| 1 TARGET A BETTER NOW JP Morgan Healthcare Conference January 9 - 12, 2023 Nasdaq: IMGN |
| 2 FORWARD - LOOKING STATEMENTS This presentation includes forward - looking statements regarding ImmunoGen’s current expectations related to : the commercialization of ELAHERE, the design and potential success of 420 study, pivekimab sunirine, IMGC 936 , and IMGN 151 preclinical and clinical studies and regulatory pathways, including the timing of initiating and receiving data from, as well as the likelihood of success of, the studies for these product candidates, including studies that are intended to support regulatory approval of ELAHERE, in addition to the accelerated approval of ELAHERE, and pivekimab ; the timing and outcome of the Company’s anticipated interactions with regulatory authorities ; the potential of ELAHERE to become a standard of care ; the potential of ELAHERE to become a combination agent of choice ; the presentation of preclinical and clinical events related to the Company's product candidates, including ELAHERE, pivekimab, IMGC 936 , and IMGN 151 , as well as compendia listings for ELAHERE ; the market opportunities for the Company’s development programs ; the occurrence, timing, and outcome of other potential preclinical, clinical, and regulatory events related to ImmunoGen’s and its collaboration partners’ programs ; the Company's business and product development strategies, including the Company's expected cash runway ; and potential future collaborations .. Various factors could cause ImmunoGen’s actual results to differ materially from those discussed or implied in the forward - looking statements, and you are cautioned not to place undue reliance on these forward - looking statements, which are current only as of the date of this presentation .. We undertake no obligation to update or revise any of these forward - looking statements .. Factors that could cause future results to differ materially from such expectations include, but are not limited to : that top - line data may change as more patient data become available and are subject to audit and verification procedures ; the difficulties inherent in the development of novel biopharmaceuticals ; the results of the ongoing MIRASOL trial may fail to support full approval of ELAHERE and, if so, additional studies may be required ; the risks and uncertainties inherent in the Company’s development programs, including its preclinical and clinical studies and regulatory processes, their timing, expense, and results as well as the possibility that studies of the Company’s development programs fail to confirm the hypotheses suggested by exploratory analyses or fail to satisfy the requirements for approval by one or more regulatory agencies ; the Company’s ability to financially support its development programs ; additional market research and sources that may cause the Company’s expectations of future market opportunities for its development programs to change ; the risk that we may not be able to obtain adequate reimbursement for any approved products, including the potential for delays or additional difficulties for ELAHERE in light of the FDA granting accelerated approval ; and the risks and uncertainties associated with the scale and duration of the COVID - 19 pandemic and resulting impact on ImmunoGen’s industry and business .. A review of these and other risks can be found in the “risk factors” set forth in the Company’s Annual Report on Form 10 - K filed with the Securities and Exchange Commission (SEC) on February 28 , 2022 , the Company's Form 10 - Qs filed with the SEC on May 6 , 2022 and August 1 , 2022 , and other reports filed with the SEC and available at www .. sec .. gov and on our website at www .. ImmunoGen .. com .. In addition, as the reported cash and cash equivalents balance and ELAHERE net sales amount in this presentation are preliminary, have not been audited, and are subject to change pending completion of our audited financial statements for the year ended December 31 , 2022 , it is possible that we or our independent registered public accounting firm may identify items that require us to make adjustments to the preliminary estimated ELAHERE net sales amount and cash and cash equivalents balance, as well as our expected cash runway, and such changes could be material .. |
| 3 ABOUT IMMUNOGEN A FULLY - INTEGRATED ONCOLOGY COMPANY A Leader in the Research and Development of ADCs with 40+ Years of Expertise Clinical Pipeline of Novel ADCs for Solid Tumors and Hematologic Malignancies TARGET A BETTER NOW ADC: antibody - drug conjugate ImmunoGen technology has produced three approved products: KADCYLA ® (Roche/Genentech), SARCLISA ® (Sanofi) and ELAHERE TM (ImmunoGen) First Independent Commercial Launch in 2022 with Significant Near - Term Expansion Potential Experienced Leadership Team and Expected Cash Runway into 2024 |
