UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2011
Commission file number 0-19125
Isis Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
Delaware | | 33-0336973 |
(State or other jurisdiction of
incorporation or organization) | | (IRS Employer Identification No.) |
2855 Gazelle Court, Carlsbad, CA 92010
(Address of principal executive offices, including zip code)
760-931-9200
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, $.001 Par Value
Indicate by check mark whether the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o
Indicate by check mark whether the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer x | | Accelerated filer o |
| | |
Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The approximate aggregate market value of the voting common stock held by non-affiliates of the Registrant, based upon the last sale price of the common stock reported on The NASDAQ Global Select Market was $761,296,183 as of June 30, 2011.*
The number of shares of voting common stock outstanding as of February 21, 2012 was 100,162,511.
DOCUMENTS INCORPORATED BY REFERENCE
(To the extent indicated herein)
Portions of the Registrant’s definitive Proxy Statement to be filed on or about April 23, 2012 with the Securities and Exchange Commission in connection with the Registrant’s annual meeting of stockholders to be held on June 7, 2012 are incorporated by reference into Part III of this Report. The Exhibit Index (Item No. 15) located on pages 73 to 77 incorporates several documents by reference as indicated therein.
* Excludes 16,507,588 shares of common stock held by directors and officers and by stockholders whose beneficial ownership is known by the Registrant to exceed 10% of the common stock outstanding at June 30, 2011. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant, or that such person is controlled by or under common control with the Registrant.
FORWARD-LOOKING STATEMENTS
This report on Form 10-K and the information incorporated herein by reference includes forward-looking statements regarding our business, the therapeutic and commercial potential of our technologies and products in development, and the financial position of Isis Pharmaceuticals, Inc. and Regulus Therapeutics, our jointly-owned subsidiary. Any statement describing our goals, expectations, financial or other projections, intentions or beliefs, including the planned commercialization of KYNAMRO, is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, particularly those inherent in the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs. Our forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause our results to differ materially from those expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in this report on Form 10-K, including those identified in Item 1A entitled “Risk Factors”. Although our forward-looking statements reflect the good faith judgment of our management, these statements are based only on facts and factors currently known by us. As a result, you are cautioned not to rely on these forward-looking statements.
In this report, unless the context requires otherwise, “Isis,” “Company,” “we,” “our,” and “us” refers to Isis Pharmaceuticals and its subsidiaries.
TRADEMARKS
Isis Pharmaceuticals® is a registered trademark of Isis Pharmaceuticals, Inc.
Regulus Therapeutics™ is a trademark of Regulus Therapeutics Inc.
Vitravene® is a registered trademark of Novartis AG
KYNAMRO™ is a trademark of Genzyme Corporation
Macugen® is a registered trademark of Eyetech
CORPORATE INFORMATION
We incorporated in California in 1989, and in January 1991 we changed our state of incorporation to Delaware. Our principal offices are in Carlsbad, California. We make available, free of charge, on our website, www.isispharm.com, our reports on Forms 10-K, 10-Q, 8-K and amendments thereto, as soon as reasonably practical after we file such materials with the Securities and Exchange Commission. Any information that we include on or link to our website is not a part of this report or any registration statement that incorporates this report by reference. You may also read and copy our filings at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-732-0330. The SEC also maintains a website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
2
PART I
Item 1. Business
Overview
We are the leading company in antisense drug discovery and development, exploiting a novel drug discovery platform we created to generate a broad pipeline of first-in-class drugs. Antisense technology provides a direct route from genomics to drugs. With our highly efficient and prolific drug discovery platform we can expand our pipeline and our partners’ pipelines with antisense drugs that address significant medical needs. Our strategy is to do what we do best—to discover unique antisense drugs and develop these drugs to key clinical value inflection points. We discover and conduct early development of new drugs and, at the key clinical value inflection points, outlicense our drugs to partners. We maximize the value of the drugs we discover by putting them in the hands of leading pharmaceutical companies with late-stage development, commercialization and marketing expertise, such as Biogen Idec, Bristol-Myers Squibb, Genzyme, a Sanofi company, GlaxoSmithKline, or GSK, and Eli Lilly and Company. For instance, our partner, Genzyme, plans to commercialize our lead product, KYNAMRO™, following planned regulatory approval in 2012. We also work with a consortium of smaller companies that can exploit our drugs and technologies in areas that are outside of our core focus. As a result of our unique strategy, we can keep our organization small and focused. Our strong financial position is a result of the successful execution of our business strategy as well as our inventive and focused research and development capabilities.
Our flagship product, KYNAMRO (formerly mipomersen), is moving closer to the market for patients with severe forms of familial hypercholesterolemia, or FH, at high cardiovascular risk, who cannot reduce their low-density lipoprotein cholesterol, or LDL-C, sufficiently with currently available lipid-lowering therapies. In July 2011, Genzyme submitted a marketing application in Europe for KYNAMRO for patients with homozygous familial hypercholesterolemia, or hoFH, and severe heterozygous familial hypercholesterolemia, or severe heFH, and plans to submit the U.S. application for marketing approval for patients with hoFH in the first quarter of 2012. Genzyme plans to request priority review, if granted, in the United States and based on estimated approval times, Genzyme is preparing to launch KYNAMRO in the United States and Europe in 2012. Genzyme is also preparing to commercialize KYNAMRO in other major markets.
In addition to KYNAMRO, many of the other drugs in our pipeline are demonstrating encouraging clinical activity in a variety of diseases. In 2011 and early 2012, we and our partners reported positive data on eight drugs, initiated Phase 1 clinical studies on eight drugs and initiated Phase 2 clinical studies on three drugs. For example, we reported data from a positive Phase 1 study of ISIS-APOCIIIRx, a novel triglyceride lowering drug. In this study, we observed reductions of up to 78 percent in apoC-III protein levels and up to 44 percent in triglycerides. Clinicians consider both apoC-III and triglycerides to be independent risk factors for cardiovascular disease, and we believe a drug that significantly reduces both apoC-III and triglycerides could provide important therapeutic benefit to patients with cardiovascular disease. In addition, we have reported positive data on our anti-thrombotic drug that targets Factor XI and our drug that inhibits the production of TTR for the treatment of TTR amyloidosis, a severe and rare disease with limited therapeutic options. These data highlight the broad therapeutic activity of antisense drugs and the power of our antisense technology platform to generate drugs that address significant medical needs.
To maximize the value of our drugs and technologies, we have a multifaceted partnering strategy. We form traditional partnering alliances that enable us to discover and conduct early development of new drugs, outlicense our drugs to partners, such as Genzyme and Eli Lilly and Company, and build a broad base of license fees, milestone payments and royalty income. We also form preferred partner transactions that provide us with a vested partner, such as Biogen Idec and GSK, early in the development of a drug. In this way, we benefit in the short term from upfront option fees and development milestone payments while we maintain control over the early development of the drug. We benefit in the long term by having a knowledgeable and committed partner to license the drug at clinical proof-of-concept and by receiving regulatory milestone payments and royalties as our partner moves the drug to the market. In all of our partnerships, we benefit from the expertise our partners bring to our drugs. We also work with a consortium of smaller companies that can exploit our drugs and technologies. We call these smaller companies our satellite companies. In this way, we benefit from the disease-specific expertise of our satellite company partners, who are advancing drugs in our pipeline in areas that are outside of our core focus. In addition, we can maintain our broad RNA technology leadership through collaborations with companies such as Alnylam Pharmaceuticals, Inc., or Alnylam, and Regulus Therapeutics Inc., or Regulus, a company we jointly own focused on microRNA therapeutics. All of these different types of relationships are part of our unique business model and create near and long-term shareholder value.
The clinical successes of the drugs in our pipeline continue to create new partnering opportunities. For example, in January 2012, we formed a new strategic alliance with Biogen Idec to develop and commercialize ISIS-SMNRx to treat spinal muscular atrophy. We received a $29 million upfront payment and are eligible to receive up to $270 million in payments and double-digit royalties on sales from ISIS-SMNRx. Since 2007, our partnerships have generated an aggregate of more than $880 million in payments from upfront and licensing fees, equity purchase payments, milestone payments and research and development funding. In addition, for our current partnered programs we have the potential to earn more than $3.5 billion in future milestone payments. We also will share in the future commercial success of our inventions and drugs resulting from these partnerships through earn out, profit sharing, or royalty arrangements. Our strong financial position is a result of the successful execution of our business strategy as well as our inventive and focused research and development capabilities.
3
We protect our proprietary technologies and products through our substantial patent estate. As an innovator in RNA-targeting drug discovery and development, we design and execute our patent strategy to provide us with extensive protection for our drugs and our technology. With our ongoing research and development, we continue to add to our substantial patent estate. The patents not only protect our key assets—our technology and our drugs—they also form the basis for lucrative licensing and partnering arrangements. To date, we have generated over $400 million from our intellectual property sale and licensing program that helps support our internal drug discovery and development programs.
Below is a list of some of our key accomplishments for 2011 and early 2012.
2011 and Early 2012 Business Highlights
Drug Development Highlights
· KYNAMRO continues to advance in clinical development and move closer to the market for patients with severe forms of FH, at high cardiovascular risk, who cannot reduce their LDL-C sufficiently with currently available lipid-lowering therapies.
· Genzyme submitted a marketing application for KYNAMRO in Europe for patients with homozygous FH and severe heterozygous FH, and plans to submit a marketing application for KYNAMRO in the United States for patients with homozygous FH in the first quarter of 2012. Genzyme is also preparing to file for marketing approval in markets beyond the United States and Europe.
· Genzyme initiated the FOCUS FH study in FH patients that is designed to support potentially broadening the FH patient population beyond the first indication and support an alternative dosing regimen of three times a week dosing. Genzyme reached an agreement with the Food and Drug Administration, or FDA, on the design of the FOCUS FH study via a Special Protocol Assessment, or SPA.
· A clinical investigator presented data from an open-label extension study in patients treated with KYNAMRO for longer than one year, which demonstrated sustained reductions in all measured atherogenic lipids with a safety profile consistent with the Phase 3 studies.
· Clinical investigators presented data from two studies of KYNAMRO at the 79th European Atherosclerosis Society Congress. The data highlight the potential of KYNAMRO in lowering lipoprotein a, or Lp(a), and potentially reducing the necessity for lipid-apheresis.
· We received more than $23 million from partners in 2011 as our partners advanced drugs in development, including $10 million from GSK for advancing ISIS-TTRRx and selecting ISIS-AATRx as a development candidate.
· We and our partners reported positive clinical data on eight drugs, and added six new drugs to our pipeline.
· We and our partners initiated Phase 1 clinical studies on eight drugs and initiated Phase 2 studies on three drugs.
Corporate Highlights
· We formed a new strategic alliance with Biogen Idec to develop and commercialize ISIS-SMNRx to treat spinal muscular atrophy. We received a $29 million upfront payment and are eligible to receive up to an additional $270 million in a license fee and milestone payments and double-digit royalties on sales of ISIS-SMNRx.
· We received $4.4 million and are eligible to receive up to an additional $9.6 million in milestone payments from the sale of our equity ownership in Excaliard Pharmaceuticals, Inc., or Excaliard, to Pfizer Inc.
· We received Orphan Drug Designation and Fast Track Status in the United States for ISIS-SMNRx for the treatment of spinal muscular atrophy.
· We and CHDI Foundation, Inc. renewed our collaboration to discover and develop an antisense drug for the treatment of Huntington’s Disease.
· We and GSK expanded our collaboration by initiating a sixth program to discover and develop drugs to treat rare and infectious diseases for which we received a $3 million payment from GSK.
· We filed a patent infringement lawsuit against Santaris Pharma based upon Santaris’ commercial activities selling antisense drugs and antisense drug discovery services to several pharmaceutical companies.
Drug Discovery and Development
Introduction to Drug Discovery
Proteins are essential working molecules in a cell. Almost all human diseases result from inappropriate protein production or improper protein activity. Scientists use traditional drug discovery methods to design drugs to interact with the proteins in the body that are supporting or causing a disease. Antisense drugs are different from traditional small molecule drugs because they interrupt the production of disease-causing proteins by targeting ribonucleic acids, or RNAs. RNAs are naturally occurring molecules in the body that provide the information the cell needs to produce proteins. When our antisense drugs bind to the specific RNAs of a particular gene, they will ultimately inhibit or alter the expression of the protein encoded in the target gene.
4
Our Development Projects
We are the leader in the discovery and development of an exciting new class of drugs called antisense drugs. With our proprietary drug discovery platform we can rapidly identify drugs, providing a wealth of potential targets to treat a broad range of diseases. We focus our efforts in therapeutic areas where our drugs will work best, efficiently screening many targets in parallel and carefully selecting the best drugs. When we combine this efficiency with our rational approach to selecting disease targets, we can build a large and diverse portfolio of drugs designed to treat a variety of health conditions, including cardiovascular, metabolic, inflammatory, ocular, severe and rare diseases, and cancer. We and our partners are developing antisense drugs for systemic, intrathecal and local delivery. We expect to continue to bring new drugs into our pipeline, creating opportunities for future licensing transactions and building a broad proprietary portfolio of drugs applicable to many disease targets. We also continue to improve our scientific understanding of our drugs, including how our drugs impact the biological processes of the diseases we target.
With our expertise in discovering and characterizing novel antisense inhibitors, our scientists can optimize the properties of our antisense drugs for use with particular targets. Our scientists have made significant advances in chemistries, which we call our second-generation antisense drugs. Second-generation antisense drugs may have increased potency, stability, oral bioavailability and an improved side effect profile. Recently we selected our generation 2.5 chemistry, an advancement that we believe will further increase the potency of our drugs and make oral administration commercially feasible. We currently have two generation 2.5 drugs in development, ISIS-STAT3Rx and ISIS-FVIIRx, and we expect that some of our future drugs will also incorporate our generation 2.5 chemistry.
Our scientists have utilized our chemistry advancements to expand the therapeutic and commercial opportunities of our pipeline. These advancements, along with shared manufacturing and analytical processes, shorten our timeline from initial concept to the first human dose when compared to small molecule drugs.
The following table lists our approved product and each of our and our partners’ drug development projects, their targets, disease indications and the development status of each. Prior to Phase 3 studies, we identify our drugs by the target, such as ISIS-GCGRRx or ISIS-APOCIIIRx. For the majority of our partnered drugs, we refer to a drug by the partner’s own compound number, such as ATL1103 or iCo-007. As the drugs in our pipeline advance in clinical development, we will adopt nonproprietary names given to each drug from the United States Adopted Names Council. For example, mipomersen is a nonproprietary name that we obtained for ISIS 301012 in 2007. Once we or our partners establish a brand name, like KYNAMRO for mipomersen, we will adopt the brand name even before regulatory agencies grant marketing approval.

5
KYNAMRO (mipomersen sodium)
KYNAMRO is the most advanced drug in our pipeline. We and Genzyme are developing KYNAMRO to treat patients with severe forms of FH, at high cardiovascular risk and who cannot reduce their LDL-C sufficiently with currently available lipid-lowering therapies. KYNAMRO is a novel, first-in-class, apo-B synthesis inhibitor in development for the reduction of LDL cholesterol, or LDL-C. It is a second-generation antisense drug we discovered and licensed to Genzyme in 2008. KYNAMRO acts by decreasing the production of apolipoprotein-B, or apo-B. Apo-B provides the structural core for atherogenic lipids, including LDL-C, which carry cholesterol through the bloodstream. KYNAMRO reduces LDL-C and other key atherogenic lipids linked to cardiovascular disease by preventing their formation.
In July 2011, Genzyme submitted a marketing application for KYNAMRO in Europe for patients with hoFH and severe heFH. Genzyme plans to submit a marketing application for KYNAMRO in the United States for patients with hoFH in the first quarter of 2012. Together with Genzyme, we completed the largest clinical study conducted to date in hoFH patients to support the regulatory submissions in the United States and Europe. In all of our Phase 3 studies, patients treated with KYNAMRO experienced significant reductions in all measures of atherogenic lipids, including LDL-C, Lp(a), and apoB.
Genzyme estimates that there are approximately 40,000 patients with hoFH and severe heFH in Europe and the United States. Due to the large size of the severe heFH population in the United States, the FDA requested additional 12 month clinical data before the KYNAMRO filing for severe heFH in the United States. To address this request, Genzyme is conducting a study titled ‘evaluating the saFety and atherOgeniC lipoprotein redUction of mipomerSen in FH (FOCUS FH)’, which began in 2011. Genzyme designed the study to support potentially broadening the FH patient population beyond the first indication and to support an alternative dosing regimen. Genzyme is also preparing to file for marketing approval in markets beyond the United States and Europe.
Commercialization Strategy
Genzyme is executing a comprehensive plan to address a global commercial market that consists of patients who are in desperate need of new treatment options. Genzyme has substantial expertise in successfully marketing drugs in the U.S. and internationally for severe and rare diseases and plans to leverage its infrastructure in these markets to rapidly launch KYNAMRO upon approval. Along with preparing for an efficient marketing and sales effort for KYNAMRO, Genzyme has made significant progress raising awareness of FH and the importance of family screening to identify patients earlier. These activities include conducting continued medical educational programs to inform physicians about FH and partnering with key advocacy groups, such as the National Lipid Association, American College of Cardiology, European Atherosclerosis Society Congress, International Symposium on Atherosclerosis and the American Heart Association.
Genzyme plans to concentrate marketing and sales efforts on lipid specialists and physicians who refer patients to these specialists to quickly reach the initial patient populations for KYNAMRO in the United States and Europe. Genzyme has also established the foundation for market access and reimbursement in the United States and Europe to accelerate market penetration after launch. Genzyme is planning to launch KYNAMRO with a prefilled syringe that will provide a convenient form of administration. We believe that Genzyme has the commercial infrastructure and aptitude to successfully commercialize KYNAMRO worldwide making the drug available for patients in need. In 2011, Sanofi acquired Genzyme, and we believe that Sanofi and its global presence will aid in the rapid market expansion of KYNAMRO throughout the world.
Familial Hypercholesterolemia
Physicians diagnose patients as having FH if they have very high cholesterol, are at high cardiovascular risk and cannot reduce their LDL-C sufficiently with currently available lipid-lowering therapies. FH is a genetic disease that causes elevated LDL-C levels and family patterns of increased risk of premature heart disease and heart disease-related death. FH patients have inherited abnormalities in liver cells that are responsible for clearing LDL particles from the blood. FH is autosomal dominant, which means that all first-degree relatives of FH patients have a 50 percent chance of having the disease as well, making early detection through early screening critically important. Patients with untreated FH have a 50 percent mortality rate by age 60.
The most severe FH patients have LDL-C levels that are two to four times higher than recommended levels, even when taking multiple cholesterol-lowering medications. These people, who clinicians characterize as having severe FH, include: those who have inherited the disease from both parents, hoFH, and those who have inherited the disease from only one parent and have a severe form of the disease, severe heFH. HoFH is a rare form of FH. HoFH patients can have untreated LDL-C levels greater than 600 mg/dL or treated LDL-C levels greater than 300 mg/dL and are at very high risk for early coronary events and sudden death. Severe heFH patients comprise a small subset of heterozygous FH patients and these patients have LDL-C levels greater than 200 mg/dL with coronary artery disease or more than 300 mg/dL without coronary artery disease despite maintaining a regimen of maximally tolerated lipid-lowering therapy. For these severe FH patients, remaining options are limited and may include apheresis and liver transplant. Apheresis is a two to four hour process administered two to four times a month that mechanically separates LDL-C from the blood and it is the only therapy available on top of maximally tolerated lipid-lowering therapy.
6
Phase 3 Summary
Together with Genzyme, we have evaluated KYNAMRO in four Phase 3 studies, which met all primary, secondary and tertiary endpoints. In all four Phase 3 studies, treatment with KYNAMRO lowered LDL-C and reduced other atherogenic lipids, including apo-B, Lp(a), triglycerides and very-low density lipoprotein, or VLDL. These key lipids are generally accepted risk factors for cardiovascular disease. Two of our Phase 3 studies evaluated KYNAMRO in patients with hoFH and severe hypercholesterolemia who were on substantial lipid lowering therapy. In both of these studies, the average reduction of LDL-C was greater than 100 mg/dL.
Phase 3 Study | | Average
Baseline
LDL-C
(mg/dL) | | Average
LDL-C
Reduction
(mg/dL) | | Placebo %
Change in
LDL-C | | KYNAMRO
% Change in
LDL-C | | | |
Homozygous FH | | 426 | | -106 | | -3.3 | | -24.7 | | | |
Severe Hypercholesterolemia | | 276 | | -101 | | +13 | | -36 | | | |
In the two additional Phase 3 studies, we evaluated KYNAMRO in patients with heFH and in patients with high cholesterol at high risk for coronary heart disease who were on substantial lipid lowering therapy.
Phase 3 Study | | Average
Baseline
LDL-C
(mg/dL) | | Average
LDL-C
Reduction
(mg/dL) | | Placebo %
Change in
LDL-C | | KYNAMRO
% Change in
LDL-C | | | |
Heterozygous FH | | 153 | | -46 | | +5 | | -28 | | 45% of patients achieved LDL-C levels of less than 100 mg/dL | |
High-Risk | | 123 | | -48 | | -5 | | -37 | | 51% of patients achieved LDL-C levels of less than 70 mg/dL | |
In all studies, frequently observed adverse events were injection site reactions, flu-like symptoms and elevations in liver transaminases. In our Phase 3 experience eight percent of patients treated with KYNAMRO had persistent ALT elevations above three times the upper limit of normal. We believe the lipid-lowering profile together with the emerging safety profile of KYNAMRO will support our initial plan for KYNAMRO to treat patients who, despite using maximally tolerated lipid-lowering therapies, are far from their recommended LDL-C goal. We now have treated more than 110 patients with KYNAMRO for longer than a year, some for more than two years, in an open label extension study. In this study, we observed sustained reductions in LDL-C and all other measured atherogenic lipids with no evidence of liver toxicity, consistent with our Phase 3 experience. Preliminary data from the open label extension study suggest liver fat may stabilize or decline in patients who continue treatment beyond a year. In general, increases in ALT levels and liver fat appear to be associated with rapid and larger drops in LDL-C.
In summary, the performance of KYNAMRO has been consistent across the entire Phase 3 program, supporting our initial market focus in patients, who despite treatment with maximally tolerated lipid-lowering therapies, are far from their recommended LDL-C goal and as a result are at high risk of a cardiovascular event or death.
Cardiovascular Franchise
Cardiovascular disease is the leading cause of death in the United States. A common cause of cardiovascular disease is atherosclerosis, or premature plaque buildup, which occurs when cholesterol and inflammatory cells accumulate in blood vessels. Researchers have shown a strong correlation between high cholesterol levels and subsequent cardiovascular diseases. As such, lowering cholesterol is a key component in preventing and managing cardiovascular disease.
Cardiovascular disease is an area of focus for us. We have created a cardiovascular disease franchise comprised of drugs that target all the key components of cardiovascular disease, including various atherogenic lipids, inflammation and thrombosis, an aberrant blood clot formation responsible for most heart attacks and strokes. For example, we are developing a drug that lowers apoC-III and triglycerides, which are both independent risk factors for cardiovascular disease. Our most recent addition to our cardiovascular franchise is our drug that lowers Lp(a), another independent risk factor for cardiovascular disease. Currently available lipid-lowering therapies do not significantly lower apoC-III, triglycerides, or Lp(a). We believe that targeting apoC-III and Lp(a) could provide a complementary approach to lipid-lowering therapies, including KYNAMRO. We are also developing a drug that lowers C-reactive protein, or CRP, a protein that scientists associate with cardiovascular disease. And finally, we expanded our cardiovascular franchise to include two anti-thrombotic agents, which could offer safer more effective alternatives to anti-clotting agents currently on the market.
7
We believe antisense drugs could have a significant positive effect in patients with high cardiovascular risk. Because there are many liver-produced targets that affect the production of cholesterol particles, clotting factors and other factors that contribute to the inflammatory components of cardiovascular disease, the liver is an ideal target organ for cardiovascular disease therapies, and antisense drugs in particular. Our antisense drugs distribute to the liver and inhibit the production of many targets associated with cardiovascular risk, creating an opportunity for us to develop many complementary and effective antisense drugs for cardiovascular disease.
ISIS-CRPRx — ISIS-CRPRx is an antisense drug that targets CRP, a protein produced in the liver. CRP levels increase dramatically during inflammatory disorders, and scientists have linked excessive amounts of CRP to coronary artery disease. Furthermore, a growing body of evidence from clinical trials implicates CRP in cardiovascular disease progression. These results suggest that it may be therapeutically beneficial to significantly decrease CRP levels in patients who are at risk for coronary events. In addition, clinicians have associated elevated CRP levels with a worsening of overall outcomes in conditions such as end-stage renal disease and multiple myeloma, suggesting that lowering CRP could help these patients. CRP elevation is also evident in many other major inflammatory diseases such as Crohn’s disease and rheumatoid arthritis.
In preclinical studies, we observed that our antisense inhibitor of CRP suppressed liver and serum CRP levels. We evaluated ISIS-CRPRx in a Phase 1 study in which ISIS-CRPRx produced statistically significant reductions in CRP in the cohort of subjects that entered the study with elevated levels of CRP. All subjects tolerated ISIS-CRPRx well. Our Phase 2 plan for ISIS-CRPRx is to evaluate the drug in diseases with elevated CRP that could provide early proof-of-concept.
We are currently evaluating ISIS-CRPRx in a Phase 2 study in patients with rheumatoid arthritis and plan to initiate an additional Phase 2 study in patients with atrial fibrillation in 2012. Atrial fibrillation involves an irregular heart rate that commonly causes poor blood flow to the body.
ISIS-APOCIIIRx — ISIS-APOCIIIRx is an antisense drug we designed to reduce apolipoprotein C-III, or apoC-III, protein production and lower triglycerides. ApoC-III is responsible for triglyceride transport in the blood and is a pro-inflammatory protein and an independent cardiovascular risk factor. People who do not produce apoC-III have lower levels of triglycerides and lower instances of cardiovascular disease. ApoC-III is elevated in patients with dyslipidemia, or an abnormal concentration of lipids in the blood, and is frequently associated with multiple metabolic abnormalities, such as insulin resistance and/or metabolic syndrome. In human population studies, lower levels of apoC-III and triglycerides correlated with a lower rate of cardiovascular events. In certain populations, apoC-III mediates insulin resistance, which can make metabolic syndrome worse.
In preclinical studies, ISIS-APOCIIIRx diminished symptoms of metabolic syndrome and reduced atherosclerosis in mice. We have completed a Phase 1 study evaluating the safety and activity of ISIS-APOCIIIRx in healthy volunteers. In this study, ISIS-APOCIIIRx produced rapid, dose-dependent reductions of up to 78 percent in apoC-III protein and up to 44 percent in blood triglycerides. All subjects tolerated ISIS-APOCIIIRx well.
We plan to initiate a Phase 2 study evaluating the efficacy of ISIS-APOCIIIRx in treatment-naïve patients with very high triglycerides, greater than 500 mg/dL, in 2012.
ISIS-FXIRx — ISIS-FXIRx is an antisense drug we designed to treat clotting disorders. It targets Factor XI, a clotting factor produced in the liver that is an important component of the coagulation pathway. High levels of Factor XI increase the risk of thrombosis, a process involving aberrant blood clot formation responsible for most heart attacks and strokes. Elevated levels of Factor XI also increase the risk of venous thrombosis, a common problem after surgery, particularly major orthopedic procedures, such as knee or hip replacement. People who are deficient in Factor XI have a lower incidence of thromboembolic events with minimal increase in bleeding risk. Although currently available anticoagulants reduce the risk of thrombosis, physicians associate these anticoagulants with increased bleeding, which can be fatal.
In preclinical studies, ISIS-FXIRx demonstrated potent antithrombotic activity with no increase in bleeding compared with standard anti-clotting agents, including low molecular weight heparin, warfarin and Factor Xa inhibitors, all of which increase bleeding. We have completed a Phase 1 study evaluating the safety and activity of ISIS-FXIRx in healthy volunteers. In this study, ISIS-FXIRx produced dose-dependent statistically significant reductions of greater than 80 percent in Factor XI protein. Subjects tolerated ISIS-FXIRx well with no increase in bleeding.
We plan to initiate a Phase 2 study evaluating the efficacy of ISIS-FXIRx in 2012 in patients undergoing total knee replacement surgery.
ISIS-APOARx — ISIS-APOARx is an antisense drug we designed to reduce apolipoprotein(a) in the liver to offer a direct approach for reducing Lp(a), an independent risk factor for cardiovascular disease. Scientists associate high levels of Lp(a) with an increased risk of atherosclerosis, coronary heart disease, heart attack and stroke. Commonly prescribed lipid-lowering drugs have little or no effect on Lp(a) levels. Even patients who can control their LDL-C levels remain at high-risk of cardiovascular events if they have high levels of Lp(a). There is a significant need for a highly specific drug that can lower Lp(a). We plan to develop ISIS-APOARx to treat patients with high Lp(a) levels who are at severe risk of experiencing cardiovascular events.
We plan to conduct preclinical studies to support an investigational new drug application for ISIS-APOARx in 2012.
8
ISIS-FVIIRx — ISIS-FVIIRx is an antisense drug we designed to reduce Factor VII, a key component of the tissue factor coagulation pathway, for the treatment or prevention of thrombotic diseases. Clinicians have linked elevated levels of Factor VII activity with poor prognosis in several thrombotic diseases, such as heart attacks, and with cancer-associated thrombosis, which is the second leading cause of death in cancer patients.
In preclinical studies, antisense inhibition of Factor VII rapidly reduced Factor VII activity by more than 90 percent in three days, suggesting that physicians could use ISIS-FVIIRx in acute clinical settings, such as following surgery, to prevent patients from developing harmful blood clots. In addition, we observed no increase in bleeding with ISIS-FVIIRx, which is a common side effect of currently available anti-thrombotic drugs. ISIS-FVIIRx is the second drug to enter development as part of our strategy to create more potent and safer anti-thrombotic drugs that do not increase bleeding.
We plan to conduct preclinical studies to support an investigational new drug application for ISIS-FVIIRx in 2012.
Metabolic Franchise
Metabolic disorders are chronic diseases that affect millions of people. There is still a significant need for new therapies for these patients. According to the Centers for Disease Control and Prevention, diabetes affects more than 25 million people in the United States, or eight percent of the population, with type 2 diabetes constituting 90 to 95 percent of those cases.
Metabolic disease is a very large area of medical need and is another area in which we focus our drug discovery efforts. We now have three drugs in our pipeline to treat type 2 diabetes, each of which acts upon targets in the liver or fat tissue through a distinct mechanism to improve insulin sensitivity, reduce glucose production, or affect other metabolic aspects of this complex disease. We plan to keep building our metabolic disease franchise and we are expanding our focus beyond type 2 diabetes to obesity and nonalcoholic steatohepatitis, or NASH. NASH is a common and often asymptomatic liver disease that can cause irreversible damage to the liver, and lead to liver cirrhosis and cancer. There is significant need to reduce liver fat in patients with metabolic disease because these patients can develop NASH if they accumulate too much fat in their liver.
Our approach is to develop antisense drugs that doctors can add to existing therapies to treat diabetes and obesity. One hurdle for traditional drug development is that most traditional drugs cannot selectively target a disease-causing protein without also affecting closely related proteins, which often results in unwanted side effects. We design our antisense drugs to target the gene responsible for producing the disease-causing protein while avoiding unwanted effects on a closely related protein, thereby reducing the risk of side effects. In addition, the liver and fat cells produce many of the most important therapeutic targets for metabolic disease, and our antisense drugs distribute to the liver and fat cells and inhibit the production of key therapeutic targets in these organs.
ISIS-FGFR4Rx — ISIS-FGFR4Rx is an antisense drug that specifically reduces the production of fibroblast growth factor receptor 4, or FGFR4, in the liver and fat tissues, which decreases the body’s ability to store fat while simultaneously increasing fat burning and energy expenditure. Many anti-obesity drugs act in the brain to suppress appetite, commonly resulting in CNS side effects. However, ISIS-FGFR4Rx does not distribute to the brain or CNS and therefore should not produce any CNS side effects.
In preclinical studies, antisense inhibition of FGFR4 lowered body weight when we administered it as a single agent and in the presence or absence of a calorie-restricted diet. Additionally, inhibiting FGFR4 decreased body weight when we administered it in combination with an appetite-suppressing drug. In addition to reducing body weight, inhibiting FGFR4 demonstrated an improvement in insulin sensitivity. ISIS-FGFR4Rx is the first drug in our metabolic franchise to treat obesity and utilizes technology we in-licensed from Verva Pharmaceuticals Ltd.
We are currently evaluating ISIS-FGFR4Rx in a Phase 1 study in healthy volunteers and plan to complete this study in 2012.
9
ISIS-GCCRRx — ISIS-GCCRRx is an antisense drug that targets the glucocorticoid receptor, or GCCR. Glucocorticoid hormones effect a variety of processes throughout the body, including promoting liver glucose production and fat storage. Although scientists have long recognized inhibiting GCCR as an attractive strategy for developing therapeutics for type 2 diabetes, the side effects associated with systemic GCCR inhibition have challenged traditional drug developers. Antisense inhibitors of GCCR take advantage of the unique tissue distribution of oligonucleotides that allows the antisense drugs to inhibit glucocortocoid action primarily in liver and fat tissue. Notably, antisense drugs delivered systemically do not reduce GCCR expression in the central nervous system, or CNS, or adrenal glands, which could lead to systemic side effects. Reducing GCCR specifically in the liver and fat tissues is an attractive therapeutic approach because it lowers glucose and lipids, without causing potential side effects associated with systemic GCCR inhibition.
In preclinical studies, we showed that we can reduce GCCR specifically in the liver and fat tissues. In addition, we have shown that antisense inhibition of GCCR produced robust lowering of blood glucose, lipid levels and decreased body fat in obese animals. Therefore, we believe that doctors could use ISIS-GCCRRx in diabetic patients with moderate to severe hypoglycemia.
We are currently evaluating ISIS-GCCRRx in a Phase 1 study in healthy volunteers and plan to complete this study in 2012.
ISIS-GCGRRx — ISIS-GCGRRx is an antisense drug that targets the glucagon receptor, or GCGR, to reduce the effects of glucagon. Glucagon is a hormone that opposes the action of insulin and stimulates the liver to produce glucose, particularly in type 2 diabetes. In addition, reducing GCGR produces more active glucagon-like peptide, or GLP-1, a hormone that preserves pancreatic function and enhances insulin secretion. Therefore, we believe we can help control type 2 diabetes by using an antisense drug to reduce GCGR which will stop the liver from producing too much glucose while preserving pancreatic function.
We conducted preclinical studies in the most insulin-resistant models of type 2 diabetes. In these studies, antisense reduction of GCGR decreased excessive liver glucagon action, produced robust glucose control, reduced levels of triglycerides and helped preserve the pancreas without producing hypoglycemia.
Given the dual-action mechanism of ISIS-GCGRRx, we believe doctors could use this drug in diabetic patients with severe hyperglycemia who are not controlled with current treatments and who would benefit from a drug that significantly decreases glucose levels without producing hypoglycemia. We are currently evaluating ISIS-GCGRRx in a Phase 1 study in healthy volunteers and plan to complete this study in 2012.
ISIS-PTP1BRx — ISIS-PTP1BRx is an antisense drug that targets protein tyrosine phosphatase-1B, or PTP-1B, to treat type 2 diabetes. PTP-1B is a phosphatase that negatively regulates insulin receptor signaling and is responsible for turning off the activated insulin receptor. Reducing PTP-1B enhances insulin activity. Scientists have long recognized PTP-1B as an attractive target to treat diabetes, but due to structural similarities among closely related proteins, pharmaceutical companies have had difficulty identifying small molecule drugs with sufficient specificity to be safe. We designed ISIS-PTP1BRx to increase the body’s sensitivity to the natural hormone insulin, resulting in better glucose control for patients with type 2 diabetes. Because of its unique mechanism, ISIS-PTP1BRx may help treat type 2 diabetes without causing weight gain or hypoglycemia, also known as low blood sugar. The reductions in LDL-C produced by inhibiting PTP-1B should also provide an added benefit to patients.
Phase 2 studies of ISIS 113715 showed that inhibiting PTP-1B could help patients with type 2 diabetes. In those studies, inhibiting PTP-1B improved glucose control and reduced LDL-C in both newly diagnosed diabetic patients and in patients who were taking sulfonylureas. The patients in these studies also did not gain weight, indicating another substantial advantage in treating diabetic patients who are frequently obese and at high cardiovascular risk. The data from these studies support the development of ISIS-PTP1BRx and should allow a rapid route to clinical proof-of-concept.
We believe that physicians may use ISIS-PTP1BRx in combination with most of the other commonly used drugs to treat patients with diabetes, including insulin, GLP-1 agonists, and more traditional drugs like metformin. The clinical development plan for ISIS-PTP1BRx focuses on treating diabetic patients who are inadequately controlled on insulin, helping them utilize insulin more efficiently and treating patients who are beginning to fail oral therapies, extending the time they have before becoming dependent on insulin. We are evaluating ISIS-PTP1BRx in a Phase 1 study in healthy volunteers. We plan to complete the Phase 1 study of ISIS-PTP1BRx and begin a Phase 2 study in 2012.
ISIS-DGAT2Rx — ISIS-DGAT2Rx is an antisense drug that specifically reduces the production of diacylglycerol acyltransferase-2, or DGAT-2, a key component in the synthesis of triglycerides. By reducing DGAT2, ISIS-DGAT2Rx should reduce liver fat in patients with NASH, a common and often asymptomatic liver disease that can cause irreversible damage to the liver, and lead to liver cirrhosis and cancer. The National Institutes of Health, or NIH, estimates that NASH affects more than 20 million people in the United States and expects the number to increase as the rate of obesity rises. There are no effective therapies available for patients with NASH and current treatments consist only of lifestyle changes. In addition, because clinicians associate increases in liver fat with insulin resistance, ISIS-DGAT2Rx could also benefit patients with type 2 diabetes who are insulin resistant.
10
We plan to conduct preclinical studies to support an investigational new drug application for ISIS-DGAT2Rx in 2012.
Cancer Franchise
We are discovering and developing antisense drugs to treat cancers both internally and through our partnerships with Eli Lilly and Company and OncoGenex Technologies Inc. Cancer is an area of significant unmet medical need and an area in which our antisense technology provides us with unique advantages in discovering new drugs. Cancer is an extremely complex disease that involves a large number of targets. With our technology we can evaluate a very broad and diverse range of targets and identify their involvement in different types of cancers. Using the information we gain early in research on each of these targets, we can quickly identify the most promising targets for an anti-cancer drug. We select anti-cancer targets that provide a multi-faceted approach to treating cancer. We develop drugs to targets that are directly involved in cancer growth, migration or survival, such as our STAT3 drug. We also evaluate drugs to targets that are indirectly linked to cancer growth, such as through inflammatory processes. For example, patients with multiple myeloma with elevated levels of the inflammatory marker CRP generally have worse outcomes. We designed a drug to reduce CRP, which we plan to evaluate in multiple myeloma and other diseases.
Our cancer pipeline consists of anti-cancer antisense drugs that act upon biological targets associated with cancer progression and/or treatment resistance. Over the past couple of years, our partners presented encouraging clinical data on a number of our antisense drugs, including positive Phase 2 overall median survival data for OGX-011 and positive Phase 1 data on LY2181308 and OGX-427. We discovered or co-discovered these antisense drugs and licensed them to our partners to treat multiple types of cancer.
We believe the favorable tolerability and early evidence of clinical benefit of the anti-cancer drugs in our pipeline demonstrate how uniquely suited our technology is to create novel cancer therapeutics. Because of the early evidence of clinical benefit, cancer is one of our core therapeutic areas and an area in which we are expanding our efforts to include new targets and new treatments.
OGX-011 — OGX-011, now under license to Teva Pharmaceutical Industries Ltd., or Teva, is a second-generation antisense drug that targets clusterin, a secreted protein that acts as a cell-survival protein and is over-expressed in response to anti-cancer agents. We and OncoGenex jointly discovered and conducted the initial development of OGX-011. In December 2009, OncoGenex licensed OGX-011 to Teva as part of a global license and collaboration agreement to develop and commercialize OGX-011. Teva is studying OGX-011 for use as an adjunct therapy to enhance the effectiveness of chemotherapy. OGX-011 has shown promising results in combination with currently available chemotherapies in several tumor types. The FDA granted OGX-011 two Fast Track Designations as a treatment in combination with first-line and second-line docetaxel for progressive metastatic prostate cancer.
OncoGenex evaluated OGX-011 in five Phase 2 studies in combination with various cancer therapies for prostate cancer, non-small cell lung cancer, or NSCLC, and breast cancer. OncoGenex reported results from a randomized Phase 2 study of OGX-011 in patients with advanced metastatic castrate resistant prostate cancer, or CRPC. In this study, OncoGenex reported a median overall survival of 23.8 months in patients treated with OGX-011 plus docetaxel compared to 16.9 months for patients treated with docetaxel alone. In addition, OncoGenex reported that the unadjusted hazard ratio, a measure used to determine the difference in survival between treatment groups, was 0.61, representing a 39 percent reduction in the rate of death for patients treated with OGX-011. OncoGenex also reported that patients treated with OGX-011 in combination with docetaxel tolerated OGX-011 well.
OncoGenex has also evaluated OGX-011 in a Phase 1/2 combination study in patients with NSCLC. In January 2012, OncoGenex reported that one- and two-year survival rates were 54 percent and 30 percent, respectively, and 12 percent of patients were still alive at a median follow-up of 41 months. The median overall survival was 14.1 months and progression-free survival was 4.3 months.
Teva and OncoGenex are collaborating on a global Phase 3 clinical program in patients with advanced prostate cancer and advanced NSCLC. In 2010, OncoGenex and Teva initiated two Phase 3 clinical studies of OGX-011 in patients with prostate cancer. These studies include a Phase 3 study for second-line chemotherapy in patients with CRPC and a Phase 3 study for first-line chemotherapy in patients with metastatic CRPC. OncoGenex also announced that, together with Teva, it intends to initiate an additional Phase 3 study of OGX-011 as first-line treatment in patients with advanced, inoperable NSCLC.
ISIS-EIF4ERx — ISIS-EIF4ERx targets the gene that is responsible for producing the protein eukaryotic initiation factor-4e, or eIF-4E, which cells over-express in a variety of cancers, including prostate, lung, ovarian, liver, breast, head and neck, bladder, colon, thyroid and lymphoma. eIF-4E facilitates the synthesis of factors in the body that support the development, growth, progression and survival of cancer. In preclinical studies, we and collaborators demonstrated marked anti-cancer activity in a broad range of animal models of cancer and provided the first in vivo evidence that tumor growth may be more susceptible to eIF-4E inhibition than growth of normal tissue. Targeting eIF-4E has been of great interest to the pharmaceutical industry and the oncology community. However the pharmaceutical industry considers eIF-4E a difficult protein to target with traditional pharmaceutical approaches.
Eli Lilly and Company completed a Phase 1 study of ISIS-EIF4ERx in patients with cancer that showed that the subjects tolerated the drug well at doses up to 1200 mg per week. Eli Lilly and Company has rights to license ISIS-EIF4ERx from us on predefined terms.
In 2010, we initiated a Phase 2 program of ISIS-EIF4ERx in patients with NSCLC and prostate cancer. The endpoints for both studies include progression-free survival, overall survival, response rates, time to progression and the reduction of a variety of biomarkers. We plan to expand our Phase 2 program over time to evaluate ISIS-EIF4ERx in other cancers that over-express eIF-4E.
LY2181308 — LY2181308 is an antisense drug that targets survivin, which plays a role in cancer cell death and is one of the most commonly over expressed proteins in cancers. We licensed our anti-cancer drug, LY2181308, to Eli Lilly and Company as part of the companies’ antisense drug discovery research collaboration in cancer. The researchers involved in this collaboration have shown that using LY2181308 to inhibit the expression of survivin inhibits the growth of cancer cells. Since normal cells in the body do not express survivin, we expect that this drug may have fewer side effects than traditional chemotherapy.
11
Eli Lilly and Company is evaluating LY2181308 as a combination therapy in two separate randomized Phase 2 studies. The first study is in patients with advanced metastatic NSCLC who failed first line chemotherapy treatment, and the second study is in patients with hormone refractory prostate cancer who received docetaxel for first line chemotherapy. In addition, Eli Lilly and Company has completed a proof-of-concept Phase 2a study in patients with refractory acute myeloid leukemia. Eli Lilly and Company plans to complete its Phase 2 studies in patients with cancer in 2012.
OGX-427 — OGX-427 is a second-generation antisense drug targeting heat shock protein 27, or Hsp27, which is a cell survival protein that cells over produce in response to many cancer treatments, including hormone ablation therapy, chemotherapy and radiation therapy. Studies have shown that increased Hsp27 production is prevalent in many human cancers, including prostate, NSCLC, breast, ovarian, bladder, renal, pancreatic, multiple myeloma and liver cancers. Studies have also linked increased Hsp27 production to faster rates of cancer progression, treatment resistance and shorter survival duration.
OncoGenex is evaluating OGX-427 in patients with cancer. In June 2010, OncoGenex reported results from a Phase 1 study of OGX-427 in patients with a variety of cancers. In this study, patients treated with OGX-427 as a monotherapy and in combination with docetaxel tolerated the drug well. In addition, OGX-427, when used as a single agent, demonstrated declines in circulating tumor cells at all doses and in all types of cancer OncoGenex evaluated. OGX-427 also demonstrated evidence of reduction in tumor markers defined as declines of prostate-specific antigen, or PSA, levels in prostate cancer and cancer-antigen-125 levels in ovarian cancer.
In February 2012, OncoGenex reported preliminary results from a Phase 1 study in patients with superficial bladder cancer. In this study, OncoGenex reported that treatment with OGX-427 resulted in a trend towards decreased levels of Hsp27 and increased tumor cell death rates.
OncoGenex is also evaluating OGX-427 in two Phase 2 studies, one in men with metastatic CRPC and one as a first-line treatment for metastatic bladder cancer. In February 2012, OncoGenex reported preliminary results from a Phase 2 study in patients with CRPC. In this study, OncoGenex reported that treatment with OGX-427 in combination with prednisone resulted in a higher number of patients without disease progression at 12 weeks and greater declines in prostate-specific antigen, or PSA, and circulating tumor cells compared to patients treated with prednisone alone.
OncoGenex plans to initiate a Phase 2 study of OGX-427 in combination with abiraterone in men with CRPC.
ISIS-STAT3Rx — We designed ISIS-STAT3Rx to treat cancer by inhibiting the production of a gene critical for tumor cell growth and survival. Signal transducer and activator of transcription 3, or STAT3, is over-active in a variety of cancers, including brain, lung, breast, bone, liver and multiple myeloma and promotes tumor cell growth and prevents cell death.
In preclinical studies, ISIS-STAT3Rx demonstrated antitumor activity in animal models of human cancer with an attractive safety profile. We plan to evaluate ISIS-STAT3Rx in a variety of cancers in which scientists believe STAT3 plays a key role, such as liver cancers and multiple myeloma. We plan to begin a Phase 1 study evaluating ISIS-STAT3Rx in 2012.
12
Severe & Rare Disease Franchise
We are pursuing the discovery and development of antisense drugs for severe and rare diseases in which there is a need for new treatment options. According to the NIH there are approximately 5,000 to 8,000 rare diseases, many life-threatening or fatal. Unfortunately there are few effective therapeutics available to treat many of these severe and rare diseases. Since most severe and rare diseases are genetic or have a genetic component, parents often pass the disease to their children, creating a legacy of the disease and resulting in profound effects on the family. In some cases, disease onset is characterized by the presence of a protein that, through a genetic defect, cannot function properly in the cell.
We are focused on developing antisense drugs to treat severe and rare diseases. Due to the severe nature of these diseases and the lack of available treatments, there is an opportunity for more flexible development paths to the market. This means that, in many cases, the studies necessary for us to demonstrate proof-of-concept with a particular drug may also be the studies that complete our marketing registration package. This allows us a relatively rapid path to market with potential new treatments for devastating and often fatal diseases.
ATL1103 — ATL1103 is an antisense drug that targets the growth hormone receptor, or GHr, a receptor that, when inhibited, reduces the level of circulating insulin-like growth factor-1, or IGF-1, produced in the liver. IGF-1 is a hormone that contributes to various diseases, including acromegaly, an abnormal growth disorder of organs, face, hands and feet, as well as diabetic retinopathy, a common disease of the eye and a leading cause of blindness, diabetic nephropathy of the kidney and certain forms of cancer. In preclinical studies, ATL1103 demonstrated significant reductions in IGF-1 levels in the blood and inhibition of neovascularization, or new blood vessels, in the eye in a mouse retinopathy model.
