Exhibit 99.1

Exhibit 99.1
SCINTILLA
Corporate Presentation
September 2016

Disclaimer
Scintilla Pharmaceuticals, Inc. (“Scintilla”) is a subsidiary of Sorrento Therapeutics Inc. (the “Company”), a publicly traded company on the
Nasdaq Stock Market (“SRNE”). Certain statements contained in this presentation or in other documents of the Company, along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. These statements can be identified by the fact that they do not relate strictly to historic or current facts. They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial perfor- mance or condition. These statements are based upon the current beliefs and expectations of the Company’s management and are subject to signifi- cant risks and uncertainties. Statements regarding future action, future performance and/or future results including, without limitation, those relating to the timing for completion, and results of, scheduled or addition- al clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formula- tions and products and regulatory filings related to the same, partnering and collaboration opportunities, and Scintilla’s proposed acquisition of Semnur
Pharmaceuticals, Inc. may differ from those set forth in the forward-looking statements. Peak sales, market size and incidence estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate.
The Company assumes no obligation to update forward-looking state- ments as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10-K, 10-Q and 8-K reports.
NASDAQ:SRNE
Because actual results are affected by these and other potential risks, con- tingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward-looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identifies some of these factors in its Securities and Exchange Commission (“SEC”) filings on Forms 10-K, 10-Q and 8-K, and investors are advised to consult the Compa- ny’s filings for a more complete listing of risk factors, contingencies and uncertainties effecting the Company and its business and financial perfor- mance.
Scintilla™, Sorrento™, G-MAB™, CAR.TNK™, TNK Therapeutics™, and the Sorrento logo are trademarks owned by Sorrento Therapeutics, Inc.
All other trademarks and trade names are the property of their respective owners.
Logo—http://photos.prnewswire.com/prnh/20150105/167173LOGO
SCINTILLA
2

Company Overview
3

Vision and Mission
SCINTILLA
Our Vision
To significantly advance treatments and outcomes for pain patients with innovative, non-opioid pain management solutions
Our Mission
To develop and commercialize a new generation of pain medications targeting moderate to severe pain with improved efficacy and reduced risk of opioid side-effects and abuse potential, meeting significant unmet patient and healthcare system needs
4

Scintilla Overview
Scintilla Pharmaceuticals is a majority-owned subsidiary of Sorrento Therapeutics Announced proposed acquisition of Semnur Pharmaceuticals in August 2016
Portfolio of Two Investigational Product Candidates Spanning Interventional
Pain Spectrum
SP-102: first non-opioid, epidural steroid injectable potentially for the SP-102 treatment of Lumbosacral radicular pain
– Phase 1 / 2 trial in chronic back pain completed dosing
– IND filing target in Q1 2017
– Projected to begin Pivotal Phase 3 clinical trials in 2017
RTX: a novel non-opioid molecule targeted for the treatment of intractable cancer pain
– Phase 1 / 2 clinical trials confirmed activity consistent with animal models
– Cancer Pain IND target early 2017 RTX
– Scheduled to begin Phase 2 clinical trials in 2017
– Orphan Drug Designation
Semnur
Pharmaceuticals
SCINTILLA
Sorrento
Sorrento
5

Investment Highlights
Innovative, Non-opioid Pain Management Portfolio Large, Established, yet Underserved Target Markets
Worldwide Commercial Rights to All Product Candidates
Strong Proprietary Platform with High Barriers to Entry Established Reimbursement Access
Two Products with Blockbuster Potential Poised to Replace
Current Standard of Care
Sorrento
6

The Right Team to Deliver Long-Term Value
25+ years of research & development corporate expertise
Henry Ji, PhD Chairman Management experience as founder and executive officer of numerous biotechnology companies
CEO, Sorrento Therapeutics
Jaisim Shah Chief Executive Officer 25+ years of pharma and biotech corporate, commercial and product development expertise
CEO, Semnur Pharma; CBO, Elevation; CBO, PDL BioPharma; VP, Bristol-Myers
20+ years of clinical development at pharmas and biotechs focused in pain and
Dmitri Lissin, MD CNS therapeutic areas
Chief Medical Officer
VP Clinical, Xenoport; VP Clinical, Durect
Glen Sato, JD 20+ years of financial and legal expertise at public biotechs and legal firms
Legal / Interim Chief Financial Officer Support Partner, Cooley; CFO and GC, Exelixis & PDL BioPharma
Suketu Desai, PhD 20+ years of manufacturing / CMC development at pharma / biotech
Chief Technology Officer VP Manufacturing / CMC, Allergan; VP Cephalon / Teva 20 years of research and operations experience at pharma / biotech
Bryan Jones, PhD VP, Operations VP Operations, Sorrento; Senior Director, Amylin
7

The Pain Market is Expanding and Underserved
Chronic pain affects 116 million, or almost one- in-three, Americans (1)
Costs the United States approximately $560 to $635 billion annually (2)
Nearly 30 million patients suffer from lower back pain in the U.S. (3)
Greater than 80% of cancer patients have uncontrolled pain during course or disease (4)
Pain likely a major reason for hospitalizations
Growing government, physician and patient backlash against opioid-based products
High rate of addiction
Myriad of serious side effects (respiratory depression, GI)
Not suitable for long term use
Scintilla Focuses on a Broad Segment of the Pain Market
Pain
Acute Chronic
Chronic
Chronic Non-Malignant malignant Pain Pain
Scintilla Focus
(1) ASIPP, NIH, Snarr, Jared. Risks, Benefits, and Complications from Epidural Steroid Injections: A Case Report. AANA Journal Volume 75, No. 3. June, 2007. http://www.aana.com/newsandjournal/documents/snarr183-188.pdf; (2) Institute of Medicine.
(3) Decisions Resources Group. Chronic Pain: Disease Landscape and Forecast. 2016. (4) Datamonitor December 2009.

