CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THE EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. SUCH EXCLUDED INFORMATION HAS BEEN MARKED WITH “[***]”.
Isis Pharmaceuticals, Inc.
Janssen Biotech Inc.
ARTICLE 1.
ARTICLE 2.
ARTICLE 3.
ARTICLE 4.
ARTICLE 5.
ARTICLE 6. FINANCIAL PROVISIONS
*[***].
ARTICLE 7.
ARTICLE 8.
ARTICLE 9.
except, in each case above, to the extent such Claim arose out of or resulted from or is attributable to any acts or omissions of Isis or its Affiliates, licensees, Sublicensees or contractors, and its or their respective directors, officers, employees and agents or other circumstance in each case for which Isis has an indemnity obligation pursuant to Section 9.2.
except, in each case above, to the extent such Claim arose out of or resulted from or is attributable to any acts or omissions of JBI or its Affiliates, licensees, Sublicensees or contractors and its or their respective directors, officers, employees and agents or other circumstance, in each case for which JBI has an indemnity obligation pursuant to Section 9.1.
ARTICLE 10.
The period from the Effective Date until the date of expiration of this Agreement pursuant to this Section 10.1 is the “Agreement Term.”
(a) JBI asserts invalidity as a defense in any court proceeding bought by Isis asserting infringement of a granted Patent within the Isis Core Technology Patents, Isis Manufacturing and Analytical Patents, or [***]; or
(b) JBI (i) acquires a Third Party that has an existing challenge, whether in a court or administrative proceeding, against a granted Patent within the Isis Core Technology Patents, Isis Manufacturing and Analytical Patents, or Isis Formulation Patents or (ii) licenses a product for which Isis has an existing challenge, whether in a court or administrative proceeding, against [***].
| 10.2.6 | Termination for Insolvency. Either Party may terminate this Agreement if, at any time, the other Party files in any court or agency pursuant to any statute or regulation of any state or country a petition in bankruptcy or insolvency or for reorganization or for an arrangement or for the appointment of a receiver or trustee of the Party or of substantially all of its assets; or if the other Party proposes a written agreement of composition or extension of substantially all of its debts; or if the other Party will be served with an involuntary petition against it, filed in any insolvency proceeding, and such petition will not be dismissed within 90 days after the filing thereof; or if the other Party will propose or be a party to any dissolution or liquidation; or if the other Party will make an assignment of substantially all of its assets for the benefit of creditors. |
| 10.3 | Consequences of Expiration or Termination of the Agreement. |
| 10.3.1 | In General. If this Agreement expires or is terminated by a Party in accordance with this ARTICLE 10 at any time and for any reason, the following terms will apply to any Drug Discovery Program that is the subject of such expiration or termination: |
| (a) | Return of Information and Materials. The Parties will return (or destroy, as directed by the other Party) all data, files, records and other materials containing or comprising the other Party’s Confidential Information, except to the extent such Confidential Information is necessary or useful to conduct activities under a surviving Drug Discovery Program. Notwithstanding the foregoing, the Parties will be permitted to retain one copy of such data, files, records, and other materials for archival and legal compliance purposes. |
| (b) | Accrued Rights. Termination or expiration of this Agreement for any reason will be without prejudice to any rights or financial compensation that will have accrued to the benefit of a Party prior to such termination or expiration. Such termination or expiration will not relieve a Party from obligations that are expressly indicated to survive the termination or expiration of this Agreement. For purposes of clarification, milestone payments under ARTICLE 6 accrue as of the date the applicable Milestone Event is achieved even if the payment is not due at that time. |
| (c) | Survival. The following provisions of this Agreement will survive the expiration or termination of this Agreement: Section 4.1.2(c) (Effect of Termination on Sublicenses), Section 4.2.2, Section 6.11.3 (Records Retention), Section 6.12 (Audits), Section 7.1.1 (Isis Technology and JBI Technology), Section 7.1.2 (Agreement Technology), Section 8.4 (Disclaimer), ARTICLE 9 (Indemnification; Insurance), Section 10.2.5 (Termination for Insolvency), Section 10.3 (Consequences of Expiration or Termination of the Agreement), ARTICLE 11 (Confidentiality), ARTICLE 12 (Miscellaneous) and Appendix 1 (Definitions) (to the extent definitions are embodied in the foregoing listed Articles and Sections). |
| 10.3.2 | Perpetual, Royalty-Free Non-Exclusive License. If JBI has exercised its Option for a particular Drug Discovery Program, then upon expiration of the Royalty Period in all countries in which the applicable Products are being or have been sold, Isis will and hereby does grant to JBI a perpetual, nonexclusive, worldwide, royalty-free, fully paid-up, sublicensable license under the Isis Know-How to Manufacture, Develop and Commercialize any Product under such Drug Discovery Program. |
| 10.3.3 | Termination Before Option Exercise. If this Agreement expires or is terminated by a Party in accordance with this ARTICLE 10 before Option exercise, then, in addition to the terms set forth in Section 10.3.1, the following terms will apply to each Drug Discovery Program that is the subject of such expiration or termination: |
| (a) | JBI’s Option under Section 3.1 will expire and Isis will be free to Develop and Commercialize Compounds included in such Drug Discovery Program on its own or with a Third Party. |
| (b) | Neither Party will have any further obligations under Section 2.1 of this Agreement with respect to the terminated Drug Discovery Program(s). |
| (c) | To the extent requested by Isis, JBI will promptly transfer to Isis all data, results and information (including JBI’s Confidential Information and any regulatory documentation (including drafts)) related to the terminated Drug Discovery Program(s) in the possession of JBI and its contractors to the extent such data, results and information were generated by or on behalf of JBI under this Agreement. |
| (d) | Except as explicitly set forth in Section 10.3.1(a), Section 10.3.1(b) or Section 10.3.1(c), JBI will have no further rights and Isis will have no further obligations with respect to each terminated Drug Discovery Program. |
| 10.3.4 | Termination After Option Exercise. If this Agreement is terminated by a Party in accordance with this ARTICLE 10 after Option exercise, then, in addition to the terms set forth in Section 10.3.1, the following terms will apply to any Pre-Clinical Development Program that is the subject of such termination: |
| (a) | The applicable licenses granted by Isis to JBI under this Agreement will terminate and JBI, its Affiliates and Sublicensees will cease selling the applicable Products. |
| (b) | Neither Party will have any further obligations under Section 2.1 of this Agreement with respect to the terminated Pre-Clinical Development Program(s). |
| (c) | Except as explicitly set forth in Section 10.3.1(a), JBI will have no further rights and Isis will have no further obligations with respect to the terminated Pre-Clinical Development Program. |
| (d) | If (y) JBI terminates the Agreement under Section 10.2.1 (JBI’s Termination for Convenience) or (z) Isis terminates this Agreement under Section 10.2.2(b) (Isis’ Right to Terminate) or Section 10.2.3 (Remedies for Failure to Use Commercially Reasonable Efforts), then the following additional terms will also apply solely with respect to the terminated Pre-Clinical Development Program(s): |
| (i) | JBI will grant to Isis a sublicensable, worldwide, royalty bearing exclusive license or sublicense, as the case may be, to all JBI Technology Controlled by JBI as of the date of such reversion that Covers the applicable Discontinued Product(s) solely as necessary to Develop, make, have made, use, sell, offer for sale, have sold, import and otherwise Commercialize the applicable Discontinued Product(s) in the Field (such license will be sublicensable by Isis in accordance with Section 4.1.2, mutatis mutandis); |
| (ii) | For each Discontinued Product for which JBI, its Affiliate or Sublicensee has [***], Isis or any sublicensee or collaborator shall pay to JBI a royalty on net sales made by Isis or its Affiliates or sublicensee of such Discontinued Product according to the following: (a) if neither [***] prior to termination: [***]% of Net Sales, (b) if JBI, its Affiliate or Sublicensee [***] for such Discontinued Product prior to termination: [***]% of Net Sales, (c) if JBI, its Affiliate or Sublicensee [***] for such Discontinued Product prior to termination: [***]% of Net Sales, and (d) if JBI, its Affiliate or Sublicensee [***] for such Discontinued Product prior to termination: [***]% of Net Sales; provided (A) if (i) Isis enters an arms-length license agreement with a Third Party with respect to a Discontinued Product and (ii) the definition of Net Sales is different in such license agreement than as described above, then, the Parties will use the definition described in the Third Party license for the calculation of royalties under this Section 10.3.4(d)(ii); and (B) Sections 6.8.2, 6.10, 6.12 and 6.14 will govern the payment of royalties from Isis to JBI under this Section 10.3.4(d)(ii), mutatis mutandis. |
| (iii) | JBI will assign to Isis any Product-Specific Patent Rights and Isis’ interest in any Jointly-Owned Program Patents that, in each case relate to the applicable Discontinued Product(s) previously assigned by Isis to JBI under this Agreement; |
| (iv) | JBI will transfer to Isis for use with respect to the Development and Commercialization of the applicable Discontinued Product(s), any Know-How data, results, regulatory information, filings, and files in the possession of JBI as of the date of such reversion to the extent related to such Discontinued Product(s), and any other information or material specified in Section 4.4; |
| (v) | JBI will license to Isis any trademarks that are specific to a Discontinued Product(s) solely for use with such Discontinued Product(s), in accordance with Section 4.1.5, mutatis mutandis; provided, however, that in no event will JBI have any obligation to license to Isis any trademarks used by JBI both in connection with the Product and in connection with the sale of any other product or service, including any JBI- or JBI-formative marks; and |
| (vi) | Isis will control and be responsible for all aspects of the Prosecution and Maintenance of all Jointly-Owned Program Patents arising from the terminated Pre-Clinical Development Program (or the corresponding Drug Discovery Program), and JBI will provide Isis with (and will instruct its counsel to provide Isis with) all of the information and records in JBI’s and its counsel’s possession related to the Prosecution and Maintenance of such Jointly-Owned Program Patents; provided, however, if Isis intends to abandon any such Jointly-Owned Program Patents without first filing a continuation or substitution, then Isis will notify JBI of such intention at least 60 days before such Patent Right will become abandoned, and JBI will have the right, but not the obligation, to assume responsibility for the Prosecution and Maintenance thereof at its own expense with counsel of its own choice. |
