Searchable text section of graphics shown above
[LOGO]
Developing RNA Drugs and Technologies to Improve the Lives and Health of Patients
April 2006
ISIS 301012:
The Reduction of Atherogenic Lipids in Subjects with Hypercholesterolemia
Mark Wedel, MD, JD
Senior Vice President and CMO
Isis Pharmaceuticals
Carlsbad, CA
April 2006
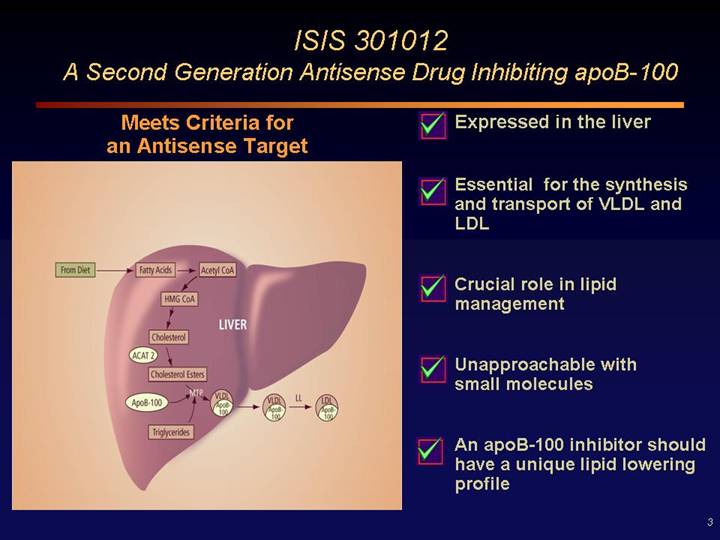
ISIS 301012
A Second Generation Antisense Drug Inhibiting apoB-100
Meets Criteria for
an Antisense Target
[GRAPHIC]
• Expressed in the liver
• Essential for the synthesis and transport of VLDL and LDL
• Crucial role in lipid management
• Unapproachable with small molecules
• An apoB-100 inhibitor should have a unique lipid lowering profile
3
ISIS 301012: Phase 1 Results
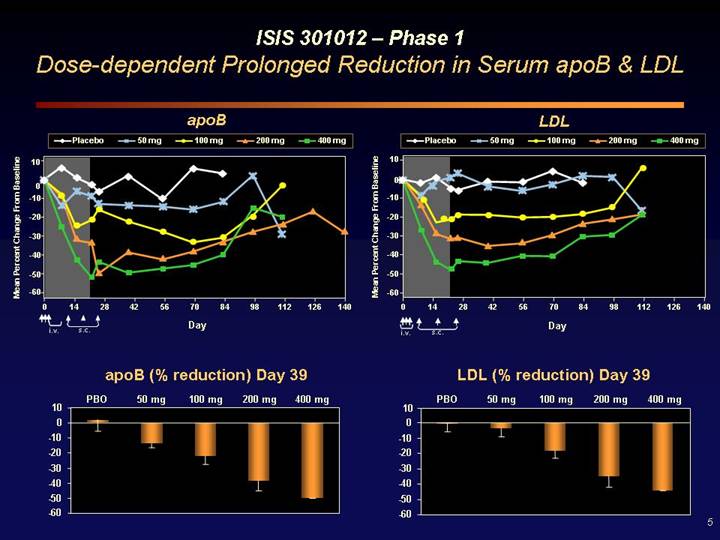
ISIS 301012 – Phase 1
Dose-dependent Prolonged Reduction in Serum apoB & LDL
apoB | | LDL |
| | |
[CHART] | | [CHART] |
| | |
apoB (% reduction) Day 39 | | LDL (% reduction) Day 39 |
| | |
[CHART] | | [CHART] |
5
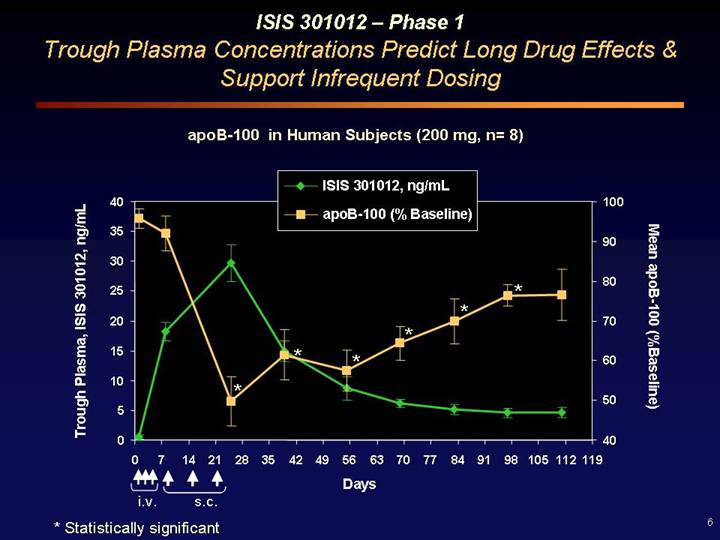
ISIS 301012 – Phase 1
Trough Plasma Concentrations Predict Long Drug Effects & Support Infrequent Dosing
apoB-100 in Human Subjects (200 mg, n= 8)
