
C C on f on f i i den t de n t i i a l a l : : F F o r or I I n n t t e e r r na l nal S S C C I I L E L E X X P P h ha r ar m m a c a c eu t eu t i i c c a l a l s s U U s e se O n On l l y y 1 1 Scilex Holding Company Innovative Leader in Non - Opioid Pain Therapeutics December 2021 Exhibit 99.1
THIS DOCUMENT CONTAINS PROPRIETARY INFORMATION THAT IS THE PROPERTY OF THE COMPANY . NEITHER THIS DOCUMENT, NOR THE PROPRIETARY INFORMATION CONTAINED HEREIN, SHALL BE PUBLISHED, REPRODUCED, COPIED, DISCLOSED OR USED FOR ANY OTHER PURPOSE, OTHER THAN THE REVIEW AND CONSIDERATION OF THIS DOCUMENT . Safe Harbor Statements Forward - Looking Statements Certain statements contained in this corporate presentation (this “Presentation”), along with certain statements that may be made by management of Scilex Holding Company (together with its subsidiaries, “ Scilex ”) orally in presenting this material, are or may be considered “forward - looking statements” as defined in the Private Securities Litigation Reform Act of 1995. These statements can be identified by the fact that they do not relate strictly to historic or current facts. They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “potential,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition. Scilex cautions that these statements are based upon the current beliefs and expectations of Scilex’s management and are subject to significant risks, uncertainties and assumptions. Statements regarding future actions, future performance and/or future results including, without limitation, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of Scilex’s formulations and products and regulatory filings related to the same, financial projections and targets, business strategy, plans and objectives for future operations, and statements regarding the proposed business combination (the “Proposed Business Combination”) between Scilex and Vickers Vantage Corp. I (the “SPAC”) may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements. In light of these risks, uncertainties and assumptions, the forward - looking events and circumstances discussed in this Presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements. Accordingly, you should not rely upon forward - looking statements as predictions of future events. Scilex Scilex Holding Company

THIS DOCUMENT CONTAINS PROPRIETARY INFORMATION THAT IS THE PROPERTY OF THE COMPANY . NEITHER THIS DOCUMENT, NOR THE PROPRIETARY INFORMATION CONTAINED HEREIN, SHALL BE PUBLISHED, REPRODUCED, COPIED, DISCLOSED OR USED FOR ANY OTHER PURPOSE, OTHER THAN THE REVIEW AND CONSIDERATION OF THIS DOCUMENT . undertakes no obligation to update publicly or revise any forward - looking statements for any reason after the date of this Presentation or to conform these statements to actual results or to changes in Scilex’s expectations. Industry and Market Data Certain data in this Presentation was obtained from various external sources, and neither Scilex nor its affiliates, advisers or representatives has verified such data with independent sources. Accordingly, neither Scilex nor any of its affiliates, advisers or representatives makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the date of this Presentation. Such data involves risks and uncertainties and is subject to change based on various factors. Trademarks The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of Scilex . Important Information and Where to Find It This Presentation references the Proposed Business Combination between Scilex and the SPAC. This Presentation does not constitute an offer to sell or exchange, or the solicitation of an offer to buy or exchange, any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, sale or exchange would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. In connection with the transaction described herein, contingent upon execution of the proposed merger agreement for the business combination (the “Merger Agreement”), the SPAC would file relevant materials with the SEC, including a registration statement on Form S - 4, which will include a proxy statement/prospectus. Scilex Holding Company

THIS DOCUMENT CONTAINS PROPRIETARY INFORMATION THAT IS THE PROPERTY OF THE COMPANY . NEITHER THIS DOCUMENT, NOR THE PROPRIETARY INFORMATION CONTAINED HEREIN, SHALL BE PUBLISHED, REPRODUCED, COPIED, DISCLOSED OR USED FOR ANY OTHER PURPOSE, OTHER THAN THE REVIEW AND CONSIDERATION OF THIS DOCUMENT . Investors and security holders of the SPAC are urged to read these materials (including any amendments or supplements thereto) and any other relevant documents in connection with the transaction that the SPAC files with the SEC when, and if, they become available because they will contain important information about the SPAC, Scilex and the proposed transaction. The preliminary proxy statement/prospectus, the definitive proxy statement/prospectus and other relevant materials in connection with the transaction (when and if they become available), and any other documents filed by the SPAC with the SEC, may be obtained free of charge at the SEC’s website ( www.sec.gov ). The documents filed by the SPAC with the SEC also may be obtained free of charge upon written request to: Vickers Vantage Corp. I, 85 Broad Street, 16th Floor, New York, NY 10004. Participants in the Solicitation If the parties execute the proposed Merger Agreement, the SPAC and its directors and executive officers may be deemed participants in the solicitation of proxies from SPAC’s shareholders with respect to the Proposed Business Combination. Information about the SPAC’s directors and executive officers and a description of their interests in the SPAC will be included in the proxy statement/prospectus for the Proposed Business Combination and would be available at the SEC’s website (www.sec.gov). Additional information regarding the interests of such participants will be contained in the proxy statement/prospectus for the Proposed Business Combination when available. Scilex Holding Company

THIS DOCUMENT CONTAINS PROPRIETARY INFORMATION THAT IS THE PROPERTY OF THE COMPANY . NEITHER THIS DOCUMENT, NOR THE PROPRIETARY INFORMATION CONTAINED HEREIN, SHALL BE PUBLISHED, REPRODUCED, COPIED, DISCLOSED OR USED FOR ANY OTHER PURPOSE, OTHER THAN THE REVIEW AND CONSIDERATION OF THIS DOCUMENT . Scilex and its directors and executive officers may also be deemed to be participants in the solicitation of proxies from the shareholders of the SPAC in connection with the Proposed Business Combination. Information about Scilex’s directors and executive officers and information regarding their interests in the Proposed Business Combination will be included in the proxy statement/prospectus for the Proposed Business Combination when available. Non - Solicitation This Presentation is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securities or in respect of the potential transaction and shall not constitute an offer to sell or a solicitation of an offer to buy the securities of the SPAC, Scilex or the combined company, nor shall there be any sale of any such securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of the Securities Act of 1933, as amended. Scilex Holding Company

Agenda 1) Scilex Holding Corporate Overview 2) ZTlido® 1.8% Update 3) SP - 102 (S EMDEXA ) for Lumbar Radicular Pain / Sciatica 4) SP - 103 (3X ZTlido) for Low Back Pain 5) DBR Low Dose Naltrexone (LDN) for Fibromyalgia 6) Company Summary Scilex Holding Company

Scilex Holding Company C onf i dent i a l : F or Inte r nal S C IL E X P ha r m a c eut i ca l s U se On l y 1 0 Corporate Overview Scilex Holding Company

