UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
| | | |
[ X ] | | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the quarterly period ended March 31, 2004
OR
| | | |
[ ] | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the transition period from to .
Commission File No. 0-18734
AVANIR PHARMACEUTICALS
(Exact name of registrant as specified in its charter)
| | | |
| California | | 33-0314804 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
| 11388 Sorrento Valley Road, San Diego, California | | 92121 |
| (Address of principal executive offices) | | (Zip Code) |
(858) 622-5200
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter periods that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES [X] NO [ ]
Indicate by check mark whether the registrant is an accelerated filer (as defined in Rule 12b-2 of the Exchange Act). YES [X] NO [ ]
As of May 5, 2004, the registrant had 71,494,608 shares of Class A common stock issued and outstanding.
PART I. FINANCIAL INFORMATION
Item 1. FINANCIAL STATEMENTS
INDEPENDENT ACCOUNTANTS’ REPORT
Board of Directors and Shareholders
AVANIR Pharmaceuticals
We have reviewed the accompanying consolidated balance sheet of AVANIR Pharmaceuticals and subsidiary (the “Company”) as of March 31, 2004 and the related consolidated statements of operations and cash flows for the three-month and six-month periods ended March 31, 2004 and 2003. These interim financial statements are the responsibility of the Company’s management.
We conducted our review in accordance with standards established by the American Institute of Certified Public Accountants. A review of interim financial information consists principally of applying analytical procedures to financial data and of making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with auditing standards generally accepted in the United States of America, the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion.
Based on our review, we are not aware of any material modifications that should be made to such consolidated interim financial statements for them to be in conformity with accounting principles generally accepted in the United States of America.
We have previously audited, in accordance with auditing standards generally accepted in the United States of America, the consolidated balance sheet of the Company as of September 30, 2003 and the related consolidated statements of operations, shareholders’ equity and cash flows for the year then ended (not presented herein); and in our report dated December 19, 2003, we expressed an unqualified opinion on those financial statements. In our opinion, the information set forth in the accompanying consolidated balance sheet as of September 30, 2003 is fairly stated, in all material respects, in relation to the consolidated balance sheet from which it has been derived.
DELOITTE & TOUCHE LLP
San Diego, California
May 14, 2004
3
AVANIR Pharmaceuticals
CONSOLIDATED BALANCE SHEETS (UNAUDITED)
| | | | | | | | | |
| | | March 31, | | September 30, |
| | | 2004
| | 2003
|
ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 7,910,493 | | | $ | 12,198,408 | |
| Short-term investments in securities | | | 4,565,121 | | | | 3,888,798 | |
| Accounts receivable | | | 178,900 | | | | 271,681 | |
| Inventory | | | 12,404 | | | | 210,854 | |
| Prepaid expenses | | | 1,193,814 | | | | 1,564,315 | |
| | | |
| | | |
| |
| Total current assets | | | 13,860,732 | | | | 18,134,056 | |
| Investments in securities | | | 103,514 | | | | 513,486 | |
| Restricted investments in securities | | | 856,597 | | | | 856,597 | |
| Property and equipment, net | | | 7,056,976 | | | | 7,742,005 | |
| Intangible assets, net | | | 2,650,615 | | | | 2,131,209 | |
| Other assets | | | 257,965 | | | | 267,904 | |
| | | |
| | | |
| |
| TOTAL ASSETS | | $ | 24,786,399 | | | $ | 29,645,257 | |
| | | |
| | | |
| |
LIABILITIES AND SHAREHOLDERS’ EQUITY (DEFICIT) | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 2,969,819 | | | $ | 2,853,604 | |
| Accrued expenses and other liabilities | | | 3,045,386 | | | | 1,806,058 | |
| Accrued compensation and payroll taxes | | | 697,781 | | | | 626,154 | |
| Notes payable | | | 150,964 | | | | 244,805 | |
| Current portion of deferred revenue | | | 1,755,768 | | | | 1,841,865 | |
| Current portion of capital lease obligations | | | 150,348 | | | | 142,354 | |
| | | |
| | | |
| |
| Total current liabilities | | | 8,770,066 | | | | 7,514,840 | |
| Deferred revenue, net of current portion | | | 19,906,368 | | | | 20,900,776 | |
| Capital lease obligations, net of current portion | | | 115,113 | | | | 192,410 | |
| | | |
| | | |
| |
| Total liabilities | | | 28,791,547 | | | | 28,608,026 | |
| | | |
| | | |
| |
| Contingencies (Note 13) | | | | | | | | |
| Shareholders’ equity (deficit): | | | | | | | | |
| Preferred stock — no par value, 10,000,000 and 9,999,500 shares authorized as of March 31, 2004 and September 30, 2003, respectively: | | | | | | | | |
| Series C Junior Participating - 1,000,000 shares authorized; no shares issued or outstanding | | | — | | | | — | |
| Common stock — no par value: | | | | | | | | |
| Class A - 200,000,000 and 99,288,000 shares authorized as of March 31, 2004 and September 30, 2003, respectively; 71,494,608 and 65,816,434 shares issued and outstanding as of March 31, 2004 and September 30, 2003, respectively | | | 105,253,977 | | | | 97,286,433 | |
| Class B — None and 712,000 shares authorized as of March 31, 2004 and September 30, 2003, respectively; none and 13,500 shares issued and outstanding as of March 31, 2004 and September 30, 2003, respectively (convertible into Class A common stock) | | | — | | | | 8,395 | |
| Accumulated deficit | | | (109,221,816 | ) | | | (96,251,049 | ) |
| Accumulated other comprehensive loss | | | (37,309 | ) | | | (6,548 | ) |
| | | |
| | | |
| |
| Total shareholders’ equity (deficit) | | | (4,005,148 | ) | | | 1,037,231 | |
| | | |
| | | |
| |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY (DEFICIT) | | $ | 24,786,399 | | | $ | 29,645,257 | |
| | | |
| | | |
| |
See notes to consolidated financial statements.
4
AVANIR Pharmaceuticals
CONSOLIDATED STATEMENTS OF OPERATIONS (UNAUDITED)
| | | | | | | | | | | | | | | | | |
| | | Three months ended | | Six months ended |
| | | March 31,
| | March 31,
|
| | | 2004
| | 2003
| | 2004
| | 2003
|
REVENUES: | | | | | | | | | | | | | | | | |
| Research contracts and licenses | | $ | 267,500 | | | $ | — | | | $ | 270,500 | | | $ | 50,000 | |
| Royalties and sale of royalty rights (Note 7) | | | 347,017 | | | | 481,450 | | | | 885,513 | | | | 1,074,745 | |
| Government research grants | | | 241,320 | | | | 140,193 | | | | 439,531 | | | | 298,604 | |
| Product sales | | | — | | | | — | | | | 769,938 | | | | 17,400 | |
| | | |
| | | |
| | | |
| | | |
| |
| Total revenues | | | 855,837 | | | | 621,643 | | | | 2,365,482 | | | | 1,440,749 | |
| | | |
| | | |
| | | |
| | | |
| |
OPERATING EXPENSES: | | | | | | | | | | | | | | | | |
| Research and development | | | 5,372,641 | | | | 4,860,280 | | | | 10,736,324 | | | | 8,471,902 | |
| General and administrative | | | 1,510,332 | | | | 1,210,567 | | | | 2,918,228 | | | | 2,289,513 | |
| Sales and marketing | | | 722,086 | | | | 606,339 | | | | 1,580,597 | | | | 1,031,468 | |
| Cost of product sales | | | — | | | | — | | | | 210,090 | | | | 3,102 | |
| | | |
| | | |
| | | |
| | | |
| |
| Total operating expenses | | | 7,605,059 | | | | 6,677,186 | | | | 15,445,239 | | | | 11,795,985 | |
| | | |
| | | |
| | | |
| | | |
| |
LOSS FROM OPERATIONS | | | (6,749,222 | ) | | | (6,055,543 | ) | | | (13,079,757 | ) | | | (10,355,236 | ) |
| Interest income | | | 56,726 | | | | 85,128 | | | | 112,268 | | | | 153,150 | |
| Other income | | | 11,722 | | | | 6,013 | | | | 19,369 | | | | 11,859 | |
| Interest expense | | | (9,719 | ) | | | (12,761 | ) | | | (20,527 | ) | | | (23,335 | ) |
| | | |
| | | |
| | | |
| | | |
| |
LOSS BEFORE INCOME TAXES | | | (6,690,493 | ) | | | (5,977,163 | ) | | | (12,968,647 | ) | | | (10,213,562 | ) |
| Provision for income taxes | | | (62 | ) | | | (771 | ) | | | (2,120 | ) | | | (2,371 | ) |
| | | |
| | | |
| | | |
| | | |
| |
NET LOSS | | $ | (6,690,555 | ) | | $ | (5,977,934 | ) | | $ | (12,970,767 | ) | | $ | (10,215,933 | ) |
| | | |
| | | |
| | | |
| | | |
| |
NET LOSS ATTRIBUTABLE TO COMMON SHAREHOLDERS: | | | | | | | | | | | | | | | | |
| Net loss | | $ | (6,690,555 | ) | | $ | (5,977,934 | ) | | $ | (12,970,767 | ) | | $ | (10,215,933 | ) |
| Dividends on redeemable convertible preferred stock | | | — | | | | (6,250 | ) | | | — | | | | (12,500 | ) |
| Accretion of discount related to redeemable convertible preferred stock | | | — | | | | (4,509 | ) | | | — | | | | (9,118 | ) |
| | | |
| | | |
| | | |
| | | |
| |
NET LOSS ATTRIBUTABLE TO COMMON SHAREHOLDERS | | $ | (6,690,555 | ) | | $ | (5,988,693 | ) | | $ | (12,970,767 | ) | | $ | (10,237,551 | ) |
| | | |
| | | |
| | | |
| | | |
| |
NET LOSS PER SHARE: | | | | | | | | | | | | | | | | |
BASIC AND DILUTED | | $ | (0.09 | ) | | $ | (0.10 | ) | | $ | (0.18 | ) | | $ | (0.18 | ) |
| | | |
| | | |
| | | |
| | | |
| |
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | | | | | | | | | | | | | | | | |
BASIC AND DILUTED | | | 71,285,690 | | | | 58,352,406 | | | | 71,248,769 | | | | 58,324,174 | |
| | | |
| | | |
| | | |
| | | |
| |
See notes to consolidated financial statements.
5
AVANIR Pharmaceuticals
CONSOLIDATED STATEMENTS OF CASH FLOWS (UNAUDITED)
| | | | | | | | | |
| | | Six Months Ended |
| | | March 31,
|
| | | 2004
| | 2003
|
OPERATING ACTIVITIES: | | | | | | | | |
| Net loss | | $ | (12,970,767 | ) | | $ | (10,215,933 | ) |
| Adjustments to reconcile net loss to net cash provided by (used for) operating activities: | | | | | | | | |
| Depreciation and amortization | | | 794,261 | | | | 383,954 | |
| Compensation paid with stock options | | | 29,471 | | | | 181,706 | |
| Loss on disposal of assets | | | — | | | | 1,100 | |
| Changes in assets and liabilities: | | | | | | | | |
| Accounts receivable | | | 92,781 | | | | 880,429 | |
| Inventory | | | 198,450 | | | | 3,102 | |
| Prepaid expenses and other assets | | | 380,440 | | | | (264,070 | ) |
| Accounts payable | | | 116,215 | | | | 230,861 | |
| Accrued expenses and other liabilities | | | 1,239,328 | | | | (61,438 | ) |
| Accrued compensation and payroll taxes | | | 71,627 | | | | 111,188 | |
| Deferred revenue | | | (1,080,505 | ) | | | 19,588,555 | |
| | | |
| | | |
| |
| Net cash provided by (used for) operating activities | | | (11,128,699 | ) | | | 10,839,454 | |
| | | |
| | | |
| |
INVESTING ACTIVITIES: | | | | | | | | |
| Investments in securities | | | (997,112 | ) | | | (706,382 | ) |
| Proceeds from sales and maturities of investments in securities | | | 700,000 | | | | 2,100,000 | |
| Patent costs | | | (556,852 | ) | | | (234,306 | ) |
| Construction in progress | | | — | | | | (2,986,803 | ) |
| Purchases of property and equipment | | | (71,786 | ) | | | (782,525 | ) |
| | | |
| | | |
| |
| Net cash used for investing activities | | | (925,750 | ) | | | (2,610,016 | ) |
| | | |
| | | |
| |
FINANCING ACTIVITIES: | | | | | | | | |
| Proceeds from issuance of common stock and warrants | | | 7,929,678 | | | | 85,350 | |
| Dividends paid on preferred stock | | | — | | | | (12,500 | ) |
| Proceeds from issuance of notes payable | | | 159,918 | | | | — | |
| Payments on notes payable and capital lease obligations | | | (323,062 | ) | | | (279,951 | ) |
| | | |
| | | |
| |
| Net cash provided by (used for) financing activities | | | 7,766,534 | | | | (207,101 | ) |
| | | |
| | | |
| |
| Net increase (decrease) in cash and cash equivalents | | | (4,287,915 | ) | | | 8,022,337 | |
| Cash and cash equivalents at beginning of period | | | 12,198,408 | | | | 8,630,547 | |
| | | |
| | | |
| |
| Cash and cash equivalents at end of period | | $ | 7,910,493 | | | $ | 16,652,884 | |
| | | |
| | | |
| |
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | | | | |
| Interest paid | | $ | 20,527 | | | $ | 23,335 | |
| Income taxes paid | | $ | 2,120 | | | $ | 2,371 | |
See notes to consolidated financial statements.