Antisense Therapeutics Limited, or ATL, is developing ATL1103. ATL has completed a Phase 1 study in healthy volunteers demonstrating that ATL1103 was safe and well tolerated and plans to evaluate ATL1103 in a Phase 2 study in patients with acromegaly in 2012.
ISIS-SMNRx — ISIS-SMNRx is an antisense drug we designed to treat spinal muscular atrophy, or SMA, a severe motor-neuron disease that is the leading genetic cause of infant mortality. SMA affects approximately 30,000 to 35,000 patients in the United States, Europe and Japan. One in 50 people, approximately six million people in the United States, are carriers of the SMA gene. Carriers experience no symptoms and do not develop the disease. When both parents are carriers, however, there is a one in four chance that their child will have SMA.
SMA is caused by a loss of, or defect in, the survival motor neuron 1, or SMN1, gene leading to a decrease in the protein, survival motor neuron, or SMN. SMN is critical to the health and survival of nerve cells in the spinal cord that are responsible for neuro-muscular growth and function. The severity of SMA correlates with the amount of SMN protein. Infants with Type I SMA, the most severe life-threatening form, produce very little SMN protein and have a shortened life expectancy. Children with Type II and Type III SMA have greater amounts of SMN protein and have less severe, but still life-altering, forms of SMA.
We designed ISIS-SMNRx to potentially treat all types of childhood SMA by altering the splicing of a closely related gene, SMN2, that leads to the increased production of fully functional SMN protein. The FDA granted Orphan Drug Designation with Fast Track Status to ISIS-SMNRx for the treatment of patients with SMA. In December 2011, we initiated the first Phase 1 clinical study evaluating ISIS-SMNRx in children with SMA. The Phase 1 study of ISIS-SMNRx is a single-dose, dose-escalation study we designed to assess the safety, tolerability and pharmacokinetic profile of the drug in children with SMA between the ages of two and 14 who are medically stable. In this study, the study physicians will administer ISIS-SMNRx intrathecally as a single injection directly into the spinal fluid. We plan to complete this study in 2012.
In January 2012, we and Biogen Idec entered into a preferred partner alliance that provides Biogen Idec an option to develop and commercialize ISIS-SMNRx. Under the agreement, we received an upfront fee and are responsible for developing ISIS-SMNRx. Biogen Idec has the option to license ISIS-SMNRx upon completion of the earlier of the first successful Phase 2/3 study, or the completion of two Phase 2/3 studies. We will receive milestone payments from Biogen Idec as ISIS-SMNRx advances through development. We acknowledge support from the following organizations for this program: Muscular Dystrophy Association, SMA Foundation, and Families of Spinal Muscular Atrophy. We have licensed intellectual property from Cold Spring Harbor Laboratory and the University of Massachusetts Medical School.
ISIS-SOD1Rx — ISIS-SOD1Rx is an antisense drug that targets superoxide dismutase, or SOD1, a protein associated with an inherited, aggressive form of amyotrophic lateral sclerosis, or ALS. The FDA granted ISIS-SOD1Rx Orphan Drug designation for the treatment of ALS. Because antisense drugs do not cross the blood-brain barrier, we can administer the drug directly into the cerebral spinal fluid. Clinicians call this type of administration intrathecal injection and commonly use this route of administration for a variety of drugs.
Researchers reported in the Journal of Clinical Investigation that treatment with ISIS-SOD1Rx prolonged life in rats that showed many symptoms of ALS. By delivering our drug directly to the fluid that circulates within the CNS, we and our collaborators lowered production of the mutant protein in neurons and surrounding cells.
The ALS Association and the Muscular Dystrophy Association provided funding for ISIS-SOD1Rx. Additionally, as part of our alliance with Genzyme, Genzyme has a right of first negotiation to license ISIS-SOD1Rx from us. We are evaluating ISIS-SOD1Rx in a Phase 1 study in patients with the familial form of ALS and plan to report data from this study in 2012.
13
ISIS-TTRRx — ISIS-TTRRx is an antisense drug we designed to treat transthyretin amyloidosis, or TTR amyloidosis, a severe and rare genetic disease in which the patient inherits a mutant gene that produces a misfolded form of TTR, which progressively accumulates in tissues. In patients with TTR amyloidosis, both the mutant and normal forms of TTR can build up as fibrils in tissues, including heart, peripheral nerves, and the gastrointestinal tract. The presence of TTR fibrils interferes with the normal functions of these tissues, and as the TTR protein fibrils enlarge more tissue damage occurs and the disease worsens.
There are two common types of TTR amyloidosis, familial amyloid cardiomyopathy, or FAC, which affects more than 40,000 patients worldwide, and familial amyloid polyneuropathy, or FAP, which affects more than 10,000 patients worldwide. Patients with FAC have TTR build up in the heart muscle and succumb to heart failure approximately five to six years after symptom onset. Patients with FAP have TTR build up in peripheral nerve tissue leading to the loss of nerve function and wasting.
We designed ISIS-TTRRx to inhibit the production of all forms of TTR, and to offer an alternative approach to treat all types of TTR-related amyloidosis. ISIS-TTRRx is the first drug to enter development under our preferred partner alliance with GSK. Because we did not disclose the target of ISIS-TTRRx at the time we selected the drug as a development candidate, we previously referred to ISIS-TTRRx as ISIS-GSK1Rx. We are responsible for developing the drug through Phase 2 proof-of-concept, at which time GSK has the option to license ISIS-TTRRx from us. We will continue to receive milestone payments from GSK as ISIS-TTRRx advances through development.
We completed a Phase 1 study evaluating the safety and activity of ISIS-TTRRx in healthy volunteers. In this study, ISIS-TTRRx produced rapid, dose-dependent reductions of up to 80 percent in plasma TTR protein. Subjects treated with ISIS-TTRRx generally tolerated the drug well. We plan to initiate a clinical study evaluating the efficacy of ISIS-TTRRx in patients with FAP in 2012.
ISIS-AATRx — ISIS-AATRx is an antisense drug that reduces production of alpha-1 antitrypsin, or AAT, for the treatment of liver disease in patients with alpha-1 antitrypsin deficiency, or AATD. AATD is a genetic disease in which the patient does not produce normal AAT, a protein primarily produced in the liver that protects lung tissue from damage. AATD affects 1 out of every 2,500 people in the United States and can lead to severe liver disease, including liver scarring, cirrhosis and liver cancer.
Patients with AATD inherit a mutant gene from one or both parents. Physicians characterize patients who inherit a mutant gene from both parents as having severe AATD. Approximately 10 percent of infants and 15 percent of adults with severe AATD experience liver damage due to progressive accumulation of misfolded AAT protein in the liver. There are currently no available therapies for patients with AATD-associated liver disease, and liver transplantation is the only available option for patients who develop severe liver dysfunction due to accumulation of mutant AAT protein. Symptoms of AATD-associated liver disease can manifest as early as infancy, and AATD is the most common genetic disease requiring pediatric liver transplantation. The Alpha-1 Association estimates that approximately 10 to 15 percent of all liver transplant candidates have AATD.
ISIS-AATRx is the second drug to enter development under our preferred partner alliance with GSK. We are responsible for developing ISIS-AATRx through Phase 2 proof-of-concept, at which time GSK has the option to license ISIS-AATRx from us. We will continue to receive milestone payments from GSK as ISIS-AATRx advances through development. We believe that ISIS-AATRx offers a unique approach to treat AATD-associated liver disease. We plan to initiate a Phase 1 study in 2012.
Other Drugs in Development
The broad applicability of our antisense technology allows us to create promising drugs in a variety of disease areas. We have successfully developed novel drugs and licensed them to highly focused satellite companies that have the specific expertise and resources to continue developing these drugs. Together with our partners we continue to advance drugs in clinical development that are outside of our core therapeutic areas. For instance, our partner, Excaliard, presented data from three Phase 2 studies demonstrating that EXC 001 reduced scarring in patients.
14
Vitravene, or fomivirsen — In August 1998, the FDA approved Vitravene, an antisense drug that we discovered and developed, to treat cytomegalovirus, or CMV, retinitis in AIDS patients. Novartis Ophthalmics AG, our worldwide distribution partner for this drug, launched Vitravene in November 1998. New anti-HIV drugs, particularly protease inhibitors and combination treatment regimens, have prolonged survival in HIV-infected individuals. This has resulted in a decline in mortality from AIDS, accompanied by a decline in the incidence of many opportunistic infections, including CMV retinitis. As a result, Novartis no longer markets Vitravene. Vitravene demonstrates that we can meet FDA and European regulatory requirements for safety and efficacy, and for the commercial manufacture of antisense drugs.
Alicaforsen — Under license to Atlantic Pharmaceuticals Limited, alicaforsen is an antisense drug that targets intercellular adhesion molecule 1, or ICAM-1. ICAM-1 is over-expressed in a wide variety of inflammatory disorders, including ulcerative colitis and pouchitis. Ulcerative colitis, or UC, is an inflammatory bowel disease, or IBD, of the colon, a part of the large intestine, and pouchitis is an inflammation of the surgically constructed internal pouch created in UC patients who have had their diseased colons removed.
In 2007, we licensed alicaforsen to Atlantic Pharmaceuticals for pouchitis, UC and other inflammatory diseases. The FDA and European Medicines Agency, or EMA, have since granted alicaforsen Orphan Drug Designation for the treatment of pouchitis in the United States and Europe, respectively. Atlantic Pharmaceuticals currently supplies alicaforsen in response to physicians’ requests under international Named Patient Supply regulations for patients with IBD. Atlantic Pharmaceuticals is currently pursuing opportunities to fund further development of alicaforsen.
ATL1102 — ATL1102 is an antisense drug that ATL is developing for the treatment of multiple sclerosis, or MS. ATL1102 inhibits CD49d, a subunit of Very Late Antigen-4, or VLA-4. Studies in animal models have demonstrated that inhibiting VLA-4 positively affects a number of inflammatory diseases, including MS. We licensed ATL1102 to ATL in December 2001 and in February 2008, ATL licensed ATL1102 to Teva. In 2008, ATL and Teva reported Phase 2a results of ATL1102 showing significantly reduced disease activity in patients with relapsing remitting MS. In 2010, Teva terminated its agreement with ATL and returned ATL1102 back to ATL. ATL is seeking a partner to continue developing ATL1102 in patients with MS.
EXC 001 — EXC 001 is an antisense drug that targets connective tissue growth factor, or CTGF, a growth factor that is over-expressed in damaged skin or tissue following a traumatic event. We co-discovered EXC 001 and licensed it to Excaliard for the local treatment of fibrotic diseases, including scarring. In November 2011, Pfizer Inc. acquired Excaliard and plans to continue to develop EXC 001. Fibrosis represents a significant and expanding area of unmet medical need where antisense drugs could offer a unique advantage as anti-fibrotic agents.
Excaliard reported the successful completion of three Phase 2 studies of EXC 001. In one Phase 2 study, treatment with EXC 001 produced a significant improvement in the appearance of scarring in patients who had revision surgery for excessive scarring. In another Phase 2 study, treatment with EXC 001 also reduced the severity of fine line scars and accelerated resolution of scarring compared to placebo. In a third Phase 2 study, treatment with EXC 001 produced a dose-dependent reduction in CTGF and inhibition of CTGF-induced collagen and other pro-fibrotic genes. In all three studies, Excaliard reported that treatment with EXC 001 produced statistically significant reductions in scar severity compared to placebo and patients tolerated EXC 001 well with no important drug related adverse effects observed. Pfizer Inc. plans to continue developing EXC 001 to address unmet medical needs in patient groups who suffer from excessive skin scarring.
iCo-007 — iCo-007 is an antisense drug that targets c-Raf kinase. In preclinical studies, clinicians associated antisense inhibition of c-Raf kinase with a reduction in the formation and leakage of new blood vessels in the eye, suggesting inhibiting c-Raf kinase can help patients with diabetic macular edema and diabetic retinopathy. Diabetic retinopathy is one of the leading causes of blindness in people in the United States, and a high percentage of type 1 diabetics have evidence of retinopathy by age 20. Additionally up to 21 percent of people with type 2 diabetes have retinopathy at the time of the first diagnosis of diabetes, and most will eventually develop some degree of retinopathy over time. We discovered iCo-007 and licensed it to iCo Therapeutics Inc., or iCo, for the treatment of various eye diseases that occur as complications of diabetes.
In May 2010, investigators evaluating iCo-007 in patients with diffuse diabetic macular edema presented positive results from the Phase 1 study showing that subjects tolerated iCo-007 well. In this study, a number of individuals exhibited a decrease of central macular edema compared to baseline using an analytical method called optical coherence tomography. In August 2011, iCo initiated a Phase 2 study on iCo-007 in patients with diabetic macular edema.
Plazomicin —Plazomicin, formerly ACHN-490, is a next-generation aminoglycoside drug that Achaogen, Inc. is developing for the treatment of multi-drug resistant gram-negative bacterial infections. Aminoglycosides are a group of antibiotics that inhibit bacterial protein synthesis and that clinicians use to treat serious bacterial infections. Achaogen discovered plazomicin based on technology licensed from us.
15
Plazomicin has displayed broad-spectrum activity in animals against multi-drug resistant gram-negative bacteria that cause systemic infections, including E. coli, and against methicillin-resistant staphylococcus aureus, or MRSA. In preclinical studies, plazomicin demonstrated an acceptable safety profile and the potential for once-daily dosing. Achaogen has completed a Phase 1 study of plazomicin in healthy volunteers and is evaluating plazomicin in a Phase 2 study in patients with complicated urinary tract infections or acute kidney infections. Achaogen plans to complete this Phase 2 study in 2012.
Antisense Technology
Our core technology programs can support multiple target-based antisense research programs without significantly increasing costs. We can design our antisense drugs to target a broad range of diseases, efficiently producing a proprietary portfolio of drugs that can interrupt the production of disease-causing proteins without disrupting other proteins that are necessary for the body’s normal functions. We are currently pursuing antisense drug discovery programs focused on various cardiovascular, metabolic, severe and rare disease, and other diseases as well as cancer.
Genes contain the information necessary to produce proteins. A gene is made up of nucleoside bases: Adenine, Thymine, Guanine, and Cytosine, commonly known as A, T, G and C, which are linked together to form a two-stranded structure that resembles a twisted ladder, known as deoxyribonucleic acid, or DNA. The nucleotides on one side of the ladder bind weakly to complementary nucleotides on the other strand according to specific rules; for example, A pairs with T and G pairs with C, creating the ladder’s rungs. Scientists call this highly specific nucleotide pairing hybridization. The sequence or order of these nucleotides establishes the cell’s recipes for making proteins. Each protein’s instructions reside in a corresponding segment of DNA known as a gene.
When a cell transcribes information from a DNA gene into messenger RNA, or mRNA, the two complementary strands of the DNA partly uncoil. One strand acts as a template and information stored in the DNA strand is copied into a complementary mRNA. mRNA then carries the information to cellular structures called ribosomes, the cell’s factories for manufacturing proteins. The ribosome reads the encoded information, the mRNA’s nucleotide sequence, and in doing so, strings together amino acids to form a specific protein. This process is called translation. Antisense technology interrupts the cell’s protein production process by preventing the RNA instructions from reaching the ribosome, thus inhibiting the synthesis of the protein. The mRNA sequence of nucleotides that carries the information for protein production is called the ‘sense’ strand. The complementary nucleotide chain that binds specifically to the sense strand is called the “antisense” strand. We use the information contained in mRNA to design chemical structures, called antisense oligonucleotides or antisense drugs, which resemble DNA and RNA and are the complement of mRNA. These potent antisense drugs inhibit the production of disease-causing proteins. Specifically, almost all of our antisense drugs in development cause a cellular enzyme called ribonuclease H1, or RNase H1, to degrade the target mRNA. The drug itself remains intact during this process, so it can remain active against additional target mRNA molecules and repeatedly trigger their degradation. Our antisense drugs can selectively bind to an mRNA that codes for a specific protein and will not bind to closely related RNAs, providing a level of specificity that is better than traditional drugs. As a result, we can design antisense drugs that selectively inhibit the disease-causing member of the group without interfering with those members of the group necessary for normal bodily functions. This unique specificity means that antisense drugs may be less toxic than traditional drugs because we can design them to minimize the impact on unintended targets.
Further, the design of antisense compounds is less complex, more rapid and more efficient than traditional drug discovery approaches directed at protein targets. Traditional drug design requires companies to identify a small molecule that will interact with protein structures to affect the disease-causing process. Since predicting which small molecules will do this has proven to be difficult, traditional drug discovery involves testing hundreds of thousands of small molecules for their ability to interfere with protein function. As a result, traditional drug discovery is a labor intensive, low probability endeavor. In contrast, we design our antisense compounds to bind to mRNA through well understood processes. We can design prototype antisense drugs as soon as we identify the sequence for the target mRNA.
Using proprietary antisense oligonucleotides to identify what a gene does, called gene functionalization, and then determining whether a specific gene is a good target for drug discovery, called target validation, are the first steps in our drug discovery process. We use our proprietary antisense technology to generate information about the function of genes and to determine the value of genes as drug discovery targets. Furthermore, because of the nature of antisense drugs, the very molecules we design for gene functionalization and target validation experiments may become our lead drug candidates. This efficiency represents a unique advantage of our antisense drug discovery process. Antisense core technology is the function within Isis that is responsible for advancing antisense technology. Through the efforts of our scientists in the antisense core technology group, we have produced second generation antisense drugs that have increased potency and stability. We combine our core technology programs in medicinal chemistry, RNA biochemistry, and molecular and cellular biology with molecular target-focused drug discovery efforts to design drugs. The goal of our target-based research programs is to identify antisense drugs to treat diseases for which there are large commercial markets or for which there is a need for better drugs. In addition, our research programs focus on the planned advancement of our technology for future antisense drugs. In 2010, we selected our generation 2.5 chemistry, an advancement that we believe will increase the potency of our drugs and make oral administration commercially feasible. We expect that these generation 2.5 drugs will constitute some of our future drugs and serve as follow-on compounds to some of our current drugs in development. Currently both our ISIS-STAT3Rx and ISIS-FVIIRx drugs incorporate our generation 2.5 chemistry, and we plan to initiate a clinical study using ISIS-STAT3Rx in 2012.
16
Other Antisense Mechanisms
Splicing
Splicing is a normal cellular mechanism that the cell uses to produce many different, but closely related proteins from a single gene by varying the processing of the RNA. Splicing occurs on the precursor mRNA, or pre-mRNA, which includes sequences that encode for proteins and regions that are unnecessary for making proteins. Before the protein-producing mRNA is produced, the cell deletes the regions that are unnecessary for making proteins from the pre-mRNA strand. Scientists call the natural process that removes these regions and re-forms the finished mRNA ‘splicing’. Through the splicing process, the cell can create many diverse proteins from a single gene and splicing accounts for most of the diversity in proteins in the cell. In fact, of the approximately 25,000 genes in the human genome, approximately 90% have alternative splice forms. Alternative splicing can produce proteins that are involved in disease. In some cases, using antisense technology, we can direct alternate splicing to produce a deficient protein critical for normal cellular function to correct for a genetic defect. Examples of diseases we could potentially treat using antisense modulation of splicing include SMA, cystic fibrosis and Duchenne’s muscular dystrophy.
We design antisense drugs to control splicing to make one protein versus another. In 2010, we identified an antisense drug, ISIS-SMNRx, to treat SMA. SMA is a splicing disease and the leading genetic cause of infant mortality. The discovery of ISIS-SMNRx resulted from a research collaboration between our scientists and scientists at Cold Spring Harbor Laboratory. In earlier published research, we and our collaborators at Cold Spring Harbor Laboratory demonstrated the feasibility of using our antisense technology to control splicing for the treatment of SMA. In December 2011, we initiated the first study to utilize an alternative splicing mechanism in patients with SMA.
Our progress in controlling splicing to treat disease demonstrates the diversity of our technology and the potential to utilize many different antisense approaches to treat disease.
RNAi
In addition to advancing our RNase H mediated antisense drugs and core chemistries, we are also working to better understand the therapeutic utility of other antisense mechanisms, including RNA interference, or RNAi. For some of this research we work with satellite companies, including Alnylam.
RNAi is an antisense mechanism that uses small interfering RNA, or siRNA, to target mRNA sequences. Most companies approach siRNA using double-stranded oligonucleotides that exploit a cellular protein complex called the RNA-induced silencing complex, or RISC, to bind to the mRNA and to prevent the production of a disease-causing protein. We have a strong and growing intellectual property position in RNAi methodology and oligonucleotide chemistry for siRNA therapeutics. We have licensed our patents for this technology to Alnylam for double-stranded siRNA therapeutics.
At present, other companies who are developing double-stranded siRNA drugs administer the drugs either locally or systemically. If scientists systemically administer double-stranded siRNA drugs, these drugs require complex formulations to achieve sufficient delivery. We have identified the critical drug design elements required to achieve RNAi activity with a single-stranded RNAi drug. We have also made substantial progress in optimizing these design elements to ensure that they survive long enough under physiological conditions to produce the desired activity in animals without the need for complex formulations. As a result, we have created single-stranded RNAi compounds that, when we administer systemically, distribute in a manner similar to our second-generation RNase H antisense drugs, without requiring the complex formulation or delivery vehicle typically necessary for double-stranded RNAi oligonucleotides. These new single-stranded RNAi drug designs are an exciting advancement in RNAi technology.
New Antisense Targets
MicroRNAs
There are many different types of RNA that exist within the body, including pre-mRNAs and mRNAs. Our antisense technology is not limited to RNA sequences that translate into proteins, but rather we can apply the principals of our technology to develop drugs that target other RNAs, such as microRNAs.
17
MicroRNAs are small, RNA molecules, typically 20 to 25 nucleotides in length, which do not encode proteins but instead work as natural antisense sequences that scientists believe regulate the expression of approximately one-third of all human genes. To date, scientists have identified more than 700 microRNAs in the human genome, and have shown that the absence or presence of specific microRNAs in various cells is associated with specific human diseases, including cancer, viral infection, metabolic disorders and inflammatory disease. MicroRNAs themselves may be drug targets. For instance, if a single microRNA can change the expression of a protein that may be involved in disease, then inhibiting this microRNA could provide a therapeutic benefit. Alternately, physicians could use microRNAs as drugs themselves, where increasing the cell concentration of a particular microRNA could modulate the expression of a particular protein. To fully exploit the therapeutic opportunities of targeting microRNAs, we and Alnylam established Regulus as a company focused on the discovery, development and commercialization of microRNA-based therapeutics.
Collaborative Arrangements and Licensing Agreements
Partnership Strategy
Overview
Our partnership strategy has allowed us to build a development pipeline of 26 drugs, to create a broad base of potential license fees, milestone payments, royalties, profit sharing and earn out payments and to control our drug development expenses. In this way, we remain a focused and efficient research and development organization that can continue to discover new drugs and expand ours and our partners’ pipelines.
Through the efficiency of our drug discovery platform we can develop drugs to almost any gene target. We concentrate on developing antisense drugs in our core therapeutic areas of cardiovascular, metabolic, severe and rare diseases and cancer. To maximize the value of our drugs and technologies, we have a multifaceted partnering strategy. We form traditional partnering alliances that enable us to discover and conduct early development of new drugs, outlicense our drugs to partners and build a broad base of license fees, milestone payments and royalty income. We also form preferred partner transactions that provide us with a vested partner early in the development of a drug. In this way, we benefit in the short term from upfront option fees and development milestone payments while we maintain control over the early development of the drug. We benefit in the long term by having a knowledgeable and committed partner to license the drug at clinical proof-of-concept and by receiving regulatory milestone payments and royalties as our partner moves the drug to the market. We also work with a consortium of smaller companies that can exploit our drugs and technologies outside our primary areas of focus. We call these smaller companies our satellite companies. In this way, we benefit from the disease-specific expertise of our satellite company drug discovery partners, who are advancing drugs in our pipeline in areas that are outside of our core focus. Through this strategy we can expand the therapeutic range of antisense drugs into disease areas that need new and innovative treatment options.
In addition we form partnerships focused on developing and advancing certain RNA-targeting therapeutic technologies. These partnerships take advantage of our dominant intellectual property estate, and leverage our own investments in our core technologies. These collaborations typically involve a cross-license between us and our partner and allow us to participate in newly emerging approaches to RNA-targeting therapeutics and augment our active programs in these areas.
Our partnerships fall into several categories, including traditional pharmaceutical alliances and licenses, and satellite company collaborators, external project funding alliances, and technology and intellectual property sales and licensing. We discuss each of these categories in more detail below, along with the relevant partnerships.
Traditional Pharmaceutical Alliances and Licensing
We have a strong history of establishing alliances with pharmaceutical industry leaders. We form traditional partnering alliances that enable us to discover and conduct early development of new drugs, outlicense our drugs to partners, and build a broad base of license fees, milestone payments and royalty income. We also form preferred partner transactions that provide us with a vested partner early in the development of a drug. In this way, we benefit in the short term from upfront option fees and development milestone payments while we maintain control over the early development of the drug. We benefit in the long term by having a knowledgeable and committed partner to license the drug at clinical proof-of-concept and by receiving regulatory milestone payments and royalties as our partner moves the drug to the market. In all of our partnerships, we benefit from the expertise our partners bring to our drugs. By coupling our partnering activity with our efficient drug discovery technology we can develop the majority of our drugs in our core therapeutic areas through early proof-of-concept ourselves prior to licensing.
Biogen Idec
In January 2012, we entered into a global collaboration agreement with Biogen Idec to develop and commercialize ISIS-SMNRx for the treatment of SMA. Because SMA is a severe and rare disease, we may have a relatively rapid path to market.
18
Under the terms of the agreement, we received an upfront payment of $29 million and are eligible to receive up to $45 million in substantive milestone payments associated with the clinical development of ISIS-SMNRx prior to licensing. We are responsible for global development of ISIS-SMNRx through the completion of Phase 2/3 registrational clinical trials. Biogen Idec has the option to license ISIS-SMNRx until completion of the first successful Phase 2/3 trial. If Biogen Idec exercises its option, it will pay us a license fee and will assume global development, regulatory and commercialization responsibilities. We may also receive up to $150 million in substantive milestone payments if Biogen Idec achieves pre-specified regulatory milestones. We will earn the next milestone payment of $18 million if we initiate the first Phase 2/3 study for ISIS-SMNRx. In addition, we will receive up to double-digit royalties on sales of ISIS-SMNRx if Biogen Idec successfully develops and commercializes ISIS-SMNRx after option exercise.
Bristol-Myers Squibb
In May 2007, we entered into a collaboration agreement with Bristol-Myers Squibb to discover, develop and commercialize novel antisense drugs targeting proprotein convertase subtilisin/kexin type 9, or PCSK9. As part of the collaboration, Bristol-Myers Squibb selected the first development candidate, BMS-PCSK9Rx, to move into development. In addition to the $15 million upfront fee, we earned $8 million in milestone payments related to the development of BMS-PCSK9Rx. The collaboration ended in December 2011, and we regained the rights to discover and develop antisense drugs to target PCSK9.
During 2011, 2010 and 2009, we earned revenue of $2.4 million, $12.2 million and $9.1 million, respectively, from Bristol-Myers Squibb, which represented 2 percent, 11 percent and 8 percent, respectively, of our total revenue for those years.
Eli Lilly and Company
In August 2001, we formed a broad strategic relationship with Eli Lilly and Company, which included a joint research collaboration. As part of the collaboration, Eli Lilly and Company licensed LY2181308, an antisense inhibitor of survivin, and LY2275796, an antisense inhibitor of eIF-4E. Eli Lilly and Company is responsible for the development of LY2181308.
As of December 31, 2011, we had earned $4.1 million in license fees and milestone payments related to the continued development of LY2181308. We may receive additional substantive milestone payments aggregating up to $25 million, including up to $5 million for the achievement of development milestones, up to $8 million for the achievement of regulatory milestones and up to $12 million for the achievement of commercialization milestones. In addition, we will receive royalties on future product sales of the drug. We will earn the next milestone payment of $5 million if Eli Lilly and Company initiates a Phase 3 study of LY2181308.
In December 2009, we reacquired LY2275796, which we renamed ISIS-EIF4ERx, and we are continuing to develop the drug. Eli Lilly and Company has the right to reacquire ISIS-EIF4ERx on predefined terms prior to the initiation of Phase 3 development. However, if we publicly disclose the results from a Phase 2 clinical study of ISIS-EIF4ERx:
· Eli Lilly and Company may license ISIS-EIF4ERx on the predefined terms;
· Eli Lilly and Company may tell us it is not interested in licensing ISIS-EIF4ERx, in which case we may license ISIS-EIF4ERx to another partner; or
· Eli Lilly and Company may offer to license ISIS-EIF4ERx on terms that are lower than the predefined terms, in which case we may license ISIS-EIF4ERx to another partner so long as the licensing terms we reach with the new partner are better than terms offered by Eli Lilly and Company and we have not publicly disclosed any results from a new clinical study of ISIS-EIF4ERx prior to reaching the agreement with the new partner.
During 2009, we earned revenue from our relationship with Eli Lilly and Company totaling $75,000. During 2011 and 2010, we did not earn any revenue from our relationship with Eli Lilly and Company.
Genzyme Corporation, a Sanofi company
In January 2008, we entered into a strategic alliance with Genzyme focused on the licensing and co-development of KYNAMRO and a research relationship. The license and co-development agreement provides Genzyme with exclusive worldwide rights for all therapeutic purposes to our patents and know-how related to KYNAMRO, including the key product related patents described in the “Patents and Proprietary Rights” section under “ApoB 100 and KYNAMRO” on page 30 of this report, and their foreign equivalents pending or granted in various countries outside the United States, including in the European Union via the European Patent Convention, Japan, Canada, Australia, South Africa and India. In addition, we agreed that we would not develop or commercialize another oligonucleotide-based compound designed to modulate apo-B by binding to the mRNA encoding apo-B, throughout the world.
19
The transaction, which closed in June 2008, included a $175 million licensing fee, a $150 million equity investment in our stock in which we issued Genzyme five million shares of our common stock, and a share of worldwide profits on KYNAMRO and follow-on drugs ranging from 30 percent to 50 percent of all commercial sales. We may also receive over $1.5 billion in substantive milestone payments if Genzyme achieves pre-specified events, including up to $750 million for the achievement of regulatory milestones and up to $825 million for the achievement of commercialization milestones. The next milestone payment we could earn under our agreement with Genzyme is $25 million when the FDA accepts the New Drug Application, or NDA, for KYNAMRO.
Under this alliance, Genzyme is responsible for the continued development and commercialization of KYNAMRO. We agreed to supply the active pharmaceutical ingredient for KYNAMRO for the Phase 3 clinical trials and initial commercial launch. Genzyme is responsible for manufacturing the finished drug product for KYNAMRO, including the initial commercial launch supply, and, if approved, Genzyme will be responsible for the long term supply of KYNAMRO drug substance and finished drug product. As part of the agreement, we contributed the first $125 million in funding for the development costs of KYNAMRO, and now we and Genzyme share development costs equally. In addition, as part of our alliance, Genzyme has a first right of negotiation for ISIS-SOD1Rx.
The license and co-development agreement for KYNAMRO will continue in perpetuity unless we or Genzyme terminate it earlier under the following situations:
· Genzyme may terminate the license and co-development agreement at any time by providing written notice to Isis;
· We may terminate the license and co-development agreement on a country-by-country basis or in its entirety upon Genzyme’s uncured failure to use commercially reasonable efforts to develop and commercialize KYNAMRO in the United States, France, Germany, Italy, Spain, the United Kingdom, Japan and Canada; and
· Either we or Genzyme may terminate the license and co-development agreement upon the other party’s uncured failure to perform a material obligation under the agreement.
Upon termination of the license and co-development agreement, the license we granted to Genzyme for KYNAMRO will terminate and Genzyme will stop selling the product. In addition, if Genzyme voluntarily terminates the agreement or we terminate the agreement in a country or countries for Genzyme’s failure to develop and commercialize KYNAMRO, then the rights to KYNAMRO will revert back to us and we may develop and commercialize KYNAMRO in the countries that are the subject of the termination, subject to a royalty payable to Genzyme.
If we are the subject of an acquisition, then within 180 days following the acquisition, Genzyme may elect to purchase all of our rights to receive payments under the KYNAMRO license and co-development agreement for a purchase price to be mutually agreed to by us and Genzyme, or, if we cannot agree, a fair market value price determined by an independent investment banking firm.
Genzyme has agreed to monthly limits on the number of shares it can sell of the Company’s stock that it purchased in February 2008. In addition, Genzyme has agreed that until the earlier of the 10 year anniversary of the KYNAMRO license and co-development agreement or the date Genzyme holds less than two percent of our issued and outstanding common stock, Genzyme will not acquire any additional shares of our common stock without our consent.
The price Genzyme paid for our common stock represented a significant premium over the fair value of our common stock. We are amortizing this $100 million premium along with the $175 million licensing fee that we received in the second quarter of 2008 ratably into revenue until June 2012, which represents the end of our performance obligation based on the current research and development plan. During 2011, 2010 and 2009, we earned revenue of $72.3 million, $66.9 million and $66.4 million, respectively, primarily related to the upfront payments we received from Genzyme, which represented 73 percent, 62 percent and 55 percent, respectively, of our total revenue for those years.
GlaxoSmithKline
In March 2010, we entered into a strategic alliance with GSK, which covers up to six programs, in which we use our antisense drug discovery platform to seek out and develop new drugs against targets for rare and serious diseases, including infectious diseases and some conditions causing blindness. This alliance allows us to control and facilitate rapid development of drugs while still being eligible to receive milestone payments as we advance these drugs in clinical development.
As of December 31, 2011, we have received $53 million from GSK, including the $35 million upfront payment, $15 million in development milestone payments and the $3 million payment we received in May 2011 when GSK expanded the collaboration by initiating a sixth program.
20
We are also eligible to receive on average up to $20 million in milestone payments per program up to Phase 2 proof-of-concept. GSK has the option to license drugs from these programs at Phase 2 proof-of-concept, and will be responsible for all further development and commercialization. If GSK successfully develops all six programs for one or more indications and achieves pre-agreed sales targets, we could receive license fees and substantive milestone payments of more than $1.4 billion, including up to $358.5 million for the achievement of development milestones, up to $581.5 million for the achievement of regulatory milestones and up to $495 million for the achievement of commercialization milestones. We will earn the next milestone payment of $2 million if we demonstrate in-vivo efficacy for the next drug discovery target. In addition, we will receive up to double-digit royalties on sales from any product that GSK successfully commercializes under this alliance.
During 2011 and 2010, we earned revenue of $17.7 million and $10.3 million, respectively, from our relationship with GSK, which represented 18 percent and nine percent, respectively, of our total revenue for those years.
Ortho-McNeil-Janssen Pharmaceuticals, Inc., formerly Ortho-McNeil, Inc.
In September 2007, we entered into a collaboration with Ortho-McNeil-Janssen Pharmaceuticals, or OMJP, to discover, develop and commercialize antisense drugs to treat metabolic diseases, including type 2 diabetes. As part of the collaboration, we granted OMJP worldwide development and commercialization rights to two of our diabetes programs, our GCGR and GCCR programs. The collaboration ended and we regained the rights to drugs from both of these programs. We are developing more potent inhibitors for our GCGR and GCCR programs, which we identified as part of our collaboration with OMJP.
During 2009 we earned revenue of $18.4 million under this collaboration, which represented 15 percent of our total revenue for that year. During 2011 and 2010, we did not earn any revenue from our relationship with OMJP.
Satellite Company Collaborations
Through our satellite company collaborations, we expand the reach and potential of RNA-targeting therapeutics into disease areas that are outside of our core focus and advance certain RNA-targeting therapeutic technologies. We refer to these companies as our satellite companies, and this strategy as our satellite company strategy. These relationships provide us with partners who are focused in a particular disease area and who share the common goal of advancing our drugs. In these partnerships, we typically own equity in the company, often as part of the licensing agreement and we also retain the potential to earn milestone payments and royalties.
In addition to our satellite company partners that are advancing RNA-targeting therapeutics, we have satellite company partners who take advantage of our dominant intellectual property estate and leverage our own investments in our core technologies to advance RNA-targeting technologies. These partnerships typically involve a cross-license between us and our partner and allow us to participate in newly emerging approaches to RNA-targeting technologies and augment our active programs in these areas.
The value of this strategy is evident in the broad pipeline of drugs we and our partners are developing to treat a large range of diseases. Using their resources and their expertise, our partners are instrumental in driving the development of antisense drugs that we discovered or co-discovered but fall outside our main areas of focus. For example, our satellite company partner, Excaliard, licensed from us a drug, EXC 001, to treat scarring. Excaliard advanced EXC 001 into clinical development and recently completed three Phase 2 studies. In these studies, local administration of EXC 001 significantly reduced scar severity in patients. In 2011, Pfizer Inc. acquired Excaliard and obtained all of Excaliard’s rights to EXC 001. We received $4.4 million and we are eligible to receive up to an additional $9.6 million from the sale of our equity ownership in Excaliard. Under our agreement with Excaliard, we are also eligible to receive substantive milestone payments totaling up to $47.7 million as Excaliard advances EXC 001. We will receive royalties on antisense drugs that Excaliard develops and commercializes. Pfizer’s acquisition of Excaliard exemplifies the significant value of our strategy to partner antisense drugs. In summary, we believe that our satellite company strategy allows us to realize opportunities outside of our therapeutic focus while our committed and knowledgeable drug development partner incurs the cost of development and assumes the risk.
Achaogen, Inc.
In 2006, we exclusively outlicensed to Achaogen, Inc. specific know-how, patents and patent applications relating to aminoglycosides. In exchange, Achaogen agreed to certain payment obligations related to aminoglycosides Achaogen developed. Aminoglycosides are a class of small molecule antibiotics that inhibit bacterial protein synthesis and that physicians use to treat serious bacterial infections. Achaogen is developing plazomicin, an aminoglycoside Achaogen discovered based on the technology we licensed to Achaogen. Plazomicin has displayed broad-spectrum activity in animals against multi-drug resistant gram-negative bacteria that cause systemic infections, including E. coli. The compound has also demonstrated activity against methicillin-resistant staphylococcus aureus, or MRSA.
21
In connection with the license, Achaogen issued to us $1.5 million of Achaogen Series A Preferred Stock. Since early 2009, we have received $3 million from Achaogen, $500,000 which was in Achaogen securities, as Achaogen has advanced plazomicin in development. In addition, assuming Achaogen successfully develops and commercializes the first two drugs under our agreement, we may receive payments totaling up to $46.3 million for the achievement of key clinical, regulatory and sales events. We will also receive royalties on sales of drugs resulting from the program. Achaogen is solely responsible for the continued development of plazomicin. During 2010 and 2009, we earned $2 million and $500,000, respectively, in revenue from our relationship with Achaogen, which does not include any revenue from the equity we received from Achaogen. During 2011, we did not earn any revenue from our relationship with Achaogen. At December 31, 2011 and 2010, we owned less than 10 percent of Achaogen’s equity.
Alnylam Pharmaceuticals, Inc.
In March 2004, we entered into a strategic alliance with Alnylam to develop and commercialize RNAi therapeutics. Under the terms of the agreement, we exclusively licensed to Alnylam our patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry for double-stranded RNAi therapeutics in exchange for a $5 million technology access fee, participation in fees for Alnylam’s partnering programs, as well as future milestone and royalty payments from Alnylam. For each drug Alnylam develops under this alliance, we may receive up to $3.4 million in substantive milestone payments, including up to $1.1 million for the achievement of development milestones and $2.3 million for regulatory milestones. In December 2010, we earned a $375,000 milestone payment from Alnylam for the initiation of a Phase 1 study in their TTR program. We will earn the next milestone payment of $750,000 if Alnylam initiates a Phase 3 study for TTR. We retained rights to a limited number of double-stranded RNAi therapeutic targets and all rights to single-stranded RNAi, or ssRNAi, therapeutics. We also made a $10 million equity investment in Alnylam at the time of the agreement. During 2007, 2006 and 2005, we sold our holdings of Alnylam stock resulting in aggregate net cash proceeds of $12.2 million and a net gain on investments of $6.2 million. At December 31, 2011, we did not own any shares of Alnylam.
In turn, Alnylam nonexclusively licensed to us its patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry to research, develop and commercialize ssRNAi therapeutics and to research double-stranded RNAi compounds. We also received a license to develop and commercialize double-stranded RNAi drugs targeting a limited number of therapeutic targets on a nonexclusive basis. If we develop or commercialize an RNAi-based drug using Alnylam’s technology, we will pay Alnylam milestone payments and royalties. For each drug, the potential milestone payments to Alnylam total $3.4 million, which we will pay if we achieve specified development and regulatory events. As of December 31, 2011, we did not have an RNAi-based drug in clinical development. Our Alnylam alliance provides us with an opportunity to realize substantial value from our pioneering work in antisense mechanisms and oligonucleotide chemistry and is an example of our strategy to participate in all areas of RNA-targeting drug discovery.
In April 2009, we and Alnylam amended our strategic collaboration and license agreement to form a new collaboration focused on the development of ssRNAi technology. Under the terms of the amended collaboration and license agreement, Alnylam paid us an upfront license fee of $11 million plus $2.6 million of research and development funding in 2009 and 2010. In November 2010, Alnylam terminated the ssRNAi research program and we recognized $4.9 million of revenue from the upfront fee that we were amortizing into revenue over the research term. As a result, any licenses to ssRNAi products we granted to Alnylam under the agreement, and any of Alnylam’s obligations to pay milestone payments, royalties or sublicense payments to us for ssRNAi products under the agreement, terminated. We continue to advance the development of ssRNAi technology and during the course of the collaboration, we made improvements in the activity of ssRNAi compounds, including increased efficacy and potency and enhanced distribution.
As of December 31, 2011, we had earned a total of $37.1 million from Alnylam resulting from sublicenses of our technology for the development of RNAi therapeutics that Alnylam has granted to pharmaceutical partners.
During 2011, 2010 and 2009, we earned revenue from our relationship with Alnylam totaling $375, 000, $10.3 million and $5.0 million, respectively, representing less than one percent, nine percent and four percent, respectively, of our total revenue for those years.
Antisense Therapeutics Limited
In December 2001, we licensed ATL1102 to ATL, an Australian company publicly traded on the Australian Stock Exchange and in February 2008, ATL licensed ATL1102 to Teva Pharmaceutical Industries Ltd. From early 2008 until early 2010, when Teva terminated the licensing agreement for ATL1102, we earned $3.4 million as Teva advanced the development of ATL1102. In March 2010, Teva terminated the licensing agreement for ATL1102 and returned rights to the drug to ATL.
22
In addition to ATL1102, ATL is currently developing ATL1103 for growth and sight disorders. ATL1103 is a product of our joint antisense drug discovery and development collaboration. Over the last two years, ATL has raised nearly $3 million that it is using to advance ATL1103. ATL pays us for access to our antisense expertise and for research and manufacturing services we may provide to ATL. In October 2009, we agreed to manufacture ATL1103 drug product for ATL in return for 18.5 million shares of ATL’s common stock. Additionally, ATL will pay royalties to us on sales of ATL1102 and ATL1103. We may also receive a portion of the fees ATL receives if it licenses ATL1102 or ATL1103.
During 2011, 2010 and 2009, we earned revenue of $210,000, $35,000 and $401,000, respectively, from our relationship with ATL. At December 31, 2011 and 2010, we owned less than 10 percent of ATL’s equity.
Atlantic Pharmaceuticals Limited, formerly Atlantic Healthcare (UK) Limited
In March 2007, we licensed alicaforsen to Atlantic Pharmaceuticals, a UK-based specialty pharmaceutical company founded in 2006, which is developing alicaforsen for the treatment of UC and other inflammatory diseases. Atlantic Pharmaceuticals is initially developing alicaforsen for pouchitis, a UC indication, followed by UC and other inflammatory diseases. In exchange for the exclusive, worldwide license to alicaforsen, we received a $2 million upfront payment from Atlantic Pharmaceuticals in the form of equity. In September 2010, we participated in Atlantic Pharmaceuticals’ financing by agreeing to sell to Atlantic Pharmaceuticals alicaforsen drug substance in return for shares of Atlantic Pharmaceuticals’ common stock. At December 31, 2011 and 2010 we owned approximately 12 percent of Atlantic Pharmaceuticals’ equity. Because realization of the upfront equity payment is uncertain, we recorded a full valuation allowance.
Under the agreement, we could receive substantive milestone payments totaling up to $1.4 million for the achievement of regulatory milestones for multiple indications. We will earn the next milestone payment of $600,000 if Atlantic Pharmaceuticals submits an NDA for alicaforsen with the FDA. We will also receive royalties on product sales of alicaforsen. Atlantic Pharmaceuticals is solely responsible for the continued development of alicaforsen. In 2010, Atlantic Pharmaceuticals announced that in response to requests received from healthcare professionals, it was to supply alicaforsen under international Named Patient Supply regulations for patients with IBD. Atlantic Pharmaceuticals is currently pursuing opportunities to fund further development of alicaforsen.
During 2011, 2010 and 2009, we did not earn any revenue from our relationship with Atlantic Pharmaceuticals.
AVI BioPharma, Inc., formerly Ercole Biotech, Inc.
In May 2003, we and Ercole entered an agreement in which each party cross-licensed its respective intellectual property related to alternative RNA splicing. As part of the agreement, we granted Ercole an additional license to some of our chemistry patents. Assuming Ercole successfully develops and commercializes a drug incorporating the splicing technology or chemistry we licensed to Ercole, we may receive payments totaling up to $21 million for the achievement of key clinical, regulatory and sales events. We will also receive royalties on sales of these drugs. Ercole is solely responsible for the continued development of its drugs.
Similarly, if we successfully develop and commercialize a drug incorporating the splicing technology Ercole licensed to us, we will pay Ercole up to $21 million for the achievement of key clinical, regulatory and sales events and will also pay royalties to Ercole on sales of these drugs. We currently do not have a drug incorporating Ercole’s technology in clinical development.
In March 2008, AVI BioPharma, Inc. acquired Ercole’s rights and obligations under the collaboration agreement. During 2011, 2010 and 2009, we did not earn any revenue from our relationship with Ercole
Excaliard Pharmaceuticals, Inc., a wholly owned subsidiary of Pfizer Inc.
In November 2007, we entered into a collaboration with Excaliard to discover and develop antisense drugs for the local treatment of fibrotic diseases, including scarring. We granted Excaliard an exclusive worldwide license for the development and commercialization of certain antisense drugs. Excaliard made an upfront payment to us in the form of equity and paid us $1 million in cash for the licensing of an antisense oligonucleotide drug targeting CTGF that is activated during skin scarring following the wound healing process.
In 2010 and 2011, we participated in Excaliard’s financings at nominal amounts to maintain our ownership percentage. In December 2011, Pfizer Inc. acquired Excaliard. We received $4.4 million and we are eligible to receive up to an additional $9.6 million from the sale of our equity ownership in Excaliard to Pfizer Inc. In addition, we will continue to be eligible for milestone and royalty payments under our licensing agreement for EXC 001. Assuming Excaliard successfully develops and commercializes drugs it licenses from us, we may receive substantive milestone payments totaling up to $47.7 million for the achievement of key development and regulatory milestones, including up to $7.7 million for the achievement of development milestones and up to $40 million for the achievement of regulatory milestones. We will earn the next milestone payment of $1.5 million upon initiation of a Phase 3 study for EXC 001. We will also receive royalties on antisense drugs that Excaliard develops and commercializes. We may also receive a portion of the fees Excaliard receives if it licenses drugs from our collaboration.
23
At December 31, 2011, we owned no equity in Excaliard, and we have no remaining performance obligations. During 2011 we did not earn any revenue from our relationship with Excaliard. During 2010 and 2009, we earned revenue of $3,000 and $290,000, respectively, from our relationship with Excaliard.
iCo Therapeutics Inc.
In August 2005, we granted a license to iCo for the development and commercialization of iCo-007. iCo is developing iCo-007 for the treatment of various eye diseases caused by the formation and leakage of new blood vessels such as diabetic macular edema and diabetic retinopathy. iCo paid us a $500,000 upfront fee and may pay us substantive milestone payments totaling up to $48.4 million for the achievement of development and regulatory milestones for multiple indications, including up to $7.9 million for the achievement of development milestones and up to $40.5 million for the achievement of regulatory milestones. We will receive the next milestone payment of $4 million if iCo initiates a Phase 3 study for iCo-007. In addition, we will receive royalties on any product sales of this drug. Under the terms of the agreement, iCo is solely responsible for the development and commercialization of the drug. In September 2007, iCo initiated Phase 1 clinical trials for iCo-007 and we earned a milestone payment of $1.25 million in the form of 936,875 shares of iCo’s common stock.
