Exhibit 99.1

NEUROSURGERY OPHTHALMOLOGY QUALITY. PERFORMANCE. INNOVATION. Investor Presentation April 2015

Certain statements made in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. This presentation may include statements concerning management’s expectations of future financial results, potential business, potential acquisitions, government agency approvals, additional indications and therapeutic applications for medical devices, as well as their outcomes, clinical efficacy and potential markets and similar statements, all of which are forward looking. Forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from predicted results. For a discussion of such risks and uncertainties, please refer to the information set forth under “Risk Factors” included in Synergetics USA, Inc.’s Annual Report on Form 10-K for the year ended July 31, 2014, and information contained in subsequent filings with the Securities and Exchange Commission. These forward looking statements are made based upon our current expectations and we undertake no duty to update information provided in this presentation. Safe Harbor Statement *

Overview Corporate Information Market Information NASDAQ: SURGMarket Cap: $138.8mm52 Week Range: $2.93 – $5.50Shares Outstanding: 25.6 mmInstitutional Ownership: 57.81%Russell Microcap Index * Synergetics USA, Inc. is a medical device company focused in the fast-growing ophthalmology and neurosurgery marketsFormed through a reverse merger of Synergetics, Inc. and Valley Forge Scientific Corp. in 2005Synergetics, Inc. was founded in 1991 and Valley Forge was founded in 1980Corporate Headquarters: O’Fallon, MOManufacturing Facilities: O’Fallon, MO, California and Redditch, UK *Source: NASDAQ, as of 03/25/15.

Track Record of Growth * *Fiscal Year 2013 gross and operating margins are displayed on a non-GAAP basis to exclude inventory write-down. Fiscal Year 2014 operating margins are displayed on a non-GAAP basis to exclude exit costs. See non-GAAP reconciliation table at the end of this presentation for additional details.

FY 2014 Revenue Mix Ophthalmic sales represent our largest and highest margin business.In the U.S., we sell ophthalmic surgical products directly to end-users at hospitals, ambulatory surgery centers and surgeon offices throughout the country.Internationally, we sell and distribute ophthalmic surgical products in over 50 countries, including four emerging markets.We serve as a marketing partner and have key OEM relationships with J&J’s Codman division and Stryker for neurosurgery products. *

Overall Strategy Drive Accelerating Growth in our Ophthalmology BusinessDeliver Improved Profitability through our Enterprise-Wide Continuous Improvement InitiativesManage our Neurosurgery and OEM Businesses for Stable Growth and Strong Cash FlowDemonstrate Consistent, Solid Financial PerformanceGrowth through Strategic Acquisitions *
Sterimedix Acquisition We purchased all of the outstanding shares of Sterimedix Limited for net cash consideration of $13.2 million.Sterimedix is a private manufacturer of cannulas, needles and other disposable products for ophthalmic and aesthetic procedures.Sterimedix generated $7.9 million in sales in its fiscal year ending December 31, 2014 with 80% of sales coming from the ophthalmic market and the balance coming from aesthetics. *

Sterimedix Acquisition (Cont’d.) Sterimedix is anticipated to grow approximately 16% on a constant currency basis for fiscal year 2015.We plan to let Sterimedix continue to operate its business as a largely independent operation going forward.We expect the acquisition to improve operating and financial results for our entire international business in addition to providing diversification of our reported results towards international sales. *

Products *

Anterior Products * I/A Hand Pieces Injection Cannulas

Posterior Products * Injection Cannulas Flute Handle Cannulas Heavy Liquid Infusion Handles Scleral Markers Infusion Lines Corneal Fixation/Incision Templates

Aesthetic Products * Dermal Filler Cannula Fat Transfer Cannula Sharp Needle Cannula from Sterimedix. A world leader in single-use surgical products

Ophthalmic Surgical Market *

Recent DevelopmentsOphthalmology Launched 2nd generation VersaVIT™ vitrectomy machine in the second half of June 2014Maturing product/challenging environment has pressured revenue growth in base ophthalmic business in recent quartersInternal focus on improving operational excellence and enterprise-wide continuous improvement initiativesM.I.S.S. Ophthalmics LTD acquisitionKing of Prussia plant closure“Biggest Loser” exerciseScrap reduction *

Ophthalmic Surgical Market * *Source: Synergetics USA annual report on Form 10-K for period ended July 31, 2012.

