
Louise Rodino-Klapac Senior Vice President, Gene Therapy Sarepta Therapeutics, Inc. October 4, 2019 Clinical Update: srp-9003 Beta-Sarcoglycanopathy Gene Therapy program Limb-Girdle Muscular Dystrophy Type 2E FUNCTIONAL DATA Exhibit 99.2
Forward-Looking Statements This presentation contains "forward-looking statements." Any statements that are not statements of historical fact may be deemed to be forward-looking statements. Words such as “believe,” “anticipate,” “plan,” “expect,” “will,” “may,” “intend,” “prepare,” “look,” “potential,” “possible” and similar expressions are intended to identify forward-looking statements. These forward-looking statements include statements relating to the safety profile of SRP-9003 seen to date supporting the ability to dose escalate; the potential benefits of the AAVrh74 vector and the MHCK7 promoter; our clinical programs in LGMD; and our plans regarding dose escalation, selection of final dose for registration trial and engaging with global regulatory agencies to discuss pivotal trial designs. These forward-looking statements involve risks and uncertainties, many of which are beyond Sarepta’s control. Actual results could materially differ from those stated or implied by these forward-looking statements as a result of such risks and uncertainties. Known risk factors include the following: success in preclinical testing and early clinical trials, especially if based on a small patient sample, does not ensure that later clinical trials will be successful, and initial results from a clinical trial do not necessarily predict final results; the data presented in this presentation may not be consistent with the final data set and analysis thereof or result in a safe or effective treatment benefit; different methodologies, assumptions and applications Sarepta utilizes to assess particular safety or efficacy parameters may yield different statistical results, and even if Sarepta believes the data collected from clinical trials of its product candidates are positive, these data may not be sufficient to support approval by the FDA or foreign regulatory authorities; Sarepta’s ongoing research and development efforts may not result in any viable treatments suitable for clinical research or commercialization due to a variety of reasons, some of which may be outside of Sarepta’s control, including possible limitations of Company financial and other resources, manufacturing limitations that may not be anticipated or resolved for in a timely manner, and regulatory, court or agency decisions, such as decisions by the United States Patent and Trademark Office with respect to patents that cover our product candidates; and even if Sarepta’s programs result in new commercialized products, Sarepta may not achieve any significant revenues from the sale of such products; and those risks identified under the heading “Risk Factors” in Sarepta’s most recent Annual Report on Form 10-K for the year ended December 31, 2018 or most recently filed Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) as well as other SEC filings made by the Company which you are encouraged to review. Any of the foregoing risks could materially and adversely affect the Company’s business, results of operations and the trading price of Sarepta’s common stock. You should not place undue reliance on forward-looking statements. Sarepta does not undertake any obligation to publicly update its forward-looking statements based on events or circumstances after the date hereof, except to the extent required by applicable law or SEC rules.
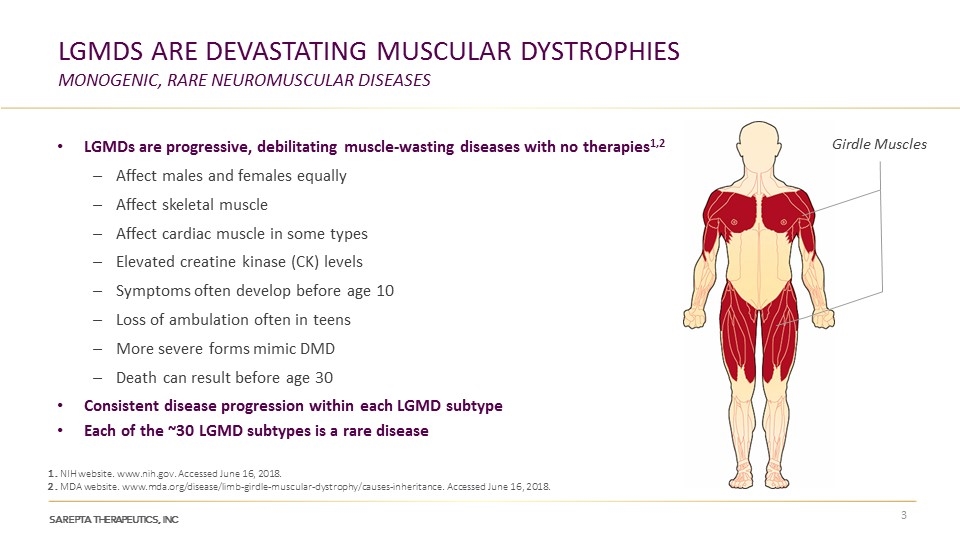
LGMDs Are Devastating Muscular Dystrophies monogenic, rare neuromuscular diseases LGMDs are progressive, debilitating muscle-wasting diseases with no therapies1,2 Affect males and females equally Affect skeletal muscle Affect cardiac muscle in some types Elevated creatine kinase (CK) levels Symptoms often develop before age 10 Loss of ambulation often in teens More severe forms mimic DMD Death can result before age 30 Consistent disease progression within each LGMD subtype Each of the ~30 LGMD subtypes is a rare disease 1. NIH website. www.nih.gov. Accessed June 16, 2018. 2. MDA website. www.mda.org/disease/limb-girdle-muscular-dystrophy/causes-inheritance. Accessed June 16, 2018. Max Girdle Muscles
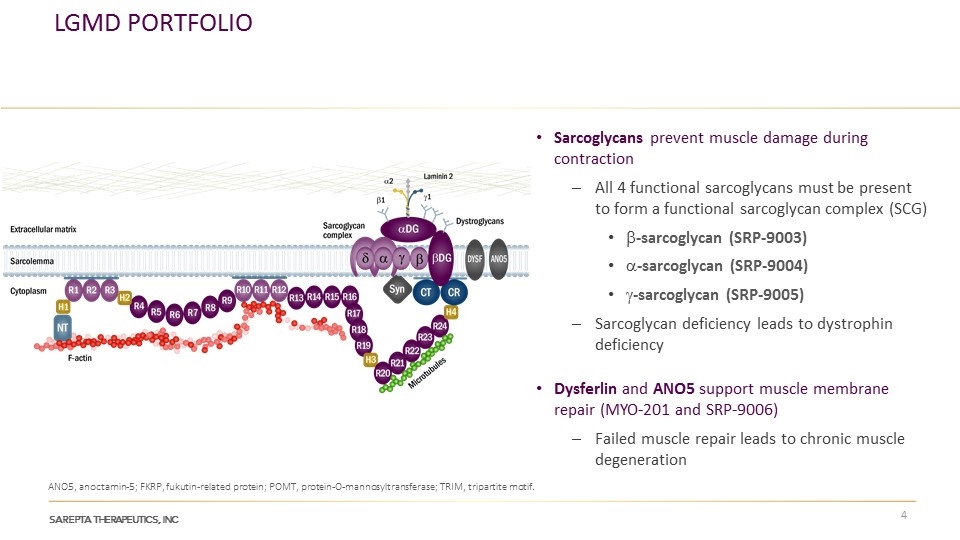
LGMD Portfolio Sarcoglycans prevent muscle damage during contraction All 4 functional sarcoglycans must be present to form a functional sarcoglycan complex (SCG) b-sarcoglycan (SRP-9003) a-sarcoglycan (SRP-9004) g-sarcoglycan (SRP-9005) Sarcoglycan deficiency leads to dystrophin deficiency Dysferlin and ANO5 support muscle membrane repair (MYO-201 and SRP-9006) Failed muscle repair leads to chronic muscle degeneration ANO5, anoctamin-5; FKRP, fukutin-related protein; POMT, protein-O-mannosyltransferase; TRIM, tripartite motif.
