|
Exhibit 99.1 
|
Exhibit 99.1
Spectrum Pharmaceuticals
Analyst Day 2015
Safe Harbor Statement
This presentation contains forward-looking statements regarding future events and the future performance of Spectrum Pharmaceuticals that involve risks and uncertainties that could cause actual results to differ materially. These statements are based on management’s current beliefs and expectations. These statements include but are not limited to statements that relate to our business and its future, our strategy, the success of our drug candidates, the safety and efficacy of our drug products, product approvals, market potential, product sales, revenue, development, regulatory and approval timelines, product launches, product acquisitions, capital resources and any statements that relate to the intent, belief, plans or expectations of Spectrum or its management, or that are not a statement of historical fact.
Risks that could cause actual results to differ include the possibility that our existing and new drug candidates may not prove safe or effective, the possibility that our existing and new drug candidates may not receive approval from the FDA and other regulatory agencies in a timely manner or at all, the possibility that our existing and new drug candidates, if approved, may not be more effective, safer or more cost efficient than competing drugs, the possibility that price and other competitive pressures may make the marketing and sale of our drugs not commercially feasible, the possibility that our efforts to acquire or in-license and develop additional drug candidates may fail, our lack of sustained revenue history, our limited experience in establishing strategic alliances, our limited marketing experience, our customer concentration, the possibility for fluctuations in customer orders, evolving market dynamics, our dependence on third parties for clinical trials, manufacturing, distribution, information and quality control and other risks that are described in further detail in the Company’s reports filed with the Securities and Exchange Commission. We do not plan to update any such forward-looking statements and expressly disclaim any duty to update the information contained in this presentation except as required by law.
2
Analyst Day Agenda
Overall Business Strategy Dr. Raj Shrotriya
Financial Review Kurt Gustafson
Operational Highlights Joe Turgeon
Research & KOL Presentations Dr. Lee Allen
Commercial Perspective Tom Riga
Questions & Answers Team
Introduction
Rajesh Shrotriya
Chairman and CEO
Spectrum’s Focus & Overview
AT SPECTRUM PHARMACEUTICALS
“WE BRING OUR EXPERTISE AND PASSION FOR EXCELLENCE TO ACQUIRE, DEVELOP AND COMMERCIALIZE PHARMACEUTICALS FOR UNMET MEDICAL NEEDS WHILE BUILDING VALUE FOR OUR SHAREHOLDERS.”
5
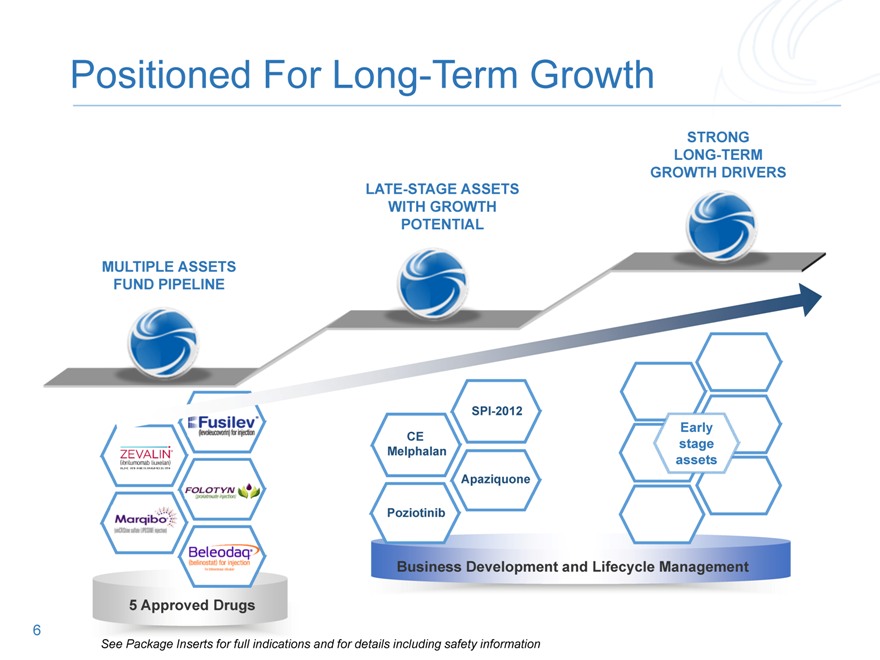
Positioned For Long-Term Growth
STRONG LONG-TERM
GROWTH DRIVERS
MULTIPLE ASSETS
FUND
Your text here
Your text here
Your text here
Your text here
LATE-STAGE ASSETS
WITH GROWTH
POTENTIAL
CE
Melphalan
Your text here
Poziotinib
Business Development and Lifecycle Management
Your text here
Apaziquone
stage
assets
See Package Inserts for full indications and for details including safety information
Financial Review
Kurt Gustafson
Executive Vice President, Chief Financial Officer
and Principal Accounting Officer
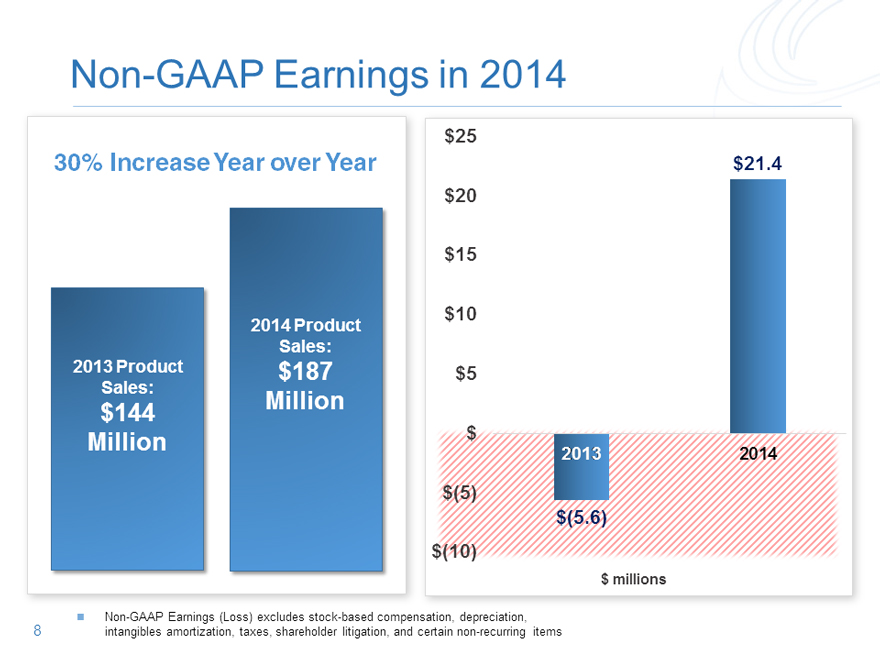
Non-GAAP Earnings in 2014
$25
30% Increase Year over Year $21.4
$20
$15
2014 Product $10
Sales:
2013 Product $187 $5
Sales:
$144 Million
Million $ 2013 2014
$(5)
$(5.6)
$(10)
Non-GAAP Earnings (Loss) excludes stock-based compensation, depreciation, 8 intangibles amortization, taxes, shareholder litigation, and certain non-recurring items
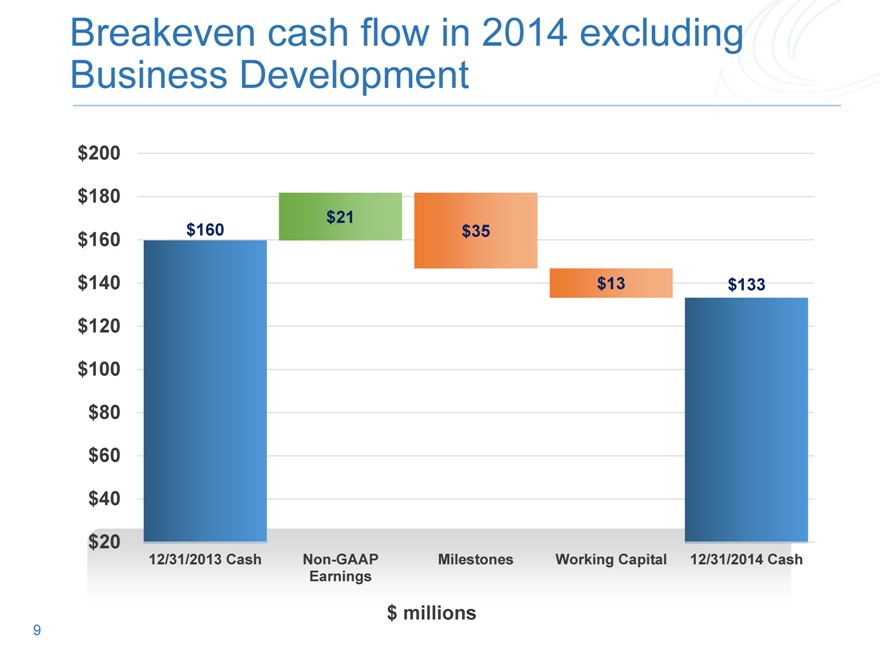
Breakeven cash flow in 2014 excluding Business Development
$200
$180
$21
$160 $160 $35
$140 $13 $133
$120
$100
$80
$60
$40
$20
12/31/2013 Cash Non-GAAP Milestones Working Capital 12/31/2014 Cash
Earnings
$ millions
9
Operational Highlights
Joseph Turgeon
President and Chief Operating Officer
Key Recent Milestones
2Q 2014 3Q 2014 4Q 2014 1Q 2015 TODAY
11
Analyst Day Highlights
12
Spectrum is Positioned For Long-Term Growth
SPI-2012: Late-stage drug targeting blockbuster market
CE Melphalan: Under FDA review PDUFA date: October 23, 2015
Apaziquone: Late-stage drug could satisfy unmet need in bladder cancer
Poziotinib: A promising Phase 2 pan-HER inhibitor
13
Positioned For Long-Term Growth
STRONG LONG-TERM
GROWTH DRIVERS LATE-STAGE ASSETS
WITH GROWTH POTENTIAL
MULTIPLE ASSETS
Your text here CE
stage
Melphalan Your text here
Your text here assets
Apaziquone
Your text here Your text here Poziotinib Your text here
Business Development and Lifecycle Management
See Package Inserts for full indications and for details including safety information
14
Research and KOL Presentations
Lee Allen, MD PhD
Chief Medical Officer
Spectrum’s Medical Development Group Aggressively Driving Portfolio Advancement
PROMISING FUTURE
STRATEGIC DEVELOPMENT
THE
DELIVERING
Multiple pipeline assets
Experienced TEAM
Proven track record of delivery
16
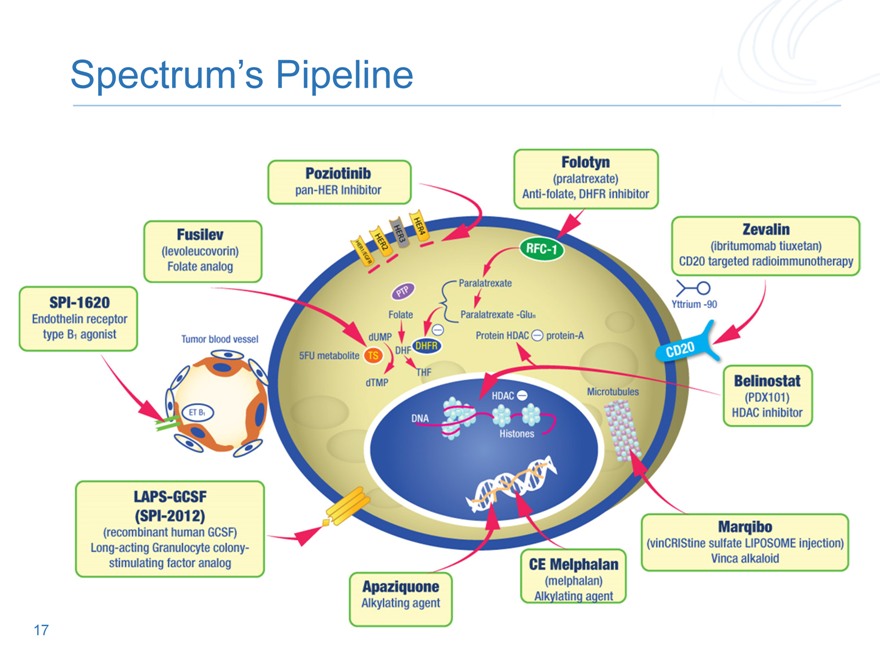
Spectrum’s Pipeline
17
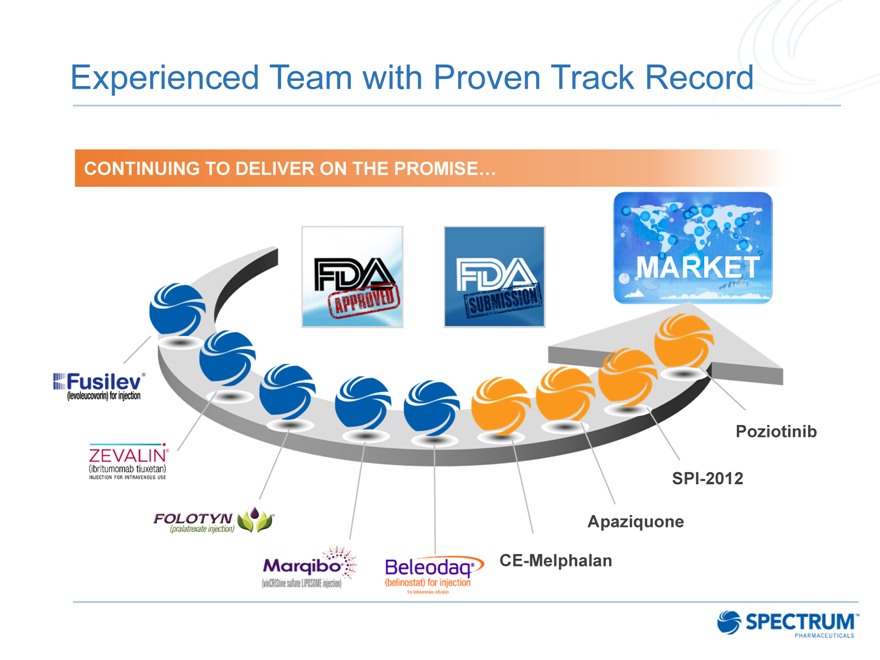
Experienced Team with Proven Track Record
CONTINUING TO DELIVER ON THE PROMISE…
Poziotinib
SPI-2012 Apaziquone CE-Melphalan
KOL Presentations
SPI-2012
Novel biologic with blockbuster potential to mitigate neutropenia
CE Melphalan
Propylene glycol-free Melphalan with an improved stability profile for the treatment of Multiple Myeloma
Apaziquone
Next generation alkylating agent addressing an unmet medical need for the treatment of Non Muscle Invasive Bladder Cancer
Poziotinib
Pan-HER inhibitor with strong early data in breast cancer and activity in other solid tumors
Dr. Jeff Vacirca
Dr. Parameswaran Hari
Dr. Fred Witjes
Dr. Francisco Esteva
Spectrum Pharmaceuticals
Analyst Day 2015
SPI-2012
Jeffrey L. Vacirca
MD, FACP
SPI-2012
CEO, Managing Partner & Chief of Clinical Research at North Shore Hematology/Oncology Associates
Medical Director for ION/ASBSG
Vice-President, Community Oncology Alliance
CEO & President National Translational Research Group.
Our Involvement With SPI-2012 Phase 2 Studies
22
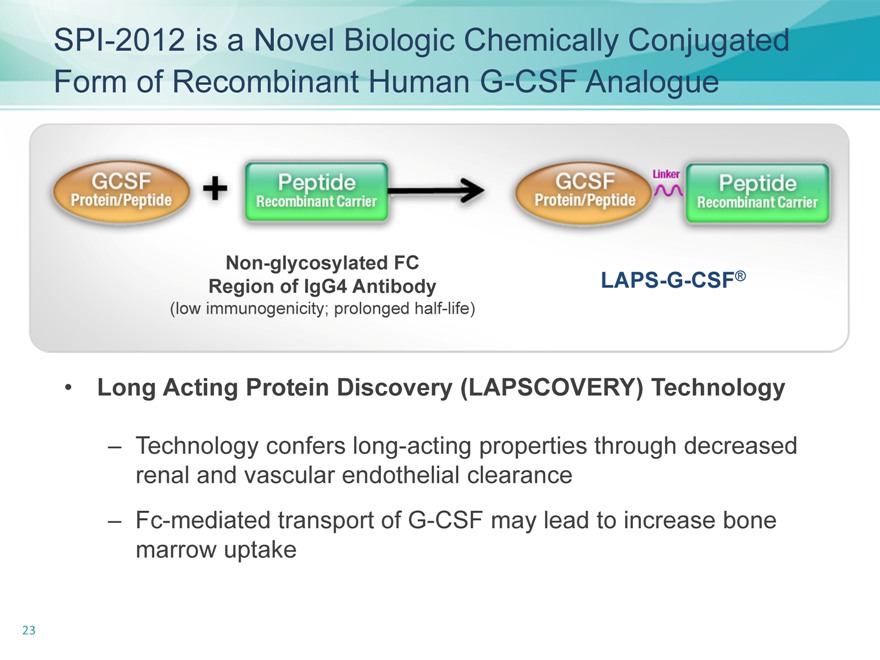
SPI-2012 is a Novel Biologic Chemically Conjugated Form of Recombinant Human G-CSF Analogue
Non-glycosylated FC
Region of IgG4 Antibody
(low immunogenicity; prolonged half-life)
LAPS-G-CSF®
Long Acting Protein Discovery (LAPSCOVERY) Technology
– Technology confers long-acting properties through decreased renal and vascular endothelial clearance
– Fc-mediated transport of G-CSF may lead to increase bone marrow uptake
23
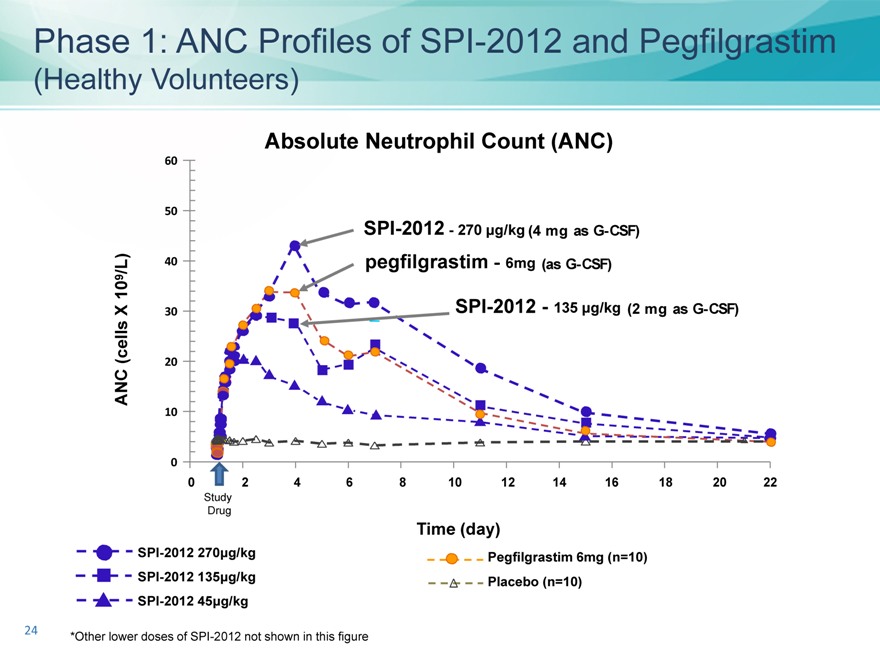
Phase 1: ANC Profiles of SPI-2012 and Pegfilgrastim
(Healthy Volunteers)
Absolute Neutrophil Count (ANC)
60
50
SPI-2012—270 ìg/kg (4 mg as G-CSF)
/L) 40 pegfilgrastim ?6mg (as G-CSF)
10 9
X 30 SPI-2012—135 ìg/kg (2 mg as G-CSF)
(cells 20 ANC
10
0
0 2 4 6 8 10 12 14 16 18 20 22
Study Drug
Time (day)
SPI-2012 270ìg/kg Pegfilgrastim 6mg (n=10) SPI-2012 135ìg/kg Placebo (n=10) SPI-2012 45ìg/kg
*Other lower doses of SPI-2012 not shown in this figure
24
SPI-2012 Phase 2 Study Summary
