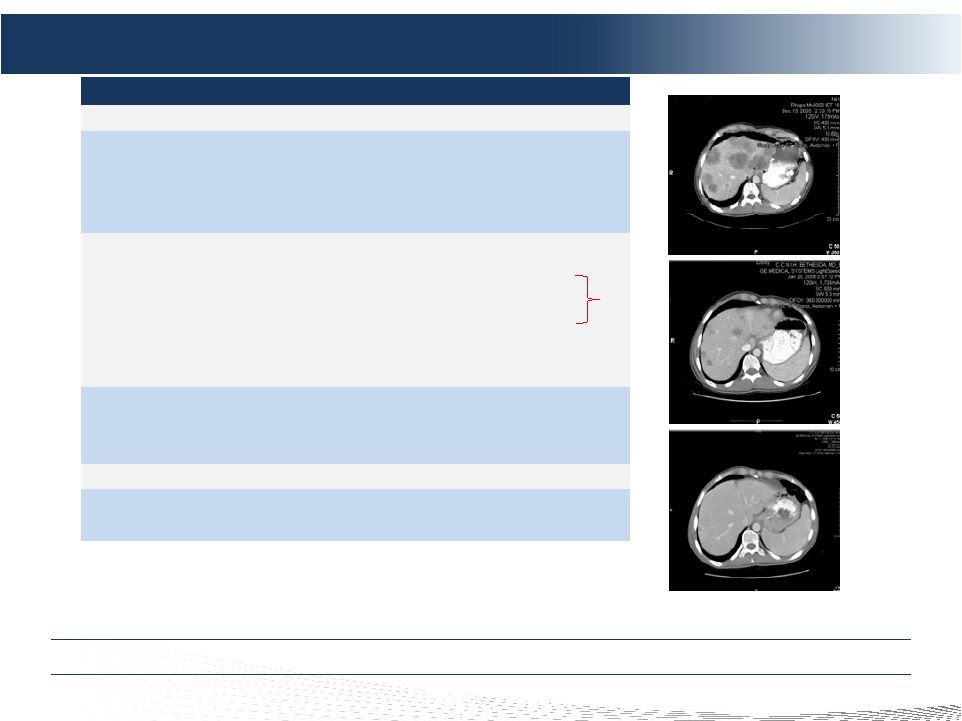
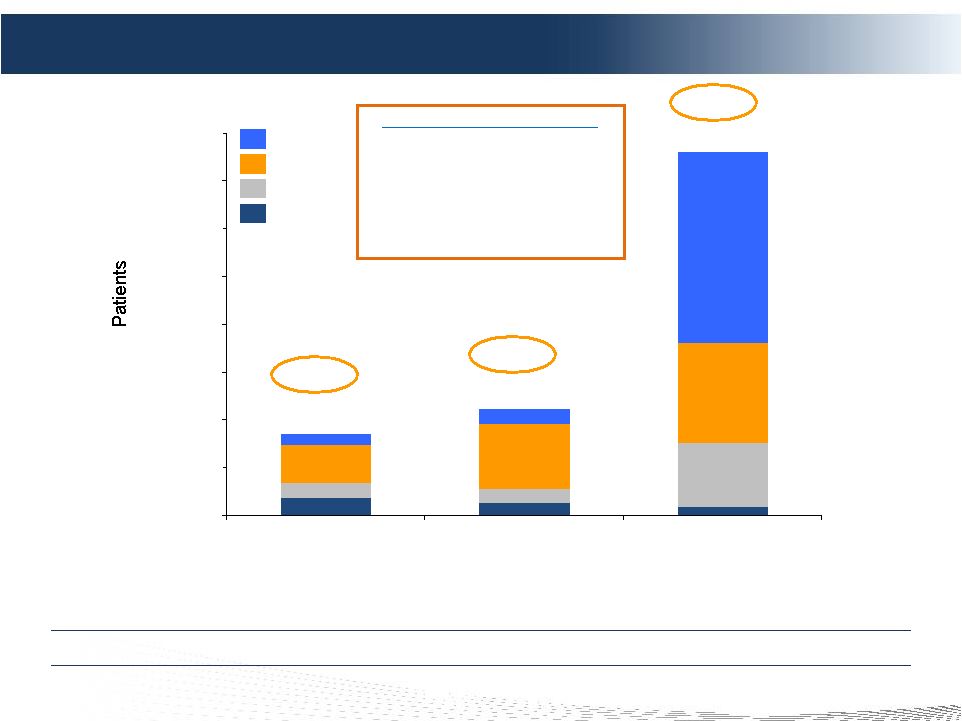
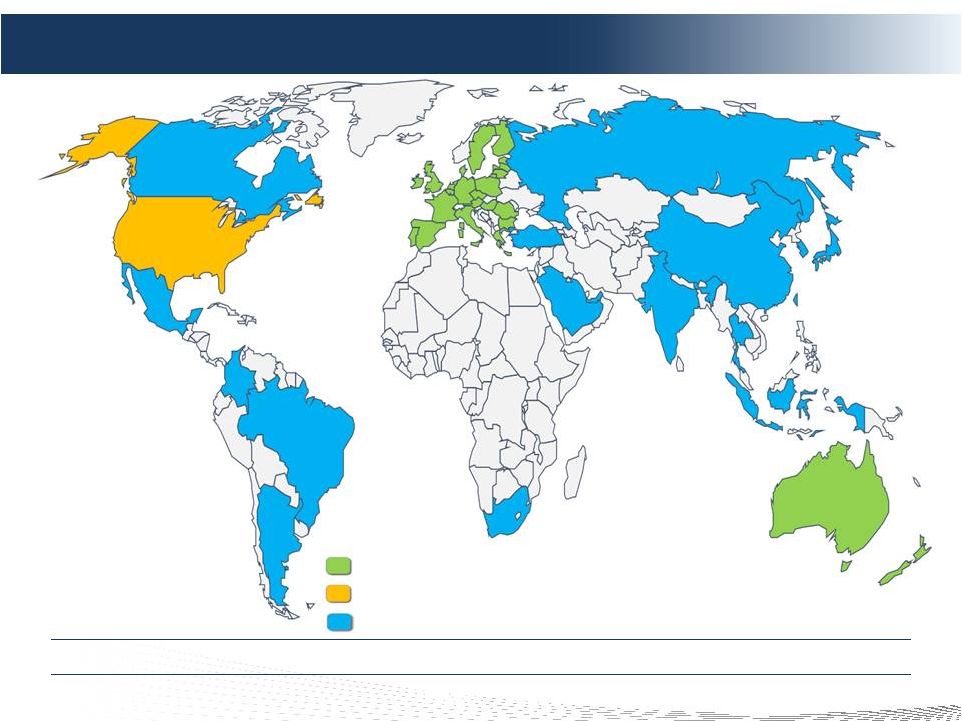
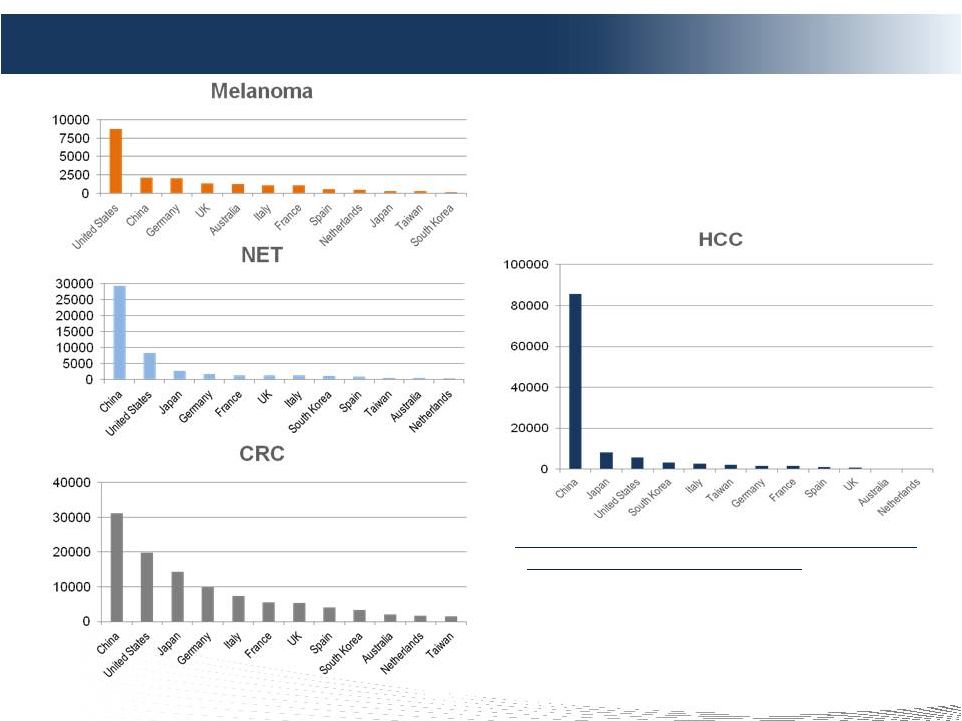
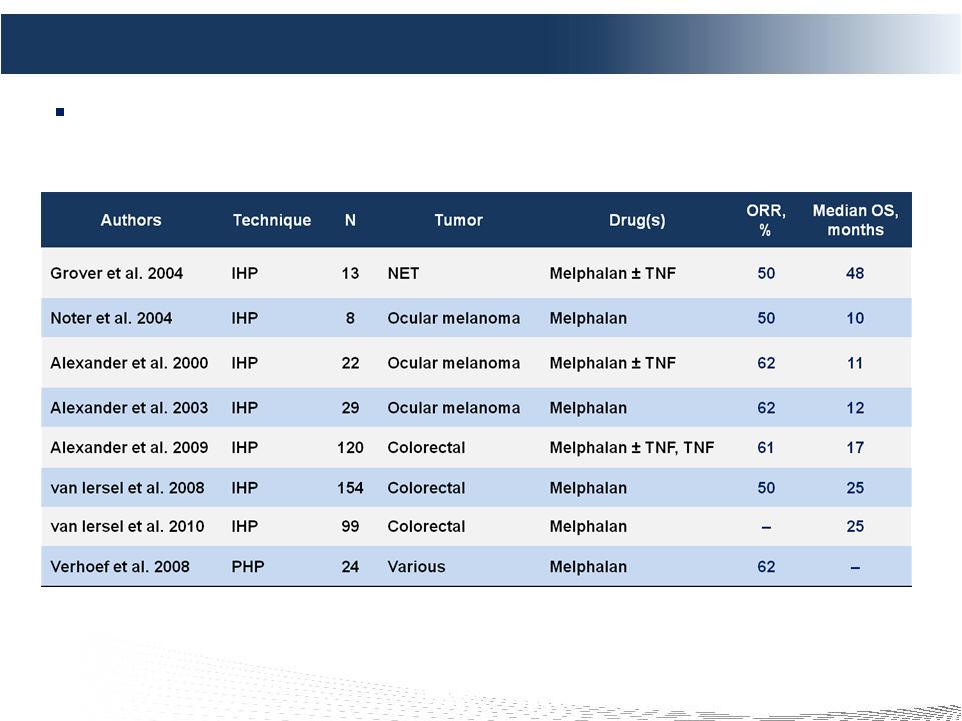
2 DELCATH SYSTEMS, INC Forward-looking Statements Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This presentation contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: timing of completion of the FDA’s review of our NDA, the extent to which the FDA may request additional information or data and our ability to provide the same in a timely manner, acceptability of the Phase 1, 2 and 3 clinical trial data by the FDA, FDA approval of the Company's NDA for the treatment of metastatic ocular melanoma to the liver, adoption, use and resulting sales, if any, for the chemosaturation system in the United States, adoption, use and resulting sales, if any, for the