price is based) over the period of the grants.
The following table summarizes information about options outstanding and outstanding and exercisable on September 30, 2002:
Range of Exercise Prices
| | Options Outstanding
| | Options Outstanding
and Exercisable
|
| | Number of
Shares
| | Weighted
Average
Remaining
Contractual
Life
| | Weighted
Average
Exercise
Price
| | Number of
Shares
| | Weighted
Average
Exercise
Price
|
$ 5.01-$10.00 | | 1,608,330 | | 2.3 | | $ | 7.31 | | 1,608,330 | | $ | 7.31 |
$10.01-$15.00 | | 168,881 | | 4.0 | | $ | 12.33 | | 168,881 | | $ | 12.33 |
$15.01-$20.00 | | 4,342,315 | | 5.7 | | $ | 19.47 | | 4,157,270 | | $ | 19.52 |
$20.01-$25.00 | | 3,192,389 | | 8.0 | | $ | 23.30 | | 1,076,946 | | $ | 22.43 |
$25.01-$30.00 | | 2,527,740 | | 9.3 | | $ | 25.10 | | 110,600 | | $ | 25.10 |
1988, 1990 and 1993 Plans
No options may be granted under the plans after ten years from the date the plans are approved by the shareholders of the Company. Options granted pursuant to the plans shall be either incentive options, which are intended to meet the requirements of section 422 of the Code, or nonstatutory options. The exercise price of the options is determined by the Compensation Committee. The exercise price of any incentive option shall not be less than the fair market value per share of the Common Stock on the date of the grant of such option. An optionee under the plans must pay the full option price of an option either (a) in cash or its equivalent, (b) with the Compensation Committee’s consent, by delivering previously acquired shares of Common Stock having a fair market value at the time of the exercise equal to the total option price, (c) with the Compensation Committee’s consent, by a cashless exercise as permitted under The Federal Reserve Board’s Regulation T, or (d) in any combination of the foregoing.
In general, options granted under the 1990 Plan after May 14, 1992, and under the 1993 Plan, vest in equal annual installments on each of the first four anniversaries following the date of grant. The Company made significant management changes in connection with the Spin-Off, including a change in the Chief Executive Officer, Chief Financial Officer and General Counsel. The Board of Directors and the Compensation Committee amended certain stock options previously granted to each of the executive officers so replaced to provide for the vesting of any unvested portion of the options granted to each of them in April of 1998. These options were also amended to provide for a five-year period (rather than a three month period) to exercise the options after termination of employment. The amendments to these options had no earnings impact because the options had no intrinsic value (i.e. there was no positive spread between the market price and exercise price of the option shares) at the time of the amendment.
Outside Directors’ Plans
The 1994 Outside Directors’ Plan provided for the automatic granting of nonstatutory stock options to those of the Company’s directors who qualified as “outside directors” at the time of grant. Following each annual meeting of shareholders prior to September 30, 1998, the plan’s expiration date, each outside director was
F-36
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
automatically granted an option to purchase 12,000 shares of Common Stock at an exercise price equal to the fair market value of the Common Stock on the date of grant. Each option granted under the 1994 Outside Directors’ Plan became exercisable six months after the date of grant, regardless of whether the grantee was still a director of the Company on such date. All rights to exercise an option granted under the 1994 Outside Directors’ Plan terminate upon the earlier of ten years from the date of grant or two years from the date the grantee ceases to be a director of the Company. The exercise price must be paid in full at the time of exercise, and such payment may be made in cash, by delivering shares of Common Stock which the optionee or the optionee’s spouse or both have beneficially owned for at least six months prior to the time of exercise, or through a combination of cash and such delivered Common Stock.
The 1999 Outside Directors’ Plan provided for the automatic granting of nonstatutory stock options to those of the Company’s directors who qualified as “outside directors” at the time of grant. Following each annual meeting of shareholders beginning in 1999, when the plan was approved by the shareholders, until the annual meeting of shareholders in 2002, when the 2001 Equity Incentive Plan was approved by the shareholders and replaced the 1999 Outside Directors’ Plan as to future grants, each outside director was automatically granted an option to purchase 12,000 shares of Common Stock at an exercise price equal to the fair market value of the Common Stock on the date of grant. Each option granted under the 1999 Outside Directors’ Plan is exercisable immediately upon grant. All rights to exercise an option granted under the 1999 Outside Directors’ Plan terminate upon the earlier of ten years from the date of grant or two years from the date the grantee ceases to be a director of the Company. The exercise price must be paid in full at the time of exercise, and such payment may be made in cash, by delivering shares of Common Stock which the optionee or the optionee’s spouse or both have beneficially owned for at least six months prior to the time of exercise, or through a combination of cash and such delivered Common Stock.
2001 Equity Incentive Plan
On December 7, 2001, the Board of Directors approved and adopted the Apogent Technologies Inc. 2001 Equity Incentive Plan and on January 28, 2002 the shareholders approved the plan. The Equity Incentive Plan replaces the 1993 Plan and the 1999 Outside Directors’ Plan as to future grants and awards. The principal objectives of the Equity Incentive Plan are to promote the success and enhance the value of the Company by linking the personal interests of participants to those of Company shareholders and providing participants with annual and long-term incentives for outstanding performance. Options granted pursuant to this plan may be either incentive options, which are intended to meet the requirements of section 422 of the code, or nonstatutory options. The exercise price of the options is determined by the Compensation Committee. The exercise price of any incentive option shall not be less than the fair market value per share of the Common Stock on the date of the grant of such option. An optionee under the plans must pay the full option price of an option either (a) in cash or its equivalent, (b) with the Compensation Committee’s consent, by delivering previously acquired shares of Common Stock having a fair market value at the time of the exercise equal to the total option price, (c) with the Compensation Committee’s consent, by a cashless exercise as permitted under The Federal Reserve Board’s Regulation T, or (d) in any combination of the foregoing. In general, options granted to employees vest over a four-year period following the date of grant, and options granted to directors vest immediately.
The Company has adopted the disclosure provisions of SFAS No. 148, “Accounting for Stock-Based Compensation—Transition and Disclosure,” which is an amendment of SFAS No. 123, “Accounting for Stock-Based Compensation,” and continues to apply Accounting Principles Board Opinion No. 25 and related interpretations in accounting for its stock plans. If the Company had elected to recognize compensation cost for
F-37
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
all of the plans based upon the fair value at the grant dates for awards under those plans, consistent with the method prescribed by SFAS No. 123, net income and earnings per share would have been changed to the pro forma amounts indicated below:
| | | Six Months Ended
March 31,
| | Year Ended September 30,
|
| | | 2003
| | | 2002
| | 2002
| | 2001
| | 2000
|
Pro forma net income | | $ | (33,770 | ) | | $ | 40,317 | | $ | 108,760 | | $ | 84,057 | | $ | 116,847 |
Basic pro forma earnings per share | | | (0.32 | ) | | | 0.38 | | | 1.02 | | | 0.80 | | | 1.12 |
Diluted pro forma earnings per share | | | (0.32 | ) | | | 0.37 | | | 1.00 | | | 0.78 | | | 1.09 |
The fair value of the Company’s stock options used to compute pro forma net income and earnings per share disclosures is the estimated present value at grant date using the Black-Scholes option pricing model with the following weighted average assumptions:
| | | 2003
| | 2002
| | 2001
| | 2000
|
Volatility | | 27.29% | | 27.29% | | 35.80% | | 35.0% |
Risk-free interest rate | | 3.69% | | 3.69% | | 5.66% | | 6.50% |
Expected holding period | | 8.0 years | | 8.0 years | | 7.8 years | | 7.8 years |
Dividend yield | | 0% | | 0% | | 0% | | 0% |
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions, including the expected stock price volatility. Because the Company’s options have characteristics significantly different from traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in the opinion of management, the existing models do not necessarily provide a reliable single value of its options and may not be representative of the future effects on reported net income or the future stock price of the Company. The weighted average estimated fair value of employee stock options granted in 2002, 2001, and 2000 was $10.25, $9.25, and $14.54, respectively. For purposes of pro forma disclosure, the estimated fair value of the options is amortized to expense over the options’ vesting period.
Employee Stock Purchase Plan
On January 28, 2002, the shareholders approved the Company’s Employee Stock Purchase Plan (ESPP). The ESPP consists of a series of overlapping 6-month offering periods. Eligible employees may purchase Company Common Stock through payroll deductions at a price equal to 85% of the lower of the fair market value of the Common Stock at the beginning of each offering period or end of such period. Participation is limited to 10% of an employee’s eligible compensation not to exceed amounts allowed by the Internal Revenue Code. As of March 31, 2003, 1,600,000 shares of Common Stock were authorized and 1,557,777 shares are available for future issuance under the ESPP.
Equity Rights
As of March 31, 2003, the Company holds 220 shares of treasury stock for delivery to equity right holders who have not yet surrendered their certificates. Equity right holders are entitled to receive 4.375 shares of Common Stock upon surrender of such certificates.
15. Segment Information
We recently realigned our lines of business for financial reporting purposes. Our three former business segments (clinical diagnostics, labware and life sciences, and laboratory equipment) have been reclassified into
F-38
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
two business segments: clinical group and research group. The clinical group business segment is the former clinical diagnostics business segment. The research group business segment is composed of the former labware and life sciences and laboratory equipment business segments.
