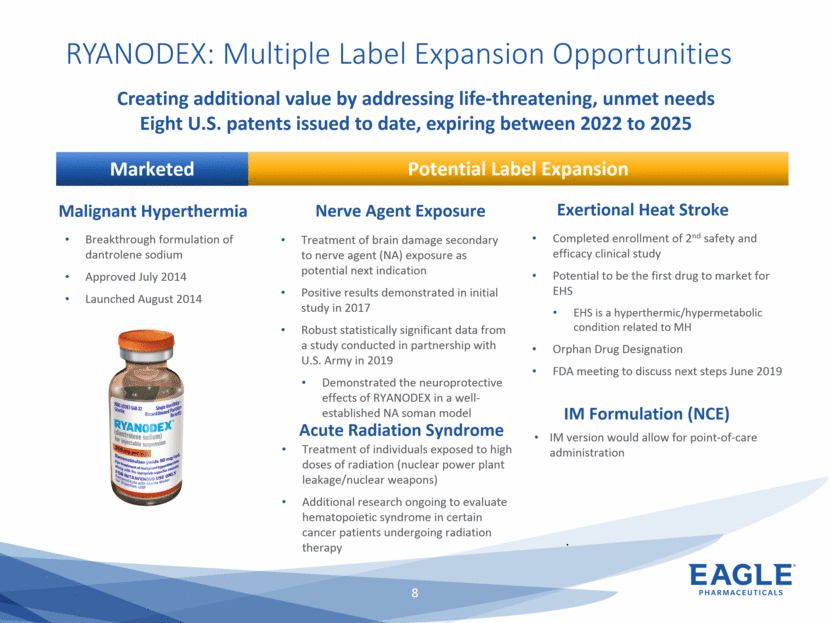
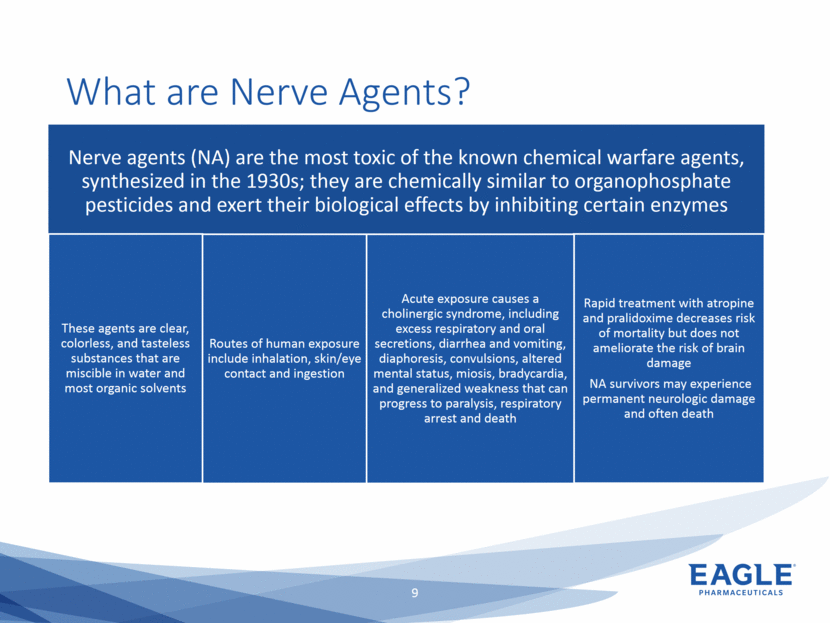
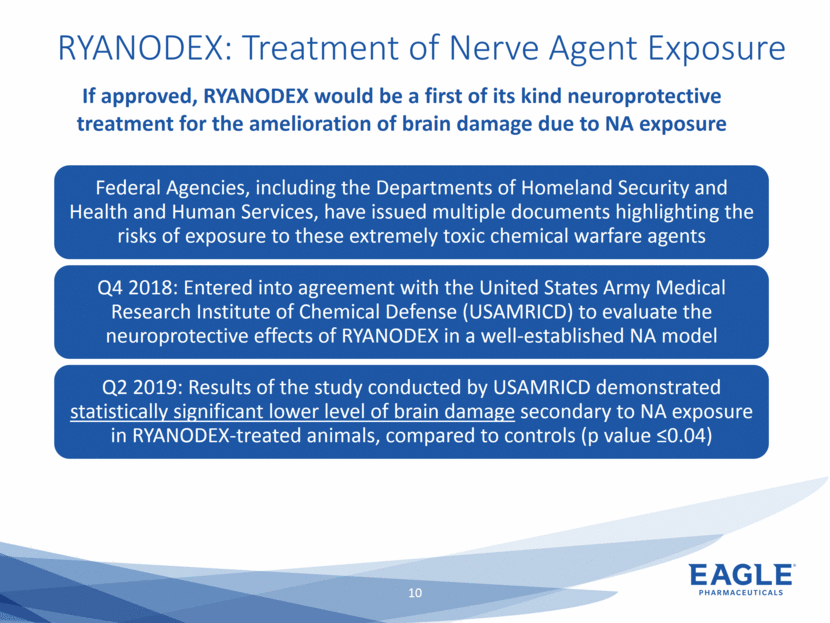
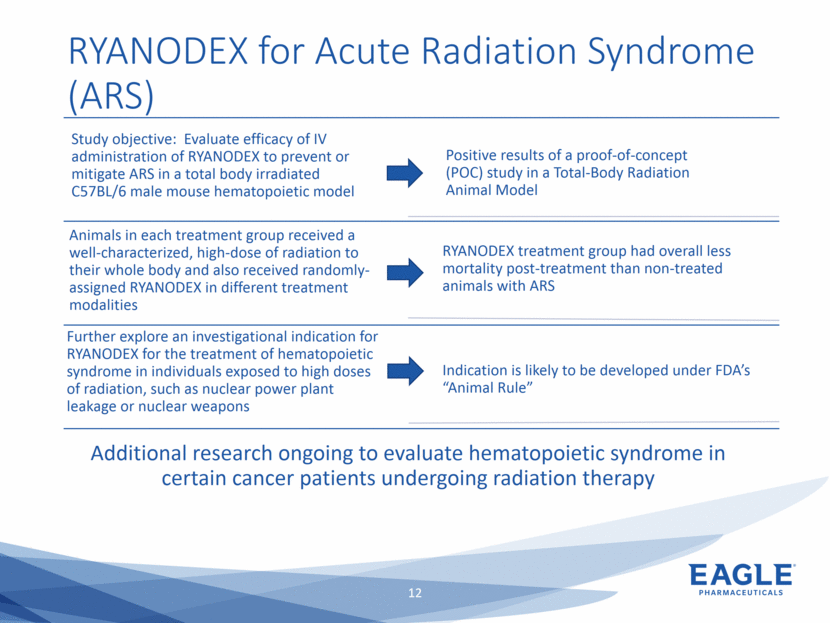
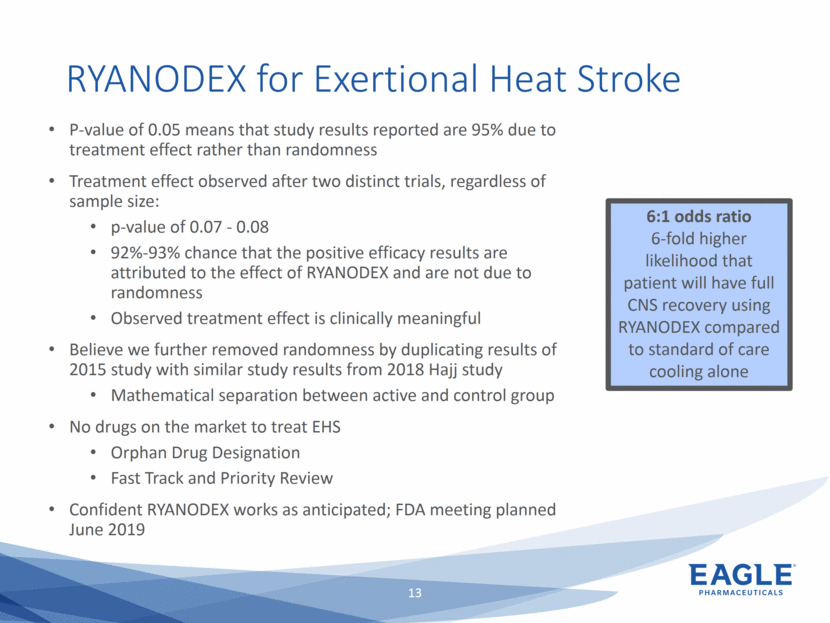
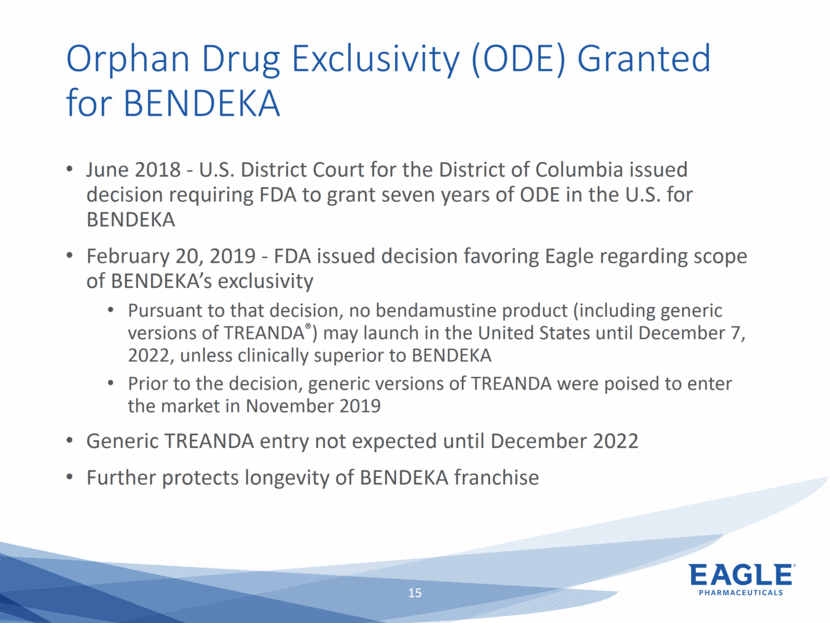
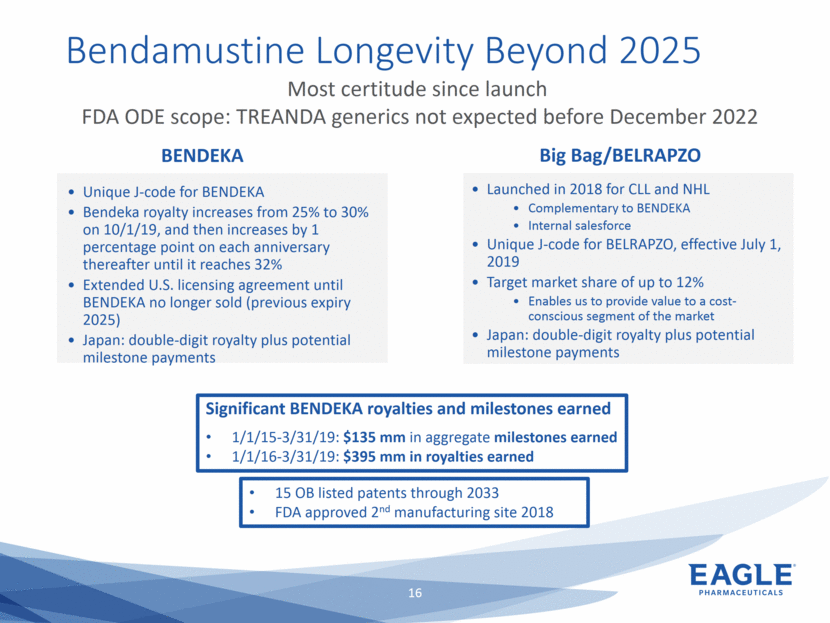
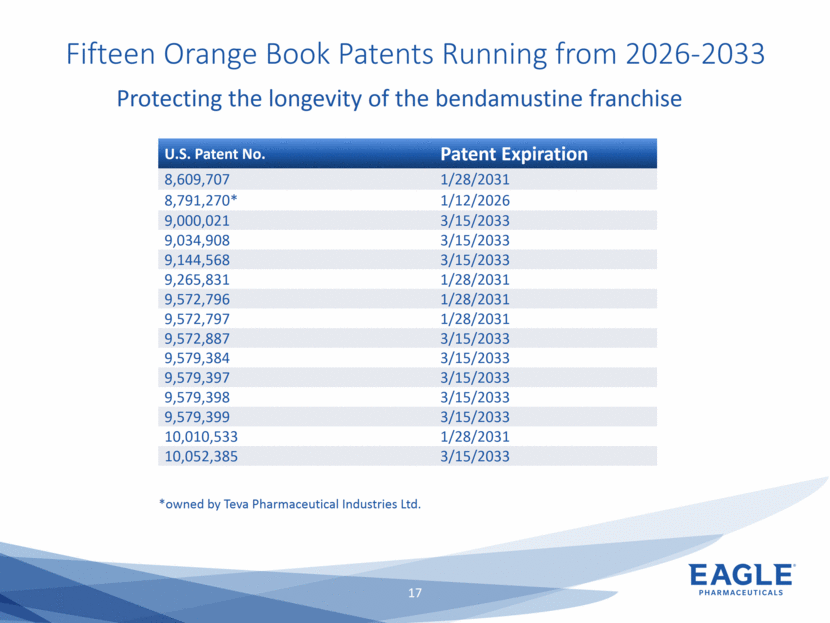
Forward Looking Statements This presentation contains forward-looking information within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, and other securities laws. Forward-looking statements are statements that are not historical facts. Words such as “will,” “underway,” “allow,” “expect(ed),” “pursuing,” “may,” “would,” “addressing,” “creating,” “intends,” “anticipate(s),” “plan,” “partner,” “could,” “enables,” “potential(ly),” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding future events such as: the continued commercial performance of our marketed products, including but not limited to Bendeka, which is marketed by our partner Teva, Ryanodex, which we market ourselves, as well as our ability to replicate our marketing successes for our other product candidates such as Ryanodex for Exertional Heat Stroke (EHS) or other additional indications, our