Exhibit 99.1
| TARGET A BETTER NOW Nasdaq: IMGN J.P. Morgan Healthcare Conference January 11-14, 2021 Exhibit 99.1 |
| 2 This presentation includes forward-looking statements regarding ImmunoGen’s expectations related to the design and potential success of ImmunoGen’s mirvetuximab soravtansine and IMGN632 clinical studies and regulatory pathways, including the timing of initiating and receiving data from, as well as the likelihood of success of, the studies to support approval of mirvetuximab and IMGN632; the occurrence, timing, and outcome of other potential preclinical, clinical, and regulatory events related to ImmunoGen’s and its collaboration partners’ programs; and potential future collaborations. For these statements, ImmunoGen claims the protection of the safe harbor for forward-looking statements provided by the Private Securities Litigation Reform Act of 1995. Various factors could cause ImmunoGen’s actual results to differ materially from those discussed or implied in the forward-looking statements, and you are cautioned not to place undue reliance on these forward-looking statements, which are current only as of the date of this presentation. Factors that could cause future results to differ materially from such expectations include, but are not limited to, the risks and uncertainties inherent in the Company’s development programs, including clinical studies and regulatory processes, their timings and results, including the possibility that studies of mirvetuximab fail to confirm the hypotheses suggested by the exploratory analyses of the FORWARD I data, and the risks and uncertainties associated with the scale and duration of the COVID-19 pandemic and resulting impact on ImmunoGen’s industry and business.A review of these risks can be found in the “Risk Factors” set forth in Exhibit 99.1 to ImmunoGen’s current report on Form 8-k, filed with the Securities and Exchange Commission on December 18, 2020 and subsequent documents filed with the Securities and Exchange Commission. FORWARD-LOOKING STATEMENTS |
| 3 POISED TO BECOME A FULLY - INTEGRATED ONCOLOGY COMPANY WITH TWO PRODUCTS ON THE MARKET BY THE END OF 2022 ACCELERATED PATH FOR MIRVETUXIMAB IN PROC PIVOTAL DATA: Q3 2021 POTENTIAL APPROVAL: 2022 INNOVATIVE EARLIER STAGE CANDIDATES AND ADVANCED ADC TECHNOLOGY ANTICIPATED COMPENDIA LISTINGS FOR MIRVETUXIMAB COMBINATIONS PATH TO FULL APPROVAL FOR IMGN632 IN BPDCN PIVOTAL DATA: 12-18 MONTH S POTENTIAL APPROVAL: 2022 WHY IMMUNOGEN? PROC: platinum-resistant ovarian cancer; BPDCN: blastic plasmacytoid dendritic cell neoplasm; ADC: antibody-drug conjugate 3 EXPERIENCED LEADERSHIP TEAM AND STRONG CASH POSITION |
