Filing pursuant to Rule 425 under the
Securities Act of 1933, as amended
Deemed filed under Rule 14a-6(b) under the
Securities Exchange Act of 1934, as amended
Filer: Allergan, Inc.
Subject Company: Allergan, Inc.
Form S-4 File No. 333-201242

| DATE: | February 10, 2015 | |
| TO: | All Employees of Actavis and Allergan | |
| FROM: | Philippe Schaison, EVP and President, U.S. Medical Aesthetics | |
| RE: | U.S. Medical Aesthetics Proposed Organizational Announcement |
As we lead the way in Growth Pharma, we have been diligently determining the right structure, processes, and ways of working to enable us to improve our effectiveness and efficiency. Our past successes will fuel our future achievements as we continue to deliver the highest value for our stockholders by making a positive impact on our physicians, customers, consumers, and patients.
In assessing the right choices we had to make difficult tradeoffs. These tradeoffs focus on key areas we believe will drive future success with a bias for action. Bringing energy to everything we do, we must unlock the potential and entrepreneurial spirit of each and every employee in the new, combined company moving forward.
U.S. Medical Aesthetics Leadership Team (following the close of the merger)
Our leadership focus will be to develop and execute on strategies that drive top and bottom line growth. Each leadership member will report directly to me having accountability for their own therapeutic or functional area, as well as, a dual responsibility for maximizing portfolio performance for our entire division. Additionally, the leadership team will include representation from HR, Finance, Legal, and Compliance.
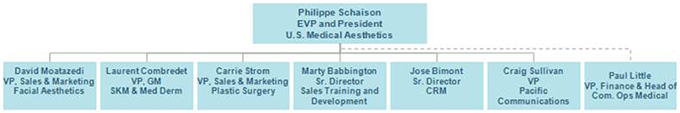

Brand teams will be organized into three therapeutic areas (see chart below). Each therapeutic area will continue to operate as an integrated sales and marketing team based in Irvine. SkinMedica and Medical Dermatology will report into one business unit leader to ensure increased alignment with our Dermatology customers. These two sales forces will operate separately as they do today. We have operated under this model successfully and this approach will enable us to maintain business continuity and customer focus. We have created a new Center of Excellence for Field Sales Training which will enable a deeper and broader use of resources and consistency in developing our valued field-based colleagues. In addition, our Customer Engagement Center of Excellence will continue to function with focus on protecting and growing our customer and physician engagement utilization and programs. Pacific Communications will now report to U.S. Medical Aesthetics and will continue to operate under its current agency model.
Carrie Strom Plastic Surgery | David Moatazedi Facial Aesthetics | Laurent Combredet SkinMedica & Medical Dermatology | ||
 |  |  | ||
 |  |  | ||
 |  | |||
 |
David Moatazedi will lead the sales and marketing team for Facial Aesthetics with the following direct reports:
| — | Tod Reed, VP, Sales |
| — | Colleen McKenna, VP, Marketing |
| — | Lisa Otis, Sr. Manager, Portfolio Marketing |
| — | Mike Jafar, Sr. Director, Strategic Communications and Marketing |
Laurent Combredet will lead SkinMedica and Medical Dermatology with the following direct reports:
| — | Ron Menezes, VP, Sales |
| — | Erin Liberto, VP, Marketing |
| — | Rahul Mehta, VP, R&D |
| — | Adelle Walker, Sr. Director, Strategic Communications and Physician Education Programs |
| — | Peter Feher, Sr. Director, Planning and Integration |