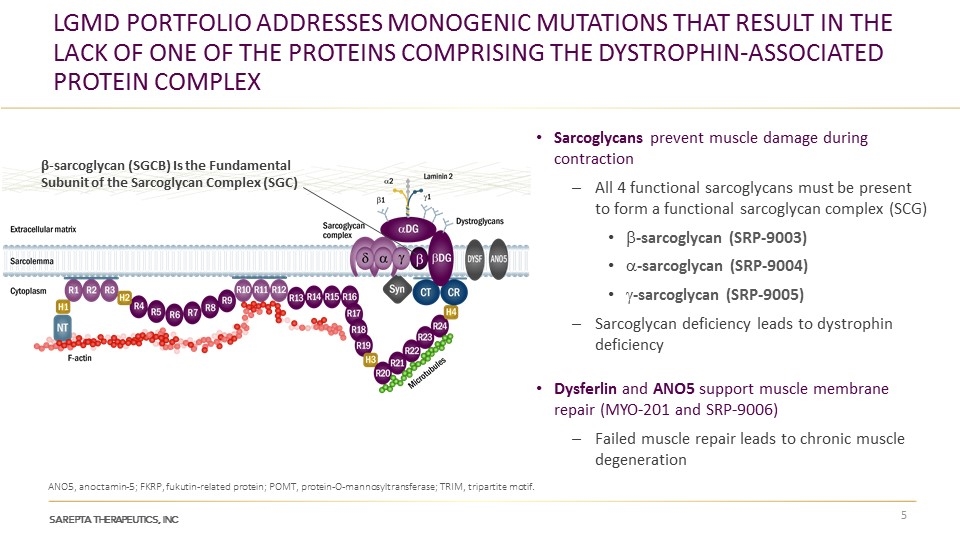
LGMD Portfolio Addresses monogenic Mutations that result in the lack of one of the Proteins Comprising the Dystrophin-associated Protein Complex ANO5, anoctamin-5; FKRP, fukutin-related protein; POMT, protein-O-mannosyltransferase; TRIM, tripartite motif. β-sarcoglycan (SGCB) Is the Fundamental Subunit of the Sarcoglycan Complex (SGC) Sarcoglycans prevent muscle damage during contraction All 4 functional sarcoglycans must be present to form a functional sarcoglycan complex (SCG) b-sarcoglycan (SRP-9003) a-sarcoglycan (SRP-9004) g-sarcoglycan (SRP-9005) Sarcoglycan deficiency leads to dystrophin deficiency Dysferlin and ANO5 support muscle membrane repair (MYO-201 and SRP-9006) Failed muscle repair leads to chronic muscle degeneration
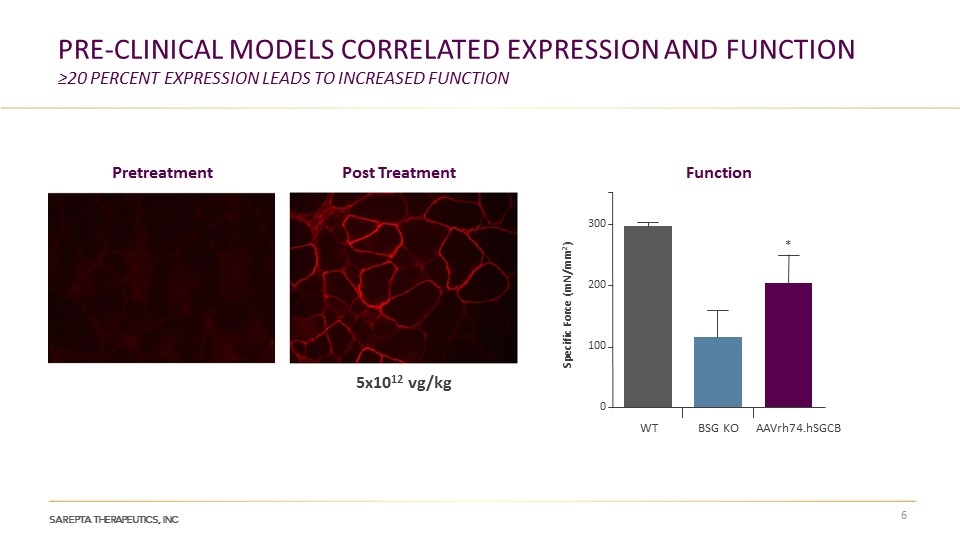
Pre-clinIcal models correlateD expression and function ≥20 percent expression leads to increased function Pretreatment Post Treatment Function WT BSG KO AAVrh74.hSGCB 5x1012 vg/kg 300 200 100 0 Specific Force (mN/mm2) *

LGMD2E Phase I/II Study: Cohort 1 (n=3)

LGMD TYPE 2E Open-label Trial Design Up to 6 subjects with LGMD Cohort 1: 3 subjects; 4-15 years of age, 5x1013 vg/kg AAVrh74.MHCK7.SGCB systemic delivery Inclusion criteria A confirmed SGCB mutation in both alleles Negative for AAVrh74 antibodies >40% of Normal 100 meter walk test 60-day needle muscle biopsy Prednisone 1 day prior to gene transfer, 30 days 1 mg/kg, taper