| (e) | If Isis terminates this Agreement due to JBI’s material breach or JBI terminates this Agreement for convenience, upon Isis’ written request pursuant to a mutually agreed supply agreement, JBI will sell to Isis any bulk API, Clinical Supplies and Finished Drug Product in JBI’s possession at the time of such termination, at a price equal to JBI’s cost at the time of manufacture. |
| (f) | To the extent requested by Isis, JBI will promptly assign to Isis any manufacturing agreements identified by Isis solely to the extent related to the applicable Discontinued Products to which JBI is a party. |
CONFIDENTIALITY
| 11.1 | Confidentiality; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, the Parties agree that, during the Agreement Term and for five years thereafter, the receiving Party (the “Receiving Party”) and its Affiliates will keep confidential and will not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement any confidential or proprietary information or materials, patentable or otherwise, in any form (written, oral, photographic, electronic, magnetic, or otherwise) which is disclosed to it by the other Party (the “Disclosing Party”) or its Affiliates or otherwise received or accessed by a Receiving Party in the course of performing its obligations or exercising its rights under this Agreement, including trade secrets, Know-How, inventions or discoveries, proprietary information, formulae, processes, techniques and information relating to the past, present and future marketing, financial, and research and development activities of any product or potential product or useful technology of the Disclosing Party or its Affiliates and the pricing thereof (collectively, “Confidential Information”). |
| 11.2 | Prior Confidentiality Agreement Superseded. As of the Effective Date, this Agreement supersedes the Confidential Disclosure Agreement executed by Isis and JBI on July 30, 2014 (including any and all amendments thereto). All information exchanged between the Parties under such Confidential Disclosure Agreement will be deemed Confidential Information hereunder and will be subject to the terms of this ARTICLE 11. |
| 11.3 | Authorized Disclosure. Except as expressly provided otherwise in this Agreement, a Receiving Party or its Affiliates may use and disclose to Third Parties Confidential Information of the Disclosing Party as follows: (i) solely in connection with the performance of its obligations or exercise of rights granted or reserved in this Agreement under confidentiality provisions no less restrictive than those in this Agreement, provided, that Confidential Information may be disclosed by a Receiving Party to a governmental entity or agency without requiring such entity or agency to enter into a confidentiality agreement; (ii) to the extent reasonably necessary to file or prosecute patent, copyright and trademark applications (subject to Section 11.4 below), complying with applicable governmental regulations, obtaining Approvals, conducting Pre-Clinical Studies or Clinical Studies, marketing the Product, or as otherwise required by applicable law, regulation, rule or legal process (including the rules of the SEC and any stock exchange); provided, however, that if a Receiving Party or any of its Affiliates is required by law or regulation to make any such disclosure of a Disclosing Party’s Confidential Information it will, except where impracticable for necessary disclosures, give reasonable advance notice to the Disclosing Party of such disclosure requirement and will use its reasonable efforts to secure confidential treatment of such Confidential Information required to be disclosed; (iii) in communication with actual or potential lenders, investors, merger partners, acquirers, consultants, or professional advisors on a need-to-know basis, in each case under confidentiality provisions no less restrictive than those of this Agreement; (iv) to the extent such disclosure is required to comply with existing expressly stated contractual obligations owed to such Party’s or its Affiliates’ licensor with respect to any intellectual property licensed to the other Party under this Agreement; or (v) as mutually agreed to in writing by the Parties. |
| 11.4 | Press Release; Publications; Disclosure of Agreement. |
| 11.4.1 | Announcement of Transaction. On or promptly after the Effective Date, the Parties will issue a public announcement of the execution of this Agreement in form and substance mutually agreed by the Parties and included in Schedule 11.4. |
| 11.4.2 | Other Disclosures. Except to the extent required to comply with applicable law, regulation, rule or legal process or as otherwise permitted in accordance with this Section 11.4, neither Party nor such Party’s Affiliates will make any public announcements, press releases or other public disclosures concerning a Drug Discovery Program, a Product, this Agreement or the terms or the subject matter hereof without the prior written consent of the other, which will not be unreasonably withheld, conditioned or delayed. |
| 11.4.3 | Use of Name. Except as set forth in Section 11.4.8, neither Party will use the other Party’s name in a press release or other publication without first obtaining the prior consent of the Party to be named. |
| 11.4.4 | Notice of Significant Events. Each party will immediately notify (and provide as much advance notice as possible, but at a minimum two Business Days advance notice to) the other Party of any event materially related to a Product (including in such notice any disclosure of starting/stopping of a Clinical Study, clinical data or results, material regulatory discussions, filings, Approval or JBI’s sales projections) so the Parties may analyze the need for or desirability of publicly disclosing or reporting such event. |
| 11.4.5 | JBI Disclosures After Option Exercise. After Option if JBI intends to make a press release or similar public communication disclosing regulatory discussions, the efficacy or safety data or results related to such Product or JBI’s sales projections, (i) JBI will submit such proposed communication to Isis for review at least two Business Days in advance of such proposed public disclosure, (ii) Isis will have the right to review and recommend changes to such communication, and (iii) JBI will in good faith consider any changes that are timely recommended by Isis. |
| 11.4.6 | Scientific or Clinical Presentations. The Parties agree to use Commercially Reasonable Efforts to control public scientific disclosures of results of the Development activities under this Agreement to prevent any potential adverse effect of any premature public disclosure of such results. The Parties will establish a procedure for publication review and each Party will first submit to the other Party through the Joint Patent Committee an early draft of all such publications or presentations, whether they are to be presented orally or in written form, at least 45 days prior to submission for publication including to facilitate the publication of any summaries of Clinical Studies data and results as required on the clinical trial registry of each respective Party. Each Party will review such proposed publication in order to avoid the unauthorized disclosure of a Party’s Confidential Information and to preserve the patentability of inventions arising from the Drug Discovery Programs. If, during such 45-day period, the other Party informs such Party that its proposed publication contains Confidential Information of the other Party, then such Party will delete such Confidential Information from its proposed publication. In addition, if at any time during such 45-day period, the other Party informs such Party that its proposed publication discloses inventions made by either Party in the course of the Development under this Agreement that have not yet been protected through the filing of a patent application, or the public disclosure of such proposed publication could be expected to have a material adverse effect on any Patent Rights or Know-How solely owned or Controlled by such other Party, then such Party will either (i) delay such proposed publication for up to 60 days from the date the other Party informed such Party of its objection to the proposed publication, to permit the timely preparation and first filing of patent application(s) on the information involved or (ii) remove the identified disclosures prior to publication. |
| 11.4.7 | Subsequent Disclosure. Notwithstanding the foregoing, to the extent information regarding this Agreement or the Product has already been publicly disclosed, either Party (or its Affiliates) may subsequently disclose the same information to the public without the consent of the other Party. |
| 11.4.8 | Acknowledgment. JBI will acknowledge in any press release, public presentation or publication regarding the collaboration or a Product, Isis’ role in discovering and developing the Product, that the Product is under license from Isis and otherwise acknowledge Isis’ contributions, and Isis’ stock ticker symbol (Nasdaq: ISIS). Isis may include the Product (and identify JBI as its partner for the Product) in Isis’ drug pipeline. |
MISCELLANEOUS
| 12.1.1 | General. The Parties recognize that a dispute may arise relating to this Agreement (“Dispute”). Except as set forth in Section 12.1.5 any Dispute, including Disputes that may involve the parent company, subsidiaries, or affiliates under common control of any Party, shall be resolved in accordance with this Section 12. |
| 12.1.2 | Continuance of Rights and Obligations During Pendency of Dispute Resolution. If there are any Disputes in connection with this Agreement, including Disputes related to termination of this Agreement under Section 10, all rights and obligations of the Parties shall continue until such time as any Dispute has been resolved in accordance with the provisions of this Section 12. |
| 12.1.3 | Escalation. Subject to Section 12.1.5, any claim, Dispute, or controversy as to the breach, enforcement, interpretation or validity of this Agreement will be referred to the Global Therapeutic Area Head, Immunology of JBI and the Chief Operating Officer of Isis (the “Executives”) for attempted resolution. In the event the Executives are unable to resolve such Dispute within 30 days of such Dispute being referred to them, then, upon the written request of either Party to the other Party, the Dispute shall be subject to arbitration in accordance with Section 12.1.4, except as expressly set forth in Section 12.1.5 or Section 12.3. |
| (a) | If the Parties fail to resolve the Dispute through Escalation, and a Party desires to pursue resolution of the Dispute, the Dispute shall be submitted by either Party for resolution in arbitration pursuant to the then current CPR Non-Administered Arbitration Rules (“CPR Rules”) (www.cpradr.org), except where they conflict with these provisions, in which case these provisions control. The arbitration will be held in Chicago, Illinois. All aspects of the arbitration shall be treated as confidential. |
| (b) | The arbitrators will be chosen from the CPR Panel of Distinguished Neutrals, unless a candidate not on such panel is approved by both Parties. Each arbitrator shall be a lawyer with at least 15 years of experience with a law firm or corporate law department of over 25 lawyers or who was a judge of a court of general jurisdiction. To the extent that the Dispute requires special expertise, the Parties will so inform CPR prior to the beginning of the selection process. |
| (c) | The arbitration tribunal shall consist of three arbitrators, of whom each Party shall designate one in accordance with the “screened” appointment procedure provided in CPR Rule 5.4. The chair will be chosen in accordance with CPR Rule 6.4. |
| (d) | If, however, the aggregate award sought by the Parties is less than $5 million and equitable relief is not sought, a single arbitrator shall be chosen in accordance with the CPR Rules. |