The Company’s operating subsidiaries are engaged primarily in the manufacture and sale of laboratory products in the United States and other countries. The Company’s products are categorized in the business segments of: the clinical group and the research group. A description of the business segments follows:
Clinical Group
Our clinical group manufactures and sells products primarily to clinical and commercial laboratories and to scientific research and industrial customers. These products are used in a number of diagnostic applications, including specimen collection, specimen transportation, drug testing, therapeutic drug monitoring, infectious disease detection, and glucose tolerance testing, other applications include anatomical pathology (histology and cytology) and immunohistochemistry, with an emphasis on cancer applications. Clinical group products include:
| | — | microscope slides, cover glass, and glass tubes and vials; |
| | — | histology and immunochemistry instrumentation; |
| | — | sample vials used in diagnostic testing; |
| | — | diagnostic reagents; and |
| | — | other products used in detecting causes of various infectious diseases, conditions, and therapeutic drugs or drugs of abuse. |
Research Group
Our research group manufactures, distributes and sells products primarily to the research and clinical life sciences industries. Applications of these products include general everyday laboratory uses as well as genomics, proteomics, high-throughput screening for drug discovery, combinatorial chemistry, cell culture, filtration, and liquid handling. In addition, this segment manufactures, distributes, and sells basic laboratory equipment used by medical, pharmaceutical, and scientific laboratories. Research group products include:
| | — | reusable plastic and glass products (e.g., bottles, carboys, graduated ware, beakers, flasks, and plastic bottles for consumer use); |
| | — | disposable plastic and glass products; |
| | — | products for critical packaging applications; |
| | — | environmental and safety containers; |
| | — | liquid handling automation products; |
| | — | autosampler vials and seals used in chromatography analysis; |
F-39
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
| | — | various consumable products for use in applications of cell culture, filtration, molecular biology, cryopreservation, immunology, electrophoresis, liquid handling, genomics, and high-throughput screening for pharmaceutical drug discovery; and |
| | — | heating, cooling, shaking, stirring, mixing, and temperature control instruments. |
Inter-business segment sales are not material. Information on these business segments is summarized as follows:
| | | Clinical
Group
| | Research
Group
| | Eliminations
| | | Total(a)
|
Six Months Ended March 31, 2003 | | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | | |
External customer | | $ | 251,930 | | $ | 282,506 | | $ | — | | | $ | 534,436 |
Intersegment | | | 5,110 | | | 282 | | | (5,392 | ) | | | — |
Total revenues | | | 257,040 | | | 282,788 | | | (5,392 | ) | | | 534,436 |
Gross profit | | | 117,867 | | | 138,203 | | | — | | | | 256,070 |
Selling general and administrative | | | 58,288 | | | 81,984 | | | — | | | | 140,272 |
Operating income | | | 59,579 | | | 56,219 | | | — | | | | 115,798 |
Segment assets | | | 982,228 | | | 1,002,502 | | | — | | | | 1,984,730 |
| | | | |
Six Months Ended March 31, 2002 | | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | | |
External customer | | $ | 228,383 | | $ | 260,679 | | $ | — | | | $ | 489,062 |
Intersegment | | | 2,932 | | | 416 | | | (3,348 | ) | | | — |
Total revenues | | | 231,315 | | | 261,095 | | | (3,348 | ) | | | 489,062 |
Gross profit | | | 110,235 | | | 128,708 | | | — | | | | 238,943 |
Selling general and administrative | | | 51,978 | | | 71,558 | | | — | | | | 123,536 |
Operating income | | | 58,257 | | | 57,150 | | | — | | | | 115,407 |
| | | | |
2002 | | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | | |
External customer | | $ | 473,388 | | $ | 554,525 | | $ | — | | | $ | 1,027,913 |
Intersegment | | | 6,803 | | | 566 | | | (7,369 | ) | | | — |
Total revenues | | | 480,191 | | | 555,091 | | | (7,369 | ) | | | 1,027,913 |
Gross profit | | | 228,647 | | | 277,247 | | | — | | | | 505,894 |
Selling general and administrative | | | 105,261 | | | 152,693 | | | — | | | | 257,954 |
Operating income | | | 123,386 | | | 124,554 | | | — | | | | 247,940 |
Depreciation and amortization | | | 26,095 | | | 29,319 | | | — | | | | 55,414 |
Interest income | | | 601 | | | 472 | | | — | | | | 1,073 |
Interest expense | | | 18,892 | | | 22,918 | | | — | | | | 41,810 |
Segment assets | | | 998,320 | | | 1,037,765 | | | — | | | | 2,036,085 |
Expenditures for property, plant and equipment | | | 29,570 | | | 34,060 | | | — | | | | 63,630 |
F-40
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
| | | Clinical
Group
| | | Research
Group
| | | Eliminations
| | | Total(a)
| |
| | | | |
2001 | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | |
External customer | | 431,193 | | | 507,626 | | | — | | | 938,819 | |
Intersegment | | 7,001 | | | 1,132 | | | (8,133 | ) | | — | |
Total revenues | | 438,194 | | | 508,758 | | | (8,133 | ) | | 938,819 | |
Gross profit | | 214,250 | | | 253,252 | | | — | | | 467,502 | |
Selling general and administrative | | 110,720 | | | 145,765 | | | — | | | 256,485 | |
Operating income | | 103,530 | | | 107,486 | | | — | | | 211,016 | |
Depreciation and amortization | | 35,161 | | | 38,187 | | | — | | | 73,348 | |
Interest income | | 558 | | | 355 | | | — | | | 913 | |
Interest expense | | 19,548 | | | 30,185 | | | — | | | 49,733 | |
Segment assets | | 899,036 | | | 929,044 | | | — | | | 1,828,080 | |
Expenditures for property, plant and equipment | | 22,780 | | | 27,340 | | | — | | | 50,120 | |
| | | | |
2000 | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | |
External customer | | 390,097 | | | 453,470 | | | — | | | 843,567 | |
Intersegment | | 5,436 | | | 1,203 | | | (6,639 | ) | | — | |
Total revenues | | 395,153 | | | 455,053 | | | (6,639 | ) | | 843,567 | |
Gross profit | | 190,756 | | | 226,807 | | | — | | | 417,563 | |
Selling general and administrative | | 102,026 | | | 131,992 | | | — | | | 234,018 | |
Operating income | | 88,730 | | | 94,815 | | | — | | | 183,545 | |
Depreciation and amortization | | 28,416 | | | 33,975 | | | — | | | 62,391 | |
Interest income | | 397 | | | 299 | | | — | | | 696 | |
Interest expense | | (9,998 | ) | | (40,282 | ) | | — | | | (50,280 | ) |
Segment assets | | 831,213 | | | 813,049 | | | — | | | 1,644,262 | |
Expenditures for property, plant and equipment | | 17,847 | | | 23,477 | | | — | | | 41,324 | |
| (a) | Includes the elimination of intercompany and corporate office activity. |
The Company’s international operations are conducted principally in Europe. Inter-geographic sales are made at prices approximating market.
| | | 2002
| | 2001
| | 2000
|
Net Sales: | | | | | | | | | |
United States: | | | | | | | | | |
Customers | | $ | 736,839 | | $ | 694,003 | | $ | 632,989 |
Inter-geographic | | | 73,810 | | | 64,035 | | | 55,616 |
| | |
|
| |
|
| |
|
|
| | | | 810,649 | | | 758,038 | | | 688,605 |
| | |
|
| |
|
| |
|
|
Europe: | | | | | | | | | |
Customers | | | 178,448 | | | 149,788 | | | 126,969 |
Inter-geographic | | | 56,473 | | | 38,271 | | | 28,059 |
| | |
|
| |
|
| |
|
|
| | | | 234,921 | | | 188,059 | | | 155,028 |
| | |
|
| |
|
| |
|
|
F-41
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
| | | 2002
| | | 2001
| | | 2000
| |
All other areas: | | | | | | | | | | | | |
Customers | | | 112,626 | | | | 95,027 | | | | 83,609 | |
Inter-geographic | | | 10,674 | | | | 7,893 | | | | 8,934 | |
| | |
|
|
| |
|
|
| |
|
|
|
| | | | 140,996 | | | | 102,920 | | | | 92,543 | |
Inter-geographic sales | | | (140,957 | ) | | | (110,199 | ) | | | (92,609 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Total net sales | | $ | 1,027,913 | | | $ | 938,818 | | | $ | 843,567 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net Property: | | | | | | | | | | | | |
United States | | $ | 204,815 | | | $ | 177,834 | | | $ | 168,472 | |
Europe | | | 64,847 | | | | 44,911 | | | | 38,766 | |
All other areas | | | 1,231 | | | | 942 | | | | 856 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total net property | | $ | 270,893 | | | $ | 223,687 | | | $ | 208,094 | |
| | |
|
|
| |
|
|
| |
|
|
|
Major customer information:
During 2002, 2001, and 2000, one customer, Fisher Scientific, accounted for 10% or more of the Company’s net revenues. During 2002 and 2001, another customer, VWR, accounted for 10% or more of the Company’s net revenues. The table below lists by segment the 2002, 2001 and 2000 sales to Fisher Scientific and the 2002 and 2001 sales to VWR.
