Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 11, 2009
MILLER DIVERSIFIED CORPORATION
(Exact name of registrant as specified in its charter)
| Nevada | 000-19001 | 84-1070932 | ||
| (State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification No.) |
| 3101 W. Hallandale Boulevard Suite 100 Hallandale, FL | 33009 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:305-749-2676
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Table of Contents
Item 1.01 Entry into a Material Definitive Agreement.
On September 1, 2009 Miller Diversified, Corp. (“we,” “Miller” or the “Company”) entered into a definitive agreement (the “Agreement”) with Smoke Anywhere USA, Inc., a Florida corporation (“Smoke”) whereby the Company will acquire 100% of the issued and outstanding shares of Smoke Anywhere USA, Inc., as a result of the transaction Smoke will become a wholly-owned subsidiary of the Company.
Item 2.01 Completion of Acquisition or Disposition of Assets.
On November 5, 2009, Miller Diversified Corporation, a Nevada corporation (“we,” “Miller” or the “Company”), Smoke Holdings, Inc., a Florida corporation (“Merger Sub”) and Smoke Anywhere USA, Inc. (“Smoke”), a privately-held Florida corporation, completed, subject to certain post closing undertakings, its Acquisition Agreement and Plan of Merger (the “Agreement,”) pursuant to which Miller, through its wholly-owned subsidiary, Merger Sub, acquired Smoke. The resulting share ownership structure is accomplished in three stages; 1) an initial issuance of 20,670,000 shares of the company’s common stock which were issued to the Smoke Shareholders from the company’s authorized but unissued shares, of which 2,074,640 of the company’s shares of common stock were contributed to Miller by Vapeco Holdings, Inc., the company’s majority controlling shareholder (“Vapeco”) and then reissued by Miller in the transaction; 2) a 2.5:1 reverse stock split and 3) a subsequent issuance resulting in the final share ownership of the company with the Smoke shareholders owning 82.68% or 49,610,613 shares of the company’s common stock and Vapeco owning 15.19% or 9,113,966 shares of the company’s common stock.
As soon as practicable following the Closing, Miller (which shall then be controlled by the Smoke Shareholders) shall, as necessary, prepare and file with the SEC preliminary proxy materials, or an information statement, for a stockholders’ meeting or consent procedure in order to consider and approve a 2.5 for 1 reverse split of the Miller Common Stock and amend the company’s articles of incorporation to increase the number of shares the company is authorized to issue.
The transaction contemplated by the Agreement was intended to be a “tax-free” reorganization pursuant to the provisions of Section 351 and 368 of the Internal Revenue Code of 1986, as amended and could only be effected through the cooperation of Vapeco, including but not limited to its contribution of shares needed to consummate the transaction. As a result of the transaction Vapeco’s ownership in the company was reduced from approximately 52% of the company’s common stock to 15.19%. Insofar as the transaction contemplated by the Agreement involves an interested director transaction within the meaning of NRS 78.140, management believes that the transaction is fair to the company and the company will benefit by acquiring an operating business, after several years of inactivity.
For accounting purposes, this transaction was being accounted for as a reverse merger, since the stockholders of Smoke own a majority of the issued and outstanding shares of common stock of the Company, and the directors and executive officers of Smoke now own an control in excess of eighty percent of the company’s outstanding stock.
Item 3.01 Unregistered Sales of Equity Securities.
In connection with the Company’s acquisition of the common stock of Smoke, the Company issued 20,670,000 shares of common stock of Miller to the SMOKE Shareholders, of which 2,074,640 of the Miller shares of Common Stock were loaned to the Company by, the Company’s controlling shareholder. Pursuant to the Acquisition Agreement and Plan of Merger, the company will issue an additional 50,000,000 shares, subsequent to certain corporate actions as set forth above and elsewhere herein.
Item 5.01 Changes in Control of Registrant.
In connection with the acquisition of the Smoke shares of common stock and its operating business, the former shareholders of Smoke, subsequent to the Transaction acquired approximately 83% of the Company’s shares of common stock, which resulted in a change of control of the registrant. (Please see item 5.06 below.)
2
Item 5.06 Change in Shell Company Status.
TABLE OF CONTENTS
| PAGE | ||||||||
| 4 | ||||||||
| 15 | ||||||||
| 34 | ||||||||
| 37 | ||||||||
| 37 | ||||||||
| 38 | ||||||||
| 38 | ||||||||
| 40 | ||||||||
| 40 | ||||||||
| 41 | ||||||||
| 42 | ||||||||
| 42 | ||||||||
| 42 | ||||||||
| 43 | ||||||||
| 43 | ||||||||
| 43 | ||||||||
| Exhibit 2.0 | ||||||||
| Exhibit 15 | ||||||||
| Exhibit 21 | ||||||||
| Exhibit 24 | ||||||||
| Exhibit 31.1 | ||||||||
| Exhibit 32.1 | ||||||||
CAUTIONARY STATEMENTS REGARDING FORWARD-LOOKING STATEMENTS
In this registration statement we make a number of statements, referred to as “forward-looking statements,” which are intended to convey our expectations or predictions regarding the occurrence of possible future events or the existence of trends and factors that may impact our future plans and operating results. These forward-looking statements are derived, in part, from various assumptions and analyses we have made in the context of our current business plan and information currently available to us and in light of our experience and perceptions of historical trends, current conditions and expected future developments and other factors we believe to be appropriate in the circumstances. You can generally identify forward-looking statements through words and phrases such as “seek,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “budget,” “project,” “may be,” “may continue,” “may likely result,” and similar expressions. When reading any forward looking statement, you should remain mindful that actual results or developments may vary substantially from those expected as expressed in or implied by that statement for a number of reasons or factors, such as those relating to:
| • | our ability to successfully sell our products to our customers; | ||
| • | our ability to attract the qualified personnel to implement our growth strategies; | ||
| • | our ability to develop sales and marketing capabilities; | ||
| • | the accuracy of our estimates and projections; | ||
| • | our ability to fund our short-term and long-term financing needs; |
3
Table of Contents
| • | our ability to gain the necessary regulatory approvals, as applicable, to market our products; | ||
| • | changes in our business plan and corporate strategies; and | ||
| • | other risks and uncertainties discussed in greater detail in the sections of this prospectus, including those captioned “Risk Factors.” |
Each forward-looking statement should be read in context with, and with an understanding of, the various other disclosures concerning our Company and our business made elsewhere in this registration statement, as well as other public reports filed with the Securities and Exchange Commission. You should not place undue reliance on any forward-looking statement as a prediction of actual results or developments. We are not obligated to update or revise any forward-looking statement contained in this registration statement to reflect new events or circumstances unless and to the extent required by applicable law. Unless the context requires otherwise, “Miller,” “Company,” “registrant,” “we,” “us,” and “our” and similar terms refer to Miller Diversified Corp. and our operating subsidiary Smoke Anywhere USA, Inc. which we may refer to as “Smoke,” “Smoke Anywhere,” or “Smoke Anywhere USA.”
INFORMATION REQUIRED IN REGISTRATION STATEMENT
Item 1. Description of Business
Overview
Miller Diversified Corp was incorporated in 1987 as a Nevada corporation, the company operated in the commercial cattle feeding business until October 31, 2003 when the company sold substantially all of its assets and became a discontinued operation. The Company has since been operating as a shell company as defined in Rule 12b-2 of the Exchange Act.
On July 15, 2009 the Company entered into a binding letter of intent to acquire Smoke Anywhere USA, Inc. (“Smoke”) in a reverse triangular merger (the “Merger.”) The parties subsequently entered into a definitive agreement on September 1, 2009. Pursuant to the Merger Smoke and Vapeco Holdings, Inc. a Florida corporation, and a wholly owned subsidiary of Miller Diversified, formed specifically for the purpose of effecting this transaction, merged with Smoke Anywhere USA and as a result of the merger Smoke Anywhere, the surviving entity became a wholly owned subsidiary of Miller and is our sole operating business.
The company is a marketer and distributor of personal vaporizers, under the Fity-One™, Krave™, EZ Smoker™ and Green Puffer™ brands. Personal vaporizers are electronic devices that vaporize a liquid solution, which provide users an experience akin to smoking without actual combustion and as such no smoke or noxious odor is dispelled from the device. The most common form of personal vaporizers are “electronic cigarettes” whose solution constituents are primarily propylene glycol, nicotine, and tobacco flavorings or essences.
The company currently sells its personal vaporizers internationally and domestically through distributors, wholesalers and direct to consumers through its websites and direct response television marketing efforts.
Our products are relatively new to market, as is the industry in which we operate. Regulations are emerging that will shape the manner and form in which we operate our business and develop our business plans and strategies going forward. We expect that our products will be regulated by the U.S. Food and Drug Administration (FDA) and other foreign governmental agencies for those respective countries in which we sell our products, and that complying with said regulations will be a critical element of our business operations and success.
Our website is located at www.smoke51.com
4
Table of Contents
Product
Personal vaporizers are electronic devices, the functional elements of which are integrated into a stainless steel shell, include: a small plastic cartridge that contains an absorbent material that is moistened with a propylene glycol liquid solution, which may or may not contain, nicotine, tobacco flavoring or other flavor essences, an electronic airflow sensor, a heating element, referred to as the atomizer that vaporizes the liquid in the mouthpiece so that it can be inhaled, a rechargeable lithium-ion battery which powers the device and certain electronic components such as a timed cutoff switch to prevent overheating, an LED to signal activation of the device and an external charger, to recharge the battery.
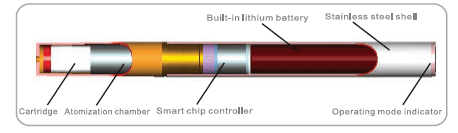
Image presented for illustrative purposes only.
When a user draws air through the device, the air flow is detected by a sensor, which activates a heating element that vaporizes the solution stored in the mouthpiece, the solution is then vaporized and it is this vapor that is inhaled by the user, for pulmonary and or oral (buccal/mucosal) absorption. The solution depending on the model may or may not contain nicotine, the flavoring if any, is also based on the particular cartridge and may contain tobacco flavoring or menthol flavoring, in addition to propylene glycol.
We sell two and three piece, in addition to disposable personal vaporizers, the distinction between two and here piece personal vaporizers is the construct of the unit, the number of core parts and the performance and actual use of the device. We also market, USB, home and car charging devices; in addition to several varieties of cartridges with varying formulations, including but not limited to zero nicotine with no flavoring, zero nicotine with tobacco and menthol flavors, in addition to the standard electronic cigarette filter which contains tobacco flavoring and varying degrees of nicotine. We sell our products in a kit, or as separate components, we also offer for sale replacement cartridges to be used with our non-disposable personal vaporizer when the cartridges become depleted.
Our Product Line
We market and distribute four distinct personal vaporizer products, which include:
EZsmoker.Ezsmoker is our no-nicotine product offering which we sell through direct response television marketing and our Ezsmoker.com website. Our EZsmoker campaign is based on a subscription model where we offer the vaporizer for free contingent on an initial purchase of replacement cartridges and subscribing for a monthly cartridge replacement service. EZsmoker cartridges are offered in tobacco and menthol flavor. Our EZsmoker product website is located at www.ezsmoker.com
Fifty-One.Fifty one is our traditional electronic cigarette product offering, available in 2 or 3 piece units and competes with other electronic cigarette products sold by our competitors. The Fifty-One is sold primarily at retail locations and is sold through our distributor network. We also sell our Fifty-One product online at www.smoke51.com
Krave.Krave is our one piece disposable personal vaporizer, it is our most affordable product offering. The Krave personal vaporizer is available in either tobacco or menthol flavors and is available to the public solely through retail channels. Our Krave product is featured at www.kraveit.com
Green Puffer.We have not yet begun to sell the Green Puffer personal vaporizer, however we plan to market the product based on our personal vaporizers environmentally friendly characteristics. Our Green Puffer website can be found at www.greenpuffer.com
5
Table of Contents
The Market for Our Products
We currently sell our products through multiple sales and distribution channels, both domestic and internationally. Our products are sold in retail locations, over the Internet and through our direct response television marketing campaigns. To date we have sold approximately 93,000 personal vaporizer kits and approximately 1,450,000 replacement cartridges to a total of approximately 35,000 customers. 675 of our customers are wholesale and/or retailer operators, 18 are distributors and we have enrolled approximately 708 independent Internet affiliates who drive traffic and sales to our internet websites.
We believe that the market for personal vaporizers is substantial and will continue to grow both domestically and abroad. Moreover if we are able to gain FDA approval to market our products as smoking cessation aids, our market will include the $3 billion per year smoking cessation drug and OTC market, according to ReportLinker a UK based research firm.
We estimate that demand for our products comes predominantly from smokers who may find our products appealing, according to the World Health Organization;
| • | There are 1.1 billion smokers in the world today, and if current trends continue, that number is expected to increase to 1.6 billion by the year 2025. | ||
| • | China is home to 300 million smokers who consume approximately 1.7 trillion cigarettes a year, or 3 million cigarettes a minute. | ||
| • | Worldwide, approximately 10 million cigarettes are purchased a minute, 15 billion are sold each day, and upwards of 5 trillion are produced and used on an annual basis. |
Our Opportunity
We believe that a market for our products exists among persons who may be seeking an alternative or replacement to cigarettes, pipes, cigars, and other similar products. We believe our product is appealing due to the fact:
| • | Fire Safe Cigarette— our products do not require matches or a lighter there is no flame and no combustion of actual tobacco leaf, our products don’t burn and as such the risk of accidental fire is drastically reduced. | ||
| • | Greater Freedom of Use— our product does not emit actual smoke users of personal vaporizers may typically use our products in places where smoking is not allowed. | ||
| • | No Smoke, No Tar.— our products contain no smoke and no tar and don’t emit the harsh and sometimes offensive or noxious odor emitted from the combustion of tobacco. | ||
| • | Green Alternative— there is less waste associated with our product, disposable waste in the form of extinguished cigarettes and discarded filters, in addition to the waste associated with the continual burn of traditional cigarettes, pipes etc. as opposed to the fact that our product is only activated when used. |
Fire Safe Cigarette.Our products do not burn, there is no combustion or open flame associated with our products, instead our products are powered by a lithium ion battery that heat a filament to between 40ºC and 65ºC and vaporizes a non-combustible solution, which greatly mitigates the risk of accidental fires caused by cigarettes. According to the U.S. Fire Administration, an entity of the Department of Homeland Security’s Federal Emergency Management Agency, almost 1,000 smokers and non-smokers are killed in home fires [caused by cigarettes] each year. And Remarked by Senator Dick Durbin (D-Illinois) Congressional record S3439- April 25, 2002 “Cigarette-ignited fires accounted for an estimated 140,800 fires in the United States. Such fires cause more than 900 deaths and 2,400 injuries each year. More than $400 million in property damage reported is due to a fire caused by a cigarette. According to the National Fire Protection Association, one out of every four fire deaths in the United States are attributed to tobacco products—by far the leading cause of civilian deaths in fires. Overall, the Consumer Product Safety Commission estimates that the cost of the loss of human life and personal property from not having a fire-safe cigarette standard is approximately $4.6 billion per year.”
6
Table of Contents
Greater Freedom of Use.Several states have passed laws restricting smoking in public places, private workplaces, in restaurants and other public, and private establishments. Our products to date have not been subject to the same restrictions, with the exception of Suffolk county New York, on use as combustion based tobacco products. And so long as our products can be used where combustion based products cannot, our opportunity for sales shall continue to exist and develop.
No Smoke, No Tar.
For people that want the feel of smoking without the tar or smoke associated with cigarettes. Our products offer an alternative.
No Smoke.According to the National Institute on Drug Abuse, although nicotine is addictive and can be toxic if ingested in high doses, it does not cause cancer—other chemicals are responsible for most of the severe health consequences of tobacco use. Tobacco smoke is a complex mixture of chemicals such as carbon monoxide, tar, formaldehyde, cyanide, and ammonia—many of which are known carcinogens. Carbon monoxide increases the chance of cardiovascular diseases. Tar exposes the user to an increased risk of lung cancer, emphysema, and bronchial disorders.
Secondhand smoke, also known as environmental tobacco smoke, consists of exhaled smoke and smoke given off by the burning end of tobacco products. According to CDC, approximately 38,000 deaths per year can be attributed to secondhand smoke.(Centers for Disease Control and Prevention. Smoking and Tobacco Use—Fact Sheet: Health Effects of Cigarette Smoking. Updated January 2008. Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/health_effects.htm.)
No Tar.Our products contain no tar, the common name for the resinous partially combusted particulate matter produced by the burning of tobacco and other plant material in the act of smoking. Tar in tobacco cigarettes is a major cause of lung cancer, emphysema and bronchitis. The toxins from the tar can damage lung cells that keep tumors from forming. Cigarette tar also damages cilia in the lungs, which protect the lining of the lungs. In addition to the discoloring of teeth, tar can cause periodontitis, a gum disease that can result in the loss of teeth.
Nor do our products contain acetaldehyde which according to studies may enhance nicotine’s effects on the brain (Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 30:705—712, 2005)or ammonia compounds which to boost the effect of nicotine on smokers and increases the nicotine potency of cigarettes by increasing the amount of nicotine contained in the vapor smokers inhale.
It is important to note that nicotine is derived from tobacco and as a result you can and should expect that like all natural tobacco and tobacco extracts, nicotine included, will contain certain carcinogens, specifically tobacco specific impurities and nitrosamines. (See Government Regulation: FDA Analysis of Electronic Cigarettes.)
Green Alternative.According to The World Health Organization Five trillion cigarette filters which weigh approximately 2 billion pounds are discarded each year and it is estimated that trillions of filters, filled with toxic chemicals from tobacco smoke, make their way into our environment as discarded waste yearly. The core of most cigarette filters — the part that looks like white cotton, is actually a form of plastic called cellulose acetate. By itself, cellulose acetate is very slow to degrade in our environment. Depending on the conditions of the area the cigarette butt is discarded in, it can take 18 months to 10 years for a cigarette filter to decompose. Moreover, used cigarette filters are full of toxins known as tar, and those chemicals leach into the ground and waterways, damaging living organisms that contact them.
Personal vaporizers contain no tar, our filters are refillable and are recyclable and are powered by lithium ion rechargeable batteries, which once they expire can be safely disposed of or recycled. Our products don’t idly burn when not used, they are only activated when a user activates the air flow sensor by drawing on the product and as a result generate less waste. We believe our products provide long-term economical savings to its users and are a greener choice for the environment.
7
Table of Contents
Distribution and Sales
The distribution and sales strategy for our products is tailored to the characteristics of each market, whether it be geographical or demographical.
