UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the Fiscal Year Ended December 31, 2016 | |
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the Transition Period From to | |
Commission file number 1-10235
IDEX CORPORATION
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 36-3555336 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
| 1925 West Field Court, Lake Forest, Illinois | 60045 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number:
(847) 498-7070
Securities Registered Pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered | |
| Common Stock, par value $.01 per share | New York Stock Exchange and Chicago Stock Exchange | |
Securities Registered Pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer þ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company ¨ | |||
| (Do not check if a smaller reporting company) | ||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
The aggregate market value, as of the last business day of the registrant’s most recently completed second fiscal quarter, of the common stock (based on the June 30, 2016 closing price of $82.10) held by non-affiliates of IDEX Corporation was $6,235,379,567.
The number of shares outstanding of IDEX Corporation’s common stock, par value $.01 per share, as of February 14, 2017 was 76,248,604.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the proxy statement with respect to the IDEX Corporation 2017 annual meeting of stockholders (the “2017 Proxy Statement”) are incorporated by reference into Part III of this Form 10-K.
Table of Contents
| PART I. | ||
| Item 1. | ||
| Item 1A. | ||
| Item 1B. | ||
| Item 2. | ||
| Item 3. | ||
| Item 4. | ||
| PART II. | ||
| Item 5. | ||
| Item 6. | ||
| Item 7. | ||
| Item 7A. | ||
| Item 8. | ||
| Item 9. | ||
| Item 9A. | ||
| Item 9B. | ||
| PART III. | ||
| Item 10. | ||
| Item 11. | ||
| Item 12. | ||
| Item 13. | ||
| Item 14. | ||
| PART IV. | ||
| Item 15. | ||
| Item 16. | Form 10-K Summary | |
PART I
Cautionary Statement Under the Private Securities Litigation Reform Act
This report contains “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. These statements may relate to, among other things, capital expenditures, acquisitions, cost reductions, cash flow, revenues, earnings, market conditions, global economies and operating improvements, and are indicated by words or phrases such as “anticipate,” “estimate,” “plans,” “expects,” “projects,” “forecasts,” “should,” “could,” “will,” “management believes,” “the company believes,” “the company intends,” and similar words or phrases. These statements are subject to inherent uncertainties and risks that could cause actual results to differ materially from those anticipated at the date of this report. The risks and uncertainties include, but are not limited to, the following: economic and political consequences resulting from terrorist attacks and wars; levels of industrial activity and economic conditions in the U.S. and other countries around the world; pricing pressures, other competitive factors and levels of capital spending in certain industries, all of which could have a material impact on order rates and IDEX Corporation’s results, particularly in light of the low levels of order backlogs it typically maintains; its ability to make acquisitions and to integrate and operate acquired businesses on a profitable basis; the relationship of the U.S. dollar to other currencies and its impact on pricing and cost competitiveness; political and economic conditions in foreign countries in which the company operates; interest rates; capacity utilization and the effect this has on costs; labor markets; market conditions and material costs; and developments with respect to contingencies, such as litigation and environmental matters. The forward-looking statements included here are only made as of the date of this report, and management undertakes no obligation to publicly update them to reflect subsequent events or circumstances, except as may be required by law. Investors are cautioned not to rely unduly on forward-looking statements when evaluating the information presented here.
| Item 1. | Business. |
IDEX Corporation (“IDEX,” the “Company,” “us,” “our,” or “we”) is a Delaware corporation incorporated on September 24, 1987. The Company is an applied solutions business that sells an extensive array of pumps, valves, flow meters and other fluidics systems and components and engineered products to customers in a variety of markets around the world. All of the Company’s business activities are carried out through wholly-owned subsidiaries.
The Company has three reportable business segments: Fluid & Metering Technologies (“FMT”), Health & Science Technologies (“HST”) and Fire & Safety/Diversified Products (“FSDP”). Within our three reportable segments, the Company maintains thirteen platforms, where we focus on organic growth and strategic acquisitions. Each of our thirteen platforms is also a reporting unit, where we annually test for goodwill impairment.
During the fourth quarter of 2016, the Company reorganized certain of its reporting units to align with changes in management and as a result of certain divestitures as well as to align with how management will run the business going forward as follows:
•Moved the Richter and Aegis businesses from the previous Industrial reporting unit to the Valves reporting unit;
•Moved the Trebor business from the previous Industrial reporting unit to the Water reporting unit;
| • | Replaced the previous Industrial reporting unit with the Pumps reporting unit, which now includes Viking and Warren Rupp; |
| • | Combined the Scientific Fluidics and IDEX Optics & Photonics reporting units into the Scientific Fluidics & Optics reporting unit; and |
| • | Combined the Fire Suppression and Rescue reporting units into the Fire & Safety reporting unit. |
The Fluid & Metering Technologies segment contains the Energy (comprised of Corken, Faure Herman, Liquid Controls, SAMPI, and Toptech), Valves (comprised of Alfa Valvole, Richter, and Aegis), Water (comprised of Pulsafeeder, Knight, ADS, Trebor, and iPEK), Pumps (comprised of Viking and Warren Rupp), and Agriculture (comprised of Banjo) platforms. The Health & Science Technologies segment contains the Scientific Fluidics & Optics (comprised of Eastern Plastics, Rheodyne, Sapphire Engineering, Upchurch Scientific, ERC, CiDRA Precision Services, CVI Melles Griot, Semrock, and AT Films), Sealing Solutions (comprised of Precision Polymer Engineering, FTL Seals Technology, Novotema, and SFC Koenig), Gast, Micropump, and Material Processing Technologies (comprised of Quadro, Fitzpatrick, Microfluidics, and Matcon) platforms. The Fire & Safety/Diversified Products segment is comprised of the Fire & Safety (comprised of Class 1, Hale, Godiva, Akron Brass, AWG Fittings, Dinglee, Hurst Jaws of Life, Lukas, and Vetter), Band-It, and Dispensing platforms.
IDEX believes that each of its reporting units is a leader in its product and service areas. The Company also believes that its strong financial performance has been attributable to its ability to design and engineer specialized quality products, coupled with its ability to identify and successfully consummate and integrate strategic acquisitions.
1
FLUID & METERING TECHNOLOGIES SEGMENT
The Fluid & Metering Technologies segment designs, produces and distributes positive displacement pumps, valves, flow meters, injectors, and other fluid-handling pump modules and systems and provides flow monitoring and other services for the food, chemical, general industrial, water & wastewater, agriculture and energy industries. Fluid & Metering Technologies application-specific pump and metering solutions serve a diverse range of end markets, including industrial infrastructure (fossil fuels, refined & alternative fuels, and water & wastewater), chemical processing, agriculture, food & beverage, pulp and paper, transportation, plastics and resins, electronics and electrical, construction & mining, pharmaceutical and bio-pharmaceutical, machinery and numerous other specialty niche markets.
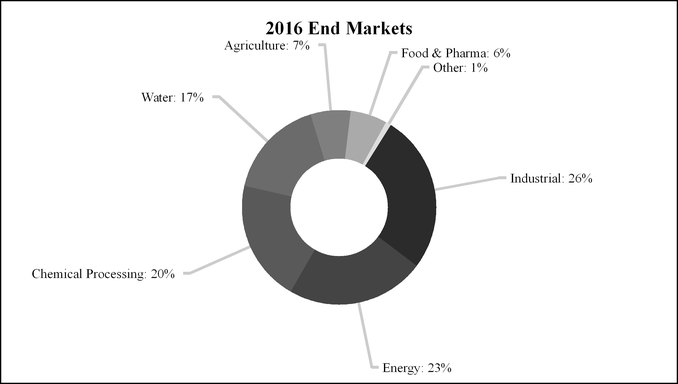
Fluid & Metering Technologies accounted for 40%, 43% and 42% of IDEX’s sales in 2016, 2015 and 2014, respectively, with approximately 44% of its 2016 sales to customers outside the U.S. The segment accounted for 44%, 43% and 43% of IDEX’s operating income in 2016, 2015 and 2014, respectively.
Energy. Energy consists of the Company’s Corken, Faure Herman, Liquid Controls, SAMPI, and Toptech businesses. Energy is a leading supplier of flow meters, electronic registration and control products, rotary vane and turbine pumps, reciprocating piston compressors, and terminal automation control systems. Applications for Liquid Controls and SAMPI consist of positive displacement flow meters, electronic, registration and control products, including mobile and stationary metering installations for wholesale and retail distribution of petroleum and liquefied petroleum gas, aviation refueling, and industrial metering and dispensing of liquids and gases. Corken products consist of positive-displacement rotary vane pumps, single and multistage regenerative turbine pumps, and small horsepower reciprocating piston compressors. Toptech supplies terminal automation hardware and software to control and manage inventories, as well as transactional data and invoicing, to customers in the oil, gas and refined-fuels markets. Faure Herman is a leading supplier of ultrasonic and helical turbine flow meters used in the custody transfer and control of high value fluids and gases. Energy maintains facilities in Lake Bluff, Illinois (Liquid Controls products); Longwood, Florida and Zwijndrecht, Belgium (Toptech products); Oklahoma City, Oklahoma (Corken products); La Ferté Bernard, France (Faure Herman products); and Altopascio, Italy (SAMPI products). Approximately 50% of Energy’s 2016 sales were to customers outside the U.S.
Valves. Valves consists of the Company’s Alfa Valvole, Richter, and Aegis businesses. Valves is a leader in the design, manufacture and sale of specialty valve products for use in the chemical, petro-chemical, energy and sanitary markets as well as a leading producer of fluoroplastic lined corrosion-resistant magnetic drive and mechanical seal pumps, shut-off, control and safety valves for corrosive, hazardous, contaminated, pure and high-purity fluids. Alfa Valvole’s products are used in various industrial fields for fluid control, in both gas and liquid form, in all sectors of plant engineering, cosmetics, detergents, food
2
industry, electric energy, pharmaceutical, chemical plants, petrochemical plants, oil, heating/air conditioning and also on ships, ferries and marine oil platforms. Richter’s products offer superior solutions for demanding and complex pump applications in the process industry. Aegis produces specialty chemical processing valves for use in the chemical, petro-chemical, chlor-alkali, and pulp & paper industries. Valves maintains operations in Casorezzo, Italy (Alfa Valvole products); Cedar Falls, Iowa, Kempen, Germany, and Suzhou, China (Richter products); and Geismar, Louisiana (Aegis products). Approximately 81% of Valves’ 2016 sales were to customers outside the U.S.
Water. Water consists of the Company’s ADS, iPEK, Knight, Trebor, and Pulsafeeder businesses. Water is a leading provider of metering technology, flow monitoring products and underground surveillance services for wastewater markets, alloy and non-metallic gear pumps, peristaltic pumps, transfer pumps, as well as dispensing equipment for industrial laundries, commercial dishwashing and chemical metering. ADS’s products and services provide comprehensive integrated solutions that enable industry, municipalities and government agencies to analyze and measure the capacity, quality and integrity of wastewater collection systems, including the maintenance and construction of such systems. iPEK supplies remote controlled systems used for infrastructure inspection. Knight is a leading manufacturer of pumps and dispensing equipment for industrial laundries, commercial dishwashing and chemical metering. Trebor is a leader in high-purity fluid handling products, including air-operated diaphragm pumps and deionized water-heating systems. Trebor products are used in the manufacturing of semiconductors, disk drives and flat panel displays. Pulsafeeder products (which also include OBL products) are used to introduce precise amounts of fluids into processes to manage water quality and chemical composition, as well as peristaltic pumps. Its markets include water & wastewater treatment, oil & gas, power generation, pulp & paper, chemical and hydrocarbon processing, and swimming pools. Water maintains operations in Huntsville, Alabama and various other locations in the United States and Australia (ADS products and services); Hirschegg, Austria and Sulzberg, Germany (iPEK products); Rochester, New York, Punta Gorda, Florida, and Milan, Italy (Pulsafeeder products); Salt Lake City, Utah (Trebor products); Irvine, California, Mississauga, Ontario, Canada, and Lewes, England (Knight products); and a maquiladora in Ciudad Juarez, Chihuahua, Mexico (Knight products). Approximately 38% of Water’s 2016 sales were to customers outside the U.S.
Pumps. Pumps consists of the Company’s Viking and Warren Rupp businesses. Pumps is a leading manufacturer of rotary internal gear, external gear, vane and rotary lobe pumps, custom-engineered OEM pumps, strainers, gear reducers and engineered pump systems. Viking’s products consist of external gear pumps, strainers and reducers, and related controls used for transferring and metering thin and viscous liquids sold under the Viking and Wright Flow brands. Viking products primarily serve the chemical, petroleum, pulp & paper, plastics, paints, inks, tanker trucks, compressor, construction, food & beverage, personal care, pharmaceutical and biotech markets. Warren Rupp products (which also include Pumper Parts and Versa-Matic products) are used for abrasive and semisolid materials as well as for applications where product degradation is a concern or where electricity is not available or should not be used. Warren Rupp products, which include air-operated double diaphragm pumps, primarily serve the chemical, paint, food processing, electronics, construction, utilities, oil & gas, mining and industrial maintenance markets. Pumps maintains operations in Cedar Falls, Iowa (Viking and Wright Flow products); Eastbourne, England (Wright Flow products); Shannon, Ireland (Viking and Blagdon products); and Mansfield, Ohio (Warren Rupp products). Pumps primarily uses independent distributors to market and sell its products. Approximately 40% of Pumps’ 2016 sales were to customers outside the U.S.
Agriculture. Agriculture consists of the Company’s Banjo business. Banjo is a provider of special purpose, severe-duty pumps, valves, fittings and systems used in liquid handling. Banjo is based in Crawfordsville, Indiana with distribution facilities in Didam, The Netherlands and Valinhos, Brazil, and its products are used in agriculture and industrial applications. Approximately 13% of Banjo’s 2016 sales were to customers outside the U.S.
HEALTH & SCIENCE TECHNOLOGIES SEGMENT
The Health & Science Technologies segment designs, produces and distributes a wide range of precision fluidics, rotary lobe pumps, centrifugal and positive displacement pumps, roll compaction and drying systems used in beverage, food processing, pharmaceutical and cosmetics, pneumatic components and sealing solutions, including very high precision, low-flow rate pumping solutions required in analytical instrumentation, clinical diagnostics and drug discovery, high performance molded and extruded, biocompatible medical devices and implantables, air compressors used in medical, dental and industrial applications, optical components and coatings for applications in the fields of scientific research, defense, biotechnology, aerospace, telecommunications and electronics manufacturing, laboratory and commercial equipment used in the production of micro and nano scale materials, precision photonic solutions used in life sciences, research and defense markets, and precision gear and peristaltic pump technologies that meet exacting original equipment manufacturer specifications.
3
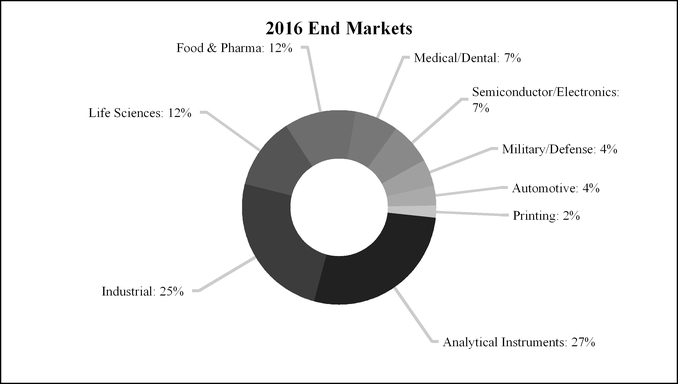
Health & Science Technologies accounted for 35%, 36% and 35% of IDEX’s sales in 2016, 2015 and 2014, respectively, with approximately 55% of its 2016 sales to customers outside the U.S. The segment accounted for 31%, 33% and 31% of IDEX’s operating income in 2016, 2015 and 2014, respectively.
Scientific Fluidics & Optics. Scientific Fluidics & Optics consists of the Company’s Eastern Plastics, Rheodyne, Sapphire Engineering, Upchurch Scientific, ERC, CiDRA Precision Services, CVI Melles Griot, Semrock, and AT Films (including Precision Photonics products) businesses. Eastern Plastics products, which consist of high-precision integrated fluidics and associated engineered manifolds, are used in a broad set of end markets including medical diagnostics, analytical instrumentation, and laboratory automation. Rheodyne products consist of injectors, valves, fittings and accessories for the analytical instrumentation market. These products are used by manufacturers of high pressure liquid chromatography (“HPLC”) equipment servicing the pharmaceutical, biotech, life science, food & beverage, and chemical markets. Sapphire Engineering and Upchurch Scientific products consist of fluidic components and systems for the analytical, biotech and diagnostic instrumentation markets, such as fittings, precision-dispensing pumps and valves, tubing and integrated tubing assemblies, filter sensors and other micro-fluidic and nano-fluidic components, as well as advanced column hardware and accessories for the high performance liquid chromatography market. The products produced by Sapphire Engineering and Upchurch Scientific primarily serve the pharmaceutical, drug discovery, chemical, biochemical processing, genomics/proteomics research, environmental labs, food/agriculture, medical lab, personal care, and plastics/polymer/rubber production markets. ERC manufactures gas liquid separations and detection solutions for the life science, analytical instrumentation and clinical chemistry markets. ERC’s products consist of in-line membrane vacuum degassing solutions, refractive index detectors and ozone generation systems. CiDRA Precision Services’ products consist of microfluidic components serving the life science, health and industrial market. CVI Melles Griot is a global leader in the design and manufacture of precision photonic solutions used in the life sciences, research, semiconductor, security and defense markets. CVI Melles Griot’s innovative products are focused on the generation, control and productive use of light for a variety of key science and industrial applications. Products consist of specialty lasers and light sources, electro-optical components, specialty shutters, opto-mechanical assemblies and components. In addition, CVI Melles Griot produces critical components for life science research, electronics manufacturing, military and other industrial applications including lenses, mirrors, filters and polarizers. These components are utilized in a number of important applications such as spectroscopy, cytometry (cell counting), guidance systems for target designation, remote sensing, menology and optical lithography. Semrock is a provider of optical filters for biotech and analytical instrumentation in the life sciences markets. Semrock’s optical filters are produced using state-of-the-art manufacturing processes which enable it to offer its customers significant improvements in instrument performance and reliability. AT Films specializes in optical components and coatings for applications in the fields of scientific research, defense, aerospace, telecommunications and electronics manufacturing. AT Films’ core competence is the design and manufacture of filters,
4
splitters, reflectors and mirrors with the precise physical properties required to support their customers’ most challenging and cutting-edge optical applications. The Precision Photonics portion of its business specializes in optical components and coatings for applications in the fields of scientific research, aerospace, telecommunications and electronics manufacturing. Scientific Fluidics & Optics has facilities in Bristol, Connecticut (Eastern Plastics products); Rohnert Park, California (Rheodyne products); Middleboro, Massachusetts (Sapphire Engineering products); Oak Harbor, Washington (Upchurch Scientific products); Kawaguchi, Japan (ERC products); Wallingford, Connecticut (CiDRA Precision Services products); Albuquerque, New Mexico; Carlsbad, California; Rochester, New York; Leicester, England; and Didam, The Netherlands (CVI Melles Griot products); Rochester, New York (Semrock products); and Boulder, Colorado (AT Films products). Approximately 53% of Scientific Fluidics & Optics’ 2016 sales were to customers outside the U.S.
Sealing Solutions. Sealing Solutions consists of the Company’s Precision Polymer Engineering, FTL Seals Technology, Novotema, and SFC Koenig businesses. Precision Polymer Engineering is a provider of proprietary high performance seals and advanced sealing solutions for a diverse range of global industries and applications, including hazardous duty, analytical instrumentation, semiconductor, process technologies, oil & gas, pharmaceutical, electronics, and food applications. Precision Polymer Engineering is headquartered in Blackburn, England with an additional manufacturing facility in Brenham, Texas. FTL Seals Technology, located in Leeds, England, specializes in the design and application of high integrity rotary seals, specialty bearings, and other custom products for the mining, power generation, and marine markets. Novotema, located in Villongo, Italy, is a leader in the design, manufacture and sale of specialty sealing solutions for use in the building products, gas control, transportation, industrial and water markets. SFC Koenig is a producer of highly engineered expanders and check valves for critical applications across the transportation, hydraulic, aviation and medical markets. SFC Koenig is based in Dietikon, Switzerland, with additional facilities in North Haven, Connecticut; Illerrieden, Germany; and Suzhou, China. Approximately 78% of Sealing Solutions’ 2016 sales were to customers outside the U.S.
Gast. The Gast business is a leading manufacturer of air-moving products, including air motors, low-range and medium-range vacuum pumps, vacuum generators, regenerative blowers and fractional horsepower compressors. Gast products are used in a variety of long-life applications requiring a quiet, clean source of moderate vacuum or pressure. Gast products primarily serve the medical equipment, environmental equipment, computers and electronics, printing machinery, paint mixing machinery, packaging machinery, graphic arts, and industrial manufacturing markets. Based in Benton Harbor, Michigan, Gast also has a logistics and commercial center in Redditch, England. Approximately 25% of Gast’s 2016 sales were to customers outside the U.S.
Micropump. Micropump, headquartered in Vancouver, Washington, is a leader in small, precision-engineered, magnetically and electromagnetically driven rotary gear, piston and centrifugal pumps. Micropump products are used in low-flow abrasive and corrosive applications. Micropump products primarily serve the continuous ink-jet printing, medical equipment, chemical processing, pharmaceutical, refining, laboratory, electronics, textiles, peristaltic metering pumps, analytical process controllers and sample preparation systems markets. Approximately 76% of Micropump’s 2016 sales were to customers outside the U.S.
Material Processing Technologies. Material Processing Technologies consists of the Company’s Quadro, Fitzpatrick, Microfluidics, and Matcon businesses. Quadro is a leading provider of particle control solutions for the pharmaceutical and bio-pharmaceutical markets. Based in Waterloo, Canada, Quadro’s core capabilities include fine milling, emulsification and special handling of liquid and solid particulates for laboratory, pilot phase and production scale processing. Fitzpatrick is a global leader in the design and manufacture of process technologies for the pharmaceutical, food and personal care markets. Fitzpatrick designs and manufactures customized size reduction, roll compaction and drying systems to support their customers’ product development and manufacturing processes. Fitzpatrick is headquartered in Elmhurst, Illinois. Microfluidics is a global leader in the design and manufacture of laboratory and commercial equipment used in the production of micro and nano scale materials for the pharmaceutical and chemical markets. Microfluidics is the exclusive producer of the Microfluidizer family of high shear fluid processors for uniform particle size reduction, robust cell disruption and nanoparticle creation. Microfluidics is based in Waterloo, Canada and has offices in Newton, Massachusetts. Matcon is a global leader in material processing solutions for high value powders used in the manufacture of pharmaceuticals, food, plastics, and fine chemicals. Matcon’s innovative products consist of the original cone valve powder discharge system and filling, mixing and packaging systems, all of which support its customers’ automation and process requirements. These products are critical to its customers’ need to maintain clean, reliable and repeatable formulations of prepackaged foods and pharmaceuticals while helping them achieve lean and agile manufacturing. Matcon is located in Evesham, England. Approximately 63% of Material Processing Technologies’ 2016 sales were to customers outside the U.S.
5
FIRE & SAFETY/DIVERSIFIED PRODUCTS SEGMENT
The Fire & Safety/Diversified Products segment produces firefighting pumps and controls, apparatus valves, monitors, nozzles, rescue tools, lifting bags and other components and systems for the fire and rescue industry, engineered stainless steel banding and clamping devices used in a variety of industrial and commercial applications, and precision equipment for dispensing, metering and mixing colorants and paints used in a variety of retail and commercial businesses around the world.
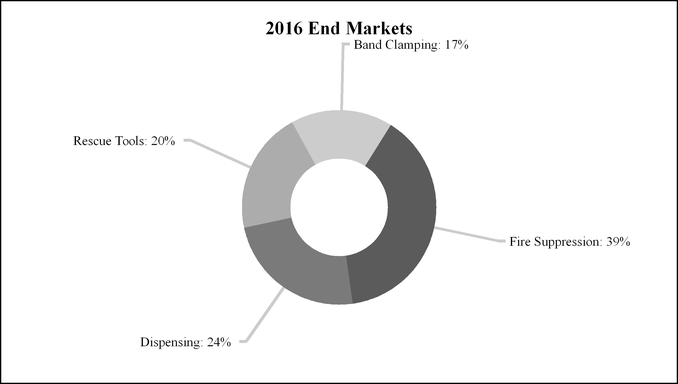
The Fire & Safety/Diversified Products segment accounted for 25%, 21% and 23% of IDEX’s sales in 2016, 2015 and 2014, respectively, with approximately 51% of its 2016 sales to customers outside the U.S. The segment accounted for 25%, 24% and 26% of IDEX’s operating income in 2016, 2015 and 2014, respectively.
Fire & Safety. Fire & Safety consists of the Company’s Class 1, Hale, Godiva, Akron Brass, AWG Fittings, Dinglee, Hurst Jaws of Life, Lukas, and Vetter businesses, which produce truck-mounted and portable fire pumps, stainless steel valves, monitors, apparatus valves, nozzles, foam and compressed air foam systems, pump modules and pump kits, electronic controls and information systems, conventional and networked electrical systems, mechanical components for the fire, rescue and specialty vehicle markets, hydraulic, battery, gas and electric-operated rescue equipment, hydraulic re-railing equipment, hydraulic tools for industrial applications, recycling cutters, pneumatic lifting and sealing bags for vehicle and aircraft rescue, environmental protection and disaster control, and shoring equipment for vehicular or structural collapse. Fire & Safety’s customers are OEMs as well as public and private fire and rescue organizations. Fire & Safety maintains facilities in Ocala, Florida (Class 1 and Hale products); Warwick, England (Godiva products); Wooster and Columbus, Ohio (Akron Brass and Weldon products); Ballendorf, Germany (AWG Fittings products); Shelby, North Carolina (Hurst Jaws of Life products); Tianjin, China (Dinglee products); Erlangen, Germany (Lukas products); and Zulpich, Germany (Vetter products). Approximately 48% of Fire & Safety’s 2016 sales were to customers outside the U.S.
Band-It. Band-It is a leading producer of high-quality stainless steel banding, buckles and clamping systems. The BAND-IT brand is highly recognized worldwide. Band-It products are used for securing exhaust system heat and sound shields, industrial hose fittings, traffic signs and signals, electrical cable shielding, identification and bundling, and in numerous other industrial and commercial applications. Band-It products primarily serve the automotive, transportation equipment, oil & gas, general industrial maintenance, electronics, electrical, communications, aerospace, utility, municipal and subsea marine markets. Band-It is based in Denver, Colorado, with additional operations in Staveley, England. Approximately 38% of Band-It’s 2016 sales were to customers outside the U.S.
6
Dispensing. Dispensing produces precision equipment for dispensing, metering and mixing colorants and paints used in a variety of retail and commercial businesses around the world. Dispensing is a global supplier of precision-designed tinting, mixing, dispensing and measuring equipment for auto refinishing and architectural paints. Dispensing products are used in retail and commercial stores, hardware stores, home centers, department stores, automotive body shops as well as point-of-purchase dispensers. Dispensing maintains facilities in Sassenheim, The Netherlands; Wheeling, Illinois; Unanderra, Australia; and Milan, Italy, as well as IDEX shared manufacturing facilities in India and China. Approximately 65% of Dispensing’s 2016 sales were to customers outside the U.S.
INFORMATION APPLICABLE TO THE COMPANY’S BUSINESS IN GENERAL AND ITS SEGMENTS
Competitors
The Company’s businesses participate in highly competitive markets. IDEX believes that the principal points of competition are product quality, price, design and engineering capabilities, product development, conformity to customer specifications, quality of post-sale support, timeliness of delivery, and effectiveness of our distribution channels.
Principal competitors of the Fluid & Metering Technologies segment are the Pump Solutions Group (Maag, Blackmer and Wilden products) of Dover Corporation (with respect to pumps and small horsepower compressors used in liquified petroleum gas distribution facilities, rotary gear pumps, and air-operated double-diaphragm pumps); Milton Roy LLC (with respect to metering pumps and controls); and Tuthill Corporation (with respect to rotary gear pumps).
Principal competitors of the Health & Science Technologies segment are the Thomas division of Gardner Denver, Inc. (with respect to vacuum pumps and compressors); Thermo Scientific Dionex products (with respect to analytical instrumentation); Parker Hannifin (with respect to sealing devices); Valco Instruments Co., Inc. (with respect to fluid injectors and valves); and Gooch & Housego PLC (with respect to electro-optic and precision photonics solutions used in the life sciences market).
The principal competitors of the Fire & Safety/Diversified Products segment are Waterous Company, a unit of American Cast Iron Pipe Company (with respect to truck-mounted firefighting pumps); Holmatro, Inc. (with respect to rescue tools); Corob S.p.A. (with respect to dispensing and mixing equipment for the paint industry); and Panduit Corporation (with respect to stainless steel bands, buckles and clamping systems).
Customers
The principal customers for our products are discussed immediately above by product category in each segment. None of our customers in 2016 accounted for more than two percent of net sales.
Employees
At December 31, 2016, the Company had 7,158 employees. Approximately 9% of employees were represented by labor unions, with various contracts expiring through June 2020. Management believes that the Company has a positive relationship with its employees. The Company historically has been able to renegotiate its collective bargaining agreements satisfactorily, with its last work stoppage in March 1993.
Suppliers
The Company manufactures many of the parts and components used in its products. Substantially all materials, parts and components purchased by the Company are available from multiple sources.
Inventory and Backlog
The Company regularly and systematically adjusts production schedules and quantities based on the flow of incoming orders. Backlogs typically are limited to one to one and a half months of production. While total inventory levels also may be affected by changes in orders, the Company generally tries to maintain relatively stable inventory levels based on its assessment of the requirements of the various industries served.
Raw Materials
The Company uses a wide variety of raw materials which are generally available from a number of sources. As a result, shortages from any single supplier have not had, and are not likely to have a material impact on operations.
7
Shared Services
The Company has production facilities in Suzhou, China and Vadodara, India that support multiple business units. IDEX also has personnel in China, India, Dubai, Mexico, Latin America and Singapore that provide sales and marketing, product design and engineering, and sourcing support to its business units, as well as personnel in various locations in South America, the Middle East, Korea and Japan to support sales and marketing efforts of IDEX businesses in those regions.
Segment Information
For segment financial information for the years 2016, 2015 and 2014, including financial information about foreign and domestic sales and operations, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Note 11 of the Notes to Consolidated Financial Statements in Part II, Item 8, “Financial Statements and Supplementary Data.”
Executive Officers of the Registrant
Set forth below are the names of the executive officers of the Company, their ages, years of service, the positions held by them, and their business experience during the past five years.
| Name | Age | Years of Service | Position | |||
| Andrew K. Silvernail | 46 | 8 | Chairman of the Board and Chief Executive Officer | |||
| William K. Grogan | 38 | 5 | Senior Vice President and Chief Financial Officer | |||
| Eric D. Ashleman | 49 | 8 | Senior Vice President and Chief Operating Officer | |||
| Denise R. Cade | 54 | 1 | Senior Vice President, General Counsel and Corporate Secretary | |||
| Daniel J. Salliotte | 50 | 12 | Senior Vice President-Corporate Strategy, Mergers & Acquisitions and Treasury | |||
| Michael J. Yates | 51 | 11 | Vice President and Chief Accounting Officer | |||
| Jeffrey D. Bucklew | 46 | 5 | Senior Vice President-Chief Human Resources Officer | |||
| James MacLennan | 53 | 5 | Senior Vice President-Chief Information Officer | |||
Mr. Silvernail has served as Chief Executive Officer since August 2011 and as Chairman of the Board since January 2012. Prior to that, Mr. Silvernail was Vice President-Group Executive Health & Science Technologies, Global Dispensing and Fire & Safety/Diversified Products from January 2011 to August 2011. From February 2010 to December 2010, Mr. Silvernail was Vice President-Group Executive Health & Sciences Technologies and Global Dispensing. Mr. Silvernail joined IDEX in January 2009 as Vice President-Group Executive Health & Science Technologies.
Mr. Grogan has served as Senior Vice President and Chief Financial Officer since January 2017. Prior to that, Mr. Grogan served as Vice President of Finance, Operations from July 2015 through January 2017. From January 2012 through July 2015, Mr. Grogan was Vice President-Finance for the Company’s Health & Science Technologies and Fire & Safety/Diversified Products segments.
Mr. Ashleman has served as Senior Vice President and Chief Operating Officer since July 2015. Prior to that, Mr. Ashleman served as the Vice President-Group Executive of the Company’s Health & Science Technologies and Fire & Safety/Diversified Products segments from January 2014 through July 2015 and President-Group Executive of the Company’s Fire & Safety/Diversified Products segment from 2011 through January 2014. Mr. Ashleman joined IDEX in 2008 as the President of Gast Manufacturing.