Over the course of our relationship, iCo has paid us in a combination of cash, common stock and convertible notes. In February 2009, iCo completed a CAD $1.3 million financing to fund the completion of its Phase 1 clinical study of iCo-007. We participated in the financing at a nominal amount to maintain our ownership percentage. In January 2010, we exercised the warrants we held to purchase 1.1 million shares of iCo’s common stock and as a result our ownership in iCo at December 31, 2011 and 2010 was approximately 12 percent. During 2011, 2010 and 2009 we earned revenue of $7,000, $7,000 and $14,000, respectively, from our relationship with iCo.
OncoGenex Technologies Inc., a subsidiary of OncoGenex Pharmaceuticals Inc.
In November 2001, we established a drug development collaboration with OncoGenex, a biotechnology company committed to the development of cancer therapeutics for patients with drug resistant and metastatic cancers, to co-develop and commercialize OGX-011, an anti-cancer antisense drug that targets clusterin. In July 2008, we and OncoGenex amended the co-development agreement pursuant to which OncoGenex became solely responsible for the costs, development and commercialization of OGX-011. In exchange, OncoGenex agreed to pay us royalties on sales of OGX-011 and to share consideration it receives from licensing OGX-011 to a third party, except for consideration OncoGenex receives for the fair market value of equity and reimbursement of research and development expenses.
Under the amended agreement, we assigned to OncoGenex our rights in the patents claiming the composition and therapeutic methods of using OGX-011 and granted OncoGenex a worldwide, nonexclusive license to our know-how and patents covering our core antisense technology and manufacturing technology solely for use with OGX-011. The key product-related patent that we assigned to OncoGenex was U.S. Patent number 6,900,187 having an expiration date of at least 2020; and the key core antisense technology patents we licensed OncoGenex are U.S. Patent number 7,919,472 having an expiration date of 2026 and its foreign equivalents pending in Australia, Canada, the European Patent Convention and Japan. In addition, we agreed that so long as OncoGenex or its commercialization partner is using commercially reasonable efforts to develop and commercialize OGX-011, we will not research, develop or commercialize an antisense compound designed to modulate clusterin. The amended agreement will continue until OncoGenex or its commercialization partner is no longer developing or commercializing OGX-011 or until we terminate the agreement for OncoGenex’s uncured failure to make a payment required under the agreement.
In December 2009, OncoGenex granted Teva the exclusive worldwide right and license to develop and commercialize any products containing OGX-011 and related compounds, with OncoGenex having an option to co-promote OGX-011 in the United States and Canada, for which we received $10 million of the upfront payment OncoGenex received from Teva. We are also eligible to receive 30 percent of up to $370 million in payments OncoGenex may receive from Teva in addition to royalties on sales of OGX-011 ranging between 3.88 percent and seven percent. Under the agreement, this royalty is due on a country-by-country basis until the later of ten years following the first commercial sale of OGX-011 in the relevant country, and the expiration of the last patent we assigned or licensed to OncoGenex that covers the making, using or selling of OGX-011 in such country.
24
To facilitate the execution and performance of OncoGenex’s agreement with Teva, we and OncoGenex amended our license agreement primarily to give Teva the ability to cure any future potential breach by OncoGenex under our agreement. As part of this amendment, OncoGenex agreed that if OncoGenex is the subject of a change of control with a third party, where the surviving entity immediately following such change of control has the right to develop and sell OGX-011, then a payment of $20 million will be due and payable to us 21 days following the first commercial sale of the product in the United States. Any non-royalty payments OncoGenex previously paid to us are creditable towards the $20 million payment, so as a result of the $10 million payment we received from OncoGenex related to its license to Teva, the remaining amount owing in the event of a change of control as discussed above is a maximum of $10 million.
In August 2003, we and OncoGenex entered into a separate collaboration and license agreement for the development of a second-generation antisense anti-cancer drug, OGX-225. OncoGenex is responsible for all development costs and activities, and we have no further performance obligations. OncoGenex issued to us $750,000 of OncoGenex securities as payment for an upfront fee. In addition, OncoGenex will pay us substantive milestone payments totaling up to $3.5 million for the achievement of development and regulatory milestones, including up to $1.5 million for the achievement of development milestones and up to $2 million for the achievement of regulatory milestones. In addition, we will receive royalties on future product sales of the drug. As of December 31, 2011, OncoGenex had not achieved any milestone events related to OGX-225. We will earn the next milestone payment of $500,000 if OncoGenex initiates a Phase 2 study for OGX-225.
In January 2005, we entered into a further agreement with OncoGenex to allow for the development of an additional second-generation antisense anti-cancer drug. Under the terms of the agreement, OncoGenex is responsible for all development costs and activities, and we have no further performance obligations. In April 2005, OncoGenex selected OGX-427 to develop under the collaboration. OncoGenex will pay us substantive milestone payments totaling up to $5.8 million for the achievement of key development and regulatory milestones, including up to $1.3 million for the achievement of development milestones and up to $4.5 million for the achievement of regulatory milestones. In addition, we will receive royalties on future product sales of the drug. In January 2011, we earned a $750,000 milestone payment related to OncoGenex’s Phase 2 trial in men with metastatic prostate cancer. We will earn the next milestone payment of $1.3 million if OncoGenex initiates a Phase 3 study for OGX-427.
During 2009, we sold the OncoGenex common stock we owned resulting in net cash proceeds of $2.8 million. We no longer own any shares of OncoGenex. During 2011 and 2009, we earned $750,000 and $11.4 million, respectively, in revenue from our relationship with OncoGenex. During 2010, we did not earn any revenue from our relationship with OncoGenex.
Xenon Pharmaceuticals Inc.
In November 2010, we established a collaboration with Xenon to discover and develop antisense drugs as novel treatments for the common disease anemia of inflammation, or AI. AI is the second most common form of anemia worldwide and physicians associate a wide variety of conditions including infection, cancer and chronic inflammation with AI. We received an upfront payment in the form of a convertible promissory note from Xenon to discover and develop antisense drugs to the targets hemojuvelin and hepcidin. In addition to license and option fees, we are eligible to receive development and commercial milestone payments and royalties on sales of drugs licensed to Xenon under the collaboration and a portion of sublicense revenue. If Xenon identifies a development candidate, Xenon may take an exclusive license for the development and worldwide commercialization for that development candidate.
Under our collaboration agreement with Xenon we may receive up to $300 million in substantive milestone payments for multiple indications upon the achievement of pre-specified events, including up to $30 million for the achievement of development milestones, up to $150 million for the achievement of regulatory milestones and up to $120 million for the achievement of commercialization milestones. We will earn the next milestone payment of $2 million if Xenon selects a development candidate. During 2011, we earned revenue of $80,000, and during 2010 we did not earn any revenue from our relationship with Xenon.
Regulus Therapeutics Inc.
In September 2007, we and Alnylam established Regulus as a company focused on the discovery, development and commercialization of microRNA-targeting therapeutics. Regulus combines the strengths and assets of our and Alnylam’s technologies, know-how, and intellectual property relating to microRNA-based therapeutics. In addition, Regulus has assembled a strong leadership team with corporate management, business and scientific expertise, a board of directors that includes industry leaders in drug discovery and development, and a scientific advisory board that consists of world-class scientists including some of the foremost authorities in the field of microRNA research.
Regulus is addressing therapeutic opportunities that arise from alterations in microRNA expression. Since microRNAs may act as master regulators of the genome, affecting the expression of multiple genes in a disease pathway, microRNA therapeutics define a new platform for drug discovery and development and microRNAs may also prove to be an attractive new biomarker tool for characterizing diseases. Regulus focuses its drug discovery and development efforts in numerous therapeutic areas, including cancer, fibrosis, metabolic disorders and inflammatory disorders.
25
We and Alnylam granted Regulus exclusive licenses to our intellectual property for microRNA therapeutic applications, and Alnylam made an initial investment in Regulus of $10 million in 2007 to balance both companies’ ownership. In early 2009, Regulus raised $20 million in a Series A preferred stock financing in which we and Alnylam were the sole and equal investors in the financing. In October 2010, Sanofi invested $10 million in Regulus, valuing Regulus at more than $130 million. From this investment Sanofi acquired less than 10 percent ownership of Regulus, leaving us with approximately 46 percent ownership. Alnylam owns the remaining equity. We and Alnylam retain rights to develop and commercialize, on pre-negotiated terms, microRNA therapeutic products that Regulus decides not to develop either by itself or with a partner.
Regulus exclusively controls many of the early fundamental patent portfolios in the microRNA field, including the “Tuschl III”, “Sarnow” and “Esau” patent series. Our “Crooke” patent estate provides Regulus exclusive rights to product compositions and methods of treatment in the field of microRNA-targeting therapeutics. The Regulus patent estate also includes claims to specific microRNA compositions that are optimized for therapeutic use, as well as therapeutic uses of these microRNA compositions, and exclusive rights to Isis’ and Alnylam’s chemical modification intellectual property estates for microRNA applications. In total, Regulus’ intellectual property portfolio includes early fundamental intellectual property in the field of microRNA and over 900 filed patent applications pertaining to chemical modifications of oligonucleotides for therapeutic applications, of which over 600 patents have been issued.
As a result of a new accounting standard we adopted at the beginning of 2010, we no longer include Regulus’ revenue and operating expenses in our operating results. See Note 1, Organization and Significant Accounting Policies, for a more detailed explanation of this change.
Regulus’ Collaboration with GSK
In April 2008, Regulus entered into a strategic alliance with GSK to discover, develop and commercialize novel microRNA-targeting therapeutics to treat inflammatory diseases such as rheumatoid arthritis and IBD, and in February 2010, Regulus and GSK expanded this alliance to include microRNA therapeutics targeting microRNA 122, or miR-122, for the treatment of hepatitis C virus infection, or HCV. The alliance utilizes Regulus’ expertise and intellectual property position in the discovery and development of microRNA-targeting therapeutics and provides GSK with an option to license drug candidates directed at four different microRNA targets, including miR-122 for HCV. Regulus is responsible for the discovery and development of the microRNA antagonists through completion of clinical proof of concept, unless GSK chooses to exercise its option earlier. After exercise of the option, GSK will have an exclusive license to drugs Regulus develops under each program for the relevant microRNA target for further development and commercialization on a worldwide basis. Regulus will have the right to further develop and commercialize any microRNA therapeutics that GSK chooses not to develop or commercialize.
Regulus received $28 million in upfront payments from GSK, including $18 million in option fees and two $5 million convertible promissory notes. The notes plus interest will convert into Regulus common stock in the future if Regulus achieves a minimum level of financing with institutional investors. In addition, we and Alnylam are guarantors of the notes, and if the notes do not convert or if Regulus does not repay the notes by February 2013, we, Alnylam and Regulus may elect to repay the notes plus interest with shares of each company’s common stock or cash. Regulus is eligible to receive from GSK up to $144.5 million in development, regulatory and sales milestone payments for each of the four microRNA-targeting drugs discovered and developed as part of the alliance. Regulus and GSK have identified three of the four microRNA-targets under this collaboration, for which Regulus has received milestone payments. In May 2009, Regulus received a $500,000 discovery milestone payment from its collaboration with GSK for demonstrating a pharmacological effect in immune cells by specific microRNA inhibition. In June 2011, Regulus earned a $500,000 milestone for a second target selection under its GSK collaboration. In addition, Regulus would receive from GSK tiered royalties up to double digits on worldwide sales of drugs resulting from the alliance.
Regulus’ Collaboration with Sanofi
In June 2010, Regulus established a collaboration with Sanofi to discover, develop and commercialize microRNA therapeutics, initially focused on fibrosis. The alliance represents the largest microRNA partnership formed to date, valued at potentially over $750 million. The alliance includes $640 million of potential future milestone payments in addition to a $25 million upfront fee, a $10 million equity investment in Regulus that Sanofi made in October 2010 and annual research support for three years with the option to extend two additional years. In addition, Regulus is eligible to receive royalties on microRNA therapeutic products that Sanofi commercializes. Sanofi also received an option for a broader technology alliance that provides Regulus certain rights to participate in development and commercialization of resulting products. If exercised, this three-year option is worth up to an additional $50 million to Regulus. We and Alnylam are each eligible to receive 7.5 percent of the upfront payment and all potential milestone payments, in addition to royalties on product sales. As a result, in July 2010 we received from Regulus a payment of $1.9 million representing 7.5 percent of the $25 million upfront fee.
26
External Project Funding
We are pursuing discovery and development projects that provide us with new therapeutic applications for antisense drugs through, for example, direct delivery to the CNS. These programs represent opportunities for us and our technology. In some cases, we fund these studies through support from our partners or disease advocacy groups and foundations. For example, we receive external funding support for our ALS and Huntington’s disease programs.
CHDI Foundation, Inc.
In August 2011, we renewed our collaboration with CHDI, which we initially entered into in November 2007, to provide us with funding for the discovery and development of an antisense drug for the treatment of Huntington’s disease. CHDI’s funding builds upon an earlier successful collaboration between us and CHDI, in which CHDI funded proof-of-concept studies that demonstrated the feasibility of using antisense drugs to treat Huntington’s disease. Under the terms of the new collaboration, we will receive funding from CHDI to identify and conduct IND-enabling studies on an antisense drug targeting the huntingtin gene. If we grant a license to a third party to commercialize our Huntington’s disease program, we and CHDI will evenly split the proceeds from the license until CHDI has recouped its funding together with a return determined at a predefined interest rate. Thereafter, we will keep the full amount of any additional proceeds. In addition, CHDI reimbursed us for approximately $1.6 million of research-related expenses we incurred after the earlier collaboration ended in 2010, which we are amortizing over the period of our performance obligation. During 2011 and 2009, we earned revenue of $2.4 million and $1.7 million, respectively, from our relationship with CHDI. In 2010, we did not earn any revenue from our relationship with CHDI.
ALS Association; The Ludwig Institute; Center for Neurological Studies; Muscular Dystrophy Association
In October 2005, we entered into a collaboration agreement with the Ludwig Institute, the Center for Neurological Studies and researchers from these institutions to discover and develop antisense drugs in the areas of ALS and other neurodegenerative diseases. Under this agreement, we agreed to pay the Ludwig Institute and Center for Neurological Studies modest milestone payments and royalties on any antisense drugs resulting from the collaboration. The researchers from the Ludwig Institute and Center for Neurological Studies, through funding from the ALS Association and the Muscular Dystrophy Association, conducted IND-enabling preclinical studies of ISIS-SOD1Rx. The ALS Association and the Muscular Dystrophy Association also provided funding to offset the costs of the Phase 1 study of ISIS-SOD1Rx. Except for the funding the ALS Association and the Muscular Dystrophy Association provided, we control and are responsible for funding the continued development of ISIS-SOD1Rx.
Intellectual Property Sale and Licensing Agreements
We have a broad patent portfolio covering our products and technologies. We believe our patent estate represents the largest and most valuable nucleic acid therapeutics-oriented patent estate in the pharmaceutical industry. While the principal purpose of our intellectual property portfolio is to protect our products and those of our pharmaceutical and satellite company partners described above, our intellectual property is a strategic asset that we are exploiting to generate near-term revenues and that we expect will also provide us with revenue in the future. We have an active intellectual property sales and licensing program in which we sell or license aspects of our intellectual property to companies. Through this program, we also license our non-antisense patents as we did with Eyetech Pharmaceuticals, Inc. To date, we have generated more than $400 million from our intellectual property sale and licensing program that helps support our internal drug discovery and development programs.
Out-Licensing Arrangements; Royalty Sharing Agreements; Sales of IP
Abbott Molecular Inc.
In January 2009, we sold our former subsidiary, Ibis Biosciences, to Abbott Molecular Inc., or AMI, pursuant to a stock purchase agreement for a total acquisition price of $215 million plus the earn out payments described below.
Under the stock purchase agreement, AMI will pay us earn out payments equal to a percentage of Ibis’ revenue related to sales of Ibis systems, including instruments, assay kits and successor products, from the date of the acquisition closing through December 31, 2025. The earn out payments will equal five percent of Ibis’ cumulative net sales over $140 million and up to $2.1 billion, and three percent of Ibis’ cumulative net sales over $2.1 billion. AMI may reduce these earn out payments from five percent to as low as 2.5 percent and from three percent to as low as 1.5 percent, respectively, upon the occurrence of certain events. During 2011, 2010 and 2009 we did not earn any revenue from our relationship with AMI.
27
Eyetech Pharmaceuticals, Inc.
In December 2001, we licensed to Eyetech certain of our patents necessary for Eyetech to develop, make and commercialize Macugen, a non-antisense drug for use in the treatment of ophthalmic diseases, that Eyetech is developing and commercializing with Pfizer Inc. Eyetech paid us a $2 million upfront fee and agreed to pay us for the achievement of pre-specified events and royalty payments in exchange for non-exclusive, worldwide rights to the intellectual property licensed from us. During 2004, we earned $4 million in payments, and our license with Eyetech may also generate additional payments aggregating up to $2.8 million for the achievement of specified regulatory events with respect to the use of Macugen for each additional therapeutic indication. Prior to 2010, we had assigned our rights to receive royalties for Macugen to Drug Royalty Trust 3. During 2009, because of our agreement with Drug Royalty Trust 3, we did not earn any revenue from our relationship with Eyetech. In 2011 and 2010, we earned $790,000 and $567,000, respectively, of revenue related to royalties for Macugen under our license to Eyetech.
Roche Molecular Systems
In October 2000, we licensed some of our novel chemistry patents to Roche Molecular Systems, a business unit of Roche Diagnostics, for use in the production of Roche Molecular Systems’ diagnostic products. The royalty-bearing license grants Roche Molecular Systems non-exclusive worldwide access to some of our proprietary chemistries in exchange for initial and ongoing payments from Roche Molecular Systems to us. In April 2011, we expanded our relationship with Roche Molecular Systems by granting Roche Molecular Systems a non-exclusive license to additional technology for research and diagnostic uses. During 2011, 2010 and 2009, we earned revenue of $828,000, $1.8 million and $1.3 million, respectively, from our relationship with Roche Molecular Systems.
In-Licensing Arrangements
Idera Pharmaceuticals, Inc., formerly Hybridon, Inc.
We have an agreement with Idera under which we acquired an exclusive license to all of Idera’s antisense chemistry and delivery technology related to our second generation antisense drugs and to double-stranded siRNA therapeutics. Idera retained the right to practice its licensed antisense patent technologies and to sublicense their technologies to collaborators under certain circumstances. In addition, Idera received a non-exclusive license to our suite of RNase H patents. During 2011, 2010 and 2009 we earned revenue of $10,000, $20,000 and $10,000, respectively, from our relationship with Idera.
Integrated DNA Technologies, Inc.
In March 1999, we further solidified our intellectual property leadership position in antisense technology by licensing certain antisense patents from Integrated DNA Technologies, Inc., or IDT, a leading supplier of antisense inhibitors for research. The patents we licensed from IDT are useful in functional genomics and in making our second-generation chemistry. We expect these patents will expire in February 2013. Under the license, we paid IDT $4.9 million in license fees in 2001 and we will pay royalties on sales of any drugs utilizing the technology we licensed from IDT until the patents expire.
University of Massachusetts
We have a license agreement with the University of Massachusetts under which we acquired an exclusive license to the University of Massachusetts’ patent rights related to ISIS-SMNRx. If we successfully develop and commercialize a drug incorporating the technology we licensed from the University of Massachusetts, we will pay milestone payments to the University of Massachusetts totaling up to $500,000 for the achievement of key clinical and regulatory milestones. In addition, we will pay the University of Massachusetts a portion of any sublicense revenue we receive from sublicensing its technology, and a royalty on sales of ISIS-SMNRx in the United States if our product incorporates the technology we licensed from the University of Massachusetts.
Verva Pharmaceuticals Ltd.
We have a license agreement with Verva under which we acquired an exclusive license to Verva’s antisense patent rights related to ISIS-FGFR4Rx. If we successfully develop and commercialize a drug incorporating the technology Verva licensed to us, we will pay milestone payments to Verva totaling up to $6.1 million for the achievement of key patent, clinical, and regulatory milestones. If we convert our license from an exclusive license to a nonexclusive license we could significantly reduce the milestone payments due to Verva. In addition, we will also pay royalties to Verva on sales of ISIS-FGFR4Rx if our product incorporates the technology we licensed from Verva.
28
Cold Spring Harbor Laboratory
We have a collaboration and license agreement with the Cold Spring Harbor Laboratory under which we acquired an exclusive license to the Cold Spring Harbor Laboratory’s patent rights related to ISIS-SMNRx. If we successfully develop and commercialize a drug incorporating the technology we licensed from the Cold Spring Harbor Laboratory, we will pay milestone payments to the Cold Spring Harbor Laboratory totaling up to $800,000 for the achievement of key clinical and regulatory milestones. In addition, we will pay the Cold Spring Harbor Laboratory a portion of any sublicense revenue we receive from sublicensing the Cold Spring Harbor Laboratory’s technology, and a royalty on sales of ISIS-SMNRx if our product incorporates the technology we licensed from the Cold Spring Harbor Laboratory.
Manufacturing
In the past, except for small quantities, it was generally expensive and difficult to produce chemically modified oligonucleotides like the antisense drugs we use in our research and development programs. As a result, we have dedicated significant resources to develop ways to improve manufacturing efficiency and capacity. Since we can use variants of the same nucleotide building blocks and the same type of equipment to produce our oligonucleotide drugs, we found that the same techniques we used to efficiently manufacture one oligonucleotide drug could help improve the manufacturing processes for many other oligonucleotide drugs. By developing several proprietary chemical processes to scale up our manufacturing capabilities, we have greatly reduced the cost of producing oligonucleotide drugs. For example, we have significantly reduced the cost of raw materials through improved yield efficiency, while at the same time increasing our capacity to make the drugs. Through both our internal research and development programs and collaborations with outside vendors we may achieve even greater efficiency and further cost reductions.
Due to the growing numbers of our antisense drug development partners and the clinical successes of our antisense drugs, including KYNAMRO, in 2009 we increased our manufacturing capacity by upgrading and optimizing the efficiency of our manufacturing facility. Genzyme has submitted a marketing application for approval of KYNAMRO in Europe and plans to submit a marketing application for approval of KYNAMRO in the United States. If approved, the increased capacity of our manufacturing facility will provide the supply of drug substance we believe is necessary for the initial launch of KYNAMRO.
We rely on Genzyme to manufacture the finished drug product for KYNAMRO, including the initial launch supply. Genzyme has contracted with a contract manufacturing organization to prepare the finished vials of drug product for KYNAMRO, and plans to prepare the finished pre-filled syringes for KYNAMRO at one of its own manufacturing facilities. In addition, after the initial launch supply of drug substance for KYNAMRO, Genzyme will be responsible for the long-term supply of KYNAMRO drug substance and finished drug product.
Our drug substance manufacturing facility is located in an approximately 28,704 square foot building in Carlsbad, California. We lease this building under a lease that has an initial term ending on December 31, 2031 with an option to extend the lease for up to four additional five-year periods. In addition, we have an approximately 25,792 square foot building that houses support functions for our manufacturing activities. We lease this facility under a lease that has an initial term ending in June 2021 with an option to extend the lease for up to two additional five-year periods.
As part of our collaborations we may agree to manufacture clinical trial materials and/or commercial supply for our partners. For example, in the past we have manufactured clinical supply materials for ATL, Bristol-Myers Squibb, Eli Lilly and Company, Genzyme, iCo, OncoGenex and Teva. We believe we have sufficient manufacturing capacity to meet our current and future obligations under existing agreements with our partners for commercial, research and clinical needs, as well as meet our current internal research and clinical needs. We believe that we have, or will be able to develop or acquire, sufficient supply capacity to meet our anticipated needs. We also believe that with reasonably anticipated benefits from increases in scale and improvements in chemistry, we can manufacture antisense drugs at commercially competitive prices.
29
Patents and Proprietary Rights
Our success depends, in part, on our ability to obtain patent protection for our products in the United States and other countries. As of February 13, 2012, we owned or exclusively licensed approximately 1,500 issued patents worldwide.
We own or control patents that provide exclusivity for products in our pipeline and patents that provide exclusivity for our core technology in the field of antisense more generally. Our core technology patents include claims to chemically-modified nucleosides and oligonucleotides as well as antisense drug designs utilizing these chemically-modified nucleosides. These core claims are each independent of specific therapeutic target, nucleic acid sequence, or clinical indication. We also own a large number of patents claiming specific antisense compounds having nucleic acid sequences complementary to therapeutic target nucleic acids, independent of the particular chemical modifications incorporated into the antisense compound. Most importantly, we seek and obtain issued patent claims for each of our drugs. For example, for each of our drugs, we file and obtain claims covering each drug’s nucleic acid sequence and precise drug design. In sum, we maintain our competitive advantage in the field of antisense technology by protecting our core platform technology, which applies to most of our drugs, and by creating multiple layers of patent protection for each of our specific drugs in development.
Type of Patent Claim | | Breadth | | Description |
Chemically Modified Nucleosides and Oligonucleotides
Antisense Drug Design Motifs Therapeutic Methods
Antisense Sequence
Drug Composition | | Broadly Applicable 
Specific | | Drug Design Motif, Target and sequence independent
Target and sequence independent
Sequence independent
Chemistry independent
Specific claim to drug candidates |
Chemically Modified Nucleosides and Oligonucleotides
The most broadly-applicable of our patents are those that claim modified nucleosides and oligonucleotides comprising the modified nucleosides that we incorporate into our antisense drugs to increase their therapeutic efficacy. Nucleosides and chemically-modified nucleosides are the basic building blocks of our antisense drugs, therefore claims that cover any oligonucleotide incorporating one of our proprietary modified nucleosides can apply to a wide array of antisense mechanisms of action as well as several therapeutic targets. Of particular note are our patents covering our proprietary 2’-O-(2-methoxy) ethyl modified nucleosides, incorporated into nearly all of our development compounds, as well as our lead candidate modification for our generation 2.5 compounds, the constrained-ethyl nucleosides, or cEt, nucleosides. In June 2011, Santaris opposed our granted patent in Europe drawn to cEt containing nucleotides and oligonucleotides and we intend to vigorously defend our patent in these proceedings. The following are some of our patents in this category:
30
Jurisdiction | | Patent No. | | Title | | Expiration | | Description of Claims |
US | | 5,914,396 | | 2’-O-MODIFIED NUCLEOSIDES AND PHOSPHORAMIDITES | | 2016 | | Covers MOE nucleosides and oligonucleotides containing said nucleotides. |
US | | 7,101,993 | | OLIGONUCLEOTIDES CONTAINING 2’O-MODIFIED PURINES | | 2023 | | Covers certain MOE nucleosides and oligonucleotides containing said nucleotides. |
US | | 7,399,845 | | 6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers our cEt nucleoside analog and oligonucleotides containing these nucleoside analogs. |
US | | 7,741,457 | | 6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers our cEt nucleoside analog and oligonucleotides containing these nucleoside analogs. |
US | | 8,022,193 | | 6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers our cEt nucleoside analog and oligonucleotides containing these nucleoside analogs. |
US | | 7,569,686 | | COMPOUNDS AND METHODS FOR SYNTHESIS OF BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers methods of synthesizing our cEt nucleoside analog. |
EP | | EP1984381 | | 6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers our cEt nucleoside analog and oligonucleotides containing these nucleoside analogs. |
US | | 7,547,684 | | 6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers our cEt nucleoside analog and oligonucleotides containing these nucleoside analogs. |
US | | 7,666,854 | | 6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers our cEt nucleoside analog and oligonucleotides containing these nucleoside analogs. |
Antisense Drug Design Motifs
MOE Gapmers
Other Isis patents claim oligonucleotides comprising specific antisense drug design motifs, or patterns of nucleoside modifications at specified positions in the oligonucleotide. Patent claims covering our antisense drug design motifs are independent of nucleic acid sequence, so they cover oligonucleotides having the recited motif, regardless of cellular target or clinical indication. The claimed motifs generally confer properties that optimize oligonucleotides for a particular antisense mechanism of action, such as RNase H, RNAi, or splicing. We have designed oligonucleotides incorporating motifs, which we refer to as chimeric compounds or gapmers to exploit the RNase H mechanism to achieve target RNA reduction. Almost all of our drugs, including KYNAMRO, contain this gapmer antisense drug design motif. In fact, we own a U.S. patent that covers each of our second generation antisense drugs until March of 2023. EP0618925 and EP1695979 are the European family members of U.S. Patent No. 6,326,199 which we are enforcing against Santaris Pharma (see Legal Proceedings on page 45). Santaris opposed unsuccessfully the ‘925 Patent and now Santaris has recently filed an opposition against the ‘979 Patent which was recently granted. We also have issued patents covering other gapmer drug designs, including our generation 2.2 drug designs which optimize gap size and overall length of the oligonucleotide and methods of lowering a target RNA in an animal with these gapmer compositions. The following patents are some examples of our patents in this category:
31
Jurisdiction | | Patent/
Application
No. | | Title | | Expiration | | Description of Claims |
US | | 7,015,315 | | GAPPED OLIGONUCLEOTIDES | | 2023 | | Covers 2’-O-alkyl-O-alkyl gapmer oligonucleotides. |
EP | | EP0618925 | | GAPPED 2’ MODIFIED PHOSPHOROTHIOATE OLIGONUCLEOTIDES | | 2012 | | Covers 2’-substituted phosphorothioate gapmer oligonucleotides. |
EP | | EP1695979 | | GAPPED 2’ MODIFIED OLIGONUCLEOTIDES | | 2012 | | Covers modified phosphorothioate gapmer oligonucleotides. |
US | | 7,919,472 | | ENHANCED ANTISENSE OLIGONUCLEOTIDES | | 2026 | | Covers methods of lowering a target RNA in an animal with a MOE gapmer with a DNA gap of 12 to 18 nucleotides. |
EP | | EP2021472 | | COMPOUNDS AND METHODS FOR MODULATING GENE EXPRESSION | | 2027 | | Short gapmer oligonucleotides, 10 to 14 nucleotides in length, with bicyclic nucleosides, which includes locked nucleic acids, in the wings for the treatment of cardiovascular or metabolic disorders |
Bicyclic Nucleoside Gapmer Oligonucleotides
In addition, we have pursued claims to antisense drug design motifs incorporating bicyclic nucleoside analogs, which include locked nucleic acids, or LNAs. In June 2011, the European Patent Office, or EPO, granted our claims drawn to short gapmer oligonucleotides, 10 to 14 nucleotides in length, with bicyclic nucleosides, which includes locked nucleic acids, in the wings for the treatment of cardiovascular or metabolic disorders. We have also successfully obtained issued patent claims covering gapmer antisense drug design motifs that incorporate our cEt modified nucleosides. The following patents are some examples of our issued patents and allowed patent applications in this category:
Jurisdiction | | Patent/
Application
No. | | Title | | Expiration | | Description of Claims |
EP | | EP2021472 | | COMPOUNDS AND METHODS FOR MODULATING GENE EXPRESSION | | 2027 | | Short gapmer oligonucleotides, 10 to 14 nucleotides in length, with bicyclic nucleosides, which includes locked nucleic acids, in the wings for the treatment of cardiovascular or metabolic disorders |
US | | 7,750,131 | | 6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS | | 2027 | | Covers cEt containing gapmer compounds |
Therapeutic Methods of Treatment and Antisense Drug Sequences
In addition to our broad core patents, we also own more than 600 patents, worldwide, with claims to antisense sequences and compounds directed to particular therapeutically important targets or methods of achieving clinical endpoints using these antisense compounds. Target patents may also include claims reciting the specific nucleic acid sequences utilized by our products, independent of chemical modifications and motifs. In addition, our product specific patents typically include claims combining specific nucleic acid sequences with nucleoside modifications and motifs. In this way, we seek patent claims narrowly tailored to protect our products specifically, in addition to the broader core antisense patents described above.
32
ApoB 100 and KYNAMRO
In 2008, we obtained patent claims in the United States drawn to the use of both single-stranded and double-stranded antisense drugs complementary to any site of the mRNA of human apoB, regardless of chemistry or antisense mechanism of action. The patent provides broad protection of the Isis-Genzyme apoB franchise, including KYNAMRO and potential future follow-on compounds. Similar claims granted in Australia and Japan in 2009 and 2010, respectively. We and Genzyme obtained issued claims to the specific antisense sequence and chemical composition of KYNAMRO in the United States and India. The issued U.S. claims should protect KYNAMRO from generic competition in the United States until at least 2025. We are also pursuing additional patent applications designed to protect KYNAMRO in these and other jurisdictions including in the European Union via the European Patent Convention, Canada, Australia and Japan. The table below lists the key issued patent claims designed to protect KYNAMRO in the applicable jurisdiction:
Jurisdiction | | Patent No. | | Title | | Expiration | | Description of Claims |
US | | 7,407,943 | | ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION | | 2021 | | Methods of inhibiting expression of apoB, decreasing serum cholesterol, decreasing lipoprotein levels, decreasing serum triglycerides in a human with an antisense compound 12 to 30 nucleotide in length and 100% complementary to human apoB. |
Australia | | 2002-326481 | | ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION | | 2021 | | An isolated oligonucleotide compound 12 to 30 nucleobases in length 100% complementary to at least a 12-nucleobase portion of a nucleic acid molecule having nucleotides 151-12820 of SEQ ID 3 (apoB) which is not a ribozyme and use of such compound in therapy. |
Japan | | 4471650 | | ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION | | 2021 | | Use of an antisense oligonucleotide 12 to 30 nucleobases in length and 100% complementary to human apoB having one or more modifications and inhibiting expression of apoB by at least 90% in primary hepatocytes when present at a concentration of 300 nM for preparation of a medicament for decreasing serum cholesterol, and decreasing lipoprotein levels in a human. |
US | | 7,511,131 | | ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION | | 2025 | | Antisense sequence and composition of matter of KYNAMRO. |
India | | 219847 | | ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION | | 2023 | | Antisense sequence and composition of matter of KYNAMRO. |
RNAi Motifs and Mechanisms - The Crooke Patents
The Crooke Patents, which are the result of the early work by Dr. Crooke and co-workers exploring oligonucleotides that activate double-stranded ribonucleases, or dsRNases, cover chemically-modified, RNA-containing oligonucleotides and methods for exploiting the RNAi pathway with these oligonucleotides until June 2016. We licensed the Crooke Patents to Alnylam for the development of double-stranded therapeutics and to Regulus for the development of microRNA-targeting therapeutics. These patents also provide us with exclusivity in the field of ssRNAi compounds, in which we have made great strides to progress this approach toward a viable therapeutic platform. The following patents have issued out of the Crooke Patent family:
33
Jurisdiction | | Patent No. | | Title | | Expiration | | Description of Claims |
US | | 5,898,031 | | OLIGORIBONUCLEOTIDES FOR CLEAVING RNA | | 2016 | | Oligonucleotides comprising regions of RNA nucleosides and regions of nucleosides having stabilizing chemical modifications. Such oligonucleotides are suitable for use in single- and double-stranded applications. |
US | | 6,107,094 | | OLIGORIBONUCLEOTIDES FOR CLEAVING RNA | | 2016 | | Compounds and methods that use oligonucleotides having both RNA nucleosides and chemically modified nucleosides, including methods that rely on a dsRNAse to reduce target RNA and compounds having nucleosides with improved affinity and/or stability. |
US | | 7,432,249 | | OLIGORIBONUCLEOTIDES FOR CLEAVING RNA | | 2016 | | Pharmaceutical compositions comprising a diluent or carrier and a single-stranded antisense oligonucleotide having a plurality of RNA nucleosides and at least one sugar modification. |
US | | 7,432,250 | | OLIGORIBONUCLEOTIDES FOR CLEAVING RNA | | 2016 | | Methods for treating a patient by administering an antisense compound having a plurality of RNA nucleosides and at least one sugar modification. |
US | | 7,629,321 | | OLIGORIBONUCLEOTIDES FOR CLEAVING RNA | | 2016 | | Methods for cleaving a target RNA in a cell by contacting the cell with a single-stranded antisense compound having a plurality of RNA nucleosides and at least one sugar modification. |
US | | 7,695,902 | | OLIGORIBONUCLEOTIDES FOR CLEAVING RNA | | 2016 | | Methods of activating a dsRNase by contacting the dsRNase with a double-stranded antisense oligonucleotide where at least one strand has a plurality of RNA nucleosides and at least one sugar modification. The methods may be performed inside a cell. |
Manufacturing Patents
We also own patents claiming methods of manufacturing and purifying oligonucleotides. These patents claim methods for improving oligonucleotide drug manufacturing, including processes for large-scale oligonucleotide synthesis and purification. These methods allow us to manufacture oligonucleotides at lower cost by, for example, eliminating expensive manufacturing steps.
Government Regulation
Regulation by government authorities in the United States and other countries is a significant component in the development, manufacture and commercialization of pharmaceutical products and services. In addition to regulations enforced by the FDA and relevant foreign regulatory authorities, we are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential future federal, state and local regulations.
Extensive regulation by United States and foreign governmental authorities governs our manufacture, development and potential sale of therapeutics. In particular, pharmaceutical products are subject to nonclinical and clinical testing, as well as other approval requirements, by the FDA in the United States under the Federal Food, Drug and Cosmetic Act and other laws and by comparable agencies in those foreign countries in which we conduct business. Various federal, state and foreign statutes also govern or influence the manufacture, safety, labeling, storage, record keeping, marketing and quality of our products. State, local, and other authorities also regulate pharmaceutical manufacturing facilities and procedures.
In conjunction with obtaining approval of Vitravene, we successfully passed the manufacturing pre-approval inspection by the FDA and European regulatory authorities. Approval of each new drug, including KYNAMRO, will require a rigorous manufacturing pre-approval inspection by regulatory authorities.
34
Competition
Our Business in General
For many of their applications, our drugs will compete with existing therapies for market share. In addition, there are a number of companies pursuing the development of oligonucleotide-based technology and the development of pharmaceuticals utilizing this technology. These companies include specialized pharmaceutical firms and large pharmaceutical companies acting either independently or together with biopharmaceutical companies.
Our products under development address numerous markets. The diseases our drugs target for which we may receive regulatory approval will determine our competition. For certain of our products, an important factor in competition may be the timing of market introduction of competitive products. Accordingly, the relative speed with which we can develop products, complete the clinical trials and approval processes and supply commercial quantities of the products to the market are important competitive factors. We expect to compete among products approved for sale based on a variety of factors, including, among other things, product efficacy, safety, reliability, availability, price, reimbursement and patent position.
KYNAMRO
In July 2011, Genzyme submitted a marketing application for KYNAMRO in Europe for patients with hoFH and severe heFH. Genzyme plans to file an NDA with the FDA in the United States for patients with hoFH in the first quarter of 2012. Apheresis and maximally tolerated lipid-lowering therapies, including statins, are the standard of care for these homozygous FH and severe heterozygous FH patients. Apheresis is a two to four hour process administered two to four times a month that mechanically separates LDL-C from the blood. Because apheresis is an invasive, time-consuming procedure conducted only in specialty centers, it can be difficult for patients to receive this treatment.
In addition, we expect that therapies currently in development may compete with KYNAMRO in this initial market. Other than KYNAMRO, the most advanced therapy currently in development is Aegerion Pharmaceuticals’ lomitapide, a small molecule drug that limits secretion of cholesterol and triglycerides from the intestines and the liver. Aegerion is initially developing lomitapide as an oral, once-a-day treatment for patients with homozygous FH. Aegerion is currently evaluating lomitapide in a Phase 3 study in 29 patients with homozygous FH. In earlier studies evaluating lomitapide, patients discontinued use of lomitapide at a high rate due to gastrointestinal adverse events, such as diarrhea, nausea and vomiting. In addition, some patients experienced elevations in liver enzymes and increased mean levels of fat in the liver, or hepatic fat, both of which Aegerion states it has observed in its ongoing Phase 3 clinical trial of lomitapide. Aegerion also states that patients in its ongoing Phase 3 trial have also experienced adverse gastrointestinal events. Aegerion states that before they submit an NDA for lomitapide to the FDA in 2012 they must complete additional clinical and non-clinical studies to assess various other aspects of lomitapide. Aegerion has stated it plans to submit an NDA and Marketing Authorization Application, or MAA, before the end of the first quarter 2012.
We believe that the overall profile of KYNAMRO provides significant competitive advantage over potential competitors. In our clinical experience with KYNAMRO, we have seen substantial reductions in LDL-C as well as reductions in other atherogenic lipids linked to cardiovascular disease, including apoB, Lp(a), triglycerides and VLDL. In our Phase 3 studies that evaluated KYNAMRO in more than 250 patients, the most common adverse events patients observed were injection site reactions and flu-like symptoms. We also observed elevations in liver transaminases and moderate median increases in liver fat that appeared to be associated with greater reductions in apoB. We believe that this safety profile supports our initial market opportunity in patients who cannot currently reach their recommended LDL-C goal. KYNAMRO is administered by injection once weekly while patients take lomitapide orally once daily. If KYNAMRO’s product profile is not advantageous when compared to an oral drug, some patients may prefer the oral drug over KYNAMRO. Factors affecting a product’s profile may include, efficacy, side effects, pricing and reimbursement.
Aegerion has no commercial partner for lomitapide and Aegerion has stated that it will need to build its own sales, marketing and commercial infrastructure. Our partner, Genzyme, has extensive experience in bringing medicines to patients with severe and rare diseases with an existing global commercial infrastructure in the cardiovascular community in Europe. In addition, we believe that Sanofi and its global presence will aid in the rapid expansion of KYNAMRO into markets throughout the world. In the United States, Genzyme also intends to capitalize on its existing sales and marketing infrastructure within specialized medical communities.
35
Employees
As of February 13, 2012, we employed approximately 281 people in all of our functions, excluding manufacturing and related departments, which employed approximately 56 people. A significant number of our management and professional employees have had prior experience with pharmaceutical, biotechnology or medical product companies. Collective bargaining agreements do not cover any of our employees, and management considers relations with our employees to be good.
Executive Officers of Isis
The following sets forth certain information regarding our executive officers as of February 8, 2012:
Name | | Age | | Position |
Stanley T. Crooke, M.D., Ph.D. | | 66 | | Chairman, Chief Executive Officer and President |
B. Lynne Parshall, J.D. | | 56 | | Director, Chief Operating Officer, Chief Financial Officer and Secretary |
C. Frank Bennett, Ph.D. | | 55 | | Senior Vice President, Antisense Research |
Richard S. Geary, Ph.D. | | 54 | | Senior Vice President, Development |
Brett Monia, Ph.D. | | 51 | | Senior Vice President, Drug Discovery and Corporate Development |
STANLEY T. CROOKE, M.D., Ph.D.
Chairman, Chief Executive Officer and President
Dr. Crooke is a founder of Isis and has been Chief Executive Officer and a Director since January 1989. He was elected Chairman of the Board in February 1991. Prior to founding Isis, from 1980 until January 1989, Dr. Crooke was employed by SmithKline Beckman Corporation, a pharmaceutical company, where his titles included President of Research and Development of SmithKline and French Laboratories.
B. LYNNE PARSHALL, J.D.
Director, Chief Operating Officer, Chief Financial Officer and Secretary
Ms. Parshall has served as a Director of Isis since September 2000. She was promoted to Chief Operating Officer in December 2007 and previously served as an Executive Vice President since December 1995. She has served as our Chief Financial Officer since June 1994, and our Secretary since November 1991. From February 1993 to December 1995, she was a Senior Vice President of Isis, and from November 1991 to February 1993, she was a Vice President of Isis. Prior to joining Isis, Ms. Parshall practiced law at Cooley LLP, outside counsel to Isis, where she was a partner from 1986 to 1991. Ms. Parshall is a member of the American, California and San Diego bar associations.
C. FRANK BENNETT, Ph.D.
Senior Vice President, Antisense Research
Dr. Bennett was promoted to Senior Vice President, Antisense Research in January 2006. From June 1995 to January 2006, Dr. Bennett served as our Vice President, Research. From March 1993 to June 1995, he was Director, Molecular Pharmacology, and from May 1992 to March 1993, he was an Associate Director in our Molecular and Cellular Biology department. Prior to joining Isis in 1989, Dr. Bennett was employed by SmithKline and French Laboratories in various research positions. He is an external member of the Scientific Advisory Board of Experimental Therapeutics Center in Singapore.
RICHARD S. GEARY, Ph.D.
Senior Vice President, Development
Dr. Geary was promoted to Senior Vice President, Development in August 2008. From August 2003 to August 2008, Dr. Geary served as our Vice President, Preclinical Development. From November 1995 to August 2003, he held various positions within the Preclinical Development department. Prior to joining Isis in 1995, Dr. Geary was Senior Research Scientist and Group Leader for the bioanalytical and preclinical pharmacokinetics group in the Applied Chemistry Department at Southwest Research Institute.
36
BRETT MONIA, Ph.D.
Senior Vice President, Drug Discovery and Corporate Development
Dr. Monia was promoted to Senior Vice President, Drug Discovery and Corporate Development in January 2012. From February 2009 to January 2012, Dr. Monia served as our Vice President, Drug Discovery and Corporate Development and from October 2000 to February 2009, he served as our Vice President, Preclinical Drug Discovery. From October 1989 to October 2000 he held various positions within our Molecular Pharmacology department.
Item 1A. Risk Factors
Investing in our securities involves a high degree of risk. You should consider carefully the following information about the risks described below, together with the other information contained in this report and in our other public filings in evaluating our business. If any of the following risks actually occur, our business could be materially harmed, and our financial condition and results of operations could be materially and adversely affected. As a result, the trading price of our securities could decline, and you might lose all or part of your investment.
Risks Associated with our Drug Discovery and Development Business
If we or our partners fail to obtain regulatory approval for our drugs, including KYNAMRO, we cannot sell them.
We cannot guarantee that any of our drugs, including KYNAMRO, will be safe and effective, or will be approved for commercialization. We and our partners must conduct time-consuming, extensive and costly clinical studies to show the safety and efficacy of each of our drugs, including KYNAMRO, before a drug can be approved for sale. We must conduct these studies in compliance with Food and Drug Administration, or FDA, regulations and with comparable regulations in other countries.
We and our partners may not obtain necessary regulatory approvals on a timely basis, if at all, for any of our drugs, including KYNAMRO. Even though Genzyme has filed for marketing approval for KYNAMRO in Europe and plans to file for marketing approval for KYNAMRO in the United States, it is possible that regulatory agencies will not approve KYNAMRO for marketing. If the FDA or another regulatory agency believes that we or our partners have not sufficiently demonstrated the safety or efficacy of any of our drugs, including KYNAMRO, the agency will not approve the specific drug or will require additional studies, which can be time consuming and expensive and which will delay commercialization of the drug.
Failure to receive marketing approval for our drugs, including KYNAMRO, or delays in these approvals could prevent or delay commercial introduction of the drug, and, as a result, could negatively impact our ability to generate revenue from product sales.
If the results of clinical testing indicate that any of our drugs, including KYNAMRO, are not suitable for commercial use we may need to abandon one or more of our drug development programs.
Drug discovery and development has inherent risks and the historical failure rate for drugs is high. Antisense drugs are a relatively new approach to therapeutics. If we cannot demonstrate that our drugs, including KYNAMRO, are safe and effective for human use, we may need to abandon one or more of our drug development programs.
In the past, we have invested in clinical studies of drugs that have not met the primary clinical end points in their Phase 3 studies. Similar results could occur with any additional clinical studies for KYNAMRO and in clinical studies for our other drugs. If any of our drugs in clinical studies, including KYNAMRO, does not show sufficient efficacy in patients with the targeted indication, it could negatively impact our development and commercialization goals for the drug and our stock price could decline.
Even if our drugs are successful in preclinical and human clinical studies, the drugs may not be successful in late-stage clinical studies.
Successful results in preclinical or initial human clinical studies, including the Phase 3 results for KYNAMRO and the Phase 2 results for some of our other drugs in development, may not predict the results of subsequent clinical studies, including subsequent studies of KYNAMRO. There are a number of factors that could cause a clinical study to fail or be delayed, including:
· the clinical study may produce negative or inconclusive results;
· regulators may require that we hold, suspend or terminate clinical research for noncompliance with regulatory requirements;
37
· we, our partners, the FDA or foreign regulatory authorities could suspend or terminate a clinical study due to adverse side effects of a drug on subjects in the trial;
· we may decide, or regulators may require us, to conduct additional preclinical testing or clinical studies;
· enrollment in our clinical studies may be slower than we anticipate;
· the cost of our clinical studies may be greater than we anticipate; and
· the supply or quality of our drugs or other materials necessary to conduct our clinical studies may be insufficient, inadequate or delayed.
