Exhibit 99.1

Exhibit 99.1
The women’s health Company Presentation to Prospective Lenders
HOLOGIC®
July 2012

The women’s health company
Agenda
Executive Summary Transaction Merits Company Overview Key Credit Strengths Historical Financial Review
Appendix A: Non-GAAP Adjustments
Appendix B: Disclaimers
HOLOGIC

The women’s health company
Transaction Summary
On April 30, 2012 Hologic, Inc. (“Hologic” or the “Company”) announced the acquisition of all of the outstanding shares of Gen-Probe, Inc. (“Gen-Probe”) for $82.75 per share in cash for a total equity purchase price of approximately $3.9 billion
The 100% cash transaction will be funded through available cash and additional financing. Hologic plans to syndicate:
— $300 million Revolving Credit Facility (expected to be unused at close)
— $1,000 million of Term Loan A
— $1,750 million of Term Loan B
— $750 million of Senior Notes
The acquisition is subject to certain closing conditions including approval by Gen-Probe shareholders and regulatory authorities
— On June 18, 2012 the waiting period under Hart-Scott-Rodino expired, satisfying a condition to closing
— Expected closing in early August
As of LTM 31-Mar-2012, on a pro forma adjusted basis, the combined Company generated:
— $2,449 million of revenue
— $897 million of pro forma adjusted EBITDA, including $75 million of indentified cost savings
The combination of Hologic and Gen-Probe combines two leading diagnostic franchises with best-in-class technologies
HOLOGIC
The womens health company
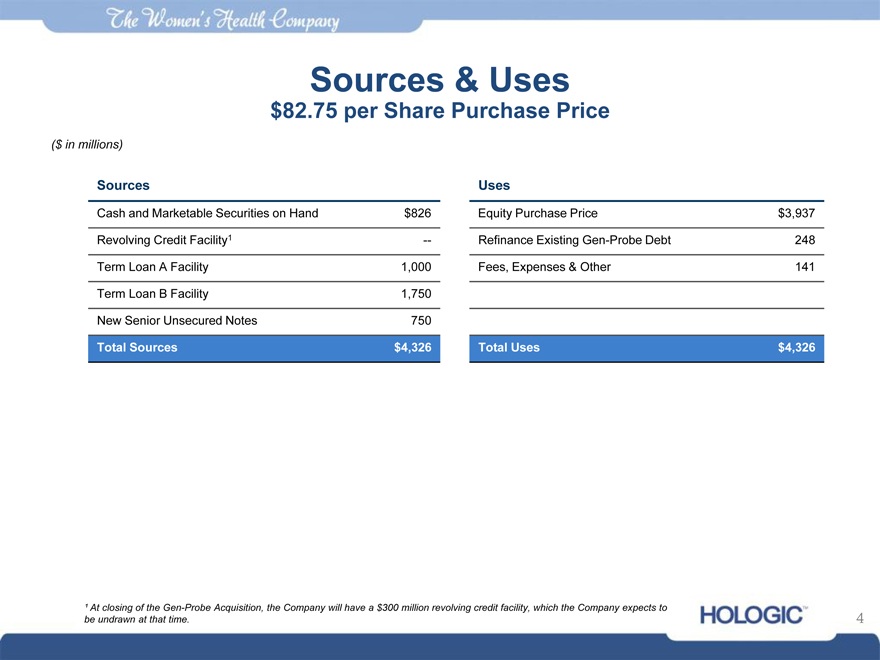
Sources & Uses
$82.75 per Share Purchase Price
($ in millions)
Sources Uses
Cash and Marketable Securities on Hand $826 Equity Purchase Price $3,937
Revolving Credit Facility1 — Refinance Existing Gen-Probe Debt 248
Term Loan A Facility 1,000 Fees, Expenses & Other 141
Term Loan B Facility 1,750
New Senior Unsecured Notes 750
Total Sources $4,326 Total Uses $4,326
¹ At closing of the Gen-Probe Acquisition, the Company will have a $300 million revolving credit facility, which the Company expects to be undrawn at that time.
HOLOGIC
The women’s health company
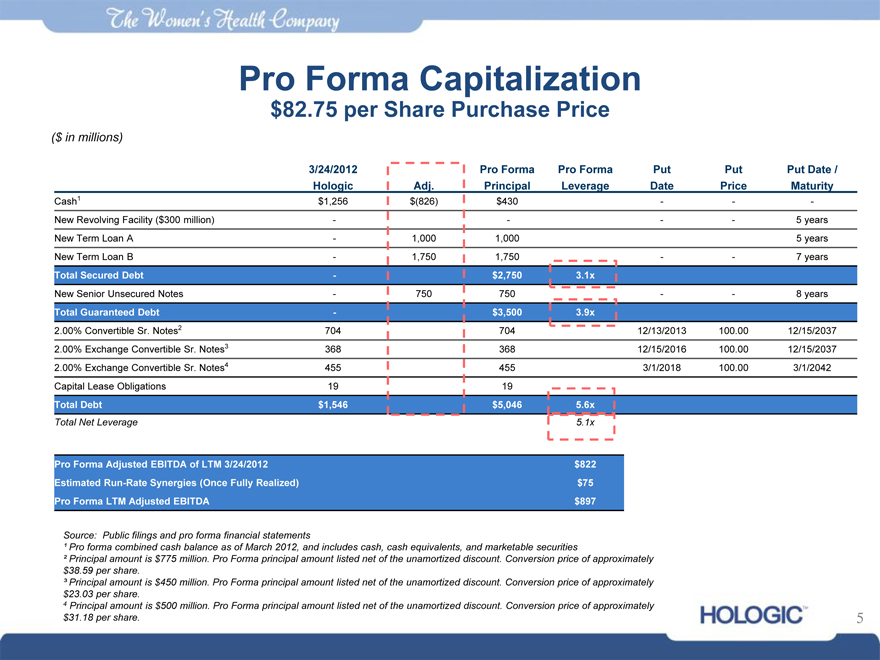
Pro Forma Capitalization
$82.75 per Share Purchase Price
($ in millions)
3/24/2012 Pro Forma Pro Forma Put Put Put Date /
Hologic Adj. Principal Leverage Date Price Maturity
Cash1 $1,256 $(826) $430 — — -
New Revolving Facility ($300 million) — — — — 5 years
New Term Loan A — 1,000 1,000 5 years
New Term Loan B — 1,750 1,750 — — 7 years
Total Secured Debt — $2,750 3.1x
New Senior Unsecured Notes — 750 750 — — 8 years
Total Guaranteed Debt — $3,500 3.9x
2.00% | | Convertible Sr. Notes2 704 704 12/13/2013 100.00 12/15/2037 |
2.00% | | Exchange Convertible Sr. Notes3 368 368 12/15/2016 100.00 12/15/2037 |
2.00% | | Exchange Convertible Sr. Notes4 455 455 3/1/2018 100.00 3/1/2042 |
Capital Lease Obligations 19 19
Total Debt $1,546 $5,046 5.6x
Total Net Leverage 5.1x
Pro Forma Adjusted EBITDA of LTM 3/24/2012 $822
Estimated Run-Rate Synergies (Once Fully Realized) $75
Pro Forma LTM Adjusted EBITDA $897
Source: Public filings and pro forma financial statements
¹ Pro forma combined cash balance as of March 2012, and includes cash, cash equivalents, and marketable securities
² Principal amount is $775 million. Pro Forma principal amount listed net of the unamortized discount. Conversion price of approximately
$38.59 per share.
³ Principal amount is $450 million. Pro Forma principal amount listed net of the unamortized discount. Conversion price of approximately
$23.03 per share.
4 Principal amount is $500 million. Pro Forma principal amount listed net of the unamortized discount. Conversion price of approximately
$31.18 per share.
HOLOGIC

The women’s health company
Agenda
Executive Summary
Transaction Merits Company Overview Key Credit Strengths Historical Financial Review
Appendix A: Non-GAAP Adjustments
Appendix B: Disclaimers
HOLOGIC
The women’s health company
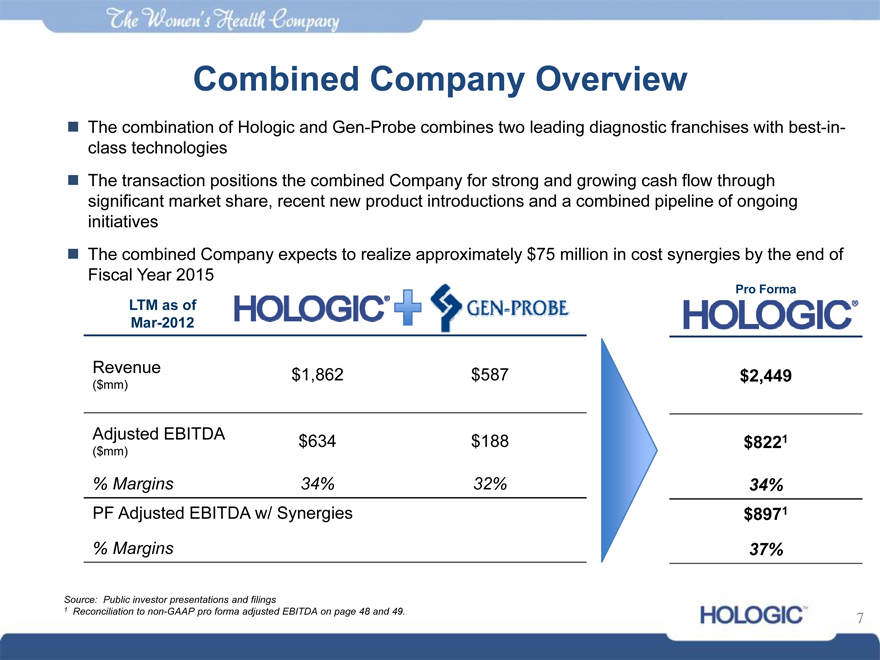
Combined Company Overview
The combination of Hologic and Gen-Probe combines two leading diagnostic franchises with best-in-class technologies
The transaction positions the combined Company for strong and growing cash flow through significant market share, recent new product introductions and a combined pipeline of ongoing initiatives
The combined Company expects to realize approximately $75 million in cost synergies by the end of Fiscal Year 2015
LTM as of Mar-2012
Pro Forma
Revenue $1,862 $587 $2,449
($mm)
Adjusted EBITDA $634 $188 $8221
($mm)
% Margins 34% 32% 34%
PF Adjusted EBITDA w/ Synergies $8971
% Margins 37%
Source: Public investor presentations and filings
1 | | Reconciliation to non-GAAP pro forma adjusted EBITDA on page 48 and 49. |
HOLOGIC