| | | Fisher Scientific
| | VWR
|
| | | 2002
| | 2001
| | 2000
| | 2002
| | 2001
|
Clinical Group | | $ | 48,649 | | $ | 34,876 | | $ | 39,286 | | $ | 12,229 | | $ | 10,162 |
Research Group | | | 92,254 | | | 85,516 | | | 78,536 | | | 100,999 | | | 83,361 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total | | $ | 140,903 | | $ | 120,392 | | $ | 117,822 | | $ | 113,228 | | $ | 93,523 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
F-42
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
16. Quarterly Financial Information (Unaudited)
| | | First
Quarter
| | | Second
Quarter
| | | Third
Quarter
| | | Fourth
Quarter
| | Total
Year
| |
2002 | | | | | | | | | | | | | | | | | | | |
Net sales | | | | | | | | | | | | | | | | | | | |
Clinical Group | | $ | 109,752 | | | $ | 118,686 | | | $ | 122,781 | | | $ | 122,169 | | $ | 473,388 | |
Research Group | | | 124,221 | | | | 136,402 | | | | 144,411 | | | | 149,491 | | | 554,525 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
| | | $ | 233,973 | | | $ | 255,088 | | | $ | 267,192 | | | $ | 271,660 | | $ | 1,027,913 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Gross profit | | $ | 114,285 | | | $ | 124,658 | | | $ | 130,447 | | | $ | 136,504 | | $ | 505,894 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Income from continuing operations | | | 29,063 | | | | 31,419 | | | | 33,947 | | | | 35,738 | | | 130,167 | |
Discontinued operation | | | 908 | | | | (12,470 | ) | | | 2,126 | | | | 418 | | | (9,018 | ) |
Income before extraordinary items | | | 29,971 | | | | 18,949 | | | | 36,073 | | | | 36,156 | | | 121,149 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net income | | $ | 29,971 | | | $ | 18,949 | | | $ | 36,073 | | | $ | 36,156 | | $ | 121,149 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Basic income per common share: | | | | | | | | | | | | | | | | | | | |
Income from continuing operations | | $ | 0.27 | | | $ | 0.30 | | | $ | 0.32 | | | $ | 0.34 | | $ | 1.22 | |
Discontinued operation | | | 0.01 | | | | (0.12 | ) | | | 0.02 | | | | 0.00 | | | (0.08 | ) |
Net income per common share | | | 0.28 | | | | 0.18 | | | | 0.34 | | | | 0.34 | | | 1.14 | |
Diluted income per common share: | | | | | | | | | | | | | | | | | | | |
Income from continuing operations | | $ | 0.27 | | | $ | 0.29 | | | $ | 0.31 | | | $ | 0.33 | | $ | 1.20 | |
Discontinued operation | | | 0.01 | | | | (0.11 | ) | | | 0.02 | | | | 0.00 | | | (0.08 | ) |
Net income per common share | | | 0.28 | | | | 0.17 | | | | 0.33 | | | | 0.34 | | | 1.11 | |
| | | | | |
2001 | | | | | | | | | | | | | | | | | | | |
Net sales | | | | | | | | | | | | | | | | | | | |
Clinical Group | | $ | 100,163 | | | $ | 106,975 | | | $ | 111,744 | | | $ | 112,311 | | $ | 431,193 | |
Research Group | | | 112,089 | | | | 125,443 | | | | 134,121 | | | | 135,973 | | | 507,626 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
| | | $ | 212,252 | | | $ | 232,418 | | | $ | 245,865 | | | $ | 248,284 | | $ | 938,819 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Gross profit | | $ | 104,469 | | | $ | 117,146 | | | $ | 121,481 | | | $ | 124,405 | | $ | 467,501 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Income from continuing operations | | | 20,583 | | | | 27,536 | | | | 25,359 | | | | 27,926 | | | 101,404 | |
Discontinued operation | | | (9,375 | ) | | | 1,815 | | | | 2,726 | | | | 1,477 | | | (3,357 | ) |
Income before extraordinary items | | | 11,208 | | | | 29,351 | | | | 28,085 | | | | 29,403 | | | 98,047 | |
Extraordinary items | | | (745 | ) | | | — | | | | (1,361 | ) | | | — | | | (2,106 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net income | | $ | 10,456 | | | $ | 29,352 | | | $ | 26,742 | | | $ | 29,391 | | $ | 95,941 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Basic income per common share: | | | | | | | | | | | | | | | | | | | |
Income from continuing operations | | $ | 0.20 | | | $ | 0.26 | | | $ | 0.24 | | | $ | 0.26 | | $ | 0.96 | |
Discontinued operation | | | (0.09 | ) | | | 0.02 | | | | 0.03 | | | | 0.01 | | | (0.03 | ) |
Extraordinary items | | | (0.01 | ) | | | — | | | | (0.01 | ) | | | — | | | (0.02 | ) |
Net income per common share | | | 0.10 | | | | 0.28 | | | | 0.25 | | | | 0.28 | | | 0.91 | |
Diluted income per common share: | | | | | | | | | | | | | | | | | | | |
Income from continuing operations | | $ | 0.19 | | | $ | 0.26 | | | $ | 0.23 | | | $ | 0.26 | | $ | 0.94 | |
Discontinued operation | | | (0.09 | ) | | | 0.02 | | | | 0.03 | | | | 0.01 | | | (0.03 | ) |
Extraordinary items | | | (0.01 | ) | | | — | | | | (0.01 | ) | | | — | | | (0.02 | ) |
Net income per common share | | | 0.10 | | | | 0.27 | | | | 0.25 | | | | 0.27 | | | 0.89 | |
F-43
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In thousands except share and per share data)
17. Condensed Consolidating Financial Information
The Company’s material U.S. subsidiaries are guarantors of its Revolving Credit Facility, 8% Senior Notes, and senior convertible contingent debt securities (CODES). Each of the subsidiary guarantors is 100% owned by the Company. The guarantees are full and unconditional as well as joint and several.
Below are the condensed consolidating balance sheets as of March 31, 2003 and September 30, 2002, statements of operations for the three and six months ended March 31, 2003 and 2002, and statements of cash flows for the six months ended March 31, 2003 and 2002, of Apogent Technologies Inc. and its subsidiaries, reflecting the subsidiary guarantors for the Revolving Credit Facility, 8% Senior Notes, and CODES. For guarantors acquired during the period, the results of operations are included from the date of acquisition.
Certain general corporate expenses have not been allocated to the subsidiaries, and are included under the Apogent Technologies Inc. heading. As a matter of course, the Company retains certain assets and liabilities at the corporate level that are not allocated to the subsidiaries including, but not limited to, certain employee benefit, insurance, and tax liabilities. Income tax provisions for the subsidiaries are typically recorded using an estimate and finalized in total with an adjustment recorded at the corporate level. Certain debt under which Apogent Technologies Inc. is listed as the debtor has been allocated to the Guarantor subsidiaries. Intercompany balances include receivables/payables incurred in the normal course of business in addition to investments and loans transacted between subsidiaries or with Apogent Technologies Inc.
F-44
CONDENSED CONSOLIDATING BALANCE SHEETS
| | | As of March 31, 2003 (unaudited)
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Assets | | | | | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 16,868 | | | $ | — | | | $ | 6,418 | | | $ | (5,880 | ) | | $ | 17,406 | |
Accounts receivable, net | | | — | | | | 134,952 | | | | 41,989 | | | | — | | | | 176,941 | |
Inventories, net | | | 1,264 | | | | 155,815 | | | | 50,229 | | | | — | | | | 207,308 | |
Other current assets | | | 65,216 | | | | 12,096 | | | | 7,661 | | | | — | | | | 84,973 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total current assets | | | 83,348 | | | | 302,863 | | | | 106,297 | | | | (5,880 | ) | | | 486,628 | |
Property, plant and equipment, net | | | 12,105 | | | | 182,211 | | | | 71,973 | | | | — | | | | 266,289 | |
Intangible assets, net | | | 10,205 | | | | 942,136 | | | | 202,608 | | | | — | | | | 1,154,949 | |
Investment in subsidiaries | | | 2,032,306 | | | | 143,440 | | | | 34,695 | | | | (2,210,441 | ) | | | — | |
Other assets | | | 66,622 | | | | 9,194 | | | | 1,048 | | | | — | | | | 76,864 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total assets | | $ | 2,204,586 | | | $ | 1,579,844 | | | $ | 416,621 | | | $ | (2,216,321 | ) | | $ | 1,984,730 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| | | | | |
Liabilities and Shareholders’ Equity | | | | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 379 | | | $ | 39,180 | | | $ | 12,602 | | | $ | (5,880 | ) | | $ | 46,281 | |
Short-term debt and current portion of long-term debt | | | — | | | | 16,145 | | | | 88 | | | | — | | | | 16,233 | |
Income taxes payable | | | 4,687 | | | | 39,630 | | | | 7,393 | | | | (2,378 | ) | | | 49,332 | |
Accrued expenses and other current liabilities | | | 29,557 | | | | 13,639 | | | | 37,894 | | | | — | | | | 81,090 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total current liabilities | | | 34,623 | | | | 108,594 | | | | 57,977 | | | | (8,258 | ) | | | 192,936 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Long-term debt, less current portion | | | — | | | | 697,482 | | | | 38 | | | | — | | | | 697,520 | |
Securities lending agreement | | | 58,579 | | | | — | | | | — | | | | — | | | | 58,579 | |
Deferred income taxes | | | 105,364 | | | | 3,669 | | | | 14,367 | | | | — | | | | 123,400 | |
Other liabilities | | | 12,716 | | | | 2,866 | | | | 1,446 | | | | — | | | | 17,028 | |
Net intercompany payable/(receivable) | | | 805,824 | | | | (1,018,236 | ) | | | 212,376 | | | | 36 | | | | — | |
Commitments and contingent liabilities | | | — | | | | — | | | | — | | | | — | | | | — | |
Shareholders’ equity | | | | | | | | | | | | | | | | | | | | |
Preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | |
Common stock | | | 1,067 | | | | — | | | | — | | | | — | | | | 1,067 | |
Equity rights | | | — | | | | — | | | | — | | | | — | | | | — | |
Additional paid-in-capital | | | 250,155 | | | | 2,134,398 | | | | 77,902 | | | | (2,191,828 | ) | | | 270,627 | |
Retained earnings (deficit) | | | 1,031,112 | | | | (348,982 | ) | | | 55,283 | | | | (16,271 | ) | | | 721,142 | |
Accumulated other comprehensive income (loss) | | | (10,859 | ) | | | 53 | | | | (2,768 | ) | | | — | | | | (13,574 | ) |
