Filed Pursuant to Rule 424(b)(5)
Registration No. 333-163454
The information in this prospectus supplement is not complete and may be changed. This prospectus supplement and the accompanying prospectus are not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated August 9, 2010
PROSPECTUS SUPPLEMENT
(To Prospectus dated January 26, 2010)

HQ SUSTAINABLE MARITIME INDUSTRIES, INC.
[ ] Shares of Common Stock
Warrants to Purchase up to [ ] Shares of Common Stock
Pursuant to this prospectus supplement and the accompanying prospectus, we are offering [ ] units, consisting of (i) [ ] shares of our common stock, par value $0.001 per share, and (ii) warrants to purchase up to [ ] shares of common stock. Each unit consists of one share of our common stock and a warrant to purchase 0.5 shares of common stock at an initial exercise price of $[ ] per share. The sales price for each unit is $[ ]. The warrants will be exercisable during the period beginning six months after the date of issue and ending on the fifth anniversary of the date of issue. In this prospectus supplement, we refer to the shares and warrants collectively as the “units.” The shares of common stock and warrants comprising the units will be issued separately.
Our common stock trades on the NYSE Amex under the symbol “HQS.” The last reported sale price of our common stock on the NYSE Amex on August [ ], 2010 was $[ ] per share. There is no established public trading market for the offered warrants and we do not expect a market to develop. In addition, we do not intend to apply for listing of the warrants on any national securities exchange or automated quotation system.
Investing in our common stock involves risk. See “Risk Factors” beginning on page S-23 of this prospectus supplement and page 8 of the accompanying prospectus. You should carefully read this prospectus supplement and the accompanying prospectus, together with the documents we incorporate by reference, before you invest in any of our securities.
We will receive the proceeds from all securities sold in this offering less underwriting discounts and commissions, and less other expenses we incur in connection with the offering of the units.
| | | | | | |
| | | Per Unit | | Total |
Price to the public | | $ | [ ] | | $ | [ ] |
Underwriting discounts and commissions | | $ | [ ] | | $ | [ ] |
Proceeds, before expenses, to us | | $ | [ ] | | $ | [ ] |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriter expects to deliver the securities being offered pursuant to this prospectus supplement and the accompanying prospectus on or about August [ ], 2010.
Ladenburg Thalmann & Co. Inc.
The date of this prospectus supplement is August [ ], 2010.
TABLE OF CONTENTS
You should rely on the information contained in this prospectus supplement, the accompanying prospectus and in the documents incorporated by reference in this prospectus supplement. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any jurisdiction where their offer or sale is not permitted. You should assume that the information appearing in this prospectus supplement is accurate only at the date on the front cover of this prospectus supplement, regardless of the time of delivery of this prospectus supplement or of any sale of the securities. Our business, financial condition, results of operations and prospects may have changed since the date indicated on the front cover of this prospectus supplement.
This prospectus supplement contains summaries of certain provisions contained in some of the documents described herein, and reference is made to the actual documents filed with the United States Securities and Exchange Commission, or SEC, for complete information. Copies of some of the documents referred to herein have been filed or will be filed or incorporated by reference as exhibits to our SEC filings, and you may obtain copies of those documents as described below under “Where You Can Find More Information.”
Additional information, including our financial statements for the years ended December 31, 2009 and 2008 and the notes related thereto, is incorporated by reference to our periodic reports filed with the SEC.
i
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of this offering. The second part, the accompanying prospectus, gives more general information, some of which may not apply to this offering. You should read this entire prospectus supplement, as well as the accompanying prospectus and the documents incorporated by reference that are described under “Information Incorporated by Reference” in this prospectus supplement and the accompanying prospectus. In the event that the description of the offering varies between this prospectus supplement and the accompanying prospectus, you should rely on the information contained in this prospectus supplement.
You should rely only on the information contained or incorporated by reference in this prospectus supplement, the accompanying prospectus and any free writing prospectus prepared by or on behalf of us, or information to which we have referred you. We have not, and the underwriter has not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it.
We are not, and the underwriter is not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted.
You should assume that the information appearing in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference is accurate only as of the respective dates of those documents in which the information is contained. Our business, financial condition, results of operations and prospects may have changed since those dates.
We obtained statistical data, market data and other industry data and forecasts used throughout, or incorporated by reference in, this prospectus supplement and the accompanying prospectus from market research, publicly available information and industry publications. Industry publications generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy and completeness of the information.
Unless otherwise indicated or unless the context otherwise requires, all references in this prospectus supplement and the accompanying prospectus to “HQS,” the “Company”, “we,” “our” and “us” refer to HQ Sustainable Maritime Industries, Inc. and all of its subsidiaries and affiliated companies including Hainan Quebec Ocean Fishing Co. Ltd., or “HQOF,” and Jiahua Marine Bio-Product Company Limited, or “Jiahua Marine,” on a consolidated basis.
S-1
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus and the documents we incorporate by reference in this prospectus contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These statements involve known and unknown risks, uncertainties and other important factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| | • | | our marketing, sales and distribution initiatives in North America, Europe and China; |
| | • | | our estimates of production, market sizes and anticipated consumption of our marine bio and healthcare products and our aquaculture products; |
| | • | | our new organic feed mill and our current and future processing facilities; |
| | • | | our ability to enter into and maintain cooperative farming arrangements and other partnerships with respect to our marine bio products and the performance of our partners under such arrangements; |
| | • | | our relationship with local governments in Hainan Province and the potential support and financing they may provide to us; |
| | • | | our estimates of future performance and growth potential; |
| | • | | our ability to obtain sufficient capital to fund our operations and expansion plans; and |
| | • | | our operating income, future revenue, expenses, capital requirements and needs for additional financing. |
In some cases, you can identify forward-looking statements by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “would” and similar expressions intended to identify forward-looking statements. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Because of these risks and uncertainties, the forward-looking events and circumstances discussed in this prospectus supplement and the accompanying prospectus may not transpire.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. You should read this document, any supplements to this document and the documents that we reference in this prospectus supplement and the accompanying prospectus with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we do not undertake any obligation to update or revise any forward-looking statements contained in this prospectus supplement and the accompanying prospectus, whether as a result of new information, future events or otherwise.
S-2
PROSPECTUS SUPPLEMENT SUMMARY
The following summary highlights selected information contained elsewhere in this prospectus supplement, the accompanying prospectus or incorporated herein by reference. This summary does not contain all the information you should consider before investing in our common stock. You should read the entire prospectus supplement, the accompanying prospectus, any applicable free writing prospectus, as well as the information incorporated by reference carefully, especially the discussion of “Risk Factors” and our consolidated financial statements and the related notes, before deciding to invest in our securities. In this prospectus supplement and the accompanying prospectus, when we use phrases such as “we,” “us,” “our,” “HQS” or “our company,” we are referring to HQ Sustainable Maritime Industries, Inc. and all of its subsidiaries and affiliated companies as a whole, unless it is clear from the context that any of these terms refer only to HQ Sustainable Maritime Industries, Inc.
About Our Company
We are a multi-national company with our headquarters and primary sales offices based in Seattle, Washington. We are a vertically integrated aquatic product producer, processor, and farmer (e.g. through cooperative arrangements with pond farmers), with operations in the People’s Republic of China (“PRC”) of all natural tilapia, other aquatic products, and marine bio and healthcare products. We use state-of-the-art and environment-friendly technologies in our production, processing, and cooperative farm operations. Our facilities are currently certified according to the Hazard Analysis and Critical Control Point (“HACCP”) standards, currently assigned an European Union (“EU”) code required for exporting aquatic products to the EU, and are currently certified in accordance with the Aquaculture Certification Counsel (“ACC”) standards. Our products are sold principally to customers in North America, Europe and Asia. Our current sales activity is primarily directed to distributors within the PRC, rather than within the U.S.
Established in 1999, our aquatic farming and processing operations are in Hainan Province, an island in the South China Sea, which is situated within the most desirable latitude for raising tilapia. The Chinese government has designated Hainan Province in southern China a “green” province, where only environmentally friendly agri-food related industry and tourism are permitted. We purchase and process farm bred and farm raised tilapia through cooperative supply arrangements with local farmers and cooperatives. Our supply cooperatives, under our guidance, use feed formulated by us to optimize the all natural growth of farm raised tilapia. Starting in 2009, we began supplying the farmers and cooperatives with feed formulated from our feed processing mill. We believe our tilapia products have achieved such a high level of purity that we market these products as all natural tilapia. In addition, we purchase and process ocean-caught aquatic products through cooperative supply arrangements with local fisherman for our marine bio healthcare products.
In August 2004, the company acquired Hainan Jiahua Marine BioProducts Co. Ltd., or Jiahua Marine, which develops, produces and sells marine bio and healthcare products, including nutraceutical products for the enrichment of our feed formulas, exclusively in China. The principal products of Jiahua Marine are manufactured from fish by-products (tilapia and shark) that include shark cartilage capsules and shark liver oil products that are distributed exclusively in China. The products undergo substantial independent laboratory testing administered by the Ministry of Health in China, which has resulted in a PRC National Certification for these products. These products have various perceived medicinal and health benefits. As part of HQS’s vertical integration plan with respect to tilapia, Jiahua Marine introduced new bio and healthcare products made from tilapia in 2009. Following changes in China legislation on food safety standards, which became effective on June 1, 2009, distribution of our health products have expanded and our sales have increased. We expect our health and bio-products business to continue to grow in the future as a result of this legislation. Further, Jiahua Marine’s health products recently have been rebranded to under the “Omojo” brand to celebrate its 10th anniversary.
S-3
In order to maintain the high quality of our products and to position ourselves for attaining completely organic production certification in the future, we have completed the construction of our own feed mill and processing plant for the production of floating feed formulations. We produce the feed using grains grown without chemical fertilizers, free of antibiotics and fishmeal, and use feed additives manufactured in our nutraceutical plant. We plan for this expanded production to satisfy our own demand through the 20,000 mu (or 3,294 acres) of production in Wenchang and Qionghai, as well as to manufacture feed for others’ farmed operations in Hainan, such as shrimp and other farmed species. We also are in the process of constructing a new re-processing plant and a new breeding and genetics facility, which are discussed in detail below.
From our headquarters in Seattle, Washington, we market and brand our high-quality, all natural tilapia products under the name TILOVEYA®. In early 2009, we introduced a new family of frozen tilapia meals under the brand name “Lillian’s Healthy Gourmet.” This new family of products includes an organic, natural and regular line of frozen tilapia meals.
We are incorporated in Delaware. The address of our Unites States headquarters is Melbourne Towers, 1511 Third Avenue, Suite 788, Seattle, Washington 98101 and our telephone number is (206) 621-9888. Our website is located atwww.hqfish.com. The information contained on our website or that can be accessed through our website does not constitute part of this prospectus supplement.
Industry Overview
Aquaculture Industry
Aquaculture is the farming of aquatic animals or plants. Aquaculture is the fastest growing segment in the food production system and has been for the past two decades. According to a recent study by the World Food and Agriculture Organization (“FAO”) published March 2, 2009, world fisheries production reached a new high of 143.6 million metric tonnes in 2006, including farmed and ocean caught product. The contribution of aquaculture to the world fisheries production in 2006 was 51.7 million tonnes of fish, which is 36% of world fisheries production, up from 3.6% in 1970. Global aquaculture accounted for 6% of the fish available for human consumption in 1970. In 2006 global aquaculture accounted for 47% of the fish available for human consumption according to the FAO. The FAO report also describes that over half of the global aquaculture in 2006 was freshwater finfish. Based on the FAO’s projections, it is estimated that in order to maintain the current level of per capita consumption, global aquaculture production will need to reach in excess of 80 million tonnes of fish by 2050.
As the availability of sites for aquaculture is becoming increasingly limited and the ability to develop non-agricultural land is often restricted, the competition to develop additional aquaculture production systems is intensifying. As the intensification for aquaculture production systems increases, the demand for institutional support, services and skilled persons is anticipated to increase, along with the demand for more knowledge-based aquaculture education and training as aquaculture becomes more important worldwide. China remains the largest producer of aquaculture products throughout the world with fisheries in China reportedly producing approximately 41.3 million tonnes of fish in 2004. Within the global aquaculture industry, China accounted for 71.0% of the world’s supply of fish for direct human consumption and 29.9% of the total production by live weight in 2002.
Tilapia Industry
In 2005, according to the American Tilapia Association (ATA), tilapia production worldwide was second in volume to carp, and it is projected by the ATA that tilapia will become the most important aquaculture crop in this century, potentially reaching $5.0 billion in global sales by 2010. Commercial production of tilapia has become popular in many countries around the world. Touted as the new white fish to replace the depleted ocean
S-4
stocks of cod, pollock, and hake, world tilapia production continues to rise and at least 100 countries currently raise tilapia, with the PRC being the largest producer. The American Tilapia Association further reports that world production of tilapia products reached approximately 2.5 million metric tonnes in 2007, of which China produced the dominant share of 45.0%.
One of the major outlets for Chinese-produced tilapia has been, and should continue to be, the United States. The following chart reflects the increase in per-capita consumption of tilapia in pounds in the United States in relation to other traditional types of seafood.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Consumer Consumption Per Capita (lbs) |
| 2000 | | 2001 | | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 (est.) |
| Tuna | | 3.5 | | Shrimp | | 3.4 | | Shrimp | | 3.7 | | Shrimp | | 4.0 | | Shrimp | | 4.2 | | Shrimp | | 4.1 | | Shrimp | | 4.4 | | Shrimp | | 4.1 | | Shrimp | | 4.0 |
| Shrimp | | 3.2 | | Tuna | | 2.9 | | Tuna | | 3.1 | | Tuna | | 3.4 | | Tuna | | 3.4 | | Tuna | | 3.1 | | Tuna | | 2.9 | | Tuna | | 2.7 | | Tuna | | 2.6 |
| Pollock | | 1.6 | | Salmon | | 2.0 | | Salmon | | 2.0 | | Salmon | | 2.2 | | Salmon | | 2.2 | | Salmon | | 2.4 | | Salmon | | 2.0 | | Salmon | | 2.3 | | Salmon | | 2.2 |
| Salmon | | 1.5 | | Pollock | | 1.2 | | Pollock | | 1.1 | | Pollock | | 1.7 | | Pollock | | 1.7 | | Pollock | | 1.5 | | Pollock | | 1.6 | | Pollock | | 1.7 | | Pollock | | 1.6 |
| Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.0 | | Tilapia | | 1.0 | | Tilapia | | 1.1 | | Tilapia | | 1.2 |
| Cod | | 0.8 | | Cod | | 0.6 | | Cod | | 0.7 | | Cod | | 0.6 | | Tilapia | | 0.7 | | Tilapia | | 0.8 | | Catfish | | 1.0 | | Catfish | | 1.0 | | Catfish | | 1.0 |
| Clams | | 0.5 | | Clams | | 0.5 | | Crabs | | 0.6 | | Crabs | | 0.6 | | Cod | | 0.6 | | Cod | | 0.6 | | Crabs | | 0.7 | | Crabs | | 0.7 | | Crabs | | 0.7 |
| Crabs | | 0.4 | | Crabs | | 0.4 | | Clams | | 0.5 | | Tilapia | | 0.5 | | Crabs | | 0.6 | | Crabs | | 0.6 | | Cod | | 0.5 | | Cod | | 0.5 | | Clams | | 0.4 |
| Flatfish | | 0.4 | | Flatfish | | 0.4 | | Tilapia | | 0.4 | | Clams | | 0.5 | | Clams | | 0.5 | | Clams | | 0.4 | | Clams | | 0.4 | | Clams | | 0.4 | | Cod | | 0.4 |
| Scallops | | 0.3 | | Tilapia | | 0.4 | | Flatfish | | 0.4 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 |
| Tilapia | | 0.3 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following chart reflects the increase in imported tilapia to the United States during the periods indicated.
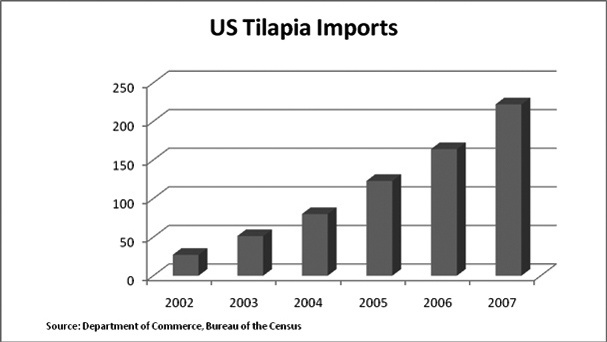
Separately, according to theInternational Trade Report produced in 2005 by the United States Department of Agriculture, or the USDA, U.S. per-capita seafood consumption has remained around 15 billion pounds
S-5
through the late 1980s and 1990s; it is expected to increase as farm-raised products become cheaper. Currently, the United States consumes nearly 12 billion pounds of fish a year. By 2025, demand for seafood is projected to grow by another 4.4 billion pounds (2 million metric tonnes) above what is consumed today. In addition, it is estimated that by 2020, 50% of the U.S. seafood supply will come from aquaculture. Presently, more than 70% of the seafood consumed in the United States is imported, and at least 40% of that is farm-raised. Major changes in U.S. population, along with shifting demographics and economic growth, will alter the U.S. seafood market over the next decade, affecting the selection of products consumed. It is expected that fresh and frozen fish products will account for a growing share of overall seafood consumption, with shrimp remaining at the top. By 2020, shrimp, salmon, tilapia, and catfish will be the top four seafood products consumed.
According to Globefish.org, during the past ten years, tilapia captured by fisheries have stabilized at 0.6 million metric tonnes, while their aquaculture production has grown from 0.55 million metric tonnes to 2.0 million metric tonnes. Tilapia is one of the top five seafood imports in the world. In 2005, more than $2.4 billion of tilapia was sold worldwide according to the FAO Fishstat 2007. In 2005, tilapia moved up to the fourth-ranked most popular seafood after farm-raised shrimp, tuna and Atlantic salmon in terms of aquaculture products imported into the United States. The United States is now the world’s largest consumer of tilapia after China, having imported 437 thousand metric tonnes of tilapia in 2007. In terms of volume, frozen whole round fish ranks first, followed by frozen fillets and, lastly, fresh fillets.
Health Benefits as the Driving Force for Growth in Tilapia Industry
The growing consumer demand for seafood is largely evolving from a new public awareness regarding its health and nutritional benefits. The USDA pyramid guidelines continue to support frequent fish consumption, and the USDA recently completed a highly technical nutritional analysis report about tilapia for the general public. The USDA’s Agriculture Research Service Lab reports that tilapia is moderate in polyunsaturated fatty acid (0.387 g/100g raw, 0.600 g/100g cooked), moderate in omega 3 fatty acids (0.141g/100g raw, 0.220g/100g cooked) and low in mercury (0.010 parts per million, which we refer to as PPM), which is considered to be non-detectable. With the increased awareness of the health concerns surrounding mercury, tilapia’s low mercury levels (0.010 PPM) distinguish tilapia favorably from other types of fish with higher mercury levels, such as swordfish (0.976 PPM), mackerel (0.730 PPM) and yellow fin tuna (0.325 PPM).
Over-the-Counter Marine Bio and Healthcare Products
Due to its aging population, China is demographically attractive for healthcare companies. According to the Chinese Ministry of Health’s 2009 figures, of the 1.3 billion people in China less than 8% are 65 or older; however, by 2050 that proportion is expected to rise to 24%. It is expected as well that Chinese will benefit from double-digit annual growth in disposable incomes during this period. We anticipate that this will lead to increasing use of western therapeutics and “over the counter” remedies. In addition, the market place is highly fragmented with more than 4,000 manufacturers of such remedies in China, most of whom lack scale and access to capital. From 2009 to 2011, we believe approximately $124 billion will be allocated to healthcare expenditure in China. HQS has benefited from regulatory consolidation that resulted in the imposition of higher Chinese Good Manufacturing Practice (“GMP”) standards in 2007 and intends to be a leader in its emphasis on the highest quality standards.
The marine bio and healthcare products industry in China is also sizable, with approximately $6 billion in sales, which still constitutes only 3% of the world market. Currently, overall sales of these products in China have fallen slightly, as compared to the previous year, as consumers gravitate toward branded products meeting international quality standards and with proven benefit for consumers, and away from less known brands and traditional remedies.
S-6
In general, sales of marine bio and healthcare products are made through retail and direct sales channels. Direct sales in China are relatively new, and restrictions on direct sales imposed on large foreign companies have been implemented in China, which has allowed for a growth in sales for PRC-based direct sales companies. These restrictions require foreign direct sellers to manufacture their products and to capitalize their businesses in China. Several companies have met these requirements, including HQS through its PRC-subsidiaries, and growth in this sector is expected to be strong in the next few years.