Our sales and distribution channels are:
| • | Internet affiliate marketing through independent sales persons. | ||
| • | Direct Sales and Distribution, where we have set up our own distribution directly to retailers. | ||
| • | Single independent distributors who are responsible for distribution within a single market. | ||
| • | Exclusive Territory and Exclusive Channel Distribution, where distributors have an exclusive territory within a country or an exclusive right to sell within a distribution channel (e.g. gas station.) | ||
| • | Distribution through wholesalers, where we supply either national or regional wholesalers who then service retailers. | ||
| • | Internet/E-commerce Sales, where we sell directly to end users through one of our internet websites and or landing pages. | ||
| • | Direct response television marketing. |
Our distribution and sales channels are supported by internal sales and customer service personnel.
We generate sales and leads through domestic and international trade-shows, telesales, internet marketing, internet affiliates and direct response television marketing. We depend on a network of internal and external sales representatives to maintain and grow our business and the revenues we generate.
Recently Facebook, a leading social network has restricted the placement of advertisement for electronic cigarettes on its website, while we don’t advertise on Facebook, certain of our independent internet sales affiliate may, which may reduce their ability to effectively market our products and generate sales and leads, moreover if other web properties follow suit, we will have to rely on alternative internet marketing strategies to generate visits to our website which may have an effect on our ability to continue to drive internet sales.
Competition
Numerous vendors sell products that compete with ours; the nature of our competitors is varied as the market is highly fragmented and the barriers to entry into the business are low. Our direct competitors sell products that are substantially similar to ours and through the same channels through which we sell our personal vaporizer products. We compete with these direct competitors for sales through distributors, wholesalers and retailers, including but not limited to national chain stores, tobacco shops, gas stations, travel stores, shopping mall kiosks, in addition to direct to public sales through the internet, mail order and telesales.
As a general matter, we have access to and market and sell the similar personal vaporizers as our competitors and since we sell our products at substantially similar prices as our competitors. Accordingly, the key competitive factors for us and other suppliers of personal vaporizers are the quality of service to customers, the scope and effectiveness of marketing efforts, including media advertising campaigns and, increasingly, the ability to identify and develop new sources of customers.
8
Table of Contents
Part of our business strategy focuses on the establishment of contractual relationships with distributors. We are aware that competitors in the industry also are seeking to enter into such contractual relationships. In many cases, competitors for such contracts may have far greater management, human, and financial resources than we do for entering into such contracts and for attracting distributor relationships.
Certain of our competitors may have better control of their supply and distribution, be, better established, larger and better financed than our Company, however we believe that as a public company we will have better access to capital, management and resources needed to build our business and pursue any regulatory approvals that may be needed in connection with future sales of our products, as the law demands it.
Additionally, we compete with cigarette companies as our products deliver nicotine like traditional cigarette products; moreover, tobacco companies like R.J. Reynolds have filed a patent application for a personal vaporizer-like device which they refer to as a “Tobacco-Containing Smoking Article.” If R. J. Reynolds or other tobacco companies endeavor to compete against us in the electronic cigarette and personal vaporizer business, or in the alternative should we receive the proper approvals to allow us to market our products as a smoking cessation aid, we will find ourselves competing with not only the world’s largest tobacco companies but the world’s largest pharmaceutical companies as well. Referred to respectively asbig tobaccoandbig pharma;both big pharma and big tobacco have limitless resources with which to compete against us.
If we were to gain FDA regulatory approval to market our products as a smoking cessation aid or nicotine replacement therapy, we would need to demonstrate similar if not greater efficacy of our product over the existing smoking cessation aids and nicotine replacement therapies and their respective track records. Moreover, we would need to be competitive on price and our product would need to be easier to use. Furthermore, in order to be sold as a drug, our products may require prescriptions to be bought and as such we would be competing against our drug company competitors in our efforts to persuade doctors that our product is a better alternative to the current drug company offerings, in order to ensure doctors will write prescriptions for our products.
Both pharmaceutical and tobacco companies have far greater resources than us and we would be hard pressed to compete successfully against either industry’s participants.
Source and Availability of Raw Materials
We believe that an adequate supply of product and raw materials will be available to us as needed and from multiple sources and suppliers.
Trademarks
We have filed trademark applications for our brands, Krave™, EZsmoker™, Fifty-One™ and Green Puffer™, these trade names which we have established and used in commerce are at various stages of the trademark application process, none of which have been awarded to us to date, however we continue to invest in marketing and promoting these brands.
Government Regulation
Our industry faces intense scrutiny and regulatory uncertainty. The uncertainty is centered in part on whether electronic cigarettes are a drug, a drug and a medical device or a tobacco product. The FDA has asserted that electronic cigarettes are a drug and medical device and are required to be approved under the Federal Food, Drug and Cosmetic Act (FFDCA) as such. Electronic cigarette marketers contend that the device is a tobacco product and should be regulated as such under the FDA’s new jurisdiction, under the Family Smoking Prevention and Tobacco Control Act (FSPTCA.)
In early 2009 the FDA issued import alert 66-41 and as a result US Customs has from time to time temporarily and in some instances indefinitely detained products sent to us by our Chinese suppliers. If the FDA and or customs modifies the import alert from a its current form which allows US Customs’ discretion to release our products to us to a mandatory and definitive hold we will no longer be able to ensure a supply of product for US sales.
9
Table of Contents
A law suit has been filed against the FDA challenging its jurisdiction over electronic cigarettes, the case is Smoking Everywhere, Inc. v. the U.S. Food and Drug Administration et. al. and is in front of the U.S. District Court for the District of Columbia. The lawsuit was filed in response to the issuance by the FDA, of import alert 66-41 issued in early 2009 which sought to restrict the importation and the subsequent detainment of certain electronic cigarette shipments belonging to the plaintiff, by U.S. Customs. The plaintiffs contend in part that at the time of the issuance of the import alert the FDA did not have jurisdiction over tobacco products, which it asserts electronic cigarettes are. Moreover the plaintiff alleges that the FDA did not follow proper procedure in issuing the import alert. The FDA argues that, notwithstanding the fact that the nicotine used in the products is derived from tobacco; the intended use, labeling and advertising materials serve to exclude the product from coverage of a tobacco product and require the product be regulated as a drug. The outcome of this case will likely establish whether the import alert will stand and whether electronic cigarettes and personal vaporizers will be regulated as tobacco products or as drugs and medical devices.
If the Court finds that electronic cigarettes are tobacco products, we will be subject to federal and state labeling, marketing and advertising laws specific to tobacco products, in addition to any and all applicable federal and state taxes, our products will also be regulated by the FDA under FSPTCA, which grants the authority to the FDA to regulate the ingredients in tobacco products, including but not limited to any and all flavorings except for menthol and the amount of nicotine, which under FSPTCA it can not reduce to zero. Moreover under FSPTCA the FDA cannot ban cigarettes or other tobacco products. FSPTCA also empowers the FDA to establish an approval procedure for the introduction of new tobacco products to market and regulates the manner and to whom tobacco products are sold.
If the Court finds that electronic cigarettes are drugs, as defined by the FFDCA and or if the FDA is empowered by the Court to use its discretion, by weighing the total facts and circumstances on a case by case basis with respect to how our products are marketed, labeled and sold; in addition to the products intended use and determines that our products are in fact drugs and medical devices, we will likely be required, in order to continue to market our products, to seek regulatory approval in accordance with the FDA’s prescribed processes for the introduction of a new drug and medial device.
The FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The FFDCA and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, record-keeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
Pharmaceutical product development in the U.S. typically involves non-clinical laboratory and animal tests, the submission to the FDA of a notice of claimed investigational exemption or an Investigational New Drug application (“IND,”) which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Non-clinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the non-clinical tests must comply with federal regulations and requirements including good laboratory practices. The results of non-clinical testing are submitted to the FDA as part of an IND along with other information including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol. Long term non-clinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not commented on or questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
10
Table of Contents
Clinical trials must be conducted in compliance with federal regulations, good clinical practices or GCP, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase I, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses and, if possible, early evidence on effectiveness. Phase II usually involves trials in a limited patient population, to determine the effectiveness of the drug for a particular indication or indications, dosage tolerance and optimum dosage, and identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase II evaluations, Phase III trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all non-clinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture, and controls. The cost of preparing and submitting an NDA is substantial. Under federal law, the submission of most NDAs is additionally subject to a substantial application user fee, for which the Company anticipates qualifying for a waiver from having to pay as a small business submitting its first NDA for approval, and the manufacturer and/or sponsor under an approved new drug application are also subject to annual product and establishment user fees. These fees are typically increased annually.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of new drug applications. Most such applications for non-priority drug products are reviewed within ten months. The review process may be extended by the FDA for three additional months to consider certain information or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it often follows such recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current good manufacturing practices is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
11
Table of Contents
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy and may impose other conditions, including labeling restrictions which can materially affect the potential market and profitability of the drug. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
The Hatch-Waxman Act
The approval process described above is premised on the applicant being the owner of, or having obtained a right of reference to, all of the data required to prove the safety and effectiveness of a drug product. This type of marketing application, sometimes referred to as a “full” or “stand-alone” NDA, is governed by Section 505(b)(1) of the FFDCA. A Section 505(b)(1) NDA contains full reports of investigations of safety and effectiveness, which includes the results of non-clinical studies and clinical trials, together with detailed information on the manufacture and composition of the product, in addition to other information.
Other Regulatory Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. The FDA may also require as a condition of approval for drugs with significant safety issues, implementation of a Risk Evaluation and Mitigation Strategy (REMS). Such strategy may include “Black Box” warnings, limitations on promotion and distribution, and periodic testing of patients on the drug to monitor whether administration of the drug continues to be safe and effective for the patient.
Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase IV testing, risk minimization action plans, and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control as well as drug manufacture, packaging, and labeling procedures must continue to conform to current good manufacturing practices, or cGMPs, after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Fast Track Designation
Under the fast track program, the sponsor of a new drug candidate intended for the treatment of a serious or life-threatening condition and which demonstrates the potential to address unmet medical needs for the condition may request the FDA to designate the drug candidate as a fast track drug concurrent with or after the filing of the IND for the drug candidate. The FDA must determine if the drug candidate qualifies for fast track designation within 60 days of receipt of the sponsor’s request. Once the FDA designates a drug as a fast track product, it is required to facilitate the development and expedite the review of that drug.
12
Table of Contents
In addition to other benefits such as the ability to use surrogate endpoints and have greater interactions with FDA, FDA may initiate review of sections of a fast track drug’s NDA before the application is complete. This rolling review is available if the applicant provides and the FDA approves a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, the fast track designation may be withdrawn by the FDA if it believes that the designation is no longer supported by data emerging in the clinical trial process.
Priority Review
Under FDA policies, a drug candidate is eligible for priority review, or review within a six-month time frame from the time a complete NDA is submitted, if the drug candidate is intended for the treatment, diagnosis or prevention of a serious or life-threatening condition, demonstrates the potential to address an unmet medical need, or provides a significant improvement compared to marketed drugs.
Anti-Kickback, False Claims Laws & The Prescription Drug Marketing Act
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer.
Physician Drug Samples
As part of the sales and marketing process, pharmaceutical companies frequently provide samples of approved drugs to physicians. The Prescription Drug Marketing Act, or the PDMA, imposes requirements and limitations upon the provision of drug samples to physicians, as well as prohibits states from licensing distributors of prescription drugs unless the state licensing program meets certain federal guidelines that include minimum standards for storage, handling and record keeping. In addition, the PDMA sets forth civil and criminal penalties for violations.
Health Care Reform
There are currently ongoing initiatives in Congress to reform the healthcare system, including the cost, delivery and availability of pharmaceutical products to patients, and these efforts are expected to be actively supported by President Obama. There can be no assurance as to how — or if — these initiatives will pass and as to whether they will affect our business. Further, recent efforts by Congress and President Obama to pass stimulus legislation in an attempt to help the economy include funding for research projects through NIH. While there can be no assurance, these efforts may help companies like us or our competitors obtain the funding for some of our studies.
13
Table of Contents
Foreign regulations
Any marketing of our designated products outside of the United States will be contingent on receiving approval from the various regulatory authorities. Foreign regulatory systems, although they vary from country to country, include risks similar to those associated with FDA regulation in the U.S. Under the European Union regulatory system, applications for drug approval may be submitted either in a centralized or decentralized manner. Under the centralized procedure, a single application to the European Medicines Agency leads to an approval granted by the European Commission which permits marketing of the product throughout the European Union. The decentralized procedure provides for mutual recognition of nationally approved decisions and is used for products that do not comply with requirements for the centralized procedure. Under the decentralized procedure, the holders of national marketing authorization in one of the countries within the European Union may submit further applications to other countries within the European Union, who will be requested to recognize the original authorization based on an assessment report provided by the country in which marketing authorization is held.
As with FDA approval, we may not be able to secure regulatory approvals in certain European countries in a timely manner, if at all. Additionally, as in the U.S., post-approval regulatory requirements would apply to any products that are approved in Europe, and failure to comply with such obligations could have a material adverse effect on our ability to successfully commercialize any product.
Outside of the European Union, we are subject to widely varying foreign obligations, which may be quite different from those of the FDA, governing clinical studies, product registration and approval and pharmaceutical sales. Whether or not FDA approval has been received, we must obtain separate approval for products by the comparable regulatory authorities of foreign countries prior to the commencement of marketing our products in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. In addition, under current U.S. law, there are significant restrictions on the export of products not approved by the FDA, depending on the country involved and the status of the product in that country.
FDA Analysis of Electronic Cigarettes
On May 4, 2009 the FDA conducted a study which it released to the public on July 22, 2009, wherein the FDA reported its results from tests it conducted on a variety of electronic cigarettes marketed by two companies and found that certain of the liquid solutions contained Tobacco Specific Nitrosamines (TSNA,) which are know carcinogens found in cigarettes and smokeless tobacco products. The FDA went on to say that that while the analytes were detected they were detected “at a level less than the limit-of-quantitation.” Moreover, the FDA identified possible tobacco specific impurities at the following limits of detection ;Cotinine 20 parts-per-billion (ppb.) Anabasine 10 ppb; myosmine 69 ppb; β-nicotyrine 170 ppb — present but at less than the level of the Nicotrol specification, (Nicotrol is a well known and approved Nicotine replacement therapy device, marketed by Pharmacia & Upjohn and was used as a control in the study.) The study detected, in one of the nineteen samples tested, diethylene glycol at a level of one percent.
While our products were not specifically tested by the FDA and the May 4, 2009 study does not relate to our products in particular; the FDA’s public announcements relating to its study have publicly cast electronic cigarettes in a negative light and have contributed to public concern over the safety of electronic cigarette products and have contributed to certain state actions that have had the effect of restricting sales.
For example, the attorney general of the State of Oregon has entered a settlement with a chain of travel stores, the terms of which restrict the sale of products by companies Njoy and Smoking Everywhere, the two brands tested by the FDA, from selling their products into its state. The Oregon attorney general has stated that the Njoy and Smoking Everywhere products are prohibited from being sold in that state until such time as the FDA approves the product or the companies demonstrate to the attorney general’s satisfaction that the products are safe for human use.
14
Table of Contents
In California, Governor Arnold Schwarzenegger vetoed bill S.B. 400, which sought to categorize electronic cigarettes, a drug. Passage of this bill into law would have restricted the sale of electronic cigarettes in the state of California until such time as electronic cigarettes were approved or cleared by the federal Food and Drug Administration.
In Suffolk county New York, a local law was passed that seeks to expand the prohibition of the sale of tobacco products to include “e-cigarettes” and “liquid nicotine” both defined terms, to persons under nineteen years of age. Electronic cigarettes has also been added to the County’s definition of smoking under section 437 and restricts the use of electronic cigarettes in places where the use of cigarettes is prohibited.
Employees
We employ 12 full-time and 1 part-time employee. Our employees are not unionized and we believe we have good relations with our employees.
Item 1A. RISK FACTORS
Risks Related to Our Business
We are a Development Stage Company. Our Limited Operating History Makes it Difficult to Evaluate Our Future Performance.
We are a development stage company and as such, we have a limited operating history upon which you can evaluate our current business and our prospects. The likelihood of our future success must be viewed in light of the problems, expenses, difficulties, delays and complications often encountered in the operation of a new business, where failures of new companies are common. We are subject to the risks inherent in the ownership and operation of a development stage company, including regulatory setbacks and delays, fluctuations in expenses, competition and government regulation. If we fail to address these risks and uncertainties our business, results of operations, financial condition and prospects would be adversely affected.
Operating Results; We Have Only Been in Operations Since March of 2008.
We were formed as a Florida corporation in March 2008. Our operating history since inception has shown an upward trend to both the total number of sales and the sales volume, We have generated $4,484,729 in revenues at a gross profit of $2,311,111 and a net income of $403,052 from operations for the nine months ended September 30, 2009, however we can give no assurances that our sales will continue to increase in the future and as such, it may be difficult to evaluate our business prospects. Moreover, we may experience seasonality in results of operations as a result, we believe that quarter-to-quarter comparisons of our results of operations may not be a fair indicator and should not be relied upon as a measure of future performance.
We Cannot Predict Our Future Capital Needs And We May Not Be Able To Secure Additional Financing.
We believe that the cash we have on hand, together with anticipated revenues from operations will be sufficient to meet our presently anticipated working capital and capital expenditure requirements for existing operations for at least the next twelve months, however our belief is based on our operating plan which in turn is based on assumptions, which may prove to be incorrect. As a result, our financial resources may not be sufficient to satisfy our capital requirements for this period.
Moreover and at such time, should we initiate efforts to pursue an investigational new drug application (IND) with the United States Food and Drug Administration (FDA) that will allow us to market certain of our products within the approved regulatory scheme, as set forth by the FDA for nicotine based personal vaporizers to be sold as a smoking cessation aid/ device or as otherwise required to gain FDA approval for certain products that may require regulatory approvals to be sold, we will likely require additional funding to begin the necessary clinical, non-clinical trials and the approval process that we believe we will be required to complete before we are in a position to file an NDA for the product.
15
Table of Contents
Further, we may require additional working capital to support our operations during any regulatory period wherein we may be precluded from marketing certain of our products. We do not presently have the funds needed to complete all the necessary trials to gain such U.S. and foreign approvals. We have not yet developed or initiated our trials and thus we cannot estimate the ultimate costs of these trials, and we will need additional funding to pay such costs.
We expect to raise any required additional funds through public or private equity offerings, debt financings, corporate collaborations, governmental research grants may in some cases be available to us. We may also seek to raise additional capital to fund additional product development efforts, even if we have sufficient funds for our planned operations.
There can be no assurance that any such required additional funding will be available to us at all or available on terms acceptable to us. Further, we currently have no credit facility or similar financing currently available. And any debt financing, if available, may involve restrictive covenants, which may limit our operating flexibility with respect to certain business matters. If additional funds are raised through the issuance of equity securities, the percentage ownership of our existing stockholders will be reduced and our stockholders will experience additional dilution in net tangible book value per share. If adequate funds are not available on acceptable terms, we may be unable to successfully market our products, take advantage of future opportunities, repay debt obligations as they become due or respond to competitive pressures, any and all of which would have an adverse effect on our business.
