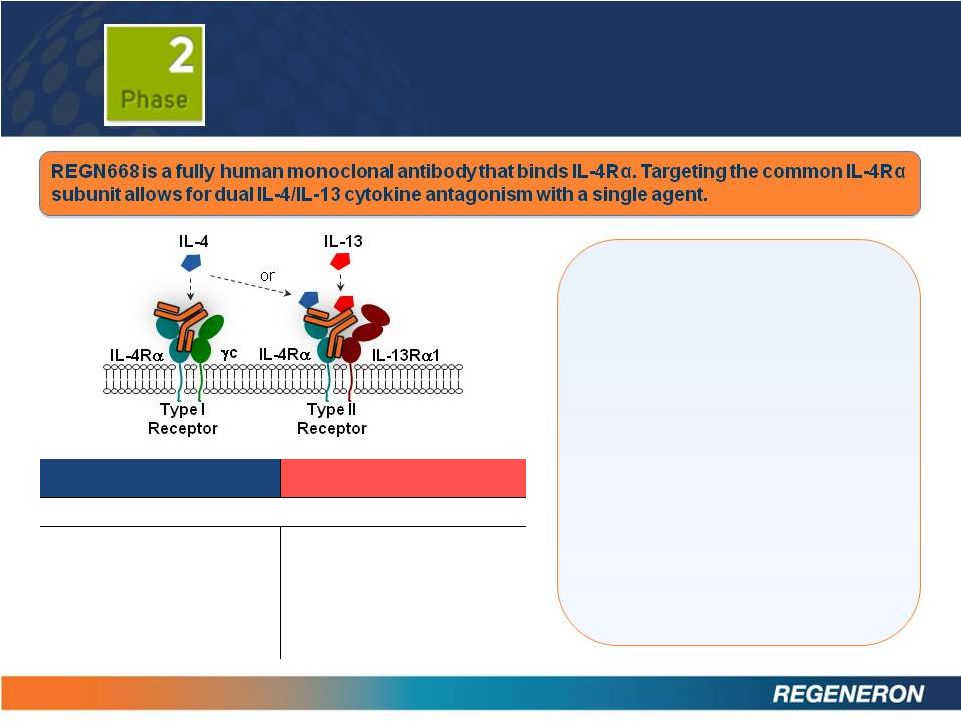
2 SAFE HARBOR STATEMENT Except for historical information, the matters contained in this presentation may constitute forward-looking statements that involve risks and uncertainties, including risks and uncertainties related to product development and clinical trials, unforeseen safety issues resulting from the administration of products and product candidates in patients, uncertainties related to the need for regulatory and other government approvals, government regulations, risks related to third party patents and proprietary technology, litigation, the need for additional capital, uncertainty of market acceptance of Regeneron’s products and product candidates, the ability of the Company to meet any of its sales or other financial projections, the receipt of future payments, the continuation of business partnerships, and additional risks detailed from time to time in Regeneron’s filings with the Securities and Exchange Commission (SEC). Please refer to Regeneron’s Form 10-K for the year ended December 31, 2011 and its Form 10-Q for the quarter ended September 30, 2012 for additional information on these risks and uncertainties and for other information related to our business. Because forward-looking statements involve risks and uncertainties, actual results may differ materially from current results expected by Regeneron. Regeneron is providing this information as of the original date of this presentation and expressly disclaims any duty to update any information contained in these materials, including without limitation any sales forecasts and any other forward-looking statements. |