We believe that nutraceutical supplements in the feed industry is a sector that has strong growth potential, as the importance of aquaculture and aquaculture feeds increases. We manufacture actualized feeds, which involves choosing additives to be included in the feed, such as vitamin E, that promote health in fish, thus reducing the need for curative measures such as antibiotics. Once ingested and present in the flesh of the fish, vitamin E increases the shelf life of the fish and also introduces an additional source of vitamin E to its consumers. Other similar nutraceutical supplements can also be used.
Our Products
Tilapia
Tilapia is currently farmed throughout many countries around the world because this fish is disease-resistant, reproduces easily, eats a wide variety of feeds and tolerates a variety of poor water quality even water with low dissolved oxygen levels. Although there are many species of tilapia, only a few species are farmed for human consumption. The most common species that is farmed commercially is the black, or Nile, tilapia that is grown in ponds, cages or rice fields.
In response to increasing demand for tilapia, we export from our subsidiary in the PRC varying quantities of tilapia in different forms to the United States, Europe, Asia and other regions. In 2007 we launched a marketing and distribution initiative in the United States and Europe with our TILOVEYA® brand tilapia. We continue to promote our TILOVEYA® brand with several leading distributors and retailers in Europe and the United States. We also entered into an exclusive agreement with the Beijing division of Newly Weds® Foods, Inc. to introduce an innovative line of battered and breaded flavored TILOVEYA® fillet for the Chinese consumer. In addition, we co-developed a process and natural flavoring giving tilapia the taste, texture and aroma of fresh ocean-caught fish. We will process such products without the drawbacks of ocean-caught product such as ocean waste and, ecological management. The tilapia products sold by us are mainly in the following forms: whole round frozen, gutted and scaled, boneless-skinless tilapia fillets and, more recently, we began selling boneless-skin-on tilapia fillets.
In 2008 and 2009, we developed a family of frozen tilapia meal products under the brand name of Lillian’s Healthy Gourmet. The family of products will have three lines focusing on organic, natural and regular frozen meals. These meals are processed by a variety of manufacturing facilities in North America including Marsan Foods located in Toronto, Canada. Marsan is the top Canadian processor of private label entrées and branded meal products. The Company has extended brokerage and distribution arrangements across the United States and is continuing to roll out products and expand its range of value-added products from 12 ounce meals to 8 ounce meals and adding flavors. Other value-added products are in the pipeline using our tilapia products catering to an all-natural value-added market.
Our principal operating subsidiary with respect to our seafood products is Hainan Quebec Ocean Fishing Co. Ltd., a company organized in the PRC (“HQOF”).
Marine Bio and Healthcare Products
Since the August 2004 acquisition of our wholly-owned subsidiary, Hainan Jiahua Marine BioProducts Co. Ltd. (Jiahua Marine), a Chinese limited liability company, we have also been engaged in the development,
S-7
production, and sales of marine bio and healthcare products in the PRC. We acquired Jiahua Marine pursuant to a purchase agreement with Sino-Sult Canada Limited (SSC), a Canadian limited liability company, and Sealink Wealth Limited (Sealink), a British Virgin Islands limited liability company, a wholly-owned subsidiary of SSC and the then sole shareholder of Jiahua Marine.
The principal products of Jiahua Marine are manufactured from fish by-products (tilapia and non-endangered shark) that include shark cartilage capsules and shark liver oil products which are distributed exclusively in China. We currently produce over 25 products all of which were developed internally. The products undergo substantial independent laboratory testing in China administered by the Ministry of Health in China and have resulted in a PRC National Certification for these products. These products have various perceived medicinal and health benefits. As part of our vertical integration plan for tilapia, Jiahua Marine has introduced new bio and healthcare products made from tilapia in 2008. These products include calcium supplement tablets made from the bones of tilapia and collagen facial creams made from the skin and head of the tilapia.
These products have medicinal and health benefits that include:(1) increasing the efficacy of the immune system, correcting blood acidity, (2) reducing fatigue, (3) improving absorption of oxygen into the blood, (4) activating the anti-cancer cells (a major component of the innate immune system), (5) strengthening of bones, and (6) increasing subcutaneous moisture which has anti-wrinkle qualities. All of our healthcare products have undergone stringent independent laboratory testing in China. These tests are administered by the Ministry of Health in China. As part of this process, several laboratories are selected at random from a pool of top university laboratories to conduct tests which must confirm the results and claims of the applicant company. The applicant company can make claims about the health benefits of its products only after such stringent, independent validation of its claims by selected laboratories have been verified. Our claims in respect of our healthcare products have been rigorously tested and proven in China through the above process. Clinical trials and laboratory testing in China by selected university laboratories have resulted in a PRC National Certification of Excellence.
As part of the HQS’s vertical integration plan for tilapia, Jiahua Marine has introduced and will continue to introduce new bio and healthcare products made from tilapia in 2010. The products introduced thus far include; (1) Shalitong Capsules, which are made from the amino acids of tilapia and help in the digestive functions of the body; (2) Xueyuan Capsules vitamin supplement including collagen polypeptides and vitamins; (3) Yubaobaokang Capsules, which contain iron supplements and increase appetite; and (4) Cephalin Capsules made with tilapia brain extract that improve brain function and memory functions and help resist the aging process of the brain.
Shark cartilage—This product is highly alkalescent; it contains chondroitin sulfate and calcium and impacts the human body positively in the following ways:
| | • | | increases efficiency of immune system and activates NK cells associated with combating cancer, as sharks are cancer free; and |
| | • | | reduces blood acidity, thus improving: |
S-8
Shark liver oil—This product is rich in squalene and other nutrients, to which we add vitamins D and E, and impacts the human body positively in the following ways:
| | • | | improves absorption of oxygen in the body; |
| | • | | eliminates fatigue; and |
| | • | | improves health, through the high levels of omega 3 oils. |
In addition to the marine bio and healthcare products that we currently produce, we started manufacturing nutraceuticals generated from palm oil, and other natural or organic matters to enrich feed formulations for tilapia and shrimp farmed in the Hainan area. The enriched feed will have a longer shelf life, and we expect that the benefits of the enriched feed will pass to the end users of our tilapia products, the ultimate customers. These ingredients help improve general health, growth, feed conversion and meat quality of fish and shrimp. Our marine bio and healthcare products processing plant is located in the Wenchang City of Hainan Province and has two production lines, a powder-product line and an oil-product line. These production lines are suited for the manufacture of nutraceutical components. The plant is equipped with a specific gravity molecular separator and accessory equipment for the manufacture of nutraceutical products that serve as feed additives in the production of feed, including tilapia and shrimp feed.
These products are currently sold throughout China, are naturally derived from ocean-harvested by-products and are winners of Science and Technology Progress Awards in China.
Jiahua Marine also had established a long-term relationship with the Qingdao University of Oceanography for research and training relating to the production of our marine bio and healthcare products up to 2006. Currently, HQOF and Jiahua Marine are in preliminary discussions with other universities in the PRC for further research and development.
Health Product Store Opportunity
Through laboratory testing and the use of documented and original research, Jiahua Marine has developed a unique range of marine bio and healthcare products. The products undergo substantial independent laboratory testing in China administered by the Ministry of Health in China and have resulted in a PRC National Certification for these products. These products have various perceived medicinal and health benefits. As part of the Company’s vertical integration plan of tilapia, Jiahua Marine introduced new bio and healthcare products made from tilapia in 2009. These products include calcium supplement tablets made from the bones of tilapia and collagen facial creams made from the skin and head of the tilapia. Currently, the Company is analyzing a proposal to develop its own flagship health product store in China and ten other stores in tier one cities throughout China, as well as the opportunity to enter into a franchise license to foster a franchisee founded network of health product stores. The Company believes that this and the creation of larger, “superstores” will give consumers access to its award winning marine aquatic-themed health products.
Proposed Equity Offering and Listing
On June 7, 2010, our board of directors authorized management to plan and prepare for a public offering of equity securities of a newly formed, wholly owned subsidiary (“NewCo”) that will hold our health and bio-products segment. Our board also authorized management to plan and prepare for the listing of such equity securities on The Stock Exchange of Hong Kong Limited (“HKEX”) or another stock exchange. NewCo will act as a holding company for, and own 100% of the common stock of, Jiahua Marine, our wholly owned subsidiary engaged in the manufacturing and selling of marine bio and healthcare products in China.
S-9
The offering will be limited to a minority stake of no more than 35% of NewCo’s equity securities. Following the completion of the offering, the Company will retain at least 65% of the outstanding shares of NewCo and will continue to be its controlling stockholder. The primary purposes of the offering and the listing are to (i) unlock the value of Jiahua Marine’s business by allowing direct public investment in Jiahua Marine; (ii) increase the overall value of the Company by capturing the expected valuation premium given to the Company’s marine bio and healthcare products segment by Asian investors; (iii) create a public market for NewCo’s common stock; (iv) obtain additional capital for the Company through the net proceeds of the offering; (v) support Jiahua Marine’s business growth by providing a vehicle through which it may have future access to the public capital markets; and (vi) improve the Company’s competitive position by further strengthening our financial condition.
The offering and stock exchange listing remain subject to a number of conditions, including, among others:
| | • | | Approval from our board of directors; |
| | • | | Regulatory approval from Hong Kong securities regulators and other applicable regulators; and |
| | • | | HKEK approval for listing of NewCo’s common stock. |
Shrimp
Our principal shrimp product is the white shrimp. Our shrimp is exported to various countries in the following forms: head-on shell-on shrimps, headless shell on shrimps, peeled tail-on shrimps, peeled and deveined shrimps, peeled and undeveined shrimps. All orders can be packaged in accordance with the requirements of the buyer, either block or individually quick frozen.
Our Principal Competitive Strengths
We believe we have the following principal competitive strengths:
| | • | | Quality All Natural Tilapia Products.We produce all natural tilapia products and have developed a farming system that avoids the use of antibiotics, hormones and other potentially toxic chemicals. Our tilapia is raised in ponds of pure rain water collected for aquaculture. Two other species of fish are introduced into the ponds to maintain the ponds’ health naturally. One of the species is a bottom feeder that cleans up all the waste at the bottom of the ponds (carp), and the other species is a predator fish that eliminates all of the unhealthy fish (snakehead). We formulate feed without fishmeal and produce feed supplements in our healthcare products processing plant to enrich this feed. It is our policy to raise all natural tilapia to distinguish our company from other tilapia producers. The latitude and the pristine environment of the Hainan province of China have provided us with what we believe to be the optimal conditions for all natural aquaculture production. |
| | • | | Vertically Integrated Operations. Our newly completed feed mill is an important additional step towards our goal of complete vertical integration of our operations, which will allow us to further control and monitor the quality of our aquaculture products, as well as control our costs. The local farmers that we have cooperative arrangements with use our production methods and allow us to constantly monitor the production process for highly consistent production that results in high quality products at the time of harvest. |
| | • | | Environmental and Quality Assurances.We are a leader in environmental and quality assurances of aquaculture products. We have adopted and implemented stringent quality control measures and procedures throughout the production process, in order to comply with the various environmental and quality standards, such as the HACCP and the EU import standards. We are also certified in accordance with the ACC standards and positioning ourselves for completely organic production certification of our tilapia products in accordance with market demands. We use state-of-the-art |
S-10
| | technologies in our farming, feed formulation and processing operations. We have adopted modern and environmentally friendly and responsible technology in our production and processing of tilapia, shrimp, and marine bio and healthcare products, which we believe have been recognized as such through the certifications our plants possess. |
| | • | | Strategic Location in Hainan Province, China. Our processing facilities are geographically well-positioned in Hainan Province to leverage favorable climatic conditions, abundant water supply, pristine environment and a readily available source of labor for our processing plant. We are also located near a seaport, near the city of Wenchang, and our processing facilities are conveniently located near the farmers from whom we obtain our supply of tilapia and shrimp. |
| | • | | International and Domestic Sales and Marketing Efforts.Our Seattle office allows us to differentiate our TILOVEYA® brand and marketing initiative of our all natural tilapia products. Following the success of the TILOVEYA® brand, we introduced in 2009 a family of frozen tilapia meal products under the brand name of “Lillian’s Healthy Gourmet.” The family of products will have three lines focusing on organic, natural and regular frozen meals. The “Lillian’s Healthy Gourmet” brand will help us continue to establish our products as the all natural tilapia products of choice both domestically and internationally. Sales from both the Seattle and China offices complement our multi-national sales efforts to become a world leader in all natural tilapia products. |
| | • | | An Established Track Record and Brand Name in the Industry.We have an established track record and recognized brand name in the industry and have received numerous awards and certifications, which we believe reflect the success of the company in distinguishing itself from its competition. |
| | • | | Competitive Cost Structure.We benefit from competitive cost structures due to the lower labor costs in China as compared to other companies that produce similar products. |
Our Growth Strategies
Our objective is to become the world leader in vertically integrated production, processing and raising of all natural tilapia products. This includes the use of tilapia by-products to increase the range and variety of our marine bio and healthcare products, with a primary focus on increasing our own seafood products. To achieve this goal, we intend to implement the following strategies:
| | • | | Our newly completed large-scale feed mill to supply our existing and anticipated new cooperative fish farmers with our fish food formula. We anticipate that the new feed mill will help increase our aquatic profit margin, help guarantee our product quality and further vertically integrate our operations; |
| | • | | We intend to achieve completely organic production of our tilapia products and to pursue organic certification of our farms as market demand dictates; |
| | • | | We plan to expand direct and retail sales of our health products in China and internationally and to add other products we currently have in the development pipeline; |
| | • | | We plan to expand our development of health products by using the by-products of tilapia, which will help increase the overall aquatic products’ gross margins; |
| | • | | We plan to expand our cooperative farming arrangements to increase the availability of tilapia to meet anticipated growth in demand; and |
| | • | | We plan to construct our own tilapia farmed ponds to improve growth time and quality of our product and further vertically integrate our operations. |
The Company plans to expand production of value added seafood products in a new re-processing production facility in close proximity to our current production facility. The Company sees increasing demand in
S-11
China for value added seafood products and believes this new re-processing plant will help the Company expand its production capacity to meet this increased demand. We commenced construction of this new re-processing plant early this year and expect to complete construction in the first half of 2011.
In addition, the Company has undertaken the development of a fry breeding and genetics facility to target improvements in the growth rate of Tilapia. The opportunity to improve the genetics of its Tilapia and improve growth rates will help the Company take a leadership position as a low cost producer of high quality Tilapia. We acquired the land for our fry breeding and genetics facility at the end of 2009 and began construction early this year. We expect to complete construction by the end of the third quarter or early fourth quarter of this year.
Manufacturing and Production
Marine Bio and Healthcare Products
Our plant for processing marine bio and healthcare products consists of two production lines: a powder-product line and an oil-product line. The production lines are equipped with a complete set of imported and domestic made devices, including a vacuum frozen dryer for bio-products, a molecular distillation device, a micro-disintegrator, a packing machine and test instruments. Our raw material treatment workshops (e.g. work area) includes an extraction workshop, a freezing and drying workshop, a powder distillation workshop, and a finished product workshop for our powder line. Our pre-treatment workshops include a cooling and filtration workshop, a molecular distillation workshop, a supplemental items workshop and a capsule workshop for our oil line.
This plant is equipped with a molecular distillation device, which produces a variety of refined oil products including vitamin E used for human consumption and as a supplement to tilapia and other feeds. These vitamins are processed from natural products, such as palm oil. This plant has been certified in accordance with the China National Health Inspection program as a Chinese Good Manufacturing Practice (GMP) by the Hainan Provincial Health Bureau. We believe that our nutraceutical business is closely connected to our planned expansion including the completion of our own feed mill described below. Actualized feed additives provide health benefits to the fish, such as increasing health and avoiding the use of curative measures involving antibiotics. The consumers of these fish products in turn benefit from increased shelf life of the fish products, and from the additional sources of vitamin E resulting from the consumption of our products.
Aquaculture Products
Our plant for processing aquatic products is a Canadian designed facility located in Hainan, China. We operate six processing lines, which consist of two filleting lines, two whole round fish processing lines (principally tilapia which is gutted, scaled and gilled), and two block processing lines . This processing plant is capable of processing an average of approximately 40,000 metric tonnes (additional capacity of 10,000 metric tonnes was completed in November 2009) per year of whole round fish (principally, tilapia), 10,000 metric tonnes per year of fillet tilapia and 3,000 metric tonnes per year of all forms of shrimp. Such capacity may become inadequate to meet our projected demand from our existing customers and the national retail food service chains targeted by us. In 2009 we completed a large scale organic feed mill, to supply our existing and anticipated new cooperative fish farmers with our fish food formula. We have completed taste trials with several national retail food service chains, and we expect to phase in deliveries in the second half of 2010. The scale of production is a critical factor for such chains, and we expect that expanded production will enable us to enter into larger supply arrangements with several chains.
Construction of Feed Mill and Processing Plant
In order to maintain the high quality of our products, we have constructed our own feed mill that began operations in late 2009 for the production of organic, extruded (floating) feed formulations. This type of feed is
S-12
the most efficient feed for our farming operations. In early 2010, we began production of the feed, and also began using feed additives manufactured in our nutraceutical plant. The feed formulations will be prepared with the benefit of the latest technologies to assure a minimum of toxicity. The feed will be enriched using vitamin E, as well as naturally sourced amino acids, which provide actualized benefits to the fish and the consumers thereof. The floating feed formulations will reduce waste in the aquaculture reservoirs, thus reducing the requirement for chemicals to stabilize reservoir health. The feed mill will allow us to continue our vertically integrated production strategy, ensuring quality control throughout the entire production and processing cycle. We plan to partner with other parties, as appropriate, to produce the optimum formulation of feed. Presently, there is no high quality floating or organic feed production in Hainan. This expanded production will satisfy our own demand, as well as to manufacture feed for other farmed operations in Hainan such as shrimp and other farmed species. The plant can manufacture some 100,000 metric tones annually of feed.
We expect that the new processing plant will provide for value added production, allowing us to make fish sticks and fish patties, as well as the fillets and whole round products that we currently manufacture. We expect that it will take approximately nine to 12 months to build the new processing plant.
Distribution Channels
Our current sales activity is primarily directed to distributor within the People’s Republic of China (“PRC”), rather than within the U.S. At the present time, we sell more than 90% of our seafood products in China to Asian clients, who then distribute the products principally in the United States and Europe, and we sell all of our marine bio and healthcare products exclusively in the PRC. Through our Seattle office, we are able to work directly with wholesale and retail buyers. The programs established with retail distributors are rather different than with wholesalers. Retailers require product introduction and marketing support and pay differently than wholesalers. The latter generally take delivery of product ex-plant and pay through a letter of credit, while the former take delivery in the United States and pay on negotiated terms.
The Seattle office is charged with maintaining existing accounts and introducing our all natural tilapia products to a new target market of retail and food service industry purchasers. We introduced our family of products branded “Lillian’s Healthy Gourmet” throughout North America in 2009. We are currently selling our all natural tilapia products to the European market through distributors and retailers. We are actively seeking to expand our distributor network in Europe for our TILOVEYA® brand.
Currently, all of our marine bio and healthcare products are sold in the PRC. Through our subsidiary Jiahua Marine, we currently sell a range of healthcare products under the brand name “Jiahua” in the PRC.
Our China sales are principally marketed through our offices in Wenchang to customers that include domestic supermarkets, airlines, hotels and local distributors. Direct sales of healthcare products target tourists in various popular destinations in China, such as Sanya, Beihai and the Three Gorges project. Seminars are organized for these tourists that usually result in the purchase of our products. These products are also sold in various chain stores, over the internet, and through mail order sales throughout China.
Advertising and Marketing
Our sales and marketing team consists of ten members that are under the overall supervision of Mr. Harry Wang Hua, our Chief Operating Officer. Our sales and marketing team is responsible for establishing our sales and distribution networks both domestically and internationally, promoting our image and product awareness and maintaining our customer relationships. As part of his duties, Mr. Harry Wang Hua leads our plant management
S-13
teams for both marine bio and healthcare products and aquaculture products. The Seattle personnel are responsible for increasing awareness and focus of our branding and marketing initiative, the rollout of our all natural TILOVEYA® brand, and the rollout of our family of products under the Lillian’s Healthy Gourmet brand.
We believe that our principal operating subsidiary, HQOF, is the only vertically integrated PRC based producer present at the international seafood shows (e.g.,Brussels Seafood Show and Boston Seafood Expo). Participation in these industry events enables us to establish high level and immediate contacts with potential buyers. Buyer preferences and our response to these preferences, as well as prices and response to quality and quantity concerns, can be promptly addressed without the usual screening and middleman costs. We plan to aggressively market our products throughout North America, Europe and Asia through such shows.
In February 2006, we established our corporate headquarters, marketing and sales office in Seattle, Washington, thus creating a strong presence in the U.S. market. This office allows us to increase awareness of the importance of our all natural product focus and to reap the benefits of more direct sales, increasing our overall sales, market penetration and profitability. We expect to also be able to broaden the scope of our products to cater to additional seafood purchasing requirements.
