UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One) |
|
| |
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
|
|
|
|
| For the fiscal year ended December 31, 2007 |
|
|
|
|
|
|
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
|
|
|
|
| For the transition period to |
|
|
Commission file number: 0-23150
IBIS TECHNOLOGY CORPORATION
(Exact name of registrant as specified in its charter)
Massachusetts (State or other jurisdiction |
| 04-2987600 |
|
|
|
32 Cherry Hill Drive, Danvers, MA |
| 01923 |
|
|
|
Registrant’s telephone number, including area code: (978) 777-4247 | ||
Securities registered pursuant to Section 12(b) of the Exchange Act:
None.
Securities registered pursuant to Section 12(g) of the Exchange Act:
Common Stock, $.008 Par Value Per Share
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company.
Large Accelerated Filer o Accelerated Filer o Non-Accelerated Filer o Smaller Reporting Company x
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the registrant’s voting and non-voting common equity held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed fourth fiscal quarter (based on the last reported sale price on the Nasdaq National Market of such date) was $6,156,517.
As of May 22, 2008, the registrant had 14,317,482 of common stock outstanding.
PART I
Special Note Regarding Forward-Looking Statements
This Form 10-K contains express or implied forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 that relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology, such as “may,” “will,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “intend,” “potential” or “continue” or the negative of such terms or other comparable terminology, although not all forward-looking statements contain such terms. In addition, these forward-looking statements include, but are not limited to, statements regarding, among other things: (i) the Company’s ability to conduct its operations in a manner consistent with its current plan and to obtain additional implanter orders to continue as a going concern, (ii) regaining compliance with the minimum bid price requirement and the continued inclusion of the Company’s Common Stock on the Nasdaq Global Market or transfer to the Nasdaq Stock Market; (iii) the Company’s expectations regarding future orders for i2000 implanters, (iv) the Company’s expectations regarding it’s strategic alternatives, including the potential sale of the Company, (v) the continued employment of key management and technical personnel, and attaining implanter improvements to the degree and in the timeframe necessary to meet our customer’s expectations, (vi) the timing of our major customer’s ramping to production quantities on the i2000 implanter and the sustained production worthiness of the i2000 implanter, (vii) the reliance on a single or small number of large customers, interest in and demand for, and market acceptance of, the Company’s SIMOX-SOI technology including the Company’s implanters, (viii) the involvement generally of the silicon wafer manufacturing industry in the SOI wafer market and the ability of the wafer manufacturer’s to produce sufficiently low cost SIMOX-SOI wafers utilizing both our SIMOX equipment and technology, as well as other equipment manufacturer’s tools, (ix) the timing and likelihood of revenue recognition on orders for the Company’s implanters, (x) the Company’s belief that wafer manufacturers will become the primary suppliers of SIMOX-SOI wafers to the chipmaking industry, (xi) the throughput and production capacity of the i2000 implanter for manufacturing 300-mm SIMOX-SOI wafers, attaining implanter improvements to the degree and in the timeframe necessary to meet customer expectations, and the ability of the i2000 implanter to achieve acceptable production yields, (xii) the Company’s plan to focus on supplying implanters to wafer manufacturers, (xiii) the ability to operate with existing capital resources or to secure financing and to continue as a going concern and (xiv) the Company’s expectation of having sufficient cash for operations. Such statements are neither promises nor guarantees but rather are subject to risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. Such risks and uncertainties include, but are not limited to: future continued migration to SOI technology and market acceptance of SIMOX; the level of demand for the Company’s products; limited customers and products; lack of order backlog and visibility to the timing of new orders; the Company’s ability to pursue and maintain further strategic relationships, partnerships and alliances with third parties; loss of key personnel; the Company’s ability to protect its proprietary technology; the potential trends in the semiconductor industry generally; the ease with which the i2000 can be installed and qualified in fabrication facilities; the likelihood that implanters, if ordered, will be qualified and accepted by customers without substantial delay, modification, or cancellation, in whole or in part; the likelihood and timing of revenue recognition on such transactions; the impact of rapidly changing technology; the possibility of the impact of competitive products, technologies and pricing; the impact of rapidly changing technology; the possibility of further asset impairment and resulting charges; equipment capacity and supply constraints or difficulties; the Company’s limited history in selling implanters; general economic conditions; and other risks and uncertainties described elsewhere in this Form 10-K and in the Company’s Securities and Exchange Commission filings from time to time. All information set forth in this Form 10-K is as of the date of this Form 10-K, and Ibis undertakes no duty to update this information, unless required by law.
Item 1. BUSINESS
Introduction
Ibis Technology Corporation (“Ibis”) develops, manufactures and markets SIMOX-SOI implantation equipment for the worldwide semiconductor industry. SIMOX, which stands for Separation by IMplantation of OXygen, is a form of silicon-on-insulator, or SOI, technology that creates an insulating oxide barrier below the top surface of a silicon wafer through implantation and annealing. Our proprietary oxygen implanters produce SIMOX-SOI wafers by implanting oxygen atoms just below the surface of a silicon wafer to create a very thin layer of silicon dioxide between the thin operating region of the transistor at the surface and the underlying silicon wafer itself. The buried layer of silicon dioxide acts as an insulator for the devices fabricated on the surface of the silicon wafer and reduces the electrical current leakage which otherwise slows integrated circuit performance, and/or increases the loss of power during circuit operation. The buried layer of
2
silicon dioxide also helps to reduce the heat generated by the transistors. Through this process our customers can produce integrated circuits, which we believe, offer significant advantages over circuits constructed on conventional silicon wafers. We believe that these advantages include:
· substantially improved speed for microprocessors and other logic integrated circuits,
· reduced power consumption,
· reduced soft error rate,
· Compatibility with a higher temperature operating environment, and
· lower temperature operation
We believe these characteristics make SIMOX-SOI wafers, and the finished integrated circuits, well-suited for many commercial applications, including:
· servers and workstations,
· portable and desktop computers,
· entertainment devices such as TVs and game consoles,
· wireless communications and battery powered feature rich hand held devices including cell phones, and
· harsh-environment electronics.
When Ibis began operations in 1988, much of our revenue was derived from research and development contracts and sales of wafers for military applications. Over the years, there was a shift in revenue to sales of SIMOX-SOI wafers for commercial applications and the nature of our business had evolved through stages where previously our revenue was at times primarily derived from selling wafers for evaluation purposes, and at other times it was primarily derived from equipment sales. This often occurs when developing and promoting a fundamental new technology, especially as it relates to the semiconductor industry embracing any change that affects fabrication operations. In mid-2004 we exited the wafer manufacturing business to concentrate our efforts on supplying equipment and process technology to our equipment customers, the major silicon manufacturers. We did this having advanced our primary goal of establishing SIMOX-SOI as an important SOI technology with the potential to be the low cost, high volume offering. We intend to continue to work with the major wafer manufacturers to support the market acceptance of 300mm SIMOX technology through continuing process research and development in conjunction with our customers. We believe this effort will directly support the wafer manufacturers’ decision to purchase our equipment.
Our fundamental SIMOX-SOI technology has been developed, refined, and tested over the last dozen years. In 2002, Ibis introduced the current-generation of SIMOX-SOI technology, which included our second generation oxygen implanter (i2000ä) and the modified low dose (“MLD”) SIMOX wafer process which was licensed to us by IBM. The i2000’s flexibility, automation and operator-friendly controls allow this tool to produce a wide range of SIMOX-SOI wafer products using different manufacturing processes, including Advantox® MLD and Advantox MLD-UT wafers. We believe the ability of the i2000 implanter to produce twelve-inch (or 300mm) SIMOX-SOI wafers using different processes from standard to the latest MLD process positions us to capitalize on the growing SOI market. In early 2004, we received an order valued at approximately $7.0 million for an i2000 SIMOX oxygen implanter from a major silicon wafer manufacturer. During the third quarter of 2004 this tool was accepted by the customer. In early January 2005, we received a $6.0 million order for an i2000 SIMOX oxygen implanter from SUMCO, another major silicon wafer manufacturer. During the first quarter of 2006, this tool was accepted by the customer. In October of 2005, we received a second order valued at $7.0 million for an i2000 SIMOX implanter from SUMCO and negotiated a master purchase agreement that will govern the general commercial terms of potential future orders from SUMCO. We shipped this implanter in April of 2006, with final customer acceptance and associated revenue recognition taking place in the August of 2006.
We were incorporated in Massachusetts in October 1987 and commenced operations in January 1988. Our executive offices are located at 32 Cherry Hill Drive, Danvers, Massachusetts 01923 and our telephone number is (978) 777-4247. Our web site is located at www.ibis.com. We make our periodic reports on
3
Form 10-K, Form 10-Q and Form 8-K (and any amendments to those reports) available on the web site, free of charge, as soon as reasonably practicable after these reports are filed with or furnished to the Securities and Exchange Commission. We have not incorporated by reference into this document the information on our web site and you should not consider it to be a part of this document. Our web site address is included in the document as an inactive textual reference only. The public can also obtain access to such reports at the Securities and Exchange Commission’s Public Reference Room at 450 Fifth Street, NW, Washington, DC 20549, by calling the SEC at 1-800-SEC-0330 or by accessing the SEC’s website, which is www.sec.gov. Unless the context otherwise requires, the terms “Ibis”, “we”, “us”, and “our” refer to Ibis Technology Corporation.
Our Strategies
The Company announced in February 2008 that it hired an investment banker to explore strategic alternatives, including the potential sale of the Company, while it continues to focus on its operational objectives. The Company took this step as a result of our recurring losses from operations and net capital deficiency, and the current lack of visibility for additional orders for implanter equipment. Since we began selling implanters in 1996, we have only sold a total of eight Ibis 1000 oxygen implanters at an average sale price of approximately $4.0 million each and four i2000 oxygen implanters at a selling price between $6.0 and $8.0 million. The most recent order received was in October 2005, for which the Company recognized revenue in the quarter ended September 30, 2006. The Company did not record implanter revenue during 2007 and as of April 10, 2008, does not have an order for an implanter from our customers. At present time, only one of the four major wafer manufacturers are a current prospective customer. The Company currently does not have a backlog for implanters, nor does it have visibility to the timing of the next order from any customer.
As the Company continues to assess its strategic alternatives, subject to the availability of resources, Ibis will continue with its primary objective to be the dominant supplier of oxygen implantation equipment to the world’s silicon wafer manufacturers. Our primary emphasis will continue to be on implanter sales and support. We also plan on continuing process development for SIMOX-SOI wafers in partnership with our equipment customers to hasten the adoption and broaden the market acceptance of SIMOX-SOI. Key elements of our strategies for achieving this objective include:
· Capitalizing on Fundamental Trends in Semiconductor Manufacturing. We believe that semiconductor manufacturers face an increasing demand for faster integrated circuit speed, reduced power consumption, smaller feature size and immunity to soft failure errors, which are changes in logic state due to exposure to radiation. In addition, heat generation caused by current leakage has become a major problem at the 65 nm feature size and will continue to increase in importance at the 45 and 32 nm technology nodes as production comes on stream over the next four years. In our experience, these manufacturers prefer to satisfy the demand with minimal additions or modifications to their existing equipment base. We believe that SIMOX-SOI technology will be a leading alternative in addressing these requirements and that there will be a continuous migration of SOI wafer manufacturing into the major silicon wafer suppliers. We reach this conclusion for a number of reasons. First, we believe that tremendous price pressure exists on commodity type products, such as silicon wafers. Because the starting wafer represents a significant component of the SOI wafer cost, silicon wafer manufacturers should have a natural cost structure advantage leading to a higher gross margin, and therefore can manage such pricing pressures better than stand-alone SOI producers that do not also produce the silicon wafer itself. Second, we expect that the pricing pressures will encourage silicon wafer manufacturers to seek out higher margin products, like SOI wafers, to increase their margins. Third, we believe that silicon wafer manufacturers have traditionally developed proprietary intellectual property in silicon materials science, which can be applied to designing optimal starting wafers for SOI production. This should give them an advantage in both minimizing wafer cost and maximizing SOI wafer quality and yield. Fourth, our experience suggests that silicon wafer manufacturers already have a well-developed infrastructure for the manufacture, sale and marketing of large volumes of substrates. Lastly, we believe that there is greater efficiency in producing the SOI
4
wafer as part of the wafer manufacturers existing product flow, specifically avoiding the need to re-package, re-clean, re-inspect and re-ship substrates twice, once as starting silicon wafers, and a second time as SOI wafers. Therefore, as a result of this thought process, we expect our ultimate customers will be drawn principally from these silicon wafer manufacturers and we plan to focus a majority of our technical and marketing resources on the leading silicon wafer manufacturers and our major key customers in the semiconductor industry who are the leaders in the adoption of SOI technology. We expect that implanter sales to chipmakers should be minimal, and focused on SOI processes, which the chipmaker wishes to keep proprietary, such as selective (or patterned) SIMOX, or other specialty substrates.
· Pursuing Strategic Marketing, Manufacturing and Development Alliances. We intend to continue to pursue relationships through which third parties will provide assistance with joint research and development opportunities on both process and equipment. In January 2003, we entered into an agreement with IBM to develop an enhanced, modified low-dose (“MLD”) process for the manufacture of SIMOX-SOI wafers.
· Enhancing and Extending Current Product Offerings. We intend to continue to use our resources and our strategic partners’ technical expertise to improve our existing equipment products, expand our core product functionality, add products to our existing product line and further advance our process technology. Our implanter research and development programs are aimed at improving quality, and increasing throughput which results in reducing the cost of SIMOX-SOI wafers. We are also exploring several alternative technologies that could utilize our engineering expertise, and represent either product extensions or potentially new applications of our technology.
· Increasing our SIMOX-SOI Equipment Manufacturing Capacity. Going forward, we intend to gauge SIMOX-SOI equipment demand from the silicon wafer manufacturers and adjust our equipment manufacturing capacity accordingly. We currently have capacity to build approximately 10 implanters per year in our existing manufacturing space.
Marketing, Sales and Customers
Over the last several years, Ibis had focused on integrating SIMOX-SOI wafers into commercial applications. We believe that commercial shipments of our wafers had been used principally for evaluation purposes or pilot production in products, including microprocessors, gate arrays, ASICs (application specific integrated circuits), and memories (DRAMs, SRAMs, etc.). We believe that one of our customers is providing SIMOX-SOI wafers for commercial production and that a number of our potential customers are sampling SIMOX wafers or are developing prototype products.
Our primary focus more recently has been on getting the silicon wafer manufacturers to embrace SIMOX-SOI technology. Where we have succeeded, we have accomplished this through joint research and development programs, the use of sales representative agreements, and the provision of SIMOX-SOI wafer foundry services to our customers. We intend to assist the wafer manufacturers in becoming the producers of SIMOX-SOI wafers by selling and servicing oxygen implanters along with continuing to improve SIMOX wafer processing technology via our SIMOX process engineering group.
In August 2004, upon exiting the wafer manufacturing business Ibis cancelled its Sales Representative Agreement with MEMC for SIMOX SOI wafers. We believe that by canceling this agreement we have opened new market opportunities with the other silicon wafer manufacturers for the sale of our i2000 oxygen implanters. We also believe this has allowed Ibis to work closely as an independent, non-competitive resource with several of the leading wafer manufacturers regarding SIMOX-SOI process improvements.
The following table sets forth, in thousands of dollars, the amount of revenue derived from our significant customers during the fiscal years ended December 31, 2005, 2006 and 2007, as well as the percent of our revenue represented by these customers’ purchases (in thousands):
5
|
| 2005 |
| 2006 |
| 2007 |
| |||||||||
Customer |
| Dollars |
| Percent |
| Dollars |
| Percent |
| Dollars |
| Percent |
| |||
IBM |
| $ | 74 |
| 12 | % | $ | 4 |
| — |
| — |
| — |
| |
Simgui |
| — |
| — |
| $ | 10 |
| — |
| $ | 64 |
| 7 | % | |
SEH |
| — |
| — |
| — |
| — |
| $ | 18 |
| 2 | % | ||
Nissin Electric |
| $ | 279 |
| 46 | % | $ | 369 |
| 3 | % | $ | 372 |
| 39 | % |
Tokyo Iovenus |
| $ | 162 |
| 27 | % | $ | 92 |
| 1 | % | $ | 265 |
| 28 | % |
SUMCO |
| — |
| — |
| $ | 13,325 |
| 95 | % | $ | 178 |
| 19 | % | |
Axcelis |
| — |
| — |
| $ | 175 |
| 1 | % | $ | 50 |
| 5 | % | |
The revenue from SUMCO in 2007 was for the balance of the relocation of an i2000 implanter from their Noda facility to Yonezawa and for services on the two implanters they own. The revenue from Nissin in 2007 was from license royalties, the revenue from Tokyo Iovenus in 2007 was from the sale of parts, and the revenue from Axcelis in 2007 was for the additional payment on the purchase of a license option. The revenue from SUMCO in 2006 was for the purchase of two i2000 implanters for a total of approximately $13 million, and for the relocation of one of these systems from their Noda facility to Yonezawa. The revenue from Nissin in 2006 was from license royalties, the revenue from Tokyo Iovenus in 2006 was from the sale of parts, and the revenue from Axcelis in 2006 was for the purchase of a license option. The revenue from IBM in 2005 was for service and parts, the revenue from Nissin in 2005 was from license royalties, and the revenue from Tokyo Iovenus in 2005 was from the sale of parts.
Sales to overseas customers in 2005, 2006 and 2007 were 81%, 99% and 94% of total revenue, respectively. In 2005, sales to Japan were 81% of total revenue, which was primarily attributable to Nissin Electric Co. Ltd., license royalties. In 2006, sales to Japan were 99% of total revenue, which was primarily attributed to SUMCO Corporation, implanter sales. In 2007, sales to Japan were 88% of total revenue, which was primarily attributed Nissin Electric Co. Ltd. for license royalties and Tokyo Iovenus for service revenue.
Strategic Alliances
Ibis has entered into a number of strategic alliances that we believe enable us to better address our target market, to advance our technology more effectively, and to match our technical developments and expansion to the needs of our key customers. We believe that strategic alliances with existing and potential customers which are essential to developing a worldwide commercial market for our SIMOX-SOI implanters.
We have a long-standing relationship with SUMCO which began as a sales distribution arrangement, progressed to a joint research and development effort, and ultimately evolved into SUMCO’s purchase of an Ibis 1000 oxygen implanter in order to establish a Japanese-based manufacturing facility for SIMOX-SOI wafers. This implanter was installed in SUMCO’s wafer manufacturing facility in Chiba, Japan in July 2001. In 1999, we completed an agreement to license our standard and Advantox SIMOX-SOI wafer fabrication process to SUMCO. Under this agreement we received an initial royalty fee and are entitled to future royalties based on a percentage of SUMCO’s sales of Advantox SIMOX-SOI wafers that are manufactured using the licensed process. In January 2005 and October 2005, SUMCO ordered i2000 SIMOX oxygen implanters which were subsequently shipped, and for which final customer acceptance was received in 2006. Revenue was also recognized for both i2000s upon final acceptance during 2006.
In January 2003, we announced the signing of a Joint Development Agreement with IBM. The objective of the agreement was to develop an enhanced, MLD process for the manufacture of SIMOX-SOI wafers, which are used as the starting material in the manufacture of advanced integrated circuits (“ICs”). Aimed at producing lower-cost, higher quality SIMOX-SOI wafers with thinner top silicon layers, the joint development work was being conducted at both Ibis and IBM. We believe that both companies brought extensive expertise and experience regarding SIMOX-SOI technology to the joint effort. IBM, a pioneer in the development and adoption of SOI technology, developed the original MLD process for high quality, low cost SIMOX-SOI wafers. IBM then licensed Ibis to manufacture SIMOX-SOI wafers using the production-proven
6
MLD process for sale to IBM and all other Ibis customers. Our implanters can operate using both the MLD process and other processes as well, including non-proprietary processes.
In February 2004, we announced the signing of a service agreement with Tokyo Iovenus based in Tokyo, Japan. This agreement provides for local training of Japanese service engineers for our oxygen implanter customers in Japan.
Research and Development
Ibis continues to have active research and development programs in both equipment and wafer process technology. For the past four years a primary focus has been developing implanter technology and advanced process capability to produce 300 mm SIMOX wafers. This required the development of a new generation oxygen implanter, the i2000, and the procurement and qualification of annealing, cleaning, and metrology tools for completing the full SIMOX process at 300 mm.
The proprietary i2000 was designed to support the volume production of high quality SIMOX-SOI 300 mm wafers for the global semiconductor industry. To minimize process risks, the i2000 duplicates the process environment of the Ibis 1000. However, it incorporates a number of features designed to improve throughput and reduce costs. These include increased beam current, faster wafer handling, off-hub wafer cooling, and modular construction, which we believe will enable improved serviceability and diagnostics, while simplifying the assembly and shipping of the machine. We also believe that the simpler beam line design of the i2000 also offers extensive capabilities, facilitating the manufacture of the Advantox product portfolio. We believe that taken together, these features significantly increase productivity of the i2000 over the Ibis 1000. Finally, the i2000 is designed to be far more fab friendly than the Ibis 1000. It is designed to be bulkhead or ballroom mounted in the clean room, offers front-opening unified pod (FOUP) capability and meets SEMI safety and ergonomic guidelines. We also believe that the i2000’s improved automation and operator-friendly controls will improve product yield and afford ease-of-use. Our plans are to improve the i2000 in terms of both quality (reduced particles) and quantity (increased throughput) of as-implanted SIMOX wafers, in order to provide continuous improvement to the cost of ownership for our customers.
Our wafer technology R&D has concentrated on enhancing the range of potential commercial applications for Ibis’ SIMOX-SOI wafers by:
· Refining techniques to produce SIMOX-SOI wafers of higher quality. In the fall of 2004 we announced a 3x to 4x reduction in the silicon roughness of the SIMOX MLD;
· Jointly developing a technology for manufacturing high resistivity SIMOX-SOI wafers for mixed signal and radio frequency (“RF”) applications with a major silicon wafer manufacturer. With this alliance, we developed advancements in SIMOX wafer manufacturing, including reduced wafer cost, scalability to 300 mm and stability of the material’s high resistivity characteristic through thermal cycling common in integrated circuit manufacturing. We filed a joint patent application with SEH entitled Method of Producing a High Resistivity SIMOX Silicon Substrate in May 2003. As of December 31, 2006 this patent has been granted;
· Processing strained silicon (another emerging wafer-materials technology) for use in SOI (“SSOI” wafers), an innovation enabling a further significant boost of device speed for complementary metal oxide semiconductor (“CMOS”) products. Improved electron mobility in strained silicon leads to an increased drive current in MOS devices and is complemented by benefits provided by SOI, such as reduction of parasitic capacitances in CMOS devices. We assist our customers in their development of SSOI SIMOX wafers; and
· Responding to specific customer requirements and emerging industry trends, such as the development of our Advantox MLD-UT (ultra thin) product line to address requirements for fully depleted devices. The term “fully depleted” describes a MOS transistor structure in which the depletion region under normal operation extends as far as a buried insulator layer. We believe that ultra-thin SOI wafers provide superior results, especially in terms of increased power efficiency
7
and heat reduction in the operation of fully depleted substrate transistors for next generation semiconductor devices.
During the fiscal years ended December 31, 2005, 2006 and 2007, Ibis’ internally funded research and development expenses were approximately $6.0 million, $5.4 million and $3.8 million or 995%, 39% and 398% of our revenues, respectively.
Competition
We believe we face three general sources of competition: (1) direct SIMOX-SOI competition, (2) competing SOI technologies, and (3) competing non-SOI technologies.
Among direct SIMOX-SOI competitors, we believe we are presently the only manufacturer of SIMOX-SOI implanters. To our knowledge, Hitachi, Ltd. of Japan had been the only other company manufacturing SIMOX implanters and has sold a limited number of tools in prior years. We believe that in early 2004, Hitachi exited this business. We are not aware of plans by any of the major ion implant manufacturers, including Hitachi, to design and develop oxygen ion implanters, but they may already have such plans, or may develop them in the future. We believe that it would take one to three years to develop such an implanter.
We also believe that SUMCO, and Simgui are manufacturing or marketing SIMOX wafers. We expect that the availability of SIMOX-SOI wafers from silicon wafer manufacturers will help address customer concerns about adequate sources of supply and their desire to purchase all of their silicon wafer requirements (e.g., bulk silicon, epitaxial, strained silicon and SOI wafers) from the same company. Our objective is to be the dominant supplier of SIMOX implanters to the world’s silicon wafer manufacturers so they can, in turn, efficiently and cost-effectively supply SOI wafers to the global semiconductor industry. In addition, we believe that these wafer manufacturers would be potential equipment customers for our implanters.
The second source of competition for us is the development of alternative SOI materials. The approach that most directly competes with SIMOX is thin-film bonded SOI wafers. The majority of SOI wafers are produced with this technology. SOITEC, a French-based company that spun off from LETI, a French government research lab, uses a bonded method. The thin-film bonded approach uses two silicon wafers, one or both having a thermally-grown oxide layer, which are first bonded together to form the silicon/silicon dioxide/silicon structure. A majority of one of the wafers is removed or separated from the double-wafer structure, and the remaining portion serves as the device layer of the SOI wafer. The most popular method is to transfer the thin layer using wafer splitting techniques, allowing the rest of the wafer to be reclaimed and reused. Regions of stress are first created using implantation and/or epitaxial growth prior to the bonding step. The wafer is split along the stress interface by the application of heat (SOITEC’s Smartcut® process), a gas jet (Silicon Genesis’ process), or a water jet (Canon’s ELTRAN® process). SEH also offers a thin SOI Unibond® wafer manufactured with the SmartCut® process, which is licensed from SOITEC. Our evidence to date suggests that both SIMOX and bonded wafers perform equally well. We believe, however, that the SIMOX process can result in an inherently lower manufacturing cost in higher volume production. We also believe that, at this stage in the market’s development, multiple SOI suppliers will help accelerate the adoption of SOI technology.
The third source of competition is derived from alternative non-SOI technologies designed to obtain benefits similar to those of SOI, including improvements to existing technologies. Significant resources are continually expended to improve epitaxial and conventional bulk silicon wafers.
The semiconductor industry has demonstrated its resourcefulness in improving materials through creative circuit design and manufacturing techniques, thereby extending the useful life of conventional substrates, and we cannot be sure that it will not continue to do so. The relatively lower cost of these substrates provides an incentive to the semiconductor industry to improve existing material without moving to new, more advanced substrates. In addition, complex variations of more conventional approaches, such as elaborate circuit structures built on conventional silicon substrates, and compound materials (such as silicon-germanium,
8
gallium-arsenide, indium phosphide, etc.), are other alternative substrate choices. Strained silicon and silicon super lattices are technologies that can be used to increase the operating speed of computer chips, such as microprocessors. Strain can be applied locally (at the fab) or globally (at the wafer manufacturer). These technologies can be applied to bare wafers, SIMOX SOI wafers or Bonded SOI wafers. The spacing between silicon atoms is stretched – or strained - farther apart, allowing holes or electrons to flow with less resistance, leading to chips that are faster, as reported by IBM. Silicon superlattices are thin layers inserted in the channel region of the transistor that provide low impedance for current flow horizontally in the channel region just below the gate, and higher impedance for vertical current flow into the regions further below the surface. The emergence of strained silicon and silicon superlattices in wafer-materials technology will lead to comparisons with SOI, among other emerging wafer-materials technologies. Although strained silicon, superlattices and SOI are wafer-material technologies that increase chip speed; they work in different – and complementary – ways and if combined can provide additional benefits.
Strained silicon and silicon superlattices increase transistor speed by increasing the mobility of holes or electrons traveling through the channel region near the silicon surface. On the other hand, SOI increases transistor speed by reducing parasitic capacitances associated with source and drain junctions. Therefore, we believe strained silicon, silicon superlattices, and SOI (as applied through either SIMOX or Bonded SOI) are complementary and mutually enhancing - not competing – technologies, although one technology may be adopted without the other. Similarly, in the area of reducing wasteful power, high-k dielectrics with metal gates and SOI address different sources of wasteful power consumption. The high-k dielectric/metal gate combinations lower power losses by reducing charge leakage through the gate dielectric. (While the gate dielectric may be similar or even identical for all transistor types, different metals/metal stacks are used for NMOS vs. PMOS gates to ensure they perform similarly as they did with doped polysilicon gates in larger geometry nodes.) SOI lowers power losses by blocking the conduction paths to the bulk silicon and between adjacent devices. We believe the real wave of the future will be combining these complementary technologies – strained silicon/silicon superlattice + high-k gate dielectric/metal gates + SOI – to create the highest speed, lowest-power competitive combinations, much like the way copper interconnects, low k dielectric materials and SOI substrates have been combined.
Backlog
As of April 10, 2008, Ibis did not have any orders for implanters. All customer orders are subject to modification or cancellation by the customers. Backlog can, and often does fluctuate significantly based upon, among other matters, the timing and receipt of orders and subsequent tool shipments. Therefore, variations in backlog may not represent a fair indication of future business trends.
Patents and Proprietary Rights
Ibis’s success is dependent in part upon certain proprietary technologies and core intellectual property. Ibis has been awarded a number of patents and has a number of pending patent applications. For example, we added seven patents to our intellectual property portfolio during 2005, five, including international patents, during 2006 and two patents during 2007. We have several more patents pending relating to our proprietary i2000 oxygen implanter or the SIMOX fabrication process. Additionally, we diligently monitor our research and development process to identify inventions that warrant pursuing patent protection.
Notwithstanding our patent portfolio strategy, we rely largely upon trade secret protection and confidentiality and proprietary information agreements to safeguard our proprietary technology. Towards this end, all of our employees currently are required to execute confidentiality agreements pursuant to which they agree to assign to us all patent rights and technical or other information developed by them during their employment with us and also agree not to disclose any trade secret or confidential information without our prior written consent.
Despite the efforts we take to protect our proprietary technologies and core intellectual property, the use of contractual, statutory and common law protections offer only limited protections. We cannot ensure that patents will issue from our pending applications or from any future applications or that, if issued, any claims
9
allowed will be sufficiently broad to protect our technology. In addition, we cannot ensure that any patents that have been or may be issued will not be challenged, invalidated or circumvented or that any rights granted by those patents would protect our proprietary rights. Failure of any patents to protect our technology may make it easier for our competitors to offer equivalent or superior technology. In addition, unauthorized parties may attempt to copy or otherwise misappropriate aspects of our products or services, or to obtain or use information that we regard as proprietary. Even if a competitor’s products were to infringe patents owned or licensed by us, it would be very costly for us to enforce our rights in an enforcement action, which would also divert funds and resources which otherwise could be used in our operations. Furthermore, third parties may also independently develop similar technology without breach of our proprietary rights.