| 4 BRINGING ANTIBODY - DRUG CONJUGATES TO CANCER PATIENTS STRATEGIC PRIORITIES F U R T H E R STRENGTHEN BALANCE SHEET AND EXPAND CAPABILITIES T H R O U G H PARTNERSHIPS ADVANCE PORTFOLIO OF EARLIER STAGE PRODUCT CANDIDATES WITH A FOCUS ON PATH TO FULL APPROVAL FOR I M G N 6 3 2 C O M P L E T E MIRVETUXIMAB REGISTRATION STUDIES AND PURSUE OPPORTUNITIES TO MOVE INTO EARLIER LINES OF THERAPY |
| SOMEONE YOU KNOW HAS BEEN DIAGNOSED WITH OVARIAN CANCER … WHAT’S NEXT FOR HER? 5 |
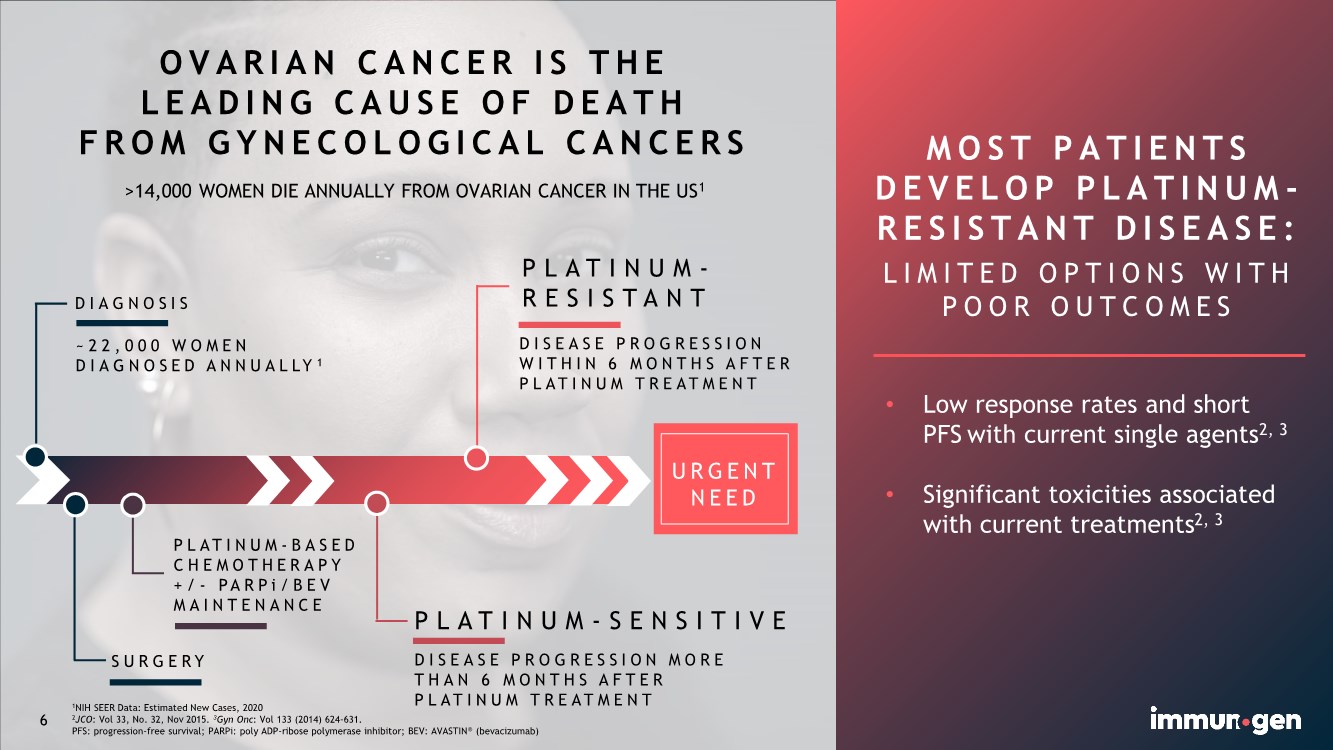
| U R G E N T NEED 1NIH SEER Data: Estimated New Cases, 2020 2JCO: Vol 33, No. 32, Nov 2015. 3Gyn Onc: Vol 133 (2014) 624-631. PFS: progression-free survival; PARPi: poly ADP-ribose polymerase inhibitor; BEV: AVASTIN® (bevacizumab) OVARIAN CANCER IS THE LEADING CAUSE OF DEATH FROM GYNECOLOGICAL CANCERS >14,000 WOMEN DIE ANNUALLY FROM OVARIAN CANCER IN THE US1 6 DISEASE PROGRESSION WITHIN 6 MONTHS AFTER PLATINUM TREATMENT PLATINUM- RESISTANT PLATINUM- B A S E D CHEMOTHERAPY +/- P A R P i / B E V MAINTENANCE PLATINUM-SENSITIVE DISEASE PROGRESSION MORE THAN 6 MONTHS AFTER PLATINUM TREATMENT SURGERY DIAGNOSIS ~22,000 WOMEN DIAGNOSED ANNUALLY 1 MOST PATIENTS DEVELOP PLATINUM - RESISTANT DISEASE: LIMITED OPTIONS WITH POOR OUTCOMES • Low response rates and short PFS with current single agents2, 3 • Significant toxicities associated with current treatments2, 3 |
| MIRVETUXIMAB SORAVTANSINE 7 DESIGNED TO DISPLACE CHEMOTHERAPY TO DELIVER MORE GOOD DAYS FOR WOMEN WITH OVARIAN CANCER KEY ATTRIBUTES • Novel ADC with distinct FRα-binding antibody, cleavable linker, and maytansinoid DM4 payload • Favorable tolerability profile • Demonstrated activity in patients with FRα-positive platinum-resistant and platinum-sensitive ovarian cancer1 • Sizeable safety database; studied in more than 700 patients DEVELOPMENT STRATEGY • Seek initial label as monotherapy in platinum-resistant ovarian cancer • Move into earlier lines of therapy and become the combination agent of choice in ovarian cancer • Lever cooperative groups and ISTs to generate complementary data in ovarian and endometrial cancers 1ASCO 2017 Poster; Moore, K., et al. ASCO 2019 Poster; O’Malley, D., et al. ESMO 2019 Poster; Moore, K., et al. ASCO 2020 Poster; Gilbert, L., et al. ESMO 2020 Poster; O’Malley, D., et al. FRα: folate receptor alpha; IST: investigator sponsored trial |