Outcome measures Primary endpoint ≥ 20% b-sarcoglycan expression Safety Secondary endpoints, including: Decrease in CK Functional endpoints North Star Assessment for LGMD (NSAD) 100m 10m 4 stairs Time to rise

LGMD2E Study Expression RESULTS: Cohort 1 (n=3)
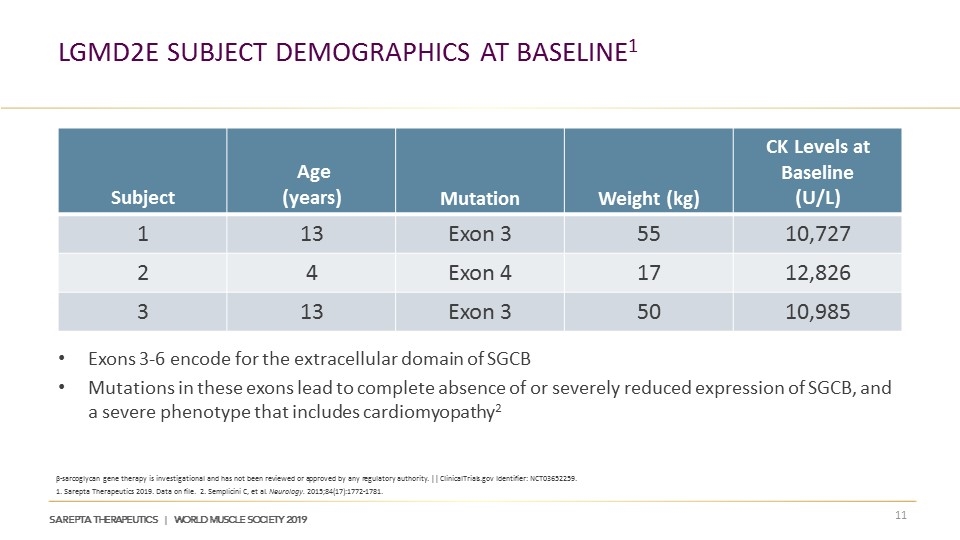
LGMD2E Subject Demographics at Baseline1 Exons 3-6 encode for the extracellular domain of SGCB Mutations in these exons lead to complete absence of or severely reduced expression of SGCB, and a severe phenotype that includes cardiomyopathy2 Subject Age (years) Mutation Weight (kg) CK Levels at Baseline (U/L) 1 13 Exon 3 55 10,727 2 4 Exon 4 17 12,826 3 13 Exon 3 50 10,985 β-sarcoglycan gene therapy is investigational and has not been reviewed or approved by any regulatory authority. || ClinicalTrials.gov Identifier: NCT03652259. 1. Sarepta Therapeutics 2019. Data on file. 2. Semplicini C, et al. Neurology. 2015;84(17):1772-1781.
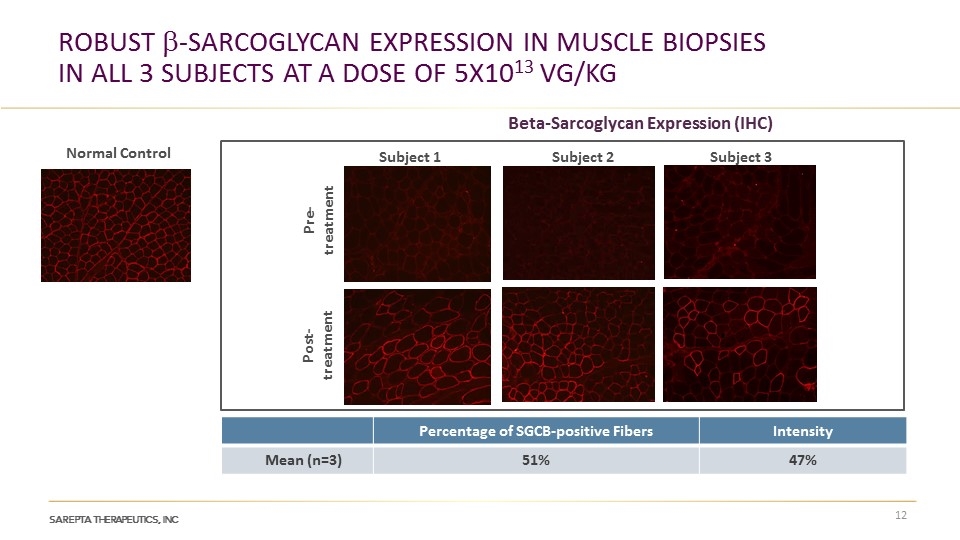
Robust b-Sarcoglycan Expression in Muscle Biopsies in All 3 Subjects at a dose of 5x1013 vg/kg Percentage of SGCB-positive Fibers Intensity Mean (n=3) 51% 47% Normal Control Subject 2 Subject 1 Subject 3 Beta-Sarcoglycan Expression (IHC) Post-treatment Pre-treatment
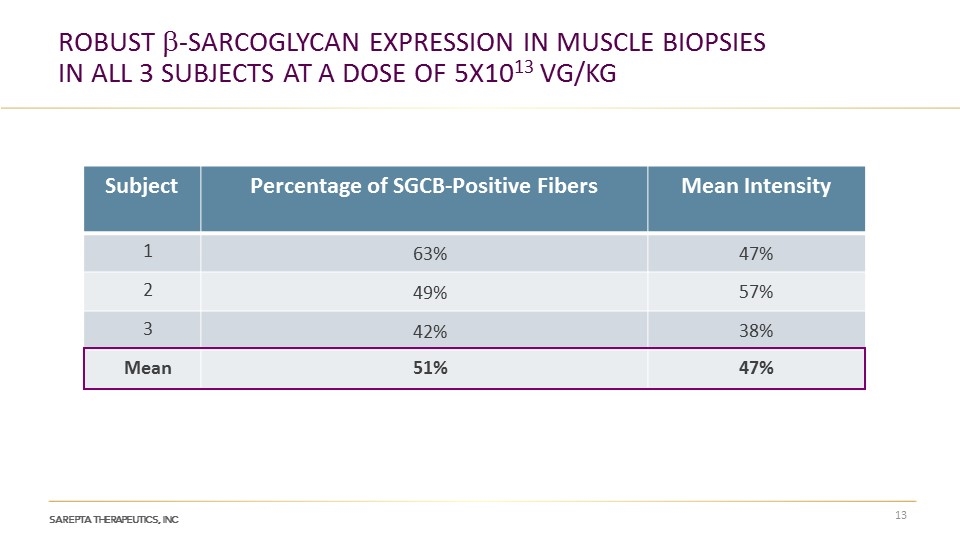
Robust b-Sarcoglycan Expression in Muscle Biopsies in All 3 Subjects at a dose of 5x1013 vg/kg Subject Percentage of SGCB-Positive Fibers Mean Intensity 1 63% 47% 2 49% 57% 3 42% 38% Mean 51% 47%
