Exhibit 99.1
2016 ANNUAL REPORT EAGLE PHARMACEUTICALS, INC. (Nasdaq: EGRX)

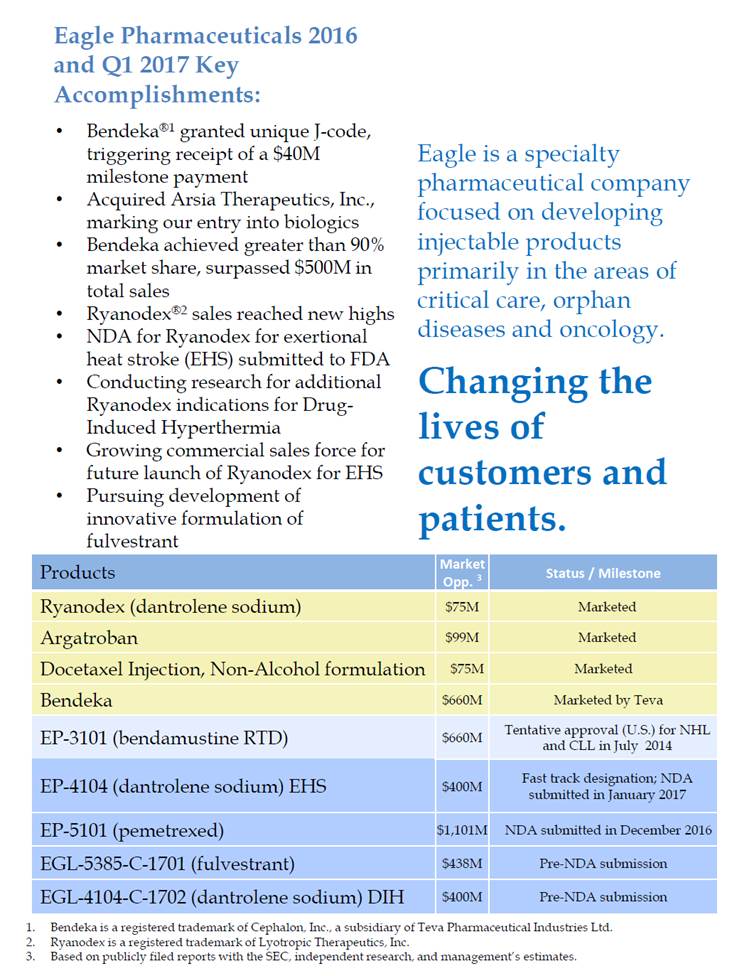
Eagle Pharmaceuticals 2016 and Q1 2017 Key Accomplishments: • Bendeka®1 granted unique J-code, triggering receipt of a $40M milestone payment Acquired Arsia Therapeutics, Inc., marking our entry into biologics Bendeka achieved greater than 90% market share, surpassed $500M in total sales Ryanodex®2 sales reached new highs NDA for Ryanodex for exertional heat stroke (EHS) submitted to FDA Conducting research for additional Ryanodex indications for Drug-Induced Hyperthermia Growing commercial sales force for future launch of Ryanodex for EHS Pursuing development of innovative formulation of fulvestrant Eagle is a specialty pharmaceutical company focused on developing injectable products primarily in the areas of critical care, orphan diseases and oncology. Changing the lives of • • • • • • customers patients. and • and CLL in July 2014 submitted in January 2017 1. 2. 3. Bendeka is a registered trademark of Cephalon, Inc., a subsidiary of Teva Pharmaceutical Industries Ltd. Ryanodex is a registered trademark of Lyotropic Therapeutics, Inc. Based on publicly filed reports with the SEC, independent research, and management’s estimates. Products Market Opp. 3 Status / Milestone Ryanodex (dantrolene sodium) $75M Marketed Argatroban $99M Marketed Docetaxel Injection, Non-Alcohol formulation $75M Marketed Bendeka $660M Marketed by Teva EP-3101 (bendamustine RTD) $660M Tentative approval (U.S.) for NHL EP-4104 (dantrolene sodium) EHS $400M Fast track designation; NDA EP-5101 (pemetrexed) $1,101M NDA submitted in December 2016 EGL-5385-C-1701 (fulvestrant) $438M Pre-NDA submission EGL-4104-C-1702 (dantrolene sodium) DIH $400M Pre-NDA submission
To my fellow stockholders:
2016 was another transformative year for Eagle. Adding to our already strong R&D, formulation and clinical capabilities, we enhanced our commercial capabilities, delivering an important commercial achievement with the successful launch of Bendeka® with our marketing partner, Teva Pharmaceutical Industries, Ltd. We drove record sales of Ryanodex® for malignant hyperthermia (MH), and our work to expand the label indication of Ryanodex to include exertional heat stroke (EHS) has resulted in Priority Review by the U.S. Food and Drug Administration (FDA) of the New Drug Application (NDA) we ultimately submitted in January 2017. We also advanced multiple other projects within our R&D program and broadened the scope of our business to include Eagle Biologics, marking our entry into the development of biobetters.