Strong Pipeline with Breakthrough Potential
Positioning
Market Opportunity
Development Milestones
SP-102
Potentially the first approved preservative and surfactant free, steroid indicated for epidural administration to treat lumbar radiculopathy
– Novel gel formulation designed to have a prolonged residency time at the site of injection
– Non-particulate, preservative and surfactant free
Progressive disease – often leading to expensive back surgery Current therapies provide limited pain relief and have potential for serious side effects
No currently approved injectable product for epidural administration Estimated ~10 million epidural steroid administrations per year in US alone (2) Potentially significant reduction in opioid use
Phase 1 / 2 trial in chronic back pain completed dosing IND filing target in Q1 2017 Projected to begin Pivotal Phase 3 clinical trials in 2017
RTX
Novel non-opioid potential for treatment of intractable cancer pain
Potentially single injection efficacy with longer term benefit Pain Reduction Potentially significant reduction in opioid use Increase Quality of Life, particularly mobility
More than 80% of cancer patients experience uncontrolled cancer- related pain during course of disease Chemotherapy induced neuropathic pain seen in up to 1/2 of patients with a cost of management of $2.3 billion(1) Potential to replace current costly invasive treatments (nerve stimulation, intrathecal opioids, radiotherapy) Can be used in addition to opiates
Phase 1 / 2 clinical trials confirmed activity consistent with animal models Cancer Pain IND target early 2017 Scheduled to begin Phase 2 clinical trials in 2017 Filing for Breakthrough designation, if granted, would allow for rapid approval Orphan drug status granted
Lema et al 2011
NEJM July 3, 2014 Editorial: Epidural Glucocorticoid Injections in Patients with Lumbar Spinal Stenosis; Gunnar B.J. Andersson, M.D., Ph.D.
9

Significant Near Term Clinical Milestone Targets for Scintilla Programs
Multiple clinical milestones for all programs expected in the next 12 months
– Mid to late stage trials commencing for SP-102 & RTX programs
In addition, potential corporate development milestones may also be achieved with potential collaborations or licensing in ex-US territory in 2017
Program 2016 2017 2018
Period 2H 1H 2H 1H 2H
Phase 1 / 2 Phase 3
SP-102 Phase 3 Start Phase 3
Complete Results
IND and
Phase 1 / 2
RTX Phase 1 / 2 Phase 2
Results
Start
10

SP-102: Innovative Non-opioid Injectable for Chronic Pain
11
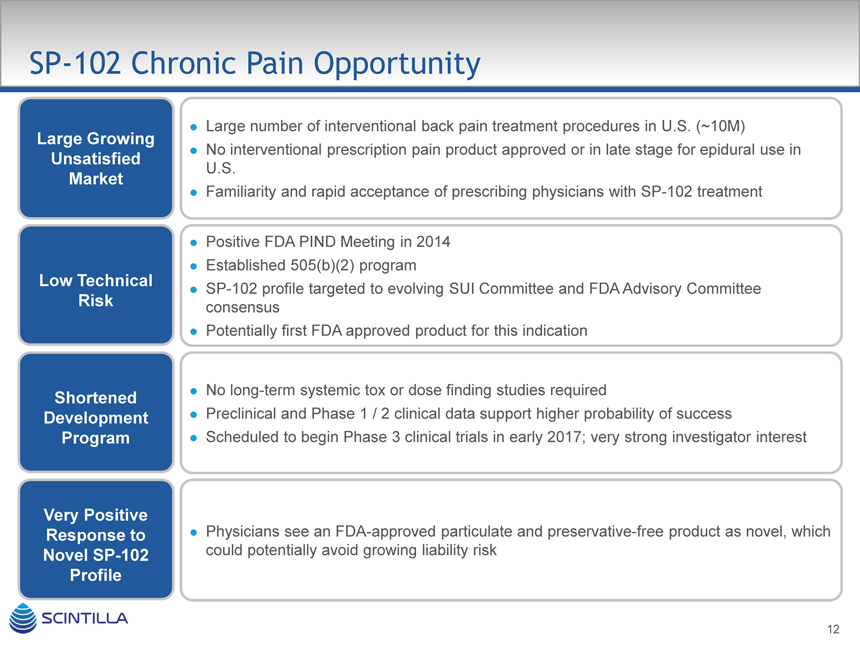
SP-102 Chronic Pain Opportunity
Large Growing
Unsatisfied
Market
Low Technical
Risk
Shortened
Development
Program
Very Positive
Response to
Novel SP-102
Profile
Large number of interventional back pain treatment procedures in U.S. (~10M)
No interventional prescription pain product approved or in late stage for epidural use in U.S.
Familiarity and rapid acceptance of prescribing physicians with SP-102 treatment
Positive FDA PIND Meeting in 2014 Established 505(b)(2) program
SP-102 profile targeted to evolving SUI Committee and FDA Advisory Committee consensus Potentially first FDA approved product for this indication
No long-term systemic tox or dose finding studies required
Preclinical and Phase 1 / 2 clinical data support higher probability of success
Scheduled to begin Phase 3 clinical trials in early 2017; very strong investigator interest
Physicians see an FDA-approved particulate and preservative-free product as novel, which could potentially avoid growing liability risk
12