Ms. Cade has served as Senior Vice President, General Counsel and Corporate Secretary since joining IDEX in October 2015. Prior to joining IDEX, Ms. Cade was Senior Vice President, General Counsel, Corporate Secretary and Chief Compliance Officer for SunCoke Energy, Inc. from March 2011 to October 2015 and held various roles at PPG Industries before joining SunCoke.
Mr. Salliotte has served as Senior Vice President-Corporate Strategy, Mergers & Acquisitions and Treasury since February 2011. Mr. Salliotte joined IDEX in October 2004 as Vice President-Strategy and Business Development.
Mr. Yates has served as Vice President and Chief Accounting Officer since February 2010, and served as interim Chief Financial Officer from September 2016 to December 2016. Mr. Yates joined IDEX as Vice President-Controller in October 2005.
8
Mr. Bucklew has served as the Senior Vice President-Chief Human Resources Officer since joining IDEX in March 2012. Prior to joining IDEX, Mr. Bucklew served as the Vice President of Human Resources for Accretive Health from March 2009 to March 2012.
Mr. MacLennan has served as the Senior Vice President-Chief Information Officer since joining IDEX in March 2012. Prior to joining IDEX, Mr. MacLennan had a dual role as CIO for Pactiv LLC and Vice President of IT for Reynolds Services Inc.
The Company’s executive officers are elected at a meeting of the Board of Directors immediately following the annual meeting of stockholders, and they serve until the meeting of the Board immediately following the next annual meeting of stockholders, or until their successors are duly elected and qualified or until their death, resignation or removal.
Public Filings
Copies of the Company’s annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports are made available free of charge at www.idexcorp.com as soon as reasonably practicable after being filed electronically with the United States Securities and Exchange Commission (the “SEC”). Our reports are also available free of charge on the SEC’s website, www.sec.gov. Information on the Company’s website is not incorporated into this Form 10-K.
9
Item 1A. Risk Factors.
For an enterprise as diverse and complex as the Company, a wide range of factors present risks to the Company and could materially affect future developments and performance. In addition to the factors affecting specific business operations identified in connection with the description of our operations and the financial results of our operations elsewhere in this report, the most significant of these factors are as follows:
Changes in U.S. or International Economic Conditions Could Adversely Affect the Sales and Profitability of Our Businesses.
In 2016, 50% of the Company’s sales were derived from domestic operations while 50% were derived from international operations. The Company’s largest end markets include life sciences and medical technologies, fire and rescue, oil & gas, paint and coatings, chemical processing, agriculture, water & wastewater treatment and optical filters and components. A slowdown in the U.S. or global economy and, in particular, any of these specific end markets could reduce the Company’s sales and profitability.
Change to Political and Economic Conditions in the U.S. and Foreign Countries in Which We Operate Could Adversely Affect Our Business.
In 2016, approximately 50% of our total sales were to customers outside the U.S. We expect our international operations and export sales to continue to be significant for the foreseeable future. Our sales from international operations and our sales from export are both subject in varying degrees to risks inherent in doing business outside the U.S. These risks include the following:
| • | possibility of unfavorable circumstances arising from host country laws or regulations; |
| • | risks of economic instability; |
| • | currency exchange rate fluctuations and restrictions on currency repatriation; |
| • | potential negative consequences from changes to taxation policies; |
| • | disruption of operations from labor and political disturbances; |
| • | withdrawal from or renegotiation of international trade agreements and other restrictions on the trade between the United States and other countries; |
| • | changes in tariff and trade barriers and import or export licensing requirements; and |
| • | political instability, terrorism, insurrection or war. |
Any of these events could have an adverse impact on our business and operations.
Our Inability to Continue to Develop New Products Could Limit Our Sales Growth.
Our ability to continue to grow organically is tied in large part to our ability to continue to develop new products.
Our Growth Strategy Includes Acquisitions and We May Not be Able to Make Acquisitions of Suitable Candidates or Integrate Acquisitions Successfully.
Our historical growth has included, and our future growth is likely to continue to include, acquisitions. We intend to continue to seek acquisition opportunities both to expand into new markets and to enhance our position in existing markets throughout the world. We may not be able to successfully identify suitable candidates, negotiate appropriate acquisition terms, obtain financing needed to consummate those acquisitions, complete proposed acquisitions or successfully integrate acquired businesses into our existing operations. In addition, any acquisition, once successfully integrated, may not perform as planned, be accretive to earnings, or otherwise prove beneficial to us.
Acquisitions involve numerous risks, including the assumption of undisclosed or unindemnified liabilities, difficulties in the assimilation of the operations, technologies, services and products of the acquired companies and the diversion of management’s attention from other business concerns. In addition, prior acquisitions have resulted in, and future acquisitions could result in, the incurrence of substantial additional indebtedness and other expenses.
The Markets We Serve are Highly Competitive and this Competition Could Reduce our Sales and Operating Margins.
Most of our products are sold in competitive markets. Maintaining and improving our competitive position will require continued investment by us in manufacturing, engineering, quality standards, marketing, customer service and support, and our distribution networks. We may not be successful in maintaining our competitive position. Our competitors may develop
10
products that are superior to our products, or may develop methods of more efficiently and effectively providing products and services or may adapt more quickly than us to new technologies or evolving customer requirements. Pricing pressures may require us to adjust the prices of our products to stay competitive. We may not be able to compete successfully with our existing competitors or with new competitors. Failure to continue competing successfully could reduce our sales, operating margins and overall financial performance.
We are Dependent on the Availability of Raw Materials, Parts and Components Used in Our Products.
While we manufacture certain parts and components used in our products, we require substantial amounts of raw materials and purchase some parts and components from suppliers. The availability and prices for raw materials, parts and components may be subject to curtailment or change due to, among other things, suppliers’ allocations to other purchasers, interruptions in production by suppliers, changes in exchange rates and prevailing price levels. Any change in the supply of, or price for, these raw materials or parts and components could materially affect our business, financial condition, results of operations and cash flow.
Significant Movements in Foreign Currency Exchange Rates May Harm Our Financial Results.
We are exposed to fluctuations in foreign currency exchange rates, particularly with respect to the Euro, Swiss Franc, Canadian Dollar, British Pound, Indian Rupee and Chinese Renminbi. Any significant change in the value of the currencies of the countries in which we do business against the U.S. Dollar could affect our ability to sell products competitively and control our cost structure, which could have a material adverse effect on our results of operations. For additional detail related to this risk, see Part II, Item 7A, “Quantitative and Qualitative Disclosure About Market Risk.”
Fluctuations in Interest Rates Could Adversely Affect Our Results of Operations and Financial Position.
Our profitability may be adversely affected during any periods of unexpected or rapid increases in interest rates. We maintain a revolving credit facility, which bears interest at either an alternate base rate or an adjusted LIBOR rate plus, in each case, an applicable margin based on the Company's senior, unsecured, long-term debt rating. A significant increase in LIBOR would significantly increase our cost of borrowings. For additional detail related to this risk, see Part II, Item 7A, "Quantitative and Qualitative Disclosure About Market Risk."
An Unfavorable Outcome of Any of Our Pending Contingencies or Litigation Could Adversely Affect Us.
We currently are involved in legal and regulatory proceedings. Where it is reasonably possible to do so, we accrue estimates of the probable costs for the resolution of these matters. These estimates are developed in consultation with outside counsel and are based upon an analysis of potential results, assuming a combination of litigation and settlement strategies. It is possible, however, that future operating results for any particular quarter or annual period could be materially affected by changes in our assumptions or the effectiveness of our strategies related to these proceedings. For additional detail related to this risk, see Item 3, “Legal Proceedings.”
Our Intangible Assets, Including Goodwill, are a Significant Portion of Our Total Assets and a Write-off of Our Intangible Assets or Goodwill Would Adversely Impact Our Operating Results and Significantly Reduce Our Net Worth.
Our total assets reflect substantial intangible assets, primarily goodwill and identifiable intangible assets. At December 31, 2016, goodwill and intangible assets totaled $1,632.6 million and $435.5 million, respectively. These assets result from our acquisitions, representing the excess of the purchase price over the fair value of the tangible net assets we have acquired. Annually, or when certain events occur that require a more current valuation, we assess whether there has been an impairment in the value of our goodwill and identifiable intangible assets. If future operating performance at one or more of our reporting units were to fall significantly below forecasted levels, we could be required to reflect, under current applicable accounting rules, a non-cash charge to operating income for an impairment. Any determination requiring the write-off of a significant portion of our goodwill or identifiable intangible assets would adversely impact our results of operations and net worth. See Note 4 in Part II, Item 8, “Financial Statements and Supplementary Data” for further discussion on goodwill and intangible assets.
A Significant or Sustained Decline in Commodity Prices, Including Oil, Could Negatively Impact the Levels of Expenditures by Certain of Our Customers.
Demand for our products depends, in part, on the level of new and planned expenditures by certain of our customers. The level of expenditures by our customers is dependent on, among other factors, general economic conditions, availability of credit, economic conditions within their respective industries and expectations of future market behavior. Volatility in commodity prices, including oil, can negatively affect the level of these activities and can result in postponement of capital
11
spending decisions or the delay or cancellation of existing orders. The ability of our customers to finance capital investment and maintenance may also be affected by the conditions in their industries. Reduced demand for our products could result in the delay or cancellation of existing orders or lead to excess manufacturing capacity, which unfavorably impacts our absorption of fixed manufacturing costs. This reduced demand could have a material adverse effect on our business, financial condition and results of operations.
Our Success Depends on Our Executive Management and Other Key Personnel.
Our future success depends to a significant degree on the skills, experience and efforts of our executive management and other key personnel and their ability to provide the Company with uninterrupted leadership and direction. The loss of the services of any of our executive officers or a failure to provide adequate succession plans for key personnel could have an adverse impact. The availability of highly qualified talent is limited, and the competition for talent is robust. However, we provide long-term equity incentives and certain other benefits for our executive officers which provide incentives for them to make a long-term commitment to our Company. Our future success will also depend on our ability to have adequate succession plans in place and to attract, retain and develop qualified personnel. A failure to efficiently replace executive management members and other key personnel and to attract, retain and develop new qualified personnel could have an adverse effect on our operations and implementation of our strategic plan.
Our Business Operations May Be Adversely Affected by Information Systems Interruptions or Intrusion.
We depend on various information technologies throughout our Company to administer, store and support multiple business activities. If these systems are damaged, cease to function properly, or are subject to cyber-security attacks, such as those involving unauthorized access, malicious software and/or other intrusions, we could experience production downtimes, operational delays, other detrimental impacts on our operations or ability to provide products and services to our customers, the compromising of confidential or otherwise protected information, destruction or corruption of data, security breaches, other manipulation or improper use of our systems or networks, financial losses from remedial actions, loss of business or potential liability, and/or damage to our reputation. While we attempt to mitigate these risks by employing a number of measures, including employee training, technical security controls, and maintenance of backup and protective systems, our systems, networks, products and services remain potentially vulnerable to known or unknown threats, any of which could have a material adverse effect on our business, financial condition or results of operations.
Failure To Comply with the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act or Other Applicable Anti-bribery Laws Could Have an Adverse Effect on Our Business.
The U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act and similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments for the purpose of obtaining or retaining business. Recent years have seen a substantial increase in anti-bribery law enforcement activity with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the SEC, increased enforcement activity by non-U.S. regulators and increases in criminal and civil proceedings brought against companies and individuals. Our policies mandate compliance with all anti-bribery laws. However, we operate in certain countries that are recognized as having governmental and commercial corruption. Our internal control policies and procedures may not always protect us from reckless or criminal acts committed by our employees or third-party intermediaries. Violations of these anti-bribery laws may result in criminal or civil sanctions, which could have a material adverse effect on our business, financial condition and results of operations.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
The Company’s principal plants and offices have an aggregate floor space area of approximately 4.7 million square feet, of which 2.9 million square feet (62%) is located in the U.S. and approximately 1.8 million square feet (38%) is located outside the U.S., primarily in Germany (9%), U.K. (8%), Italy (6%), China (4%), India (3%), Canada (2%) and The Netherlands (2%). Management considers these facilities suitable and adequate for the Company’s operations. Management believes the Company can meet demand increases over the near term with its existing facilities, especially given its operational improvement initiatives that usually increase capacity. The Company’s executive office occupies 36,588 square feet of leased space in Lake Forest, Illinois and 4,420 square feet of leased space in Chicago, Illinois.
12
Approximately 3.3 million square feet (70%) of the principal plant and office floor area is owned by the Company, and the balance is held under lease. Approximately 1.7 million square feet (36%) of the principal plant and office floor area is held by business units in the Fluid & Metering Technologies segment; 1.4 million square feet (30%) is held by business units in the Health & Science Technologies segment; and 1.3 million square feet (28%) is held by business units in the Fire & Safety/Diversified Products segment. The remaining 0.3 million square feet include the executive office as well as shared services locations.
Item 3. Legal Proceedings.
The Company and six of its subsidiaries are presently named as defendants in a number of lawsuits claiming various asbestos-related personal injuries and seeking money damages, allegedly as a result of exposure to products manufactured with components that contained asbestos. These components were acquired from third party suppliers, and were not manufactured by the Company or any of the defendant subsidiaries. To date, the majority of the Company’s settlements and legal costs, except for costs of coordination, administration, insurance investigation and a portion of defense costs, have been covered in full by insurance subject to applicable deductibles. However, the Company cannot predict whether and to what extent insurance will be available to continue to cover its settlements and legal costs, or how insurers may respond to claims that are tendered to them. Claims have been filed in jurisdictions throughout the United States. Most of the claims resolved to date have been dismissed without payment. The balance have been settled for various insignificant amounts. Only one case has been tried, resulting in a verdict for the affected business unit. No provision has been made in the financial statements of the Company for these asbestos-related claims, other than for insurance deductibles in the ordinary course, and the Company does not currently believe these claims will have a material adverse effect on the Company’s business, financial position, results of operations or cash flows.
The Company is also party to various other legal proceedings arising in the ordinary course of business, none of which is expected to have a material adverse effect on its financial condition, results of operations or cash flows.
Item 4. Mine Safety Disclosures.
Not applicable.
13
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
The principal market for the Company’s common stock is the New York Stock Exchange, but the common stock is also listed on the Chicago Stock Exchange. As of February 14, 2017, there were approximately 7,030 stockholders of record of our common stock and there were 76,248,604 shares outstanding.
The high and low sales prices of the common stock per share and the dividends paid per share during the last two years are as follows:
| 2016 | 2015 | ||||||||||||||||||||||
| High | Low | Dividends | High | Low | Dividends | ||||||||||||||||||
| First Quarter | $ | 84.05 | $ | 67.20 | $ | 0.32 | $ | 78.85 | $ | 69.44 | $ | 0.28 | |||||||||||
| Second Quarter | 87.18 | 77.93 | 0.34 | 80.31 | 73.80 | 0.32 | |||||||||||||||||
| Third Quarter | 95.33 | 79.91 | 0.34 | 79.61 | 66.88 | 0.32 | |||||||||||||||||
| Fourth Quarter | 95.76 | 82.05 | 0.34 | 79.59 | 69.40 | 0.32 | |||||||||||||||||
Our payment of dividends in the future will be determined by our Board of Directors and will depend on business conditions, our earnings and other factors.
For information pertaining to securities authorized for issuance under equity compensation plans and the related weighted average exercise price, see Part III, Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
The Company’s purchases of common stock during the quarter ended December 31, 2016 are as follows:
| Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs(1) | Maximum Dollar Value that May Yet be Purchased Under the Plans or Programs(1) | |||||||||
| October 1, 2016 to October 31, 2016 | — | $ | — | — | $ | 580,010,084 | |||||||
| November 1, 2016 to November 30, 2016 | — | — | — | 580,010,084 | |||||||||
| December 1, 2016 to December 31, 2016 | — | — | — | 580,010,084 | |||||||||
| Total | — | $ | — | — | $ | 580,010,084 | |||||||
| (1) | On December 1, 2015, the Company’s Board of Directors approved an increase of $300.0 million in the authorized level of repurchases of common stock. This followed the prior Board of Directors approved repurchase authorization of $400.0 million that was announced by the Company on November 6, 2014. These authorizations have no expiration date. |
14
Performance Graph. The following table compares total stockholder returns over the last five years to the Standard & Poor’s (the “S&P”) 500 Index, the S&P Midcap Industrials Sector Index and the Russell 2000 Index assuming the value of the investment in our common stock and each index was $100 on December 31, 2011. Total return values for our common stock, the S&P 500 Index, S&P Midcap Industrials Sector Index and the Russell 2000 Index were calculated on cumulative total return values assuming reinvestment of dividends. The stockholder return shown on the graph below is not necessarily indicative of future performance.
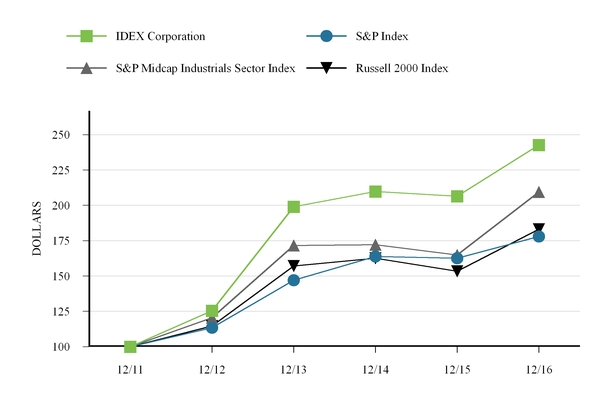
| 12/11 | 12/12 | 12/13 | 12/14 | 12/15 | 12/16 | |||||||||||||
| IDEX Corporation | $ | 100.00 | $ | 125.38 | $ | 199.00 | $ | 209.75 | $ | 206.44 | $ | 242.68 | ||||||
| S&P 500 Index | $ | 100.00 | $ | 113.41 | $ | 146.98 | $ | 163.72 | $ | 162.53 | $ | 178.02 | ||||||
| S&P Midcap 400 Industrials Sector Index | $ | 100.00 | $ | 120.51 | $ | 171.67 | $ | 172.18 | $ | 164.81 | $ | 209.44 | ||||||
| Russell 2000 Index | $ | 100.00 | $ | 114.63 | $ | 157.05 | $ | 162.60 | $ | 153.31 | $ | 183.17 | ||||||
15
Item 6. Selected Financial Data.(1)
| (Dollars in thousands, except per share data) | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||
| RESULTS OF OPERATIONS | |||||||||||||||||||
| Net sales | $ | 2,113,043 | $ | 2,020,668 | $ | 2,147,767 | $ | 2,024,130 | $ | 1,954,258 | |||||||||
| Gross profit | 930,767 | 904,315 | 949,315 | 873,364 | 803,700 | ||||||||||||||
| Selling, general and administrative expenses | 498,994 | 479,408 | 504,419 | 477,851 | 444,490 | ||||||||||||||
| Loss (gain) on sale of businesses - net | 22,298 | (18,070 | ) | — | — | — | |||||||||||||
| Restructuring expenses | 3,674 | 11,239 | 13,672 | — | 32,473 | ||||||||||||||
| Asset impairments | — | — | — | — | 198,519 | ||||||||||||||
| Operating income | 405,801 | 431,738 | 431,224 | 395,513 | 128,218 | ||||||||||||||
| Other (income) expense - net | (8,327 | ) | (2,243 | ) | (3,111 | ) | 178 | (236 | ) | ||||||||||
| Interest expense | 45,616 | 41,636 | 41,895 | 42,206 | 42,250 | ||||||||||||||
| Provision for income taxes | 97,403 | 109,538 | 113,054 | 97,914 | 48,574 | ||||||||||||||
| Net income | 271,109 | 282,807 | 279,386 | 255,215 | 37,630 | ||||||||||||||
Earnings per share (2) | |||||||||||||||||||
| — basic | $ | 3.57 | $ | 3.65 | $ | 3.48 | $ | 3.11 | $ | 0.45 | |||||||||
| — diluted | $ | 3.53 | $ | 3.62 | $ | 3.45 | $ | 3.09 | $ | 0.45 | |||||||||
| Weighted average shares outstanding | |||||||||||||||||||
| — basic | 75,803 | 77,126 | 79,715 | 81,517 | 82,689 | ||||||||||||||
| — diluted | 76,758 | 77,972 | 80,728 | 82,489 | 83,641 | ||||||||||||||
| Year-end shares outstanding | 76,441 | 76,535 | 78,766 | 81,196 | 82,727 | ||||||||||||||
| Cash dividends per share | $ | 1.36 | $ | 1.28 | $ | 1.12 | $ | 0.89 | $ | 0.80 | |||||||||
| FINANCIAL POSITION | |||||||||||||||||||
| Current assets | $ | 822,721 | $ | 862,684 | $ | 1,075,791 | $ | 990,953 | $ | 881,865 | |||||||||
| Current liabilities | 309,158 | 309,597 | 411,968 | 304,609 | 291,427 | ||||||||||||||
| Current ratio | 2.7 | 2.8 | 2.6 | 3.3 | 3.0 | ||||||||||||||
Operating working capital (3) | 396,739 | 370,213 | 366,209 | 350,881 | 373,704 | ||||||||||||||
Total assets (4) | $ | 3,154,944 | $ | 2,805,443 | $ | 2,903,463 | $ | 2,881,118 | $ | 2,777,821 | |||||||||
Total borrowings (4) | 1,015,281 | 840,794 | 859,345 | 767,417 | 779,007 | ||||||||||||||
| Shareholders’ equity | 1,543,894 | 1,443,291 | 1,486,451 | 1,572,989 | 1,464,998 | ||||||||||||||
| PERFORMANCE MEASURES AND OTHER DATA | |||||||||||||||||||
| Percent of net sales: | |||||||||||||||||||
| Gross profit | 44.0 | % | 44.8 | % | 44.2 | % | 43.1 | % | 41.1 | % | |||||||||
| Selling, general and administrative expenses | 23.6 | % | 23.7 | % | 23.5 | % | 23.6 | % | 22.7 | % | |||||||||
| Operating income | 19.2 | % | 21.4 | % | 20.1 | % | 19.5 | % | 6.6 | % | |||||||||
| Income before income taxes | 17.4 | % | 19.4 | % | 18.3 | % | 17.4 | % | 4.4 | % | |||||||||
| Net income | 12.8 | % | 14.0 | % | 13.0 | % | 12.6 | % | 1.9 | % | |||||||||
| Capital expenditures | $ | 38,242 | $ | 43,776 | $ | 47,997 | $ | 31,536 | $ | 35,520 | |||||||||
| Depreciation and amortization | 86,892 | 78,120 | 76,907 | 79,334 | 78,312 | ||||||||||||||
Return on average assets (5) | 9.1 | % | 9.9 | % | 9.7 | % | 9.0 | % | 1.3 | % | |||||||||
Borrowings as a percent of capitalization (5) | 39.7 | % | 36.8 | % | 36.6 | % | 32.8 | % | 34.7 | % | |||||||||
Return on average shareholders’ equity (5) | 18.2 | % | 19.3 | % | 18.3 | % | 16.8 | % | 2.5 | % | |||||||||
| Employees at year end | 7,158 | 6,801 | 6,712 | 6,787 | 6,717 | ||||||||||||||
| Record holders at year end | 7,030 | 6,760 | 6,500 | 6,500 | 6,700 | ||||||||||||||
NON-GAAP MEASURES (6) | |||||||||||||||||||
| EBITDA | $ | 501,020 | $ | 512,101 | $ | 511,242 | $ | 474,669 | $ | 206,766 | |||||||||
| EBITDA margin | 23.7 | % | 25.3 | % | 23.8 | % | 23.5 | % | 10.6 | % | |||||||||
| Adjusted EBITDA | $ | 530,546 | $ | 505,270 | $ | 524,914 | $ | 474,669 | $ | 437,758 | |||||||||
Adjusted EBITDA margin | 25.1 | % | 25.0 | % | 24.4 | % | 23.5 | % | 22.4 | % | |||||||||
| Adjusted operating income | $ | 435,327 | $ | 424,907 | $ | 444,896 | $ | 395,513 | $ | 359,210 | |||||||||
| Adjusted operating margin | 20.6 | % | 21.0 | % | 20.7 | % | 19.5 | % | 18.4 | % | |||||||||
Adjusted net income | $ | 288,373 | $ | 277,229 | $ | 288,823 | $ | 255,215 | $ | 224,067 | |||||||||
Adjusted earnings per share | $ | 3.75 | $ | 3.55 | $ | 3.57 | $ | 3.09 | $ | 2.68 | |||||||||
| (1) | For additional detail, see Notes to Consolidated Financial Statements in Part II, Item 8, “Financial Statements and Supplementary Data.” |
| (2) | Calculated by applying the two-class method of allocating earnings to common stock and participating securities as required by Accounting Standards Codification (“ASC”) 260, Earnings Per Share. |
| (3) | Operating working capital is defined as inventory plus accounts receivable minus accounts payable. |
| (4) | In the fourth quarter of fiscal year 2015, the Company adopted Accounting Standards Update 2015-03 regarding simplifying the presentation of debt issuance costs. The update was applied retrospectively to all periods presented in accordance with the provisions of the update. Refer to Note 1 for additional information related to ASU 2015-03 in the Notes to Consolidated Financial Statements in Part II, Item 8, “Financial Statements and Supplementary Data.” |
| (5) | Return on average assets is calculated as: Net income / (Current year Total assets + Prior year Total assets) / 2; Borrowings as a percent of capitalization is calculated as: (Long-term borrowings + Short-term borrowings) / (Long-term borrowings + Short-term borrowings + Total shareholders’ equity); Return on average shareholders’ equity is calculated as Net Income / (Current year Total shareholders’ equity + Prior year Total shareholders’ equity) / 2 |
| (6) | Set forth below are reconciliations of Adjusted operating income, Adjusted net income, Adjusted EPS, EBITDA and Adjusted EBITDA to the comparable measures of net income and operating income, as determined in accordance with generally accepted accounting principles in the U.S. (“U.S. GAAP”). We have reconciled Adjusted operating income to Operating income; Adjusted net income to Net income; Adjusted EPS to EPS; consolidated EBITDA, segment EBITDA, adjusted EBITDA, and adjusted segment EBITDA to net income. The reconciliation of segment EBITDA to net income was performed on a consolidated basis due to the fact that we do not allocate consolidated interest expense or the consolidated provision for income taxes to our segments. |
Management uses Adjusted operating income, Adjusted net income, and Adjusted EPS as metrics by which to measure performance of the Company since they exclude items that are not reflective of ongoing operations, such as gains/losses on the sale of businesses, restructuring expenses, and pension settlements. Management also supplements its U.S. GAAP financial statements with adjusted information to provide investors with greater insight, transparency, and a more comprehensive understanding of the information used by management in its financial and operational decision making.
EBITDA means earnings before interest, income taxes, depreciation and amortization. Given the acquisitive nature of the Company which results in a higher level of amortization expense from recently acquired businesses, management uses EBITDA as an internal operating metric to provide another representation of the businesses’ performance across our three segments and for enterprise valuation purposes. EBITDA is also used to calculate certain financial covenants, as discussed in Note 5 of the Notes to Consolidated Financial Statements in Part II, Item 8, “Financial Statements and Supplementary Data.” In addition, EBITDA has been adjusted for items that are not reflective of ongoing operations, such as gains/losses on the sale of businesses, restructuring expenses, and pension settlements to arrive at Adjusted EBITDA. Management believes that Adjusted EBITDA is useful as a performance indicator of ongoing operations. We believe that Adjusted EBITDA is also useful to some investors as an indicator of the strength and performance of the Company and its segments’ ongoing business operations and a way to evaluate and compare operating performance and value companies within our industry. The definition of Adjusted EBITDA used here may differ from that used by other companies.
The non-GAAP financial measures disclosed by the Company should not be considered a substitute for, or superior to, financial measures prepared in accordance with U.S. GAAP. The financial results prepared in accordance with U.S. GAAP and the reconciliations from these results should be carefully evaluated.