Sustainable Farming
The concept of sustainable development has been popularized by the 1987 World Commission on Environment and Development. It defined sustainable development as meeting the needs of the present generation, without compromising the needs of future generations. The idea of sustainability has caught up with aquaculture partly because of pressure from environmental groups. In 1998, the Holmenkollen Guidelines for Sustainable Aquaculture were formulated. These guidelines recommended, among other things, that new technologies and management procedures should be utilized so that the quality and quantity of aquaculture products is improved and the risk of adverse effects on the environment and on the livelihood of other people, including future generations, is reduced. The guidelines also recommended (1) strict compliance with the internationally agreed food safety, environmental safety and ethical criteria if genetically modified organisms or hormones are utilized in the production, as well as (2) giving priority to the development of integrated fish farming and of sources for animal feed other than fish protein and fish lipid. We fully endorse the idea of sustainable farming and implement it in our operations through our cooperative supply arrangements.
Organic Farming
We believe that organic farming may be considered to be the next step after sustainable farming. Organic farming is a trend towards simple and moderate farming methods that are inherently sustainable. Organic farming advocates against the use of hormones and certain drugs, genetically modified organisms, very intensive culture systems, use of fish meal from the fish meal industry and oils from animals. As the market demand for sustainable and environmentally sustainable practices increases, the aquaculture industry is in the process of adjusting to such demand by starting to offer organic products. Further, we believe that many companies are finding that the use of waste streams from aquaculture can be used as a nutrient source for the culture of other aquatic flora and fauna, as well as land based agriculture.
We believe that operating costs for organic culture should be lower than intensive farming, with the feed remaining the key operating cost. We have determined that organic production of tilapia is technically possible in Hainan Province. Further, our preliminary evaluation of farm production economics (such as feed costs, feed conversion ratios, growth rates and yields) as they relate to expected market demand indicates that we could engage in organic farming profitably. In late 2009, we finished construction of a large-scale organic feed mill and began pursuing organic certification of our farms in order to commence organic tilapia production.
S-14
We anticipate that our tilapia products will meet organic standards as defined by The US Department of Agriculture.
In January of 2008 the Company received the Certification attesting to the organic standards for its tilapia production, processing, labeling, marketing and management system. The Company obtained this Certification following an initial annual audit conducted by Beijing Continental Hengtong Certification Co., Ltd. (CHTC), a certifier authorized by The China National Accreditation Service for Conformity (CNAS) and the Certification and Accreditation Administration of the PRC (CNCA).
Our Cooperative Supply Arrangements
We purchase and process farm-bred and ocean caught aquatic products through cooperative supply arrangements with local fishermen and cooperatives. Our farmed tilapia products come from approximately 1,647 pond acres of farms situated in the Wenchang area of Hainan. These farms are grouped through cooperatives to supply us with the highest quality tilapia in accordance with the sustainable farming standards described above.
We strive to implement the principles underlying sustainable farming and elements of organic farming in our cooperative supply arrangements with local fishermen and cooperatives, from which we purchase and process farm-bred and ocean caught aquatic products. Under our related cooperatives, or collaboration agreements, the farmers or holders of the concession retain their proprietary status, while agreeing to operate under planned and scheduled practices put forward by our company as cooperative partner of the concessions. The farmers are trained to our standards for deploying appropriate feeds and using poly-culture techniques, while we monitor compliance with these standards on an on-going basis. The farmers are also required to agree to treat waste water responsibly as a nutrient rich fertilizer for the vegetable fields maintained by neighboring farmers.
We typically have between five and ten cooperative supply agreements, which number may vary from time to time. We believe that these cooperative supply arrangements provide mutual benefits to the parties involved, as they help increase revenues of the local farmers, while ensuring a stable supply of quality raw tilapia to us.
Trademarks and Patents
We have the following patents on our products: Chinese Patent Number 460000X340-2001—Shark Cartilage; Chinese Patent Number 460000X131-2001—Shark Cartilage; Chinese Patent Number 460000X338-2001—Shark Liver Oil; and Patent Number 460000X342-2001—Shark Liver Oil. These patents are for a 20 year period and will expire in 2021. Two products are produced from refined shark cartilage and two from shark liver, both harvested from non-endangered shark species.
We consider our service marks, trademarks, trade secrets, patents and similar intellectual property to be critical to our success. We rely on trademark, patent and trade secret law, as well as confidentiality and license agreements with our employees, customers, partners and others to protect our proprietary rights. We have received patent protection and applied for trademark protection for our products in the PRC. We have also applied for trademark protection in the U.S., as described below, in connection with our branding of all natural tilapia. Effective trademark, service mark, patent and trade secret protection may not be available in every country in which we sell or may in the future sell our products, and our competitors may independently develop formulations and processes that are substantially equivalent or superior to our own.
TILOVEYA®
In May 2006, we introduced our new all natural tilapia brand TILOVEYA® at the European Seafood Exposition in Brussels, the largest seafood show in the world. This brand is designed to celebrate the health benefits of our tilapia produced in Hainan Province, China. Our freshwater tilapia products are made without
S-15
hormones, antibiotics and free of heavy metals and other toxins associated with ocean sourced products. The United States Patent and Trademark Office (USPTO) has issued registrations with respect to the following trademarks as used in connection with our branding of all natural tilapia:
| | • | | Registration Number 3313536 for the TILOVEYA® mark; and |
| | • | | Registration Number 3304756 for the TILOVEYA® Logo. |
Lillian’s Healthy Gourmet®
In August of 2008, we developed the concept of a family of products under the brand of Lillian’s Healthy Gourmet. This family of products is based on the concept of our all natural tilapia products produced in Hainan Province, China. Individuals should be able to eat a convenient, healthy seafood meal at a reasonable price, while maintaining their demanding life style. This family of products is designed to meet the need for a convenient, healthy seafood meal at a reasonable price. The USPTO has allowed the following applications for trademark registrations in connection with our branding of the Lillian’s Healthy Gourmet family of products, and registrations will issue once we submit, and the USPTO accepts, affidavits that we are using these marks in commerce:
| | • | | Application Serial Number 77614994 for the LILLIAN’S HEALTHY GOURMET mark; |
| | • | | Application Serial Number 77614989 for the LILLIAN’S ORGANIC GOURMET mark; and |
| | • | | Application Serial Number 77614981 for the LILLIAN’S KITCHEN GOURMET mark. |
Competition
In general, the aquaculture industry is intensely competitive and highly fragmented. The PRC aquaculture industry is further open to competition from local and overseas operators engaged in aquaculture and from other captured fish producers. We compete with various companies, many of which are developing or can be expected to develop products similar to ours. For example, 8th Sea—The Organic Seafood Company currently produces and processes tilapia fillets in Brazil’s Parana state. Our main aquaculture products (tilapia and shrimp) are also facing competition from some other PRC-based aquaculture producers. Some of the other aquaculture processing companies in Hainan Province have obtained certifications similar to those we possess. However, we believe that the competition from such producers is minimal because, to the best of our knowledge, there are no competitors in Hainan Province that have a similar operating scale and production capacity, or that have developed the vertically integrated business model under which we operate.
Many of our competitors are more established than we are, and have significantly greater financial, technical, marketing and other resources than we presently possess. Some of our competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements, and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We intend to create greater brand awareness for our brand name(s) so that we can successfully compete with our competitors. We cannot assure you that we will be able to compete effectively or successfully with current or future competitors, or that the competitive pressures we face will not harm our business.
With respect to potential new competitors, although there are no formal barriers to entry for engaging in similar aquaculture processing production and activities in the PRC, we believe that the high infrastructure costs associated with developing and constructing processing plants and facilities does pose a barrier to potential competitors. Fish farms are tied directly to a processing plant and a new processing plant must either enlist new
S-16
farms or build its own. Furthermore, measures addressing environmental considerations, such as water quality and waste water processing requirements, are costly to deploy on a greenfield site and are not readily available to all new companies. Accordingly, potential competitors have to mobilize extensive resources in order to maintain a presence similar to ours.
Government Regulation
Our company complies with various national, provincial and local environmental protection laws and regulations, as well as certifications and inspections relating to the quality control of our production and the environmental and social impact of our operations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Also, all of our healthcare products have met stringent independent testing by selected university laboratories in China. Our costs of compliance with applicable environmental laws are minimal. Penalties would be levied upon us if we fail to adhere to and maintain our compliance with the applicable environmental regulations in the PRC. Such failure has not occurred in the past, and we generally do not anticipate that it may occur in the future, although no assurance can be given in this regard.
HACCP Standards
Our facilities are certified in accordance with the Hazard Analysis Critical Control Points, or HACCP, standards for exporting aquatic products to the United States. The HACCP standards are developed by the FDA pursuant to the FDA’s HACCP regulation, Title 21, Code of Federal Regulations, part 123, and are used by the FDA to help ensure food safety and control sanitary standards. These standards focus on monitoring the quality of production and sanitation measures in processing plants for food products, and also take into account the environmental and social impact of the operations of the certified company. Compliance with the HACCP procedures is mandatory, and the successful implementation of these procedures depends on the design and performance of facilities and equipment, and excellent quality control and hygiene practices. HACCP conducts sample laboratory testing on our processed aquatic products to ensure no forbidden substances are present in them. Laboratory testing of our processed aquatic products was initiated by the HACCP in compliance with strict quarantine guidelines imposed by domestic export control government agencies and foreign import control government agencies.
In addition, our facilities continuously pass USDA inspection.
ACC Standards
We have completed the process of certification of our processing plant in China in relation to shrimp and tilapia processing in accordance with the Aquaculture Certification Counsel, Inc., or the ACC standards. The ACC standards are considered super HACCP standards, as they also take into account various environmental and social issues. ACC certification is required by many large retailers in the United States.
The ACC is a U.S.-based, non-governmental body established to certify social, environmental and food safety standards at aquaculture facilities throughout the world. The ACC uses a certification system that combines site inspections and effluent sampling with sanitary controls, therapeutic controls and traceability. Part of the ACC’s mission is to help educate the aquaculture public regarding the benefits of applying best aquaculture practices and the advancing scientific technology that directs them. The ACC believes that, by implementing such standards, seafood producers can better meet the demands of the growing global market for safe, wholesome seafood produced in an environmentally and socially responsible manner. The ACC offers a primarily process, rather than product, certification, with an orientation toward seafood buyers. Successful participation in the ACC program is visually represented by limited use of a Best Aquaculture Practices certification mark. The ACC currently certifies only shrimp hatcheries, farms and processing plants.
S-17
Assignment of EU Code
Our facilities have been assigned a European Union (EU) approval registration, referred to as an EU Code, required for exporting aquatic products to the EU. This requirement applies to production both inside and outside of the EU, and defines the applicable standards of the EEC for handling, processing, storing and transporting fish. Our aquatic products processing plant in China must meet or exceed these standards every year, in order to maintain the assigned EU Code. The assignment of the EU Code to us, and our ability to maintain it on an annual basis, evidence the fact that our products meet the EU importable food standards set by the relevant inspection agencies.
ISO Certification
The Company received ISO 9001 and 22000 certifications for both the seafood and feed mill plants in the fourth quarter of 2009.
| | • | | ISO 9001 is the internationally recognized standard for the quality management of businesses. It applies to the processes that create and control the products and services an organization supplies. It prescribes systematic control of activities to ensure that the needs and expectations of customers are met. It is designed and intended to apply to virtually any product or service, made by any process anywhere in the world. |
| | • | | ISO 22000 specifies requirements for a food safety management system where an organization in the food chain needs to demonstrate its ability to control food safety hazards in order to ensure that food is safe at the time of human consumption. It is applicable to all organizations, regardless of size, which are involved in any aspect of the food chain and want to implement systems that consistently provide safe products. |
Product Liability Insurance
We have purchased general commercial liability insurance, which provides adequate aggregate product liability insurance based on industry standards. However, there is a possibility that our customers, or the ultimate buyers of our products, may have adverse reactions to the tilapia and other aquatic products or marine bio and healthcare products that we process and sell. Any such adverse reaction may result in actual or potential product liability claims against us, which may not be covered by our insurance or, if covered, may be significantly higher than the insurance amount. Such actual or potential product liability claims may have an adverse effect on our reputation and profitability.
Government Regulation in China
Aquaculture producers in the PRC have to comply with the environmental protection laws and regulations promulgated by the national and local governments of the PRC. Such rules and regulations include, among others, Environmental Protection Law of the PRC, Ocean Environmental Protection Law of the PRC, Regulations on Administration over Dumping of Wastes in the Ocean of the PRC, Ocean Aquatic Industry Administration Regulation, Fishing License Administration Regulation, Regulations on Administration of Hygiene Registration of Exported Food Manufacturers, and Regulations on Administration of Quality Control of Food Processors.
In addition, HACCP and sanitary programs in China in accordance with the FDA’s HACCP standards are verified by the China Inspection and Quarantine Office, or CIQ, which is a branch of the State Administration for Entry-Exit Inspection and Quarantine of the PRC and, in our case, also by the Hainan Entry-Exit Inspection and
S-18
Quarantine Bureau of the PRC. In addition, the CIQ evaluates the compliance by our processing plant with the EU standards described above under “—Assignment of EU Code.” As a result of such review, our aquatic products processing plant in China has received a CIQ certificate. The CIQ certificate must be renewed on an annual basis.
Our Work with the Hainan Province
We have enjoyed close collaboration with the local government as we conduct our operations in the Hainan Province. We have continued to expand our operations in that area and to foster close ties with the local government through the construction of our large-scale organic feed mill, which was completed in late 2009. See “Prospectus Supplement Summary—Manufacturing and Production—Construction of Feed Mill and Processing Plant.” The success of our operations in the PRC depends in part on the continued investment by Hainan Province in the development of the local aquaculture industry. While there can be no assurances that such investment will continue, we believe that it should continue for the following reasons. The central government of China has limited Hainan Province to two areas of economic activity, agri-food and tourism. The resulting focus of the Hainan Province on the agri-food and tourism sectors creates a strong potential synergy with private sector companies intent on further development of these sectors. Part of the attraction for investors is the low tax rate in Hainan. In addition, foreign companies setting up new ventures in Hainan do not pay any tax for the two first years of profitable operations, then pay 7.5% for the following three years and 15% thereafter. For this reason, we plan to continue to structure and conduct our operations in China through the use of separate subsidiaries, held by foreign holding companies which are separate and distinct from holding companies already incorporated. In turn, these holding companies are held by HQS. Under these arrangements, we are not considered involved in joint ventures, but rather in wholly owned foreign enterprises, under the local law. Government support for such ventures meeting local needs is positive, and we believe our operations have already demonstrated our ability to channel this support in the manner favorable to our business and Hainan. We believe that not having a joint venture with the local government is the best way to minimize the potential for government interference and to maximize government support, and we plan to continue to conduct our business in China accordingly.
Furthermore, on March 17, 2007, a new PRC Enterprise Income Tax Law (EIT) was promulgated and introduced a new uniform tax regime in the PRC. The EIT became effective on January 1, 2008. That new law provides, amongst other issues, that income derived from preliminary processing of fishery products and processing of agricultural products will be exempt from the EIT tax rate. Starting in 2008, our existing fish processing unit and our feed mill plant both benefit from a 0% tax rate. With regards to our nutraceutical unit, the income tax rate, under the new law, will increase by approximately 2% yearly until it reaches a maximum of 25% in 2012.
Employees
Through our subsidiaries, we currently employ approximately 620 employees, all of whom are full-time employees. They are located predominantly in the PRC. Of our key employees, Harry Wang Hua, He Jian Bo and Wang Fu Hai are located in China and are fully dedicated to our China operations. In addition, Lillian Wang Li, Norbert Sporns, and William Sujian contribute to both our China operations and our U.S. operations, depending on the needs of our business over time.
In addition, during the high season, we hire up to 100 part-time employees. We typically pay our local employees much higher wages than the required minimum wages, in order to attract and retain key employees. We have employment agreements with many of our full-time employees. None of our employees are covered by a collective bargaining agreement, and we believe our employee relations are good.
S-19
Properties
We own three processing plants located in Wenchang, Hainan Province, PRC, and the related manufacturing equipment, office equipment and motor vehicles. We use one plant to process the seafood products we produce, a second plant to process our marine bio and healthcare products and since the last quarter of 2009, a third plant to process our fish feed products.
In addition, we currently lease corporate premises for our United States headquarters located in Seattle, Washington, consisting of approximately 4,170 square feet, from Doncaster Investments NV, Inc. The term of the related lease is sixty months, which commenced on December 1, 2005 and will end on December 1, 2010. Our base rental payment under the lease is $6,950 per month for 2009 and 2010. In 2010, we will move our headquarters to California. We plan to use our new headquarters as, among other things, the center of our United States direct sales effort. In connection with this move, we leased approximately 5,400 square feet of office space with a base monthly rental of approximately $11,000.
Our properties are in good condition and are sufficient to meet our needs at this time. We do not plan to obtain additional space in the foreseeable future for the above cited plants, but we intend to build or acquire additional processing facilities in the near future.
Principal Shareholders
The following table sets forth certain information regarding beneficial ownership of common stock as of June 30, 2010 by:
| | • | | each person known to us to own beneficially more than 5 percent, in the aggregate, of the outstanding shares of our common stock; |
| | • | | each of our chief executive officer and our other two most highly compensated executive officers; and |
| | • | | all executive officers and directors as a group. |
S-20
The number of shares beneficially owned and the percent of shares outstanding are based on 14,681,002 shares outstanding as of August 9, 2010. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Except as otherwise noted below, the address of each of the shareholders in the table is c/o HQ Sustainable Maritime Industries, Inc., 1511 Third Avenue, Suite 788, Seattle, Washington 98101.
