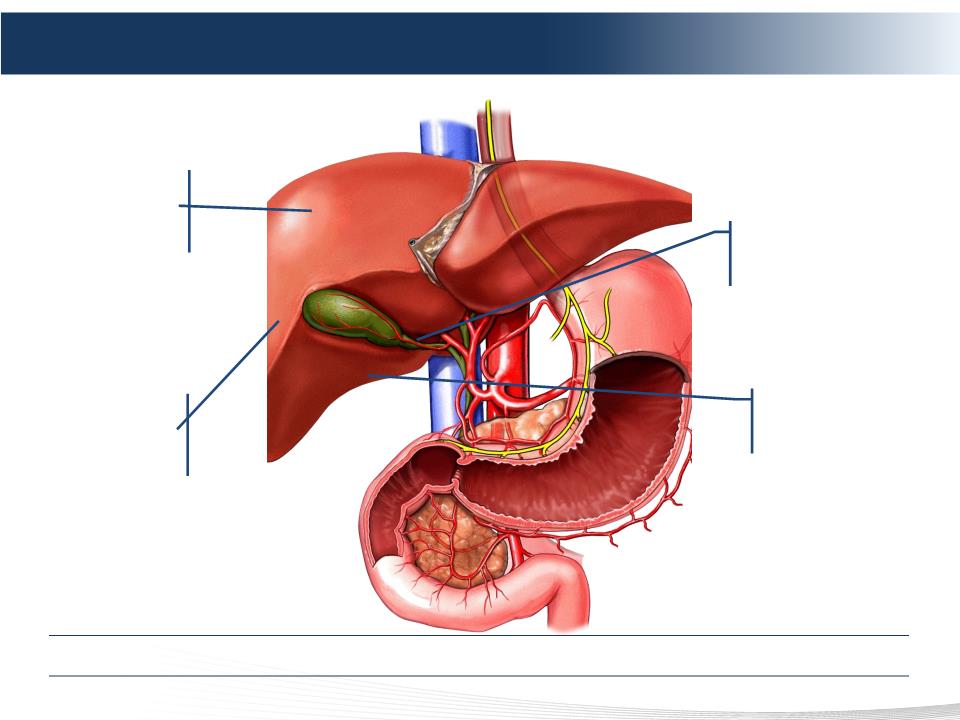
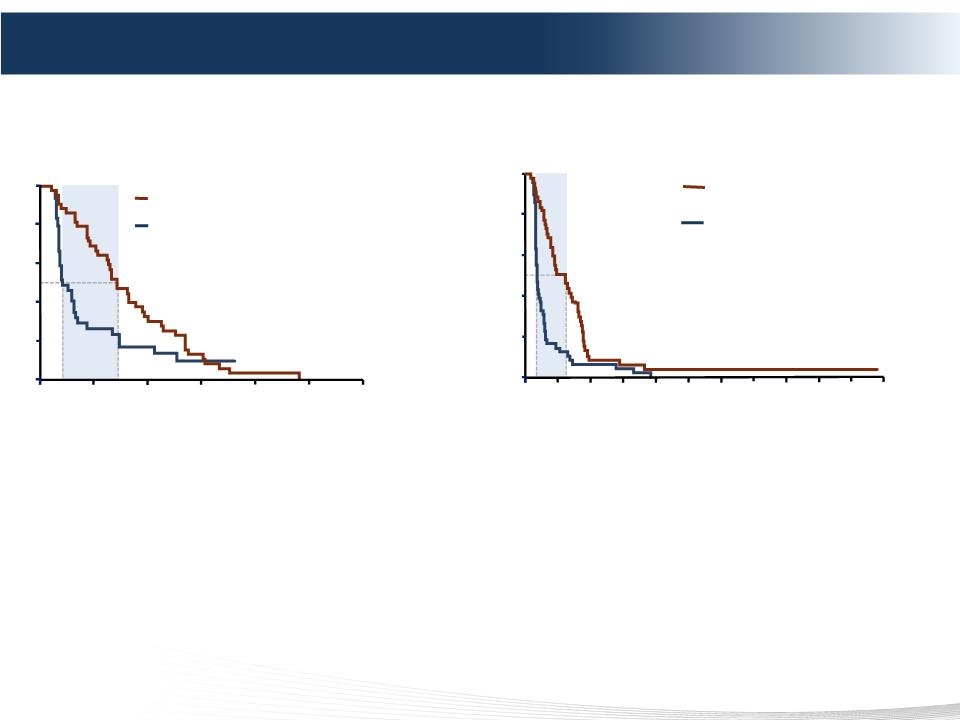
2 DELCATH SYSTEMS, INC
Forward-looking Statements
This presentation contains forward-looking statements, within the meaning of the federal securities laws, related to future
events and future financial performance which include statements about our expectations, beliefs, plans, objectives,
intentions, goals, strategies, assumptions and other statements that are not historical facts. Forward-looking statements
are subject to known and unknown risks and uncertainties and are based on potentially inaccurate assumptions, which
could cause actual results to differ materially from expected results, performance or achievements expressed or implied
by statements made herein. Our actual results could differ materially from those anticipated in forward-looking statements
for many reasons, including, but not limited to, uncertainties relating to: the timing and results of future clinical trials
including without limitation the OM, HCC, ICC, and mCRC trials in the Company’s Clinical Development Program, clinical
adoption, use and resulting sales, if any, for the CHEMOSAT system in Europe, our ability to obtain reimbursement for
the CHEMOSAT system in various markets including without limitation Germany and the United Kingdom, our ability to
successfully commercialize the Melphalan/HDS system and the potential of the Melphalan/HDS system as a treatment for
patients with primary and metastatic disease in the liver, the Company's ability to satisfy the requirements of the FDA's
Complete Response Letter relating to the ocular melanoma indication and the timing of the same, approval of the
Melphalan/HDS system by the US FDA, submission and acceptance of the phase 3 trial publication, approval of the
current or future Melphalan/HDS system for delivery and filtration of melphalan or other chemotherapeutic agents for
various indications in the US and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability
to successfully enter into strategic partnership and distribution arrangements in foreign markets and the timing and
revenue, if any, of the same, uncertainties relating to the timing and results of research and development projects, and
uncertainties regarding our ability to obtain financial and other resources for any clinical trials, research, development,
and commercialization activities. These factors, and others, are discussed from time to time in our filings with the
Securities and Exchange Commission including the section entitled ‘‘Risk Factors’’ in our most recent Annual Report on
Form 10-K and our Reports on Form 10-Q and Form 8-K.