New Product Faces Intense Media Attention and Public Pressure
Our product is new to the marketplace and since its introduction certain members of the media, politicians, government regulators and advocate groups, including independent doctors have called for an outright ban of all “electronic cigarettes,” pending regulatory review and a demonstration of safety. A ban of this type would likely have the effect of terminating our United States’ sales and marketing efforts, of certain products which we may currently market or have plans to market in the future. Such a ban would also likely cause public confusion as to which products are the subject of the ban and which are not and would have a material adverse effect on our business, financial condition and performance.
The Market For Our Products Is Uncertain And Is Still Evolving.
Personal vaporizers, having recently been introduced to market, are at an early stage of development and are evolving rapidly and are characterized by an increasing number of market entrants. Our future revenues and any future profits are substantially dependent upon the widespread acceptance and use of personal vaporizers. Rapid growth in the use of, and interest in, personal vaporizers is a recent phenomenon, and may not continue on a lasting basis. The demand and market acceptance for these products is subject to a high level of uncertainty.
We Market a Single Class of Products, Which may be Subject to Certain Government Regulations, Whose Approval We may or may not be Able to Achieve.
Personal Vaporizers, which are our sole product offering, commonly referred to as “electronic cigarettes” are new to the marketplace and may be subject to regulation as a drug, a medical device, a drug and medical device and or as a tobacco product. Most personal vaporizers are sold as a means of delivering nicotine to the body. The Food and Drug Administration (“FDA”) is the regulatory agency which oversees drugs, medical devices and tobacco; however at present it is unclear which, if any regulatory process is required to market, and sell personal vaporizers. To date the FDA has not established a definitive policy regulating “electronic cigarettes” but is reviewing cases on a case by case basis. We intend to use reasonable efforts to file for the appropriate approvals to allow us to sell our product in the United States, however we have no indication that at present we will be able to afford to pursue regulatory approval and that if we are able to pursue said approval we have no assurances that the outcome of said approval process will result in our products being approved by the FDA. Moreover, if the FDA establishes a regulatory process that we are unable or unwilling to comply with our business, results of operations, financial condition and prospects would be adversely affected. (See section “Government Regulation.”)
16
Table of Contents
Our Products Contain Nicotine Which is Considered to be a Highly Addictive Substance.
Certain of our products contain nicotine, a chemical found in cigarettes and other tobacco products which is considered to be highly addictive. The Family Smoking Prevention and Tobacco Control Act, empowers the FDA to regulate the amount of nicotine found in tobacco products, but may not require the reduction of nicotine yields of a tobacco product to zero. Any FDA regulation may require us to reformulate, recall and or discontinue certain of the products we may sell from time to time, which may have a material adverse effect on our ability to market our products and have a material adverse effect on our business, financial condition, results of operations, cash flows and/or future prospects.
We May Not Successfully Commercialize Our Personal Vaporizers.
We began marketing our personal vaporizers in September, 2008 and have generated approximately $4,993,447 in revenues through our efforts. We derive revenues thorough: distributor sales, selling to wholesalers, direct to retail distribution and through direct sales to customers over the Internet and through television sales. Our success depends on our ability to continue to serve our existing customers and by attracting new customers.
Moreover, our ability to expand and commercialize our products outside of the United States is critical to our business success. Our inability to continue to generate revenues through our sales channels both at home and abroad would have a material adverse effect on our business, prospects, financial condition and results of operations.
Our Business may be Affected if we are Taxed Like Other Tobacco Products or if we are Required to Collect and Remit Sales Tax on Certain of our Internet Sales
Presently our products are not taxed like cigarettes or other tobacco products, all of which have faced significant increases in the amount of taxes collected on the sale of their products. Should state and federal governments and or taxing authorities impose taxes similar to those levied against cigarettes and tobacco products on our products, it may have a material adverse effect on the demand for our products. Moreover we may be unable to establish the systems and processes needed to track and submit the taxes we collect through internet sales, which would limit our ability to market our products through our websites which would have a material adverse effect on our revenues, operation and financial condition.
States such as New York, Hawaii, Rhode Island and North Carolina have begun collecting taxes on Internet sales where companies have used independent contractors in those states to solicit sales from residents of that state. The requirement to collect track and remit taxes based on independent affiliate sales may require us to increase our prices, which may effect demand for our products or conversely reduce our net profit margin; either of which would have a material adverse effect on our revenues, financial condition and operating results.
Downturns In The Economy May Affect the demand for our products and our Financial Performance
Personal vaporizers are new to market and may be regarded by users as a novelty item and expendable as such demand for our products may be extra sensitive to economic conditions. When economic conditions are prosperous, discretionary spending increases; conversely, when economic conditions are unfavorable, discretionary spending declines. Any significant decline in general corporate conditions or the economy that affect consumer spending could have a material adverse effect on the Company’s business and consequently, upon an investment in the Common Stock of our Company.
17
Table of Contents
The World Health Organization (WHO) does not consider electronic cigarettes to be a legitimate therapy for smokers trying to quit smoking
The WHO has stated that there is currently insufficient scientific evidence to establish electronic cigarettes as a legitimate smoking cessation aid. If we or others in our industry or the scientific and medical community are unable to demonstrate the “safety” of our products, their effectiveness as a smoking cessation aid or as a “reduced harm” alternative to smoking. We may face the same Governmental actions aimed at cigarettes and other tobacco products. The tobacco industry expects significant regulatory developments to take place over the next few years, driven principally by the World Health Organization’s Framework Convention on Tobacco Control (“FCTC”). The FCTC is the first international public health treaty on tobacco, and its objective is to establish a global agenda for tobacco regulation with the purpose of reducing initiation of tobacco use and encouraging cessation. Regulatory initiatives that have been proposed, introduced or enacted include:
| • | the levying of substantial and increasing tax and duty charges; | ||
| • | restrictions or bans on advertising, marketing and sponsorship; | ||
| • | the display of larger health warnings, graphic health warnings and other labeling requirements; | ||
| • | restrictions on packaging design, including the use of colors and generic packaging; | ||
| • | restrictions or bans on the display of tobacco product packaging at the point of sale, and restrictions or bans on cigarette vending machines; | ||
| • | requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and other smoke constituents levels; | ||
| • | requirements regarding testing, disclosure and use of tobacco product ingredients; | ||
| • | increased restrictions on smoking in public and work places and, in some instances, in private places and outdoors; | ||
| • | elimination of duty free allowances for travelers; and | ||
| • | encouraging litigation against tobacco companies. |
Operating income could be significantly affected by any significant decrease in demand for our products, any significant increase in the cost of complying with new regulatory requirements.
We may be Unable to Anticipate Changes in Consumer Preferences or to Respond to Consumer Behavior Influenced by Economic Downturns.
Our business is subject to changes in consumer preferences, which may be influenced by local economic conditions. To be successful, we must:
| • | promote brand equity successfully; | ||
| • | anticipate and respond to new consumer trends; | ||
| • | develop new products and markets and broaden brand portfolios; | ||
| • | improve productivity; and | ||
| • | be able to protect or enhance margins through price increases. |
In periods of economic uncertainty, consumers may tend to purchase lower price brands or alternatives, and the volume of our higher priced products and our profitability could suffer accordingly.
18
Table of Contents
We may Become Dependent on Foreign Sales to Maintain Our Business
If the FDA or other state or Federal government agencies restrict or prohibit the sale of personal vaporizers in the United States, in part or in whole, our ability to maintain our business is dependent on our ability to successfully commercialize our product and brands in foreign jurisdictions where our product can be sold. Our inability to establish distribution in foreign jurisdictions, specifically those that allow for the sale of personal vaporizers will deprive us of the operating revenue we require to fund any domestic regulatory approval effort and continue to maintain our business operations.
Foreign Commercialization Will Result in Additional Costs and Expenses.
Commercializing our product in foreign countries will likely require us to expend additional resources which may reduce our profit margins; additional expenses including but not limited to, local language advertising and marketing materials, packaging, translating product documentation, additionally we may be required to retain foreign counsel to ensure compliance with foreign laws, regulations and taxing regimes, in addition to drafting and enforcing contracts with foreign distributors.
If we fail to commercialize our products in foreign jurisdictions we may find ourselves at a competitive disadvantage not only in those foreign jurisdictions but in each market in which we have a presence. If we are unable to be competitive we risk losing market share, a decrease in operating revenues all of which would have a material adverse effect on our financial condition and our ability to operate our business.
Our Success is Dependent Upon Our Marketing Efforts.
We have limited marketing experience in marketing personal vaporizers and limited financial, personnel and other resources to undertake extensive marketing activities. If we are unable to generate significant market awareness for our products and our brands our operations may not generate sufficient revenues for us to execute our business plan, generate revenues and achieve profitable operations.
If we are to gain FDA Regulatory Approval to Market our Products, We will need to develop marketing, distribution and production capabilities or relationships to be successful.
We do not currently have the pharmaceutical marketing, distribution or production capabilities required to generate sales of our candidate products, to do so we must either acquire or develop an internal marketing force with technical expertise and with supporting documentation capabilities, or make arrangements with third parties to perform these services for us. The acquisition and development of a pharmaceutical marketing and distribution infrastructure will require substantial resources and compete for available resources with our product development efforts. To the extent that we enter into marketing and distribution arrangements with third parties, our revenues will depend on the efforts of others. If we fail to enter into such agreements, or if we fail to develop our own marketing and distribution channels, we would experience delays in product sales and incur increased costs.
Similarly, should we gain regulatory approval to market our candidate products. we may be required to contract with an FDA approved manufacturing facility. We have no assurances that we will be able to contract with manufacturing providers that will meet our requirements, and or pass FDA inspection. Moreover, if any third party fails to perform on a timely basis we may not be able to find a suitable replacement. If we cannot obtain a sufficient supply of product, it would have a material adverse effect on our ability to successfully operate our business.
We Rely on the Efforts of Our Outside Independent Sales Force to Generate Sales
We rely, in part, on the efforts of our independent sales distributors to purchase and distribute our product to wholesalers and or retailers to generate revenues. No single distributor currently accounts for a material percentage of our revenues and we believe that should any of these relationships terminate we would be able to find suitable replacements, however any change in distributors or our ability to timely replace any given distributor would have a material adverse effect on our business, prospects, financial condition and results of operations.
19
Table of Contents
We rely, in part, on the efforts of outside independent salespersons and Internet sales affiliates to generate sales for our company. No single independent salesperson of Internet affiliate currently accounts for a material percentage of our revenues and we believe that should any of these relationships terminate we would be able to find suitable replacements, however any loss of these independent sales persons or our Internet sales affiliates could have a material adverse effect on our business, prospects, financial condition and results of operations.
We May Not Be Able to Adapt to Trends In our Industry
We may not be able to adapt as the personal vaporizer industry and customer demand evolves; whether attributable to regulatory constraints, mis-management or a lack of financial resources or, our failure to respond in a timely manner; to new technologies, customer preferences, changing market conditions or new developments in our industry. Any of the failures to adapt or inabilities described herein or otherwise would have a material adverse effect on our business, prospects, financial condition and results of operations.
Existing or Pending Patents Could Prevent Us From Operating Our Business In Its Present Form.
Ruyan, a Chinese company, has made certain public claims as to their ownership of a Chinese patent relating to an “Atomizing Electronic Cigarette.” We currently purchase our products from Chinese manufacturers other than Ruyan. Should Ruyan’s patent be valid and enforceable and cover the devices we purchase from our suppliers, we may be forced to pay more for our products or we may be cutoff from our supply. We may also face a potential action by Ruyan, which we may be forced to defend and which we may ultimately lose. Should any of these events occur, they are likely to have a material adverse effect on our ability to operate our business as a going concern.
R. J. Reynolds one of the largest tobacco companies in the world has filed a patent application for a “Tobacco-Containing Smoking Article.” If R.J. Reynolds patent is awarded and our products are found to be infringing on their patent, our business, prospects, financial condition and results of operations could be materially and adversely affected.
Neither Ruyan or R.J. Reynolds has contacted us regarding any possible infringement of their intellectual property rights nor has any party commenced or threatened to commence any legal action against us. If we are required to participate in litigation we may not have the resources to fund the required litigation costs, which may adversely affect our business prospects, financial condition and results of operations.
In the event that either Ruyan or R. J. Reynolds’ patents are enforceable against us, we may be required to obtain a license to the covered intellectual property or substantially modify or redesign our existing product line in order to continue operations. We can offer no assurance that a license would be available on acceptable terms or at all, or that we will be able to revise our business model economically, efficiently or at all.
We Depend On Third Party Suppliers and Manufacturers For Our Personal Vaporizer Products.
We do not own or control our supply chain our suppliers or our suppliers’ suppliers, therefore we are unable to control or ensure our supply of products or the consistency of those products. We depend on third-party suppliers and manufacturers for our personal vaporizer products, which includes, but is not limited to, our electrical components, technology, flavorings and essences. Our customers associate certain characteristics of our products including the weight, feel, draw, flavor, packaging and other unique attributes of our products to the brands we market, distribute and sell. Any interruption in supply and or consistency of our products may harm our relationships and goodwill with customers, and have a materially adverse affect on our cash flow and our operations.
Although we believe that several alternative sources for our products are available, any failure to obtain the components, chemicals constituents and manufacturing services necessary for the production of our products would have a material adverse effect on our business and prevent us from timely execution of our business plan and may result in additional expenditures of time and money in seeking viable new sources of supply and manufacturer alternatives.
20
Table of Contents
Moreover our inability to replicate those certain characteristics of our products which our customers associate and enjoy, which are unique to our brands, may cause a loss of customer loyalty, patronage and goodwill and which may have a material adverse effect on our business.
We Use Chinese Manufacturers for the Production of Our Products
Our suppliers and product manufacturers are based in China. Certain Chinese factories and the products they export have recently been the source of safety concerns and recalls, which is generally attributed to lax regulatory, quality control and safety standards. Should Chinese factories continue to draw public criticism for exporting unsafe products, whether those products relate to us or not we may be adversely and materially affected by the stigma associated with Chinese production, which would effect our business operation, our revenues and our financial projections and prospects.
Moreover, products manufactured by our Chinese suppliers that are not considered safe and or those products that do not comply with U.S. safety and health standards may cause significant harm and or death to persons who use the product and subject us to liability and potential legal claims and cause injury to our reputation, goodwill and operating results.
Product Exchanges, Returns, Warranty Claims, Defect and Recalls May Adversely Affect Our Business
Any and all products are subject to customer service claims, malfunctions and defects, which may subject us to requests for product exchanges, returns, warranty claims and recalls. If we are unable to maintain a certain degree of quality control of our products we will incur costs of replacing and or recalling our products and servicing our customers. Any product returns, exchanges, and or recalls we may make will have a material adverse effect on our business, our operations and our profitability and will likely result in the loss of customers and goodwill.
Moreover products that do not meet our quality control standards and or those products that do not comply with U.S. safety and health standards or that may be defective may reduce the effectiveness, enjoyment and or cause harm to property, person and or death to persons who use the product. Any such instance will likely result in claims against us and potentially subject us to liability and legal claims which may cause injury to our reputation, goodwill and operating results.
We May Be Unable To Promote And Maintain Our Brands.
We believe that establishing and maintaining the brand identities of our products is a critical aspect of attracting and expanding a large client base. Promotion and enhancement of our brands will depend largely on our success in continuing to provide high quality products. If our customers and end users do not perceive our products to be of high quality, or if we introduce new products or enter into new business ventures that are not favorably received by our customers and end users, we will risk diluting our brand identities and decreasing their attractiveness to existing and potential customers.
Moreover, in order to attract and retain customers and to promote and maintain our brand equity in response to competitive pressures, we may have to increase substantially our financial commitment to creating and maintaining a distinct brand loyalty among our customers. If we incur significant expenses in an attempt to promote and maintain our brands, our profitability will likely be impaired.
We Depend on Our Intellectual Property Rights to Distinguish our Brands and Products
Our current intellectual property rights are limited to Copyright, Trademark and common law claims of use which are established by registration, publication or use. We have filed trademark applications for our brands, Krave, EZsmoker, Fifty-One and Green Puffer, these trade names which we have established and used in commerce are at various stages of the trademark application process, however we expect to be awarded trademarks on each and have not been contacted by any third parties with respect to our use of these marks.
21
Table of Contents
If we are unable to trademark our brands or if others assert claims to the right to our marks which are synonymous to our brands, we will be adversely and materially harmed as a result of our inability to continue to use the marks that have become associated with our products, the existence of any confusion by and between our products and others and a loss of any and all goodwill we have developed as a result of our investment in our brands.
Our current and future business activities, products and brands may infringe upon the proprietary rights of others, and third parties may assert infringement claims against us. Any such claims and resulting litigation could subject us to significant liability for damages and could result in the invalidation of our proprietary rights. Even if not meritorious, such claims could be time-consuming, expensive to defend and could result in the diversion of our management’s time and attention. In addition, this diversion of managerial resources could have a material adverse effect on our business, prospects, financial condition and results of operations.
We Expect that New Products and/or Brands We Develop will Expose Us to Risks That May be Difficult to Identify Until Such Products and/or Brands are Launched.
We are currently developing, and in the future will continue to develop, new products and brands, the risks of which will be difficult to ascertain until these products and or brands are commercially launched. For example, we are developing a new look and feel to our personal vaporizer, in addition to different formulations, flavors, potencies, packaging and distribution channels. Any negative events or results that may arise as we develop new products or brands may adversely affect our reputation, business, financial condition and results of operations.
We Depend On The Efforts Of our Management. Our Management Team Lacks Experience In Managing A Public Company and the Obligations Incident to Being a Public Company Will Place Significant Demands on Our Management.
Our officers lack experience in running a public company. Our success is substantially dependent on the performance of our executive officers. In particular, our success depends substantially on the continued efforts of our executive officers and our Board of Directors.
As a public reporting company, we are required to comply with the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC, including periodic reports, disclosures and more complex accounting rules. As directed by Section 404 of Sarbanes-Oxley, the SEC adopted rules requiring public companies to include a report of management on a company’s internal control over financial reporting in their Annual Report on Form 10-K. In addition, the independent registered public accounting firm auditing our financial statements must attest to and report on the effectiveness of our internal control over financial reporting. Based on current rules, we are required to report under Section 404(a) of Sarbanes-Oxley regarding the effectiveness of our internal control over financial reporting. If we are unable to conclude that we have effective internal control over our financial reporting as required by Section 404(a), investors could lose confidence in the reliability of our financial statements, which could result in a decrease in the value of our common stock. Further, commencing with the 2009 fiscal year, our auditors will be required, under Section 404(b) of Sarbanes-Oxley, to report on the effectiveness of our internal control over financial reporting.
Currently, we do not have key person life insurance on our executive officers or board members and may be unable to obtain such insurance in the near future due to high cost or other reasons. We also do not have written employment agreements with any of these key personnel. The loss of the services of any of our executive officers/key employees could have a material adverse effect on our business, if we are unable to find suitable replacements.