| 4 ELAHERE: FIRST AND ONLY ADC APPROVED IN OVARIAN CANCER • ELAHERE granted accelerated approval by FDA for the treatment of PROC on November 14 • Inclusion of ELAHERE monotherapy and in combination with bevacizumab in NCCN guidelines and compendium • Completed enrollment in MIRASOL with top - line data expected early 2023 • Continued enrollment in PICCOLO for patients with FR α - high recurrent PSOC • Initiated 2 combination studies in PSOC: Trial 0420 in FR α - low, medium, and high patients and GLORIOSA for maintenance in FR α - high patients PIVEKIMAB SUNIRINE: CD123 TARGETING ADC • Reported initial data from pivotal CADENZA trial in frontline BPDCN; aligned with FDA that efficacy evaluable population will be in de novo patients • Presented safety and efficacy findings for pivekimab in combination with venetoclax and azacitidine in patients with R/R and frontline AML in our 4 th consecutive oral session at ASH 2022 • Partnered with Gilead to evaluate pivekimab in combination with magrolimab in R/R AML IMGC936: FIRST - IN - CLASS ADAM9 - TARGETING ADC • Completed Phase 1 dose escalation; initiated expansion cohorts in TNBC and NSCLC IMGN151: FOLLOW - ON CANDIDATE FOR FRα - TARGETING FRANCHISE • Initiated Phase 1 study FINANCIALS • ~$275M in cash and cash equivalents on hand as of December 31 • Expect current cash, combined with anticipated product and collaboration revenues, will fund operations into 2024 AML: acute myeloid leukemia; ASH: American Society of Hematology; BPDCN: blastic plasmacytoid dendritic cell neoplasm; FDA: US Food and Drug Administration; FR α : folate receptor alpha; ISTs: investigator - sponsored trials; NCCN: National Comprehensive Cancer Network; NSCLC: non – small cell lung cancer; PROC: platinum - resistant ovarian cancer; PSOC: platinum - sensitive ovarian cancer; R/R: relapsed/refractory; TNBC: triple - negative breast cancer RECENT ACCOMPLISHMENTS SIGNIFICANTLY ADVANCED THE BUSINESS IN 2022 4 TARGET A BETTER NOW |
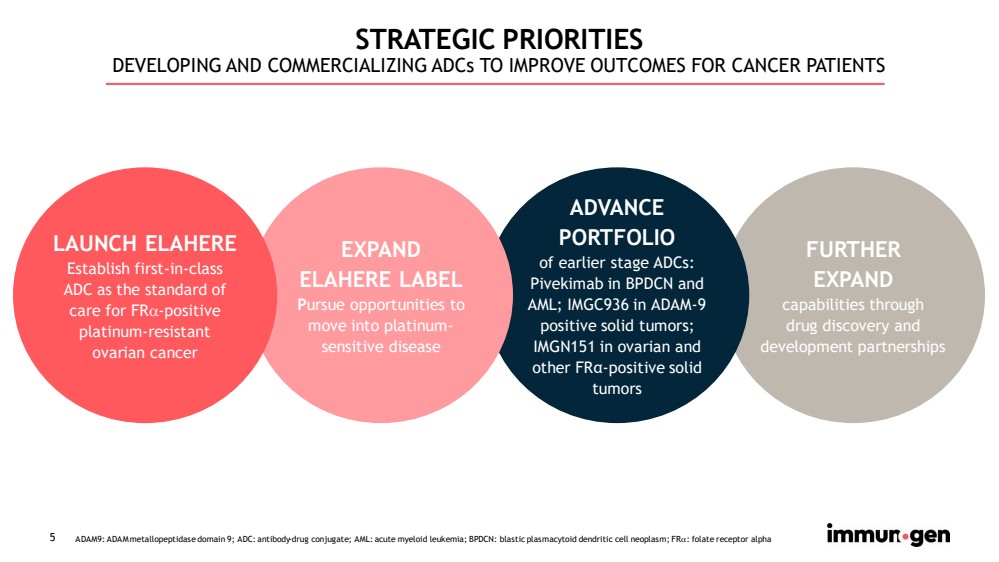
| 5 STRATEGIC PRIORITIES DEVELOPING AND COMMERCIALIZING ADCs TO IMPROVE OUTCOMES FOR CANCER PATIENTS ADAM9: ADAM metallopeptidase domain 9; ADC: antibody - drug conjugate; AML: acute myeloid leukemia; BPDCN: blastic plasmacytoid dendritic cell neoplasm; FR : folate receptor alpha FURTHER EXPAND capabilities through drug discovery and development partnerships ADVANCE PORTFOLIO of earlier stage ADCs: Pivekimab in BPDCN and AML; IMGC936 in ADAM - 9 positive solid tumors; IMGN151 in ovarian and other FR α - positive solid tumors EXPAND ELAHERE LABEL P ursue opportunities to move into platinum - sensitive disease LAUNCH ELAHERE Establish first - in - class ADC as the standard of care for FR - positive platinum - resistant ovarian cancer |
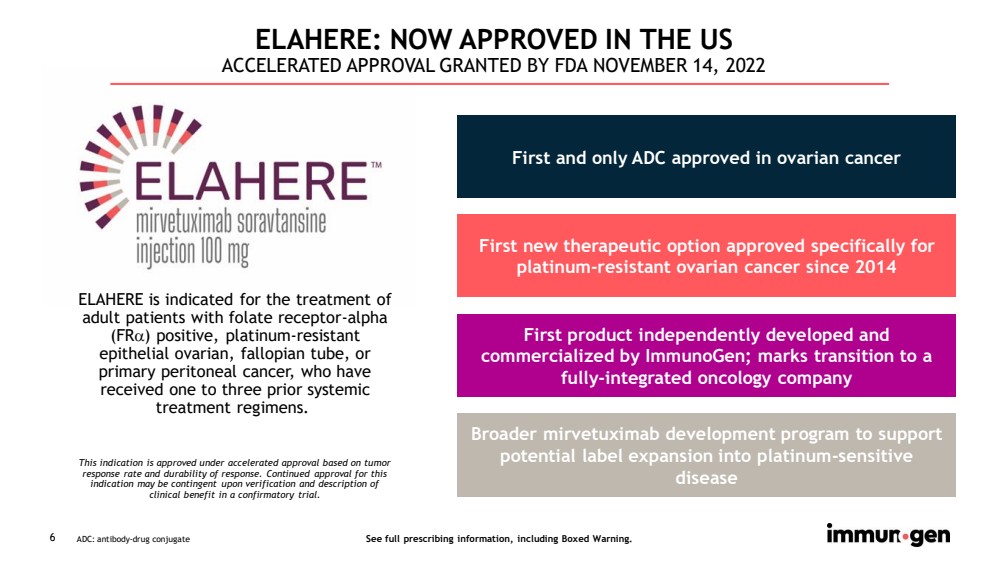
| 6 ELAHERE is indicated for the treatment of adult patients with folate receptor - alpha (FR ) positive, platinum - resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have received one to three prior systemic treatment regimens. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. ELAHERE: NOW APPROVED IN THE US ACCELERATED APPROVAL GRANTED BY FDA NOVEMBER 14, 2022 First and only ADC approved in ovarian cancer First new therapeutic option approved specifically for platinum - resistant ovarian cancer since 2014 First product independently developed and commercialized by ImmunoGen; marks transition to a fully - integrated oncology company Broader mirvetuximab development program to support potential label expansion into platinum - sensitive disease ADC: antibody - drug conjugate See full prescribing information, including Boxed Warning. |
| 7 ELAHERE LAUNCH IMPERATIVES FR : folate receptor alpha Redefine expectations for positive treatment outcomes in ovarian cancer with ELAHERE Ensure a positive physician experience based on education and guidance for patient management Seek broad payer access and reimbursement and deliver a seamless patient experience Support adoption of early FR testing and establish standards for in - house and centralized testing GOAL: ESTABLISH ELAHERE AS THE STANDARD OF CARE IN FR POSITIVE PATIENTS |
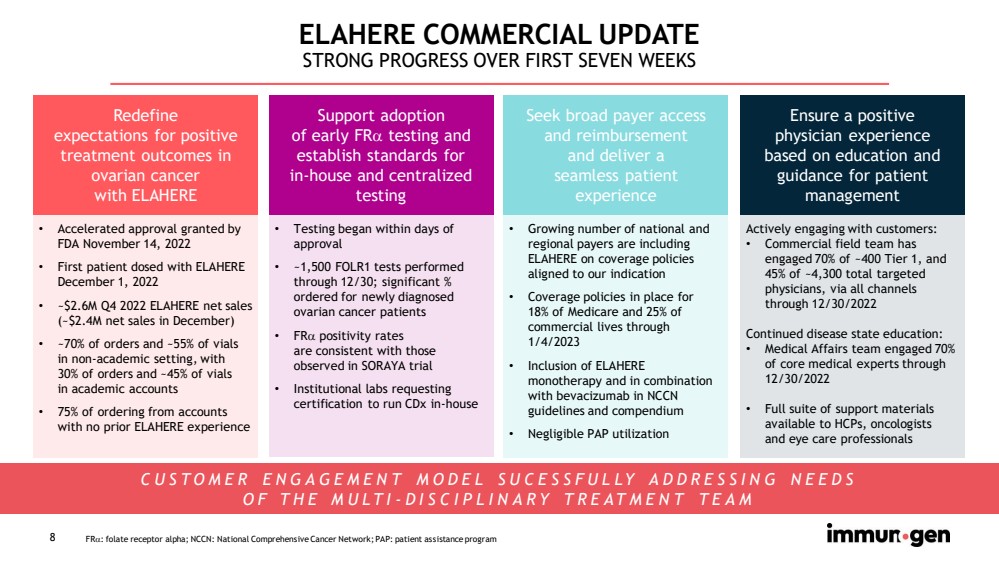
| 8 ELAHERE COMMERCIAL UPDATE STRONG PROGRESS OVER FIRST SEVEN WEEKS Redefine expectations for positive treatment outcomes in ovarian cancer with ELAHERE Ensure a positive physician experience based on education and guidance for patient management Seek broad payer access and reimbursement and deliver a seamless patient experience Support adoption of early FR testing and establish standards for in - house and centralized testing FR : folate receptor alpha; NCCN: National Comprehensive Cancer Network; PAP: patient assistance program • Accelerated approval granted by FDA November 14, 2022 • First patient dosed with ELAHERE December 1, 2022 • ~$2.6M Q4 2022 ELAHERE net sales (~$2.4M net sales in December) • ~70% of orders and ~55% of vials in non - academic setting, with 30% of orders and ~45% of vials in academic accounts • 75% of ordering from accounts with no prior ELAHERE experience • Testing began within days of approval • ~1,500 FOLR1 tests performed