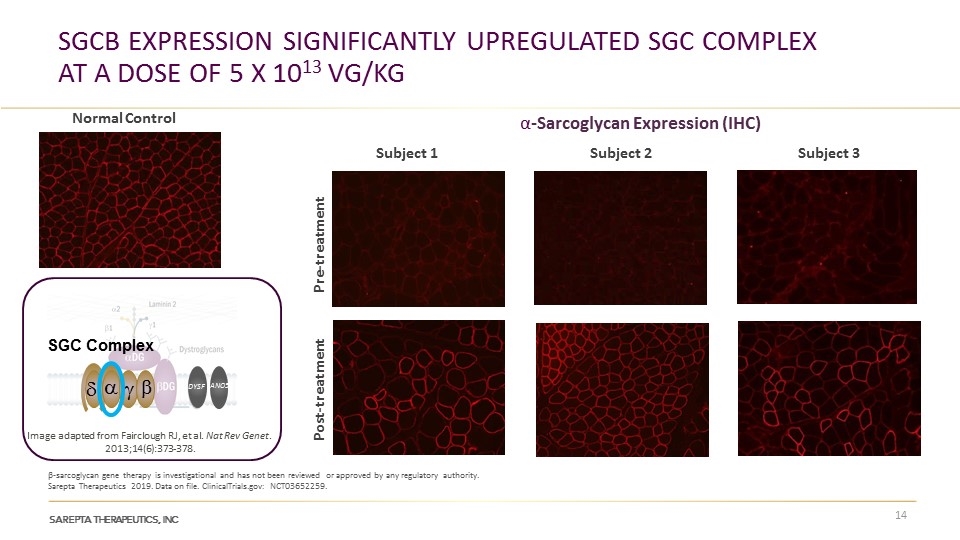
SGCB Expression significantly upregulated sgc complex at a dose of 5 x 1013 vg/kg β-sarcoglycan gene therapy is investigational and has not been reviewed or approved by any regulatory authority. Sarepta Therapeutics 2019. Data on file. ClinicalTrials.gov: NCT03652259. Normal Control d a g b SGC Complex ANO5 DYSF Image adapted from Fairclough RJ, et al. Nat Rev Genet. 2013;14(6):373-378. Subject 1 Post-treatment Pre-treatment Subject 2 Subject 3 α-Sarcoglycan Expression (IHC)
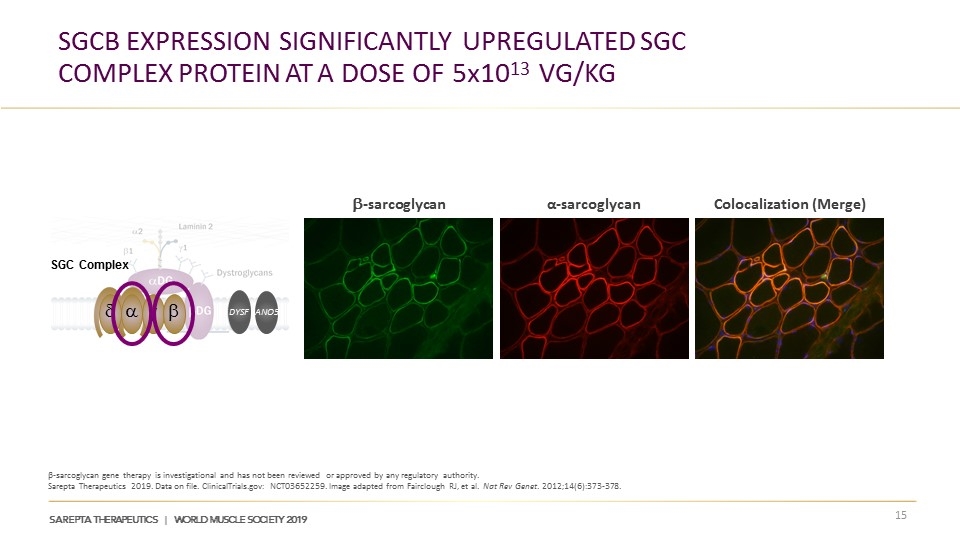
SGCB Expression Significantly Upregulated SGC Complex protein at a Dose of 5x1013 vg/kg β-sarcoglycan gene therapy is investigational and has not been reviewed or approved by any regulatory authority. Sarepta Therapeutics 2019. Data on file. ClinicalTrials.gov: NCT03652259. Image adapted from Fairclough RJ, et al. Nat Rev Genet. 2012;14(6):373-378. b-sarcoglycan α-sarcoglycan Colocalization (Merge) d a g b SGC Complex ANO5 DYSF
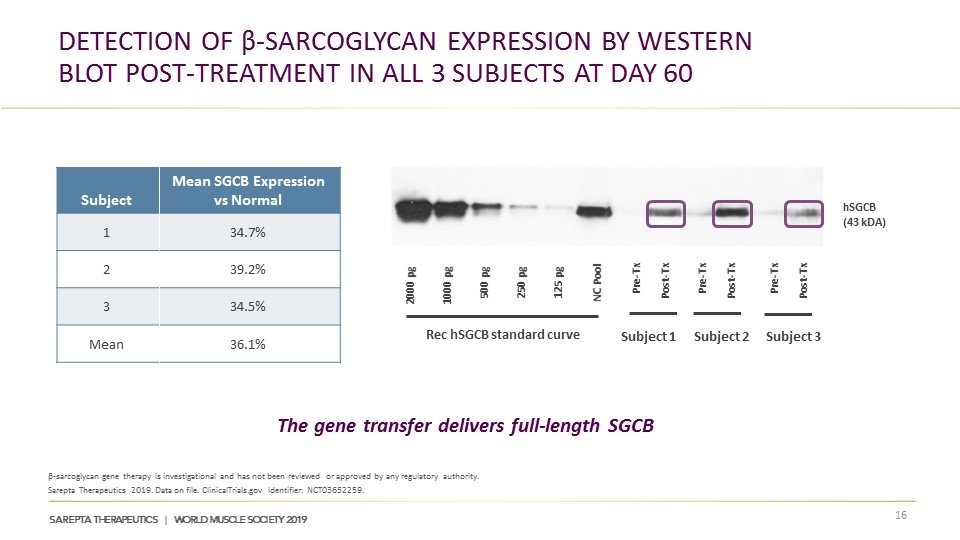
Detection of β-sarcoglycan Expression by Western Blot Post-treatment in All 3 Subjects at Day 60 β-sarcoglycan gene therapy is investigational and has not been reviewed or approved by any regulatory authority. Sarepta Therapeutics 2019. Data on file. ClinicalTrials.gov Identifier: NCT03652259. The gene transfer delivers full-length SGCB Subject Mean SGCB Expression vs Normal 1 34.7% 2 39.2% 3 34.5% Mean 36.1% 2000 pg 1000 pg 500 pg 250 pg 125 pg NC Pool Pre-Tx Post-Tx Pre-Tx Post-Tx Pre-Tx Post-Tx Rec hSGCB standard curve hSGCB (43 kDA) Subject 1 Subject 2 Subject 3