Any failure or delay in our clinical studies, including any further studies under our development program for KYNAMRO, could reduce the commercial potential or viability of our drugs.
Even if approved, KYNAMRO and any of our other drugs may be subject to regulatory limitations.
Following approval of a drug, we and our partners must comply with comprehensive government regulations regarding how we manufacture, market and distribute drug products. Even if approved, we may not obtain the labeling claims necessary or desirable for successfully commercializing our drug products, including KYNAMRO. The FDA and foreign regulatory authorities have the authority to impose significant restrictions on an approved drug product through the product label and on advertising, promotional and distribution activities. If approved, the FDA or a foreign regulatory authority may condition approval on the performance of post-approval clinical studies or patient monitoring, which could be time consuming and expensive. If the results of such post-marketing studies are not satisfactory, the FDA or a foreign regulatory authority may withdraw marketing authorization or may condition continued marketing on commitments from us or our partners that may be expensive and/or time consuming to fulfill. In addition, if we or others identify side effects after any of our drug products are on the market, or if manufacturing problems occur subsequent to regulatory approval, we may lose regulatory approval, or we may need to conduct additional clinical studies and/or change the labeling of our drug products including KYNAMRO.
If the market does not accept KYNAMRO or our other drugs, we are not likely to generate revenues or become consistently profitable.
If KYNAMRO or any of our other drugs is approved for marketing, our success will depend upon the medical community, patients and third party payors accepting our drug as medically useful, cost-effective and safe. Even if the FDA or foreign regulatory authorities approve KYNAMRO or our other drugs for commercialization, doctors may not use our drugs to treat patients. For example, we currently have one commercially approved drug, Vitravene, a treatment for CMV retinitis in AIDS patients, which our partner is no longer marketing due to a dramatic decline in the incidence of CMV retinitis in AIDS patients. We and our partners may not successfully commercialize additional drugs.
Additionally, in many of the markets where we may sell our drugs in the future, if we cannot agree with the government regarding the price we can charge for our drugs, then we may not be able to sell our drugs in that market.
The degree of market acceptance for KYNAMRO, and any of our other drugs, depends upon a number of factors, including the:
· receipt and scope of regulatory approvals;
· establishment and demonstration in the medical and patient community of the efficacy and safety of our drugs and their potential advantages over competing products;
· cost and effectiveness of our drugs compared to other available therapies;
· patient convenience of the dosing regimen for our drugs; and
· reimbursement policies of government and third-party payors.
38
Based on the profile of our drugs, physicians, patients, patient advocates, payors or the medical community in general may not accept and/or use any drugs that we may develop. In addition, cost control initiatives by governments or third party payors could decrease the price that we receive for KYNAMRO or our other drugs or increase patient coinsurance to a level that makes KYNAMRO or our other drugs unaffordable.
We depend on our collaboration with Genzyme for the development and commercialization of KYNAMRO.
We have entered into a collaborative arrangement with Genzyme to develop and commercialize KYNAMRO.
We entered into this collaboration primarily to:
· fund some of our development activities for KYNAMRO;
· seek and obtain regulatory approvals for KYNAMRO; and
· successfully commercialize KYNAMRO.
In general, we cannot control the amount and timing of resources that Genzyme devotes to our collaboration. If Genzyme fails to further develop and commercialize KYNAMRO, or if Genzyme’s efforts are not effective, our business may be negatively affected. We are relying on Genzyme to obtain marketing approvals for and successfully commercialize KYNAMRO. Our collaboration with Genzyme may not continue or result in the successful commercialization of KYNAMRO. Genzyme can terminate our collaboration at any time. If Genzyme stopped developing or commercializing KYNAMRO, we would have to seek additional sources for funding and may have to delay or reduce our development and commercialization programs for KYNAMRO. If Genzyme does not successfully commercialize KYNAMRO, we may receive limited or no revenues for KYNAMRO. In addition, Sanofi recently acquired Genzyme which could disrupt Genzyme or distract it from performing its obligations under our collaboration.
If Genzyme cannot manufacture finished drug product for KYNAMRO or the post-launch supply of the active drug substance for KYNAMRO, KYNAMRO may not achieve or maintain commercial success.
We believe that our manufacturing facility has sufficient capacity to supply the drug substance necessary for the initial commercial launch of KYNAMRO, if approved. However, we rely on Genzyme to manufacture the finished drug product for KYNAMRO, including the initial commercial launch supply. In addition, if approved, Genzyme will be responsible for the long term supply of both KYNAMRO drug substance and finished drug product. Genzyme may not be able to reliably manufacture KYNAMRO drug substance and drug product to support KYNAMRO’s long term commercialization. If Genzyme cannot reliably manufacture KYNAMRO drug substance and drug product, KYNAMRO may not achieve or maintain commercial success, which will harm our ability to generate revenue.
If we cannot manufacture our drugs or contract with a third party to manufacture our drugs at costs that allow us to charge competitive prices to buyers, we cannot market our products profitably.
To successfully commercialize any of our drugs, we or our partner would need to establish large-scale commercial manufacturing capabilities either on our own or through a third party manufacturer. In addition, as our drug development pipeline increases and matures, we will have a greater need for clinical trial and commercial manufacturing capacity. We have limited experience manufacturing pharmaceutical products of the chemical class represented by our drugs, called oligonucleotides, on a commercial scale for the systemic administration of a drug. There are a small number of suppliers for certain capital equipment and raw materials that we use to manufacture our drugs, and some of these suppliers will need to increase their scale of production to meet our projected needs for commercial manufacturing. Further, we must continue to improve our manufacturing processes to allow us to reduce our drug costs. We may not be able to manufacture our drugs at a cost or in quantities necessary to make commercially successful products.
Also, manufacturers, including us, must adhere to the FDA’s current Good Manufacturing Practices regulations and similar regulations in foreign countries, which the applicable regulatory authorities enforce through facilities inspection programs. We and our contract manufacturers may not comply or maintain compliance with Good Manufacturing Practices, or similar foreign regulations. Non-compliance could significantly delay or prevent our receipt of marketing approval for our drugs, including KYNAMRO, or result in enforcement action after approval that could limit the commercial success of our drugs, including KYNAMRO.
39
If our drug discovery and development business fails to compete effectively, our drugs will not contribute significant revenues.
Our competitors engage in all areas of drug discovery throughout the world, are numerous, and include, among others, major pharmaceutical companies and specialized biopharmaceutical firms. Other companies engage in developing antisense technology. Our competitors may succeed in developing drugs that are:
· priced lower than our drugs;
· safer than our drugs;
· more effective than our drugs; or
· more convenient to use than our drugs.
These competitive developments could make our drugs, including KYNAMRO, obsolete or non-competitive.
Certain of our partners are pursuing other technologies or developing other drugs either on their own or in collaboration with others, including our competitors, to treat the same diseases our own collaborative programs target. Competition may negatively impact a partner’s focus on and commitment to our drugs and, as a result, could delay or otherwise negatively affect the commercialization of our drugs, including KYNAMRO.
Many of our competitors have substantially greater financial, technical and human resources than we do. In addition, many of these competitors have significantly greater experience than we do in conducting preclinical testing and human clinical studies of new pharmaceutical products and in obtaining FDA and other regulatory approvals of products for use in health care. Accordingly, our competitors may succeed in obtaining regulatory approval for products earlier than we do. Marketing and sales capability is another factor relevant to the competitive position of our drugs, and we will rely on our partners to provide this capability.
Regarding KYNAMRO, some competitors are pursuing a development strategy that competes with our strategy for KYNAMRO. Other companies are currently developing products that could compete with KYNAMRO. For example, products such as microsomal triglyceride transfer protein inhibitors, or MTP inhibitors, and other lipid lowering drugs other companies are developing could potentially compete with KYNAMRO. For example, Aegerion is currently evaluating its MTP inhibitor in a Phase 3 study in homozygous FH patients. Our revenues and financial position will suffer if KYNAMRO receives regulatory approval, but cannot compete effectively in the marketplace.
We depend on third parties to conduct our clinical studies for our drugs and any failure of those parties to fulfill their obligations could adversely affect our development and commercialization plans.
We depend on independent clinical investigators, contract research organizations and other third-party service providers to conduct our clinical studies for our drugs and expect to continue to do so in the future. For example, Medpace is the primary clinical research organization for clinical studies for KYNAMRO. We rely heavily on these parties for successful execution of our clinical studies, but do not control many aspects of their activities. For example, the investigators are not our employees. However, we are responsible for ensuring that these third parties conduct each of our clinical studies in accordance with the general investigational plan and approved protocols for the study. Third parties may not complete activities on schedule, or may not conduct our clinical studies in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations or a termination of our relationship with these third parties could delay or prevent the development, approval and commercialization of our drugs, including KYNAMRO.
Risks Associated with our Businesses as a Whole
We have incurred losses, and our business will suffer if we fail to consistently achieve profitability in the future.
Because drug discovery and development requires substantial lead-time and money prior to commercialization, our expenses have generally exceeded our revenue since we were founded in January 1989. As of December 31, 2011, we had an accumulated deficit of approximately $841.5 million and stockholders’ equity of approximately $171.4 million. Most of the losses resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. Most of our revenue has come from collaborative arrangements, with additional revenue from research grants and the sale or licensing of our patents, as well as interest income. We have had only one product, Vitravene, approved for commercial use, but our exclusive distribution partner for this product no longer markets this product. We may incur additional operating losses over the next several years, and these losses may increase if we cannot increase or sustain revenue. We may not successfully develop any additional products or achieve or sustain future profitability.
40
Since corporate partnering is a key part of our strategy to fund the development and commercialization of our development programs, if any of our collaborative partners fail to fund our collaborative programs, or if we cannot obtain additional partners, we may have to delay or stop progress on our drug development programs.
To date, corporate partnering has played a key role in our strategy to fund our development programs and to add key development resources. We plan to continue to rely on additional collaborative arrangements to develop and commercialize our unpartnered drugs. However, we may not be able to negotiate favorable collaborative arrangements for these drug programs. If we cannot continue to secure additional collaborative partners, our revenues could decrease and the development of our drugs could suffer.
Our corporate partners are developing and/or funding many of the drugs in our development pipeline, including ATL, Atlantic Pharmaceuticals, Biogen Idec, iCo, Eli Lilly and Company, Genzyme, GSK, OncoGenex, Pfizer, and Teva Pharmaceutical Industries Ltd. If any of these pharmaceutical companies stops developing and/or funding these drugs, our business could suffer and we may not have, or be willing to dedicate, the resources available to develop these drugs on our own.
Our collaborators can terminate their relationships with us under certain circumstances, many of which are outside of our control. In the past, based on the disappointing results of Phase 3 clinical studies, we had a partner discontinue its investment in one of our drugs.
Even with funding from corporate partners, if our partners do not effectively perform their obligations under our agreements with them, it would delay or stop the progress of our drug development programs.
In addition to receiving funding, we enter into collaborative arrangements with third parties to:
· conduct clinical studies;
· seek and obtain regulatory approvals; and
· manufacture, market and sell our drugs.
Once we have secured a collaborative arrangement to further develop and commercialize one of our drug development programs, such as our collaborations with Genzyme, GSK, and Biogen Idec, these collaborations may not continue or result in commercialized drugs, or may not progress as quickly as we first anticipated.
For example, a collaborator such as Genzyme, GSK or Biogen Idec, could determine that it is in its financial interest to:
· pursue alternative technologies or develop alternative products that may be competitive with the drug that is part of the collaboration with us;
· pursue higher-priority programs or change the focus of its own development programs; or
· choose to devote fewer resources to our drugs than it does for its own drugs.
If any of these occur, it could affect our partner’s commitment to the collaboration with us and could delay or otherwise negatively affect the commercialization of our drugs, including KYNAMRO.
If we do not progress in our programs as anticipated, the price of our securities could decrease.
For planning purposes, we estimate and may disclose the timing of a variety of clinical, regulatory and other milestones, such as when we anticipate a certain drug will enter the clinic, when we anticipate completing a clinical study, or when we anticipate filing an application for marketing approval. We base our estimates on present facts and a variety of assumptions. Many underlying assumptions are outside of our control. If we do not achieve milestones in accordance with our or our investors’ expectations, including milestones for approval of KYNAMRO, the price of our securities would likely decrease.
41
For example, in April 2008 the FDA provided guidance regarding approval requirements for KYNAMRO. The FDA indicated that reduction of LDL-C is an acceptable surrogate endpoint for accelerated approval of KYNAMRO for use in patients with HoFH. The FDA required us to include data from two preclinical studies for carcinogenicity in the HoFH filing, which studies we have now completed. The FDA also indicated that for broader indications in high risk, high cholesterol patients the FDA would require an outcome study. This FDA guidance caused us to revise our development plans and timelines such that in July 2011 Genzyme filed for marketing approval in Europe for the treatment of patients with HoFH and patients with severe HeFH and plans to file for marketing approval for the treatment of patients with HoFH in the United States in the first quarter of 2012.
If we cannot protect our patents or our other proprietary rights, others may compete more effectively against us.
Our success depends to a significant degree upon whether we can continue to develop and secure intellectual property rights to proprietary products and services. However, we may not receive issued patents on any of our pending patent applications in the United States or in other countries. In addition, the scope of any of our issued patents may not be sufficiently broad to provide us with a competitive advantage. Furthermore, our issued patents or patents licensed to us may be successfully challenged, invalidated or circumvented so that our patent rights would not create an effective competitive barrier or revenue source.
Intellectual property litigation could be expensive and prevent us from pursuing our programs.
From time to time we have to defend our intellectual property rights. In the event of an intellectual property dispute, we sometimes need to litigate to defend our rights or assert them against others. Disputes can involve arbitration, litigation or proceedings declared by the United States Patent and Trademark Office or the International Trade Commission or foreign patent authorities. Intellectual property litigation can be extremely expensive, and this expense, as well as the consequences should we not prevail, could seriously harm our business. For example, in September 2011 we filed a patent infringement lawsuit against Santaris Pharma A/S and Santaris Pharma A/S Corp. in the United States District Court of the Southern District of California. This lawsuit may be costly and may not be resolved in our favor.
If a third party claims that our drugs or technology infringe its patents or other intellectual property rights, we may have to discontinue an important product or product line, alter our products and processes, pay license fees or cease certain activities. We may not be able to obtain a license to needed intellectual property on favorable terms, if at all. There are many patents issued or applied for in the biotechnology industry, and we may not be aware of patents or patent applications held by others that relate to our business. This is especially true since patent applications in the United States are filed confidentially for the first 18 months. Moreover, the validity and breadth of biotechnology patents involve complex legal and factual questions for which important legal issues remain unresolved.
If we fail to obtain timely funding, we may need to curtail or abandon some of our programs.
Many of our drugs are undergoing clinical studies or are in the early stages of research and development. All of our drug programs will require significant additional research, development, preclinical and/or clinical testing, regulatory approval and/or commitment of significant additional resources prior to their commercialization. As of December 31, 2011, we had cash, cash equivalents and short-term investments equal to $343.7 million. If we do not meet our goals to commercialize KYNAMRO or our other drugs, or to license our drugs and proprietary technologies, we will need additional funding in the future. Our future capital requirements will depend on many factors, such as the following:
· marketing approval and successful commercial launch of KYNAMRO;
· changes in existing collaborative relationships and our ability to establish and maintain additional collaborative arrangements;
· continued scientific progress in our research, drug discovery and development programs;
· the size of our programs and progress with preclinical and clinical studies;
· the time and costs involved in obtaining regulatory approvals;
· competing technological and market developments, including the introduction by others of new therapies that address our markets; and
· the profile and launch timing of our drugs.
42
If we need additional funds, we may need to raise them through public or private financing. Additional financing may not be available at all or on acceptable terms. If we raise additional funds by issuing equity securities, the shares of existing stockholders will be diluted and the price, as well as the price of our other securities, may decline. If adequate funds are not available or not available on acceptable terms, we may have to cut back on one or more of our research, drug discovery or development programs. For example, in January 2005 we terminated the development of two lower priority drugs, ISIS 14803 and ISIS 104838. Alternatively, we may obtain funds through arrangements with collaborative partners or others, which could require us to give up rights to certain of our technologies or drugs.
The loss of key personnel, or the inability to attract and retain highly skilled personnel, could make it more difficult to run our business and reduce our likelihood of success.
We are dependent on the principal members of our management and scientific staff. We do not have employment agreements with any of our executive officers that would prevent them from leaving us. The loss of our management and key scientific employees might slow the achievement of important research and development goals. It is also critical to our success that we recruit and retain qualified scientific personnel to perform research and development work. We may not be able to attract and retain skilled and experienced scientific personnel on acceptable terms because of intense competition for experienced scientists among many pharmaceutical and health care companies, universities and non-profit research institutions. In addition, failure to succeed in clinical studies may make it more challenging to recruit and retain qualified scientific personnel.
If the price of our securities continues to be highly volatile, this could make it harder for you to liquidate your investment and could increase your risk of suffering a loss.
The market price of our common stock, like that of the securities of many other biopharmaceutical companies, has been and is likely to continue to be highly volatile. These fluctuations in our common stock price may significantly affect the trading price of our securities. During the 12 months preceding December 31, 2011, the market price of our common stock ranged from $6.25 to $10.45 per share. Many factors can affect the market price of our securities, including, for example, fluctuations in our operating results, announcements of collaborations, clinical study results, technological innovations or new products being developed by us or our competitors, governmental regulation, regulatory approval, developments in patent or other proprietary rights, public concern regarding the safety of our drugs and general market conditions.
We are exposed to potential product liability claims, and insurance against these claims may not be available to us at a reasonable rate in the future or at all.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing, marketing and sale of therapeutic products. We have clinical study insurance coverage and commercial product liability insurance coverage. However, this insurance coverage may not be adequate to cover claims against us, or be available to us at an acceptable cost, if at all. Regardless of their merit or eventual outcome, products liability claims may result in decreased demand for our drug products, injury to our reputation, withdrawal of clinical study volunteers and loss of revenues. Thus, whether or not we are insured, a product liability claim or product recall may result in losses that could be material.
Because we use biological materials, hazardous materials, chemicals and radioactive compounds, if we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development and manufacturing activities involve the use of potentially harmful biological materials as well as materials, chemicals and various radioactive compounds that could be hazardous to human health and safety or the environment. We store these materials and various wastes resulting from their use at our facilities in Carlsbad, California pending ultimate use and disposal. We cannot completely eliminate the risk of contamination, which could cause:
· interruption of our research, development and manufacturing efforts;
· injury to our employees and others;
· environmental damage resulting in costly clean up; and
· liabilities under federal, state and local laws and regulations governing health and human safety, as well as the use, storage, handling and disposal of these materials and resultant waste products.
In such an event, we may be held liable for any resulting damages, and any liability could exceed our resources. Although we carry insurance in amounts and types that we consider commercially reasonable, we do not have insurance coverage for losses relating to an interruption of our research, development or manufacturing efforts caused by contamination, and the coverage or coverage limits of our insurance policies may not be adequate. If our losses exceed our insurance coverage, our financial condition would be adversely affected.
43
We depend on Regulus for development of our microRNA technology.
Regulus is a jointly owned company that we and Alnylam established to focus on discovering, developing, and commercializing microRNA therapeutics. We exclusively licensed to Regulus our intellectual property rights covering microRNA technology. Regulus operates as an independent company, governed by a board of directors. We and Alnylam can elect an equal number of directors to serve on the Regulus Board. Regulus researches and develops microRNA projects and programs pursuant to an operating plan that its board approves. However, Regulus and its employees are ultimately responsible for researching and developing our microRNA technology. If Regulus is not successful, the value of our microRNA technology would be harmed and we would lose part or all of our investment in Regulus.
If a natural or man-made disaster strikes our research, development or manufacturing facilities, it could delay our progress developing and commercializing our drugs.
We manufacture our research and clinical supplies in a manufacturing facility located in Carlsbad, California. The facilities and the equipment we use to research, develop and manufacture our drugs would be costly to replace and could require substantial lead time to repair or replace. Our facilities may be harmed by natural or man-made disasters, including, without limitation, earthquakes, floods, fires and acts of terrorism; and if our facilities are affected by a disaster, our development and commercialization efforts would be delayed. Although we possess insurance for damage to our property and the disruption of our business from casualties, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
Provisions in our certificate of incorporation, other agreements and Delaware law may prevent stockholders from receiving a premium for their shares.
Our certificate of incorporation provides for classified terms for the members of our board of directors. Our certificate also includes a provision that requires at least 66 2¤3 percent of our voting stockholders to approve a merger or certain other business transactions with, or proposed by, any holder of 15 percent or more of our voting stock, except in cases where certain directors approve the transaction or certain minimum price criteria and other procedural requirements are met.
Our certificate of incorporation also requires that any action required or permitted to be taken by our stockholders must be taken at a duly called annual or special meeting of stockholders and may not be taken by written consent. In addition, only our board of directors, chairman of the board or chief executive officer can call special meetings of our stockholders. We have in the past, and may in the future, implement a stockholders’ rights plan, also called a poison pill, which could make it uneconomical for a third party to acquire our company on a hostile basis. These provisions, as well as Delaware law and other of our agreements, may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over then current market prices, and may limit the ability of our stockholders to approve transactions that they think may be in their best interests. In addition, our board of directors has the authority to fix the rights and preferences of, and issue shares of preferred stock, which may have the effect of delaying or preventing a change in control of our company without action by our stockholders.
The provisions of our convertible subordinated notes could make it more difficult or more expensive for a third party to acquire us. Upon the occurrence of certain transactions constituting a fundamental change, holders of the notes will have the right, at their option, to require us to repurchase all of their notes or a portion of their notes, which may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over the then current market prices.
In addition, our collaboration agreement with Genzyme regarding KYNAMRO provides that if we are acquired, Genzyme may elect to purchase all of our rights to receive payments under the KYNAMRO collaboration agreement for a purchase price to be mutually agreed to by us and Genzyme, or, if we cannot agree, a fair market value price determined by an independent investment banking firm. This provision may make it more difficult or complicated for us to enter into an acquisition agreement with a potential acquirer.
Future sales of our common stock in the public market could adversely affect the trading price of our securities.
Future sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect trading prices of our securities. For example, we have registered for resale our 25/8 percent convertible subordinated notes, including the approximately 11.1 million shares issuable upon conversion of the notes. The addition of any of these shares into the public market may have an adverse effect on the price of our securities.
44
Our business is subject to changing regulations for corporate governance and public disclosure that has increased both our costs and the risk of noncompliance.
Each year we are required to evaluate our internal controls systems in order to allow management to report on and our Independent Registered Public Accounting Firm to attest to, our internal controls as required by Section 404 of the Sarbanes-Oxley Act. As a result, we continue to incur additional expenses and divert our management’s time to comply with these regulations. In addition, if we cannot continue to comply with the requirements of Section 404 in a timely manner, we might be subject to sanctions or investigation by regulatory authorities, such as the SEC, the Public Company Accounting Oversight Board, or PCAOB, or The Nasdaq Global Market. Any such action could adversely affect our financial results and the market price of our common stock.
The SEC and other regulators have continued to adopt new rules and regulations and make additional changes to existing regulations that require our compliance. On July 21, 2010, the Dodd-Frank Wall Street Reform and Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation-related provisions in the Dodd-Frank Act that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business.
Negative conditions in the global credit markets and financial services and other industries may adversely affect our business.
The global credit markets, the financial services industry, the U.S. capital markets, and the U.S. economy as a whole have been experiencing a period of substantial turmoil and uncertainty characterized by unprecedented intervention by the U.S. federal government and the failure, bankruptcy, or sale of various financial and other institutions. The impact of these events on our business and the severity of the economic crisis are uncertain. It is possible that the crisis in the global credit markets, the U.S. capital markets, the financial services industry and the U.S. economy may adversely affect our business, vendors and prospects as well as our liquidity and financial condition. More specifically, our insurance carriers and insurance policies covering all aspects of our business may become financially unstable or may not be sufficient to cover any or all of our losses and may not continue to be available to us on acceptable terms, or at all.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
As of February 13, 2012, we occupied three buildings in Carlsbad, California totaling approximately 231,000 square feet of laboratory, manufacturing and office space, including a 28,704 square foot facility, which houses our manufacturing suites for our drug development business built to meet Good Manufacturing Practices. We lease all three buildings under lease agreements. The leases on the 176,000 square foot facility that we use for laboratory and office space for our drug development business and our 28,704 square foot manufacturing facility expire in 2031 and have four five-year options to extend. Under these lease agreements, we have the option to purchase the facilities, independent of each other at the end of each year from 2016 through 2020, and at the end of 2026 and 2031. To support our manufacturing activities we lease 25,792 square feet of laboratory, office and storage space adjacent to our manufacturing facility. The lease has an initial term ending in June 2021 with an option to extend the lease for up to two five-year periods.
Item 3. Legal Proceedings
In September 2011, we filed a patent infringement lawsuit against Santaris Pharma A/S and Santaris Pharma A/S Corp. in the United States District Court of the Southern District of California. Our infringement lawsuit alleges that Santaris’ activities providing antisense drugs and antisense drug discovery services to several pharmaceutical companies infringes U.S. Patent No. 6,326,199, entitled “Gapped 2’ Modified Oligonucleotides” and U.S. Patent No. 6,066,500, entitled “Antisense Modulation of Beta Catenin Expression.” In the lawsuit we are seeking monetary damages and an injunction enjoining Santaris from conducting or participating in the infringing activities. In December 2011, Santaris filed an answer to our complaint, denying our allegations, and seeking a declaration from the court that Santaris has not, and does not, infringe the patents we asserted against Santaris in the suit. In January 2012, Santaris filed a motion for summary judgment asking the court to decide as a matter of law that Santaris’ activities do not infringe the patents we assert in the suit.
In January 2012, Alnylam Pharmaceuticals, Inc. filed a patent infringement lawsuit against Tekmira Pharmaceuticals Corporation in the U.S. District Court of the District of Massachusetts. Alnylam’s lawsuit alleges Tekmira has infringed a number of issued patents related to siRNA and LNP technologies, including: U.S. Patent No. 7,695,902; U.S. Patent No. 6,858,225; U.S. Patent No. 6,815,432; U.S. Patent No. 6,534,484; U.S. Patent No. 6,586,410; and, U.S. Patent No. 6,858,224. Under Alnylam’s contractual right to enforce Isis’ patent U.S. Patent No. 7,695,902, Alnylam joined us to the suit as a co-plaintiff.
Item 4. Mine Safety Disclosures
Not applicable.
45
PART II
Item 5. Market for Registrant’s Common Equity and Related Stockholder Matters and Issuer Repurchases of Equity Securities
Our common stock is traded publicly through The Nasdaq Global Select Market under the symbol “ISIS.” The following table presents quarterly information on the price range of our common stock. This information indicates the high and low sale prices reported by The Nasdaq Global Select Market. These prices do not include retail markups, markdowns or commissions.
| | HIGH | | LOW | |
2011 | | | | | |
First Quarter | | $ | 10.45 | | $ | 8.52 | |
Second Quarter | | $ | 9.49 | | $ | 8.25 | |
Third Quarter | | $ | 9.36 | | $ | 6.55 | |
Fourth Quarter | | $ | 8.67 | | $ | 6.25 | |
2010 | | | | | |
First Quarter | | $ | 11.82 | | $ | 8.59 | |
Second Quarter | | $ | 11.27 | | $ | 8.46 | |
Third Quarter | | $ | 10.19 | | $ | 7.59 | |
Fourth Quarter | | $ | 10.63 | | $ | 7.86 | |
As of February 21, 2012, there were approximately 814 stockholders of record of our common stock. We have never paid dividends and do not anticipate paying any dividends in the foreseeable future.
Set forth below is a table and chart comparing the total return on an indexed basis of $100 invested on December 31, 2006 in our common stock, the NASDAQ Composite Index (total return) and the NYSE Arca Biotechnology Index. The total return assumes reinvestment of dividends.
Performance Graph (1)
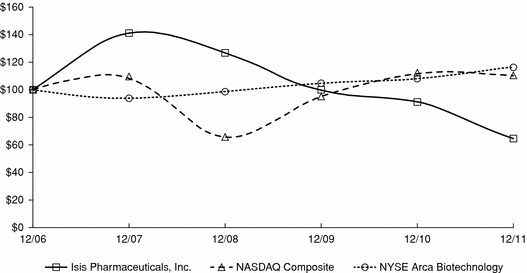
| | Dec-06 | | Dec-07 | | Dec-08 | | Dec-09 | | Dec-10 | | Dec-11 | |
Isis Pharmaceuticals, Inc. | | $ | 100 | | $ | 142 | | $ | 128 | | $ | 100 | | $ | 91 | | $ | 65 | |
NASDAQ Composite Index | | $ | 100 | | $ | 110 | | $ | 66 | | $ | 95 | | $ | 112 | | $ | 111 | |
NYSE Arca Biotechnology Index | | $ | 100 | | $ | 94 | | $ | 99 | | $ | 105 | | $ | 109 | | $ | 117 | |
(1) This section is not “soliciting material,” is not deemed “filed” with the SEC, is not subject to the liabilities of Section 18 of the Exchange Act and is not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
46
Item 6. Selected Consolidated Financial Data (in thousands, except per share amounts):
| | Years Ended December 31, | |
| | 2011 | | 2010 | | 2009 | | 2008 | | 2007 | |
Consolidated Statement of Operations Data: | | | | | | | | | | | |
Revenue(1) | | $ | 99,086 | | $ | 108,473 | | $ | 121,600 | | $ | 107,190 | | $ | 58,344 | |
Research and development expenses(1) | | $ | 157,397 | | $ | 145,160 | | $ | 134,623 | | $ | 106,439 | | $ | 78,204 | |
Net loss from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders(1)(2) | | $ | (84,801 | ) | $ | (61,251 | ) | $ | (30,562 | ) | $ | (9,785 | ) | $ | (10,264 | ) |
Net income (loss) attributable to Isis Pharmaceuticals, Inc. common stockholders(2) | | $ | (84,801 | ) | $ | (61,251 | ) | $ | 155,066 | | $ | (18,172 | ) | $ | (141,604 | ) |
Basic and diluted net loss per share from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders(1)(2) | | $ | (0.85 | ) | $ | (0.62 | ) | $ | (0.31 | ) | $ | (0.10 | ) | $ | (0.12 | ) |
Basic and diluted net income (loss) per share attributable to Isis Pharmaceuticals, Inc. common stockholders(2) | | $ | (0.85 | ) | $ | (0.62 | ) | $ | 1.58 | | $ | (0.19 | ) | $ | (1.69 | ) |
Shares used in computing basic and diluted net income (loss) per share | | | 99,656 | | | 99,143 | | | 98,109 | | | 94,566 | | | 83,739 | |
47
| | As of December 31, | |
| | 2011 | | 2010 | | 2009 | | 2008 | | 2007 | |
Consolidated Balance Sheet: | | | | | | | | | | | |
Cash, cash equivalents and short-term investments(3)(4) | | $ | 343,664 | | $ | 472,353 | | $ | 574,312 | | $ | 490,998 | | $ | 193,719 | |
Working capital(3)(4) | | $ | 284,027 | | $ | 377,247 | | $ | 484,682 | | $ | 393,685 | | $ | 147,669 | |
Total assets(4) | | $ | 484,894 | | $ | 550,477 | | $ | 657,184 | | $ | 572,776 | | $ | 257,216 | |
Long-term debt and other obligations, less current portion(3)(4) | | $ | 232,924 | | $ | 199,175 | | $ | 243,675 | | $ | 300,697 | | $ | 135,426 | |
Accumulated deficit(4) | | $ | (841,488 | ) | $ | (756,687 | ) | $ | (696,150 | ) | $ | (851,216 | ) | $ | (833,044 | ) |
Noncontrolling interest in Regulus Therapeutics Inc.(4) | | $ | — | | $ | — | | $ | 10,343 | | $ | 4,737 | | $ | 9,371 | |
Noncontrolling interest in Ibis Biosciences, Inc. | | $ | — | | $ | — | | $ | — | | $ | 32,419 | | $ | — | |
Investment in Regulus Therapeutics Inc.(4) | | $ | 4,424 | | 870 | | $ | — | | $ | — | | $ | — | |
Stockholders’ equity | | $ | 171,434 | | $ | 244,542 | | $ | 302,065 | | $ | 147,380 | | $ | 59,585 | |
(1) As a result of the sale of Ibis to AMI in 2009, we have adjusted our revenue; research and development expenses; net loss from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders; and net loss per share from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders to reflect Ibis’ results of operations as discontinued operations in 2009, 2008 and 2007.
(2) Our net loss from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders, net income (loss) attributable to Isis Pharmaceuticals, Inc. common stockholders and the related per share information include a charge of $125.3 million related to excess purchase price over carrying value of noncontrolling interest in Symphony GenIsis, Inc. in 2007.
(3) As a result of the sale of Ibis to AMI, we have adjusted our cash, cash equivalents and short-term investments balance; working capital; and long-term debt and other obligations balance at December 31, 2008 and our working capital at December 31, 2007 to reflect Ibis’ assets and liabilities as assets and liabilities from discontinued operations.
(4) Beginning in the first quarter of 2010, as a result of adopting a new accounting standard, we changed the way we account for our variable interest in Regulus. We adopted the new standard on a prospective basis; therefore, beginning in the first quarter of 2010, we deconsolidated Regulus from our consolidated financial statements and began to account for our ownership interest in Regulus using the equity method of accounting. We no longer include Regulus’ revenue and operating expenses in our operating results. Instead we include our share of Regulus’ operating results on a separate line in our consolidated statement of operations called “Equity in net loss of Regulus Therapeutics Inc.” On our consolidated balance sheet, we present our investment in Regulus on a separate line in the non-current liabilities section called “Investment in Regulus Therapeutics Inc.” We have not reclassified amounts in the prior period financial statements to conform to the current period presentation. For additional information, see Note 2, Investment in Regulus Therapeutics Inc.
48
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
We are the leading company in antisense drug discovery and development, exploiting a novel drug discovery platform we created to generate a broad pipeline of first-in-class drugs. Antisense technology provides a direct route from genomics to drugs. With our highly efficient and prolific drug discovery platform we can expand our pipeline and our partners’ pipelines with antisense drugs that address significant medical needs. Our strategy is to do what we do best—to discover unique antisense drugs and develop these drugs to key clinical value inflection points. We discover and conduct early development of new drugs and, at the key clinical value inflection points, outlicense our drugs to partners. We maximize the value of the drugs we discover by putting them in the hands of leading pharmaceutical companies with late-stage development, commercialization and marketing expertise, such as Biogen Idec, Bristol-Myers Squibb, Genzyme, a Sanofi company, GlaxoSmithKline, or GSK, and Eli Lilly and Company. For instance, our partner, Genzyme, plans to commercialize our lead product, KYNAMRO™, following planned regulatory approval in 2012. We also work with a consortium of smaller companies that can exploit our drugs and technologies in areas that are outside of our core focus. As a result of our unique strategy, we can keep our organization small and focused. Our strong financial position is a result of the successful execution of our business strategy as well as our inventive and focused research and development capabilities.
Our flagship product, KYNAMRO (formerly mipomersen), is moving closer to the market for patients with severe forms of FH, at high cardiovascular risk, who cannot reduce their low-density lipoprotein cholesterol, or LDL-C, sufficiently with currently available lipid-lowering therapies. In July 2011, Genzyme submitted a marketing application in Europe for KYNAMRO for patients with homozygous familial hypercholesterolemia, or hoFH, and severe heterozygous familial hypercholesterolemia, or severe heFH, and plans to submit the U.S. application for marketing approval for patients with hoFH in the first quarter of 2012. Genzyme plans to request priority review, if granted, in the United States and based on estimated approval times, Genzyme is preparing to launch KYNAMRO in the United States and Europe in 2012. Genzyme is also preparing to commercialize KYNAMRO in other major markets.
To maximize the value of our drugs and technologies, we have a multifaceted partnering strategy. We form traditional partnering alliances that enable us to discover and conduct early development of new drugs, outlicense our drugs to partners, such as Genzyme and Eli Lilly and Company, and build a broad base of license fees, milestone payments and royalty income. We also form preferred partner transactions that provide us with a vested partner, such as Biogen Idec and GSK, early in the development of a drug. In this way, we benefit in the short term from upfront option fees and development milestone payments while we maintain control over the early development of the drug. We benefit in the long term by having a knowledgeable and committed partner to license the drug at clinical proof-of-concept and by receiving regulatory milestone payments and royalties as our partner moves the drug to the market. In all of our partnerships, we benefit from the expertise our partners bring to our drugs. We also work with a consortium of smaller companies that can exploit our drugs and technologies. We call these smaller companies our satellite companies. In this way, we benefit from the disease-specific expertise of our satellite company partners, who are advancing drugs in our pipeline in areas that are outside of our core focus. In addition, we can maintain our broad RNA technology leadership through collaborations with companies such as Alnylam Pharmaceuticals, Inc., or Alnylam, and Regulus Therapeutics Inc., or Regulus, a company we jointly own focused on microRNA therapeutics. All of these different types of relationships are part of our unique business model and create near and long-term shareholder value.
The clinical successes of the drugs in our pipeline continue to create new partnering opportunities. For example, in January 2012, we formed a new strategic alliance with Biogen Idec to develop and commercialize ISIS-SMNRx to treat spinal muscular atrophy. We received a $29 million upfront payment and are eligible to receive up to $270 million in payments and double-digit royalties on sales from ISIS-SMNRx. Since 2007, our partnerships have generated an aggregate of more than $880 million in payments from upfront and licensing fees, equity purchase payments, milestone payments and research and development funding. In addition, for our current partnered programs we have the potential to earn more than $3.5 billion in future milestone payments. We also will share in the future commercial success of our inventions and drugs resulting from these partnerships through earn out, profit sharing, or royalty arrangements. Our strong financial position is a result of the successful execution of our business strategy as well as our inventive and focused research and development capabilities.
We protect our proprietary technologies and products through our substantial patent estate. As an innovator in RNA-targeting drug discovery and development, we design and execute our patent strategy to provide us with extensive protection for our drugs and our technology. With our ongoing research and development, we continue to add to our substantial patent estate. The patents not only protect our key assets—our technology and our drugs—they also form the basis for lucrative licensing and partnering arrangements. To date, we have generated over $400 million from our intellectual property sale and licensing program that helps support our internal drug discovery and development programs.
49
Business Segments
Prior to 2011, we reported our results in two separate segments: Drug Discovery and Development and Regulus. Beginning in 2011, we no longer consider Regulus as an operating segment because our chief decision making officer no longer reviews Regulus’ operating results for purposes of making resource allocations. Therefore we now only operate in, and will provide financial information and results for, our Drug Discovery and Development operations.
In our Drug Discovery and Development operations we are exploiting a novel drug discovery platform we created to generate a broad pipeline of first-in-class drugs for us and our partners. With our proprietary drug discovery platform we can rapidly identify drugs, providing a wealth of potential targets to treat a broad range of diseases. We focus our efforts in therapeutic areas where our drugs will work best, efficiently screening many targets in parallel and carefully selecting the best drugs. When we combine this efficiency with our rational approach to selecting disease targets we can build a large and diverse portfolio of drugs to treat a variety of health conditions, including cardiovascular, metabolic, inflammatory, ocular, severe and rare diseases, and cancer. We currently have 26 drugs in development. Our partners are developing, with our support, 10 of these 26 drugs, which substantially reduces our development costs.
Critical Accounting Policies
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the United States of America. As such, we make certain estimates, judgments and assumptions that we believe are reasonable, based upon the information available to us. These judgments involve making estimates about the effect of matters that are inherently uncertain and may significantly impact our quarterly or annual results of operations and financial condition. Each quarter, our senior management discusses the development, selection and disclosure of such estimates with our audit committee of our board of directors. In the following paragraphs, we describe the specific risks associated with these critical accounting policies. For all of these policies, we caution that future events rarely develop exactly as one may expect, and that best estimates routinely require adjustment.
Historically, our estimates have been accurate as we have not experienced any material differences between our estimates and our actual results. The significant accounting policies, which we believe are the most critical to aid in fully understanding and evaluating our reported financial results, require the following:
· Assessing the propriety of revenue recognition and associated deferred revenue;
· Determining the proper valuation of investments in marketable securities and other equity investments;
· Assessing the recoverability of long-lived assets, including property and equipment, intellectual property and licensed technology;
· Determining the proper valuation of inventory;
· Determining the appropriate cost estimates for unbilled preclinical studies and clinical development activities;
· Estimating our net deferred income tax asset valuation allowance;
· Determining when we are the primary beneficiary for entities that we identify as variable interest entities;
· Determining the fair value of convertible debt without the conversion feature; and
· Determining the fair value of stock-based compensation, including the expected life of the option, the expected stock price volatility over the term of the expected life and estimated forfeitures.
Descriptions of these critical accounting policies follow.
Revenue Recognition
We generally recognize revenue when we have satisfied all contractual obligations and are reasonably assured of collecting the resulting receivable. We are often entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue under current accounting rules. In those instances in which we have received payment from our customers in advance of recognizing revenue, we include the amounts in deferred revenue on our consolidated balance sheet.
50
Research and Development Revenue Under Collaborative Agreements
On January 1, 2011, we adopted an accounting standard, which amended the criteria to identify separate units of accounting for revenue arrangements with multiple deliverables. The new guidance replaces the concept of allocating revenue among deliverables in a multiple-element revenue arrangement according to fair value with an allocation based on estimated selling price. The new standard is applicable on a prospective basis to agreements we entered into or materially modified after January 1, 2011. The adoption of the standard did not impact our financial position or results of operations as of and for the year ended December 31, 2011 as we did not enter into or materially modify any multiple-element arrangements during that period. However, the adoption of this standard may result in revenue recognition for future agreements that is different from our existing multiple-element arrangements.
For agreements that we entered into or materially modified prior to the adoption of the revised multiple element guidance, we recognize revenue from each element of the arrangement as long as we can determine a standalone value for the delivered element and fair value for the undelivered elements, we have completed our obligation to deliver or perform on that element and we are reasonably assured of collecting the resulting receivable.
We often enter into collaborations with multiple deliverables under which we receive non-refundable upfront payments. For collaborations where we determine that there is a single unit of accounting because our research and development efforts are directly tied to the upfront payment, we recognize revenue related to upfront payments ratably over our estimated period of performance relating to the term of the contractual arrangements. Occasionally, we must estimate our period of performance when the agreements we entered into do not clearly define such information. The revenue we recognize could be materially different if different estimates prevail. We have made estimates of our continuing obligations on several agreements, including our collaborations with ATL, Bristol-Myers Squibb, Genzyme, GSK, Eli Lilly and Company, OncoGenex and Pfizer Inc. Our collaborative agreements typically include a research and/or development project plan that includes the activities the agreement requires each party to perform during the collaboration and the party responsible for performing them. We estimate the period of time over which we will complete the activities for which we are responsible and use that period of time as our period of performance for purposes of revenue recognition and amortize revenue over such period. If our collaborators ask us to continue performing work in a collaboration beyond the initial period of performance, we extend our amortization period to correspond to the new extended period of performance. The revenue we recognize could be materially different if different estimates prevail. We have made estimates of our continuing obligations on several agreements. Adjustments to performance periods and related adjustments to revenue amortization periods have had a material impact on our revenue on only one occasion. When Alnylam terminated the companies’ ssRNAi research program in November 2010, we recognized as revenue $4.9 million, which was the remaining deferred revenue from the upfront fee that we were amortizing into revenue over the research term.
As part of our Genzyme strategic alliance, in February 2008 Genzyme made a $150 million equity investment in us by purchasing five million shares of our common stock at $30 per share. The price Genzyme paid for our common stock represented a significant premium over the fair value of our stock. We accounted for this premium as deferred revenue and are amortizing it along with the $175 million licensing fee that we received in June 2008 ratably into revenue until June 2012, which represents the end of our performance obligation based on the current research and development plan.
Our collaborations often include contractual milestones, which typically relate to the achievement of pre-specified development, regulatory and commercialization events. These three categories of milestone events reflect the three stages of the life-cycle of our drugs, which we describe in more detail in the following paragraph.
Prior to the first stage in the life-cycle of our drugs, we perform a significant amount of work using our proprietary antisense technology to design chemical compounds that interact with specific genes that are good targets for drug discovery. From these research efforts, we hope to identify a development candidate. The designation of a development candidate is the first stage in the life-cycle of our drugs. A development candidate is a chemical compound that has demonstrated the necessary safety and efficacy in preclinical animal studies to warrant further study in humans. During the first step of the development stage, we or our partners study our drugs in IND-enabling studies, which are animal studies intended to support an Investigational New Drug, or IND, application and/or the foreign equivalent. An approved IND allows us or our partners to study our development candidate in humans. If the regulatory agency approves the IND, we or our partners initiate Phase 1 clinical trials in which we typically enroll a small number of healthy volunteers to ensure the development candidate is safe for use in patients. If we or our partners determine that a development candidate is safe based on the Phase 1 data, we or our partners initiate Phase 2 studies that are generally larger scale studies in patients with the primary intent of determining the efficacy of the development candidate. The final step in the development stage is Phase 3 studies to gather the necessary safety and efficacy data to request marketing approval from the FDA and/or foreign equivalents. The Phase 3 studies typically involve large numbers of patients and can take up to several years to complete. If the data gathered during the trials demonstrates acceptable safety and efficacy results, we or our partner will submit an application to the FDA and/or its foreign equivalents for marketing approval. This stage of the drug’s life-cycle is the regulatory stage. If a drug achieves marketing
51
approval, it moves into the commercialization stage, during which our partner will market and sell the drug to patients. Although our partner will ultimately be responsible for marketing and selling the drug, our efforts to discover and develop a drug that is safe, effective and reliable contributes significantly to our partner’s ability to successfully sell the drug. The FDA and its foreign equivalents have the authority to impose significant restrictions on an approved drug through the product label and on advertising, promotional and distribution activities. Therefore, our efforts designing and executing the necessary animal and human studies are critical to obtaining claims in the product label from the regulatory agencies that would allow our partner to successfully commercialize our drug. Further, the patent protection afforded our drugs as a result of our initial patent applications and related prosecution activities in the United States and foreign jurisdictions are critical to our partner’s ability to sell our drugs without competition from generic drugs. The potential sales volume of an approved drug is dependent on several factors including the size of the patient population, market penetration of the drug, and the price charged for the drug.
Generally, the milestone events contained in our partnership agreements coincide with the progression of our drugs from development, to regulatory approval and then to commercialization. The process of successfully discovering a new development candidate, having it approved and ultimately sold for a profit is highly uncertain. As such, the milestone payments we may earn from our partners involve a significant degree of risk to achieve. Therefore, as a drug progresses through the stages of its life-cycle, the value of the drug generally increases.
Development milestones in our partnerships may include the following types of events:
· Designation of a development candidate. Following the designation of a development candidate, generally, IND-enabling animal studies for a new development candidate take 12 to 18 months to complete;
· Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete;
· Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete;
· Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete.
Regulatory milestones in our partnerships may include the following types of events:
· Filing of regulatory applications for marketing approval such as a NDA in the United States or MAA in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
· Marketing approval in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain approval from the applicable regulatory agency.
Commercialization milestones in our partnerships may include the following types of events:
· First commercial sale in a particular market, such as in the United States or Europe.
· Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
We assess whether a substantive milestone exists at the inception of our agreements. When a substantive milestone is achieved, we recognize revenue related to the milestone payment. For our existing licensing and collaboration agreements in which we are involved in the discovery and/or development of the related drug or provide the partner with ongoing access to new technologies we discover, we have determined that all future development, regulatory and commercialization milestones are substantive. For example, for our strategic alliance with GSK we are using our antisense drug discovery platform to seek out and develop new drugs against targets for rare and serious diseases. Alternatively, we provide on-going access to our technology to Alnylam to develop and commercialize RNA interference, or RNAi, therapeutics. We consider milestones for both of these collaborations to be substantive. For those agreements that do not meet the following criteria, we do not consider the future milestones to be substantive. In evaluating if a milestone is substantive we consider whether:
· Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
· The achievement of the milestone involves substantive effort and can only be achieved based in whole or part on our performance or the occurrence of a specific outcome resulting from our performance;
· The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
· There is no future performance required to earn the milestone; and
· The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
52
If any of these conditions are not met, we will defer recognition of the milestone payment and recognize it as revenue over the estimated period of performance, if any. In 2011, OncoGenex Pharmaceuticals Inc. initiated a Phase 2 trial of OGX-427 in men with metastatic prostate cancer. Also in 2011, we initiated a Phase 1 clinical study on ISIS-TTRRX, the first drug selected as part of our collaboration with GSK and we selected ISIS-AATRX as the second development candidate as part of our collaboration. We consider milestones related to progression of a drug through the development stage of its life cycle to be substantive milestones because the level of effort and inherent risk associated with these events is high. Therefore, we recognized the entire $750,000 milestone payment from OncoGenex and two $5 million milestone payments from GSK in 2011. Further information about our collaborative arrangements can be found in Note 8, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Licensing and Royalty Revenue
We often enter into agreements to license our proprietary patent rights on an exclusive or non-exclusive basis in exchange for license fees and/or royalties. We generally recognize as revenue immediately those licensing fees and royalties for which we have no significant future performance obligations and are reasonably assured of collecting the resulting receivable.