through 12/30; significant % ordered for newly diagnosed ovarian cancer patients • FR positivity rates are consistent with those observed in SORAYA trial • Institutional labs requesting certification to run CDx in - house • Growing number of national and regional payers are including ELAHERE on coverage policies aligned to our indication • Coverage policies in place for 18% of Medicare and 25% of commercial lives through 1/4/2023 • Inclusion of ELAHERE monotherapy and in combination with bevacizumab in NCCN guidelines and compendium • Negligible PAP utilization Actively engaging with customers: • Commercial field team has engaged 70% of ~400 Tier 1, and 45% of ~4,300 total targeted physicians, via all channels through 12/30/2022 Continued disease state education: • Medical Affairs team engaged 70% of core medical experts through 12/30/2022 • Full suite of support materials available to HCPs, oncologists and eye care professionals CUSTOMER ENGAGEMENT MODEL SUCESSFULLY ADDRESSING NEEDS OF THE MULTI - DISCIPLINARY TREATMENT TEAM |
| 9 Goal: Move into Platinum - Sensitive Disease and Become the Combination Agent of Choice in Ovarian Cancer • Single - arm Phase 2 trial for mirvetuximab in FR α - high patients with PSOC • Enrollment ongoing • ORR data by year - end 2023; potential for label expansion in 2024 MIRVETUXIMAB IN DEVELOPMENT FOR PSOC MONOTHERAPY • Randomized Phase 3 trial for mirvetuximab + bevacizumab maintenance in FR α - high PSOC • Open for enrollment • Single - arm Phase 2 trial for mirvetuximab + carboplatin followed by mirvetuximab continuation in FR α - low, medium, and high patients with PSOC • Open for enrollment • Designed to inform a potential path to registration in recurrent PSOC TRIAL 420 MIRVETUXIMAB + BEVACIZUMAB MIRVETUXIMAB + CARBOPLATIN MIRVETUXIMAB IN DEVELOPMENT FOR COMBINATION REGIMENS ELAHERE DEVELOPMENT STRATEGY FOR GEOGRAPHIC AND LABEL EXPANSION • Phase 3 randomized trial for mirvetuximab in FRα - high patients with PROC • Enrollment completed mid - 2022 • Expect top - line data early 2023 • Designed to support full approval in the US and EU PHASE 3 RANDOMIZED CONFIRMATORY STUDY ImmunoGen’s investigational products have not been approved by the U.S. Food and Drug Administration or other regulatory auth ori ties. The safety and efficacy of the investigational products have not been established. See full prescribing information, including Boxed Warning. FR : folate receptor alpha; ORR: overall response rate; PSOC: platinum - sensitive ovarian cancer; PROC: platinum - resistant ovarian cancer |
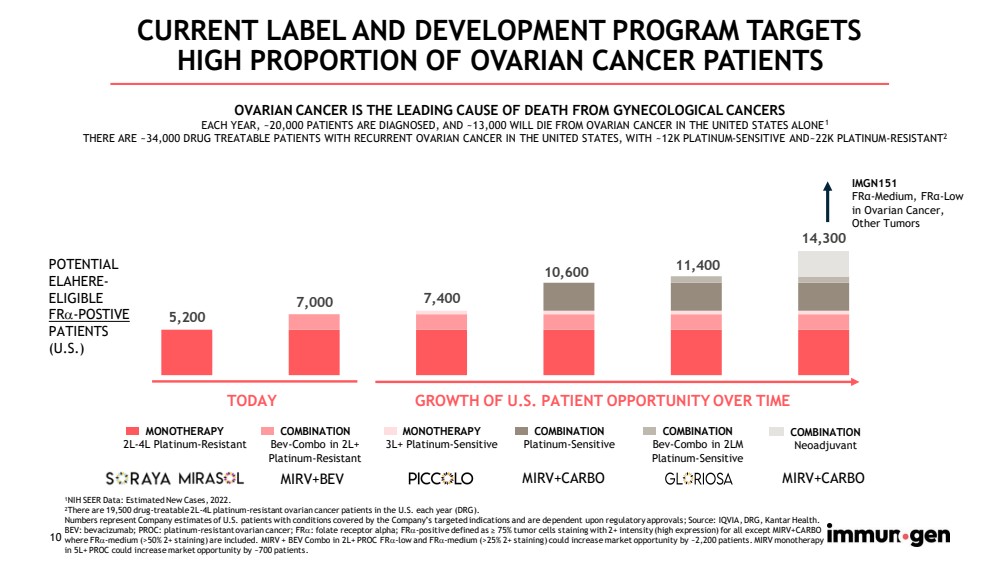
| 10 5,200 7,000 7,400 10,600 11,400 14,300 CURRENT LABEL AND DEVELOPMENT PROGRAM TARGETS HIGH PROPORTION OF OVARIAN CANCER PATIENTS POTENTIAL ELAHERE - ELIGIBLE FR - POSTIVE PATIENTS (U.S.) MONOTHERAPY 2L - 4L Platinum - Resistant COMBINATION Bev - Combo in 2L+ Platinum - Resistant MONOTHERAPY 3L+ Platinum - Sensitive COMBINATION Neoadjuvant COMBINATION Bev - Combo in 2LM Platinum - Sensitive COMBINATION Platinum - Sensitive IMGN151 FR α - Medium, FR α - Low in Ovarian Cancer, Other Tumors OVARIAN CANCER IS THE LEADING CAUSE OF DEATH FROM GYNECOLOGICAL CANCERS EACH YEAR, ~20,000 PATIENTS ARE DIAGNOSED, AND ~13,000 WILL DIE FROM OVARIAN CANCER IN THE UNITED STATES ALONE 1 THERE ARE ~34,000 DRUG TREATABLE PATIENTS WITH RECURRENT OVARIAN CANCER IN THE UNITED STATES, WITH ~12K PLATINUM - SENSITIVE AND~2 2K PLATINUM - RESISTANT 2 MIRV+CARBO MIRV+BEV MIRV+CARBO GROWTH OF U.S. PATIENT OPPORTUNITY OVER TIME TODAY 1 NIH SEER Data: Estimated New Cases, 2022. 2 T here are 19,500 drug - treatable 2L - 4L platinum - resistant ovarian cancer patients in the U.S. each year (DRG). Numbers represent Company estimates of U.S. patients with conditions covered by the Company’s targeted indications and are de pen dent upon regulatory approvals; Source: IQVIA, DRG, Kantar Health. BEV: bevacizumab; PROC: platinum - resistant ovarian cancer; FR : folate receptor alpha; FR - positive defined as ≥ 75% tumor cells staining with 2+ intensity (high expression) for all except MIRV+CARBO where FR - medium (>50% 2+ staining) are included. MIRV + BEV Combo in 2L+ PROC FR - low and FR - medium (>25% 2+ staining) could increase market opportunity by ~2,200 patients. MIRV monotherapy in 5L+ PROC could increase market opportunity by ~700 patients. |
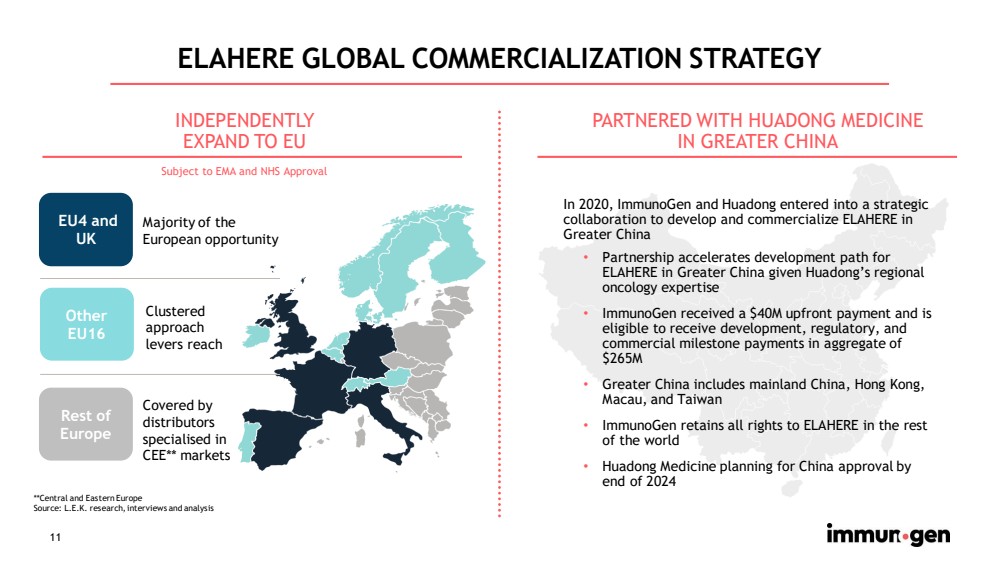
| 11 ELAHERE GLOBAL COMMERCIALIZATION STRATEGY • Partnership accelerates development path for ELAHERE in Greater China given Huadong’s regional oncology expertise • ImmunoGen received a $40M upfront payment and is eligible to receive development, regulatory, and commercial milestone payments in aggregate of $265M • Greater China includes mainland China, Hong Kong, Macau, and Taiwan • ImmunoGen retains all rights to ELAHERE in the rest of the world • Huadong Medicine planning for China approval by end of 2024 **Central and Eastern Europe Source: L.E.K. research, interviews and analysis PARTNERED WITH HUADONG MEDICINE IN GREATER CHINA In 2020, ImmunoGen and Huadong entered into a strategic collaboration to develop and commercialize ELAHERE in Greater China INDEPENDENTLY EXPAND TO EU EU4 and UK Other EU16 Rest of Europe Majority of the European opportunity Clustered approach levers reach Covered by distributors specialised in CEE** markets Subject to EMA and NHS Approval |