Our ability to implement our strategy of attracting and retaining employees may be impaired by the uncertainty in our business due to the FDA’s public statements.
Recent FDA statements with respect to electronic cigarette products, the uncertainty of present and future regulations, including our ability to gain regulatory approval to market our products is likely to injure our ability to compete for talented employees and managers, who may be drawn to more established companies and as a result, we may be unable to attract talented employees, managers, consultants and contractors to help us grow our business.
22
Table of Contents
We may Encounter Difficulties in Managing Our Growth, Which Would Adversely Affect Our Results of Operations.
If we are successful in growing our business we will need to significantly expand our operations, which could put significant strain on our management and our operational and financial resources. To manage future growth, we will need to hire, train, and manage additional employees. Concurrent with expanding our operational and marketing capabilities, we will also need to increase our product development activities. We may not be able to support, financially or otherwise, future growth, or hire, train, motivate, and manage the required personnel. Our failure to manage growth effectively could limit our ability to achieve our goals.
Our success in managing our growth will depend in part on the ability of our executive officers to continue to implement and improve our operational, management, information and financial control systems and to expand, train and manage our employee base, and particularly to attract, expand, train, manage and retain a sales force to market our products on acceptable terms. Our inability to manage growth effectively could cause our operating costs to grow at a faster pace than we currently anticipate, and could have a material adverse effect on our business, financial condition, results of operations and prospects.
We Face a Risk of Product Liability Claims and may not be Able to Obtain Adequate Insurance.
Our business exposes us to potential liability risks that may arise from the clinical testing, manufacture, and sale of our products. Substantial damage awards in certain jurisdictions against pharmaceutical and tobacco companies based on claims for injuries allegedly caused by the use of pharmaceutical and tobacco products. Liability claims may be expensive to defend and result in large judgments against us. We currently carry liability insurance, however there is no assurance that it will continue to be available to us at an affordable price if at all. Our insurance may not reimburse us, or the coverage may not be sufficient to cover claims made against us. We cannot predict any or all of the possible harms or side effects that may result from the use of our current products or any future products and, therefore, the amount of insurance coverage we currently hold may not be adequate to cover all liabilities we might incur. If we are sued for any injury allegedly caused by our products, our liability could exceed our ability to pay the liability. Whether or not we are ultimately successful in any adverse litigation, such litigation could consume substantial amounts of our financial and managerial resources, all of which could have a material adverse effect on our business, financial condition, results of operations, prospects and stock price.
We Face Substantial And Increasing Competition.
We face intense competition from direct and indirect competitors, including“big pharma,”“big tobacco”and other known and established or yet to be formed personal vaporizer and electronic cigarette companies, each of whom pose a competitive threat to our current business and future prospects. We expect competition to intensify in the future. Certain of these companies are either currently competing with us or are focusing significant resources on providing products that will compete with our personal vaporizer product offerings in the future.
Our principal competitors can be classified into three main categories: 1) pharmaceutical companies; 2) tobacco companies; and 3) other personal vaporizer and electronic cigarette companies.
Pharmaceutical companies market smoking cessation aids and alternative nicotine delivery products such as Glaxo SmithKline that marketNicorette® stop smoking chewing gum Nicoderm® the stop smoking patch andZyban® a sustained release tablet , Pfizer that marketsChantix® andNicotrol® the nicotine inhaler,
Tobacco companies, including Phillip Morris, R. J. Reynolds, and Lorillard who currently offer traditional tobacco products and may introduce new tobacco based cigarettes and smoking devices (eg. the “tobacco containing smoking article” covered by patent # 20080092912 as filed by R.J. Reynolds, one of the worlds largest tobacco companies.) We also face competition from smaller tobacco companies that are much larger, better funded and more established than us.
Electronic cigarette companies, that currently market competing products, which include but are not limited to, Njoy, Smoking Everywhere and Smoke Free Innotech, a publicly traded company. Moreover these competitors may, like us may seek regulatory approvals to market their products and these competitors may succeed in obtaining FDA approval for products more rapidly than we can.
23
Table of Contents
There can be no assurance that we will be able to compete successfully against any of the aforementioned competitors, who likely have far greater resources, capital, experience, market penetration, sales and distribution channels than us. We have no assurances that we will be able to compete with these formidable competitors and that we will be successful in operating our business and ever achieving profitability. Our inability to successfully compete against these or any of our competitors will have a material adverse effect our business, results of operations and financial condition.
Litigation and Government regulation will dictate who will be our direct competitors and how we can market our products, if at all.
The manner in which we are able to sell, market and distribute our products will likely be a result of new and existing U.S. FDA regulations, and how those regulations effect us will likely be determined by a judgment from the Federal district court for the District of Columbia and or other appellate courts.
If a court of competent jurisdiction and or the FDA determines that our product is a smoking cessation device or a nicotine replacement product and assuming we gain regulatory approval and or otherwise are able and required to market our products as drug products, we will face intense competition from large pharmaceutical companies with far greater resources, capital, experience, market penetration, sales and distribution channels than us. We have no assurances that we will be able to compete with these formidable competitors and that we will be successful in operating our business and ever achieving profitability.
If a court of competent jurisdiction and or the FDA determines we are a tobacco product and assuming we gain regulatory approval and or otherwise are able and required to market our products as tobacco products, we will face intense competition from tobacco companies with far greater resources, capital, experience, market penetration, sales and distribution channels than us. We have no assurances that we will be able to compete with these formidable competitors and that we will be successful in operating our business and ever achieving profitability.
We Face Competition from Foreign Importers Who Do Not Comply With Government Regulation
We face competition from foreign sellers of personal vaporizers who may illegally ship their products in to the United States for direct delivery to customers. These market participants will not have the added cost and expense of complying with U.S. regulations and taxes and as a result will be able to offer their product at a more competitive price than us and potentially capture market share. Moreover, should we be unable to sell certain of our products during any regulatory approval process we have no assurances that we will be able to recapture those customers that we lost to our foreign domiciled competitors during any “blackout” periods, wherein we were not permitted to sell our products. This competitive disadvantage may have a material adverse impact on our ability to compete with competitors, which may result in a loss of revenue and market share and hamper our ability to generate revenue and continue to operate as a going concern.
Restrictions On the Use of Our Products may Reduce the Attractiveness and Demand for Our Personal Vaporizers
Our product since it emits no smoke and no smell can be used in places where the use of traditional tobacco products, exclusive of smokeless tobacco is prohibited. Should city, state or federal regulators, municipalities, local governments and private industry likewise restrict the use of our personal vaporizer products from use in those same places where the use of tobacco products is prohibited, our customers may reduce or otherwise cease using our products entirely, which would have a material adverse effect on our business, financial condition and performance.
Liability for Improper Marketing, Medical Claims and Labeling
As a distributor and marketer of a product that the FDA may assert is a smoking cessation device and or a tobacco product, the Company faces potential fines, sanctions, administrative actions, penalties, and other liability for: improper labeling, making improper claims, referencing or publishing to its websites, marketing materials, advertisements, testimonials or representations that certain of our products have the ability or potential to treat, cure or otherwise improve a medical condition, and or provide a healthier alternative to other more traditional tobacco products.
24
Table of Contents
Moreover, if the FDA asserts we are a tobacco product, we may be required to follow federal and state tobacco labeling laws, and could face potential fines, sanctions, administrative actions, penalties and other liability either civil and or criminal for any violations thereof.
We discovered that one or more unrelated persons posted their personal unsolicited accounts and experiences with our products to one of our websites which upon notice and inspection we removed as the accounts could be construed as an improper medical claim.
Any violation of law with respect to the company’s marketing materials, and or labeling could expose the Company to liability including but not limited to fines, sanctions, administrative actions, penalties, civil actions and or criminal prosecution. And although the Company maintains general liability insurance, the Company’s insurance may not cover potential claims of this type or may not be adequate to indemnify the Company for all liability that may be imposed. In addition any imposition of liability that is not covered by insurance, is in excess of insurance coverage could have a material adverse effect on the Company’s business, results of operations and financial condition. (See “Government Regulation.”)
Internet Security Poses a Risk To Our E-Commerce Sales.
At present we generate significant revenues through the sale of our products through our websites. We manage our websites and e-commerce platform internally and as a result any compromise of our security or misappropriation of proprietary information could have a material adverse effect on our business, prospects, financial condition and results of operations. We rely on encryption and authentication technology licensed from other companies to provide the security and authentication necessary to effect secure Internet transmission of confidential information, such as credit and other proprietary information. Advances in computer capabilities, new discoveries in the field of cryptography or other events or developments may result in a compromise or breach of the technology used by us to protect client transaction data. Anyone who is able to circumvent our security measures could misappropriate proprietary information or cause material interruptions in our operations. We may be required to expend significant capital and other resources to protect against security breaches or to minimize problems caused by security breaches. To the extent that our activities or the activities of others involve the storage and transmission of proprietary information, security breaches could damage our reputation and expose us to a risk of loss or litigation and possible liability. For example the storage and loss of credit card numbers, that may reside on our servers and be used directly by us or by our service suppliers (ex. merchant account processors). Our security measures may not prevent security breaches. Our failure to prevent these security breaches may result in consumer distrust and may result in a loss of sales and resultantly a loss of revenues.
Credit Card Payment Processors and Merchant Account Risk
We accept credit cards as a means of payment for the sale of our products. Two of our credit card processors, have recently, prompted by public statements made by the FDA with regard to the “illegality” of electronic cigarettes, have alerted us that they would no longer willing or able to process credit card transactions on our behalf for our electronic cigarette products. If we are unable to find suitable replacement providers or an alternative method of payment for our customers or these credit card processing companies continue to hold our funds, our cash-flow will be constrained and our sales may be effected which may have a material adverse effect on our performance, financial condition and results of operations.
The Former Shareholder of Smoke Anywhere USA Are Controlling Stockholders, Our Stockholders May Be Unable To Affect Corporate Activity Without The Support Of These Individuals.
The former shareholders of Smoke Anywhere USA, Inc. as a result of our reverse merger collectively own a majority of our outstanding common stock and, therefore, are able to control all matters requiring approval of our stockholders. Accordingly, our other stockholders may not be able to effect corporate action after this offering without the supporting vote of one or more of these individuals.
25
Table of Contents
Our Earnings Could be Adversely Affected by Currency Exchange Rates and Currency Devaluations.
The bulk of our revenues are currently generated in U.S. dollars, however our manufacturers and suppliers are located in China. Fluctuations in exchange rates between our respective currencies could result in higher production and supply costs to us which would have an adverse effect on our profit margins and our business operation if we re not willing or able to pass those costs on to our customers or effectively hedge our currency exposure.
Moreover, if we attempt to hedge our risk in the currency markets and are unsuccessful and or if our competitors are more successful arbitraging the currency risk we may find ourselves at a competitive disadvantage to other market participants which would have a material adverse effect on our business operations.
Risks Related To Government Regulation
We have not yet applied for any regulatory approval in the United States or any foreign jurisdiction for our product candidates. The regulatory approval process is lengthy, and we may not be able to obtain all of the regulatory approvals required to manufacture and commercialize our product candidates.
We have not applied for any regulatory approval in the United States or any foreign jurisdiction for any of our products. We are not resolved as to which if any of our products require FDA approval and which products may require FDA approval to be sold or continue to be sold. To obtain regulatory approval of a product candidate, we must demonstrate to the satisfaction of the applicable regulatory agency that such product candidate is safe and effective for its intended uses. The type and magnitude of the testing required for regulatory approval varies depending on the product candidate and the disease or condition for which it is being developed. In addition, in the U.S. we must show that the facilities used to produce the product candidate are in compliance with cGMP. We will also have to meet similar regulations in any foreign country where we may seek to market and distribute our products. In general, these requirements mandate that manufacturers follow elaborate design, testing, control, documentation and other quality assurance procedures throughout the entire manufacturing process. The process of obtaining regulatory approvals typically takes several years and requires the expenditure of substantial capital and other resources. Despite the time, expense and resources invested by us in the approval process, we may not be able to demonstrate that our product candidates are safe and effective, in which event we would not receive the regulatory approvals required to market them.
The FDA and other regulatory authorities generally approve products for particular indications. While our current focus is a cigarette alternative, we may also intend to seek to market certain of our products as a “reduced harm” cigarette and or a smoking cessation aid. We may not be approved for any or all of the indications that we request, which would limit the indications for which we can promote it and adversely impact our ability to generate revenues. We may be required to conduct costly, post-marketing follow-up studies if FDA requests additional information.
A Ruling in a Federal District Court and any Subsequent Appeals, Will Dictate What Regulations We Are Required to Follow, if Any in Marketing Certain of Our Products.
A case currently being heard in the U.S District Court for the District of Columbia titled Smoking Everywhere, Inc. v. U.S. Food and Drug Administration et. al. case # 1:2009cv00771 filed April 28, 2009 before Judge Richard J. Leon, is pending. A decision by the court may offer guidance on the legal status and classification of electronic cigarettes, personal vaporizers and products delivering nicotine, derived from tobacco, through non-traditional means. The courts findings may serve in defining which regulatory processes and regime that personal vaporizer and electronic cigarette companies will be required to follow in order to bring these products to market. The courts ruling will likely have a significant and material impact on our business model.
26
Table of Contents
The Family Smoking Prevention and Tobacco Control Act Grants the FDA Authority to Regulate Tobacco Products and How they are Marketed and Sold.
On June 22, 2009 the Family Smoking Prevention and Tobacco Control Act (the “Act”) was signed into law. The effect of this legislation on our business is presently unknown and may place limits on our ability to market and or distribute our products and maintain or bring new products to market. The legislation may impose costly and resource intensive processes to gain regulatory approval and there is no certainty that we will have the capital, resources necessary to comply with the regulation or that we would be ultimately successful in receiving the necessary approvals to continue marketing our product in the United States.
Specifically the Act grants the FDA the authority to regulate tobacco products including but not limited to how they are marketed, the level of nicotine and the method for introducing new tobacco products to market. The legislation eliminates all flavoring other than menthol, yet under the legislation the FDA is not empowered to ban certain tobacco products or require that the nicotine in tobacco products be reduced to zero. While the legislation does not mention electronic cigarettes, it does suggest that nicotine and natural tobacco flavoring are tobacco products under the law and as such electronic cigarettes may be covered under this Act.
In the event a court of competent jurisdiction or the FDA declares certain of our products, namely electronic cigarettes, tobacco products, we would thereafter be required to comply with the Act and the rules promulgated thereunder, in addition to any existing and future tobacco laws and taxing regimes. The imposition of a tobacco tax on our products would make our products more expensive and less competitive with products that carry no tax or a lesser tax. Imposing a tax on our products would make our product more expensive to consumers and could have an adverse effect on the demand for our product and consequently our revenues.
If our product is recognized as a tobacco product, we would become subject to current and future tobacco labeling laws and laws restricting the sale of our product to persons under 18. While we currently do not market our products to minors, unintentional violations may subject us to fines and penalties.
Until the FDA establishes the regulatory processes and regime as provided for by the Act, we do not know how and to what degree we will be regulated. Moreover, if the FDA establishes a regulatory process that we are unable or unwilling to comply with our business, results of operations, financial condition and prospects would be adversely and materially affected. See section “Government Regulation.”
The FDA Regulates How Products are Marketed and Used
The FDA regulates claims to diagnose, mitigate, prevent, treat or cure a disease. And the FDA in Smoking Everywhere v. FDA contends that theIntended Use ofelectronic cigarettes is sufficient for the FDA to assert jurisdiction. If claims made at large by our competitors and third parties, arise to the level to subject us to FDA regulation we may determine it is necessary to change our business model and product to differentiate our product(s) and band(s) so as not to be confused with those companies who improperly market their products. If we are found to have improperly sold or marketed our products in violation of FDA rules, laws or policies, we may be subject to disciplinary, regulatory or administrative actions, fines and or sanctions which may have a material adverse effect on our operations, financial results and business prospects.
The FDA Regulates Drugs and Medical Devices, If Our Products are Considered Either or Both We may be subject to Regulation and May in Fact have Violated Federal law in our Previous Sales and Marketing Efforts.
We have been engaged in marketing and selling efforts of personal vaporizers and electronic cigarettes since the 4th quarter of 2008. We may have unknowingly and without intent failed to comply with certain regulations relating to our product and the means by which we market and sell our products. Our efforts may have been in violation of existing laws which may subject us to enforcement actions, sanctions, fines, administrative action or other penalties, all which would have a material adverse effect on our financial condition, performance and results of operations.
27
Table of Contents
Regulations on the Transportation of Lithium Ion Batteries may Affect Our Business
The Air Line Pilots Association International (ALPA) is calling on the U.S. government to prohibit shipments of lithium-ion batteries on cargo and passenger planes pending new regulations, in light of recent incidents including a battery pack for an electric bicycle and more recently lithium ion batteries in a shipment of electronic cigarettes may have been a contributing factor of a fire on a Fedex cargo plane. In 2004, the regulators at Pipeline and Hazardous Materials Safety Administration (PHMSA) banned the shipment of bulk non-rechargeable lithium batteries on passenger jets. Rechargeable lithium-ion batteries are not as flammable and can be put out with fire extinguishers, but the NTSB has issued a series of recommendations calling for tighter regulation and testing of the batteries. Our products are powered by rechargeable lithium ion batteries and We currently utilize air shipping in addition to sea based container shipping to import our products from their country or origin. If additional restrictions are put in place which limit our ability to import our products by air freight, it could have an adverse effect on our supply chain, our inventory management procedures and processes and our ability to fill orders and service our clients in a timely manner, which could have a material adverse effect on our performance, operating results and our financial condition.
If We Have Improperly Marketed and Distributed Certain of Our Products in Violation of FDA Regulations We may be Subject to Disciplinary, Actions, Administrative Actions, Sanctions and Fines.
We may be subject to disciplinary, administrative and or regulatory actions if the FDA and or a court of proper jurisdiction determines that our products or the means by which we marketed and sold our products was effected without the proper regulatory approvals. Any such disciplinary, regulatory or administrative actions, fines and or sanctions which may have a material adverse effect on our operations, financial results and business prospects.
The FDA has Issued an Import Alert Which has Limited Our Ability to Import Certain of Our Products
As a result of FDA import alert 66-41 US Customs has from time to time temporarily and in some instances indefinitely detained products sent to us by our Chinese suppliers. If the FDA and or customs modifies the import alert from its current form which allows US customs’ discretion to release our products to us, to a mandatory and definitive hold we will no longer be able to ensure a supply of product for US sales, which will have material adverse effect on our ability to generate revenues, as domestic sales currently account for a significant portion of our revenue.
Moreover as a result of the import alert, approximately three hundred thousand dollars of product is being detained by US customs. We are actively working with our attorneys and customs brokers to have the product released to us or returned to its origin, or diverted to other jurisdictions from which we can market, sell and distribute our products. We are presently unable to predictably determine if and when customs will hold additional and subsequent shipments of our products, in addition certain components of our product may be at risk of spoiling, if we are unable to resolve the detainment of our products and ensure the ability to predictably import our products, our performance, financial condition and results of operations will be materially and adversely effected.