[CHART]
* Statistically significant
6
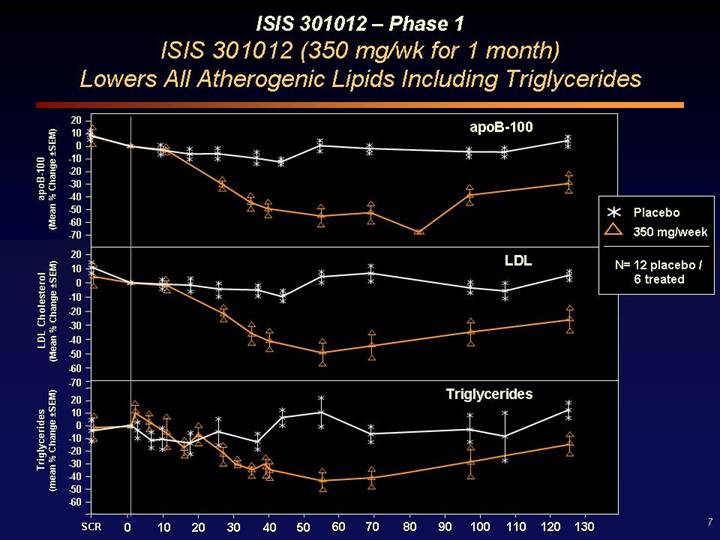
ISIS 301012 – Phase 1
ISIS 301012 (350 mg/wk for 1 month)
Lowers All Atherogenic Lipids Including Triglycerides
[CHART]
7
ISIS 301012 – Phase 2a
Study Objectives
• Safety & tolerability
• Efficacy
• Statistical significance
• Dose dependence
• apoB
• LDL
• Other atherogenic lipids
• PK/PD correlation
8
ISIS 301012 – Phase 2a
Study Design
• n = 10 subjects per cohort (total 50 subjects)
• Concomitant placebo subjects (1:4)
• Dosing duration: 3 months
• Target population: hypercholesterolemic subjects failing to reach target on diet alone
• Dosing cohorts:
• 50 mg/wk
• 100 mg/wk
• 200 mg/wk
• midpoint safety/efficacy review
• 300 mg/wk
• 400 mg/wk
9
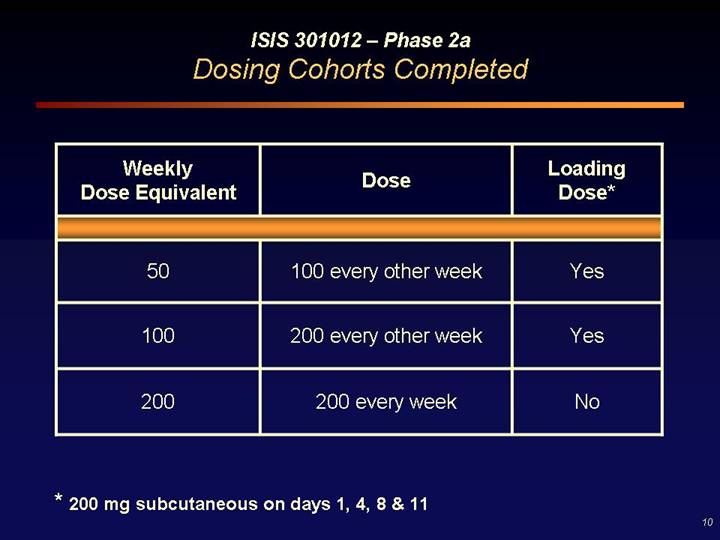
ISIS 301012 – Phase 2a
Dosing Cohorts Completed
Weekly
Dose Equivalent | | Dose | | Loading
Dose* |
| | | | |
50 | | 100 every other week | | Yes |
| | | | |
100 | | 200 every other week | | Yes |
| | | | |
200 | | 200 every week | | No |
* 200 mg subcutaneous on days 1, 4, 8 & 11
10
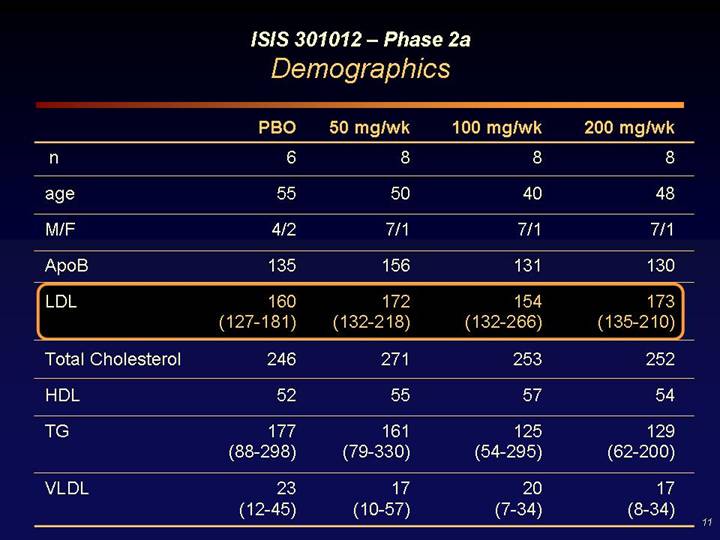
ISIS 301012 – Phase 2a
Demographics