Valuation of Investments
We consider all liquid investments with maturities of 90 days or less when purchased to be cash equivalents. Our short-term investments have initial maturities of greater than 90 days from date of purchase. We classify our short-term investments as “available-for-sale” and carry them at fair market value based upon prices for identical or similar items on the last day of the fiscal period. We record unrealized gains and losses as a separate component of stockholders’ equity and include net realized gains and losses in gain (loss) on investments. We use the specific identification method to determine the cost of securities sold.
We use a three-tier fair value hierarchy to prioritize the inputs used in our fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets, which includes our money market funds and treasury securities classified as available-for-sale securities and equity securities in publicly-held biotechnology companies; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable, which includes our fixed income securities and commercial paper classified as available-for-sale securities; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions. The majority of our securities have been classified as Level 2. To estimate the fair value of securities classified as Level 2, we utilize the services of a fixed income pricing provider that uses an industry standard valuation model, which is based on a market approach. We validate the fair value of securities from our pricing provider by understanding the pricing models they used and comparing their assessment of the fair value of our Level 2 investments to the fair value from the custodians of our Level 2 investments. Our pricing provider and custodians use similar techniques to derive fair value for Level 2 securities. We may also validate the fair value by obtaining market values from other pricing sources.
We have equity investments in privately- and publicly-held biotechnology companies that we have received as part of a technology license or collaboration agreement. We hold ownership interests of less than 20 percent in each of the respective companies except Regulus, our jointly owned subsidiary. In determining if and when a decrease in market value below our cost in our equity positions is temporary or other-than-temporary, we examine historical trends in the stock price, the financial condition of the company, near term prospects of the company and our current need for cash. We record unrealized gains and losses related to temporary declines in the publicly-held companies as a separate component of stockholders’ equity and account for securities in the privately-held companies, except for Regulus, under the cost method of accounting because we own less than 20 percent and do not have significant influence in their operations. Most of the cost method investments we hold are in early stage biotechnology companies and realization of our equity position in those companies is uncertain. In those circumstances we record a full valuation allowance. When we determine that a decline in value in either a public or private investment is other-than-temporary, we recognize an impairment loss in the period in which the other-than-temporary decline occurs.
During 2011, we recognized a $4.2 million net gain on investments primarily consisting of a $4.4 million gain we recorded in the fourth quarter of 2011 from our ownership interest in Excaliard when they were acquired by Pfizer Inc. During 2010, we recognized a $713,000 loss on investments primarily consisting of an $880,000 non-cash loss primarily related to the other-than-temporary impairment of our equity investment in ATL. See further discussion about our investments in these satellite companies in Note 8, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
53
Valuation of Long-Lived Assets
We evaluate long-lived assets, which include property, plant and equipment, patent costs, and licenses acquired from third parties, for impairment on at least a quarterly basis and whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. During this process, we review our property and equipment listings, pending domestic and international patent applications, domestic and international issued patents, and licenses we have acquired from other parties to determine if any impairment is present. We consider the following factors:
· Evidence of decreases in market value;
· Changes in the extent or manner in which we use an asset;
· Adverse changes in legal factors or in the business climate that would affect the value of an asset;
· An adverse action or assessment by a regulator;
· An accumulation of costs significantly in excess of amounts originally expected to acquire or construct an asset;
· Current period operating or cash flow loss combined with a history of operating or cash flow losses associated with an asset used for the purpose of producing revenue; and
· Challenges or potential challenges to our existing patents, the likelihood that the United States Patent and Trademark Office, or foreign equivalent, will issue an application and the scope of our issued patents.
We recorded a charge of $1.9 million, $1.5 million and $696,000 for the years ended December 31, 2011, 2010 and 2009, respectively, primarily related to the write-down of intangible assets to their estimated net realizable values.
Valuation of Inventory
We capitalize the costs of raw materials that we purchase for use in producing our drugs because until we use these raw materials they have alternative future uses. We include in inventory raw material costs for drugs that we manufacture for our partners under contractual terms and that we use primarily in our clinical development activities and drug products. We can use each of our raw materials in multiple products and, as a result, each raw material has future economic value independent of the development status of any single drug. For example, if one of our drugs failed, we could use the raw materials allocated for that drug to manufacture our other drugs. We expense these costs when we deliver the drugs to our partners, or as we provide these drugs for our own clinical trials. We reflect our inventory on the balance sheet at the lower of cost or market value under the first-in, first-out method. We review inventory periodically and reduce the carrying value of items we consider to be slow moving or obsolete to their estimated net realizable value. We consider several factors in estimating the net realizable value, including shelf life of raw materials, alternative uses for our drugs and clinical trial materials and historical write-offs.
Estimated Liability for Clinical Development Costs
We record accrued liabilities related to expenses for which service providers have not yet billed us related to products or services that we have received, specifically related to ongoing preclinical studies and clinical trials. These costs primarily relate to third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator grants. We have multiple drugs in concurrent preclinical studies and clinical trials at several clinical sites throughout the world. In order to ensure that we have adequately provided for ongoing preclinical and development costs during the period in which we incur such costs, we maintain an accrual to cover these expenses. We update our estimate for this accrual on at least a quarterly basis. The assessment of these costs is a subjective process that requires judgment. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual amounts.
Valuation Allowance for Net Deferred Tax Assets
We record a valuation allowance to offset our net deferred tax assets because we are uncertain that we will realize these net tax assets. Except for 2009, we have had net losses since inception, and as a result, we have established a 100 percent valuation allowance for our net deferred tax asset. If we determine that we are able to realize a portion or all of these deferred tax assets in the future, we will record an adjustment to the valuation allowance.
54
Segment Information
Prior to 2011, we reported our results in two separate segments: Drug Discovery and Development and Regulus. Beginning in the first quarter of 2011, we no longer consider Regulus as an operating segment because our chief decision making officer no longer reviews Regulus’ operating results for purposes of making resource allocations. Therefore we only provide financial information and results for our Drug Discovery and Development operations.
Convertible Debt
We account for convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) by separating the liability and equity components of the instruments in a manner that reflects our nonconvertible debt borrowing rate when we recognize interest expense in subsequent periods. As a result, we assigned a value to the debt component of our 25/8 percent convertible notes equal to the estimated fair value of a similar debt instrument without the conversion feature, which resulted in us recording the debt at a discount. We are amortizing the resulting debt discount over the life of the debt as additional non-cash interest expense utilizing the effective interest method. At adoption, we retrospectively implemented the presentation and disclosure requirements to all periods presented in our consolidated financial statements. For additional information, see Note 5, Long-Term Obligations and Commitments, in the Notes to the Consolidated Financial Statements.
Determining the fair value of the debt component requires the use of accounting estimates and assumptions. These estimates and assumptions are judgmental in nature and could have a significant impact on the determination of the debt component, and the associated non-cash interest expense. We determine the carrying amount of the liability component by measuring the fair value of a similar debt instrument that does not have the conversion feature. If no similar debt instrument exists, we estimate fair value by using assumptions that market participants would use in pricing a debt instrument, including market interest rates, credit standing, yield curves and volatilities.
Consolidation of Variable Interest Entities
On January 1, 2010, we adopted an accounting standard, which replaced the quantitative-based risks and rewards calculation for determining which enterprise, if any, has a controlling financial interest in a variable interest entity. The new approach focuses on identifying which enterprise has the power to direct the activities of a variable interest entity that most significantly impacts the variable interest entity’s economic performance and (1) the obligation to absorb losses of the variable interest entity or (2) the right to receive benefits from the variable interest entity. As a result of adopting this new accounting standard, we were required to change the way we account for our variable interest in Regulus. Since we and Alnylam share equally the ability to impact Regulus’ economic performance, we are no longer the primary beneficiary of Regulus. We adopted the new standard on a prospective basis, therefore beginning in the first quarter of 2010, we deconsolidated Regulus from our consolidated financial statements and began to account for our ownership interest in Regulus using the equity method of accounting. We no longer include Regulus’ revenue and operating expenses in our operating results. Instead we include our share of Regulus’ operating results on a separate line in our consolidated statement of operations called “Equity in net loss of Regulus Therapeutics Inc.” On our consolidated balance sheet, we present our investment in Regulus on a separate line in the non-current liabilities section called “Investment in Regulus Therapeutics Inc.” We have not reclassified amounts in the prior period financial statements to conform to the current period presentation. For additional information, see Note 2, Investment in Regulus Therapeutics Inc., in the Notes to the Consolidated Financial Statements.
Stock-Based Compensation
We utilize the Black-Scholes model and assumptions discussed in Note 6, Stockholders’ Equity, in the Notes to the Consolidated Financial Statements, for estimating the fair value of the stock-based awards we grant. We recognize compensation expense for all stock-based payment awards using the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), an entity recognizes compensation expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period. We base our risk-free interest rate assumption on observed interest rates appropriate for the term of our employee stock options and our Employee Stock Purchase Plan, or ESPP. We base the dividend yield assumption on our history and expectation of dividend payouts. We have not paid dividends in the past and do not expect to in the future. We use an average of the historical stock price volatility of our stock to calculate the expected volatility assumption required for the Black-Scholes model. The expected term of stock options granted represents the period of time that we expect them to be outstanding.
We estimate the expected term of options granted based on historical exercise patterns. We estimate forfeitures based on historical experience. There were no material changes to our estimated forfeitures for 2011, 2010 and 2009.
55
We account for stock options we grant to non-employees, which consist primarily of options we grant to consultants, by estimating their fair value. We remeasure the fair value at each reporting period until the stock option vests. We recognize the expense over the period of time we require the non-employee to perform services.
As of December 31, 2011, total unrecognized compensation cost related to non-vested stock-based compensation plans was $7.6 million. We will adjust the total unrecognized compensation cost for future changes in estimated forfeitures. We expect to recognize this cost over a weighted average period of 1.3 years.
Results of Operations
Years Ended December 31, 2011 and December 31, 2010
Revenue
Total revenue for the year ended December 31, 2011 was $99.1 million compared to $108.5 million for 2010. Our revenue fluctuates based on the nature and timing of payments under agreements with our partners, including license fees, milestone-related payments and other payments. For example, revenue in 2011 included $17.7 million in revenue from GSK, compared to $10.3 million in 2010, primarily due to the timing of milestone payments. This increase in revenue was offset by less revenue from Bristol-Myers Squibb and Alnylam compared to 2010 because we were no longer amortizing the upfront fees. Revenue in 2011 also included $5.8 million of commercial revenue for drug substance that we sold to Genzyme to support the commercial launch of KYNAMRO.
Research and Development Revenue Under Collaborative Agreements
Research and development revenue under collaborative agreements for the year ended December 31, 2011 was $96.2 million compared to $102.9 million for 2010. Lower revenue in 2011 compared to 2010 was primarily due to the timing of milestone payments and less amortization of upfront fees. Milestones earned from GSK in 2011 included a $5 million milestone in the second quarter of 2011 for the initiation of a Phase 1 study for ISIS-TTRRx and a $5 million milestone in the fourth quarter of 2011 for designating ISIS-AATRx as a development candidate.
Licensing and Royalty Revenue
Our revenue from licensing activities and royalties for the year ended December 31, 2011 was $2.9 million compared to $5.6 million for 2010. The decrease primarily related to $1.9 million of sublicense revenue we earned from Regulus in the second quarter of 2010 related to its strategic alliance with Sanofi.
Operating Expenses
Operating expenses for the year ended December 31, 2011 were $170.2 million compared to $156.8 million for 2010. Our operating expenses in 2011 reflected moderately higher development costs associated with our maturing pipeline of drugs. These increases were offset by lower non-cash compensation expense related to stock options resulting from a decrease in the average price of Isis’ stock in 2011. While we will share development expenses for KYNAMRO equally with Genzyme beginning in 2012, many of the drugs in our pipeline will enter later-stage clinical development. Therefore, we expect our operating expenses to be slightly higher next year.
In order to analyze and compare our results of operations to other similar companies, we believe that it is important to exclude non-cash compensation expense related to stock options from our operating expenses. We believe non-cash compensation expense is not indicative of our operating results or cash flows from our operations. Further, we internally evaluate the performance of our operations excluding it.
56
Research and Development Expenses
Our research and development expenses consist of costs for antisense drug discovery, antisense drug development, manufacturing and operations and R&D support costs.
The following table sets forth information on research and development costs (in thousands):
| | Year Ended
December 31, | |
| | 2011 | | 2010 | |
Research and development expenses | | $ | 148,870 | | $ | 135,012 | |
Non-cash compensation expense related to stock options | | 8,527 | | 10,148 | |
| | | | | |
Total research and development | | $ | 157,397 | | $ | 145,160 | |
For the year ended December 31, 2011, we incurred total research and development expenses of $148.9 million compared to $135.0 million for 2010. Research and development expenses in 2011 reflected moderately higher costs associated with our maturing pipeline of drugs offset by lower costs associated with the completion of the KYNAMRO Phase 3 program that supports the initial regulatory filings. While we will share development expenses for KYNAMRO equally with Genzyme beginning in 2012, many of the drugs in our pipeline will enter later-stage clinical development. Therefore, we expect our drug development expenses to be moderately higher next year. All amounts discussed exclude non-cash compensation expense related to stock options.
Antisense Drug Discovery
We use our proprietary antisense technology to generate information about the function of genes and to determine the value of genes as drug discovery targets. We use this information to direct our own antisense drug discovery research, and that of our antisense drug discovery partners. Antisense drug discovery is also the function within Isis that is responsible for advancing antisense core technology.
As we continue to advance our antisense technology, we are investing in our drug discovery programs to expand our and our partners’ drug pipelines. We anticipate that our existing relationships and collaborations, as well as prospective new partners, will continue to help fund our research programs and contribute to the advancement of the science by funding core antisense technology research.
Our antisense drug discovery expenses were as follows (in thousands):
| | Year Ended
December 31, | |
| | 2011 | | 2010 | |
Antisense drug discovery | | $ | 32,618 | | $ | 33,175 | |
Non-cash compensation expense related to stock options | | 2,433 | | 2,941 | |
| | | | | |
Total antisense drug discovery | | $ | 35,051 | | $ | 36,116 | |
Antisense drug discovery costs were $32.6 million for the year ended December 31, 2011, and decreased slightly compared to $33.2 million for 2010. Both amounts exclude non-cash compensation expense related to stock options.
57
Antisense Drug Development
The following table sets forth research and development expenses for our major antisense drug development projects (in thousands):
| | Year Ended
December 31, | |
| | 2011 | | 2010 | |
KYNAMRO | | $ | 13,719 | | $ | 25,807 | |
Other antisense development products | | 47,395 | | 29,907 | |
Development overhead costs | | 5,708 | | 4,713 | |
Non-cash compensation expense related to stock options | | 2,908 | | 3,207 | |
| | | | | |
Total antisense drug development | | $ | 69,730 | | $ | 63,634 | |
Antisense drug development expenditures were $66.8 million for the year ended December 31, 2011 compared to $60.4 million for 2010. Both amounts exclude non-cash compensation expense related to stock options. The higher expenses in 2011 were primarily due to moderately higher costs associated with our maturing pipeline of drugs as these drugs move forward to more advanced stages of development, including into larger, longer clinical studies. These increases were offset by lower costs associated with the completion of the KYNAMRO Phase 3 program that supports the initial regulatory filings. While our share of KYNAMRO development expenses will be shared equally with Genzyme in 2012, many of the drugs in our pipeline will enter later-stage clinical development. Therefore, we expect our drug development expenditures to be moderately higher next year.
We may conduct multiple clinical trials on a drug candidate, including multiple clinical trials for the various indications we may be studying. Furthermore, as we obtain results from trials we may elect to discontinue clinical trials for certain drug candidates in certain indications in order to focus our resources on more promising drug candidates or indications. Our Phase 1 and Phase 2 programs are clinical research programs that fuel our Phase 3 pipeline. When our products are in Phase 1 or Phase 2 clinical trials, they are in a dynamic state where we continually adjust the development strategy for each product. Although we may characterize a product as “in Phase 1” or “in Phase 2,” it does not mean that we are conducting a single, well-defined study with dedicated resources. Instead, we allocate our internal resources on a shared basis across numerous products based on each product’s particular needs at that time. This means we are constantly shifting resources among products. Therefore, what we spend on each product during a particular period is usually a function of what is required to keep the products progressing in clinical development, not what products we think are most important. For example, the number of people required to start a new study is large, the number of people required to keep a study going is modest and the number of people required to finish a study is large. However, such fluctuations are not indicative of a shift in our emphasis from one product to another and cannot be used to accurately predict future costs for each product. And, because we always have numerous products in preclinical and early stage clinical research, the fluctuations in expenses from product to product, in large part, offset one another. If we partner a drug, it may affect the size of a trial, its timing, its total cost and the timing of the related cost. Our partners are developing, with our support, 10 of our 26 drug candidates, which substantially reduces our development costs. As part of our collaboration with Genzyme, we have transitioned the majority of the development responsibility for KYNAMRO to Genzyme. In 2011, we satisfied our $125 million development funding obligation. We and Genzyme now share development costs equally. Our shared funding will end when the program is profitable.
Manufacturing and Operations
Expenditures in our manufacturing and operations function consist primarily of personnel costs, specialized chemicals for oligonucleotide manufacturing, laboratory supplies and outside services. This function is responsible for providing drug supplies to antisense drug discovery and antisense drug development, including the analytical testing to satisfy good laboratory and good manufacturing practices requirements.
Our manufacturing and operations expenses were as follows (in thousands):
| | Year Ended
December 31, | |
| | 2011 | | 2010 | |
Manufacturing and operations | | $ | 19,506 | | $ | 17,513 | |
Non-cash compensation expense related to stock options | | 1,101 | | 1,425 | |
| | | | | |
Total manufacturing and operations | | $ | 20,607 | | $ | 18,938 | |
58
Manufacturing and operations expenses for the year ended December 31, 2011 were $19.5 million compared to $17.5 million for 2010, both amounts exclude non-cash compensation expense related to stock options. The increase in expenses was a result of increases in the cost of raw materials used to manufacture our generation 2.5 compounds, services provided by third parties and personnel costs to support our expanded clinical development programs including KYNAMRO.
R&D Support
In our research and development expenses, we include support costs such as rent, repair and maintenance for buildings and equipment, utilities, depreciation of laboratory equipment and facilities, amortization of our intellectual property, information technology costs, procurement costs and waste disposal costs. We call these costs R&D support costs.
The following table sets forth information on R&D support costs (in thousands):
| | Year Ended
December 31, | |
| | 2011 | | 2010 | |
Personnel costs | | $ | 8,665 | | $ | 8,153 | |
Occupancy | | 9,446 | | 6,587 | |
Depreciation and amortization | | 7,894 | | 6,394 | |
Insurance | | 884 | | 922 | |
Other | | 3,035 | | 1,840 | |
Non-cash compensation expense related to stock options | | 2,085 | | 2,576 | |
| | | | | |
Total R&D support costs | | $ | 32,009 | | $ | 26,472 | |
R&D support costs for the year ended December 31, 2011 were $29.9 million compared to $23.9 million for 2010, both amounts exclude non-cash compensation expense related to stock options. The increase in expenses in 2011 compared to 2010 primarily relates to one-time occupancy and relocation costs associated with the move to our new facility, additional depreciation costs and property taxes we recorded in 2011 for our new facility, and a reduction in the costs we allocated to Regulus in 2011 compared to 2010. When Regulus moved to a separate facility in the second half of 2010, we significantly reduced the amount we were charging them for facilities and support.
General and Administrative Expenses
General and administrative expenses include corporate costs required to support our company, our employees and our stockholders. These costs include personnel and outside costs in the areas of legal, human resources, investor relations, and finance. Additionally, we include in general and administrative expenses such costs as rent, repair and maintenance of buildings and equipment, depreciation, utilities, information technology and procurement costs that we need to support the corporate functions listed above.
The following table sets forth information on general and administrative expenses (in thousands):
| | Year Ended
December 31, | |
| | 2011 | | 2010 | |
General and administrative expenses | | $ | 11,471 | | $ | 9,658 | |
Non-cash compensation expense related to stock options | | 1,318 | | 2,011 | |
| | | | | |
Total general and administrative | | $ | 12,789 | | $ | 11,669 | |
General and administrative expenses for the year ended December 31, 2011 were $11.5 million compared to $9.7 million for 2010. The increase in expenses in 2011 compared to 2010 primarily relates to higher personnel costs, one-time occupancy and relocation costs associated with the move to our new facility, and a reduction in the amount we charged to Regulus for general and administrative support. All amounts discussed exclude non-cash compensation expense related to stock options.
59
Equity in Net Loss of Regulus Therapeutics Inc.
Our equity in net loss of Regulus for the year ended December 31, 2011 was $3.6 million compared to $2.2 million for 2010. In 2010, our equity in net loss of Regulus included a $4.7 million gain we recorded to reflect an increase in the valuation of Regulus and the change in our ownership percentage due to the $10 million investment Sanofi made in Regulus valuing Regulus at more than $130 million. The increase in our equity in net loss of Regulus in 2011 is primarily due to this gain.
Investment Income
Investment income for the year ended December 31, 2011 totaled $2.4 million compared to $3.4 million for 2010. The decrease in investment income was primarily due to a lower average return on our investments resulting from the current market conditions and a lower average cash balance.
Interest Expense
Interest expense for the year ended December 31, 2011 totaled $16.7 million compared to $13.2 million for 2010. The increase in interest expense in 2011 is a result of additional non-cash interest expense we recorded for the long-term liability associated with our new facility. See Note 5, Long-Term Obligations and Commitments, in the Notes to the Consolidated Financial Statements for additional information about the accounting treatment for our new facility.
Gain (Loss) on Investments, Net
Net gain on investments for the year ended December 31, 2011 was $4.2 million compared to a net loss on investments of $713,000 for 2010. The net gain on investments in 2011 consists primarily of a $4.4 million gain we recorded in the fourth quarter of 2011 from our ownership interest in Excaliard when they were acquired by Pfizer Inc. The net loss on investments in 2010 primarily consisted of an $880,000 non-cash loss related to the other-than-temporary impairment of our equity investment in ATL. See further discussion about our investments in these satellite companies in Note 8, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Net Loss and Net Loss Per Share attributable to Isis Pharmaceuticals, Inc. Common Stockholders
Net loss attributable to Isis Pharmaceuticals, Inc. common stockholders for the year ended December 31, 2011 was $84.8 million compared to $61.3 million for 2010. Basic and diluted net loss per share for the year ended December 31, 2011 was $0.85 per share compared to $0.62 per share for 2010. Our net loss for 2011 increased compared to 2010 primarily due to an increase in our net operating loss, interest expense, and in our share of Regulus’ net loss, all of which we discuss above.
Net Operating Loss Carryforward
At December 31, 2011, we had federal and California tax net operating loss carryforwards of approximately $510.6 million and $428.1 million, respectively. We also had federal and California research credit carryforwards of approximately $42.9 million and $16.0 million, respectively. Our federal and California tax loss carryforwards expire at various dates starting in 2014, unless we utilize them before then. Our net operating loss and tax credit carryforwards may be subject to an annual limitation regarding utilization against taxable income in future periods due to “change of ownership” provisions of the Tax Reform Act of 1986. We believe that such limitation will not have a material adverse impact on the benefits that may arise from our net operating loss and tax credit carryforwards.
Years Ended December 31, 2010 and December 31, 2009
Revenue
Total revenue for the year ended December 31, 2010 was $108.5 million compared to $121.6 million for 2009. Our revenue fluctuates based on the nature and timing of payments under agreements with our partners, including license fees, milestone-related payments and other payments. We earned new revenue in 2010 in the form of an upfront fee from our new partnership with GSK, which we are amortizing through the first quarter of 2015, milestone payments from GSK, Bristol-Myers Squibb, and Achaogen, and sublicensing income from Regulus’ collaboration with Sanofi. Additionally, when Alnylam terminated the ssRNAi research program in November 2010, we recognized $4.9 million of revenue from the upfront fee that we were amortizing into revenue over the research term. Our revenue in 2010 decreased compared to 2009 principally because the amortization of the upfront fee from our Ortho-McNeil-Janssen Pharmaceuticals, Inc., or OMJP, collaboration ended in the third quarter of 2009. In addition, revenue decreased by $3.0 million because we were no longer including Regulus’ revenue in our 2010 revenue.
60
Drug Discovery & Development
Research and Development Revenue Under Collaborative Agreements
Research and development revenue under collaborative agreements for the year ended December 31, 2010 was $102.9 million compared to $108.1 million for 2009. Although we recognized $13.4 million of revenue for the milestone payments we received in 2010, $5.3 million of amortization of revenue related to the upfront payment we received from GSK in 2010 and $4.9 million of additional revenue when Alnylam terminated the ssRNAi research program in 2010, our revenue in 2010 compared to 2009 decreased. The decrease was primarily because revenue from our collaboration with OMJP ended in the third quarter of 2009. Research and development revenue also decreased by $3.0 million because we are no longer including Regulus’ revenue in our 2010 revenue.
Licensing and Royalty Revenue
Our revenue from licensing activities and royalties for the year ended December 31, 2010 was $5.6 million compared to $13.5 million for 2009. The decrease primarily related to the $10 million sublicensing revenue we earned from OncoGenex in the fourth quarter of 2009 when OncoGenex entered into a strategic alliance with Teva.
Operating Expenses
Operating expenses for the year ended December 31, 2010 were $156.8 million compared to $149.1 million for 2009. The higher expenses in 2010 were primarily due to increased costs related to advancing KYNAMRO towards its initial regulatory filings for marketing approval, maturing and expanding our pipeline, and implementing generation 2.5 chemistry. The increase in costs was offset in part by an $11.7 million decrease because we did not include Regulus’ operating expenses in our 2010 operating expenses.
In order to analyze and compare our results of operations to other similar companies, we believe that it is important to exclude non-cash compensation expense related to stock options from our operating expenses. We believe non-cash compensation expense is not indicative of our operating results or cash flows from our operations. Further, we internally evaluate the performance of our operations excluding it.
Research and Development Expenses
The following table sets forth information on research and development costs (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
Research and development expenses | | $ | 135,012 | | $ | 123,646 | |
Non-cash compensation expense related to stock options | | 10,148 | | 10,977 | |
| | | | | |
Total research and development | | $ | 145,160 | | $ | 134,623 | |
For the year ended December 31, 2010, we incurred total research and development expenses of $135.0 million compared to $123.6 million for 2009. The higher expenses in 2010 were primarily due to an increase in costs associated with advancing KYNAMRO, maturing and expanding our pipeline, and implementing generation 2.5 chemistry. The increase in costs was offset in part by a $9.1 million decrease because we did not include Regulus’ operating expenses in our 2010 operating expenses. All amounts discussed exclude non-cash compensation expense related to stock options.
61
Antisense Drug Discovery
Our antisense drug discovery expenses were as follows (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
Antisense drug discovery | | $ | 33,175 | | $ | 27,535 | |
Non-cash compensation expense related to stock options | | 2,941 | | 3,067 | |
| | | | | |
Total antisense drug discovery | | $ | 36,116 | | $ | 30,602 | |
Antisense drug discovery costs were $33.2 million for the year ended December 31, 2010 compared to $27.5 million for 2009. Both amounts exclude non-cash compensation expense related to stock options. The higher expenses in 2010 were primarily due to our planned investment to expand our pipeline by adding three to five new drugs a year and additional spending to identify our generation 2.5 chemistry. These activities resulted in an increase in personnel, laboratory supplies and research services provided by third parties in 2010.
Antisense Drug Development
The following table sets forth research and development expenses for our major antisense drug development projects (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
KYNAMRO | | $ | 25,807 | | $ | 26,909 | |
Other antisense development products | | 29,907 | | 17,472 | |
Development overhead costs | | 4,713 | | 4,253 | |
Non-cash compensation expense related to stock options | | 3,207 | | 3,578 | |
| | | | | |
Total antisense drug development | | $ | 63,634 | | $ | 52,212 | |
Antisense drug development expenditures were $60.4 million for the year ended December 31, 2010 compared to $48.6 million for 2009. Both amounts exclude non-cash compensation expense related to stock options. We attribute the increase to the expansion of our drug pipeline.
Manufacturing and Operations
Our manufacturing and operations expenses were as follows (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
Manufacturing and operations | | $ | 17,513 | | $ | 14,415 | |
Non-cash compensation expense related to stock options | | 1,425 | | 1,440 | |
| | | | | |
Total manufacturing and operations | | $ | 18,938 | | $ | 15,855 | |
Manufacturing and operations expenses for the year ended December 31, 2010 were $17.5 million compared to $14.4 million for 2009, both amounts exclude non-cash compensation expense related to stock options. The increase in expenses was primarily a result of an increase in personnel costs and services provided by third parties to support our expanded clinical development programs including KYNAMRO and depreciation expense related to the upgrades made to our manufacturing facility to prepare to manufacture KYNAMRO for launch.
62
R&D Support
The following table sets forth information on R&D support costs (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
Personnel costs | | $ | 8,153 | | $ | 7,859 | |
Occupancy | | 6,587 | | 7,230 | |
Depreciation and amortization | | 6,394 | | 6,379 | |
Insurance | | 922 | | 903 | |
Other | | 1,840 | | 2,739 | |
Non-cash compensation expense related to stock options | | 2,576 | | 3,058 | |
| | | | | |
Total R&D support costs | | $ | 26,472 | | $ | 28,168 | |
R&D support costs for the year ended December 31, 2010 were $23.9 million compared to $25.1 million for 2009, both amounts exclude non-cash compensation expense related to stock options. The decrease in expenses primarily relates to lease modification fees that we paid in September 2009. Other R&D support costs also decreased because we no longer include Regulus’ R&D support costs in our 2010 operating expenses.
General and Administrative Expenses
The following table sets forth information on general and administrative expenses (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
General and administrative expenses | | $ | 9,658 | | $ | 12,107 | |
Non-cash compensation expense related to stock options | | 2,011 | | 2,408 | |
| | | | | |
Total general and administrative | | $ | 11,669 | | $ | 14,515 | |
General and administrative expenses for the year ended December 31, 2010 were $9.7 million compared to $12.1 million for 2009. The decrease primarily related to Regulus’ general and administrative expenses of $2.5 million in 2009, which we did not include in our 2010 operating expenses. All amounts discussed exclude non-cash compensation expense related to stock options.
Equity in Net Loss of Regulus Therapeutics Inc.
Beginning in the first quarter of 2010, as a result of adopting a new accounting standard, we concluded that we were no longer the primary beneficiary of Regulus. As such we no longer include Regulus’ revenue and operating expenses in our operating results. Instead we have presented our share of Regulus’ operating results on a separate line in our consolidated statement of operations called “Equity in net loss of Regulus Therapeutics Inc.” Prior to the adoption of the new accounting standard, we consolidated Regulus’ financial results on a line-by-line basis. See Note 1, Organization and Significant Accounting Policies, and Note 2, Investment in Regulus Therapeutics Inc., in the Notes to the Consolidated Financial Statements for a more detailed explanation of this change.
Our equity in net loss of Regulus for the year ended December 31, 2010 was $2.2 million. Our share of Regulus’ 2010 net loss was offset by a $4.7 million gain we recorded in October 2010 to reflect an increase in the valuation of Regulus and the change in our ownership percentage due to the $10 million investment Sanofi made in Regulus valuing Regulus at more than $130 million. We had the option to adopt the new accounting standard on a retrospective or prospective basis. We chose to adopt it prospectively, therefore we did not adjust our prior period results. If we had retrospectively adopted the new standard, our share of equity in net loss of Regulus for the year ended December 31, 2009 would have been $6.1 million, which included $1.7 million in losses that would have been previously suspended. Under the equity method of accounting, we are required to suspend losses if the carrying amount of our investment in Regulus exceeds the amount of funding we are required to provide. The decrease in our equity in net loss of Regulus is primarily related to the gain we recognized in 2010 when Sanofi made its investment offset by an increase in Regulus’ 2010 operating expenses. We discuss expenses related to Regulus in a separate section below.
63
Investment Income
Investment income for the year ended December 31, 2010 totaled $3.4 million compared to $6.4 million for 2009. The decrease in investment income was primarily due to a lower average return on our investments resulting from the current market conditions and a lower average cash balance.
Interest Expense
Interest expense for the year ended December 31, 2010 totaled $13.2 million and was slightly higher compared to $12.7 million for 2009.
Gain (Loss) on Investments, net
Net loss on investments for the year ended December 31, 2010 was $713,000 compared to a gain on investments of $2.1 million for 2009. The net loss on investments in 2010 primarily consisted of an $880,000 non-cash loss related to the other-than-temporary impairment of our equity investment in ATL. The gain on investments in 2009 reflected a $2.5 million gain when we sold all of the common stock of OncoGenex that we owned and a $574,000 gain that we realized on our available-for-sale securities, offset by a $1.0 million valuation allowance we recorded in November 2009 related to our investment in Altair. Because realization of our Altair investment was uncertain we recorded a full valuation allowance. See further discussion about our investment in Altair in Note 8, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Income Tax Expense
Even though we finished 2009 with a net loss from continuing operations, we had taxable income, which was primarily a result of the significant upfront payments that we received from our strategic alliance with Genzyme in 2008 and the gain we recognized on the sale of Ibis to AMI in early 2009. We recorded income tax expense as part of our financial results from continuing operations of $3.2 million for the year ended December 31, 2009. In 2010, we recorded $92,000 of income tax expense related to our 2009 tax return.
Net loss from Continuing Operations attributable to Isis Pharmaceuticals, Inc. Common Stockholders
The following table sets forth computations for our net loss from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
Net loss from continuing operations, including income tax expense | | $ | (61,251 | ) | $ | (34,956 | ) |
Net loss attributable to noncontrolling interest in Regulus Therapeutics Inc. | | — | | 4,394 | |
Net loss from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders | | $ | (61,251 | ) | $ | (30,562 | ) |
Net loss from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders for the year ended December 31, 2010 was $61.3 million compared to $30.6 million for 2009. The increase in our net loss from continuing operations was primarily due to the following:
· $29.6 million increase in our loss from operations, excluding Regulus, as described above;
· $2.1 million decrease in our share of Regulus’ net loss;
· $3.0 million decrease in investment income; and
· $3.0 million decrease in gain (loss) on investments.
Net Income from Discontinued Operations
Since we sold Ibis to AMI and Ibis met the criteria for a component of an entity, we reflect Ibis as a discontinued operation on our financial statements. Accordingly, we have presented the operating results of Ibis in our Consolidated Statements of Operations as discontinued operations and we have reclassified all prior periods. Net income from discontinued operations, net of tax, in 2009 was $185.6 million, and primarily consisted of the $202.5 million gain less $16.8 million of income taxes.
64
Net Income (Loss) and Net Income (Loss) Per Share attributable to Isis Pharmaceuticals, Inc. Common Stockholders
Net loss attributable to Isis Pharmaceuticals, Inc. common stockholders for the year ended December 31, 2010 was $61.3 million compared to net income of $155.1 million for 2009. Basic and diluted net loss per share for the year ended December 31, 2010 was $0.62 per share compared to basic and diluted net income of $1.58 per share for 2009. Net income and net income per share in 2009 primarily consisted of the $185.6 million gain, net of tax, which we recognized when we sold Ibis to AMI in the first quarter of 2009.
Net Operating Loss Carryforward
At December 31, 2010, we had federal, California and foreign tax net operating loss carryforwards of approximately $402.0 million, $315.1 million and $1.1 million, respectively. We also had federal and California research credit carryforwards of approximately $38.2 million and $12.9 million, respectively.
Regulus Therapeutics
Regulus’ revenue for the year ended December 31, 2010 was $8.6 million compared to $3.0 million for 2009. The increase primarily relates to the amortization of the $25 million upfront payment Regulus received from Sanofi in July 2010 and the $3 million upfront license fee Regulus received from GSK in February 2010 for its HCV alliance targeting miR-122.
The following table sets forth information on Regulus’ operating expenses (in thousands):
| | Year Ended
December 31, | |
| | 2010 | | 2009 | |
Research and development expenses | | $ | 19,775 | | $ | 9,147 | |
General and administrative expenses | | 3,721 | | 2,490 | |
Non-cash compensation expense related to stock options | | 603 | | 99 | |
| | | | | |
Total Regulus operating expenses | | $ | 24,099 | | $ | 11,736 | |
Operating expenses for Regulus were $23.5 million for the year ended December 31, 2010 compared to $11.6 million for 2009, both amounts exclude non-cash compensation expense related to stock options. The increase primarily related to Regulus’ efforts to build its team to support its internal microRNA programs, the efforts associated with its GSK collaboration, and the $3.8 million of sublicense fees paid to us and Alnylam from Regulus’ strategic alliance with Sanofi.
Liquidity and Capital Resources
We have financed our operations with revenue primarily from research and development collaborative agreements. Additionally, we have earned revenue from the sale or licensing of our intellectual property. We have also financed our operations through the sale of our equity securities and the issuance of long-term debt. From our inception through December 31, 2011, we have earned approximately $1.0 billion in revenue from contract research and development and the sale and licensing of our intellectual property. From the time we were founded through December 31, 2011, we have raised net proceeds of approximately $824.1 million from the sale of our equity securities and we have borrowed approximately $568.5 million under long-term debt arrangements to finance a portion of our operations.
At December 31, 2011, we had cash, cash equivalents and short-term investments of $343.7 million and stockholders’ equity of $171.4 million. In comparison, we had cash, cash equivalents and short-term investments of $472.4 million and stockholders’ equity of $244.5 million at December 31, 2010. At December 31, 2011, we had consolidated working capital of $284.0 million compared to $377.2 million at December 31, 2010. The decrease in cash and working capital primarily relates to cash used for our operations.
As of December 31, 2011, our debt and other obligations totaled $218.8 million compared to $144.3 million at December 31, 2010. The increase was primarily related to a $59.8 million increase in other long-term liabilities that we recorded related to the lease of our new facility, which we describe below, $8.6 million of non-cash amortization of the debt discount we recorded in 2011, which increased the carrying value of our 25/8 percent convertible notes, and an additional draw down of $1.6 million on our equipment financing arrangement offset by $5.7 million of principal payments we made in 2011 on our equipment financing arrangement. For additional information, see Note 5, Long-Term Obligations and Commitments, in the Notes to the Consolidated Financial Statements.
65
The following table summarizes our contractual obligations as of December 31, 2011. The table provides a breakdown of when obligations become due. We provide a more detailed description of the major components of our debt in the paragraphs following the table:
| | Payments Due by Period (in millions) | |
Contractual Obligations
(selected balances described below) | | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | After
5 years | |
25/8 percent Convertible Subordinated Notes (principal and interest payable) | | $ | 171.1 | | $ | 4.3 | | $ | 166.8 | | $ | — | | $ | — | |
New Facility Rent Payments | | $ | 149.5 | | $ | 5.8 | | $ | 12.0 | | $ | 12.7 | | $ | 119.0 | |
Equipment Financing Arrangements (principal and interest payable) | | $ | 5.6 | | $ | 3.4 | | $ | 2.2 | | $ | — | | $ | — | |
Other Obligations (principal and interest payable) | | $ | 1.4 | | $ | 0.1 | | $ | 0.1 | | $ | 0.1 | | $ | 1.1 | |
Capital Lease | | $ | 0.8 | | $ | 0.2 | | $ | 0.4 | | $ | 0.2 | | $ | — | |
Operating Leases | | $ | 28.9 | | $ | 1.4 | | $ | 2.8 | | $ | 2.7 | | $ | 22.0 | |
Total | | $ | 357.3 | | $ | 15.2 | | $ | 184.3 | | $ | 15.7 | | $ | 142.1 | |
Our contractual obligations consist primarily of our publicly traded convertible debt. In addition, we also have facility leases, equipment financing arrangements and other obligations.
In January 2007, we completed a $162.5 million convertible debt offering, which raised proceeds of approximately $157.1 million, net of $5.4 million in issuance costs. We included the issuance costs in our balance sheet and are amortizing these costs to interest expense over the life of the debt. The $162.5 million convertible subordinated notes mature in 2027 and bear interest at 25/8 percent, which is payable semi-annually. The 25/8 percent notes are convertible, at the option of the note holders, into approximately 11.1 million shares of our common stock at a conversion price of $14.63 per share. We can redeem these notes at a redemption price equal to 100.75 percent of the principal amount between February 15, 2012 and February 14, 2013; 100.375 percent of the principal amount between February 15, 2013 and February 14, 2014; and 100 percent of the principal amount thereafter. Holders of the 25/8 percent notes may also require us to repurchase the 25/8 percent notes on February 15, 2014, February 15, 2017 and February 15, 2022, and upon the occurrence of certain defined conditions, at 100 percent of the principal amount of the 25/8 percent notes plus unpaid interest.
In October 2008, we entered into a loan agreement related to an equipment financing and in September 2009, we amended the loan agreement to increase the aggregate maximum amount of principal we could draw under the agreement. Under the amended loan agreement, we could borrow up to $18.4 million in principal to finance the purchase of equipment until July 2011. Each draw down under the loan agreement has a term of three years, with principal and interest payable monthly. We calculated interest on amounts we borrowed under the loan agreement based upon the three year interest rate swap at the time we made each draw down plus four percent. We are using the equipment purchased under the loan agreement as collateral. In June 2011, we drew down an additional $1.6 million in principal under the loan agreement. As of December 31, 2011, we had drawn down $18.3 million in principal under this loan agreement at a weighted average interest rate of 6.19 percent. The carrying balance under this loan agreement at December 31, 2011 and 2010 was $5.3 million and $9.4 million, respectively. We will continue to use equipment lease financing as long as the terms remain commercially attractive.
In March 2010, we entered into a lease agreement with an affiliate of BioMed Realty, L.P. Under the lease, BioMed constructed a new facility in Carlsbad, California. The lease has an initial term of 20 years with an option to extend the lease for up to four five-year periods. Our rent under the new lease is based on a percentage of the total construction costs spent by BioMed to acquire the land and build the new facility. The leases on our former primary research and development facilities expired at the end of 2011. Rather than invest in costly renovations to these facilities, we chose to consolidate the majority of our operations in a new leased facility that Biomed constructed. To make our move as efficient as possible, we requested access to the new facility prior to the completion of construction. To gain early access, we agreed to modify our lease with BioMed to accept additional responsibility. As a result, accounting rules required us to record the cost of the facility as a fixed asset with a corresponding liability. In the third quarter of 2011, we consolidated the majority of our operations into the new facility. Therefore, beginning in the third quarter of 2011, we began depreciating the building over its economic life and our rent payments, which began on January 1, 2012, will decrease the liability over the term of the lease.
In addition to contractual obligations, we had outstanding purchase orders as of December 31, 2011 for the purchase of services, capital equipment and materials as part of our normal course of business.
66
We plan to continue to enter into collaborations with partners to provide for additional revenue to us and we may incur additional cash expenditures related to our obligations under any of the new agreements we may enter into. We currently intend to use our cash, cash equivalents and short-term investments to finance our activities. However, we may also pursue other financing alternatives, like issuing additional shares of our common stock, issuing debt instruments, refinancing our existing debt, or securing lines of credit. Whether we use our existing capital resources or choose to obtain financing will depend on various factors, including the future success of our business, the prevailing interest rate environment and the condition of financial markets generally.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
We are exposed to changes in interest rates primarily from our long-term debt arrangements and, secondarily, investments in certain short-term investments. We primarily invest our excess cash in highly liquid short-term investments of the U.S. Treasury and reputable financial institutions, corporations, and U.S. government agencies with strong credit ratings. We typically hold our investments for the duration of the term of the respective instrument. We do not utilize derivative financial instruments, derivative commodity instruments or other market risk sensitive instruments, positions or transactions to manage exposure to interest rate changes. Accordingly, we believe that, while the securities we hold are subject to changes in the financial standing of the issuer of such securities, we are not subject to any material risks arising from changes in interest rates, foreign currency exchange rates, commodity prices, equity prices or other market changes that affect market risk sensitive instruments.
Item 8. Financial Statements and Supplementary Data
We filed our consolidated financial statements and supplementary data required by this item as exhibits hereto, and listed them under Item 15(a)(1) and (2), and incorporate them herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
There have been no reported disagreements on any matter of accounting principles or procedures or financial statement disclosure in 2011 with our Independent Registered Public Accounting Firm.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
Based on our evaluation as of the end of the period covered by this report on Form 10-K, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or Exchange Act) were effective as of December 31, 2011 to ensure that information required to be disclosed in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rules 13a-15(f). Our internal control over financial reporting is a process designed under the supervision of our chief executive officer and chief financial officer to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with United States generally accepted accounting principles.
As of December 31, 2011, our management, with the participation of the chief executive officer and chief financial officer, assessed the effectiveness of our internal control over financial reporting based on the criteria for effective internal control over financial reporting established in “Internal Control—Integrated Framework,” issued by the Committee of Sponsoring Organizations, or COSO, of the Treadway Commission. Based on the assessment, our management determined that we maintained effective internal control over financial reporting as of December 31, 2011.
Ernst & Young LLP, an independent registered public accounting firm, audited the effectiveness of our internal control over financial reporting as of December 31, 2011, as stated in their attestation report, which is included elsewhere herein.
Changes in Internal Control over Financial Reporting
The above evaluation did not identify any change in our internal control over financial reporting that occurred during our latest fiscal quarter and that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
67
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Isis Pharmaceuticals, Inc.
We have audited Isis Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Isis Pharmaceuticals, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Isis Pharmaceuticals, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Isis Pharmaceuticals, Inc. as of December 31, 2011 and 2010, and the related statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2011 of Isis Pharmaceuticals, Inc. and our report dated February 29, 2012 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP |
| |
San Diego, California | |
February 29, 2012 | |
68
Item 9B. Other Information
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
We incorporate by reference the information required by this Item with respect to directors and the Audit Committee from the information under the caption “ELECTION OF DIRECTORS,” including in particular the information under “Nominating, Governance and Review Committee” and “Audit Committee,” contained in our definitive Proxy Statement (the “Proxy Statement”), which we will file on or about April 23, 2012 with the Securities and Exchange Commission in connection with the solicitation of proxies for our 2012 Annual Meeting of Stockholders to be held on June 7, 2012.
We incorporate by reference the required information concerning our Code of Ethics from the information under the caption “Code of Ethics and Business Conduct” contained in the Proxy Statement. We have filed our Code of Ethics as an exhibit to our Report on Form 10-K for the year ended December 31, 2009.
Item 1, Part I of this Report contains information concerning our executive officers. We incorporate by reference the information required by this Item concerning compliance with Section 16(a) of the Securities Exchange Act of 1934, as amended, from the information under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” contained in the Proxy Statement.
Item 11. Executive Compensation
We incorporate by reference the information required by this item to the information under the caption “EXECUTIVE COMPENSATION,” “Compensation Committee Interlocks and Insider Participation” and “COMPENSATION COMMITTEE REPORT” contained in the Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
We incorporate by reference the information required by this item to the information under the captions “SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT” contained in the Proxy Statement.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth information regarding outstanding options and shares reserved for future issuance under our equity compensation plans as of December 31, 2011.
Plan Category | | Number of Shares
to be Issued
Upon Exercise of
Outstanding Options | | Weighted Average
Exercise Price of
Outstanding Options | | Number of Shares
Remaining
Available for
Future Issuance | |
Equity compensation plans approved by stockholders(a) | | 8,165,400 | | $ | 10.62 | | 5,667,733 | (c) |
Equity compensation plans not approved by stockholders(b) | | 2,556,537 | | $ | 13.82 | | — | |
| | | | | | | |
Total | | 10,721,937 | | $ | 11.39 | | 5,667,733 | |
(a) Consists of four Isis plans: 1989 Stock Option Plan, 2002 Non-Employee Directors’ Stock Option Plan, 2011 Equity Incentive Plan and ESPP.
(b) Consists of the 2000 Broad-Based Equity Incentive Plan, more fully described below. The 2000 Broad-Based Equity Incentive Plan expired on January 5, 2010.
(c) Of these shares, 191,088 remained available for purchase under the ESPP as of December 31, 2011. The ESPP incorporates an evergreen formula pursuant to which on January 1 of each year, we automatically increase the aggregate number of shares reserved for issuance under the plan by 150,000 shares.
