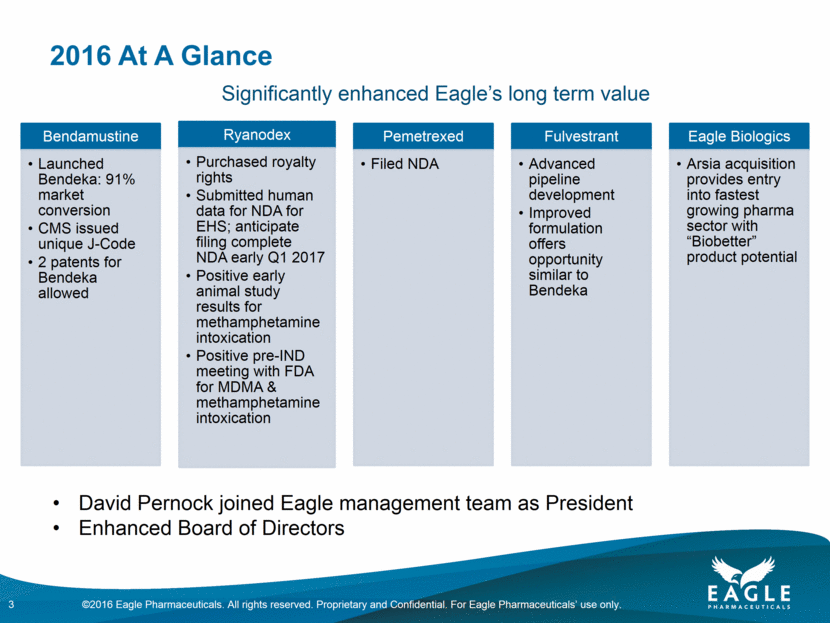
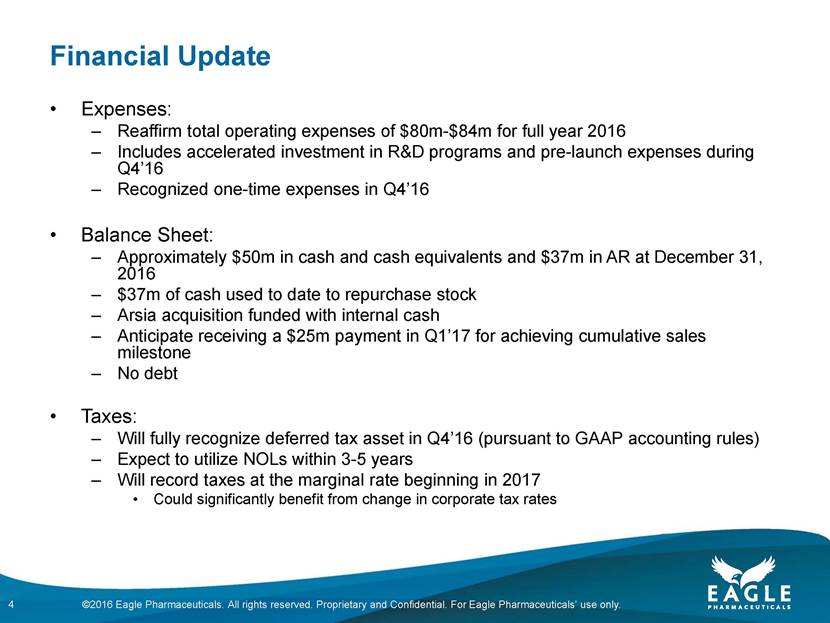
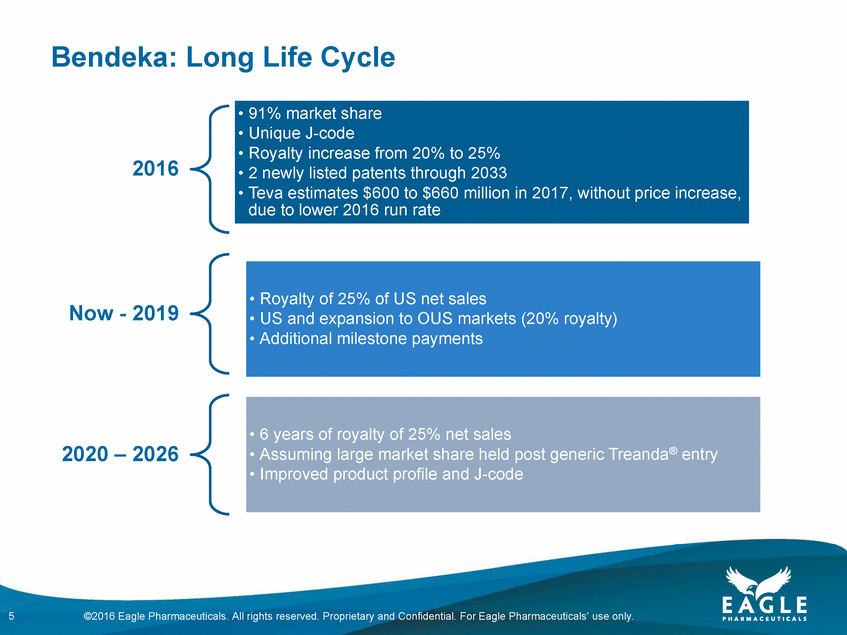
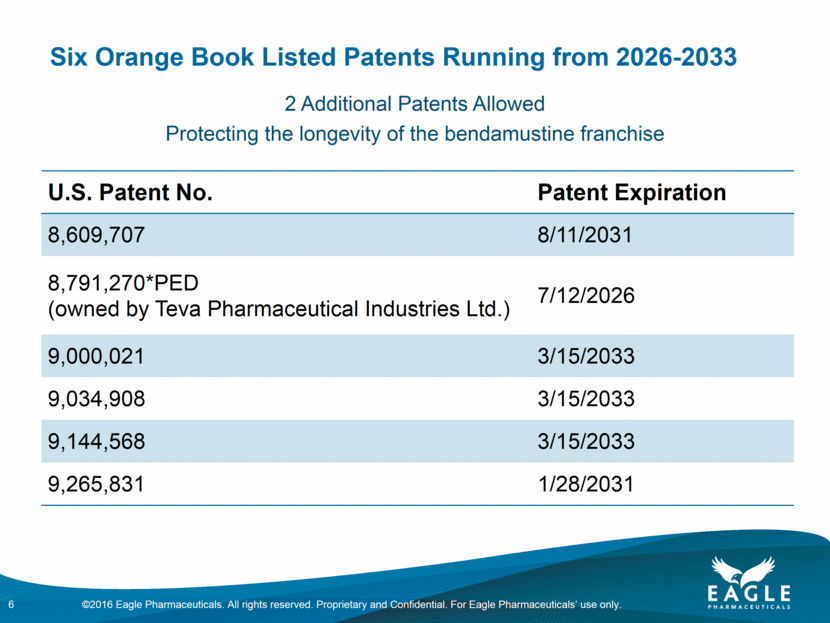
Forward Looking Statements This presentation contains forward-looking information within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, and other securities laws. Forward-looking statements are statements that are not historical facts. Words such as “will,” “determined,” “confirming,” “underway,” ramping up,” “agreement,” “allows,” “well positioned,” “expect,” “may,” “intends,” “anticipate(s),” “plan,” “enables,” “potentially,” “look forward,” “on track,” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding future events including, but not limited to: the reporting of our cash and cash equivalents as December 31, 2016, as well as our expenses and revenue for the quarter ended December 31, 2016, the continued commercial performance of our marketed products, including BENDEKA, which is marketed by our partner Cephalon, and RYANODEX, which we market ourselves as well as our ability to replicate our marketing successes for our other product candidates either through joint or direct marketing efforts; the lack of a need for human safety and efficacy data for the submission of an NDA for Ryanodex and the adequacy of the regulatory pathway to complete an NDA submission; the Company’s share repurchase authorization and timing and ability to continue to repurchase shares of the Company’s common stock under a share repurchase program; the business path forward for the Company beyond 2020; the label expansions of Ryanodex for EHS patients and for the treatment of ecstasy and methamphetamine intoxication; the strength of the Company’s cash position and the ability to optimize the deployment of capital and take advantage of market opportunities; the potential of the Company’s pipeline to drive value beyond 2020; the contribution of the Ryanodex portfolio to the Company’s growth; the timing of Ryanodex for EHS entering the market; and the advancement of any of the Company’s product candidates including fulvestrant and pemetrexed, through the development process including FDA approval and the ability of any such products to have commercial success and to access significant new markets. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond Eagle’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. Such risks include, but are not limited to: whether our animal studies will support the safety and efficacy of Ryanodex for the treatment of EHS and ecstasy and methamphetamine intoxication; whether the FDA will ultimately approve Ryanodex for these indications; whether the FDA will approve our application for pemetrexed, and, if filed, fulvestrant; fluctuations in the trading volume and market price of shares of the Company's common stock, general business and market conditions and management's determination of alternative needs and uses of the Company's cash resources which may affect the Company's share repurchase program; the success of our commercial relationship with Teva and the parties’ ability to work effectively together; whether Eagle and Teva will successfully perform their respective obligations under the license agreement; difficulties or delays in manufacturing; the availability and pricing of third party sourced products and materials; the outcome of litigation involving any of our products or that may have an impact on any of our products, successful compliance with FDA and other governmental regulations applicable to product approvals, manufacturing facilities, products and/or businesses; general economic conditions; the strength and enforceability of our intellectual property rights or the rights of third parties; competition from other pharmaceutical and biotechnology companies; the timing of product launches; the successful marketing of our products; the risks inherent in the early stages of drug development and in conducting clinical trials; and other factors that are discussed in Eagle’s Annual Report on Form 10-K for the year ended December 31, 2015, and its other filings with the U.S. Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. All financial estimates are subject to completion of the Company’s financial closing procedures. Eagle’s independent registered public accounting firm, BDO USA LLP, has not audited, reviewed or compiled such estimates, and they are not a comprehensive statement of the Eagle’s financial results for the year or quarter ended December 31, 2016. Eagle’s actual results may differ materially from these estimates as a result of the completion of Eagle’s financial closing procedures, final adjustments and other developments arising between now and the time that Eagle’s financial results are finalized. 2 ©2016 Eagle Pharmaceuticals. All rights reserved. Proprietary and Confidential. For Eagle Pharmaceuticals’ use only.