Revenue totaled $189.5 million in 2016, supported by Bendeka royalty income and growth in sales of Ryanodex. Net income was $81.5 million in 2016, or $5.24 per basic and $4.96 per diluted share. Our balance sheet remains strong. We closed 2016 with $52.8 million in cash and cash equivalents, no debt and $151.2 million in stockholders’ equity.
Key achievements during and subsequent to 2016 include:
Bendeka
Net sales of our rapidly infused bendamustine product, launched in December 2015 with Teva, have exceeded $500 million and achieved greater than 90% market share as of March 31, 2017. We have earned $110 million in milestone payments from Teva related to: FDA approval, Bendeka net sales in excess of $500 million and the granting of a unique J-code for Bendeka by the Centers for Medicare & Medicaid Services in recognition of the unique formulation and delivery mechanism of the drug. In addition, our royalty percentage increased to 25% of Bendeka net sales as a result of the J-code decision. With the product’s differentiated profile, and the protection of 14 issued and 13 Orange Book listed patents, we expect Bendeka to be an important contributor to Eagle’s earnings for years to come.
Ryanodex
Sales of our FDA-approved treatment for MH reached new highs this year. We believe this improvement reflects the efforts of our dedicated sales force in communicating the message about its effectiveness, and we expect to see continued sales momentum for the current indication. We are also focused on potential label expansion opportunities that we believe would significantly increase the value of Ryanodex and enhance the longevity of the product. This is why we have submitted an NDA for Ryanodex for EHS to the FDA and are conducting research in collaboration with the National Institutes of Health/National Institute on Drug Abuse for the treatment of MDMA (Ecstasy) and methamphetamine intoxication. We believe, if approved, Ryanodex in combination with the current standard of care, can lead to improved patient outcomes for those
experiencing EHS, a leading cause of death among high school and college athletes as well as members of our military. With continued effort and focus, Ryanodex could potentially be the first drug approved by the FDA for the treatment of this sudden and unpredictable disorder that constitutes a medical emergency and can result in severe multi-organ dysfunction and death.
To prepare for the potential launch of Ryanodex for EHS, we continue to grow our commercial team. We are very optimistic about the future of this franchise.
R&D Pipeline
We are pursuing development of an innovative formulation of fulvestrant and are proud of the work we are doing here. This is Eagle’s first product completely developed in-house, showcasing our formulation and clinical know-how. The currently marketed formulation requires two intramuscular injections to deliver the monthly recommended dose, contains castor oil, and a warning has been added to the label concerning painful injections, sciatica, neuropathic pain, and peripheral neuropathy. The injections can last one to two minutes each. Our formulation contains no castor oil and only requires one monthly injection, allowing for alternating the injection site every month. Therefore, our belief is that we can eliminate the current warning in the label by conducting the appropriate clinical trial. As a result of these improvements, we hope to achieve a unique J-code. We are hopeful that we will complete clinical trials this year and submit our NDA in 2018.
We also submitted an NDA for our ready-to-use pemetrexed formulation in December 2016, which was recently accepted for filing. It has a Prescription Drug User Fee Act (PDUFA) target date of October 30, 2017.
Eagle Biologics, Inc.
