
Exhibit 99.1

Forward-Looking Statements
This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform
Act of 1995. Forward-looking statements include statements that address activities, events or developments that Bradley
expects, believes or anticipates will or may occur in the future, such as Bradley’s plans to in-license, develop and launch
new and enhanced products with long-term intellectual property protection or other significant barriers to market entry,
sales and earnings estimates, other predictions of financial performance, timing of payments on indebtedness, launches
by Bradley of new products, market acceptance of Bradley's products, and the achievement of initiatives to enhance
corporate governance and long-term shareholder value. Forward-looking statements are based on Bradley's experience
and perception of current conditions, trends, expected future developments and other factors it believes are appropriate
under the circumstances and are subject to numerous risks and uncertainties, many of which are beyond Bradley's
control. These risks and uncertainties include Bradley's ability to: launch VEREGEN™ and ELESTRIN™ during 2007;
predict the safety and efficacy of these products in a commercial setting; estimate sales; maintain adequate inventory of
levels; comply with the restrictive covenants under its credit facility; refinance its credit facility; access the capital
markets on attractive terms or at all; favorably resolve the pending SEC informal inquiry; maintain or increase sales of its
products; or effectively react to other risks and uncertainties described from time to time in Bradley's SEC filings, such
as fluctuation of quarterly financial results, estimation of product returns, chargebacks, rebates and allowances,
concentration of customers, reliance on third party manufacturers and suppliers, litigation or other proceedings (including
the pending class action and shareholder derivative lawsuits), government regulation, stock price volatility and ability to
achieve strategic initiatives to enhance long-term shareholder value. Further, Bradley cannot accurately predict the
impact on its business of the approval, introduction, or expansion by competitors of generic or therapeutically equivalent
or comparable versions of Bradley's products or of any other competing products. In addition, actual results may differ
materially from those projected. Bradley undertakes no obligation to publicly update any forward-looking statement,
whether as a result of new information, future events or otherwise.

Company Overview
Unique specialty pharmaceutical company committed to
adding value
Founded: 1985, Daniel Glassman, President and CEO
Operating segments:
Dermatology
Podiatry
Gastroenterology
OB/GYN
May 2003, BDY lists on NYSE from Nasdaq
Employees(1): 301
(1) As of December 31, 2006

Strong track record of sales growth
2006 Net Sales: $144.8 million
CAGR(1) : 33.4% since 2001
Experienced Management Team
Company Overview
(1) Compound annual growth rate (CAGR) from 2001 to 2006
Name
Title
Experience (yrs)
Daniel Glassman
President and CEO
36
Brent Lenczycki
VP, Chief Financial Officer
10
Bradley Glassman
SVP, Sales & Marketing
10
Alan Goldstein
VP, Corporate Development
21
Ralph Landau, Ph.D.
VP, Chief Scientific Officer
14