| | | | | | | | | | | | | | | |
Beneficial Owner | | Number of
Shares of
Common
Stock | | | Percent of Common
Stock
Beneficially Owned | | | Number of Shares
of Series A
Preferred Stock | | | Percent of Series A
Preferred Stock
Beneficially Owned | | | Percentage
of Total
Voting
Power | |
Norbert Sporns | | 796,761 | (1) | | 5.4 | % | | 24,000 | (2) | | 24.0 | % | | 21.6 | % |
Lillian Wang Li | | 828,918 | (1) | | 5.6 | % | | 25,000 | (2) | | 25.0 | % | | 22.5 | % |
Harry Wang Hua | | 1,639,993 | (1) | | 11.2 | % | | 51,000 | (2) | | 51.0 | % | | 45.9 | % |
Andrew Intrater (3) | | 5,248 | | | Less than 1 | % | | | | | | | | Less than 1 | % |
Fred Bild | | 10,568 | | | Less than 1 | % | | | | | | | | Less than 1 | % |
Daniel Too | | 12,443 | | | Less than 1 | % | | | | | | | | Less than 1 | % |
Kevin M. Fitzsimmons | | 0 | | | Less than 1 | % | | | | | | | | Less than 1 | % |
Jean-Pierre Dallaire | | 10,000 | | | Less than 1 | % | | | | | | | | Less than 1 | % |
All such directors and executive officers as a group (8 persons) | | 3,303,931 | | | 22.5 | % | | 100,000 | | | 100.0 | % | | 90.1 | % |
| | | | | |
Five Percent Shareholders (Other than Directors and named executive officers) | | | | | | | | | | | | | | | |
River Road Asset Management, LLC (4) | | 907,350 | | | 6.2 | % | | | | | | | | Less than 1 | % |
The Tail Wind Fund Ltd. (5) | | 778,698 | | | 5.3 | % | | | | | | | | Less than 1 | % |
| (1) | Beneficially owns the shares indicated, which are owned of record by Red Coral Group Limited and Sino-Sult Canada (S.S.C.) Limited. Each of Mr. Sporns, Ms. Wang and Mr. Wang own respectively 24%, 25% and 51% of the issued capital of Red Coral Group Limited and Sino-Sult Canada (S.S.C.) Limited and share voting and investment power over the shares held by Red Coral Group Limited and Sino-Sult Canada (S.S.C.) Limited. |
| (2) | Beneficially owns the shares indicated, which are owned of record by Sino-Sult Canada (S.S.C.) Limited. Each of Mr. Sporns, Ms. Wang and Mr. Wang own respectively 24%, 25% and 51% of the issued capital of Sino-Sult Canada (S.S.C.) Limited and share voting and investment power over the shares held by Sino-Sult Canada (S.S.C.) Limited. Each share of Series A Preferred Stock has the right to the voting power equal to that of 1,000 shares of the Company’s Common Stock. The voting power of the Series A Preferred Shares remains the same and is not adjusted for any reverse split, namely each share of Series A Preferred Stock continues to have the voting power of 1,000 shares of Common Stock. Each share of Series A Preferred Stock is convertible at a rate of 2 shares of Common Stock for 1 share of Preferred Stock at the option of the beneficial holder, which has been adjusted to reflect a 1 for 20 reverse stock split effectuated on January 31, 2007 to 0.1 shares of Common Stock for 1 share of Series A Preferred Stock. |
| (3) | The address of this stockholder is c/o Renova U.S. Management LLC, 153 E. 53rd Street, 58th Floor, New York, New York 10022. |
| (4) | Based on a Schedule 13G/A filed by River Road Asset Management, LLC with the SEC on February 16, 2010. The address of the indicated holder is 462 S. 4th Street, Suite 1600, Louisville, KY 40202. |
| (5) | Based on a Schedule 13G/A filed by The Tail Wind Fund Ltd. with the SEC on February 9, 2010. Tail Wind Advisory & Management Ltd., a UK corporation authorized and regulated by the Financial Services Authority of Great Britain (“TWAM”), is the investment manager for The Tail Wind Fund Ltd., and David Crook is the CEO and controlling stockholder of TWAM. Each of TWAM and David Crook expressly disclaims any equitable or beneficial ownership of the shares held by The Tail Wind Fund Ltd. The address of the indicated holder is Windemere House, 404 East Bay Street, P. O. Box SS-5539, Nassau, Bahamas. |
S-21
THE OFFERING
Common stock offered by us | [ ] shares of common stock. |
Warrants offered by us | Warrants to purchase up to [ ] shares of common stock. Each Warrant has an exercise price of $[ ] per share, and is exercisable during the period beginning six months after the date of issue and ending on the fifth anniversary of the date of issue. This prospectus supplement also relates to the offering of the shares of common stock issuable upon exercise of the Warrants. |
Approximate number of shares of common stock to be outstanding after this offering | [ ] shares of common stock.(1) |
Offering price | $[ ] per unit, which consists of one share of our common stock and a warrant to purchase 0.5 shares of common stock. |
Exchange listing | Our shares of common stock are listed on the NYSE Amex under the symbol “HQS.” |
Use of proceeds | We will receive net proceeds from this offering of approximately $[ ], after deducting underwriter’s discounts and commissions and estimated offering expenses paid and payable by us. We intend to use the net proceeds from this offering for general corporate purposes, including investments in our Marine Bio and Healthcare Products segment. Pending the application of the net proceeds, we intend to invest the net proceeds in investment-grade, interest-bearing securities. |
Transfer Agent and Registrar | American Stock Transfer & Trust Company, LLC serves as transfer agent and registrar for our common stock. |
Risk Factors | Investing in our securities involves risk. See “Risk Factors” beginning on page S-[ ] of this prospectus supplement and in “Item 1A Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2010, and other information included or incorporated by reference in this prospectus supplement and the accompanying prospectus for a discussion of factors you should carefully consider before deciding to invest in our securities. |
| (1) | The number of shares of common stock outstanding after this offering is based on [ ] shares outstanding as of August [ ], 2010, and does not give effect to (i) [ ] shares of common stock underlying stock options we have granted and outstanding and [ ] shares of common stock available for future equity awards under our equity incentive plans, each as of August [ ], 2010, (ii) 10,000 shares of common stock underlying outstanding shares of our Series A preferred stock as of August [ ], 2010, (iii) [ ] shares of common stock underlying outstanding warrants as of August [ ], 2010, and (iv) [ ] shares of common stock underlying the warrants offered hereby. |
S-22
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information contained in this prospectus supplement and accompanying prospectus and in the documents incorporated by reference into this prospectus supplement and the accompanying prospectus, before investing in our securities. If any of the events anticipated by the risks described below occur, our results of operations and financial condition could be adversely affected which could result in a decline in the value of our securities, causing you to lose all or part of your investment.
Risks Relating To This Offering
This offering will dilute current stockholders.
We are issuing up to [ ] shares of our common stock which represent approximately [ ]% of the shares of our common stock outstanding immediately prior to the sale of such shares (not including shares that may be issued upon exercise of the warrants). Thus, this offering will dilute current stockholders, will have a dilutive effect on earnings per share and may adversely affect the market price of our common stock. Stockholders would experience additional dilution if we issue shares of common stock pursuant to the warrants that we are issuing in this offering.
There is no public market for the warrants being offered by this prospectus supplement.
There is no established trading market for the warrants being offered by this prospectus supplement and we do not expect a market to develop. In addition, we do not intend to apply to list the warrants on any national securities exchange or automated quotation system. Without an active market, the liquidity of the warrants will be limited.
We will have broad discretion in applying the net proceeds of this offering and may not use these proceeds in ways that will enhance the market value of our common stock.
We have broad discretion in applying the net proceeds we will receive in this offering. Such proceeds may be used for general corporate purposes, including investments in our Marine Bio and Healthcare Products segment. For more information, see “Use of Proceeds.” As part of your investment decision, you will not be able to assess or direct how we apply these net proceeds.
Risks Relating To Our Business
We rely on cooperative suppliers and any adverse changes in these relationships may adversely affect us.
We have developed a network of aquaculture farmers in Hainan Province for the supply of tilapia and shrimp by entering into cooperative supply agreements. Pursuant to the cooperative supply agreements, we are assured the necessary supply of aquatic products that meet our quality standards. Smooth continual operation of this cooperative network is essential in controlling our costs, meeting quality standards and the timely fulfillment of our customer orders. Any adverse change to our cooperative network, including any early termination or non-renewal of any supply agreement or any failure of suppliers to fulfill their obligations under the supply agreements, could have a material adverse effect on our business model, operations and competitiveness.
We may require additional capital in the future, which may not be available on favorable terms or at all.
Our future capital requirements will depend on many factors, including industry and market conditions, our ability to successfully implement our branding and marketing initiatives and expansion of our production capabilities. We anticipate that we may need to raise additional funds in order to grow our business and implement our business strategy. We anticipate that any such additional funds would be raised through equity or
S-23
debt financings. In addition, we may enter into a revolving credit facility or a term loan facility with one or more syndicates of lenders. Any equity or debt financing, if available at all, may be on terms that are not favorable to us. Even if we are able to raise capital through equity or debt financings, as to which there can be no assurance, the interest of existing stockholders in our company will be diluted, and the securities we issue may have rights, preferences and privileges that are senior to those of our common stock or may otherwise materially and adversely affect the holdings or rights of our existing stockholders. If we cannot obtain adequate capital, we may not be able to fully implement our business strategy, and our business, results of operations and financial condition would be adversely affected. In addition, we have and will continue to raise additional capital through private placements or registered offerings, in which broker-dealers will be engaged. The activities of such broker-dealers are highly regulated and we cannot assure that the activities of such broker-dealers will not violate relevant regulations and generate liabilities despite our expectation otherwise.
We depend on the availability of additional human resources for future growth.
We have recently experienced a period of significant growth in our sales volume. We believe that continued expansion is essential for us to remain competitive and to capitalize on the growth potential of our business. Such expansion may place a significant strain on our management and operations and financial resources. As our operations continue to grow, we will have to continually improve our management, operational and financial systems, procedures and controls, and other resources infrastructure, and expand our workforce. There can be no assurance that our existing or future management, operating and financial systems, procedures and controls will be adequate to support our operations, or that we will be able to recruit, retain and motivate our personnel. Further, there can be no assurance that we will be able to establish, develop or maintain the business relationships beneficial to our operations, or to do so or to implement any of the above activities in a timely manner. Failure to manage our growth effectively could have a material adverse effect on our business and the results of our operations and financial condition.
We depend on our key management personnel, and the loss of any of their services could materially adversely affect us.
Our operations are dependent upon the experience and expertise of a small number of key management personnel, which includes Lillian Wang Li, our Chairman of the Board, Norbert Sporns, our Chief Executive Officer and President, and Harry Wang Hua, our Chief Operating Officer. Lillian Wang Li and Harry Wang Hua are brother and sister, and Ms. Wang Li is married to Norbert Sporns. Although we have obtained life insurance on Ms. Wang Li and have begun the process of obtaining life insurance on Mr. Sporns, the loss of the services of any of these key personnel for any reason would likely have a material adverse effect on our business, and the results of our operations and financial condition, or could delay or prevent us from fully implementing our business strategy.
A few of our customers account for a significant portion of our business.
We have derived, and over the near term we expect to continue to derive, a significant portion of our sales from a limited number of customers. For example, our five largest customers accounted for a total of 40.6% of our consolidated sales for the year ended December 31, 2009, and they are all related to the aquaculture product segment. At June 30, 2010, approximately 42.3% of our trade receivables was from transactions with these five largest customers. The loss of any of these customers or non-payment of outstanding amounts due to the company from these customers could materially and adversely affect our results of operations, financial position and liquidity.
We may be unable to continue to take advantage of the seasonal pricing fluctuation in sales of our products, and we may be adversely affected by the seasonal fluctuation in the prices we earn for our products.
We have experienced seasonal fluctuation in the prices we earn for our products, generally in the range of 15 to 20%. Pricing fluctuation occurs during the winter season when fish farms in the northern part of the PRC
S-24
suspend production due to cold weather conditions. These weather related disruptions in supply permit us to increase the sales prices of our tilapia products. However, there can be no assurance that such premium pricing, benefiting our profitability, can be maintained in the future. Other factors, such as an increase in the cost of feed, might also adversely impact on the cost of fish and lessen our margins and profitability.
Any adverse changes in the supply of our tilapia and other raw materials, including contamination or disease or increased costs of raw materials, may adversely affect our operations or reduce our margins or profits.
We are dependent on the availability of raw materials from Hainan Province and the oceans in that region. The supply of these raw materials can be adversely affected by any material change in the climatic or environmental conditions in and around Hainan Province. In addition, if there is contamination resulting from disease, pollution or other foreign substances, our supply of raw materials could be jeopardized or disrupted. The shortage or lack of raw materials and any consequential change in their cost would, in turn, have a material adverse effect on the cost on our operations and margins and our ability to provide products to our customers.
Any actual contamination of our products resulting from processing, packaging or transit of our products, or negative press from contamination experienced by other companies in our industry may adversely affect our operations or reduce our margins or profits.
We actively seek to control the quality of our products and avoid risk of contamination in our processing, packaging and distribution of such products; however no quality control program is guaranteed to be completely effective. We are dependent on others for the reliable safe transportation of our products to the market place and the quality of our final product as experienced by the consumer may be impacted by disruptions in the transit process beyond our control. In addition, if our competitors experience problems with contamination of their products, even if we do not concurrently suffer similar adverse events, publicity of such problems could negatively impact our reputation. Actual contamination or reports of industry problems with contamination or poor quality may have a material adverse effect on our operations, including an increase in product liability claims, higher quality control and transport costs, reduced margins and decreased consumer interest in our products.
We may be adversely affected by the fluctuation in raw material prices and selling prices of our products.
Neither our products nor the raw materials we use have experienced any significant price fluctuations since we began operation, but there is no assurance that they will not be subject to future price fluctuations or pricing control. The products and raw materials we use may experience price volatility caused by events such as market fluctuations or changes in governmental programs. The market price of these raw materials may also experience significant upward adjustment, if, for instance, there is a material under-supply or over-demand in the market. These price changes may ultimately result in increases in the selling prices of our products, and may, in turn, adversely affect our sales volume, revenue and operating profit.
We could be adversely affected by the occurrence of natural disasters in Hainan Province.
From time to time, Hainan Province experiences typhoons, particularly from June through September of any given year. Natural disasters could impede operations, damage infrastructure necessary to our operations or adversely affect the logistical services to and from Hainan Province. Even though we currently have insurance against damages caused by natural disasters, including typhoons, accidents or similar events, the occurrence of natural disasters in Hainan Province could adversely affect our business, the results of our operations, prospects and financial condition, through business disruptions and / or any losses in excess of our policy limits.
S-25
Intense competition from existing and new entities may adversely affect our revenues and profitability.
In general, the aquaculture industry is intensely competitive and highly fragmented. We compete with various companies, many of which are developing or can be expected to develop products similar to ours. For example, 8th Sea–The Organic Seafood Company currently produces and processes tilapia fillets in Brazil’s Parana state. Many of our competitors are more established than we are and have significantly greater financial, technical, marketing and other resources than we presently possess. Some of our competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We intend to create greater awareness for our brand name in an attempt to successfully compete with our competitors. We cannot assure you that we will be able to compete effectively or successfully with current or future competitors or that the competitive pressures we face will not harm our business.
Our operating subsidiaries must comply with environmental protection laws that could adversely affect our profitability.
We are required to comply with the environmental protection laws and regulations promulgated by the national and local governments of the PRC. Some of these regulations govern the level of fees payable to government entities providing environmental protection services and the prescribed standards relating to the discharge of effluent, or liquid waste. Yearly inspections of waste treatment systems require the payment of a license fee which could become a penalty fee if standards are not maintained. Currently, our plant treats all of its effluent completely to level one, which is consistent with releasing potable water back to the environment, and there is currently no charge being levied. Although our production technologies allow us to efficiently control the level of pollution resulting from our production process, and notwithstanding the fact that we have received evidence of compliance with environmental protection requirements from government authorities, due to the nature of our business, effluent wastes are unavoidably generated in the aquaculture production processes. If we fail to comply with any of these environmental laws and regulations in the PRC, depending on the types and seriousness of the violation, we may be subject to, among other things, warning from relevant authorities, imposition of fines, specific performance and/or criminal liability, forfeiture of profits made, being ordered to close down our business operations and suspension of relevant permits.
Climate change and related regulatory responses may impact our business.
Climate change as a result of emissions of greenhouse gases is a significant topic of discussion and may generate U.S. federal and other regulatory responses in the near future, including the imposition of a so-called “cap and trade” system. It is impracticable to predict with any certainty the impact of climate change on our business or the regulatory responses to it, although we recognize that they could be significant. The most direct impact is likely to be an increase in energy costs, which would increase slightly our operating costs, primarily through increased utility and transportations costs. However, it is too soon for us to predict with any certainty the ultimate impact, either directionally or quantitatively, of climate change and related regulatory responses.
To the extent that climate change increases the risk of natural disasters or other disruptive events in the areas in which we operate, we could be harmed. While we maintain business recovery plans that are intended to allow us to recover from natural disasters or other events that can be disruptive to our business, our plans may not fully protect us from all such disasters or events.
Our operations, revenue and profitability could be adversely affected by changes in laws and regulations in the countries where we do business.
The governments of countries into which we sell our products, including the United States, Canada and the European Union, from time to time, consider regulatory proposals relating to raw materials, food safety and
S-26
markets, and environmental regulations, which, if adopted, could lead to disruptions in distribution of our products and increase our operational costs, which, in turn, could affect our profitability. To the extent that we increase our product prices as a result of such changes, our sales volume and revenues may be adversely affected.
Furthermore, these governments may change import regulations or impose additional taxes or duties on certain Chinese imports from time to time. For example, in 2004, the United States government imposed heavy tariffs of more than 100% on certain Chinese shrimp exporters. Similar regulations and fees or new regulatory developments may have a material adverse impact on our operations, revenue and profitability. If one or more of the countries into which we sell our products bars the import or sale of fish or related products from China, our available market would shrink significantly, adversely impacting our results of operations and growth potential.
Our business could be adversely affected by the recent negative public reports on seafood imported from China.
In June 2007, the U.S. Food and Drug Administration issued an alert report on the sale of five types of farm-raised seafood from China in the United States because of unapproved chemical residues. The five types of farm-raised seafood are shrimp, catfish, eel, basa and dace. As a result, in order for the seafood to be sold in the United States, importers must provide independent testing that shows the seafood does not contain the unapproved residues. Although tilapia is not included in the list and we believe our main seafood product, which is tilapia, does not contain any of the unapproved residues, it is possible that our business may be adversely impacted as a result of the negative public reports on seafood imported from China.
There could be changes in the policies of the PRC government that may adversely affect our business.
The aquaculture industry in the PRC is subject to policies implemented by the PRC government. The PRC government may, for instance, impose control over aspects of our business such as distribution of raw materials, product pricing and sales. If the raw materials used by us or our products become subject to any form of government control, then depending on the nature and extent of the control and our ability to make corresponding adjustments, there could be a material adverse effect on our business and operating results.
Separately, our business and operating results also could be adversely affected by changes in policies of the Chinese government such as: changes in laws, regulations or the interpretation thereof; confiscatory taxation; restrictions on currency conversion, imports on sources of supplies; or the expropriation or nationalization of private enterprises. Although the Chinese government has been pursuing economic reform policies for approximately two decades to liberalize the economy and introduce free market aspects, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting China’s political, economic and social life.
Compliance with changing regulation of corporate governance and public disclosure will result in additional expenses and pose challenges for our management team.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated thereunder, the Sarbanes-Oxley Act and SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the U.S. public markets. Our management team will need to devote significant time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities.
Certain political and economic considerations relating to PRC could adversely affect our company.
The PRC is passing from a planned economy to a market economy. The Chinese government has confirmed that economic development will follow a model of market economy under socialism. While the PRC government
S-27
has pursued economic reforms since its adoption of the open-door policy in 1978, a large portion of the PRC economy is still operating under five-year plans and annual state plans adopted by the government that set down national economic development goals. Through these plans and other economic measures, such as control on foreign exchange, taxation and restrictions on foreign participation in the domestic market of various industries, the PRC government exerts considerable direct and indirect influence on the economy. Many of the economic reforms are unprecedented or experimental for the PRC government, and are expected to be refined and improved. Other political, economic and social factors can also lead to further readjustment of such reforms. This refining and readjustment process may not necessarily have a positive effect on our operations or future business development. Our operating results may be adversely affected by changes in the PRC’s economic and social conditions as well as by changes in the policies of the PRC government, which we may not be able to foresee, such as changes in laws and regulations (or the official interpretation thereof), measures which may be introduced to control inflation, changes in the rate or method of taxation, and imposition of additional restrictions on currency conversion.
The recent nature and uncertain application of many PRC laws applicable to us create an uncertain environment for business operations and they could have a negative effect on us.
The PRC legal system is a civil law system. Unlike the common law system, such as the legal system used in the United States, the civil law system is based on written statutes in which decided legal cases have little value as precedents. In 1979, the PRC began to promulgate a comprehensive system of laws and has since introduced many laws and regulations to provide general guidance on economic and business practices in the PRC and to regulate foreign investment. Progress has been made in the promulgation of laws and regulations dealing with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. The promulgation of new laws, changes of existing laws and the abrogation of local regulations by national laws could have a negative impact on our business and business prospects. In addition, as these laws, regulations and legal requirements are relatively recent, their interpretation and enforcement involve significant uncertainty.
If relations between the United States and China worsen, we may be unable to serve a portion of our customer base, the value of our securities could decline and we may have difficulty accessing the U.S. capital markets.
At various times during recent years, the United States and China have had disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and China could adversely affect our ability to continue to sell to U.S. customers, the value of our securities and our ability to access U.S. capital markets.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of the PRC. Currently, the Renminbi is not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency denominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of loans and corporate debt obligations denominated in foreign currencies.
The PRC government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain of our expenses as they come due.
S-28
The fluctuation of the Renminbi may materially and adversely affect your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. Any significant revaluation of the Renminbi may materially and adversely affect our cash flows, revenues and financial condition. For example, to the extent that we need to convert U.S. dollars we receive from this offering of our securities into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar could have a material adverse effect on our business, financial condition and results of operations.
Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making dividend payments on our common or preferred stock or for other business purposes and the U.S. dollar appreciates against the Renminbi, the U.S. dollar equivalent of the Renminbi we convert would be reduced. Any significant devaluation of Renminbi may reduce our operation costs in U.S. dollars but may also reduce our earnings in U.S. dollars. In addition, the depreciation of significant U.S. dollar denominated assets could result in a charge to our income statement and a reduction in the value of these assets.
Commencing from July 21, 2005, China has adopted a managed floating exchange rate regime based on market demand and supply with reference to a basket of currencies. The exchange rate of the U.S. dollar against the RMB was adjusted from approximately RMB 8.28 per U.S. dollar to approximately RMB 8.11 per U.S. dollar on July 21, 2005. Since then, The People’s Bank of China administers and regulates the exchange rate of U.S. dollar against RMB taking into account demand and supply of RMB, as well as domestic and foreign economic and financial conditions.
In addition, there can be no assurance that we will be able to obtain sufficient foreign exchange to pay dividends or satisfy other foreign exchange requirements in the future. We currently do not intend to pay dividends.
It may be difficult to effect service of process and enforcement of legal judgments upon our Company and our officers and directors because some of them reside outside the United States.
As our operations are presently based in China and some of our key directors and officers reside outside the United States, service of process on our key directors and officers may be difficult to effect within the United States. Also, substantially all of our assets are located outside the United States and any judgment obtained in the United States against us may not be enforceable outside the United States. We have appointed Norbert Sporns, our Chief Executive Officer and President, as our agent to receive service of process in any action against our company in the United States.