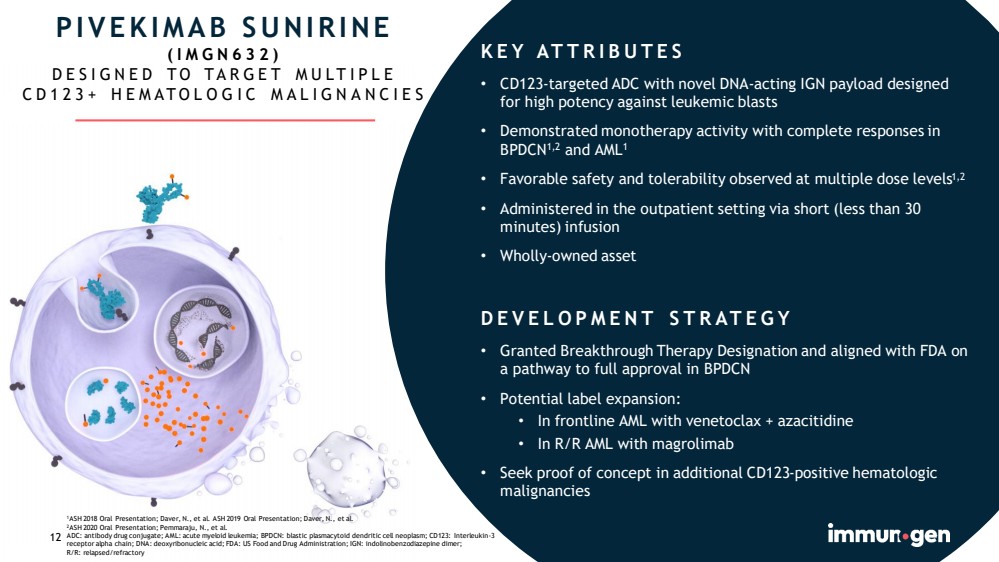
| 12 PIVEKIMAB SUNIRINE (IMGN632) DESIGNED TO TARGET MULTIPLE CD123+ HEMATOLOGIC MALIGNANCIES 1 ASH 2018 Oral Presentation; Daver , N., et al. ASH 2019 Oral Presentation; Daver , N., et al. 2 ASH 2020 Oral Presentation; Pemmaraju, N., et al. ADC: antibody drug conjugate; AML: acute myeloid leukemia; BPDCN: blastic plasmacytoid dendritic cell neoplasm; CD123: Interleukin - 3 receptor alpha chain; DNA: deoxyribonucleic acid; FDA: US Food and Drug Administration; IGN: indolinobenzodiazepine dimer ; R/R: relapsed/refractory 12 KEY ATTRIBUTES • CD123 - targeted ADC with novel DNA - acting IGN payload designed for high potency against leukemic blasts • Demonstrated monotherapy activity with complete responses in BPDCN 1,2 and AML 1 • Favorable safety and tolerability observed at multiple dose levels 1,2 • Administered in the outpatient setting via short (less than 30 minutes) infusion • Wholly - owned asset DEVELOPMENT STRATEGY • Granted Breakthrough Therapy Designation and aligned with FDA on a pathway to full approval in BPDCN • Potential label expansion: • In frontline AML with venetoclax + azacitidine • In R/R AML with magrolimab • Seek proof of concept in additional CD123 - positive hematologic malignancies |
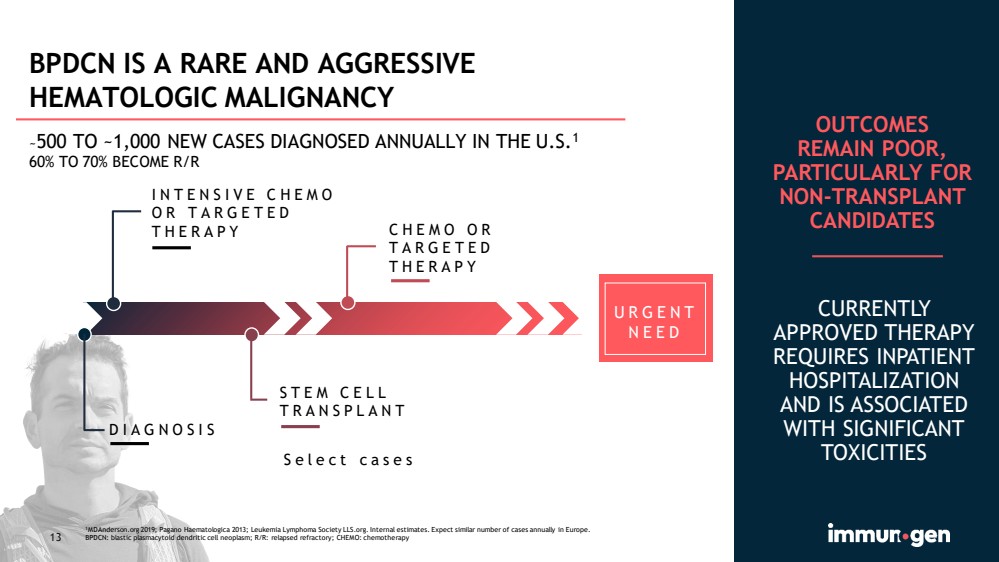
| 13 INTENSIVE CHEMO OR TARGETED THERAPY DIAGNOSIS STEM CELL TRANSPLANT CHEMO OR TARGETED THERAPY Select cases CURRENTLY APPROVED THERAPY REQUIRES INPATIENT HOSPITALIZATION AND IS ASSOCIATED WITH SIGNIFICANT TOXICITIES URGENT NEED BPDCN IS A RARE AND AGGRESSIVE HEMATOLOGIC MALIGNANCY ~ 500 TO ~1,000 NEW CASES DIAGNOSED ANNUALLY IN THE U.S. 1 60% TO 70% BECOME R/R OUTCOMES REMAIN POOR, PARTICULARLY FOR NON - TRANSPLANT CANDIDATES 1 MDAnderson.org 2019; Pagano Haematologica 2013; Leukemia Lymphoma Society LLS.org. Internal estimates. Expect similar number of cases annually in Europe. BPDCN: blastic plasmacytoid dendritic cell neoplasm; R/R: relapsed refractory; CHEMO: chemotherapy |
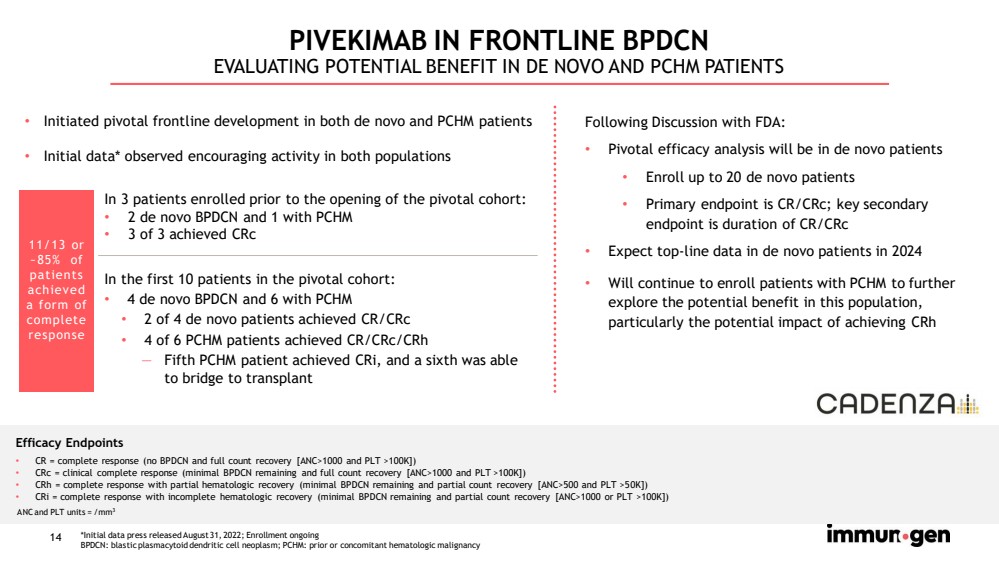
| 14 In the first 10 patients in the pivotal cohort: • 4 de novo BPDCN and 6 with PCHM • 2 of 4 de novo patients achieved CR/ CRc • 4 of 6 PCHM patients achieved CR/ CRc / CRh — Fifth PCHM patient achieved CRi , and a sixth was able to bridge to transplant PIVEKIMAB IN FRONTLINE BPDCN EVALUATING POTENTIAL BENEFIT IN DE NOVO AND PCHM PATIENTS Following Discussion with FDA: • Pivotal efficacy analysis will be in de novo patients • Enroll up to 20 de novo patients • Primary endpoint is CR/ CRc ; key secondary endpoint is duration of CR/ CRc • Expect top - line data in de novo patients in 2024 • Will continue to enroll patients with PCHM to further explore the potential benefit in this population, particularly the potential impact of achieving CRh Efficacy Endpoints • CR = complete response (no BPDCN and full count recovery [ANC>1000 and PLT >100K]) • CRc = clinical complete response (minimal BPDCN remaining and full count recovery [ANC>1000 and PLT >100K]) • CRh = complete response with partial hematologic recovery (minimal BPDCN remaining and partial count recovery [ANC>500 and PLT >5 0K ]) • CRi = complete response with incomplete hematologic recovery (minimal BPDCN remaining and partial count recovery [ANC>1000 or PLT > 100K]) In 3 patients enrolled prior to the opening of the pivotal cohort: • 2 de novo BPDCN and 1 with PCHM • 3 of 3 achieved CRc 11/13 or ~85% of patients achieved a form of complete response ANC and PLT units = /mm 3 *Initial data press released August 31, 2022; Enrollment ongoing BPDCN: blastic plasmacytoid dendritic cell neoplasm; PCHM: prior or concomitant hematologic malignancy • Initiated pivotal frontline development in both de novo and PCHM patients • Initial data* observed encouraging activity in both populations |
| 15 AML IS AN AGGRESSIVE HEMATOLOGIC MALIGNANCY ~20,000 PEOPLE DIAGNOSED WITH AML AND ~11,000 DIE ANNUALLY IN THE U.S. 1 FIT PATIENTS 2 Approximately half of patients are “fit” enough to undergo intensive chemotherapy and transplant with curative intent Median survival: 2 - 4 years UNFIT PATIENTS 2 Approximately half of patients are “unfit” to undergo intensive chemotherapy and are appropriate for lower intensity therapy (e.g., VEN+AZA) Median survival: 1 - 2 years DIAGNOSIS Decisions about fitness for chemotherapy must be made quickly RELAPSE 2,3 Up to 80% of patients are refractory to initial treatment or relapse within 2 years, with few treatment options available including various chemotherapy regimens and, for few patients, transplant Median survival: 9 months – 2 years UNMET NEED IN AML REMAINS HIGH WHILE VEN+AZA HAS LED TO IMPROVED OUTCOMES IN UNFIT PATIENTS, SURVIVAL AFTER VEN+AZA FAILURE IS POOR AT ~ 2 TO 3 MONTHS 4 URGENT NEED 15 1 NIH SEER Data: Estimated New Cases and Deaths in 2021. 2 Dinardo, C., et al. ASH “How to Treat” Series, 2020. 3 Lima, M., et al. Blood Reviews 2021. 4 Maiti, A., et al. Hematologica 2021. AML: acute myeloid leukemia; VEN: VENCLEXTA ® (venetoclax); AZA: VIDAZA ® (azacitidine) VIDAZA ® , and VENCLEXTA® are registered trademarks of their respective owners. |