| 1. Reconciliations of Consolidated EBITDA | ||||||||||||||||||||
| For the Years Ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Net income | $ | 271,109 | $ | 282,807 | $ | 279,386 | $ | 255,215 | $ | 37,630 | ||||||||||
| + Provision for income taxes | 97,403 | 109,538 | 113,054 | 97,914 | 48,574 | |||||||||||||||
| + Interest expense | 45,616 | 41,636 | 41,895 | 42,206 | 42,250 | |||||||||||||||
| + Depreciation and amortization | 86,892 | 78,120 | 76,907 | 79,334 | 78,312 | |||||||||||||||
| EBITDA | 501,020 | 512,101 | 511,242 | 474,669 | 206,766 | |||||||||||||||
| + Restructuring expenses | 3,674 | 11,239 | 13,672 | — | 32,473 | |||||||||||||||
| + Loss (gain) on sale of businesses - net | 22,298 | (18,070 | ) | — | — | — | ||||||||||||||
| + Pension settlement | 3,554 | — | — | — | — | |||||||||||||||
| + Asset impairments | — | — | — | — | 198,519 | |||||||||||||||
| Adjusted EBITDA | $ | 530,546 | $ | 505,270 | $ | 524,914 | $ | 474,669 | $ | 437,758 | ||||||||||
| Net sales | $ | 2,113,043 | $ | 2,020,668 | $ | 2,147,767 | $ | 2,024,130 | $ | 1,954,258 | ||||||||||
| EBITDA margin | 23.7 | % | 25.3 | % | 23.8 | % | 23.5 | % | 10.6 | % | ||||||||||
| Adjusted EBITDA margin | 25.1 | % | 25.0 | % | 24.4 | % | 23.5 | % | 22.4 | % | ||||||||||
| 2. Reconciliations of Segment EBITDA | ||||||||||||||||||||||||||||||||||||
| For the Years Ended December 31, | ||||||||||||||||||||||||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||||||||||||||||||
| FMT | HST | FSDP | FMT | HST | FSDP | FMT | HST | FSDP | ||||||||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||||||||||
| EBITDA | $ | 242,892 | $ | 200,980 | $ | 135,400 | $ | 233,008 | $ | 200,953 | $ | 123,249 | $ | 243,899 | $ | 196,019 | $ | 138,067 | ||||||||||||||||||
| + Restructuring expenses | 932 | 1,117 | 1,425 | 7,090 | 3,408 | 576 | 6,413 | 4,912 | 1,034 | |||||||||||||||||||||||||||
| + Pension settlement | 2,032 | — | 540 | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Adjusted EBITDA | $ | 245,856 | $ | 202,097 | $ | 137,365 | $ | 240,098 | $ | 204,361 | $ | 123,825 | $ | 250,312 | $ | 200,931 | $ | 139,101 | ||||||||||||||||||
| Net sales | $ | 849,101 | $ | 744,809 | $ | 520,009 | $ | 860,792 | $ | 738,996 | $ | 423,915 | $ | 899,588 | $ | 752,021 | $ | 502,749 | ||||||||||||||||||
| EBITDA margin | 28.6 | % | 27.0 | % | 26.0 | % | 27.1 | % | 27.2 | % | 29.1 | % | 27.1 | % | 26.1 | % | 27.5 | % | ||||||||||||||||||
| Adjusted EBITDA margin | 29.0 | % | 27.1 | % | 26.4 | % | 27.9 | % | 27.7 | % | 29.2 | % | 27.8 | % | 26.7 | % | 27.7 | % | ||||||||||||||||||
| 3. Reconciliations of Consolidated Reported-to-Adjusted Operating Income and Margin | ||||||||||||||||||||
| For the Years Ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Operating income | $ | 405,801 | $ | 431,738 | $ | 431,224 | $ | 395,513 | $ | 128,218 | ||||||||||
| + Restructuring expenses | 3,674 | 11,239 | 13,672 | — | 32,473 | |||||||||||||||
| + Loss (gain) on sale of businesses - net | 22,298 | (18,070 | ) | — | — | — | ||||||||||||||
| + Asset impairments | — | — | — | — | 198,519 | |||||||||||||||
| + Pension settlement | 3,554 | — | — | — | — | |||||||||||||||
| Adjusted operating income | $ | 435,327 | $ | 424,907 | $ | 444,896 | $ | 395,513 | $ | 359,210 | ||||||||||
| Net sales | $ | 2,113,043 | $ | 2,020,668 | $ | 2,147,767 | $ | 2,024,130 | $ | 1,954,258 | ||||||||||
| Operating margin | 19.2 | % | 21.4 | % | 20.1 | % | 19.5 | % | 6.6 | % | ||||||||||
| Adjusted operating margin | 20.6 | % | 21.0 | % | 20.7 | % | 19.5 | % | 18.4 | % | ||||||||||
| 4. Reconciliations of Segment Reported-to-Adjusted Operating Income and Margin | ||||||||||||||||||||||||||||||||||||
| For the Years Ended December 31, | ||||||||||||||||||||||||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||||||||||||||||||
| FMT | HST | FSDP | FMT | HST | FSDP | FMT | HST | FSDP | ||||||||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||||||||||
| Operating income | $ | 214,242 | $ | 153,722 | $ | 121,888 | $ | 204,506 | $ | 157,948 | $ | 115,745 | $ | 216,886 | $ | 152,999 | $ | 130,494 | ||||||||||||||||||
| + Restructuring expenses | 932 | 1,117 | 1,425 | 7,090 | 3,408 | 576 | 6,413 | 4,912 | 1,034 | |||||||||||||||||||||||||||
| + Pension settlement | 2,032 | — | 540 | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Adjusted operating income | $ | 217,206 | $ | 154,839 | $ | 123,853 | $ | 211,596 | $ | 161,356 | $ | 116,321 | $ | 223,299 | $ | 157,911 | $ | 131,528 | ||||||||||||||||||
| Net sales | $ | 849,101 | $ | 744,809 | $ | 520,009 | $ | 860,792 | $ | 738,996 | $ | 423,915 | $ | 899,588 | $ | 752,021 | $ | 502,749 | ||||||||||||||||||
| Operating margin | 25.2 | % | 20.6 | % | 23.4 | % | 23.8 | % | 21.4 | % | 27.3 | % | 24.1 | % | 20.3 | % | 26.0 | % | ||||||||||||||||||
| Adjusted operating margin | 25.6 | % | 20.8 | % | 23.8 | % | 24.6 | % | 21.8 | % | 27.4 | % | 24.8 | % | 21.0 | % | 26.2 | % | ||||||||||||||||||
| 5. Reconciliations of Reported-to-Adjusted Net Income and EPS | ||||||||||||||||||||
| For the Years Ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Net income | $ | 271,109 | $ | 282,807 | $ | 279,386 | $ | 255,215 | $ | 37,630 | ||||||||||
| + Restructuring expenses | 3,674 | 11,239 | 13,672 | — | 32,473 | |||||||||||||||
| + Tax impact on restructuring expenses | (1,299 | ) | (3,586 | ) | (4,235 | ) | — | (9,547 | ) | |||||||||||
| + Loss (gain) on sale of businesses | 22,298 | (18,070 | ) | — | — | — | ||||||||||||||
| + Tax impact on loss (gain) on sale of businesses | (9,706 | ) | 4,839 | — | — | — | ||||||||||||||
| + Asset impairments | — | — | — | — | 198,519 | |||||||||||||||
| +Tax impact on asset impairments | — | — | — | — | (35,008 | ) | ||||||||||||||
| + Pension settlement | 3,554 | — | — | — | — | |||||||||||||||
| + Tax impact on pension settlement | (1,257 | ) | — | — | — | — | ||||||||||||||
| Adjusted net income | $ | 288,373 | $ | 277,229 | $ | 288,823 | $ | 255,215 | $ | 224,067 | ||||||||||
| EPS | $ | 3.53 | $ | 3.62 | $ | 3.45 | $ | 3.09 | $ | 0.45 | ||||||||||
| + Restructuring expenses | 0.05 | 0.14 | 0.17 | — | 0.39 | |||||||||||||||
| + Tax impact on restructuring expenses | (0.02 | ) | (0.04 | ) | (0.05 | ) | — | (0.11 | ) | |||||||||||
| + Loss (gain) on sale of businesses | 0.29 | (0.23 | ) | — | — | — | ||||||||||||||
| + Tax impact on loss (gain) on sale of businesses | (0.13 | ) | 0.06 | — | — | — | ||||||||||||||
| + Asset impairments | — | — | — | — | 2.37 | |||||||||||||||
| +Tax impact on asset impairments | — | — | — | (0.42 | ) | |||||||||||||||
| + Pension settlement | 0.05 | — | — | — | — | |||||||||||||||
| + Tax impact on pension settlement | (0.02 | ) | — | — | — | — | ||||||||||||||
| Adjusted EPS | $ | 3.75 | $ | 3.55 | $ | 3.57 | $ | 3.09 | $ | 2.68 | ||||||||||
| Diluted weighted average shares | 76,758 | 77,972 | 80,728 | 82,489 | 83,641 | |||||||||||||||
| 6. Reconciliations of EBITDA to Net Income (dollars in thousands) | ||||||||||||||||||||
| For the Year Ended December 31, 2016 | ||||||||||||||||||||
| FMT | HST | FSDP | Corporate | IDEX | ||||||||||||||||
| Operating income (loss) | $ | 214,242 | $ | 153,722 | $ | 121,888 | $ | (84,051 | ) | $ | 405,801 | |||||||||
| - Other (income) expense - net | (192 | ) | (1,960 | ) | (1,556 | ) | (4,619 | ) | (8,327 | ) | ||||||||||
| + Depreciation and amortization | 28,458 | 45,298 | 11,956 | 1,180 | 86,892 | |||||||||||||||
| EBITDA | 242,892 | 200,980 | 135,400 | (78,252 | ) | 501,020 | ||||||||||||||
| - Interest expense | 45,616 | |||||||||||||||||||
| - Provision for income taxes | 97,403 | |||||||||||||||||||
| - Depreciation and amortization | 86,892 | |||||||||||||||||||
| Net income | $ | 271,109 | ||||||||||||||||||
| Net sales (eliminations) | $ | 849,101 | $ | 744,809 | $ | 520,009 | $ | (876 | ) | $ | 2,113,043 | |||||||||
| Operating margin | 25.2 | % | 20.6 | % | 23.4 | % | n/m | 19.2 | % | |||||||||||
| EBITDA margin | 28.6 | % | 27.0 | % | 26.0 | % | n/m | 23.7 | % | |||||||||||
| 7. Reconciliations of EBITDA to Net Income (dollars in thousands) | ||||||||||||||||||||
| For the Year Ended December 31, 2015 | ||||||||||||||||||||
| FMT | HST | FSDP | Corporate | IDEX | ||||||||||||||||
| Operating income (loss) | $ | 204,506 | $ | 157,948 | $ | 115,745 | $ | (46,461 | ) | $ | 431,738 | |||||||||
| - Other (income) expense - net | (840 | ) | (178 | ) | (1,453 | ) | 228 | (2,243 | ) | |||||||||||
| + Depreciation and amortization | 27,662 | 42,827 | 6,051 | 1,580 | 78,120 | |||||||||||||||
| EBITDA | 233,008 | 200,953 | 123,249 | (45,109 | ) | 512,101 | ||||||||||||||
| - Interest expense | 41,636 | |||||||||||||||||||
| - Provision for income taxes | 109,538 | |||||||||||||||||||
| - Depreciation and amortization | 78,120 | |||||||||||||||||||
| Net income | $ | 282,807 | ||||||||||||||||||
| Net sales (eliminations) | $ | 860,792 | $ | 738,996 | $ | 423,915 | $ | (3,035 | ) | $ | 2,020,668 | |||||||||
| Operating margin | 23.8 | % | 21.4 | % | 27.3 | % | n/m | 21.4 | % | |||||||||||
| EBITDA margin | 27.1 | % | 27.2 | % | 29.1 | % | n/m | 25.3 | % | |||||||||||
| 8. Reconciliations of EBITDA to Net Income (dollars in thousands) | ||||||||||||||||||||
| For the Year Ended December 31, 2014 | ||||||||||||||||||||
| FMT | HST | FSDP | Corporate | IDEX | ||||||||||||||||
| Operating income (loss) | $ | 216,886 | $ | 152,999 | $ | 130,494 | $ | (69,155 | ) | $ | 431,224 | |||||||||
| - Other (income) expense - net | (560 | ) | (542 | ) | (990 | ) | (1,019 | ) | (3,111 | ) | ||||||||||
| + Depreciation and amortization | 26,453 | 42,478 | 6,583 | 1,393 | 76,907 | |||||||||||||||
| EBITDA | 243,899 | 196,019 | 138,067 | (66,743 | ) | 511,242 | ||||||||||||||
| - Interest expense | 41,895 | |||||||||||||||||||
| - Provision for income taxes | 113,054 | |||||||||||||||||||
| - Depreciation and amortization | 76,907 | |||||||||||||||||||
| Net income | $ | 279,386 | ||||||||||||||||||
| Net sales (eliminations) | $ | 899,588 | $ | 752,021 | $ | 502,749 | $ | (6,591 | ) | $ | 2,147,767 | |||||||||
| Operating margin | 24.1 | % | 20.3 | % | 26.0 | % | n/m | 20.1 | % | |||||||||||
| EBITDA margin | 27.1 | % | 26.1 | % | 27.5 | % | n/m | 23.8 | % | |||||||||||
| 9. Reconciliation of the Change in Net Sales to Net Organic Sales | ||||||||||||||||||||||||||||||||||||
| For the Years Ended December 31, | ||||||||||||||||||||||||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||||||||||||||||||
| FMT | HST | FSDP | IDEX | FMT | HST | FSDP | IDEX | FMT | HST | FSDP | IDEX | |||||||||||||||||||||||||
| Change in net sales | (1 | )% | 1 | % | 23 | % | 5 | % | (4 | )% | (2 | )% | (16 | )% | (6 | )% | 3 | % | 5 | % | 13 | % | 6 | % | ||||||||||||
| - Net impact from acquisitions/divestitures | 1 | % | 3 | % | 27 | % | 7 | % | 2 | % | 2 | % | — | % | 2 | % | 1 | % | — | % | — | % | 1 | % | ||||||||||||
| - Impact from FX | (1 | )% | (1 | )% | (1 | )% | (1 | )% | (4 | )% | (3 | )% | (6 | )% | (4 | )% | — | % | 1 | % | — | % | — | % | ||||||||||||
| Change in organic net sales | (1 | )% | (1 | )% | (3 | )% | (1 | )% | (2 | )% | (1 | )% | (10 | )% | (4 | )% | 2 | % | 4 | % | 13 | % | 5 | % | ||||||||||||
16
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
2016 Overview and Outlook
IDEX is an applied solutions company specializing in fluid and metering technologies, health and science technologies, and fire, safety and other diversified products built to customers’ specifications. IDEX’s products are sold in niche markets to a wide range of industries throughout the world. Accordingly, IDEX’s businesses are affected by levels of industrial activity and economic conditions in the U.S. and in other countries where it does business and by the relationship of the U.S. dollar to other currencies. Levels of capacity utilization and capital spending in certain industries and overall industrial activity are important factors that influence the demand for IDEX’s products.
The Company has three reportable business segments: Fluid & Metering Technologies, Health & Science Technologies and Fire & Safety/Diversified Products. Within our three reportable segments, the Company maintains thirteen platforms, where we focus on organic growth and strategic acquisitions. Each of our thirteen platforms is also a reporting unit, where we annually test for goodwill impairment.
The Fluid & Metering Technologies segment contains the Energy (comprised of Corken, Faure Herman, Liquid Controls, SAMPI, and Toptech), Valves (comprised of Alfa Valvole, Richter, and Aegis), Water (comprised of Pulsafeeder, Knight, ADS, Trebor, and iPEK), Pumps (comprised of Viking and Warren Rupp), and Agriculture (comprised of Banjo) platforms. The Health & Science Technologies segment contains the Scientific Fluidics & Optics (comprised of Eastern Plastics, Rheodyne, Sapphire Engineering, Upchurch Scientific, ERC, CiDRA Precision Services, CVI Melles Griot, Semrock, and AT Films), Sealing Solutions (comprised of Precision Polymer Engineering, FTL Seals Technology, Novotema, and SFC Koenig), Gast, Micropump, and Material Processing Technologies (comprised of Quadro, Fitzpatrick, Microfluidics, and Matcon) platforms. The Fire & Safety/Diversified Products segment is comprised of the Fire & Safety (comprised of Class 1, Hale, Akron Brass, AWG Fittings, Godiva, Dinglee, Hurst Jaws of Life, Lukas, and Vetter), Band-It, and Dispensing platforms.
The Fluid & Metering Technologies segment designs, produces and distributes positive displacement pumps, flow meters, valves, injectors, and other fluid-handling pump modules and systems and provides flow monitoring and other services for the food, chemical, general industrial, water & wastewater, agriculture and energy industries. The Health & Science Technologies segment designs, produces and distributes a wide range of precision fluidics, rotary lobe pumps, centrifugal and positive displacement pumps, roll compaction and drying systems used in beverage, food processing, pharmaceutical and cosmetics, pneumatic components and sealing solutions, including very high precision, low-flow rate pumping solutions required in analytical instrumentation, clinical diagnostics and drug discovery, high performance molded and extruded, biocompatible medical devices and implantables, air compressors used in medical, dental and industrial applications, optical components and coatings for applications in the fields of scientific research, defense, biotechnology, life sciences, aerospace, telecommunications and electronics manufacturing, laboratory and commercial equipment used in the production of micro and nano scale materials, precision photonic solutions used in life sciences, research and defense markets, and precision gear and peristaltic pump technologies that meet exacting original equipment manufacturer specifications. The Fire & Safety/Diversified Products segment produces firefighting pumps and controls, valves, monitors, nozzles, rescue tools, lifting bags and other components and systems for the fire and rescue industry, engineered stainless steel banding and clamping devices used in a variety of industrial and commercial applications, and precision equipment for dispensing, metering and mixing colorants and paints used in a variety of retail and commercial businesses around the world.
Our 2016 financial results were as follows:
| • | Sales of $2.1 billion increased 5%, reflecting a 1% decrease in organic sales (excluding acquisitions, divestitures and foreign currency translation), a 1% decrease due to foreign currency translation, and a 7% increase due to acquisitions/divestitures. |
| • | Operating income of $405.8 million and operating margin of 19.2% were down 6% and 220 basis points, respectively, from the prior year. |
| • | Net income decreased 4% to $271.1 million. |
| • | Diluted EPS of $3.53 decreased $0.09 or 2% compared to 2015. |
Our 2016 financial results, adjusted for $3.7 million of restructuring costs, a $3.6 million pension settlement charge and a $22.3 million loss on sale of businesses, compared to our 2015 financial results adjusted for $11.2 million of restructuring costs and an $18.1 million gain on sale of a business are as follows (these non-GAAP measures have been reconciled to U.S. GAAP measures in Item 6, “Selected Financial Data”):
| • | Adjusted operating income of $435.3 million and adjusted operating margin of 20.6% were up 2% and down 40 basis points, respectively, from the prior year. |
17
| • | Adjusted net income increased 4% to $288.4 million. |
| • | Adjusted EPS of $3.75 was 6% higher than prior year adjusted EPS of $3.55. |
Overall, we remain cautious due to the uncertainty within the global economy and the global political environment and project 1 to 2 percent organic growth in 2017. We expect to deliver full year 2017 EPS of $3.87 to $3.95.
Results of Operations
The following is a discussion and analysis of our results of operations for each of the three years in the period ended December 31, 2016. For purposes of this Item, reference is made to the Consolidated Statements of Operations in Part II, Item 8, “Financial Statements and Supplementary Data.” Segment operating income excludes unallocated corporate operating expenses. Management’s primary measurements of segment performance are sales, operating income, and operating margin.
In the following discussion, and throughout this report, references to organic sales, a non-GAAP measure, refers to sales from continuing operations calculated according to generally accepted accounting principles in the United States but excludes (1) the impact of foreign currency translation and (2) sales from acquired or divested businesses during the first twelve months of ownership or divestiture. The portion of sales attributable to foreign currency translation is calculated as the difference between (a) the period-to-period change in organic sales and (b) the period-to-period change in organic sales after applying prior period foreign exchange rates to the current year period. Management believes that reporting organic sales provides useful information to investors by helping identify underlying growth trends in our business and facilitating easier comparisons of our revenue performance with prior and future periods and to our peers. The Company excludes the effect of foreign currency translation from organic sales because foreign currency translation is not under management’s control, is subject to volatility and can obscure underlying business trends. The Company excludes the effect of acquisitions and divestitures because the nature, size, and number of acquisitions and divestitures can vary dramatically from period to period and between the Company and its peers and can also obscure underlying business trends and make comparisons of long-term performance difficult.
Performance in 2016 Compared with 2015
| (In thousands) | 2016 | 2015 | Change | ||||||||
| Net sales | $ | 2,113,043 | $ | 2,020,668 | 5 | % | |||||
| Operating income | 405,801 | 431,738 | (6 | )% | |||||||
| Operating margin | 19.2 | % | 21.4 | % | (220 | ) | bps | ||||
Sales in 2016 were $2.1 billion, a 5% increase from last year. This increase reflects a 1% decrease in organic sales, a 1% decrease from foreign currency translation and a 7% increase from acquisitions/divestitures (Acquisitions: SFC Koenig - September 2016; AWG Fittings - July 2016; Akron Brass - March 2016; CiDRA Precision Services - July 2015; Alfa Valvole - June 2015 and Novotema - June 2015. Divestitures: CVI Korea - December 2016; IETG - October 2016; CVI Japan - September 2016; Hydra-Stop - July 2016 and Ismatec - July 2015). Sales to customers outside the U.S. represented approximately 50% of total sales in both 2016 and 2015.
In 2016, Fluid & Metering Technologies contributed 40% of sales and 44% of operating income; Health & Science Technologies contributed 35% of sales and 31% of operating income; and Fire & Safety/Diversified Products contributed 25% of sales and 25% of operating income.
Gross profit of $930.8 million in 2016 increased $26.5 million, or 3%, from 2015, while gross margin decreased 80 basis points to 44.0% in 2016 from 44.8% in 2015. The increase in gross profit is primarily a result of increased sales volume as a result of acquisitions, while the margin decrease is mainly attributable to $14.7 million of fair value inventory step up charges from 2016 acquisitions compared to $3.4 million from 2015 acquisitions.
SG&A expenses increased to $499.0 million in 2016 from $479.4 million in 2015. The $19.6 million increase is mainly attributable to $41.4 million of incremental costs from new acquisitions and $3.6 million of pension settlement charges in 2016, partially offset by current year divestitures and cost savings from prior year restructuring actions. As a percentage of sales, SG&A expenses were 23.6% for 2016 and 23.7% for 2015.
During 2016, the Company recorded a $22.3 million pre-tax loss on the sale of businesses related to the four divestitures during the year (Hydra-Stop - July 2016; CVI Japan - September 2016; IETG - October 2016; and CVI Korea - December 2016), compared to the $18.1 million pre-tax gain on the sale of a business in 2015 (Ismatec - July 2015).
18
During 2016, the Company recorded pre-tax restructuring expenses totaling $3.7 million as part of initiatives that support the implementation of key strategic efforts designed to facilitate long-term, sustainable growth through cost reduction actions primarily consisting of employee reductions and facility rationalization. In 2015, the Company recorded $11.2 million of restructuring expenses mainly attributable to employee severance from headcount reductions across all three segments and corporate.
Operating income of $405.8 million in 2016 decreased from $431.7 million in 2015, primarily resulted from the impact of the four divestitures in 2016 and the associated loss compared to the one divestiture in 2015 and the associated gain as well as the $3.6 million pension settlement charge in 2016 and the incremental fair value inventory step-up charges related to the 2016 acquisitions, partially offset by the reversal of $4.7 million of contingent consideration related to a 2015 acquisition and lower restructuring costs recorded in 2016 compared to 2015. Operating margin of 19.2% in 2016 was down 220 basis points from 21.4% in 2015 primarily due to the loss on the sale of businesses in 2016 compared to a gain on the sale of a business in 2015, and the 2016 pension settlement, partially offset by productivity improvements and lower restructuring costs year over year.
Other (income) expense increased $6.1 million from income of $2.2 million in 2015 to income of $8.3 million in 2016 mainly due to $4.7 million of foreign currency transaction gains on intercompany loans that were established in conjunction with the SFC Koenig acquisition.
Interest expense increased to $45.6 million in 2016 from $41.6 million in 2015. The increase was primarily due to the $200 million series of Senior Notes issued in 2016 and higher borrowings outstanding on the Revolving Facility.
The provision for income taxes is based upon estimated annual tax rates for the year applied to federal, state and foreign income. The provision for income taxes decreased to $97.4 million in 2016 compared to $109.5 million in 2015. The effective tax rate decreased to 26.4% in 2016 compared to 27.9% in 2015, due to tax benefits on the divestitures of CVI Korea and CVI Japan, certain return-to-provision adjustments and the early adoption of ASU 2016-09 and the related tax effects of share based payments now recognized as a reduction to income tax expense. These adjustments were offset by the incurrence of additional foreign withholding taxes, the prior year revaluation of the Italian deferred tax liability related to the reduction in the Italian statutory tax rate and tax expense on the divestiture of the Hydra-Stop product line and the prior year divestiture of the Ismatec product line as well as the mix of global pre-tax income among jurisdictions.
Net income for the year of $271.1 million decreased from the $282.8 million in 2015. Diluted earnings per share in 2016 of $3.53 decreased $0.09 from $3.62 in 2015.
Fluid & Metering Technologies Segment
| (In thousands) | 2016 | 2015 | Change | ||||||||
| Net sales | $ | 849,101 | $ | 860,792 | (1 | )% | |||||
| Operating income | 214,242 | 204,506 | 5 | % | |||||||
| Operating margin | 25.2 | % | 23.8 | % | 140 | bps | |||||
Sales of $849.1 million decreased $11.7 million, or 1%, in 2016 compared with 2015. This decrease reflected a 1% decline in organic sales, a 1% increase from acquisitions (Alfa Valvole - June 2015) and 1% of unfavorable foreign currency translation. In 2016, sales were flat domestically and decreased approximately 3% internationally. Sales to customers outside the U.S. were approximately 44% of total segment sales in both 2016 and 2015.
Sales within our Energy platform increased compared to 2015 primarily due to strength within the aviation market, partially offset by continued weakness in the propane and oil and gas markets as well as challenges in the mobile end market. Sales within our Pumps platform (formerly Industrial) decreased compared to 2015 due to weakness in the North American industrial distribution market. Sales within the Water platform decreased due to the divestitures of Hydra-Stop and IETG and slowing demand in the chemical end market, partially offset by increased municipal spending. Sales within our Agriculture platform increased year over year due to increased demand in the second half of 2016 from both OEMs and distributors in anticipation of the 2017 planting season. Sales within the Valves platform, which was created in the third quarter of 2015, increased as a result of the full year impact of the Alfa Valvole acquisition, offset by a challenging oil & gas market and overall weakness in the European market.
Operating income and operating margin of $214.2 million and 25.2%, respectively, were higher than the $204.5 million and 23.8%, respectively, recorded in 2015, primarily due to the full year impact of the Alfa Valvole acquisition as well as productivity initiatives, partially offset by lower volume.
19
Health & Science Technologies Segment
| (In thousands) | 2016 | 2015 | Change | ||||||||
| Net sales | $ | 744,809 | $ | 738,996 | 1 | % | |||||
| Operating income | 153,722 | 157,948 | (3 | )% | |||||||
| Operating margin | 20.6 | % | 21.4 | % | (80 | ) | bps | ||||
Sales of $744.8 million increased $5.8 million, or 1%, in 2016 compared with 2015. This increase reflected a 1% decrease in organic sales, a 3% increase from acquisitions / divestitures (Acquisitions: SFC Koenig - September 2016; CiDRA Precision Services - July 2015 and Novotema - May 2015. Divestitures: CVI Korea - December 2016 and CVI Japan - September 2016) and 1% of unfavorable foreign currency translation. In 2016, sales decreased 1% domestically and increased 3% internationally. Sales to customers outside the U.S. were approximately 55% of total segment sales in both 2016 and 2015.
Sales within our Scientific Fluidics & Optics platform were down year over year due to a slowed demand in the industrial and laser optics end markets as well as the impact of the CVI Japan and CVI Korea divestitures in 2016 and the Ismatec divestiture in 2015 partially offset by strong demand in the core biotech and in-vitro diagnostic markets coupled with the full year impact of the CiDRA Precision Services acquisition and a strong semiconductor market. Sales within our Material Processing Technologies platform decreased compared to 2015 due to challenges in the North American markets which offset strength in the European and Indian pharma markets. Sales within our Sealing Solutions platform increased compared to 2015 due to the full year impact of the Novotema acquisition in 2015, the 2016 acquisition of SFC Koenig and continued strength in the semiconductor markets, partially offset by pressure in the oil & gas market. Sales in our Gast and Micropump platforms decreased year over year due to continued softness in the North American industrial distribution markets.
Operating income and operating margin of $153.7 million and 20.6%, respectively, in 2016 were down from $157.9 million and 21.4%, respectively, in 2015, primarily due to the inventory step-up charges related to the SFC Koenig acquisition, the incremental impact of divestitures, partially offset by volume increases.
Fire & Safety/Diversified Products Segment
| (In thousands) | 2016 | 2015 | Change | ||||||||
| Net sales | $ | 520,009 | $ | 423,915 | 23 | % | |||||
| Operating income | 121,888 | 115,745 | 5 | % | |||||||
| Operating margin | 23.4 | % | 27.3 | % | (390 | ) | bps | ||||
Sales of $520.0 million increased $96.1 million, or 23%, in 2016 compared with 2015. This increase reflected a 3% decline in organic sales, a 27% increase due to acquisitions (AWG Fittings - July 2016 and Akron Brass - March 2016) and 1% of unfavorable foreign currency translation. In 2016, sales increased 28% domestically and 18% internationally. Sales to customers outside the U.S. were approximately 51% of total segment sales in 2016 compared with 52% in 2015.
Sales within our Dispensing platform increased year over year due to a strong Asian market and the overall strength of the X-Smart product sales, partially offset by the foreign currency impact caused by the strength of the U.S. dollar and challenges within the European markets. Sales decreased in our Band-It platform compared to 2015 as a result of declines in the oil & gas market, offset by strength in the transportation industry and the rebound of the European and Asian markets. Sales within our Fire & Safety platform increased compared to 2015 primarily due to the Akron Brass and AWG Fittings acquisitions as well as increased sales due to new product development, partially offset by project delays in Asia and large projects in Europe in 2015 which did not reoccur.
Operating income of $121.9 million was higher than the $115.7 million in 2015, while operating margin of 23.4% was lower than the 27.3% in 2015, primarily due to the dilutive impact of acquisitions on margins and the inventory step-up charges related to the Akron Brass and AWG Fittings acquisitions. The higher operating income is primarily related to the impact of 2016 acquisitions.
20
Performance in 2015 Compared with 2014
| (In thousands) | 2015 | 2014 | Change | ||||||||
| Net sales | $ | 2,020,668 | $ | 2,147,767 | (6 | )% | |||||
| Operating income | 431,738 | 431,224 | — | % | |||||||
| Operating margin | 21.4 | % | 20.1 | % | 130 | bps | |||||
Sales in 2015 were $2.0 billion, a 6% decrease from 2014. This decrease reflects a 4% decrease in organic sales, a 4% decrease from foreign currency translation and a 2% increase from acquisitions (CiDRA Precision Services — July 2015; Alfa Valvole — June 2015; Novotema — May 2015 and Aegis — April 2014). Sales to customers outside the U.S. represented approximately 50% of total sales in both 2015 and 2014.
In 2015, Fluid & Metering Technologies contributed 43% of sales and 43% of operating income; Health & Science Technologies contributed 36% of sales and 33% of operating income; and Fire & Safety/Diversified Products contributed 21% of sales and 24% of operating income.
Gross profit of $904.3 million in 2015 decreased $45.0 million, or 5%, from 2014, while gross margins increased 60 basis points to 44.8% in 2015 from 44.2% in 2014. The margin increase is mainly attributable to benefits from productivity initiatives, partially offset by decreased sales volume.
SG&A expenses decreased to $479.4 million in 2015 from $504.4 million in 2014. The $25.0 million decrease is mainly attributable to a reduction in volume-related expenses of $35.1 million, partially offset by approximately $10.1 million of incremental costs from new acquisitions. As a percentage of sales, SG&A expenses were 23.7% for 2015 and 23.5% for 2014.
During 2015, the Company recorded pre-tax restructuring expenses totaling $11.2 million compared to $13.7 million recorded in 2014. The restructuring expenses for both years were mainly attributable to employee severance related to head count reductions across all three segments and corporate.
Operating income of $431.7 million in 2015 increased slightly from the $431.2 million recorded in 2014, primarily reflecting improved productivity offset by decreased volumes. Operating margin of 21.4% in 2015 was up 130 basis points from 20.1% in 2014 primarily due to the gain on the sale of the Ismatec product line and productivity improvements.
Other (income) expense decreased $0.9 million from other income of $3.1 million in 2014 to $2.2 million of income in 2015 mainly due to mark-to-market gains in available for sale securities in 2014 compared to losses in 2015.
Interest expense decreased slightly to $41.6 million in 2015 from $41.9 million in 2014. The decrease was primarily due to the maturation of the 2.58% Senior Euro Notes, partially offset by a higher balance on the Revolving Facility.
The provision for income taxes is based upon estimated annual tax rates for the year applied to federal, state and foreign income. The provision for income taxes decreased to $109.5 million in 2015 compared to $113.1 million in 2014. The effective tax rate decreased to 27.9% in 2015 compared to 28.8% in 2014, due to the revaluation of the Italian deferred tax liability related to the reduction in the Italian statutory tax rate, the disposition of the Ismatec product line and the mix of global pre-tax income among jurisdictions.
Net income for the year of $282.8 million increased from the $279.4 million earned in 2014. Diluted earnings per share in 2015 of $3.62 increased $0.17 from $3.45 in 2014.
Fluid & Metering Technologies Segment
| (In thousands) | 2015 | 2014 | Change | ||||||||
| Net sales | $ | 860,792 | $ | 899,588 | (4 | )% | |||||
| Operating income | 204,506 | 216,886 | (6 | )% | |||||||
| Operating margin | 23.8 | % | 24.1 | % | (30 | ) | bps | ||||
Sales of $860.8 million decreased $38.8 million, or 4%, in 2015 compared with 2014. This decrease reflected a 2% decline in organic sales, a 2% increase from acquisitions (Alfa Valvole — June 2015 and Aegis — April 2014) and 4% of unfavorable foreign currency translation. In 2015, sales decreased approximately 3% domestically and 5% internationally. Sales to customers outside the U.S. were approximately 44% of total segment sales in 2015, compared with 45% in 2014.
21
Sales within our Energy platform decreased compared to 2014 primarily due to the fall in oil prices and the related delay in large capital projects in Europe and the Middle East. Sales within our Pumps platform (formerly Industrial) similarly decreased compared to 2014 due to the fall in oil & gas prices, but also due to the weakening of the North American industrial distribution market. This decrease was partially offset by an increase in European chemical project activity. Sales within our Agriculture platform decreased as OEM and after-market distribution sales fell significantly due to depressed commodity prices and lower farm incomes. The slight sales decrease in the Water platform was driven by weakness in North American industrial markets, offset by growth in the global municipal markets and share gains from new products. Sales in the Valves platform, which was created in the third quarter of 2015, increased as a result of the Alfa acquisition.
Operating income and operating margin of $204.5 million and 23.8%; respectively, were lower than the $216.9 million and 24.1%; respectively, recorded in 2014, primarily due to the lower sales volume.
Health & Science Technologies Segment
| (In thousands) | 2015 | 2014 | Change | ||||||||
| Net sales | $ | 738,996 | $ | 752,021 | (2 | )% | |||||
| Operating income (loss) | 157,948 | 152,999 | 3 | % | |||||||
| Operating margin | 21.4 | % | 20.3 | % | 110 | bps | |||||
Sales of $739.0 million decreased $13.0 million, or 2%, in 2015 compared with 2014. This decrease reflected a 1% decline in organic sales, a 2% increase from acquisitions (CiDRA Precision Services — July 2015 and Novotema — May 2015) and 3% unfavorable foreign currency translation. In 2015, sales decreased 3% domestically and 1% internationally. Sales to customers outside the U.S. were approximately 55% of total segment sales in 2015 compared with 54% in 2014.
Sales within our Scientific Fluidics & Optics platform increased as demand from the core biotech, in-vitro diagnostic and analytical instrumentation markets grew and remained consistently strong through the year, partially offset by from slow demand in the industrial and laser optical end markets. Sales within our Material Processing Technologies platform decreased compared to 2014 due to softer orders in the first half of the year, as general spending on large capital projects declined. Sales within our Sealing Solutions platform increased compared to 2014 due to the acquisition of Novotema and strong growth in the semiconductor markets, partially offset by declines in the oil & gas market. Sales in our Gast platform decreased compared to 2014 due to softness in North American industrial distribution markets. Sales in our Micropump platform decreased compared to 2014 due to softness in Asian printing markets, and declines in the North American industrial distribution market.
Operating income and operating margin of $157.9 million and 21.4%, respectively, in 2015 were up from $153.0 million and 20.3%, respectively, recorded in 2014, primarily due to productivity initiatives, partially offset by lower volume.
Fire & Safety/Diversified Products Segment
| (In thousands) | 2015 | 2014 | Change | ||||||||
| Net sales | $ | 423,915 | $ | 502,749 | (16 | )% | |||||
| Operating income | 115,745 | 130,494 | (11 | )% | |||||||
| Operating margin | 27.3 | % | 26.0 | % | 130 | bps | |||||
Sales of $423.9 million decreased $78.8 million, or 16%, in 2015 compared with 2014. This decrease reflected a 10% decline in organic sales and 6% unfavorable foreign currency translation. In 2015, sales decreased 12% domestically and 19% internationally. Sales to customers outside the U.S. were approximately 52% of total segment sales in 2015, compared with 54% in 2014.
