QuickLinks -- Click here to rapidly navigate through this document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 20-F
(Mark One)
| o | | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR |
ý |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE FISCAL YEAR ENDED DECEMBER 31, 2002 |
OR |
o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number: 0-17434 |
DRAXIS HEALTH INC.
(Exact name of Registrant as specified in its charter)
PROVINCE OF ONTARIO, CANADA
(Jurisdiction of incorporation or organization)
6870 GOREWAY DRIVE, 2nd FLOOR, MISSISSAUGA, ONTARIO, CANADA L4V 1P1
(Address of principal executive offices)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
Title of each class
NONE |
|
Name of each exchange on which registered
NONE |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
COMMON SHARES
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
NONE
(Title of Class)
Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of the period covered by the annual report.
Shares—37,098,690 (as of 12/31/02)
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ý No o
Indicate by check mark which financial statement item the Registrant has elected to follow.
Item 17 o Item 18 ý
| Item 1. | | Identity of Directors, Senior Management and Advisers | | 1 |
| Item 2. | | Offer Statistics and Expected Timetable | | 1 |
| Item 3. | | Key Information | | 1 |
| Item 4. | | Information on the Company | | 10 |
| Item 5. | | Operating and Financial Review and Prospects | | 42 |
| Item 6. | | Directors, Senior Management and Employees | | 57 |
| Item 7. | | Major Shareholders and Related Party Transactions | | 74 |
| Item 8. | | Financial Information | | 75 |
| Item 9. | | The Offer and Listing | | 75 |
| Item 10. | | Additional Information | | 78 |
| Item 11. | | Quantitative and Qualitative Disclosures About Market Risk | | 86 |
| Item 12. | | Description of Securities Other Than Equity Securities | | 87 |
| Item 13. | | Defaults, Dividends Arrearages and Delinquencies | | 87 |
| Item 14. | | Material Modifications to the Rights of Security Holders and Use of Proceeds | | 87 |
| Item 15. | | Controls and Procedures | | 87 |
| Item 17. | | Financial Statements | | 87 |
| Item 18. | | Financial Statements | | 87 |
| Item 19. | | Exhibits | | 87 |
Item 1. Identity of Directors, Senior Management and Advisers
Not Applicable
Item 2. Offer Statistics and Expected Timetable
Not Applicable
Item 3. Key Information
DRAXIS Health Inc. ("DRAXIS" or the "Company") is an integrated specialty pharmaceutical company with core competencies in two segments: the development, production, marketing and distribution of radiopharmaceuticals through DRAXIMAGE Inc. ("DRAXIMAGE") and the provision of contract pharmaceutical manufacturing services, specializing in liquid and freeze-dried injectables and other sterile products through DRAXIS Pharma Inc. ("DPI"). The Company believes that both DRAXIMAGE and DPI have significant long-term growth potential and has invested considerable financial and management resources in developing these businesses.
We also engage in Canadian pharmaceutical sales and marketing through DRAXIS Pharmaceutica ("DRAXIS Pharmaceutica"), which operates as a division of DRAXIS. DRAXIS has the strategic intent to divest DRAXIS Pharmaceutica. Accordingly, commencing with the quarter ended December 31, 2001, the results of operations of DRAXIS Pharmaceutica have been reported as discontinued operations. Commencing in the second quarter of 2002, discontinued operations no longer include revenues and expenses directly attributable to Alertec.
Our subsidiary Deprenyl Animal Health, Inc. ("DAHI") receives licensing and royalty revenue related to Anipryl, a companion animal health product.
Our registered and principal office is located at 6870 Goreway Drive, 2nd Floor, Mississauga, Ontario, L4V 1P1.
The selected data set forth in the following table are expressed in U.S. dollars and in accordance with U.S. generally accepted accounting principles ("U.S. GAAP"). All data presented below should be read in conjunction
1
with, and is qualified in its entirety by, reference to the audited Consolidated Financial Statements and Notes thereto for the year ended December 31, 2002.
| | U.S. GAAP
(in thousands of U.S. dollars except share related data)
|
|---|
| | 2002
| | 2001
| | 2000
| | 1999
| | 1998
|
|---|
| Operations | | | | | | | | | | | | | | | |
| | Revenues | | $ | 38,640 | | $ | 33,903 | | $ | 29,721 | | $ | 24,591 | | $ | 30,643 |
| | Operating income (loss) | | | 2,894 | | | 1,773 | | | 36 | | | (1,638 | ) | | 5,027 |
| | Income (loss) from continuing operations before cumulative effect of accounting change | | | 3,020 | | | (1,015 | ) | | 363 | | | (860 | ) | | 3,378 |
| | (Loss) income from discontinued operations, net of taxes | | | (834 | ) | | (569 | ) | | (801 | ) | | (4,610 | ) | | 339 |
| | Cumulative effect of accounting change, net of tax | | | — | | | — | | | (19,900 | ) | | — | | | — |
| | |
| |
| |
| |
| |
|
| | Net income (loss) | | $ | 2,186 | | $ | (1,584 | ) | $ | (20,338 | ) | $ | (5,470 | ) | $ | 3,717 |
| | |
| |
| |
| |
| |
|
Basic income (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | From continuing operations before cumulative effect of accounting change | | $ | 0.08 | | $ | (0.03 | ) | $ | 0.01 | | | (0.02 | ) | | 0.11 |
| | From discontinued operations | | | (0.02 | ) | | (0.02 | ) | | (0.02 | ) | | (0.14 | ) | | 0.01 |
| | From cumulative effect of accounting change | | | — | | | — | | | (0.55 | ) | | — | | | — |
| | |
| |
| |
| |
| |
|
| Basic income (loss) per share | | | 0.06 | | | (0.05 | ) | | (0.56 | ) | | (0.16 | ) | | 0.12 |
| | |
| |
| |
| |
| |
|
Financial Position at December 31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Cash and cash equivalents | | | 4,899 | | | 5,602 | | | 4,420 | | | 2,016 | | | 2,393 |
| | Total assets | | | 67,951 | | | 64,360 | | | 67,307 | | | 60,647 | | | 56,649 |
| | Long-term debt | | | 12,726 | | | 8,060 | | | 9,890 | | | 14,476 | | | 11,861 |
| | Common shareholders' equity | | | 20,227 | | | 16,878 | | | 20,808 | | | 38,116 | | | 34,677 |
| | Book value per common share | | | 0.55 | | | 0.46 | | | 0.57 | | | 1.07 | | | 1.07 |
Share Information |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Number of shares outstanding at year end | | | 37,098,690 | | | 36,613,434 | | | 36,565,102 | | | 35,557,366 | | | 32,280,524 |
| | Weighted average number of shares outstanding—basic | | | 36,981,985 | | | 36,587,794 | | | 36,324,199 | | | 33,825,654 | | | 31,950,704 |
Overview
This Annual Report (Form 20-F) contains forward-looking statements (within the meaning of the Securities Exchange Act of 1934, as amended) and information that are based on management's beliefs, as well as assumptions made by and information currently available to management. When used in this Annual Report (Form 20-F), the words "anticipate," "estimate," "believe," "expect," "potential," "intend," "designed" and "should" and similar expressions are intended to identify forward-looking statements. These forward-looking statements necessarily make numerous assumptions with respect to industry performance, general business, economic and regulatory conditions, access to markets and materials and other matters, all of which are inherently subject to significant uncertainties and contingencies and many of which are beyond the Company's control. Should one or more of these risks or uncertainties materialise, or should underlying assumptions prove incorrect, actual results may vary materially from those anticipated, estimated or projected. The Company believes that any of the following risk factors could cause the Company's actual results to differ from those that may have been or may be projected in forward-looking statements made by or on behalf of the Company from time to time. The forward-looking statements in this Annual Report (Form 20-F) are contained principally under Items 4 and 5.
RISK FACTORS
The following list of factors may not be exhaustive, as we operate in a rapidly changing business, and new risk factors emerge from time to time. We cannot predict such risk factors, nor can we assess the impact, if any, of such risk factors on our business or the extent to which any factor, or combination of factors, may cause actual
2
results to differ materially from those projected in any forward-looking statements. Accordingly, do not rely on forward-looking statements as a prediction of actual results.
The following summarizes the major risks and uncertainties facing us:
WE MAY NOT BE ABLE TO GET TIMELY REGULATORY APPROVAL FOR OUR PRODUCTS.
Preclinical studies and clinical trials, as well as the manufacturing and marketing of our existing and potential products, are subject to extensive regulation by the Health Products and Food Branch of Health Canada ("HPFB") and other authorities in Canada and by numerous federal, state and local government authorities in the United States, including the Food and Drug Administration ("FDA"). Similar regulatory requirements exist in Europe and other countries. To the extent we choose to explore foreign markets, we may rely on foreign licensees to obtain regulatory approvals in such countries. The commercialization of certain of our products will be subject to rigorous preclinical and clinical testing and other premarket approval requirements by the FDA, HPFB and similar authorities in other foreign countries. Any failure or delay by us, our collaborators or licensees to comply with applicable requirements or obtain regulatory approvals for our products could adversely affect the marketing of products developed or licensed by us and our ability to receive product or royalty revenue.
The regulatory process, which includes preclinical studies and clinical trials of each compound to establish its safety and efficacy, takes many years and requires the expenditure of substantial resources. Moreover, if regulatory approval of a drug or diagnostic product is granted, such approval may entail limitations on the indicated uses for which it may be marketed. Failure to comply with applicable regulatory requirements can, among other things, result in suspension of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. Further, government policy may change, and additional government regulations may be established that could prevent or delay regulatory approvals for our products. In addition, a marketed drug and its manufacturer are subject to continual review. Later discovery of previously unknown problems with the product or manufacturer may result in restrictions on such product or manufacturer, including withdrawal of the product from the market.
We manufacture, or expect to manufacture, many active pharmaceutical ingredients and advanced pharmaceutical intermediates that are used in our own and our customers' drug products. The final drug products in which the pharmaceutical ingredients and advanced pharmaceutical intermediates are used, however, are subject to regulation for safety and efficacy by the FDA, HPFB and other jurisdictions, as the case may be. Such products must be approved by such agencies before they can be commercially marketed. The process of obtaining regulatory clearance for marketing is uncertain, costly and time consuming. We cannot predict how long the necessary regulatory approvals will take or whether our customers will ever obtain such approval for their products. To the extent that we and/or our customers do not obtain the necessary regulatory approvals for marketing new products, our product sales could be adversely affected.
WE MAY NOT BE ABLE TO OBTAIN AND ENFORCE EFFECTIVE PATENTS TO PROTECT OUR PROPRIETARY RIGHTS FROM USE BY COMPETITORS, AND THE PATENTS OF OTHER PARTIES COULD REQUIRE US TO STOP USING OR TO ACQUIRE A LICENSE FOR CONDUCTING CERTAIN BUSINESS ACTIVITIES, AND OUR COMPETITIVE POSITION AND PROFITABILITY COULD SUFFER AS A RESULT.
Our success will depend, in part, on our ability to obtain, enforce and maintain patent protection for our technology in Canada, the United States and other countries. We cannot assure you that patents will issue from any pending applications or that claims now or in the future, if any, allowed under issued patents will be sufficiently broad to protect our technology. In addition, no assurance can be given that any patents issued to or licensed by us will not be challenged, invalidated, infringed or circumvented, or that the rights granted thereunder will provide continuing competitive advantages to us. The patent positions of pharmaceutical and biotechnology firms, including us, are generally uncertain and involve complex legal and factual questions. In addition, we do not know whether any of our current research endeavours will result in the issuance of patents in Canada, the United States, or elsewhere, or if any patents already issued will provide significant proprietary protection or will be circumvented or invalidated. Since patent applications in the United States and Canada are maintained in secrecy for at least 18 months from the date of filing, and since publication of discoveries in the scientific or patent literature tends to lag behind actual discoveries by several months, we cannot be certain that we were the first to create inventions claimed by pending patent applications or that we were the first to file patent applications for such inventions. Certain patents related to our products have expired. Loss of patent protection could lead to
3
generic competition for these products, and others in the future, which would materially and adversely affect the financial prospects for these products and, to a lesser extent, the Company.
Our commercial success will also depend in part on our not infringing patents or proprietary rights of others and not breaching the licenses granted to us. The degree of patent protection afforded to pharmaceutical or biotechnological inventions around the world is uncertain and varies significantly between different countries. There can be no assurance that we will be able to obtain a license to any third-party technology or patents that we may require to conduct our business or that such technology or patents can be licensed at a reasonable cost. Failure by us or our collaborators to obtain a license to any technology or patents that we may need to commercialize our technologies or products may result in delays in marketing our proposed products or the inability to proceed with the development, manufacture or sale of products requiring such licenses and may have a material adverse effect on us.
We have been required to, and may continue to be required to, engage in litigation and other patent proceedings to enforce patents issued to us, to defend our right, title and interest to patents related to our products and to determine the scope and validity of other parties' proprietary rights. These proceedings can be costly, and if the outcome of any such proceedings is adverse to us, we could lose the right to sell some of our products or could be required to pay damages.
We also rely on unpatented trade secrets, improvements and know-how to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our corporate partners, collaborators, employees and consultants. These agreements could be breached, and we may not have adequate remedies for any breach. Further, our trade secrets could otherwise become known or be independently discovered by competitors.
IF OUR COLLABORATIVE AND COMMERCIAL RELATIONSHIPS WITH THIRD PARTIES ON WHOM WE RELY ARE UNSUCCESSFUL, OUR BUSINESS MAY SUFFER.
To be successful, we must continually establish and maintain strategic relationships with leaders in a number of biotechnology and pharmaceutical industry segments. This is critical to success because such relationships enable us to extend the reach of our products and sales in various jurisdictions, generate additional revenue and develop and deploy new products in various marketplaces. Entering into strategic relationships is complicated as some of our current and future strategic partners may decide to compete with us in some or all of the markets or refuse to fulfil or honor their contractual obligations to us.
We have entered into a number of product in-licensing and out-licensing arrangements in which our business and financial success is dependent on third parties. In many of our product in-licensing arrangements, the product licensor is responsible for developing a body of data upon which we can base a submission to the HPFB, FDA or other regulatory authority. The interests of the other party to each of these agreements may not be or remain consistent with our interests, and our collaborators may not succeed in developing a body of data that can form the basis of regulatory approval. Should our collaborators fail to develop such body of data to enable us to obtain the requisite regulatory approvals or otherwise fail to honor their commitments to us or meet our expectations, our business, financial condition and results of operations may be materially and adversely affected. In addition, we cannot control the amount and timing of resources our collaborators devote to the products to which they have rights or to the subject matters of our agreements with them generally. The agreements may be terminated by our collaborators in certain circumstances.
To the extent we enter into product out-licensing arrangements for the marketing or distribution of our own products with collaborative partners, any revenues we receive will depend upon the efforts of third parties. There can be no assurance that any third party will market our products successfully or that any third-party collaboration will be on terms favorable to us. If any marketing partner does not market a product successfully, our business might be materially and adversely affected. Because revenues from our collaboration agreements with Pfizer Inc. ("Pfizer") relating to Anipryl have been a primary source of income for the Company, our business could be materially and adversely affected if Pfizer does not continue to market Anipryl successfully.
A SIGNIFICANT PORTION OF OUR BUSINESS IS DEPENDENT ON A SMALL NUMBER OF KEY CUSTOMERS.
As at December 31, 2002, the Company's two largest customers represented 19% and 17% respectively of our total revenues. The termination by either of these customers of its relationship with us would have a material adverse effect on our business, financial condition and results of operations.
4
IF OUR COLLABORATORS, EMPLOYEES, OR CONSULTANTS DISCLOSE OUR CONFIDENTIAL INFORMATION TO OTHERS DESPITE CONFIDENTIALITY AGREEMENTS IN PLACE, OUR BUSINESS MAY SUFFER.
Our practice is to require our employees, collaborators, consultants and outside scientific advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships. These agreements provide that all confidential information developed or made known to the individual during the course of the individual's relationship with us is to be kept confidential and not disclosed to third parties, subject to certain specific limited exceptions. In the case of employees, the agreements provide that all inventions conceived by the individual shall be our exclusive property. These agreements, however, may not provide meaningful protection for our trade secrets or adequate remedies in the event of unauthorized use or disclosure of such information.
WE ARE SUBJECT TO REGULATION BY GOVERNMENTS IN MANY JURISDICTIONS AND, IF WE DO NOT COMPLY WITH HEALTHCARE, MANUFACTURING, NUCLEAR SAFETY AND ENVIRONMENTAL REGULATIONS, OUR EXISTING AND FUTURE OPERATIONS MAY BE CURTAILED, AND WE COULD BE SUBJECT TO LIABILITY.
The HPFB, FDA and other governmental regulators have increased requirements for drug purity and have increased environmental burdens upon the pharmaceutical industry. Because pharmaceutical drug manufacturing is a highly regulated industry, requiring significant documentation and validation of manufacturing processes and quality control assurance prior to approval of the facility to manufacture a specific drug, there can be considerable transition time between the initiation of a contract to manufacture a product and the actual initiation of manufacture of that product. Any lag time in the initiation of a contract to manufacture product and the actual initiation of manufacture at our facilities could cause us to lose profits or incur liabilities.
Products manufactured by us will have to comply with the FDA's current Good Manufacturing Practices ("cGMP") and other FDA, HPFB or European guidelines and regulations. Products containing radioactive isotopes will have to comply with the guidelines and regulations of the Canadian Nuclear Safety Commission in Canada and the Nuclear Regulatory Commission in the U.S. and with other similar regulations in other countries. Additionally, certain of our customers may require us to adhere to additional manufacturing standards, even if not required by the FDA or other regulatory authorities. Compliance with cGMP regulations requires manufacturers to expend time, money and effort in production, and to maintain precise records and quality control to ensure that the product meets applicable specifications and other requirements. The FDA and other regulators periodically inspect drug-manufacturing facilities to ensure compliance with applicable cGMP requirements. If we fail to comply with the cGMP requirements, we may become subject to possible regulatory action and manufacturing at the facility could consequently be suspended causing possible loss of profit, regulatory fines and third-party liability. Any one of these events could have a material adverse effect on our business, financial condition and results of operations.
The FDA may also require the submission of any lot of a particular product for inspection. If the lot product fails to meet the FDA requirements, then the FDA could take any of the following actions: (i) restrict the release of the product; (ii) suspend manufacturing of the specific lot of the product; (iii) order a recall of the lot of the product; or (iv) order a seizure of the lot of the product.
Canadian and United States federal, state, local and provincial regulations govern extensively the use, manufacture, storage, handling, transport and disposal of hazardous material and associated waste products. Although we believe that the operations at our facilities comply in all material respects with the applicable environmental laws in Canada and the United States, we cannot completely eliminate the risk of substantial environmental liabilities. Any failure by us or any of our subsidiaries to comply with the present or future environmental laws in Canada, the United States or elsewhere, could result in any of the following: (i) cessation of portions or all of our or our subsidiaries' operations; (ii) imposition of fines; (iii) restrictions on our or our subsidiaries' ability to carry on or expand our operations; (iv) significant expenditures by us in order to comply with environmental laws and regulations; or (v) liabilities in excess of our resources. Any of these sanctions could have a material adverse effect on our business, financial condition and results of operations.
We have in place facilities and procedures designed to reduce and, to the extent possible, eliminate the risk of environmental contamination resulting from the processing of raw materials and, more specifically, from the radiopharmaceutical business of our subsidiary DRAXIMAGE and the manufacturing business of our subsidiary DPI. We also have in place a regular maintenance program to ensure continued compliance with all applicable environmental regulations.
5
COMPETITION FROM MANUFACTURERS AND MARKETERS OF GENERIC DRUGS MAY REDUCE OUR REVENUE AND PROFITS.
Our brand name drugs face competition from generic drugs, which can have adverse effects upon sales. We have attempted to mitigate the impact of generic competition by diversifying our product lines and entering into agreements to share in the profits from the sales of generic versions of our products. However, competition from generics could result in lower revenues and margins.
OUR BUSINESS COULD BE HARMED IF THERE WERE A DISPUTE OR DISRUPTION WITH OUR UNIONISED EMPLOYEES.
Although our subsidiary DPI currently has a good relationship with the United Food & Commercial Workers International Union, Local 291 (AFL-CIO), there can be no assurance that future labor difficulties will not arise. Should such difficulties arise which lead to significant grievance issues and/or a labor strike, such events might have a material adverse effect on our business.
FACTORS BEYOND OUR CONTROL COULD CAUSE INTERRUPTION IN OUR OPERATIONS, WHICH WOULD ADVERSELY AFFECT OUR REPUTATION IN THE MARKETPLACE AND OUR RESULTS OF OPERATIONS.
��To succeed, we must be able to operate our manufacturing facilities without interruption and deliver radiopharmaceutical products in a timely manner. We could suffer an interruption caused by damage from a variety of sources, many of which are not within our control, including, fire, flood and other natural disasters, power loss and telecommunication failure, disruption to transportation systems, software and hardware errors, failures or crashes and similar disruptions, or undue delays or restrictions with cross border shipments. Any significant interruptions in our operations or our ability to deliver time-sensitive products would damage our reputation in the marketplace and have a negative impact on our results of operations.
ALTHOUGH WE CARRY INSURANCE, A SUCCESSFUL LIABILITY CLAIM COULD NEGATIVELY IMPACT OUR BUSINESS.
The use of any of our unapproved products under development and the sale of any approved products may expose us to liability claims resulting from the use of these products. Such claims might be made directly by consumers, healthcare providers or by pharmaceutical companies or others selling such products. We currently have liability insurance, but we cannot provide assurance that we will be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to this potential liability. We might not be able to maintain or obtain additional commercially reasonable product liability insurance for any products approved for marketing. A successful product liability claim or a series of claims brought against us could have a material adverse effect on our business, financial condition or results of operations. Even unsuccessful product liability claims could result in the expenditure of funds in litigation and the diversion of management time and resources and could damage our reputation and impair the marketability of our products.
IF THE MARKET DOES NOT ACCEPT OUR PRODUCTS, OUR BUSINESS COULD BE HARMED.
There can be no assurance that any of our products in development or products recently launched will achieve market acceptance. The degree of market acceptance will depend upon a number of factors, including the receipt of regulatory approvals, the establishment and demonstration in the medical community of the clinical efficacy and safety of the products, the establishment and demonstration of the potential advantages over existing and new diagnostic and treatment methods and the reimbursement policies of government and third-party payors. There can be no assurance that physicians, patients, payors or the medical community in general will accept and utilize any existing or new products that may be developed or manufactured by us.
We anticipate that we will face increased competition in the future as new products enter the market and advanced technologies become available. There can be no assurance that existing products or new products developed by our competitors will not be more effective, or be more effectively marketed and sold, than any that may be developed or sold by us. Competitive products may render our products obsolete and uncompetitive prior to recovering research, development or commercialization expenses incurred with respect to any such products.
Many of our existing or potential competitors, particularly large pharmaceutical companies, have substantially greater financial, technical and human resources than do we. In addition, many of these competitors have significantly greater experience in undertaking research, preclinical studies and human clinical trials of new pharmaceutical products, obtaining regulatory approvals and manufacturing and marketing such products.
6
Accordingly, our competitors may succeed in commercializing products more rapidly or effectively, which could have a material adverse effect on our business, financial condition or results of operations.
FAILURE OF ONE OF OUR CURRENT OR FUTURE CLINICAL TRIALS COULD HAVE A MATERIALLY NEGATIVE IMPACT ON OUR FUTURE PROSPECTS.
The development of new products is subject to a number of significant risks. Potential products that appear to be promising in various stages of development may not reach the market for a number of reasons. Such reasons include the possibilities that the potential product will be found ineffective or unduly toxic during preclinical or clinical trials, fail to receive necessary regulatory approvals, be difficult to manufacture on a large scale, be uneconomical to market or not achieve market acceptance, or be precluded from commercialization by proprietary rights of third parties. Certain products we are attempting to develop have never been manufactured on a commercial scale, and there can be no assurance that such products can be manufactured at a cost or in a quantity to render such products commercially viable. Production of such products may require the development of new manufacturing technologies and expertise. The impact on our business in the event that new manufacturing technologies and expertise would have to be developed is uncertain. Many of our potential products will require significant additional research and development efforts and significant additional preclinical and clinical testing, prior to any commercial use. There can be no assurance that we will successfully meet any of these technological challenges, or others that may arise in the course of development.
Before obtaining regulatory approval for the commercial sale of any product under development, we must demonstrate through preclinical studies and clinical trials that the product is safe and efficacious. The results from preclinical studies and clinical trials may not be totally predictive of results obtained in larger clinical trials, and there can be no assurance that our or any collaborators' clinical trials will demonstrate safety and efficacy, achieve regulatory approvals or result in marketable products. A number of companies in the biotechnology and pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after achieving promising results in earlier trials. Failure to successfully complete clinical trials on a timely basis could have an adverse effect on our future business, financial condition and results of operations.
OUR PROFIT DEPENDS IN PART ON REIMBURSEMENT POLICIES AND REGULATIONS OF GOVERNMENT HEALTH ADMINISTRATION AUTHORITIES, PRIVATE HEALTH INSURERS AND OTHER ORGANIZATIONS.
The business and financial condition of pharmaceutical companies will continue to be affected by the efforts of governments and third-party payors to contain or reduce the costs of healthcare through various means. For example, in certain markets, including Canada, pricing or profitability of prescription pharmaceuticals, medical devices and diagnostic products is subject to government control. In the United States there have been, and we expect that there will continue to be, a number of federal and state proposals to implement similar government controls. In addition, an increasing emphasis on managed healthcare in the United States has increased and will continue to increase the pressure on pharmaceutical pricing. While we cannot predict whether such legislative or regulatory proposals will be adopted or the effects such proposals or managed care efforts may have on our business, the announcement and/or adoption of such proposals or efforts could have a material adverse effect on our business and financial condition and that of our current and prospective corporate partners. Accordingly, our ability to establish strategic alliances may be adversely affected. In addition, in Canada, the United States and elsewhere, sales of prescription pharmaceutical products and radiopharmaceutical products are dependent, in part, on the availability of reimbursement to the consumer from third-party payors, such as government and private insurance plans. Third-party payors are increasingly challenging the prices charged for medical products and services. To the extent we succeed in bringing new products to market, there can be no assurance that these products will be considered cost-effective and reimbursement to consumers will be available or will be sufficient to allow the sale of these products on a competitive basis. The Patented Medicine Prices Review Board, which monitors and controls prices of patented drug products marketed in Canada, may assert jurisdiction over our products under development which may limit the prices that can be charged for such products. We may not be able to obtain prices for our products under development that will make them commercially viable.
OUR FUTURE SUCCESS DEPENDS ON OUR ABILITY TO GROW, AND IF WE ARE UNABLE TO MANAGE OUR GROWTH EFFECTIVELY, WE MAY INCUR UNEXPECTED EXPENSES AND BE UNABLE TO MEET OUR CUSTOMERS' REQUIREMENTS.
We will need to maintain, expand and upgrade our manufacturing facilities and operations to execute current commercial obligations to customers, widen our customer base and increase manufacturing efficiencies over the
7
next few years. We cannot be certain that DPI will be able to enter into enough lucrative third-party contracts to increase its profitability. If we do secure advantageous agreements, we cannot be certain that our employees, systems, procedures, controls and existing space will be adequate to support expansion of its operations. Our future operating results will depend on the ability of our officers and key employees to manage changing business conditions and to implement and improve our technical, administrative, financial control and reporting systems and operational excellence in order to achieve established business objectives. An unexpectedly large increase in the volume of manufacturing business or the number of orders placed by customers may require us to expand and further upgrade our facilities and the technology related to our manufacturing. We may not be able to project the rate of timing of such increases or customer demands accurately or to expand and upgrade our facilities and supporting systems and infrastructure to accommodate such increases. Difficulties in managing future growth and meeting customers' expanded requirements could have a significant negative impact on our business and its profitability.
OUR FINANCIAL RESULTS MAY FLUCTUATE, AND OUR FUTURE REVENUE AND PROFITABILITY ARE UNCERTAIN.
Our ability to achieve and maintain profitability in the foreseeable future depends on the commercial success of our products and services. Because we will be launching or are in the process of launching new products in new markets, revenues are difficult to predict and may fluctuate substantially from period to period. In addition, product development programs will require substantial additional investment, including the cost of clinical trials, obtaining additional regulatory approvals, if necessary, and marketing and sales expenses associated with potential new product introductions. Our manufacturing facility will continue to require capital investment in order to maintain, upgrade and expand its operations. The success of our subsidiaries DRAXIMAGE and DPI will rely significantly on the ability of both companies to maintain and to increase substantially their manufacturing capabilities to satisfy customer demand. There can be no assurance that, or if so when, we will successfully develop, receive regulatory approvals for, or manufacture or market, any new products for our own marketing purposes, or for third parties. DPI may fail to secure sufficiently lucrative third-party manufacturing contracts to increase its profitability. The research, development, production and marketing of new products will require the application of considerable technical and financial resources by us and our collaborators, while revenues that are generated by such products, if successfully developed and marketed, may not be realized for several years. We may not be able to sustain profitability. We may require external financing to complete certain aspects of our strategic plan, and external sources of capital may not be available at an acceptable cost. Should Société générale de financement du Québec ("SGF") divest its interest in DPI, or the shareholder relationship between us and SGF become strained, there may be a negative effect on us and our manufacturing facility operations.
IF WE CANNOT ADAPT TO CHANGING TECHNOLOGIES, OUR PRODUCTS AND SERVICES MAY BECOME OBSOLETE AND OUR BUSINESS COULD SUFFER.
Because the biotechnology and pharmaceutical industry is characterized by rapid technology change and obsolescence, we may be unable to anticipate changes in our current and potential customer requirements that could make our existing technology obsolete. Our success will depend, in part, on our ability to continue to enhance our existing products and services, develop new technology that addresses the increasing sophistication and varied needs of our respective customers, license leading technologies and respond to technological advances and emerging industry standards and practices on a timely and cost-effective basis. The development of our proprietary technology and investing in certain niche markets entails significant technical and business risks. We may not be successful in using our new technologies or exploiting our niche markets effectively or adapting our businesses to evolving customer requirements or emerging industry standards.
OUR COMMON SHARE PRICE HAS BEEN, AND IS LIKELY TO CONTINUE TO BE, VOLATILE.
The market prices for the securities of pharmaceutical and biotechnology companies, including ours, have historically been highly volatile, and the market has, from time to time, experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. Factors such as fluctuations in our operating results, announcements of competing technological innovations or new therapeutic products by our competitors, clinical trial results, governmental regulation, developments in patent or other proprietary rights, public concern as to the safety of drugs developed by us or others and general market conditions can have an adverse effect on the market price of our shares. In particular, the realization of any of the risks described herein could have a material adverse impact on such market price. Sales of substantial amounts of our shares in the public market, or the perception that such sales will occur, could also adversely affect the market price of our shares and make it more difficult in the future for us to raise funds through equity offerings.
8
WE ARE EXPOSED TO EXCHANGE RATE FLUCTUATIONS WHICH COULD NEGATIVELY AFFECT OUR BUSINESS.
A substantial portion of our revenues are now, and are expected to continue to be, realized in US dollars. Our operating expenses are primarily paid in Canadian dollars. Fluctuations in the exchange rate between the US dollar and the Canadian dollar may have a material effect on our results of operations. In particular, we may be adversely affected by a significant strengthening of the Canadian dollar against the US dollar. We do not currently use derivative instruments to hedge our foreign exchange risk and currently have no plans to do so in the near future.
IF WE LOSE THE SERVICES OF KEY PERSONNEL, WE MAY BE UNABLE TO REPLACE THEM, AND OUR BUSINESS COULD BE NEGATIVELY AFFECTED.
Our success depends on the retention of principal members of our management and scientific staff and on our ability to continue to attract, motivate and retain additional key personnel. The market for retaining and obtaining such key personnel is intensely competitive and the loss of the services of key personnel or the failure to recruit necessary additional personnel could materially and adversely affect operations and our research and development efforts.
ALTHOUGH WE CONSIDER THE COMPANY TO HAVE GOOD DEFENCES TO SUCH ACTIONS, SHOULD CURRENT LAWSUITS AGAINST US SUCCEED, WE COULD INCUR A SUBSTANTIAL LOSS.
While we believe the Company has good defences to the Knoll, Innovations Foundation and University of Toronto proceedings (see "Legal/Arbitration Proceedings" under Item 8), these disputes may not be resolved in our favour. It is possible that a court or arbitration tribunal may find us to be in breach of certain agreements, or of infringing validly issued patents of third parties or practicing the intellectual property of others. In that event, in addition to the cost of defending the underlying proceedings, we may have to pay license fees, additional royalties and/or damages and may be ordered to assign certain Anipryl-related patents and be prohibited from conducting certain activities. Under such circumstances, we could incur substantial loss and our business could be negatively affected.
9
Item 4. Information on the Company
DRAXIS is an integrated specialty pharmaceutical company with core competencies in two segments: the development, production, marketing and distribution of radiopharmaceuticals through DRAXIMAGE and the provision of contract pharmaceutical manufacturing services, specializing in liquid and freeze-dried injectables and other sterile products through DPI. The Company believes that both DRAXIMAGE and DPI have significant long-term growth potential and has invested considerable financial and management resources in developing these businesses.
DRAXIS also has a Canadian pharmaceutical sales and marketing division which operates under the name DRAXIS Pharmaceutica. DRAXIS has made the strategic decision to divest DRAXIS Pharmaceutica in order to focus on its two core businesses.
In addition, DRAXIS has continuing financial interests associated with its collaboration agreements with Pfizer Inc. with respect to Anipryl and GlaxoSmithKline ("GSK") with respect to the SpectroPharm line of dermatology products.
The Company's consolidated operations are integrated across research, product development, manufacturing, sales and marketing, as well as the in-licensing and commercial development of pharmaceutical products.
DRAXIS is currently emerging from a transitional phase of its development. To date, the Company has been reliant on income earned from Anipryl. For the year ended December 31, 2002, the Company's collaboration agreement with respect to Anipryl was the Company's primary source of income. In the near term, however, the Company expects to lessen this reliance as it develops a diversified profit base from its other business units. In particular, the Company expects radiopharmaceuticals and contract manufacturing to become major sources of longer-term revenue and earnings.
The common shares of DRAXIS are listed on the Toronto Stock Exchange (ticker symbol DAX) and on NASDAQ (ticker symbol DRAX).
The Company's registered and head office is located at 6870 Goreway Drive, 2nd Floor, Mississauga, Ontario, Canada L4V 1P1. Telephone: (905) 677-5500. Fax: (905) 677-5494.
The Company's manufacturing, research and development facilities are located at 16751 Trans-Canada Road, Kirkland, Quebec, Canada H9H 4J4.
The Company's website is: www.draxis.com.
History and Development of the Company
The Company was incorporated under the name Deprenyl Research Limited on October 13, 1987 under the Canada Business Corporations Act. The Company was founded principally to engage in the marketing in Canada of prescription pharmaceuticals discovered, developed or acquired by Chinoin Pharmaceutical and Chemical Works Co., Ltd., the first of which was Eldepryl (selegiline; 1-deprenyl), for the treatment of Parkinson's disease. The Company changed its name to DRAXIS Health Inc. in May 1994.
The Company completed an initial public offering of its common shares in February 1988.
Beginning in 1990, the Company expanded on its knowledge and experience with selegiline by initiating directly on its own behalf, as well as through contract research arrangements, studies designed to investigate the potential of selegiline for companion animal use. This initiative ultimately resulted in the formation of its subsidiary DAHI, through which the Company developed and commercialized a companion animal health product, Anipryl.
In 1991 the Company began to invest in the applications of aminolevulinic acid photodynamic therapy which resulted in the formation of a subsidiary, DUSA Pharmaceuticals, Inc. ("DUSA"). Through a series of transactions from 1991 to 1996, the Company divested all of its interest in DUSA.
By late 1992, the Company faced considerable uncertainty due to the impending threat of generic competition for Eldepryl which, at that time, still accounted for almost 100% of the Company's revenues. Faced with this as well as other business challenges, in 1992 the Company began the process of recruiting new senior management to develop and implement a new strategic business plan for the Company to (i) address the generic threat to its lead drug by expanding and diversifying its established Canadian pharmaceuticals franchise; (ii) strengthen the
10
Company's financial position; and (iii) diversify from its historical Canadian base through the acquisition of niche pharmaceutical products and/or manufacturing-oriented businesses with international growth potential.
Between 1993 and 1999 the Company implemented the following corporate development initiatives:
Canadian Pharmaceuticals
- •
- December 1993—extension of the Company's Eldepryl franchise through an agreement with Novopharm Limited with respect to Novo-Selegiline, a generic version of Eldepryl. In September 1998, this agreement was renewed for a further five-year period to September 2003.
- •
- 1993 to 1999—acquisition and licensing of several prescription and non-prescription products for the Canadian market, predominantly products for neurological disorders.
Financial Position
- •
- March 1996—disposition of the Company's remaining interest in DUSA for net proceeds of $6.8 million.
- •
- April 1996—public offering of 3,000,000 common shares generating net proceeds of $8.5 million.
Diversification
- •
- November 1996—acquisition of the shares of DAHI that the Company did not previously own through a mandatory share exchange transaction valued at $17.4 million.
- •
- July 1997—$8.6 million acquisition of the Company's radiopharmaceutical business which began operations through DRAXIMAGE.
- •
- December 1997—licensing of Anipryl to Pfizer in return for milestone payments, royalties, a manufacturing supply agreement and a research collaboration. To December 31, 2002 $40.6 million has been received in milestone payments, royalties and royalty-related payments from Pfizer.
- •
- May 1998—$11.1 million acquisition of the Company's pharmaceutical manufacturing facility in Kirkland, Quebec, which operates as DRAXIS Pharma Inc.
Beginning in 1998 the Company embarked on a capital expansion plan directed primarily toward construction of the Company's new lyophilization line, the construction of radiopharmaceutical manufacturing facilities and the subsequent expansion of the radiopharmaceutical operation.
In late 1999 and early 2000, the Company undertook a strategic review of its operations from the perspective of enhancing shareholder value. As a result of this review, the Company resolved to focus its financial and management resources on its radiopharmaceutical and contract manufacturing businesses.
During 2000, the Company divested its dermatology product lines for aggregate proceeds of $9.0 million. The largest transaction in this regard was the sale of the SpectroPharm line of products to Block Drug Inc. (now part of GlaxoSmithKline Consumer Healthcare).
In February 2000, the Company established a strategic alliance with SGF with the sale of a 34.1% equity interest in DPI to SGF and members of DPI's management team.
During 2001 the Company received U.S. and Canadian regulatory approvals for the expanded radiopharmaceutical manufacturing facility and FDA acceptance to manufacture sterile lyophilized and sterile injectable products.
In March 2001 the Company in-licensed INFECTON, a technetium 99m-based radiopharmaceutical for imaging infection.
Also during 2001, DRAXIMAGE launched BrachySeed I-125 in the U.S. and Canada, and received regulatory approvals in the U.S. and Canada for BrachySeed Pd-103.
In 2001, the Company further implemented its strategic plan by (i) initiating the production of Bracco Diagnostic Inc.'s sodium iodide I-131 radiotherapy capsules; (ii) signing a long-term supply agreement to manufacture several GlaxoSmithKline predominantly sterile products for multiple international markets; and (iii) amending the Anipryl licensing agreement with Pfizer resulting in a $3.1 million cash payment to the Company plus return of product rights outside of North America.
11
During 2002 the Company achieved a number of significant accomplishments, including:
- •
- Record financial results from continuing operations:
- •
- Consolidated revenues of $38.6 million, an increase of 14.0% over 2001.
- •
- Consolidated net income from continuing operations before non-recurring items of $0.077 per share, an increase of 24.2% over 2001.
- •
- Consolidated earnings before interest, taxes, depreciation, amortization, research and development and non-recurring items ("EBITDARD") of $8.1 million, an increase of 46.7% over 2001.
- •
- Regulatory approvals of manufacturing facilities:
- •
- U.S. and Canadian regulatory approval to transfer production of DRAXIMAGE's line of lyophilized medical imaging products to the Company's own production facility.
- •
- U.S. regulatory approval to commence production at DPI of an established injectable product for GlaxoSmithKline for the U.S. market.
- •
- U.S. regulatory approval to manufacture solid dosage form products at DPI.
- •
- Subsequent to year end, U.K. regulatory approval to manufacture multiple sterile and non-sterile products at DPI.
- •
- Continued growth together with the realignment of the Company's U.S. sales and marketing strategy for BrachySeed, the Company's proprietary second generation radioactive brachytherapy implant:
- •
- Increased U.S. and Canadian market acceptance of the iodine version of BrachySeed which was launched in 2001.
- •
- U.S. launch of the palladium version of BrachySeed and the subsequent suspension of shipments pending the development of a revised marketing strategy for this product.
- •
- Early in 2003, commencement of direct sales of BrachySeed in the U.S.
- •
- Underwritten public sale by two corporate shareholders of 4.2 million common shares, representing approximately 11.4% of issued and outstanding shares.
- •
- In January 2003, commencement of a Phase I trial for INFECTON, a technetium-99m-based radiopharmaceutical for imaging infection.
- •
- In January 2003, U.S. regulatory approval of a new radiotherapeutic kit product for the treatment of thyroid cancer and hyperthyroidism.
Business Strategy
The Company believes that both DRAXIMAGE and DPI have significant long-term growth potential and has invested considerable financial and management resources in developing these businesses including:
- •
- $6.5 million of investments establishing the first of two sterile lyophilization (freeze-drying) units at DPI to provide in-house manufacturing of the Company's radiopharmaceutical imaging kits as well as third-party contract manufacturing services (a second unit announced in April 2002 will result in the tripling of lyophilization capacity);
- •
- $4.7 million of investments in the construction and later expansion of DRAXIMAGE's radiopharmaceutical production facilities and capabilities, including its robotic manufacturing lines;
- •
- $0.8 million of investments to enhance DPI's and DRAXIMAGE's capabilities and regulatory compliance;
- •
- $12.0 million planned investment in a three-year capital plan at DPI announced in April 2002, including expansion of the Company's existing lyophilization capacity; and
- •
- the continued development of the DRAXIMAGE product pipeline including: BrachySeed, Fibrimage, Amiscan, and INFECTON.
12
These initiatives are consistent with the Company's general business strategy to:
- •
- Focus on specialty pharmaceutical markets in which the Company can develop and sustain competitive advantage—
- •
- DRAXIS has refocused its operations on its two core businesses, radiopharmaceuticals and specialty pharmaceutical contract manufacturing. Both businesses target highly differentiated, specialized markets which feature high barriers to entry including regulatory requirements and specialized manufacturing standards.
- •
- Develop new pharmaceutical products and services consistent with the Company's strengths and capabilities
- •
- DRAXIS has established a growing franchise in the highly differentiated radiopharmaceutical segment with a portfolio of over 40 currently marketed products plus five new products in development. In addition to its radiopharmaceutical platform, the Company's expanding lyophilization capability positions it to offer new services to take advantage of growth opportunities in contract manufacturing.
- •
- Pursue growth opportunities with international market potential
- •
- The Company is leveraging its capabilities, experience and regulatory compliance in radiopharmaceuticals and manufacturing to expand its product and service offerings in the United States and other international markets.
- •
- Leverage alliances with business partners
- •
- The Company is pursuing multiple opportunities with new and existing business partners. Meeting the strict quality and service standards of such partners positions the Company to expand on these relationships in both its radiopharmaceuticals and pharmaceutical contract manufacturing businesses.
The Company's primary operational focus in 2003 will continue to be on: (i) improving near-term financial and operational performance of its radiopharmaceutical and manufacturing businesses through increasing sales of existing products and services, improving manufacturing efficiency and effectiveness, and obtaining regulatory approvals; and (ii) securing and advancing its base for long-term growth through the development of its existing product pipeline as well as identifying and capitalizing on additional new business opportunities that are consistent with the Company's capabilities and that contribute to the long-term value of the Company.