Scilex Holding Company Vickers Strengths Strong deal structuring, investing and M&A experience within the fin - tech space Significant private and public investment experience History of working within highly regulated industries requiring vast and rigorous due diligence efforts Necessary experience in working with international governments and government regulators Scilex LOI Scilex Holding Company ( Scilex ), a subsidiary of Sorrento Therapeutics, Inc. (Nasdaq: SRNE), and Vickers Vantage Corp I (Nasdaq: VCKA) signed a letter of intent for a proposed business combination, which provides for a pre - transaction equity value of Scilex of approximately $1.5 billion, subject to adjustment, with expected gross proceeds of up to $140 million Scilex Business Combination with Vickers Vantage Corp I Vickers Vantage Corp. I IPO Overview: Gross Proceeds: $138.0 Million IPO Date: January 6, 2021 Sponsor: Vickers Venture Partners About Vickers Venture Partners Founded in 2005 Deep technology focused global VC $432 Million invested to - date across six funds (1) Gross Value across six funds circa $1 Billion (1) Global footprint with international transaction and diligence experience 21 investment professionals across sectors and geographies, almost all with advanced degrees in their respective specialties Note: (1) As of 30 June 2021. Scilex Holding Company
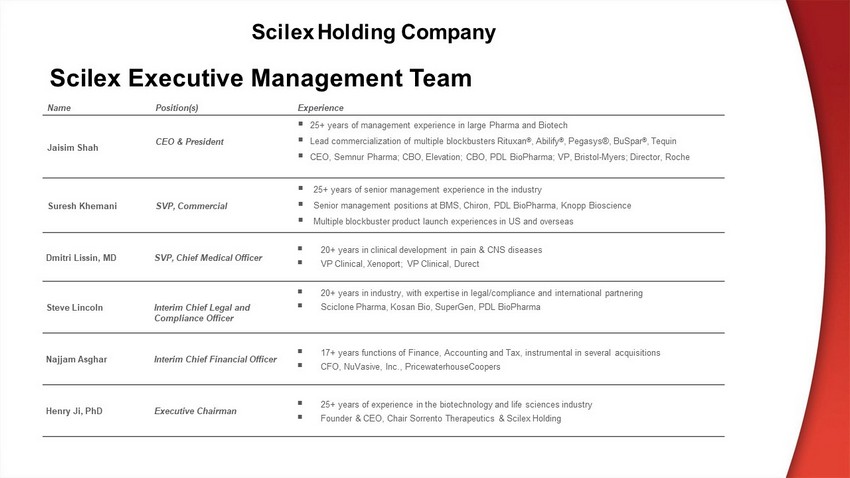
Name Position(s) Experience Jaisim Shah CEO & President ▪ 2 5 + years of management experience in large Pharma and Biotech ▪ Lead commercialization of multiple blockbusters Rituxan ® , Abilify ® , Pegasys®, BuSpar ® , Tequin ▪ CEO, Semnur Pharma; CBO , Elevation; CBO , PDL BioPharma; VP , Bristol - Myers ; Dir ector, Roche Suresh Khemani SVP, Commercial ▪ 25+ years of senior management experience in the industry ▪ Senior management positions at BMS, Chiron, PDL BioPharma , Knopp Bioscience ▪ Multiple blockbuster product launch experiences in US and overseas Dmitri Lissin, MD SVP, Chief Medical Officer ▪ 20+ years in clinical development in pain & CNS diseases ▪ VP Clinical, Xenoport; VP Clinical, Durect S teve Lincoln Interim Chief Legal and Compliance Officer ▪ 2 0 + years in industry , with expertise in legal/compliance and international partnering ▪ Sciclone Pharma, Kosan Bio, SuperGen , PDL BioPharma Najjam Asghar Interim Chief Financial Officer ▪ 17+ years functions of Finance, Accounting and Tax, instrumental in several acquisitions ▪ CFO, NuVasive, Inc., PricewaterhouseCoopers Henry Ji , PhD Executive Chairman ▪ 25+ years of experience in the biotechnology and life sciences industry ▪ Founder & CEO, Chair Sorrento Therapeutics & Scilex Holding Scilex Executive Management Team Scilex Holding Company
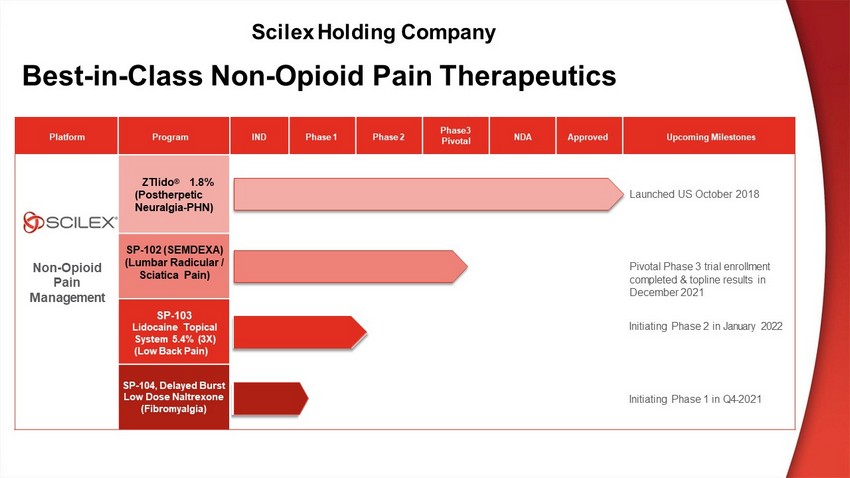
Platform Program IND P h as e 1 P h as e 2 P h ase3 P i v o t al NDA Approved U p c o m in g M il es t o n es N on - O p i o i d P a i n M a n a g e m e n t ZTlido ® 1 . 8 % (Postherpetic Neuralgia - PHN) Laun c h e d U S October 2018 SP - 102 (S EMDEXA ) (Lumbar Radicular / Sciatica Pain) P i vot a l P ha s e 3 t r i a l en r o l l m ent co m p l et e d & top li n e r e s u l t s in December 2021 SP - 103 Lidocaine Topical S ys t e m 5 . 4 % ( 3 X) (Low Back Pain) In i t i a t i n g P ha s e 2 i n January 2022 SP - 104 , D e l a y e d B u r s t Low Dose Naltrexone (Fibromyalgia) In i t i a t i n g P ha s e 1 i n Q4 - 2021 Best - in - Class Non - Opioid Pain Therapeutics Scilex Holding Company

Scilex Holding Company 2021 Scilex Business Update In April 2021, Scilex received a supplemental new drug application ( sNDA ) approval from the FDA for ZTlido to expand the label for use in water stress conditions In 2020, ZTlido had US net sales ~$27MM, which represents a ~35% growth rate YoY 2019 ● Scilex is currently projecting strong sales growth in 2021 ZTlido in the US. ● Discussions ongoing for registering/partnering ex - US rights to ZTlido and other pipeline programs SP - 102 (SEMDEXA) pivotal Phase 3 trial completed enrollment in July 2021, with highly significant positive topline results released in December 2021. Fast Track status granted by FDA Executed a LOI/term sheet for business combination with a SPAC (Vickers Vantage Corp. I) for a pre - transaction equity value of approximately $1.5 billion, subject to adjustment, and expected total proceeds of $140 million SP - 103 (3x formulation of ZTlido ) Phase 2 trial acute back pain on track to commence in January 2022 SP - 104 (Delayed Burst Low Dose Naltrexone) Phase 1 clinical trials began in Q4 - 2021 Scilex Holding Company
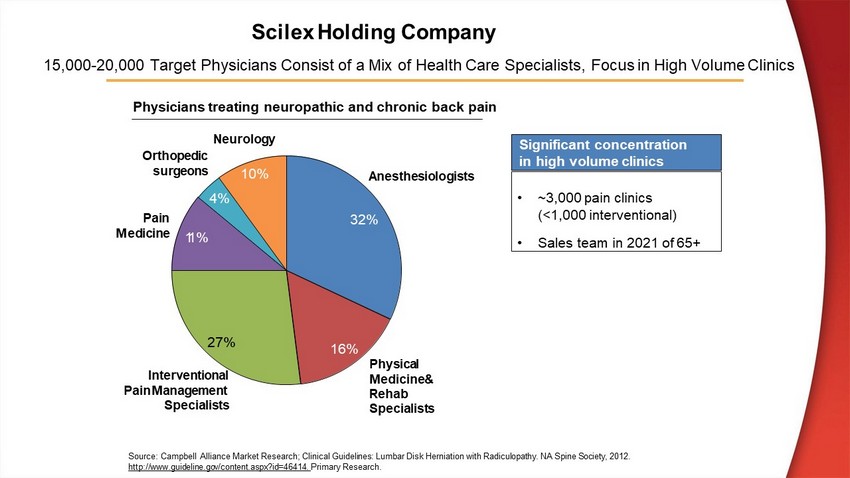
15,000 - 20,000 T arget P hysician s C onsist of a M ix of Health Care S pecialists, Focus in H igh V olume C linics Source: Campbell Alliance Market Research; Clinical Guidelines: Lumbar Disk Herniation with Radiculopathy. NA Spine Society, 2012. http://www.guideline.gov/content.aspx?id=46414. Primary Research. 32% 16% 27% 1 1% 4% 10% A n est h es i o l og i st s Physical Medicine& Rehab S p ec i a l i s t s I n t e r v e n t i on a l Pain Management Specialists Pa i n M e d i c i n e Neurology O r t hop e d i c s u r g e o n s • ~3,000 pain clinics (<1,000 interventional) • Sales team in 2021 of 65+ Physicians treating neuropathic and chronic back pain Significant concentration in high volume clinics Scilex Holding Company

Scilex Holding Company Headquarters - 960 San Antonio Road, Palo Alto CA 94303 - Satellite Office: 4955 Directors Place, San Diego, CA 92121 Scilex Holding Company