Hepatic CHEMOSAT delivery system in the EEA, our ability to successfully commercialize the chemosaturation system in various markets and the potential of the chemosaturation system as a treatment for patients with cancers in the liver, the timing and our ability to successfully enter into strategic partnership and distribution arrangements in foreign markets including Australia and key Asian markets and resulting sales, if any, from the same, patient outcomes using the Generation 2 system, approval of the current or future chemosaturation system for other indications and/or for use with various chemotherapeutic agents, actions by the FDA or other foreign regulatory agencies, our ability to obtain reimbursement for the CHEMOSAT system in various markets, submission and publication of the Phase II and III clinical trial data, the timing and results of research and development projects, the timing and results of future clinical trials including the initiation of clinical trials in key Asian markets with the Hepatic CHEMOSAT Delivery System device for intra-hepatic arterial delivery and extracorporeal filtration of doxorubicin, approval of the Hepatic CHEMOSAT Delivery System to delver and filter doxorubicin in key Asian markets and adoption, sales, if any, and patient outcomes using the same, the timing, price and use, if any, of the committee equity financing facility with Terrapin, the timing and use, if any, of the line of credit from SVB and our ability to access this facility and uncertainties regarding our ability to obtain financial and other resources for any research, development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward- looking statements to reflect events or circumstances after the date they are made. |