A description of the principal markets in which the Company operates and a breakdown of total revenues by category of activity and geographic market for the three most recent financial years is included in Note 23 of the Company's Consolidated Financial Statements and Notes beginning on page F-1.
The Company's two main operating businesses, radiopharmaceuticals and pharmaceutical contract manufacturing, are not characterized by significant seasonality. Plant operations, however, are generally reduced or shut down once a year for approximately two to four weeks in order to conduct regular required maintenance of facilities.
DRAXIMAGE's principal raw materials are radioactive isotopes that are used to label other compounds that act as carriers or molecular targeting agents. The isotopes are obtained from companies and agencies that are licensed by governmental regulators to produce purified radioactive chemicals. Radioactive materials, by their nature, decay over time—some rapidly and some more slowly depending on specific isotopes. Therefore, DRAXIMAGE receives shipments of chemical grade radioisotopes several times each week and converts these to finished products within days or weeks. While prices of selected isotopes can be somewhat volatile over the medium term, DRAXIMAGE endeavours to negotiate long-term supply contracts with appropriate suppliers, especially for the more important isotopes such as radioactive iodine.
DPI, as a manufacturer of pharmaceutical products, obtains its chemical raw materials from approved chemical suppliers of both active pharmaceutical ingredients ("API") and inactive or excipient ingredients such as fillers, stabilizers, preservative agents and colouring agents. In some instances the API is supplied by the customer. DPI conducts full quality control testing of all ingredients and packaging materials before they are incorporated into the finished product. Prices of principal API are not generally volatile although prices can vary between alternative suppliers.
13
Radiopharmaceuticals
DRAXIMAGE discovers, develops, manufactures, and markets diagnostic imaging and therapeutic radiopharmaceutical products for the global nuclear medicine marketplace. Nuclear medicine entails the use of radioactive agents (radiopharmaceuticals) for imaging and therapeutic purposes. The energy released as these products decay can be harnessed to: (i) elicit a visual representation of various organs and tissues (nuclear imaging), or (ii) destroy cancerous cells (radiotherapy). Products currently marketed by DRAXIMAGE include a line of lyophilized technetium-99m kits used in nuclear imaging procedures, a line of imaging and therapeutic products labelled with a variety of isotopes including radioiodine, and BrachySeed, second generation iodine-125 and palladium-103 brachytherapy implants. DRAXIMAGE also has a number of products in development including three technetium-99m-based diagnostic imaging products: Fibrimage for imaging deep vein thrombosis currently in Phase III clinical trials, Amiscan for the early diagnosis of acute myocardial infarct currently in Phase II clinical trials, and INFECTON for imaging infection currently in Phase I.
Founded in 1950 as a division of Charles E. Frosst & Co., the predecessor business to DRAXIMAGE pioneered and became a leader in the medical application of nuclear technology after assuming the development function from Atomic Energy of Canada Ltd. Following the 1965 acquisition of Charles E. Frosst & Co. by Merck Inc. ("Merck"), the business continued operations as a division of Merck's Canadian subsidiary, Merck Frosst Canada and Co. ("Merck Frosst"), until its acquisition by DRAXIS in 1997. As part of the acquisition, the Company retained all of the business's managers, scientists and employees as well as its existing products and intellectual property.
The acquisition of this business was consistent with the Company's strategy of acquiring speciality pharmaceutical platforms with potential for expansion into global markets. DRAXIMAGE is the only company that manufactures radiopharmaceuticals in Canada.
DRAXIMAGE and its predecessor have a long history of technological and scientific progress in the field of radiopharmaceuticals. Notable achievements include: the development of lyophilized kits for the in-situ preparation of technetium-99m ("Tc-99m") radiopharmaceutical products for nuclear medical imaging ("Tc-99m Kits"); the development of chelates for the indium/yttrium and technetium/rhenium groups of metals; and development of stabilisers for use in iodinated radiopharmaceuticals which resulted in DRAXIMAGE being one of the few companies to market iodinated products that do not require refrigeration.
Key business development transactions involving DRAXIMAGE have included:
- •
- July 1997—$8.6 million acquisition of the radiopharmaceutical division of Merck Frosst which began operations through DRAXIMAGE;
- •
- June 1998—establishment of a strategic alliance with Molecular Targeting Technology, Inc. ("MTTI") to develop, manufacture and market potential novel imaging agents. The first opportunity being pursued under this alliance is Amiscan, an agent for the imaging of acute myocardial infarct;
- •
- October 1999—licensing from Isogenic Science Ltd. ("Isogen") of the rights to Isogen's proprietary technology related to radioactive brachytherapy implants ("BrachySeed") for the treatment of various localized cancers;
- •
- December 2000—entering into license, distribution and supply agreements for BrachySeed for the U.S. market with Cytogen Corporation;
- •
- March 2001—licensing from British Technology Group ("BTG") of the rights to INFECTON, an agent for imaging infection;
- •
- October 2001—long-term manufacturing supply agreement to produce Bracco Diagnostics Inc.'s sodium iodide I-131 radiotherapy capsules for the U.S. market;
- •
- March 2003—five-year non-exclusive product distribution agreement with Cardinal Health, Inc. for a new radioactive iodine I-131 kit product for the U.S.; and
- •
- April 2003—termination of license, distribution and supply agreements with Cytogen Corporation for BrachySeed for the U.S. market.
14
DRAXIMAGE's growth strategy is to leverage its history and experience in the research, development, manufacture and distribution of radiopharmaceutical products and services and to capitalize on its recently expanded manufacturing capabilities. In particular, DRAXIMAGE's business strategy includes:
- •
- Increasing sales of radioactive products, particularly in the U.S., facilitated by the recent expansion of its radiopharmaceutical manufacturing facility and the acceptance of that facility by U.S. regulatory agencies;
- •
- Increasing international sales of its existing Tc-99m Kits facilitated by the increase in manufacturing capacity following regulatory approval of DPI's lyophilization manufacturing line;
- •
- Timely, cost-effective development of its portfolio of innovative imaging and therapeutic radiopharmaceutical products; and
- •
- Identifying and capitalizing on additional new business opportunities which are consistent with DRAXIMAGE's capabilities.
Overview of Radiopharmaceuticals
The medical specialty of nuclear medicine involves the use of short-lived radioisotopes for both diagnostic imaging and therapeutic applications. Pharmaceutical products used in such applications are commonly called radiopharmaceuticals. Radiopharmaceuticals represent a well-defined, growing niche market with high barriers to entry, including regulatory approval, specialized manufacturing standards, access to supply and critical delivery logistics.
Frost and Sullivan, a pharmaceutical industry consulting firm, estimates that the U.S. radiopharmaceuticals market will continue to demonstrate double-digit growth rates. The total U.S. market reached more than $900 million in 2001 with over 80% of revenues from the diagnostics sector, strongly led by cardiology diagnostics. Total diagnostic procedures performed in the U.S. reached nearly 14 million in 2001, with an estimated 90% of dosages being distributed through radiopharmacies. Frost and Sullivan forecasts that the U.S. diagnostic radiopharmaceuticals market could reach more than $1 billion by 2004 and that the total U.S. radiopharmaceuticals market could grow to exceed $1.6 billion by the end of 2006.
The diagnostic imaging applications of nuclear medicine normally involve the intravenous administration of a radiopharmaceutical consisting of a targeting molecule, which has a particular binding affinity for a specific organ or tissue, linked to a low intensity, gamma-emitting radioisotope such as Tc-99m. Following administration, the radiopharmaceutical with its radioactive isotope concentrates at the biological target which is then imaged using a scanning device such as a gamma camera. A gamma camera consists of a scintillation crystal which, when placed over a region of the body, is capable of detecting gamma rays emitted by radionuclides in underlying tissues. This imaging modality is also referred to as gamma scintigraphy. The gamma camera and its associated computerised peripheral devices accurately measure the radiation being emitted by the radioisotope and so provide both a visual picture of the target as well as quantitative data concerning the distribution of radioactivity.
A nuclear medicine image provides both static and functional, or dynamic, information about a biological target thereby revealing functional and morphological information normally unobtainable by other imaging techniques such as X-ray, magnetic resonance imaging, computer tomography, and ultrasound, all of which provide primarily anatomical information.
Nuclear medical imaging procedures are well-established and trusted medical procedures with many years of safe and effective use. Most procedures are non-invasive, are performed with a minimum of discomfort and inconvenience to the patient and are normally less costly and involve less risk than alternative techniques.
In the U.S., it is estimated by the Nuclear Medicine Industry Association that one in three hospital stays involves at least one nuclear medicine procedure, aggregating in excess of 40,000 such procedures per day, 80—90% of which involve the use of Tc-99m as the radioactive isotope.
15
Increasingly, therapeutic radiopharmaceuticals are being used and new products are being developed for the treatment of disease, particularly for the treatment of various cancers. Examples of currently marketed therapeutic radiopharmaceuticals, include:
- •
- Various sodium iodide I-131 products for the treatment of thyroid cancer and hyperthyroidism;
- •
- Brachytherapy implants for the treatment of prostate and other forms of localized cancer;
- •
- Phosphorous-32 for the palliation of bone pain for patients with advanced bone metastases; and
- •
- I-131 meta-Iodobenzylguanidine (MIBG) for the treatment of neuroblastoma.
New generations of anti-cancer agents based on targeting molecules such as peptides, proteins, metabolites and monoclonal antibodies that have binding affinities to the surface of cancerous cells are being developed to be linked to more potent, higher intensity, beta-emitting radioisotopes such as yttrium-90, rhenium-186, holmiun-166 and lutetium-199. There is a growing body of scientific evidence that molecules, thus bound, will permit the delivery of therapeutic quantities of radiation directly to malignant cells.
Proprietary technology and know-how within DRAXIMAGE are particularly applicable to the discovery and development of the new generations of innovative radiotherapeutic products. The Company is currently in preclinical development of Somatostatin Therapy which is being developed for the treatment of neuroendocrine tumours, lymphoma, carcinoid and small cell lung cancer.
Competition
The radiopharmaceutical field is highly specialized, and there are significant barriers to entry, especially in the therapeutic area. The barriers include meeting pharmaceutical product and nuclear regulatory requirements, having the ability to handle radioactive materials and establishing facilities and procedures to manufacture in a sterile lyophilized or freeze-dried form, which involves manufacturing standards higher than those typically employed for most pharmaceutical products. In addition, manufacturing and distribution logistics systems must be well-integrated and supportive to ensure that radiopharmaceutical products can be produced rapidly and delivered to the end-user in a timely manner with the prescribed level of strength.
Companies with significant radiopharmaceutical operations include: Bristol-Myers Squibb Company, Amersham plc, Tyco International Ltd., Schering AG, and Bracco SpA. In addition, there are a number of companies that are developing and/or marketing other radiopharmaceutical products including: MDS Nordion Inc., Immunomedics, Inc., IDEC Pharmaceuticals Corporation and NeoRx Corporation.
BrachySeed implants are sold into a competitive marketplace that includes the established market leader, Amersham plc. Other competitors in the U.S. market include Theragenics Corporation, North American Scientific, Inc., UroCor, Inc., Mentor Corporation, Implant Sciences Corporation and Cardinal Health, Inc.
Marketed Products
DRAXIMAGE's products are divided into two groups: (i) radioactive products, which are in a radioactive and ready-to-use form when shipped to customers, and (ii) non-radioactive products (predominantly Tc-99m Kits), which are sold in lyophilized (freeze-dried), non-radioactive form consisting of sterile, pyrogen-free complexes of chemical and/or biological substances. Tc-99m Kits are reconstituted and labelled with Tc-99m in the nuclear medicine laboratory of a hospital or a unit-dose service supplier prior to use.
Radioactive products, which are shipped by DRAXIMAGE with the radioactive isotope already incorporated, are distributed to the nuclear medicine departments of hospitals or diagnostic clinics in a ready-to-use form.
16
The following table summarises DRAXIMAGE's radioactive products:
Product
| | Indication
| | Principle
Markets
|
|---|
| Imaging Products | | | | |
| |
51 Cr-Chromic Chloride Injection |
|
Diagnosis of gastroenteropathy |
|
Canada |
| | 51 Cr-Sodium Chromate Injection, USP | | Measurement of blood volume | | Canada |
| | 57 Co-Cyanocobalamin Capsule USP | | Diagnosis of Vitamin-B12 malabsorption | | Canada |
| | 57 Co-Cyanocobalamin Solution USP | | Diagnosis of Vitamin-B12 malabsorption | | Canada |
| | 57 Co-Standard Cobalt Reference Solution | | Reference for57Co-Cyanocobalamin | | Canada |
| | 111 In-Diethylene Triamine Pentacetic Acid Injection (DTPA) | | Cisternography | | Canada |
| | 111 In-Indium Chloride Sterile Solution | | Labelling of peptides and antibodies | | Canada |
| | 131 I-Sodium Iodide Diagnostic Capsules and Solutions, Oral and Intravenous | | Imaging of thyroid disorders | | Canada |
| | 131 I-metaIodobenzylguanidine | | Adrenal imaging | | Canada |
| | 131 I-Iodinated Serum Albumin Injection, USP | | Measurement of blood and plasma volume | | Canada |
| | 131 I-Iodohippuric Sodium Injection, USP | | Measurement of renal plasma flow | | Canada |
| | 125 I-Iodinated Albumin Injection, USP | | Measurement of blood and plasma volume | | Canada |
| | 133 Xe-Xenon Gas | | Lung ventilation studies | | Canada |
| Therapeutic Products | | | | |
| |
125 I BrachySeed |
|
Treatment of various localized cancers |
|
U.S./Canada/
South America |
| | 103 Pd BrachySeed | | Treatment of various localized cancers | | U.S./Canada |
| | 131 I-Sodium Iodide Therapeutic Capsules and Solutions, Oral and Intravenous | | Treatment of thyroid disorders | | Canada |
| | Kit for the Preparation of Sodium Iodide I-131 Capsules and Solution USP Therapeutic—Oral | | Treatment of thyroid disorders | | U.S. |
| | 131 I-meta-Iodobenzylguanidine (MIBG) | | Treatment of neuroblastoma | | Canada/Europe |
| | 32 P-Sodium Phosphate Solution, USP | | Treatment of bone pain | | Canada |
| Other | | | | Canada |
| |
131 I-Sodium Iodine Solution—Chemical |
|
Radiochemical synthesis |
|
Canada |
| | 125 I-Sodium Iodide Solution—Chemical | | Radiochemical synthesis | | Canada |
| | 131 I-Iodinated Human Serum Albumin | | Radiochemical | | U.S./Europe |
| | 125 I-Iodinated Human Serum Albumin | | Radiochemical | | U.S./Europe |
During the second half of 2001, DRAXIMAGE entered into a third-party manufacturing contract to supply sodium iodide I-131 radiotherapy capsules for Bracco Diagnostics Inc. for the U.S. market for the treatment of thyroid cancer and hyperparathyroidism.
In January 2003, DRAXIMAGE received FDA approval to produce and market a new radiopharmaceutical kit product for the preparation of Sodium Iodide I-131 Capsules and Oral Solution for the treatment of thyroid
17
cancer and hyperthyroidism. This was the first such product on the market that allows physicians and radiopharmacists to prepare an FDA-approved I-131 gelatine capsule or oral solution. In March 2003, DRAXIMAGE launched the new kit product and initiated shipments to the Nuclear Pharmacy Services group of Cardinal Health, Inc., under a five-year, non-exclusive distribution agreement for the product for the United States and its possessions.
BrachySeed is a uniquely designed, second-generation radioactive brachytherapy implant developed by DRAXIMAGE. The unique design of BrachySeed brings a high level of accuracy, precision and safety to sealed source implant surgery. Each BrachySeed is robotically manufactured at the DRAXIMAGE facility.
One of the key segments of the radiopharmaceuticals market is brachytherapy—the implantation of radioactive "seeds" for the treatment of localized cancers of the prostate and other organs. These seeds can contain either iodine-125 or palladium-103, depending on the status of the cancer and the required duration of treatment. The seeds deliver a concentrated, defined radiation dose that destroys cancer cells while minimizing damage to surrounding tissues. This targeted strategy is particularly important for organs such as the prostate, which is nestled between the bladder and the rectum. The procedure involves placement of approximately 100-150 of these seeds into a locally anaesthetised patient.
DRAXIMAGE's Iodine-125 implant was approved by the FDA in the second half of 2000 and launched into the Canadian and U.S. markets during the second quarter of 2001. The palladium-103 product was approved by the FDA in the fourth quarter of 2001 and launched during the second quarter of 2002, but shipments were subsequently suspended pending development of a revised marketing strategy. The palladium isotope releases its energy over a shorter time-span, and tends to be used in the treatment of more aggressive cancers. DRAXIS' brachytherapy seed utilizes a patented design that delivers a more uniform distribution of radiation than its competitors' products, which can result in the use of relatively fewer BrachySeed implants per procedure.
In early 2003 DRAXIMAGE implemented a strategy to market brachytherapy products directly to U.S. customers drawing on the expertise and resources of its established customer service system, following the ending of license, distribution and supply agreements with Cytogen Corporation. The move to the end-user marketing of brachytherapy implants is part of a trend that has become evident throughout the brachytherapy industry. This trend is in part as a result of a change in the reimbursement policy of the U.S. Center for Medicare and Medicaid Services (CMS) as of January 2003, whereby the fee for a prostate cancer treatment procedure now includes the cost of implants. DRAXIMAGE established a direct brachytherapy marketing and sales group, including product management, in-house sales/telemarketing, customer service support, secure website ordering and sales representatives in the U.S.
In a report in 2000, Frost & Sullivan forecasted that the brachytherapy market in the U.S. would grow from $160 million in 1999 (base year) to $360 million by 2006, a compound annual growth rate (1999-2006) of 12.0%. With the trend towards earlier diagnosis and treatment of prostate cancer, brachytherapy is expected to gain an increasingly larger market share for the treatment of localized prostate cancer, currently believed to be approximately 50-55%.
In addition, DRAXIMAGE is developing BrachySeed product enhancements to support expanded sales of BrachySeed. Examples of such value-added enhancements include: pre-sterilization of BrachySeed implants and delivery of BrachySeed in pre-loaded needles and cartridges.
DRAXIMAGE believes that BrachySeed offers important advantages over its competitors including:
- •
- a patented design resulting in a nearly spherical dispersion of the radiation field, delivering uniform doses of radiation to the affected area;
- •
- double encapsulation of the radioisotope for additional patient safety;
- •
- less then 2% variation between individual seeds in an order compared to the industry standard of 5—7%; and
- •
- excellent visualisation by fluoroscopy because of an internal platinum/iridium marker.
The nearly spherical dispersion of the radiation field provides a more uniform dosimetry to reduce "cold spots," areas within the target organ not reached by the radiation. Such "cold spots" may occur when
18
brachytherapy seeds do not provide symmetrical and spherical fields of radiation. This also permits the implantation of 8-10% fewer seeds to achieve the same dosimetry.
In 2002, DRAXIMAGE became the exclusive Canadian distributor for Mick Radio-Nuclear Instruments, Inc., the leading manufacturer of brachytherapy equipment.
DRAXIMAGE currently markets the following non-radioactive products:
Product
| | Indication
| | Principle
Markets
|
|---|
| Tc-99m Kits | | | | |
| | 99m Tc-MarcoAggregated Albumin Kit ("MAA") | | Diagnosis of lung perfusion | | U.S./Canada |
| | 99m Tc-Diethylene TriaminePentacetic Acid Kit ("DTPA") Stabilized with p-Aminobenzoic Acid | | Diagnosis of kidney function, blood pool imaging and lung ventilation function | | U.S./Canada |
| | 99m Tc-Calcium Glucoheptonate Kit | | Diagnosis of kidney function | | Canada |
| | 99m Tc-MethyleneDiphosphonic Acid Kit ("MDP") Stabilized with p-Aminobenzoic Acid | | Diagnosis of inflammatory and neoplastic bone disease | | U.S./Canada |
| | 99m Tc-Calcium Gluceptate Kit | | Diagnosis of kidney function and red blood cell imaging agent | | U.S./Canada |
| Other | | | | |
| | Stannous Gluconate | | Antibody labelling | | Canada |
| | Sodium Carbonate/Bicarbonate Solution | | Antibody labelling | | Canada |
| | Acetic Acid/HCI Solution | | Antibody labelling | | Canada |
| | Sterile Empty Vial | | Antibody labelling | | Canada |
| | A-C-D Solution Special Formula | | Use with51 Cr-Sodium Chromate | | Canada |
| | Intrinsic Factor Capsule | | Use with57 Co-Cyanocobalamin | | Canada |
| | Quality Control Kit | | Nuclear pharmacy quality control kit | | Canada |
DRAXIMAGE's major non-radioactive products are its MDP, DTPA and MAA Tc-99m diagnostic imaging Kits. While these products would technically be classified as generic pharmaceutical products, DRAXIMAGE's versions are proprietary and are sold under the DRAXIMAGE trademark or that of its local distributors. All three of these products have a reputation of high quality and enjoy a high degree of acceptance with DRAXIMAGE's customers, in part due to a proprietary formulation that provides additional shelf life following reconstitution compared to competitive products.
Prior to 2002, DRAXIMAGE outsourced the production of its Tc-99m Kits. Following FDA approval of the Company's own lyophilization production facilities in early 2002, the resulting increased manufacturing capacity provided the opportunity to increase sales volumes of these products into existing markets in the U.S. and Canada and to build new markets in as well as Europe and Asia, territories where product approvals already are either in place or expected to be granted in the near future.
19
Products under Development
DRAXIMAGE has the following products under development:
Product
| | Status
| | Indication
|
|---|
| Imaging Products | | | | |
| | Fibrimage | | Phase III | | Deep venous thrombosis imaging |
| | Amiscan | | Phase II | | Myocardial infarct imaging |
| | INFECTON | | Phase I | | Infection imaging |
| | Somatoscan | | Preclinical | | Imaging of neuroendocrine tumours, lymphoma, carcinoid and small cell lung cancer |
| Therapeutic Products | | | | |
| | Somatostatin Therapy | | Preclinical | | Treatment of neuroendocrine tumours, lymphoma, carcinoid and small cell lung cancer |
Description—Fibrimage is a Tc-99m Kit for the imaging of active thrombus in deep vein thrombosis ("DVT"). In DVT, a blood clot (or thrombus) forms in the veins, normally in the lower legs, thighs or pelvic areas. Patients with DVT are at risk of pulmonary embolism, which occurs when a clot associated with DVT breaks free and travels to the lungs obstructing blood flow. The Council on Thrombosis estimates that in North America, approximately two million people are affected by DVT each year of which approximately 600,000 result in pulmonary embolism.
Mechanism of Action—Fibrimage is based on linking Tc-99m to the fibrin binding domain ("FBD") of human fibronectin, a recombinant polypeptide with high binding affinity for active fibrin dimer, the primary component of actively forming venous thrombus. Fibrimage is thus able to positively image DVT and to distinguish acute (i.e. active) thrombus from chronic (i.e. inactive) thrombus.
Technology Partner—Recombinant FBD was developed by Bio-Technology General Corp. and is licensed to DRAXIMAGE on an exclusive, world-wide basis.
Regulatory Status—Fibrimage entered Phase III clinical trials in Canada in early 2000 to assess diagnostic accuracy in symptomatic patients with either an initial or recurrent episode of DVT. The phase III studies are designed to address the accuracy, sensitivity, specificity and positive and negative predictive values of Fibrimage compared to standard diagnostic tests. The Phase III trials for Fibrimage have been hampered by the lack of availability of clinical trial materials and by the need to revise the chemistry and manufacturing sections of existing filings. Phase II studies of Fibrimage were completed in July 1999 and interim results from the Phase II studies were reported at the Society of Nuclear Medicine meeting held in Los Angeles in June 1999. Based on interim results of the investigators, lead by Dr. Raymond Taillefer of the Centre Hospitalier du l'Université du Montréal Hôtel Dieu Campus, observers concluded that Fibrimage is a new and very promising radiopharmaceutical for the detection of DVT.
Competing Modalities—Traditional approaches to the diagnosis of DVT have included Doppler ultrasound and contrast venography.
Potential Advantages Of Fibrimage—DRAXIMAGE believes that nuclear medicine imaging with Fibrimage will offer a number of advantages over existing imaging modalities including:
- •
- The ability to image within two hours post injection;
- •
- The ability to distinguish old chronic, stabilised (passive) thrombus from new active (life threatening) thrombus;
- •
- High degree of sensitivity and specificity; and
- •
- Lack of pain, degree of invasiveness and risk of side effect associated with venography.
Fibrimage is considered by DRAXIMAGE to be an attractive alternative to Doppler ultrasound in diagnosing DVT in certain areas, such as below the knee and in the pelvis, where ultrasound is less efficacious. DRAXIMAGE believes that the only competitive radiopharmaceutical imaging product on the market at this time is AcuTect, marketed by Berlex Laboratories, Inc. DRAXIMAGE believes that Fibrimage will compete well
20
against AcuTect because Fibrimage binds to fibrin dimer, which comprises 75-80% of an actively forming blood clot whereas AcuTect binds to platelets.
Description—Amiscan is a formulation of technetium glucarate that is being developed as a sensitive indicator of acute myocardial infarct ("AMI" or heart attack). AMI occurs when blood supply to a portion of the heart is reduced or completely cut off due to an obstruction in one of the coronary arteries. As a result, heart muscle is deprived of oxygen (hypoxic insult) and changes from fat metabolism to carbohydrate metabolism, thus causing uptake of technetium glucarate. Definitive diagnosis of AMI early in the process is often difficult for clinicians. An imaging agent that will localize quickly and specifically in areas of acute necrosis could provide critical diagnostic information. The American Heart Association, in its Heart Disease and Stroke Statistics—2003 Update, noted that in the U.S. in 2000 there were 6,865,000 outpatient department visits and 4,397,000 visits to emergency departments with a primary diagnosis of cardiovascular disease. The prevalence (an estimate of how many people have a disease at a given point in time) of AMI is 7,600,000, and it is estimated that 20—25% of suspected AMI cases are equivocal or difficult to diagnose. Harrison's Principles of Internal Medicine (13th Ed.) notes that 15-20% of patients present to the emergency department with symptoms other than chest pain. About 6-21% of patients with acute infarct have normal electrocardiograms.
Mechanism of Action—It has been shown in animal models and independent human trials in Europe that Amiscan is selectively taken up in hypoxic heart tissue. The exact mechanism of intracellular accumulation of the technetium glucarate and the exact structure of the resulting radiolabelled compound are not known. Subcellular distribution studies in experimental AMI have shown about 75% incorporation of technetium glucarate in nuclear fractions, the remainder in cytosol and mitochondrial fractions and about 83% of nuclear-incorporated material is associated with nucleoproteins (histones). The hypothesis has been made that intracellular accumulation is simply associated with disruption of the cell and nuclear membranes (an event occurring early after irreversible hypoxic insult). This product is, therefore, configured as an infarct-avid radiolabelled agent to permit detection and localization of AMI within a few hours of onset with minimal delay between injection and imaging.
In 1999, the American Hospital Association (JAMA, Vol. 282, No. 23, 1999) reported that nuclear imaging could help physicians determine whether patients with suspected acute cardiac ischemia have normal cardiac blood flow and may safely be sent home rather than admitted to hospital.
Technology Partner—DRAXIMAGE developed Amiscan in conjunction with MTTI pursuant to a strategic alliance established in June 1998. Under the terms of that alliance it was contemplated that DRAXIMAGE and MTTI will co-market Amiscan in the U.S.
Regulatory Status—DRAXIMAGE filed an Investigational New Drug Application for Amiscan with both the FDA and the Canadian HPFB in May 2000. The Phase I study was conducted by Dr. Raymond Taillefer of the Centre Hospitalier du l'Université du Montréal Hôtel Dieu. In February 2001, DRAXIMAGE received permission from the FDA and the HPFB to initiate a Phase II clinical study for Amiscan. The timely conduct of the Phase II trials has been hampered by the lack of availability of clinical trial materials during 2001 and 2002 and slow patient enrolment. The Phase II study is currently being pursued at leading nuclear medicine centres in Canada and the United States.
Competing Diagnostic Modalities—The Company is unaware of any currently approved products that would compete directly with Amiscan's mechanism of action. Current heart perfusion agents such as Cardiolite from Bristol-Myers Squibb Company and Myoview from Amersham plc have the ability to distinguish normally perfused tissue from hypoxic tissue but the images are not specifically related to hypoxia, are limited to only imaging the absence of perfusion and may require multiple scans.
Potential Advantages Of Amiscan—Amiscan is expected to offer distinct advantages in diagnosis of AMI. However, more extensive clinical trials are required to confirm the preliminary results obtained with Amiscan.
21
Description—INFECTON, a complex of Tc-99m and the anti-bacterial agent, ciprofloxacin, is a novel agent for the specific imaging of infection. INFECTON acts by binding directly to infecting organisms and so is highly specific for imaging infection. It is anticipated that INFECTON will be used to image a number of serious medical conditions, including fever of unknown origin, osteomyelitis (bone infection), wound infection, abdominal abscess, pneumonia, appendicitis, and tuberculosis. Many of these conditions are difficult to diagnose even by invasive means, or by surgery.
Technology Partner—INFECTON was discovered by Drs. Britton and Solanki at St. Bartholomew's Hospital in London, England. In March 2001, DRAXIMAGE entered into an agreement with BTG. The agreement gives DRAXIMAGE exclusive rights to manufacture and sell INFECTON in the U.S., Canada, South America and Europe.
Regulatory Status—INFECTON's proof of principle was established in a clinical trial sponsored by the International Atomic Energy Agency involving over 573 patients in which INFECTON displayed excellent results with sensitivity of 88.3%, specificity of 86.5%, and an accuracy of 87.6%. The clinical study showed that the agent achieves up to 96% specificity in detecting bacteria in the lung, bones and tissues—areas where other diagnostics have failed in the past.
In January 2003, DRAXIMAGE initiated a Phase I clinical trial of INFECTON, conducted by Dr. Raymond Taillefer at Hôpital Hotel-Dieu at the Centre Hospitalier du l'Université du Montréal. The study involved 10 healthy subjects in an open-label, single-center, single-dose safety pharmacokinetic and radiation dosimetry study. Whole body images were taken at various intervals after injection of INFECTON to visualize distribution of the imaging agent throughout the body over time. The Phase I study was completed in February with the report to be submitted in May 2003, in anticipation of Phase II studies later in 2003.
Competing Diagnostic Modalities—The most specific current procedure for medical imaging of inflammation due to infection involves removing blood from the patient, isolating white blood cells in the patient's blood, radiolabelling the white blood cells with an isotope such as indium-111 or with technetium coupled to a carrier molecule and then injecting the white blood cells back into the patient. These white blood cells localize around the site of the infection and emit radiation which can be detected using a gamma camera. White blood cells are not highly specific and cannot readily distinguish infection from inflammation. However, Palatin Technologies, Inc. is developing LeuTech, a technetium-labelled monoclonal antibody that binds to white blood cells, for diagnosing equivocal appendicitis and for detection of other infections including osteomyelitis, pulmonary infection, fever of unknown origin, inflammatory bowel disease and post-surgical infection.
Potential Advantages of INFECTON—Preliminary investigations suggest the possibility that INFECTON may be the first radiopharmaceutical capable of distinguishing inflammation from infection.
Description—Somatoscan and Somatostatin Therapy are radiolabelled somatostatin peptides derived from a synthetic peptide based on MK-678, originally developed by Merck, which shows a high binding affinity for the somatostatin receptors expressed in neuroendocrine tumours, lymphoma, carcinoid and small cell lung cancer. This peptide is being developed as both a diagnostic imaging agent (Somatoscan) and therapeutic product (Somatostatin Therapy) and will permit the identification and treatment of primary tumours and the correct diagnosis of metastatic lesions.
Technology Partner—Somatoscan and Somatostatin Therapy are based on innovative research by DRAXIMAGE and the University of Pennsylvania from which DRAXIMAGE has an exclusive world-wide licence for development and commercialisation.
Regulatory Status—Preclinical evaluation of Somatoscan are in progress toward the filing of an application to initiate clinical testing during 2003. A pilot study involving over 30 patients using an iodine-131-MK-678 demonstrated good uptake in all of the target tumour types. DRAXIMAGE's clinical studies will be designed to confirm Somatoscan's earlier successful results observed with the iodine-131 formulation. Additional preclinical studies to scale up synthesis of the drug substance and establish a viable formulation of Somatostatin Therapy is currently being conducted at DRAXIMAGE.
Competing Products—There are two approved somatostatin-based imaging products in the marketplace: OctreoScan from Tyco International Ltd. and NeoTect from Berlex Laboratories Inc. Both of these molecules
22
offer similar information but OctreoScan is based on indium-111 and is more expensive than Tc-99m-based NeoTect and must be delivered in radioactive form. The Company is also aware that development work is also being done on other somatostatin-based cancer therapy products that would compete with Somatostatin Therapy.
Potential Advantages Of Somatoscan—DRAXIMAGE believes that by enabling the same molecule to be used for both diagnosis and therapy, physicians will have a greater ability to deliver therapeutic quantities of radiation specifically to well-defined targets in the body.
DRAXIMAGE holds an option to acquire Canadian marketing and global manufacturing rights to Ovarex, an anti-ovarian cancer vaccine currently in late stage clinical development by AltaRex Corp.
DRAXIMAGE has also conducted preclinical development of atrial natriuretic peptide ("ANP"). ANP is a natural peptide produced in the atrium of the heart and is intended for development as an imaging agent for kidney structure and function. Radiolabelled ANP has demonstrated high binding affinity to receptors in both the lung and kidney. Experimental results in animals have shown that ANP is effective in the detection of kidney dysfunction associated with such diseases as diabetes, essential hypertension and reno-vascular hypertension induced by the stenosis of renal arteries. ANP is based on innovative research by DRAXIMAGE, the Clinical Research Institute of Montréal and the Research Institute of Hôtel Dieu de Montréal, from which DRAXIMAGE has an exclusive world-wide licence.
Description—Paclitaxel is a novel, anti-tumour agent referred to in some scientific and medical literature as "taxol." Paclitaxel is currently marketed under the brand name Taxol by Bristol-Myers Squibb Co. In January 1997, DRAXIS acquired from Mylan the exclusive Canadian marketing rights to the Mylan formulation of paclitaxel. In June 1998, DRAXIS filed with the HPFB a New Drug Submission in Canada for paclitaxel and the HPFB has completed its review process. Prior to approval, DRAXIS must comply with Canadian Patented Drugs Regulations in respect of generic competition. Mylan is currently assessing the status of patent infringement litigation in Canada regarding paclitaxel and the Company has agreed to defer launch in Canada until such issues have been clarified.
DRAXIS and Mylan will share the profits from marketing and selling paclitaxel in Canada according to a formula agreed to between the parties. Mylan has obtained from Phytogen Life Sciences Inc. of Vancouver, British Columbia, the exclusive right to manufacture, market and distribute a formulated dosage form of paclitaxel in Canada, the United States and Mexico, using Phytogen produced bulk fine chemical paclitaxel.
Manufacturing
DRAXIMAGE manufactures its radioactive products in a cGMP compliant manufacturing facility supported by a full quality control department licensed by regulatory agencies in Canada and the U.S.
In 2001, DRAXIMAGE completed the expansion to its radiopharmaceutical production area in anticipation of increasing sales volumes of its radiopharmaceutical line in the U.S. and other markets. This expansion has approximately doubled the size of the original facility to 19,000 square feet.
Most of the radioisotopes used to produce radiopharmaceuticals are supplied under a long-term supply agreement to DRAXIMAGE primarily, but not exclusively, by MDS Nordion, a subsidiary of MDS Inc. and the world's largest supplier of chemical grade isotopes for use as medical radioisotopes.
A key distinguishing characteristic of the radiopharmaceuticals business is the requirement for a sophisticated logistics system. Radiopharmaceuticals, by their nature, decay continually over time thereby losing their potency. Therefore manufacturing and product delivery systems must be well co-ordinated to ensure that the level of radioactivity present in the product supplied to the physician is correct at the time of administration.
23
DRAXIMAGE's Tc-99m Kits are manufactured currently in the DPI lyophilization facility which was accepted by the FDA in October 2001. Subsequent manufacturing site transfer approvals for the Tc-99m Kits were received during the period December 2001 to March 2002.
DPI's initial lyophilizer is expected to have the production capacity to approximately double the supply of Tc-99m Kits to DRAXIMAGE as compared to the previous outsourced manufacturer.
In May 2000, DRAXIMAGE received Canadian regulatory approval to market its Iodine-125 BrachySeed for treating prostate cancer and in August 2000 received FDA approval to market the implant in the U.S.
In September and October 2000, DRAXIMAGE was approved respectively by the U.S. Nuclear Regulatory Commission and the Canadian Nuclear Safety Commission with respect to its BrachySeed implant.
In the period June-July 2001, DRAXIMAGE received FDA and Health Canada regulatory approval for its Palladium-103 BrachySeed.
In the period December 2001 through March 2002, DRAXIMAGE was approved by the FDA to transfer production of its line of lyophilized medical imaging products in-house to the DRAXIS lyophilization production facility.
In January 2003, DRAXIMAGE received FDA approval to produce and market a new radiotherapeutic kit product for the preparation of Sodium Iodide I-131 Capsules and Oral Solution.
Sales and Marketing
At the present time, DRAXIMAGE's products are marketed primarily in the U.S. and Canada. As many of the products marketed by DRAXIMAGE have global approvals, it is expected that non-North American based revenues will gain greater prominence in the future. The most active growth areas are expected to be Europe and South America.
DRAXIMAGE sells Tc-99m Kits in the U.S. through several distributors including Cardinal Health, Inc., which operates the largest chain of radiopharmacies (170 U.S. outlets servicing approximately 50% of the market), Tyco Healthcare division of Tyco International Ltd., Amersham Health business of Amersham plc, CIS bio international (a subsidiary of Schering AG) and United Pharmacy Partners Inc.
Tc-99m Kits sold in the U.S. are marketed under the DRAXIMAGE trademark with the exception of selected Tc-99m Kits sold through Amersham Health and CIS bio international, which carry the distributor's trade name.
From December 2000 until January 2003, BrachySeed was distributed in the U.S. on an exclusive basis by Cytogen. In January 2003, DRAXIMAGE notified Cytogen that it was exercising its rights, under its license and supply agreements with Cytogen, to terminate Cytogen's exclusivity with regard to the marketing and distribution of BrachySeed implants in the United States as a result of Cytogen's continued failure to meet the minimum annual sales requirements set out in the agreements. Cytogen subsequently unilaterally terminated each of the license and distribution and supply agreements with DRAXIMAGE.
In April 2003, DRAXIMAGE and Cytogen reached agreement to end the license and distribution and supply agreements and to co-ordinate efforts for an orderly transition of the U.S. brachytherapy business.
In Canada, DRAXIMAGE currently markets most of its products directly to end-users through a co-operative agreement with the Canadian subsidiary of Bristol-Myers Squibb Company, pursuant to which the two companies' sales forces promote the two companies' non-competitive product lines. This arrangement allows for enhanced coverage of the Canadian market while reducing administrative and shipping costs.
DRAXIMAGE markets BrachySeed in Canada directly to hospitals and healthcare buying groups through its own sales force and marketing resources.
24
For many years, DRAXIMAGE has been the primary Canadian supplier of iodine-131 and iodine-125 labelled radiopharmaceuticals, including solutions and capsules used primarily for the diagnosis and treatment of thyroid gland disorders and the diagnosis of kidney and lung dysfunction.
The radiopharmaceutical market in Europe is characterised by strong regional fragmentation which gives the leading market share to the individual manufacturer located in each one of the major countries (i.e. Amersham Health in the UK, Tyco Healthcare in Holland and CIS bio in France). DRAXIMAGE's marketing activities in Europe are presently limited to the distribution of some of its diagnostic products primarily into northern European regional markets.
Research and Development
DRAXIMAGE conducts both basic research on its own products and development work on in-licensed products and technology developed by other firms, predominantly in the biotechnology field. DRAXIMAGE applies its chelating expertise and technologies to link these compounds with radioisotopes to create innovative diagnostic and therapeutic radiopharmaceuticals.
DRAXIMAGE also provides labelling technology for other companies for use with monoclonal antibodies and peptides. The Company is also working on the development of novel therapeutic uses of radioactivity.
DRAXIMAGE personnel have extensive experience developing and optimising formulations applicable to the lyophilization manufacturing processes, used in the production of cold Tc-99m Kit products.
Employees
As at December 31, 2002 DRAXIMAGE had 72 employees broken down as follows: general management and administration 13, quality operations 14, manufacturing 30, and research and development 15.
Patents
Most of DRAXIMAGE's products are covered by patents held by either DRAXIMAGE or licensed in from third parties. DRAXIMAGE has numerous patents issued and allowed and patent applications pending in the United States, Canada, Europe and other selected countries. For example, DRAXIMAGE has various U.S. issued patents related to chelates for radiopharmaceutical applications, and process patents for the preparation of certain radiopharmaceuticals. In addition, DRAXIMAGE has licensed from licensors certain U.S. patents covering its pipeline products and products already in the United States market, such as BrachySeed.
DRAXIMAGE also relies on trade secrets, know-how and other proprietary information to protect its current products and technologies. To protect DRAXIMAGE's rights in these areas, it requires all licensors, licensees and significant employees to enter into confidentiality agreements. There could be no assurance, however, that these agreements will provide meaningful protection to DRAXIMAGE's patents, trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure of such patents, trade secrets, know-how or other proprietary information.
Contract Manufacturing (DRAXIS Pharma Inc.)
DPI is a contract pharmaceutical manufacturer with capabilities in a broad range of dosage forms, specializing in liquid and lyophilized (freeze-dried) injectables and other sterile products. Operating out of a cGMP-compliant 247,000 square-foot facility located in Montreal, Canada, DPI manufactures pharmaceutical products for DRAXIMAGE, as well as for over 15 other pharmaceutical clients for many international jurisdictions.
Key business development transactions involving DPI have included:
- •
- May 1998—$11.1 million acquisition of DPI's pharmaceutical manufacturing facility;
- •
- February 2000—Sale of 34.1% equity interest to SGF and DPI senior management for net proceeds of $5.4 million;
- •
- March 2000—Signing of a five-year manufacturing supply agreement with Warner Lambert Consumer Healthcare, Division of Pfizer Canada Inc.; and
25
- •
- December 2001—Signing of a long-term manufacturing and supply agreement for the renewal and expansion of DPI's existing contract manufacturing relationship with GSK.
In April 2002, the Company announced a three-year, $12 million capital plan at DPI including a tripling of the Company's existing lyophilization capacity. The capital plan also includes new sterile manufacturing capabilities to support recently announced contracts, improvements to production line efficiency, and improvements to infrastructure and supporting systems to maintain the DPI facility at the forefront of regulatory compliance.
Since DRAXIS acquired DPI, DPI's revenues have risen from $6.1 million for the eight month period ended December 31, 1998 to $20.9 million for the year ended December 31, 2002.
DPI's business goal is to become a leading supplier of high value-added, high-margin contract manufacturing services. Key components of DPI's strategy to achieve this goal include:
- •
- obtaining and maintaining all required regulatory approvals for DPI's manufacturing facility;
- •
- securing additional manufacturing contracts with existing and new customers;
- •
- focusing on DPI's distinctive capabilities in sterile and lyophilized product manufacturing;
- •
- providing manufacturing and related services to DRAXIS' other businesses; and
- •
- focusing on operational excellence, including regulatory compliance, cost effectiveness and customer service.
26
Overview of Pharmaceutical Contract Manufacturing
The value of the manufacturing portion of the $400 billion global pharmaceutical market was estimated by CIBC World Markets to be $50 billion in 2001, with approximately $13 billion outsourced.
The secondary, or dosage-form, manufacturing segment that is currently outsourced was further estimated to be approximately $5.5 billion with an anticipated near-term growth in the 10%—25% range.