| | PBO | | 50 mg/wk | | 100 mg/wk | | 200 mg/wk | |
n | | 6 | | 8 | | 8 | | 8 | |
age | | 55 | | 50 | | 40 | | 48 | |
M/F | | 4/2 | | 7/1 | | 7/1 | | 7/1 | |
ApoB | | 135 | | 156 | | 131 | | 130 | |
LDL | | 160
(127-181) | | 172
(132-218) | | 154
(132-266) | | 173
(135-210) | |
Total Cholesterol | | 246 | | 271 | | 253 | | 252 | |
HDL | | 52 | | 55 | | 57 | | 54 | |
TG | | 177
(88-298) | | 161
(79-330) | | 125
(54-295) | | 129
(62-200) | |
VLDL | | 23
(12-45) | | 17
(10-57) | | 20
(7-34) | | 17
(8-34) | |
11
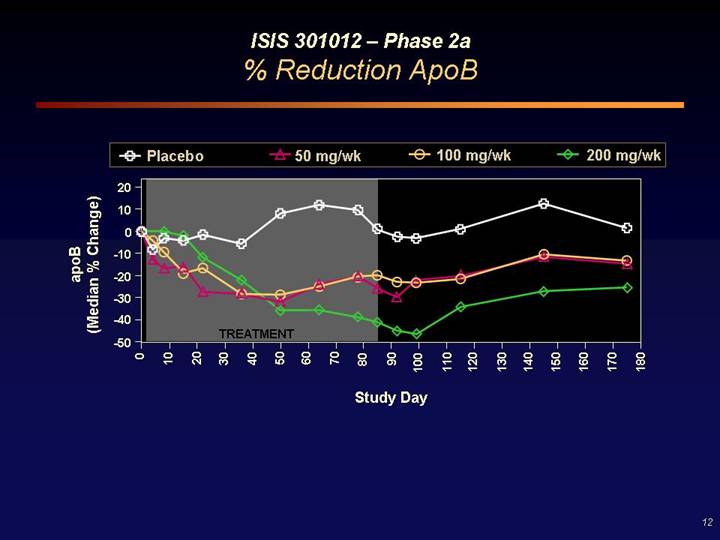
ISIS 301012 – Phase 2a
% Reduction ApoB
[CHART]
12
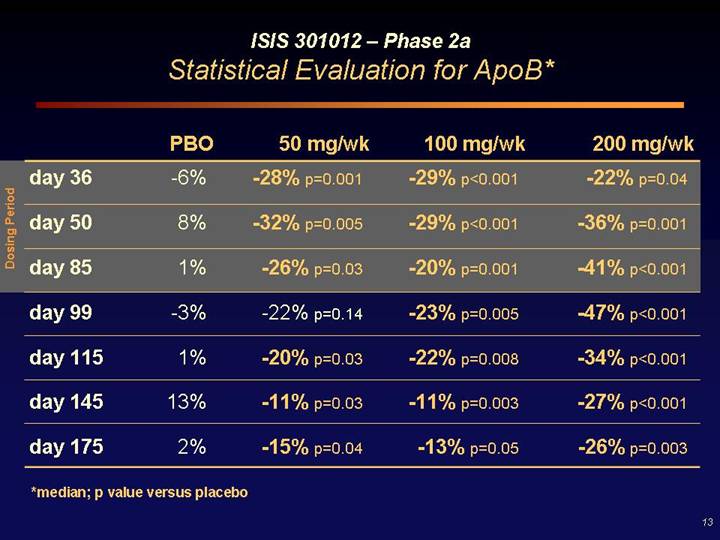
ISIS 301012 – Phase 2a
Statistical Evaluation for ApoB*
Dosing Period | | PBO | | 50 mg/wk | | 100 mg/wk | | 200 mg/wk | |
day 36 | | -6 | % | -28% p=0.001 | | -29% p<0.001 | | -22% p=0.04 | |
day 50 | | 8 | % | -32% p=0.005 | | -29% p<0.001 | | -36% p=0.001 | |
day 85 | | 1 | % | -26% p=0.03 | | -20% p=0.001 | | -41% p<0.001 | |
day 99 | | -3 | % | -22% p=0.14 | | -23% p=0.005 | | -47% p<0.001 | |
day 115 | | 1 | % | -20% p=0.03 | | -22% p=0.008 | | -34% p<0.001 | |
day 145 | | 13 | % | -11% p=0.03 | | -11% p=0.003 | | -27% p<0.001 | |