Company Overview
Net Sales
2004
2005
2006
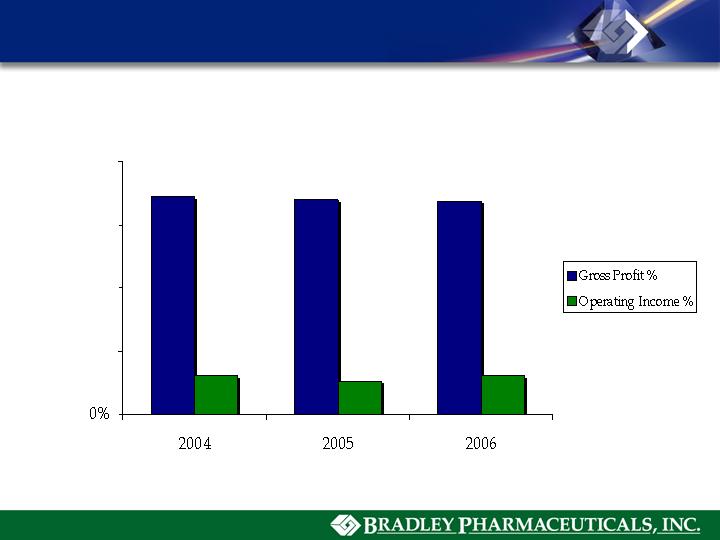
Gross Profit & Operating Income(1)
(1) Operating income is net sales minus cost of sales, SG&A, R&D and D&A expense (excludes interest expense, interest income
and income tax expense).
(2) Includes $3,475,000 compensation cost for share-based payment arrangements, $2,100,000 in legal and advisor fees relating to
proxy contest, and $8,500,000 in R&D expense relating to MediGene AG and BioSante Pharmaceuticals license agreements
100%
Company Overview
(2)
86.2%
85.1%
84.5%
15.7%
13.8%
15.8%
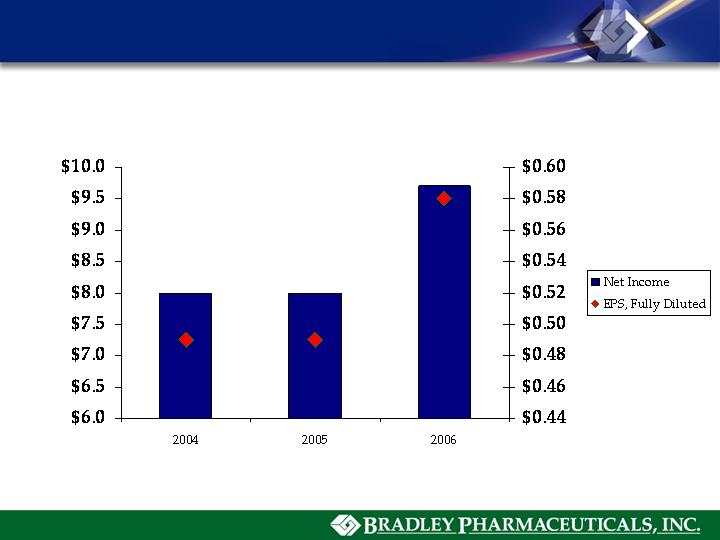
Net Income and EPS, Fully Diluted(1)
(1) 2004, 2005, 2006 EPS fully diluted calculations based on 18,410,000, 17,950,000, and 16,550,000 diluted shares.
(2) Includes $13,475,000 compensation cost for share-based payment arrangements, $2,100,000 in legal and advisor fees relating to
proxy contest, and $8,500,000 in R&D expense relating to MediGene AG and BioSante Pharmaceuticals license agreements
Company Overview
(2)

Product Returns and Inventory Improvement Initiatives
Continue efforts to reduce wholesaler inventory levels
Improve effective dating of products – In progress
Expiration date testing
Optimize product production schedules
Raised minimum dating requirements prior to shipping
Shift product mix to patent-protected products – In progress
Longer lifecycles
Lower risk of generic intrusion
Company Overview

Success built on core marketing and sales expertise
Company Overview
Late-stage product candidates that upon approval
leverage our sales and marketing capabilities
New products through lifecycle management and
new indications for existing brands
Brands that fill unmet physician and patient needs

