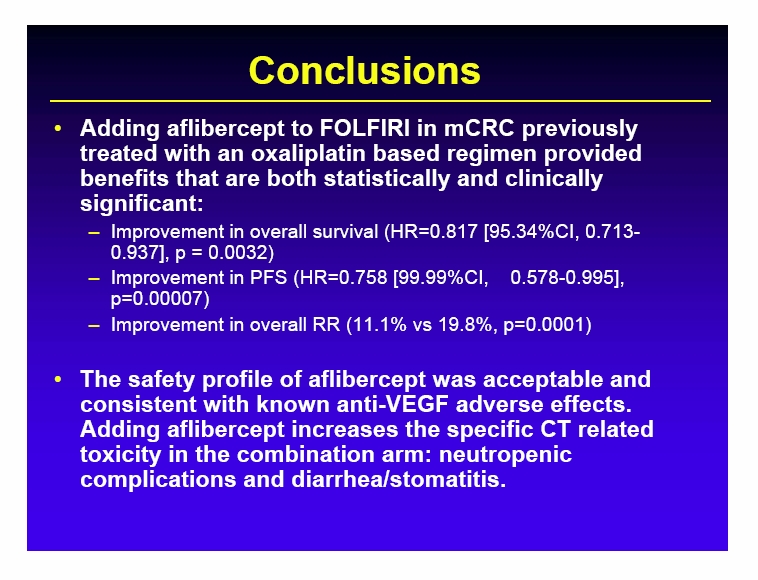
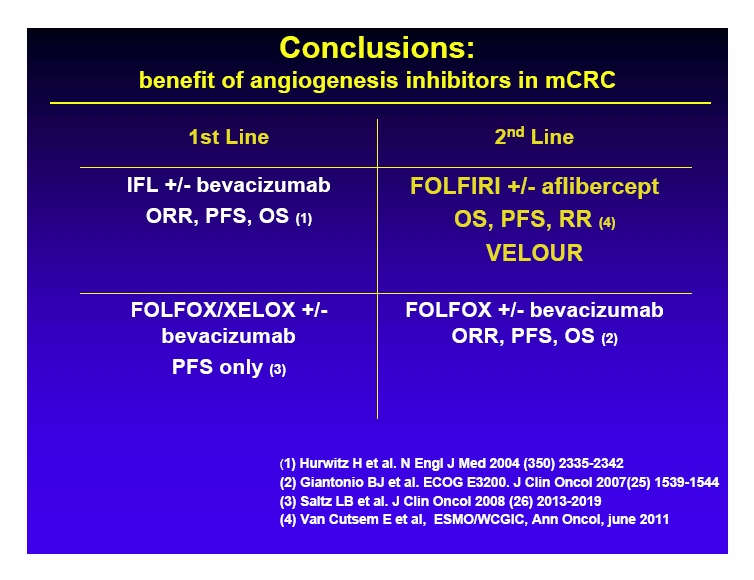
Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
REGN similar filings
- 22 Aug 11 Regulation FD Disclosure
- 17 Aug 11 Regeneron Announces Review of Biologics License Application for EYLEA™ (aflibercept injection) Extended by Three Months by FDA
- 28 Jul 11 Results of Operations and Financial Condition
- 27 Jun 11 Regulation FD Disclosure
- 21 Jun 11 Other Events
- 15 Jun 11 Departure of Directors or Certain Officers
- 3 May 11 Regeneron Reports First Quarter 2011 Financial and Operating Results
Filing view
External links