25
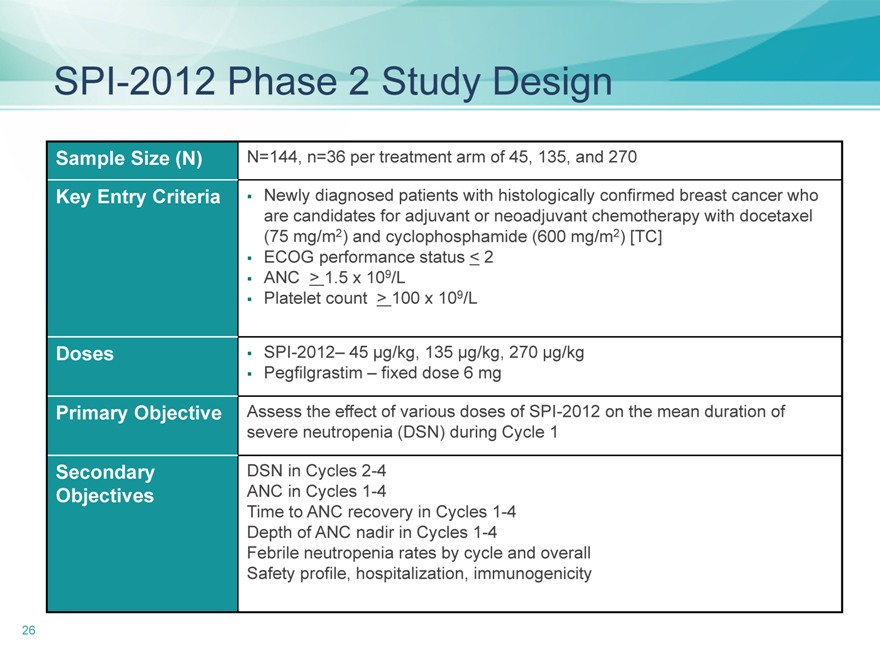
SPI-2012 Phase 2 Study Design
Sample Size (N)
Key Entry Criteria
Doses
Primary Objective
Secondary
Objectives
N=144, n=36 per treatment arm of 45, 135, and 270
Newly diagnosed patients with histologically confirmed breast cancer who
are candidates for adjuvant or neoadjuvant chemotherapy with docetaxel
(75 mg/m2) and cyclophosphamide (600 mg/m2) [TC]
ECOG performance status < 2
ANC > 1.5 x 109/L
Platelet count > 100 x 109/L
SPI-2012– 45 µg/kg, 135 µg/kg, 270 µg/kg
Pegfilgrastim – fixed dose 6 mg
Assess the effect of various doses of SPI-2012 on the mean duration of
severe neutropenia (DSN) during Cycle 1
DSN in Cycles 2-4
ANC in Cycles 1-4
Time to ANC recovery in Cycles 1-4
Depth of ANC nadir in Cycles 1-4
Febrile neutropenia rates by cycle and overall
Safety profile, hospitalization, immunogenicity
26
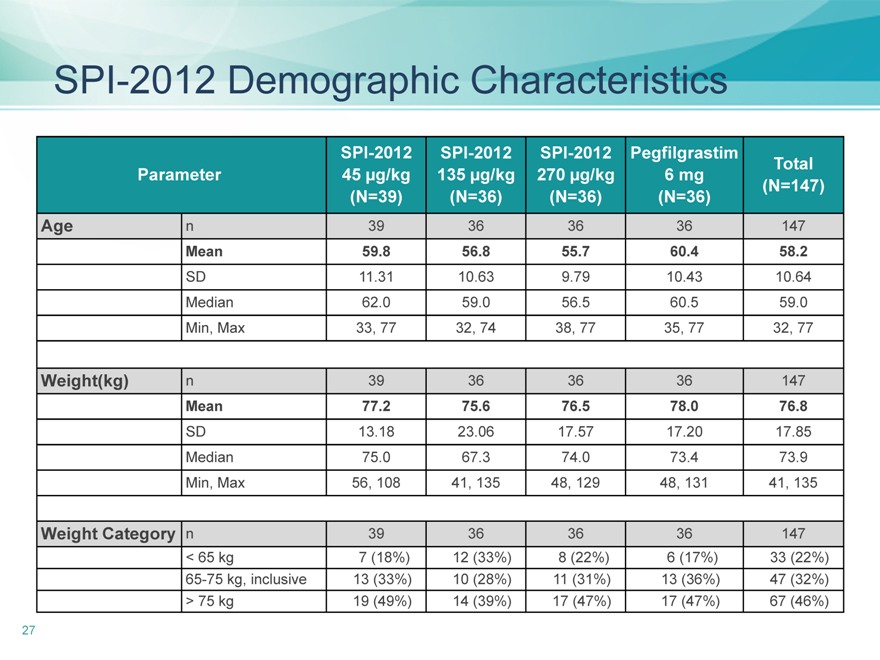
SPI-2012 Demographic Characteristics
SPI-2012 SPI-2012 SPI-2012 Pegfilgrastim Total
Parameter 45 µg/kg 135 µg/kg 270 µg/kg 6 mg(N=147)
(N=39)(N=36)(N=36)(N=36)
Age n39 36 36 36 147
Mean 59.8 56.8 55.7 60.4 58.2
SD 11.31 10.63 9.79 10.43 10.64
Median 62.0 59.0 56.5 60.5 59.0
Min, Max 33, 77 32, 74 38, 77 35, 77 32, 77
Weight(kg) n 39 36 36 36 147
Mean 77.2 75.6 76.5 78.0 76.8
SD 13.18 23.06 17.57 17.20 17.85
Median 75.0 67.3 74.0 73.4 73.9
Min, Max 56, 108 41, 135 48, 129 48, 131 41, 135
Weight Category n 39 36 36 36 147
< 65 kg 7 (18%) 12 (33%) 8 (22%) 6 (17%) 33 (22%)
65-75 kg, inclusive 13 (33%) 10 (28%) 11 (31%) 13 (36%) 47 (32%)
> 75 kg 19 (49%) 14 (39%) 17 (47%) 17 (47%) 67 (46%)
27
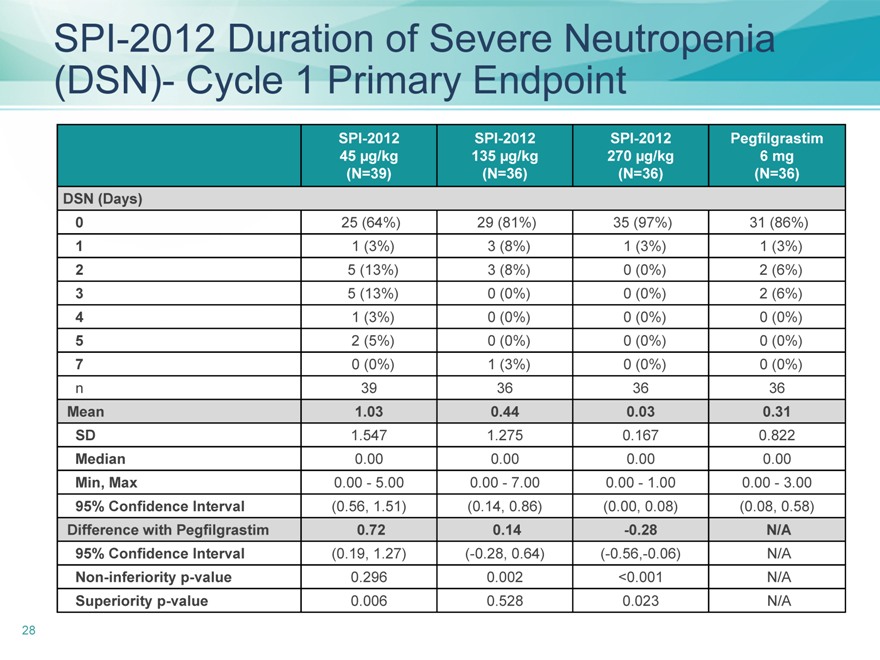
SPI-2012 Duration of Severe Neutropenia (DSN)- Cycle 1 Primary Endpoint
SPI-2012 SPI-2012 SPI-2012 Pegfilgrastim
45 | | µg/kg 135 µg/kg 270 µg/kg 6 mg |
(N=39)(N=36)(N=36)(N=36)
DSN (Days)
0 25 (64%) 29 (81%) 35 (97%) 31 (86%)
1 | | 1 (3%) 3 (8%) 1 (3%) 1 (3%) |
2 | | 5 (13%) 3 (8%) 0 (0%) 2 (6%) |
3 | | 5 (13%) 0 (0%) 0 (0%) 2 (6%) |
4 | | 1 (3%) 0 (0%) 0 (0%) 0 (0%) |
5 | | 2 (5%) 0 (0%) 0 (0%) 0 (0%) |
7 | | 0 (0%) 1 (3%) 0 (0%) 0 (0%) |
n 39 36 36 36
Mean 1.03 0.44 0.03 0.31
SD 1.547 1.275 0.167 0.822
Median 0.00 0.00 0.00 0.00
Min, Max 0.00—5.00 0.00—7.00 0.00—1.00 0.00—3.00
95% Confidence Interval(0.56, 1.51)(0.14, 0.86)(0.00, 0.08)(0.08, 0.58)
Difference with Pegfilgrastim 0.72 0.14 -0.28 N/A
95% Confidence Interval(0.19, 1.27)(-0.28, 0.64)(-0.56,-0.06) N/A
Non-inferiority p-value 0.296 0.002 <0.001 N/A
Superiority p-value 0.006 0.528 0.023 N/A
28
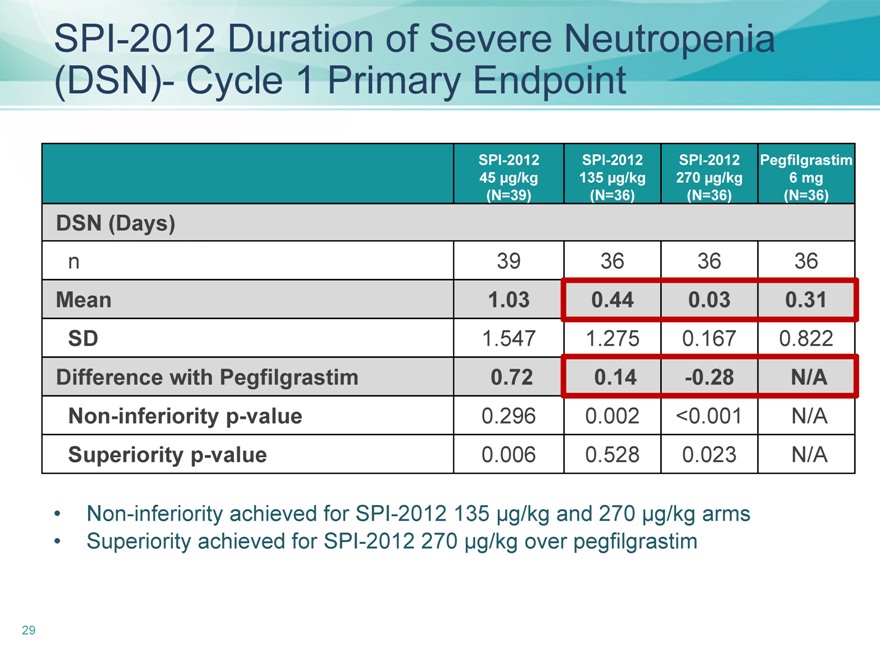
SPI-2012 Duration of Severe Neutropenia (DSN)- Cycle 1 Primary Endpoint
SPI-2012 SPI-2012 SPI-2012 Pegfilgrastim
45 | | µg/kg 135 µg/kg 270 µg/kg 6 mg |
(N=39)(N=36)(N=36)(N=36)
DSN (Days)
n 39 36 36 36
Mean 1.03 0.44 0.03 0.31
SD 1.547 1.275 0.167 0.822
Difference with Pegfilgrastim 0.72 0.14 -0.28 N/A
Non-inferiority p-value 0.296 0.002 <0.001 N/A
Superiority p-value 0.006 0.528 0.023 N/A
Non-inferiority achieved for SPI-2012 135 µg/kg and 270 µg/kg arms
Superiority achieved for SPI-2012 270 µg/kg over pegfilgrastim
29
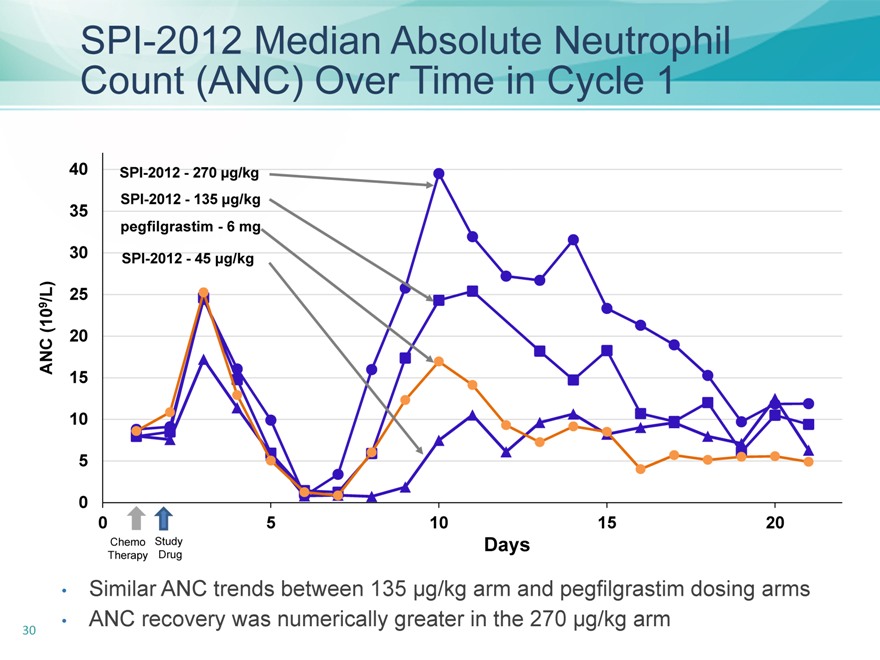
SPI-2012 Median Absolute Neutrophil Count (ANC) Over Time in Cycle 1
40 SPI-2012—270 ìg/kg SPI-2012—135 ìg/kg
35 pegfilgrastim—6 mg
30 SPI-2012—45 ìg/kg
/L) 25 9 (10 ANC 20 15
10
0
0 5 10 15 20
Chemo Study Days Therapy Drug
Similar ANC trends between 135 µg/kg arm and pegfilgrastim dosing arms
ANC recovery was numerically greater in the 270 µg/kg arm
30
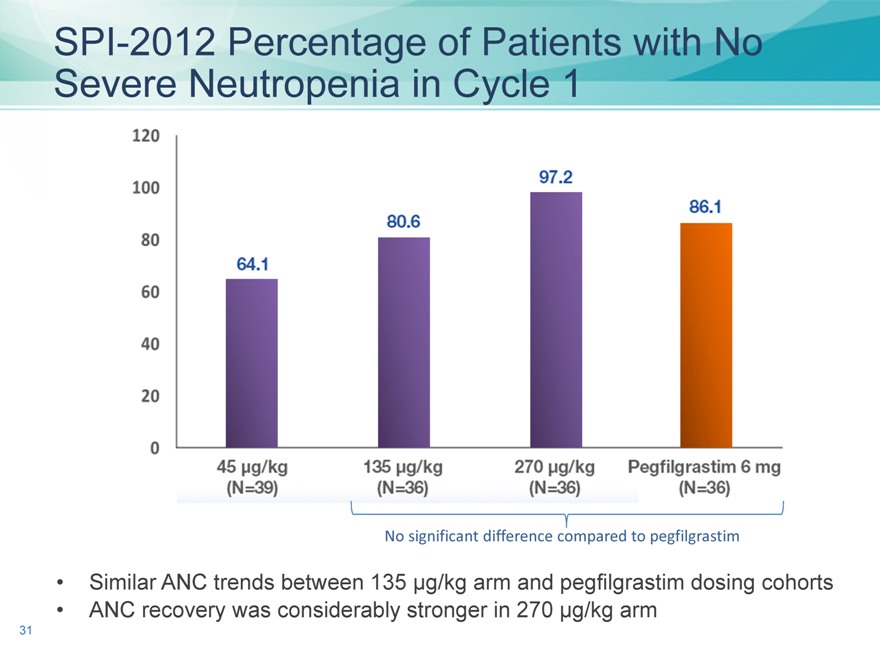
SPI-2012 Percentage of Patients with No Severe Neutropenia in Cycle 1
No significant difference compared to pegfilgrastim
Similar ANC trends between 135 µg/kg arm and pegfilgrastim dosing cohorts
ANC recovery was considerably stronger in 270 µg/kg arm
31
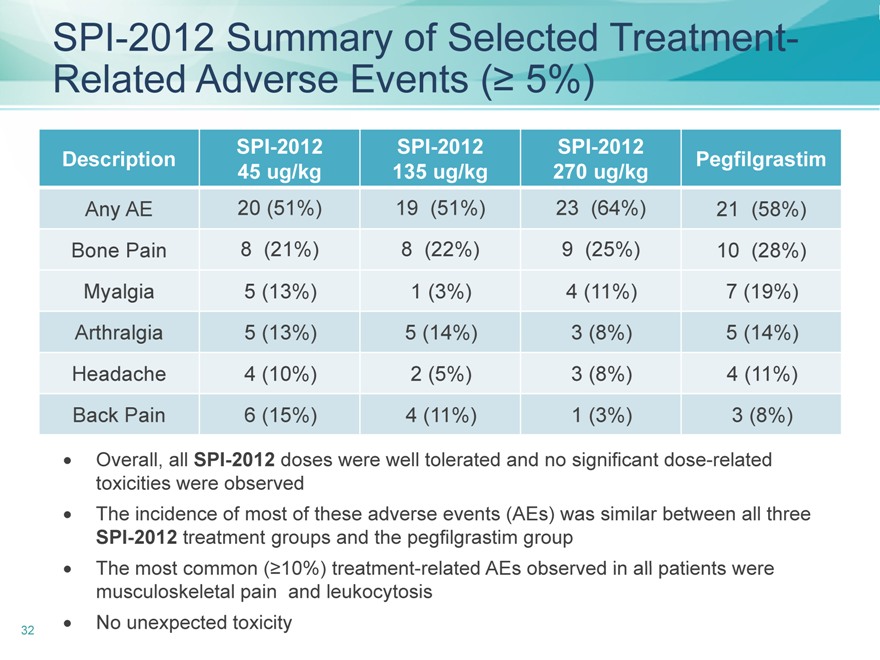
SPI-2012 Summary of Selected Treatment-Related Adverse Events (? 5%)
SPI-2012 SPI-2012 SPI-2012
Description Pegfilgrastim
45 | | ug/kg 135 ug/kg 270 ug/kg |
Any AE 20 (51%) 19(51%) 23(64%) 21(58%)
Bone Pain 8(21%) 8(22%) 9(25%) 10(28%)
Myalgia 5(13%) 1(3%) 4 (11%) 7(19%)
Arthralgia 5(13%) 5 (14%) 3(8%) 5(14%)
Headache 4(10%) 2(5%) 3(8%) 4 (11%)
Back Pain 6(15%) 4 (11%) 1(3%) 3 (8%)
Overall, all SPI-2012 doses were well tolerated and no significant dose-related toxicities were observed? The incidence of most of these adverse events (AEs) was similar between all three SPI-2012 treatment groups and the pegfilgrastim group? The most common (?10%) treatment-related AEs observed in all patients were musculoskeletal pain and leukocytosis
No unexpected toxicity
32
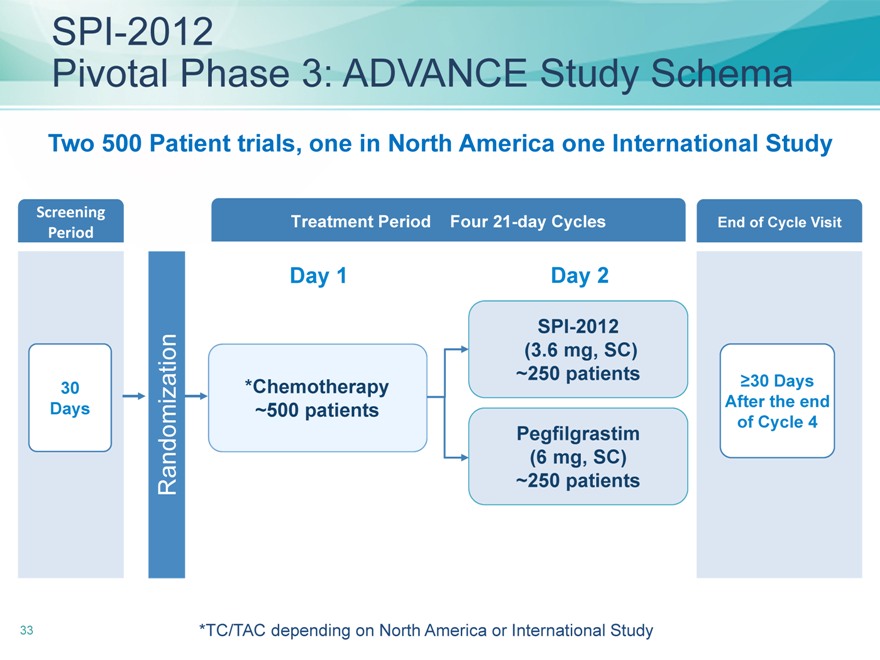
SPI-2012
Pivotal Phase 3: ADVANCE Study Schema
Two 500 Patient trials, one in North America one International Study
Screening Treatment Period Four 21-day Cycles End of Cycle Visit
Period
Day 1 Day 2
SPI-2012
30*Chemotherapy
250 patients ?30 Days
Days
500 patients After the end
Pegfilgrastim of Cycle 4
(6 mg, SC)
Randomization
250 patients
*TC/TAC depending on North America or International Study
33
SPI-2012
Clinical Summary to Date
Over 230 patients treated to date
Innovative biologic based on elegant science
Non-inferior efficacy to pegfilgrastim
Comparable safety profile to pegfilgrastim
Low immunogenicity
Novel biologic expands patient options
34
Captisol-Enabled™ Melphalan
Parameswaran Hari,
MD, MRCP, MS
Captisol-Enabled™ Melphalan
Armand J. Quick/William F. Stapp Professor of Hematology Medical College of Wisconsin
Director of the Adult Blood and Marrow Transplant Program
Section Head of Hematologic Malignancies and Transplantation
Division of Hematology and Oncology
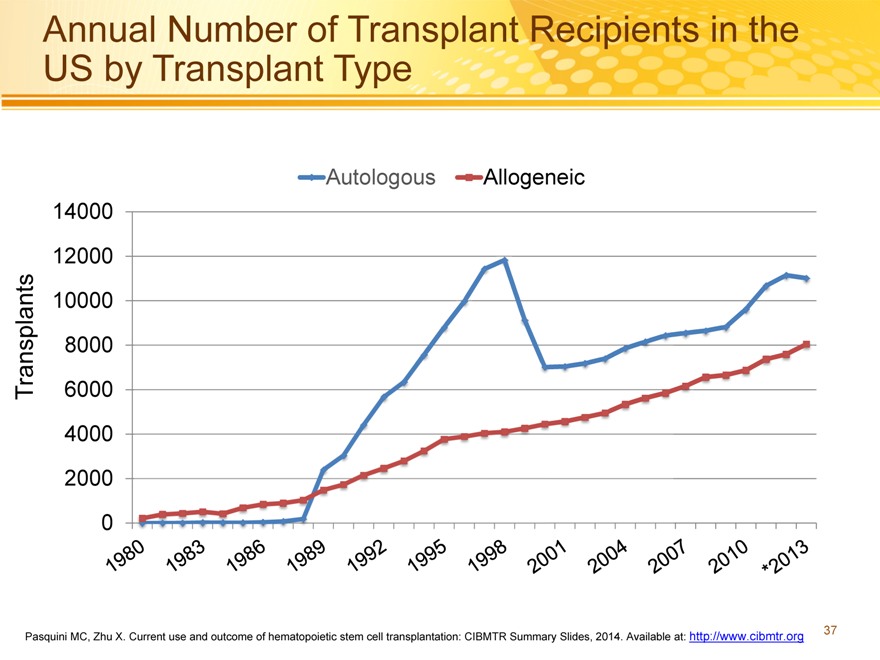
Annual Number of Transplant Recipients in the US by Transplant Type
Autologous Allogeneic 14000 12000 10000 8000
Transplants 6000 4000 2000 0
Pasquini MC, Zhu X. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2014. Available at: http://www.cibmtr.org
37
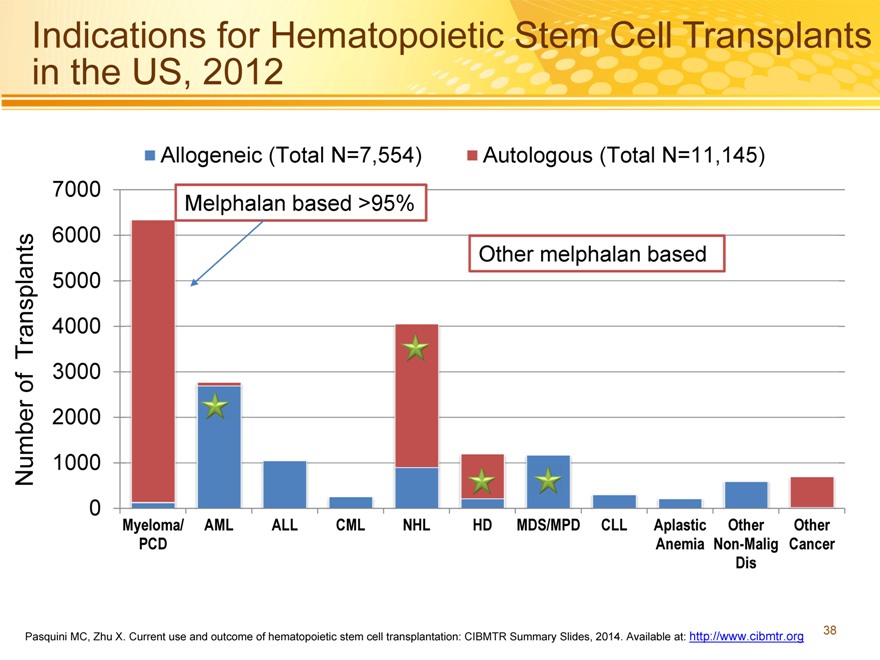
Indications for Hematopoietic Stem Cell Transplants in the US, 2012
Allogeneic (Total N=7,554) Autologous (Total N=11,145) 7000 Melphalan based >95% 6000 Other melphalan based 5000
Transplants 4000
3000 of
2000
Number 1000
0 Myeloma/ AML ALL CML NHL HD MDS/MPD CLL Aplastic Other Other PCD Anemia Non-Malig Cancer Dis
Pasquini MC, Zhu X. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2014. Available at: http://www.cibmtr.org
38
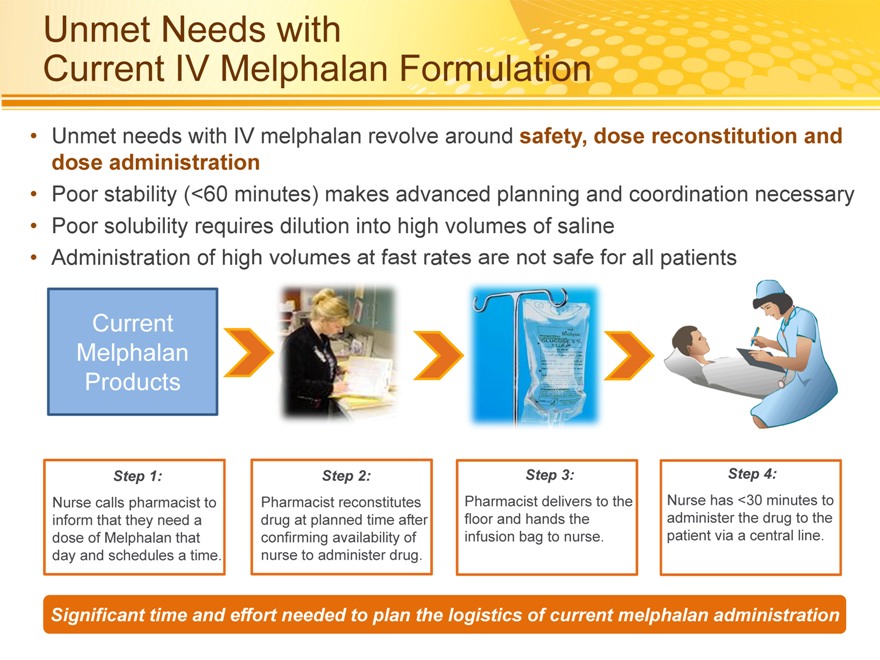
Unmet Needs with
Current IV Melphalan Formulation
Unmet needs with IV melphalan revolve around safety, dose reconstitution and dose administration
Poor stability (<60 minutes) makes advanced planning and coordination necessary
Poor solubility requires dilution into high volumes of saline
Administration of high volumes at fast rates are not safe for all patients
Current Melphalan Products
Step 1:
Nurse calls pharmacist to inform that they need a dose of Melphalan that day and schedules a time.
Step 2:
Pharmacist reconstitutes drug at planned time after confirming availability of nurse to administer drug.
Step 3:
Pharmacist delivers to the floor and hands the infusion bag to nurse.
Step 4:
Nurse has <30 minutes to administer the drug to the patient via a central line.