2011 Global Retinal Surgery Device Market * *Source: Synergetics USA quarterly report on Form 10-Q for period ended April 30, 2013. Market Size = $935 million*Synergetics products compete in ~22% of the retinal device market (shaded in black)

2014 Global Retinal Surgery Device Market Estimated Market Size = $1.22 Billion* Implied Annual Growth = ~7% Synergetics products compete in ~69% of the retinal device market (shaded in black)17 Hemostasis $25M Light Pipes $39M Cryosurgery $39M Scleral Buckles $17M Light Sources $7M Vitrectomy Packs $348M Retinal Lasers $264M Vitrectomy Machines $235M Instruments $153M Tamponades $50M Retinal Laser Probes $40M *Source: Synergetics USA quarterly report on Form 10-Q for period ended April 30, 2013. Market Scope data estimates that the vitreoretinal market will grow approximately 7 percent to $1.2 billion in 2014, as compared to 2013

ASC vs. Hospital Ambulatory Surgery Center (ASC) Physicians control care for patientsTypically owned by surgeons or corporationsMore efficient – less time wastedSpecialized (ophtho, ortho, etc.)Highly focused on profitabilityLower costs to patients and government (2014 vitrectomy reimbursement rate of $1,655) Hospital Out-Patient Department (HOPD) Challenging patient