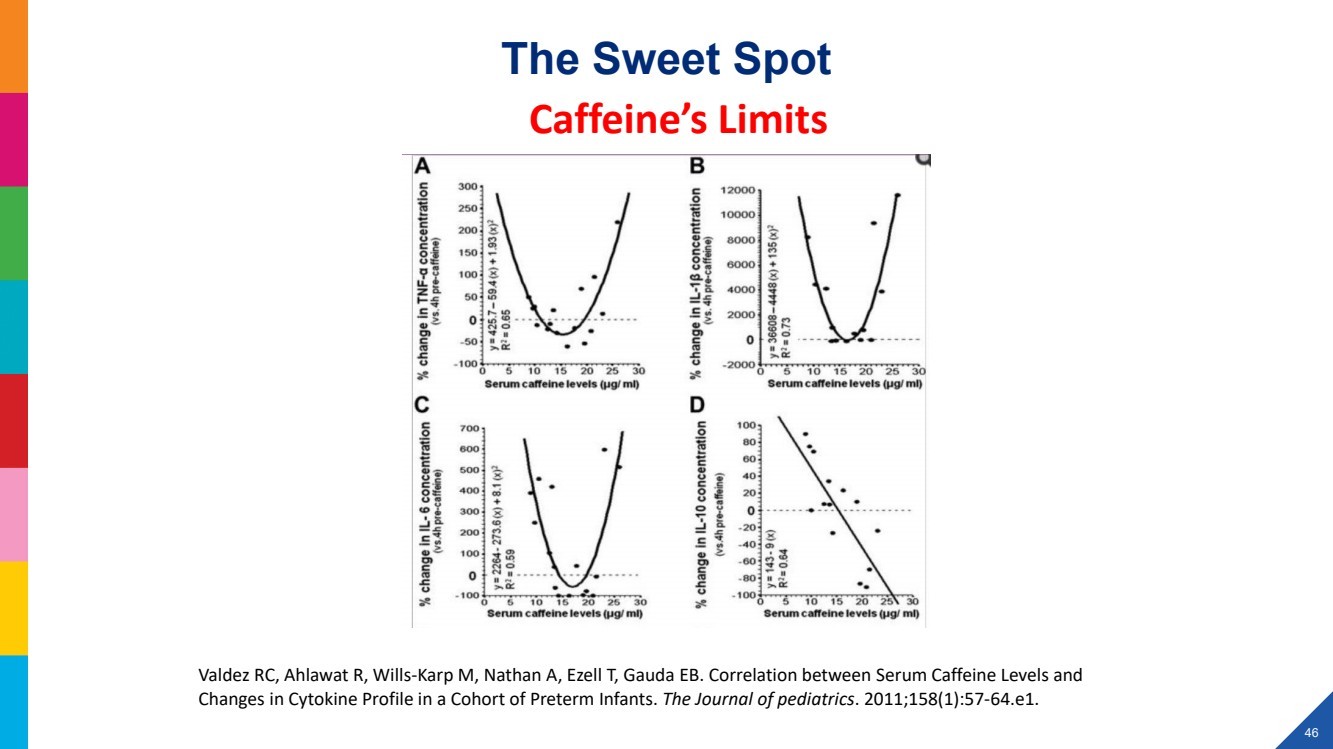
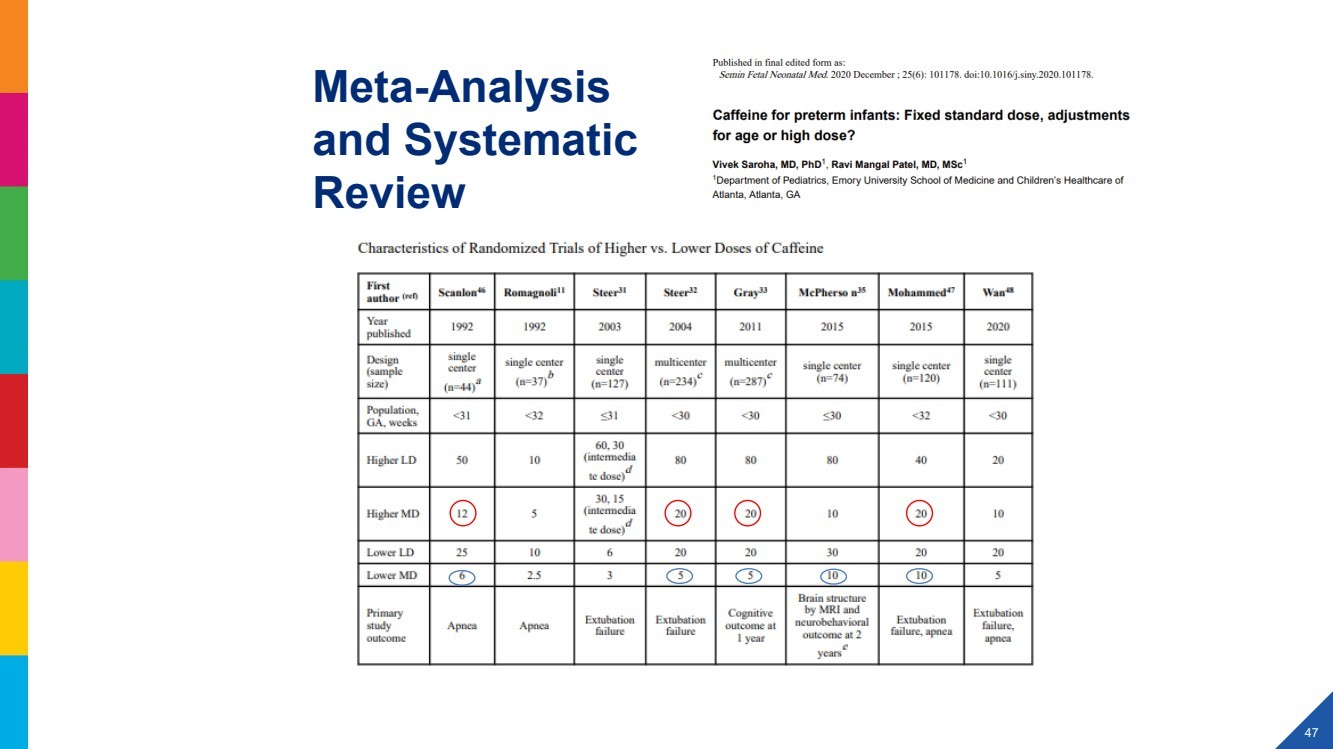
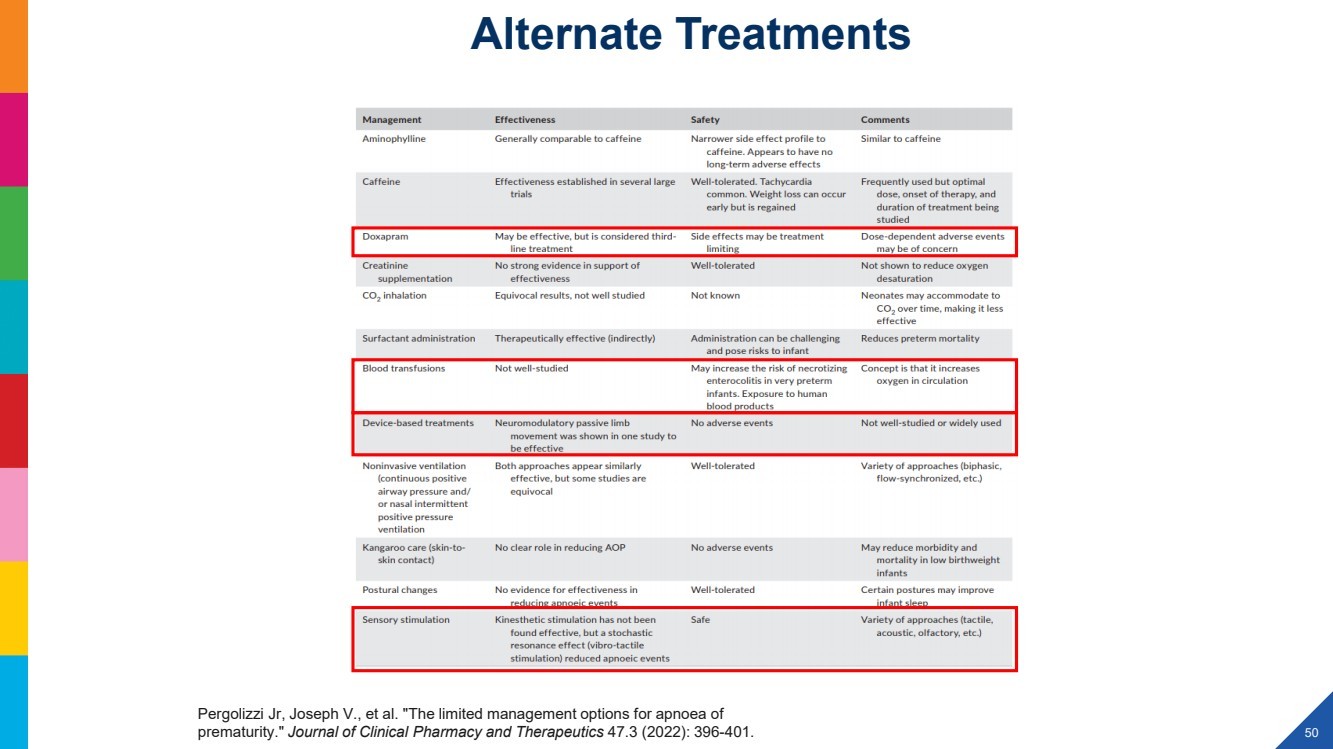
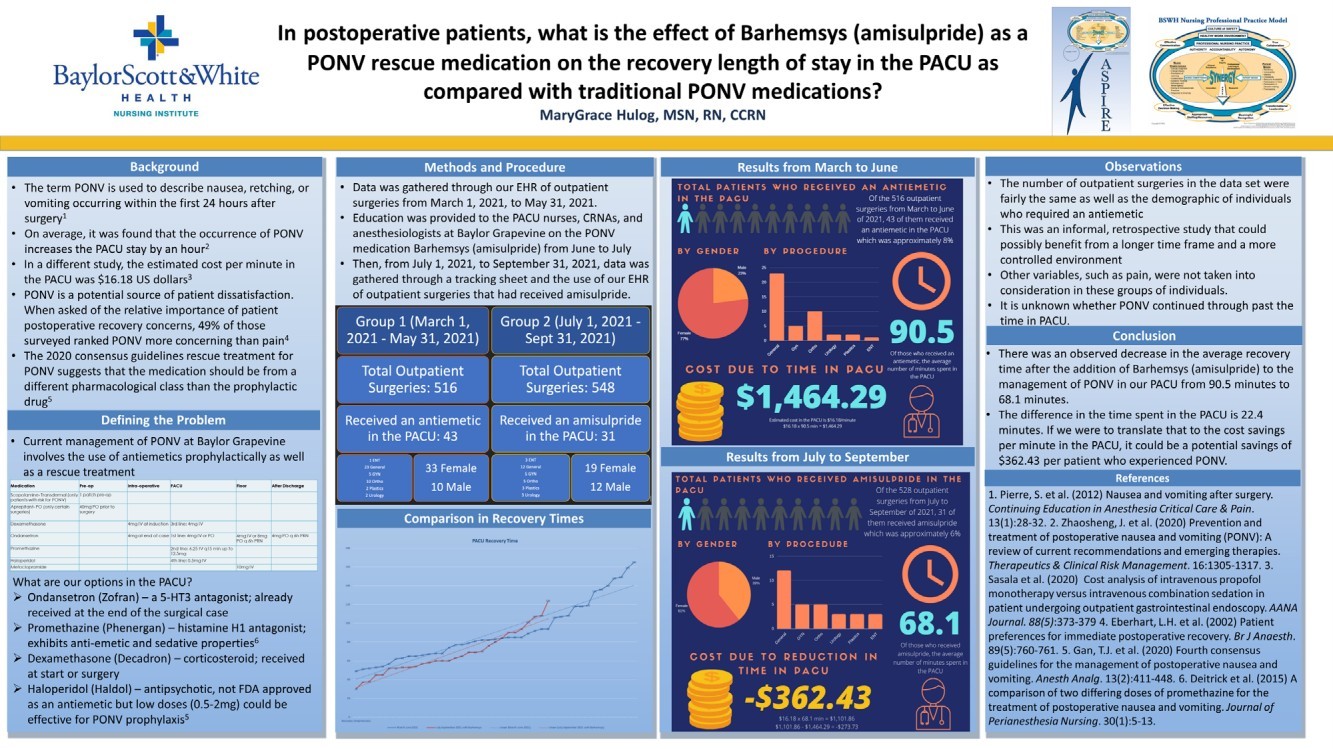
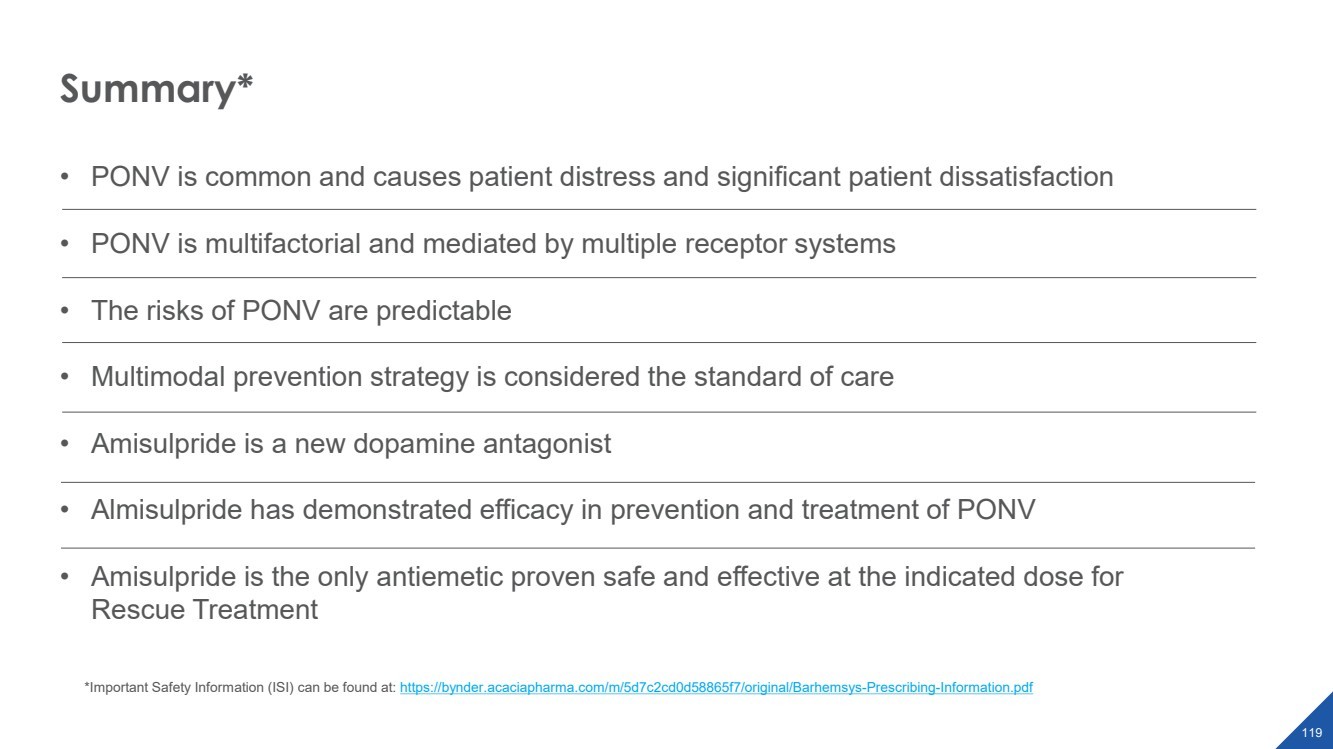
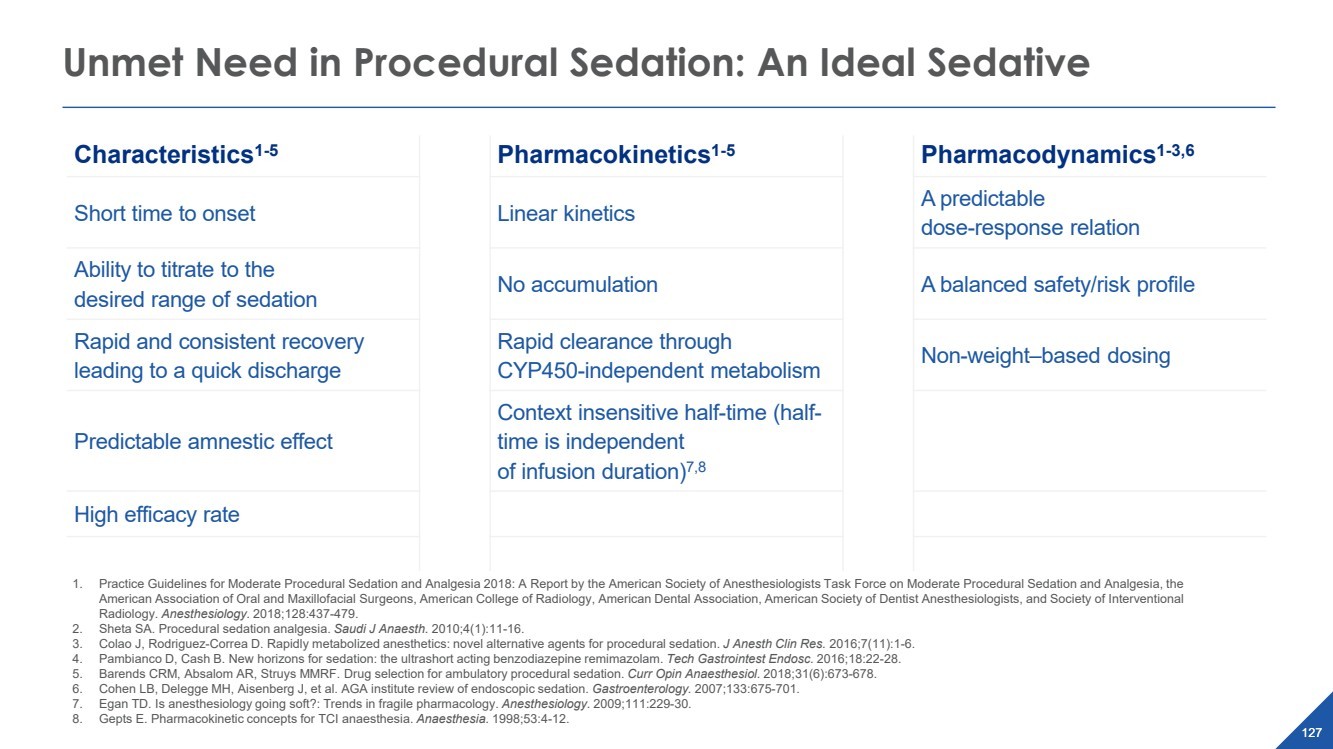
| 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 2 Forward-Looking StatementsThis presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, and other securities law. Forward-looking statements are statements that are not historical facts. Words and phrases such as anticipated, forward, will, would, could, should, may, remain, potential, prepare, expected, believe, plan, near future, belief, guidance, estimate, and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements with respect to: the Companys development programs, products and pipeline; any further investments in Enalare and Enalares development programs; the potential exercise of the Companys option to acquire all of Enalares outstanding shares; the ability of the Companys products to address challenges faced by healthcare providers and hospitals today; the Companys ability to achieve revenue growth; the potential for the Company to transition into a diversified pharmaceutical company with a portfolio of branded, first-in-class assets; the Companys and Enalares ability to obtain and maintain regulatory approval of its products and product candidates; the Company's clinical development plan for its product candidates, including the number and timing of development initiatives or new indications for the Companys product candidates; the ability of the Companys and Enalares products and product candidates; the development of, potential benefits of and expected regulatory activities and matters with respect to the product