The reasons pharmaceutical companies outsource manufacturing include:
- •
- Productivity benefits—plant utilization rates in the pharmaceutical industry are declining and have created increased need to lower operating costs;
- •
- Moving assets off the balance sheet—contract manufacturers are a solution to high levels of industry assets tied up in under-utilized facilities;
- •
- Leveraging external expertise—third-party contractors provide a logical route to specialist services and key technical niches; and
- •
- Pharmaceutical industry consolidation—continuing mergers will likely force rationalization of manufacturing capacity.
The Company believes that there is currently a significant global shortage of sterile lyophilization capacity and that this sector offers significantly higher margin growth potential for DPI due to its technical complexity and its increasing demand as a favoured dosage form for biotechnology products in particular.
DPI believes that the key competitive factors in the contract manufacturing industry include the reliability of supply, quality of product, strict compliance with governmental regulations, capacity availability, competitive pricing and the technical and manufacturing ability to produce a full range of quantities—from small pilot batches often required for clinical trials to larger commercial quantities.
Competition
DPI competes with pharmaceutical companies with in-house manufacturing capabilities as well as third-party contract manufacturers including: Abbott Laboratories, Boehringer Ingelheim, Cardinal Health, Inc., DPT Laboratories, Haupt Pharma AG, Patheon Inc. and DSM Pharmaceuticals, Inc.
Manufacturing Capabilities
DPI is a customer-driven pharmaceutical contract manufacturing company that is positioned to manufacture a variety of dosage forms. DPI is one of a few existing full-scale pharmaceutical contract manufacturing facilities in Canada that has FDA-approved sterile manufacturing and sterile lyophilization capabilities.
Plant operations are organised into four manufacturing areas, supported by packaging and warehousing and distribution functions.
Lyophilization is the preferred dosage form for a broad range of sterile products that are unstable in liquid form. Lyophilization is a complex process of freeze-drying where a liquid solution is frozen under vacuum and all water is removed, leaving behind a stable dry sterile powder that has a relatively long shelf life and is easily reconstituted into a liquid form prior to use. Products delivered in a lyophilized dosage form include injectable pharmaceuticals, vaccines, biotechnology proteins or peptides and diagnostic products.
DPI's existing sterile manufacturing capabilities were recently enhanced by the addition of sterile lyophilization, which became fully operational and approved by the FDA in the latter half of 2001. This fully automated line includes a washer, depyrogenation tunnel, in-line filling machine, robot loaders and unloaders, the first freeze-drier and capper. This first freeze-drier has a capacity based on 11 square meters (120 square feet) of shelf space while the planned second unit, at twice that capacity, will essentially triple overall capacity once it is operational.
In 2002, the Company announced a three-year, $12 million capital plan for DPI including a tripling of DPI's existing lyophilization capacity, new sterile manufacturing capabilities to support recently announced contracts,
27
improvements to production line efficiency, and improvements to infrastructure and supporting systems to maintain DPI at the forefront of regulatory compliance.
The second lyophilizer, with 24 square metres (254 square feet) of freeze-drying shelf space, will be incorporated into DPI's existing lyophilization facility. The specialized facility was originally designed to readily accommodate this second lyophilizer at a cost significantly less than that of the initial installation with minimal disruption to ongoing production. The two units, both of which are supplied by BOC Edwards, will provide total annual capacity equivalent to five to six million 10 mL-vials of lyophilized product.
DPI has scheduled the installation of the second lyophilizer and an additional autoclave for the latter half of 2003. These major upgrades had originally been planned for December 2002—January 2003, but following a detailed review it was concluded that it would be more appropriate to defer the installations to late 2003. The revised installation schedule is not expected to result in any loss of new business opportunities.
The Sterile Products Department ("SPD") includes preparation and pharmaceutical areas with manufacturing, filling and inspection rooms for the production of injectable liquids in ampoules and vials, topicals and sterile ophthalmic ointments. DRAXIS believes that DPI possesses one of the most modern facilities of its kind in Canada approved for the manufacture of sterile prescription pharmaceuticals for Canada, the United States and other global markets.
Including the new lyophilization line, SPD covers approximately 17,100 square feet and is designed for segregated operations complemented by secure access controls.
Computerised systems are utilised for both topical and automated ointment lines in order to optimise process control. Both of these lines are closed-loop systems to ensure sterile integrity. The sterile ointment system utilises a patented automated system to place tubes on the fillers, thereby minimising human intervention.
The processes that are incorporated in the operation include aseptic manufacturing and filling; terminal sterilization; clean in place ("CIP"); and sterilized in place ("SIP"). The dosage forms/product types manufactured include solutions in ampoules and vials, suspensions in drop dose form and ointments in tubes. The department currently has the capacity to fill vials ranging in size from 5 ml to 30 ml, ampoules ranging in size from 1 ml to 10 ml and 3.5 g tubes.
Ointments, Creams and Liquids
The 16,000 square foot Ointments, Creams and Liquids ("OCL") Department offers substantial flexibility in production scale. Production utilises a gravity-fed system and incorporates segregated wash and clean storage areas for equipment.
Batch capability in the OCL Department varies from 200 to 18,000 litres and incorporates four dedicated packaging lines. Interconnecting tanks can be utilised where required, thereby providing production flexibility. Fully automatic validated clean-in-place systems ensure the purity of each customer's product. Product is pumped to two of the packaging lines and gravity fed to the other two lines. Dosage forms/product types manufactured include creams, ointments, lotions, syrups, shampoos, gels, suspensions and mouthwashes.
The Solid Dosage Department covers approximately 10,300 square feet of space and is comprised of two suites for granulating powders prior to compression and four isolated compression suites where tablets and caplets of various forms and sizes are manufactured. The first granulation suite is designed for large batch blending, granulating and drying; while the second granulation suite is equipped with smaller scale equipment that can be used for small production or pilot batches.
Each granulation and compression suite has its own testing equipment and cleaning area. The rooms are isolated and have purpose-built airflow systems to contain powder.
Dosage forms and product types that are manufactured in this department include tablets in bottles and blisters, caplets in bottles and blisters, and powders.
28
�� The Packaging Department covers approximately 51,000 square feet and incorporates an open space with movable separations that provide a flexible packaging area featuring several packaging lines. Segregated zones are defined for de-boxing, filling and secondary packaging. Bar coding insures complete control of all packaging components.
Within the department there are packaging lines for ointments and creams, lines for tablets (bottles and blisters) and a sterile product automatic inspection and packaging line. One packaging area is dedicated to small-quantity tablet filling. The inspection of finished sterile products includes automated inspection by a leak pinhole detector. An automatic inspection machine has been validated to detect particles in sterile products packaged in vials and ampoules. These products may also be inspected manually.
Several packaging types can be accommodated, including: jars, bottles (glass and plastic), blisters, vials and ampoules. The department handles a wide variety of product types including caplets, tablets, gravity fed creams, ointments and liquids.
Regulatory
Manufacturing operations at DPI, from receipt of raw material and packaging ingredients, through compounding, dosage form processing, filling, labelling, packaging to final product release by quality control are all conducted in accordance with cGMP requirements and other appropriate international regulatory standards. Regular inspections of facilities, production processes, control and validation systems as well as employee training programs are conducted by the Company, by U.S., Canadian and international governmental regulatory agencies and by customer inspection teams throughout the year. The Company maintains a distinct internal team to provide effective liaison with various external inspection groups.
DPI achieved a consistent record of regulatory approvals during 2002 and into 2003 as tangible evidence of its commitment to regulatory compliance and quality operations.
- •
- In October 2001, subsequent to an inspection during mid-2001, the DPI facility was accepted by the FDA to manufacture sterile lyophilized and sterile liquid injectable products.
- •
- In March 2002, DPI received approval from the FDA to transfer the production of previously outsourced DRAXIMAGE sterile lyophilized products to DPI.
- •
- In the second quarter of 2002, U.S. regulatory approval was received for the manufacturing site transfer of an established GSK sterile injectable product destined for the U.S. market.
- •
- In October 2002, DPI was accepted by the FDA to manufacture solid dosage form products, such as capsules and tablets, for the U.S. market.
- •
- In January 2003, DPI received approval from the U.K. Medicines Control Agency to manufacture several products destined for the U.K. and major European markets, including products being transferred to DPI from GSK.
- •
- In March 2003, DPI was accepted by the FDA as an additional manufacturing site for Hectorol Injection (doxercalciferol) under a CBE-30 supplemental application submitted by Bone Care International, Inc. ("Bone Care").
All regulatory, quality control and quality assurance activities at DPI are under the direction of a Director of Quality who reports directly to the president of DPI.
Warehousing and Distribution
The warehousing and distribution facilities of the DPI plant allow additional manufacturing flexibility and efficiency. A five-tier pallet-racking warehouse has full height of 29 feet, covers approximately 48,900 square feet of space and has six shipping and receiving docks. The warehouse has separately locked areas including refrigeration units to control sensitive raw materials and finished goods.
In November 2002, DPI commissioned its new raw material storage and dispensing area, which improved cGMP compliance and provided an improved environment for the handling and dispensing of raw materials. This installation will improve the flow of material and personnel and is equipped with a new ventilation system and state-of-the-art dust containment technology.
29
Customers
DPI manufactures prescription and non-prescription pharmaceutical products for more than 15 pharmaceutical companies (excluding the Company) pursuant to manufacturing supply contracts. Currently, DPI's two major customers are Warner-Lambert Consumer Healthcare, Division of Pfizer Canada Inc. and GSK.
In March 2000, DPI entered into a five-year manufacturing and supply agreement with Warner-Lambert Consumer Healthcare, Division of Pfizer Canada Inc. covering several non-prescription products for the Canadian market including, Polysporin, Sudafed, Actifed and Zincofax. Manufacturing of these products commenced in early 2000.
In December 2001, DPI finalized supply and related agreements with GSK for the renewal and expansion of an existing contract manufacturing relationship between the companies. The products being transferred to DPI are all established, sterile products currently marketed by GSK in multiple international markets. In 2002, an additional, established sterile product was added under this contract. U.S. regulatory approval for the site transfer of this product, the first under the new DPI/GSK contract destined for the U.S. market, was received and commercial production of this product commenced in the second quarter of 2002. During 2002, site transfer and related activities associated with the other GSK products continued at a high level. Manufacturing revenues for these products are expected to ramp-up gradually through 2003 and into 2004.
In the second quarter of 2002, DPI was designated by Axcan Pharma Inc., as a commercial manufacturing site for its lyophilized biological product, Photofrin photodynamic therapy used to selectively palliate, cure or prevent various forms of tumourous cancers.
In the first quarter of 2003, DPI was named by Bone Care as an additional manufacturing site for Hectorol Injection and commercial shipments of the sterile injectable product commenced in March 2003.
Commencing in early 2002, DPI became the FDA-approved manufacturing site for DRAXIMAGE's lyophilized Tc-99m Kits. In addition, the Company intends to qualify DPI as the manufacturing site for Anipryl. DPI also provides warehousing, distribution and related services to DRAXIS Pharmaceutica.
Employees
As at December 31, 2002, DPI had 213 employees consisting of: 37 managerial employees, 70 salaried non-managerial employees and 106 unionised hourly employees. The unionised hourly employees are represented by the United Food and Commercial Workers International Union, Local 291P. The collective agreement between DPI and the union was negotiated early in 1998 with an initial five-year term running from May 1, 1998 to April 30, 2003. Negotiations were initiated with the union in early 2003 for renewal of the collective agreement beyond April 30, 2003. DPI has a good relationship with the union.
During September 2002, Mr. Jim Garner, the Company's Senior Vice President, Finance and Chief Financial Officer, was appointed to the additional position of Acting President of DPI following the resignation of the previous incumbent. DRAXIS subsequently commenced a formal search process to identify candidates for a permanent president of DPI and in May 2003 announced the appointment of Mr. John E.M. Durham as the new President of DPI, effective June 2, 2003.
Equity Partners
In February 2000, SGF and senior management of DPI acquired a non-controlling equity interest through the subscription of treasury shares of DPI for aggregate net proceeds of $5.4 million. As a result of this subscription, the Company's equity interest in DPI was reduced to 65.9%. The Company's current equity interest in DPI is 66.9%.
The shareholders of DPI are governed by the terms of a unanimous shareholders agreement that contains provisions standard to an investment of this nature, such as veto rights on certain decisions, restrictions on disposition of shares, rights of first refusal, piggy back rights and put and call provisions. The Company has call rights, which, if exercised, would assure SGF, depending on the call right being exercised, the fair market value of its shares or the greater of the fair market value of its shares plus a specified premium and of a specified
30
minimum return on its investment. SGF's put rights are exercisable beginning in February 2005 or upon the occurrence of a hostile take-over of the Company. If SGF were to exercise its put rights, it would be entitled to the fair market value of its shares, as determined by an independent business valuator.
SGF's sole shareholder is the Government of Québec. SGF makes equity investments notably in the forest products, metals and minerals, chemicals, petrochemicals and plastics, health, high technology, agri-food, industrial logistics, machinery and equipment, transportation and recreational tourism industries in the Province of Québec. SGF does not seek to take control of entities in which it invests but rather holds only minority interests.
DPI's three-year, $12 million capital plan is being financed through a combination of up to $7.4 million from the Company, SGF and certain management shareholders, up to $3.0 million in debt funding from Investissement Québec and the remaining portion from internally generated funds.
Other Collaboration Agreements
The Company has continuing financial interests associated with its collaboration agreements with Pfizer with respect to Anipryl and GlaxoSmithKline Consumer Healthcare with respect to the SpectroPharm line of products.
Beginning in 1990, the Company expanded on its knowledge and experience with selegiline by initiating directly on its own behalf, as well as through contract research arrangements, studies designed to investigate the potential of selegiline for companion animal use. This initiative ultimately resulted in the formation of its subsidiary DAHI, through which the Company developed and commercialized a companion animal health product, Anipryl.
Anipryl is a selegiline product developed for use in veterinary prescriptive applications, particularly for use in dogs. The two indications for which Anipryl is currently approved are canine Cushing's disease and canine cognitive dysfunction syndrome ("CDS").
Cushing's syndrome refers to increased blood cortisol and the presence of one or more typical clinical signs, such as change in appetite, obesity, frequent urination, abdominal distension, loss of hair, lethargy and other behavioural changes. Canine Cushing's disease is the form of the disorder that is due to primary hyperfunction of the pituitary gland. Approximately 15,000 cases of Cushing's disease are diagnosed each year in Canada and 150,000 in the United States.
CDS, sometimes known as "Old Dog Syndrome," refers to the onset in elderly dogs of behavioural problems unrelated to a generalised medical condition such as neoplasia, infection, or organ failure. Typical signs of this disorder can include: confusion, disorientation, decreased activity, changes in sleep/wake cycles, loss of house training and loss of interest in or ability to interact with its owner and environment. Approximately 150,000 older dogs in Canada and 1.5 million older dogs in the United States are diagnosed with CDS each year.
From March 1991 to November 1996, DAHI's common shares were publicly traded on NASDAQ. In November 1996, the Company took DAHI private in a mandatory share exchange transaction.
In December 1997, the Company entered into an alliance with Pfizer whereby Pfizer was granted a perpetual exclusive license to market, sell and distribute Anipryl in exchange for non-refundable fees, royalties based on the worldwide sales of Anipryl, a manufacturing and supply agreement and a research collaboration.
In December 1999, the Company and Pfizer Inc. amended the terms of the alliance (the "First Amendment") whereby $9.0 million of potential additional non-refundable fees were eliminated in exchange for the Company receiving additional regulatory support for a potential new indication and additional manufacturing data. These potential additional non-refundable fees would have become payable if Pfizer had exercised its rights to acquire product registrations following regulatory approval of Anipryl in designated European countries.
In April 2001, the Company received a payment of $1.5 million in respect of minimum royalty entitlements for the first three-year period ended December 31, 2000.
In December 2001, the Company and Pfizer Inc. further amended the term of the alliance (the "Second Amendment") whereby the Company received a payment of $3.1 million in respect of minimum royalty entitlements for the second and third three-year periods ended December 31, 2001 and 2002 and modifications to future royalty entitlements. The Second Amendment also resulted in all rights to Anipryl outside of North
31
America reverting back to the Company, forfeiture of any additional minimum royalty entitlements and the termination of any future collaborative research on new indications or formulations for Anipryl.
Up to December 31, 2002, the Company received a total of $28.1 million in non-refundable fees and $12.5 million in royalties and royalty-related payments.
Under the amended arrangement, the Company will not be entitled to receive any additional non-refundable fees but will continue to earn royalties on Pfizer's sales of Anipryl in the United States and Canada. The $28.1 million of non-refundable fees already received from Pfizer have been deferred and are being recognised as revenue on a straight-line basis over the period to December 31, 2006.
The Company currently does not intend to pursue any additional or expanded indications for Anipryl.
Anipryl is currently approved for sale in the following jurisdictions:
Canada—the Canadian Bureau of Veterinary Affairs ("BVA") approved Anipryl for the control of clinical signs associated with canine Cushing's disease and CDS in October 1995 and February 1997, respectively;
United States—the FDA granted regulatory approval to market and sell Anipryl in the United States for the treatment of canine Cushing's disease and CDS in June 1997 and September 1997, respectively;
Australia—In December 1998, the National Registration Authority granted regulatory approval to market and sell Anipryl in Australia for the control of clinical signs associated with canine Cushing's disease and CDS;
New Zealand—In May 2000, the New Zealand Animal Remedies Board granted regulatory approval to market and sell Anipryl in New Zealand for the control of clinical signs associated with canine Cushing's disease and CDS; and
Brazil—In October 2000, the Brazilian Ministerio da Agricultura e de Abastecimento granted regulatory approval to market and sell Anipryl in Brazil for the control of clinical signs associated with canine Cushing's disease and CDS.
In 1997, DAHI filed in Europe for regulatory approval of Anipryl by the decentralised procedure. This procedure allows DAHI to file the Anipryl submission in a country of its choice, and to designate five additional member states of the European Union as the countries that will review and approve the regulatory submissions. DAHI has chosen the United Kingdom as the country in which to file the initial Anipryl applications. Following approval to market Anipryl in the UK, filings will be made in the five member states. These five member states normally have 90 days to respond to these submissions. Following their approval of Anipryl, the UK Veterinary Medicine Directorate will act as DAHI's advocate in this procedure.
DRAXIS may continue to seek veterinary regulatory approvals for Anipryl in other jurisdictions, as appropriate.
Sales and Marketing
Under the terms of the amended arrangement, Pfizer will continue to market and sell Anipryl in the United States and Canada.
DRAXIS intends to seek Anipryl marketing partnerships for countries outside North America.
Manufacturing
DAHI's primary supplier of selegiline for the production of Anipryl is Chinoin, and DAHI purchases its supply of selegiline under a supply agreement with Chinoin dated October 1, 1990, as amended (the "Chinoin Supply Agreement"). In 1995, DAHI developed the data required to qualify an alternative source of supply for selegiline and the BVA and the FDA have accepted the data.
Under its supply agreement with Pfizer, DAHI is entitled to designate a third-party supplier or to manufacture Anipryl itself in a qualified facility.
Until recently, Anipryl was produced by a third-party contract manufacturer. During 2000, Pfizer qualified one of its own facilities to manufacture Anipryl for the North American marketplace.
32
The Company intends to qualify DPI to manufacture Anipryl upon receipt of regulatory approval to transfer the manufacturing site to DPI. The application for the site transfer has been submitted and the transfer is expected to be completed during 2003.
Competition
The animal health marketplace is served, generally, by veterinary, agricultural or animal health divisions of large international pharmaceutical and chemical companies that are involved in research and development activities. Products resulting from their activities, may, in the future, compete directly with Anipryl.
The Company is not aware of any other HPFB, BVA or FDA approved product available at this time that competes directly with Anipryl. In the United States, the 1988 generic animal drug law offers marketing protection from veterinary generic applicants in the United States for a period of five years, until 2002, in the case of Anipryl. However, that law does not prevent other companies from repeating the full clinical New Drug Application process to seek FDA approval for a bio-equivalent product, nor does it prohibit human generic versions of Anipryl from being sold to veterinarians. Any such competitor, including sellers of a human or veterinary generic selegiline, would be subject to DAHI's United States and international patent rights.
No significant competition for Anipryl from products approved for veterinary or human use has been experienced to date in Canada or the United States. However, there are two other treatments available that have not been approved in Canada or the United States to treat canine Cushing's disease but may be used off-label for canine Cushing's disease—Lysodren (mitotane) by Bristol-Myers Squibb Co., which was approved for use in the treatment of human inoperable cancer of the adrenal gland, and Nizoral (ketoconazole) by Johnson & Johnson Inc. These competitive treatments work by selectively killing the outer layer of the adrenal gland, thereby limiting production of corticosteroid. Notwithstanding such off-label uses, the human generic version of Eldepryl is not approved for the treatment of canine Cushing's disease or CDS in Canada, the United States or elsewhere. The dosage required for dogs suffering from canine Cushing's disease and CDS, in most cases, is much higher than the human 5mg dose for selegiline. Veterinarians will have an incentive to prescribe the product actually approved for veterinary use that can be dispensed from their offices. Lastly, patents for the use of Anipryl for the treatment of dogs with conditions including canine Cushing's disease and CDS have now issued in Canada, the United States and other jurisdictions. DAHI is positioned to enforce its proprietary patent rights and defend itself against infringement by other parties.
Ceva Santé Animale S.A. ("Ceva") currently markets a veterinary selegiline product under the brand name Selgian in certain European countries for treating behavioural problems of emotional origin in dogs. The Company believes that Ceva's product may infringe its patent rights in Europe. See Patents.
Patents
In September 1992, the United States Patent and Trademark Office issued a patent to DAHI entitled "Use of l-deprenyl for Retention of Specific Physiological Functions." The patent claims specific uses of l-deprenyl for use in treating dogs. Similar patents have also issued to DAHI in Australia, New Zealand and at the European Patent Office. Six additional United States patents have also issued to DAHI. These patents cover various veterinary pharmaceutical uses of l-deprenyl. In March 1996, DAHI received from the United States Patent and Trademark Office a Notice of Allowance covering Anipryl to extend the life expectancy of dogs. DAHI has filed similar patent applications in Canada, Japan and other jurisdictions. In February 1997, the Canadian Intellectual Property Office issued a patent to the Company for the use of Anipryl in the treatment of dogs with conditions including canine Cushing's disease and CDS. In March 1997, the European Patent Office ("EPO") issued a patent to DAHI for the use of Anipryl for retarding the normal age-dependent deterioration of the cognitive process in dogs, also known as CDS.
The European CDS patent is subject to an opposition procedure initiated by Ceva. DAHI submitted its response to the opposition in the second quarter of 1998 and made further submissions in 1999 and 2000. In December 2001, the EPO on oral hearing upheld DAHI's European CDS patent. Ceva has appealed this ruling.
In August 1996, the United States Patent Office issued a patent to Ceva relating to the use of selegiline for treating behavioural disorders with change of mood in dogs and cats. A similar patent application was filed in 1994 with the Canadian Intellectual Property Office. A similar patent application in Europe was allowed in April 2000. Ceva's European patent is subject to an opposition procedure by DAHI. In December 2001, the EPO upheld Ceva's patent. DAHI has appealed this ruling.
33
In May 2000, the Company entered into an arrangement with Block Drug Company (Canada) Limited, now part of GlaxoSmithKline Consumer Healthcare, with respect to the SpectroPharm line of non-prescription dermatology products, which included the sale of product rights by the Company in exchange for a non-refundable fee, the acquisition of inventory on hand, a supply agreement and a technical services arrangement. The $8.2 million received by the Company in respect of the SpectroPharm product rights has been deferred and is being recognised as revenue on a straight-line basis over the period to January 31, 2005.
Discontinued Operations (DRAXIS Pharmaceutica)
In January 2002, DRAXIS announced it had entered into a binding letter of intent with Elan Corporation, plc ("Elan") wherein Elan agreed to acquire substantially all of the operations, product rights and other assets and obligations of DRAXIS Pharmaceutica. In June 2002, the terms of the proposed sale were modified to exclude the Canadian rights to Alertec, a currently marketed product, plus non-marketed Hectorol and paclitaxel. However, in August 2002, Elan notified DRAXIS that it would not proceed with the purchase of DRAXIS Pharmaceutica. In March 2003, DRAXIS amended its License, Distribution and Supply Agreement with affiliates of Elan to return to Elan the Canadian rights for several of Elan's neurology products that were yet to be approved in Canada in exchange for a cash payment to DRAXIS of $6.5 million. DRAXIS believes that this transaction will facilitate the divestiture of the remaining assets of DRAXIS Pharmaceutica.
As options regarding potential divestiture of assets are explored, the Company will continue to manage and operate DRAXIS Pharmaceutica to enhance shareholder value consistent with the Company's strategic focus on its core radiopharmaceutical and specialty contract manufacturing businesses.
Commencing with the quarter ended December 31, 2001, the results of operations of DRAXIS Pharmaceutica have been reported as discontinued operations. Commencing in the second quarter of 2002, discontinued operations no longer include revenues and expenses directly attributable to Alertec.
DRAXIS Pharmaceutica markets and sells in-licensed pharmaceutical products in Canada with a current focus in neurology.
DRAXIS Pharmaceutica has focused primarily on innovative pharmaceutical products in specialty therapeutic areas where it could leverage its experience in regulatory affairs, the management of clinical trials, interfacing with the Patented Medicine Prices Review Board ("PMPRB"), private and public reimbursement, and pharmaceutical marketing and sales.
Key business development transactions involving DRAXIS Pharmaceutica have included:
- •
- July 1996—$0.7 million acquisition of TICAN Pharmaceuticals Inc. and its portfolio of prescription and non-prescription dermatology products;
- •
- February 1997—$6.8 million acquisition of Spectro-Pharm Inc. including five non-prescription dermatology products;
- •
- September 1997—signing of a framework agreement with Mylan Pharmaceuticals Inc. ("Mylan") for an ongoing collaboration pursuant to which DRAXIS Pharmaceutica would introduce in Canada Mylan products identified by the parties from time to time that fit the Company's strategic niches. The only product currently included under this arrangement is Mylan's formulation of the anti-cancer drug paclitaxel;
- •
- May 1998—renewal of an exclusive licensing agreement with Eli Lilly Canada Inc. ("Lilly") with respect to the Parkinson's drug Permax for a licensing fee of up to $6.1 million paid over a 10-year period;
- •
- June 1999—licensing of the exclusive Canadian rights to eight neurology products from Elan for an up-front licensing fee of $12.0 million;
- •
- May 2000—divestiture of the Company's dermatology product lines for aggregate net proceeds of $9.0 million including the sale of the SpectroPharm line of products to Block Drug Inc. (now part of GlaxoSmithKline Consumer Healthcare).
Overview of Canadian Pharmaceutical In-Licensing Market
Due to financial and other considerations, multinational pharmaceutical companies may elect not to market directly certain of their products in Canada. In those situations, multinationals often choose to out-license their
34
products to local firms that in turn, engage in marketing and sales activities. DRAXIS Pharmaceutica established its business to benefit from such out-licensing activities.
DRAXIS Pharmaceutica competes in its niche markets with various integrated and non-integrated pharmaceutical companies that license and distribute prescription drugs in Canada. Companies with which DRAXIS Pharmaceutica may directly compete for Canadian product rights include Biovail Corporation and Paladin Labs Inc. Many of the division's competitors have significant financial and other resources, experience and expertise in research and development, manufacturing, testing, obtaining regulatory approvals, marketing and distribution of pharmaceutical products in the Canadian marketplace.
Marketed Products
The following products are marketed in Canada by DRAXIS Pharmaceutica:
| |
| |
|
|---|
Product
| | Indication
| | Licensing Partner
|
| Permax | | Parkinson's disease | | Lilly |
| Alertec | | Narcolepsy | | Lafon/Cephalon |
| Zanaflex | | Spasticity | | Elan |
| Diastat | | Epilepsy | | Xcel |
| Mysoline | | Anti-convulsant | | Elan |
| Eldepryl | | Parkinson's disease | | Somerset |
| Novo-Selegiline | | Parkinson's disease | | Novopharm |
Description—Permax (pergolide mesylate) is a D1 and D2 dopamine receptor agonist that is used either alone or as adjunctive therapy to levodopa in the management of Parkinson's disease. In contrast to other available dopamine agonists, Permax is the only long acting dopamine agonist that stimulates both D1 and D2 receptors.
Licensing Partner—In 1994, DRAXIS acquired an exclusive sublicense from Lilly to market Permax in Canada. In May 1998, DRAXIS renewed its exclusive sublicense with Lilly. Pursuant to the renewal, Lilly will continue to manufacture Permax and to supply it exclusively to DRAXIS for marketing and distribution in Canada for a further 10-year period expiring on December 31, 2008, with automatic yearly renewals thereafter.
Description—Alertec (modafinil) is a non-amphetamine that improves wakefulness without significant cardiovascular effects in patients with narcolepsy. In numerous controlled clinical trials, which have been supported by over four years of commercialisation in North America, Alertec has demonstrated a high degree of clinical efficacy and an excellent safety profile. Narcolepsy is a primary sleep disorder characterised by uncontrolled episodes of falling asleep at unexpected times and under unexpected conditions. Prior to the 1999 approval of Alertec, no treatment for narcolepsy had been approved in Canada since 1959. Alternative therapies for the treatment of narcolepsy, such as amphetamine-like stimulants, may have undesirable side effects such as overstimulation, nervousness and insomnia. In addition, such alternative therapies also have proven abuse potential and the development of tolerance, which results in increasing dosages to maintain therapeutic effectiveness. The Notice of Compliance recommended that Alertec be considered a controlled drug.
Licensing Partner—In November 1992, DRAXIS entered into a license agreement with Laboratoire L. Lafon ("Lafon") for the right to market in Canada any product containing the compound modafinil. DRAXIS has agreed to purchase from Lafon all quantities of modafinil that it may require during the term of the agreement, and Lafon has agreed to supply such product or allow DRAXIS to take over the manufacture of such product. The agreement terminates in Canada 15 years after the date of the last HPFB approval obtained for a dosage form of and/or a new indication for Alertec in Canada. In 2001, Lafon was acquired by Cephalon, Inc.
Description—Zanaflex (tizanidine hydrochloride) is a short-acting alpha-2 adrenergic agonist indicated for the management of spasticity. Spasticity, a common complication of central nervous system disorders such as multiple sclerosis, spinal cord injury, stroke, cerebral palsy and brain injury, is characterised by abnormal increases in muscle tone (tension). The condition causes stiffness or rigidity, restricting normal movement and produces painful
35
muscle spasms that are frequently debilitating. The Multiple Sclerosis Society of Canada estimates that there are approximately 50,000 Canadians with multiple sclerosis and the International Campaign for Cures of Spinal Cord Injury Paralysis (ICCP) further estimates there are 30,000 Canadians with some form of spinal cord injury. It has been suggested (Young, R R et al. Current issues in spasticity management. The Neurologist 1997; 3:261-279) that approximately 67% require medication to control spasticity.
Licensing Partner—Zanaflex is licensed from Elan for a term to December 31, 2008. Under the terms of the license agreement, Elan supplies the manufactured product to DRAXIS and provides liaison assistance with respect to regulatory and marketing issues for the Canadian market.
Description—Diastat (diazepam rectal gel) is a unique formulation of diazepam designed to control breakthrough epileptic seizures in the management of selected refractory patients with epilepsy on stable regimes of anti-epilepsy drugs who require intermittent use of diazepam to control bouts of seizure activity. It is available in the form of a special prefilled, single-dose delivery system that is administered rectally by a parent or caregiver. Epilepsy Canada indicates that approximately 300,000 Canadians suffer from epilepsy or seizure disorders and that approximately 30% of these patients do not obtain satisfactory seizure control with current anti-convulsants.
Licensing Partner—Diastat was originally licensed from Elan for a term to December 31, 2008. In March 2001, Elan transferred, with the Company's consent, the Canadian rights for Diastat to Xcel Pharmaceuticals, Inc., ("Xcel") a speciality pharmaceutical company. Under the terms of this agreement, Xcel assumed Elan's responsibility to supply the manufactured product to the Company and provide liaison assistance with respect to regulatory and marketing issues for the Canadian market.
Description—Mysoline (primidone) is an anti-convulsant drug to treat epilepsy and seizure disorders. Epilepsy, the term used to describe a variety of seizure disorders, is one of the most common disorders of the central nervous system, affecting more than 300,000 Canadians according to Epilepsy Canada. Characterised by the tendency to have recurrent seizures, epilepsy is when the electrical balance in the brain is disturbed. When this occurs, the corresponding physical reaction to the increased electrical activity is the seizure.
Licensing Partner—Mysoline is licensed from Elan and had previously been marketed in Canada by another pharmaceutical company. DRAXIS assumed responsibility for marketing and selling Mysoline in Canada in January 2000 with Wyeth Ayerst Canada Inc. supplying product and Elan providing liaison assistance for regulatory and marketing issues. The product is no longer manufactured in Canada and sales are not expected to continue once existing inventory is depleted, which is expected near the end of 2003.
Description—Eldepryl (selegiline hydrochloride) is a selective monoamine oxidase-type B ("MAO-B") inhibitor used in the treatment of Parkinson's disease. MAO-B is an enzyme that degrades the structure of certain compounds, such as dopamine, which regulate various physiological functions, including certain aspects of central nervous system activity. Dopamine is a neurotransmitter that facilitates movement, posture, balance and walking. Eldepryl has several common or generic names, including selegiline hydrochloride, selegiline and l-deprenyl.
Licensing Partner—Under a sublicense agreement dated February 9, 1988 (the "Somerset License") with Somerset Pharmaceuticals, Inc., DRAXIS acquired the exclusive right to market Eldepryl in Canada. The Somerset License will expire upon the expiration of Somerset's license with Chinoin, which expires on November 22, 2003 and may be renewed for successive five-year terms with the agreement of the parties thereto.
Description—Novo-Selegiline (selegiline hydrochloride) is a generic, selective MAO-B inhibitor used in the treatment of Parkinson's disease and was developed in partnership with Novopharm Limited ("Novopharm") (now beneficially owned by Teva Pharmaceutical Industries Ltd.) in response to the genericization of the Canadian selegiline hydrochloride molecule. Novo-Selegiline competes for selegiline prescriptions with Eldepryl and five other generic selegiline products. Including sales of Eldepryl, the Company estimates that DRAXIS has approximately 50% of the Canadian selegiline market.
36
Licensing Partner—In December 1993, DRAXIS entered into a five-year distribution agreement with Novopharm with respect to the sale of Novo-Selegiline. Under the agreement, DRAXIS currently records all sales of Novo-Selegiline as revenues and retains 50% of the net profit therefrom. In addition, Novopharm retains responsibility for all aspects of the Canadian marketing and distribution of Novo-Selegiline as the Company's sole Canadian distributor. In September 1998, Novopharm agreed to extend the agreement on the same terms and conditions for a further five years from its expiry date in December 1998.
Recently Approved Unlaunched Products
DRAXIS currently has Canadian regulatory approval for, but has not yet commercially launched the following products:
Product
| | Indication
| | Licensing Partner
|
|---|
| Hectorol | | Secondary hyperparathyroidism | | Bone Care |
| Levulan Kerastick | | Actinic keratoses | | DUSA |
Description—Hectorol (doxercalciferol, a synthetic D-hormone analogue derived from Vitamin D2) is indicated for the reduction of elevated parathyroid hormone ("PTH") levels in the management of secondary hyperparathyroidism in patients undergoing chronic renal dialysis. The consequences of poor PTH management are often severe, and in some long-term cases, life threatening. Elevated PTH contributes directly to many of the pathophysiological conditions seen in chronic renal failure patients. Elevated PTH plays a role in the development of left ventricular hypertrophy, myocardial fibrosis and abnormal lipid metabolism. Elevated PTH levels have also the well-known negative effect on bone growth and turn over, conditions collectively referred to as renal osteodystrophy.
Recent Developments—In May 2001, DRAXIS obtained from the HPFB a Notice of Compliance approving the oral formulation of Hectorol in the treatment of secondary hyperparathyroidism. Product is available through wholesale suppliers in Canada but to date the formal market launch has not been initiated.
Licensing Partner—In March 1990, Bone Care granted to DRAXIS an exclusive Canadian license to market Hectorol for treating osteoporosis. In March 1996, the license was extended to all other metabolic bone diseases, including renal osteodystrophy and secondary hyperparathyroidism. The license also covers all know-how developed by or on behalf of Bone Care relating to the use of Hectorol for those indications. The initial term of the license agreement expires on the expiration of the last of all Canadian patent application and patents issuing therefrom which Bone Care owns or controls related to Hectorol for all indications licensed to the Company. The term renews automatically thereafter from year to year unless terminated under certain conditions. Under its license agreement with Bone Care, Bone Care is required to provide reasonable technical and other assistance, including all data and information Bone Care submits to the FDA and any other regulatory agency to support its request for such approval in the U.S. DRAXIS has agreed to purchase its requirements of Hectorol from Bone Care.
37
Description—The information set forth in this paragraph and the third paragraph below is derived from public information disclosed by DUSA. DUSA, formerly an affiliate of DRAXIS, has the world-wide rights to make, have made, use and sell products capable of producing protoporphyrin IX precursors, including 5-aminolevulinic acid ("ALA") for administration with subsequent exposure to controlled lights under a license (the "PARTEQ License") from PARTEQ Research & Development Innovations, the licensing arm of Queen's University, Canada. Under the terms of the PARTEQ License, DUSA assumed responsibility for clinical development and regulatory submissions relating to commercial exploitation of such products. In general, the methods and processes employing ALA with light to treat or detect a variety of conditions are known as photodynamic therapy ("PDT") or photodetection ("PD"). PDT/PD is a two-step treatment. The first step involves the application of a drug (termed a photosensitiser) that collects in specific cells. The second step involves the activation of the photosensitiser by controlled exposure to a selective light source. In PDT, energy from the light activates the photosensitiser, which destroys or alters the sensitised cells. In PD, the activated photosensitiser emits energy in the form of light, making the sensitised cells fluoresce, or "glow." In PDT, the appropriate combination of drug and light can affect target tissue with relatively minimal damage to surrounding tissue. See DUSA public disclosure for current patent information.
Recent Developments—In June 2001, DRAXIS obtained from the HPFB a Notice of Compliance granting approval to market Levulan Kerastick Photodynamic Therapy (PDT) in Canada for the treatment of Actinic Keratoses.
Licensing Partner—In October 1991, DUSA, PARTEQ and DRAXIS entered into an agreement (the "Assignment Agreement") under which DUSA assigned to DRAXIS its rights and obligations under the PARTEQ License insofar as they relate to Canada. In addition, DUSA has agreed to disclose to DRAXIS, on an ongoing basis, any technology available to DUSA and relating to the subject matter of the PARTEQ License that would assist DRAXIS in developing the Canadian market. As of March 11, 1998, DUSA and PARTEQ amended and restated the PARTEQ License (the "Amended PARTEQ License"). DUSA has the right to terminate the Amended PARTEQ License with or without cause upon 90 days' notice. The Amended PARTEQ License is effective through the expiration date of the latest United States patent made subject to the Amended PARTEQ License. PARTEQ, DUSA and DRAXIS revised the Assignment Agreement to conform its terms to the Amended PARTEQ License.
Principal Markets
DRAXIS Pharmaceutica markets products in Canada only.
Employees
As at December 31, 2002, DRAXIS Pharmaceutica had 16 employees broken down as follows: general management and administration 4, new product development 2, marketing 4, and sales 6.
Distribution
DPI provides warehousing, distribution and related services to DRAXIS Pharmaceutica.
Patents
Patents have been issued, or applied for, in Canada covering many of the products and technologies licensed from strategic partners, including those that are under development. A number of the Company's strategic partners have also filed numerous product patent applications in several other countries in relation to the products marketed by DRAXIS Pharmaceutica.
Government Regulation
The Company's business is governed by a variety of industry-specific statutes and regulations in Canada, the United States and other countries.
38
Drug Approval Process
In Canada, pharmaceutical research, development and marketing activities are regulated by the Food and Drugs Act (Canada) and the rules and regulations made under that Act. The Food and Drugs Act (Canada) is administered under the Therapeutic Products Programme of the HPFB, which regulates the use and sale of diagnostic and therapeutic products in Canada. Regulations imposed by federal and local authorities in the United States, where regulation is carried out by the FDA, also significantly regulate the Company's activities.
In Canada, clinical trials of new pharmaceutical products involve three phases, conducted under what is known as Investigational New Drug Submissions. In Phase I, the product's safety is assessed during clinical trials involving healthy volunteers. In Phase II, the product's efficacy, dosage and safety are tested on a small number of patients with known disease. In Phase III, controlled clinical trials are conducted in which the product is administered to a larger number of patients with known disease, and in which further information relating to the safety and efficacy is gathered. Further, in Phase III, the effectiveness of the product is, in certain cases, compared to that of accepted methods of treatment. If clinical studies establish that the product has value, an applicant files a New Drug Submission with the HPFB to obtain marketing approval for the product. The New Drug Submission includes a comprehensive summary and analysis of the results of the clinical trials, information relating to proposed labelling and packaging materials, and data relating to the proposed manufacturing and quality control procedures. If the New Drug Submission is found to be satisfactory, the HPFB issues a Drug Identification Number and a Notice of Compliance permitting sale of the new product in Canada.
In the United States, new drugs require FDA approval of a marketing application (i.e., a New Drug Application or a Product License Application) prior to commercial sale. To obtain marketing approval, data from adequate and well-controlled clinical investigations, demonstrating to the FDA's satisfaction a new drug's safety and effectiveness for its intended use, are required. Such data is generated in studies conducted under an Investigational New Drug Application. Clinical studies are characterised as Phase I, Phase II and Phase III trials. In a marketing application, the applicant must also demonstrate the identity, potency, quality and purity of the active ingredients of the product involved and the stability of these ingredients. Further, the manufacturing facilities, equipment, processes and quality controls for the new drug must comply with prescribed cGMP for drugs or biologic products, both in a pre-licensing inspection and in subsequent periodic inspections after licensing. Failure to demonstrate that a drug can be commercially manufactured in accordance with strict regulatory guidelines will preclude approval of the drug. Regulatory approvals must be received before moving the production of a product to a new manufacturing site, and such approvals may cause delays in the production, supply and marketing of a product.
The process of completing clinical trials and obtaining regulatory approvals for a new drug will, in general, take a number of years and may require the expenditure of substantial resources. Once a New Drug Application/New Drug Submission or Product License Application is submitted, there can be no assurance that the HPFB or FDA will review and approve the application in a timely manner. In certain limited circumstances, the HPFB will permit a New Drug Submission to be subject to a priority review. The HPFB's Priority Review Process allows for a faster review to make available promising drug products for life-threatening or severely debilitating conditions for which there are few effective therapies already on the market. Even after initial approval has been obtained, further studies, including post-marketing studies, may be required to provide additional data on safety necessary to gain approval for the use of the product as a treatment for clinical indications other than those for which the product was initially tested. The HPFB and FDA may also require post-marketing surveillance programs to monitor a product's side effects. Results of post-marketing programs may limit or expand the further marketing of products. A serious safety or effectiveness problem involving an approved new drug may result in HPFB or FDA action requiring withdrawal of the product from the market and possible civil action.
The Special Access Program of the Therapeutic Products Directorate of HPFB is responsible for authorizing the sale of pharmaceutical, biologic and radiopharmaceutical products that are not yet approved in Canada to treat patients in emergencies who have a serious or life-threatening illness when conventional therapies have failed or are unsuitable.
Medical devices such as DRAXIMAGE's BrachySeed do not require the detailed approval process for therapeutic drugs. If the device is "substantially equivalent" to one currently marketed, as is the case with BrachySeed, then the approval period can be as short as 90 days since no clinical trials are required. In the United States this process is called a 510-K approval.
39
Outside of Canada and the United States, the regulatory approval process for the manufacture and sale of pharmaceuticals varies from country to country and the time required may be longer or shorter than that required for HPFB or FDA approval. To the extent it chooses to explore foreign markets, the Company may rely on foreign licensees to obtain regulatory approval for marketing its products in foreign countries.