69
Description of 2000 Broad-Based Equity Incentive Plan
We adopted the 2000 Broad-Based Equity Incentive Plan, or the 2000 Plan, to provide our employees, officers, directors and consultants an opportunity to benefit from increases in the value of our common stock through the granting of non-statutory stock options, stock bonuses and rights to purchase restricted stock. At the time we adopted the 2000 Plan, we were not required to seek the approval of our stockholders. The Board has delegated administration of the 2000 Plan to the Compensation Committee of the Board, and the Compensation Committee has delegated administration of the 2000 Plan to the Non-Management Stock Option Committee with respect to certain option grants to employees who are not our executive officers. The Board has the power to construe and interpret the 2000 Plan and, subject to the provisions of the 2000 Plan, to select the persons to whom stock awards are to be made, to designate the number of shares to be covered by each stock award, to establish vesting schedules, to specify the exercise price and the type of consideration to be paid to us upon exercise or purchase.
As of December 31, 2011, the 2000 Plan had 5,990,000 shares authorized for issuance, options to purchase an aggregate of 2,556,537 shares were granted and outstanding under the 2000 Plan, option holders had exercised options to purchase an aggregate of 3,170,502 shares under the 2000 Plan, and no shares remained available for grant thereunder. The 2000 Plan expired on January 5, 2010, so we may no longer grant new options under the 2000 Plan.
Options granted under the 2000 Plan generally have a term of seven or ten years, have an exercise price equal to the fair market value at the time of grant, can only be exercised with a cash payment and vest at the rate of 25 percent per year after the first year and then at the rate of 2.08 percent per month thereafter during the option holder’s employment or service as a consultant, employee or director. If any change is made in the common stock subject to the 2000 Plan, or subject to any stock award, without the receipt of consideration by us (through merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or other transaction not involving the receipt of consideration by us), we will adjust the outstanding stock awards appropriately in the class(es) and number of securities and price per share of common stock subject to such outstanding stock awards. Our Board will make such adjustments, and its determination will be final, binding and conclusive. We will not treat the conversion of any of our convertible securities as a transaction without receipt of consideration.
In the event of our dissolution or liquidation, all outstanding stock awards will terminate immediately prior to such event.
In the event of:
· a sale, lease or other disposition of all or substantially all of our assets;
· a merger or consolidation in which we are not the surviving corporation; or
· reverse merger in which we are the surviving corporation but the shares of common stock outstanding immediately preceding the merger are converted by virtue of the merger into other property, whether in the form of securities, cash or otherwise;
then any surviving corporation or acquiring corporation will assume any stock awards outstanding under the 2000 Plan or will substitute similar stock awards (including an award to acquire the same consideration paid to the stockholders in the transaction for those outstanding under the 2000 Plan). In the event any surviving corporation or acquiring corporation refuses to assume such stock awards or to substitute similar stock awards for those outstanding under the 2000 Plan, then with respect to stock awards held by participants whose continuous service has not terminated, we will accelerate the vesting of such stock awards in full and the stock awards will terminate if not exercised (if applicable) at or prior to such event.
Item 13. Certain Relationships and Related Transactions, and Director Independence
We incorporate by reference the information required by this item to the information under the captions “Independence of the Board of Directors” and “Certain Relationships and Related Transactions” contained in the Proxy Statement.
Item 14. Principal Accountant Fees and Services
We incorporate by reference the information required by this item to the information under the caption “RATIFICATION OF SELECTION OF INDEPENDENT AUDITORS” contained in the Proxy Statement.
70
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Index to Financial Statements
We submitted the consolidated financial statements required by this item in a separate section beginning on page F-1 of this Report.
(a)(2) Index to Financial Statement Schedules
We omitted these schedules because they are not required, or are not applicable, or the required information is shown in the consolidated financial statements or notes thereto.
(a)(3) Index to Exhibits
See Index to Exhibits beginning on page 73.
(b) Exhibits
We listed the exhibits required by this Item under Item 15(a)(3).
(c) Financial Statement Schedules
None.
71
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 29th day of February, 2012.
| ISIS PHARMACEUTICALS, INC. |
| |
| By: | /s/ STANLEY T. CROOKE |
| | Stanley T. Crooke, M.D., Ph.D. |
| | Chairman of the Board, President and Chief Executive Officer (Principal executive officer) |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Stanley T. Crooke and B. Lynne Parshall, or any of them, his or her attorney-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Report, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signatures | | Title | | Date |
| | Chairman of the Board, President, and Chief Executive Officer | | |
/s/ STANLEY T. CROOKE | | | February 29, 2012 |
Stanley T. Crooke, M.D., Ph.D. | | (Principal executive officer) | | |
| | | | |
| | Director, Chief Operating Officer, Chief Financial Officer and Secretary | | |
/s/ B. LYNNE PARSHALL | | | February 29, 2012 |
B. Lynne Parshall, J.D. | | (Principal financial and accounting officer) | | |
| | | | |
/s/ SPENCER R. BERTHELSEN | | Director | | February 29, 2012 |
Spencer R. Berthelsen, M.D. | | | | |
| | | | |
/s/ JOSEPH KLEIN | | Director | | February 29, 2012 |
Joseph Klein, III. | | | | |
| | | | |
/s/ FREDERICK T. MUTO | | Director | | February 29, 2012 |
Frederick T. Muto, Esq. | | | | |
| | | | |
/s/ JOHN C. REED, M.D. PH.D. | | Director | | February 29, 2012 |
John C. Reed, M.D., Ph.D. | | | | |
| | | | |
/s/ JOSEPH H. WENDER | | Director | | February 29, 2012 |
Joseph H. Wender | | | | |
72
INDEX TO EXHIBITS
Exhibit
Number | | Description of Document |
3.1 | | Amended and Restated Certificate of Incorporation filed June 19, 1991.(1) |
| | |
3.2 | | Certificate of Amendment to Restated Certificate of Incorporation filed May 3, 2006.(3) |
| | |
3.3 | | Amended and Restated Bylaws.(18) |
| | |
4.1 | | Certificate of Designation of the Series C Junior Participating Preferred Stock.(17) |
| | |
4.2 | | Specimen Common Stock Certificate.(1) |
| | |
4.3 | | Stock Purchase Agreement between the Registrant and Genzyme Corporation dated January 7, 2008. (8) |
| | |
4.4 | | Indenture, dated January 23, 2007, between the Registrant and Wells Fargo Bank, N.A., a national banking association, as trustee, including Form of 25/8 percent Convertible Subordinated Note due 2027.(14) |
| | |
4.5 | | Registration Rights Agreement, dated January 23, 2007, among the Registrant and the Initial Purchasers identified therein.(14) |
| | |
10.1 | | Form of Indemnification Agreement entered into between the Registrant and its Directors and Officers with related schedule.(1) |
| | |
10.2* | | Registrant’s 1989 Stock Option Plan, as amended. (36) |
| | |
10.3* | | Registrant’s Amended and Restated Employee Stock Purchase Plan.(20) |
| | |
10.4 | | Form of Employee Assignment of Patent Rights.(1) |
| | |
10.5* | | Registrant’s 2000 Broad-Based Equity Incentive Stock Option Plan and related form of option agreement.(10) |
| | |
10.6 | | Drug Development and License Option Agreement dated December 2, 2009 between the Registrant and Eli Lilly and Company. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(32) |
| | |
10.7 | | Patent Rights Purchase Agreement between the Registrant and Gilead Sciences, Inc., dated December 18, 1998. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(9) |
| | |
10.8 | | Amendment #1 to the Product Development and Commercialization Agreement between Regulus Therapeutics Inc. and Glaxo Group Limited dated February 24, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (33) |
| | |
10.9 | | Collaboration and License Agreement between the Registrant and Hybridon, Inc., dated May 24, 2001. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(19) |
| | |
10.10 | | License and Co-Development Agreement between the Registrant and Genzyme Corporation dated June 24, 2008. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (12) |
| | |
10.11 | | Amendment #1 to the Research, Development and License Agreement dated May 11, 2011 by and between the Registrant and Glaxo Group Limited. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (15) |
| | |
10.12 | | Amended and Restated Collaboration and License Agreement between the Registrant and Antisense Therapeutics Ltd dated February 8, 2008. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(8) |
| | |
10.13 | | Amended and Restated License Agreement between the Registrant and Atlantic Pharmaceuticals Limited dated November 30, 2009. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (32) |
73
10.14 | | Exclusive License and Nonexclusive Option Agreement between Regulus Therapeutics Inc. and Glaxo Group Limited dated February 24, 2010 . Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (33) |
| | |
10.15 | | Amended and Restated License Agreement dated July 2, 2008 between the Registrant and OncoGenex Technologies Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (22) |
| | |
10.16 | | Lease Agreement between the Registrant and BMR-Gazelle Court LLC dated March 30, 2010 . Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (33) |
| | |
10.17 | | Second Amendment to Lease Agreement dated May 15, 2011 between the Registrant and BMR-Gazelle Court LLC, with First Amendment to Lease Agreement included. (15) |
| | |
10.18 | | Registrant’s Restated Isis Pharmaceuticals, Inc. 10b5-1 Trading Plan dated September 30, 2005.(23) |
| | |
10.19* | | Registrant’s Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan, as amended.(36) |
| | |
10.20* | | Registrant’s Form of 2002 Non-Employee Directors’ Stock Option Agreement.(29) |
| | |
10.21 | | Product Development and Commercialization Agreement between Regulus Therapeutics LLC and Glaxo Group Limited dated April 17, 2008. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (12) |
| | |
10.22* | | Amended and Restated Severance Agreement dated December 3, 2008 between the Registrant and Stanley T. Crooke. (21) |
| | |
10.23* | | Amended and Restated Severance Agreement dated December 3, 2008 between the Registrant and B. Lynne Parshall. (21) |
| | |
10.24 | | Amended and Restated Strategic Collaboration and License Agreement dated April 28, 2009 between the Registrant and Alnylam Pharmaceuticals, Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(28) |
| | |
10.25* | | Isis Pharmaceuticals, Inc. 2011 Equity Incentive Plan (25) |
| | |
10.26 | | Loan Agreement dated October 15, 2008 between the Registrant and RBS Asset Finance, Inc. (31) |
| | |
10.27* | | Form of Option Agreement for Options granted under the 2011 Equity Incentive Plan. (38) |
| | |
10.28* | | Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the 2011 Equity Incentive Plan. (38) |
| | |
10.29 | | Second Amendment to Lease Agreement between the Registrant and BMR-2282 Faraday Avenue LLC dated March 30, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (33) |
| | |
10.30* | | Form of Option Agreement for Options Granted after March 8, 2005 under the 1989 Stock Option Plan.(16) |
| | |
10.31* | | Form of Option Agreement for Options Granted after March 8, 2005 under the 2000 Broad-Based Equity Incentive Plan.(16) |
| | |
10.32* | | Form of Option Agreement for Options Granted after March 8, 2005 under the 2002 Non-Employee Director’s Stock Option Plan.(16) |
| | |
10.33 | | Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated March 30, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (33) |
| | |
10.34 | | Collaboration and License Agreement dated June 17, 2010 between sanofi-aventis and Regulus Therapeutics Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (34) |
74
10.35 | | First Amendment to Loan Agreement between the Registrant and RBS Asset Finance, Inc. dated September 30, 2009.(32) |
| | |
10.36 | | Lease Agreement dated September 6, 2005 between the Registrant and BMR-2282 Faraday Avenue LLC.(23) |
| | |
10.37 | | Second Amended and Restated Collaboration Agreement dated August 5, 2005 between the Registrant and Eli Lilly and Company. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(23) |
| | |
10.38 | | Non-Exclusive Technology Alliance and Option Agreement dated June 17, 2010 between sanofi-aventis and Regulus Therapeutics Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (34) |
| | |
10.39 | | Stock Purchase Agreement dated December 17, 2008, among the Registrant, Ibis Biosciences, Inc. and Abbott Molecular Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(31) |
| | |
10.40 | | Purchase Agreement, dated January 17, 2007, among the Registrant and the Initial Purchasers identified therein.(14) |
| | |
10.41 | | Collaboration and License Agreement between the Registrant and Bristol-Myers Squibb Company dated May 8, 2007. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (7) |
| | |
10.42 | | Research Agreement dated August 10, 2011 between the Registrant and CHDI Foundation, Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (4) |
| | |
10.43 | | Founding Investor Rights Agreement among the Registrant, Alnylam Pharmaceuticals, Inc. and Regulus Therapeutics Inc. dated January 1, 2009. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(6) |
| | |
10.44 | | Amended and Restated License and Collaboration Agreement among the Registrant, Alnylam Pharmaceuticals, Inc. and Regulus Therapeutics LLC dated January 1, 2009. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.(6) |
| | |
10.45 | | Amendment No. 1 to Amended and Restated License Agreement between the Registrant and OncoGenex Technologies Inc. dated December 18, 2009.(32) |
| | |
10.46 | | Amendment Number One to the Amended and Restated License and Collaboration Agreement dated June 10, 2010 among the Registrant, Alnylam Pharmaceuticals, Inc. and Regulus Therapeutics Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (34) |
| | |
10.47 | | Amendment Number One to the Founding Investor Rights Agreement dated June 7, 2010 among the Registrant, Alnylam Pharmaceuticals, Inc. and Regulus Therapeutics Inc. (34) |
| | |
10.48 | | First Amendment to Collaboration Research and License Agreement dated July 27, 2010 between Bristol Myers Squibb Company and the Registrant. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (35) |
| | |
10.49 | | Second Amendment to Loan Agreement dated November 15, 2010 between the Registrant and RBS Asset Finance, Inc. (30) |
| | |
14.1 | | Registrant’s Code of Ethics and Business Conduct.(21) |
| | |
21.1 | | List of Subsidiaries for the Registrant. |
| | |
23.1 | | Consent of Independent Registered Public Accounting Firm. |
| | |
24.1 | | Power of Attorney.(37) |
| | |
31.1 | | Certification by Chief Executive Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
75
31.2 | | Certification by Chief Financial Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | |
32.1 | | Certification Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | |
99.2 | | Form of Confidentiality Agreement.(11) |
| | |
101 | | The following financial statements from the Isis Pharmaceuticals, Inc. Annual Report on Form 10-K for the year ended December 31, 2011, formatted in Extensive Business Reporting Language (XBRL): (i) consolidated balance sheets, (ii) consolidated statements of operations, (iii) consolidated statements of stockholders’ equity, (iv) consolidated statements of cash flows, and (v) notes to consolidated financial statements (tagged as blocks of text). |
(1) | | Filed as an exhibit to the Registrant’s Registration Statement on Form S-1 (No. 33-39640) or amendments thereto and incorporated herein by reference. |
| | |
(2) | | Filed as an exhibit to Registrant’s Current Report on Form 8-K, filed with the SEC on April 15, 2009 and incorporated herein by reference. |
| | |
(3) | | Filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2006. |
| | |
(4) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2011 and incorporated herein by reference. |
| | |
(5) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2007 and incorporated herein by reference. |
| | |
(6) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2009 and incorporated herein by reference. |
| | |
(7) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2007 and incorporated herein by reference. |
| | |
(8) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2008 and incorporated herein by reference. |
| | |
(9) | | Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1998 and incorporated herein by reference. |
| | |
(10) | | Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1999 and incorporated herein by reference. |
| | |
(11) | | Filed as an exhibit to the Registrant’s Registration Statement on Form S-3 (No. 333-71911) or amendments thereto and incorporated herein by reference. |
| | |
(12) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008 and incorporated herein by reference. |
| | |
(13) | | Filed as an exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 5, 2006 and incorporated herein by reference. |
| | |
(14) | | Filed as an exhibit to Registrant’s Current Report on Form 8-K dated January 24, 2007 and incorporated herein by reference. |
| | |
(15) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2011 and incorporated herein by reference. |
| | |
(16) | | Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2004 and incorporated herein by reference. |
| | |
(17) | | Filed as an exhibit to Registrant’s Report on Form 8-K dated December 8, 2000 and incorporated herein by reference. |
76
(18) | | Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 14, 2011 and incorporated herein by reference. |
| | |
(19) | | Filed as an exhibit to the Registrant’s report on Form 10-Q as amended for the quarter ended June 30, 2001 and incorporated herein by reference. |
| | |
(20) | | Filed as an exhibit to Registrant’s Notice of Annual Meeting and Proxy Statement for the 2009 Annual Meeting of Stockholders, filed with the SEC on April 20, 2008, and incorporated herein by reference. |
| | |
(21) | | Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 5, 2008 and incorporated herein by reference. |
| | |
(22) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2008 and incorporated herein by reference. |
| | |
(23) | | Filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005 and incorporated herein by reference. |
| | |
(24) | | Filed as an exhibit to the Registrant’s Report on Form 8-K filed January 4, 2002 and incorporated herein by reference. |
| | |
(25) | | Filed as an exhibit to the Registrant’s Notice of 2011Annual Meeting of Stockholders and Proxy Statement filed with the SEC on April 28, 2011, and incorporated herein by reference. |
| | |
(26) | | Filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005 and incorporated herein by reference. |
| | |
(27) | | Filed as an exhibit to Registrant’s Current Report on Form 8-K dated April 7, 2005 and incorporated herein by reference. |
| | |
(28) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2009 and incorporated herein by reference. |
| | |
(29) | | Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2001 and incorporated herein by reference. |
| | |
(30) | | Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2010 and incorporated herein by reference. |
| | |
(31) | | Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008 and incorporated herein by reference. |
| | |
(32) | | Filed as an exhibit to the Registrant’s Annual Report as Form 10-K for the year ended December 31, 2009 and incorporated herein by reference. |
| | |
(33) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010 and incorporated herein by reference. |
| | |
(34) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 and incorporated herein by reference. |
| | |
(35) | | Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2010 and incorporated herein by reference. |
| | |
(36) | | Filed as an exhibit to Registrant’s Notice of Annual Meeting and Proxy Statement for the 2010 Annual Meeting of Stockholders, filed with the SEC on April 16, 2010, and incorporated herein by reference. |
| | |
(37) | | Filed as part of this Annual Report on Form 10-K for the year ended December 31, 2010, reference is made to page 70. |
| | |
(38) | | Filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed with the SEC on August 8, 2011, and incorporated herein by reference. |
| | |
* | | Indicates management compensatory plans and arrangements as required to be filed as exhibits to this Report pursuant to Item 14(c). |
77
ISIS PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Isis Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Isis Pharmaceuticals, Inc. as of December 31, 2011 and 2010, and the related consolidated statements of operations, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Isis Pharmaceuticals, Inc. at December 31, 2011 and 2010, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2011, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 1 to the consolidated financial statements, the Company changed its method of accounting for variable interest entities as a result of the adoption of the amendments to the FASB Accounting Standards Codification resulting from Accounting Standards Update No. 2009-17, Consolidations (Topic 810): Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities, effective January 1, 2010.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Isis Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 29, 2012 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Diego, California
February 29, 2012
F-2
ISIS PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share data)
| | December 31, | |
| | 2011 | | 2010 | |
ASSETS | | | | | |
Current assets: | | | | | |
Cash and cash equivalents | | $ | 65,477 | | $ | 70,052 | |
Short-term investments | | 278,187 | | 402,301 | |
Contracts receivable | | 6,921 | | 1,242 | |
Inventories | | 4,139 | | 2,484 | |
Other current assets | | 5,415 | | 7,058 | |
Total current assets | | 360,139 | | 483,137 | |
Property, plant and equipment, net | | 96,615 | | 35,703 | |
Licenses, net | | 9,036 | | 12,288 | |
Patents, net | | 16,259 | | 15,821 | |
Deposits and other assets | | 2,845 | | 3,528 | |
Total assets | | $ | 484,894 | | $ | 550,477 | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | |
Current liabilities: | | | | | |
Accounts payable | | $ | 8,300 | | $ | 6,523 | |
Accrued compensation | | 9,183 | | 6,831 | |
Accrued liabilities | | 18,655 | | 12,389 | |
Current portion of long-term obligations | | 3,390 | | 5,645 | |
Current portion of deferred contract revenue | | 36,584 | | 74,502 | |
Total current liabilities | | 76,112 | | 105,890 | |
Long-term deferred contract revenue | | 17,474 | | 50,413 | |
25/8 percent convertible subordinated notes | | 141,448 | | 132,895 | |
Long-term obligations, less current portion | | 4,125 | | 5,720 | |
Long-term financing liability for leased facility | | 69,877 | | 10,147 | |
Investment in Regulus Therapeutics Inc. | | 4,424 | | 870 | |
Total liabilities | | 313,460 | | 305,935 | |
Stockholders’ equity: | | | | | |
Common stock, $0.001 par value; 200,000,000 shares authorized, 100,042,976 and 99,393,780 shares issued and outstanding at December 31, 2011 and 2010, respectively | | 100 | | 99 | |
Additional paid-in capital | | 1,013,592 | | 1,000,181 | |
Accumulated other comprehensive income (loss) | | (770 | ) | 949 | |
Accumulated deficit | | (841,488 | ) | (756,687 | ) |
Total stockholders’ equity | | 171,434 | | 244,542 | |
Total liabilities and stockholders’ equity | | $ | 484,894 | | $ | 550,477 | |
See accompanying notes.
F-3
ISIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except for per share amounts)
| | Years Ended December 31, | |
| | 2011 | | 2010 | | 2009 | |
Revenue: | | | | | | | |
Research and development revenue under collaborative agreements | | $ | 96,190 | | $ | 102,921 | | $ | 108,131 | |
Licensing and royalty revenue | | 2,896 | | 5,552 | | 13,469 | |
Total revenue | | 99,086 | | 108,473 | | 121,600 | |
| | | | | | | |
Expenses: | | | | | | | |
Research and development | | 157,397 | | 145,160 | | 134,623 | |
General and administrative | | 12,789 | | 11,669 | | 14,515 | |
Total operating expenses | | 170,186 | | 156,829 | | 149,138 | |
| | | | | | | |
Loss from operations | | (71,100 | ) | (48,356 | ) | (27,538 | ) |
| | | | | | | |
Other income (expense): | | | | | | | |
Equity in net loss of Regulus Therapeutics Inc. | | (3,554 | ) | (2,228 | ) | — | |
Investment income | | 2,414 | | 3,370 | | 6,361 | |
Interest expense | | (16,732 | ) | (13,232 | ) | (12,672 | ) |
Gain (loss) on investments, net | | 4,182 | | (713 | ) | 2,084 | |
| | | | | | | |
Loss from continuing operations, before income tax expense | | (84,790 | ) | (61,159 | ) | (31,765 | ) |
| | | | | | | |
Income tax expense | | (11 | ) | (92 | ) | (3,191 | ) |
| | | | | | | |
Net loss from continuing operations | | (84,801 | ) | (61,251 | ) | (34,956 | ) |
| | | | | | | |
Discontinued operations: | | | | | | | |
Loss from discontinued operations | | — | | — | | (29 | ) |
Gain on sale of Ibis Biosciences, Inc., net of tax | | — | | — | | 185,657 | |
Net income from discontinued operations, net of tax | | — | | — | | 185,628 | |
| | | | | | | |
Net income (loss) | | (84,801 | ) | (61,251 | ) | 150,672 | |
| | | | | | | |
Net loss attributable to noncontrolling interest in Regulus Therapeutics Inc. | | — | | — | | 4,394 | |
| | | | | | | |
Net income (loss) attributable to Isis Pharmaceuticals, Inc. common stockholders | | $ | (84,801 | ) | $ | (61,251 | ) | $ | 155,066 | |
| | | | | | | |
Basic and diluted net income (loss) per share: | | | | | | | |
Net loss from continuing operations attributable to Isis Pharmaceuticals, Inc. common stockholders | | $ | (0.85 | ) | $ | (0.62 | ) | $ | (0.31 | ) |
Net income from discontinued operations | | — | | — | | 1.89 | |
Basic and diluted net income (loss) attributable to Isis Pharmaceuticals, Inc. common stockholders | | $ | (0.85 | ) | $ | (0.62 | ) | $ | 1.58 | |
Shares used in computing basic and diluted net income (loss) per share | | 99,656 | | 99,143 | | 98,109 | |
See accompanying notes.
F-4
ISIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
Years Ended December 31, 2011, 2010 and 2009
(In thousands)
| | Isis Pharmaceuticals, Inc. Stockholders’ Equity | | | | | |
| | Common stock | | Additional
paid in | | Accumulated
other
comprehensive | | Accumulated | | Noncontrolling Interests | | Total
stockholders’ | |
Description | | Shares | | Amount | | capital | | income | | deficit | | Regulus | | Ibis | | Equity | |
Balance at December 31, 2008 | | 97,172 | | $ | 97 | | $ | 960,361 | | $ | 982 | | $ | (851,216 | ) | $ | 4,737 | | $ | 32,419 | | $ | 147,380 | |
Comprehensive income: | | | | | | | | | | | | | | | | | |
Net income (loss) | | — | | — | | — | | — | | 155,066 | | (4,394 | ) | — | | 150,672 | |
Change in unrealized gains, net of $0.8 million of tax expense | | — | | — | | — | | 2,819 | | — | | — | | — | | 2,819 | |
Reclassification adjustment for realized gains included in net income | | — | | — | | — | | (1,648 | ) | — | | — | | — | | (1,648 | ) |
Comprehensive income | | | | | | | | | | | | | | | | 151,843 | |
Options exercised and employee stock purchase plan issuances | | 1,670 | | 2 | | 13,154 | | — | | — | | — | | — | | 13,156 | |
Warrants exercised | | 9 | | — | | — | | — | | — | | — | | — | | — | |
Excess tax benefits on share-based compensation | | — | | — | | 278 | | — | | — | | — | | — | | 278 | |
Share-based compensation expense | | — | | — | | 11,827 | | — | | — | | — | | — | | 11,827 | |
Sale of Ibis to AMI | | — | | — | | — | | — | | — | | — | | (32,419 | ) | (32,419 | ) |
Alnylam’s capital contribution to noncontrolling interest | | — | | — | | — | | — | | — | | 10,000 | | — | | 10,000 | |
Balance at December 31, 2009 | | 98,851 | | $ | 99 | | $ | 985,620 | | $ | 2,153 | | $ | (696,150 | ) | $ | 10,343 | | $ | — | | $ | 302,065 | |
Adoption of accounting standard to deconsolidate Regulus Therapeutics Inc. | | — | | — | | (1,954 | ) | — | | 714 | | (10,343 | ) | — | | (11,583 | ) |
Comprehensive loss: | | | | | | | | | | | | | | | | | |
Net loss | | — | | — | | — | | — | | (61,251 | ) | — | | — | | (61,251 | ) |
Change in unrealized losses | | — | | — | | — | | (1,342 | ) | — | | — | | — | | (1,342 | ) |
Reclassification adjustment for realized losses included in net loss | | — | | — | | — | | 138 | | — | | — | | — | | 138 | |
Comprehensive loss | | | | | | | | | | | | | | | | (62,455 | ) |
Options exercised and employee stock purchase plan issuances | | 475 | | — | | 4,356 | | — | | — | | — | | — | | 4,356 | |
Warrants exercised | | 68 | | — | | — | | — | | — | | — | | — | | — | |
Share-based compensation expense | | — | | — | | 12,159 | | — | | — | | — | | — | | 12,159 | |
Balance at December 31, 2010 | | 99,394 | | $ | 99 | | $ | 1,000,181 | | $ | 949 | | $ | (756,687 | ) | $ | — | | $ | — | | $ | 244,542 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | |
Net loss | | — | | — | | — | | — | | (84,801 | ) | — | | — | | (84,801 | ) |
Change in unrealized losses | | — | | — | | — | | (1,719 | ) | — | | — | | — | | (1,719 | ) |
Comprehensive loss | | | | | | | | | | | | | | | | (86,520 | ) |
Options exercised and employee stock purchase plan issuances | | 646 | | 1 | | 3,566 | | — | | — | | — | | — | | 3,567 | |
Warrants exercised | | 3 | | — | | — | | — | | — | | — | | — | | — | |
Share-based compensation expense | | — | | — | | 9,845 | | — | | — | | — | | — | | 9,845 | |
Balance at December 31, 2011 | | 100,043 | | $ | 100 | | $ | 1,013,592 | | $ | (770 | ) | $ | (841,488 | ) | $ | — | | $ | — | | $ | 171,434 | |
See accompanying notes.
F-5
ISIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | Years Ended December 31, | |
| | 2011 | | 2010 | | 2009 | |
Operating activities: | | | | | | | |
Net income (loss) | | $ | (84,801 | ) | $ | (61,251 | ) | $ | 150,672 | |
Adjustments to reconcile net income (loss) to net cash used in operating activities: | | | | | | | |
Depreciation | | 6,594 | | 4,840 | | 3,935 | |
Amortization of patents | | 1,938 | | 1,961 | | 3,024 | |
Amortization of licenses | | 3,252 | | 2,376 | | 2,344 | |
Amortization of premium on investments, net | | 5,410 | | 5,075 | | 2,026 | |
Amortization of debt issuance costs | | 507 | | 507 | | 507 | |
Amortization of 25/8 percent convertible subordinated notes discount | | 8,553 | | 7,795 | | 7,107 | |
Share-based compensation expense | | 9,845 | | 12,159 | | 13,385 | |
Equity in net loss of Regulus Therapeutics Inc. | | 3,554 | | 2,228 | | — | |
Gain from sale of Ibis Biosciences, Inc. to Abbott Molecular Inc. | | — | | — | | (185,657 | ) |
Gain from the sale of property, plant and equipment | | — | | (72 | ) | — | |
(Gain) loss on investments, net | | (4,182 | ) | 713 | | (2,084 | ) |
Non-cash losses related to patents, licensing and property, plant and equipment | | 1,924 | | 1,512 | | 696 | |
Excess tax benefits on share-based compensation | | — | | — | | (278 | ) |
Changes in operating assets and liabilities: | | | | | | | |
Contracts receivable | | (5,679 | ) | 10,479 | | (6,778 | ) |
Inventories | | (1,655 | ) | 284 | | (17 | ) |
Other current and long-term assets | | 914 | | (943 | ) | (955 | ) |
Accounts payable | | 875 | | 1,325 | | (3,652 | ) |
Accrued compensation | | 2,352 | | 394 | | (4,371 | ) |
Income taxes payable | | — | | (7,178 | ) | (10,013 | ) |
Deferred rent | | 382 | | — | | — | |
Accrued liabilities | | 6,273 | | 1,013 | | 4,318 | |
Deferred contract revenue | | (70,857 | ) | (46,810 | ) | (82,650 | ) |
Net cash used in operating activities | | (114,801 | ) | (63,593 | ) | (108,441 | ) |
Investing activities: | | | | | | | |
Purchases of short-term investments | | (371,108 | ) | (530,137 | ) | (776,381 | ) |
Proceeds from the sale of short-term investments | | 488,918 | | 577,533 | | 578,886 | |
Purchases of property, plant and equipment | | (7,331 | ) | (13,237 | ) | (13,414 | ) |
Proceeds from the sale of property, plant and equipment | | — | | 185 | | — | |
Proceeds from land sold to BioMed | | — | | 10,147 | | — | |
Reduction of cash due to deconsolidation of Regulus Therapeutics Inc. upon adoption of a new accounting standard | | — | | (16,228 | ) | — | |
Acquisition of licenses and other assets, net | | (3,667 | ) | (4,319 | ) | (2,880 | ) |
Purchases of strategic investments, net of proceeds received | | (359 | ) | (250 | ) | (1,349 | ) |
Proceeds from the sale of strategic investments | | 4,445 | | — | | 2,848 | |
Net cash provided by (used in) investing activities | | 110,898 | | 23,694 | | (212,290 | ) |
Financing activities: | | | | | | | |
Proceeds from issuance of equity | | 3,567 | | 4,356 | | 13,156 | |
Excess tax benefits on share-based compensation | | — | | — | | 278 | |
Proceeds from equipment financing arrangement | | 1,625 | | 4,694 | | 6,394 | |
Principal payments on debt and capital lease obligations | | (5,864 | ) | (4,354 | ) | (2,827 | ) |
Proceeds from sale of Ibis Biosciences, Inc to Abbott Molecular Inc. | | — | | — | | 175,000 | |
Proceeds from Alnylam’s capital contribution to Regulus Therapeutics Inc. | | — | | — | | 10,000 | |
Net cash (used in) provided by financing activities | | (672 | ) | 4,696 | | 202,001 | |
Net decrease in cash and cash equivalents | | (4,575 | ) | (35,203 | ) | (118,730 | ) |
Cash and cash equivalents at beginning of year | | 70,052 | | 105,255 | | 223,985 | |
Cash and cash equivalents at end of year | | $ | 65,477 | | $ | 70,052 | | $ | 105,255 | |
Supplemental disclosures of cash flow information: | | | | | | | |
Interest paid | | $ | 4,804 | | $ | 4,889 | | $ | 4,883 | |
Income taxes paid, net of refund received | | $ | 2 | | $ | 7,270 | | $ | 13,205 | |
Supplemental disclosures of non-cash investing and financing activities: | | | | | | | |
Amounts accrued for capital and patent expenditures | | $ | 902 | | $ | 922 | | $ | 870 | |
Capital lease obligations | | $ | — | | $ | 770 | | $ | — | |
Capitalized costs and financing liability associated with leased facility | | $ | 59,730 | | $ | — | | $ | — | |
See accompanying notes
F-6
ISIS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Significant Accounting Policies
Basis of Presentation
The consolidated financial statements include the accounts of Isis Pharmaceuticals, Inc. (“we”, “us” or “our”) and our wholly owned subsidiary, Symphony GenIsis, Inc., which is currently inactive. In addition to our wholly owned subsidiary, our consolidated financial statements include our equity investment in Regulus Therapeutics Inc. (formerly Regulus Therapeutics LLC), an entity we identified as a variable interest entity. As a result of adopting a new accounting standard for identifying which enterprise has the power to direct activities of a variable interest entity, we concluded that, beginning in 2010, we were no longer the primary beneficiary of Regulus. As such we have presented our share of Regulus’ operating results on a separate line in our consolidated statement of operations called “Equity in net loss of Regulus Therapeutics Inc.” On our consolidated balance sheet, we have presented our investment in Regulus on a separate line in the non-current liabilities section called “Investment in Regulus Therapeutics Inc.” Prior to the adoption of the new accounting standard in 2010, we were the primary beneficiary of Regulus and as such we consolidated Regulus’ financial results on a line-by-line basis. We have not reclassified amounts in the prior period financial statements to conform to the current year presentation.
As a result of completing the sale of Ibis Biosciences, Inc. to Abbott Molecular Inc., or AMI, in January 2009, we have presented Ibis’ financial position and results of operations separately as discontinued operations in our consolidated financial statements.
Organization and business activity
We incorporated in California on January 10, 1989. In conjunction with our initial public offering, we reorganized as a Delaware corporation in April 1991. We were organized principally to develop human therapeutic drugs using antisense technology.
Basic and diluted net income (loss) per share
We compute basic net income (loss) per share by dividing the net income (loss) by the weighted-average number of common shares outstanding during the period. As we incurred a loss from continuing operations for the years ended December 31, 2011, 2010 and 2009, we did not include the following diluted common equivalent shares in the computation of diluted net income (loss) from continuing operations per share because the effect would be anti-dilutive:
· 25/8 percent convertible subordinated notes;
· GlaxoSmithKline convertible promissory notes;
· Dilutive stock options; and
· Warrants issued to Symphony GenIsis Holdings LLC.
In April 2011, Symphony GenIsis Holdings LLC exercised the remaining warrants.
Revenue Recognition
We generally recognize revenue when we have satisfied all contractual obligations and are reasonably assured of collecting the resulting receivable. We are often entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue under current accounting rules. In those instances in which we have received payment from our customers in advance of recognizing revenue, we include the amounts in deferred revenue on our consolidated balance sheet.
Research and development revenue under collaborative agreements
On January 1, 2011, we adopted an accounting standard, which amended the criteria to identify separate units of accounting for revenue arrangements with multiple deliverables. The new guidance replaces the concept of allocating revenue among deliverables in a multiple-element revenue arrangement according to fair value with an allocation based on estimated selling price. The new standard is applicable on a prospective basis to agreements we enter into or materially modify after January 1, 2011. Our adoption of the standard did not impact our financial position or results of operations as of and for the year ended December 31, 2011 as we did not enter into or materially modify any multiple-element arrangements during that period. However, our adoption of this standard may result in revenue recognition for future agreements that is different from our existing multiple-element arrangements.
F-7
For agreements that we entered into or materially modified prior to the adoption of the revised multiple element guidance, we recognize revenue from each element of the arrangement as long as we can determine a standalone value for the delivered element and fair value for the undelivered elements, we have completed our obligation to deliver or perform on that element and we are reasonably assured of collecting the resulting receivable.
We often enter into collaborations with multiple deliverables under which we receive non-refundable upfront payments. For collaborations where we determine that there is a single unit of accounting because our research and development efforts are directly tied to the upfront payment, we recognize revenue related to upfront payments ratably over our estimated period of performance relating to the term of the contractual arrangements. Occasionally, we must estimate our period of performance when the agreements we entered into do not clearly define such information. Our collaborative agreements typically include a research and/or development project plan that includes the activities the agreement requires each party to perform during the collaboration and the party responsible for performing them. We estimate the period of time over which we will complete the activities for which we are responsible and use that period of time as our period of performance for purposes of revenue recognition and amortize revenue over such period. If our collaborators ask us to continue performing work in a collaboration beyond the initial period of performance, we extend our amortization period to correspond to the new extended period of performance. The revenue we recognize could be materially different if different estimates prevail. We have made estimates of our continuing obligations on several agreements. Adjustments to performance periods and related adjustments to revenue amortization periods have had a material impact on our revenue on only one occasion. When Alnylam Pharmaceuticals, Inc. terminated the companies’ single-stranded RNAi, or ssRNAi, research program in November 2010, we recognized as revenue $4.9 million, which was the remaining deferred revenue from the upfront fee that we were amortizing into revenue over the research term.
As part of our strategic alliance with Genzyme, a Sanofi company, in February 2008 Genzyme made a $150 million equity investment in us by purchasing five million shares of our common stock at $30 per share. The price Genzyme paid for our common stock represented a significant premium over the fair value of our stock. We accounted for this premium as deferred revenue and are amortizing it along with the $175 million licensing fee that we received in June 2008 ratably into revenue until June 2012, which represents the end of our performance obligation based on the current research and development plan.
Our collaborations often include contractual milestones, which typically relate to the achievement of pre-specified development, regulatory and commercialization events. These three categories of milestone events reflect the three stages of the life-cycle of our drugs, which we describe in more detail in the following paragraph.
Prior to the first stage in the life-cycle of our drugs, we perform a significant amount of work using our proprietary antisense technology to design chemical compounds which interact with specific genes that are good targets for drug discovery. From these research efforts, we hope to identify a development candidate. The designation of a development candidate is the first stage in the life-cycle of our drugs. A development candidate is a chemical compound that has demonstrated the necessary safety and efficacy in preclinical animal studies to warrant further study in humans. During the first step of the development stage, we or our partners study our drugs in IND-enabling studies, which are animal studies intended to support an Investigational New Drug, or IND, application and/or the foreign equivalent. An approved IND allows us or our partners to study our development candidate in humans. If the regulatory agency approves the IND, we or our partners initiate Phase 1 clinical trials in which we typically enroll a small number of healthy volunteers to ensure the development candidate is safe for use in patients. If we or our partners determine that a development candidate is safe based on the Phase 1 data, we or our partners initiate Phase 2 studies that are generally larger scale studies in patients with the primary intent of determining the efficacy of the development candidate. The final step in the development stage is Phase 3 studies to gather the necessary safety and efficacy data to request marketing approval from the Food and Drug Administration, or FDA and/or foreign equivalents. The Phase 3 studies typically involve large numbers of patients and can take up to several years to complete. If the data gathered during the trials demonstrates acceptable safety and efficacy results, we or our partner will submit an application to the FDA and/or its foreign equivalents for marketing approval. This stage of the drug’s life-cycle is the regulatory stage. If a drug achieves marketing approval, it moves into the commercialization stage, during which our partner will market and sell the drug to patients. Although our partner will ultimately be responsible for marketing and selling the drug, our efforts to discover and develop a drug that is safe, effective and reliable contributes significantly to our partner’s ability to successfully sell the drug. The FDA and its foreign equivalents have the authority to impose significant restrictions on an approved drug through the product label and on advertising, promotional and distribution activities. Therefore, our efforts designing and executing the necessary animal and human studies are critical to obtaining claims in the product label from the regulatory agencies that would allow our partner to successfully commercialize our drug. Further, the patent protection afforded our drugs as a result of our initial patent applications and related prosecution activities in the United States and foreign jurisdictions are critical to our partner’s ability to sell our drugs without competition from generic drugs. The potential sales volume of an approved drug is dependent on several factors including the size of the patient population, market penetration of the drug, and the price charged for the drug.
F-8
Generally, the milestone events contained in our partnership agreements coincide with the progression of our drugs from development, to regulatory approval and then to commercialization. The process of successfully discovering a new development candidate, having it approved and ultimately sold for a profit is highly uncertain. As such, the milestone payments we may earn from our partners involve a significant degree of risk to achieve. Therefore, as a drug progresses through the stages of its life-cycle, the value of the drug generally increases.
Development milestones in our partnerships may include the following types of events:
· Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete;
· Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete;
· Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete;
· Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete.
Regulatory milestones in our partnerships may include the following types of events:
· Filing of regulatory applications for marketing approval such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
· Marketing approval in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain approval from the applicable regulatory agency.
Commercialization milestones in our partnerships may include the following types of events:
· First commercial sale in a particular market, such as in the United States or Europe.
· Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
We assess whether a substantive milestone exists at the inception of our agreements. When a substantive milestone is achieved, we recognize revenue related to the milestone payment. For our existing licensing and collaboration agreements in which we are involved in the discovery and/or development of the related drug or provide the partner with ongoing access to new technologies we discover, we have determined that all future development, regulatory and commercialization milestones are substantive. For example, for our strategic alliance with GlaxoSmithKline, or GSK, we are using our antisense drug discovery platform to seek out and develop new drugs against targets for rare and serious diseases. Alternatively, we provide on-going access to our technology to Alnylam to develop and commercialize RNA interference, or RNAi, therapeutics. We consider milestones for both of these collaborations to be substantive. For those agreements that do not meet the following criteria, we do not consider the future milestones to be substantive. In evaluating if a milestone is substantive we consider whether:
· Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
· The achievement of the milestone involves substantive effort and can only be achieved based in whole or part on our performance or the occurrence of a specific outcome resulting from our performance;
· The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
· There is no future performance required to earn the milestone; and
· The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
If any of these conditions are not met, we will defer recognition of the milestone payment and recognize it as revenue over the estimated period of performance, if any. In 2011, OncoGenex Pharmaceuticals Inc. initiated a Phase 2 trial of OGX-427 in men with metastatic prostate cancer. Also in 2011, we initiated a Phase 1 clinical study on ISIS-TTRRX, the first drug selected as part of our collaboration with GSK and we selected ISIS-AATRX as the second development candidate as part of our that collaboration. We consider milestones related to progression of a drug through the development stage of its life cycle to be substantive milestones because the level of effort and inherent risk associated with these events is high. Therefore, we recognized the entire $750,000 milestone payment from OncoGenex and two $5 million milestone payments from GSK in 2011. Further information about our collaborative arrangements can be found in Note 8, Collaborative Arrangements and Licensing Agreements.
F-9
Licensing and royalty revenue
We often enter into agreements to license our proprietary patent rights on an exclusive or non-exclusive basis in exchange for license fees and/or royalties. We generally recognize as revenue immediately those licensing fees and royalties for which we have no significant future performance obligations and are reasonably assured of collecting the resulting receivable.
Research and development expenses
We expense research and development costs as we incur them. Included in research and development expenses are costs associated with our collaboration agreements. For the years ended December 31, 2011, 2010 and 2009, research and development costs of approximately $26.3 million, $44.6 million, and $57.1 million, respectively, were related to collaborative research and development arrangements.
Concentration of credit risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash equivalents, short-term investments and receivables. We place our cash equivalents and certain of our short-term investments with reputable financial institutions. We primarily invest our excess cash in commercial paper and debt instruments of the U.S. Treasury and financial institutions, corporations, and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody’s, Standard & Poor’s or Fitch, respectively. We and our audit committee establish guidelines relative to credit ratings, diversification and maturities that seek to maintain safety and liquidity.
Cash, cash equivalents and short-term investments
We consider all liquid investments with maturities of 90 days or less when we purchase them to be cash equivalents. Our short-term investments have initial maturities of greater than 90 days from date of purchase. We classify our short-term investments as “available-for-sale” and carry them at fair market value based upon prices for identical or similar items on the last day of the fiscal period. We record unrealized gains and losses as a separate component of stockholders’ equity and include net realized gains and losses in gain (loss) on investments. We use the specific identification method to determine the cost of securities sold.
We have equity investments in privately- and publicly-held biotechnology companies that we have received as part of a technology license or collaboration agreement. We hold ownership interests of less than 20 percent in each of the respective companies except Regulus, our jointly owned subsidiary. In determining if and when a decrease in market value below our cost in our equity positions is temporary or other-than-temporary, we examine historical trends in the stock price, the financial condition of the company, near term prospects of the company and our current need for cash. We record unrealized gains and losses related to temporary declines in the publicly-held companies as a separate component of stockholders’ equity and account for securities in the privately-held companies, except for Regulus, under the cost method of accounting because we own less than 20 percent and do not have significant influence in their operations. Most of the cost method investments we hold are in early stage biotechnology companies and realization of our equity position in those companies is uncertain. In those circumstances we record a full valuation allowance. When we determine that a decline in value in either a public or private investment is other-than-temporary, we recognize an impairment loss in the period in which the other-than-temporary decline occurs.
Inventory valuation
We capitalize the costs of raw materials that we purchase for use in producing our drugs because until we use these raw materials they have alternative future uses. We include in inventory raw material costs for drugs that we manufacture for our partners under contractual terms and that we use primarily in our clinical development activities and drug products. We can use each of our raw materials in multiple products and, as a result, each raw material has future economic value independent of the development status of any single drug. For example, if one of our drugs failed, we could use the raw materials allocated for that drug to manufacture our other drugs. We expense these costs when we deliver the drugs to our partners, or as we provide these drugs for our own clinical trials. We reflect our inventory on the balance sheet at the lower of cost or market value under the first-in, first-out method. We review inventory periodically and reduce the carrying value of items we consider to be slow moving or obsolete to their estimated net realizable value. We consider several factors in estimating the net realizable value, including shelf life of raw materials, alternative uses for our drugs and clinical trial materials and historical write-offs. We did not record any inventory write-offs for the years ended December 31, 2011, 2010 and 2009. Total inventory, which consisted of raw materials, was $4.1 million and $2.5 million as of December 31, 2011 and 2010, respectively.
F-10
Property, plant and equipment
We carry our property, plant and equipment at cost, which consists of the following (in thousands):
| | December 31, | |
| | 2011 | | 2010 | |
Equipment and computer software | | $ | 42,422 | | $ | 38,685 | |
Building and building systems | | 48,431 | | — | |
Land improvements | | 2,822 | | — | |
Leasehold improvements | | 34,839 | | 25,147 | |
Furniture and fixtures | | 5,323 | | 2,550 | |
| | 133,837 | | 66,382 | |
Less accumulated depreciation | | (47,420 | ) | (40,826 | ) |
| | 86,417 | | 25,556 | |
Land | | 10,198 | | 10,147 | |
| | | | | |
| | $ | 96,615 | | $ | 35,703 | |
We depreciate our property, plant and equipment on the straight-line method over estimated useful lives as follows:
Computer software and hardware | | 3 years | |
Manufacturing Equipment | | 10 years | |
Other Equipment | | 5-7 years | |
Furniture and fixtures | | 5-10 years | |
Building | | 40 years | |
Building systems and improvements | | 10-25 years | |
Land improvements | | 20 years | |
We depreciate our leasehold improvements using the shorter of the estimated useful life or remaining lease term.
Licenses
We obtain licenses from third parties and capitalize the costs related to exclusive licenses. We amortize capitalized licenses over their estimated useful life or term of the agreement, which for current licenses is between approximately three years and 15 years. The cost of our licenses at December 31, 2011 and 2010 was $36.2 million. Accumulated amortization related to licenses was $27.2 million and $23.9 million at December 31, 2011 and 2010, respectively. Based on existing licenses, estimated amortization expense related to licenses is $2.4 million and $2.0 million for the years ending December 31, 2012 and 2013, respectively, $1.9 million for each of the years ending December 31, 2014 and 2015, and $0.8 million for the year ended December 31, 2016.