day 175 | | 2 | % | -15% p=0.04 | | -13% p=0.05 | | -26% p=0.003 | |
*median; p value versus placebo
13
ISIS 301012 – Phase 2a
LDL % Reduction
[CHART]
14
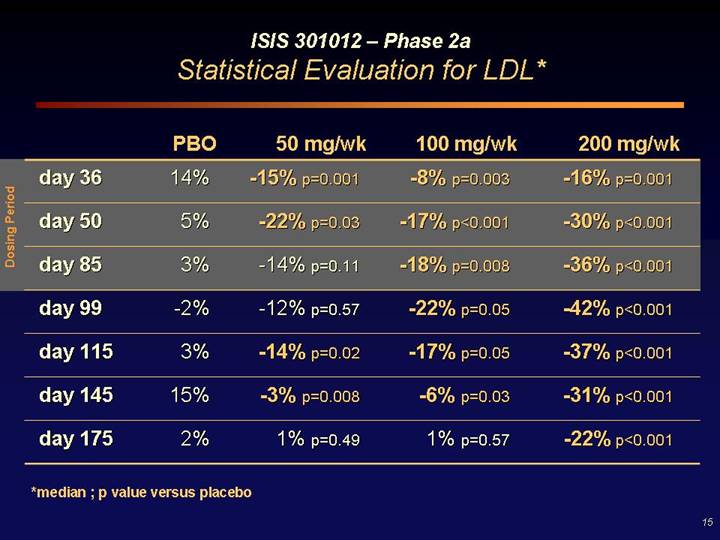
ISIS 301012 – Phase 2a
Statistical Evaluation for LDL*
Dosing Period | | PBO | | 50 mg/wk | | 100 mg/wk | | 200 mg/wk | |
day 36 | | 14 | % | -15% p=0.001 | | -8% p=0.003 | | -16% p=0.001 | |
day 50 | | 5 | % | -22% p=0.03 | | -17% p<0.001 | | -30% p<0.001 | |
day 85 | | 3 | % | -14% p=0.11 | | -18% p=0.008 | | -36% p<0.001 | |
day 99 | | -2 | % | -12% p=0.57 | | -22% p=0.05 | | -42% p<0.001 | |
day 115 | | 3 | % | -14% p=0.02 | | -17% p=0.05 | | -37% p<0.001 | |
day 145 | | 15 | % | -3% p=0.008 | | -6% p=0.03 | | -31% p<0.001 | |
day 175 | | 2 | % | 1% p=0.49 | | 1% p=0.57 | | -22% p<0.001 | |
*median ; p value versus placebo
15
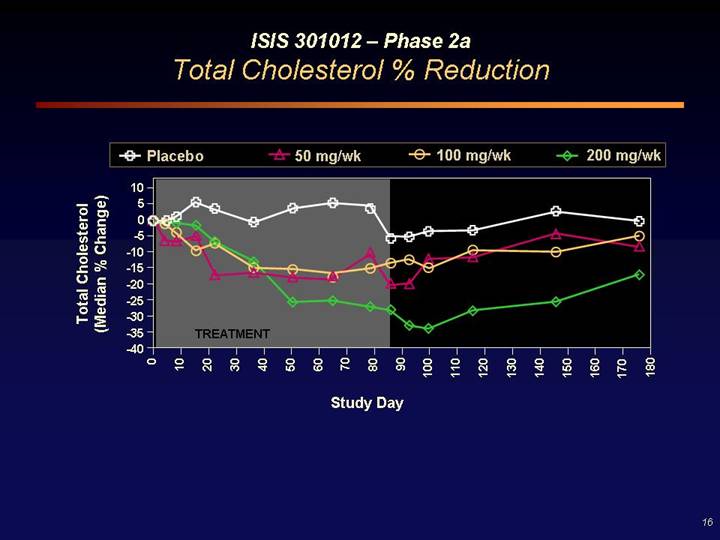
ISIS 301012 – Phase 2a
Total Cholesterol % Reduction
[CHART]
16
ISIS 301012 – Phase 2a
Non-HDL % Reduction
[CHART]
17
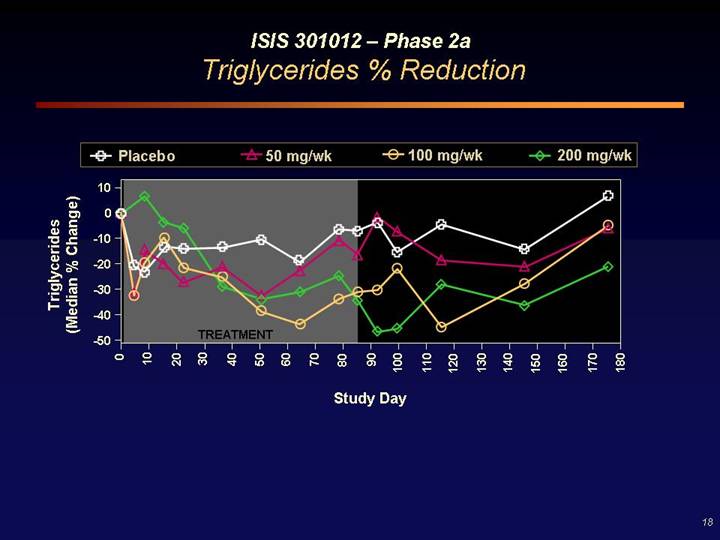
ISIS 301012 – Phase 2a
Triglycerides % Reduction
[CHART]
18
ISIS 301012 – Phase 2a
Duration of Effect on LDL*
Post Dosing