pemetrexed candidate, or our fulvestrant candidate, either through joint or direct marketing efforts; Eagle’s ability to advance Ryanodex in the treatment of Acute Radiation Syndrome (ARS); Eagle’s plans to continue to evaluate the data and conduct further research with respect to Ryanodex in the treatment of ARS; successful compliance with FDA and other governmental regulations applicable to our products and businesses; the label expansions of Ryanodex for EHS patients and for the treatment of neurological impact and nerve agent exposure; our ability to protect the longevity of the bendamustine franchise; the strength of our cash position and the ability to optimize the deployment of capital and take advantage of market opportunities; the continued year over year growth of our revenue, EBITDA, adjusted non-GAAP earnings per share and profit margins; the continued growth of the global biologics market and our ability to use Arsia Therapeutics (now Eagle Biologics) to enter into the biologics market and to effectively carry out our strategy in this new market; the contribution of the Ryanodex portfolio to our growth; the timing of Ryanodex for EHS obtaining FDA approval, if ever, and entering the market; the advancement of any of our other product candidates including, but not limited to, fulvestrant and pemetrexed, through the development process including FDA approval and the ability of any such products to have commercial success and to access significant new markets; the Company’s plans to finance and consummate the stock repurchase program, including the accelerated share repurchase (ASR); and the anticipated outcome of the stock repurchase program, including the ASR. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond our control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. Such risks include, but are not limited to: whether the FDA will ultimately approve Ryanodex for the treatment of EHS and neurological impact of nerve agent exposure; whether we can continue to make progress with the development of fulvestrant, whether our bendamustine product offering will achieve the anticipated market share; fluctuations in the trading column and market price of shares of our common stock; difficulties or delays in manufacturing; the availability and pricing of third party sourced products and materials; the outcome of litigation involving any of our products or product candidates or that may have an impact on any of our products or product candidates, successful compliance with FDA and other governmental regulations applicable to product approvals, manufacturing facilities, products and/or businesses; the strength and enforceability of our intellectual property rights or the rights of third parties; competition from other pharmaceutical and biotechnology companies; the timing of product launches; the successful marketing of our products; the risks inherent in the early stages of drug development and in conducting pre-clinical studies and clinical trials; the possibility that the study results with respect to Ryanodex may be inaccurate or incomplete; management’s determination of alternative needs and uses of our cash resources; the impact of general economic, industry, or political conditions in the United States or internationally; the performance of financial markets, the fluctuation of interest rates; and other factors that are discussed in our Annual Report on Form 10-K for the year ended December 31, 2018, our Quarterly Reports on Form 10-Q, and our other filings with the U.S. Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events.