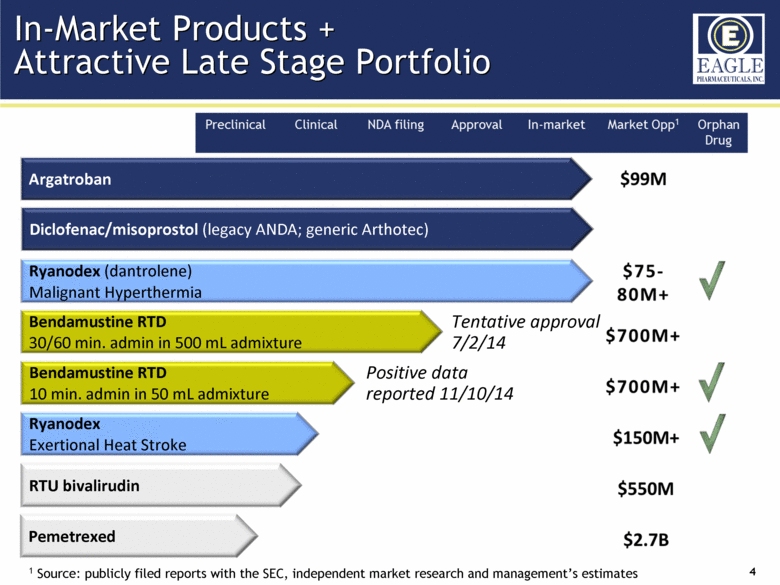
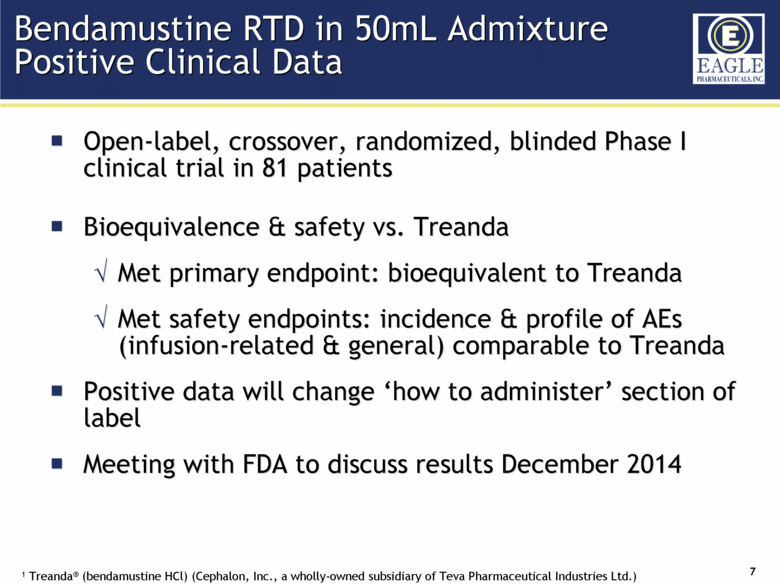
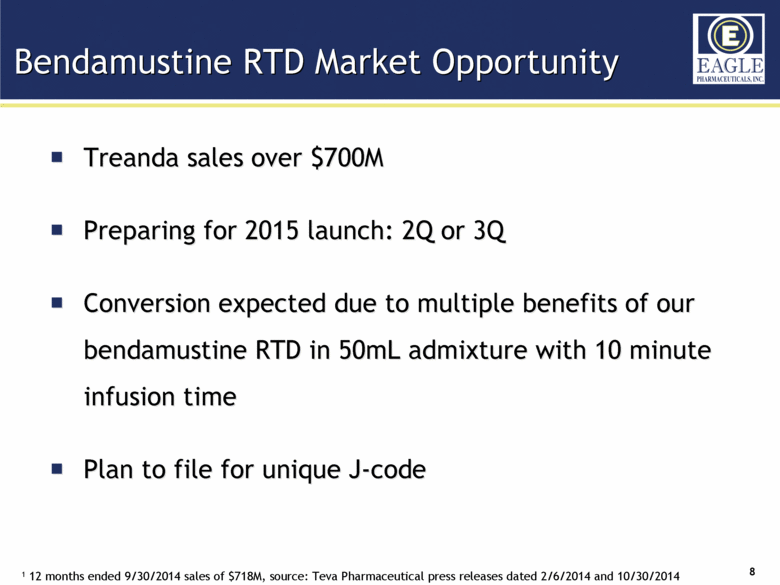
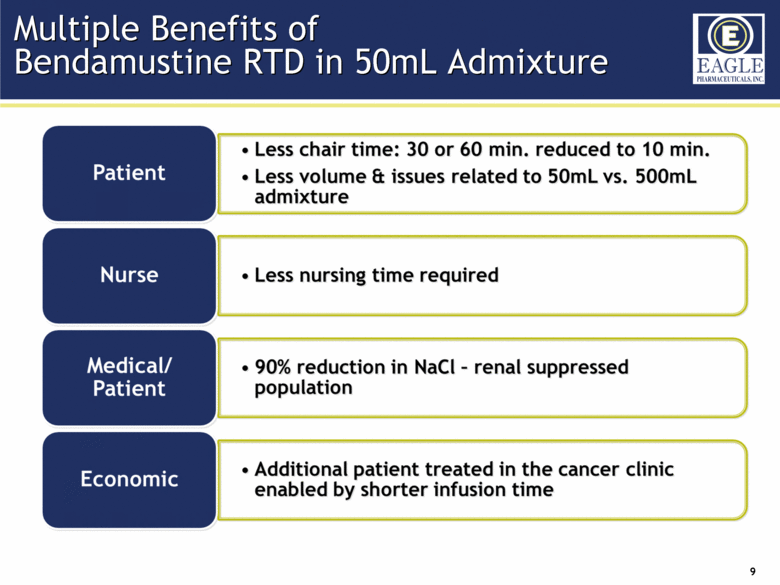
| Forward Looking Statements 2 This presentation contains certain forward-looking information about Eagle Pharmaceuticals that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “strong,” “upcoming,” “prepared” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our therapeutic products; our ability to expand our long-term business opportunities; financial projections and estimates and their underlying assumptions; and future performance. All such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the Company’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, but are not limited to: whether the FDA will approve our NDA for our bendamustine product and other pharmaceutical products in development and when such approval might be granted; our ability to successfully complete the overall safety assessment for the bendamustine HCl rapidly infused product candidate on time, if at all, and whether the results of the safety assessment will establish the safety of the product or demonstrate an adverse safety profile; whether we will successfully market our products; our ability to successfully compete with other pharmaceutical and biotechnology companies; the development of, and our ability to take advantage of, the market for our therapeutic products; the strength and enforceability of our intellectual property rights; the outcome of pending litigation; general economic conditions; and the other risk factors included in our final prospectus filed with the Securities and Exchange Commission on February 13, 2014 relating to our Registration Statement on Form S-1 (File No. 333-192984) for our IPO. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. |