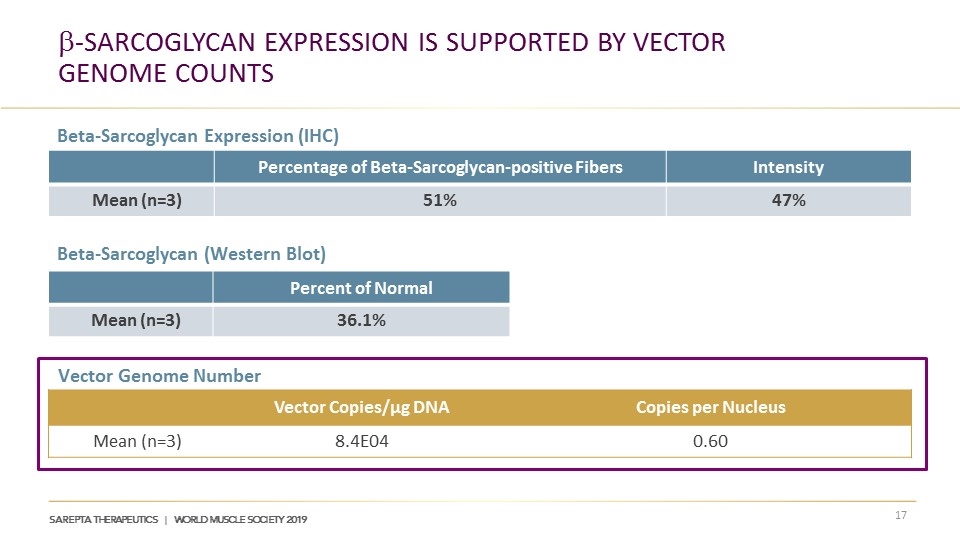
b-Sarcoglycan Expression is Supported by Vector Genome CountS Percentage of Beta-Sarcoglycan-positive Fibers Intensity Mean (n=3) 51% 47% Percent of Normal Mean (n=3) 36.1% Vector Copies/μg DNA Copies per Nucleus Mean (n=3) 8.4E04 0.60 Beta-Sarcoglycan Expression (IHC) Vector Genome Number Beta-Sarcoglycan (Western Blot)

LGMD2E Study Functional data summary: Cohort 1 (n=3)
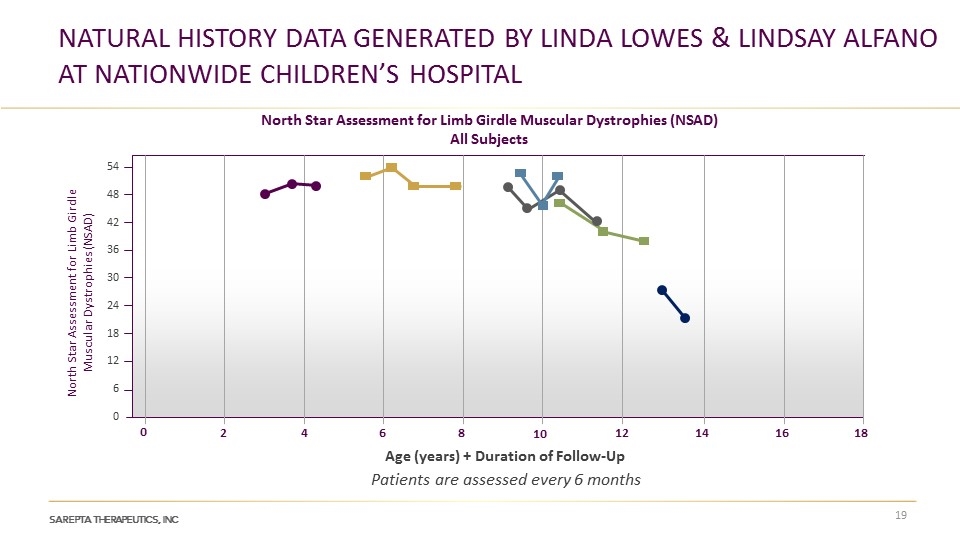
NATURAL HISTORY DATA generated by linda lowes & LINDSAY ALFANO at Nationwide Children’s Hospital 54 48 42 36 30 24 18 12 6 0 Age (years) + Duration of Follow-Up 0 2 4 6 12 16 14 8 10 18 North Star Assessment for Limb Girdle Muscular Dystrophies (NSAD) All Subjects North Star Assessment for Limb Girdle Muscular Dystrophies (NSAD) Patients are assessed every 6 months
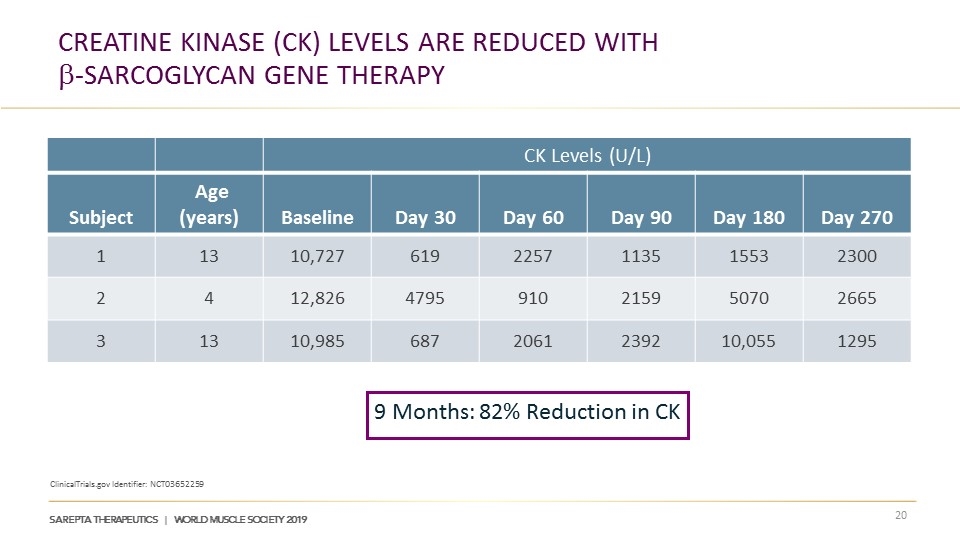
Creatine Kinase (CK) Levels ARE reduced with b-Sarcoglycan GENE Therapy ClinicalTrials.gov Identifier: NCT03652259 CK Levels (U/L) Subject Age (years) Baseline Day 30 Day 60 Day 90 Day 180 Day 270 1 13 10,727 619 2257 1135 1553 2300 2 4 12,826 4795 910 2159 5070 2665 3 13 10,985 687 2061 2392 10,055 1295 9 Months: 82% Reduction in CK
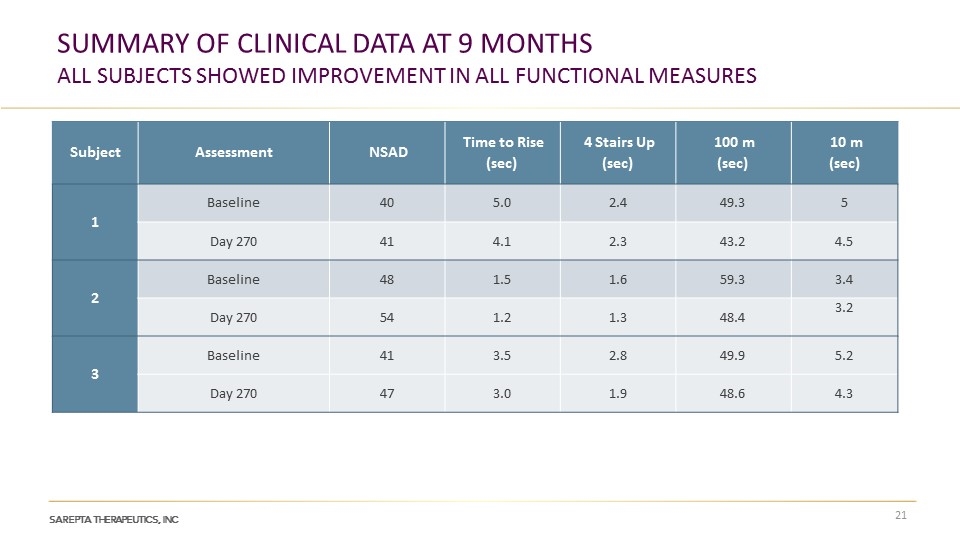