6
AVANIR Pharmaceuticals
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
1. BASIS OF PRESENTATION
AVANIR Pharmaceuticals (“AVANIR,” “we” or the “Company”) has prepared the unaudited consolidated financial statements in this quarterly report in accordance with the instructions to Form 10-Q adopted under the Securities Exchange Act of 1934. Certain information and note disclosures normally included in consolidated financial statements prepared in accordance with accounting principles generally accepted in the United States have been condensed or omitted pursuant to such rules and regulations. These unaudited consolidated financial statements should be read with our audited consolidated financial statements and the accompanying notes included in our Annual Report on Form 10-K for the fiscal year ended September 30, 2003. In our opinion, all adjustments (consisting only of normal recurring adjustments) necessary to present a fair statement of our financial position as of March 31, 2004, and the results of operations for the three-month and six-month periods ended March 31, 2004 and 2003, have been made. The results of operations for the three-month and six-month periods ended March 31, 2004 are not necessarily indicative of the results for the fiscal year ending September 30, 2004 or any future periods.
2. STOCK-BASED COMPENSATION
Statement of Financial Accounting Standards No. 123 (“SFAS No. 123”) encourages, but does not require, companies to record compensation cost for stock-based employee compensation at fair value. The Company has chosen to continue to account for stock-based compensation using the intrinsic value method prescribed in Accounting Principles Board (“APB”) Opinion No. 25, “Accounting for Stock Issued to Employees,” and related interpretations for all periods presented. Accordingly, compensation cost for stock options is measured as the excess, if any, of the fair value of the Company’s stock at the date of the grant over the amount an employee must pay to acquire the stock.
If compensation cost had been determined based upon the fair value at the grant date for awards under the stock option plans consistent with the methodology prescribed under SFAS No. 123, the Company’s net loss would have been higher by approximately $262,000 and $552,000 for the three-month and six-month periods ended March 31, 2004 and approximately $395,000 and $868,000 for the three-month and six-month periods ended 2003, respectively, as summarized in the following table.
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended March 31,
| | Six Months Ended March 31,
|
| | | 2004
| | 2003
| | 2004
| | 2003
|
| Net loss attributable to common shareholders, as reported | | $ | (6,690,555 | ) | | $ | (5,988,693 | ) | | $ | (12,970,767 | ) | | $ | (10,237,551 | ) |
| Add: Stock-based employee compensation included in reported net loss attributable to common shareholders | | | 12,227 | | | | 132,570 | | | | 24,069 | | | | 175,743 | |
| Deduct: Total stock-based employee compensation expense determined under fair value based method for all awards | | | (274,054 | ) | | | (527,883 | ) | | | (576,106 | ) | | | (1,043,735 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| Pro forma net loss attributable to common shareholders | | $ | (6,952,382 | ) | | $ | (6,384,006 | ) | | $ | (13,522,804 | ) | | $ | (11,105,543 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| Net loss per share: | | | | | | | | | | | | | | | | |
| Basic and diluted — as reported | | $ | (0.09 | ) | | $ | (0.10 | ) | | $ | (0.18 | ) | | $ | (0.18 | ) |
| Basic and diluted — pro forma | | $ | (0.10 | ) | | $ | (0.11 | ) | | $ | (0.19 | ) | | $ | (0.19 | ) |
The Company accounts for stock options granted to non-employees using the fair value method. Compensation expense for options granted to non-employees has been determined in accordance with
7
Emerging Issues Task Force (“EITF”) No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services,” as the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. For the purpose of determining compensation expense for stock options granted to non-employees, all of the Company’s directors are considered to be employees.
3. RECLASSIFICATIONS
Certain amounts from prior periods have been reclassified to conform to the current period presentation.
4. BALANCE SHEET DETAILS
The following tables provide details of selected balance sheet items.
Property and equipment.Property and equipment consist of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | March 31, 2004
| | September 30, 2003
|
| | | Gross | | | | | | | | | | Gross | | | | |
| | | Carrying | | Accumulated | | | | | | Carrying | | Accumulated | | |
| | | Value
| | Depreciation
| | Net
| | Value
| | Depreciation
| | Net
|
| Research and development equipment | | $ | 3,597,372 | | | $ | (1,657,429 | ) | | $ | 1,939,943 | | | $ | 3,544,343 | | | $ | (1,331,544 | ) | | $ | 2,212,799 | |
| Computer equipment and related software | | | 997,979 | | | | (466,569 | ) | | | 531,410 | | | | 1,000,206 | | | | (383,512 | ) | | | 616,694 | |
| Leasehold improvements | | | 5,018,345 | | | | (746,057 | ) | | | 4,272,288 | | | | 5,000,147 | | | | (445,435 | ) | | | 4,554,712 | |
| Office equipment, furniture and fixtures | | | 552,650 | | | | (239,315 | ) | | | 313,335 | | | | 549,864 | | | | (192,064 | ) | | | 357,800 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total property and equipment | | $ | 10,166,346 | | | $ | (3,109,370 | ) | | $ | 7,056,976 | | | $ | 10,094,560 | | | $ | (2,352,555 | ) | | $ | 7,742,005 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Depreciation expense related to property and equipment was approximately $381,000 and $757,000 for the three-month and six-month periods ended March 31, 2004 and $171,000 and $342,000 for the same periods, respectively, in the prior year.
Intangible assets.Intangible assets with indefinite useful lives consist of costs of trademarks for AVANIR and Xenerex and similar names intended for use or potential use in the United States and the rest of the world. The Company reviews intangible assets with indefinite useful lives at least annually for impairment, following the guidelines established in Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (SFAS No. 142). There were no impairments to trademarks during the three-month and six-month periods ended March 31, 2004 and 2003. Intangible assets with finite lives are amortized over their useful lives and are evaluated for impairment whenever events or changes in circumstances indicate that their carrying value may not be recoverable under SFAS No. 144, “Accounting for Impairment or Disposal of Long-lived Assets.” AVANIR’s amortizable intangible assets consist of the costs of patents, patent applications, and licenses.
8
Intangible assets, consisting of both intangible assets with finite and indefinite useful lives, are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | March 31, 2004
| | September 30, 2003
|
| | | Gross | | | | | | | | | | Gross | | | | |
| | | Carrying | | Accumulated | | | | | | Carrying | | Accumulated | | |
| | | Value
| | Amortization
| | Net
| | Value
| | Amortization
| | Net
|
| Intangible assets with finite lives: | | | | | | | | | | | | | | | | | | | | | | | | |
| Patent applications pending | | $ | 1,966,276 | | | $ | — | | | $ | 1,966,276 | | | $ | 1,464,859 | | | $ | — | | | $ | 1,464,859 | |
| Patents | | | 908,633 | | | | (290,970 | ) | | | 617,663 | | | | 855,799 | | | | (254,657 | ) | | | 601,142 | |
| Licenses | | | 42,461 | | | | (14,515 | ) | | | 27,946 | | | | 42,461 | | | | (13,383 | ) | | | 29,078 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total intangible assets with finite lives | | | 2,917,370 | | | | (305,485 | ) | | | 2,611,885 | | | | 2,363,119 | | | | (268,040 | ) | | | 2,095,079 | |
| Intangible assets with indefinite useful lives | | | 38,730 | | | | — | | | | 38,730 | | | | 36,130 | | | | — | | | | 36,130 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total intangible assets | | $ | 2,956,100 | | | $ | (305,485 | ) | | $ | 2,650,615 | | | $ | 2,399,249 | | | $ | (268,040 | ) | | $ | 2,131,209 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Amortization expense related to amortizable intangible assets was approximately $20,000 and $37,000 for the three-month and six-month periods ended March 31, 2004 and $20,000 and $36,000 for the same periods, respectively, in the prior year. Based solely on the amortizable intangible assets as of March 31, 2004, the estimated annual amortization expense of intangible assets for the fiscal years ending September 30 is shown in the following table. Actual amortization expense to be reported in future periods could differ from these estimates as a result of acquisitions, divestitures, and other relevant factors.
| | | | | |
Amortization Expense
|
Fiscal year ending September 30,
2004 (Remaining six months) | | $ | 37,178 | |
| 2005 | | | 72,182 | |
| 2006 | | | 72,188 | |
| 2007 | | | 70,360 | |
| 2008 | | | 70,515 | |
| Thereafter | | | 323,186 | |
| | | |
| |
| Subtotal | | | 645,609 | |
| Patent applications pending | | | 1,966,276 | |
| | | |
| |
| Total | | $ | 2,611,885 | |
| | | |
| |
5. USE OF ESTIMATES
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
6. INVENTORY
Inventory is stated at the lower of cost (first-in, first-out) or market. Inventory consists of only the raw material docosanol, which is the active ingredient in docosanol 10% cream. Docosanol in its present form as stored by the Company has a substantial shelf life, a relatively stable value and long-term use, and carries a low risk of becoming excess inventory or obsolete. AVANIR does not own or store any docosanol 10% cream in its finished product form. The Company and one of its licensees receive raw
9
materials from a single supplier. AVANIR also supplies several other licensees with raw materials from the same supplier. The inability of a sole supplier to fulfill supply requirements of the Company or its licensees could materially impact future operating results.
7. DEFERRED REVENUE
On December 24, 2002, AVANIR sold an undivided interest in its Abreva® license agreement with SB Pharmco Puerto Rico, Inc. (“SB” or “GlaxoSmithKline”) to Drug Royalty USA, Inc. (“Drug Royalty USA”) for $24.1 million. We recorded the net proceeds of the transaction as deferred revenue, to be recognized as revenue over the life of the license agreement, because of our ongoing involvement in earning future revenues under our license agreement with SB. The following table sets forth the deferred revenue balances for the Drug Royalty USA agreement and other agreements as of March 31, 2004 and September 30, 2003. The portion of deferred revenue classified as a current liability represents the amount AVANIR expects to realize as revenue within the next 12 months.
| | | | | | | | | | | | | |
| | | Drug Royalty | | Other | | |
| | | USA Agreement
| | Agreements
| | Total
|
| Deferred revenue as of September 30, 2003 | | $ | 22,509,308 | | | $ | 233,333 | | | $ | 22,742,641 | |
| Changes during period: | | | | | | | | | | | | |
| Additions to deferred revenue | | | — | | | | 50,000 | | | | 50,000 | |
| Recognized as revenue during period | | | (880,505 | ) | | | (250,000 | ) | | | (1,130,505 | ) |
| | | |
| | | |
| | | |
| |
| Deferred revenue as of March 31, 2004 | | $ | 21,628,803 | | | $ | 33,333 | | | $ | 21,662,136 | |
| | | |
| | | |
| | | |
| |
| Classified as: | | | | | | | | | | | | |
| Current portion of deferred revenue | | $ | 1,722,435 | | | $ | 33,333 | | | $ | 1,755,768 | |
| Deferred revenue, net of current portion | | | 19,906,368 | | | | — | | | | 19,906,368 | |
| | | |
| | | |
| | | |
| |
| Total deferred revenue | | $ | 21,628,803 | | | $ | 33,333 | | | $ | 21,662,136 | |
| | | |
| | | |
| | | |
| |
8. REVENUE RECOGNITION
Performance-based license fees.We recognize revenues from license fees when the performance requirements have been met, the fee is fixed or determinable, and collection of fees is probable. We defer revenues and recognize them ratably over the life of the agreement when we have continuing obligations to perform under the agreement.