FDA Pre-IND Meeting (2014)
FDA expressed interest in supporting an ESI product with good safety profile
FDA focus on local tox; did not express concerns on systemic tox with API and excipient FDA stressed marketed injectable steroids are not approved for epidural use but are used extensively and patients are treated with 3 – 6 injections/year; they are aware of the safety issues associated with steroid particulates and preservatives causing rare neurologic deficits Potential for reducing size of safety database for SP-102 is dependent on tox study and comparative PK study outcome and 1st Phase 3 clinical trial results
– Agree to 505(b)2 application
– Need bridging (comparative bioavailability) study to reference drug
– Pediatric Study Plan needed prior to NDA submission
– Safety Follow-Up – “6 month follow-up safety evaluation, evaluation of any safety issues identified in the nonclinical studies, and evaluation of safety issues that were the subject of FDA’s Drug Safety Communication regarding epidural steroid injections”
– Primary endpoint at 4 weeks, secondary at 12 weeks
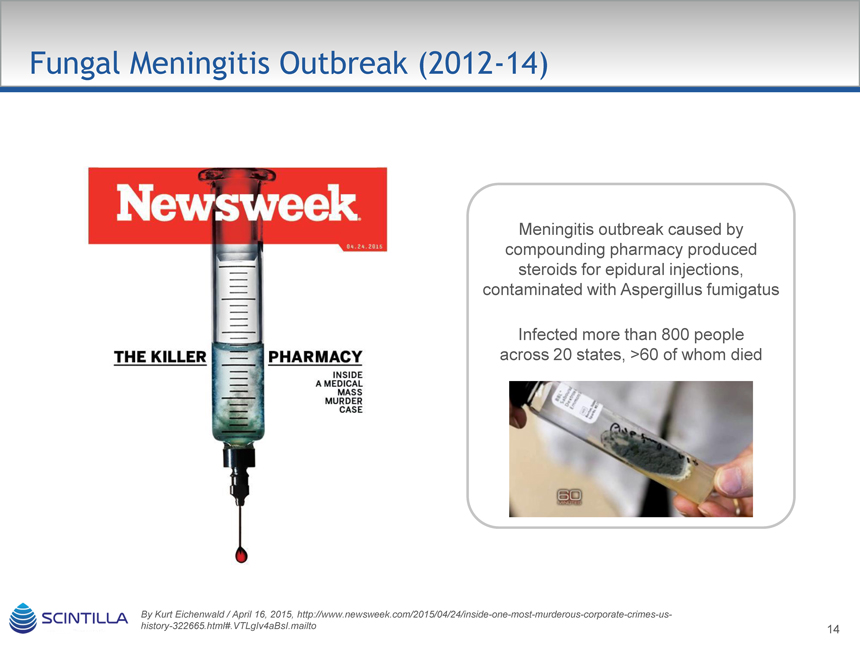
Fungal Meningitis Outbreak (2012-14)
Newsweek
04.24.2016.
THE KILLER PHARMACY
INSIDE
A MEDICAL
MASS
MURDER
CASE
Meningitis outbreak caused by compounding pharmacy produced steroids for spidural injections, contaminated with Aspergillus fumigatus
Infected more than 800 people across 20 states, >60 of whom died
By Kurt Eichenwald / April 16, 2015, http://www.newsweek.com/2015/04/24/inside-one-most-murderous-corporate-crimes-us-history-322665.html#.VTLgIv4aBsI.mailto
14
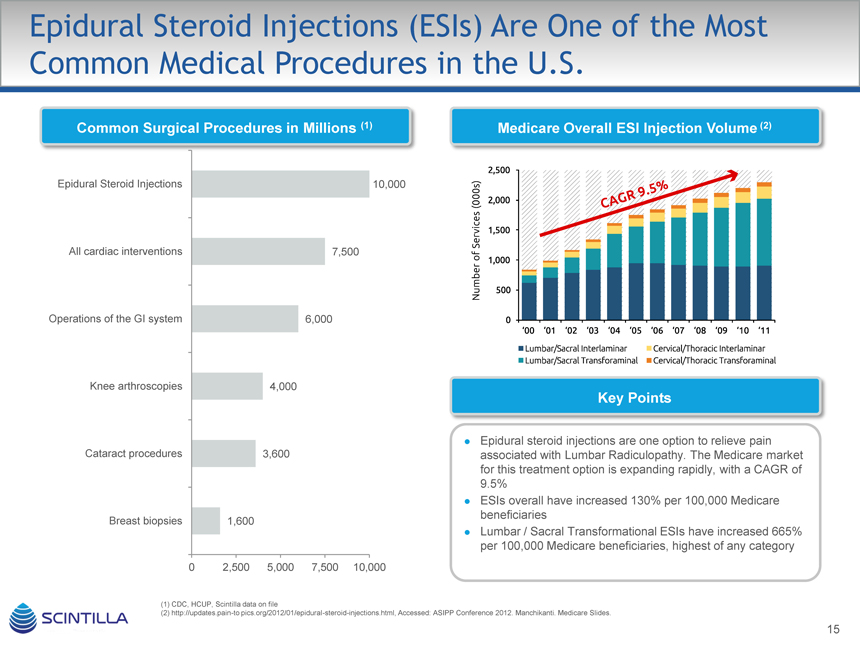
Epidural Steroid Injections (ESIs) Are One of the Most Common Medical Procedures in the U.S.
Common Surgical Procedures in Millions (1)
Epidural Steroid Injections 10,000 All cardiac interventions 7,500 Operations of the GI system 6,000 Knee arthroscopies 4,000 Cataract procedures 3,600 Breast biopsies 1,600
0 2,500 5,000 7,500 10,000
CDC, HCUP, Scintilla data on file
http://updates.pain-to pics.org/2012/01/epidural-steroid-injections.html, Accessed: ASIPP Conference 2012. Manchikanti. Medicare Slides.
Medicare Overall ESI Injection Volume (2)
Key Points
Epidural steroid injections are one option to relieve pain associated with Lumbar Radiculopathy. The Medicare market for this treatment option is expanding rapidly, with a CAGR of 9.5% ESIs overall have increased 130% per 100,000 Medicare beneficiaries Lumbar / Sacral Transformational ESIs have increased 665% per 100,000 Medicare beneficiaries, highest of any category
15
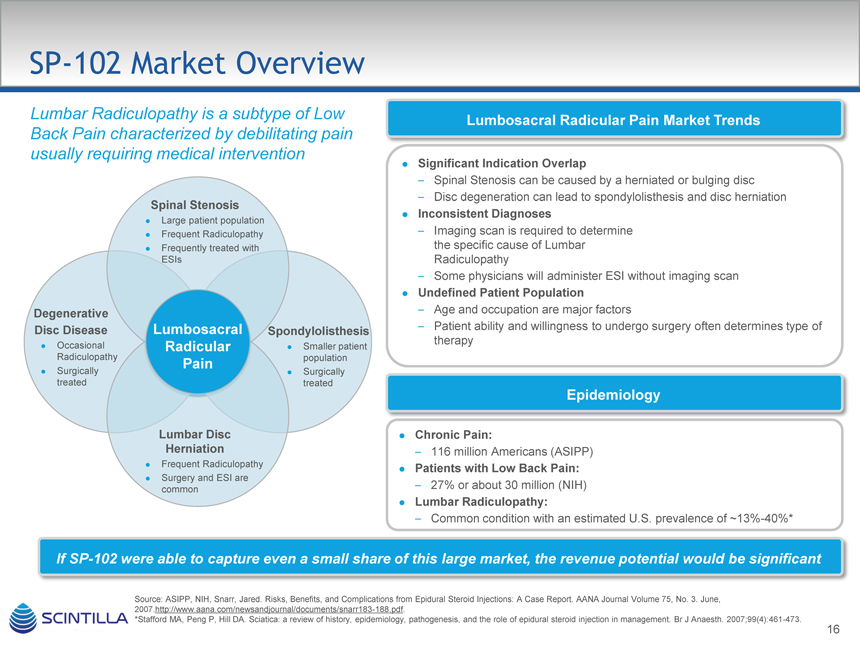
SP-102 Market Overview
Lumbar Radiculopathy is a subtype of Low Back Pain characterized by debilitating pain usually requiring medical intervention
Spinal Stenosis
Large patient population Frequent Radiculopathy Frequently treated with ESIs
Degenerative
Disc Disease Lumbosacral Spondylolisthesis
Occasional Radicular Smaller patient Radiculopathy population
Pain
Surgically Surgically treated treated
Lumbar Disc Herniation
Frequent Radiculopathy Surgery and ESI are common
Lumbosacral Radicular Pain Market Trends
Significant Indication Overlap
– Spinal Stenosis can be caused by a herniated or bulging disc
– Disc degeneration can lead to spondylolisthesis and disc herniation
Inconsistent Diagnoses
– Imaging scan is required to determine the specific cause of Lumbar Radiculopathy
– Some physicians will administer ESI without imaging scan
Undefined Patient Population
– Age and occupation are major factors
– Patient ability and willingness to undergo surgery often determines type of therapy
Epidemiology
Chronic Pain:
– 116 million Americans (ASIPP)
Patients with Low Back Pain:
– 27% or about 30 million (NIH)
Lumbar Radiculopathy:
– Common condition with an estimated U.S. prevalence of ~13%-40%*
If SP-102 were able to capture even a small share of this large market, the revenue potential would be significant
Source: ASIPP, NIH, Snarr, Jared. Risks, Benefits, and Complications from Epidural Steroid Injections: A Case Report. AANA Journal Volume 75, No. 3. June, 2007.http://www.aana.com/newsandjournal/documents/snarr183-188.pdf.
*Stafford MA, Peng P, Hill DA. Sciatica: a review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007;99(4):461-473.
16