Significant time and effort needed to plan the logistics of current melphalan administration
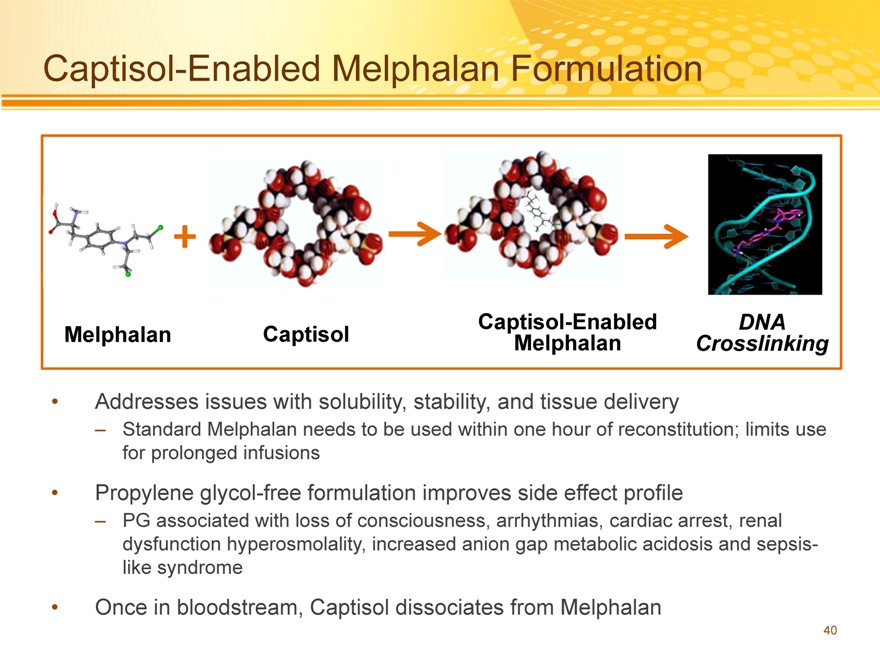
Captisol-Enabled Melphalan Formulation
+
Captisol-Enabled DNA Melphalan Captisol Melphalan Crosslinking
Addresses issues with solubility, stability, and tissue delivery
– Standard Melphalan needs to be used within one hour of reconstitution; limits use for prolonged infusions
Propylene glycol-free formulation improves side effect profile
– PG associated with loss of consciousness, arrhythmias, cardiac arrest, renal dysfunction hyperosmolality, increased anion gap metabolic acidosis and sepsis-like syndrome
Once in bloodstream, Captisol dissociates from Melphalan
40
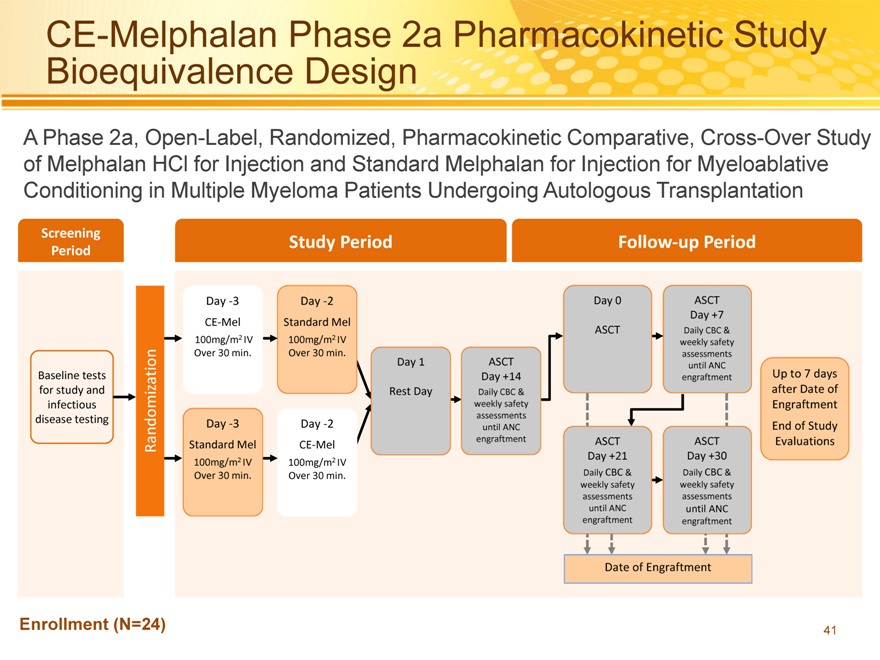
CE-Melphalan Phase 2a Pharmacokinetic Study Bioequivalence Design
A Phase 2a, Open-Label, Randomized, Pharmacokinetic Comparative, Cross-Over Study of Melphalan HCl for Injection and Standard Melphalan for Injection for Myeloablative Conditioning in Multiple Myeloma Patients Undergoing Autologous Transplantation
Screening Study Period Follow?up Period
Period
Day ?3 Day ?2 Day 0 ASCT
Day +7
CE?Mel Standard Mel
ASCT Daily CBC &
100mg/m2 IV 100mg/m2 IV weekly safety
Over 30 min. Over 30 min. assessments
Day 1 ASCT until ANC
Baseline tests Day +14 engraftment Up to 7 days
for study and Rest Day Daily CBC & after Date of
infectious weekly safety Engraftment
assessments
disease testing Day ?3 Day ?2 until ANC End of Study
Randomization Standard Mel CE?Mel engraftment ASCT ASCT Evaluations
100mg/m2 IV 100mg/m2 IV Day +21 Day +30
Over 30 min. Over 30 min. Daily CBC & Daily CBC &
weekly safety weekly safety
assessments assessments
until ANC until ANC
engraftment engraftment
Date of Engraftment
Enrollment (N=24)
41
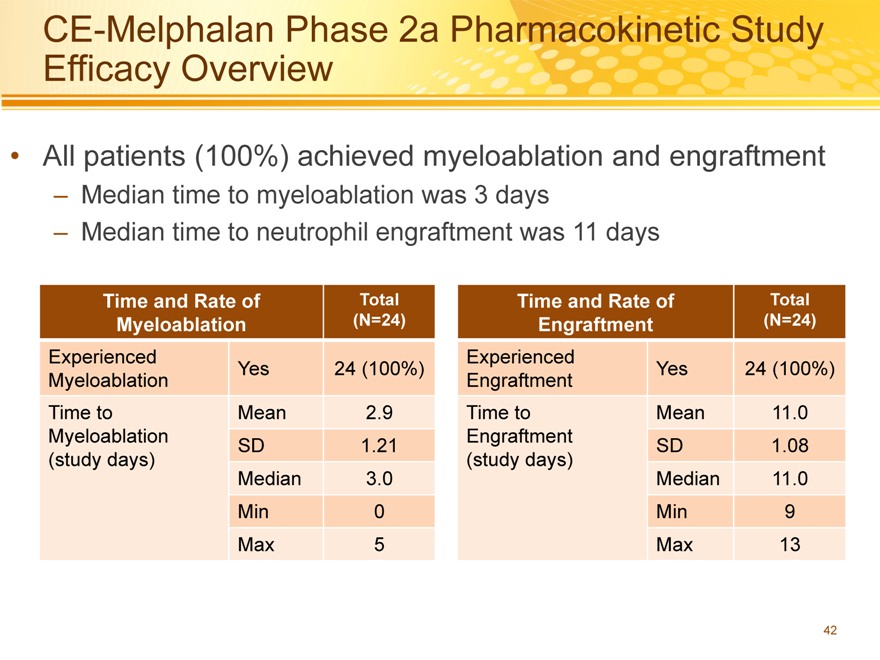
CE-Melphalan Phase 2a Pharmacokinetic Study Efficacy Overview
All patients (100%) achieved myeloablation and engraftment
– Median time to myeloablation was 3 days
– Median time to neutrophil engraftment was 11 days
Time and Rate of Total Time and Rate of Total
Myeloablation(N=24) Engraftment(N=24)
Experienced Experienced
Yes 24 (100%) Yes 24 (100%)
Myeloablation Engraftment
Time to Mean 2.9 Time to Mean 11.0
Myeloablation Engraftment
SD 1.21 SD 1.08
(study days)(study days)
Median 3.0 Median 11.0
Min 0 Min 9
Max 5 Max 13
42
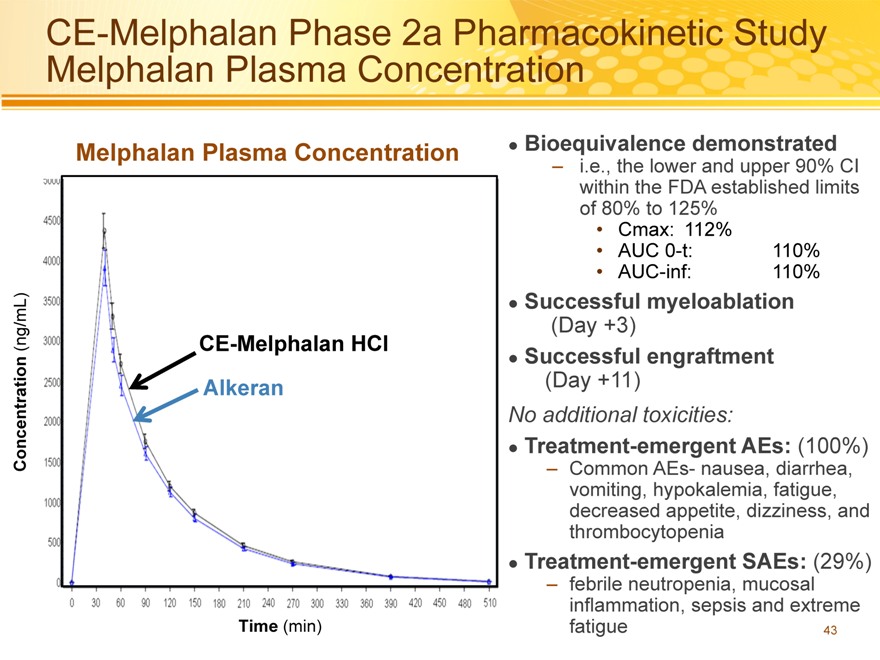
CE-Melphalan Phase 2a Pharmacokinetic Study Melphalan Plasma Concentration
Bioequivalence demonstrated
– i.e., the lower and upper 90% CI within the FDA established limits of 80% to 125%
Cmax: 112%
AUC 0-t: 110%
AUC-inf: 110%
Successful myeloablation
(Day +3)
Successful engraftment
(Day +11)
No additional toxicities:
Treatment-emergent AEs: (100%)
– Common AEs- nausea, diarrhea, vomiting, hypokalemia, fatigue, decreased appetite, dizziness, and thrombocytopenia
Treatment-emergent SAEs: (29%)
– febrile neutropenia, mucosal inflammation, sepsis and extreme fatigue 43
Melphalan Plasma Concentration
(ng/mL) CE-Melphalan HCl
Concentration Alkeran
Time (min)
43
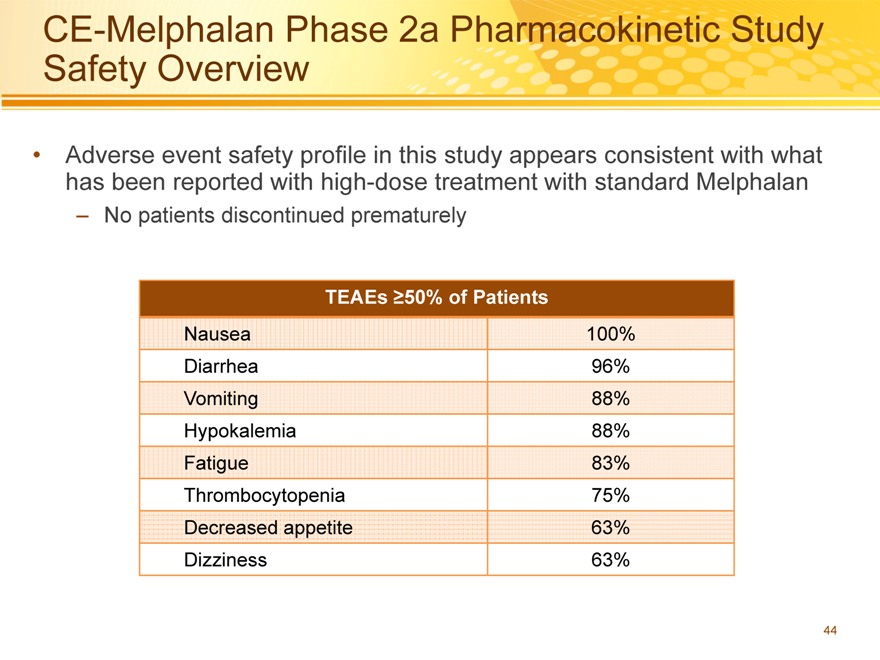
CE-Melphalan Phase 2a Pharmacokinetic Study Safety Overview
Adverse event safety profile in this study appears consistent with what has been reported with high-dose treatment with standard Melphalan
– No patients discontinued prematurely
TEAEs ?50% of Patients
Nausea 100%
Diarrhea 96%
Vomiting 88%
Hypokalemia 88%
Fatigue 83%
Thrombocytopenia 75%
Decreased appetite 63%
Dizziness 63%
44
CE-Melphalan Phase 2a
Pharmacokinetic Study Summary
Results from the Phase 2a study (N=24):
CE-Melphalan is bioequivalent to standard Melphalan
– CE-Melphalan (propylene glycol-free) peak and systemic exposure were slightly greater than with standard Melphalan
The efficacy and safety profile were consistent with that already established for high-dose Melphalan conditioning with ASCT for MM
– 100% of patients achieved myeloablation and engraftment
– Most frequent AEs included fatigue, nausea, and hypokalemia
CE-Melphalan has improved stability and is propylene glycol-free
– More stable following reconstitution
– Longer infusion times/higher doses possible with CE-Melphalan (propylene glycol-free) could improve response to treatment
45
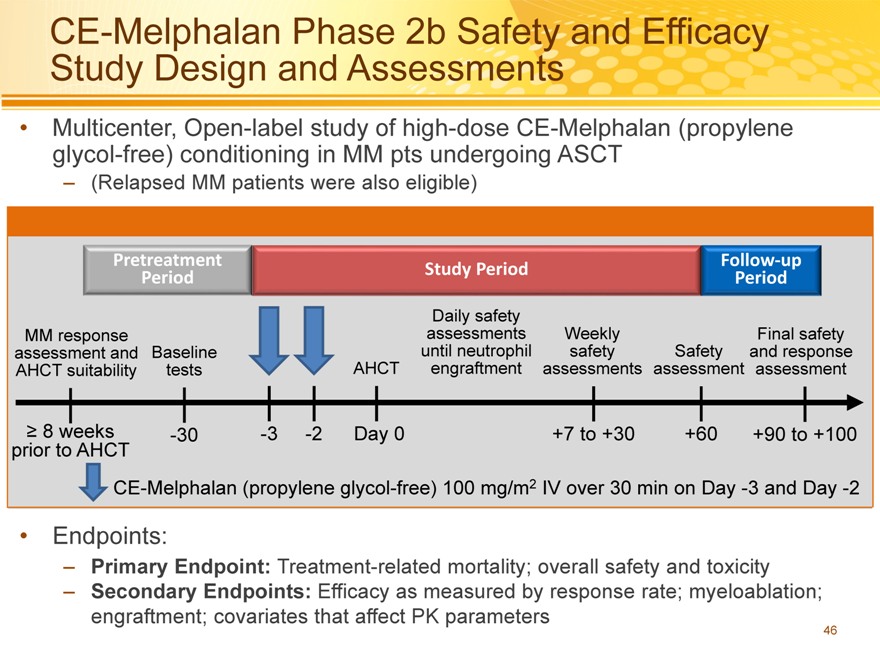
CE-Melphalan Phase 2b Safety and Efficacy Study Design and Assessments
Multicenter, Open-label study of high-dose CE-Melphalan (propylene glycol-free) conditioning in MM pts undergoing ASCT
– (Relapsed MM patients were also eligible)
Pretreatment Followup
Study Period
Period Period
Daily safety
MM response assessments Weekly Final safety
assessment and Baseline until neutrophil safety Safety and response
AHCT suitability tests AHCT engraftment assessments assessment assessment
8 weeks -30 -3 -2 Day 0 +7 to +30 +60 +90 to +100
prior to AHCT
CE-Melphalan (propylene glycol-free) 100 mg/m2 IV over 30 min on Day -3 and Day -2
Endpoints:
– Primary Endpoint: Treatment-related mortality; overall safety and toxicity
– Secondary Endpoints: Efficacy as measured by response rate; myeloablation; engraftment; covariates that affect PK parameters
46
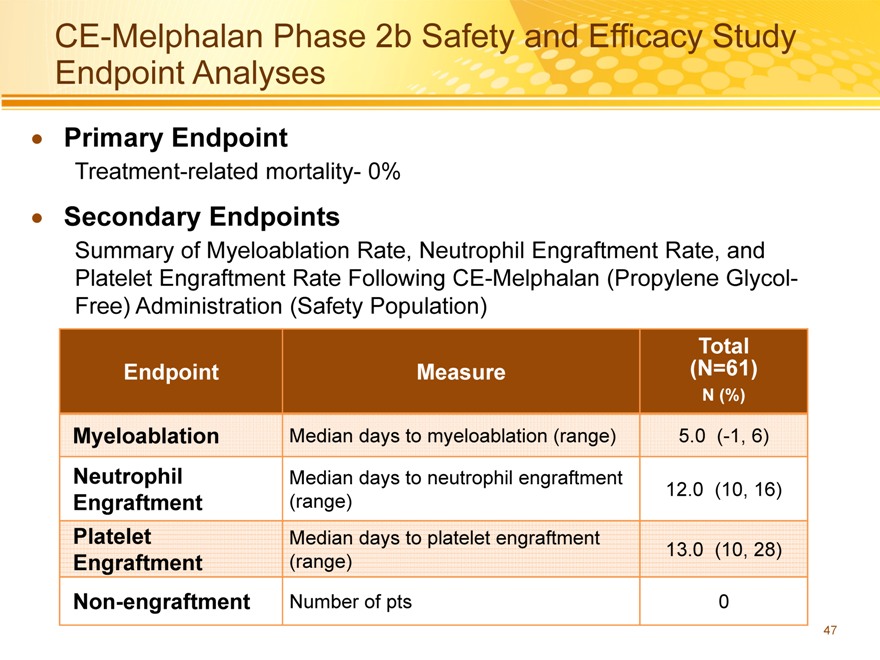
CE-Melphalan Phase 2b Safety and Efficacy Study Endpoint Analyses
Primary Endpoint
Treatment-related mortality- 0%
Secondary Endpoints
Summary of Myeloablation Rate, Neutrophil Engraftment Rate, and Platelet Engraftment Rate Following CE-Melphalan (Propylene Glycol-Free) Administration (Safety Population)
Total
Endpoint Measure(N=61)
N (%)
Myeloablation Median days to myeloablation (range) 5.0(-1, 6)
Neutrophil Median days to neutrophil engraftment
Engraftment(range) 12.0(10, 16)
Platelet Median days to platelet engraftment
Engraftment(range) 13.0(10, 28)
Non-engraftment Number of pts 0
47
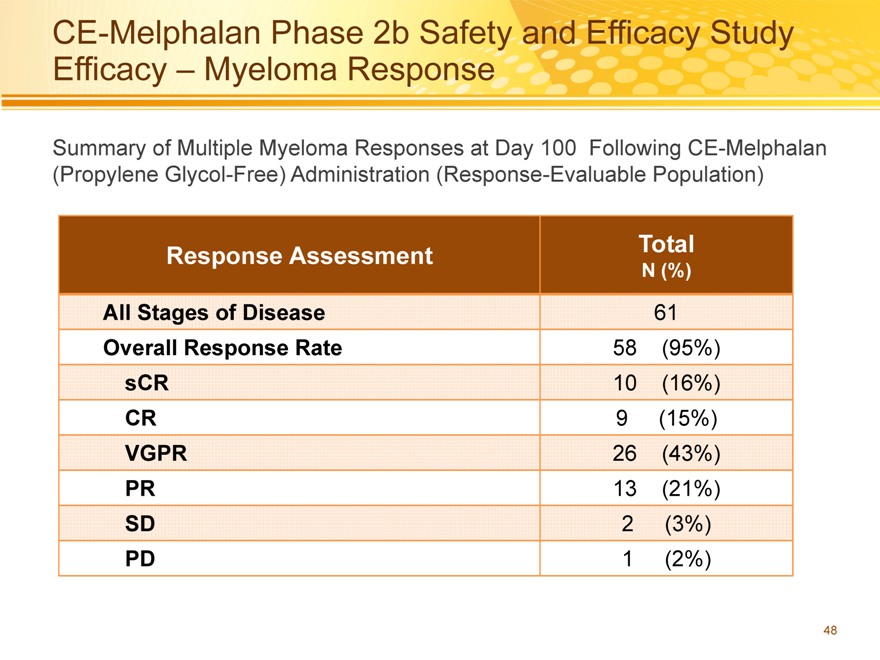
CE-Melphalan Phase 2b Safety and Efficacy Study Efficacy – Myeloma Response
Summary of Multiple Myeloma Responses at Day 100 Following CE-Melphalan (Propylene Glycol-Free) Administration (Response-Evaluable Population)
Response Assessment Total
N (%)
All Stages of Disease 61
Overall Response Rate 58(95%)
sCR 10(16%)
CR 9(15%)
VGPR 26(43%)
PR 13(21%)
SD 2(3%)
PD 1(2%)
48
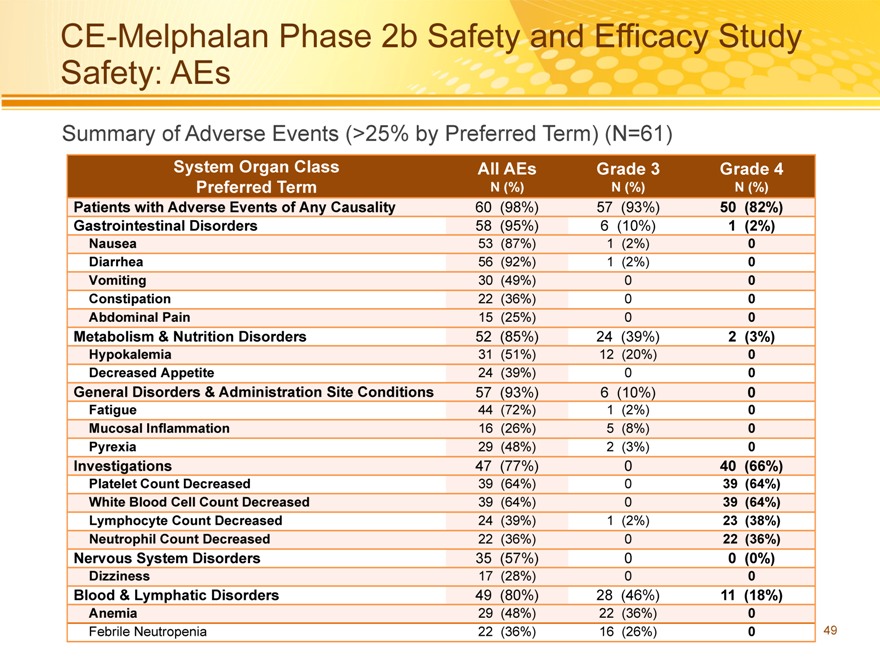