Patents
We capitalize costs consisting principally of outside legal costs and filing fees related to obtaining patents. We review our capitalized patent costs regularly to ensure that they include costs for patents and patent applications that have future value. We evaluate patents and patent applications that we are not actively pursuing and write off any associated costs. We amortize patent costs over their estimated useful lives of 10 years, beginning with the date the United States Patent and Trademark Office, or foreign equivalent, issues the patent. The weighted average remaining amortizable life of issued patents was 2.6 years and 2.5 years at December 31, 2011 and 2010, respectively. In 2011, 2010 and 2009, we recorded a non-cash charge of $1.9 million, $1.5 million and $696,000, respectively, which we included in research and development expenses, related to the write-down of our patent costs to their estimated net realizable values.
F-11
The cost of our patents at December 31, 2011 and 2010 was $29.9 million and $33.3 million, respectively. Accumulated amortization related to patents was $13.7 million and $17.5 million at December 31, 2011 and 2010, respectively. Based on existing patents, estimated amortization expense related to patents is as follows:
Years Ending December 31, | | Amortization | |
| | (in millions) | |
| | | |
2012 | | $ | 1.5 | |
2013 | | $ | 1.0 | |
2014 | | $ | 0.8 | |
2015 | | $ | 0.6 | |
2016 | | $ | 0.6 | |
Fair value of financial instruments
We have estimated the fair value of our financial instruments. The amounts reported for cash, accounts receivable, accounts payable and accrued expenses approximate the fair value because of their short maturities. We report our investment securities at their estimated fair value based on quoted market prices for identical or similar instruments.
Long-lived assets
We evaluate long-lived assets, which include property, plant and equipment, patent costs, and licenses acquired from third parties, for impairment on at least a quarterly basis and whenever events or changes in circumstances indicate that we may not be able to recover the carrying amount of such assets. We recorded a charge of $1.9 million, $1.5 million and $696,000 for the years ended December 31, 2011, 2010 and 2009, respectively, related to the write-down of intangible assets.
Equity method of accounting
On January 1, 2010, we adopted an accounting standard, which replaced the quantitative-based risks and rewards calculation for determining which enterprise, if any, has a controlling financial interest in a variable interest entity. The new approach focuses on identifying which enterprise has the power to direct the activities of a variable interest entity that most significantly impacts the variable interest entity’s economic performance and (1) the obligation to absorb losses of the variable interest entity or (2) the right to receive benefits from the variable interest entity. As a result of adopting this new accounting standard, we were required to change the way we account for our variable interest in Regulus. Since we and Alnylam Pharmaceuticals, Inc. share equally the ability to impact Regulus’ economic performance, we are no longer the primary beneficiary of Regulus. We adopted the new standard on a prospective basis, therefore beginning in the first quarter of 2010, we deconsolidated Regulus from our consolidated financial statements and began to account for our ownership interest in Regulus using the equity method of accounting. We no longer include Regulus’ revenue and operating expenses in our operating results. Instead we include our share of Regulus’ operating results on a separate line in our consolidated statement of operations called “Equity in net loss of Regulus Therapeutics Inc.” On our consolidated balance sheet, we present our investment in Regulus on a separate line in the non-current liabilities section called “Investment in Regulus Therapeutics Inc.” We have not reclassified amounts in the prior period financial statements to conform to the current period presentation.
Under the equity method of accounting, we are required to suspend recognizing losses if the carrying amount of our investment in Regulus exceeds the amount of funding we are required to provide to Regulus. Since we and Alnylam are guarantors of both of the convertible notes that Regulus issued to GSK we will continue to recognize losses in excess of our net investment in Regulus up to the principal plus accrued interest we guaranteed, which at December 31, 2011 was $5.5 million.
Use of estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
F-12
Consolidation of variable interest entities
We identify entities as variable interest entities either: (1) that do not have sufficient equity investment at risk to permit the entity to finance its activities without additional subordinated financial support, or (2) in which the equity investors lack an essential characteristic of a controlling financial interest. We perform ongoing qualitative assessments of our variable interest entities to determine whether we have a controlling financial interest in the variable interest entity and therefore are the primary beneficiary. As of December 31, 2011 and 2010, we had collaborative arrangements with six and eight entities, respectively, that we considered to be variable interest entities. We are not the primary beneficiary for any of these entities as we do not have both the power to direct the activities that most significantly impact the economic performance of our variable interest entities and the obligation to absorb losses or the right to receive benefits from our variable interest entities that could potentially be significant to the variable interest entities. For 2009, our consolidated financial statements included one variable interest entity, Regulus, for which we were the primary beneficiary. As a result of adopting the new accounting standard related to our investment in Regulus in 2010, we deconsolidated Regulus because we were no longer the primary beneficiary of Regulus. See Note 2, Investment in Regulus Therapeutics Inc., for additional details.
Stock-based compensation
We estimate the fair value of stock-based payment awards on the date of grant using an option-pricing model. We recognize the value of the portion of the award that we ultimately expect to vest as expense over the requisite service period as stock-based compensation expense in our consolidated statements of operations. We recognize compensation expense for all stock-based payment awards using the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), an entity recognizes compensation expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period. We reduce stock-based compensation expense for estimated forfeitures at the time of grant and revise in subsequent periods if actual forfeitures differ from those estimates.
We utilize the Black-Scholes model as our method of valuing stock-based awards we granted. On the grant date, we use our stock price and assumptions regarding a number of highly complex and subjective variables to determine the estimated fair value of stock-based payment awards. These variables include, but are not limited to, our expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. Option-pricing models were developed for use in estimating the value of traded options that have no vesting or hedging restrictions and are fully transferable. Because our employee stock options have certain characteristics that are significantly different from traded options, and because changes in the subjective assumptions can materially affect the estimated value, in management’s opinion, the existing valuation models may not provide an accurate measure of the fair value of our employee stock options. Although we determine the estimated fair value of employee stock options using an option-pricing model, that value may not be indicative of the fair value observed in a willing buyer/willing seller market transaction.
We account for stock options we grant to non-employees, which consist of options we grant to consultants, by estimating their fair value. We remeasure the fair value at each reporting period until the stock option vests. We recognize the expense over the period of time we require the non-employee to perform services.
See Note 6, Stockholders’ Equity, for additional information regarding our share-based compensation plans.
Comprehensive income (loss)
We display comprehensive income (loss) and its components as part of our full set of consolidated financial statements. Comprehensive income (loss) is comprised of net income (loss) and certain changes in stockholders’ equity that we exclude from net income (loss). We include unrealized holding gains and losses, net of taxes, and reclassification adjustment for realized gains and losses on our available-for-sale securities, which we report separately in stockholders’ equity, in accumulated other comprehensive income (loss). We include comprehensive income (loss) for the years ended December 31, 2011, 2010 and 2009 in our consolidated statements of stockholders’ equity.
Convertible debt
We account for our 25/8 percent convertible notes by separating the liability and equity components of the instruments in a manner that reflects our nonconvertible debt borrowing rate when we recognize interest expense in subsequent periods. As a result, we assigned a value to the debt component of our 25/8 percent convertible notes equal to the estimated fair value of a similar debt instrument without the conversion feature, which resulted in us recording the debt at a discount. We are amortizing this debt discount over the life of the debt as additional non-cash interest expense utilizing the effective interest method. For additional information, see Note 5, Long-Term Obligations and Commitments.
F-13
Segment information
Prior to 2011, we reported our results in two separate segments: Drug Discovery and Development and Regulus. Beginning in the first quarter of 2011, we no longer consider Regulus as an operating segment because our chief decision making officer no longer reviews Regulus’ operating results for purposes of making resource allocations. Therefore we only provide financial information and results for our Drug Discovery and Development operations.
Fair Value Measurements
We use a three-tier fair value hierarchy to prioritize the inputs used in our fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets, which includes our money market funds and treasury securities classified as available-for-sale securities and equity securities in publicly-held biotechnology companies; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable, which includes our fixed income securities and commercial paper classified as available-for-sale securities; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions. The majority of our securities have been classified as Level 2. To estimate the fair value of securities classified as Level 2, we utilize the services of a fixed income pricing provider that uses an industry standard valuation model, which is based on a market approach. We validate the fair value of securities from our pricing provider by understanding the pricing model they used and comparing their assessment of the fair value of our Level 2 investments to the fair value from the custodians of our Level 2 investments. Our pricing provider and custodians use similar techniques to derive fair value for Level 2 securities. At December 31, 2011, we had no securities that we classified as Level 3.
We measure the following major security types at fair value on a recurring basis. We break down the inputs used to measure fair value for these assets at December 31, 2011 and 2010 as follows (in thousands):
| | At December 31,
2011 | | Quoted Prices in
Active Markets
(Level 1) | | Significant Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
Cash equivalents (1) | | $ | 58,892 | | $ | 55,893 | | $ | 2,999 | | $ | — | |
Corporate debt securities (2) | | 166,922 | | — | | 166,922 | | — | |
Debt securities issued by U.S. government agencies (2) | | 80,440 | | — | | 80,440 | | — | |
Debt securities issued by the U.S. Treasury (2) | | 2,356 | | 2,356 | | — | | — | |
Debt securities issued by states of the United States and political subdivisions of the states (2) | | 28,469 | | — | | 28,469 | | — | |
Equity securities (3) | | 1,282 | | 1,282 | | — | | — | |
Total | | $ | 338,361 | | $ | 59,531 | | $ | 278,830 | | $ | — | |
| | At December 31,
2010 | | Quoted Prices in
Active Markets
(Level 1) | | Significant Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
Cash equivalents (1) | | $ | 68,618 | | $ | 50,379 | | $ | 18,239 | | $ | — | |
Corporate debt securities (2) | | 244,228 | | — | | 244,228 | | — | |
Debt securities issued by U.S. government agencies (2) | | 127,041 | | — | | 127,041 | | — | |
Debt securities issued by the U.S. Treasury (2) | | 24,040 | | 24,040 | | — | | — | |
Debt securities issued by states of the United States and political subdivisions of the states (2) | | 6,992 | | — | | 6,992 | | — | |
Equity securities (3) | | 2,011 | | 2,011 | | — | | — | |
Total | | $ | 472,930 | | $ | 76,430 | | $ | 396,500 | | $ | — | |
(1) Included in cash and cash equivalents on our consolidated balance sheet.
(2) Included in short-term investments on our consolidated balance sheet.
(3) Included in other current assets on our consolidated balance sheet.
F-14
Income Taxes
We use the asset and liability method of accounting for income taxes. Under the asset and liability method, deferred tax assets and liabilities reflect the impact of temporary differences between amounts of assets and liabilities for financial reporting purposes and such amounts as measured under enacted tax laws. We record a valuation allowance to offset any net deferred tax assets if, based upon the available evidence, it is more likely than not that we will not recognize some or all of the deferred tax assets.
In our financial statements, we recognize the impact of an uncertain income tax position on our income tax returns at the largest amount that the relevant taxing authority is more-likely-than-not to sustain upon audit. If we feel that the likelihood of sustaining an uncertain income tax position is less than 50 percent, we do not recognize it.
Impact of recently issued accounting standards
In May 2011, the FASB amended its authoritative guidance on the measurement and disclosure for fair value measurements. The amendment clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. The guidance is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2011 and is effective for our fiscal year beginning January 1, 2012. We do not expect the adoption of this guidance to have a material impact on our financial statements.
In June 2011, the FASB amended its authoritative guidance on the presentation of comprehensive income. Under the amendment, companies have the option to present the components of net income and other comprehensive income either in a single continuous statement of comprehensive income or in separate but consecutive statements. This amendment eliminates the currently available option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. The amendment does not change the items that companies must report in other comprehensive income or when companies must reclassify an item of other comprehensive income to net income. In December 2011, the FASB issued an update that defers the presentation requirement for other comprehensive income reclasifications on the face of the financial statements. The guidance is effective retrospectively for fiscal years, and interim periods within those years, beginning after December 15, 2011 and is effective for our fiscal year beginning January 1, 2012. As this guidance relates to presentation only, the adoption of this guidance will not have any other effect on our financial statements.
2. Investment in Regulus Therapeutics Inc.
In September 2007, we and Alnylam established Regulus as a company focused on the discovery, development and commercialization of microRNA-targeting therapeutics. Regulus combines our and Alnylam’s technologies, know-how, and intellectual property relating to microRNA-based therapeutics.
We and Alnylam each granted Regulus exclusive licenses to our respective intellectual property for microRNA therapeutic applications, as well as certain early fundamental patents in the microRNA field. Alnylam made an initial investment of $10 million in Regulus to balance both companies’ ownership. In October 2010, Sanofi invested $10 million in Regulus. From this investment Sanofi acquired less than 10 percent ownership of Regulus, leaving us with approximately 46 percent ownership. Alnylam owns the remaining equity. Regulus operates as an independent company with a separate board of directors, scientific advisory board and management team. We and Alnylam retain rights to develop and commercialize on pre-negotiated terms microRNA therapeutic products that Regulus decides not to develop either by itself or with a partner.
Regulus Collaborations
Regulus’ Collaboration with Sanofi
In June 2010, Regulus established a collaboration with Sanofi to discover, develop, and commercialize microRNA therapeutics, initially focused on fibrosis. The alliance represents the largest microRNA partnership formed to date, valued at potentially over $750 million. The alliance includes a $25 million upfront fee, a $10 million equity investment in Regulus that Sanofi made in October 2010 and annual research support for three years with the option to extend two additional years. In addition, Regulus is eligible to receive royalties on microRNA therapeutic products that Sanofi commercializes. Sanofi also received an option for a broader technology alliance that provides Regulus certain rights to participate in development and commercialization of resulting products. If exercised, this three-year option is worth up to an additional $50 million to Regulus. We and Alnylam are each eligible to receive 7.5 percent of the upfront payment and all potential milestone payments, in addition to royalties on product sales. As a result, in July 2010 we received from Regulus a payment of $1.9 million representing 7.5 percent of the $25 million upfront fee.
F-15
Regulus’ Collaboration with GSK
In April 2008, Regulus entered into a strategic alliance with GSK to discover, develop and commercialize novel microRNA-targeting therapeutics to treat inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease, and in February 2010, Regulus and GSK expanded this alliance to include microRNA therapeutics targeting microRNA 122, or miR-122, for the treatment of hepatitis C virus infection, or HCV. The alliance utilizes Regulus’ expertise and intellectual property position in the discovery and development of microRNA-targeting therapeutics and provides GSK with an option to license drug candidates directed at four different microRNA targets, including miR-122 for HCV. Regulus received $28 million in upfront payments from GSK, including $18 million in option fees and two $5 million convertible promissory notes. The notes plus interest will convert into Regulus common stock in the future if Regulus achieves a minimum level of financing with institutional investors. In addition, we and Alnylam are guarantors of the notes issued to GSK, and if the notes do not convert or if Regulus does not repay the notes by February 2013, we, Alnylam and Regulus may elect to repay the notes plus interest with shares of each company’s common stock or cash. In 2009, Regulus earned revenue of $3.0 million related to its collaboration with GSK, which we included in our 2009 consolidated revenue as Regulus was a consolidated subsidiary in 2009.
Equity method of accounting
On January 1, 2010, as a result of adopting the new accounting standard for identifying which enterprise has the power to direct activities of a variable interest entity, we prospectively changed the way we account for our variable interest in Regulus. Since we and Alnylam share the ability to impact Regulus’ economic performance, we are no longer the primary beneficiary of Regulus. Beginning in 2010, we deconsolidated Regulus from our consolidated financial statements and began to account for our ownership interest in Regulus using the equity method of accounting.
In October 2010, Sanofi invested $10 million in Regulus. From this investment Sanofi acquired less than 10 percent ownership of Regulus, leaving us with approximately 46 percent ownership. Under the equity method of accounting, when Regulus issued shares to Sanofi we recorded a gain of $4.7 million and adjusted the carrying value of our investment in Regulus to reflect the increased valuation of Regulus and our new ownership percentage.
Under the equity method of accounting, we will suspend recognizing losses if the carrying amount of our investment in Regulus exceeds the amount of funding we provided to Regulus. Since we and Alnylam are guarantors of both of the convertible notes that Regulus issued to GSK, we will continue to recognize losses in excess of our net investment in Regulus up to the principal plus accrued interest we guaranteed, which was $5.5 million at December 31, 2011. At December 31, 2011, the total carrying value of our investment in Regulus is a liability of $4.4 million.
Summarized financial information for Regulus is as follows (in thousands):
| | Year Ended December 31, | |
| | 2011 | | 2010 | | 2009 | |
Net revenues | | $ | 13,789 | | $ | 8,601 | | $ | 3,013 | |
Operating expenses | | 20,926 | | 24,099 | | 11,789 | |
Loss from operations | | (7,137 | ) | (15,498 | ) | (8,776 | ) |
Other income (expense) | | (259 | ) | (91 | ) | 13 | |
Income tax benefit (expense) | | (206 | ) | 30 | | (141 | ) |
Net loss | | $ | (7,602 | ) | $ | (15,559 | ) | $ | (8,904 | ) |
| | December 31, | |
| | 2011 | | 2010 | |
Current assets | | $ | 38,666 | | $ | 55,175 | |
Non-current assets | | 4,215 | | 4,528 | |
Total assets | | 42,881 | | 59,703 | |
| | | | | |
Current liabilities | | 12,850 | | 14,729 | |
Non-current liabilities | | 28,834 | | 36,978 | |
Total liabilities | | 41,684 | | 51,707 | |
| | | | | |
Net assets | | $ | 1,197 | | $ | 7,996 | |
F-16
3. Discontinued Operations
In January 2009, AMI completed its acquisition of Ibis for a total purchase price of $215 million. Since we sold Ibis to AMI and Ibis met the criteria for a component of an entity, we reflect Ibis as a discontinued operation. Accordingly, we have presented the operating results of Ibis in our consolidated statements of operations as discontinued operations. Net income from discontinued operations for the year ended December 31, 2009 primarily consisted of the $202.5 million gain related to the sale of Ibis to AMI less $16.8 million of income tax expense.
The components of discontinued operations for the year ended December 31, 2009 is as follows (in thousands):
Revenue | | $ | — | |
Total operating expenses | | 35 | |
Loss from operations | | (35 | ) |
Loss attributed to noncontrolling interest in Ibis Biosciences, Inc. | | 6 | |
Loss from discontinued operations | | (29 | ) |
Gain on sale of Ibis Biosciences, Inc., net of tax | | 185,657 | |
Net income from discontinued operations, net of tax | | $ | 185,628 | |
We do not have any assets and liabilities from discontinued operations in our accompanying consolidated balance sheets at December 31, 2011 and 2010. We have not separately classified cash flows from discontinued operations in our consolidated statement of cash flows.
4. Investments
As of December 31, 2011, we have primarily invested our excess cash in commercial paper and debt instruments of the U.S. Treasury and financial institutions, corporations, and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody’s, Standard & Poor’s (S&P) or Fitch, respectively. We have established guidelines relative to diversification and maturities that maintain safety and liquidity. We periodically review and modify these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity.
The following table summarizes the contract maturity of the available-for-sale securities we held as of December 31, 2011:
One year or less | | 65 | % |
After one year but within two years | | 29 | % |
After two years but within three years | | 6 | % |
Total | | 100 | % |
In April 2011, S&P affirmed the ‘AAA/A-1+’ rating on the sovereign credit rating of the United States. At the same time, however, S&P lowered the outlook of the long-term rating to ‘Negative’ from ‘Stable.’ In July 2011, Moody’s placed the AAA bond rating for the United States on review for a possible downgrade. The actions taken by S&P and Moody’s pertain primarily to the long-term challenges associated with the United States’ budget deficits and rising indebtedness. As illustrated above, we primarily invest our excess cash in short-term instruments with 94 percent of our available-for-sale securities having a maturity of less than two years. Therefore, the action taken by S&P and Moody’s did not impact the carrying value of our available-for-sale securities at December 31, 2011.
At December 31, 2011, we had an ownership interest of less than 20 percent in each of three private companies and two public companies with which we conduct business. The companies are Santaris Pharma A/S (formerly Pantheco A/S), Achaogen Inc., and Atlantic Pharmaceuticals Limited, which are privately-held and Antisense Therapeutics Limited, or ATL, and iCo Therapeutics Inc., which are publicly-traded. We account for securities in the privately-held companies under the cost method of accounting and we classify the securities in the publicly-traded companies as available-for-sale. During 2011, we recognized a $4.2 million net gain on investments primarily consisting of a $4.4 million gain we recorded in the fourth quarter of 2011 from our ownership interest in Excaliard when they were acquired by Pfizer Inc. See further discussion about our investments in these satellite companies in Note 8, Collaborative Arrangements and Licensing Agreements.
F-17
The following is a summary of our investments (in thousands):
| | | | | | | | Other-Than- | | | |
| | | | | | | | Temporary | | | |
| | Amortized | | Unrealized | | Impairment | | Estimated | |
December 31, 2011 | | Cost | | Gains | | Losses | | Loss | | Fair Value | |
Short-term investments: | | | | | | | | | | | |
Corporate debt securities | | $ | 109,842 | | $ | 13 | | $ | (255 | ) | $ | — | | $ | 109,600 | |
Debt securities issued by U.S. government agencies | | 53,723 | | 35 | | (5 | ) | — | | 53,753 | |
Debt securities issued by the U.S. Treasury | | 2,353 | | 3 | | — | | — | | 2,356 | |
Debt securities issued by states of the United States and political subdivisions of the states | | 16,141 | | 4 | | (3 | ) | — | | 16,142 | |
Total securities with a maturity of one year or less | | 182,059 | | 55 | | (263 | ) | — | | 181,851 | |
Corporate debt securities | | 57,632 | | 21 | | (331 | ) | — | | 57,322 | |
Debt securities issued by U.S. government agencies | | 26,754 | | — | | (67 | ) | — | | 26,687 | |
Debt securities issued by states of the United States and political subdivisions of the states | | 12,331 | | 19 | | (23 | ) | — | | 12,327 | |
Total securities with a maturity of more than one year | | 96,717 | | 40 | | (421 | ) | — | | 96,336 | |
Subtotal | | $ | 278,776 | | $ | 95 | | $ | (684 | ) | $ | — | | $ | 278,187 | |
Equity securities: | | | | | | | | | | | |
Current portion (included in Other current assets) | | $ | 1,538 | | $ | 624 | | $ | — | | $ | (880 | ) | $ | 1,282 | |
Long-term portion (included in Deposits and other assets) | | 625 | | — | | — | | — | | 625 | |
Subtotal | | $ | 2,163 | | $ | 624 | | $ | — | | $ | (880 | ) | $ | 1,907 | |
| | $ | 280,939 | | $ | 719 | | $ | (684 | ) | $ | (880 | ) | $ | 280,094 | |
| | | | | | | | Other-Than- | | | |
| | | | | | | | Temporary | | | |
| | Amortized | | Unrealized | | Impairment | | Estimated | |
December 31, 2010 | | Cost | | Gains | | Losses | | Loss | | Fair Value | |
Short-term investments: | | | | | | | | | | | |
Corporate debt securities | | $ | 196,010 | | $ | 294 | | $ | (41 | ) | $ | — | | $ | 196,263 | |
Debt securities issued by U.S. government agencies | | 119,890 | | 53 | | (34 | ) | — | | 119,909 | |
Debt securities issued by the U.S. Treasury | | 24,030 | | 10 | | — | | — | | 24,040 | |
Debt securities issued by states of the United States and political subdivisions of the states | | 6,989 | | 3 | | — | | — | | 6,992 | |
Total securities with a maturity of one year or less | | 346,919 | | 360 | | (75 | ) | — | | 347,204 | |
Corporate debt securities | | 47,842 | | 167 | | (44 | ) | — | | 47,965 | |
Debt securities issued by U.S. government agencies | | 7,139 | | 4 | | (11 | ) | — | | 7,132 | |
Total securities with a maturity of more than one year | | 54,981 | | 171 | | (55 | ) | — | | 55,097 | |
Subtotal | | $ | 401,900 | | $ | 531 | | $ | (130 | ) | $ | — | | $ | 402,301 | |
Equity securities: | | | | | | | | | | | |
Current portion (included in Other current assets) | | $ | 1,538 | | $ | 1,353 | | $ | — | | $ | (880 | ) | $ | 2,011 | |
Long-term portion (included in Deposits and other assets) | | 625 | | — | | — | | — | | 625 | |
Subtotal | | $ | 2,163 | | $ | 1,353 | | $ | — | | $ | (880 | ) | $ | 2,636 | |
| | $ | 404,063 | | $ | 1,884 | | $ | (130 | ) | $ | (880 | ) | $ | 404,937 | |
F-18
Investments we consider to be temporarily impaired at December 31, 2011 are as follows (in thousands):
| | | | Less than 12 months of
temporary��impairment | |
| | Number of
Investments | | Estimated
Fair Value | | Unrealized
Losses | |
Corporate debt securities | | 59 | | $ | 118,695 | | $ | (586 | ) |
Debt securities issued by U.S. government agencies | | 10 | | 33,685 | | (72 | ) |
Debt securities issued by states of the United States and political subdivisions of the states | | 5 | | 9,685 | | (26 | ) |
Total temporarily impaired securities | | 74 | | $ | 162,065 | | $ | (684 | ) |
We believe that the decline in value of these securities is temporary and primarily related to the change in market interest rates since purchase. We believe it is more likely than not that we will be able to hold these securities to maturity. Therefore we anticipate full recovery of their amortized cost basis at maturity.
5. Long-Term Obligations and Commitments
Long-term obligations consisted of the following (in thousands):
| | December 31, | |
| | 2011 | | 2010 | |
25/8 percent convertible subordinated notes | | $ | 141,448 | | $ | 132,895 | |
Long-term financing liability for leased facility | | 69,877 | | 10,147 | |
Equipment financing arrangement | | 5,325 | | 9,440 | |
Leases and other obligations | | 2,190 | | 1,925 | |
Total | | $ | 218,840 | | $ | 154,407 | |
Less: current portion | | (3,390 | ) | (5,645 | ) |
| | | | | |
Total Long-Term Obligations | | $ | 215,450 | | $ | 148,762 | |
Convertible Subordinated Notes
In January 2007, we completed a $162.5 million convertible debt offering, which raised proceeds of approximately $157.1 million, net of $5.4 million in issuance costs. We included the issuance costs in our balance sheet and are amortizing these costs to interest expense over the life of the debt. The $162.5 million convertible subordinated notes mature in 2027 and bear interest at 25/8 percent, which is payable in cash semi-annually. The 25/8 percent notes are convertible, at the option of the note holders, into approximately 11.1 million shares of our common stock at a conversion price of $14.63 per share. At December 31, 2011 and 2010, the principal and accrued interest payable on the notes was $162.5 million and $1.6 million, respectively. The fair value based on quoted market prices was $151.1 million and $162.3 million, at December 31, 2011 and 2010, respectively. We did not include the effect of the conversion of the note into our common stock in the computation of diluted net loss from continuing operations per share because the effect would have been anti-dilutive.
We will be able to redeem the 25/8 percent notes at a redemption price equal to 100.75 percent of the principal amount between February 15, 2012 and February 14, 2013; 100.375 percent of the principal amount between February 15, 2013 and February 14, 2014; and 100 percent of the principal amount thereafter. Holders of the 25/8 percent notes may also require us to repurchase these notes on February 15, 2014, February 15, 2017 and February 15, 2022, and upon the occurrence of certain defined conditions, at 100 percent of the principal amount of the 25/8 percent notes being repurchased plus unpaid interest.
We account for the 25/8 percent notes using an accounting standard which requires us to assign a value to our convertible debt equal to the estimated fair value of a similar debt instrument without the conversion feature that results in us recording our convertible debt at a discount. We are amortizing the resulting debt discount over the expected life of the debt as additional non-cash interest expense. Using a combination of the present value of the debt’s cash flows and a Black-Scholes valuation model, we determined that our nonconvertible debt borrowing rate for the 25/8 percent notes was 9.3 percent. Interest expense for the year ended December 31, 2011, 2010 and 2009 included $8.6 million, $7.8 million and $7.1 million, respectively, of non-cash interest expense related to the amortization of the debt discount.
F-19
Equipment Financing Arrangement
In October 2008, we entered into a loan agreement related to an equipment financing and in September 2009, we amended the loan agreement to increase the aggregate maximum amount of principal we could draw under the agreement. Under the amended loan agreement, we could borrow up to $18.4 million in principal to finance the purchase of equipment until July 2011. Each draw down under the loan agreement has a term of three years, with principal and interest payable monthly. We calculated interest on amounts we borrowed under the loan agreement based upon the three year interest rate swap at the time we made each draw down plus four percent. We are using the equipment purchased under the loan agreement as collateral. In 2011, we drew down an additional $1.6 million in principal under the loan agreement. As of December 31, 2011, we had drawn down $18.3 million in principal under this loan agreement at a weighted average interest rate of 6.19 percent. The carrying balance under this loan agreement at December 31, 2011 and 2010 was $5.3 million and $9.4 million, respectively.
Capital Lease
In 2010, we entered into a lease agreement associated with the purchase of certain office equipment. Since the lease contains a bargain purchase option, we classified it as a capital lease. At December 31, 2011 and 2010 we had approximately $656,000 and $773,000, respectively, outstanding under the lease. The lease bears interest at a rate of 5.14 percent and has a term of five years. We include the office equipment related to this capital lease in our property, plant and equipment. At December 31, 2011 and 2010, this equipment had a net book value of $585,000 and $705,000, respectively, which included $228,000 and $65,000, respectively, of accumulated depreciation.
Maturity Schedules
Annual debt and other obligation maturities, including fixed and determinable interest, at December 31, 2011 are as follows (in thousands):
2012 | | $ | 7,938 | |
2013 | | 6,325 | |
2014 | | 163,087 | |
2015 | | 230 | |
2016 | | 60 | |
Thereafter | | 1,140 | |
Subtotal | | $ | 178,780 | |
Less: current portion | | (3,390 | ) |
Less: fixed and determinable interest | | (9,941 | ) |
Less: debt discount | | (21,051 | ) |
Deferred rent | | 1,175 | |
Total | | $ | 145,573 | |
Operating Leases
We lease certain office equipment as well as office and laboratory space under non-cancelable operating leases with terms through December 2031. We are located in three buildings in Carlsbad, California, including a 176,000 square foot leased facility that is accounted for as a financing obligation discussed below. We currently occupy approximately 231,000 square feet of laboratory and office space, including a 28,704 square foot facility, which houses our manufacturing suites for our drug development business built to meet Good Manufacturing Practices, and a 25,792 square foot building adjacent to our manufacturing facility which we use for laboratory and office space and to support our manufacturing activities. The lease for our 28,704 square foot manufacturing facility expires in 2031 and has four five-year options to extend. Under the lease agreement we have the option to purchase the facility at the end of each year from 2016 through 2020, and at the end of 2026 and 2031. The lease for the 25,792 square foot facility has an initial term ending in June 2021 with an option to extend the lease for up to two five-year periods.
F-20
Annual future minimum payments under operating leases as of December 31, 2011 are as follows (in thousands):
| | Operating
Leases | |
2012 | | $ | 1,406 | |
2013 | | 1,423 | |
2014 | | 1,389 | |
2015 | | 1,332 | |
2016 | | 1,380 | |
Thereafter | | 21,979 | |
Total minimum payments | | $ | 28,909 | |
Rent expense for the years ended December 31, 2011, 2010 and 2009 was $4.6 million, $4.3 million and $4.6 million, respectively. In connection with certain of our leases, we recognize rent expense on a straight line basis over the lease term resulting in a deferred rent balance of $1.2 million and $793,000 at December 31, 2011 and 2010, respectively.
New Facility Lease Obligation
The leases on our former primary research and development facilities expired at the end of 2011. Rather than invest in costly renovations to these facilities, we chose to consolidate the majority of our operations in a new 176,000 square foot leased facility that BioMed Realty, L.P. constructed. To make our move to the new facility as efficient as possible, we requested access to the new facility prior to the completion of construction. To gain early access, we agreed to modify our lease with BioMed to accept additional responsibility. As a result, we recorded the costs for the facility as a fixed asset and we also recorded a corresponding liability. In July 2011, we took possession of the new facility. Therefore beginning in the third quarter of 2011, we began depreciating the cost of the facility over its economic useful life. At December 31, 2011, the facility and associated parcel of land had a net book value of $71.5 million, which included $945,000 of accumulated depreciation. Our rent payments began on January 1, 2012 and will decrease the liability over the term of the lease.
In conjunction with the lease agreement with BioMed, we purchased a parcel of land for $10.1 million and subsequently sold it to BioMed. Since we have the option to purchase the facility, including the land, we have continuing involvement in the land, which requires us to account for the purchase and sale of the land as a financing transaction. As such, our property, plant and equipment at December 31, 2011 and 2010 included the value of the land. Additionally, we have recorded a corresponding amount in our non-current liabilities as a long-term financing obligation. Since land is not a depreciable asset, the value of the land and financing obligation we recorded will not change until we exercise our purchase option or the lease terminates.
The lease on this new facility expires in 2031 and has four five-year options to extend. Under the lease agreement we have the option to purchase the facility and land at the end of each year from 2016 through 2020, and at the end of 2026 and 2031.
Annual future rent payments for the new facility as of December 31, 2011 are as follows (in thousands):
| | New
Facility Lease | |
2012 | | $ | 5,829 | |
2013 | | 5,829 | |
2014 | | 6,179 | |
2015 | | 6,179 | |
2016 | | 6,551 | |
Thereafter | | 119,000 | |
Total minimum payments | | $ | 149,567 | |
6. Stockholders’ Equity
Preferred Stock
We are authorized to issue up to 15,000,000 shares of “blank check” Preferred Stock. As of December 31, 2011, there were no shares of our Series A Convertible Exchangeable five percent Preferred Stock or Series B Convertible Exchangeable five percent Preferred Stock outstanding. We have designated Series C Junior Participating Preferred Stock but have no issued or outstanding shares as of December 31, 2011.
In December 2010, our Preferred Share Purchase Rights Plan expired.
F-21
Common Stock
At December 31, 2011 and 2010, we had 200,000,000 shares of common stock authorized, of which 100,042,976 and 99,393,780 were issued and outstanding, respectively. As of December 31, 2011, total common shares reserved for future issuance were approximately 21,833,053.
We issued 646,000 and 475,000 shares of common stock for stock option exercises and the Employee Stock Purchase Plan (“ESPP”) purchases for the years ending December 31, 2011 and 2010, respectively. We received net proceeds from these transactions of $3.6 million and $4.4 million in 2011 and 2010, respectively.
Stock Option Plans
1989 Stock Option Plan
In June 1989, our Board of Directors adopted, and the stockholders subsequently approved, a stock option plan that, as amended, provides for the issuance of non-qualified and incentive stock options for the purchase of up to 20,000,000 shares of common stock to our employees, directors, and consultants. The plan expires in January 2014. The 1989 Plan does not allow us to grant stock bonuses or restricted stock awards and prohibits us from repricing any options outstanding under the plan unless our stockholders approve the repricing. Options vest over a four-year period, with 25 percent exercisable at the end of one year from the date of the grant and the balance vesting ratably thereafter. Options we granted after May 26, 2004 have a term of seven years while options we granted before May 26, 2004 have a term of ten years. At December 31, 2011, a total of 7,520,400 options were outstanding, of which options to purchase 4,442,537 shares were exercisable, and 3,277,145 shares were available for future grant under the 1989 plan.
2000 Broad Based Equity Incentive Plan
In January 2000, we adopted the 2000 Broad-Based Equity Incentive Plan (the “2000 Plan”), which, as amended, provided for the issuance of non-qualified stock options for the purchase of up to 5,990,000 shares of common stock to our employees, directors, and consultants. Typically options expire seven or ten years from the date of grant. Options granted under this plan generally vest over a four-year period, with 25 percent exercisable at the end of one year from the date of the grant and the balance vesting ratably thereafter. At December 31, 2011, a total of 2,556,537 options were outstanding, of which 2,258,342 shares were exercisable, and no shares were available for future grant under the 2000 Plan. The 2000 Plan expired on January 5, 2010, so we may no longer grant new options under the 2000 Plan.
Change of Control Under 1989 Plan and 2000 Plan
With respect to both the 1989 Plan and 2000 Plan, in the event of:
· a sale, lease or other disposition of all or substantially all of our assets;
· a merger or consolidation in which we are not the surviving corporation; or
· reverse merger in which we are the surviving corporation but the shares of common stock outstanding immediately preceding the merger are converted by virtue of the merger into other property, whether in the form of securities, cash or otherwise,
then any surviving corporation or acquiring corporation will assume any stock awards outstanding under the 2000 Plan and the 1989 Plan or will substitute similar stock awards (including an award to acquire the same consideration paid to the shareholders in the transaction for those outstanding under the 2000 Plan and the 1989 Plan). In the event any surviving corporation or acquiring corporation refuses to assume such stock awards or to substitute similar stock awards for those outstanding under the 2000 Plan and the 1989 Plan, then with respect to stock awards held by participants whose continuous service has not terminated, such stock awards automatically vest in full and the stock awards will terminate if not exercised (if applicable) at or prior to such event.
2011 Equity Incentive Plan
In March 2011, our Board of Directors adopted, and the stockholders subsequently approved, a stock option plan that provides for the issuance of stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, and performance cash awards. The plan provides for the purchase of up to 2,000,000 shares of common stock for issuance to our employees, directors, and consultants. The plan expires in June 2021. The 2011 Plan does not allow us to reduce the exercise price of any outstanding stock options or stock appreciation rights or cancel any outstanding stock options or stock appreciation rights that
F-22
have an exercise price or strike price greater than the current fair market value of the common stock in exchange for cash or other stock awards unless our stockholders approve such action. Currently we anticipate awarding only options and restricted stock units awards to our employees, directors and consultants. Under the 2011 Plan, stock options cannot vest in a period of less than two years and restricted stock unit awards cannot vest in a period of less than three years. In January 2012, we granted restricted stock unit awards to our employees under the 2011 Plan which vest annually over a four year period. At December 31, 2011, no options or restricted stock units awards were outstanding or exercisable, and 2,000,000 shares were available for future grant under the 2011 Plan.
Under the 2011 Plan, we may issue a stock award with additional acceleration of vesting and exercisability upon or after a change in control. In the absence of such provisions, no such acceleration will occur. The stock options and restricted stock unit awards we issue to our chief executive officer and chief operating officer will accelerate upon a change of control, as defined in the 2011 Plan.
Corporate Transactions and Change in Control under 2011 Plan
In the event of certain significant corporate transactions, our Board of Directors has the discretion to take one or more of the following actions with respect to outstanding stock awards under the 2011 Plan:
· arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring entity (or its parent company);
· arrange for the assignment of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award to the surviving or acquiring corporation (or its parent company);
· accelerate the vesting and exercisability of a stock award followed by the termination of the stock award;
· arrange for the lapse of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award;
· cancel or arrange for the cancellation of a stock award, to the extent not vested or not exercised prior to the effective date of the corporate transaction, in exchange for cash consideration, if any, as the Board, in its sole discretion, may consider appropriate; and
· arrange for the surrender of a stock award in exchange for a payment equal to the excess of (a) the value of the property the holder of the stock award would have received upon the exercise of the stock award, over (b) any exercise price payable by such holder in connection with such exercise.
2002 Non-Employee Directors’ Stock Option Plan
In September 2001, our Board of Directors adopted, and the stockholders subsequently approved, an amendment and restatement of the 1992 Non-Employee Directors’ Stock Option Plan, which provides for the issuance of non-qualified stock options to our non-employee directors. The name of the resulting plan is the 2002 Non-Employee Directors’ Stock Option Plan (the “2002 Plan”). The 2002 Plan provides for the issuance of stock options to our non-employee directors for the purchase of up to 1,000,000 shares of our common stock. Options under this plan expire ten years from the date of grant. Options granted become exercisable in four equal annual installments beginning one year after the date of grant. At December 31, 2011, a total of 645,000 options were outstanding, 457,500 of the shares issued were exercisable and 199,500 shares were available for future grant under the 2002 Plan.
Employee Stock Purchase Plan
In June 2009, our Board of Directors adopted, and the stockholders subsequently approved, the amendment and restatement of the 2000 ESPP and we reserved an additional 150,000 shares of common stock for issuance thereunder. In each of the subsequent years, we reserved an additional 150,000 shares of common stock for the ESPP resulting in a total of 2,124,596 million shares authorized in the plan as of December 31, 2011. The ESPP permits full-time employees to purchase common stock through payroll deductions (which cannot exceed 10 percent of each employee’s compensation) at the lower of 85 percent of fair market value at the beginning of the purchase period or the end of each six-month purchase period. Under the amended and restated ESPP, employees must hold the stock they purchase for a minimum of six months from the date of purchase beginning with the offering ending in January 1, 2010. During 2011, employees purchased and we issued to employees 98,218 shares under the ESPP at prices ranging from $7.79 to $7.91 per share. At December 31, 2011, 191,088 shares were available for purchase under the ESPP.
Stock Option Activity and Stock-Based Compensation Expense
The following table summarizes the stock option activity for the year ended December 31, 2011 (in thousands, except per share and contractual life data):
| | Number of
Shares | | Weighted
Average Exercise
Price
Per Share | | Average
Remaining
Contractual Term | | Aggregate
Intrinsic
Value | |
| | | | | | (Years) | | | |
Outstanding at December 31, 2010 | | 9,811 | | $ | 11.54 | | | | | |
Granted | | 2,029 | | $ | 9.92 | | | | | |
Exercised | | (479 | ) | $ | 5.84 | | | | | |
Cancelled/forfeited/expired | | (639 | ) | $ | 13.18 | | | | | |
| | | | | | | | | |
Outstanding at December 31, 2011 | | 10,722 | | $ | 11.39 | | 3.81 | | $ | 2,259 | |
| | | | | | | | | |
Exercisable at December 31, 2011 | | 7,158 | | $ | 11.57 | | 2.90 | | $ | 2,245 | |
The weighted-average estimated fair values of options granted were $4.85, $5.53 and $7.27 for the years ended December 31, 2011, 2010 and 2009, respectively. The total intrinsic value of options exercised during the years ended December 31, 2011, 2010 and 2009 were $686,000, $905,000 and $9.2 million, respectively, which we determined as of the date of exercise. The amounts of cash received from the exercise of stock options were $2.8 million, $3.3 million and $11.9 million for the years ended December 31, 2011, 2010 and 2009, respectively. For the year ended December 31, 2011, the weighted-average fair value of options exercised was $7.27. As of December 31, 2011, total unrecognized compensation cost related to non-vested stock-based compensation plans was $7.6 million. We will adjust the total unrecognized compensation cost for future changes in estimated forfeitures. We expect to recognize this cost over a weighted average period of 1.3 years.
F-23
Stock-based Valuation and Compensation Expense Information
The following table summarizes stock-based compensation expense related to employee and non-employee stock options and the ESPP for the year ended December 31, 2011, 2010 and 2009 (in thousands), which was allocated as follows:
| | Year Ended December 31, | |
| | 2011 | | 2010 | | 2009 | |
Research and development | | $ | 8,527 | | $ | 10,148 | | $ | 10,977 | |
General and administrative | | 1,318 | | 2,011 | | 2,408 | |
Non-cash compensation expense related to stock options included in continuing operations | | 9,845 | | 12,159 | | 13,385 | |
Non-cash compensation (benefit) expense related to stock options included in discontinued operations | | — | | — | | (1,558 | ) |
Total | | $ | 9,845 | | $ | 12,159 | | $ | 11,827 | |
Determining Fair Value
Valuation. We utilize the Black-Scholes model as our method of valuing stock-based awards we have granted. We recognize the value of the portion of the award that we ultimately expect to vest as expense over the requisite service period as stock-based compensation expense in our consolidated statements of operations. We recognize compensation expense for all stock-based payment awards using the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), an entity recognizes compensation expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period.
We estimated the fair value of each stock option grant and the ESPP purchase rights on the date of grant using the Black-Scholes model with the following weighted-average assumptions (annualized percentages), which vary based on type of plan, for the years ended December 31, 2011, 2010 and 2009:
Employee Stock Options:
| | December 31, | |
| | 2011 | | 2010 | | 2009 | |
Risk-free interest rate | | 2.3 | % | 2.7 | % | 1.9 | % |
Dividend yield | | 0.0 | % | 0.0 | % | 0.0 | % |
Volatility | | 52.4 | % | 55.5 | % | 56.8 | % |
Expected life | | 5.3 years | | 5.1 years | | 4.9 years | |
Board of Director Stock Options:
| | December 31, | |
| | 2011 | | 2010 | | 2009 | |
Risk-free interest rate | | 2.9 | % | 2.7 | % | 3.4 | % |
Dividend yield | | 0.0 | % | 0.0 | % | 0.0 | % |
Volatility | | 52.8 | % | 57.7 | % | 61.5 | % |
Expected life | | 7.8 years | | 7.8 years | | 7.7 years | |
F-24
ESPP:
| | December 31, | |
| | 2011 | | 2010 | | 2009 | |
Risk-free interest rate | | 0.1 | % | 0.2 | % | 0.3 | % |
Dividend yield | | 0.0 | % | 0.0 | % | 0.0 | % |
Volatility | | 34.9 | % | 47.8 | % | 56.5 | % |
Expected life | | 6 months | | 6 months | | 6 months | |
Risk-Free Interest Rate. We base the risk-free interest rate assumption on observed interest rates appropriate for the term of our stock option plans or ESPP.
Dividend Yield. We base the dividend yield assumption on our history and expectation of dividend payouts. We have not paid dividends in the past and do not expect to in the future.
Volatility. We use an average of the historical stock price volatility of our stock for the Black-Scholes model. We computed the historical stock volatility based on the expected term of the awards.
Expected Life. The expected term of stock options we have granted represents the period of time that we expect them to be outstanding. For the 2002 Plan, we estimated the expected term of options we have granted based on historical exercise patterns. For the 1989 Plan and 2000 Plan, we estimated the expected term of options granted subsequent to January 1, 2008, based on historical exercise patterns. The expected term for stock options we have granted prior to January 1, 2008 was a derived output of the simplified method.
Forfeitures. We reduce stock-based compensation expense for estimated forfeitures. We estimate forfeitures at the time of grant and revise, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We estimate forfeitures based on historical experience. Our historical forfeiture estimates have not been materially different from our actual forfeitures.
Warrants
In April 2006, we granted the members of Symphony GenIsis Holdings LLC warrants to purchase 4.25 million shares of common stock at an exercise price of $8.93 per share. In April 2011, Symphony GenIsis Holdings LLC exercised the remaining warrants.
7. Income Taxes
We have net deferred tax assets relating primarily to net operating loss carryforwards, or NOL’s, and research and development tax credit carryfowards. Subject to certain limitations, we may use these deferred tax assets to offset taxable income in future periods. Since we have a history of losses and the likelihood of future profitability is not assured, we have provided a full valuation allowance for the deferred tax assets in our balance sheet as of December 31, 2011. If we determine that we are able to realize a portion or all of these deferred tax assets in the future, we will record an adjustment to increase their recorded value and a corresponding adjustment to increase income or additional paid in capital, as appropriate, in that same period.
Primarily as a result of the significant upfront funding that we received from our strategic alliance with Genzyme in 2008 and the gain we recognized on the sale of Ibis to AMI in January 2009, we had a substantial amount of taxable income in 2009. To reduce our tax liability, we offset a portion of the taxable income with our 2009 loss from continuing operations. We also used some of our NOL’s to reduce our federal income taxes in 2009. The tax law changes that were enacted with the 2008/2009 California Budget suspended our ability to use NOL’s to offset our California tax expense for 2009. However, we offset our California income tax liability to the fullest extent allowed under the tax regulations with our research and development tax credit carryforwards, which California tax regulations limit to 50 percent of our California liability. After using all of our allowable losses and tax credits to reduce our tax liability, our 2009 tax expense was $20.0 million.
We were required to allocate our 2009 tax expense between discontinued operations and continuing operations in our consolidated statement of operations. Accordingly, we recorded tax expense of $3.2 million in continuing operations and $16.8 million in discontinued operations in 2009.
We are subject to taxation in the United States and various state jurisdictions. Our tax years for 1993 and forward are subject to examination by the U.S. tax authorities and our tax years for 1989 and forward are subject to examination by the California tax authorities due to the carryforward of unutilized net operating losses and research and development credits. Our tax years for 2001, 2002, 2006 and 2007 are currently being audited by California’s Franchise Tax Board. We do not expect that the results of these examinations will have a material effect on our financial condition or results of operations.