A Focus on Non-narcotic Pain Management Will Drive the Growth of Epidural Steroid Injections
Prescription opioid abuse is at epidemic proportions in the U.S.1
Current Problem Additionally, research shows that opioids do not provide clinically meaningful pain relief in patients with low
back pain2
Multi-modal analgesia is the use of two or more analgesic agents or techniques to improve pain
Multi-Modal Pain management while minimizing risk of adverse events
Multiple medical organizations recommend multi-modal analgesia for chronic pain management including
Management the American Society of Anesthesiologists, American Society of Regional Anesthesia & the American
Academy of Orthopedic Surgeons
Potential for The SP-102 clinical program is intended to demonstrate its utility as a key adjunct treatment for lumbar
SP-102 radiculopathy and potential as a new pain management standard
“Consultants, ASA members and ASRA members strongly agree that epidural steroid injections with or without local anesthetics should be used for radicular pain or radiculopathy”
— American Society of Anesthesiology Practice Guidelines for Chronic Pain Management3
1. Center for Disease Control and Prevention. Increases in Drug and Opioid Overdose Deaths 200-20014. MMWR 2015; 64; 1-5.
2. Efficacy, Tolerability and Dose Effects of Opioid Analgesics for Low Back Pain. JAMA Internal Medicine. 2016 Jul 1; 176
3. Practice Guidelines for Chronic Pain Management. Anesthesiology. 2010; 112: No 4 Apr 2010. 17
17

Chronic Back Pain:
Steroid Injections Bolded Warnings
Less
Used Currently Used
Drug Formulation Potential Issues
The safety and effectiveness of epidural administration
Methylprednisolone acetateof corticosteroids have not been established and
Depomedrol • Contains polyethylene glycol + benzylcorticosteroids are not approved for this use
alcohol• Benzyl alcohol neurotoxicity
The safety and effectiveness of epidural administration
Kenalog • Triamcinolone acetonideof corticosteroids have not been established and
Contains benzyl alcoholcorticosteroids are not approved for this use
• Neurotoxicity of benzyl alcohol
Betamethasone acetate 50% &The safety and effectiveness of epidural administration
Soluspan Betamethasone sodium phosphate 50%of corticosteroids have not been established and
Contains benzalkonium chloride &corticosteroids are not approved for this use
EDTA• Neurotoxicity of benzalkonium chloride & EDTA
The safety and effectiveness of epidural administration
Dexamethasone sodium phosphateof corticosteroids have not been established and
Decadron • Benzyl alcoholcorticosteroids are not approved for this use
• Benzyl alcohol neurotoxicity
All marketed products have bolded label warnings: “serious neurologic events, some resulting in death, have been reported with epidural injection” and that the “safety and effectiveness of epidural administration of corticosteroids have not been established”
All current ESI products have preservatives and surfactants
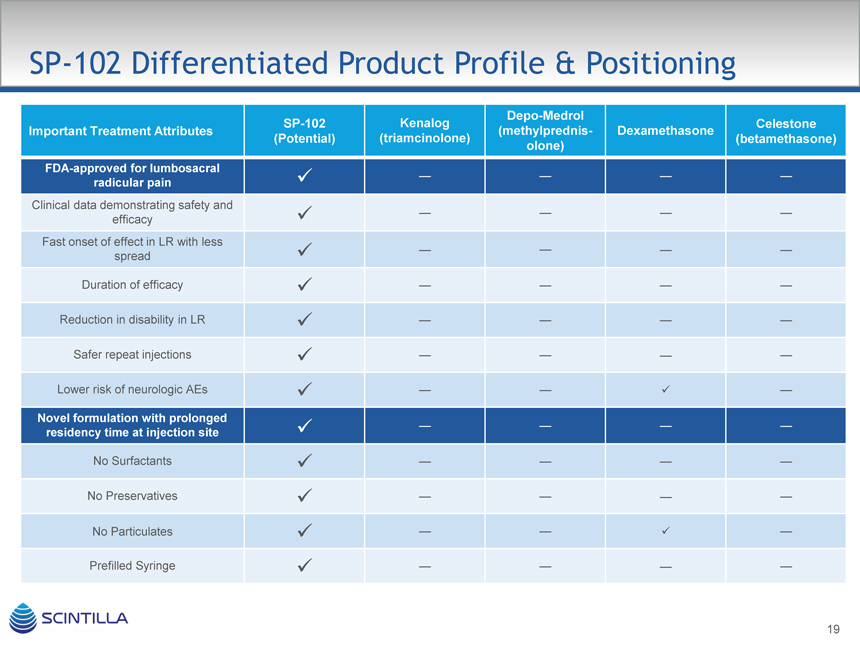
SP-102 Differentiated Product Profile & Positioning
Depo-Medrol
SP-102 KenalogCelestone
Important Treatment Attributes (methylprednis-Dexamethasone
(Potential) (triamcinolone)(betamethasone)
olone)
FDA-approved for lumbosacral
radicular pain ————
Clinical data demonstrating safety and
efficacy ————
Fast onset of effect in LR with less
spread ————
Duration of efficacy ————
Reduction in disability in LR ————
Safer repeat injections ————
Lower risk of neurologic AEs —— —
Novel formulation with prolonged
residency time at injection site ————
No Surfactants ————
No Preservatives ————
No Particulates —— —
Prefilled Syringe ————