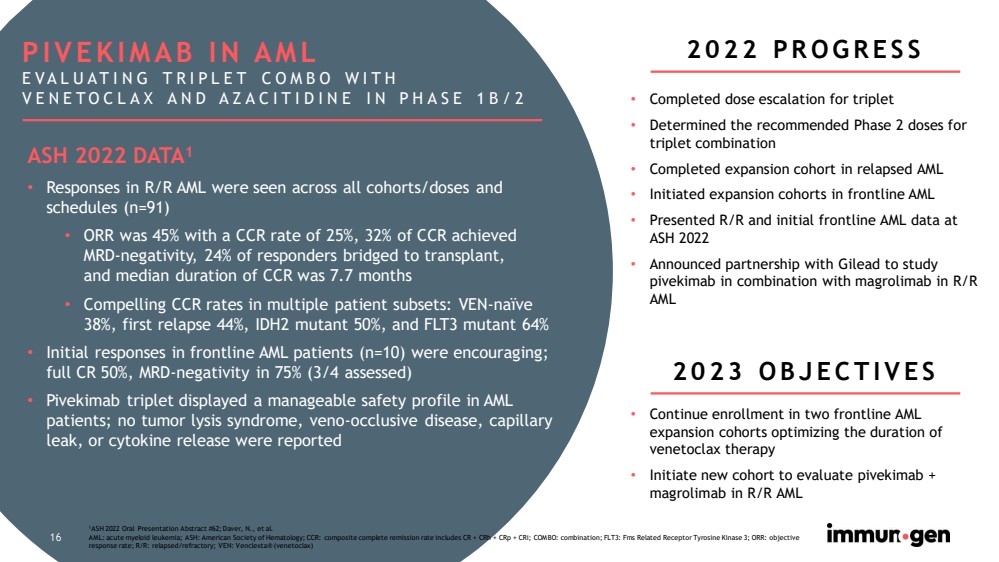
| 16 1 ASH 2022 Oral Presentation Abstract #62; Daver , N., et al. AML: acute myeloid leukemia; ASH: American Society of Hematology; CCR: composite complete remission rate includes CR + CRh + CRp + CRi ; COMBO: combination; FLT3: Fms Related Receptor Tyrosine Kinase 3; ORR: objective response rate; R/R: relapsed/refractory; VEN: Venclexta ® (venetoclax) • Completed dose escalation for triplet • Determined the recommended Phase 2 doses for triplet combination • Completed expansion cohort in relapsed AML • Initiated expansion cohorts in frontline AML • Presented R/R and initial frontline AML data at ASH 2022 • Announced partnership with Gilead to study pivekimab in combination with magrolimab in R/R AML 16 PIVEKIMAB IN AML EVALUATING TRIPLET COMBO WITH VENETOCLAX AND AZACITIDINE IN PHASE 1B/2 2022 PROGRESS ASH 2022 DATA 1 • Responses in R/R AML were seen across all cohorts/doses and schedules (n=91) • ORR was 45% with a CCR rate of 25%, 32% of CCR achieved MRD - negativity, 24% of responders bridged to transplant, and median duration of CCR was 7.7 months • Compelling CCR rates in multiple patient subsets: VEN - naïve 38%, first relapse 44%, IDH2 mutant 50%, and FLT3 mutant 64% • Initial responses in frontline AML patients (n=10) were encouraging; full CR 50%, MRD - negativity in 75% (3/4 assessed) • Pivekimab triplet displayed a manageable safety profile in AML patients; no tumor lysis syndrome, veno - occlusive disease, capillary leak, or cytokine release were reported 2023 OBJECTIVES • Continue enrollment in two frontline AML expansion cohorts optimizing the duration of venetoclax therapy • Initiate new cohort to evaluate pivekimab + magrolimab in R/R AML |
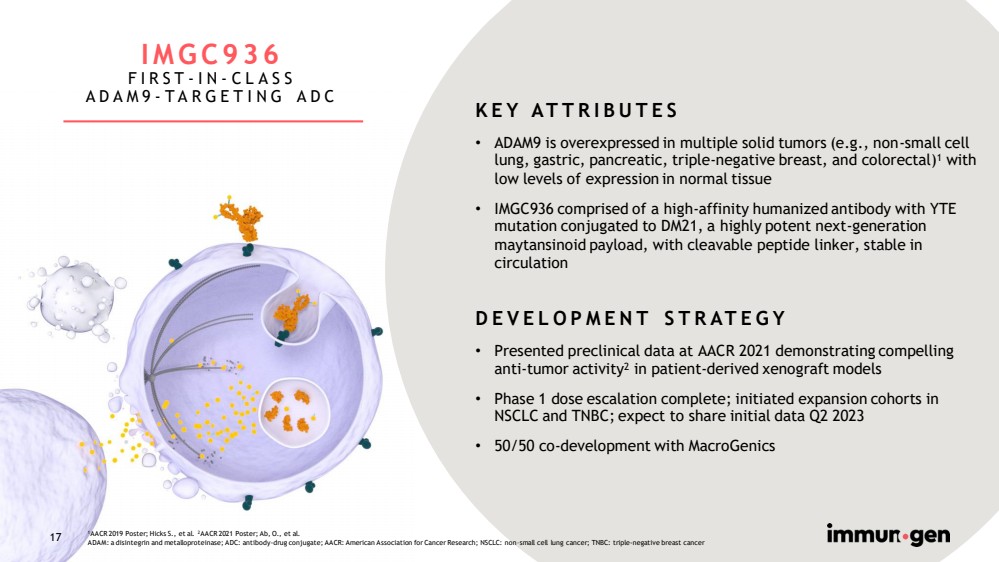
| 17 IMGC936 FIRST - IN - CLASS ADAM9 - TARGETING ADC KEY ATTRIBUTES • ADAM9 is overexpressed in multiple solid tumors (e.g., non - small cell lung, gastric, pancreatic, triple - negative breast, and colorectal) 1 with low levels of expression in normal tissue • IMGC936 comprised of a high - affinity humanized antibody with YTE mutation conjugated to DM21, a highly potent next - generation maytansinoid payload, with cleavable peptide linker, stable in circulation DEVELOPMENT STRATEGY • Presented preclinical data at AACR 2021 demonstrating compelling anti - tumor activity 2 in patient - derived xenograft models • Phase 1 dose escalation complete; initiated expansion cohorts in NSCLC and TNBC; expect to share initial data Q2 2023 • 50/50 co - development with MacroGenics 1 AACR 2019 Poster; Hicks S., et al. 2 AACR 2021 Poster; Ab, O., et al. ADAM: a disintegrin and metalloproteinase; ADC: antibody - drug conjugate; AACR: American Association for Cancer Research; NSCLC: non – small cell lung cancer; TNBC: triple - negative breast cancer 17 |
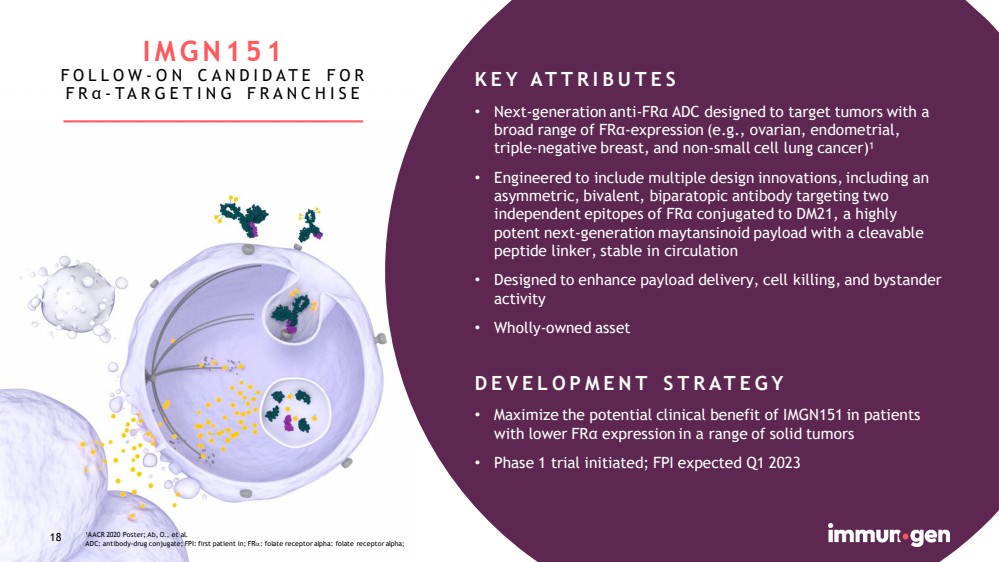
| 18 1 AACR 2020 Poster; Ab, O., et al. ADC: antibody - drug conjugate; FPI: first patient in; FR : folate receptor alpha : folate receptor alpha; 18 KEY ATTRIBUTES • Next - generation anti - FRα ADC designed to target tumors with a broad range of FRα - expression (e.g., ovarian, endometrial, triple - negative breast, and non - small cell lung cancer) 1 • Engineered to include multiple design innovations, including an asymmetric, bivalent, biparatopic antibody targeting two independent epitopes of FRα conjugated to DM21, a highly potent next - generation maytansinoid payload with a cleavable peptide linker, stable in circulation • Designed to enhance payload delivery, cell killing, and bystander activity • Wholly - owned asset DEVELOPMENT STRATEGY • Maximize the potential clinical benefit of IMGN151 in patients with lower FR α expression in a range of solid tumors • Phase 1 trial initiated; FPI expected Q1 2023 IMGN151 FOLLOW - ON CANDIDATE FOR FR α - TARGETING FRANCHISE |
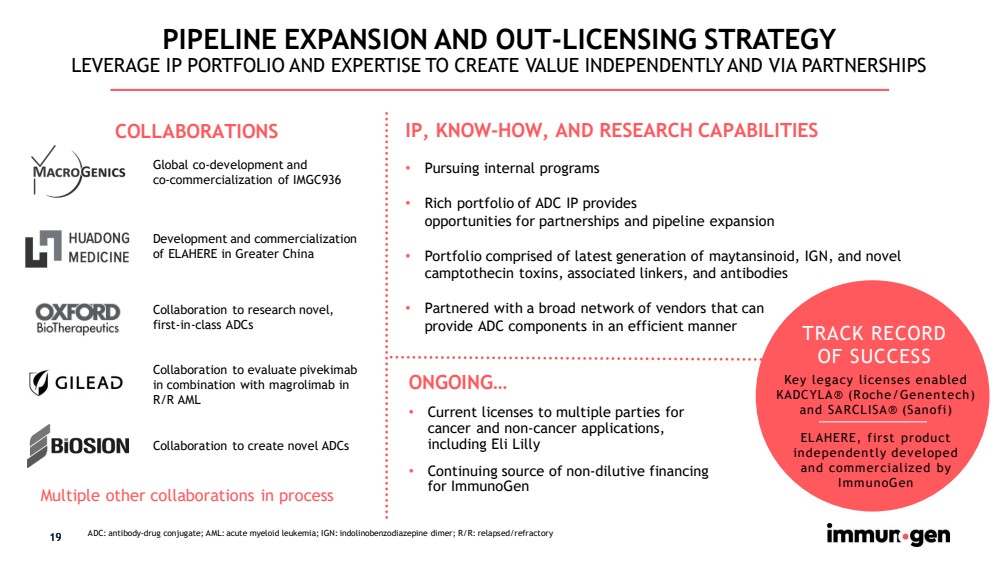
| 19 PIPELINE EXPANSION AND OUT - LICENSING STRATEGY LEVERAGE IP PORTFOLIO AND EXPERTISE TO CREATE VALUE INDEPENDENTLY AND VIA PARTNERSHIPS Development and commercialization of ELAHERE in Greater China Global co - development and co - commercialization of IMGC936 IP, KNOW - HOW, AND RESEARCH CAPABILITIES • Pursuing internal programs • Rich portfolio of ADC IP provides opportunities for partnerships and pipeline expansion • Portfolio comprised of latest generation of maytansinoid , IGN, and novel camptothecin toxins, associated linkers, and antibodies • Partnered with a broad network of vendors that can provide ADC components in an efficient manner 19 ADC: antibody - drug conjugate; AML: acute myeloid leukemia; IGN: indolinobenzodiazepine dimer; R/R: relapsed/refractory Collaboration to research novel, first - in - class ADCs Multiple other collaborations in process COLLABORATIONS ONGOING… • Current licenses to multiple parties for cancer and non - cancer applications, including Eli Lilly • Continuing source of non - dilutive financing for ImmunoGen TRACK RECORD OF SUCCESS Key legacy licenses enabled KADCYLA® (Roche/Genentech) and SARCLISA® (Sanofi) ELAHERE, first product independently developed and commercialized by ImmunoGen Collaboration to create novel ADCs Collaboration to evaluate pivekimab in combination with magrolimab in R/R AML |