The women’s health company
Combination of Leading Domestic Franchises
The Gen-Probe Fit
Compelling Strategic Rationale Delivers Operational Strength
• #1 Stand-alone player in Molecular • Proven track record of new product
Diagnostics development
• Strengthens and enhances Hologic’s • Accelerates top- and bottom-line growth
Diagnostics franchise
• Cost synergies
• Enables further integration of cytology with
molecular diagnostics • Revenue synergies – significant potential
upside
• Provides access to best-in-class
automation in molecular diagnostics • Complementary sales force and products
• Streamlines development programs • Global distribution for Gen-Probe products
• Enhances recurring revenues stream with • Expand product development pipeline
additional disposables products
• Strong growth and margin profile
Source: Public investor presentations and filings
HOLOGIC
The women’s health company
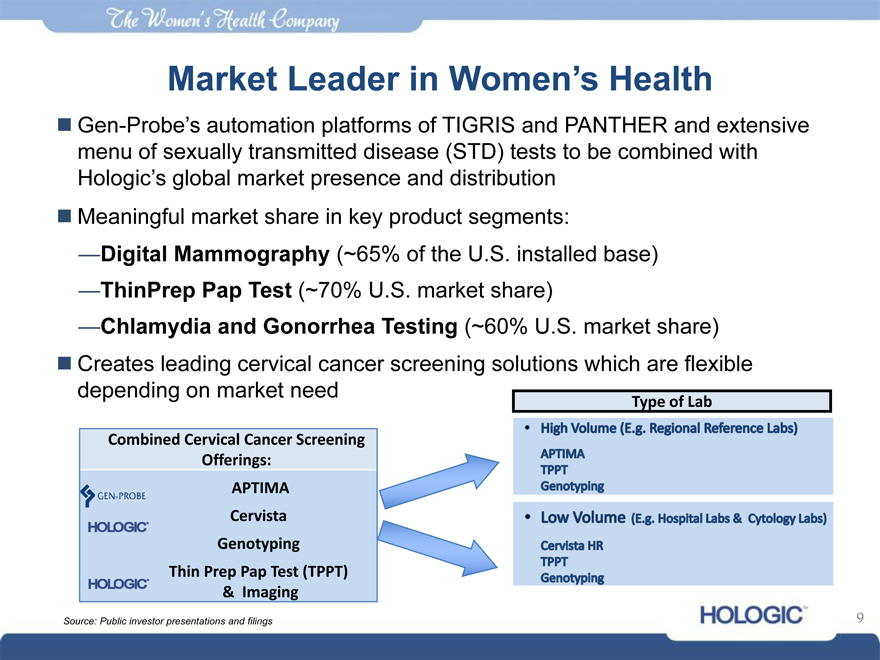
Market Leader in Women’s Health
Gen-Probe’s automation platforms of TIGRIS and PANTHER and extensive menu of sexually transmitted disease (STD) tests to be combined with Hologic’s global market presence and distribution
Meaningful market share in key product segments:
—Digital Mammography (~65% of the U.S. installed base)
—ThinPrep Pap Test (~70% U.S. market share)
—Chlamydia and Gonorrhea Testing (~60% U.S. market share)
Creates leading cervical cancer screening solutions which are flexible depending on market need
Combined Cervical Cancer Screening Offerings: APTIMA
Cervista Genotyping Thin Prep Pap Test (TPPT)
& Imaging
Type of Lab
High volume (E.g. Regional Reference Labs)
APTIM
TPPT
Genotyping
Low Volume (E.g Hospital Labs & Cytology Labs)
Cervista HR
TPPT
Genotyping
Source: Public investor presentations and filings
HOLOGIC
9
The women’s health company
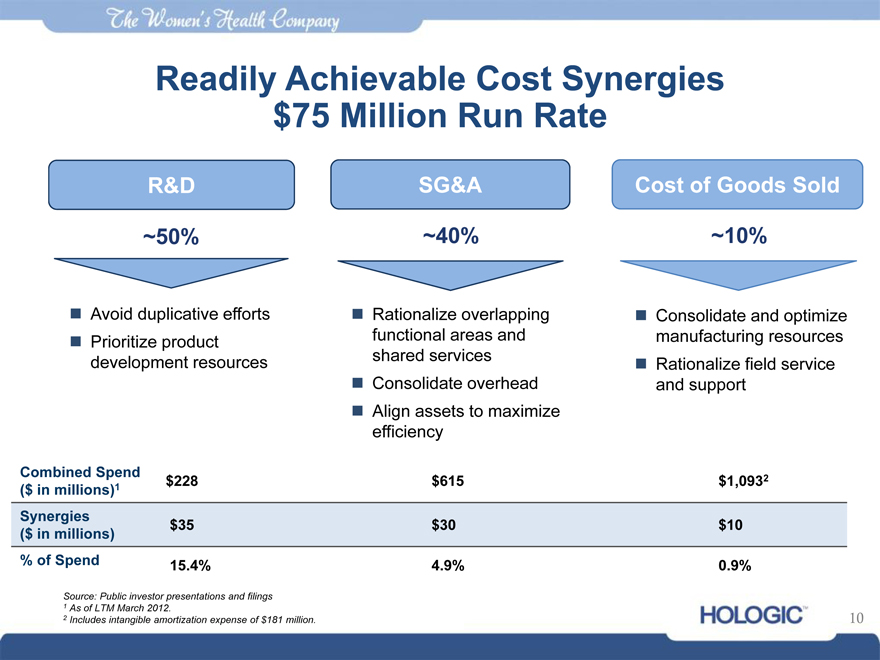
Readily Achievable Cost Synergies
$75 Million Run Rate
R&D SG&A Cost of Goods Sold
~50% ~40% ~10%
Avoid duplicative efforts
Prioritize product development resources
Rationalize overlapping functional areas and shared services
Consolidate overhead
Align assets to maximize efficiency
Consolidate and optimize manufacturing resources
Rationalize field service and support
Combined Spend $228 $615 $1,0932
($ in millions)1
Synergies $35 $30 $10
($ in millions)
% of S 15.4% 4.9% 0.9%
Source: Public investor presentations and filings
2 | | Includes intangible amortization expense of $181 million. |
HOLOGIC
10
The women’s health company
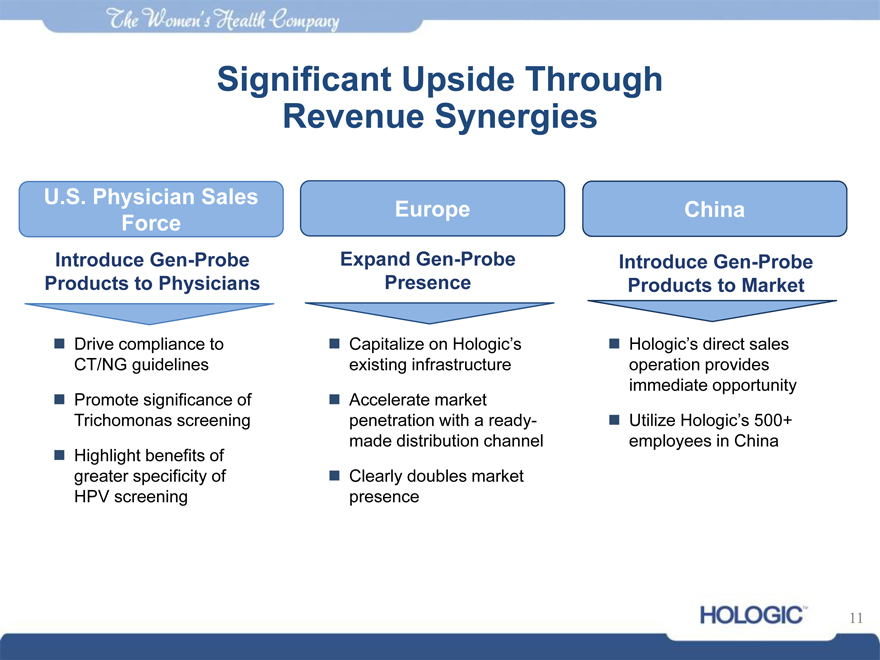
Significant Upside Through Revenue Synergies
U.S. Physician Sales
Europe China Force
Introduce Gen-Probe Expand Gen-Probe Introduce Gen-Probe Products to Physicians Presence Products to Market
Drive compliance to CT/NG guidelines
Promote significance of Trichomonas screening
Highlight benefits of greater specificity of HPV screening
Capitalize on Hologic’s existing infrastructure
Accelerate market penetration with a ready-made distribution channel
Clearly doubles market presence
Hologic’s direct sales operation provides immediate opportunity
Utilize Hologic’s 500+ employees in China
HOLOGIC
11
The Women’s Health Company
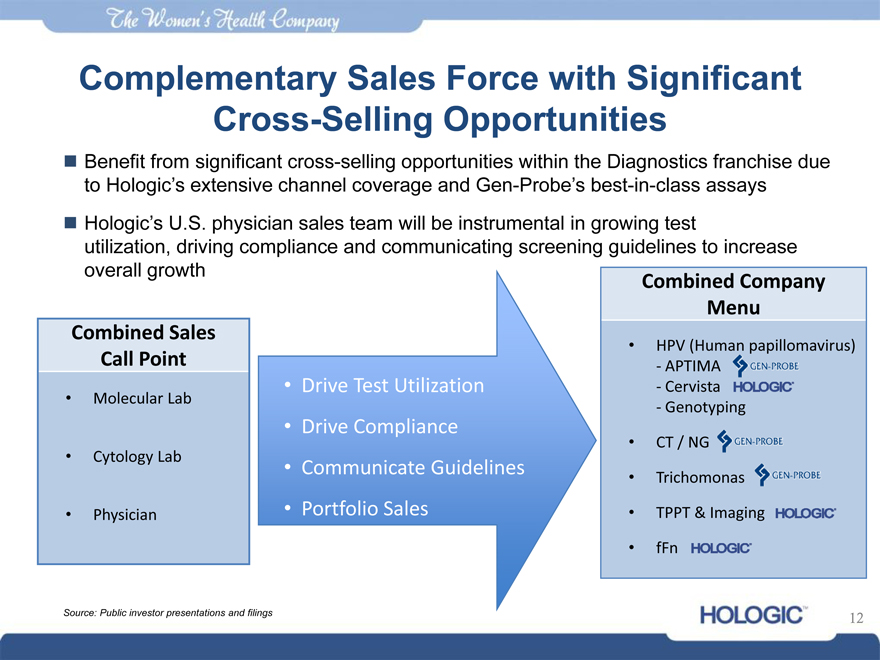
Complementary Sales Force with Significant Cross-Selling Opportunities
Benefit from significant cross-selling opportunities within the Diagnostics franchise due to Hologic’s extensive channel coverage and Gen-Probe’s best-in-class assays
Hologic’s U.S. physician sales team will be instrumental in growing test utilization, driving compliance and communicating screening guidelines to increase overall growth
Combined Sales Call Point
• Molecular Lab
• Cytology Lab
• Physician
• Drive Test Utilization
• Drive Compliance
• Communicate Guidelines
• Portfolio Sales
Menu
Combined Company
• HPV (Human papillomavirus)
- APTIMA
- Cervista
- Genotyping
• CT / NG
• Trichomonas
• TPPT & Imaging • fFn
Source: Public investor presentations and filings
HOLOGIC
12

The Women’ss Health Company
Roadmap to Completion
Management Team Focused on Seamless Integration
Integration teams report to CEO
Retention of Gen-Probe senior management team and San Diego presence (under way)
Continuity of well-recognized Gen-Probe brand
Regulatory authorities approvals
—On June 18, 2012 the waiting period under Hart-Scott-Rodino expired
Gen-Probe shareholder vote
Expected close August 2012
Source: Public investor presentations and filings
HOLOGIC
13