flow (pre, intra, post)Staff not specialized and are trained to handle multiple specialtiesPatient frustrations – parking, long walks to OR, confusing, etc.Equipped to handle more difficult proceduresHigher costs to patients and government (2014 vitrectomy reimbursement rate of $2,820) *
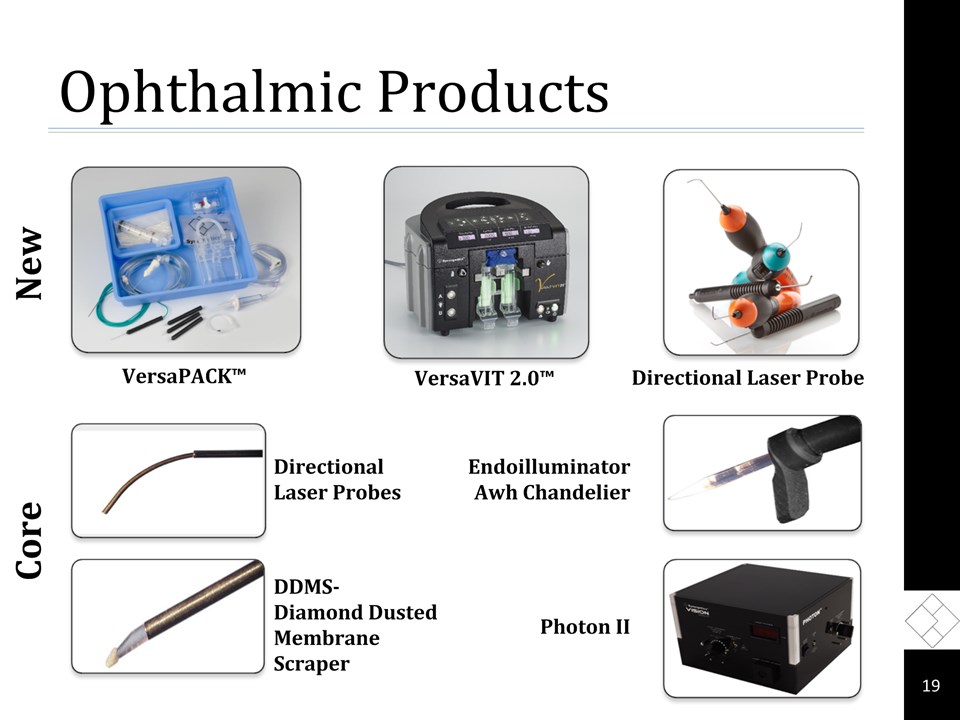
Ophthalmic Products Core VersaPACK™ VersaVIT 2.0™ Directional Laser Probes DDMS-Diamond Dusted Membrane Scraper Endoilluminator Awh Chandelier Photon II New * Directional Laser Probe
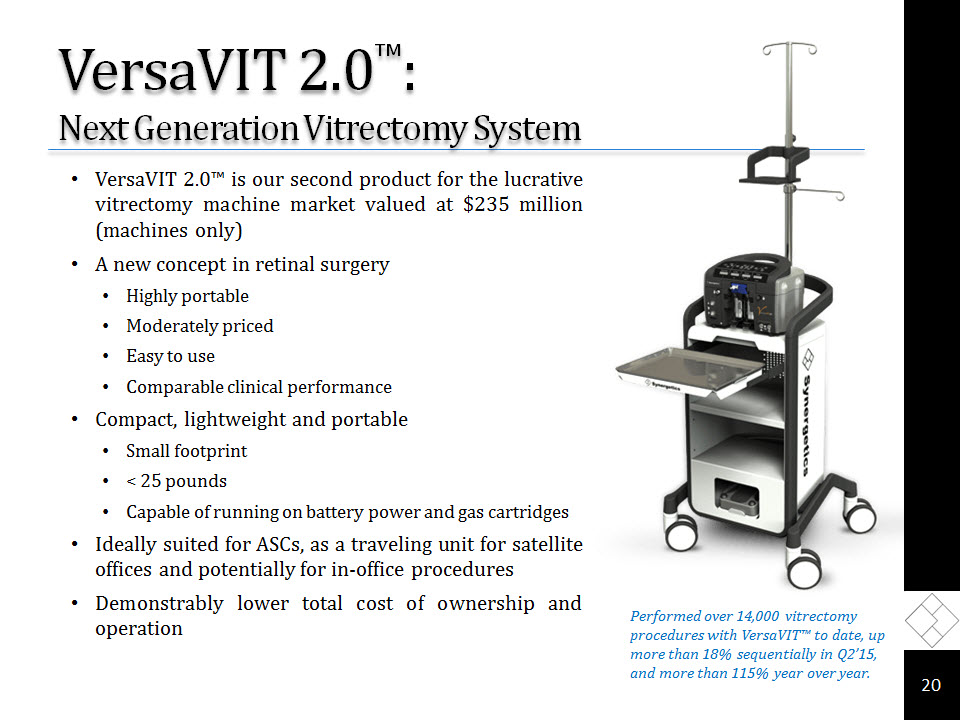
VersaVIT 2.0™: Next Generation Vitrectomy System VersaVIT 2.0™ is our second product for the lucrative vitrectomy machine market valued at $235 million (machines only)A new concept in retinal surgery Highly portableModerately priced Easy to useComparable clinical performanceCompact, lightweight and portableSmall footprint< 25 pounds Capable of running on battery power and gas cartridgesIdeally suited for ASCs, as a traveling unit for satellite offices and potentially for in-office proceduresDemonstrably lower total cost of ownership and operation * Performed over 14,000 vitrectomy procedures with VersaVIT™ to date, up more than 18% sequentially in Q2’15, and more than 115% year over year.