Treasury stock (at cost) | | | (83,995 | ) | | | — | | | | — | | | | — | | | | (83,995 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total shareholders’ equity | | | 1,187,480 | | | | 1,785,469 | | | | 130,417 | | | | (2,208,099 | ) | | | 895,267 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total liabilities and shareholders’ equity | | $ | 2,204,586 | | | $ | 1,579,844 | | | $ | 416,621 | | | $ | (2,216,321 | ) | | $ | 1,984,730 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
F-45
CONDENSED CONSOLIDATING BALANCE SHEETS (Continued)
| | | As of September 30, 2002
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Assets | | | | | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 19,889 | | | $ | — | | | $ | 3,991 | | | $ | (7,553 | ) | | $ | 16,327 | |
Accounts receivable, net | | | — | | | | 148,637 | | | | 38,313 | | | | — | | | | 186,950 | |
Inventories, net | | | 1,263 | | | | 157,386 | | | | 51,413 | | | | (6,065 | ) | | | 203,997 | |
Other current assets | | | 20,703 | | | | 12,098 | | | | 6,954 | | | | (503 | ) | | | 39,252 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total current assets | | | 41,855 | | | | 318,121 | | | | 100,671 | | | | (14,121 | ) | | | 446,526 | |
Property, plant and equipment, net | | | 12,592 | | | | 193,008 | | | | 65,293 | | | | — | | | | 270,893 | |
Intangible assets, net | | | 12,025 | | | | 994,596 | | | | 236,492 | | | | — | | | | 1,243,113 | |
Investment in subsidiaries | | | 2,090,958 | | | | 57,712 | | | | (1,185 | ) | | | (2,147,485 | ) | | | — | |
Other assets | | | 64,018 | | | | 10,561 | | | | 974 | | | | — | | | | 75,553 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total assets | | $ | 2,221,448 | | | $ | 1,573,998 | | | $ | 402,245 | | | $ | (2,161,606 | ) | | $ | 2,036,085 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| | | | | |
Liabilities and Shareholders’ Equity | | | | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 316 | | | $ | 49,178 | | | $ | 11,838 | | | $ | (7,553 | ) | | $ | 53,779 | |
Short-term debt and current portion of long-term debt | | | — | | | | 25,336 | | | | 10,656 | | | | — | | | | 35,992 | |
Income taxes payable | | | 45,102 | | | | — | | | | 10,240 | | | | (2,278 | ) | | | 53,064 | |
Accrued expenses and other current liabilities | | | 32,603 | | | | 26,574 | | | | 14,389 | | | | — | | | | 73,566 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total current liabilities | | | 78,021 | | | | 101,088 | | | | 47,123 | | | | (9,831 | ) | | | 216,401 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Long-term debt, less current portion | | | — | | | | 634,995 | | | | 25 | | | | — | | | | 635,020 | |
Securities lending agreement | | | 60,183 | | | | — | | | | — | | | | — | | | | 60,183 | |
Deferred income taxes | | | 116,568 | | | | 1,190 | | | | 14,342 | | | | — | | | | 132,100 | |
Other liabilities | | | 12,525 | | | | 3,284 | | | | 1,434 | | | | — | | | | 17,243 | |
Net intercompany payable/(receivable) | | | 748,402 | | | | (960,623 | ) | | | 216,305 | | | | (4,084 | ) | | | — | |
Commitments and contingent liabilities | | | — | | | | — | | | | — | | | | — | | | | — | |
Shareholders’ equity | | | | | | | | | | | | | | | | | | | | |
Preferred stock | | | — | | | | — | | | | — | | | | — | | | | — | |
Common stock | | | 1,070 | | | | — | | | | — | | | | — | | | | 1,070 | |
Equity rights | | | — | | | | — | | | | — | | | | — | | | | — | |
Additional paid-in-capital | | | 251,210 | | | | 2,070,551 | | | | 78,793 | | | | (2,128,872 | ) | | | 271,682 | |
Retained earnings (deficit) | | | 991,114 | | | | (269,931 | ) | | | 50,547 | | | | (22,939 | ) | | | 748,791 | |
Accumulated other comprehensive loss | | | (17,659 | ) | | | (6,556 | ) | | | (6,324 | ) | | | 4,120 | | | | (26,419 | ) |
Treasury stock (at cost) | | | (19,986 | ) | | | — | | | | — | | | | — | | | | (19,986 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total shareholders’ equity | | | 1,205,749 | | | | 1,794,064 | | | | 123,016 | | | | (2,147,691 | ) | | | 975,138 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total liabilities and shareholders’ equity | | $ | 2,221,448 | | | $ | 1,573,998 | | | $ | 402,245 | | | $ | (2,161,606 | ) | | $ | 2,036,085 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
F-46
CONDENSED CONSOLIDATING BALANCE SHEETS (Continued)
| | | As of September 30, 2001
| |
| | | Apogent
Technologies
| | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Assets | | | | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 4,145 | | $ | — | | | $ | 10,699 | | | $ | (5,652 | ) | | $ | 9,192 | |
Accounts receivable, net | | | — | | | 146,981 | | | | 36,297 | | | | — | | | | 183,278 | |
Inventories, net | | | 1,263 | | | 136,906 | | | | 33,335 | | | | (4,068 | ) | | | 167,436 | |
Other current assets | | | 14,099 | | | 13,458 | | | | 5,969 | | | | (406 | ) | | | 33,120 | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total current assets | | | 19,507 | | | 297,345 | | | | 86,300 | | | | (10,126 | ) | | | 393,026 | |
Property, plant and equipment, net | | | 9,553 | | | 169,032 | | | | 45,102 | | | | — | | | | 223,687 | |
Intangible assets, net | | | 7,003 | | | 913,651 | | | | 219,680 | | | | — | | | | 1,140,334 | |
Investment in subsidiaries | | | 1,593,800 | | | 46,461 | | | | — | | | | (1,640,261 | ) | | | — | |
Other assets | | | 58,605 | | | 11,543 | | | | 885 | | | | — | | | | 71,033 | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total assets | | $ | 1,688,468 | | $ | 1,438,032 | | | $ | 351,967 | | | $ | (1,650,387 | ) | | $ | 1,828,080 | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| | | | | |
Liabilities and Shareholders’ Equity | | | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 787 | | $ | 46,802 | | | $ | 11,885 | | | $ | (5,652 | ) | | $ | 53,822 | |
Short-term debt and current portion of long-term debt | | | 378 | | | 73,228 | | | | 36 | | | | — | | | | 73,642 | |
Income taxes payable | | | 33,432 | | | — | | | | 6,638 | | | | (1,323 | ) | | | 38,747 | |
Accrued expenses and other current liabilities | | | 32,873 | | | 28,603 | | | | 14,968 | | | | — | | | | 76,444 | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total current liabilities | | | 67,470 | | | 148,633 | | | | 33,527 | | | | (6,975 | ) | | | 242,655 | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Long-term debt, less current portion | | | — | | | 583,765 | | | | 23 | | | | — | | | | 583,788 | |
Securities lending agreement | | | 55,072 | | | — | | | | — | | | | — | | | | 55,072 | |
Deferred income taxes | | | 68,264 | | | 20,778 | | | | 12,031 | | | | — | | | | 101,073 | |
Other liabilities | | | 3,231 | | | 2,453 | | | | 1,318 | | | | — | | | | 7,002 | |
Net intercompany payable/(receivable) | | | 375,705 | | | (599,911 | ) | | | 224,169 | | | | 37 | | | | — | |
Commitments and contingent liabilities | | | — | | | — | | | | — | | | | — | | | | — | |
Shareholders’ equity | | | | | | | | | | | | | | | | | | | |
Preferred stock | | | — | | | — | | | | — | | | | — | | | | — | |
Common stock | | | 1,059 | | | — | | | | — | | | | — | | | | 1,059 | |
Equity rights | | | — | | | — | | | | — | | | | — | | | | — | |
Additional paid-in-capital | | | 234,166 | | | 1,561,854 | | | | 80,265 | | | | (1,621,648 | ) | | | 254,637 | |
Retained earnings (deficit) | | | 880,299 | | | (279,540 | ) | | | 48,684 | | | | (21,801 | ) | | | 627,642 | |
Accumulated other comprehensive loss | | | 3,202 | | | — | | | | (48,050 | ) | | | — | | | | (44,848 | ) |
Treasury stock (at cost) | | | — | | | — | | | | — | | | | — | | | | — | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total shareholders’ equity | | | 1,118,726 | | | 1,282,314 | | | | 80,899 | | | | (1,643,449 | ) | | | 838,490 | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total liabilities and shareholders’ equity | | $ | 1,688,468 | | $ | 1,438,032 | | | $ | 351,967 | | | $ | (1,650,387 | ) | | $ | 1,828,080 | |
| | |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
F-47
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS
| | | For the Six Months Ended March 31, 2003 (unaudited)
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Net sales | | $ | — | | | $ | 447,851 | | | $ | 127,339 | | | $ | (40,754 | ) | | $ | 534,436 | |
Cost of sales | | | — | | | | 238,943 | | | | 78,668 | | | | (39,245 | ) | | | 278,366 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | — | | | | 208,908 | | | | 48,671 | | | | (1,509 | ) | | | 256,070 | |
Selling, general and administrative expenses | | | 16,324 | | | | 93,943 | | | | 30,005 | | | | — | | | | 140,272 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | (16,324 | ) | | | 114,965 | | | | 18,666 | | | | (1,509 | ) | | | 115,798 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | — | | | | (20,666 | ) | | | (126 | ) | | | — | | | | (20,792 | ) |
Other, net | | | — | | | | (795 | ) | | | (228 | ) | | | — | | | | (1,023 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income before income taxes and discontinued operations | | | (16,324 | ) | | | 93,504 | | | | 18,312 | | | | (1,509 | ) | | | 93,983 | |
Income taxes | | | (6,509 | ) | | | 34,129 | | | | 6,684 | | | | — | | | | 34,304 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations | | | (9,815 | ) | | | 59,375 | | | | 11,628 | | | | (1,509 | ) | | | 59,679 | |
Loss from discontinued operations, net of tax | | | — | | | | (56,940 | ) | | | (30,388 | ) | | | — | | | | (87,328 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | (9,815 | ) | | $ | 2,435 | | | $ | (18,760 | ) | | $ | (1,509 | ) | | $ | (27,649 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| |
| | | For the Six Months Ended March 31, 2002 (unaudited)
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Net sales | | $ | — | | | $ | 417,482 | | | $ | 105,281 | | | $ | (33,701 | ) | | $ | 489,062 | |
Cost of sales | | | — | | | | 221,025 | | | | 61,534 | | | | (32,440 | ) | | | 250,119 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | — | | | | 196,457 | | | | 43,747 | | | | (1,261 | ) | | | 238,943 | |