Scilex Holding Company C onf i dent i a l : F or Inte r nal S C IL E X P ha r m a c eut i ca l s U se On l y 1 0 Scilex Pharmaceuticals ZTlido 1.8% for Post - Herpetic Neuralgia (PHN) Scilex Holding Company
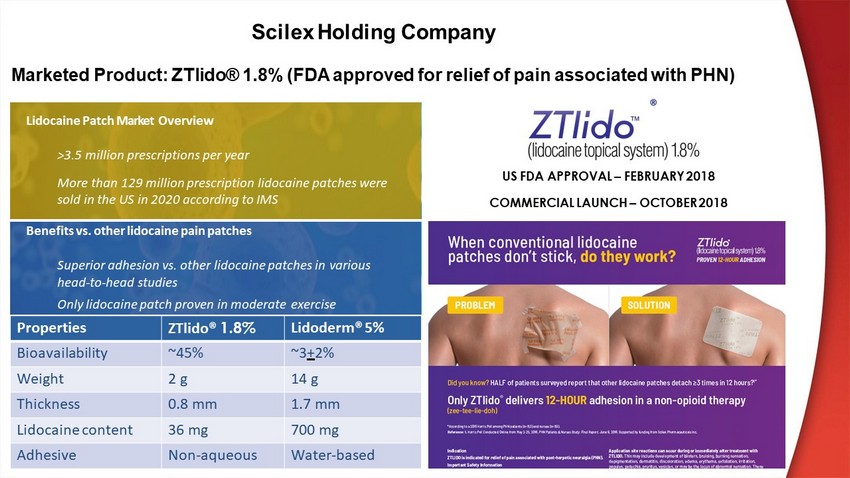
US FDA APPROVAL – FEBRUARY 2018 COMMERCIAL LAUNCH – OCTOBER 2018 Marketed Product: ZTlido® 1.8% (FDA approved for relief of pain associated with PHN ) Lidocaine Patch Market Overview >3 .5 million prescriptions per year More than 129 million prescription lidocaine patches were sold in the US in 2020 according to IMS Benefits vs. other lidocaine pain patches Superior adhesion vs. other lidocaine patches in various head - to - head studies Only lidocaine patch proven in moderate exercise Properties ZTlido® 1.8% Lidoderm® 5% Bioavailability ~45% ~3 + 2% Weight 2 g 14 g Thickness 0.8 mm 1.7 mm Lidocaine content 36 mg 700 mg Adhesive Non - aqueous Water - based ® Scilex Holding Company
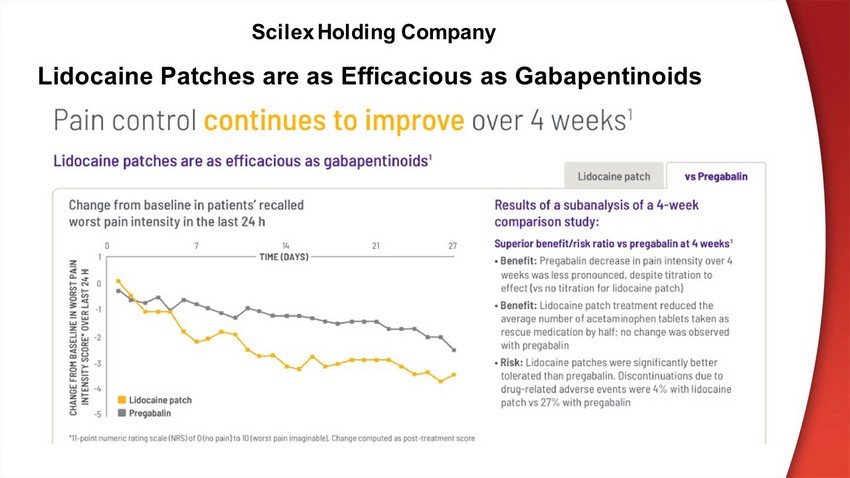
Scilex Holding Company Lidocaine Patches are as Efficacious as Gabapentinoids
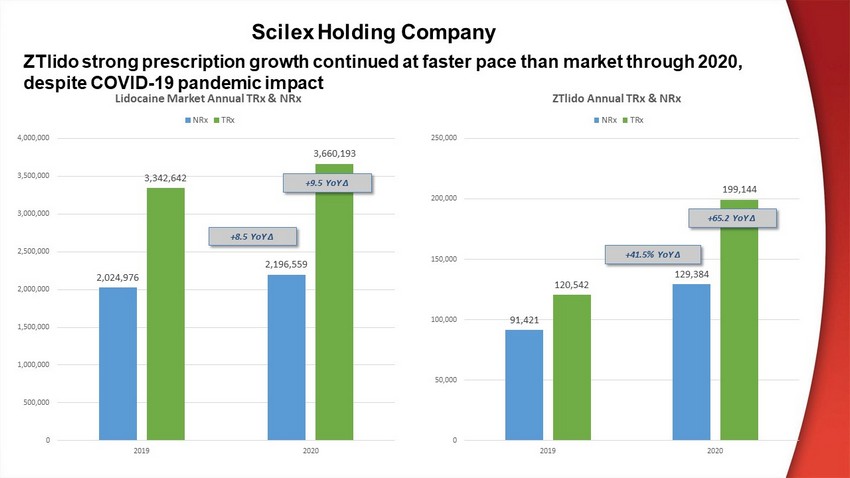
Scilex Holding Company 91,421 129,384 120,542 199,144 0 50,000 100,000 150,000 200,000 250,000 2019 2020 ZTlido Annual TRx & NRx NRx TRx ZTlido strong prescription growth continued at faster pace than market through 2020, despite COVID - 19 pandemic impact +65.2 YoY ∆ +41.5% YoY ∆ 2,024,976 2,196,559 3,342,642 3,660,193 0 500,000 1,000,000 1,500,000 2,000,000 2,500,000 3,000,000 3,500,000 4,000,000 2019 2020 Lidocaine Market Annual TRx & NRx NRx TRx +9.5 YoY ∆ +8.5 YoY ∆
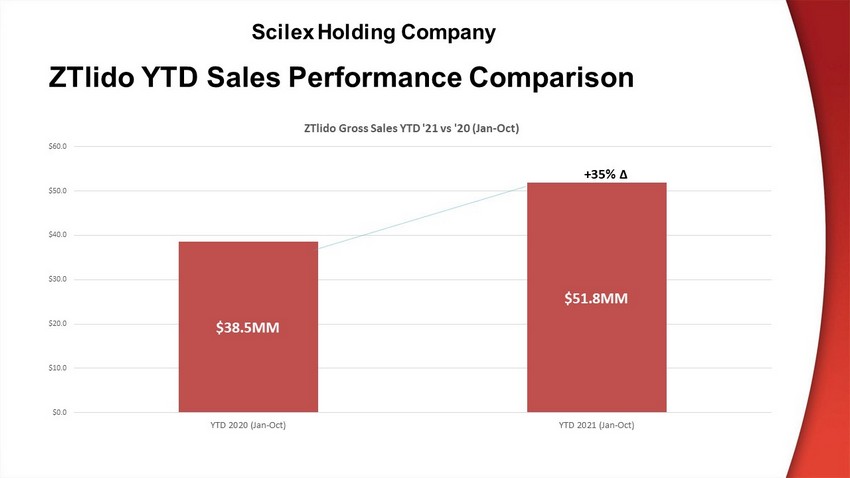
$38.5 MM $51.8 MM $0.0 $10.0 $20.0 $30.0 $40.0 $50.0 $60.0 YTD 2020 (Jan-Oct) YTD 2021 (Jan-Oct) ZTlido Gross Sales YTD '21 vs '20 (Jan - Oct) ZTlido YTD Sales Performance Comparison +35 % ∆ Scilex Holding Company
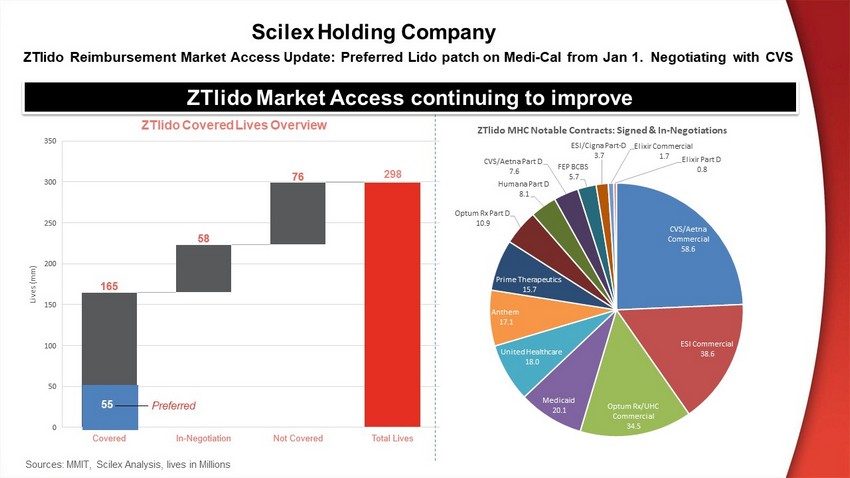
Scilex Holding Company ZTlido Reimbursement Market Access Update: Preferred Lido patch on Medi - Cal from Jan 1. Negotiating with CVS ZTlido Market Access continuing to improve Sources: MMIT, Scilex Analysis, lives in Millions 298 Preferred 55 CVS/Aetna Commercial 58.6 ESI Commercial 38.6 Optum Rx/UHC Commercial 34.5 Medicaid 20.1 United Healthcare 18.0 Anthem 17.1 Prime Therapeutics 15.7 Optum Rx Part D 10.9 Humana Part D 8.1 CVS/Aetna Part D 7.6 FEP BCBS 5.7 ESI/Cigna Part - D 3.7 Elixir Commercial 1.7 Elixir Part D 0.8 ZTlido MHC Notable Contracts: Signed & In - Negotiations

ZTlido Global Intellectual Property Family 1 – Expiring in 2031 ● U.S. 9,283,174 – Non - aqueous patch comprising lidocaine – Orange Book listed ● U.S. 9,925,264 – Methods of treating pain using patch – Orange Book listed ● U.S. 9,931,403 – Non - aqueous patch comprising lidocaine – Orange Book listed ● U.S. 10,765,749 – Non - aqueous patch comprising lidocaine – Orange Book listed ● 1 pending U.S. application ● Ex - U.S. applications in: AT, BE, BR, CA, CH, DE, EP, ES, FR, GB, IT, JP, MX, NL, TW , HK (granted patent in bold ) Family 2 – Expiring in 2031 ● U.S. 10,765,640 – Non - aqueous patch comprising lidocaine (stretch support) – Orange Book listed ● 1 pending U.S. application ● Ex - U.S. applications in: AT, BE, BG, BR, CA, CH, CZ, DE, EE, EP, ES, FI, FR, GB, GR, HK, HR, IE, IT, JP, LT, LU, MC, MK, MX , NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TR, ZA (granted patent in bold ) Family 3 – Expiring in 2036 ● Ex - U.S. applications in: AU, BR, CA, CN, ID, IL, IN, JP, KR, MX, MY, NZ, PA, PE, PH, RU, SG, UA, ZA (granted patent in bold ) Scilex Holding Company