Royalty revenues.We recognize royalty revenues from license agreements when earned. Net sales figures used for calculating royalties due the Company can often include deductions for costs of unsaleable returns, managed care charge-backs, cash discounts, freight and warehousing, and miscellaneous write-offs, which may vary over the course of the license agreement.
Revenues from sale of royalty rights.In agreements where we have sold our rights to future royalties under license agreements and we maintain continuing involvement in earning such royalties, we defer revenues and recognize them over the life of the license agreement. For example, in the sale of an undivided interest of our Abreva license agreement to Drug Royalty USA, revenue recognition is being determined under the units-of-revenue method. Under this method, the amount of deferred revenue to be recognized as revenue in each period is calculated by multiplying (i) the ratio of the royalty payments due to Drug Royalty USA for the period to the total remaining royalties that we expect SB will pay Drug Royalty USA over the term of the agreement, by (ii) the unamortized deferred revenue amount.
Revenue arrangements with multiple deliverables.In certain circumstances, we enter into revenue arrangements whereby we are obligated to deliver to the customer multiple products and/or services (multiple deliverables). Such arrangements could include antibody generation services agreements and other forms of research collaborations. In these transactions, we allocate the total revenue to be earned under the arrangement among the various elements based on their relative fair value. In the case of
10
antibody generation services, the allocation is based on objective, customer-specific evidence of fair value. We recognize revenue related to the delivered products or services only if: (i) the above performance or service criteria are met; (ii) any undelivered products or services are not essential to the functionality of the delivered products or services; (iii) payment for the delivered products or services is not contingent upon delivery of the remaining products or services; (iv) we have an enforceable claim to receive the amount due in the event we do not deliver the undelivered products or services; and (v) as discussed above, there is evidence of the fair value for each of the undelivered products or services.
Government research grant revenues.We recognize revenues from government research grants during the period in which the related expenditures are incurred.
9. INVESTMENTS
The following tables summarize the Company’s investments in securities:
| | | | | | | | | | | | | | | | | |
| | | | | | | Gross | | Gross | | |
| | | Amortized | | Unrealized | | Unrealized | | Fair |
| | | Cost
| | Gains (1)
| | Losses (1)
| | Value
|
| As of March 31, 2004: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | $ | 1,356,597 | | | $ | 10,217 | | | $ | — | | | $ | 1,366,814 | |
| Adjustable rate mutual fund (2) | | | 3,014,365 | | | | — | | | | (40,566 | ) | | | 2,973,799 | |
| Government debt securities | | | 1,191,579 | | | | — | | | | (6,960 | ) | | | 1,184,619 | |
| | | |
| | | |
| | | |
| | | |
| |
| Total | | $ | 5,562,541 | | | $ | 10,217 | | | $ | (47,526 | ) | | $ | 5,525,232 | |
| | | |
| | | |
| | | |
| | | |
| |
| Reported as: | | | | | | | | | | | | | | | | |
| Short-term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | $ | 4,565,121 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| Long-term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | | 103,514 | | | | | | | | | | | | | |
| Restricted investments (3) | | | 856,597 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| Long-term investments | | | 960,111 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| Total | | $ | 5,525,232 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | Gross | | Gross | | |
| | | Amortized | | Unrealized | | Unrealized | | Fair |
| | | Cost
| | Gains (4)
| | Losses (4)
| | Value
|
| As of September 30, 2003: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | $ | 2,056,597 | | | $ | 15,575 | | | $ | — | | | $ | 2,072,172 | |
| Adjustable rate mutual fund (2) | | | 3,014,365 | | | | — | | | | (18,723 | ) | | | 2,995,642 | |
| Government debt securities | | | 194,467 | | | | — | | | | (3,400 | ) | | | 191,067 | |
| | | |
| | | |
| | | |
| | | |
| |
| Total | | $ | 5,265,429 | | | $ | 15,575 | | | $ | (22,123 | ) | | $ | 5,258,881 | |
| | | |
| | | |
| | | |
| | | |
| |
| Reported as: | | | | | | | | | | | | | | | | |
| Short-term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | $ | 3,888,798 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| Long-term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | | 513,486 | | | | | | | | | | | | | |
| Restricted investments (3) | | | 856,597 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| Long-term investments | | | 1,370,083 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| Total | | $ | 5,258,881 | | | | | | | | | | | | | |
| | | |
| | | | | | | | | | | | | |
| (1) | | Gross unrealized gains of $10,217 and gross unrealized losses of $47,526 on government securities, the adjustable rate mutual fund, and certificates of deposit represent an accumulated net unrealized loss of $37,309, which is reported as “accumulated other comprehensive loss” on the consolidated balance sheet as of March 31, 2004. |
| |
| (2) | | Represents an investment in a mutual fund that invests primarily in adjustable rate mortgage-backed securities. |
| |
| (3) | | Restricted investments amounting to $856,597 as of both March 31, 2004 and September 30, 2003 represent amounts pledged to our bank as collateral for letters of credit issued in connection with our leases of office and laboratory space. |
11
| | | |
| |
| (4) | | Gross unrealized gains of $15,575 and gross unrealized losses of $22,123 on government securities, the adjustable rate mutual fund, and certificates of deposit represent an accumulated net unrealized loss of $6,548, which is reported as “accumulated other comprehensive loss” on the consolidated balance sheet as of September 30, 2003. |
10. COMPUTATION OF NET LOSS PER COMMON SHARE
We compute basic net loss per common share by dividing the net loss attributable to common shareholders by the weighted-average number of common shares outstanding during the period (“Basic EPS Method”). We compute diluted net loss per common share by dividing the net loss attributable to common shareholders by the weighted-average number of common and dilutive common equivalent shares outstanding during the period (“Diluted EPS Method”). Dilutive common equivalent shares consist of shares issuable upon exercise of stock options and warrants and conversion of preferred stock. In the accompanying consolidated statements of operations, we have presented our net loss per share for the three-month and six-month periods ended March 31, 2004 and 2003 using the Basic EPS Method and the Diluted EPS Method. The shares of common stock issuable upon exercise of stock options and warrants and conversion of preferred stock under the Diluted EPS Method were excluded from the three-month and six-month periods ended March 31, 2004 and 2003, as the inclusion of such shares would have been anti-dilutive.
For the three-month and six-month periods ended March 31, 2004 and 2003, options to purchase 5,250,357 and 6,450,676 shares of common stock, respectively, were excluded from the computation of diluted net loss per share, as the inclusion of such shares would be antidilutive. For the three-month and six-month periods ended March 31, 2004 and 2003, warrants to purchase 5,442,911 and 1,031,350 shares of common stock, respectively, were excluded from the computation of diluted net loss per share, as the inclusion of such shares would be antidilutive.
11. SHAREHOLDERS’ EQUITY (DEFICIT)
Preferred stock. Preferred stock consists of Series C Junior Participating Preferred Stock, of which none is outstanding.
Class A common stock.During the three-month period ended March 31, 2004, we issued an aggregate of 263,239 shares of Class A common stock in connection with the exercise of stock purchase warrants (154,971 shares) and employee stock options (108,268 shares) for cash in the aggregate amount of $384,826, representing a weighted average price per share of $1.46. Also during the period the Class J warrant holder exercised its rights to acquire 19,119 shares of Class A common stock using a “cashless” exercise option. The market price on the date of exercise of the Class J warrant was $1.70 per share. The market price was $0.65 per share greater than the $1.05 per share exercise price of the warrant. Accordingly, the holder surrendered to us the warrant to purchase 30,881 shares that had an aggregate value of approximately $20,075 based on the difference in the market price on the tender date and the exercise price, times the number of shares purchasable under the warrant.
On December 5, 2003, we sold and issued 5,382,316 shares of Class A common stock and five-year warrants to purchase an additional 3,229,389 shares of Class A common stock to several accredited investors and received $8,019,652 in gross proceeds. The exercise price of the warrants is $1.75 per share. The financing transaction was made pursuant to the terms of a securities purchase agreement that provided each investor with a five-year warrant to purchase six-tenths of a share of Class A common stock for every share of Class A common stock purchased under the agreement. The effect of the financing transaction was an increase in cash and shareholders’ equity in the amount of $7,544,852, after taking into effect the costs of the transaction. The placement agent was paid $415,000 in fees and expenses, and was issued a five-year warrant to purchase 161,470 shares of the Company’s Class A common stock at $1.75 per share, in connection with this transaction.
12
Class B common stock conversions.During the three-month period ended March 31, 2004, 3,500 shares of Class B common stock were converted to 3,500 shares of Class A common stock. As of March 31, 2004 and September 30, 2003 there were none and 13,500 shares of Class B common stock outstanding, respectively.
12. RECENT ACCOUNTING PRONOUNCEMENTS
In January 2003, the Financial Accounting Standards Board (“FASB”) issued FASB Interpretation No. 46 (“FIN 46”), “Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51.” FIN 46 requires the consolidation of certain variable interest entities by the primary beneficiary of the entity if the equity investment at risk is not sufficient to permit the entity to finance its activities without additional subordinated financial support from other parties or if the equity investors lack the characteristics of a controlling financial interest. FIN 46 was effective immediately for variable interest entities created or acquired after January 31, 2003. For variable interest entities created or acquired prior to February 1, 2003, the provisions of FIN 46, as amended, were applicable to entities deemed special purpose entities effective on December 31, 2003 and were applicable to entities not deemed special purpose entities effective on March 31, 2004. The application of the provisions of FIN 46 did not have a material impact on the Company’s financial statements.
13. CONTINGENCIES
In the ordinary course of business, we may face various claims brought by third parties, including claims relating to the safety or efficacy of our products. Any of these claims could subject us to costly litigation and, while we generally believe that we have adequate insurance to cover many different types of liabilities, our insurance carriers may deny coverage or our policy limits may be inadequate to fully satisfy any damage awards or settlements. If this were to happen, the payment of any such awards could have a material adverse effect on our operations and financial position. Additionally, any such claims, whether or not successful, could damage our reputation and business. Management believes the outcome of currently identified claims and lawsuits will not have a material adverse effect on the Company’s operations or financial position.
| | | |
Item 2. | | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
This quarterly report on Form 10-Q contains forward-looking statements concerning future events and performance of our company. You should not rely excessively on these forward-looking statements, because they are only predictions based on our current expectations and assumptions. When used in this report, the words “intend,” “estimate,” “anticipate,” “believe,” “plan” or “expect” and similar expressions as they related to AVANIR are included to identify forward-looking statements. Many factors could cause our actual results to differ materially from those indicated in these forward-looking statements. You should review carefully the factors identified in this report under the caption, “Risk Factors that Might Affect Future Results,” and in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission (“SEC”). We disclaim any obligation to update or announce revisions to any forward-looking statements to reflect actual events or developments.
EXECUTIVE OVERVIEW
We are a drug discovery and development company focused primarily on novel treatments for chronic diseases. We have one product that has been approved by the U.S. Food and Drug Administration (“FDA”) for the treatment of cold sores, docosanol 10% cream (sold as Abreva® by our marketing partner, GlaxoSmithKline Consumer Healthcare, in North America), and have several product candidates in clinical development. Our late-stage product candidate, NeurodexTM, is in Phase III clinical development for the treatment of pseudobulbar affect (“PBA”), also known as pathological laughing or crying. Neurodex is also in Phase II clinical development for the treatment of neuropathic pain. A
13
potential product for allergy and asthma, AVP-13358, is in Phase I clinical development. We also have preclinical research programs targeting inflammatory diseases, atherosclerosis and cancer. Our preclinical research and drug discovery programs are focused primarily on small molecules that can be taken orally as therapeutic treatments. Using our proprietary XenerexTM technology, we are also conducting research to develop injectable human monoclonal antibody products for infectious diseases, such as anthrax and cytomegalovirus, and for other therapeutic applications.
The following graph illustrates the development status of our product candidates.
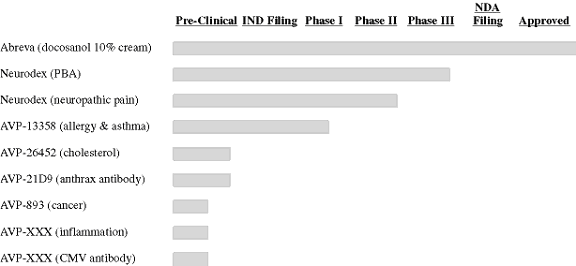
The continued development of our product candidates, and the potential launch of a new drug, will require substantial additional capital. We intend to partner with pharmaceutical companies that can help fund our research and potential product launch in exchange for sharing in the rights to commercialize new drugs. We have licensed certain rights to docosanol 10% cream and continue to seek licensees for that product and potential products in our pipeline. Research collaborations also represent an important way to achieve our development goals by sharing the risks and the opportunities that come from such development efforts.