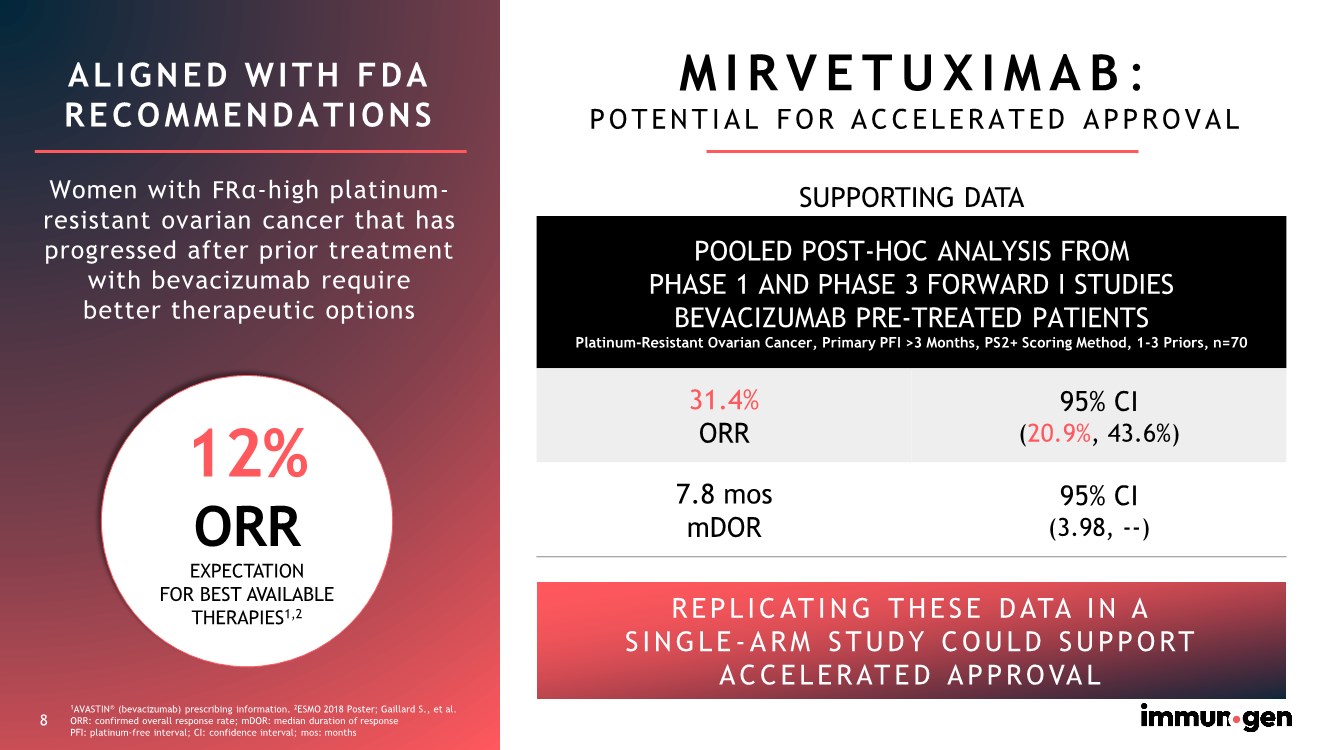
| 8 ALIGNED WITH FDA RECOMMENDATIONS Women with FRα-high platinum- resistant ovarian cancer that has progressed after prior treatment with bevacizumab require better therapeutic options MIRVETUXIMAB: POTENTIAL FOR ACCELERATED APPROVAL SUPPORTING DATA POOLED POST-HOC ANALYSIS FROM PHASE 1 AND PHASE 3 FORWARD I STUDIES BEVACIZUMAB PRE-TREATED PATIENTS Platinum-Resistant Ovarian Cancer, Primary PFI >3 Months, PS2+ Scoring Method, 1-3 Priors, n=70 31.4% ORR 95% CI (20.9%, 43.6%) 7.8 mos mDOR 95% CI (3.98, --) REPLICATING THESE DATA IN A SINGLE- ARM STUDY COULD SUPPORT ACCELERATED APPROVAL 1AVASTIN® (bevacizumab) prescribing information. 2ESMO 2018 Poster; Gaillard S., et al. ORR: confirmed overall response rate; mDOR: median duration of response PFI: platinum-free interval; CI: confidence interval; mos: months 8 12% ORR EXPECTATION FOR BEST AVAILABLE THERAPIES1,2 |
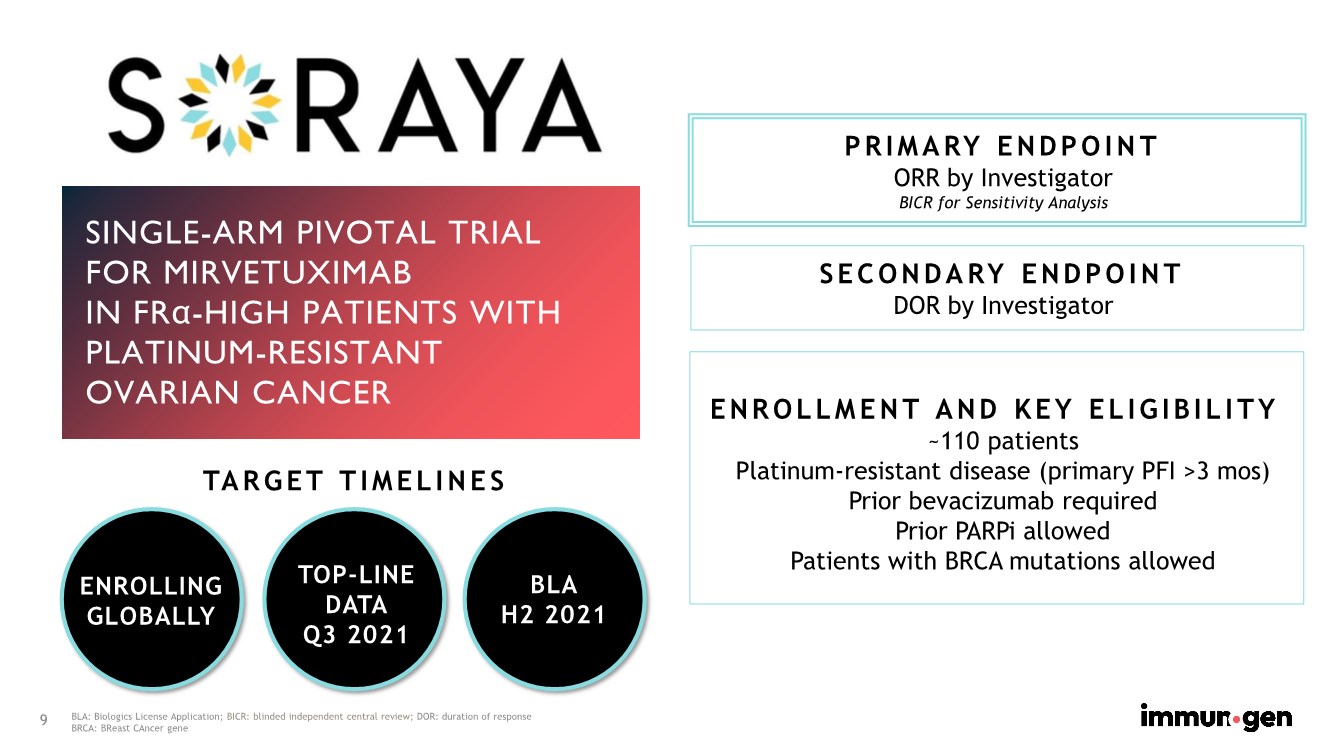
| 9 SINGLE-ARM PIVOTAL TRIAL FOR MIRVETUXIMAB IN FRα-HIGH PATIENTS WITH PLATINUM-RESISTANT OVARIAN CANCER PRIMARY ENDPOINT ORR by Investigator BICR for Sensitivity Analysis SECONDARY ENDPOINT DOR by Investigator ENROLLING GLOBALLY TARGET TIMELINES ENROLLMENT AND KEY ELIGIBILITY ~110 patients Platinum-resistant disease (primary PFI >3 mos) Prior bevacizumab required Prior PARPi allowed Patients with BRCA mutations allowed TOP-LINE DATA Q3 2021 BLA H2 2021 BLA: Biologics License Application; BICR: blinded independent central review; DOR: duration of response BRCA: BReast CAncer gene |
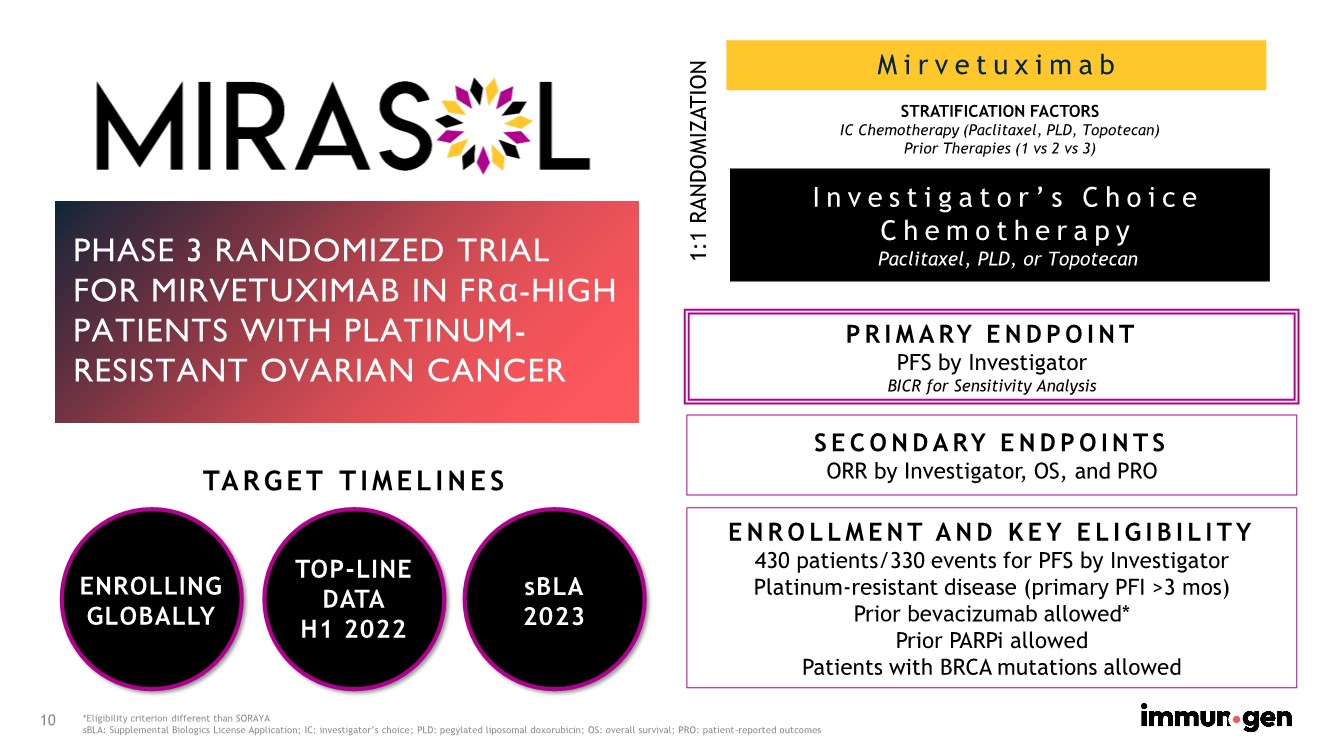
| 10 Mirvetuximab PHASE 3 RANDOMIZED TRIAL FOR MIRVETUXIMAB IN FRα-HIGH PATIENTS WITH PLATINUM- RESISTANT OVARIAN CANCER PRIMARY ENDPOINT PFS by Investigator BICR for Sensitivity Analysis *Eligibility criterion different than SORAYA sBLA: Supplemental Biologics License Application; IC: investigator’s choice; PLD: pegylated liposomal doxorubicin; OS: overall survival; PRO: patient-reported outcomes SECONDARY ENDPOINTS ORR by Investigator, OS, and PRO Investigator’s Choice Chemotherapy Paclitaxel, PLD, or Topotecan STRATIFICATION FACTORS IC Chemotherapy (Paclitaxel, PLD, Topotecan) Prior Therapies (1 vs 2 vs 3) 1:1 RANDOMIZATION ENROLLMENT AND KEY ELIGIBILITY 430 patients/330 events for PFS by Investigator Platinum-resistant disease (primary PFI >3 mos) Prior bevacizumab allowed* Prior PARPi allowed Patients with BRCA mutations allowed ENROLLING GLOBALLY TARGET TIMELINES TOP-LINE DATA H1 2022 sBLA 2023 |