ZTlido sales continue s to show impressive growth 2 years after launch (growth of 35% in 2020, projected strong growth in 2021) Effective September 1, 2021, ZTlido ® (lidocaine topical system) 1.8% has been added to multiple formularies, including two national PBMs (United Health and Optum Rx), a national health plan and two regional health plans – thereby expanding coverage by up to additional 33 million lives. ZTlido now on >55% of MHC formularies (>1 65 MM US lives covered) Expecting additional lives covered in 20 22 on commercial, Medicare part D and Medicaid formularies to >200MM covered lives Scilex has WW rights to ZTlido (ex - Japan) and patch technology for other APIs , patent protected through 203 1 in the US . Regulatory discussions ongoing in multiple ex - US markets ZTlido Summar y

Scilex Holding Company C onf i dent i a l : F or Inte r nal S C IL E X P ha r m a c eut i ca l s U se On l y 1 0 SP - 102 (S EMDEXA) for Lumbar Radicular Sciatica Pain Scilex Holding Company
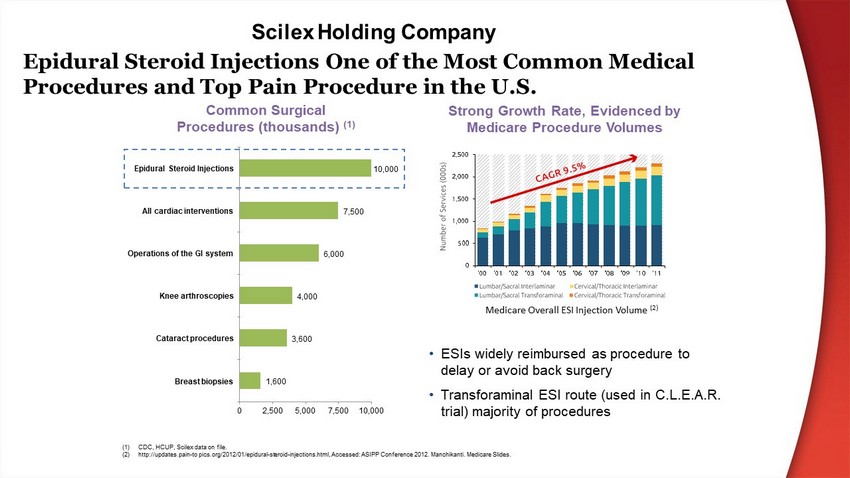
Epidural Steroid Injections One of the Most Common Medical Procedures and Top Pain Procedure in the U.S. • ESIs widely reimbursed as procedure to delay or avoid back surgery • Transforaminal ESI route (used in C.L.E.A.R. trial) majority of procedures Common Surgical Procedures (thousands) (1) Strong Growth Rate, Evidenced by Medicare Procedure Volumes 1 ,6 00 3 ,6 00 4 ,0 00 6 ,0 00 7 ,5 00 10 ,0 00 0 2 , 5 00 5 , 0 00 7 , 5 00 1 0 ,0 00 Breast biopsies Cataract procedures Knee arthroscopies Operations of the GI system All cardiac interventions Epidural Steroid Injections (1) CDC, HCUP, Scilex data on file . (2) http://updates.pain - to pics.org/2012/01/epidural - steroid - injections.html, Accessed: ASIPP Conference 2012. Manchikanti. Medicare Slides. Medicare Overall ESI Injection Volume (2) Scilex Holding Company

“ Consultants, ASA members, and ASRA members strongly agree that eipidural steroid injections with or without local anesthetics should be used for radicular pain or radiculopathy.” - American Society of Anesthesiology Practice Guidelines for Chronic Pain Management 3 Prescription opioid abuse is at epidemic proportions in the U.S. 1 Additionally, research shows that opioids do not provide clinically meaningful pain relief in patients with low back and radicular pain 2 1. Center for Disease Control and Prevention. Increases in Drug and Opioid Overdose Deaths 20 0 0 - 20014. MMWR 2015; 64; 1 - 5. 2. Efficacy, Tolerability and Dose Effects of Opioid Analgesics for Low Back Pain. JAMA Internal Medicine. 2016 Jul 1; 176 3. Practice Guidelines for Chronic Pain Management. Anesthesiology. 2010; 112: No 4 Apr 2010. Current P r oblem Multi - Modal Pain M anage m ent M ul t ipl e M edi c a l O r gani z a t ion s R e c o mm en d M ul t i - m oda l A nalge s i a for chronic pain management , including the American Society of Anesthesiologists, American Society of Regional Anesthesia & the American Academy of Orthopedic Surgeons. Potential for SP - 102 The SP - 102 clinical program is intended to demonstrate its utility as a key adjunct treatment for lumbar radiculopathy and potential as a new pain management standard Focus on Non - narcotic Pain Management Driving ESI Growth Scilex Holding Company

Important Environmental Factors Fungal Meningitis Outbreak with ESI (2013 - 14) Scilex Holding Company

CDC Data: Drug Overdose Deaths Top 100K for First Time 26 Scilex Holding Company

Well - tolerated. Key viscous excipient, long h i s t or y o f u s e i n c l ud i n g s a f e t y and safer repeat injections Fast acting onset of effect with less spread Potent non - particulate steroid (injectable dexamethasone sodium phosphate viscous gel) Pre - filled syringe for epidural use Gel formulation for extended local release and substantial magnitude of pain relief No preservatives, no surfactants, no particulates. Non - opioid and non - addictive Projected 24 month shelf life SP - 102: On - Track to be the First Novel Epidural Steroid Formulation with an FDA - approved Label to Treat Sciatica