Selling, general and administrative expenses | | | 14,941 | | | | 83,352 | | | | 25,243 | | | | — | | | | 123,536 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | (14,941 | ) | | | 113,105 | | | | 18,504 | | | | (1,261 | ) | | | 115,407 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | — | | | | (20,590 | ) | | | 12 | | | | — | | | | (20,578 | ) |
Other, net | | | — | | | | 504 | | | | 64 | | | | — | | | | 568 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income before income taxes and discontinued operations | | | (14,941 | ) | | | 93,019 | | | | 18,580 | | | | (1,261 | ) | | | 95,397 | |
Income taxes | | | (5,930 | ) | | | 34,045 | | | | 6,800 | | | | — | | | | 34,915 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations | | | (9,011 | ) | | | 58,974 | | | | 11,780 | | | | (1,261 | ) | | | 60,482 | |
Loss from discontinued operations, net of tax | | | — | | | | (11,040 | ) | | | (522 | ) | | | — | | | | (11,562 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | (9,011 | ) | | $ | 47,934 | | | $ | 11,258 | | | $ | (1,261 | ) | | $ | 48,920 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
F-48
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS (Continued)
| | | For the Year Ended September 30, 2002
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Net sales | | $ | — | | | $ | 882,184 | | | $ | 221,801 | | | $ | (76,072 | ) | | $ | 1,027,913 | |
Cost of sales | | | — | | | | 465,019 | | | | 131,075 | | | | (74,075 | ) | | | 522,019 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | — | | | | 417,165 | | | | 90,726 | | | | (1,997 | ) | | | 505,894 | |
Selling, general and administrative expenses | | | 26,717 | | | | 177,100 | | | | 54,137 | | | | — | | | | 257,954 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | (26,717 | ) | | | 240,065 | | | | 36,589 | | | | (1,997 | ) | | | 247,940 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | — | | | | (40,673 | ) | | | (64 | ) | | | — | | | | (40,737 | ) |
Other, net | | | (5,455 | ) | | | 3,463 | | | | 100 | | | | — | | | | (1,892 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income before income taxes and discontinued operation | | | (32,172 | ) | | | 202,855 | | | | 36,625 | | | | (1,997 | ) | | | 205,311 | |
Income taxes | | | (13,496 | ) | | | 75,106 | | | | 13,533 | | | | — | | | | 75,144 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income (loss) from continuing operations | | | (18,676 | ) | | | 127,749 | | | | 23,092 | | | | (1,997 | ) | | | 130,167 | |
Loss from discontinued operations, net of tax | | | — | | | | (7,683 | ) | | | (1,335 | ) | | | — | | | | (9,018 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | (18,676 | ) | | $ | 120,066 | | | $ | 21,757 | | | $ | (1,997 | ) | | $ | 121,149 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
| |
| | | For the Year Ended September 30, 2001
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Net sales | | $ | — | | | $ | 816,934 | | | $ | 175,538 | | | $ | (53,653 | ) | | $ | 938,819 | |
Cost of sales | | | — | | | | 423,111 | | | | 101,613 | | | | (53,406 | ) | | | 471,318 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | — | | | | 393,823 | | | | 73,925 | | | | (247 | ) | | | 467,501 | |
Selling, general and administrative expenses | | | 24,947 | | | | 187,032 | | | | 44,506 | | | | — | | | | 256,485 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | (24,947 | ) | | | 206,791 | | | | 29,419 | | | | (247 | ) | | | 211,016 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | — | | | | (48,886 | ) | | | 66 | | | | — | | | | (48,820 | ) |
Other, net | | | 3,636 | | | | 1,930 | | | | (886 | ) | | | — | | | | 4,680 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income before income taxes, discontinued operations and extraordinary item | | | (21,311 | ) | | | 159,835 | | | | 28,599 | | | | (247 | ) | | | 166,876 | |
Income taxes | | | (4,005 | ) | | | 59,183 | | | | 10,294 | | | | — | | | | 65,472 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations before extraordinary item | | | (17,306 | ) | | | 100,652 | | | | 18,305 | | | | (247 | ) | | | 101,404 | |
Discontinued operations, net of tax | | | (11,805 | ) | | | 8,006 | | | | 442 | | | | — | | | | (3,357 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income before extraordinary item | | | (29,111 | ) | | | 108,658 | | | | 18,747 | | | | (247 | ) | | | 98,047 | |
Extraordinary item | | | (2,106 | ) | | | — | | | | — | | | | — | | | | (2,106 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | (31,217 | ) | | $ | 108,658 | | | $ | 18,747 | | | $ | (247 | ) | | $ | 95,941 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
F-49
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS (Continued)
| | | For the Year Ended September 30, 2000
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | | Consolidated
| |
Net sales | | $ | — | | | $ | 740,310 | | | $ | 147,628 | | | $ | (44,371 | ) | | $ | 843,567 | |
Cost of sales | | | — | | | | 381,856 | | | | 87,533 | | | | (43,385 | ) | | | 426,004 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Gross profit | | | — | | | | 358,454 | | | | 60,095 | | | | (986 | ) | | | 417,563 | |
Selling, general and administrative expenses | | | 9,563 | | | | 187,934 | | | | 36,521 | | | | — | | | | 234,018 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Operating income | | | (9,563 | ) | | | 170,520 | | | | 23,574 | | | | (986 | ) | | | 183,545 | |
Other income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | (78 | ) | | | (44,024 | ) | | | (5,482 | ) | | | — | | | | (49,584 | ) |
Other, net | | | 776 | | | | 1,453 | | | | (1,431 | ) | | | — | | | | 798 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income before income taxes and discontinued operations | | | (8,865 | ) | | | 127,949 | | | | 16,661 | | | | (986 | ) | | | 134,759 | |
Income taxes | | | (2,595 | ) | | | 48,033 | | | | 8,624 | | | | (287 | ) | | | 53,775 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Income from continuing operations | | | (6,270 | ) | | | 79,919 | | | | 8,037 | | | | (699 | ) | | | 80,987 | |
Discontinued operations, net of tax | | | 41,597 | | | | 5,740 | | | | — | | | | — | | | | 47,337 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net income | | $ | 35,327 | | | $ | 85,656 | | | $ | 8,037 | | | $ | (699 | ) | | $ | 128,321 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
F-50
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS
| | | For the Six Months Ended March 31, 2003
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | Consolidated
| |
Cash flows provided by operating activities: | | $ | 62,689 | | | $ | (3,725 | ) | | $ | (2,000 | ) | | $ | — | | $ | 56,964 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | (619 | ) | | | (14,256 | ) | | | (7,685 | ) | | | — | | | (22,560 | ) |
Proceeds from sales of property, plant and equipment | | | — | | | | 383 | | | | 175 | | | | — | | | 558 | |
Net payments for businesses acquired | | | — | | | | (21,567 | ) | | | — | | | | — | | | (21,567 | ) |
Other investing activities | | | — | | | | 1,546 | | | | — | | | | — | | | 1,546 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash used in investing activities | | | (619 | ) | | | (32,762 | ) | | | (7,510 | ) | | | — | | | (42,023 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | |
Proceeds from long-term debt | | | — | | | | 323,100 | | | | — | | | | — | | | 323,100 | |
Principal payments on long-term debt | | | — | | | | (283,808 | ) | | | | | | | — | | | (283,808 | ) |
Proceeds from the exercise of stock options and stock purchase program | | | 3,272 | | | | — | | | | — | | | | — | | | 3,272 | |
Purchase of treasury stock | | | (69,328 | ) | | | — | | | | — | | | | — | | | (69,328 | ) |
Other | | | 965 | | | | — | | | | 993 | | | | — | | | 1,958 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | (65,091 | ) | | | 39,292 | | | | 993 | | | | — | | | (24,806 | ) |
Effect of exchange rate on cash and cash equivalents | | | — | | | | — | | | | 10,944 | | | | — | | | 10,944 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net increase (decrease) in cash and cash equivalents | | | (3,021 | ) | | | 1,673 | | | | 2,427 | | | | — | | | 1,079 | |
Cash and cash equivalents at beginning
of period | | | 19,889 | | | | (7,553 | ) | | | 3,991 | | | | — | | | 16,327 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash and cash equivalents at end
of period | | $ | 16,868 | | | $ | (5,880 | ) | | $ | 6,418 | | | $ | — | | $ | 17,406 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Supplemental disclosures of cash flow information | | | | | | | | | | | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | | | | | | | | | | | |
Interest | | $ | — | | | $ | 20,812 | | | $ | 175 | | | $ | — | | $ | 20,987 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Income taxes | | $ | 30,359 | | | $ | — | | | $ | 6,966 | | | $ | — | | $ | 37,325 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Capital lease obligations incurred | | $ | — | | | $ | 58 | | | $ | 10 | | | $ | — | | $ | 68 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
F-51
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS (Continued)
| | | For the Six Months Ended March 31, 2002
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | Consolidated
| |
Cash flows provided by operating activities: | | $ | 13,563 | | | $ | 57,458 | | | $ | 12,457 | | | $ | — | | $ | 83,478 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | (1,040 | ) | | | (11,269 | ) | | | (4,790 | ) | | | — | | | (17,099 | ) |
Proceeds from sales of property, plant and equipment | | | — | | | | 113 | | | | 155 | | | | — | | | 268 | |
Net payments for businesses acquired | | | — | | | | (113,780 | ) | | | — | | | | — | | | (113,780 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash used in investing activities | | | (1,040 | ) | | | (124,936 | ) | | | (4,635 | ) | | | — | | | (130,611 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | |
Proceeds from long-term debt | | | — | | | | 479,500 | | | | — | | | | — | | | 479,500 | |
Principal payments on long-term debt | | | — | | | | (387,988 | ) | | | (12 | ) | | | — | | | (388,000 | ) |
Proceeds from the exercise of stock options | | | 4,790 | | | | — | | | | — | | | | — | | | 4,790 | |
Other | | | (3,345 | ) | | | (5,232 | ) | | | — | | | | — | | | (8,577 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | 1,445 | | | | 86,280 | | | | (12 | ) | | | — | | | 87,713 | |
Effect of exchange rate on cash and cash equivalents | | | — | | | | — | | | | (7,499 | ) | | | — | | | (7,499 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net increase in cash and cash equivalents | | | 13,968 | | | | 18,802 | | | | 311 | | | | — | | | 33,081 | |
Cash and cash equivalents at beginning
of period | | | 4,145 | | | | (5,509 | ) | | | 10,699 | | | | — | | | 9,335 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash and cash equivalents at end
of period | | $ | 18,113 | | | $ | 13,293 | | | $ | 11,010 | | | $ | — | | $ | 42,416 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Supplemental disclosures of cash flow information | | | | | | | | | | | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | | | | | | | | | | | |
Interest | | $ | — | | | $ | 17,654 | | | $ | 165 | | | $ | — | | $ | 17,819 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Income taxes | | $ | 12,961 | | | $ | 390 | | | $ | 5,754 | | | $ | — | | $ | 19,105 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Capital lease obligations incurred | | $ | — | | | $ | 117 | | | $ | — | | | $ | — | | $ | 117 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
F-52
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS (Continued)
| | | For the Year Ended September 30, 2002
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | Consolidated
| |
Cash flows provided by operating activities: | | $ | 26,319 | | | $ | 154,629 | | | $ | 11,202 | | | $ | — | | $ | 192,150 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | (9,413 | ) | | | (33,802 | ) | | | (20,415 | ) | | | — | | | (63,630 | ) |
Proceeds from sales of property, plant and equipment | | | 2,981 | | | | — | | | | 2,026 | | | | — | | | 5,007 | |
Net payments for businesses acquired | | | — | | | | (139,735 | ) | | | — | | | | — | | | (139,735 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash used in investing activities | | | (6,432 | ) | | | (173,537 | ) | | | (18,389 | ) | | | — | | | (198,358 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | |
Proceeds from long-term debt | | | — | | | | 658,500 | | | | — | | | | — | | | 658,500 | |
Principal payments on long-term debt | | | — | | | | (646,089 | ) | | | — | | | | — | | | (646,089 | ) |
Proceeds from the exercise of stock options | | | 10,293 | | | | — | | | | — | | | | — | | | 10,293 | |
Other | | | (13,811 | ) | | | (8,259 | ) | | | — | | | | — | | | (22,070 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | (3,518 | ) | | | 4,152 | | | | — | | | | — | | | 634 | |
Effect of exchange rate on cash and cash equivalents | | | (625 | ) | | | 12,855 | | | | 479 | | | | — | | | 12,709 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net increase (decrease) in cash and cash equivalents | | | 15,744 | | | | (1,901 | ) | | | (6,708 | ) | | | — | | | 7,135 | |
Cash and cash equivalents at beginning of year | | | 4,145 | | | | (5,652 | ) | | | 10,699 | | | | — | | | 9,192 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash and cash equivalents at end of year | | $ | 19,889 | | | $ | (7,553 | ) | | $ | 3,991 | | | $ | — | | $ | 16,327 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Supplemental disclosures of cash flow information | | | | | | | | | | | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | | | | | | | | | | | |
Interest | | $ | — | | | $ | 39,350 | | | $ | 341 | | | $ | — | | $ | 39,691 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Income taxes | | $ | 31,010 | | | $ | — | | | $ | 8,503 | | | $ | — | | $ | 39,513 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Capital lease obligations incurred | | $ | — | | | $ | 334 | | | $ | — | | | $ | — | | $ | 334 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
F-53
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS (Continued)
| | | For the Year Ended September 30, 2001
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | Consolidated
| |
Cash flows (used by) provided by operating activities: | | $ | (53,740 | ) | | $ | 221,287 | | | $ | 11,846 | | | $ | — | | $ | 179,393 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | (8,320 | ) | | | (32,243 | ) | | | (9,557 | ) | | | — | | | (50,120 | ) |
Proceeds from sales of property, plant and equipment | | | 10,212 | | | | 2,076 | | | | 169 | | | | — | | | 12,457 | |
Net cash inflow from SDS | | | 46,394 | | | | — | | | | — | | | | — | | | 46,394 | |
Net payments for businesses acquired | | | — | | | | (161,208 | ) | | | (2,311 | ) | | | | | | (163,519 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash provided by (used in) investing activities | | | 48,286 | | | | (191,375 | ) | | | (11,699 | ) | | | — | | | (154,788 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | |
Proceeds from long-term debt | | | — | | | | 1,158,008 | | | | — | | | | — | | | 1,158,008 | |
Principal payments on long-term debt | | | — | | | | (1,184,273 | ) | | | (41 | ) | | | — | | | (1,184,314 | ) |
Proceeds from the exercise of stock options | | | 6,631 | | | | — | | | | — | | | | — | | | 6,631 | |
Other | | | (4,118 | ) | | | (6,721 | ) | | | — | | | | — | | | (10,839 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | 2,513 | | | | (32,986 | ) | | | (41 | ) | | | — | | | (30,514 | ) |
Effect of exchange rate on cash and cash equivalents | | | — | | | | — | | | | 2,690 | | | | | | | 2,690 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net (decrease) increase in cash and cash equivalents | | | (2,941 | ) | | | (3,073 | ) | | | 2,796 | | | | — | | | (3,218 | ) |
Cash and cash equivalents at beginning of year | | | 7,086 | | | | (2,577 | ) | | | 7,902 | | | | — | | | 12,411 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash and cash equivalents at end of year | | $ | 4,145 | | | $ | (5,649 | ) | | $ | 10,698 | | | $ | — | | $ | 9,192 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Supplemental disclosures of cash flow information | | | | | | | | | | | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | | | | | | | | | | | |
Interest | | $ | — | | | $ | 36,767 | | | $ | 365 | | | $ | — | | $ | 37,132 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Income taxes | | $ | 35,629 | | | $ | 638 | | | $ | 6,803 | | | $ | — | | $ | 43,070 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Capital lease obligations incurred | | $ | 36 | | | $ | 59 | | | $ | 9 | | | $ | — | | $ | 104 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
F-54
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS (Continued)
| | | For the Year Ended September 30, 2000
| |
| | | Apogent
Technologies
| | | Guarantor
Subsidiaries
| | | Non
Guarantor
Subsidiaries
| | | Eliminations
| | Consolidated
| |
Cash flows provided by operating activities: | | $ | 8,446 | | | $ | 104,703 | | | $ | 2,226 | | | $ | — | | $ | 115,375 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | (1,007 | ) | | | (32,654 | ) | | | (7,663 | ) | | | — | | | (41,324 | ) |
Security purchased | | | — | | | | — | | | | — | | | | — | | | — | |
Proceeds from sales of property, plant and equipment | | | — | | | | 871 | | | | 53 | | | | — | | | 924 | |
Other investing activities | | | (2,600 | ) | | | — | | | | — | | | | — | | | (2,600 | ) |
Net cash inflow from SDS | | | 58,098 | | | | — | | | | — | | | | — | | | 58,098 | |
Net payments for businesses acquired | | | (82,348 | ) | | | (130,992 | ) | | | 6,187 | | | | — | | | (207,153 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash used in investing activities | | | (27,857 | ) | | | (163,944 | ) | | | (1,423 | ) | | | — | | | (192,055 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | |
Proceeds from long-term debt | | | — | | | | 332,640 | | | | — | | | | — | | | 332,640 | |
Principal payments on long-term debt | | | — | | | | (274,712 | ) | | | (58 | ) | | | — | | | (274,770 | ) |
Securities lending agreement | | | 3,544 | | | | — | | | | — | | | | — | | | 3,544 | |
Proceeds from the exercise of stock options | | | 12,599 | | | | — | | | | — | | | | — | | | 12,599 | |
Other | | | 3,646 | | | | — | | | | — | | | | — | | | 3,646 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | 19,789 | | | | 57,928 | | | | (58 | ) | | | — | | | 77,659 | |
Effect of exchange rate on cash and cash equivalents | | | — | | | | — | | | | (969 | ) | | | — | | | (969 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Net increase (decrease) in cash and cash equivalents | | | 378 | | | | (144 | ) | | | (224 | ) | | | — | | | 10 | |
Cash and cash equivalents at beginning of year | | | 6,708 | | | | (2,433 | ) | | | 8,126 | | | | — | | | 12,401 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Cash and cash equivalents at end of year | | $ | 7,086 | | | $ | (2,577 | ) | | $ | 7,902 | | | $ | — | | $ | 12,411 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Supplemental disclosures of cash flow information | | | | | | | | | | | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | | | | | | | | | | | |
Interest | | $ | — | | | $ | 55,509 | | | $ | 324 | | | $ | — | | $ | 55,833 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Income taxes | | $ | 40,544 | | | $ | 319 | | | $ | 1,549 | | | $ | — | | $ | 42,412 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
Capital lease obligations incurred | | $ | — | | | $ | 25 | | | $ | — | | | $ | — | | $ | 25 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
| |
|
|
|
F-55
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 20. Indemnification of Directors and Officers.