CE-Melphalan Phase 2b Safety and Efficacy Study Safety: AEs
Summary of Adverse Events (>25% by Preferred Term) (N=61)
System Organ Class All AEs Grade 3 Grade 4
Preferred Term N (%) N (%) N (%)
Patients with Adverse Events of Any Causality 60(98%) 57(93%) 50(82%)
Gastrointestinal Disorders 58(95%) 6(10%) 1(2%)
Nausea 53(87%) 1(2%) 0
Diarrhea 56(92%) 1(2%) 0
Vomiting 30(49%) 0 0
Constipation 22(36%) 0 0
Abdominal Pain 15(25%) 0 0
Metabolism & Nutrition Disorders 52(85%) 24(39%) 2(3%)
Hypokalemia 31(51%) 12(20%) 0
Decreased Appetite 24(39%) 0 0
General Disorders & Administration Site Conditions 57(93%) 6(10%) 0
Fatigue 44(72%) 1(2%) 0
Mucosal Inflammation 16(26%) 5(8%) 0
Pyrexia 29(48%) 2(3%) 0
Investigations 47(77%) 0 40(66%)
Platelet Count Decreased 39(64%) 0 39(64%)
White Blood Cell Count Decreased 39(64%) 0 39(64%)
Lymphocyte Count Decreased 24(39%) 1(2%) 23(38%)
Neutrophil Count Decreased 22(36%) 0 22(36%)
Nervous System Disorders 35(57%) 0 0(0%)
Dizziness 17(28%) 0 0
Blood & Lymphatic Disorders 49(80%) 28(46%) 11(18%)
Anemia 29(48%) 22(36%) 0
Febrile Neutropenia 22(36%) 16(26%) 0
49
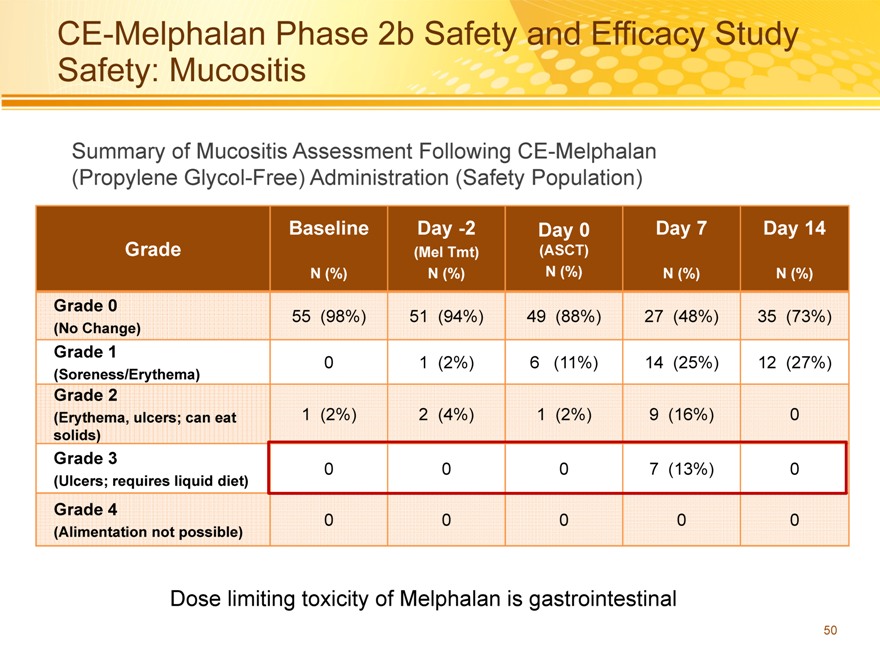
CE-Melphalan Phase 2b Safety and Efficacy Study Safety: Mucositis
Summary of Mucositis Assessment Following CE-Melphalan (Propylene Glycol-Free) Administration (Safety Population)
Baseline Day -2 Day 0 Day 7 Day 14
Grade(Mel Tmt)(ASCT)
N (%) N (%) N (%) N (%) N (%)
Grade 0 55(98%) 51(94%) 49(88%) 27(48%) 35(73%)
(No Change)
Grade 1 0 1(2%) 6(11%) 14(25%) 12(27%)
(Soreness/Erythema)
Grade 2
(Erythema, ulcers; can eat 1(2%) 2(4%) 1(2%) 9(16%) 0
solids)
Grade 3 0 0 0 7(13%) 0
(Ulcers; requires liquid diet)
Grade 4 0 0 0 0 0
(Alimentation not possible)
Dose limiting toxicity of Melphalan is gastrointestinal
50
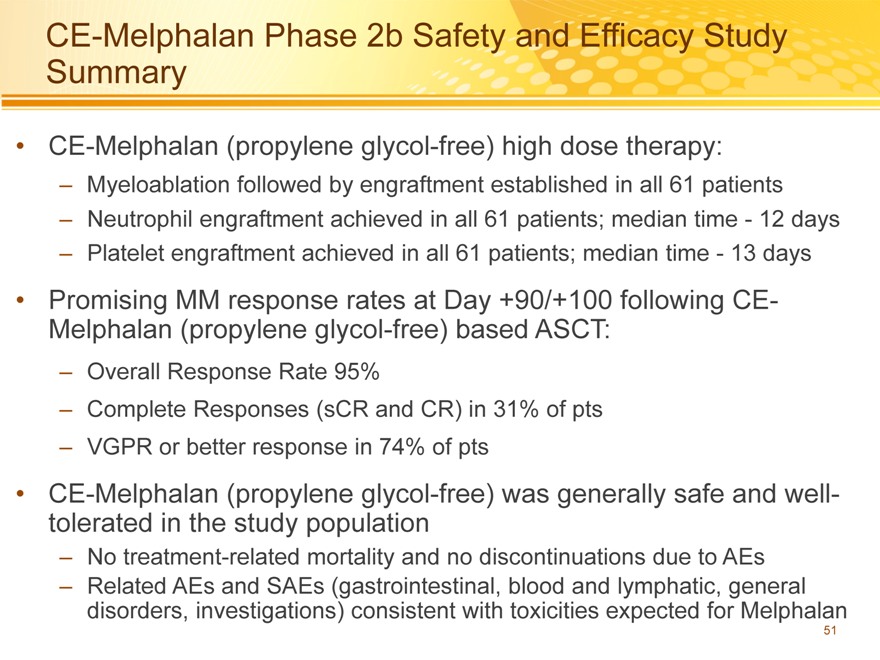
CE-Melphalan Phase 2b Safety and Efficacy Study Summary
CE-Melphalan (propylene glycol-free) high dose therapy:
– Myeloablation followed by engraftment established in all 61 patients
– Neutrophil engraftment achieved in all 61 patients; median time—12 days
– Platelet engraftment achieved in all 61 patients; median time—13 days
Promising MM response rates at Day +90/+100 following CE-Melphalan (propylene glycol-free) based ASCT:
– Overall Response Rate 95%
– Complete Responses (sCR and CR) in 31% of pts
– VGPR or better response in 74% of pts
CE-Melphalan (propylene glycol-free) was generally safe and well-tolerated in the study population
– No treatment-related mortality and no discontinuations due to AEs
– Related AEs and SAEs (gastrointestinal, blood and lymphatic, general disorders, investigations) consistent with toxicities expected for Melphalan
51
Apaziquone
Bladder Cancer and Apaziquone
Spectrum Investors Day
Friday March 13, 2015
Prof Dr. Fred Witjes
Dept Urology
RadboudUMC, Nijmegen
Epidemiology versus Awareness
54
Prostate cancer
55
Testicular cancer
56
Bladder cancer
57
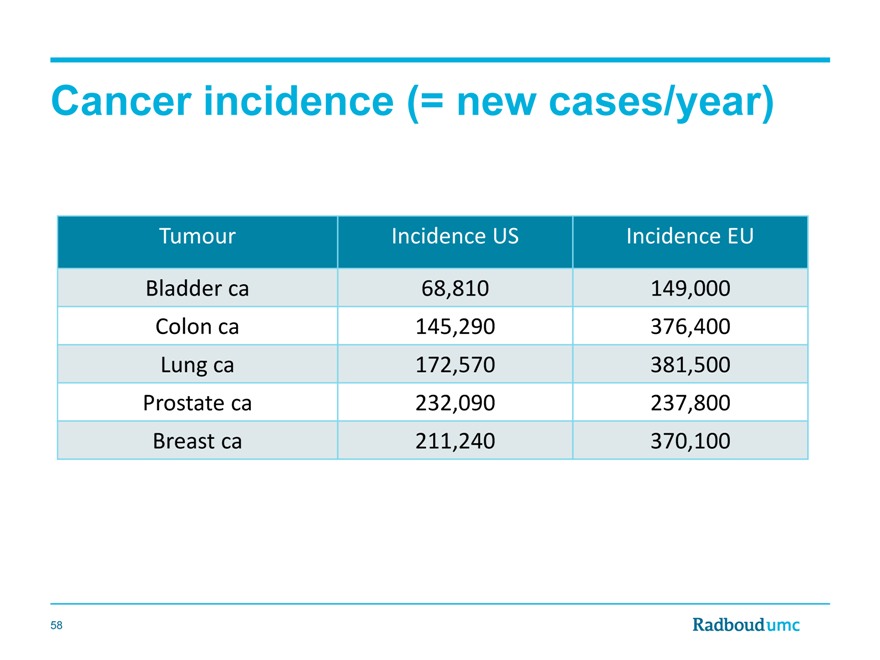
Cancer incidence (= new cases/year)
Tumour Incidence US Incidence EU
Bladder ca 68,810 149,000
Colon ca 145,290 376,400
Lung ca 172,570 381,500
Prostate ca 232,090 237,800
Breast ca 211,240 370,100
58
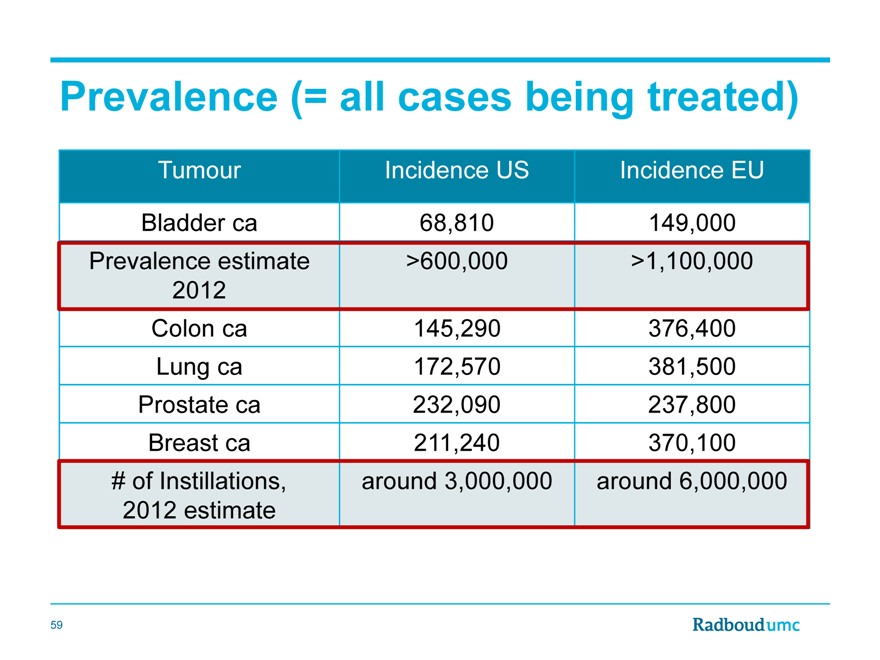
Prevalence (= all cases being treated)
Tumour Incidence US Incidence EU
Bladder ca 68,810 149,000
Prevalence estimate >600,000 >1,100,000
2012
Colon ca 145,290 376,400
Lung ca 172,570 381,500
Prostate ca 232,090 237,800
Breast ca 211,240 370,100
# of Instillations, around 3,000,000 around 6,000,000
2012 estimate
59
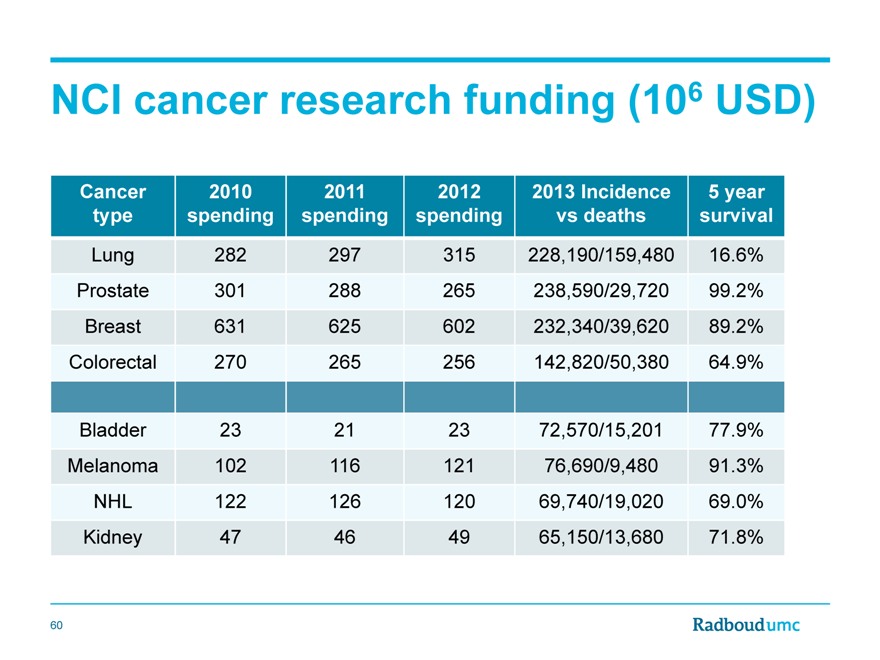
NCI cancer research funding (106 USD)
Cancer 2010 2011 2012 2013 Incidence 5 year
type spending spending spending vs deaths survival
Lung 282 297 315 228,190/159,480 16.6%
Prostate 301 288 265 238,590/29,720 99.2%
Breast 631 625 602 232,340/39,620 89.2%
Colorectal 270 265 256 142,820/50,380 64.9%
Bladder 23 21 23 72,570/15,201 77.9%
Melanoma 102 116 121 76,690/9,480 91.3%
NHL 122 126 120 69,740/19,020 69.0%
Kidney 47 46 49 65,150/13,680 71.8%
60
Conclusion 1: bladder cancer
- Is very prevalent and costly
- Has limited awareness and funding
- Is a large potential market
61
Let’s focus on NMIBC
(non muscle invasive bladder cancer)
62
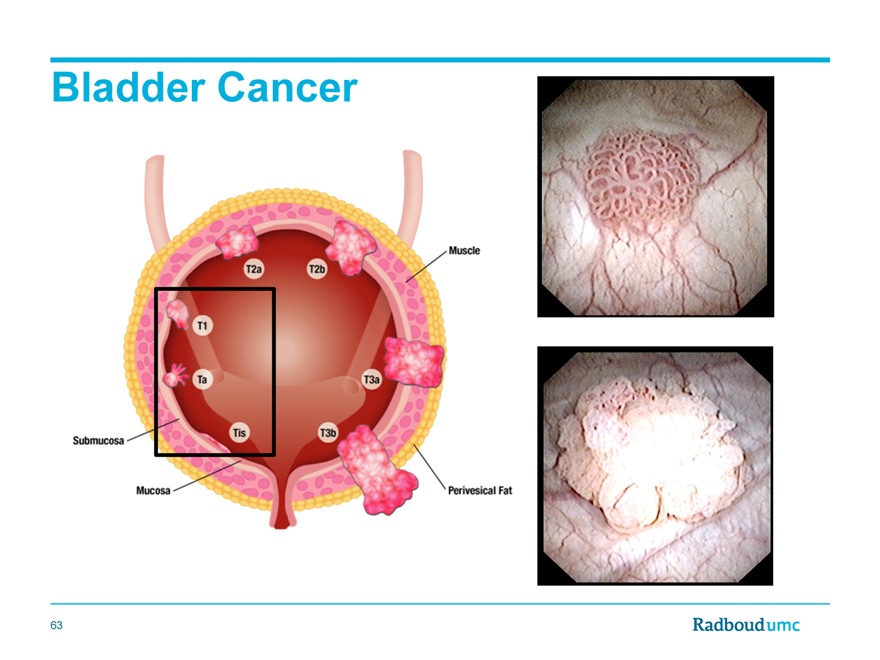
Bladder Cancer
63
The unmet needs in NMIBC
1) The operation
64
Guideline for the management of nonmuscle invasive bladder cancer (Stages Ta, T1, and Tis): 2007 update
M. Craig Hall,* Sam S. Chang,† Guido Dalbagni, Raj Som Pruthi, John Derek Seigne,‡ Eila Curlee Skinner, J. Stuart Worf, Jr.§ and Paul F. Schellhammer
From the American Urological Association Education and Research, Inc.
Standard: Under most circumstances, eradication of tumors should be performed.
[Based on Panel consensus]
65
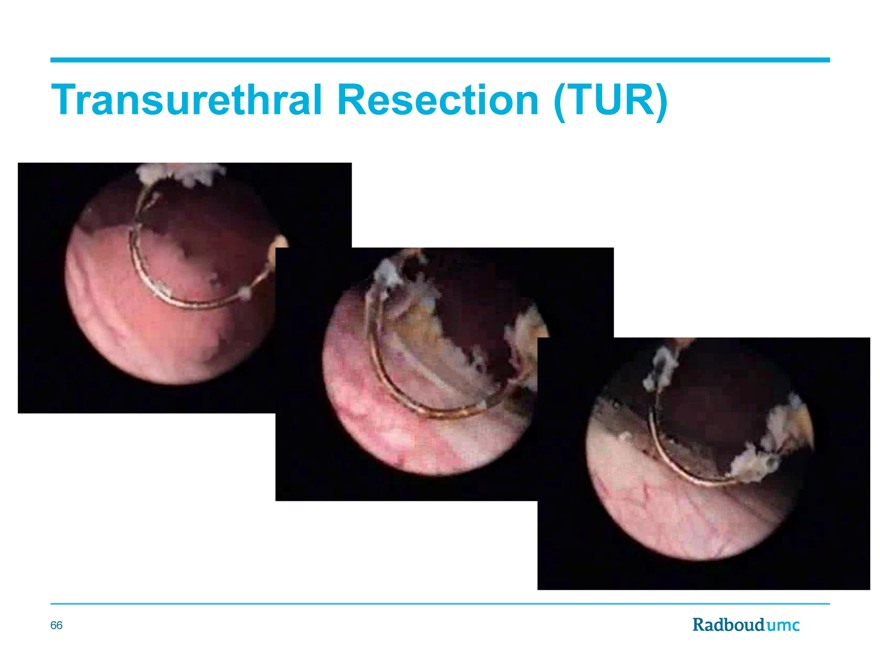
Transurethral Resection (TUR)
66
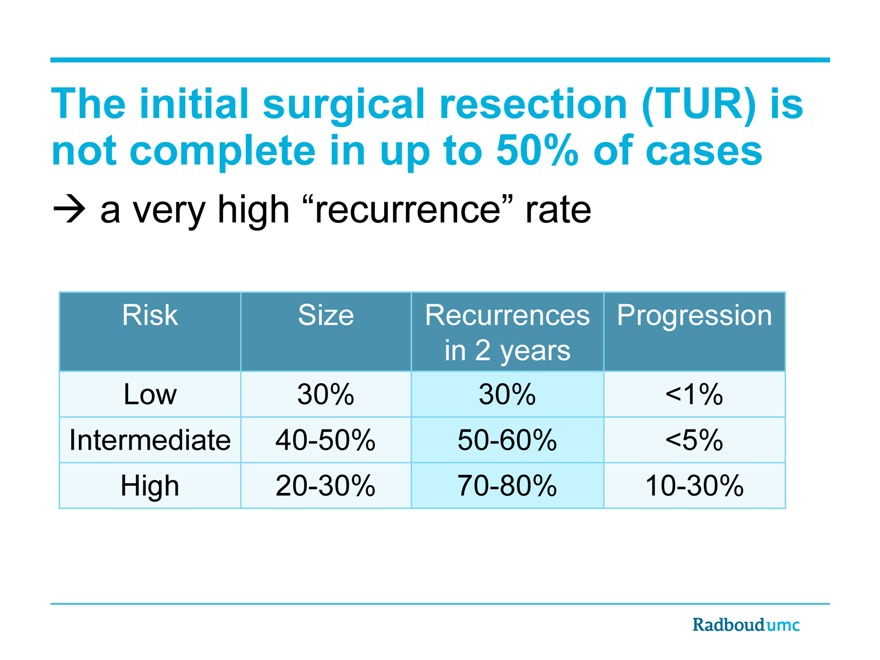
The initial surgical resection (TUR) is not complete in up to 50% of cases
a very high “recurrence” rate
Risk Size Recurrences Progression
in 2 years
Low 30% 30% <1%
Intermediate 40-50% 50-60% <5%
High 20-30% 70-80% 10-30%
The unmet needs in NMIBC
2) Adjuvant Treatment
68
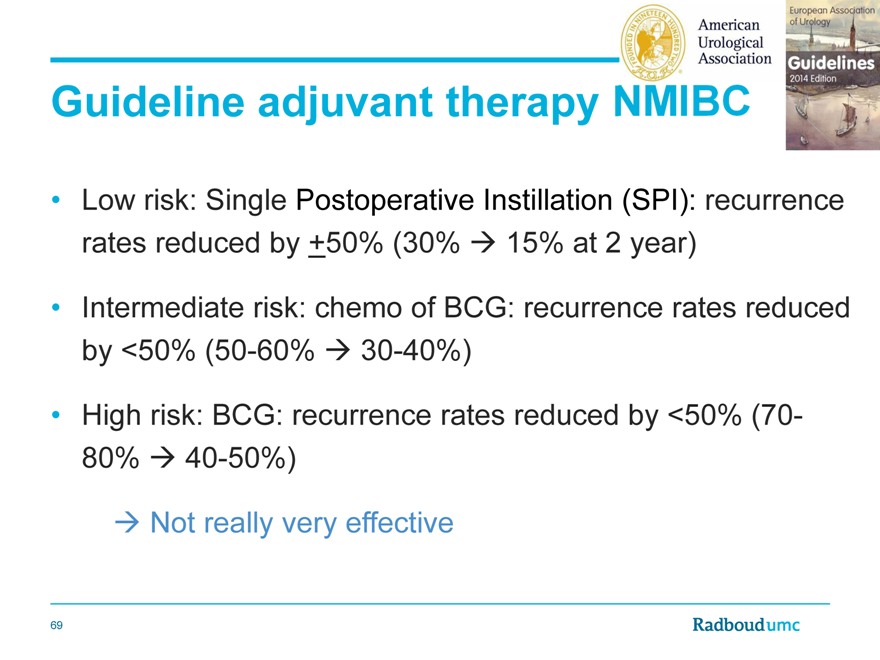
Guideline adjuvant therapy
Low risk: Single Postoperative Instillation (SPI): recurrence rates reduced by +50% (30% 15% at 2 year)
Intermediate risk: chemo of BCG: recurrence rates reduced by <50% (50-60% 30-40%)
High risk: BCG: recurrence rates reduced by <50% (70-80% 40-50%)
Not really very effective
69
And
70
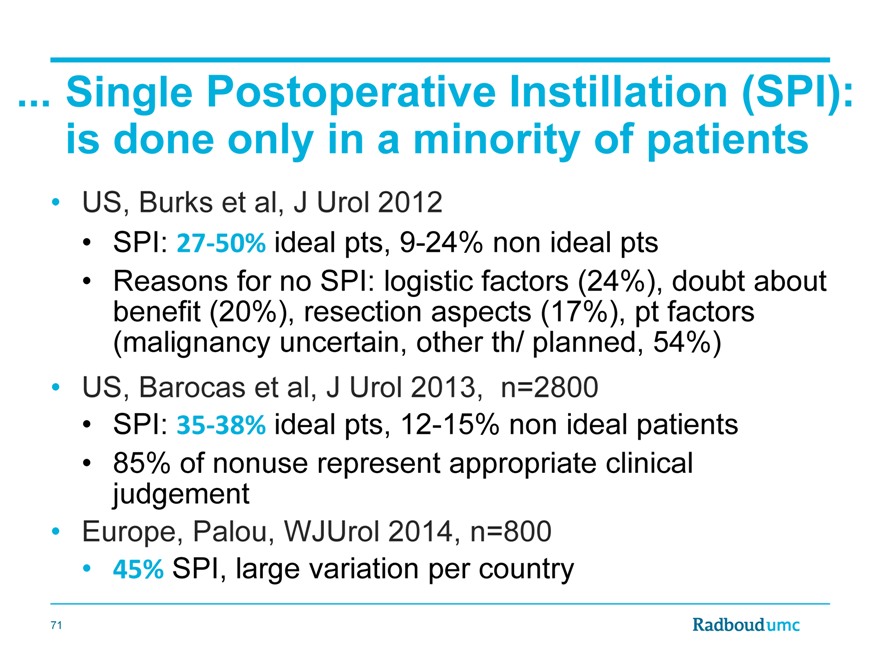
. Single Postoperative Instillation (SPI): is done only in a minority of patients
US, Burks et al, J Urol 2012
SPI: 27?50% ideal pts, 9-24% non ideal pts
Reasons for no SPI: logistic factors (24%), doubt about benefit (20%), resection aspects (17%), pt factors (malignancy uncertain, other th/ planned, 54%)
US, Barocas et al, J Urol 2013, n=2800
SPI: 35?38% ideal pts, 12-15% non ideal patients
85% of nonuse represent appropriate clinical judgement
Europe, Palou, WJUrol 2014, n=800
45% SPI, large variation per country
71
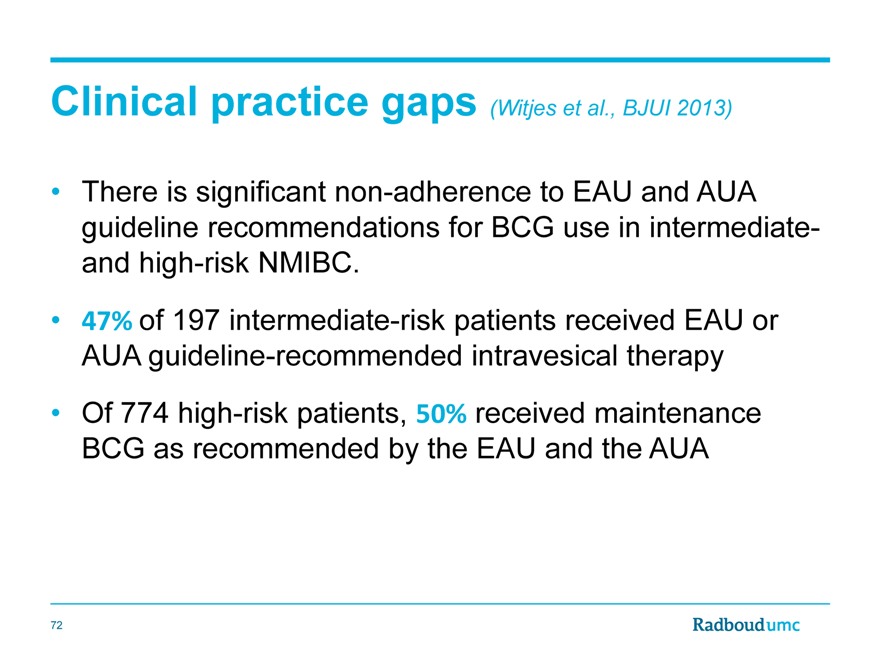
Clinical practice gaps (Witjes et al., BJUI 2013)
There is significant non-adherence to EAU and AUA guideline recommendations for BCG use in intermediate-and high-risk NMIBC.