C onf i dent i a l : F or Inte r nal S C IL E X P ha r m a c eut i ca l s U se On l y 2 7 SEMDEXA – Product Development & Regulatory Scilex Holding Company
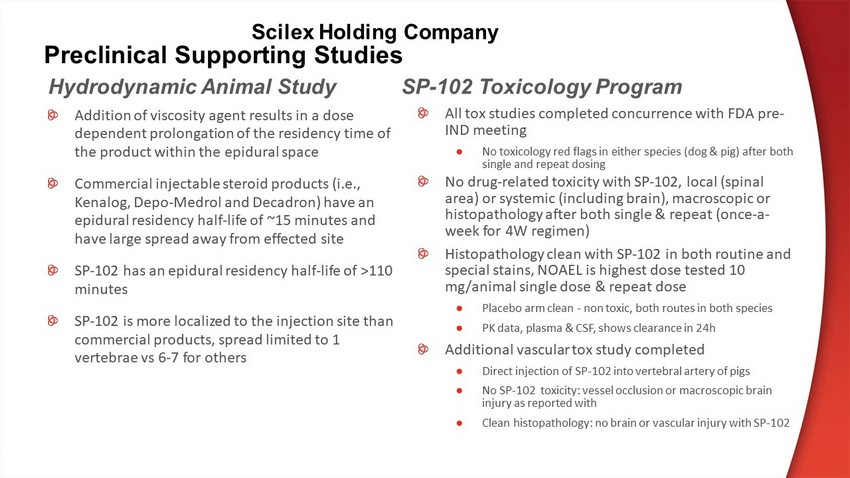
Preclinical Supporting Studies Hydrodynamic Animal Study All tox studies completed concurrence with FDA pre - IND meeting ● No toxicology red flags in either species (dog & pig) after both single and repeat dosing No drug - related toxicity with SP - 102, local (spinal area) or systemic (including brain), macroscopic or histopathology after both single & repeat (once - a - week for 4W regimen) Histopathology clean with SP - 102 in both routine and special stains, NOAEL is highest dose tested 10 mg/animal single dose & repeat dose ● Placebo arm clean - non toxic, both routes in both species ● PK data, plasma & CSF, shows clearance in 24h Additional vascular tox study completed ● Direct injection of SP - 102 into vertebral artery of pigs ● No SP - 102 toxicity: vessel occlusion or macroscopic brain injury as reported with ● Clean histopathology: no brain or vascular injury with SP - 102 SP - 102 Toxicology Program Addition of viscosity agent results in a dose dependent prolongation of the residency time of the product within the epidural space Commercial injectable steroid products (i.e., Kenalog , Depo - Medrol and Decadron ) have an epidural residency half - life of ~15 minutes and have large spread away from effected site SP - 102 has an epidural residency half - life of >110 minutes SP - 102 is more localized to the injection site than commercial products, spread limited to 1 vertebrae vs 6 - 7 for others Scilex Holding Company
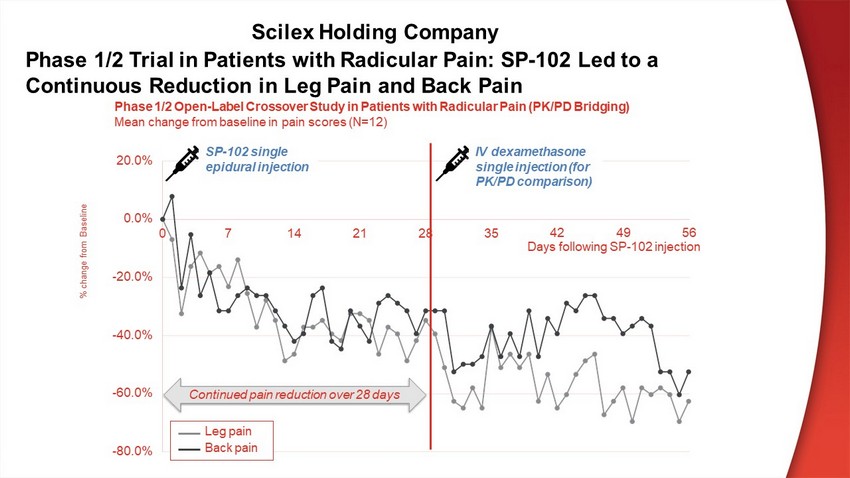
- 80 . 0% - 60 . 0% - 40 . 0% - 20 . 0% 0 . 0% 20 . 0 % 0 7 1 4 2 1 2 8 3 5 Phase 1/2 Open - Label Crossover Study in Patients with Radicular Pain (PK/PD Bridging) Mean change from baseline in pain scores (N=12) 42 49 56 Days following SP - 102 injection SP - 102 single e p i du r a l i n j ec t i o n IV dexamethasone single injection (for PK/PD comparison) Leg pain Back pain Continued pain reduction over 28 days Phase 1/2 Trial in Patients with Radicular Pain: SP - 102 Led to a Continuous Reduction in Leg Pain and Back Pain % change from Baseline Scilex Holding Company
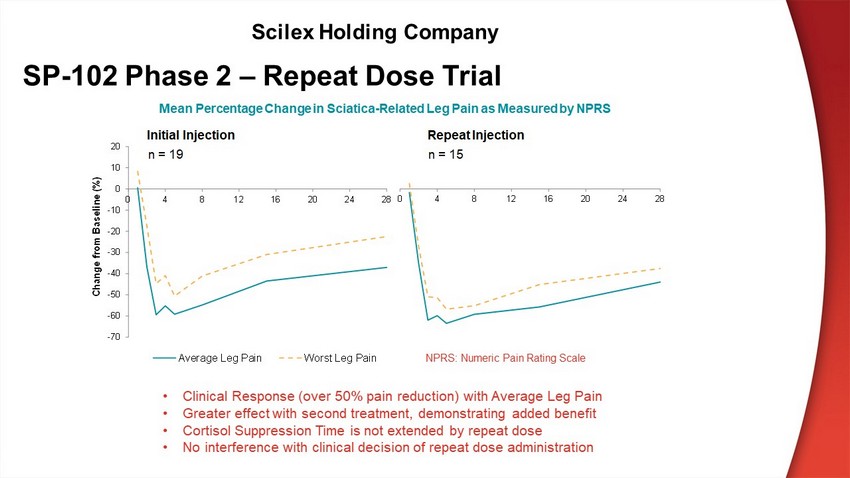
NPRS: Numeric Pain Rating Scale • Clinical Response (over 50% pain reduction) with Average Leg Pain • Greater effect with second treatment, demonstrating added benefit • Cortisol Suppression Time is not extended by repeat dose • No interference with clinical decision of repeat dose administration SP - 102 Phase 2 – Repeat Dose Trial Scilex Holding Company

Scilex Holding Company SP - 102 Pivotal Phase 3 C.L.E.A.R. Trial Topline Results

SP - 102 C.L.E.A.R. Phase 3 Trial Objectives Primary objective: ● Evaluate the analgesic effect on average leg pain (as measured by the Numeric Pain Rating Scale in the affected leg) following a single transforaminal (TF) injection of SP - 102 compared to an intramuscular injection of placebo over 4 weeks. Secondary objectives: ● Evaluate degree of disability over time as measured by the Oswestry Disability Index (ODI). ● Characterize the change of the subject’s radiculopathy symptoms and overall condition using PainDETECT, Brief Pain Inventory – Short Form (BPI - SF), Clinical Global Impression of Change (CGIC), and Patient Global Impression of Change (PGIC). ● Evaluate the safety of single and repeat SP - 102 TF injections. Scilex Holding Company
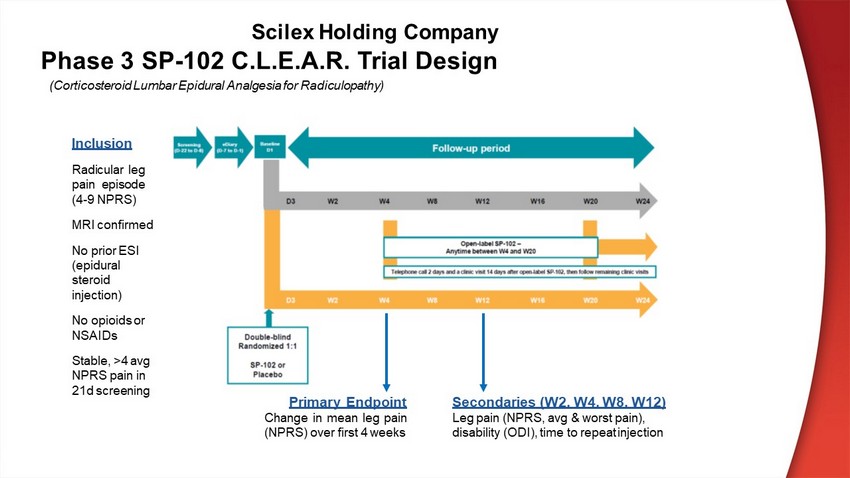
Phase 3 SP - 102 C.L.E.A.R. Trial Design Inclusion Radicular leg pain episode ( 4 - 9 NPRS) MRI confirmed No prior ESI (epidural steroid injection) No opioids or NSAIDs Stable, >4 avg NPRS pain in 21d screening Primary Endpoint Change in mean leg pain (NPRS) over first 4 weeks Secondaries (W2, W4, W8, W12) Leg pain (NPRS, avg & worst pain), disability (ODI), time to repeat inject ion (Corticosteroid Lumbar Epidural Analgesia for Radiculopathy) Scilex Holding Company
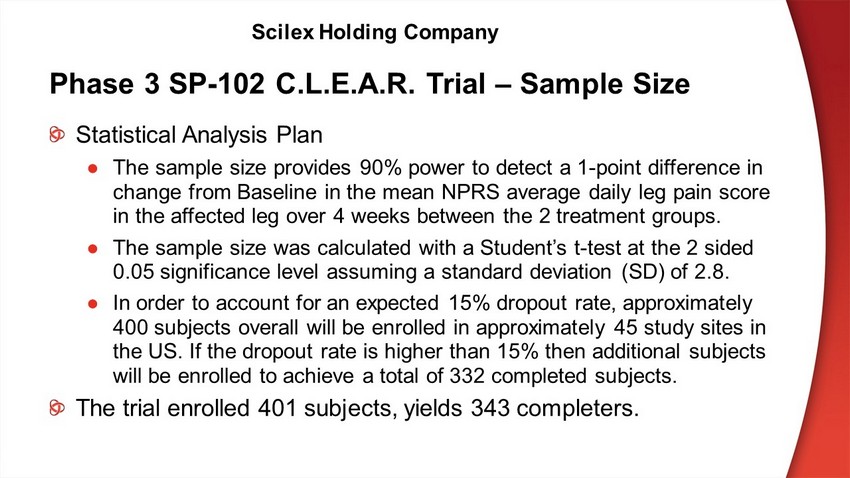
Phase 3 SP - 102 C.L.E.A.R. Trial – Sample Size Statistical Analysis Plan ● The sample size provides 90% power to detect a 1 - point difference in change from Baseline in the mean NPRS average daily leg pain score in the affected leg over 4 weeks between the 2 treatment groups. ● The sample size was calculated with a Student’s t - test at the 2 sided 0.05 significance level assuming a standard deviation (SD) of 2.8. ● In order to account for an expected 15% dropout rate, approximately 400 subjects overall will be enrolled in approximately 45 study sites in the US. If the dropout rate is higher than 15% then additional subjects will be enrolled to achieve a total of 332 completed subjects. The trial enrolled 401 subjects, yields 343 completers. Scilex Holding Company
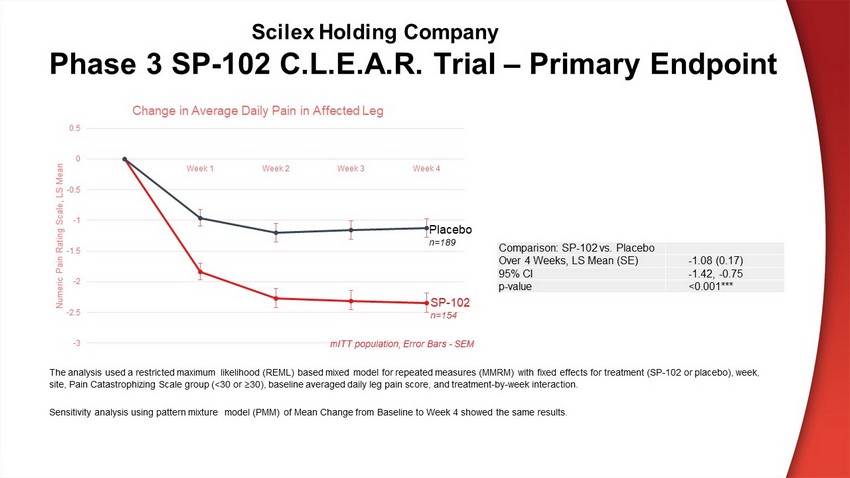
Phase 3 SP - 102 C.L.E.A.R. Trial – Primary Endpoint Comparison: SP - 102 vs. Placebo Over 4 Weeks, LS Mean (SE) - 1.08 (0.17) 95% CI - 1.42, - 0.75 p - value <0.001*** -3 -2.5 -2 -1.5 -1 -0.5 0 0.5 Week 1 Week 2 Week 3 Week 4 Numeric Pain Rating Scale, LS Mean Change in Average Daily Pain in Affected Leg Placebo n=189 SP - 102 n=154 The analysis used a restricted maximum likelihood (REML) based mixed model for repeated measures (MMRM) with fixed effects fo r t reatment (SP - 102 or placebo), week, site, Pain Catastrophizing Scale group (<30 or ≥30), baseline averaged daily leg pain score, and treatment - by - week interaction. Sensitivity analysis using pattern mixture model (PMM) of Mean Change from Baseline to Week 4 showed the same results. mITT population, Error Bars - SEM Scilex Holding Company