SUMMARY OF CLINICAL DATA AT 9 MONTHS ALL SUBJECTS SHOWED IMPROVEMENT IN ALL FUNCTIONAL MEASURES Subject Assessment NSAD Time to Rise (sec) 4 Stairs Up (sec) 100 m (sec) 10 m (sec) 1 Baseline 40 5.0 2.4 49.3 5 Day 270 41 4.1 2.3 43.2 4.5 2 Baseline 48 1.5 1.6 59.3 3.4 Day 270 54 1.2 1.3 48.4 3.2 3 Baseline 41 3.5 2.8 49.9 5.2 Day 270 47 3.0 1.9 48.6 4.3
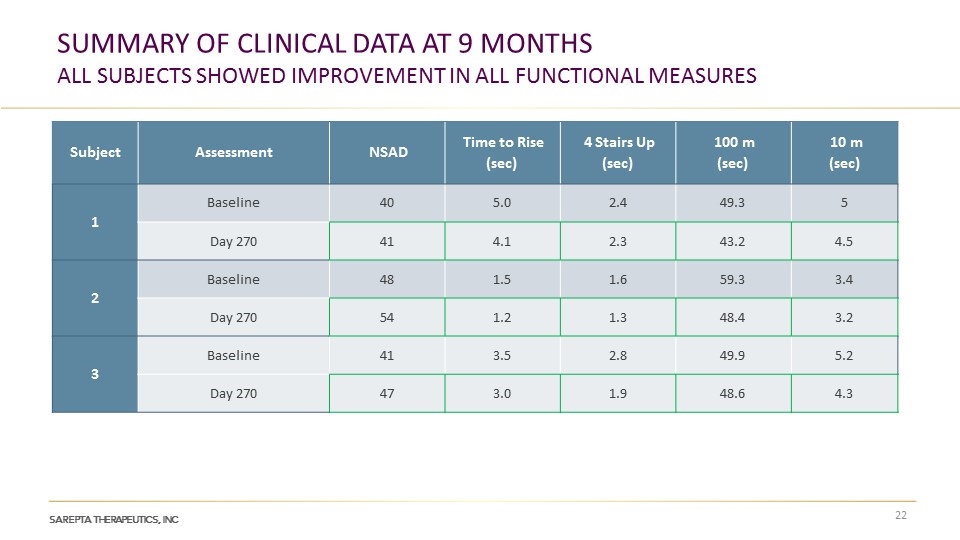
SUMMARY OF CLINICAL DATA AT 9 MONTHS ALL SUBJECTS SHOWED IMPROVEMENT IN ALL FUNCTIONAL MEASURES Subject Assessment NSAD Time to Rise (sec) 4 Stairs Up (sec) 100 m (sec) 10 m (sec) 1 Baseline 40 5.0 2.4 49.3 5 Day 270 41 4.1 2.3 43.2 4.5 2 Baseline 48 1.5 1.6 59.3 3.4 Day 270 54 1.2 1.3 48.4 3.2 3 Baseline 41 3.5 2.8 49.9 5.2 Day 270 47 3.0 1.9 48.6 4.3
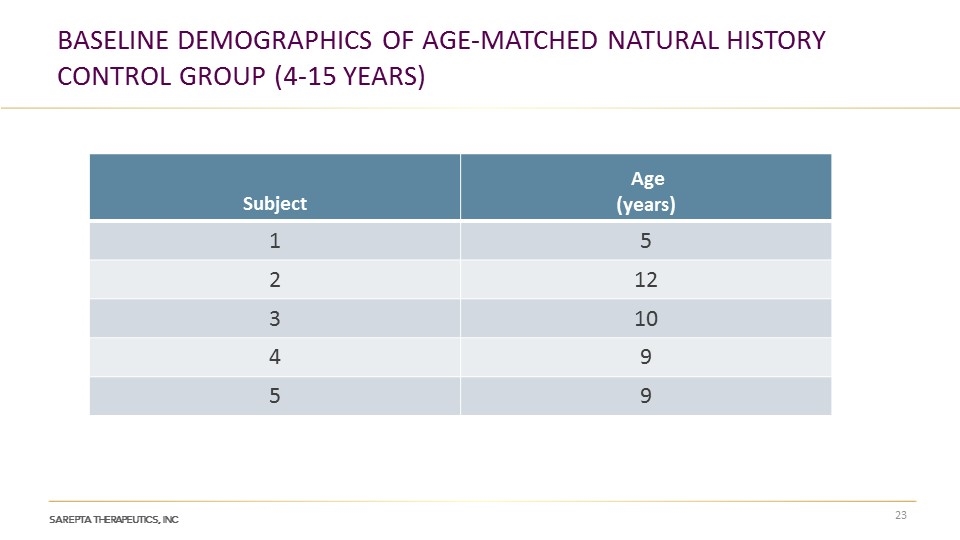
Baseline Demographics of age-matched natural history control group (4-15 years) Subject Age (years) 1 5 2 12 3 10 4 9 5 9
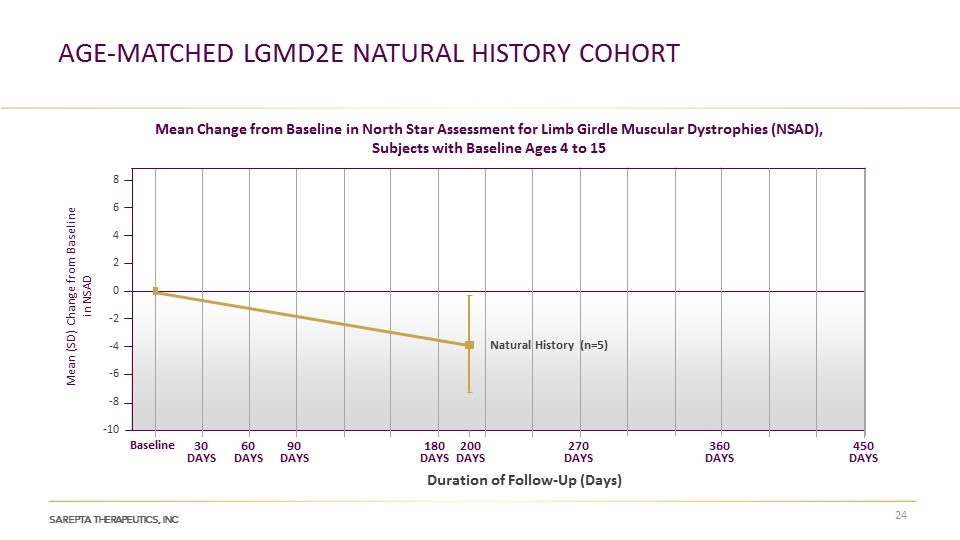
age-matched LGMD2E Natural history Cohort Mean Change from Baseline in North Star Assessment for Limb Girdle Muscular Dystrophies (NSAD), Subjects with Baseline Ages 4 to 15 Mean (SD) Change from Baseline in NSAD 8 6 4 2 0 -2 -4 -6 -8 -10 Natural History (n=5) Baseline 30 DAYS 60 DAYS 90 DAYS 180 DAYS 270 DAYS 200 DAYS 360 DAYS 450 DAYS Duration of Follow-Up (Days)
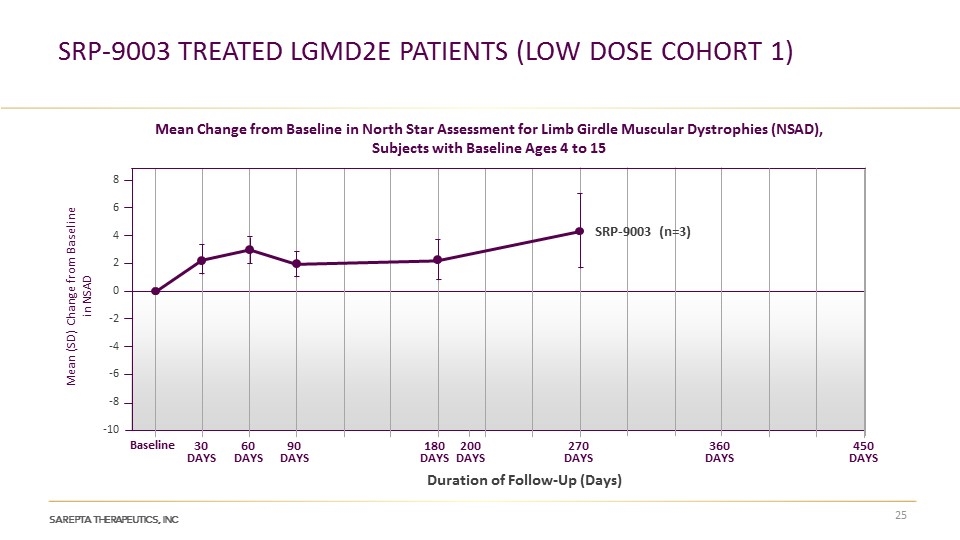
SRP-9003 treated LGMD2E patients (Low dose cohort 1) Mean Change from Baseline in North Star Assessment for Limb Girdle Muscular Dystrophies (NSAD), Subjects with Baseline Ages 4 to 15 Mean (SD) Change from Baseline in NSAD 8 6 4 2 0 -2 -4 -6 -8 -10 Baseline 30 DAYS 60 DAYS 90 DAYS 180 DAYS 270 DAYS 200 DAYS 360 DAYS 450 DAYS SRP-9003 (n=3) Duration of Follow-Up (Days)

