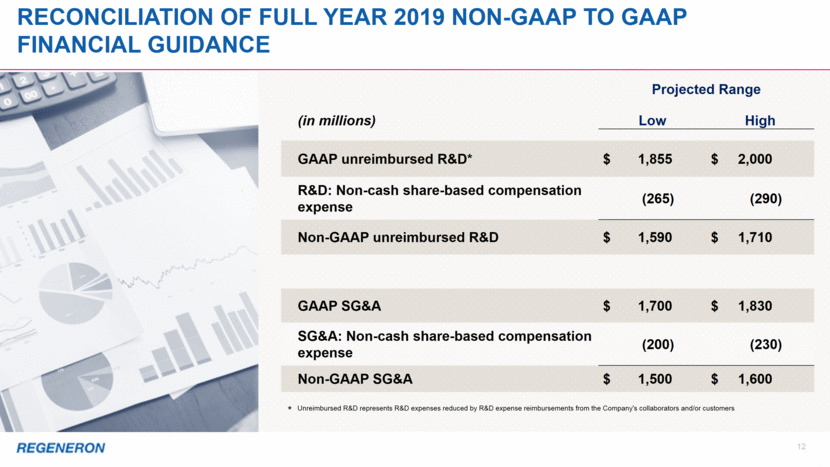
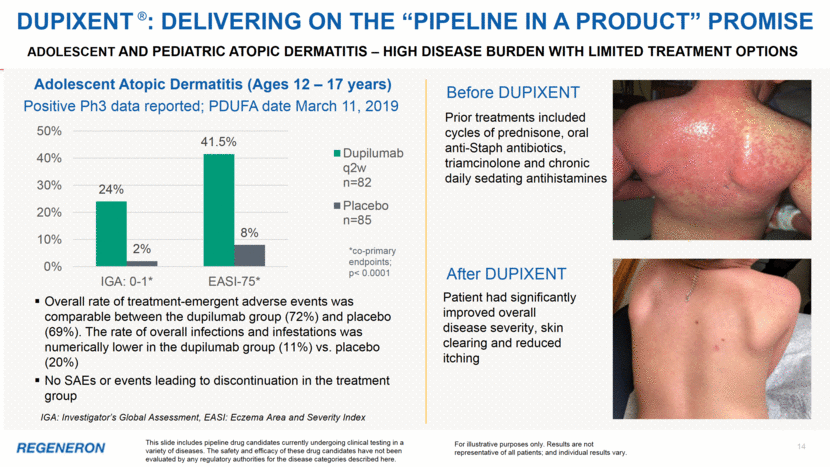
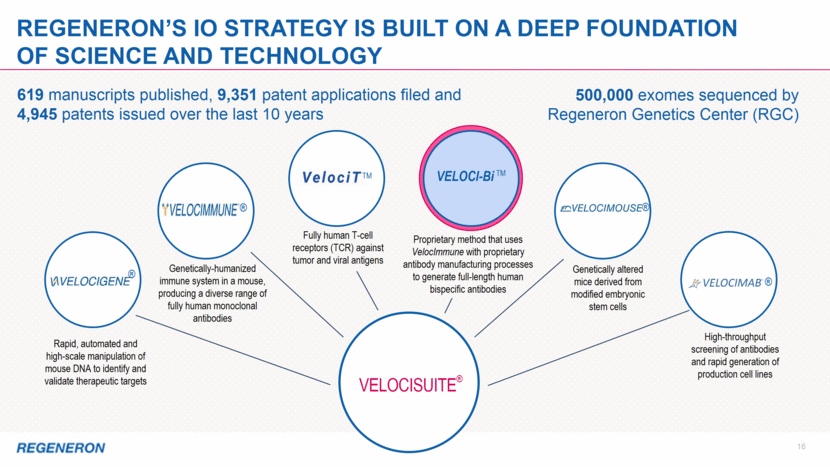
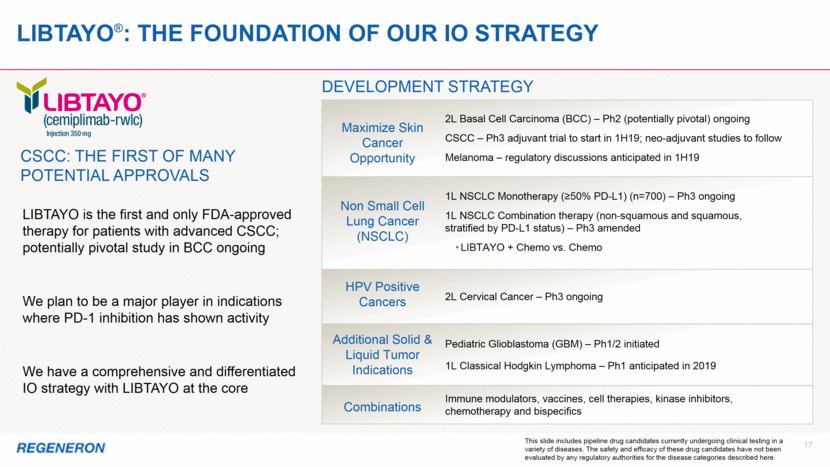
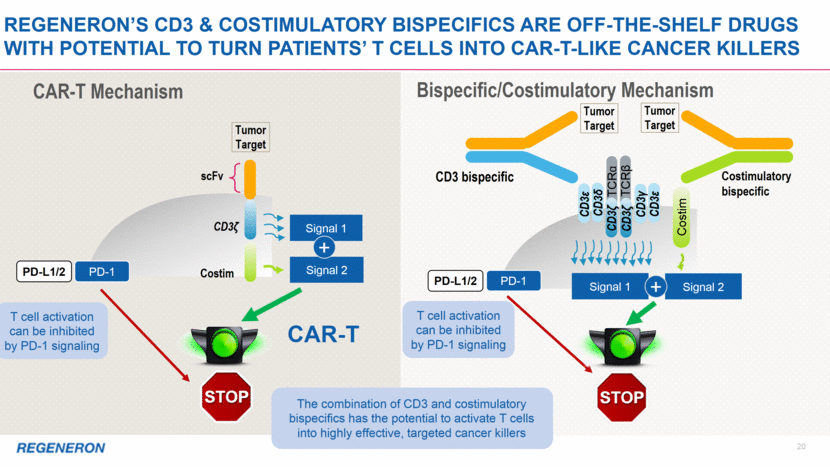
2 Note regarding FORWARD-LOOKING STATEMENTs and non-gaap financial measures This presentation includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of Regeneron’s products, product candidates, and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, Dupixent® (dupilumab) Injection, Praluent® (alirocumab) Injection, Kevzara® (sarilumab) Injection, Libtayo® (cemiplimab) Injection, fasinumab, evinacumab, Regeneron’s immuno-oncology programs (including its costimulatory bispecific portfolio), Regeneron’s earlier-stage product candidates, and the use of human genetics in Regeneron’s research programs; the extent to which the results from Regeneron’s research programs or preclinical testing may lead to advancement of product candidates to clinical trials or therapeutic applications; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron’s late-stage product candidates and new indications for marketed products, including without limitation EYLEA, Dupixent, Praluent, Kevzara, Libtayo, fasinumab, and evinacumab; the likelihood and timing of achieving any of the anticipated milestones described in this presentation; the extent to which the results from the research and development programs conducted by Regeneron or its collaborators may be replicated in other studies and lead to therapeutic applications; ongoing regulatory obligations and oversight impacting Regeneron’s marketed products (such as EYLEA, Dupixent, Praluent, Kevzara, and Libtayo), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s products and product candidates; competing drugs and product candidates that may be superior to Regeneron’s products and product candidates; uncertainty of market acceptance and commercial success of Regeneron’s products and product candidates and the impact of studies (whether conducted by Regeneron or others and whether mandated or voluntary) on the commercial success of Regeneron's products and product candidates; the availability and extent of reimbursement of the Company’s products from third-party payers, including private payer healthcare and insurance programs, health maintenance organizations, pharmacy benefit management companies, and government programs such as Medicare and Medicaid; coverage and reimbursement determinations by such payers and new policies and procedures adopted by such