Commercialize
Commercialize
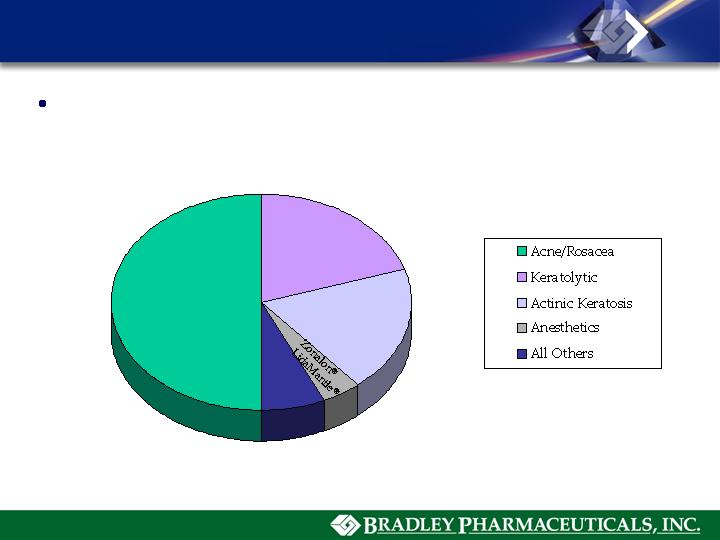
Adoxa®
Rosula ®
Zoderm®
Solaraze® Gel
KeralacTM
KerolTM
Carmol® 40
Doak Dermatologics - Major Brands
2006 Net Sales = $114.5 M
48.5%
18.4%
20.6%
5.0%
7.5%
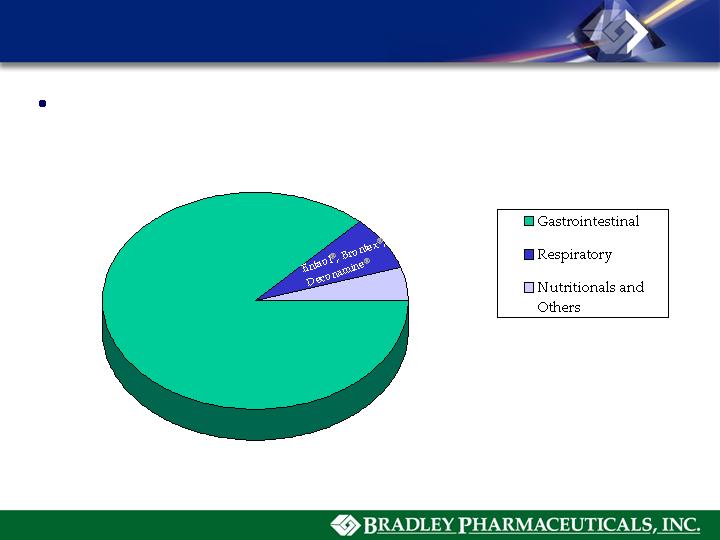
Pamine®
AnaMantle HC®
Flora-Q ®
Glutofac ZX/MX ®
Kenwood Therapeutics - Major Brands
Commercialize
2006 Net Sales = $30.3 M
85.5%
6.8%
7.7%

Synergistic Promotion and Sales Execution
Commercialize
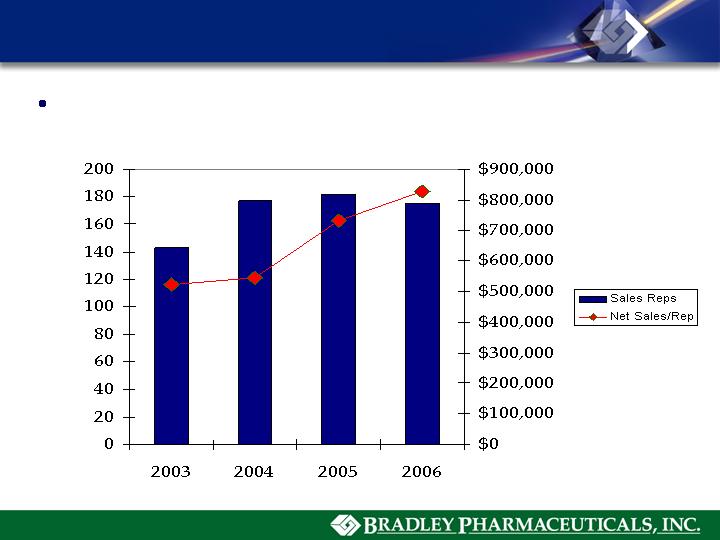
Sales Force Productivity
National Field Force Focused on “QAD” Selling Model
Commercialize