F-25
The provision for taxes on earnings from continuing operations, were as follows (in thousands):
| | Year Ended December 31, | |
| | 2011 | | 2010 | |
Current: | | | | | |
| | | | | |
Federal | | $ | — | | $ | (73 | ) |
State | | 11 | | 165 | |
| | 11 | | 92 | |
Deferred: | | | | | |
| | | | | |
Federal | | — | | — | |
State | | — | | — | |
Foreign | | — | | — | |
| | — | | — | |
| | | | | |
Income Tax Expense | | $ | 11 | | $ | 92 | |
The reconciliation between the Company’s effective tax rate on income from continuing operations and the statutory U.S. tax rate is as follows (in thousands):
| | Year Ended December 31, | |
| | 2011 | | 2010 | | 2009 | |
| | | | | | | | | | | | | |
Pre tax income | | $ | (84,790 | ) | | | $ | (61,251 | ) | | | $ | (31,765 | ) | | |
| | | | | | | | | | | | | |
Statutory rate | | (29,677 | ) | 35.0 | % | (21,438 | ) | 35.0 | % | (11,118 | ) | 35.0 | % |
State income tax net of federal benefit | | (4,870 | ) | 5.7 | % | (3,518 | ) | 5.7 | % | (1,825 | ) | 5.7 | % |
Net change in federal valuation allowance | | 41,136 | | (48.5 | )% | 26,869 | | (43.9 | )% | 12,275 | | (38.6 | )% |
Tax credits | | (4,202 | ) | 5.0 | % | (3,175 | ) | 5.2 | % | 3,401 | | (10.7 | )% |
Expired NOL’s | | — | | — | | — | | — | | (879 | ) | 2.8 | % |
Noncontrolling interest | | 1,448 | | (1.7 | )% | 908 | | (1.5 | )% | 3,562 | | (11.2 | )% |
Deferred tax true-up | | (4,236 | ) | 5.0 | % | — | | — | | — | | — | |
Other | | 412 | | (0.5 | )% | 446 | | (0.7 | )% | (2,225 | ) | 7.0 | % |
Effective rate | | $ | 11 | | (0.1 | )% | $ | 92 | | (0.2 | )% | 3,191 | | (10.0 | )% |
| | | | | | | | | | | | | | | | |
Significant components of our deferred tax assets and liabilities as of December 31, 2011 and 2010 are as follows (in thousands):
| | Year Ended December 31, | |
| | 2011 | | 2010 | |
Deferred Tax Assets: | | | | | |
Net operating loss carryovers | | $ | 195,399 | | $ | 148,688 | |
R&D credits | | 44,970 | | 38,989 | |
Capitalized R&D | | 23,212 | | 26,932 | |
Deferred revenue | | 20,541 | | 38,409 | |
Accrued restructuring | | 10,888 | | 10,888 | |
Other | | 25,606 | | 19,396 | |
Total deferred tax assets | | $ | 320,616 | | $ | 283,302 | |
| | | | | |
Deferred Tax Liabilities: | | | | | |
Convertible debt | | $ | (9,426 | ) | $ | (12,275 | ) |
Intangible and capital assets | | (3,702 | ) | (5,156 | ) |
Net deferred tax asset | | $ | 307,488 | | $ | 265,871 | |
Valuation allowance | | (307,488 | ) | (265,871 | ) |
| | | | | |
Net deferreds | | $ | — | | $ | — | |
F-26
The deferred tax assets and liabilities shown above do not include certain deferred tax assets at December 31, 2011 and 2010 that arose directly from (or the use of which was postponed by) tax deductions related to equity compensation in excess of compensation recognized for financial reporting. Those deferred tax assets include non-qualified stock options and incentive stock options we issued. We will increase stockholders’ equity by approximately $10.3 million if and when we ultimately realize such deferred tax assets. We use tax return ordering for purposes of determining when excess tax benefits have been realized.
At December 31, 2011, we had federal and California tax net operating loss carryforwards of approximately $510.6 million and $428.1 million, respectively. The Federal and California tax loss carryforwards will expire at various dates starting in 2014, unless we use them before then. We also have federal and California research and development tax credit carryforwards of approximately $41.1 million and $16.0 million, respectively and federal Orphan Drug credits of $1.8 million. The Federal research and development tax credit carryforwards began expiring in 2004 and will continue to expire unless we use them before then. The California research and development tax credit carryforwards are available indefinitely. The difference between the tax loss carryforwards for federal and California purposes is attributable to the capitalization of research and development expenses for California tax purposes and the shorter carryforward periods related to the state loss carryforwards.
We analyze filing positions in all of the federal and state jurisdictions where we are required to file income tax returns, and all open tax years in these jurisdictions to determine if we have any uncertain tax positions on any of our income tax returns. We recognize the impact of an uncertain tax position on an income tax return at the largest amount that the relevant taxing authority is more-likely-than not to sustain upon audit. We do not recognize uncertain income tax positions if they have less than 50 percent likelihood of being sustained.
The following table summarizes the gross amounts of unrecognized tax benefits without regard to reduction in tax liabilities or additions to deferred tax assets and liabilities if such unrecognized tax benefits were settled.
Reconciliation of unrecognized tax benefits (in thousands):
Unrecognized tax benefits balance at January 1, 2011 | | $ | 8,968 | |
Decrease for prior period tax positions | | (97 | ) |
Increase for current period tax positions | | 963 | |
Unrecognized tax benefits balance at December 31, 2011 | | $ | 9,834 | |
The balance of unrecognized tax benefits at December 31, 2011 of $9.8 million are tax benefits that, if we recognize them, would not impact our effective tax rates as long as they remain subject to a full valuation allowance. The net effect on the deferred tax assets and corresponding decrease in the valuation allowance at December 31, 2011 resulting from unrecognized tax benefits is $742,000. We have not recognized any accrued interest and penalties related to unrecognized tax benefits during the year ended December 31, 2011 due to our NOL and research credit carryforwards. We do not foresee any material changes to unrecognized tax benefits within the next twelve months. We recognize interest and/or penalties related to income tax matters in income tax expense.
8. Collaborative Arrangements and Licensing Agreements
Traditional Pharmaceutical Alliances and Licensing
Biogen Idec
In January 2012, we entered into a global collaboration agreement with Biogen Idec to develop and commercialize ISIS-SMNRx for the treatment of spinal muscular atrophy, or SMA. Because SMA is a severe and rare disease, we may have a relatively rapid path to market.
Under the terms of the agreement, we received an upfront payment of $29 million and are eligible to receive up to $45 million in substantive milestone payments associated with the clinical development of ISIS-SMNRx prior to licensing. We are responsible for global development of ISIS-SMNRx through the completion of Phase 2/3 registrational clinical trials. Biogen Idec has the option to license ISIS-SMNRx until completion of the first successful Phase 2/3 trial. If Biogen Idec exercises its option, it will pay us a license fee and will assume global development, regulatory and commercialization responsibilities. We may also receive up to $150 million in substantive milestone payments if Biogen Idec achieves pre-specified regulatory milestones. We will earn the next milestone payment of $18 million if we initiate the first Phase 2/3 study for ISIS-SMNRx. In addition, we will receive up to double-digit royalties on sales of ISIS-SMNRx if Biogen Idec successfully develops and commercializes ISIS-SMNRx after option exercise.
F-27
Bristol-Myers Squibb
In May 2007, we entered into a collaboration agreement with Bristol-Myers Squibb to discover, develop and commercialize novel antisense drugs targeting proprotein convertase subtilisin/kexin type 9, or PCSK9. As part of the collaboration, Bristol-Myers Squibb selected the first development candidate, BMS-PCSK9Rx, to move into development. In addition to the $15 million upfront fee, we earned $8 million in milestone payments related to the development of BMS-PCSK9Rx. The collaboration ended in December 2011, and we regained the rights to discover and develop antisense drugs to target PCSK9.
During 2011, 2010 and 2009, we earned revenue of $2.4 million, $12.2 million and $9.1 million, respectively, from Bristol-Myers Squibb, which represented 2 percent, 11 percent and 8 percent, respectively, of our total revenue for those years. Our balance sheet at December 31, 2011 included deferred revenue of $126,000 related to our relationship with Bristol-Myers Squibb.
Eli Lilly and Company
In August 2001, we formed a broad strategic relationship with Eli Lilly and Company, which included a joint research collaboration. As part of the collaboration, Eli Lilly and Company licensed LY2181308, an antisense inhibitor of survivin, and LY2275796, an antisense inhibitor of eIF-4E, or eukaryotic initiation factor-4E. Eli Lilly and Company is responsible for the development of LY2181308.
As of December 31, 2011, we had earned $4.1 million in license fees and milestone payments related to the continued development of LY2181308. We may receive additional substantive milestone payments aggregating up to $25 million, including up to $5 million for the achievement of development milestones, up to $8 million for the achievement of regulatory milestones and up to $12 million for the achievement of commercialization milestones. In addition, we will receive royalties on future product sales of the drug. We will earn the next milestone payment of $5 million if Eli Lilly and Company initiates a Phase 3 study of LY2181308.
In December 2009, we reacquired LY2275796, which we renamed ISIS-EIF4ERx, and we are continuing to develop the drug. Eli Lilly and Company has the right to reacquire ISIS-EIF4ERx on predefined terms prior to the initiation of Phase 3 development. However, if we publicly disclose the results from a Phase 2 clinical study of ISIS-EIF4ERx:
· Eli Lilly and Company may license ISIS-EIF4ERx on the predefined terms;
· Eli Lilly and Company may tell us it is not interested in licensing ISIS-EIF4ERx, in which case we may license ISIS-EIF4ERx to another partner; or
· Eli Lilly and Company may offer to license ISIS-EIF4ERx on terms that are lower than the predefined terms, in which case we may license ISIS-EIF4ERx to another partner so long as the licensing terms we reach with the new partner are better than terms offered by Eli Lilly and Company and we have not publicly disclosed any results from a new clinical study of ISIS-EIF4ERx prior to reaching the agreement with the new partner.
During 2009, we earned revenue from our relationship with Eli Lilly and Company totaling $75,000. During 2011 and 2010, we did not earn any revenue from our relationship with Eli Lilly and Company.
Genzyme Corporation, a Sanofi company
In January 2008, we entered into a strategic alliance with Genzyme focused on the licensing and co-development of KYNAMRO and a research relationship. The license and co-development agreement provides Genzyme with exclusive worldwide rights for all therapeutic purposes to our patents and know-how related to KYNAMRO, including the key product related patents, and their foreign equivalents pending or granted in various countries outside the United States, including in the European Union via the European Patent Convention, Japan, Canada, Australia, South Africa and India. In addition, we agreed that we would not develop or commercialize another oligonucleotide-based compound designed to modulate apolipoprotein B-100 by binding to the messenger RNA, or mRNA, encoding apolipoprotein B-100, throughout the world.
The transaction, which closed in June 2008, included a $175 million licensing fee, a $150 million equity investment in our stock in which we issued Genzyme five million shares of our common stock, and a share of worldwide profits on KYNAMRO and follow-on drugs ranging from 30 percent to 50 percent of all commercial sales. We may also receive over $1.5 billion in substantive milestone payments if Genzyme achieves pre-specified events, including up to $750 million for the achievement of regulatory milestones and up to $825 million for the achievement of commercialization milestones. The next milestone payment we could earn under our agreement with Genzyme is $25 million when the FDA accepts the NDA for KYNAMRO.
F-28
Under this alliance, Genzyme is responsible for the continued development and commercialization of KYNAMRO. We agreed to supply the active pharmaceutical ingredient for KYNAMRO for the Phase 3 clinical trials and initial commercial launch. Genzyme is responsible for manufacturing the finished drug product for KYNAMRO, including the initial commercial launch supply, and, if approved, Genzyme will be responsible for the long term supply of KYNAMRO drug substance and finished drug product. As part of the agreement, we contributed the first $125 million in funding for the development costs of KYNAMRO and now we and Genzyme share development costs equally. In addition, as part of our alliance, Genzyme has a first right of negotiation for ISIS-SOD1Rx.
The license and co-development agreement for KYNAMRO will continue in perpetuity unless we or Genzyme terminate it earlier under the following situations:
· Genzyme may terminate the license and co-development agreement at any time by providing written notice to Isis;
· We may terminate the license and co-development agreement on a country-by-country basis or in its entirety upon Genzyme’s uncured failure to use commercially reasonable efforts to develop and commercialize KYNAMRO in the United States, France, Germany, Italy, Spain, the United Kingdom, Japan and Canada; and
· Either we or Genzyme may terminate the license and co-development agreement upon the other party��s uncured failure to perform a material obligation under the agreement.
Upon termination of the license and co-development agreement, the license we granted to Genzyme for KYNAMRO will terminate and Genzyme will stop selling the product. In addition, if Genzyme voluntarily terminates the agreement or we terminate the agreement in a country or countries for Genzyme’s failure to develop and commercialize KYNAMRO, then the rights to KYNAMRO will revert back to us and we may develop and commercialize KYNAMRO in the countries that are the subject of the termination, subject to a royalty payable to Genzyme.
If we are the subject of an acquisition, then within 180 days following the acquisition, Genzyme may elect to purchase all of our rights to receive payments under the KYNAMRO license and co-development agreement for a purchase price to be mutually agreed to by us and Genzyme, or, if we cannot agree, a fair market value price determined by an independent investment banking firm.
Genzyme has agreed to monthly limits on the number of shares it can sell of the Company’s stock that it purchased in February 2008. In addition, Genzyme has agreed that until the earlier of the 10 year anniversary of the KYNAMRO license and co-development agreement or the date Genzyme holds less than two percent of our issued and outstanding common stock, Genzyme will not acquire any additional shares of our common stock without our consent.
The price Genzyme paid for our common stock represented a significant premium over the fair value of our common stock. We are amortizing this $100 million premium along with the $175 million licensing fee that we received in the second quarter of 2008 ratably into revenue until June 2012, which represents the end of our performance obligation based on the current research and development plan. During 2011, 2010 and 2009, we earned revenue of $72.3 million, $66.9 million and $66.4 million, respectively, primarily related to the upfront payments we received from Genzyme, which represented 73 percent, 62 percent and 55 percent, respectively, of our total revenue for those years. Our balance sheets at December 31, 2011 and 2010 included deferred revenue of $27.7 million and $94.1 million, respectively, related to our relationship with Genzyme.
GlaxoSmithKline
In March 2010, we entered into a strategic alliance with GSK, which covers up to six programs, in which we use our antisense drug discovery platform to seek out and develop new drugs against targets for rare and serious diseases, including infectious diseases and some conditions causing blindness. This alliance allows us to control and facilitate rapid development of drugs while still being eligible to receive milestone payments as we advance these drugs in clinical development.
As of December 31, 2011, we have received $53 million from GSK, including the $35 million upfront payment, $15 million in development milestone payments and the $3 million payment we received in May 2011 when GSK expanded the collaboration by initiating a sixth program.
We are also eligible to receive on average up to $20 million in milestone payments per program up to Phase 2 proof-of-concept. GSK has the option to license drugs from these programs at Phase 2 proof-of-concept, and will be responsible for all further development and commercialization. If GSK successfully develops all six programs for one or more indications and achieves pre-agreed sales targets, we could receive license fees and substantive milestone payments of more than $1.4 billion, including up to $358.5 million for the achievement of development milestones, up to $581.5 million for the achievement of regulatory milestones and up to $495 million for the achievement of commercialization milestones. We will earn the next milestone payment of $2 million if we demonstrate in-vivo efficacy for the next drug discovery target. In addition, we will receive up to double-digit royalties on sales from any product that GSK successfully commercializes under this alliance.
F-29
During 2011 and 2010, we earned revenue of $17.7 million and $10.3 million, respectively, from our relationship with GSK, which represented 18 percent and nine percent, respectively, of our total revenue for those years. Our balance sheets at December 31, 2011 and 2010 included deferred revenue of $25.3 million and $29.8 million, respectively, related to the upfront and expansion payments.
Ortho-McNeil-Janssen Pharmaceuticals, Inc., formerly Ortho-McNeil, Inc.
In September 2007, we entered into a collaboration with Ortho-McNeil-Janssen Pharmaceuticals, or OMJP, to discover, develop and commercialize antisense drugs to treat metabolic diseases, including type 2 diabetes. As part of the collaboration, we granted OMJP worldwide development and commercialization rights to two of our diabetes programs, our glucagon receptor, or GCGR, and glucocorticoid receptor, or GCCR, programs. The collaboration ended and we regained the rights to drugs from both of these programs. We are developing more potent inhibitors for our GCGR and GCCR programs, which we identified as part of our collaboration with OMJP.
During 2009 we earned revenue of $18.4 million under this collaboration, which represented 15 percent of our total revenue for that year. During 2011 and 2010, we did not earn any revenue from our relationship with OMJP.
Satellite Company Collaborations
Achaogen, Inc.
In 2006, we exclusively outlicensed to Achaogen, Inc. specific know-how, patents and patent applications relating to aminoglycosides. In exchange, Achaogen agreed to certain payment obligations related to aminoglycosides Achaogen developed. Aminoglycosides are a class of small molecule antibiotics that inhibit bacterial protein synthesis and that physicians use to treat serious bacterial infections. Achaogen is developing plazomicin, an aminoglycoside Achaogen discovered based on the technology we licensed to Achaogen. Plazomicin has displayed broad-spectrum activity in animals against multi-drug resistant gram-negative bacteria that cause systemic infections, including E. coli. The compound has also demonstrated activity against methicillin-resistant staphylococcus aureus, or MRSA.
In connection with the license, Achaogen issued to us $1.5 million of Achaogen Series A Preferred Stock. Since early 2009, we have received $3 million from Achaogen, $500,000 which was in Achaogen securities, as Achaogen has advanced plazomicin in development. In addition, assuming Achaogen successfully develops and commercializes the first two drugs under our agreement, we may receive payments totaling up to $46.3 million for the achievement of key clinical, regulatory and sales events. We will also receive royalties on sales of drugs resulting from the program. Achaogen is solely responsible for the continued development of plazomicin. During 2010 and 2009, we earned $2 million and $500,000, respectively, in revenue from our relationship with Achaogen, which does not include any revenue from the equity we received from Achaogen. During 2011, we did not earn any revenue from our relationship with Achaogen. At December 31, 2011 and 2010, we owned less than 10 percent of Achaogen’s equity.
Alnylam Pharmaceuticals, Inc.
In March 2004, we entered into a strategic alliance with Alnylam to develop and commercialize RNA interference, or RNAi, therapeutics. Under the terms of the agreement, we exclusively licensed to Alnylam our patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry for double-stranded RNAi therapeutics in exchange for a $5 million technology access fee, participation in fees for Alnylam’s partnering programs, as well as future milestone and royalty payments from Alnylam. For each drug Alnylam develops under this alliance, we may receive up to $3.4 million in substantive milestone payments, including up to $1.1 million for the achievement of development milestones and $2.3 million for regulatory milestones. In December 2010, we earned a $375,000 milestone payment from Alnylam for the initiation of a Phase 1 study in their transthyretin, or TTR, program. We will earn the next milestone payment of $750,000 if Alnylam initiates a Phase 3 study for TTR. We retained rights to a limited number of double-stranded RNAi therapeutic targets and all rights to single-stranded RNAi, or ssRNAi, therapeutics. We also made a $10 million equity investment in Alnylam at the time of the agreement. During 2007, 2006 and 2005, we sold our holdings of Alnylam stock resulting in aggregate net cash proceeds of $12.2 million and a net gain on investments of $6.2 million. At December 31, 2011, we did not own any shares of Alnylam.
In turn, Alnylam nonexclusively licensed to us its patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry to research, develop and commercialize ssRNAi therapeutics and to research double-stranded RNAi compounds. We also received a license to develop and commercialize double-stranded RNAi drugs targeting a limited number of therapeutic targets on a nonexclusive basis. If we develop or commercialize an RNAi-based drug using Alnylam’s technology, we will pay Alnylam milestone payments and royalties. For each drug, the potential milestone payments to Alnylam total $3.4 million, which we will pay if we achieve specified development and regulatory events. As of December 31, 2011, we did not have an RNAi-based drug in clinical development. Our Alnylam alliance provides us with an opportunity to realize substantial value from our pioneering work in antisense mechanisms and oligonucleotide chemistry and is an example of our strategy to participate in all areas of RNA-targeting drug discovery.
F-30
In April 2009, we and Alnylam amended our strategic collaboration and license agreement to form a new collaboration focused on the development of ssRNAi technology. Under the terms of the amended collaboration and license agreement, Alnylam paid us an upfront license fee of $11 million plus $2.6 million of research and development funding in 2009 and 2010. In November 2010, Alnylam terminated the ssRNAi research program and we recognized $4.9 million of revenue from the upfront fee that we were amortizing into revenue over the research term. As a result, any licenses to ssRNAi products we granted to Alnylam under the agreement, and any of Alnylam’s obligations to pay milestone payments, royalties or sublicense payments to us for ssRNAi products under the agreement, terminated. We continue to advance the development of ssRNAi technology and during the course of the collaboration, we made improvements in the activity of ssRNAi compounds, including increased efficacy and potency and enhanced distribution.
As of December 31, 2011, we had earned a total of $37.1 million from Alnylam resulting from sublicenses of our technology for the development of RNAi therapeutics that Alnylam has granted to pharmaceutical partners.
During 2011, 2010 and 2009, we earned revenue from our relationship with Alnylam totaling $375,000, $10.3 million and $5.0 million, respectively, representing less than one percent, nine percent and four percent, respectively, of our total revenue for those years.
Antisense Therapeutics Limited
In December 2001, we licensed ATL1102 to ATL, an Australian company publicly traded on the Australian Stock Exchange and in February 2008, ATL licensed ATL1102 to Teva Pharmaceutical Industries Ltd. From early 2008 until early 2010, when Teva terminated the licensing agreement for ATL1102, we earned $3.4 million as Teva advanced the development of ATL1102. In March 2010, Teva terminated the licensing agreement for ATL1102 and returned rights to the drug to ATL.
In addition to ATL1102, ATL is currently developing ATL1103 for growth and sight disorders. ATL1103 is a product of our joint antisense drug discovery and development collaboration. Over the last two years, ATL has raised nearly $3 million that it is using to advance ATL1103. ATL pays us for access to our antisense expertise and for research and manufacturing services we may provide to ATL. In October 2009, we agreed to manufacture ATL1103 drug product for ATL in return for 18.5 million shares of ATL’s common stock. Additionally, ATL will pay royalties to us on sales of ATL1102 and ATL1103. We may also receive a portion of the fees ATL receives if it licenses ATL1102 or ATL1103.
At December 31, 2011 and 2010, we owned less than 10 percent of ATL’s equity. During 2011, 2010 and 2009, we earned revenue of $210,000, $35,000, and $401,000, respectively, from our relationship with ATL. Our balance sheet at December 31, 2010 included deferred revenue of $210,000 related to our relationship with ATL.
Atlantic Pharmaceuticals Limited, formerly Atlantic Healthcare (UK) Limited
In March 2007, we licensed alicaforsen to Atlantic Pharmaceuticals, a UK-based specialty pharmaceutical company founded in 2006, which is developing alicaforsen for the treatment of ulcerative colitis, or UC, and other inflammatory diseases. Atlantic Pharmaceuticals is initially developing alicaforsen for pouchitis, a UC indication, followed by UC and other inflammatory diseases. In exchange for the exclusive, worldwide license to alicaforsen, we received a $2 million upfront payment from Atlantic Pharmaceuticals in the form of equity. In September 2010, we participated in Atlantic Pharmaceuticals’ financing by agreeing to sell to Atlantic Pharmaceuticals alicaforsen drug substance in return for shares of Atlantic Pharmaceuticals’ common stock. At December 31, 2011 and 2010, we owned approximately 12 percent of Atlantic Pharmaceuticals’ equity. Because realization of the upfront equity payment is uncertain, we recorded a full valuation allowance.
Under the agreement, we could receive substantive milestone payments totaling up to $1.4 million for the achievement of regulatory milestones for multiple indications. We will earn the next milestone payment of $600,000 if Atlantic Pharmaceuticals submits an NDA for alicaforsen with the FDA. We will also receive royalties on product sales of alicaforsen. Atlantic Pharmaceuticals is solely responsible for the continued development of alicaforsen. In 2010, Atlantic Pharmaceuticals announced that in response to requests received from healthcare professionals, it was to supply alicaforsen under international Named Patient Supply regulations for patients with inflammatory bowel disease, or IBD. Atlantic Pharmaceuticals is currently pursuing opportunities to fund further development of alicaforsen. During 2011, 2010 and 2009, we did not earn any revenue from our relationship with Atlantic Pharmaceuticals.
F-31
AVI BioPharma, Inc., formerly Ercole Biotech, Inc.
In May 2003, we and Ercole entered an agreement in which each party cross-licensed its respective intellectual property related to alternative RNA splicing. As part of the agreement, we granted Ercole an additional license to some of our chemistry patents. Assuming Ercole successfully develops and commercializes a drug incorporating the splicing technology or chemistry we licensed to Ercole, we may receive payments totaling up to $21 million for the achievement of key clinical, regulatory and sales events. We will also receive royalties on sales of these drugs. Ercole is solely responsible for the continued development of its drugs.
Similarly, if we successfully develop and commercialize a drug incorporating the splicing technology Ercole licensed to us, we will pay Ercole up to $21 million for the achievement of key clinical, regulatory and sales events and will also pay royalties to Ercole on sales of these drugs. We currently do not have a drug incorporating Ercole’s technology in clinical development.
In March 2008, AVI BioPharma, Inc. acquired Ercole’s rights and obligations under the collaboration agreement. As a result of our collaboration agreement with Ercole, as part of the acquisition, we received a warrant to purchase 238,228 shares of AVI’s common stock at an exercise price of $0.1679 per share, and a warrant to purchase 207,757 shares of AVI’s common stock at an exercise price of $3.61 per share. During 2011, 2010 and 2009, we did not earn any revenue from our relationship with Ercole.
Excaliard Pharmaceuticals, Inc., a wholly owned subsidiary of Pfizer Inc.
In November 2007, we entered into a collaboration with Excaliard to discover and develop antisense drugs for the local treatment of fibrotic diseases, including scarring. We granted Excaliard an exclusive worldwide license for the development and commercialization of certain antisense drugs. Excaliard made an upfront payment to us in the form of equity and paid us $1 million in cash for the licensing of an antisense oligonucleotide drug targeting expression of connective tissue growth factor, or CTGF, that is activated during skin scarring following the wound healing process.
In 2010 and 2011, we participated in Excaliard’s financings at nominal amounts to maintain our ownership percentage. In December 2011, Pfizer Inc. acquired Excaliard. We received $4.4 million and we are eligible to receive up to an additional $9.6 million from the sale of our equity ownership in Excaliard to Pfizer Inc. In addition, we will continue to be eligible for milestone and royalty payments under our licensing agreement for EXC 001. Assuming Excaliard successfully develops and commercializes drugs it licenses from us, we may receive substantive milestone payments totaling up to $47.7 million for the achievement of key development and regulatory milestones, including up to $7.7 million for the achievement of development milestones and up to $40 million for the achievement of regulatory milestones. We will earn the next milestone payment of $1.5 million upon initiation of a Phase 3 study for EXC 001. We will also receive royalties on antisense drugs that Excaliard develops and commercializes. We may also receive a portion of the fees Excaliard receives if it licenses drugs from our collaboration.
At December 31, 2011, we owned no equity in Excaliard, and we have no remaining performance obligations. During 2011 we did not earn any revenue from our relationship with Excaliard. During 2010 and 2009, we earned revenue of $3,000 and $290,000, respectively, from our relationship with Excaliard.
iCo Therapeutics Inc.
In August 2005, we granted a license to iCo for the development and commercialization of iCo-007. iCo is developing iCo-007 for the treatment of various eye diseases caused by the formation and leakage of new blood vessels such as diabetic macular edema and diabetic retinopathy. iCo paid us a $500,000 upfront fee and may pay us substantive milestone payments totaling up to $48.4 million for the achievement of development and regulatory milestones for multiple indications, including up to $7.9 million for the achievement of development milestones and up to $40.5 million for the achievement of regulatory milestones. We will receive the next milestone payment of $4 million if iCo initiates a Phase 3 study for iCo-007. In addition, we will receive royalties on any product sales of this drug. Under the terms of the agreement, iCo is solely responsible for the development and commercialization of the drug. In September 2007, iCo initiated Phase 1 clinical trials for iCo-007 and we earned a milestone payment of $1.25 million in the form of 936,875 shares of iCo’s common stock.
Over the course of our relationship, iCo has paid us in a combination of cash, common stock and convertible notes. In February 2009, iCo completed a CAD $1.3 million financing to fund the completion of its Phase 1 clinical study of iCo-007. We participated in the financing at a nominal amount to maintain our ownership percentage. In January 2010, we exercised the warrants we held to purchase 1.1 million shares of iCo’s common stock and as a result our ownership in iCo at December 31, 2011 and 2010 was approximately 12 percent. During 2011, 2010 and 2009 we earned revenue of $7,000, $7,000 and $14,000, respectively, from our relationship with iCo.
F-32
OncoGenex Technologies Inc., a subsidiary of OncoGenex Pharmaceuticals Inc.
In November 2001, we established a drug development collaboration with OncoGenex, a biotechnology company committed to the development of cancer therapeutics for patients with drug resistant and metastatic cancers, to co-develop and commercialize OGX-011, an anti-cancer antisense drug that targets clusterin. In July 2008, we and OncoGenex amended the co-development agreement pursuant to which OncoGenex became solely responsible for the costs, development and commercialization of OGX-011. In exchange, OncoGenex agreed to pay us royalties on sales of OGX-011 and to share consideration it receives from licensing OGX-011 to a third party, except for consideration OncoGenex receives for the fair market value of equity and reimbursement of research and development expenses.
Under the amended agreement, we assigned to OncoGenex our rights in the patents claiming the composition and therapeutic methods of using OGX-011 and granted OncoGenex a worldwide, nonexclusive license to our know-how and patents covering our core antisense technology and manufacturing technology solely for use with OGX-011. The key product-related patent that we assigned to OncoGenex was U.S. Patent number 6,900,187 having an expiration date of at least 2020; and the key core antisense technology patents we licensed OncoGenex are U.S. Patent number 7,919,472 having an expiration date of 2026 and its foreign equivalents pending in Australia, Canada, the European Patent Convention and Japan. In addition, we agreed that so long as OncoGenex or its commercialization partner is using commercially reasonable efforts to develop and commercialize OGX-011, we will not research, develop or commercialize an antisense compound designed to modulate clusterin. The amended agreement will continue until OncoGenex or its commercialization partner is no longer developing or commercializing OGX-011 or until we terminate the agreement for OncoGenex’s uncured failure to make a payment required under the agreement.
In December 2009, OncoGenex granted Teva the exclusive worldwide right and license to develop and commercialize any products containing OGX-011 and related compounds, with OncoGenex having an option to co-promote OGX-011 in the United States and Canada, for which we received $10 million of the upfront payment OncoGenex received from Teva. We are also eligible to receive 30 percent of up to $370 million in payments OncoGenex may receive from Teva in addition to royalties on sales of OGX-011 ranging between 3.88 percent and seven percent. Under the agreement, this royalty is due on a country- by-country basis until the later of ten years following the first commercial sale of OGX-011 in the relevant country, and the expiration of the last patent we assigned or licensed to OncoGenex that covers the making, using or selling of OGX-011 in such country.
To facilitate the execution and performance of OncoGenex’s agreement with Teva, we and OncoGenex amended our license agreement primarily to give Teva the ability to cure any future potential breach by OncoGenex under our agreement. As part of this amendment, OncoGenex agreed that if OncoGenex is the subject of a change of control with a third party, where the surviving entity immediately following such change of control has the right to develop and sell OGX-011, then a payment of $20 million will be due and payable to us 21 days following the first commercial sale of the product in the United States. Any non-royalty payments OncoGenex previously paid to us are creditable towards the $20 million payment, so as a result of the $10 million payment we received from OncoGenex related to its license to Teva, the remaining amount owing in the event of a change of control as discussed above is a maximum of $10 million.
In August 2003, we and OncoGenex entered into a separate collaboration and license agreement for the development of a second-generation antisense anti-cancer drug, OGX-225. OncoGenex is responsible for all development costs and activities, and we have no further performance obligations. OncoGenex issued to us $750,000 of OncoGenex securities as payment for an upfront fee. In addition, OncoGenex will pay us substantive milestone payments totaling up to $3.5 million for the achievement of development and regulatory milestones, including up to $1.5 million for the achievement of development milestones and up to $2 million for the achievement of regulatory milestones. In addition, we will receive royalties on future product sales of the drug. As of December 31, 2011, OncoGenex had not achieved any milestone events related to OGX-225. We will earn the next milestone payment of $500,000 if OncoGenex initiates a Phase 2 study for OGX-225.
In January 2005, we entered into a further agreement with OncoGenex to allow for the development of an additional second-generation antisense anti-cancer drug. Under the terms of the agreement, OncoGenex is responsible for all development costs and activities, and we have no further performance obligations. In April 2005, OncoGenex selected OGX-427 to develop under the collaboration. OncoGenex will pay us substantive milestone payments totaling up to $5.8 million for the achievement of key development and regulatory milestones, including up to $1.3 million for the achievement of development milestones and up to $4.5 million for the achievement of regulatory milestones. In addition, we will receive royalties on future product sales of the drug. In January 2011, we earned a $750,000 milestone payment related to OncoGenex’s Phase 2 trial in men with metastatic prostate cancer. We will earn the next milestone payment of $1.3 million if OncoGenex initiates a Phase 3 study for OGX-427.
During 2009, we sold the OncoGenex common stock we owned resulting in net cash proceeds of $2.8 million. We no longer own any shares of OncoGenex. During 2011 and 2009, we earned $750,000 and $11.4 million, respectively, in revenue from our relationship with OncoGenex. During 2010, we did not earn any revenue from our relationship with OncoGenex. Our balance sheet at December 31, 2010 included deferred revenue of $750,000 related to our relationship with OncoGenex.
F-33
Xenon Pharmaceuticals Inc.
In November 2010, we established a collaboration with Xenon to discover and develop antisense drugs as novel treatments for the common disease anemia of inflammation, or AI. AI is the second most common form of anemia worldwide and physicians associate a wide variety of conditions including infection, cancer and chronic inflammation with AI. We received an upfront payment in the form of a convertible promissory note from Xenon to discover and develop antisense drugs to the targets hemojuvelin and hepcidin. In addition to license and option fees, we are eligible to receive development and commercial milestone payments and royalties on sales of drugs licensed to Xenon under the collaboration and a portion of sublicense revenue. If Xenon identifies a development candidate, Xenon may take an exclusive license for the development and worldwide commercialization for that development candidate.
Under our collaboration agreement with Xenon we may receive up to $300 million in substantive milestone payments for multiple indications upon the achievement of pre-specified events, including up to $30 million for the achievement of development milestones, up to $150 million for the achievement of regulatory milestones and up to $120 million for the achievement of commercialization milestones. We will earn the next milestone payment of $2 million if Xenon selects a development candidate. During 2011, we earned revenue of $80,000 and during 2010 we did not earn any revenue from our relationship with Xenon.
External Project Funding
CHDI Foundation, Inc.
In August 2011, we renewed our collaboration with CHDI, which we initially entered into in November 2007, to provide us with funding for the discovery and development of an antisense drug for the treatment of Huntington’s disease. CHDI’s funding builds upon an earlier successful collaboration between us and CHDI, in which CHDI funded proof-of-concept studies that demonstrated the feasibility of using antisense drugs to treat Huntington’s disease. Under the terms of the new collaboration, we will receive funding from CHDI to identify and conduct IND-enabling studies on an antisense drug targeting the huntingtin gene. If we grant a license to a third party to commercialize our Huntington’s disease program, we and CHDI will evenly split the proceeds from the license until CHDI has recouped its funding together with a return determined at a predefined interest rate. Thereafter, we will keep the full amount of any additional proceeds. In addition, CHDI reimbursed us for approximately $1.6 million of research-related expenses we incurred after the earlier collaboration ended in 2010, which we are amortizing over the period of our performance obligation. During 2011 and 2009, we earned revenue of $2.4 million and $1.7 million, respectively, from our relationship with CHDI. In 2010, we did not earn any revenue from our relationship with CHDI. Our balance sheet at December 31, 2011 included deferred revenue of $568,000 related to our relationship with CHDI.
ALS Association; The Ludwig Institute; Center for Neurological Studies; Muscular Dystrophy Association
In October 2005, we entered into a collaboration agreement with the Ludwig Institute, the Center for Neurological Studies and researchers from these institutions to discover and develop antisense drugs in the areas of amyotrophic lateral sclerosis, or ALS, and other neurodegenerative diseases. Under this agreement, we agreed to pay the Ludwig Institute and Center for Neurological Studies modest milestone payments and royalties on any antisense drugs resulting from the collaboration. The researchers from the Ludwig Institute and Center for Neurological Studies, through funding from the ALS Association and the Muscular Dystrophy Association, conducted IND-enabling preclinical studies of ISIS-SOD1RX. The ALS Association and the Muscular Dystrophy Association also provided funding to offset the costs of the Phase 1 study of ISIS-SOD1RX. Except for the funding the ALS Association and the Muscular Dystrophy Association provided, we control and are responsible for funding the continued development of ISIS-SOD1RX.
F-34
Intellectual Property Sale and Licensing Agreements
Out-Licensing Arrangements; Royalty Sharing Agreements; Sales of IP
Abbott Molecular Inc.
In January 2009, we sold our former subsidiary, Ibis Biosciences, to AMI pursuant to a stock purchase agreement for a total acquisition price of $215 million plus the earn out payments described below.
Under the stock purchase agreement, AMI will pay us earn out payments equal to a percentage of Ibis’ revenue related to sales of Ibis systems, including instruments, assay kits and successor products, from the date of the acquisition closing through December 31, 2025. The earn out payments will equal five percent of Ibis’ cumulative net sales over $140 million and up to $2.1 billion, and three percent of Ibis’ cumulative net sales over $2.1 billion. AMI may reduce these earn out payments from five percent to as low as 2.5 percent and from three percent to as low as 1.5 percent, respectively, upon the occurrence of certain events. During 2011, 2010 and 2009 we did not earn any revenue from our relationship with AMI.
Eyetech Pharmaceuticals, Inc.
In December 2001, we licensed to Eyetech certain of our patents necessary for Eyetech to develop, make and commercialize Macugen, a non-antisense drug for use in the treatment of ophthalmic diseases, that Eyetech is developing and commercializing with Pfizer Inc. Eyetech paid us a $2 million upfront fee and agreed to pay us for the achievement of pre-specified events and royalty payments in exchange for non-exclusive, worldwide rights to the intellectual property licensed from us. During 2004, we earned $4 million in payments, and our license with Eyetech may also generate additional payments aggregating up to $2.8 million for the achievement of specified regulatory events with respect to the use of Macugen for each additional therapeutic indication. Prior to 2010, we had assigned our rights to receive royalties for Macugen to Drug Royalty Trust 3. During 2009, because of our agreement with Drug Royalty Trust 3, we did not earn any revenue from our relationship with Eyetech. In 2011 and 2010, we earned $790,000 and $567,000, respectively, of revenue related to royalties for Macugen under our license to Eyetech.
Roche Molecular Systems
In October 2000, we licensed some of our novel chemistry patents to Roche Molecular Systems, a business unit of Roche Diagnostics, for use in the production of Roche Molecular Systems’ diagnostic products. The royalty-bearing license grants Roche Molecular Systems non-exclusive worldwide access to some of our proprietary chemistries in exchange for initial and ongoing payments from Roche Molecular Systems to us. In April 2011, we expanded our relationship with Roche Molecular Systems by granting Roche Molecular Systems a non-exclusive license to additional technology for research and diagnostic uses. During 2011, 2010 and 2009, we earned revenue of $828,000, $1.8 million and $1.3 million, respectively, from our relationship with Roche Molecular Systems. Our balance sheet at December 31, 2011 included deferred revenue of $300,000 related to our agreements with Roche Molecular Systems.
In-Licensing Arrangements
Idera Pharmaceuticals, Inc., formerly Hybridon, Inc.
We have an agreement with Idera under which we acquired an exclusive license to all of Idera’s antisense chemistry and delivery technology related to our second generation antisense drugs and to double-stranded siRNA therapeutics. Idera retained the right to practice its licensed antisense patent technologies and to sublicense their technologies to collaborators under certain circumstances. In addition, Idera received a non-exclusive license to our suite of ribonuclease H, or RNase H, patents. During 2011, 2010 and 2009 we earned revenue of $10,000, $20,000 and $10,000, respectively, from our relationship with Idera.
Integrated DNA Technologies, Inc.
In March 1999, we further solidified our intellectual property leadership position in antisense technology by licensing certain antisense patents from Integrated DNA Technologies, Inc., or IDT, a leading supplier of antisense inhibitors for research. The patents we licensed from IDT are useful in functional genomics and in making our second-generation chemistry. We expect these patents will expire in February 2013. Under the license, we paid IDT $4.9 million in license fees in 2001 and we will pay royalties on sales of any drugs utilizing the technology we licensed from IDT until the patents expire.
F-35
University of Massachusetts
We have a license agreement with the University of Massachusetts under which we acquired an exclusive license to the University of Massachusetts’ patent rights related to ISIS-SMNRx. If we successfully develop and commercialize a drug incorporating the technology we licensed from the University of Massachusetts, we will pay milestone payments to the University of Massachusetts totaling up to $500,000 for the achievement of key clinical and regulatory milestones. In addition, we will pay the University of Massachusetts a portion of any sublicense revenue we receive from sublicensing its technology, and a royalty on sales of ISIS-SMNRx in the United States if our product incorporates the technology we licensed from the University of Massachusetts.
Verva Pharmaceuticals Ltd.
We have a license agreement with Verva under which we acquired an exclusive license to Verva’s antisense patent rights related to ISIS-FGFR4Rx. If we successfully develop and commercialize a drug incorporating the technology Verva licensed to us, we will pay milestone payments to Verva totaling up to $6.1 million for the achievement of key patent, clinical, and regulatory milestones. If we convert our license from an exclusive license to a nonexclusive license we could significantly reduce the milestone payments due to Verva. In addition, we will also pay royalties to Verva on sales of ISIS-FGFR4Rx if our product incorporates the technology we licensed from Verva.
Cold Spring Harbor Laboratory
We have a collaboration and license agreement with the Cold Spring Harbor Laboratory under which we acquired an exclusive license to the Cold Spring Harbor Laboratory’s patent rights related to ISIS-SMNRx. If we successfully develop and commercialize a drug incorporating the technology we licensed from the Cold Spring Harbor Laboratory, we will pay milestone payments to the Cold Spring Harbor Laboratory totaling up to $800,000 for the achievement of key clinical and regulatory milestones. In addition, we will pay the Cold Spring Harbor Laboratory a portion of any sublicense revenue we receive from sublicensing the Cold Spring Harbor Laboratory’s technology, and a royalty on sales of ISIS-SMNRx if our product incorporates the technology we licensed from the Cold Spring Harbor Laboratory.
9. Segment Information and Concentration of Business Risk
Segment Information
Prior to 2011, we reported our results in two separate segments: Drug Discovery and Development and Regulus. Beginning in 2011, we no longer consider Regulus as an operating segment because our chief decision making officer no longer reviews Regulus’ operating results for purposes of making resource allocations. Therefore we now only operate in, and will provide financial information and results for, our Drug Discovery and Development operations.
Our Drug Discovery and Development operations generate revenue from collaborations with corporate partners and from licensing proprietary patent rights. Revenue from collaborations with corporate partners may consist of upfront payments, funding for research and development activities, milestone payments and royalties or profit sharing payments. Our proprietary technology to discover and characterize novel antisense inhibitors has enabled our scientists to modify the properties of our antisense drugs for optimal use with particular targets and thus, to produce a broad proprietary portfolio of drugs applicable to many disease targets.
Concentrations of Business Risk
We have historically funded our operations from collaborations with corporate partners and a relatively small number of partners have accounted for a significant percentage of our revenue. Revenue from significant partners, which is defined as 10 percent or more of our total revenue, was as follows:
| | 2011 | | 2010 | | 2009 | |
Partner A | | 73 | % | 62 | % | 55 | % |
Partner B | | 2 | % | 11 | % | 8 | % |
Partner C | | 0 | % | 0 | % | 15 | % |
Partner D | | 18 | % | 9 | % | 2 | % |
Contract receivables from one significant partner comprised approximately 85 percent of our contract receivables at December 31, 2011. Contract receivables from two significant partners comprised approximately 30 percent and 15 percent of our contract receivables at December 31, 2010. Included in our contract receivables at December 31, 2010 was $544,000, representing 44 percent of our contract receivables, due from Regulus.
F-36
10. Employee Post Employment Benefits
We have an employee 401(k) salary deferral plan, covering all employees. Employees may make contributions by withholding a percentage of their salary up to the IRS annual limit ($16,500 and $22,000 in 2011 for employees under 50 years old and over 50 years old, respectively). We made approximately $487,000, $449,000 and $450,000 in matching contributions for the years ended December 31, 2011, 2010 and 2009, respectively.
11. Legal Proceedings
In September 2011, we filed a patent infringement lawsuit against Santaris Pharma A/S and Santaris Pharma A/S Corp. in the United States District Court of the Southern District of California. Our infringement lawsuit alleges that Santaris’ activities providing antisense drugs and antisense drug discovery services to several pharmaceutical companies infringes U.S. Patent No. 6,326,199, entitled “Gapped 2’ Modified Oligonucleotides” and U.S. Patent No. 6,066,500, entitled “Antisense Modulation of Beta Catenin Expression.” In the lawsuit we are seeking monetary damages and an injunction enjoining Santaris from conducting or participating in the infringing activities. In December 2011, Santaris filed an answer to our complaint, denying our allegations, and seeking a declaration from the court that Santaris has not, and does not, infringe the patents we asserted against Santaris in the suit. In January 2012, Santaris filed a motion for summary judgment asking the court to decide as a matter of law that Santaris’ activities do not infringe the patents we assert in the suit.
In January 2012, Alnylam Pharmaceuticals, Inc. filed a patent infringement lawsuit against Tekmira Pharmaceuticals Corporation in the U.S. District Court of the District of Massachusetts. Alnylam’s lawsuit alleges Tekmira has infringed a number of issued patents related to siRNA and LNP technologies, including: U.S. Patent No. 7,695,902; U.S. Patent No. 6,858,225; U.S. Patent No. 6,815,432; U.S. Patent No. 6,534,484; U.S. Patent No. 6,586,410; and, U.S. Patent No. 6,858,224. Under Alnylam’s contractual right to enforce Isis’ patent U.S. Patent No. 7,695,902, Alnylam joined us to the suit as a co-plaintiff.
12. Quarterly Financial Data (Unaudited)
The following financial information reflects all normal recurring adjustments, which are, in the opinion of management, necessary for a fair statement of the results of the interim periods. Summarized quarterly data for the years ended December 31, 2011 and 2010 are as follows (in thousands, except per share data).
| | First
Quarter | | Second
Quarter | | Third
Quarter | | Fourth
Quarter | |
2011 Quarters | | | | | | | | | |
Revenue | | $ | 21,147 | | $ | 24,823 | | $ | 20,713 | | $ | 32,403 | |
Operating expenses | | 37,255 | | 38,883 | | 43,029 | | 51,019 | |
Loss from operations | | (16,108 | ) | (14,060 | ) | (22,316 | ) | (18,616 | ) |
Net loss attributable to Isis Pharmaceuticals, Inc. common stockholders | | $ | (19,994 | ) | $ | (17,889 | ) | $ | (26,882 | ) | $ | (20,036 | ) |
Basic and diluted net loss attributable to Isis Pharmaceuticals, Inc. common stockholders(1) | | $ | (0.20 | ) | $ | (0.18 | ) | $ | (0.27 | ) | $ | (0.20 | ) |
| | First
Quarter | | Second
Quarter | | Third
Quarter | | Fourth
Quarter | |
2010 Quarters | | | | | | | | | |
Revenue | | $ | 29,926 | | $ | 23,503 | | $ | 28,624 | | $ | 26,420 | |
Operating expenses | | 34,806 | | 42,175 | | 37,571 | | 42,277 | |
Loss from operations | | (4,880 | ) | (18,672 | ) | (8,947 | ) | (15,857 | ) |
Net loss attributable to Isis Pharmaceuticals, Inc. common stockholders | | $ | (9,658 | ) | $ | (25,154 | ) | $ | (12,454 | ) | $ | (13,985 | ) |
Basic and diluted net loss attributable to Isis Pharmaceuticals, Inc. common stockholders(1) | | $ | (0.10 | ) | $ | (0.25 | ) | $ | (0.13 | ) | $ | (0.14 | ) |
(1) We computed net loss per share independently for each of the quarters presented. Therefore, the sum of the quarterly net loss per share will not necessarily equal the total for the year.
F-37