The Women’s Health Company
Agenda Executive Summary Transaction Merits Company Overview Key Credit Strengths Historical Financial Review
Appendix A: Non-GAAP Adjustments
Appendix B: Disclaimers
HOLOGIC
14
The women’s health company
Overview of Hologic Business Description
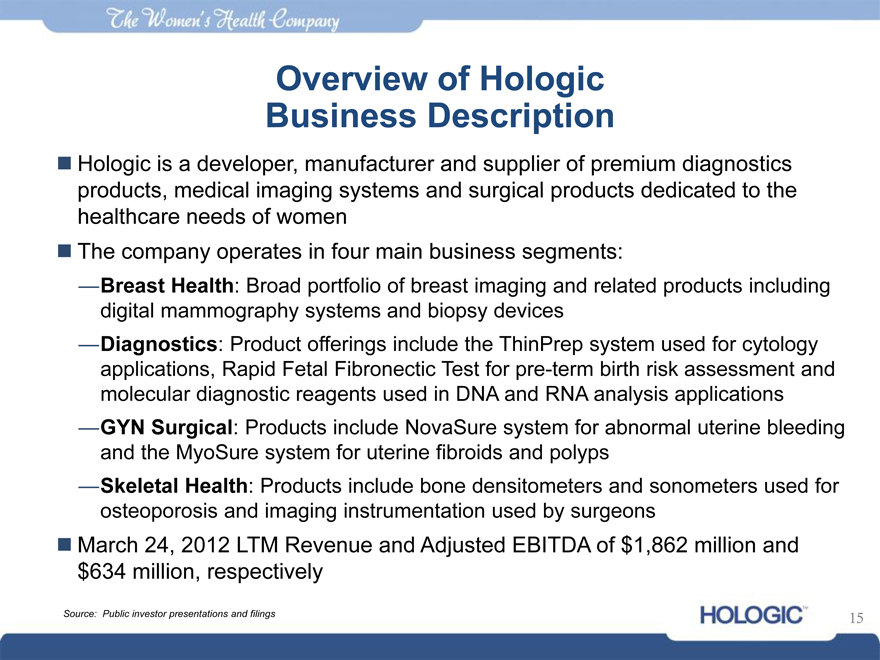
Hologic is a developer, manufacturer and supplier of premium diagnostics products, medical imaging systems and surgical products dedicated to the healthcare needs of women
The company operates in four main business segments:
—Breast Health: Broad portfolio of breast imaging and related products including digital mammography systems and biopsy devices
—Diagnostics: Product offerings include the ThinPrep system used for cytology applications, Rapid Fetal Fibronectic Test for pre-term birth risk assessment and molecular diagnostic reagents used in DNA and RNA analysis applications
—GYN Surgical: Products include NovaSure system for abnormal uterine bleeding and the MyoSure system for uterine fibroids and polyps
—Skeletal Health: Products include bone densitometers and sonometers used for osteoporosis and imaging instrumentation used by surgeons
March 24, 2012 LTM Revenue and Adjusted EBITDA of $1,862 million and $634 million, respectively
Source: Public investor presentations and filings
HOLOGIC
15
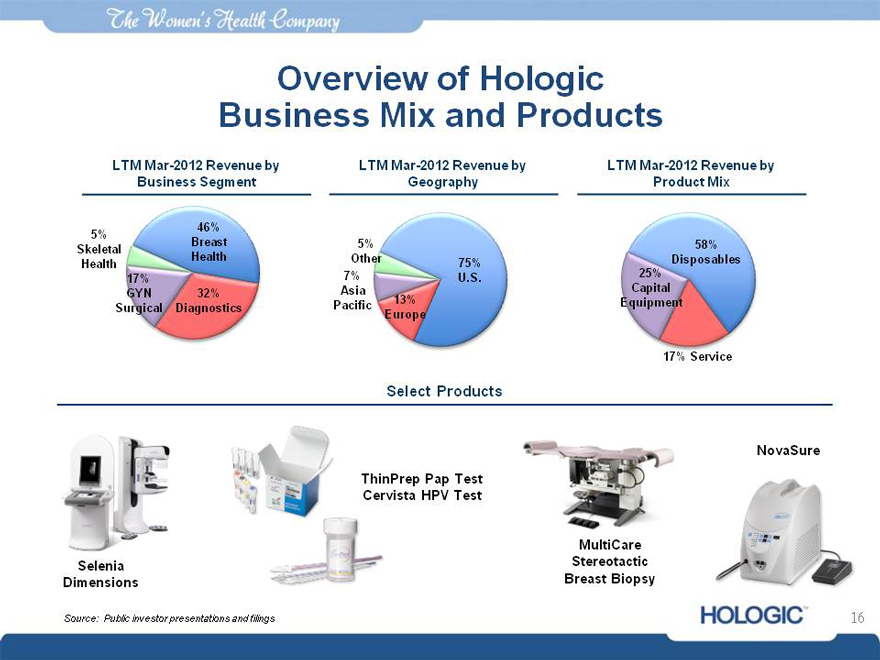
The women’s health company
Overview of Hologic Business Mix and Products
LTM Mar-2012 Revenue by LTM Mar-2012 Revenue by LTM Mar-2012 Revenue by Business Segment Geography Product Mix
5% Skeletal Health
46% Breast Health
17% GYN
Surgical
32% Diagnostics
5%
Other 75%
7% U.S.
Asia
Pacific 13% Europe
58% Disposables 25% Capital Equipment
17% Service
Select Products
Selenia Dimensions
ThinPrep Pap Test Cervista HPV Test
MultiCare Stereotactic Breast Biopsy
NovaSure
Source: Public investor presentations and filings
HOLOGIC
16
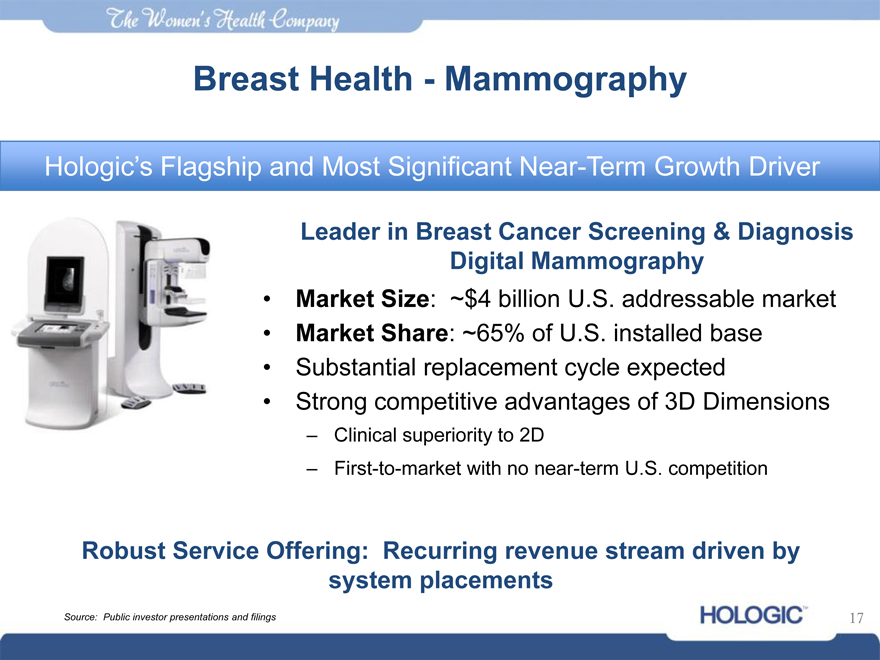
The womens health company
Breast Health—Mammography
Hologic’s Flagship and Most Significant Near-Term Growth Driver
Leader in Breast Cancer Screening & Diagnosis Digital Mammography Market Size: ~$4 billion U.S. addressable market Market Share: ~65% of U.S. installed base Substantial replacement cycle expected Strong competitive advantages of 3D Dimensions
– Clinical superiority to 2D
– First-to-market with no near-term U.S. competition
Robust Service Offering: Recurring revenue stream driven by system placements
Source: Public investor presentations and filings
HOLOGIC
17
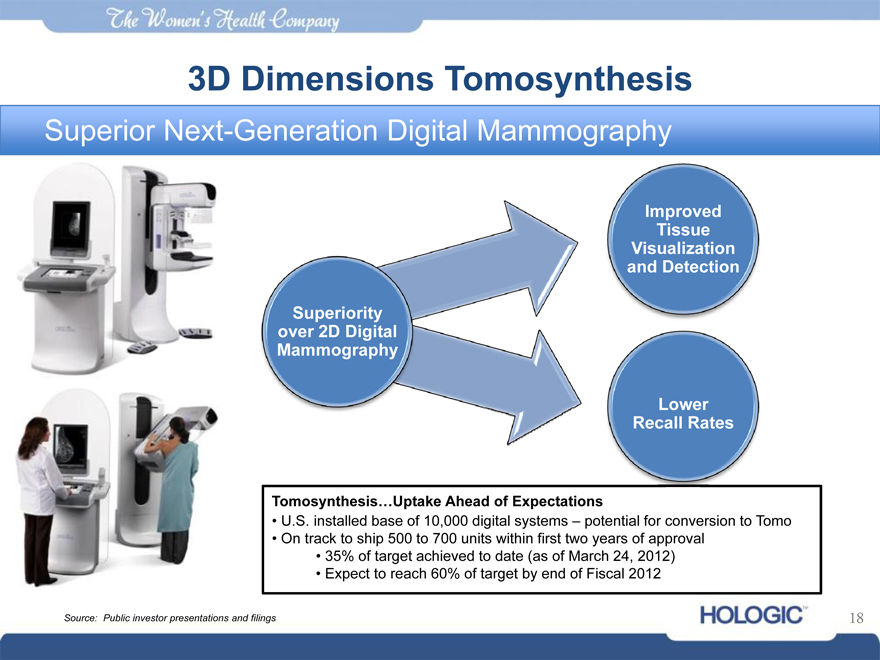
The womens health company
3D Dimensions Tomosynthesis
Superior Next-Generation Digital Mammography
Superiority over 2D Digital Mammography
Improved Tissue Visualization and Detection
Lower Recall Rates
Tomosynthesis…Uptake Ahead of Expectations
• U.S. installed base of 10,000 digital systems – potential for conversion to Tomo
• On track to ship 500 to 700 units within first two years of approval
• 35% of target achieved to date (as of March 24, 2012)
• Expect to reach 60% of target by end of Fiscal 2012
Source: Public investor presentations and filings
HOLOGIC
18
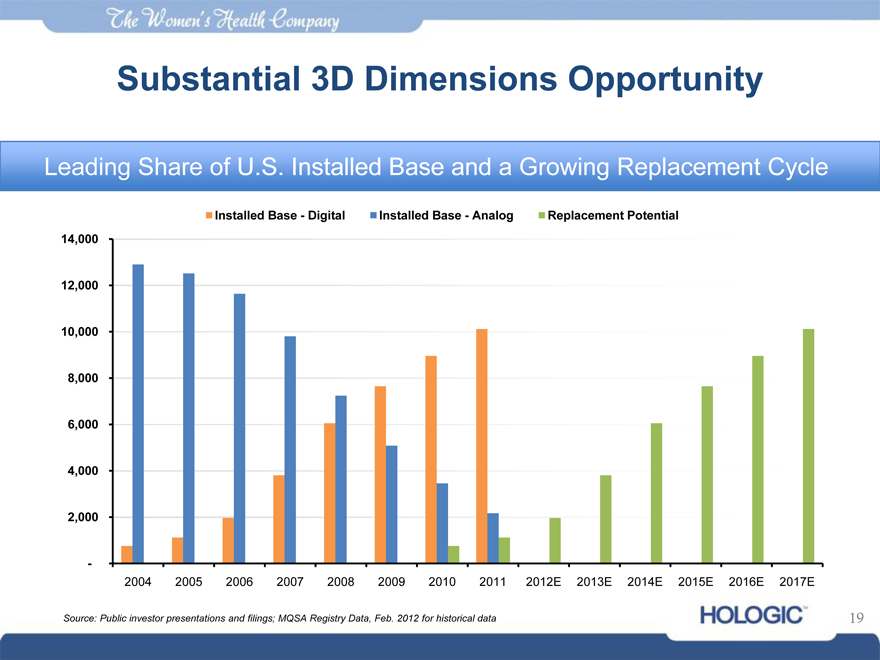
Substantial 3D Dimensions Opportunity
Leading Share of U.S. Installed Base and a Growing Replacement Cycle
Installed Base—Digital Installed Base—Analog Replacement Potential
14,000
12,000
10,000
8,000
6,000
4,000
2,000
-
2004 2005 2006 2007 2008 2009 2010 2011 2012E 2013E 2014E 2015E 2016E 2017E
Source: Public investor presentations and filings; MQSA Registry Data, Feb. 2012 for historical data 19
Fundamental Factors Key to Adoption of
3D Dimensions
#1: Ongoing Clinical Validation of the Technology
Oslo study
– Over 12,000 patients imaged
– Significant increase in cancer detection rates
FDA trial data—submitted for publication by Dr. Betty Rafferty, Mass General Hospital
#2: Competition Among Regional Imaging Providers for
Local Market Share Leadership
#3: Establish Code for 3D Dimensions Reimbursement
Source: Public investor presentations and filings 20
Diagnostics
ThinPrep: Steady US Business with Upside Potential Internationally
Cervical Cancer Screening & Diagnosis
ThinPrep Pap Test & Related Instrumentation
• U.S. market share ~ 70%
• Significant EBITDA contributor
• Growth opportunity OUS, especially in China and other emerging markets
Molecular Diagnostics: Significant Contributor to Global Diagnostics Segment Growth
HPV Screening & Diagnosis
Cervista HPV HR & 16/18 Genotyping
• Utilizes powerful ThinPrep franchise with physician / lab sales infrastructure
HPV Screening & Diagnosis
• Serves mid and low volume lab segments
• Recent approvals in U.S., Europe and China
Source: Public investor presentations and filings 21
GYN Surgical
Addressing Substantial Unmet Medical Needs for Women Globally
Abnormal Uterine Bleeding
NovaSure
• Clear leader with ~ 60-65% current U.S. share of a potential future $1B+ global market opportunity
• Standard of care
Uterine Fibroids and Polyps
MyoSure
• Double-digit annual growth expected to continue
• Minimally-invasive technology
• Maintains uterine form and function
Source: Public investor presentations and filings 22