Changes in Governmental Regulation May Affect the Countries in Which we Sell Our products
Foreign jurisdiction have varying policies and laws with respect to the use of personal vaporizers that vaporize nicotine, countries such as the United Kingdom do not restrict its use while other countries such as Thailand have instituted a total ban. If countries such as the United Kingdom reverse their stance or should other countries who have a neutral stance move towards prohibition, it will have a direct impact on our ability to market our products and will have a material adverse effect on our business.
Actions by the FDA Adverse to Our Company and Our Products may Restrict our Ability to do Business Domestically and Internationally.
The United States Food and Drug Administration is the largest and most pervasive health regulator in the world, should we be unable to comply with FDA regulations or should the FDA refuse registration of our products and or should the FDA ban or prohibit the sale and or marketing of our products, other regulators from different countries may assume the same position with respect to our product, causing us substantial harm and raise questions with respect to our ability to continue to operate our business in its current form or at all.
28
Table of Contents
Our Products Contain Two Components each and or both of which may require FDA approvals to be Marketed
Our products have two functional components, the electrical device, which contains the air flow sensor, microchip, lithium ion battery, the heating element and other electrical components and the liquid solution which is may or may not contain nicotine or a placebo. The FDA may assert that the liquid solution and the electronic device are 2 separate and distinct components each requiring separate regulatory approvals, which would could result in additional fees, costs, studies, trials and time. We intend to use reasonable efforts to file for the appropriate approvals to allow us to sell our product in the United States however we have no indication that at present we will be able to afford to pursue regulatory approval and that if we are able to pursue said approval we have no assurances that the outcome of said approval process will result in our products being approved by the FDA. Moreover, if the FDA establishes a regulatory process that we are unable or unwilling to comply with our business, results of operations, financial condition and prospects would be adversely affected. See section “Government Regulation.”
If our third-party suppliers or contract manufacturers do not maintain appropriate standards of manufacturing in accordance with cGMP and other manufacturing regulations, our development and commercialization activities could suffer significant interruptions or delays.
We rely, and intend to continue to rely, on third-party suppliers and contract manufacturers to provide us with materials for our clinical trials and commercial-scale production of our products. These suppliers and manufacturers must continuously adhere to cGMP as well as any applicable corresponding manufacturing regulations outside of the U.S. In complying with these regulations, we and our third-party suppliers and contract manufacturers must expend significant time, money and effort in the areas of design and development, testing, production, record-keeping and quality control to assure that our products meet applicable specifications and other regulatory requirements. Failure to comply with these requirements could result in an enforcement action against us, including warning letters, the seizure of products, suspension or withdrawal of approvals, shutting down of production and criminal prosecution. Any of these third-party suppliers or contract manufacturers will also be subject to audits by the FDA and other regulatory agencies. If any of our third-party suppliers or contract manufacturers fail to comply with cGMP or other applicable manufacturing regulations, our ability to develop and commercialize our products could suffer significant interruptions and delays.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured the product ourselves, including:
| • | reliance on the third party for regulatory compliance and quality assurance; | ||
| • | reliance on the continued financial viability of the third parties; | ||
| • | limitations on supply availability resulting from capacity and scheduling constraints of the third parties; | ||
| • | impact on our reputation in the marketplace if manufacturers of our products, once commercialized, fail to meet the demands of our customers; | ||
| • | the possible breach of the manufacturing agreement by the third party because of factors beyond our control; and | ||
| • | the possible termination or non-renewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us. |
If any of our contract manufacturers fail to achieve and maintain appropriate manufacturing standards, patients using our product candidates could be injured or die, resulting in product liability claims. Even absent patient injury, we may be subject to product recalls, product seizures or withdrawals, delays or failures in testing or delivery, cost overruns or other problems that could seriously harm our business or profitability.
29
Table of Contents
Risks Related to FDA Regulation
Our Product development Efforts may fail.
| Development of our product candidates is subject to risks of failure. For example: | |||
| • | The nicotine based personal vaporizer may be found to be ineffective or unsafe, or fail to receive necessary regulatory approvals; | ||
| • | Our personal vaporizers may be uneconomical to market or take substantially longer to obtain necessary regulatory approvals than anticipated; or | ||
| • | competitors may market equivalent or superior products. |
As a result, our product development activities may not result in any safe, effective and commercially viable products, and we may not be able to commercialize our products successfully. Our failure to develop safe, effective, and commercially viable products would have a material adverse effect on our business, prospects, results of operations and financial condition.
Failure can occur at any stage of our product development efforts.
We will only obtain regulatory approval to commercialize synthetic nicotine, nicotine derived from tobacco and or tobacco based personal vaporizers, if we can demonstrate to the satisfaction of the FDA (or the equivalent foreign regulatory authorities) in adequate and well-controlled clinical studies that the product(s) is safe and effective for its intended use and that it otherwise meets approval requirements. A failure of one or more clinical or non-clinical studies can occur at any stage of product development. We may experience numerous unforeseen events during, or as a result of, testing that could delay or prevent us from obtaining regulatory approval for or commercializing these certain products, including but not limited to:
| • | regulators or institutional review boards, which are commonly called IRBs, may not authorize us to commence a clinical trial or conduct a clinical trial at a prospective trial site; | |
| • | conditions may be imposed upon us by the FDA regarding the scope or design of our clinical trials, or we may be required to resubmit our clinical trial protocols to IRBs for re-inspection due to changes in the regulatory environment; | |
| • | the number of subjects required for our clinical trials may be larger than we anticipate, patient enrollment may take longer than we anticipate, or patients may drop out of our clinical trials at a higher rate than we anticipate; | |
| • | we may have to suspend or terminate one or more of our clinical trials if we, regulators, or IRBs determine that the participants are being subjected to unreasonable health risks; | |
| • | our third-party contractors or clinical investigators may fail to comply with regulatory requirements or fail to meet their contractual obligations to us in a timely manner; | |
| • | our tests may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional testing; and | |
| • | the costs of our clinical and/or non-clinical trials may be greater than we anticipate. |
30
Table of Contents
We rely on third parties to conduct our clinical trials, and if they do not perform their obligations to us we may not be able to obtain approval for Certain of Our Products.
We do not have the ability to conduct our clinical trials independently. In the event we conduct clinical trials of certain of our products We will rely on academic institutions and other third-party research organizations to assist us in designing, managing, monitoring and otherwise carrying out our clinical trials. Accordingly, we do not have control over the timing or other aspects of our clinical trials. If these third parties do not successfully carry out their duties, both our clinical trials and our business may be materially adversely affected. While we believe that there are numerous third parties that can assist us with our clinical trials, if the third parties with which we contract do not perform, our product development efforts would likely be delayed by any such change, and our efforts would likely be more expensive.
Although we intend to rely on third parties to manage the data from these clinical trials, we are responsible for confirming that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. Moreover, the FDA and foreign regulatory agencies will require us to comply with regulations and standards, commonly referred to as good clinical practice, for conducting, recording and reporting the results of clinical trials to assure that the data and the results are credible and accurate and that the trial participants are adequately protected. Our reliance on third parties does not relieve us of these obligations and requirements, and we may fail to obtain regulatory approval for our candidate products if these requirements are not met.
If Our Non-Clinical or Clinical Trials are Unsuccessful or Significantly Delayed, Our Ability to Commercialize Our Product Candidates May be Impaired.
Before we can obtain regulatory approval for the sale of our product candidates, we may have to conduct, at our own expense, non-clinical tests in animals order to support the safety of certain of our products. Non-clinical testing is expensive, difficult to design and implement, can take several years to complete and is uncertain as to outcome. While the scope of the required Phase I clinical trials are currently uncertain, Our non-clinical tests may produce negative or inconclusive results, and on the basis of such results, we may decide, or regulators may require us, to halt ongoing clinical trials or conduct additional non-clinical testing.
Additionally, we will likely be required to conduct clinical trials demonstrating the efficacy and safety of certain our products in humans. Even if the results of our clinical trials are promising, our product candidates may subsequently fail to meet the safety and efficacy standards required to obtain regulatory approvals. Future clinical trials for our products may not be successfully completed or may take longer than anticipated because of any number of factors, including potential delays in the start of the trial, an inability to recruit clinical trial participants at the expected rate, failure to demonstrate safety and efficacy, unforeseen safety issues, or unforeseen governmental or regulatory delays.
Moreover, any clinical trials we might develop and implement may not be completed in a timely manner or at all and our product candidates may not be found to be safe and effective, and may not be approved by regulatory authorities for its proposed use. Further, regulatory authorities and IRBs that must approve and monitor the safety of each clinical study may suspend a clinical study at any time if the patients participating in such study are deemed to be exposed to any unacceptable health risk. We may also choose to suspend clinical trials and studies if we become aware of any such risks.
In other countries where our product candidates or any other product we develop may be marketed, we will also be subject to regulatory requirements governing human clinical studies and marketing approval for drugs. The requirements governing the conduct of clinical studies, product licensing, pricing and reimbursement varies widely from country to country.
We Have Not Conducted any Clinical or Non-Clinical Testing for Our Product Candidates and We are not Certain at this Time Which Clinical or Non-Clinical Tests the FDA Will Require with Respect to any NDA that We may File.
The FDA will require us to submit data from non-clinical testing for our product candidates before approving our product. We do not yet know what non-clinical tests will be required or whether any non-clinical tests will begin as planned, will need to be restructured or will be completed on schedule, if at all. We do not know whether the non-clinical tests that we undertake, if conducted, will be acceptable to the FDA nor have not initiated discussions with the FDA in this regard.
31
Table of Contents
Despite Our Efforts We may Fail to Gain Regulatory Approval For our Products
The FDA approval process as further described in the below section titled “Government Regulation” is both capital and time intensive and we have no assurances that we will have the resources to pursue the regulatory process and if we do there are no assurances that we will be able to complete the approval process that our products will ultimately gain approval for sale. If we are unable to gain regulatory approval for our products, our operations, financial results and business prospects would be adversely effected and our ability to continue to operate would be severely impaired.
Even if we are successful at winning regulatory approval from the FDA, a prescription may be required to buy our products.
Drug products are sold either over the counter (OTC) or by prescription; in the event we win regulatory approval from the FDA, the FDA may still be require that our products be sold by prescription, from a licensed and practicing medical doctor. If our product requires a prescription to be sold it would limit our ability to sell our products, increase our marketing and distribution costs and would have a material adverse effect on our financial performance and require us to change our business model and sell our products through new distribution channels.
Post-approval marketing of our products will be subject to substantial government regulation. Failure to comply with these regulations could result in fines and withdrawal of approvals.
Even if our products receive regulatory approvals, we will be subject to extensive ongoing government regulation. The FDA or other regulatory authorities may impose strict limitations on the distribution, marketing, and use for a product, impose a Risk Evaluation and Mitigation Strategy (REMS), that could include further restrictions on distribution and use, subsequently withdraw approval or take other actions against us or our products for many reasons, including subsequent discoveries of previously unknown problems or safety issues with the product. Also, based on subsequent events or other circumstances that may come to our attention, we may voluntarily take action to limit the marketing or use of one or more of our products. We may also be required to conduct additional post-approval non-clinical or clinical studies.
We are subject to inspection and market surveillance by regulatory authorities for compliance with regulations that prohibit the promotion of a medical product for a purpose or indication other than those for which approval has been granted. While a medical product manufacturer may not promote a product for such “off-label” use, doctors are allowed, in the exercise of their professional judgment in the practice of medicine, to use a product in ways not approved by regulatory authorities. Regulatory authorities have broad enforcement power, and any failure by us to comply with manufacturing or marketing regulations could result in penalties, including warning letters, fines, partial or total suspension of production, product recalls or seizures, withdrawals of previously approved marketing approvals or applications, and criminal prosecutions.
Risks Related to Ownership of Our Stock
We intend to Amend Our Articles of Incorporation to Authorize Us To Authorize and Issue Additional Shares Of Stock.
We are currently authorized to, and have issued to 25,000,000 shares of our common stock, moreover, pursuant to our recent acquisition of Smoke Anywhere USA, Inc. Moreover, we have undertaken to, as soon as practicable, with the consent of the owners of the majority of our shares of common stock, to amend our articles of incorporation to authorize up to 250,000,000 shares of common stock; of which 50,000,000 shares will be issued immediately in connection with the Smoke acquisition and additional shares of our common stock may be issued by our board of directors for such consideration as they may consider sufficient without seeking stockholder approval. The issuance of additional shares of common stock in the future will reduce the proportionate ownership and voting power of current stockholders.
Your Percentage Ownership of our Common Shares may be Diluted by Future Share Issuances
To the extent we issue new shares to fund acquisitions, to raise additional capital, to compensate employees and other persons your percentage ownership of our shares will be diluted.
32
Table of Contents
We Do Not Intend To Pay Future Cash Dividends.
We currently do not anticipate paying cash dividends on our common stock at any time in the near future. We may never pay cash dividends or distributions on our common stock. Any credit agreements which we may enter into with institutional lenders may restrict our ability to pay dividends. Whether we pay cash dividends in the future will be at the discretion of our board of directors and will be dependent upon our financial condition, results of operations, capital requirements and any other factors that the board of directors decides is relevant.
Our Common Stock is Illiquid And Should A Market For Our Securities Develop The Price Of Our Securities May Be Volatile.
Our shares are currently listed on the National Quotation Bureaus Pink Sheets, the market for our securities is and will likely remain illiquid. This means that as an investor you will likely have a difficult time selling our Common Stock at market. Furthermore because of the small amount of shares that will be outstanding and or in the public float, the market price of our common stock may experience significant volatility. Other factors that may contribute to volatility should a market for our Common Stock develop are, our quarterly results, announcements by us or our competitors regarding acquisitions or dispositions, loss of existing clients, new procedures or technology, litigation, changes in general conditions in the economy and general market conditions could cause the market price of the common stock to fluctuate substantially. In addition, the stock market has experienced significant price and volume fluctuations that have particularly affected the trading prices of equity securities of many technology and Internet companies. Frequently, these price and volume fluctuations have been unrelated to the operating performance of the affected companies.
Future Sales Of Our Common Stock May Depress Our Stock Price.
If our controlling stockholders sell substantial amounts of our common stock in the public market following this merger or at any time in the future, the market price of our common stock could fall. Subsequent to post closing undertakings, restructurings and new issuances, there will be approximately 60,000,000 shares of common stock outstanding.
Existing Shareholders beneficially hold approximately 25,000,000 Shares of which approximately 3,576,829 shares are in the public float and are eligible to be sold free of any restrictions. All other Shares are “restricted” as defined in Rule 144 under the Securities Act (“Rule 144”). Since the company has previously been designated a shell company , the “restricted” Shares must be held for one year from the date of this filing prior to being eligible to be sold pursuant to Rule 144. The Company can make no prediction as to the effect, if any, that sale of Shares, or the availability of Shares for future sale, will have on the market price of the Shares prevailing from time to time. Sales of substantial amounts of Shares in the public market, or the perception that such sales could occur, could depress prevailing market prices for the Shares. Such sales may also make it more difficult for the Company to sell equity securities or equity-related securities in the future at a time and price which it deems appropriate.
Broker-Dealers may be discouraged from effecting transactions in our common stock because they may be considered a “Penny Stock” and are subject to the applicable Penny Stock rules.
Rules 15g-1 through 15g-9 promulgated under the Exchange Act impose sales practice and disclosure requirements on certain brokers-dealers who engage in certain transactions involving a “penny stock.” Subject to certain exceptions, a penny stock generally includes any non-NASDAQ equity security that has a market price of less than $5.00 per share. There is currently no established price quotation for our shares, however, we expect that initial quotations will not exceed $5.00 and there is the possibility that the quoted shares price may never exceed $5.00, and that our common stock will be deemed penny stock for the purposes of the Exchange Act. The additional sales practice and disclosure requirements imposed upon brokers-dealers may discourage broker-dealers from effecting transactions in our common stock, which could severely limit the market liquidity of the stock and impede the sale of our stock in the secondary market. Specifically, any broker-dealer selling penny stock to anyone other
33
Table of Contents
than an established customer or “accredited investor,” generally, an individual with net worth in excess of $1,000,000 or an annual income exceeding $200,000, or $300,000 together with his or her spouse, must make a special suitability determination for the purchaser and must receive the purchaser’s written consent to the transaction prior to sale, unless the broker-dealer or the transaction is otherwise exempt. In addition, the penny stock regulations require the broker-dealer to deliver, prior to any transaction involving a penny stock, a disclosure schedule prepared by the United States Securities and Exchange Commission relating to the penny stock market, unless the broker-dealer or the transaction is otherwise exempt. A broker-dealer is also required to disclose commissions payable to the broker-dealer and the registered representative and current quotations for the securities. Finally, a broker-dealer is required to send monthly statements disclosing recent price information with respect to the penny stock held in a customer’s account and information with respect to the limited market in penny stocks.
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
Our Management’s Discussion and Analysis should be read in conjunction with our financial statements included in this prospectus.
Forward-Looking Statements
This current report contains forward-looking statements and information relating to us that are based on the beliefs of our management as well as assumptions made by, and information currently available to, our management. When used in this report, the words “believe,” “anticipate,” “expect,” “estimate,” “intend”, “plan” and similar expressions, as they relate to us or our management, are intended to identify forward-looking statements. These statements reflect management’s current view of us concerning future events and are subject to certain risks, uncertainties and assumptions, including among many others: a general economic downturn; a downturn in the securities markets; federal or state laws or regulations having an adverse effect on proposed transactions that we desire to effect; Securities and Exchange Commission regulations which affect trading in the securities of “penny stocks,”; and other risks and uncertainties. Should any of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this report as anticipated, estimated or expected. The accompanying information contained herein, including, without limitation, the information set forth under the heading “Management’s Discussion and Analysis and Plan of Operation” identifies important additional factors that could materially adversely affect actual results and performance. You are urged to carefully consider these factors. All forward-looking statements attributable to us are expressly qualified in their entirety by the foregoing cautionary statement. The terms “Miller Diversified,” “Miller,” “we,” “us,” “our,” and the “Company” refer to Miller Diversified Corporation and the terms “Smoke Anywhere USA,” and “Smoke” refer to our wholly owned subsidiary Smoke Anywhere USA, Inc.”
Recent Developments
Purchase Business Combination:
On September 1, 2009 Miller Diversified, Corp. (“we,” “Miller” or the “Company”) entered into a definitive agreement (the “Agreement”) with Smoke Anywhere USA, Inc., a Florida corporation (“Smoke”) whereby the Company will acquire 100% of the issued and outstanding shares of Smoke Anywhere USA, Inc., as a result of the transaction Smoke will become a wholly-owned subsidiary of the Company. On November 5, 2009, Miller and Smoke, completed, subject to certain post closing undertakings, the transaction. The transaction contemplated by the Agreement was intended to be a “tax-free” reorganization pursuant to the provisions of Section 351 and 368 of the Internal Revenue Code of 1986, as amended. For accounting purposes, this transaction was being accounted for as a reverse merger, since the stockholders of Smoke own a majority of the issued and outstanding shares of common stock of the Company, and the directors and executive officers of Smoke now own an control in excess of eighty percent of the company’s outstanding stock.