Patient 1: 100m Running

Patient 2: Trunk control

PATIENT 3: Getting up from Sitting
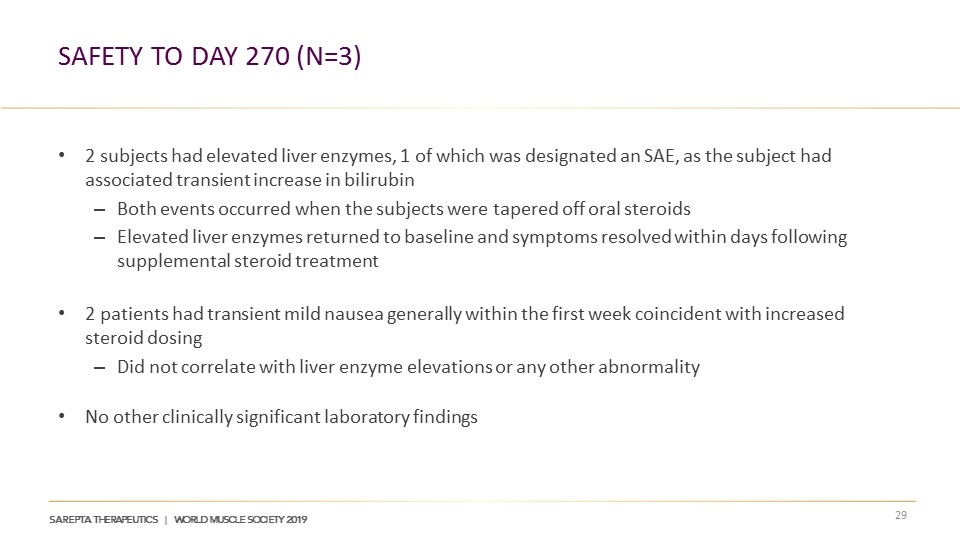
Safety TO Day 270 (n=3) 2 subjects had elevated liver enzymes, 1 of which was designated an SAE, as the subject had associated transient increase in bilirubin Both events occurred when the subjects were tapered off oral steroids Elevated liver enzymes returned to baseline and symptoms resolved within days following supplemental steroid treatment 2 patients had transient mild nausea generally within the first week coincident with increased steroid dosing Did not correlate with liver enzyme elevations or any other abnormality No other clinically significant laboratory findings
Summary Construct optimized for use in LGMD AAVrh74 efficiently transduces all muscle types Low pre-existing immunity for AAVrh74 MHCK7 promoter allows for cardiac and skeletal transgene muscle expression Preliminary clinical results Widespread beta-sarcoglycan expression across all patients at a systemic dose of 5x1013 vg/kg Substantial reduction in CK Consistent improvement in all functional measures in all patients Safety profile supports dose escalation