| (e) | Candidates for the arbitrator position(s) may be interviewed by representatives of the Parties in advance of their selection, provided that all Parties are represented. |
| (f) | The Parties agree to select the arbitrator(s) within 45 days of initiation of the arbitration. The hearing will be concluded within nine (9) months after selection of the arbitrator(s) and the award will be rendered within 60 days of the conclusion of the hearing, or of any post hearing briefing, which briefing will be completed by both sides within 45 days after the conclusion of the hearing. In the event the Parties cannot agree upon a schedule, then the arbitrator(s) shall set the schedule following the time limits set forth above as closely as practical. |
| (g) | The hearing will be concluded in ten hearing days or less. Multiple hearing days will be scheduled consecutively to the greatest extent possible. A transcript of the testimony adduced at the hearing shall be made and shall be made available to each Party. |
| (h) | The arbitrator(s) shall be guided, but not bound, by the CPR Protocol on Disclosure of Documents and Presentation of Witnesses in Commercial Arbitration (www.cpradr.org) (“Protocol”). The Parties will attempt to agree on modes of document disclosure, electronic discovery, witness presentation, etc. within the parameters of the Protocol. If the Parties cannot agree on discovery and presentation issues, the arbitrator(s) shall decide on presentation modes and provide for discovery within the Protocol, understanding that the Parties contemplate reasonable discovery. |
| (i) | The arbitrator(s) shall decide the merits of any Dispute in accordance with the law governing this Agreement, without application of any principle of conflict of laws that would result in reference to a different law. The arbitrator(s) may not apply principles such as “amiable compositeur” or “natural justice and equity.” |
| (j) | The arbitrator(s) are expressly empowered to decide dispositive motions in advance of any hearing and shall endeavor to decide such motions as would a United States District Court Judge sitting in the jurisdiction whose substantive law governs. |
| (k) | The arbitrator(s) shall render a written opinion stating the reasons upon which the award is based. The Parties consent to the jurisdiction of the United States District Court for the district in which the arbitration is held for the enforcement of these provisions and the entry of judgment on any award rendered hereunder. Should such court for any reason lack jurisdiction, any court with jurisdiction may act in the same fashion. |
| (l) | Each Party has the right to seek from the appropriate court provisional remedies such as attachment, preliminary injunction, replevin, etc. to avoid irreparable harm, maintain the status quo, or preserve the subject matter of the Dispute. Rule 14 of the CPR Rules does not apply to this Agreement. |
| (m) | EXCEPT IN THE CASE OF COURT ACTIONS PERMITTED BY SECTION 12.1.5 AND FOR CLAIMS NOT SUBJECT TO ARBITRATION PURSUANT TO SECTION 12.1.4 AS SET FORTH IN SECTION 12.1.5, EACH PARTY HERETO WAIVES: (1) ITS RIGHT TO TRIAL OF ANY ISSUE BY JURY, (2) WITH THE EXCEPTION OF RELIEF MANDATED BY STATUTE, ANY CLAIM TO PUNITIVE, EXEMPLARY, MULTIPLIED, INDIRECT, CONSEQUENTIAL OR LOST PROFITS/REVENUES DAMAGES, AND (3) ANY CLAIM FOR ATTORNEY FEES, COSTS AND PREJUDGMENT INTEREST. |
| (n) | Each Party will bear its own attorney’s fees, costs, and disbursements arising out of the arbitration, and will pay an equal share of the fees and costs of the arbitrator; provided, however, the arbitrator will be authorized to determine whether a Party is the prevailing party, and if so, to award to that prevailing party reimbursement for any or all of its reasonable attorneys’ fees, costs and disbursements (including, for example, expert witness fees and expenses, photocopy charges, travel expenses, etc.), and/or the fees and costs of the Administrator and the arbitrator. |
| 12.1.5 | Injunctive Relief; Court Actions. Notwithstanding anything to the contrary in this Agreement, each Party will be entitled to seek from any court of competent jurisdiction, in addition to any other remedy it may have at law or in equity, injunctive or other equitable relief in the event of an actual or threatened breach of this Agreement by the other Party, without the posting of any bond or other security, and such an action may be filed and maintained notwithstanding any ongoing discussions between the Parties or any ongoing arbitration proceeding. The Parties agree that in the event of a threatened or actual material breach of this Agreement injunctive or equitable relief would be appropriate remedy. In addition, either Party may bring an action in any court of competent jurisdiction to resolve disputes pertaining to the validity, construction, scope, enforceability, infringement or other violations of Patent Rights or other intellectual property rights, and no such claim will be subject to arbitration pursuant to Section 12.1.4. |
| 12.2 | Governing Law; Jurisdiction; Venue; Service of Process. This Agreement and any Dispute will be governed by and construed and enforced in accordance with the laws of the State of New York, U.S.A., without reference to conflicts of laws principles. |
| 12.3 | Recovery of Losses. Neither Party will be entitled to recover any Losses relating to any matter arising under one provision of this Agreement to the extent that such Party has already recovered Losses with respect to such matter pursuant to other provisions of this Agreement (including recoveries under Section 9.1 or Section 9.2, and the offsets under Section 6.9.3(c)). Except for the offsets and credits explicitly set forth in Section 6.12, and Section 6.9.3(b) neither Party will have the right to set off any amount it is owed or believes it is owed against payments due or payable to the other Party under this Agreement. |
| 12.4 | Assignment and Successors. Neither this Agreement nor any obligation of a Party hereunder may be assigned by either Party without the prior written consent of the other, which will not be unreasonably withheld, delayed or conditioned, except that (i) Isis may assign or transfer its rights to receive payments under this Agreement (but no liabilities), without JBI’s consent, to an Affiliate or to a Third Party in connection with a payment factoring transaction, and (ii) each Party may assign this Agreement and the rights, obligations and interests of such Party hereunder, without the other Party’s consent to any Third Party purchaser of all or substantially all of its assets or all or substantially all of its assets to which this Agreement relates or to any successor corporation resulting from any merger, consolidation, share exchange or other similar transaction with a Third Party, provided that in the event of any such transaction (whether this Agreement is actually assigned or is assumed by the acquiring Third Party or the successor corporation (as applicable) by operation of law (e.g., in the context of a reverse triangular merger)), intellectual property rights of the acquiring Third Party that existed prior to such transaction shall not be included in the technology licensed hereunder or otherwise subject to this Agreement; provided that if JBI transfers or assigns this Agreement to [***] described in this Agreement, then JBI (or such Affiliate), will [***] due Isis under ARTICLE 6 for the [***] (defined below) such that Isis receives [***]. |
The [***].
To the extent Isis utilizes [***] in any year, Isis will [***] to JBI [***]. To assist JBI in determining when [***] pursuant to the foregoing sentence, beginning with the first Annual tax return for the year in which JBI [***] payment under this Section 12.4, and each year thereafter (including, for clarity, all years in which Isis [***], Isis will provide JBI with Isis’ Annual tax returns (federal and state) and, in years in which Isis utilizes [***], supporting documentation for such [***]. Notwithstanding the foregoing, if the [***].
12.4.1 Termination of Reporting Obligations Upon Isis Change of Control. If there is a change in control of Isis, JBI, at its discretion, may terminate all reporting obligations regarding the Development and/or Commercialization of any Products including reporting under the Information Sharing Committee, except in all cases JBI will continue to provide the reports, audit rights and other information required under Sections 6.10, 6.11, 6.12 and 6.13.
| 12.5 | Force Majeure. No Party will be held responsible to the other Party nor be deemed to be in default under, or in breach of any provision of, this Agreement for failure or delay in performing any obligation of this Agreement when such failure or delay is due to force majeure, and without the fault or negligence of the Party so failing or delaying. For purposes of this Agreement, force majeure means a cause beyond the reasonable control of a Party, which may include acts of God; acts, regulations, or laws of any government; war; terrorism; civil commotion; fire, flood, earthquake, tornado, tsunami, explosion or storm; pandemic; epidemic and failure of public utilities or common carriers. In such event the Party so failing or delaying will immediately notify the other Party of such inability and of the period for which such inability is expected to continue. The Party giving such notice will be excused from such of its obligations under this Agreement as it is thereby disabled from performing for so long as it is so disabled for up to a maximum of 90 days, after which time the Parties will negotiate in good faith any modifications of the terms of this Agreement that may be necessary to arrive at an equitable solution, unless the Party giving such notice has set out a reasonable timeframe and plan to resolve the effects of such force majeure and executes such plan within such timeframe. To the extent possible, each Party will use reasonable efforts to minimize the duration of any force majeure. |
| 12.6 | Notices. Any notice or request required or permitted to be given under or in connection with this Agreement will be deemed to have been sufficiently given if in writing and personally delivered or sent by certified mail (return receipt requested), facsimile transmission (receipt verified), or overnight express courier service (signature required), prepaid, to the Party for which such notice is intended, at the address set forth for such Party below: |
| If to Isis, addressed to: | Isis Pharmaceuticals, Inc. |
2855 Gazelle Court
Carlsbad, CA 92010
Attention: Chief Operating Officer
Fax: 760-918-3592
| with a copy to: | Isis Pharmaceuticals, Inc. |
2855 Gazelle Court
Carlsbad, CA 92010
Attention: General Counsel
Fax: 760-268-4922
| If to JBI, addressed to: | Janssen Research & Development, LLC |
Murray McKinnon, PhD1400 McKean Road
Spring House, PA 19477
Mmckinno2@its.jnj.com
with a copy to:
Chief Patent Counsel
Johnson & Johnson
One Johnson & Johnson Plaza
New Brunswick, NJ 08933
Attn: Brian Carey
or to such other address for such Party as it will have specified by like notice to the other Party; provided that notices of a change of address will be effective only upon receipt thereof. If delivered personally or by facsimile transmission, the date of delivery will be deemed to be the date on which such notice or request was given. If sent by overnight express courier service, the date of delivery will be deemed to be the next Business Day after such notice or request was deposited with such service. If sent by certified mail, the date of delivery will be deemed to be the third Business Day after such notice or request was deposited with the U.S. Postal Service.