VersaVIT 2.0™ vs. the Competition * VersaVIT™ vs. ACCURUS® (25lbs vs. 90lbs) CONSTELLATION® Vision System CONSTELLATION® Vision System and ACCURUS® are registered trademarks of Alcon® Laboratories, a division of Novartis

VersaVIT 2.0™: Strategic Growth Plan & Progress Update Introduced on June 4, 2014Commercial launch in the second half of June targeting two primary segments: high volume ASC facilities that perform the majority of vitrectomy proceduresselect teaching institutions U.S. Market: 22 direct sales reps; International markets: hybrid distribution of direct and dealers Anticipate progress towards broader market adoption of our next generation vitrectomy technology in fiscal 2015 *

Ophthalmology Product Video *

Neurosurgery Market *

Recent DevelopmentsNeurosurgery Our neurosurgery partnerships remain strong – sales increased 8.3% year-over-year during fiscal 2014. Our Codman contract has been renewed.A majority of the business is consumables.We launched “slim” and irrigating bipolar forceps in September.Our King of Prussia plant closed during Q2 2015. *

Neurosurgery Overview We provide best-in-class neurosurgical technologies.Ultrasonic aspirators Disposable tips and tubingElectrosurgical generators Disposable bipolar forceps We have strong partnerships.J&J’s Codman division distributes our electrosurgical generators and bipolar forceps.Stryker distributes our ultrasonic aspirator disposables.Long-term relationships with Codman and Stryker provide stable annual growth, attractive operating margins and high barriers to entry. *
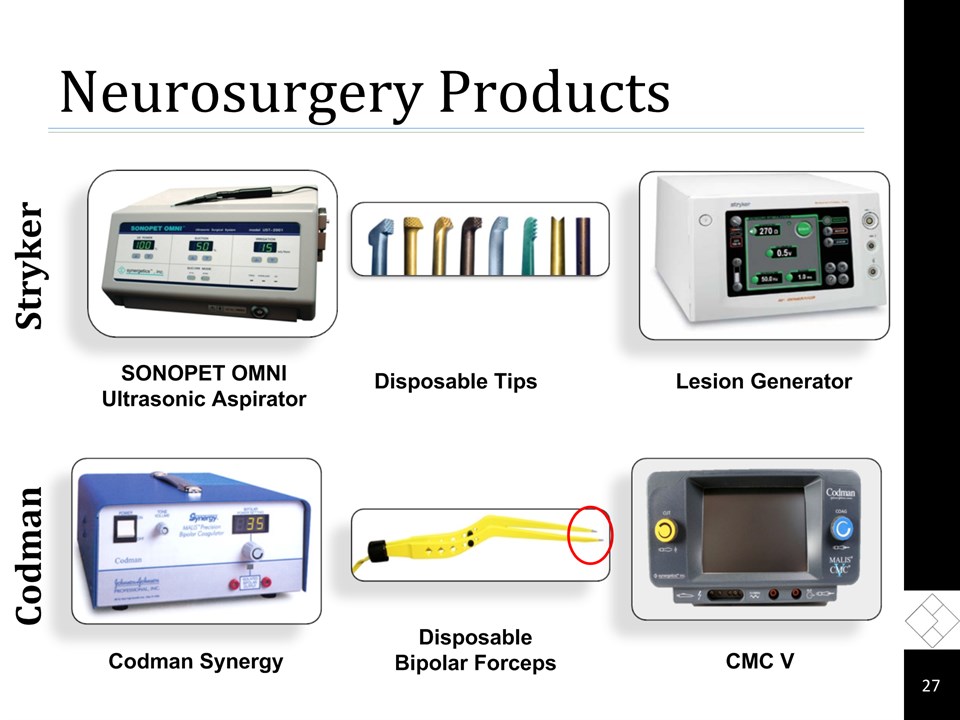
Neurosurgery Products * Codman Stryker Lesion Generator SONOPET OMNI Ultrasonic Aspirator Disposable Tips Codman Synergy Disposable Bipolar Forceps CMC V

Neurosurgery Product Video *

Financials *

Financial Comparison – Quarterly * Net sales from Ophthalmic represent all sales of ophthalmic devices from direct sales representatives, distribution partners and OEMs. Recognition of deferred revenue of $322,000 from Alcon, Inc. is included in this category for the three months ended January 31, 2015 and 2014, respectively.Net sales from Neurosurgery represent sales of electrosurgery generators, disposable bipolar forceps and related accessories and royalties from Codman; multi-channel generators, disposable ultrasonic tips and related accessories to Stryker; and certain neurosurgery disposables sold through distribution. Many of the products we sell to our neurosurgery OEM customers are shipped to their non-U.S. customers in various countries around the world, but are included in our domestic revenues.Other net sales represent all sales of aesthetic devices and other miscellaneous revenues. Non-operating adjustments include: exit costs (pre-tax costs of $657,000 and $514,000 for the three months ended January 31, 2015 and January 31, 2014, respectively) and Sterimedix acquisition related costs (pre-tax $204,000 for the three months ended January 31, 2015). See non-GAAP reconciliation slides at the end of this presentation for additional details. N/M Not Meaningful