47% of 197 intermediate-risk patients received EAU or AUA guideline-recommended intravesical therapy
Of 774 high-risk patients, 50% received maintenance BCG as recommended by the EAU and the AUA
72
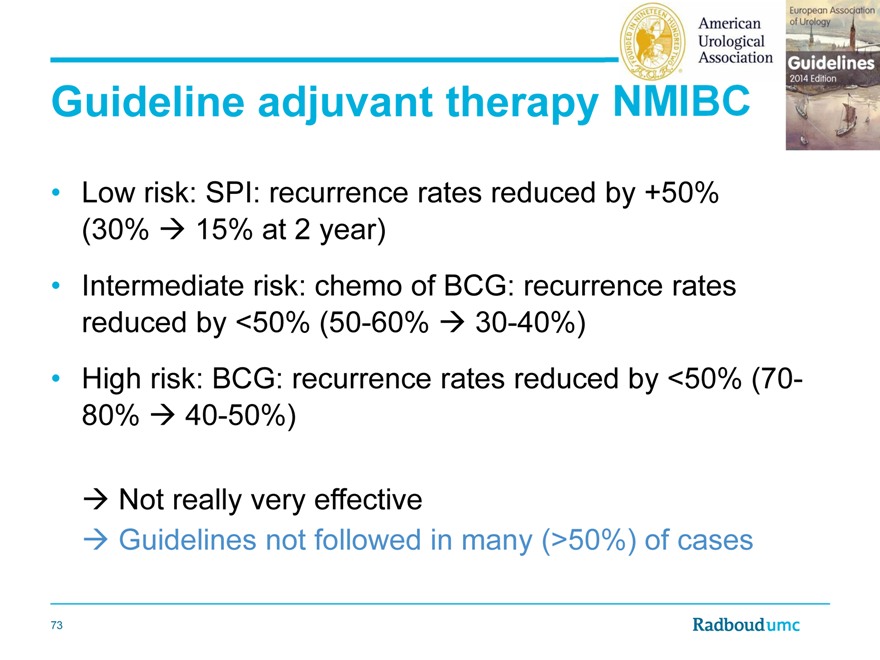
Guideline adjuvant therapy
Low risk: SPI: recurrence rates reduced by +50% (30% 15% at 2 year)
Intermediate risk: chemo of BCG: recurrence rates reduced by <50% (50-60% 30-40%)
High risk: BCG: recurrence rates reduced by <50% (70-80% 40-50%)
Not really very effective
Guidelines not followed in many (>50%) of cases
73
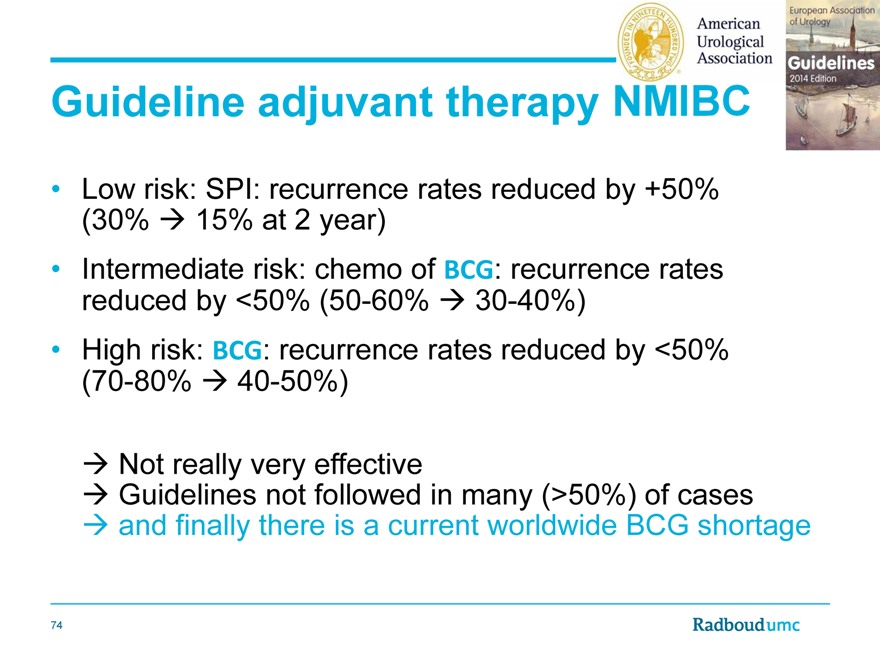
Guideline adjuvant therapy
Low risk: SPI: recurrence rates reduced by +50% (30% 15% at 2 year)
Intermediate risk: chemo of BCG: recurrence rates reduced by <50% (50-60% 30-40%)
High risk: BCG: recurrence rates reduced by <50% (70-80% 40-50%)
Not really very effective
Guidelines not followed in many (>50%) of casesand finally there is a current worldwide BCG shortage
74
Conclusion 2: treatment NMIBC
Results are not very good many recurrences
Reasons are
TUR is not a good operation (we cut into the tumor!)
Guideline advise treatments are not very effective
Guidelines are not followed well
BCG shortage
Therefore there is a Large Unmet Medical Need
75
New developments…
76
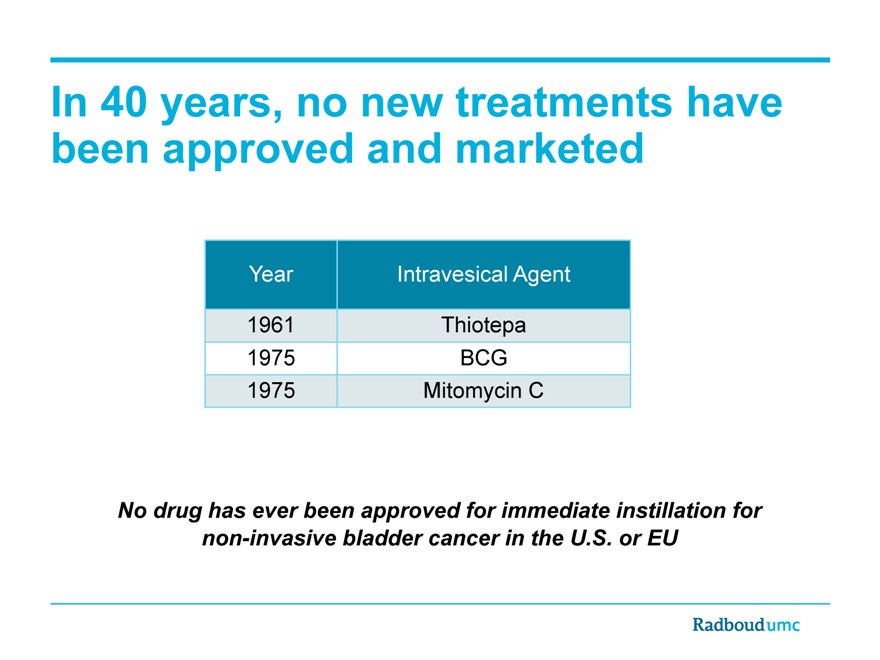
In 40 years, no new treatments have been approved and marketed
Year Intravesical Agent
1961 Thiotepa
1975 BCG
1975 Mitomycin C
No drug has ever been approved for immediate instillation for non-invasive bladder cancer in the U.S. or EU
Apaziquone
78
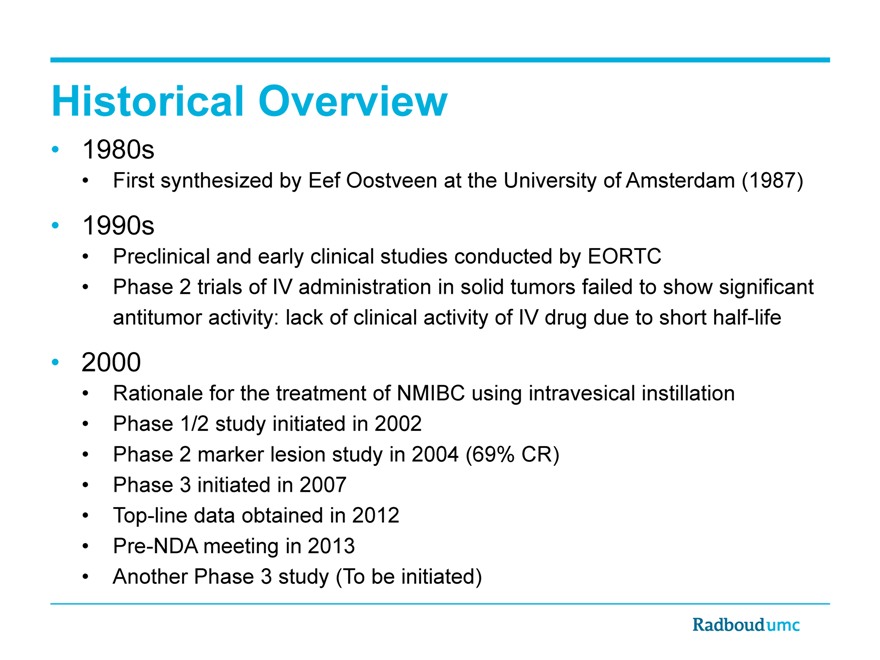
Historical Overview
1980s
First synthesized by Eef Oostveen at the University of Amsterdam (1987)
1990s
Preclinical and early clinical studies conducted by EORTC
Phase 2 trials of IV administration in solid tumors failed to show significant antitumor activity: lack of clinical activity of IV drug due to short half-life
2000
Rationale for the treatment of NMIBC using intravesical instillation
Phase 1/2 study initiated in 2002
Phase 2 marker lesion study in 2004 (69% CR)
Phase 3 initiated in 2007
Top-line data obtained in 2012
Pre-NDA meeting in 2013
Another Phase 3 study (To be initiated)
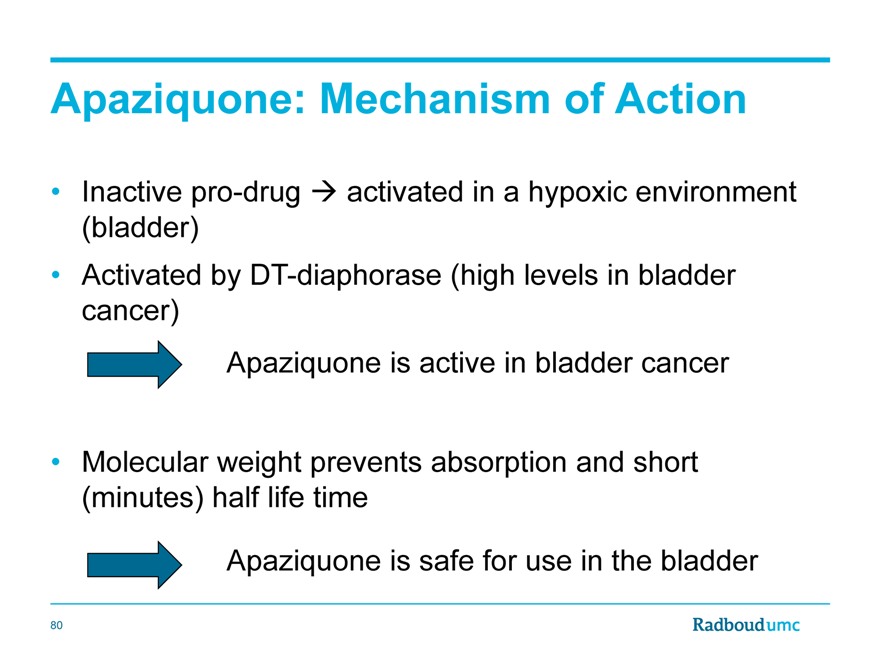
Apaziquone: Mechanism of Action
Inactive pro-drug activated in a hypoxic environment (bladder)
Activated by DT-diaphorase (high levels in bladder cancer)
Apaziquone is active in bladder cancer
Molecular weight prevents absorption and short (minutes) half life time
Apaziquone is safe for use in the bladder
80
In Vitro Data (vdHeijden et al. J Urol 2005)
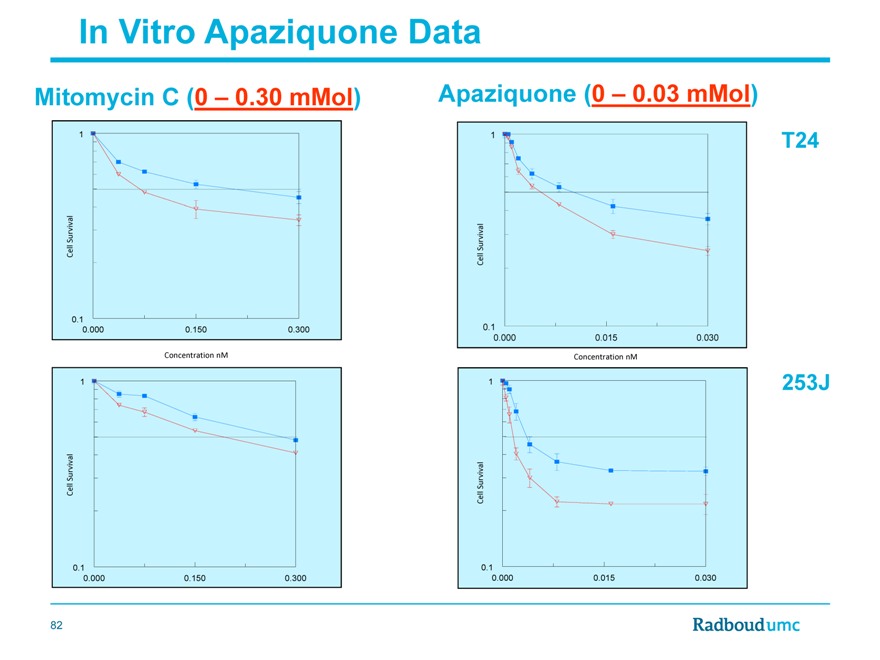
On an average Apaziquone is 26 times more effective (range 6 to 78 times) than MMC
81
In Vitro Apaziquone Data
Mitomycin C (0 – 0.30 mMol)
Survival Cell
0.1
0.000 0.150 0.300
Concentration nM
Survival Cell
0.1
0.000 0.150 0.300
Apaziquone (0 – 0.03 mMol)
Survival Cell
0.1
0.000 0.015 0.030
Concentration nM
Survival Cell
0.1
0.000 0.015 0.030
82
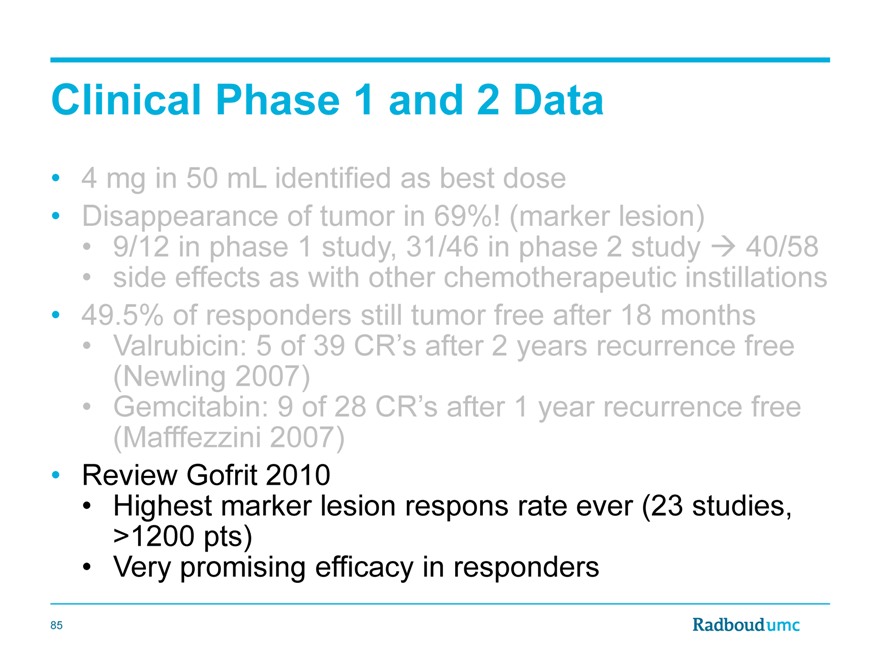
Clinical Phase 1 and 2 Data
4 | | mg in 50 mL identified as best dose |
Disappearance of tumor in 69%! (marker lesion)
9/12 in phase 1 study, 31/46 in phase 2 study40/58 side effects as with other chemotherapeutic instillations
49.5% of responders still tumor free after 18 months
Valrubicin: 5 of 39 CR’s after 2 years recurrence free (Newling 2007)
Gemcitabin: 9 of 28 CR’s after 1 year recurrence free (Mafffezzini 2007)
Review Gofrit 2010
Highest marker lesion respons rate ever (23 studies, >1200 pts)
Very promising efficacy in responders
83
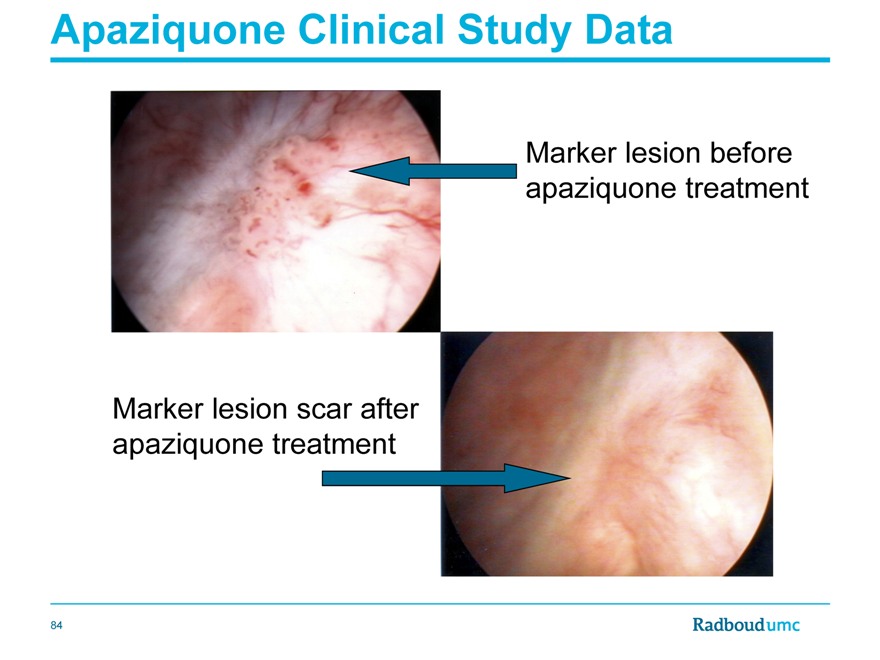
Apaziquone Clinical Study Data
Marker lesion before apaziquone treatment
Marker lesion scar after apaziquone treatment
84
Clinical Phase 1 and 2 Data
4 | | mg in 50 mL identified as best dose |
Disappearance of tumor in 69%! (marker lesion)
9/12 in phase 1 study, 31/46 in phase 2 study40/58 side effects as with other chemotherapeutic instillations
49.5% of responders still tumor free after 18 months
Valrubicin: 5 of 39 CR’s after 2 years recurrence free (Newling 2007)
Gemcitabin: 9 of 28 CR’s after 1 year recurrence free (Mafffezzini 2007)
Review Gofrit 2010
Highest marker lesion respons rate ever (23 studies, >1200 pts)
Very promising efficacy in responders
85
Marker lesion experiments in bladder cancer – what have we learned?