50 mg/week | | 0.4 months |
| | |
100 mg/week | | 2.0 months |
| | |
200 mg/week | | 5.2 months (projected) |
*Return to 90% of baseline
19
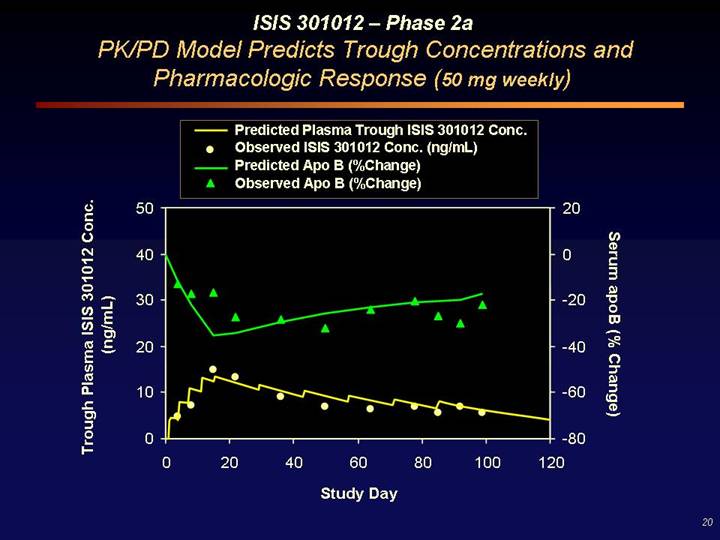
ISIS 301012 – Phase 2a
PK/PD Model Predicts Trough Concentrations and Pharmacologic Response (50 mg weekly)
[CHART]
20
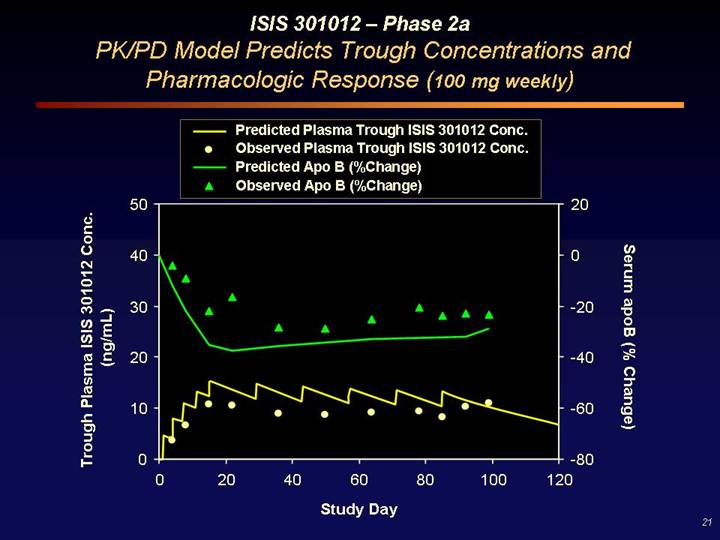
ISIS 301012 – Phase 2a
PK/PD Model Predicts Trough Concentrations and
Pharmacologic Response (100 mg weekly)
[CHART]
21
ISIS 301012 – Phase 2a
PK/PD Model Predicts Trough Concentrations and
Pharmacologic Response (200 mg weekly)
[CHART]
22
ISIS 301012 – Phase 2a
Safety
Serious Adverse Events:
• One unrelated SAE (encephalitis)
Adverse Events:
• Injection site reactions: Mild transient painless erythema
• No impact on compliance
• One subject with transient ALT rise (3.4x ULN) 60 days post last dose
• No dose response relationship for LFT elevations of any level
• No other laboratory or clinical abnormalities observed
23
ISIS 301012 – Phase 2a
Conclusions
• Low weekly subcutaneous doses of ISIS 301012 lower all atherogenic lipids & triglycerides and are well tolerated
• The effects of ISIS 30102 are predictable