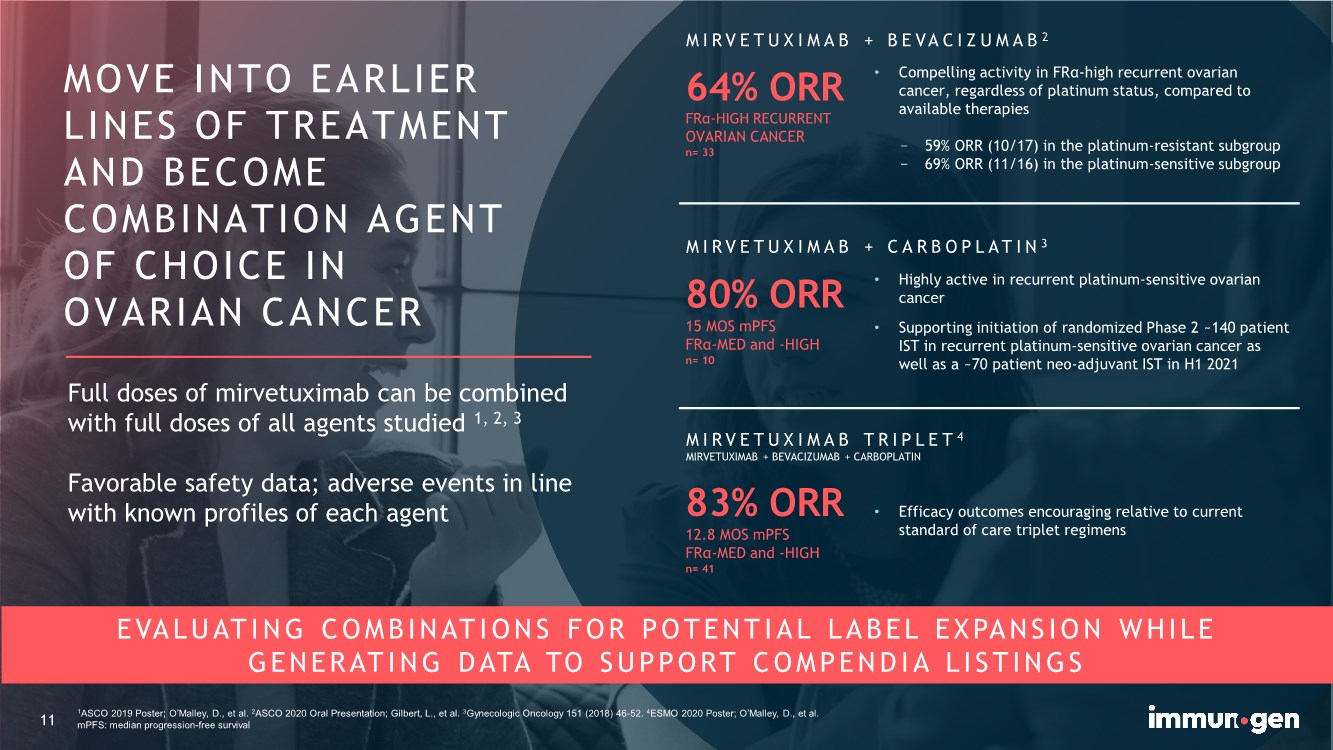
| 11 1ASCO 2019 Poster; O’Malley, D., et al. 2ASCO 2020 Oral Presentation; Gilbert, L., et al. 3Gynecologic Oncology 151 (2018) 46-52. 4ESMO 2020 Poster; O’Malley, D., et al. mPFS: median progression-free survival EVALUATING COMBINATIONS FOR POTENTIAL LABEL EXPANSION WHILE GENERATING DATA TO SUPPORT COMPENDIA LISTINGS MOVE INTO EARLIER LINES OF TREATMENT AND BECOME COMBINATION AGENT OF CHOICE IN OVARIAN CANCER 11 64% ORR FRα-HIGH RECURRENT OVARIAN CANCER n= 33 MIRVETUXIMAB + BEVACIZUMAB 2 MIRVETUXIMAB + CARBOPLATIN 3 • Compelling activity in FRα-high recurrent ovarian cancer, regardless of platinum status, compared to available therapies − 59% ORR (10/17) in the platinum-resistant subgroup − 69% ORR (11/16) in the platinum-sensitive subgroup • Highly active in recurrent platinum-sensitive ovarian cancer • Supporting initiation of randomized Phase 2 ~140 patient IST in recurrent platinum-sensitive ovarian cancer as well as a ~70 patient neo-adjuvant IST in H1 2021 80% ORR 15 MOS mPFS FRα-MED and -HIGH n= 10 MIRVETUXIMAB TRIPLET 4 MIRVETUXIMAB + BEVACIZUMAB + CARBOPLATIN 83% ORR 12.8 MOS mPFS FRα-MED and -HIGH n= 41 • Efficacy outcomes encouraging relative to current standard of care triplet regimens Full doses of mirvetuximab can be combined with full doses of all agents studied 1, 2, 3 Favorable safety data; adverse events in line with known profiles of each agent |
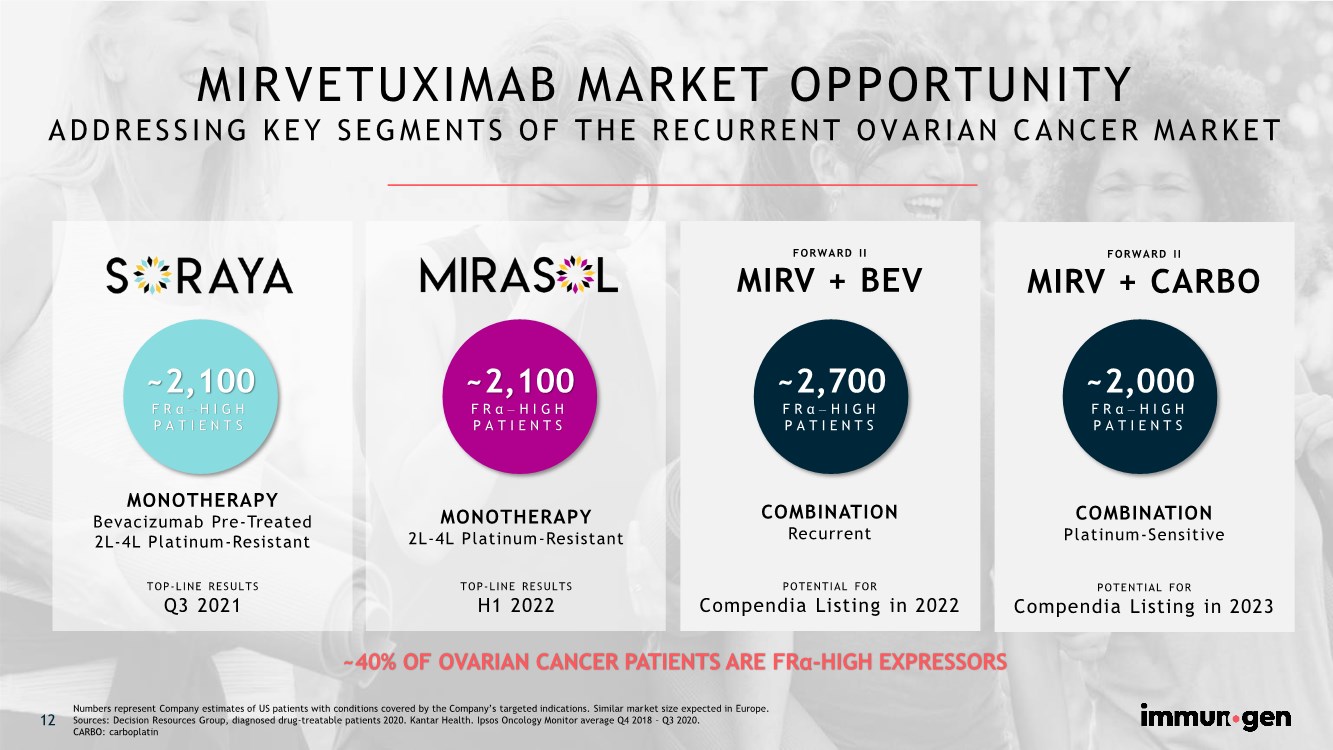
| 12 MIRVETUXIMAB MARKET OPPORTUNITY ADDRESSING KEY SEGMENTS OF THE RECURRENT OVARIAN CANCER MARKET ~40% OF OVARIAN CANCER PATIENTS ARE FRα-HIGH EXPRESSORS MONOTHERAPY Bevacizumab Pre-Treated 2L-4L Platinum-Resistant TOP-LINE RESULTS Q3 2021 ~2,100 FRα -HIGH PATIENTS FORWARD II MIRV + CARBO COMBINATION Platinum-Sensitive POTENTIAL FOR Compendia Listing in 2023 MONOTHERAPY 2L-4L Platinum-Resistant TOP-LINE RESULTS H1 2022 ~2,100 FRα -HIGH PATIENTS FORWARD II MIRV + BEV COMBINATION Recurrent POTENTIAL FOR Compendia Listing in 2022 ~2,000 FRα -HIGH PATIENTS ~2,700 FRα -HIGH PATIENTS 12 Numbers represent Company estimates of US patients with conditions covered by the Company’s targeted indications. Similar market size expected in Europe. Sources: Decision Resources Group, diagnosed drug-treatable patients 2020. Kantar Health. Ipsos Oncology Monitor average Q4 2018 – Q3 2020. CARBO: carboplatin |