| 20 VALUE CREATION OPPORTUNITIES IN 2023 ESTABLISH ELAHERE AS THE STANDARD OF CARE IN FR POSITIVE PATIENTS • Progress pivotal CADENZA study in frontline BPDCN ADAM: a disintegrin and metalloproteinase; ADC: antibody - drug conjugate; AML: acute myeloid leukemia; BPDCN: blastic plasmacytoid dendritic cell neoplasm; FR α : folate receptor alpha; MAA: Marketing Authorization Application; NCCN: National Comprehensive Cancer Network ; R/R: relapsed/refractory • IMGN936: First - in - class ADAM9 - Targeting ADC; Phase 1 dose escalation complete; expand cohorts in NSCLC and TNBC; initial data expected in Q2 PIVEKIMAB TO ADDRESS UNMET NEED IN BPDCN and AML ADVANCE EARLIER - STAGE PIPELINE • Support label expansion into platinum - sensitive disease • Initiate combination cohort with magrolimab in R/R AML in collaboration with Gilead • IMGN151: Pursue dose escalation for next generation FR targeting ADC to build upon ELAHERE franchise • Continue to drive and expand commercial uptake in platinum - resistant setting • Report top - line data from the Phase 3 Confirmatory Study (MIRASOL) and file MAA to support initial EU approval • Continue enrollment in frontline AML expansion cohorts optimizing the duration of venetoclax therapy |
| 21 TARGET A BETTER NOW APPENDIX |
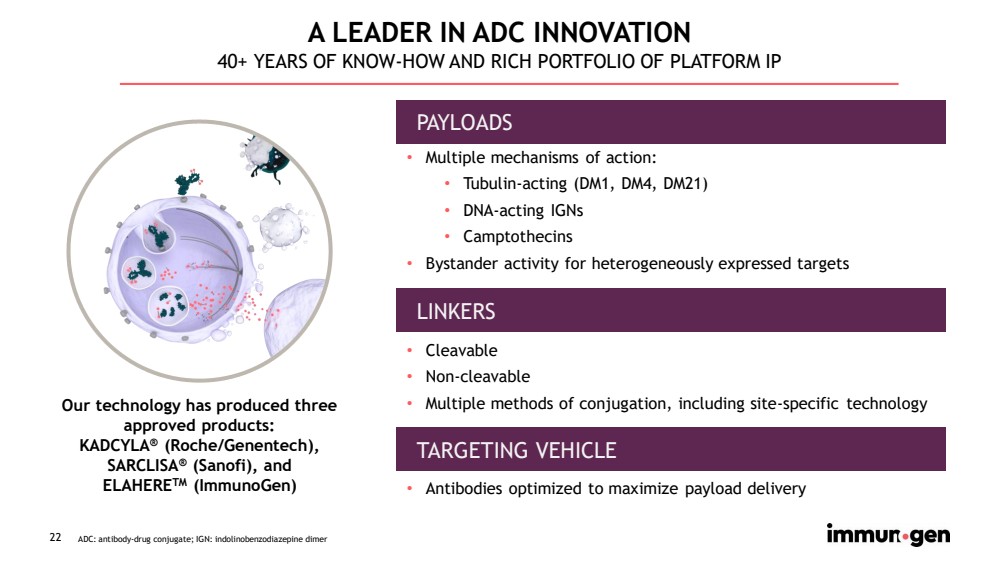
| 22 A LEADER IN ADC INNOVATION 40+ YEARS OF KNOW - HOW AND RICH PORTFOLIO OF PLATFORM IP • Multiple mechanisms of action: • Tubulin - acting (DM1, DM4, DM21) • DNA - acting IGNs • Camptothecins • Bystander activity for heterogeneously expressed targets • Cleavable • Non - cleavable • Multiple methods of conjugation, including site - specific technology • Antibodies optimized to maximize payload delivery PAYLOADS LINKERS ADC: antibody - drug conjugate; IGN: indolinobenzodiazepine dimer TARGETING VEHICLE Our technology has produced three approved products: KADCYLA ® (Roche/Genentech), SARCLISA ® (Sanofi), and ELAHERE TM (ImmunoGen) |
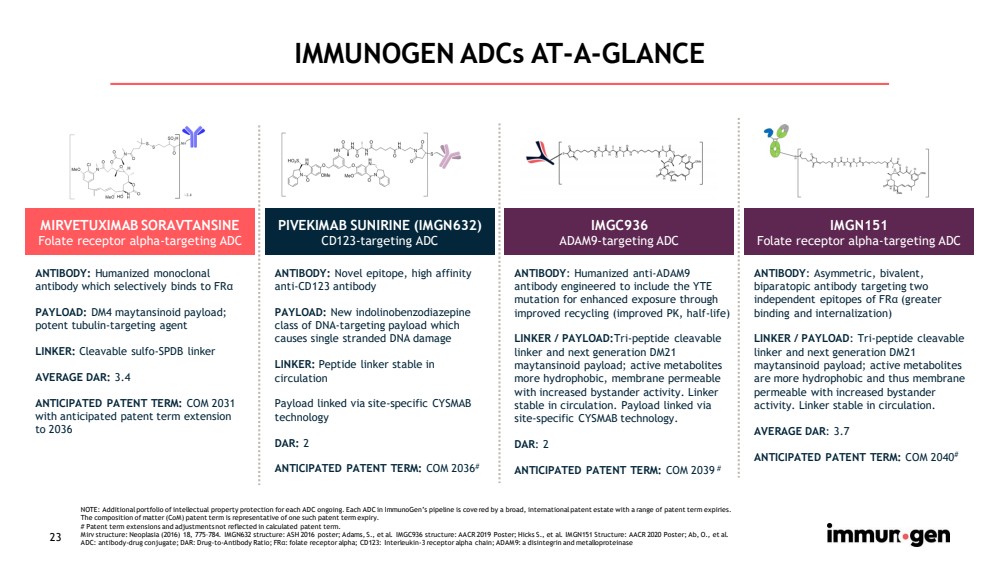
| 23 IMMUNOGEN ADCs AT - A - GLANCE MIRVETUXIMAB SORAVTANSINE Folate receptor alpha - targeting ADC PIVEKIMAB SUNIRINE (IMGN632) CD123 - targeting ADC IMGC936 ADAM9 - targeting ADC IMGN151 Folate receptor alpha - targeting ADC ANTIBODY: Hu manized monoclonal antibody which selectively binds to FR α PAYLOAD: DM4 maytansinoid payload; potent tubulin - targeting agent LINKER: Cleavable sulfo - SPDB linker AVERAGE DAR: 3.4 ANTICIPATED PATENT TERM: COM 2031 with anticipated patent term extension to 2036 ANTIBODY: Novel epitope, high affinity anti - CD123 antibody PAYLOAD: N ew indolinobenzodiazepine class of DNA - targeting payload which causes single stranded DNA damage LINKER: Peptide linker stable in circulation Payload linked via site - specific CYSMAB technology DAR: 2 ANTICIPATED PATENT TERM: COM 2036 # ANTIBODY : Humanized anti - ADAM9 antibody engineered to include the YTE mutation for enhanced exposure through improved recycling (improved PK, half - life) LINKER / PAYLOAD: Tri - peptide cleavable linker and next generation DM21 maytansinoid payload; active metabolites more hydrophobic, membrane permeable with increased bystander activity. Linker stable in circulation. Payload linked via site - specific CYSMAB technology. DAR : 2 ANTICIPATED PATENT TERM: COM 2039 # ANTIBODY : Asymmetric, bivalent, biparatopic antibody targeting two independent epitopes of FR α (greater binding and internalization) LINKER / PAYLOAD : Tri - peptide cleavable linker and next generation DM21 maytansinoid payload; active metabolites are more hydrophobic and thus membrane permeable with increased bystander activity. Linker stable in circulation. AVERAGE DAR : 3.7 ANTICIPATED PATENT TERM: COM 2040 # NOTE: Additional portfolio of intellectual property protection for each ADC ongoing. Each ADC in ImmunoGen’s pipeline is cove red by a broad, international patent estate with a range of patent term expiries. The composition of matter ( CoM ) patent term is representative of one such patent term expiry. # Patent term extensions and adjustments not reflected in calculated patent term. Mirv structure: Neoplasia (2016) 18, 775 – 784. IMGN632 structure: ASH 2016 poster; Adams, S., et al. IMGC936 structure: AACR 2019 Poster; Hicks S., et al. IMGN151 Structure: AACR 2020 Poster; Ab, O., et al. ADC: antibody - drug conjugate; DAR: Drug - to - Antibody Ratio; FR α: folate receptor alpha; CD123: Interleukin - 3 receptor alpha chain; ADAM9: a disintegrin and metalloproteinase |