candidates of the Company and Enalare; the potential therapeutic and economicbenefits of the Companys and Enalares products and product candidates; potential commercial opportunities, addressable markets, patient populations and settings for the Companys and Enalares products and product candidates; the achievement of milestones and deliverables; the potential use of ENA-001 to help preterm infants with respiratory conditions; the ability of ENA-001 and other products and product candidates to address unmet clinical needs, including for patients with post-operative respiratory depression and in combatting community drug overdose; CAL02s ability to neutralize virulence factors produced by bacteria that are commonly associated severe pneumonia; the potential of CAL02 to be a medical breakthrough and offer unique therapeutic benefits to seriously ill patients, potentially improving the treatment regimen for patients with severe community-acquired pneumonia, shortening the duration of illness and improving patient outcomes; the Companys expectations for the design and timing of the planned CAL02Phase 2 study, including with respect to enrollment and site selection and the timing thereof; potential regulatory exclusivity, CAL02s potential eligibility for fast track and breakthrough therapy designations and the potential for a CAL02 new drug applicationfor the treatment of SCABP to qualify for priority review; the ability of hospital environmental trends to bolster the value proposition of the Companys acute care portfolio, including of Barhemsys and Byfavo; the ability of Barhemsys to reduce overall hospital stays;the strategic fit of Barhemsys and Byfavo with the Companys specialized hospital-based salesforce; the Companys