Sales within our Dispensing platform decreased due to the benefit of large projects in the first half of the prior year and softness in Asian markets. The sales decrease in our Band-It platform was driven by the decline of upstream oil & gas sales, due to depressed prices, slightly offset by continued strength in the North American transportation markets. Sales within our Fire & Safety platform decreased due to prior year trailer sales for North American power production facilities, a lack of project orders in China and North America, and continued decision delays on municipal projects in Europe and Asia.
Operating income of $115.7 million was lower than the $130.5 million recorded in 2014, while operating margin of 27.3% was higher than the 26.0% recorded in 2014, primarily due to favorable mix within the Dispensing platform along with productivity improvements across the entire segment, partially offset by lower volume.
22
Liquidity and Capital Resources
Operating Activities
Cash flows from operating activities increased $39.6 million, or 11.0%, to $399.9 million in 2016, primarily due to the impact of current year acquisitions, lower bonus payments, early adoption of ASU 2016-09, and improved operating working capital performance, partially offset by higher prepaid income taxes in 2016. At December 31, 2016, working capital was $513.6 million and the Company’s current ratio was 2.66 to 1. At December 31, 2016, the Company’s cash and cash equivalents totaled $236.0 million, of which $190.3 million was held outside of the United States.
Investing Activities
Cash flows used in investing activities increased $298.7 million to $509.2 million in 2016, primarily as a result of an additional $315.0 million of cash paid for acquisitions, partially offset by $11.4 million of higher proceeds from the sale of businesses and $5.5 million of lower capital expenditures.
Cash flows from operations were more than adequate to fund capital expenditures of $38.2 million and $43.8 million in 2016 and 2015, respectively. Capital expenditures were generally for machinery and equipment that improved productivity, although a portion was for business system technology, replacement of equipment, and construction of new facilities. Management believes that the Company has ample capacity in its plants and equipment to meet demand increases for future growth in the intermediate term.
The Company acquired Akron Brass in March 2016 for cash consideration of $221.4 million; AWG Fittings in July 2016 for cash consideration of $47.5 million (€42.8 million); and SFC Koenig in September 2016 for cash consideration of $241.1 million (€215.9 million). The purchase prices for the 2016 acquisitions were funded with both cash on hand and borrowings under the Company’s revolving facilities. The Company acquired Novotema in May 2015 for cash consideration of $61.1 million (€56 million); Alfa Valvole in June 2015 for cash consideration of $112.6 million (€99.8 million); and CPS in July 2015 for cash consideration of $19.5 million and non-cash contingent consideration valued at $4.7 million. The entire purchase price for all of the 2015 acquisitions was funded with cash on hand.
Financing Activities
Cash flows from financing activities increased from $295.5 million of cash used in financing activities in 2015 to $46.5 million of cash provided by financing activities in 2016, primarily as a result of the Company paying off the $88.4 million balance on the 2.58% Senior Euro Notes in 2015, a reduction of $153.6 million of purchases of common stock in 2016, and the issuance of $200 million of Senior Notes in 2016.
On June 13, 2016, the Company completed a private placement of $100 million aggregate principal amount of 3.20% Senior Notes due June 13, 2023 and $100 million aggregate principal amount of 3.37% Senior Notes due June 13, 2025 (collectively, the “Notes”) pursuant to a Note Purchase Agreement, dated June 13, 2016 (the “Purchase Agreement”). Each series of Notes bears interest at the stated amount per annum, which is payable semi-annually in arrears on each June 13th and December 13th. The Notes are unsecured obligations of the Company and rank pari passu in right of payment with all of the Company’s other unsecured, unsubordinated debt. The Company may at any time prepay all, or any portion of the Notes; provided that such portion is greater than 5% of the aggregate principal amount of Notes then outstanding. In the event of a prepayment, the Company will pay an amount equal to par plus accrued interest plus a make-whole amount. In addition, the Company may repurchase the Notes by making an offer to all holders of the Notes, subject to certain conditions.
The Company maintains a revolving credit facility (the “Revolving Facility”), which is a $700.0 million unsecured, multi-currency bank credit facility expiring on June 23, 2020. At December 31, 2016, there was $169.6 million outstanding under the Revolving Facility and $9.2 million of outstanding letters of credit, resulting in a net available borrowing capacity under the Revolving Facility at December 31, 2016 of $521.2 million. Borrowings under the Revolving Facility bear interest, at either an alternate base rate or an adjusted LIBOR rate plus, in each case, an applicable margin. This applicable margin is based on the Company’s senior, unsecured, long-term debt rating and can range from .005% to 1.50%. Based on the Company’s credit rating at December 31, 2016, the applicable margin was 1.10%, resulting in a weighted average interest rate of 1.50% at December 31, 2016. Interest is payable (a) in the case of base rate loans, quarterly, and (b) in the case of LIBOR rate loans, on the maturity date of the borrowing, or quarterly from the effective date for borrowings exceeding three months. The Company may request increases in the lending commitments under the Credit Agreement, but the aggregate lending commitments pursuant to such increases may not exceed $350.0 million. An annual Revolving Facility fee, also based on the Company’s credit rating, is currently 15 basis points and is payable quarterly.
On June 9, 2015, the Company paid the balance of the 2.58% Senior Euro Notes, upon its maturity, using cash on hand.
On December 9, 2011, the Company completed a public offering of $350.0 million 4.2% senior notes due December 15, 2021 (“4.2% Senior Notes”). The net proceeds from the offering of $346.2 million, after deducting a $0.9 million issuance
23
discount, a $2.3 million underwriting commission and $0.6 million offering expenses, were used to repay $306.0 million of outstanding bank indebtedness, with the balance used for general corporate purposes. The 4.2% Senior Notes bear interest at a rate of 4.2% per annum, which is payable semi-annually in arrears on each June 15 and December 15. The Company may redeem all or part of the 4.2% Senior Notes at any time prior to maturity at the redemption prices set forth in the Note Indenture governing the 4.2% Senior Notes. The Company may issue additional debt from time to time pursuant to the Indenture. The Indenture and 4.2% Senior Notes contain covenants that limit the Company’s ability to, among other things, incur certain liens securing indebtedness, engage in certain sale-leaseback transactions, and enter into certain consolidations, mergers, conveyances, transfers or leases of all or substantially all the Company’s assets. The terms of the 4.2% Senior Notes also require the Company to make an offer to repurchase the 4.2% Senior Notes upon a change of control triggering event (as defined in the Indenture) at a price equal to 101% of their principal amount plus accrued and unpaid interest, if any.
On December 6, 2010, the Company completed a public offering of $300.0 million 4.5% senior notes due December 15, 2020 (“4.5% Senior Notes”). The net proceeds from the offering of $295.7 million, after deducting a $1.6 million issuance discount, a $1.9 million underwriting commission and $0.8 million offering expenses, were used to repay $250.0 million of outstanding bank indebtedness, with the balance used for general corporate purposes. The 4.5% Senior Notes bear interest at a rate of 4.5% per annum, which is payable semi-annually in arrears on each June 15 and December 15. The Company may redeem all or a portion of the 4.5% Senior Notes at any time prior to maturity at the redemption prices set forth in the Note Indenture governing the 4.5% Senior Notes. The Company may issue additional debt from time to time pursuant to the Indenture. The Indenture and 4.5% Senior Notes contain covenants that limit the Company’s ability to, among other things, incur certain liens securing indebtedness, engage in certain sale-leaseback transactions, and enter into certain consolidations, mergers, conveyances, transfers or leases of all or substantially all the Company’s assets. The terms of the 4.5% Senior Notes also require the Company to make an offer to repurchase the 4.5% Senior Notes upon a change of control triggering event (as defined in the Indenture) at a price equal to 101% of their principal amount plus accrued and unpaid interest, if any.
There are two key financial covenants that the Company is required to maintain in connection with the Revolving Facility and the Notes, a minimum interest coverage ratio of 3.0 to 1 and a maximum leverage ratio of 3.50 to 1. At December 31, 2016, the Company was in compliance with both of these financial covenants, as the Company’s interest coverage ratio was 11.51 to 1 and the leverage ratio was 1.91 to 1. There are no financial covenants relating to the 4.5% Senior Notes or 4.2% Senior Notes; however, both are subject to cross-default provisions.
On December 1, 2015 the Company’s Board of Directors approved an increase of $300.0 million in the authorized level for repurchases of common stock. Repurchases under the program will be funded with future cash flow generation or borrowings available under the Revolving Facility. During 2016, the Company purchased a total of 0.7 million shares at a cost of $55.0 million compared to 2.8 million shares purchased in 2015 at a cost of $210.5 million, of which $2.3 million was settled in January 2016. As of December 31, 2016, there was $580 million of repurchase authorization remaining.
The Company believes current cash, cash from operations and cash available under the Revolving Facility will be sufficient to meet its operating cash requirements, planned capital expenditures, interest and principal payments on all borrowings, pension and postretirement funding requirements, authorized share repurchases and annual dividend payments to holders of the Company’s common stock for the next twelve months. Additionally, in the event that suitable businesses are available for acquisition upon acceptable terms, the Company may obtain all or a portion of the financing for these acquisitions through the incurrence of additional borrowings. As of December 31, 2016, $169.6 million was outstanding under the Revolving Facility, with $9.2 million of outstanding letters of credit, resulting in net available borrowing capacity under the Revolving Facility at December 31, 2016 of approximately $521.2 million.
Contractual Obligations
Our contractual obligations include pension and postretirement medical benefit plans, rental payments under operating leases, payments under capital leases, and other long-term obligations arising in the ordinary course of business. There are no identifiable events or uncertainties, including the lowering of our credit rating, which would accelerate payment or maturity of any of these commitments or obligations.
The following table summarizes our significant contractual obligations and commercial commitments at December 31, 2016, and the future periods in which such obligations are expected to be settled in cash. In addition, the table reflects the timing of principal and interest payments on outstanding borrowings. Additional detail regarding these obligations is provided in the Notes to Consolidated Financial Statements in Part II, Item 8, “Financial Statements and Supplementary Data.”
24
| Payments Due by Period | Total | Less Than 1 Year | 1-3 Years | 3-5 Years | More Than 5 Years | ||||||||||||||
| (In thousands) | |||||||||||||||||||
Borrowings (1) | $ | 1,209,173 | $ | 38,408 | $ | 76,816 | $ | 877,354 | $ | 216,595 | |||||||||
| Operating lease obligations | 73,157 | 16,923 | 23,759 | 13,369 | 19,106 | ||||||||||||||
Capital lease obligations (2) | 1,307 | 1,055 | 252 | — | — | ||||||||||||||
Purchase obligations (3) | 114,103 | 108,994 | 3,573 | 518 | 1,018 | ||||||||||||||
| Pension and post-retirement obligations | 108,761 | 14,394 | 20,897 | 21,420 | 52,050 | ||||||||||||||
Total contractual obligations (4) | $ | 1,506,501 | $ | 179,774 | $ | 125,297 | $ | 912,661 | $ | 288,769 | |||||||||
| (1) | Includes interest payments based on contractual terms and current interest rates for variable debt. |
| (2) | Consists primarily of tangible personal property leases. |
| (3) | Consists primarily of inventory commitments. |
| (4) | Comprises liabilities recorded on the balance sheet of $1,110.5 million, and obligations not recorded on the balance sheet of $396.0 million. |
Critical Accounting Policies
We believe that the application of the following accounting policies, which are important to our financial position and results of operations, require significant judgments and estimates on the part of management. For a summary of all of our accounting policies, including the accounting policies discussed below, see Note 1 of the Notes to Consolidated Financial Statements in Part II, Item 8, “Financial Statements and Supplementary Data.”
Revenue recognition — The Company recognizes revenue when persuasive evidence of an arrangement exists, delivery has occurred, the sales price is fixed or determinable, and collectability of the sales price is reasonably assured. For product sales, delivery does not occur until the products have been shipped and risk of loss has been transferred to the customer. Revenue from services is recognized when the services are provided or ratably over the contract term. Some arrangements with customers may include multiple deliverables, including the combination of products and services. In such cases, the Company has identified these as separate elements in accordance with ASC 605-25, Revenue Recognition-Multiple-Element Arrangements, and recognizes revenue consistent with the policy for each separate element based on the relative selling price method. Revenues from certain long-term contracts are recognized on the percentage-of-completion method. Percentage-of-completion is measured principally by the percentage of costs incurred to date for each contract to the estimated total costs for such contract at completion. Provisions for estimated losses on uncompleted long-term contracts are made in the period in which such losses are determined. Due to uncertainties inherent in the estimation process, it is reasonably possible that completion costs, including those arising from contract penalty provisions and final contract settlements, will be revised in the near-term. Such revisions to costs and income are recognized in the period in which the revisions are determined.
The Company records allowances for discounts, product returns and customer incentives at the time of sale as a reduction of revenue as such allowances can be reliably estimated based on historical experience and known trends. The Company also offers product warranties and accrues its estimated exposure for warranty claims at the time of sale based upon the length of the warranty period, warranty costs incurred and any other related information known to the Company.
Goodwill, long-lived and intangible assets — The Company evaluates the recoverability of certain noncurrent assets utilizing various estimation processes. An impairment of a long-lived asset exists when the asset’s carrying amount exceeds its fair value, and is recorded when the carrying amount is not recoverable through future operations. An impairment of an indefinite-lived intangible asset or goodwill exists when the carrying amount of the intangible asset or goodwill exceeds its fair value. Assessments of possible impairments of long-lived or indefinite-lived intangible assets or goodwill are made if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. Additionally, testing for possible impairments of recorded indefinite-lived intangible asset balances and goodwill is performed annually. On October 31, or more frequently if triggering events occur, the Company compares the fair value of its reporting units to the carrying value of each reporting unit to determine if a goodwill impairment exists. The amount and timing of impairment charges for these assets require the estimation of future cash flows to determine the fair value of the related assets. In 2016 and 2015, the Company concluded that certain long-lived assets had a fair value that was less than the carrying value of the assets, resulting in $0.2 million and $0.8 million, respectively, of long-lived asset impairment charges.
25
The Company’s business acquisitions result in recording goodwill and other intangible assets, which affect the amount of amortization expense and possible impairment expense that the Company will incur in future periods. The Company follows the guidance prescribed in ASC 350, Goodwill and Other Intangible Assets, to test goodwill and intangible assets for impairment. The Company determines the fair value of each reporting unit utilizing an income approach (discounted cash flows) weighted 50% and a market approach (consisting of a comparable public company multiples methodology) weighted 50%. To determine the reasonableness of the calculated fair values, the Company reviews the assumptions to ensure that neither the income approach nor the market approach yielded significantly different valuations.
The key assumptions are updated every year for each reporting unit for the income and market approaches used to determine fair value. Various assumptions are utilized including forecasted operating results, annual operating plans, strategic plans, economic projections, anticipated future cash flows, the weighted average cost of capital, market data and market multiples. The assumptions that have the most significant effect on the fair value calculations are the weighted average cost of capital, market multiples, forecasted EBITDA, and terminal growth rates. The 2016 and 2015 ranges for these three assumptions utilized by the Company are as follows:
| Assumptions | 2016 Range | 2015 Range | ||
| Weighted average cost of capital | 9.0% to 12.0% | 9.5% to 13.0% | ||
| Market multiples | 9.5x to 17.5x | 7.5x to 14.0x | ||
| Terminal growth rates | 3.0% to 3.5% | 3.0% to 3.5% | ||
In assessing the fair value of the reporting units, the Company considers both the market approach and the income approach. Under the market approach, the fair value of the reporting unit is determined by the respective trailing twelve month EBITDA and the forward looking 2017 EBITDA (generally 50% each), based on multiples of comparable public companies. The market approach is dependent on a number of significant management assumptions including forecasted EBITDA and selected market multiples. Under the income approach, the fair value of the reporting unit is determined based on the present value of estimated future cash flows. The income approach is dependent on a number of significant management assumptions including estimates of operating results, capital expenditures, net working capital requirements, long-term growth rates and discount rates. Weighting was equally attributed to both the market and income approaches (50% each) in arriving at the fair value of the reporting units.
In addition to performing our annual impairment test, we also performed interim impairment tests due to the divestitures in the third and fourth quarters of 2016 as well as the reorganization of certain reporting units. As a result of these impairment tests, the Company concluded that the reporting units had fair values in excess of their carrying values.
The Banjo trade name is an indefinite-lived intangible asset which is tested for impairment on an annual basis in accordance with ASC 350 or more frequently if events or changes in circumstances indicate that the asset might be impaired. The Company uses the relief-from-royalty method, a form of the income approach. The relief-from-royalty method is dependent on a number of significant management assumptions, including estimates of revenues, royalty rates and discount rates.
The Akron Brass trade name is an indefinite-lived intangible asset that was acquired as a result of the Akron Brass acquisition in March 2016 and is tested for impairment on an annual basis in accordance with ASC 350 or more frequently if events or changes in circumstances indicate that the asset might be impaired. The Company uses the relief-from-royalty method, a form of the income approach. The relief-from-royalty method is dependent on a number of significant management assumptions, including estimates of revenues, royalty rates and discount rates.
In 2016 and 2015, there were no triggering events or changes in circumstances that would have required a review other than as of our annual test date. Based on the results of our measurement as of October 31, 2016, the fair value of the Banjo trade name was more than 25% in excess of the carrying value and the fair value of the Akron Brass trade name was near its carrying value as a result of the acquisition of this business in March 2016.
Defined benefit retirement plans — The plan obligations and related assets of the defined benefit retirement plans are presented in Note 15 of the Notes to Consolidated Financial Statements in Part II, Item 8, “Financial Statements and Supplementary Data.” Level 1 assets are valued using unadjusted quoted prices for identical assets in active markets. Level 2 assets are valued using quoted prices or other observable inputs for similar assets. Level 3 assets are valued using unobservable inputs, but reflect the assumptions market participants would use in pricing the assets. Plan obligations and the annual pension expense are determined by consulting with actuaries using a number of assumptions provided by the Company. Key assumptions in the determination of the annual pension expense include the discount rate, the rate of salary increases, and the estimated future return on plan assets. To the extent actual amounts differ from these assumptions and estimated amounts, results could be adversely affected.
26
The Society of Actuaries releases annual updates to mortality tables, which update life expectancy assumptions. IDEX adopts these annual updates and, in consideration of these tables, we modified the mortality assumptions used in determining our pension and post-retirement benefit obligations as of December 31, 2016, which will have a related impact on our annual benefit expense in future years. New mortality tables may result in additional funding requirements dependent upon the funded status of our plans. These expectations presume all other assumptions remain constant and there are no changes to applicable funding regulations.
Changes in the discount rate assumptions will impact the (gain) loss amortization and interest cost components of the projected benefit obligation (“PBO”), which in turn, may impact the Company’s funding decisions if the PBO exceeds plan assets. Each 100 basis point increase in the discount rate will cause a corresponding decrease in the PBO of approximately $27 million based upon the December 31, 2016 data. Each 100 basis point decrease in the discount rate will cause a corresponding increase in the PBO of approximately $33 million based upon the December 31, 2016 data.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
The Company is subject to market risk associated with changes in foreign currency exchange rates and interest rates. The Company may, from time to time, enter into foreign currency forward contracts and interest rate swaps on its debt when it believes there is a financial advantage in doing so. A treasury risk management policy, adopted by the Board of Directors, describes the procedures and controls over derivative financial and commodity instruments, including foreign currency forward contracts and interest rate swaps. Under the policy, the Company does not use financial or commodity derivative instruments for trading purposes, and the use of these instruments is subject to strict approvals by senior officers. Typically, the use of derivative instruments is limited to foreign currency forward contracts and interest rate swaps on the Company’s outstanding long-term debt.
Foreign Currency Exchange Rates
The Company’s foreign currency exchange rate risk is limited principally to the Euro, Swiss Franc, British Pound, Canadian Dollar, Indian Rupee and Chinese Renminbi. The Company manages its foreign exchange risk principally through invoicing customers in the same currency as the source of products. The foreign currency transaction (gains) losses for the years ending December 31, 2016, 2015 and 2014 were $(6.2) million, $(0.1) million, and $0.9 million, respectively, and are reported within Other (income) expense-net on the Consolidated Statements of Operations.
Interest Rate Fluctuations
The Company’s interest rate exposure is primarily related to its $1,020.9 million of total debt outstanding at December 31, 2016. Approximately 17% of the debt is priced at interest rates that float with the market. A 50 basis point movement in the interest rate on the floating rate debt would result in an approximate $0.8 million annualized increase or decrease in interest expense and cash flows. The remaining debt is fixed rate debt.
27
Item 8. Financial Statements and Supplementary Data.
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America, and as defined in Exchange Act Rule 13a-15(f).
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. Because of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting.
Management has used the framework set forth in the report entitled “Internal Control — Integrated Framework” (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission to assess the effectiveness of the Company’s internal control over financial reporting. Based on that assessment, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2016.
The Company completed the acquisitions of Akron Brass Holding Corporation in March 2016, AWG Fittings GmbH in July 2016 and SFC Koenig AG in August 2016. Due to the timing of the acquisitions, management has excluded these acquisitions from our evaluation of effectiveness of internal controls over financial reporting. This exclusion represented 6.3% of net sales and 19.4% of total assets of the Company for the year ended December 31, 2016. The effectiveness of the Company’s internal control over financial reporting as of December 31, 2016, has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report which appears herein.
28
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of IDEX Corporation
We have audited the internal control over financial reporting of IDEX Corporation and subsidiaries (the “Company”) as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. As described in Management’s Report on Internal Control Over Financial Reporting, management excluded from its assessment the internal control over financial reporting at Akron Brass Holding Corporation, which was acquired in March 2016; AWG Fittings GmbH, which was acquired in July 2016; and SFC Koenig AG, which was acquired in August 2016. These exclusions collectively represented 6.3% of net sales and 19.4% of total assets of the Company for the year ended December 31, 2016. Accordingly, our audit did not include the internal control over financial reporting at Akron Brass Holding Corporation, AWG Fittings GmbH, and SFC Koenig AG. The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed by, or under the supervision of, the company's principal executive and principal financial officers, or persons performing similar functions, and effected by the company's board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the year ended December 31, 2016 of the Company and our report dated February 23, 2017 expressed an unqualified opinion on those financial statements.
| /s/ DELOITTE & TOUCHE LLP | |
| Chicago, Illinois | |
| February 23, 2017 | |
29
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of IDEX Corporation
We have audited the accompanying consolidated balance sheets of IDEX Corporation and subsidiaries (the "Company") as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive income, shareholders' equity, and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of IDEX Corporation and subsidiaries as of December 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company's internal control over financial reporting as of December 31, 2016, based on the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 23, 2017 expressed an unqualified opinion on the Company's internal control over financial reporting.
| /s/ DELOITTE & TOUCHE LLP | |
| Chicago, Illinois | |
| February 23, 2017 | |
30
IDEX CORPORATION
CONSOLIDATED BALANCE SHEETS
| As of December 31, | |||||||
| 2016 | 2015 | ||||||
(In thousands except share and per share amounts) | |||||||
| ASSETS | |||||||
| Current assets | |||||||
| Cash and cash equivalents | $ | 235,964 | $ | 328,018 | |||
| Receivables — net | 272,813 | 260,000 | |||||
| Inventories | 252,859 | 239,124 | |||||
| Other current assets | 61,085 | 35,542 | |||||
| Total current assets | 822,721 | 862,684 | |||||
| Property, plant and equipment — net | 247,816 | 240,945 | |||||
| Goodwill | 1,632,592 | 1,396,529 | |||||
| Intangible assets — net | 435,504 | 287,837 | |||||
| Other noncurrent assets | 16,311 | 17,448 | |||||
| Total assets | $ | 3,154,944 | $ | 2,805,443 | |||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | |||||||
| Current liabilities | |||||||
| Trade accounts payable | $ | 128,933 | $ | 128,911 | |||
| Accrued expenses | 152,852 | 153,672 | |||||
| Short-term borrowings | 1,046 | 1,087 | |||||
| Dividends payable | 26,327 | 25,927 | |||||
| Total current liabilities | 309,158 | 309,597 | |||||
| Long-term borrowings | 1,014,235 | 839,707 | |||||
| Deferred income taxes | 166,427 | 110,483 | |||||
| Other noncurrent liabilities | 121,230 | 102,365 | |||||
| Total liabilities | 1,611,050 | 1,362,152 | |||||
| Commitments and contingencies (Note 8) | |||||||
| Shareholders’ equity | |||||||
| Preferred stock: | |||||||
| Authorized: 5,000,000 shares, $.01 per share par value; Issued: none | — | — | |||||
| Common stock: | |||||||
| Authorized: 150,000,000 shares, $.01 per share par value; Issued: 90,200,951 shares at December 31, 2016 and 90,151,131 shares at December 31, 2015 | 902 | 902 | |||||
| Additional paid-in capital | 697,213 | 679,623 | |||||
| Retained earnings | 1,834,739 | 1,666,680 | |||||
| Treasury stock at cost: 13,760,266 shares at December 31, 2016 and 13,616,592 shares at December 31, 2015 | (787,307 | ) | (757,416 | ) | |||
| Accumulated other comprehensive loss | (201,653 | ) | (146,498 | ) | |||
| Total shareholders’ equity | 1,543,894 | 1,443,291 | |||||
| Total liabilities and shareholders’ equity | $ | 3,154,944 | $ | 2,805,443 | |||
See Notes to Consolidated Financial Statements.
31
IDEX CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Years Ended December 31, | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands except per share amounts) | |||||||||||
| Net sales | $ | 2,113,043 | $ | 2,020,668 | $ | 2,147,767 | |||||
| Cost of sales | 1,182,276 | 1,116,353 | 1,198,452 | ||||||||
| Gross profit | 930,767 | 904,315 | 949,315 | ||||||||
| Selling, general and administrative expenses | 498,994 | 479,408 | 504,419 | ||||||||
| Loss (gain) on sale of businesses - net | 22,298 | (18,070 | ) | — | |||||||
| Restructuring expenses | 3,674 | 11,239 | 13,672 | ||||||||
| Operating income | 405,801 | 431,738 | 431,224 | ||||||||
| Other (income) expense - net | (8,327 | ) | (2,243 | ) | (3,111 | ) | |||||
| Interest expense | 45,616 | 41,636 | 41,895 | ||||||||
| Income before income taxes | 368,512 | 392,345 | 392,440 | ||||||||
| Provision for income taxes | 97,403 | 109,538 | 113,054 | ||||||||
| Net income | $ | 271,109 | $ | 282,807 | $ | 279,386 | |||||
| Earnings per common share: | |||||||||||
| Basic earnings per common share | $ | 3.57 | $ | 3.65 | $ | 3.48 | |||||
| Diluted earnings per common share | $ | 3.53 | $ | 3.62 | $ | 3.45 | |||||
| Share data: | |||||||||||
| Basic weighted average common shares outstanding | 75,803 | 77,126 | 79,715 | ||||||||
| Diluted weighted average common shares outstanding | 76,758 | 77,972 | 80,728 | ||||||||
See Notes to Consolidated Financial Statements.
32
IDEX CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| For the Years Ended December 31, | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Net income | $ | 271,109 | $ | 282,807 | $ | 279,386 | |||||
| Other comprehensive income (loss) | |||||||||||
| Reclassification adjustments for derivatives, net of tax | 4,361 | 4,531 | 4,510 | ||||||||
| Pension and other postretirement adjustments, net of tax | 3,049 | 9,415 | (16,459 | ) | |||||||
| Foreign currency translation adjustments | |||||||||||
| Cumulative translation adjustment | (76,822 | ) | (63,441 | ) | (77,024 | ) | |||||
| Reclassification of foreign currency translation to earnings upon sale of businesses | 14,257 | (4,725 | ) | — | |||||||
| Other comprehensive income (loss) | (55,155 | ) | (54,220 | ) | (88,973 | ) | |||||
| Comprehensive income | $ | 215,954 | $ | 228,587 | $ | 190,413 | |||||
See Notes to Consolidated Financial Statements.
33
IDEX CORPORATION
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
Common Stock and Additional Paid-In Capital | Retained Earnings | Accumulated Other Comprehensive Income (Loss) | Treasury Stock | Total Shareholders’ Equity | |||||||||||||||||||||||
Cumulative Translation Adjustment | Retirement Benefits Adjustments | Cumulative Unrealized Gain (Loss) on Derivatives | |||||||||||||||||||||||||
| (In thousands except share and per share amounts) | |||||||||||||||||||||||||||
| Balance, December 31, 2013 | $ | 608,658 | $ | 1,293,740 | $ | 52,211 | $ | (23,857 | ) | $ | (31,659 | ) | $ | (326,104 | ) | $ | 1,572,989 | ||||||||||
| Net income | — | 279,386 | — | — | — | — | 279,386 | ||||||||||||||||||||
| Cumulative translation adjustment | — | — | (77,024 | ) | — | — | — | (77,024 | ) | ||||||||||||||||||
| Net change in retirement obligations (net of tax benefit of $6,852) | — | — | — | (16,459 | ) | — | — | (16,459 | ) | ||||||||||||||||||
| Net change on derivatives designated as cash flow hedges (net of tax of $2,713) | — | — | — | — | 4,510 | — | 4,510 | ||||||||||||||||||||
| Issuance of 571,751 shares of common stock from issuance of unvested shares, exercise of stock options and deferred compensation plans (net of tax of $3,425) | 23,195 | — | — | — | — | — | 23,195 | ||||||||||||||||||||
| Repurchase of 2,970,461 shares of common stock | — | (222,487 | ) | (222,487 | ) | ||||||||||||||||||||||
| Share-based compensation | 16,598 | — | — | — | — | — | 16,598 | ||||||||||||||||||||
| Unvested shares surrendered for tax withholding | — | — | — | — | — | (4,952 | ) | (4,952 | ) | ||||||||||||||||||
| Cash dividends declared — $1.12 per common share outstanding | — | (89,305 | ) | — | — | — | — | (89,305 | ) | ||||||||||||||||||
| Balance, December 31, 2014 | $ | 648,451 | $ | 1,483,821 | $ | (24,813 | ) | $ | (40,316 | ) | $ | (27,149 | ) | $ | (553,543 | ) | $ | 1,486,451 | |||||||||
| Net income | — | 282,807 | — | — | — | — | 282,807 | ||||||||||||||||||||
| Cumulative translation adjustment | — | — | (68,166 | ) | — | — | — | (68,166 | ) | ||||||||||||||||||
| Net change in retirement obligations (net of tax of $3,842) | — | — | — | 9,415 | — | — | 9,415 | ||||||||||||||||||||
| Net change on derivatives designated as cash flow hedges (net of tax of $2,499) | — | — | — | — | 4,531 | — | 4,531 | ||||||||||||||||||||
| Issuance of 685,501 shares of common stock from issuance of unvested shares, exercise of stock options and deferred compensation plans (net of tax of $3,794) | 14,545 | — | — | — | — | 9,937 | 24,482 | ||||||||||||||||||||
| Repurchase of 2,811,002 shares of common stock | — | — | — | — | — | (210,551 | ) | (210,551 | ) | ||||||||||||||||||
| Share-based compensation | 17,529 | — | — | — | — | — | 17,529 | ||||||||||||||||||||
| Unvested shares surrendered for tax withholding | — | — | — | — | — | (3,259 | ) | (3,259 | ) | ||||||||||||||||||
| Cash dividends declared — $1.28 per common share outstanding | — | (99,948 | ) | — | — | — | — | (99,948 | ) | ||||||||||||||||||
| Balance, December 31, 2015 | $ | 680,525 | $ | 1,666,680 | $ | (92,979 | ) | $ | (30,901 | ) | $ | (22,618 | ) | $ | (757,416 | ) | $ | 1,443,291 | |||||||||
| Net income | — | 271,109 | — | — | — | — | 271,109 | ||||||||||||||||||||
| Cumulative translation adjustment | — | — | (62,565 | ) | — | — | — | (62,565 | ) | ||||||||||||||||||
| Net change in retirement obligations (net of tax of $2,107) | — | — | — | 3,049 | — | — | 3,049 | ||||||||||||||||||||
| Net change on derivatives designated as cash flow hedges (net of tax of $2,490) | — | — | — | — | 4,361 | — | 4,361 | ||||||||||||||||||||
| Issuance of 594,919 shares of common stock from issuance of unvested shares, performance share units and exercise of stock options (net of tax of $5,305) | 253 | — | — | — | — | 29,987 | 30,240 | ||||||||||||||||||||
| Repurchase of 738,593 shares of common stock | — | — | — | — | — | (54,950 | ) | (54,950 | ) | ||||||||||||||||||
| Share-based compensation | 17,337 | — | — | — | — | — | 17,337 | ||||||||||||||||||||
| Unvested shares surrendered for tax withholding | — | — | — | — | — | (4,928 | ) | (4,928 | ) | ||||||||||||||||||
| Cash dividends declared — $1.36 per common share outstanding | — | (103,050 | ) | — | — | — | — | (103,050 | ) | ||||||||||||||||||
| Balance, December 31, 2016 | $ | 698,115 | $ | 1,834,739 | $ | (155,544 | ) | $ | (27,852 | ) | $ | (18,257 | ) | $ | (787,307 | ) | $ | 1,543,894 | |||||||||
See Notes to Consolidated Financial Statements.