Financial Comparison – 6 Months * Net sales from Ophthalmic represent all sales of ophthalmic devices from direct sales representatives, distribution partners and OEMs. Recognition of deferred revenue of $644,000 from Alcon, Inc. is included in this category for the six months ended January 31, 2015 and 2014, respectively.Net sales from Neurosurgery represent sales of electrosurgery generators, disposable bipolar forceps and related accessories and royalties from Codman; multi-channel generators, disposable ultrasonic tips and related accessories to Stryker; and certain neurosurgery disposables sold through distribution. Many of the products we sell to our neurosurgery OEM customers are shipped to their non-U.S. customers in various countries around the world, but are included in our domestic revenues.Other net sales represent all sales of aesthetic devices and other miscellaneous revenues. Non-operating adjustments include: exit costs (pre-tax costs of $719,000 and $514,000 for the six months ended January 31, 2015 and January 31, 2014, respectively) and Sterimedix acquisition related costs (pre-tax $290,000 for the six months ended January 31, 2015). See non-GAAP reconciliation slides at the end of this presentation for additional details. N/M Not Meaningful
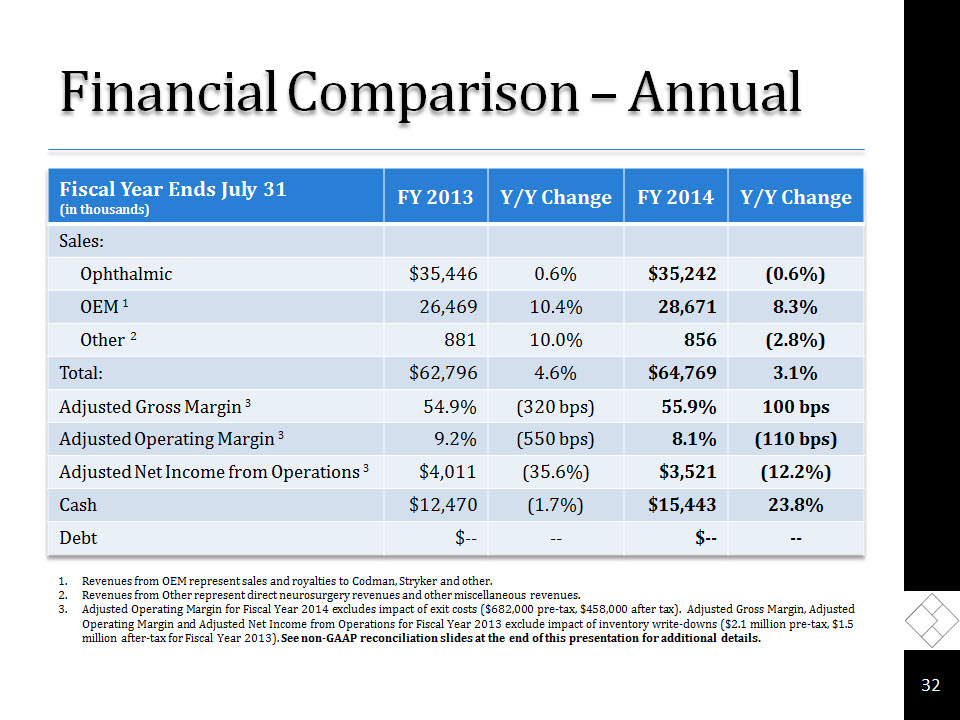
Financial Comparison – Annual * Revenues from OEM represent sales and royalties to Codman, Stryker and other.Revenues from Other represent direct neurosurgery revenues and other miscellaneous revenues. Adjusted Operating Margin for Fiscal Year 2014 excludes impact of exit costs ($682,000 pre-tax, $458,000 after tax). Adjusted Gross Margin, Adjusted Operating Margin and Adjusted Net Income from Operations for Fiscal Year 2013 exclude impact of inventory write-downs ($2.1 million pre-tax, $1.5 million after-tax for Fiscal Year 2013). See non-GAAP reconciliation slides at the end of this presentation for additional details.

Financial Comparison – Market vs. Distribution * Net sales from Ophthalmic represent all sales of ophthalmic devices from direct sales representatives, distribution partners and OEMs. Recognition of deferred revenue of $322,000 and $644,000 from Alcon, Inc. is included in this category for the three and six months ended January 31, 2015 and 2014, respectively.Net sales from Neurosurgery represent sales of electrosurgery generators, disposable bipolar forceps and related accessories and royalties from Codman; multi-channel generators, disposable ultrasonic tips and related accessories to Stryker; and certain neurosurgery disposables sold through distribution. Many of the products we sell to our neurosurgery OEM customers are shipped to their non-U.S. customers in various countries around the world, but are included in our domestic revenues.Other net sales represent all sales of aesthetic devices and other miscellaneous revenues.Net sales from Ophthalmic represent sales of ophthalmic devices from direct sales representatives and distribution partners.Net sales from OEM represent sales of electrosurgery generators, disposable bipolar forceps and related accessories and royalties from Codman; multichannel generators, disposable ultrasonic tips and related accessories to Stryker; and sales of certain disposable products. Recognition of deferred revenues of $322,000 and $644,000 from Alcon, Inc. is included in this category for the three and six months ended January 31, 2015 and 2014, respectively. Many of the products that the Company sells to its neurosurgery OEM customers are shipped to their non-U.S. customers in various countries around the world, but are included in the Company’s domestic revenues.Other net sales represent direct neurosurgery revenues and other miscellaneous revenues.