Risks Relating to our Common Stock
There are a large number of shares underlying our Series A preferred stock and warrants that may be available for future sale and the sale of these shares may depress the market price of our common stock.
As of August 9, 2010, we had 14,681,002 shares of common stock issued and outstanding. We currently have outstanding Class B warrants to purchase up to 114,583 shares of our common stock, and stock purchase warrants to purchase up to 30,000 shares of our common stock issued in connection with the November 2006 financing. In addition, we currently have 100,000 shares of Series A preferred stock issued and outstanding, and the holders of our Series A preferred stock have an option right to convert each share of Series A Preferred Stock into 0.1 shares of our common stock. These shares, including all of the shares issuable upon conversion of our Series A preferred stock and upon exercise of our warrants, may be sold into the market place currently. The sale of these shares may adversely affect the market price of our common stock.
S-29
Our principal stockholders, current executive officers and directors own a significant percentage of our company and will be able to exercise significant influence over our company.
Our executive officers and directors and principal stockholders together beneficially own approximately 90.08% of the total voting power of our outstanding voting capital stock, primarily as a result of their ownership of Series A preferred stock of our company which, while convertible into shares of our common stock on a 0.1-for-1 basis, carries 1,000 votes per Series A preferred share rather than the 1 vote per share of our common stock. The Series A preferred stock and the common stock vote together as one class on all matters to be voted upon by the stockholders. In particular, our three largest stockholders, Mr. Sporns, Ms. Wang Li and Mr. Wang Hua, are family members who share approximately 90.05% of the total voting power of our company. Ms. Wang Li is the wife of Mr. Sporns and Mr. Wang Hua is the brother of Ms. Wang Li. These stockholders will be able to determine the composition of our board of directors, will retain the voting power to approve all matters requiring stockholder approval and will continue to have significant influence over our affairs. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the market price of the common stock or prevent our stockholders from realizing a premium over the market prices for their shares of common stock.
Investors may experience dilution from any exercise of warrants.
We currently have outstanding Class B warrants to purchase up to 114,583 shares of our common stock and stock purchase warrants to purchase up to 30,000 shares of our common stock. The Class B warrants expire on January 25, 2011 and the stock purchase warrants expire on December 28, 2011.
S-30
USE OF PROCEEDS
We estimate that the net proceeds from this offering will be approximately $[ ] after deducting underwriter’s discounts and commissions and estimated offering expenses paid or payable by us. We intend to use the net proceeds from the sale of our securities under this prospectus supplement for general corporate purposes, including investments in our Marine Bio and Healthcare Products segment. Pending the application of the net proceeds, we intend to invest the net proceeds in investment-grade, interest-bearing securities.
S-31
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2010:
| | • | | on an actual basis; and |
| | • | | on an as adjusted basis to effect our sale of [ ] shares of common stock in this offering, based on the public offering price of $[ ] per share, and after deducting underwriter’s discounts and commissions and estimated offering expenses paid or payable by us, assuming no exercise of the warrants. |
This table should be read in conjunction with our financial statements and the related notes, which are incorporated by reference in this prospectus supplement and the accompanying prospectus.
| | | | | | |
| | | June 30, 2010 |
| | | Actual | | As Adjusted |
| | | (in thousands) |
Cash and cash equivalents | | $ | 43,839,971 | | $ | [ ] |
| | | | | | |
| | |
Indebtedness: | | | | | | |
Bank indebtedness | | | — | | $ | [ ] |
Other notes payable | | | 6,510,044 | | | [ ] |
| | | | | | |
Total indebtedness | | | 6,510,044 | | | [ ] |
| | | | | | |
| | |
Shareholders’ equity: | | | | | | |
Preferred stock, $0.001 par value, 10,000,000 shares authorized, 100,000 issued and outstanding | | | 100 | | | [ ] |
Common stock, $0.001 par value, 200,000,000 shares authorized, 14,681,002 shares of common stock shares issued and outstanding actual; and [ ] shares issued and outstanding, as adjusted | | | 14,681 | | | [ ] |
Additional capital | | | 79,329,349 | | | [ ] |
Accumulated other comprehensive income | | | 9,791,334 | | | [ ] |
Retained earnings | | | 18,418,397 | | | [ ] |
Appropriation of Retained Earnings | | | 7,640,155 | | | [ ] |
| | | | | | |
Total shareholders’ equity | | | 115,194,016 | | | [ ] |
| | | | | | |
| | |
Total capitalization | | $ | 121,704,060 | | $ | [ ] |
S-32
DESCRIPTION OF SECURITIES
General
In this offering, we are offering a maximum of [ ] units, consisting of [ ] shares of common stock and warrants to purchase up to [ ] shares of common stock. Each unit consists of one share of common stock and warrants to purchase 0.5 shares of common stock at an initial exercise price of $[ ] per share. This prospectus supplement also relates to the offering of shares of our common stock issuable upon the exercise, if any, of the warrants issued in this offering. The sales price for each unit is $[ ]. The shares of common stock and warrants comprising the units will be issued separately.
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Common Stock” starting on page 14 of the accompanying prospectus.
Warrants
The material terms and provisions of the warrants being offered pursuant to this prospectus supplement and the accompanying prospectus are summarized below. This summary is subject to, and qualified in its entirety by, the form of warrant, which will be provided to the investors in this offering and will be filed as an exhibit to a Current Report on Form 8-K.
The warrants will provide for an exercise price of $[ ] per share and will be exercisable at the option of the holder during the period beginning six months after the date of issue and ending on the fifth anniversary of the date of issue. The exercise price of the warrants will be subject to adjustment in the case of stock splits, stock dividends, combinations of shares and similar recapitalization transactions. The holder will not have the right to exercise any portion of the warrant if the holder, together with its affiliates, would, subject to limited exceptions, beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after the exercise. The holder may elect to change this beneficial ownership limitation from 4.99% to 9.99% of the number of shares of our common stock outstanding immediately after the exercise upon 61 days’ prior written notice to us.
The warrant holders must surrender payment in cash of the exercise price of the shares being acquired upon exercise of the warrants. If, however, we are unable to offer and sell the shares underlying these warrants pursuant to this prospectus supplement due to the ineffectiveness of the registration statement of which this prospectus supplement is a part, then the warrants may be exercised on a “net” or “cashless” basis.
S-33
UNDERWRITING
We have entered into an underwriting agreement with Ladenburg Thalmann & Co. Inc. with respect to the offering set forth in this prospectus supplement. Under the terms of an underwriting agreement, which we will file as an exhibit to a current report on Form 8-K, we have agreed to sell to the underwriter, and the underwriter has agreed to purchase from us, in each case subject to certain closing conditions, (i) an aggregate of [ ] shares (the “Shares”) of common stock, $0.001 par value per share (the “Common Stock”) of the Company and (ii) warrants (the “Warrants”) to purchase up to an aggregate of [ ] shares of Common Stock. The Shares and the Warrants shall be sold in units (the “Units”), each Unit consisting of one (1) Share and [ ] of a Warrant, each whole Warrant exercisable for one (1) Warrant Share at $[ ] per share.
The underwriting agreement provides that the obligation of the underwriter to purchase the Shares and the Warrants offered hereby is subject to certain conditions and that the underwriter is obligated to purchase all of the Shares and Warrants offered hereby if any of the Shares or Warrants are purchased.
Commissions and Expenses
The underwriter proposes to offer to the public the Shares and Warrants purchased pursuant to the underwriting agreement at the public offering price on the cover page of this prospectus supplement. After the Shares and Warrants are released for sale to the public, the underwriter may change the offering price and other selling terms at various times. In connection with the sale of the Shares and Warrants to be purchased by the underwriter, the underwriter will be deemed to have received compensation in the form of underwriting commissions and discounts. The underwriter’s commissions and discounts will be [ ]% of the proceeds, or $[ ] per share of Common Stock, from Shares and Warrants sold to purchasers. In addition, we shall reimburse the underwriter for its reasonable expenses in conjunction with the offering, including travel expenses and reasonable expenses of its legal counsel. Pursuant to a requirement by the Financial Industry Regulatory Authority, or FINRA, the maximum compensation to be received by any FINRA member or independent broker/dealer generally, as calculated consistent with FINRA’s rules, may not be greater than 8.0% of the gross proceeds received by us from the sale of any securities registered pursuant to SEC Rule 415.
We estimate the total expenses of the offering to us, excluding underwriting commissions and discounts, but including reimbursement of underwriter’s expenses payable by us, to be $[ ].
The following table shows the public offering price, underwriting discount and proceeds before expenses to us.
| | | | | | |
| | | Per Share | | Total |
Public offering price | | $ | | | $ | |
Underwriting discounts and commissions paid by us | | $ | | | $ | |
Proceeds, before expenses, to us | | $ | | | $ | |
S-34
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments which the underwriter or such other indemnified parties may be required to make in respect of any such liabilities.
Electronic Distribution
A prospectus supplement and the accompanying prospectus in electronic format may be made available on the Internet sites or through other online services maintained by the underwriter or by its affiliates. In those cases, prospective investors may view offering terms online and prospective investors may be allowed to place orders online.
Other than the prospectus supplement and the accompanying prospectus in electronic format, the information on the underwriter’s web site and any information contained in any other web site maintained by the underwriter is not part of the prospectus supplement, the accompanying prospectus or the registration statement of which this prospectus supplement and the accompanying prospectus forms a part, has not been approved and/or endorsed by us or the underwriter in its capacity as underwriter and should not be relied upon by investors.
Other Relationships
The underwriter and its affiliates have provided, and may in the future provide, various investment banking, commercial banking and other financial services for us for which services they have received, and may receive in the future, customary fees.
S-35
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon for us by Troutman Sanders LLP, New York, New York.
EXPERTS
The consolidated financial statements for the years ended December 31, 2009 and 2008, incorporated in this prospectus by reference from our Annual Report on Form 10-K, and the effectiveness of our internal control over financial reporting have been audited by Schwartz Levitsky Feldman LLP, an independent registered public accounting firm, as stated in their reports which are incorporated herein by reference. Such consolidated financial statements have been so incorporated in reliance upon the reports of such firm given upon their authority as experts in accounting and auditing.
The consolidated financial statements for the year ended December 31, 2007 have been audited by EFP Rotenberg, LLP, as successor to Rotenberg & Co., LLP, an independent registered public accounting firm, as stated in their report which is incorporated herein by reference. Such consolidated financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-3 and an amendment thereto with the SEC. This prospectus supplement and accompanying prospectus, which are a part of the registration statement, do not contain all of the information contained in the registration statement. Because some information is omitted, you should refer to the registration statement, as amended, and its exhibits for additional information. For example, the descriptions in this prospectus supplement and accompanying prospectus regarding the contents of any of our contracts, agreements or other documents are not necessarily complete and you should refer to the exhibits attached to the registration statement or incorporated by reference for copies of the actual contract, agreement or other document. You may obtain a copy of these documents from the SEC at its address or web site, which are provided below.
We file annual reports, quarterly reports, current reports, proxy statements and other information with the SEC. You may read and copy any of our SEC filings at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may call the SEC at 1-800-SEC-0330 for further information about the Public Reference Room. Our SEC filings are also available to the public on the SEC’s web site at www.sec.gov.
S-36
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference” information from some of our other SEC filings. This means that we can disclose information to you by referring you to those other filings, and the information incorporated by reference is considered to be part of this prospectus supplement. In addition, some information that we file with the SEC after the date of this prospectus supplement will automatically update, and in some cases supersede, the information contained or otherwise incorporated by reference in this prospectus supplement and the accompanying prospectus. The following documents, which we filed with the Securities and Exchange Commission, are incorporated by reference in this prospectus supplement:
| | (a) | Our Annual Report on Form 10-K for the fiscal year ended December 31, 2009 (as filed on March 15, 2010); |
| | (b) | Our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2010 (as filed on May 10, 2010); |
| | (c) | Our Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2010 (as filed on August 9, 2010); |
| | (d) | Our Current Report on Form 8-K filed on March 8, 2010; |
| | (e) | Our Schedule 14C filed on July 7, 2010; and |
| | (f) | The description of our common stock contained in our registration statement on Form 8-A filed on May 16, 2007 with the SEC under Section 12 of the Securities Exchange Act of 1934, including any amendment or report filed for the purpose of updating such description. |
Also incorporated by reference into this prospectus supplement are all documents that we may file with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act after the date of this prospectus supplement and prior to the termination of this offering. These documents include periodic reports, such as annual reports on Form 10-K, quarterly reports on Form 10-Q, and current reports on Form 8-K, as well as proxy statements. Pursuant to General Instruction B of Form 8-K, any information submitted under Item 2.02, Results of Operations and Financial Condition, or Item 7.01, Regulation FD Disclosure, of Form 8-K is not deemed to be “filed” for the purpose of Section 18 of the Exchange Act, and we are not subject to the liabilities of Section 18 with respect to information submitted under Item 2.02 or Item 7.01 of Form 8-K. We are not incorporating by reference any information submitted under Item 2.02 or Item 7.01 of Form 8-K into any filing under the Securities Act or the Exchange Act or into this prospectus supplement. Any statement contained herein or in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement.
You may request copies of these filings, at no cost, by writing to or calling our Investor Relations department at:
HQ Sustainable Maritime Industries, Inc.
Melbourne Towers, 1511 Third Avenue
Suite 788
Seattle, WA 98101
Telephone: (206) 621-9888
Information is also available on our website atwww.hqfish.com. Information contained in, or accessible through, our website is not incorporated by reference into this prospectus supplement.
S-37
PROSPECTUS
$50,000,000
HQ SUSTAINABLE MARITIME INDUSTRIES, INC.
Common Stock
Preferred Stock
Warrants
Units
We may offer and sell from time to time:
| | • | | shares of our common stock; |
| | • | | shares of our preferred stock; |
| | • | | warrants to purchase common stock and/or preferred stock; and |
| | • | | units consisting of two or more of these classes or series of securities. |
We may sell any combination of these securities in one or more offerings, up to an aggregate initial offering price of $50,000,000, on terms to be determined at the time of the offering. We may sell the securities to or through underwriters, directly to investors or through agents. We also may sell common stock upon conversion of preferred stock or common stock or preferred stock upon the exercise of warrants.
Each time we offer securities, we will provide a prospectus supplement containing more specific information about the particular securities and offering and attach it to this prospectus. The prospectus supplements also may add, update or change information contained in this prospectus. This prospectus may not be used to offer or sell securities without a prospectus supplement that includes a description of the method and terms of the offering.
Our common stock trades on the NYSE Amex Stock Exchange (“NYSE Amex”) under the symbol “HQS.” The last reported sale price of our common stock on the NYSE Amex on January 26, 2010 was $7.10 per share.
Investing in our securities involves a high degree of risk. SeeRISK FACTORS beginning on page 8.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is January 26, 2010.
TABLE OF CONTENTS
You should rely on the information contained in this prospectus, in any applicable prospectus supplement and in the documents incorporated by reference in this prospectus. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any jurisdiction where their offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only at the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the securities. Our business, financial condition, results of operations and prospects may have changed since the date indicated on the front cover of this prospectus.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, and reference is made to the actual documents filed with the United States Securities and Exchange Commission for complete information. Copies of some of the documents referred to herein have been filed or will be filed or incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under “Where You Can Find More Information.”
i
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission (the “SEC”) utilizing a shelf registration process. Under this shelf registration statement, we may sell common stock, preferred stock, warrants or units from time to time in one or more offerings up to a total public offering price of $50,000,000. This prospectus provides you with a general description of the securities we may offer.
Each time we sell securities, we will provide a prospectus supplement containing specific information about the terms of the securities and the offering. The prospectus supplement may add, update or change information contained in this prospectus and may include a discussion of any risk factors or other special considerations that apply to the securities. If there is any inconsistency between the information in this prospectus and a prospectus supplement, you should rely on the information in that prospectus supplement. Before making an investment decision, it is important for you to read and consider the information contained in this prospectus and any prospectus supplement, together with the additional information described under the heading “Where You Can Find More Information.”
You should rely only upon the information contained in this prospectus, any prospectus supplement and the registration statement of which this prospectus is a part. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it.
We are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted.
You should assume the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date. This prospectus is based on information provided by us and other sources that we believe are reliable. We have summarized certain documents and other information in a manner we believe to be accurate, but we refer you to the actual documents for a more complete understanding of what we discuss in this prospectus. In making an investment decision, you must rely on your own examination of our business and the terms of the offering, including the merits and risks involved.
We obtained statistical data, market data and other industry data and forecasts used throughout, or incorporated by reference in, this prospectus from market research, publicly available information and industry publications. Industry publications generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy and completeness of the information. Similarly, while we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of the information. We have not sought the consent of the sources to refer to their reports appearing or incorporated by reference in this prospectus.
1
PROSPECTUS SUMMARY
The following summary highlights selected information contained elsewhere in this prospectus or incorporated herein by reference. This summary does not contain all the information you should consider before investing in our securities. You should read the entire prospectus carefully, especially the discussion of “Risk Factors” and our consolidated financial statements and the related notes, before deciding to invest in our securities. In this prospectus, when we use phrases such as “we,” “us,” “our,” “HQS” or “our company,” we are referring to HQ Sustainable Maritime Industries, Inc. and all of its subsidiaries and affiliated companies as a whole, unless it is clear from the context that any of these terms refer only to HQ Sustainable Maritime Industries, Inc.
About Our Company
We are a multi-national company with our headquarters and primary sales offices based in Seattle, Washington. We are a vertically integrated aquatic product producer, processor, and farmer (e.g. through cooperative arrangements with pond farmers), with operations in the People’s Republic of China, or the PRC, of all natural tilapia, other aquatic products, and marine bio and healthcare products. We use state-of-the-art and environment-friendly technologies in our production, processing, and cooperative farm operations. Our facilities are currently certified according to the Hazard Analysis and Critical Control Point (“HACCP”) standards, currently assigned a European Union (“EU”) code required for exporting aquatic products to the EU, and are currently certified in accordance with the Aquaculture Certification Counsel (“ACC”) standards. Our products are sold principally to customers in North America, Europe and Asia. Our current sales activity is primarily directed to distributors within the PRC, rather than within the U.S.
Established in 1999, our aquatic farming and processing operations are in Hainan Province, an island in the South China Sea, which is situated within the most desirable latitude for raising tilapia. The Chinese government has designated Hainan Province in southern China a “green” province, where only environmentally friendly agri-food related industry and tourism are permitted. We purchase and process farm bred and farm raised tilapia through cooperative supply arrangements with local farmers and cooperatives. Our supply cooperatives, under our guidance, use feed formulated by us to optimize the all natural growth of farm raised tilapia. Starting in 2009, we will supply the farmers and cooperatives the feed formulation from our feed processing mill. We believe our tilapia products have achieved such a high level of purity that we market these products as all natural tilapia. In addition, we purchase and process ocean-caught aquatic products through cooperative supply arrangements with local fisherman for our marine bio healthcare products.
In August 2004, the company acquired Hainan Jiahua Marine BioProducts Co. Ltd., or Jiahua Marine, which develops, produces and sells marine bio and healthcare products, including nutraceutical products for the enrichment of our feed formulas, exclusively in China. The principal products of Jiahua Marine are manufactured from fish by-products (tilapia and shark) that include shark cartilage capsules and shark liver oil products that are distributed exclusively in China. The products undergo substantial independent laboratory testing administered by the Ministry of Health in China, which has resulted in a PRC National Certification for these products. These products have various perceived medicinal and health benefits. As part of HQS’s vertical integration plan with respect to tilapia, Jiahua Marine introduced new bio and healthcare products made from tilapia in 2009.
In order to maintain the high quality of our products and to position ourselves for attaining completely organic production certification in the future, we have completed the construction of our own feed mill and processing plant for the production of floating feed formulations. We produce the feed using grains grown without chemical fertilizers, free of antibiotics and fishmeal, and use feed additives manufactured in our nutraceutical plant. We plan for this expanded production to satisfy our own demand through the 20,000 mu (or 3,294 acres) of production in Wenchang and Qionghai, as well as to manufacture feed for others’ farmed operations in Hainan, such as shrimp and other farmed species.
2
From our headquarters in Seattle, Washington, we market and brand our high-quality, all natural tilapia products under the name TILOVEYA®. In early 2009, we introduced a new family of frozen tilapia meals under the brand name “Lillian’s Healthy Gourmet.” This new family of products includes an organic, natural and regular line of frozen tilapia meals.
We are incorporated in Delaware. The address of our Unites States headquarters is Melbourne Towers, 1511 Third Avenue, Suite 788, Seattle, Washington 98101 and our telephone number is (206) 621-9888. Our website is located atwww.hqfish.com. The information contained on our website or that can be accessed through our website does not constitute part of this prospectus.