Oswestry Disability Index The gold standard for measuring degree of disability and estimating quality of life in a person with low back pain. Contains 10 topics concerning intensity of pain, lifting, ability to care for oneself, ability to walk, ability to sit, sexual function, ability to stand, social life, sleep quality, and ability to travel. Each question scored 0 - 5, where 5 indicating most severe disability. All scores are summed, multiplied by 2 to obtain the Index (range 0 to 100). Zero is equated with no disability and 100 is the maximum disability possible. The ODI obtained at Screening, Baseline, W4, W12, and W24. Scilex Holding Company
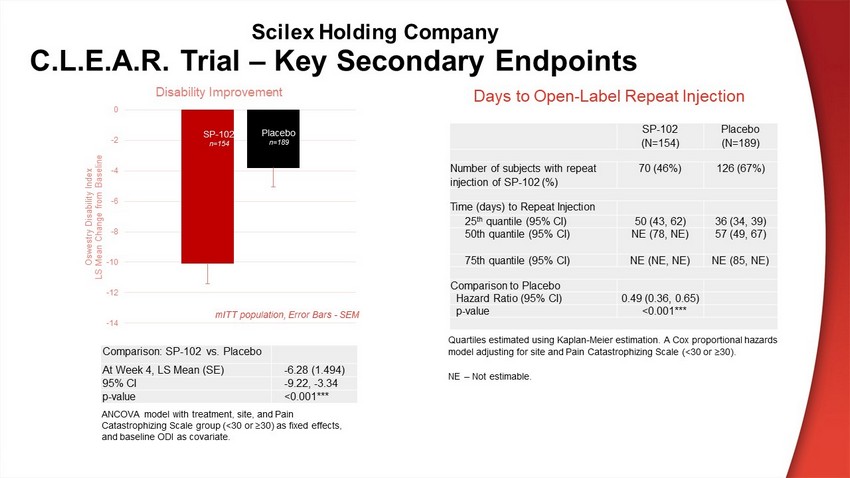
C.L.E.A.R. Trial – Key Secondary Endpoints Comparison: SP - 102 vs. Placebo At Week 4, LS Mean (SE) - 6.28 (1.494) 95% CI - 9.22, - 3.34 p - value <0.001*** SP - 102 (N=154) Placebo (N=189) Number of subjects with repeat injection of SP - 102 (%) 70 (46%) 126 (67%) Time (days) to Repeat Injection 25 th quantile (95% CI) 50 (43, 62) 36 (34, 39) 50th quantile (95% CI) NE (78, NE) 57 (49, 67) 75th quantile (95% CI) NE (NE, NE) NE (85, NE) Comparison to Placebo Hazard Ratio (95% CI) 0.49 (0.36, 0.65) p - value <0.001*** -14 -12 -10 -8 -6 -4 -2 0 Oswestry Disability Index LS Mean Change from Baseline Disability Improvement mITT population, Error Bars - SEM SP - 102 n=154 Placebo n=189 Days to Open - Label Repeat Injection ANCOVA model with treatment, site, and Pain Catastrophizing Scale group (<30 or ≥30) as fixed effects, and baseline ODI as covariate. Quartiles estimated using Kaplan - Meier estimation. A Cox proportional hazards model adjusting for site and Pain Catastrophizing Scale (<30 or ≥30). NE – Not estimable. Scilex Holding Company
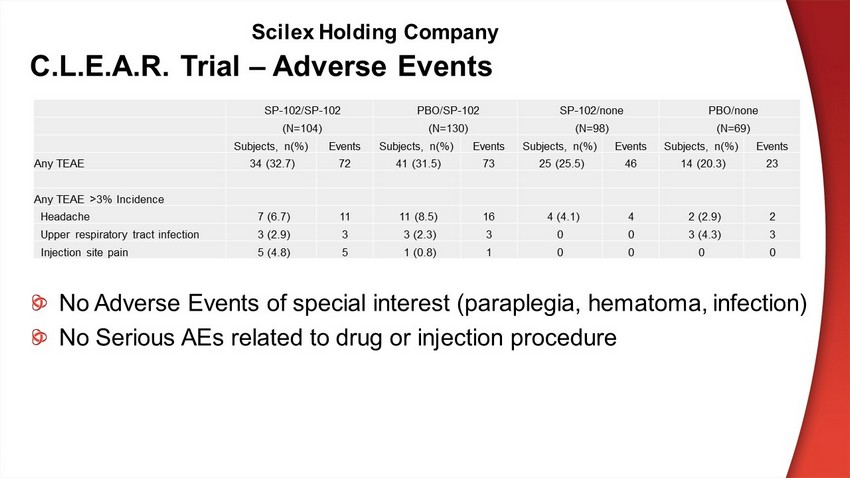
C.L.E.A.R. Trial – Adverse Events SP - 102/SP - 102 PBO/SP - 102 SP - 102/none PBO/none (N=104) (N=130) (N=98) (N=69) Subjects, n(%) Events Subjects, n(%) Events Subjects, n(%) Events Subjects, n(%) Events Any TEAE 34 (32.7) 72 41 (31.5) 73 25 (25.5) 46 14 (20.3) 23 Any TEAE >3% Incidence Headache 7 (6.7) 11 11 (8.5) 16 4 (4.1) 4 2 (2.9) 2 Upper respiratory tract infection 3 (2.9) 3 3 (2.3) 3 0 0 3 (4.3) 3 Injection site pain 5 (4.8) 5 1 (0.8) 1 0 0 0 0 No Adverse Events of special interest (paraplegia, hematoma, infection) No Serious AEs related to drug or injection procedure Scilex Holding Company

Phase 3 SP - 102 C.L.E.A.R. Trial - Conclusions The trial met primary and key secondary endpoints with statistical significance Demonstrated clear safety profile of SP - 102 Achieved all study objectives Next steps in 2022 ● Apply for Breakthrough Therapy Designation with FDA ● Request pre - NDA meeting with the FDA ● Advisory Board meeting following complete data analysis in March 2022 ● Prepare for publication of results in prestigious journals (NEJM, JAMA, etc ), presentations at ASRA, ASIPP, SIS, AAPM physician annual conferences Scilex Holding Company

Commercial supply chain in place ▪ API : Dexamethasone sodium phosphate supplier in place ▪ Novel excipient : Supply from leading commercial provider, 20 - year supply agreement for specific type for use ▪ Drug product : Commercial manufacturer in place, FDA experienced with biocompatible excipient, sterile injectables, and high viscosity pre - filled syringe filling CMC Activities to Submission ▪ Current 20L scale – planning scale - up to 120L in 2021 (acceptable scale for commercial) ▪ Process validation / registration / commercial batches at 120L – 2022 ▪ Current stability indicates pre - filled injectable syringe with 2 year shelf life ▪ Gross margin projected to be well in the range Scilex has developed significant know - how and expertise in formulation and manufacturing of sterile viscous gel The SP - 102 Manufacturing On - Track for NDA Submission to FDA in 2023 Scilex Holding Company

SP - 102 ( SEMDEXA ) Global Intellectual Property Patent family expiring in 2036 ● U.S. 10,117,938 - methods of treating inflammation and/or pain ● U.S. 10,500,284 – composition of matter and syringe ● U.S. 11,020,485 – composition of matter ● 1 pending U.S. application Ex - U.S. applications in AT , AU , BE , BR, CA, CH , CN , DE , EP , ES , FR , GB , HK , IN, IL , IT , JP , KR, MX, NL , NZ, TW (granted patent in bold ) Scilex Holding Company