The drug approval process for veterinary pharmaceuticals is similar to the process for obtaining approvals for human pharmaceuticals. To receive regulatory approval, a new animal drug must successfully complete a number of development phases. The phases include establishing safety and efficacy in target species as well as the establishment of manufacturing procedures and final product labelling. The final phase of this process includes controlled clinical trials in which the drug is administered to a larger number of such animals, and in which further information relating to safety and efficacy is gathered. Following the clinical trials, the drug sponsor submits an application to the appropriate regulatory agency for marketing approval.
Drug Marketing
Prescription drug products generally are made known by manufacturers through advertisements to healthcare professionals and visits to such professionals known as "detailing." Product-specific advertising to the general public is permitted, subject to certain regulations and industry guidelines.
An increasing percentage of sales of prescription pharmaceuticals relate to sales of products which are paid, in whole or in part, by government or private insurance drug plans. Many governments have established regimes to control drug pricing at the retail pharmacy level. In addition, there have been, and the Company expects that there will continue to be, an increasing number of proposals to implement government and other third-party payer restrictions upon the pricing of prescription pharmaceuticals as a result of continuing efforts to contain or reduce the costs of healthcare throughout North America. See Item 3: Key Information—Risk Factors.
In most provinces of Canada, there is a drug benefit formulary. A formulary lists the drugs for which a provincial government will reimburse qualifying persons and the prices at which the government will reimburse such persons. There is not complete uniformity between provinces; however, provincial governments generally will reimburse the lowest available price of the generic versions of any drug listed on the province's formulary list. The formularies can also provide for drug substitution, even for patients who do not qualify for government reimbursement. The effect of these provincial formulary regimes is to encourage the sale of lower-priced generic versions of pharmaceutical products.
In the case of new veterinary "prescription" pharmaceuticals, no sales may be made by drug manufacturers directly to the public. Instead, a prescription drug is initially available only through veterinarians who generally sell it from their offices. Veterinary prescription drugs generally are promoted by manufacturers through advertisements to veterinarians and sales visits to animal health clinics. Product-specific advertising to the general public is permitted, subject to certain regulations and industry guidelines.
There are no government price controls or reimbursement plans in the veterinary pharmaceutical marketplace. There are a few private pet insurance plans; however, these plans do not represent a significant portion of the veterinary market. Accordingly, the veterinary marketplace is not subject to the cost containment measures that are prevalent in the human pharmaceutical market.
Patent Protection and Price Controls
Companies that have invented human or veterinary drugs can apply for patent protection virtually world-wide, subject to strict rules relating to timing, subject matter and the scope of protection sought. Patents can cover many aspects of a pharmaceutical product, including the drug itself, processes for preparing the drug, delivery systems and new uses. Patents do not, however, guarantee that the owner of the patent or its licensee can utilise the patented invention because there may be pre-existing governing rights. While a patent permits the owner or its licensee to prevent others from doing what is covered by the patent, competitors are always free to market products that do not infringe the particular patent, provided such competitors otherwise comply with health regulatory requirements.
40
Historically, pharmaceutical companies have relied heavily upon patents to protect proprietary positions on drug products. The Company's policy is to protect its technology, inventions and improvements by, among other things, filing patent applications for technology it considers important to the development of its business. The Company also relies upon trade secrets, know-how and licensing opportunities to develop and maintain its competitive position.
Under United States patent law, with respect to inventions made prior to January 1, 1996, a patent is issued to the person who made the invention first, rather than to the first person to file an application thereafter, as is common in other countries. In determining who is entitled to a United States patent on a particular technology, events in the United States prior to January 1, 1996 may be important.
The Patent Act (Canada) provides remedies for patent infringement. In addition to the standard legal action for patent infringement, in 1993, the Canadian government enacted Regulations under the Patent Act (Canada) whereby a competitor proposing a generic version for a drug which has been marketed in Canada under a Notice of Compliance must serve a Notice of Allegation on the originator of the drug before the competitor may be granted a Notice of Compliance in respect of its generic drug. The originator of the drug may apply to the Federal Court of Canada for an order prohibiting the Minister of National Health and Welfare from issuing a Notice of Compliance until the issue of patent infringement has been resolved.
The Canadian Government has also established the PMPRB, which monitors and controls prices of patented drug products marketed in Canada by persons holding, or licensed under, one or more patents relating to drug products. For a patented drug product, the PMPRB will approve an introductory price (based on a comparative analysis) and will require that the price not be increased each year thereafter by more than the annual increase of the Canadian Consumer Price Index. As such, the existence of one or more patents relating to a drug product, while providing some level of proprietary protection for the product, also triggers a governmental price control regime which significantly impacts on the Canadian pharmaceutical industry's ability to set pricing.
Drug Manufacturing
Pharmaceutical companies are required to submit as part of their New Drug Submission in Canada, or as part of their New Drug Application in the United States, detailed descriptions regarding the proposed manufacturing and packaging process and manufacturers in respect of a particular drug. As a result, a decision to manufacture or package products in a facility other than that originally approved under the New Drug Application or New Drug Submission (as may be the case with contracting manufacturing outsourcing), can result in significant delays in production.
Pharmaceutical manufacturing facilities are subject to strict quality control standards including cGMP. Production processes within a facility are subject to one-time validation testing, as well as periodic review. In the case of sterile product manufacturing, including lyophilized products, the standards are even higher than for the manufacturing of ointments, creams and liquids. The manufacture of radioactive drugs is subject not only to cGMP but also the environmental safety, handling and transportation requirements of the Canadian Nuclear Safety Commission ("CNSC") and the United States Nuclear Regulatory Commission ("NRC"). There are no issues with respect to radioactive waste disposal since all of the isotopes used in nuclear medicine are short-lived and can be easily stored on site until decayed and then disposed of.
The FDA, HPFB, CNSC and NRC conduct regular audits of the Company's facilities to ensure compliance with cGMP and other statutory requirements. See Item 3: Key Information—Risk Factors.
41
Organizational Structure
The following chart illustrates the corporate organization and jurisdictions of the Company and its significant affiliates as at April 14, 2003:
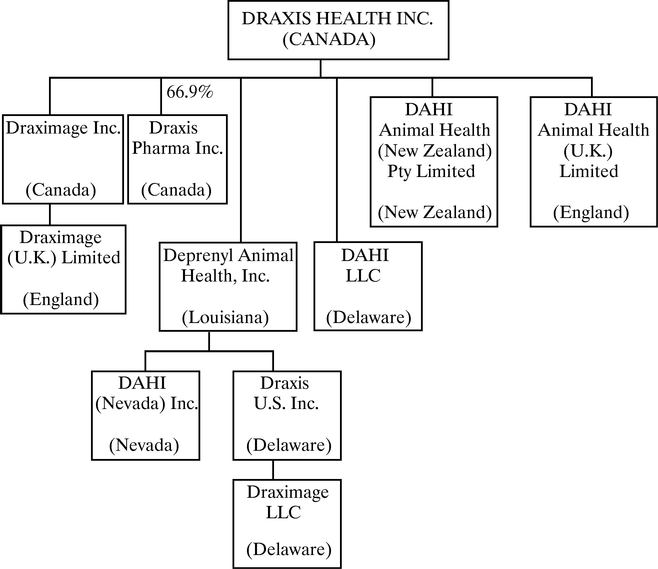
All of the above-depicted companies are wholly owned subsidiaries of DRAXIS Health Inc. except for Draxis Pharma Inc., 66.9% of the shares of which are held by DRAXIS Health Inc.
Property, Plants and Equipment
The Company's operating facility is its owned 247,000 square foot pharmaceutical manufacturing facility which houses DRAXIMAGE and DPI. The facility is located at 16751 Trans-Canada Road, Kirkland, Quebec, Canada, H9H 4J4.
The Company believes all of the above-noted properties are suitable and adequate for its business purposes as presently contemplated.
For information concerning the Company's capital expenditures and methods of financing, please see Item 5: Operating and Financial Review and Prospects.
Item 5. Operating and Financial Review and Prospects
The following discussion and analysis of the Company's financial condition and results of operations should be read in conjunction with the Company's consolidated audited financial statements and notes thereto for the year ended December 31, 2002.
All amounts referred to herein are expressed in U.S. dollars and are in accordance with U.S. generally accepted accounting principles ("GAAP"), unless otherwise indicated.
DRAXIS is a specialty pharmaceutical company focused on the development, production, marketing and distribution of radiopharmaceuticals through DRAXIMAGE and the provision of pharmaceutical contract manufacturing services, specializing in liquid and freeze-dried injectables and other sterile products through DPI.
42
The Company believes that its radiopharmaceutical business, acquired in 1997, and its manufacturing business, acquired in 1998, have significant long-term growth potential. Accordingly, the Company has invested considerable financial and management resources toward the development of these businesses including:
- •
- $11.9 million invested at DPI prior to 2002 included a plant expansion and the establishment of sterile lyophilization (freeze-drying) capabilities to provide in-house manufacturing of the Company's radiopharmaceutical imaging kits as well as third-party contract manufacturing services;
- •
- In 2002, DPI initiated a three-year $12.0 million capital plan, including a tripling of lyophilization capacity;
- •
- $6.5 million has been invested to date in the construction and subsequent expansion of DRAXIMAGE's radiopharmaceutical production facilities and capabilities; and
- •
- $5.7 million has been invested for research and the development of the DRAXIMAGE product pipeline including: BrachySeed, Fibrimage, Amiscan, and INFECTON.
These initiatives are consistent with the Company's general business strategy of:
- •
- Focusing on specialty pharmaceutical markets in which DRAXIS can develop and sustain competitive advantage;
- •
- Developing new pharmaceutical products and services that exploit the Company's existing strengths, capabilities and intellectual property;
- •
- Pursuing growth opportunities with international market potential; and
- •
- Leveraging alliances with business partners, when appropriate.
The Company's primary operational focus continues to be: (i) improving near-term financial and operational performance of its radiopharmaceutical and manufacturing businesses through increasing sales of existing products and services, improving manufacturing efficiency and effectiveness, and obtaining additional regulatory approvals; and (ii) securing and advancing its base for long-term growth through the development of its existing product pipeline as well as identifying new business opportunities that are consistent with the Company's capabilities and that contribute to the long-term value of the Company.
In 1999, the Company initiated a strategic review of its operations from the perspective of enhancing shareholder value. Following completion of this review, the Company resolved to focus on its radiopharmaceutical and manufacturing platforms. Consistent with this strategic decision, the Company divested its dermatology product lines in 2000 and is continuing to pursue the possible sale of its Canadian prescription pharmaceutical sales and marketing business DRAXIS Pharmaceutica.
The Company achieved a number of significant accomplishments in 2002 including:
- •
- Record financial results from continuing operations:
- •
- Consolidated revenues of $38.6 million, an increase of 14.0% over 2001.
- •
- Consolidated net income from continuing operations before non-recurring items of $0.077 per share, an increase of 24.2% over 2001.
- •
- Consolidated earnings before interest, taxes, depreciation, amortization, research and development and non-recurring items ("EBITDARD") of $8.1 million, an increase of 46.7% over 2001.
- •
- Regulatory approvals of manufacturing facilities:
- •
- U.S. and Canadian regulatory approval to transfer production of DRAXIMAGE's line of lyophilized medical imaging products to the Company's own production facility.
- •
- U.S. regulatory approval to commence production at DPI of an established injectable product for GlaxoSmithKline.
- •
- U.S. regulatory approval to manufacture solid dosage form products at DPI.
- •
- Subsequent to year end, U.K. regulatory approval to manufacture multiple sterile and non-sterile products at DPI.
43
- •
- Continued growth together with the realignment of the Company's U.S. sales and marketing strategy for BrachySeed, the Company's proprietary second generation radioactive brachytherapy implant:
- •
- Increased U.S. and Canadian market acceptance of the iodine version of BrachySeed which was launched in 2001.
- •
- U.S. launch of the palladium version of BrachySeed and the subsequent suspension of shipments pending the development of a revised marketing strategy for this product.
- •
- Subsequent to year end, commencement of direct sales of BrachySeed in the U.S.
- •
- Subsequent to year end, commencement of a Phase I trial for INFECTON, a technetium-99m-based radiopharmaceutical for imaging infection.
- •
- Subsequent to year end, U.S. regulatory approval of a new radiotherapeutic kit product for the treatment of thyroid cancer and hyperthyroidism.
- •
- Underwritten public sale by two corporate shareholders of 4.2 million common shares, representing approximately 11.4% of issued and outstanding shares.
- •
- Completion in December 2002, of a bank credit facility providing for up to $3.2 million of additional borrowing capacity.
In 2002 the Company indicated that its target for compound annual growth in revenues over the next two to three years would be approximately 50% for its radiopharmaceutical business and 15% for its manufacturing business and that both businesses were expected to experience increased revenues and operating profitability in 2002.
The Company experienced record radiopharmaceutical results in 2002 with revenues of $10.2 million and EBITDARD of $2.7 million representing increases over 2001 of 46.0% and 51.9%, respectively.
The Company also experienced record manufacturing results in 2002 with revenues of $20.9 million, a 2.4% increase over 2001, and EBITDA of $0.6 million representing an increase over the $0.1 million in 2001. The lower than expected growth in manufacturing revenues was largely attributable to delays in the receipt of regulatory approvals for new product introductions.
The Company adopted U.S. GAAP and U.S. dollars as its primary financial reporting convention, beginning with the first quarter of 2001. This change was influenced by the Company's desire to better meet the needs of shareholders in assessing the Company's financial performance by following accounting practices which are consistent with the majority of its customers and peer companies.
A summary of the change in accounting policies adopted by the Company in the preparation of its consolidated financial statements is included in Note 3 to the 2002 audited consolidated financial statements.
Commencing with the quarter ended December 31, 2001, the results of operations of DRAXIS Pharmaceutica have been reported as discontinued operations. Commencing with the second quarter of 2002, discontinued operations no longer include revenues and expenses directly attributable to Alertec. Information for prior periods has been reclassified to reflect this change.
In conjunction with the commencement of reporting the results of operations of DRAXIS Pharmaceutica as a discontinued operation, the Company has modified the definition of its business segments. Commencing in the fourth quarter of 2001, the Company's results of operations have been reported within three segments: Radiopharmaceuticals, Manufacturing, and Corporate and Other.
44
For a summary of recent pronouncements, see Note 28 to the Company's 2002 consolidated financial statements.
The terms EBITDARD, EBITDA (pre R&D), EBITDA and net income and earnings per share before non-recurring items used herein do not have standardized meanings prescribed by U.S. GAAP and therefore may not be comparable to similar measures used by other companies. DRAXIS uses such terms as measures to assess the operating performance of its on-going businesses and believes that most shareholders and investors prefer such measures, since they are consistent with industry practice for analyzing operating performance. Such measures are used consistently and explicitly defined, and excluded charges are clearly identified. Such measures should not be construed as the equivalent of net cash flows from operating activities.
45
Consolidated Results of Operations(1)
| | Years ended December 31,
| |
|---|
| | 2002
| | 2001
| | 2000
| |
|---|
| | (in thousands of U.S. dollars except share related data) (U.S. GAAP)
| |
|---|
| Revenues | | | | | | | | | | |
| | Product sales | | $ | 30,338 | | $ | 27,151 | | $ | 22,589 | |
| | Royalty and licensing | | | 8,302 | | | 6,752 | | | 7,132 | |
| | |
| |
| |
| |
| | | $ | 38,640 | | $ | 33,903 | | $ | 29,721 | |
| | |
| |
| |
| |
| Product gross margin | | $ | 6,934 | | $ | 5,106 | | $ | 4,214 | |
| | % of Product sales revenues | | | 22.9 | % | | 18.8 | % | | 18.7 | % |
| Royalty and licensing revenue | | | 8,302 | | | 6,752 | | | 7,132 | |
| SG&A | | | (7,182 | )(2) | | (6,369 | ) | | (7,531 | ) |
| | % of Product sales revenues | | | -23.7 | % | | -23.5 | % | | -33.3 | % |
| | |
| |
| |
| |
| EBITDA(3) (pre-R&D) | | | 8,054 | | | 5,489 | | | 3,815 | |
| | % of Total revenues | | | 20.8 | % | | 16.2 | % | | 12.8 | % |
| R & D | | | (1,996 | ) | | (1,280 | ) | | (1,027 | ) |
| | |
| |
| |
| |
| EBITDA(3) | | | 6,058 | | | 4,209 | | | 2,788 | |
| | % of Total revenues | | | 15.7 | % | | 12.4 | % | | 9.4 | % |
| Depreciation and amortization | | | (2,804 | ) | | (2,436 | ) | | (2,318 | ) |
| Non-recurring items | | | | | | | | | | |
| —Income tax recovery | | | 418 | | | — | | | — | |
| —Offering costs | | | (251 | ) | | — | | | — | |
| —Revaluation of tax assets | | | — | | | (3,300 | ) | | — | |
| —Restructuring charges | | | — | | | — | | | (434 | ) |
| —Other income | | | — | | | — | | | 411 | |
| —Cumulative effect of accounting change | | | — | | | — | | | (19,900 | ) |
| Financial | | | | | | | | | | |
| —Foreign exchange translation | | | (12 | ) | | 378 | | | (21 | ) |
| —Other | | | (268 | ) | | (403 | ) | | (616 | ) |
| Income tax recovery (provision)(4) | | | (373 | ) | | 251 | | | 215 | |
| Minority interest | | | 252 | | | 286 | | | 338 | |
| Loss from discontinued operations | | | (834 | ) | | (569 | ) | | (801 | ) |
| | |
| |
| |
| |
| Net income (loss) | | $ | 2,186 | | $ | (1,584 | ) | $ | (20,338 | ) |
| | |
| |
| |
| |
| Net income (loss) | | | | | | | | | | |
| —From continuing operations before non-recurring items | | $ | 2,853 | | $ | 2,285 | | $ | 386 | |
| —Non-recurring items | | | 167 | | | (3,300 | ) | | (19,923 | ) |
| —From discontinued operations | | | (834 | ) | | (569 | ) | | (801 | ) |
| | |
| |
| |
| |
| | | $ | 2,186 | | $ | (1,584 | ) | $ | (20,338 | ) |
| | |
| |
| |
| |
| Basic income (loss) per share | | | | | | | | | | |
| —From continuing operations before non-recurring items | | $ | 0.077 | | $ | 0.062 | | $ | 0.011 | |
| —Non-recurring items | | | 0.004 | | | (0.090 | ) | | (0.548 | ) |
| —From discontinued operations | | | (0.023 | ) | | (0.016 | ) | | (0.022 | ) |
| | |
| |
| |
| |
| | | $ | 0.058 | | $ | (0.044 | ) | $ | (0.559 | ) |
| | |
| |
| |
| |
- (1)
- Commencing with the quarter ended December 31, 2001, the results of operations of DRAXIS Pharmaceutica have been reported as discontinued operations. Commencing in the second quarter of 2002 discontinued operations no longer include revenues and expenses directly attributable to Alertec. Information for prior periods has been reclassified to reflect this change.
- (2)
- Excludes offering costs of $360 ($251, after tax).
- (3)
- Income from continuing operations before non-recurring items, depreciation and amortization, interest, income taxes and minority interest. This earnings measure does not have a standardized meaning prescribed by U.S. GAAP and therefore may not be comparable to similar measures used by other companies. Such measures should not be construed as the equivalent of net cash flows from operating activities.
- (4)
- Excludes tax impact of non-recurring items.
46
Consolidated revenues from continuing operations for the year ended December 31, 2002 of $38,640,000 were a record level for the Company representing an increase of 14.0% over 2001. An 11.7% increase in product sales coupled with a 23.0% increase in royalty and licensing revenue accounted for the overall increase.
Product gross margin in 2002 improved to 22.9% of product sales from continuing operations from 18.8% for the same period in 2001 due to a change in revenue mix.
Selling, general and administration expenses associated with continuing operations was 23.7% of product sales from continuing operations in 2002, relatively unchanged as compared to the 23.5% of product sales from continuing operations in 2001.
EBITDARD from continuing operations of $8,054,000 in 2002 was an annual record for the Company, representing an increase of 46.7% compared to 2001. Expressed as a percentage of total revenues, EBITDARD increased to 20.8% in 2002 from 16.2% in 2001.
Research and development expenditures associated with continuing operations increased 55.9% in 2002 as compared to 2001 due to increased staffing and additional clinical development activity involving the Company's pipeline of innovative radiopharmaceutical products.
Depreciation and amortization expense associated with continuing operations increased 15.1% compared with 2001 following the commencement of depreciation charges on completed capital projects.
Net financial items associated with continuing operations in 2002 resulted in a higher expense in 2002 due to a strengthening Canadian dollar in 2002 compared with 2001, partially offsetting the impact of lower interest rates.
Minority interest in 2002 contributed positively to net income by $252,000, a $34,000 decline compared to 2001 due to the reduction in DPI's net loss.
Net loss from discontinued operations in 2002 was $834,000 as compared to $569,000 in 2001 due to higher operating expenses including severance costs related to the restructuring of the Company's corporate scientific and regulatory affairs group, partly offset by higher product gross margins.
The only significant non-recurring items in 2002 were net after-tax offering costs of $251,000 and a tax recovery of $418,000. The only significant non-recurring item in 2001 was a $3,300,000 charge associated with the revaluation of the Company's income tax assets.
In December 2001, the Company filed amended U.S. returns for its 1998, 1999 and 2001 taxation years. In June 2002 the Company was notified by the U.S. Internal Revenue Service that its revised filing position had been accepted and related refunds aggregating $418,000 were recognized in the second quarter of 2002 as a reduction in income tax expense.
In June 2001, the Governments of Canada and Ontario enacted legislation implementing gradual reductions in their respective corporate income tax. Following full implementation of the reductions, the effective tax rate applicable to the Company's Canadian operations will decline to approximately 31%. Accordingly, in 2001, the Company recorded a non-cash charge of $3,300,000 to reduce the carrying value of its deferred income taxes. Although this development caused the Company to reduce the carrying value of its income tax assets, future periods will benefit from a significant reduction in Canadian income tax rates.
Excluding non-recurring items, for the twelve month period ended December 31, 2002 the Company recorded an income tax expense (expressed as a percentage of pre-tax earnings) of 12.5% as compared to a recovery of 14.4% in 2001. During 2002, the Company had indicated that it had expected its 2002 effective tax rate to be in the 20-25% range. The lower actual rate for 2002 was due to an unforeseen shift in profit mix in the later half of 2002.
Net income from continuing operations before non-recurring items increased 24.9% compared with 2001. Basic earnings per share from continuing operations before non-recurring items in 2002 increased 24.2% over 2001 (on the same basis) to $0.077 per share in 2002.
The weighted average number of common shares outstanding in 2002 increased 1.1% to 36,981,985, over 2001 due to the exercise of options and employee participation shares.
47
Consolidated revenues from continuing operations for the year ended December 31, 2001 of $33,903,000 were an annual record for the Company, representing an increase of 14.1% over the $29,721,000 in 2000. A 20.2% increase in product sales was partly offset by a 5.3% decline in royalty and licensing revenue due to a decline in the amount of additional Anipryl minimum royalty earned in 2001 as compared to the $1,456,000 earned in 2000.
Cost of goods sold decreased in 2001 to 81.2% of product sales from continuing operations from 81.3% for the same period in 2000 due to a change in revenue mix.
Selling, general and administration expenses associated with continuing operations of $6,369,000, or 23.5% of product sales from continuing operations, in 2001 represented a substantial decline as compared to the $7,531,000, or 33.3% of product sales from continuing operations, in 2000.
EBITDARD from continuing operations of $5,489,000 in 2001 was an annual record for the Company, representing an increase of 43.9% compared to $3,815,000 in 2000.
Research and development expenditures associated with continuing operations increased to $1,280,000 in 2001 as compared to $1,027,000 in 2000 due to increased development activity involving the Company's radiopharmaceutical product pipeline and non-recurring charges totalling $196,000 associated with license payments for INFECTON and BrachySeed.
The only significant non-recurring item in 2001 was a $3,300,000 charge associated with the revaluation of the Company's income tax assets. Non-recurring items in 2000 included a $19,900,000 charge representing the cumulative effect of the change in accounting policy, a $434,000 restructuring charge related to the divestiture of the Company's dermatology product lines and a $411,000 gain on the sale of dermatology product rights.
Depreciation and amortization expense associated with continuing operations of $2,436,000 in 2001 increased 5.1% as compared to 2000, following the commencement of depreciation charges on completed capital projects.
Net financial items associated with continuing operations in 2001 were an expense of $25,000 as compared to expense of $637,000 for 2000. 2001 results were positively impacted by $378,000 in foreign exchange gains arising from the stronger U.S. dollar relative to the Canadian dollar.
Minority interest in 2001 contributed positively to net income by $286,000, a $52,000 decline compared to 2000 due to the reduction in DPI's net losses.
Revenues from discontinued operations was the major factor contributing to a $232,000 improvement in the net loss from discontinued operations, from a loss of $801,000 in 2000 to a loss of $569,000 in 2001.
In 2001 the Company recorded an income tax expense associated with continuing operations of $3,049,000 as compared to an income tax benefit of $215,000 in 2000.
In June 2001, the Governments of Canada and Ontario enacted legislation implementing gradual reductions in their respective corporate income tax. Following full implementation of the reductions, the effective tax rate applicable to the Company's Canadian operations will decline to approximately 31%. Accordingly, in 2001, the Company recorded a non-cash charge of $3,300,000 to reduce the carrying value of its deferred income taxes. Although this development caused the Company to reduce the carrying value of its income tax assets, future periods will benefit from a significant reduction in Canadian income tax rates.
Excluding the $3,300,000 non-recurring charge, in 2001 the income tax benefit would have been $410,000. The change in the effective tax rate from 2000 to 2001 is largely attributable to a change in the mix of income across tax jurisdictions in which the Company operates and reconciling differences between income for accounting and tax purposes.
Net income from continuing operations, before the charge associated with revaluation of tax assets, of $2,285,000, or $0.062 per share, for 2001 represents a $1,899,000, or $0.051 per share, improvement from income of $386,000, or $0.011 per share, in 2000 before the charge associated with the cumulative effect of the change in accounting policy.
The weighted average number of common shares outstanding in 2001 increased to 36,587,794, a 0.7% increase over 2000 due to the exercise of options.
48
| | Years Ended December 31,
| |
|---|
| | 2002
| | 2001
| | 2000
| |
|---|
| | (in thousands of U.S. dollars)
(U.S. GAAP)
| |
|---|
| Revenues | | | | | | | | | | |
| Product sales | | $ | 9,704 | | $ | 6,763 | | $ | 5,951 | |
| Royalty and licensing | | | 451 | | | 192 | | | — | |
| | |
| |
| |
| |
| | | $ | 10,155 | | $ | 6,955 | | $ | 5,951 | |
EBITDARD |
|
$ |
2,702 |
|
$ |
1,779 |
|
$ |
1,360 |
|
| Research and development | | | (1,996 | ) | | (1,280 | ) | | (1,027 | ) |
| | |
| |
| |
| |
| EBITDA | | | 706 | | | 499 | | | 333 | |
| Depreciation and amortization | | | (716 | ) | | (623 | ) | | (547 | ) |
| | |
| |
| |
| |
| (Loss) income from operations | | $ | (10 | ) | $ | (124 | ) | $ | (214 | ) |
| | |
| |
| |
| |
Radiopharmaceuticals and radiotherapy devices are the focus of the Company's radiopharmaceutical subsidiary, DRAXIMAGE, which discovers, develops, manufactures and markets diagnostic imaging and therapeutic radiopharmaceutical products for the global marketplace. Products currently marketed by DRAXIMAGE include a line of lyophilized technetium-99m kits used in nuclear imaging procedures, a line of imaging and therapeutic products labelled with a variety of isotopes including radioiodine, and BrachySeed, second generation iodine-125 and palladium-103 brachytherapy implants. DRAXIMAGE has a number of products in late-stage development including Fibrimage, currently in Phase III, for imaging deep vein thrombosis; Amiscan, currently in Phase II, for the early diagnosis of acute myocardial infarct; and INFECTON, currently in Phase I clinical development, for imaging infection. DRAXIMAGE is also developing a somatostatin-based peptide that has potential for cancer imaging and therapy.
Highlights for the Company's radiopharmaceutical segment in 2002 included:
- •
- Record financial results:
- •
- Revenues of $10.2 million, an increase of 46.0% over 2001
- •
- EBITDARD of $2.7 million, an increase of 51.9% over 2001
- •
- U.S. and Canadian regulatory approval to transfer production of DRAXIMAGE's line of lyophilized medical imaging products to the Company's own production facility.
- •
- Increased U.S. and Canadian market acceptance of the iodine version of BrachySeed which was launched in 2001.
- •
- U.S. launch of the palladium version of BrachySeed and the subsequent suspension of shipments pending the development of a revised marketing strategy for this product.
- •
- Subsequent to year end, commencement of direct sales of BrachySeed in the U.S.
- •
- Subsequent to year end, commencement of a Phase I trial for INFECTON, a technetium-99m-based radiopharmaceutical for imaging infection.
- •
- Subsequent to year end, U.S. regulatory approval of a new radiotherapeutic kit product for the treatment of thyroid cancer and hyperthyroidism.
In January 2003, DRAXIMAGE served notice to its BrachySeed licensee in the U.S. that it was exercising its contractual rights to terminate the licensee's marketing exclusivity provisions as a result of the licensee's continued failure to meet minimum annual sales requirements. DRAXIMAGE assured its licensee at this time that in all other respects contractual relations between the parties remained in effect in accordance with the provisions of the license and supply agreements, including the licensee's continued right to market and distribute BrachySeed in the U.S. on a non-exclusive basis. The licensee subsequently notified DRAXIMAGE that it was unilaterally terminating all contractual agreements between the companies alleging breach by DRAXIMAGE. DRAXIMAGE has disputed the licensee's right to terminate the agreements, denies any breach, and maintains that the licensee has repudiated the agreements.
DRAXIMAGE has established full control over the marketing, sales and distribution of BrachySeed in the U.S and has taken steps to assure its BrachySeed customers that there will be no disruption in the supply of
49
BrachySeed I-125 implants. DRAXIMAGE is considering several options for the U.S. market, including continued direct sales as well as the possibility of establishing new marketing and distribution relationships.
Comparison of 2002 to 2001
Total revenues for the radiopharmaceutical segment in 2002 of $10,155,000 were an annual record representing an increase of 46.0% over 2001. The 43.5% increase in product sales was primarily attributable to increased sales of Sodium Iodide I-131 radiotherapy capsules, which were launched in the fourth quarter of 2001, diagnostic imaging products, and BrachySeed implants.
Revenues in 2001 were negatively affected by supply disruptions associated with the previously outsourced supply of the Company's line of lyophilized diagnostic imaging products. For the year ended December 31, 2002, U.S. sales of these products increased 21.4% as compared to 2001.
During 2002 DRAXIMAGE completed the installation of three new BrachySeed production lines at its FDA-approved facility, incorporating robotic assembly units for efficient production and greater product quality.
In the second quarter of 2002, the Company received a $1,000,000 non-refundable fee related to BrachySeed Pd-103 bringing the total of non-refundable fees received from its U.S. BrachySeed licensee to $2,000,000. Up to December 31, 2002, non-refundable fees under this collaboration have been deferred and recognized as revenue on a straight-line basis over the period to December 31, 2010. Future treatment of this deferred revenue source will depend on the outcome of the dispute between DRAXIMAGE and its BrachySeed licensee in the U.S.
EBITDARD for 2002 for this segment of $2,702,000, or 26.6% of revenues, was an annual record, representing an increase of 51.9%, compared to income of $1,779,000, or 25.6% of revenues, in 2001.
Research and development expenditures for this segment increased to $1,996,000 in 2002 as compared to $1,280,000 in 2001 due to increased development activity involving the Company's radiopharmaceutical product pipeline.
For the year ended December 31, 2002, BrachySeed net contribution to EBITDA was approximately break-even on $1.8 million of revenues. Included in 2002 results were $1.0 million of inventory losses associated with the palladium version of BrachySeed. In December 2002, manufacturing and sale of BrachySeed Pd-103 were suspended to reduce inventory losses which had been incurred for a period of several months during which production, based on its U.S. licensee's projected sales, significantly exceeded actual sales. DRAXIMAGE is exploring alternatives for the possible re-introduction of this product.
Depreciation and amortization expense for this segment increased 14.9% from 2001 following the commencement of depreciation of the expanded radiopharmaceutical production facility.
In 2002, the Company strengthened its radiopharmaceutical management team with the addition of Dr. George Desypris as Director of Clinical Development and Mr. Giovanni Venditti as Director of Sales and Marketing. In 2001, DRAXIMAGE added Dr. Edward Bump as Director of Research.
Comparison of 2001 to 2000
Total revenues for the radiopharmaceutical segment in 2001 of $6,955,000 were an annual record representing an increase of 16.9% over the $5,951,000 in 2000. The 13.6% increase in product sales was primarily attributable to U.S. sales of BrachySeed I-125 and Sodium Iodide I-131 radiotherapy capsules which were launched in 2001 and increased sales of diagnostic imaging kits.
Revenues in both 2000 and 2001 were negatively affected by supply disruptions associated with the previously outsourced supply of the Company's line of lyophilized diagnostic imaging products.
EBITDARD for this segment of $1,779,000 for 2001 was an annual record, representing an increase of $419,000, or 30.8%, compared to income of $1,360,000 in 2000.
Research and development expenditures for this segment increased to $1,280,000 in 2001 as compared to $1,027,000 in 2000 due to increased development activity involving the Company's radiopharmaceutical product pipeline and non-recurring charges totalling $196,000 associated with license payments for INFECTON and BrachySeed.
50
Depreciation and amortization expense for this segment of $623,000 in 2001 increased 13.9% from $547,000 in 2000 following the commencement of depreciation of the recently expanded radiopharmaceutical production facility.
Manufacturing
| | Years Ended December 31,
| |
|---|
| | 2002
| | 2001
| | 2000
| |
|---|
| | (in thousands of U.S. dollars)
(U.S. GAAP)
| |
|---|
| Revenues | | | | | | | | | | |
| Product sales | | $ | 20,946 | | $ | 20,460 | | $ | 15,477 | |
| | |
| |
| |
| |
| EBITDA | | $ | 627 | | $ | 149 | | $ | (200 | ) |
| Depreciation and amortization | | | (1,166 | ) | | (867 | ) | | (807 | ) |
| | |
| |
| |
| |
| Loss from operations | | $ | (539 | ) | $ | (718 | ) | $ | (1,007 | ) |
| | |
| |
| |
| |
Manufacturing comprises the Company's manufacturing subsidiary, DPI, and product sales of Anipryl produced for Pfizer Inc. DPI is a pharmaceutical contract manufacturer with capabilities in a broad range of dosage forms, specializing in liquid and lyophilized (freeze-dried) injectables and other sterile products. Operating out of a cGMP-compliant 247,000 square-foot facility located in Montreal, Canada, DPI manufactures pharmaceutical products for DRAXIMAGE, as well as for over 15 other pharmaceutical clients for many international jurisdictions.
Highlights for the Company's manufacturing segment in 2002 included:
- •
- Record financial results:
- •
- Revenues of $20.9 million, an increase of 2.4% over 2001
- •
- EBITDA of $627,000 compared with $149,000 for 2001, and improved EBITDA margin to 3.0% from 0.7%.
- •
- Initiation of a three-year, $12 million capital plan, with a tripling of DPI's existing lyophilization capacity and related financing arrangements including additional participation by DPI's strategic partner, Société générale de financement du Québec.
- •
- U.S. and Canadian regulatory approval to transfer production of DRAXIMAGE's line of lyophilization medical imaging products to the Company's own production facility.
- •
- U.S. regulatory approval to commence production at DPI of an established injectable product for GlaxoSmithKline.
- •
- U.S. regulatory approval to manufacture solid dosage form products at DPI.
- •
- Subsequent to year end, U.K. regulatory approval to manufacture multiple sterile and non-sterile products at DPI.
- •
- Several new business opportunities.
During 2002, Mr. Jim Garner, the Company's Senior Vice President, Finance and Chief Financial Officer, was appointed to the additional position of Acting President of DPI following the resignation of the previous incumbent. The Company has commenced a formal search process to identify potential candidates for a permanent president of DPI.
Revenues for the twelve month period ended December 31, 2002 were flat despite increased new business, the commencement of shipments of lyophilized products principally to DRAXIMAGE and increased Anipryl product sales to Pfizer Inc., all of which was offset by lower volumes of lower margin, legacy products.
DPI's regular summer shut-down was completed in July 2002. The entire sterile area, including lyophilization, was affected by the 2002 shutdown. This resulted in slower than anticipated ramp up in sales in the third quarter and much of the fourth quarter of 2002.
51
In 2002 the Company announced that it had received approval from the FDA to transfer the production of previously outsourced DRAXIMAGE lyophilized imaging products to DPI. Ramp-up of lyophilization production in 2002 was slower than anticipated due to production start-up issues in this new manufacturing area.
In December 2001 DPI finalized supply and related agreements with GlaxoSmithKline for the renewal and expansion of an existing contract manufacturing relationship between the companies. The products being transferred to DPI are all established, sterile products currently marketed by GlaxoSmithKline in multiple international markets.
In 2002 an additional, established sterile product was added under this contract. U.S. regulatory approval for the site transfer of this product, the first under the new DPI/GlaxoSmithKline contract destined for the U.S. market, was received and commercial production of this product commenced in the second quarter of 2002.
During 2002, site transfer and related activities associated with the other GlaxoSmithKline products continued at a high level. Manufacturing revenues for these products are now expected to ramp up gradually through 2003 and into 2004.
In January 2003, DPI received formal approval from the U.K. Medicines Control Agency to manufacture several products destined for the U.K. and major European markets. The authorization includes products being transferred to DPI under the GlaxoSmithKline contract.
In 2002, DPI was selected by Bone Care International Inc. to be a manufacturer of Hectorol Injection, an FDA approved D-hormone product used in the treatment of secondary hyperparathyroidism in chronic renal dialysis patients.
Also in 2002, DPI was designated by Axcan Pharma Inc. as a commercial manufacturing site for its lyophilized biological product, Photofrin photodynamic therapy used to selectively palliate, cure or prevent various forms of malignant cancers.
EBITDA for this segment of $627,000 for 2002 was an annual record representing an increase of $478,000 from $149,000 in 2001. The EBITDA margin for this segment increased to 3.0% for 2002 compared to 0.7% in 2001. The improvement in operating profitability was attributable to higher margin products.
Depreciation and amortization expense for this segment increased 34.5% from 2001 following the commencement of depreciation charges on completed capital projects.
Total revenues for the manufacturing segment in 2001 of $20,460,000 were an annual record representing an increase of 32.2% over the $15,477,000 in 2000. The increase was primarily attributable to increased volumes under established contracts, new product introductions and increased service revenues associated with new product introductions.
EBITDA for this segment of $149,000 for 2001 was an annual record representing an increase of $349,000 from a loss of $200,000 in 2000, in line with increased revenues.
Depreciation and amortization expense for this segment of $867,000 in 2001 increased 7.4% from $807,000 in 2000 following the commencement of depreciation charges on completed capital projects.
52
Corporate and Other
| | Years Ended December 31,
| |
|---|
| | 2002
| | 2001
| | 2000
| |
|---|
| | (in thousands of U.S.dollars)
(U.S. GAAP)
| |
|---|
| Revenues | | | | | | | | | | |
| Product sales(1) | | $ | (312 | ) | $ | (72 | ) | $ | 1,161 | |
| Royalty and licensing | | | 7,851 | | | 6,560 | | | 7,132 | |
| | |
| |
| |
| |
| | | $ | 7,539 | | $ | 6,488 | | $ | 8,293 | |
| | |
| |
| |
| |
| EBITDA | | $ | 4,725 | | $ | 3,561 | | $ | 2,221 | |
| Depreciation and amortization | | | (922 | ) | | (946 | ) | | (964 | ) |
| Non-recurring items: | | | | | | | | | | |
| | Offering costs | | | (360 | ) | | — | | | — | |
| | |
| |
| |
| |
| Income from operations | | $ | 3,443 | | $ | 2,615 | | $ | 1,257 | |
| | |
| |
| |
| |
- (1)
- Net of inter-segment sales.
Corporate and Other comprises deferred revenues, royalties and expenses associated with the Company's business agreements with Pfizer Inc. with respect to Anipryl, royalties and expenses from GlaxoSmithKline Consumer Healthcare with respect to the SpectroPharm line of products, revenues and directly attributable expenses associated with Alertec, non-allocated corporate expenses and inter-segment eliminations. The Company follows a policy of not allocating its central corporate expenses to its operating business segments.
Royalty and licensing revenue in this segment in 2002 increased 19.7% as compared to 2001. The increase was attributable to increased minimum royalty amounts from Pfizer Inc. with respect to Anipryl.
EBITDA for this segment, before non-recurring items, in 2002 increased $1,164,000 over 2001 levels due to increased minimum royalty amounts from Pfizer Inc. with respect to Anipryl, combined with a higher contribution from Alertec offsetting inter-segment eliminations.
This segment incurred non-recurring charges of $360,000 ($251,000 after tax) of costs associated with the public offering of shares during the second quarter of 2002.
Depreciation and amortization expense for this segment in 2002 declined slightly compared to 2001.
Inter-segment sales in 2001 totalled $72,000. In 2000, dermatology product sales, net of inter-segment sales, were $591,000. The Company did not record any dermatology product revenues in 2001.
Royalty and licensing revenue in this segment in 2001 of $6,560,000 represented a decrease of 8.0% as compared to $7,132,000 in 2000. The decrease was attributable to a decline in the amount of additional Anipryl minimum royalty earned in 2001 as compared to the $1,456,000 earned in 2000 partly offset by the impact of deferred revenue accounting for the SpectroPharm transaction. The net proceeds from the May 2000 sale of the SpectroPharm line of products were deferred and are being recognized as revenue on a straight-line basis over the period to February 2005.
EBITDA for this segment, before non-recurring items, in 2001 increased $1,340,000 over 2000 levels due to the net impact of the deferred revenue accounting for the SpectroPharm transaction and improved margins with respect to Alertec partially offset by the decline in the amount of additional Anipryl minimum royalty earned in 2001 as compared to the $1,456,000 earned in 2000.
Non-recurring items in 2000 included a $434,000 restructuring charge related to the divestiture of the Company's dermatology product lines.
Depreciation and amortization expense for this segment of $946,000 in 2001 was largely unchanged as compared to 2000.
53
Critical Accounting Policies and Estimates
The foregoing discussion and analysis of the financial condition and results of operations is based upon the Company's consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of consolidated financial statements requires the Company to make estimates and judgements that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, the Company evaluates its estimates and makes adjustments as appropriate. Actual results may differ from these estimates under different assumptions or conditions.
A summary of the significant accounting policies and methods used by the Company in the preparation of its consolidated financial statements is included in Note 2 to the 2002 audited consolidated financial statements. The Company believes the following critical accounting policies affect its more significant judgements and estimates used in the preparation of its consolidated financial statements.
Recognition of Licensing Revenue
License and other forms of non-refundable fees received pursuant to collaboration agreements are accounted for according to the related contractual agreements. In general, such fees are deferred and recognized on a straight-line basis over the lesser of the contract period and the estimated term over which contractual benefits are expected to be derived. If payment of such fees is contingent upon future performance obligations of the Company or other future events, revenue recognition of such amounts is deferred and recognized upon completion of the specific event.
Deferred Tax Assets
Realization of the net deferred tax assets is dependent on the Company's ability to generate sufficient taxable income in certain tax jurisdictions. Management believes that it is more likely than not that the assets will be realized, based on forecasted income. On a quarterly basis, the estimates and assumptions underlying the forecasted income are reviewed by management to determine whether additional valuation allowances are warranted.