In connection with the Company’s acquisition of the common stock of Smoke, the Company issued 20,670,000 shares of common stock of Miller to the SMOKE Shareholders, of which 2,074,640 of the Miller shares of Common Stock were loaned to the Company by, the Company’s controlling shareholder. Pursuant to the Acquisition Agreement and Plan of Merger, the company will issue an additional 50,000,000 shares, subsequent to certain corporate actions as set forth above and elsewhere herein.
34
Table of Contents
Restricted Inventories
In early 2009 the FDA issued import alert 66-41, and subsequent thereto US Customs has from time to time temporarily and in some instances indefinitely detained products sent to us by our Chinese suppliers. If the FDA and or customs modifies the import alert from a its current form which allows US customs’ discretion to release our products to us to a mandatory and definitive hold we will no longer be able to ensure a supply of product for US sales. At present we have two shipments of product that are subject to regulatory holds, one shipment has been detained by customs and another shipment is in our possession but subject to an FDA hold. The value of these two lots of inventory is approximately $500,000.
Critical Accounting Policies
This discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these consolidated financial statements requires us to make significant estimates and judgments that affect the amounts reported in the consolidated financial statements and the accompanying notes. These items are regularly monitored and analyzed by management for changes in facts and circumstances, and material changes in these estimates could occur in the future. Changes in estimates are recorded in the period in which they become known. The estimates are based on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from the estimates.
Revenue Recognition
We recognize revenue from product sales when the persuasive evidence of an arrangement exists, delivery has occurred and collect-ability is reasonably assured. Product sales and shipping revenues, net of promotional discounts, rebates, and return allowances, are recorded when the products are shipped and title passes to customers. Retail items sold to customers are made pursuant to sales contracts that generally provide for transfer of both title and risk of loss upon our delivery to the carrier. Return allowances, which reduce product revenue by our best estimate of expected product returns, are estimated using historical experience. Revenue from product sales and services rendered is recorded net of sales taxes.
Taxes
We record valuation allowances against our deferred tax assets. Realization of deferred tax assets (such as net operating loss carry-forwards) is dependent on future taxable earnings and is therefore uncertain. At least quarterly, we assess the likelihood that our deferred tax asset balance will be recovered from future taxable income. To the extent we believe that recovery is not likely, we establish a valuation allowance against our deferred tax asset, which increases our income tax expense in the period when such determination is made.
In addition, we do not plan to record U.S. income tax expense for foreign earnings that we have determined to be indefinitely reinvested offshore, thus reducing our overall income tax expense. The amount of earnings designated as indefinitely reinvested offshore is based upon the actual deployment of such earnings in our offshore assets and our expectations of the future cash needs of our U.S. and foreign entities. Income tax considerations are also a factor in determining the amount of foreign earnings to be indefinitely reinvested offshore.
We carefully review all factors that drive the ultimate disposition of foreign earnings determined to be reinvested offshore, and apply stringent standards to overcoming the presumption of repatriation. Despite this approach, because the determination involves our future plans and expectations of future events, the possibility exists that amounts declared as indefinitely reinvested offshore may ultimately be repatriated. For instance, the actual cash needs of our U.S. entities may exceed our current expectations, or the actual cash needs of our foreign entities may be less than our current expectations. This would result in additional income tax expense in the year we determined that amounts were no longer indefinitely reinvested offshore. Conversely, our approach may also result in a determination that accumulated foreign earnings (for which U.S. income taxes have been provided) will be indefinitely reinvested offshore. In this case, our income tax expense would be reduced in the year of such determination.
35
Table of Contents
On an interim basis, we estimate what our effective tax rate will be for the full fiscal year. The estimated annual effective tax rate is then applied to the year-to-date pre-tax income excluding infrequently occurring or unusual items, to determine the year-to-date tax expense. The income tax effects of infrequent or unusual items are recognized in the interim period in which they occur. As the fiscal year progresses, we continually refine our estimate based upon actual events and earnings by jurisdiction during the year. This continual estimation process periodically results in a change to our expected effective tax rate for the fiscal year. When this occurs, we adjust the income tax provision during the quarter in which the change in estimate occurs so that the year-to-date provision equals the expected annual rate.
We account for uncertain tax positions on a quarterly basis, we reevaluate the probability that a tax position will be effectively sustained and the appropriateness of the amount recognized for uncertain tax positions based on factors including changes in facts or circumstances, changes in tax law, settled audit issues and new audit activity. Changes in our assessment may result in the recognition of a tax benefit or an additional charge to the tax provision in the period our assessment changes. We recognize interest and penalties related to income tax matters in income tax expense.
Other Contingencies
In the ordinary course of business, we are involved in legal proceedings regarding contractual and employment relationships, product liability claims, trademark rights, and a variety of other matters. We record contingent liabilities resulting from claims against us, including related legal costs, when a loss is assessed to be probable and the amount of the loss is reasonably estimable. Assessing probability of loss and estimating probable losses requires analysis of multiple factors, including in some cases judgments about the potential actions of third party claimants and courts. Recorded contingent liabilities are based on the best information available and actual losses in any future period are inherently uncertain. If future adjustments to estimated probable future losses or actual losses exceed our recorded liability for such claims, we would record additional charges as other (income) expense, net during the period in which the actual loss or change in estimate occurred. In addition to contingent liabilities recorded for probable losses, we disclose contingent liabilities when there is a reasonable possibility that the ultimate loss will materially exceed the recorded liability. Currently, we do not believe that any of our pending legal proceedings or claims will have a material impact on our financial position or results of operations.
Results of Operations for the Nine Months Ended September 30, 2009 Compared to the Nine Months ended September 30, 2008
During the nine months ended September 30, 2009 we had $4,484,729 in revenues. This was an increase of$4,337,247 or approximately 3,040% from the nine months ended September 30, 2008. This increase is a result of the fact that we were founded on March 24, 2008 and were developing the business and our operations.
Our operating expenses increased by $1,527,732 to $1,642,334 for the nine months ended September 30, 2009. This was an increase of 1,433%, as compared to operating expenses of $114,602 for the nine months ended September 30, 2008. Our operating expenses for the nine months ended September 30, 2009 consisted of general and administrative expenses of $1,642,334 compared to general and administrative expenses of $114,602 for the period ended September 30, 2008.
During the nine months ended September 30, 2009 we had $0 in research and development costs. This was unchanged from the period ended September 30, 2008.
36
Table of Contents
We had net income of $403,052 for the nine months ended September 30, 2009, as compared to net loss of $58,559 for the nine months ended September 30, 2008. The increase in net income was due to an increase in revenue, as discussed above.
Liquidity and Capital Resources
During the nine months ended September 30, 2009 we had total assets of $1,275,442.
During the nine months ended September 30, 2009 we had total liabilities of $503,644 as compared to total liabilities of $29,084 for the nine months ended September 30, 2008.
We had retained earnings of $386,298 and total stockholders’ equity of $771,798 as of September 30, 2009.
Our net cash provided by operating activities was $10,603 for the nine months end September 30, 2009 which included net income of $403,052, and accounts payable and other accrued liabilities of $230,024. Net cash used in operating activities for the nine months ended September 30, 2008 was $178,998, which included a net loss of $58,559, the acquisition and purchase of inventories in the amount of $123,308 and accounts payable and other accrued liabilities of $2,869.
Cash flows from operations were sufficient to fund our requirements during this period.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Item 3. Properties.
Our principal executive offices are located at 3101 Hallandale Beach Boulevard #100, Pembroke Park, Florida 33009. We are currently leasing those premises for $2,756 per month for a term that will expire on December 31, 2009.
We lease additional warehouse space from certain of our controlling shareholders, at a price which we believe is more favorable than we could otherwise secure. We believe that existing facilities are suitable and adequate for our current needs. If we require additional space, we believe that we will be able to secure such space on commercially reasonable terms without undue operational disruption.
37
Table of Contents
Item 4. Security Ownership of Certain Beneficial Owners and Management.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Security ownership of certain beneficial owners.The following table provides the names and addresses of each person known to own directly or beneficially more than 5% of our outstanding Common Stock (as determined in accordance with Rule 13d-3 under the Exchange Act) as of November 5th, 2009.
| Shares | Percentage of | |||||||
| Beneficially | Beneficial | |||||||
| Name and address of beneficial owner | Owned(1)(4) | Ownership(1) | ||||||
5% Stockholders | ||||||||
| Adam Frija | 1,860,300 | 7.44 | % | |||||
| Isaac Galazan | 4,237,350 | 16.95 | % | |||||
| Jeffrey Holman | 2,343,978 | 9.38 | % | |||||
| Doron Ziv | 2,893,800 | 11.58 | % | |||||
Vapeco Holdings | — | — | % | |||||
Named Executive Officers | ||||||||
| Kevin Frija | — | 0 | % | |||||
| All Current Officers and Directors As a Group (1 person) | 0 | % | ||||||
| (1) | Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Except as indicated by footnote, and subject to the community property laws where applicable, to the Company’s knowledge the persons named in the table above have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them. Unless otherwise indicated, the address for each person is the Company’s address at 3101 W. Hallandale Boulevard #100, Hallandale, Florida 33009. | |
| (2) | On November 5, 2009 there were 25,000,000 shares of our common stock outstanding and no shares of preferred stock issued and outstanding. We have no outstanding stock options or warrants. | |
| (3) | In determining the percent of voting stock owned by a person (a) the numerator is the number of shares of common stock beneficially owned by the person, including shares the beneficial ownership of which may be acquired within 60 days upon the exercise of options or warrants or conversion of convertible securities, and (b) the denominator is the total of (i) the 25,000,000 shares of common stock outstanding, or 60,000,000 shares of common stock post closing undertakings, which includes a 2.5:1 reverse stock split (ii) any shares of common stock which the person has the right to acquire within 60 days upon the exercise of options or warrants or conversion of convertible securities. Neither the numerator nor the denominator includes shares which may be issued upon the exercise of any other options or warrants or the conversion of any other convertible securities. | |
| (4) | Subsequent to the post closing undertakings , which includes a 2.5:1 reverse stock split and additional issuances resulting in 60,000,000 shares of common stock outstanding; Mr . Adam Frija will own 4,64,720, Mr. Galazan will own 10,169,640, Mr. Ziv will own, 6,945,120, of the company’s common stock respectively, the percentages of shares beneficially owned percentages will remain unchanged. Subsequent to the post closing issuances,Vapeco Holdings will own 4,799,966 which represents an 8% beneficial ownership, and Mr. Kevin Frija will own, 4,314,000 which represents a 7.19% beneficial ownership. |
Item 5. Directors and Executive Officers.
Directors
The names of our directors and certain information about them, as of July 27, 2009, are set forth below:
| Name of Director | Age | Position with Company | Director since | |||
| Kevin Frija | 37 | President, Chief Executive Officer and | June 9, 2009 | |||
| Chairman of the Board of Directors |
Kevin Frija
Mr. Frija has served as chairman of the board of directors and the sole director of the company since June 9,2009. Mr. Frija also serves as the company’s president and chief executive officer. Mr. Frija is responsible for the day to day operations of the company, including but not limited to managing the company’s employees, establishing and implementing operational procedures, marketing and sales strategies and the creation and implementation of our business strategies. Mr. Frija is also a principal of the In Gear Companies a network of businesses with operations and interests in apparel, licensing and consumer products. Mr. Frija is principally responsible for strategic acquisitions and business development.
38
Table of Contents
Item 6. Executive Compensation.
EXECUTIVE COMPENSATION
SUMMARY COMPENSATION TABLE
| Nonequity | Nonqualified | |||||||||||||||||||||||||||||||||||
| incentive | deferred | |||||||||||||||||||||||||||||||||||
| Name & Principal | Stock | Option | plan | compensation | All Other | |||||||||||||||||||||||||||||||
| Position(1)(2) | Year | Salary $ | Bonus $ | Awards $ | Awards $ | compensation $ | earnings $ | Compensation $ | Total $ | |||||||||||||||||||||||||||
Kevin Frija PEO, PAO and Director | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
James E. Miller former CEO | 2008 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
| 2007 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| 2006 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| 2005 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
Option Grants in Last Fiscal Year
There were no options, restricted stock, or restricted stock units granted to the Named Executive Officers during fiscal year 2008.
Arrangements with Named Executive Officers
Effective October 1, 2009 the Company entered into an employment agreement with Mr. Kevin Frija, PEO, PAO and president of the company. The agreement provides for the payment of $72,000 in annual cash compensation and an award of 600,000 stock options which shall vest monthly on a pro-rata basis. Mr. Frija shall be eligible to participate in any employee benefit programs, stock option plan or other equity incentive or bonus plan that the company may subsequently adopt.
Termination of Employment and Change of Control Agreement
In the event any of the Named Executive Officers or other covered employees or consultants, as determined by the company, are terminated without cause or if they should resign for “Good Reason,” those options, restricted stock, and restricted stock units outstanding that are not yet vested as of the date of such termination or resignation will vest in full. Generally, “Cause” is defined to include a felony conviction, willful disclosure of confidential information or willful and continued failure to perform his or her employment duties. “Good Reason” includes resignation of employment as a result of a substantial diminution in position or duties, or an adverse change in title or reduction in annual base salary.
The Company has no arrangement with any of its Named Executive Officers, employees, consultants or other persons, which results or will result from the change of control of the Company
Directors
We do not currently have any non-employee directors and no additional compensation is currently paid to Mr. Frija in connection with his directorship over and above his employee based compensation.
39
Table of Contents
Item 7. Certain Relationships and Related Transactions, and Director Independence.
The Company has entered into indemnity agreements its sole officer and director which provides, among other things, that the Company will indemnify such officer or director, under the circumstances and to the extent provided for therein, for expenses, judgments, fines and settlements such officer or director may be required to pay in actions or proceedings which they are or may be made a party by reason of their position as a director, officer or other agent of the Company, and otherwise to the full extent permitted under Nevada law and the Amended and Restated Bylaws of the Company.
A familial relationship exists by and between our president Kevin Frija and certain of the Smoke Anywhere USA shareholders. Hannah Frija and Adam Frija are respectively the mother and brother of Kevin Frija and were minority shareholders of Smoke Anywhere USA, prior to the acquisition by and between Smoke Anywhere and the Company. Ralph Frija, Kevin Frija’s father acquired an interest in the company from a Smoke Anywhere shareholder, subsequent to the execution of the acquisition agreement. Moreover, Jacob Levy and Kevin Frija are equity and operational partners in numerous other business ventures. Mr. Levy and his family and Mr Frija’s family members were minority shareholders in Smoke owning an aggregate of 23% of the Smoke shares at the time of the acquisition.
We currently contract for warehousing and certain fulfillment services with a company that is jointly owned by our president, Kevin Frija and our shareholder Jacob Levy. Services are rendered on an at will basis and can be cancelled at anytime without notice. We believe that we are receiving more favorable terms than we would be able to receive from an unrelated third party.
Prior to the completion of the acquisition agreement by and between the company and Smoke Anywhere USA, Kevin Frija, our president served as the president of Vapeco Holdings, Inc., our controlling shareholder. Mr. Frija has since resigned from Vapeco but will receive approximately 4,314,000 shares or 7.19% of our total outstanding common stock as issued subsequent to the completion of our 2.5 for 1 reverse split.
Insofar as the transaction contemplated by the Agreement involves an interested director transaction within the meaning of NRS 78.140, management believes that the transaction is fair to the company and the company will benefit by acquiring an operating business, after several years of inactivity.
Director Independence
The company is currently traded on the National Quotation Bureaus Pink Sheets. The Pink Sheets does not have any director independence requirements. Mr. Firja is presently our sole executive officer and director and as such we do not have an independent board of directors.
We have entered into an offer letter agreement with our chief executive officer that, among other things, provides for certain severance and change of control benefits. For a description of this agreement, see “—Executive Compensation”
We have indemnification agreements with each of our current director, officer and some employees.
Other than as described above under this section “Certain Relationships and Related Transactions,” since the beginning of our last fiscal year, we have not entered into any transactions, nor are there any currently proposed transactions, between us and a related party where the amount involved exceeds, or would exceed, $120,000, and in which any related person had or will have a direct or indirect material interest. We believe the terms of the transactions described above were comparable to terms we could have obtained in arm’s length dealings with unrelated third parties.
40
Table of Contents
Item 8. Legal Proceedings.
We are not currently involved in legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition or results of operations. We may become involved in material legal proceedings in the future. We are not aware of any proceeding to which any of our directors, officers, affiliates or security holders are a party adverse to us or have a material interest adverse to us.
Item 9. Market Price of and Dividends on the Registrant’s Common Equity and Related Stockholder Matters.
The Company’s’ securities are traded on the National Quotation Bureaus’ Pink Sheets, however there is not an established public trading market for the company’s securities. The following prices do not reflect the planned 2.5 for 1 reverse stock split.
| High | Low | |||||||
2009 | ||||||||
1st Quarter | 0.03 | 0.015 | ||||||
2nd Quarter | 0.19 | 0.015 | ||||||
3rd Quarter | 0.30 | 0.10 | ||||||
4th Quarter | 0.51 | 0.18 | ||||||
2008 | ||||||||
1st Quarter | 0.07 | 0.04 | ||||||
2nd Quarter | 0.045 | 0.03 | ||||||
3rd Quarter | 0.045 | 0.025 | ||||||
4th Quarter | 0.03 | 0.02 | ||||||
2007 | ||||||||
3rd Quarter | 0.063 | 0.05 | ||||||
4th Quarter | 0.05 | 0.04 | ||||||
Holders.There are approximately 1,425 beneficial holders of the Company’s common stock.
Penny Stock Considerations.Our common stock will be considered a “penny stock” as defined in certain rules under the Exchange Act. Our shares thus will be subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in certain transactions involving a penny stock. In general, a security which is not quoted on NASDAQ or has a market price of less than $5 per share where the issuer does not have in excess of $2,000,000 in net tangible assets is considered a penny stock. The Commission’s Rule 15g-9 regarding penny stocks imposes additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally persons with net worth in excess of $1,000,000 or an annual income exceeding $200,000 or $300,000 jointly with their spouse). For transactions covered by the rules, the broker-dealer must make a special suitability determination for the purchaser and receive the purchaser’s written agreement to the transaction prior to the sale. Thus, the rules affect the ability of broker-dealers to sell our common stock should they wish to do so because of the adverse effect that the rules have upon liquidity of penny stocks. Unless the transaction is exempt under the rules, under the Securities Enforcement Remedies and Penny Stock Reform Act of 1990, broker-dealers effecting customer transactions in penny stocks are required to provide their customers with (i) a risk disclosure document; (ii) disclosure of current bid and ask quotations if any; (iii) disclosure of the compensation of the broker-dealer and its sales personnel in the transaction; and (iv) monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, various state securities laws impose restrictions on transferring penny stocks.