SP-102 Clinical Utility of Product Concept is Consistent With Pain Specialists Needs
Physicians reacted favorably to the SP-102 product profile, giving the steroid an average score of 6.2 on a favorability scale of 1 – 7
Overall Reaction
Steroid A’s stated duration of effect, non-particulate form, and immediate onset of action were the most often cited benefits by physicians On a favorability scale of 1 – 7, seven physicians scored it with a 7, four with a 6, and four with 5 Cost was cited often as the only negative aspect of the profile An 8 week duration of effect would increase favorability in some physicians, but decrease favorability in others due to less revenue caused by lower volume of injections
“It’s novel and disruptive. The non-particulate and non-preservative aspects are exciting”
— Anesthesiologist
Steroid A’s Favorability N-15
7 6 543210
6.3 6.1 6.36.2
Anesthesiologists PMR Specialists Other Specialists All Specialists
Specialty Group
“Cost may be high, but if eliminated need for 2nd injection may be well worth it”
— Anesthesiologist
20
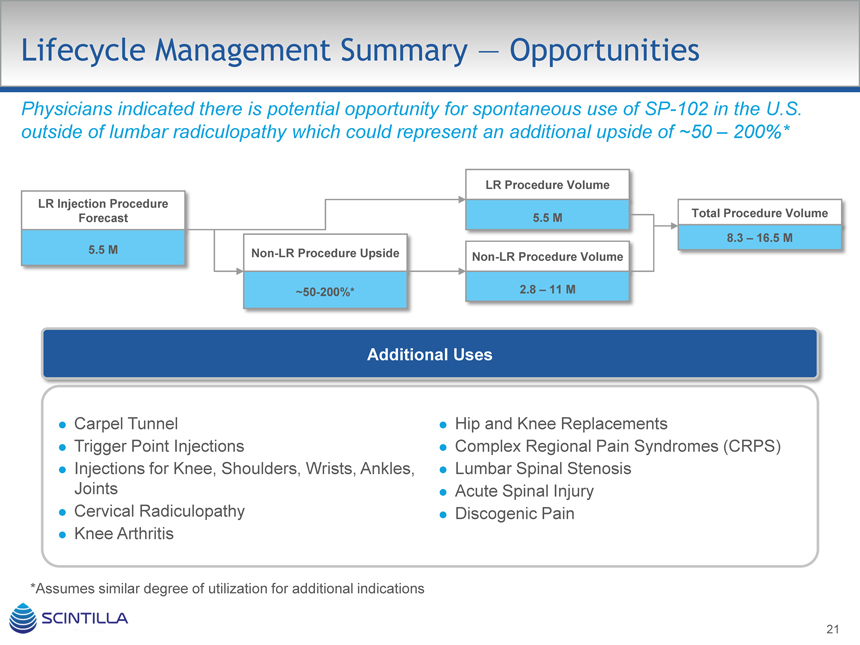
Lifecycle Management Summary — Opportunities
Physicians indicated there is potential opportunity for spontaneous use of SP-102 in the U.S. outside of lumbar radiculopathy which could represent an additional upside of ~50 – 200%*
LR Procedure Volume LR Injection Procedure
Forecast 5.5 M Total Procedure Volume
8.3 – 16.5 M
5.5 M Non-LR Procedure Upside
Non-LR Procedure Volume
~50-200%* 2.8 – 11 M
Additional Uses
Carpel Tunnel
Trigger Point Injections
Injections for Knee, Shoulders, Wrists, Ankles, Joints
Cervical Radiculopathy
Knee Arthritis
Hip and Knee Replacements
Complex Regional Pain Syndromes (CRPS)
Lumbar Spinal Stenosis
Acute Spinal Injury
Discogenic Pain
*Assumes similar degree of utilization for additional indications
21

SP-102 Toxicology Program Design Plan
Identical programs in >100 Beagle dogs and Mini-Pigs (each program costs approximately $2m)
Multiple arm GLP study using 2 routes:
SD Epidural Control (saline) 1 mL
SD Epidural Placebo 0.4 mL
SD Epidural Placebo 1 mL
SD Epidural Low dose:
mg in 0.4 mL
SD Epidural Med dose:
mg in 0.4 mL
SD Epidural High dose:
mg in 1 mL
SD Intrathecal control (saline)
SD Intrathecal low dose
SD Intrathecal Med Dose
SD Intrathecal high dose
Multi-dose epidural control (once a week for 4 weeks)
Multi-dose epidural med dose (once a week for 4 weeks)
22