Liquidity and Capital Resources
| | Years Ended December 31,
|
|---|
| | 2002
| | 2001
| | 2000
|
|---|
| | (in thousands of U.S. dollars)
(U.S. GAAP)
|
|---|
| Cash and cash equivalents | | $ | 4,899 | | $ | 5,602 | | $ | 4,420 |
| Non-financial working capital (net)(1) | | $ | 6,284 | | $ | 8,106 | | $ | 10,363 |
| Total debt | | $ | 13,610 | | $ | 9,726 | | $ | 11,225 |
| | |
| |
| |
|
- (1)
- Excluding cash and cash equivalents, bank loan and current portions of deferred revenues and long-term debt and customer deposits.
Cash and cash equivalents at December 31, 2002 totalled $4,899,000 as compared with $5,602,000 as at December 31, 2001 and $4,420,000 as at December 31, 2000.
The Company follows a policy of investing its surplus cash resources in high quality, liquid, short-term commercial paper and government treasury bills and money market mutual funds which invest in high quality short-term securities. As at December 31, 2002 and December 31, 2001 there were no restrictions on the flow of these funds nor have any of these funds been committed in any way. As at December 31, 2000, $341,000 was held as a deposit against representations provided by the Company in connection with the SpectroPharm transaction and was released in February 2001. There are certain standard financial liquidity ratio requirements pursuant to DPI's term loan as well as terms of the DPI shareholders' agreement that could restrict the free flow of funds from one subsidiary of the Company to another.
Cash flows used in continuing operations, before changes in working capital, in 2002, were $1,773,000 as compared to usage of $2,306,000 in 2001 and $3,746,000 in 2000. The increase related to improved EBITDA.
Cash flows used in discontinued operations in 2002 were $439,000 as compared to a cash inflow of $75,000 in 2001. The decrease in 2002 was due to the decline in EBITDA from discontinued operations. Cash flows from
54
discontinued operations increased to an inflow of $75,000 in 2001 from a usage of $881,000 in 2000 due to improved EBITDA.
The Company had $18,994,000, $24,615,000 and $26,264,000 of deferred revenues as at December 31, 2002, December 31, 2001 and December 31, 2000, respectively. The decline in 2002 was attributable to amortization during the year, partly offset by the addition of a $1,000,000 non-refundable fee related to BrachySeed Pd-103. The change in 2001 was attributable to amortization during the year, partly offset by the addition of a portion of the payment received in December 2001 regarding Anipryl.
Deferred revenue amortization, which represents a source of non-cash earnings for the Company, increased to $6,568,000 in 2002 as compared to $4,783,000 in 2001 and $4,234,000 in 2000. The increase in 2002 was due to commencement of amortization of non-refundable fees related to BrachySeed in 2001 and 2002. The increase in 2001 was largely due to the full year effect of amortization arising from the SpectroPharm transaction.
Non-financial working capital declined to $6,284,000 as at December 31, 2002 from $8,106,000 as at December 31, 2001 and $10,363,000 as at December 31, 2000 due to lower current deferred income taxes and increased accounts payable. The decline in 2001 was due to lower inventories and accounts receivable and higher accounts payable.
Cash flows used in investing activities, excluding changes in deferred revenue, totalled $5,450,000 in 2002, $5,288,000 in 2001 and $384,000 in 2000.
Capital expenditures in 2002 of $5,390,000 increased from $5,363,000 in 2001 and $1,126,000 in 2000. Virtually all capital expenditures in 2002 are attributable to DPI's previously announced capital plan. The increase in 2001 was attributable to increased spending in the Company's manufacturing and radiopharmaceutical businesses.
The Company did not make any acquisitions in 2002, 2001 or 2000.
Net proceeds from the divestiture of the Company's dermatology product lines in 2000 totalled $8,911,000, of which $8,169,000 was capitalized as deferred revenue.
In March 2002, co-incident with the announcement of DPI's new capital plan, the Company entered into a number of financing arrangements which will provide for up to $7,400,000 in debt and equity from DPI's existing shareholders and up to $3,000,000 in debt financing from Investissement Québec.
Total debt at December 31, 2002 increased to $13,610,000 from $9,726,000 at December 31, 2001 due to additional bank facilities completed during December 2002 and the initial debt drawdown pursuant to DPI's new financing arrangement partially offset by scheduled term debt repayments. Total debt at December 31, 2001 was $9,726,000 compared to $11,225,000 at December 31, 2000.
As at December 31, 2002, the Company's debt was comprised as follows:
- •
- CDN$1,500,000 secured revolving credit facility of which $Nil was drawn at December 31, 2002 (DRAXIS Health Inc.);
- •
- CDN$3,500,000 secured revolving credit facility of which $884,000 was drawn at December 31, 2002 (DPI);
- •
- $2,220,000 secured bank term loan (DRAXIS Health Inc.);
- •
- $5,167,000 secured bank term loan (DPI);
- •
- $1,081,000 secured subordinated term loan from Investissement Québec (DPI);
- •
- $1,976,000 unsecured subordinated term loan from SGF (DPI); and
- •
- $2,283,000 unsecured subordinated obligation related to the in-licensing of Permax (DRAXIS Health Inc.).
As at December 31, 2001, the Company's debt was comprised of two DPI bank loans, a $5,295,000 term loan and a CDN$3,500,000 revolving credit facility, of which $1,666,000 was drawn at December 31, 2001, and a $2,765,000 unsecured obligation related to the in-licensing of Permax.
The Company was in compliance with all lending covenants as at December 31, 2002, except with respect to a minimum working capital requirement under the DRAXIS Health Inc. credit agreements. A waiver has been
55
received with respect to the non-compliance to this covenant. The Company was in compliance with all lending covenants as at December 31, 2001 and 2000.
Proceeds from the issuance of treasury common shares by the Company attributable to the exercise of options and employee participation shares generated $844,000, $83,000 and $2,308,000 in 2002, 2001 and 2000, respectively. In 2002, the Company received proceeds of $792,000 from the issuance of treasury shares by DPI, net of the repurchase of shares held by minority interest. In 2000, the Company received net proceeds of $5,375,000 from the issuance of treasury shares by DPI.
In December 1999, the Company received regulatory approval from the Toronto Stock Exchange for a stock repurchase plan to repurchase for cancellation up to 1.83 million common shares. In December 2001 the plan was renewed and subsequently terminated on December 18, 2002. No shares were acquired under the plan in 2001 or 2002.
For information on the maturity profile of the Company's debt, see Note 20 to the Company's Consolidated Financial Statements and Notes, beginning on page F-1.
Commitments
The following table summarises the Company's major contractual cash obligations as of December 31, 2002:
| | Years Ended December 31,
|
|---|
| | 2003
| | 2004
| | 2005
| | 2006
| | 2007
|
|---|
| | (in thousands of U.S. dollars)
|
|---|
| Long-term debt | | $ | 2,158 | | $ | 1,598 | | $ | 2,471 | | $ | 1,329 | | $ | 3,304 |
| Operating leases | | $ | 252 | | $ | 145 | | $ | 49 | | $ | 10 | | | — |
| | |
| |
| |
| |
| |
|
In addition to the above, the Company is obligated to make certain royalty payments based on related product sales and milestone payments based on the achievement of certain specified events.
The Company is party to a shareholders' agreement which granted SGF and DPI's management shareholders the right to obligate the Company to purchase their shareholdings in DPI under certain circumstances. Further details of this commitment are included Note 24 to the 2002 consolidated financial statements.
Outlook
The Company's primary operational focus for 2003 continues to be: (i) improving near-term financial and operational performance of its radiopharmaceutical and manufacturing businesses through increasing sales of existing products and services, improving manufacturing efficiency and effectiveness, and obtaining additional regulatory approvals; and (ii) securing and advancing its base for long-term growth through the development of its existing product pipeline as well as identifying new business opportunities that are consistent with the Company's capabilities and that contribute to the long-term value of the Company.
The Company's longer term objective for its radiopharmaceutical business is to leverage its existing business base and record of regulatory approvals to become a significant North American and international competitor in this highly specialized market through a combination of increasing sales of existing products, the identification and commercialization of additional product opportunities, development of its new product pipeline and possible acquisitions, consistent with its well-defined business focus.
DRAXIMAGE, coming off of a strong 2002 with 46% revenue growth, is expected to continue to grow in 2003 through increasing sales of existing products in the U.S., introduction of new products into the U.S., including its recently approved Sodium Iodide I-131 kit, as well as expansion into non-North American jurisdictions.
Sales of BrachySeed implants are not expected to increase in 2003 at the same rate experienced in 2002. Product sales may decline somewhat during the transition period, following the repudiation of agreements by the Company's former U.S. BrachySeed licensee, while alternative marketing, sales and distribution strategies for BrachySeed are developed and implemented. Management expects that continued emphasis on quality, customer service and pricing will be the key factors for the future success for BrachySeed.
The outlook for EBITDARD margins in the radiopharmaceutical segment in 2003 is positive as a result of increased new product sales and the reduction of inventory losses related to BrachySeed Pd-103.
56
In 2003 and beyond the Company expects to increase its investment in the development of its radiopharmaceutical product pipeline.
The Company believes that long-term growth potential for DRAXIMAGE should be divided between the potential for its five innovative radiopharmaceutical products currently under development and opportunities excluding these products. Although each of DRAXIMAGE's five innovative products under development addresses substantial unmet market needs and thus has considerable potential, given the inherent uncertainties associated with new drug development, it is difficult to predict if, or when, any of these products will achieve commercialization.
Excluding the potential impact of the five innovative radiopharmaceutical products currently under development, the Company's target is to achieve radiopharmaceutical revenues within the next five years of $30 million to $35 million (representing more than three times its 2002 base) together with improving profitability margins.
The Company's longer term objective for its manufacturing business is to leverage its existing capabilities and its excellence in regulatory compliance to increase third-party contract manufacturing revenues while managing costs to achieve improving levels of profitability associated with increased capacity utilization. Capacity utilization at DPI varies by production flow, but on average during 2002 was approximately 25%-35% based on current capabilities.
DPI's near-term focus is on the successful execution of the several new business opportunities it has secured or is currently negotiating, including the GlaxoSmithKline contract, revenues from which are expected to ramp-up gradually through 2003 and into 2004.
In 2003, DPI will also be focused on the successful execution of its three-year, $12 million capital plan. Installation of the second lyophilizer, which will triple DPI's current capacity in this highly specialized area, is now scheduled for the third quarter of 2003 and is expected to become operational approximately one year after installation.
The Company's target is to achieve manufacturing revenues within the next five years from its Montreal facility of $40 million to $50 million (representing more than two times its 2002 base) together with improving profitability margins.
In general, the timing of regulatory approvals will be the major factor determining the rate of revenue and earnings growth for both the Company's radiopharmaceutical and manufacturing businesses.
In 2003, the Company's Corporate and Other segment is expected to experience a decline in revenues and earnings following the completion of revenue recognition in 2002 associated with a non-recurring Anipryl payment received in 2001.
In 2002 the Company received several non-binding offers related to the acquisition of DRAXIS Pharmaceutica and is currently engaged in advanced negotiations regarding a potential transaction. As divestiture options are explored, DRAXIS will continue to manage and operate this division to enhance shareholder value consistent with the Company's strategic focus on its core radiopharmaceutical and specialty contract manufacturing businesses.
Management expects operating cash flow, before changes in non-financial working capital, to be positive in 2003.
Capital expenditures in 2003 are expected to increase over 2002 levels due to the execution of DPI's capital plan.
With the Company's current cash balances, reduced operating cash requirements and established financing arrangements, management expects to have sufficient liquidity available to fund the Company's cash requirements in 2003. Any investments or acquisitions of businesses, products or technologies may require additional funding.
Item 6. Directors, Senior Management and Employees
The following is a list of the current directors and senior officers of the Company, their municipalities of residence, their current position with the Company and their principal occupations:
57
| Martin Barkin | | Toronto, ON | | President, Chief Executive Officer and Chief
Operating Officer |
Director since May 19, 1992 and employee of the Company since March, 1992.
Dr. Barkin, MD, B.Sc. (MED.), MA, FRCSC, is the President, Chief Executive Officer and Chief Operating Officer of the Company. He also serves on the Boards of Viventia Biotech Inc. and Bone Care and is Chairman of the Board of Sunnybrook & Women's College Health Sciences Centre. Dr. Barkin came to the Company in 1992 from KPMG where he was Partner and National Practice Leader for Health Care (1991-1992). Before that, he was Deputy Minister of Health for the Province of Ontario (1987-1991). Dr Barkin is a surgical specialist in Urology, a former Research Scientist at University of Toronto and Division Chief at Sunnybrook Health Sciences Centre.
| Leslie L. Dan | | Toronto, ON | | Director |
Director since December 10, 1993.
Mr. Dan is Chairman of Novopharm, one of Canada's leading pharmaceutical companies. Prior to April 5, 2000, Mr. Dan was Chairman and Chief Executive Officer of Novopharm.
| George M. Darnell | | Coral Gables, FL | | Director |
Director since November 26, 1996.
Mr. Darnell is a former senior executive of Baxter Corporation and has 35 years of U.S. and international experience in the diagnostic industry.
| Rolf H. Henel | | Wayne, NJ | | Director |
Director since August 14, 2002
Mr. Henel is the retired President of Cyanamid International Lederle Division. He currently serves as an advisor to the healthcare industry and is a partner in Naimark & Associates, a healthcare consulting firm. He is also a director and member of the Audit Committee of both SciClone Pharmaceuticals and Penwest Pharmaceuticals.
| Brian M. King | | Kenora, ON | | Chairman and Director |
Director since May 26, 1994.
Mr. King is the Chairman of the Board of the Company. He is the former Chairman and Chief Executive Officer of Connaught Biosciences, Inc. He is also a director of Onex Corporation, the VenGrowth Investment Funds and the Health Care and Biotechnology Venture Fund.
| Samuel Sarick | | Toronto, ON | | Director |
Director since March 31, 1989.
Mr. Sarick is President of Samuel Sarick Limited, a company with long-standing involvement in commercial property development. He is also a director with The Goldfarb Corporation.
| Stewart D. Saxe | | Toronto, ON | | Director |
Director since November 11, 1987.
Mr. Saxe is an international Partner with the law firm of Baker & McKenzie. He is certified by the Law Society of Upper Canada as a specialist in labour law and as a Human Resources Professional by the Human Resources Professionals Association of Ontario.
| Bruce W. Simpson | | Fort Worth, TX | | Director |
Director since August 14, 2002.
Mr. Simpson served as the President of the Genpharm division of E Merck, the President and CEO of Medeva Pharmaceuticals in the United States and in senior management positions at Fisons Corporation. He
58
currently heads a private consulting firm specializing in marketing and business development within the healthcare industry.
| John A. Vivash | | Toronto, ON | | Director |
Director since November 17, 1998.
Mr. Vivash was formerly President and Chief Executive Officer of CIBC Securities Inc. He also was President and Chief Executive Officer of Fidelity Investments Canada Limited and Manulife Securities International Ltd. He is currently President and Chief Executive Officer of Tesseract Financial Inc. (formerly Vivash Consulting Inc.), a financial services consultancy.
| Jim A.H. Garner | | Toronto, ON | | Officer |
Officer since September 1996.
Mr. Garner, CA, B. COMM., MBA, CBVA, joined DRAXIS in September 1996 and is currently the Senior Vice President, Finance and Chief Financial Officer of the Company. Since September 2002, he has also served as Acting President of DPI. Immediately prior to joining DRAXIS, Mr. Garner was Director of Finance at Algoma Steel Inc. Mr. Garner's other experience includes ten years in investment banking with RBC Dominion Securities and Rothschild Canada. Mr. Garner holds a B. Comm. from Queen's University and an MBA from IMD (Lausanne, Switzerland). He is also a Chartered Accountant and a Chartered Business Valuator.
| Dan Brazier | | Stouffville, ON | | Officer |
Officer since August 1998.
Mr. Brazier, HON. B. COMM., joined DRAXIS in August 1998 as President of SpectroPharm Dermatology and now holds the position of President of DRAXIS Pharmaceutica. Prior to joining the Company, Mr. Brazier was the Director of Skin Care at Allergan Inc. Mr. Brazier also held positions in Marketing Management and Sales Management in his 10 years with Allergan. From 1980-1989, Mr. Brazier held Marketing and Sales positions with Bausch & Lomb, Stafford Foods and Canada Packers. Mr. Brazier holds an Honors B. Comm., majoring in Marketing and Finance.
| Jack A. Carter | | Toronto, ON | | Officer |
Officer since September 1998.
Mr. Carter, B.A., C.H.R.P., joined DRAXIS in September 1998 as Vice President Human Resources following a lengthy relationship with the Company in a Consultant capacity. Immediately prior to joining DRAXIS, Mr. Carter was the President of a Human Resource Consulting firm, specializing in strategic human resource issues within the Canadian Pharmaceutical Industry. Prior to this Mr. Carter has gained significant experience as Vice President Human Resources for Sterling Drug/Kodak, Director of Human Resources for the Baxter Corporation, and Director of Human Resources for Johnson & Johnson. Mr. Carter also served on the founding Board of Directors of the Human Resources Professionals Association of Ontario, and was nominated to the "Council of Human Resource Executives" for The Conference Board of Canada.
| Douglas M. Parker | | Toronto, ON | | Officer |
Officer since April 1999.
Mr. Parker, B.A., LL.B., joined DRAXIS as Director, Legal Affairs in April 1999 and became General Counsel & Secretary in June 2000. Prior to joining DRAXIS, Mr. Parker was a corporate associate with the Toronto law firm of Beard, Winter. Mr. Parker holds an Honours Bachelor of Arts from Trinity College, University of Toronto and a law degree from Queen's University.
| Richard Flanagan | | Montreal, QC | | Officer |
Officer since September 1997.
Dr. Flanagan, B.Sc., Ph.D., MCIC, is President of DRAXIMAGE. He has been with the Company since its founding in July of 1997 and with the same division since 1980 when it was part of Merck. Dr. Flanagan served as Executive Vice President of DRAXIMAGE until September 1, 2001 when he was named President. Prior to
59
joining Merck, Dr. Flanagan was an Adjunct Professor in the Faculty of Pharmacy at the University of Alberta. Dr. Flanagan is the author of thirty scientific papers and is listed as inventor on twelve patents. Dr. Flanagan obtained his Ph.D. in Organic Chemistry from the University of Alberta and a B.Sc. from the University College Dublin, Ireland. He is a Killam Fellow and received the Hugh Ryan Memorial Gold Medal as an undergraduate. Dr. Flanagan has served as an advisor to the International Atomic Energy Agency and has sat on grant review committees in Canada and the United States.
| Jerry Ormiston | | Toronto, ON | | Officer |
Officer since May 2001.
Mr. Ormiston, B.SC., MBA, joined DRAXIS in May 2001 as Executive Director, Investor Relations following a two-year stint as a senior consultant in a Toronto investor relations firm working with small and mid-sized entrepreneurial companies on capital markets communications strategies. Prior to that he was at Allelix Biopharmaceuticals for 12 years—four as Manager of Investor Relations and eight in the business development area. He has 25 years experience in the healthcare industry in pharmaceuticals and biotechnology. His assignments over those years have included positions in business development, marketing, corporate communications, production management, quality assurance and regulatory affairs.
Terms of office as director and/or officer are for one year or until the next Annual Meeting of the Shareholders and subsequent Meeting of the Board of Directors, respectively.
There is no family relationship between any of the executive officers. Officers of the Company serve at the pleasure of the Board of Directors.
60
The following table sets forth all annual and long-term compensation for services in all capacities to the Company and its subsidiaries for the fiscal years ended December 31, 2002, 2001 and 2000 in respect of each of the individuals who were, at December 31, 2002, the Chief Executive Officer or other named executive officers (as defined in the Regulations to the Securities Act (Ontario)) of the Company and who received salary and bonus in excess of CDN$100,000 in the 2002 fiscal year.
| |
| | Annual Compensation
| |
| |
|
|---|
| |
| |
| |
| |
| |
| | Other Annual
Compensation(1)(2)
| | Long-Term Compensation Awards
|
|---|
| |
| | Salary
| | Bonus
|
|---|
| |
| |
| | Issuance
of EPSPP
Shares
Series "C"
(#)
|
|---|
Name & Principal Position
| | Year
| | Cash
(CDN$)
| | DSU
Election(3)
(CDN$)
| | Cash
(CDN$)
| | DSU
Election(3)
(CDN$)
| | (CDN$)
| | Securities
Under Options
Granted
(#)(4)
|
|---|
Martin Barkin, MD
President & Chief Executive Officer | | 2002
2001
2000 | | 301,600
297,000
298,083 | | 75,400
73,000
66,917 | | nil
109,500
75,000 |
(5) | —
nil
55,000 | | 309,943
32,490
38,346 | (6)(7)
| 225,000
120,000
400,000 | | nil
nil
nil |
Jim A.H. Garner
Senior Vice President, Finance & Chief Financial Officer(9), Acting President, Draxis Pharma Inc. |
|
2002
2001
2000 |
|
226,250
180,000
163,334 |
|
nil
20,000
36,667 |
|
30,000
45,000
16,000 |
|
10,000
15,000
16,000 |
|
87,597
nil
nil |
(7)(8)
|
25,000
60,000
90,000 |
|
nil
nil
nil |
Richard J. Flanagan
President, DRAXIMAGE |
|
2002
2001
2000 |
|
180,000
153,493
140,240 |
|
nil
nil
nil |
|
25,000
10,000
6,300 |
|
nil
nil
nil |
|
nil
nil
nil |
|
40,000
70,000
nil |
|
nil
nil
nil |
Dan Brazier
President, DRAXIS Pharmaceutica |
|
2002
2001
2000 |
|
165,000
165,000
165,000 |
|
nil
nil
nil |
|
49,500
62,700
59,400 |
|
nil
nil
nil |
|
nil
nil
nil |
|
20,000
nil
nil |
|
nil
nil
nil |
Jack A. Carter
Vice President, Human Resources |
|
2002
2001
2000 |
|
130,000
130,000
130,000 |
|
nil
nil
nil |
|
30,000
24,375
17,550 |
|
nil
nil
nil |
|
21,961
nil
41,094 |
(10)
(11) |
10,000
55,000
15,000 |
|
nil
nil
nil |
- (1)
- "Other Annual Compensation" does not exceed the lesser of CDN$50,000 or 10% of the annual salary and bonus for the fiscal year, except as noted.
- (2)
- This column excludes amounts related to subscription proceeds advanced by the Company in connection with the original 1995 issuance of Participation Shares, Series A (CDN$150,000 to Dr. Barkin). On February 15, 2000 the Company's common share price had not achieved the specified threshold price for conversion of the Participation Shares, Series A, and accordingly any remaining outstanding shares were cancelled and the original subscription advances were deemed as income for Canadian tax purposes. See "Incentive Plans—Employee Participation Share Purchase Plan."
- (3)
- Amounts in these columns relate to the portions of salary and bonus elected to be received in the form of Deferred Share Units. See "Incentive Plans—Deferred Share Unit Plan."
- (4)
- Option grants in subsidiaries of the Company are reported under "Subsidiary Long-Term Incentive Plans."
- (5)
- This amount includes one-time special bonus awards to Dr. Barkin (CDN$75,000) to offset a portion of the negative tax consequences experienced by participants in connection with the cancellation of the original 1995 issuance of Participation Shares, Series A.
- (6)
- This amount includes the aggregate value realised from the exercise of options in the amount of CDN$289,125 for 2002.
- (7)
- Dr. Barkin exercised and held on March 12, 2002, 225,000 expiring stock options, and Mr. Garner exercised and held on May 21, 2002, 40,000 expiring stock options. See "Incentive Plans—Stock Option Plan."
- (8)
- This amount includes the aggregate value realised from the exercise of options in the amount of $76,500 for 2002.
- (9)
- Mr. Garner's base salary in 2002 was $200,000. For the period September 5, 2002 to December 31, 2002, he held the position of Acting President of Draxis Pharma Inc., for which he received additional compensation of $26,250 in 2002.
- (10)
- This amount includes the aggregate value realized from the exercise of options in the amount of $16,450 for 2002.
- (11)
- This amount includes the aggregate value realized from the exercise of options in the amount of $38,600 for 2000.
61
Incentive Plans
The Company's philosophy is to link employee compensation to the success of the Company and to emphasise "at risk" employee compensation. The Company does not provide any form of pension program to its executive officers.
The Company has implemented the following plans in an effort to achieve "at risk" employee compensation.
The Company has an established guideline limiting the aggregate number of common shares that can be issued at any point in time, either through the exercise of options or the conversion of Participation Shares, to 13% of the Company's outstanding common shares. As at March 31, 2003, the number of common shares so issuable was 9.5%.
Intention—This plan was approved by shareholders on February 3, 1988 to permit the Board of Directors to grant options to purchase common shares to directors, officers, employees and arm's-length consultants of the Company and its subsidiaries so as to link corporate compensation to enhanced shareholder value.
Maximum Common Shares Issuable—The shareholders have authorized 7,500,000 common shares for issuance under this plan.
Options Available for Future Grants—1,271,528 as at March 31, 2003.
Percentage of Common Shares represented by unexercised options of Named Executive Officers—4.7%.
Aggregate Option Grants to Named Executive Officers in 2002—320,000.
The following table sets forth individual exercises of options by the following named executive officers during the financial year ended December 31, 2002 and the financial year end value of unexercised options:
Name
| | Securities
Acquired on
Exercise
| | Aggregate Value
Realised
| | Unexercised Options at
December 31, 2002
(#)
Exercisable/
Unexercisable
| | Value of Unexercised in-
the-Money Options at
December 31, 2002
(CDN$)
Exercisable/
Unexercisable
|
|---|
| Martin Barkin | | 225,000 | | 289,125 | (1) | 424,292/438,333 | | nil/nil |
| Dan Brazier | | nil | | nil | | 100,000/20,000 | | nil/nil |
| Jim A.H. Garner | | 40,000 | | 76,400 | (2) | 335,000/95,000 | | nil/nil |
| Richard Flanagan | | nil | | nil | | 43,333/86,667 | | nil/nil |
| Jack Carter | | 10,000 | | 16,450 | | 48,333/51,667 | | nil/nil |
- (1)
- Dr. Barkin exercised 225,000 expiring options on March 12, 2002 and has retained the underlying shares at his own expense.
- (2)
- Mr. Garner exercised 40,000 expiring options on May 21, 2002 and has retained the underlying shares at his own expense.
Intention—To align further the interests of senior management with those of shareholders by increasing management shareholdings at minimal cost to the Company.
Mechanism—Eligible participants in this plan are entitled to elect yearly to receive up to 20% of base salary and up to 100% of any bonus paid in respect of that year in Deferred Share Units in lieu of cash compensation. An election must be made by December 1 of each year in respect of base salary and bonus for the next year. The elected amount is converted to a number of Deferred Share Units equal to the elected amount divided by the closing price of the common shares on the Toronto Stock Exchange or The NASDAQ National Market System on December 31 of each year in which the election is made, based on a purchase commitment as of December 1 of the prior year. This plan is administered by the Board of Directors of the Company.
62
Participants—Members of senior management designated by the Compensation Committee.
Redemption of Deferred Share Units—Participants are not entitled to receive any Deferred Share Units until cessation of employment with the Company for any reason. The value of each Deferred Share Unit, redeemable by the participant, will be equivalent to the market value of a common share at the time of redemption. The Deferred Share Units must be redeemed no later than the end of the first calendar year commencing after the date of cessation of employment.
Named Executive Officer Elections for 2001, 2002 and 2003—The following table summarises the elections under this plan by named executive officers:
| | DSU Elections
|
|---|
| | 2001
| | 2002
| | 2003
|
|---|
| | Salary
| | Bonus
| | Salary
| | Bonus
| | Salary
| | Bonus
|
|---|
| | %
| | CDN$
| | %
| | CDN$
| | %
| | CDN$
| | %
| | CDN$
| | %
| | CDN$
| | %
| | CDN$
|
|---|
| Martin Barkin | | 20 | | 73,000 | | nil | | nil | | 20 | | 75,400 | | 100 | | nil | | 20 | | 75,400 | | 50 | | TBD |
| Jim Garner | | 10 | | 20,000 | | 25 | | 15,000 | | nil | | nil | | 25 | | 10,000 | | 5 | | 10,500 | | 25 | | TBD |
As at April 7, 2003, Dr. Barkin and Mr. Garner held 106,323 and 39,512 DSUs, respectively.
Employee Group Registered Retirement Savings Plan
Intention—To provide a cost-sharing program for employees of the Company and its subsidiaries by way of a corporate sponsored registered retirement savings plan ("RSP") matching mechanism for employee retirement.
Mechanism—Commencing 12 months after continuous employment, employees of the Company and its subsidiaries are entitled to receive a discretionary payment from the Company which matches employee contributions on a dollar-for-dollar basis towards an employee's RSP, up to a maximum of 5% of the employee's eligible income ("normal" straight time annual earnings or salary, not to include overtime payments, bonus or any other form of exceptional payment). The corporate contribution to this plan remains under the ownership and control of the employee and is not subject to any lock-in provisions. The plan is administered by a committee. Subject to the investment choices approved by the committee, employees control the investment of both employee and the Company.
Participants—Participation is open to all full and part time permanent Canadian employees of the Company and its subsidiaries, other than designated senior management employees.
Commencement Date—The plan commenced on January 1, 2002, but employee matching contributions are not required for 2002 and 2003.
Subsidiary Long-term Incentive Plans
Intention—To align further the interests of senior management of the Company's wholly-owned subsidiary, DRAXIMAGE, with increasing the value of the subsidiary and therefore enhancing shareholder value of the Company as a whole.
Mechanism—The terms of this plan provide that, subject to the achievement of certain conditions, the Company will make payments to plan participants in the form of cash and/or the Company's common shares, at the Company's option, based on increases in the fair market value of DRAXIMAGE's equity in excess of the Company's acquisition cost.
Participants—Selected members of senior management of DRAXIMAGE as designated by the Company. Dr. Richard Flanagan participates in this plan.
Intention—To align further the interests of senior management of the Company's subsidiary, DPI, with increasing the value of the subsidiary and therefore enhancing shareholder value of the Company as a whole.
Mechanism—Eligible participants in this plan subscribe for treasury shares of DPI at a pre-determined initial subscription price. This initial subscription price is funded 20% by participants and 80% by way of a loan by DPI.
63
For each DPI share acquired by a participant, the participant receives a ten-year share purchase warrant to purchase an additional DPI treasury share at the original subscription price. These warrants only vest if certain pre-established DPI earning hurdles are achieved.
Loan Terms—Loans for the initial share subscription are interest free, non-recourse to the participants and repaid after a two-year non-payment period followed by amortization on a quarterly basis over a ten-year period. The security for the loans are the DPI shares issued to the participants pledged in favour of DPI until re-payment is made in full, with standard events of default.
Participants—Selected members of the senior management of DPI designated by the shareholders of DPI. No named executive officer participates in this plan. Mr. Dwight Gorham, former President of DPI, resigned from his position with the Company in September 2002, resulting in a return of his initial subscription price proceeds and the cancellation of his DPI shares.
Discontinued Plans
In late 2002, the Board of Directors undertook a comprehensive review of the Company's various incentive plans in the context of current best practices and the requirements of the Sarbanes-Oxley Act of 2002.
As a result of this review, the Board of Directors decided to discontinue certain incentive plans of the Company effective December 4, 2002, subject to appropriate grandfathering provisions related to outstanding plan participation. There was no participation in these plans in 2002.
The details of these plans are listed below.
This plan was originally implemented in 1991, and subsequently amended in 1998 and in 2001, to provide an incentive to all employees of the Company by giving them a direct interest in the Company's growth and development. In 2002, the Board of Directors replaced this plan with a registered retirement savings program. No common shares were granted under the plan to employees in 2002.
Eligible participants in this plan were entitled yearly to purchase common shares of the Company equal to not more than to 40% of base salary, funded by an interest bearing loan from the Company, with the common shares to be acquired by open market purchase by a trustee on behalf of the participants. The purchase price is equal to the closing price of the common shares on the TSX or NASDAQ on December 31 of each year, based on a purchase commitment as of December 1 of the prior year. This plan has been cancelled by the Board.
Each Participation Share is equal to a fraction of a common share depending on the performance of the common share from the date the Participation Share is issued to the date of conversion of the Participation Share. To minimize dilution to shareholders compared to a stock option plan, common shares are issued only to the extent that the stock price has appreciated since the date the Participation Shares are issued rather than on a per option exercise basis. The value and subscription price of such common shares is determined with the assistance of an independent valuator and the subscription amount is loaned to the participant on a non-recourse, interest-free basis by the Company. Certain restrictions on transferability apply and redemption rights are retained by the Company. Participation Shares generally vest over a four-year period. The loaned funds are repayable to the Company on conversion of vested Participation Shares. Participation Shares were viewed as advantageous over other forms of compensation for various reasons including mild dilution impact, lack of need for participants to sell shares to convert and favourable participant tax treatment. No further Participation Shares will be issued by the Company.
64
The following table sets forth the 2002 financial year end value of the named executive officers' unconverted Participation Shares, on an aggregated basis:
| | Unconverted Participation Shares(1) at December 31, 2002
| | Value of Unconverted in-the-Money Participation Shares at December 31, 2002
(CDN$)
|
|---|
Name
|
|---|
| | Convertible
| | Unconvertible
| | Convertible
| | Unconvertible
|
|---|
| Martin Barkin | | 147,000 | | 98,000 | | nil | | nil |
| Jim A.H. Garner | | 45,000 | | 30,000 | | nil | | nil |
| Dan Brazier | | 15,000 | | 10,000 | | nil | | nil |
- (1)
- These Participation Shares, Series "C" were issued on May 12, 1999 for $0.50 per Participation Share subscription price.
65
Termination of Employment and Employment Contracts
Commencing in 1988, as executive officers joined the Company, DRAXIS entered into employment agreements with certain of these individuals. The agreements provide for the compensation in the amounts set forth under "Executive Compensation—Summary Compensation Table." In the event of his termination of employment without cause, Dr. Barkin is entitled to receive a payment equal to three times his annual remuneration. In the event of his termination of employment following a change of control of the Company, Dr. Barkin is entitled to receive a payment equal to five times his annual remuneration. In the event of his termination of employment without cause, Mr. Garner is entitled to receive a payment equal to two times his annual remuneration. In the event of termination of employment following a change of control of DRAXIS, Mr. Garner is entitled to receive a payment equal to three times his annual remuneration. In the event of his termination of employment without cause, Mr. Brazier or Mr. Carter or Dr. Flanagan, as the case may be, is entitled to receive a payment equal to his annual remuneration. In the event of his termination of employment following a change of control of DRAXIS or DRAXIS Pharmaceutica, Mr. Brazier is entitled to receive a payment equal to two times his annual remuneration.
Compensation of Directors
The compensation paid to each independent director of the Company is an annual retainer of CDN$12,500 plus a meeting fee of CDN$1,500 for each Board and committee meeting attended, some via teleconference. In addition, the Chairs of committees receive an annual retainer of CDN$1,500. For the year ended December 31, 2002, Mr. Brian King received CDN$60,000 as non-executive Chairman of the Board. He did not otherwise receive meeting fees as a director. In December 2001, under the Company's Stock Option Plan, each of the directors (excluding the President and Chief Executive Officer) was awarded 15,000 options as partial compensation for services rendered as directors. The Chairman was awarded an additional 5,000 options. It is contemplated that an award of the same number of options will occur annually on each January 1 based on the average price of the common shares on the TSX for the five trading days immediately preceding January 1 in each year.
From time to time, special committees of the Board of Directors are appointed to consider special issues, in particular, issues which could potentially involve related party transactions and the adequacy and form of the compensation of Directors. Compensation for work on such committees is set based on the amount of work involved.
The directors, other than Dr. Barkin, do not have service contracts with the Company or any of its subsidiaries providing for benefits upon termination of employment.
Minimum Shareholding Requirements
In 2001, the Board of Directors established a corporate policy requiring each current Board member, to acquire over the next three years (or in the case of future Board members, within three years of appointment) shares in the Company equal to five times his or her annual cash compensation.
Director shareholdings as of March 31, 2003
Director
| | Shares Owned
| | % Owned of total issued and
outstanding common shares
| |
|---|
| Martin Barkin | | 561,848 | (1) | 1.5 | |
| Leslie Dan | | 18,356 | | <1 | |
| George Darnell | | 16,000 | | <1 | |
| Rolf H. Henel | | 10,000 | | <1 | |
| Brian King | | 80,750 | | <1 | |
| Sam Sarick | | 1,070,904 | | 2.9 | |
| Stewart Saxe | | 126,485 | | <1 | |
| Bruce W. Simpson | | nil | | nil | |
| John Vivash | | 15,000 | | <1 | |
| | |
| |
| |
| Total | | 1,899,343 | | 5.1 | % |
- (1)
- In addition, Dr. Barkin owns 98,198 Deferred Share Units of the Company. See "Incentive Plans—Deferred Share Unit Plan."
66
Senior Management shareholdings as of March 31, 2003
Senior Management
| | Shares Owned
| | % Owned of total issued and
outstanding common shares
| |
|---|
| Martin Barkin | | 561,848 | (1) | 1.5 | |
| Jim A.H. Garner | | 115,863 | | <1 | |
| Dan Brazier | | 15,949 | | <1 | |
| Jerry Ormiston | | nil | | <1 | |
| Jack A. Carter | | 6,526 | | <1 | |
| Douglas M. Parker | | 9,753 | | <1 | |
| Richard Flanagan | | 15,576 | | <1 | |
| | |
| |
| |
| Total | | 725,515 | | 2.0 | % |
- (1)
- In addition, Dr. Barkin owns 98,198 Deferred Share Units of the Company. See "Incentive Plans—Deferred Share Unit Plan."
67
Director outstanding options as of April 30, 2003
Director
| | # of Options
| | Exercise Price
| | Expiration Date
|
|---|
| Martin Barkin | | 40,000
33,750
33,750
400,000
10,125
120,000
225,000 | | CDN$3.05
US$1.48
US$1.85
CDN$3.07
US$3.02
CDN$3.60
CDN$4.00 | | August 12, 2003
May 20, 2004
September 20, 2004
April 18, 2005
June 13, 2006
July 5, 2006
February 5, 2007 |
|
| Leslie L. Dan | | 10,000
10,000
10,000
15,000
15,000 | | CDN$3.64
CDN$1.63
CDN$2.91
CDN$4.19
CDN$2.33 | | December 31, 2003
December 31, 2004
December 31, 2005
December 31, 2006
December 31, 2007 |
|
| George M. Darnell | | 10,000
10,000
33,750
10,000
10,125
15,000
15,000 | | CDN$3.64
CDN$1.63
US$1.76
CDN$2.91
US$3.02
CDN$4.19
CDN$2.33 | | December 31, 2003
December 31, 2004
June 13, 2005
December 31, 2005
June 13, 2006
December 31, 2006
December 31, 2007 |
|
| Rolf H. Henel | | 10,000
5,000
15,000 | | CDN$5.12
CDN$3.66
CDN$2.33 | | April 30, 2007
August 13, 2007
December 31, 2007 |
|
| Brian M. King | | 15,000
15,000
15,000
20,000
20,000 | | CDN$3.64
CDN$1.63
CDN$2.91
CDN$4.19
CDN$2.33 | | December 31, 2003
December 31, 2004
December 31, 2005
December 31, 2006
December 31, 2007 |
|
| Samuel Sarick | | 10,000
10,000
10,000
10,125
15,000
15,000 | | CDN$3.64
CDN$1.63
CDN$2.91
US$3.02
CDN$4.19
CDN$2.33 | | December 31, 2003
December 31, 2004
December 31, 2005
June 13, 2006
December 31, 2006
December 31, 2007 |
|
| Stewart D. Saxe | | 10,000
33,750
10,000
10,000
10,125
15,000
15,000 | | CDN$3.64
US$1.85
CDN$1.63
CDN$2.91
US$3.02
CDN$4.19
CDN$2.33 | | December 31, 2003
September 20, 2004
December 31, 2004
December 31, 2005
June 13, 2006
December 31, 2006
December 31,2007 |
|
| Bruce W. Simpson | | 10,000
5,000
15,000 | | CDN$5.12
CDN$3.66
CDN$2.33 | | April 30, 2007
August 13, 2007
December 31, 2007 |
|
| John A. Vivash | | 10,000
10,000
10,000
15,000
15,000 | | CDN$3.64
CDN$1.63
CDN$2.91
CDN$4.19
CDN$2.33 | | December 31, 2003
December 31, 2004
December 31, 2005
December 31, 2006
December 31, 2007 |
|
| Total | | 1,405,500 | | | | |
68
Senior Management outstanding options as of April 30, 2003
Senior Management
| | # of Options
| | Exercise Price
| | Expiration Date
|
|---|
| Martin Barkin | | 40,000
33,750
33,750
400,000
10,125
120,000
225,000 | | CDN$3.05
US$1.48
US$1.85
CDN$3.07
US$3.02
CDN$3.60
CDN$4.00 | | August 12, 2003
May 20, 2004
September 20, 2004
April 18, 2005
June 13, 2006
July 5, 2006
February 5, 2007 |
|
| Jim A.H. Garner | | 30,000
40,000
50,000
60,000
225,000
25,000
40,000 | | CDN$3.05
CDN$2.49
CDN$3.27
CDN$3.60
CDN$4.40
CDN$3.66
CDN$2.32 | | August 11, 2003
February 8, 2005
December 5, 2005
July 5, 2006
September 4, 2006
August 13, 2007
December 31, 2007 |
|
| Dan Brazier | | 100,000
20,000
30,000 | | CDN$3.05
CDN$2.50
CDN$2.32 | | August 11, 2003
October 31,2007
December 31, 2007 |
|
| Jack A. Carter | | 15,000
55,000
10,000
20,000 | | CDN$3.27
CDN$3.60
CDN$3.66
CDN$2.32 | | December 6, 2005
July 5, 2006
August 13, 2007
December 31, 2007 |
|
| Richard Flanagan | | 20,000
70,000
40,000
20,000 | | CDN$3.05
CDN$3.60
CDN$3.66
CDN$2.32 | | August 11, 2003
July 5, 2006
August 13, 2007
December 31, 2007 |
|
| Jerry Ormiston | | 25,000
50,000
25,000
10,000
15,000 | | CDN$3.00
CDN$3.15
CDN$3.60
CDN$3.66
CDN$2.32 | | May 29, 2006
June 19, 2006
July 5, 2006
August 13, 2007
December 31, 2007 |
|
| Douglas M. Parker | | 6,667
30,000
15,000
20,000
35,000
30,000 | | CDN$2.84
CDN$3.37
CDN$3.27
CDN$3.60
CDN$3.66
CDN$2.32 | | April 7, 2004
May 11, 2005
December 6, 2005
July 5, 2006
August 13, 2007
December 31, 2007 |
|
| Total | | 1,994,292 | | | | |
The Board of Directors of the Company believes that sound corporate governance practices are essential to the well being of the Company and its shareholders, and that these practices should be reviewed regularly to ensure that they are appropriate. A description of the Company's corporate governance practices follows. The Board of Directors has carefully considered and reviewed the guidelines for effective corporate governance issued by the Toronto Stock Exchange ("TSX Guidelines"), the corporate governance requirements of the Sarbanes-Oxley Act of 2002, and the proposed reforms of corporate governance listing standards of NASDAQ. The Board of Directors believes that the Company's corporate governance practices are well-aligned with the recommendations contained therein.
69
The mandate of the Board of Directors is to supervise the management of the business and affairs of the Company. In light of that responsibility, the Board reviews, discusses and approves various matters related to the Company's operations, strategic direction and organizational structure, where required, and involves itself jointly with management in ensuring the creation of shareholder value and the serving of the best interests of the Company. The Board of Directors has responsibility for such matters as:
- (a)
- the strategic planning process (including approval of strategic business plans);
- (b)
- the identification of the principal risks of the Company's business and ensuring the implementation of appropriate systems to manage such risks;
- (c)
- succession planning, including the appointing and monitoring of senior management;
- (d)
- the review of the Company's communications policy;
- (e)
- the integrity of the Company's internal control and management information systems; and
- (f)
- the supervision of management of the Company's operations.