Carrie Strom will lead the sales and marketing team for Plastic Surgery with the following direct reports:
| — | Jeff Ehrhardt, VP, Sales |
| — | Amber Brown, Sr. Product Manager |
| — | Kristie Halls, Sr. Product Manager |
| — | Kristin Black, Product Manager |
| — | Heath Ponder, Sr. Reimbursement Manager |
| — | Breck Bair, Sr. Strategic Communications Manager |
Marty Babbington will lead the corporate and field sales training for all U.S. Medical Aesthetics with the following direct reports:
| — | Kelly Wright, Manager, National Training |
| — | Vincent Parras, Manager, National Training |
| — | Elene Eilopulos, Manager, National Training |
| — | Renee Bowers, Manager, Field Training |
| — | Jennifer Lawicki, Manager, Field Training |
| — | Ryan Skeeters, Manager, Field Training |
| — | Laine Marnelakis, Manager, Field Training |
| — | Susan Hughes-Hawkins, Manager, Field Training |
| — | Faith Bourgoyne, Manager, Field Training |
| — | Ann Stutzman, Manager, Field Training |
| — | Mysha Suguitan, Associate Field Specialist |
Jose Bimont will lead customer engagement and portfolio programs with the following direct reports:
| — | Heidi Shurtz, Sr. Product Manager |
| — | Philip Irvine, Product Manager |
| — | Richard Shaner, Product Manager |
Craig Sullivan will lead our Pacific Communications agency with the following direct reports:
| — | Pete Siegel, Creative Director |
| — | Henry Lee, Director, Client Services |
| — | Joseph Abiad, Director, Finance and Operations |
| — | James Marlin, Executive Manager, Agency Services |
| — | Ryan Orsini, Director, Client Services |
| — | Kun Yang Kim, Director, Client Services |
| — | Patrick Macke, Creative Digital Director |

Shared Commercial Services
Shared Commercial Services will be an integrated Center of Excellence providing support across the U.S. Branded Pharma and U.S. Medical Aesthetics teams. This includes business analytics, business insights including market research, marketing operations, sales analytics, sales operations, forecasting, and managed care teams. This function will be a bi-coastal operation with team members co-located with their respective businesses, wherever appropriate. This will maximize service and support to our internal customers. Our U.S. Medical Aesthetics Customer Service Center will remain in Austin.
As we move forward with our competitive model, we will change the way we work to ensure we make better, focused choices. It will be vital to our success that we operate, work and behave as one team. We must make clear decisions about the programs we undertake, where we spend our resources, and how we leverage these resources across the business units. By being smart in our choices, I believe that our, new combined company as a whole and, specifically, U.S. Medical Aesthetics has many exciting years ahead.
Finally, I would like to express my sincere thanks and gratitude to each and every one of you for your many contributions in making our organization a success. We had a phenomenal 2014 in growing U.S. Medical Aesthetics to new heights and there is no doubt in my mind that 2015 will be even better.
Cautionary Statement Regarding Forward-Looking Statements
Statements contained in this communication that refer to Actavis’ or Allergan’s estimated or anticipated future results, including estimated synergies, or other non-historical facts are forward-looking statements that reflect Actavis’ or Allergan’s current perspective of existing trends and information as of the date of this communication. Forward looking statements generally will be accompanied by words such as “anticipate,” “believe,” “plan,” “could,” “should,” “estimate,” “expect,” “forecast,” “outlook,” “guidance,” “intend,” “may,” “might,” “will,” “possible,” “potential,” “predict,” “project,” or other similar words, phrases or expressions. Such forward-looking statements include, but are not limited to, statements about the benefits of the Allergan acquisition, including future financial and operating results, Actavis’ or Allergan’s plans, objectives, expectations and intentions and the expected timing of completion of the transaction. It is important to note that Actavis’ and Allergan’s respective goals and expectations are not predictions of actual performance. Actual results may differ materially from Actavis’ or Allergan’s current expectations depending upon a number of factors affecting Actavis’ business, Allergan’s business and risks associated with acquisition transactions. These factors include, among others, the inherent uncertainty associated with financial projections; restructuring in connection with, and successful closing of, the Allergan acquisition; subsequent integration of the Allergan acquisition and the ability to recognize the anticipated synergies and benefits of the Allergan acquisition; the ability to obtain required regulatory approvals for the transaction (including the approval of antitrust authorities necessary to complete the acquisition), the timing of obtaining such approvals and the risk that such approvals may result in the imposition of conditions that could adversely affect the combined company or the expected benefits of the transaction; the ability to obtain the requisite Allergan and Actavis shareholder approvals; the risk that a condition to closing of the Allergan acquisition may not be satisfied on a timely basis or at all; the failure of the proposed transaction to close for any other reason; risks relating to the value of the Actavis shares to be issued in the transaction; the anticipated size of the markets and continued demand for Actavis’ and Allergan’s products; the impact of competitive products and pricing; access to available financing (including financing for the acquisition or refinancing of debt) on a timely basis and on reasonable terms; the risks of fluctuations in foreign currency exchange rates; the risks and uncertainties normally incident to the