Summary SP - 102 (SEMDEXA) N o FDA approved products to treat Sciatica/chronic back pain. All currently used off - label products have FDA warnings not to use Scilex will meet with FDA to discuss NDA filing for SEMDEXA in 2022 Breakthrough Designation filing with Phase 3 results in 2022 Priority Review filing at time of NDA filing in 2022/23 Scilex has experience with launch of pain management products and obtaining approval on Managed Health Care/ Medicare/Medicaid formularies Scilex has WW rights to SEMDEXA, patent protected through 2036 in US and major markets Scilex Holding Company

C onf i dent i a l : F or Inte r nal S C IL E X P ha r m a c eut i ca l s U se On l y 3 4 SEMDEXA – Commercial Strategy & Outlook Scilex Holding Company
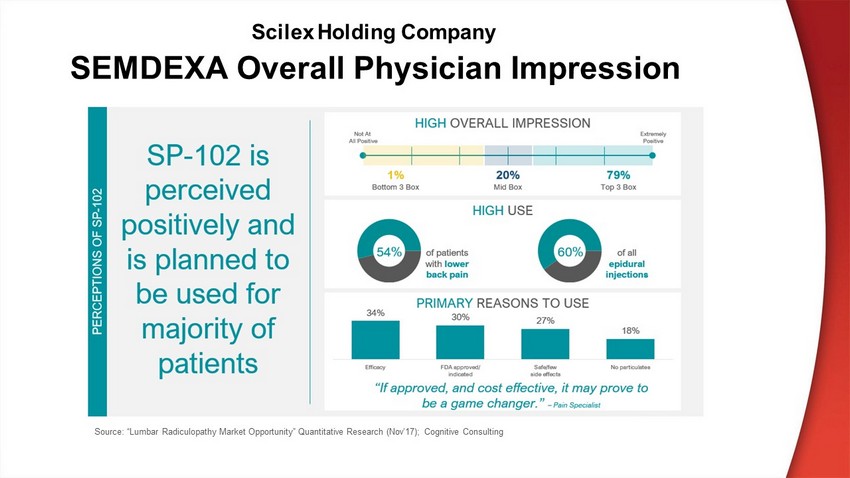
Source: “Lumbar Radiculopathy Market Opportunity” Quantitative Research (Nov’17); Cognitive Consulting SEMDEXA Overall Physician Impression Scilex Holding Company
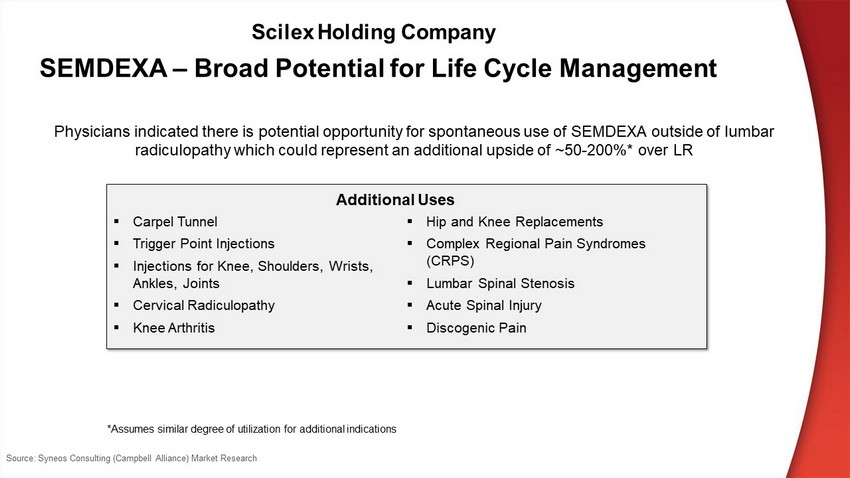
SEMDEXA – Broad Potential for Life Cycle Management ▪ Carpel Tunnel ▪ Trigger Point Injections ▪ Injections for Knee, Shoulders, Wrists, Ankles, Joints ▪ Cervical Radiculopathy ▪ K ne e A r t hr i t is ▪ Hip and Knee Replacements ▪ Complex Regional Pain Syndromes (CRPS) ▪ Lumbar Spinal Stenosis ▪ Acute Spinal Injury ▪ Discogenic Pain Physicians indicated there is potential opportunity for spontaneous use of SEMDEXA outside of lumbar radiculopathy which could represent an additional upside of ~50 - 200%* over LR Additional Uses *Assumes similar degree of utilization for additional indications Source: Syneos Consulting (Campbell Alliance) Market Research Scilex Holding Company

SP - 102 Reimbursement Scenario SP - 102 is expected to be used in the Office setting as well as in Ambulatory Surgical Centers and Hospital Outpatient clinics Scilex expects SP - 102 to secure favorable pricing and reimbursement due to its strong product profile, meeting important unmet medical needs in pain management and lack of other approved products ● As the only ESI that is preservative and particulate free and FDA approved option for lumbar radiculopathy, SP - 102 will have a strong case for reimbursement by payers Reimbursement consultants believe SP - 102 will receive a carve out reimbursement due to its clinical profile and as the only FDA approved ESI with a unique formulation that is effective and safe Scilex has engaged reimbursement experts and healthcare experts to ensure strong reimbursement at launch Scilex Holding Company
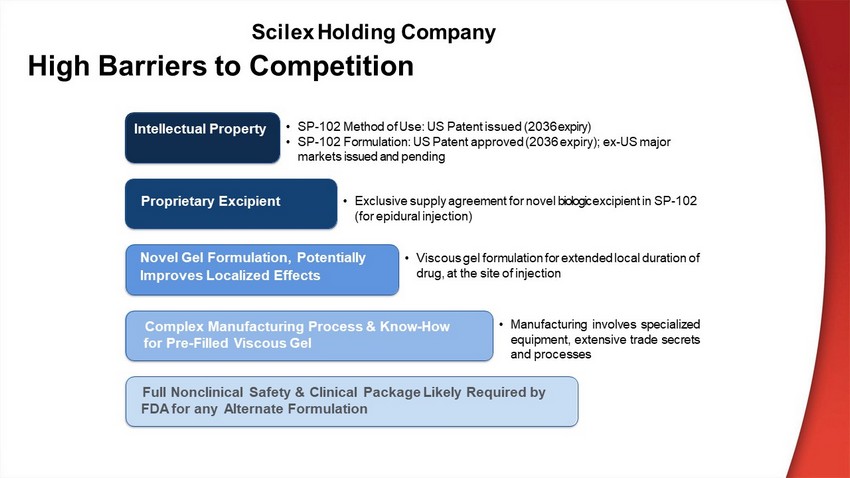
Intellectual Property P r op r i eta r y E xc i p i e n t Complex Manufacturing Process & Know - How for Pre - Filled Viscous Gel Full Nonclinical Safety & Clinical Package Likely Required by F D A f o r a n y A l te r n at e Fo r m u l a t i on • SP - 102 Method of Use: US Patent issued (2036 expiry) • SP - 102 Formulation: US Patent approved (2036 expiry); ex - US major m ar k e t s i ss ue d an d pend i ng • Exclusive supply agreement for novel biologic excipient in SP - 102 (for epidural injection) • Viscous gel formulation for extended local duration of drug, at the site of injection • Manufacturing involves specialized equ i p m en t , e x t en s ive t rad e s e c re t s and processes SP - 102 for Lumbar Radic Novel Gel Formulation, Potentially Improves Localized Effects High Barriers to Competition Scilex Holding Company

Market with high unmet need & no FDA approved products to treat sciatica pain Scilex will meet with FDA to discuss NDA for S EMDEXA in 2022 and pursue B reakthrough D esignation and P riority R eview applications SEMDEXA is a potential blockbuster product with market potential of $3 - 5 billion in peak annual sales in the US Target audience for S EMDEXA reached using sales team (65+) currently promoting ZTlido Scilex has global rights to SEMDEXA , patent protected through 2036 in US and major markets Summary – SP - 102 (SEMDEXA) Scilex Holding Company