| 13 ROBUST DATA IN MORE THAN 700 PATIENTS • Strong and consistent efficacy data in FRα-high patients • Favorable tolerability profile • Selection assay identifies patients most likely to benefit SORAYA: POTENTIAL PATH TO ACCELERATED APPROVAL • Enrolling patients globally • Top-line data expected in Q3 2021 • BLA expected by the end of 2021 with potential for accelerated approval in 2022 MIRASOL: DESIGNED TO PROVIDE DATA TO SUPPORT FULL APPROVAL • Enrolling patients globally • Top-line data expected in H1 2022 • Potential for full approval in 2023 COMBINING TO DEVELOP MIRVETUXIMAB FOR EARLIER LINES OF THERAPY • Mature MIRV + BEV data in recurrent ovarian cancer to be presented at ASCO 2021 • Planning for label expansion and compendia listings PREPARING FOR COMMERCIALIZATION • Pre-commercial activities underway in the US • Strategic collaboration with Huadong established to develop and commercialize mirvetuximab in mainland China, Hong Kong, Macau, and Taiwan MIRVETUXIMAB FOR OVARIAN CANCER |
| SOMEONE YOU KNOW HAS BEEN DIAGNOSED W I T H B P D C N … WHAT’S NEXT? 14 |
| 15 OUTCOMES REMAIN POOR, PARTICULARLY FOR NON- TRANSPLANT CANDIDATES CURRENTLY APPROVED THERAPIES REQUIRE INPATIENT HOSPITALIZATION AND ARE ASSOCIATED WITH SIGNIFICANT TOXICITIES B P D C N I S A RARE AND AGGRESSIVE HEMATOLOGIC MALIGNANCY ~500-1,000 NEW CASES DIAGNOSED ANNUALLY IN THE US1 60% TO 70% BECOME R/R S T E M C E L L TRANSPLANT DIAGNOSIS U R G E N T NEED SELECT CASES 1MDAnderson.org 2019; Pagano Haematologica 2013; Leukemia Lymphoma Society LLS.org. Internal estimates. Expect similar number of cases annually in Europe. R/R: relapsed refractory I N T E N S I V E C H E M O O R TARGETED THERAPY C H E M O O R TARGETED THERAPY |
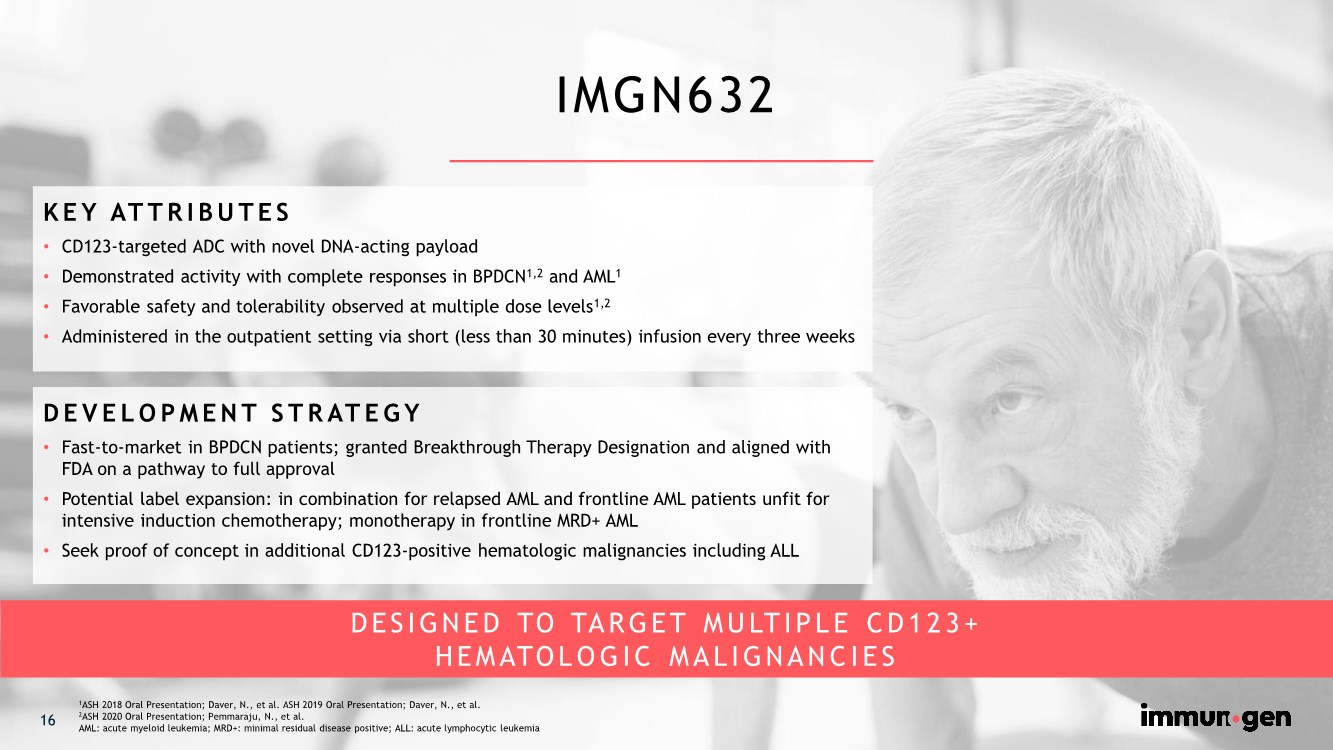
