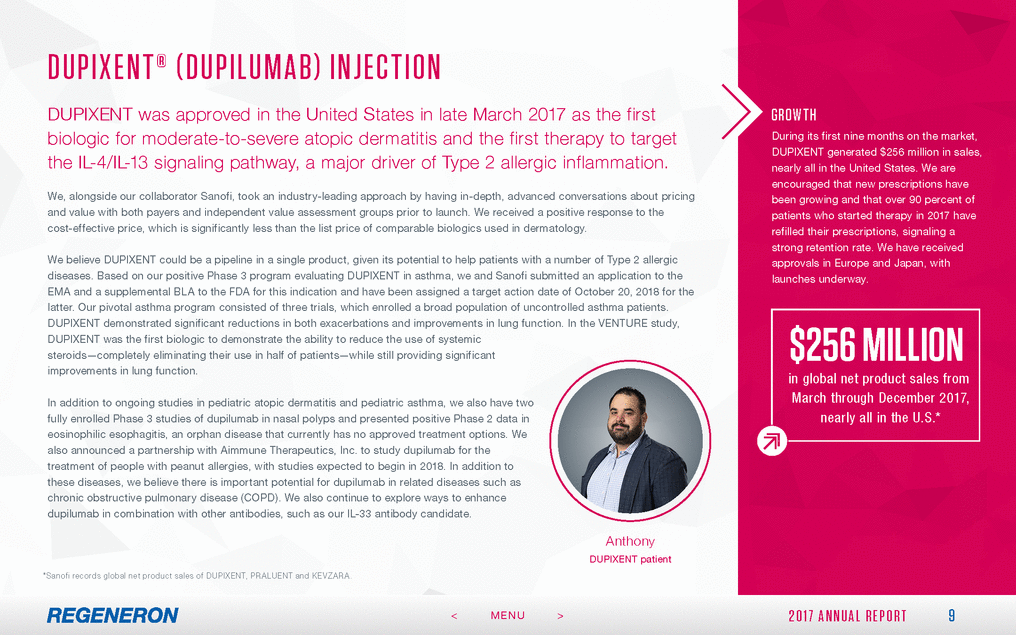
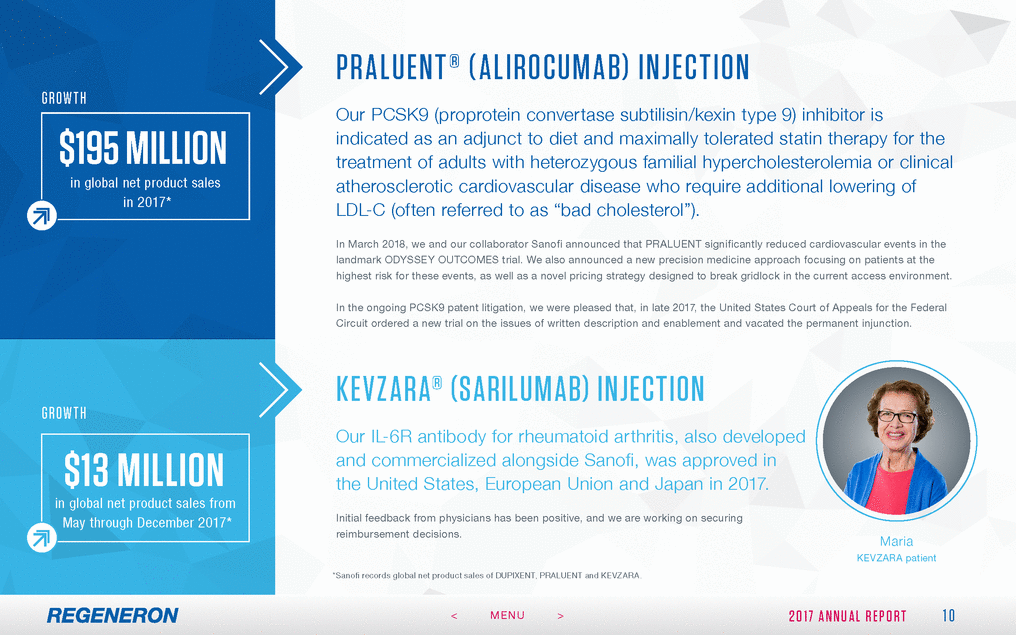
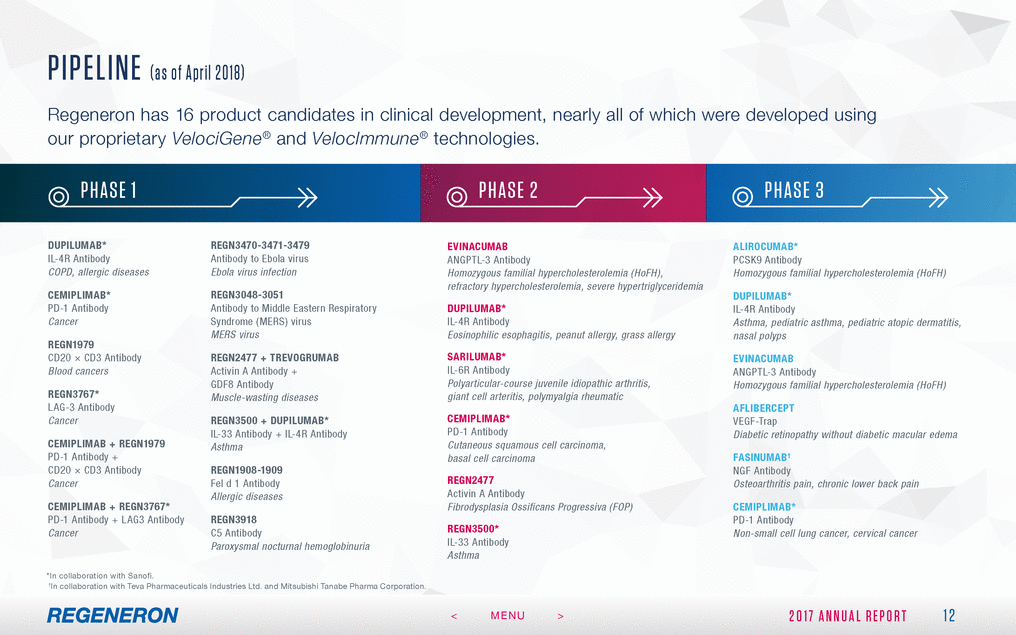
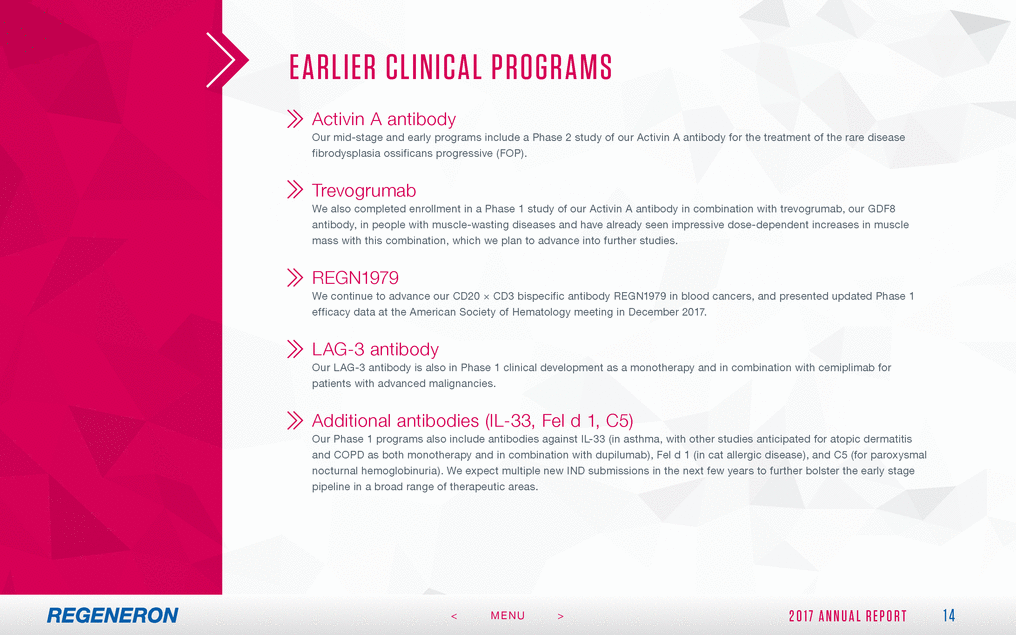
FORWA RD - LOOK ING S TAT EMEN T S A ND NON - GA A P FIN A NCIA L ME ASURES This Annual Report includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (where applicable, together with its subsidiaries, “Regeneron” or the “Company” ), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing and possible success and therapeutic applications of Regeneron’s products, product candidates and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, DUPIXENT® (dupilumab) Injection, PRALUENT® (alirocumab) Injection, KEVZARA® (sarilumab) Injection, cemiplimab, fasinumab and evinacumab; the likelihood and timing of achieving any of Regeneron’s anticipated clinical development milestones; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron’s late-stage product candidates and new indications for marketed products, including without limitation EYLEA, DUPIXENT, PRALUENT, KEVZARA, cemiplimab, fasinumab and evinacumab; the extent to which the results from the research and development programs conducted by Regeneron or its collaborators may be replicated in other studies and lead to therapeutic applications; ongoing regulatory obligations and oversight impacting Regeneron’s marketed products (such as EYLEA, DUPIXENT, PRALUENT and KEVZARA), research and clinical programs and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s products and product candidates; competing drugs and product candidates that may be superior to Regeneron’s products and product candidates; uncertainty of market acceptance and commercial success of Regeneron’s products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, suppliers or other third parties to perform filling, finishing, packaging, labeling, distribution and other steps related to Regeneron’s products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing and selling products; the ability of Regeneron to meet any of its financial projections or guidance, and changes to the assumptions underlying those projections or guidance; the potential for any license or collaboration agreement, including Regeneron’s agreements with Sanofi, Bayer and Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), to be cancelled or terminated without any further product success; and risks associated with intellectual property of others and pending or future litigation relating thereto, including without limitation the patent litigation proceedings relating to PRALUENT, the ultimate outcome of any such litigation proceedings and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results and financial condition. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2017, including in the section thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, whether as a result of new information, future events or otherwise. 27 2 0 1 7 A N N U A L R E P O R T < MENU >