We expect that our development and operational costs will continue to exceed revenues from existing sources at least until the end of 2005. We will have to raise significant amounts of additional capital to finance our research and development activities and operations if we are unable to enter into partnership or collaborative arrangements with pharmaceutical companies that will share our drug development costs. If we are unable to raise capital as needed to fund our operations, or if we are unable to enter into collaborative arrangements, then we may need to slow the rate of development of some of our programs or sell the rights to one or more our drug candidates.
Our most significant product license to date is with GlaxoSmithKline for the right to sell Abreva in North America for the treatment of cold sores. In December 2002, we sold an undivided interest in our license with GlaxoSmithKline to Drug Royalty USA for $24.1 million, under which we retained one-half of all royalties from GlaxoSmithKline on annual Abreva sales in excess of $62 million. Although we have received the $24.1 million in sale proceeds under this contract, the revenues have been deferred in accordance with accounting principles generally accepted in the United States and we are recognizing a portion of the revenue each quarter through April 2014.
We are seeking additional licensees for docosanol 10% cream in Europe and Japan and, in November 2003, the Swedish Medical Products Agency approved docosanol 10% cream for marketing as a non-prescription, over-the-counter (“OTC”) topical treatment for cold sores. Sweden is acting as the Reference Member State for obtaining further regulatory approvals of docosanol 10% cream in the
14
European Union. We expect that the Swedish approval should facilitate regulatory approval in additional countries in the European Union through what is known as the “mutual recognition process.” We also believe that European regulatory approvals will make the drug more attractive for licensing.
In March 2004, we completed enrollment in our Phase III clinical trial for Neurodex in the treatment of PBA. If the results of the 90-day patient trials are favorable, we expect to submit a new drug application (“NDA”) to the FDA for the drug in the second half of the 2004 calendar year. We are in the process of completing enrollment in an open-label safety study of Neurodex in the treatment of PBA, which is expected to be substantially complete by the time of our projected NDA submission. If the FDA approves the product, we may license Neurodex for distribution in the United States and other countries, or elect to market the drug directly with a co-promotion partner. If we choose to market the drug directly, we will need to raise additional capital to fund the creation of a sales force and provide working capital related to manufacturing, promotion and sales of the product. If we are able to obtain approval from the FDA in the second half of 2005 to market Neurodex, and launch Neurodex shortly thereafter, we expect that revenues from sales of the product will begin to contribute operating cash flows in 2006. See “Risk Factors That Might Affect Future Operations — Risks Relating to our Business.”
Regarding our other clinical and preclinical development programs, we intend to develop those programs to Phase II “proof of concept” and then license those programs to major pharmaceutical companies. We are pursuing this strategy for these programs because we do not have sufficient human or capital resources to carry out all phases of clinical development.
Our offices and research facilities are located at 11388 Sorrento Valley Road, San Diego, California 92121. Our telephone number is (858) 622-5200 and our e-mail address is info@Avanir.com. Additional information about AVANIR can be found on our website, atwww.avanir.com,and in our periodic and current reports filed with the Securities and Exchange Commission (“SEC”). Copies of our current and periodic reports filed with the SEC are available at the SEC Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549, and online atwww.sec.govand our website atwww.avanir.com.
RESULTS OF OPERATIONS —
COMPARISON OF THREE MONTHS ENDED MARCH 31, 2004 AND 2003
Revenues
Revenues in the second quarter of fiscal 2004 amounted to $856,000 including $268,000 from research contracts and licenses, $345,000 from the recognition of deferred revenue relating to the sale of an undivided interest in our Abreva® license agreement to Drug Royalty USA (see Note 7, “Deferred Revenue,” in the accompanying consolidated financial statements), and $241,000 from government research grants. Revenues from research contracts and licenses included $200,000 from completion of antibody development work for DNAX Research, Inc. Revenues in the second quarter of fiscal 2003 amounted to $622,000, including $481,000 from the recognition of deferred revenue relating to the sale of an undivided interest in our Abreva license agreement to Drug Royalty USA and $140,000 from government research grants.
During fiscal 2004, we expect to recognize approximately $1.7 million in revenues from the recognition of deferred revenue relating to the sale of an undivided interest in our Abreva license agreement to Drug Royalty USA and the DNAX Research contract, approximately $800,000 in sales of the active ingredient docosanol to licensees, and approximately $800,000 in revenues from government research grants. As of March 31, 2004, approximately one-half of deferred revenues and revenues from government research grants had been recognized out of projected amounts for fiscal 2004. Also, as of March 31, 2004, substantially all of projected fiscal 2004 revenues from the sales of docosanol had been achieved.
Projected fiscal 2004 revenues from new sources, such as license fees, milestone payments, and royalties on foreign sales of licensed products, will depend substantially on the timing of approval decisions for docosanol 10% cream by foreign regulatory agencies and our ability to enter into additional license arrangements and achieve milestones under those arrangements. Such arrangements could be in the form
15
of licensing or partnering agreements for docosanol 10% cream or for our other product development programs for the treatment of PBA, neuropathic pain, allergy and asthma, and cholesterol. Many of our product development programs could take years of additional development before they reach the stage of licensing, if ever, by other pharmaceutical companies.
Revenue-generating Contracts
Commercial contracts that remained active at the end of the second quarter of fiscal 2004 include five docosanol 10% cream (e.g. Abreva) license agreements, one antibody license and one Neurodex sublicense. The GlaxoSmithKline license arrangement has been our most significant revenue source, representing approximately 40% and 77% of total revenues for the three months ended March 31, 2004 and 2003, respectively.
Expenses
Operating expenses. Total operating expenses were $7.6 million in the second quarter of fiscal 2004, compared to $6.7 million in the same period in fiscal 2003. The 14% increase in operating expenses was primarily caused by an $899,000 increase in spending for Phase III clinical development and the open label study of Neurodex for the treatment of PBA, reflecting substantially higher rates of enrollment than during the same period a year ago. Research and development programs accounted for 71% of total operating expenses for the three months ended March 31, 2004. Sales and marketing expenses for the three months ended March 31, 2004, increased by 19% over the same period a year ago, representing additional medical education and awareness programs for PBA. These and other costs are more fully described below.
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended | | Six Months Ended |
| | | March 31,
| | March 31,
|
| | | 2004
| | 2003
| | 2004
| | 2003
|
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 71 | % | | | 73 | % | | | 70 | % | | | 72 | % |
| General and administrative | | | 20 | % | | | 18 | % | | | 19 | % | | | 19 | % |
| Sales and marketing | | | 9 | % | | | 9 | % | | | 10 | % | | | 9 | % |
| Cost of sales | | | — | % | | | — | % | | | 1 | % | | | — | % |
| | | |
| | | |
| | | |
| | | |
| |
| Total operating expenses | | | 100 | % | | | 100 | % | | | 100 | % | | | 100 | % |
| | | |
| | | |
| | | |
| | | |
| |
Research and development (“R&D”) expenses. R&D expenses were $5.4 million in the second fiscal quarter of 2004, compared to $4.9 million in the same period a year ago. The Phase III clinical trial for Neurodex in the treatment of PBA in patients with multiple sclerosis (MS) accounted for approximately 45% of all R&D spending in the second quarter of fiscal 2004. Costs related to a Phase I single rising dose study of AVANIR’s lead compound for the treatment of allergies and asthma accounted for approximately 16% of total R&D spending in the second quarter of fiscal 2004. The balance of R&D spending was for other programs, including preclinical research related to inflammation and cholesterol-lowering compounds and antibody research programs. We expect R&D spending will continue at the current rate through the next several quarters as we proceed towards completion of the Phase III clinical trial and open label study of Neurodex and continue the Phase I clinical trials of AVP-13358 and preclinical research related to cholesterol reduction and inflammation.
In the second quarter of fiscal 2003, approximately 31% of R&D expenses were related to the Phase III clinical trial for Neurodex in the treatment of PBA in patients with MS. Approximately 26% of R&D expenses were related to completion of toxicology and other preclinical research related to treatments for allergies and asthma. The balance of R&D spending was divided primarily among research related to inflammation, cholesterol-lowering compounds, and antibody research programs.
Pharmaceutical R&D programs, by their very nature, require a substantial amount of financial and human resources and there is no assurance that any drug candidate will be approved for marketing by domestic and/or foreign regulatory agencies. The later stages of clinical development typically require
16
substantially greater funds for development than the earlier stages. Thus, for many of our larger development programs, we intend to license our technology or partner with other pharmaceutical companies that have substantially greater financial resources. We expect that our licensees will continue to develop and fund those projects and pay us up-front license fees, milestone payments, and royalties on product sales if those products are successfully developed and approved for marketing by the FDA and foreign regulatory agencies. We caution that many of our development efforts could experience delays, setbacks and failures, with no assurance that any of our clinical research or our potential licensees or partners will ever reach the stage of submitting an NDA to the FDA or that any NDA will be approved. The following table sets forth the status of, and costs attributable to, our proprietary research and clinical development programs.
17
Research and Development Projects and Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended | | Six Months Ended | | | | |
| | | March 31,
| | March 31,
| | Inception Through | | Estimated Cost to License |
| | | 2004 (1)
| | 2003 (1)
| | 2004 (1)
| | 2003 (1)
| | March 31, 2004 (1)(2)
| | or Complete Project (1)
|
Major Company-Funded Projects: | | | | | | | | | | | | | | | | | | | | | | | | |
| Develop Neurodex for FDA marketing approval in treating PBA. Estimated timing to complete remaining phases of project necessary to submit an NDA to FDA: 0.5 year | | $ | 2,428,453 | | | $ | 1,529,437 | | | $ | 4,198,307 | | | $ | 2,103,395 | | | $ | 14,660,810 | | | $ | 4M | |
| Develop Neurodex for neuropathic pain. Phase II open-label study completed | | | 510,298 | | | | 611,845 | | | | 1,105,660 | | | | 710,784 | | | | 5,862,833 | | | | (3 | ) |
| Development program for allergy and asthma (IgE regulator). Estimated timing to complete Phase IIa and license the product: up to 2 years | | | 882,574 | | | | 1,247,157 | | | | 1,949,868 | | | | 2,483,614 | | | | 16,452,869 | | | $ | 15M | |
| Preclinical anti-inflammatory research program (MIF inhibitor). Estimated timing to complete Phase IIa and to license the product: up to 4 years | | | 453,851 | | | | 391,256 | | | | 1,066,636 | | | | 1,017,831 | | | | 6,112,497 | | | $ | 23M | |
| Preclinical cholesterol reduction program. Estimated timing to complete proof of concept and license product: up to 3.5 years | | | 237,059 | | | | 181,320 | | | | 460,424 | | | | 408,983 | | | | 2,361,203 | | | $ | 27M | |
Government-Funded Projects: | | | | | | | | | | | | | | | | | | | | | | | | |
| Preclinical research, primarily for potential treatments for genital herpes and anthrax. Estimated timing to complete the various projects varies from one to two years | | | 337,039 | | | | 185,946 | | | | 562,872 | | | | 357,002 | | | | 1,645,926 | | | $ | 1.0M | (4) |
Other Research Projects: | | | | | | | | | | | | | | | | | | | | | | | | |
| Other projects involving monoclonal antibodies and docosanol. The costs and timing to complete these projects are unpredictable because of the uncertainty of outcomes of our research | | | 523,367 | | | | 713,319 | | | | 1,392,557 | | | | 1,390,293 | | | | 12,790,146 | | | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | | | |
| Total | | $ | 5,372,641 | | | $ | 4,860,280 | | | $ | 10,736,324 | | | $ | 8,471,902 | | | $ | 59,886,284 | | | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | | | |
| (1) | | Each project includes allocation of laboratory occupancy costs. “M” refers to millions. Estimated costs and timing to complete the projects are subject to the availability of funds. |
| |
| (2) | | Inception dates are on or after October 1, 1998, at which time we began identifying and tracking program costs. |
| |
| (3) | | Licensing and/or co-promotion alternatives are being explored whereby the partner would fund Phase III clinical trials. See “Research and Development Program Status and Plans.” Program expenditures for Neurodex for the treatment of neuropathic pain during each of the listed periods include allocated costs of certain research programs that are shared with Neurodex for PBA. |
| |
| (4) | | Represents the remaining balance of State and Federal research grant awards totaling $2.1 million for research related to genital herpes, anthrax, cytomegalovirus (“CMV”) and a blood donor program. |
18
Research and Development Program Status and Plans
Neurodex for the treatment of PBA.On March 31, 2004, we completed enrollment totaling 150 patients in a Phase III clinical trial of Neurodex for the treatment of PBA in patients with MS and we closed the study to any further enrollments. Given the 90-day study period, the last patient should complete the clinical trial at the end of June 2004. We also recently surpassed one-half the targeted enrollment in an open-label safety study for the treatment of PBA in a broader pool of patients who could have any one of several neurodegenerative diseases. Prior to engaging in these studies, we completed the initial Phase III clinical trial of PBA in patients with amyotrophic lateral sclerosis (ALS), in May 2002. Our goal is to submit an NDA to the FDA in the second half of the 2004 calendar year.