| 12.7 | ISIS Reporting of This Agreement. Isis shall provide JBI with at least [***] ([***]) days written notice of any disclosure of this document to a Third Party or to a governmental authority. The Parties agree to promptly convene to discuss such disclosure and discuss, inter alia, the subject matter that may be redacted prior to such submission. Notwithstanding the foregoing, Isis may (i) disclose this Agreement to Isis’ legal counsel, auditors, and other professional advisors on a need-to-know basis, in each case where such advisors have agreed to confidentiality provisions no less restrictive than those of this Agreement, and (ii) may disclose the publicly available redacted version of this Agreement once such redacted version has been filed publicly with the SEC. |
| 12.8 | Export Clause. Each Party acknowledges that the laws and regulations of the United States restrict the export and re-export of commodities and technical data of United States origin. Each Party agrees that it will not export or re-export restricted commodities or the technical data of the other Party in any form without the appropriate United States and foreign government licenses. |
| 12.9 | Waiver. Neither Party may waive or release any of its rights or interests in this Agreement except in writing. The failure of either Party to assert a right hereunder or to insist upon compliance with any term or condition of this Agreement will not constitute a waiver of that right or excuse a similar subsequent failure to perform any such term or condition. No waiver by either Party of any condition or term in any one or more instances will be construed as a continuing waiver or subsequent waiver of such condition or term or of another condition or term. |
| 12.10 | Severability. If any provision hereof should be held invalid, illegal or unenforceable in any jurisdiction, the Parties will negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and all other provisions hereof will remain in full force and effect in such jurisdiction and will be liberally construed in order to carry out the intentions of the Parties hereto as nearly as may be possible. Such invalidity, illegality or unenforceability will not affect the validity, legality or enforceability of such provision in any other jurisdiction. |
| 12.11 | Entire Agreement. This Agreement, together with the Schedules and Appendices hereto, sets forth all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties and supersedes and terminates all prior agreements and understanding between the Parties. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties other than as set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement will be binding upon the Parties hereto unless reduced to writing and signed by the respective authorized officers of the Parties. |
| 12.12 | Independent Contractors. Nothing herein will be construed to create any relationship of employer and employee, agent and principal, partnership or joint venture between the Parties. Each Party is an independent contractor. Neither Party will assume, either directly or indirectly, any liability of or for the other Party. Neither Party will have the authority to bind or obligate the other Party, and neither Party will represent that it has such authority. |
| 12.13 | Interpretation. Except as otherwise explicitly specified to the contrary, (a) references to a section, exhibit or schedule means a section of, or schedule or exhibit to this Agreement, unless another agreement is specified, (b) the word “including” (in its various forms) means “including without limitation,” (c) the words “shall” and “will” have the same meaning, (d) references to a particular statute or regulation include all rules and regulations thereunder and any predecessor or successor statute, rules or regulation, in each case as amended or otherwise modified from time to time, (e) words in the singular or plural form include the plural and singular form, respectively, (f) references to a particular Person include such Person’s successors and assigns to the extent not prohibited by this Agreement, (g) unless otherwise specified, “$” is in reference to United States dollars, and (h) the headings contained in this Agreement, in any exhibit or schedule to this Agreement and in the table of contents to this Agreement are for convenience only and will not in any way affect the construction of or be taken into consideration in interpreting this Agreement. |
| 12.14 | Books and Records. Any books and records to be maintained under this Agreement by a Party or its Affiliates or Sublicensees will be maintained in accordance with U.S. Generally Accepted Accounting Principles (or any successor standard), consistently applied. |
| 12.15 | Further Actions. Each Party will execute, acknowledge and deliver such further instruments, and do all such other acts, as may be necessary or appropriate in order to carry out the expressly stated purposes and the clear intent of this Agreement. |
| 12.16 | Construction of Agreement. The terms and provisions of this Agreement represent the results of negotiations between the Parties and their representatives, each of which has been represented by counsel of its own choosing, and neither of which has acted under duress or compulsion, whether legal, economic or otherwise. Accordingly, the terms and provisions of this Agreement will be interpreted and construed in accordance with their usual and customary meanings, and each of the Parties hereto hereby waives the application in connection with the interpretation and construction of this Agreement of any rule of law to the effect that ambiguous or conflicting terms or provisions contained in this Agreement will be interpreted or construed against the Party whose attorney prepared the executed draft or any earlier draft of this Agreement. |
| 12.17 | Supremacy. In the event of any express conflict or inconsistency between this Agreement and any Schedule or Appendix hereto, the terms of this Agreement will apply. The Parties understand and agree that the Schedules identifying the Licensed Technology are not intended to be the final and complete embodiment of any terms or provisions of this Agreement, and are to be updated from time to time during the Agreement Term, as appropriate and in accordance with the provisions of this Agreement. |
| 12.18 | Counterparts. This Agreement may be signed in counterparts, each of which will be deemed an original, notwithstanding variations in format or file designation which may result from the electronic transmission, storage and printing of copies of this Agreement from separate computers or printers. Facsimile signatures and signatures transmitted via electronic mail in PDF format will be treated as original signatures. |
| 12.19 | Compliance with Laws. Each Party will, and will ensure that its Affiliates and Sublicensees will, comply with all relevant laws and regulations in exercising its rights and fulfilling its obligations under this Agreement. |
[SIGNATURE PAGE FOLLOWS]
* - * - * - *
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their representatives thereunto duly authorized as of the Effective Date.
| ISIS PHARMACEUTICALS, INC. | |
| |
| By: | /s/ B. Lynne Parshall | |
Name:
| B. Lynne Parshall | |
Title:
| Chief Operating Officer | |
Signature Page to Research Collaboration, Option and License Agreement
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their representatives thereunto duly authorized as of the Effective Date.
| JANSSEN BIOTECH INC. | |
| |
| By: | /s/ John Wilson | |
| | | |
Name:
| John Wilson | |
| | | |
Title:
| Vice President, Janssen Biotech Inc | |
Signature Page to Research Collaboration, Option and License Agreement
List of Appendices and Schedules
Appendix 1 – Definitions |
| |
Appendix 2 – Development Candidate Checklist |
| |
Appendix 3 – J&J Universal Calendar |
| |
Schedule 1.6.1 – JRC Governance |
| |
Schedule 1.6.5 – Alliance Management Activities |
| |
Schedule 4.4.4 �� Isis’ Fully Absorbed Cost of Goods Methodology |
| |
Schedule 5.2 – Specific Performance Milestone Events |
| |
Schedule 6.9.1 – Certain Isis In-License Agreements |
| |
Schedule 8.2.2(a) – Isis Core Technology Patents |
| |
Schedule 8.2.2(b) – Isis Manufacturing and Analytical Patents |
| |
Schedule 8.2.2(c) – Isis Product-Specific Patents |
| |
Schedule 8.2.2(d) – Isis Formulation Patents |
| |
Schedule 11.4 – Press Release |
| |
Schedule 8.2.2(e) – Prior Agreements |
DEFINITIONS
For purposes of this Agreement, the following capitalized terms will have the following meanings:
“Acceptance” means, with respect to an NDA, MAA or JNDA filed for a Product, (a) in the United States, the receipt of written notice from the FDA in accordance with 21 C.F.R. §314.101(a)(2) that such NDA is officially “filed,” (b) in the European Union, receipt by JBI of written notice of acceptance by the EMA of such MAA for filing under the centralized European procedure in accordance with any feedback received from European Regulatory Authorities; provided that if the centralized filing procedure is not used, then Acceptance will be determined upon the acceptance of such MAA by the applicable Regulatory Authority in a Major Country in the EU, and (c) in Japan, receipt by JBI of written notice of acceptance of filing of such JNDA from the Koseisho (i.e., the Japanese Ministry of Health and Welfare, or any successor agency thereto).
“Additional Core IP” means Third Party intellectual property that is necessary to [***]; provided Additional Core IP does not include any Patent Rights claiming (or intellectual property related to) [***].
“Affiliate” of an entity means any corporation, firm, partnership or other entity which directly or indirectly through one or more intermediaries controls, is controlled by or is under common control with a Party to this Agreement. An entity will be deemed to control another entity if it (i) owns, directly or indirectly, at least 50% of the outstanding voting securities or capital stock (or such lesser percentage which is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) of such other entity, or has other comparable ownership interest with respect to any entity other than a corporation; or (ii) has the power, whether pursuant to contract, ownership of securities or otherwise, to direct the management and policies of the entity. For clarity, Regulus Therapeutics Inc. will not be deemed an “Affiliate” of Isis for the purposes of this Agreement under any circumstances.
“Agreement” has the meaning set forth in the Preamble of this Agreement.
“Agreement Term” has the meaning set forth in Section 10.1.
“Alliance Manager” has the meaning set forth in Section 1.6.5.
“Annual” means the period covering a Calendar Year or occurring once per Calendar Year, as the context requires.
“API” means the bulk active pharmaceutical ingredient manufactured in accordance with GMP (unless expressly stated otherwise) for a Product.
“Applicable Law” or “Law” means all applicable laws, statutes, rules, regulations and other pronouncements having the effect of law of any federal, national, multinational, state, provincial, county, city or other political subdivision, agency or other body, domestic or foreign, including any applicable rules, regulations, guidelines, or other requirements of the Regulatory Authorities that may be in effect from time to time.
“Approval” means, with respect to a Product in any regulatory jurisdiction, approval from the applicable Regulatory Authority sufficient for the manufacture, distribution, use, marketing and sale of such Product in such jurisdiction in accordance with Applicable Laws. In jurisdictions where the applicable Regulatory Authority sets the pricing or reimbursement authorizations necessary for the general marketing and sale of such Product in the marketplace, Approval will not be deemed to have occurred if the final approval to market and sell such Product is being withheld because JBI (or its Affiliate or Sublicensee) and the Regulatory Authority have not yet determined pricing or reimbursement even if all other approvals, licenses, registrations or authorizations necessary for marketing, sale or use of such Product in such jurisdiction have been obtained. “Approval” does not include authorization by a Regulatory Authority to conduct named patient, compassionate use or other similar activities.
“ASO” means a single-stranded or double-stranded oligonucleotide compound, or analog, variant, mimic, or mimetic thereof, having a sequence that is at least six bases long and is designed to hybridize to a nucleic acid transcript via the binding, partially or wholly, of such compound to the nucleic acid transcript.
“Audit Report” has the meaning set forth in Section 6.12.
“Autoimmune Diseases” is any of a number of diseases characterized by abnormal functioning of the immune system which causes the immune system to attack the body’s own tissues. Crohn’s disease and ulcerative Colitis which are inflammatory bowel diseases are included as autoimmune diseases for purposes of this Agreement.
“Bankruptcy Code” has the meaning set forth in Section 7.12.
“Breaching Party” means the Party that is believed by the Non-Breaching Party to be in material breach of this Agreement.
“Business Day” means any day other than a Saturday or Sunday on which banking institutions in New York, New York are open for business.