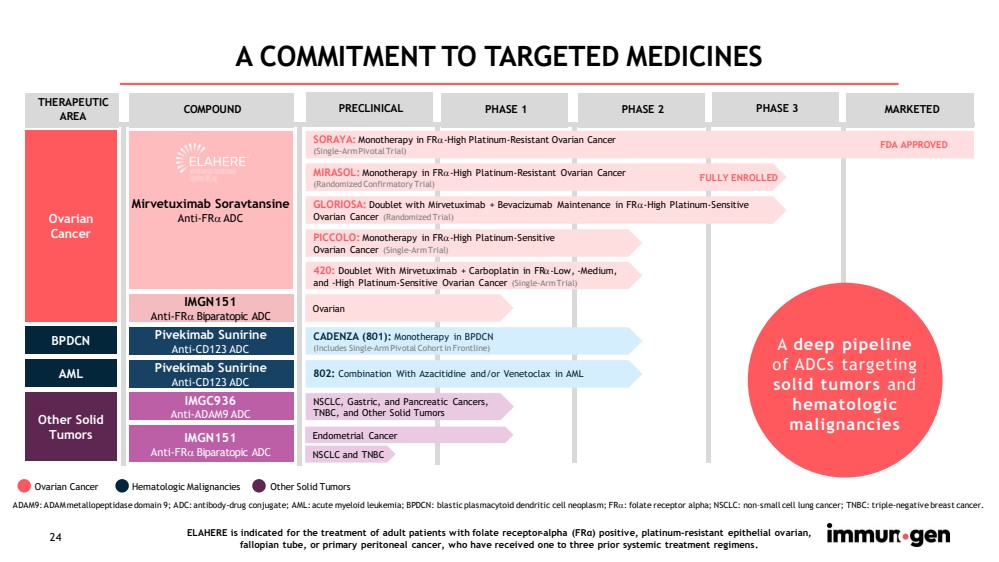
| 24 A COMMITMENT TO TARGETED MEDICINES Ovarian Cancer Hematologic Malignancies Other Solid Tumors ADAM9: ADAM metallopeptidase domain 9; ADC: antibody - drug conjugate; AML: acute myeloid leukemia; BPDCN: blastic plasmacytoid dendritic cell neoplasm; FR : folate receptor alpha; NSCLC: non – small cell lung cancer; TNBC: triple - negative breast cancer. COMPOUND PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKETED Mirvetuximab Soravtansine Anti - FR ADC SORAYA: Monotherapy in FR - High Platinum - Resistant Ovarian Cancer (Single - Arm Pivotal Trial) FDA APPROVED MIRASOL: Monotherapy in FR - High Platinum - Resistant Ovarian Cancer (Randomized Confirmatory Trial) GLORIOSA: Doublet with Mirvetuximab + Bevacizumab Maintenance in FR - High Platinum - Sensitive Ovarian Cancer (Randomized Trial) PICCOLO: Monotherapy in FR - High Platinum - Sensitive Ovarian Cancer (Single - Arm Trial) 420: Doublet With Mirvetuximab + Carboplatin in FR - Low, - Medium, and - High Platinum - Sensitive Ovarian Cancer (Single - Arm Trial) IMGC936 Anti - ADAM9 ADC NSCLC, Gastric, and Pancreatic Cancers, TNBC, and Other Solid Tumors IMGN151 Anti - FR Biparatopic ADC Endometrial Cancer Pivekimab Sunirine Anti - CD123 ADC CADENZA (801): Monotherapy in BPDCN (Includes Single - Arm Pivotal Cohort in Frontline) THERAPEUTIC AREA Ovarian Cancer Other Solid Tumors Pivekimab Sunirine Anti - CD123 ADC IMGN151 Anti - FR Biparatopic ADC Ovarian BPDCN AML FULLY ENROLLED A deep pipeline of ADCs targeting solid tumors and hematologic malignancies ELAHERE is indicated for the treatment of adult patients with folate receptor - alpha (FRα) positive, platinum - resistant epithelia l ovarian, fallopian tube, or primary peritoneal cancer, who have received one to three prior systemic treatment regimens .. 802: Combination With Azacitidine and/or Venetoclax in AML NSCLC and TNBC |
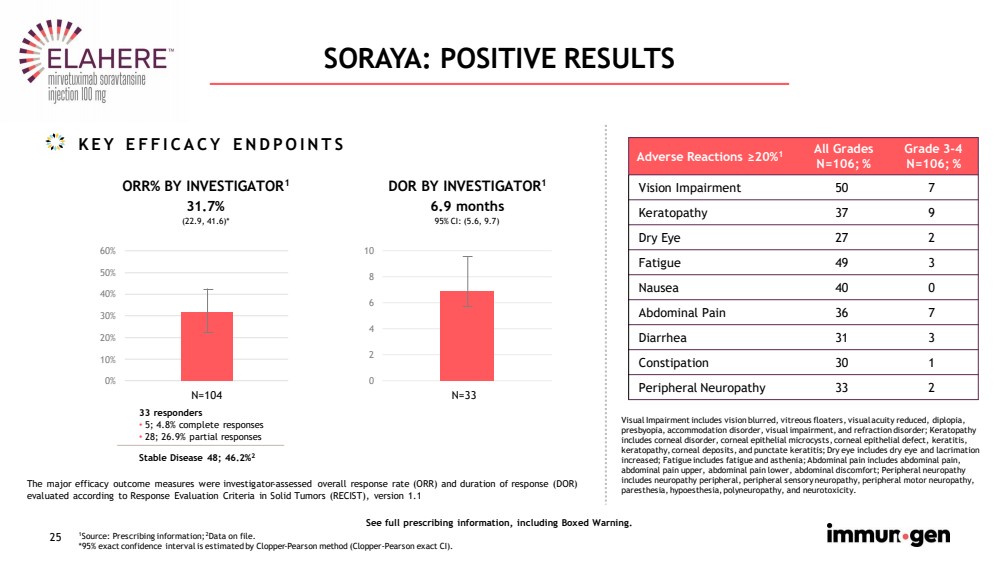
| 25 SORAYA: POSITIVE RESULTS 1 Source: Prescribing information; 2 Data on file. *95% exact confidence interval is estimated by Clopper - Pearson method (Clopper - Pearson exact CI). KEY EFFICACY ENDPOINTS ORR% BY INVESTIGATOR 1 0% 10% 20% 30% 40% 50% 60% DOR BY INVESTIGATOR 1 33 responders • 5; 4.8% complete responses • 28; 26.9% partial responses 31.7% 0 2 4 6 8 10 6.9 months 95% CI: ( 5.6, 9.7) N=104 (22.9, 41.6)* Adverse Reactions ≥20% 1 All Grades N=106; % Grade 3 - 4 N=106; % Vision Impairment 50 7 Keratopathy 37 9 Dry Eye 27 2 Fatigue 49 3 Nausea 40 0 Abdominal Pain 36 7 Diarrhea 31 3 Constipation 30 1 Peripheral Neuropathy 33 2 See full prescribing information, including Boxed Warning. The major efficacy outcome measures were investigator - assessed overall response rate (ORR) and duration of response (DOR) evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 Visual Impairment includes vision blurred, vitreous floaters, visual acuity reduced, diplopia, presbyopia, accommodation disorder, visual impairment, and refraction disorder; Keratopathy includes corneal disorder, corneal epithelial microcysts, corneal epithelial defect, keratitis, keratopathy, corneal deposits, and punctate keratitis; Dry eye includes dry eye and lacrimation increased; Fatigue includes fatigue and asthenia ; Abdominal pain includes abdominal pain, abdominal pain upper , abdominal pain lower , abdominal discomfort ; Peripheral neuropathy includes neuropathy peripheral , peripheral sensory neuropathy , peripheral motor neuropathy , paresthesia , hypoesthesia , polyneuropathy , and neurotoxicity .. N=33 Stable Disease 48; 46.2% 2 |
| CONFIDENTIAL 26 Mirvetuximab PHASE 3 RANDOMIZED TRIAL FOR MIRVETUXIMAB IN FR α - HIGH PATIENTS WITH PLATINUM - RESISTANT OVARIAN CANCER *Eligibility criterion different than SORAYA BICR: blinded independent central review; BRCA: BReast CAncer gene; FR α : folate receptor alpha; IC: investigator’s choice; ORR: objective response rate; OS: overall survival; PARPi : poly ADP - ribose polymerase inhibitor; PFI: platinum - free interval; PFS: progression - free survival; PLD: pegylated liposomal doxorubicin; PRO: pa tient - reported outcomes Investigator’s Choice Chemotherapy Paclitaxel, PLD, or Topotecan STRATIFICATION FACTORS IC Chemotherapy (Paclitaxel, PLD, Topotecan) Prior Therapies (1 vs 2 vs 3) 1:1 RANDOMIZATION PRIMARY ENDPOINT PFS by Investigator BICR for Sensitivity Analysis SECONDARY ENDPOINTS ORR by Investigator, OS, and PRO ENROLLMENT AND KEY ELIGIBILITY 430 patients/330 events for PFS by Investigator Platinum - resistant disease (primary PFI >3 months) 1 to 3 prior lines of therapy Prior bevacizumab* and prior PARPi allowed Patients with BRCA mutations allowed Confirmatory trial with potential to support full approval in the US and a marketing application in the EU • Enrollment completed mid - 2022 • Expect top - line data early 2023 |
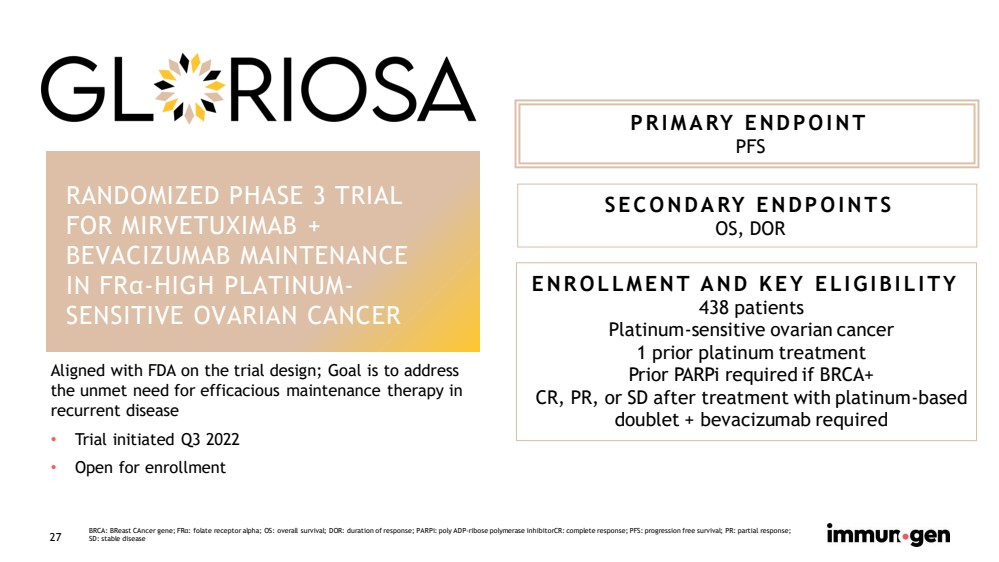