Neurodex for the treatment of neuropathic pain.This program is in the Phase II stage of clinical development. At present, we are evaluating alternatives for continued development of Neurodex in the treatment of neuropathic pain, including continuing development on our own or with the funding of a potential co-promotion partner or potential licensee. In mid-2003, we successfully completed a four-week open-label dose escalation study of Neurodex in patients with painful diabetic neuropathy. There can be no assurances as to the timing of a license or co-promotion arrangement, if any.
Development program for allergy and asthma (IgE regulator).We recently completed a Phase I clinical trial of our asthma/allergy drug, AVP-13358, in 54 healthy volunteers. The placebo-controlled study was intended to assess safety, tolerability and pharmacokinetics following a single oral administration. Results of the study suggest AVP-13358 was well tolerated at all doses. The study also demonstrated AVP-13358 was detectable in the bloodstream at all doses administered and remains in circulation long enough to allow potentially once or twice daily dosing. AVP-13358 is a novel drug molecule discovered by AVANIR scientists that is intended to inhibit the production of IgE, a well-known mediator of allergy and asthma. Phase I trials are intended to establish safety of the drug in single rising doses in healthy volunteers. These trials will be followed by Phase Ib clinical trials using rising multi-doses in healthy patients. Assuming the successful completion of Phase I clinical trials and adequate funds to continue development without a partner, we intend to continue development of the drug through Phase IIa. Assuming the results of such additional studies are favorable, we expect to license the drug to a larger pharmaceutical company for further development.
Cholesterol reduction. We are engaged in preclinical research focused on certain types of small molecules that can be taken orally as a potential treatment of hyper-cholesterolemia (high cholesterol). Cholesterol is transported in the blood in large cholesterol-rich particles called lipoproteins. Generally, low-density lipoproteins (LDL) deliver the cholesterol to peripheral tissues including aorta and coronary arteries, whereas high-density lipoproteins (HDL) remove excess cholesterol from peripheral tissues, and transport it to the liver for elimination. The process is also known as reverse cholesterol transport (“RCT”). We are evaluating a series of compounds that, in animal modes, reduce the fatty deposits in the blood vessel walls (commonly called plaque) typically associated with atherosclerosis. We estimate the costs to develop selected compounds to the stage of being able to demonstrate the proof of concept and to license the compound to be $27 million.
Anti-inflammatory research program (MIF inhibitor). We are currently conducting research on several potential drug candidates capable of inhibiting or blocking the activity of macrophage migration inhibitory factor (“MIF”). Our research indicates that MIF may serve as a potential drug target in a variety of diseases, including rheumatoid arthritis, Crohn’s disease, colitis and asthma. Assuming we are successful in finding a lead compound and submitting an investigational new drug application (“IND”) to the FDA, and successfully completing some of the initial studies, we intend to license the compound to another pharmaceutical company for further development. Any delays to our product development
19
timeline or in submitting an IND could cause a similar delay in the timing of when we expect that we could license our MIF technology.
Government research contracts.AVANIR has been engaged in research funded by $2.1 million in government research grants. We recently completed two government-funded research programs – the development of a blood donor program intended to find high-affinity antibodies to infectious diseases and research on potential antibodies to cytomegalovirus. Approximately $1.0 million in funds remains to be spent throughout 2004 and 2005 under existing government research grants for research on docosanol based formulations for the treatment of genital herpes and for the development of antibodies to anthrax toxins.
Monoclonal antibodies research. Two of AVANIR’s most potent anthrax antibodies, AVP 21D9 and AVP 22G12, appear unique both in mechanism of action and in terms of the binding site on the anthrax toxin. AVP 21D9 is currently in preclinical development for use as a prophylactic and therapeutic drug to treat anthrax infections. Ultimately, AVANIR plans to submit an IND application seeking clearance from the FDA to begin clinical trials to evaluate the safety, tolerability, and pharmacology of AVP 21D9 in healthy human subjects. Much of our work targeting infectious diseases has been funded by government research grants. Because all of our research is at a very early preclinical stage of development and is unpredictable in terms of the outcome, we are unable to predict the cost and timing for development of any antibody or drug.
General and administrative expenses. General and administrative expenses amounted to $1.5 million in the second quarter of fiscal 2004, representing a 25% increase over expenses of $1.2 million in the same period in the prior year. Higher expenses were primarily attributable to a $257,000 increase in expenses related to securities compliance and implementation of requirements under the Sarbanes-Oxley Act of 2002 and a $102,000 increase in building occupancy costs, including rent, utilities, maintenance and insurance resulting from the utilization of additional office space in the current period, partially offset by a $21,700 reduction in investor relations expenses due primarily to discontinuation of certain contract services. The Company expects that general and administrative expenses will remain at about the same level over the next two quarters.
Sales and marketing expenses. During the second quarter of fiscal 2004, sales and marketing expenses were $722,000, compared to $606,000 in the same period a year ago. Higher expenses in the second fiscal quarter of 2004 were related primarily to the addition of approximately $203,000 in medical education and awareness programs for PBA and pre-launch planning activities for Neurodex. We expect that sales and marketing expenses will continue at the current rate for at least the next quarter and increase thereafter if Phase III clinical trial results for Neurodex are favorable.
Net Loss
For the second quarter of fiscal 2004, the net loss attributable to common shareholders was $6.7 million, compared to $6.0 million for the same period a year ago. The basic and diluted net loss per share was $0.09 for the second quarter of fiscal 2004, compared to $0.10 for the same period a year ago. A higher number of shares outstanding during the three months ended March 31, 2004 accounted for the lower per share loss compared to the same period in the prior year. We expect to continue to pursue our drug development strategy focused on the development of Neurodex, followed by other programs in earlier stages of development that are in large therapeutic areas and that have significant partnering and licensing potential. To help fund and develop our products, we intend to seek licensees and partners to share the costs of development. Some of these potential license arrangements could materially change our outlook for future revenues and costs. However, the timing of such potential arrangements is unpredictable.
20
RESULTS OF OPERATIONS –
Comparison of Six Months Ended March 31, 2004 and 2003
Revenues
Revenues for the first six months of fiscal 2004 amounted to $2.4 million, compared to $1.4 million for the same period last year. Revenues for the first six months of fiscal year 2004 included $881,000 in revenue that the Company recognized from the sale of certain rights to future Abreva royalties to Drug Royalty USA, $770,000 in sales of the raw material docosanol, and $440,000 from government research grants. Revenues for the first six months of fiscal year 2003 included $1.1 million in revenue that the Company recognized from the sale of certain rights to future Abreva royalties to Drug Royalty USA, $299,000 from government research grants and $17,000 in sales of the raw material docosanol.
Expenses
Operating Expenses. Total operating expenses were $15.4 million in the first six months of fiscal 2004, compared to $11.8 million in the same period in fiscal 2003. The 31% increase in operating expenses was primarily caused by a 27% increase in spending on research and development programs. Research and development programs accounted for 70% and 72% of total operating expenses for the first six months of fiscal 2004 and 2003, respectively. General and administrative expenses accounted for 19% of total operating expenses for both the first six months of fiscal 2004 and 2003. Sales and marketing expenses accounted for 10% and 9% of total operating expenses for the first six months of fiscal 2004 and 2003, respectively. These and other costs are more fully described below.
Research and Development Expenses. Research and development (R&D) expenses were $10.7 million in the first six months of fiscal 2004, compared to $8.5 million in the same period a year ago. The clinical trials of Neurodex in the treatment of pseudobulbar affect (PBA) in patients with multiple sclerosis accounted for 39% of all R&D spending in the first six months of fiscal 2004. Costs associated primarily with completion of toxicology, filing an IND and initiating Phase I clinical trials of AVANIR’s lead compound for the treatment of allergies and asthma accounted for 18% of total R&D spending in the first six months of fiscal 2004. The balance of R&D spending was for other programs, including pre-clinical research related to inflammation and cholesterol-lowering compounds and antibody research programs.
In the first six months of fiscal 2003, costs associated with completion of toxicology, filing an IND and initiating Phase I clinical trials of AVANIR’s lead compound for the treatment of allergies and asthma accounted for 29% of total R&D spending. The clinical trials of Neurodex in the treatment of PBA in patients with MS and in the treatment of neuropathic pain accounted for 25% and 8%, respectively, of all R&D spending in the first six months of fiscal 2003. The balance of R&D spending was for other programs, including pre-clinical research related to inflammation and cholesterol-lowering compounds and antibody research programs.
General and Administrative Expenses. General and administrative expenses amounted to $2.9 million in the first six months of fiscal 2004, representing a 27% increase over expenses of $2.3 million in the same period in the prior year. Higher expenses were attributable to a $321,000 increase related to human resource programs, a $203,000 increase related to securities compliance and the Sarbanes-Oxley Act of 2002, and a $190,000 increase in building occupancy costs, including rent, utilities, maintenance and insurance, resulting from the utilization of additional office space in the current period, partially offset by a $70,000 reduction in outside services for investor relations.
21
Sales and Marketing Expenses. During the first six months of fiscal 2004, sales and marketing expenses were $1.6 million, compared to $1.0 million in the same period a year ago. Higher expenses in the first six months of fiscal 2004 were primarily related to the addition of medical education and awareness programs for PBA and pre-launch planning activities for Neurodex.
Net Loss
For the first six months of fiscal 2004, the net loss attributable to common shareholders was $13.0 million, compared to $10.2 million for the same period a year ago. The basic and diluted net loss per share was $0.18 for both the first six months of fiscal 2004 and 2003.
LIQUIDITY AND CAPITAL RESOURCES
As of March 31, 2004, we had cash, cash equivalents, and investments in securities totaling $13.4 million and a net working capital balance of $5.1 million. As of September 30, 2003, we had cash, cash equivalents and investments in securities of $17.5 million and a net working capital balance of $10.6 million. Explanations of net cash provided by or used for operating, investing and financing activities are provided below.
| | | | | | | | | | | | | |
| | | March 31, | | Decrease | | September 30, |
| | | 2004
| | During Period
| | 2003
|
| Cash, cash equivalents and investments in securities | | $ | 13,435,725 | | | $ | (4,021,564 | ) | | $ | 17,457,289 | |
| Cash and cash equivalents | | $ | 7,910,493 | | | $ | (4,287,915 | ) | | $ | 12,198,408 | |
| Net working capital | | $ | 5,090,666 | | | $ | (5,528,550 | ) | | $ | 10,619,216 | |
| | | | | | | | | | | | | |
| | | Six months ended | | Change | | Six months ended |
| | | March 31, | | Between | | March 31, |
| | | 2004
| | Periods
| | 2003
|
| Net cash provided by (used for) operating activities | | $ | (11,128,699 | ) | | $ | (21,968,153 | ) | | $ | 10,839,454 | |
| Net cash used for investing activities | | | (925,750 | ) | | | 1,684,266 | | | | (2,610,016 | ) |
| Net cash provided by (used for) financing activities | | | 7,766,534 | | | | 7,973,635 | | | | (207,101 | ) |
| | | |
| | | |
| | | |
| |
| Net increase (decrease) in cash and cash equivalents | | $ | (4,287,915 | ) | | $ | (12,310,252 | ) | | $ | 8,022,337 | |
| | | |
| | | |
| | | |
| |
Operating activities. Net cash used for operating activities amounted to $11.1 million in the first six months of fiscal 2004, compared to $10.8 million in cash provided by operating activities during the same period a year ago. For the first six months of fiscal 2004, the net loss of $13.0 million primarily accounted for the cash used for operating activities. Also during the period, inventory declined by $198,000, representing the cost of docosanol sold to various licensees, and accounts receivable increased by $93,000, representing services provided under government grants for which payments were received after the quarter ended. We intend to replenish our docosanol inventory later in the fiscal year. The net cash provided by operating activities in the first six months of fiscal 2003 includes $24.1 million in cash, net of transaction costs, received from Drug Royalty USA on December 24, 2002, from the sale of an undivided interest in our Abreva® license agreement with GlaxoSmithKline. The sale of rights to future Abreva royalties was recorded as deferred revenue on the date of sale. (See Note 7, “Deferred Revenue” in the accompanying notes to the consolidated financial statements.)