• Dose and schedule dependent
• Pharmacokinetics consistent & predictable
• Pharmacodynamics highly correlated with pharmacokinetics
• No clinical evidence of fat malabsorption or steatosis
24
ISIS 301012
Next Steps
• Evaluate effects of 300 and 400mg per week for three months in patients with high cholesterol
• Evaluate effects of ISIS 301012 in combination with statins
• Initially for 5 weeks
• Then 6 months
• Evaluate the effects of ISIS 301012 in patients with Familial Hypercholesterolemia (FH)
• Define induction and maintenance doses of ISIS 301012 in longer term trials
• Continue to define profile
• Initiation of surrogate outcome studies
25
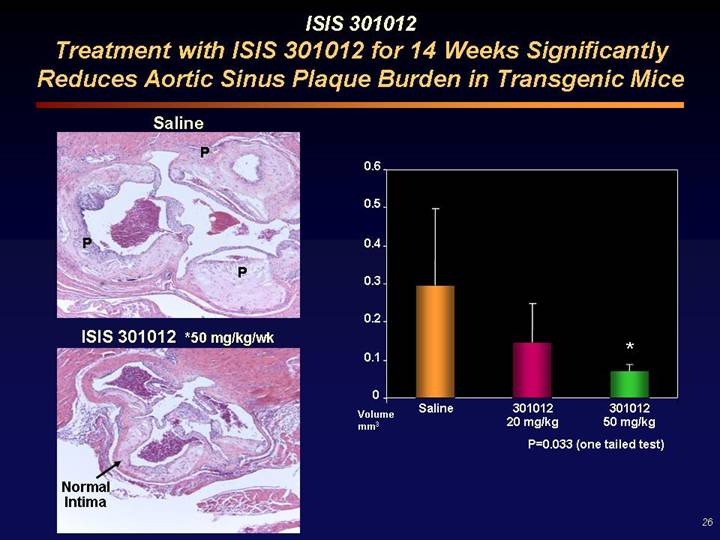
ISIS 301012
Treatment with ISIS 301012 for 14 Weeks Significantly Reduces Aortic Sinus Plaque Burden in Transgenic Mice
Saline | | |
[GRAPHIC] | | |
| | [CHART] |
ISIS 301012 *50 mg/kg/wk | | |
[GRAPHIC] | | |
26
ISIS 301012
Clinical Development Strategy
• We intend for ISIS 301012 to be:
• The drug of choice for patients at risk who are unable to achieve target levels on statins & ezetimibe
• Initially in patients with FH
• Ultimately in polygenic hypercholesterolemia