At Eagle, we remain focused on the goal of identifying and providing drug products that address needs in the existing market and deliver improved experiences for patients and health care providers. Toward that end, in 2016 we took steps to extend the reach of our business into the exciting and growing field of biologics. With our acquisition of Arsia Therapeutics, Inc. (renamed Eagle Biologics, Inc.), we intend to pursue opportunities to apply the Eagle model to the growing field of biologics by improving delivery and partnering with an innovator or with those developing biosimilars.
We believe that a strong and diverse product portfolio, protected by a solid patent estate, is essential to delivering important new therapies to patients and building long-term value for Eagle stockholders. We will continue to scale our investment in R&D in accordance with our growth, and extend this approach into biologics as opportunities arise.
We are proud of the work we have accomplished in creating products that improve people’s lives, the value we are building at Eagle and the multiple opportunities we will continue to advance. We look forward to continuing to unlock the value in our pipeline. Thank you to all of our employees for your dedication and commitment to making Eagle
a truly exceptional company. Thank you, to our stockholders, for your continued support. We are delighted that you have decided to take this journey with us.
Sincerely, | |
| |

| |
| |
Scott Tarriff | |
Chief Executive Officer | |
Eagle Pharmaceuticals, Inc. | |
BOARD OF DIRECTORS Michael Graves Chairman of the Board Scott Tarriff Chief Executive Officer Sander Flaum Steven Ratoff Douglas L. Braunstein Robert Glenning Richard A. Edlin EXECUTIVE OFFICERS & MANAGEMENT TEAM Scott Tarriff Chief Executive Officer, Director David M. Pernock President & Chief Commercial Officer Adrian J. Hepner, MD, Ph.D. Executive Vice President and Chief Medical Officer Steven L. Krill Ph.D. Executive Vice President and Chief Scientific Officer David E. Riggs Chief Financial Officer John W. LaRocca Executive Vice President and General Counsel Daniel J. O’Connor Executive Vice President, Biologics and Corporate Development | | REGISTRAR & TRANSFER AGENT American Stock Transfer & Trust Company 6201 15th Avenue
Brooklyn, NY 11219 718-921-8200 SECURITIES & RELATED INFORMATION The Company’s common stock is listed on the Nasdaq Stock Exchange under the symbol “EGRX”. ANNUAL MEETING The annual meeting of stockholders will be held on Tuesday, June 20, 2017 at 10 a.m. in the offices of our outside counsel, Cooley LLP, The Grace Building, 1114 Avenue of the Americas, New York, NY 10036-7798. STOCKHOLDER INFORMATION Stockholders, investors, and analysts interested in corporate information may contact: In-Site Communications Lisa M. Wilson 212-452-2793 WEBSITE Information about Eagle Pharmaceuticals, Inc. may be obtained on our website at www.eagleus.com. Investors interested in Eagle Pharmaceuticals, Inc. stock quotes, news releases, SEC filings and other corporate information may click on the Investors link on our website. |
Safe Harbor Statement
This annual report contain forward-looking statements regarding Eagle Pharmaceuticals’ strategic direction, prospects and future results, which statements are indicated by the words “may,” “could,” “will,” “can,” “plans,” “believes,” “intend,” “expects,” “potential,” “should,” “remain” and similar expressions. These include all statements relating to expected financial performance and future business or product developments, such as sales and marketing efforts in connection with Bendeka and Ryanodex, as well as development efforts around fulvestrant and pemetrexed and Eagle Pharmaceuticals’ entry into biologics. Management believes that these forward-looking statements are reasonable as and when made on April 28, 2017. However, such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause actual results to differ materially from those projected in the forward-looking statements. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date on which they are made. These statements are based on management’s current expectations and Eagle Pharmaceuticals does not undertake any responsibility to revise or update any forward-looking statements contained herein, except as expressly required by law. For a discussion of certain risks and uncertainties associated with Eagle Pharmaceuticals’ forward-looking statements, please review Eagle Pharmaceuticals’ reports filed with the SEC, including, but not limited to, its Annual Report on Form 10-K for the fiscal year ended December 31, 2016.
Eagle Pharmaceuticals, Inc. 50 Tice Boulevard Suite 315 Woodcliff Lake, NJ 07677 Phone: 201.326.5300 Fax: 201.391.2430 Email: info@eagleus.com www.eagleus.com