Investing activities. Net cash used for investing activities during the first six months of fiscal 2004 amounted to $926,000, including investments in securities totaling $997,000, purchases of property and equipment of $72,000 and patent costs of $557,000, partially offset by sales and maturities of investments totaling $700,000. Net cash used for investing activities during the first six months of fiscal 2003 amounted to $2.6 million, including construction in progress totaling $3.0 million on additional leased occupancy space, investments in securities totaling $706,000, purchases of property and equipment of $783,000 and patent costs of $234,000, partially offset by sales and maturities of investments totaling $2.1 million. Capital expenditures were substantially lower in the second quarter of
22
fiscal 2004, compared to the same period a year ago when the Company was engaged in making leasehold improvements to additional office and laboratory space.
Financing activities. Net cash provided by financing activities amounted to $7.8 million during the first six months of fiscal 2004, consisting primarily of $7.5 million received from the sale of Class A common stock and warrants in December 2003. Net cash used for financing activities amounted to $207,000 in the first six months of fiscal 2003, consisting primarily of payments on notes and capital lease obligations.
To continue to fund the development of our new drug candidates and technology platforms during at least the next two years, we expect to continue to pursue various alternatives for raising capital. For example, in April 2004, we filed a shelf registration statement on Form S-3 with the SEC that would enable the Company, in one or more offerings, to sell an aggregate of up to $50 million in Class A common stock from time to time over a period of up to two years. The nature and terms of any offering will be established at the time of the offering and set forth in a supplemental filing with the SEC. Other potential alternatives that we are considering for raising capital include, but are not limited to, partnering arrangements where partners share development costs, issuance of debt or equity securities, and licensing or sales of any of our platform technologies or new drug candidates.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of financial statements in accordance with accounting principles generally accepted in the United States of America requires that management make a number of assumptions and estimates that affect the reported amounts of assets, liabilities, revenues and expenses in our consolidated financial statements and accompanying notes. Management bases its estimates on historical information and assumptions believed to be reasonable. Although these estimates are based on management’s best knowledge of current events and circumstances that may impact the Company in the future, actual results may differ from these estimates.
Our critical accounting policies are those that affect our financial statements materially and involve a significant level of judgment by management. Our critical accounting policies regarding revenue recognition are in the following areas: milestone payments in license agreements, royalties on licensed products, sale of rights to future royalties, and recognition of revenues in research contracts. Our critical accounting policies regarding expense recognition are principally related to research contracts. Our critical accounting policies also include the valuation of long-lived and intangible assets.
Milestone Payments in License Agreements
We recognize revenues from license fees when the performance requirements have been met, the fee is fixed or determinable, and collection of fees is probable. We defer revenues and recognize them over the life of the agreement when we have continuing obligations to perform under the agreement.
Our largest and most significant license agreement is with SB Pharmco Puerto Rico, Inc., a subsidiary of GlaxoSmithKline (“GlaxoSmithKline”). On March 31, 2000, we transferred the rights to manufacture, use, and sell Abreva in the United States and Canada to GlaxoSmithKline and gave them full control, authority and responsibility over research, development, regulatory compliance activities (including actions required to obtain appropriate government approvals), and commercialization of the product in those territories. GlaxoSmithKline has achieved and paid all of the performance milestones under the agreement. With regard to the milestones, we have no further performance obligations. Future revenues, if any, to be earned under the GlaxoSmithKline agreement will come solely from royalty revenues.
We expect to enter into additional license agreements in the future. We expect that each license agreement will have its own set of circumstances and terms of performance. We will consider the
23
specific facts and circumstances of each license agreement to determine the appropriate revenue recognition for such items, including nonrefundable up-front fees and milestone payments and taking into consideration when the earnings process is complete and collection is reasonably assured.
Royalties on Licensed Products
We recognize royalty revenues from our licensed products based on the reported sales by our licensees and computed in accordance with the specific terms of the license agreements. Since the launch of Abreva in October 2000 through March 31, 2004, substantially all of our royalties have come from GlaxoSmithKline. We have entered into additional license agreements that contain royalties as a source of revenues, and we expect to enter into additional similar license agreements with foreign-based companies.
Sales of Rights to Future Royalties
In fiscal 2003, we sold an undivided interest in our license agreement with GlaxoSmithKline to Drug Royalty USA for $24.1 million. Because of our ongoing involvement with GlaxoSmithKline in earning future royalty revenues, we recorded the amount received from Drug Royalty USA, net of costs of the transaction and forgiveness of certain advances, as deferred revenue. The amount recorded as deferred revenue is being recognized as revenue under the units-of-revenue method over the period from October 2002 through April 2014. The portion of deferred revenue classified as a current liability represents the amount that we expect to realize as revenue within the next 12 months. (See Note 7, “Deferred Revenue,” in the accompanying consolidated financial statements.)
Recognition of Revenues in Research Contracts
In certain circumstances, we may enter into research contracts or collaborations that have obligations to deliver to the customer multiple products and/or services (multiple deliverables) in exchange for fees or milestone payments. Such contracts could include antibody generation services agreements and other forms of research collaborations, as discussed below.
Antibody generation services.As of March 31, 2004, we were engaged in work on one research collaboration agreement that had research initiation fees. In this type of agreement, the customer provides us with the target antigens. We then perform research services to develop potential antibodies for those antigens. If we are able to estimate the period of service in the contract in advance of beginning the work, then we recognize such research initiation fees ratably as revenue over the estimated period of service. If we are unable to identify the period of service in the contract in advance of beginning the work, we defer research initiation fees and recognize such fees as revenue once we have completed our efforts to create the antibodies. In the research phases of the research collaboration agreement, we may receive payment either to start a research phase or to complete a research phase (including receipt by the customer of the deliverable). We recognize revenue once the product has been delivered, because the earnings process is then complete and an exchange has been made. Factors taken into consideration in recognizing revenues include the following:
| • | | The performance criteria have been met; |
| • | | Any undelivered products or services are not essential to the functionality of the delivered products or services; and |
| • | | Payment for the delivered products or services is not contingent on delivery of the remaining products or services. |
Other research contracts.As with all our research contracts, including the up-front initiation fee, we defer revenue recognition until services have been rendered or products (e.g. developed antibodies) are
24
delivered. The milestones established within the contract are typically set to approximate the effort associated with the completion of each phase.
Recognition of Expenses in Research Contracts
Pursuant to management’s assessment of the progress that has been made on clinical trials and services provided in other research contracts, we recognize expenses as the services are provided. Several of our contracts extend across multiple reporting periods. For example, contracts with organizations that conduct our Phase III clinical trial of Neurodex for the treatment of PBA in patients with multiple sclerosis and our Neurodex open label study, extend beyond one year. Such contracts require an assessment of the work that has been completed during the period, including measurement of progress, analysis of data that justifies the progress, and finally, management’s judgment.
Valuation of Long-Lived and Intangible Assets
We assess the impairment of identifiable intangible assets and long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors we consider important that could trigger an impairment review include the following:
| | • | | a significant underperformance relative to expected historical or projected future operating results; |
| |
| | • | | a significant change in the manner of our use of the acquired asset or the strategy for our overall business; and/or |
| |
| | • | | a significant negative industry or economic trend. |
When we determine that the carrying value of intangible assets or long-lived assets are not recoverable based upon the existence of one or more of the above indicators of impairment, we may be required to record impairment charges for these assets that have not been previously recorded.
RECENT ACCOUNTING PRONOUNCEMENTS
See Note 12, “Recent Accounting Pronouncements” in the accompanying consolidated financial statements for a discussion of recent accounting pronouncements and their effect, if any, on the Company.
RISK FACTORS THAT MIGHT AFFECT FUTURE OPERATIONS
Risks Relating to Our Business
We have a history of losses and we may never achieve or maintain profitability.
To date, we have experienced significant operating losses in funding the research, development and clinical testing of our drug candidates and we expect to continue to incur substantial operating losses through at least 2005. As of March 31, 2004, our accumulated deficit was approximately $109 million and our shareholders’ deficit was $4.0 million. To achieve profitability, we would need to generate significant additional revenue with positive gross margins. Although we are seeking to negotiate revenue-generating licenses and/or co-promotion arrangements for docosanol 10% cream, Neurodex and other product candidates, we may not be successful in doing so, and any such arrangements may not generate the anticipated revenue. Additionally, our sales and marketing and general and administrative expenses are expected to increase over the next several quarters. These increases in expenses may not be offset by new or increased revenue, and, as a result, we may not achieve or maintain profitability.
25
We expect to complete our Phase III clinical trial soon for Neurodex and any negative results in this trial could harm our stock price.
In March 2004, we announced that we had completed enrollment in our Phase III clinical trial for Neurodex, our late-stage candidate for the treatment of pseudobulbar affect. We expect to announce preliminary data from this trial later in 2004. Neurodex is our most developed drug candidate and we believe it represents the most immediate opportunity to produce significant revenue for the Company. If the clinical trial data are negative or inconclusive, then we may be required to conduct one or more additional clinical trials and may not be able to seek regulatory approval to market the compound. If this were to happen, we expect our stock price would be negatively affected.
Assuming favorable results from our Phase III trial for Neurodex in Multiple Sclerosis patients with PBA, we must submit our results to the FDA for review and approval prior to U.S. commercialization. Any delay in the regulatory review or approval process may harm our prospects and could harm our stock price.
Assuming positive results from our Phase III trial of Neurodex in patients suffering from PBA with Multiple Sclerosis, we will have to seek FDA approval prior to commercialization in the United States. Any delays in our submission or in the FDA’s review or approval would delay market launch and increase our cash requirements and could increase the volatility of our stock price and result in additional operating losses.
The process of obtaining FDA approval often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. FDA’s review and approval of the actual NDA application is expensive and uncertain. If we submit an NDA for Neurodex, the FDA must decide whether to accept or reject the submission for filing. The FDA’s official filing of an NDA begins the application’s substantive review. The FDA may refuse to file an NDA for review for many reasons, including if the submission contains insufficient data to demonstrate efficacy and safety. We cannot be certain that our NDA submission would be accepted for filing and reviewed by the FDA or that we would be able to respond to any requests during the review period in a timely manner without delaying potential action on our request for approval. We also cannot be certain that Neurodex will receive a favorable recommendation from any FDA advisory committees or be approved for marketing by the FDA. Even if the FDA grants us marketing approval for Neurodex, we cannot be certain that we will be able to obtain the labeling claims necessary or desirable for the promotion of the product. In addition, delays in approvals or rejections of marketing applications may be based upon many factors, including regulatory requests for additional analyses, reports, data and/or studies, regulatory questions regarding data and results, changes in regulatory policy during the period of product development and/or the emergence of new information regarding our products or other products.
We expect that we will need to raise additional capital to fund ongoing operations through at least 2005. If we are unable to raise additional capital, we may be forced to curtail operations. If we succeed in raising additional capital through a licensing or financing transaction, it may affect our stock price and future revenues.
In order to maintain sufficient cash and investments to fund future operations, we will need to raise additional capital. We expect to seek to raise additional capital over the next 12 to 24 months through various alternatives, including licensing or sales of our technologies and drug candidates and through the sale of shares of our Class A common stock.
| | • | | If we raise capital through licensing or sales of one or more of our technologies and drug candidates, then we may lose an opportunity for product sales if a product is successfully developed, approved by the FDA and marketed. If we license any of our technologies or drug candidates, then the development of that product or technology may no longer be in our control. A licensee might not ever reach any of the milestones in a license agreement and we would not earn any additional payments in such an event. Further, if we sell any of our technologies or drug candidates, the sales price may not cover our investment in such technology or drug candidate. |
| | • | | If we raise capital by issuing additional shares of Class A common stock at a price per share less than the then-current market price per share, then the value of the shares of Class A common stock outstanding may be diluted or reduced. Further, even if we were to sell shares of common stock at prices equal to or higher than the current market price, the issuance of additional shares may depress the market price of our Class A common stock and dilute your voting rights in the Company. |
| | • | | We may not be able raise capital on terms that we find acceptable, if at all. If we are unable to raise additional capital to fund future operations, then we might have to reduce operations or defer or abandon one or more of our clinical or preclinical research programs. Any of these actions could be expected to have an adverse effect on our stock price. |
Our stock price is highly volatile and you may not be able to sell your shares at or above the price you pay for them.