There are five regularly scheduled meetings per year. However, the Board of Directors also meets as frequently as the need arises to consider opportunities or major transactions.
There were eleven meetings of the Board of Directors in 2002.
The Board of Directors has reviewed the composition of the Board to determine which of the directors may be considered "unrelated" within the meaning of that term as set out in the TSX Guidelines. A director who is free from any interest and any business or other relationship which could, or could reasonably be perceived to, materially interfere with the director's ability to act with a view to the best interests of the Company, other than interests arising from shareholdings, is considered to be an unrelated director.
The Board of Directors, currently composed of nine members, has considered the relationship of each of the directors to the Company and has determined that eight of the nine directors are unrelated to the Company. Dr. Martin Barkin, the President and Chief Executive Officer of the Company, is considered to be a related director since he is an employee of the Company.
The Board believes that Dr. Barkin is sensitive to conflicts of interest and excuses himself from deliberations and voting in appropriate circumstances. He brings a skill set and knowledge base that is beneficial to the Company and his participation as a director contributes to the effectiveness of the Board of Directors.
The Board has adopted a practice that the Chair of the Board and the CEO of the Company are separate individuals.
The Board also has adopted a policy of meeting without any management present (including the CEO) at every Board meeting.
The Board of Directors has three standing committees: the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee. To maintain appropriate independence, no members of management sit on any of the three standing committees.
From time to time, special committees of the Board of Directors are appointed to consider special issues, in particular, any issues which could potentially involve related party transactions.
Board committees and individual Board members engage independent consultants and outside advisors at the expense of the Company, where reasonable and appropriate, to assist them in discharging their responsibilities.
The Audit Committee is responsible for reviewing the Company's financial reporting procedures and internal controls. The Committee is also responsible for reviewing quarterly financial statements and the annual financial
70
statements prior to their approval by the full Board of Directors and communicating regularly with the Company's external auditors.
The Company believes the oversight responsibility of the Committee provides a key stewardship role for the Committee in the Company's financial disclosure issues, internal controls, risk management, corporate finance and related matters.
In reviewing the audited financial statements of the Company, the Committee discusses the quality, not just the acceptability, of the accounting principles, the reasonableness of significant judgements, and the clarity of disclosure in the financial statements. In addition, the Committee discusses with the Company's independent auditors the overall scope and plans for their audit. The Committee meets with the external auditors, with and without management present, to discuss the results of their examination and the overall quality of the Company's financial reporting. The Committee also carefully reviews evolving Canadian and U.S. audit committee regulations and best practices to ensure corporate alignment with the spirit and intent of such practices.
In February 2003, the Board of Directors approved a detailed charter for the Audit Committee. The charter specifies the purpose of the Committee is to assist the Board of Directors with its oversight responsibilities by:
- •
- Reviewing the integrity of the consolidated financial statements of the Company;
- •
- Recommending to the Board the appointment of the independent auditor and reviewing the independent auditor's qualifications and independence;
- •
- Reviewing the performance of the Company's independent auditors;
- •
- Reviewing the timely compliance by the Company with all legal and regulatory requirements for audit and related financial functions of the Company;
- •
- Reviewing financial information contained in public filings of the Company prior to filing;
- •
- Reviewing earnings announcements of the Company prior to release to the public;
- •
- Reviewing the Company's systems of and compliance with internal financial controls; and
- •
- Reviewing the Company's auditing, accounting and financial reporting processes.
The Committee is composed of four directors, all of whom are unrelated directors. The current members of the Audit Committee are Messrs. Samuel Sarick (Chair), Leslie Dan, George Darnell and Rolf Henel. Mr. Henel is the Company's designated "Audit Committee Financial Expert."
The Compensation Committee oversees overall corporate policy with respect to compensation and benefits and makes recommendations to the Board of Directors on, among other things, the compensation of senior executives. In assessing compensation issues, the Committee reviews and examines in detail the performance of senior management. Each of the four members of the Committee is an unrelated director. The current members of the Compensation Committee are Messrs. Brian King (Chair), Stewart Saxe, Bruce Simpson and John Vivash.
The Nominating and Corporate Governance Committee is responsible for recommending annually the members of the Board proposed for election to the Board of Directors, recommending new candidates for Board membership, monitoring Board composition and suggesting appropriate changes. It seeks on behalf of shareholders well qualified candidates to nominate as directors (individuals who provide a balance in terms of their backgrounds and experience in different industries and professions). It is also responsible for making recommendations to the full Board with respect to developments in the area of corporate governance and the practices of the Board.
Each of the four members of the Committee is an unrelated director. The current members of the Nominating and Corporate Governance Committee are Messrs. John Vivash (Chair), George Darnell, Rolf Henel and Bruce Simpson.
71
In addition to those matters that must by law be approved by the Board of Directors, certain other significant matters relating to the business and affairs of the Company require prior approval of the Board. These matters are:
- (a)
- the approval of the quarterly financial statements and earnings announcements;
- (b)
- the approval of the Company's strategic business plans and directions;
- (c)
- any related party transaction or any transaction involving any officer or director, regardless of materiality, such as the approval of the Company's lease of its Goreway Drive premises; and
- (d)
- any disposition or expenditure in excess of CDN$1,000,000.
In orienting new members to the Board of Directors, members are provided with an opportunity to visit the Company's facilities and to meet with management and other members of the Board to discuss and understand the business. In addition, detailed documentation is provided relating to the current business plan and current policies of the Company.
The Board of Directors discusses regularly the effectiveness of its meetings and decision making process by considering and assessing from time to time the performance of the Board relating to its effectiveness, size, compensation policies and the assessment of management performance.
On an annual basis, the Company's President and CEO circulates a strategic plan which is discussed and, if appropriate, adopted by the Board of Directors. This strategic plan forms the basis of the corporate objectives which the CEO is responsible for meeting. The Compensation Committee of the Board of Directors assesses the CEO's performance and recommends to the Board as a whole, his compensation. Specifically, the Compensation Committee's assessment of the CEO's performance considers matters such as the development of appropriate strategic direction and action plans for the Company, meeting key objectives, identification of significant issues and challenges facing the Company and the development of appropriate solutions to address such issues and challenges.
The Company maintains an investor relations department headed by an Executive Director, Investor Relations, which reports directly to the President and CEO of the Company. In addition, investor relations experts are retained from time to time by the Company to advise on issues and best practices for investor relations. The Company communicates regularly with its shareholders through annual and quarterly reports. At the Company's annual meetings of shareholders, a full opportunity is afforded for shareholders to ask questions concerning the Company's business. Each shareholder and investor inquiry receives a prompt response from the investor relations department or an appropriate officer of the Company. Information about the Company, including annual reports, interim financial reports and recent news releases, are also available on the Company's Internet home page at www.draxis.com. In addition, the Company provides the opportunity for investors in both Canada and the U.S. to pose questions to senior management, including the CEO, the Chief Financial Officer and the Executive Director, Investor Relations through public forums such as conference calls and webcasts.
Management is responsible for the day-to-day operations of the Company and is expected to implement the approved strategic business plan within the context of authorized budgets and corporate policies and procedures. The information which management provides to the Board of Directors is critical. Management is expected to report regularly to the Board of Directors in a comprehensive, accurate and timely fashion on the business and affairs of the Company. The Board of Directors monitors the nature of the information requested by and provided to it so that it can effectively identify issues and opportunities for the Company.
72
As at December 31, 2002, the Company and its subsidiaries employed a total of 315 employees. DPI is the largest employer with 213 employees, all located in Montréal, Québec. DRAXIMAGE employs 72 people all in Montréal, Québec. The Company itself employs 30 people, the majority of whom are located in the Province of Ontario. At December 31, 2002 and 2001, the Company and its subsidiaries had 315 and 313 employees, respectively.
73
Item 7. Major Shareholders and Related Party Transactions
The Company is not aware of any company, foreign government or natural or legal person that owns or controls the Company, directly or indirectly.
As of March 31, 2003, the officers and directors of the Company as a group owned 2,063,010 shares of the Company's common stock, which represents 5.6% of the outstanding shares. To our knowledge, no other person beneficially owns, directly or indirectly, or exercises control over our common shares carrying more than five percent (5%) of the votes attached to all of those shares.
As of April 7, 2003, 17,985,003 common shares (48.5% of the outstanding common shares) were held of record by 584 shareholders whose registered addresses were in the United States. The Company has no knowledge of any other Shares held beneficially in the United States.
As of April 7, 2003, there were a total of 1,236 shareholders of record holding 37,098,690 common shares issued and outstanding.
To our knowledge, there are no arrangements, the operation of which may, at a subsequent date, result in a change in control.
The building housing the Company's premises at 6870 Goreway Drive in Mississauga, Ontario is owned 50% by Samuel Sarick Limited, a corporation wholly owned by Mr. Sarick, a director of the Company. The original lease for the premises was entered into on April 25, 1994 between Samuel Sarick Limited, Kentlake Construction Limited and Anec Investments Limited, as landlord, and the Company, as tenant. The lease renewal was negotiated between the Company and Samuel Sarick Limited on an arm's-length basis with the assistance of Colliers International retained by the Company and was renewed with Board approval effective May 1, 1999 for a period of five years. The Company believes that the lease terms are consistent with arm's-length comparables and that the annual rent payable of CDN$172,000 is at fair market value.
The Company has made loans to certain key management personnel in connection with its incentive plans and as inducements to enter into employment agreements. Details of loans currently outstanding are set forth in the table below.
| |
| | Largest Amount Outstanding During 2002 Fiscal Year(1)
| | Amount Outstanding as at March 31, 2003(1)
| |
| |
|
|---|
Name & Principal
Position
| | Involvement of Issuer or Subsidiary
| | Fully
Recourse
Interest
Bearing
(EPP)
(CDN$)
| | Other
Employee
Share
Participation
Plans
(CDN$)
| | Fully
Recourse
Interest
Bearing
(EPP)
(CDN$)
| | Other
Employee
Share
Participation
Plans
(CDN$)
| | Financially Assisted
Securities Purchased
During the 2002
Fiscal Year(1)(#)
| | Security for Indebtedness as at March 31, 2003(1)(#)
|
|---|
Martin Barkin, MD
President & Chief Executive Officer | | Lender | | 100,000 | | 193,549 | | 100,000 | | 157,989 | | nil | | 60,790 common shares of the Company; 245,000 EPSPP shares, Series C |
Jim Garner
Senior Vice President, Finance & Chief Financial Officer | | Lender | | 75,000 | | 77,680 | | 75,000 | | 57,464 | | nil | | 39,462 common shares of the Company; 75,000 EPSPP shares, Series C |
Dan Brazier
President, Draxis Pharmaceutica | | Lender | | nil | | 44,869 | | nil | | 20,592 | | nil | | 3,375 common shares of the Company; 25,000 EPSPP shares, Series C |
Jack Carter(2)
Vice President, Human Resources | | Lender | | 104,000 | | 20,268 | | nil | | 10,863 | | 41,348 | | 4,531 common shares of the Company |
Richard Flanagan
President, DRAXIMAGE | | Lender | | 30,000 | | 28,186 | | 30,000 | | 13,766 | | nil | | 19,507 common shares of the Company |
Douglas Parker(3)
General Counsel & Secretary | | Lender | | 22,000 | | 11,432 | | 22,000 | | 6,491 | | nil | | 9,808 common shares of the Company |
- (1)
- These figures take into account indebtedness pursuant to the Employee Stock Ownership Plan, the Employee Participation Share Purchase Plan, the Equity Purchase Plan and the DPI Long-term Incentive Plan:
Employee Stock Ownership Plan: through an interest free loan mechanism, each employee of the Company, including each named executive officer, is granted annually a number of common shares of the Company equal to 7% of total cash compensation. See "Discontinued Plans—Stock Ownership Plan;"
74
Employee Participation Share Purchase Plan: subscription proceeds were loaned to each named executive officer on a non-recourse, interest free basis. Amounts referenced in this table include any outstanding loans relating to Participation Shares, Series A, Series B and Series C. See "Discontinued Plans—Employee Participation Share Purchase Plan;" and
Equity Purchase Plan: the Company loans funds to participating named executive officers on a full recourse, interest bearing basis. See "Discontinued Plans—Equity Purchase Plan."
- (2)
- In addition, a loan of $20,000 was made to Mr. Carter in October 2001. This loan bears interest at Canada Customs and Revenue's prescribed rate with the principal repayable in 72 equal semi-monthly installments, deducted from regular salary, and interest payable quarterly.
- (3)
- In addition, an education tuition loan of $21,000 was made to Mr. Parker in December 2002. This loan bears interest at Canada Customs and Revenue's prescribed rate with the principal repayable in 72 equal semi-monthly installments, deducted from regular salary, and interest payable quarterly.
Item 8. Financial Information
The Company's Consolidated Financial Statements are attached as pages F-1 through F-24. There have been no significant changes since the date of the financial statements.
From time to time, the Company becomes involved in legal proceedings and claims which arise in the ordinary course of business. With the exception of the two matters described below related to Anipryl, the Company considers that the ultimate liability with respect to any known actions will not materially affect the business, financial position, results of operations or cash flows of the Company.
In 1998, a Canadian legal proceeding was launched against the Company and DAHI by a former consultant, Jozsef Knoll, claiming royalty entitlements based on the net profit from sales of Anipryl. Total damages claimed are $100 million. The plaintiff also claims entitlement to certain shares of DAHI. The plaintiff has taken no steps in the last two years to move the claim forward and may not do so. The Company believes that it has good defences to this claim and that it is without merit and has and will continue to vigorously defend itself.
Since 2000, the Company and DAHI have been involved in U.S. and Canadian legal proceedings with the University of Toronto and the University of Toronto Innovations Foundation. One dispute relates to the terms of a 1992 license agreement under which Innovations Foundation is claiming entitlement to a portion of the consideration earned by DAHI with respect to Anipryl. The second dispute relates to a 1988 contract research agreement under which the University of Toronto is claiming a declaration of ownership and an order for assignment of patents and damages related to certain Anipryl-related intellectual property. The damages claimed in the proceedings are in the range of CDN $100 million. In July 2002, the U.S. Court of Appeals ruled that the U.S. proceedings with University of Toronto Innovations Foundation should be stayed pending arbitration of this dispute in Ontario. While both claims are at a very preliminary stage and the matters at issue are factually and legally complex, the Company believes that it has good defences to the claims made by the University of Toronto and the University of Toronto Innovations Foundation and considers the claims to be without merit and is vigorously defending itself.
Item 9. The Offer and Listing
The Company's common shares are listed on The Toronto Stock Exchange (the "TSX") under the symbol "DAX." The following table sets forth, in Canadian dollars, the per share high and low sales prices on the TSX for the five most recent full financial years:
Years
| | High
| | Low
|
|---|
| 1998 | | 5.10 | | 2.30 |
| 1999 | | 4.57 | | 1.41 |
| 2000 | | 5.95 | | 2.05 |
| 2001 | | 4.40 | | 2.11 |
| 2002 | | 5.60 | | 2.00 |
75
The following table sets forth, in Canadian dollars, the per share high and low sales prices on the TSX by fiscal quarter for 2002 and 2001.
| | High
| | Low
|
|---|
| 2002 | | | | |
| First Quarter | | 4.98 | | 3.60 |
| Second Quarter | | 5.68 | | 3.75 |
| Third Quarter | | 4.15 | | 2.51 |
| Fourth Quarter | | 3.00 | | 2.00 |
|
|
High
|
|
Low
|
|---|
| 2001 | | | | |
| First Quarter | | 3.98 | | 2.70 |
| Second Quarter | | 3.90 | | 2.35 |
| Third Quarter | | 3.75 | | 2.11 |
| Fourth Quarter | | 4.40 | | 2.52 |
The following table sets forth, in Canadian dollars, the per share high and low sales prices on the TSX for the most recent six months:
Last 6 months
| | High
| | Low
|
|---|
| November 2002 | | 2.70 | | 2.30 |
| December 2002 | | 2.59 | | 2.30 |
| January 2003 | | 2.50 | | 1.70 |
| February 2003 | | 2.25 | | 1.75 |
| March 2003 | | 2.00 | | 1.61 |
| April 2003 | | 2.02 | | 1.72 |
The common shares are quoted on the NASDAQ National Market System ("NASDAQ") in the United States under the symbol "DRAX." The following table sets forth, in U.S. dollars, the per share high bid and low asked prices on NASDAQ for the five most recent full financial years:
Years
| | High
| | Low
|
|---|
| 1998 | | 3.53 | | 1.56 |
| 1999 | | 3.12 | | 0.97 |
| 2000 | | 4.62 | | 1.03 |
| 2001 | | 2.79 | | 1.45 |
| 2002 | | 3.67 | | 1.29 |
The following table sets forth, in U.S. dollars, the per share high and low asked prices on NASDAQ in U.S. dollars, by fiscal quarter for 2002 and 2001:
2002
| | High
| | Low
|
|---|
| First Quarter | | 3.14 | | 2.22 |
| Second Quarter | | 3.67 | | 2.45 |
| Third Quarter | | 2.72 | | 1.59 |
| Fourth Quarter | | 1.90 | | 1.29 |
2001
|
|
High
|
|
Low
|
|---|
| First Quarter | | 2.63 | | 1.69 |
| Second Quarter | | 2.55 | | 1.50 |
| Third Quarter | | 2.45 | | 1.45 |
| Fourth Quarter | | 2.79 | | 1.60 |
76
The following table sets forth, in U.S. dollars, the per share high and low sales prices on NASDAQ for the most recent six months:
Last 6 months
| | High
| | Low
|
|---|
| November 2002 | | 1.74 | | 1.42 |
| December 2002 | | 1.62 | | 1.44 |
| January 2003 | | 1.66 | | 1.10 |
| February 2003 | | 1.50 | | 1.14 |
| March 2003 | | 1.35 | | 1.09 |
| April 2003 | | 1.40 | | 1.18 |
77
Item 10. Additional Information
The Company's Articles of Amalgamation are on file with the Corporations Directorate of Industry Canada under Corporation Number 357288-9. The Articles of Amalgamation do not include a stated purpose and do not place any restrictions on the business that the Company may carry on.
The minimum number of directors of the Company is one (1) and the maximum number is twelve (12). In accordance with the Company's bylaws and the Canada Business Corporations Act (the "Act"), a majority of its directors must be residents of Canada. In order to serve as a director, a person must be a natural person at least 18 years of age, of sound mind and not bankrupt. Neither the Articles of Amalgamation, bylaws, nor the Act, impose any mandatory retirement requirements for directors.
Under the Company's bylaws and the Articles of Amalgamation, a director of the Company need not be a shareholder. However, in 2001 the Board of Directors established a corporate policy requiring each current Board member to acquire over the next three years (or in the case of future Board members, within three years of appointment) shares in the Company equal to five times his or her annual compensation.
A director who is a party to, or who is a director or officer of, or has a material interest in, any person who is a party to a material contract or transaction or proposed material contract or transaction with the Company must disclose to the Company the nature and extent of his or her interest at the time and in the manner provided by the Act. The Act prohibits such a director from voting on any resolution to approve the contract or transaction unless the contract or transaction:
- •
- is an arrangement by way of security for money lent to or obligations undertaken by the director for the benefit of the Company or an affiliate;
- •
- relates primarily to his or her remuneration as a director, officer, employee or agent of the Company or an affiliate;
- •
- is for indemnity or insurance for director's liability as permitted by the Act; or
- •
- is with an affiliate.
The Board of Directors may, on behalf of the Company and without authorization of our shareholders:
- •
- borrow money upon the credit of the Company;
- •
- issue, reissue, sell or pledge debt obligations of the Company;
- •
- give a guarantee on behalf of the Company to secure performance of an obligation of any person; and
- •
- mortgage, hypothecate, pledge or otherwise create a security interest in all or any property of the Company, owned or subsequently acquired, to secure any obligation of the Company.
The Act prohibits the giving of a guarantee to any shareholder, director, officer or employee of the Company or of an affiliated corporation or to an associate of any such person for any purpose or to any person for the purpose of or in connection with a purchase of a share issued or to be issued by the Company or its affiliates, where there are reasonable grounds for believing that the Company is or, after giving the guarantee, would be unable to pay its liabilities as they become due, or the realisable value of the Company's assets in the form of assets pledged or encumbered to secure a guarantee, after giving the guarantee, would be less than the aggregate of the Company's liabilities and stated capital of all classes.
These borrowing powers may be varied by the Company's bylaws or its Articles of Amalgamation. However, the Company's bylaws and Articles of Amalgamation do not contain any restrictions on or variations of these borrowing powers.
The Company's Articles of Amalgamation authorize the issuance of an unlimited number of common shares (37,098,690 of which are outstanding as of April 7, 2003), an unlimited number of preferred shares (none of which are issued and outstanding), and an unlimited number of Employee Participation Shares (2,000,000 of which have
78
been issued and 345,000 remain outstanding as of April 7, 2003). The Articles of Amalgamation do not authorize the issuance of any other class of shares.
The holders of the common shares of the Company are entitled to receive notice of and to attend all meetings of the shareholders of the Company and have one vote for each common share held at all meetings of shareholders. The directors are elected at each annual meeting of shareholders and do not stand for re-election at staggered intervals.
The holders of common shares are entitled to receive dividends and the Company will pay dividends, as and when declared by the Board of Directors, out of moneys properly applicable to the payment of dividends, in such amount and in such form as the Board of Directors may from time to time determine, subject to the rights of the holders of any other class of shares of the Company entitled to receive dividends in priority to or rateably with the holders of the common shares, and all dividends which the Board of Directors may declare on the common shares shall be declared and paid in equal amounts per share on all common shares at the time outstanding.
In the event of the dissolution, liquidation or winding-up of the Company, whether voluntary or involuntary, or any other distribution of assets of the Company among its shareholders for the purpose of winding-up its affairs, the holders of the common shares shall, subject to the rights of the holders of any other class or shares of the Company entitled to receive the assets of the Company upon such distribution in priority to or rateably with the holders of the common shares, be entitled to participate rateably in any distribution of the assets of the Company.
The preferred shares of the Company may at any time or from time to time be issued in one or more series. No preferred shares of the Company have been issued to date. Preferred shares shall be entitled to preference over the common shares and any other shares of the Company ranking junior to the preferred shares and the distribution of assets in the event of any liquidation and common dissolution or winding-up of the Company, whether voluntary or involuntary, or other distribution of the assets of the Company among its shareholders for the purpose of winding-up its affairs. The preferred shares of each series shall rank in parity with the preferred shares of every other series with respect to priority in the payment of dividends and in the distribution of assets in the event of liquidation, dissolution or winding-up of the Company.
Subject to the provisions relating to any particular series, the Company may redeem the whole or any part of the preferred shares on any one or more series outstanding from time to time at such price or prices as may be applicable to such series by given at least thirty (30) days' prior notice in writing of the intention of the Company to redeem such shares to each person who at the date of giving such notice is the registered holder of preferred shares to be redeemed.
On February 16, 1995, the Board of Directors of the Company authorized the issuance of 975,000 Series A Participation Shares; on December 18, 1995, the Board of Directors of the Company authorized the issuance of 555,000 Series B Participation Shares and on May 12, 1999, the Board of Directors of the Company authorized the issuance of 470,000 Series C Participation Shares. The Employee Participation Share Purchase Plan (the "EP Plan") was discontinued effective December 4, 2002, though participation shares previously issued remain outstanding.
Each participation share entitles the holder to receive cash dividends, if any, as may from time to time be declared payable thereon, at the same time as dividends, if any, are paid on the common shares of the Company, in an amount for each participation share of a particular series which is equal to the proportion of the amount of the dividend declared on each common share that the subscription price of such participation share is of the fair market value at the date of issuance of such participation share.
In the event of a proposal, order or resolution for the liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary; or on the "Automatic Conversion Date" (as defined in the Articles of Amalgamation and the terms of the EP Plan); or at the option of the holder on a "Conversion Date" (as defined in the Articles of Amalgamation and the terms of the EP Plan) other than the "Automatic Conversion Date" (as defined in the Articles of Amalgamation and the terms of the EP Plan), the participation shares will become
79
common shares, and the number of common shares a holder receives on conversion is the number of common shares which is determined by multiplying the number of participation shares held, or in the case of conversion on a Conversion Date other than the Automatic Conversion Date, the number of participation shares which the holder elects to convert, by a fraction:
- 1.
- the numerator of which shall be the "EP Share Value"(as defined in the Articles of Amalgamation and the terms of the EP Plan) of a participation share of a particular series as at the earlier of the "Automatic Conversion Date" and a "Conversion Date," as the case may be; and
- 2.
- the denominator of which shall be the "Fair Market Value of a Common Share" (as defined in the Articles of Amalgamation and the terms of the EP Plan) as at the earlier of the two dates described in 1; provided that no fraction common shares will be issued and no payment will be made in respect of fractional common shares.
On May 16, 2002, the shareholders of the Company approved a Shareholder Rights Plan Agreement (the "Rights Plan"), the principal terms of which are as follows:
The Rights Plan became effective on April 23, 2002 (the "Effective Date") and was approved by shareholders of the Company at its Annual and Special Meeting of shareholders held on May 16, 2002.
The term of the Rights Plan is ten years, subject to reconfirmation by shareholders at every third annual meeting.
One right (a "Right") attaches to each outstanding common share.
The Rights will separate from the shares to which they are attached and will become exercisable at the time (the "Separation Time") that is ten trading days after the earlier of a person having acquired, or the commencement, announcement or other date determined by the Board of Directors in respect of a take-over bid to acquire, 20% or more of the common shares, other than by an acquisition pursuant to a take-over bid permitted by the Rights Plan (a "Permitted Bid").
The acquisition of Beneficial Ownership (as defined in the Rights Plan) by any person (an "Acquiring Person"), including others acting in concert, of 20% or more of the common shares, other than by way of a Permitted Bid, is referred to as a "Flip-in Event." Under the Rights Plan, there are certain exceptions to that rule, including (i) the Company or a subsidiary of the Company, (ii) a person who acquires 20% or more of the outstanding common shares through, among other things, a share redemption or a Permitted Bid, (iii) an underwriter or selling group member during the course of a public distribution; or (iv) investment and fund managers, trust companies and other persons who are managing investment funds, pension funds or plans, estates or accounts on behalf of another person, provided they are not making, or are not part of a group making, a take-over bid. Any Rights held by an Acquiring Person on or after the earlier of the Separation Time or the first date of public announcement by the Company or an Acquiring Person that an Acquiring Person has become such will become void upon the occurrence of a Flip-in Event.
Under the Rights Plan, a bidder is entitled to "lock up" any shareholder of the Company without triggering the Rights Plan so long as the lock-up agreement is a "soft" lock up, meaning the locked-up shareholder is entitled to tender to a higher offer (so long as the higher offer is not required under the lock-up agreement to be more than 7% higher than the first offer).
Ten trading days after the occurrence of the Flip-in Event, the Rights (other than those held by the Acquiring Person) will permit the holder to purchase, for the exercise price of the Rights, common shares having a value based on the then-prevailing market price) equal to twice such exercise price (i.e., at a 50% discount). The exercise price of the Rights will be equal to five times the prevailing market price at the Separation Time.
80
The issue of the Rights is not initially dilutive. Upon a Flip-in Event occurring and the Rights separating from the attached shares, reported earnings per common share on a fully diluted or non-diluted basis may be affected. Holders of Rights who do not exercise their Rights upon the occurrence of a Flip-in Event may suffer substantial dilution.
Prior to the Separation Time, the Rights will be evidenced by a legend imprinted on certificates for common shares issued from and after the Effective Date. Rights are also attached to such shares outstanding on the Effective Date, although share certificates issued prior to that date will not bear such a legend. Prior to the Separation Time, Rights will not be transferable separately from the attached shares. From and after the Separation Time, the Rights will be evidenced by Rights certificates which will be transferable and traded separately from the shares.
The requirements of a Permitted Bid include the following:
- •
- the take-over bid must be made by way of a take-over bid circular to all holders of common shares;
- •
- the take-over bid must not permit common shares tendered pursuant to the take-over bid to be taken up prior to the expiry of a period of not less than 60 days and only if at such time more than 50% of the common shares held by shareholders other than the bidder, its affiliates, associates and persons acting jointly or in concert with the bidder (the "Independent Shareholders") have been tendered pursuant to the take-over bid and not withdrawn; and
- •
- if more than 50% of the common shares held by Independent Shareholders are tendered to the take-over bid within the 60-day period, the bidder must make a public announcement of that fact and the take-over bid must remain open for deposits of common shares for an additional 10 business days from the date of such public announcement.
The Rights Plan allows a competing Permitted Bid (a "Competing Permitted Bid") to be made while a Permitted Bid is in existence. A Competing Permitted Bid must satisfy all the requirements of a Permitted Bid except that it may expire on the same date as the Permitted Bid, subject to the requirement that it be outstanding for a minimum period of 35 days.
The Board of Directors may, prior to a Flip-in Event, waive the dilutive effects of the Rights Plan in respect of a particular Flip-in Event (an "Exempt Acquisition") that would result from a take-over bid made by way of a take-over bid circular to all holders of common shares, provided that in such circumstances the Board shall be deemed to have waived the application of the Rights Plan to any other Flip-in Event occurring as a result of a take-over bid made by way of a take-over bid circular to all holders of common shares prior to the expiry of any take-over bid in respect of which the Rights Plan has been waived. The Board of Directors may also waive the Rights Plan in respect of a particular Flip-in Event that has occurred through inadvertence, provided that the Acquiring Person that inadvertently triggered such Flip-in Event reduces its beneficial holdings to less than 20% of the outstanding voting shares of the Company within 14 days or such other period as may be specified by the Board of Directors.
At any time prior to the occurrence of a Flip-in Event, the Board of Directors may with the prior approval of the holders of the common shares or the Rights redeem all, but not less than all, of the outstanding Rights at a price of CDN$0.000001 each. Rights will be deemed to have been redeemed by the Board of Directors following completion of a Permitted Bid, Competing Permitted Bid or Exempt Acquisition.
The Company is authorized to make amendments to the Rights Plan to correct any clerical or typographical error or, subject to subsequent ratification by shareholders or Rights holders, to maintain the validity of the Rights
81
Plan as a result of changes in law or regulation. Other amendments or supplements to the Rights Plan may be made with the prior approval of shareholders or Rights holders.
In order to change the rights of our shareholders, the Company would need to amend its Articles of Amalgamation to effect the change. Such an amendment would require the approval of holders of two-thirds of the common shares cast at a duly called special meeting. For certain amendments such as those creating of a class of preferred shares, a shareholder is entitled under the Act to dissent in respect of such a resolution amending the Articles of Amalgamation and, if the resolution is adopted and the Company implements such changes, demand payment of the fair value of its common shares.
An annual meeting of shareholders is held each year for the purpose of considering the financial statements and reports, electing directors, appointing auditors and for the transaction of other business as may be brought before the meeting. The Board of Directors has the power to call a special meeting of shareholders at any time.
Notice of the time and place of each meeting of shareholders must be given not less than 21 days, nor more than 50 days, before the date of each meeting to each director, to the auditor and to each shareholder who at the close of business on the record date for notice is entered in the securities register as the holder of one or more shares carrying the right to vote at the meeting. Notice of meeting of shareholders called for any other purpose other than consideration of the minutes of an earlier meeting, financial statements and auditor's report, election of directors and reappointment of the incumbent auditor, must state the nature of the business in sufficient detail to permit the shareholder to form a reasoned judgement on and must state the text of any special resolution or bylaw to be submitted to the meeting.
The only persons entitled to be present at a meeting of shareholders are those entitled to vote, the directors of the Company and the auditor of the Company. Any other person may be admitted only on the invitation of the chairman of the meeting or with the consent of the meeting. In circumstances where a court orders a meeting of shareholders, the court may direct how the meeting may be held, including who may attend the meeting.
Neither Canadian law nor the Company's Articles of Amalgamation or bylaws limit the right of a non-resident to hold or vote common shares of the Company, other than as provided in the Investment Canada Act (the "Investment Act"). The Investment Act generally prohibits implementation of a direct reviewable investment by an individual, government or agency thereof, corporation, partnership, trust or joint venture that is not a "Canadian," as defined in the Investment Act (a "non-Canadian"), unless, after review, the minister responsible for the Investment Act is satisfied or is deemed to be satisfied that the investment is likely to be of net benefit to Canada. An investment in the common shares of the Company by a non-Canadian (other than a "WTO Investor," as defined below) would be reviewable under the Investment Act if it were an investment to acquire direct control of the Company, and the book value of the assets of the Company were CDN$5.0 million or more (provided that immediately prior to the implementation of the investment the Company was not controlled by WTO Investors). An investment in common shares of the Company by a WTO Investor (or by a non-Canadian other than a WTO Investor if, immediately prior to the implementation of the investment, the Company was controlled by WTO Investors) would be reviewable under the Investment Act if it were an investment to acquire direct control of the Company (in 2003) and the value of the assets of the Company equalled or exceeded CDN$223.0 million. A non-Canadian, whether a WTO Investor or otherwise, would be deemed to acquire control of the Company for purposes of the Investment Act if he or she acquired a majority of the common shares of the Company. The acquisition of less than a majority, but at least one-third of the shares, would be presumed to be an acquisition of control of the Company, unless it could be established that the Company was not controlled in fact by the acquirer through the ownership of the shares. In general, an individual is a WTO Investor if he or she is a "national" of a country (other than Canada) that is a member of the World Trade Organisation ("WTO Member") or has a right of permanent residence in a WTO Member. A corporation or other entity will be a "WTO Investor" if it is a "WTO Investor-controlled entity," pursuant to detailed rules set out in the Investment
82
Act. The United States is a WTO Member. Certain transactions involving the Company's common shares would be exempt from the Investment Act, including:
- (a)
- an acquisition of the shares if the acquisition were made in the ordinary course of that person's business as a trader or dealer in securities;
- (b)
- an acquisition of control of the Company in connection with the realization of a security interest granted for a loan or other financial assistance and not for any purpose related to the provisions of the Investment Act; and
- (c)
- an acquisition of control of the Company by reason of an amalgamation, merger, consolidation or corporate reorganization, following which the ultimate direct or indirect control in fact of the Company, through the ownership of voting interests, remains unchanged.
In November 1997 the Company entered into a global alliance with Pfizer and granted Pfizer a perpetual exclusive license to market, sell and distribute Anipryl in exchange for non-refundable fees, royalties based on world-wide sales of Anipryl, a manufacturing and supply agreement and a research collaboration. The terms of the alliance were amended in December 1999 and again in December 2001. See "Item 4: Other Collaboration Agreements" and "Item 18: Financial Statements." The definitive agreements governing this global alliance are as follows:
- •
- Master Agreement dated November 12, 1997 between DRAXIS, DAHI and Pfizer (Exhibit 4.1);
- •
- License Agreement dated November 12, 1997 between DAHI and Pfizer (Exhibit 4.2); and
- •
- Letter agreement dated December 22, 1999 between DAHI and Pfizer (Exhibit 4.3); and
- •
- Second Amendment dated December 18, 2001 between DRAXIS, DAHI and Pfizer (Exhibit 4.4).
On May 30, 2001, an employment agreement was entered into between the Company and Mr. Jerry Ormiston, Executive Director, Investor Relations of the Company. Pursuant to this agreement, Mr. Ormiston is paid a gross salary of CDN$130,000 per annum and is entitled to an annual discretionary bonus as determined by the Compensation Committee. This agreement also has certain specific termination provisions. In event of his termination of employment without cause, Mr. Ormiston is entitled to receive a payment equal to his annual remuneration; in the event of Mr. Ormiston's termination of employment following a change of control of the Company, Mr. Ormiston is entitled to receive a payment equal to two (2) times his annual remuneration. See "Item 6: Directors, Senior Management and Employees Termination of Employment Contracts." This agreement is Exhibit 4.27 to this annual report.
On March 28, 2002, a Subscription Agreement was entered into among the Company, DPI and SGF Santé Inc. ("SGF Santé"). The Company and SGF Santé subscribed for 577,402 common shares and 279,930 common shares, respectively, in the capital of DPI at a subscription price of CDN$1.50 per share. The aggregate number of shares subscribed for by the Company and SGF Santé represented 4.16% of the outstanding common shares of DPI on a non-diluted basis. The aggregate subscription price was CDN$1,286,000. This agreement is Exhibit 4.8 to this annual report.
On October 24, 2002, the Company entered into a First Amendment to the Subscription Agreement among the Company, DPI and SGF Santé, which provides for additional share subscriptions by the Company and SGF Santé in three separate and equal tranches. On October 24, 2002, the Company and SGF Santé subscribed for 748,319 common shares and 362,792 common shares, respectively, in the capital of DPI at a subscription price of CDN$1.50 per share. The aggregate number of shares subscribed for by the Company and SGF Santé represented 5.18% of the outstanding common shares of DPI on a non-diluted basis. The aggregate subscription price was CDN$1,666,666.50. On December 31, 2002, the Company and SGF Santé subscribed for 748,319 common shares and 362,792 common shares, respectively, in the capital of DPI at a subscription price of CDN$1.50 per share. The aggregate number of shares subscribed for by the Company and SGF Santé represented 4.93% of the outstanding common shares of DPI on a non-diluted basis. The aggregate subscription price was CDN$1,666,666.50. On March 28, 2003, the Company and SGF Santé subscribed for 748,319 common shares and 362,792 common shares, respectively, in the capital of DPI at a subscription price of CDN$1.50 per share. The aggregate number of shares subscribed for by the Company and SGF Santé represented 4.70% of the outstanding
83
common shares of DPI on a non-diluted basis. The aggregate subscription price was CDN$1,666,666.50. This amendment is Exhibit 4.29 to this annual report.
The Company entered into an Amended and Restated Unanimous Shareholders' Agreement dated as of March 28, 2002, among the Company, SGF Santé, DPI, Dwight Gorham, Mohammed Barkat and Michel Sauvageau with respect to DPI, which grants SGF Santé the right to obligate the Company to purchase its shareholdings in DPI anytime after February 18, 2005. The agreement contains additional put and call provisions, as well as restrictions on disposition of shares, rights of first refusal and piggy back rights. This agreement is Exhibit 4.9 to this annual report.
On March 28, 2002, the Company intervened with respect to a Loan Agreement between DPI and Investissement Québec whereby DPI received a term loan in the maximum principal amount of CDN$4,800,000. The loan matures on March 28, 2008 or on such later date as the lender may agree. The loan is subject to mandatory prepayments in each quarter beginning with the second anniversary of the first disbursement. The interest rate is the Canadian prime rate plus 2.50%. This agreement is Exhibit 4.13 to this annual report.
On March 28, 2002, a Loan Agreement was entered into among the Company, DPI and SGF Santé whereby the Company and SGF Santé will make available to DPI term loans in an initial principal amount of CDN$9,139,335. The loan matures on March 28, 2007 or on such later date as the lenders may agree. The interest rate is the Canadian prime rate plus 1.50%. This agreement is Exhibit 4.14 to this annual report.
The shareholders of the Company approved at the May 16, 2002 Annual and Special Meeting a shareholders rights plan for the Company as set out in a Shareholder Rights Plan Agreement dated April 23, 2002 between the Company and Computershare Trust Company of Canada as trustee. This plan is substantially similar to one that has been in place at the Company since April 1997. This plan is aimed at allowing more time for the Board of Directors to ensure that shareholders are fully compensated for the value of their common shares in the event of a take-over offer for the Company. See "Item 10: Additional Information, Shareholder Rights Plan." This agreement is Exhibit 4.28 to this annual report.
Canada has no system of exchange controls. There are no exchange restrictions on borrowing from foreign countries or on the remittance of dividends, interest, royalties and similar payments, management fees, loan repayments, settlement of trade debts or the repatriation of capital. There are no limits on the rights of non-Canadians to exercise voting rights on their common shares of the Company.
The following summary describes the principal Canadian federal income tax considerations generally applicable under the Income Tax Act (Canada) (the "ITA") to a holder of the Company's common shares who has never been resident or deemed to have been resident in Canada for the purposes of the ITA and who holds common shares as capital property and does not hold or use and is not deemed to hold or use such common shares in or in the course of carrying on a business in Canada. The summary is based on the current provisions of the ITA, the regulations thereunder in force on the date hereof, all proposed amendments to the ITA and regulations publicly announced by the Minister of Finance (Canada) prior to the date hereof (the "Amendments"), and on the understanding of counsel of the current published administrative and assessing practices of the Canada Customs and Revenue Agency. This summary is not exhaustive of all possible Canadian federal income tax considerations, and except as mentioned above, does not take into account any prospective changes to the tax law of Canada, whether occasioned by legislative, governmental, or judicial action. Further, the summary does not take account of foreign or provincial tax legislation or considerations that may vary from the Canadian federal income tax implications described herein. This summary does not purport to be, nor should it be construed as, legal or tax-related advice to any holder of common shares. Each holder of common shares should consult his or her own tax advisor.
Dividends, including stock and cash dividends and certain distributions and redemptions deemed to be dividends under the ITA, on the common shares paid to non-residents of Canada will be subject to Canadian withholding tax at a rate of twenty-five percent (25%). The applicable rate of withholding may, however, be reduced by virtue of the terms of any applicable tax treaty. Under the terms of the Canada-U.S. Income Tax
84
Convention, 1980 (the "Treaty"), the rate which is generally applicable to United States resident individuals who are beneficial owners of common shares in respect of which there is paid or credited or deemed by the ITA to be paid or credited Canadian-source dividends is fifteen percent (15%); in certain cases where the holder is a company owning more than ten percent (10%) of the common shares, five percent (5%) for dividends paid. Under the Treaty, certain tax-exempt entities that are resident in the United States may be exempt from Canadian withholding taxes, including any withholding tax levied in respect of dividends received on the common shares.
Dividends, including stock and cash dividends and certain distributions and redemptions deemed to be dividends under the ITA, on the common shares of the Company paid to residents of Canada will be included in computing their income and will be subject to the gross-up and dividend tax credit rules normally applicable to taxable dividends paid by taxable Canadian corporations. A holder that is a corporation may be liable to pay refundable tax under Part IV of the ITA. However, a public corporation that is not controlled, whether because of a beneficial interest in one or more trusts or otherwise, by or for the benefit of an individual (other than a trust) or related group of individuals (other than trusts) will not be liable to pay refundable tax under Part IV of the ITA.
In the case of a holder of common shares of the Company that is a corporation, the amount of any capital loss otherwise determined resulting from the disposition of a common share of the Company may be reduced by the amount of dividends previously received or deemed to have been received thereon. Any such restriction will not occur where the corporate holder owned the common share of the Company for 365 days or longer and such holder (together with any persons with whom it did not deal with at arm's length) did not own more than five percent (5%) of the shares of any class or series of shares at the time the relevant dividends were received or deemed to have been received. Analogous rules apply where a corporation is a member of a partnership or a beneficiary of a trust, which owns common shares of the Company.
Capital gains arising as a consequence of the disposition of common shares (other than to the Company) by a resident of the United States will normally be exempt from taxation under the ITA except to the extent that such shares constitute or are deemed to constitute taxable Canadian property ("TCP") (as defined in the ITA), the gain on which would not otherwise be exempt under the terms of the Treaty. In general, the common shares will not constitute TCP of a non-resident holder unless (i) such holder holds the common shares as capital property and uses them in carrying on a business in Canada, (ii) the holder is a non-resident insurer who holds the common shares as capital property and holds or uses such shares in the course of carrying on an insurance business in Canada in a particular taxation year, or (iii) at any time in the five-year period immediately preceding the disposition, not less than twenty-five percent (25%) of the issued shares of any class of the capital stock of the Company belonged to such holder, to persons not dealing at arm's length with him or to such holder and persons not dealing at arm's length with him. For the purposes of the twenty-five percent (25%) test, a person holding an option to acquire common shares or other securities convertible into or exchangeable for common shares, or otherwise having an interest in common shares, will be considered to own such shares. Special rules apply to deem certain partnership interests, where fifty percent (50%) or more of the value of the underlying partnership's property is represented by TCP, to be TCP. Consequently, a resident of the United States who disposes of such partnership interests will be required to comply with certain notification procedures, including obtaining a certificate from Canada Customs and Revenue Agency in respect of the disposition.