R&D Investment Drives Innovation
Steady Pace of Recent New Product Approvals
U.S. FDA approvals and clearances
• 3D Dimensions Tomosynthesis (PMA)
• Cervista HPV High-Throughput Automation (PMA)
• Sentinelle Prostate coils (510k)
• Sentinelle 16-channel breast coils (510k)
• Trident Specimen Radiography system (510k)
International approvals and clearances
• Serenity digital mammography system (SFDA – China)
• Cervista HPV HR test (SFDA – China)
• C-View (CE Marking)
• Cervista MTA Medium-Throughput Automation (CE Marking)
Source: Public investor presentations and filings 23
Overview of Gen-Probe Business Description
Gen-Probe is a global leader in blood screening and women’s health through the development, manufacturing and marketing of molecular diagnostic products and services
The company operates in two main business segments:
—Clinical Diagnostics: Market leading position in NAT assays for the detection of chlamydia and gonorrhea (CT/NG). Products include amplified APTIMA products
—Blood Screening: Develops reagents and instrumentation for blood screening applications (PROCLEIX assays). Applications in HIV, WNV, HCA and HBA screening of blood, plasma, organs and tissues
Fully automated TIGRIS and PANTHER systems provide differentiated competitive advantages
Best-in-class assays with leading market shares
March 31, 2012 LTM Revenue and Adjusted EBITDA of $587 million and $188 million, respectively
Source: Public investor presentations and filings 24
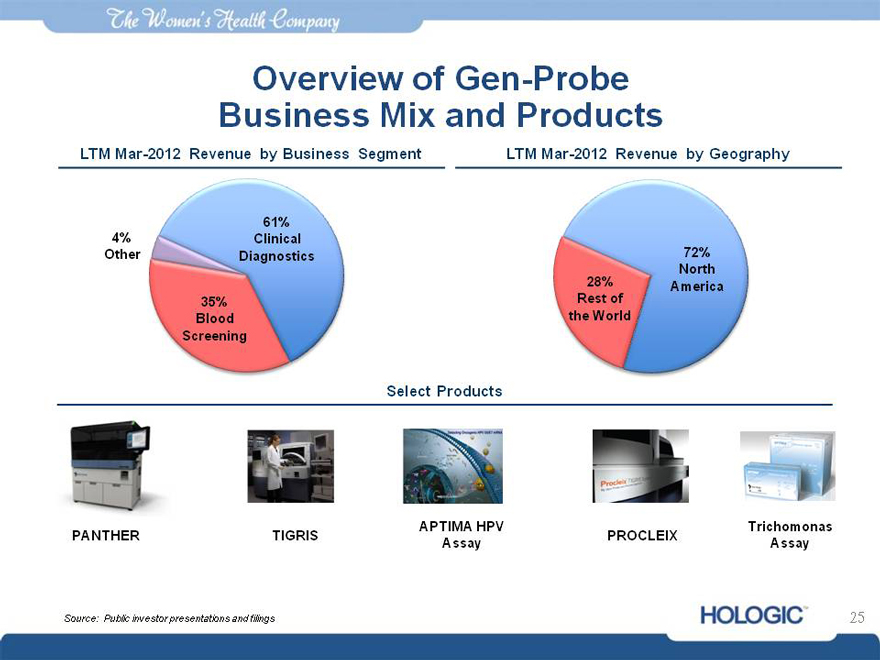
Overview of Gen-Probe Business Mix and Products
LTM Mar-2012 Revenue by Business Segment LTM Mar-2012 Revenue by Geography
61%
4% Clinical
Other Diagnostics 72%
North
28% America
35% Rest of
Blood the World
Screening
Select Products
APTIMA HPV Trichomonas
PANTHER TIGRIS PROCLEIX
Assay Assay
Source: Public investor presentations and filings 25
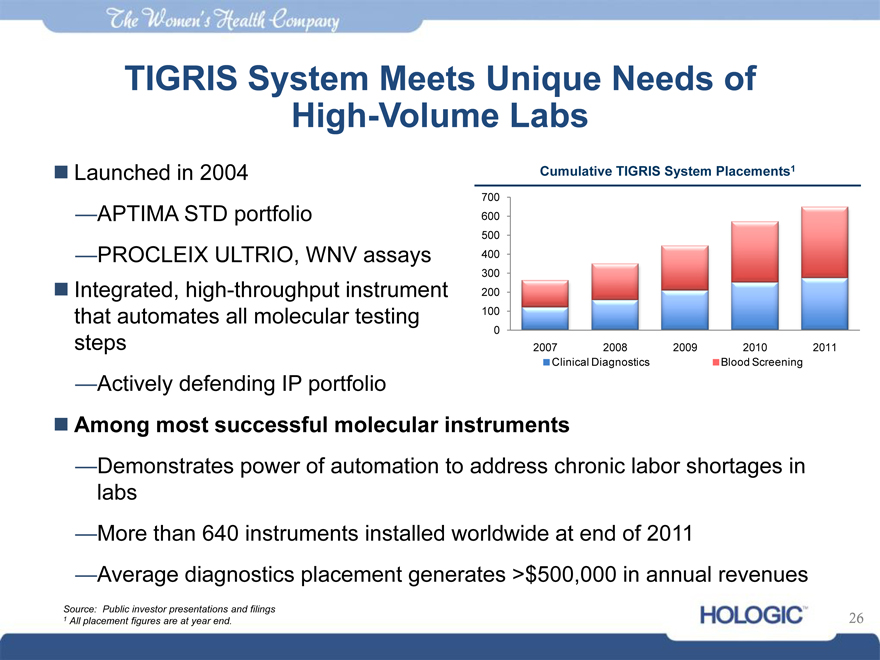
TIGRIS System Meets Unique Needs of
High-Volume Labs
Launched in 2004 Cumulative TIGRIS System Placements1
700
—APTIMA STD portfolio 600
500
—PROCLEIX ULTRIO, WNV assays 400
300
Integrated, high-throughput instrument 200
that automates all molecular testing 100
0
steps 2007 2008 2009 2010 2011
Clinical Diagnostics Blood Screening
—Actively defending IP portfolio
Among most successful molecular instruments
—Demonstrates power of automation to address chronic labor shortages in
labs
—More than 640 instruments installed worldwide at end of 2011
—Average diagnostics placement generates >$500,000 in annual revenues
Source: Public investor presentations and filings
1 All placement figures are at year end. 26
PANTHER System Offers Increased Flexibility and Functionality
Offers automation to low- and mid-volume labs
—Drives growth from menu expansion
—Provides opportunity to consolidate platforms
—Increases access to global markets
—Provides full automation to current users of DTS system
Builds on success of TIGRIS system while improving:
—Flexibility, functionality and cost
Capabilities of chemistry, immunoassay systems provide unique competitive differentiation
Commercial Status
—Launched in Europe in late 2010
– Achieved 2011 placement goal, assay revenue growing steadily and contributing to overall product sales growth
—Approved in U.S. for application with APTIMA COMBO 2 in May 2012
Source: Public investor presentations and filings 27
Gen-Probe STD Franchise Remains Robust
Chlamydia and gonorrhea are most common bacterial STDs
—CDC reported 1.3 million cases of Chlamydia in 2010, most for any condition
—HEDIS data indicates that less than half of sexually active women are tested
$492 million market in 2011
—$344 million in U.S., +3%
—$148 million ex-U.S., +10%
APTIMA COMBO 2 assay, on TIGRIS and PANTHER systems, remains a growth driver
—Sustainable competitive advantage based on best combination of sensitivity, specificity, reproducibility, sample flexibility and scalable automation
—Gen-Probe estimates they have leading global market share in Chlamydia
Source: and Public investor Gonorrhea presentation and filings. testing Data are 2011 with year-end ~60% Gen-Probe of estimates U.S. and ~49% of worldwide markets
28
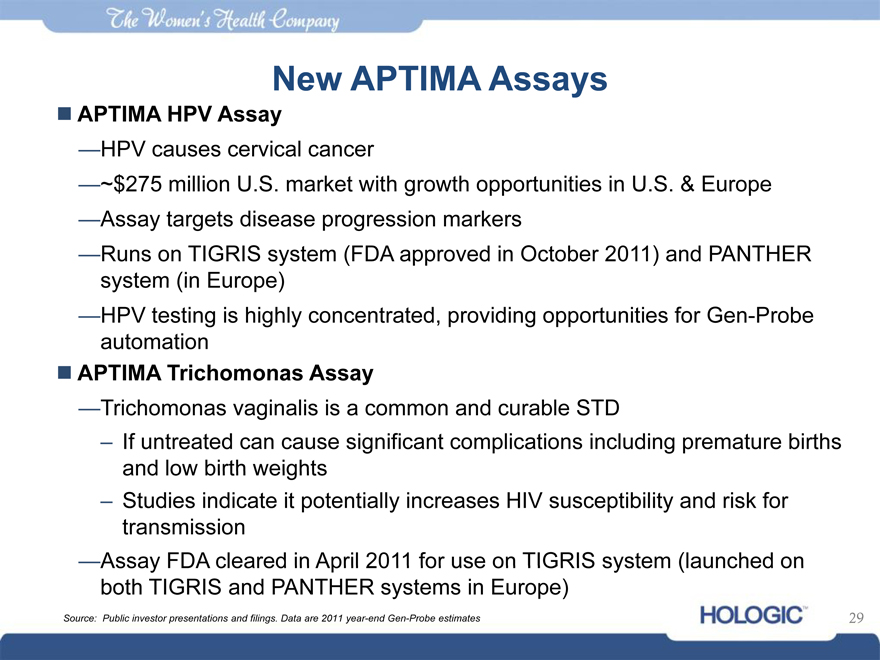
New APTIMA Assays
APTIMA HPV Assay
—HPV causes cervical cancer
—~$275 million U.S. market with growth opportunities in U.S. & Europe
—Assay targets disease progression markers
—Runs on TIGRIS system (FDA approved in October 2011) and PANTHER system (in Europe)
—HPV testing is highly concentrated, providing opportunities for Gen-Probe automation
APTIMA Trichomonas Assay
—Trichomonas vaginalis is a common and curable STD
– If untreated can cause significant complications including premature births and low birth weights
– Studies indicate it potentially increases HIV susceptibility and risk for transmission
—Assay FDA cleared in April 2011 for use on TIGRIS system (launched on both TIGRIS and PANTHER systems in Europe)
Source: Public investor presentations and filings. Data are 2011 year-end Gen-Probe estimates 29
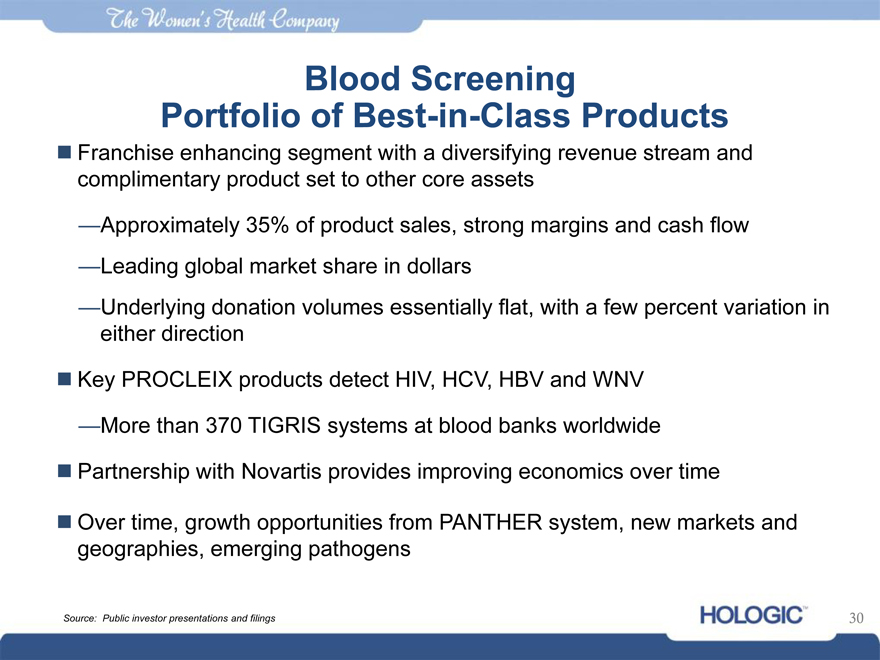
Blood Screening
Portfolio of Best-in-Class Products
Franchise enhancing segment with a diversifying revenue stream and complimentary product set to other core assets
—Approximately 35% of product sales, strong margins and cash flow
—Leading global market share in dollars
—Underlying donation volumes essentially flat, with a few percent variation in either direction
Key PROCLEIX products detect HIV, HCV, HBV and WNV
—More than 370 TIGRIS systems at blood banks worldwide
Partnership with Novartis provides improving economics over time
Over time, growth opportunities from PANTHER system, new markets and geographies, emerging pathogens
Source: Public investor presentations and filings 30
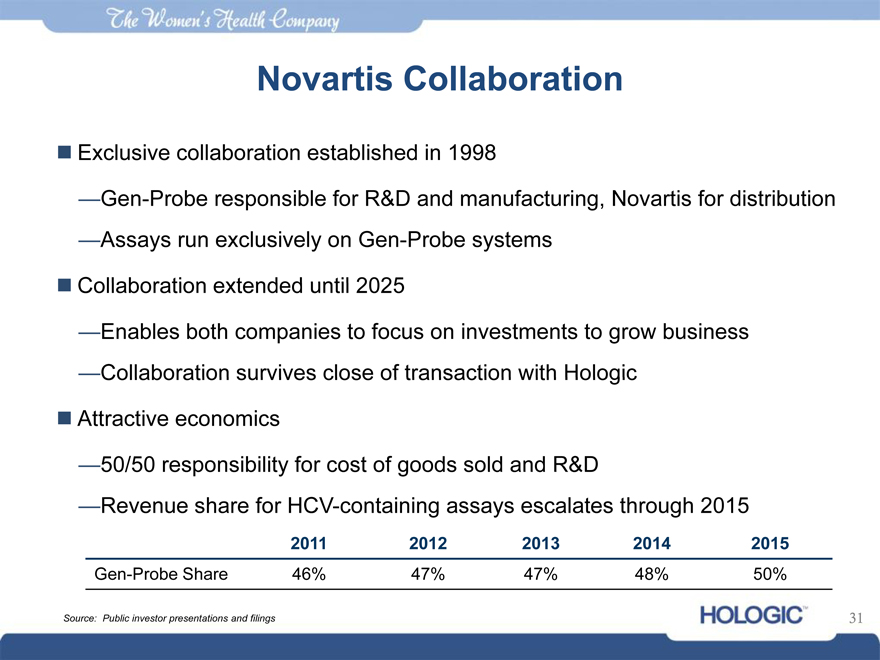
Novartis Collaboration
Exclusive collaboration established in 1998
—Gen-Probe responsible for R&D and manufacturing, Novartis for distribution
—Assays run exclusively on Gen-Probe systems
Collaboration extended until 2025
—Enables both companies to focus on investments to grow business
—Collaboration survives close of transaction with Hologic
Attractive economics
—50/50 responsibility for cost of goods sold and R&D
—Revenue share for HCV-containing assays escalates through 2015
2011 2012 2013 2014 2015
Gen-Probe Share 46% 47% 47% 48% 50%
Source: Public investor presentations and filings 31