34
IDEX CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended December 31, | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Cash flows from operating activities | |||||||||||
| Net income | $ | 271,109 | $ | 282,807 | $ | 279,386 | |||||
| Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||
| Loss (gain) on sale of fixed assets | (28 | ) | (114 | ) | (351 | ) | |||||
| Loss (gain) on sale of businesses - net | 22,298 | (18,070 | ) | — | |||||||
| Asset impairments | 205 | 795 | 2,473 | ||||||||
| Depreciation and amortization | 37,854 | 35,694 | 33,720 | ||||||||
| Amortization of intangible assets | 49,038 | 42,426 | 43,187 | ||||||||
| Amortization of debt issuance expenses | 1,295 | 1,612 | 1,723 | ||||||||
| Share-based compensation expense | 20,326 | 20,048 | 20,717 | ||||||||
| Deferred income taxes | (17,308 | ) | (339 | ) | (8,593 | ) | |||||
| Excess tax benefit from share-based compensation | — | (5,265 | ) | (6,275 | ) | ||||||
| Non-cash interest expense associated with forward starting swaps | 6,851 | 7,030 | 7,223 | ||||||||
| Pension settlement | 3,554 | — | — | ||||||||
| Changes in (net of the effect from acquisitions and divestitures): | |||||||||||
| Receivables | 302 | 8,832 | (11,110 | ) | |||||||
| Inventories | 32,747 | 4,557 | (7,821 | ) | |||||||
| Other current assets | (22,006 | ) | (2,728 | ) | (5,201 | ) | |||||
| Trade accounts payable | 73 | (2,828 | ) | (2,466 | ) | ||||||
| Accrued expenses | (5,470 | ) | (16,672 | ) | 23,760 | ||||||
| Other — net | (923 | ) | 2,536 | (2,411 | ) | ||||||
| Net cash flows provided by operating activities | 399,917 | 360,321 | 367,961 | ||||||||
| Cash flows from investing activities | |||||||||||
| Purchases of property, plant and equipment | (38,242 | ) | (43,776 | ) | (47,997 | ) | |||||
| Acquisition of businesses, net of cash acquired | (510,001 | ) | (195,013 | ) | (25,443 | ) | |||||
| Proceeds from fixed asset disposals | 49 | 894 | 1,460 | ||||||||
| Proceeds from sale of businesses, net of cash sold | 39,064 | 27,677 | — | ||||||||
| Other — net | (69 | ) | (273 | ) | (280 | ) | |||||
| Net cash flows (used in) investing activities | (509,199 | ) | (210,491 | ) | (72,260 | ) | |||||
| Cash flows from financing activities | |||||||||||
| Borrowings under revolving credit facilities | 501,529 | 414,032 | 165,014 | ||||||||
| Proceeds from issuance of 3.20% Senior Notes | 100,000 | — | — | ||||||||
| Proceeds from issuance of 3.37% Senior Notes | 100,000 | — | — | ||||||||
| Payment of 2.58% Senior Euro Notes | — | (88,420 | ) | — | |||||||
| Payments under revolving credit facilities | (520,125 | ) | (333,630 | ) | (61,951 | ) | |||||
| Debt issuance costs | (246 | ) | (1,739 | ) | — | ||||||
| Dividends paid | (102,650 | ) | (96,172 | ) | (85,726 | ) | |||||
| Proceeds from stock option exercises | 30,240 | 19,217 | 17,161 | ||||||||
| Excess tax benefit from share-based compensation | — | 5,265 | 6,275 | ||||||||
| Purchases of common stock | (57,272 | ) | (210,822 | ) | (219,893 | ) | |||||
| Unvested shares surrendered for tax withholding | (4,928 | ) | (3,259 | ) | (4,952 | ) | |||||
| Net cash flows provided by (used in) financing activities | 46,548 | (295,528 | ) | (184,072 | ) | ||||||
| Effect of exchange rate changes on cash and cash equivalents | (29,320 | ) | (35,421 | ) | (42,121 | ) | |||||
| Net increase (decrease) in cash | (92,054 | ) | (181,119 | ) | 69,508 | ||||||
| Cash and cash equivalents at beginning of year | 328,018 | 509,137 | 439,629 | ||||||||
| Cash and cash equivalents at end of period | $ | 235,964 | $ | 328,018 | $ | 509,137 | |||||
| Supplemental cash flow information | |||||||||||
| Cash paid for: | |||||||||||
| Interest | $ | 37,067 | $ | 33,502 | $ | 32,565 | |||||
| Income taxes | 109,399 | 112,613 | 122,295 | ||||||||
| Significant non-cash activities: | |||||||||||
| Contingent consideration for acquisition | — | 4,705 | — | ||||||||
See Notes to Consolidated Financial Statements.
35
IDEX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Significant Accounting Policies
Business
IDEX is an applied solutions company specializing in fluid and metering technologies, health and science technologies, and fire, safety and other diversified products built to customers’ specifications. IDEX’s products are sold in niche markets to a wide range of industries throughout the world. The Company’s products include industrial pumps, compressors, flow meters, injectors and valves, and related controls for use in a wide variety of process applications; precision fluidics solutions, including pumps, valves, degassing equipment, corrective tubing, fittings, and complex manifolds, optical filters and specialty medical equipment and devices for use in life science applications; precision-engineered equipment for dispensing, metering and mixing paints; and engineered products for industrial and commercial markets, including fire and rescue, transportation equipment, oil & gas, electronics, and communications. These activities are grouped into three reportable segments: Fluid & Metering Technologies, Health & Science Technologies and Fire & Safety/Diversified Products.
Principles of Consolidation
The consolidated financial statements include the Company and its subsidiaries. All intercompany transactions and accounts have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities, and reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. The principal areas of estimation reflected in the financial statements are revenue recognition, sales returns and allowances, allowance for doubtful accounts, inventory valuation, recoverability of long-lived assets, income taxes, product warranties, contingencies and litigation, insurance-related items, defined benefit retirement plans and purchase accounting related to acquisitions.
Revenue Recognition
The Company recognizes revenue when persuasive evidence of an arrangement exists, delivery has occurred, the sales price is fixed or determinable, and collectability of the sales price is reasonably assured. For product sales, delivery does not occur until the products have been shipped and risk of loss has been transferred to the customer. Revenue from services is recognized when the services are provided or ratably over the contract term. Some arrangements with customers may include multiple deliverables, including the combination of products and services. In such cases, the Company has identified these as separate elements in accordance with Accounting Standards Codification (“ASC”) 605-25, Revenue Recognition-Multiple-Element Arrangements, and recognizes revenue consistent with the policy for each separate element based on the relative selling price method. Revenues from certain long-term contracts are recognized on the percentage-of-completion method. Percentage-of-completion is measured principally by the percentage of costs incurred to date for each contract to the estimated total costs for such contract at completion. Provisions for estimated losses on uncompleted long-term contracts are made in the period in which such losses are determined. Due to uncertainties inherent in the estimation process, it is reasonably possible that completion costs, including those arising from contract penalty provisions and final contract settlements, will be revised in the near-term. Such revisions to costs and income are recognized in the period in which the revisions are determined.
The Company records allowances for discounts, product returns and customer incentives at the time of sale as a reduction of revenue as such allowances can be reliably estimated based on historical experience and known trends. The Company also offers product warranties and accrues its estimated exposure for warranty claims at the time of sale based upon the length of the warranty period, warranty costs incurred and any other related information known to the Company.
Shipping and Handling Costs
Shipping and handling costs are included in Cost of sales and are recognized as a period expense during the period in which they are incurred.
Advertising Costs
Advertising costs of $15.3 million, $16.1 million and $14.5 million for 2016, 2015 and 2014, respectively, are expensed as incurred within Selling, general and administrative expenses.
36
Cash and Cash Equivalents
The Company considers all highly liquid instruments purchased with an original maturity of 90 days or less to be cash and cash equivalents.
Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses as a result of customers’ inability to make required payments. Management evaluates the aging of the accounts receivable balances, the financial condition of its customers, historical trends and the time outstanding of specific balances to estimate the amount of accounts receivable that may not be collected in the future and records the appropriate provision.
Inventories
The Company states inventories at the lower of cost or market. Cost, which includes material, labor, and factory overhead, is determined on a FIFO basis. We make adjustments to reduce the cost of inventory to its net realizable value, if required, for estimated excess, obsolescence or impaired balances. Factors influencing these adjustments include changes in market demand, product life cycle and engineering changes.
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount, as measured by comparing their net book value to the projected undiscounted future cash flows generated by their use. A long-lived asset impairment exists when the carrying amount of the asset exceeds its fair value. The amount and timing of impairment charges for these assets require the estimation of future cash flows to determine the fair value of the related assets. Impaired assets are recorded at their estimated fair value based on a discounted cash flow analysis. In 2016, 2015, and 2014, the Company concluded that certain long-lived assets had a fair value that was less than the carrying value of the assets, resulting in $0.2 million, $0.8 million and $2.5 million, respectively, of long-lived asset impairment charges.
Goodwill and Indefinite-Lived Intangible Assets
In accordance with ASC 350, Goodwill and Other Intangible Assets, the Company reviews the carrying value of goodwill and indefinite-lived intangible assets annually on October 31, or if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. The Company evaluates the recoverability of these assets based on the estimated fair value of each of the thirteen reporting units and the indefinite-lived intangible assets. See Note 4 for a further discussion on goodwill and intangible assets.
Borrowing Expenses
Expenses incurred in securing and issuing debt are capitalized and included as a reduction of Long-term borrowings. These amounts are amortized over the life of the related borrowing and the related amortization is included in Interest expense.
Earnings per Common Share
Earnings per common share (“EPS”) is computed by dividing net income by the weighted average number of shares of common stock (basic) plus common stock equivalents (diluted) outstanding during the year. Common stock equivalents consist of stock options, which have been included in the calculation of weighted average shares outstanding using the treasury stock method, restricted stock, performance share units, and shares issuable in connection with certain deferred compensation agreements (“DCUs”).
ASC 260, Earnings per Share, concludes that all outstanding unvested share-based payment awards that contain rights to nonforfeitable dividends participate in undistributed earnings with common shareholders. If awards are considered participating securities, the Company is required to apply the two-class method of computing basic and diluted earnings per share. The Company has determined that its outstanding shares of restricted stock are participating securities. Accordingly, EPS was computed using the two-class method prescribed by ASC 260. Net income attributable to common shareholders for the purpose of calculating EPS was reduced by $0.5 million, $0.8 million and $1.3 million in 2016, 2015 and 2014, respectively.
37
Basic weighted average shares outstanding reconciles to diluted weighted average shares outstanding as follows:
| 2016 | 2015 | 2014 | ||||||
| (In thousands) | ||||||||
| Basic weighted average common shares outstanding | 75,803 | 77,126 | 79,715 | |||||
| Dilutive effect of stock options, restricted stock, performance share units and DCUs | 955 | 846 | 1,013 | |||||
| Diluted weighted average common shares outstanding | 76,758 | 77,972 | 80,728 | |||||
Options to purchase approximately 0.9 million, 0.9 million and 0.5 million shares of common stock in 2016, 2015 and 2014, respectively, were not included in the computation of diluted EPS because the effect of their inclusion would have been antidilutive.
Share-Based Compensation
The Company accounts for share-based payments in accordance with ASC 718, Compensation-Stock Compensation. Accordingly, the Company expenses the fair value of awards made under its share-based compensation plans. That cost is recognized in the consolidated financial statements over the requisite service period of the grants. See Note 13 for further discussion on share-based compensation.
Depreciation and Amortization
Property and equipment are stated at cost, with depreciation and amortization provided using the straight-line method over the following estimated useful lives:
| Land improvements | 8 to 12 years |
| Buildings and improvements | 8 to 30 years |
| Machinery, equipment and other | 3 to 12 years |
| Office and transportation equipment | 3 to 10 years |
Certain identifiable intangible assets are amortized over their estimated useful lives using the straight-line method. The estimated useful lives used in the computation of amortization of identifiable intangible assets are as follows:
| Patents | 5 to 17 years |
| Trade names | 10 to 20 years |
| Customer relationships | 6 to 20 years |
| Unpatented technology and other | 6 to 20 years |
Research and Development Expenditures
Costs associated with research and development are expensed in the period incurred and are included in Cost of sales. Research and development expenses, which include costs associated with developing new products and major improvements to existing products, were $39.4 million, $33.6 million and $36.8 million in 2016, 2015 and 2014, respectively.
Foreign Currency
The functional currency of substantially all operations outside the United States is the respective local currency. Accordingly, those foreign currency balance sheet accounts have been translated using the exchange rates in effect as of the balance sheet date. Income statement amounts have been translated using the average exchange rate for the year. The gains and losses resulting from changes in exchange rates from year to year have been reported in Accumulated other comprehensive income (loss) in the Consolidated Balance Sheets. The foreign currency transaction losses (gains) for the periods ending December 31, 2016, 2015 and 2014 were $(6.2) million, $(0.1) million, and $0.9 million, respectively, and are reported within Other (income) expense-net on the Consolidated Statements of Operations. Of the $6.2 million reported as foreign currency transaction gains for the period ending December 31, 2016, $4.7 million was due to intercompany loans established in conjunction with the SFC Koenig acquisition.
38
Income Taxes
Income tax expense includes United States, state, local and international income taxes. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial reporting and the tax basis of existing assets and liabilities and for loss carryforwards. The tax rate used to determine the deferred tax assets and liabilities is the enacted tax rate for the year and manner in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized.
Concentration of Credit Risk
The Company is not dependent on a single customer as its largest customer accounted for less than 2% of net sales for all years presented.
Recently Adopted Accounting Standards
In March 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-09, Improvements to Employee Share-Based Payment Accounting, which simplifies several aspects of the accounting for employee share-based payment transactions, including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as classification in the statement of cash flows. The Company elected to early adopt this standard in the quarter ended March 31, 2016. The Company applied this standard prospectively and thus, prior periods have not been adjusted. The impact of the adoption resulted in the following:
| • | The Company recorded a tax benefit of $6.8 million within Provision for income taxes for the year ended December 31, 2016, related to the excess tax benefit on stock options, restricted stock and performance share units. Prior to adoption this amount would have been recorded as a reduction of additional paid-in capital. The adoption of this standard could create volatility in the Company’s effective tax rate going forward. |
| • | The Company elected not to change our policy on accounting for forfeitures and continued to estimate the total number of awards for which the requisite service period will not be rendered. |
| • | The Company no longer reclassifies the excess tax benefit from operating activities to financing activities in the statement of cash flows. |
| • | The Company excluded the excess tax benefits from the assumed proceeds available to repurchase shares in the computation of our diluted earnings per share for the year ended December 31, 2016. This increased our diluted weighted average common shares outstanding by 127 thousand shares for the year ended December 31, 2016. |
In April 2015, the FASB issued ASU 2015-03, Interest-Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs, which simplifies the presentation of debt issuance costs. Under ASU 2015-03, an entity presents such costs in the balance sheet as a direct deduction from the related debt liability rather than as an asset. Amortization of the costs is reported as interest expense. This standard is effective for fiscal years beginning after December 15, 2015. The Company elected to early adopt this guidance effective in the fourth quarter of fiscal year 2015.
In April 2014, the FASB issued ASU 2014-08, Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity, which includes amendments that change the requirements for reporting discontinued operations. Under the new guidance, only disposals representing a strategic shift in operations with a major effect on the organization’s operations and financial results should be presented as discontinued operations. Additionally, the ASU requires expanded disclosures about disposal transactions that do not meet the discontinued operations criteria. The Company adopted the standard effective January 1, 2015 and the adoption did not impact the consolidated financial position, results of operations or cash flows of the Company. The Company concluded that none of the divestitures that took place during the years ended December 31, 2016 and 2015 met the new criteria for reporting discontinued operations. The Company did include required disclosures of disposals of components of an entity in Note 2.
New Accounting Pronouncements
In January 2017, the FASB issued ASU 2017-04, Simplifying the Test for Goodwill Impairment, which eliminates Step 2 from the goodwill impairment test. Under this ASU, if the carrying amount of a reporting unit exceeds its fair value, an impairment loss will be recognized in an amount equal to the excess, limited to the total amount of goodwill allocated to the reporting unit. This ASU also eliminated the requirements for any reporting unit with a zero or negative carrying amount to perform a qualitative
39
assessment and, if it fails that qualitative test, to perform Step 2 of the goodwill impairment test. In addition, companies will be required to disclose the amount of goodwill allocated to each reporting unit with a zero or negative carrying amount of net assets. The update is effective for annual and any interim impairment tests for periods beginning after December 15, 2019, and early adoption is permitted. The Company does not believe the guidance will have a material impact on our consolidated financial statements.
In October 2016, the FASB issued ASU 2016-16, Intra-Entity Transfers of Assets Other Than Inventory, which amends ASC 740, Income Taxes. This ASU requires that the income tax consequences of an intra-entity asset transfer other than inventory are recognized at the time of the transfer. An entity will continue to recognize the income tax consequences of an intercompany transfer of inventory when the inventory is sold to a third party. The update is effective for financial statements issued for fiscal years beginning after December 15, 2017, and early adoption is permitted. The ASU requires adoption on a modified-retrospective basis through a cumulative adjustment to retained earnings at the beginning of the period of adoption. The Company is currently assessing the impact that adopting this new accounting standard will have on its consolidated financial statements and footnote disclosures.
In August 2016, the FASB issued ASU 2016-15, Classification of Certain Cash Receipts and Cash Payments (a consensus of the FASB Emerging Issues Task Force). This ASU addresses the following eight specific cash flow issues: Debt prepayment or debt extinguishment costs; settlement of zero-coupon debt instruments or other debt instruments with coupon interest rates that are insignificant in relation to the effective interest rate of the borrowing; contingent consideration payments made after a business combination; proceeds from the settlement of insurance claims; proceeds from the settlement of corporate-owned life insurance policies (including bank-owned life insurance policies); distributions received from equity method investees; beneficial interests in securitization transactions; and separately identifiable cash flows and application of the predominance principle. This standard is effective for fiscal years beginning after December 15, 2017. The Company does not believe the guidance will have a material impact on our consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases, which sets out the principles for the recognition, measurement, presentation and disclosure of leases for both parties to a contract (i.e. lessees and lessors). The standard introduces a new lessee model that will require most leases to be recorded on the balance sheet and eliminates the required use of bright line tests in current U.S. GAAP for determining lease classification. The new standard requires lessors to account for leases using an approach that is substantially equivalent to existing guidance for sales-type leases, direct financing leases and operating leases. This standard is effective for fiscal years beginning after December 15, 2018 and interim periods within those fiscal years. Companies are permitted to adopt the standard early and a modified retrospective application is required. The Company is currently evaluating the impact of adopting the new guidance on our consolidated financial statements.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers, which will replace numerous requirements in U.S. GAAP, including industry-specific requirements, and provide companies with a new five-step model for recognizing revenue from contracts with customers. Under ASU 2014-09, an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This ASU also requires disclosures sufficient to enable users to understand the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers, including qualitative and quantitative disclosures about contracts with customers, significant judgments and changes in judgments, and assets recognized from the costs to obtain or fulfill a contract. This standard is effective for fiscal years beginning after December 15, 2017, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption. The FASB has also issued the following standards which clarify ASU 2014-09 and have the same effective date as the original standard: ASU 2016-08, Revenue from Contracts with Customers: Principal versus Agent Considerations (Reporting Revenue Gross versus Net); ASU 2016-10, Revenue from Contracts with Customers: Identifying Performance Obligations and Licensing; ASU 2016-12, Revenue from Contracts with Customers: Narrow-Scope Improvements and Practical Expedients; and ASU 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers.
In 2016, we established an implementation team and analyzed the impact of the standard by surveying business units and reviewing contracts to identify potential differences that may result from applying the requirements of the new standard. We have made significant progress on our contract reviews during 2016 and the first quarter of 2017. While we are continuing to assess all potential impacts of the new standard, we currently believe that the most significant potential change relates to contracts for the development, manufacture and sale of customized products in our Health & Science Technologies segment. Due to the complexity of certain contracts in our Health & Science Technologies segment, the actual revenue recognition treatment required under the standard will be dependent on contract-specific terms. However, under the new standard we expect revenue recognition
40
to remain substantially unchanged as the contract reviews support the recognition of revenue at a point in time, which is consistent with our current revenue recognition model. We also expect revenue related to the Fluid & Metering Technologies segment and the Fire & Safety/Diversified Products segment to remain substantially unchanged. The implementation team has reported these initial findings and progress of the project to the Audit Committee. The Company is still evaluating the impact of the new guidance on our consolidated financial statements and has not yet determined the method by which we will adopt the standard in 2018.
2. Acquisitions and Divestitures
All of the Company’s acquisitions have been accounted for under ASC 805, Business Combinations. Accordingly, the accounts of the acquired companies, after adjustments to reflect fair values assigned to assets and liabilities, have been included in the Company’s consolidated financial statements from their respective dates of acquisition. The results of operations of the acquired companies have been included in the Company’s consolidated results since the date of each acquisition. Supplemental pro forma information has not been provided as the acquisitions did not have a material impact on the Company’s consolidated results of operations individually or in the aggregate.
2016 Acquisitions
On March 16, 2016, the Company acquired the stock of Akron Brass Holding Corporation (“Akron Brass”), a producer of a large array of engineered life–safety products for the safety and emergency response markets, which includes apparatus valves, monitors, nozzles, specialty lighting, electronic vehicle–control systems and firefighting hand tools. The business was acquired to complement and create synergies with our existing Hale, Class 1, and Godiva businesses. Headquartered in Wooster, Ohio, Akron Brass had annual revenues in its most recent fiscal year of approximately $120 million and operates in our Fire & Safety/Diversified Products segment. Akron Brass was acquired for cash consideration of $221.4 million. The purchase price was funded with borrowings under the Company’s revolving facilities. Goodwill and intangible assets recognized as part of the transaction were $124.6 million and $90.4 million, respectively. The goodwill is not deductible for tax purposes.
On July 1, 2016, the Company acquired the stock of AWG Fittings GmbH (“AWG Fittings”), a producer of engineered products for the safety and emergency response markets, including valves, monitors and nozzles. The business was acquired to complement and create synergies with our existing Hale, Class 1, Godiva and Akron Brass businesses. Headquartered in Ballendorf, Germany, AWG Fittings had annual revenues in its most recent fiscal year of approximately $40 million and operates in our Fire & Safety/Diversified Products segment. AWG Fittings was acquired for cash consideration of $47.5 million (€42.8 million). The purchase price was funded with cash on hand. Goodwill and intangible assets recognized as part of the transaction were $22.0 million and $10.3 million, respectively. The goodwill is not deductible for tax purposes.
On August 31, 2016, the Company acquired the stock of SFC Koenig AG (“SFC Koenig”), a producer of highly engineered expanders and check valves for critical applications across the transportation, hydraulic, aviation and medical markets. Headquartered in Dietikon, Switzerland, SFC Koenig had annual revenues in its most recent fiscal year of approximately $63 million and operates in our Health & Science Technologies segment. SFC Koenig was acquired for cash consideration of $241.1 million (€215.9 million). The purchase price was funded with cash on hand and borrowings under the Company’s revolving facilities. Goodwill and intangible assets recognized as part of the transaction were $143.7 million and $117.0 million, respectively. The goodwill is not deductible for tax purposes.
The Company made initial allocations of the purchase price for the Akron Brass, AWG Fittings and SFC Koenig acquisitions as of the acquisition date based on its understanding of the fair value of the acquired assets and assumed liabilities. These nonrecurring fair value measurements are classified as Level 3 in the fair value hierarchy. As the Company obtains additional information about these assets and liabilities and learns more about the newly acquired businesses, we will refine the estimates of fair value and more accurately allocate the purchase price. Only items identified as of the acquisition date are considered for subsequent adjustment. The Company is in the process of finalizing purchase price allocations for the Akron Brass, AWG Fittings and SFC Koenig acquisitions and will make appropriate adjustments to the purchase price allocations prior to the completion of the measurement period, as required.
41
The preliminary allocation of the acquisition costs to the assets acquired and liabilities assumed, based on their estimated fair values at their respective acquisition dates, are as follows:
| Akron Brass | AWG Fittings | SFC Koenig | Total | |||||||||||||
| (In thousands) | ||||||||||||||||
| Accounts receivable | $ | 14,523 | $ | 5,952 | $ | 9,425 | $ | 29,900 | ||||||||
| Inventory | 29,157 | 11,766 | 21,247 | 62,170 | ||||||||||||
| Other assets, net of cash acquired | 446 | 565 | 4,501 | 5,512 | ||||||||||||
| Property, plant and equipment | 12,195 | 6,847 | 5,125 | 24,167 | ||||||||||||
| Goodwill | 124,643 | 22,031 | 143,719 | 290,393 | ||||||||||||
| Intangible assets | 90,400 | 10,279 | 116,998 | 217,677 | ||||||||||||
| Deferred income taxes | — | 3,515 | — | 3,515 | ||||||||||||
| Total assets acquired | 271,364 | 60,955 | 301,015 | 633,334 | ||||||||||||
| Current liabilities | (7,081 | ) | (5,017 | ) | (10,088 | ) | (22,186 | ) | ||||||||
| Deferred income taxes | (36,439 | ) | — | (41,370 | ) | (77,809 | ) | |||||||||
| Other noncurrent liabilities | (6,445 | ) | (8,444 | ) | (8,449 | ) | (23,338 | ) | ||||||||
| Net assets acquired | $ | 221,399 | $ | 47,494 | $ | 241,108 | $ | 510,001 | ||||||||
Acquired intangible assets consist of trade names, customer relationships and unpatented technology. The goodwill recorded for the acquisitions reflects the strategic fit, revenue and earnings growth potential of these businesses.
Of the $217.7 million of acquired intangible assets, $28.8 million was assigned to the Akron Brass trade name and is not subject to amortization. The acquired intangible assets and weighted average amortization periods are as follows:
| (In thousands, except weighted average life) | Total | Weighted Average Life | |||
| Trade names | $ | 14,078 | 15 | ||
| Customer relationships | 134,519 | 13 | |||
| Unpatented technology | 40,280 | 13 | |||
| Amortized intangible assets | 188,877 | ||||
| Indefinite lived - Akron Brass trade name | 28,800 | ||||
| Total acquired intangible assets | $ | 217,677 | |||
The Company incurred $4.7 million of acquisition-related transaction costs in 2016. These costs were recorded in Selling, general and administrative expenses and were related to completed transactions, pending transactions and potential transactions, including transactions that ultimately were not completed. The Company also incurred $14.7 million of non-cash acquisition fair value inventory step-up charges associated with the completed 2016 acquisitions. These charges were recorded in Cost of sales.
2015 Acquisitions
On May 29, 2015, the Company acquired the stock of Novotema, SpA (“Novotema”), a leader in the design, manufacture and sale of specialty sealing solutions for use in the building products, gas control, transportation, industrial and water markets. The business was acquired to complement and create synergies with our existing Sealing Solutions platform. Located in Villongo, Italy, Novotema operates in our Health & Science Technologies segment. Novotema was acquired for cash consideration of $61.1 million (€56 million). The entire purchase price was funded with cash on hand. Goodwill and intangible assets recognized as part of this transaction were $34.3 million and $20.0 million, respectively. The $34.3 million of goodwill is not deductible for tax purposes.
On June 10, 2015, the Company acquired the stock of Alfa Valvole, S.r.l (“Alfa Valvole”), a leader in the design, manufacture and sale of specialty valve products for use in the chemical, petro-chemical, energy and sanitary markets. The business was acquired to expand our valve capabilities. Located in Casorezzo, Italy, Alfa Valvole operates in our Fluid & Metering Technologies segment. Alfa Valvole was acquired for cash consideration of $112.6 million (€99.8 million). The entire purchase price was funded with cash on hand. Goodwill and intangible assets recognized as part of this transaction were $69.6 million and $32.1 million, respectively. The $69.6 million of goodwill is not deductible for tax purposes.
42
On July 1, 2015, the Company acquired the membership interests of CiDRA Precision Services, LLC (“CPS” or “CiDRA Precision Services”), a leader in the design, manufacture and sale of microfluidic components serving the life science, health and industrial markets. The business was acquired to provide a critical building block to our emerging microfluidic and nanofludics capabilities. Located in Wallingford, Connecticut, CPS operates in our Health & Science Technologies segment. CPS was acquired for an aggregate purchase price of $24.2 million, consisting of $19.5 million in cash and contingent consideration valued at $4.7 million as of the opening balance sheet date. The contingent consideration was based on the achievement of financial objectives during the 12-month period following the close. Based on potential outcomes, the undiscounted amount of all the future payments that the Company could have been required to make under the contingent consideration arrangement was between $0 and $5.5 million. During the six months ended June 30, 2016, the Company re-evaluated the contingent consideration arrangement and fully reversed the $4.7 million liability based on CPS’s actual operating results from July 1, 2015 to June 30, 2016. The $4.7 million reversal was recognized as a benefit within Selling, general and administrative expenses, of which $3.7 million was recognized in March 2016 and the remaining $1.0 million was recognized in June 2016. The entire purchase price was funded with cash on hand. Goodwill and intangible assets recognized as part of this transaction were $9.7 million and $12.3 million, respectively. The $9.7 million of goodwill is deductible for tax purposes.
On December 1, 2015, the Company acquired the assets of a complementary product line within our Fluid & Metering Technologies segment. The purchase price and goodwill associated with this transaction were $1.9 million and $0.7 million, respectively.
The purchase prices for Novotema, Alfa Valvole and CPS have been allocated to the assets acquired and liabilities assumed based on the estimated fair values at the date of the acquisitions.
The allocation of the acquisition costs to the assets acquired and liabilities assumed, based on their estimated fair values at their respective acquisition dates, is as follows:
| Novotema | Alfa Valvole | CPS | Other | Total | |||||||||||||||
| (In thousands) | |||||||||||||||||||
| Accounts receivable | $ | 8,029 | $ | 13,487 | $ | 945 | $ | — | $ | 22,461 | |||||||||
| Inventory | 2,886 | 11,036 | 442 | 1,102 | 15,466 | ||||||||||||||
| Other assets, net of cash acquired | 1,866 | 3,367 | 79 | — | 5,312 | ||||||||||||||
| Property, plant and equipment | 11,844 | 8,395 | 1,105 | — | 21,344 | ||||||||||||||
| Goodwill | 34,316 | 69,568 | 9,739 | 748 | 114,371 | ||||||||||||||
| Intangible assets | 20,011 | 32,058 | 12,290 | — | 64,359 | ||||||||||||||
| Total assets acquired | 78,952 | 137,911 | 24,600 | 1,850 | 243,313 | ||||||||||||||
| Current liabilities | (7,760 | ) | (11,279 | ) | (420 | ) | — | (19,459 | ) | ||||||||||
| Deferred income taxes | (7,803 | ) | (12,622 | ) | — | — | (20,425 | ) | |||||||||||
| Other noncurrent liabilities | (2,291 | ) | (1,420 | ) | — | — | (3,711 | ) | |||||||||||
| Net assets acquired | $ | 61,098 | $ | 112,590 | $ | 24,180 | $ | 1,850 | $ | 199,718 | |||||||||
Acquired intangible assets consist of trade names, customer relationships and unpatented technology. The goodwill recorded for the acquisitions reflects the strategic fit, revenue and earnings growth potential of these businesses.
The acquired intangible assets and weighted average amortization periods are as follows:
| (In thousands, except weighted average life) | Total | Weighted Average Life | |||
| Trade names | $ | 9,247 | 15 | ||
| Customer relationships | 44,401 | 12 | |||
| Unpatented technology | 10,711 | 8 | |||
| Total acquired intangible assets | $ | 64,359 | |||
43
The Company incurred $2.6 million of acquisition-related transaction costs in 2015. These costs were recorded in Selling, general and administrative expense and were related to completed transactions, pending transactions and potential transactions, including transactions that ultimately were not completed. The Company also incurred $3.4 million of non-cash acquisition fair value inventory charges in 2015. These charges were recorded in Cost of sales.
2014 Acquisitions
On April 28, 2014, the Company acquired the stock of Aegis Flow Technologies (“Aegis”), a producer of specialty chemical processing valves for use in the chemical, petro-chemical, chlor-alkali, and pulp/paper industries. Located in Geismar, Louisiana, Aegis operates in our Fluid & Metering Technologies segment. Aegis was acquired for cash consideration of approximately $25 million. The entire purchase price was funded with borrowings under the Company’s revolving facilities. Goodwill and intangible assets recognized as part of this transaction were $7.7 million and $8.8 million, respectively. The $7.7 million of goodwill is deductible for tax purposes.
The purchase price for Aegis has been allocated to the assets acquired and liabilities assumed based on the estimated fair values at the date of the acquisition.