To the extent that the common shares do constitute TCP of the non-resident holder and the disposition thereof results in a capital gain, such holder will normally be obliged to recognise that gain and pay tax thereon pursuant to the provisions of the ITA. However, the Treaty will normally apply to exempt the United States resident holder of common shares from such tax, provided that the value of such shares is not derived principally from real property and/or interests therein situate in Canada at the time the common shares are disposed of and provided such holder does not have and has not had within the 12-month period preceding the disposition a permanent establishment or fixed base available to such holder in Canada.
On a disposition or deemed disposition of a common share of the Company a resident of Canada holder will receive a capital gain (or capital loss) equal to the amount by which the proceeds of disposition for the common
85
share exceeded (or are less than) the aggregate of any cost of disposition and the adjusted cost base to the holder of the common share of the Company immediately before the disposition. Pursuant to the ITA, a holder of common shares of the Company who is a resident of Canada will be required to include in income one-half (1/2) of the amount of any capital gain and may deduct one-half (1/2) of the amount of any capital loss against taxable capital gains realised by the holder in the year of the disposition. Allowable capital losses in excess of taxable capital gains may be carried back and deducted in any of the three proceeding years or carried forward and deducted in any following year against taxable capital gains realised in such years to the extent and under the circumstances described in the ITA and the Amendments.
A Canadian-controlled private corporation will also be subject to a refundable tax of sixty-six and two-thirds percent (662/3%) on certain investment income, including taxable capital gains realised on the disposition of common shares of the Company, that will be refundable when the corporation pays taxable dividends (at a rate of CDN$1.00 for every CDN$3.00 of taxable dividend paid).
A capital loss realised by a holder of common shares of the Company that is a corporation, a partnership of which a corporation is a member or a trust of which a corporation is a beneficiary may be reduced by the amount of dividends received in certain circumstances. Capital gains received by an individual may give rise to a liability for alternative minimum tax.
The Company is subject to the information requirements of the U.S. Securities Exchange Act of 1934, as amended. In accordance with these requirements, the Company files reports and other information with the United States Securities and Exchange Commission. These materials, including this Annual Report and the exhibits thereto, may be inspected and copied at the Commission's Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549 and at the Commission's regional offices at 500 West Madison Street, Suite 1400, Chicago, Illinois 60661. Copies of the materials may be obtained from the Public Reference Room of the Commission at 450 Fifth Street, N.W., Washington, D.C. 20549 at prescribed rates. The public may obtain information on the operation of the Commission's Public Reference Room by calling the Commission in the United States at 1-800-SEC-0330. The Commission also maintains a web site at http://www.sec.gov that contains reports, proxy statements and other information regarding registrants that file electronically with the Commission. The Company's annual reports and some of the other information submitted by the Company to the Commission may be accessed through this web site. In addition, material filed by the Company can be inspected at the offices of the NASDAQ at 9801 Washingtonian Blvd., Gaithersburg, MD 20878, and on the Canadian Securities Administrators' electronic filing system, SEDAR, accessible at the web site http://www.sedar.
Item 11. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to financial market risks, including changes in foreign currency exchange rates, interest rates on investments and debt obligations. We do not use derivative financial instruments for speculative or trading purposes.
Inflation has not had a significant impact on our results of operations.
We operate internationally, however a substantial portion of our revenue and expense activities and capital expenditures are transacted in Canadian dollars. We do not believe we have a material exposure to foreign currency risk because of the relative stability of the Canadian dollar in relation to the U.S. dollar. A 10% adverse change in foreign currency exchange rates would not have a material effect on our consolidated results of operations, financial position, or cash flows.
The primary objective of our investment policy is the protection of principal, and accordingly we invest in high-grade commercial paper and government securities with varying maturities, but typically less than 90 days. As it is our intent and policy to hold these investments until maturity, we do not have a material exposure to interest rate risk. Therefore, a 100 basis-point adverse change in interest rates would not have a material effect on our investment portfolio.
86
We are exposed to interest rate risk on borrowings from our term bank loan facility and unsecured obligation. The term bank loan facility bears interest based on Canadian dollar prime rate and the unsecured obligation bears interest based on Bank of Canada rate. Based on projected advances under both facilities, a 100 basis-point adverse change in interest rates would not have a material effect on our financial position.
Item 12. Description of Securities Other Than Equity Securities
Not Applicable
87
PART II.
Item 13. Defaults, Dividends Arrearages and Delinquencies
Not Applicable
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Not Applicable
Item 15. Controls and Procedures
Based on their evaluation of the Company's disclosure controls and procedures as of a date within 90 days of the filing of this Annual Report, the President and Chief Executive Officer and the Senior Vice President, Finance and Chief Financial Officer have concluded that such controls and procedures are effective.
There were no significant changes in the Company's internal controls or in other factors that could significantly affect such controls subsequent to the date of their evaluation.
Item 17. Financial Statements
Not Applicable
Item 18. Financial Statements
The following financial statements are filed as part of this annual report on Form 20-F.
Management's Report |
|
F-1 |
| Report of Deloitte & Touche LLP | | F-2 |
| Consolidated statements of operations | | F-3 |
| Consolidated balance sheets | | F-4 |
| Consolidated statements of shareholders' equity | | F-5 |
| Consolidated statements of cash flows | | F-6 |
| Notes to the consolidated financial statements | | F-7 |
Item 19. Exhibits
Exhibit No.
| | Description
|
|---|
| 1.1 | | Articles of Amalgamation of DRAXIS Health Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
1.2 |
|
By-law No. 1 of DRAXIS Health Inc. (formerly Deprenyl Research Limited) (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.1 |
|
Master Agreement dated November 12, 1997 among DRAXIS Health Inc., Deprenyl Animal Health Inc. and Pfizer Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.2 |
|
License Agreement dated November 12, 1997 between Deprenyl Animal Health Inc. and Pfizer Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.3 |
|
Letter Agreement dated December 22, 1999 between DRAXIS Health Inc., Deprenyl Animal Health Inc. and Pfizer Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
| | | |
87
4.4 |
|
Second Amendment dated December 18, 2001 to the Master Agreement, as amended December 22, 1999, License Agreement, Research Agreement, U.S. and Canada Manufacturing and Supply Agreement and International Manufacturing and Supply Agreement between Pfizer Inc., Deprenyl Animal Health, Inc. and DRAXIS Health Inc. dated November 12, 1997 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.5 |
|
License, Distribution and Supply Agreement dated June 17, 1999 between DRAXIS Health, Inc., Elan Pharma (International) Limited and Elan Pharmaceuticals, Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.6 |
|
Amending Agreement dated March 31, 2003 between DRAXIS Health, Inc., Elan Pharma International Limited and Elan Pharmaceuticals, Inc. |
4.7 |
|
Subscription Agreement dated February 18, 2000 among SGF Santé Inc. and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.8 |
|
Subscription Agreement dated March 28, 2002 among SGF Santé Inc., DRAXIS Health Inc. and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.9 |
|
Amended and Restated Unanimous Shareholders' Agreement dated March 28, 2002 among DRAXIS Health Inc., SGF Santé Inc., DRAXIS Pharma Inc., Dwight Gorham, Mohammed Barkat and Michel Sauvageau (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.10 |
|
Term Loan Agreement dated June 9, 1998 between National Bank of Canada and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.11 |
|
Credit Agreement dated February 18, 2000 between National Bank of Canada and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.12 |
|
Amendment to Credit Facilities for DRAXIS Pharma Inc. dated March 28, 2002 between National Bank of Canada and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.13 |
|
Loan Agreement dated March 28, 2002 among DRAXIS Pharma Inc., SGF Santé Inc. and DRAXIS Health Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.14 |
|
Loan Agreement dated March 28, 2002 among DRAXIS Pharma Inc., Investissement Québec and DRAXIS Health Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.15 |
|
Stock Option Plan of DRAXIS Health Inc., as amended, dated August 26, 1998 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.16 |
|
DRAXIS Health Inc. Employee Stock Ownership Plan, amended and restated, Effective December 1, 1998 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.17 |
|
DRAXIS Health Inc. Employee Participation Share Purchase Plan, Effective February 16, 1995 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.18 |
|
DRAXIS Health Inc. Deferred Share Unit Plan for Employees (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
| | | |
88
4.19 |
|
DRAXIS Health Inc. Equity Purchase Plan (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.20 |
|
DRAXIS Retirement Savings Program |
4.21 |
|
Employment Agreement dated April 15, 1999 between DRAXIS Health Inc. and Dr. Martin Barkin (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.22 |
|
Amendment dated June 14, 2000 to Employment Agreement dated April 15, 1999 between DRAXIS Health Inc. and Dr. Martin Barkin (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.23 |
|
Employment Agreement dated September 3, 1996 between DRAXIS Health Inc. and James A. H. Garner (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.24 |
|
Employment Agreement dated October 18, 2000 between DRAXIS Health Inc. and Jack A. Carter (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.25 |
|
Employment Agreement dated April 15, 1999 between DRAXIS Health Inc. and Dan Brazier (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.26 |
|
Employment Agreement dated October 11, 2000 between DRAXIS Health Inc. and Douglas Parker (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.27 |
|
Employment Agreement dated May 14, 2001 between DRAXIS Health Inc. and Jerry Ormiston (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.28 |
|
Shareholder Rights Plan Agreement dated April 23, 2002 between DRAXIS Health Inc. and Computershare Trust Company of Canada as trustee (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.29 |
|
First Amendment to Subscription Agreement dated October 24, 2002 among SGF Santé Inc., DRAXIS Health Inc. and DRAXIS Pharma Inc. |
8.1 |
|
List of Subsidiaries |
99.1 |
|
Certification required by Section 906 of the Sarbanes-Oxley Act of 2002. |
89
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
|
DRAXIS HEALTH INC. |
Date: May 14, 2003 |
|
By: |
|
/s/ JIM A.H. GARNER
Senior Vice President, Finance and
Chief Financial Officer |
|
|
By: |
|
/s/ MARTIN BARKIN, M.D.
President and Chief Executive Officer |
90
EXHIBIT INDEX
DRAXIS HEALTH INC.
Form 20-F Annual Report
Exhibit No.
| | Description
|
| 1.1 | | Articles of Amalgamation of DRAXIS Health Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
1.2 |
|
By-law No. 1 of DRAXIS Health Inc. (formerly Deprenyl Research Limited) (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.1 |
|
Master Agreement dated November 12, 1997 among DRAXIS Health Inc., Deprenyl Animal Health Inc. and Pfizer Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.2 |
|
License Agreement dated November 12, 1997 between Deprenyl Animal Health Inc. and Pfizer Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.3 |
|
Letter Agreement dated December 22, 1999 between DRAXIS Health Inc., Deprenyl Animal Health Inc. and Pfizer Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.4 |
|
Second Amendment dated December 18, 2001 to the Master Agreement, as amended December 22, 1999, License Agreement, Research Agreement, U.S. and Canada Manufacturing and Supply Agreement and International Manufacturing and Supply Agreement between Pfizer Inc., Deprenyl Animal Health, Inc. and DRAXIS Health Inc. dated November 12, 1997 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.5 |
|
License, Distribution and Supply Agreement dated June 17, 1999 between DRAXIS Health, Inc., Elan Pharma (International) Limited and Elan Pharmaceuticals, Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.6 |
|
Amending Agreement dated March 31, 2003 between DRAXIS Health, Inc., Elan Pharma International Limited and Elan Pharmaceuticals, Inc. |
4.7 |
|
Subscription Agreement dated February 18, 2000 among SGF Santé Inc. and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.8 |
|
Subscription Agreement dated March 28, 2002 among SGF Santé Inc., DRAXIS Health Inc. and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.9 |
|
Amended and Restated Unanimous Shareholders' Agreement dated March 28, 2002 among DRAXIS Health Inc., SGF Santé Inc., DRAXIS Pharma Inc., Dwight Gorham, Mohammed Barkat and Michel Sauvageau (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.10 |
|
Term Loan Agreement dated June 9, 1998 between National Bank of Canada and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.11 |
|
Credit Agreement dated February 18, 2000 between National Bank of Canada and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.12 |
|
Amendment to Credit Facilities for DRAXIS Pharma Inc. dated March 28, 2002 between National Bank of Canada and DRAXIS Pharma Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
| | | |
91
4.13 |
|
Loan Agreement dated March 28, 2002 among DRAXIS Pharma Inc., SGF Santé Inc. and DRAXIS Health Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.14 |
|
Loan Agreement dated March 28, 2002 among DRAXIS Pharma Inc., Investissement Québec and DRAXIS Health Inc. (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.15 |
|
Stock Option Plan of DRAXIS Health Inc., as amended, dated August 26, 1998 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.16 |
|
DRAXIS Health Inc. Employee Stock Ownership Plan, amended and restated, Effective December 1, 1998 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.17 |
|
DRAXIS Health Inc. Employee Participation Share Purchase Plan, Effective February 16, 1995 (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.18 |
|
DRAXIS Health Inc. Deferred Share Unit Plan for Employees (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.19 |
|
DRAXIS Health Inc. Equity Purchase Plan (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.20 |
|
DRAXIS Retirement Savings Program |
4.21 |
|
Employment Agreement dated April 15, 1999 between DRAXIS Health Inc. and Dr. Martin Barkin (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.22 |
|
Amendment dated June 14, 2000 to Employment Agreement dated April 15, 1999 between DRAXIS Health Inc. and Dr. Martin Barkin (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.23 |
|
Employment Agreement dated September 3, 1996 between DRAXIS Health Inc. and James A. H. Garner (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.24 |
|
Employment Agreement dated October 18, 2000 between DRAXIS Health Inc. and Jack A. Carter (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.25 |
|
Employment Agreement dated April 15, 1999 between DRAXIS Health Inc. and Dan Brazier (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.26 |
|
Employment Agreement dated October 11, 2000 between DRAXIS Health Inc. and Douglas Parker (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2000, filed on June 29, 2001 (SEC file no. 000-17434)) |
4.27 |
|
Employment Agreement dated May 14, 2001 between DRAXIS Health Inc. and Jerry Ormiston (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.28 |
|
Shareholder Rights Plan Agreement dated April 23, 2002 between DRAXIS Health Inc. and Computershare Trust Company of Canada as trustee (incorporated herein by reference to the Company's Annual Report on Form 20-F for the year ended December 31, 2001, filed on May 20, 2002 (SEC file no. 000-17434)) |
4.29 |
|
First Amendment to Subscription Agreement dated October 24, 2002 among SGF Santé Inc., DRAXIS Health Inc. and DRAXIS Pharma Inc |
8.1 |
|
List of Subsidiaries |
99.1 |
|
Certification required by Section 906 of the Sarbanes-Oxley Act of 2002 |
92
Management's Report
The Company's management is responsible for preparing the accompanying consolidated financial statements in conformity with United States generally accepted accounting principles ("GAAP"). In preparing these consolidated financial statements, management selects accounting policies and uses its judgement and best estimates, as appropriate in the circumstances. Management has determined such amounts on a reasonable basis in order to ensure that the financial statements are presented fairly, in all material respects.
The Company maintains a system of internal accounting controls designed to provide reasonable assurance, at a reasonable cost, that assets are safeguarded and that transactions are executed and recorded in accordance with the Company's policies. This system is supported by policies and procedures for key business activities, by the hiring of qualified staff and by a continuous planning and monitoring program.
Deloitte & Touche LLP has been engaged by the Company's shareholders to audit the consolidated financial statements. During the course of their audit, Deloitte & Touche LLP reviewed the Company's system of internal controls to the extent necessary to render their opinion on the consolidated financial statements.
The Board of Directors is responsible for ensuring that management fulfills its responsibility for financial reporting and is ultimately responsible for reviewing and approving the financial statements. The Board carries out the responsibility principally through its Audit Committee. The members of the Audit Committee are outside Directors. The Committee considers, for review by the Board of Directors and approval by the shareholders, the engagement or reappointment of the external auditors. Deloitte & Touche LLP has full and free access to the Audit Committee.
Management acknowledges its responsibility to provide financial information that is representative of the Company's operations, is consistent and reliable, and is relevant for the informed evaluation of the Company's activities.
For the year ended December 31, 2001, the Company switched from Canadian GAAP to U.S. GAAP as its primary reporting convention. Financial statements prepared in accordance with Canadian GAAP are available to all shareholders.
Martin Barkin, MD, FRCSC
President and Chief Executive Officer | | Jim Garner, CA
Senior Vice President, Finance and Chief Financial Officer |
Mississauga, Ontario
February 4, 2003, except for Note 27, which is as of April 8, 2003. |
F-1
Independent Auditors' Report
To the Shareholders of
DRAXIS Health Inc.
We have audited the consolidated balance sheets of DRAXIS Health Inc. as at December 31, 2002 and 2001 and the consolidated statements of operations, shareholders' equity and cash flows for each of the years in the three-year period ended December 31, 2002. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in Canada and the United States of America. Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for the opinion expressed.
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of the Company as at December 31, 2002 and 2001 and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2002 in accordance with generally accepted accounting principles in the United States of America.
On February 4, 2003, except for Note 27, which is as of April 8, 2003, we reported separately to shareholders of the Company, on our audits, where we expressed an opinion without reservation on the financial statements for the same periods prepared in accordance with generally accepted accounting principles in Canada.
Chartered Accountants
Montreal, Quebec
February 4, 2003, except for Note 27, which is as of April 8, 2003.
F-2
DRAXIS HEALTH INC.
Consolidated Statements of Operations
Years ended December 31
(in thousands of U.S. dollars except share related data)
| | 2002
| | 2001
| | 2000
| |
|---|
| REVENUES | | | | | | | | | | |
| | Product sales | | $ | 30,338 | | $ | 27,151 | | $ | 22,589 | |
| | Royalty and licensing | | | 8,302 | | | 6,752 | | | 7,132 | |
| | |
| |
| |
| |
| | | | 38,640 | | | 33,903 | | | 29,721 | |
| | |
| |
| |
| |
| EXPENSES | | | | | | | | | | |
| | Cost of goods sold | | | 23,404 | | | 22,045 | | | 18,375 | |
| | Selling, general and administration | | | 7,542 | | | 6,369 | | | 7,531 | |
| | Research and development | | | 1,996 | | | 1,280 | | | 1,027 | |
| | Depreciation and amortization | | | 2,804 | | | 2,436 | | | 2,318 | |
| | Restructuring charges (Note 5) | | | — | | | — | | | 434 | |
| | |
| |
| |
| |
| | | | 35,746 | | | 32,130 | | | 29,685 | |
| | |
| |
| |
| |
| Operating income | | | 2,894 | | | 1,773 | | | 36 | |
| Interest expense, net (Note 6) | | | (280 | ) | | (25 | ) | | (637 | ) |
| Other income (Note 7) | | | — | | | — | | | 411 | |
| | |
| |
| |
| |
| Income (loss) before undernoted | | | 2,614 | | | 1,748 | | | (190 | ) |
| Income taxes (Note 8) | | | (154 | ) | | 3,049 | | | (215 | ) |
| Minority interest | | | 252 | | | 286 | | | 338 | |
| | |
| |
| |
| |
| Income (loss) from continuing operations before cumulative effect of accounting change | | | 3,020 | | | (1,015 | ) | | 363 | |
| Loss from discontinued operations, net of taxes (Note 9) | | | (834 | ) | | (569 | ) | | (801 | ) |
| Cumulative effect of accounting change, net of taxes | | | — | | | — | | | (19,900 | ) |
| | |
| |
| |
| |
| Net income (loss) | | $ | 2,186 | | $ | (1,584 | ) | $ | (20,338 | ) |
| | |
| |
| |
| |
Basic income (loss) per share (Note 10) |
|
|
|
|
|
|
|
|
|
|
| | from continuing operations before cumulative effect of accounting change | | $ | 0.08 | | $ | (0.03 | ) | $ | 0.01 | |
| | from discontinued operations | | | (0.02 | ) | | (0.02 | ) | | (0.02 | ) |
| | from cumulative effect of accounting change | | | — | | | — | | | (0.55 | ) |
| | |
| |
| |
| |
| | | $ | 0.06 | | $ | (0.05 | ) | $ | (0.56 | ) |
| | |
| |
| |
| |
Diluted income (loss) per share (Note 10) |
|
|
|
|
|
|
|
|
|
|
| | from continuing operations before cumulative effect of accounting change | | $ | 0.08 | | $ | (0.03 | ) | $ | 0.01 | |
| | from discontinued operations | | | (0.02 | ) | | (0.02 | ) | | (0.02 | ) |
| | from cumulative effect of accounting change | | | — | | | — | | | (0.55 | ) |
| | |
| |
| |
| |
| | | $ | 0.06 | | $ | (0.05 | ) | $ | (0.56 | ) |
| | |
| |
| |
| |
APPROVED BY THE BOARD
See the accompanying notes to the Consolidated Financial Statements
F-3
DRAXIS HEALTH INC.
Consolidated Balance Sheets
December 31
(in thousands of U.S. dollars except share related data)
| | 2002
| | 2001
| |
|---|
| ASSETS | | | | | | | |
CURRENT |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 4,899 | | $ | 5,602 | |
| | Accounts receivable (Note 11) | | | 7,934 | | | 7,472 | |
| | Inventories (Note 12) | | | 6,134 | | | 5,272 | |
| | Prepaid expenses | | | 415 | | | 570 | |
| | Deferred income taxes, net (Note 8) | | | 990 | | | 2,917 | |
| | |
| |
| |
| | | | 20,372 | | | 21,833 | |
Property, plant and equipment, net (Note 13) |
|
|
26,054 |
|
|
22,294 |
|
| Goodwill, net (Note 14) | | | 556 | | | 551 | |
| Intangible assets, net (Note 15) | | | 7,724 | | | 9,539 | |
| Other assets | | | 627 | | | 995 | |
| Deferred income taxes, net (Note 8) | | | 12,618 | | | 9,148 | |
| | |
| |
| |
| | | $ | 67,951 | | $ | 64,360 | |
| | |
| |
| |
LIABILITIES |
|
|
|
|
|
|
|
CURRENT |
|
|
|
|
|
|
|
| | Bank indebtedness (Note 16) | | $ | 884 | | $ | 1,666 | |
| | Accounts payable and accrued liabilities (Note 17) | | | 9,189 | | | 8,125 | |
| | Current portion of deferred revenues (Note 19) | | | 5,142 | | | 6,476 | |
| | Current portion of long-term debt (Note 20) | | | 2,158 | | | 1,446 | |
| | Customer deposits | | | 2,314 | | | — | |
| | |
| |
| |
| | | | 19,687 | | | 17,713 | |
Deferred revenues (Note 19) |
|
|
13,852 |
|
|
18,139 |
|
| Long-term debt (Note 20) | | | 10,568 | | | 6,614 | |
| Customer deposits | | | — | | | 1,966 | |
| Minority interest | | | 3,617 | | | 3,050 | |
| | |
| |
| |
| | | | 47,724 | | | 47,482 | |
| | |
| |
| |
| Commitments and contingencies (Note 24) | | | | | | | |
SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
| Common stock, without par value of unlimited shares authorized, 37,098,690 and 36,613,434 issued and outstanding at December 31, 2002 and 2001, respectively | | | 60,652 | | | 59,781 | |
| Additional paid-in capital | | | 15,550 | | | 15,476 | |
| Employee participation shares; 2,000,000 shares authorized (Note 22 c) | | | 140 | | | 166 | |
| | Less: loans receivable | | | (140 | ) | | (166 | ) |
| Warrants (Note 22 a) | | | — | | | 74 | |
| Deficit | | | (48,683 | ) | | (50,869 | ) |
| Accumulated other comprehensive loss | | | (7,292 | ) | | (7,584 | ) |
| | |
| |
| |
| | | | 20,227 | | | 16,878 | |
| | |
| |
| |
| | | $ | 67,951 | | $ | 64,360 | |
| | |
| |
| |
See the accompanying notes to the Consolidated Financial Statements
F-4
DRAXIS HEALTH INC.
Consolidated Statements of Shareholders' Equity
December 31
(in thousands of U.S. dollars except share related data)
| | 2002
| | 2001
| | 2000
| |
|---|
| Common Stock (Number of Shares) | | | | | | | | | | |
| Balance, beginning of period | | | 36,613,434 | | | 36,565,102 | | | 35,557,366 | |
| | Exercise of warrants | | | — | | | — | | | 600,000 | |
| | Exercise of options | | | 468,168 | | | 48,332 | | | 382,582 | |
| | Exercise of employee participation shares | | | 17,088 | | | — | | | 125,154 | |
| | Repurchased for cancellation | | | — | | | — | | | (100,000 | ) |
| | |
| |
| |
| |
| Balance, end of period | | | 37,098,690 | | | 36,613,434 | | | 36,565,102 | |
| | |
| |
| |
| |
| Common Stock | | | | | | | | | | |
| Balance, beginning of period | | $ | 59,781 | | $ | 59,698 | | $ | 57,163 | |
| | Exercise of warrants | | | — | | | — | | | 1,883 | |
| | Exercise of options | | | 817 | | | 83 | | | 787 | |
| | Exercise of employee participation shares | | | 54 | | | — | | | — | |
| | Repurchased for cancellation | | | — | | | — | | | (135 | ) |
| | |
| |
| |
| |
| Balance, end of period | | $ | 60,652 | | $ | 59,781 | | $ | 59,698 | |
| | |
| |
| |
| |
| Additional Paid-In Capital | | | | | | | | | | |
| Balance, beginning of period | | $ | 15,476 | | $ | 15,476 | | $ | 14,099 | |
| | Fair value associated with expired warrants | | | 74 | | | — | | | — | |
| | Gain on reduction of ownership in subsidiary (Note 21) | | | — | | | — | | | 1,377 | |
| | |
| |
| |
| |
| Balance, end of period | | $ | 15,550 | | $ | 15,476 | | $ | 15,476 | |
| | |
| |
| |
| |
| Employee Participation Shares | | | | | | | | | | |
| Balance, beginning of period | | $ | 166 | | $ | 166 | | $ | 351 | |
| | Exercise of employee participation shares | | | (26 | ) | | — | | | (185 | ) |
| | |
| |
| |
| |
| Balance, end of period | | $ | 140 | | $ | 166 | | $ | 166 | |
| | |
| |
| |
| |
| Employee Participation Shares-Loans Receivable | | | | | | | | | | |
| Balance, beginning of period | | $ | (166 | ) | $ | (166 | ) | $ | (351 | ) |
| | Exercise of employee participation shares | | | 26 | | | — | | | 185 | |
| | |
| |
| |
| |
| Balance, end of period | | $ | (140 | ) | $ | (166 | ) | $ | (166 | ) |
| | |
| |
| |
| |
| Warrants | | | | | | | | | | |
| Balance, beginning of period | | $ | 74 | | $ | 74 | | $ | 437 | |
| | Exercise of warrants | | | — | | | — | | | (363 | ) |
| | Expiry of warrants | | | (74 | ) | | — | | | — | |
| | |
| |
| |
| |
| Balance, end of period | | $ | — | | $ | 74 | | $ | 74 | |
| | |
| |
| |
| |
| Deficit | | | | | | | | | | |
| Balance, beginning of period | | $ | (50,869 | ) | $ | (49,285 | ) | $ | (28,819 | ) |
| | Net income (loss) | | | 2,186 | | | (1,584 | ) | | (20,338 | ) |
| | Common shares purchased for cancellation | | | — | | | — | | | (128 | ) |
| | |
| |
| |
| |
| Balance, end of period | | $ | (48,683 | ) | $ | (50,869 | ) | $ | (49,285 | ) |
| | |
| |
| |
| |
| Accumulated Other Comprehensive Income (Loss) | | | | | | | | | | |
| Balance, beginning of period | | $ | (7,584 | ) | $ | (5,155 | ) | $ | (4,764 | ) |
| | Other comprehensive income (loss) | | | 292 | | | (2,429 | ) | | (391 | ) |
| | |
| |
| |
| |
| Balance, end of period | | | (7,292 | ) | | (7,584 | ) | | (5,155 | ) |
| | |
| |
| |
| |
| | Total shareholders' equity | | $ | 20,227 | | $ | 16,878 | | $ | 20,808 | |
| | |
| |
| |
| |
| Comprehensive Income (Loss) | | | | | | | | | | |
| | Foreign currency translation adjustments | | $ | 292 | | $ | (2,429 | ) | $ | (391 | ) |
| | |
| |
| |
| |
| Other comprehensive income (loss) | | | 292 | | | (2,429 | ) | | (391 | ) |
| Net income (loss) | | | 2,186 | | | (1,584 | ) | | (20,338 | ) |
| | |
| |
| |
| |
| Total comprehensive income (loss) | | $ | 2,478 | | $ | (4,013 | ) | $ | (20,729 | ) |
| | |
| |
| |
| |
See the accompanying notes to the Consolidated Financial Statements
F-5
DRAXIS HEALTH INC.
Consolidated Statements Of Cash Flows
Years ended December 31
(in thousands of U.S. dollars)
| | 2002
| | 2001
| | 2000
| |
|---|
| CASH FLOWS (USED IN) FROM OPERATING ACTIVITIES | | | | | | | | | | |
| Net income (loss) from continuing operations before cumulative effect of accounting change | | $ | 3,020 | | $ | (1,015 | ) | $ | 363 | |
| | Adjustments to reconcile net income (loss) from continuing operations to net cash (used in) from operating activities | | | | | | | | | | |
| | Amortization of deferred revenues | | | (6,568 | ) | | (4,783 | ) | | (4,234 | ) |
| | Depreciation and other amortization | | | 2,804 | | | 2,436 | | | 2,318 | |
| | Stock compensation | | | 28 | | | — | | | — | |
| | Other income (Note 7) | | | — | | | — | | | (411 | ) |
| | Deferred income taxes | | | (1,181 | ) | | 872 | | | (1,550 | ) |
| | Minority interest | | | (252 | ) | | (286 | ) | | (339 | ) |
| | Other | | | 376 | | | 470 | | | 392 | |
| Changes in operating assets and operating liabilities | | | | | | | | | | |
| | Accounts receivable | | | (401 | ) | | (991 | ) | | (1,192 | ) |
| | Inventories | | | (835 | ) | | 675 | | | (1,719 | ) |
| | Income taxes | | | (235 | ) | | 1,592 | | | (18 | ) |
| | Prepaid expenses | | | (185 | ) | | 495 | | | (912 | ) |
| | Accounts payable and accrued liabilities | | | 1,245 | | | 1,850 | | | (101 | ) |
| | Current portion of deferred revenues | | | — | | | 1,629 | | | 1,686 | |
| | |
| |
| |
| |
| | | | (2,184 | ) | | 2,944 | | | (5,717 | ) |
| | |
| |
| |
| |
| CASH FLOWS (USED IN) FROM INVESTING ACTIVITIES | | | | | | | | | | |
| | Expenditures for property, plant and equipment | | | (5,390 | ) | | (5,363 | ) | | (1,126 | ) |
| | (Decrease) increase in intangible assets | | | (60 | ) | | 75 | | | — | |
| | Increase in deferred revenues | | | 899 | | | 1,722 | | | 6,938 | |
| | Proceeds from disposition of product rights, net (Note 7) | | | — | | | — | | | 742 | |
| | |
| |
| |
| |
| | | | (4,551 | ) | | (3,566 | ) | | 6,554 | |
| | |
| |
| |
| |
| CASH FLOWS (USED IN) FROM FINANCING ACTIVITIES | | | | | | | | | | |
| | Proceeds from bank indebtedness | | | 382 | | | 434 | | | 1,381 | |
| | Repayment of bank indebtedness | | | (1,190 | ) | | — | | | (2,522 | ) |
| | Proceeds from long-term debt | | | 5,243 | | | — | | | — | |
| | Repayment of long-term debt | | | (172 | ) | | (762 | ) | | (4,046 | ) |
| | Proceeds from customer deposits | | | 709 | | | 2,000 | | | — | |
| | Repayment of customer deposits | | | (232 | ) | | — | | | — | |
| | Exercise of warrants and options | | | 844 | | | 83 | | | 2,308 | |
| | Common shares purchased for cancellation | | | — | | | — | | | (262 | ) |
| | Issue of common shares by subsidiary to minority interest (Note 21) | | | 969 | | | — | | | 5,375 | |
| | Repurchase of common shares by subsidiary from minority interest | | | (177 | ) | | — | | | — | |
| | |
| |
| |
| |
| | | | 6,376 | | | 1,755 | | | 2,234 | |
| | |
| |
| |
| |
| | Effect of foreign exchange rate changes on cash and cash equivalents | | | 95 | | | (26 | ) | | 214 | |
| | |
| |
| |
| |
| | Net cash (used in) from continuing operations | | | (264 | ) | | 1,107 | | | 3,285 | |
| | Net cash (used in) from discontinued operations | | | (439 | ) | | 75 | | | (881 | ) |
| | |
| |
| |
| |
| | Net (decrease) increase in cash and cash equivalents | | | (703 | ) | | 1,182 | | | 2,404 | |
| | Cash and cash equivalents, beginning of period | | | 5,602 | | | 4,420 | | | 2,016 | |
| | |
| |
| |
| |
| | Cash and cash equivalents, end of period | | $ | 4,899 | | $ | 5,602 | | $ | 4,420 | |
| | |
| |
| |
| |
Additional Information |
|
|
|
|
|
|
|
|
|
|
| | Interest paid | | $ | 417 | | $ | 634 | | $ | 676 | |
| | Income taxes paid | | $ | 1,791 | | $ | 336 | | $ | 1,474 | |
| | |
| |
| |
| |
See the accompanying notes to the Consolidated Financial Statements
F-6
DRAXIS HEALTH INC.
Notes to the Consolidated Financial Statements
(in thousands of U.S. dollars except share related data)
1. Nature of Operations
DRAXIS Health Inc. ("DRAXIS" or the "Company") is an integrated pharmaceutical company focused in two specialty segments: the development, production, marketing and distribution of radiopharmaceuticals through its wholly owned subsidiary DRAXIMAGE Inc. ("DRAXIMAGE") and the provision of contract pharmaceutical manufacturing services, specializing in liquid and freeze-dried injectables and other sterile products through its 66.9%-owned subsidiary DRAXIS Pharma Inc. ("DPI"). The Company's common shares are listed on NASDAQ and the Toronto Stock Exchange.
2. Significant Accounting Policies
The Company has prepared these consolidated financial statements in U.S. dollars and in accordance with generally accepted accounting principles ("GAAP") in the United States.
Consolidated financial statements prepared in U.S. dollars and in accordance with Canadian GAAP are available to shareholders and filed with various regulatory authorities.
- (b)
- Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its subsidiary companies with provision for minority interests. The Company's effective interest in the voting equity share capital of its principal subsidiaries is 100%, except in the case of DPI, for which it is 66.9% (2001-65.9%; 2000-65.9%). All significant intercompany transactions and balances are eliminated on consolidation.
Minority interest represents the minority shareholders' proportionate share of equity and net income or loss of DPI.
The preparation of the Company's consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Estimates are used when accounting for items and matters such as long-term contracts, allowance for uncollectible accounts receivable, inventory obsolescence, product warranty, amortization, employee benefits, taxes, provisions, acquired research and development and contingencies.
- (e)
- Reporting Currency and Foreign Currency Translation
The Company reports its consolidated financial statements in U.S. dollars. The financial statements of the parent company and its non-U.S. subsidiaries are translated into U.S. dollars in accordance with Statement of Financial Accounting Standards ("SFAS") No. 52, "Foreign Currency Translation." Asset and liability accounts are translated at the rate of exchange prevailing at the balance sheet date. Shareholders' equity accounts are translated at the applicable historical rate. Revenue and expense accounts are translated at the average rate of exchange for the period. The cumulative foreign currency translation adjustment is reported as a component of accumulated other comprehensive loss in shareholders' equity. The net change in the cumulative foreign currency translation adjustment in the periods presented is primarily due to fluctuations in the exchange rates between the Company's reporting currency and the Canadian dollar.
Product Sales—The Company recognizes revenue, net of trade discounts and allowances, when evidence of an arrangement exists, delivery has occurred or services rendered, the price is fixed or determinable and collectability is reasonably assured.
Amounts received from customers as prepayments for products to be shipped in the future are reported as customer deposits.
F-7
Service revenues are recognized at the time of performance or proportionately over the term of the contract, as appropriate.
Royalty and Licensing—Royalty revenue is recognized on an accrual basis in accordance with contractual agreements when all significant contractual obligations have been satisfied, the amounts are determinable and collection is reasonably assured. Royalty revenue is net of amounts owing to sublicensees where the Company is simply acting as an agent for the sublicensee.
License and other forms of non-refundable fees received pursuant to collaboration agreements are accounted for according to the related contractual agreements. In general, such fees are deferred and recognized on a straight-line basis over the lesser of the contract period and the estimated term over which contractual benefits are expected to be derived. If payment of such fees is contingent upon future performance obligations of the Company or other future events, revenue recognition of such amounts is deferred and recognized upon completion of the specific event.
- (g)
- Research and Development
In accordance with SFAS No. 2, "Accounting for Research and Development Costs," research and development costs are expensed in the period in which they are incurred. Acquired research and development having no alternative future use is written off at the time of acquisition. The cost of intangibles that are purchased from others for a particular research and development project that have no alternative future use is written off at the time of acquisition.
The liability method of accounting for income taxes is used in accordance with SFAS No. 109, "Accounting for Income Taxes." Under this method, deferred tax assets and liabilities are recognized for the differences between the financial statement and income tax bases of assets and liabilities, and for operating losses and tax credit carryforwards. A valuation allowance is provided for the portion of deferred tax assets which are more likely than not to be unrealized. Deferred tax assets and liabilities are measured using the enacted tax rates and laws.
- (i)
- Cash and Cash Equivalents
All highly liquid investments with original maturities of three months or less are classified as cash and cash equivalents. The fair value of cash and cash equivalents approximates the amounts shown in the financial statements.
Inventories are comprised of raw materials, work-in-process and finished goods. Raw materials are valued at the lower of cost on a moving average basis and replacement cost. Work-in-progress is valued at the lower of cost and net realizable value and includes material, direct labour, and related manufacturing overhead costs. Finished goods are valued at the lower of cost on a first-in, first-out basis and net realizable value and includes all related manufacturing and packaging costs.
- (k)
- Property, Plant and Equipment
Property, plant and equipment are reported at cost, less accumulated depreciation. The Company provides for depreciation using the following methods and applying rates estimated to amortize the cost over the useful life of the assets:
| Building | | straight-line over 25 years |
| Equipment | | 20%-30% declining balance and straight-line over 5-10 years |
Expenditures for construction of assets incurred prior to productive use are reflected as assets under construction. Depreciation commences when an asset is substantially completed and ready for productive use.
Effective with the adoption of SFAS No. 142, "Goodwill and Other Intangible Assets," on January 1, 2002, goodwill is no longer subject to amortization over its estimated useful life. Goodwill is subject to at least an annual assessment of impairment by applying a fair-value-based test. The Company completed its assessment for goodwill impairment and has determined that goodwill is not impaired.
F-8
Acquired intangible assets which do not have regulatory approval and for which there are no alternative uses are expensed as acquired in-process research and development, and those that have regulatory approval are capitalized.
Intangible assets with finite lives are reported at cost, less accumulated amortization. The Company does not have any intangible assets with indefinite lives. The Company provides for amortization on a straight-line basis on the following estimated useful lives:
| Patents and trademarks | | 10 years |
| Licenses | | 9-15 years |
The Company uses a discounted cash flow model to value the assessments of impairment that requires assumptions about the timing and amount of future cash flows, risk, the cost of capital and terminal values. Intangible assets are reviewed for impairment on an annual basis. The Company completed its assessment for intangible assets impairment and has determined that intangible assets are not impaired.
- (n)
- Stock-based Compensation
Under the provisions of SFAS No. 123, "Accounting for Stock Compensation," companies can either measure the compensation cost of equity instruments issued under employee compensation plans using a fair-value-based method or can continue to recognize compensation cost using the intrinsic value method under the provisions of Accounting Principles Board Opinion ("APB") No. 25, "Accounting for Stock Issued to Employees." However, if the provisions of APB No. 25 are applied, pro forma disclosure of net income (loss) and earnings (loss) per share must be presented in the financial statements as if the fair value method had been applied. For all periods presented, the Company recognized compensation costs under the provisions of APB No. 25, and the Company has provided the expanded disclosure required by SFAS No. 123.
3. Accounting Changes
- (a)
- Goodwill and Other Intangible Assets
Effective January 1, 2002, the Company adopted the new recommendations of the SFAS with respect to Statement No. 142, "Goodwill and Other Intangible Assets." Under SAFS No. 142, which can only be applied prospectively, goodwill and other intangible assets with indefinite lives are no longer amortized, but are tested for impairment upon adoption of the new standard and at least annually thereafter. The Company has assessed its goodwill by applying the prescribed method of comparing the fair value of its reporting unit to its carrying value and determined that there has been no goodwill impairment. The Company does not have any intangible assets with indefinite lives.
The following is a reconciliation of net income (loss) to reflect the impact of no longer amortizing goodwill effective January 1, 2002.
| | 2002
| | 2001
| | 2000
| |
|---|
| Net income (loss), as reported | | $ | 2,186 | | $ | (1,584 | ) | $ | (20,338 | ) |
| Amortization expense on goodwill | | | — | | | 103 | | | 107 | |
| | |
| |
| |
| |
| | Net income (loss), adjusted | | $ | 2,186 | | $ | (1,481 | ) | | (20,231 | ) |
| | |
| |
| |
| |
Effective January 1, 2002, the Company adopted the new recommendations of the SFAS with respect to Statement No. 141, "Business Combinations." Under SFAS No. 141, if certain criteria are met upon the initial adoption, reclassifications between goodwill and other intangible assets are required for any business combinations before July 1, 2001. The implementation of SFAS No. 141 resulted in reclassifications between goodwill and intangible assets for 2002 and the comparative periods.
Effective January 1, 2000, the Company changed its policy with respect to revenue recognition of non-refundable fees received in connection with collaboration agreements, whereby such fees are now deferred and recognized as revenue rateably over the contract period. This new policy is consistent with the guidelines
F-9
contained in the U.S. Securities and Exchange Commission Staff Accounting Bulletin No. 101, "Revenue Recognition in Financial Statements," dated December 1999. In 2000, $19,900 or $0.55 per share, representing the cumulative effect of this change in policy, was charged to earnings. Previously, such fees had been recognized as revenue, based on contractual entitlements and when receipt was reasonably assured.
4. Government Assistance
During the year, the Company recognized government assistance of $Nil (2001-$Nil; 2000-$211) related to creation of permanent jobs by DPI. This assistance was recorded as a reduction of selling, general and administration expenses.
The agreement governing the government assistance contains a contingency clause which would require repayment of funding if certain conditions are not met. The Company believes that it is compliant with these conditions.
5. Restructuring Charges
| | 2002
| | 2001
| | 2000
|
|---|
| Severance | | $ | — | | $ | — | | $ | 434 |
| | |
| |
| |
|
During 2000, the Company recorded a restructuring charge to cover costs associated with the divestiture of its dermatology product lines. These restructuring activities were completed in 2000 and the full amount of the reserve was utilized. No costs in excess of the provision have been incurred in subsequent periods.
6. Interest Expense
| | 2002
| | 2001
| | 2000
| |
|---|
| Interest income | | $ | 155 | | $ | 164 | | $ | 397 | |
| Financing expense | | | (422 | ) | | (567 | ) | | (1,013 | ) |
| Foreign exchange gain (loss) | | | (13 | ) | | 378 | | | (21 | ) |
| | |
| |
| |
| |
| | | $ | (280 | ) | $ | (25 | ) | $ | (637 | ) |
| | |
| |
| |
| |
7. Other Income
| | 2002
| | 2001
| | 2000
|
|---|
| Gain on disposition of product rights | | $ | — | | $ | — | | $ | 411 |
| | |
| |
| |
|
During 2000, the Company disposed of rights to certain dermatology products for proceeds of $742 and realized a gain of $411 on this transaction.