The market price of our Class A common stock has been, and is likely to continue to be, highly volatile. The following factors, among others, could have a significant impact on the market price of our Class A common stock:
| | • | | our success or failure in entering into license and/or co-promotion arrangements for our products and product candidates; |
26
| | • | | unfavorable or delayed announcements by us regarding clinical trial results or results of operations, or favorable announcements by our competitors; |
| | • | | delays in meeting goals or performance milestones by us or our marketing partners; |
| | • | | comments made by securities analysts, including changes in, or failure to achieve, financial estimates or milestones; |
| |
| | • | | announcements of financing transactions and/or future sales of equity or debt securities; |
| | • | | announcements by us of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| | • | | sales of our Class A common stock by our directors, officers or significant shareholders; and |
| | • | | market and economic conditions. |
In addition, the market for biotechnology and pharmaceutical stocks has experienced significant price and volume fluctuations that are frequently unrelated to operating performance. This price volatility is often more pronounced for companies with a low stock price and a small market capitalization, such as ours. These broad market and industry factors might seriously harm the market price of our Class A common stock, regardless of our operating performance.
A significant decline in our stock price, or other factors, could also result in our Class A common stock being delisted from the American Stock Exchange and could initiate litigation, including a securities class action lawsuit. Such a lawsuit against us could result in substantial costs, potential liabilities and the diversion of management’s attention and resources.
Developing and testing a drug candidate is a very expensive and time-consuming process that may not ultimately lead to a marketable product.
The drug development process is lengthy and capital-intensive. Since September 1998, we have spent approximately $60 million in preclinical and clinical studies researching the safety and efficacy of our drug candidates and potential drug candidates. If any of our drug candidates fail to demonstrate the desired safety and efficacy, we may abandon the development of the compound, in which event we would not recover our expenditures incurred to date for that compound. If a compound appears to be safe and effective in preclinical studies, we may decide to proceed with human clinical trials. The full complement of clinical trials required to obtain regulatory approval for a new drug may involve tens-of-millions of dollars. Because of our limited financial resources, we may be required to license the compound to a pharmaceutical company with greater financial resources in order to complete development of the drug. We may be unable to find a large pharmaceutical company interested in licensing the drug or, if we do locate such a licensee, that the proposed license terms will be acceptable to the Company. In the event that we are unable to find a large pharmaceutical partner or licensee on acceptable terms, we may be forced to abandon one or more of our drug candidates.
We expect our quarterly operating results to fluctuate significantly from period-to-period for a number of reasons.
Historically, we have had only limited recurring revenue. As a result, operating results have been, and will continue to be, subject to significant quarterly fluctuations based on a variety of factors, including:
| | • | | Co-promotion or License Arrangements— We are currently seeking co-promotion or |
27
| | | | licensing partners for docosanol 10% cream and Neurodex, as well as for our compounds targeting IgE (allergy and asthma), MIF (inflammation) and apolipoprotein A1 (cholesterol). It is difficult to predict whether any of these discussions will result in a partnering or license arrangement and what the financial terms of such an arrangement might be. If we do enter into any such arrangements, the recognition of revenue under that arrangement may depend on the efforts and performance of our licensees or partners in reaching milestones that are outside of our control. Such milestones may include specific events, such as regulatory approval, product launch, the passage of time, or reaching a sales threshold. |
| |
| | • | | Limited Rights to Future Abreva Royalties— In December 2002 we sold to Drug Royalty USA the rights to a substantial portion of our future royalty revenues from sales of Abreva by GlaxoSmithKline. We will not receive any future royalty payments unless and until annual Abreva wholesale sales exceed $62 million, at which time we will receive one-half of the stated royalty rate on any excess sales. We expect that any royalty payments on these excess sales, if any, would occur only once a year, after the end of each calendar year. |
| | • | | Concentration of Significant Customers, Suppliers and Industries— Milestone payments, royalties earned, and revenues recognized from the sale of rights to royalties from a single licensee (GlaxoSmithKline) accounted for approximately 73% and 95% of our fiscal 2003 and 2002 revenues, respectively. We have now received all of the milestone payments from GlaxoSmithKline for North America. With the sale of our Abreva royalty rights to Drug Royalty USA, future royalty payments from GlaxoSmithKline will come exclusively from our remaining 50% share of Abreva royalties on contract sales in excess of $62 million a year. Additionally, we purchase our raw materials from a sole foreign supplier that has been approved for manufacture by the FDA. Any disturbances or delays in the manufacture of the raw materials could seriously and adversely affect our business. |
| | • | | Acquisitions/Alliances— If, in the future, we acquire technologies, products, or businesses, or we form alliances with companies requiring technology investments or commitments, we will face a number of risks to our business. The risks that we may encounter include those associated with integrating operations, personnel, and technologies acquired or licensed, and the potential for unknown liabilities of the acquired business. Our business and operating results on a quarterly basis could be adversely affected if any of our acquisition or alliance activities, to the extent they exist in the future, are not successful. |
AVANIR and its licensees may not be successful in obtaining regulatory approval of docosanol 10% cream immediately as an over-the-counter (“OTC”) product in the rest of the world, or in licensing, marketing and selling the product in foreign countries.
Currently, docosanol 10% cream is approved for sale in the United States, Canada, Korea, Israel and Sweden and we are currently seeking regulatory approval in various other countries in the European Union and intend to seek approval through a licensee in Japan. AVANIR and its licensees face a wide variety of risks in foreign countries in obtaining regulatory approval and in marketing and selling docosanol 10% cream, including:
| | • | | Regulatory approval requirements differ by country, and obtaining approvals to market the drug in foreign countries may be difficult to obtain, may require additional costly and time consuming clinical trials, or may require prescription status first before obtaining sufficient experience to warrant approval as an OTC product; |
| | • | | Building product awareness of a new drug, whether prescription or OTC, among customers or retail store decision makers may require a substantial amount of product promotion, which |
28
| | | | does not guarantee success; |
| | • | | Consumers may not perceive that docosanol 10% cream is superior to existing and potentially new OTC products for oral herpes; |
| | • | | Acceptance of docosanol 10% cream in the OTC consumer market may not be widespread; and |
| |
| | • | | Potential price erosion could occur due to competitive products and responses to our product’s introduction. |
Foreign sales of docosanol 10% cream and other potential products are subject to various foreign trade risks.
Our license agreement with GlaxoSmithKline is for the United States and Canada. We also have exclusive license agreements for docosanol 10% cream for Israel, South Korea, Italy and Egypt. We are holding discussions with other potential licensees for marketing and selling docosanol 10% cream in other countries not already licensed. However, we may not finalize any license or distribution arrangements for other territories on a timely basis or on favorable terms, if at all. Further, our foreign licensees expose us to various foreign trade risks relating to development and marketing of docosanol 10% cream. We may arrange for contracts in the future for the manufacture, marketing and distribution of docosanol 10% cream overseas by foreign licensees, which will be substantially outside our control. Even if we are able to obtain experienced licensees in foreign markets, specific risks that could impact significantly our potential revenues on foreign sales include:
| | • | | difficulties in obtaining regulatory approval of docosanol 10% cream in foreign countries; |
| | • | | changes in the regulatory and competitive environments in foreign countries; |
| | • | | changes in a specific country’s or region’s political or economic conditions, including related to terrorism; |
| | • | | difficulty in finding foreign partners with sufficient capital to effectively launch, market and promote the product; |
| | • | | difficulties in managing operations across disparate geographic areas; |
| | • | | fluctuations in foreign currency exchange rates; |
| | • | | prices of competitive products; |
| | • | | difficulties associated with enforcing agreements through foreign legal systems; |
| | • | | trade protection measures, including customs duties and export quotas; and |
| | • | | foreign tax withholding laws. |
29
The Board of Directors has the authority to effect a reverse stock split within a stated range until March 18, 2005. If implemented, the reverse stock split may negatively affect the price and liquidity of our Class A common stock.
At our 2004 Annual Meeting of Shareholders, the Board of Directors received the authority to implement, within its discretion and for a period of one year, a reverse split of our Class A common stock within a range of 1:2 to 1:12.5. If the Board of Directors were to effect a reverse stock split, the bid price of the Class A common stock may not continue at a level in proportion to the reduction in the number of outstanding shares resulting from the reverse stock split. For example, if the Board of Directors decided to implement a reverse stock split at a ratio of 1-for-5, that the post-split market price of our Class A common stock may not be at least five times the pre-split price. Accordingly, the total market capitalization of our Class A common stock after a reverse stock split, if implemented, could be lower than the total market capitalization before the proposed reverse stock split. Additionally, the liquidity of our Class A common stock could be affected adversely by the reduced number of shares outstanding after the reverse stock split.
Our inability to attract and retain key management and scientific personnel could negatively affect our business.
Our success depends on the performance of a small core staff of key management and scientific employees with biotechnology experience. Given our small staff size and programs currently under development, we depend substantially on our ability to hire, train, retain and motivate high quality personnel, especially our scientists and management team in this field. If we were to lose one or more of our key scientists, then we would likely lose some portion of our institutional knowledge and technical know-how, potentially causing a substantial delay in one or more of our development programs until adequate replacement personnel could be hired and trained. Other than our chief executive officer, our executives do not have employment agreements. We do not have “key person” life insurance policies for any of our executives. The industry in which we compete has a high level of employee mobility and aggressive recruiting of skilled personnel. This type of environment creates intense competition for qualified personnel, particularly in product research and development, sales and marketing, and accounting and finance.
Our patents may be challenged and our pending patents may be denied. Either result would seriously jeopardize our ability to compete in the intended markets for our proposed products.
We rely substantially on the protection of our intellectual property through our ownership or control of 85 issued patents and 254 patent applications. Patents and patent applications owned or controlled by the Company are for docosanol-related products and technologies, Neurodex, compounds capable of regulating the target IgE in controlling symptoms of allergy and asthma, compounds capable of regulating the target MIF in the treatment of inflammatory diseases, compounds targeting apolipoprotein A1 for the treatment of cholesterol, and Xenerex technologies for developing monoclonal antibodies. Because of the competitive nature of the biopharmaceutical industry, we cannot assure you that:
| | • | | the claims in any pending patent applications will be allowed or that patents will be granted; |
| | • | | present and future competitors will not develop similar or superior technologies independently, duplicate our technologies or design around the patented aspects of our technologies; |
| | • | | our proposed technologies will not infringe other patents or rights owned by others, including licenses that may not be available to us; |
30
| | • | | any of our issued patents will provide us with significant competitive advantages; or |
| | • | | challenges will not be instituted against the validity or enforceability of any patent that we own or, if instituted, that these challenges will not be successful. |
Our inability to obtain or maintain patent protections for our products in foreign markets may negatively affect our financial condition.
The process for the approval of patent applications in foreign countries may differ significantly from the process in the U.S. These differences may delay our plans to market and sell docosanol 10% cream and other products in the international marketplace. Approval in one country does not indicate that approval will be obtained in other countries. The patent authorities in each country administer that country’s laws and regulations relating to patents independently of the laws and regulations of any other country and we must seek and obtain the patents separately. Our inability to obtain or maintain patent protections for docosanol 10% cream and other products in foreign markets would severely hamper our ability to generate international sales from our first product and other products still under development.
We are dependent on third parties to manufacture our drug and drug-candidate compounds. The failure of these third parties to perform successfully could harm our business.
We have utilized, and intend to continue utilizing, third parties to manufacture docosanol 10% cream, Neurodex and our other drug candidates. We have no experience in manufacturing and do not have any manufacturing facilities. Currently, we have only a single supplier for docosanol 10% cream and we do not have any long-term supply agreements in place with this manufacturer. Although we and GlaxoSmithKline maintain a strategic reserve of docosanol 10% cream to mitigate against a short-term supply disruption, any sustained disruption of our supply could result in shipping and sales delays and additional costs that could harm our operations.
Developing new pharmaceutical products for human use involves product liability risks, for which we currently have limited insurance coverage.