SP-102 Preclinical Study Results
Hydrodynamic Study Results
Addition of viscosity agent results in a dose dependent prolongation of the residency time of the product within the epidural space Commercial injectable steroid products (i.e., Kenalog, Depo-Medrol and Decadron) have an epidural residency half-life of ~15 minutes and have large spread away from effected site Has an epidural residency half-life of >110 minutes Is more localized to the injection site than commercial products, spread limited to one vertebrae in 1st hour for greater local effect vs 6-7 with commercial products
Toxicology Studies Completed in Concurrence with FDA Pre-IND Meeting Minutes
No drug-related toxicity, local (spinal area) or systemic (including brain), macroscopic or histopathology after both single & repeat (once-a-week for 4W regimen) Histopathology clean in both routine and special stains, NOAEL is highest dose tested 10 mg / animal single dose & 2 mg repeat dose
– Placebo arm clean – non toxic, both routes in both species
Additional Vascular tox study mentioned by FDA also completed successfully
– Direct injection into vertebral artery of pigs
– No toxicity: vessel occlusion or macroscopic brain injury as reported with Depo-Medrol, clean histopathology: no brain or vascular injury
23

SP-102 Clinical Development Plan Overview
Corporate Clinical Strategy is based on a two stage approach…
Stage 1: IND to pivotal Phase 3 decision
– Phase 1 / 2 trial in chronic back pain completed dosing
– Adequate and well-controlled Phase 3 efficacy trial #1 (USA)
– Open-label, long-term safety study
– Phase 3 open label safety study
Stage 2: Commercial scale-up decision through NDA
– Adequate and well-controlled efficacy trial #2
– Pediatric study (plan to request deferral to conduct post-marketing)
24
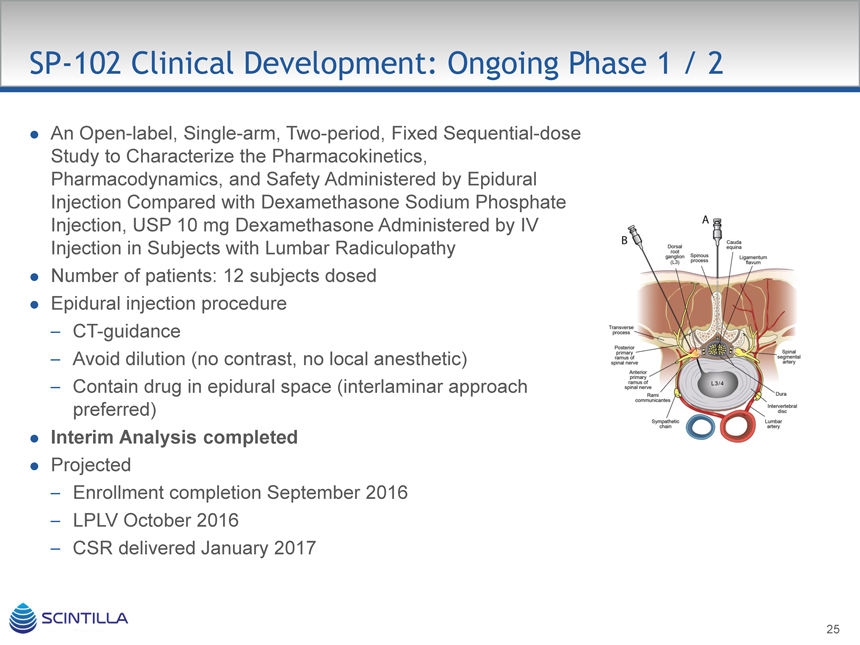
SP-102 Clinical Development: Ongoing Phase 1 / 2
An Open-label, Single-arm, Two-period, Fixed Sequential-dose Study to Characterize the Pharmacokinetics, Pharmacodynamics, and Safety Administered by Epidural Injection Compared with Dexamethasone Sodium Phosphate Injection, USP 10 mg Dexamethasone Administered by IV
Injection in Subjects with Lumbar Radiculopathy Number of patients: 12 subjects dosed Epidural injection procedure
– CT-guidance
– Avoid dilution (no contrast, no local anesthetic)
– Contain drug in epidural space (interlaminar approach preferred)
Interim Analysis completed
Projected
– Enrollment completion September 2016
– LPLV October 2016
– CSR delivered January 2017
25

SP-102 Clinical Development: C.L.E.A.R. Phase 3 Objectives
Corticosteroid Lumbar Epidural Analgesia for Radiculopathy (C.L.E.A.R.)
Evaluate the analgesic effect on leg pain following a single transforaminal epidural injection compared to a single sham injection of placebo saline (0.9% sodium chloride, 2 mL) in subjects with unilateral lumbosacral radiculopathy Evaluate opioid consumption as compared to placebo Evaluate pain at 4 weeks and degree of disability as measured by the Oswestry Disability Index (ODI) Characterize duration of analgesic effect Evaluate the safety and tolerability of multiple epidural injections Indication
– Lumbosacral Radicular Pain Selection Criteria
– Clinical symptoms consistent with the MRI diagnosis of nerve root compression secondary to a pathologic disc condition (e.g., herniated disc, annular tear or foreaminal stenosis)
– Leg pain ( 5, 9) present for at least 2 months and not more than 9 months, intensity being equal or worse than any concurrent lower back pain
26

High Barriers to Entry Protect SP-102
Intellectual Property
Filed IP filing claiming novel formulations (viscosity, dissolution profile, stability, residency time) and method of treating
U.S. Application No. 14/162,625 (“Pharmaceutical Formulation”) filed January 23, 2014 (with priority to U.S.
Provisional Application No. 61/755723 (January 23, 2013) and No. 61/776617 (March 11, 2013). Published December 4, 2014
U.S. Provisional Application No. 62/106,045 (“Pharmaceutical Formulation”) filed January 21, 2015
Barriers to Competition
Generics
– Use of specific excipient in formulation (not in label) will require determination and repeat local tox studies, required for increase in residency time for target indications
– Long term Supply and Exclusivity agreement with key excipient pharma provider completed
– Patents claiming proprietary formulation, method-of-use possible in Orange Book
– FDA requirement for expensive local tox, PK, efficacy/safety studies for new route Manufacturing
– Trade secrets, substantial know-how learned over past few years with formulation steroids and viscous solution gels
27