The allocation of the acquisition costs to the assets acquired and liabilities assumed, based on their estimated fair values at the acquisition date, is as follows:
| (In thousands) | |||
| Accounts receivable | $ | 1,147 | |
| Inventory | 6,230 | ||
| Other current assets, net of cash acquired | 232 | ||
| Property, plant and equipment | 2,988 | ||
| Goodwill | 7,711 | ||
| Intangible assets | 8,770 | ||
| Total assets acquired | 27,078 | ||
| Total liabilities assumed | (1,633 | ) | |
| Net assets acquired | $ | 25,445 | |
Acquired intangible assets consist of trade names, customer relationships and unpatented technology. The goodwill recorded for the acquisition reflects the strategic fit, revenue and earnings growth potential of this business.
The acquired intangible assets and weighted average amortization periods are as follows:
| (In thousands, except weighted average life) | Total | Weighted Average Life | |||
| Trade names | $ | 3,304 | 15 | ||
| Customer relationships | 4,393 | 14 | |||
| Unpatented technology | 1,073 | 8 | |||
| Total acquired intangible assets | $ | 8,770 | |||
The Company incurred $1.7 million of acquisition-related transaction costs in 2014. These costs were recorded in Selling, general and administrative expense and were related to completed transactions, pending transactions and potential transactions, including transactions that ultimately were not completed. The Company incurred $1.3 million of non-cash acquisition fair value inventory charges in 2014. These charges were recorded in Cost of sales.
44
2016 Divestitures
The Company periodically reviews its operations for businesses which may no longer be aligned with its strategic objectives and focus on core business and customers. Any resulting gain or loss recognized due to divestitures is recorded within Loss (gain) on sale of businesses - net.
On July 29, 2016, the Company completed the sale of its Hydra-Stop product line for $15.0 million in cash, resulting in a pre-tax gain on the sale of $5.8 million. In addition, the Company can earn up to $2 million based on the achievement of financial objectives for net sales in 2016 and 2017. The Company recorded $2.8 million of income tax expense associated with this transaction during the year ended December 31, 2016. The results of Hydra-Stop were reported within the Fluid & Metering Technologies segment and generated $7.5 million of revenues in 2016 through the date of sale.
On September 9, 2016, the Company completed the sale of its Melles Griot KK (“CVI Japan”) subsidiary for $17.5 million in cash, resulting in a pre-tax loss on the sale of $7.9 million. The Company recorded $3.4 million of income tax benefit associated with this transaction during the year ended December 31, 2016. The results of CVI Japan were reported within the Health & Science Technologies segment and generated $13.1 million of revenues in 2016 through the date of sale.
On October 10, 2016, the Company completed the sale of its IETG and 40Seven subsidiaries for $2.7 million in cash, resulting in a pre-tax loss on the sale of $4.2 million. There was no income tax impact associated with this transaction. The results of IETG and 40Seven were reported within the Fluid & Metering Technologies segment and generated $8.3 million of revenues in 2016 through the date of sale.
On December 30, 2016, the Company completed the sale of its Korea Electro-Optics Co., Ltd. (“CVI Korea”) subsidiary for $3.8 million in cash, resulting in a pre-tax loss on the sale of $16.0 million. The Company recorded $9.1 million of income tax benefit associated with this transaction during the year ended December 31, 2016. The results of CVI Korea were reported within the Health & Science Technologies segment and generated $11.7 million of revenues in 2016 through the date of sale.
2015 Divestiture
On July 31, 2015, the Company completed the sale of its Ismatec product line for $27.7 million in cash, resulting in a pre-tax gain on the sale of $18.1 million. The Company recorded $4.8 million of income tax expense associated with this transaction during the year ended December 31, 2015. The results of Ismatec were reported in the Health & Science Technologies segment and generated $5.3 million of revenues in 2015 through the date of sale.
45
3. Balance Sheet Components
| December 31, | |||||||
| 2016 | 2015 | ||||||
| (In thousands) | |||||||
| RECEIVABLES | |||||||
| Customers | $ | 275,250 | $ | 262,304 | |||
| Other | 5,641 | 5,508 | |||||
| Total | 280,891 | 267,812 | |||||
| Less allowance for doubtful accounts | 8,078 | 7,812 | |||||
| Total receivables — net | $ | 272,813 | $ | 260,000 | |||
| INVENTORIES | |||||||
| Raw materials and components parts | $ | 154,278 | $ | 141,671 | |||
| Work in process | 34,832 | 32,387 | |||||
| Finished goods | 63,749 | 65,066 | |||||
| Total | $ | 252,859 | $ | 239,124 | |||
| PROPERTY, PLANT AND EQUIPMENT | |||||||
| Land and improvements | $ | 33,883 | $ | 34,343 | |||
| Buildings and improvements | 169,261 | 157,946 | |||||
| Machinery, equipment and other | 328,779 | 331,146 | |||||
| Office and transportation equipment | 98,355 | 97,250 | |||||
| Construction in progress | 10,373 | 13,377 | |||||
| Total | 640,651 | 634,062 | |||||
| Less accumulated depreciation and amortization | 392,835 | 393,117 | |||||
| Total property, plant and equipment — net | $ | 247,816 | $ | 240,945 | |||
| ACCRUED EXPENSES | |||||||
| Payroll and related items | $ | 67,600 | $ | 67,209 | |||
| Management incentive compensation | 16,339 | 12,599 | |||||
| Income taxes payable | 8,808 | 3,836 | |||||
| Insurance | 9,416 | 9,505 | |||||
| Warranty | 5,628 | 7,936 | |||||
| Deferred revenue | 12,607 | 9,885 | |||||
| Restructuring | 3,893 | 6,636 | |||||
| Liability for uncertain tax positions | 1,366 | 3,498 | |||||
| Accrued interest | 1,663 | 1,230 | |||||
| Contingent consideration for acquisition | — | 4,705 | |||||
| Other | 25,532 | 26,633 | |||||
| Total accrued expenses | $ | 152,852 | $ | 153,672 | |||
| OTHER NONCURRENT LIABILITIES | |||||||
| Pension and retiree medical obligations | $ | 93,604 | $ | 76,190 | |||
| Liability for uncertain tax positions | 2,623 | 4,252 | |||||
| Deferred revenue | 2,442 | 3,763 | |||||
| Other | 22,561 | 18,160 | |||||
| Total other noncurrent liabilities | $ | 121,230 | $ | 102,365 | |||
46
The valuation and qualifying account activity for the years ended December 31, 2016, 2015 and 2014 is as follows:
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
ALLOWANCE FOR DOUBTFUL ACCOUNTS (1) | |||||||||||
| Beginning balance January 1 | $ | 7,812 | $ | 6,961 | $ | 5,841 | |||||
| Charged to costs and expenses, net of recoveries | 1,425 | 1,556 | 2,643 | ||||||||
| Utilization | (1,585 | ) | (1,009 | ) | (1,195 | ) | |||||
| Currency translation and other | 426 | 304 | (328 | ) | |||||||
| Ending balance December 31 | $ | 8,078 | $ | 7,812 | $ | 6,961 | |||||
| (1) | Includes provision for doubtful accounts, sales returns and sales discounts granted to customers. |
4. Goodwill and Intangible Assets
The changes in the carrying amount of goodwill for 2016 and 2015, by reportable business segment, were as follows:
Fluid & Metering Technologies | Health & Science Technologies | Fire & Safety/ Diversified Products | Total | ||||||||||||
| (In thousands) | |||||||||||||||
| Goodwill | $ | 544,870 | $ | 713,285 | $ | 263,753 | $ | 1,521,908 | |||||||
| Accumulated goodwill impairment losses | (20,721 | ) | (149,820 | ) | (30,090 | ) | (200,631 | ) | |||||||
| Balance at January 1, 2015 | 524,149 | 563,465 | 233,663 | 1,321,277 | |||||||||||
| Foreign currency translation | (11,318 | ) | (6,155 | ) | (12,509 | ) | (29,982 | ) | |||||||
| Acquisitions | 71,939 | 43,508 | — | 115,447 | |||||||||||
| Disposition of businesses | — | (10,213 | ) | — | (10,213 | ) | |||||||||
| Balance at December 31, 2015 | 584,770 | 590,605 | 221,154 | 1,396,529 | |||||||||||
| Foreign currency translation | (5,951 | ) | (23,559 | ) | (7,972 | ) | (37,482 | ) | |||||||
| Acquisitions | — | 143,719 | 146,674 | 290,393 | |||||||||||
| Disposition of businesses | (3,759 | ) | (12,013 | ) | — | (15,772 | ) | ||||||||
| Acquisition adjustments | (1,623 | ) | 547 | — | (1,076 | ) | |||||||||
| Balance at December 31, 2016 | $ | 573,437 | $ | 699,299 | $ | 359,856 | $ | 1,632,592 | |||||||
ASC 350 requires that goodwill be tested for impairment at the reporting unit level on an annual basis and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of the reporting unit below its carrying value. Goodwill represents the purchase price in excess of the net amount assigned to assets acquired and liabilities assumed.
Goodwill and other acquired intangible assets with indefinite lives were tested for impairment as of October 31, 2016, the Company’s annual impairment date. In assessing the fair value of the reporting units, the Company considers both the market approach and the income approach. Under the market approach, the fair value of the reporting unit is determined by the respective trailing twelve month EBITDA and the forward looking 2017 EBITDA (generally 50% each), based on multiples of comparable public companies. The market approach is dependent on a number of significant management assumptions including forecasted EBITDA and selected market multiples. Under the income approach, the fair value of the reporting unit is determined based on the present value of estimated future cash flows. The income approach is dependent on a number of significant management assumptions including estimates of operating results, capital expenditures, net working capital requirements, long-term growth rates and discount rates. Weighting was equally attributed to both the market and the income approaches (50% each) in arriving at the fair value of the reporting units.
47
In addition to performing our annual impairment test, we also performed interim impairment tests due to the divestitures in the third and fourth quarters of 2016 as well as the reorganization of certain reporting units. As a result of these impairment tests, the Company concluded that the reporting units had fair values in excess of their carrying values.
The following table provides the gross carrying value and accumulated amortization for each major class of intangible asset at December 31, 2016 and 2015:
| At December 31, 2016 | At December 31, 2015 | ||||||||||||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Net | Weighted Average Life | Gross Carrying Amount | Accumulated Amortization | Net | |||||||||||||||||||
| (In thousands) | (In thousands) | ||||||||||||||||||||||||
| Amortized intangible assets: | |||||||||||||||||||||||||
| Patents | $ | 9,856 | $ | (6,635 | ) | $ | 3,221 | 11 | $ | 10,202 | $ | (6,175 | ) | $ | 4,027 | ||||||||||
| Trade names | 113,428 | (42,653 | ) | 70,775 | 16 | 110,658 | (38,696 | ) | 71,962 | ||||||||||||||||
| Customer relationships | 369,087 | (161,065 | ) | 208,022 | 12 | 257,071 | (144,134 | ) | 112,937 | ||||||||||||||||
| Non-compete agreements | — | — | — | 3 | 794 | (775 | ) | 19 | |||||||||||||||||
| Unpatented technology | 106,747 | (44,516 | ) | 62,231 | 12 | 78,562 | (42,745 | ) | 35,817 | ||||||||||||||||
| Other | 6,527 | (6,172 | ) | 355 | 10 | 6,554 | (5,579 | ) | 975 | ||||||||||||||||
| Total amortized intangible assets | 605,645 | (261,041 | ) | 344,604 | 463,841 | (238,104 | ) | 225,737 | |||||||||||||||||
| Indefinite-lived intangible assets: | |||||||||||||||||||||||||
| Banjo trade name | 62,100 | — | 62,100 | 62,100 | — | 62,100 | |||||||||||||||||||
| Akron Brass trade name | 28,800 | — | 28,800 | — | — | — | |||||||||||||||||||
| Total intangible assets | $ | 696,545 | $ | (261,041 | ) | $ | 435,504 | $ | 525,941 | $ | (238,104 | ) | $ | 287,837 | |||||||||||
The Banjo trade name is an indefinite-lived intangible asset which is tested for impairment on an annual basis in accordance with ASC 350 or more frequently if events or changes in circumstances indicate that the asset might be impaired. The Company uses the relief-from-royalty method, a form of the income approach, to determine the fair value of the Banjo trade name. The relief-from-royalty method is dependent on a number of significant management assumptions, including estimates of revenues, royalty rates and discount rates.
The Akron Brass trade name is an indefinite-lived intangible asset that was acquired as a result of the Akron Brass acquisition in March 2016 and is tested for impairment on an annual basis in accordance with ASC 350 or more frequently if events or changes in circumstances indicate that the asset might be impaired. The Company uses the relief-from-royalty method, a form of the income approach, to determine the fair value of the Akron Brass trade name. The relief-from-royalty method is dependent on a number of significant management assumptions, including estimates of revenues, royalty rates and discount rates.
In 2016 and 2015, there were no events that occurred or circumstances that changed that would have required a review other than as of our annual test date. Based on the results of our measurement as of October 31, 2016, the fair value of the Banjo trade name was more than 25% in excess of the carrying value and the fair value of the Akron Brass trade name was near its carrying value as a result of the acquisition of this business in March 2016.
Amortization of intangible assets was $49.0 million, $42.4 million and $43.2 million in 2016, 2015 and 2014, respectively. Based on the intangible asset balances as of December 31, 2016, amortization expense is expected to approximate $44.8 million in 2017, $36.5 million in 2018, $33.1 million in 2019, $31.7 million in 2020 and $30.8 million in 2021.
48
5. Borrowings
Borrowings at December 31, 2016 and 2015 consisted of the following:
| 2016 | 2015 | ||||||
| (In thousands) | |||||||
| Revolving Facility | $ | 169,579 | $ | 195,000 | |||
| 4.5% Senior Notes, due December 2020 | 300,000 | 300,000 | |||||
| 4.2% Senior Notes, due December 2021 | 350,000 | 350,000 | |||||
| 3.2% Senior Notes, due June 2023 | 100,000 | — | |||||
| 3.37% Senior Notes, due June 2025 | 100,000 | — | |||||
| Other borrowings | 1,294 | 2,436 | |||||
| Total borrowings | 1,020,873 | 847,436 | |||||
| Less current portion | 1,046 | 1,087 | |||||
| Less deferred debt issuance costs | 4,399 | 5,203 | |||||
| Less unaccreted debt discount | 1,193 | 1,439 | |||||
| Total long-term borrowings | $ | 1,014,235 | $ | 839,707 | |||
On June 13, 2016, the Company completed a private placement of $100 million aggregate principal amount of 3.20% Senior Notes due June 13, 2023 and $100 million aggregate principal amount of 3.37% Senior Notes due June 13, 2025 (collectively, the “Notes”) pursuant to a Note Purchase Agreement, dated June 13, 2016 (the “Purchase Agreement”). Each series of Notes bears interest at the stated amount per annum, which is payable semi-annually in arrears on each June 13th and December 13th. The Notes are unsecured obligations of the Company and rank pari passu in right of payment with all of the Company’s other unsecured, unsubordinated debt. The Company may at any time prepay all, or any portion of the Notes; provided that such portion is greater than 5% of the aggregate principal amount of Notes then outstanding. In the event of a prepayment, the Company will pay an amount equal to par plus accrued interest plus a make-whole amount. In addition, the Company may repurchase the Notes by making an offer to all holders of the Notes, subject to certain conditions.
The Purchase Agreement contains certain covenants that restrict the Company’s ability to, among other things, transfer or sell assets, incur indebtedness, create liens, transact with affiliates and engage in certain mergers or consolidations or other change of control transactions. In addition, the Company must comply with a leverage ratio and interest coverage ratio, as further described below, and the Purchase Agreement also limits the outstanding principal amount of priority debt that may be incurred by the Company to 15% of consolidated assets. The Purchase Agreement provides for customary events of default. In the case of an event of default arising from specified events of bankruptcy or insolvency, all of the outstanding Notes will become due and payable immediately without further action or notice. In the case of payment event of default, any holder of the Notes affected thereby may declare all the Notes held by it due and payable immediately. In the case of any other event of default, a majority of the holders of Notes may declare all of the Notes to be due and payable immediately.
On June 23, 2015, the Company entered into a credit agreement (the “Credit Agreement”) along with certain of its subsidiaries, as borrowers (the “Borrowers”), Bank of America, N.A., as administrative agent, swing line lender and an issuer of letters of credit, with other agents party thereto. The Credit Agreement replaces the Company’s existing five-year $700 million credit agreement, dated as of June 27, 2011, which was due to expire on June 27, 2016.
The Credit Agreement consists of a revolving credit facility (the “Revolving Facility”) in an aggregate principal amount of $700 million, with a final maturity date of June 23, 2020. The maturity date may be extended under certain conditions for an additional one-year term. Up to $75 million of the Revolving Facility is available for the issuance of letters of credit. Additionally, up to $50 million of the Revolving Facility is available to the Company for swing line loans, available on a same-day basis.
Proceeds of the Revolving Facility are available for use by the Borrowers for acquisitions, working capital and other general corporate purposes, including refinancing existing debt of the Company and its subsidiaries. The Company may request increases in the lending commitments under the Credit Agreement, but the aggregate lending commitments pursuant to such increases may not exceed $350 million. The Company has the right, subject to certain conditions set forth in the Credit Agreement, to designate
certain foreign subsidiaries of the Company as borrowers under the Credit Agreement. In connection with any such designation,
the Company is required to guarantee the obligations of any such subsidiaries.
49
Borrowings under the Credit Agreement bear interest at either an alternate base rate or an adjusted LIBOR rate plus, in each case, an applicable margin. Such applicable margin is based on the Company’s senior, unsecured, long-term debt rating and can range from .005% to 1.50%. Based on the Company’s credit rating at December 31, 2016, the applicable margin was 1.10% resulting in a weighted average interest rate of 1.50% at December 31, 2016. Interest is payable (a) in the case of base rate loans, quarterly, and (b) in the case of LIBOR rate loans, on the maturity date of the borrowing, or quarterly from the effective date for borrowings exceeding three months.
The Credit Agreement requires payment to the lenders of a facility fee based upon (a) the amount of the lenders’ commitments under the credit facility from time to time and (b) the applicable corporate credit ratings of the Company. Voluntary prepayments of any loans and voluntary reductions of the unutilized portion of the commitments under the credit facility are permissible without penalty, subject to break funding payments and minimum notice and minimum reduction amount requirements.
The negative covenants include, among other things, limitations (each of which is subject to customary exceptions for financings of this type) on our ability to grant liens; enter into transactions resulting in fundamental changes (such as mergers or sales of all or substantially all of the assets of the Company); restrict subsidiary dividends or other subsidiary distributions; enter into transactions with the Company’s affiliates; and incur certain additional subsidiary debt.
The Credit Agreement also contains customary events of default (subject to grace periods, as appropriate) including among others: nonpayment of principal, interest or fees; breach of the representations or warranties in any material respect; breach of the financial, affirmative or negative covenants; payment default on, or acceleration of, other material indebtedness; bankruptcy or insolvency; material judgments entered against the Company or any of its subsidiaries; certain specified events under the Employee Retirement Income Security Act of 1974, as amended; certain changes in control of the Company; and the invalidity or unenforceability of the Credit Agreement or other documents associated with the Credit Agreement.
At December 31, 2016, $169.6 million was outstanding under the Revolving Facility, with $9.2 million of outstanding letters of credit, resulting in net available borrowing capacity under the Revolving Facility at December 31, 2016 of approximately $521.2 million.
On December 9, 2011, the Company completed a public offering of $350.0 million 4.2% senior notes due December 15, 2021 (“4.2% Senior Notes”). The net proceeds from the offering of $346.2 million, after deducting a $0.9 million issuance discount, a $2.3 million underwriting commission and $0.6 million offering expenses, were used to repay $306.0 million of outstanding bank indebtedness, with the balance used for general corporate purposes. The 4.2% Senior Notes bear interest at a rate of 4.2% per annum, which is payable semi-annually in arrears on each June 15th and December 15th. The Company may redeem all or a portion of the 4.2% Senior Notes at any time prior to maturity at the redemption prices set forth in the Note Indenture governing the 4.2% Senior Notes. The Company may issue additional debt from time to time pursuant to the Indenture. The Indenture and 4.2% Senior Notes contain covenants that limit the Company’s ability to, among other things, incur certain liens securing indebtedness, engage in certain sale-leaseback transactions, and enter into certain consolidations, mergers, conveyances, transfers or leases of all or substantially all the Company’s assets. The terms of the 4.2% Senior Notes also require the Company to make an offer to repurchase the 4.2% Senior Notes upon a change of control triggering event (as defined in the Indenture) at a price equal to 101% of their principal amount plus accrued and unpaid interest, if any.
On December 6, 2010, the Company completed a public offering of $300.0 million 4.5% senior notes due December 15, 2020 (“4.5% Senior Notes”). The net proceeds from the offering of $295.7 million, after deducting a $1.6 million issuance discount, a $1.9 million underwriting commission and $0.8 million offering expenses, were used to repay $250.0 million of outstanding bank indebtedness, with the balance used for general corporate purposes. The 4.5% Senior Notes bear interest at a rate of 4.5% per annum, which is payable semi-annually in arrears on each June 15th and December 15th. The Company may redeem all or a portion of the 4.5% Senior Notes at any time prior to maturity at the redemption prices set forth in the Note Indenture governing the 4.5% Senior Notes. The Company may issue additional debt from time to time pursuant to the Indenture. The Indenture and 4.5% Senior Notes contain covenants that limit the Company’s ability to, among other things, incur certain liens securing indebtedness, engage in certain sale-leaseback transactions, and enter into certain consolidations, mergers, conveyances, transfers or leases of all or substantially all the Company’s assets. The terms of the 4.5% Senior Notes also require the Company to make an offer to repurchase the 4.5% Senior Notes upon a change of control triggering event (as defined in the Indenture) at a price equal to 101% of their principal amount plus accrued and unpaid interest, if any.
Other borrowings of $1.3 million at December 31, 2016 consisted primarily of debt at international locations maintained for working capital purposes. Interest is payable on the outstanding debt balances at rates ranging from 1.0% to 2.8% per annum.
There are two key financial covenants that the Company is required to maintain in connection with the Revolving Facility and the Notes, a minimum interest coverage ratio of 3.0 to 1 and a maximum leverage ratio of 3.50 to 1, which is the ratio of the
50
Company’s consolidated total debt to its consolidated EBITDA. At December 31, 2016, the Company was in compliance with both of these financial covenants. There are no financial covenants relating to the 4.5% Senior Notes or 4.2% Senior Notes; however, both are subject to cross-default provisions.
Total borrowings at December 31, 2016 have scheduled maturities as follows:
| (In thousands) | |||
| 2017 | $ | 1,046 | |
| 2018 | 239 | ||
| 2019 | 9 | ||
| 2020 | 469,579 | ||
| 2021 | 350,000 | ||
| Thereafter | 200,000 | ||
| Total borrowings | $ | 1,020,873 | |
6. Derivative Instruments
As of December 31, 2016 and 2015, the Company did not have any interest rate or foreign exchange contracts outstanding. The type of cash flow hedges the Company has entered into includes interest rate exchange agreements that effectively convert a portion of floating-rate debt to fixed-rate debt and are designed to reduce the impact of interest rate changes on future interest expense.
The effective portion of gains or losses on interest rate exchange agreements is reported in accumulated other comprehensive income (loss) in shareholders’ equity and reclassified into net income in the same period or periods in which the hedged transaction affects net income. The remaining gain or loss in excess of the cumulative change in the present value of future cash flows or the hedged item, if any, is recognized into net income during the period of change. See Note 14 for the amount of loss reclassified into income for interest rate contracts for the years ended December 31, 2016, 2015 and 2014.
Fair values relating to derivative financial instruments reflect the estimated amounts that the Company would receive or pay to sell or buy the contracts based on quoted market prices of comparable contracts at each balance sheet date.
On April 15, 2010, the Company entered into a forward starting interest rate contract with a notional amount of $300.0 million with a settlement date in December 2010. This contract was entered into in anticipation of the issuance of the 4.5% Senior Notes and was designed to lock in the market interest rate as of April 15, 2010. In December 2010, the Company settled and paid this interest rate contract for $31.0 million. The $31.0 million is being amortized into interest expense over the 10 year term of the 4.5% Senior Notes, which results in an effective interest rate of 5.8%.
On July 12, 2011, the Company entered into a forward starting interest rate contract with a notional amount of $350.0 million and a settlement date of September 30, 2011. This contract was entered into in anticipation of the issuance of the 4.2% Senior Notes and was designed to lock in the market interest rate as of July 12, 2011. On September 29, 2011, the Company settled this interest rate contract for $34.7 million with a payment made on October 3, 2011. Simultaneously, the Company entered into a separate interest rate contract with a notional amount of $350.0 million and a settlement date of February 28, 2012. The contract was entered into in anticipation of the expected issuance of the 4.2% Senior Notes and was designed to maintain the market rate as of July 12, 2011. In December 2011, the Company settled and paid the September interest rate contract for $4.0 million, resulting in a total settlement of $38.7 million. Of the $38.7 million, $0.8 million was recognized as other expense in 2011 and the balance of $37.9 million is being amortized into interest expense over the 10 year term of the 4.2% Senior Notes, which results in an effective interest rate of 5.3%.
The amount of expense reclassified into interest expense for interest rate contracts for the years ended December 31, 2016, 2015 and 2014 is $6.9 million, $7.0 million and $7.2 million, respectively.
Approximately $6.7 million of the pre-tax amount included in accumulated other comprehensive loss in shareholders’ equity at December 31, 2016 will be recognized to net income over the next 12 months as the underlying hedged transactions are realized.
51
7. Fair Value Measurements
ASC 820, “Fair Value Measurements and Disclosures,” defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The standard utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
| • | Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities. |
| • | Level 2: Inputs, other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. |
| • | Level 3: Unobservable inputs that reflect the reporting entity’s own assumptions. |
The following table summarizes the basis used to measure the Company’s financial assets (liabilities) at fair value on a recurring basis in the balance sheets at December 31, 2016 and 2015:
| Basis of Fair Value Measurements | |||||||||||||||
| Balance at December 31, 2016 | Level 1 | Level 2 | Level 3 | ||||||||||||
| (In thousands) | |||||||||||||||
| Available for sale securities | $ | 5,369 | $ | 5,369 | $ | — | $ | — | |||||||
| Basis of Fair Value Measurements | |||||||||||||||
| Balance at December 31, 2015 | Level 1 | Level 2 | Level 3 | ||||||||||||
| (In thousands) | |||||||||||||||
| Money market investments | $ | 21,931 | $ | 21,931 | $ | — | $ | — | |||||||
| Available for sale securities | 4,794 | 4,794 | — | — | |||||||||||
| Contingent consideration | (4,705 | ) | — | — | (4,705 | ) | |||||||||
There were no transfers of assets or liabilities between Level 1 and Level 2 in 2016 or 2015.
In determining the fair value of the contingent consideration potentially due on the acquisition of CPS, the Company used probability weighted estimates of EBITDA during the earn-out period. The $4.7 million represented management’s best estimate of the liability as of the opening balance sheet date and December 31, 2015, based on a range of outcomes of CPS’s 12 month operating results, from July 1, 2015 to June 30, 2016. During the six months ended June 30, 2016, the Company re-evaluated the contingent consideration arrangement and fully reversed the $4.7 million liability based on CPS’s actual operating results from July 1, 2015 to June 30, 2016. The $4.7 million reversal was recognized as a benefit within Selling, general and administrative expenses.
The carrying value of our cash and cash equivalents, accounts receivable, accounts payable and accrued expenses approximates their fair values because of the short term nature of these instruments. At December 31, 2016, the fair value of the outstanding indebtedness under our Revolving Facility, 3.2% Senior Notes, 3.37% Senior Notes, 4.5% Senior Notes and 4.2% Senior Notes, based on quoted market prices and current market rates for debt with similar credit risk and maturity, was approximately $1,029.9 million compared to the carrying value of $1,018.4 million. This fair value measurement is classified as Level 2 within the fair value hierarchy since it is determined based upon significant inputs observable in the market, including interest rates on recent financing transactions to entities with a credit rating similar to ours.
8. Commitments and Contingencies
The Company leases certain office facilities, warehouses and data processing equipment under operating leases. Rental expense totaled $18.6 million, $18.9 million and $19.2 million in 2016, 2015 and 2014, respectively.
52
The aggregate future minimum lease payments for operating and capital leases as of December 31, 2016 were as follows:
| Operating | Capital | ||||||
| (In thousands) | |||||||
| 2017 | $ | 16,923 | $ | 1,055 | |||
| 2018 | 14,238 | 243 | |||||
| 2019 | 9,521 | 9 | |||||
| 2020 | 7,668 | — | |||||
| 2021 | 5,701 | — | |||||
| 2022 and thereafter | 19,106 | — | |||||
| $ | 73,157 | $ | 1,307 | ||||
Warranty costs are provided for at the time of sale. The warranty provision is based on historical costs and adjusted for specific known claims. A rollforward of the warranty reserve is as follows:
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Beginning balance January 1 | $ | 7,936 | $ | 7,196 | $ | 4,888 | |||||
| Provision for warranties | 1,828 | 4,788 | 6,220 | ||||||||
| Claim settlements | (3,539 | ) | (3,864 | ) | (3,823 | ) | |||||
| Other adjustments, including acquisitions, divestitures and currency translation | (597 | ) | (184 | ) | (89 | ) | |||||
| Ending balance December 31 | $ | 5,628 | $ | 7,936 | $ | 7,196 | |||||
The Company is party to various legal proceedings arising in the ordinary course of business, none of which are expected to have a material effect on its business, financial condition, results of operations or cash flow.
9. Common and Preferred Stock
On December 1, 2015 the Company’s Board of Directors approved an increase in the authorized level for repurchases of common stock by $300.0 million. Repurchases under the program will be funded with future cash flow generation or borrowings available under the Revolving Facility. During 2016, the Company purchased a total of 0.7 million shares at a cost of $55.0 million, compared to 2.8 million shares purchased at a cost of $210.5 million in 2015, of which $2.3 million was settled in January 2016. As of December 31, 2016, there was $580 million of repurchase authorization remaining.
At December 31, 2016 and 2015, the Company had 150 million shares of authorized common stock, with a par value of $.01 per share, and five million shares of authorized preferred stock, with a par value of $.01 per share. No preferred stock was issued as of December 31, 2016 and 2015.
10. Income Taxes
Pretax income for 2016, 2015 and 2014 was taxed in the following jurisdictions:
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| U.S. | $ | 265,260 | $ | 285,399 | $ | 275,334 | |||||
| Foreign | 103,252 | 106,946 | 117,106 | ||||||||
| Total | $ | 368,512 | $ | 392,345 | $ | 392,440 | |||||
53
The provision (benefit) for income taxes for 2016, 2015 and 2014, was as follows:
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Current | |||||||||||
| U.S. | $ | 67,668 | $ | 73,059 | $ | 77,454 | |||||
| State and local | 4,503 | 6,188 | 7,133 | ||||||||
| Foreign | 42,540 | 30,630 | 37,060 | ||||||||
| Total current | 114,711 | 109,877 | 121,647 | ||||||||
| Deferred | |||||||||||
| U.S. | (6,249 | ) | 7,125 | (3,176 | ) | ||||||
| State and local | (331 | ) | (1,017 | ) | (1,708 | ) | |||||
| Foreign | (10,728 | ) | (6,447 | ) | (3,709 | ) | |||||
| Total deferred | (17,308 | ) | (339 | ) | (8,593 | ) | |||||
| Total provision for income taxes | $ | 97,403 | $ | 109,538 | $ | 113,054 | |||||
Deferred tax assets (liabilities) at December 31, 2016 and 2015 were:
| 2016 | 2015 | ||||||
| (In thousands) | |||||||
| Employee and retiree benefit plans | $ | 42,950 | $ | 37,393 | |||
| Capital loss carryforwards | 18,668 | — | |||||
| Depreciation and amortization | (238,321 | ) | (185,321 | ) | |||
| Inventories | 11,519 | 12,615 | |||||
| Allowances and accruals | 9,338 | 12,528 | |||||
| Interest rate exchange agreement | 10,442 | 12,948 | |||||
| Other | (90 | ) | 2,800 | ||||
| Total gross deferred tax (liabilities) | (145,494 | ) | (107,037 | ) | |||
| Capital loss valuation allowance | (18,668 | ) | — | ||||
| Total deferred tax (liabilities), net of valuation allowances | $ | (164,162 | ) | $ | (107,037 | ) | |
The deferred tax assets and liabilities recognized in the Company’s Consolidated Balance Sheets as of December 31, 2016 and 2015 were:
| 2016 | 2015 | ||||||
| (In thousands) | |||||||
| Noncurrent deferred tax asset — Other noncurrent assets | $ | 2,265 | $ | 3,446 | |||
| Noncurrent deferred tax liabilities — Deferred income taxes | (166,427 | ) | (110,483 | ) | |||
| Net deferred tax liabilities | $ | (164,162 | ) | $ | (107,037 | ) | |
54
The provision for income taxes differs from the amount computed by applying the statutory federal income tax rate to pretax income. The computed amount and the differences for 2016, 2015 and 2014 are as follows:
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Pretax income | $ | 368,512 | $ | 392,345 | $ | 392,440 | |||||
| Provision for income taxes | |||||||||||
| Computed amount at statutory rate of 35% | $ | 128,979 | $ | 137,321 | $ | 137,354 | |||||
| State and local income tax (net of federal tax benefit) | 4,070 | 5,033 | 4,875 | ||||||||
| Taxes on non-U.S. earnings-net of foreign tax credits | (6,666 | ) | (11,663 | ) | (9,378 | ) | |||||
| Effect of flow-through entities | (8,735 | ) | (8,358 | ) | (9,018 | ) | |||||
| U.S. business tax credits | (1,665 | ) | (1,273 | ) | (1,680 | ) | |||||
| Domestic activities production deduction | (9,043 | ) | (6,521 | ) | (7,489 | ) | |||||
| Deferred tax effect of foreign tax rate change | — | (2,636 | ) | — | |||||||
| Capital loss on divestitures | (23,444 | ) | — | — | |||||||
| Valuation allowance | 17,973 | — | — | ||||||||
| Share-based payments | (6,520 | ) | — | — | |||||||
| Other | 2,454 | (2,365 | ) | (1,610 | ) | ||||||
| Total provision for income taxes | $ | 97,403 | $ | 109,538 | $ | 113,054 | |||||
The Company has $670 million and $715 million of undistributed earnings of non-U.S. subsidiaries as of December 31, 2016 and 2015, respectively. No deferred U.S. income taxes have been provided on these earnings as they are considered to be reinvested for an indefinite period of time or will be repatriated when it is tax effective to do so. If these amounts were distributed to the U.S., in the form of dividends or otherwise, the Company would be subject to additional U.S. income taxes, which could be material. Determination of the amount of unrecognized deferred income tax liabilities on these earnings is not practicable because of the complexities with the hypothetical calculation, and the amount of liability, if any, is dependent on circumstances if and when remittance occurs. During the years ended December 31, 2016, 2015 and 2014, the Company repatriated $28.8 million, $14.3 million and $6.5 million of foreign earnings, respectively, resulting in $2.7 million of incremental tax expense, $0.3 million of incremental income tax expense and $0.2 million of incremental tax benefit, respectively. These repatriations represent distributions of current year earnings and distributions from liquidating subsidiaries and do not impact our representation that the undistributed earnings are permanently invested.