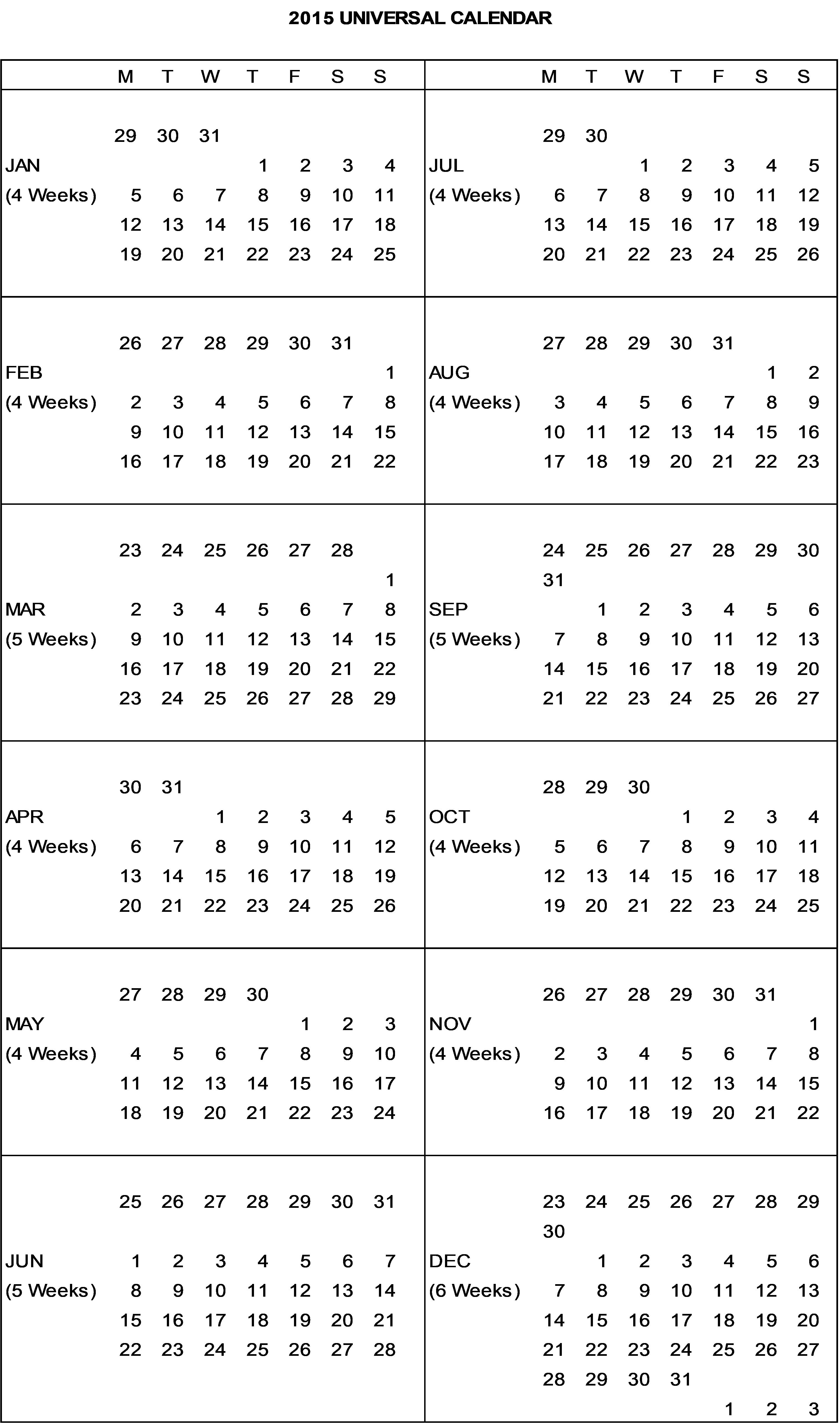
“Calendar Quarter” means a financial quarter based on the J&J Universal Calendar for that year (a copy of which is attached hereto as Appendix 3) and is used for JBI’s internal and external reporting purposes; provided, however, that the first Calendar Quarter for the first Calendar Year extends from the Effective Date to the end of the then current Calendar Quarter and the last Calendar Quarter extends from the first day of such Calendar Quarter until the effective date of the termination or expiration of the Agreement.
“Calendar Year” means a year based on the J&J Universal Calendar for that year. The Last Calendar Year of the Term begins on the first day of the J&J Universal Calendar Year for the year during which termination or expiration of the Agreement will occur, and the last day of such Calendar Year will be the effective date of such termination or expiration.
“Claims” has the meaning set forth in Section 9.1.
“Clinical Study” or “Clinical Studies” means a Phase I Clinical Trial, Phase II Clinical Trial, Phase III Clinical Trial or Phase IV Clinical Trial, or such other study in humans that is conducted in accordance with good clinical practices and is designed to generate data in support or maintenance of an NDA, MAA or other similar marketing application.
“Clinical Supplies” means API and finished drug Product for use in a Clinical Study.
“CMO” means a Third Party contract manufacturer Manufacturing API, Clinical Supplies or Finished Drug Product for any purpose under this Agreement.
“Collaboration Target” means a gene target designated as a Collaboration Target pursuant to Section 1.2.
“Commercialize,” “Commercialization” or “Commercializing” means any and all activities directed to marketing, promoting, detailing, distributing, importing, having imported, exporting, having exported, selling or offering to sell a Product following receipt of Approval for such Product in the applicable country, including conducting pre-and post-Approval activities, including studies reasonably required to increase the market potential of the Product and studies to provide improved formulation and Product delivery, and launching and promoting such Product in each country.
“Commercializing Party” means (a) JBI, with respect to a Product that is being Developed and Commercialized by or on behalf of JBI, its Affiliates or Sublicensees hereunder, and (b) Isis, with respect to a Discontinued Product that is being Developed and Commercialized by or on behalf of Isis, its Affiliates or Sublicensees hereunder.
“Commercially Reasonable Efforts” means the carrying out of discovery, research, development or commercialization activities using good-faith commercially reasonable and diligent efforts that the applicable Party would reasonably devote to a compound or product of similar market potential or profit potential at a similar stage in development or product life resulting from its own research efforts, based on conditions then prevailing and taking into account, without limitation, issues of safety and efficacy, regulatory authority-approved labeling, product profile, the competitiveness of alternative products in the marketplace, the likely timing of the product’s entry into the market, the patent and other proprietary position, the likelihood of Approval and other relevant scientific, technical and commercial factors. Without limiting any of the foregoing, Commercially Reasonable Efforts as it applies to JBI’s Development or Commercialization of a Product hereunder includes the use of Commercially Reasonable Efforts to perform the “General Activities” described in Schedule 5.2, and Commercially Reasonable Efforts as it applies to Isis’ Development of a Product hereunder includes use of Commercially Reasonable Efforts to adhere to the activities and timelines set forth in each Drug Discovery Plan and Development Plan.
“Competitive Infringement” has the meaning set forth in Section 7.5.1.
“Completion of PoC” means, on a Product-by-Product basis, when JBI receives the primary end-point data generated under the statistical analysis plan of the first PoC Study.
“Compound” means on a Drug Discovery Program-by-Drug Discovery Program basis, any ASO that is designed to bind to the RNA that encodes the applicable Collaboration Target, where such ASO is discovered by Isis prior to or in the performance of the Drug Discovery Plan, including each Development Candidate under such Drug Discovery Program.
“Confidential Information” has the meaning set forth in Section 11.1. “Confidential Information” does not include information that:
| (a) | was in the lawful knowledge and possession of the Receiving Party or its Affiliates prior to the time it was disclosed to, or learned by, the Receiving Party or its Affiliates, or was otherwise developed independently by the Receiving Party or its Affiliates, as evidenced by written records kept in the ordinary course of business, or other documentary proof of actual use by the Receiving Party or its Affiliates; |
| (b) | was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party or its Affiliates; |
| (c) | became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the Receiving Party or its Affiliates in breach of this Agreement; or |
| (d) | was disclosed to the Receiving Party or its Affiliates, other than under an obligation of confidentiality, by a Third Party who had no obligation to the Disclosing Party or its Affiliates not to disclose such information to others. |
“Control” or “Controlled” means possession of the ability to grant a license or sublicense hereunder without violating the terms of any agreement with any Third Party; provided, however, that if a Party has a right to grant a license or sublicense, with respect to an item of intellectual property to the other Party only upon payment of compensation (including milestones or royalties) to a Third Party (“Third Party Compensation”) (other than Isis Supported Pass-Through Costs in the case of Isis, and other than JBI Supported Pass-Through Costs in the case of JBI), then the first Party will be deemed to have “Control” of the relevant item of intellectual property only if the other Party agrees to bear the cost of such Third Party Compensation. Notwithstanding anything to the contrary under this Agreement, with respect to any Third Party that becomes an Affiliate of a Party after the Effective Date (including a Third Party acquirer), no intellectual property of such Third Party will be included in the licenses granted hereunder by virtue of such Third Party becoming an Affiliate of such Party.
“Cover,” “Covered” or “Covering” means, with respect to a patent, that, but for a license under such patent, the act of making, using or selling would infringe a Valid Claim included in such patent, or in the case of a patent that is a patent application, would infringe a Valid Claim in such patent application if it were to issue as a patent.
“CREATE Act” means the Cooperative Research and Technology Enhancement Act of 2004, 35 U.S.C. § 103(c)(2)-(c)(3).
“CTD” has the meaning set forth in Section 5.4.1.
“Currency Hedge Rate(s)” is calculated as a weighted average hedge rate of the outstanding external foreign currency forward hedge contract(s) of Johnson & Johnson’s Global Treasury Services Center (GTSC) and its Affiliates with third party banks. The hedge contract(s) is entered into to protect the transactional foreign exchange risk exposures of JBI by reducing the impact of foreign currency volatility through a systematic buildup of a yearly Currency Hedge Rate(s).
“Develop,” “Developing” or “Development” means with respect to a Product, any and all discovery, characterization, or preclinical (including IND-Enabling Toxicology Studies), clinical, or regulatory activity with respect to the Product to seek Approval (including the submission of all necessary filings with applicable Regulatory Authorities to support such preclinical and clinical activities and Approval), including human clinical trials conducted after Approval of the Product to seek Approval for additional indications for the Product.
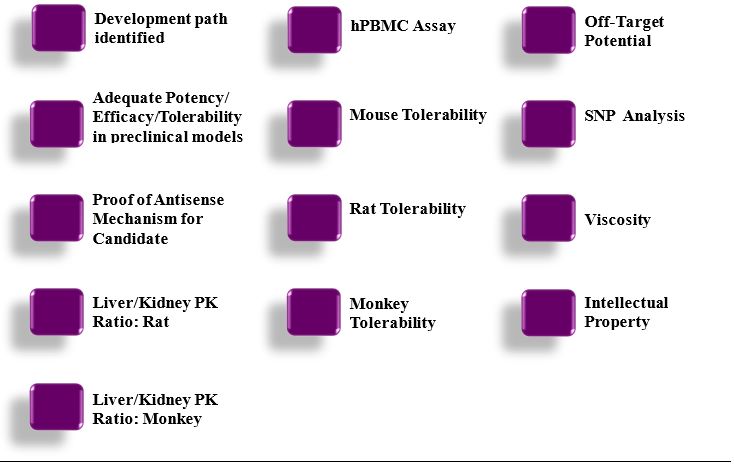
“Development Candidate” means a Compound that is reasonably determined by Isis’ Research Management Committee in accordance with Isis’ standard procedures for designating development candidates (and giving good faith consideration to the input of JBI’s representatives on the JRC) as ready to start IND-Enabling Toxicology Studies. The checklist Isis uses as of the Effective Date when reviewing potential development candidates for approval is attached hereto as Appendix 2.
“Development Candidate Data Package” means, with respect to a Development Candidate: [***].
“Development Plan” has the meaning set forth in Section 1.3.2(b).
“Disclosing Party” has the meaning set forth in Section 11.1.
“Discontinued Product” means a Product that is the subject of a termination under this Agreement.
“Dispositive Rejection Condition” has the meaning set forth in Section 1.2.3.
“Dispute” means any dispute arising between the Parties relating to, arising out of or in any way connected with this Agreement or any term or condition hereof, or the performance by either Party of its obligations hereunder, whether before or after termination of this Agreement that cannot be resolved by the Parties.
“Drug Discovery Plan” has the meaning set forth in Section 1.3.2(b).
“Drug Discovery Program” has the meaning set forth in Section 1.2.
“Drug Discovery Term” has the meaning set forth in Section 1.5.1.