| IMGN632 16 1ASH 2018 Oral Presentation; Daver, N., et al. ASH 2019 Oral Presentation; Daver, N., et al. 2ASH 2020 Oral Presentation; Pemmaraju, N., et al. AML: acute myeloid leukemia; MRD+: minimal residual disease positive; ALL: acute lymphocytic leukemia DESIGNED TO TARGET MULTIPLE CD123+ HEMATOLOGIC MALIGNANCIES KEY ATTRIBUTES • CD123-targeted ADC with novel DNA-acting payload • Demonstrated activity with complete responses in BPDCN1,2 and AML1 • Favorable safety and tolerability observed at multiple dose levels1,2 • Administered in the outpatient setting via short (less than 30 minutes) infusion every three weeks DEVELOPMENT STRATEGY • Fast-to-market in BPDCN patients; granted Breakthrough Therapy Designation and aligned with FDA on a pathway to full approval • Potential label expansion: in combination for relapsed AML and frontline AML patients unfit for intensive induction chemotherapy; monotherapy in frontline MRD+ AML • Seek proof of concept in additional CD123-positive hematologic malignancies including ALL |
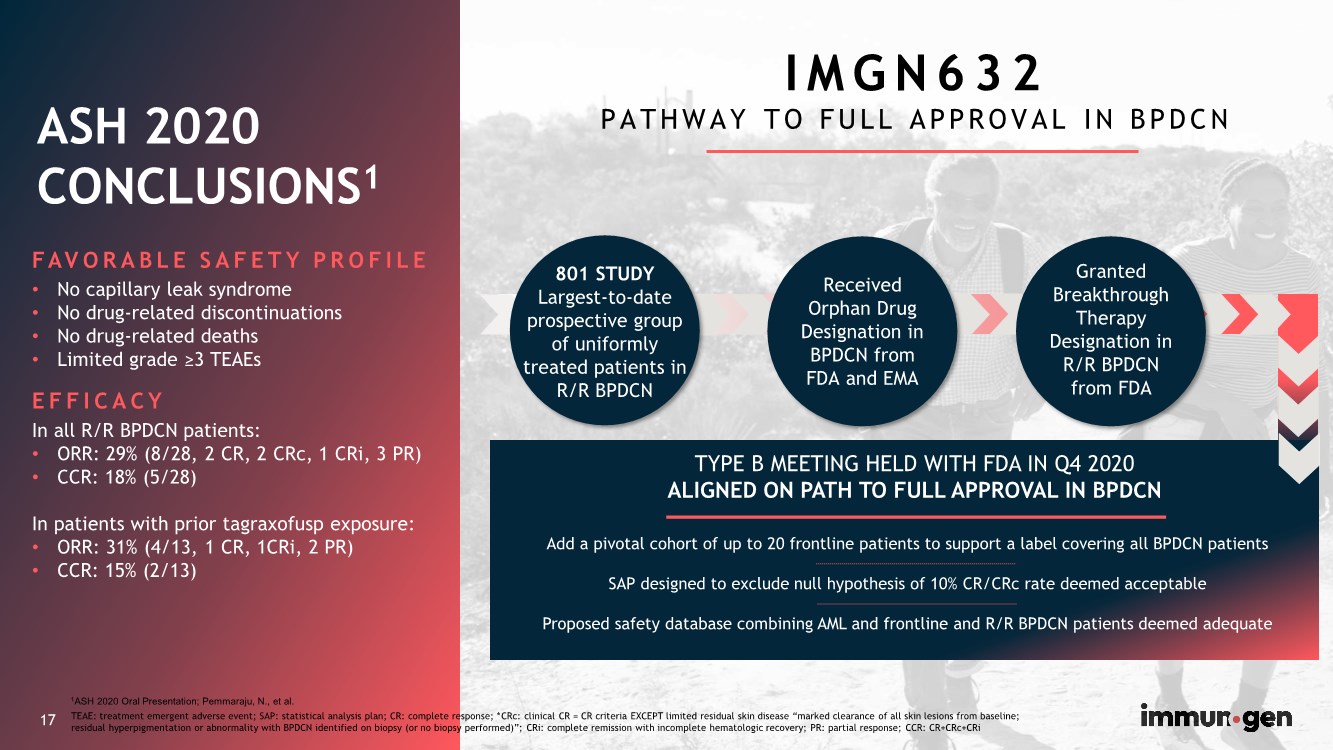
| 17 FAVORABLE SAFETY PROFILE • No capillary leak syndrome • No drug-related discontinuations • No drug-related deaths • Limited grade ≥3 TEAEs EFFICACY In all R/R BPDCN patients: • ORR: 29% (8/28, 2 CR, 2 CRc, 1 CRi, 3 PR) • CCR: 18% (5/28) In patients with prior tagraxofusp exposure: • ORR: 31% (4/13, 1 CR, 1CRi, 2 PR) • CCR: 15% (2/13) ASH 2020 CONCLUSIONS1 17 TYPE B MEETING HELD WITH FDA IN Q4 2020 ALIGNED ON PATH TO FULL APPROVAL IN BPDCN Add a pivotal cohort of up to 20 frontline patients to support a label covering all BPDCN patients SAP designed to exclude null hypothesis of 10% CR/CRc rate deemed acceptable Proposed safety database combining AML and frontline and R/R BPDCN patients deemed adequate 801 STUDY Largest-to-date prospective group of uniformly treated patients in R/R BPDCN Granted Breakthrough Therapy Designation in R/R BPDCN from FDA Received Orphan Drug Designation in BPDCN from FDA and EMA I M G N 6 3 2 PATHWAY TO FULL APPROVAL IN BPDCN TEAE: treatment emergent adverse event; SAP: statistical analysis plan; CR: complete response; *CRc: clinical CR = CR criteria EXCEPT limited residual skin disease “marked clearance of all skin lesions from baseline; residual hyperpigmentation or abnormality with BPDCN identified on biopsy (or no biopsy performed)”; CRi: complete remission with incomplete hematologic recovery; PR: partial response; CCR: CR+CRc+CRi 1ASH 2020 Oral Presentation; Pemmaraju, N., et al. |
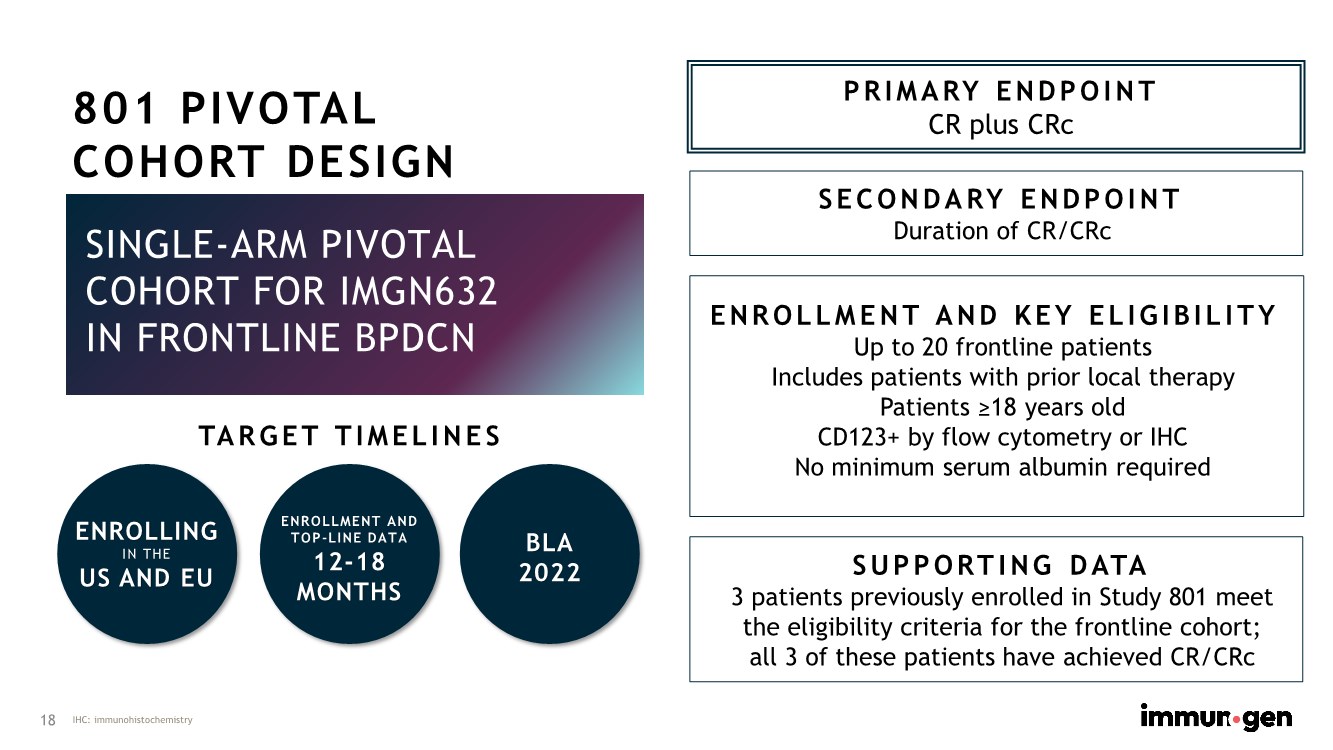