In addition to our efforts to develop proprietary technology, historically we have also supplemented and commercialized our intellectual property through the grant and receipt of licenses. For example, Ibis has an exclusive worldwide sublicense to the proprietary beam scanning system developed and patented by a consultant of ours during the development of the Ibis 1000. Our beam scanning system sublicense agreement also grants us certain rights to further sublicense the beam scanning system for certain applications other than oxygen implantation. Pursuant to these rights, we have entered into four non-exclusive sublicense agreements that permit the respective sub-licensees to manufacture, use and sell implantation machines incorporating the beam scanning system so long as such machines are not designed for the production of oxygen implanted wafers. Each sub-licensee has paid us a non-refundable option fee upon signing an agreement and pays an initial license fee when it exercises its option to use the licensed technology. In addition, each sub-licensee will pay a royalty fee with respect to each implantation machine using this beam scanning system manufactured, used or sold after its option fee and initial license fee has been applied. License fees received by us from sub-licenses are to be shared on a substantially equal basis with the licensor of the beam scanning system. As of December 31, 2007, Ibis had received approximately $3.2 million in net license fees, after deducting amounts paid to the licensor.
Ibis also obtained in 1994 an exclusive license to technology that facilitates the presentation of wafers to ion beams developed by Superion Limited, a United Kingdom corporation. Through December 31, 2007, Ibis has paid $0.6 million for license fees for implantation machines that have been manufactured by us. Under the terms of this agreement, Superion Limited has retained the right to utilize the technology for uses not involving oxygen implantation of silicon or other semiconductor materials. During 2001, this agreement was modified to incorporate i2000 implantation machines. Ibis also entered into a sublicense agreement during 2001 which gives our customer a royalty-bearing, non-exclusive license to utilize this technology for ion implantation machines, excluding oxygen implanters.
During 1999, we completed an agreement to license our standard and Advantox SIMOX-SOI wafer fabrication process to SUMCO. The agreement consisted of an initial royalty fee. Future royalties shall be payable based on a percentage of SUMCO’s SIMOX-SOI wafers sold which are manufactured using the licensed process.
Furthermore, in 2000, we licensed from IBM the right to manufacture and sell SIMOX-SOI wafers, using IBM’s proprietary MLD SIMOX process, to IBM and to all our other customers. Under the royalty-bearing license agreement, we were able to use IBM’s process to produce MLD SIMOX-SOI wafers which we marketed as Advantox MLD. Advantox MLD wafers were broadly marketed to integrated circuit manufacturers looking to accelerate their SOI adoption process. Under the agreement we granted IBM rights to our patents utilized in the modified low dose, or MLD process. Although we believe that other SIMOX-SOI wafer processing methods exist and are being used today, our existing or potential equipment customers that intend to use the MLD process to manufacture SIMOX-SOI wafers would be required to license this technology directly from IBM. Two major silicon wafer manufacturers have already licensed this technology from IBM and others have developed their own SIMOX processes. No assurances can be given that the remaining equipment customers that intend to use the MLD process and IBM would come to terms acceptable to both parties in a timely manner, or at all. These MLD process license issues may effect the timing of placement of customer orders in the future if customers plan to license this technology.
10
Finally, during 2001 we licensed our Advantox 50 and 150 SIMOX wafer fabrication processes to Simgui. Ibis received the initial license fee from Simgui in January 2003, and the technology transfer took place in the first quarter ended March 31, 2003. License revenue of approximately $0.5 million was recognized in that quarter.
Government Regulation
Ibis is subject to a variety of federal, state and local environmental regulations related to the storage, treatment, discharge or disposal of chemicals used in its operations and to the exposure of our personnel to occupational hazards. Although we believe that we have all permits necessary to conduct our business, the failure to comply with present or future regulations could result in fines being imposed on us, suspension of production, or a cessation of operations. Our future activities may result in our being subject to additional regulations. Such regulations could require us to acquire significant equipment or to incur other substantial expenses to comply with regulations. Any failure by us to control the use of, or to restrict adequately the discharge of, hazardous substances or to properly control other occupational hazards could subject us to substantial financial liabilities.
Certain technologies associated with Ibis’ implanters are subject to export regulations administered by the U.S. Department of Commerce. Accordingly, Ibis may be required to secure U.S. export licenses with respect to sales of implanters or transfers of technologies to end users in certain foreign countries. There can be no assurance that if necessary, Ibis will be able to secure such licenses in a timely manner, or at all.
Manufacturing and Supplies
Ibis manufactures its oxygen implanters from standard components and from components manufactured in-house or by other vendors according to our design specifications. Most raw materials and components not produced by us are available from more than one supplier. However, certain raw materials, components and subassemblies are obtained from a limited group of suppliers and at least one major subsystem is a long lead time, sole source component. Semiconductor equipment is a growth industry and is very cyclical in nature, so if our suppliers experience an increase in demand from other semiconductor equipment manufacturers with much higher volumes than us, the lead-time and/or price for some of our components may increase. Although we have sought to reduce our dependence on these limited source suppliers and we have not experienced significant production delays due to unavailability or delay in procurement of component parts or raw materials to date, increased market demand for the materials supplied by, or disruption or termination of, certain of these sources could occur and such increased demand, disruptions, or termination could have a material adverse effect on our business and results of operations.
Employees
As of December 31, 2007, we employed 20 persons on a full-time basis. In January 2007 certain executive and senior level management personnel accepted voluntary salary reductions that ranged from 10% to 50% of their salaries. These reductions are anticipated to remain in place until market conditions warrant further review. None of our employees are represented by a labor union and we believe our relations with our employees are good.
Item 1A. RISK FACTORS
Our Registered Public Acoounting Firm Has Expressed Substantial Doubt About Our Ability to Continue as a Going Concern.
In their report dated May 22, 2008, our registered public accounting firm stated that our financial statements for the fiscal year ended December 31, 2007 were prepared assuming we would continue as a going concern; however our recurring losses and accumulated deficit raise substantial doubts about our ability to continue as a going concern. Our ability to continue as a going concern is an issue raised as a result of our recurring losses from operations and net capital deficiency and is subject to our ability to secure additional
11
orders of our implanter equipment or, in the alternative, identify and consummate a strategic transaction, including the potential sale of the Company, or other strategic alternative.
Our Efforts to Identify, Evaluate and Consummate a Transaction or Other Initiative to Increase the Value of the Company for our Stockholders May Not Be Successful. Should We Consummate a Strategic Transaction or Other Strategic Alternative, There is No Guarantee that Our Stockholders will Realize Greater Value For, or Preserve Existing Value of, Their Shares of the Company.
In February 2008, we announced the engagement of the investment bank BlueLake Partners, LLC for the purpose of assessing strategic alternatives for Ibis, including a potential sale of the Company and/or its assets. We can give no assurance that we will identify an alternative that allows our stockholders to realize an increase in the value of the Company’s stock. We also can give no assurance that a transaction or other strategic alternative, once identified, evaluated and consummated, will provide greater value to our stockholders than that reflected in the current stock price. In fact, if we are unable to recognize revenue through the sale of our products pursuant to our current operating plan, and if we are unable to identify a viable strategic alternative, we may be unable to continue as a going concern. Further, in pursuit of the highest value for shareholders, our Board also may consider alternatives that include a liquidation of our assets and resulting cash distribution per share after payment of outstanding liabilities. In such case, any per-share cash liquidation distribution could be less than the purchase price per share that stockholders may have paid for their Company securities.
We Have Received Only Limited Orders for Our Oxygen Implanter Equipment. If We Are Unable to Secure Additional Orders Within a Reasonable Timeframe in the Future, or Otherwise Obtain Offsetting Revenue, We may Be Unable to Continue to Conduct Operations in a Manner Consistent with Our Current Plan.
Since the Company began selling implanters in 1996, only sold a total of eight Ibis 1000 oxygen implanters have been sold at an average sale price of approximately $4.0 million each and four i2000 oxygen implanters at a selling price between $6.0 and $8.0 million. The most recent order received was in October 2005, for which the Company recognized revenue in the quarter ended September 30, 2006. The Company did not record implanter revenue during 2007 and as of April 10, 2008, the Company does not have an order for an implanter from its customers. At present time, only one of the four major wafer manufacturers is counted as a current prospective customer. Although the sale of one implanter would generally represent a substantial portion of the Company’s annual revenue, there is no expectation to sell more than a limited number of implanters in the near future. The Company currently does not have a backlog for implanters, nor is there visibility to the timing of the next order from any customer. The Company’s failure to obtain any orders for its implanters in the foreseeable future, notwithstanding the belief that sufficient cash remains to support current operations through 2008, or to obtain other sources of offsetting revenue, would have a material adverse impact on the Company’s business and hinder its ability to continue as a going concern. The Company’s inability to generate revenue likely will result in the inability to continue to invest in research development and manufacturing capabilities, hinder the Company’s ability to pursue and maintain relationships, partnerships and alliances with third parties, result in a loss of key personnel by way of workforce reduction or by voluntary termination of employment, limit the Company’s ability to secure financing on acceptable terms, if at all, and create greater volatility in the trading of its common stock.
The Commercial Market for SIMOX-SOI Technology is Still Developing and May Never Fully Develop.
The sources of our revenue have shifted from primarily research and development contracts and sales of SIMOX-SOI wafers for commercial applications to sales and support of oxygen implantation equipment. We are aware of only a few commercial manufacturers that are using SIMOX-SOI wafers in low volume production for a limited number of products. The performance advantages of SIMOX-SOI wafers may never be realized commercially and a commercial market for SIMOX-SOI wafers may never fully develop which in turn would adversely affect the sales of our oxygen implanters. The failure of major semiconductor manufacturers and /or major silicon wafer manufacturers to adopt SIMOX-SOI technology would adversely affect, and may prevent, the adoption of this technology by others.
12
We Have Relied Heavily on Sales to One Customer. We Expect to Rely on Sales to a Limited Number of Customers Which May Cause Sales to Vary Significantly from Quarter to Quarter Causing Our Operating Results to Fluctuate.
We have derived all of our sales of wafer manufacturing equipment from one customer over the last two years. The details for the last three years can be seen in section 1 under the title: Marketing, Sales and Customers in this Annual Report on Form 10-K. Ibis expects that we will continue to rely on a relatively small number of customers as sources of revenue in the foreseeable future. The loss of one or more of these major customers and our failure to obtain other sources of offsetting revenue would have a material adverse impact on our business and hinder our ability to continue as a going concern. In addition, any downturn in these customers’ business or the industry in which these customers operate could result in a significant decrease in any sales of our implanters to these customers, which would have an adverse effect on our business.
We May Need Substantial Additional Capital to Continue Operations in the Future.
The Company’s management believes that it will have sufficient cash resources to support current operations until at least June 2009 with the receipt of the proceeds of approximately $5.3 million from the Company’s sale of common stock and warrants pursuant to a financing agreement made in 2007 with Special Situations Funds. This expectation however, is based on the Company’s current operating plan and general sales outlook, each of which may change rapidly.
We intend to continue to invest in our research, development and manufacturing capabilities. Changes in technology or sales growth beyond currently established capabilities may require further investment. As a result, we may need to raise substantial additional capital in the future. We have previously financed our working capital requirements through:
· equity financings, including warrant and option exercises,
· equipment lines of credit,
· a working capital line of credit,
· a term loan,
· sale-leaseback arrangements,
· collaborative relationships,
· wafer product and equipment sales, and
· government contracts.
There can be no assurance, however, that our actual needs will not exceed expectations or that we will be able to fund our operations on a long-term basis in the absence of other sources. There also can be no assurance that any additional required longer term financing will be available through additional bank borrowings, debt or equity offerings or otherwise, or that if such financing is available, that it will be available on terms acceptable to us. If future financing is not available or is not available on a timely basis or on acceptable terms, we may not be able to fund our future needs, which would seriously harm our business and results of operations and our ability to continue as a going concern. In addition, if we raise additional funds through the sale of equity or convertible debt securities, the value of our common stock outstanding may be diluted. We may also have to issue securities that have rights, preferences and privileges senior to our common stock.
The Company Received Notice from the Nasdaq Stock Market that at April 16, 2008 Nasdaq had not received the Company’s Form 10-K for the fiscal year ended December 31, 2007, as required by Marketplace Rule 4310(c)(14).
On March 31, 2008 the Company requested and received a 15 day extension to file its Annual Report 10K to allow additional time to conduct an impairment analysis with respect to Ibis’ assets, as permitted by Rule 12b-25.
13
On April 16, 2008, the Company received a letter from the Nasdaq Stock Market advising that it had not received the Company’s Form 10-K for the fiscal year ended December 31, 2007, as required by Marketplace Rule 4310(c)(14). Accordingly, unless the Company requests an appeal of this determination, trading of the Company’s common stock will be suspended at the opening of business on April 25, 2008, and a Form 25-NSE will be filed with the Securities and Exchange Commission (the “SEC”), which will remove the Company’s securities from listing and registration on The Nasdaq Stock Market. The Company believes that, as a result of filing this Form 10-K, it is now in compliance with Marketplace Rule 4310(c)(14).
The Company Received Notice from the Nasdaq Stock Market that its Bid Price is Below the Minimum Share Price Requirement.
On December 10, 2007, the Company received a letter from the Nasdaq Stock Market advising that for the previous 30 consecutive business days, the bid price of the Company’s common stock (the “Common Stock”) had closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq Global Market pursuant to Nasdaq Marketplace Rule 4450(a)(5).
Nasdaq stated in its letter that in accordance with Nasdaq Marketplace Rule 4450(e)(2), the Company will be provided 180 calendar days, or until June 9, 2008, to regain compliance with the minimum bid price requirement. The bid price of the Common Stock must close at $1.00 per share or more for a minimum of 10 consecutive business days to achieve compliance.
If the Company does not regain compliance with the minimum bid price requirement by June 9, 2008, the Nasdaq staff will provide the Company with written notification that the Common Stock will be delisted from the Nasdaq Global Market. At that time, the Company may appeal the delisting determination to a Nasdaq Listings Qualifications Panel pursuant to applicable Nasdaq rules. Alternatively, Nasdaq Marketplace Rule 4450(i) may permit the Company to transfer the Common Stock to the Nasdaq Capital Market if the Common Stock satisfies all criteria, other than compliance with the minimum bid price requirement, for initial inclusion on such market. In the event of such a transfer, the Nasdaq Marketplace Rules provide that the Company will be afforded an additional 180 calendar days to comply with the minimum bid price requirement while listed on the Nasdaq Capital Market. If the Company’s common stock does not qualify for, or is subsequently delisted from the Nasdaq Capital Market, investors may have difficulty converting their investment into cash efficiently. The price of our common stock and the ability of holders to sell such stock would be adversely affected, and we would be required to comply with the initial listing requirements to be re-listed on Nasdaq.
The issuance of shares in connection with the February 2007 financing and the exercise of the related warrants will dilute the value of our shares of common stock and could cause the price of our shares of common stock to decline.
Prior to the February 2007 financing, there were 10,915,481 shares outstanding and there were 370,784 shares of common stock reserved for issuance under our equity incentive and stock purchase plans. In connection with the first closing of the two tranche financing, we issued 1,400,000 shares of common stock and warrants to purchase 1,124,434 shares of common stock, including warrants to purchase 74,434 shares of common stock issued to TN Capital Equities, Ltd., which served as placement agent, and its designees. At the second closing, we sold an additional 1,978,377 shares of common stock and warrants to purchase 1,588,966 shares of common stock, including 105,185 to the placement agent and its designees. As of May 22, 2008, Special Situation Funds and its affiliates beneficially own approximately 41% of our capital stock, all of which was purchased in the February 2007 financing.
The exercise of the warrants issued in the February 2007 financing would result in dilution in the value of the shares of the Company’s outstanding common stock and the voting power represented thereby. In addition, the exercise price of the warrants may be lowered under the price adjustment provisions in the event of a “dilutive issuance,” that is, if we issue common stock at any time prior to their maturity as a per share price below such conversion or exercise price, either directly or in connection with the issuance of securities that are convertible into, or exercisable for, shares of our common stock. A reduction in the exercise price may result in the issuance of a significant number of additional shares upon the exercise of the warrants.
14
The warrants do not establish a “floor” that would limit reductions in such conversion price or exercise price. The downward adjustment of the exercise price of these warrants could result in further dilution in the value of the shares of our outstanding common stock and the voting power represented thereby.
No prediction can be made as to the effect, if any, that future sales of shares of our common stock, or the availability of shares for future sale, will have on the market price of our common stock prevailing from time to time. Sales of substantial amounts of shares of our common stock in the public market, or the perception that such sales could occur, may adversely affect the market price of our common stock and may make it more difficult for us to sell our equity securities in the future at a time and price which we deem appropriate.
To the extent the security-holders who have purchased our common shares and the holders of our warrants exercise such securities and then sell the shares of our common stock they receive upon exercise, our stock price may decrease due to the additional amount of shares available in the market. The subsequent sales of these shares could encourage short sales by our security holders and others, which could place further downward pressure on our stock price. Moreover, holders of these warrants may hedge their positions in our common stock by shorting our common stock, which could further adversely affect our stock price.
To the extent the selling security-holders who have purchased our common shares and the holders of our warrants exercise such securities and then sell the shares of our common stock they receive upon exercise, our stock price may decrease due to the additional amount of shares available in the market. The subsequent sales of these shares could encourage short sales by our security-holders and others, which could place further downward pressure on our stock price. Moreover, holders of these warrants may hedge their positions in our common stock by shorting our common stock, which could further adversely affect our stock price.
We Have Significant Losses and May Never Be Able to Sustain Profitability.
We experienced a net loss of $9.2 million in 2005, net income of $0.4 million in 2006 and a net loss of $8.5 million in 2007, respectively. As of December 31, 2007, we had an accumulated deficit of $90.2 million. Net losses may continue for the foreseeable future. Although we have had profitable quarterly operating results from time to time, we may not be able to achieve sustained profitability.
Revenue Recognition and Cash Payments from Customers Depend on a Manufacturing and Customer Qualification and Acceptance Process that is Complex, Lengthy and Costly.
In the semiconductor industry customers regularly require equipment manufacturers to qualify the equipment at the customer’s site. The time required to customer-qualify an implanter at a customer’s site is very difficult to predict because the qualification process for each of our implanters is complex, lengthy and costly and varies depending on the customer’s varying specifications. The manufacturing and qualification process for each implanter requires us to construct and the customer qualify the machine at our premises, disassemble the machine for transportation, and reassemble and re-qualify it at the customer’s premises. During this qualification period, we invest significant resources and dedicate substantial production and technical personnel to achieve acceptance of the implanter. A customer will not accept the implanter until it has successfully produced wafers to exact specifications at the customer’s premises. Even very small differences in the customer’s environment or initially imperceptible changes that may occur to the implanter during the transportation and reassembly of the implanter at the customer’s site can cause a large percentage of wafers produced by the implanter to be rejected, which would delay the acceptance of the implanter by the customer. Historically, we have experienced delays in achieving customer acceptance. Delays or difficulties in our manufacturing and qualification process could increase manufacturing and warranty costs and adversely affect our relationships with our customers. In addition, because we do not recognize revenue on the sale of an implanter until it is delivered and qualified by the customer, any delay in qualification would result in a delay in our ability to recognize revenue from the sale and receipt of final payment. Historically it has taken approximately nine to eighteen months from our receipt of our order to build, ship and obtain customer acceptance of our implanters.
15
We Expect Our Quarterly Revenue and Operating Results to Fluctuate Significantly.
We anticipate that our revenue and operating results are likely to vary significantly from quarter to quarter in the foreseeable future, and it is likely that in future quarters our operating results may from time to time be below the expectations of public market analysts or investors. Our stock price has been volatile and if we fail to meet expectations of public market analysts or investors, the price of our common stock would likely decrease. Further, customers may cancel or revise orders at any time prior to delivery. These ordering patterns most likely will result in significant quarterly fluctuations in our revenue and operating results, and accordingly in our share price. In addition, because we have only sold a limited number of implanters to date on an irregular basis, the recognition of revenue from the sale of even one implanter is likely to result in a significant increase in the revenue for that quarter. A number of other factors, many of which are discussed in more detail in other risk factors, may also cause variations in our results of operations and share price, including:
· lack of orders,
· cancellations of orders and shipment delays and rescheduling,
· new product introductions, which often result in a mismatching of research and development expenses and recognition of revenue, and
· economic conditions and capital spending in the semiconductor industry and in other industries in which our customers operate.
A high percentage of our expenses are essentially fixed in the short term. As a result, if we experience delays in generating and recognizing revenue, our quarterly operating results are likely to be seriously harmed. Due to this, as well as to the cyclicality of the semiconductor industry and other factors, we believe that quarter-to-quarter comparisons of our operating results will not be meaningful. You should not rely on our results for one quarter as any indication of our future performance.
Competitors and Competing Technologies May Render Some or All of Our Products or Future Products Noncompetitive or Obsolete Which Would Result in a Write-down for Impaired or Obsolete Assets.
The semiconductor industry is highly competitive and has been characterized by rapid and significant technological advances. A number of established semiconductor and materials manufacturers, including certain of our customers, have expended significant resources in developing improved wafer substrates. Our competitors or others, many of which have substantially greater financial, technical and other resources than we do, may succeed in developing technologies and products that are equal to or more effective than any which we are developing, which could render our technology obsolete or noncompetitive. In addition to competition from other manufacturers of SOI wafers, we face competition from manufacturers using bulk silicon and epitaxial wafer technology, and compound materials technology such as silicon-germanium, gallium-arsenide and indium phosphide and SOI technology. Although we believe that SIMOX-SOI wafers offer integrated circuit performance advantages, semiconductor manufacturers may develop improvements to existing bulk silicon, epitaxial or strained silicon wafer technology, and competing compound materials or SOI technologies may be more successfully developed, which would eliminate or diminish the performance advantages of SIMOX-SOI wafers which in turn would diminish the demand for our oxygen implanters. Further, in addition to the SIMOX implanter other equipment must be purchased and implemented in order to complete the SIMOX wafer manufacturing process. This other equipment can involve substantial cost that can increase the overall cost of SIMOX-SOI wafers.
If semiconductor manufacturers fail to adopt SIMOX technology during the current or subsequent process cycle (such cycles typically last two to three years), widespread adoption of SIMOX technology may never materialize, our technology may become obsolete and we may be required to recognize an additional material impairment loss in the future.
In addition, although we are aware of no other company manufacturing oxygen implant equipment, other major semiconductor implant equipment manufacturers could develop a less expensive oxygen implanter with superior technology. Our ability to compete with other manufacturers of semiconductor implanters,
16
manufacturers of competing SOI wafers, as well as with bulk silicon, epitaxial, strained silicon and compound materials wafer manufacturers, will depend on numerous factors within and outside our control, including:
· the success and timing of our product introductions and those of our competitors,
· product distribution,
· customer support,
· sufficiency of funding available to us, and
· the price, quality and performance of competing products and technologies.
We Must Continually Improve Existing Products, Design and Sell New Products and Manage the Costs of Research and Development in Order to Compete Effectively.
The semiconductor industry is characterized by rapid technological change, evolving industry standards and continuous improvements in products and required customer specifications. Due to the constant changes in our markets, our future success depends on our ability to improve our manufacturing processes, improve existing products and develop new products. For example, our oxygen implanters must remain competitive on the basis of cost of ownership, process performance and evolving customer needs. To remain competitive we must continually introduce oxygen implanters with higher capacity, better production yields and the ability to process larger wafer sizes.
The commercialization of new products involves, among other requirements, substantial expenditures in research and development, production and marketing. We may be unable to successfully design or manufacture these new products and may have difficulty penetrating new markets. Because it is generally not possible to predict the amount of time required and the costs involved in achieving certain research, development and engineering objectives, actual development costs may exceed budgeted amounts and estimated product development schedules may be extended. Our business may be materially and adversely affected if:
· we are unable to improve our existing products on a timely basis,
· our new products are not introduced on a timely basis,
· we incur budget overruns or delays in our research and development efforts, or
· our new products experience reliability or quality problems.
The Sales Cycle for Our Oxygen Implanter Equipment is Lengthy and Complex and Certain Third Party Licensing Agreements May Cause Significant Delays.
Our customers expend significant efforts in evaluating and qualifying our implanters before they place orders with us. Since we began selling implanters in 1996, we have only sold a total of eight Ibis 1000 oxygen implanters at an average sale price of approximately $4.0 million each and four i2000 oxygen implanters at a selling price between $6.0 and $8.0 million. The sales cycle typically goes from equipment demonstration, equipment specification negotiations, formal quotation, contract negotiations and receipt of order and could take up to one year or longer. In addition, our potential equipment customers that would like to use the MLD process, owned by IBM, to manufacture SIMOX-SOI wafers using our implanters would be required to license this technology directly from IBM. We believe two silicon wafer manufacturers have already licensed this technology from IBM and others have developed their own SIMOX processes. Our potential equipment customers may wish to secure this license prior to giving us an order for equipment and these negotiations between IBM and our customer are beyond our control and no assurances are given that our customers and IBM would come to terms acceptable to both parties in a timely manner, or at all. These MLD process license issues may affect the timing of placement of customer orders in the future if customers plan to license this technology. We do not expect to sell more than a limited number of implanters in the near future. The sale of one implanter would generally represent a substantial portion of our annual revenue. Accordingly, the delay in the receipt of orders, manufacture or delivery of even one unit or the modification, change or cancellation of any such order would have a material adverse effect on our quarterly and annual results of operations.
17
Our Implanters and Associated Technology are Subject to Export Regulations, Which Could Prevent or Delay the Sale of Such Products in Foreign Countries.
Certain technologies associated with our implanters are subject to export regulations administered by the U.S. Department of Commerce. Accordingly, we may be required to secure U.S. export licenses with respect to sales of implanters or transfers of technologies to end users in certain foreign countries. This requirement could result in significant delays in, or the prevention of, sales of implanters or transfers of technology or other such technical data to customers in certain foreign countries. For example, the sale of an Ibis 1000 implanter and the corresponding transfer of technology to Simgui required an export license which took approximately one year to secure. There can be no assurance that if necessary, we will be able to secure such licenses in the future in a timely manner, or at all.
The Loss of Key Members of Our Scientific and Management Staff Could Delay and May Prevent the Achievement of Our Research, Development and Business Objectives.
Our President and Chief Executive Officer, Martin J. Reid, and other current officers and key members of our scientific staff are responsible for areas such as product development and improvements, and process improvement research, which are important to our specialized scientific business. The loss of, and failure to promptly replace, any member of this group could significantly delay and may prevent the achievement of our research, development and business objectives. While we have entered into an employment agreement with our Chairman, under certain circumstances he may be able to terminate his employment with us. Furthermore, although our employees are subject to certain confidentiality and non-competition obligations, our key personnel may terminate their employment at any time and may become employed by a competitor. The current composition of Ibis management and of its board of directors is subject to change and should not be unduly relied upon.
Changes in Accounting Standards Regarding Stock Option Plans Could Limit the Desirability of Granting Stock Options, Which Could Harm Our Ability to Attract and Retain Employees, and Have Also Negatively Impacted Our Results of Operations.
On December 2004, the Financial Accounting Standards Board issued FASB Statement No. 123R, Share Based Payment, which requires all companies to treat the fair value of stock options granted to employees as an expense. As a result of this standard, effective for periods beginning after January 1, 2006, we and other companies are required to record a compensation expense equal to the fair value of each stock option granted. This amounted to an expense of $0.4 million in each of the fiscal years ending December 31, 2006 and December 31, 2007. FAS 123(R) requirements reduce the attractiveness of granting stock options because of the additional expense associated with these grants, which have impacted and will continue to negatively impact our results of operations. Nevertheless, stock options are an important employee recruitment and retention tool, and we may not be able to attract and retain key personnel if we reduce the scope of our employee stock option program. In addition, this accounting standard could negatively impact our ability to use stock options as an employee recruitment and retention tool in the future.
We May Not Be Able to Successfully Produce Our Products on a Large-Scale.
We have limited manufacturing experience and have only manufactured limited quantities of oxygen implanters. To be successful, our products must be manufactured in commercial quantities, at acceptable costs. We may not be able to make the transition to high volume commercial production successfully. Future production in commercial quantities may create technical and financial challenges for us. Any difficulty or delay in constructing additional implanters, if needed, could have a material adverse effect on our business.
18
Our Latest Products Have Not Been Used in Large-Scale Long-Term Production and Consequently They May Not Be Able to Perform at the Availability Levels Expected for Continuous (7 Days per Week / 24 Hours per Day) Operation. (and May Be Subject to Unknown and Undetected Hardware or Software Failures).
Semiconductor equipment is subject to stringent mean time between failure (MBTF) and similar quality and reliability requirements. Although our equipment has previously been used in our own production environment for manufacturing SIMOX SOI wafers, and has been used in limited manufacturing runs in our customers sites, as our equipment is exposed to long-term extended production processing on an automated basis, previously unknown and undetected hardware or software failures or combinations thereof may occur. Such failures could adversely affect our relationship with our customers.
We May Not Be Able to Use All of Our Existing or Future Manufacturing Capacity at a Profitable Level.
At times we may have the capacity to produce more oxygen implantation machines than we have orders for at such times. During such idle time we would continue to be responsible for the fixed costs of our facility and maintaining personnel, which could have a material adverse effect on our business.
We May Not Successfully Form or Maintain Desirable Strategic Alliances.
We believe we will need to form or maintain alliances with strategic partners for the manufacturing, marketing and distribution of our products. We may enter into these strategic alliances to satisfy customer demand and to address possible customer concerns regarding our being a sole source supplier. The limited number of reliable sources of supply other than Ibis may adversely affect or delay the integration of SIMOX-SOI wafers in mainstream commercial applications. We may not be successful in maintaining alliances or in forming and maintaining other alliances, including satisfying our contractual obligations with our strategic partners, and our partners may not devote adequate resources to manufacture, market and distribute these products successfully or may attempt to compete with us.
We May Have Difficulty Obtaining the Materials and Components Needed to Produce Our Products, and At Least One Major Component Has Only One Source.
Ibis manufactures its oxygen implanters from standard components and from components manufactured in-house or by other vendors according to our design specifications. Most raw materials and components not produced by us are available from more than one supplier. However, certain raw materials, components and subassemblies are obtained from a limited group of suppliers and at least one major component has a sole source. If we are unable to obtain such materials and components on a timely basis and on acceptable terms, if at all, our ability to complete orders could be significantly delayed and our business and results from operations could be materially and adversely affected. Semiconductor equipment is a growth industry and is very cyclical in nature, so if our suppliers experience an increase in demand from other semiconductor equipment manufacturers with much higher volumes than us, the lead-time and/or price for some of our components may increase. Although we have sought to reduce our dependence on these limited source suppliers and we have not experienced significant production delays due to unavailability or delay in procurement of component parts or raw materials to date, increased market demand for materials from, or disruption or termination of, certain of these sources could occur and such increased demand, disruptions, or termination could have a material adverse effect on our business and results of operations.
We May Not Be Able to Protect Our Patents and Proprietary Technology.
Our ability to compete effectively with other companies will depend, in part, on our ability to maintain the proprietary nature of our technology. Although we have been awarded or have filed applications for a number of patents in the U.S. and foreign countries, those patents may not provide meaningful protection, or pending patents may not be issued. As part of the charge for impairment of assets in 2007, we wrote-down $0.3 million in patent expenditures previously carried on the balance sheet. Our competitors in both the U.S. and foreign countries, many of which have substantially greater resources and have made substantial investments in competing technologies, may have or may obtain patents that will prevent, limit or interfere with our ability to make and sell our products or infringe on our patents. The defense and prosecution of patent suits is both costly and time-consuming, even if the outcome is favorable to us. In addition, there is an inherent
19
unpredictability regarding obtaining and enforcing patents. An adverse outcome in the defense of a patent suit could:
· subject us to significant liabilities to third parties,
· require disputed rights to be licensed from third parties, or
· require us to cease selling our products.