86
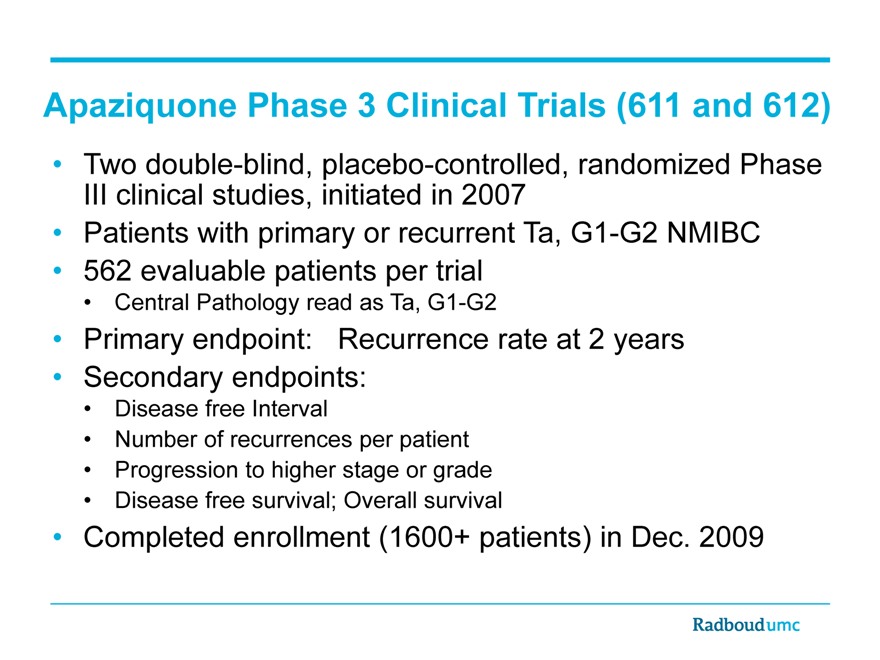
Apaziquone Phase 3 Clinical Trials (611 and 612)
Two double-blind, placebo-controlled, randomized Phase III clinical studies, initiated in 2007
Patients with primary or recurrent Ta, G1-G2 NMIBC
562 evaluable patients per trial
Central Pathology read as Ta, G1-G2
Primary endpoint: Recurrence rate at 2 years
Secondary endpoints:
Disease free Interval
Number of recurrences per patient
Progression to higher stage or grade
Disease free survival; Overall survival
Completed enrollment (1600+ patient) in Dec. 2009
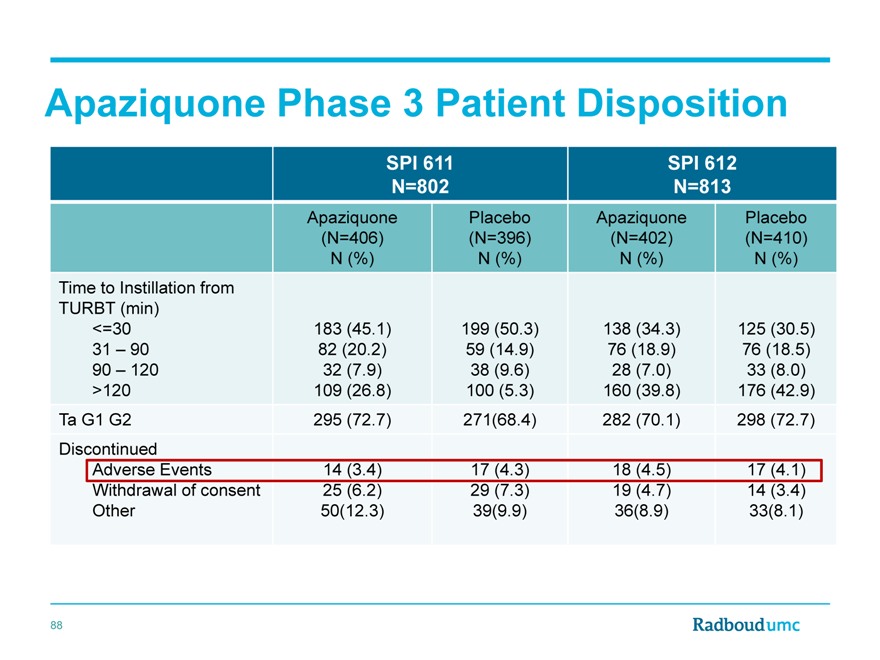
Apaziquone Phase 3 Patient Disposition
SPI 611 SPI 612
N=802 N=813
Apaziquone Placebo Apaziquone Placebo
(N=406)(N=396)(N=402)(N=410)
N (%) N (%) N (%) N (%)
Time to Instillation from
TURBT (min)
<=30 183(45.1) 199 (50.3) 138(34.3) 125(30.5)
31 | | – 90 82 (20.2) 59 (14.9) 76 (18.9) 76 (18.5) |
90 | | – 120 32(7.9) 38 (9.6) 28(7.0) 33(8.0) |
>120 109(26.8) 100 (5.3) 160(39.8) 176(42.9)
Ta G1 G2 295(72.7) 271(68.4) 282(70.1) 298 (72.7)
Discontinued
Adverse Events 14(3.4) 17 (4.3) 18(4.5) 17(4.1)
Withdrawal of consent 25(6.2) 29 (7.3) 19(4.7) 14(3.4)
Other 50(12.3) 39(9.9) 36(8.9) 33(8.1)
88
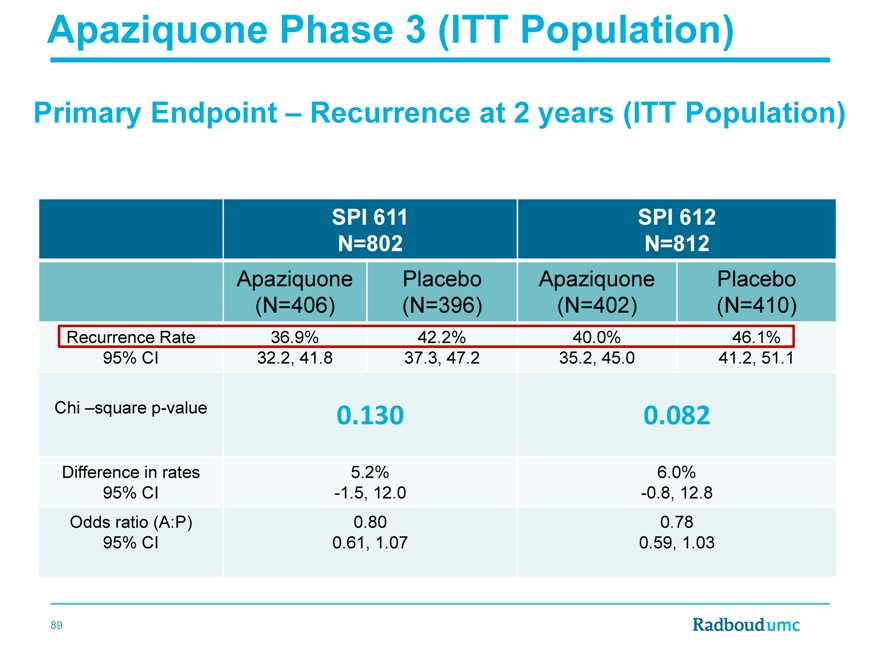
Apaziquone Phase 3 (ITT Population)
Primary Endpoint – Recurrence at 2 years (ITT Population)
SPI 611 SPI 612
N=802 N=812
Apaziquone Placebo Apaziquone Placebo
(N=406)(N=396)(N=402)(N=410)
Recurrence Rate 36.9% 42.2% 40.0% 46.1%
95% CI 32.2, 41.8 37.3, 47.2 35.2, 45.0 41.2, 51.1
Chi –square p-value 0.130 0.082
Difference in rates 5.2% 6.0%
95% CI -1.5, 12.0 -0.8, 12.8
Odds ratio (A:P) 0.80 0.78
95% CI 0.61, 1.07 0.59, 1.03
89
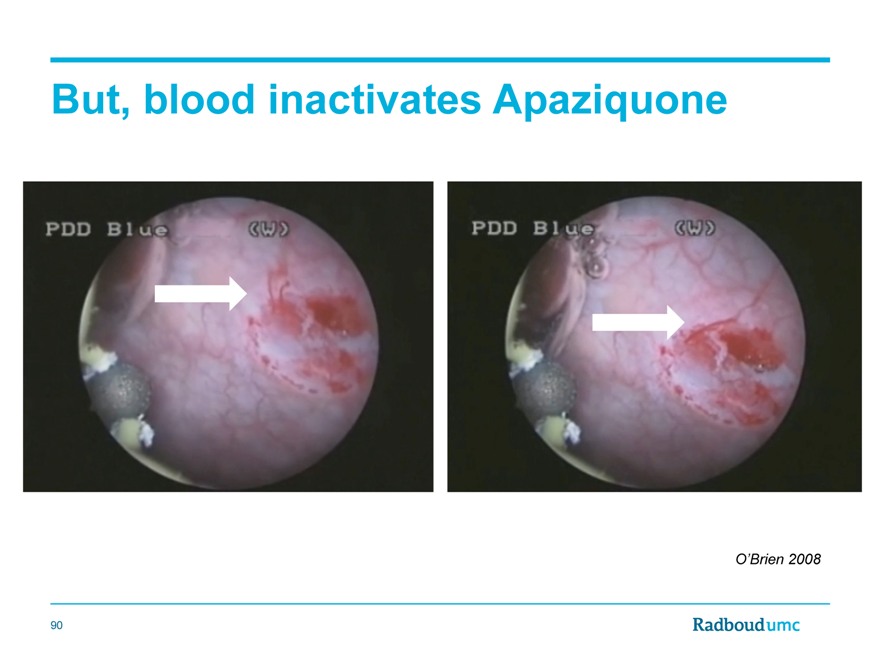
But, blood inactivates Apaziquone
O’Brien 2008
90
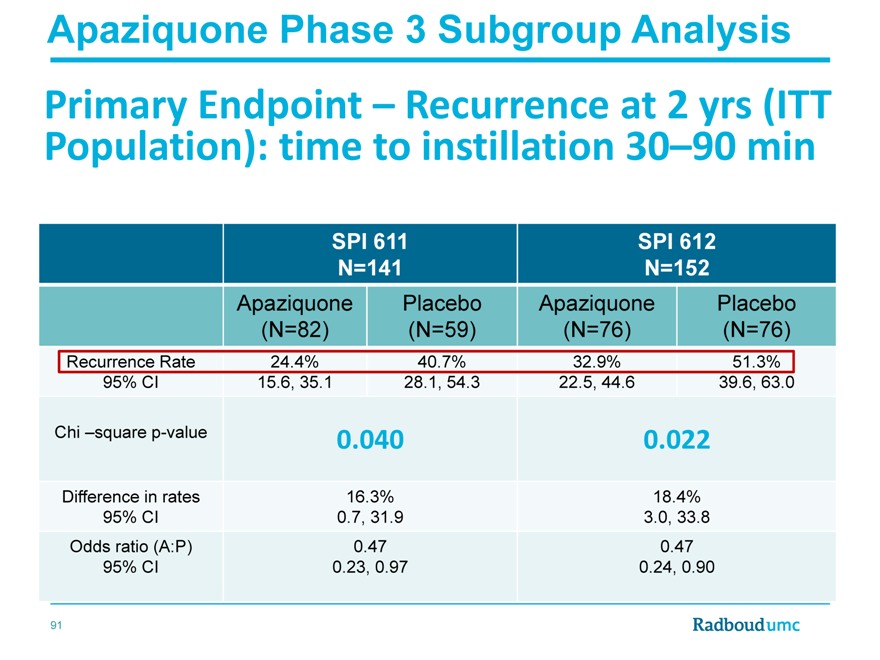
Apaziquone Phase 3 Subgroup Analysis
Primary Endpoint – Recurrence at 2 yrs (ITT Population): time to instillation 30–90 min
SPI 611 SPI 612
N=141 N=152
Apaziquone Placebo Apaziquone Placebo
(N=82)(N=59)(N=76)(N=76)
Recurrence Rate 24.4% 40.7% 32.9% 51.3%
95% CI 15.6, 35.1 28.1, 54.3 22.5, 44.6 39.6, 63.0
Chi –square p-value 0.040 0.022
Difference in rates 16.3% 18.4%
95% CI 0.7, 31.9 3.0, 33.8
Odds ratio (A:P) 0.47 0.47
95% CI 0.23, 0.97 0.24, 0.90
91
Apaziquone Phase 3 Combined Analysis
611 and 612
Apaziquone Placebo Apaziquone Placebo
(N=577)(N=569)(N=117)(N=100)
All 30 – 90 min
Recurrence rate 38.8% 45.5% 28.2% 50.0%
95% CI 34.8, 42.9 41.4, 49.7 20.3, 37.3 39.8, 60.2
Chi-square test p=0.022 p=0.001
Difference in RR 6.7% 21.8%
95% CI 1.0, 12.4 9.0, 34.5
Odds ratio (A:P) 0.76 0.39
95% CI 0.60, 0.96 0.22, 0.69
92
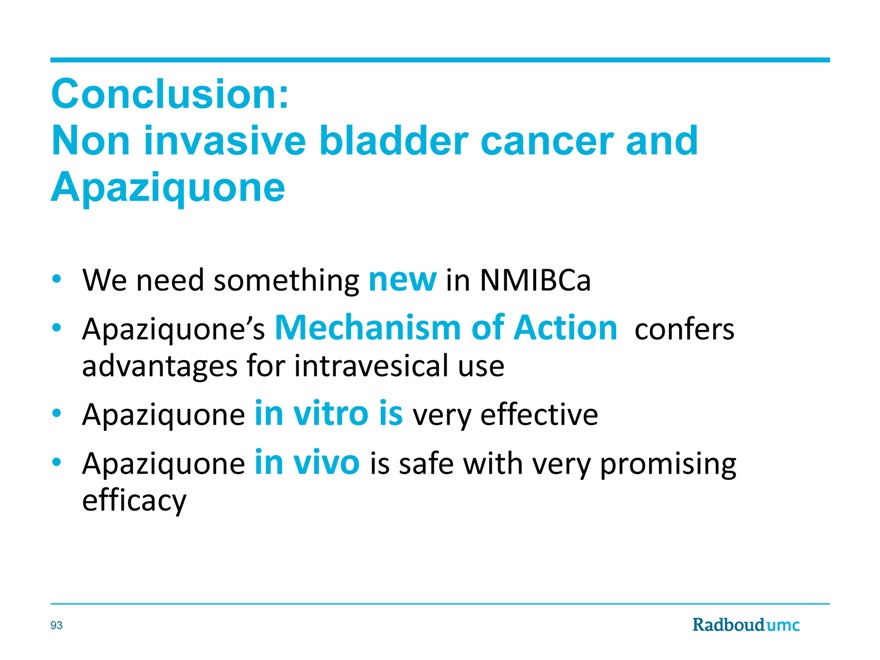
Conclusion:
Non invasive bladder cancer and Apaziquone
We need something new in NMIBCa
Apaziquone’s Mechanism of Action confers advantages for intravesical use
Apaziquone in vitro is very effective
Apaziquone in vivo is safe with very promising efficacy
93
Poziotinib
Francisco Esteva
MD, PhD
Associate Director of Clinical Investigation
Director, Breast Medical Oncology Program
Laura and Isaac Perlmutter Cancer Center
New York University, Langone,
New York, NY
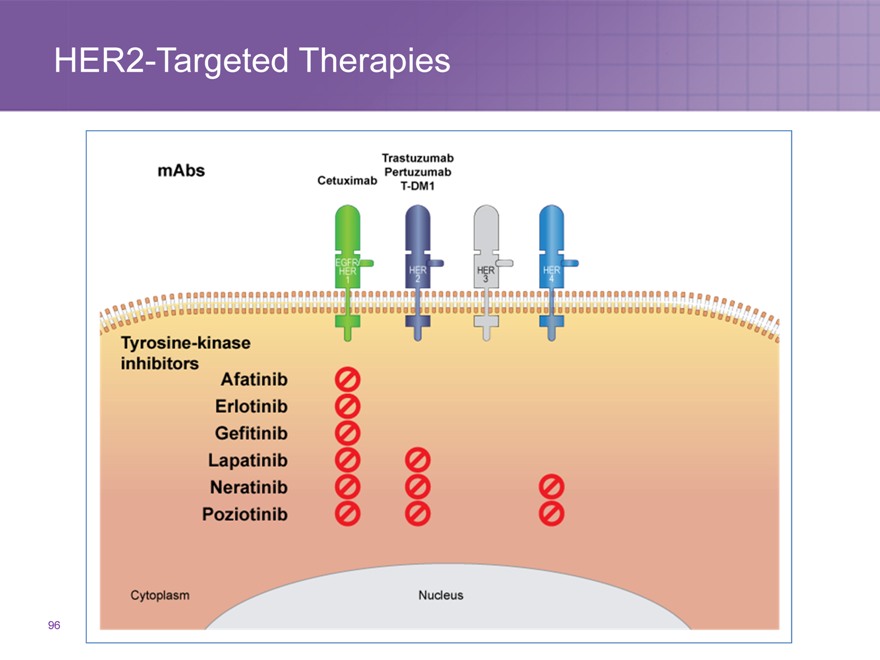
HER2-Targeted Therapies
96
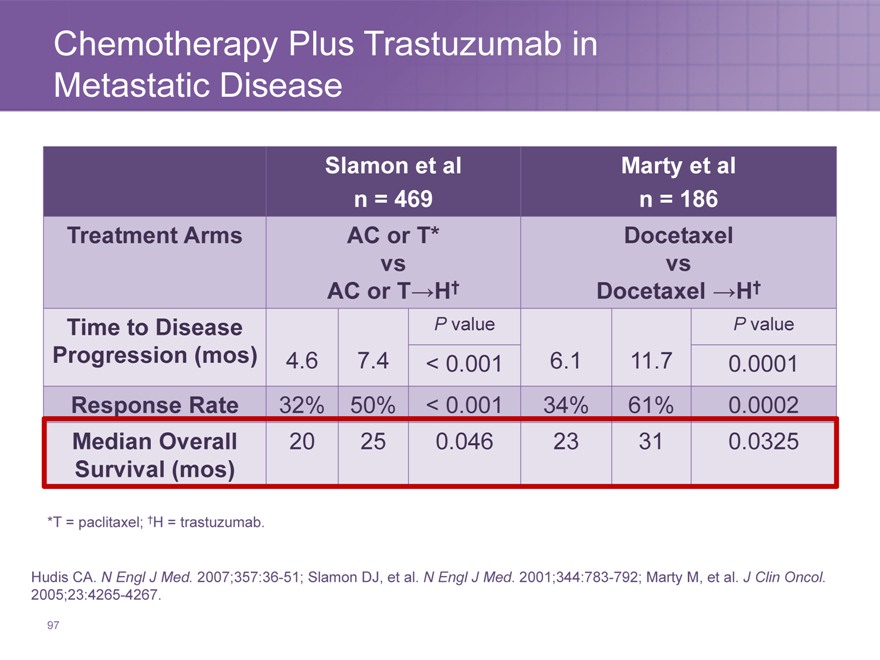
Chemotherapy Plus Trastuzumab in Metastatic Disease
Slamon et al Marty et al
n = 469 n = 186
Treatment Arms AC or T* Docetaxel
vs vs
AC or T?H† Docetaxel ?H†
Time to Disease P value P value
Progression (mos) 4.6 7.4 < 0.001 6.1 11.7 0.0001
Response Rate 32% 50% < 0.001 34% 61% 0.0002
Median Overall 20 25 0.046 23 31 0.0325
Survival (mos)
*T = paclitaxel; †H = trastuzumab.
Hudis CA. N Engl J Med. 2007;357:36-51; Slamon DJ, et al. N Engl J Med. 2001;344:783-792; Marty M, et al. J Clin Oncol. 2005;23:4265-4267.
97
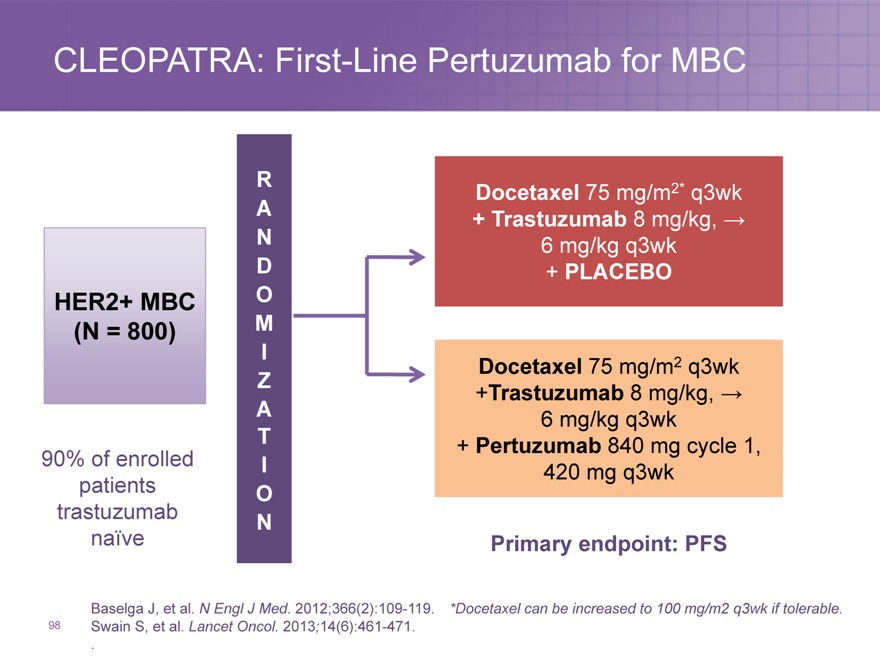
CLEOPATRA: First-Line Pertuzumab for MBC
R A N D
HER2+ MBC O
(N = 800) M
I Z A T
90% of enrolled I patients O trastuzumab
N naïve
Baselga J, et al. N Engl J Med. 2012;366(2):109-119.
Swain S, et al. Lancet Oncol. 2013;14(6):461-471. .
98
Docetaxel 75 mg/m2* q3wk
+ Trastuzumab 8 mg/kg, ? 6 mg/kg q3wk
+ PLACEBO
Docetaxel 75 mg/m2 q3wk
+Trastuzumab 8 mg/kg, ? 6 mg/kg q3wk
+ Pertuzumab 840 mg cycle 1, 420 mg q3wk
Primary endpoint: PFS
*Docetaxel can be increased to 100 mg/m2 q3wk if tolerable.
CLEOPATRA: First-Line Pertuzumab for HER2+ Metastatic Breast Cancer—Overall Survival
100
90 Ptz + T + D: median 56.5 months
80 Pla + T + D: median 40.8 months 70 60
% OS, 50
40 30
20 HR=0.68
(95% CI, 0.56-0.84) ? 15.7 months
10 P=0.0002
90% of enrolled 0
patients 0 1020304050 60 70
trastuzumab Time, Months
Number naïve at risk
Primary endpoint: PFS
Ptz + T + D 402 371 318 268 226 104 28 1 Pla + T + D 406 350 289 230 179 91 23 0
Swain S. ESMO. 2014
99
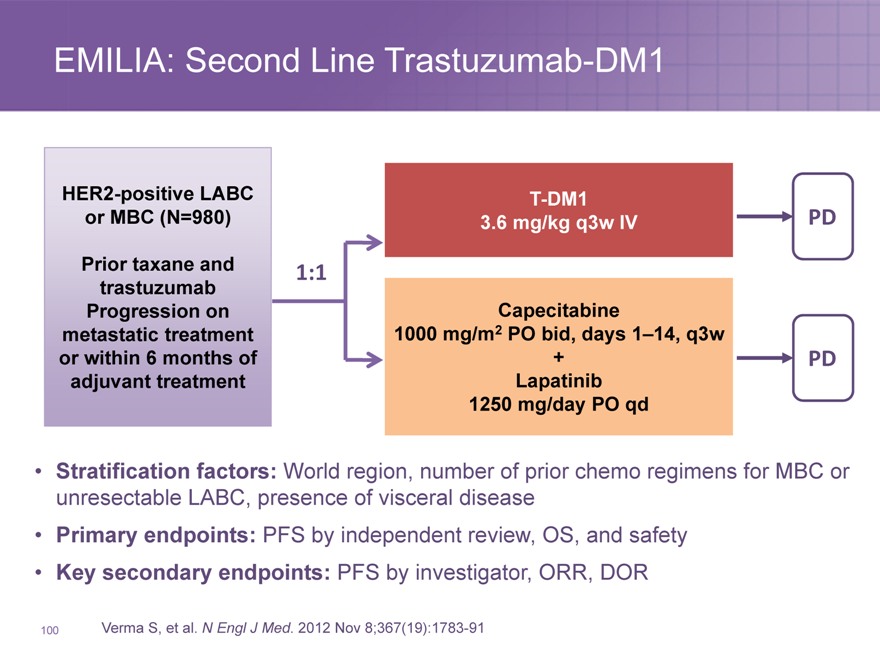
EMILIA: Second Line Trastuzumab-DM1
HER2-positive LABC T-DM1
or MBC (N=980) 3.6 mg/kg q3w IV PD
Prior taxane and 1:1
trastuzumab
Progression on Capecitabine
metastatic treatment 1000 mg/m2 PO bid, days 1–14, q3w
or within 6 months of + PD
adjuvant treatment Lapatinib
1250 mg/day PO qd
Stratification factors: World region, number of prior chemo regimens for MBC or unresectable LABC, presence of visceral disease
Primary endpoints: PFS by independent review, OS, and safety