Leverage Dynamic Physician Relationships
Kenwood Therapeutics
Gastroenterology and Colon Rectal Surgery
Headquarters Physician Territory
Major Conference Exhibitor
Digestive Disease Week
American College of Gastroenterology
Sponsored more than 60 GE specialty society meetings
OB/GYN
Major Conference Exhibitor
American College of Obstetricians and Gynecologists
Sponsored four regional OB/GYN specialty society meetings
Commercialize

Leverage Dynamic Physician Relationships
Doak Dermatologics
Podiatry
“See the Podiatrist” Campaign
Major Sponsor, Superbones/Superskin™ CME
Pharmacists
“Profiles in Pharmacy” Campaign
Leaders in Community Pharmacy Advisory Board
Physicians Assistants/Dermatology
CME Sponsorship
Major Supporter, SDPA
Commercialize

Leverage Dynamic Physician Relationships
Doak Dermatologics
Dermatology
Headquarters Physician Territory
Key Opinion Leader Endorsements
Skin Cancer Screening Tour
Conducted more than 5,500 free screenings
Generated more than 55 million media impressions
regarding importance of seeing a dermatologist
Commercialize

Growing, Patent-Protected Brands
Solaraze® Gel (diclofenac sodium 3%)
Indication: Actinic Keratosis
Strong brand growth
2006 Net Sales: $23.6 million, up 80% over 2005
Estimated market share: 17.5% (1)
Safety and efficacy:
90% mean decrease in target lesions vs. baseline
58% of patients experienced 100% AK lesion clearance based on
Target Lesion Number Score vs. baseline
Well-tolerated with few side-effects
Additional clinical studies in progress
Patent expiration: 2014 - 2016
Commercialize
(1) Wolters Kluwer, based on 2006 TRx

Develop

Develop
Develop Brands Through Lifecycle Management
Introduce new products and line extensions filling unmet
patient and physician needs

Develop Brands Through Clinical Support
Doak Dermatologics - Current and on-going studies:
Keralac™ Nail Gel:
Brittle Nails (Dr. Scher)
Dystrophic Nails (Dr. Robbins)
Solaraze® Gel:
Effectiveness Post Cryosurgery, multi-center (Dr. Rigel)
AK of the Lip Study (Dr. Nelson)
Proof of Concept, new indications (Dr. Elewski, Dr. Wolf, Dr.
Kaplan and Dr. Draelos)
Zoderm®: Acne Tolerability/Efficacy, multi-center
Kenwood Therapeutics - Current and on-going studies:
Flora-Q®:
IBD, maintenance of remission
Diarrhea-predominant IBS
Develop

In-License

Merger/Acquisition/Licensing Activity – Last 5 Yrs
Merger/Acquisition/Licensing Activity – Prior
In-License
Company
Transaction
Adams Respiratory
ENTSOL®
BioSante
ElestrinTM
Lundbeck
Flora-Q®
MediGene AG
VeregenTM
Company
Transaction
Mission Pharmacal
AnaMantle HC®
Quintiles
Bioglan
SkyePharma
Solaraze®
Company
Transaction
Bayer
LidaMantle®
Berlex
Deconamine®
Doak Pharmacal
Doak Pharmacal
Hoechst
Duadacin®
Kenwood Labs
Kenwood Labs
Procter & Gamble
Brontex®
Roche
Carmol®
Company
Transaction
Sandoz
AcidMantle®
Schering-Plough
Tyzine®, Nitroglyn® , Ircon ®,
Ipsatol®
Upjohn
Pamine®, Adeflor®
Upsher-Smith
Lubrin®
Tsumora
Trans-Ver-Sal ®, Glandosane®