We also rely in large part on unpatented proprietary technology and others, including strategic partners, may independently develop the same or similar technology or otherwise obtain access to our proprietary technology. To protect our rights in these areas, we currently require all of our employees to enter into confidentiality agreements. However, these agreements may not provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use, misappropriation or disclosure of such trade secrets, know-how or other proprietary information.
Others may claim that our technology infringes on their proprietary rights. Any infringement claims, even if without merit, can be time consuming and expensive to defend and may divert management’s attention and resources. If successful, they could also require us to enter into costly royalty or licensing agreements. A successful claim of product infringement against us and our inability to license the infringed or similar technology could adversely affect our business.
If We Do Not Comply With All Applicable Environmental Regulations, We May be Subject to Fines and Other Sanctions.
We are subject to a variety of federal, state and local environmental regulations related to the storage, treatment, discharge or disposal of chemicals used in our operations and to the exposure of our personnel to occupational hazards. Although we believe that we have all permits necessary to conduct our business, the failure to comply with present or future regulations could result in fines being imposed on us, suspension of production or a cessation of operations. Our future activities may result in our being subject to additional regulations. Such regulations could require us to acquire significant equipment or to incur other substantial expenses to comply with regulations. Our failure to control the use of, or to restrict adequately the discharge of, hazardous substances or properly control other occupational hazards could subject us to substantial financial liabilities.
Our Stock Price is Highly Volatile.
The market prices for securities of high tech companies have been volatile. This volatility has significantly affected the market prices for these securities for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our common stock. The market price for our common stock has fluctuated significantly. Since January 1, 1999, our stock price has fluctuated from a high of $135.00 to a low of $0.25. It is likely that the market price of our stock will continue to fluctuate in the future. Events or factors that may have a significant impact on our business and on the market price of our common stock include the following:
· quarterly fluctuations in operating results,
· difficulty in forecasting future results,
· announcements by us or our present or potential competitors,
· technological innovations or new commercial products or services by us or our competitors,
· the timing of receipt of orders and / or customer acceptance from major customers,
· product mix,
· product obsolescence,
· shifts in customer demand,
· our ability to manufacture and ship products on a cost-effective and timely basis,
· market acceptance of new and enhanced versions of our implanters,
· the evolving and unpredictable nature of the markets for the products incorporating SIMOX-SOI wafers,
20
· the amount of research and development expenses associated with new or enhanced products or implanters
· the cyclical nature of the semiconductor industry, and its current negative outlook
· general market conditions.
Concentrated Ownership of Shares by One Shareholder and Its Affiliates Could Affect the Price of Our Common Stock.
As of May 22, 2008, our largest known shareholders include Special Situations Fund III QP, L.P and affiliates (“SSF”), which own an aggregate of 3,378,377 shares, or approximately 24% of our outstanding shares, as well as warrants to purchase an additional 2,533,781 shares. At present, SSF’s ownership may have the effect of delaying, deferring or preventing a change in control of our company, or may directly or indirectly effect a change in control of our company. Moreover, the disposition by SSF of a substantial amount of its shares of our common stock in the open market could have an adverse impact on the market for our common stock.
Future Issuances of Preferred Stock May Diminish the Rights of Our Common Stockholders.
Our board of directors has the authority to approve the issue of up to 2.0 million shares of preferred stock and to determine the price, rights, privileges and other terms of these shares. The board of directors may exercise this authority without the approval of the stockholders. The rights of the holders of common stock may be adversely affected by the rights of the holders of any preferred stock that may be issued in the future.
Anti-takeover Provisions in Our Charter and Bylaws and Provisions of Massachusetts Law Could Make an Acquisition of the Business by a Third-Party Difficult.
Our restated articles of organization, as amended and restated bylaws and the Massachusetts Business Corporation Law contain certain provisions that may make a third-party acquisition of us difficult, including:
· a classified board of directors, with three classes of directors each serving a staggered three-year term,
· the ability of the board of directors to issue preferred stock, and
· a 75% super-majority shareholder vote to amend certain provisions of our articles of organization and bylaws.
Limitations on Effectiveness of Controls.
The Company’s management, including the Chief Executive Officer and President and the Chief Financial Officer, does not expect that our internal controls will prevent all errors and intentional misrepresentations. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and no assurance can be given that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or intentional conduct may occur and not be detected.
21
If We Fail to Maintain an Effective System of Internal Control over Financial Reporting and Disclosure Controls and Procedures, We May Be Unable to Accurately Report our Financial Results and Comply with the Reporting Requirements Under the Exchange Act.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”), we are required to include our management’s report on internal control over financial reporting beginning with our annual report on Form 10-K for the fiscal year ending December 31, 2007. Accordingly, we have completed our compliance for management’s report and management has concluded that the Company did not have effective controls in its financial reporting process as of December 31, 2007. A discussion of the material weaknesses that have been identified can be found in Item 9A, Disclosure Controls and Procedures, together with the Company’s remediation plan. Although we believe that the consolidated financial statements included in this Form 10-K present fairly, in all material respects, our financial position, results of operations and cash flow for the periods presented in conformity with GAAP, and we are taking the remedial steps described in Item 9A with respect to the identified material weaknesses, we cannot assure you that additional material weaknesses in our internal control over financial reporting will not be identified in the future. Any further inability on the part of the Company to maintain effective internal controls over financial reporting could, among other things, cause us to file our periodic reports with the SEC in an untimely manner, require us to incur additional costs, divert management resources or result in a material misstatement of the Company’s annual or interim financial statements that would not be prevented or detected on a timely basis.
Compliance with the requirements of Section 404 is expensive and time-consuming. If we fail to complete this evaluation in a timely manner, we could be subject to regulatory scrutiny and a loss of public confidence in our internal control over financial reporting. In addition, any failure to establish and maintain an effective system of disclosure controls and procedures could cause our current and potential stockholders and customers to lose confidence in our financial reporting and disclosure required under the Exchange Act, which could adversely affect our business.
Evolving Regulation of Corporate Governance and Public Disclosure May Result in Additional Expenses and Continuing Uncertainty.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and related SEC regulations as well as the listing standards of the NASDAQ Stock Market, are creating uncertainty for public companies. We continually evaluate and monitor developments with respect to new and proposed rules and cannot predict or estimate the amount of the additional costs we may incur or the timing of such costs. These new or changed laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We are committed to maintaining high standards of corporate governance and public disclosure. As a result, we have invested resources to comply with evolving laws, regulations and standards. This investment may result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and we may be harmed.
Item 2. PROPERTIES
Ibis’ corporate office and manufacturing site are located at a leased facility in Danvers, Massachusetts. The equipment manufacturing business and the engineering and wafer processing research and development efforts are housed in approximately 32,000 square feet which includes a modernized clean-room that contains metrology equipment, cleaning equipment and an implantation equipment manufacturing and service area. The lease on the space we currently occupy was renewed during 2006 and will expire on June 30, 2011, and contains an option to renew for five years.
Item 3. LEGAL PROCEEDINGS
On April 26, 2007, the United Stares District Court in the District of Massachusetts approved a settlement agreement in the consolidated securities class action captioned In re Ibis Technology Securities Litigation, Civil Action No. 04-10446 RCL (D. Mass.). The Company and its Chairman of the Board, Martin J. Reid, and the plaintiffs reached an agreement in principle to settle the securities class action, which the Company announced on October 2, 2006. Thereafter, the parties executed a formal settlement agreement and submitted it for Court approval. The settlement provided for a payment to the plaintiffs of $1.9 million. The Court’s April 26, 2007 Order, certified the proposed class for settlement purposes only, approved the parties’ settlement agreement, and dismissed the securities class action with prejudice and in its entirety. The settlement was funded entirely by the Company’s insurance carrier and did not have a material adverse affect on our business, results of operations and financial condition.
On April 10, 2007, the Company announced that the shareholder derivative action filed in February 2004 against certain of the Company’s directors and officers: Louis F. Matheson, Jr. v. Martin J. Reid et al., Civ. Act. No. 04-10341 (RCL) (D. Mass) had been voluntarily dismissed by the plaintiff. The notice of dismissal filed by the plaintiff dismissed the action with prejudice. As set forth in the notice, the plaintiff received a $55,000 payment following the dismissal in consideration of plaintiff’s analysis of information provided by the Company regarding, among other things, the Company’s contention that the litigation should be dismissed for failure to make a demand on the Board of Directors before filing suit as required by Massachusetts law. The payment was funded entirely by the Company’s insurance carrier.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted to stockholders during the fourth quarter of the year ended December 31, 2007.
22
PART II
Item 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Ibis’ Common Stock began trading on May 20, 1994 on the Nasdaq SmallCap Market and on the Boston Stock Exchange. Prior to May 20, 1994, there was no public market for the Common Stock or any other securities of Ibis. On April 4, 1996, Ibis commenced trading on the Nasdaq National Market and is currently trading on the Nasdaq Global Market. Our Common Stock is traded under the symbol “IBIS.” The following table sets forth, for the periods so indicated the high and low sale prices for the Common Stock as reported by the Nasdaq National Market or Nasdaq Global Market, as applicable.
|
| Common Stock |
| ||||
|
| High |
| Low |
| ||
|
|
|
|
|
| ||
2006: |
|
|
|
|
| ||
First Quarter |
| $ | 4.50 |
| $ | 2.58 |
|
Second Quarter |
| $ | 3.78 |
| $ | 2.20 |
|
Third Quarter |
| $ | 3.65 |
| $ | 1.99 |
|
Fourth Quarter |
| $ | 3.56 |
| $ | 1.32 |
|
2007: |
|
|
|
|
| ||
First Quarter |
| $ | 1.86 |
| $ | 1.28 |
|
Second Quarter |
| $ | 1.65 |
| $ | 1.29 |
|
Third Quarter |
| $ | 1.54 |
| $ | 0.96 |
|
Fourth Quarter |
| $ | 1.35 |
| $ | 0.25 |
|
23
Stock Performance Graph
The following graph compares the annual cumulative total stockholders return (assuming reinvestment of dividends) from investing $100 on December 31, 2001 and plotted at the end of each fiscal year thereafter, in each of (i) the Company’s Common Stock, (ii) the Nasdaq Stock Market, and (iii) the Media General Financial Services SIC Code Index 3674-Semiconductors, Related Devices-which consists of other companies on the Common Stock, and no dividends are included in the representation of the Company’s performance. The stock price performance on the graph below is not necessarily indicative of future price performance.
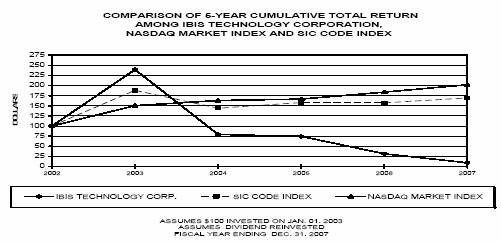
COMPARISON OF CUMULATIVE TOTAL RETURN OF ONE OR MORE
COMPANIES, PEER GROUPS, INDUSTRY INDEXES AND/OR BROAD MARKETS
FISCAL YEAR ENDING
COMPANY/INDEX/MARKET |
| 12/31/02 |
| 12/31/03 |
| 12/31/04 |
| 12/30/05 |
| 12/29/06 |
| 12/31/07 |
| ||||||
Ibis Technology Corp |
| $ | 100.00 |
| $ | 239.36 |
| $ | 79.15 |
| $ | 74.47 |
| $ | 31.49 |
| $ | 9.15 |
|
Semiconductors, Related Device |
| $ | 100.00 |
| $ | 188.19 |
| $ | 144.02 |
| $ | 157.87 |
| $ | 157.25 |
| $ | 169.42 |
|
NASDAQ Market Index |
| $ | 100.00 |
| $ | 150.36 |
| $ | 163.00 |
| $ | 166.58 |
| $ | 183.68 |
| $ | 201.91 |
|
The Graph is not “soliciting material” under Regulation 14A or 14C of the rules promulgated under the Securities Exchange Act of 1934, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. Information used in the graph was obtained from Hemscott, Inc., a source believed to be reliable, but the company is not responsible for any errors or omissions in such information
24
Stockholders
As of May 22, 2008, there were approximately 144 stockholders of record of the 14,317,482 outstanding shares of Common Stock and approximately 4,071 beneficial owners of the Common Stock.
Dividends
Ibis has never declared or paid any dividends and does not anticipate paying such dividends on its Common Stock in the foreseeable future. Ibis currently intends to retain any future earnings for use in its business. The payment of any future dividends will be determined by the Board of Directors in light of conditions then existing, including our financial condition and requirements, future prospects, restrictions in financing agreements, business conditions and other factors deemed relevant by the Board of Directors.
25
Item 6. SELECTED FINANCIAL DATA
The selected financial data presented below under the captions “Statement of Operations Data” and “Balance Sheet Data” for, and as of the end of each of the years in the five-year period ended December 31, 2007, are derived from the financial statements of Ibis, which have been audited by KPMG LLP, independent registered public accounting firm. The audited balance sheets at December 31, 2006 and 2007 and the related statements of operations, stockholders equity and cash flows for each of the years in the three-year period ended December 31, 2007 and the independent registered public accounting firm’s report thereon, are included elsewhere in this Annual Report on Form 10-K. The data set forth below should be read in conjunction with Ibis’ financial statements, the related notes thereto and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Annual Report on Form 10-K. The historical results are not necessarily indicative of the operating results to be expected in the future.
|
| Years Ended December 31, |
| |||||||||||||
|
| 2003 |
| 2004 |
| 2005 |
| 2006 |
| 2007 |
| |||||
|
| (In thousands, except for per share data) |
| |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
|
|
| |||||
License and other revenue |
| $ | 660 |
| $ | 391 |
| $ | 324 |
| $ | 560 |
| $ | 426 |
|
Equipment revenue |
| 8,782 |
| 7,535 |
| 278 |
| 13,427 |
| 525 |
| |||||
Total revenue (2) |
| 9,442 |
| 7,926 |
| 602 |
| 13,987 |
| 951 |
| |||||
Cost of license and other revenue |
| 45 |
| 15 |
| — |
| — |
| — |
| |||||
Cost of equipment revenue |
| 4,331 |
| 4,722 |
| 750 |
| 5,893 |
| 2,214 |
| |||||
Total cost of revenue |
| 4,376 |
| 4,737 |
| 750 |
| 5,893 |
| 2,214 |
| |||||
Gross profit (loss) |
| 5,066 |
| 3,189 |
| (148 | ) | 8,094 |
| (1,263 | ) | |||||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
| |||||
General and administrative |
| 2,337 |
| 2,221 |
| 2,217 |
| 2,401 |
| 2,005 |
| |||||
Marketing and selling |
| 1,236 |
| 1,521 |
| 1,319 |
| 1,096 |
| 578 |
| |||||
Research and development |
| 5,381 |
| 5,329 |
| 5,993 |
| 5,396 |
| 3,789 |
| |||||
Impairment of long lived assets |
| — |
| — |
| — |
| — |
| 1,603 |
| |||||
Total operating expenses |
| 8,954 |
| 9,071 |
| 9,529 |
| 8,893 |
| 7,975 |
| |||||
Income (loss) from operations |
| (3,888 | ) | (5,882 | ) | (9,677 | ) | (799 | ) | (9,238 | ) | |||||
Total other income |
| 27 |
| 242 |
| 218 |
| 1,205 |
| 699 |
| |||||
Income (loss) before income taxes |
| (3,861 | ) | (5,640 | ) | (9,459 | ) | 406 |
| (8,539 | ) | |||||
Income tax expense (benefit) |
| (8 | ) | 1 |
| 1 |
| 1 |
| 1 |
| |||||
Income (loss) from continuing operations |
| (3,853 | ) | (5,641 | ) | (9,460 | ) | 405 |
| (8,540 | ) | |||||
Discontinued operations (2): |
|
|
|
|
|
|
|
|
|
|
| |||||
Loss from discontinued operations |
| (17,597 | ) | (3,179 | ) | — |
| — |
| — |
| |||||
Loss on disposal |
| — |
| (2,099 | ) | 215 |
| — |
| — |
| |||||
Income (loss) from discontinued operations |
| (17,597 | ) | (5,278 | ) | 215 |
| — |
| — |
| |||||
Net income (loss) |
| $ | (21,450 | ) | $ | (10,919 | ) | $ | (9,245 | ) | $ | 405 |
| $ | (8,540 | ) |
Income (loss) from continuing operations per common share (1) |
| $ | (0.40 | ) | $ | (0.53 | ) | $ | (0.88 | ) | $ | 0.04 |
| $ | (0.64 | ) |
Net Income (loss) per common share (1) |
| $ | (2.21 | ) | $ | (1.02 | ) | $ | (0.86 | ) | $ | 0.04 |
| $ | (0.64 | ) |
Weighted average common shares outstanding |
| 9,728 |
| 10,666 |
| 10,738 |
| 10,853 |
| 13,411 |
| |||||
Weighted average common shares outstanding basic |
| 9,728 |
| 10,666 |
| 10,738 |
| 10,980 |
| 13,411 |
| |||||
|
| As of December 31, |
| |||||||||||||
|
| 2003 |
| 2004 |
| 2005 |
| 2006 |
| 2007 |
| |||||
|
| (In thousands) |
| |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
Working capital |
| $ | 12,607 |
| $ | 12,415 |
| $ | 5,145 |
| $ | 7,494 |
| $ | 7,304 |
|
Total assets |
| 35,343 |
| 22,283 |
| 19,992 |
| 13,789 |
| 9,763 |
| |||||
Long-term debt, less current portion |
| — |
| — |
| — |
| — |
| — |
| |||||
Total liabilities |
| 4,226 |
| 1,863 |
| 8,695 |
| 1,534 |
| 732 |
| |||||
Shareholders’ equity |
| 31,117 |
| 20,420 |
| 11,297 |
| 12,255 |
| 9,031 |
| |||||
26
(1) Computed on the basis described for net earnings (loss) per common share in Note 2(g) of Notes to Financial Statements.
(2) Historically, much of the Company’s revenue was derived from research and development contracts and sales of wafers for military and commercial applications. In mid-2004, we discontinued the wafer manufacturing portion of our business in order to focus exclusively on our equipment business. Wafer product sales, as well as wafer revenue reported in subsequent periods, are reported net of associated costs, as loss from discontinued operations or other income after 2005. We will maintain a research and development effort relating to wafers for our equipment improvement programs. This revision to our business strategy could materially affect the comparability of the information reflected in the above selected financial data.
27
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with the Financial Statements of Ibis (including Notes thereto) and Selected Financial Data included elsewhere in this Annual Report on Form 10-K.
OVERVIEW
Ibis Technology Corporation (“Ibis”) was formed in October 1987 and commenced operations in January 1988. Ibis’ initial activities consisted of producing and selling SIMOX-SOI wafers and conducting research and development activities. This effort led to the development of a proprietary oxygen implanter, the Ibis 1000, which we began selling in 1996, the next generation implanter, the i2000ä, which we began selling in 2002, and also to other proprietary process technology.
Initially, much of our revenue was derived from research and development contracts and sales of wafers for military applications. Over the years, the Company decided to focus its business operations and sales strategy on the manufacture and sale of our implanter equipment products and de-emphasized the sale of wafers given that, among other things, we believed that the wafer manufacturing companies were in the best position to manufacture SIMOX-SOI wafers using our implanter equipment in light of their expertise and operating efficiencies. As we announced on July 21, 2004, we exited the wafer manufacturing business. Wafer product sales, as well as wafer revenue reported in subsequent periods, are reported net of associated costs, as loss from discontinued operations or other income after 2005 on our income statement. The Company believes that its decision to discontinue the wafer business permits broader strategic collaboration efforts between Ibis and the wafer manufacturers. We reach this conclusion for a number of reasons. First, we believe that tremendous price pressure exists on commodity type products, such as silicon wafers, and this pressure is already eroding future price expectations of SOI wafers. Because the starting wafer represents a significant component of the SOI wafer cost, we believe that silicon wafer manufacturers should have a natural cost structure advantage leading to a higher gross margin, and therefore should be able to manage such price pressure better than stand-alone SOI producers that do not also produce the silicon wafer itself. Second, we expect that the price pressure will encourage silicon wafer manufacturers to seek out higher margin products, like SOI wafers, to increase their margins. Third, we believe that silicon wafer manufacturers have traditionally developed proprietary intellectual property in silicon materials science, which can be applied to designing optimal starting wafers for SOI production. We believe that this should give them an advantage in both minimizing wafer cost and maximizing SOI wafer quality and yield. Fourth, our experience suggests that silicon wafer manufacturers already have a well-developed infrastructure for manufacturing, sale and marketing of large volumes of substrates. Lastly, we believe that there is greater efficiency in producing the SOI wafer as part of the wafer manufacturers existing product flow, specifically avoiding the need to repackage, re-clean, re-inspect and re-ship substrates twice, once as starting silicon wafers, and a second time as SOI wafers. Therefore, as a result of these trends, we expect our ultimate customers will be drawn from these silicon wafer manufacturers and we plan to focus a majority of our technical and marketing resources on the sale of implanters to the leading silicon wafer manufacturers and our key customers in the semiconductor industry who we believe are the leaders in the adoption of SOI technology. We expect that implanter sales to chipmakers should be minimal, and that these sales will be focused on SOI processes that the chipmaker wishes to keep proprietary, such as selective (or patterned) SIMOX, or other specialty substrates.
Our fundamental SIMOX-SOI technology has been developed, refined, and tested over the last dozen years. In 2002, we introduced the current generation of SIMOX-SOI technology, which included our second-generation oxygen implanter (i2000ä) and the MLD wafer process which was licensed to us by IBM. We believe that the i2000’s flexibility, automation and operator-friendly controls allow this tool to produce a wide range of SIMOX-SOI wafer products using a range of manufacturing processes, including Advantox® MLD and Advantox MLD-UT wafers. We also believe the ability of the i2000 implanter to produce twelve-inch (or 300 mm) SIMOX-SOI wafers coupled with the MLD process positions us to capitalize on the growing SOI market. In 1999, we commenced a program to design and develop the i2000, introduced it in March 2002 and began shipping 300 mm wafers implanted from this machine shortly thereafter. Customers who purchase the i2000 can utilize more than one SIMOX wafer manufacturing process on the implanter including the IBM
28
IBIS TECHNOLOGY CORPORATION
PART I - ITEM 2
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATION
MLD process, when licensed, as well as other SIMOX-SOI wafer manufacturing processes that do not require the IBM license.
In their report dated May 22, 2008, our registered public accounting firm stated that our financial statements for the fiscal year ended December 31, 2007 were prepared assuming we would continue as a going concern; however, the Company’s recurring losses and accumulated deficit raise substantial doubts about its ability to continue as a going concern. The Company’s ability to continue as a going concern is an issue raised as a result of our recurring losses from operations and net capital deficiency and is subject to our ability to secure additional orders of our implanter equipment or, in the alternative, identify and consummate a strategic transaction, including the potential a sale of the Company, or other strategic alternative.
Since we began selling implanters in 1996, we have only sold a total of eight Ibis 1000 oxygen implanters at an average sale price of approximately $4.0 million each and four i2000 oxygen implanters at a selling price between $6.0 and $8.0 million. Because we have sold only a limited number of implanters to date on an irregular basis, the recognition of revenue from the sale of even one implanter is likely to result in a significant increase in the revenue during that quarter. We recognize implanter revenue in accordance with SAB 104, which includes, among other criteria, the shipment and final customer acceptance of the implanter at the customer’s location. As a result, deferral of implanter revenue will be recorded on our balance sheet until the Company is able to meet these criteria The most recent order received was in October 2005, for which the Company recognized revenue in the quarter ended September 30, 2006. The Company did not record implanter revenue during 2007 and as of April 10, 2008, does not have an order for an implanter from its customers. At present time, only one of the four major wafer manufacturers are a current prospective customer. The Company currently does not have a backlog for implanters, nor does it have visibility to the timing of the next order from any customer. In the fourth quarter of 2007, we recorded an obsolescence charge to inventory reflecting the current lack of marketability for the Company’s implanters. If we are unable to sell the implanters through a normal sales process to our customers, the Company may seek to find alternative methods to monetize the inventory.
In February 2008, the Company announced the engagement of the investment bank BlueLake Partners, LLC for the purpose of assessing strategic alternatives for Ibis, including a potential sale of the Company and/or its assets. No decision to date has been made as to whether Ibis will engage in a transaction or transactions resulting from its consideration of strategic alternatives, and no assurance can be given that any transaction or transactions will occur or, if undertaken, the terms or timing of such a transaction.
Critical Accounting Policies
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that have a significant impact on the results we report in our financial statements. Some of our accounting policies require us to make difficult and subjective judgments, often as a result of the need to make estimates on matters that are inherently uncertain. Our most critical accounting policies include: revenue recognition, inventory valuation and reserves, and the assessment of long-lived asset impairment. Actual results may differ from these estimates under different assumptions or conditions. Below, we discuss these policies further, as well as the estimates and judgments involved.
Revenue Recognition. We recognize revenue from equipment sales and the sales of spare parts when all of the following criteria have been met: (1) evidence exists that the customer is bound to the transaction; (2) the product has been delivered to the customer and, when applicable, the product has been installed and accepted
29
by the customer; (3) the sales price to the customer has been fixed or is determinable; and (4) collectibility of the sale price is reasonably assured. We recognize revenue from implanter sales upon acceptance at the customer’s site. Revenue derived from contracts and services is recognized upon performance. Significant management judgments and estimates must be made and used in connection with revenue recognized in any period. Management analyzes various factors, including a review of specific transactions, historical experience, credit worthiness of customers and current market and economic conditions. Changes in judgment based upon these factors could impact the timing and amount of revenue and cost recognized.
Inventory Valuation and Reserves. Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out (“FIFO”) cost method. Our policy for the valuation of inventory, including the determination of obsolete or excess inventory, requires us to forecast the future demand for our products within specific time horizons, generally twelve months or less. If our forecasted demand for specific products is greater than actual demand and we fail to reduce manufacturing output accordingly, we could be required to record additional inventory reserves, which would have a negative impact on our gross margin. For the year ended December 31, 2007, the Company recorded a $1.8 million increase in reserves charged to cost of goods for potential obsolescence of inventory. We also reserve for obsolescence when engineering changes or other technological advances indicate that obsolescence has occurred.
Valuation of Long-Lived Assets. Ibis reviews the valuation of long-lived assets, including property and equipment and licenses, under the provisions of SFAS No. 144, Accounting for Impairment or Disposal of Long-Lived Assets. Management is required to assess the recoverability of its long-lived assets or asset groups whenever events and circumstances indicate that the carrying value may not be recoverable. Based on current conditions, factors we consider important and that could trigger an impairment review include the following:
· Significant underperformance relative to expected historical or projected future operating results,
· Significant changes in the manner of our use of the acquired assets or the strategy of our overall business,
· Significant negative industry or economic trends,
· Significant decline in our stock price for a sustained period, and
· Our market capitalization relative to book value.
In accordance with SFAS No. 144, when the Company determine that the carrying value of applicable long-lived assets may not be recoverable based upon the existence of one or more of the above indicators of impairment, an evaluation takes place regarding whether the carrying amount of the asset exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of that asset or asset group. If such a circumstance exists, the Company would measure an impairment loss to the extent the carrying amount of the particular long-lived asset or group exceeds its fair value. The Company would determine the fair value based on either a projected discounted cash flow method using a discount rate determined by our management to be commensurate with the risk inherent in our current business model, or estimated fair value based on comparable sales prices for similar used equipment. We recorded an impairment loss on certain long-lived assets used in our manufacturing process, as well as certain of our patents for the fourth quarter of 2007 of $1.6 million.
Results of Operations
Fiscal Year Ended December 31, 2007 Compared to Fiscal Year Ended December 31, 2006
License and Other Revenue. License and other revenue for the fiscal year ended December 31, 2007 was $0.5 million compared to $0.6 million for the fiscal year ended December 31, 2006, a decrease of $0.1 million,
30
or 24%. This decrease is primarily attributable to a new sublicense agreement valued at approximately $0.2 million related to equipment technology in the year ended December 31, 2006.
Equipment Revenue. Equipment revenue represents revenue recognized from the sale of implanters, spare parts and field service revenue. Equipment revenue decreased to $0.5 million for the fiscal year ended December 31, 2007 from $13.4 million for the fiscal year ended December 31, 2006, a decrease of $12.9 million. Equipment revenue in 2006 included revenue recognized for two i2000 implanters for $13.0 million as compared to no implanter revenue recognition in 2007. Field service revenue accounted for $0.2 million, or 37% of equipment revenue for the fiscal year ended December 31, 2007 as compared to $0.3 million, or 2% of equipment revenue for the fiscal year ended December 31, 2006. Sales of spare parts accounted for $0.3 million, or 63% of equipment revenue for the fiscal year ended December 31, 2007 as compared to $0.1 million, or 1% of equipment revenue for the fiscal year ended December 31, 2006. Sales of spare parts fluctuate depending on customer demand and when the warranties expire on individual pieces of equipment. Warranty expense is calculated on our anticipated replacement costs for equipment accepted by our customers over a one or two year contract period.
Total Sales and Revenue. Total sales and revenue for the fiscal year ended December 31, 2007 was approximately $1.0 million, a decrease of $13.0 million, from $14.0 million for the fiscal year ended December 31, 2006. The decrease is due to revenue recognition for two i2000 implanters of $13.0 million for the fiscal year ended December 31, 2006 along with decreased license revenue of $0.1 million and decreased service revenue of $0.1 million which was offset by an increase in spare parts revenue of $0.2 million for the fiscal year ended December 31, 2007.
Total Cost of Sales and Revenue. Cost of license and other revenue consists of labor and materials expended during the year. There were no license costs or costs for other revenue during the period.
Cost of equipment revenue represents the cost of equipment including manufacturing labor, the cost for spare parts, and the cost of labor incurred for field service. Cost of equipment revenue also includes $1.8 million for the increase in inventory reserves for potential obsolescence of the Company’s inventory. Cost of equipment revenue for the fiscal year ended December 31, 2007 was $2.2 million, as compared to $5.9 million for the fiscal year ended December 31, 2006, a decrease of $3.7 million. This decrease of $3.7 million was due to the costs associated with the recognition of revenue for the two i2000 implanters during the year ended December 31, 2006, offset by the increase in inventory reserves in the year ended December 31, 2007.
The gross margin for all sales was a negative 133% for the fiscal year ended December 31, 2007, as compared to a positive gross margin of 58% for the fiscal year ended December 31, 2006. The decrease in the gross margin for all sales is attributable to the approximate gross margin of 55% for the two i2000 implanter sales recognized in the fiscal year ended December 31, 2006, the inventory reserve increase of $1.8 million in the fiscal year ended December 31, 2007 for inventory obsolescence and $0.6 million reduction in cost from the expiration of warranties on two i2000 implanters in the fiscal year ended December 31, 2006 as compared to the $0.4 million reduction in cost from the expiration of warranties on two i2000 implanters in the fiscal year ended December 31, 2007.