A reconciliation of the beginning and ending amount of unrecognized tax benefits for 2016, 2015 and 2014 is as follows:
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Beginning balance January 1 | $ | 7,228 | $ | 3,619 | $ | 5,124 | |||||
| Gross increase due to non-U.S. acquisitions | — | 3,772 | — | ||||||||
| Gross increases for tax positions of prior years | 201 | 1,256 | 834 | ||||||||
| Gross decreases for tax positions of prior years | (93 | ) | — | (51 | ) | ||||||
| Settlements | (2,014 | ) | (667 | ) | (2,057 | ) | |||||
| Lapse of statute of limitations | (1,547 | ) | (752 | ) | (231 | ) | |||||
| Ending balance December 31 | $ | 3,775 | $ | 7,228 | $ | 3,619 | |||||
55
We recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2016, 2015 and 2014, we had approximately $0.1 million, $0.2 million and $0.7 million, respectively, of accrued interest related to uncertain tax positions. As of December 31, 2016, 2015 and 2014, we had approximately $0.1 million, $0.3 million and $0.3 million, respectively, of accrued penalties related to uncertain tax positions.
The total amount of unrecognized tax benefits that would affect our effective tax rate if recognized is $1.8 million, $3.0 million and $2.9 million as of December 31, 2016, 2015 and 2014, respectively. The tax years 2010-2015 remain open to examination by major taxing jurisdictions. Due to the potential for resolution of federal, state and foreign examinations, and the expiration of various statutes of limitation, it is reasonably possible that the Company’s gross unrecognized tax benefits balance may change within the next 12 months by a range of zero to $1.4 million.
The Company had net operating loss and credit carry forwards related to prior acquisitions for U.S. federal purposes at December 31, 2016 and 2015 of $3.5 million and $4.8 million, respectively. The federal net operating loss carry forwards are available for use against the Company’s consolidated federal taxable income and expire between 2021 and 2028. For non-U.S. purposes, the Company had net operating loss carry forwards at December 31, 2016 and 2015 of $25.6 million and $0.7 million, respectively, the majority of which relates to acquisitions. The entire balance of the non-U.S. net operating losses is available to be carried forward. At December 31, 2016 and 2015, the Company had U.S. state net operating loss and credit carry forwards of approximately $33.1 million and $27.0 million, respectively. If unutilized, the U.S. state net operating loss will expire between 2019 and 2036. At December 31, 2016 and 2015, the Company recorded a valuation allowance against the deferred tax asset attributable to the U.S. state net operating loss of $1.3 million and $1.0 million, respectively.
The Company had a capital loss carryover for U.S. federal purposes at December 31, 2016 of approximately $70.1 million. U.S. capital loss carryovers can be carried back three years and forward five years, thus, if unutilized, the federal capital loss carryover will expire in 2021. At December 31, 2016, the Company recorded a valuation allowance against the deferred tax asset attributable to the federal capital loss carryover of $18.7 million. At December 31, 2016, the Company had U.S. state capital loss carryovers of approximately $70.1 million. If unutilized, the U.S. state capital loss carryovers will expire between 2021 and 2031. At December 31, 2016, the Company recorded a valuation allowance against the deferred tax assets attributable to the state capital loss carryovers of $0.7 million. At December 31, 2016 and 2015, the Company had a foreign capital loss carry forward of approximately $0.7 million and $0.9 million, respectively. The foreign capital loss can be carried forward indefinitely. At both December 31, 2016 and 2015, the Company has a full valuation allowance against the deferred tax asset attributable to the foreign capital loss.
11. Business Segments and Geographic Information
IDEX has three reportable business segments: Fluid & Metering Technologies, Health & Science Technologies and Fire & Safety/Diversified Products.
The Fluid & Metering Technologies segment designs, produces and distributes positive displacement pumps, flow meters, injectors, and other fluid-handling pump modules and systems and provides flow monitoring and other services for the food, chemical, general industrial, water & wastewater, agriculture and energy industries. The Health & Science Technologies segment designs, produces and distributes a wide range of precision fluidics, rotary lobe pumps, centrifugal and positive displacement pumps, roll compaction and drying systems used in beverage, food processing, pharmaceutical and cosmetics, pneumatic components and sealing solutions, including very high precision, low-flow rate pumping solutions required in analytical instrumentation, clinical diagnostics and drug discovery, high performance molded and extruded, biocompatible medical devices and implantables, air compressors used in medical, dental and industrial applications, optical components and coatings for applications in the fields of scientific research, defense, biotechnology, aerospace, telecommunications and electronics manufacturing, laboratory and commercial equipment used in the production of micro and nano scale materials, precision photonic solutions used in life sciences, research and defense markets, and precision gear and peristaltic pump technologies that meet exacting original equipment manufacturer specifications. The Fire & Safety/Diversified Products segment produces firefighting pumps, valves and controls, rescue tools, lifting bags and other components and systems for the fire and rescue industry, engineered stainless steel banding and clamping devices used in a variety of industrial and commercial applications, and precision equipment for dispensing, metering and mixing colorants and paints used in a variety of retail and commercial businesses around the world.
Information on the Company’s business segments is presented below based on the nature of products and services offered. The Company evaluates performance based on several factors, of which sales and operating income are the primary financial measures. Intersegment sales are accounted for at fair value as if the sales were to third parties.
56
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| NET SALES | |||||||||||
| Fluid & Metering Technologies | |||||||||||
| External customers | $ | 848,708 | $ | 859,945 | $ | 898,530 | |||||
| Intersegment sales | 393 | 847 | 1,058 | ||||||||
| Total segment sales | 849,101 | 860,792 | 899,588 | ||||||||
| Health & Science Technologies | |||||||||||
| External customers | 744,380 | 737,011 | 747,186 | ||||||||
| Intersegment sales | 429 | 1,985 | 4,835 | ||||||||
| Total segment sales | 744,809 | 738,996 | 752,021 | ||||||||
| Fire & Safety/Diversified Products | |||||||||||
| External customers | 519,955 | 423,712 | 502,051 | ||||||||
| Intersegment sales | 54 | 203 | 698 | ||||||||
| Total segment sales | 520,009 | 423,915 | 502,749 | ||||||||
| Intersegment eliminations | (876 | ) | (3,035 | ) | (6,591 | ) | |||||
| Total net sales | $ | 2,113,043 | $ | 2,020,668 | $ | 2,147,767 | |||||
OPERATING INCOME (LOSS) (1) | |||||||||||
| Fluid & Metering Technologies | $ | 214,242 | $ | 204,506 | $ | 216,886 | |||||
| Health & Science Technologies | 153,722 | 157,948 | 152,999 | ||||||||
| Fire & Safety/Diversified Products | 121,888 | 115,745 | 130,494 | ||||||||
Corporate office (2) | (84,051 | ) | (46,461 | ) | (69,155 | ) | |||||
| Total operating income | 405,801 | 431,738 | 431,224 | ||||||||
| Interest expense | 45,616 | 41,636 | 41,895 | ||||||||
| Other (income) expense - net | (8,327 | ) | (2,243 | ) | (3,111 | ) | |||||
| Income before taxes | $ | 368,512 | $ | 392,345 | $ | 392,440 | |||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| ASSETS | |||||||||||
| Fluid & Metering Technologies | $ | 1,065,670 | $ | 1,125,266 | $ | 1,026,238 | |||||
| Health & Science Technologies | 1,266,036 | 1,108,302 | 1,101,155 | ||||||||
| Fire & Safety/Diversified Products | 705,735 | 448,867 | 510,841 | ||||||||
Corporate office (3) | 117,503 | 123,008 | 265,229 | ||||||||
| Total assets | $ | 3,154,944 | $ | 2,805,443 | $ | 2,903,463 | |||||
DEPRECIATION AND AMORTIZATION (4) | |||||||||||
| Fluid & Metering Technologies | $ | 28,458 | $ | 27,662 | $ | 26,453 | |||||
| Health & Science Technologies | 45,298 | 42,827 | 42,478 | ||||||||
| Fire & Safety/Diversified Products | 11,956 | 6,051 | 6,583 | ||||||||
| Corporate office and other | 1,180 | 1,580 | 1,393 | ||||||||
| Total depreciation and amortization | $ | 86,892 | $ | 78,120 | $ | 76,907 | |||||
| CAPITAL EXPENDITURES | |||||||||||
| Fluid & Metering Technologies | $ | 16,389 | $ | 22,846 | $ | 18,215 | |||||
| Health & Science Technologies | 15,665 | 13,104 | 19,161 | ||||||||
| Fire & Safety/Diversified Products | 5,945 | 5,804 | 6,761 | ||||||||
| Corporate office and other | 243 | 2,022 | 3,860 | ||||||||
| Total capital expenditures | $ | 38,242 | $ | 43,776 | $ | 47,997 | |||||
57
| (1) | Segment operating income (loss) excludes net unallocated corporate operating expenses. |
| (2) | 2016 includes a $22.3 million loss on the sale of businesses - net and 2015 includes an $18.1 million gain on the sale of a business. |
| (3) | 2014 balance has been reclassified to conform to the current presentation for ASU 2015-03. |
| (4) | Excludes amortization of debt issuance expenses. |
Information about the Company’s operations in different geographical regions for the years ended December 31, 2016, 2015 and 2014 is shown below. Net sales were attributed to geographic areas based on location of the customer and no country outside the U.S. was greater than 10% of total revenues.
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| NET SALES | |||||||||||
| U.S. | $ | 1,067,333 | $ | 1,015,277 | $ | 1,068,758 | |||||
| North America, excluding U.S. | 84,836 | 85,852 | 95,917 | ||||||||
| Europe | 517,179 | 490,435 | 527,975 | ||||||||
| Asia | 340,624 | 325,507 | 337,668 | ||||||||
| Other | 103,071 | 103,597 | 117,449 | ||||||||
| Total net sales | $ | 2,113,043 | $ | 2,020,668 | $ | 2,147,767 | |||||
| LONG-LIVED ASSETS — PROPERTY, PLANT AND EQUIPMENT | |||||||||||
| U.S. | $ | 152,504 | $ | 144,508 | $ | 139,702 | |||||
| North America, excluding U.S. | 1,533 | 643 | 814 | ||||||||
| Europe | 71,681 | 69,082 | 54,088 | ||||||||
| Asia | 21,793 | 26,498 | 24,912 | ||||||||
| Other | 305 | 214 | 27 | ||||||||
| Total long-lived assets — net | $ | 247,816 | $ | 240,945 | $ | 219,543 | |||||
58
12. Restructuring
During the fourth quarter of 2016, the third and fourth quarters of 2015 and the fourth quarter of 2014, the Company recorded restructuring costs as a part of restructuring initiatives that support the implementation of key strategic efforts designed to facilitate long-term, sustainable growth through cost reduction actions, primarily consisting of employee reductions and facility rationalization. The costs incurred related to these initiatives were included in Restructuring expenses in the Consolidated Statements of Operations while the related accruals were included in Accrued expenses in the Consolidated Balance Sheets. Severance costs primarily consisted of severance benefits through payroll continuation, COBRA subsidies, outplacement services, conditional separation costs and employer tax liabilities, while exit costs primarily consisted of asset disposals or impairments and lease exit costs.
2016 Initiative
During 2016 the Company recorded pre-tax restructuring expenses totaling $3.7 million related to the 2016 restructuring initiative. These expenses consisted of employee severance related to employee reductions across various functional areas. The 2016 restructuring initiative included severance benefits for 129 employees. Severance payments are expected to be substantially paid by the end of 2017 using cash from operations. The Company expects an additional $2 to $3 million of pre-tax restructuring expenses in the first quarter of 2017 related to the 2016 initiative.
Pre-tax restructuring expenses, comprised solely of severance costs, by segment for 2016 are as follows:
| Total Restructuring Costs | |||
| (In thousands) | |||
| Fluid & Metering Technologies | $ | 932 | |
| Health & Science Technologies | 1,117 | ||
| Fire & Safety/Diversified Products | 1,425 | ||
| Corporate/Other | 200 | ||
| Total restructuring costs | $ | 3,674 | |
2015 Initiative
During 2015 the Company recorded pre-tax restructuring expenses totaling $11.2 million related to the 2015 restructuring initiative. These expenses consisted of employee severance related to employee reductions across various functional areas. The 2015 restructuring initiative included severance benefits for 208 employees. Severance payments are expected to be paid by March 31, 2017 using cash from operations.
Pre-tax restructuring expenses, comprised solely of severance costs, by segment for 2015 are as follows:
| Total Restructuring Costs | |||
| (In thousands) | |||
| Fluid & Metering Technologies | $ | 7,090 | |
| Health & Science Technologies | 3,408 | ||
| Fire & Safety/Diversified Products | 576 | ||
| Corporate/Other | 165 | ||
| Total restructuring costs | $ | 11,239 | |
2014 Initiative
During 2014 the Company recorded pre-tax restructuring expenses in the fourth quarter totaling $13.7 million related to the 2014 restructuring initiative. These expenses consisted of employee severance related to employee reductions across various functional areas as well as exit costs and asset impairments. The 2014 restructuring initiative included severance benefits for 217 employees. Severance payments were fully paid by the end of 2015 using cash from operations.
59
Pre-tax restructuring expenses by segment for 2014 were as follows:
| Severance Costs | Exit Costs and Asset Impairments | Total | |||||||||
| (In thousands) | |||||||||||
| Fluid & Metering Technologies | $ | 6,413 | $ | — | $ | 6,413 | |||||
| Health & Science Technologies | 3,520 | 1,392 | 4,912 | ||||||||
| Fire & Safety/Diversified Products | 908 | 126 | 1,034 | ||||||||
| Corporate/Other | 1,313 | — | 1,313 | ||||||||
| Total restructuring costs | $ | 12,154 | $ | 1,518 | $ | 13,672 | |||||
Restructuring accruals of $3.9 million and $6.6 million at December 31, 2016 and 2015, respectively, are reflected in Accrued expenses in our Consolidated Balance Sheets as follows:
| Restructuring Initiatives | |||
| (In thousands) | |||
| Balance at January 1, 2015 | $ | 6,056 | |
| Restructuring expenses | 11,239 | ||
| Payments, utilization and other | (10,659 | ) | |
| Balance at December 31, 2015 | 6,636 | ||
| Restructuring expenses | 3,674 | ||
| Payments, utilization and other | (6,417 | ) | |
| Balance at December 31, 2016 | $ | 3,893 | |
13. Share-Based Compensation
The Company maintains two share-based compensation plans for executives, non-employee directors and certain key employees that authorize the granting of stock options, restricted stock, performance share units, and other types of awards consistent with the purpose of the plans. The number of shares authorized for issuance under the Company’s plans as of December 31, 2016 totaled 15.6 million, of which 5.4 million shares were available for future issuance. The Company’s policy is to recognize compensation cost on a straight-line basis, assuming forfeitures, over the requisite service period for the entire award.
Stock Options
Stock options granted under IDEX plans are generally non-qualified and are granted with an exercise price equal to the market price of the Company’s stock at the date of grant. The majority of the options issued to employees become exercisable in four equal installments, beginning one year from the date of grant, and generally expire 10 years from the date of grant. Stock options granted to non-employee directors cliff vest after one year.
Weighted average option fair values and assumptions for the period are as follows:
| Years Ended December 31, | |||||
| 2016 | 2015 | 2014 | |||
| Weighted average fair value of grants | $18.56 | $20.32 | $19.52 | ||
| Dividend yield | 1.69% | 1.45% | 1.27% | ||
| Volatility | 29.70% | 29.90% | 30.36% | ||
| Risk-free interest rate | 0.53% - 2.49% | 0.24% - 2.82% | 0.12% - 4.65% | ||
| Expected life (in years) | 5.91 | 5.93 | 5.89 | ||
60
The assumptions are as follows:
| • | The Company estimated volatility using its historical share price performance over the contractual term of the option. |
| • | The Company uses historical data to estimate the expected life of the option. The expected life assumption for the years ended December 31, 2016, 2015 and 2014 is an output of the Binomial lattice option-pricing model, which incorporates vesting provisions, rate of voluntary exercise and rate of post-vesting termination over the contractual life of the option to define expected employee behavior. |
| • | The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the contractual life of the option. For the years ended December 31, 2016, 2015 and 2014, we present the range of risk-free one-year forward rates, derived from the U.S. treasury yield curve, utilized in the Binomial lattice option-pricing model. |
| • | The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the contractual life of the option. |
A summary of the Company’s stock option activity as of December 31, 2016, and changes during the year ended December 31, 2016 is presented as follows:
| Stock Options | Shares | Weighted Average Price | Weighted-Average Remaining Contractual Term | Aggregate Intrinsic Value | ||||||||
| Outstanding at January 1, 2016 | 2,266,433 | $ | 54.05 | 6.58 | $ | 51,918,028 | ||||||
| Granted | 570,250 | 75.35 | ||||||||||
| Exercised | (675,525 | ) | 44.44 | |||||||||
| Forfeited/Expired | (173,212 | ) | 72.30 | |||||||||
| Outstanding at December 31, 2016 | 1,987,946 | $ | 61.83 | 6.84 | $ | 56,144,876 | ||||||
| Vested and expected to vest at December 31, 2016 | 1,884,401 | $ | 61.06 | 6.74 | $ | 54,671,036 | ||||||
| Exercisable at December 31, 2016 | 945,265 | $ | 48.53 | 5.17 | $ | 39,252,480 | ||||||
The intrinsic value for stock options outstanding and exercisable is defined as the difference between the market value of the Company’s common stock as of the end of the period and the grant price. The total intrinsic value of options exercised in 2016, 2015 and 2014 was $26.5 million, $16.9 million and $20.0 million, respectively. In 2016, 2015 and 2014, cash received from options exercised was $30.2 million, $19.2 million and $17.2 million, respectively, while the actual tax benefit realized for the tax deductions from stock options exercised totaled $9.6 million, $6.1 million and $7.3 million, respectively.
Total compensation cost for stock options is recorded in the Consolidated Statements of Operations as follows:
| Years Ended December 31, | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Cost of goods sold | $ | 427 | $ | 543 | $ | 581 | |||||
| Selling, general and administrative expenses | 6,561 | 6,488 | 6,245 | ||||||||
| Total expense before income taxes | 6,988 | 7,031 | 6,826 | ||||||||
| Income tax benefit | (2,213 | ) | (2,208 | ) | (2,194 | ) | |||||
| Total expense after income taxes | $ | 4,775 | $ | 4,823 | $ | 4,632 | |||||
As of December 31, 2016 there was $10.8 million of total unrecognized compensation cost related to stock options that is expected to be recognized over a weighted-average period of 1.4 years.
Restricted Stock
Restricted stock awards generally cliff vest after three years for employees and non-employee directors. Unvested restricted stock carries dividend and voting rights and the sale of the shares is restricted prior to the date of vesting. Dividends are paid on restricted stock awards and their fair value is equal to the market price of the Company’s stock at the date of the
61
grant. A summary of the Company’s restricted stock activity as of December 31, 2016, and changes during the year ending December 31, 2016 is as follows:
| Restricted Stock | Shares | Weighted-Average Grant Date Fair Value | ||||
| Unvested at January 1, 2016 | 272,755 | $ | 65.90 | |||
| Granted | 76,970 | 78.86 | ||||
| Vested | (105,382 | ) | 51.90 | |||
| Forfeited | (26,445 | ) | 74.61 | |||
| Unvested at December 31, 2016 | 217,898 | $ | 76.19 | |||
Total compensation cost for restricted stock is recorded in the Consolidated Statements of Operations as follows:
| Years Ended December 31, | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Cost of goods sold | $ | 390 | $ | 341 | $ | 369 | |||||
| Selling, general and administrative expenses | 4,401 | 5,213 | 6,182 | ||||||||
| Total expense before income taxes | 4,791 | 5,554 | 6,551 | ||||||||
| Income tax benefit | (1,410 | ) | (1,604 | ) | (1,630 | ) | |||||
| Total expense after income taxes | $ | 3,381 | $ | 3,950 | $ | 4,921 | |||||
As of December 31, 2016 there was $5.3 million of total unrecognized compensation cost related to restricted stock that is expected to be recognized over a weighted-average period of 1.1 years.
Cash-Settled Restricted Stock
The Company also maintains a cash-settled share based compensation plan for certain employees. Cash-settled restricted stock awards generally cliff vest after three years. Dividend equivalents are paid on certain cash-settled restricted stock awards. A summary of the Company’s unvested cash-settled restricted stock activity as of December 31, 2016, and changes during the year ending December 31, 2016 is as follows:
| Cash-Settled Restricted Stock | Shares | Weighted-Average Fair Value | ||||
| Unvested at January 1, 2016 | 110,860 | $ | 76.61 | |||
| Granted | 41,060 | 90.06 | ||||
| Vested | (36,660 | ) | 72.91 | |||
| Forfeited | (11,470 | ) | 90.06 | |||
| Unvested at December 31, 2016 | 103,790 | $ | 90.06 | |||
62
Total compensation cost for cash-settled restricted stock is recorded in the Consolidated Statements of Operations as follows:
| Years Ended December 31, | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Cost of goods sold | $ | 764 | $ | 753 | $ | 1,384 | |||||
| Selling, general and administrative expenses | 2,224 | 1,765 | 2,735 | ||||||||
| Total expense before income taxes | 2,988 | 2,518 | 4,119 | ||||||||
| Income tax benefit | (419 | ) | (355 | ) | (603 | ) | |||||
| Total expense after income taxes | $ | 2,569 | $ | 2,163 | $ | 3,516 | |||||
At December 31, 2016 and 2015, the Company has $3.0 million and $3.2 million, respectively, included in Accrued expenses in the Consolidated Balance Sheets and $2.4 million and $1.8 million, respectively, included in Other non-current liabilities.
Performance Share Units
Beginning in 2013 the Company granted performance share units to selected key employees that may be earned based on IDEX total shareholder return over the three-year period following the date of grant. Performance share units are expected to be made annually and are paid out at the end of a three-year period based on the Company’s performance. Performance is measured by determining the percentile rank of the total shareholder return of IDEX common stock in relation to the total shareholder return of the S&P Midcap 400 Industrial Group (for awards granted prior to 2016) or the Russell Midcap Index (for awards granted in 2016) for the three-year period following the date of grant. The payment of awards following the three-year award period will be based on performance achieved in accordance with the scale set forth in the plan agreement and may range from 0 percent to 250 percent of the initial grant. A target payout of 100 percent is earned if total shareholder return is equal to the 50th percentile of the peer group. Performance share units earn dividend equivalents for the award period, which will be paid to participants with the award payout at the end of the period based on the actual number of performance share units that are earned. Payments made at the end of the award period will be in the form of stock for performance share units and will be in cash for dividend equivalents. The Company’s performance share awards are considered performance condition awards and the grant date fair value of the awards, based on a Monte Carlo simulation model, is expensed ratably over the three-year term of the awards. The Company granted approximately 0.1 million of performance share units in each of 2016, 2015 and 2014.
Weighted average performance share unit fair values and assumptions for the period specified are as follows:
| Years Ended December 31, | |||||
| 2016 | 2015 | 2014 | |||
| Weighted average fair value of grants | $111.42 | $95.07 | $94.55 | ||
| Dividend yield | —% | —% | —% | ||
| Volatility | 17.99% | 19.14% | 26.41% | ||
| Risk-free interest rate | 0.89% | 1.01% | 0.65% | ||
| Expected life (in years) | 2.86 | 2.86 | 2.88 | ||
The assumptions are as follows:
| • | The Company estimated volatility using its historical share price performance over the remaining performance period as of the grant date. |
| • | The Company uses a Monte Carlo simulation model that uses an expected life commensurate with the performance period. As a result, the expected life of the performance share units was assumed to be the period from the grant date to the end of the performance period. |
| • | The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant with a term commensurate with the remaining performance period. |
63
| • | Total Shareholder Return is determined assuming that dividends are reinvested in the issuing entity over the performance period, which is mathematically equivalent to utilizing a 0% dividend yield. |
A summary of the Company’s performance share unit activity as of December 31, 2016, and changes during the year ending December 31, 2016 is as follows:
| Performance Share Units | Shares | Weighted-Average Grant Date Fair Value | ||||
| Unvested at January 1, 2016 | 146,275 | $ | 94.80 | |||
| Granted | 85,130 | 111.42 | ||||
| Vested | (63,325 | ) | 94.55 | |||
| Forfeited | (31,025 | ) | 99.52 | |||
| Unvested at December 31, 2016 | 137,055 | $ | 104.18 | |||
Awards that vested in 2016 will result in 89,288 shares being issued in 2017.
Total compensation cost for performance share units is as follows:
| Years Ended December 31, | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Cost of goods sold | $ | — | $ | — | $ | — | |||||
| Selling, general and administrative expenses | 5,559 | 4,946 | 3,220 | ||||||||
| Total expense before income taxes | 5,559 | 4,946 | 3,220 | ||||||||
| Income tax benefit | (1,859 | ) | (1,670 | ) | (1,081 | ) | |||||
| Total expense after income taxes | $ | 3,700 | $ | 3,276 | $ | 2,139 | |||||
As of December 31, 2016 there was $6.3 million of total unrecognized compensation cost related to performance shares that is expected to be recognized over a weighted-average period of 1.0 year.
14. Other Comprehensive Income (Loss)
The components of Other comprehensive income (loss) are as follows:
| For the Year Ended December 31, 2016 | For the Year Ended December 31, 2015 | ||||||||||||||||||||||
| Pre-tax | Tax | Net of tax | Pre-tax | Tax | Net of tax | ||||||||||||||||||
| (In thousands) | |||||||||||||||||||||||
| Foreign currency translation adjustments | |||||||||||||||||||||||
| Cumulative translation adjustment | $ | (76,822 | ) | $ | — | $ | (76,822 | ) | $ | (63,441 | ) | $ | — | $ | (63,441 | ) | |||||||
| Reclassification of foreign currency translation to earnings upon sale of business | 14,257 | — | 14,257 | (4,725 | ) | — | (4,725 | ) | |||||||||||||||
| Foreign currency translation adjustments | (62,565 | ) | — | (62,565 | ) | (68,166 | ) | — | (68,166 | ) | |||||||||||||
| Pension and other postretirement adjustments | |||||||||||||||||||||||
| Net gain (loss) arising during the year | (1,927 | ) | 789 | (1,138 | ) | 8,318 | (2,411 | ) | 5,907 | ||||||||||||||
| Amortization/recognition of settlement loss | 7,083 | (2,896 | ) | 4,187 | 4,939 | (1,431 | ) | 3,508 | |||||||||||||||
| Pension and other postretirement adjustments | 5,156 | (2,107 | ) | 3,049 | 13,257 | (3,842 | ) | 9,415 | |||||||||||||||
| Reclassification adjustments for derivatives | 6,851 | (2,490 | ) | 4,361 | 7,030 | (2,499 | ) | 4,531 | |||||||||||||||
| Total other comprehensive income (loss) | $ | (50,558 | ) | $ | (4,597 | ) | $ | (55,155 | ) | $ | (47,879 | ) | $ | (6,341 | ) | $ | (54,220 | ) | |||||
64
| For the Year Ended December 31, 2014 | |||||||||||||||||
| Pre-tax | Tax | Net of tax | |||||||||||||||
| (In thousands) | |||||||||||||||||
| Foreign currency translation adjustments | |||||||||||||||||
| Cumulative translation adjustment | $ | (77,024 | ) | $ | — | $ | (77,024 | ) | |||||||||
| Pension and other postretirement adjustments | |||||||||||||||||
| Net gain (loss) arising during the year | (26,424 | ) | 7,767 | (18,657 | ) | ||||||||||||
| Amortization/recognition of settlement loss | 3,113 | (915 | ) | 2,198 | |||||||||||||
| Pension and other postretirement adjustments, net | (23,311 | ) | 6,852 | (16,459 | ) | ||||||||||||
| Reclassification adjustments for derivatives | 7,223 | (2,713 | ) | 4,510 | |||||||||||||
| Total other comprehensive income (loss) | $ | (93,112 | ) | $ | 4,139 | $ | (88,973 | ) | |||||||||
Amounts reclassified from accumulated other comprehensive income (loss) to net income are summarized as follows:
| For the Years Ended December 31, | ||||||||||||||
| 2016 | 2015 | 2014 | Income Statement Caption | |||||||||||
| Foreign currency translation: | ||||||||||||||
| Reclassification upon sale of business | $ | 14,257 | $ | (4,725 | ) | $ | — | Loss (gain) on sale of businesses - net | ||||||
| Total before tax | 14,257 | (4,725 | ) | — | ||||||||||
| Provision for income taxes | — | — | — | |||||||||||
| Total net of tax | $ | 14,257 | $ | (4,725 | ) | $ | — | |||||||
| Pension and other postretirement plans: | ||||||||||||||
| Amortization of service cost | $ | 7,083 | $ | 4,939 | $ | 3,113 | Selling, general and administrative expense | |||||||
| Total before tax | 7,083 | 4,939 | 3,113 | |||||||||||
| Provision for income taxes | (2,896 | ) | (1,431 | ) | (915 | ) | ||||||||
| Total net of tax | $ | 4,187 | $ | 3,508 | $ | 2,198 | ||||||||
| Derivatives: | ||||||||||||||
| Reclassification adjustments | $ | 6,851 | $ | 7,030 | $ | 7,223 | Interest expense | |||||||
| Total before tax | 6,851 | 7,030 | 7,223 | |||||||||||
| Provision for income taxes | (2,490 | ) | (2,499 | ) | (2,713 | ) | ||||||||
| Total net of tax | $ | 4,361 | $ | 4,531 | $ | 4,510 | ||||||||
15. Retirement Benefits
The Company sponsors several qualified and nonqualified pension plans and other postretirement plans for its employees. The Company uses a measurement date of December 31 for its defined benefit pension plans and post retirement medical plans. The Company employs the measurement date provisions of ASC 715, Compensation-Retirement Benefits, which require the measurement date of plan assets and liabilities to coincide with the sponsor’s year end.
During 2016, the Company offered a voluntary lump-sum pension payment opportunity to certain terminated vested U.S. pension plan participants. Total lump-sum payments of $11.0 million were made for those participants electing to receive lump sums using pension plan assets. The Company recognized pretax settlement losses of $3.5 million in the fourth quarter of 2016 for those plans where the settlement payment exceeded the sum of the plans' service and interest costs.
The following table provides a reconciliation of the changes in the benefit obligations and fair value of plan assets over the two-year period ended December 31, 2016, and a statement of the funded status at December 31 for both years.