Future Clinical Development: Dose Escalation to Identify Registrational Trial Dose Next Steps: Dose Escalation: 4-fold increase Final dose for registration trial will be selected from 2 doses studied Engagement with global regulatory agencies to discuss pivotal trial designs AAVrh74.MHCK7.SGCB (SRP-9003) β-sarcoglycan gene therapy is investigational and has not been reviewed or approved by any regulatory authority. Sarepta Therapeutics 2019. Data on file.
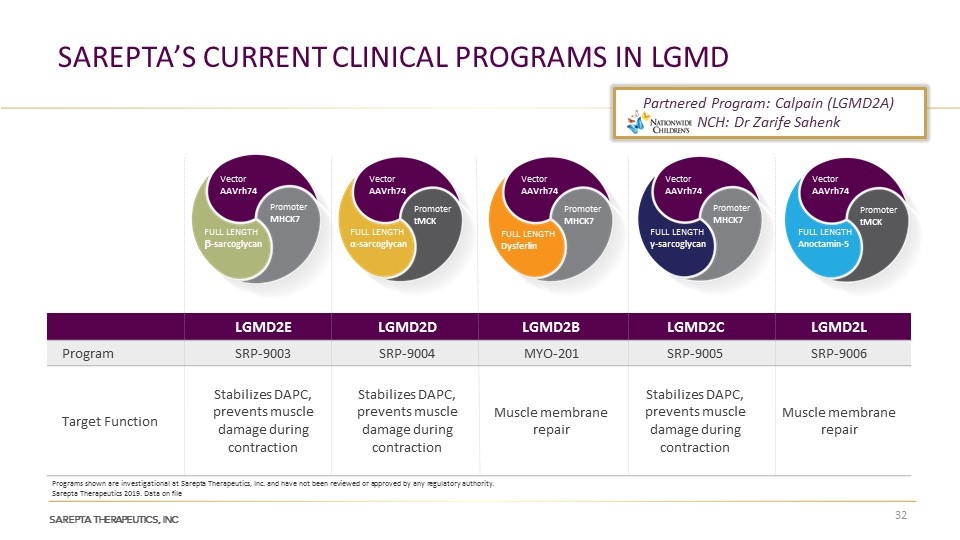
LGMD2E LGMD2D LGMD2B LGMD2C LGMD2L Program SRP-9003 SRP-9004 MYO-201 SRP-9005 SRP-9006 Target Function Stabilizes DAPC, prevents muscle damage during contraction Stabilizes DAPC, prevents muscle damage during contraction Muscle membrane repair Stabilizes DAPC, prevents muscle damage during contraction Muscle membrane repair Vector AAVrh74 Promoter MHCK7 FULL LENGTH b-sarcoglycan Vector AAVrh74 FULL LENGTH Dysferlin Promoter MHCK7 Vector AAVrh74 FULL LENGTH γ-sarcoglycan Promoter MHCK7 Vector AAVrh74 FULL LENGTH α-sarcoglycan Promoter tMCK Vector AAVrh74 FULL LENGTH Anoctamin-5 Promoter tMCK Partnered Program: Calpain (LGMD2A) NCH: Dr Zarife Sahenk Sarepta Therapeutics 2019. Data on file Programs shown are investigational at Sarepta Therapeutics, Inc. and have not been reviewed or approved by any regulatory authority. Sarepta’s Current Clinical Programs in LGMD

Questions & ANswers

Louise Rodino-Klapac Senior Vice President, Gene Therapy Sarepta Therapeutics, Inc. October 4, 2019 Clinical Update: srp-9003 Beta-Sarcoglycanopathy Gene Therapy program Limb-Girdle Muscular Dystrophy Type 2E FUNCTIONAL DATA

