Key secondary endpoints: PFS by investigator, ORR, DOR
Verma S, et al. N Engl J Med. 2012 Nov 8;367(19):1783-91
100
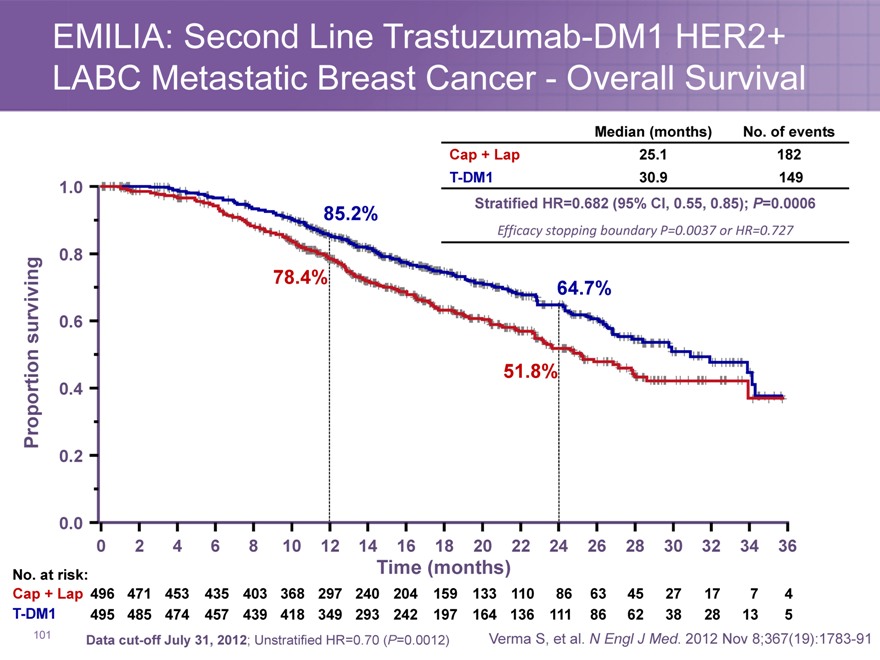
EMILIA: Second Line Trastuzumab-DM1 HER2+ LABC Metastatic Breast Cancer—Overall Survival
Median (months) No. of events
Cap + Lap 25.1 182
85.2% | | Stratified HR=0.682 (95% CI, 0.55, 0.85); P=0.0006 |
Efficacy stopping boundary P=0.0037 or HR=0.727
0.8
surviving 0.6
51.8%
Proportion 0.4
0.2
0.0
0 2 4 6 8 1012 1416182022 24262830 323436
No. at risk: Time (months)
Cap + Lap 496 471 453 435 403 368 297 240 204 159 133 110 86 63 45 27 17 7 4
T-DM1 495 485 474 457 439 418 349 293 242 197 164 136 111 86 62 38 28 13 5
Data cut-off July 31, 2012; Unstratified HR=0.70 (P=0.0012) Verma S, et al. N Engl J Med. 2012 Nov 8;367(19):1783-91
101
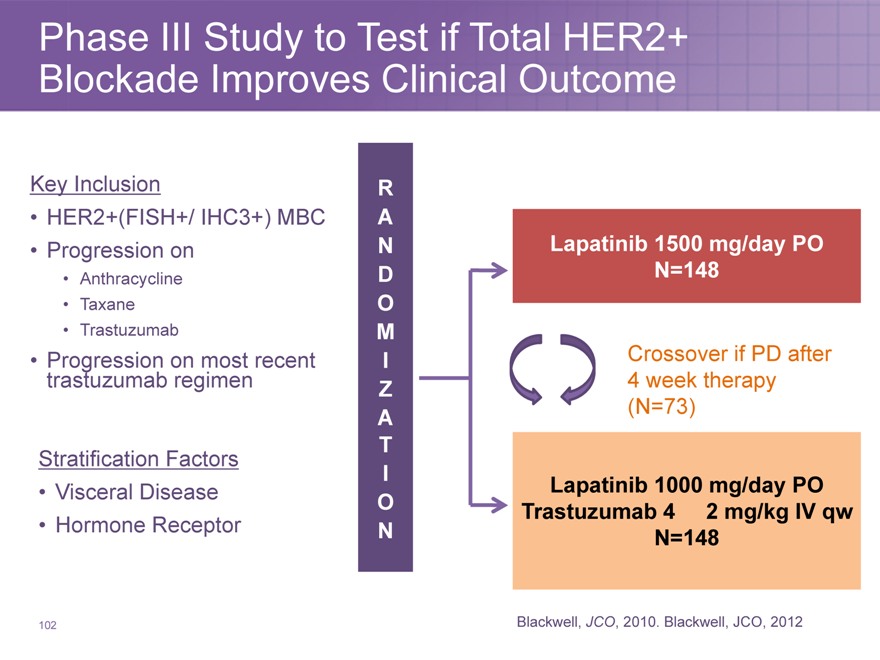
Phase III Study to Test if Total HER2+ Blockade Improves Clinical Outcome
Key Inclusion
HER2+(FISH+/ IHC3+) MBC
Progression on
Anthracycline
Taxane
Trastuzumab
Progression trastuzumab on regimen most recent
Stratification Factors
Visceral Disease
Hormone Receptor
R
A
N Lapatinib 1500 mg/day PO
D N=148
O
M
I Crossover if PD after
Z 4 week therapy
A(N=73)
T
I Lapatinib 1000 mg/day PO
O Trastuzumab 4 2 mg/kg IV qw
N N=148
Blackwell, JCO, 2010. Blackwell, JCO, 2012
102
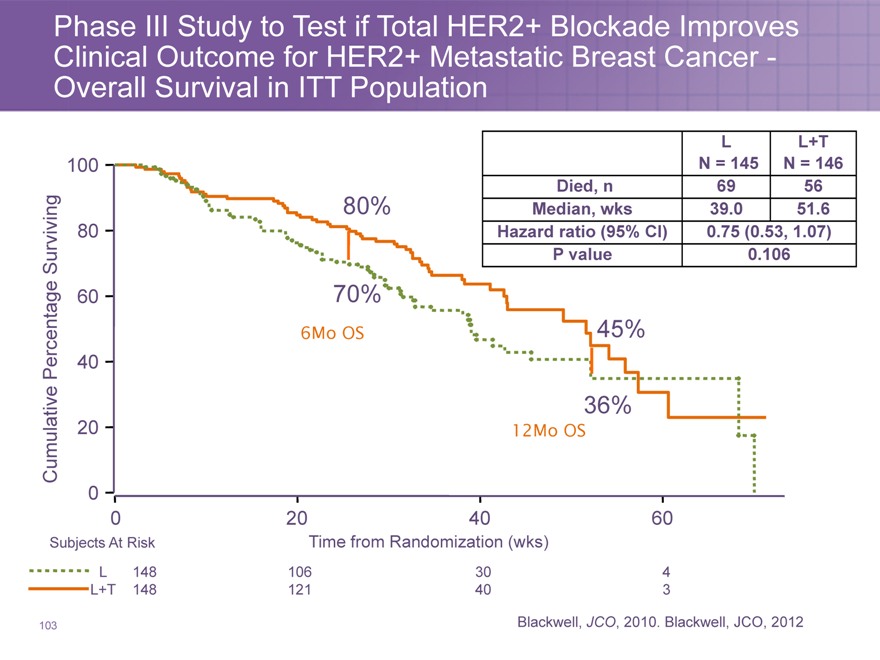
Phase III Study to Test if Total HER2+ Blockade Improves Clinical Outcome for HER2+ Metastatic Breast Cancer -Overall Survival in ITT Population
L L+T
100 N = 145 N = 146
Died, n 69 56
80% Median, wks 39.0 51.6
80 | | Hazard ratio (95% CI) 0.75 (0.53, 1.07) |
Surviving P value 0.106
6Mo OS 45%
Percentage 40
36%
Cumulative 20 12Mo OS
0
020 40 60
Subjects At Risk Time from Randomization (wks)
L 148 106 30 4
L+T 148 121 40 3
Blackwell, JCO, 2010. Blackwell, JCO, 2012
103
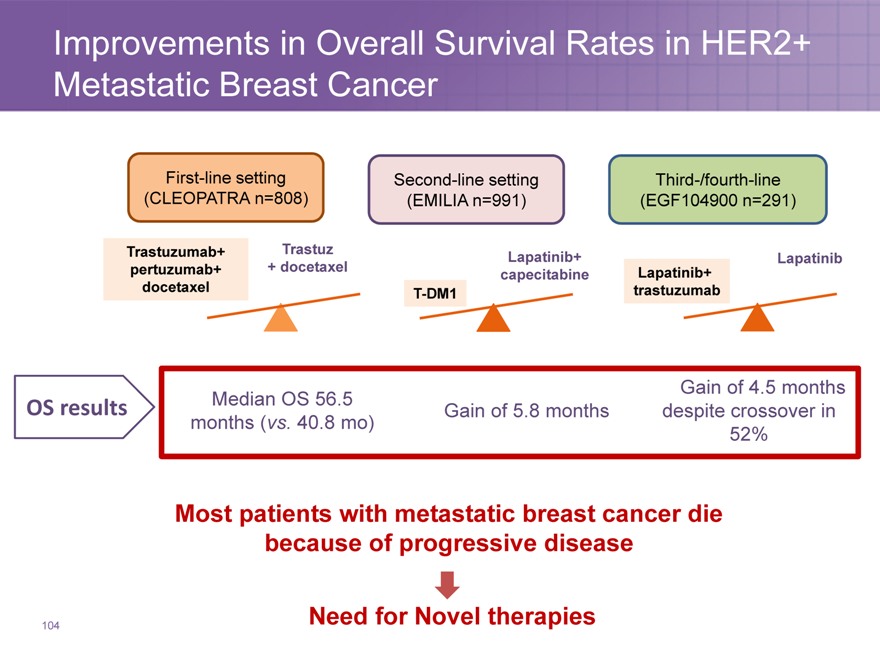
Improvements in Overall Survival Rates in HER2+ Metastatic Breast Cancer
First-line setting Second-line setting Third-/fourth-line
(CLEOPATRA n=808)(EMILIA n=991)(EGF104900 n=291)
Trastuzumab+ Trastuz Lapatinib+ Lapatinib
pertuzumab+ + docetaxel capecitabine Lapatinib+
docetaxel T-DM1 trastuzumab
Median OS 56.5 Gain of 4.5 months
OS results Gain of 5.8 months despite crossover in
months (vs. 40.8 mo) 52%
Most patients with metastatic breast cancer die because of progressive disease
Need for Novel therapies
104
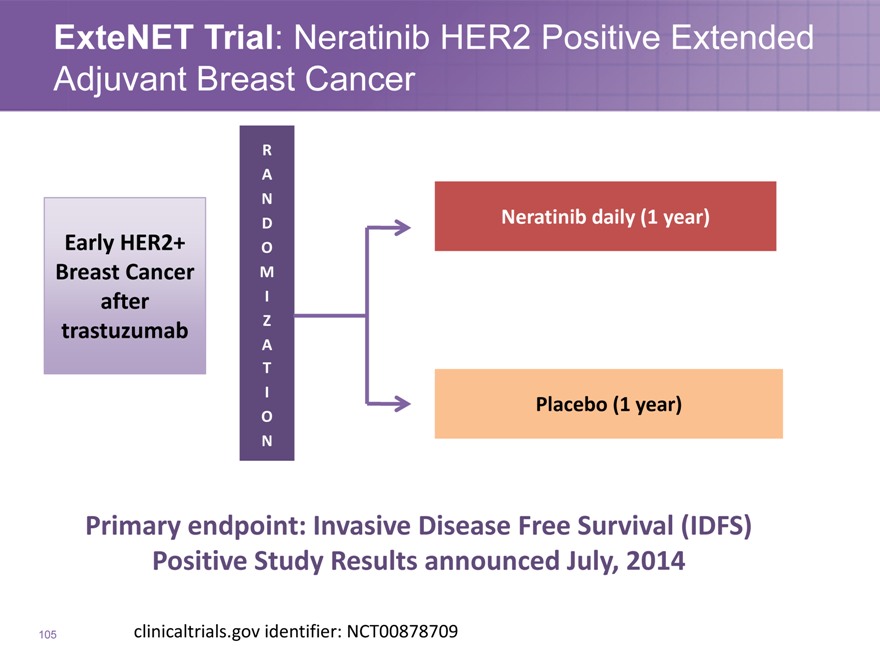
ExteNET Trial: Neratinib HER2 Positive Extended Adjuvant Breast Cancer
Early HER2+
Breast Cancer
after
trastuzumab
R
A
N
D
O
M
I
Z
A
T
I
O
N
Neratinib daily (1 year)
Placebo (1 year)
Primary endpoint: Invasive Disease Free Survival (IDFS) Positive Study Results announced July, 2014
clinicaltrials.gov identifier: NCT00878709
105
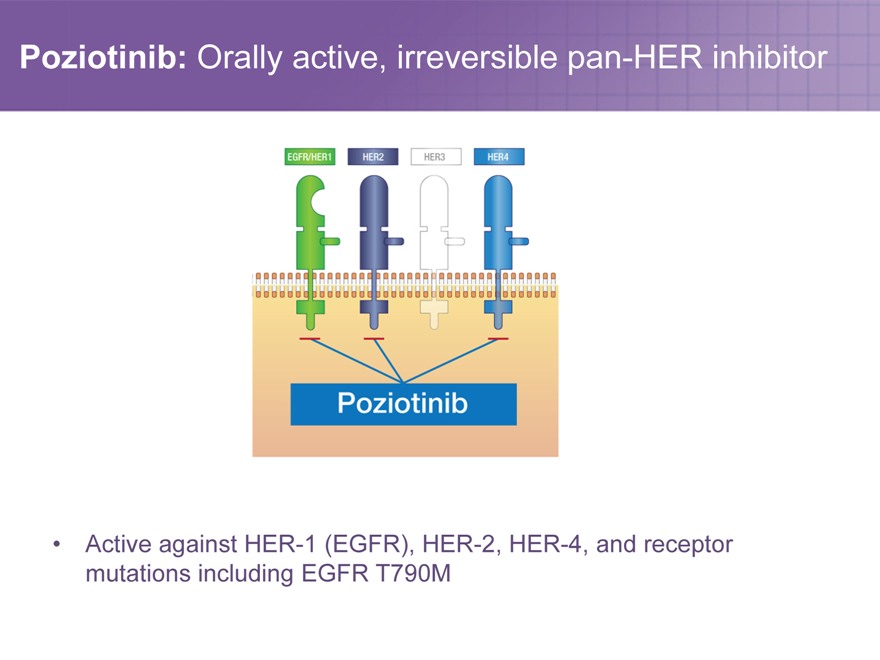
Poziotinib: Orally active, irreversible pan-HER inhibitor
Active against HER-1 (EGFR), HER-2, HER-4, and receptor mutations including EGFR T790M
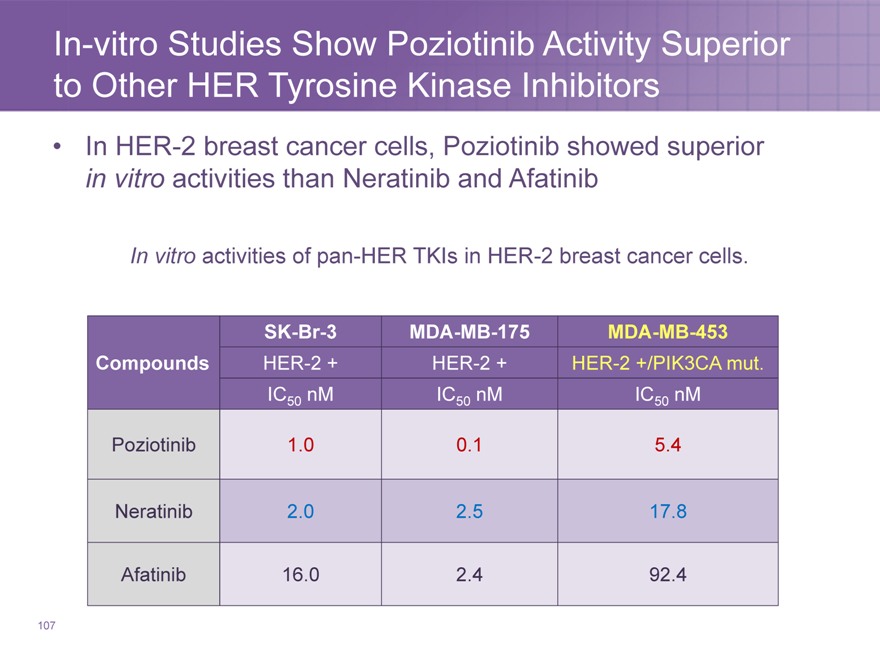
In-vitro Studies Show Poziotinib Activity Superior to Other HER Tyrosine Kinase Inhibitors
In HER-2 breast cancer cells, Poziotinib showed superior in vitro activities than Neratinib and Afatinib
In vitro activities of pan-HER TKIs in HER-2 breast cancer cells.
SK-Br-3 MDA-MB-175 MDA-MB-453
Compounds HER-2 + HER-2 + HER-2 +/PIK3CA mut.
IC50 nM IC50 nM IC50 nM
Poziotinib 1.0 0.1 5.4
Neratinib 2.0 2.5 17.8
Afatinib 16.0 2.4 92.4
107
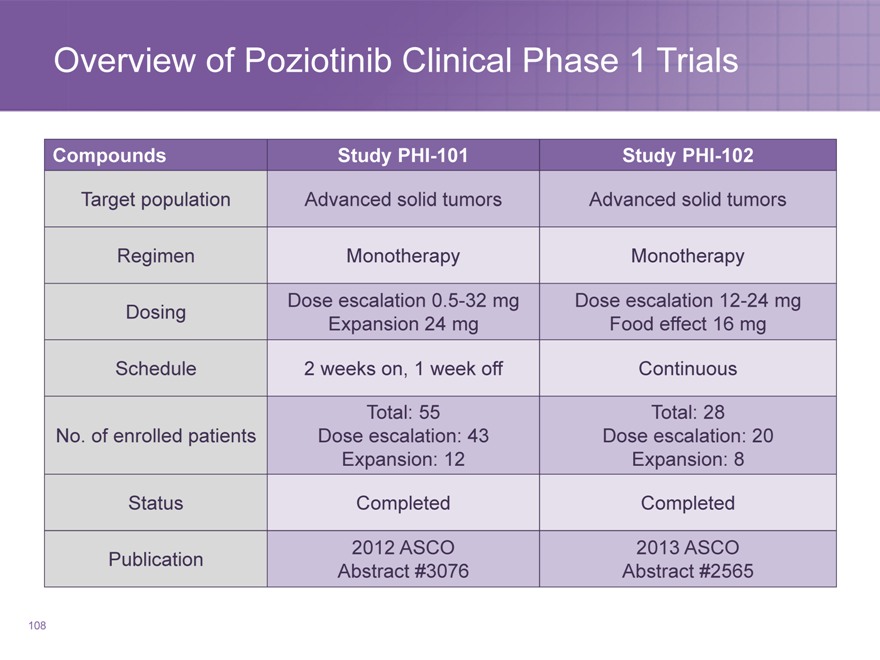
Overview of Poziotinib Clinical Phase 1 Trials
Compounds Study PHI-101 Study PHI-102
Target population Advanced solid tumors Advanced solid tumors
Regimen Monotherapy Monotherapy
Dose escalation 0.5-32 mg Dose escalation 12-24 mg
Dosing Expansion 24 mg Food effect 16 mg
Schedule 2 weeks on, 1 week off Continuous
Total: 55 Total: 28
No. of enrolled patients Dose escalation: 43 Dose escalation: 20
Expansion: 12 Expansion: 8
Status Completed Completed
2012 ASCO 2013 ASCO
Publication Abstract #3076 Abstract #2565
108
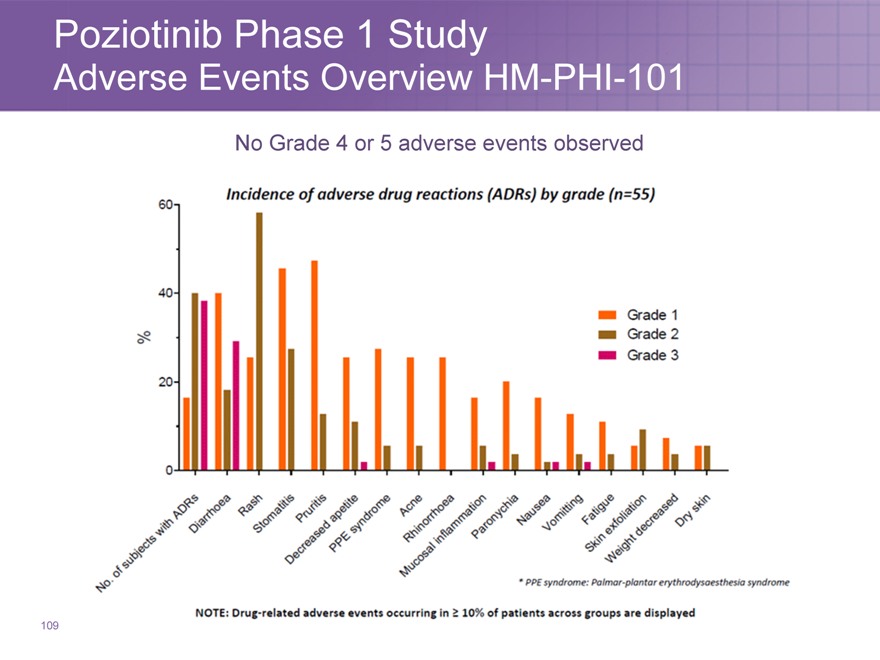
Poziotinib Phase 1 Study
Adverse Events Overview HM-PHI-101
No Grade 4 or 5 adverse events observed
109
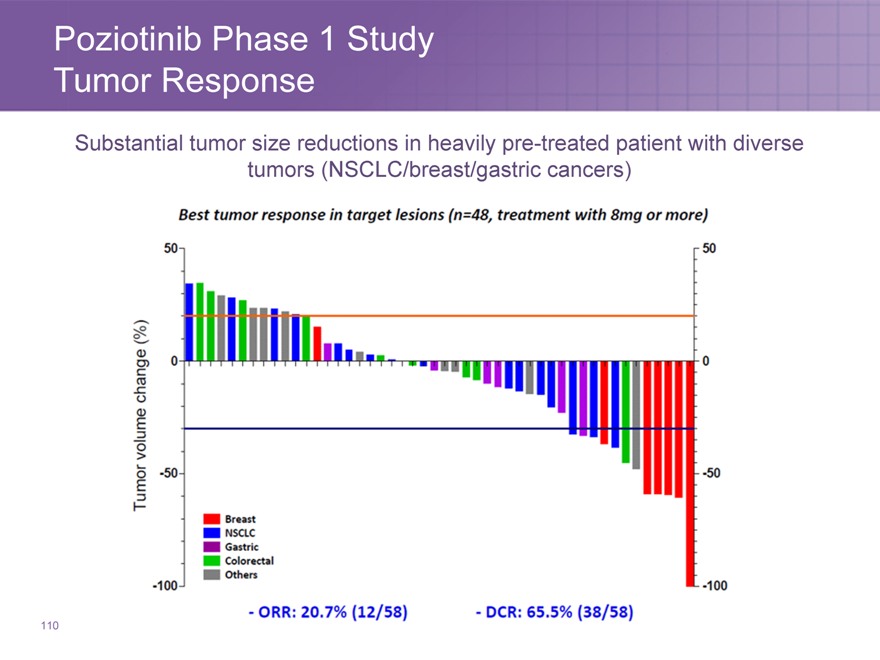
Poziotinib Phase 1 Study Tumor Response
Substantial tumor size reductions in heavily pre-treated patient with diverse tumors (NSCLC/breast/gastric cancers)
110
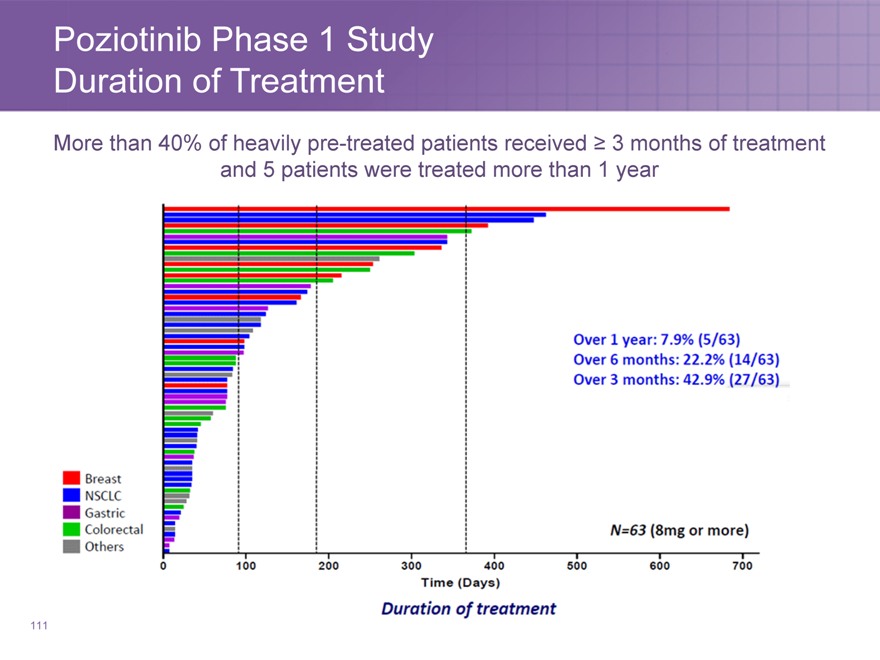
Poziotinib Phase 1 Study
Duration of Treatment
More than 40% of heavily pre-treated patients received ? 3 months of treatment and 5 patients were treated more than 1 year
111
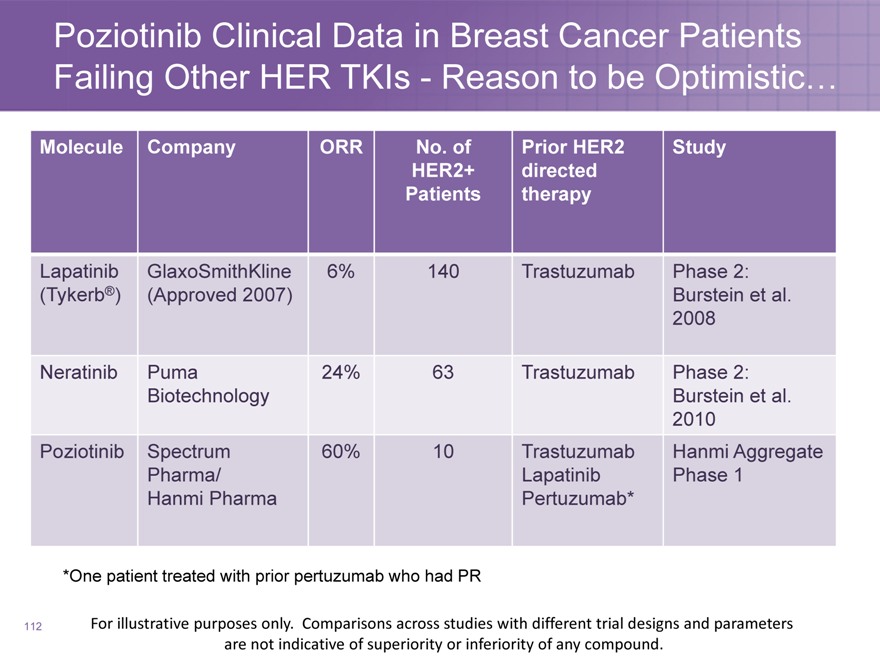
Poziotinib Clinical Data in Breast Cancer Patients Failing Other HER TKIs—Reason to be Optimistic…
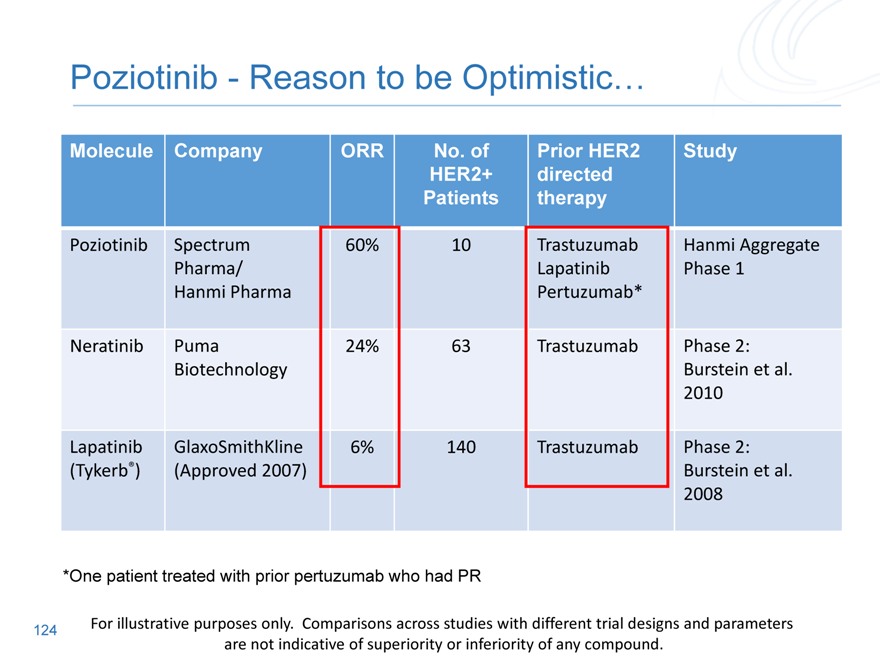
Molecule Company ORR No. of Prior HER2 Study
HER2+ directed
Patients therapy
Lapatinib GlaxoSmithKline 6% 140 Trastuzumab Phase 2:
(Tykerb®)(Approved 2007) Burstein et al.
2008
Neratinib Puma 24% 63 Trastuzumab Phase 2:
Biotechnology Burstein et al.
2010
Poziotinib Spectrum 60% 10 Trastuzumab Hanmi Aggregate
Pharma/ Lapatinib Phase 1
Hanmi Pharma Pertuzumab*
*One patient treated with prior pertuzumab who had PR
112
For illustrative purposes only. Comparisons across studies with different trial designs and parameters are not indicative of superiority or inferiority of any compound.