| 18 SINGLE-ARM PIVOTAL COHORT FOR IMGN632 IN FRONTLINE BPDCN PRIMARY ENDPOINT CR plus CRc SECONDARY ENDPOINT Duration of CR/CRc ENROLLING IN THE US AND EU TARGET TIMELINES ENROLLMENT AND KEY ELIGIBILITY Up to 20 frontline patients Includes patients with prior local therapy Patients ≥18 years old CD123+ by flow cytometry or IHC No minimum serum albumin required ENROLLMENT AND TOP-LINE DATA 12-18 MONTHS BLA 2022 IHC: immunohistochemistry 801 PIVOTAL COHORT DESIGN SUPPORTING DATA 3 patients previously enrolled in Study 801 meet the eligibility criteria for the frontline cohort; all 3 of these patients have achieved CR/CRc |
| 19 1ASH 2020 Poster; Kuruvilla, V., et al. VEN: venetoclax; AZA: azacitidine EVALUATING COMBINATION DOUBLET AND TRIPLET REGIMENS OF IMGN632 PLUS AZACITIDINE AND/OR VENETOCLAX PRE- CLINICAL COMBINATION DATA 1 • IMGN632+VEN+AZA triplet significantly improved survival compared to VEN+AZA doublet in CD123+ AML patient-derived xenograft models • Triplet demonstrated significant improvement in survival in a model sensitive to VEN+AZA • In two models refractory to VEN+AZA, triplet demonstrated the potential to overcome VEN+AZA resistance PATH FORWARD • Actively enrolling R/R AML patients in a Phase 1b/2 dose escalation and expansion study • Working to determine recommended Phase 2 dose and schedule for combination regimens • No dose limiting toxicities reported, including none in the triplet cohort ANTICIPATE INITIAL COMBINATION DATA IN MID-2021 IMGN632 IN AML 19 |
| 20 ADVANCING OUR PORTFOLIO OF EARLIER STAGE PRODUCT CANDIDATES 20 1AACR 2019 Poster; Hicks S., et al. ADAM: a disintegrin and metalloproteinase • First-in-class ADAM9-targeting therapy • ADAM9 is overexpressed in multiple solid tumors (e.g., non-small cell lung, gastric, pancreatic, triple- negative breast, and colorectal)1 but has a low level of expression in normal tissues/cells • Comprised of high-affinity humanized antibody with YTE mutation conjugated to DM21, a highly potent next-generation maytansinoid payload, with a stable peptide linker • 50/50 co-development with MacroGenics; first patient dosed November 2020 IMGC936 CONTINUED INNOVATION GENERATING DIFFERENTIATED ADCs |
| 21 21 1AACR 2020 Poster; Ab, O., et al. IND: investigational new drug application • Next-generation anti-FRα ADC designed to have improved activity against tumors with a broad range of FRα-expression (e.g., ovarian, endometrial, triple-negative breast, and non-small cell lung cancer)1 • Comprised of a bivalent, biparatopic antibody targeting two independent epitopes of FRα conjugated to DM21, a highly potent next-generation maytansinoid payload, with a stable peptide linker • In preclinical development; IND expected by end of 2021 IMGN151 CONTINUED INNOVATION GENERATING DIFFERENTIATED ADCs ADVANCING OUR PORTFOLIO OF EARLIER STAGE PRODUCT CANDIDATES |