65
| Pension Benefits | Other Benefits | ||||||||||||||||||||||
| 2016 | 2015 | 2016 | 2015 | ||||||||||||||||||||
| U.S. | Non-U.S. | U.S. | Non-U.S. | ||||||||||||||||||||
| (In thousands) | |||||||||||||||||||||||
| CHANGE IN BENEFIT OBLIGATION | |||||||||||||||||||||||
| Obligation at January 1 | $ | 98,476 | $ | 58,063 | $ | 102,312 | $ | 69,488 | $ | 20,400 | $ | 22,855 | |||||||||||
| Service cost | 1,016 | 1,627 | 1,279 | 1,506 | 601 | 673 | |||||||||||||||||
| Interest cost | 3,043 | 1,429 | 3,770 | 1,734 | 811 | 833 | |||||||||||||||||
| Plan amendments | — | — | 113 | — | — | — | |||||||||||||||||
| Benefits paid | (3,140 | ) | (2,023 | ) | (3,985 | ) | (2,448 | ) | (718 | ) | (622 | ) | |||||||||||
| Actuarial loss (gain) | 1,987 | 6,844 | (5,013 | ) | (6,909 | ) | (1,990 | ) | (2,966 | ) | |||||||||||||
| Currency translation | — | (6,988 | ) | — | (5,308 | ) | 52 | (373 | ) | ||||||||||||||
| Settlements | (11,126 | ) | (819 | ) | — | — | — | — | |||||||||||||||
| Acquisition/Divestiture | — | 29,491 | — | — | 5,480 | — | |||||||||||||||||
| Other | — | 140 | — | — | — | — | |||||||||||||||||
| Obligation at December 31 | $ | 90,256 | $ | 87,764 | $ | 98,476 | $ | 58,063 | $ | 24,636 | $ | 20,400 | |||||||||||
| CHANGE IN PLAN ASSETS | |||||||||||||||||||||||
| Fair value of plan assets at January 1 | $ | 77,575 | $ | 20,645 | $ | 79,687 | $ | 22,152 | $ | — | $ | — | |||||||||||
| Actual return on plan assets | 6,740 | 2,470 | (2,587 | ) | 205 | — | — | ||||||||||||||||
| Employer contributions | 3,639 | 1,974 | 4,460 | 1,837 | 718 | 622 | |||||||||||||||||
| Benefits paid | (3,140 | ) | (2,023 | ) | (3,985 | ) | (2,448 | ) | (718 | ) | (622 | ) | |||||||||||
| Currency translation | — | (4,108 | ) | — | (1,101 | ) | — | — | |||||||||||||||
| Settlements | (11,126 | ) | (819 | ) | — | — | — | — | |||||||||||||||
| Acquisition/Divestiture | — | 14,307 | — | — | — | — | |||||||||||||||||
| Other | — | 140 | — | — | — | — | |||||||||||||||||
| Fair value of plan assets at December 31 | $ | 73,688 | $ | 32,586 | $ | 77,575 | $ | 20,645 | $ | — | $ | — | |||||||||||
| Funded status at December 31 | $ | (16,568 | ) | $ | (55,178 | ) | $ | (20,901 | ) | $ | (37,418 | ) | $ | (24,636 | ) | $ | (20,400 | ) | |||||
| COMPONENTS ON THE CONSOLIDATED BALANCE SHEETS | |||||||||||||||||||||||
| Current liabilities | $ | (729 | ) | $ | (1,005 | ) | $ | (743 | ) | $ | (875 | ) | $ | (1,044 | ) | $ | (911 | ) | |||||
| Other noncurrent liabilities | (15,839 | ) | (54,173 | ) | (20,158 | ) | (36,543 | ) | (23,592 | ) | (19,489 | ) | |||||||||||
| Net liability at December 31 | $ | (16,568 | ) | $ | (55,178 | ) | $ | (20,901 | ) | $ | (37,418 | ) | $ | (24,636 | ) | $ | (20,400 | ) | |||||
The accumulated benefit obligation (“ABO”) for all defined benefit pension plans was $176.7 million and $150.4 million at December 31, 2016 and 2015, respectively.
The weighted average assumptions used in the measurement of the Company’s benefit obligation at December 31, 2016 and 2015 were as follows:
| U.S. Plans | Non-U.S. Plans | ||||||||||
| 2016 | 2015 | 2016 | 2015 | ||||||||
| Discount rate | 3.91 | % | 4.12 | % | 1.76 | % | 2.99 | % | |||
| Rate of compensation increase | 4.00 | % | 4.00 | % | 2.29 | % | 2.98 | % | |||
66
The pretax amounts recognized in Accumulated other comprehensive income (loss) on the Consolidated Balance Sheets as of December 31, 2016 and 2015 were as follows:
| Pension Benefits | Other Benefits | ||||||||||||||||||||||
| 2016 | 2015 | 2016 | 2015 | ||||||||||||||||||||
| U.S. | Non-U.S. | U.S. | Non-U.S | ||||||||||||||||||||
| (In thousands) | |||||||||||||||||||||||
| Prior service cost (credit) | $ | 110 | $ | (77 | ) | $ | 135 | $ | (38 | ) | $ | (849 | ) | $ | (1,215 | ) | |||||||
| Net loss | 27,860 | (17,643 | ) | 33,461 | 15,330 | (3,852 | ) | (2,197 | ) | ||||||||||||||
| Total | $ | 27,970 | $ | (17,720 | ) | $ | 33,596 | $ | 15,292 | $ | (4,701 | ) | $ | (3,412 | ) | ||||||||
The amounts in Accumulated other comprehensive income (loss) on the Consolidated Balance Sheet as of December 31, 2016, that are expected to be recognized as components of net periodic benefit cost during 2017 are as follows:
U.S. Pension Benefit Plans | Non-U.S. Pension Benefit Plans | Other Benefit Plans | Total | ||||||||||||
| (In thousands) | |||||||||||||||
| Prior service cost (credit) | $ | 24 | $ | 9 | $ | (366 | ) | $ | (333 | ) | |||||
| Net loss | 2,542 | 1,493 | (427 | ) | 3,608 | ||||||||||
| Total | $ | 2,566 | $ | 1,502 | $ | (793 | ) | $ | 3,275 | ||||||
The components of, and the weighted average assumptions used to determine, the net periodic benefit cost for the plans in 2016, 2015 and 2014 are as follows:
| Pension Benefits | |||||||||||||||||||||||
| 2016 | 2015 | 2014 | |||||||||||||||||||||
| U.S. | Non-U.S. | U.S. | Non-U.S. | U.S. | Non-U.S. | ||||||||||||||||||
| (In thousands) | |||||||||||||||||||||||
| Service cost | $ | 1,016 | $ | 1,627 | $ | 1,279 | $ | 1,506 | $ | 1,162 | $ | 1,331 | |||||||||||
| Interest cost | 3,043 | 1,429 | 3,770 | 1,734 | 4,037 | 2,345 | |||||||||||||||||
| Expected return on plan assets | (4,777 | ) | (993 | ) | (4,910 | ) | (1,114 | ) | (5,430 | ) | (1,297 | ) | |||||||||||
| Settlement loss recognized | 3,339 | 215 | — | — | — | — | |||||||||||||||||
| Net amortization | 3,226 | 1,008 | 3,422 | 1,931 | 2,187 | 1,400 | |||||||||||||||||
| Net periodic benefit cost | $ | 5,847 | $ | 3,286 | $ | 3,561 | $ | 4,057 | $ | 1,956 | $ | 3,779 | |||||||||||
| Other Benefits | |||||||||||
| 2016 | 2015 | 2014 | |||||||||
| (In thousands) | |||||||||||
| Service cost | $ | 601 | $ | 673 | $ | 714 | |||||
| Interest cost | 811 | 833 | 932 | ||||||||
| Net amortization | (705 | ) | (414 | ) | (474 | ) | |||||
| Net periodic benefit cost | $ | 707 | $ | 1,092 | $ | 1,172 | |||||
| U.S. Plans | Non-U.S. Plans | ||||||||||||||||
| 2016 | 2015 | 2014 | 2016 | 2015 | 2014 | ||||||||||||
| Discount rate | 4.12 | % | 3.78 | % | 4.61 | % | 2.99 | % | 2.66 | % | 4.03 | % | |||||
| Expected return on plan assets | 6.50 | % | 6.50 | % | 7.00 | % | 4.58 | % | 5.19 | % | 5.83 | % | |||||
| Rate of compensation increase | 4.00 | % | 4.00 | % | 4.00 | % | 2.98 | % | 3.00 | % | 3.14 | % | |||||
67
The pretax change recognized in Accumulated other comprehensive income (loss) on the Consolidated Balance Sheet in 2016 is as follows:
| Pension Benefits | Other Benefits | ||||||||||
| U.S. | Non-U.S. | ||||||||||
| (In thousands) | |||||||||||
| Net gain (loss) in current year | $ | (23 | ) | $ | (5,367 | ) | $ | 1,990 | |||
| Prior service cost | — | — | — | ||||||||
| Amortization of prior service cost (credit) | 24 | (15 | ) | (366 | ) | ||||||
| Amortization of net loss (gain) | 6,541 | 1,239 | (340 | ) | |||||||
| Exchange rate effect on amounts in OCI | — | 1,468 | 5 | ||||||||
| Total | $ | 6,542 | $ | (2,675 | ) | $ | 1,289 | ||||
The discount rates for our plans are derived by matching the plan’s cash flows to a yield curve that provides the equivalent yields on zero-coupon bonds for each maturity. The discount rate selected is the rate that produces the same present value of cash flows.
In selecting the expected rate of return on plan assets, the Company considers the historical returns and expected returns on plan assets. The expected returns are evaluated using asset return class, variance and correlation assumptions based on the plan’s target asset allocation and current market conditions.
Prior service costs are amortized on a straight-line basis over the average remaining service period of active participants. Gains and losses in excess of 10% of the greater of the benefit obligation or the market value of assets are amortized over the average remaining service period of active participants.
Costs of defined contribution plans were $10.1 million, $10.3 million and $9.1 million for 2016, 2015 and 2014, respectively.
The Company, through its subsidiaries, participates in certain multi-employer pension plans covering approximately 381 participants under U.S. collective bargaining agreements. None of these plans are considered individually significant to the Company as contributions to these plans totaled $1.3 million, $1.0 million, and $1.0 million for 2016, 2015 and 2014, respectively.
For measurement purposes, a 6.58% weighted average annual rate of increase in the per capita cost of covered health care benefits was assumed for 2016. The rate was assumed to decrease gradually each year to a rate of 4.50% for 2037, and remain at that level thereafter. Assumed health care cost trend rates have an effect on the amounts reported for the health care plans. A 1% increase in the assumed health care cost trend rates would increase the service and interest cost components of the net periodic benefit cost by $0.2 million and the health care component of the accumulated postretirement benefit obligation by $2.1 million. A 1% decrease in the assumed health care cost trend rate would decrease the service and interest cost components of the net periodic benefit cost by $0.1 million and the health care component of the accumulated postretirement benefit obligation by $1.8 million.
68
Plan Assets
The Company’s pension plan weighted average asset allocations at December 31, 2016 and 2015, by asset category, were as follows:
| U.S. Plans | Non-U.S. Plans | ||||||||||
| 2016 | 2015 | 2016 | 2015 | ||||||||
| Equity securities | 44 | % | 49 | % | 24 | % | 36 | % | |||
| Fixed income securities | 43 | % | 49 | % | 26 | % | 44 | % | |||
Cash/Commingled Funds/Other (1) | 13 | % | 2 | % | 50 | % | 20 | % | |||
| Total | 100 | % | 100 | % | 100 | % | 100 | % | |||
The basis used to measure the defined benefit plans’ assets at fair value at December 31, 2016 and 2015 is summarized as follows:
| Basis of Fair Value Measurement | |||||||||||||||
Outstanding Balances | Level 1 | Level 2 | Level 3 | ||||||||||||
| As of December 31, 2016 | (In thousands) | ||||||||||||||
| Equity | |||||||||||||||
| U.S. Large Cap | $ | 15,345 | $ | 15,345 | $ | — | $ | — | |||||||
| U.S. Small / Mid Cap | 8,920 | 7,111 | 1,809 | — | |||||||||||
| International | 16,282 | 10,647 | 5,635 | — | |||||||||||
| Fixed Income | |||||||||||||||
| U.S. Intermediate | 10,014 | 9,943 | 71 | — | |||||||||||
| U.S. Short Duration | 10,160 | 10,160 | — | — | |||||||||||
| U.S. High Yield | 9,343 | 7,924 | 1,419 | — | |||||||||||
| International | 10,310 | 3,627 | 6,683 | — | |||||||||||
Other Commingled Funds (1) | 14,180 | — | — | 14,180 | |||||||||||
| Cash and Equivalents | 10,382 | 9,660 | 722 | — | |||||||||||
| Other | 1,338 | — | 1,338 | — | |||||||||||
| $ | 106,274 | $ | 74,417 | $ | 17,677 | $ | 14,180 | ||||||||
| (1) Other commingled funds represent pooled institutional investments in non-U.S. plans. | |||||||||||||||
69
| Basis of Fair Value Measurement | |||||||||||||||
| Outstanding Balances | Level 1 | Level 2 | Level 3 | ||||||||||||
| As of December 31, 2015 | (In thousands) | ||||||||||||||
| Equity | |||||||||||||||
| U.S. Large Cap | $ | 23,465 | $ | 23,465 | $ | — | $ | — | |||||||
| U.S. Small / Mid Cap | 10,184 | 7,482 | 2,702 | — | |||||||||||
| International | 11,986 | 7,786 | 4,200 | — | |||||||||||
| Fixed Income | |||||||||||||||
| U.S. Intermediate | 15,000 | 15,000 | — | — | |||||||||||
| U.S. Short Duration | 8,935 | 8,935 | — | — | |||||||||||
| U.S. High Yield | 7,758 | 6,922 | 836 | — | |||||||||||
| International | 15,249 | 7,241 | 8,008 | �� | |||||||||||
| Cash and Equivalents | 1,829 | 1,829 | — | — | |||||||||||
| Other | 3,836 | — | 3,836 | — | |||||||||||
| $ | 98,242 | $ | 78,660 | $ | 19,582 | $ | — | ||||||||
Equities that are valued using quoted prices are valued at the published market prices. Equities in a common collective trust or a registered investment company that are valued using significant other observable inputs are valued at the net asset value (“NAV”) provided by the fund administrator. The NAV is based on the value of the underlying assets owned by the fund minus its liabilities. Fixed income securities that are valued using significant other observable inputs are valued at prices obtained from independent financial service industry-recognized vendors.
Investment Policies and Strategies
The investment objective of the plan, consistent with prudent standards for preservation of capital and maintenance of liquidity, is to earn the highest possible total rate of return consistent with the plan’s tolerance for risk. The general asset allocation guidelines for plan assets are that “equities” will constitute from 40% to 60% of the market value of total fund assets with a target of 44%, and “fixed income” obligations, including cash, will constitute from 40% to 60% with a target of 56%. The term “equities” includes common stock, convertible bonds and convertible stock. The term “fixed income” includes preferred stock and/or contractual payments with a specific maturity date. The Company strives to maintain asset allocations within the designated ranges by conducting periodic reviews of fund allocations and plan liquidity needs, and rebalancing the portfolio accordingly. Diversification of assets is employed to ensure that adverse performance of one security or security class does not have an undue detrimental impact on the portfolio as a whole. Diversification is interpreted to include diversification by type, characteristic and number of investments, as well as by investment style of designated investment fund managers. No restrictions are placed on the selection of individual investments by the investment fund managers. The total fund performance and the performance of the investment fund managers is reviewed on a regular basis, using appointed professional independent advisors. As of December 31, 2016 and 2015, there were no shares of the Company’s stock held in plan assets.
Cash Flows
The Company expects to contribute approximately $5.8 million to its defined benefit plans and $0.1 million to its other postretirement benefit plans in 2017. The Company also expects to contribute approximately $10.0 million to its defined contribution plan and $8.4 million to its 401(k) savings plan in 2017.
Estimated Future Benefit Payments
The future estimated benefit payments for the next five years and the five years thereafter are as follows: 2017 — $14.4 million; 2018 — $10.6 million; 2019 — $10.3 million; 2020 — $10.8 million; 2021 — $10.6 million; 2022 to 2026 — $52.1 million.
70
16. Quarterly Results of Operations (Unaudited)
The unaudited quarterly results of operations for the years ended December 31, 2016 and 2015 are as follows:
| 2016 Quarters | 2015 Quarters | ||||||||||||||||||||||||||||||
| First | Second | Third | Fourth | First | Second | Third | Fourth | ||||||||||||||||||||||||
| (In thousands, except per share amounts) | |||||||||||||||||||||||||||||||
| Net sales | $ | 502,572 | $ | 549,696 | $ | 530,356 | $ | 530,419 | $ | 502,198 | $ | 514,881 | $ | 503,791 | $ | 499,798 | |||||||||||||||
| Gross profit | 223,335 | 244,058 | 230,889 | 232,485 | 226,041 | 231,615 | 223,260 | 223,399 | |||||||||||||||||||||||
| Operating income | 102,557 | 112,976 | 108,857 | 81,411 | 101,757 | 109,909 | 121,813 | 98,259 | |||||||||||||||||||||||
| Net income | 68,130 | 75,759 | 69,873 | 57,347 | 65,954 | 69,585 | 79,505 | 67,763 | |||||||||||||||||||||||
| Basic EPS | $ | 0.90 | $ | 1.00 | $ | 0.92 | $ | 0.75 | $ | 0.84 | $ | 0.89 | $ | 1.03 | $ | 0.89 | |||||||||||||||
| Diluted EPS | $ | 0.89 | $ | 0.99 | $ | 0.91 | $ | 0.75 | $ | 0.84 | $ | 0.89 | $ | 1.02 | $ | 0.88 | |||||||||||||||
| Basic weighted average shares outstanding | 75,749 | 75,690 | 75,819 | 75,955 | 77,996 | 77,466 | 76,831 | 76,211 | |||||||||||||||||||||||
| Diluted weighted average shares outstanding | 76,699 | 76,674 | 76,880 | 76,806 | 78,856 | 78,297 | 77,646 | 77,091 | |||||||||||||||||||||||
| (1) Quarterly data includes acquisition of Novotema (June 2015), Alfa Valvole (June 2015), CiDRA Precision Services (July 2015), Akron Brass (March 2016), AWG Fittings (July 2016) and SFC Koenig (September 2016) from the date of acquisition. Quarterly data also includes the results of Ismatec (July 2015), Hydra-Stop (July 2016), CVI Japan (September 2016), IETG (October 2016) and CVI Korea (December 2016) through the date of disposition. | |||||||||||||||||||||||||||||||
17. Subsequent Events
On January 31, 2017 the Company entered into four forward contracts with a combined notional value of €180 million and a settlement date of March 31, 2017. These contracts were executed to minimize the earnings impact due to foreign currency fluctuations between the Swiss Franc and the Euro associated with certain intercompany loans that were established in conjunction with the SFC Koenig acquisition.
71
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
The Company maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed in the Company’s Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including its Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
As required by SEC Rule 13a-15(b), the Company carried out an evaluation, under the supervision and with the participation of the Company’s management, including the Company’s Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of the Company’s disclosure controls and procedures as of the end of the period covered by this report. Based on the foregoing, the Company’s Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2016.
Management’s Report on Internal Control Over Financial Reporting appearing on page 35 of this report is incorporated into this Item 9A by reference.
There has been no change in the Company’s internal control over financial reporting during the Company’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
Item 9B. Other Information.
None.
72
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Information under the headings “Election of Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance,” and the information under the subheading “Information Regarding the Board of Directors and Committees,” in the 2017 Proxy Statement is incorporated into this Item 10 by reference. Information regarding executive officers of the Company is located in Part I, Item 1, of this report under the caption “Executive Officers of the Registrant.”
The Company has adopted a Code of Business Conduct and Ethics applicable to the Company’s directors, officers (including the Company’s principal executive officer, principal financial officer and principal accounting officer) and employees. The Code of Business Conduct and Ethics, along with the Audit Committee Charter, Nominating and Corporate Governance Committee Charter, Compensation Committee Charter and Corporate Governance Guidelines are available on the Company’s website at www.idexcorp.com under “Investor Relations.” In the event we amend or waive any of the provisions of the Code of Business Conduct and Ethics applicable to our principal executive officer, principal financial officer or principal accounting officer, we intend to disclose the same on the Company’s website.
Item 11. Executive Compensation.
Information under the heading “Executive Compensation” in the 2017 Proxy Statement is incorporated into this Item 11 by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
Information under the heading “Security Ownership” in the 2017 Proxy Statement is incorporated into this Item 12 by reference.
Equity Compensation Plan Information
Information with respect to the Company’s equity compensation plans as of December 31, 2016 is as follows:
| Plan Category | Number of Securities To be Issued Upon Exercise of Outstanding Options, Warrants and Rights | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans(1) | ||||||
| Equity compensation plans approved by the Company’s stockholders | 2,400,384 | $ | 61.83 | 5,382,493 | |||||
(1) Includes an indeterminate number of shares underlying deferred compensation units (“DCUs”) granted under the Directors Deferred Compensation Plan and Deferred Compensation Plan for Non-officer Presidents which are issuable under the Company’s Incentive Award Plan. Also includes an indeterminate number of shares underlying DCUs granted under the Deferred Compensation Plan for Officers, which shares are issuable under the Incentive Award Plan. The number of DCUs granted under these plans is determined by dividing the amount deferred by the closing price of the common stock the day before the date of deferral. The DCUs are entitled to receive dividend equivalents which are reinvested in DCUs based on the same formula for investment of a participant’s deferral.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information under the heading “Information Regarding the Board of Directors and Committees” in the 2017 Proxy Statement is incorporated into this Item 13 by reference.
Item 14. Principal Accountant Fees and Services.
Information under the heading “Principal Accountant Fees and Services” in the 2017 Proxy Statement is incorporated into this Item 14 by reference.
73
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(A) 1. Financial Statements
Consolidated financial statements filed as part of this report are listed under Part II. Item 8. “Financial Statements and Supplementary Data.”
2. Financial Statement Schedules
Financial statement schedules are omitted because they are not applicable, not required, or because the required information is included in the Consolidated Financial Statements of the Company or the Notes thereto.
3. Exhibits
The exhibits filed with this report are listed on the “Exhibit Index.”
(B) Exhibit Index
Reference is made to the Exhibit Index beginning on page 83 hereof.
74
Item 16. Form 10-K Summary.
None.
75
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| IDEX CORPORATION | ||
| By: | /s/ WILLIAM K. GROGAN | |
| William K. Grogan | ||
| Senior Vice President and Chief Financial Officer | ||
Date: February 23, 2017
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ ANDREW K. SILVERNAIL | Chairman of the Board and Chief Executive Officer (Principal Executive Officer) | |||
| Andrew K. Silvernail | February 23, 2017 | |||
| /s/ WILLIAM K. GROGAN | Senior Vice President and Chief Financial Officer (Principal Financial Officer) | |||
| William K. Grogan | February 23, 2017 | |||
| /s/ MICHAEL J. YATES | Vice President and Chief Accounting Officer (Principal Accounting Officer) | |||
| Michael J. Yates | February 23, 2017 | |||
| /s/ MARK A. BUTHMAN | Director | |||
| Mark A. Buthman | February 23, 2017 | |||
| /s/ WILLIAM M. COOK | Director | |||
| William M. Cook | February 23, 2017 | |||
| /s/ KATRINA L. HELMKAMP | Director | |||
| Katrina L. Helmkamp | February 23, 2017 | |||
| /s/ ERNEST J. MROZEK | Director | |||
| Ernest J. Mrozek | February 23, 2017 | |||
| /s/ DAVID C. PARRY | Director | |||
| David C. Parry | February 23, 2017 | |||
| /s/ LIVINGSTON L. SATTERTHWAITE | Director | |||
| Livingston L. Satterthwaite | February 23, 2017 | |||
| /s/ CYNTHIA J. WARNER | Director | |||
| Cynthia J. Warner | February 23, 2017 | |||
76
Exhibit Index
Exhibit Number | Description | ||
| 3.1 | Restated Certificate of Incorporation of IDEX Corporation (incorporated by reference to Exhibit No. 3.1 to the Registration Statement on Form S-1 of IDEX, et al., Registration No. 33-21205, as filed on April 21, 1988) | ||
| 3.1(a) | Amendment to Restated Certificate of Incorporation of IDEX Corporation (incorporated by reference to Exhibit No. 3.1 (a) to the Quarterly Report of IDEX on Form 10-Q for the quarter ended March 31, 1996, Commission File No. 1-10235) | ||
| 3.1(b) | Amendment to Restated Certificate of Incorporation of IDEX Corporation (incorporated by reference to Exhibit No. 3.1 (b) to the Current Report of IDEX on Form 8-K filed March 24, 2005, Commission File No. 1-10235) | ||
| 3.2 | Amended and Restated By-Laws of IDEX Corporation (incorporated by reference to Exhibit No. 3.1 to the Current Report of IDEX on Form 8-K filed November 14, 2011, Commission File No. 1-10235) | ||
| 4.1 | Specimen Certificate of Common Stock of IDEX Corporation (incorporated by reference to Exhibit No. 4.3 to the Registration Statement on Form S-2 of IDEX, et al., Registration No. 33-42208, as filed on September 16, 1991) | ||
| 4.2 | Credit Agreement, dated as of June 23, 2015, among IDEX Corporation, Bank of America N.A. as Agent and Issuing Bank, and the Other Financial Institutions Party Hereto (incorporated by reference to Exhibit 10.1 to the Current Report of IDEX on Form 8-K filed June 25, 2015, Commission File No. 1-10235) | ||
| 4.3 | Indenture between IDEX Corporation and Wells Fargo Bank, National Association, as Trustee, dated as of December 6, 2010 (Debt Securities) (incorporated by reference to Exhibit No. 4.1 to the Current Report of IDEX on Form 8-K filed December 7, 2010, Commission File No. 1-10235) | ||
| 4.4 | First Supplemental Indenture between IDEX Corporation and Wells Fargo Bank, National Association, as Trustee, dated as of December 6, 2010 (as to 4.5% Senior Notes due 2020) (incorporated by reference to Exhibit No. 4.2 to the Current Report of IDEX on Form 8-K filed December 7, 2010, Commission File No. 1-10235) | ||
| 4.5 | Second Supplemental Indenture between IDEX Corporation and Wells Fargo Bank, National Association, as Trustee, dated as of December 13, 2011 (as to 4.2% Senior Notes due 2021) (incorporated by reference to Exhibit No. 4.1 to the Current Report of IDEX on Form 8-K filed December 14, 2011, Commission File No. 1-10235) | ||
| 4.6 | Note Purchase Agreement, dated June 13, 2016, between IDEX Corporation and the Purchasers listed in Schedule A thereto (incorporated by reference in Exhibit No. 4.1 to the Current Report of IDEX on Form 8-K filed June 15, 2016, Commission File No. 1-10235) | ||
| 10.1** | Revised and Restated IDEX Management Incentive Compensation Plan for Key Employees Effective January 1, 2013 (incorporated by reference to Exhibit 10.2 to the Current Report of IDEX on Form 8-K filed February 20, 2013, Commission File No. 1-10235) | ||
| 10.2** | Form of Indemnification Agreement of IDEX Corporation (incorporated by reference to Exhibit No. 10.23 to the Registration Statement on Form S-1 of IDEX, et al., Registration No. 33-28317, as filed on April 26, 1989, Commission File No. 1-10235) | ||
| 10.3** | IDEX Corporation Amended and Restated Stock Option Plan for Outside Directors, adopted by resolution of the Board of Directors dated as of November 20, 2003 (incorporated by reference to Exhibit 10.6 (a) to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2003, Commission File No. 1-10235) | ||
| 10.4** | IDEX Corporation Incentive Award Plan (as amended and restated) (incorporated by reference to Appendix A of the Proxy Statement of IDEX on Schedule 14A, filed March 5, 2010, Commission File No. 1-10235) | ||
| 10.5** | Employment Agreement dated November 8, 2015 between IDEX Corporation and Andrew K. Silvernail (incorporated by reference to Exhibit No. 10.1 to the Current Report of IDEX Corporation on Form 8-K filed February 19, 2016, Commission File No. 1-10235) | ||
77
Exhibit Number | Description | ||
| 10.6** | Third Amended and Restated IDEX Corporation Directors Deferred Compensation Plan (incorporated by reference to Exhibit No. 10.30 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2010, Commission File No. 1-10235) | ||
| 10.7** | IDEX Corporation Supplemental Executive Retirement and Deferred Compensation Plan (incorporated by reference to Exhibit No. 10.31 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2010, Commission File No. 1-10235) | ||
| 10.8** | Letter Agreement between IDEX Corporation and Daniel Salliotte, dated September 30, 2010 (incorporated by reference to Exhibit No. 10.17 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2012, Commission File No. 1-10235) | ||
| 10.9** | Letter Agreement between IDEX Corporation and Jeffrey Bucklew, dated January 16, 2012 (incorporated by reference to Exhibit No. 10.16 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2013, Commission File No. 1-10235) | ||
| 10.10** | Letter Agreements between IDEX Corporation and Eric Ashleman, dated January 14, 2008 and February 12, 2014 (incorporated by reference to Exhibit No. 10.14 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2014, Commission File No. 1-10235) | ||
| 10.11** | Form of IDEX Corporation Restricted Stock Award Agreement effective February 2015 (incorporated by reference to Exhibit No. 10.16 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2014, Commission File No. 1-10235) | ||
| 10.12** | Form of IDEX Corporation Stock Option Agreement effective February 2015 (incorporated by reference to Exhibit No. 10.17 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2014, Commission File No. 1-10235) | ||
| 10.13** | Form of IDEX Corporation Restricted Stock Unit Award Agreement effective February 2015 (incorporated by reference to Exhibit No. 10.18 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2014, Commission File No. 1-10235) | ||
| 10.14** | Form of IDEX Corporation Restricted Stock Unit Award Agreement - Cash Settled effective February 2015 (incorporated by reference to Exhibit No. 10.19 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2015, Commission File No. 1-10235) | ||
| 10.15** | Form of IDEX Corporation Performance Share Unit Award Agreement effective February 2015 (incorporated by reference to Exhibit No. 10.20 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2015, Commission File No. 1-10235) | ||
| 10.16** | Form of IDEX Corporation Restricted Stock Unit Agreement for Directors effective February 2015 (incorporated by reference to Exhibit No. 10.21 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2014, Commission File No. 1-10235) | ||
| 10.17** | Form of IDEX Corporation Stock Option Agreement effective February 2015 (incorporated by reference to Exhibit No. 10.22 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2014, Commission File No. 1-10235) | ||
| 10.18** | Form of IDEX Corporation Restricted Stock Award Agreement effective February 2015 (incorporated by reference to Exhibit No. 10.23 to the Annual Report of IDEX on Form 10-K for the year ended December 31, 2014, Commission File No. 1-10235) | ||
| 10.19** | Letter Agreement between IDEX Corporation and Brett Finley, dated December 18, 2015. (incorporated by reference to Exhibit No. 10.23 to the Annual Report of IDEX Corporation on Form 10-K for the fiscal year ended December 31, 2015, Commission File No. 1-10235) | ||
| 10.20** | Letter Agreement between IDEX Corporation and Denise Cade, dated September 24, 2015. (incorporated by reference to Exhibit No. 10.24 to the Annual Report of IDEX Corporation on Form 10-K for the fiscal year ended December 31, 2015, Commission File No. 1-10235) | ||
| 10.21 | Stock Purchase Agreement, dated February 4, 2016, by and among IDEX Corporation, Premier Farnell PLC, Celdis Limited, Premier Farnell Corp. and Akron Brass Holding Corp. (incorporated by reference to Exhibit No. 10.25 to the Annual Report of IDEX Corporation on Form 10-K for the fiscal year ended December 31, 2015, Commission File No. 1-10235) | ||
| 10.22** | Letter Agreement between IDEX Corporation and William K. Grogan, dated December 30, 2016. | ||
78
| Exhibit Number | Description | ||
| 12 | Ratio of Earnings to Fixed Charges | ||
| 21 | Subsidiaries of IDEX | ||
| 23 | Consent of Deloitte & Touche LLP | ||
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14 (a) or Rule 15d-14 (a) | ||
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14 (a) or Rule 15d-14 (a) | ||
| ***32.1 | Certification pursuant to Section 1350 of Chapter 63 of Title 18 of the United States Code | ||
| ***32.2 | Certification pursuant to Section 1350 of Chapter 63 of Title 18 of the United States Code | ||
| ****101 | The following materials from IDEX Corporation’s Annual Report on Form 10-K for the year ended December 31, 2016 formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Balance Sheets at December 31, 2016 and 2015, (ii) the Consolidated Statements of Operations for the three years ended December 31, 2016, (iii) the Consolidated Statements of Comprehensive Income for the three years ended December 31, 2016, (iv) the Consolidated Statements of Shareholders’ Equity for the three years ended December 31, 2016, (v) the Consolidated Statements of Cash Flows for the three years ended December 31, 2016, and (vi) Notes to the Consolidated Financial Statements. | ||
| ** | Management contract or compensatory plan or agreement. | |
| *** | Furnished herewith. | |
| **** | In accordance with Rule 406T of Regulation S-T, the XBRL related information in Exhibit 101 to this Annual Report on Form 10-K shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, and shall not be part of any registration statement or other document filed under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such filing. | |
79