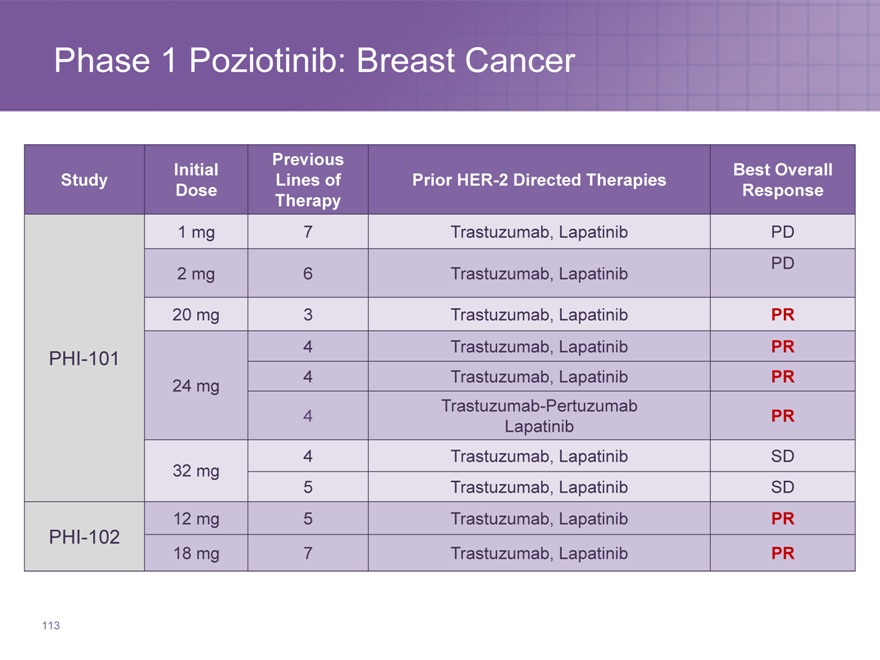
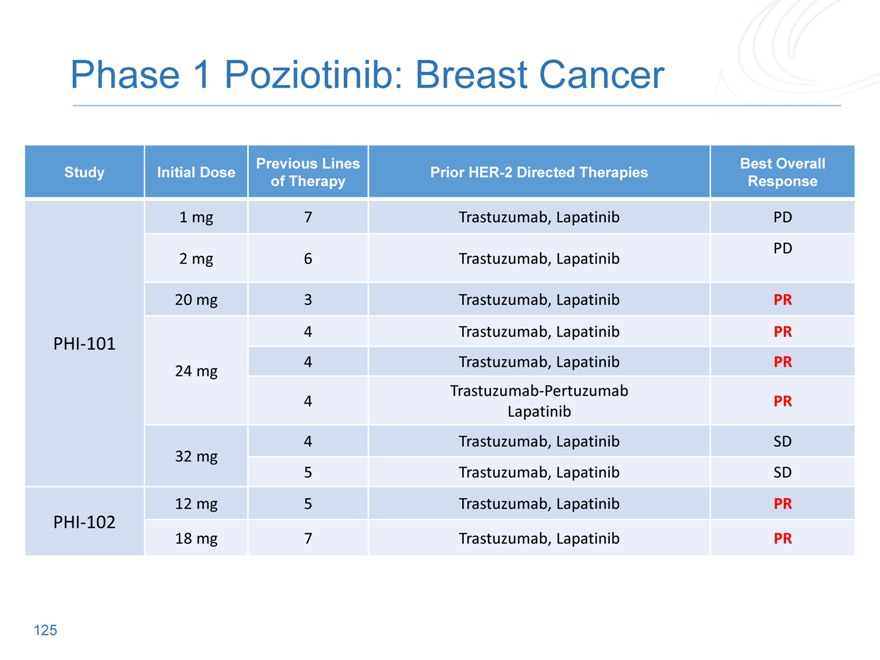
Phase 1 Poziotinib: Breast Cancer
Previous
Initial Best Overall
Study Lines of Prior HER-2 Directed Therapies
Dose Response
Therapy
1 | | mg 7 Trastuzumab, Lapatinib PD |
2 | | mg 6 Trastuzumab, Lapatinib PD |
20 | | mg 3 Trastuzumab, Lapatinib PR |
PHI-101 4 Trastuzumab, Lapatinib PR
24 | | mg 4 Trastuzumab, Lapatinib PR |
Trastuzumab-Pertuzumab
Lapatinib
4 | | Trastuzumab, Lapatinib SD |
5 | | Trastuzumab, Lapatinib SD |
12 | | mg 5 Trastuzumab, Lapatinib PR |
PHI-102
18 | | mg 7 Trastuzumab, Lapatinib PR |
113
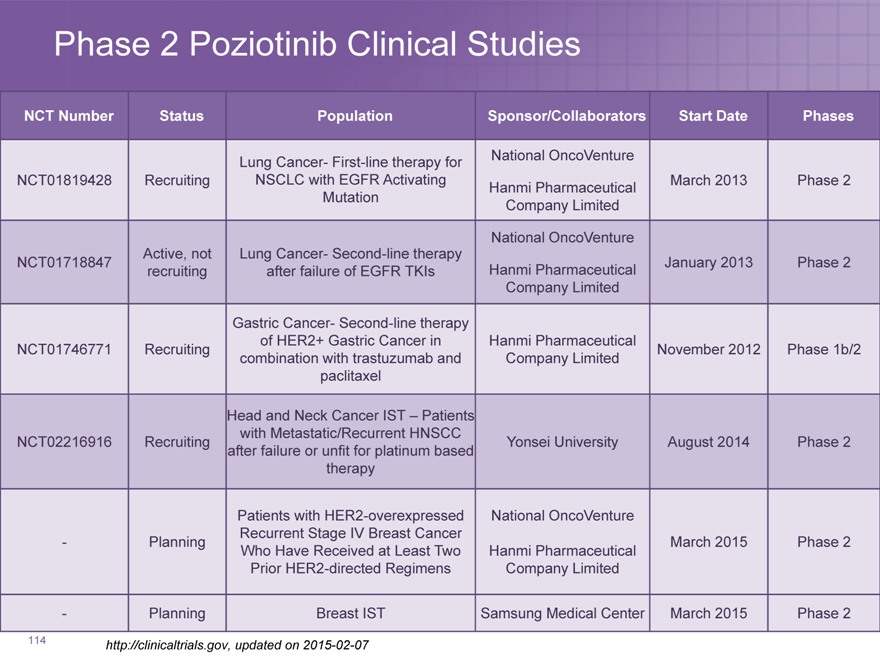
Phase 2 Poziotinib Clinical Studies
NCT Number Status Population Sponsor/Collaborators Start Date Phases
Lung Cancer- First-line therapy for National OncoVenture
NCT01819428 Recruiting NSCLC with EGFR Activating March 2013 Phase 2
Hanmi Pharmaceutical
Mutation Company Limited
National OncoVenture
Active, not Lung Cancer- Second-line therapy
NCT01718847 January 2013 Phase 2
recruiting after failure of EGFR TKIs Hanmi Pharmaceutical
Company Limited
Gastric Cancer- Second-line therapy
of HER2+ Gastric Cancer in Hanmi Pharmaceutical
NCT01746771 Recruiting November 2012 Phase 1b/2
combination with trastuzumab and Company Limited
paclitaxel
Head and Neck Cancer IST – Patients
with Metastatic/Recurrent HNSCC
NCT02216916 Recruiting Yonsei University August 2014 Phase 2
after failure or unfit for platinum based
therapy
Patients with HER2-overexpressed National OncoVenture
Recurrent Stage IV Breast Cancer
—Planning March 2015 Phase 2
Who Have Received at Least Two Hanmi Pharmaceutical
Prior HER2-directed Regimens Company Limited
—Planning Breast IST Samsung Medical Center March 2015 Phase 2
http://clinicaltrials.gov, updated on 2015-02-07
114
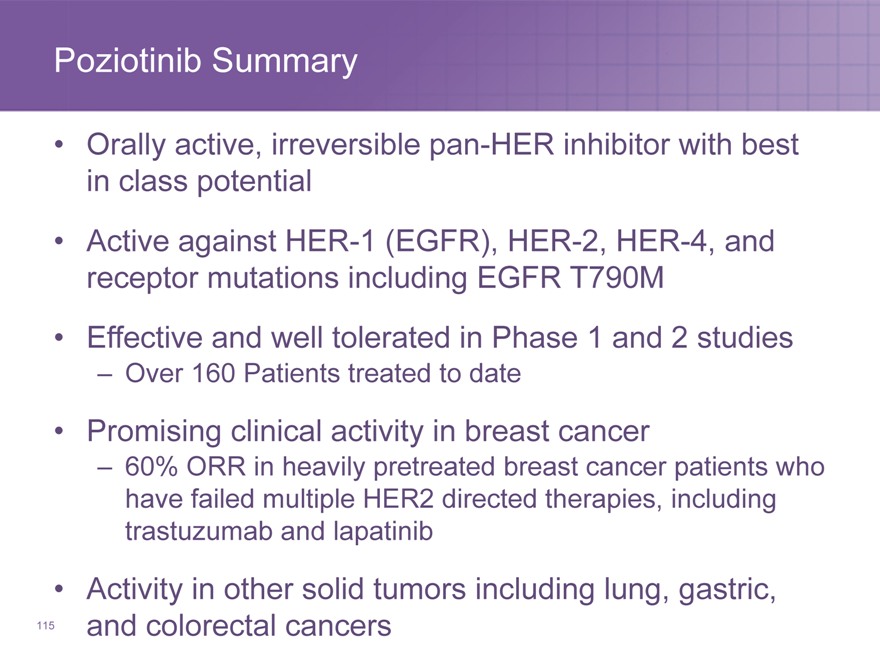
Poziotinib Summary
Orally active, irreversible pan-HER inhibitor with best in class potential
Active against HER-1 (EGFR), HER-2, HER-4, and receptor mutations including EGFR T790M
Effective and well tolerated in Phase 1 and 2 studies
– Over 160 Patients treated to date
Promising clinical activity in breast cancer
– 60% ORR in heavily pretreated breast cancer patients who have failed multiple HER2 directed therapies, including trastuzumab and lapatinib
Activity in other solid tumors including lung, gastric,
and colorectal cancers
115
Commercial Overview
Tom Riga
Senior Vice President and Chief Commercial Officer
Commercial Perspective
117
Positioned For Long-Term Growth
STRONG LONG-TERM GROWTH DRIVERS
LATE-STAGE ASSETS WITH GROWTH POTENTIAL
MULTIPLE ASSETS FUND PIPELINE
Early Stage assets
CE Melphalan
Apaziquone
Poziotinib
SPI-2012
Business Development and Lifecycle Management
See Package inserts for full indications and for details including safety information
5 Approved Drugs
118
See Package Inserts for full indications and for details including safety information
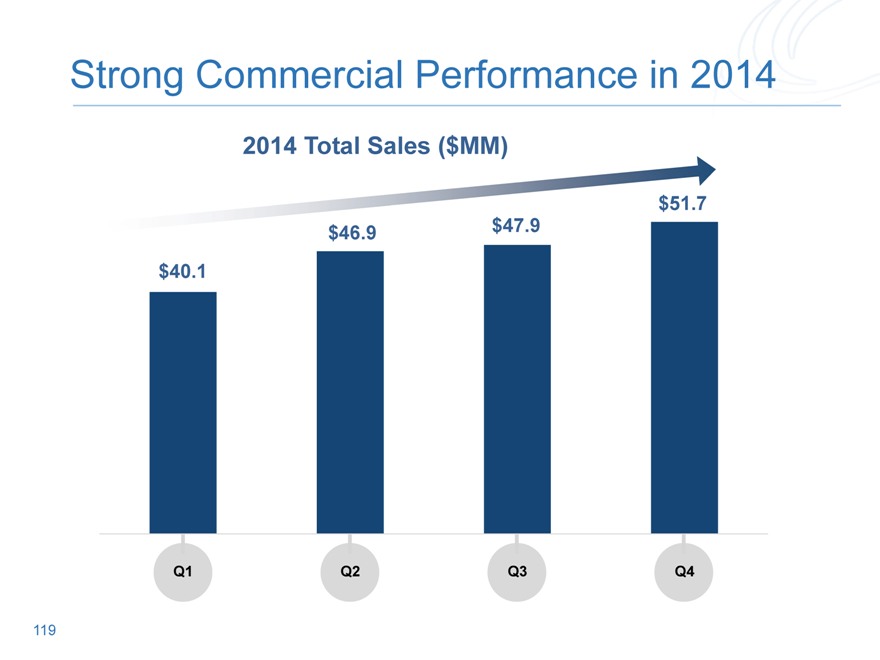
Strong Commercial Performance in 2014
2014 Total Sales ($MM)
$40.1
Q1
Q2
Q3
Q4
119
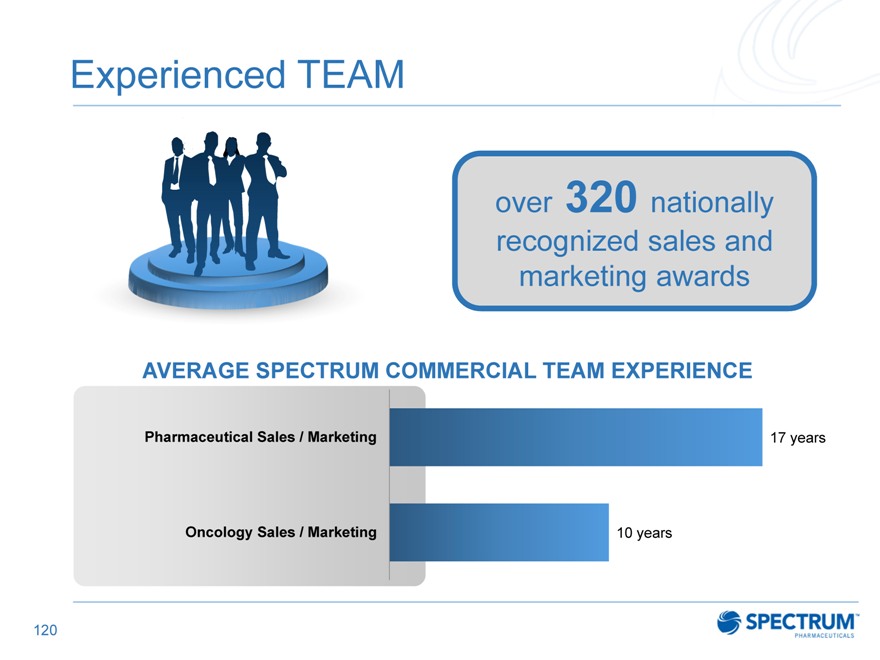
Experienced TEAM
over 320 nationally recognized sales and marketing awards
AVERAGE SPECTRUM COMMERCIAL TEAM EXPERIENCE
Pharmaceutical Sales / Marketing 17 years
Oncology Sales / Marketing 10 years
120
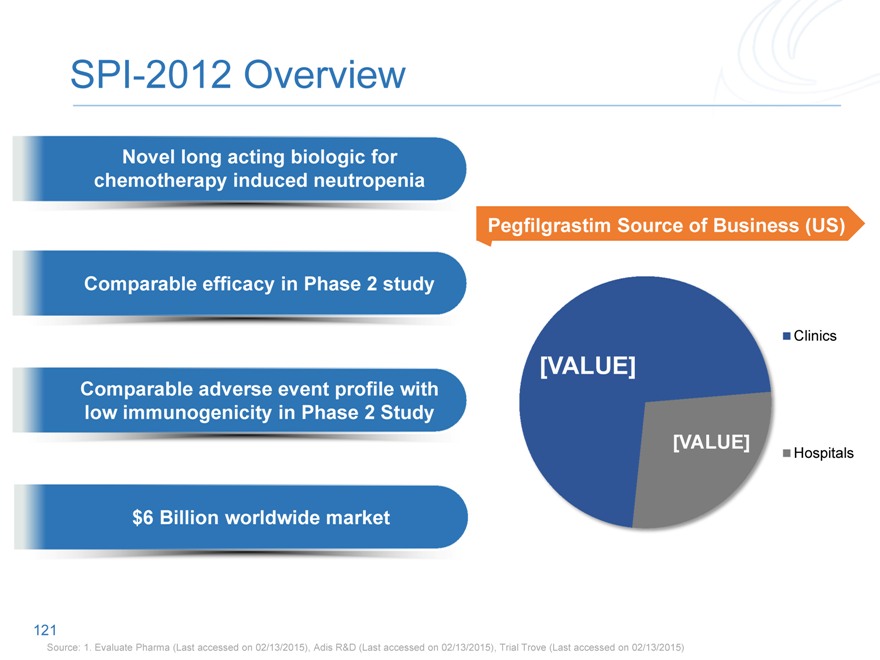
SPI-2012 Overview
Novel long acting biologic for chemotherapy induced neutropenia
Pegfilgrastim Source of Business (US)
Comparable efficacy in Phase 2 study
Clinics
[VALUE]
Comparable adverse event profile with low immunogenicity in Phase 2 Study
[VALUE]
Hospitals
$6 Billion worldwide market
121
Source: 1. Evaluate Pharma (Last accessed on 02/13/2015), Adis R&D (Last accessed on 02/13/2015), Trial Trove (Last accessed on 02/13/2015)
Capitalizing on Our Potential Blockbuster
TRUSTED PARTNERSHIPS
122
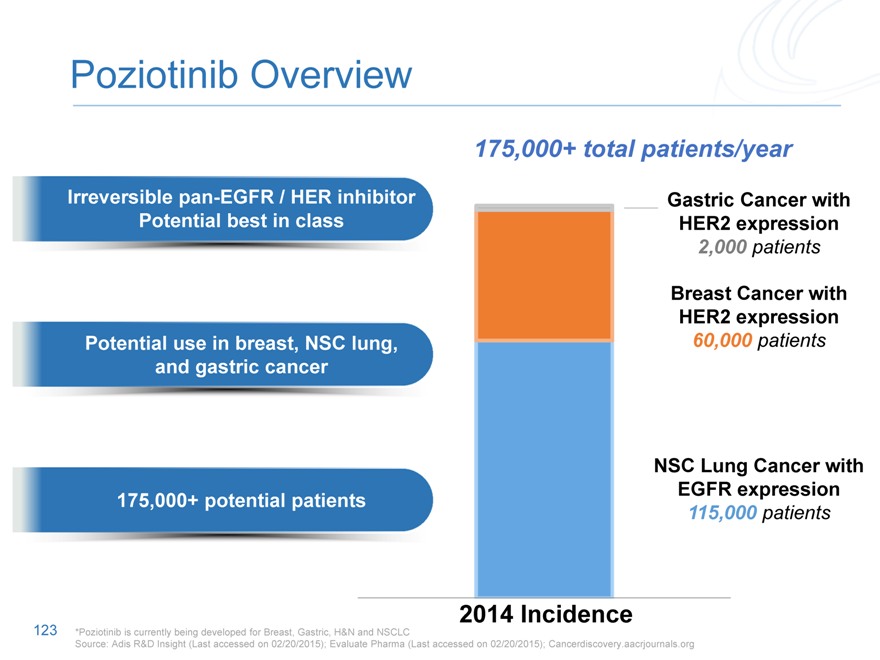
Poziotinib Overview
175,000+ total patients/year
Irreversible pan-EGFR / HER inhibitor Gastric Cancer with
Potential best in class HER2 expression
2,000 patients
Breast Cancer with
HER2 expression
Potential use in breast, NSC lung, 60,000 patients
and gastric cancer
175,000+ potential patients
NSC Lung Cancer with
EGFR expression
115,000 patients
2014 Incidence
123
*Poziotinib is currently being developed for Breast, Gastric, H&N and NSCLC
Source: Adis R&D Insight (Last accessed on 02/20/2015); Evaluate Pharma (Last accessed on 02/20/2015); Cancerdiscovery.aacrjournals.org
Poziotinib—Reason to be Optimistic…
Molecule Company ORR No. of Prior HER2 Study
HER2+ directed
Patients therapy
Poziotinib Spectrum 60% 10 Trastuzumab Hanmi Aggregate
Pharma/ Lapatinib Phase 1
Hanmi Pharma Pertuzumab*
Neratinib Puma 24% 63 Trastuzumab Phase 2:
Biotechnology Burstein et al.
2010
Lapatinib GlaxoSmithKline 6% 140 Trastuzumab Phase 2:
(Tykerb®)(Approved 2007) Burstein et al.
2008
*One patient treated with prior pertuzumab who had PR
124
For illustrative purposes only. Comparisons across studies with different trial designs and parameters are not indicative of superiority or inferiority of any compound.
Phase 1 Poziotinib: Breast Cancer
Previous Lines Best Overall
Study Initial Dose Prior HER-2 Directed Therapies
of Therapy Response
1 mg 7 Trastuzumab, Lapatinib PD
PD
2 mg 6 Trastuzumab, Lapatinib
20 mg 3 Trastuzumab, Lapatinib PR
4 | | Trastuzumab, Lapatinib PR |
PHI?101
24 mg 4 Trastuzumab, Lapatinib PR
Trastuzumab?Pertuzumab
Lapatinib
4 | | Trastuzumab, Lapatinib SD |
32 mg
5 | | Trastuzumab, Lapatinib SD |
12 mg 5 Trastuzumab, Lapatinib PR
PHI?102
18 mg 7 Trastuzumab, Lapatinib PR
125
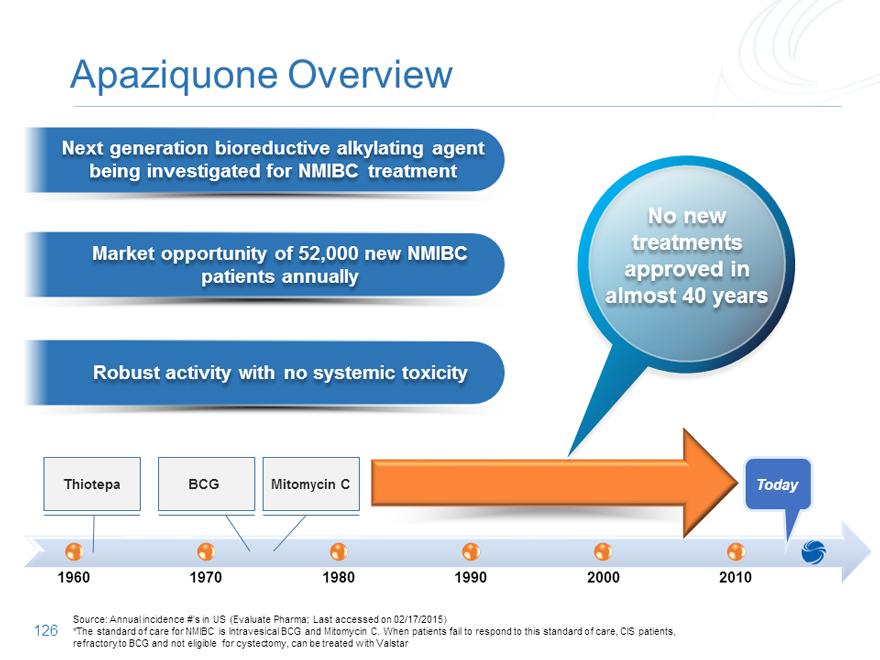
Apaziquone Overview
Next generation bioreductive alkylating agent being investigated for NMIBC treatment
No new treatments
Market opportunity of 52,000 new NMIBC patients annually approved in almost 40 years
Robust activity with no systemic toxicity
Thiotepa BCG Mitomycin C Today
1960 1970 1980 1990 2000 2010
Source: Annual incidence #’s in US (Evaluate Pharma; Last accessed on 02/17/2015)
*The standard of care for NMIBC is Intravesical BCG and Mitomycin C. When patients fail to respond to this standard of care, CIS patients,
refractory to BCG and not eligible for cystectomy, can be treated with Valstar
126
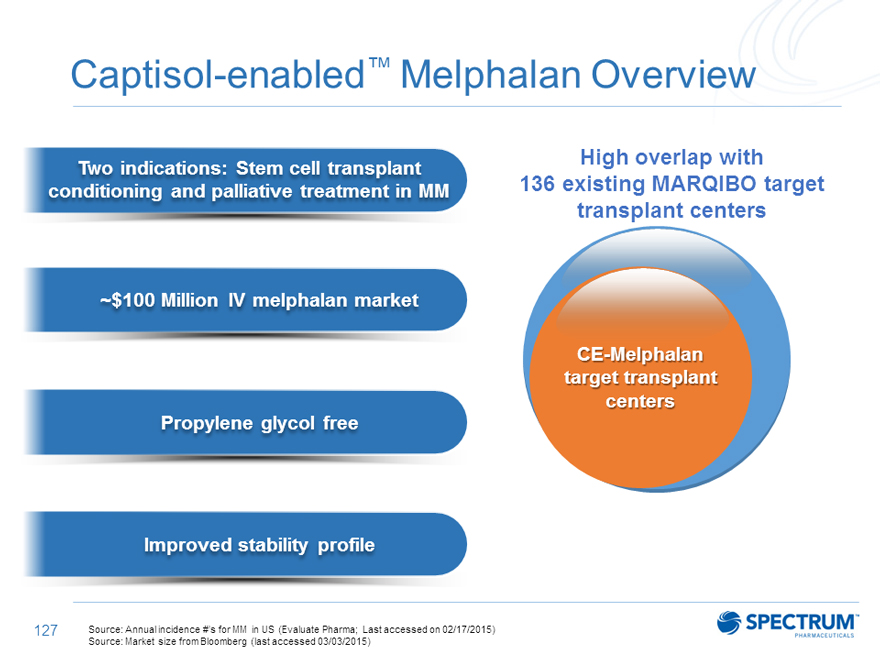
Captisol-enabled™ Melphalan Overview
Two indications: Stem cell transplant High overlap with
conditioning and palliative treatment in MM 136 existing MARQIBO target
transplant centers
~$100 Million IV melphalan market
CE-Melphalan target transplant centers Propylene glycol free
Improved stability profile
Source: Annual incidence #’s for MM in US (Evaluate Pharma; Last accessed on 02/17/2015) Source: Market size from Bloomberg (last accessed 03/03/2015)
127
Positioned Well for Long-Term Growth
Experienced team that is executing today
Two near?term NDA’s
Game changing pipeline with two potential blockbuster drugs
128
Summary
Rajesh Shrotriya
Chairman and CEO
Analyst Day Summary
Two Near-Term NDAs and Two Potential Blockbusters
SPI-2012 is a novel biologic with blockbuster potential to mitigate neutropeniaPoziotinib has strong early data, validated MOA, targets large indicationsCE Melphalan is propylene glycol-free with an improved stability profile for the treatment of Multiple Myeloma: PDUFA: October 23, 2015Apaziquone is a next generation alkylating agent addressing an unmet medical need
STRONG
LONG-TERM
GROWTH DRIVERS
LATE-STAGE ASSETS
WITH GROWTH
POTENTIAL
MULTIPLE ASSETS
FUND PIPELINE
130
Q&A
131
132