Patent-Protected Brands
VeregenTM (sinecatechins)
In-licensed U.S. rights from MediGene AG
Indication: Treatment of external genital and perianal warts
FDA approval: 10/31/06
Hypothesized mode of action (1) includes anti-inflammatory,
antiviral, anti-oxidant and immune modulatory
Market considerations:
Launch timing: Fourth quarter 2007
Doak Dermatologics: 1st position detail to dermatologists
Kenwood Therapeutics: 1st position detail to high-decile HPV
prescribing OB/GYN and primary care physicians
U.S. market size (2): $200 million
Patent expiration: 2017
(1) Source: MediGene AG
(2) Wolters Kluwer, retail and mail order TRx dollars, discounted 20%
In-License

Patent-Protected Brands
VeregenTM : Advantages over Aldara ®
Clears newly emerging warts
Relatively high clearance in males
Relatively low recurrence rates
Summary of Combined Pivotal Data
Aldara®(1)
VeregenTM
Source: MediGene AG
13 – 27.5%(3)
6.2%
Recurrence Rate:
Not Investigated
50% (F 72%, M 33%)
54.9% (F 61%, M 49%)
56.7% (F 64%, M 50%)
Efficacy(2) :
Clearance of all warts
Clearance of baseline warts
(1) Aldara® pivotal data; no head-to-head comparison
(2) Based on the intent-to-treat population
(3) 13% reported in pivotal studies; higher numbers reported in publications
In-License

Patent-Protected Brands
VeregenTM : Better Safety Profile vs. Aldara ®
Systemic side effects unlikely
Skin reactions mild, and decrease with time
No requirements for drug holidays
Summary of Combined Pivotal Data
Aldara® (1)
VeregenTM
Source: MediGene AG
•3x/week
•3x/day (compliance 90%)
•Potential for 2x/day regimen
(additional studies required)
Dosage/
Administration:
Systemic side effects reported in
some patients
Severe reactions reported with
3x/week application (Integrated
“drug holidays”) in dosing
program
Systemic side effects highly
unlikely
Majority of local reactions mild to
moderate and reduce with time. No
requirements for drug holidays
Safety:
(1) Aldara® pivotal data; no head-to-head comparison
In-License

Patent-Protected Brands
ElestrinTM (estradiol) Gel
In-Licensed U.S. rights from BioSante Pharmaceuticals
Indication: Treatment of moderate-to-severe vasomotor
symptoms in menopausal women
FDA approval: 12/15/06
Market considerations:
Transdermal gel offering the lowest effective dose of estradiol
Launch timing: June 2007
Kenwood Therapeutics: 1st position detail to high-decile
estradiol prescribing OB/GYN and primary care physicians
U.S. market size(1) : $1.3 billion, with transdermal segment
expected to grow > $300 million over the next several years
Patent expiration: 2017-2021
(1) Wolters Kluwer, retail and mail order TRx dollars, discounted 20%
In-License
Patent-Protected Brands
ElestrinTM Gel:
Advantages over oral estradiol therapies
Reduced risk of thromboembolic events
Advantages over estradiol patches
Less irritating
Cosmetically appealing
Dose titration possible with metered dose dispenser
Advantages over other topical estradiol products
In-License
Unknown
Unknown
Spray bottle
Spray
EvamistTM(1)
Both thighs
Not determined
Satchet
Emulsion
Estrasorb®
Full arm
Not determined
Metered pump
Gel
Estrogel®
Upper arm
12.5µg
Metered pump
Gel
ElestrinTM
Application
Site
Lowest Effective
Dose
Packaging
Formulation
Product
(1) NDA filed October 2006

Successful track record of strong sales and profits
Experienced senior management team
Well established relationships with key specialty
physician opinion leaders and physician audiences
Winning strategy for creating value built upon core
marketing and sales expertise, which enables us to:
Commercialize brands that fill unmet patient and physician
needs
Develop new products through lifecycle management of
existing brands
In-License late-stage, patent-protected product candidates that
upon approval leverage our sales and marketing capabilities
Growing portfolio of patent-protected drugs
Why Bradley Pharmaceuticals

4/4/07





