“Effective Date” has the meaning set forth in the Preamble of this Agreement.
“EMA” means the European Medicines Agency and any successor entity thereto.
“European Union” or “EU” means each and every country or territory that is officially part of the European Union.
“Executives” has the meaning set forth in Section 12.1.1.
“FDA” means the United States Food and Drug Administration and any successor entity thereto.
“Field” means, except as may be limited under Section 4.1.4, the prophylactic or therapeutic use or form of administration of a Product for any indication.
“Finished Drug Product” means any drug product containing API as an active ingredient in finished bulk form for the Development or Commercialization by a Party under this Agreement.
“First Commercial Sale” means with respect to a Product, the first sale of such Product by JBI, its Affiliate or its Sublicensee to a Third Party in a particular country after Approval of the Product has been obtained in such country.
“Follow-On Agreement” has the meaning set forth in Section 2.1.2.
“Follow-On Compound” means, with respect to a given Compound for a given Collaboration Target, any ASO (other than the Development Candidate for such Collaboration Target) that is designed to bind to the RNA that encodes such Collaboration Target discovered by or on behalf of Isis following exercise of the applicable Option by JBI.
“FTE” means a total of 47 weeks or 1880 hours per year of work on the Development, Manufacturing or Commercialization of a Product carried out by employees of a Party having the appropriate relevant expertise to conduct such activities.
“FTE Rate” Means for a given Calendar Year the rate that Isis charges for a full time equivalent [***].
“Fully Absorbed Cost of Goods” means the reasonable and necessary internal and third party costs with no mark-up incurred by Isis in making or acquiring of product as determined using the methodology set forth in Schedule 4.4.4 fairly applied and as employed on a consistent basis throughout Isis’ operations and shall not include inter-company profits among Isis and its Affiliates. .
“GCP” means the then current standards for clinical trials for pharmaceuticals, as set forth in the United States Code of Federal Regulations, ICH guidelines and applicable regulations, laws or rules as promulgated thereunder.
“Generic Product” means, with respect to a particular Product in a country, a generic or biosimilar pharmaceutical product, that is not produced, licensed or owned by JBI or any of its Affiliates, that:(a) contains the same, or a bioequivalent of the, active ingredient as a Product; and (b) is approved for use in such country by a regulatory authority through a regulatory pathway by referencing clinical data first submitted for obtaining regulatory approval for such Product. Generic Product includes any pharmaceutical products obtained via a bioequivalence or bioavailability showing such as those covered by section 505(b)(2) or under 505(j) of the U.S. Federal Food, Drug, and Cosmetic Act or an equivalent outside the United States.
“GLP” means the then-current good laboratory practice standards promulgated or endorsed by the FDA as defined in 21 C.F.R. Part 58, and comparable foreign regulatory standards.
“GMP” means current Good Manufacturing Practices as specified in the United States Code of Federal Regulations, ICH Guideline Q7A, or equivalent laws, rules, or regulations of an applicable Regulatory Authority at the time of manufacture.
“[***].
“IND” means an Investigational New Drug Application (as defined in the Food, Drug and Cosmetic Act, as amended) filed with the FDA or its foreign counterparts.
“IND-Enabling Toxicology Studies” means the pharmacokinetic and toxicology studies required to meet the requirements for filing an IND. IND-Enabling Toxicology Studies do not include chronic toxicology studies or reproductive toxicology studies.
“Indemnitee” has the meaning set forth in Section 9.3.
“Indication” means distinct, well-categorized disease or condition in humans for which a separate marketing authorization (or amendment to a marketing authorization) is required.
“Initiation” or “Initiate” means, with respect to any IND-Enabling Toxicology Study, dosing of the first animal subject in such IND-Enabling Toxicology Study and, with respect to any Clinical Study, dosing of the first human subject in such Clinical Study.
“Integrated Development Plan” or “IDP” has the meaning set forth in Section 5.3.
“Isis” has the meaning set forth in the Preamble of this Agreement.
“
Isis Core Technology Patents” means all Patent Rights owned, used, developed by, or licensed to Isis or its Affiliates, in each case to the extent Controlled by Isis or its Affiliates on the Effective Date or at any time during the Agreement Term, claiming subject matter generally applicable to ASOs, other than Isis Product-Specific Patents or Isis Manufacturing and Analytical Patents. A list of Isis Core Technology Patents as of the Effective Date is set forth on
Schedule 8.2.2(a) attached hereto.
“Isis Formulation Patents” means the Patent Rights listed on Schedule 8.2.2(d) attached hereto.
“Isis In-License Agreements” has the meaning set forth in Section 6.9.1(a).
“Isis Internal ASO Safety Database” has the meaning set forth in Section 5.6.
“Isis Know-How” means any Know-How, including any Jointly-Owned Program Know-How and Isis Program Know-How, owned, used, developed by, or licensed to Isis or its Affiliates, in each case to the extent Controlled by Isis or its Affiliates on the Effective Date or at any time during the Agreement Term. Isis Know-How does not include the Isis Manufacturing and Analytical Know-How.
“Isis Manufacturing and Analytical Know-How” means Know-How, including Jointly-Owned Program Know-How, that relates to the synthesis or analysis of a Product regardless of sequence or chemical modification, owned, used, developed by, or licensed to Isis or its Affiliates, in each case to the extent Controlled by Isis or its Affiliates on the Effective Date or at any time during the Agreement Term. Isis Manufacturing and Analytical Know-How does not include the Isis Know-How.
“Isis Manufacturing and Analytical Patents” means Patent Rights, including Jointly-Owned Program Patents, that claim methods and materials used in the synthesis or analysis of a Product regardless of sequence or chemical modification, owned, used, developed by, or licensed to Isis or its Affiliates, in each case to the extent Controlled by Isis or its Affiliates on the Effective Date or at any time during the Agreement Term. A list of Isis Manufacturing and Analytical Patents as of the Effective Date is set forth on Schedule 8.2.2(b) attached hereto. Isis Manufacturing and Analytical Patents do not include the Isis Product-Specific Patents or the Isis Core Technology Patents.
“Isis Platform Technology” has the meaning set forth in Section 8.2.2.
“Isis Product-Specific Patents” means all Product-Specific Patents, in each case to the extent Controlled by Isis or its Affiliates on the Effective Date or at any time during the Agreement Term. A list of Isis Product-Specific Patents as of the Effective Date is set forth on Schedule 8.2.2(c) attached hereto.
“Isis Program Know-How” has the meaning set forth in Section 7.1.2.
“Isis Program Patents” has the meaning set forth in Section 7.1.2.
“Isis Supported Pass-Through Costs” means [***].
“JBI” has the meaning set forth in the Preamble of this Agreement.
“JBI Royalty” has the meaning set forth in Section 6.8.1.
“JBI Know-How” means any Know-How owned, used, developed by, or licensed to JBI or its Affiliates, in each case to the extent Controlled by JBI or its Affiliates on the Effective Date or at any time during the Agreement Term, but specifically excluding the JBI Program Know-How.
“JBI Patents” means any Patent Rights included in the JBI Technology.
“JBI Product-Specific Patents” means all Product-Specific Patents owned, used, developed by, or licensed to JBI or its Affiliates, in each case to the extent Controlled by JBI or its Affiliates on the Effective Date or at any time during the Agreement Term.
“JBI Program Know-How” has the meaning set forth in Section 7.1.2.
“JBI Program Patents” has the meaning set forth in Section 7.1.2.
“JBI Program Technology” has the meaning set forth in Section 7.1.2.
“JBI-Prosecuted Patents” has the meaning set forth in Section 7.2.4.
“JBI Supported Pass-Through Costs” means [***].
“JBI Technology” means the JBI Program Technology, Jointly-Owned Program Technology, JBI Product-Specific Patents and any trademarks described in Section 4.1.5, owned, used, developed by, or licensed to JBI or its Affiliates that is necessary or useful to Develop, register, Manufacture or Commercialize a Product.
“Japan NDA” or “JNDA” means the Japanese equivalent of an NDA filed with the Koseisho (i.e., the Japanese Ministry of Health and Welfare, or any successor agency thereto).
“JNDA Approval” means the Approval of a JNDA by the Koseisho (i.e., the Japanese Ministry of Health and Welfare, or any successor agency thereto) for the applicable Product in Japan including pricing.
“Joint Patent Committee” or “JPC” has the meaning set forth in Section 7.1.3(a).
“Jointly-Owned Program Know-How” has the meaning set forth in Section 7.1.2.
“Jointly-Owned Program Patents” has the meaning set forth in Section 7.1.2.
“Jointly-Owned Program Technology” has the meaning set forth in Section 7.1.2.
“JRC” has the meaning set forth in Section 1.6.1.
“Know-How” means inventions, technical information, know-how and materials, including technology, data, compositions, formulas, biological materials, assays, reagents, constructs, compounds, discoveries, procedures, processes, practices, protocols, methods, techniques, results of experimentation or testing, knowledge, trade secrets, skill and experience, in each case whether or not patentable or copyrightable, and in each case that are unpatented.
“Lead Party” has the meaning set forth in Section 7.4.1.
“Licensed Know-How” means Isis Manufacturing and Analytical Know-How, and Isis Know-How. For clarity, Licensed Know-How does not include any Know-How covering delivery devices.
“Licensed Patents” means the Isis Product-Specific Patents, Isis Core Technology Patents, Isis Manufacturing and Analytical Patents, Isis Formulation Patents and Isis’ interest in Jointly-Owned Program Patents. For clarity, Licensed Patents do not include any Patent Rights claiming formulation technology or delivery devices unless such Patent Rights are included in the Jointly-Owned Program Patents.
“Licensed Technology” means, on a Product-by-Product basis, any and all Licensed Patents, Licensed Know-How, and any trademarks described in Section 4.1.5, to the extent necessary or useful to Develop, register, Manufacture or Commercialize such Product.
“Losses” has the meaning set forth in Section 9.1.
“MAA” means, with respect to a particular Product, a marketing authorization application filed with the EMA after completion of Clinical Studies (excluding Phase IV Clinical Trials) to obtain Approval for such Product under the centralized European filing procedure or, if the centralized EMA filing procedure is not used, filed using the applicable procedures in any European Union country.
“MAA Approval” means, with respect to a particular Product, the Approval of an MAA by the EMA for such Product in any country in the EU including pricing.
“Major Market” means any of the following countries: the United States, Japan, the United Kingdom, Germany, France, Italy and Spain.
“Manufacture” or “Manufactured” or “Manufacturing” means any activity involved in or relating to the manufacturing, quality control testing (including in-process, release and stability testing), releasing or packaging, for pre-clinical and clinical purposes, of API or a Product in finished form.