RTX: First-in-class Therapeutic for
Intractable Pain
28

Product Overview: RTX
Ultra potent TRPV1 agonist selectively targeting afferent neurons in chronic pain states Highly specific: affects only TRPV1 expressing nerves (A and C fibers) RTX vs. capsaicin Mechanism of – 18 Billion Scoville units
Action 2-5000
– Jalapeno
– Dorsal horn = > 12,000 times capsaicin
Pharmacological sequence of effects in vivo TRPV1 receptor activation depolarization apoptosis
Potentially permanent clinically meaningful analgesia regardless of type of pain (nociceptive, visceral and neuropathic) Efficacy Concomitant reduction in opiate use Improvement in QoL and function
Safety Alteration of heat sensation in the targeted area
No effect on normal acute pain perception / sensation or muscle function
Targeted single injection for treatment of intractable pain Dosing One injection
Patient need not discontinue current analgesic medications prior to injection
29

RTX Cancer Pain Epidemiology Overview
Intractable cancer pain is a subset of cancer pain usually requiring significant medical intervention RTX is positioned to replace costly interventional pain management procedures
M Cancer Prevalence
M Cancer Pain
M Moderate – Severe Pain
80% of cancer patients experience moderate to severe pain during the course of their disease progression.
WHO estimates 10 – 20% of cancer pain patients cannot be adequately managed EVEN with optimal opioid therapy (4)
1. NIH / NCI and Scintilla internal research
Van den Beuken-van Everdingen et al. 2007.
Ross et al. 20006 and Scintilla KOL market research
Datamonitor December 2009
Estimated Patients 2019
Cancer Pain
– 18 million Americans (1) Patients with Pain
– 53% or about 10 million (2) Moderate-Severe Cancer Pain
– 33% or about 3.1 million (3)
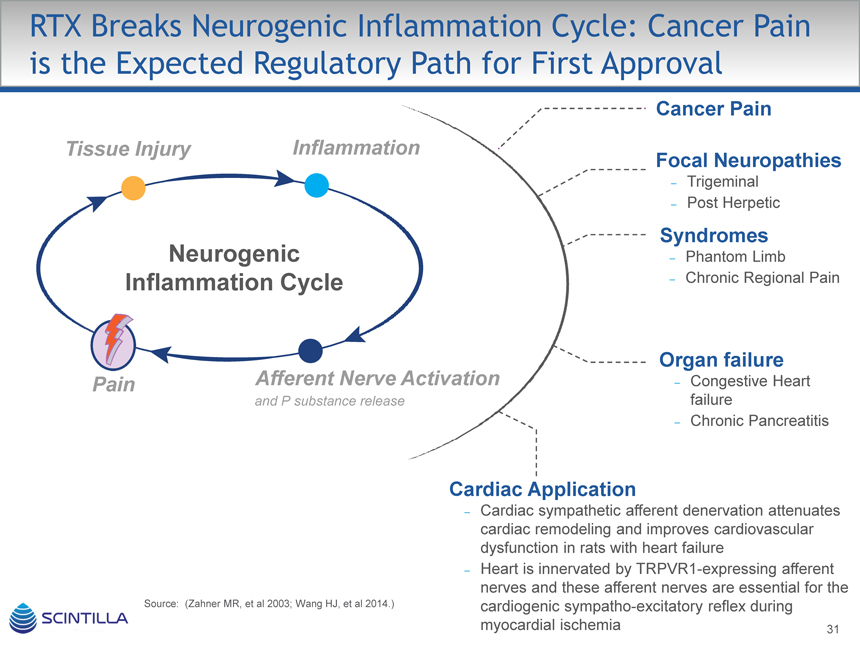
RTX Breaks Neurogenic Inflammation Cycle: Cancer Pain is the Expected Regulatory Path for First Approval
Cancer Pain
Tissue Injury Inflammation
Focal Neuropathies
– Trigeminal
– Post Herpetic
Syndromes
Neurogenic – Phantom Limb
Inflammation Cycle – Chronic Regional Pain
Organ failure
Pain Afferent Nerve Activation – Congestive Heart
and P substance release failure
– Chronic Pancreatitis
Cardiac Application
– Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure
– Heart is innervated by TRPVR1-expressing afferent nerves and these afferent nerves are essential for the Source: (Zahner MR, et al 2003; Wang HJ, et al 2014.) cardiogenic sympatho-excitatory reflex during myocardial ischemia 31