Our Principal Competitive Strengths
We believe we have the following principal competitive strengths:
| | • | | Quality All Natural Tilapia Products.We produce all natural tilapia products and have developed a farming system that avoids the use of antibiotics, hormones and other potentially toxic chemicals. Our tilapia is raised in ponds of pure rain water collected for aquaculture. Two other species of fish are introduced into the ponds to maintain the ponds’ health naturally. One of the species is a bottom feeder that cleans up all the waste at the bottom of the ponds (carp), and the other species is a predator fish that eliminates all of the unhealthy fish (snakehead). We formulate feed without fishmeal and produce feed supplements in our healthcare products processing plant to enrich this feed. It is our policy to raise all natural tilapia to distinguish our company from other tilapia producers. The latitude and the pristine environment of the Hainan province of China have provided us with what we believe to be the optimal conditions for all natural aquaculture production. |
| | • | | Vertically Integrated Operations. Our newly completed feed mill is an important additional step towards our goal of complete vertical integration of our operations, which will allow us to further control and monitor the quality of our aquaculture products, as well as control our costs. The local farmers that we have cooperative arrangements with use our production methods and allow us to constantly monitor the production process for highly consistent production that results in high quality products at the time of harvest. |
| | • | | Environmental and Quality Assurances.We are a leader in environmental and quality assurances of aquaculture products. We have adopted and implemented stringent quality control measures and procedures throughout the production process, in order to comply with the various environmental and quality standards, such as the HACCP and the EU import standards. We are also certified in accordance with the ACC standards and positioning ourselves for completely organic production certification of our tilapia products in accordance with market demands. We use state-of-the-art technologies in our farming, feed formulation and processing operations. We have adopted modern and environmentally friendly and responsible technology in our production and processing of tilapia, shrimp, and marine bio and healthcare products, which we believe have been recognized as such through the certifications our plants possess. |
| | • | | Strategic Location in Hainan Province, China. Our processing facilities are geographically well-positioned in Hainan Province to leverage favorable climatic conditions, abundant water supply, pristine environment and a readily available source of labor for our processing plant. We are also located near a seaport, near the city of Wenchang, and our processing facilities are conveniently located near the farmers from whom we obtain our supply of tilapia and shrimp. |
| | • | | International and Domestic Sales and Marketing Efforts.Our Seattle office allows us to differentiate our TILOVEYA® brand and marketing initiative of our all natural tilapia products. Following the success of the TILOVEYA® brand, we introduced in 2009 a family of frozen tilapia meal products under the brand name of “Lillian’s Healthy Gourmet.” The family of products will have three lines focusing on organic, natural and regular frozen meals. The “Lillian’s Healthy Gourmet” brand will help |
3
| | us continue to establish our products as the all natural tilapia products of choice both domestically and internationally. Sales from both the Seattle and China offices complement our multi-national sales efforts to become a world leader in all natural tilapia products. |
| | • | | An Established Track Record and Brand Name in the Industry.We have an established track record and recognized brand name in the industry and have received numerous awards and certifications, which we believe reflects the success of the company in distinguishing itself from its competition. |
| | • | | Competitive Cost Structure.We benefit from competitive cost structures due to the lower labor costs in China as compared to other companies that produce similar products. |
Our Growth Strategies
Our objective is to become the world leader in vertically integrated production, processing and raising of all natural tilapia products. This includes the use of tilapia by-products to increase the range and variety of our marine bio and healthcare products, with a primary focus on increasing our own seafood products. To achieve this goal, we intend to implement the following strategies:
| | • | | Our newly completed large-scale feed mill to supply our existing and anticipated new cooperative fish farmers with our fish food formula. We anticipate that the new feed mill will help increase our aquatic profit margin, help guarantee our product quality and further vertically integrate our operations; |
| | • | | We intend to achieve completely organic production of our tilapia products and to pursue organic certification of our farms as market demand dictates; |
| | • | | We plan to expand direct and retail sales of our health products in China and internationally and to add other products we currently have in the development pipeline; |
| | • | | We plan to expand our development of health products by using the by-products of tilapia, which will help increase the overall aquatic products’ gross margins; |
| | • | | We plan to expand our cooperative farming arrangements to increase the availability of tilapia to meet anticipated growth in demand; |
| | • | | We plan to construct our own tilapia farmed ponds to improve growth time and quality of our product and further vertically integrate our operations; |
| | • | | We plan to continue to expand our production and processing facilities in China to satisfy the anticipated growing demand for our products; |
| | • | | Our Seattle office allows us to increase awareness of the importance of our all natural products and to benefit from more direct sales. The Seattle office also allows us to expand our distribution options in North America and Europe by broadening the variety of products we offer to cater to the demands of our customers; and |
| | • | | We plan to expand our product offerings, including our family of frozen tilapia meals. We will continue our branding and marketing initiatives in North America and Europe to introduce our new family of products to major retail and food service chains. |
Industry Overview
Aquaculture Industry
Aquaculture is the farming of aquatic animals or plants. Aquaculture is also the world’s fastest growing segment in the food production system and has been for the past two decades. According to a recent study by the World Food and Agriculture Organization (“FAO”) published on March 2, 2009, world fisheries production reached a new high of 143.6 million metric tonnes in 2006, including farmed and ocean caught product. The
4
contribution of aquaculture to the world fisheries production in 2006 was 51.7 million tonnes of fish, which is 36 percent of world fisheries production up from 3.6 percent in 1970. Global aquaculture accounted for 6 percent of the fish available for human consumption in 1970. In 2006 global aquaculture accounted for 47 percent of the fish available for human consumption according to the FAO. The FAO report also describes that over half of the global aquaculture in 2006 was freshwater finfish. Based on the FAO’s projections, it is estimated that in order to maintain the current level of per capita consumption, global aquaculture production will need to reach in excess of 80 million tonnes of fish by 2050.
As the availability of sites for aquaculture is becoming increasingly limited and the ability to develop non-agricultural land is often restricted, the competition to develop additional aquaculture production systems is intensifying. As the intensification for aquaculture production systems increases, the demand for institutional support, services and skilled persons is anticipated to increase, along with the demand for more knowledge-based aquaculture education and training as aquaculture becomes more important worldwide. China remains the largest producer of aquaculture products throughout the world with fishes in China reportedly producing approximately 41.3 million tonnes of fish in 2004. Within the global aquaculture industry, China accounted for 71.0% of the world’s supply of fish for direct human consumption and 29.9% of the total production by live weight in 2002.
Tilapia Industry
In 2005, according to the American Tilapia Association (“ATA”), tilapia production worldwide was second in volume to carp, and it is projected by the ATA that tilapia will become the most important aquaculture crop in this century, potentially reaching $5.0 billion in global sales by 2010. Commercial production of tilapia has become popular in many countries around the world. Touted as the “new white fish” to replace the depleted ocean stocks of cod, pollock, and hake, world tilapia production continues to rise and at least 100 countries currently raise tilapia, with the PRC being the largest producer. The American Tilapia Association further reports that world production of tilapia products reached approximately 2.5 million metric tonnes in 2007, of which China produced the dominant share of 45.0 percent.
One of the major outlets for Chinese-produced tilapia has been, and should continue to be, the United States. The following chart reflects the increase in per-capita consumption of tilapia in pounds in the United States in relation to other traditional types of seafood.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Consumer Consumption Per Capita (lbs) |
| 2000 | | 2001 | | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 (est.) |
| Tuna | | 3.5 | | Shrimp | | 3.4 | | Shrimp | | 3.7 | | Shrimp | | 4.0 | | Shrimp | | 4.2 | | Shrimp | | 4.1 | | Shrimp | | 4.4 | | Shrimp | | 4.1 | | Shrimp | | 4.0 |
| Shrimp | | 3.2 | | Tuna | | 2.9 | | Tuna | | 3.1 | | Tuna | | 3.4 | | Tuna | | 3.4 | | Tuna | | 3.1 | | Tuna | | 2.9 | | Tuna | | 2.7 | | Tuna | | 2.6 |
| Pollock | | 1.6 | | Salmon | | 2.0 | | Salmon | | 2.0 | | Salmon | | 2.2 | | Salmon | | 2.2 | | Salmon | | 2.4 | | Salmon | | 2.0 | | Salmon | | 2.3 | | Salmon | | 2.2 |
| Salmon | | 1.5 | | Pollock | | 1.2 | | Pollock | | 1.1 | | Pollock | | 1.7 | | Pollock | | 1.7 | | Pollock | | 1.5 | | Pollock | | 1.6 | | Pollock | | 1.7 | | Pollock | | 1.6 |
| Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.1 | | Catfish | | 1.0 | | Tilapia | | 1.0 | | Tilapia | | 1.1 | | Tilapia | | 1.2 |
| Cod | | 0.8 | | Cod | | 0.6 | | Cod | | 0.7 | | Cod | | 0.6 | | Tilapia | | 0.7 | | Tilapia | | 0.8 | | Catfish | | 1.0 | | Catfish | | 1.0 | | Catfish | | 1.0 |
| Clams | | 0.5 | | Clams | | 0.5 | | Crabs | | 0.6 | | Crabs | | 0.6 | | Cod | | 0.6 | | Cod | | 0.6 | | Crabs | | 0.7 | | Crabs | | 0.7 | | Crabs | | 0.7 |
| Crabs | | 0.4 | | Crabs | | 0.4 | | Clams | | 0.5 | | Tilapia | | 0.5 | | Crabs | | 0.6 | | Crabs | | 0.5 | | Cod | | 0.5 | | Cod | | 0.5 | | Clams | | 0.4 |
| Flatfish | | 0.4 | | Flatfish | | 0.4 | | Tilapia | | 0.4 | | Clams | | 0.5 | | Clams | | 0.5 | | Clams | | 0.4 | | Clams | | 0.4 | | Clams | | 0.4 | | Cod | | 0.4 |
| Scallops | | 0.3 | | Tilapia | | 0.4 | | Flatfish | | 0.4 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 | | Scallops | | 0.3 |
| Tilapia | | 0.3 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
5
The following chart reflects the increase in imported tilapia to the United States during the periods indicated.

Separately, according to the International Trade Report produced in 2005 by the United States Department of Agriculture, or the USDA,“U.S. per-capita seafood consumption has remained around 15 billion pounds through the late 1980s and 1990s; it is expected to increase as farm-raised products become cheaper. Currently, the United States consumes nearly 12 billion pounds of fish a year. By 2025, demand for seafood is projected to grow by another 4.4 billion pounds (2 million metric tonnes) above what is consumed today. In addition, it is estimated that by 2020, 50 percent of the U.S. seafood supply will come from aquaculture. Presently, more than 70 percent of the seafood consumed in the United States is imported, and at least 40 percent of that is farm-raised. Major changes in U.S. population, along with shifting demographics and economic growth, will alter the U.S. seafood market over the next decade, affecting the selection of products consumed. It is expected that fresh and frozen fish products will account for a growing share of overall seafood consumption, with shrimp remaining at the top. By 2020, shrimp, salmon, tilapia, and catfish will be the top four seafood products consumed…”
According to Globefish.org, during the past ten years, tilapia captured by fisheries have stabilized at 0.6 million metric tonnes, while their aquaculture production has grown from 0.55 million metric tonnes to 2.0 million metric tonnes. Tilapia is one of the top five seafood imports in the world. In 2005, more than $2.4 billion of tilapia was sold worldwide according to the FAO Fishstat 2007. In 2005, tilapia moved up to the fourth-ranked most popular seafood after farm-raised shrimp, tuna and Atlantic salmon in terms of aquaculture products imported into the United States. The United States is now the world’s largest consumer of tilapia after China, having imported 437 thousand metric tonnes of tilapia in 2007. In terms of volume, frozen whole round fish ranks first, followed by frozen fillets and then fresh fillets.
The growing consumer demand for seafood is largely evolving from a new public awareness regarding its health and nutritional benefits. The USDA “pyramid” guidelines continue to support frequent fish consumption, and the USDA recently completed a highly technical nutritional analysis report about tilapia for the general public. The USDA’s Agriculture Research Service Lab reports that tilapia is moderate in polyunsaturated fatty
6
acid (0.387 g/100g raw, 0.600 g/100g cooked), moderate in omega 3 fatty acids (0.141g/100g raw, 0.220g/100g cooked) and low in mercury (0.010 parts per million, which we refer to as PPM), which is considered to be non-detectable. With the increased awareness of the health concerns surrounding mercury, tilapia’s low mercury levels (0.010 PPM) distinguish tilapia favorably from other types of fish with higher mercury levels, such as swordfish (0.976 PPM), mackerel (0.730 PPM) and yellow fin tuna (0.325 PPM).
Over-the-Counter Marine Bio and Healthcare Products
As in the United States, because of the aging population, China is demographically attractive for healthcare companies. According to China’s Ministry of Health 2009 figures, of the 1.3 billion people in China currently, less than 8 percent of China’s population is 65 or older; however, by 2050 that proportion is expected to rise to 24 percent. It is expected as well that Chinese will benefit from double-digit annual growth in disposable incomes during this period. We anticipate that this will lead to increasing use of western therapeutics and “over the counter” remedies. In addition, the market place is highly fragmented with more than 4,000 manufacturers of such remedies in China, most of whom lack scale and access to capital. From 2009 to 2011, we believe approximately $124 billion will be allocated to healthcare expenditure in China. HQS has benefited from regulatory consolidation that resulted in the imposition of higher Chinese Good Manufacturing Practice (“GMP”) standards in 2007 and intends to be a leader in its emphasis on the highest quality standards.
The marine bio and healthcare products industry in China is also sizable, with approximately $6 billion in sales, which still constitutes only 3 percent of the world market. Currently, overall sales of these products in China have fallen slightly as compared to the previous year, as consumers gravitate toward branded products meeting international quality standards and with proven benefit for consumers, and away from less known brands and traditional remedies.
In general, sales of marine bio and healthcare products are made through retail and direct sales channels. Direct sales in China are relatively new, and restrictions on direct sales imposed on large foreign companies have been implemented in China, which has allowed for a growth in sales for PRC-based direct sales companies. These restrictions require foreign direct sellers to manufacture their products and to capitalize their businesses in China. Several companies have met these requirements, including HQS through its PRC-subsidiaries, and growth in this sector is expected to be strong in the next few years.
We believe that nutraceutical supplements in the feed industry is a sector that has strong growth potential, as the importance of aquaculture and aquaculture feeds increases. We manufacture actualized feeds, which involves choosing additives to be included in the feed, such as vitamin E, that promote health in fish, thus reducing the need for curative measures such as antibiotics. Once ingested and present in the flesh of the fish, vitamin E increases the shelf life of the fish and also introduces an additional source of vitamin E to its consumers. Other similar nutraceutical supplements can also be used.
7
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information contained in this prospectus and in the documents incorporated by reference into this prospectus, before investing in our securities. If any of the events anticipated by the risks described below occur, our results of operations and financial condition could be adversely affected which could result in a decline in the value of our securities, causing you to lose all or part of your investment.
Risks Relating To Our Business
We rely on cooperative suppliers and any adverse changes in these relationships may adversely affect us.
We have developed a network of aquaculture farmers in Hainan Province for the supply of tilapia and shrimp by entering into cooperative supply agreements. Pursuant to the cooperative supply agreements, we are assured the necessary supply of aquatic products that meet our quality standards. Smooth continual operation of this cooperative network is essential in controlling our costs, meeting quality standards and the timely fulfillment of our customer orders. Any adverse change to our cooperative network, including any early termination or non-renewal of any supply agreement or any failure of suppliers to fulfill their obligations under the supply agreements, could have a material adverse effect on our business model, operations and competitiveness.
We may require additional capital in the future, which may not be available on favorable terms or at all.
Our future capital requirements will depend on many factors, including industry and market conditions, our ability to successfully implement our branding and marketing initiatives and expansion of our production capabilities. We anticipate that we may need to raise additional funds in order to grow our business and implement our business strategy. We anticipate that any such additional funds would be raised through equity or debt financings. In addition, we may enter into a revolving credit facility or a term loan facility with one or more syndicates of lenders. Any equity or debt financing, if available at all, may be on terms that are not favorable to us. Even if we are able to raise capital through equity or debt financings, as to which there can be no assurance, the interest of existing stockholders in our company will be diluted, and the securities we issue may have rights, preferences and privileges that are senior to those of our common stock or may otherwise materially and adversely affect the holdings or rights of our existing stockholders. If we cannot obtain adequate capital, we may not be able to fully implement our business strategy, and our business, results of operations and financial condition would be adversely affected. In addition, we have and will continue to raise additional capital through private placements or registered offerings, in which broker-dealers will be engaged. The activities of such broker-dealers are highly regulated and we cannot assure that the activities of such broker-dealers will not violate relevant regulations and generate liabilities despite our expectation otherwise.
We depend on the availability of additional human resources for future growth.
We have recently experienced a period of significant growth in our sales volume. We believe that continued expansion is essential for us to remain competitive and to capitalize on the growth potential of our business. Such expansion may place a significant strain on our management and operations and financial resources. As our operations continue to grow, we will have to continually improve our management, operational and financial systems, procedures and controls, and other resources infrastructure, and expand our workforce. There can be no assurance that our existing or future management, operating and financial systems, procedures and controls will be adequate to support our operations, or that we will be able to recruit, retain and motivate our personnel. Further, there can be no assurance that we will be able to establish, develop or maintain the business relationships beneficial to our operations, or to do so or to implement any of the above activities in a timely manner. Failure to manage our growth effectively could have a material adverse effect on our business and the results of our operations and financial condition.
8
We depend on our key management personnel, and the loss of any of their services could materially adversely affect us.
Our operations are dependent upon the experience and expertise of a small number of key management personnel, which includes Lillian Wang Li, our Chairman of the Board, Norbert Sporns, our Chief Executive Officer and President, and Harry Wang Hua, our Chief Operating Officer. Lillian Wang Li and Harry Wang Hua are brother and sister, and Ms. Wang Li is married to Norbert Sporns. Although we have obtained life insurance on Ms. Wang Li and have begun the process of obtaining life insurance on Mr. Sporns, the loss of the services of any of these key personnel for any reason would likely have a material adverse effect on our business, and the results of our operations and financial condition, or could delay or prevent us from fully implementing our business strategy.
A few of our customers account for a significant portion of our business.
We have derived, and over the near term we expect to continue to derive, a significant portion of our sales from a limited number of customers. For example, our five largest customers accounted for a total of 38.6 percent of our consolidated sales for the year ended December 31, 2008, and they are all related to the aquaculture product segment. At December 31, 2008, approximately 46.3 percent of our trade receivables was from transactions with these five largest customers. The loss of any of these customers or non-payment of outstanding amounts due to the company from these customers could materially and adversely affect our results of operations, financial position and liquidity.
We may be unable to continue to take advantage of the seasonal pricing fluctuation in sales of our products, and we may be adversely affected by the seasonal fluctuation in the prices we earn for our products.
We have experienced seasonal fluctuation in the prices we earn for our products, generally in the range of 15 to 20%. Pricing fluctuation occurs during the winter season when fish farms in the northern part of the PRC suspend production due to cold weather conditions. These weather related disruptions in supply permit us to increase the sales prices of our tilapia products. However, there can be no assurance that such premium pricing, benefiting our profitability, can be maintained in the future. Other factors, such as an increase in the cost of feed, might also adversely impact on the cost of fish and lessen our margins and profitability.
Any adverse changes in the supply of our tilapia and other raw materials, including contamination or disease or increased costs of raw materials, may adversely affect our operations or reduce our margins or profits.
We are dependent on the availability of raw materials from Hainan Province and the oceans in that region. The supply of these raw materials can be adversely affected by any material change in the climatic or environmental conditions in and around Hainan Province. In addition, if there is contamination resulting from disease, pollution or other foreign substances, our supply of raw materials could be jeopardized or disrupted. The shortage or lack of raw materials and any consequential change in their cost would, in turn, have a material adverse effect on the cost on our operations and margins and our ability to provide products to our customers.
Any actual contamination of our products resulting from processing, packaging or transit of our products, or negative press from contamination experienced by other companies in our industry may adversely affect our operations or reduce our margins or profits.
We actively seek to control the quality of our products and avoid risk of contamination in our processing, packaging and distribution of such products; however no quality control program is guaranteed to be completely effective. We are dependent on others for the reliable safe transportation of our products to the market place and the quality of our final product as experienced by the consumer may be impacted by disruptions in the transit process beyond our control. In addition, if our competitors experience problems with contamination of their products, even if we do not concurrently suffer similar adverse events, publicity of such problems could
9
negatively impact our reputation. Actual contamination or reports of industry problems with contamination or poor quality may have a material adverse effect on our operations, including an increase in product liability claims, higher quality control and transport costs, reduced margins and decreased consumer interest in our products.
We may be adversely affected by the fluctuation in raw material prices and selling prices of our products.