Agenda
Executive Summary Transaction Merits Company Overview Key Credit Strengths Historical Financial Review
Appendix A: Non-GAAP Adjustments
Appendix B: Disclaimers 32

Key Credit Strengths
Market Leader in Key Products Diversified and Balanced Revenue Mix Best-in-Class Technologies Broad International Presence Experienced and Proven Management Team
Significant Free Cash Flow Generation Drives Expected Deleveraging
Attractive Credit Profile with Meaningful Equity Value
33
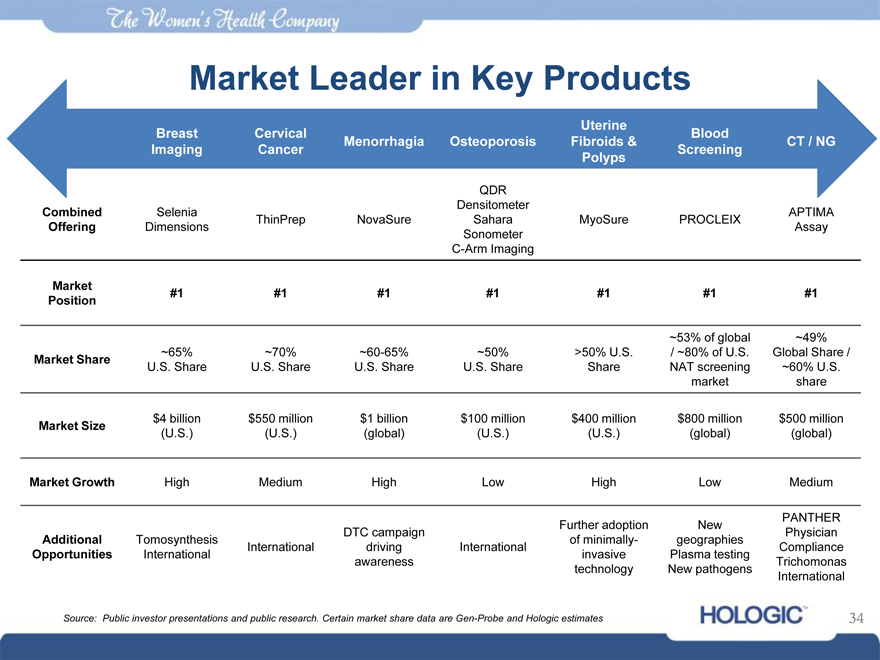
Market Leader in Key Products
Uterine
Breast Cervical Blood
Menorrhagia Osteoporosis Fibroids & CT / NG
Imaging Cancer Screening
Polyps
QDR
Densitometer
Combined Selenia APTIMA
ThinPrep NovaSure Sahara MyoSure PROCLEIX
Offering Dimensions Assay
Sonometer
C-Arm Imaging
Market #1 #1 #1 #1 #1 #1 #1
Position
~53% of global ~49%
~65% ~70% ~60-65% ~50% >50% U.S. / ~80% of U.S. Global Share /
Market Share U.S. Share U.S. Share U.S. Share U.S. Share Share NAT screening ~60% U.S.
market share
Market Size $4 billion $550 million $1 billion $100 million $400 million $800 million $500 million
(U.S.) (U.S.) (global) (U.S.) (U.S.) (global) (global)
Market Growth High Medium High Low High Low Medium
Further adoption New PANTHER
DTC campaign Physician
Additional Tomosynthesis of minimally- geographies
International driving International Compliance
Opportunities International invasive Plasma testing
awareness Trichomonas
technology New pathogens International
Source: Public investor presentations and public research. Certain market share data are Gen-Probe and Hologic estimates
34
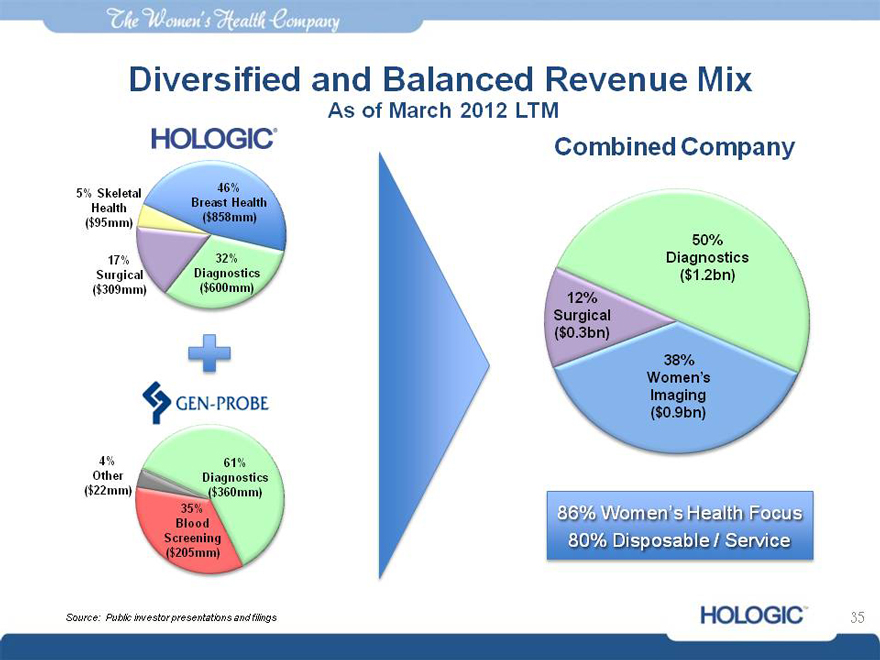
Diversified and Balanced Revenue Mix
As of March 2012 LTM
Combined Company
5% Skeletal 46%
Health Breast Health
($95mm) ($858mm)
17% 32%
Surgical Diagnostics
($309mm) ($600mm)
4% 61%
Other Diagnostics
($22mm) ($360mm)
35%
Blood
Screening
($205mm)
Source: Public investor presentations and filings
50% Diagnostics
( $1.2bn) 12% Surgical
($0.3bn)
38% Women’ s Imaging
($0.9bn)
86% Women’s Health Focus 80% Disposable / Service
35
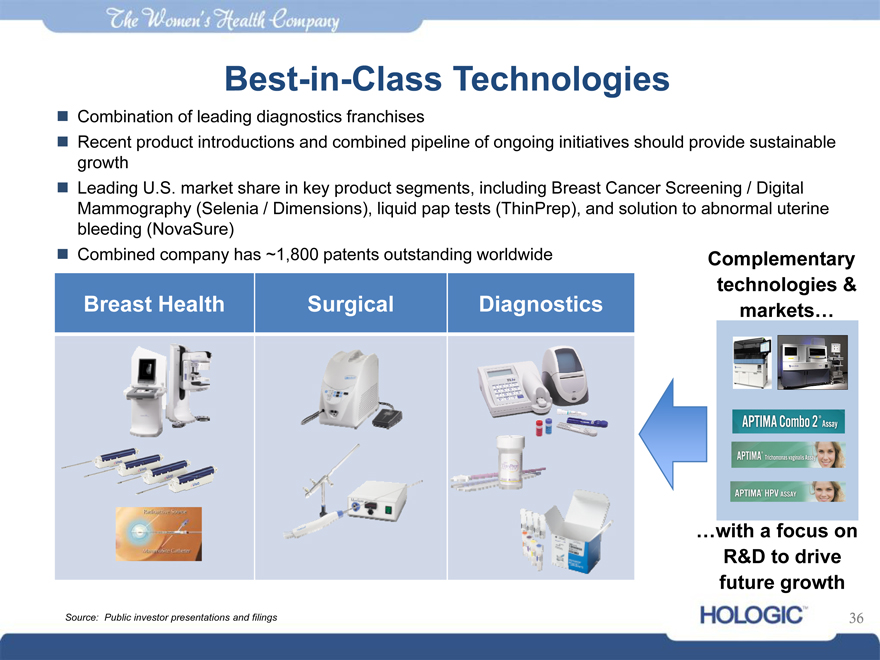
Best-in-Class Technolo iesg
Combination of leading diagnostics franchises
Recent product introductions and combined pipeline of ongoing initiatives should provide sustainable
growth
Leading U.S. market share in key product segments, including Breast Cancer Screening / Digital
Mammography (Selenia / Dimensions), liquid pap tests (ThinPrep), and solution to abnormal uterine
bleeding (NovaSure)
Combined company has ~1,800 patents outstanding worldwide Complementary
technologies &
Breast Health Surgical Diagnostics markets…
…with a focus on R&D to drive future growth
Source: Public investor presentations and filings
36
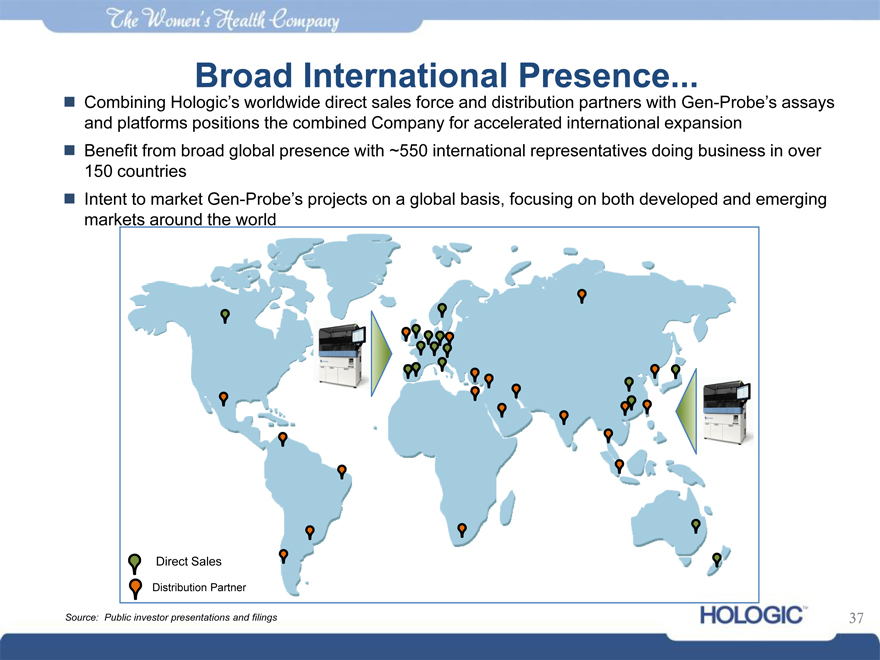
Broad International Presence
Combining Hologic’s worldwide direct sales force and distribution partners with Gen-Probe’s assays and platforms positions the combined Company for accelerated international expansion Benefit from broad global presence with ~550 international representatives doing business in over 150 countries Intent to market Gen-Probe’s projects on a global basis, focusing on both developed and emerging markets around the world
Direct Sales
Distribution Partner
Source: Public investor presentations and filings
37