41
Table of Contents
Preferred Stock.We have authorized preferred stock consisting of 1,000,000 shares par value $2.00 per share. No shares of preferred stock are issued and outstanding.
Dividends.To date, we have not declared or paid any dividends on our outstanding shares. We currently do not anticipate paying any cash dividends in the foreseeable future on our Common Stock. Although we intend to retain our earnings to finance our operations and future growth, our Board of Directors will have discretion to declare and pay dividends in the future. Payment of dividends in the future will depend upon our earnings, capital requirements and other factors, which our Board of Directors may deem relevant.
Equity Compensation Plans.The Company does not presently have any equity compensation plans.
Item 10. Recent Sales of Unregistered Securities.
The Company in consideration of its acquisition of Smoke Anywhere USA, Inc. has issued 20,670,000 shares of its common stock to the former shareholders of Smoke Anywhere. Pursuant to the acquisition agreement by and between the company and Smoke, the company is obligated to issue an additional 50,000,000 on a fully diluted basis after the company completes its 2.5:1 reverse split.
Item 11. Description of Registrant’s Securities to be Registered.
DESCRIPTION OF CAPITAL STOCK
Authorized Capital Stock
We have authorized capital stock consisting of 25,000,000 shares of Common Stock, $0.001 par value per share (“Common Stock”). As of November 6, 2009, we had 25,000,000 shares of Common Stock issued and outstanding.
Common Stock
The holders of common stock are entitled to be informed on all matters to be voted on by stockholders with voting shares, including elections of directors, and the holders of such shares currently possess all voting power. The holders of common stock will be entitled to such dividends as may be declared from time to time by the Board of Directors from funds legally available therefore. In the event of our dissolution, liquidation or winding up, holders of our shares of common stock will be entitled to receive, pro rata, all assets available for distribution to such holders after payment of all liabilities, subject to prior rights of any outstanding preferred stock. The holders of our common stock have no preemptive rights to purchase newly issued securities.
The company’s common stock is quoted on the National Quotation Bureau’s Pink Sheets under the symbol “MILR”.
Item 12. Indemnification of Directors and Officers.
Our By-Laws provide for the indemnification of directors, officers, employees and agents of the Company to the fullest extent provided by the Business Corporation Law of the State of Nevada. These sections generally provide that the Company may indemnify any person who was or is a party to any threatened, pending or completed action, suit or proceeding whether civil, criminal, administrative or investigative except for an action by or in right of the corporation by reason of the fact that he or she is or was a director, officer, employee or agent of the corporation. Generally, no indemnification may be made where the person has been determined to be negligent or guilty of misconduct in the performance of his or her duties to the Company.
At present, there is no pending litigation or proceeding involving a director, officer, employee or agent of the Corporation where indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that may result in a claim for indemnification by any director or officer.
42
Table of Contents
The Company’s By-Laws provide that it will indemnify its Directors and Officers, and hold them harmless to the fullest extent legally permissible under the Business Corporation Law of the State of Nevada. These rights of indemnification shall not be exclusive of any other right which any Officer or Director may have or may thereafter acquire under any by-law, agreement, vote of stockholders, provision of law or otherwise.
The By-Laws require the Company to pay all expenses, liability and losses (including attorney fees, judgments, fines and amounts paid or to be paid in the settlement of a matter), which are reasonably incurred or suffered by the Officer or Director. Any expenses of the Officer or Director incurred in defending a civil or criminal action, suit or proceeding must be paid by the Company as they are incurred in an advance of the final determination of the action, suit or proceeding upon receipt of an undertaking by or on behalf of the Director or Officer to repay the amount if it is ultimately determined by a court of competent jurisdiction that he or she is not entitled to be indemnified by the Corporation.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 (the “Act”) may be permitted to directors, officers and controlling persons of the small business issuer pursuant to the foregoing provisions, or otherwise, the small business issuer has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
Item 13. Financial Statements and Supplementary Data.
Not Applicable to Smaller reporting Companies.
Item 14. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 15. Financial Statements and Exhibits.
(a) Financial Statements of business acquired.
The required financial statements of Smoke Anywhere USA, Inc. for the periods specified in Rule 3-05(b) of Regulation S-X are included herein. This Current Report includes the audited financial statements of Smoke Anywhere USA, Inc. for the year ended December 31, 2008 and a review of the nine months ended September 30, 2009.
(b) Pro Forma Financial Information.
The required Pro Forma financial statements of Smoke Anywhere USA, Inc. are included herein. This Current Report on Form 8-K includes the unaudited pro forma consolidated financial information of Miller Diversified Corporation and Smoke Anywhere USA, Inc.
(c) Exhibits
43
Table of Contents
Paritz & Company, P.A.
SMOKE ANYWHERE USA, INC.
FINANCIAL STATEMENTS
WITH
REPORT OF INDEPENDENT REGISTERED ACCOUNTING FIRM
WITH
REPORT OF INDEPENDENT REGISTERED ACCOUNTING FIRM
PERIOD FROM INCEPTION (MARCH 24, 2008)
TO DECEMBER 31, 2008
TO DECEMBER 31, 2008
44
Table of Contents
REPORT OF INDEPENDENT REGISTERED ACCOUNTING FIRM
Board of Directors
Smoke Anywhere USA, Inc.
Hallandale, Florida
Smoke Anywhere USA, Inc.
Hallandale, Florida
We have audited the accompanying balance sheet of Smoke Anywhere USA, Inc. (the “Company”) as of December 31, 2008 and the related statements of operations, stockholders’ equity and cash flows for the period from inception (March 24, 2008) to December 31, 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with auditing standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Smoke Anywhere USA, Inc., as of December 31, 2008 and the results of its operations and its cash flows for the period from inception (March 24, 2008) to December 31, 2008 in conformity with accounting principles generally accepted in the United States of America.
Hackensack, New Jersey
September 15, 2009
September 15, 2009
45
Table of Contents
SMOKE ANYWHERE USA, INC.
BALANCE SHEET
DECEMBER 31, 2008
ASSETS | ||||
CURRENT ASSETS: | ||||
| Cash | $ | 311,762 | ||
| Vendor deposits | 60,000 | |||
| Inventories | 11,313 | |||
| Sundry current assets | 4,755 | |||
TOTAL CURRENT ASSETS | 387,830 | |||
TOTAL ASSETS | $ | 387,830 | ||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||
CURRENT LIABILITIES: | ||||
| Accounts payable and accrued expenses | $ | 7,894 | ||
| Customer deposits | 21,190 | |||
TOTAL CURRENT LIABILITIES | 29,084 | |||
STOCKHOLDERS’ EQUITY: | ||||
| Common stock, par value $1, 100 shares authorized, issued and outstanding | 100 | |||
| Additional paid-in capital | 375,400 | |||
| Accumulated deficit | (16,754 | ) | ||
TOTAL STOCKHOLDERS’ EQUITY | 358,746 | |||
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | 387,830 | ||
See notes to financial statements
46
Table of Contents
SMOKE ANYWHERE USA, INC.
STATEMENT OF OPERATIONS
FROM INCEPTION (MARCH 24, 2008) TO DECEMBER 31, 2008
REVENUE | $ | 508,718 | ||
COSTS AND EXPENSES: | ||||
| Cost of sales | 285,780 | |||
| Selling, general and administrative | 239,692 | |||
TOTAL COSTS AND EXPENSES | 525,472 | |||
NET LOSS | $ | (16,754 | ) | |
| NET LOSS PER COMMON SHARE | (167.54 | ) | ||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES | 100 | |||
See notes to financial statements
47
Table of Contents
SMOKE ANYWHERE USA, INC.
STATEMENT OF STOCKHOLDERS’ EQUITY
| Additional | ||||||||||||||||
| Common | Paid-In | |||||||||||||||
| Shares | Capital | Deficit | Total | |||||||||||||
BALANCE — MARCH 24, 2008 (INCEPTION) | $ | — | $ | — | $ | — | $ | — | ||||||||
| Issuance of common stock | 100 | 375,400 | — | 375,500 | ||||||||||||
| Net loss | — | — | (16,754 | ) | (16,754 | ) | ||||||||||
BALANCE — DECEMBER 31, 2008 | $ | 100 | $ | 375,400 | $ | (16,754 | ) | $ | 358,746 | |||||||
See notes to financial statements
48
Table of Contents
SMOKE ANYWHERE USA, INC.
STATEMENTS OF CASH FLOWS
FROM INCEPTION (MARCH 24, 2008) TO DECEMBER 31, 2008
OPERATING ACTIVITIES: | ||||
| Net loss | $ | (16,754 | ) | |
Changes in operating assets and liabilities: | ||||
| Vendor deposits | (60,000 | ) | ||
| Purchase of inventory | (11,313 | ) | ||
| Sundry current assets | (4,755 | ) | ||
| Accounts payable | 7,894 | |||
| Customer deposits | 21,190 | |||
NET CASH USED IN OPERATING ACTIVITIES | (63,738 | ) | ||
FINANCING ACTIVITIES: | ||||
| Issuance of common stock | 375,500 | |||
NET CASH PROVIDED BY FINANCING ACTIVITIES | 375,500 | |||
INCREASE IN CASH AND CASH — END OF PERIOD | $ | 311,762 | ||
See notes to financial statements
49
Table of Contents
SMOKE ANYWHERE USA, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2008
| 1 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES | |
| Business description | ||
| Smoke Anywhere USA, Inc. (the “Company”) was formed on March 24, 2008 and is incorporated under the laws of Florida. The company is a marketer and distributor of personal vaporizers, under the Fity-One™, Krave™, EZ Smoker™ and Green Puffer™ brands. | ||
| Use of estimates in the preparation of financial statements | ||
| The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of net revenue and expenses during each reporting period. Actual results could differ from those estimates. | ||
| Cash | ||
| The Company maintains cash balances at various financial institutions. Accounts at each institution are insured by the Federal Deposit Insurance Corporation up to $250,000. The Company’s accounts at these institutions may, at times, exceed the Federally insured limits. The Company has not experienced any losses in such accounts. | ||
| Inventories | ||
| Inventories, consisting of merchandise purchased for resale, are valued at the lower of cost (determined on the first-in, first-out basis) or market (replacement cost). | ||
| Revenue recognition | ||
| The Company recognizes revenue from product sales when the persuasive evidence of an arrangement exists ,delivery has occurred and collect-ability is reasonably assured. Product sales and shipping revenues, net of promotional discounts, rebates, and return allowances, are recorded when the products are shipped and title passes to customers. Retail items sold to customers are made pursuant to sales contracts that generally provide for transfer of both title and risk of loss upon our delivery to the carrier. Return allowances, which reduce product revenue by our best estimate of expected product returns, are estimated using historical experience. Revenue from product sales and services rendered is recorded net of sales taxes. | ||
| Deferred income taxes | ||
| The Company accounts for income taxes in accordance with Statement of Financial Accounting Standards No. 109 (SFAS 109) which requires that deferred tax assets and liabilities be recognized for future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. In addition, SFAS 109 requires recognition of future tax benefits, such as carry-forwards, to the extent that realization of such benefits is more likely than not and that a valuation allowance be provided when it is more likely than not that some portion of the deferred tax asset will not be realized. | ||
| Related Party | ||
| The Company currently contract for warehousing and certain fulfillment services with a company that is jointly owned by our president, Kevin Frija and our shareholder Jacob Levy. Services are rendered on an at will basis and can be cancelled at anytime without notice. We believe that we are receiving more favorable terms than we would be able to receive from an unrelated third party. |
50
Table of Contents
| Recent Accounting Pronouncements |
| As of January 1, 2006, SFAS No. 123R,Share-Based Payment, became effective for all companies and addresses the accounting for share-based payment transactions. SFAS No. 123R eliminates the ability to account for share-based compensation transactions using APB No. 25, and generally requires instead that such transactions be accounted and recognized in the statement of operations based on their fair value. The Company does not maintain a stock option plan and, therefore, this pronouncement has no impact on these financial statements. |
| In September 2006, the FASB issued SFAS No. 157 (“SFAS 157”), “Fair Value Measurements”. SFAS 157 defines fair value, establishes a framework for measuring fair value under GAAP, and expands disclosures about fair value measurements. This statement is effective for financial statements issued for fiscal years beginning after November 15, 2007. In February 2008, the FASB agreed to delay the effective date of SFAS 157 for all non-financial assets and non-financial liabilities, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis, to fiscal years beginning after November 15, 2008. As of December 31, 2007, the Company’s fair values of its financial assets and liabilities, which consist of cash and cash equivalents, accounts payable and notes payable, approximate their carrying amount due to the short period of time to maturity. |
| In February 2007, the FASB issued SFAS No. 159 (“SFAS 159”), “The Fair Value Option for Financial Assets and Financial Liabilities — Including an Amendment of FASB Statement No. 115”. This statement provides companies with an option to measure, at specified election dates, many financial instruments and certain other items at fair value that are not currently measured at fair value. A company that adopts SFAS 159 will report unrealized gains and losses on items for which the fair value option has been elected in earnings at each subsequent reporting date. This statement also establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar types of assets and liabilities. This statement is effective for fiscal years beginning after November 15, 2007. The Company is currently evaluating the effect that the adoption of SFAS 159 will have on its results of operations and financial position. |
| In December 2007, FASB issued SFAS No. 160 (“SFAS 160”),“Interests in Consolidated Financial Statements — an amendment of ARB No. 51”, which impacts the accounting for minority interest in the consolidated financial statements of filers. The statement requires the reclassification of minority interest to the equity section of the balance sheet and the results from operations attributed to minority interest to be included in net income. The related minority interest impact on earnings would then be disclosed in the summary of other comprehensive income. The statement is applicable for all fiscal years beginning on or after December 15, 2008 and earlier adoption is prohibited. The adoption of this standard will require prospective treatment. The Company is currently evaluating the effect that the adoption of SFAS 160 will have on its results of operations and financial position. However, the adoption of SFAS 160 is not expected to have a material impact on the Company’s financial statements. |
51
Table of Contents
| In December 2007, FASB issued SFAS No. 141R (“SFAS 141R”),“Business Combinations”, which impacts the accounting for business combinations. The statement requires changes in the measurement of assets and liabilities required in favor of a fair value method consistent with the guidance provided in SFAS 157 (see above). Additionally, the statement requires a change in accounting for certain acquisition related expenses and business adjustments which no longer are considered part of the purchase price. Adoption of this standard is required for fiscal years beginning after December 15, 2008. Early adoption of this standard is not permitted. The statement requires prospective application for all acquisitions after the date of adoption. The Company is currently evaluating the effect that the adoption of SFAS 141R will have on its results of operations and financial position. However, the adoption of SFAS 141R is not expected to have a material impact on the Company’s financial statements. |
| In April 2008, the FASB issued FSP FAS 142-3, “Determination of the Useful Life of Intangible Assets”. This guidance is intended to improve the consistency between the useful life of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets”, and the period of expected cash flows used to measure the fair value of the asset under SFAS No. 141R when the underlying arrangement includes renewal or extension of terms that would require substantial costs or result in a material modification to the asset upon renewal or extension. Companies estimating the useful life of a recognized intangible asset must now consider their historical experience in renewing or extending similar arrangements or, in the absence of historical experience, must consider assumptions that market participants would use about renewal or extension as adjusted for SFAS No. 142’s entity-specific factors. This standard is effective for fiscal years beginning after December 15, 2008, and is applicable to the Company’s fiscal year beginning January 1, 2009. The Company does not anticipate that the adoption of this FSP will have an impact on its results of operations or financial condition |
| In May 2008, the FASB issued Statement of Financial Accounting Standards No. 162, “The Hierarchy of Generally Accepted Accounting Principles” (“Statement No. 162”). Statement No. 162 identifies the sources of accounting principles and the framework for selecting the principles used in preparation of the financial statements of non-governmental entities that are presented in conformity with U.S. GAAP (the GAAP hierarchy). Statement No. 162 will become effective sixty days following the Securities and Exchange Commission’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411, “The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles”. The adoption of Statement No. 162 is not expected to materially impact the Company’s financial position or results of operations. |
52
Table of Contents
SMOKE ANYWHERE USA, INC.