Wisconsin Law. Apogent Technologies Inc. is incorporated under the Wisconsin Business Corporation Law (the AWBCL@).
Under Section 180.0851(1) of the WBCL, Apogent is required to indemnify a director or officer, to the extent such person is successful on the merits or otherwise in the defense of a proceeding, for all reasonable expenses incurred in the proceeding if such person was a party because he or she was a director or officer of Apogent Technologies Inc. In all other cases, Apogent is required by Section 180.0851(2) to indemnify a director or officer against liability incurred in a proceeding to which such person was a party because he or she was a director or officer of Apogent, unless it is determined that he or she breached or failed to perform a duty owed to Apogent and the breach or failure to perform constitutes:
| | $ | a willful failure to deal fairly with Apogent or its shareholders in connection with a matter in which the director or officer has a material conflict of interest; |
| | $ | a violation of criminal law, unless the director or officer had reasonable cause to believe his or her conduct was lawful or no reasonable cause to believe his or her conduct was unlawful; |
| | $ | a transaction from which the director or officer derived an improper personal profit; or |
Section 180.0858(1) provides that, subject to certain limitations, the mandatory indemnification provisions do not preclude any additional right to indemnification or allowance of expenses that a director or officer may have under Apogent=s articles of incorporation, bylaws, any written agreement or a resolution of the board of directors or shareholders.
Section 180.0859 of the WBCL provides that it is the public policy of the State of Wisconsin to require or permit indemnification, allowance of expenses and insurance to the extent required or permitted under Sections 180.0850 to 180.0858 of the WBCL, for any liability incurred in connection with a proceeding involving a federal or state statute, rule or regulation regulating the offer, sale or purchase of securities.
Section 180.0828 of the WBCL provides that, with certain exceptions, a director is not liable to a corporation, its shareholders, or any person asserting rights on behalf of the corporation or its shareholders, for damages, settlements, fees, fines, penalties or other monetary liabilities arising from a breach of, or failure to perform, any duty resulting solely from his or her status as a director, unless the person asserting liability proves that the breach or failure to perform constitutes any of the four exceptions to mandatory indemnification under Section 180.0851(2) referred to above.
Under Section 180.0833 of the WBCL, directors of Apogent against whom claims are asserted with respect to the declaration of improper dividends or distributions to shareholders or certain other improper acts which they approved are entitled to contribution from other directors who approved such actions and from shareholders who knowingly accepted an improper dividend or distribution, as provided therein.
Bylaws. Article VIII of Apogent=s bylaws contains provisions that generally parallel the indemnification provisions of the WBCL and cover certain procedural matters not dealt with in the WBCL. Furthermore, certain officers of Apogent are also officers of subsidiaries of Apogent and, as a result, such officers may be entitled to indemnification pursuant to provisions of such subsidiaries= governing corporate laws, articles of incorporation and bylaws. Apogent has also executed an indemnity agreement with each of its directors and certain of its officers which provides certain indemnity rights to such individuals.
II-1
Insurance. Directors and officers of Apogent are covered by directors= and officers= liability insurance under which they are insured (subject to certain exceptions and limitations specified in the policy) against expenses and liabilities arising out of proceedings to which they are parties by reason of being or having been directors or officers, including liabilities under the Securities Act of 1933.
Item 21. Exhibits and Financial Statement Schedules .
See Exhibit Index following the Signatures page in this registration statement, which Exhibit Index is incorporated herein by reference.
Item 22. Undertakings.
The undersigned registrants hereby undertake (in accordance with the corresponding lettered undertakings in Item 512 of Regulation S-K):
(a) Rule 415 Offering. (1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| | (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| | (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the ACalculation of Registration Fee@ table in the effective registration statement; |
| | (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
Provided, however, that paragraphs (a)(1)(i) and (a)(1)(ii) above do not apply if the registration statement is on Form S-3 or Form S-8 or Form F-3, and the information required to be included in a post-effective amendment by those paragraphs is contained in periodic reports filed with or furnished to the Commission by the registrants pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
| | (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| | (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
(b) That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) of Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(g) (1) That prior to any public reoffering of the securities registered hereunder through use of a prospectus which is part of this registration statement, by any person or party who is deemed to be an underwriter within the
II-2
meaning of Rule 145(c), the issuer undertakes that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other Items of the applicable form.
| | (2) | That every prospectus: (i) that is filed pursuant to paragraph (g)(i) immediately preceding, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Act and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the registration statement and will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act of 1933, each such post effective amendment shall be deemed to be a new registration statement relating to the securities offering therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
(h) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrants pursuant to the provisions referred to in Item 20 of this registration statement, or otherwise, the registrants have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrants of expenses incurred or paid by a director, officer or controlling person of the registrants in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrants will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrants hereby further undertake (in accordance with the corresponding lettered undertakings in Item 22 of Form S-4):
(b) To respond to requests for information that is incorporated by reference into this prospectus pursuant to Items 4, 10(b), 11, or 13 of this Form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request.
(c) To supply by means of a post-effect amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective.
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
APOGENT TECHNOLOGIES INC. |
| |
By: | | /s/ FRANK H. JELLINEK, JR.
|
| | | Frank H. Jellinek, Jr. President and Chief Executive Officer |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ FRANK H. JELLINEK, JR.
Frank H. Jellinek, Jr. | | President and Chief Executive Officer and Director (principal executive officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Chief Financial Officer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ WILLIAM H. BINNIE
William H. Binnie | | Director |
| |
/s/ DON H. DAVIS, JR.
Don H. Davis, Jr. | | Director |
| |
/s/ CHRISTOPHER L. DOERR
Christopher L. Doerr | | Director |
| |
/s/ STEPHEN R. HARDIS
Stephen R. Hardis | | Director |
| |
/s/ R. JEFFREY HARRIS
R. Jeffrey Harris | | Director |
| |
/s/ JOE L. ROBY
Joe L. Roby | | Director |
| |
/s/ RICHARD W. VIESER
Richard W. Vieser | | Director |
| |
/s/ KENNETH F. YONTZ
Kenneth F. Yontz | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-1
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-4 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
APPLIED BIOTECH, INC. |
| |
By: | | /s/ JEFFREY A. KONECKE
|
| | | Jeffrey A. Konecke
President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ JEFFREY A. KONECKE
Jeffrey A. Konecke | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-2
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
APOGENT FINANCE COMPANY |
| |
By: | | /s/ MICHAEL K. MICHAUD
|
| | | Michael K. Michaud
President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | President and Treasurer (principal executive officer, principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-3
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
APOGENT HOLDING COMPANY |
| |
By: | | /s/ MICHAEL K. BRESSON
|
| | | Michael K. Bresson
President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
| APOGENT SERVICE CORPORATION |
| |
By: | | /s/ MICHAEL K. BRESSON
|
| | | Michael K. Bresson
President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
APOGENT TRANSITION CORP. |
| |
By: | | /s/ FRANK H. JELLINEK, JR.
|
| | | Frank H. Jellinek, Jr.
President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ FRANK H. JELLINEK, JR.