payers; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, suppliers, or other third parties to perform filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s products and product candidates; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the assumptions underlying those projections or guidance, including financial guidance relating to Sanofi collaboration revenue, non-GAAP unreimbursed R&D, non-GAAP SG&A, effective tax rate, and capital expenditures; risks associated with intellectual property of other parties and pending or future litigation relating thereto, including without limitation the patent litigation proceedings relating to EYLEA, Dupixent, and Praluent, the ultimate outcome of any such litigation proceeding, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition; and the potential for any license or collaboration agreement, including Regeneron’s agreements with Sanofi, Bayer, and Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), to be cancelled or terminated without any further product success. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2017 and its Form 10-Q for the quarterly period ended September 30, 2018, including in each case in the section thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise. This presentation uses non-GAAP unreimbursed R&D and non-GAAP SG&A, which are financial measures that are not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). These non-GAAP financial measures are computed by excluding certain non-cash and other items from the related GAAP financial measure. Non-GAAP adjustments also include the income tax effect of reconciling items. The Company makes such adjustments for items the Company does not view as useful in evaluating its operating performance. For example, adjustments may be made for items that fluctuate from period to period based on factors that are not within the Company’s control, such as the Company’s stock price on the dates share-based grants are issued. Management uses these and other non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides forecasts to investors on this basis. Additionally, such non-GAAP measures provide investors with an enhanced understanding of the financial performance of the Company’s core business operations. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company’s non-GAAP financial measures may not be comparable with non-GAAP information provided by other companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP. A reconciliation of the Company's full year 2019 non-GAAP to GAAP financial guidance is provided on slide 12.