Neither our products nor the raw materials we use have experienced any significant price fluctuations since we began operation, but there is no assurance that they will not be subject to future price fluctuations or pricing control. The products and raw materials we use may experience price volatility caused by events such as market fluctuations or changes in governmental programs. The market price of these raw materials may also experience significant upward adjustment, if, for instance, there is a material under-supply or over-demand in the market. These price changes may ultimately result in increases in the selling prices of our products, and may, in turn, adversely affect our sales volume, revenue and operating profit.
We could be adversely affected by the occurrence of natural disasters in Hainan Province.
From time to time, Hainan Province experiences typhoons, particularly from June through September of any given year. Natural disasters could impede operations, damage infrastructure necessary to our operations or adversely affect the logistical services to and from Hainan Province. Even though we currently have insurance against damages caused by natural disasters, including typhoons, accidents or similar events, the occurrence of natural disasters in Hainan Province could adversely affect our business, the results of our operations, prospects and financial condition, through business disruptions and/or any losses in excess of our policy limits.
Intense competition from existing and new entities may adversely affect our revenues and profitability.
In general, the aquaculture industry is intensely competitive and highly fragmented. We compete with various companies, many of which are developing or can be expected to develop products similar to ours. For example, 8th Sea–The Organic Seafood Company currently produces and processes tilapia fillets in Brazil’s Parana state. Many of our competitors are more established than we are and have significantly greater financial, technical, marketing and other resources than we presently possess. Some of our competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We intend to create greater awareness for our brand name in an attempt to successfully compete with our competitors. We cannot assure you that we will be able to compete effectively or successfully with current or future competitors or that the competitive pressures we face will not harm our business.
Our operating subsidiaries must comply with environmental protection laws that could adversely affect our profitability.
We are required to comply with the environmental protection laws and regulations promulgated by the national and local governments of the PRC. Some of these regulations govern the level of fees payable to government entities providing environmental protection services and the prescribed standards relating to the discharge of effluent, or liquid waste. Yearly inspections of waste treatment systems require the payment of a license fee which could become a penalty fee if standards are not maintained. Currently, our plant treats all of its effluent completely to level one, which is consistent with releasing potable water back to the environment, and there is currently no charge being levied. Although our production technologies allow us to efficiently control the level of pollution resulting from our production process, and notwithstanding the fact that we have received evidence of compliance with environmental protection requirements from government authorities, due to the nature of our business, effluent wastes are unavoidably generated in the aquaculture production processes. If we fail to comply with any of these environmental laws and regulations in the PRC, depending on the types and
10
seriousness of the violation, we may be subject to, among other things, warning from relevant authorities, imposition of fines, specific performance and/or criminal liability, forfeiture of profits made, being ordered to close down our business operations and suspension of relevant permits.
Our operations, revenue and profitability could be adversely affected by changes in laws and regulations in the countries where we do business.
The governments of countries into which we sell our products, including the United States, Canada and the European Union, from time to time, consider regulatory proposals relating to raw materials, food safety and markets, and environmental regulations, which, if adopted, could lead to disruptions in distribution of our products and increase our operational costs, which, in turn, could affect our profitability. To the extent that we increase our product prices as a result of such changes, our sales volume and revenues may be adversely affected.
Furthermore, these governments may change import regulations or impose additional taxes or duties on certain Chinese imports from time to time. For example, in 2004, the United States government imposed heavy tariffs of more than 100 percent on certain Chinese shrimp exporters. Similar regulations and fees or new regulatory developments may have a material adverse impact on our operations, revenue and profitability. If one or more of the countries into which we sell our products bars the import or sale of fish or related products from China, our available market would shrink significantly, adversely impacting our results of operations and growth potential.
Our business could be adversely affected by the recent negative public reports on seafood imported from China.
In June 2007, the U.S. Food and Drug Administration issued an alert report on the sale of five types of farm-raised seafood from China in the United States because of unapproved chemical residues. The five types of farm-raised seafood are shrimp, catfish, eel, basa and dace. As a result, in order for the seafood to be sold in the United States, importers must provide independent testing that shows the seafood does not contain the unapproved residues. Although tilapia is not included in the list and we believe our main seafood product, which is tilapia, does not contain any of the unapproved residues, it is possible that our business may be adversely impacted as a result of the recent negative public reports on seafood imported from China.
There could be changes in the policies of the PRC government that may adversely affect our business.
The aquaculture industry in the PRC is subject to policies implemented by the PRC government. The PRC government may, for instance, impose control over aspects of our business such as distribution of raw materials, product pricing and sales. If the raw materials used by us or our products become subject to any form of government control, then depending on the nature and extent of the control and our ability to make corresponding adjustments, there could be a material adverse effect on our business and operating results.
Separately, our business and operating results also could be adversely affected by changes in policies of the Chinese government such as: changes in laws, regulations or the interpretation thereof; confiscatory taxation; restrictions on currency conversion, imports on sources of supplies; or the expropriation or nationalization of private enterprises. Although the Chinese government has been pursuing economic reform policies for approximately two decades to liberalize the economy and introduce free market aspects, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting China’s political, economic and social life.
Certain political and economic considerations relating to PRC could adversely affect our company.
The PRC is passing from a planned economy to a market economy. The Chinese government has confirmed that economic development will follow a model of market economy under socialism. While the PRC government
11
has pursued economic reforms since its adoption of the open-door policy in 1978, a large portion of the PRC economy is still operating under five-year plans and annual state plans adopted by the government that set down national economic development goals. Through these plans and other economic measures, such as control on foreign exchange, taxation and restrictions on foreign participation in the domestic market of various industries, the PRC government exerts considerable direct and indirect influence on the economy. Many of the economic reforms are unprecedented or experimental for the PRC government, and are expected to be refined and improved. Other political, economic and social factors can also lead to further readjustment of such reforms. This refining and readjustment process may not necessarily have a positive effect on our operations or future business development. Our operating results may be adversely affected by changes in the PRC’s economic and social conditions as well as by changes in the policies of the PRC government, which we may not be able to foresee, such as changes in laws and regulations (or the official interpretation thereof), measures which may be introduced to control inflation, changes in the rate or method of taxation, and imposition of additional restrictions on currency conversion.
The recent nature and uncertain application of many PRC laws applicable to us create an uncertain environment for business operations and they could have a negative effect on us.
The PRC legal system is a civil law system. Unlike the common law system, such as the legal system used in the United States, the civil law system is based on written statutes in which decided legal cases have little value as precedents. In 1979, the PRC began to promulgate a comprehensive system of laws and has since introduced many laws and regulations to provide general guidance on economic and business practices in the PRC and to regulate foreign investment. Progress has been made in the promulgation of laws and regulations dealing with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. The promulgation of new laws, changes of existing laws and the abrogation of local regulations by national laws could have a negative impact on our business and business prospects. In addition, as these laws, regulations and legal requirements are relatively recent, their interpretation and enforcement involve significant uncertainty.
If relations between the United States and China worsen, we may be unable to serve a portion of our customer base, the value of our securities could decline and we may have difficulty accessing the U.S. capital markets.
At various times during recent years, the United States and China have had disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and China could adversely affect our ability to continue to sell to U.S. customers, the value of our securities and our ability to access U.S. capital markets.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of the PRC. Currently, the Renminbi is not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency denominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of loans and corporate debt obligations denominated in foreign currencies.
The PRC government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain of our expenses as they come due.
12
The fluctuation of the Renminbi may materially and adversely affect your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. Any significant revaluation of the Renminbi may materially and adversely affect our cash flows, revenues and financial condition. For example, to the extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar could have a material adverse effect on our business, financial condition and results of operations.
Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making dividend payments on our common or preferred stock or for other business purposes and the U.S. dollar appreciates against the Renminbi, the U.S. dollar equivalent of the Renminbi we convert would be reduced. Any significant devaluation of Renminbi may reduce our operation costs in U.S. dollars but may also reduce our earnings in U.S. dollars. In addition, the depreciation of significant U.S. dollar denominated assets could result in a charge to our income statement and a reduction in the value of these assets.
Commencing from July 21, 2005, China has adopted a managed floating exchange rate regime based on market demand and supply with reference to a basket of currencies. The exchange rate of the US dollar against the RMB was adjusted from approximately RMB 8.28 per US dollar to approximately RMB 8.11 per US dollar on July 21, 2005. Since then, The People’s Bank of China administers and regulates the exchange rate of US dollar against RMB taking into account demand and supply of RMB, as well as domestic and foreign economic and financial conditions.
In addition, there can be no assurance that we will be able to obtain sufficient foreign exchange to pay dividends or satisfy other foreign exchange requirements in the future. We currently do not intend to pay dividends.
It may be difficult to effect service of process and enforcement of legal judgments upon our company and our officers and directors because some of them reside outside the United States.
As our operations are presently based in China and some of our key directors and officers reside outside the United States, service of process on our key directors and officers may be difficult to effect within the United States. Also, substantially all of our assets are located outside the United States and any judgment obtained in the United States against us may not be enforceable outside the United States. We have appointed Norbert Sporns, our Chief Executive Officer and President, as our agent to receive service of process in any action against our company in the United States.
Risks Relating to our Common Stock
There are a large number of shares underlying our Series A preferred stock and warrants that may be available for future sale and the sale of these shares may depress the market price of our common stock.
As of December 1, 2009, we had 14,657,163 shares of common stock issued and outstanding. We currently have outstanding Class B warrants to purchase up to 114,583 shares of our common stock and stock purchase warrants to purchase up to 30,000 shares of our common stock issued in connection with the November 2006 financing. In addition, we currently have 100,000 shares of Series A preferred stock issued and outstanding, and the holders of our Series A preferred stock have an option right to convert each share of Series A Preferred Stock into 0.1 shares of our common stock. These shares, including all of the shares issuable upon conversion of our Series A preferred stock and upon exercise of our warrants, may be sold into the market place currently. The sale of these shares may adversely affect the market price of our common stock.
Our principal stockholders, current executive officers and directors own a significant percentage of our company and will be able to exercise significant influence over our company.
Our executive officers and directors and principal stockholders together beneficially own approximately 90.10% of the total voting power of our outstanding voting capital stock, primarily as a result of their ownership
13
of Series A preferred stock of our company which, while convertible into shares of our common stock on a 0.1-for-1 basis, carries 1,000 votes per Series A preferred share rather than the 1 vote per share of our common stock. The Series A preferred stock and the common stock vote together as one class on all matters to be voted upon by the stockholders. In particular, our three largest stockholders, Mr. Sporns, Ms. Wang Li and Mr. Wang Hua, are family members who share approximately 90.06% of the total voting power of our company. Ms. Wang Li is the wife of Mr. Sporns and Mr. Wang Hua is the brother of Ms. Wang Li. These stockholders will be able to determine the composition of our board of directors, will retain the voting power to approve all matters requiring stockholder approval and will continue to have significant influence over our affairs. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the market price of the common stock or prevent our stockholders from realizing a premium over the market prices for their shares of common stock.
Investors may experience dilution from any exercise of warrants.
We currently have outstanding Class B warrants to purchase up to 114,583 shares of our common stock and stock purchase warrants to purchase up to 30,000 shares of our common stock. The Class B warrants expire on January 25, 2011 and the stock purchase warrants expire on December 28, 2011.
14
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, any prospectus supplement, and the documents we incorporate by reference in this prospectus contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements involve known and unknown risks, uncertainties and other important factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| | • | | our marketing, sales and distribution initiatives in North America, Europe and China; |
| | • | | our estimates of production, market sizes and anticipated consumption of our marine bio and healthcare products and our aquaculture products; |
| | • | | our new organic feed mill and our current and future processing facilities; |
| | • | | our ability to enter into and maintain cooperative farming arrangements and other partnerships with respect to our marine bio products and the performance of our partners under such arrangements; |
| | • | | our relationship with local governments in Hainan Province and the potential support and financing they may provide to us; |
| | • | | our estimates of future performance and growth potential; |
| | • | | our ability to obtain sufficient capital to fund our operations and expansion plans; and |
| | • | | our operating income, future revenue, expenses, capital requirements and needs for additional financing. |
In some cases, you can identify forward-looking statements by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “would” and similar expressions intended to identify forward-looking statements. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. These risks and uncertainties include, among others, those set forth above under the heading “Risk Factors,” and in the other documents that we file with the SEC. There are also other risks and uncertainties that we may not describe, generally because we currently do not perceive them to be material, which could cause actual results to differ materially from our expectations. Because of these risks and uncertainties, the forward-looking events and circumstances discussed in this prospectus and in any prospectus supplement may not transpire.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. You should read this document, any supplements to this document and the documents that we reference in this prospectus with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we do not undertake any obligation to update or revise any forward-looking statements contained in this prospectus and any supplements to this prospectus, whether as a result of new information, future events or otherwise.
15
RATIO OF EARNINGS TO FIXED CHARGES
The following table sets forth our ratio of earnings to fixed charges for the periods shown. For purposes of computing the ratio, earnings represent the sum of income from continuing operations before taxes plus fixed charges. Fixed charges represent interest expense and amortization of premiums and discounts related to indebtedness. We did not pay any preferred stock dividends during the periods shown. Consequently, the ratio of earnings to fixed charges and preferred stock dividends is the same as the ratio of earnings to fixed charges for the periods shown.
| | | | | | | | | | | | |
| | | Nine Months
Ended
September 30,
2009 | | Fiscal Year Ended December 31, |
| | | | 2008 | | 2007 | | 2006 | | 2005 | | 2004 (1) |
Ratio of Earnings to Fixed Changes | | 10.02 | | 5.86 | | 2.20 | | 1.36 | | 11.80 | | 0.44 |
| (1) | The amount of the deficiency for the fiscal year ended December 31, 2004 was $229,590. |
USE OF PROCEEDS
Unless otherwise provided in the applicable prospectus supplement, we intend to use the net proceeds from the sale of our securities under this prospectus for general corporate purposes, including investments in long-term assets to complete our integration objective in line with our business plan. We will set forth in the prospectus supplement our intended use for the net proceeds received from the sale of our securities. Pending the application of the net proceeds, we intend to invest the net proceeds in investment-grade, interest-bearing securities.
DESCRIPTION OF COMMON STOCK
The following description summarizes the material terms and provisions of our common stock that we may offer from time to time. The following summary description of our common stock is based on the provisions of our certificate of incorporation and by-laws, which are incorporated by reference, and the applicable provisions of Delaware General Corporation Law. In connection with an offering of our common stock, we will describe the specific terms of the common stock and the offering in a prospectus supplement. The information below, and any description of common stock in a prospectus supplement, is only a summary and is subject to and qualified in its entirety by reference to our certificate of incorporation and by-laws and the applicable provisions of Delaware General Corporation Law.
General
Authorized. We currently have authority to issue up to 200,000,000 shares of common stock, par value $0.001 per share.
Voting. Each holder of common stock is entitled to one vote for each share owned on all matters voted upon by stockholders. In addition, our Series A preferred stock is entitled to vote on all matters to be voted on by holders of our common stock. The Series A preferred stock is entitled to 1,000 votes per share. A majority vote is required for all actions to be taken by stockholders, except that a plurality is required for the election of directors. The common stock has no preemptive rights, no cumulative voting rights and no redemption, sinking fund or conversion provisions.
Dividends. Holders of common stock are entitled to receive dividends, if and when declared by our board of directors, out of funds legally available for such purpose, subject to the dividend and liquidation rights of any preferred stock that may then be outstanding, including, but not limited to, the Series A preferred stock.
16
We have never declared or paid any cash dividends on our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. In addition, as stipulated by the relevant laws and regulations for enterprises operating in the PRC, Hainan Quebec Ocean Fishing Co. Ltd. and Jiahua Marine are required to make annual appropriations to two reserve funds, consisting of the statutory surplus and public welfare funds, which amounts are not available for the payment of dividends. As a result, we do not anticipate paying cash dividends on our common stock in the foreseeable future and we may not have sufficient funds to legally pay dividends.
Liquidation and Dissolution. In the event we liquidate, dissolve or wind-up our operations, the holders of the common stock are entitled to share equally and ratably in our assets, if any, remaining after the payment of all our debts and liabilities and the liquidation preference of any shares of preferred stock that may then be outstanding.
Fully Paid and Nonassessable. All shares of our outstanding common stock are fully paid and nonassessable and any additional shares of common stock that we issue will be fully paid and nonassessable.
Listing. Our common stock is listed on the NYSE Amex under the symbol “HQS.”
Transfer Agent and Registrar. The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, 59 Maiden Lane, Plaza Level, New York, NY 10038.
Outstanding Convertible Securities
As of December 1, 2009, we had 10,000,000 shares of preferred stock authorized and 100,000 shares of Series A preferred stock issued and outstanding, and the holders of our Series A preferred stock have an option right to convert each share of Series A Preferred Stock into 0.1 shares of our common stock. The issuance of preferred stock could have the effect of restricting dividends on the common stock, diluting the voting power for the common stock, impairing the liquidation rights of the common stock or delaying or preventing a change in control without further action by the stockholders. Our preferred stock is described below under “Description of Preferred Stock.” In addition, as of December 1, 2009 we had outstanding Class B warrants to purchase up to 114,583 shares of our common stock and stock purchase warrants to purchase up to 30,000 shares of our common stock. Our outstanding warrants are described below under “Description of Warrants—Outstanding Warrants.”
Anti-Takeover Effects of Provisions of Delaware Law, Our Certificate of Incorporation and By-laws
Some provisions of Delaware General Corporation Law, our certificate of incorporation and our by-laws contain provisions that could make the following transactions more difficult: acquisition of us by means of a tender offer; acquisition of us by means of a proxy contest or otherwise; or removal of our incumbent officers and directors.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Business Combinations. We are subject to the provisions of Section 203 of Delaware General Corporation Law. Subject to certain exceptions, Section 203 prohibits a Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination”
17
includes mergers, asset sales and other transactions resulting in a financial benefit to the interested stockholder. Subject to exceptions, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within the prior three years did own, 15% or more of the corporation’s voting stock.
Undesignated Preferred Stock. The ability to authorize undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of our company.
Stockholder Action; Special Meeting of Stockholders. Our by-laws provide that action required or permitted to be taken by our stockholders at an annual meeting of stockholders may only be taken if it is properly brought before the meeting. Our by-laws also provide that special meetings of stockholders may only be called by the chairman of our board or by a majority of our board of directors. These provisions could have the effect of delaying until the next stockholders’ meeting stockholder actions which are favored by the holders of a majority of our outstanding voting securities.
Advance Notice Requirements for Stockholder Proposals and Director Nominations. Our by-laws provide that nominations for election to our board of directors may be made either by our board of directors (or a proxy committee appointed by the board of directors) or by any stockholder who complies with specified notice provisions. Our by-laws contain similar advance notice provisions for stockholder proposals for action at stockholder meetings. These provisions prevent stockholders from making nominations for directors and stockholder proposals from the floor at any stockholder meeting and require any stockholder making a nomination or proposal to submit the name of the nominees for board seats or the stockholder proposal, together with specified information about the nominee or any stockholder proposal, prior to the meeting at which directors are to be elected or action is to be taken. These provisions ensure that stockholders have adequate time to consider nominations and proposals before action is required, and they may also have the effect of delaying stockholder action.
Limitation of Liability and Indemnification Provisions
Our certificate of incorporation contains provisions permitted under Delaware General Corporation Law relating to the liability of directors. The provisions eliminate a director’s liability for monetary damages for a breach of fiduciary duty, except to the extent that the elimination or limitation of such liability is not permitted by Delaware General Corporation Law. The limitation of liability described above does not alter the liability of our directors and officers under federal securities laws. Furthermore, our certificate of incorporation contains provisions to indemnify our directors and officers to the fullest extent permitted by Delaware General Corporation Law.
18
DESCRIPTION OF PREFERRED STOCK
The following description summarizes the general provisions of our preferred stock that we may offer from time to time. The following summary description of our preferred stock is based on the provisions of our certificate of incorporation and by-laws, which are incorporated by reference, and the applicable provisions of Delaware General Corporation Law. In connection with an offering of our preferred stock, our board will adopt a certificate of designations that sets forth the terms and conditions of the particular series of preferred stock and we will describe the specific terms of the preferred stock and the offering in a prospectus supplement. We also will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the certificate of designations. The information below, and any description of preferred stock in a prospectus supplement, is only a summary and is subject to and qualified in its entirety by reference to the certificate of designations for the particular series of preferred stock, as well as to our certificate of incorporation and by-laws and the applicable provisions of Delaware General Corporation Law.
General
Under the terms of our certificate of incorporation, our board of directors has the authority, without further action by the stockholders and subject to the limits imposed by Delaware General Corporation Law, to issue up to 10,000,000 shares of preferred stock, par value $0.001 per share, in one or more series Prior to issuance of shares of each series of our undesignated preferred stock, our board of directors will adopt resolutions and file a Certificate of Designations with the Secretary of State of the State of Delaware, fixing for each such series the designations, powers, preferences, rights, qualifications, limitations and restrictions of the shares of such series.