The testing, marketing, and sale of pharmaceutical products involves the risk of product liability claims by consumers and other third parties. We maintain product liability insurance coverage for our clinical trials in the amount of $5 million per incident and $5 million in the aggregate. However, product liability claims can be high in the pharmaceutical industry and our insurance may not sufficiently cover our actual liabilities. If a suit against our business or proposed products is successful, then the lack or insufficiency of insurance coverage could affect materially and adversely our business and financial condition. Furthermore, various distributors of pharmaceutical products require minimum product liability insurance coverage before their purchase or acceptance of products for distribution. Failure to satisfy these insurance requirements could impede our ability to achieve broad distribution of our proposed products.
Abreva faces competition from a number of existing and well-established products and the companies that market their products.
We have the opportunity to earn royalties on Abreva product wholesale sales if sales exceed $62 million a year. Abreva competes with several other products for oral-facial herpes currently on the market in the U.S., as well as other products or potential products that are or may be under development or undergoing FDA review. Most of the competing products are manufactured by companies having substantial financial resources, research and development facilities and manufacturing and marketing experience. Even with Abreva being marketed by one of the world’s largest consumer healthcare companies, GlaxoSmithKline, not all competitive responses and the impacts of those responses can be foreseen.
31
Business interruptions could adversely affect our business.
Our operations are vulnerable to interruption by fire, earthquake, power loss, telecommunications failure, and other events beyond our control. For example, during 2001 and again in 2003, we experienced electrical power outages lasting several hours. The loss of electrical power for any significant periods of time could adversely affect our ability to conduct experiments and could also harm our vendors. Further, we could lose valuable data made to date in experiments currently underway. We have mitigated the severity of power losses by installing emergency power equipment, which we have used on several occasions to supply electricity in the areas that we consider to be the most critical to our operations.
However, the emergency power units do not cover all of our electrical needs and, further, they might not operate properly in the event of a power loss.
Our financial results could be affected by potential changes in the accounting rules governing the recognition of stock-based compensation expense.
We measure compensation expense for our employee stock compensation plans under the intrinsic value method of accounting prescribed by Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees.” In addition, we provide pro forma disclosures of our operating results in our Notes to Consolidated Financial Statements as if the fair value method of accounting had been applied in accordance with SFAS No. 123, “Accounting for Stock-based Compensation.” Had we accounted for our compensation expense under the fair value method of accounting prescribed by SFAS No. 123, the charges would have been significantly higher, by approximately $1,554,000, $2,176,000, and $1,540,000 during fiscal 2003, 2002, and 2001, respectively. Recently, the Financial Accounting Standards Board proposed changes to accounting rules concerning the recognition of stock option compensation expense. If these proposals are implemented, we and other companies would be required to measure compensation expense using the fair value method, which would adversely affect our results of operations by reducing our income or increasing our losses by an amount equal to the difference in the two measurement methods.
Provisions in our charter documents and our shareholder rights plan could prevent or delay a change of control in a transaction that offers our shareholders a premium for their stock.
Certain provisions of our charter documents may have an anti-takeover effect and could discourage a third party from acquiring control of us without approval of our board of directors. Additionally, we adopted a Shareholder Rights Plan in 1999 for the purpose of requiring bidders to negotiate with our Board of Directors before attempting a takeover of our company. Although the Shareholder Rights Plan is intended to prevent coercive or low offers, it could have the effect of discouraging, delaying or preventing a third party from acquiring us in a transaction that may otherwise offer a premium over our then-current stock price.
Risks Relating to Our Industry
The pharmaceutical industry is highly competitive and most of our competitors are larger and have greater resources. As a result, we face significant competitive hurdles.
The pharmaceutical and biotechnology industries are highly competitive and subject to significant and rapid technological change. We compete with hundreds of companies that develop and market products and technologies in similar areas as our research. For example, docosanol 10% cream faces worldwide competition from the following products:
| | • | | OTC monograph preparations, including Carmex®, Zilactin®, Campho®, Orajel®, |
32
| | • | | Zovirax® acyclovir (oral and topical) and Valtrex® valacyclovir (oral) prescription products marketed by Biovail Corporation and GlaxoSmithKline, respectively, and |
| | • | | Famvir® famciclovir (oral) and Denavir® penciclovir (topical) prescription products marketed by Novartis. |
Our competitors may have specific expertise and technologies that are better than ours and many of these companies, either alone or together with their research partners, have substantially greater financial resources, larger research and development staffs, and substantially greater experience than we do. Accordingly, our competitors may develop superior products or may develop competing products more rapidly. If we commence commercial sales of our products, we will also be competing with respect to manufacturing efficiencies and marketing capabilities, areas in which we have limited or no direct experience.
Our industry is highly regulated and our failure or inability to comply with government regulations regarding the development, production, testing, manufacturing and marketing of our products may adversely affect our operations.
Governmental authorities in the U.S., including the FDA, and other countries highly regulate the development, production, testing, manufacturing and marketing of pharmaceutical products. The clinical testing and regulatory approval process can take a number of years and requires the expenditure of substantial resources. Failure to obtain, or delays in obtaining, these approvals will adversely affect our business operations, including our ability to commence marketing of any of the proposed products. We may find it necessary to use a significant portion of our financial resources for research and development and the clinical trials necessary to obtain these approvals for our proposed products. We will continue to incur costs of development without any assurance that we will ever obtain regulatory approvals for any of our products under development. Additionally, we cannot predict the extent to which adverse governmental regulation might arise from future U.S. or foreign legislative or administrative action. Moreover, we cannot predict with accuracy the effects of any future changes in the regulatory approval process and in the domestic health care system for which we develop our products, or the costs of on-going compliance regulations after marketing approval has been obtained. Future changes could affect adversely the time frame required for regulatory review, our financial resources, and the sale prices of our proposed products, if approved for sale.
Companies in our industry must protect their intellectual property rights and operate without infringing on or misappropriating the proprietary rights of others. Our inability to do so could be costly and could significantly affect our business prospects.
Biotechnology companies such as AVANIR rely heavily on intellectual property rights to protect their innovations. Although we attempt to protect these rights by regularly filing patent applications and requiring all employees to sign confidentiality agreements, we cannot assure you that patents will issue, that secrecy obligations will be honored, or that others will not independently develop similar or superior technology. Additionally, if our consultants, key employees or other third parties apply to our projects technological information independently developed by them or by others, then disputes may arise as to the ownership rights of these innovations.
Even if we successfully preserve our intellectual property rights, other biotechnology or pharmaceutical companies may allege that our technology infringes on their rights. Intellectual property litigation is costly, and, even if we were to prevail in such a dispute, the cost of such litigation would adversely affect our business, financial condition and results of operations. Litigation is also time consuming and would
33
divert management’s attention and resources away from our operations and other activities. If we were not to prevail in any litigation, in addition to any damages we would have to pay, we could be required to stop the infringing activity or obtain a license. Any required license might not be available to us on acceptable terms. Some licenses might be non-exclusive, and our competitors could have access to the same technology licensed to us. If we were to fail to obtain a required license or were unable to design around a competitor’s patent, we would be unable to sell or continue to develop some of our products, which would have a material adverse affect on our business, financial condition and results of operations.
Companies in our industry are frequently subjected to product liability claims, which can be very costly to defend against.
In the ordinary course of business, biotechnology and pharmaceutical companies face various claims brought by third parties, including claims relating to the safety or efficacy of products. Any of these claims could subject us to costly litigation and, while we generally believe that we have adequate insurance to cover many different types of liabilities, our insurance carriers may deny coverage or our policy limits may be inadequate to fully satisfy any damage awards or settlements. If this were to happen, the payment of any such awards could have a material adverse effect on our operations and financial position. Additionally, any such claims, whether or not successful, could damage our reputation and business.
| | | |
Item 3. | | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest rate sensitivity
We are exposed to market risks related to changes in interest rates. Our investment portfolio consists primarily of fixed income instruments with an average duration of seven-tenths of a year as of March 31, 2004 (2.0 years as of September 30, 2003). The primary objective of our investments in debt securities is to preserve principal while achieving attractive yields, without significantly increasing risk. We carry some investments that we intend to hold to maturity and others that we classify as available-for-sale. These available-for-sale securities are subject to interest rate risk. In general, we would expect that the volatility of this portfolio would increase as its duration increases.
Foreign currency exchange rate sensitivity
Because substantially all of our revenue, expense, and capital purchasing activities are transacted in U.S. dollars, our exposure to foreign currency exchange rates is immaterial. However, in the future we could face increasing exposure to foreign currency exchange rates as we expand international distribution of docosanol 10% cream. Until such time as we are faced with material amounts of foreign currency exchange rate risks, we do not plan to use derivative financial instruments, which can be used to hedge such risks. We will evaluate the use of derivative financial instruments to hedge our exposures as the needs and risks should arise.
Item 4. CONTROLS AND PROCEDURES
The Company’s Chief Executive Officer and Chief Financial Officer carried out an evaluation of the effectiveness of the Company’s “disclosure controls and procedures” (as defined in the Securities Exchange Act of 1934 Rules 13a-15(e) and 15(d)-15(e)), as of the end of the period covered by this report. Based on that evaluation, these officers have concluded that the Company’s disclosure controls and procedures were adequate and designed to ensure that material information relating to the Company and the Company’s consolidated subsidiary would be made known to them by others within those entities.
34
PART II OTHER INFORMATION
Items 1-3. NOT APPLICABLE
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
The Annual Meeting of Shareholders of AVANIR Pharmaceuticals was convened on March 18, 2004, at 10:00 a.m. local time. There were issued and outstanding on January 20, 2004, the record date, 71,266,550 shares of Class A common stock, each share being entitled to one vote, constituting all of the outstanding voting securities of the Company. There were present at the meeting in person or by proxy, shareholders of the Company who were the holders of 67,078,172 votes of the Company’s common stock entitled to vote thereat, constituting a quorum. The following proposals received the number of votes set forth below:
| | | | | | | | | | | | | | | | | | | |
Directors
| | | | For
| | Withhold
|
| | | Dennis J. Carlo, Ph.D. | | | 54,379,138 | | | | 12,670,389 | |
| | | Kenneth E. Olson | | | 56,434,715 | | | | 10,614,812 | |
| | | Gerald J. Yakatan, Ph.D. | | | 55,944,517 | | | | 11,105,010 | |
| |
Proposals
| | | | For
| | Against
| | Abstain
| | | | |
| 2. | | Restate Articles of Incorporation | | | 57,907,905 | | | | 7,728,256 | | | | 1,442,011 | | | | | |
| 3. | | Increase Authorized Class A | | | | | | | | | | | | | | | | |
| | | Common Stock | | | 50,585,585 | | | | 15,913,111 | | | | 579,475 | | | | | |
| 4. | | Approve Reverse Stock Split | | | | | | | | | | | | | | | | |
| | | Within Stated Range | | | 49,307,358 | | | | 17,225,442 | | | | 545,372 | | | | | |
| 5. | | Ratification of Deloitte & | | | | | | | | | | | | | | | | |
| | | Touche LLP as Independent Auditors | | | 61,547,468 | | | | 4,279,960 | | | | 1,250,744 | | | | | |
Item 6. EXHIBITS AND REPORTS ON FORM 8-K
(a) Exhibits
| | 15.0 | | Letter on unaudited interim financial information |
| |
| | 31.1 | | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 2002 |
| |
| | 31.2 | | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 2002 |
| |
| | 32.1 | | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 2002 |
| |
| | 32.2 | | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 2002 |
(b) Reports on Form 8-K
On February 12, 2004, the registrant filed a current report on Form 8-K furnishing, under Items 7 and 12, a press release announcing the results of operations for the quarter ended December 31, 2003.
On April 5, 2004, the registrant filed a current report on Form 8-K disclosing, under Item 5 that it had amended and restated its Articles of Incorporation for the purposes of restating certain earlier amendments, eliminating certain classes and series of stock no longer outstanding, and giving effect to an increase in the authorized number of shares of Class A common stock, all of which were approved at the Annual Meeting of Shareholders held on March 18, 2004.
35
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | |
Signature
| | Title
| | Date
|
/s/Gerald J. Yakatan, Ph.D.
Gerald J. Yakatan, Ph.D. | | President and Chief
Executive Officer
(Principal Executive Officer) | | May 14, 2004 |
/s/Gregory P. Hanson, CMA
Gregory P. Hanson, CMA | | Vice President, Finance and Chief
Financial Officer
(Principal Financial and Accounting Officer) | | May 14, 2004 |
36
EXHIBIT INDEX
| | | |
Exhibit No.
| | Description
|
| 15.0 | | Letter on unaudited interim financial information |
| 31.1 | | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 2002 |
| 31.2 | | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 2002 |
| 32.1 | | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 2002 |
| 32.2 | | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 2002 |
37