“Milestone Event” means a Pre-Licensing Milestone Event or a Post-Licensing Milestone Event, as the case may be.
“Minimum Third Party Payments” means [***].
“NDA” means a New Drug Application filed with the FDA after completion of Clinical Studies to obtain Approval for a Product in the United States.
“NDA Approval” means the Approval of an NDA by the FDA for a Product in the U.S.
“Negotiation Period” has the meaning set forth in Section 2.1.2.
“
Net Sales” means the gross amounts invoiced on sales of a Product by JBI or any of its Affiliates or sublicensees to a Third Party purchaser in an arms-length transaction, less the following customary deductions, determined in accordance with US generally accepted accounting principles and standard internal policies and procedures and accounting standards consistently applied throughout Johnson & Johnson, to the extent specifically and solely allocated to such Product and actually taken, paid, accrued, allowed, included or allocated based on good faith estimates in the gross sales prices with respect to such sales (and consistently applied as set forth below):
| a) | normal and customary trade, cash and/or quantity discounts, allowances, and credits allowed or paid, in the form of deductions actually allowed or fees actually paid with respect to sales of such Product (to the extent not already reflected in the amount invoiced) excluding commissions for commercialization; |
| b) | excise taxes, use taxes, tariffs, sales taxes and customs duties, and/or other government charges imposed on the sale of Product to the extent included in the price and separately itemized on the invoice price (but specifically excluding, for clarity, any income taxes assessed against the income arising from such sale) (including VAT, but only to the extent that such VAT taxes are not reimbursable or refundable); |
| c) | outbound freight, shipment and insurance costs to the extent included in the price and separately itemized on the invoice price; |
| d) | compulsory payments and cash rebates related to the sales of such Product paid to a Governmental Authority (or agent thereof) pursuant to governmental regulations by reason of any national or local health insurance program or similar program, to the extent allowed and taken; including Government levied fees as a result of Healthcare Reform policies |
| e) | retroactive price reductions, credits or allowances actually granted upon rejections or returns of Product, including for recalls or damaged good and billing errors; and |
| f) | rebates, chargebacks, and discounts (or equivalent thereof) actually granted to managed health care organizations, pharmacy benefit managers (or equivalent thereof), federal, state/provincial, local or other governments, or their agencies or purchasers, reimbursers, or trade customers. |
The sales of Products arising from named patient, compassionate use, or other similar programs will not be considered a First Commercial Sale for purposes of calculating the Royalty Period.
All aforementioned deductions shall only be allowable to the extent they are commercially reasonable and shall be determined, on a country-by-country basis, as incurred in the ordinary course of business in type and amount consistent with the Party’s, the Affiliate’s, or Third Party sublicensee’s (as the case may be) business practices consistently applied across its product lines and accounting standards and verifiable based on the Johnson & Johnson sales reporting system. All such discounts, allowances, credits, rebates, and other deductions shall be fairly and equitably allocated to Product and other products of the Party and its Affiliates and sublicensees such that Product does not bear a disproportionate portion of such deductions.
The following shall be excluded for the purposes of calculating royalties or sales milestones:
| a) | Sales of Product by and between JBI and its Affiliates and sublicenses so long as such Product is subsequently resold to a Third-party end user where such resale to such Third-party end user is included in Net Sales |
| b) | Sales of Product for the use in conducting clinical trials, pre-clinical studies or other research or development activities in a country in order to obtain Regulatory Approval of Product in such country |
| c) | Product provided free of charge for a bona fide charitable purpose |
| d) | Product used for commercially reasonable free sampling programs. |
| e) | Sales of Product free of charge for Compassionate |
| f) | Sales of Product for Named Patient Sales where such Product is sold at a significant discount to the proposed price for the Product following Approval. |
In the event Product(s) are sold in combination with other products or services from JBI, its Affiliates or sublicensees and the customer receives a specific discount for such “bundling” of products (for clarity, this situation describes bundling of two or more separate products, each in finished dosage form, and not a fixed combination of two active pharmaceutical ingredients), the Net Sales of the said Product(s), for the purposes of determining royalty payments, shall be determined by multiplying the relevant Net Sales by the fraction, A/(A+B) where A is the weighted (by sales volume) average sale price in a particular country of the Product(s) in the previous Calendar Year when sold separately and B is the weighted average sale price in that country in the previous Calendar Year of the other product sold separately. In the event that such average sale price cannot be determined for either of the Product(s) or the other product(s) it has been sold with, in combination, then for purposes of determining the royalty payments, JBI will propose a reasonable good faith estimate of the fair market value of each component (and JBI will provide Isis a justification and support for such estimates) which will be substituted for the weighted average sales price for each such product in the formula above. If JBI, its Affiliate or a Sublicensee receives non-monetary consideration for a Product, Net Sales are calculated based on the fair market value of that consideration.
“New Third Party Licenses” has the meaning set forth in Section 8.3.2.
“Non-Breaching Party” means the Party that believes the Breaching Party is in material breach of this Agreement.
“Option” has the meaning set forth in Section 3.1.
“Option Deadline” has the meaning set forth in Section 3.1.
“Option Period” means, with respect to a Drug Discovery Program, the period beginning on the date when the applicable Collaboration Target was designated and ending on the expiration or earlier termination of the Option with respect to such Drug Discovery Program.
“Out-of-Pocket Expenses” means the amounts paid to Third Party vendors or contractors, for services or materials provided by them directly in the performance of activities to the extent such services or materials apply directly to the activities under this agreement.
“Party” or “Parties” means JBI and Isis individually or collectively.
“Patent Costs” means the reasonable fees and expenses paid to outside legal counsel, and filing, maintenance and other reasonable Out-of-Pocket expenses paid to Third Parties, incurred in connection with the Prosecution and Maintenance of Patent Rights.
“Patent Rights” means (a) patents, patent applications and similar government-issued rights protecting inventions in any country or jurisdiction however denominated, (b) all priority applications, divisionals, continuations, substitutions, continuations-in-part of and similar applications claiming priority to any of the foregoing, and (c) all patents and similar government-issued rights protecting inventions issuing on any of the foregoing applications, together with all registrations, reissues, renewals, re-examinations, confirmations, supplementary protection certificates, and extensions of any of (a), (b) or (c).
“Permitted Licenses” means (1) licenses granted by Isis before or after the Effective Date to any Third Party under the Isis Core Technology Patents, the Isis Manufacturing and Analytical Patents, or the Isis Manufacturing and Analytical Know-How (but not under the Isis Product-Specific Patents) to (a) use oligonucleotides (or supply oligonucleotides to end users) solely to conduct pre-clinical research, or (b) enable such Third Party to manufacture or formulate oligonucleotides, where (i) such Third Party is primarily engaged in providing contract manufacturing or services and is not primarily engaged in drug discovery, development or commercialization of therapeutics; and (ii) Isis does not assist such Third Party to identify, discover or make a Compound or Product; and (2) material transfer, collaboration or sponsored research agreements with academic collaborators or non-profit institutions solely to conduct noncommercial research.
“Person” will mean any corporation, limited or general partnership, limited liability company, joint venture, trust, unincorporated association, governmental body, authority, bureau or agency, any other entity or body, or an individual.
“Phase I Clinical Trial” means a human clinical trial that is intended to initially evaluate the safety, metabolism and pharmacokinetics of a therapeutic agent that would otherwise satisfy the requirements of 21 C.F.R. 312.21(a) or an equivalent clinical trial in a country in the Territory other than the United States.
“Phase II Clinical Trial” means a human clinical trial, for which the primary endpoints include a determination of safety, dose ranges or an indication of efficacy of a therapeutic in patients being studied as described in 21 C.F.R. §312.21(b), or an equivalent clinical trial in a country in the Territory other than the United States, and that is prospectively designed to generate sufficient data (if successful) to commence pivotal clinical trials.
“Phase III Clinical Trial” means a human clinical trial (regardless of whether actually designated as “Phase III”) that is prospectively designed, along with other Phase III Clinical Trials, to demonstrate statistically whether a therapeutic is safe and effective for use in humans in the indication being investigated as described in 21 C.F.R. §312.21(c), or an equivalent clinical trial in a country in the Territory other than the United States.
“Phase IV Clinical Trial” means, with respect to a Product, (a) any Clinical Study conducted to satisfy a requirement of a Regulatory Authority in order to maintain a Regulatory Approval for such Product or (b) any Clinical Study conducted after the first Regulatory Approval in the same disease state for which such Product received Regulatory Approval other than for purposes of obtaining Regulatory Approval.
“Plan” means a Drug Discovery Plan and/or Development Plan, as applicable.
“PoC Study” means a study conducted during a Phase II Clinical Trial designed to give preliminary evidence of efficacy and safety for a Product.
“Post-Licensing Milestone Event” has the meaning set forth in Section 6.4.
“Pre-Clinical Studies” means in vitro and in vivo studies of a Product, not in humans, including those studies conducted in whole animals and other test systems, designed to determine the toxicity, bioavailability, and pharmacokinetics of such Product and whether such Product has a desired effect.
“
Pre-Licensing Milestone Event” has the meaning set forth in Section 6.2.
“Prior Agreements” means the Agreements listed on Schedule 8.2.2(e) attached hereto.
“Proceeding” means an action, suit or proceeding.
“Product” means, on a Drug Discovery Program-by-Drug Discovery Program basis, a finished drug product containing a unique and specific Compound as an active pharmaceutical ingredient. Each Product shall contain a different specific Compound (s).
“
Product-Specific Patents” means Patent Rights Controlled by a Party or any of its Affiliates on or after the Effective Date, including any Program Patents, claiming (i) the specific composition of matter of a Product, or (ii) methods of using a Product as a prophylactic or therapeutic;
provided however, Patent Rights Controlled by Isis or any of its Affiliates that (y) include claims that are directed to subject matter applicable to ASOs in general, or (z) include an ASO, the sequence of which targets the RNA that encodes a Collaboration Target and the RNA of a gene that does not encode a Collaboration Target, will not be considered Product-Specific Patents, and in the case of (y) and (z), such Patent Rights will be considered Isis Core Technology Patents.
“Program Patents” has the meaning set forth in Section 7.1.2.
“Prosecution and Maintenance” or “Prosecute and Maintain” means, with regard to a Patent Right, the preparing, filing, prosecuting and maintenance of such Patent Right, as well as handling re-examinations, reissues, and requests for patent term extensions with respect to such Patent Right, together with the conduct of interferences, the defense of oppositions and other similar proceedings with respect to the particular Patent Right. For clarification, “Prosecution and Maintenance” or “Prosecute and Maintain” will not include any other enforcement actions taken with respect to a Patent Right.
“Receiving Party” has the meaning set forth in Section 11.1.