Frank H. Jellinek, Jr. | | President (principal executive officer of the registrant) |
| |
/s/ Michael K. Michaud
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-6
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-4 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
BARNSTEAD THERMOLYNE CORPORATION |
| |
By: | | /s/ DUNCAN R. ROSS
|
| | | Duncan R. Ross
President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ DUNCAN R. ROSS
Duncan R. Ross | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ BRENDA K. HOEFLER
Brenda K. Hoefler | | Vice President–Finance (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-7
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
BT CANADA HOLDINGS INC. |
| |
| By: | | /s/ MICHAEL K. MICHAUD |
| |
|
| | | Michael K. Michaud President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | President and Treasurer (principal executive officer, principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
CAPITOL VIAL, INC. |
| |
By: | | /s/ MARK F. STUPPY |
| |
|
| | | Mark F. Stuppy President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MARK F. STUPPY
Mark F. Stuppy | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-9
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
CHASE SCIENTIFIC GLASS, INC. |
| |
By: | | /s/ STEPHEN MUNZER |
| |
|
| | | Stephen Munzer President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ STEPHEN MUNZER
Stephen Munzer | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ DAVID BALES
David Bales | | Vice President–Finance (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-10
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
CONSOLIDATED TECHNOLOGIES, INC. |
| |
By: | | /s/ ROBERT WOLF |
| |
|
| | | Robert Wolf President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ ROBERT WOLF
Robert Wolf | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ LINDA M. LECHNER
Linda M. Lechner | | Vice President–Finance and Controller (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-11
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
ERIE SCIENTIFIC COMPANY |
| |
By: | | /s/ MARK F. STUPPY |
| |
|
| | | Mark F. Stuppy President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MARK F. STUPPY
Mark F. Stuppy | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ GERALD PRIOR
Gerald Prior | | Controller (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-12
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
ERIE SCIENTIFIC COMPANYOF PUERTO RICO |
| |
By: | | /s/ MARK F. STUPPY�� |
| |
|
| | | Mark F. Stuppy President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MARK F. STUPPY
Mark F. Stuppy | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ GERALD PRIOR
Gerald Prior | | Controller (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-13
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
ERIE UK HOLDING COMPANY |
| |
By: | | /s/ MICHAEL K. MICHAUD |
| |
|
| | | Michael K. Michaud President and Treasurer |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | President and Treasurer (principal executive officer, principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-14
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
EVER READY THERMOMETER CO., INC. |
| |
By: | | /s/ TIMOTHY B. WEISS |
| |
|
| | | Timothy B. Weiss President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ TIMOTHY B. WEISS
Timothy B. Weiss | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ VITO LILOIA
Vito LiLoia | | Accounting Manager (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-15
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
FOREFRONT DIAGNOSTICS, INC. |
| |
By: | | /s/ JEFFREY A. KONECKE |
| |
|
| | | Jeffrey A. Konecke President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ JEFFREY A. KONECKE
Jeffrey A. Konecke | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-16
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
GENEVAC, INC. |
| |
By: | | /s/ HARRY COLE |
| |
|
| | | Harry Cole President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ HARRY COLE
Harry Cole | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ DEBRA INGER
Debra Inger | | Vice President and Controller (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-17
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
G&P LABWARE HOLDINGS INC. |
| |
By: | | /s/ ROBERT V. AHLGREN |
| |
|
| | | Robert V. Ahlgren President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ ROBERT V. AHLGREN
Robert V. Ahlgren | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ KAREN DALLY
Karen Dally | | Vice President (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-18
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
LAB-LINE INSTRUMENTS, INC. |
| |
By: | | /s/ TIMOTHY B. WEISS |
| |
|
| | | Timothy B. Weiss President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ TIMOTHY B. WEISS
Timothy B. Weiss | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ PHILIP T. REID
Philip T. Reid | | Controller (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-19
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
LAB VISION CORPORATION |
| |
By: | | /s/ GLENN K. TAKAYAMA |
| |
|
| | | Glenn K. Takayama President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ GLENN K. TAKAYAMA
Glenn K. Takayama | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ BRIAN WOOD
Brian Wood | | Chief Financial Officer (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-20
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
MARSH BIO PRODUCTS, INC. |
| |
By: | | /s/ JAMES MARSH
|
| | | James Marsh President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ JAMES MARSH
James Marsh | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ GARY SCHNEIDER
Gary Schneider | | Vice President and Chief Financial Officer (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-21
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
MATRIX TECHNOLOGIES CORPORATION |
| |
By: | | /s/ P. GEORGE KALMAKIS
|
| | | P. George Kalmakis President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ P. GEORGE KALMAKIS
P. George Kalmakis | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ JOHN L. STOWELL
John L. Stowell | | Executive Vice President and Chief Financial Officer (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-22
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
METAVAC LLC |
| |
By: | | /s/ MICHAEL KESSLER
|
| | | Michael Kessler President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
| | | Signature
| | Title
|
| |
/s/ MICHAEL KESSLER
Michael Kessler | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
THE NAUGATUCK GLASS COMPANY | | |
| | |
| By: | | /s/ MICHAEL K. BRESSON
| | Sole Member |
| | | Michael K. Bresson | |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-23
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
MICROGENICS CORPORATION |
| |
By: | | /s/ YUH-GENG TSAY
|
| | | Yuh-Geng Tsay President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ YUH-GENG TSAY
Yuh-Geng Tsay | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ LINDA M. LECHNER
Linda M. Lechner | | Vice President Administration and Chief Financial Officer (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-24
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
MOLECULAR BIOPRODUCTS, INC. |
| |
By: | | /s/ ROBERT ARNOLD
|
| | | Robert Arnold President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ ROBERT ARNOLD
Robert Arnold | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ ANGELA M. EGNER
Angela M. Egner | | Controller (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-25
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
NALGE NUNC INTERNATIONAL CORPORATION |
| |
By: | | /s/ CRAIG M. JACK
|
| | | Craig M. Jack President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ CRAIG M. JACK
Craig M. Jack | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ JEAN C. MUCENSKI
Jean C. Mucenski | | Vice President—Finance (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-26
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
NATIONAL SCIENTIFIC COMPANY |
| |
By: | | /s/ GORDON HAMNETT
|
| | | Gordon Hamnett President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ GORDON HAMNETT
Gordon Hamnett | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ JEAN C. MUCENSKI
Jean C. Mucenski | | Vice President—Finance (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-27
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
THE NAUGATUCK GLASS COMPANY |
| |
By: | | /s/ HAROLD A. RACEVICIUS
|
| | | Harold A. Racevicius President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ HAROLD A. RACEVICIUS
Harold A. Racevicius | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ HARRY X. CASHIN, III
Harry X. Cashin, III | | Director of Finance (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-28
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
NEOMARKERS, INC. |
| |
By: | | /s/ GLENN K. TAKAYAMA
|
| | | Glenn K. Takayama President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ GLENN K. TAKAYAMA
Glenn K. Takayama | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-29
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
NERL DIAGNOSTICS CORPORATION |
| |
By: | | /s/ DONALD H. MCGLORY, JR.
|
| | | Donald H. McGlory, Jr. President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ DONALD H. MCGLORY, JR.
Donald H. McGlory, Jr. | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ DOUGLAS FISHER
Douglas Fisher | | Controller/Operations Manager (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-30
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
OWL SEPARATION SYSTEMS, INC. |
| |
By: | | /s/ STEPHEN NORTON
|
| | | Stephen Norton President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ STEPHEN NORTON
Stephen Norton | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ KEITH BLESSINGTON
Keith Blessington | | Manager of Accounting (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-31
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
REMEL INC. |
| |
By: | | /s/ SUSANNE GARAY
|
| | | Susanne Garay President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ SUSANNE GARAY
Susanne Garay | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ ROB CHESTNUT
Rob Chestnut | | Vice President—Finance (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-32
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
RICHARD-ALLAN SCIENTIFIC COMPANY |
| |
By: | | /s/ DAVID P. SANFORD
|
| | | David P. Sanford President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ DAVID P. SANFORD
David P. Sanford | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ BRIAN WOOD
Brian Wood | | Vice President—Finance (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-33
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
ROBBINS SCIENTIFIC CORPORATION |
| |
By: | | /s/ GARY E. NELSON
|
| | | Gary E. Nelson President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ GARY E. NELSON
Gary E. Nelson | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer of the registrant) |
| |
/s/ JOHN L. STOWELL
John L. Stowell | | Vice President and Chief Financial Officer (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-34
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
SAMCO SCIENTIFIC CORPORATION |
| |
By: | | /s/ MARK F. STUPPY
|
| | | Mark F. Stuppy President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MARK F. STUPPY
Mark F. Stuppy | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (.incipal financial officer of the registrant) |
| |
/s/ HARRY MANOUSHAGIAN
Harry Manoushagian | | Controller (principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-35
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
SEPARATION TECHNOLOGY, INC. |
| |
By: | | /s/ JAMI MEEKS
|
| | | Jami Meeks President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ JAMI MEEKS
Jami Meeks | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-36
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Portsmouth, State of New Hampshire, on July 16, 2003.
SERADYN, INC. |
| |
By: | | /s/ MARK ROBERTS
|
| | | Mark Roberts President |
Power of Attorney. Each person whose signature appears below constitutes and appoints, Frank H. Jellinek, Jr., Dennis Brown and Michael K. Bresson, and each of them, his true and lawful attorneys-in-fact and agents, for him and in his name, place and stead in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission and any other regulatory authority, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.*
Signature
| | Title
|
| |
/s/ MARK ROBERTS
Mark Roberts | | President (principal executive officer of the registrant) |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Treasurer (principal financial officer and principal accounting officer of the registrant) |
| |
/s/ DENNIS BROWN
Dennis Brown | | Director |
| |
/s/ MICHAEL K. MICHAUD
Michael K. Michaud | | Director |
| |
/s/ MICHAEL K. BRESSON
Michael K. Bresson | | Director |
| * | Each of these signatures is affixed as of July 16, 2003. |
S-37
APOGENT TECHNOLOGIES INC.
(the “Registrant”) and its Guarantor Subsidiaries
(Commission File No. 1-11091)
EXHIBIT INDEX
TO
FORM S-4 REGISTRATION STATEMENT
Exhibit Number
| | | Description
| | Incorporated Herein By Reference To
| | Filed Herewith
|
| | | |
| 3.1 | (a) | | Restated Articles of Incorporation of the Registrant | | Exhibit 3.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2000. | | |
| | | |
| 3.1 | (b) | | Articles of Amendment containing Certificate of Designation, Preferences and Rights of Series A Preferred Stock | | Exhibit 3.1(b) to the Registrant’s Form 10-K filed for the fiscal year ended September 30, 2000. | | |
| | | |
| 3.2 | | | Bylaws of the Registrant, as amended as of January 30, 2001 | | Exhibit 3.2 to the Registrant’s Quarterly Report on Form 10-Q for the period ended December 31, 2000. | | |
| | | |
| 4.1 | | | Indenture, dated June 2, 2003, between Apogent Technologies Inc., the Subsidiary Guarantors parties thereto, and The Bank of New York, as Trustee | | | | X |
| | | |
| 4.2 | | | Registration Rights Agreement, dated as of June 2, 2003, among Apogent Technologies Inc., the Subsidiary Guarantors and Lehman Brothers Inc., Creidt Suisse First Boston LLC and J.P. Morgan Securities Inc., (collectively, as Representative of several Initial Purchasers) | | | | X |
| | | |
| 4.3 | | | Purchase Agreement, dated June 2, 2003, between Apogent Technologies, the Subsidiary Guarantors and Lehman Brothers Inc., Credit Suisse First Boston LLC and J.P. Morgan Securities Inc. (collectively, as Representatives of the several Initial Purchasers) | | | | X |
| | | |
| 5 | | | Opinion letter of Quarles & Brady LLP as to the legality of the securities being registered | | | | X |
| | | |
| 12 | | | Statements regarding computation of ratio of earnings to fixed charges | | | | X |
| | | |
| 21 | | | Subsidiaries of the Registrant | | | | X |
| | | |
| 23.1 | | | Consent of KPMG LLP | | | | X |
| | | |
| 23.2 | | | Consent of Ernst & Young LLP | | | | X |
| | | |
| 24 | | | Power of Attorney | | | | See Signature
pages |
| | | |
| 25 | | | Statement of Eligibility on Form T-1 of The Bank of New York | | | | X |
| | | |
| 99.1 | | | Letter of Transmittal | | | | X |
| | | |
| 99.2 | | | Notice of Guaranteed Delivery | | | | X |