With Significant Emerging Market Opportunity
2011 Investments and Progress in China
Two recent acquisitions in 2011 increased our footprint in China
TCT Medical – Market leading distributor of ThinPrep
Healthcome – Manufacturer of low-cost mammography systems
China expected to be major growth driver over the next five years
– Improving healthcare standards for the emerging middle class
– Hospital construction fueling demand for capital equipment
Enhancing the infrastructure to sell Hologic’s existing and newly-approved products
ThinPrep (existing)
Cervista HPV HR test, NovaSure and Serenity Digital Mammography system (recently approved)
Utilizing the model to further expand into other emerging markets Introduce Gen-Probe’s products on a global basis, focusing on both developed and emerging markets around the world
Source: Public investor presentations and filings
38
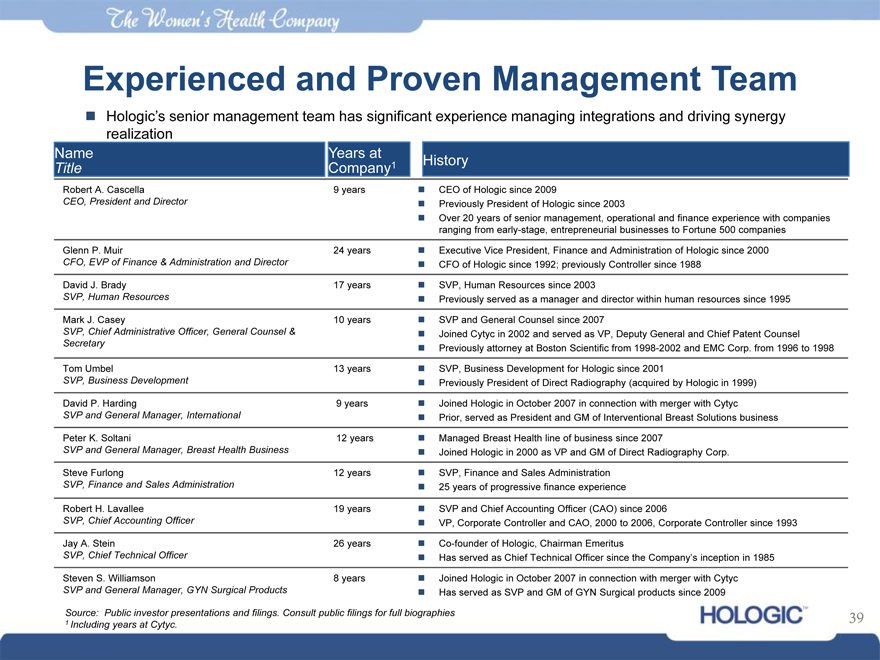
Experienced and Proven Management Team
Hologic’s senior management team has significant experience managing integrations and driving synergy realization
Name Years at
Title Company1
Robert A. Cascella 9 years
CEO, President and Director
Glenn P. Muir 24 years
CFO, EVP of Finance & Administration and Director
David J. Brady 17 years
SVP, Human Resources
Mark J. Casey 10 years
SVP, Chief Administrative Officer, General Counsel &
Secretary
Tom Umbel 13 years
SVP, Business Development
David P. Harding 9 years
SVP and General Manager, International
Peter K. Soltani 12 years
SVP and General Manager, Breast Health Business
Steve Furlong 12 years
SVP, Finance and Sales Administration
Robert H. Lavallee 19 years
SVP, Chief Accounting Officer
Jay A. Stein 26 years
SVP, Chief Technical Officer
Steven S. Williamson 8 years
SVP and General Manager, GYN Surgical Products
History
CEO of Hologic since 2009
Previously President of Hologic since 2003
Over 20 years of senior management, operational and finance experience with companies
ranging from early-stage, entrepreneurial businesses to Fortune 500 companies
Executive Vice President, Finance and Administration of Hologic since 2000
CFO of Hologic since 1992; previously Controller since 1988
SVP, Human Resources since 2003
Previously served as a manager and director within human resources since 1995
SVP and General Counsel since 2007
Joined Cytyc in 2002 and served as VP, Deputy General and Chief Patent Counsel
Previously attorney at Boston Scientific from 1998-2002 and EMC Corp. from 1996 to 1998
SVP, Business Development for Hologic since 2001
Previously President of Direct Radiography (acquired by Hologic in 1999)
Joined Hologic in October 2007 in connection with merger with Cytyc
Prior, served as President and GM of Interventional Breast Solutions business
Managed Breast Health line of business since 2007
Joined Hologic in 2000 as VP and GM of Direct Radiography Corp.
SVP, Finance and Sales Administration
25 years of progressive finance experience
SVP and Chief Accounting Officer (CAO) since 2006
VP, Corporate Controller and CAO, 2000 to 2006, Corporate Controller since 1993
Co-founder of Hologic, Chairman Emeritus
Has served as Chief Technical Officer since the Company’s inception in 1985
Joined Hologic in October 2007 in connection with merger with Cytyc
Has served as SVP and GM of GYN Surgical products since 2009
Source: Public investor presentations and filings. Consult public filings for full biographies
1 | | Including years at Cytyc. |
39

Significant Free Cash Flow Generation Drives Expected Deleveraging
Pro Forma LTM net leverage of 5.4x1, including synergies, expected to decline to below 5.0x by fiscal year-end
Cash flow should allow for rapid deleveraging of acquisition debt, with the goal of returning to pre-transaction leverage ratio within 3 years Hologic has historically maintained a disciplined approach to balance sheet management as demonstrated by significant debt paydown post the Cytyc and Third Wave acquisitions in calendar 2007 and 2008, respectively
Hologic Historical Net Leverage Profile ($ in millions)
4.2x 4.1x
3.6x
2.7x
2.0x
1.7x
6/30/2007 6/30/2008 FY 2008 FY 2009 FY 2010 FY 2011
Total Net Debt $2,220 $2,205 $2,133 $1,636 $1,234 $1,033 EBITDA $531 $536 $600 $603 $605 $611
Mar. 2012 LTM Operating Cash Flow less CapEx ($ in millions)
$390
$126
$516
Combined
Source: Public filings, public investor presentations
Note: September Hologic Fiscal Year End. 6/30/2007 pro forma for Cytyc acquisition. 6/30/2008 pro forma for Third Wave acquisition. Red represents amount of added leverage from the Cytyc acquisition in 2007 (took leverage from ~0.0x to 4.1x). Leverage multiples from 2007 and 2008 public marketing materials for the associated capital raises.
1 | | Calculated using the principal amount of outstanding convertible senior notes rather than book value. |
40
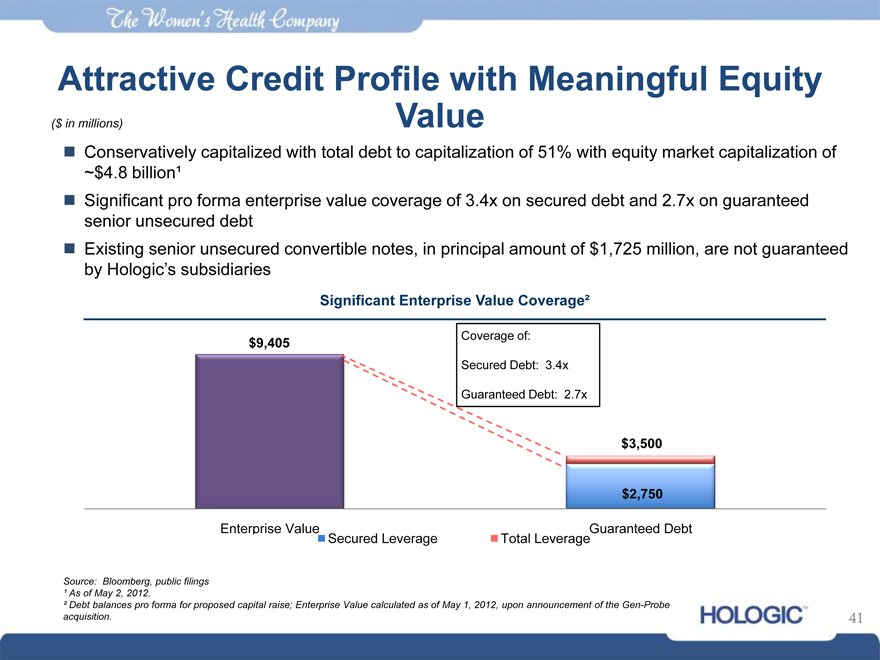
Attractive Credit Profile with Meaningful Equity
($ in millions) Value
Conservatively capitalized with total debt to capitalization of 51% with equity market capitalization of
~$4.8 billion¹
Significant pro forma enterprise value coverage of 3.4x on secured debt and 2.7x on guaranteed senior unsecured debt Existing senior unsecured convertible notes, in principal amount of $1,725 million, are not guaranteed by Hologic’s subsidiaries
Significant Enterprise Value Coverage²
$9,405
Coverage of: Secured Debt: 3.4x Guaranteed Debt: 2.7x
$3,500
Enterprise Value
Secured Leverage
Total Leverage
Guaranteed Debt
Source: Bloomberg, public filings
¹ As of May 2, 2012.
² Debt balances pro forma for proposed capital raise; Enterprise Value calculated as of May 1, 2012, upon announcement of the Gen-Probe acquisition.
41