pharmaceutical industry, including product liability claims and the availability of product liability insurance on reasonable terms; the difficulty of predicting the timing or outcome of pending or future litigation or government investigations; periodic dependence on a small number of products for a material source of net revenue or income; variability of trade buying patterns; changes in generally accepted accounting principles; risks that the carrying values of assets may be negatively impacted by future events and circumstances; the timing and success of product launches; the difficulty of predicting the timing or outcome of product development efforts and regulatory agency approvals or actions, if any; market acceptance of and continued demand for Actavis’ and Allergan’s products; costs and efforts to defend or enforce intellectual property rights; difficulties or delays in manufacturing; the availability and pricing of third party sourced products and materials; successful compliance with governmental regulations applicable to Actavis’ and Allergan’s facilities, products and/or businesses; changes in the laws and regulations affecting, among other things, pricing and reimbursement of pharmaceutical products; changes in tax laws or interpretations that could increase Actavis’ consolidated tax liabilities; the loss of key senior management or scientific staff; and such other risks and uncertainties detailed in Actavis’ and Allergan’s respective periodic public filings with the Securities and Exchange Commission, including but not limited to Actavis’ Annual Report on Form 10-K for the year ended December 31, 2013, as amended by Actavis’ Current Reports on Form 8-K filed on May 20, 2014 and December 5, 2014, Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2014, in Warner Chilcott Limited’s Registration Statement on Form S-4 effective as of October 15, 2014, and the related prospectus, Allergan’s Annual Report on Form 10-K for the year ended December 31, 2013 and Quarterly Report on Form 10-Q for the quarterly period ended on September 30, 2014, and from time to time in Actavis’ and Allergan’s respective other investor communications. Except as expressly required by law, each of Actavis and Allergan disclaim any intent or obligation to update or revise these forward-looking statements.
Important Information for Investors and Shareholders
This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. In connection with the proposed merger between Actavis and Allergan, Actavis has filed with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-4, including Amendment No. 1 thereto, that contains a joint proxy statement of Actavis and Allergan that also constitutes a prospectus of Actavis. The registration statement was declared effective by the SEC on January 26, 2015. Each of Actavis and Allergan commenced mailing the joint proxy statement/prospectus to its shareholders or its stockholders on January 28, 2015. INVESTORS AND SECURITY HOLDERS OF ACTAVIS AND ALLERGAN ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS THAT HAVE BEEN FILED OR WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION. Investors and security holders are able to obtain free copies of the registration statement and the joint proxy statement/prospectus and other documents filed with the SEC by Actavis and Allergan through the website maintained by the SEC athttp://www.sec.gov. Copies of the documents filed with the SEC by Actavis are available free of charge on Actavis’ internet website atwww.Actavis.com or by contacting Actavis’ Investor Relations Department at (862) 261-7488. Copies of the documents filed with the SEC by Allergan are available free of charge on Allergan’s internet website atwww.Allergan.com or by contacting Allergan’s Investor Relations Department at (714) 246-4766.
Participants in the Merger Solicitation
Actavis, Allergan, their respective directors and certain of their executive officers and employees may be considered participants in the solicitation of proxies in connection with the proposed transaction. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of the Actavis and Allergan shareholders in connection with the proposed merger is set forth in the joint proxy statement/prospectus. Information about the directors and executive officers of Allergan is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 26, 2014 and certain of its Current Reports on Form 8-K. Information about the directors and executive officers of Actavis is set forth in Actavis’ proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 28, 2014 and certain of Actavis’ Current Reports on Form 8-K. Additional information regarding the participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, is contained in the joint proxy statement/prospectus filed with the above-referenced registration statement on Form S-4 and other relevant materials to be filed with the SEC when they become available.

 | www.ourwinningway.com |