General and Administrative Expenses. General and administrative expenses for the fiscal year ended December 31, 2007 were $2.0 million as compared to $2.4 million for the fiscal year ended December 31, 2006, a decrease of $0.4 million or 16%. This is due to $0.4 million in decreased payroll and payroll related expenses from headcount reductions and reduced salaries.
31
Marketing and Selling Expenses. Marketing and selling expenses for the fiscal year ended December 31, 2007 were $0.6 million as compared to $1.1 million for the fiscal year ended December 31, 2006, a decrease of $0.5 million, or 47%. The decrease in marketing and selling expenses is a result of decreased payroll and payroll related expenses of $0.5 million from headcount reductions and labor costs charged out for consulting at a neighboring business in the fiscal year ended December 31, 2007.
Research and Development Expenses. Internally funded research and development expenses decreased by $1.6 million, or 30% to $3.8 million for the fiscal year ended December 31, 2007 from $5.4 million for the fiscal year ended December 31, 2006. This decrease was due to a reduction in wafer research and development costs of $0.7 million, which was the result of $0.3 million from reduced payroll and payroll related expenses from reduced headcount and reduced salaries along with $0.1 million of reduced wafer testing material, $0.1 million of reduced utility expense and $0.1 million in reduced depreciation and amortization. In addition, for the equipment research and development area, which reduced spending by $0.9 million, $0.5 million was from reduced payroll and payroll related expenses from headcount reductions and reduced salaries, reduced R&D material of $0.3 million and reduced utilities and equipment repair and maintenance of $0.1 million.
Impairment of Long-Lived Assets. During the fourth quarter ended December 31, 2007 a number of unexpected developments occurred which impacted our assets, including changes in the projected cash flow generation and our projected utilization of the assets within our revised plans. As a result of our subsequent impairment analysis under the provisions of SFAS No. 144, Accounting for Impairment or Disposal of Long-Lived Assets we recognized an impairment charge of approximately $1.6 million. Of this total impairment, approximately $1.3 million related to fixed assets and the balance of $0.3 million was associated with certain patents.
Other Income. Total other income for the fiscal year ended December 31, 2007 was $0.7 million as compared to $1.2 million for the fiscal year ended December 31, 2006, a decrease of $0.5 million. The decrease in total other income is attributed to $0.8 million received from the expiration of an option to place an order for an additional implanter prior to the end of 2006. This was offset by an increase of $0.3 million as a result of outside consulting provided to a neighboring business under a staffing services agreement in the fiscal year ended December 31, 2007.
Taxes. The Company had federal net operating loss and general business credit carryovers of approximately $91.6 million and $1.2 million, respectively, at December 31, 2007, that may be used to offset future taxable income through 2027. State net operating loss and credit carryovers of $44.1 million and $1.2 million, respectively, have varying expiration dates. Included in the total deferred tax assets, offset by the valuation allowance, was $3.2 million related to the net operating loss carryover resulting from the exercise of employee stock options the tax benefit of which, when recognized, will be accounted for as a credit to additional paid-in capital rather than a reduction of income tax expense. Net operating loss carryovers and other tax attributes may be limited due to past or future changes in ownership interests.
Fiscal Year Ended December 31, 2006 Compared to Fiscal Year Ended December 31, 2005
License and Other Revenue. License and other revenue for the fiscal year ended December 31, 2006 was $0.6 million compared to $0.3 million for the fiscal year ended December 31, 2005, an increase of $0.3 million, or 73%. This increase is primarily attributable to a new sublicense agreement valued at $0.2 million related to equipment technology in the year ended December 31, 2006.
32
Equipment Revenue. Equipment revenue represents revenue recognized from the sale of implanters, spare parts and field service revenue. Equipment revenue increased to $13.4 million for the fiscal year ended December 31, 2006 from $0.3 million for the fiscal year ended December 31, 2005, an increase of $13.1 million. Equipment revenue in 2006 included revenue recognized for two i2000 implanters for $13.0 million as compared to no implanter revenue recognition in 2005.
Field service revenue accounted for $0.3 million, or 2% of equipment revenue for the fiscal year ended December 31, 2006 as compared to $0.1 million, or 34% of equipment revenue for the fiscal year ended December 31, 2005. Sales of spare parts accounted for $0.1 million, or 1% of equipment revenue for the fiscal year ended December 31, 2006 as compared to $0.2 million, or 66% of equipment revenue, for the fiscal year ended December 31, 2005. Sales of spare parts fluctuate depending on customer demand and when the warranties expire on individual pieces of equipment. Warranty expense is calculated on our anticipated replacement costs for equipment accepted by our customers over a one or two year contract period.
Total Sales and Revenue. Total sales and revenue for the fiscal year ended December 31, 2006 was $14.0 million, an increase of $13.4 million, from $0.6 million for the fiscal year ended December 31, 2005. The increase is due to revenue recognition for two i2000 implanters of $13.0 million for the fiscal year ended December 31, 2006 along with increased license revenue of $0.2 million and increased service revenue of $0.2 million which was offset by a decrease in spare parts revenue of $0.1 million.
Total Cost of Sales and Revenue. Cost of license and other revenue consists of labor and materials expended during the twelve month period. There were no license costs or costs for other revenue during the period.
Cost of equipment revenue represents the cost of equipment including manufacturing labor, the cost for spare parts, and the cost of labor incurred for field service. Cost of equipment revenue for the fiscal year ended December 31, 2006 was $5.9 million, as compared to $0.8 million for the fiscal year ended December 31, 2005, an increase of $5.1 million. This increase of $5.1 million was primarily due to the costs associated with the recognition of revenue for the two i2000 implanters during the period ended December 31, 2006.
The gross margin for all sales was a positive 58% for the fiscal year ended December 31, 2006, as compared to a negative gross margin of 25% for the fiscal year ended December 31, 2005. This increase in the gross margin for all sales is attributable to the approximate gross margin of 55% for the two i2000 implanter sales recognized in the fiscal year ended December 31, 2006 and $0.6 million reduction in cost from the expiration of warranties on two i2000 implanters in the fiscal year ended December 31, 2006.
General and Administrative Expenses. General and administrative expenses for the fiscal year ended December 31, 2006 were $2.4 million as compared to $2.2 million for the fiscal year ended December 31, 2005, an increase of $0.2 million or 8%. This is due to $0.2 million in stock based compensation expense which impacted the financial statements in compliance with the new accounting rule in the fiscal year ended December 31, 2006.
Marketing and Selling Expenses. Marketing and selling expenses for the fiscal year ended December 31, 2006 were $1.1 million as compared to $1.3 million for the fiscal year ended December 31, 2005, a decrease of $0.2 million, or 17%. The decrease in marketing and selling expenses is a result of decreased payroll and payroll related expenses of $0.2 million from headcount reductions.
Research and Development Expenses. Internally funded research and development expenses decreased by $0.6 million, or 10% to $5.4 million for the fiscal year ended December 31, 2006 from $6.0 million for the fiscal year ended December 31, 2005. This decrease was due to a reduction in wafer research
33
and development costs of $0.3 million. In addition, reduced payroll and payroll related expenses of $0.3 million offset by $0.1 million associated with FAS 123(R) stock based compensation expense contributed to these results for the fiscal year ended December 31, 2006.
Other Income. Total other income for the fiscal year ended December 31, 2006 was $1.2 million as compared to $0.2 million for the fiscal year ended December 31, 2005, an increase of $1.0 million. The increase in total other income is attributed to $0.8 million received from the expiration of an option to place an order for an additional implanter prior to the end of 2006. In addition, interest income increased by $0.1 million due to the increase in interest rates and miscellaneous income increased by $0.1 million from the sales of excess material and equipment.
Discontinued Operations. There was no gain or loss shown for the fiscal year ended December 31, 2006. For the fiscal year ended December 31, 2005, the Company recognized a gain on disposal of $0.2 million consisting primarily of the sale of excess equipment, sale of wafer materials previously written off and an accrual adjustment related to sales tax.
Taxes. The Company had federal net operating loss and general business credit carryovers of approximately $87.1 million and $1.2 million, respectively, at December 31, 2006, that may be used to offset future taxable income through 2026. State net operating loss and credit carryovers of $52.4 million and $1.2 million, respectively, have varying expiration dates. Included in the total deferred tax assets, offset by the valuation allowance, was $3.2 million related to the net operating loss carryover resulting from the exercise of employee stock options the tax benefit of which, when recognized, will be accounted for as a credit to additional paid-in capital rather than a reduction of income tax expense. Net operating loss carryovers and other tax attributes may be limited in the event of certain changes in ownership interests. The Company recorded and paid Massachusetts and California excise and corporate tax which in aggregate was $1,256 for 2006.
Liquidity and Capital Resources
Our financial statements for the fiscal year ended December 31, 2007 were prepared assuming the Company would continue as a going concern; however, the Company’s recurring losses and accumulated deficit raise substantial doubts about its ability to continue as a going concern. The Company’s ability to continue as a going concern is an issue raised as a result of limited equipment sales, recurring losses from operations and accumulated deficit and is subject to the Company’s ability to secure additional orders of our implanter equipment or, in the alternative, identify and consummate a strategic transaction, including the potential a sale of the Company, or other strategic alternative.
As of December 31, 2007, the Company had cash and cash equivalents of $5.8 million, including the gross proceeds from the financing of $5.3 million from the sale of 3.4 million shares of common stock and 2.5 million common stock warrants in the year ended December 31, 2007.
During the fiscal year ended December 31, 2007, the Company used $3.9 million of cash for operating activities compared to $2.1 million in 2006. To date, working capital requirements have been funded primarily through debt (capital leases) and equity financings, including the 2007 financing discussed below. The principal uses of cash during the fiscal year ended December 31, 2007, as described in the Statement of Cash Flows in this 10K, were to fund operations. At December 31, 2007, the Company had commitments to purchase approximately $0.2 million in material. Headcount for the year ended December 31, 2007 was 20 employees.
34
Cash flow projections developed by management indicate the Company believes it will have sufficient cash resources to support current operations until at least June 2009. However, since it began selling implanters in 1996, only a total of eight Ibis 1000 oxygen implanters have been sold at an average sale price of approximately $4.0 million each and four i2000 oxygen implanters at a selling price between $6.0 and $8.0 million. The most recent order received was in October 2005, for which the Company recognized revenue in the quarter ended September 30, 2006. The Company did not record implanter revenue during 2007 and as of April 10 2008, does not have an order for an implanter from our customers. At present time, only one of the four major wafer manufacturers can be viewed as a current prospective customer. The Company currently does not have a backlog for implanters, nor does it have visibility to the timing of the next order from any customer. Our failure to obtain any orders for implanters in the foreseeable future, notwithstanding our belief that we have sufficient cash for the 2008 fiscal year, or to obtain other sources of offsetting revenue, will have a material adverse impact on the Company’s business and hinder its ability to continue as a going concern. The Company’s continuing inability to generate revenue likely will result in its inability to continue to invest in research development and manufacturing capabilities, hinder its ability to pursue and maintain relationships, partnerships and alliances with third parties, result in a loss of key personnel by way of workforce reduction or by voluntary termination of employment, limit its ability to secure financing on acceptable terms, if at all, and create greater volatility in the trading of the Company’s common stock.
In January 2007, the Company announced that to conserve cash it was reducing its operating expenses by approximately 40% which involved cost cutting measures consisting of headcount reductions, consulting at a neighboring business for a minimum of six months under a staffing services agreement, salary reductions and reducing consumable expenses. On February 16, 2007, the Company entered into a purchase agreement pursuant to which it agreed to sell unregistered shares of its common stock and warrants exercisable for common stock to Special Situation Funds for an aggregate purchase price of $5.3 million in a two-tranche financing. The first closing, at which the Company received approximately $2.2 million in proceeds, occurred on February 20, 2007. Pursuant to stockholder approval, received at the Company Annual Meeting on May 10, 2007, the Company sold the remainder of the securities at a second closing, at which the Company received approximately $3.1 million in proceeds. In connection with the transaction, the Company also entered into a registration rights agreement with the investors. The Company paid a placement fee to a placement agent, of approximately $0.3 million, which represents a cash fee equal to 5% of the gross proceeds and warrants exercisable for common stock with a dollar value equal to 5% of the aggregate gross proceeds received by the Company in both closings of the financing. The Company also issued to the placement agent an aggregate of 179,619 warrants, exercisable for common stock, with a dollar value equal to 5% of the aggregate gross proceeds. Total transaction costs associated with the financing were approximately $0.4 million, providing net cash to the Company of $4.9 million. All of the securities were sold in a private placement pursuant to Regulation D of the Securities Act.
On December 10, 2007, the Company received a letter from the Nasdaq Stock Market advising that for the previous 30 consecutive business days, the bid price of the Company’s common stock (the “Common Stock”) had closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq Global Market pursuant to Nasdaq Marketplace Rule 4450(a)(5).
Nasdaq stated in its letter that in accordance with Nasdaq Marketplace Rule 4450(e)(2), the Company will be provided 180 calendar days, or until June 9, 2008, to regain compliance with the minimum bid price requirement. The bid price of the Common Stock must close at $1.00 per share or more for a minimum of 10 consecutive business days to achieve compliance.
If the Company does not regain compliance with the minimum bid price requirement by June 9, 2008,
35
the Nasdaq staff will provide the Company with written notification that the Common Stock will be delisted from the Nasdaq Global Market. At that time, the Company may appeal the delisting determination to a Nasdaq Listings Qualifications Panel pursuant to applicable Nasdaq rules. Alternatively, Nasdaq Marketplace Rule 4450(i) may permit the Company to transfer the Common Stock to the Nasdaq Capital Market if the Common Stock satisfies all criteria, other than compliance with the minimum bid price requirement, for initial inclusion on such market.
In February 2008, the Company announced the engagement of the investment bank, BlueLake Partners, LLC for the purpose of assessing strategic alternatives for Ibis, including a potential sale of the Company and/or its assets. The Company can give no assurance that it will identify an alternative that allows stockholders to realize an increase in the value of the Company’s stock. The Company can also give no assurance that a transaction or other strategic alternative, once identified, evaluated and consummated, will provide greater value to stockholders than that reflected in the current stock price. In fact, if the Company is unable to recognize revenue through the sale of our products pursuant to its current operating plan, and if it is unable a viable strategic alternative is not identified, the Company may be unable to continue as a going concern. Further, in pursuit of the highest value for shareholders, the Board of Directors also may consider alternatives that include a liquidation of assets and resulting cash distribution per share after payment of outstanding liabilities. In such case, any per-share cash liquidation distribution could be less than the purchase price per share that stockholders may have paid for their Company securities.
On March 31, 2008 the Company requested and received a 15 day extension to files its Annual Report 10K to additional time to conduct an impairment analysis with respect to Ibis’ assets, as permitted by Rule 12b-25.
On April 16, 2008, the Company received a letter from the Nasdaq Stock Market advising that it had not received the Company’s Form 10-K for the fiscal year ended December 31, 2007, as required by Marketplace Rule 4310(c)(14). Accordingly, unless the Company requests an appeal of this determination, trading of the Company’s common stock will be suspended at the opening of business on April 25, 2008, and a Form 25-NSE will be filed with the SEC, which will remove the Company’s securities from listing and registration on The Nasdaq Stock Market. The Company believes that, as a result of filing this Form 10-K, it is now in compliance with Marketplace Rule 4310(c)(14).
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as defined in Regulation S-K Section 303(a)(4)(ii).
Contractual Obligations
We have no significant contractual obligations not fully recorded on our Balance Sheets or fully disclosed in the Notes to our Financial Statements. We have no off-balance sheet arrangements as defined in Regulation S-K Section 303(a)(4)(ii).
At December 31, 2007, our outstanding contractual obligations included:
|
| Payment due by period |
| ||||||||||||||||
Contractual Obligations |
| Total |
| Less than |
| 1 |
| 2 |
| 3-5 |
| More |
| ||||||
Minimum operating lease payments |
| $ | 1,329,230 |
| $ | 400,164 |
| $ | 375,308 |
| $ | 369,456 |
| $ | 184,302 |
| $ | — |
|
36
Additional information regarding our financial commitments at December 31, 2007 is provided in the Notes to our Financial Statements. See “Notes to Financial Statements, Note 8, Commitments and Contingencies”.
Effects Of Inflation
Ibis believes that over the past three years inflation has not had a significant impact on our sales or operating results.
New Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“SFAS 157”) which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles and expands disclosures about fair value measurements. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007 and interim periods within those fiscal years. We do not expect the adoption of SFAS No. 157 to have a material impact on our financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities — Including an Amendment of FASB Statement No. 115” (“SFAS No. 159”). SFAS No. 159 permits entities to choose to measure many financial instruments and certain other items at fair value and is effective as of the beginning of the Company’s fiscal year beginning after November 15, 2007. The Company is currently evaluating the potential impact of SFAS No. 159 on its financial position and results of operations.
37
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The exposure of market risk associated with risk-sensitive instruments is not material to Ibis, as we do not transact our sales denominated in other than United States dollars, invest primarily in short-term commercial paper, hold our investments until maturity and have not entered into hedging transactions.
38
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
IBIS TECHNOLOGY CORPORATION
Item 8. Index to Financial Statements and Financial Statement Schedule
Financial Statements:
| Page |
|
|
Report of Independent Registered Public Accounting Firm | 40 |
Balance Sheets as of December 31, 2006 and 2007 | 41 |
Statements of Operations for the Years Ended December 31, 2005, 2006 and 2007 | 42 |
Statements of Stockholders’ Equity for the Years Ended December 31, 2005, 2006 and 2007 | 43 |
Statements of Cash Flows for the Years Ended December 31, 2005, 2006 and 2007 | 44 |
Notes to Financial Statements | 45-66 |
|
|
Schedule: |
|
Report of Independent Registered Public Accounting Firm on Financial Statement Schedule |
|
Schedule II – Valuation and Qualifying Accounts for the Years Ended December 31, 2005, 2006 and 2007 |
|
39
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
of Ibis Technology Corporation:
We have audited the accompanying balance sheets of Ibis Technology Corporation as of December 31, 2006 and 2007, and the related statements of operations, stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2007. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Ibis Technology Corporation as of December 31, 2006 and 2007, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2007, in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As described in note 3 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plan with regard to these matters, are also discussed in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As described in note 2(k) to the financial statements, the Company adopted Statement of Financial Accounting Standards No. 123 (revised 2004), Share-based Payment, effective January 1, 2006.
|
| /s/ KPMG LLP | |||
|
|
|
|
|
|
Boston, Massachusetts |
|
|
|
|
|
May 22, 2008 |
|
|
|
|
|
40
IBIS TECHNOLOGY CORPORATION
BALANCE SHEETS
December 31, 2006 and 2007
|
| 2006 |
| 2007 |
| ||
|
|
|
|
|
| ||
Assets |
|
|
|
|
| ||
Current Assets: |
|
|
|
|
| ||
Cash and cash equivalents |
| $ | 4,812,477 |
| $ | 5,770,072 |
|
Accounts receivable, trade, net (notes 4 and 17) |
| 349,154 |
| 308,768 |
| ||
Inventories, net (note 5 and 7) |
| 3,575,296 |
| 1,790,394 |
| ||
Prepaid expenses and other current assets |
| 290,780 |
| 165,925 |
| ||
Total current assets |
| 9,027,707 |
| 8,035,159 |
| ||
Property and equipment (notes 6, 7, and 9) |
| 24,728,824 |
| 19,598,897 |
| ||
Less: Accumulated depreciation and amortization |
| (20,744,772 | ) | (18,026,667 | ) | ||
Net property and equipment |
| 3,984,052 |
| 1,572,230 |
| ||
Patents and other assets, net (note 7 and 8) |
| 777,082 |
| 155,444 |
| ||
Total assets |
| $ | 13,788,841 |
| $ | 9,762,833 |
|
|
|
|
|
|
| ||
Liabilities and Stockholders’ Equity |
|
|
|
|
| ||
Current Liabilities: |
|
|
|
|
| ||
Accounts payable |
| $ | 409,705 |
| $ | 260,372 |
|
Accrued liabilities (notes 10 and 11) |
| 973,994 |
| 471,219 |
| ||
Deferred revenue (note 12) |
| 150,000 |
| — |
| ||
Total current liabilities |
| 1,533,699 |
| 731,591 |
| ||
|
|
|
|
|
| ||
Commitments and contingencies (notes 9, 12 and 17) |
|
|
|
|
| ||
|
|
|
|
|
| ||
Stockholders’ Equity (notes 15 and 16): |
|
|
|
|
| ||
Undesignated preferred stock, $.01 par value |
|
|
|
|
| ||
Authorized 2,000,000 shares; none issued |
| — |
| — |
| ||
Common stock, $.008 par value |
|
|
|
|
| ||
Authorized 50,000,000 shares; issued and outstanding 10,915,481 shares and 14,317,482 shares in 2006 and 2007, respectively |
| 87,324 |
| 114,540 |
| ||
Additional paid-in capital |
| 93,798,393 |
| 99,087,089 |
| ||
Accumulated deficit |
| (81,630,575 | ) | (90,170,387 | ) | ||
Total stockholders’ equity |
| 12,255,142 |
| 9,031,242 |
| ||
Total liabilities and stockholders’ equity |
| $ | 13,788,841 |
| $ | 9,762,833 |
|
See accompanying notes to financial statements.
41
IBIS TECHNOLOGY CORPORATION
STATEMENTS OF OPERATIONS
Years ended December 31, 2005, 2006, 2007
|
| 2005 |
| 2006 |
| 2007 |
| |||
|
|
|
|
|
|
|
| |||
License and other revenue (note 13) |
| $ | 324,621 |
| $ | 560,384 |
| $ | 425,924 |
|
Equipment revenue |
| 277,684 |
| 13,427,035 |
| 525,267 |
| |||
Total net sales and revenue (note 17) |
| 602,305 |
| 13,987,419 |
| 951,191 |
| |||
|
|
|
|
|
|
|
| |||
Cost of license and other revenue |
| — |
| — |
| 257 |
| |||
Cost of equipment revenue (note 5) |
| 750,472 |
| 5,893,600 |
| 2,214,619 |
| |||
Total cost of sales and revenue |
| 750,472 |
| 5,893,600 |
| 2,214,876 |
| |||
|
|
|
|
|
|
|
| |||
Gross profit (loss) |
| (148,167 | ) | 8,093,819 |
| (1,263,685 | ) | |||
|
|
|
|
|
|
|
| |||
Operating expenses: |
|
|
|
|
|
|
| |||
General and administrative |
| 2,216,748 |
| 2,400,645 |
| 2,004,614 |
| |||
Marketing and selling |
| 1,318,979 |
| 1,095,844 |
| 578,001 |
| |||
Research and development |
| 5,993,350 |
| 5,396,321 |
| 3,789,164 |
| |||
Impariment of long-lived assets (note 7) |
| — |
| — |
| 1,602,407 |
| |||
Total operating expenses |
| 9,529,077 |
| 8,892,810 |
| 7,974,186 |
| |||
|
|
|
|
|
|
|
| |||
Loss from operations |
| (9,677,244 | ) | (798,991 | ) | (9,237,871 | ) | |||
|
|
|
|
|
|
|
| |||
Other income (expense): |
|
|
|
|
|
|
| |||
Interest income |
| 208,818 |
| 304,008 |
| 313,326 |
| |||
Interest expense |
| (536 | ) | — |
| — |
| |||
Other (note 18) |
| 9,671 |
| 901,124 |
| 385,533 |
| |||
Total other income |
| 217,953 |
| 1,205,132 |
| 698,859 |
| |||
|
|
|
|
|
|
|
| |||
Income (loss) from continuing operations before income taxes |
| (9,459,291 | ) | 406,141 |
| (8,539,012 | ) | |||
Income tax expense (note 14) |
| 1,256 |
| 1,256 |
| 800 |
| |||
Income (loss) from continuing operations |
| (9,460,547 | ) | 404,885 |
| (8,539,812 | ) | |||
|
|
|
|
|
|
|
| |||
Discontinued operations (note 19): |
|
|
|
|
|
|
| |||
Gain (loss) on disposal |
| 215,242 |
| — |
| — |
| |||
Income (loss) from discontinued operations |
| 215,242 |
| — |
| — |
| |||
|
|
|
|
|
|
|
| |||
Net income (loss) |
| $ | (9,245,305 | ) | $ | 404,885 |
| $ | (8,539,812 | ) |
|
|
|
|
|
|
|
| |||
Earnings (loss) per share: |
|
|
|
|
|
|
| |||
Basic and diluted |
|
|
|
|
|
|
| |||
Continuing operations |
| $ | (0.88 | ) | $ | 0.04 |
| $ | (0.64 | ) |
Discontinued operations |
| 0.02 |
| — |
| — |
| |||
|
|
|
|
|
|
|
| |||
Net income (loss) |
| $ | (0.86 | ) | $ | 0.04 |
| $ | (0.64 | ) |
|
|
|
|
|
|
|
| |||
Weighted average common shares outstanding: |
|
|
|
|
|
|
| |||
Basic |
| 10,737,924 |
| 10,853,304 |
| $ | 13,410,938 |
| ||
Diluted |
| 10,737,924 |
| 10,979,783 |
| $ | 13,410,938 |
| ||
See accompanying notes to financial statements.
42
IBIS TECHNOLOGY CORPORATION
STATEMENTS OF STOCKHOLDERS’ EQUITY
Years ended December 31, 2005, 2006 and 2007
|
| Common |
| Additional |
| Accumulated |
| Total |
| ||||
|
|
|
|
|
|
|
|
|
| ||||
Balances at December 31, 2004 |
| $ | 85,757 |
| $ | 93,124,259 |
| $ | (72,790,155 | ) | $ | 20,419,861 |
|
|
|
|
|
|
|
|
|
|
| ||||
Exercise of stock options |
| — |
| 8,550 |
| — |
| 8,550 |
| ||||
Employee Stock Purchase Plan |
| 771 |
| 112,945 |
| — |
| 113,716 |
| ||||
Net loss |
| — |
| — |
| (9,245,305 | ) | (9,245,305 | ) | ||||
|
|
|
|
|
|
|
|
|
| ||||
Balances at December 31, 2005 |
| 86,528 |
| 93,245,754 |
| (82,035,460 | ) | 11,296,822 |
| ||||
|
|
|
|
|
|
|
|
|
| ||||
Exercise of stock options |
| 8 |
| 1,386 |
| — |
| 1,394 |
| ||||
Share-based compensation |
| — |
| 398,599 |
| — |
| 398,599 |
| ||||
Employee Stock Purchase Plan |
| 788 |
| 152,654 |
| — |
| 153,442 |
| ||||
Net income |
| — |
| — |
| 404,885 |
| 404,885 |
| ||||
|
|
|
|
|
|
|
|
|
| ||||
Balances at December 31, 2006 |
| 87,324 |
| 93,798,393 |
| (81,630,575 | ) | 12,255,142 |
| ||||
|
|
|
|
|
|
|
|
|
| ||||
Common stock issued, net of issuance costs |
| 27,027 |
| 4,850,426 |
| — |
| 4,877,453 |
| ||||
Share-based compensation |
| — |
| 410,276 |
| — |
| 410,276 |
| ||||
Employee Stock Purchase Plan |
| 189 |
| 27,994 |
| — |
| 28,183 |
| ||||
Net loss |
| — |
| — |
| (8,539,812 | ) | (8,539,812 | ) | ||||
|
|
|
|
|
|
|
|
|
| ||||
Balances at December 31, 2007 |
| $ | 114,540 |
| $ | 99,087,089 |
| $ | (90,170,387 | ) | $ | 9,031,242 |
|
See accompanying notes to financial statements.
43
IBIS TECHNOLOGY CORPORATION
STATEMENTS OF CASH FLOWS
Years ended December 31, 2005, 2006, 2007
|
| 2005 |
| 2006 |
| 2007 |
| |||
|
|
|
|
|
|
|
| |||
Cash flows from operating activities: |
|
|
|
|
|
|
| |||
Net income (loss) |
| $ | (9,245,305 | ) | $ | 404,885 |
| $ | (8,539,812 | ) |
Less: income from discontinued operations |
| 215,242 |
| — |
| — |
| |||
Income (loss) from continuing operations |
| (9,460,547 | ) | 404,885 |
| (8,539,812 | ) | |||
|
|
|
|
|
|
|
| |||
Adjustments to reconcile net income (loss) to net cash used in operating activities: |
|
|
|
|
|
|
| |||
Share-based compensation expense |
| — |
| 394,776 |
| 410,276 |
| |||
Depreciation and amortization |
| 1,746,989 |
| 1,535,856 |
| 1,511,659 |
| |||
Impairment of long-lived assets |
| — |
| — |
| 1,602,407 |
| |||
Changes in operating assets and liabilities: |
|
|
|
|
|
|
| |||
Accounts receivable, trade |
| 117,023 |
| (258,276 | ) | 40,386 |
| |||
Inventories |
| (651,483 | ) | 2,705,177 |
| 1,784,902 |
| |||
Prepaid expenses and other current assets |
| (42,302 | ) | 324,922 |
| 124,855 |
| |||
Accounts payable |
| (162,004 | ) | 178,834 |
| (149,333 | ) | |||
Accrued liabilities and deferred revenue |
| 7,240,174 |
| (7,340,701 | ) | (652,775 | ) | |||
Net cash used in operating activities of continuing operations |
| (1,212,150 | ) | (2,054,527 | ) | (3,867,435 | ) | |||
Cash flows from investing activities: |
|
|
|
|
|
|
| |||
Additions to property and equipment, net |
| (149,648 | ) | (41,804 | ) | (11,840 | ) | |||
Other assets |
| 247,988 |
| (102,902 | ) | (68,766 | ) | |||
Net cash provided (used) in investing activities of continuing operations |
| 98,340 |
| (144,706 | ) | (80,606 | ) | |||
Cash flows from financing activities: |
|
|
|
|
|
|
| |||
Proceeds from sales of common stock, net of issuance costs |
| — |
| — |
| 4,877,453 |
| |||
Exercise of stock options, warrants and Employee Stock Purchase Plan |
| 122,266 |
| 154,836 |
| 28,183 |
| |||
Net cash provided by financing activities of continuing operations |
| 122,266 |
| 154,836 |
| 4,905,636 |
| |||
Discontinued operations (see note 19): |
|
|
|
|
|
|
| |||
Operating cash flows |
| 45,297 |
| — |
| — |
| |||
Investing cash flows |
| 77,049 |
| — |
| — |
| |||
Net cash provided (used) in discontinued operations |
| 122,346 |
| — |
| — |
| |||
Net increase (decrease) in cash and cash equivalents |
| (869,198 | ) | (2,044,397 | ) | 957,595 |
| |||
Cash and cash equivalents, beginning of year |
| 7,726,072 |
| 6,856,874 |
| 4,812,477 |
| |||
Cash and cash equivalents, end of year |
| $ | 6,856,874 |
| $ | 4,812,477 |
| $ | 5,770,072 |
|
|
|
|
|
|
|
|
| |||
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
| |||
Cash paid during the year for interest |
| $ | 536 |
| $ | — |
| $ | — |
|
See accompanying notes to financial statements.