INTERIM FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDING SEPTEMBER 30, 2009
53
Table of Contents
SMOKE ANYWHERE USA, INC
BALANCE SHEET
September 30, 2009
| September 30, 2009 | December 31, 2008 | |||||||
| (Unaudited) | (Audited) | |||||||
ASSETS | ||||||||
CURRENT ASSETS: | ||||||||
| Cash | $ | 332,365 | $ | 311,762 | ||||
| Inventories | 799,744 | 11,313 | ||||||
| Vendor deposits | 143,333 | 60,000 | ||||||
| Sundry current assets | 4,755 | |||||||
TOTAL CURRENT ASSETS AND ASSETS | 1,275,442 | 387,830 | ||||||
LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
CURRENT LIABILITIES | ||||||||
| Accounts payable & accrued expenses | 237,918 | 7,894 | ||||||
| Taxes payable | 265,726 | — | ||||||
| Customer deposits | — | 21,190 | ||||||
TOTAL CURRENT LIABILITIES | 503,644 | 29,084 | ||||||
SHAREHOLDERS’ EQUITY: | ||||||||
| Common Stock | 100 | 100 | ||||||
| Additional paid-in capital | 385,400 | 375,400 | ||||||
| Retained earnings (deficit) | 386,298 | (16,754 | ) | |||||
TOTAL SHAREHOLDERS’ EQUITY | 771,798 | 358,746 | ||||||
TOTAL LIABILITIES & SHAREHOLDERS’ EQUITY | $ | 1,275,442 | $ | 387,830 | ||||
See notes to financial statements
54
Table of Contents
SMOKE ANYWHERE USA, INC
STATEMENTS OF OPERATIONS
| NINE MONTHS ENDED | THREE MONTHS ENDED | |||||||||||||||
| SEPTEMBER 30, | SEPTEMBER 30, | |||||||||||||||
| 2009 | 2008 | 2009 | 2008 | |||||||||||||
REVENUE | $ | 4,484,729 | $ | 147,482 | $ | 1,437,062 | $ | 147,482 | ||||||||
COST OF SALES | 2,173,618 | 91,439 | 548,829 | 91,439 | ||||||||||||
GROSS PROFIT | 2,311,111 | 56,043 | 888,233 | 56,043 | ||||||||||||
COSTS AND EXPENSES: | ||||||||||||||||
| Selling, general & administrative | 1,642,334 | 114,602 | 945,776 | 87,730 | ||||||||||||
TOTAL COSTS AND EXPENSES | 1,642,334 | 114,602 | 945,776 | 87,730 | ||||||||||||
| NET INCOME (LOSS) BEFORE INCOME TAX EXPENSE | 668,777 | (58,559 | ) | (57,543 | ) | (31,687 | ) | |||||||||
INCOME TAX EXPENSE (CREDIT) | 265,725 | — | (14,780 | ) | — | |||||||||||
NET INCOME (LOSS) | $ | 403,052 | $ | (58,559 | ) | $ | (42,763 | ) | $ | (31,687 | ) | |||||
| BASIC & DILUTED NET INCOME (LOSS) PER COMMON SHARE | $ | 4,030.52 | $ | (585.59 | ) | $ | (427.63 | ) | $ | (316.87 | ) | |||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES | 100 | 100 | 100 | 100 | ||||||||||||
See notes to financial statements
55
Table of Contents
SMOKE ANYWHERE USA, INC
STATEMENTS OF CASH FLOWS
| NINE MONTHS ENDED | ||||||||
| SEPTEMBER 30, | ||||||||
| 2009 | 2008 | |||||||
OPERATING ACTIVITIES: | ||||||||
| Net income(loss) | 403,052 | (58,559 | ) | |||||
Changes in operating assets and liabilities: | ||||||||
| Inventories | (788,431 | ) | (123,308 | ) | ||||
| Vendor deposits | (83,333 | ) | — | |||||
| Sundry current assets | 4,755 | — | ||||||
| Accounts payable & accrued expenses | 230,024 | 2,869 | ||||||
| Accrued taxes | — | — | ||||||
| Customer deposits | (21,190 | ) | — | |||||
NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | (255,123 | ) | (178,998 | ) | ||||
FINANCING ACTIVITIES: | ||||||||
| Issuance of common stock | — | 331,300 | ||||||
| Additional investment | 10,000 | — | ||||||
NET CASH(USED IN) BY FINANCING ACTIVITIES | 10,000 | 331,300 | ||||||
| INCREASE IN CASH | (245,123 | ) | 152,302 | |||||
CASH — BEGINNING OF PERIOD | 311,762 | — | ||||||
CASH — END OF PERIOD | 66,639 | 152,302 | ||||||
See notes to financial statements
56
Table of Contents
SMOKE ANYWHERE USA, INC
STATEMENT of STOCKHOLDERS’ EQUITY
| Additional | Retained | |||||||||||||||
| Common | Paid In | Earnings | ||||||||||||||
| Shares | Capital | (Deficit) | Total | |||||||||||||
| Balance — March 24, 2008 | $ | — | $ | — | $ | — | $ | — | ||||||||
| Issuance of common stock | 100 | 375,400 | — | 375,500 | ||||||||||||
| Net loss from the period of inception (March 24, 2008) through December 31, 2008 | — | — | (16,754 | ) | (16,754 | ) | ||||||||||
Balance — December 31, 2008 | 100 | 375,400 | (16,754 | ) | 358,746 | |||||||||||
| Additional investment | — | 10,000 | — | 10,000 | ||||||||||||
| Net income for the nine months ending September 30, 2009 | — | — | 403,052 | 403,052 | ||||||||||||
Balance — December 31, 2009 | 100 | 385,400 | 386,298 | 771,798 | ||||||||||||
See notes to financial statements
57
Table of Contents
SMOKE ANYWHERE USA, INC.
NOTES TO FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
| 1 | INTERIM FINANCIAL STATEMENTS | |
| The unaudited financial statements as of September 30, 2009 and for the three and nine months ended September 30, 2009 and 2008 have been prepared in accordance with accounting principles generally accepted in the United States for interim financial information and with instructions to Regulation S-X. In the opinion of management, the unaudited financial statements have been prepared on the same basis as the annual financial statements and reflect all adjustments, which include only normal recurring adjustments, necessary to present fairly the financial position as of September 30, 2009 and the results of operations and cash flows for the periods ended September 30, 2009 and 2008. The financial data and other information disclosed in these notes to the interim financial statements related to these periods are unaudited. The results for the three and nine months ended September 30, 2009 is not necessarily indicative of the results to be expected for any subsequent quarter of the entire year ending December 31, 2009. The balance sheet at December 31, 2008 has been derived from the audited financial statements at that date. | ||
| Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States have been condensed or omitted pursuant to the Securities and Exchange Commission’s rules and regulations. These unaudited financial statements should be read in conjunction with our audited financial statements and notes thereto for the year ended December 31, 2008 as included in our report on Form 10-K. |
| 1. | The Company has evaluated subsequent events through the filing date of this Form 8-k and determined that there were no subsequent events to recognize or disclose in these financial statements. |
| 2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES | |
| Business description | ||
| Smoke Anywhere USA, Inc. (the “Company”) was formed on March 24, 2008 and is incorporated under the laws of Florida. The company is a marketer and distributor of personal vaporizers, under the Fity-One™, Krave™, EZ Smoker™ and Green Puffer™ brands. | ||
| Use of estimates in the preparation of financial statements | ||
| The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of net revenue and expenses during each reporting period. Actual results could differ from those estimates. | ||
| Cash | ||
| The Company maintains cash balances at various financial institutions. Accounts at each institution are insured by the Federal Deposit Insurance Corporation up to $250,000. The Company’s accounts at these institutions may, at times, exceed the Federally insured limits. The Company has not experienced any losses in such accounts. |
58
Table of Contents
| Inventories | ||
| Inventories, consisting of merchandise purchased for resale, are valued at the lower of cost (determined on the first-in, first-out basis) or market (replacement cost). | ||
| Revenue recognition | ||
| The Company recognizes revenue from product sales when the persuasive evidence of an arrangement exists ,delivery has occurred and collect-ability is reasonably assured. Product sales and shipping revenues, net of promotional discounts, rebates, and return allowances, are recorded when the products are shipped and title passes to customers. Retail items sold to customers are made pursuant to sales contracts that generally provide for transfer of both title and risk of loss upon our delivery to the carrier. Return allowances, which reduce product revenue by our best estimate of expected product returns, are estimated using historical experience. Revenue from product sales and services rendered is recorded net of sales taxes. | ||
| Deferred income taxes | ||
| The Company accounts for income taxes in accordance with the authoritative guidance on deferred income taxes which requires that deferred tax assets and liabilities be recognized for future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. In addition, SFAS 109 requires recognition of future tax benefits, such as carry-forwards, to the extent that realization of such benefits is more likely than not and that a valuation allowance be provided when it is more likely than not that some portion of the deferred tax asset will not be realized. | ||
| Related Party | ||
| The Company currently contract for warehousing and certain fulfillment services with a company that is jointly owned by our president, Kevin Frija and our shareholder Jacob Levy. Services are rendered on an at will basis and can be cancelled at anytime without notice. We believe that we are receiving more favorable terms than we would be able to receive from an unrelated third party. | ||
| Advertising Expense | ||
| Advertising costs are charged to the statement of operations as incurred. The advertising cost for the nine months ended September 30, 2009 is $217,416. | ||
| 3. | SUBSEQUENT EVENTS | |
| We have evaluated events after the date of these financial statements through October 25, 2009, the date that these statements were available to be issued. There were no material subsequent events as of that date. |
59
Table of Contents
| Recent Accounting Pronouncements | ||
| As of January 1, 2006, the FASB issued a standard on Share Based Payment which became effective for all companies and addresses the accounting for share-based payment transactions. This standard eliminates the ability to account for share-based compensation transactions using APB No. 25, and generally requires instead that such transactions be accounted and recognized in the statement of operations based on their fair value. The Company does not maintain a stock option plan and, therefore, this pronouncement has no impact on these financial statements. | ||
| In September 2006, the FASB issued a standard offering guidance on, “Fair Value Measurements”. This standard defines fair value, establishes a framework for measuring fair value under GAAP, and expands disclosures about fair value measurements. This statement is effective for financial statements issued for fiscal years beginning after November 15, 2007. In February 2008, the FASB agreed to delay the effective date of the standard for all non-financial assets and non-financial liabilities, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis, to fiscal years beginning after November 15, 2008. As of December 31, 2007, the Company’s fair values of its financial assets and liabilities, which consist of cash and cash equivalents, accounts payable and notes payable, approximate their carrying amount due to the short period of time to maturity. | ||
| In February 2007, the FASB issued a standard addressing , “The Fair Value Option for Financial Assets and Financial Liabilities “. This statement provides companies with an option to measure, at specified election dates, many financial instruments and certain other items at fair value that are not currently measured at fair value. A company that adopts this standard will report unrealized gains and losses on items for which the fair value option has been elected in earnings at each subsequent reporting date. This statement also establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar types of assets and liabilities. This statement is effective for fiscal years beginning after November 15, 2007. The Company is currently evaluating the effect that the adoption of the standard will have on its results of operations and financial position. | ||
| In December 2007, FASB issued guidance on,“Interests in Consolidated Financial Statements”, which impacts the accounting for minority interest in the consolidated financial statements of filers. The statement requires the reclassification of minority interest to the equity section of the balance sheet and the results from operations attributed to minority interest to be included in net income. The related minority interest impact on earnings would then be disclosed in the summary of other comprehensive income. The statement is applicable for all fiscal years beginning on or after December 15, 2008 and earlier adoption is prohibited. The adoption of this standard will require prospective treatment. The Company is currently evaluating the effect that the adoption of the standard will have on its results of operations and financial position. However, adopting the standard is not expected to have a material impact on the Company’s financial statements. | ||
| In December 2007, FASB issued a standard covering“Business Combinations”, which impacts the accounting for business combinations. The statement requires changes in the measurement of assets and liabilities required in favor of a fair value method consistent with the guidance provided in on “Fair Value Measurements” (see above). Additionally, the statement requires a change in accounting for certain acquisition related expenses and business adjustments which no longer are considered part of the purchase price. Adoption of this standard is required for fiscal years beginning after December 15, 2008. Early adoption of this standard is not permitted. The statement requires prospective application for all acquisitions after the date of adoption. The Company is currently evaluating the effect that the adoption of the standard will have on its results of operations and financial position. However, the adopting this standard is not expected to have a material impact on the Company’s financial statements. |
60
Table of Contents
| In April 2008, the FASB issued a standard addressing the, “Determination of the Useful Life of Intangible Assets”. This guidance is intended to improve the consistency between the useful life of a recognized intangible asset, and the period of expected cash flows used to measure the fair value of the asset when the underlying arrangement includes renewal or extension of terms that would require substantial costs or result in a material modification to the asset upon renewal or extension. Companies estimating the useful life of a recognized intangible asset must now consider their historical experience in renewing or extending similar arrangements or, in the absence of historical experience, must consider assumptions that market participants would use about renewal or extension as adjusted for entity-specific factors. This standard is effective for fiscal years beginning after December 15, 2008, and is applicable to the Company’s fiscal year beginning January 1, 2009. The Company does not anticipate that the adoption of this FSP will have an impact on its results of operations or financial condition | ||
| In May 2008, the FASB issued a statement addressing, “The Hierarchy of Generally Accepted Accounting Principles”. The standard identifies the sources of accounting principles and the framework for selecting the principles used in preparation of the financial statements of non-governmental entities that are presented in conformity with U.S. GAAP (the GAAP hierarchy). The Standard will become effective sixty days following the Securities and Exchange Commission’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411, “The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles”. The adoption of the standard is not expected to materially impact the Company’s financial position or results of operations. | ||
| In June 2009, the FASB issued theCodification and the Hierarchy of Generally Accepted Accounting Principles (“GAAP”)(“Codification”). The purpose of the Codification is to provide a single source of authoritative U.S. GAAP. The Codification was effective for the Company in the third quarter of 2009. As the Codification was not intended to change or alter existing GAAP, the adoption of the Codification did not have a material effect on the Company’s financial statements. |
61
Table of Contents
PRO FORMA FINANCIAL STATEMENTS
MILLER DIVERSIFIED CORPORATION
Unaudited Pro Forma Condensed Financial
The following unaudited pro forma condensed financial statements of Miller Diversified Corporation have been prepared to indicate how the financial statements of Miller Diversified Corporation might have looked if the merger of Smoke Anywhere USA, Inc. into a subsidiary of Miller Diversified Corporation and the transactions related to the merger had occurred as of September 1, 2009
The acquisition has been accounted for as a reverse acquisition under the purchase method of accounting for business combinations. The combination of the two companies is recorded as a recapitalization of the Company pursuant to which Smoke Anywhere USA, Inc. is treated as the accounting acquirer and Miller Diversified Corporation is the legal acquirer. The pro forma condensed financial statements have been prepared using the historical financial statements of Miller Diversified Corporation as of and for the year ended August 31, 2008 and of Smoke Anywhere USA, Inc. as of and for the quarter ended September 30, 2009. The adjustments to those historical statements in the pro forma presentation reflect the following events:
| a) | The acquisition of Smoke Anywhere USA, Inc. by Miller Diversified Corporation, as if it had occurred on September 1, 2009. |
| b) | The Company issued 20,670,000 shares of common stock of Miller to the SMOKE Shareholders, of which 2,074,640 of the Miller shares of Common Stock were loaned to the Company by, the Company’s controlling shareholder. Pursuant to the Acquisition Agreement and Plan of Merger, the company will issue an additional 50,000,000 shares, subsequent to certain corporate actions as set forth above and elsewhere herein. |
The pro forma condensed financial statements should be read in conjunction with the historical financial statements of Miller Diversified Corporation and Smoke Anywhere USA, Inc. The pro forma condensed financial statements are presented for illustrative purposes only and are not intended to be indicative of the actual financial condition or results of operations that Miller Diversified Corporation would have realized had the merger occurred on September 1, 2009 Also, the pro forma financial statements are not indicative of the financial condition or results of operations of Miller Diversified Corporation that may be reported in the future.
62
Table of Contents
MILLER DIVERSIFIED CORPORATION\
PRO FORMA BALANCE SHEET
PRO FORMA BALANCE SHEET
| MILLER | SMOKE | |||||||||||||||
| DIVERSIFIED | ANYWHERE | PRO FORMA | ||||||||||||||
| CORPORATION | USA, INC. | ADJUSTMENTS | PRO FORMA | |||||||||||||
ASSETS | ||||||||||||||||
CURRENT ASSETS: | ||||||||||||||||
| Cash | $ | — | $ | 332,365 | $ | — | $ | 332,365 | ||||||||
| Inventories | — | 799,744 | — | 799,744 | ||||||||||||
| Vendor deposits | — | 143,333 | — | 143,333 | ||||||||||||
TOTAL ASSETS | — | 1,275,442 | — | 1,275,442 | ||||||||||||
LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||||||||
CURRENT LIABILITIES | ||||||||||||||||
| Accounts payable & accrued expenses | 750 | 237,918 | (750 | )(a) | 237,918 | |||||||||||
| Taxes payable | — | 265,726 | — | 265,726 | ||||||||||||
TOTAL CURRENT LIABILITIES | 750 | 503,644 | (750 | ) | 503,644 | |||||||||||
SHAREHOLDERS’ EQUITY: | ||||||||||||||||
| Common Stock | 640 | 100 | (100 | )(b) | 60,000 | |||||||||||
| 5,764 | (c) | |||||||||||||||
| 18,596 | (d) | |||||||||||||||
| 35,000 | (g) | |||||||||||||||
| Additional paid-in capital | 2,679,553 | 385,400 | 100 | (b) | 325,500 | |||||||||||
| (5,764 | ) (c) | |||||||||||||||
| (2,680,943 | )(e) | |||||||||||||||
| 750 | (a) | |||||||||||||||
| (18,596 | )(d) | |||||||||||||||
| (35,000 | )(g) | |||||||||||||||
| Retained earnings | (2,680,943 | ) | 386,298 | 2,680,943 | (e) | 386,298 | ||||||||||
TOTAL SHAREHOLDERS’ EQUITY | (750 | ) | 771,798 | 750 | 771,798 | |||||||||||
TOTAL LIABILITIES & SHAREHOLDERS’ EQUITY | $ | — | $ | 1,275,442 | $ | — | $ | 1,275,442 | ||||||||
| (a) | Represents the waiving of the Accrued expense on Miller Diversified Corporation | |
| (b) | Represents the elimination of Smoke Anywhere common stock | |
| (c) | To correct the par value amount of Miller Diversified Corporation common stock | |
| (d) | Represents 18,595,360 shares of Smoke Anywhere USA Inc. common stock loaned to Miller Diversified Corporation | |
| (e) | Adjustments of equity accounts to reflect merger | |
| (g) | To affect the subsequent reverse merger and subsequent re-issuance |
63
Table of Contents
MILLER DIVERSIFIED CORPORATION\
PRO FORMA STATEMENT OF OPERATIONS
PRO FORMA STATEMENT OF OPERATIONS
| MILLER | SMOKE | |||||||||||||||
| DIVERSIFIED | ANYWHERE | PRO FORMA | ||||||||||||||
| CORPORATION | USA INC. | ADJUSTMENTS | PRO FORMA | |||||||||||||
REVENUE | $ | — | $ | 4,484,729 | $ | — | $ | 4,484,729 | ||||||||
COST OF SALES | — | 2,173,618 | — | 2,173,618 | ||||||||||||
GROSS PROFIT | ||||||||||||||||
COSTS AND EXPENSES: | — | |||||||||||||||
| Selling, general & administrative | — | 1,642,334 | — | — | ||||||||||||
TOTAL COSTS AND EXPENSES | — | 1,642,334 | — | — | ||||||||||||
NET INCOME BEFORE INCOME TAX EXPENSE | 668,777 | — | ||||||||||||||
INCOME TAX EXPENSE | 265,726 | — | — | |||||||||||||
NET INCOME | $ | — | $ | 403,051 | $ | — | $ | 403,051 | ||||||||
| Basic and diluted income per share based on 25,000,000 shares outstanding | $ | 0.02 | ||||||||||||||
64
Table of Contents
Item 9.01 Financial Statements and Exhibits.
INDEX OF EXHIBITS
| Exhibit | ||
| Number | Description | |
| 2.0 | Plan of acquisition, reorganization, arrangement, liquidation or succession | |
| 3.1 | Articles of Incorporation, Bylaws and Amendments (except the Amendment described in 3.2 below) thereto (incorporated by reference to Exhibit 3.1 to Registrant’s Registration Statement No. 33-26285 on January 10, 1986). | |
| 3.2 | Amendment to Articles of Incorporation dated January 22, 1990; providing for 1:250 reverse stock split and reduction in number of authorized shares (incorporated by reference to Exhibit 3.2 to Registrant’s Registration Statement No. 33-40461 on May 6, 1991). | |
| 15 | Consent of Paritz & Company P.A., Independent Registered Public Accounting Firm | |
| 21 | Subsidiaries of the registrant | |
| 24 | Power of attorney | |
| 31.1 | 31.1 Certification of Principal Executive Officer and Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1 | Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. ss.1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| Miller Diversified Corp. | ||||
| Date: November 6, 2009 | By: | /s/ Kevin Frija | ||
| Kevin Frija | ||||
| PEO, PAO and Chairman of the Board of Directors | ||||
65