“Regulatory Approval” means the approval necessary for the commercial manufacture, distribution, marketing, promotion, offer for sale, use, import, export, and sale of a pharmaceutical product in a jurisdiction regulated by a Regulatory Authority.
“Regulatory Authority” means any governmental authority, including the FDA, EMA or Koseisho (i.e., the Japanese Ministry of Health and Welfare, or any successor agency thereto), that has responsibility for granting any licenses or approvals or granting pricing or reimbursement approvals necessary for the marketing and sale of a Product in any country.
“Research” means conducting the research activities with Compounds as set forth in each Drug Discovery Plan, including pre-clinical research and lead optimization, but specifically excluding Development and Commercialization. When used as a verb, “Researching” means to engage in Research.
“RMC” means Isis’ Research Management Committee, or any successor committee.
“ROFN Period” has the meaning set forth in Section 2.1.2.
“ROFN Termination Event” has the meaning set forth in Section 2.1.2.
“Royalty Period” has the meaning set forth in Section 6.8.2(a).
“Sales Milestone Event” has the meaning provided in Section 6.7.
“[***].
“Specific Performance Milestone Event” has the meaning set forth in Section 5.2.
“Step-In Party” has the meaning set forth in Section 7.4.1.
“Sublicensee” means a Third Party to whom a Party or its Affiliates or Sublicensees has granted a sublicense or license under any Licensed Technology or JBI Technology, as the case may be, licensed to such Party in accordance with the terms of this Agreement.
“Substitution Fee” means $[***] per substituted target to be paid by JBI following Isis’ acceptance of JBI’s proposed substitute gene target under Section 1.2.5 .
“Supplemental Information” has the meaning provided in Section 1.3.5.
“Target Nomination Period” has the meaning set forth in Section 1.2.1.
[***] “Third Party” means a Person or entity other than the Parties or their respective Affiliates.
“Third Party Obligations” means any financial and non-financial encumbrances, obligations, restrictions, or limitations imposed by an agreement between Isis and a Third Party (including the Isis In-License Agreements) that relate to a Product, a Collaboration Target, including field or territory restrictions, covenants, milestone payments, diligence obligations, sublicense revenue, royalties, or other payments.
“United States” or “U.S.” means the fifty states of the United States of America and all of its territories and possessions and the District of Columbia.
“Valid Claim” means a claim (i) of any issued, unexpired United States or foreign Patent Right, which will not, in the country of issuance, have been donated to the public, disclaimed, nor held invalid or unenforceable by a court of competent jurisdiction in an unappealed or unappealable decision, or (ii) of any United States or foreign patent application within a Patent Right, which will not, in the country in question, have been cancelled, withdrawn, abandoned nor been pending for more than six years, not including in calculating such six-year period time in which such application is in interference or opposition or similar proceedings or time in which a decision of an examiner is being appealed. Notwithstanding the foregoing, on a country-by-country basis, a patent application pending for more than six years will not be considered to have any Valid Claim for purposes of this Agreement unless and until a patent meeting the criteria set forth in clause (i) above with respect to such application issues.
Appendix 2
Development Candidate Checklist
Appendix 3
J&J Universal Calendar
Schedule 1.6.1
JRC Governance
| (a) | The JRC will determine the JRC operating procedures, including frequency of meetings (at least quarterly), location of meetings, and responsibilities for agendas and minutes. The JRC will codify these operating procedures in the written minutes of the first meeting. |
| (b) | The JRC may hold meetings in person or by audio or video conference as determined by the JRC; but at least two meetings per year will be in person (one held at Isis’ facilities, and the other held at JBI’s facilities in the U.S.). Alliance Managers will attend JRC meetings as participating non-members. In addition, upon prior approval of the other Party, each Party may invite its employees or consultants to attend JRC meetings, including any subject matter expert(s) with valuable knowledge of Collaboration Targets or the diseases associated with such Collaboration Targets. |
| (c) | The co-chairs will be responsible for ensuring that activities occur as set forth in this Agreement, including ensuring that JRC meetings occur, JRC recommendations are properly reflected in the minutes, and any dispute is given prompt attention and resolved in accordance with Section 1.6.3, Section 7.1.3 and Section 12.1, as applicable. |
| (d) | The JRC members from the same Party will collectively have one vote. The JRC will strive to make recommendations with approval of both Isis members and JBI members, and record such recommendations in the minutes of the applicable JRC meeting. |
| (e) | The JRC may form subcommittees and working groups as it determines in order to carry out its activities under this Agreement, all of which will dissolve when the JRC dissolves. |
Schedule 1.6.5
Alliance Management Activities
Each Alliance Manager is responsible for:
| (a) | Promoting the overall health of the relationship between the Parties; |
| (b) | Developing a mutually agreed alliance launch plan covering any activities and systems that the Parties need to implement within the first 100 days after the Effective Date to support the Drug Discovery Programs; |
| (c) | Organizing JRC meetings, including agendas, drafting minutes, and publishing final minutes; |
| (d) | Supporting the co-chairs of the JRC with organization of meetings, information exchange, meeting minutes, and facilitating dispute resolution as necessary; |
| (e) | Preparing status and progress reports on the above as determined necessary by the JRC; |
| (f) | Ensuring compliance in maintaining the Isis Internal ASO Safety Database as outlined in Section 5.6; |
| (g) | Ensuring proper approval of publications prior to submission as required in Section 11.4; and |
| (h) | Understanding and communicating the components contained in the relationship-management document provided by Isis to JBI, to assist JBI in understanding and complying with the contractual obligations under the Isis In-License Agreements after Option exercise. |
Schedule 4.4.4
Isis’ Fully Absorbed Cost of Goods Methodology
[***]
Schedule 5.2
JBI’s Development and Commercialization Activities
[***]
Schedule 6.9.1
Certain Isis In-License Agreements
(Relevant to the Drug Discovery Programs as of the Effective Date)
[***]
Schedule 8.2.2(a)
Isis Core Technology Patents
[***]
Schedule 8.2.2(b)
Isis Manufacturing and Analytical Patents
[***]
Schedule 8.2.2(c)
Isis Product-Specific Patents
[***]
Schedule 8.2.2(d)
Isis Formulation Patents
[***]
Schedule 8.2.2(e)
Prior Agreements
[***]
Schedule 11.4 – Press Release
ISIS PHARMACEUTICALS ANNOUNCES COLLABORATION WITH JANSSEN TO DISCOVER AND DEVELOP RNA-TARGETED THERAPEUTICS FOR AUTOIMMUNE DISEASES IN THE GI TRACT
-- Collaboration Combines Isis’ RNA-Targeted Technology with Expertise of Janssen in Autoimmune Disorders and Therapeutic Formulation --
Carlsbad, Calif., Jan. [5], 2015 –Isis Pharmaceuticals, Inc. (NASDAQ: ISIS) today announced that the company has entered into a global collaboration with Janssen Biotech, Inc. (Janssen) to discover and develop antisense drugs to treat autoimmune disorders of the gastrointestinal (GI) tract. The collaboration brings together Isis’ RNA-targeted technology platform and the expertise of Janssen in autoimmune disorders and therapeutic formulation to discover and develop antisense drugs that can be locally administered, including oral delivery, to treat autoimmune disorders in the GI tract.
“We are excited to be working with Janssen to apply our drug discovery and development efforts in this therapeutic area. This collaboration broadens the utility of our drug discovery technology to new targets in the GI tract and expands the administration of antisense drugs to local delivery, including oral delivery, to the gut,” said B. Lynne Parshall, chief operating officer at Isis Pharmaceuticals. “We are the leader in RNA-targeted therapeutics and our innovation and the successes of our pipeline drugs enable us to form collaborations, like this one, with leaders in specific therapeutic areas. This partnering strategy ensures that we have access to resources that support and enhance our drug discovery efforts and also provides us with collaborators, like Janssen, who are uniquely capable of conducting development, marketing and commercial efforts for these drugs. .”
Under the terms of the agreement, which covers three programs, Isis will receive $35 million in upfront payments, including a payment to initiate human lead optimization on the first collaboration target. Isis is eligible to receive nearly $800 million in development, regulatory and sales milestone payments and license fees for these programs. In addition, Isis will receive tiered royalties that on average are double-digits on sales from any product that is successfully commercialized. Janssen has the option to license a drug from each of the programs once a development candidate is identified. If Janssen exercises its option, it will assume global development, regulatory and commercialization responsibilities.
ABOUT ISIS PHARMACEUTICALS, INC.
Isis is exploiting its leadership position in RNA-targeted technology to discover and develop novel drugs for its product pipeline and for its partners. Isis’ broad pipeline consists of 34 drugs to treat a wide variety of diseases with an emphasis on cardiovascular, metabolic, severe and rare diseases, including neurological disorders, and cancer. Isis’ partner, Genzyme, is commercializing Isis’ lead product, KYNAMRO®, in the United States and other countries for the treatment of patients with homozygous FH. Isis has numerous drugs in Phase 3 development in severe and rare and cardiovascular diseases. These include a ISIS-APOCIIIRx, a drug Isis is developing to treat patients with severely high triglycerides, such as patients with familial chylomicronemia syndrome; ISIS-TTRRx, a drug Isis is developing with GSK to treat patients with the polyneuropathy form of TTR amyloidosis; and, ISIS-SMNRx, a drug Isis is developing with Biogen Idec to treat infants and children with spinal muscular atrophy, a severe and rare neuromuscular disease. Isis’ patents provide strong and extensive protection for its drugs and technology. Additional information about Isis is available at www.isispharm.com.
ISIS PHARMACEUTICALS’ FORWARD-LOOKING STATEMENT
This press release includes forward-looking statements regarding Isis’ alliance with Janssen, Isis’ research, development and commercial opportunities in developing antisense drugs to treat inflammatory bowel disease. Any statement describing Isis’ goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, particularly those inherent in the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs. Isis’ forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Isis’ forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Isis. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Isis’ programs are described in additional detail in Isis’ annual report on Form 10-K for the year ended December 31, 2013, and its most recent quarterly report on Form 10-Q, which are on file with the SEC. Copies of these and other documents are available from the Company.
In this press release, unless the context requires otherwise, “Isis,” “Company,” “we,” “our,” and “us” refers to Isis Pharmaceuticals and its subsidiaries.
Isis Pharmaceuticals® is a registered trademark of Isis Pharmaceuticals, Inc. KYNAMRO® is a registered trademark of Genzyme Corporation.
Isis Pharmaceuticals’ Contacts:
D. Wade Walke, Ph.D. Vice President, Corporate Communications and Investor Relations 760-603-2741 | Amy Blackley, Ph.D. Associate Director, Corporate Communications 760-603-2772 |
###