44
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS
December 31, 2006 and 2007
(1) Nature of Business and Organization
Ibis Technology Corporation (the “Company”) was incorporated in October 1987 for the purpose of supplying silicon-on-insulator (SOI) wafers formed by SIMOX (Separation by Implantation of Oxygen) technology. SIMOX-SOI wafers were manufactured by the Company using a specialized oxygen ion implanter, which was developed and manufactured by the Company and is integrated with other specialized processes and characterization equipment. The Company is the leading manufacturer of high current oxygen implanters and began selling these oxygen implanters in 1996.
(2) Summary of Significant Accounting Policies
(a) Cash and Cash Equivalents
Cash equivalents represent highly liquid investments with original maturities of three months or less.
(b) Inventory Valuation and Reserves
Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out (“FIFO”) cost method. Our policy for the valuation of inventory, including the determination of obsolete or excess inventory, requires us to forecast the future demand for our products within specific time horizons, generally twelve months or less. If our forecasted demand for specific products is greater than actual demand and we fail to reduce manufacturing output accordingly, we could be required to record additional inventory reserves, which would have a negative impact on our gross margin. We have reserved for obsolescence when engineering changes or other technological advances indicate that obsolescence has occurred.
(c) Property and Equipment and Impairment of Long-Lived Assets
Property and equipment is stated at cost. Depreciation is provided using the straight-line method over the estimated useful lives of the respective assets, ranging from three to eight years. Machinery and equipment is depreciated over eight years while furniture and fixtures are depreciated over three years. Amortization of leasehold improvements is provided using the straight-line method over the lesser of the life of the lease, or the estimated useful life of the asset. Ibis reviews the valuation of long-lived assets, including property and equipment and licenses, under the provisions of SFAS No. 144, Accounting for Impairment or Disposal of Long-Lived Assets. The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. If such a circumstance exists, the Company would measure an impairment loss to the extent the carrying amount of the particular long-lived asset or group exceeds its fair value. The Company would determine the fair value based on either a projected discounted cash flow method using a discount rate determined by management to be commensurate with the risk inherent in the current business model, or estimated fair value based on comparable sales prices for similar used equipment.
(d) Patents and Other Assets
Other assets consist principally of deposits, prepaid royalties and licenses. Patents and prepaid royalties are amortized over five years using the straight-line method. Licenses are amortized over seven years using the straight-line method.
45
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS
(e) Revenue Recognition
The Company recognizes revenue from equipment sales and the sales of spare parts when all of the following criteria have been met: (1) evidence exists that the customer is bound to the transaction; (2) the product has been delivered to the customer and, when applicable, the product has been installed and accepted by the customer; (3) the sales price to the customer has been fixed or is determinable; and (4) collectibility of the sale price is reasonably assured. The Company recognizes revenue from implanter sales upon acceptance at the customer’s site. Revenue derived from contracts and services is recognized upon performance. Provisions for anticipated losses are made in the period in which such losses become determinable.
(f) Research and Development
Research and development costs are charged to expense as incurred.
(g) Net Income (Loss) Per Common Share
Net income (loss) per share of common stock is computed based upon the weighted average number of shares outstanding during each period and including the dilutive effect, if any, of stock options and warrants. Statement of Financial Accounting Standards No. 128, “Earnings per Share”, establishes standards for computing and presenting earnings per share and applies to entities with publicly held common stock or potential common stock. This Statement simplifies the standards for computing earnings per share previously found in APB Opinion No. 15, Earnings per Share, and makes them comparable to international EPS standards. It replaces the presentation of primary EPS with a presentation of basic EPS. It also requires dual presentation of basic and diluted EPS on the face of the income statement for all entities with complex capital structures and requires a reconciliation of the numerator and denominator of the basic EPS computation to the numerator and denominator of the diluted EPS computation and requires the presentation of basic and diluted earnings (loss) per share for all periods presented. As the Company was in a net loss position for the years ended December 31, 2005 and December 31, 2007, common stock equivalents of 74,590 and zero for the years ended December 31, 2005, and 2007, respectively, were excluded from the diluted loss per share calculation as they would be antidilutive. As a result, diluted loss per share is the same as basic loss per share for 2005 and 2007.
The reconciliation of the denominators of the basic and diluted net income (loss) per common share for the Company’s net income (loss) is as follows:
46
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
|
| Years ended December 31, |
| ||||||||||||||||
|
| 2005 |
| 2006 |
| 2007 |
| ||||||||||||
|
| Diluted |
| Basic |
| Diluted |
| Basic |
| Diluted |
| Basic |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Income (loss) from: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Continuing operations |
| $ | (9,460,547 | ) | $ | (9,460,547 | ) | $ | 404,885 |
| $ | 404,885 |
| $ | (8,539,812 | ) | $ | (8,539,812 | ) |
Discontinued operations |
| 215,242 |
| 215,242 |
| — |
| — |
| — |
| — |
| ||||||
Net income (loss) |
| $ | (9,245,305 | ) | $ | (9,245,305 | ) | $ | 404,885 |
| $ | 404,885 |
| $ | (8,539,812 | ) | $ | (8,539,812 | ) |
Weighted average common shares outstanding |
| 10,737,924 |
| 10,737,924 |
| 10,853,304 |
| 10,853,304 |
| 13,410,938 |
| 13,410,938 |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Potential common share equivalents |
| — |
|
|
| 126,479 |
|
|
| — |
|
|
| ||||||
Weighted average shares outstanding |
| 10,737,924 |
|
|
| 10,979,783 |
|
|
| 13,410,938 |
|
|
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Earning (loss) per common share and share equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Continuing operations |
| $ | (0.88 | ) | $ | (0.88 | ) | $ | 0.04 |
| $ | 0.04 |
| $ | (0.64 | ) | $ | (0.64 | ) |
Discontinued operations |
| 0.02 |
| 0.02 |
| — |
| — |
| — |
| — |
| ||||||
Net income (loss) |
| $ | (0.86 | ) | $ | (0.86 | ) | $ | 0.04 |
| $ | 0.04 |
| $ | (0.64 | ) | $ | (0.64 | ) |
(h) Issuance Costs
Common stock issuance costs are netted against additional paid-in capital.
(i) Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingencies at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results may differ from these estimates. Management exercises judgment and relies on estimates in recognizing revenue, valuing inventory, accruing certain liabilities, determining the measurement of share-based compensation, assessing long-lived asset impairment, and estimating the useful lives of long-lived assets.
(j) Fair Value of Financial Instruments
Financial instruments of the Company consist of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities. The carrying amount of these financial instruments approximates fair value.
(k) Share-Based Compensation
On January 1, 2006, the Company adopted Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment,”(“SFAS 123(R)”) which requires the measurement and recognition of compensation expense for all share-based payment awards made to employees and directors including employee stock options, employee stock purchases related to the 2000 Employee Stock Purchase Plan (“the ESPP”), restricted stock and other special equity awards based on grant-date estimated fair values. SFAS 123(R) supersedes the Company’s previous accounting under Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB 25”) for periods beginning in 2006. In March 2005, the Securities and Exchange Commission issued SAB 107 relating to SFAS 123(R). The Company has applied the provisions of SAB 107 in its adoption of SFAS 123(R).
47
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
The Company’s financial statements for the fiscal years ended December 31, 2006 and December 31, 2007 reflect the impact of SFAS 123(R). In accordance with the modified prospective transition method, the Company’s financial statements for prior periods have not been restated to reflect, and do not include, the impact of SFAS 123(R). Share-based compensation expense recognized under SFAS 123(R) for the years ended December 31, 2006 and December 31, 2007 was $0.4 million or $0.04 per share and $0.4 million or $0.03 per share, respectively, which consisted of share-based compensation expense related to employee stock options and the employee stock purchase plan. Of this 2006 expense, fifty four percent came from options issued prior to December 31, 2005 and are valued using the accrual method, which is consistent with prior periods, while twenty three percent came from options issued in 2006 and are valued using the straight-line prorated method, which was adopted in 2006 under the new guidance, and twenty three percent came from the valuation of shares issued under the employee stock purchase plan. Of this 2007 expense, twenty percent came from options issued prior to December 31, 2005 and are valued using the accrual method, which is consistent with prior periods, while seventy three percent came from options issued in 2006 and 2007 and are valued using the straight-line prorated method, which was adopted in 2006 under the new guidance, and seven percent came from the valuation of shares issued under the employee stock purchase plan. There was no share-based compensation expense related to employee stock options or employee stock purchases recognized during the fiscal year ended December 31, 2005 because the Company elected not to adopt the recognition provisions, and instead provided footnote disclosure permissible under Statement of Financial Accounting Standards No. 123, “Accounting for Stock-Based Compensation” (“SFAS 123”).
SFAS 123(R) requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in the Company’s Statement of Operations. Prior to the adoption of SFAS 123(R), the Company accounted for share-based awards to employees and directors using the intrinsic value method in accordance with APB 25, as allowed under SFAS 123. Under the intrinsic value method, share-based compensation expense had not been recognized in the Company’s statement of operations when the exercise price of the Company’s stock options granted to employees and directors equaled or exceeded the fair market value of the underlying stock at the date of grant.
Share-based compensation expense recognized during the period is based on the value of the portion of share-based payment awards that is ultimately expected to vest during the period. Share-based compensation expense recognized in the Company’s Statement of Operations for the fiscal years ended December 31, 2006 and December 31, 2007 included compensation expenses for share-based payment awards granted prior to, but not yet vested as of December 31, 2005, based on the grant date fair value estimated in accordance with the pro forma provisions of SFAS 123, and compensation expense for the share-based payment awards granted subsequent to December 31, 2005 based on the grant date fair value estimated in accordance with the provisions of SFAS 123(R).
As share-based compensation expense recognized in the Statement of Operations for the fiscal years ended December 31, 2006 and December 31, 2007 is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Upon adoption of SFAS 123(R), the Company elected to retain its method of valuation for share-based awards granted beginning in 2006 using the Black-Scholes option–pricing model (“Black–Scholes model”) which was also previously used for the Company’s pro forma information required under SFAS 123. The Company’s determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by the Company’s stock price as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to the Company’s expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors.
48
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
(l) Accounting for Income Taxes
The Company accounts for income taxes under Statement of Financial Accounting Standards No. 109, “Accounting for Income Taxes”, (SFAS 109). This Statement establishes financial accounting and reporting standards for the effects of income taxes that result from an enterprise’s activities during the current and preceding years.
In July 2006, the Financial Accounting Standards Board (FASB) issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes – an interpretation of FASB Statement No. 109” (“FIN 48”), which clarifies the accounting and disclosure for uncertainty in tax positions, as defined. FIN 48 seeks to reduce the diversity in practice associated with certain aspects of the recognition and measurement related to accounting for income taxes. This interpretation is effective for fiscal years beginning after December 15, 2006 and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken, or expected to be taken, in a tax return, and provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosures and transition. As a result of the implementation of FIN 48, the Company recorded no adjustment for unrecognized income tax benefits. At the adoption date of January 1, 2007, the Company had no unrecognized tax benefits. Ibis classifies any interest and penalties related to income taxes as components of income tax expense.
(m) Reclassifications
Certain prior year amounts have been reclassified to conform to current year presentation. These reclassifications had no effect on the Company’s reported net loss or financial position.
(n) New Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“SFAS 157”) which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles and expands disclosures about fair value measurements. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007 and interim periods within those fiscal years. We do not expect the adoption of SFAS No. 157 to have a material impact on our financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities — Including an Amendment of FASB Statement No. 115” (“SFAS No. 159”). SFAS No. 159 permits entities to choose to measure many financial instruments and certain other items at fair value and is effective as of the beginning of the Company’s fiscal year beginning after November 15, 2007. The Company is currently evaluating the potential impact of SFAS No. 159 on its financial position and results of operations.
(3) Liquidity
The Company’s financial statements for the fiscal year ended December 31, 2007 were prepared assuming the Company would continue as a going concern; however, the Company’s recurring losses and accumulated deficit raise substantial doubts about its ability to continue as a going concern. The Company’s ability to continue as a going concern is an issue raised as a result of recurring losses from operations and net capital deficiency and is subject to the Company’s ability to secure additional orders of our implanter equipment or, in the alternative, identify and consummate a strategic transaction, including the potential a sale of the Company, or other strategic alternative.
49
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
As of December 31, 2007, the Company had cash and cash equivalents of $5.8 million, including the gross proceeds from the financing of $5.3 million from the sale of 3.4 million shares of common stock and 2.5 million common stock warrants in the year ended December 31, 2007.
During the year ended December 31, 2007, the Company used $3.9 million of cash for operating activities of continuing operations as compared to $2.1 million in 2006. To date, the Company’s working capital requirements have been funded primarily through debt (capital leases) and equity financings, including the 2007 financing discussed below. The principal uses of cash during the fiscal year ended December 31, 2007, were to fund operations. At December 31, 2007, the Company had commitments to purchase approximately $0.2 million in material. Headcount for the year ended December 31, 2007 was 20 employees.
Cash flow projections developed by management indicate the Company believes it will have sufficient cash resources to support current operations through 2008. However, since the Company began selling implanters in 1996, the Company has only sold a total of eight Ibis 1000 oxygen implanters at an average sale price of approximately $4.0 million each and four i2000 oxygen implanters at a selling price between $6.0 and $8.0 million. The most recent order received was in October 2005, for which the Company recognized revenue in the quarter ended September 30, 2006. The Company did not record implanter revenue during 2007 and as of April 10, 2008, does not have an order for an implanter. At present time, only one of the four major wafer manufacturers are counted as a current prospective customer. The Company currently does not have a backlog for implanters, nor does it have visibility to the timing of the next order from any customer. The Company’s failure to obtain any orders for implanters in the foreseeable future, notwithstanding the belief that the Company has sufficient cash for the 2008 fiscal year, or to obtain other sources of offsetting revenue, will have a material adverse impact on the Company’s business and hinder its ability to continue as a going concern. The Company’s continuing inability to generate revenue likely will result in the inability to continue to invest in research development and manufacturing capabilities, hinder the ability to pursue and maintain relationships, partnerships and alliances with third parties, result in a loss of key personnel by way of workforce reduction or by voluntary termination of employment, limit its ability to secure financing on acceptable terms, if at all, and create greater volatility in the trading of the Company’s common stock.
In January 2007, the Company announced that to conserve cash it was reducing its operating expenses by approximately 40% which involved cost cutting measures consisting of headcount reductions, consulting at a neighboring business for a minimum of six months under a staffing services agreement, salary reductions and reducing consumable expenses. On February 16, 2007, the Company entered into a purchase agreement pursuant to which it agreed to sell unregistered shares of its common stock and warrants exercisable for common stock to Special Situation Funds for an aggregate purchase price of $5.3 million in a two-tranche financing. The first closing, at which the Company received approximately $2.2 million in proceeds, occurred on February 20, 2007. Pursuant to stockholder approval, received at the Company Annual Meeting on May 10, 2007, the Company sold the remainder of the securities at a second closing, at which the Company received approximately $3.1 million in proceeds. In connection with the transaction, the Company also entered into a registration rights agreement with the investors. The Company paid a placement fee to a placement agent, of approximately $0.3 million, which represents a cash fee equal to 5% of the gross proceeds and warrants exercisable for common stock with a dollar value equal to 5% of the aggregate gross proceeds received by the company in both closings of the financing. The Company also issued to the placement agent an aggregate of 179,619 warrants, exercisable for common stock, with a dollar value equal to 5% of the aggregate gross proceeds. Total transaction costs associated with the financing were approximately $0.4 million, providing net cash to the Company of $4.9 million. All of the securities were sold in a private placement pursuant to Regulation D of the Securities Act.
On December 10, 2007, the Company received a letter from the Nasdaq Stock Market advising that for the previous 30 consecutive business days, the bid price of the Company’s common stock (the “Common
50
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
Stock”) had closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq Global Market pursuant to Nasdaq Marketplace Rule 4450(a)(5).
Nasdaq stated in its letter that in accordance with Nasdaq Marketplace Rule 4450(e)(2), the Company will be provided 180 calendar days, or until June 9, 2008, to regain compliance with the minimum bid price requirement. The bid price of the Common Stock must close at $1.00 per share or more for a minimum of 10 consecutive business days to achieve compliance.
If the Company does not regain compliance with the minimum bid price requirement by June 9, 2008, the Nasdaq staff will provide the Company with written notification that the Common Stock will be delisted from the Nasdaq Global Market. At that time, the Company may appeal the delisting determination to a Nasdaq Listings Qualifications Panel pursuant to applicable Nasdaq rules. Alternatively, Nasdaq Marketplace Rule 4450(i) may permit the Company to transfer the Common Stock to the Nasdaq Capital Market if the Common Stock satisfies all criteria, other than compliance with the minimum bid price requirement, for initial inclusion on such market.
In February 2008, the Company announced the engagement of the investment bank BlueLake Partners, LLC for the purpose of assessing strategic alternatives for Ibis, including a potential sale of the Company and/or its assets. The Company can give no assurance that it will identify an alternative that allows stockholders to realize an increase in the value of the Company’s stock. There can also be no assurance that a transaction or other strategic alternative, once identified, evaluated and consummated, will provide greater value to the stockholders than that reflected in the current stock price. In fact, if the Company is unable to recognize revenue through the sale of its products pursuant to the current operating plan, and if a viable strategic alternative is not identified, the Company may be unable to continue as a going concern. Further, in pursuit of the highest value for shareholders, the Board of Directors may also consider alternatives that include a liquidation of assets and the resulting cash distribution per share after payment of outstanding liabilities. In such case, any per-share cash liquidation distribution could be less than the purchase price per share that stockholders may have paid for the Company’s securities.
(4) Accounts Receivable
Accounts receivable consisted of the following at December 31:
|
| 2006 |
| 2007 |
| ||
|
|
|
|
|
| ||
Accounts receivable, trade |
| $ | 160,822 |
| $ | 110,125 |
|
Accounts receivable, other |
| 188,332 |
| 198,643 |
| ||
|
| $ | 349,154 |
| $ | 308,768 |
|
(5) Inventories
Inventories consisted of the following at December 31:
|
| 2006 |
| 2007 |
| ||
|
|
|
|
|
| ||
Raw materials |
| $ | 1,445,182 |
| $ | 328,802 |
|
Work in process |
| 2,130,114 |
| 1,461,592 |
| ||
Total equipment inventory |
| $ | 3,575,296 |
| $ | 1,790,394 |
|
Equipment inventory consists of i2000 parts and/or implanters under construction or otherwise available for resale. For the year ended December 31, 2007, the Company recorded a $1.8 million increase in reserves charged to cost of goods for potential obsolescence of inventory.
51
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
At December 31, 2007, the Company had commitments to purchase approximately $0.2 million in material.
52
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
(6) Property and Equipment
Property and equipment consisted of the following at December 31:
|
| 2006 |
| 2007 |
| ||
|
|
|
|
|
| ||
Machinery and equipment |
| $ | 19,448,571 |
| $ | 17,029,136 |
|
Furniture and fixtures |
| 376,618 |
| 376,618 |
| ||
Leasehold improvements |
| 4,903,635 |
| 2,193,143 |
| ||
|
| $ | 24,728,824 |
| $ | 19,598,897 |
|
In 2007, the Company impaired approximately $1.3 million of property and equipment. See note 7.
(7) Impairment of Long-Lived Assets
During the fourth quarter ended December 31, 2007, a number of unexpected events occurred which impacted our assets, including changes in cash flow generation and our projected utilization of the assets within our revised plans. As a result of our impairment analysis under the provisions of SFAS No. 144, Accounting for Impairment or Disposal of Long-Lived Assets we recognized an impairment charge of approximately $1.6 million. Of this total impairment, approximately $1.3 million related to fixed assets and the balance of $0.3 million related to certain patents. The Company recognized these charges as part of operating expense in the business segment for SIMOX equipment.
(8) Other Assets
In December 2000, the Company entered into a royalty-bearing license agreement which gives the Company the right to manufacture SIMOX-SOI wafers using the licensed process. Much of the R&D development work is still being done using this licensed process in support of implanter and process improvements. Warrants were issued in connection with this agreement. The cost of the license agreement, including cash paid and the fair value of the warrants issued, was $2.3 million and is included in other assets at December 31, 2006 and December 31, 2007, net of accumulated amortization of $2.0 million and $2.3 million, respectively. The warrants associated with this transaction expired in December 2005 unexercised.
(9) Commitments and Contingencies
(a) Leases
Ibis’ corporate office and manufacturing site are located at a leased facility in Danvers, Massachusetts. The equipment manufacturing business and the engineering and wafer processing research and development effort are housed in approximately 32,000 square feet which includes a modernized cleanroom that contains metrology equipment, cleaning equipment and an implantation equipment manufacturing and service area. The lease was renewed during 2006 and will expire on June 30, 2011, and contains an option to renew for five years. The Company has no significant contractual obligations not fully recorded on its Balance Sheets or fully disclosed in the Notes to its Financial Statements. The Company has no off-balance sheet arrangements.
53
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
At December 31, 2007, the Company’s contractual obligations included:
|
| Payment due by period |
| |||||||||||
Contractual Obligations |
| Total |
| Less than |
| 2 |
| 3 |
| 4 |
| More |
| |
Minimum operating lease payments |
| $ | 1,329,230 |
| 400,164 |
| 375,308 |
| 369,456 |
| 184,302 |
| — |
|
Rent expense was approximately $0.5 million, $0.3 million and $0.3 million for the years ended December 31, 2005, 2006 and 2007, respectively.
(b) Contingencies
On April 26, 2007, the United Stares District Court in the District of Massachusetts approved a settlement agreement in the consolidated securities class action captioned In re Ibis Technology Securities Litigation, Civil Action No. 04-10446 RCL (D. Mass.). The Company and its Chairman of the Board, Martin J. Reid, and the plaintiffs reached an agreement in principle to settle the securities class action, which the Company announced on October 2, 2006. Thereafter, the parties executed a formal settlement agreement and submitted it for Court approval. The settlement provided for a payment to the plaintiffs of $1.9 million. The Court’s April 26, 2007 Order, certified the proposed class for settlement purposes only, approved the parties’ settlement agreement, and dismissed the securities class action with prejudice and in its entirety. The settlement was funded entirely by the Company’s insurance carrier and did not have a material adverse affect on our business, results of operations and financial condition.
On April 10, 2007, the Company announced that the shareholder derivative action filed in February 2004 against certain of the Company’s directors and officers: Louis F. Matheson, Jr. v. Martin J. Reid et al., Civ. Act. No. 04-10341 (RCL) (D. Mass) had been voluntarily dismissed by the plaintiff. The notice of dismissal filed by the plaintiff dismissed the action with prejudice. As set forth in the notice, the plaintiff will receive a $55,000 payment following the dismissal in consideration of plaintiff’s analysis of information provided by the Company regarding, among other things, the Company’s contention that the litigation should be dismissed for failure to make a demand on the Board of Directors before filing suit as required by Massachusetts law. The payment was funded entirely by the Company’s insurance carrier.
(10) Accrued Liabilities
Current accrued liabilities were as follows at December 31:
|
| 2006 |
| 2007 |
| ||
|
|
|
|
|
| ||
Accrued vacation |
| $ | 156,213 |
| $ | 129,903 |
|
Accrued warranty |
| 435,979 |
| — |
| ||
Accrued payroll |
| 128,343 |
| 39,401 |
| ||
Accrued expenses |
| 253,459 |
| 301,915 |
| ||
Total |
| $ | 973,994 |
| $ | 471,219 |
|
54
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
(11) Accrued Warranty
At the time that revenue is recognized for the sale of an implanter a liability for warranty is also established. An estimate of the warranty cost is made based on the number of years involved and the Company’s prior experience. As material and labor is used during the warranty period the liability is reduced based on these charges. At the end of the warranty term, the balance in the liability is eliminated and adjusted through cost of sales, the account charged at inception. A reconciliation of warranty liability for the years ended December 31, 2005, 2006 and 2007 is as follows:
Warranty balance December 31, 2004 |
| $ | 708,830 |
|
Expense incurred - 2005 |
| (31,061 | ) | |
Initiated - 2005 |
| — |
| |
Expired - 2005 |
| (24,636 | ) | |
Warranty balance December 31, 2005 |
| $ | 653,133 |
|
Expense incurred - 2006 |
| (160,939 | ) | |
Initiated - 2006 |
| 608,220 |
| |
Expired - 2006 |
| (664,435 | ) | |
Warranty balance December 31, 2006 |
| $ | 435,979 |
|
Expense incurred - 2007 |
| (45,391 | ) | |
Initiated - 2007 |
| — |
| |
Expired - 2007 |
| (390,588 | ) | |
Warranty balance December 31, 2007 |
| $ | — |
|
(12) Deferred Revenue
Deferred revenue at December 31, 2006 was $150,000 in deposits for service from SUMCO and zero dollars at December 31, 2007.
(13) License Agreements
The Company obtained an exclusive sublicense in the field of oxygen implantation to the proprietary beam scanning system developed by a consultant to the Company during the development of the first Ibis 1000 implanter. The beam scanning system sublicense agreement also grants the Company certain rights to further sublicense the technology for certain applications. The Company received $0.3 million, $0.5 million and $0.4 million in 2005, 2006 and 2007, respectively, for non-refundable option fees or royalty fees in accordance with non-exclusive sublicense agreements.
55
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
(14) Income Taxes
The income tax provision (benefit) attributable to continuing operations consists of the following:
|
| Years ended December 31, |
| |||||||
|
| 2005 |
| 2006 |
| 2007 |
| |||
Current: |
|
|
|
|
|
|
| |||
Federal |
| $ | — |
| $ | — |
| $ | — |
|
State |
| 1,256 |
| 1,256 |
| 800 |
| |||
Deferred: |
|
|
|
|
|
|
| |||
Federal |
| — |
| — |
| — |
| |||
State |
| — |
| — |
| — |
| |||
Total |
| $ | 1,256 |
| $ | 1,256 |
| $ | 800 |
|
State income tax expense (benefit), which consists of Massachusetts and California excise and corporate taxes. Income tax expense differs from the amount computed by applying the statutory federal income tax rate of 34% to the income (loss) before income taxes from continuing operations as follows:
|
| 2005 |
| 2006 |
| 2007 |
| |||
|
|
|
|
|
|
|
| |||
Computed “expected” tax expense (benefit) |
| $ | (3,216,586 | ) | $ | 142,444 |
| $ | (2,038,139 | ) |
State income taxes, net of federal tax benefit |
| 829 |
| 26,441 |
| (376,632 | ) | |||
Other |
| — |
| — |
| 2,252 |
| |||
Losses (benefited)/not benefited |
| 3,217,013 |
| (167,629 | ) | 2,413,319 |
| |||
|
| $ | 1,256 |
| $ | 1,256 |
| $ | 800 |
|
The tax effects of temporary differences that give rise to significant portions of deferred tax assets and liabilities are presented below at December 31:
|
| 2006 |
| 2007 |
| ||
|
|
|
|
|
| ||
Deferred tax assets: |
|
|
|
|
| ||
Net operating loss carryovers |
| $ | 34,586,000 |
| $ | 33,902,000 |
|
Accruals not currently deductible for tax purposes |
| 1,607,000 |
| 2,027,000 |
| ||
General business tax credit carryovers |
| 2,374,000 |
| 1,926,000 |
| ||
Impairment reserves |
| 1,906,000 |
| 1,765,000 |
| ||
SFAS 123R equity compensation |
| 173,000 |
| 323,000 |
| ||
Less: Valuation allowance |
| (38,715,000 | ) | (38,452,000 | ) | ||
Total deferred tax assets |
| 1,931,000 |
| 1,491,000 |
| ||
Deferred tax liabilities: |
|
|
|
|
| ||
Property and equipment, principally due to differences in depreciation |
| (1,735,000 | ) | (1,428,000 | ) | ||
Patents |
| (196,000 | ) | (63,000 | ) | ||
Net deferred tax assets |
| $ | — |
| $ | — |
|
56
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
Total valuation allowance decreased by $2.0 million and decreased by $0.3 million for the years ended December 31, 2006 and 2007, respectively. The Company has recorded a valuation allowance against its net deferred tax assets for 2006 and 2007, since management believes that, after considering all the available objective evidence, both positive and negative, historical and prospective, with the greater weight given to historical evidence, it is more likely than not that these assets will not be realized in the foreseeable future.
The Company had federal net operating loss and general business credit carryovers of approximately $91.6 million and $1.2 million, respectively, at December 31, 2007, with varying expiration dates through 2027 that may be used to offset future taxable income. State net operating loss and credit carryovers of $44.1 million and $1.2 million, respectively, have varying expiration dates. Included in the total deferred tax assets, offset by the valuation allowance, was $3.2 million related to the net operating loss carry forward resulting from the exercise of employee stock options, the tax benefit of which, will be accounted for as a credit to additional paid-in capital rather than a reduction of income tax expense when the deductions reduce current taxes payable.
The Company adopted the provisions of FASB Interpretation No. 48 (“FIN 48”) Accounting for Uncertainty in Income Taxes, an interpretation of FASB Statement No. 109 (“SFAS 109”) on January 1, 2007. As a result of the implementation of FIN 48, the Company recorded no adjustment for unrecognized income tax benefits. At the adoption date of January 1, 2007 and also at December 31, 2007, the Company had no unrecognized tax benefits. The Company does not expect that the total amount of unrecognized tax benefits will significantly increase in the next twelve months.
The Company recognises interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2007, the Company had no accrued interest or penalties related to uncertain tax positions. The tax years 2000 to 2006 remain open to examination by the major taxing jurisdictions to which we are subject. Preceding years also remain open to examination by U.S. federal and state revenue authorities to the extent of future utilization of net operating losses (NOLs) and research and development (R&D) tax credits generated in each preceding year.
Utilization of NOL and R&D credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future as provided by Section 382 of the Internal Revenue Code of 1986 and similar state provisions. These ownership changes may limit the amount of NOL and R&D credit carryforwards that can be utilized annually to offset future taxable income and tax. In general, an ownership change, as defined in Section 382, results from transactions which increase the ownership of certain 5% or greater shareholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. Since the Company’s formation, the Company has raised capital through the issuance of capital stock on several occasions which, combined with the purchasing shareholders’ subsequent disposition of those shares, may have resulted in a change of control, as defined in Section 382, or could result in a change of control in the future upon subsequent disposition. The Company has not currently completed a study to assess whether a change of control has occurred or whether there have been multiple changes of control since the Company’s formation. If the Company has experienced a change of control at any time since formation, or if Ibis experiences a change of control in the future, utilization of the NOL and R&D credit carryforwards would be subject to an annual limitation under Section 382. Any limitation may result in expiration of all or a portion of the NOL or R&D credit carryforwards before utilization. Until a study is completed and any limitation known, no amounts are being presented as an uncertain tax position under FIN 48.
(15) Capitalization
The Company has 50,000,000 shares of common stock and 2,000,000 shares of preferred stock (“Undesignated Preferred Stock”) authorized. At December 31, 2007, 50,368 common shares were reserved for issuance upon exercise of options outstanding under the Company’s 1993 Employee, Director and Consultant Stock Option Plan. At December 31, 2007, the Company also had 1,301,532 common shares
57
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
reserved for issuance upon exercise of options outstanding under the Company’s 1997 Employee, Director and Consultant stock option plan. At December 31, 2007, the Company also had 1,500,000 common shares reserved for issuance for grant under the Company’s 2007 Employee, Director and Consultant Stock Option Plan. At December 31, 2007, the Company had 146,038 common shares reserved for issuance under the Company’s 2000 Employee Stock Purchase Plan.