Agenda
Executive Summary Transaction Merits Company Overview Key Credit Strengths
Historical Financial Review
Appendix A: Non-GAAP Adjustments
Appendix B: Disclaimers
42
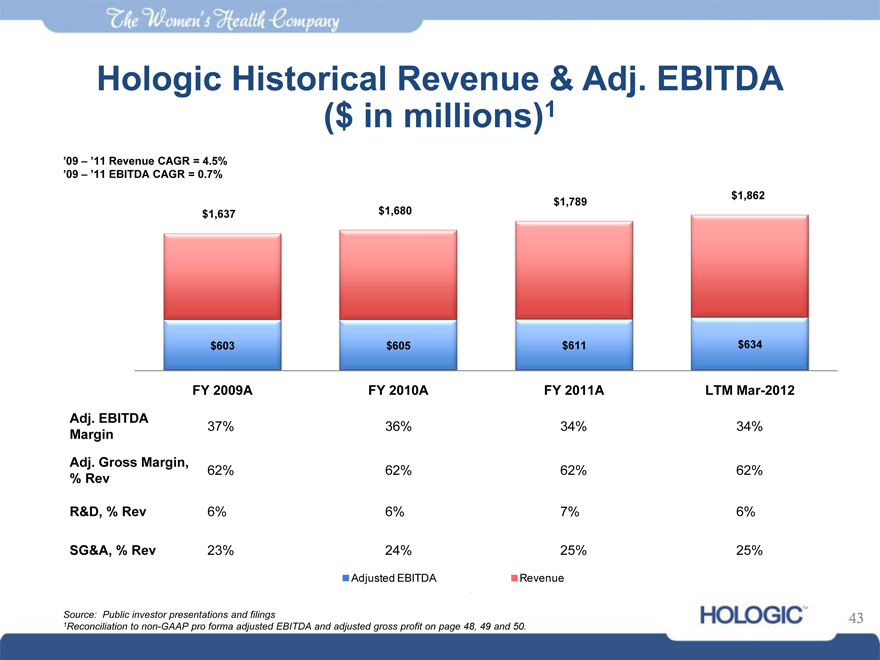
Hologic Historical Revenue & Adj. EBITDA ($ in millions)1
‘09 – ‘11 Revenue CAGR = 4.5% ‘09 – ‘11 EBITDA CAGR = 0.7%
$1,637 $1,680
$1,789
$1,862
$603
$605
$611
$634
FY 2009A FY 2010A FY 2011A LTM Mar-2012
Adj. EBITDA Margin
Adj. Gross Margin,
% Rev R&D, % Rev
SG&A, % Rev
37% 62% 6% 23%
36% 62% 6% 24%
34% 62% 7% 25%
34% 62% 6% 25%
Adjusted EBITDA Revenue
Source: Public investor presentations and filings
1Reconciliation to non-GAAP pro forma adjusted EBITDA and adjusted gross profit on page 48, 49 and 50.
43
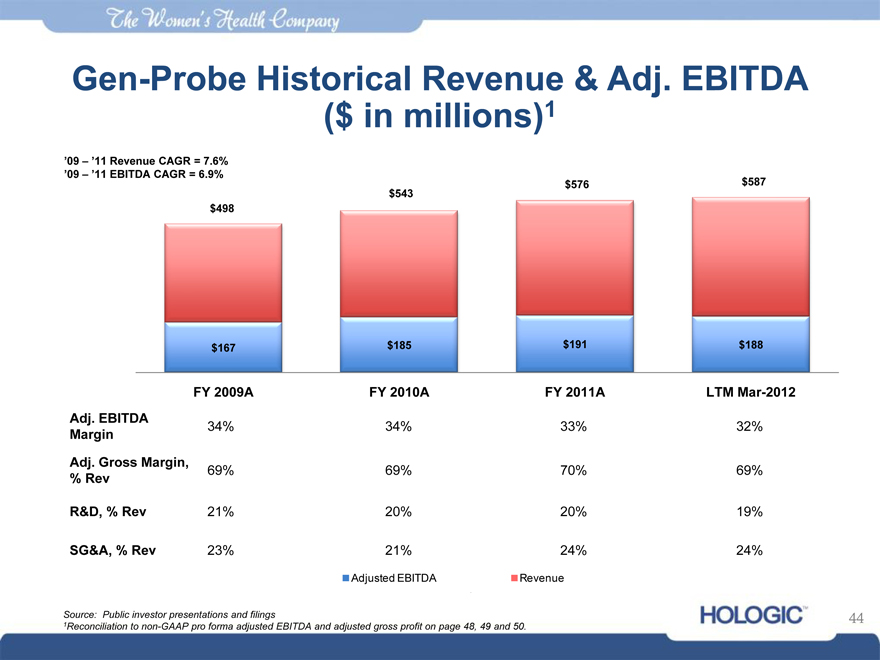
Gen-Probe Historical Revenue & Adj. EBITDA ($ in millions)1
‘09 – ‘11 Revenue CAGR = 7.6% ‘09 – ‘11 EBITDA CAGR = 6.9%
$498
$167
$543
$185
$576
$191
$587
$188
FY 2009A FY 2010A FY 2011A LTM Mar-2012
Adj. EBITDA Margin
Adj. Gross Margin,
% Rev R&D, % Rev
SG&A, % Rev
34% 69% 21% 23%
34% 69% 20% 21%
33% 70% 20% 24%
32% 69% 19% 24%
Revenue
Adjusted EBITDA
Source: Public investor presentations and filings
1Reconciliation to non-GAAP pro forma adjusted EBITDA and adjusted gross profit on page 48, 49 and 50.
44
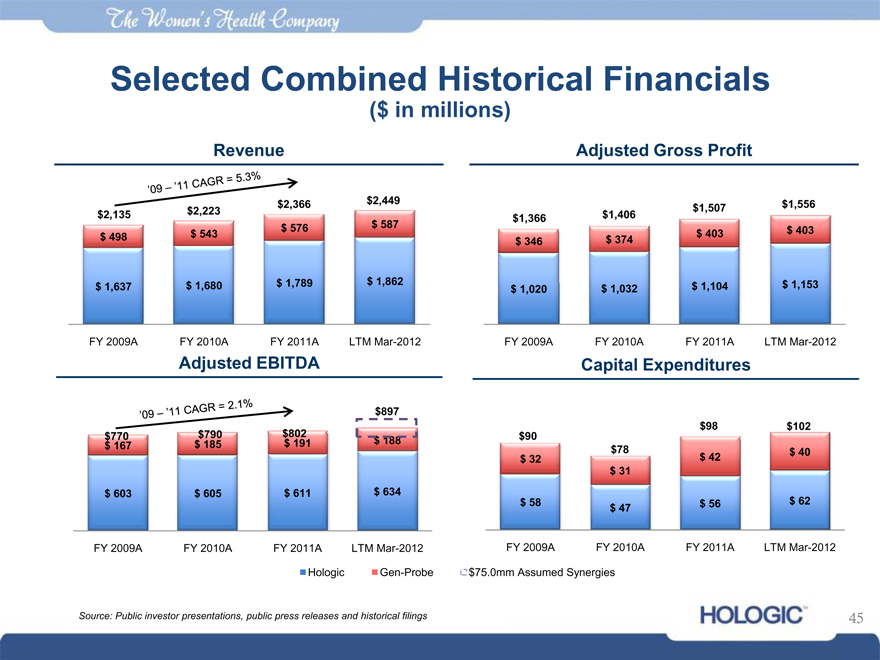
Selected Combined Historical Financials
($ in millions)
Revenue
‘09-’11 CAGR = 5.3%
$2,135 $ 498
$ 1,637
$2,223
$ 543
$ 1,680
$2,366
$ 576
$ 1,789
$2,449
$ 587
$ 1,862
FY 2009A FY 2010A FY 2011A LTM Mar-2012
$1,366
$ 346
$ 1,020
$1,406
$ 374
$ 1,032
$1,507
$ 403
$ 1,104
$1,556
$ 403
$ 1,153
Adjusted Gross Profit
FY 2009A FY 2010A FY 2011A LTM Mar-2012
Adjusted EBITDA
‘09-’11 CAGR = 2.1%
$770 $ 167
$ 603
$790 $ 185
$ 605
$802 $ 191
$ 611
$897
$ 188
$ 634
FY 2009A FY 2010A FY 2011A LTM Mar-2012
Capital Expenditures
$90 $ 32 $ 58
$78 $ 31 $ 47
$98
$ 42
$ 56
$102
$ 40
$ 62
FY 2009A FY 2010A FY 2011A LTM Mar-2012
$75.0mm Assumed Synergies
Gen-Probe
Hologic
Source: Public investor presentations, public press releases and historical filings
45

Agenda
Executive Summary Transaction Merits Company Overview Key Credit Strengths Historical Financial Review Financing Overview Questions & Answers
Appendix A: Non-GAAP Adjustments
Appendix B: Disclaimers
46