| CONFIDENTIAL 27 RANDOMIZED PHASE 3 TRIAL FOR MIRVETUXIMAB + BEVACIZUMAB MAINTENANCE IN FR α - HIGH PLATINUM - SENSITIVE OVARIAN CANCER PRIMARY ENDPOINT PFS SECONDARY ENDPOINTS OS, DOR ENROLLMENT AND KEY ELIGIBILITY 438 patients Platinum - sensitive ovarian cancer 1 prior platinum treatment Prior PARPi required if BRCA+ CR, PR, or SD after treatment with platinum - based doublet + bevacizumab required BRCA: BReast CAncer gene; FR α : folate receptor alpha; OS: overall survival; DOR: duration of response; PARPi : poly ADP - ribose polymerase inhibitorCR : complete response; PFS: progression free survival; PR: partial response; SD: stable disease Aligned with FDA on the trial design; Goal is to address the unmet need for efficacious maintenance therapy in recurrent disease • Trial initiated Q3 2022 • Open for enrollment |
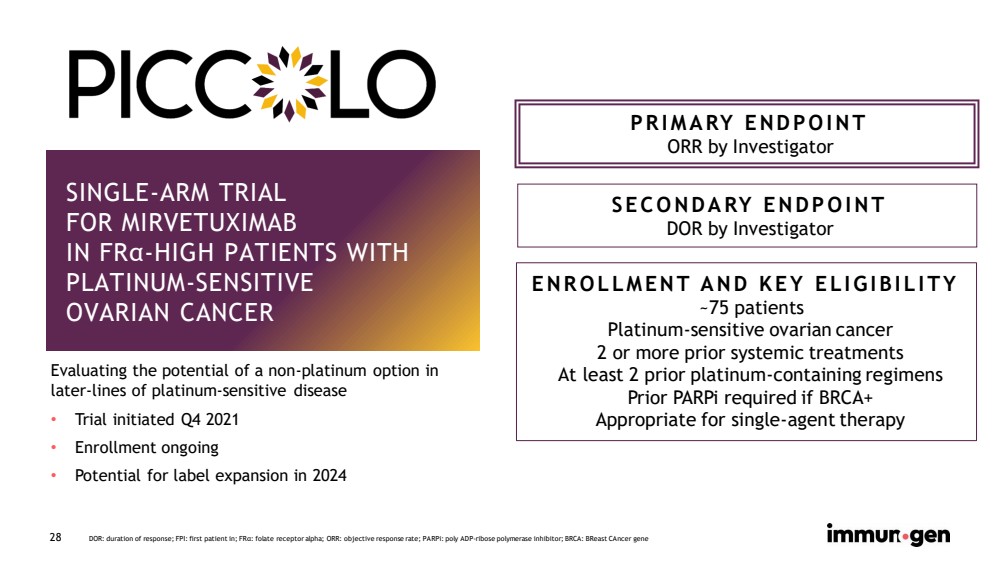
| CONFIDENTIAL 28 SINGLE - ARM TRIAL FOR MIRVETUXIMAB IN FR α - HIGH PATIENTS WITH PLATINUM - SENSITIVE OVARIAN CANCER PRIMARY ENDPOINT ORR by Investigator SECONDARY ENDPOINT DOR by Investigator ENROLLMENT AND KEY ELIGIBILITY ~75 patients Platinum - sensitive ovarian cancer 2 or more prior systemic treatments At least 2 prior platinum - containing regimens Prior PARPi required if BRCA+ Appropriate for single - agent therapy DOR: duration of response; FPI: first patient in; FR α : folate receptor alpha; ORR: objective response rate; PARPi : poly ADP - ribose polymerase inhibitor; BRCA: BReast CAncer gene Evaluating the potential of a non - platinum option in later - lines of platinum - sensitive disease • Trial initiated Q4 2021 • Enrollment ongoing • Potential for label expansion in 2024 |
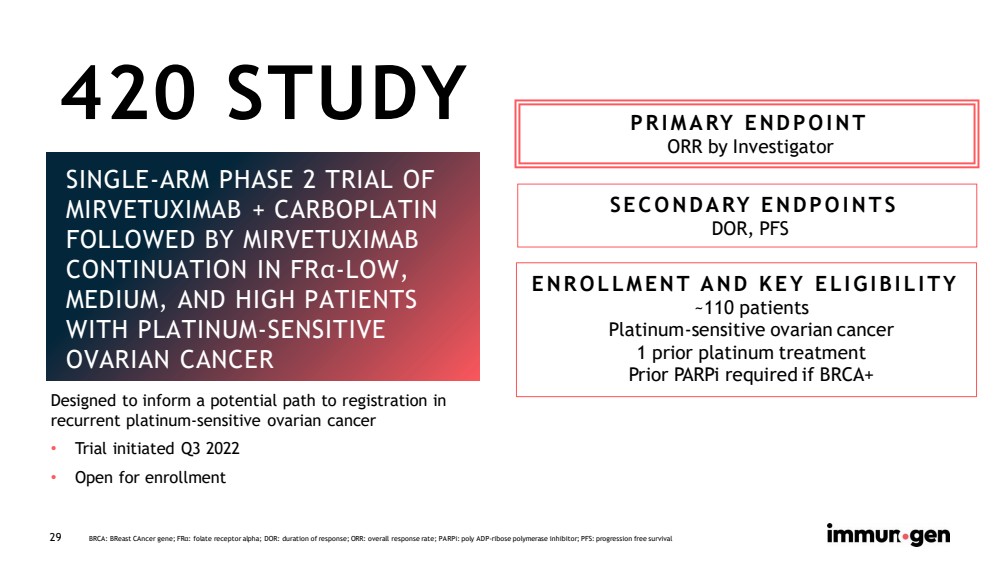
| CONFIDENTIAL 29 SINGLE - ARM PHASE 2 TRIAL OF MIRVETUXIMAB + CARBOPLATIN FOLLOWED BY MIRVETUXIMAB CONTINUATION IN FR α - LOW, MEDIUM, AND HIGH PATIENTS WITH PLATINUM - SENSITIVE OVARIAN CANCER PRIMARY ENDPOINT ORR by Investigator SECONDARY ENDPOINTS DOR, PFS ENROLLMENT AND KEY ELIGIBILITY ~110 patients Platinum - sensitive ovarian cancer 1 prior platinum treatment Prior PARPi required if BRCA+ BRCA: BReast CAncer gene; FR α : folate receptor alpha; DOR: duration of response; ORR: overall response rate; PARPi : poly ADP - ribose polymerase inhibitor; PFS: progression free survival 420 STUDY Designed to inform a potential path to registration in recurrent platinum - sensitive ovarian cancer • Trial initiated Q3 2022 • Open for enrollment |
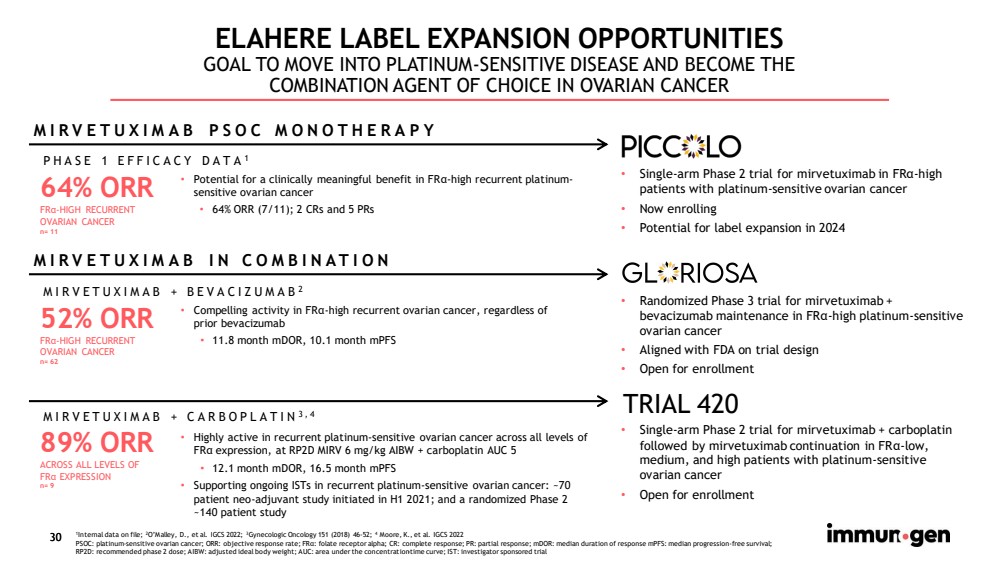
| 30 ELAHERE LABEL EXPANSION OPPORTUNITIES GOAL TO MOVE INTO PLATINUM - SENSITIVE DISEASE AND BECOME THE COMBINATION AGENT OF CHOICE IN OVARIAN CANCER 89% ORR ACROSS ALL LEVELS OF FR α EXPRESSION n= 9 64% ORR FR α - HIGH RECURRENT OVARIAN CANCER n= 11 MIRVETUXIMAB + BEVACIZUMAB 2 MIRVETUXIMAB + CARBOPLATIN 3,4 • Highly active in recurrent platinum - sensitive ovarian cancer across all levels of FR α expression, at RP2D MIRV 6 mg/kg AIBW + carboplatin AUC 5 • 12.1 month mDOR , 16.5 month mPFS • Supporting ongoing ISTs in recurrent platinum - sensitive ovarian cancer: ~70 patient neo - adjuvant study initiated in H1 2021; and a randomized Phase 2 ~140 patient study PHASE 1 EFFICACY DATA 1 • Potential for a clinically meaningful benefit in FR α - high recurrent platinum - sensitive ovarian cancer • 64% ORR (7/11); 2 CRs and 5 PRs MIRVETUXIMAB IN COMBINATION MIRVETUXIMAB PSOC MONOTHERAPY • Single - arm Phase 2 trial for mirvetuximab in FR α - high patients with platinum - sensitive ovarian cancer • Now enrolling • Potential for label expansion in 2024 • Randomized Phase 3 trial for mirvetuximab + bevacizumab maintenance in FR α - high platinum - sensitive ovarian cancer • Aligned with FDA on trial design • Open for enrollment • Single - arm Phase 2 trial for mirvetuximab + carboplatin followed by mirvetuximab continuation in FR α - low, medium, and high patients with platinum - sensitive ovarian cancer • Open for enrollment TRIAL 420 1 Internal data on file; 2 O’Malley, D., et al. IGCS 2022; 3 Gynecologic Oncology 151 (2018) 46 - 52; 4 Moore, K., et al. IGCS 2022 PSOC: platinum - sensitive ovarian cancer; ORR: objective response rate; FR α : folate receptor alpha; CR: complete response; PR: partial response; mDOR : median duration of response mPFS : median progression - free survival; RP2D: recommended phase 2 dose; AIBW: adjusted ideal body weight; AUC: area under the concentration - time curve; IST: investigato r sponsored trial 30 52% ORR FR α - HIGH RECURRENT OVARIAN CANCER n= 62 • Compelling activity in FR α - high recurrent ovarian cancer, regardless of prior bevacizumab • 11.8 month mDOR , 10.1 month mPFS |
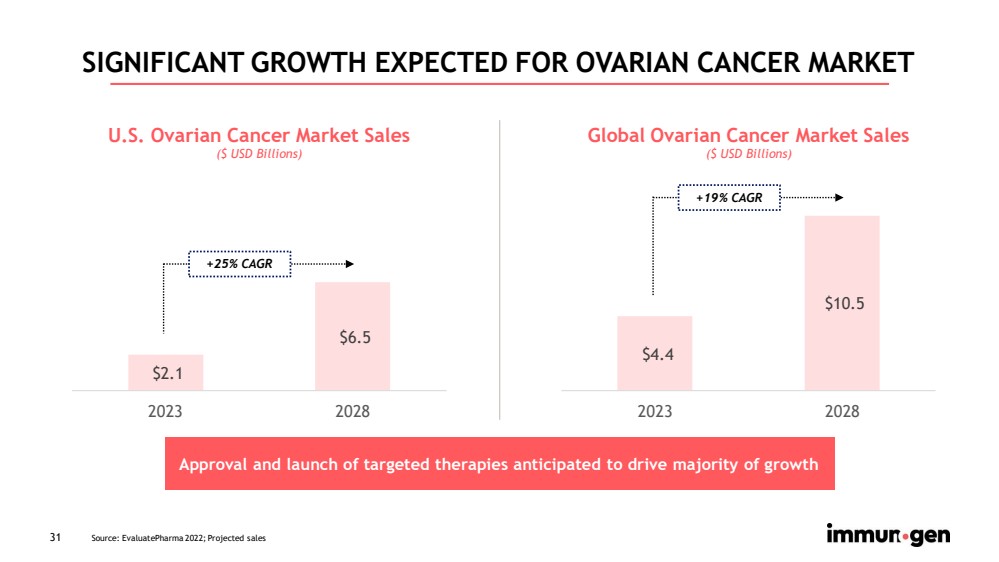
| 31 SIGNIFICANT GROWTH EXPECTED FOR OVARIAN CANCER MARKET $2.1 $6.5 2023 2028 U.S. Ovarian Cancer Market Sales ($ USD Billions) +25% CAGR Approval and launch of targeted therapies anticipated to drive majority of growth $4.4 $10.5 2023 2028 Global Ovarian Cancer Market Sales ($ USD Billions) +19% CAGR Source: EvaluatePharma 2022; Projected sales |