Investment Rationale Key medical device manufacturer supplying ophthalmic and neurosurgery markets with leading technologiesRetinal surgery, a compelling segment of ophthalmologyNew product introductions – foremost of which is the VersaVIT 2.0™ vitrectomy machine – drives total Company revenue growth over long termBusiness model fueled by the combination of high margin disposables and innovative capital equipment Long-term profit margin opportunities driven by improving operational efficiency and continuous improvement initiatives *

Management Team David M. Hable – President, CEOOver 30 years of progressive responsibility in sales, marketing, new business development and general management in the medical device industry. Over 20 years with J&J/Codman. Pamela Boone – Executive Vice President, CFOPreviously served as CFO, VP and Corporate Controller for Maverick Tube Corporation. 30 years of financial expertise.Michael Fanning – Vice President, SalesOver 20 years in sales and management roles, working in service, medical device and manufacturing sectors.Jason Stroisch – Vice President, Marketing & TechnologyOver 15 years in the medical device industry covering engineering, international sales and marketing management roles.Joan Kraus – Vice President, Regulatory Affairs / Quality AssurancePreviously served as Senior Director Global Compliance for Teleflex Medical. Over 25 years in quality systems and process improvement roles working in medical devices, manufacturing, and distribution sectors. *
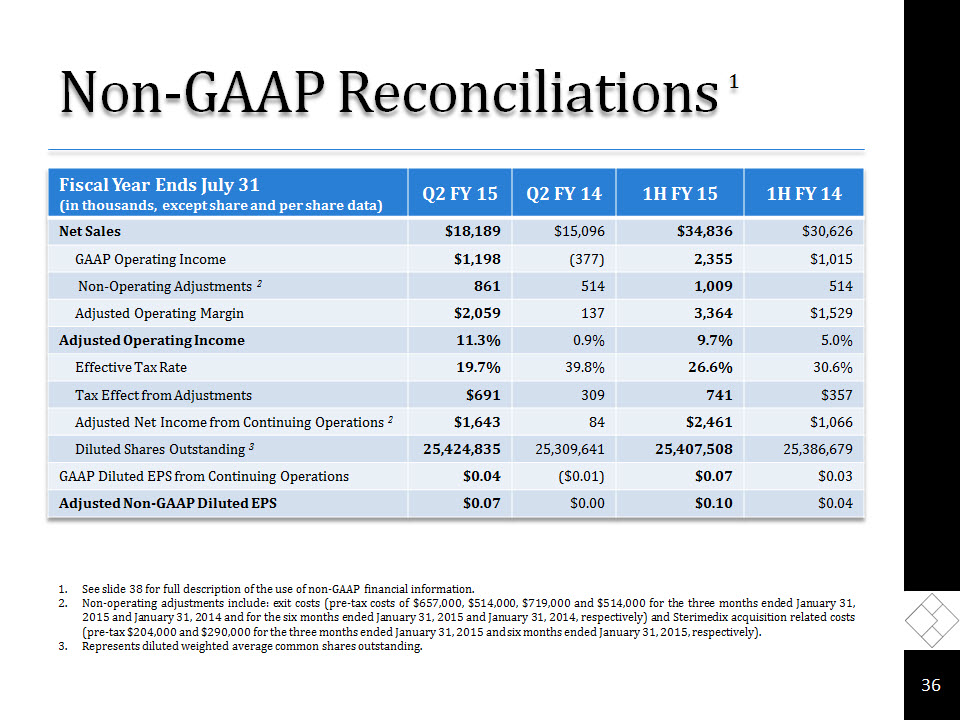
Non-GAAP Reconciliations 1 * See slide 38 for full description of the use of non-GAAP financial information.Non-operating adjustments include: exit costs (pre-tax costs of $657,000, $514,000, $719,000 and $514,000 for the three months ended January 31, 2015 and January 31, 2014 and for the six months ended January 31, 2015 and January 31, 2014, respectively) and Sterimedix acquisition related costs (pre-tax $204,000 and $290,000 for the three months ended January 31, 2015 and six months ended January 31, 2015, respectively).Represents diluted weighted average common shares outstanding.
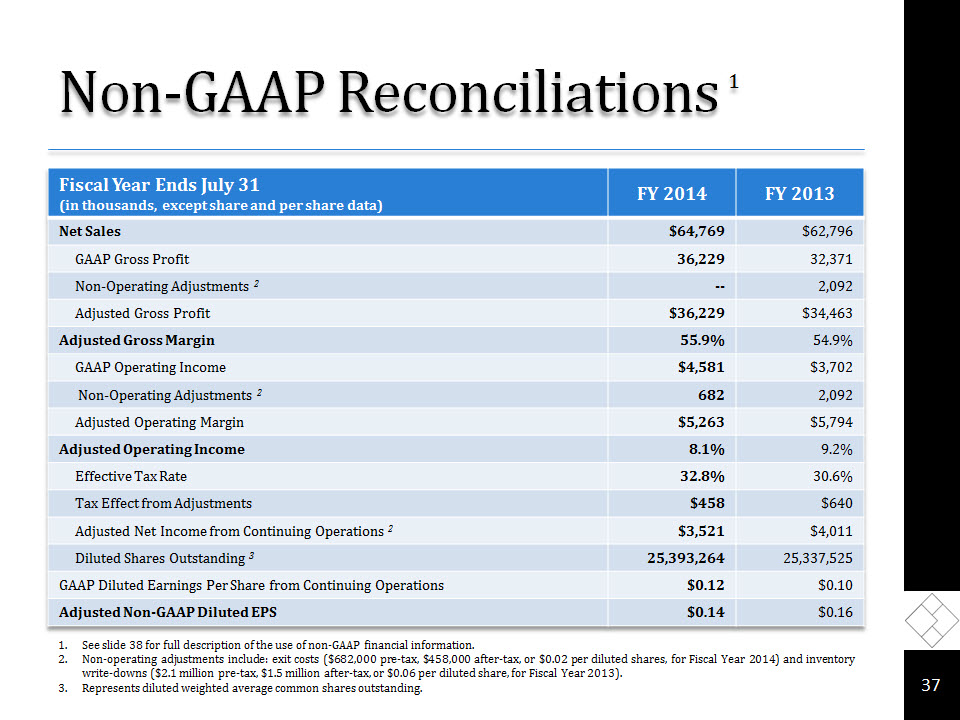
Non-GAAP Reconciliations 1 * See slide 38 for full description of the use of non-GAAP financial information.Non-operating adjustments include: exit costs ($682,000 pre-tax, $458,000 after-tax, or $0.02 per diluted shares, for Fiscal Year 2014) and inventory write-downs ($2.1 million pre-tax, $1.5 million after-tax, or $0.06 per diluted share, for Fiscal Year 2013).Represents diluted weighted average common shares outstanding.