C on f i d e n t i a l : F or Inte r nal S C ILEX Pha r m a c e ut i c a l s U se On l y 31 SP - 103 for Low Back Pain & Delayed Release LDN for Fibromyalgia Scilex Holding Company
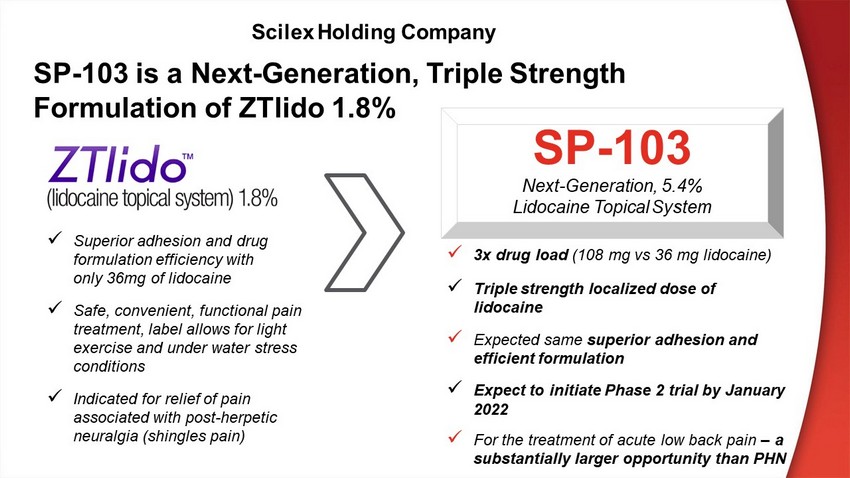
x 3x drug load (108 mg vs 36 mg lidocaine) x Triple strength localized dose of lidocaine x Expected same superior adhesion and efficient formulation x Expect to initiate Phase 2 trial by January 2022 x For the treatment of acute low back pain – a substantially larger opportunity than PHN SP - 103 Next - Generation, 5.4% Lidocaine Topical System x Superior adhesion and drug formulation efficiency with only 36mg of lidocaine x Safe, convenient, functional pain treatment , label allows for light exercise and under water stress conditions x Indicated for relief of pain associated with post - herpetic neuralgia (shingles pain) SP - 103 is a Next - Generation, Triple Strength Formulation of ZTlido 1.8% Scilex Holding Company

SP - 104 Delayed Burst Low Dose Naltrexone (LDN) – Fibromyalgia Fibromyalgia is a long - term condition that causes pain all over the body and affects ~2% of general population ( 5 - 8 million patients, 80 - 90% women) Low Dose Naltrexone (LDN) efficacy well documented • Routinely used off - label to treat multiple types of chronic pain – fibromyalgia, complex regional pain, and other indications. Demonstrated efficacy in multiple independent investigator - initiated trials Problems with current formulations of Naltrexone • There are no low - dose non - compounded forms of naltrexone commercially available (< 5 mg/day) • Poor compliance due to immediate release undesirable effects including hyperalgesia, dysphoria, nausea, anxiety, and insomnia with current formulations • Physician hesitancy for off - label prescriptions due to dysphoric effects of naltrexone as well as complications of dose titrating with limited compounding pharmacy supply • The few treatments approved for Fibromyalgia are marginally effective with unpleasant side - effects • Phase 1 SP - 104 program of delayed burst release LDN underway • Phase 2 trial in Fibromyalgia scheduled for 2022 Scilex Holding Company

C onf i dent i a l : F or Inte r nal S C IL E X P ha r m a c eut i ca l s U se On l y 4 6 S umm a r y Scilex Holding Company
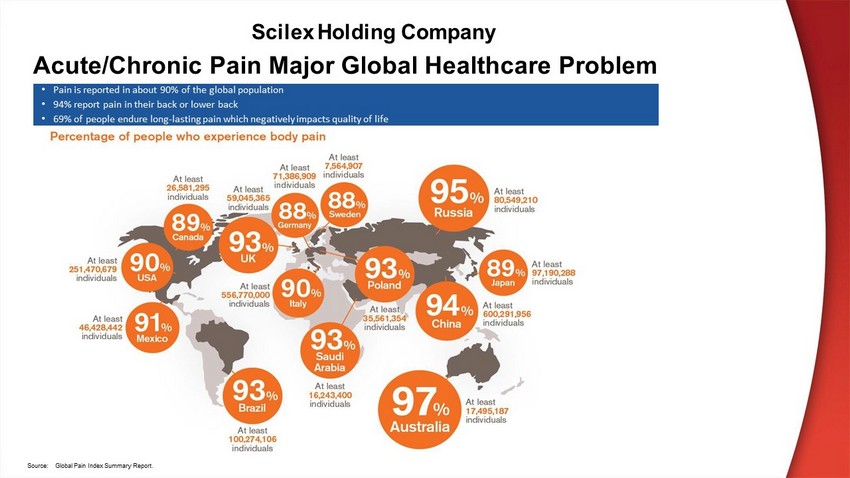
Acute/Chronic Pain Major Global Healthcare Problem • Pain is reported in about 90% of the global population • 94% report pain in their back or lower back • 69% of people endure long - lasting pain which negatively impacts quality of life S ou r c e : Gl oba l P a i n I nde x S u mm a r y R epo r t . Scilex Holding Company
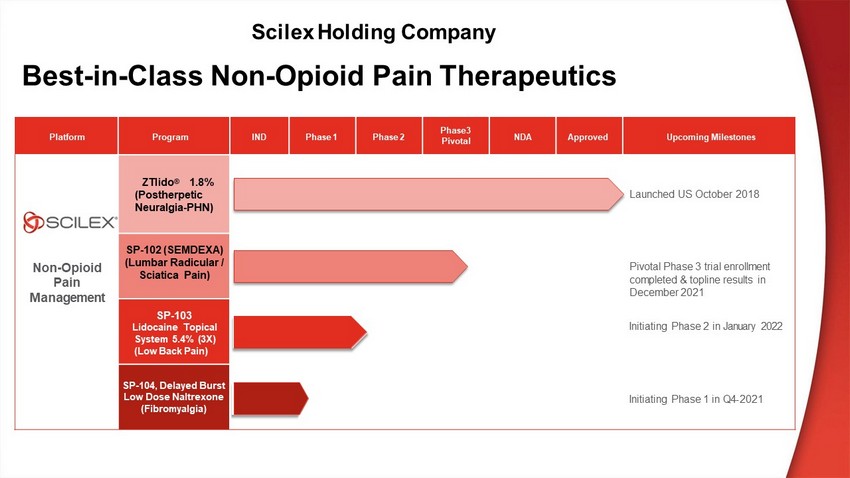
Platform Program IND P h as e 1 P h as e 2 P h ase3 P i v o t al NDA Approved U p c o m in g M il es t o n es N on - O p i o i d P a i n M a n a g e m e n t ZTlido ® 1 . 8 % (Postherpetic Neuralgia - PHN) Laun c h e d U S October 2018 SP - 102 (S EMDEXA ) (Lumbar Radicular / Sciatica Pain) P i vot a l P ha s e 3 t r i a l en r o l l m ent co m p l et e d & top li n e r e s u l t s in December 2021 SP - 103 Lidocaine Topical S ys t e m 5 . 4 % ( 3 X) (Low Back Pain) In i t i a t i n g P ha s e 2 i n January 2022 SP - 104 , D e l a y e d B u r s t Low Dose Naltrexone (Fibromyalgia) In i t i a t i n g P ha s e 1 i n Q4 - 2021 Best - in - Class Non - Opioid Pain Therapeutics Scilex Holding Company
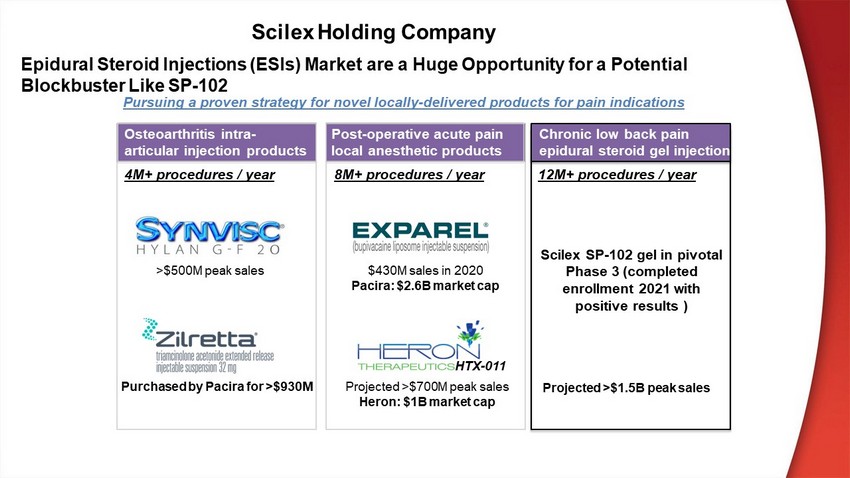
Epidural Steroid Injections (ESIs) Market are a Huge Opportunity for a Potential Blockbuster Like SP - 102 Osteoarthritis intra - articular injection products Post - operative acute pain local anesthetic products 4M+ procedures / year 8M+ procedures / year 12M+ procedures / year Chronic low back pain epidural steroid gel injection Scilex SP - 102 gel in pivotal Phase 3 (completed enrollment 2021 with positive results ) >$500M peak sales Purchased by Pacira for >$930M $430M sales in 2020 Pacira : $2.6B market cap Projected >$700M peak sales Heron: $1B market cap Pursuing a proven strategy for novel locally - delivered products for pain indications HTX - 011 Projected >$1.5B peak sales Scilex Holding Company

Scilex Holding Summary Commercial non - opioid pain management company with 3 clinical stage programs in large markets with very high unmet need Launched rapidly growing ZTlido (lidocaine topical system 1.8%) in 2018 with in - house commercial and sales team Semnur Pharma merged with Scilex in 2019 and lead program SP - 102 for sciatica back pain, has blockbuster potential, Fast Track Designation; Phase 3 trial completed enrollment and positive results Former Investors and partners include leading global venture capital firms and Itochu a global conglomerate. Currently Scilex is a subsidiary of Sorrento Therapeutics, Inc. SP - 102 SP - 103 SP - 104
























