On February 16, 2007, the Company entered into a purchase agreement pursuant to which it agreed to sell unregistered shares of its common stock and warrants exercisable for common stock to Special Situation Funds for an aggregate purchase price of $5.3 million in a two-tranche financing. In connection with the first closing, we issued 1,400,000 shares of common stock and warrants to purchase 1,124,434 shares of common stock, for which the Company received approximately $2.2 million in proceeds, on February 20, 2007. Pursuant to stockholder approval, received at the Company Annual Meeting on May 10, 2007, at the second closing, the Company sold an additional 1,978,377 shares of common stock and warrants to purchase 1,588,966 shares of common stock for which the Company received approximately $3.1 million in proceeds. In connection with the transaction, the Company also entered into a registration rights agreement with the investors. The Company paid a placement fee to a placement agent, of approximately $0.3 million, which represents a cash fee equal to 5% of the gross proceeds and warrants exercisable for common stock with a dollar value equal to 5% of the aggregate gross proceeds received by the company in both closings of the financing. The Company also issued to the placement agent an aggregate of 179,619 warrants, exercisable for common stock, with a dollar value equal to 5% of the aggregate gross proceeds. Total transaction costs associated with the financing were approximately $0.4 million, providing net cash to the Company of $4.9 million. All of the securities were sold in a private placement pursuant to Regulation D of the Securities Act.
(16) Stock Plans and Warrants
(a) Stock Option Plans
In December 1993, the Board of Directors and stockholders approved the adoption of the Company’s 1993 Employee, Director and Consultant Stock Option Plan which provided for the issuance of options to purchase up to 250,000 shares of common stock to employees, consultants and non-employee directors. In May 1996, the stockholders increased to 750,000 shares the aggregate number of shares that may be granted under this plan.
In October 1997, the Board of Directors approved the adoption of the Company’s 1997 Employee, Director and Consultant Stock Option Plan (the “1997 Plan”) which provides for the issuance of options to purchase up to 750,000 shares of common stock of the Company to employees, consultants and non-employee directors. The stockholders approved the Plan at the May 1998 Annual Stockholders Meeting. In February 2001, the Board of Directors approved an amendment to the 1997 Plan to increase the aggregate number of shares reserved for issuance to 1,350,000. The stockholders approved this amendment at the May 2001 Annual Stockholders Meeting. In February 2004, the Board of Directors approved an amendment to the 1997 Plan to increase the aggregate number of shares reserved for issuance to 1,650,000. The stockholders approved this amendment at the May 2004 Annual Stockholders Meeting. In March 2006, the Board of directors approved an amendment to the 1997 plan to increase the aggregate number of shares reserved for issuance to 1,950,000. The stockholders approved this amendment at the May 2006 Annual Stockholders Meeting.
In May 2007, the Board of Directors and stockholders approved the adoption of the Company’s 2007 Employee, Director and Consultant Stock Option Plan which provided for the issuance of options to purchase up to 1,500,000 shares of common stock to employees, consultants and non-employee directors.
The Company has stock-based compensation plans under which employees and directors may be granted options to purchase common stock. Options are generally granted with exercise prices at not less than the fair market value on the grant date, generally vest over 4 years and expire in 10 years after the grant date. As of December 31, 2007 a total of 4.7 million shares had been authorized for grant under the Company’s
58
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
stock-based compensation plans. The number of common shares reserved for granting of future awards to employees and directors under these plans was 1.5 million at December 31, 2007. The remaining unrecognized compensation expense on stock options at December 31, 2007 was $0.5 million. The weighted average period over which the cost is expected to be recognized is approximately 1.5 years.
As of December 31, 2007, the Company had four equity compensation plans under which our equity securities have been authorized for issuance to our employees and/or directors: the 2000 Employee Stock Purchase Plan, as amended (see note 16), the 1993 Employee, Director and Consultant Stock Option Plan, as amended (the 1993 plan); the 1997 Employee, Directors and Consultant Stock Option Plan, as amended; and the 2007 Employee Director and Consultant Equity Plan, (the 2007 plan). The 1993 Plan and the 1997 Plan have expired and there are no options available for issuance; however, the 1993 Plan and the 1997 Plan continue to govern all options, awards and other grants granted and outstanding under the 1993 Plan and the 1997 Plan, respectively.
Distribution and Dilutive Effect of Options
The following table illustrates the grant dilution and exercise dilution for the years ended December 31:
|
| 2005 |
| 2006 |
| 2007 |
|
|
|
|
|
|
|
|
|
Shares of common stock outstanding |
| 10,816,029 |
| 10,915,481 |
| 14,317,482 |
|
|
|
|
|
|
|
|
|
Granted |
| 304,500 |
| 201,721 |
| 612,500 |
|
Canceled/forfeited |
| (331,473 | ) | (167,573 | ) | (614,985 | ) |
Expired |
| — |
| — |
| — |
|
Net options granted (cancelled) |
| (26,973 | ) | 34,148 |
| (2,485 | ) |
|
|
|
|
|
|
|
|
Grant dilution (1) |
| 0.0 | % | 0.3 | % | 0.0 | % |
Exercised |
| — |
| 1,025 |
| — |
|
Exercised dilution (2) |
| — |
| 0.0 | % | 0.0 | % |
(1) The percentage for grant dilution is computed based on net options granted as a percentage of shares of common stock outstanding.
(2) The percentage for exercise dilution is computed based on options exercised as a percentage of shares of common stock outstanding.
Basic and diluted shares outstanding for the years ended December 31, 2005, 2006 and 2007 were 10,737,924, 10,812,514, 10,853,304, 10,979,783, 13,410,938 and 13,410,938 shares respectively. During the year ending December 31, 2007, the dilutive effect of in the money employee stock options was zero shares or 0.0% of the basic shares outstanding based on the Company’s average share price of $1.23 as all options were below this average share price.
59
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
A summary of stock option activity under the plans is as follows:
|
| Options Outstanding |
|
|
| |||
|
| Options Available |
| Shares |
| Weighted average |
| |
|
|
|
|
|
|
|
| |
Balance outstanding as December 31, 2004 |
| 158,218 |
| 1,348,235 |
| $ | 14.06 |
|
Granted |
| (304,500 | ) | 304,500 |
| 1.46 |
| |
Exercised |
| — |
| — |
| — |
| |
Cancelled/forfeited |
| 331,473 |
| (331,473 | ) | 12.95 |
| |
Expired |
| — |
| — |
| — |
| |
Additional shares reserved |
| — |
| — |
| — |
| |
Balance outstanding as December 31, 2005 |
| 185,191 |
| 1,321,262 |
| $ | 11.43 |
|
Granted |
| (201,721 | ) | 201,721 |
| 3.17 |
| |
Exercised |
| — |
| (1,025 | ) | 1.36 |
| |
Cancelled/forfeited |
| 167,573 |
| (167,573 | ) | 16.42 |
| |
Expired |
| (10,117 | ) | — |
| — |
| |
Additional shares reserved |
| 300,000 |
| — |
| — |
| |
Balance outstanding as December 31, 2006 |
| 440,926 |
| 1,354,385 |
| $ | 9.59 |
|
Granted |
| (612,500 | ) | 612,500 |
| 1.47 |
| |
Exercised |
| — |
| — |
| — |
| |
Cancelled/forfeited |
| 614,985 |
| (614,985 | ) | 6.43 |
| |
Expired |
| (443,411 | ) | — |
| — |
| |
Additional shares reserved |
| 1,500,000 |
| — |
| — |
| |
Balance outstanding as December 31, 2007 |
| 1,500,000 |
| 1,351,900 |
| $ | 7.35 |
|
The following table summarizes information concerning outstanding and exercisable options as of December 31, 2007:
Range of |
| Number |
| Weighted |
| Weighted |
| Aggregate |
| Options |
| Weighted |
| |||
$.01 - 6.00 |
| 932,997 |
| 6.8 |
| $ | 2.53 |
| $ | 0 |
| 475,907 |
| $ | 3.08 |
|
$6.01 - 9.00 |
| 127,704 |
| 4.7 |
| $ | 7.90 |
|
|
| 115,204 |
| $ | 7.91 |
| |
$9.01 - 13.50 |
| 163,499 |
| 2.4 |
| $ | 10.65 |
|
|
| 163,499 |
| $ | 10.65 |
| |
$13.51 - 20.26 |
| 30,000 |
| 2.6 |
| $ | 18.14 |
|
|
| 30,000 |
| $ | 18.14 |
| |
$20.27 - 30.37 |
| 8,250 |
| 1.8 |
| $ | 23.35 |
|
|
| 8,250 |
| $ | 23.35 |
| |
$30.38 - 45.55 |
| 11,250 |
| 2.3 |
| $ | 37.24 |
|
|
| 11,250 |
| $ | 37.24 |
| |
$45.56 - 68.32 |
| 77,200 |
| 1.9 |
| $ | 46.35 |
|
|
| 77,200 |
| $ | 46.35 |
| |
$68.33 - 98.71 |
| 1,000 |
| 0.2 |
| $ | 89.94 |
|
|
| 1,000 |
| $ | 89.94 |
| |
|
| 1,351,900 |
|
|
|
|
| $ | 0 |
| 882,310 |
|
|
| ||
The aggregate intrinsic value in the preceding table represents the total pretax intrinsic value, based on the Company’s closing stock price of $0.43 as of December 31, 2007, which would have been received by the option holders had all option holders exercised their options as of that date. Since all options that were exercisable were at a price higher than the closing price at December 31, 2007, the intrinsic value is zero. The
60
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
fair value of stock options vested at December 31, 2006 and 2007 was $4.9 million and $3.6 million, respectively. The total number of in-the-money options exercisable as of December 31, 2007 was zero. As of December 31, 2007, 882,310 outstanding options were exercisable, and the weighted average exercise price was $10.13.
For the years ended December 31, 2006 and 2007, there were 201,721, and 612,500 options granted at the weighted average fair value of $0.5 million and $0.7 million, respectively. There were 155,577, and 275,321 options that vested at the weighted average fair value of $0.4 million and $0.6 million, respectively. The intrinsic value of options exercised were $1,456 and zero, respectively.
The Company had non-vested options of 497,785 shares with a weighted average fair value of $1.1 million at January 1, 2007. At December 31, 2007, there were non-vested options of 469,590 shares at the weighted average fair value of $0.8 million.
Valuation and Expense Information under SFAS 123(R)
The following table summarizes share-based compensation expense related to employee stock options and employee stock purchases under SFAS 123(R) for the year ended December 31, 2006 and December 31, 2007 which was allocated as follows:
|
| 2006 |
| 2007 |
| ||
|
|
|
|
|
| ||
Cost of sales |
| $ | 18,672 |
| $ | 26,276 |
|
General and administration |
| 213,807 |
| 179,581 |
| ||
Marketing and selling |
| 44,762 |
| 56,508 |
| ||
Research and development |
| 117,536 |
| 147,911 |
| ||
Stock based compensation expense included in operation expense |
| $ | 394,777 |
| $ | 410,276 |
|
As of December 31, 2007, the Company capitalized zero stock-based compensation in inventory. As of December 31, 2006, the Company capitalized $4.0 thousand of stock-based compensation in inventory. The Company did not recognize any tax benefits on the stock-based compensation recorded in the periods based on the current tax status of the Company.
61
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
The table below reflects reported net loss per share, basic and diluted for the year ended December 31, 2005 compared with the pro forma information as required by SFAS 123(R).
|
| Year ended |
| |
|
|
|
| |
Net loss as reported for prior periods (1) |
| $ | (9,245,305 | ) |
|
|
|
| |
Stock-based compensation expense related to employee stock options and employee stock purchases (2) |
| (717,379 | ) | |
|
|
|
| |
Net loss including the effect of stock-based compensation expense (3) |
| $ | (9,962,684 | ) |
|
|
|
| |
Per share information, basic and diluted: |
|
|
| |
Net loss as reported for prior periods (1) |
| $ | (0.86 | ) |
Net loss including the effect of stock-based compensation expense |
| $ | (0.93 | ) |
(1) Net loss and net loss per share for the year ended December 31, 2005 did not include stock-based compensation expense related to employee stock options and employee stock purchases under SFAS 123 because the Company did not adopt the recognition provisions of SFAS 123.
(2) Stock-based compensation expense is calculated on the pro forma application of SFAS 123 as previously disclosed in the notes to the Financial Statements.
(3) Net loss and net loss per share represents pro forma information based on SFAS 123 as previously disclosed in the notes to the Financial Statements.
Pro Forma Information Under SFAS 123 for Periods Prior to Fiscal 2007
The weighted-average estimated fair value of employee stock options granted during the year ended December 31, 2005 was $1.05 using the Black Scholes option-pricing model with the following weighted average assumptions:
|
| 2005 |
|
|
|
|
|
Expected Volatility |
| 97.62 | % |
Risk Free Interest Rate |
| 2.91 | % |
Dividend yield |
| 0.00 |
|
Expected option life (10 year contractual life option) |
| 4.00 |
|
For purposes of pro forma disclosures under SFAS 123, the estimated fair value of the options is assumed to be amortized to expense over the options’ vesting period.
The weighted-average estimated fair value of employee stock options granted during the twelve months ended December 31, 2006 and December 31, 2007 was $2.69 and $1.21 per share using the Black Scholes option-pricing model with the following weighted-average assumptions:
62
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
|
| 2006 |
| 2007 |
|
|
|
|
|
|
|
Expected Volatility |
| 102.49 | % | 97.32 | % |
Risk Free Interest Rate |
| 4.70 | % | 4.87 | % |
Dividend yield |
| 0.00 |
| 0.00 |
|
Expected option life (10 year contractual life option) |
| 6.25 |
| 6.25 |
|
The expected life of employee stock options is the anticipated average time that an option will be outstanding. The Company chose to use the simplified method provided under SAB107 to estimate the expected term for “plain vanilla” stock options. The expected term as calculated under the simplified method is 6.25 years.
The Company determined the expected volatility of the stock price by using a period equal to the expected life of the stock options, 6.25 years. Volatility was measured as the standard deviation of the difference in the natural logarithms of the stock over the expected life of the options using the daily closing stock price. This resulted in the volatility of 102.49 percent for the year ended December 31, 2006 and 97.32 percent for the year ended December 31, 2007.
The risk-free interest rate assumption is based upon observed treasury bill interest rates (risk free) appropriate for the expected term of the Company’s employee stock options.
As share-based compensation expense recognized in the Statement of Operations for the year ended December 31, 2006 and December 31, 2007 is actually based on awards ultimately expected to vest, it has been reduced for annualized estimated forfeitures of 14.18% and 33.26%, respectively. SFAS 123(R ) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience.
(b) Employee Stock Purchase Plan
On February 24, 2000, the Board of Directors adopted the Ibis Technology Corporation 2000 Employee Stock Purchase Plan (the “Purchase Plan”) pursuant to which a total of 300,000 shares of the Company’s Common Stock may be sold to eligible employees of the Company at a 15% discount from the market value of the shares. The stockholders approved the plan at the May 2000 Annual Stockholders Meeting. On February 17, 2005, the Board of Directors approved an amendment to the 2000 Employee Stock Purchase Plan to increase by 300,000 shares the aggregate number of shares of Common stock that can be sold to eligible employees. The stockholders approved the amendment at the May 2005 Annual Stockholders Meeting. Under the terms of the Purchase Plan, employees may elect to have up to 15% of their base earnings withheld to purchase these shares during each offering period, which is a six-month period. The purchase price under the Purchase Plan is 85% of the lesser of the market price on the beginning or the end of the offering period. Approximately 51% of eligible employees participated in the Purchase Plan, 51% in 2005, 51% in 2006 and 66% in 2007. During 2005, 2006, and 2007 the Company sold 96,434, 98,427 and 23,624 shares, respectively, to employees under the Purchase Plan.
(c) Warrants
In February 2007, in connection with the first closing of a two step financing agreement, the Company issued 1,050,000 Common Stock warrants exercisable for Common Stock at a strike price of $1.50 per share. Additionally, the Company issued 1,483,781 Common Stock warrants exercisable for Common Stock, also at a strike price of $1.50 per share, in a second closing held in May 2007.
63
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
In connection with the transaction, the Company also entered into a Registration Rights Agreement with the Investors. The Company paid a placement fee to a placement agent, which includes a cash fee equal to 5% of the Company’s gross proceeds from the agreement, and warrants of 74,434 and 105,185 exercisable for Common Stock with a dollar value also equal to 5% of the Company’s gross proceeds from the agreement All of the securities were sold in a private placement pursuant to Regulation D of the Securities Act of 1933, as amended (the “Act”), solely to accredited investors, as defined in Rule 501 of the Act.
(17) Significant Customers and Concentration of Business Risk
The Company sells its implanters to a limited number of semiconductor manufacturers primarily in the United States and the Pacific Rim.
Significant customer revenue is shown in dollar amounts and as a percentage of total revenue as follows:
Year Ended |
| Significant |
| Amount |
| % |
| |
|
|
|
|
|
|
|
| |
December 31, 2005 |
| — |
| $ | — |
| — | % |
December 31, 2006 |
| 1 |
| $ | 13,325,000 |
| 95 | % |
December 31, 2007 |
| — |
| $ | — |
| — | % |
Accounts receivable from significant customers was zero at December 31, 2005, $150,000 at December 31, 2006, and zero at December 31, 2007.
Export sales to unaffiliated customers in 2005, 2006, and 2007 were 81%, 99% and 94% of total revenues, respectively.
During 2005, 2006 and 2007, the Company purchased substantially all of its raw materials, components and subassemblies for its implanters from a limited group of suppliers. Disruption or termination of certain of these sources could occur and such disruptions could have a material adverse effect on the Company’s business and results of operations.
(18) Other Income
In 2006, the Company recognized a gain in other income of approximately $0.8 million, which is the result of the expiration of an option to place an order for an additional implanter prior to the end of 2006. This payment was received in the fiscal year ended December 31, 2007. In 2007, approximately $0.3 million of contract work done at a neighboring business is shown as Other Income.
(19) Discontinued Operations
In mid-2004, the Company discontinued the wafer manufacturing portion of the business in order to focus exclusively on the equipment business. Wafer product sales, as well as wafer revenue reported in subsequent periods are reported net of associated costs, as loss from discontinued operations or other income after 2005. For the year ending December 31, 2005, the Discontinued Operations generated a net gain of $0.2 million. The net gain of $0.2 million was the result of the reduction of $0.1 million of expenses associated with the closure of the wafer operation and $0.1 million from scrap recovery. For the period ending December 31, 2006 and December 31, 2007 there was no discontinued operations gain or loss recognized by the Company. In 2005 and 2006, the Company has separately disclosed the operating, investing, and financing portions of the cash flows attributable to discontinued operations.
64
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
(20) Industry Segments
The Company’s reportable segments are SIMOX Equipment and Other Products or Services. For purposes of segment reporting, equipment spares and field service revenue are combined and reported as SIMOX Equipment. Government contracts, other services and license revenue are combined and reported as Other Products or Services.
The accounting policies of the operating segments are the same as those described in the summary of significant accounting policies. The Company generally evaluates operating performance based on income or loss before interest and taxes.
The table below provides information for the years ended December 31, 2005, 2006, and 2007 pertaining to the Company’s two industry segments after the discontinuance of the wafer manufacturing business.
|
| SIMOX |
| Other Products |
| Total |
| |||
Net Revenue |
|
|
|
|
|
|
| |||
Year ended December 31, 2005 |
| $ | 277,684 |
| $ | 324,621 |
| $ | 602,305 |
|
Year ended December 31, 2006 |
| 13,427,035 |
| 560,384 |
| 13,987,419 |
| |||
Year ended December 31, 2007 |
| 525,267 |
| 425,924 |
| 951,191 |
| |||
|
|
|
|
|
|
|
| |||
Operating Income (Loss) |
|
|
|
|
|
|
| |||
Year ended December 31, 2005 |
| (7,785,117 | ) | 324,621 |
| (7,460,496 | ) | |||
Year ended December 31, 2006 |
| 1,041,270 |
| 560,384 |
| 1,601,654 |
| |||
Year ended December 31, 2007 |
| (7,658,924 | ) | 425,667 |
| (7,233,257 | ) | |||
|
|
|
|
|
|
|
| |||
Assets |
|
|
|
|
|
|
| |||
Year ended December 31, 2005 |
| 12,937,175 |
| 79,628 |
| 13,016,803 |
| |||
Year ended December 31, 2006 |
| 8,630,273 |
| 188,332 |
| 8,818,605 |
| |||
Year ended December 31, 2007 |
| 3,656,813 |
| 204,701 |
| 3,861,514 |
| |||
|
|
|
|
|
|
|
| |||
Capital Expenditures |
|
|
|
|
|
|
| |||
Year ended December 31, 2005 |
| 149,648 |
| — |
| 149,648 |
| |||
Year ended December 31, 2006 |
| 41,804 |
| — |
| 41,804 |
| |||
Year ended December 31, 2007 |
| 3,706 |
| — |
| 3,706 |
| |||
|
|
|
|
|
|
|
| |||
Depreciation and Amortization |
|
|
|
|
|
|
| |||
Year ended December 31, 2005 |
| 1,671,836 |
| — |
| 1,671,836 |
| |||
Year ended December 31, 2006 |
| 1,474,900 |
| — |
| 1,474,900 |
| |||
Year ended December 31, 2007 |
| 1,445,719 |
| — |
| 1,445,719 |
| |||
65
IBIS TECHNOLOGY CORPORATION
NOTES TO FINANCIAL STATEMENTS – (Continued)
The table below provides the reconciliation of reportable segment operating income (loss), assets, capital expenditures, and depreciation and amortization to the Company’s totals.
|
| Years ended December 31, |
| |||||||
|
| 2005 |
| 2006 |
| 2007 |
| |||
Segment Reconciliation |
|
|
|
|
|
|
| |||
Net Income (Loss): |
|
|
|
|
|
|
| |||
Total operating income (loss) for reportable segments |
| $ | (7,460,496 | ) | $ | 1,601,654 |
| $ | (7,233,257 | ) |
Corporate general and administrative expenses |
| (2,216,748 | ) | (2,400,645 | ) | (2,004,614 | ) | |||
Net other income |
| 217,953 |
| 1,205,132 |
| 698,859 |
| |||
Income tax expense |
| 1,256 |
| 1,256 |
| 800 |
| |||
Income (loss) from discontinued operations |
| 215,242 |
| — |
| — |
| |||
Net loss |
| $ | (9,242,793 | ) | $ | 404,885 |
| $ | (8,539,812 | ) |
|
|
|
|
|
|
|
| |||
Assets: |
|
|
|
|
|
|
| |||
Total assets for reportable segments |
| $ | 13,016,803 |
| $ | 8,818,605 |
| $ | 3,861,514 |
|
Cash & cash equivalents not allocated to segments |
| 6,856,874 |
| 4,812,477 |
| 5,770,072 |
| |||
Other unallocated assets |
| 118,711 |
| 157,759 |
| 131,247 |
| |||
Total assets |
| $ | 19,992,388 |
| $ | 13,788,841 |
| $ | 9,762,833 |
|
|
|
|
|
|
|
|
| |||
Capital Expenditures: |
|
|
|
|
|
|
| |||
Total capital expenditures for reportable segments |
| 149,648 |
| 41,804 |
| 3,706 |
| |||
Corporate capital expenditures |
| — |
| — |
| 8,134 |
| |||
Total capital expenditures |
| $ | 149,648 |
| $ | 41,804 |
| $ | 11,840 |
|
|
|
|
|
|
|
|
| |||
Depreciation and Amortization: |
|
|
|
|
|
|
| |||
Total depreciation and amortization for reportable segements |
| $ | 1,671,836 |
| $ | 1,474,900 |
| $ | 1,445,719 |
|
Corporation depreciation and amortization |
| 75,153 |
| 60,956 |
| 65,940 |
| |||
Total depreciation and amortization |
| $ | 1,746,989 |
| $ | 1,535,856 |
| $ | 1,511,659 |
|
(21) Selected Quarterly Financial Data (Unaudited)
The table below provides information for the years 2006 and 2007.
|
| First |
| Second |
| Third |
| Fourth |
| ||||
2006 |
|
|
|
|
|
|
|
|
| ||||
Total sales and revenue |
| $ | 6,260,822 |
| $ | 42,411 |
| $ | 7,223,863 |
| $ | 460,323 |
|
Gross profit (loss) |
| 3,588,030 |
| (11,073 | ) | 4,266,614 |
| 250,248 |
| ||||
Profit (loss) from continuing operations |
| 1,364,886 |
| (2,166,062 | ) | 2,237,233 |
| (1,031,172 | ) | ||||
Net income (loss) |
| 1,364,886 |
| (2,166,062 | ) | 2,237,233 |
| (1,031,172 | ) | ||||
Net income (loss) per common share |
| 0.12 |
| (0.20 | ) | 0.21 |
| (0.09 | ) | ||||
2007 |
|
|
|
|
|
|
|
|
| ||||
Total sales and revenue |
| $ | 330,063 |
| $ | 265,130 |
| $ | 193,009 |
| $ | 162,989 |
|
Gross profit (loss) |
| 214,225 |
| 60,751 |
| 14,003 |
| (1,552,664 | ) | ||||
Loss from continuing operations |
| (1,469,758 | ) | (1,446,740 | ) | (1,290,630 | ) | (4,332,684 | ) | ||||
Net loss |
| (1,469,758 | ) | (1,446,740 | ) | (1,290,630 | ) | (4,332,684 | ) | ||||
Net loss per common share |
| (0.14 | ) | (0.11 | ) | (0.09 | ) | (0.30 | ) | ||||
66
Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
Item 9A. | CONTROLS AND PROCEDURES |
a) Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report, management of the Company, with the participation of the Chief Executive Office and the Chief Financial Officer, evaluated the effectiveness of the design and operation of the Company’s disclosure controls and procedures (defined in Rule 13a-15e) of the Securities and Exchange Act of 1934, as amended) as of December 31, 2007. Based upon this evaluation, the Chief Executive Officer and the Chief Financial Officer have concluded that the Company’s disclosure controls and procedures were not effective as of December 31, 2007 because of the material weakness described in internal control over financial reporting disclosed in 9A(b).
b) Management’s Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Securities and Exchange Act of 1934, as amended. The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and preparation of published financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2007. In making its evaluation, the Company used the criteria set forth in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Based on this evaluation, management has concluded that internal control over financial reporting was ineffective as of December 31, 2007 as a result of the material weakness described below.
The Company did not have effective controls in its financial reporting process. Specifically:
· The Company did not maintain effective policies and procedures to ensure a sufficiently detailed management review of the preliminary financial statements including consideration of the impact of non-routine transactions on the financial statements. These deficiencies resulted in material adjustments to inventory reserves; property, plant and equipment and patents, errors in the income tax disclosures, and omissions of required disclosures in the Company’s 2007 preliminary financial statements which were corrected prior to issuance.
The Company’s independent registered public accounting firm did not issue an auditor’s report on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2007.
67
c) Changes in Internal Control over Financial Reporting
There have been no changes in the Company’s internal control over financial reporting in the quarter ended December 31, 2007 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
To address the material weakness described above, the Company currently is evaluating its accounting processes and timelines for quality review of the Company's financial statements and related disclosures. The Company has also enhanced procedures to help ensure that the proper accounting for all complex, non-routine transactions are researched, detailed in memoranda and reviewed by both the Chief Financial Officer and the Chief Executive Officer prior to recording and before the issuance of financial statements. These procedures increase (i) the required level of documentation with respect to assumptions supporting certain accounting conclusions, including without limitation, with respect to any impairment analysis, and (ii) the required level of review performed with respect to the reasonableness of such assumptions. In addition, beginning in the second quarter of 2008 and continuing until such time as management determines such action is no longer relevant or necessary, each quarter our Chief Financial Officer expects to prepare a summary and analysis of any change in our operations, forecasts of customer orders, overall financial position and strategic alternatives under consideration by management to be used in connection with any further asset impairment analysis.
Management recognizes that these enhancements require continual monitoring and evaluation for effectiveness. The development of these actions is an interactive process and will evolve as the Company continues to evaluate and improve our internal controls over financial reporting. Management will review progress on these activities on a consistent and ongoing basis at the senior management level in conjunction with our Audit Committee.
68
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors
The names of the Company’s current directors and certain information about them are set forth below:
Name |
| Age |
| Position |
|
|
|
|
|
Martin J. Reid |
| 66 |
| Chairman, President and Chief Executive Officer |
Dimitri Antoniadis, Ph.D. |
| 61 |
| Director |
Robert L. Gable(1) |
| 77 |
| Director |
Leslie B. Lewis(1)(2) |
| 67 |
| Director |
Donald F. McGuinness |
| 75 |
| Director |
Lamberto Raffaelli(2) |
| 57 |
| Director |
Cosmo S. Trapani(2) |
| 69 |
| Director |
(1) Member of the Compensation Committee
(2) Member of the Audit Committee
The following is a brief summary of the background of each director of the Company:
Martin J. Reid joined Ibis in December 1997 as President and Chief Executive Officer and as a director. In May 2000, Mr. Reid was elected to serve as Chairman of the Company’s Board of Directors. On November 20, 2006, Mr. Reid assumed the role of Executive Chairman and remained Chairman of the Board. On December 4, 2007, Mr. Reid resumed his position as President and Chief Executive Officer and Chairman of the Board upon the unexpected death of the Company’s then-current President and Chief Executive Officer, Charles McKenna. From 1991 to 1996, Mr. Reid was President and Chief Executive Officer of Alpha Industries (now known as Skyworks Solutions), a publicly held manufacturer of a broad range of Gallium Arsenide products and silicon integrated circuits for the semiconductor industry. He served as a director of Alpha Industries from 1985 to January 1998 and of Asahi America from 1997 to November 1999, which merged with Asahi Organic Chemical in December 1999.
Dimitri Antoniadis, Ph.D. was appointed to the Board of Directors in 1996. He is the Ray and Maria Stata Professor of Electrical Engineering at Massachusetts Institute of Technology (MIT) and has been a member of the faculty since 1978.
Robert L. Gable was appointed to the Board of Directors in 1997. He was a director and advisor (November 1998—October 1999), Chairman (June 1990—July 1998) and Chief Executive Officer (June 1990—October 1997) of Unitrode Corporation, a publicly held company that was acquired by Texas Instruments in October 1999.
Leslie B. Lewis was appointed to the Board of Directors in 1998. From 1985 to 2002, he was President of Asahi America, Inc., a publicly held company, which merged with Asahi Organic Chemical in December 1999. He was Chief Executive Officer of Asahi from 1989 to 2003 and Chairman from 1996 to 2002. In January 2004 Mr. Lewis was appointed Operations Partner of Watermill Ventures, a private strategic investment firm.
Donald F. McGuinness was appointed to the Board of Directors in 1996. He was Chairman, President and Chief Executive Officer (November 1989 to February 1999) of White Electronic Designs, Inc., a publicly held company that was acquired by Bowmar Instrument Corporation in October 1998.
Lamberto Raffaelli was appointed to the Board of Directors in 1998. In May 2001, he founded privately held LNX Corporation and serves as President and Chief Executive Officer. From 1994 to December 2000, he was President and Chief Executive Officer of Arcom, Inc., which was acquired by Quadrant-Vectron, a division of Dover Corporation International, in September 1999.