1 Use of Non-GAAP Financial Information We measure our performance primarily through our operating profit. In addition to our consolidated financial statements presented in accordance with GAAP, management uses certain non-GAAP measures, including adjusted gross margin, adjusted operating margin, adjusted EPS and adjusted net income from operations, to measure our operating performance. We provide a definition of the components of these measurements and reconciliation to the most directly comparable GAAP financial measure. These non-GAAP measures are presented to enhance an understanding of our operating results and are not intended to represent cash flow or results of operations. The use of these non-GAAP measures provides an indication of our ability to service debt and measure operating performance. We believe these non-GAAP measures are useful in evaluating our operating performance compared to other companies in our industry, and are beneficial to investors, potential investors and other key stakeholders, including creditors who use this measure in their evaluation of performance. These non-GAAP measures are not in accordance with, or an alternative to, measures prepared in accordance with GAAP and may be different from non-GAAP measures used by other companies. In addition, these non-GAAP measures are not based on any comprehensive set of accounting rules or principles. Non-GAAP measures have limitations in that they do not reflect all of the amounts associated with the Company’s results of operations as determined in accordance with GAAP. These measures should only be used to evaluate our results of operations in conjunction with the corresponding GAAP measures. *

NEUROSURGERY OPHTHALMOLOGY QUALITY. PERFORMANCE. INNOVATION. Investor Presentation April 2015 3845 Corporate Centre DriveO’Fallon, MO 63368(636) 939-5100www.synergeticsusa.com