RTX POC: Canine Bone Cancer Pain
Preclinical Findings
Permanent Reduction In Pain Measured at Week 14
No Side Effects As Seen With Opiates
Or High Dose NSAIDS Such As Sedation, Constipation Or Nausea mm) 100 to
Could Sense Normal Pain Signals
(0 VAS
No Loss Of Muscle Function
Fully Alert Post-treatment
Return Of “Normal” Personalities
Reported By Owners
Source: Brown et al, Anesthesiology 2005; 103:1052-9
60 n=18
Score 50
40 p=0.0001
Pain for all time points
30
Observational 20
10 n=18 n=8 n=5 n=4
0 2 6 10 14
Weeks
32
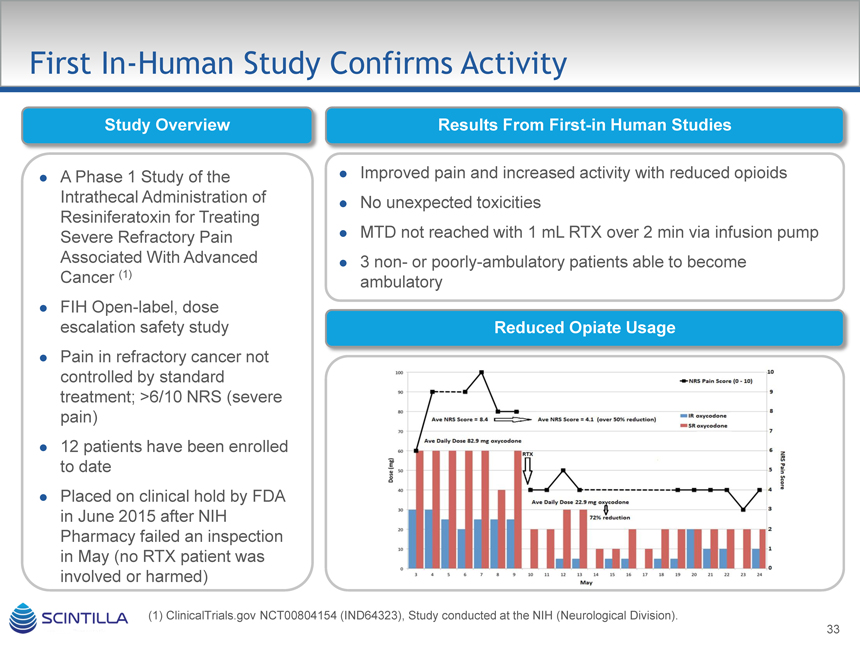
First In-Human Study Confirms Activity
Study Overview Results From First-in Human Studies
A Phase 1 Study of the Intrathecal Administration of Resiniferatoxin for Treating Severe Refractory Pain Associated With Advanced Cancer (1)
FIH Open-label, dose escalation safety study
Pain in refractory cancer not controlled by standard treatment; >6/10 NRS (severe pain)
12 patients have been enrolled to date
Placed on clinical hold by FDA in June 2015 after NIH
Pharmacy failed an inspection in May (no RTX patient was involved or harmed)
Improved pain and increased activity with reduced opioids
No unexpected toxicities
MTD not reached with 1 mL RTX over 2 min via infusion pump
3 non- or poorly-ambulatory patients able to become ambulatory
Reduced Opiate Usage
(1) ClinicalTrials.gov NCT00804154 (IND64323), Study conducted at the NIH (Neurological Division).

Switch to Epidural
Epidural injection into or near the dorsal root ganglion (DRG) for unilateral or diffuse pain
Intrathecal injection into the cerebrospinal fluid space (CSF) targeting both DRGs and dorsal horn neurons
Note: Green = Substance P as marker of Afferent nerves.
Untreated Intrathecal Epidural
34

Human Program Status
GMP API completed (3 registration batches) CMC Commercial supply chain established Investigational drug product available
Mutagenicity studies completed
Tox
Lumbar GLP completed, Pathology and reports ongoing
Cancer Pain
– IND planned early 2017
– Epidural MED study 2017
Clinical,
Cardiovascular
Preclinical &
– Mini Pig study at UCLA ongoing
Regulatory
– 2nd Hypertension Model at Nebraska ongoing
– Pre-IND meeting with Cardio Division, Q1 2017

Current Treatment Cost
Estimated Cost of Patient Controlled Morphine Treatment*
Year 2000Today**
Pump Rental, medications, refill charges, and supplies (Per Day) $154 $228
Per Month $4,625$6,845
6 Month treatment $27,750$41,070
Top 5 Reasons for Unscheduled Readmission to a Cancer Center
(N=1,351)
Fever 15%
Sepsis ** Assuming 3% increase per year 11%
Uncontrolled Pain 8%
Dehydration 6%
Pneumonia 5%
Admission Cost of Uncontrolled Pain $8,025$11,877
Total Cost of Uncontrolled Pain (6 month window with one admission) $35,775 $52,947
Source: MSA Internal Research of the following:
* The Cost of Comfort: Economics of Pain Management in Oncology, Betty Ferrell, City of Hope, ONE
Publication.2000, Vol 1. No. 9:56-61
** Assuming 3% increase per year
36

Market Research(1)
Initial Price Point
Our evaluation suggests that the market will support a potential price range of $15,000 – $30,000 for RTX therapy for pain management in the terminal, intractable pain, cancer patient
Based on Key Study Findings
Current coverage of comparator therapies (IT drug infusions) at a cost ($60 – 100K in first year) that is predictably higher than this range for the targeted population Payers are not expected to limit coverage or access for effective pain management in the terminal population Payers have supported palliative care and quality of life improvements in this population Payers have given a favorable impression of the early clinical profile and results with RTX in this population A majority of payers consulted have suggested that a range of $10 – $30K is within a coverage
“comfort zone” considering the cost of IT drug therapy, surgical and neurolytic procedural options in this population
(1) Study approved by Scintilla and prepared by Alliance Life Sciences.
37

RTX Marketing Opportunities
Opportunities
Inadequate treatment for cancer patients with intractable pain
Innovative, non-opioid, non-addictive analgesic that works by selectively killing the neurons that are responsible for cancer pain while leaving other neurons intact Superior efficacy and safety (TBD) profile with potential competitive cost advantage over opioids
– With long-term use, opioids gradually lose their effectiveness and patients need progressively higher doses to get pain relief but at the cost of a host of debilitating side effects, including impaired consciousness, nausea, vomiting and constipation. In addition, for patients who do not get relief from morphine, there are not a lot of options

Significant Near Term Clinical Milestones for Scintilla Programs
Multiple clinical milestones for all programs expected in the next 12 months
– Mid to late stage trials commencing for SP-102 & RTX programs
In addition, potential corporate development milestones may also be achieved with potential collaborations or licensing in ex-US territory related to SP-102 and RTX in 2017
Program 2016 20172018
Period 2H 1H2H1H2H
Phase 1 / 2 Phase 3
SP-102 Phase 3 Start Phase 3
Complete Results
IND and Phase 1 / 2
RTX Phase 1 / 2 Phase 2
StartResults
39

Financing and Use of Proceeds
~$100 M Private Placement of Common Stock
– Fund clinical trials and NDA submissions
– Pipeline additions
– Key hires across departments
– Commercial launch prep
– Payment to shareholders related to Semnur acquisition
– General corporate purposes

Investment Highlights
Innovative, Non-opioid Pain Management Portfolio Large, Established, yet Underserved Target Markets
Worldwide Commercial Rights to All Product Candidates
Strong Proprietary Platform with High Barriers to Entry Established Reimbursement Access
Two Products with Blockbuster Potential Poised to Replace
Current Standard of Care
41








