69
Cosmo S. Trapani was appointed to the Board of Directors in 2003. From 1990 to 1999 he was Executive Vice President and Chief Financial Officer of Unitrode Corporation, a publicly held company. From 1999 to 2000 he was Senior Vice President and Chief Financial Officer of Circor International, a publicly held company and from 2001 to 2003 was Vice President and Chief Financial Officer of PRI Automation, a publicly held company. Mr. Trapani serves on the Board of Directors of Hittite Microwave Corporation, a publicly traded diversified electronics company.
Executive Officers
The names of, and certain information regarding, current executive officers of the Company who are not also directors, are set forth below. Except for the President, Chief Executive Officer and Chairman, who has an employment agreement with the Company, all executive officers serve at the pleasure of the Board of Directors.
Name |
| Age |
| Position |
|
|
|
|
|
William J. Schmidt |
| 53 |
| Chief Financial Officer, Treasurer and Secretary |
Robert P. Dolan |
| 48 |
| Vice President of Wafer Technology |
William J. Schmidt joined the Company and was appointed Chief Financial Officer and Treasurer and Secretary in May 2004. Prior to joining Ibis, Mr. Schmidt was Vice President of Finance and Corporate Controller from April 2002 to May 2004 at High Voltage Engineering Corporation, a private equity based corporation focused in the capital equipment markets for semiconductors, instruments and industrial automation. Prior to joining High Voltage, from March 2000 to May 2002, Mr. Schmidt served as Vice President of Finance for PRI Automation (Brooks) and held senior level financial positions including Vice President of Finance and CFO roles at the operations of several leading technology companies including, PerkinElmer and Philips Electronics Corporation. Mr. Schmidt holds multiple Bachelors degree’s from West Virginia University and an MBA in Accounting and Taxation from Fairleigh Dickinson University (NJ).
Robert P. Dolan joined the Company in 1988 as Production Manager. In January 1997, he was appointed Vice President of Wafer Manufacturing. In August 2004, he was appointed Vice President of Wafer Technology. The Wafer Technology Group was formed after discontinuance of wafer operations to continue to work with our customers to improve the SIMOX process.
Stockholder Recommendations of Director Nominees
The Company’s By-laws include a procedure whereby its stockholders can nominate persons for election to the Board of Directors. The Board of Directors will consider director candidates recommended by the Company’s stockholders in a similar manner as those recommended by members of management or other directors, provided the stockholder submitting such nomination has complied with the procedures set forth in its By-laws. To date, the Company has not received any recommended nominees from any non-management stockholder or group of stockholders that beneficially owns more than five percent of its voting stock.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires the Company’s directors and officers, and persons who own more than 10% of the Company’s Common Stock, to file with the Securities and Exchange Commission (the “SEC”) initial reports of beneficial ownership and reports of changes in beneficial ownership of the Common Stock and other equity securities of the Company. Officers, directors and greater than 10% beneficial owners are required by SEC regulation to furnish the Company with copies of all Section 16(a) forms they file.
To the Company’s knowledge, based solely on review of the copies of such reports furnished to the Company and written representations that no other reports were required, during the fiscal year ended December 31, 2007, all Section 16(a) filing requirements applicable to its officers, directors and greater than 10% beneficial owners were complied with on a timely basis.
70
Audit Committee
The Audit Committee has three members and currently consists of Lamberto Raffaelli, Leslie B. Lewis and Cosmo S. Trapani, none of whom is an officer or employee of the Company. The Audit Committee met four times in 2007. The Audit Committee reviews the engagement of the Company’s independent registered public accounting firm, reviews quarterly and annual financial statements, considers matters relating to accounting policy and internal controls and reviews the scope of annual audits. A detailed listing of the Audit Committee’s responsibilities is set forth in the Company’s Audit Committee Charter adopted by the Board of Directors. All of the members of the Audit Committee, as evaluated by the Board of Directors in its business judgment, are independent directors within the meaning of Rule 4200(a)(15) of the Nasdaq Marketplace Rules and is independent pursuant to the rules of the Securities and Exchange Commission for the purposes of being an independent member of the Audit Committee. In addition, the Board of Directors has determined that Mr. Trapani qualifies as an “audit committee financial expert” under the rules of the SEC. Stockholders should understand that this designation is a disclosure requirement of the SEC related to Mr. Trapani’s experience and understanding with respect to certain accounting and auditing matters. The designation does not impose on Mr. Trapani any duties, obligations or liabilities that are greater than are generally imposed on him as a member of the Audit Committee and the Board of Directors, and his designation as an audit committee financial expert pursuant to the SEC requirement does not affect the duties, obligations or liabilities of any other member of the Audit Committee or the Board of Directors. The charter for the audit committee can be found on the “Corporate Governance” page located at the Investor Center tab of our website, www.ibis.com, free of charge.
Code of Ethical Conduct
The Company has adopted a Code of Ethical Conduct that applies to all officers, directors, employees and consultants. The Code of Ethical Conduct is intended to comply with Item 406 of Regulation S-K of the Securities Exchange Act of 1934 and with applicable Nasdaq Marketplace Rules. The Company’s Code of Ethical Conduct and its Financial Information Integrity Policy are available on the Company’s web site at www.ibis.com, free of charge. The Company intends to disclose any amendment to or waiver of a provision of the Code of Ethical Conduct that applies to its principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, by posting such information on its web site.
Item 11. EXECUTIVE COMPENSATION
Executive Compensation Disclosure and Analysis
The Compensation Committee of the Board of Directors is responsible for discharging the Board’s responsibilities relating to the compensation of our named executive officers, as well as our other officers and key employees, and, in this regard, has the responsibility to establish a compensation policy for officers and key employees designed to (i) enhance the profitability of the Company and increase shareholder value, (ii) reward officers and key employees for their contribution to our growth and profitability, and (iii) provide competitive compensation that will attract and retain qualified officers and key employees.
The individuals who served as our Chief Executive Officer (specifically Martin Reid and Charles McKenna) and Chief Financial Officer (specifically William Schmidt) during fiscal 2007, as well as other individuals (specifically Robert Dolan) included in the Summary Compensation Table on page 74, are referred to as “named executive officers” throughout this CD&A.
Compensation Policy
Subject to variation where appropriate, the compensation policy for officers and key employees includes:
· base salary, which is determined on an annual basis,
· annual or other time-based cash incentive compensation, and
71
· long-term incentive compensation in the forms of equity participation and other awards.
In setting compensation packages for the named executive officers, the Compensation Committee considers principles of internal equity with respect to each executive’s role relative to other executives and key employees within the Company, market competitiveness, short and long-term performance goals, and rewarding management’s collective role in the Company’s success. Although we seek to structure our compensation program to aggressively attract and retain key personnel, compensation is not set against any defined peer group in an effort to compete for talent at top prices. Given the Company’s ongoing strategic transitioning, the Compensation Committee has determined that external benchmarking is inappropriate at this time. Rather, a significant portion of our compensation program is based on internal benchmarking, including progress toward customer orders and customer technical servicing, which requires the application of judgment and subjective determination of both individual and Company performance. The Compensation Committee is comfortable with the benchmarking data and other supporting information provided to the Compensation Committee by the Company’s management and believes it is adequately experienced and equipped to address the relevant issues. The Compensation Committee has not, to date, used a third-party compensation consultant. The Compensation Committee believes that outside consultants are unnecessary at this time because of the limited straight-forward elements of the current compensation program.
Elements of Compensation
There is no pre-established policy or target for the allocation between either cash and non-cash or short-term and long-term incentive compensation, however a significant percentage of total compensation is allocated to incentives as a result of the philosophy discussed in this CD&A. On an annual basis, the Compensation Committee determines the appropriate level and mix of incentive compensation. The named executive officers will realize more income from such incentive compensation as a result of the positive performance of the Company. In some circumstances, the Company may establish incentive plans to reward individual performance against established goals or in connection with an employee retention plan. Historically, and in fiscal 2007 and 2006, the Compensation Committee granted a majority of total compensation to executive officers and key employees in the form of a base salary. In 2006, the only named executive officer to receive any additional cash incentive compensation received a bonus equal to 20% of his total base salary. In 2007, no additional cash incentive compensation was awarded.
Base Salary. The compensation philosophy of the Company is to maintain executive base salaries at a competitive level to enable the Company to attract and retain executive and key employee talent needed to accomplish the Company’s goals. In determining appropriate base salary levels and, to a lesser extent, other compensation elements, the Compensation Committee considers the scope of responsibility, prior experience and past accomplishments, as well as historical practices within the Company, each vis-à-vis other executives and key employees. Economic and business conditions affecting the Company are also considerations. Accordingly, the Compensation Committee has maintained base salaries at fairly low levels to conserve cash. Periodic adjustments in base salary may be merit based with respect to individual performance or tied to the Company’s financial condition or other competitive factors. As discussed below, at times, certain named executive officers have voluntarily agreed to reduce their base salaries in connection with Company’s cost-cutting initiatives.
Annual Incentive Bonuses. The Compensation Committee, in its discretion, may establish cash incentive programs and otherwise award bonuses to executive officers. More than any other element, bonus decisions are influenced by economic and business conditions affecting the Company and, as a result, the Compensation Committee has approved only limited cash bonuses to the named executive officers in recent years. It is anticipated that bonus awards in the future will be tied to obtained product orders.
Stock Options. The Compensation Committee believes that granting stock options on an ongoing basis provides officers with a strong economic interest in maximizing stock price appreciation over the long term. We believe that the practice of granting stock options is critical to retaining and recruiting the key talent necessary at all employee levels to ensure the Company’s continued success. Historically options have been granted to all permanent employees under the Second Amended and Restated 1997 Employee, Director and
72
Consultant Equity Plan, and particularly to key employees likely to contribute significantly to the Company. Such options granted after October 23, 2007 have been made pursuant to the 2007 Employee, Director and Consultant Equity Plan. In determining the size of an option grant to a named executive officer, the Compensation Committee considers not only competitive market factors, changes in responsibility and the executive officer’s achievement of pre-established goals, but also the number, term and vesting of options previously granted to the officer. The Compensation Committee may also consider an executive’s total compensation package, or changes made thereto, when determining whether to make an option grant.
Executive officers, as well as other officers and employees, receive a grant of stock options upon employment with the Company. Stock options are awarded at an exercise price equal to the fair market value of our common stock on the date of grant, as defined in the plan pursuant to which the options are issued, and typically vest one quarter per year over a four year period. The Compensation Committee typically approves stock option grants annually at the meeting coinciding with the annual meeting of stockholders. However, the Compensation Committee has the discretion to make additional periodic stock option grants at its discretion, including without limitation, to offset salary reductions or in lieu of base salary increases or bonus consideration.
Benefits. The named executive officers are entitled to participate in benefit plans which are generally available to all employees, including health, dental, life, accidental disability and dismemberment and for each of these benefit plans, the Company makes contributions to the premiums paid to the Plans. The Company does not offer any kind of deferred compensation, nor does it offer retirement benefits other than a 401(k) defined contribution plan. The Company typically does not match contributions made by executive officers and other employees to the 401(k) plan. Named executive officers are also encouraged to participate in the Company’s Employee Stock Purchase Plan, through which employees may purchase shares at a 15% discount from fair market value.
Change of Control Arrangements. The Company has entered into certain Change of Control agreements with Mr. Reid and Mr. Schmidt. It is the Company’s belief that the interests of our shareholders will be best served if the interests of our executive officers are aligned with them, and providing change of control benefits should eliminate, or at least reduce, the reluctance of the executive officers to pursue potential change of control transactions that may be in the best interests of the shareholders.
Role of Executive Officers in Setting Compensation Decisions
Regarding most compensation matters, Mr. Reid historically has provided recommendations to the Compensation Committee relying on his personal experience serving in the capacity of President and Chief Executive Officer with respect to evaluating the contribution of our other executive officers. Through November 2007, compensation recommendations also included input from Dr. McKenna, our then-current President and Chief Executive Officer. The Compensation Committee considers, but retains the right to accept, reject or modify such recommendations. Although Mr. Reid attends portion of the meetings of the Compensation Committee, neither he nor any other member of management is present during executive sessions of the Compensation Committee. Moreover, Mr. Reid is not present when decisions with respect to his compensation are made. The Compensation Committee may, but does not currently delegate any of its functions to others in setting compensation for the named executive officers, provided, however, that management may be responsible for the implementation of certain compensation programs established by the Compensation Committee. The Company does not currently engage any consultant related to executive and/or director compensation matters.
Compensation of Named Executive Officers
On November 28, 2005, base salaries for Messrs. Reid, McKenna, Schmidt and Dolan were set at $214,240, $214,240, $161,200 and $146,600, respectively, marking a 4% increase from salaries set earlier in 2005. These November 2005 base salaries remained unchanged throughout 2006. The decision to maintain the
73
base salary rate did not reflect a negative view of the named executive officers’ performance, but rather the Compensation Committee determined that the base salaries provided adequate compensation given the Company’s limited cash flow.
The Compensation Committee determined that there would continue to be no increases Company-wide in base salaries for fiscal 2007. In furtherance of these cost reductions, on January 8, 2007, the Compensation Committee approved voluntary reductions in base salaries for Dr. McKenna and Mr. Reid, reducing their base salaries by 50% to for 2007. Similar voluntary reductions were approved for Mr. Schmidt, reducing his salary by 33%, and for Mr. Dolan, reducing his salary by 20% for fiscal 2007. In light of these reductions, Messrs. McKenna, Schmidt and Dolan received grants of stock options. It is anticipated that the salary reductions will continue until such time as the Company significantly increases its product orders.
Payments of bonuses are at the discretion of the Compensation Committee and, in fiscal 2006, no bonus awards were paid to the named executive officers other than to Mr. Dolan. The bonus payment, which was made in two equal installments, was in the aggregate equal to 20% of Mr. Dolan’s 2006 base salary and was intended to reward Mr. Dolan for his efforts in connection with customer acceptance of an i2000 implanter during the year, developments of certain i2000 implanter specifications for additional implanters and retention of key process group staff. The first bonus award was paid in the first quarter of 2006 based on Mr. Dolan’s contribution for that quarter, and the second payment was made in January, 2007 for achievement over the remainder of 2006. Similar awards were made to three other key employees within the Company. The Compensation Committee did not award any cash bonus to Mr. Dolan, or to any of the other named executive officers in 2007, and such bonuses are not anticipated for 2008 other than in the event of a significant increase in product orders.
The only awards of stock options to the named executive officers in fiscal 2006 were grants of 100,000 and 25,000 incentive stock options made to Mr. Reid and Mr. Schmidt, respectively, in March of 2006. These grants were made in an effort to equitably award equity incentive compensation vis-à-vis the other named executive officers. Dr. McKenna had received options in 2005 in connection with his consulting services and upon becoming an officer of the Company and Mr. Dolan was the recipient of a grant in connection with a Company-wide option grant in May 2005. Neither Mr. Reid nor Mr. Schmidt received any stock options in fiscal 2005.
In November 2006, as part of the Company’s efforts to conserve cash, the Company initiated an option grant in lieu of salary increase program, whereby employees rather than receiving a salary increase with respect to 2007 base salary compensation, were instead granted the equivalent of five percent of their 2006 base salary in the form of an option grant with one year vesting. The program was designed as part of an overall retention effort, as well as to improve morale, and provide the opportunity for employees to potentially earn in excess of five percent of their base salary if the Company’s operating performance improved and was reflected in the Company’s stock price. The named executive officers did not participate in the option grant in lieu of salary increase program.
In light of the voluntary reductions in salary by each of the named executive officers for fiscal 2007, in January 2007, the Compensation Committee approved grants to Messrs. McKenna, Schmidt and Dolan of 110,000, 30,000 and 30,000, respectively. The options were intended to qualify as incentive stock options and vest over three years. The grants were made in an effort to align the named executive officers’ total compensation package with prior years and provide alternative compensation in light of the voluntary salary reductions.
Accounting and Tax Implications
On December 2004, the Financial Accounting Standards Board issued FASB Statement No. 123(R), Share Based Payment, which requires all companies to treat the fair value of stock options granted to employees as an expense. As a result of this standard, effective for periods beginning after January 1, 2006, we are required to record a compensation expense equal to the fair value of each stock option granted. SFAS 123(R)
74
requirements reduce the attractiveness of granting stock options because of the additional expense associated with these grants, which have impacted and will continue to negatively impact our results of operations. Nevertheless, the Compensation Committee has determined that stock options are an important employee recruitment and retention tool, and, to date, we have not reduced the scope of our stock option program.
The Compensation Committee has reviewed and considered Section 162(m) of the Internal Revenue Code, which generally disallows a tax deduction of more than $1 million that is paid to certain executive officers. Given the general range of salaries and bonuses for the named executive officers, as well as other officers and employees, the threshold is unlikely to be reached in the foreseeable future.
The following table shows compensation information for fiscal 2007 and 2006 for the named executive officers.
Summary Compensation Table
(a) |
| (b) |
| (c) |
| (d) |
| (e) |
| (f) |
| (g) |
|
Name and Principal |
| Year |
| Salary |
| Bonus |
| Option |
| All Other |
| Total ($) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Martin J. Reid, Executive Chairman, |
| 2007 | (3) | 111,240 |
| — |
| 108,868 |
| 6,081 |
| 226,189 |
|
Chairman of the Board |
| 2006 | (4) | 214,240 |
| — |
| 120,850 |
| 6,081 |
| 341,171 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Charles M. McKenna, PhD, President and |
| 2007 | (5) | 97,566 |
| — |
| 78,280 |
| — |
| 175,846 |
|
Chief Executive Officer |
| 2006 | (6) | 214,240 |
| — |
| 34,148 |
| 3,160 |
| 251,548 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
William J. Schmidt, CFO Treasurer and |
| 2007 |
| 109,200 |
| — |
| 88,921 |
| 809 |
| 198,930 |
|
Clerk |
| 2006 |
| 161,200 |
| — |
| 73,835 |
| 809 |
| 235,844 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Robert P. Dolan, Vice President of Wafer |
| 2007 |
| 118,440 |
| — |
| 25,773 |
| 475 |
| 144,688 |
|
Technology |
| 2006 |
| 146,640 |
| 29,328 |
| 17,389 |
| 475 |
| 193,166 |
|
(1) The amounts in column (e) reflect the dollar amount recognized for financial statement reporting for the fiscal years ended December 31, 2007 and December 31, 2006 and computed in accordance with SFAS 123(R), of incentive stock option awards granted pursuant to the Second Amended and Restated 1997 Employee, Director and Consultant Equity Plan and thus include a portion of the SFAS 123(R) value of awards granted in and prior to 2006. The SFAS 123(R) valuations reflected in column (e) disregard the estimate of forfeitures related to service-based vesting conditions and, thus, assume zero forfeitures. Other assumptions used in the calculation of this amount are included in footnote 2(k) to the Company’s audited financial statements for the fiscal year ended December 31, 2007, included in this Annual Report on Form 10-K.
(2) The amounts in column (f) reflect the value attributable to group term life insurance benefits in excess of $50,000 per year. The company provides all employees with Group Term Life (GTL). GTL to $50K is considered a non-taxable benefit. The cost of the premium over 50K is calculated based on age and added to an employees’ income.
75
(3) Mr. Reid resumed his position as President, Chief Executive Officer and Chairman of the Board on December 4, 2007 following the unexpected death of then-current President and Chief Executive officer, Dr. McKenna. Prior to such appointment Mr. Reid served as Executive Chairman. The amount shown in column (c) is the salary paid to Mr. Reid as Executive Chairman. His appointment as President and Chief Executive Officer did not result in any changes to his compensation.
(4) Mr. Reid served as President, Chief Executive Officer and Chairman of the Board until November 20, 2006, at which time he became Executive Chairman. The amount shown in column (c) is the salary paid to Mr. Reid as President, Chief Executive Officer and Chairman of the Board until such time and salary paid to Mr. Reid in his capacity as Executive Chairman after such time.
(5) Dr. McKenna served as President and Chief Executive Officer until his unexpected death on November 2, 2007. The amount shown in column (c) is the salary paid to Dr. McKenna in such capacity.
(6) Dr. McKenna served as Executive Vice President and Chief Operating Officer until November 20, 2006, at which time he became President and Chief Executive Officer. The amount shown in column (c) is the salary paid to Dr. McKenna as Executive Vice President and Chief Operating Officer until such time and salary paid to Dr. McKenna in his capacity as President and Chief Executive Officer after such time.
The following table shows grants of plan-based awards for fiscal 2007 for the named executive officers.
Grants of Plan-Based Awards
Name |
| Grant |
| All Other |
| Exercise or |
| Market Price |
| Grant Date |
|
(a) |
| (b) |
| (c) |
| (d) |
| (e) |
| (f) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Martin J. Reid |
| — |
| — |
| — |
| — |
| — |
|
Charles M. McKenna |
| 1/17/07 |
| 110,000 |
| 1.52 |
| 1.57 |
| 141,966 |
|
William J. Schmidt |
| 1/17/07 |
| 30,000 |
| 1.52 |
| 1.57 |
| 38,718 |
|
Robert P. Dolan |
| 1/17/07 |
| 30,000 |
| 1.52 |
| 1.57 |
| 38,718 |
|
|
| 7/2/07 |
| 15,000 |
| 1.53 |
| 1.48 |
| 17,585 |
|
(1) The option grants made to the named executive officers in 2007 are intended to qualify as incentive stock options and, as such, each option was issued with an exercise price equal to 100% of fair market value. Pursuant to the 1997 Plan, from which all of the options to the named executive officers were granted, “fair market value” means the closing or last price of the common stock on The Nasdaq Stock Market for the trading day immediately preceding the grant date. The amounts in column (e) represent the closing market price on the grant date.
(2) The amounts reflected in column (f) are the grant date fair values computed in accordance with FAS 123(R). Regardless of the value placed on a stock option on the grant date, the actual value of the option will depend on the market value of the common stock at such future date as the option is exercised.
76
The following table shows outstanding equity awards for fiscal 2007 for named executive officers.
Outstanding Equity Awards at 2007 Fiscal Year-End
Name |
| Number of |
| Number of |
| Option |
| Option Expiration |
|
(a) |
| (b) |
| (c) |
| (d) |
| (e) |
|
|
|
|
|
|
|
|
|
|
|
Martin J. Reid |
| 96,000 | (1) | — |
| 9.375 |
| Expired |
|
|
| 50,000 | (2) | — |
| 11.75 |
| 2/4/2009 |
|
|
| 50,000 | (2) | — |
| 46.25 |
| 1/4/2010 |
|
|
| 6,000 | (2) | — |
| 20.00 |
| 1/4/2011 |
|
|
| 30,000 | (2) | — |
| 7.98 |
| 8/7/2011 |
|
|
| 4,083 | (2) | — |
| 9.79 |
| 12/3/2011 |
|
|
| 30,000 | (2) | — |
| 6.00 |
| 6/17/2012 |
|
|
| 15,000 | (2) | — |
| 4.75 |
| 2/6/2013 |
|
|
| 9,517 | (3) | — |
| 9.01 |
| 8/26/2013 |
|
|
| 6,000 | (4) | — |
| 10.70 |
| 9/1/2013 |
|
|
| 37,500 | (2) | 12,500 | (2) | 3.63 |
| 8/24/2014 |
|
|
| 50,000 | (2) | 50,000 | (2) | 3.45 |
| 3/7/2016 |
|
|
|
|
|
|
|
|
|
|
|
Charles M. McKenna |
| 5,842 | (5) | — |
| 1.26 |
| 11/3/2008 |
|
|
| 51,472 | (6) | — |
| 1.73 |
| 11/3/2008 |
|
|
| 29,031 | (7) | — |
| 1.52 |
| 11/3/2008 |
|
|
|
|
|
|
|
|
|
|
|
William J. Schmidt |
| 37,500 | (2) | 12,500 | (2) | 7.88 |
| 5/10/2014 |
|
|
| 5,250 | (2) | 1,750 | (2) | 3.63 |
| 8/24/2014 |
|
|
| 12,500 | (2) | 12,500 | (2) | 3.45 |
| 3/7/2016 |
|
|
| 10,000 | (1) | 20,000 | (1) | 1.52 |
| 1/17/2017 |
|
|
|
|
|
|
|
|
|
|
|
Robert P. Dolan |
| 8,500 | (2) | — |
| 11.75 |
| 2/4/2009 |
|
|
| 17,000 | (2) | — |
| 46.25 |
| 1/4/2010 |
|
|
| 6,000 | (2) | — |
| 20.00 |
| 1/4/2011 |
|
|
| 11,500 | (2) | — |
| 7.98 |
| 8/7/2011 |
|
|
| 2,015 | (2) | — |
| 9.79 |
| 12/3/2011 |
|
|
| 10,000 | (2) | — |
| 6.00 |
| 6/17/2012 |
|
|
| 5,000 | (2) | — |
| 4.75 |
| 2/6/2013 |
|
|
| 4,017 | (3) | — |
| 9.01 |
| 8/26/2013 |
|
|
| 5,625 | (2) | 1,875 | (2) | 3.63 |
| 8/24/2014 |
|
|
| 7,500 | (2) | 7,500 | (2) | 1.36 |
| 5/2/2015 |
|
|
| 10,000 | (1) | 20,000 | (1) | 1.52 |
| 1/17/2017 |
|
|
| 3,750 | (8) | 14,625 | (8) | 1.53 |
| 7/2/2017 |
|
(1) Options granted have a vesting schedule of one-third per year over three years and a ten year term.
(2) Options granted have a vesting schedule of 25% per year over four years and a ten year term.
(3) Options granted on August 26, 2003 have a vesting schedule of 50% per year over two years and a 10 year term. Options were granted as part of an expense reduction which included mandatory salary reductions for all employees who earned $60,000 or more per year.
77
(4) Options granted on September 1, 2003, have a one year vesting and a ten year term. Options were granted as part of an expense reduction which included voluntary salary reductions.
(5) Options granted pursuant to a consulting agreement dated May 11, 2005; 2,500 options vested on July 1, 2005, 2500 options vested on July 1, 2007, and 842 prorated options vested upon the participants death. The options are exercisable within a one year period following the participant’s death.
(6) Options granted have a vesting schedule of 25% per year over four years and a ten year term. Ninety thousand options were granted on July 20, 2005; 22,500 options vested on July 20, 2006, 22,500 options vested on July 20, 2007, and 6,472 prorated options vested upon the participant’s death. The options are exercisable within a one year period following the participant’s death.
(7) Options granted have a vesting schedule of one-third per year over three years and a ten year term. One hundred and ten thousand options were granted on January 17, 2007; 29,031 prorated options vested upon the participant’s death. The options are exercisable within a one year period following the participant’s death.
(8) Options granted have a vesting schedule of 25% each six months over two years and a ten year term.
Employment Contracts and Change of Control Arrangements
The Company has an employment agreement with Martin J. Reid. This agreement, dated November 12, 2003, as amended, provides that Mr. Reid is to serve as Executive Chairman and Chief Executive Officer until December 31, 2008, at an initial annual base salary of $220,000, subject to change from time to time as determined by action of the Board of Directors and except as voluntarily reduced by Mr. Reid. The agreement also provides that Mr. Reid will be paid bonuses as are determined from time to time by the Board or its Compensation Committee in its discretion, taking into account, among other factors, Mr. Reid’s performance and the Company’s performance. In the event that Mr. Reid’s employment is terminated by the Company without cause or in certain other circumstances, Mr. Reid will be paid at his then annual base salary rate for a period of 12 months following the date of such termination and he will be entitled to be paid for the cost of 12 months of health benefits. Mr. Reid may terminate his employment at any time, but will forfeit certain benefits if he does not provide the Company with at least 60 days prior written notice. This agreement also contains a one-year post termination non-competition provision.
In 2005, the Company entered into a Restated Change of Control Agreement with Martin J. Reid and a Change of Control Agreement with William J. Schmidt. In the event that Mr. Reid’s employment is terminated within two years of a Change of Control (as defined in his agreement) of the Company, he will be paid a lump sum equal to twice the highest annual salary for the preceding three year period. In the event that Mr. Schmidt’s employment is terminated within two years of a Change of Control (as defined in his agreement) of the Company, he will be paid a lump sum equal to one times his highest annual salary for the preceding three year period. Annual salary includes base salary and bonus and excludes reimbursements and amounts attributable to stock options and other non-cash compensation. Under the agreements, each of Mr. Reid and Mr. Schmidt will also be provided health benefits for a period of time following termination or until the date they become eligible for such coverage offered by a subsequent employer if earlier. Mr. Reid is entitled to these benefits for two years and Mr. Schmidt is entitled to these benefits for one year. These severance compensation and benefits replace, and are provided in lieu of, any severance compensation and benefits that may be provided under any other agreement. “Change of Control” in both agreements is deemed to occur upon (i) a sale or transfer of all or substantially all of the Company’s assets; (ii) a merger or consolidation in which the Company is not the continuing or surviving corporation or the Company’s voting stockholders do not continue to hold 50% of the voting stock of the continuing or surviving corporation, or (iii) any person, together with affiliates and associates, acquires, directly or indirectly, securities representing 30% of the then outstanding shares of common stock or the combined voting power of all then outstanding securities having the right to vote in an election of the Board of Directors of the Company.
78
All of the Company’s employees are subject to certain confidentiality and non-competition obligations. Each employee has also agreed that all inventions, discoveries and developments which may be used in the Company’s business and that are developed by such employee during his or her employment with the Company are the Company’s property and the employee will assign his or her rights therein to the Company.
In the event of an “Acquisition” of the Company (as defined in the 1993 and 1997 Plans), the administrator of the Plans may either (i) make appropriate provision for the continuation of outstanding options, (ii) provide for accelerated vesting and/or exercise of outstanding options on certain conditions, or (iii) terminate all options in exchange for a cash consideration payment in connection with such Acquisition.
Compensation of Directors
The following table shows the compensation of the Company’s directors:
Name (1) |
| Fees |
| Option |
| Total |
|
(a) |
| (b) |
| (c) |
| (d) |
|
|
|
|
|
|
|
|
|
Dimitri Antoniadis, Ph.D. |
| 3,000 |
| 2,109 |
| 5,109 |
|
Robert L. Gable |
| 4,250 |
| 2,109 |
| 6,359 |
|
Leslie B. Lewis |
| 2,750 |
| 2,109 |
| 4,859 |
|
Donald F. McGuinness |
| 4,250 |
| 2,109 |
| 6,359 |
|
Lamberto Raffaelli |
| 3,750 |
| 2,109 |
| 5,859 |
|
Cosmo S. Trapani |
| 6,000 |
| 2,109 |
| 8,109 |
|
(1) Martin J. Reid, the Company’s Executive Chairman, is not included in this table, as he is an employee of the Company and thus receives no compensation for his service as Director. The compensation received by Mr. Reid as an employee of the Company is shown in the Summary Compensation Table on page 74 of this Form 10-K.