marketing, product development, partnering and growth strategy, including relating to the commercialization of Barhemsys and Byfavo, and the ability of Acacias technology and know-how to help the Company achieve its strategy; the ability of Barhemsys, Byfavo and Landiolol to address unmet clinical needs; the ability of Barhemsys to offer significant economic savings to hospitals and ambulatory centers; the ability of Byfavo to offer potential health economic benefits and enable shorter procedure times and greater patient throughput; the potential market opportunity for the Companys products or product candidates, including for Barhemsys, Byfavo or Landiolol; expected patient volumes; the progress and success of the Companys launch of any products; the period of marketing exclusivity forproducts or product candidates, including CAL02; the timing, scope or likelihood and timing of regulatory filings and approvals from the FDA for the Companys product candidates and the Companys ability to maintain regulatory approval of its products and product candidates; the Company's clinical development plan for the product candidates; the implementation of certain healthcare reform measures; the ability of the Company to obtain and maintain coverage and adequate reimbursement for its products; the successofthe Company's collaborations with its strategic partners and the timing and results of these partners preclinical studies and clinical trials, and the Companys potential earnings potential through such collaborations; the Company's plans and ability to advance the product candidate in its pipeline; potential opportunities for, and the Companys ability to complete, business development transactions, in a timely manner, on favorable terms to the Company, or at all; the sufficiency of the Companys cash flows and capital resources and expectations with respect to deployment of cash resources; and the Companys ability to achieve expected future financial performance and results. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predictand generally beyond the Companys control, that could cause actual results to differ materially from those expressed in, or impliedor projected by, the forward-looking information and statements. Such risks and uncertainties include, but are not limited to: the risk that the anticipated benefits of the Companys recently completed transaction with Acacia are not realized; the ability of Enalaretoachieve milestones and deliverables under the BARDA agreement and otherwise accelerate and achieve successful results in the development of ENA-001; the impacts of the COVID-19 pandemic and geopolitical events such as the conflict in Ukraine, including disruption or impact in the sales of the Company's marketed products, interruptions or other adverse effects to clinical trials,delays in regulatory review, manufacturing and supply chain interruptions, adverse effects on healthcare systems, disruption in the operations of the Company's third party partners and disruption of the global economy, and the overall impact of the COVID-19 pandemicor other events on the Company's business, financial condition and results of operations; macroeconomic conditions, including rising inflation and uncertain credit and financial markets; whether the Company will incur unforeseen expenses or liabilities or other market factors; whether the Company will successfully implement its development plan for its product candidates; delay in or failuretoobtain regulatory approval of the Company's or its partners product candidates; whether the Company can successfully market and commercialize its product candidates; the success of the Company's relationships with its partners; the availability and pricingof third party sourced products and materials; the outcome of litigation involving any of its products or that may have an impact on any of our products; successful compliance with the FDA and other governmental regulations applicable to product approvals, manufacturing facilities, products and/or businesses; general economic conditions, including the potential adverse effects of public healthissues, including the COVID-19 pandemic and geopolitical events, on economic activity and the performance of the financial markets generally; the strength and enforceability of the Company's intellectual property rights or the rights of third parties; competition from other pharmaceutical and biotechnology companies and the potential for competition from generic entrants into the market; the risksinherent in the early stages of drug development and in conducting clinical trials; factors in addition to the foregoing that may impact the Companys financial projects and guidance, including among other things, any potential business development transactions, acquisitions, restructurings or legal settlements, in addition to any unanticipated factors, that may cause the Companys actual results and outcomes to materially differ from its projections and guidance; and those risks and uncertainties identified in the Risk Factors sections of the Company's Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission (the SEC) on March 8, 2022, the Companys Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, filedwith the SEC on May 9, 2022, the Companys Quarterly Report on Form 10-Q for the quarter ended June 30, 2022, filed with the SEC on August 9, 2022, the Companys Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, filed with the SEC on November 9, 2022 and its other subsequent filings with the SEC. Readers are cautioned not to place undue reliance on theseforward-looking statements. All forward-looking statements contained in this press release speak only as of the date on which they were made. Except to the extent required by law, the Company undertakes no obligation to update such statements to reflectevents that occur or circumstances that exist after the date on which they were made.This presentation includes statistical and other industry and market data that the Company obtained from industry publications and research, surveys and studies conducted by third parties or us. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guaranteethe accuracy or completeness of such information. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While the Company believes these industry publications and third-party research, surveys and studies are reliable, the Company has not independently verified such data. The industry in which the Company operates is subject to a high degree of uncertainty, change and risk due to a variety of factors, which could cause results to differ materially from those expressed in the estimates made by the independent parties and by the Company.This presentation includes statements and commentary of independent third parties, including key opinion leaders and Enalare,which are strictly the views, opinions and expectations of such third parties and are not the responsibility of the Company. |