Subject to limitations prescribed by the Delaware General Corporation Law, our certificate of incorporation and our by-laws, our board of directors is authorized to fix the number of shares constituting each series of preferred stock and the designations, powers, preferences, rights, qualifications, limitations and restrictions of the shares of such series, including such provisions as may be desired concerning voting, redemption, dividends, dissolution or the distribution of assets, conversion or exchange, and such other subjects or matters as may be fixed by resolution of the board of directors. Each series of preferred stock that we offer under this prospectus will, when issued, be fully paid and nonassessable and will not have, or be subject to, any preemptive or similar rights.
We will describe in the applicable prospectus supplement the terms of the preferred stock being offered, including the following:
| | • | | the title and stated value of the preferred stock; |
| | • | | the number of shares of the preferred stock offered, the liquidation preference per share and the purchase price of the preferred stock; |
| | • | | the dividend rate(s), period(s) and/or payment date(s) or the method(s) of calculation for dividends, if applicable; |
| | • | | whether dividends shall be cumulative or non-cumulative and, if cumulative, the date from which dividends on the preferred stock shall accumulate; |
| | • | | the procedures for any auction and remarketing, if any, for the preferred stock; |
| | • | | the provisions for a sinking fund, if any, for the preferred stock; |
| | • | | the provisions for redemption, if applicable, of the preferred stock; |
| | • | | any listing of the preferred stock on any securities exchange or market; |
| | • | | the terms and conditions, if applicable, upon which the preferred stock will be convertible into common stock, including the conversion price (or its manner of calculation) and conversion period; |
| | • | | voting rights, if any, of the preferred stock; |
19
| | • | | a discussion of any material and/or special U.S. federal income tax considerations applicable to the preferred stock; |
| | • | | the relative ranking and preferences of the preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of our affairs; |
| | • | | any limitations on issuance of any class or series of preferred stock ranking senior to or on a parity with the preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of our affairs; and |
| | • | | any other specific terms, powers, preferences, rights, qualifications, limitations or restrictions on the preferred stock. |
Outstanding Preferred Stock
Authorized. Of the 10,000,000 shares of preferred stock we are authorized to issue, 100,000 shares have been designated as Series A preferred stock. All of the 100,000 shares of Series A preferred stock are issued and outstanding.
Voting. Each holder of shares of our Series A preferred stock is entitled to 1,000 votes per share on all matters to be voted on by holders of our common stock;provided, however, that the vote or consent of the holders of at least a majority of the shares of Series A preferred stock then outstanding is required for us to (1) authorize, create or issue, or increase the authorized number of shares of, any class or series of capital stock ranking prior to or on a parity with the Series A preferred stock either as to dividends or liquidation; (2) authorize, create or issue any class or series of our stock other than the common stock; (3) authorize any reclassification of the Series A preferred stock; (4) authorize, create or issue any securities convertible into or exercisable for capital stock prohibited by clauses (1) through (3) above; (5) amend our certificate of incorporation; or (6) enter into any disposal, merger or reorganization involving 20% of our total capitalization.
Conversion Rights. The holders of our Series A preferred stock have an optional right to convert each share of Series A preferred stock into 0.1 shares of our common stock.
Dividends. Subject to the rights of the holders of any class or series of capital stock ranking senior to or on parity with the Series A preferred stock, the holders of our Series A preferred stock have the right to receive cumulative dividends when and as declared by the board of directors.
Liquidation. The holders of our Series A preferred stock have the right to receive a liquidation preference of $1.00 per share of Series A preferred stock, plus an amount equal to all dividends accrued and unpaid, in preference to the holders of our common stock or any other class or series of capital stock ranking junior to the Series A preferred stock. This liquidation preference will be adjusted to reflect any stock dividend, stock distribution or stock split with respect to the Series A preferred stock.
Reacquired Shares. Any shares of Series A preferred stock acquired by us will be retired and not reissued.
20
DESCRIPTION OF WARRANTS
The following description summarizes the general provisions of our warrants that we may offer from time to time. In connection with an offering of warrants, our board will adopt a warrant agreement and warrant certificates that set forth the terms and conditions of the particular series of warrants and we will describe the specific terms of the warrants and the offering in a prospectus supplement. We also will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of warrant agreement and warrant certificate. The information below, and any description of warrants in a prospectus supplement, is only a summary and is subject to and qualified in its entirety by reference to the warrant agreement and warrant certificate for the particular series of warrants.
General
We may issue warrants to purchase preferred stock (“preferred stock warrants”) or common stock (“common stock warrants,” and collectively with the preferred stock warrants, “warrants”). We may issue warrants independently or together with any other securities we offer pursuant to a prospectus supplement and the warrants may be attached or separate from the securities.
We will evidence each series of warrants by warrant certificates that we will issue under a separate agreement. We may enter into an agreement with a warrant agent. If we elect to do so, the warrant agent will act solely as our agent in connection with the warrants and will not assume any obligation or relationship of agency or trust for or with any registered holders of warrants or beneficial owners of warrants. We will indicate the name and address and other information regarding the warrant agent in the applicable prospectus supplement relating to a particular series of warrants if we elect to use a warrant agent.
We will describe in the applicable prospectus supplement the terms of the preferred stock warrants and common stock warrants being offered, including the following:
| | • | | the title of the warrants; |
| | • | | the securities for which the warrants are exercisable; |
| | • | | the price or prices at which the warrants will be issued; |
| | • | | the number of the warrants issued with each share of preferred stock or common stock; |
| | • | | any provisions for adjustment of the number or amount of shares of preferred stock or common stock receivable upon exercise of the warrants or the exercise price of the warrants; |
| | • | | if applicable, the date on and after which the warrants and the related preferred stock or common stock shares will be separately transferable; |
| | • | | if applicable, a discussion of the material United Stated Federal income tax considerations applicable to the exercise of the warrants; |
| | • | | any other terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants; |
| | • | | the date on which the right to exercise the warrants will commence, and the date on which the right will expire; and |
| | • | | the maximum or minimum number of the warrants which may be exercised at any time. |
Each warrant will entitle the holder of the warrant to purchase for cash at the exercise price set forth in the applicable prospectus supplement the shares of preferred stock or common stock being offered. Holders may exercise warrants at any time up to the close of business on the expiration date set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants are void.
21
Holders may exercise warrants as set forth in the prospectus supplement relating to the warrants being offered. Upon receipt of payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the prospectus supplement, we will, as soon as practicable, forward the shares of preferred stock or common stock purchasable upon the exercise. If less than all of the warrants represented by the warrant certificate are exercised, we will issue a new warrant certificate for the remaining warrants.
Outstanding Warrants
Class A and Class B Warrants
The Class A warrants expired in January 2009.
The Class B warrants can be exercised at any time until January 25, 2011. The adjusted exercise price of the Class B warrants is $6.00 per share. The number of shares of common stock or other securities at the time issuable upon exercise of such warrants will be appropriately adjusted to reflect any stock dividend, stock split, combination of shares, reclassification, recapitalization or other similar event affecting the number of outstanding shares of stock or securities. In case of any consolidation or merger by us with or into any other corporation, entity or person, or any other corporate reorganization, in which we shall not be the continuing or surviving entity of such consolidation, merger or reorganization (any such transaction being hereinafter referred to as a “Reorganization”), then, in each case, the holder of the warrants on exercise any time after the consummation or effective date of such Reorganization (such date, the “Effective Date”), shall receive, in lieu of the shares of stock or other securities at any time issuable upon the exercise of the warrants prior to the Effective Date, the stock and other securities and property (including cash) to which such holder would have been entitled upon the Effective Date if such holder had exercised the warrants immediately prior thereto.
The Class B warrants can be exercised pursuant to a cashless exercise. Under the terms of the warrants, the holders thereof agreed not to elect for a period of one (1) year from the date of issuance a cashless exercise of the warrants and also agreed not to elect a cashless exercise so long as there is an effective registration statement for the shares underlying the respective warrants. As of December 1, 2009, there were outstanding Class B warrants to purchase up to 114,583 shares of common stock.
Class C and Class D Warrants
The Class C and Class D warrants expired in April 2009.
Stock Purchase Warrants
We issued non-denominated stock purchase warrants in connection with our November 2006 financing. These stock purchase warrants have an adjusted exercise price of $5.00 per share and expire on December 28, 2011. These stock purchase warrants can be exercised on a cashless basis. As of December 1, 2009, there were outstanding non-denominated stock purchase warrants to purchase up to 30,000 shares of our common stock.
22
DESCRIPTION OF UNITS
The following description summarizes the general provisions of our units that we may offer from time to time. In connection with an offering of units, our board will adopt a unit agreement and unit certificates that set forth the terms and conditions of the particular series of units and we will describe the specific terms of the units and the offering in a prospectus supplement. We also will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of unit agreement and unit certificate. The information below, and any description of units in a prospectus supplement, is only a summary and is subject to and qualified in its entirety by reference to the unit agreement and unit certificate for the particular series of units.
General
We may issue, in one more series, units comprised of shares of our common stock or preferred stock and warrants to purchase common stock or preferred stock or any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
We may evidence units by unit certificates that we issue under a separate agreement. We may issue the units under an agreement between us and one or more unit agents. If we elect to enter into an agreement with a unit agent, the unit agent will act solely as our agent in connection with the units and will not assume any obligation or relationship of agency or trust for or with any registered holders of units or beneficial owners of units. We will indicate the name and address and other information regarding the unit agent in the applicable prospectus supplement relating to a particular series of units if we elect to use a unit agent.
We will describe in the applicable prospectus supplement the terms of the series of units being offered, including:
| | • | | the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| | • | | any provisions of the governing unit agreement that differ from those described below; and |
| | • | | any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units. |
23
PLAN OF DISTRIBUTION
We may sell the securities offered through this prospectus: (i) to or through underwriters or dealers, (ii) directly to purchasers, including our affiliates, (iii) through agents, (iv) through a block trade in which the broker or dealer engaged to handle the block will attempt to sell the securities as agent, but may position and resell a portion of the block as principal to facilitate the transaction, or (v) through a combination of any of these methods. In addition, we may issue the securities being offered by this prospectus as a dividend or distribution. The securities may be distributed at a fixed price or prices, which may be changed, market prices prevailing at the time of sale, prices related to the prevailing market prices, or negotiated prices. The prospectus supplement will describe the terms of the offering, including the following information:
| | • | | the type and amount of securities we are offering; |
| | • | | the terms of the offering; |
| | • | | any securities exchanges on which the securities may be listed; |
| | • | | the names of any underwriters, agents or dealers; |
| | • | | the name or names of any managing underwriter or underwriters; |
| | • | | the method of distribution of the securities we are offering; |
| | • | | the purchase price of the securities; |
| | • | | the net proceeds from the sale of the securities; |
| | • | | any delayed delivery arrangements; |
| | • | | any over-allotment options under which underwriters may purchase additional securities from us; |
| | • | | any underwriting discounts and commissions or agency fees and commissions and other items constituting underwriters’ or agents’ compensation; |
| | • | | any initial public offering price; and |
| | • | | any discounts or concessions allowed or reallowed or paid to dealers. |
Sales through Underwriters or Dealers
If underwriters are used in the sale, the underwriters will acquire the securities for their own account, including through underwriting, purchase, security lending or repurchase agreements with us. The underwriters may resell the securities from time to time in one or more transactions, including negotiated transactions. Underwriters may sell the securities in order to facilitate transactions in any of our other securities (described in this prospectus or otherwise), including other public or private transactions and short sales. Underwriters may offer securities to the public either through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters. Unless otherwise indicated in the prospectus supplement, the obligations of the underwriters to purchase the securities will be subject to certain conditions, and the underwriters will be obligated to purchase all the offered securities if they purchase any of them. The underwriters may change from time to time any initial public offering price and any discounts or concessions allowed or reallowed or paid to dealers.
If dealers are used in the sale of securities offered through this prospectus, we or an underwriter will sell the securities to them as principals. They may then resell the securities to the public at varying prices determined by the dealers at the time of resale. The prospectus supplement will include the names of the dealers and the terms of the transaction.
24
Direct Sales and Sales through Agents
We may sell the securities offered through this prospectus directly. In this case, no underwriters or agents would be involved. Such securities may also be sold through agents designated from time to time. The prospectus supplement will name any agent involved in the offer or sale of the offered securities and will describe any commissions payable to the agent. Unless otherwise indicated in the prospectus supplement, any agent will agree to use its reasonable best efforts to solicit purchases for the period of its appointment.
We may sell the securities directly to institutional investors or others who may be deemed to be underwriters within the meaning of the Securities Act with respect to any sale of those securities. The terms of any such sales will be described in the prospectus supplement.
Delayed Delivery Contracts
If the prospectus supplement indicates, we may authorize agents, underwriters or dealers to solicit offers from certain types of institutions to purchase securities at the public offering price under delayed delivery contracts. These contracts would provide for payment and delivery on a specified date in the future. The contracts would be subject only to those conditions described in the prospectus supplement. The applicable prospectus supplement will describe the commission payable for solicitation of those contracts.
Market Making, Stabilization and Other Transactions
Our common stock is listed on the NYSE Amex. Any common stock sold pursuant to a prospectus supplement will be eligible for listing and trading on the NYSE Amex, subject to official notice of issuance. Unless the applicable prospectus supplement states otherwise, each other class or series of securities issued will be a new issue and will have no established trading market. We may elect to list any other class or series of securities on an exchange, but we are not currently obligated to do so. Any underwriters that we use in the sale of offered securities may make a market in such securities, but may discontinue such market making at any time without notice. Therefore, we cannot assure you that the securities will have a liquid trading market.
Any underwriter also may engage in stabilizing transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Stabilizing transactions involve bids to purchase the underlying security in the open market for the purpose of pegging, fixing or maintaining the price of the securities. Syndicate covering transactions involve purchases of the securities in the open market after the distribution has been completed in order to cover syndicate short positions.
Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a syndicate covering transaction to cover syndicate short positions. Stabilizing transactions, syndicate covering transactions and penalty bids may cause the price of the securities to be higher than it would be in the absence of the transactions. The underwriters may, if they commence these transactions, discontinue them at any time.
The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. We make no representation or prediction as to the direction or magnitude of any effect that the transactions described above, if implemented, may have on the price of our securities.
Derivative Transactions and Hedging
We, the underwriters or other agents may engage in derivative transactions involving the securities. These derivatives may consist of short sale transactions and other hedging activities. The underwriters or agents may acquire a long or short position in the securities, hold or resell securities acquired and purchase options or futures on the securities and other derivative instruments with returns linked to or related to changes in the price of the securities. In order to facilitate these derivative transactions, we may enter into security lending or repurchase
25
agreements with the underwriters or agents. The underwriters or agents may effect the derivative transactions through sales of the securities to the public, including short sales, or by lending the securities in order to facilitate short sale transactions by others. The underwriters or agents also may use the securities purchased or borrowed from us or others (or, in the case of derivatives, securities received from us in settlement of those derivatives) to directly or indirectly settle sales of the securities or close out any related open borrowings of the securities.
Electronic Auctions
We also may make sales through the Internet or through other electronic means. Since we may from time to time elect to offer securities directly to the public, with or without the involvement of agents, underwriters or dealers, utilizing the Internet or other forms of electronic bidding or ordering systems for the pricing and allocation of such securities, you will want to pay particular attention to the description of that system we will provide in a prospectus supplement.
Such electronic system may allow bidders to directly participate, through electronic access to an auction site, by submitting conditional offers to buy that are subject to acceptance by us, and which may directly affect the price or other terms and conditions at which such securities are sold. These bidding or ordering systems may present to each bidder, on a so-called “real-time” basis, relevant information to assist in making a bid, such as the clearing spread at which the offering would be sold, based on the bids submitted, and whether a bidder’s individual bids would be accepted, prorated or rejected. Of course, many pricing methods can and may also be used.
Upon completion of such an electronic auction process, securities will be allocated based on prices bid, terms of bid or other factors. The final offering price at which securities would be sold and the allocation of securities among bidders would be based in whole or in part on the results of the Internet or other electronic bidding process or auction.
General Information
Agents, underwriters, and dealers may be entitled, under agreements entered into with us, to indemnification by us against specified liabilities, including liabilities under the Securities Act, or to contribution by us to payments they may be required to make in respect to such liabilities. The applicable prospectus supplement will describe the terms and conditions of indemnification or contribution. Some of our agents, underwriters, and dealers, or their affiliates, may be customers of, engage in transactions with or perform services for us, in the ordinary course of business. We will describe in the prospectus supplement the nature of any such relationship and the name of the parties involved. Any lockup arrangements will be set forth in the applicable prospectus supplement.
26
WHERE YOU CAN FIND MORE INFORMATION
We file annual reports, quarterly reports, current reports, proxy statements and other information with the SEC. You may read and copy any of our SEC filings at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may call the SEC at 1-800-SEC-0330 for further information about the Public Reference Room. Our SEC filings also are available to the public on the SEC’s web site at www.sec.gov.
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference” information from some of our other SEC filings. This means that we can disclose information to you by referring you to those other filings, and the information incorporated by reference is considered to be part of this prospectus. In addition, some information that we file with the SEC after the date of this prospectus will automatically update, and in some cases supersede, the information contained or otherwise incorporated by reference in this prospectus. The following documents, which we filed with the SEC, are incorporated by reference in this prospectus:
| | (a) | Our annual report on Form 10-K for the fiscal year ended December 31, 2008 (as filed on March 12, 2009); |
| | (b) | Our Amendment No. 1 on Form 10-K/A for the fiscal year ended December 31, 2008 (as filed on June 9, 2009); |
| | (c) | Our Amendment No. 2 on Form 10-K/A for the fiscal year ended December 31, 2008 (as filed on October 16, 2009); |
| | (d) | Our quarterly report on Form 10-Q for the fiscal quarter ended March 31, 2009 (as filed on May 11, 2009); |
| | (e) | Our quarterly report on Form 10-Q for the fiscal quarter ended June 30, 2009 (as filed on August 10, 2009); |
| | (f) | Our quarterly report on Form 10-Q for the fiscal quarter ended September 30, 2009 (as filed on November 9, 2009); |
| | (g) | Our current reports on Form 8-K filed on June 15, 2009, June 17, 2009, June 30, 2009, August 17, 2009 and October 22, 2009; and |
| | (h) | The description of our common stock contained in our registration statement on Form 8-A filed on May 16, 2007 with the SEC under Section 12 of the Exchange Act, including any amendment or report filed for the purpose of updating such description. |
Also incorporated by reference into this prospectus are all documents that we may file with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act after the date of filing of this registration statement and prior to the termination of the offering of the securities to which this prospectus relates. These documents include periodic reports, such as annual reports on Form 10-K, quarterly reports on Form 10-Q, and current reports on Form 8-K, as well as proxy statements. Pursuant to General Instruction B of Form 8-K, any information submitted under Item 2.02, Results of Operations and Financial Condition, or Item 7.01, Regulation FD Disclosure, of Form 8-K is not deemed to be “filed” for the purpose of Section 18 of the Exchange Act, and we are not subject to the liabilities of Section 18 with respect to information submitted under Item 2.02 or Item 7.01 of Form 8-K. We are not incorporating by reference any information submitted under Item 2.02 or Item 7.01 of Form 8-K into any filing under the Securities Act or the Exchange Act or into this prospectus. Any statement contained herein or in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement.
27
You may request copies of these filings, at no cost, by writing to or calling our Investor Relations department at:
HQ Sustainable Maritime Industries, Inc.
Melbourne Towers, 1511 Third Avenue
Suite 788
Seattle, WA 98101
Telephone: (206) 621-9888
LEGAL MATTERS
The validity of the securities offered by this prospectus have been passed upon for us by Jill Arlene Robbins, P.A.
EXPERTS
The consolidated financial statements for the year ended December 31, 2008, incorporated in this prospectus by reference from our Annual Report on Form 10-K, and the effectiveness of our internal control over financial reporting have been audited by Schwartz Levitsky Feldman LLP, an independent registered public accounting firm, as stated in their reports which are incorporated herein by reference. Such consolidated financial statements have been so incorporated in reliance upon the reports of such firm given upon their authority as experts in accounting and auditing.
The consolidated financial statements for the years ended December 31, 2007 and 2006 have been audited by EFP Rotenberg, LLP as successor to Rotenberg & Co., LLP, an independent registered public accounting firm, as stated in their report which is incorporated herein by reference. Such consolidated financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
28
[ ] Shares of Common Stock
Warrants to Purchase up to [ ] Shares of Common Stock

HQ SUSTAINABLE MARITIME INDUSTRIES, INC.
Common Stock
Warrants
PROSPECTUS SUPPLEMENT
August [ ], 2010