Hologic Reconciliation of GAAP Net Income to Non-GAAP Adj. Net Income and Pro Forma EBITDA
($ in millions) Year Ended Twelve Months Twelve Months Pro Forma
September September September Ended Ended
26, 2009 25, 2010 24, 2011 March 24, 2012 March 24, 2012
NET INCOME
GAAP net income($2,216.64)($62.81) $157.15 $44.30($132.79)
Adjustments
Amortization of intangible assets 206.73 226.31 235.79 242.12 425.22
Non-cash interest expense relating to convertible notes 67.67 73.13 72.91 73.60 73.60
Non-cash loss on convertible notes exchange—- 29.89 42.35 42.35
Contingent consideration—- 11.99 91.98 91.98
Adiana closure charges—— 18.28 18.28
Gain on sale of intellectual property, net—-(84.50)(12.42)(12.42)
Acquisition-related costs and other charges—2.23 2.32 2.92 5.12
Restructuring and divestiture charges (benefit) 2.00 1.58(0.07) 0.28 3.27
Litigation settlement charges—11.40 0.77 0.76 0.76
Fair value write up of acquired inventory sold 1.17 0.73 3.30
Goodwill and asset impairment charges 2,344.09 220.19—- 12.75
Other-than-temporary impairment loss on equity investments 2.24 1.10 2.45 0.35 39.48
Acquired in-process research and development—2.00 -
Income tax effect of reconciling items (1) (2)(101.91)(167.17)(96.50)(152.32)(251.41)
Non-GAAP adjusted net income $305.35 $308.68 $335.48 $352.20 $316.19
EBITDA
Non-GAAP adjusted net income $305.35 $308.68 $335.48 $352.20 $316.19
Interest expense, net, not adjusted above 66.12 52.70 39.86 39.37 234.58
Provision for income taxes 164.42 174.99 166.74 175.35 162.89
Depreciation and amortization expense, not adjusted above 67.20 68.46 68.95 67.57 108.62
Cost Reduction Initiatives (3)———75.00
Adjusted EBITDA $603.09 $604.84 $611.03 $634.49 $897.28
Source: Public filings and pro forma financial statements
Note: Column summations may be different due to rounding
1 For Years Ended September 26, 2009, September 25, 2010 and September 24, 2011: To reflect an annual effective tax rate of 35.0%, 36.2% and 33.2%, respectively, on a non-GAAP basis.
2 For Six Months Ended March 26, 2011 and March 24, 2012: To reflect an annual effective tax rate of 34% on a non-GAAP basis.
3 Represents cost-savings Hologic currently expects to achieve as a result of the Gen-Probe Acquisition. The Company estimates that they will realize approximately $75 million of cost savings by the end of fiscal year 2015.
47
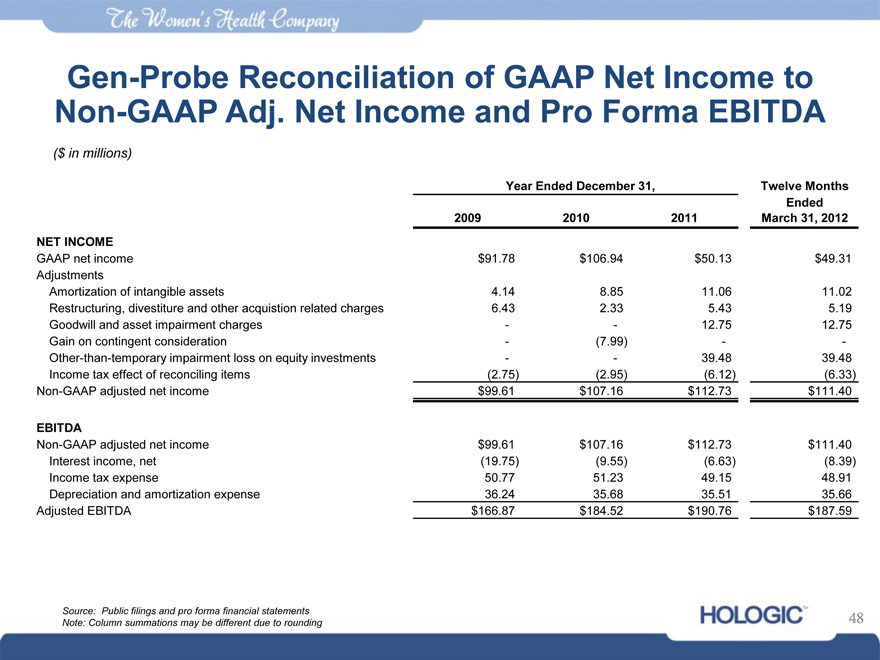
Gen-Probe Reconciliation of GAAP Net Income to Non-GAAP Adj. Net Income and Pro Forma EBITDA
($ in millions)
Year Ended December 31, Twelve Months
Ended
2009 2010 2011 March 31, 2012
NET INCOME
GAAP net income $91.78 $106.94 $50.13 $49.31
Adjustments
Amortization of intangible assets 4.14 8.85 11.06 11.02
Restructuring, divestiture and other acquistion related charges 6.43 2.33 5.43 5.19
Goodwill and asset impairment charges—- 12.75 12.75
Gain on contingent consideration -(7.99)—-
Other-than-temporary impairment loss on equity investments—- 39.48 39.48
Income tax effect of reconciling items(2.75)(2.95)(6.12)(6.33)
Non-GAAP adjusted net income $99.61 $107.16 $112.73 $111.40
EBITDA
Non-GAAP adjusted net income $99.61 $107.16 $112.73 $111.40
Interest income, net(19.75)(9.55)(6.63)(8.39)
Income tax expense 50.77 51.23 49.15 48.91
Depreciation and amortization expense 36.24 35.68 35.51 35.66
Adjusted EBITDA $166.87 $184.52 $190.76 $187.59
Source: Public filings and pro forma financial statements Note: Column summations may be different due to rounding
48
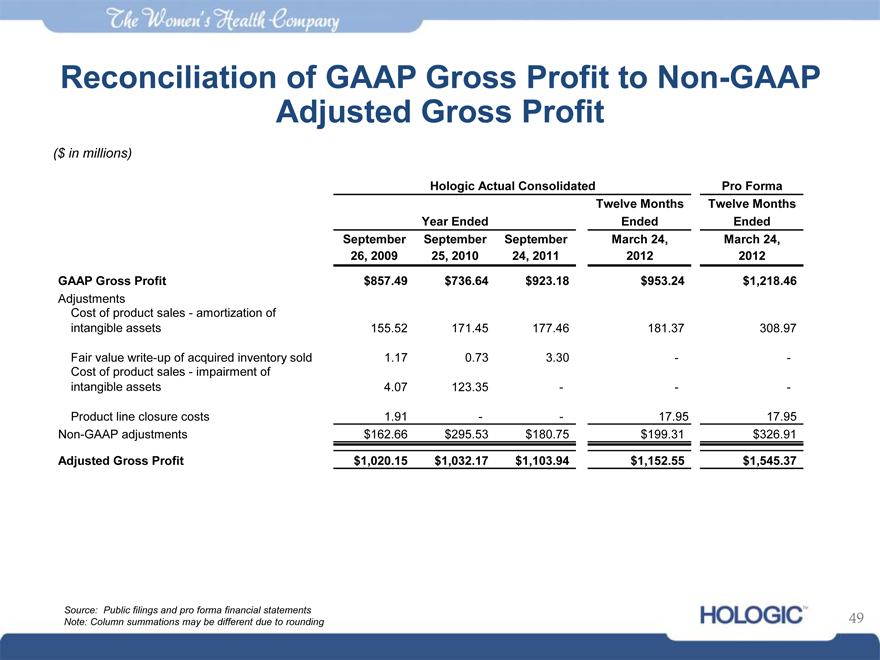
Reconciliation of GAAP Gross Profit to Non-GAAP Adjusted Gross Profit
($ in millions)
Hologic Actual Consolidated Pro Forma
Twelve Months Twelve Months
Year Ended Ended Ended
September September September March 24, March 24,
26, 2009 25, 2010 24, 2011 2012 2012
GAAP Gross Profit $857.49 $736.64 $923.18 $953.24 $1,218.46
Adjustments
Cost of product sales—amortization of
intangible assets 155.52 171.45 177.46 181.37 308.97
Fair value write-up of acquired inventory sold 1.17 0.73 3.30—-
Cost of product sales—impairment of
intangible assets 4.07 123.35——
Product line closure costs 1.91—- 17.95 17.95
Non-GAAP adjustments $162.66 $295.53 $180.75 $199.31 $326.91
Adjusted Gross Profit $1,020.15 $1,032.17 $1,103.94 $1,152.55 $1,545.37
Source: Public filings and pro forma financial statements Note: Column summations may be different due to rounding
49

Agendag
Executive Summary Transaction Overview Company Overview Key Credit Strengths Historical Financial Review
Appendix A: Non-GAAP Adjustments
50

Forward-Looking Statements
This presentation contains forward-looking information that involves risks and uncertainties, including statements about Hologic’s plans, objectives, expectations and intentions. Such statements include, without limitation, statements about the timing of the completion of the transaction, the anticipated benefits thereof, including anticipated future financial and operating results of the combined company, the expected permanent financing for the transaction, other of Hologic’s plans, objectives, expectations and intentions, and other statements that are not historical facts. Forward-looking statements may contain words such as “expect,” “believe,” “may,” “can,” “should,” “will,” “forecast,” “anticipate,” or similar expressions (including their use in the negative), and include assumptions that underlie such statements. These forward-looking statements are subject to known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements, including but not limited to: the ability of the parties to consummate the proposed merger in a timely manner or at all; satisfaction of the conditions precedent to consummation of the proposed merger, including the ability to secure regulatory approvals in a timely manner or at all, and approval by Gen-Probe’s stockholders; uncertainties relating to litigation (including pending and future Gen-Probe shareholder lawsuits related to the proposed merger); successful completion of anticipated financing arrangements; Hologic’s ability to successfully and timely integrate Gen-Probe’s operations, product lines, technologies and employees, and realize synergies from the proposed transaction; unknown, underestimated or undisclosed commitments or liabilities; effects of purchase accounting that may be different from expectations; the level of demand for the combined company’s products; the ability of the combined company to develop, deliver and support a broad range of products, develop new products, expand its markets and/or develop new markets; and the ability of the combined company to attract, motivate and retain key employees. Moreover, the combined business may be adversely affected by future legislative, regulatory, or tax changes as well as other economic, business and/or competitive factors. The risks included above are not exhaustive. Other factors that could adversely affect the combined company’s business and prospects are described in Hologic’s and Gen-Probe’s filings with the Securities and Exchange Commission. Hologic expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any such statements presented herein to reflect any change in expectations or any change in events, conditions or circumstances on which any such statements are based.
51

Trademark Notice
Hologic is a trademark of Hologic, Inc. Other trademarks, logos, and slogans registered or used by Hologic and its divisions and Subsidiaries in the United States and other countries include, but are not limited to, the following: Adiana, Aegis, Affirm, ATEC, Aquilex, Celero, Cellient, Cervista, Citra, C-View, Dimensions, DigitalNow, Discovery, Explorer, Eviva, Fluoroscan, Imagechecker, Inplex, Interlace, Invader, MammoPad, MammoSite, MultiCare, MyoSure, NovaSure, PreservCyt, QDR, QCette, Rapid fFN, Sahara, SecurView, SecurMark, SecurXchange, Selenia, Sentinelle, Serenity, Smartvalve, Suresound, StereoLoc, ThinPrep, THS, TCT, TLI IQ, Trident, Trimark, Urocyte.
ACCUPROBE, AMPLIFIED MTD, APTIMA, APTIMA COMBO 2, DKA, DTS, ELUCIGENE, GASDIRECT, GEN-PROBE, GTI DIAGNOSTICS, HPA, LIFECODES, PACE, PANTHER, PROADENO, PRODESSE, PROFAST, PROFLU, PROGASTRO, PROGENSA, TEPNEL, TIGRIS, TMA and Gen- Probe’ s other logos and trademarks are the property of Gen-Probe Incorporated or its subsidiaries. PROCLEIX and ULTRIO are trademarks of Novartis Vaccines & Diagnostics, Inc. XMAP is a trademark of Luminex Corporation.
All other brand names or trademarks appearing in this offering circular are the property of their respective holders. Hologic’s use or display of other parties’ trademarks, trade dress or products in this offering circular does not imply that Hologic or Gen-Probe has a relationship with, or endorsement or sponsorship of, the trademark or trade dress owners.
52
Use of Non-GAAP Financial Measures
Hologic has presented the following non-GAAP financial measures in this presentation: adjusted net income, adjusted gross profit and adjusted EBITDA of each of Hologic and Gen-Probe, and pro forma adjusted net income and adjusted EBITDA of the combined company.
The Company defines its non-GAAP adjusted net income to exclude (i) the amortization of intangible assets, (ii) acquisition-related charges and effects (comprised of (a) adjustments for changes in the fair value of the contingent consideration liabilities initially recorded as part of the purchase price of an acquisition as required by GAAP, and (b) contingent consideration that is tied to continuing employment of the former shareholders and employees which is recorded as compensation expense), transaction costs, charges associated with the write-up of acquired inventory to fair value, and the effects of lost revenue related to the write-up of accounts receivable to fair value, (iii) non-cash interested expense related to amortization of the debt discount for convertible debt securities, (iv) divestiture and restructuring charges, (v) non-cash loss on exchange of convertible notes, (vi) litigation settlement charges (benefits), (vii) other-than-temporary impairment losses on equity investments, (viii) other one-time, nonrecurring, unusual or infrequent charges, expenses or gains that may not be indicative of our core business results, and (ix) income taxes related to such adjustments.
The Company defines adjusted EBITDA as our non-GAAP adjusted net income plus interest expense, net, income taxes, and depreciation expense included in our non-GAAP adjusted net income.
The Company defines adjusted gross profit as consolidated revenues less cost of product sales and cost of services and other revenues, but excludes “cost of product sales-amortization of intangible assets” and “cost of product sales-impairment of intangible assets,” and non-recurring or infrequent items such as the purchase accounting effects resulting from the write-up of accounts receivable and inventory to fair value, other acquisition related charges, and impairment and other charges related to closure of facilities or abandonment of product lines.
The reconciliations of these historical non-GAAP measures to each of Hologic’s and Gen-Probe’s GAAP financial measures for the periods presented, are set forth on slide 48, 49 and 50.
The Company believes the use of non-GAAP adjusted net income and non-GAAP adjusted gross profit are useful to investors by eliminating certain of the more significant effects of its acquisitions and related activities, noncash charges resulting from the application of GAAP to convertible debt instruments with cash settlement features, charges related to debt extinguishment losses, equity investment impairments, litigation settlements, and divestiture and restructuring initiatives. These measures also reflect how the Company manages its businesses internally. In addition to the adjustments set forth in the calculation of the Company’s non-GAAP adjusted net income, its non-GAAP adjusted EBITDA eliminates the effects of financing, income taxes and the accounting effects of capital
spending. As with the items eliminated in its calculation of non-GAAP adjusted net income, these items may vary for different companies for reasons unrelated to the overall operating performance of a company’s business. When analyzing the Hologic’s, Gen-Probe’s and the pro forma combined company’s operating performance, investors should not consider these non-GAAP financial measures as a substitute for net income and gross profit prepared in accordance with GAAP.
53




















