F-10
8. Income Taxes
| | 2002
| | 2001
| | 2000
| |
|---|
| The components of income tax expense are as follows: | | | | | | | | | | |
| | Current | | $ | 1,144 | | $ | 1,254 | | $ | 947 | |
| | Deferred | | | (1,298 | ) | | 1,795 | | | (1,162 | ) |
| | |
| |
| |
| |
| | | $ | (154 | ) | $ | 3,049 | | $ | (215 | ) |
| | |
| |
| |
| |
The reported income tax expense differs from the expected amount calculated by applying the Company's Canadian combined federal and provincial tax rate to income (loss) before income tax expense. The reasons for this difference and the related tax effects are as follows:
| | 2002
| | 2001
| | 2000
| |
|---|
| Income (loss) before income taxes from continuing operations | | $ | 2,614 | | $ | 1,748 | | $ | (190 | ) |
| Canadian combined federal and provincial tax rate | | | 36.4 | % | | 37.5 | % | | 38.3 | % |
| | |
| |
| |
| |
| Expected income tax expense (recovery) | | $ | 951 | | $ | 656 | | $ | (73 | ) |
Increase (decrease) resulting from: |
|
|
|
|
|
|
|
|
|
|
| | Foreign tax rate differences | | | (90 | ) | | (191 | ) | | (80 | ) |
| | Effects on deferred income taxes from reduction in Canadian income tax rates | | | 178 | | | 3,300 | | | — | |
| | Recovery of prior years' U.S. taxes | | | (418 | ) | | — | | | — | |
| | Non-taxable portion of capital gains | | | — | | | — | | | (107 | ) |
| | Unrecognized income tax benefit of losses | | | (658 | ) | | (870 | ) | | (607 | ) |
| | Goodwill and other amortization | | | 253 | | | 286 | | | 174 | |
| | Investment tax credits | | | (495 | ) | | (283 | ) | | (236 | ) |
| | Other | | | 125 | | | 151 | | | 714 | |
| | |
| |
| |
| |
| | | $ | (154 | ) | $ | 3,049 | | $ | (215 | ) |
| | |
| |
| |
| |
Deferred income tax assets have been provided as follows:
| | 2002
| | 2001
| |
|---|
| Loss carryforwards and investment tax credits | | $ | 7,007 | | $ | 6,079 | |
| Expenses not currently deductible for tax purposes | | | 328 | | | 260 | |
| Deferred revenue | | | 3,028 | | | 3,474 | |
| Tax value of property, plant and equipment in excess of net book value | | | 859 | | | 526 | |
| Tax value of intangible assets in excess of net book value | | | 4,545 | | | 4,499 | |
| | |
| |
| |
| Total deferred tax assets | | | 15,767 | | | 14,838 | |
| Valuation allowance | | | (2,159 | ) | | (2,773 | ) |
| | |
| |
| |
| Net deferred tax assets | | $ | 13,608 | | $ | 12,065 | |
| | |
| |
| |
| Deferred income tax assets are classified as follows: | | | | | | | |
| | Current | | $ | 990 | | $ | 2,917 | |
| | Non-current | | | 12,618 | | | 9,148 | |
| | |
| |
| |
| | | $ | 13,608 | | $ | 12,065 | |
| | |
| |
| |
At December 31, 2002, the Company has accumulated tax losses of $12,077 available for federal and provincial purposes in Canada, which expire from 2004 to 2009. The Company also has $1,098 of unclaimed Canadian investment tax credits, which expire from 2004 to 2012. These losses and investment tax credits can be used to offset future years' taxable income.
The Company has accumulated tax losses of $6,283 for federal and state purposes in the U.S., which expire from 2010 to 2014. Subject to certain limitations, these losses can be used to offset future years' taxable income.
F-11
9. Discontinued Operations
In 2001, the Company adopted a formal plan to dispose of its Canadian sales and marketing division, DRAXIS Pharmaceutica.
In January 2002, the Company announced that it had entered into a binding letter of intent (subject to satisfaction of various conditions) to sell DRAXIS Pharmaceutica to Elan Corporation, plc ("Elan"). On June 4, 2002, the Company announced that it had modified the terms of the proposed sales, primarily by the exclusion of the product rights to Alertec.
DRAXIS has received several non-binding offers related to the possible acquisition of DRAXIS Pharmaceutica following the August 2002 announcement that Elan had decided not to proceed with its planned acquisition of this division. The Company is currently engaged in advanced negotiations regarding a possible transaction. As options regarding potential divestiture are explored, the Company will continue to manage and operate this division to enhance shareholder value consistent with the Company's strategic focus on its core radiopharmaceutical and specialty contract manufacturing businesses. (See Note 27 under "Subsequent Events").
Pursuant to APB No. 30, "Reporting the Results of Operations—Reporting the Effects of Disposal of a Segment of a Business, and Extraordinary, Unusual and Infrequently Occurring Events and Transactions," the results of operations of DRAXIS Pharmaceutica have been reported as discontinued operations and the consolidated financial statements and notes thereto for the year ended December 31, 2002 and all comparative periods presented have been restated. In the second quarter of 2002, the Company resolved to retain ownership of the Canadian rights to Alertec and continue to market and sell Alertec in Canada itself. Accordingly, discontinued operations no longer include revenues and expenses directly attributable to Alertec and information for prior periods has been reclassified to reflect this change.
Interest expense directly attributable to license obligations included in the transaction has been allocated to the discontinued operations.
The results of discontinued operations, presented in the accompanying Consolidated Statements of Operations, were as follows:
| | 2002
| | 2001
| | 2000
| |
|---|
| Revenues | | $ | 6,844 | | $ | 6,180 | | $ | 5,764 | |
| | |
| |
| |
| |
| Net loss from discontinued operations | | $ | (834 | ) | $ | (569 | ) | $ | (801 | ) |
| | |
| |
| |
| |
10. Earnings (Loss) per Share
Earnings (loss) per share is based on the weighted average number of common shares outstanding (basic) adjusted, to the extent they are dilutive, for outstanding stock options and stock purchase warrants (diluted). The calculation of diluted earnings (loss) per share excludes any potential conversion of warrants, options and Participation Shares that would increase earnings per share or decrease a loss per share.
The following table details the weighted average number of common shares outstanding for the years ended December 31:
| | 2002
| | 2001
| | 2000
|
|---|
| Weighted average number of common shares outstanding—basic | | 36,981,985 | | 36,587,794 | | 36,324,199 |
| Weighted average effect of dilutive securities: | | | | | | |
| | Warrants | | 32,305 | | 23,133 | | 38,087 |
| | Stock option plan | | 295,866 | | — | | 187,703 |
| | Employee Participation Share Purchase Plan | | 27,723 | | — | | 23,585 |
| | |
| |
| |
|
| Weighted average number of common shares outstanding—diluted | | 37,337,879 | | 36,610,927 | | 36,573,574 |
| | |
| |
| |
|
F-12
11. Accounts Receivable
| | 2002
| | 2001
| |
|---|
| Trade | | $ | 7,671 | | $ | 7,135 | |
| Allowance for doubtful accounts | | | (154 | ) | | (132 | ) |
| Loans to employees | | | 262 | | | 469 | |
| Income taxes | | | 155 | | | — | |
| | |
| |
| |
| | | $ | 7,934 | | $ | 7,472 | |
| | |
| |
| |
12. Inventories
| | 2002
| | 2001
|
|---|
| Raw materials | | $ | 2,865 | | $ | 2,526 |
| Work-in-process | | | 1,024 | | | 741 |
| Finished goods | | | 2,245 | | | 2,005 |
| | |
| |
|
| | | $ | 6,134 | | $ | 5,272 |
| | |
| |
|
13. Property, Plant and Equipment
| | 2002
| | 2001
| |
|---|
| Land | | $ | 1,747 | | $ | 1,732 | |
| Building | | | 11,484 | | | 11,376 | |
| Equipment | | | 13,206 | | | 11,524 | |
| Assets under construction | | | 5,836 | | | 2,033 | |
| | |
| |
| |
| | | | 32,273 | | | 26,665 | |
| Accumulated depreciation | | | (6,219 | ) | | (4,371 | ) |
| | |
| |
| |
| | | $ | 26,054 | | $ | 22,294 | |
| | |
| |
| |
Depreciation of property, plant and equipment from continuing operations was $1,742, $1,250, and $1,168, for the years ended December 31, 2002, 2001 and 2000, respectively.
14. Goodwill
| | 2002
| | 2001
| |
|---|
| Goodwill | | $ | 1,011 | | $ | 1,006 | |
| Accumulated amortization | | | (455 | ) | | (455 | ) |
| | |
| |
| |
| | | $ | 556 | | $ | 551 | |
| | |
| |
| |
Amortization of goodwill from continuing operations was $Nil, $103, and $125, for the years ended December 31, 2002, 2001 and 2000, respectively.
Effective with the adoption of SFAS No. 142, "Goodwill and Other Intangible Assets," on January 1, 2002, goodwill is no longer subject to amortization over its estimated useful life (see Note 3 under "Accounting Changes"). Goodwill is subject to at least an annual assessment of impairment by applying a fair-value-based test. The Company completed its assessment for goodwill impairment and has determined that goodwill is not impaired.
F-13
15. Intangible Assets
| | 2002
|
|---|
| | Cost
| | Accumulated
Amortization
| | Net Book Value
|
|---|
| Patents and trademarks | | $ | 337 | | $ | 185 | | $ | 152 |
| Licenses | | | 18,427 | | | 10,938 | | | 7,489 |
| Other | | | 159 | | | 76 | | | 83 |
| | |
| |
| |
|
| | | $ | 18,923 | | $ | 11,199 | | $ | 7,724 |
| | |
| |
| |
|
|
|
2001
|
|---|
| | Cost
| | Accumulated
Amortization
| | Net Book Value
|
|---|
| Patents and trademarks | | $ | 335 | | $ | 150 | | $ | 185 |
| Licenses | | | 18,262 | | | 9,005 | | | 9,257 |
| Other | | | 157 | | | 60 | | | 97 |
| | |
| |
| |
|
| | | $ | 18,754 | | $ | 9,215 | | $ | 9,539 |
| | |
| |
| |
|
Amortization of intangible assets from continuing operations was $1,049, $1,103, and $1,037, for the years ended December 31, 2002, 2001 and 2000, respectively.
16. Bank Indebtedness
The Company's credit facilities as well as their interest rates were as follows:
December 31, 2002
| | Committed
| | Amount
Drawn
| | Available
| | Period-end
Rate
| |
|---|
| Credit Facilities: | | | | | | | | | | | | |
| (a)DRAXIS Pharma Inc. | | $ | 2,220 | | $ | 884 | | $ | 1,336 | | 5.5 | % |
| (b)DRAXIS Health Inc. | | | 951 | | | Nil | | | 951 | | 5.5 | % |
| | |
| |
| |
| |
| |
| | | $ | 3,171 | | $ | 884 | | $ | 2,287 | | | |
| | |
| |
| |
| |
| |
December 31, 2001
|
|
Committed
|
|
Amount
Drawn
|
|
Available
|
|
Period-end
Rate
|
|
|---|
| Credit Facility: | | | | | | | | | | | | |
| (a)DRAXIS Pharma Inc. | | $ | 2,220 | | $ | 1,666 | | $ | 554 | | 4.75 | % |
| | |
| |
| |
| |
| |
- (a)
- DPI was a party to a credit agreement with a Canadian chartered bank with respect to a revolving credit facility which provides for advances against eligible accounts receivable and inventories up to a maximum of $2,220 (CDN$3,500). The credit facility is secured by assets of DPI and bears interest at Canadian prime plus 1.0% (2001-Canadian prime plus 0.75%).
- (b)
- On December 31, 2002, the Company entered into a new $951 (CDN$1,500) credit facility with a Canadian chartered bank. This new credit facility provides for advances against eligible accounts receivable and inventories and is secured by assets of DRAXIS. Amounts may be drawn under this credit facility in either U.S. or Canadian dollars at Canadian prime plus 1.0%
F-14
17. Accounts Payable and Accrued Liabilities
| | 2002
| | 2001
|
|---|
| Trade | | $ | 6,069 | | $ | 5,155 |
| Employee related items | | | 1,894 | | | 1,647 |
| Income taxes | | | 868 | | | 864 |
| Other | | | 358 | | | 459 |
| | |
| |
|
| | | $ | 9,189 | | $ | 8,125 |
| | |
| |
|
18. Collaboration Agreements
In September 2000, the Company entered into a 10-year arrangement with Cytogen Corporation ("Cytogen") whereby Cytogen was granted an exclusive license to market, sell and distribute the Company's BrachySeed implant for the treatment of prostate cancer in the United States in exchange for non-refundable fees, royalties based on Cytogen's sales of BrachySeed and a supply agreement. Under the arrangement, the Company is entitled to receive up to $2,000 in non-refundable fees upon achievement of specified milestones of which $1,000 was received in 2002 (2001—$500; 2000—$500). Non-refundable fees received from Cytogen are deferred and recognized as revenue on a straight-line basis over the period to December 31, 2010. (See Note 27 under "Subsequent Events").
- (b)
- SpectroPharm Product Line
In May 2000, the Company entered an arrangement with GlaxoSmithKline Consumer Healthcare ("GSK"), formerly Block Drug Company (Canada) Limited, with respect to the Company's SpectroPharm line of dermatology products which included the sale of product rights to GSK in exchange for a non-refundable fee, the acquisition of inventory on hand, a supply agreement and a technical services arrangement. As a result of the Company's ongoing obligations to GSK pursuant to this arrangement, the $8,169 of net proceeds from the sale of the product rights have been deferred and are being recognized as revenue on a straight-line basis over the period to January 31, 2005.
In December 1997, the Company entered into an alliance with Pfizer, whereby Pfizer was granted a perpetual exclusive license to market, sell and distribute Anipryl in exchange for non-refundable fees, royalties based on the worldwide sales of Anipryl, a manufacturing and supply agreement and a research collaboration.
In December 1999, the Company and Pfizer amended the terms of the alliance (the "First Amendment") whereby $9,000 of potential additional non-refundable fees were eliminated in exchange for the Company receiving additional regulatory support for a potential new indication and additional manufacturing data. These potential additional non-refundable fees would have become payable if Pfizer had exercised its right to acquire product registrations following regulatory approval of Anipryl in designated European countries.
In December 2001, the Company and Pfizer further amended the terms of the alliance (the "Second Amendment") whereby the Company received a payment of $3,150 in respect of minimum royalty entitlements for the second and third three-year periods ending December 31, 2001 and 2002 and modifications to future royalty entitlements. The Second Amendment also resulted in all rights to Anipryl outside of North America reverting back to the Company, forfeiture of any additional minimum royalty entitlements and the termination of any future collaborative research on new indications or formulations for Anipryl.
Under the amended arrangement, the Company will not be entitled to receive any additional non-refundable fees. The $28,090 in non-refundable fees already received from Pfizer have been deferred and are being recognized as revenue on a straight-line basis over the period to December 31, 2006.
The portion of the $3,150 payment allocated to the modifications of future royalty entitlements has been deferred and is being recognized as revenue on a straight-line basis over the period to December 31, 2013.
The portion of the $3,150 payment referable to the minimum royalty entitlement for the three-year period ending December 31, 2001 was recognized as revenue in the fourth quarter of 2001. The portion referable to the
F-15
third three-year period ending December 31, 2002 was deferred and recognized as revenue on a straight-line basis over the four quarters of 2002.
19. Deferred Revenue
| | 2002
| | 2001
|
|---|
| BrachySeed | | $ | 1,767 | | $ | 872 |
| SpectroPharm product line | | | 7,760 | | | 7,691 |
| Anipryl | | | 29,892 | | | 29,892 |
| | |
| |
|
| | | | 39,419 | | | 38,455 |
| Less: accumulated amortization | | | 20,425 | | | 13,840 |
| | |
| |
|
| | | | 18,994 | | | 24,615 |
| Less: current portion | | | 5,142 | | | 6,476 |
| | |
| |
|
| | | $ | 13,852 | | $ | 18,139 |
| | |
| |
|
Amortization of deferred revenue totalled $6,568, $4,783, and $4,234 for the years ended December 31, 2002, 2001, and 2000, respectively.
20. Long-Term Debt
| | 2002
| | 2001
|
|---|
| DRAXIS Pharma Inc. | | | | | | |
Term bank loan, secured by the assets of DPI and bearing interest at Canadian prime plus 1.0% |
|
$ |
5,167 |
|
$ |
5,295 |
Term government loan, secured by specified assets of DPI and bearing interest at Canadian prime plus 2.5% |
|
|
1,081 |
|
|
— |
Loan from minority interest, secured by the assets of DPI and bearing interest at Canadian prime plus 1.5% |
|
|
1,975 |
|
|
— |
DRAXIS Health Inc. |
|
|
|
|
|
|
| Term bank loan, secured by the assets of DHI and bearing interest at Canadian prime plus 2.0% | | | 2,220 | | | — |
Unsecured obligation, bearing interest at the Bank of Canada rate |
|
|
2,283 |
|
|
2,765 |
| | |
| |
|
| | | | 12,726 | | | 8,060 |
| Less: current portion | | | 2,158 | | | 1,446 |
| | |
| |
|
| | | $ | 10,568 | | $ | 6,614 |
| | |
| |
|
The annual aggregate amounts of maturities on long-term debt for the next five years are as follows:
| 2003 | | $ | 2,158 |
| 2004 | | $ | 1,598 |
| 2005 | | $ | 2,471 |
| 2006 | | $ | 1,329 |
| 2007 | | $ | 3,304 |
Interest expense on long-term debt from continuing operations totalled $367, $398, and $611 for the years ended December 31, 2002, 2001, and 2000, respectively.
The fair value of the long-term debt is considered to be equivalent to its carrying value based upon consideration of borrowings with similar credit ratings and maturities.
F-16
21. Minority Interest
During 2002, the Company's wholly owned subsidiary, DPI, issued 1,018,809 common shares to SGF and members of DPI's management team in exchange for net proceeds of $969. These issuances were based on the pro-rata shareholdings of DPI prior to the issuance and accordingly there was no change in the Company's effective interest in DPI.
During 2002, the Company also repurchased and cancelled 277,778 outstanding shares of DPI held by a former employee of DPI in exchange for cash and settlement of an existing loan to the employee related to the original issuance of those shares.
During 2000, the Company's wholly owned subsidiary, DPI, issued 6,733,660 common shares to SGF and members of DPI's management team. These issuances, which reduced the Company's ownership in DPI from 100.0% to 65.9%, yielded aggregate net proceeds of $5,375 and resulted in an aggregate gain on reduction of ownership in subsidiary of $1,377. This amount is reflected as an adjustment to the shareholders' equity.
22. Shareholders' Equity
In connection with borrowings incurred related to the acquisition of the radiopharmaceutical division of Merck Frosst Canada & Co. ("MFCC"), the Company issued to a financial institution non-transferable warrants to purchase 750,000 common shares at CDN$3.70 per share on or before July 31, 2000. The number of exercisable warrants was reduced to 600,000 as a result of the early repayment of the related borrowings in 1997. In 1999, included as a component of shareholders' equity and deferred financing charges was $363, which represents the fair value of the above warrants. The fair value of the warrants was estimated at the date of issue using the Black-Scholes option-pricing model with the following assumptions: dividend yield of 0%, expected volatility of 40%, risk-free interest rate of 5.5%, and expected life of three years. As these warrants were fully exercised in 2000, the value of the warrants was accounted as cost of capital stock issued.
On November 8, 1999, in connection with the engagement of a financial advisor, the Company issued a non-assignable warrant to purchase 125,000 shares at $1.65 per share on or before November 8, 2002. Included as a component of shareholders' equity and deferred financing charges was $74, which represented the fair value of the above warrant. The fair value of the warrant was estimated at the date of issue using the Black-Scholes option-pricing model with the following assumptions: share price at date of issue of $1.375, dividend yield of 0%, expected volatility of 65%, risk-free rate of 5.8%, and expected life of three years. As these warrants expired unexercised in 2002, the value of the expired warrants was accounted as additional paid-in capital.
In aggregate, there were no warrants (2001-125,000) to purchase common shares at December 31, 2002.
The Board of Directors has adopted a stock option plan in order to provide an incentive for directors, officers and employees. The plan provides that the Board of Directors may, from time to time, at its discretion, grant to directors, officers and employees the option to purchase common shares. The Board of Directors will determine the price per common share and the number of common shares which may be allotted to each designated director, officer or employee and all other terms and conditions of the option in accordance with the applicable requirements of any relevant regulatory authority or stock exchange. These options will be exercisable for a period not exceeding 10 years from the date of the grant and generally options vest one third on each of the first, second and third anniversaries of grant.
On June 20, 2001, the Board of Directors received shareholder approval to increase the maximum number of options for issuance under the stock option plan from 5,500,000 to 7,500,000.
The Board of Directors has adopted a guideline limiting the aggregate number of common shares that can be issued at any point in time, either through the exercise of options or the conversion of Employee Participation Shares, to 13% of the Company's outstanding common shares. As at December 31, 2002 the aggregate number of shares issuable pursuant to outstanding options and Employee Participation Shares represented 8.9% of the outstanding common shares.
F-17
The following is a summary of the maximum number of common shares issuable pursuant to outstanding stock options and available for future issuance:
| | Outstanding
| |
| |
|---|
| | Available For Issuance 2002
| |
|---|
| | 2002
| | 2001
| | 2000
| |
|---|
| Balance, beginning of year | | 3,358,444 | | 3,079,527 | | 2,998,110 | | 1,890,361 | |
| Increase (decrease) resulting from: | | | | | | | | | |
| | Approved for issuance | | — | | — | | — | | — | |
| | Granted | | 750,000 | | 581,500 | | 611,500 | | (750,000 | ) |
| | Exercised | | (468,168 | ) | (48,332 | ) | (382,582 | ) | — | |
| | Cancelled | | (150,167 | ) | (63,001 | ) | (66,751 | ) | 150,167 | |
| | Expired | | (176,000 | ) | (191,250 | ) | (80,750 | ) | 176,000 | |
| | |
| |
| |
| |
| |
| Balance, end of year | | 3,314,109 | | 3,358,444 | | 3,079,527 | | 1,466,528 | |
| | |
| |
| |
| |
| |
Exercisable (vested), end of year |
|
2,102,610 |
|
2,365,654 |
|
2,116,560 |
|
|
|
| | |
| |
| |
| | | |
|
|
2002
|
|
2001
|
|
2000
|
|---|
| Weighted average exercise price of options: | | | | | | |
| | Outstanding, end of year | | CDN$3.60 | | CDN$3.42 | | CDN$3.35 |
| | Exercisable, end of year | | CDN$3.60 | | CDN$3.49 | | CDN$3.46 |
| | Granted | | CDN$3.86 | | CDN$3.44 | | CDN$3.10 |
| | Exercised | | CDN$2.78 | | CDN$2.64 | | CDN$3.01 |
| | Cancelled | | CDN$3.49 | | CDN$3.44 | | CDN$3.25 |
Range of exercise price of options: |
|
|
|
|
|
|
| | Granted | | CDN$2.50-$5.41 | | CDN$2.91-$3.60 | | CDN$2.49-$3.27 |
| | Exercised | | CDN$2.55-$4.29 | | CDN$2.55-$3.05 | | CDN$2.18-$4.40 |
| | Cancelled | | CDN$2.55-$4.42 | | CDN$2.94-$3.95 | | CDN$2.55-$3.80 |
| | |
| |
| |
|
The following table summarizes information about stock options outstanding at December 31, 2002:
| |
| | Options Outstanding
| | Options Exercisable
(Vested)
|
|---|
| |
| | Weighted Average
Remaining
Contractual Life
(In Years)
| |
|
|---|
Exercise Price
| | Number
| | Weighted
Average
Exercise Price
| | Number
| | Weighted
Average
Exercise Price
|
|---|
| CDN$1.63 - $2.00 | | 75,000 | | 2.00 | | CDN$ | 1.63 | | 75,000 | | CDN$ | 1.63 |
| CDN$2.01 - $2.50 | | 121,250 | | 2.97 | | | 2.45 | | 60,417 | | | 2.40 |
| CDN$2.51 - $3.00 | | 275,916 | | 2.12 | | | 2.87 | | 234,249 | | | 2.86 |
| CDN$3.01 - $3.50 | | 858,700 | | 1.98 | | | 3.12 | | 637,867 | | | 3.11 |
| CDN$3.51 - $4.00 | | 1,130,000 | | 3.41 | | | 3.72 | | 340,167 | | | 3.68 |
| CDN$4.01 - $4.50 | | 659,000 | | 3.57 | | | 4.30 | | 585,667 | | | 4.31 |
| CDN$4.51 - $5.41 | | 194,243 | | 2.11 | | | 5.16 | | 169,243 | | | 5.12 |
| | |
| |
| |
| |
| |
|
| | | 3,314,109 | | 2.84 | | CDN$ | 3.60 | | 2,102,610 | | CDN$ | 3.60 |
| | |
| |
| |
| |
| |
|
- (c)
- Employee Participation Share Purchase Plan
On February 16, 1995, the Company established the Employee Participation Share Purchase Plan for the directors, officers and employees of the Company to tie employee compensation more closely to shareholder value. The Employee Participation Share Purchase Plan was approved by the shareholders on June 16, 1995. The Board of Directors has provided that it would be a condition to receiving any benefit from the Employee Participation Share Purchase Plan that the share price have appreciated at least 25% from the date of issuance of any Participation Shares. The maximum number of Participation Shares issuable pursuant to the Employee Participation Share Purchase Plan is 2,000,000.
F-18
Vesting takes place over a four-year period at the rate of 20%, 20%, 20% and 40% commencing on the first anniversary of the issuance of the Participation Shares and for each of the three years thereafter, with the exception of 500,000 Participation Shares held by an officer of the Company, which vest at the rate of 10%, 20%, 30% and 40%. Vested Participation Shares are automatically convertible into shares of the Company at the election of the holder, provided that the shares have increased in value since the date of issuance of the vested Participation Shares by the aforementioned 25%. The number of common shares a Participant will receive when converting Participation Shares is determined by multiplying the number of Participation Shares held by a Participant by a fraction whose numerator is the amount by which the fair market value of a common share at the date of conversion exceeds the fair market value of a common share as at the date on which the Participation Shares were issued, and whose denominator is the fair market value of the common shares at the date of conversion. For purposes of the Employee Participation Share Purchase Plan, the fair market value of common shares at a particular time means the average of the daily high and low board lot trading prices on each of the five trading days on the Toronto Stock Exchange immediately preceding the valuation date.
On February 16, 1995, the Board of Directors of the Company authorized the issuance of 975,000 Series A Participation Shares at a subscription price of CDN$0.30 each. The average of the daily high and low board lot trading prices on each of the five trading days on the Toronto Stock Exchange immediately preceding the issuance of the Series A Participation Shares was CDN$2.45. As at December 31, 2000 all Series A Participation Shares had been either converted into common shares or cancelled by the Company.
On December 18, 1995, the Board of Directors of the Company authorized the issuance of 555,000 Series B Participation Shares at a subscription price of CDN$0.30 each. The average of the daily high and low board lot trading prices on each of the five trading days on the Toronto Stock Exchange immediately preceding the issuance of the Series B Participation Shares was CDN$2.25. As at December 31, 2000 all Series B Participation Shares had been either converted into common shares or cancelled by the Company.
On May 12, 1999, the Board of Directors of the Company authorized the issuance of 470,000 Series C Participation Shares at a subscription price of CDN$0.50 each. The average of the daily high and low board lot trading prices on each of the five trading days on the Toronto Stock Exchange immediately preceding the issuance of the Series C Participation Shares was CDN$3.24.
All outstanding Participation Shares have been issued and paid for by the employees through the issuance of a limited recourse promissory note and are secured against the shares.
Information pertaining to Participation Shares for the years ended December 31, 2002, 2001 and 2000 is set forth in the following table:
| | 2002
| | 2001
| | 2000
| |
|---|
| Outstanding, beginning of year | | 470,000 | | 470,000 | | 1,347,000 | |
| Granted | | — | | — | | — | |
| Exercised | | (75,000 | ) | — | | (292,000 | ) |
| Cancelled | | — | | — | | — | |
| Expired | | — | | — | | (585,000 | ) |
| | |
| |
| |
| |
| Outstanding, end of year | | 395,000 | | 470,000 | | 470,000 | |
| | |
| |
| |
| |
| Exercisable (vested), end of year | | 207,000 | | 188,000 | | 94,000 | |
| | |
| |
| |
| |
- (d)
- Stock-based Compensation Costs
The Company has adopted the disclosure requirements of SFAS No. 123, "Accounting for Stock-based Compensation," and as permitted under SFAS No. 123, applies APB No. 25 and related interpretations in accounting for its plan. SFAS No. 123 requires disclosure of pro forma amounts to reflect the impact if the Company had elected to adopt the optional recognition provisions of SFAS No. 123 for its stock option plans and Employee Participation Share Purchase Plan. Accordingly, the Company's net income (loss) applicable to common
F-19
shares and income (loss) per common share would have been increased to the pro forma amounts as indicated below:
| | 2002
| | 2001
| | 2000
| |
|---|
| Net income (loss), as reported | | $ | 2,186 | | $ | (1,584 | ) | $ | (20,338 | ) |
| Pro forma impact | | | (736 | ) | | (399 | ) | | (791 | ) |
| | |
| |
| |
| |
| Pro forma net income (loss) | | $ | 1,450 | | $ | (1,983 | ) | $ | (21,129 | ) |
| | |
| |
| |
| |
Basic net income (loss) per share, as reported |
|
$ |
0.058 |
|
$ |
(0.044 |
) |
$ |
(0.560 |
) |
| Pro forma impact per share | | | (0.020 | ) | | (0.011 | ) | | (0.022 | ) |
| | |
| |
| |
| |
| Pro forma net income (loss) per share (Basic) | | $ | 0.038 | | $ | (0.055 | ) | $ | (0.582 | ) |
| Pro forma net income (loss) per share (Diluted) | | $ | 0.038 | | $ | (0.055 | ) | $ | (0.582 | ) |
| | |
| |
| |
| |
The fair value of stock options used to compute pro forma net loss applicable to common shares and loss per common share disclosures is the estimated fair value at grant date using the Black-Scholes option-pricing model with the following assumptions as at December 31:
| | 2002
| | 2001
| | 2000
|
|---|
| Dividend yield | | 0.0% | | 0.0% | | 0.0% |
| Expected volatility | | 62%-64% | | 63%-66% | | 65%-68% |
| Risk-free interest rate | | 4.1%-5.6% | | 5.2%-5.6% | | 5.3%-6.3% |
| Expected option life | | 5 yrs | | 5 yrs | | 5 yrs |
| | |
| |
| |
|
The Black-Scholes option-pricing model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable and which significantly differ from the Company's stock option awards. In addition, option-pricing models require the input of highly subjective assumptions including the expected price volatility. The Company uses expected volatility rates, which are based on historical volatility rates trended into future years. Changes in the subjective input assumptions can materially affect the fair value estimate, and therefore the existing model do not necessarily provide a reliable single measure of the fair value of the Company's stock options.
- (e)
- Stock Repurchase Program
During 2002, under the stock repurchase program, the Company repurchased no common shares (2001-Nil; 2000-100,000) for cancellation at an average price of $Nil per share (2001-$Nil; 2000-$2.62) for total consideration of $Nil (2001-$Nil; 2000-$262). The excess of $Nil (2001-$Nil; 2000-$1.28) over the stated capital of the acquired shares was charged to deficit. The most recent issuer bid commenced on December 19, 2001 and terminated on December 18, 2002.
F-20
23. Segmented Information and Major Customers
For purposes of operating decision-making and assessing performance, management considers that it operates in three segments: Radiopharmaceuticals, Manufacturing, and Corporate and Other.
| | 2002
| | 2001
| | 2000
| |
|---|
| PRODUCT SALES REVENUES | | | | | | | | | | |
| Radiopharmaceuticals | | $ | 9,704 | | $ | 6,763 | | $ | 5,951 | |
| Manufacturing | | | 20,946 | | | 20,460 | | | 15,477 | |
| Corporate and Other | | | (312 | ) | | (72 | ) | | 1,161 | |
| | |
| |
| |
| |
| | | $ | 30,338 | | $ | 27,151 | | $ | 22,589 | |
| | |
| |
| |
| |
| ROYALTY AND LICENSING REVENUES | | | | | | | | | | |
| Radiopharmaceuticals | | $ | 451 | | $ | 192 | | $ | — | |
| Manufacturing | | | — | | | — | | | — | |
| Corporate and Other | | | 7,851 | | | 6,560 | | | 7,132 | |
| | |
| |
| |
| |
| | | $ | 8,302 | | $ | 6,752 | | $ | 7,132 | |
| | |
| |
| |
| |
| TOTAL REVENUES | | | | | | | | | | |
| Radiopharmaceuticals | | $ | 10,155 | | $ | 6,955 | | $ | 5,951 | |
| Manufacturing | | | 20,946 | | | 20,460 | | | 15,477 | |
| Corporate and Other | | | 7,539 | | | 6,488 | | | 8,293 | |
| | |
| |
| |
| |
| | | $ | 38,640 | | $ | 33,903 | | $ | 29,721 | |
| | |
| |
| |
| |
| SEGMENT INCOME (LOSS)(1) | | | | | | | | | | |
| Radiopharmaceuticals | | $ | 706 | | $ | 499 | | $ | 333 | |
| Manufacturing | | | 627 | | | 149 | | | (200 | ) |
| Corporate and Other | | | 4,365 | | | 3,561 | | | 2,221 | |
| | |
| |
| |
| |
| | | $ | 5,698 | | $ | 4,209 | | $ | 2,354 | |
| | |
| |
| |
| |
| DEPRECIATION AND AMORTIZATION | | | | | | | | | | |
| Radiopharmaceuticals | | $ | 716 | | $ | 623 | | $ | 547 | |
| Manufacturing | | | 1,166 | | | 867 | | | 807 | |
| Corporate and Other | | | 922 | | | 946 | | | 964 | |
| | |
| |
| |
| |
| | | $ | 2,804 | | $ | 2,436 | | $ | 2,318 | |
| | |
| |
| |
| |
| OPERATING INCOME (LOSS)(2) | | | | | | | | | | |
| Radiopharmaceuticals | | $ | (10 | ) | $ | (124 | ) | $ | (214 | ) |
| Manufacturing | | | (539 | ) | | (718 | ) | | (1,007 | ) |
| Corporate and Other | | | 3,443 | | | 2,615 | | | 1,257 | |
| | |
| |
| |
| |
| | | $ | 2,894 | | $ | 1,773 | | $ | 36 | |
| | |
| |
| |
| |
| IDENTIFIABLE ASSETS | | | | | | | | | | |
| Radiopharmaceuticals | | $ | 10,823 | | $ | 13,558 | | $ | 7,652 | |
| Manufacturing | | | 30,701 | | | 24,906 | | | 25,717 | |
| Corporate and Other | | | 26,427 | | | 25,896 | | | 33,938 | |
| | |
| |
| |
| |
| | | $ | 67,951 | | $ | 64,360 | | $ | 67,307 | |
| | |
| |
| |
| |
- (1)
- Segment income (loss) from continuing operations before depreciation and amortization, interest expense, other income, income taxes, minority interest and cumulative effect of accounting change.
F-21
- (2)
- Segment income (loss) from continuing operations before interest expense, other income, income taxes, minority interest and cumulative effect of accounting change.
| | 2002
| | 2001
| | 2000
|
|---|
| Geographic Segmentation | | | | | | | | | |
| REVENUES(3) | | | | | | | | | |
| Canada | | $ | 23,205 | | $ | 22,240 | | $ | 20,104 |
| United States | | | 15,246 | | | 11,663 | | | 9,617 |
| Other | | | 189 | | | — | | | — |
| | |
| |
| |
|
| | | $ | 38,640 | | $ | 33,903 | | $ | 29,721 |
| | |
| |
| |
|
| LONG-LIVED ASSETS(4) | | | | | | | | | |
| Canada | | $ | 34,334 | | $ | 32,384 | | $ | 32,414 |
| United States | | | — | | | — | | | — |
| | |
| |
| |
|
| | | $ | 34,334 | | $ | 32,384 | | $ | 32,414 |
| | |
| |
| |
|
- (3)
- Revenues are attributable to countries based upon the location of the customer.
- (4)
- Represents property, plant and equipment, goodwill and intangible assets that are identified with each geographic region.
| | Percentage of Total Revenue
| |
|---|
| | 2002
| | 2001
| | 2000
| |
|---|
| Customer A | | 19 | % | 23 | % | 21 | % |
| Customer B | | 17 | | 18 | | 14 | |
| | |
| |
| |
| |
| | | 36 | % | 41 | % | 35 | % |
| | |
| |
| |
| |
24. Commitments and Contingencies
The Company is committed under operating leases requiring minimum annual lease payments as follows:
| 2003 | | $ | 252 |
| 2004 | | | 145 |
| 2005 | | | 49 |
| 2006 | | | 10 |
| 2007 | | | — |
| | |
|
| | | $ | 456 |
| | |
|
Coincident with DPI's issuance of shares in 2000 (Note 21), the Company entered into a shareholders' agreement which granted SGF the right to obligate the Company to purchase its shareholdings in DPI anytime after February 18, 2005 and DPI's management shareholders the right to obligate DPI to purchase their shareholdings in DPI anytime following the tenth anniversary of the initial subscription. The price to be paid is based on the fair market value of DPI at the time of exercise. Subject to certain conditions, at the Company's option up to 40% of the SGF purchase consideration may be made in the form of the Company's common shares.
The Company is obligated to make certain royalty payments based on related product sales and milestone payments based on the achievement of certain specified events.
F-22
From time to time, the Company becomes involved in legal proceedings and claims which arise in the ordinary course of business.
With the exception of the two matters described below related to Anipryl, the Company considers that the ultimate liability with respect to any known actions will not materially affect the business, financial position, results of operations or cash flows of the Company.
- (i)
- In 1998 a Canadian legal proceeding was launched against the Company and Deprenyl Animal Health, Inc. ("DAHI"), a wholly owned subsidiary of the Company, by a former consultant claiming royalty entitlements based on the net profit from sales of Anipryl. The Company regards this claim to be without merit and is vigorously defending itself.
- (ii)
- Since 2000, the Company and DAHI have been involved in U.S. and Canadian legal proceedings with the University of Toronto and the University of Toronto Innovations Foundation. One dispute relates to the terms of a 1992 license agreement under which Innovations Foundation is claiming entitlement to a portion of the consideration earned by DAHI with respect to Anipryl. The second dispute relates to a 1990 contract research agreement under which the University of Toronto is claiming damages related to the ownership of certain Anipryl-related intellectual property. The Company considers all of the claims made by the University of Toronto and the University of Toronto Innovations Foundation to be without merit and is vigorously defending itself.
25. Related Party Transactions
Significant transactions not otherwise disclosed in the accompanying financial statements were as follows:
| | 2002
| | 2001
| | 2000
|
|---|
| Net contribution from the sales of a product by a company which is a shareholder included in loss from discontinued operations (total revenues: 2002 — $784; 2001 — $856; 2000 — $1,160) | | $ | 192 | | $ | 176 | | $ | 303 |
| Rent paid to a company jointly controlled by a member of the Board of Directors included in selling, general and administration expenses | | $ | 125 | | $ | 116 | | $ | 121 |
| | |
| |
| |
|
The aforementioned transactions are in the normal course of operations and are measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties.
In 1999, the Company sold all of its shares in Stëf International Corporation to an officer of the Company. In 2000, the Company reacquired these shares. Both transactions were valued at an identical nominal amount.
26. Financial Instruments
The fair value of cash, accounts receivable, accounts payable and accrued charges are equivalent to their carrying value because of the short-term maturity of those instruments. The fair value of long-term investments is determined based on quoted market prices. The Company is not party to any derivative instruments.
The Company is subject to credit risk through trade receivables and short-term cash investments. Credit risk with respect to trade receivables is limited given the creditworthiness of the counterparties. The Company invests its excess liquidity in high quality government securities and short-term commercial paper, bank deposits and money market mutual funds which are invested in high quality short-term securities.
The Company is subject to currency risk through its U.S. integrated foreign operations. Changes in the exchange rate may result in a decrease or increase in the foreign exchange gain or loss. The Company does not use derivative instruments to reduce its exposure to foreign currency risk.
F-23
27. Subsequent Events
On March 31, 2003 DRAXIS amended its License, Distribution and Supply Agreement with affiliates of Elan to return the Canadian rights for several of Elan's neurology products in exchange for a cash payment of $6.5 million. This transaction is the first step related to the divestiture of DRAXIS' pharmaceutical sales and marketing division.
On April 8, 2003, DRAXIMAGE reached an agreement with its licensee for terminating both the License and Distribution Agreement and the Product Manufacturing and Supply Agreement for BrachySeed implants in the U.S.
28. Recent Pronouncements
In June 2001, the Financial Accounting Standards Board (FASB) issued SFAS No. 143, "Accounting for Asset Retirement Obligations," which is effective for financial statements issued for fiscal years beginning after June 15, 2002. In November 2002, the FASB issued Interpretation No. 45, "Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others." This Interpretation requires the recognition of certain guarantees as liabilities at fair market value and is effective for guarantees issued or modified after December 31, 2002. Adoption of the provisions of the Statement and Interpretation will not have a material effect on the financial statements of the Company.
In June 2002, the FASB issued SFAS No. 146, "Accounting for Costs Associated with Exit or Disposal Activities." SFAS No. 146 requires that a liability for costs associated with an exit or disposal activity be recognized and measured initially at fair value only when the liability is incurred. SFAS No. 146 is effective for exit or disposal activities that are initiated after December 31, 2002 and will not have a material effect on the financial statements of the Company.
29. Comparative Information
The Company has reclassified certain prior years' information to conform with the current presentation format.
F-24
CERTIFICATIONS
I, Martin Barkin, M.D., certify that:
1. I have reviewed this Annual Report on Form 20-F of DRAXIS Health Inc.;
2. Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
3. Based on my knowledge, the financial statements, and other financial information included in this Annual Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this Annual Report.
4. The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in exchange Act Rules 13a-14 and 15d-14) for the registrant and have:
a. designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared;
b. evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this Annual Report (the "Evaluation Date"); and
c. presented in this Annual Report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
5. The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent function):
a. all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
b. any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
6. The registrant's other certifying officers and I have indicated in this annual report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
| Date: | | May 14, 2003
| | By: | | /s/ MARTIN BARKIN, M.D.
Martin Barkin, M.D.
President and Chief Executive Officer |
CERTIFICATIONS
I, Jim A.H. Garner, Senior Vice President, Finance and Chief Financial Officer, certify that:
1. I have reviewed this Annual Report on Form 20-F of DRAXIS Health Inc.;
2. Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
3. Based on my knowledge, the financial statements, and other financial information included in this Annual Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this Annual Report.
4. The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in exchange Act Rules 13a-14 and 15d-14) for the registrant and have:
a. designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared;
b. evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this Annual Report (the "Evaluation Date"); and
c. presented in this Annual Report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
5. The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent function):
a. all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
b. any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
6. The registrant's other certifying officers and I have indicated in this annual report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
| Date: | | May 14, 2003
| | By: | | /s/ JIM A.H. GARNER
Jim A.H. Garner
Senior Vice President, Finance and Chief Financial Officer |
QuickLinks
RISK FACTORSPART II.SIGNATURESEXHIBIT INDEX DRAXIS HEALTH INC. Form 20-F Annual ReportConsolidated Statements of OperationsConsolidated Balance SheetsConsolidated Statements of Shareholders’ EquityConsolidated Statements Of Cash Flows Years ended December 31Notes to the Consolidated Financial StatementsCERTIFICATIONS