• An alternative to statins for those who are intolerant of statins
• An alternative to statins, period
27
ISIS 301012
Anticipated Initial Product Profile
• Achieves significant reductions in cholesterol via a non-statin mechanism
• Combines safely with statins and ezetimibe
• Enables more patients to reach target lipid levels when combined with statins and ezetimibe
• Doses at convenient intervals of weekly and monthly
• Reduces serum triglycerides
28
ISIS 301012
Anticipated Initial Safety Profile
• No fat accumulation in the liver (steatosis)
• No muscle toxicity (antisense drugs do not get into muscle)
• No CNS toxicity (antisense drugs do not get into the CNS)
• No drug-drug interactions (antisense drugs do not interact with the pathway that metabolizes small molecule drugs)
• No excretion of fat in stool (steatorrhea)
29
ISIS 301012
Cardiovascular Advisory Board
• John Kastelein, MD, Amsterdam
• Thomas Michel, MD, Boston
• Steve Nissen, MD, Cleveland
• Dan Rader, MD, Philadelphia
• Paul Ridker, MD, Boston
• Evan Stein, MD, Cincinnati
• Erik Stroes, MD, Amsterdam
• Steve Young, MD, Los Angeles
• Willis Maddrey, MD, Dallas
• Bruce Bacon, MD, St. Louis
30