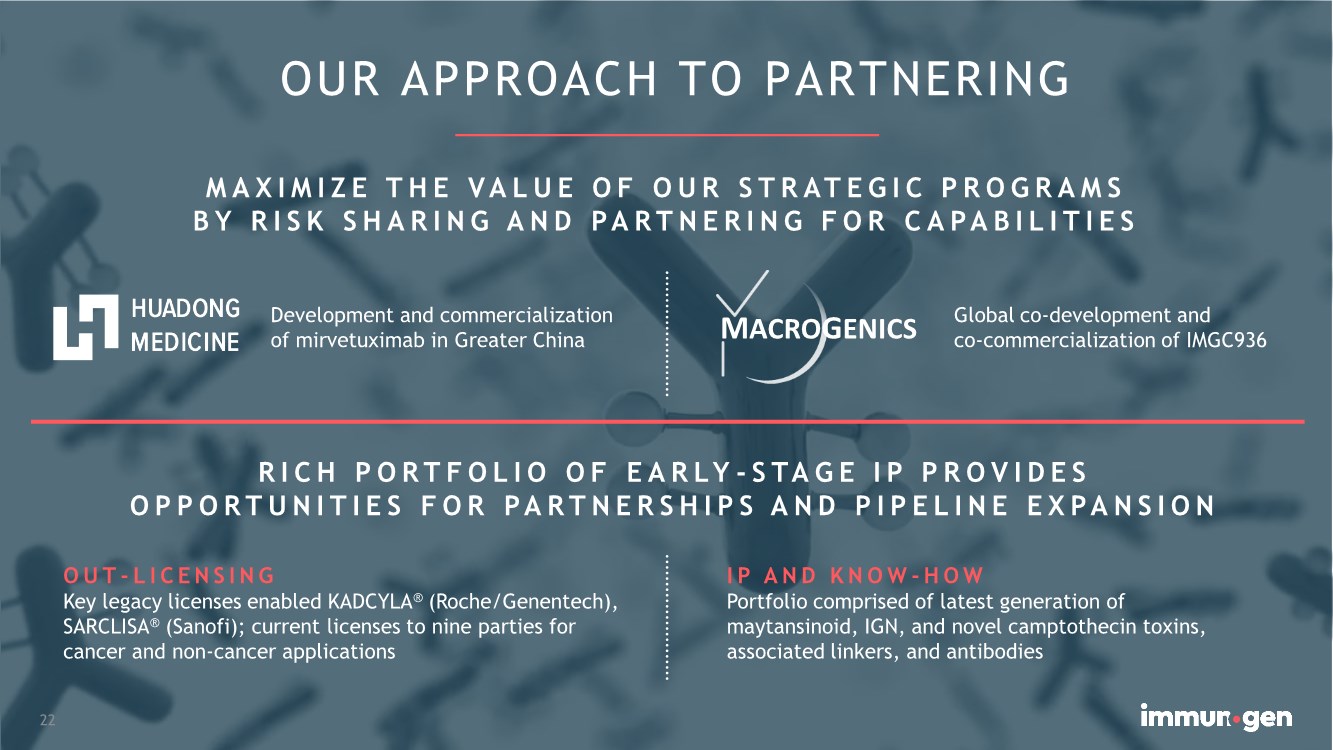
| OUR APPROACH TO PARTNERING MAXIMIZE THE VALUE OF OUR STRATEGIC PROGRAMS BY RISK SHARING AND PARTNERING FOR CAPABILITIES 22 MACROGENICS Development and commercialization of mirvetuximab in Greater China Global co-development and co-commercialization of IMGC936 OUT-LICENSING Key legacy licenses enabled KADCYLA® (Roche/Genentech), SARCLISA® (Sanofi); current licenses to nine parties for cancer and non-cancer applications RICH PORTFOLIO OF EARLY - STAGE IP PROVIDES OPPORTUNITIES FOR PARTNERSHIPS AND PIPELINE EXPANSION IP AND KNOW -HOW Portfolio comprised of latest generation of maytansinoid, IGN, and novel camptothecin toxins, associated linkers, and antibodies |
| 23 OUR PATH TO BECOMING A FULLY-INTEGRATED ONCOLOGY COMPANY MIRVETUXIMAB • Complete enrollment in pivotal SORAYA trial • Share top-line SORAYA data in Q3 2021 and submit BLA by end of 2021 • Present mature MIRV + BEV data in recurrent ovarian cancer at ASCO 2021 • Support initiation of neoadjuvant and platinum-sensitive MIRV + CARBO ISTs 2021 Milestones I M G C 9 3 6 • Continue enrollment in Phase 1 dose escalation study • Potential for initial data by late 2021 I M G N 1 5 1 • Continue pre-clinical development work • Submit IND in H2 2021 I M G N 6 3 2 • Continue enrollment in frontline and R/R BPDCN monotherapy cohorts • Present mature R/R BPDCN and initial AML combination data at ASH 2021 • Continue evaluation of triplet combination in AML and MRD+ monotherapy |
| TARGET A BETTER NOW IMPORTANT CATALYSTS IN 2021 FOR LEAD MIRVETUXIMAB PROGRAM PIVOTAL DATA IN Q3 AND BLA BY YEAR-END INNOVATIVE EARLIER STAGE CANDIDATES IN SOLID TUMORS IMGC936: FIRST-IN-CLASS ADAM9-TARGETING ADC IN THE CLINIC IMGN151: NEXT-GENERATION FRα-TARGETING ADC IND EXPECTED BY YEAR-END LEADING ADC TECHNOLOGY ADVANCING TO FULLY-INTEGRATED ONCOLOGY COMPANY POTENTIAL FOR TWO MARKETED PRODUCTS IN 2022 STRONG CASH POSITION AND EXPERIENCED MANAGEMENT TEAM 24 PATH TO FULL APPROVAL FOR IMGN632 IN BPDCN ESTABLISHED ENROLLMENT AND TOP-LINE DATA IN 12-18 MONTHS AND BLA IN 2022 |