(2) The amounts in column (c) reflect the dollar amount recognized for financial statement reporting for the fiscal year ended December 31, 2007 and computed in accordance with SFAS 123(R), of incentive stock option awards granted pursuant to the Second Amended and Restated 1997 Employee, Director and Consultant Equity Plan and thus include a portion of the SFAS 123(R) value of awards granted in and prior to 2006. The SFAS 123(R) valuations reflected in column (c) disregard the estimate of forfeitures related to service-based vesting conditions and, thus, assume zero forfeitures. Other assumptions used in the calculation of this amount are included in footnote 2(k) to the Company’s audited financial statements for the fiscal year ended December 31, 2007, included in this Annual Report on Form 10-K.
Director Compensation Policy
The Company pays each non-employee director (Dr. Antoniadis, Mr. McGuinness, Mr. Gable, Mr. Lewis, Mr. Raffaelli and Mr. Trapani) $1,000 for each meeting of the Board of Directors or meeting of a committee of the Board that each of them attends and $250 for each telephonic meeting of the Board or telephonic meeting of a committee of the Board in which they participate. In 2007, the aggregate amount of compensation and reimbursement for such expenses paid to all of these directors was approximately $24,000.
Pursuant to the 2007 Plan, each non-employee director of the Company then serving as a director is automatically granted non-qualified stock options to purchase 1,250 shares of Common Stock following each Annual Meeting of Stockholders of the Company. These options vest in full immediately prior to the next annual meeting following the date the options are granted and have an exercise price equal to the closing price of the Common Stock for the trading day immediately preceding the grant date.
79
Compensation Committee Interlocks and Insider Participation
The Compensation Committee consists of two members, Leslie B. Lewis and Robert L. Gable, neither of whom was at any time during the past fiscal year an officer or employee of the Company, was formerly an officer of the Company or had any relationship with the Company requiring disclosure herein. During the fiscal year ended December 31, 2007, no executive officer of the Company served as a (a) member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served on the Compensation Committee of the Company; (b) director of another entity, one of whose executive officers served on the Compensation Committee of the Company; or (c) member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served as a director of the Company.
COMPENSATION COMMITTEE REPORT
The Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis contained in this proxy statement. The Compensation Committee is satisfied that the Compensation Discussion and Analysis fairly and completely represents the philosophy, intent, and actions of the Compensation Committee with regard to executive compensation. We recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this proxy statement for filing with the Securities and Exchange Commission.
|
| The Compensation |
|
| Robert L. Gable |
|
| Leslie B. Lewis |
Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Description of Equity Plans
The Company maintains four equity compensation plans under which the Company’s equity securities are authorized for issuance to the Company’s employees and/or directors. These plans consist of (a) the Company’s 1993 Employee, Director and Consultant Stock Option Plan, as amended; (b) the Company’s 1997 Employee, Director and Consultant Stock Option Plan, as amended; (c) the Company’s 2000 Employee Stock Purchase Plan, as amended, and (d) Company’s 2007 Employee, Director, and Consultant Equity Plan at the Annual Meeting.
80
Each of the foregoing equity compensation plans was approved by the stockholders of the Company. The table below presents information about all of the foregoing plans as of December 31, 2007.
Plan Category |
| Number of |
| Weighted-average |
| Number of |
| |
|
| (a) |
| (b) |
| (c) |
| |
|
|
|
|
|
|
|
| |
Equity compensation plans approved by security holders (1) |
| 3,885,681 |
| $ | 3.54 |
| 1,646,038 | (2) |
Equity compensation plans not approved by security holders |
|
|
| $ |
|
|
|
|
Total |
| 3,885,681 |
| $ | 3.54 |
| 1,646,038 | (2) |
(1) The 2000 Employee Stock Purchase Plan, as amended, is not included in (a) and (b) above as the number of outstanding options and weighted-average exercise price are not determinable under the 2000 Employee Stock Purchase Plan.
(2) This amount includes 146,038 non-transferable options available for exercise as of December 31, 2007 under the 2000 Employee Stock Purchase Plan.
1993 Employee, Director and Consultant Stock Option Plan, as amended
The 1993 Plan has expired and there are no options available for issuance under this plan; however, the 1993 Plan continues to govern all options, awards and other grants granted and outstanding under the 1993 Plan. All employees, directors and consultants of the Company and its affiliates (approximately 26 people) were eligible to participate in the 1993 Plan. Options granted under the 1993 Plan were either (i) options to qualify as “incentive stock options” under Section 422 of the Code, or (ii) non-qualified stock options. Incentive stock options granted under the 1993 Plan were not granted at a price less than the closing price of the Common Stock for the trading day immediately preceding the grant date, or 110% of the fair market value in the case of employees holding 10% or more of the voting stock of the Company. Non-qualified stock options granted under the 1993 Plan were not granted at an exercise price less than the closing price of the Common Stock for the trading day immediately preceding the grant date.
A total of 750,000 shares of Common Stock were initially reserved for issuance under the 1993 Plan. As of December 31, 2007, options to purchase up to an aggregate of 50,368 shares of Common Stock were outstanding, at a weighted average exercise price of $ 11.98 per share.
Please refer to the actual text of the 1993 Plan for a more detailed description of the features of the 1993 Plan.
1997 Employee, Director and Consultant Stock Option Plan, as amended
The 1997 Plan has expired and there are no options available for issuance under this plan, however, the 1997 Plan continues to govern all options, awards and other grants granted and outstanding under the 1997 plan. All employees, directors and consultants of the Company and its affiliates (approximately 51 people) were eligible to participate in the 1997 Plan. Options granted under the 1997 Plan may be either (i) options to qualify as “incentive stock options” under Section 422 of the Code, or (ii) non-qualified stock options. Incentive stock options granted under the 1997 Plan were not granted at a price less than the closing price of the Common Stock for the trading day immediately preceding the grant date, or 110% of the fair market value
81
in the case of employees holding 10% or more of the voting stock of the Company. Non-qualified stock options granted under the 1997 Plan were not granted at an exercise price less than the closing price of the Common Stock for the trading day immediately preceding the grant date.
Upon the initial adoption of the 1997 Plan, a total of 750,000 shares of Common Stock were initially reserved for issuance. The 1997 Plan was amended by the Company’s Board of Directors and its Stockholders on three occasions in order to increase the number of shares of Common Stock reserved for issuance to its current total of 1,950,000 shares. As of December 31, 2007, options to purchase up to an aggregate of 1,301,532 shares of Common Stock were outstanding, at a weighted average exercise price of $ 7.17 per share.
Please refer to the actual text of the 1997 Plan for a more detailed description of the features of the 1997 Plan.
82
2000 Employee Stock Purchase Plan, as amended
All employees that work 20 hours or more per week, who have been continuously employed by the Company for at least three months immediately prior to any offering period and who are not considered temporary employees are eligible to participate in the Purchase Plan. Temporary employees are defined as employees who are employed by the company for a specified period of time, less than six months.
However, any employee who would own more than 5% of the voting power of the Company’s stock immediately after a grant under the Purchase Plan is not eligible to participate and no participant may purchase more than $25,000 of Common Stock, based on the undiscounted value of the Common Stock at the beginning of each offering period, in any one calendar year.
The Purchase Plan is implemented by a series of offering periods, with a new offering period starting on June 1, and December 1 of each year (or such other times as may be determined by the Board of Directors). To participate in the Plan an eligible employee will authorize the Company to deduct up to 15% of the employee’s pay, not to exceed $21,250 per year, beginning on the first day of each designated offering period. On the first business day of each offering period, each eligible employee who has elected to participate in the Purchase Plan will be granted an option to purchase shares of the Company’s Common Stock. Unless a participating employee withdraws from the Purchase Plan prior to the end of the offering period, on the last day of the offering period the option will be automatically exercised for the purchase of a number of shares of the Company’s Common Stock determined by dividing the employee’s contributions during the offering period by the lesser of (i) 85% of the fair market value of the Common Stock on the first day of the offering period, or (ii) 85% of the fair market value of the Common Stock on the last of the offering period. Under the Purchase Plan, the fair market value of a share of Common Stock on a given date shall be determined by the Board of Directors based on the closing sale price of the Common Stock for such date (or if the Common Stock is not traded on such date, on the immediately preceding trading date), as reported on the Nasdaq National Market.
A participant may, on one occasion only during an offering period, decrease the amount of payroll deductions or may withdraw from participation in the Purchase Plan at any time. If a participant withdraws from the Purchase Plan or becomes ineligible to participate in the Purchase Plan, any accumulated employee contributions are paid back to such participant.
A total of 300,000 shares of Common Stock were initially reserved for issuance under the Purchase Plan to be sold to eligible employees of the Company at a discount from the market value of the shares. The Purchase Plan was amended by the Company’s Board of Directors and its stockholders in order to increase the number of shares of Common Stock reserved for issuance to its current total of 600,000. As of December 31, 2006, non-transferable options to purchase up to an aggregate of 146,038 shares of Common Stock were outstanding. The weighted-average exercise price is not determinable under the Purchase Plan.
Please refer to the actual text of the Purchase Plan for a more detailed description of the features of the Purchase Plan.
2007 Employee, Director, and Consultant Equity Plan
All employees, directors and consultants of the Company and its affiliates (approximately 51 people) are eligible to participate in the 2007 Plan. Options granted under the 2007 Plan may be either (i) options to qualify as “incentive stock options” under Section 422 of the Code, or (ii) non-qualified stock options. Incentive stock options granted under the 2007 Plan may not be granted at a price less than the closing price of the Common Stock on the grant date, or 110% of the fair market value in the case of employees holding 10% or more of the voting stock of the Company. Non-qualified stock options granted under the 2007 Plan may not be granted at an exercise price less than the closing price of the Common Stock on the grant date.
Upon the initial adoption of the 2007 Plan, a total of 1,500,000 shares of Common Stock were initially reserved for issuance. As of December 31, 2007,no stock options, stock purchase rights or other stock-based awards have been granted under the 2007 Plan The 2007 Plan expires on March 5, 2017, if not otherwise amended.
83
Share Ownership
The following table sets forth certain information as of May 22, 2008 concerning the ownership of Common Stock by (i) each stockholder known by the Company to be the beneficial owner of more than 5% of its outstanding shares of Common Stock, (ii) each current member of the Board of Directors, (iii) each executive officer named in the Summary Compensation Table contained herein, and (iv) all current directors and executive officers as a group.
|
| Shares Beneficially |
| ||
Names and Address (1)** |
| Number |
| Percent |
|
|
|
|
|
|
|
Special Situations Fund III QP, L.P. and its affiliated funds(3)(4) |
| 5,912,148 |
| 41.3 | % |
Martin J. Reid (5) |
| 304,100 |
| 2.1 | % |
Robert P. Dolan (6) |
| 90,907 |
| * |
|
Robert L. Gable (7) |
| 46,250 |
| * |
|
Charles M. McKenna (8) |
| 110,382 |
| * |
|
William J. Schmidt (9) |
| 76,227 |
| * |
|
Leslie B. Lewis (10) |
| 37,050 |
| * |
|
Lamberto Raffaelli (11) |
| 22,000 |
| * |
|
Dimitri Antoniadis, PhD (12) |
| 11,250 |
| * |
|
Cosmo S. Trapani (13) |
| 13,500 |
| * |
|
Donald F. McGuinness (14) |
| 10,000 |
| * |
|
|
|
|
|
|
|
Executive Officers and Directors as a group (10 Persons) |
| 721,666 |
| 4.8 | % |
* Represents beneficial ownership of less than 1% of the outstanding Common Stock.
** Addresses are given for beneficial owners of more than 5% of the outstanding Common Stock only.
(1) The address for each beneficial owner is c/o Ibis Technology Corporation, 32 Cherry Hill Drive, Danvers, Massachusetts, 01923.
(2) The number of shares of Common Stock issued and outstanding on May 22, 2008 was 14,317,482. The calculation of percentage ownership for each listed beneficial owner is based upon the number of shares of Common Stock issued and outstanding at May 22, 2008, plus shares of Common Stock subject to options held by such person or group at May 22, 2008 and exercisable within 60 days thereafter. The Company believes that all the persons and entities named in the Table have the sole voting and investment power with respect to all the shares as beneficially owned by them, except as noted below.
(3) MGP Advisors Limited (“MGP”) is the general partner of the Special Situations Fund III QP, L.P. (“QP”). AWM Investment Company, Inc. (“AWM”) is the general partner of MGP and the investment adviser to QP, the Special Situations Technology Fund, L.P. (“Tech”), the Special Situations Technology Fund II, L.P. (“Tech II”) and the Special Situations Private Equity Fund, L.P. (“PE”). Austin W. Marxe and David M. Greenhouse are the principal owners of MGP and AWM. Through their control of MGP and AWM, Messrs. Marxe and Greenhouse share voting and investment control over the portfolio securities of each of QP, Tech, Tech II and PE.
(4) Includes share voting and investment control over 1,689,189 shares of Common Stock, 145,270 shares of Common Stock, 686,233 shares of Common Stock and 675,675 shares of Common Stock held by QP, Tech, Tech II and PE, respectively, as well as 1,266,891 shares of Common Stock, 108,952 shares of Common Stock, 651,182 shares of Common Stock and 506,756shares of Common Stock issuable to QP, Tech, Tech II and PE upon the exercise of warrants exercisable within 60 days of the date hereof. The interest of Austin W. Marxe and David M. Greenhouse in the shares of Common Stock and Warrants owned by QP, PE, Tech and Tech II is limited to the extent of his pecuniary interest. This information is based solely on a Form SC 13D filed with the Securities and Exchange Commission and dated May 24, 2007.
84
(5) Includes 288,100 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
6) Includes 90,907 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(7) Includes11,250 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(8) Includes 86,345 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(9) Includes 65,250 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(10) Includes 18,750 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(11) Includes 15,000 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(12) Consists of 11,250 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(13) Includes 12,500 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(14) Consists of 10,000 shares of Common Stock that may be acquired upon the exercise of options within 60 days of May 22, 2008.
(15) See footnotes 5 through 14 above (10 Persons).
85
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
In connection with the review and approval or ratification, if appropriate, of any related party transaction, the Audit Committee should consider whether the transaction will compromise the Company’s professional standards included in its Code of Ethics. In the case of any related person transaction involving an outside director or nominee for director, the Audit Committee should also consider whether the transaction will compromise the director’s status as an independent director as prescribed in the Nasdaq listing standards.
All related person transactions are required to be disclosed in the Company’s applicable filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended and related rules.
In 2007 the Company was not a participant in any transaction or series of transactions or business relationship in which the amount involved exceeded $120,000 and in which any of its officers, directors or stockholders who own more than 5% of the Company’s Common Stock had or will have a direct or indirect material interest, nor is there currently proposed any such transaction or series of transactions or business relationship.
Director Independence
The Board of Directors has determined that, with the exception of Martin J. Reid, who is the Executive Chairman, all of the members of the Board of Directors are “independent directors” within the meaning of the director independence standards of The Nasdaq Stock Market, Inc. (“Nasdaq”) and the Securities and Exchange Commission (“SEC”), including Rule 10A-3(b)(1) under the Exchange Act of 1934, as amended (the “Exchange Act”). In making this determination, the Board considered relationships, transactions and/or arrangements with each of the directors, and concluded that, other than Martin J. Reid’s position as Executive Chairman of the Company, none of the directors has any relationships with the Company that would impair his or her independence. Furthermore, the Board of Directors has determined that each member of each of the committees of the Board of Directors is independent within the meaning of Nasdaq’s and the SEC’s director independence standards.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Audit and Non Audit Fees
The following table presents fees for professional services rendered by KPMG LLP and billed to us for the audit of the Company’s annual financial statements for the years ended December 31, 2007 and December 31, 2006 and fees billed for other services rendered by KPMG LLP during those periods.
Fees |
| Fiscal 2007 |
| Fiscal 2006 |
| ||
|
|
|
|
|
| ||
Audit Fees |
| $ | 168,765 |
| $ | 205,062 |
|
Audit-Related Fees |
|
|
|
|
| ||
Tax Fees |
|
|
|
|
| ||
All Other Fees |
|
|
|
|
| ||
Totals |
| $ | 168,765 |
| $ | 205,062 |
|
Audit Fees. Annual audit fees relate to services rendered in connection with the audit of the annual financial statements included in our Form 10-K, the SAS 100 quarterly reviews of financial statements included in our Form 10-Q filings and services, including, consent procedures rendered in conjunction with other SEC filings in fiscal years 2007 and 2006 through May 22, 2008. Additional fees related to the audit of fiscal year 2007 will be billed subsequently and will be approximately $40,000.
Audit-Related Fees. Audit-related services include fees for assurance and related services by the independent registered public accounting firm that are reasonably related to the performance of the audit or the
86
review of the Company’s financial statements and are not reported as Audit Fees. No such services were provided during fiscal years 2007 and 2006.
Tax Fees. Tax services include fees for professional services rendered by the independent registered public accounting firm relating to tax compliance, tax advice and tax planning. No such services were provided during fiscal years 2007 and 2006.
All Other Fees. All other fees include all other non-audit services. No such services were provided during fiscal years 2007 and 2006.
The Audit Committee has determined that the provision of the services provided by the independent registered public accounting firm, as set forth herein, is compatible with maintaining the independence of the independent registered public accounting firm.
Policy on Audit Committee Pre-Approval of Audit and Non-Audit Services of Independent Auditor
The Audit Committee’s policy is to pre-approve all audit and allowable non-audit services provided by the independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services. The Audit Committee may delegate the authority to grant pre-approval of auditing or allowable non-audit services to one or more members of the Audit Committee. Each pre-approval decision pursuant to this delegation will be presented to the full Audit Committee at its next scheduled meeting for ratification. All audit or allowable non-audit related fees described above were approved by the Audit Committee prior to services being rendered.
The affirmative vote of a majority of the shares voted affirmatively or negatively at the Meeting is required to ratify the appointment of the independent registered public accounting firm. In the event that ratification of the appointment of KPMG LLP as the independent registered public accounting firm for the Company is not obtained at the Meeting, the Board of Directors will reconsider its appointment. If the selection of KPMG LLP as the independent registered public accounting firm of the Company is ratified, the Audit Committee in its discretion may select a different registered public accounting firm at any time during the year if it determines that such a change would be in the best interests of the Company and its stockholders.
87
Item 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULE, AND REPORTS ON FORM 8-K |
(a) | The following documents are filed as part of this Annual Report on Form 10-K. |
(1) and (2) See “Index to Financial Statements and Financial Statement Schedule” at Item 8 to this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
(3) Exhibits
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
Exhibit |
| Description |
1 | - | Underwriting Agreement dated October 16, 2003, between the Registrant and CDC Securities (Filed as Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on October 17, 2003 and incorporated herein by reference) |
|
|
|
3.1 | - | Restated Articles of Organization of Registrant (Filed as Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the Quarter Ended September 30, 2000 and incorporated herein by reference) |
|
|
|
3.1.1 | - | Articles of Amendment to the Restated Articles of Organization of the Registrant (Filed as Exhibit 3.1.1 to the Company’s Quarterly Report on Form 10-Q for the Quarter Ended September 30, 2000 and incorporated herein by reference) |
|
|
|
3.1.2 | - | Articles of Amendment to the Restated Articles of Organization of the Registrant (Filed as Exhibit 3.1.2 to the Company’s Quarterly Report on Form 10-Q for the Quarter Ended September 30, 2000 and incorporated herein by reference) |
|
|
|
3.2 | - | Restated Bylaws of Registrant (Filed as Exhibit 3.1 to the Company’s Quarterly Report on Form 10Q for the Quarter Ended September 30, 2004 and incorporated herein by reference) |
|
|
|
4.1 | - | Article 4 of Restated Articles of Organization (Filed as Exhibit 4.1*) |
|
|
|
4.2 | - | Form of Common Stock Certificate (Filed as Exhibit 4.2*) |
|
|
|
4.3 | - | Purchase Agreement dated February 16, 2007 between the Registrant and various Investors (filed as Exhibit 4.1 to our Current Report on Form 8-K filed on February 21, 2007 and incorporated herein by reference) |
|
|
|
4.4 | - | Registration Rights Agreement dated February 16, 2007 between the Registrant. and various Investors (filed as Exhibit 4.2 to our Current Report on Form 8-K filed on February 21, 2007 and incorporated herein by reference). |
|
|
|
4.5 | - | Form of Warrant to Purchase Shares of Common Stock. |
|
|
|
4.6 | - | Placement Agent Form of Warrant to Purchase Shares of Common Stock. Hilton Glavish, and Zimec, Inc. (Filed as Exhibit 10.2*) |
|
|
|
10.1 | - | Master Agreement, dated as of August 7, 1992, among the Registrant, Dr. Hilton Glavish, and Zimec, Inc. (Filed as Exhibit 10.1*) |
|
|
|
10.2 | - | Sublicense Agreement, dated December 21, 1993, among the Registrant, Dr. Hilton Glavish, and Zimec, Inc. (Filed as Exhibit 10.2*) |
|
|
|
@10.3 | - | Business Development Agreement, dated as of July 15, 1994, between the Registrant and Mitsubishi Materials Corporation (Filed as Exhibit 10.3*) |
|
|
|
10.4 | - | Lease Agreement, dated December 22, 1987, as amended, between the Registrant and Thomas J. Flatley d/b/a The Flatley Company (“Flatley”) (Filed as Exhibit 10.4*) |
|
|
|
10.4A | - | Fifth Amendment to Lease Agreement, dated February 4, 1997 between the Registrant and Flatley (Filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the Quarter ended March 31, 1997 and Incorporated herein by reference). |
|
|
|
10.5 | - | Form of Noncompetition, Nondisclosure and Assignment of Inventions Agreement between the Registrant and each current employee of the Registrant (Filed as Exhibit 10.11*) |
|
|
|
†10.6 | - | Ibis Technology Corporation 1993 Employee, Director and Consultant Stock Option Plan as amended (Filed as Exhibit 10.15 to the Company’s Quarterly Report on Form 10-Q for the Quarter Ended June 30, 1996 and Incorporated herein by reference) |
|
|
|
†10.7 | - | Form of Stock Option Agreement under 1993 Employee, Director and Consultant Stock Option Plan (Filed as Exhibit 10.16*) |
88
Exhibit |
| Description | |
10.9 | - | Exclusive Patent License Agreement, dated November 1, 1994, between the Registrant and Superion Limited (Filed as Exhibit 10.26*) | |
|
|
| |
10.10 | - | License Agreement, dated as of September 1, 1994, between the Registrant and Nissin Electric Co., Ltd. (Filed as Exhibit 10.27*) | |
|
|
| |
10.11 | - | Equipment Purchase Master Agreement, dated as of May 22, 1996, between Registrant, and IBM (Filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K/A (File No.0-13078) filed on September 12, 1996 and incorporated herein by reference). | |
|
|
| |
†10.12 | - | Amended and Restated Ibis Technology Corporation 1997 Employee, Director and Consultant Stock Option Plan (Filed as Exhibit 99.1 to the Company’s Form S-8 (File No. 333-45247) filed on January 30, 1998, as amended May 18, 2001 and June 17, 2004 and incorporated herein by reference). | |
|
|
| |
10.13 | - | Form of Stock Option Agreement under 1997 Employee, Director and Consultant Stock Option Plan. | |
|
|
| |
@10.14 | - | Licensing and Development Agreement, dated June 9, 1998, between the Registrant and IBM (Filed as Exhibit 10.41 to the Company’s Quarterly Report on Form 10-Q for the Quarter Ended June 30, 1998 and incorporated herein by reference) | |
|
|
| |
10.15 | - | Sixth Amendment to Lease dated July 16, 1998, amending Lease Agreement dated December 22, 1987 between the Company and Thomas J. Flatley d/b/a the Flatley Company (Filed as Exhibit 10.42 to the Company’s Quarterly Report on Form 10-Q for the Quarter Ended September 30, 1998 and incorporated herein by reference) | |
|
|
| |
†10.16 | - | Restated Change of Control Agreement, dated March 24, 2005, between the Registrant and Martin J. Reid. (Filed as Exhibit 10.17 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 2004 and incorporated herein by reference). | |
|
|
| |
@10.17 | - | License Agreement dated July 1, 1999, between the Registrant and Mitsubishi Materials Silicon Corporation (Filed as Exhibit 10.45 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 1999 and incorporated herein by reference) | |
|
|
| |
10.18 | - | Ibis Technology Corporation 2000 Employee Stock Purchase Plan (Filed as Exhibit 99.1 to the Company’s Form S-8 (File No. 333-36706) filed on May 10, 2000, as amended May31, 2005 and incorporated herein by reference) | |
|
|
| |
@10.19 | - | Advantox 150 License Agreement dated November 1, 2000, between the Registrant and Mitsubishi Materials Silicon Corporation (Filed as Exhibit 10.48 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 2000 and incorporated herein by reference) | |
|
|
| |
†10.20 | - | Employment Agreement, dated November 12, 2003 between the Registrant and Martin J. Reid | |
|
|
| |
@10.21 | - | License Agreement dated December 15, 2000, between the Registrant and International Business Machines Corporation (“IBM”) (Filed as Exhibit 10.50 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 2000 and incorporated herein by reference) | |
|
|
| |
10.22 | - | Patent License Agreement dated December 15, 2000, between the Registrant and IBM (Filed as Exhibit 10.51 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 2000 and incorporated herein by reference) | |
|
|
| |
@10.23 | - | Amended and Restated License Agreement dated November 14, 2002, between the Registrant and IBM (Filed as Exhibit 10.30 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 2002 and incorporated herein by reference) |
|
|
|
|
|
10.24 | - | Amendment to the Patent License Agreement dated November 14, 2002, between the Registrant and IBM (Filed as Exhibit 10.31 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 2002 and incorporated herein by reference) |
|
|
|
|
|
†10.25 | - | Change of Control Agreement, dated March 24, 2005, between the Registrant and William J. Schmidt. (Filed as Exhibit 10.31 to the Company’s Annual Report on Form 10-K for the Year Ended December 31, 2004 and incorporated herein by reference) |
|
|
|
|
|
†10.26
| - | Amended and restated Ibis Technology Corporation 1997 Employee, Directors and Consultant Stock Option Plan (Filed as Exhibit 99.1 to the Company’s Form S-8 (file No. 333-45247) filed on January 30, 1998, as amended May 18, 2001, June 17, 2004 and May 26, 2006 and incorporated herin by reference ). |
|
89
Exhibit |
| Description | |
10.27 | - | Ninth Amendment to Lease dated April 3, 2006 between the Company and Cherry Hill Corporation Center LLC amending Lease Agreement dated December 22, 1987 between the company and Thomas J. Fatly d/b/a the Flatly Company (Filed as Exhibit 10.42 to the Company’s Quarterly Report as Form 10-Q for the Quarter Ended September 30, 1998 and incorporated herin by reference.) |
|
|
|
|
|
†10.28
| - | Amendment to Employment Agreement dated November 12, 2003 between the Registrant and Martin J. Reid, (Filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on August 17, 2006 and incorporated herein by reference) |
|
|
|
|
|
†10.29 | - | 2007 Employee, Director and Consultant Equity Plan (Filed as Appendix A to the Company’s Proxy Statement filed on April 3, 2007 and incorporated herein by reference.) |
|
|
|
|
|
†10.30 | - | Extension of Employment Agreement dated November 12, 2003 between the Registrant and Martin J. Reid (Filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 7, 2007 and incorporated herein by reference.) |
|
|
|
|
|
** 11 | - | Statement regarding computation of per share income (loss) |
|
|
|
|
|
**23 | - | Report and Consent on Financial Statement Schedule of KPMG LLP |
|
|
|
|
|
**31.1 | - | CEO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
|
**31.2 | - | CFO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
|
|
|
|
|
**32.1 | - | CEO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S. Section 1350) |
|
|
|
|
|
**32.2 | - | CFO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S. Section 1350) |
|
* Previously filed with the Commission as Exhibits to, and incorporated herein by reference from, the Company’s Registration Statement filed on Form S-1, File No. 333-1174, effective April 2, 1996.
** Filed herewith.
@ Confidential treatment previously obtained from the Securities and Exchange Commission. The portions of the document for which confidential treatment has been granted are marked “Confidential” and such confidential portions have been filed separately with the Securities and Exchange Commission.
† Management contract or compensatory plan, contract or arrangement.
Where a document is incorporated by reference from a previous filing, the Exhibit number of the document in that previous filing is indicated in parentheses after the description of such document.
(B) Financial Statement Schedules
Schedule II – Valuation and Qualifying Accounts
90
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in Danvers, Massachusetts on May 22, 2008.
| IBIS TECHNOLOGY CORPORATION |
| |
|
|
| |
| By: | /s/ Martin J. Reid | |
|
| Martin J. Reid | |
| President, Chief Executive Officer and Chairman | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities indicated and on the dates indicated.
Signatures |
| Title |
| Date | |
|
|
|
|
| |
By: | /s/ Martin J. Reid |
| President, Chief Executive Officer and |
| May 22, 2008 |
| Martin J. Reid |
| Chairman (principal executive officer) |
|
|
|
| and Director |
|
| |
|
|
|
|
| |
By: | /s/ William J. Schmidt |
| Chief Financial Officer, |
| May 22, 2008 |
William J. Schmidt |
| Treasurer and Secretary, (principal financial |
|
| |
|
| and accounting officer) |
|
| |
|
|
|
|
| |
By: | /s/ Dimitri A. Antoniadis |
| Director |
| May 22, 2008 |
Dimitri A. Antoniadis, Ph.D. |
|
|
|
| |
|
|
|
|
| |
By: | /s/ Robert L. Gable |
| Director |
| May 22, 2008 |
Robert L. Gable |
|
|
|
| |
|
|
|
|
| |
By: | /s/ Leslie B. Lewis |
| Director |
| May 22, 2008 |
Leslie B. Lewis |
|
|
|
| |
|
|
|
|
| |
By: | /s/ Donald McGuinnes |
| Director |
| May 22, 2008 |
Donald McGuinness |
|
|
|
| |
|
|
|
|
| |
By: | /s/ Lamberto Raffaelli |
| Director |
| May 22, 2008 |
Lamberto Raffaelli |
|
|
|
| |
|
|
|
|
| |
By: | /s/ Cosmo S. Trapani |
| Director |
| May 22, 2008 |
Cosmo S. Trapani |
|
|
|
| |
91
SCHEDULE II
IBIS TECHNOLOGY CORPORATION
VALUATION AND QUALIFYING ACCOUNTS
For the Years Ended December 31, 2005, 2006 and 2007
Description |
| Balance at |
| Expense |
| Amounts |
| Balance at |
| |
|
|
|
|
|
|
|
|
|
| |
Allowance for Doubtful Accounts |
|
|
|
|
|
|
|
|
| |
December 31, 2005 |
| $ | 25,000 |
| — |
| — |
| 25,000 |
|
December 31, 2006 |
| 25,000 |
| (25,000 | ) | — |
| — |
| |
December 31, 2007 |
| — |
| — |
| — |
| — |
| |
|
|
|
|
|
|
|
|
|
| |
Reserve for Inventory Obsolescence |
|
|
|
|
|
|
|
|
| |
December 31, 2005 |
| $ | 3,772,000 |
| — |
| (100,000 | ) | 3,672,000 |
|
December 31, 2006 |
| 3,672,000 |
| — |
| (225,000 | ) | 3,447,000 |
| |
December 31, 2007 |
| 3,447,000 |
| 1,790,000 |
| (60,000 | ) | 5,177,000 |
| |